SUMMARY
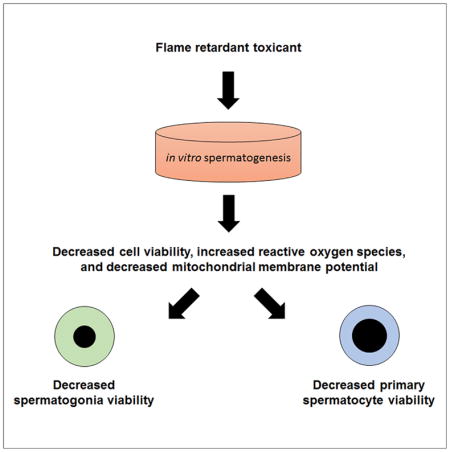
Sperm counts have rapidly declined in Western males over the past four decades. This rapid decline remains largely unexplained, but exposure to environmental toxicants provides one potential explanation for this decline. Flame retardants are highly prevalent and persistent in the environment, but many have not been assessed for their effects on human spermatogenesis. Using a human stem cell-based model of spermatogenesis, we evaluated two major flame retardants, hexabromocyclododecane (HBCDD) and tetrabromobisphenol A (TBBPA), under acute conditions simulating occupational-level exposures. Here we show that HBCDD and TBBPA are human male reproductive toxicants in vitro. Although these toxicants do not specifically affect the survival of haploid spermatids, they affect spermatogonia and primary spermatocytes through mitochondrial membrane potential perturbation and reactive oxygen species generation, ultimately causing apoptosis. Taken together, these results show that HBCDD and TBBPA affect human spermatogenesis in vitro and potentially implicate this highly prevalent class of toxicants in the decline of Western males’ sperm counts.
Graphical Abstract
INTRODUCTION
Semen parameters, including sperm counts, in the Western males have declined rapidly since the 1970s, with no indication of leveling off (Levine et al., 2017). Between 1973 and 2011, sperm counts have decreased by over 50%, with an average of a greater than 1% decline per year (Levine et al., 2017). It is uncertain if these declines are seen in other world regions owing to sparse studies in developing nations (Deonandan and Jaleel, 2012). To date, nearly 15% of couples—roughly 50 to 80 million worldwide—are estimated to experience infertility (Khosrorad et al., 2015). Of these couples, male factor infertility accounts for 30% of cases and is a contributing factor in roughly another 30% (Quaas and Dokras, 2008). Should sperm counts continue to decline, cases of infertility may continue to rise. Chemical exposure has been linked to declines in male fertility and may be responsible for declining semen parameters in the Western world (Bloom et al., 2015). Polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs), which belong to a class of chemicals known as halogenated flame retardants (FRs), have been implicated in male reproductive issues, including reduced sperm motility, abnormal sperm morphology, endocrine-disrupting activity, and changes in reproductive organs, and are hypothesized to affect male fecundity, among other concerns (Meeker and Hauser, 2010). Although these chemicals have been phased out due to their adverse impacts on human health, replacement halogenated FRs have taken their place on the market. Although advertised as safer alternatives to their predecessors, limited data exist regarding their impacts on human health, including male fertility and spermatogenesis.
Hexabromocyclododecane (HBCDD) and tetrabromobisphenol A (TBBPA) are replacement halogenated FRs that can be found as additives to products such as rigid foam insulation, textiles, high-impact polystyrene, and electrical equipment (Covaci et al., 2006; Agency, 2014; van der Veen and de Boer, 2012; Betts, 2013; United Nations Environment Programme, 2004; Stapleton et al., 2011; E.C.B. European Commission Directrate-General Joint Research Center, 2006; Schecter et al., 2012). HBCDD and TBBPA are among the most widely used FRs globally, with TBBPA accounting for 25% of the global FR demand (Peverly et al., 2014; Hu et al., 2014; E.C.B. European Commission Directrate-General Joint Research Center, 2006; Wang et al., 2015; Jarosiewicz and Bukowska, 2017). HBCDD and TBBPA have been detected in house dust of 97% and 80% of homes sampled worldwide, respectively, highlighting their widespread distribution (Schecter et al., 2012; Johnson et al., 2013; Wang et al., 2015; Meeker and Stapleton, 2010; Betts, 2013; Dodson et al., 2012). Owing to the lipophilic nature of this class of chemicals, HBCDD and TBBPA readily enter the human body through inhalation, dermal contact, or ingestion of contaminated food and have been detected in a range of human tissues including blood, adipose tissue, breast milk, and urine (Agency, Schecter et al., 2012; Johnson et al., 2013; Fromme et al., 2016b; Bjermo et al., 2017; Fromme et al., 2016a; Rawn et al., 2014b; Darnerud et al., 2011; Rawn et al., 2014a; Betts, 2013; van der Veen and de Boer, 2012; Carignan et al., 2013; E.C.B. European Commission Directrate-General Joint Research Center, 2006; Ke, 2002; Jakobsson et al., 2002; Thomsen et al., 2001; Thomsen et al., 2002a; Thomsen et al., 2002b; Agency; Blum et al., 1978; Abafe and Martincigh, 2016).
Despite the high prevalence of HBCDD and TBBPA, there is a significant lack of understanding regarding how these chemicals affect human health, particularly in individuals exposed to higher than average concentrations. The risk of occupational exposure is estimated to be upward of 70% in workers responsible for the production and processing of HBCDD (Yi et al., 2016). Similarly, industrial workers have been shown to have HBCDD in their blood with some having concentrations greater than 800 times the concentrations of HBCDD found in non-occupationally exposed populations (Thomsen et al., 2007; Li et al., 2014). Similarly, in one study that assessed the concentration of TBBPA in the blood serum of occupationally exposed workers, TBBPA was found at concentrations as high as 3.4 pmol/g (Jakobsson et al., 2002). However, some studies of the general population’s exposure have shown higher concentrations, reporting concentrations as high as 93 ng/g (0.171 μM) TBBPA in blood (Cariou et al., 2008). In addition, other halogenated FRs of similar prevalence have been reported at still higher concentrations, with the halogenated FR TDCPP detectable in human tissues at 10,490 ng/g (24.3 μM) (Liu et al., 2016).
Despite the knowledge that HBCDD and TBBPA are entering and accumulating within the bodies of occupationally exposed workers and the history the effect of their predecessors, PCBs and PBDEs, on human spermatogenesis, no studies on the impacts of HBCDD or TBBPA on human spermatogenesis have been reported. As predicted, human endocrine disruptors, HBCDD and TBBPA, have been shown to correlate with changes in human male hormonal systems (Johnson et al., 2013; Meeker and Stapleton, 2010; Yard et al., 2011; Gold et al., 1978). The US Environmental Protection Agency predicts that HBCDD is a moderate hazard to human reproductive health, including adverse effects on gamete production (Agency, 2014). However, this designation is based on reduced primordial follicles in female mice (Agency, 2014). There is evidence that TBBPA targets the testis, although no analysis of the effects of TBBPA on human spermatogenesis has been conducted for any population (Choi et al., 2011). However, in animal models, TBBPA can cause changes in genes required for spermatogenesis, and TBBPA has been shown to decrease the number of mouse spermatogonia and affect the cell cycle of spermatogenic cells in vitro (Liang et al., 2017; Zatecka et al., 2013, 2014; Linhartova et al., 2015).
There is a significant lack of understanding regarding how these highly prevalent and ubiquitous FRs affect human spermatogenesis, and ultimately, male fertility. Our laboratory has demonstrated that male human embryonic stem cells (hESCs) can be directly differentiated into spermatogonial stem cells/differentiating spermatogonia, primary and secondary spermatocytes, and haploid spermatids (Easley et al., 2012). Using this model, we previously recapitulated clinical phenotypes of two known human male reproductive toxicants: 1,2-dibromo-3-chloropropane (DBCP) and 2-bromopropane (2-BP) (Easley et al., 2015). The purpose of this study was to assess the reproductive toxicity of HBCDD and TBBPA at occupationally relevant concentrations to determine if these chemicals could affect spermatogenesis under short-term conditions. We assessed sub-cellular effects that could lead to impaired human spermatogenesis, including cell viability of spermatogenic lineages, mitochondrial membrane potential, reactive oxygen species (ROS) generation, haploid cell production, and cell cycle progression in a dose-dependent manner. Here we show that our human in vitro model identifies HBCDD and TBBPA as male reproductive toxicants by affecting viability of spermatogonia and primary spermatocytes through ROS generation and mitochondrial dysfunction. As such, we provide evidence for their potential to have a significant impact on male fertility in vivo for occupationally exposed workers and others and potentially implicate this highly prevalent class of toxicants in the decline of Western males’ sperm counts.
RESULTS
HBCDD and TBBPA Exposure Induces Apoptosis in In Vitro Spermatogenic Cells
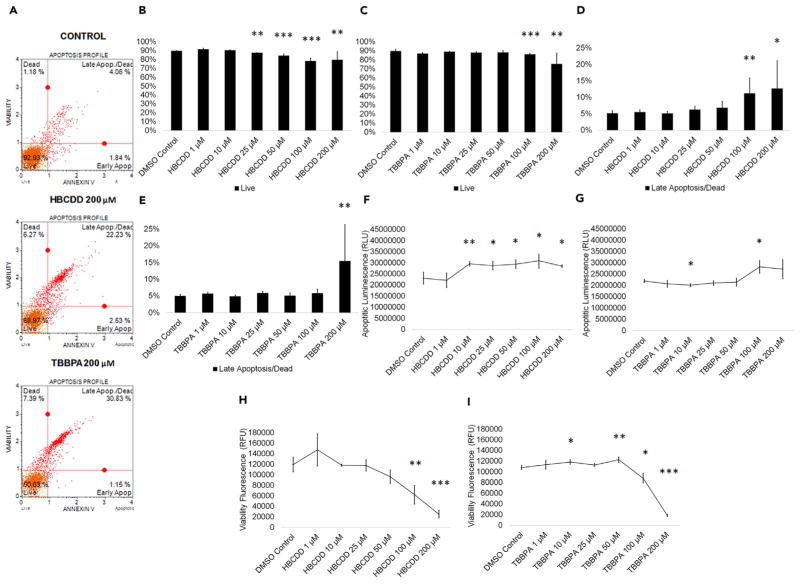
Multiple toxicants have been shown to increase apoptosis in human spermatogenic lineages, although the apoptotic effects of halogenated FRs on human spermatogenic lineages are largely unknown (Aly, 2013; Bloom et al., 2015; Aitken and Baker, 2013). Although no studies on HBCDD’s effects on spermatogenic cells have been reported, HBCDD has been shown to induce apoptosis in cultured SH-SY5Y human neuroblastoma cells (Al-Mousa and Michelangeli, 2014). Although one group showed that TBBPA caused apoptosis in testicular tissue, this cell death was attributed to Sertoli cells, whereas apoptosis in spermatogenic cell lineages was undetermined (Zatecka et al., 2013). A recent study showed that TBBPA decreased the number of mouse spermatogonia in vitro, suggesting an impact on spermatogenic cells (Liang et al., 2017). To assess the effects of these FRs on the cell viability of in vitro spermatogenic cell lineages, male hESCs were differentiated as described (Easley et al., 2012). This differentiation protocol produces a mixed population of spermatogonial stem cells/differentiating spermatogonia, primary spermatocytes, secondary spermatocytes, and haploid spermatids. After 9 days of differentiation, mixed germ cell cultures were treated for 24 hr with concentrations of HBCDD or TBBPA. Chemical concentrations of 1 μM, 10 μM, 25 μM, 50 μM, 100 μM, and 200 μM dissolved in dimethyl sulfoxide (DMSO) were chosen based on published occupationally relevant in vivo and in vitro data (Liang et al., 2017; Reistad et al., 2007; Crump et al., 2012; Liu et al., 2016; Cariou et al., 2008; Jakobsson et al., 2002; Thomsen et al., 2007; Li et al., 2014). Although the occupational exposure literature only reports concentrations as high as 25 μM, additional, higher concentrations were assessed due to the wide-ranging variability reported and to further elucidate the mechanisms of toxicity. HBCDD and TBBPA treatment groups were analyzed in comparison to a 0.2% DMSO-only treated negative control, which represents the highest concentration of DMSO used in this study, for cell viability/apoptosis. Flow cytometry analyses reported the percentage of live, early apoptotic, late apoptotic/dead, and dead cells in our in vitro cultures (Figures 1A and S1A). HBCDD and TBPPA both significantly reduced cell viability at higher concentrations, with HBCDD and TBBPA significantly reducing live cell populations at concentrations as low as 25 μM and 100 μM, and 200 μM concentration significantly decreasing viability by 11% and 16%, respectively (Figures 1B and 1C). Cells treated with HBCDD and TBBPA showed a significant increase in cells undergoing late apoptosis starting at 100 μM and 200 μM, respectively (Figures 1D and 1E). It was observed that 200 μM HBCDD and TBBPA increased late apoptotic cells by 59% and 68%, respectively (Figures 1D and 1E). Results were validated by staining HBCDD and TBBPA treatment groups with the substrates glycylphenylalanyl-aminofluorocoumarin (GF-AFC) and bis-AAF-R110 to determine apoptotic luminescence and viability fluorescence. HBCDD and TBBPA both increase apoptotic luminescence beginning at 10 and 100 μM, respectively (Figures 1F and 1G) and decrease viability fluorescence at as low as 10 and 50 μM, respectively (Figures 1H and 1I). Although they have different core structures, two other halogenated FRs, TDCPP and tris(2,3-dibromo-propyl) phosphate (TDBPP), also decrease cell viability at similar concentrations (Figures S1A–S1I). Taken together, these results show that HBCDD and TBBPA are capable of negatively affecting germ cell viability at varying concentrations, and the results with TDCPP and TDBPP suggest that this negative impact may be a characteristic of this class of chemicals.
