Abstract
Purpose
We report the first randomized trial to directly compare outcomes after passively scattered proton therapy (PSPT) versus intensity-modulated (photon) radiotherapy (IMRT), both with concurrent chemotherapy, for inoperable non-small-cell lung cancer (NSCLC) (NCT00915005). We hypothesized that PSPT exposes less lung tissue to radiation than IMRT, thereby reducing toxicity without compromising tumor control. The primary endpoints were grade ≥3 radiation pneumonitis (RP) and local failure (LF).
Patients and Methods
Eligible patients had stage IIB-IIIB NSCLC (or stage IV with a single brain metastasis or recurrent lung or mediastinal disease after surgery) who were candidates for concurrent chemoradiation therapy. Pairs of treatment plans (for IMRT and PSPT) were created for each patient. Patients were eligible for randomization only if both plans satisfied the same prespecified dose–volume constraints for organs at risk at the same tumor dose.
Results
Compared with IMRT (n=92), PSPT (n=57) exposed less lung to doses of 5–10 Gy(RBE) (absorbed dose [in Gy] × the relative biological effectiveness factor [RBE] for protons); more lung to ≥20 Gy(RBE); and less heart at all dose levels (5–80 Gy(RBE)). The grade ≥ 3 RP rate for all patients was 8.1% (6.5% IMRT, 10.5% PSPT); corresponding LF rates were 10.7%, 10.9%, and 10.5%. The posterior probability of IMRT being better than PSPT was 0.54. Exploratory analysis showed that RP+LF rates at 12 months for patients enrolled before versus after the trial midpoint were 21.1% versus 18.2% for the IMRT group (P=0.047) and 31.0% versus 13.1% for the PSPT group (P=0.027).
Conclusion
PSPT did not improve dose–volume indices for lung but did for heart. No benefit was noted in RP or LF after PSPT. Improvements in both endpoints were observed over the course of the trial.
INTRODUCTION
The current standard of care for locally advanced non–small-cell lung cancer (NSCLC), concurrent chemoradiation therapy, produces a median survival time of 28.7 months.1 Radiation-induced toxicity to normal tissues, particularly radiation pneumonitis (RP), negatively affects both survival and quality of life.1,2 We previously showed that the development of RP depends on the radiation dose to a threshold volume of lung (Vdose),3,4 and mean lung dose (MLD) is also highly predictive of RP.4,5 Advances in the planning and delivery of photon (X-ray) radiation such as intensity-modulated radiation therapy (IMRT) have reduced RP incidence by minimizing both the radiation dose and volume of lung exposed relative to 3-dimensional conformal techniques.2,6 Ostensibly, proton therapy has the potential to further reduce exposure of the lung (and presumably RP) because of the differences in physical characteristics of photons and protons—theoretically, protons allow higher, more focused radiation doses to be delivered to the tumor, with less exposure of surrounding tissues. These presumptions have led some to conclude that the superiority of protons over photons is so obvious that randomized trials comparing protons with photons would be unnecessary, inappropriate, or even unethical.7,8,9
Proton therapy is more costly than even the best currently available photon technology, and evidence demonstrating clinical benefit is increasingly demanded to justify the financial burden on the healthcare system. However, randomized trials comparing outcomes between different treatment technologies are practically nonexistent, and trials of protons vs. photons are no exception. Rather, the potential of protons vs. photons is typically assessed from treatment-plan comparisons, with validation of such results coming largely from retrospective analyses of small single-institution studies, meta-analyses of multi-institutional studies, or reviews of large databases. Although randomized controlled trials are not the only mechanism for producing high-level evidence, they are traditionally considered the “gold standard.”10
We hypothesized that proton therapy exposes significantly less lung tissue to radiation than does photon therapy, thus reducing toxicity without compromising tumor control. We tested this hypothesis in a Bayesian trial in which patients underwent adaptive randomization to IMRT or 3-dimensional passive scattering proton therapy (PSPT) for inoperable NSCLC. Adaptive randomization uses real-time assessment of accumulated outcome data to efficiently detect differences between arms and to allow the ratio of patient allocation to be adjusted based on ongoing assessment of the risk of failure so that more patients are allocated to the better treatment if a difference exists between the arms; if no difference exists, the ratio would converge to the classic 1:1 allocation.7,9,11,12
METHODS
Study design and patient eligibility and enrollment
This trial was designed to compare the toxicity and effectiveness of PSPT with that of standard IMRT, both with concurrent chemotherapy, for patients with locally advanced NSCLC (Appendix: Protocol). Patients were treated at The University of Texas MD Anderson Cancer Center and at Massachusetts General Hospital. The protocol was approved by the institutional review boards of both institutions, and all enrollees provided written informed consent to participate.
Eligibility criteria included age >18 years, Karnofsky Performance Status score ≥70, stage II–IIIB disease, or stage IV disease with a single brain metastasis, or recurrent tumor after surgical resection that could be treated definitively with concurrent chemoradiation, and baseline pulmonary function of forced expiratory volume in the first second of ≥1 liter. Patients who had received systemic chemotherapy regardless of response before enrollment were also eligible. Eligible patients consented to participate after they were evaluated and deemed suitable candidates for concurrent chemoradiation.
The primary endpoint was the first occurrence of either severe (grade ≥3) RP or local failure (LF). The choice to use two primary endpoints emphasized the importance of being free of RP, a potentially lethal form of toxicity, in addition to local disease control. From our historical data13,14 we assumed 15% RP rates at 1 year in the IMRT group and 5% in the PSPT group; we further assumed a 25% LF rate in both groups (because the prescribed dose to tumor was the same in both arms by design). With a maximum of 150 randomized and evaluable patients, we would have 81% power to detect such a difference with a one-sided type I error rate of ≥10%. The posterior probability of PSPT being better than IMRT based on the primary endpoints was to be reported. The Bayesian adaptive design was constructed to possess the desirable frequentist properties. Detailed information on assumptions for the study design and trial operating characteristics can be found in the study protocol (Appendix: Protocol).
