Summary
We investigated risk factors for severe acute lower respiratory infections (ALRI) among hospitalised children <2 years old, with a focus on the interactions between virus and age. Statistical interactions between age and respiratory syncytial virus (RSV), influenza, adenovirus (ADV), and rhinovirus on the risk of ALRI outcomes were investigated. Of 1,780 hospitalizations, 228 (12.8%) were admitted to ICU. The median (range) length of stay (LOS) in hospital was 3 (1–27) days. An increase of one month of age was associated with a decreased risk of ICU admission (rate ratio [RR] 0.94; 95% confidence intervals [CI] 0.91–0.98) and with a decrease in LOS (RR 0.96; 95% CI 0.95–0.97). Associations between RSV, influenza, ADV positivity and ICU admission and LOS were significantly modified by age. Children <5 months old were at the highest risk from RSV associated severe outcomes while children >8 months were at greater risk from influenza associated ICU admissions and long hospital stay. Children with ADV had increased LOS across all ages. In the first two years of life, the effects of different viruses on ALRI severity varies with age. Our findings help to identify specific ages that would most benefit from virus-specific interventions such as vaccines and antivirals.
Keywords: severe ALRI, children, respiratory virus
Introduction
Acute lower respiratory infections (ALRI), are major causes of morbidity and mortality among children worldwide [1]. The most frequently identified pathogen among young hospitalised children with ALRI is respiratory syncytial virus (RSV) [2, 3] however, adenovirus, rhinovirus, and influenza are also common etiological agents [4, 5]. The greatest burden of ALRI exists in low-and-middle income countries; but ALRIs are also a significant health issue in high-income countries [1, 6]. Of children hospitalised with ALRI in high-income countries, 5–20% develop very severe disease necessitating intensive care unit admission (ICU) or a prolonged hospital length of stay (LOS) [5, 7–9].
The determinants of severe ALRI among children are complex. Studies show that a combination of host and viral factors influence disease severity, yet the relative impact of each and the role of specific virus types in determining severity remains unclear [10–12]. The majority of studies investigating risk factors for severe ALRI have focused on the “individual or main effects” of a host factor or of a particular virus. Young age at infection has been frequently found to be the most significant risk factor for severe ALRI [3, 7, 8, 13–15], as it is associated with both host and viral features. For example, young age affects airway size; and also defines the naivety of a child’s immune system. However, by simply classifying young age as a risk factor for severity and not investigating the interactions between age and virus infection, the impact of specific virus types remains unclear. Such knowledge is necessary for clinical and public health interventions, such as antiviral agents and vaccines, which are virus specific.
In the current study, we aimed to identify risk factors of severe ALRI as defined by risk of ICU admission and hospital LOS among hospitalised children <2 years. Our intention was to enable a better understanding of the relationship between age and specific respiratory viruses on the risk of severe ALRI outcomes among children.
Methods
Settings and study design
This study was undertaken as part of the Southern Hemisphere Influenza Vaccine Effectiveness Research and Surveillance (SHIVERS) project, which tracked hospitalizations for acute respiratory illness at the two public hospitals serving the central, eastern, and southern Auckland region in New Zealand from 2012 to 2015. In New Zealand all acute paediatric inpatient care is provided by public hospitals. The regions are predominantly urban and include approximately 53,658 children aged < 2 years, of which 25% are Pacific, 27% Asian, 15% Māori (New Zealand’s indigenous population), and 33% are of European or other ethnicities [16]. Further information on the SHIVERS surveillance platform, including study setting, patient enrolment, and data collection are described elsewhere [17, 18].
The SHIVERS study enrolled all patients who met the World Health Organization (WHO) severe acute respiratory infection (SARI) case definition – reported fever and cough with onset in preceding 7 days (in 2012) or preceding 10 days (2013–2015). From 2013 to 2015, SHIVERS enrolment was extended to include a systematic random sample of children hospitalised with an acute respiratory complaint that did not meet the SARI case definition (i.e. cough and/or fever but not both with 10 days). Study nurses completed detailed case report forms on all enrolled patients and collected nasopharyngeal swab/aspirates. Clinician ordered laboratory investigations were added to the study dataset after validation of the respiratory virus PCR assay used at each hospital. Only results from assays with a sensitivity greater than 80% and a specificity greater than 95% were included in the study dataset. SHIVERS study data was collected and managed using REDCap electronic data capture tools [19]. Ethical approval for the SHIVERS study was obtained from the New Zealand Health and Disabilities Ethics Committee (NTX/11/11/102).
Laboratory methods
Specimens were tested for respiratory viruses (influenza, respiratory syncytial virus, parainfluenza virus 1–3, human metapneumovirus, rhinovirus, and adenovirus) using the United States Centers for Disease Control and Prevention real-time RT-PCR protocol [20, 21] or the AusDiagnostic PCR protocol and real time PCR assay at hospital laboratories [22]. A sample of viral positive specimens were further sub-typed. Further information on laboratory methods and sensitivity and specificity of assays are provided in the supplementary material (Text S1, Table S1).
