Abstract
Pontospinal noradrenergic neurons form a component of an endogenous analgesic system and represent a potential therapeutic target. We tested the principle that genetic manipulation of their excitability can alter nociception using an adenoviral vector (AVV-PRS-hKir2.1) containing a catecholaminergic-selective promoter (PRS) to retrogradely-transduce and inhibit the noradrenergic neurons projecting to the lumbar dorsal horn through the expression of a potassium channel (hKir2.1).
Expression of hKir2.1 in catecholaminergic PC12 cells hyperpolarized the membrane potential and produced a barium-sensitive inward rectification. LC neurons transduced by AVV-PRS-hKir2.1 in slice cultures also showed barium-sensitive inward rectification and reduced spontaneous firing rate (median 0.2 Hz; n=19 vs. control 1.0 Hz; n=18, P<0.05). Pontospinal noradrenergic neurons were retrogradely transduced in vivo by injection of AVV into the lumbar dorsal horn (L4–5). Rats transduced with AVV-PRS-hKir2.1 showed thermal but not mechanical hyperalgesia. Similar selective augmentation of thermal hyperalgesia was seen in the CFA-inflammatory pain model after AVV-PRS-hKir2.1. In the formalin test, rats transduced with hKir2.1 showed enhanced nocifensive behaviors (both Phase I and II, P<0.05, n=11/group) and increased c-fos positive cells in the lumbar dorsal horn. Transduction with AVV-PRS-hKir2.1 prior to spared nerve injury produced no change in tactile or cold allodynia.
Thus the selective genetic inhibition of ~150 pontospinal noradrenergic neurons produces a modality specific thermal hyperalgesia, increased nocifensive behaviors and spinal c-fos expression in the formalin test, but not in the spared nerve injury model of neuropathic pain, indicating that these neurons exert a selective tonic restraining influence on in vivo nociception.
Keywords: Pain, Descending control, Adenoviral vector, Noradrenergic, Potassium channel, Pontospinal
Introduction
The degree of pain perceived in response to a given noxious stimulus is greatly influenced by the context within which the injury is suffered (eg (Melzack et al., 1982)). In part, this variation is due to the engagement of endogenous analgesic mechanisms within the central nervous system that act to modulate pain. Since the report that electrical stimulation of the mid-brain produces profound analgesia (Reynolds, 1969), much attention has focused on descending control systems which can influence the spinal transmission of noxious inputs (Millan, 2002; Fields, 2004). The norepinephrine (NE)-containing neurons of the locus coeruleus (LC), A5 and A7 cell groups in the pons have been implicated as key components of this descending control system (Jones, 1991; Millan, 1997; Pertovaara, 2006).
These neurons send axonal projections to the spinal dorsal horn which release NE (Crawley et al., 1979; Hentall et al., 2003) that acts via α2-adrenoceptors to inhibit both primary afferents and second order projection neurons (Reddy et al., 1980; Hammond and Yaksh, 1984; North and Yoshimura, 1984; Jones and Gebhart, 1986a; Miller and Proudfit, 1990; Sonohata et al., 2004). Stimulation of the pontine noradrenergic (NAergic) cell groups has been shown to be analgesic in a number of acute pain models (Jones and Gebhart, 1986a, b; Miller and Proudfit, 1990; Jones, 1991; Yeomans et al., 1992; West et al., 1993) and they are activated in stressful situations (e.g. swim stress analgesia (Bodnar et al., 1985)). In chronic neuropathic pain models there are plastic changes in the NAergic innervation of the spinal dorsal horn and alterations in α2-adrenoceptor sensitivity (Ma and Eisenach, 2003; Hayashida et al., 2008). This endogenous pain modulating system is also important in clinical practice as several analgesic drugs are believed to act by mimicking or modulating these NAergic neurons and are used in the treatment of acute (e.g. tramadol, clonidine) and chronic pain (tricyclic antidepressants, duloxetine or intrathecal clonidine (Eisenach et al., 1995)).
Therefore the pontospinal NAergic neurons represent a potential target for therapeutic genetic intervention. We have previously demonstrated these neurons can be retrogradely-transduced using an adenoviral vector (AVV) with a catecholaminergic-selective promoter (Howorth et al., 2009). In the current study we have tested the principle that genetic manipulation of these noradrenergic neurons can alter nociception. We hypothesized that inhibiting the activity of these NAergic neurons, through the selective expression of a ‘leak’ potassium channel (hKir2.1), will enhance the responsiveness to acute and chronic noxious stimuli in vivo.
Methods
Experiments were performed on male Wistar rats (n=116, University of Bristol colony). All procedures conformed to the UK Animals (Scientific Procedures) Act 1986 and were approved by our institutional ethical review committee. Animals were group housed, with an enriched environment, on thick sawdust bedding and under a standard 12 hour light / dark cycle, with free access to food and water.
Adenoviral vector constructs and preparation
The control vector AVV-PRS-EGFP employs a 240-bp PRSx8 promoter sequence (Hwang et al., 2001) that restricts the expression of the transgene - enhanced green fluorescent protein (EGFP) - to a subset of neurons that express the Phox2 transcription factor. We have previously described its preparation (Lonergan et al., 2005) and use to express fluorophores for detailed anatomical studies of pontospinal NAergic neurons (Howorth et al., 2009).
AVV-PRS-hKir2.1 was created from a previously described lentiviral vector expression cassette which was transferred into an adenoviral backbone AVV-PRS-hKir2.1 (Duale et al., 2007). The PRSx8 promoter was employed to drive expression of both hKir2.1 a human inwardly rectifying potassium channel gene ((Kubo et al., 1993), plasmid kindly donated by Dr David C. Johns, Johns Hopkins University School of Medicine, Baltimore) and EGFP through the use of an internal ribosomal entry site. To take advantage of the bidirectional activity of the PRSx8 promoter ((Duale et al., 2007), see also (Amendola et al., 2005)) an additional copy of EGFP was placed in anti-sense orientation upstream of the PRSx8 promoter to enhance the level of EGFP fluorescence.
AVVs were obtained by homologous recombination of shuttle with helper plasmids and proliferation in HEK293 cells, followed by CsCl gradient purification, using standard techniques (Graham and Prevec, 1995). The AVV stocks were titred by an immuno-reactivity ‘spot’ assay (goat anti-hexon antiserum, Biodesign International, MA) according to previously published protocols (Bewig and Schmidt, 2000; Duale et al., 2005) and titres were determined as transducing units (TU) per ml.
Cell culture
Catecholaminergic PC12 cells were seeded on glass covers slips coated with Matrigel™ (BD Biosciences, Bedford, MA, USA) in serum-supplemented Dulbecco’s Modified Eagle Medium. The cells were transfected with shuttle plasmid DNA for either AVV-PRS-hKir2.1 or AVV-PRS-EGFP (2.5 μg/well) using SuperFect (Qiagen, Düsseldorf, Germany). Following transduction, cells were differentiated with nerve growth factor (NGF, 50 ng.ml−1). Both plasmids produced visible EGFP fluorescence in PC12 cells (typically in ~10% of PC12 cells). However, the fluorescence induced by the AVV-PRS-hKir2.1 plasmid was dimmer, as expected, given the decreased level of expression of gene products placed downstream of an IRES or upstream of a promoter in anti-sense orientation.
Slice culture
Brainstem slice cultures were prepared as described previously (Stoppini et al., 1991; Teschemacher et al., 2005; Wang et al., 2006). Briefly, Wistar rat pups (P7–8, n=45) were terminally anesthetized with halothane. The brainstem was removed and bathed in ice-cold dissection medium. Slices of the pons were cut (250 μm thick, coronal or longitudinal) in cold (4°C) sterile dissection solution using a vibratome (Vibroslice, Campden Instruments, UK) and kept on ice for 1 h. Two or three slices were plated on each organotypic culture insert (Millicell-CM, Millipore) in Optimem-1-based medium (Stoppini et al., 1991; Teschemacher et al., 2005; Wang et al., 2006) containing AVV (either AVV-PRS-EGFP alone or a 2:1 mixture of AVV-PRS-hKir2.1 : AVV-PRS-EGFP). Slices were incubated at 37°C in a 5% CO2 atmosphere. After 3 days, the plating medium was exchanged for supplemented Neurobasal medium, which was subsequently changed twice a week (Stoppini et al., 1991; Teschemacher et al., 2005; Wang et al., 2006). Slice cultures were allowed to settle for 7 days before being used for experimentation. Following electrophysiological recordings some of the organotypic slice cultures were fixed in 5% formalin for 20 minutes and then washed (x3) in 0.1M phosphate buffered 0.9% NaCl (pH 7.4, PBS). The tissue sections were teased from the supporting membrane, mounted on slides and processed for immunohistochemistry (IHC; see below).
Electrophysiology
After transduction, coverslips with PC12 cells or membranes with pontine slice cultures were transferred into the recording chamber of an upright fluorescent microscope (DMLFSA, Leica Microsystems, Heidelburg, Germany), superfused with artificial cerebrospinal fluid (in mM: NaCl (125), KCl (3), NaHCO3 (24), KH2PO4 (1.25), MgSO4 (1.25), CaCl2 (2.5) and D-glucose (10) saturated with 95% O2 / 5% CO2, pH 7.3, osmolality 290 mOsm/L, 20°C) at a rate of 1 ml.min−1. Patch pipettes (resistances of 4–7 MΩ) were filled with internal solution (in mM: K Gluconate (130), KCl (10), NaCl (10), MgCl2 (2), HEPES (10), Na2ATP (2) and Na2GTP (0.2)). All membrane potentials were corrected for a junction potential of 13 mV. EGFP expressing cells were identified using epifluorescence microscopy and then whole cell recordings were obtained under gradient contrast illumination (Dodt and Zieglgansberger, 1990). Recordings were made in current clamp mode and current pulses were injected to examine the current-voltage and current-spike frequency relationships (Axopatch 1D amplifier, Axon Instruments, USA). Data were acquired and stored using Spike 2 software (CED, Cambridge Electronic Design, UK). The threshold for spike firing was determined as the point at which the rate of change of membrane potential exceeded 7.5 V/s and all spike parameters were measured with reference to this point (using a custom written Spike2 script).