Radiation treatment planning and randomization
Each patient underwent standard radiation treatment-planning procedures, including four-dimensional computed tomography (4D CT) for motion assessment and target delineation, and development of pairs of IMRT and PSPT treatment plans for dosimetric comparison. Commercial treatment planning systems were used to create plans for each modality. Beginning in the first year of the protocol, the IMRT planning system was supplemented with an in-house automated optimization algorithm that improved plan quality.15
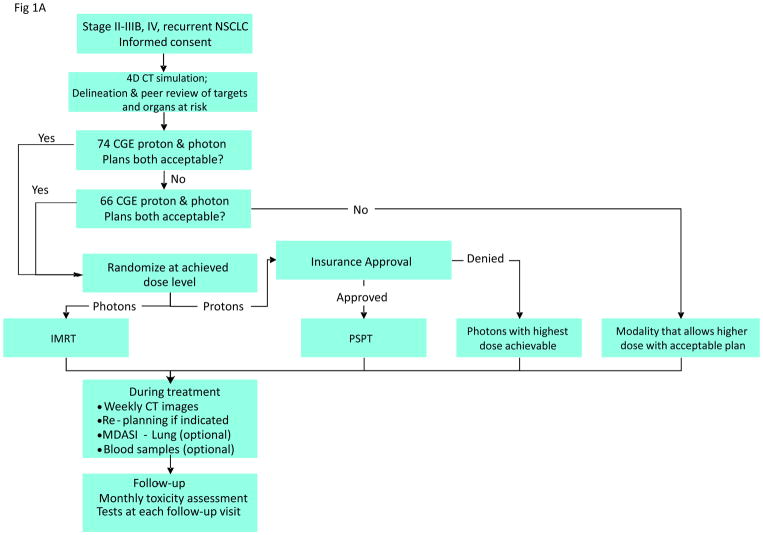
Both plans were evaluated according to prespecified dose–volume constraints (Suppl. Table S1) developed for photon radiotherapy. The prescribed tumor dose was 74 or 66 Gy(RBE), whichever could be achieved safely within these constraints. [Gy(RBE) is the unit of absorbed dose of protons; it represents the absorbed dose for photons (in Gy) multiplied by the relative biological effectiveness factor (RBE) for protons.] Patients were eligible for randomization only if both plans satisfied the constraints on lung V20 and MLD. The initial 20 patients were randomized equally. Subsequently, patients were adaptively randomized, with the randomization probability proportional to the 1-year failure rate in each of the two arms. Observed RP or LF events were updated as they occurred. Patients with un-randomizable plans (i.e., those not meeting constraints) received either PSPT or IMRT, whichever produced the acceptable plan (Fig. 1A). All patients received standard platinum- and taxane-based chemotherapy concurrent with radiotherapy, with pemetrexed allowed for adenocarcinoma. Our experience indicated that use of different chemotherapy agents routinely prescribed for NSCLC would not affect the primary endpoints tested in this study.16
Figure 1.
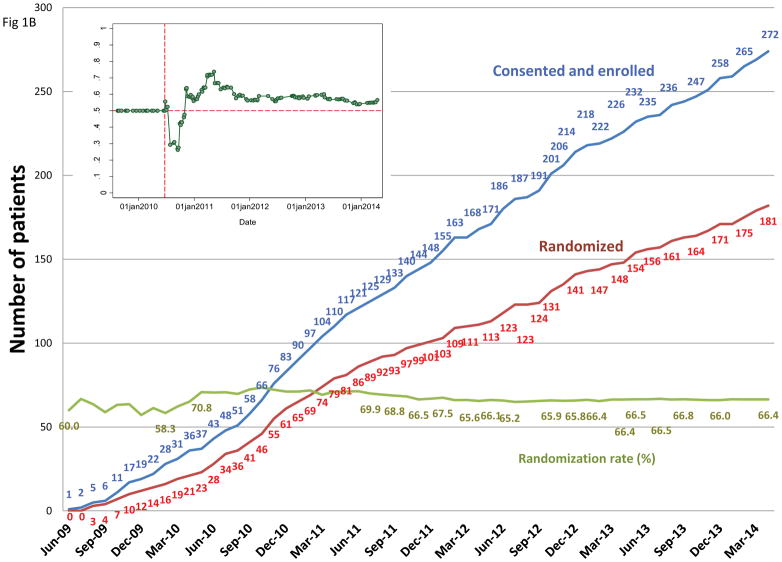
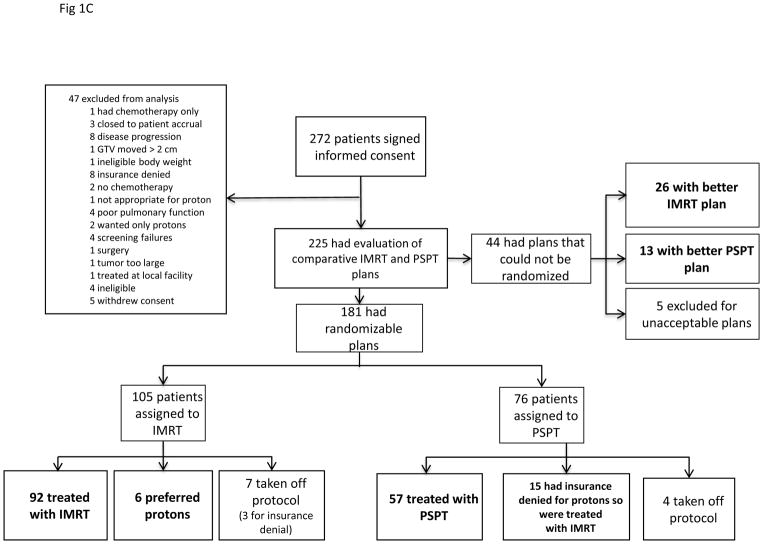
A, The adaptive-randomization process. Eligibility criteria included stage II–IIIB non-small cell lung cancer (NSCLC) or stage IV NSCLC with a single brain metastasis or isolated tumor recurrence after surgical resection; 50% of patients had disease progression after systemic chemotherapy before enrollment. All patients underwent four-dimensional computed tomography (4D CT) -based treatment simulation, and target volume contours and preliminary plans were reviewed before randomization. The prescribed dose for both sets of treatment plans (intensity-modulated [photon] radiation therapy [IMRT] and passive scattered proton therapy [PSPT]) was 74 Gy(RBE). If one of the two plans did not meet prespecified dose constraints (Suppl Table S1), the prescribed dose was reduced to 66 Gy for a second pair of plans. Patients were randomized only when both IMRT and PSPT plans met the dose-constraint standards. If one of the two plans did not meet dose constraints at the 66-Gy(RBE) level, the patient was treated with the modality that produced the acceptable dose distribution. During treatment, weekly 4D CT scans were obtained for all patients and additional treatment plans were created as needed to account for anatomic changes during treatment. To ensure that the radiation pneumonitis events (“toxicity assessment”) was noted accurately, patients were contacted weekly with a questionnaire to assess symptoms of pneumonitis. B, Cumulative patient randomization (red) and enrollment (blue) over time. Inset, posterior probability of randomization to IMRT, with the vertical dashed line representing the last date (June 22, 2010) at which the randomization probability was 0.5. The first patient was randomized on August 17, 2009 and the last patient on April 18, 2014; 60% to 67% of all patients who consented to participate were eligible for randomization. C, Trial profile. The final numbers of patients included in the analysis are shown in bold.
