Abstract
Biofilms are bacterial communities contained within an extracellular matrix, which can colonize both native tissues and artificial surfaces. In particular, indwelling medical devices and prosthetic implants are targets for biofilm formation because they facilitate bacterial attachment via host proteins that coat the foreign body. Biofilm infections are particularly challenging to treat, since they are not readily cleared by antibiotics, require invasive procedures to eradicate, and are prone to recurrence. It has been demonstrated that biofilm-derived products can actively suppress pro-inflammatory immune responses, as evident by the recruitment of myeloid-derived suppressor cells (MDSCs) and macrophage (MΦ) polarization towards an anti-inflammatory state. Recent studies have shown that alterations in leukocyte metabolism shape their inflammatory phenotype and function. For example, anti-inflammatory MΦs are biased towards oxidative phosphorylation whereas pro-inflammatory MΦs favor aerobic glycolysis. This review will compare the immune responses elicited by planktonic and biofilm bacterial infections, with a discussion on the metabolic properties of MΦs and neutrophils in response to both bacterial growth conditions.
Keywords: Bacterial biofilm, macrophage, myeloid-derived suppressor cell, neutrophil, innate immunity, immunometabolism
INTRODUCTION
Bacterial pathogens adapt to host tissues and immune defenses by shifting between planktonic and biofilm modes of growth. In general, a bacterial biofilm can be differentiated from planktonic infection by its association or attachment to a surface, which can be biotic or abiotic [1-4]. For example, infections of damaged heart valves and implanted medical devices are classified as biofilm infections. In contrast, skin and soft tissue infections and sepsis encourage planktonic bacterial growth over biofilm [1,2,5]. Biofilms are communities of bacteria embedded in an extracellular matrix, which exhibit altered growth, metabolism, and gene expression relative to planktonic cells of the same species [1,2,4,5]. For example, the metabolic heterogeneity of biofilms confers antibiotic resistance, since most available antibiotics act on dividing cells by targeting cell wall and protein synthesis, which are not effective against metabolically dormant “persister” cells in the biofilm [1-3,6]. Furthermore, biofilms elicit unique responses from the host immune system [2]. These attributes are typically not shared with planktonic infections, highlighting the distinctions between the two modes of bacterial growth.
In general, host immune responses generated during a biofilm infection are largely ineffective, which leads to chronic disease. Studies have shown that this occurs through a variety of mechanisms, which include direct killing of leukocytes (MΦs, MDSCs, and neutrophils) or reprogramming of the immune response [7-10]. This review will discuss research in murine models that has contributed to our understanding of leukocyte-biofilm interactions. Elucidating how biofilms avoid immune-mediated killing could potentially advance therapeutic options to promote pro-inflammatory responses and facilitate biofilm clearance. Recent immunometabolic studies in cancer, infection, and autoimmunity have provided insights into how the metabolic state of leukocytes shapes their inflammatory phenotype and function [11-13]. This review will provide an overview of how biofilms reprogram the immune response to promote biofilm formation and persistence, with an emphasis on MΦ metabolism. We will briefly introduce the differences in growth characteristics between planktonic and biofilm infections and the distinct immune responses elicited by each, and discuss what is known and knowledge gaps of how bacterial pathogens influence immunometabolic properties of MΦs during infection.
BACTERIAL GROWTH
Biofilms are bacterial communities that collectively represent a diverse range of physiologic states at any given time [1-4,6]. Unlike the growth of planktonic bacteria in suspension, the spatial arrangements of bacteria within an anchored biofilm shape their microenvironment, from intercellular relationships to concentration gradients. The sum of these interactions influences bacterial growth rates, metabolism, gene expression, and general biological activities within the biofilm [1,6]. The biofilm community is encased in an extracellular matrix (ECM) that supports the three-dimensional organization of bacteria, while providing protection from its environment, antibiotics, and immune-mediated clearance [1-3,6].
Biofilm gradients
Biofilm growth is driven, in part, by adaptive changes in response to environmental cues, which leads to the establishment of chemical gradients throughout its structure [1,4-6]. The formation and persistence of these gradients are influenced by diminished fluid flow within the biofilm, spatial relationships of bacteria, as well as rates of solute production, consumption, and diffusion. Generalizations can be made concerning the direction of a concentration gradient for metabolic products and substrates based on Reaction-Diffusion theory [4]. For example, metabolic products produced by the biofilm, such as carbon dioxide and acids, can quickly diffuse at the biofilm-fluid interface but do so at a slower rate in the interior [4,14]. Conversely, the availability of metabolic substrates, such as glucose, oxygen, and other nutrients, are generally greatest at the fluid-biofilm interface and are slowly depleted as they diffuse towards the center of the biofilm [14,15]. It also follows that the ability of antibiotics to penetrate a biofilm and reach an effective concentration is dependent on diffusion [14]. Furthermore, biofilms can maintain a zone of relative nutrient depletion and toxin accumulation at their margins that may affect local host cells [4]. These multi-faceted and dynamic gradients ultimately provide a spectrum of unique environments for biofilm-associated bacteria and infiltrating leukocytes that encounter biofilm infections.