Study Population
The current analysis was limited to consented children < 2 years old meeting SARI and non-SARI respiratory case definitions, who had an overnight admission with a primary or secondary discharge diagnosis of an acute lower respiratory infection (ICD-10 codes J09-J22, Supplementary Text S2) during winter seasons (April through October) between 2012 and 2015. Non-SARI respiratory children who had cough and/or fever onset > 10 days before admission were excluded to ensure only children with acute symptoms were investigated. For children with a repeat hospitalization within 14 days, only the first hospitalisation event was included. Respiratory viruses of interest were influenza, RSV, adenovirus, rhinovirus. We excluded children who were not tested for all four viruses. Comparisons of tested and non-tested children are provided in the supplementary material (Table S2). No significant difference in demographics and outcomes measures were found between the two groups.
Measurements and Covariates
Patient demographics and clinical histories collected via SHIVERS surveillance were validated and supplemented with data from the NZ Ministry of Health National Data Collections [23]. Our two measures of disease severity were ICU admission and hospital LOS in days. The primary exposures were age in months and type of respiratory virus identified (influenza, RSV, rhinovirus, and adenovirus). Other exposures that were considered relevant, due to being associated with disease severity and/or with age or virus positivity included patient demographics (sex, ethnicity), socio-economic status (SES), influenza vaccination status, co-infection, SARI case definition, and the presence of other underlying health conditions. SES was quantified using a small area level measure of household deprivation derived from the national census (NZDep2013) [24]. Underlying health conditions were grouped into three broad categories: preterm birth and/or low birth weight (<37 weeks of gestational age and/or birth weight <2500 grams), pre-existing respiratory conditions (asthma, other lung conditions, and/or a prior respiratory hospitalisation before the preceding 14 days), or other underlying conditions (congenital heart conditions, immunodeficiency, renal or hepatic disease). Further detail on sourcing and defining exposures are available in the supplementary material (Table S3).
Statistical Methods
ICU admission was modelled as a function of exposure variables using logistic regression with risk ratios derived by applying a substitution method [25, 26]. Hospital LOS was modelled as a continuous outcome using negative binomial regression. Statistical interactions between child age in months and each respiratory virus were assessed on the multiplicative scale by including main effects and interaction terms in regression models. It is recommended to measure biological interactions on both additive and multiplicative scales for dichotomous outcomes [27, 28]. Hence, for ICU admission, we tested for statistically significant interactions on an additive scale by calculating the relative excess risk due to interaction (RERI) with standard errors [29].
Crude associations were estimated for all exposure variables, in addition to interactions between each virus and age in months. Demographics, influenza vaccination status, and underlying conditions were examined as potential confounders of age and virus associations with the outcomes. Multivariable models included exposures and age-virus interactions terms that were statistically significant at P < 0.05 in crude models.
Influenza vaccination was excluded from multivariable models due to the very low number of children with a current vaccination (n=76/1780; 4.3%) and the high number of children with missing vaccination status (n=155/1780; 8.7%). Sensitivity analyses for influenza vaccination demonstrated no effect on age or viral associations (Supplementary Table S4) regardless of approach. All analysis was conducted in Stata 14.0 statistical software (Stata Corporation, College Station, TX).
Results
During four winter respiratory seasons, there were 4,360 ALRI hospitalisations among children aged <2 years old, of which 1,780 met inclusion criteria (Figure 1). Among the 1,780 ALRI hospitalizations, 228 (12.8%) were admitted to ICU (Table 1). The proportion of children admitted to ICU ranged from 8% to 15% of the four-year study period. The median days in hospital was 3 (range: 1–27), with 393 (22.1%) hospitalizations in the upper quartile of four or more days LOS (Supplementary Figure S1). Demographics, clinical characteristics, and viral results among all hospitalised cases, ICU admissions, and those with >4 days in hospital are presented in Table 1.
Figure 1.
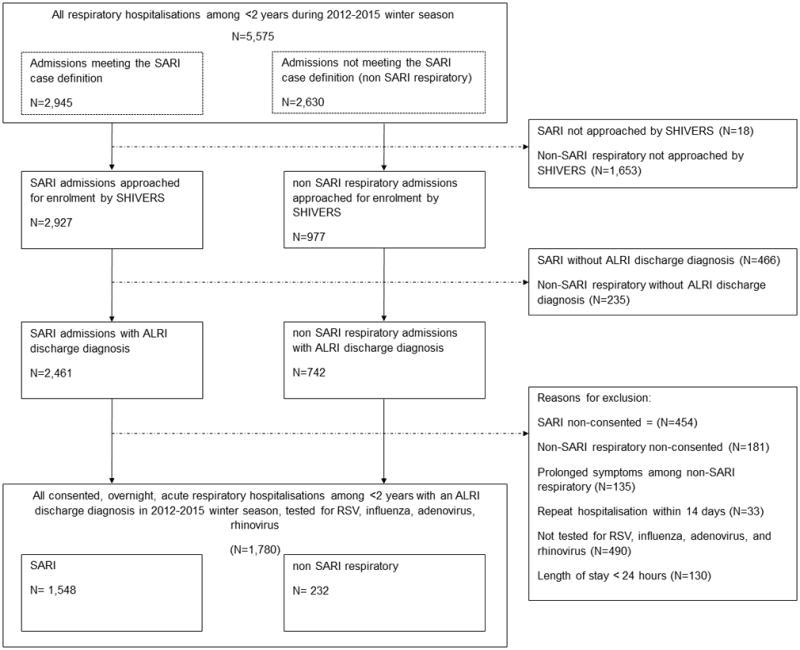
Table 1.