Dorsal horn adenoviral vector injection
The procedures for lumbar spinal injection of AVV have been described elsewhere (Howorth et al., 2009). In brief, rats (130–200g) were anesthetized (i.m. or i.p.) with ketamine (5 mg/100g, Vetalar, Pharmacia, UK) and medetomidine (30 μg/100g, Domitor, Pfizer, UK) until loss of paw withdrawal reflex. The animal was placed in a stereotaxic frame and core temperature was maintained at 37°C using a homeothermic blanket (Harvard Apparatus, UK). Aseptic surgical techniques were employed throughout.
The spinous processes of T13 and L1 were identified and a laminectomy performed to allow access to the spinal segments L4–5. Injections of AVV were made in the dorsal horn 400 μm lateral to the midline and 500 μm deep to the dorsal surface using a micro-capillary pipette (calibrated in 1 μL intervals; Sigma), with a tip diameter of 20 μm. Two pairs of bilateral injections (each 500 μm apart in the rostrocaudal axis) of AVV (500nl/per injection of 3×1010 TU/ml AVV over 2 minutes) were made into the L4–5 spinal segments. The dose of AVV administered was chosen to achieve maximal transduction of the NA neurons, based on our previous titration experiments balancing the efficacy of retrograde expression against the inflammatory response at the injection site triggered by higher viral titres (Howorth et al., 2009).
Following injections anesthesia was reversed with atipamezole (0.1 mg/100g i.p., Antisedan, Pfizer, UK) and buprenorphine was given for pain relief (2 μg/100g s.c., Temgesic, Schering-Plough, Hertfordshire, UK). Animals showed a rapid functional recovery from anesthesia and surgery and their movement appeared normal the following day. Rotarod testing before and after spinal AVV injection showed no significant difference in motor function in either the AVV-PRS-EGFP group (210±21 s vs. 191±32 s after injection, (n=7)) or the AVV-PRS-hKir2.1 group (201±18 s vs. 178±22 s after injection, (n=6), paired t-test, not significant)
Pain assays
Animals were handled daily and habituated to the testing room, equipment and observer. All experiments were done under observer-blinded conditions with concealment of the AVV identity.
Hargreaves’ and von Frey testing
Animals had tests of both thermal and mechanical nociception before and after AVV administration and following induction of sensitization. The heat pain withdrawal latencies were measured for the hindpaw using the method described by Hargreaves et al. (1988). The infrared source was directed onto the plantar surface of the paw and the time to withdrawal recorded (Ugo Basile Plantar test, Italy). Each withdrawal value was the mean of 3 tests (5 mins between tests). A 30 s cut off value was used to terminate the test and avoid tissue damage. The punctate pressure withdrawal threshold was assessed using von Frey hairs (TouchTest, Linton Instruments, UK) applied to the lateral edge of the plantar surface of the paw for 3 seconds or until paw withdrawal. Filaments were applied sequentially according to the up-down method (Dixon, 1980) to obtain a threshold value (after (Chaplan et al., 1994)) starting with the 6g filament and with an upper cut off of 26g (~10% of rat body weight, stiffer hairs simply lifted the hindpaw).
Hindpaw inflammation
Rats received bilateral lumbar spinal injections of AVV-PRS-hKir2.1 (n=7) or AVV-PRS-EGFP (n=7). Ten days later they had complete Freund’s adjuvant (CFA) injected subcutaneously to the plantar surface of the hindpaw (50 μl, 50 μg, Calbiochem, California, USA) under brief Halothane anesthesia. This produced localized inflammation, edema and sensitization of the hindpaw (as previously described (Iadarola et al., 1988)) and resulted in an increase in paw thickness (4.0±0.1 to 5.1±0.2 mm (n=14), with no significant differences between AVV groups). The dose of CFA was chosen (based on previous experience, personal communication from Lucy Donaldson) to produce a moderate degree of sensitization to facilitate the detection of any hyperalgesic effects of AVV administration (after (Wei et al., 1999)). Sensory testing (Hargreaves’ and von Frey, as above) was performed before AVV injection, immediately before CFA injection and again 2 hours following injection. Subsequently the animals were sacrificed for histology at 3 hours post CFA injection.
Formalin testing
The nociceptive behavioral response to subcutaneous formalin was assessed (Dubuisson and Dennis, 1977) following bilateral lumbar spinal injections of AVV-PRS-hKir2.1 (n=15 rats) or AVV-PRS-EGFP (n=16). Two weeks later, the animals underwent nociceptive testing and had either formalin (5% neutral buffered, n=22) or 0.9% saline (n=9) injected subcutaneously (50 μl, 30G needle) on the dorsal surface of the right hind paw. Rats were replaced in the testing chamber and the numbers of flinches and foot lifts were tallied over 1 minute periods, initially every 2 mins for the first 10 mins and then every 5 mins for the remainder of the 60 mins.
Animals were culled 2 hours after the end of the observation period to allow optimal c-fos expression and perfused with fixative (n=6 for control and n=16 for formalin test rats, protocol below). The lumbar spinal cord was removed with intact dorsal roots and ganglia to allow segmental identification. Spinal tissue was sectioned transversely on a freezing microtome (40 μm sections) and 1 section in 4 was processed for c-fos IHC (see below). The spinal c-fos expression was quantified for each animal by tallying the positive neurons from ten non-sequential, transverse spinal cord sections (40 μm) from L3–5 with the greatest numbers of c-fos positive nuclei. Counts were sub-divided into three regions corresponding to the superficial (SDH, laminas I-II) and deep dorsal horn (DDH, laminas III-VI) and the ventral horn (VH, laminas VII-IX, excluding area X) with reference to Paxinos and Watson (2005). The rostrocaudal distribution of the c-fos expression was quantitated by averaging the number of c-fos positive cells per section in dorsal and ventral horns from each spinal cord segment from L2-L6.
Chronic neuropathic pain model
Spared nerve injury
To produce a model of neuropathic hind limb pain the spared nerve injury (SNI) method was employed (Decosterd and Woolf, 2000). Rats (n=13) were anaesthetized with ketamine and medetomidine until loss of paw withdrawal. The sciatic nerve was exposed at the mid-thigh level and its branches the tibial, common peroneal, and sural nerves were identified. The tibial and peroneal nerves were ligated with 5–0 silk and cut, paying particular attention to avoid damage to the sural nerve.
Animals had sensory testing (schedule in Fig. 5) on consecutive days prior to AVV injection, one day before SNI and then on days 4, 7, 10 and 14 following SNI. Mechanical allodynia was assessed using von Frey hairs (as described previously) to obtain a threshold value. Cold allodynia was tested by the application of an acetone drop to the lateral aspect of the hindpaw. A withdrawal / flinch was scored as a positive response and the duration of paw lift was measured for each response (with an upper cut off of 1 minute). Animals were culled on day 15 (post SNI) and brain tissue was processed (see below) to confirm successful AVV transduction of the pontospinal NAergic neurons (by detection of EGFP).
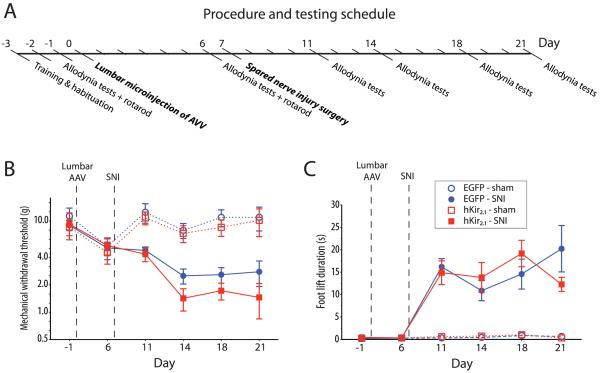
Figure 5. AVV-PRS-hKir2.1 does not increase allodynia in spared nerve injury model.
A) To assess the effect of transduction with AVV-PRS-hKir2.1 on the development of the signs of neuropathic pain, animals received lumbo-spinal injection of either AVV-PRS-hKir2.1 or AVV-PRS-EGFP one week prior to SNI. Each group was then followed for two weeks and assessed for the development of mechanical and cold allodynia. Following spinal AVV administration both groups of SNI animals developed significant (B) mechanical allodynia (von Frey hair threshold) and (C) cold allodynia (acetone drop induced paw withdrawal). Transduction of the pontospinal NAergic neurons with AVV-PRS-hKir2.1 did not significantly alter the expression of mechanical or cold allodynia compared to AVV-PRS-EGFP (two-way rmANOVA).
Rotarod testing
To assess motor function after spinal injection of AVV animals had rotarod testing (n=13, from SNI group). After training and habituation to the rotarod (2 sessions, animals were trained to remain on the rotarod for 40 seconds at 16 rpm, 5 minutes between each session) animals were tested (maximum duration 5 mins, speed increasing from 4–40 r.p.m. over 5 mins) both before and 6 days after AVV injection. The length of time the animals remained on the rotarod was recorded.