Biofilm physiology
Bacterial biofilms are known to display increased resistance to stress and have general differences in physiology compared to their planktonic counterparts. Besides the chemical gradients described above, the intercellular relationships of biofilm-associated bacteria and how they influence quorum sensing-mediated gene regulation are unique compared to planktonic organisms. For example, transcriptomic analysis comparing biofilm and planktonic P. aeruginosa [16] and S. aureus [17] demonstrated global differences in gene expression that were clearly distinct between each mode of growth. Furthermore, small molecules like cyclic-di-GMP have been shown to play a role in promoting biofilm formation and growth [18]. In general, biofilms exhibit reduced metabolism adapted to micro-aerobic growth and favor the production of ECM components. These attributes endow biofilm tolerance to metal, oxidative, and antibiotic stress [19]. In S. aureus biofilms, genes associated with responses to oxidative stress, metabolism, and toxin production are upregulated in biofilms relative to their planktonic counterparts [9,17,19]. In addition, an abundance of small RNAs are differentially expressed between biofilm and planktonic growth, which suggests that post-transcriptional regulation is another means of physiologic regulation [16,17]. Proteomic analysis of secreted proteins demonstrated that 108 of 301 proteins (36%) were significantly enriched in S. aureus biofilms compared to planktonic cultures. This included proteases and toxins, such as alpha-toxin (Hla) and leukocidin AB (LukAB) [9]. Analysis of >700 proteins from P. aeruginosa biofilms revealed that induced proteins could be grouped by their function and temporal relationship with biofilm developmental stages [20]. Other studies have reported the induction of specific genes and proteins that coincide with progressive stages of biofilm development [20,21]. Furthermore, changes in gene expression patterns can contribute to biofilm topology. For example, P. aeruginosa can stochastically express type IV pili to induce elevated stalks at specific foci in a biofilm [22]. In addition, stochastic expression of S. aureus nuclease during early biofilm growth has been implicated in biofilm establishment [23]. Together, this illustrates the differences in biofilm physiology and function compared to planktonic bacteria.
Biofilm extracellular matrix (ECM)
Although the ECM is a dynamic structure, especially during the initial stages of biofilm development, here we will focus on the ECM components of a mature biofilm [1,4]. In general, the bacterial biofilm ECM is composed of extracellular DNA, polysaccharides, and proteins, where the contributions of each to biofilm structure continue to be studied. Prior work has shown that proteinase K treatment leads to the dispersal of S. aureus and S. epidermidis biofilms [24], but growth enhancement of P. aeruginosa biofilms [25]. This suggests that the protein components of a Staphylococcal biofilm likely have direct structural contributions, whereas the contribution of proteins to P. aeruginosa biofilms is more complex. Tetz et al. found that DNase I treatment of biofilms from several bacterial species, including P. aeruginosa and S. aureus, resulted in biofilm dispersal without affecting cell viability. They also found that DNase I acted synergistically with antibiotics by promoting antibiotic penetration into the biofilm [26]. The clinical implications of this finding suggest that antibiotic efficacy may be increased by disrupting the biofilm structure.
The structural attributes of a biofilm promote adherence to biotic and abiotic surfaces while maintaining intercellular contacts. In addition, host-derived proteins can be exploited to facilitate biofilm establishment and maturation. For example, serum proteins including, fibronectin, fibrinogen, and collagen, rapidly coat foreign devices and are recognized by bacterial surface proteins, collectively referred to as Microbial Surface Components Recognizing Adhesive Matrix Molecules (MSCRAMMs), which facilitate bacterial adhesion and accumulation [27]. In addition, host-derived soluble proteins or cell components released from necrotic eukaryotic cells can also be incorporated into the biofilm ECM. For example, Pseudomonas biofilm growth is enhanced by the incorporation of neutrophil components released via NETosis or toxin-mediated neutrophil necrosis [10].
Other ECM components do not directly contribute to the ECM biomass but instead regulate the complexity and topology of the biofilm structure. For example, bacteria release nucleases, proteases, and detergent-like molecules that remodel the ECM to form channels that facilitate nutrient delivery to deeper biofilm layers. S. aureus [28] and P. aeruginosa [6] produce phenol-soluble modulins (PSMs) and rhamnolipids, respectively, which influence biofilm structure and dissemination. These molecules are multi-functional and contribute to the repertoire of virulence factors that allow biofilms to escape immune-mediated clearance, which will be discussed in greater detail below.
IMMUNE EVASION DURING BIOFILM INFECTION
Bacterial biofilms are able to subvert the host immune response by several mechanisms, which include interfering with humoral immunity, secreting toxins to impair recognition, and regulating the inflammatory status of recruited MDSCs, MΦs and neutrophils. The failure to initiate an effective immune response results in chronic biofilm infections, which require physical dissociation and removal of infected tissues/medical implants for treatment.
Humoral responses and pattern recognition receptors (PRRs)
Established biofilms have several mechanisms to protect against humoral immune attack. Recent studies have shown that Staphylococcal biofilms do not prevent the diffusion of humoral components, but instead their large biomass, as compared to planktonic bacteria, dilutes targeting antibodies and interferes with opsonophagocytosis [29]. Other humoral components, including complement and antimicrobial peptides are also targets of immune evasion during biofilm growth. For example, P. aeruginosa upregulates alkaline proteases and elastases during biofilm growth, which directly inactivate complement proteins [3]. Furthermore, the ECM can bind positively-charged antimicrobial peptides to mask the bacterial surface or inhibit activation of the alternative complement pathway, as in the case of Pseudomonas alginate [30].