Demographic and clinical characteristics of children aged <2 years hospitalised with acute lower respiratory infections in the two main public hospitals within Auckland, New Zealand 2012–2015
| All ALRI hospitalisations | ICU admission | Hospital LOS>4 | ||||
|---|---|---|---|---|---|---|
| n | (column %) | n | (column %) | n | (column %) | |
|
| ||||||
| Total | 1780 | (100.0) | 228 | (100.0) | 393 | (100.0) |
| Age | ||||||
| 0 – 2 months | 352 | (19.8) | 81 | (35.5) | 115 | (29.3) |
| 3 – 5 months | 368 | (20.7) | 44 | (19.3) | 76 | (19.3) |
| 6 – 11 months | 601 | (33.8) | 53 | (23.2) | 122 | (31.0) |
| 12 – 23 months | 459 | (25.8) | 50 | (21.9) | 80 | (20.4) |
| Ethnicity | ||||||
| Maori | 513 | (28.8) | 81 | (35.5) | 124 | (31.6) |
| Pacific | 896 | (50.3) | 95 | (41.7) | 185 | (47.1) |
| European, Asian, and Other | 371 | (20.8) | 52 | (22.8) | 84 | (21.4) |
| Sex | ||||||
| Male | 1082 | (60.8) | 146 | (64.0) | 249 | (63.4) |
| Household deprivation | ||||||
| NZ dep 1–6 (Least deprived) | 359 | (20.2) | 60 | (26.3) | 87 | (22.1) |
| NZ dep 7–8 | 282 | (15.8) | 49 | (21.5) | 62 | (15.8) |
| NZ dep 9–10 (Most deprived) | 1139 | (63.9) | 119 | (52.2) | 243 | (61.8) |
| Discharge diagnosis | ||||||
| Bronchiolitis | 928 | (52.1) | 130 | (57.0) | 196 | (49.9) |
| Pneumonia | 770 | (43.3) | 88 | (38.6) | 182 | (46.3) |
| Other ALRI b | 82 | (4.6) | 10 | (4.4) | 15 | (3.8) |
| Influenza vaccine | ||||||
| Influenza vaccine within 12 months | 76 | (4.3) | 12 | (5.3) | 21 | (5.3) |
| No influenza vaccine | 1549 | (87.0) | 198 | (86.8) | 341 | (86.8) |
| Unknown vaccination status | 155 | (8.7) | 18 | (7.9) | 31 | (7.9) |
| Treatment | ||||||
| Influenza antiviral medication | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Underlying conditions | ||||||
| Respiratory (asthma, other lung disease, and/or prior respiratory hospitalisation) | 670 | (37.6) | 115 | (50.4) | 186 | (47.3) |
| Other underlying conditions (Congenital cardiovascular, immune, renal, hepatic) | 96 | (5.4) | 26 | (11.4) | 42 | (10.7) |
| Premature birth and/or low birth weight d | 341 | (19.2) | 71 | (31.1) | 101 | (25.7) |
| Viral Infection | ||||||
| Influenza | 230 | (12.9) | 19 | (8.3) | 46 | (11.7) |
| Influenza A(H1N1)pdm09 | 68 | (3.8) | 7 | (3.1) | 15 | (3.8) |
| Influenza A(H3N2) | 97 | (5.4) | 7 | (3.1) | 18 | (4.6) |
| Influenza B | 65 | (3.7) | 5 | (2.2) | 13 | (3.3) |
| RSV | 855 | (48.0) | 102 | (44.7) | 210 | (53.4) |
| RSV-A | 316 | (17.8) | 24 | (10.5) | 65 | (16.5) |
| RSV-B | 264 | (14.8) | 21 | (9.2) | 53 | (13.5) |
| Non subtyped | 275 | (15.4) | 57 | (25.0) | 92 | (23.4) |
| Rhinovirus | 463 | (26.0) | 60 | (26.3) | 85 | (21.6) |
| Adenovirus | 301 | (16.9) | 47 | (20.6) | 83 | (21.1) |
| Adenovirus-1 | 15 | (0.8) | 3 | (1.3) | 3 | (0.8) |
| Adenovirus-7 | 17 | (1.0) | 5 | (2.2) | 12 | (3.1) |
| Adenovirus other | 17 | (1.0) | 3 | (1.3) | 5 | (1.3) |
| Non subtyped | 252 | (14.2) | 36 | (15.8) | 63 | (16.0) |
| Multiple Viruses | 332 | (18.7) | 41 | (18.0) | 88 | (22.4) |
Abbreviations: ICU, intensive care unit; LOS, hospital length of stay; ALRI, acute lower respiratory infection.
Small area level measure of household deprivation derived from variables collected at the 2013 national census (NZDep2013 Index of Deprivation).
Other ALRI includes unspecified acute lower respiratory infection (ICD-10 J22)
Preterm birth defined as <37 weeks of gestational age. Low birth weight defined as birth weight <2500 grams.
