Abstract
The substrate specificities of Trypanosoma brucei and human (HeLa) GlcNAc-PI de-N-acetylases were determined using 24 substrate analogues. The results show the following. (i) The de-N-acetylases show little specificity for the lipid moiety of GlcNAc-PI. (ii) The 3′-OH group of the GlcNAc residue is essential for substrate recognition whereas the 6′-OH group is dispensable and the 4′-OH, while not required for recognition, cannot be epimerized or substituted. (iii) The parasite enzyme can act on analogues containing βGlcNAc or aromatic N-acyl groups, whereas the human enzyme cannot. (iv) Three GlcNR-PI analogues are de-N-acetylase inhibitors, one of which is a suicide inhibitor. (v) The suicide inhibitor most likely forms a carbamate or thiocarbamate ester to an active site hydroxy-amino acid or Cys or residue such that inhibition is reversed by certain nucleophiles. These and previous results were used to design two potent (IC50 = 8 nM) parasite-specific suicide substrate inhibitors. These are potential lead compounds for the development of anti-protozoan parasite drugs.
Keywords: de-N-acetylase/glycosylphosphatidylinositol/mannosyltransferase/suicide inhibition/Trypanosoma brucei
Introduction
A significant proportion of eukaryotic cell-surface glycoproteins are attached to the plasma membrane by covalent linkage to a glycosylphosphatidylinositol (GPI) membrane anchor. The structure and biosynthesis of GPI membrane anchors and related molecules have recently been reviewed (Ferguson, 1999; Kinoshita and Inoue, 2000; McConville and Menon, 2000; Morita et al., 2000a). The basic GPI core structure attached to protein comprises NH2CH2CH2 PO4H-6Manα1-2Manα1-6Manα1-4GlcNα1-6d-myo-inositol-1-HPO4-lipid (EtNP-Man3GlcN-PI), where the lipid can be diacylglycerol, alkylacylglycerol or ceramide. This minimal GPI structure may be embellished with additional ethanolamine phosphate groups and/or carbohydrate side chains in a species- and tissue-specific manner (Ferguson et al., 1999).
Protozoa tend to express significantly higher densities of cell-surface GPI-anchored proteins than do higher eukaryotes. For example, Trypanosoma brucei, the causative agent of African sleeping sickness, expresses a dense cell-surface coat consisting of ∼5 × 106 dimers of a GPI-anchored variant surface glycoprotein. This protects the parasite from the alternative complement pathway of the host and, through antigenic variation, from specific immune responses (Cross, 1996). A variety of GPI-related structures, such as lipophosphoglycans, glycoinositolphospholipids (GIPLs) and mucin-like structures, are expressed by other trypanosomatid parasites (Ferguson, 1999, and references therein). Plasmodium (Gerold et al., 1996), Toxoplasma (Striepen et al., 1997), Trichomonas (Singh et al., 1994) and Entamoeba (Moody-Haupt et al., 2000) also have abundant GPI-anchored glycoproteins and/or GIPLs. Inhibitors able to arrest the formation of GPI-anchored proteins and/or GPI-related molecules on the plasma membrane of parasitic protozoa should prove useful in the development of anti-parasitic agents. This notion has been validated, at least for T.brucei, where disruption of the TbGPI10 gene encoding the third mannosyltransferase of GPI anchor biosynthesis has been shown to be lethal for the bloodstream form of the parasite (Ferguson, 2000; Nagamune et al., 2000). Furthermore, GIPLs appear to be essential for the survival of Leishmania (Ilgoutz et al., 1999) and Trypanosoma cruzi (Garg et al., 1997).
The sequence of events underlying GPI biosynthesis has been studied in T.brucei (Masterson et al., 1989, 1990; Menon et al., 1990a,b; Güther and Ferguson, 1995; Morita et al., 2000b), T.cruzi (Heise et al., 1996), Toxoplasma gondii (Striepen et al., 1999), Plasmodium falciparum (Gerold et al., 1999), Leishmania (Smith et al., 1997a; Ralton and McConville, 1998), Saccharomyces cerevisiae (Sütterlin et al., 1998; Flury et al., 2000) and mammalian cells (Hirose et al., 1992; Puoti and Conzelmann, 1993; Chen et al., 1998, and references therein). In all cases, GPI biosynthesis involves the addition of GlcNAc to phosphatidylinositol (PI) to give D-GlcNAcα1-6D-myo-inositol-1-HPO4-sn-1,2-diacylglycerol (GlcNAc-PI), which is then de-N-acetylated to form d-GlcNα1-6d-myo-inositol-1-HPO4-sn-1,2-diacylglycerol (GlcN-PI) (Doering et al., 1989; Milne et al., 1994; Watanabe et al., 1999, 2000). De-N-acetylation is a prerequisite for the mannosylation of GlcN-PI to form later GPI intermediates (Nakamura et al., 1997; Sharma et al., 1997). The GlcNAc-PI de-N-acetylases from protozoan and mammalian sources are similar with regard to their specificities for the acyl (R) group removed from GlcNR-PI substrates (Sharma et al., 1997), but differ with regard to their specificity for the myo-inositol residue. Thus, the trypanosomal enzyme can de-N-acetylate GlcNAc-PI containing either d- or l-myo-inositol, whereas the human (HeLa) enzyme strictly requires d-myo-inositol (Sharma et al., 1999).
Notable differences between the T.brucei and mammalian GPI biosynthetic pathways occur from GlcN-PI onwards, including the timing of inositol acylation and deacylation (Güther and Ferguson, 1995), the addition of extra ethanolamine phosphate groups to mammalian GPI anchors (Hirose et al., 1992; Puoti and Conzelmann, 1993) and fatty acid remodelling of T.brucei GPI anchors (Masterson et al., 1990). The processing of GlcN-PI is a key step in the GPI biosynthetic pathway; inositol acylation (the transfer of fatty acid to the 2-OH group of the d-myo-inositol residue) of GlcN-PI either precedes or follows a first mannosylation, as in mammalian cells and T.brucei, respectively (Güther and Ferguson, 1995; Doerrler et al., 1996; Smith et al., 1997b). This difference was exploited in the discovery of the first generation of specific inhibitors of the parasite GPI biosynthetic pathway in vitro (Smith et al., 1999). Thus, d-GlcNα1-6d-2-O-hexadecyl-myo-inositol-1-HPO4-sn-1,2-dipalmitoylglycerol [GlcN-(2-O-hexadecyl)-PI] was shown to inhibit the first mannosyltransferase (MT-1), whereas d-GlcNα1-6d-2-O-octyl-myo-inositol-1-HPO4-sn-1,2-dipalmitoylglycerol [GlcN-(2-O-octyl)-PI] and its N-acetylated version inhibited inositol acylation of Man1-3GlcN-PI and prevented the subsequent addition of an ethanolamine phosphate bridge (Smith et al., 1999). Another series of parasite-specific GPI pathway inhibitors containing l-myo-inositol inhibited T.brucei MT-1 (Smith et al., 2000), whereas a terpenoid natural product inhibited yeast and human, but not parasite, GPI biosynthesis (Sütterlin et al., 1997).
In this paper we describe the first mechanism-based suicide inhibitor of GPI biosynthesis, and combine features of this molecule with others to produce two parasite-specific suicide substrate inhibitors.
Results and discussion
Optimization of the GlcN[3H]Ac-PI de-N-acetylase assay
The release of [3H]acetate from GlcN[3H]Ac-PI by the GlcNAc-PI de-N-acetylase of the trypanosome and HeLa cell-free systems was followed for up to 4 h under different conditions. The presence of GDP-Man (1 mM), which allows the conversion of the GlcN-PI product to downstream mannosylated intermediates, stimulated trypanosomal GlcNAc-PI de-N-acetylase activity by ∼10%. The addition of CoA and an ATP-regenerating system, which allows the in situ formation of acyl-CoA and hence the formation of GlcN-(acyl)PI, stimulated the HeLa GlcNAc-PI de-N-acetylase activity by ∼15%. The addition of 1 mM GDP-Man stimulated the HeLa activity by a further 10%. Neither enzyme was affected by the addition of a 10-fold excess of GlcN-PI over GlcN[3H]Ac-PI, indicating that there is no direct feedback product inhibition. Since substrate turnover was linear only within 60–90 min, all subsequent assays to determine initial rates were performed up to 120 min in the presence of either GDP-Man for the trypanosomal system or GDP-Man and CoA/ATP-regenerating system for the HeLa cell-free system. Under optimal conditions, the specific activity of the trypanosome cell-free system de-N-acetylase for GlcN[3H]Ac-PI is almost double that of the HeLa system (Table I), which is consistent with a greater turnover rate for the protozoan GPI pathway.