Prior studies have shown that MΦs are capable of phagocytosing S. aureus from a disrupted, but not intact biofilm [10,31]. This suggests that the size of a biofilm likely represents a physical barrier, inducing a phenomenon known as “frustrated phagocytosis” [9,31,32] and the release of intracellular molecules that can lead to bystander toxicity of surrounding immune and stromal cells. In addition, S. aureus biofilms evade TLR2- and TLR9-dependent recognition and Pseudomonas has been shown to downregulate PAMP expression during biofilm development to inhibit immune recognition [10,33].
Toxins
Many biofilm toxins are regulated by quorum sensing mechanisms that are enriched during biofilm growth, and can directly kill MΦs, neutrophils, and other leukocytes to inhibit immune recognition and microbicidal activity. For example, S. aureus biofilms secrete α-hemolysin (Hla) and leukocidin AB (LukAB), which inhibit MΦ phagocytosis and promote MΦ death [9]. Furthermore, Hla facilitates immune evasion within phagosomes, preventing the intracellular killing of phagocytosed bacteria [34]. A S. aureus hla/lukAB mutant displayed significantly reduced bacterial burden and increased MΦ infiltration during orthopedic implant biofilm infection, demonstrating a complementary role for both toxins in vivo [9]. These toxins are controlled by quorum sensing mechanisms, which are disrupted following destruction of the biofilm architecture. This highlights the importance of intercellular interactions within the biofilm community, representing a communal virulence determinant [9]. Detergent-like molecules produced by biofilms can also exert direct cytotoxic activity on responding immune effector cells. For example rhamnolipids, which contribute to biofilm channel formation and dissemination in Pseudomonas, are toxic to neutrophils and host tissues surrounding biofilm infections [3]. The resulting host debris provides additional substrates for the biofilm ECM, promoting its development. Furthermore, bulky matrix components, such as alginate, may act as a virulence determinant in P. aeruginosa biofilms by inducing frustrated phagocytosis [32], similar to Staphylococcal biofilms described above.
Immune polarization
The concept of immune polarization originated from in vitro studies of MΦ activation, which led to the identification of M1 (classical) and M2 (alternative) states to describe pro-inflammatory versus anti-inflammatory attributes of MΦs, respectively [35]. However, it is now well recognized that in vivo, MΦ activation exists in a spectrum between pro-inflammatory and anti-inflammatory states, which exhibits some degree of plasticity. Here, we will discuss the immune polarization states of murine MΦs following exposure to biofilm or planktonic bacterial infections. Of note, there are important distinctions between murine and human MΦs in terms of cytokines responsible for driving polarization states and effector molecules, such as inducible nitric oxide (iNOS), which is more robustly expressed in murine compared to human MΦs [35]. For more detailed information on MΦ immune polarization states and corresponding metabolic effects, the reader is referred to several excellent reviews [11,12].
By virtue of their antibiotic resistance, induced by both mutations as well as the metabolic dormancy of bacterial subpopulations, the increasing frequency of biofilm infections highlights the importance of understanding the resultant host immune response, in hopes of developing novel therapeutic approaches. Biofilm infections employ numerous strategies to actively induce an anti-inflammatory MΦ response to promote biofilm persistence. Clinical evidence of immune deviation comes from P. aeruginosa pulmonary biofilm infections that are dominated by a Th2 response, whereas acutely infected airways are characterized by greater Th1 recruitment and IFN-β expression [36]. In addition, immune effector mechanisms differ in their utility during biofilm and planktonic infections. Recent work demonstrates that despite the importance of myeloid-derived arginase-1 in controlling S. aureus planktonic infection, arginase-1 does not play a significant role during implant-associated biofilm infections [37]. These findings provide additional evidence that biofilms are able to polarize the immune response to promote biofilm persistence.
Recent reports have shown that S. aureus biofilm infections are characterized by anti-inflammatory MΦs, MDSC expansion, and paucity of T cells in both humans and mouse models [7,8,38-40]. In contrast, planktonic S. aureus infections are generally cleared by eliciting a robust pro-inflammatory response through the activation of MΦ pattern recognition receptors, neutrophil recruitment, and T cell activation. MΦs associated with S. aureus and S. epidermidis biofilm infections are characterized by increased Arg-1 and decreased iNOS expression [7,8,38,39]. Hanke et. al. demonstrated that the MΦ activation state was critical for biofilm persistence. For example, as opposed to endogenous biofilm-associated MΦs that do not exert any anti-bacterial activity, the adoptive transfer of activated pro-inflammatory MΦs into S. aureus biofilm infections in vivo facilitated biofilm clearance. In addition, activated pro-inflammatory MΦs were able to infiltrate and phagocytose S. aureus biofilms in vitro, which was not observed with non-activated MΦs [7]. Additional mechanisms of active immune polarization by S. aureus biofilms, including the preferential accumulation of MDSCs (CD11b+Ly6GhlghLy6C+) have been identified. MDSCs are likely recruited and expanded at the site of biofilm infection due to the robust chemokine/cytokine milieu, which has been identified as a key signal for promoting MDSC activation [8,38,39,41]. Recent work has demonstrated that MDSCs are a primary source of IL-10 and maintain suppressive activity ex vivo [8,38,39]. Depletion of MDSCs with a Ly6G antibody significantly reduced biofilm burdens and increased the pro-inflammatory activity of biofilm-associated monocytes. The ability of monocytes/MΦs to promote biofilm clearance in the absence of MDSC action was indirectly demonstrated using a Gr-1 antibody, which resulted in significantly increased S. aureus burdens, since effector Ly6C monocytes and by extension, mature MΦs, were also depleted. Taken together, these results support the conclusion that biofilm-mediated MDSC recruitment regulates, in part, the antiinflammatory polarization of monocytes, effectively promoting biofilm persistence [8].