Figure 1. HBCDD and TBBPA Induce Apoptosis in Spermatogenic Cells Derived from hESCs.
(A) Flow cytometry analyses for indicating percent viable cells, percent early apoptotic cells, percent late apoptotic cells, and percent dead/necrotic cells for the highest concentration of HBCDD and TBBPA assessed. Lower left quadrant represents viable cells, lower right quadrant represents early apoptotic cells, upper right quadrant is late apoptotic/dead cells, and the upper right quadrant is dead/necrotic cells.
(B and C) Graphical representation showing that HBCDD (B) and TBBPA (C) exposure induced germ cell death in hESCs differentiated in in vitro spermatogenic conditions.
(D and E) Graphical representation showing that HBCDD (D) and TBBPA (E) exposure increased the percentage of germ cells undergoing late apoptosis/death in spermatogenic cells derived from hESCs.
(F and G) Graphical representation showing that HBCDD (F) and TBBPA (G) exposure increased apoptotic luminescence in hESCs differentiated in in vitro spermatogenic conditions.
(H and I) Graphical representation showing that HBCDD (H) and TBBPA (I) decreased viability fluorescence in hESCs differentiated in in vitro spermatogenic conditions. A total of 5,000 events were analyzed, with five replications performed for each condition for (B)–(E). Three replications were analyzed for (F)–(I). Significant changes in cell viability were determined using a 1-way analysis of variance (ANOVA) and validated via a Student’s t test, where * is p < 0.05, ** is p < 0.01, and *** is p < 0.001. Data are represented as mean ± SEM.
See also Figure S1.
HBCDD and TBBPA Negatively Affect the Viability of Spermatogonia
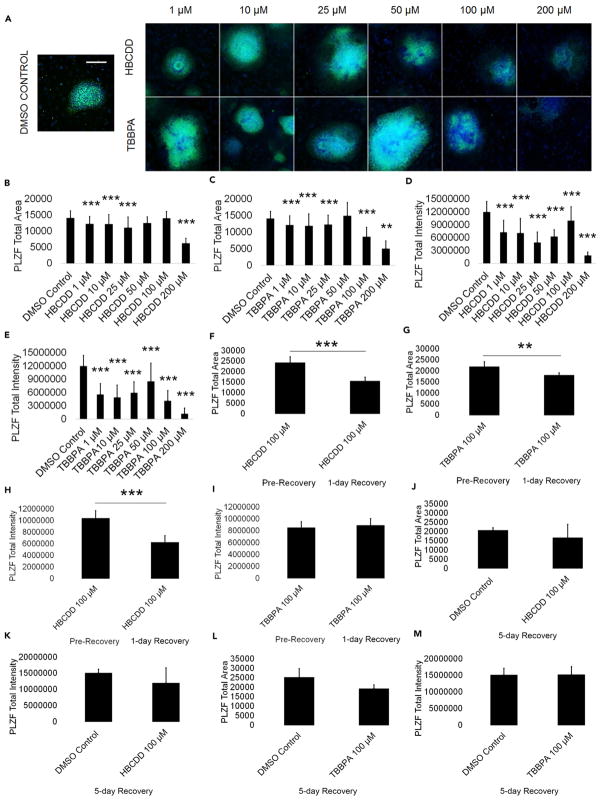
Spermatogonia are the foundation for male fertility, giving rise to primary and secondary spermatocytes, differentiating spermatids, and eventually, mature sperm capable of fertilizing an oocyte, all while maintaining their own pool through self-renewal (Phillips et al., 2010). As such, perturbations in this cell population could act to disturb the entire spermatogenesis process. To determine if spermatogonia are the cellular targets of our chemicals, we analyzed for expression of the consensus marker of stem and progenitor spermatogonia, promyelocytic leukemia zinc finger (PLZF). We have previously established PLZF as a reliable marker for spermatogonia in our in vitro model (Easley et al., 2012, 2015). Using high-content imaging and quantification, we determined that HBCDD and TBBPA both significantly reduce the total area of expression and total intensity of PLZF in our cell cultures (Figure 2A). Area measurements of PLZF+ colonies show that HBCDD and TBBPA significantly reduce PLZF+ area beginning at 1 μM (Figures 2B and 2C). With 200 μM HBCDD and TBBPA, a 56% and 64% decrease in PLZF+ area compared with DMSO-only negative control, respectively, was observed (Figures 2B and 2C). Notably, HBCDD treatment at 50 μM and 100 μM shows a PLZF+ area that is not significantly different from control (Figure 2B). Expression levels of PLZF, represented by the total intensity of PLZF+ staining, show significant reductions for HBCDD and TBBPA beginning at 1 μM (Figures 2D and 2E). At 200 μM, HBCDD and TBBPA show a significant 85% and 90% decrease in total PLZF intensity compared with 0.2% DMSO-only negative control, respectively (Figures 2D and 2E). Quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) results from the amplification of ZBTB16 (PLZF) transcripts in our in vitro model show a decreasing trend at 100 μM HBCDD and TBBPA, with ZBTB16 mRNA steady-state levels at 30% and 45% of DMSO-only negative control levels, respectively, which correlates with our staining data (Figure S2A). PLZF staining for cells treated with the FRs TDCPP and TDBPP shows a similar decrease in PLZF intensity and area beginning at 1 μM (Figures S2E–S2H). PLZF+ spermatogonia were not capable of recovery upon a 24-hr recovery period following the removal of HBCDD and TBBPA (Figures 2F–2I). PLZF area and intensity continued to significantly decline following recovery of cells treated with 100 μM HBCDD by 36% and 41%, respectively (Figures 2F and 2H). Cells treated with 100 μM TBBPA show a significant 17% decline in PLZF area but an insignificant 5% increase in PLZF intensity following a 24-hr recovery period (Figures 2G and 2I). PLZF intensity of TBBPA-treated cells remains significantly less than that of DMSO-only treated cells (Figure S2B). DMSO-only treated cells experience a 20% increase in PLZF area and intensity that is not significant during the same time period (Figures S2C and S2D). However, PLZF+ area and intensity are not statistically different from DMSO-negative control following a 5-day recovery from 100 μM HBCDD and 100 μM TBBPA exposure (Figures 2J–2M). Together, these data suggest that spermatogonia are sensitive to acute treatment with the FRs HBCDD and TBBPA at concentrations that are physiologically relevant. The differences in PLZF area and intensity recovery in HBCDD- and TBBPA-treated cells suggests differences in mechanisms of toxicity. HBCDD-treated spermatogonia viability continues to decline precipitously following initial removal of the toxicant, although cells recover following a longer recovery period. The area of spermatogonia cells continues to decline immediately following TBBPA exposure, whereas PLZF intensity shows evidence of attempted recovery after 1 day following removal of the toxicant, with the PLZF area and intensity returning to control levels after 5 days. These data indicate that recovery from acute HBCDD and TBBPA exposure is possible following a prolonged recovery period, although it is unclear if this trend would persist following repeated exposures similar to daily occupational exposure.
Figure 2. HBCDD and TBBPA Reduce PLZF+ Spermatogonia in In Vitro Spermatogenic Cultures.
(A) Representative 5X images of PLZF+ (green) and DAPI (blue)-stained colonies treated with HBCDD and TBBPA. A control image is included; scale bar, 5,000 μm. All images are taken under the same imaging conditions and parameters.
(B and C) Graphical representation showing that HBCDD (B) and TBBPA (C) reduce the average total PLZF+ area in spermatogonia derived under in vitro spermatogenic conditions.
(D and E) Graphical representation showing that HBCDD (D) and TBBPA (E) reduce the average total PLZF+ intensity in spermatogonia.
(F and G) Graphical representation showing that HBCDD (F) and TBBPA (G) exposure continues to reduce the average total PLZF+ area in spermatogonia, even after a 24-hr chemical-free recovery period.
(H and I) Graphical representation showing that HBCDD (H) and TBBPA (I) exposure continues to reduce the average total PLZF+ intensity in spermatogonia, even after a 24-hr chemical-free recovery period.
(J–M) Graphical representation showing that spermatogonia are capable of recovery following a 5-day recovery period after 100 μM TBBPA and HBCDD exposure. Five replications were performed for each condition for (B)–(E). Three replications were performed for each condition for (F)–(M). Significant changes in PLZF+ area and intensity were determined using a 1-way analysis of variance (ANOVA) and validated via a Student’s t test, where ** is p < 0.01, and *** is p < 0.001. Data are represented as mean ± SEM.
See also Figure S2.