Table I. Release of [3H]acetate from GlcN[3H]Ac-PI substrate analogues by the trypanosome and HeLa cell-free systems.
| Analogue | Trypanosome |
HeLa |
||
|---|---|---|---|---|
| Initial ratea | %b | Initial ratea | %b | |
| GlcNAc-PI | 1.35 | 100 | 0.75 | 100 |
| 3-dGlcNAc-PI | 0.00 | 0 | 0.00 | 0 |
| 4-dGlcNAc-PI | 1.40 | 103 | 0.69 | 92 |
| 6-dGlcNAc-PI | 1.53 | 108 | 0.90 | 120 |
| GlcNAc4Me-PI | 0.30 | 22 | 0.15 | 20 |
| ManNAc-PI | 0.01 | 1 | 0.00 | 0 |
| GalNAc-PI | 0.01 | 1 | 0.00 | 0 |
| GlcNAc-β-PI | 1.42 | 104 | 0.00 | 0 |
| GlcNAc-[L]-PI | 0.08 | 6 | 0.00 | 0 |
| GlcNAc-[L]-(2-O-methyl)-PI | 0.09 | 7 | 0.01 | 1 |
| GlcNAc-(2-O-methyl)-PI | 1.05 | 77 | 0.00 | 0 |
| GlcNAc-(2-O-octyl)-PI | 0.99 | 73 | 0.00 | 0 |
| GlcNAc-(2-O-hexadecyl)-PI | 0.02 | 2 | 0.00 | 0 |
| GlcNAc-I-P-C18 | 1.30 | 96 | 0.80 | 107 |
| GlcNAc-I-P-C8 | 0.24 | 18 | 0.04 | 5 |
| GlcNAc-PI(diC8) | 1.37 | 101 | 0.65 | 86 |
| GlcNAc-PI(diC18) | 1.31 | 97 | 0.89 | 118 |
| GlcNAc-PI(Me,C18) | 1.41 | 104 | 0.66 | 88 |
aThe initial reaction rates (pmol [3H]acetate released min–1 mg–1 protein) were estimated from the initial linear range of [3H]acetate release. Each time point was measured in triplicate and measurements were within ±3% of the mean value. The values given here are the mean of at least two estimates each of the initial rates; individual estimates were within ±4% of the mean value. Background levels of non-GPI-specific de-N-acetylation (4 and 1% for the trypanosomal and HeLa systems, respectively) were estimated from the initial rates of [3H]acetate released from GlcN[3H]Acα1-S-C8 and GlcN[3H]Acβ1-S-C8, which do not compete for the GlcNAc-PI de-N-acetylase. The values of the initial rates recorded in the table have been adjusted accordingly.
bInitial reaction rate relative to that for GlcNAc-PI (100%).
Comparison of the specificities of the trypanosome and HeLa GlcNAc-PI de-N-acetylases
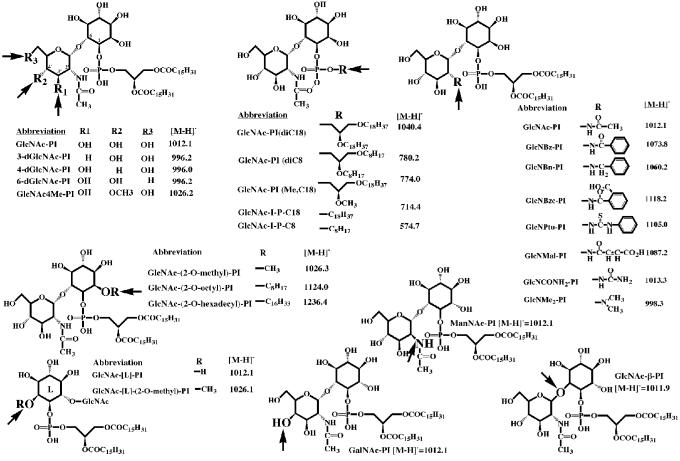
Various GlcN[3H]Ac-PI analogues (Figure 1) were tested as substrates (Table I). In both systems, the deoxy compounds 4-dGlcNAc-PI and 6-dGlcNAc-PI were de-N-acetylated at a similar rate to that of GlcNAc-PI, suggesting that the 4- and 6-OH groups of the GlcNAc residue are not involved in substrate recognition. However, the methylated analogue GlcNAc4Me-PI is de-N-acetylated at one-fifth of the rate of GlcNAc-PI, whereas GalNAc-PI (the 4′-epimer) is not a substrate in either system. This suggests that there are steric constraints in the active site that disfavor substitution or epimerization of the 4′-OH group. These results are consistent with a previous observation that substitution of the 4′-OH group with an αMan residue, as in Manα1- 4GlcNAc-PI, prevents substrate recognition by the trypanosomal de-N-acetylase (Sharma et al., 1997).
Fig. 1. Synthetic substrate analogues of GlcNAc-PI. Their abbreviated names and the m/z values of their [M-H]–1 pseudomolecular ions are indicated.
The 3-dGlcNAc-PI analogue is not a substrate (nor an inhibitor) for the de-N-acetylase of either system, implying that the 3′-OH group is a crucial hydrogen-bond acceptor or donor involved in substrate recognition by the de-N-acetylases. Neither is ManNAc-PI (the 2′-epimer) a substrate; this is not surprising since the N-acetyl group now occupies an axial orientation on the pyranose ring compared with its equatorial orientation in the natural substrate.
Surprisingly, GlcNAc-β-PI is as good a substrate as the natural α-anomer (GlcNAc-PI) for the trypanosomal enzyme, although it is not a substrate for the HeLa enzyme. Modelling studies suggest that the geometric arrangement of the 2′-acetamido, 3′-OH and phosphodiester groups is remarkably similar for both anomers, although the relative position of the inositol ring differs significantly (Figure 2). This, in turn, suggests that there are no significant interactions between the 2-, 3-, 4- and 5-OH groups of d-myo-inositol and the trypanosomal de-N-acetylase. This contrasts with the recognition of GlcN-PI by trypanosomal MT-1, which appears to make essential hydrogen bonds to the 3- and 5-OH groups of the d-myo-inositol residue (Smith et al., 2000). The absence of an interaction between the parasite de-N-acetylase and the 2-OH group of the d-myo-inositol residue can also be inferred from the ability of the trypanosomal enzyme to act on substrates alkylated at this position, notably GlcNAc-(2-O-methyl)-PI and GlcNAc-(2-O-octyl)-PI (Sharma et al., 1999) (Table I). Furthermore, the absence of an interaction between the parasite de-N-acetylase and the 2- and 4-OH groups of the d-myo-inositol residue is supported by the slow turnover of GlcNAc-[L]-PI (Table I), which can adopt a conformation similar to GlcNAc-PI apart from the orientations of the 2- and 4-OH groups (Sharma et al., 1999; Smith et al., 2000). Clearly, the HeLa cell de-N-acetylase, which recognizes neither GlcNAc-β-PI nor GlcNAc-[L]-PI (Table I), is more stringent with regard to the configurations of the glycosidic linkage and the myo-inositol residue, and it is tempting to speculate that the HeLa enzyme makes essential hydrogen bonds with the α1-6-linked d-myo-inositol residue of GlcNAc-PI.
Fig. 2. Molecular models of GlcNAcα1-6d-myo-inositol-1-HPO4-lipid and GlcNAcβ1-6d-myo-inositol-1-HPO4-lipid. The energy-minimized structures (using an unrestrained MOPAC molecular dynamics simulation at 300 K) show how similar the two isomers are with respect to the relative positions of the GlcNAc residue and the phosphodiester group. For simplicity, the lipid group in these models is represented as a simple alkyl chain.
All of the lipid variants of GlcNAc-PI (Figure 1) were good substrates for the trypanosomal and HeLa de-N-acetylases, whether presented as a glycerolipid or a single alkyl chain (Table I). However, the more hydrophobic (longer chain) analogues were better substrates in the HeLa cell-free system than in the trypanosomal cell-free system. The poorest substrate in both cell-free systems was the short-chain analogue GlcNAc-I-P-C8, presumably because it failed to interact sufficiently with the membrane bilayer in order to bring it into intimate contact with the membrane-bound de-N-acetylases. Thus, the primary role of the lipid component of GlcNAc-PI in substrate recognition appears to be to insert the substrate into the membrane. This differs from the situation for the mammalian UDP-GlcNAc:PI α1-6 GlcNAc-transferase complex, which shows specificity for the glycerolipid component of the PI acceptor substrate (Watanabe et al., 1998).