IMMUNOMETABOLISM
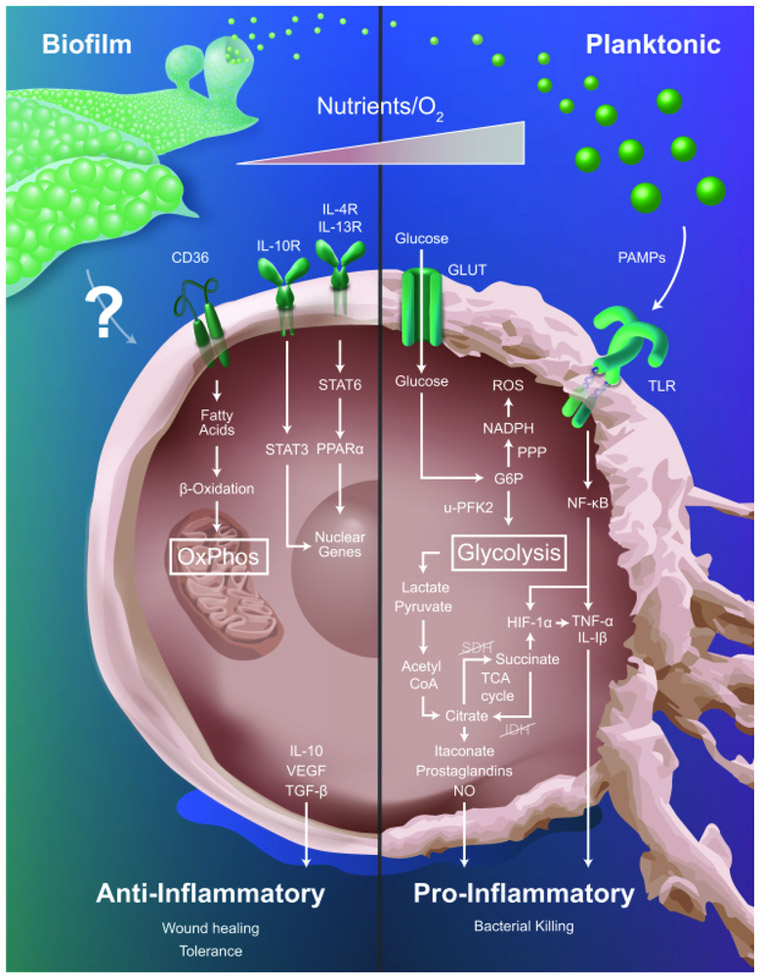
The field of immunometabolism focuses on changes in leukocyte metabolism, which ultimately govern inflammatory phenotypes [12,13]. The link between metabolic alterations and cellular function was first reported by Otto Warburg, when he observed that proliferating tumor cells relied heavily on aerobic glycolysis [42]. It has been well-established that aerobic glycolysis is a primary feature of pro-inflammatory MΦs, which is necessary to increase carbon flux through the pentose-phosphate pathway and provide precursor molecules for anabolic processes and reactive oxygen species production [12,13]. In contrast, MΦs primarily rely on oxidative phosphorylation (OxPhos) to drive their anti-inflammatory activity, with fatty acid oxidation also playing a role. In MΦs, these metabolic switches are facilitated by global changes in gene expression. For example, pro-inflammatory MΦs express U-PFK2 (ubiquitous phosphofructokinase), a highly active PFK-2 isoform and down-regulate TCA cycle enzymes, facilitating intracellular accumulation of glucose, succinate, and citrate [12,13]. In the mouse, pro-inflammatory MΦs also generate nitric oxide through upregulation of iNOS, which directly inhibits OxPhos [12]. In contrast, anti-inflammatory MΦs express PFKB1, a less active PFK-2 isoform, and upregulate CD36 to facilitate triglyceride uptake to fuel the TCA cycle [43]. In solid tumors, MΦs undergo metabolic shifts through fluctuations in oxygen, nutrient, and metabolite availability, which coincide with changes in inflammatory phenotype [44]. Similarly, S. aureus biofilms generate nutrient, proton, and oxygen gradients with the potential to metabolically reprogram MΦs and alter their inflammatory profile [4]. In this section, we will discuss studies describing the immunometabolic profiles of MΦs associated with planktonic infections and provide insight into the possible metabolic changes that occur in biofilm-associated MΦs (Figure 1).
Figure 1. Metabolic profiles influence macrophage inflammatory status.