Assessment of primary endpoints
RP was scored with the Common Terminology Criteria for Adverse Events (CTCAE) v3.0. The following factors were requred for a diagnosis of RP: receipt of radiation that included a certain volume of normal lung; radiographic changes suggesting inflammation consistent with the radiation dose distribution within 12 months after starting chemoradiation; and symptoms attributable to RP. LF was defined as failure within the planning target volume plus a ≥1 cm margin. Images used to report LF were registered with radiation dose distribution to accurately assess the location of the failure. Biopsy to confirm LF was strongly recommended (Appendix: Protocol). To ensure objectivity and consistency in reporting RP and LF, an internal Outcomes Review Committee was formed to review each event. Final RP outcomes were also reviewed and approved by a group of independent external experts.
Data analysis
Associations of categorical variables were analyzed with Fisher’s exact tests. Differences in continuous variables between two groups were analyzed with Wilcoxon rank-sum tests. Survival times were calculated from the protocol registration date to the first occurrence of the considered event. A Cox proportional hazards model was used for univariate and multivariate analysis to assess the effect of patient, tumor, and other characteristics on the endpoints.
Data were analyzed in three ways: (1) randomized and treated according to randomization (“randomized,” n=149); (2) intent-to-treat (n=173); and (3) non-randomizable (n=39), see Fig. 1C, grouped according to treatment modality received. We present the results of only the first, the “randomized” analysis; results for the intent-to-treat and non-randomizable groups are presented in the supplementary material. Competing-risks regression analysis was done according to Fine and Gray.17 For multivariate analyses, all factors with P 0.25 in univariate analysis were included in the analysis, and backwards elimination was done with the most parsimonious multivariate model presented. Subhazard ratios for the failure event of primary interest (grade ≥3 RP and LF) are reported. Competing events for the primary endpoints were distant metastases and death, from disease or other causes. In exploratory analyses, we also evaluated whether a “learning curve” (i.e., improvements in the design or delivery of radiation using either method [IMRT or PSPT]) over the course of the study may have influenced the outcomes by analyzing patients according to time of enrollment (before or after the trial midpoint [September 27, 2011]).
RESULTS
A total of 272 patients consented to participate from June 2009 through March 2014 (Fig. 1B); 47 patients were excluded before the planning process began (8 for denial of insurance coverage of proton treatment; other reasons are shown in Fig. 1C). A total of 225 enrolled patients completed the planning process, with two plans each; 181 of those patients had randomizable plans, and 149 were treated according to randomization (IMRT=92, PSPT=57). Of the 181 patients with randomizable plans, 32 were not treated according to protocol allocation because of insurance denial of PSPT (n=15; all received IMRT), insurance denial of protocol (n=3, off protocol), patient preference for PSPT (n=6), or other reasons (n=8) (Fig. 1C). Another 44 patients did not have randomizable plans and either were treated with the modality that produced the acceptable dose distribution (26 IMRT and 13 PSPT) or were not treated on protocol because a definitive radiation plan could not be achieved (n=5) (Fig. 1C). Patient- and tumor-related characteristics did not differ between groups (Table 1; Suppl. Table S2).
Table 1.