Substrate specificity of the trypanosomal de-N-acetylase for the GlcNR-PI substrate analogues
We have previously defined the substrate specificities of the trypanosomal and HeLa de-N-acetylases with respect to the nature of the acyl group (R) released from GlcNR-PI analogues (Sharma et al., 1997). Both enzymes were active when R was acetyl or propionyl, barely active when R was butyryl, isobutyryl or pentanoyl, and inactive when R was hexanoyl. The most sensitive way of assessing substrate turnover for GlcNR-PI analogues is to use the cell-free system as a coupled assay, exploiting the fact that GPI mannosylation reactions can proceed from GlcN-PI, but not from GlcNR-PI (Sharma et al., 1997). In the trypanosomal cell-free system, this assay is performed in the presence of N-ethylmaleimide (NEM), which inhibits UDP-GlcNAc:PI α1-6 GlcNAc-transferase without affecting the downstream enzymes (Milne et al., 1992). This prevents the labelling of endogenous GPI intermediates (Figure 3A, lane 1), and simplifies the interpretation of the effects resulting from the addition of synthetic substrates and substrate analogues (Smith et al., 1996).
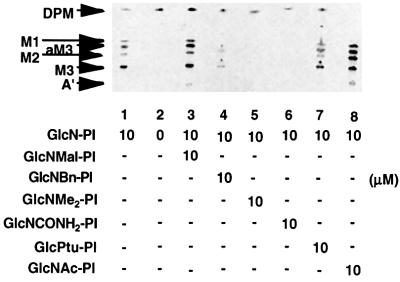
Fig. 3. GlcNR-PI analogues as substrates for the trypanosomal GlcNAc-PI de-N-acetylase. (A) The trypanosomal cell-free system was incubated with GDP-[3H]Man and NEM alone (lane 1), or together with either GlcN-PI, GlcNAc-PI or GlcNBz-PI at the concentrations indicated (lanes 2–5). (B) The trypanosomal cell-free system was incubated with GDP-[3H]Man and NEM alone (lane 1), or together with the compounds indicated at a final concentration of 10 µM (lanes 2–8). In both cases, the radiolabelled glycolipid products were analysed by HPTLC and fluorography. The products are: DPM, dolichol-phosphate-mannose; M1–3, Man1-3GlcN-PI; aM2–3, Man2-3GlcN-(acyl)PI; A′, EtNP-Man3GlcN-PI; C′, EtNP-Man3GlcN-(acyl)PI.
The addition of 10 µM GlcN-PI and GlcNAc-PI primed the production of GPI intermediates up to and including glycolipids A′ and C′ (Figure 3A, lanes 2 and 3). The products were identified by standard treatments, including digestions with jack bean α-mannosidase, phosphatidylinositol-specific phospholipase C and GPI-specific phospholipase D, and nitrous acid deamination and base hydrolysis (data not shown). Products identical to those obtained with GlcNAc-PI were formed when d-GlcN(benzoyl)α1-6d-myo-inositol-1-HPO4-sn-1,2-dipalmitoylglycerol (GlcNBz-PI) was added (Figure 3A, lanes 4 and 5). All of the mannosylated products were sensitive to nitrous acid deamination, demonstrating that they had been de-N-acylated (data not shown). The enhanced priming of the trypanosomal GPI biosynthetic pathway by GlcNAc-PI and GlcNBz-PI compared with GlcN-PI (Figure 3A, lane 2) is consistent with previous reports pointing to substrate channelling between the de-N-acetylase and MT-1 (Smith et al., 1996). The data show that GlcNBz-PI is a good substrate for the trypanosomal GlcNAc-PI de-N-acetylase. This result was unexpected in view of the poor turnover of the N-butyryl, N-isobutyryl and N-pentanoyl derivatives of GlcN-PI (Sharma et al., 1997), and suggests that the planar aromatic ring of the N-benzoyl group is beneficial for substrate recognition. The ability of the trypanosomal de-N-acetylase to turnover GlcNR-PI analogues with even larger aromatic groups [i.e. d-GlcN(2-carboxybenzoyl)α1-6d-myo-inositol-1-HPO4-sn-1,2-dipalmitoylglycerol (GlcNBzc-PI) and 2-deoxy-2-(3-phenylthioureido)-d-Glcα1-6d-myo-inositol-1-HPO4-sn-1,2-dipalmitoylglycerol (GlcPtu-PI); Figure 3B, lanes 5 and 8] supports this view, although these analogues were turned over less rapidly than GlcNAc-PI (Figure 3B, lane 2).
None of the four other GlcNR-PI analogues, i.e. d-GlcNMe2α1-6d-myo-inositol-1-HPO4-sn-1,2-dipalmitoylglycerol (GlcNMe2-PI), 2-deoxy-2-ureido-d-Glcα1- 6d - myo - inositol - 1 - HPO4 - sn - 1,2 - dipalmitoylglycerol (GlcNCONH2-PI), d-GlcNmaleoylα1-6d-myo-inositol-1-HPO4-sn-1,2-dipalmitoylglycerol (GlcNMal-PI) and d-GlcN(benzyl)α1-6d-myo-inositol-1-HPO4-sn-1,2-dipalmitoylglycerol (GlcNBn-PI), tested in the trypanosomal cell-free system was a substrate for the de-N-acetylase (Figure 3B, lanes 3, 4, 6 and 7). Of these, only GlcNCONH2-PI and GlcNMal-PI might be expected to be potential substrates since the others do not possess an N-acyl group on which the de-N-acetylase might act (Figure 1).
Inihibition of trypanosomal de-N-acetylase by GlcNR-PI substrate analogues
The GlcNR-PI analogues that were not substrates for the trypanosomal GlcNAc-PI de-N-acetylase were tested as inhibitors.
Using the indirect de-N-acetylase assay (which relies on the de-N-acetylation of exogenous GlcNAc-PI and subsequent [3H]mannosylation of the GlcN-PI produced), GlcNMal-PI and GlcNPtu-PI showed no significant inhibition when compared with the control (Figure 4A, lanes 2, 3 and 7), whereas GlcNBn-PI, GlcNMe2-PI and GlcNCONH2-PI inhibited the processing of exogenous GlcNAc-PI (Figure 4A, lanes 4–6, respectively). In this assay, inhibition could result from inhibition of the de-N-acetylase and/or MT-1. To throw light on which, if any, of these analogues inhibited the de-N-acetylase, they were pre-incubated with trypanosome membranes and the rate of [3H]acetate release from GlcN[3H]Ac-PI was measured (Figure 4B). Pre-incubation with an equimolar amount of unlabelled GlcNAc-PI or GlcNBz-PI reduced the rate of release of [3H]acetate to 50–60% of the control, as expected. By contrast, the release of [3H]acetate from GlcN[3H]Ac-PI was completely inhibited following pre-incubation with an equimolar amount of GlcNMe2-PI or GlcNCONH2-PI, demonstrating that both of these analogues inhibited the de-N-acetylase. The indirect assay was also used to assess the potency of the two inhibitors; GlcNMe2-PI gave complete inhibition at 1 µM (data not shown), whereas GlcNCONH2-PI gave complete inhibition at 80 nM with an IC50 of ∼8 nM (Figure 4C). This is the most potent inhibitor of GPI biosynthesis reported thus far.
Fig. 4. Inhibition of GPI biosynthesis in the trypanosomal cell-free system by GlcNR-PI substrate analogues. (A) The trypanosomal cell-free system was incubated with GDP-[3H]Man and NEM either alone (lane 1) or together with GlcNAc-PI (lane 2), or with GlcNAc-PI after pre-incubation with the compounds indicated (lanes 3–7). (B) The trypanosomal cell-free system was incubated with GlcN[3H]Ac-PI alone (squares) or in the presence of equimolar GlcNAc-PI (diamonds), GlcNBz-PI (circles), GlcNMe2-PI (triangles) or GlcNCONH2-PI (crosses), and the release of [3H]acetate was measured against time. (C) The trypanosomal cell-free system was incubated with GDP-[3H]Man and NEM either alone (lane 1) or with GlcNAc-PI (lane 2) or GlcNCONH2-PI (lane 3), or with GlcNAc-PI after pre-incubation with various concentrations of GlcNCONH2-PI (lanes 4–8).