Macrophages respond to planktonic infections via sensing of pathogen-associated molecular patterns (PAMPs) through Toll-like receptor (TLR) engagement. This favors aerobic glycolysis to provide TCA cycle intermediates for anabolic processes required for pro-inflammatory effector mechanisms. In contrast, biofilm infections polarize macrophages towards an anti-inflammatory state and the biofilm-derived signals that drive this process are largely unknown (indicated by question mark). Since anti-inflammatory macrophages are typified by oxidative phosphorylation (OxPhos) it is predicted that biofilm infections will bias cells towards this metabolic pathway and several receptors associated with anti-inflammatory cytokines might be involved (i.e. CD36, IL-10R, IL-4R, and IL-13R). The metabolic gradients present in the tissue microenvironment (i.e. nutrients, oxygen) also influence the pro- versus anti-inflammatory profiles of macrophages that are intimately linked to their metabolic state. G6P, glucose-6-phosphate; GLUT, glucose transporter; HIF-1α, hypoxia inducible factor-alpha; IL-1β, IDH, isocitrate dehydrogenase; interleukin-1 beta; IL-4R, interleukin-4 receptor; IL-10, interleukin-10; IL-10R, interleukin-10 receptor; IL-13R, interleukin-13 receptor; iNOS, inducible nitric oxide synthase; NADPH, nicotinamide adenine dinucleotide phosphate; NF-κB, nuclear factor-kappa B; NO, nitric oxide; PPARα, peroxisome proliferator-activated receptor alpha; PPP, pentose phosphate pathway; ROS, reactive oxygen species; SDH, succinate dehydrogenase; STAT3, signal transducer and activator of transcription 3; STAT6, signal transducer and activator of transcription 6; TCA, tricarboxylic acid cycle; TGF-β, transforming growth factor-beta; TNF-α, tumor necrosis factor-alpha; uPFK2, ubiquitous 6-phosphofructo-2-kinase/fructose bisphosphatase 2; VEGF, vascular endothelial growth factor
Mycobacterium tuberculosis provides an excellent example of how MΦ metabolism and function are intimately related. During M. tuberculosis infection, a granuloma is formed that is surrounded by activated T cells and pro-inflammatory MΦs, both of which are highly glycolytic. These MΦs curtail the growth of intracellular bacteria by secreting anti-microbial effector molecules that facilitate the transition to latent infection [45,46]. During stages of active infection MΦs display anti-inflammatory properties, including a dependence on β-oxidation and the formation of foamy MΦs. These MΦs no longer produce anti-microbial compounds, allowing expansion and dissemination of the infection [45,46]. Recent studies revealed the importance of metabolism by targeting HIF-1α, a master regulator of aerobic glycolysis. Elks et al. stabilized HIF-1α during Mycobacterium infection in zebrafish, which reduced bacterial burden and enhanced neutrophil anti-microbial function [47]. When Cardoso et al. infected HIF-1α knockout mice with Mycobacterium, the bacterial burden was significantly increased along with premature dissemination [48].
Effect of chemical gradients and nutrient availability on leukocyte metabolism
Although biofilms are generally less metabolically active than planktonic bacteria, the sheer number of organisms allows the biofilm to establish metabolic gradients by depleting glucose and oxygen from the surrounding microenvironment [49]. Furthermore, host cell lysis can increase the abundance of metabolic enzymes, such as hexokinase and indoleamine-2,3-dioxygenase, that can deplete usable glucose and amino acids, respectively [50]. Mammalian intracellular metabolic pathways are sensitively regulated by nutrient availability, including glucose, glutamine, and fatty acids. Local hypoxia and nutrient depletion from the microenvironment can reduce glycolytic rates in MΦs and promote anti-inflammatory polarization [11,12]. Local nutrient depletion likely results from contributions of both the biofilm and host cells, as it has been shown that neutrophils consume the majority of oxygen in cystic fibrosis patients [10]. Although immune shifts are possible during planktonic infections with highly metabolically active bacteria such as Staphylococcal sp., it is unlikely that planktonic infections can deplete nutrients as quickly as they are replenished in the host. Therefore, it is probable that the communal and relatively static nature of biofilms allow these metabolic gradients and zones of nutrient depletion to persist over the course of infection, having a dramatic impact on leukocyte metabolism and inflammatory phenotypes.
Effect of metabolism on leukocyte development and life span
OxPhos is more efficient than glycolysis in extracting energy from glucose. However, under conditions where glycolysis is enhanced, a concomitant increase in the pentose phosphate pathway (PPP) is generally observed to provide NADPH and ribose phosphate, which are important for biosynthesis, cell division, and other cellular functions [11,12]. An important aspect of immunity is controlling cellular life span, such as memory lymphocyte populations and terminally differentiated leukocytes responding to pathogens. Following the resolution of infection, the life span of these effector cells must be tightly regulated to prevent potential bystander pathology. Several studies have demonstrated that reliance on OxPhos metabolism supports longevity. For example, anti-inflammatory MΦs that favor OxPhos have an increased life span, whereas pro-inflammatory MΦs, which rely more on glycolysis are shorter lived [11]. This relationship is most pronounced in memory T cells, where long-lived resting T cells do not oxidize glucose but instead rely solely on fatty acid β-oxidation, whereas cytokine stimulation promotes glycolytic metabolism, with cells undergoing rapid apoptosis following cytokine withdrawal [11].