Tissue fixation
Rats were sacrificed (at 14 days, unless otherwise stated) with an overdose of pentobarbital (20 mg/100 g i.p. Euthatal, Merial Animal Health, UK) and perfused transcardially with 0.9% NaCl (1 ml/g) followed by 4% formaldehyde (Sigma) in 0.1M phosphate buffer (pH 7.4, 1 ml/g). The brain and spinal cord (including dorsal root ganglion and spinal roots) were removed and post fixed for 2 hours before overnight cryoprotection in 30% sucrose. The attached dorsal roots and ganglia enabled identification of lumbar regions (L2–6) and confirmation of correct injection targeting to L4–5 segments (failures were excluded from analysis, see supplemental Fig. 1 available at www.jneurosci.org as supplemental material). Coronal tissue sections were cut at 40 μm intervals using a freezing microtome and either serially mounted or left free floating for fluorescence IHC.
Immunohistochemistry
Tissue sections were washed (x3) in PBS, permeabilized in 50% ethanol for 30 minutes before further washing. The tissue was incubated with primary antibodies against Dopamine beta-hydroxylase (DBH), hKir2.1, c-fos or EGFP (see table 1 and (Howorth et al., 2009) for details) in PBS with 5% horse serum (HS), 0.3% Triton X-100 and 0.01% sodium azide for 24–72 hours at 5°C. Mounted sections were kept in a humidified chamber (RA Lamb, Eastbourne, UK) and free-floating sections were continuously agitated. After further washing, sections were incubated overnight with either conjugated or biotinylated secondary antibodies (all Jackson ImmunoResearch, West Grove, PA, see table 1) and diluted 1:500–1,000 with 2% HS and 0.3% Triton. Sections were washed, incubated with Streptavidin Cy3 (1:1,000 in PBS, Sigma) for 4 hours before a final wash. Negative controls were routinely run, by omitting primary antibodies. The specificity of labeling was verified by pre-incubation with either control antigen epitope peptides (hKir2.1 - intracellular, C-terminal amino acids 392–410, 1:200, Alomone Labs APC-026; c-fos N-terminus peptide amino acids 3–16, 1:10,000, sc-52P, Santa Cruz) or with slices of adrenal medulla (DBH, after (Howorth et al., 2009)). The distribution of hKir2.1 immunoreactivity in rat forebrain (in particular the piriform cortex and hippocampus) was similar to that previously reported (Karschin et al., 1996; Howe et al., 2008).
Table 1. Antibodies.
| Primaries | ||||
|---|---|---|---|---|
| Epitope | Dilution | Species | Source | Code / Batch |
| DBH | 1:5,000 | Mouse | Chemicon, Temecula, CA. | MAB308 / 24120144 |
| c-fos | 1:2,000 | Rabbit | Santa Cruz Biotechnology, CA. | sc-52 / H0105 |
| GFP | 1:4,000 | Rabbit | Invitrogen, Paisley, UK | A11122 / 56884A |
| hKir2.1 | 1:200 | Rabbit | Alomone Labs, Jerusalem, Israel | APC-026 / AN 02 |
| Secondaries (raised in donkey) | ||||
| Anti- | Tag | Dilution | Source | Code |
| Mouse | AMCA | 1:100 | Jackson | 715-156-150 |
| Rabbit | Biotinylated | 1:500-1,000 | Jackson | 711-066-152 |
Cell counts
Following in vivo spinal transduction with AVVs, successful retrograde expression was confirmed by post hoc examination of pontine tissue for EGFP positive neurons in the NAergic cell groups (A5-A7). Cell counts were determined for representative groups of AVV-PRS-hKir2.1 and AVV-PRS-EGFP animals (n=4/group) to compare the efficacy of transduction and the distribution of labeled pontospinal NAergic neurons. Neuronal somatawere counted as being present in the section only if their nucleus was visible and all counts were Abercrombie corrected, as previously described (Howorth et al., 2009).
Photomicrography
Representative images were taken on either a Zeiss Axioskop 2 with Axiocam HRC (Carl Zeiss, Hertfordshire, UK) using appropriate excitation-emission filter sets (EGFP - #10, Cy3 - #15) or on a Leica DMRBE and TCSNT confocal microscope (Leica, Wetzlar, Germany). Confocal image stacks were obtained at 0.5 – 4 μm steps for Cy3 (excitation 543 nm, emission 607 nm) and EGFP (excitation 488 nM, emission 500 – 530 nM), and each frame was averaged 4 times, to reduce background noise. Final images are maximum intensity projections, unless otherwise stated. Images were initially processed using respective company software, and prepared for presentation using Adobe Photoshop CS3 with optimisation of contrast / brightness as required and addition of annotation.
Analysis
All data are presented as mean ± standard error of mean (SEM) or median [interquartile range] as appropriate. The normality of data were assessed using the D’Agostino-Pearson test. Subsequent statistical testing was undertaken using paired and unpaired t-tests, one and two way ANOVA (with Bonferroni’s post tests) and Mann Whitney tests as appropriate. Data were analyzed using Prism (Graphpad Prism 5, San Diego, CA, USA) and differences were considered significant at P<0.05.
Results
Functional expression of hKir2.1 in a catecholaminergic cell line
Whole-cell current clamp recordings were made from EGFP-positive PC12 cells transfected with either PRS-hKir2.1 or PRS-EGFP (control) plasmid DNA. Cells expressing hKir2.1 had a hyperpolarized resting membrane potential as compared to control cells (−68±2 mV [n=16] vs. −56±2 mV [n=21], P<0.01, one way ANOVA) which was returned to the control level by the addition of barium to the perfusate (100 μM BaCl2). The injection of hyperpolarizing current pulses showed the expression of hKir2.1 was associated with a marked inward rectification that was also blocked by barium (100 μM, Supplemental Fig. 2 available at www.jneurosci.org as supplemental material) as would be anticipated if it were a consequence of functional hKir2.1 expression (Kubo et al., 1993; Johns et al., 1999).
Expression of hKir2.1 inhibits locus coeruleus neurons in vitro
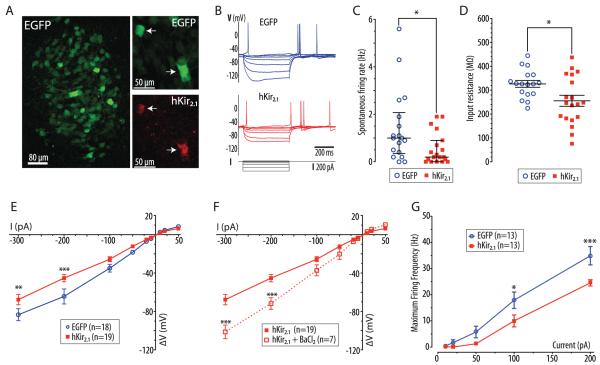
Transduction of pontine slice cultures with AVV-PRS-EGFP alone or with AVV-PRS-hKir2.1 allowed visualization of EGFP positive neurons in the LC (clearly identifiable by its dorsal position and dense packing of EGFP positive NAergic neurons, Fig. 1A). After transduction with AVV-PRS-hKir2.1, IHC showed expression of hKir2.1 in EGFP-positive neurons within the LC (Fig. 1A); notably, hKir2.1 was not detectable in LC neurons that were EGFP negative (cultures from 3 rats) or in cultures transduced with AVV-PRS-EGFP.
Figure 1. Expression of hKir2.1 inhibits locus coeruleus neurons in vitro.
A) Pontine slice culture (from p7 rat) after transduction with AVV-PRS-EGFP, showing EGFP expression in neurons of the LC. Right panels show LC neurons from a pontine slice culture transduced with AVV-PRS-EGFP and AVV-PRS-hKir2.1 (ratio 2:1) showing co-localization of EGFP and hKir2.1-immunoreactivity, (white arrows indicate hKir2.1 positive neurons).
B) Current clamp recordings from representative transduced LC neurons (control or hKir2.1-expressing) showing overlaid membrane potential responses to injected current pulses (+10 to −300 pA).
C) LC neurons transduced with AVV-PRS-hKir2.1 had a reduced frequency of spontaneous action potential discharge (median 0.2 vs. 1.0 Hz, interquartile range marked, Mann-Whitney test, * - P<0.05)
D) Expression of hKir2.1 in LC neurons lowered their input resistances (256±23 vs. 327±14 MΩ, unpaired t-test, * - P<0.05)
E) Examination of the current-voltage relationship showed that LC neurons expressing hKir2.1 had marked inward rectification (two-way ANOVA, ** - P<0.01 and *** - P<0.001).
F) This inward rectification was blocked by superfusion of barium (100 μM, two-way ANOVA, ** - P<0.01 and *** - P<0.001).
G) Neurons were hyperpolarized to −75 mV (by DC current injection) to stop spontaneous action potential discharge. The maximum spike frequency response to injected current pulses (10–200 pA, 500 ms) was plotted showing that the LC neurons expressing hKir2.1 were less excitable (* - P<0.05 and *** - P<0.001, repeated measures ANOVA).