Inihibition of trypanosomal MT-1 by GlcNR-PI substrate analogues
Next to be assessed was the ability of GlcNR-PI analogues to inhibit MT-1 and/or later enzymes in the GPI pathway. This was achieved by pre-incubating the trypanosomal cell-free system with the analogues prior to incubation with GlcN-PI and GDP-[3H]Man. GlcNMal-PI and GlcPtu-PI showed little or no inhibition of the mannosylation of GlcN-PI when compared with the control (Figure 5, lanes 1, 3 and 7). GlcNBn-PI caused significant, but incomplete inhibition, whereas GlcNMe2-PI and Glc NCONH2-PI produced complete inhibition (Figure 5, lanes 4, 5 and 6). These inhibitory effects cannot be ascribed to non-specific effects, resulting from increased concentrations of synthetic lipid in the system, since pre-incubation with an equivalent concentration of GlcNAc-PI stimulated the biosynthetic pathway (Figure 5, lane 8).
Fig. 5. GlcNR-PI inhibitors of trypanosome GPI biosynthesis inhibit MT-1 as well as GlcNAc-PI de-N-acetylase. The trypanosomal cell-free system was incubated with GDP-[3H]Man and NEM either alone (lane 2) or with GlcN-PI (lane 1), or with GlcN-PI after pre-incubation with the compounds indicated (lanes 3–8).
These data suggest that GlcNCONH2-PI, GlcNMe2-PI and, to a lesser extent, GlcNBn-PI, inhibit MT-1 as well as the de-N-acetylase. Furthermore, in a titration experiment similar to that shown in Figure 4C, but using GlcN-PI as the substrate to specifically assay MT-1, the IC50 for GlcNCONH2-PI was also ∼8 nM (data not shown). Given the evidence for substrate channelling between the two enzymes (Smith et al., 1996, 1997b), the most likely explanation for this joint inhibition is a physical overlap between the substrate/product and substrate binding sites of the de-N-acetylase and MT-1, respectively, such that occupation of the former blocks the latter.
The mechanism of inhibition of GPI biosynthesis by GlcNR-PI analogues
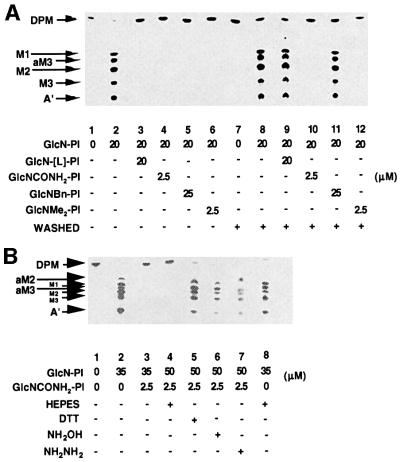
The cell-free system was pre-incubated with the known MT-1 inhibitor d-GlcNα1-6l-myo-inositol-1-HPO4-sn-1,2-dipalmitoylglycerol (GlcN-[L]-PI) (Smith et al., 2000) and each of the three de-N-acetylase/MT-1 inhibitors described herein, i.e. GlcNCONH2-PI, Glc NBn-PI and GlcNMe2-PI, prior to the addition of GlcN-PI and GDP-[3H]Man. The negative and positive controls are shown in Figure 6A (lanes 1 and 2), and the expected inhibition by the aforementioned compounds is shown in Figure 6A (lanes 3–6). When this experiment was repeated with a washing step between the pre-incubation stage and the addition of GlcN-PI, the inhibition caused by GlcN-[L]-PI and GlcNBn-PI was abolished (Figure 6A, lanes 9 and 11), suggesting that they are competitive inhibitors. However, the inhibition caused by GlcNCONH2-PI and GlcNMe2-PI was irreversible (Figure 6A, lanes 10 and 12), presumably due to a strong (possibly covalent) interaction between the enzyme and the inhibitor. Further investigation revealed that the inhibition caused by GlcNMe2-PI, but not that by GlcNCONH2-PI, could be partly reversed by washing the membranes with 2 M NaCl (data not shown), suggesting that the interaction between Glc NMe2-PI and the de-N-acetylase–MT-1 complex is non-covalent, and is likely therefore to involve charge–charge interaction(s).
Fig. 6. The reversible and irreversible inhibition of trypanosome GPI biosynthesis by GlcNR-PI analogues. (A) The trypanosomal cell-free system was incubated with GDP-[3H]Man and NEM alone (lane 1) or with GlcN-PI (lane 2), or with GlcN-PI after pre-incubation with the compounds indicated (lanes 3–6). Alternatively, membranes were pre-incubated with GDP-[3H]Man and NEM alone (lanes 7 and 8), or with GlcN-[L]-PI (lane 9), GlcNCONH2-PI (lane 10), GlcNBn-PI (lane 11) or GlcNMe2-PI (lane 12) prior to membrane washing (+) and incubation with GlcN-PI (lanes 8–12). (B) The trypanosomal cell-free system was incubated with GDP-[3H]Man and NEM alone (lane 1) or with GlcN-PI (lane 2), or with GlcN-PI after pre-incubation for 5 min with GlcNCONH2-PI (lane 3). The same membranes were also pre-incubated with (lanes 4–7) or without (lane 8) GlcNCONH2-PI, and subsequently washed with HEPES incorporation buffer alone (lanes 4 and 8) or with buffer containing DTT, hydroxylamine (NH2OH) or hydrazine (NH2NH2) (lanes 5–7), prior to incubation with GlcN-PI.
The stability of the GlcNCONH2-PI enzyme–inhibitor complex was also examined. The trypanosomal cell-free system was pre-incubated with GlcNCONH2-PI so as to allow the enzyme–inhibitor complex to form, and it was then incubated with GlcN-PI before or after washing the membranes. In both cases, inhibition was complete (compare Figure 6B, lanes 3 and 4, with the positive controls, lanes 2 and 8), again demonstrating irreversible inhibition by GlcNCONH2-PI. However, washing the GlcNCONH2-PI-treated membranes with dithiothreitol (DTT), hydroxylamine and hydrazine reversed the inhibition (Figure 6B, lanes 5–7).
Substrate specificity and inhibition of the HeLa cell de-N-acetylase
A number of the foregoing GlcNR-PI analogues were tested in the human (HeLa) cell-free system. In this system, NEM cannot be used to suppress endogenous GPI biosynthesis; consequently, some endogenous GPI glycolipids (H2 and H5) are formed as well as Dol-P-[3H]Man (Figure 7A, lane 1). The addition of GlcNAc-PI leads to the formation of significantly more H2 and H5 (Figure 7A, lanes 2 and 6), as previously described (Sharma et al., 1997; Smith et al., 1997b). The Rf of H5 produced from exogenous GlcNAc-PI is lower than that of the endogenous H5 (Figure 7A, compare lanes 1 and 2) because the dipalmitoylglycerol lipid component of the synthetic substrate is less hydrophobic than the glycerolipid component of endogenous GPI intermediates. The addition of GlcNBz-PI, GlcNCONH2-PI or GlcNMe2-PI did not stimulate the synthesis of H2 and H5 (data not shown), indicating that these compounds are not substrates for the de-N-acetylase. The result with GlcNBz-PI is in contrast to that for the trypanosome cell-free system (Figure 3A, lane 4), demonstrating that GlcNBz-PI is a parasite-specific substrate.
Fig. 7. Inhibition of HeLa cell GPI biosynthesis by GlcNR-PI analogues. (A) The HeLa cell-free system was incubated with GDP-[3H]Man alone (lane 1) or with GlcNAc-PI (lanes 2 and 6), or with GlcNAc-PI after pre-incubation with the compounds indicated (lanes 3–5). The products are: DPM, dolichol-phosphate-mannose; H2, Man1GlcN-(acyl)PI; ENDO and EXOG H5, endogenous and exogenous EtNP-Man1GlcN-(acyl)PI derived from endogenous GlcN-PI or exogenous synthetic GlcNAc-PI, respectively. (B) The HeLa cell-free system was incubated with GlcN[3H]Ac-PI alone (squares) or in the presence of equimolar GlcNAc-PI (diamonds), GlcNBn-PI (circles), GlcNMe2-PI (triangles) or GlcNCONH2-PI (crosses), and the release of [3H]acetate was measured against time.
As with the trypanosomal system, pre-incubation of the HeLa cell-free system with GlcNBn-PI, GlcNMe2-PI and GlcNCONH2-PI inhibited the processing of exogenous GlcNAc-PI, the former the least (Figure 7A, lanes 3–5). However, none of them inhibited the formation of endogenous H5, suggesting that they inhibited the de-N-acetylase rather than MT-1. The inhibitory effects could not be attributed to the concentration of synthetic lipid in the system (totalling 200 µM) since the addition of 200 µM GlcNAc-PI showed greater stimulation of the biosynthetic pathway than did 100 µM GlcNAc-PI (Figure 7A, compare lanes 2 and 6).
