Abstract
It has recently been shown that mononuclear cells from murine skeletal muscle contain the potential to repopulate all major peripheral blood lineages in lethally irradiated mice, but the origin of this activity is unknown. We have fractionated muscle cells on the basis of hematopoietic markers to show that the active population exclusively expresses the hematopoietic stem cell antigens Sca-1 and CD45. Muscle cells obtained from 6- to 8-week-old C57BL/6-CD45.1 mice and enriched for cells expressing Sca-1 and CD45 were able to generate hematopoietic but not myogenic colonies in vitro and repopulated multiple hematopoietic lineages of lethally irradiated C57BL/6-CD45.2 mice. These data show that muscle-derived hematopoietic stem cells are likely derived from the hematopoietic system and are a result not of transdifferentiation of myogenic stem cells but instead of the presence of substantial numbers of hematopoietic stem cells in the muscle. Although CD45-negative cells were highly myogenic in vitro and in vivo, CD45-positive muscle-derived cells displayed only very limited myogenic activity and only in vivo.
Stem cells are defined by their ability to self renew and differentiate into the cell types of their derivative tissue. Traditionally, it has been assumed that a stem cell derived from adult tissues can give rise only to progeny specific to that tissue type. However, this dogma has been challenged recently by a series of studies that suggest that adult tissue-derived stem cells may have the potential to differentiate into disparate cell types. For example, purified hematopoietic stem cells (HSCs), derived from whole bone marrow (WBM), have been shown to contribute to regenerating skeletal muscle (1), cardiac muscle (2), liver (3), and multiple epithelial tissues (4). In addition, stem cells from other tissues have also been proposed to differentiate outside their tissue of origin (5, 6). Although these studies have provoked new critical thinking about stem cell differentiation capacity, definitive proof of transdifferentiation remains to be established at a clonal level.
Two recent studies focused on the ability of muscle-derived cells to repopulate WBM in lethally irradiated mice. Gussoni et al. (1) reported that muscle cells fractionated on the basis of their efflux of Hoechst dye could rescue lethally irradiated recipients. Similarly, Jackson et al. (7) reported that unfractionated mononuclear muscle cells could repopulate all major blood lineages of lethally irradiated mice up to 12 weeks after transplant. In addition, when bone marrow from engrafted animals was transplanted into secondary recipients, their peripheral blood was also repopulated with muscle-derived cells, demonstrating the important property of self renewal (7).
Satellite cells are a potent myogenic stem cell population that resides in the muscle and are responsible for postnatal muscle regeneration and growth (8, 9). We proposed that satellite cells accounted for muscle-derived hematopoietic activity via transdifferentiation when introduced into the regenerative environment of bone marrow after the severe injury of myeloablative irradiation (7). However, two other models could account for the activity. Skeletal muscle could contain multiple distinct stem cell populations or a primitive precursor capable of generating both HSCs and satellite cells.
To begin to distinguish between these possibilities, we sought to further characterize the phenotype of the muscle-derived HSC (ms-HSC). Gussoni et al. reported that the muscle side population (SP) contained ms-HSCs (1). The SP phenotype is a result of efficient Hoechst dye efflux and has previously been used to purify HSCs (10). However, this phenotype does not provide clues of cellular origin. Therefore, we chose to evaluate the ms-HSCs on the basis of expression of hematopoietically relevant cell surface markers. If ms-HSCs are derived ultimately from the hematopoietic system, they should express HSC markers, whereas myogenic stem cells should not.
Murine HSCs have been extensively characterized for the expression of specific cell surface markers (11–13). Stem cell antigen-1 (Sca-1) is a marker of murine bone marrow HSCs (14). CD45 is a cell surface tyrosine phosphatase that has been found in several isoforms on all nucleated cells of hematopoietic origin including HSCs but not on any nonhematopoietic cells (15). CD45 is therefore considered to be an exclusive marker of the hematopoietic lineage. We reasoned that Sca-1 and CD45 could be used to distinguish between myogenic stem cells and HSCs. Here we show that the ms-HSCs active both in vitro and in vivo express CD45 and Sca-1. We also show that CD45-negative cells contain the bulk of the myogenic activity, and that CD45-positive cells also display only very limited myogenic potential.
Experimental Procedures
Muscle Isolation.