HBCDD and TBBPA Affect Primary Spermatocytes
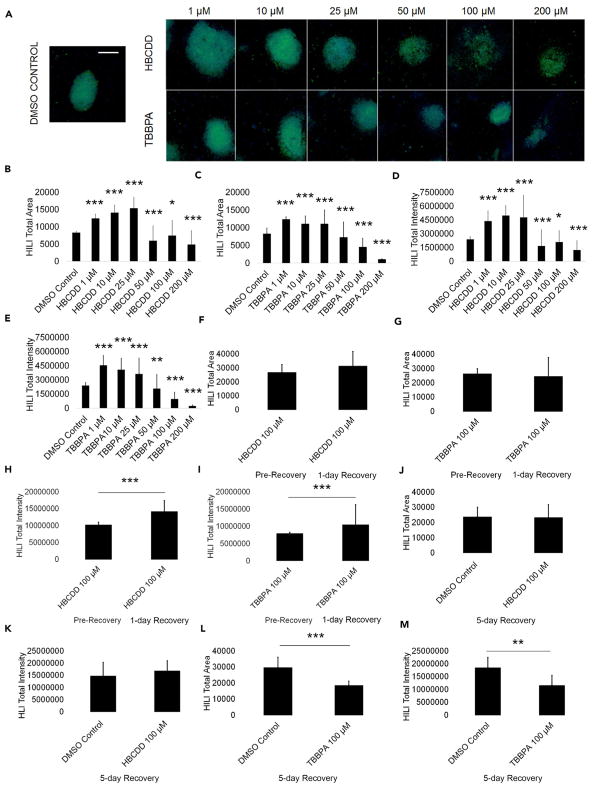
Spermatocytes are crucial to genome integrity as they undergo meiosis to give rise to haploid spermatids (Chen and Liu, 2015; Yan and McCarrey, 2009). Perturbation in this process could result in meiotic arrest and failure to progress in differentiation or inducing cell death. To assess if primary spermatocytes are also cellular targets of HBCDD and TBBPA, we analyzed for expression of the primary spermatocyte marker piwi like RNA-mediated gene silencing 2 (HILI). Using high-content imaging and quantification, we determined that HBCDD and TBBPA significantly affect HILI total area and total intensity (Figure 3A). HBCDD and TBBPA both showed significant increases in HILI+ area of 50% when compared with control at lower treatment doses (Figures 3B and 3C). TBBPA showed a steady, significant decline in HILI+ area with increasing concentration until the levels decreased to roughly 90% of control (Figure 3C). HBCDD showed a steady, significant increase in HILI+ area until 25 μM, wherein it is 85% above the HILI+ total area in 0.2% DMSO-only negative control (Figure 3B). There is an abrupt, significant decline in HBCDD HILI+ area at 50 μM, ending with levels roughly 41% of control at 200 μM (Figure 3B). Similarly, HBCDD and TBBPA significantly increased HILI+ total intensity at 1 μM, with TBBPA showing a significant, steady decline in HILI total intensity as treatment concentrations increased (Figures 3D and 3E). Initially, HILI total intensity for HBCDD- and TBBPA-treated cells was 80% and 90% more than DMSO-only negative control, respectively (Figures 3D and 3E). TBBPA HILI+ total intensity significantly declined to 9% of control levels at 200 μM (Figure 3E). HBCDD HILI+ total intensity remained above control until 50 μM, where it significantly declined (Figure 3D). HBCDD HILI+ intensity levels were lowest at 200 μM, where they were roughly 50% of control levels (Figure 3D). qRT-PCR results from the amplification of PIWIL2 (HILI) transcripts in our in vitro model showed an increasing trend for 100 μM TBBPA messenger RNA (mRNA) steady-state levels compared with control, with a roughly 80% increase that was not statistically significant (Figure S3A). HBCDD concentration of 100 μM showed a slight but insignificant decrease in PIWIL2 mRNA steady-state levels, with levels decreasing by roughly 8% (Figure S3A). Primary spermatocytes appear to fare better than spermatogonia following a 24-hr recovery period after 100 μM HBCDD and TBBPA exposure (Figures 3F–3I). HBCDD-exposed primary spermatocytes show an insignificant 17% increase in HILI area, whereas TBBPA-exposed cells show a 7% decline in area (Figures 3F and 3G). Notably, DMSO-only-treated cells show an insignificant 12% increase in HILI area (Figure S3B). HBCDD-treated cells show a significant 39% increase in HILI intensity, and TBBPA-treated cells show a significant 32% increase in HILI intensity during the 24-hr recovery period (Figures 3H and 3I). Although the increases in HILI area and intensity observed may indicate a recovery of primary spermatocytes, both HBCDD and TBBPA do cause increases in HILI area and intensity at low levels. Possibly, the mechanism activated in low-level doses is similarly present in recovering cells. This theory is highlighted by the fact that whereas cells treated with 100 μM HBCDD recover following a 5-day recovery period, cells treated with 100 μM TBBPA show a 36% decline in HILI total area and a 32% decline in HILI total intensity during the same time period (Figures 3J–3M). As such, it is possible that the increases in HILI seen are indicative of a toxic mechanism, with primary spermatocytes undergoing cell death at a time after this increase. In addition, the differences in the recovery of TBBPA- and HBCDD-treated cells further highlights a difference in the mechanisms of toxicity.
Figure 3. HBCDD and TBBPA Influence HILI Expression in Primary Spermatocytes in In Vitro Spermatogenic Cultures.
(A) Representative 5X images of HILI+ (green) and DAPI (blue)-stained colonies treated with HBCDD and TBBPA. A control image is included; scale bar, 5,000 μm. All images are taken under the same imaging conditions and parameters.
(B and C) Graphical representation showing that HBCDD (B) and TBBPA (C) exposure affects the average total HILI+ area in primary spermatocytes derived under in vitro spermatogenic conditions.
(D and E) Graphical representation showing that HBCDD (D) and TBBPA (E) exposure affects the average total HILI+ intensity in primary spermatocytes.
(F and G) Graphical representation showing that the average total HILI+ area in primary spermatocytes does not statistically change after a 24-hr chemical-free recovery period following HBCDD (F) and TBBPA (G) exposure.
(H and I) Graphical representation showing that the average total HILI+ intensity in primary spermatocytes increases following a 24-hr chemical-free recovery period after HBCDD (H) and TBBPA (I) exposure.
(J–M) Graphical representation showing that primary spermatocytes are capable of recovery following a 5-day recovery period after 100 μM HBCDD exposure (J and K), but spermatocytes do not make a full recovery following exposure to 100 μM TBBPA (L and M). Five replications were performed for each condition for (B)–(E). Three replications were performed for each condition for (F)–(M). Significant changes in HILI+ area and intensity were determined using a 1-way analysis of variance (ANOVA) and validated via a Student’s t test, where * is p < 0.05, ** is p < 0.01, and *** is p < 0.001. Data are represented as mean ± SEM.
See also Figure S3.
Finally, similar to our PLZF data, exposure of our in vitro cultures to the FRs TDCPP and TDBPP affect HILI expression by increasing the area and intensity at low levels, whereas decreasing the area and intensity at increasingly higher concentrations, again suggesting that these mechanisms of toxicity may be class-wide (Figures S3C–S3F). Although the exact details remain unclear, these data suggest that low-dose FR exposure increases HILI expression, whereas at higher doses, FR exposure may reduce HILI expression by affecting spermatocyte viability. Primary spermatocytes may be more capable of recovery following FR exposure, although the data could indicate irreversible damage and other defects that could lead to later apoptosis.
HBCDD and TBBPA Exposure Impairs Cell Cycle Progression in In Vitro Cultures but Does Not Affect Haploid Sperm Viability
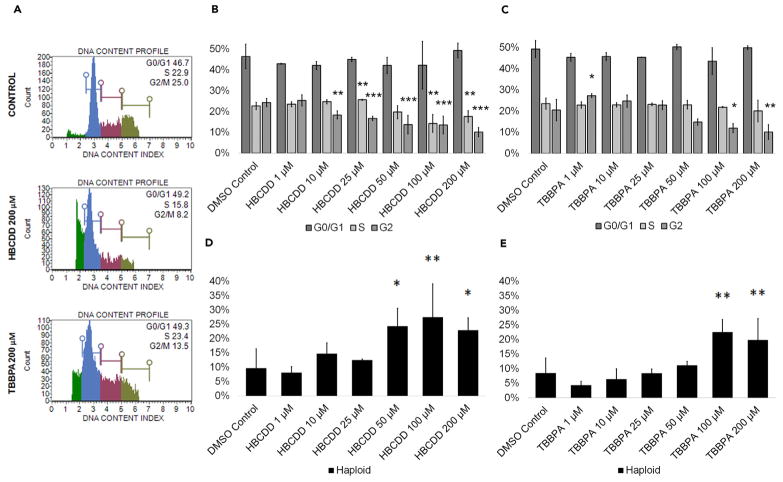
Diploid cells can arrest at multiple checkpoints for reasons varying from genetic damage to improper pairing of chromosomes, making cell cycle profiles vital indicators of cell health. Cell cycle information in regard to FR exposure during spermatogenesis is limited. In somatic cells, HBCDD has been shown to upregulate cell-cycle-related genes in LNCaP cells and may act to increase cell proliferation (Kim et al., 2016). In germ cells, TBBPA has been shown to have an adverse effect on the cell cycle in mouse spermatogonial stem cells (Liang et al., 2017). To determine how these toxicants affect in vitro spermatogenesis in a mixed population of spermatogonia, primary and secondary spermatocytes, and spermatids, cell cycle profiles of FR-exposed cells were generated by staining with propidium iodide. Flow cytometry plots were generated showing the percentage of haploid cells and cells in G0/G1, S, and G2 phases in our cultures (Figures 4A and S4A). HBCDD and TBBPA did not affect G0/G1 (Figures 4B and 4C). Similarly, TBBPA did not affect S phase (Figure 4C). However, HBCDD significantly increased cells in S phase at 25 μM by 13%, with a decrease in cells in S phase at 100 μM and 200 μM by 35% and 22%, respectively (Figure 4B). Both HBCDD and TBBPA had a significant impact on G2 (Figures 4B and 4C). HBCDD and TBBPA significantly decreased the percentage of cells in G2 beginning at 10 μM and 100 μM by as much as 56% and by 53% at 200 μM, respectively (Figures 4B and 4C). These data support our previous data in that G2 populations represent either dividing spermatogonia or meiotic primary spermatocytes. In addition, the halogenated FRs TDCPP and TDBPP similarly disrupt cell cycle progression, further highlighting a potential chemical class effect on spermatogenic cells (Figures S4B and S4C).
Figure 4. HBCDD and TBBPA Affect the Cell Cycle in Spermatogenic Cells Derived from hESCs Without Affecting Haploid Cell Viability.
(A) Flow cytometry analyses of cell cycle profiles following acute 24-hr treatment. Green, blue, purple, and beige populations on flow cytometry correspond to haploid, G0/G1, S, and G2 phases, respectively.
(B and C) Graphical representation showing that HBCDD (B) and TBBPA (C) affect the cell cycle of actively dividing hESCs differentiated in in vitro spermatogenic conditions.
(D and E) Graphical representation showing that HBCDD (B) and TBBPA (C) exposure increases the percentage of haploid cells in spermatogenic cells derived from hESCs. A total of 5,000 events were analyzed, with five replications performed for each condition. Significant changes in percentages of haploid cells and cells in G0/G1, S, and G2 phases were determined using a 1-way analysis of variance (ANOVA) and validated via a Student’s t test, where * is p < 0.05, ** is p < 0.01, and *** is p < 0.001. Data are represented as mean ± SEM.
See also Figure S4.
However, the end product of spermatogenesis is the production of haploid spermatids. Numerous environmental factors have been shown to reduce sperm counts (Wong and Cheng, 2011), and some toxicants are known to target haploid spermatids (Easley et al., 2015). However, the halogenated FRs HBCDD and TBBPA both significantly increased the percentage of haploid spermatids in our cultures beginning at 50 μM and 100 μM, respectively (Figures 4D and 4E). HBCDD and TBBPA concentrations of 100 μM significantly increased the percentage of spermatids in our cultures by 200% and 165%, respectively (Figures 4D and 4E). HBCDD treatment caused a significant decrease in haploid spermatids at 200 μM versus 100 μM by roughly 17%; however, the percentage of haploid spermatids was still greater than control (Figure 4D). Notably, TDCPP and TDBPP exposure also increased the percentage of haploid spermatids in our cultures (Figures S4D and S4E). Importantly, the increases in haploid cells seen in these assays were likely not due to increases in meiosis that drove the generation of more spermatids. Because chemical exposure occurred under acute conditions over 24 hr, percentages of haploid cells likely increased due to spermatogonia and primary spermatocytes undergoing cell death, leaving more haploid cells present in our mixed cell cultures. As such, these results again indicate that the direct targets of these toxicants are likely the actively dividing spermatogonia and primary spermatocytes undergoing meiosis. These results are of critical importance. Although spermatids are not directly targeted, thus not causing immediate infertility in an adult male, evidence suggests that exposure to these FRs at physiologically relevant concentrations could be affecting the pool of spermatogonia responsible for generating spermatids/sperm. In addition, spermatogonia and primary spermatocytes continue to be affected by HBCDD and TBBPA even after removal during recovery experiments. As such, exposure could lead to reduced fertility in populations exposed, although the potential for abnormalities in the surviving spermatids also exists.