Inhibition of GPI biosynthesis in the HeLa cell-free system by GlcNRPI analogues was investigated further using the direct de-N-acetylase assay. The membranes were pre-incubated with the GlcNR-PI analogues, and the rate of [3H]acetate released from GlcN[3H]Ac-PI was measured (Figure 7B). In the presence of an equimolar amount of unlabelled GlcNAc-PI, the rate of [3H]acetate released was halved, whereas pre-incubation with GlcNBn-PI had no effect, indicating that this analogue did not compete efficiently with GlcN[3H]Ac-PI for the HeLa de-N-acetylase. However, in agreement with the results with the trypanosomal enzyme, GlcNCONH2-PI and GlcNMe2-PI were potent inhibitors of the HeLa de-N-acetylase. This suggests that although the parasite and human enzymes show different substrate specificities, they operate by much the same reaction mechanism.
Design of parasite-specific suicide substrate inhibitors
The selective recognition by parasite GlcNAc-PI de-N-acetylase of GlcNAc-β-PI (this study) and GlcN-(2-O-octyl)-PI (Sharma et al., 1999) was combined with the suicide substrate properties of GlcNCONH2-PI to produce the compounds 2-deoxy-2-ureido-d-Glcβ1-6d-myo-inositol-1-HPO4-sn-1,2-dipalmitoylglycerol (GlcNCONH2-β- PI) and 2-deoxy-2-ureido-d-Glcα1-6d-(2-O-octyl)myo-inositol-1-HPO4-sn-1,2-dipalmitoylglycerol [GlcNCO NH2-(2-O-octyl)-PI].
The non-selective inhibitor GlcNCONH2-PI and the two novel compounds were tested as inhibitors of the trypanosomal and HeLa cell enzymes using the direct assay (measuring release of [3H]acetate from GlcN[3H]Ac-PI). In agreement with the indirect coupled assay (Figure 4C), the IC50 for GlcNCONH2-PI against the trypanosome enzyme was ∼8 nM (Figure 8A). This compound was less active against the HeLa enzyme (IC50 between 0.1 and 1 µM), but, nevertheless, inhibitory (Figure 8B). In contrast, while GlcNCONH2-β-PI and GlcNCONH2-(2-O-octyl)-PI were equally potent inhibitors as GlcNCONH2-PI for the trypanosomal enzyme (Figure 8C and E), neither of these compounds inhibited the HeLa cell enzyme at concentrations up to 100 µM (Figure 8D and F).
Fig. 8. Selective inhibition of the trypansome de-N-acetylase by GlcNCONH2-β-PI and GlcNCONH2-(2-O-octyl)-PI. The trypanosome (A, C and E) and HeLa cell (B, D and F) cell-free systems were incubated with GlcN[3H]Ac-PI after pre-incubation with various concentrations of GlcNCONH2-PI (A and B), GlcNCONH2-β-PI (C and D) or GlcNCONH2-(2-O-octyl)-PI (E and F), and the release of [3H]acetate was measured against time.
Summary
Based on the work in this paper and by others (Milne et al., 1994; Sharma et al., 1997, 1999; Smith et al., 1997b, 1999, 2000), certain structural features have been identified as being responsible for the interactions between the trypanosomal de-N-acetylase and GlcNAc-PI. The essential features are: (i) the phosphodiester group appears to be important for recognition and may interact with positively charged residue(s) on the enzyme; (ii) the 3′-OH group of the GlcNAc residue appears to be essential for substrate recognition, suggesting that it acts as a hydrogen-bond acceptor or donor; (iii) the 4′-OH group of the GlcNAc residue is not essential for substrate recognition, but epimerization, methylation or mannosylation of this position reduces or abolishes substrate turnover; (iv) the 6′-OH group of the GlcNAc residue is not essential for substrate recognition; (v) the 2-, 3-, 4- and 5-OH groups of the d-myo-inositol residue do not appear to be involved in substrate recognition by the de-N-acetylase, hence the ability of the parasite enzyme to act on GlcNAc-[L]-PI, GlcNAc-β-PI and GlcNAc-(2-O-alkyl)PI. The latter point illustrates a fundamental difference between the parasite and human (HeLa) de-N-acetylases; namely, that the human enzyme is configurationally and anomerically specific for the GlcNAcα1-6d-myo-inositol component of GlcNAc-PI, whereas the parasite enzyme is not.
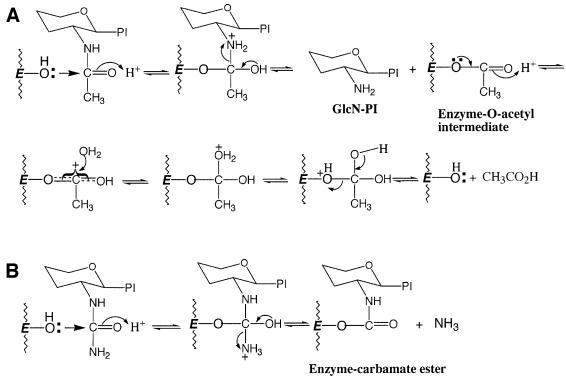
The irreversible inhibition of the trypanosomal GlcNAc-PI de-N-acetylase by GlcNCONH2-PI, and reactivation by the nucleophiles hydroxylamine, hydrazine and DTT at neutral pH, can reasonably be explained by postulating a reaction mechanism that involves either a hydroxyl or sulfhydryl group at the active site. Attack on the carbonyl group of the N-acetyl function of the GlcNAc residue by a Ser/Thr group, for example, would liberate GlcN-PI and generate an O-acetyl-enzyme intermediate (Figure 9A), which is subsequently hydrolysed to liberate acetic acid and regenerate the Ser/Thr OH group. However, loss of ammonia from the tetrahedral intermediate formed with GlcNCONH2-PI (Figure 9B) would still leave the enzyme covalently bound as a carbamate ester to the substrate analogue. This model would account for the reactivation of the enzyme on treatment with hyrdoxylamine, hydrazine and DTT, requiring nucleophilic attack on the carbonyl group of the carbamate ester to lead to C–O bond cleavage and restoration of the Ser/Thr OH group at the active site. It would also apply should the initial attack on GlcNCONH2-PI involve a Cys SH group, culminating in the formation of an enzyme-linked thiocarbamate ester. However, the insensitivity of the de-N-acetylase to sulfhydryl alkylating reagents such as iodoacetic acid, iodoacetamide and NEM suggests that a hydroxy-amino acid may be more likely. Site-directed mutagenesis studies will be performed to see which residues are essential for enzymatic activity.
Fig. 9. A proposed mechanism for the action of GlcNAc-PI de-N-acetylase. (A) A proposed mechanism for cleavage of the acetyl function from GlcNAc-PI via the formation of an O-acetyl enzyme intermediate. (B) A proposed mechanism for inhibition of the de-N-acetylase by GlcNCONH2-PI. Note: the data do not exclude the possibility that the active site residue is a hydroxy-amino acid instead of Cys.
Finally, we exploited the more fastidious nature of the human de-N-acetylase (Sharma et al., 1999; Smith et al., 1999; this study) by combining features that provide specificity for the parasite de-N-acetylase (i.e. β anomeric linkage or 2-O-alkylation of the d-myo-inositol residue) with the N-acyl function of the non-specific inhibitor GlcNCONH2-PI to produce GlcNCONH2-β-PI and GlcNCONH2-(2-O-octyl)-PI. These compounds, which are active in vitro, are leads for the design and synthesis of parasite-specific inhibitors that may be active in vivo. Such compounds could impact significantly on the development of trypanocidal drugs, since the GPI biosynthetic pathway has been validated as a therapeutic target against African trypanosomes (Ferguson, 2000; Nagamune et al., 2000).
Materials and methods
Substrates and substrate analogues
The compounds shown in Figure 1 were synthesized as previously described (Cottaz et al., 1993; Crossman et al., 1997, 1999; Borissow et al., 2001; Dix et al., 2001), except for d-GlcNα1-6l-2-O-methyl-myo-inositol-1-HPO4-sn-1,2-dipalmitoylglycerol (GlcN-[L]-(2-O-methyl)- PI), which was prepared in a similar manner to the corresponding d-myo-inositol analogue (Crossman et al., 1997), and d-GalNα1-6d-myo-inositol-1-HPO4-sn-1,2-dipalmitoylglycerol (GalN-PI), d-ManNα1-6d-myo-inositol-1-HPO4-sn-1,2-dipalmitoylglycerol (ManN-PI), d-Glc Nβ1-6d-myo-inositol-1-HPO4-sn-1,2-dipalmitoylglycerol (GlcN-β-PI), d-GlcNα1-6d-myo-inositol-1-HPO4-octadecyl (GlcN-I-P-C18) and d-GlcNα1-6d-myo-inositol-1-HPO4-octyl (GlcN-I-P-C8) (A.Crossman, M.J.Paterson and J.S.Brimacombe, details to be published elsewhere). N-acetyl derivatives of the compounds were prepared by standard procedures (Smith et al., 1996).