Children admitted to ICU were younger (median age: ICU = 4 months versus general ward = 7 months, p < 0.0001) and more likely to have an underlying condition (ICU= 93% vs general ward 62%, p < 0.0001) than those on the general ward. Children admitted to ICU were also less likely to be of Pacific ethnicity (ICU= 42% vs general ward 50%, p < 0.0001) or from the lowest SES (ICU= 52% vs general ward 64%, p < 0.0001).
Children with >4 days LOS were younger (median age: 6 months for LOS >4 vs. 7 months for LOS ≤4, p < 0.0001) and more likely to have any underlying condition (LOS>4= 84% vs LOS≤4 = 62%, p < 0.0001) than those with shorter lengths of stay. There was no significant difference in LOS by ethnicity or SES.
Of the 1780 ALRI hospitalizations, 1,479 (83.1%) were positive for at least one respiratory virus, of which 855 (48.0%) were RSV; 332 (18.7%) hospitalizations were positive for more than one respiratory virus. Crude associations for all exposures including age and viral interactions are included in the supplementary material (Table S5).
ICU admissions – Main and interactive effects of age and virus
Age had a significant main effect, with decreased ICU admission risk for every month increase in age (Risk ratio [RR]: 0.95; 95% Confidence interval [CI] 0.92–0.99) (Table 2) after adjusting for ethnicity, socio-economic status, single and co-infection with virus, and underlying conditions. The risk of ICU admission was higher for children born prematurely and/or with a low birth weight (RR: 1.78; 95% CI 1.36–2.33), those with a pre-existing respiratory conditions (RR: 1.80; 95% CI 1.40–2.33), and/or those with cardiovascular, immune, renal or hepatic conditions (RR: 2.05; 95% CI 1.36–3.09) (Table 2). The overall risk of ICU admission was also higher in hospitalizations where adenovirus (RR=1.53; 95% CI 1.12–2.09) or RSV (RR=1.55; 95% CI 1.00–2.39) were detected, while detection of influenza was associated with a decreased risk of ICU admission (RR: 0.33; 95% CI 0.12–0.89).
Table 2.
Association of intensive care unit admission and longer hospital stay with age and virus type among children aged <2 years hospitalised with acute lower respiratory infection in Auckland, New Zealand 2012–2015
| Adjusted Models | ICU admission | Prolonged hospital stay | ||||
|---|---|---|---|---|---|---|
| RR | (95% CI) | P-value | RR | (95% CI) | P-value | |
| Main effects | ||||||
| Demographics | ||||||
| Age in months | 0.95 | (0.92–0.99) | 0.016 | 0.97 | (0.96–0.97) | 0.000 |
| European/Asian/Other | 1.00 | (ref) | 1.00 | (ref) | ||
| Maori | 1.34 | (0.95–1.89) | 0.095 | 1.04 | (0.94–1.16) | 0.467 |
| Pacific | 1.01 | (0.70–1.45) | 0.957 | 0.99 | (0.89–1.10) | 0.857 |
| NZDep2013 1–6a (Least deprived) | 0.85 | (0.59–1.22) | 0.388 | 1.04 | (0.92–1.17) | 0.539 |
| NZDep2013 7–8 | 1.00 | (ref) | 1.00 | (ref) | ||
| NZDep2013 9–10 (Most deprived) | 0.53 | (0.38–0.72) | 0.000 | 0.95 | (0.86–1.05) | 0.307 |
| Underlying conditions | ||||||
| Low birth weight/premature birth | 1.78 | (1.36–2.33) | 0.000 | 1.15 | (1.05–1.27) | 0.002 |
| Respiratory condition/prior respiratory hospitalization | 1.80 | (1.40–2.33) | 0.000 | 1.20 | (1.11–1.30) | 0.000 |
| Other underlying conditions | 2.05 | (1.36–3.09) | 0.001 | 1.68 | (1.39–2.04) | 0.000 |
| Viral Infection | ||||||
| Influenza | 0.33 | (0.12–0.89) | 0.028 | 0.78 | (0.64–0.95) | 0.014 |
| RSV | 1.55 | (1.00–2.39) | 0.051 | 1.02 | (0.94–1.11) | 0.591 |
| Rhinovirus | 0.86 | (0.65–1.14) | 0.306 | 0.85 | (0.78–0.93) | 0.000 |
| Adenovirus | 1.53 | (1.12–2.09) | 0.007 | 0.98 | (0.78–1.23) | 0.861 |
| Interactive effects (scale) | ||||||
| Influenza and age in months (multiplicative) | 1.07 | (0.98–1.16) | 0.135 | 1.03 | (1.02–1.06) | 0.001 |
| Influenza and age in months (additive [RERI]) | 0.05 | (0.01–0.09) | 0.012 | |||
| RSV and age in months (multiplicative) | 0.91 | (0.86–0.97) | 0.003 | |||
| RSV and age in months (additive [RERI]) | −0.15 | (−0.3–0.01) | 0.042 | |||
| Adenovirus and age in months (multiplicative) | 1.02 | (1.01–1.05) | 0.036 | |||
| Adenovirus and age in months (additive [RERI]) | ||||||
Abbreviations: ICU = Intensive care unit; RR = risk ratio; RERI =relative excess risk owing to interaction; RSV = respiratory syncytial virus
Small area level measure of household deprivation derived from variables collected at the 2013 national census (NZDep2013 Index of Deprivation).[24]
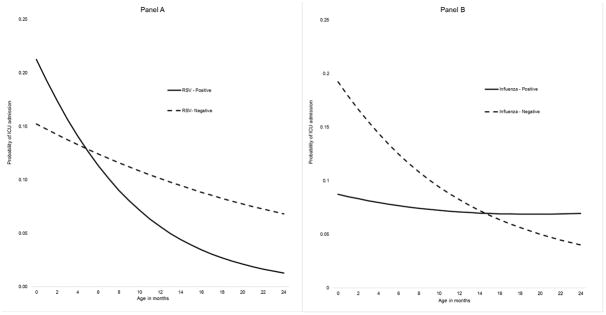
We detected significant interactions for age with RSV and influenza infections (Table 2). RSV and age had a significant negative interaction (P< 0.05), meaning the decrease in ICU admission risk with age was more pronounced among children with RSV than those without RSV infection. Among children aged <5 months, those with RSV had a higher predicted probability of ICU admission compared to children without RSV. For children aged 6–24 months, the predicted probability of ICU was inverse that of younger children – higher among children without RSV compared to RSV positive children (Figure 2 panel A, Supplementary figure S2).