TBBPA and HBCDD Exposure Decreases GSH/GSSG Ratios, whereas TBBPA Exposure Increases Reactive Oxygen Species Levels in In Vitro Spermatogenesis
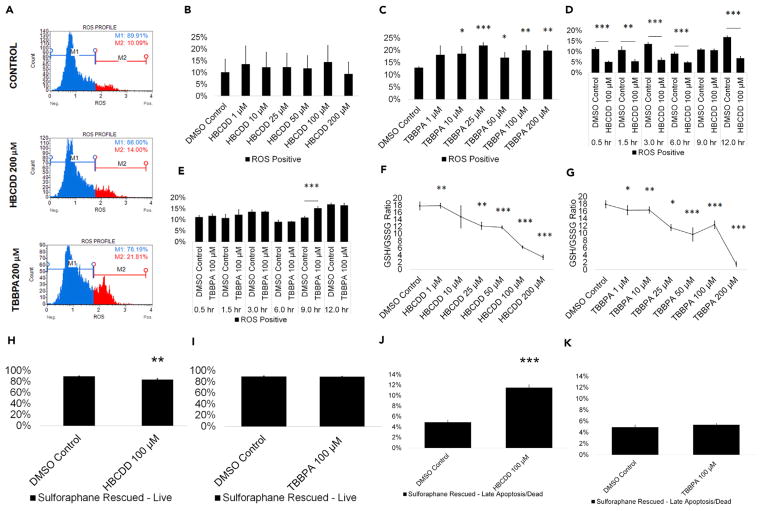
Known reproductive toxicants have been shown to induce oxidative stress (Aly, 2013; Erkekoglu and Kocer-Gumusel, 2014; Maiorino and Ursini, 2002) even in our in vitro model (Easley et al., 2015). The mammalian testis is susceptible to toxic assault by ROS (Agarwal et al., 2014), with ROS causing cell death through necrotic and apoptotic pathways (Ryter et al., 2007). As such, ROS-induced cell death in testis cells could lead to impaired male fertility. Increased ROS generation may provide a mechanism for increased germ cell death in response to halogenated FR exposure. TBBPA has been shown to increase oxidative stress in in vitro assays and to increase ROS in fish sperm (Dishaw et al., 2014; Huang et al., 2016; Linhartova et al., 2015). However, there is no information on the effects of HBCDD on ROS generation during spermatogenesis, although Chinese rare minnows exposed to HBCDD have shown an increase in ROS generation (Zhang et al., 2008). We examined whether HBCDD and TBPPA when compared with 0.2% DMSO-only negative control can increase ROS generation in our in vitro spermatogenesis model using dihydroethidium (DHE) staining. Flow cytometry profiles were generated showing the percentage of ROS-positive (ROS+; red) and ROS-negative (ROS−; blue) cells in our cultures (Figures 5A and S5A). HBCDD treatment did not cause a statistically significant increase in ROS generation at any concentration (Figure 5B). However, TBBPA treatment caused a statistically significant increase in ROS generation (ROS+ cells) beginning at 10 μM, consistent with published data and relevant to occupationally exposed populations (Figure 5C). TBBPA showed the most significant increase in ROS+ cells at 25 μM, with ROS+ cells increasing by 10% (Figure 5C). When assessed over the course of 12 hr, ROS production appears to decrease in cells treated with 100 μM HBCDD beginning within the first half hour and persisting for the entirety of the 12-hr assay (Figure 5D). During a 12-hr exposure, cells treated with 100 μM TBBPA experience a significant 46% increase in ROS at 9 hr (Figure 5E). ROS levels return to normal at 12 hr, suggesting that our in vitro cultures are still capable of processing the ROS generated by TBBPA exposure at that time (Figure 5E). However, ROS generation does still appear to be the main mechanism of cell death in TBBPA-treated cells. Following treatment of our in vitro cultures with 1 μM of the antioxidant L-sulforaphane for 12 hr, cells that were treated with 100 μM TBBPA show live cell and late apoptotic/dead cell populations similar to control (Figures 5I and 5K). However, L-sulforaphane pre-treatment does not rescue cell death caused by 100 μM HBCDD treatment, with cells showing a 6% decrease in live cells and a 135% increase in late apoptotic/dead cells (Figures 5H and 5J). This increase in apoptosis remains similar to non-rescued cells treated with 100 μM HBCDD, suggesting that ROS does not play a role in HBCDD-induced cell death (Figures 1B and 1D). We have previously used this method to rescue our in vitro cultures following exposure to the known male reproductive toxicants 2-BP and DBCP (Easley et al., 2015). These results suggest that HBCDD’s mechanism of toxicity is distinctly different from those of known reproductive toxicants, which classically induce cell death through ROS assault, as well as TBBPA.
Figure 5. TBBPA Causes ROS Production in Spermatogenic Cells Derived from hESCs, while HBCDD and TBBPA Exposure Decrease GSH/GSSG Ratios.
(A) Flow cytometry-based analysis of DHE labeling. Blue indicates ROS−. Red indicates ROS+.
(B and C) Graphical representation showing that HBCDD does not affect ROS generation in hESCs differentiated in in vitro spermatogenic conditions (B), but TBBPA exposure causes overwhelming increase in ROS production (C).
(D and E) Graphical representation showing the generation of overwhelming ROS over a 12-hr period post-exposure to 100 μM HBCDD (D) and TBBPA (E).
(F and G) Graphical representation showing that HBCDD (F) and TBBPA (G) exposure decreases the GSH/GSSG ratio of hESCs differentiated in in vitro spermatogenic conditions.
(H–K) A 12-hr pre-treatment with 1 μM L-sulforaphane rescues 100 μM TBBPA-mediated cell death (I and K) but does not rescue cell death following 100 μM HBCDD exposure (H and J). A total of 5,000 events were analyzed, with three replications performed for each condition for (B)–(C) and (F)–(G). Three replications were performed for each condition for (D)–(E) and (H)–(K). Significant changes in ROS generation, GSH/GSSG ratio, and cell viability were determined using a 1-way analysis of variance (ANOVA) and validated via a Student’s t test, where * is p < 0.05, ** is p < 0.01, and *** is p < 0.001. Data are represented as mean ± SEM.
See also Figure S5.
Results from the ROS assay were validated by assessing changes in the reduced glutathione (GSH)/oxidized glutathione (GSSG) ratios in HBCDD and TBBPA treatment groups. HBCDD and TBBPA both decrease GSH/GSSG ratios at as little as 1 μM, indicating increased ROS generation (Figures 5F and 5G). Although HBCDD and TBBPA both increase ROS as indicated by decreases in GSH/GSSG ratios, the results suggest that only TBBPA is capable of generating sufficient ROS to overwhelm the cell’s defenses in response to exposure in vitro. Exposure to TDCPP and TDBPP also fails to produce ROS capable of overwhelming spermatogenic cells but does decrease GSH/GSSG ratio similarly to HBCDD and TBBPA (Figures S5A–S5E). These results again highlight the class-wide effects that these chemicals have on in vitro spermatogenic cells and also further elucidate the different mechanisms of action between HBCDD and TBBPA.
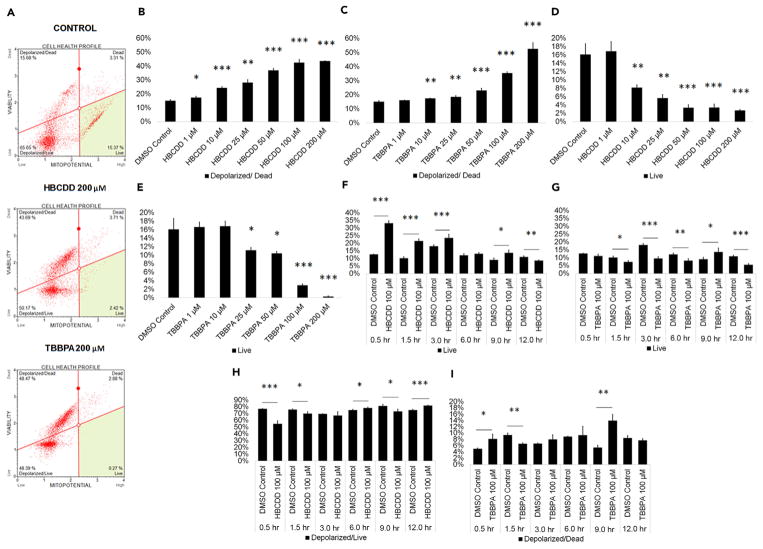
HBCDD and TBPPA Decrease Mitochondrial Membrane Potential in In Vitro Spermatogenesis Cultures
Mitochondria supply cells with energy in the form of oxidative phosphorylation that generates ATP (Attene-Ramos et al., 2013). In addition, mitochondria are required for calcium homeostasis, cell signaling, and apoptosis regulation (Attene-Ramos et al., 2013). As such, any perturbation of mitochondrial function can prove detrimental to cells such as spermatogenic cells. Mitochondria have been shown to be susceptible to early-stage effects of chemical toxicity, and multiple chemicals have been shown to decrease mitochondrial membrane potential and cause mitochondrial dysfunction (Schmidt, 2010). As such, assessing mitochondrial membrane potential could act as a valid, early assessment for cell health in our in vitro cultures. HBCDD and TBBPA have been shown to negatively affect mitochondria or impair oxidative phosphorylation in A549 and pancreatic β islet cells in vitro, respectively (An et al., 2014; Suh et al., 2017; Zhang et al., 2016). Here we examined whether HBCDD and TBPPA negatively affected mitochondrial membrane potential in our in vitro spermatogenesis model that consists of a mixed population of spermatogonia, primary and secondary spermatocytes, and spermatids. Flow cytometry plots were generated showing the percentage of live, depolarized/live, depolarized/dead, and dead cells in our cultures (Figures 6A and S6A). HBCDD significantly increased mitochondrial dysfunction beginning at 1 μM and showed a 190% increase in mitochondrial membrane depolarization and death at 200 μM (Figure 6B). TBBPA significantly increased mitochondrial dysfunction at 10 μM with nearly 250% more membrane depolarization and cell death compared with control at 200 μM (Figure 6C). Similar to our apoptosis data, HBCDD and TBBPA significantly decreased healthy, live cell populations beginning at 10 μM and 25 μM, respectively (Figures 6D and 6E). HBCDD and TBBPA significantly decreased healthy, live cells at 200 μM by 83% and 98%, respectively (Figures 6D and 6E). Similar results were seen upon treatment with TDCPP and TDBPP (Figures S6A–S6E). The mechanism by which HBCDD and TBBPA cause mitochondrial dysfunction appears to be drastically different, consistent with PLZF, HILI, and ROS assays. A shift toward live cells by 162% can be seen within half an hour of treating cells with 100 μM HBCDD (Figure 6F). As it is unlikely that HBCDD exposure drastically increases cell viability after half an hour, this shift from the norm is likely the result of mitochondrial hyperpolarization. This hyperpolarizing event occurs for approximately 9 hr post-exposure, with depolarization significantly shifting cells toward the depolarized/live quadrant at 6 and 12 hr (Figure 6H). Because mitochondria produce ROS during oxidative phosphorylation, this acute perturbation of the mitochondria may be another potential explanation for the decrease in ROS seen in HBCDD-treated cells. The viability of TBBPA-exposed cells begins decreasing at 1.5 hr post-exposure, although significant depolarization of the mitochondrial membrane and cell death occurs as quickly as 0.5 hr after TBBPA exposure (Figures 6G and 6I). This increase in depolarized/dead cells becomes more dramatic at 9 hr (Figure 6I). Because mitochondria are sensitive to ROS, it is likely that the abrupt mitochondrial membrane depolarization seen following TBBPA exposure is likely due to assault by ROS (Balaban et al., 2005). These results indicate that mitochondria are a direct target of halogenated FRs in our in vitro cultures at concentrations that are physiologically relevant, with exposure resulting in mitochondrial membrane dysfunction and increased cell death. TBBPA likely causes mitochondrial dysfunction due to ROS generation, although the mechanism by which HBCDD affects the mitochondria is less clear. Possibly, HBCDD’s unique mechanism of toxicity involves directly targeting mitochondria in spermatogenic cells.