Preparation of GlcNR-PI substrate analogues
The GlcNR-PI substrate analogues GlcNBz-PI, GlcNBzc-PI and GlcNMal-PI were prepared by dissolving GlcN-PI in tetrahydrofuran/methanol (1:1, v/v) containing 5% triethylamine, and then adding half a volume of tetrahydrofuran containing an excess of either benzoic, phthalic or maleic anhydrides, respectively. After 15 min at 0°C, the reaction was quenched with an equal volume of water. GlcPtu-PI was prepared in a similar manner using pyridine and phenyl isothiocyanate. GlcNMe2-PI was prepared by reductive methylation: to a solution of GlcN-PI in propan-1-ol/0.5 M HEPES pH 7.4 (1:1, v/v) was added formaldehyde (5%, v/v) followed by sodium cyanoborohydride (20 mM) and incubated at room temperature (RT) for 1 h. GlcNBn-PI was prepared in a similar manner to GlcNMe2-PI, except that benzaldehyde was used instead of formaldehyde. GlcNCONH2-PI analogues were prepared by mixing a solution of the corresponding GlcN-PI analogue in propan-1-ol/0.5 M HEPES pH 7.4 (1:1, v/v) with an equal volume of 10 mM sodium cyanate dissolved in the same buffer and incubated at RT for 2 h. After removal of the solvent, the GlcNR-PI substrate analogues were purified using 100 mg Isolute cartridges, as described below for GlcN[3H]Ac-PI. The identity and purity of each substrate analogue were assessed by negative-ion electrospray mass spectrometry (Figure 1), and the concentration of each stock solution was ascertained by measuring the inositol content by selected ion-monitoring gas chromatography–mass spectrometry (Ferguson, 1994).
N-[3H]acetylation and purification of the substrate analogues
GlcN-PI and analogues thereof were N-acetylated with [3H]acetic anhydride as now described for GlcN-PI. GlcN-PI (10 nmol) in 150 µl of dry tetrahydrofuran/methanol (2:1, v/v) containing 5 µl of triethyl amine was treated with 100 nmol [3H]acetic anhydride (50.0 Ci/mmol) for 30 min at RT, followed by 50 nmol of non-radioactive acetic anhydride for 30 min at RT. The reaction was quenched with 100 µl of water, whereafter GlcN[3H]Ac-PI was purified by diluting the reaction mixture with 5 ml of 5% propan-1-ol in 100 mM ammonium acetate and loading onto a pre-equilibrated 500 mg C8 Isolute cartridge. The cartridge was washed with the same buffer (≥200 ml) until radioactivity in the eluate had reached background levels. GlcN[3H]Ac-PI was eluted from the cartridge with 10 ml of 80% propan-1-ol in 100 mM ammonium acetate, which was evaporated to dryness. The residue was desalted by dissolution in butan-1-ol and washing the organic solution with water. [Analogues with short lipid chains (e.g. GlcN[3H]Ac-I-P-C8) were freeze-dried three times and residual acetic acid was removed by co-evaporation with toluene.] Purified GlcN[3H]Ac-PI was dissolved in 1 ml of butan-1-ol, and aliquots used to determine the inositol content and specific activity. The GlcN[3H]Ac-PI analogues were diluted with the corresponding non-radioactive compound to give a final specific activity of 18.2 mCi/mmol. The GlcN[3H]Ac-PI substrate analogues were analysed by HPTLC, to determine their radiochemical purity (data not shown). The deoxy-GlcN[3H]Ac-PIs and GlcN[3H]Ac4Me-PI have slightly higher Rf values than GlcN[3H]Ac-PI, as have GlcN[3H]Ac-β-PI, ManN[3H]Ac-PI and GalN[3H]Ac-PI. GlcN[3H]Ac-[L]-PI has the same Rf value as GlcN[3H]Ac-PI, while that of GlcN[3H]Ac-[L]-(2-O-methyl)-PI is slightly higher. The lipid-modified GlcN[3H]Ac-PI analogues have significantly different Rf values due to differences in the lipid component.
Preparation of trypanosomal and HeLa membranes
Trypanosoma brucei and HeLa cell membranes (cell-free systems) were prepared as described previously (Masterson et al., 1989; Smith et al., 1996, 1997b), except that HeLa aliquots were frozen at 1 × 107 and 2 × 107 cell equivalents/ml for GDP-[3H]Man labelling and GlcN[3H]Ac-PI de-N-acetylase assays, respectively.
Direct de-N-acetylase assay
Washed trypanosome membranes were suspended in incorporation buffer (Smith et al., 1996, 1997b) supplemented with NEM. The lysate was sonicated and 40 µl aliquots (5 × 107 cell equivalents) were added to reaction tubes containing either 0.5 nmol (10 000 c.p.m.) of GlcN[3H]Ac-PI or a substrate analogue in 10 µl of incorporation buffer supplemented with n-octyl β-d-glucopyranoside (n-OG) (0.3% w/v) and GDP-Man (1 mM), unless stated otherwise. After brief sonication, the reaction mixtures were incubated at 30°C.
HeLa cell lysate was thawed and supplemented as previously described (Smith et al., 1997b) with 100 µM CoA, ATP-regenerating system (100 µM ATP, 5 mM phosphocreatine and 5 U of creatine phosphokinase) and 1 mM GDP-Man, unless stated otherwise. Aliquots of 100 µl (2 × 106 cell equivalents) were added to reaction tubes containing either 1.5 nmol (30 000 c.p.m.) of GlcN[3H]Ac-PI or a substrate analogue, sonicated briefly and incubated at 35°C.
Reactions were terminated by the addition of 50 µl of propan-1-ol, followed by vortexing and snap-freezing. Each GlcN[3H]Ac-PI analogue was studied at least twice with triplicate samples at each time interval. Samples were thawed, adjusted to 1 ml with 100 µl of 1 M ammonium acetate and water, and then applied to a pre-equilibrated 100 mg C8 Isolute cartridge. The cartridge was washed with 2 ml of 5% propan-1-ol in 100 mM ammonium acetate, and the eluate (containing the released [3H]acetate) was counted for radioactivity. The unreacted GlcN[3H]Ac-PI analogue was then eluted with 2 ml of 80% propan-1-ol in 100 mM ammonium acetate, and the eluate was counted for radioactivity. All input radioactivity was accounted for.
Inhibition assays were conducted the same way, except that the membranes were pre-incubated with potential inhibitors for 5 min prior to being added to GlcN[3H]Ac-PI.
Indirect de-N-acetylase assay
The indirect assay detects the transfer of [3H]Man to de-N-acylated products and has been described previously (Smith et al., 1996, 1997b). Inhibition assays were conducted in a similar manner, except that the membranes were pre-incubated with potential inhibitors for 5 min prior to being added to GlcN-PI or GlcNAc-PI. Some inhibition assays (such as those in Figure 6A) were conducted by pre-incubation of the membranes (5 × 107 cell equivalents) with GDP-[3H]Man (0.5 µCi) and a potential inhibitor for 10 min at RT, in order to allow Dol-P-[3H]Man to be formed and inhibition to take place. Thereafter, the membranes were pelleted (16 000 g for 2 min at 4°C), resuspended in 200 µl of fresh incorporation buffer supplemented with NEM, sonicated briefly, pelleted again and resuspended in 25 µl of fresh 2× incorporation buffer supplemented with both NEM and n-OG (0.3%, w/v). The suspensions were added to an equal volume of GlcN-PI (50 µM) in n-OG (0.3%, w/v), sonicated briefly and incubated at 30°C for 1 h.
Reactivation studies
The reversibility of the inhibition by GlcNCONH2-PI was studied using an assay similar to that used to obtain the data for Figure 6B. Membranes (5 × 107 cell equivalents) were incubated in incorporation buffer with GlcNCONH2-PI (2.5 µM) for 5 min to allow inhibition of the de-N-acetylase to take place. Thereafter, the membranes were pelleted and the activity of the enzyme–inhibitor complex was analysed after brief sonication and incubation on ice for 15 min with either 100 µl of 25 mM DTT or 100 µl of 100 mM hydroxylamine or 100 µl 25 mM hydrazine (all in incorporation buffer). Following incubation, the membranes were pelleted, washed with 200 µl of fresh incorporation buffer, sonicated briefly, pelleted again and resuspended in 25 µl of fresh 2× incorporation buffer supplemented with both NEM and n-OG (0.3% w/v). The suspensions were added to an equal volume of GDP-[3H]Man (0.5 µCi) and GlcN-PI (50 µM) in n-OG (0.3% w/v), sonicated and incubated at 30°C for 1 h.