Patient, Tumor, and Radiation Characteristics Among the 149 Randomized Patients
| Characteristics | All | IMRT | PSPT | P Value |
|---|---|---|---|---|
| No. of Patients (%) | 149 (100) | 92 (61.7) | 57 (38.3) | |
| Sex | ||||
| Female | 69 (46.3) | 45 (30.2) | 24 (16.1) | 0.499 |
| Male | 80 (53.6) | 47 (31.5) | 33 (22.1) | |
| Median age, years | 66 (33–85) | 66 (33–85) | 67 (39–78) | |
| <65 | 61 (40.9) | 39 (26.2) | 22 (14.8) | 0.732 |
| ≥65 | 88 (59.1) | 53 (35.6) | 35 (23.5) | |
| Ethnicity | ||||
| Caucasian | 130 (87.2) | 80 (53.7) | 50 (33.6) | 0.764 |
| Hispanic or Latino | 7 (4.7) | 4 (2.7) | 3 (2.0) | |
| Black or African American | 10 (6.7) | 7 (4.7) | 3 (2.0) | |
| Asian | 1 (0.7) | 0 (0) | 1(0.7) | |
| Other | 1 (0.7) | 1 (0.7) | 0 (0) | |
| Karnofsky Performance | ||||
| Status score | ||||
| ≤80 | 98 (65.8) | 61 (40.9) | 37 (24.8) | 0.861 |
| ≥90 | 51 (34.2) | 31 (20.8) | 20 (13.4) | |
| Smoking History | ||||
| Never smoked | 12 (8.1) | 9 (6.0) | 3 (2.0) | 0.374 |
| Ever smoked | 137 (91.9) | 83 (55.7) | 54 (36.2) | |
| Pulmonary Function | ||||
| FEV1, L, median (range)a | 2.03 (0.58–4.25) | 2.08 (0.72–4.18) | 1.97 (0.58–4.25) | |
| FEV1, % predicted, median (range)a | 74.0 (20–131) | 75.0 (20–119) | 72 (29–131) | |
| DLCO, % predicted, median (range)b | 69.5 (27–123) | 68.5 (27–121) | 70.5 (34–123) | |
| Induction Chemotherapy | ||||
| Yes | 103 (69.1) | 61 (40.9) | 42 (28.2) | 0.368 |
| No | 46 (30.9) | 31 (20.8) | 15 (10.1) | |
| Tumor Histology | ||||
| Adenocarcinoma | 79 (53.0) | 49 (32.9) | 30 (20.1) | 0.405 |
| SCC | 48 (32.2) | 31 (20.8) | 17 (11.4) | |
| NSCLC Unspecified | 16 (10.7) | 7 (4.7) | 9 (6.0) | |
| Large Cell | 3 (2.0) | 2 (1.3) | 1 (0.7) | |
| Other | 3 (2.0) | 3 (2.0) | 0 (0) | |
| Clinical Disease Stage | ||||
| IIA/B | 14 (9.4) | 6 (4.0) | 8 (5.4) | 0.159 |
| IIIA | 65 (43.6) | 44 (29.5) | 21 (14.1) | |
| IIIB | 52 (34.9) | 28 (18.8) | 24 (16.1) | |
| IV | 8 (5.4) | 5 (3.4) | 3 (2.0) | |
| Recurrence | 10 (6.7) | 9 (6.0) | 1 (0.7) | |
| Target Volumes, median (range) | ||||
| Gross tumor volume, cm3 | 70.3 (1.9–686.6) | 66.1 (5.8–686.6) | 77.7 (1.9–673.7) | 0.141 |
| Internal target volume, cm3 | 292.7 (30.0–1384.0) | 257.66 (42.0–1316.2) | 320.7 (30.0–1384.0) | 0.055 |
| Planning target Volume, cm3 | 480.3 (76–1906.0) | 429.4 (103.9–1776.1) | 524.9 (76.0–1906.0) | 0.071 |
| Radiation Doses to Critical Organs at Risk, Gy(RBE) | ||||
| Mean lung dose | 16.5 (0.4–22.1) | 16.6 (0.4–22.7) | 16.1 (6.9–22.1) | 0.818 |
| Mean esophagus dose | 25.7 (0.04–49.9) | 23.9 (3.36–47.62) | 23.6 (0.04–49.9) | 0.717 |
| Mean heart dose | 7.4 (0.4–34.6) | 10.1 (0.6–34.6) | 5.9 (0.4–21.1) | 0.002 |
IMRT, intensity-modulated radiation therapy; PSPT, passive scattered proton therapy; FEV1, forced expiratory volume in 1 second; DCLO, diffusion capacity of the lung for carbon monoxide (normalized for Hgb; in mm CO per min per mm Hg); SCC, squamous cell carcinoma; NSCLC, non-small cell lung cancer; Gy(RBE), unit dose for proton therapy; represents [dose in Gy] × relative biological effectiveness factor [RBE].
Data available for 161 patients (98 IMRT and 63 PSPT)
Data available for 116 patients (68 IMRT and 48 PSPT)
Values are number (%) or median (range)
Radiation dose metrics by treatment modality
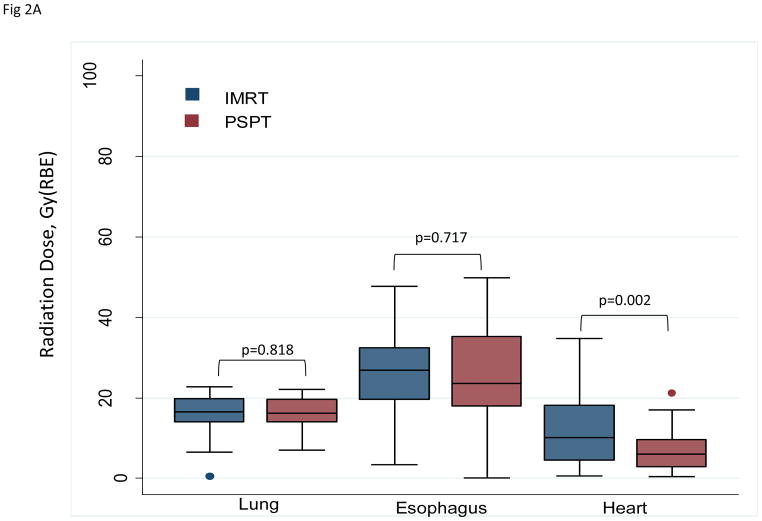
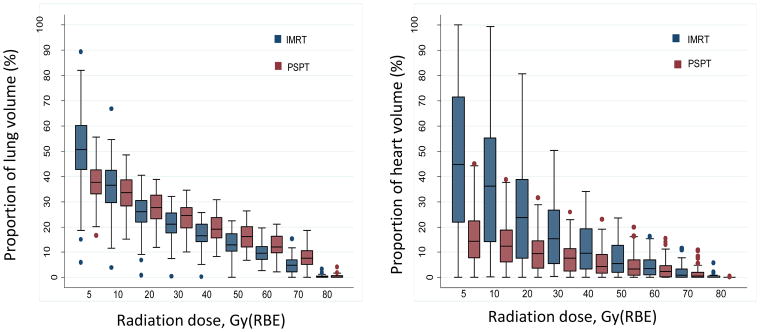
PSPT significantly reduced the mean radiation dose to the heart (P=0.002), but no differences were found between IMRT and PSPT in the mean doses to the lung or esophagus (Table 1; Fig. 2A) (Suppl. Table S2; Suppl. Fig. S1). Compared with IMRT, PSPT reduced the lung V5–10 but increased lung V20–80 (Fig. 2B, left). PSPT spared more heart than did IMRT at all dose levels measured (Fig. 2B, right). These comparisons are shown for the intent-to-treat and the non-randomizable groups in Supplementary Figure S2.