Whole-cell current clamp recordings of transduced LC neurons in slice cultures showed characteristic patterns of activity with spontaneous firing of action potentials (with long duration, a prominent inflexion on repolarization and a large afterhyperpolarisation, Fig. 1B) often driven by underlying oscillations in membrane potential (Williams et al., 1984; Williams and Marshall, 1987). Neurons transduced with AVV-PRS-hKir2.1 showed a lower frequency of spontaneous spike firing (median 0.2 [0–0.9], n=19 vs. 1.0 [0.35–2.1] Hz, n=18, P<0.05, Mann-Whitney test, Fig. 1C) and reduced input resistance (256±23 [n=19] vs. control 327±14 MΩ [n=17], unpaired t-test, P<0.05, Fig. 1D, table 2). Expression of hKir2.1 increased the inward rectification seen on hyperpolarizing current injection (Fig. 1E), which was blocked by the addition of barium to the perfusate (BaCl2, 100 μM, P<0.01, two way ANOVA, n=7, Fig. 1F). Barium superfusion increased the input resistance of LC neurons transduced with AVV-PRS-hKir2.1 (243±28 to 376±68 MΩ, n=7, P<0.05, paired t-test) but not those transduced with AVV-PRS-EGFP (366±28 to 392±118 MΩ, n=4, NS, paired t-test). No difference was seen in membrane potential or action potential parameters in neurons transduced with AVV-PRS-hKir2.1 compared to AVV-PRS-EGFP (table 2). To test for changes in excitability neurons were hyperpolarized with DC current injection to −75 mV (to stop spontaneous spiking) and then depolarizing current pulses were injected (10–200 pA, 500 ms). LC neurons transduced with AVV-PRS-hKir2.1 were significantly less excitable with a lower maximum firing frequency in response to a given current pulse (Fig. 1G).
Table 2.
| Parameter | AVV-PRS-EGFP (n=18) |
AVV-PRS-hKir2.1 (n=19) |
|---|---|---|
| Membrane potential (mV) | −58.5 [−57.2 to −64.0] |
−56.6 [−46.5 to −58.6] |
| Input resistance (MΩ) | 327±14 | 256±23 * |
| Spontaneous firing rate ( Hz) | 1.0 [0.35 to 2.1] |
0.2 * [0.0 to 0.9] |
| Spike threshold (mV) | −37±1.4 | −35±2.4 |
| Spike amplitude (mV) | 60±3 | 56±4 |
| Spike duration (⅓ max, ms) | 5.3 [3.5 to 7.2] |
5.2 [3.7 to 7.6] |
| AHP amplitude (mV) | 23 [18 to 24] |
23 [21 to 26] |
| AHP duration (ms) | 124 [81 to 181] |
182 [98 to 250] |
Comparison of membrane properties and action potential parameters of LC neurons in slice culture transduced with AVV-PRS-EGFP or AVV-PRS-hKir2.1. Data quoted as mean±SEM or median [interquartile range] and significance assessed using unpaired t-test or Mann Whitney test as appropriate.
P<0.05.
Transduction of pontospinal NAergic neurons in vivo with AVV-PRS-hKir2.1
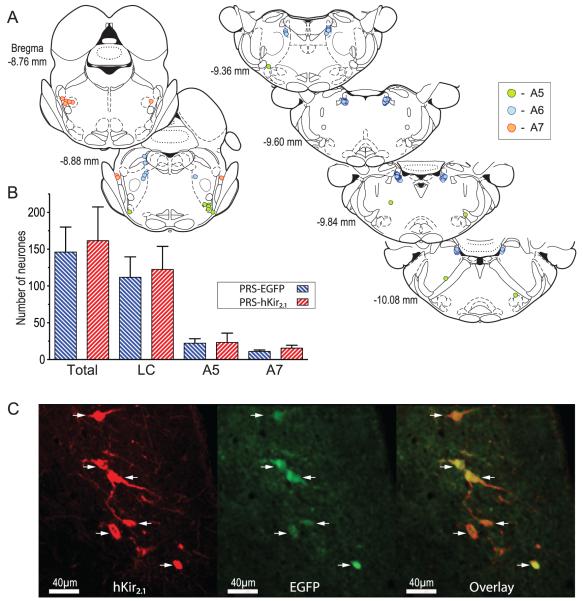
Intraparenchymal injection of either AVV-PRS-EGFP or AVV-PRS-hKir2.1 to the lumbar dorsal horn (L4–5) retrogradely transduced pontine NAergic neurons as indicated by the presence of EGFP fluorescence (Fig. 2). The numbers of transduced pontine neurons and pattern of retrograde labeling was similar to that which we previously reported (Howorth et al., 2009) and depended on the intraparenchymal administration of AVV as intentional control intrathecal injection of AVV did not transduce any neurons in the pons (nor spinal cord, n=2). There was no difference in the total number of neurons transduced with AVV-PRS-hKir2.1 compared to AVV-PRS-EGFP (162±46 vs. control 146±34, n=4 rats/group, one way ANOVA, NS) with a comparable distribution across the LC (123±31 vs. control 112±28), A5 (23±13 vs. control 22±6) and A7 (16±4 vs. control 11±2) pontine cell groups (Fig. 2B). Following transduction with AVV-PRS-hKir2.1, only pontine NAergic neurons expressing EGFP were immuno-positive for hKir2.1 (Fig. 2C). In contrast, no NAergic neurons showed hKir2.1-immunoreactivity in animals transduced with AVV-PRS-EGFP (nor in naïve animals). Expression of EGFP and hKir2.1 was detectable four weeks after transduction (the longest time point examined) indicating that AVV gene expression persisted at least for the duration of the experiments reported below.
Figure 2. Retrograde transduction of pontospinal NAergic neurons by adenoviral vectors.
A) Schematic showing the typical pattern of retrograde labeling of NAergic somata in the pons following bilateral AVV injection in the lumbar dorsal horn (taken from a single representative animal). The majority of neurons were located in the ventral LC (blue symbols) with a cluster in A7 (red) and a smattering of neurons in the A5 region (green). (Neurons plotted on sections from Paxinos & Watson 2005)
B) AVV-PRS-hKir2.1 transduced similar numbers of neurons as AVV-PRS-EGFP (n=4 rats/group), with a majority found in the LC (76%), and the remainder found in A5 (14%) or A7 (10%).
C) Following dorsal horn injection of AVV-PRS-hKir2.1 in vivo, IHC for hKir2.1 showed labeling in a subset of NAergic neurons (shown here in ventral LC). The same subset of NA neurons also showed EGFP fluorescence confirming that they had been retrogradely transduced by the AVV. The overlaid EGFP and hKir2.1 images illustrates that hKir2.1 expression was restricted to EGFP-positive neurons (arrows) and there was no evidence of hKir2.1 expression in the surrounding LC neurons.
hKir2.1 expression in pontospinal NAergic neurons causes hyperalgesia
An initial assessment of the effect of AVV transduction on baseline measures of thermal and mechanical nociception showed evidence of thermal hyperalgesia in the AVV-PRS-hKir2.1 group. Hargreaves’ hindpaw withdrawal latency decreased significantly following transduction of NA neurons with hKir2.1 (16±1 vs 13±1 s after AVV (10 days) respectively, n=11, p=0.02) but not after transduction with EGFP ((14±1 vs. 12±1 s, n = 8, NS). In contrast there was no evidence of any accompanying mechanical sensitization (assessed with von Frey hairs). To explore this further we employed the CFA inflammatory pain model and repeated the tests of mechanical and thermal nociception in two groups of AVV transfected animals (n=7/group).
As before the AVV-PRS-hKir2.1 group showed thermal hyperalgesia after vector administration (hKir2.1 17±2 vs. 14±1 s (p<0.05) vs. EGFP 16±1 vs. 16±1 s (NS), Fig. 3A) without any change in mechanical thresholds (hKir2.1 26±0 g vs. 26±1 g (NS) and EGFP 23±2 g vs. 21±3 g (NS), Fig. 3C). To take account of the altered thermal nociception after AVV administration in the AVV-PRS-hKir2.1 group, the change in hindpaw thermal withdrawal after CFA was compared against the contralateral hindpaw. Injection of CFA produced thermal sensitization in both groups of animals (difference after CFA: hKir2.1 −10±1 s, p<0.001 and EGFP −6±2 s, p<0.01, two-way rmANOVA with post hoc Bonferroni tests, Fig. 3B). Interestingly, the AVV-PRS-hKir2.1 group, which started from an already sensitized state, showed almost a 2-fold greater decrease in thermal withdrawal latency after CFA (−8.0 vs. −4.7 s, respectively). In contrast, although the mechanical withdrawal threshold was also significantly lowered after CFA (hKir2.1 − 26±1 to 21±2 g, p<0.05 vs. EGFP 21±3 to 14±3 g, p<0.01, Fig. 3C) the magnitude of this change was similar in both groups.
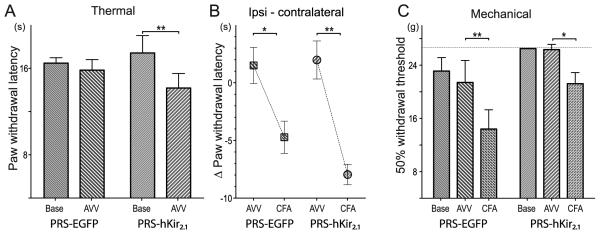
Figure 3. Inhibition of pontospinal NAergic neurons causes thermal but not mechanical hyperalgesia.
To examine the effect of inhibition of pontospinal NA neurons in a sensitized state we used the CFA inflammatory pain model in two groups of animals that had been transfected with AVV ten days earlier (n=7/group).