Enzymatic and chemical treatments of radiolabelled glycolipids and HPTLC
Digestions with jack bean α-mannosidase, glycosylphosphatidyl-inositol specific phospholipase D and phosphatidylinositol-specific phospholipase C, and base hydrolysis, deamination, N-acetylation and HPTLC were performed as previously described (Güther et al., 1994; Smith et al., 1996, 1997b).
Acknowledgments
Acknowledgements
This work was supported by a Wellcome Trust Programme Grant (054491); A.D., M.J.P. and C.N.B. thank the BBRSC for studentships.
References
- Borissow C.N., Smith,T.K., Ferguson,M.A.J. and Brimacombe,J.S. (2001) Synthesis of 3′-, 4′- and 6′-deoxy and other analogues of d-glucosaminylphosphatidylinositol. Tetrahedron Lett., 42, 121–123. [Google Scholar]
- Chen R., Walter,E.I., Parker,G., Lapurga,J.P., Millan,J.L., Ikehara,Y., Udenfriend,S. and Medof,M.E. (1998) Mammalian glycophosphat idylinositol anchor transfer to proteins and posttransfer deacylation. Proc. Natl Acad. Sci. USA, 95, 9512–9517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottaz S., Brimacombe,J.S. and Ferguson,M.A.J. (1993) Parasite glycoconjugates. Part 1. Synthesis of some early and related inter mediates in the biosynthetic pathway of glycosyl-phosphatidylinositol membrane anchors. J. Chem. Soc. Perkin Trans., 1, 2945–2951. [Google Scholar]
- Cross G.A.M. (1996) Antigenic variation in trypanosomes: secrets surface slowly. BioEssays, 18, 283–291. [DOI] [PubMed] [Google Scholar]
- Crossman A., Brimacombe,J.S. and Ferguson,M.A.J. (1997) Parasite glycoconjugates. Part 7. Synthesis of further substrate analogues of early intermediates in the biosynthetic pathway of glycosyl phosphatidylinositol membrane anchors. J. Chem. Soc. Perkin Trans., 1, 2769–2774. [Google Scholar]
- Crossman A., Brimacombe,J.S., Ferguson,M.A.J. and Smith,T.K. (1999) Synthesis of some second-generation substrate analogues of early intermediates in the biosynthetic pathway of glycosylphosphatidyl inositol membrane anchors. Carbohydr. Res., 321, 42–51. [DOI] [PubMed] [Google Scholar]
- Dix A.P., Borissow,C.N., Ferguson,M.A.J. and Brimacombe,J.S. (2001) Analogues of d-glucosaminylphosphatidylinositol: synthesis of the glycosyl donors. Tetrahedron Lett., 42, 117–119. [Google Scholar]
- Doering T.L., Masterson,W.J., Englund,P.T. and Hart,G.W. (1989) Biosynthesis of the glycosylphosphatidylinositol membrane anchor of the trypanosome variant surface glycoprotein. Origin of the non-acetylated glucosamine. J. Biol. Chem., 264, 11168–11173. [PubMed] [Google Scholar]
- Doerrler W.T., Ye,J., Falck,J.R. and Lehrman,M.A. (1996) Acylation of glucosaminyl phosphatidylinositol revisited. Palmitoyl-CoA dependent palmitoylation of the inositol residue of a synthetic dioctanoyl glucosaminyl phosphatidylinositol by hamster membranes permits efficient mannosylation of the glucosamine residue. J. Biol. Chem., 271, 27031–27038. [DOI] [PubMed] [Google Scholar]
- Ferguson M.A.J. (1994) GPI membrane anchors: isolation and analysis. In Fukuda,M. and Kobata,A. (eds), Glycobiology: A Practical Approach. IRL at Oxford University Press, Oxford, UK, pp. 349–383.
- Ferguson M.A.J. (1999) The structure, biosynthesis and functions of glycosylphosphatidylinositol anchors and the contributions of trypanosome research. J. Cell Sci., 112, 2799–2808. [DOI] [PubMed] [Google Scholar]
- Ferguson M.A.J. (2000) Glycosylphosphatidylinositol biosynthesis validated as a drug target for African sleeping sickness. Proc. Natl Acad. Sci. USA, 97, 10673–10675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson M.A.J. et al. (1999) The GPI biosynthetic pathway as a therapeutic target for African sleeping sickness. Biochim. Biophys. Acta, 1455, 327–340. [DOI] [PubMed] [Google Scholar]
- Flury I., Benachour,A. and Conzelmann,A. (2000) YLL031c belongs to a novel family of membrane proteins involved in the transfer of ethanolamine phosphate onto the core structure of glycosyl phosphatidylinositol anchors in yeast. J. Biol. Chem., 275, 24458–24465. [DOI] [PubMed] [Google Scholar]
- Garg N., Postan,M., Mensa-Wilmot,K. and Tarleton,R.L. (1997) Glycosylphosphatidylinositols are required for the development of Trypanosoma cruzi amastigotes. Infect. Immun., 65, 4055–4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerold P., Schofield,L., Blackman,M.J., Holder,A.A. and Schwarz,R.T. (1996) Structural analysis of the glycosylphosphatidylinositol membrane anchor of the merozoite surface proteins-1 and -2 of Plasmodium falciparum. Mol. Biochem. Parasitol., 75, 131–143. [DOI] [PubMed] [Google Scholar]
- Gerold P., Jung,N., Azzouz,N., Freiberg,N., Kobe,S. and Schwarz,R.T. (1999) Biosynthesis of glycosylphosphatidylinositols of Plasmodium falciparum in a cell-free incubation system: inositol acylation is needed for mannosylation of glycosylphosphatidylinositols. Biochem. J., 344, 731–738. [PMC free article] [PubMed] [Google Scholar]
- Güther M.L. and Ferguson,M.A.J. (1995) The role of inositol acylation and inositol deacylation in GPI biosynthesis in Trypanosoma brucei. EMBO J., 14, 3080–3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güther M.L., Masterson,W.J. and Ferguson,M.A.J. (1994) The effects of phenylmethylsulfonyl fluoride on inositol-acylation and fatty acid remodeling in African trypanosomes. J. Biol. Chem., 269, 18694–18701. [PubMed] [Google Scholar]
- Heise N., Raper,J., Buxbaum,L.U., Peranovich,T.M. and de Almeida,M.L. (1996) Identification of complete precursors for the glycosylphosphatidylinositol protein anchors of Trypanosoma cruzi. J. Biol. Chem., 271, 16877–16887. [DOI] [PubMed] [Google Scholar]
- Hirose S., Prince,G.M., Sevlever,D., Ravi,L., Rosenberry,T.L., Ueda,E. and Medof,M.E. (1992) Characterization of putative glycoinositol phospholipid anchor precursors in mammalian cells. Localization of phosphoethanolamine. J. Biol. Chem., 267, 16968–16974. [PubMed] [Google Scholar]
- Ilgoutz S.C., Zawadzki,J.L., Ralton,J.E. and McConville,M.J. (1999) Evidence that free GPI glycolipids are essential for growth of Leishmania mexicana. EMBO J., 18, 2746–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T. and Inoue,N. (2000) Dissecting and manipulating the pathway for glycosylphosphatidylinositol-anchor biosynthesis. Curr. Opin. Chem. Biol., 4, 632–638. [DOI] [PubMed] [Google Scholar]
- Masterson W.J., Doering,T.L., Hart,G.W. and Englund,P.T. (1989) A novel pathway for glycan assembly: biosynthesis of the glycosyl-phosphatidylinositol anchor of the trypanosome variant surface glycoprotein. Cell, 56, 793–800. [DOI] [PubMed] [Google Scholar]
- Masterson W.J., Raper,J., Doering,T.L., Hart,G.W. and Englund,P.T. (1990) Fatty acid remodeling: a novel reaction sequence in the biosynthesis of trypanosome glycosyl-phosphatidylinositol membrane anchors. Cell, 62, 73–80. [DOI] [PubMed] [Google Scholar]
- McConville M.J. and Menon,A.K. (2000) Recent developments in the cell biology and biochemistry of glycosylphosphatidylinositol lipids. Mol. Membr. Biol., 17, 1–16. [DOI] [PubMed] [Google Scholar]
- Menon A.K., Mayor,S. and Schwarz,R.T. (1990a) Biosynthesis of glycosyl-phosphatidylinositol lipids in Trypanosoma brucei: involvement of mannosyl-phosphoryldolichol as the mannose donor. EMBO J., 9, 4249–4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon A.K., Schwarz,R.T., Mayor,S. and Cross,G.A.M. (1990b) Cell-free synthesis of glycosyl-phosphatidylinositol precursors for the glycolipid membrane anchor of Trypanosoma brucei variant surface glycoproteins. Structural characterization of putative biosynthetic intermediates. J. Biol. Chem., 265, 9033–9042. [PubMed] [Google Scholar]
- Milne K.G., Ferguson,M.A.J. and Masterson,W.J. (1992) Inhibition of the GlcNAc transferase of glycosylphosphatidylinositol anchor biosynthesis in African trypanosomes. Eur. J. Biochem., 208, 309–314. [DOI] [PubMed] [Google Scholar]
- Milne K.G., Field,R.A., Masterson,W.J., Cottaz,S., Brimacombe,J.S. and Ferguson,M.A.J. (1994) Partial purification and characterisation of the N-acetylglucosaminyl-phosphatidylinositol de-N-acetylase of glycosylphosphatidylinositol anchor biosynthesis in African trypanosomes. J. Biol. Chem., 269, 16403–16408. [PubMed] [Google Scholar]
- Moody-Haupt S., Patterson,J.H., Mirelman,D. and McConville,M.J. (2000) The major surface antigens of Entamoeba histolytica trophozoites are GPI-anchored proteophosphoglycans. J. Mol. Biol., 297, 409–420. [DOI] [PubMed] [Google Scholar]
- Morita Y.S., Acosta-Serrano,A. and Englund,P.T. (2000a) The biosynthesis of GPI anchors. In Ernst,P., Sinay,P. and Hart,G. (eds), Oligosaccharides in Chemistry and Biology—A Comprehensive Handbook. Wiley-VCH, Weinheim, Germany, pp. 417–433.