Figure 2.
A, Box-and-whiskers plot of mean radiation doses to the lung, esophagus, and heart. Mean doses to the lung and esophagus were no different between the two treatment groups (intensity-modulated radiation therapy [IMRT] and passive scatter proton therapy [PSPT]), but the mean heart dose was lower for those treated with protons (P=0.002). Whiskers indicate 1.5 times the interquartile range above and below the boxes; dots represent outliers. B, Box-and-whiskers plot of distributions of dose–volume indices for the lung (right) and heart (left). The y axis is the percentage of the organ at risk (lung or heart) that received the indicated radiation dose in Gy(RBE) on the x axis. PSPT led to smaller volumes of lung being exposed to low doses (V5–V10) and larger volumes of lung exposed to ≥20 Gy(RBE). Conversely, PSPT reduced the volume of heart exposed to all dose levels measured (5–80 Gy(RBE)). Whiskers indicate 1.5 times the interquartile range above and below the boxes; dots represent outliers.
Primary endpoints by treatment modality
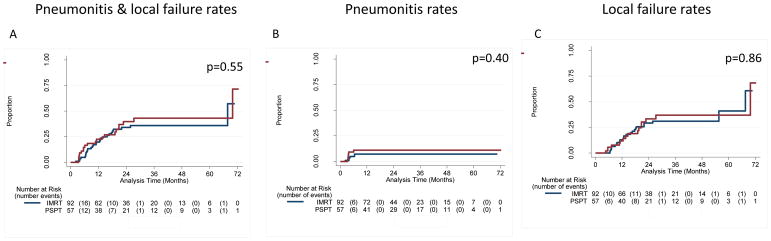
The median follow-up times for the IMRT group were 24.1 months for all patients and 36.4 months for patients alive at the time of analysis; corresponding median follow-up times for the PSPT group were 25.7 months (all patients) and 48.8 months (surviving patients). Twelve patients developed grade ≥3 RP, six in each group; at 1 year, the RP rates were 8.1% for all patients (6.5% for IMRT and 10.5% for PSPT; P=0.537). Two patients in the IMRT group had grade 5 RP; no patients in the PSPT group had grade 4 or 5 RP. Rates of LF at 12 months were 10.7% for all patients (10.9% for IMRT patients and 10.5% for PSPT patients; P=1.0); all LF rates were considerably lower than the 25% assumed in the trial design. Combined rates of RP and LF at 12 months were 17.4% after IMRT and 21.1% after PSPT (P=0.175) (Fig. 3, Suppl. Fig. S3). The median overall survival times were 29.5 months for the IMRT group and 26.1 months for the PSPT group (P=0.297) (Suppl. Fig. S4). The posterior probability of IMRT being better than PSPT was 0.54. Results from the analyses of patients grouped as intent-to-treat or non-randomizable were consistent with the results reported here (Suppl. Fig. S4).
Figure 3.
Cumulative incidence of severe (grade 3) radiation pneumonitis (RP) plus that of local failure (LF) according to treatment among patients who were randomized and treated according to randomization. A, Cumulative incidence of RP+LF for patients treated with intensity-modulated (photon) radiation therapy (IMRT; blue) or passive scatter proton therapy (PSPT; red). B and C, Cumulative incidence of RP and LF. Results were similar when patients were analyzed by intent-to-treat or by treatment received (not shown).