A) Animals transduced with AVV-PRS-hKir2.1 showed thermal hyperalgesia on Hargreaves’ testing (hKir2.1 17±2 vs. 14±1 s (P<0.05)) prior to CFA injection, this was not seen in the control animals (EGFP 16±1 vs. 16±1 s (NS)) (two-way rmANOVA with Bonferroni post tests)
B) To account for the change in baseline thermal nociception in the AVV-PRS-hKir2.1 group, the changes in ipsilateral hindpaw withdrawal latency after CFA was compared relative to the contralateral hindpaw. Injection of CFA produced thermal sensitization in both groups of animals (difference after CFA: EGFP −6±2 s, P<0.01 vs. hKir2.1 −10±1 s, P<0.001 (two-way rmANOVA with post hoc Bonferroni tests). Strikingly, the AVV-PRS-hKir2.1 group, which started from an already sensitized state, showed almost a 2-fold greater decrease in thermal withdrawal latency after CFA (hKir2.1 −8.0 s vs. EGFP −4.7 s).
C) Transduction with AVV had no significant effect on the mechanical withdrawal thresholds in either group. After CFA injection both groups of animals showed significant sensitization (EGFP P<0.01, hKir2.1 P<0.05) although the magnitude of this change was similar in both groups (EGFP −7.0 g vs. hKir2.1 −5.1 g).
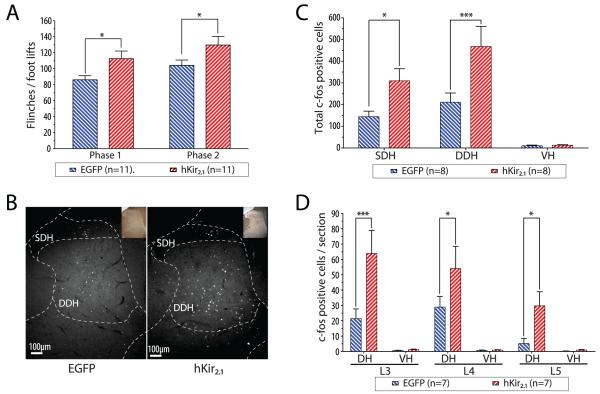
To further characterize this hyperalgesic effect we studied the effect of inhibition of pontospinal NA neurons in the formalin test (Dubuisson and Dennis, 1977) to see whether there was any alteration in nociceptive behavioural responses and spinal c-fos expression. Both groups of animals (hKir2.1 and EGFP) showed characteristic biphasic nociceptive behavioral responses to hindpaw formalin testing. Animals transduced with AVV-PRS-hKir2.1 showed significantly more flinches/paw lifts than the AVV-PRS-EGFP group in response to formalin (Fig. 4A; Phase I - 113±10 vs. control 86±5, P<0.05 and Phase II - 130±11 vs. control 104±7, P<0.05 n=11/group). This was mirrored by an increased intensity of c-fos expression in the lumbar spinal cord after formalin testing in the AVV-PRS-hKir2.1 group (Fig. 4B, 4C; superficial dorsal horn 310±56 vs. EGFP 144±26, P<0.05 and deep dorsal horn 468±93 vs. EGFP 211±42, P<0.01, c-fos positive profiles/10 sections, n=8/group). This increased c-fos expression in the AVV-PRS-hKir2.1 group was seen across lumbar segments L3-L5 (Fig. 4D), which receive sensory inputs from the hind paw, but not in segments L2 or L6 that showed negligible induction of c-fos in either group of animals (n=7/group).
Figure 4. Inhibition of pontospinal NAergic neurons causes hyperalgesia and increased spinal c-fos in the formalin test.
A) Rats transduced with either AVV showed typical biphasic nocifensive behavioral responses to the injection of formalin. Transduction with AVV-PRS-hKir2.1 produced hyperalgesia as indicated by an increase in the numbers of flinches/foot lifts in both phase I and II of the formalin test (two way ANOVA, *-P<0.05, n=11/group).
B) Formalin test-evoked expression of c-fos in the superficial and deep laminae of the lumbar spinal cord (shown here at L4). There was a marked increase in the numbers of c-fos positive cells in animals transduced with AVV-PRS-Kir2.1.
C) Transduction with AVV-PRS-hKir2.1 increased the numbers of formalin test-evoked c-fos positive cells in both the superficial and deep lumbar dorsal horn (compared to animals transduced with AVV-PRS-EGFP (n=8/group), two way ANOVA, *-P<0.05, ***-P<0.001)
D) The AVV-PRS-hKir2.1 group also showed a significant increase in the numbers of c-fos positive cells in the dorsal horn across each of the spinal segments L3-L5 (two way ANOVA, *-P<0.05 and ***-P<0.001, n=7/group).
hKir2.1 expression in pontospinal NAergic neurons does not alter neuropathic allodynia
Spared nerve injury (SNI, (Decosterd and Woolf, 2000)) produced behavioral signs of hindlimb sensitization (mechanical and cold allodynia, Fig. 5) in rats that had received prior spinal cord injections of either AVV-PRS-EGFP or AVV-PRS-hKir2.1. There was no significant difference between the vector treated groups (Fig. 5) in either mechanical allodynia (von-Frey threshold 1.4±0.4 g, n=6 vs. control 2.5±0.5 g, n=7, day 7 post SNI) or in cold allodynia (duration of paw lift 14±3 s, n=6 vs. control 11±2 s, n=7, day 7 post SNI) or at any other time point examined.
Discussion
We have developed a genetic strategy to alter the excitability of NAergic neurons projecting to the lumbar spinal cord. This has been achieved using an AVV to express a potassium channel (hKir2.1) selectively in pontospinal NAergic neurons. Expression of hKir2.1 decreased the excitability of NAergic neurons in vitro and reduced their spontaneous firing frequency. In vivo expression of hKir2.1 in around 150 pontospinal NAergic neurons produced selective thermal hyperalgesia in tests of evoked nociception (in naive and CFA inflamed hindpaws) and increased nocifensive behaviors in the formalin test accompanied by increased c-fos expression in the dorsal horn of the spinal cord. These data provide functional evidence for the tonic involvement of pontospinal NAergic neurons in the regulation of specific nociceptive behaviors and identify the retrograde transduction approach using viral vectors as a viable strategy to alter their excitability and affect nociceptive processing in vivo.
AVV can retrogradely transduce CNS cells (Akli et al., 1993; Ridoux et al., 1994) including pontospinal neurons (Liu et al., 1997b). We have refined this technique to target pontospinal catecholaminergic neurons using the cell-specific PRSx8 promoter developed by Hwang et al (2001). This approach has good efficiency for retrograde transduction of NAergic neurons (Lonergan et al., 2005; Howorth et al., 2009) and injection of this AVV to the lumbar dorsal horn identifies ~150 neurons (mostly in LC with the remainder in A7 and A5).
The LC projects to most of the neuroaxis and there has been debate over which of the pontine NAergic cell groups provides the predominant innervation to the dorsal horn (Guyenet, 1980; Westlund et al., 1983; Loughlin et al., 1986; Fritschy and Grzanna, 1990; Burnett and Gebhart, 1991; Clark and Proudfit, 1991a, b; West et al., 1993) with evidence of differences between rat strains and stock (Fritschy and Grzanna, 1990; Clark and Proudfit, 1991a; Sluka and Westlund, 1992). Therefore, to circumvent these issues, we retrogradely targeted just the NAergic neurons with terminals in the lumbar dorsal horn with the objective of modulating the activity of the neurons regulating spinal sensory transmission (minimizing interference with the other roles of the NAergic system).
Since the original description of the use of hKir2.1 to inhibit neurons in vitro (Johns et al., 1999) several studies have used hKir2.1 to suppress neuronal activity in vivo (Yu et al., 2004; Duale et al., 2007; Mizuno et al., 2007). We chose hKir2.1 to manipulate excitability because it is not normally present in pontine NAergic neurons (Karschin et al., 1996) and as they exhibit tonic firing (Guyenet, 1980) we expected to be able to demonstrate a functional phenotype. The expression of hKir2.1 in NAergic neurons in vitro produced the anticipated barium-sensitive inward rectification (Kubo et al., 1993) and reduced the spontaneous discharge rate by ~80%, comparable to that reported to produce changes in axonal mapping (Yu et al., 2004; Mizuno et al., 2007). We have previously shown that hKir2.1-mediated inhibition of NAergic A2 neurons in vivo produces functional alterations in blood pressure and fluid balance regulation (Duale et al., 2007).
Inhibition of pontospinal NAergic neurons produced a modality-specific hyperalgesia supporting the hypothesis that these neurons are a component of the endogenous analgesic system (Jones, 1991; Millan, 1997; Millan, 2002; Pertovaara, 2006). Acute stimulation of the LC or A7 produces potent analgesic effects (Jones and Gebhart, 1986a; Miller and Proudfit, 1990; Yeomans et al., 1992; West et al., 1993; Holden et al., 1999) that are reversed by intrathecal administration of α2-adrenergic antagonists. This is mediated by both a direct hyperpolarizing action and a pre-synaptic inhibition of afferent input (North and Yoshimura, 1984; Sonohata et al., 2004). Thus, there is a well-defined neural circuit for descending NAergic control of spinal nociceptive transmission.