With regard to biofilms, recent studies have demonstrated that S. aureus biofilm-associated MΦs exhibit anti-inflammatory profiles, despite elevated pro-inflammatory cytokines in infected tissues [8,38]. Cytokine levels were significantly reduced during device-associated biofilm infection in MyD88-deficient mice; however, this resulted in increased bacterial burden and dissemination, since MΦs and neutrophils lacked major TLR effector pathways necessary for killing planktonic bacteria following biofilm dispersal [51]. It is possible that elevated glycolysis-inducing cytokines could contribute to biofilm persistence by eliminating effector cells by apoptosis in combination with biofilm-derived lytic toxins until nutrients are depleted, at which point newly recruited MΦs and neutrophils are unable to be activated; however, this remains speculative. Although cytokine-induced apoptosis of MΦs and neutrophils occur during planktonic infections, the static and chronic nature of biofilms facilitates continued cytokine accumulation, likely accounting for the robust cytokine milieu associated with S. aureus biofilm infections in vivo [8,38-40].
Host-derived metabolites that influence leukocyte-biofilm interactions
TCA cycle defects in pro-inflammatory MΦs lead to the accumulation of TCA cycle intermediates, namely citrate and succinate (Figure 1). Accumulation of succinate promotes HIF-1α stabilization, epigenetic changes, and enhances IL-1β production through inhibition of α-ketoglutarate-dependent enzymes [11]. Citrate can be used to generate itaconic acid, which is an anti-microbial metabolite that inhibits the growth of planktonic Salmonella enterica and Mycobacterium tuberculosis [11]. However, the antimicrobial actions of itaconic acid have not yet been explored in the context of biofilm infections. Furthermore, citrate is used as a precursor for nitric oxide, ROS, lipid, and prostaglandin production. Since biofilm-associated MΦs are anti-inflammatory in nature, it is likely that they primarily utilize OxPhos metabolism.
CONCLUSIONS AND FUTURE PERSPECTIVES
Biofilms are communities of organisms encased in a complex extracellular matrix, which can colonize both biotic and abiotic surfaces. Biofilm infections are associated with significant morbidity and economic burden, since they are recalcitrant to antibiotic therapy and require physical removal and/or debridement of infected tissues for treatment. Biofilms can subvert the host immune response by preventing immune detection, toxin production, and polarizing MΦs towards an anti-inflammatory state, which promotes biofilm persistence in an immune competent host (Figure 1). Recent metabolic studies have shown that immune cell function and metabolism are intimately related. In terms of bacterial infection, immunometabolic studies have primarily focused on MΦs and neutrophils responding to planktonic bacteria. Given the differences in inflammatory properties of MΦs and neutrophils responding to planktonic versus biofilm infections, future studies must also consider the metabolic properties of biofilm-associated immune cells.
ACKNOWLEDGEMENTS
This work was supported by the NIH National Institute of Allergy and Infectious Disease (NIAID) 2P01AI083211 (Project 4 to T.K.). The authors thank LabRat Design for the artwork presented in Figure 1.
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Flemming HC, Wingender J, Szewzyk U, Steinberg P, Rice SA, Kjelleberg S: Biofilms: an emergent form of bacterial life. Nature reviews Microbiology 2016;14:563–575. [DOI] [PubMed] [Google Scholar]
- 2.Gries CM, Kielian T: Staphylococcal Biofilms and Immune Polarization During Prosthetic Joint Infection. The Journal of the American Academy of Orthopaedic Surgeons 2017;25 Suppl 1:S20–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mulcahy LR, Isabella VM, Lewis K: Pseudomonas aeruginosa biofilms in disease. Microbial ecology 2014;68:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stewart PS, Franklin MJ: Physiological heterogeneity in biofilms. Nature reviews Microbiology 2008;6:199–210. [DOI] [PubMed] [Google Scholar]
- 5.Archer NK, Mazaitis MJ, Costerton JW, Leid JG, Powers ME, Shirtliff ME: Staphylococcus aureus biofilms: properties, regulation, and roles in human disease. Virulence 2011;2:445–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rasamiravaka T, Labtani Q, Duez P, El Jaziri M: The formation of biofilms by Pseudomonas aeruginosa: a review of the natural and synthetic compounds interfering with control mechanisms. BioMed research international 2015;2015:759348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanke ML, Heim CE, Angle A, Sanderson SD, Kielian T: Targeting macrophage activation for the prevention and treatment of Staphylococcus aureus biofilm infections. Journal of immunology 2013;190:2159–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heim CE, Vidlak D, Scherr TD, Kozel JA, Holzapfel M, Muirhead DE, Kielian T: Myeloid-derived suppressor cells contribute to Staphylococcus aureus orthopedic biofilm infection. Journal of immunology 2014;192:3778–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scherr TD, Hanke ML, Huang O, James DB, Horswill AR, Bayles KW, Fey PD, Torres VJ, Kielian T: Staphylococcus aureus Biofilms Induce Macrophage Dysfunction Through Leukocidin AB and Alpha-Toxin. mBio 