Figure 6. HBCDD and TBBPA Depolarize the Mitochondrial Membrane to Increase Cell Death in Spermatogenic Cells Derived from hESCs.
(A) Flow cytometry analyses for indicating percent live cells, percent depolarized/live cells, percent depolarized/dead cells, and percent dead cells. Lower right quadrant represents viable cells, lower left quadrant represents depolarized/live cells, upper right quadrant is depolarized/dead cells, and the upper right quadrant is dead cells.
(B and C) Graphical representation showing that HBCDD (B) and TBBPA (C) exposure increases membrane depolarization and death in hESCs differentiated in in vitro spermatogenic conditions.
(D and E) Graphical representation showing that HBCDD (D) and TBBPA (E) decrease the percentage of healthy, live cells in hESCs differentiated in in vitro spermatogenic conditions.
(F–I) Graphical representation showing mitochondrial membrane depolarization and death and live cell percentages over a 12-hr period post-exposure to 100 μM HBCDD (F and H) and TBBPA (G and I). A total of 5,000 events were analyzed, with three replications performed for each condition. Significant changes in mitochondrial membrane potential were determined using a 1-way analysis of variance (ANOVA) and validated via a Student’s t test, where * is p < 0.05, ** is p < 0.01, and *** is p < 0.001. Data are represented as mean ± SEM.
See also Figure S6.
DISCUSSION
Few studies on the potential human health effects resulting from halogenated FR exposure exist despite evidence of widespread, everyday exposure to these compounds through direct contact or from ingestion of house dust and other contaminated sources (Weissman and Pan, 2015; Dishaw et al., 2014). In studies that have directly assessed relationships between FRs and human male fertility, some FRs have been associated with changes in male hormones, although no effects on sperm quantity or quality have ever been reported and direct changes in spermatogenesis have not been investigated (Cooper et al., 2011; Johnson et al., 2013; Meeker and Stapleton, 2010; Yard et al., 2011). Here, we show that the highly prevalent halogenated FRs HBCDD and TBBPA negatively affect the viability of human spermatogenic cell lineages in vitro at concentrations relevant to occupationally exposed workers, with HBCDD and TBBPA affecting spermatogonia and primary spermatocytes at as little as 1 μM during 24 hr of treatment.
Spermatogonia are the progenitors of primary and secondary spermatocytes, and ultimately spermatids and sperm (Neto et al., 2016). Their function involves producing sperm during a male’s post-pubertal lifetime by undergoing mitosis to replenish their own population as well as meiosis to produce male gametes (Neto et al., 2016). Spermatogonia are direct targets of the FRs we tested, with populations reduced at the lowest concentrations assessed during a 24-hr exposure. This finding suggests that males who experience long-term or acute exposure in an occupational setting could experience a depletion of their spermatogonia, which could render them infertile over time, yet to date no clinical studies have been undertaken to examine at-risk populations. Notably, occupational workers could be exposed to concentrations of HBCDD and TBBPA assessed in this study on a daily basis. The results shown in this study are the result of acute exposure, and occupationally exposed workers may see more detrimental impacts over time. These results also highlight changes that may not be visible in epidemiological data, as sperm, the most common cell type assessed in epidemiological studies, are not the direct targets of these chemicals and it is unclear how long exposure would have to occur before sperm would be directly affected. Infertility and sterility resulting from reduced populations of spermatogonia may occur long after the exposure occurs and may not be linked to this exposure, thus making these results even more relevant for assessments on occupational workers in the future. Importantly, spermatogonia are also the only spermatogenic cell lineage to exist before puberty. It has been reported that young children are exposed to higher than average concentrations of FRs, and reports indicate that this early exposure can lead to low sperm count and other reproductive disorders later in life (Bonde et al., 2016). As such, this research also has implications for childhood exposures to chemicals that could have impacts on fertility during adulthood. Finally, it is notable that spermatogonia exhibited a delayed recovery following removal of the FRs tested. Although spermatogonia did recover over time, occupational workers are exposed to these toxicants on a daily basis. As such, these chemicals may not be eliminated from their bodies long enough for recovery to occur. This finding suggests that those who have been exposed to higher concentrations of HBCDD and TBBPA may suffer irreversible damage to their fertility.
Similarly, primary spermatocytes are also affected by exposure to HBCDD and TBBPA, although the exact impacts that these chemicals have on primary spermatocytes is less clear. Primary spermatocytes express HILI, which functions in the male germline to repress transposons; regulates gene expression at the epigenetic, post-transcriptional, and translational levels; and has been implicated in chromosome synapsis during meiosis, among other important processes (Juliano et al., 2011). Significantly, HILI levels are up-regulated upon exposure to our halogenated FRs at low to moderate levels. HILI levels do decrease at higher chemical concentrations for both chemicals assessed, and studies have shown that decreases in HILI expression lead to apoptosis in primary spermatocytes (Juliano et al., 2011). Perhaps most importantly, the results of this study do not suggest that primary spermatocytes undergo cell death in response to chemical exposure to the same extent as spermatogonia. Spermatogonia are capable of recovery following HBCDD and TBBPA exposure, although primary spermatocytes do not recover from TBBPA exposure, even after a 5-day recovery.
Decreased viability of spermatogonia and primary spermatocytes occurred via apoptosis at higher chemical concentrations, highlighting a disconnect between apoptotic data and immunostaining results for PLZF, where cell populations were decreased at even the lowest concentration. Mitochondrial membrane potential data suggest that our in vitro cultures are sensitive to FR toxicants at lower concentrations, which have implications for occupationally exposed workers. Mitochondria have been called the “canary” of cell health, and our results indicate that they may be susceptible to toxicants earlier than other processes and act as an early warning system for cell health in contrast to apoptotic and ROS markers. Decreasing mitochondrial function is often paired with increasing ROS generation, although only TBBPA showed increases in ROS capable of overwhelming the cell’s defense mechanisms, with the first signs of ROS beginning to overwhelm cell defenses at 9 hr post-exposure. The mitochondrial membrane depolarization data suggest different mechanisms of toxicity for HBCDD and TBBPA despite having similar end results. HBCDD exposure causes an immediate shift to a more negative, hyperpolarized mitochondrial state that inevitably leads to depolarization and death. The results of this study point to HBCDD utilizing a mechanism of toxicity that is distinctly different from those used by recognized male reproductive toxicants, such as 2-BP and DBCP. Possibly, mitochondria may be the direct target of HBCDD, although further studies are required. TBBPA exposure, however, shows an opposite mechanism to HBCDD, with depolarization occurring in the first 1.5 hr followed by death. This depolarization is likely due to assault by ROS, as this was identified as the main mechanism of cell death for TBBPA following L-sulforaphane rescue. However, cell death, whether it is through mitochondrial dysfunction or another mechanism, may not be the only explanation for decreases in spermatogonia and primary spermatocyte populations. Although this was a 24-hr exposure, cell cycle profiles revealed that our chemicals can arrest our cultures during cell division. Alternatively, or perhaps in conjunction with cell death, it is possible that our chemicals at higher concentrations block differentiation from spermatogonia to primary and secondary spermatocytes and spermatids by arresting cells during mitosis and meiosis, although longer term studies will need to be conducted to fully elucidate this mechanism.
Our in vitro human stem cell model of spermatogenesis has revealed for the first time that the FRs HBCDD and TBBPA can directly affect human spermatogenesis. These results highlight the need for more data regarding the prevalence of these toxicants in the human system and the need for additional experiments to understand how HBCDD and TBBPA may alter spermatogenesisand male fertility, especially at persistent concentrations that are relevant to occupationally exposed workers. It must be stressed that spermatogonia and primary spermatocytes were affected at occupationally relevant concentrations after only 1 day of exposure in vitro. In addition, as semen parameters continue to plummet in the Western males with no definitive cause, further investigation into HBCDD’s and TBBPA’s potential to affect male fertility is highly recommended, as the average person is also exposed to these chemicals on a daily basis. Finally, although they have different core structures, the halogenated FRs TDCPP and TDBPP showed similar impacts on human spermatogenesis to HBCDD and TBBPA. This suggests that this class of chemicals could be as detrimental as their PCB and PBDE predecessors and stresses the need for continued studies on their potential health impacts.
METHODS
All methods can be found in the accompanying Transparent Methods supplemental file.
Supplementary Material
Figure S1. Related to Figure 1. TDCPP, TDBPP, HBCDD and TBBPA induce apoptosis in spermatogenic cells derived from hESCs. (A) Flow cytometry analyses indicating percent viable cells, percent early apoptotic cells, percent late apoptotic cells, and percent dead/necrotic cells for all concentrations of TDCPP, TDBPP, HBCDD, and TBBPA assessed. Lower left quadrant represents viable cells, lower right quadrant represents early apoptotic cells, upper right quadrant is late apoptotic/dead cells, and the upper left quadrant is dead/necrotic cells. (B–C) Graphical representation showing that TDCPP and TDBPP decrease germ cell viability in hESCs differentiated in in vitro spermatogenic conditions. (D–E) Graphical representation showing that TDCPP and TDBPP increase the percentage of germ cells undergoing late apoptosis/death in spermatogenic cells derived from hESCs. (F–G) Graphical representation showing that TDCPP and TDBPP increase apoptotic luminescence in hESCs differentiated in in vitro spermatogenic conditions. (H–I) Graphical representation showing that TDCPP and TDBPP decreased viability fluorescence in hESCs differentiated in in vitro spermatogenic conditions. 5,000 events were analyzed, with five replications performed for each condition for A–E. Three replications were analyzed for F–I. Significant changes in cell viability were determined using a 1-way analysis of variance (1-way ANOVA) and validated via a Student’s t-test, where * is p<0.05, ** is p<0.01, and *** is p<0.001. Data are represented as mean ± SEM.
Figure S2. Related to Figure 2. TDCPP, TDBPP, HBCDD, and TBBPA exposure impact PLZF expression. (A) mRNA steady state transcripts for ZBTB16 (PLZF) correspond to decreases in PLZF+ area and intensity in immunostaining data for TDCPP, TDBPP, HBCDD, and TBBPA. (B) Graphical representation showing that PLZF intensity in spermatogonia derived under in vitro spermatogenic conditions treated with TBBPA remain below control levels following a twenty-four hour, chemical-free recovery period. (C) Graphical representation showing that PLZF area in spermatogonia derived under in vitro spermatogenic control conditions remain statistically the same after a twenty four hour recovery period. (D) Graphical representation showing that PLZF intensity in spermatogonia derived under in vitro spermatogenic control conditions remain statistically the same after a twenty four hour recovery period. (E–F) Graphical representation showing that TDCPP and TDBPP reduce average PLZF+ area in spermatogonia derived under in vitro spermatogenic conditions. (G–H) Graphical representation showing that TDCPP and TDBPP reduce average PLZF+ intensity in spermatogonia. mRNA transcript levels were normalized to 0.2% DMSO-only control. Two separate replications were performed in duplicate (n=4) for each condition. Significant changes in mRNA steady state levels were determined using Bio-Rad CFX Manager™ Software (Bio-Rad, Hercules, CA). Five replications were performed for each condition for PLZF immunostaining for E–H. Three replications were performed for B–D. Significant changes in PLZF+ area and intensity were determined using a 1-way analysis of variance (1-way ANOVA) and validated via a Student’s t-test, where * is p<0.05, ** is p<0.01, and *** is p<0.001. Data are represented as mean ± SEM.