Figure 2.
In contrast, the influenza and age interaction was positive (P< 0.05). The predicted probability of ICU admission associated with influenza remained relatively flat across ages; whereas, those without influenza decreased over age. Consequently, for children >16 months, the predicted probability of ICU admission was higher for those with influenza compared to those without influenza infection (Figure 2 panel B, Supplementary Figure S3). No significant interactions were found between age and adenovirus, rhinovirus, or multiple viruses on ICU admission risk.
Long length of hospital stay – Main and interactive effects of age and virus
Age had a significant main effect, with a decrease in LOS for every month increase in age (RR: 0.97; 95% CI 0.96–0.97) (Table 2) after adjusting for ethnicity, socio-economic status, single and co-infection with virus, and underlying conditions. Children with low birth weight and/or premature gestation had an increased risk of longer LOS (RR: 1.15; 95% CI 1.05–1.27), as did those with a pre-existing respiratory conditions (RR: 1.20; 95% CI 1.11–1.30) or with cardiovascular, immune, renal or hepatic conditions (RR: 1.68; 95% CI 1.39–2.04) (Table 2). The main effects of influenza (RR=0.78; 95% CI 0.64–0.95) and rhinovirus (RR=0.85; 95% CI 0.78–0.93) were associated with a decreased LOS.
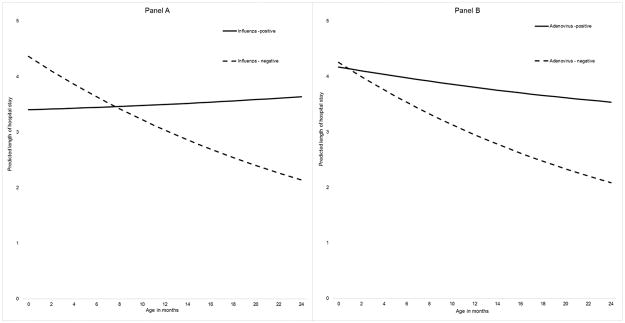
We detected a significant interactive effect between age and either influenza or adenovirus infection on LOS (Table 2). Both influenza and adenovirus infections had positive interactions with age (P< 0.05), meaning the predicted LOS for children with either infection remained elevated across ages; whereas, LOS decreased over age in hospitalisations without these infections. Among children >8 months old, those positive for influenza had a longer predicted LOS compared to those without influenza (Figure 3 panel A). Apart from the first month of life, adenovirus hospitalizations had a higher predicted LOS compared to those without adenovirus (Figure 3 panel B). No significant interactions were found between age and RSV, rhinovirus, or multiple virus detections on LOS.
Figure 3.
Discussion
In this hospital-based study in New Zealand, we found 83% of children <2 years hospitalised with ALRI to be positive for at least one respiratory virus. Younger age and underlying conditions were associated with a higher risk of ICU admission and longer hospital stay among children. Additionally, we found important joint effects on these outcomes between age in months and whether the detected virus was RSV, influenza, or adenovirus. Overall, RSV infections were associated with a higher risk of ICU admission that decreased with age. In contrast, influenza infection did not increase the risk of ICU admission or LOS overall, but was associated with an increased risk of both outcomes among older children. Finally, adenovirus was shown to be associated with an increased risk of ICU admission and longer LOS regardless of age. If these associations are causal, they identify specific age groups within the first two years of life, that would most benefit from viral-specific interventions such as antiviral treatments and vaccinations.
Previous studies have reported similar main effects for age [7, 13], underlying conditions [8, 9], and viruses [5, 14, 30] on ALRI associated ICU admission and/or LOS; however, such a detailed examination of the combined effects of age in months and laboratory confirmed viral effects on disease severity outcomes is a novel approach in the current study. Similar to other studies [5, 14, 31], RSV was the most common cause of ALRI in our seasonal study accounting for almost half of the hospitalisations, and caused more severe disease in the youngest children. We show hospitalised children <5 months to be at the greatest risk of severe disease outcomes due to RSV, and that this risk decreased rapidly with age in months. The reasons for the association between risk of severe RSV disease and very young age has been well established through immunological investigations [32–34]. Our results, ascertained from a large hospital based study, support these findings and highlight the potential value of a RSV maternal vaccine or passive immunoprophylaxis. They also emphasize the importance of extended protection among new RSV preventative strategies to at least 5 months of age among infants in order to protect them from the most severe RSV disease.