2015;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rada B: Interactions between Neutrophils and Pseudomonas aeruginosa in Cystic Fibrosis. Pathogens 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loftus RM, Finlay DK: Immunometabolism: Cellular Metabolism Turns Immune Regulator. The Journal of biological chemistry 2016;291:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Neill LA, Pearce EJ: Immunometabolism governs dendritic cell and macrophage function. The Journal of experimental medicine 2016;213:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torres A, Makowski L, Wellen KE: Immunometabolism: Metabolism fine-tunes macrophage activation. eLife 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiamco MM, Atci E, Mohamed A, Call DR, Beyenal H: Hyperosmotic Agents and Antibiotics Affect Dissolved Oxygen and pH Concentration Gradients in Staphylococcus aureus Biofilms. Applied and environmental microbiology 2017;83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stewart PS, Zhang T, Xu R, Pitts B, Walters MC, Roe F, Kikhney J, Moter A: Reaction-diffusion theory explains hypoxia and heterogeneous growth within microbial biofilms associated with chronic infections. NPJ biofilms and microbiomes 2016;2:16012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dotsch A, Eckweiler D, Schniederjans M, Zimmermann A, Jensen V, Scharfe M, Geffers R, Haussler S: The Pseudomonas aeruginosa transcriptome in planktonic cultures and static biofilms using RNA sequencing. PloS one 2012;7:e31092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Resch A, Rosenstein R, Nerz C, Gotz F: Differential gene expression profiling of Staphylococcus aureus cultivated under biofilm and planktonic conditions. Applied and environmental microbiology 2005;71:2663–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hengge R, Grundling A, Jenal U, Ryan R, Yildiz F: Bacterial Signal Transduction by Cyclic Di-GMP and Other Nucleotide Second Messengers. Journal of bacteriology 2016;198:15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Booth SC, Workentine ML, Wen J, Shaykhutdinov R, Vogel HJ, Ceri H, Turner RJ, Weljie AM: Differences in metabolism between the biofilm and planktonic response to metal stress. Journal of proteome research 2011;10:3190–3199. [DOI] [PubMed] [Google Scholar]
- 20.Southey-Pillig CJ, Davies DG, Sauer K: Characterization of temporal protein production in Pseudomonas aeruginosa biofilms. Journal of bacteriology 2005;187:8114–8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei Q, Ma LZ: Biofilm matrix and its regulation in Pseudomonas aeruginosa. International journal of molecular sciences 2013;14:20983–21005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klausen M, Aaes-Jorgensen A, Molin S, Tolker-Nielsen T: Involvement of bacterial migration in the development of complex multicellular structures in Pseudomonas aeruginosa biofilms. Molecular microbiology 2003;50:61–68. [DOI] [PubMed] [Google Scholar]
- 23.Moormeier DE, Bose JL, Horswill AR, Bayles KW: Temporal and stochastic control of Staphylococcus aureus biofilm development. mBio 2014;5:e01341–01314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elchinger PH, Delattre C, Faure S, Roy O, Badel S, Bernardi T, Taillefumier C, Michaud P: Effect of proteases against biofilms of Staphylococcus aureus and Staphylococcus epidermidis. Letters in applied microbiology 2014;59:507–513. [DOI] [PubMed] [Google Scholar]
- 25.Gilan I, Sivan A: Effect of proteases on biofilm formation of the plastic-degrading actinomycete Rhodococcus ruber C208. FEMS microbiology letters 2013;342:18–23. [DOI] [PubMed] [Google Scholar]
- 26.Tetz GV, Artemenko NK, Tetz VV: Effect of DNase and antibiotics on biofilm characteristics. Antimicrobial agents and chemotherapy 2009;53:1204–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arrecubieta C, Toba FA, von Bayern M, Akashi H, Deng MC, Naka Y, Lowy FD: SdrF, a Staphylococcus epidermidis surface protein, contributes to the initiation of ventricular assist device driveline-related infections. PLoS pathogens 2009;5:e1000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Periasamy S, Joo HS, Duong AC, Bach TH, Tan VY, Chatterjee SS, Cheung GY, Otto M: How Staphylococcus aureus biofilms develop their characteristic structure. Proceedings of the National Academy of Sciences of the United States of America 2012;109:1281–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cerca N, Jefferson KK, Oliveira R, Pier GB, Azeredo J: Comparative antibody-mediated phagocytosis of Staphylococcus epidermidis cells grown in a biofilm or in the planktonic state. Infection and immunity 2006;74:4849–4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewenza S: Extracellular DNA-induced antimicrobial peptide resistance mechanisms in Pseudomonas aeruginosa. Frontiers in microbiology 2013;4:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thurlow LR, Hanke ML, Fritz T, Angle A, Aldrich A, Williams SH, Engebretsen IL, Bayles KW, Horswill AR, Kielian T: Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. Journal of immunology 2011;186:6585–6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leid JG, Willson CJ, Shirtliff ME, Hassett DJ, Parsek MR, Jeffers AK: The exopolysaccharide alginate protects Pseudomonas aeruginosa biofilm bacteria from IFN-gamma-mediated macrophage killing. Journal of immunology 2005;175:7512–7518. [DOI] [PubMed] [Google Scholar]