Primary endpoints by time of enrollment
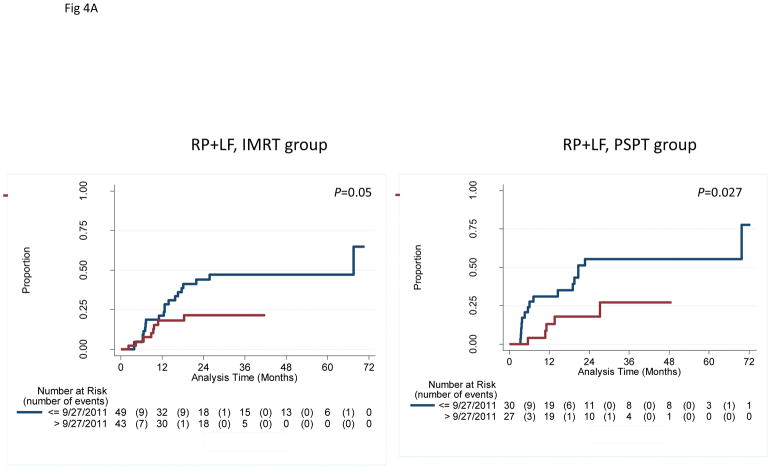
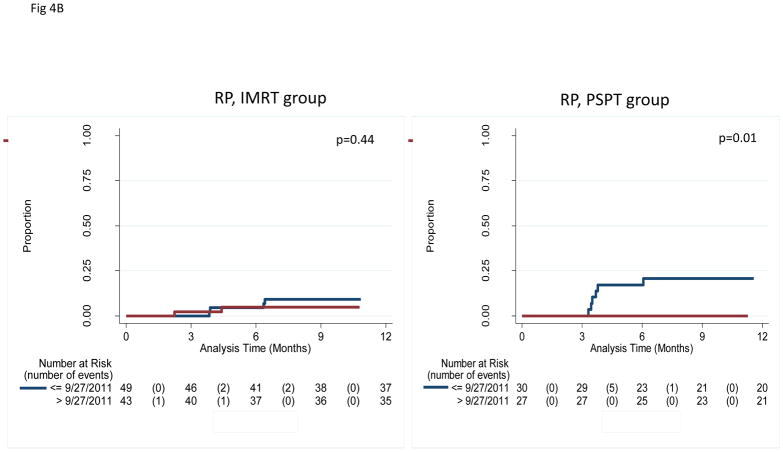
In an exploratory analysis to assess the potential influence of time of enrollment, we grouped patients as having enrolled before the study midpoint of September 27, 2011 (“early”) or afterward (“later”). No differences in clinical characteristics were noted between early vs. later patients in the IMRT group, but in the PSPT group, more patients in the later group had adenocarcinoma (14% vs. 6% in the early group, P<0.001) and the later group had considerably smaller gross tumor volumes (GTVs) (56.0 cm3 vs. 150.6 cm3 in the early group, P=0.01) (Suppl Table S3; corresponding values for the intent-to-treat and non-randomizable groups are included as Suppl. Tables S4 and S5). Moreover, the combined rates of grade ≥3 RP plus LF at 12 months also differed according to time of enrollment: in the IMRT group, those rates were 21.1% for the early group and 18.2% for the later group (P=0.047); and in the PSPT group, 31.0% early vs. 13.1% later (P=0.027) (Fig. 4A; Suppl. Fig. S5). Notably, all 6 grade ≥3 RP events in the PSPT group occurred in the early group, whereas in the IMRT group, grade ≥3 RP occurred throughout the trial (Fig. 4B; Suppl. Fig. S6).
Figure 4.
A, Cumulative incidence of radiation pneumonitis (RP) plus that of local failure (LF) among patients who were randomized and treated according to time of enrollment (early=before the midpoint of the trial September 2011 [blue lines, median follow-up time 29 months]; later=after the midpoint [red lines, median follow-up 23 months]). Patients in the “later” group had lower rates of RP+LF. Similar results were found when patients were analyzed by intent-to-treat or by treatment received (not shown). IMRT, intensity-modulated (photon) therapy; PSPT, passive scattering proton therapy. B, Cumulative incidence of RP among patients among patients who were randomized and treated according to time of enrollment. In the IMRT group, RP occurred in both the early and later enrollees; however, in the PSPT group, RP occurred only among the early enrollees. The results suggest the existence of a “learning curve” for the delivery of PSPT, with corresponding improvements in PSPT over the course of the trial.
Risk factors
In univariate analyses, combined RP and LF rates were associated with time of enrollment (early vs. later), MLD, and lung V5–20. RP was associated with time of enrollment, age, MLD, total GTV, and lung volume receiving 5–10 Gy(RBE); LF was associated only with time of enrollment. In multivariate Cox regression analyses, the only significant adverse factor for RP and LF combined (and for RP and LF individually) was time of enrollment (Table 2). Because the time of enrollment may have been a surrogate for smaller tumors and lower prescribed radiation dose (Suppl. Table S3), we repeated the multivariate Cox regression analyses excluding time of enrollment. Results of that analysis showed that MLD (P=0.044) and lung V10 (P=0.008) were adverse factors for RP and LF combined. Factors associated with RP were patient age (<65 vs. ≥65), MLD, and planning target volume. No factors were associated with LF when time of enrollment was excluded. Competing risk analyses that considered local-regional recurrence, high-grade RP, distant metastasis, cancer-related death, and other death as competing events showed no differences between IMRT and PSPT in terms of RP or LF.
Table 2.
Multivariate Cox Regression Analysis of Factors Associated with Primary Endpoint (RP+LF) for the 149 Randomized Patients
| Variable | Randomized Patients (n=149) | |
|---|---|---|
| HR (95% CI) | P Value | |
| Time of Enrollment | ||
| Before 9/27/2011 (Ref) | ||
| After 9/27/2011 | 0.35 (0.17–0.72) | 0.004 |
| Performance status | ||
| ≤80 (Ref) | ||
| ≥90 | 1.00 (0.54–1.87) | 0.997 |
| Total RT Dose | ||
| <74 Gy (Ref) | ||
| ≥74 Gy | 0.99 (0.44–2.18) | 0.971 |
| Tumor histology | ||
| Adeno (Ref) | ||
| Not Adeno | 0.99 (0.54–1.79) | 0.963 |
| RT type | ||
| IMRT (Ref) | ||
| Proton | 1.35 (0.73–2.48) | 0.34 |
| Heart mean dose | ||
| Continuous | 1.02 (0.99–1.06) | 0.234 |
DISCUSSION
This prospective randomized study, the first such study to directly compare IMRT with PSPT, revealed no statistically significant difference in our primary endpoints (grade ≥3 RP or LF) after IMRT or PSPT for patients with locally advanced NSCLC based on both Bayesian and frequentist analyses. Considerably fewer events occurred in this trial than were expected, especially grade ≥3 RP after IMRT (6.5% in the current study vs. 15% in our historical data).2,18 LF rates at 12 months were also lower than expected after either IMRT or PSPT (25% expected vs. 10.7% actual). Reasons for these improvements in outcome are actively being investigated. For example, to determine if a “learning curve” for proton plans was truly present, we generated new treatment plans for the six patients who developed RP in the “early” PSPT group to see if the dose distribution could be further optimized from the delivered plan. Early findings from this exercise suggest that the MLD in three of those six patients was lower in the new plans (not shown), supporting the existence of a learning curve.