Figure S3. Related to Figure 3. TDCPP, TDBPP, HBCDD, and TBBPA exposure impact HILI expression. (A) mRNA steady state transcripts for PIWIL2 (HILI) correspond to increases in HILI+ area and intensity in immunostaining data corresponding to certain concentrations of TDCPP, TDBPP, HBCDD, and TBBPA. (B) Graphical representation showing that HILI area in primary spermatocytes derived under in vitro spermatogenic control conditions remain statistically the same after a twenty four hour recovery period. (C–D) Graphical representation showing that TDCPP and TDBPP impact average total HILI+ area in primary spermatocytes derived under in vitro spermatogenic conditions. (E–F) Graphical representation showing that TDCPP and TDBPP impact average total HILI+ intensity in primary spermatocytes. mRNA transcript levels were normalized to 0.2% DMSO-only control. Two separate replications were performed in duplicate (n=4) for each condition. Significant changes in mRNA steady state levels were determined using Bio-Rad CFX Manager™ Software (Bio-Rad, Hercules, CA). Five replications were performed for each condition for HILI immunostaining for C–F. Three replications were performed for B. Significant changes in HILI+ area and intensity were determined using a 1-way analysis of variance (1-way ANOVA) and validated via a Student’s t-test, where * is p<0.05, ** is p<0.01, and *** is p<0.001. Data are represented as mean ± SEM.
Figure S4. Related to Figure 4. TDCPP, TDBPP, HBCDD, and TBBPA affect the cell cycle in spermatogenic cells derived from hESCs without impacting haploid cell production. (A) Flow cytometry analyses of cell cycle profiles following acute twenty-four hour treatment. Green, blue, purple, and beige populations on flow cytometry correspond to haploid, G0/G1, S, and G2 phases, respectively. (B–C) Graphical representation showing that TDCPP and TDBPP affect the cell cycle of actively dividing hESCS differentiated in in vitro spermatogenic conditions. (D–E) Graphical representation showing that TDCPP and TDBPP exposure increases the percentage of haploid cells in spermatogenic cells derived from hESCs. 5,000 events were analyzed, with five replications performed for each condition. Significant changes in percentages of haploid cells and cells in G0/G1, S phase, and G2 were determined using a 1-way analysis of variance (1-way ANOVA) and validated via a Student’s t-test, where * is p<0.05, ** is p<0.01, and *** is p<0.001. Data are represented as mean ± SEM.
Figure S5. Related to Figure 5. TDCPP, TDBPP, HBCDD, and TBBPA impact ROS production and GSH/GSSG ratios. (A) Flow cytometry analyses indicating percent ROS− and ROS+ cells for all concentrations of TDCPP, TDBPP, HBCDD, and TBBPA assessed. ROS− cells are labeled blue. ROS+ cells are labeled red. (B–C) Graphical representation showing that TDCPP and TDBPP do not overwhelmingly increase ROS+ cells in hESCs differentiated in in vitro spermatogenic conditions. (D–E) Graphical representation showing that TDCPP and TDBPP decrease the GSH/GSSG ratio of hESCs differentiated in in vitro spermatogenic conditions. 5,000 events were analyzed, with three replications performed for each condition for A–C. Three replications were performed for each condition for D–E. Significant changes in ROS generation and GSH/GSSG ratio were determined using a 1-way analysis of variance (1-way ANOVA) and validated via a Student’s t-test, where * is p<0.05, ** is p<0.01, and *** is p<0.001. Data are represented as mean ± SEM.
Figure S6. Related to Figure 6. TDCPP, TDBPP, HBCDD, and TBBPA decrease mitochondrial membrane potential and the percentage of healthy, live cells in in vitro spermatogenic cultures.. (A) Flow cytometry analyses indicating percent live cells, percent a depolarized/live cells, percent depolarized/dead cells, and percent dead cells. Lower right quadrant represents viable cells, lower left quadrant represents depolarized/live cells, upper right quadrant is depolarized/dead cells, and the upper right quadrant is dead cells. (B–C) Graphical representation showing that TDCPP and HBCDD increase membrane depolarization and death in hESCS differentiated in in vitro spermatogenic conditions. (D–E) Graphical representation showing that TDCPP and TDBPP decrease the percentage of healthy, live cells in in hESCS differentiated in in vitro spermatogenic conditions. 5,000 events were analyzed, with three replications performed for each condition. Significant changes in mitochondrial potential and live cells were determined using a 1-way analysis of variance (1-way ANOVA) and validated via a Student’s t-test, where * is p<0.05, ** is p<0.01, and *** is p<0.001. Data are represented as mean ± SEM.
HIGHLIGHTS.
Environmental toxicants may contribute to declining sperm counts in Western males
Flame retardants HBCDD and TBBPA affect spermatogonia and primary spermatocytes
HBCDD and TBBPA affect viability by affecting mitochondrial membrane potential
Acknowledgments
This work was supported by the National Science Foundation [DGE-1444932] to A.S., the National Institutes of Health [1K22 ES025418-01] to C.E., Emory University’s Atlanta Clinical & Translational Science Institute and University Research Committee (ACTSI/URC) award to C.E., and the NIH [P30 ES019776-01-A1] to G.M.
Footnotes
Supplemental Information includes Transparent Methods and six figures and can be found with this article online at https://doi.org/10.1016/j.isci.2018.04.014.
AUTHOR CONTRIBUTIONS
A.S. designed and conducted the experiments and wrote the paper. J.B., K.F., D.C.-T., B.G., and A.T. conducted the experiments. G.M. and W.M.C. designed the experiments. A.C. and C.A.E. designed the experiments, edited the paper, and served as the primary mentors to A.S.
DECLARATION OF INTERESTS
The authors declare no competing interests.
References
- Abafe OA, Martincigh BS. Determination and human exposure assessment of polybrominated diphenyl ethers and tetrabromobisphenol A in indoor dust in South Africa. Environ Sci Pollut Res Int. 2016;23:7038–7049. doi: 10.1007/s11356-015-6031-0. [DOI] [PubMed] [Google Scholar]
- Agarwal A, Virk G, Ong C, Du Plessis SS. Effect of oxidative stress on male reproduction. World J Mens Health. 2014;32:1–17. doi: 10.5534/wjmh.2014.32.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agency, U. S. E. P. SuppLemental file 1: TBBPA human biomonitoring data. [Google Scholar]
- Agency, U. S. E. P. Flame retardant alternatives for HEXABROMOCYCLODODECANE (HBCD) 2014. [Google Scholar]
- Aitken RJ, Baker MA. Causes and consequences of apoptosis in spermatozoa; contributions to infertility and impacts on development. Int J Dev Biol. 2013;57:265–272. doi: 10.1387/ijdb.130146ja. [DOI] [PubMed] [Google Scholar]
- Al-Mousa F, Michelangeli F. The sarcoplasmic-endoplasmic reticulum Ca(2+)-ATPase (SERCA) is the likely molecular target for the acute toxicity of the brominated flame retardant hexabromocyclododecane (HBCD) Chem Biol Interact. 2014;207:1–6. doi: 10.1016/j.cbi.2013.10.021. [DOI] [PubMed] [Google Scholar]
- Aly HA. Aroclor 1254 induced oxidative stress and mitochondria mediated apoptosis in adult rat sperm in vitro. Environ Toxicol Pharmacol. 2013;36:274–283. doi: 10.1016/j.etap.2013.04.006. [DOI] [PubMed] [Google Scholar]
- An J, Chen C, Wang X, Zhong Y, Zhang X, Yu Y, Yu Z. Oligomeric proanthocyanidins alleviate hexabromocyclododecane-induced cytotoxicity in HepG2 cells through regulation on ROS formation and mitochondrial pathway. Toxicol In Vitro. 2014;28:319–326. doi: 10.1016/j.tiv.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Attene-Ramos MS, Huang R, Sakamuru S, Witt KL, Beeson GC, Shou L, Schnellmann RG, Beeson CC, Tice RR, Austin CP, Xia M. Systematic study of mitochondrial toxicity of environmental chemicals using quantitative high throughput screening. Chem Res Toxicol. 2013;26:1323–1332. doi: 10.1021/tx4001754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Betts KS. Exposure to TDCPP appears widespread. Environ Health Perspect. 2013;121:a150. doi: 10.1289/ehp.121-a150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjermo H, Aune M, Cantillana T, Glynn A, Lind PM, Ridefelt P, Darnerud PO. Serum levels of brominated flame retardants (BFRs: PBDE, HBCD) and influence of dietary factors in a population-based study on Swedish adults. Chemosphere. 2017;167:485–491. doi: 10.1016/j.chemosphere.2016.10.008. [DOI] [PubMed] [Google Scholar]
- Bloom MS, Whitcomb BW, Chen Z, Ye A, Kannan K, Buck Louis GM. Associations between urinary phthalate concentrations and semen quality parameters in a general population. Hum Reprod. 2015;30:2645–2657. doi: 10.1093/humrep/dev219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum A, Gold MD, Ames BN, Jones FR, Hett EA, Dougherty RC, Horning EC, Dzidic I, Carroll DI, Stillwell RN, Thenot JP. Children absorb tris-BP flame retardant from sleepwear: urine contains the mutagenic metabolite, 2,3-dibromopropanol. Science. 1978;201:1020–1023. doi: 10.1126/science.684422. [DOI] [PubMed] [Google Scholar]
- Bonde JP, Flachs EM, Rimborg S, Glazer CH, Giwercman A, Ramlau-Hansen CH, Hougaard KS, Hoyer BB, Hærvig KK, Petersen SB, et al. The epidemiologic evidence linking prenatal and postnatal exposure to endocrine disrupting chemicals with male reproductive disorders: a systematic review and meta-analysis. Hum Reprod Update. 2016;23:104–125. doi: 10.1093/humupd/dmw036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carignan CC, McClean MD, Cooper EM, Watkins DJ, Fraser AJ, Heiger-Bernays W, Stapleton HM, Webster TF. Predictors of tris(1,3-dichloro-2-propyl) phosphate metabolite in the urine of office workers. Environ Int. 2013;55:56–61. doi: 10.1016/j.envint.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cariou R, Antignac JP, Zalko D, Berrebi A, Cravedi JP, Maume D, Marchand P, Monteau F, Riu A, Andre F, Le Bizec B. Exposure assessment of French women and their newborns to tetrabromobisphenol-A: occurrence measurements in maternal adipose tissue, serum, breast milk and cord serum. Chemosphere. 2008;73:1036–1041. doi: 10.1016/j.chemosphere.2008.07.084. [DOI] [PubMed] [Google Scholar]
- Chen SR, Liu YX. Regulation of spermatogonial stem cell self-renewal and spermatocyte meiosis by Sertoli cell signaling. Reproduction. 2015;149:R159–R167. doi: 10.1530/REP-14-0481. [DOI] [PubMed] [Google Scholar]
- Choi JS, Lee YJ, Kim TH, Lim HJ, Ahn MY, Kwack SJ, Kang TS, Park KL, Lee J, Kim ND, et al. Molecular mechanism of tetrabromobisphenol a (TBBPA)-induced target organ toxicity in Sprague-Dawley male rats. Toxicol Res. 2011;27:61–70. doi: 10.5487/TR.2011.27.2.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper EM, Covaci A, Van Nuijs AL, Webster TF, Stapleton HM. Analysis of the flame retardant metabolites bis(1,3-dichloro-2-propyl) phosphate (BDCPP) and diphenyl phosphate (DPP) in urine using liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2011;401:2123–2132. doi: 10.1007/s00216-011-5294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covaci A, Gerecke AC, Law RJ, Voorspoels S, Kohler M, Heeb NV, Leslie H, Allchin CR, De Boer J. Hexabromocyclododecanes (HBCDs) in the environment and humans: a review. Environ Sci Technol. 2006;40:3679–3688. doi: 10.1021/es0602492. [DOI] [PubMed] [Google Scholar]