Several subtractive approaches have been used to examine the functional role of the NAergic neurons in regulating nociception in vivo including intrathecal α2-antagonists (Sagen and Proudfit, 1984; Sugimoto et al., 1986; Liu et al., 1997a; Omote et al., 1998; Wei and Pertovaara, 2006), electrical ablation of pontine NAergic nuclei (Tsuruoka and Willis, 1996), targeted chemical neuro-ablation (Fasmer et al., 1986; Martin et al., 1999; Jasmin et al., 2003) or genetic knock-out studies of the α2-adrenoceptor (Stone et al., 1997) or DBH (Jasmin et al., 2002). We have built on this foundation using a viral vector to produce a neuroanatomically-focused genetic manipulation to alter the excitability of the pontospinal NAergic neurons projecting to the lumbar dorsal horn.
We show that inhibition of pontospinal NAergic neurons produces thermal hyperalgesia, in agreement with previous studies using chemical ablation (Fasmer et al., 1986; Martin et al., 1999), intrathecal administration of α2-adrenoceptor antagonists (Sagen and Proudfit, 1984) and gene knock-out to up- (Bohn et al., 2000) or down-regulate (Jasmin et al., 2002) NAergic function. The NAergic-regulation of nociceptive thresholds exhibits sensory modality specificity, as we found no alteration in mechanical nociception (like Jasmin et al. (2002)). This change in thermal nociception indicates a tonic influence of the NAergic system on nociceptive processing.
Inhibition of pontospinal NAergic neurons exaggerated the nocifensive pain behaviors displayed during the formalin test. This was associated with increased c-fos expression in the superficial and deep dorsal horn, indicating a heightened spinal responsiveness to noxious inputs. The formalin test has been shown to activate pontospinal NAergic neurons (Howorth et al., 2009) and to increase the level of spinal NE (Omote et al., 1998). Intrathecal administration of α2-antagonists augments the formalin response (Omote et al., 1998), increases the excitation of dorsal horn neurons (Green et al., 1998) and interruption of the descending monoaminergic pathways increases formalin-evoked spinal c-fos expression (Liu et al., 1997a). These findings indicate that the NAergic endogenous analgesic system is recruited during the formalin test, acting at a spinal level to suppress the nociceptive signal (also noted in inflammatory pain models (Tsuruoka and Willis, 1996; Mansikka et al., 2004)). It is therefore surprising that NAergic ablation studies have reported contrary effects on formalin-induced behaviors with hyperalgesia, hypoalgesia and no change (Fasmer et al., 1986; Martin et al., 1999; Jasmin et al., 2003). This may be because of different degrees of compensation for the ablation or because of the variable loss of NAergic neurons and projections to CNS sites after rostral toxin spread in the CSF (documented by Jasmin et al. (2003)). This illustrates a potential advantage inherent in our selective vector approach that restricts expression of hKir2.1 to only those NAergic neurons with terminals in the lumbar dorsal horn.
Expression of hKir2.1 in the pontospinal NAergic neurons prior to SNI did not have a demonstrable hyperalgesic effect. A similar lack of efficacy in neuropathic pain models has been reported by studies using ablation of NA neurons (Li et al., 2002) or intrathecal α2-antagonists (Xu et al., 1999). However, a longer-term ablation study showed a transitory increase in mechanical sensitization following nerve ligation that resolved after 120 days (Jasmin et al., 2003)). These authors suggested that the discrepancy between their findings and those of previous studies may have been because of a “floor” effect making it difficult to detect further sensitization in allodynic hindpaws (their animals had relatively moderate sensitization). Along the same lines, in nerve-injured animals that did not exhibit a neuropathic phenotype, blockade of spinal α2-receptors unmasked allodynia (Xu et al., 1999) an effect not seen in animals with a clear neuropathic phenotype. There is also evidence of a deficit in the activity of the descending NAergic system in neuropathic pain models (Viisanen and Pertovaara, 2007; Rahman et al., 2008). These results suggest that the level of ongoing tonic activity in the NAergic system may be insufficient to suppress established neuropathic allodynia but may be able to play a role in ameliorating the expression of less severe neuropathic pain phenotypes. Such a functional deficit could explain the efficacy of NAergic re-uptake inhibition in the treatment of neuropathic pain and could explain the lack of effect seen using our AVV-mediated inhibitory strategy.
Viral vectors are being developed for the treatment of neurological conditions including chronic pain (Glorioso and Fink, 2004). A variety of approaches have been explored for pain treatment including vectors targeted at primary afferent (Wilson et al., 1999; Xu et al., 2003) or spinal dorsal horn (Eaton et al., 2002) neurons, leading to the approval of phase I studies of Herpes simplex-based vectors for cancer pain (Mata et al., 2008). Thus far, no pain studies have targeted descending control systems, hence our vector strategy is novel and we demonstrate that genetic alteration of the excitability of ~150 pontospinal NAergic neurons can produce a pro-nociceptive phenotype. Several other genetic approaches have been employed to alter the excitability of central catecholaminergic neurons (Salbaum et al., 2004; Han et al., 2006; Hnasko et al., 2006). Pertinently, one of these studies also used retrograde viral transduction to rescue movement-disordered animals lacking dopamine (Hnasko et al., 2006) and others used interventions to increase the excitability of LC neurons (constitutively-active CREB (Han et al., 2006) and chlorotoxin (Salbaum et al., 2004)). This raises the prospect that targeted retrograde genetic upregulation of the pontospinal NAergic system could be employed to produce a therapeutic analgesic action.
Supplementary Material
Acknowledgements
AEP is a Wellcome Advanced Clinical Fellow. AGT is a British Heart Foundation Research Fellow. We thank Sergey Kasparov and Julian Paton for their advice, support and generous contributions to this work.
References
- Akli S, Caillaud C, Vigne E, Stratford-Perricaudet LD, Poenaru L, Perricaudet M, Kahn A, Peschanski MR. Transfer of a foreign gene into the brain using adenovirus vectors. Nat Genet. 1993;3:224–228. doi: 10.1038/ng0393-224. [DOI] [PubMed] [Google Scholar]
- Amendola M, Venneri MA, Biffi A, Vigna E, Naldini L. Coordinate dual-gene transgenesis by lentiviral vectors carrying synthetic bidirectional promoters. Nat Biotechnol. 2005;23:108–116. doi: 10.1038/nbt1049. [DOI] [PubMed] [Google Scholar]
- Bewig B, Schmidt WE. Accelerated titering of adenoviruses. Biotechniques. 2000;28:870–873. doi: 10.2144/00285bm08. [DOI] [PubMed] [Google Scholar]
- Bodnar RJ, Mann PE, Stone EA. Potentiation of cold-water swim analgesia by acute, but not chronic desipramine administration. Pharmacol Biochem Behav. 1985;23:749–752. doi: 10.1016/0091-3057(85)90066-8. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Xu F, Gainetdinov RR, Caron MG. Potentiated opioid analgesia in norepinephrine transporter knock-out mice. J Neurosci. 2000;20:9040–9045. doi: 10.1523/JNEUROSCI.20-24-09040.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett A, Gebhart GF. Characterization of descending modulation of nociception from the A5 cell group. Brain Res. 1991;546:271–281. doi: 10.1016/0006-8993(91)91491-i. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Clark FM, Proudfit HK. The projection of noradrenergic neurons in the A7 catecholamine cell group to the spinal cord in the rat demonstrated by anterograde tracing combined with immunocytochemistry. Brain Res. 1991a;547:279–288. doi: 10.1016/0006-8993(91)90972-x. [DOI] [PubMed] [Google Scholar]
- Clark FM, Proudfit HK. The projection of locus coeruleus neurons to the spinal cord in the rat determined by anterograde tracing combined with immunocytochemistry. Brain Res. 1991b;538:231–245. doi: 10.1016/0006-8993(91)90435-x. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Roth RH, Maas JW. Locus coeruleus stimulation increases noradrenergic metabolite levels in rat spinal cord. Brain Res. 1979;166:180–184. doi: 10.1016/0006-8993(79)90661-9. [DOI] [PubMed] [Google Scholar]
- Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- Dodt HU, Zieglgansberger W. Visualizing unstained neurons in living brain slices by infrared DIC-videomicroscopy. Brain Res. 1990;537:333–336. doi: 10.1016/0006-8993(90)90380-t. [DOI] [PubMed] [Google Scholar]
- Duale H, Kasparov S, Paton JF, Teschemacher AG. Differences in transductional tropism of adenoviral and lentiviral vectors in the rat brainstem. Exp Physiol. 2005;90:71–78. doi: 10.1113/expphysiol.2004.029173. [DOI] [PubMed] [Google Scholar]