Advantages based on dose distributions have often been used to justify new technologies in radiation therapy. Many treatment plan comparison studies have suggested that proton therapy could reduce volumes of normal tissues exposed to various dose levels relative to photon therapy.19 However, conclusions based on planning studies may not always be reliable. In this study, with similar MLD, PSPT was able to reduce the “low-dose bath” (lung V5–10) but PSPT exposed significantly larger lung volumes to higher doses (V20–80).
The fact that PSPT was associated with larger high-dose lung volumes presumably reflected the use of relatively large safety margins for the 3D scattering proton beams used for this trial. The high-dose volumes may have contributed to the higher-than-expected rates of RP after proton therapy. Our results also imply that the dose-volume constraints (derived from photon therapy) may not apply to the drastically different dose distribution patterns of PSPT. Either a different set of constraints is needed to guide proton treatment planning, or a unified set of constraints that applies to both photons and protons may emerge. One possibility would be to use the “effective dose” concept20 when evaluating proton lung plans or when comparing proton vs. photon dose distributions.
Proton therapy has changed considerably over the past 3 decades with the introduction of hospital-based facilities with rotational gantries to allow treatment of tumors at any anatomic site, improvements in treatment planning capability, and the development of pencil beam scanning with intensity modulation. Nevertheless, the state of the art had not advanced significantly over that time, perhaps because of the higher cost of protons. Meanwhile, the past decade has seen major improvements in IMRT, as evidenced during this trial by the lower-than-anticipated number of RP events in the IMRT arm. Specifically, introducing an automated IMRT optimization system during the first year after trial activation led to significant improvement in the potential clinical effectiveness of IMRT plans.15 Our findings suggest that evolution of technology and increasing experience in its use over the course of a technology-based trial can influence outcomes and thus could pose substantial challenges to the ability to demonstrate the clinical benefit of a new modality.
The allocation of the patients in this trial was 0.58 to 0.42 with a higher probability of being randomized to IMRT arm. The Bayesian adaptive randomization design relies on event information being updated in real time so that the ratio of allocation to treatment arms can be adjusted before the randomization of the next patient. However, the median time to the development of the events in this study was about 5 months. In the meantime, the allocation between the study arms had to continue using the adaptive randomization parameters that had already been set. The Bayesian randomization also gave greater importance to more recent events. Thus, patient allocation did not consider improvements in experience with the techniques or technologic evolution over the course of the trial.
Finally, even though this trial was not designed to test survival, the median overall survival time of 28.8 months in the current trial is consistent with a recent benchmark established from Radiation Oncology Study Group (RTOG) protocol 0617.1 This finding is encouraging, because the patients enrolled in the current protocol had less favorable disease than did the patients in RTOG 0617—our study included patients with stage IV disease and patients with recurrent disease after surgery, and about 50% of our patients had experienced disease progression during systemic therapy before being referred to a chemoradiation trial. In RTOG 0617, heart V5 and V35 independently predicted overall survival. In our study, PSPT reduced the heart volume exposed to all dose levels relative to IMRT. The importance of heart sparing for overall survival is being evaluated in a phase 3 randomized lung trial, RTOG 1308 (NCT01993810), which will provide much-needed level I evidence regarding proton therapy for lung cancer. Another important trial, a phase 2 study comparing IMRT and the next-generation proton technique, intensity-modulated proton therapy, is also ongoing (NCT01629498). Findings from these two trials will be critical for addressing the issues identified here and for providing additional evidence of the efficacy of proton beam therapy relative to photons.
Conclusions
In our study, no benefit was noted in either grade ≥3 RP or LF after PSPT, presumably because PSPT was not associated with improved lung dose–volume indices. PSPT significantly reduced heart exposure in both dose and volume, and its influence on cardiac toxicity and overall survival is under active investigation. Outcomes after both IMRT and PSPT improved over the course of this trial, but the magnitude of improvement in RP was greater and statistically more significant in the PSPT arm.
Supplementary Material
Acknowledgments
The authors are deeply indebted to their patients who volunteered to participated in this trial; to their colleagues who supported this important trial; to the research staff for their dedication and hard work; to Mr. Jaques B. Bluett for outstanding work in developing the dosimetric plans; to Mr. Mark Munsell for his endless “real-time” statistical support in conducting this trial; to Mr. Mike Hernandez for his statistical support; to Drs. Jeffery Bradley, Maria Werner-Wasik, and Charles Simone, who served on the external review board; and to Ms. Christine Wogan for her expert editorial guidance in the development and finalization of the manuscript.
Funding: Supported by National Cancer Institute grants P01 CA021230, U19 CA021239, and P30 CA016672.