- Crump D, Chiu S, Kennedy SW. Effects of tris(1,3-dichloro-2-propyl) phosphate and tris(1-chloropropyl) phosphate on cytotoxicity and mRNA expression in primary cultures of avian hepatocytes and neuronal cells. Toxicol Sci. 2012;126:140–148. doi: 10.1093/toxsci/kfs015. [DOI] [PubMed] [Google Scholar]
- Darnerud PO, Aune M, Larsson L, Lignell S, Mutshatshi T, Okonkwo J, Botha B, Agyei N. Levels of brominated flame retardants and other persistent organic pollutants in breast milk samples from Limpopo Province, South Africa. Sci Total Environ. 2011;409:4048–4053. doi: 10.1016/j.scitotenv.2011.05.054. [DOI] [PubMed] [Google Scholar]
- Deonandan R, Jaleel M. Global decline in semen quality: ignoring the developing world introduces selection bias. Int J Gen Med. 2012;5:303–306. doi: 10.2147/IJGM.S30673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishaw LV, Macaulay LJ, Roberts SC, Stapleton HM. Exposures, mechanisms, and impacts of endocrine-active flame retardants. Curr Opin Pharmacol. 2014;19:125–133. doi: 10.1016/j.coph.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson RE, Perovich LJ, Covaci A, Van Den Eede N, Ionas AC, Dirtu AC, Brody JG, Rudel RA. After the PBDE phase-out: a broad suite of flame retardants in repeat house dust samples from California. Environ Sci Technol. 2012;46:13056–13066. doi: 10.1021/es303879n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E.C.B. European Commission Directrate-General Joint Research Center, I. F. H. A. C. P. E., European Union Risk Assessment Report, European Chemicals Bureau, United Kingdom. European union risk assessment report. 2,2′,6,6′-Tetrabromo-4,4′-isopropylidenediphenol (tetrabromobisphenol-A or TBBP-A) Part II – Human health 2006 [Google Scholar]
- Easley CAT, Bradner JM, Moser A, Rickman CA, McEachin ZT, Merritt MM, Hansen JM, Caudle WM. Assessing reproductive toxicity of two environmental toxicants with a novel in vitro human spermatogenic model. Stem Cell Res. 2015;14:347–355. doi: 10.1016/j.scr.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easley CAT, Phillips BT, McGuire MM, Barringer JM, Valli H, Hermann BP, Simerly CR, Rajkovic A, Miki T, Orwig KE, Schatten GP. Direct differentiation of human pluripotent stem cells into haploid spermatogenic cells. Cell Rep. 2012;2:440–446. doi: 10.1016/j.celrep.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkekoglu P, Kocer-Gumusel B. Genotoxicity of phthalates. Toxicol Mech Methods. 2014;24:616–626. doi: 10.3109/15376516.2014.960987. [DOI] [PubMed] [Google Scholar]
- Fromme H, Becher G, Hilger B, Volkel W. Brominated flame retardants - exposure and risk assessment for the general population. Int J Hyg Environ Health. 2016a;219:1–23. doi: 10.1016/j.ijheh.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Fromme H, Hilger B, Albrecht M, Gries W, Leng G, Volkel W. Occurrenceofchlorinated and brominated dioxins/furans, PCBs, and brominated flame retardants in blood of German adults. Int J Hyg Environ Health. 2016b;219:380–388. doi: 10.1016/j.ijheh.2016.03.003. [DOI] [PubMed] [Google Scholar]
- Gold MD, Blum A, Ames BN. Another flame retardant, tris-(1,3-dichloro-2-propyl)-phosphate, and its expected metabolites are mutagens. Science. 1978;200:785–787. doi: 10.1126/science.347576. [DOI] [PubMed] [Google Scholar]
- Hu M, Li J, Zhang B, Cui Q, Wei S, Yu H. Regional distribution of halogenated organophosphate flame retardants in seawater samples from three coastal cities in China. Mar Pollut Bull. 2014;86:569–574. doi: 10.1016/j.marpolbul.2014.06.009. [DOI] [PubMed] [Google Scholar]
- Huang X, Chen C, Shang Y, Zhong Y, Ren G, Yu Z, An J. In vitro study on the biotransformation and cytotoxicity of three hexabromocyclododecane diastereoisomers in liver cells. Chemosphere. 2016;161:251–258. doi: 10.1016/j.chemosphere.2016.07.001. [DOI] [PubMed] [Google Scholar]
- Jakobsson K, Thuresson K, Rylander L, Sjodin A, Hagmar L, Bergman A. Exposure to polybrominated diphenyl ethers and tetrabromobisphenol A among computer technicians. Chemosphere. 2002;46:709–716. doi: 10.1016/s0045-6535(01)00235-1. [DOI] [PubMed] [Google Scholar]
- Jarosiewicz M, Bukowska B. Tetrabromobisphenol A - toxicity, environmental and occupational exposures. Med Pr. 2017;68:121–134. doi: 10.13075/mp.5893.00491. [DOI] [PubMed] [Google Scholar]
- Johnson PI, Stapleton HM, Mukherjee B, Hauser R, Meeker JD. Associations between brominated flame retardants in house dust and hormone levels in men. Sci Total Environ. 2013;445–446:177–184. doi: 10.1016/j.scitotenv.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano C, Wang J, Lin H. Uniting germline and stem cells: the function of Piwi proteins and the piRNA pathway in diverse organisms. Annu Rev Genet. 2011;45:447–469. doi: 10.1146/annurev-genet-110410-132541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke H. Tetrabromobisphenol a [79-94-7]: review of toxicological literature. 2002. [Google Scholar]
- Khosrorad T, Dolatian M, Riazi H, Mahmoodi Z, Alavimajd H, Shahsavari S, Bakhtiari M. Comparison of lifestyle in fertile and infertile couples in Kermanshah during 2013. Iran J Reprod Med. 2015;13:549–556. [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Nam KH, Hwang KA, Choi KC. Influence of hexabromocyclododecane and 4-nonylphenol on the regulation of cell growth, apoptosis and migration in prostatic cancer cells. Toxicol In Vitro. 2016;32:240–247. doi: 10.1016/j.tiv.2016.01.008. [DOI] [PubMed] [Google Scholar]
- Levine N, Martino-Andrade A, Mendiola J, Weksler-Derri D, Mindlis I, Pinotti R, Swan S. Temporal trends in sperm count: a systematic review and meta-regression analysis. Hum Reprod Update. 2017;23:646–659. doi: 10.1093/humupd/dmx022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Yang CQ, Jin J, Wang Y, Liu WZ, Ding WW. Correlations between HBCD and thyroid hormone concentrations in human serum from production source area. Huan Jing Ke Xue. 2014;35:3970–3976. [PubMed] [Google Scholar]
- Liang S, Yin L, Shengyang Yu K, Hofmann MC, Yu X. High-content analysis provides mechanistic insights into the testicular toxicity of bisphenol a and selected analogues in mouse spermatogonial cells. Toxicol Sci. 2017;155:43–60. doi: 10.1093/toxsci/kfw178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linhartova P, Gazo I, Shaliutina-Kolesova A, Hulak M, Kaspar V. Effects of tetrabromobisphenol A on DNA integrity, oxidative stress, and sterlet (Acipenser ruthenus) spermatozoa quality variables. Environ Toxicol. 2015;30:735–745. doi: 10.1002/tox.21953. [DOI] [PubMed] [Google Scholar]
- Liu LY, He K, Hites RA, Salamova A. Hair and nails as noninvasive biomarkers of human exposure to brominated and organophosphate flame retardants. Environ Sci Technol. 2016;50:3065–3073. doi: 10.1021/acs.est.5b05073. [DOI] [PubMed] [Google Scholar]
- Maiorino M, Ursini F. Oxidative stress, spermatogenesis and fertility. Biol Chem. 2002;383:591–597. doi: 10.1515/BC.2002.061. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Hauser R. Exposure to polychlorinated biphenyls (PCBs) and male reproduction. Syst Biol Reprod Med. 2010;56:122–131. doi: 10.3109/19396360903443658. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Stapleton HM. House dust concentrations of organophosphate flame retardants in relation to hormone levels and semen quality parameters. Environ Health Perspect. 2010;118:318–323. doi: 10.1289/ehp.0901332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neto FT, Bach PV, Najari BB, Li PS, Goldstein M. Spermatogenesis in humans and its affecting factors. Semin Cell Dev Biol. 2016;59:10–26. doi: 10.1016/j.semcdb.2016.04.009. [DOI] [PubMed] [Google Scholar]
- Peverly AA, Salamova A, Hites RA. Air is still contaminated 40 years after the Michigan Chemical plant disaster in St. Louis, Michigan Environ Sci Technol. 2014;48:11154–11160. doi: 10.1021/es502809f. [DOI] [PubMed] [Google Scholar]
- Phillips BT, Gassei K, Orwig KE. Spermatogonial stem cell regulation and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1663–1678. doi: 10.1098/rstb.2010.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaas A, Dokras A. Diagnosis and treatment of unexplained infertility. Rev Obstet Gynecol. 2008;1:69–76. [PMC free article] [PubMed] [Google Scholar]
- Rawn DF, Gaertner DW, Weber D, Curran IH, Cooke GM, Goodyer CG. Hexabromocyclododecane concentrations in Canadian human fetal liver and placental tissues. Sci Total Environ. 2014a;468–469:622–629. doi: 10.1016/j.scitotenv.2013.08.014. [DOI] [PubMed] [Google Scholar]
- Rawn DF, Ryan JJ, Sadler AR, Sun WF, Weber D, Laffey P, Haines D, Macey K, Van Oostdam J. Brominated flame retardant concentrations in sera from the Canadian health measures survey (CHMS) from 2007 to 2009. Environ Int. 2014b;63:26–34. doi: 10.1016/j.envint.2013.10.012. [DOI] [PubMed] [Google Scholar]
- Reistad T, Mariussen E, Ring A, Fonnum F. In vitro toxicity of tetrabromobisphenol-A on cerebellar granule cells: cell death, free radical formation, calcium influx and extracellular glutamate. Toxicol Sci. 2007;96:268–278. doi: 10.1093/toxsci/kfl198. [DOI] [PubMed] [Google Scholar]
- Ryter SW, Kim HP, Hoetzel A, Park JW, Nakahira K, Wang X, Choi AM. Mechanisms of cell death in oxidative stress. Antioxid Redox Signal. 2007;9:49–89. doi: 10.1089/ars.2007.9.49. [DOI] [PubMed] [Google Scholar]
- Schecter A, Szabo DT, Miller J, Gent TL, Malik-Bass N, Petersen M, Paepke O, Colacino JA, Hynan LS, Harris TR, et al. Hexabromocyclododecane (HBCD) stereoisomers in U.S. food from Dallas, Texas. Environ Health Perspect. 2012;120:1260–1264. doi: 10.1289/ehp.1204993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt CW. Unraveling environmental effects on mitochondria. Environ Health Perspect. 2010;118:A292–A297. [Google Scholar]
- Stapleton HM, Klosterhaus S, Keller A, Ferguson PL, Van Bergen S, Cooper E, Webster TF, Blum A. Identification of flame retardants in polyurethane foam collected from baby products. Environ Sci Technol. 2011;45:5323–5331. doi: 10.1021/es2007462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh KS, Choi EM, Rhee SY, Oh S, Kim SW, Pak YK, Choe W, Ha J, Chon S. Tetrabromobisphenol A induces cellular damages in pancreatic beta-cells in vitro. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2017;52:624–631. doi: 10.1080/10934529.2017.1294964. [DOI] [PubMed] [Google Scholar]
- Thomsen C, Lundanes E, Becher G. Brominated flame retardants in plasma samples from three different occupational groups in Norway. J Environ Monit. 2001;3:366–370. doi: 10.1039/b104304h. [DOI] [PubMed] [Google Scholar]
- Thomsen C, Leknes H, Lundanes E, Becher G. A new method for determination of halogenated flame retardants in human milk using solid-phase extraction. J Anal Toxicol. 2002a;26:129–137. doi: 10.1093/jat/26.3.129. [DOI] [PubMed] [Google Scholar]
- Thomsen C, Lundanes E, Becher G. Brominated flame retardants in archived serum samples from Norway: a study on temporal trends and the role of age. Environ Sci Technol. 2002b;36:1414–1418. doi: 10.1021/es0102282. [DOI] [PubMed] [Google Scholar]
- Thomsen C, Molander P, Daae HL, Janak K, Froshaug M, Liane VH, Thorud S, Becher G, Dybing E. Occupational exposure to hexabromocyclododecane at an industrial plant. Environ Sci Technol. 2007;41:5210–5216. doi: 10.1021/es0702622. [DOI] [PubMed] [Google Scholar]