In contrast, the risk of ICU admission of ALRI cases and/or longer LOS remained comparatively low for influenza and did not decrease significantly with age. In fact, influenza increased the risk of ICU admission and longer LOS among older hospitalised children (ICU: aged 16–23 months and LOS: 8–23 months). Our findings indicate that although the risk of severe disease following hospitalisation is relatively low for influenza, this risk does not change dramatically with age and may contribute more to severe outcomes among children older than 8–16 months than in very young children. Other studies from the New Zealand, UK, and US have found influenza hospitalisations and infections to be concentrated in children over six months of age [5, 35, 36] and to decline after two years of life [35]. As the most severe outcomes associated with influenza among children appear to also be concentrated in a narrow age range of >6 months to <2 years, influenza vaccination and early anti-viral treatment of children in this age group would likely provide the greatest benefit in controlling influenza associated severe outcomes.
Adenovirus infection among ALRI hospitalizations in children was also found to be significant risk factor for ICU admission and a longer LOS compared to non-adenovirus respiratory infections for most ages (Figure 3, panel b). We hypothesize that the association of adenovirus with disease severity in our study was driven by an adenovirus-7 outbreak in Auckland during 2015 [37]. Adenovirus-7 has been shown to have greater virulence than other adenovirus strains [38, 39] and contributed a larger proportion of adenovirus in ICU (Table 1). Since only a small number of our adenovirus isolates were sub-typed, we are unable to confirm this association and encourage further research in the area.
Finally, although rhinovirus was present in more than 20% of cases admitted to ICU or with prolonged hospital stay, it did not present as a significant risk factor for ALRI severity nor did it display an age-associated effect. The role of rhinovirus as a pathogen is unclear [30, 40]. Considering the wide genetic diversity of rhinovirus, investigation of specific rhinovirus sub-types would appear necessary to further understand its role in ALRI severity.
Limitations of our study are; first, the very low level of influenza vaccination coverage in our study population, which prevented us from including it as an exposure variable in our analysis. Second, although we had robust laboratory data, we had limited information on viral subtypes and bacterial pathogens, hence were unable to investigate their specific roles on severe ALRI. Third, precautionary clinician practices could be driving some of the outcomes, particularly ICU admission, however by investigating both ICU and LOS we hoped to capture a realistic picture of ALRI severity. Finally, while supportive therapies such as oxygen supplies and mechanical ventilation would have also been informative outcomes to investigate, the equipment and criteria used to provide such care changed during the course of our study period and prevented us from including them in our analysis. Strengths of this study include its large prospective design over multiple seasons with systematic identification of cases and testing for a number of respiratory viruses across a broad spectrum of ALRIs. The use of national health data to validate our measures prevented bias from self-report and allowed adjustment for important demographic factors.
Conclusions
To our knowledge, this study is the first to examine both independent main and interactive effects of child age and respiratory virus on the risk of severe ALRI outcomes. By doing so, we are able to elucidate more clearly the relationship between age and specific viruses on the risk of severe ALRI. We found very young children (< 5 months) to be at the highest risk from RSV associated severe outcomes while children > 8 months were at greater risk from influenza. Our findings suggest that in controlling severe ALRI among children <2 years, RSV specific interventions would benefit children <5 months the most, while influenza vaccination and anti-viral treatment among children >6 months to <2 years would likely have a significant impact in reducing influenza associated severe outcomes.
Supplementary Material
Acknowledgments
We appreciate the contributions of (1) research nurses at Auckland District Health Board (ADHB): Kathryn Haven, Bhamita Chand, Pamela Muponisi, Debbie Aley, Claire Sherring, Miriam Rea, Judith Barry, Tracey Bushell, Julianne Brewer, Catherine McClymont; (2) research nurses at Counties Manukau District Health Board (CMDHB): Shirley Laurence, Shona Chamberlin, Reniza Ongcoy, Kirstin Davey, Emilina Jasmat, Maree Dickson, Annette Western, Olive Lai, Sheila Fowlie, Faasoa Aupa’au, Louise Robertson; (3) Researchers at the WHO National Influenza Centre, Institute of Environmental Science and Research (ESR): P. Kawakami, S. Walker, Robyn Madge, Amanda des Barres; (4) Researchers at the ADHB Laboratory (Fahimeh Rahnama), the CMDHB Laboratory (Helen Qiao, Fifi Tse, Mahtab Zibaei, Tirzah Korrapadu, Louise Optland, Cecilia Dela Cruz), and Labtests Laboratory in Auckland.
Financial support: The SHIVERS (Southern Hemisphere Influenza and Vaccine Effectiveness Research and Surveillance) project is funded by US Department of Health and Human Services, Centers for Disease Control and Prevention (CDC) (1U01IP000480-01). Support in kind is provided by the Ministry of Health. The funding resource has no role in study design; collection, analysis or interpretation of data; writing of reports; nor decision to submit papers for publication. The views expressed are the opinions of the authors and not necessarily the CDC.