Footnotes
AUTHOR CONTRIBUTIONS
Conception and design: Zhongxing Liao, J Jack Lee, Ritsuko Komaki, Daniel R Gomez, Michael S O’Reilly, Frank V Fossella, George R Blumenschein Jr, John V Heymach, Ara A Vaporciyan, Stephen Swisher, Noah Chan Choi, Thomas F Delaney, Stephen M Hahn, James D Cox, Charles S Lu, Radhe Mohan
Collection and assembly of data: Zhongxing Liao, Daniel R Gomez, Michael S O’Reilly, Frank V Fossella, George R Blumenschein Jr, John V Heymach, Ara A Vaporciyan, Stephen Swisher, Noah Chan Choi, Thomas F Delaney, Stephen M Hahn, James D Cox, Charles S Lu, Radhe Mohan
Data analysis and interpretation: Zhongxing Liao, J Jack Lee, Pamela K Allen, Radhe Mohan
Manuscript writing: All authors
Final approval of manuscript: All authors
References
- 1.Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncology. 2015;16(2):187–199. doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liao ZX, Komaki RR, Thames HD, Jr, et al. Influence of technologic advances on outcomes in patients with unresectable, locally advanced non-small-cell lung cancer receiving concomitant chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2010;76(3):775–781. doi: 10.1016/j.ijrobp.2009.02.032. [DOI] [PubMed] [Google Scholar]
- 3.Liao ZX, Travis EL, Tucker SL. Damage and morbidity from pneumonitis after irradiation of partial volumes of mouse lung. Int J Radiat Oncol Biol Phys. 1995;32(5):1359–70. doi: 10.1016/0360-3016(94)00660-D. [DOI] [PubMed] [Google Scholar]
- 4.Wang S, Liao Z, Wei X, et al. Analysis of clinical and dosimetric factors associated with treatment-related pneumonitis (TRP) in patients with non-small-cell lung cancer (NSCLC) treated with concurrent chemotherapy and three-dimensional conformal radiotherapy (3D-CRT) Int J Radiat Oncol Biol Phys. 2006;66(5):1399–407. doi: 10.1016/j.ijrobp.2006.07.1337. [DOI] [PubMed] [Google Scholar]
- 5.Marks LB, Bentzen SM, Deasy JO, et al. Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S70–6. doi: 10.1016/j.ijrobp.2009.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murshed H, Liu HH, Liao Z, et al. Dose and volume reduction for normal lung using intensity-modulated radiotherapy for advanced-stage non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;58(4):1258–67. doi: 10.1016/j.ijrobp.2003.09.086. [DOI] [PubMed] [Google Scholar]
- 7.Glimelius B, Montelius A. Proton beam therapy - do we need the randomised trials and can we do them? Radiother Oncol. 2007;83:105–9. doi: 10.1016/j.radonc.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Goitein M, Cox JD. Should randomized clinical trials be required for proton radiotherapy? J Clin Oncol. 2008;26(2):175–6. doi: 10.1200/JCO.2007.14.4329. [DOI] [PubMed] [Google Scholar]
- 9.Bentzen SM. Randomized controlled trials in health technology assessment: overkill or overdue? Radiother Oncol. 2008;86:142–7. doi: 10.1016/j.radonc.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bothwell LE, Podolsky SH. The emergence of the randomized, controlled trial. N Engl J Med. 2016;375(6):501–4. doi: 10.1056/NEJMp1604635. [DOI] [PubMed] [Google Scholar]
- 11.Suit H, Goldberg S, Niemierko A, et al. Proton beams to replace photon beams in radical dose treatments. Acta Oncol. 2003;42(8):800–8. doi: 10.1080/02841860310017676. [DOI] [PubMed] [Google Scholar]
- 12.Lee JJ, Chen N, Yin G. Worth adapting? Revisiting the usefulness of outcome-adaptive randomization. Clin Cancer Res. 2012;18(17):4498–507. doi: 10.1158/1078-0432.CCR-11-2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yom SS, Liao Z, Liu HH, et al. Initial evaluation of treatment-related pneumonitis in advanced-stage non-small-cell lung cancer patients treated with concurrent chemotherapy and intensity-modulated radiotherapy. Int J Radiat Biol Oncol Phys. 2007;68:94–102. doi: 10.1016/j.ijrobp.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 14.Sejpal S, Komaki R, Tsao A, et al. Early findings on toxicity of proton beam therapy with concurrent chemotherapy for nonsmall cell lung cancer. Cancer. 2011;117(13):3004–13. doi: 10.1002/cncr.25848. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Li X, Quan EM, Pan X, Li Y. A methodology for automatic intensity-modulated radiation treatment planning for lung cancer. Phys Med Biol. 2011;56:3873–3893. doi: 10.1088/0031-9155/56/13/009. [DOI] [PubMed] [Google Scholar]
- 16.Jin H, Tucker SL, Liu HH, Wei X, Yom SS, Wang S, Komaki R, Chen Y, Martel MK, Mohan R, Cox JD, Liao Z. Dose-volume thresholds and smoking status for the risk of treatment-related pneumonitis in inoperable non-small cell lung cancer treated with definitive radiotherapy. Radiother Oncol. 2009;91(3):427–32. doi: 10.1016/j.radonc.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Statist Assoc. 1999;94:496–509. [Google Scholar]
- 18.Jiang ZQ, Yang K, Komaki R, et al. Long-term clinical outcome of intensity-modulated radiotherapy for inoperable non-small cell lung cancer: the MD Anderson experience. Int Int J Radiat Oncol Biol Phys. 2006;65(4):1087–96. doi: 10.1016/j.ijrobp.2011.06.1963. [DOI] [PubMed] [Google Scholar]
- 19.Chang JY, Zhang X, Wang X, et al. Significant reduction of normal tissue dose by proton radiotherapy compared with three-dimensional conformal or intensity-modulated radiation therapy in stage I or stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;65(4):1087–96. doi: 10.1016/j.ijrobp.2006.01.052. [DOI] [PubMed] [Google Scholar]
- 20.Tucker SL, Mohan R, Liengsawangwong R, Martel MK, Liao Z. Predicting pneumonitis risk: a dosimetric alternative to mean lung dose. Int J Radiat Oncol Biol Phys. 2013;85(2):522–7. doi: 10.1016/j.ijrobp.2012.03.052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.