- Morita Y.S., Acosta-Serrano,A., Buxbaum,L.U. and Englund,P.T. (2000b) Glycosyl-phosphatidylinositol myristoylation in African trypanosomes. New intermediates in the pathway for fatty acid remodeling. J. Biol. Chem., 275, 14147–14154. [DOI] [PubMed] [Google Scholar]
- Nagamune K. et al. (2000) Critical roles of glycosylphosphatidylinositol for Trypanosoma brucei. Proc. Natl Acad. Sci. USA, 97, 10336–10341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura N., Inoue,N., Watanabe,R., Takahashi,M., Takeda,J., Stevens,V.L. and Kinoshita,T. (1997) Expression cloning of PIG-L, a candidate N-acetylglucosaminyl-phosphatidylinositol deacetylase. J. Biol. Chem., 272, 15834–15840. [DOI] [PubMed] [Google Scholar]
- Puoti A. and Conzelmann,A. (1993) Characterization of abnormal free glycophosphatidylinositols accumulating in mutant lymphoma cells of classes B, E, F and H. J. Biol. Chem., 268, 7215–7224. [PubMed] [Google Scholar]
- Ralton J.E. and McConville,M.J. (1998) Delineation of three pathways of glycosylphosphatidylinositol biosynthesis in Leishmania mexicana. Precursors from different pathways are assembled on distinct pools of phosphatidylinositol and undergo fatty acid remodeling. J. Biol. Chem., 273, 4245–4257. [DOI] [PubMed] [Google Scholar]
- Sharma D.K., Smith,T.K., Crossman,A., Brimacombe,J.S. and Ferguson,M.A.J. (1997) Substrate specificity of the N-acetylglucosaminyl-phosphatidylinositol de-N-acetylase of glycosylphosphatidylinositol membrane anchor biosynthesis in African trypanosomes and human cells. Biochem. J., 328, 171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D.K., Smith,T.K., Weller,C.T., Crossman,A., Brimacombe,J.S. and Ferguson,M.A.J. (1999) Differences between the trypanosome and human GlcNAc-PI de-N-acetylases of glycosylphosphatidyl inositol membrane anchor biosynthesis. Glycobiology, 9, 415–422. [DOI] [PubMed] [Google Scholar]
- Singh B.N., Beach,D.H., Lindmark,D.G. and Costello,C.E. (1994) Identification of the lipid moiety and further characterization of the novel lipophosphoglycan-like glycoconjugates of Trichomonas vaginalis and Trichomonas foetus. Arch. Biochem. Biophys., 309, 273–280. [DOI] [PubMed] [Google Scholar]
- Smith T.K., Cottaz,S., Brimacombe,J.S. and Ferguson,M.A.J. (1996) Substrate specificity of the dolichol phosphate mannose:glucosaminyl phosphatidylinositol α1-4 mannosyltransferase of the glycosylphos phatidylinositol biosynthetic pathway of African trypanosomes. J. Biol. Chem., 271, 6476–6482. [DOI] [PubMed] [Google Scholar]
- Smith T.K., Milne,F.C., Sharma,D.K., Crossman,A., Brimacombe,J.S. and Ferguson,M.A.J. (1997a) Early steps in glycosylphos phatidylinositol biosynthesis in Leishmania major. Biochem. J., 326, 393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T.K., Sharma,D.K., Crossman,A., Dix,A., Brimacombe,J.S. and Ferguson,M.A.J. (1997b) Parasite and mammalian GPI biosynthetic pathways can be distinguished using synthetic substrate analogues. EMBO J., 16, 6667–6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T.K., Sharma,D.K., Crossman,A., Brimacombe,J.S. and Ferguson,M.A.J. (1999) Selective inhibitors of the glycosylphosphatidylinositol biosynthetic pathway of Trypanosoma brucei.EMBO J., 18, 5922–5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T.K., Paterson,M.J., Crossman,A., Brimacombe,J.S. and Ferguson,M.A.J. (2000) Parasite-specific inhibition of the glycosylphos phatidylinositol biosynthetic pathway by stereoisomeric substrate analogues. Biochemistry, 39, 11801–11807. [DOI] [PubMed] [Google Scholar]
- Striepen B., Zinecker,C.F., Damm,J.B., Melgers,P.A., Gerwig,G.J., Koolen,M., Vliegenthart,J.F.G., Dubremetz,J.F. and Schwarz,R.T. (1997) Molecular structure of the low molecular weight antigen of Toxoplasma gondii: a glucose α1-4 N-acetylgalactosamine makes free glycosyl-phosphatidylinositols highly immunogenic. J. Mol. Biol., 266, 797–813. [DOI] [PubMed] [Google Scholar]
- Striepen B., Dubremetz,J.-F. and Schwarz,R.T. (1999) Glucosylation of glycosylphosphatidylinositol membrane anchors: identification of uridine diphosphate-glucose as direct donor for side chain modification in Toxoplasma gondii using carbohydrate analogues. Biochemistry, 38, 1478–1487. [DOI] [PubMed] [Google Scholar]
- Sütterlin C., Horvath,A., Gerold,P., Schwarz,R.T., Wang,Y., Dreyfuss,M. and Riezman,H. (1997) Identification of a species-specific inhibitor of glycosylphosphatidylinositol synthesis. EMBO J., 16, 6374–6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sütterlin C., Escribano,M.V., Gerold,P., Maeda,Y., Mazon,M.J., Kinoshita,T., Schwarz,R.T. and Riezman,H. (1998) Saccharomyces cerevisiae GPI10, the functional homologue of human PIG-B, is required for glycosylphosphatidylinositol-anchor synthesis. Biochem. J., 332, 153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe R., Inoue,N., Westfall,B., Taron,C.H., Orlean,P., Takeda,J. and Kinoshita,T. (1998) The first step of glycosylphosphatidylinositol biosynthesis is mediated by a complex of PIG-A, PIG-H, PIG-C and GPI1. EMBO J., 17, 877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe R., Ohishi,K., Maeda,Y., Nakamura,N. and Kinoshita,T. (1999) Mammalian PIG-L and its yeast homologue Gpi12p are N-acetylglucosaminylphosphatidylinositol de-N-acetylases essential in glycosylphosphatidylinositol biosynthesis. Biochem. J., 339, 185–192. [PMC free article] [PubMed] [Google Scholar]
- Watanabe R., Murakami,Y., Marmor,M.D., Inoue,N., Maeda,Y., Hino,J., Kangawa,K., Julius,M. and Kinoshita,T. (2000) Initial enzyme for glycosylphosphatidylinositol biosynthesis requires PIG-P and is regulated by DPM2. EMBO J., 19, 4402–4411. [DOI] [PMC free article] [PubMed] [Google Scholar]