- Duale H, Waki H, Howorth P, Kasparov S, Teschemacher AG, Paton JF. Restraining influence of A2 neurons in chronic control of arterial pressure in spontaneously hypertensive rats. Cardiovasc Res. 2007;76:184–193. doi: 10.1016/j.cardiores.2007.06.018. [DOI] [PubMed] [Google Scholar]
- Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977;4:161–174. doi: 10.1016/0304-3959(77)90130-0. [DOI] [PubMed] [Google Scholar]
- Eaton MJ, Blits B, Ruitenberg MJ, Verhaagen J, Oudega M. Amelioration of chronic neuropathic pain after partial nerve injury by adeno-associated viral (AAV) vector-mediated over-expression of BDNF in the rat spinal cord. Gene Ther. 2002;9:1387–1395. doi: 10.1038/sj.gt.3301814. [DOI] [PubMed] [Google Scholar]
- Eisenach JC, DuPen S, Dubois M, Miguel R, Allin D, The Epidural Clonidine Study Group Epidural clonidine analgesia for intractable cancer pain. Pain. 1995;61:391–399. doi: 10.1016/0304-3959(94)00209-W. [DOI] [PubMed] [Google Scholar]
- Fasmer OB, Berge OG, Tveiten L, Hole K. Changes in nociception after 6-hydroxydopamine lesions of descending catecholaminergic pathways in mice. Pharmacol Biochem Behav. 1986;24:1441–1444. doi: 10.1016/0091-3057(86)90207-8. [DOI] [PubMed] [Google Scholar]
- Fields H. State-dependent opioid control of pain. Nat Rev Neurosci. 2004;5:565–575. doi: 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Grzanna R. Demonstration of two separate descending noradrenergic pathways to the rat spinal cord: evidence for an intragriseal trajectory of locus coeruleus axons in the superficial layers of the dorsal horn. J Comp Neurol. 1990;291:553–582. doi: 10.1002/cne.902910406. [DOI] [PubMed] [Google Scholar]
- Glorioso JC, Fink DJ. Herpes vector-mediated gene transfer in treatment of diseases of the nervous system. Annu Rev Microbiol. 2004;58:253–271. doi: 10.1146/annurev.micro.58.030603.123709. [DOI] [PubMed] [Google Scholar]
- Graham FL, Prevec L. Methods for construction of adenovirus vectors. Molecular Biotechnology. 1995;3:207–220. doi: 10.1007/BF02789331. [DOI] [PubMed] [Google Scholar]
- Green GM, Lyons L, Dickenson AH. Alpha2-adrenoceptor antagonists enhance responses of dorsal horn neurones to formalin induced inflammation. Eur J Pharmacol. 1998;347:201–204. doi: 10.1016/s0014-2999(98)00217-9. [DOI] [PubMed] [Google Scholar]
- Guyenet PG. The coeruleospinal noradrenergic neurons: anatomical and electrophysiological studies in the rat. Brain Res. 1980;189:121–133. doi: 10.1016/0006-8993(80)90012-8. [DOI] [PubMed] [Google Scholar]
- Hammond DL, Yaksh TL. Antagonism of stimulation-produced antinociception by intrathecal administration of methysergide or phentolamine. Brain Res. 1984;298:329–337. doi: 10.1016/0006-8993(84)91432-x. [DOI] [PubMed] [Google Scholar]
- Han MH, Bolanos CA, Green TA, Olson VG, Neve RL, Liu RJ, Aghajanian GK, Nestler EJ. Role of cAMP response element-binding protein in the rat locus ceruleus: regulation of neuronal activity and opiate withdrawal behaviors. J Neurosci. 2006;26:4624–4629. doi: 10.1523/JNEUROSCI.4701-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hayashida K, Clayton BA, Johnson JE, Eisenach JC. Brain derived nerve growth factor induces spinal noradrenergic fiber sprouting and enhances clonidine analgesia following nerve injury in rats. Pain. 2008;136:348–355. doi: 10.1016/j.pain.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentall ID, Mesigil R, Pinzon A, Noga BR. Temporal and spatial profiles of pontine-evoked monoamine release in the rat’s spinal cord. J Neurophysiol. 2003;89:2943–2951. doi: 10.1152/jn.00608.2002. [DOI] [PubMed] [Google Scholar]
- Hnasko TS, Perez FA, Scouras AD, Stoll EA, Gale SD, Luquet S, Phillips PE, Kremer EJ, Palmiter RD. Cre recombinase-mediated restoration of nigrostriatal dopamine in dopamine-deficient mice reverses hypophagia and bradykinesia. Proc Natl Acad Sci U S A. 2006;103:8858–8863. doi: 10.1073/pnas.0603081103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden JE, Schwartz EJ, Proudfit HK. Microinjection of morphine in the A7 catecholamine cell group produces opposing effects on nociception that are mediated by alpha1- and alpha2-adrenoceptors. Neuroscience. 1999;91:979–990. doi: 10.1016/s0306-4522(98)00673-3. [DOI] [PubMed] [Google Scholar]
- Howe MW, Feig SL, Osting SM, Haberly LB. Cellular and subcellular localization of Kir2.1 subunits in neurons and glia in piriform cortex with implications for K+ spatial buffering. J Comp Neurol. 2008;506:877–893. doi: 10.1002/cne.21534. [DOI] [PubMed] [Google Scholar]
- Howorth PW, Teschemacher AG, Pickering AE. Retrograde adenoviral vector targeting of nociresponsive pontospinal noradrenergic neurons in the rat in vivo. J Comp Neurol. 2009;512:141–157. doi: 10.1002/cne.21879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang DY, Carlezon WA, Jr., Isacson O, Kim KS. A high-efficiency synthetic promoter that drives transgene expression selectively in noradrenergic neurons. Hum Gene Ther. 2001;12:1731–1740. doi: 10.1089/104303401750476230. [DOI] [PubMed] [Google Scholar]
- Iadarola MJ, Brady LS, Draisci G, Dubner R. Enhancement of dynorphin gene expression in spinal cord following experimental inflammation: stimulus specificity, behavioral parameters and opioid receptor binding. Pain. 1988;35:313–326. doi: 10.1016/0304-3959(88)90141-8. [DOI] [PubMed] [Google Scholar]
- Jasmin L, Boudah A, Ohara PT. Long-term effects of decreased noradrenergic central nervous system innervation on pain behavior and opioid antinociception. J Comp Neurol. 2003;460:38–55. doi: 10.1002/cne.10633. [DOI] [PubMed] [Google Scholar]
- Jasmin L, Tien D, Weinshenker D, Palmiter RD, Green PG, Janni G, Ohara PT. The NK1 receptor mediates both the hyperalgesia and the resistance to morphine in mice lacking noradrenaline. Proc Natl Acad Sci U S A. 2002;99:1029–1034. doi: 10.1073/pnas.012598599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns DC, Marx R, Mains RE, O’Rourke B, Marban E. Inducible genetic suppression of neuronal excitability. J Neurosci. 1999;19:1691–1697. doi: 10.1523/JNEUROSCI.19-05-01691.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SL. Descending noradrenergic influences on pain. Prog Brain Res. 1991;88:381–394. doi: 10.1016/s0079-6123(08)63824-8. [DOI] [PubMed] [Google Scholar]
- Jones SL, Gebhart GF. Characterization of coeruleospinal inhibition of the nociceptive tail-flick reflex in the rat: mediation by spinal alpha 2-adrenoceptors. Brain Res. 1986a;364:315–330. doi: 10.1016/0006-8993(86)90844-9. [DOI] [PubMed] [Google Scholar]
- Jones SL, Gebhart GF. Quantitative characterization of ceruleospinal inhibition of nociceptive transmission in the rat. J Neurophysiol. 1986b;56:1397–1410. doi: 10.1152/jn.1986.56.5.1397. [DOI] [PubMed] [Google Scholar]
- Karschin C, Dissmann E, Stuhmer W, Karschin A. IRK(1–3) and GIRK(1–4) inwardly rectifying K+ channel mRNAs are differentially expressed in the adult rat brain. J Neurosci. 1996;16:3559–3570. doi: 10.1523/JNEUROSCI.16-11-03559.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo Y, Baldwin TJ, Jan YN, Jan LY. Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature. 1993;362:127–133. doi: 10.1038/362127a0. [DOI] [PubMed] [Google Scholar]
- Li X, Conklin D, Ma W, Zhu X, Eisenach JC. Spinal noradrenergic activation mediates allodynia reduction from an allosteric adenosine modulator in a rat model of neuropathic pain. Pain. 2002;97:117–125. doi: 10.1016/s0304-3959(02)00011-8. [DOI] [PubMed] [Google Scholar]
- Liu RJ, Wang R, Nie H, Zhang RX, Qiao JT, Dafny N. Effects of intrathecal monoamine antagonists on the nociceptive c-Fos expression in a lesioned rat spinal cord. Int J Neurosci. 1997a;91:169–180. doi: 10.3109/00207459708986374. [DOI] [PubMed] [Google Scholar]
- Liu Y, Himes BT, Moul J, Huang W, Chow SY, Tessler A, Fischer I. Application of recombinant adenovirus for in vivo gene delivery to spinal cord. Brain Res. 1997b;768:19–29. doi: 10.1016/s0006-8993(97)00587-8. [DOI] [PubMed] [Google Scholar]
- Lonergan T, Teschemacher AG, Hwang DY, Kim KS, Pickering AE, Kasparov S. Targeting brain stem centers of cardiovascular control using adenoviral vectors: impact of promoters on transgene expression. Physiol Genomics. 2005;20:165–172. doi: 10.1152/physiolgenomics.00120.2004. [DOI] [PubMed] [Google Scholar]
- Loughlin SE, Foote SL, Bloom FE. Efferent projections of nucleus locus coeruleus: topographic organization of cells of origin demonstrated by three-dimensional reconstruction. Neuroscience. 1986;18:291–306. doi: 10.1016/0306-4522(86)90155-7. [DOI] [PubMed] [Google Scholar]
- Ma W, Eisenach JC. Chronic constriction injury of sciatic nerve induces the up-regulation of descending inhibitory noradrenergic innervation to the lumbar dorsal horn of mice. Brain Res. 2003;970:110–118. doi: 10.1016/s0006-8993(03)02293-5. [DOI] [PubMed] [Google Scholar]