Abbreviations
- ALRI
acute lower respiratory infection
- ICU
intensive care unit admission
- LOS
hospital length of stay
- RSV
Respiratory Syncytial virus
- ADV
Adenovirus
Footnotes
Financial Disclosure Statement: The authors have no financial relationships relevant to this article to disclose.
Conflict of Interest Statement: The authors have no conflicts of interest relevant to this article to disclose
Authorship
Namrata Prasad assisted in study data management, conceptualised the study carried out the analyses, drafted the initial manuscript, and approved the final manuscript as submitted.
Adrian A. Trenholme coordinated study data collection and management, provided guidance for the analyses, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Q. Sue Huang, the SHIVERS principal investigator, developed the data collection instrument, coordinated study implementation, conceptualized the study, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Mark G. Thompson provided guidance for the analyses, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Nevil Pierse provided guidance for the analyses, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Marc-Alain Widdowson developed the data collection instrument, coordinated study implementation, and approved the final manuscript as submitted
Timothy Wood developed the data collection instrument, coordinated study implementation, and approved the final manuscript as submitted
Ruth Seeds, the SHIVERS project coordinator, developed the data collection instrument, coordinated study implementation and data collection at all sites, reviewed the manuscript, and approved the final manuscript as submitted.
Susan Taylor, coordinated study implementation and data collection at all sites and approved the final manuscript as submitted.
Cameron C. Grant coordinated study data collection and management, reviewed and revised the manuscript, and approved the final manuscript as submitted.
E. Claire Newbern, assisted in study data management, conceptualized the study, provided guidance for the analyses, reviewed and revised the manuscript, and approved the final manuscript as submitted.
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
References
- 1.Nair H, et al. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. The Lancet. 2013;381(9875):1380–1390. doi: 10.1016/S0140-6736(12)61901-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nair H, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. The Lancet. 2010;375(9725):1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sala KA, et al. Factors associated with disease severity in children with bronchiolitis. Journal of Asthma. 2015;52(3):268–272. doi: 10.3109/02770903.2014.956893. [DOI] [PubMed] [Google Scholar]
- 4.Pavia AT. Viral infections of the lower respiratory tract: old viruses, new viruses, and the role of diagnosis. Clinical Infectious Diseases. 2011;52(suppl_4):S284–S289. doi: 10.1093/cid/cir043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trenholme AA, et al. Respiratory virus detection during hospitalisation for lower respiratory tract infection in children under 2 years in South Auckland, New Zealand. Journal of Paediatrics and Child Health. 2017 doi: 10.1111/jpc.13529. [DOI] [PubMed] [Google Scholar]
- 6.Ehlken B, et al. Economic impact of community-acquired and nosocomial lower respiratory tract infections in young children in Germany. European Journal of Pediatrics. 2005;164(10):607–615. doi: 10.1007/s00431-005-1705-0. [DOI] [PubMed] [Google Scholar]
- 7.Damore D, et al. Prospective multicenter bronchiolitis study: predicting intensive care unit admissions. Academic Emergency Medicine. 2008;15(10):887–894. doi: 10.1111/j.1553-2712.2008.00245.x. [DOI] [PubMed] [Google Scholar]
- 8.Wang EE, Law BJ, Stephens D. Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC) prospective study of risk factors and outcomes in patients hospitalized with respiratory syncytial viral lower respiratory tract infection. The Journal of Pediatrics. 1995;126(2):212–219. doi: 10.1016/s0022-3476(95)70547-3. [DOI] [PubMed] [Google Scholar]
- 9.Willson DF, et al. Complications in infants hospitalized for bronchiolitis or respiratory syncytial virus pneumonia. The Journal of Pediatrics. 2003;143(5):142–149. doi: 10.1067/s0022-3476(03)00514-6. [DOI] [PubMed] [Google Scholar]
- 10.Collins PL, Graham BS. Viral and host factors in human respiratory syncytial virus pathogenesis. Journal of Virology. 2008;82(5):2040–2055. doi: 10.1128/JVI.01625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rouse BT, Sehrawat S. Immunity and immunopathology to viruses: what decides the outcome? Nature Reviews: Immunology. 2010;10(7):514–526. doi: 10.1038/nri2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tregoning JS, Schwarze J. Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clinical Microbiology Reviews. 2010;23(1):74–98. doi: 10.1128/CMR.00032-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaiser SV, et al. Risk factors for prolonged length of stay or complications during pediatric respiratory hospitalizations. Hospital Pediatrics. 2015;5(9):461–473. doi: 10.1542/hpeds.2014-0246. [DOI] [PubMed] [Google Scholar]
- 14.Marguet C, et al. In very young infants severity of acute bronchiolitis depends on carried viruses. PloS One. 2009;4(2):e4596. doi: 10.1371/journal.pone.0004596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Opavsky MA, Stephens D, Wang EE-L. Testing models predicting severity of respiratory syncytial virus infection on the PICNIC RSV database. Archives of Pediatrics and Adolescent Medicine. 1995;149(11):1217–1220. doi: 10.1001/archpedi.1995.02170240035005. [DOI] [PubMed] [Google Scholar]