- United Nations Environment Programme, I. L. O., World health organization. Flame Retardants: Tris(Chloropropyl) Phosphate And Tris(2-Chloroethyl) Phosphate. 2004. [Google Scholar]
- van der Veen I, de Boer J. Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis. Chemosphere. 2012;88:1119–1153. doi: 10.1016/j.chemosphere.2012.03.067. [DOI] [PubMed] [Google Scholar]
- Wang W, Abualnaja KO, Asimakopoulos AG, Covaci A, Gevao B, Johnson-Restrepo B, Kumosani TA, Malarvannan G, Minh TB, Moon HB, et al. A comparative assessment of human exposure to tetrabromobisphenol A and eight bisphenols including bisphenol A via indoor dust ingestion in twelve countries. Environ Int. 2015;83:183–191. doi: 10.1016/j.envint.2015.06.015. [DOI] [PubMed] [Google Scholar]
- Weissman TA, Pan YA. Brainbow: new resources and emerging biological applications for multicolor genetic labeling and analysis. Genetics. 2015;199:293–306. doi: 10.1534/genetics.114.172510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EW, Cheng CY. Impacts of environmental toxicants on male reproductive dysfunction. Trends Pharmacol Sci. 2011;32:290–299. doi: 10.1016/j.tips.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, McCarrey JR. Sex chromosome inactivation in the male. Epigenetics. 2009;4:452–456. doi: 10.4161/epi.4.7.9923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yard EE, Terrell ML, Hunt DR, Cameron LL, Small CM, McGeehin MA, Marcus M. Incidence of thyroid disease following exposure to polybrominated biphenyls and polychlorinated biphenyls, Michigan, 1974–2006. Chemosphere. 2011;84:863–868. doi: 10.1016/j.chemosphere.2011.06.020. [DOI] [PubMed] [Google Scholar]
- Yi S, Liu JG, Jin J, Zhu J. Assessment of the occupational and environmental risks of hexabromocyclododecane (HBCD) in China. Chemosphere. 2016;150:431–437. doi: 10.1016/j.chemosphere.2016.01.047. [DOI] [PubMed] [Google Scholar]
- Zatecka E, Castillo J, Elzeinova F, Kubatova A, Ded L, Peknicova J, Oliva R. The effect of tetrabromobisphenol A on protamine content and DNA integrity in mouse spermatozoa. Andrology. 2014;2:910–917. doi: 10.1111/j.2047-2927.2014.00257.x. [DOI] [PubMed] [Google Scholar]
- Zatecka E, Ded L, Elzeinova F, Kubatova A, Dorosh A, Margaryan H, Dostalova P, Peknicova J. Effect of tetrabromobisphenol A on induction of apoptosis in the testes and changes in expression of selected testicular genes in CD1 mice. Reprod Toxicol. 2013;35:32–39. doi: 10.1016/j.reprotox.2012.05.095. [DOI] [PubMed] [Google Scholar]
- Zhang J, Williams TD, Chipman JK, Viant MR. Defensive and adverse energy-related molecular responses precede tris (1, 3-dichloro-2-propyl) phosphate cytotoxicity. J Appl Toxicol. 2016;36:649–658. doi: 10.1002/jat.3194. [DOI] [PubMed] [Google Scholar]
- Zhang X, Yang F, Zhang X, Xu Y, Liao T, Song S, Wang J. Induction of hepatic enzymes and oxidative stress in Chinese rare minnow (Gobiocypris rarus) exposed to waterborne hexabromocyclododecane (HBCDD) Aquat Toxicol. 2008;86:4–11. doi: 10.1016/j.aquatox.2007.07.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Related to Figure 1. TDCPP, TDBPP, HBCDD and TBBPA induce apoptosis in spermatogenic cells derived from hESCs. (A) Flow cytometry analyses indicating percent viable cells, percent early apoptotic cells, percent late apoptotic cells, and percent dead/necrotic cells for all concentrations of TDCPP, TDBPP, HBCDD, and TBBPA assessed. Lower left quadrant represents viable cells, lower right quadrant represents early apoptotic cells, upper right quadrant is late apoptotic/dead cells, and the upper left quadrant is dead/necrotic cells. (B–C) Graphical representation showing that TDCPP and TDBPP decrease germ cell viability in hESCs differentiated in in vitro spermatogenic conditions. (D–E) Graphical representation showing that TDCPP and TDBPP increase the percentage of germ cells undergoing late apoptosis/death in spermatogenic cells derived from hESCs. (F–G) Graphical representation showing that TDCPP and TDBPP increase apoptotic luminescence in hESCs differentiated in in vitro spermatogenic conditions. (H–I) Graphical representation showing that TDCPP and TDBPP decreased viability fluorescence in hESCs differentiated in in vitro spermatogenic conditions. 5,000 events were analyzed, with five replications performed for each condition for A–E. Three replications were analyzed for F–I. Significant changes in cell viability were determined using a 1-way analysis of variance (1-way ANOVA) and validated via a Student’s t-test, where * is p<0.05, ** is p<0.01, and *** is p<0.001. Data are represented as mean ± SEM.
Figure S2. Related to Figure 2. TDCPP, TDBPP, HBCDD, and TBBPA exposure impact PLZF expression. (A) mRNA steady state transcripts for ZBTB16 (PLZF) correspond to decreases in PLZF+ area and intensity in immunostaining data for TDCPP, TDBPP, HBCDD, and TBBPA. (B) Graphical representation showing that PLZF intensity in spermatogonia derived under in vitro spermatogenic conditions treated with TBBPA remain below control levels following a twenty-four hour, chemical-free recovery period. (C) Graphical representation showing that PLZF area in spermatogonia derived under in vitro spermatogenic control conditions remain statistically the same after a twenty four hour recovery period. (D) Graphical representation showing that PLZF intensity in spermatogonia derived under in vitro spermatogenic control conditions remain statistically the same after a twenty four hour recovery period. (E–F) Graphical representation showing that TDCPP and TDBPP reduce average PLZF+ area in spermatogonia derived under in vitro spermatogenic conditions. (G–H) Graphical representation showing that TDCPP and TDBPP reduce average PLZF+ intensity in spermatogonia. mRNA transcript levels were normalized to 0.2% DMSO-only control. Two separate replications were performed in duplicate (n=4) for each condition. Significant changes in mRNA steady state levels were determined using Bio-Rad CFX Manager™ Software (Bio-Rad, Hercules, CA). Five replications were performed for each condition for PLZF immunostaining for E–H. Three replications were performed for B–D. Significant changes in PLZF+ area and intensity were determined using a 1-way analysis of variance (1-way ANOVA) and validated via a Student’s t-test, where * is p<0.05, ** is p<0.01, and *** is p<0.001. Data are represented as mean ± SEM.
Figure S3. Related to Figure 3. TDCPP, TDBPP, HBCDD, and TBBPA exposure impact HILI expression. (A) mRNA steady state transcripts for PIWIL2 (HILI) correspond to increases in HILI+ area and intensity in immunostaining data corresponding to certain concentrations of TDCPP, TDBPP, HBCDD, and TBBPA. (B) Graphical representation showing that HILI area in primary spermatocytes derived under in vitro spermatogenic control conditions remain statistically the same after a twenty four hour recovery period. (C–D) Graphical representation showing that TDCPP and TDBPP impact average total HILI+ area in primary spermatocytes derived under in vitro spermatogenic conditions. (E–F) Graphical representation showing that TDCPP and TDBPP impact average total HILI+ intensity in primary spermatocytes. mRNA transcript levels were normalized to 0.2% DMSO-only control. Two separate replications were performed in duplicate (n=4) for each condition. Significant changes in mRNA steady state levels were determined using Bio-Rad CFX Manager™ Software (Bio-Rad, Hercules, CA). Five replications were performed for each condition for HILI immunostaining for C–F. Three replications were performed for B. Significant changes in HILI+ area and intensity were determined using a 1-way analysis of variance (1-way ANOVA) and validated via a Student’s t-test, where * is p<0.05, ** is p<0.01, and *** is p<0.001. Data are represented as mean ± SEM.
Figure S4. Related to Figure 4. TDCPP, TDBPP, HBCDD, and TBBPA affect the cell cycle in spermatogenic cells derived from hESCs without impacting haploid cell production. (A) Flow cytometry analyses of cell cycle profiles following acute twenty-four hour treatment. Green, blue, purple, and beige populations on flow cytometry correspond to haploid, G0/G1, S, and G2 phases, respectively. (B–C) Graphical representation showing that TDCPP and TDBPP affect the cell cycle of actively dividing hESCS differentiated in in vitro spermatogenic conditions. (D–E) Graphical representation showing that TDCPP and TDBPP exposure increases the percentage of haploid cells in spermatogenic cells derived from hESCs. 5,000 events were analyzed, with five replications performed for each condition. Significant changes in percentages of haploid cells and cells in G0/G1, S phase, and G2 were determined using a 1-way analysis of variance (1-way ANOVA) and validated via a Student’s t-test, where * is p<0.05, ** is p<0.01, and *** is p<0.001. Data are represented as mean ± SEM.
Figure S5. Related to Figure 5. TDCPP, TDBPP, HBCDD, and TBBPA impact ROS production and GSH/GSSG ratios. (A) Flow cytometry analyses indicating percent ROS− and ROS+ cells for all concentrations of TDCPP, TDBPP, HBCDD, and TBBPA assessed. ROS− cells are labeled blue. ROS+ cells are labeled red. (B–C) Graphical representation showing that TDCPP and TDBPP do not overwhelmingly increase ROS+ cells in hESCs differentiated in in vitro spermatogenic conditions. (D–E) Graphical representation showing that TDCPP and TDBPP decrease the GSH/GSSG ratio of hESCs differentiated in in vitro spermatogenic conditions. 5,000 events were analyzed, with three replications performed for each condition for A–C. Three replications were performed for each condition for D–E. Significant changes in ROS generation and GSH/GSSG ratio were determined using a 1-way analysis of variance (1-way ANOVA) and validated via a Student’s t-test, where * is p<0.05, ** is p<0.01, and *** is p<0.001. Data are represented as mean ± SEM.
Figure S6. Related to Figure 6. TDCPP, TDBPP, HBCDD, and TBBPA decrease mitochondrial membrane potential and the percentage of healthy, live cells in in vitro spermatogenic cultures.. (A) Flow cytometry analyses indicating percent live cells, percent a depolarized/live cells, percent depolarized/dead cells, and percent dead cells. Lower right quadrant represents viable cells, lower left quadrant represents depolarized/live cells, upper right quadrant is depolarized/dead cells, and the upper right quadrant is dead cells. (B–C) Graphical representation showing that TDCPP and HBCDD increase membrane depolarization and death in hESCS differentiated in in vitro spermatogenic conditions. (D–E) Graphical representation showing that TDCPP and TDBPP decrease the percentage of healthy, live cells in in hESCS differentiated in in vitro spermatogenic conditions. 5,000 events were analyzed, with three replications performed for each condition. Significant changes in mitochondrial potential and live cells were determined using a 1-way analysis of variance (1-way ANOVA) and validated via a Student’s t-test, where * is p<0.05, ** is p<0.01, and *** is p<0.001. Data are represented as mean ± SEM.