- Mansikka H, Lahdesmaki J, Scheinin M, Pertovaara A. Alpha(2A) adrenoceptors contribute to feedback inhibition of capsaicin-induced hyperalgesia. Anesthesiology. 2004;101:185–190. doi: 10.1097/00000542-200407000-00029. [DOI] [PubMed] [Google Scholar]
- Martin WJ, Gupta NK, Loo CM, Rohde DS, Basbaum AI. Differential effects of neurotoxic destruction of descending noradrenergic pathways on acute and persistent nociceptive processing. Pain. 1999;80:57–65. doi: 10.1016/s0304-3959(98)00194-8. [DOI] [PubMed] [Google Scholar]
- Mata M, Hao S, Fink DJ. Applications of gene therapy to the treatment of chronic pain. Curr Gene Ther. 2008;8:42–48. doi: 10.2174/156652308783688527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzack R, Wall PD, Ty TC. Acute pain in an emergency clinic: latency of onset and descriptor patterns related to different injuries. Pain. 1982;14:33–43. doi: 10.1016/0304-3959(82)90078-1. [DOI] [PubMed] [Google Scholar]
- Millan MJ. The role of descending noradrenergic and serotoninergic pathways in the modulation of nociception: focus on receptor multiplicity. In: Dickenson A, Besson JM, editors. The Pharmacology of Pain. Springer; Berlin: 1997. pp. 385–446. [Google Scholar]
- Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Miller JF, Proudfit HK. Antagonism of stimulation-produced antinociception from ventrolateral pontine sites by intrathecal administration of alpha-adrenergic antagonists and naloxone. Brain Res. 1990;530:20–34. doi: 10.1016/0006-8993(90)90653-s. [DOI] [PubMed] [Google Scholar]
- Mizuno H, Hirano T, Tagawa Y. Evidence for activity-dependent cortical wiring: formation of interhemispheric connections in neonatal mouse visual cortex requires projection neuron activity. J Neurosci. 2007;27:6760–6770. doi: 10.1523/JNEUROSCI.1215-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA, Yoshimura M. The actions of noradrenaline on neurones of the rat substantia gelatinosa in vitro. J Physiol. 1984;349:43–55. doi: 10.1113/jphysiol.1984.sp015141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omote K, Kawamata T, Kawamata M, Namiki A. Formalin-induced nociception activates a monoaminergic descending inhibitory system. Brain Res. 1998;814:194–198. doi: 10.1016/s0006-8993(98)01086-5. [DOI] [PubMed] [Google Scholar]
- Pertovaara A. Noradrenergic pain modulation. Prog Neurobiol. 2006;80:53–83. doi: 10.1016/j.pneurobio.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Rahman W, D’Mello R, Dickenson AH. Peripheral nerve injury-induced changes in spinal alpha(2)-adrenoceptor-mediated modulation of mechanically evoked dorsal horn neuronal responses. J Pain. 2008;9:350–359. doi: 10.1016/j.jpain.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Reddy SV, Maderdrut JL, Yaksh TL. Spinal cord pharmacology of adrenergic agonist-mediated antinociception. J Pharmacol Exp Ther. 1980;213:525–533. [PubMed] [Google Scholar]
- Reynolds DV. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science. 1969;164:444–445. doi: 10.1126/science.164.3878.444. [DOI] [PubMed] [Google Scholar]
- Ridoux V, Robert JJ, Zhang X, Perricaudet M, Mallet J, Le Gal La Salle G. Adenoviral vectors as functional retrograde neuronal tracers. Brain Res. 1994;648:171–175. doi: 10.1016/0006-8993(94)91919-4. [DOI] [PubMed] [Google Scholar]
- Sagen J, Proudfit HK. Effect of intrathecally administered noradrenergic antagonists on nociception in the rat. Brain Res. 1984;310:295–301. doi: 10.1016/0006-8993(84)90152-5. [DOI] [PubMed] [Google Scholar]
- Salbaum JM, Cirelli C, Walcott E, Krushel LA, Edelman GM, Tononi G. Chlorotoxin-mediated disinhibition of noradrenergic locus coeruleus neurons using a conditional transgenic approach. Brain Res. 2004;1016:20–32. doi: 10.1016/j.brainres.2004.03.078. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Westlund KN. Spinal projections of the locus coeruleus and the nucleus subcoeruleus in the Harlan and the Sasco Sprague-Dawley rat. Brain Res. 1992;579:67–73. doi: 10.1016/0006-8993(92)90742-r. [DOI] [PubMed] [Google Scholar]
- Sonohata M, Furue H, Katafuchi T, Yasaka T, Doi A, Kumamoto E, Yoshimura M. Actions of noradrenaline on substantia gelatinosa neurones in the rat spinal cord revealed by in vivo patch recording. J Physiol. 2004;555:515–526. doi: 10.1113/jphysiol.2003.054932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone LS, MacMillan LB, Kitto KF, Limbird LE, Wilcox GL. The alpha2a adrenergic receptor subtype mediates spinal analgesia evoked by alpha2 agonists and is necessary for spinal adrenergic-opioid synergy. J Neurosci. 1997;17:7157–7165. doi: 10.1523/JNEUROSCI.17-18-07157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Sugimoto M, Kuraishi Y, Satoh M, Takagi H. Involvement of medullary opioid-peptidergic and spinal noradrenergic systems in the regulation of formalin-induced persistent pain. Neuropharmacology. 1986;25:481–485. doi: 10.1016/0028-3908(86)90171-1. [DOI] [PubMed] [Google Scholar]
- Teschemacher AG, Paton JF, Kasparov S. Imaging living central neurones using viral gene transfer. Adv Drug Deliv Rev. 2005;57:79–93. doi: 10.1016/j.addr.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Tsuruoka M, Willis WD., Jr. Bilateral lesions in the area of the nucleus locus coeruleus affect the development of hyperalgesia during carrageenan-induced inflammation. Brain Res. 1996;726:233–236. [PubMed] [Google Scholar]
- Viisanen H, Pertovaara A. Influence of peripheral nerve injury on response properties of locus coeruleus neurons and coeruleospinal antinociception in the rat. Neuroscience. 2007;146:1785–1794. doi: 10.1016/j.neuroscience.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Wang S, Teschemacher AG, Paton JF, Kasparov S. Mechanism of nitric oxide action on inhibitory GABAergic signaling within the nucleus tractus solitarii. FASEB J. 2006;20:1537–1539. doi: 10.1096/fj.05-5547fje. [DOI] [PubMed] [Google Scholar]
- Wei F, Dubner R, Ren K. Nucleus reticularis gigantocellularis and nucleus raphe magnus in the brain stem exert opposite effects on behavioral hyperalgesia and spinal Fos protein expression after peripheral inflammation. Pain. 1999;80:127–141. doi: 10.1016/s0304-3959(98)00212-7. [DOI] [PubMed] [Google Scholar]
- Wei H, Pertovaara A. Spinal and pontine alpha2-adrenoceptors have opposite effects on pain-related behavior in the neuropathic rat. Eur J Pharmacol. 2006;551:41–49. doi: 10.1016/j.ejphar.2006.08.064. [DOI] [PubMed] [Google Scholar]
- West WL, Yeomans DC, Proudfit HK. The function of noradrenergic neurons in mediating antinociception induced by electrical stimulation of the locus coeruleus in two different sources of Sprague-Dawley rats. Brain Res. 1993;626:127–135. doi: 10.1016/0006-8993(93)90571-4. [DOI] [PubMed] [Google Scholar]
- Westlund KN, Bowker RM, Ziegler MG, Coulter JD. Noradrenergic projections to the spinal cord of the rat. Brain Res. 1983;263:15–31. doi: 10.1016/0006-8993(83)91196-4. [DOI] [PubMed] [Google Scholar]
- Williams JT, Marshall KC. Membrane properties and adrenergic responses in locus coeruleus neurons of young rats. J Neurosci. 1987;7:3687–3694. doi: 10.1523/JNEUROSCI.07-11-03687.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JT, North RA, Shefner SA, Nishi S, Egan TM. Membrane properties of rat locus coeruleus neurones. Neuroscience. 1984;13:137–156. doi: 10.1016/0306-4522(84)90265-3. [DOI] [PubMed] [Google Scholar]
- Wilson SP, Yeomans DC, Bender MA, Lu Y, Goins WF, Glorioso JC. Antihyperalgesic effects of infection with a preproenkephalin-encoding herpes virus. Proc Natl Acad Sci U S A. 1999;96:3211–3216. doi: 10.1073/pnas.96.6.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Kontinen VK, Kalso E. Endogenous noradrenergic tone controls symptoms of allodynia in the spinal nerve ligation model of neuropathic pain. Eur J Pharmacol. 1999;366:41–45. doi: 10.1016/s0014-2999(98)00910-8. [DOI] [PubMed] [Google Scholar]
- Xu Y, Gu Y, Xu GY, Wu P, Li GW, Huang LY. Adeno-associated viral transfer of opioid receptor gene to primary sensory neurons: a strategy to increase opioid antinociception. Proc Natl Acad Sci U S A. 2003;100:6204–6209. doi: 10.1073/pnas.0930324100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans DC, Clark FM, Paice JA, Proudfit HK. Antinociception induced by electrical stimulation of spinally projecting noradrenergic neurons in the A7 catecholamine cell group of the rat. Pain. 1992;48:449–461. doi: 10.1016/0304-3959(92)90098-V. [DOI] [PubMed] [Google Scholar]
- Yu CR, Power J, Barnea G, O’Donnell S, Brown HE, Osborne J, Axel R, Gogos JA. Spontaneous neural activity is required for the establishment and maintenance of the olfactory sensory map. Neuron. 2004;42:553–566. doi: 10.1016/s0896-6273(04)00224-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.