- 16.Statistics New Zealand. [Accessed 12 June 2017];Census district health board tables. 2013 ( http://www.stats.govt.nz/Census/2013-census/data-tables/dhb-tables.aspx)
- 17.Huang QS, et al. Southern Hemisphere Influenza and Vaccine Effectiveness Research and Surveillance. Influenza Other Respiratory Viruses. 2015;9(4):179–190. doi: 10.1111/irv.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang QS, et al. Implementing hospital-based surveillance for severe acute respiratory infections caused by influenza and other respiratory pathogens in New Zealand. Western Pacific Surveillance and Response Journal. 2014;5(2):23–30. doi: 10.5365/WPSAR.2014.5.1.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris PA, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shu B, et al. Design and performance of the CDC real-time reverse transcriptase PCR swine flu panel for detection of 2009 A (H1N1) pandemic influenza virus. Journal of Clinical Microbiology. 2011;49(7):2614–2619. doi: 10.1128/JCM.02636-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim C, et al. Comparison of nasopharyngeal and oropharyngeal swabs for the diagnosis of eight respiratory viruses by real-time reverse transcription-PCR assays. PloS One. 2011;6(6):e21610. doi: 10.1371/journal.pone.0021610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szewczuk E, et al. Rapid semi-automated quantitative multiplex tandem PCR (MT-PCR) assays for the differential diagnosis of influenza-like illness. BMC Infectious Diseases. 2010;10:113. doi: 10.1186/1471-2334-10-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.New Zealand Ministry of Health. [Accessed 14 June 2017];National collections and surveys. ( http://www.health.govt.nz/nz-health-statistics/national-collections-and-surveys/collections)
- 24.Atkinson J, Salmond C, Crampton P. NZDep2013 Index Of Deprivation. Wellington: Department of Public Health, University of Otago; 2014. [Google Scholar]
- 25.Diaz-Quijano FA. A simple method for estimating relative risk using logistic regression. BMC Medical Research Methodology. 2012;12(1):14. doi: 10.1186/1471-2288-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cummings P. Methods for estimating adjusted risk ratios. Stata Journal. 2009;9(2):175. [Google Scholar]
- 27.VanderWeele TJ, Knol MJ. A tutorial on interaction. Epidemiologic Methods. 2014;3(1):33–72. [Google Scholar]
- 28.Knol MJ, VanderWeele TJ. Recommendations for presenting analyses of effect modification and interaction. International Journal of Epidemiology. 2012;41(2):514–520. doi: 10.1093/ije/dyr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knol MJ, et al. Estimating interaction on an additive scale between continuous determinants in a logistic regression model. International Journal of Epidemiology. 2007;36(5):1111–1118. doi: 10.1093/ije/dym157. [DOI] [PubMed] [Google Scholar]
- 30.Mansbach JM, et al. Prospective multicenter study of bronchiolitis: predicting safe discharges from the emergency department. Pediatrics. 2008;121(4):680–688. doi: 10.1542/peds.2007-1418. [DOI] [PubMed] [Google Scholar]
- 31.Franz A, et al. Correlation of viral load of respiratory pathogens and co-infections with disease severity in children hospitalized for lower respiratory tract infection. Journal of Clinical Virology. 2010;48(4):239–245. doi: 10.1016/j.jcv.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watkiss ER. Pathogenesis of respiratory syncytial virus. Current Opinion in Virology. 2012;2(3):300–305. doi: 10.1016/j.coviro.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 33.Polack FP, et al. The cysteine-rich region of respiratory syncytial virus attachment protein inhibits innate immunity elicited by the virus and endotoxin. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(25):8996–9001. doi: 10.1073/pnas.0409478102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johansson C. Respiratory syncytial virus infection: an innate perspective. F1000Research. 2016:5. doi: 10.12688/f1000research.9637.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nicholson KG, et al. Rates of hospitalisation for influenza, respiratory syncytial virus and human metapneumovirus among infants and young children. Vaccine. 2006;24(1):102–108. doi: 10.1016/j.vaccine.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 36.Glezen WP, et al. Influenza virus infections in infants. The Pediatric Infectious Disease Journal. 1997;16(11):1065–1068. doi: 10.1097/00006454-199711000-00012. [DOI] [PubMed] [Google Scholar]
- 37.Institute of Environmental Science and Research Ltd (ESR) [Accessed 15 May 2017];Virology Annual Report. 2015 ( https://surv.esr.cri.nz/PDF_surveillance/Virology/VirAnnRpt/VirAnn2015.pdf)
- 38.Carballal G, et al. Adenovirus type 7 associated with severe and fatal acute lower respiratory infections in Argentine children. BMC Pediatrics. 2002;2(1):6. doi: 10.1186/1471-2431-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang L, et al. Adenovirus serotype 7 associated with a severe lower respiratory tract disease outbreak in infants in Shaanxi Province, China. Virology Journal. 2011;8(1):23. doi: 10.1186/1743-422X-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Papadopoulos NG, et al. Association of rhinovirus infection with increased disease severity in acute bronchiolitis. American Journal of Respiratory and Critical Care Medicine. 2002;165(9):1285–1289. doi: 10.1164/rccm.200112-118BC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.