- 33.Bernthal NM, Pribaz JR, Stavrakis AI, Billi F, Cho JS, Ramos RI, Francis KP, Iwakura Y, Miller LS: Protective role of IL-1beta against post-arthroplasty Staphylococcus aureus infection. Journal of orthopaedic research : official publication of the Orthopaedic Research Society 2011;29:1621–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koziel J, Chmiest D, Bryzek D, Kmiecik K, Mizgalska D, Maciag-Gudowska A, Shaw LN, Potempa J: The Janus face of alpha-toxin: a potent mediator of cytoprotection in staphylococci-infected macrophages. Journal of innate immunity 2015;7:187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez FO, Gordon S: The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000prime reports 2014;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moser C, Kjaergaard S, Pressler T, Kharazmi A, Koch C, Hoiby N: The immune response to chronic Pseudomonas aeruginosa lung infection in cystic fibrosis patients is predominantly of the Th2 type. APMIS : acta pathologica, microbiologica, et immunologica Scandinavica 2000;108:329–335. [DOI] [PubMed] [Google Scholar]
- 37.Yamada KJ, Heim CE, Aldrich AL, Gries CM, Staudacher AG, Kielian T: Arginase-1 expression in myeloid cells regulates S. aureus planktonic but not biofilm infection. Infection and immunity 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heim CE, Vidlak D, Kielian T: Interleukin-10 production by myeloid-derived suppressor cells contributes to bacterial persistence during Staphylococcus aureus orthopedic biofilm infection. Journal of leukocyte biology 2015;98:1003–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heim CE, Vidlak D, Scherr TD, Hartman CW, Garvin KL, Kielian T: IL-12 promotes myeloid-derived suppressor cell recruitment and bacterial persistence during Staphylococcus aureus orthopedic implant infection. Journal of immunology 2015;194:3861–3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heim CE, Vidlak D, Odvody J, Hartman CW, Garvin KL, Kielian T: Human prosthetic joint infections are associated with myeloid-derived suppressor cells (MDSCs): Implications for infection persistence. Journal of orthopaedic research : official publication of the Orthopaedic Research Society 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heim CE, Hanke ML, Kielian T: A mouse model of Staphylococcus catheter-associated biofilm infection. Methods in molecular biology 2014;1106:183–191. [DOI] [PubMed] [Google Scholar]
- 42.Hsu PP, Sabatini DM: Cancer cell metabolism: Warburg and beyond. Cell 2008;134:703–707. [DOI] [PubMed] [Google Scholar]
- 43.Feingold KR, Shigenaga JK, Kazemi MR, McDonald CM, Patzek SM, Cross AS, Moser A, Grunfeld C: Mechanisms of triglyceride accumulation in activated macrophages. Journal of leukocyte biology 2012;92:829–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Overmeire E, Stijlemans B, Heymann F, Keirsse J, Morias Y, Elkrim Y, Brys L, Abels C, Lahmar Q, Ergen C, Vereecke L, Tacke F, De Baetselier P, Van Ginderachter JA, Laoui D: M-CSF and GM-CSF Receptor Signaling Differentially Regulate Monocyte Maturation and Macrophage Polarization in the Tumor Microenvironment. Cancer research 2016;76:35–42. [DOI] [PubMed] [Google Scholar]
- 45.Benoit M, Desnues B, Mege JL: Macrophage polarization in bacterial infections. Journal of immunology 2008;181:3733–3739. [DOI] [PubMed] [Google Scholar]
- 46.Kim MJ, Wainwright HC, Locketz M, Bekker LG, Walther GB, Dittrich C, Visser A, Wang W, Hsu FF, Wiehart U, Tsenova L, Kaplan G, Russell DG: Caseation of human tuberculosis granulomas correlates with elevated host lipid metabolism. EMBO molecular medicine 2010;2:258–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elks PM, Brizee S, van der Vaart M, Walmsley SR, van Eeden FJ, Renshaw SA, Meijer AH: Hypoxia inducible factor signaling modulates susceptibility to mycobacterial infection via a nitric oxide dependent mechanism. PLoS pathogens 2013;9:e1003789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cardoso MS, Silva TM, Resende M, Appelberg R, Borges M: Lack of the Transcription Factor Hypoxia-Inducible Factor 1alpha (HIF-1alpha) in Macrophages Accelerates the Necrosis of Mycobacterium avium-Induced Granulomas. Infection and immunity 2015;83:3534–3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilde AD, Snyder DJ, Putnam NE, Valentino MD, Hammer ND, Lonergan ZR, Hinger SA, Aysanoa EE, Blanchard C, Dunman PM, Wasserman GA, Chen J, Shopsin B, Gilmore MS, Skaar EP, Cassat JE: Bacterial Hypoxic Responses Revealed as Critical Determinants of the Host-Pathogen Outcome by TnSeq Analysis of Staphylococcus aureus Invasive Infection. PLoS pathogens 2015;11:e1005341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hirayama A, Kami K, Sugimoto M, Sugawara M, Toki N, Onozuka H, Kinoshita T, Saito N, Ochiai A, Tomita M, Esumi H, Soga T: Quantitative metabolome profiling of colon and stomach cancer microenvironment by capillary electrophoresis time-of-flight mass spectrometry. Cancer research 2009;69:4918–4925. [DOI] [PubMed] [Google Scholar]
- 51.Hanke ML, Angle A, Kielian T: MyD88-dependent signaling influences fibrosis and alternative macrophage activation during Staphylococcus aureus biofilm infection. PloS one 2012;7:e42476. [DOI] [PMC free article] [PubMed] [Google Scholar]