Experiments were performed with a single cell suspension of muscle-derived cells prepared as previously described (7, 16) with slight modifications. The gastrocnemius, soleus, and plantaris were excised from multiple C57BL/6-CD45.1, C57BL/6 ROSA26, or C57/MlacZ 6- to 8-week-old mice. Bones and tendons were removed, and the muscle tissue was thoroughly minced and then digested at 37°C with 0.2% collagenase type II-filtered (Worthington) for 30 min, followed by 0.25% trypsin (GIBCO) for 30 min. The tissue was triturated briefly by using a 10-ml pipette and then passed through a 70-μm filter. Cells were collected by centrifugation, resuspended in 3 ml of Hanks' balanced salt solution (HBSS) (GIBCO), overlaid onto a Percoll (Amersham Pharmacia Pharmacia Biotech) gradient [3 ml of 70% Percoll overlaid with 3 ml of 40% Percoll diluted with 1× phosphate-buffered solution (GIBCO)], and then immediately centrifuged at 770 × g for 20 min with the brake off at 25°C. The cells were then removed from the 40/70% Percoll interface and resuspended in DMEM with 1% glutamine (vol/vol), 2% FCS (vol/vol), and antibiotics (GIBCO). Cells were counted by hand by using a hemocytometer; typical preparations yielded 1 × 106 to 2 × 106 cells/mouse.
Methylcellulose Cultures.
Muscle-derived cells were purified as described above and plated at various concentrations in 3 ml of Methocult GF m3434 (StemCell Technologies, Vancouver). Colony number was assessed at day 9 after plating, and colonies were identified as myeloid via Wright–Giemsa staining (Sigma).
Flow Cytometry and Magnetic Enrichment.
Muscle-derived cells were purified as described above and stained with CD45-phycoerythrin (30-F11, PharMingen) and Sca-1-biotin (E13–161.7, PharMingen), followed by streptavidin-allophyocyanin (APC) (Molecular Probes), c-kit-FITC (2B81, PharMingen), or CD34-biotin (RAM34, PharMingen), followed by streptavidin-APC. Flow cytometric analysis was performed by using a two-laser instrument, FACScan (Beckton Dickinson).
For magnetic enrichment, muscle-derived cells were purified as described above and stained with either Sca-1-biotin or CD45-biotin (30-F11, PharMingen) followed by staining with a 1/10 dilution of streptavidin-conjugated microbeads (Miltenyi Biotec, Auburn, CA). Cells were then suspended to ≈12 × 103 cells/μl in DMEM (1% glutamine, 2% FCS, and antibiotics) and fractionated into positive and negative populations by using an LS-positive enrichment column followed by an AS depletion column (Miltenyi Biotec). Cells were fractionated by using a VarioMACS magnet (Miltenyi Biotec).
Cells were also fractionated by using fluorescent activated cell sorting (FACS). Muscle-derived cells were stained with Sca-1-biotin and CD45-FITC (30-F11 PharMingen), followed by staining with streptavidin-phycoerythrin (Molecular Probes). Cell sorting was performed on a triple-laser instrument (MoFlow, Cytomation, Fort Collins, CO).
Bone Marrow Transplantation.
Bone marrow transplantation was performed as previously described (7). Muscle-derived cells were purified from 6- to 8-week-old C57BL/6-CD45.1 mice and fractionated as described above. Cells were suspended at various concentrations in 250 μl of HBSS (Table 1) and transplanted via retroorbital injection into 6- to 12-week-old C57BL/6-CD45.2 recipients that had received 11 Gy of irradiation. Recipients also received 2 × 105 nucleated WBM cells prepared from 6- to 12-week-old C57BL/6-CD45.2 animals. Animals were anesthetized during injections by using isoflurane and maintained on acidified water and autoclaved food.
Table 1.
Summary of hematopoietic reconstitution from enriched muscle-derived cells
| Experiment | Injected sample | No. of cells/recipient | Reconstitution*
|
Average CD45.1 engraftment (%)
|
||
|---|---|---|---|---|---|---|
| 6 weeks | Late† | 6 weeks | Late† | |||
| 1 | Sca-1+ | 53,000 | 2/3 | 2/3 | 19 | 37 |
| Sca-1− | 157,000 | 0/4 | 0/4 | 0 | 0 | |
| 2 | Sca-1+ | 298,000 | 4/4 | 3/3‡ | 15 | 6.4 |
| Sca-1− | 96,000 | 0/1 | 0/1 | 0 | 0 | |
| 3 | Sca-1+ | 167,000 | 3/4 | 2/4 | 7 | 4 |
| Sca-1− | 123,000 | 1/5 | 0/5 | 2 | 0 | |
| 4 | CD45+ | 164,000 | 4/4 | 2/2‡ | 4.5 | 3 |
| CD45− | 72,500 | 0/2 | 0/2 | 0 | 0 | |
| 5 | CD45+ | 200,000 | 4/4 | 2/3‡ | 9 | 1 |
| CD45− | 151,000 | 0/5 | 0/4‡ | 0 | 0 | |
| 6 | CD45+ | 200,000 | 4/4 | 4/4 | 13 | 12 |
| CD45− | 196,000 | 0/4 | 0/4 | 0 | 0 | |
| 7 | CD45+ | 246,000 | 5/5 | 5/5 | 18 | 23 |
| CD45− | 150,000 | 0/5 | 0/4‡ | 0 | 0 | |
Reconstitution is defined as multilineage contribution greater than 1%. Values are expressed as number of positive animals/number of total animals transplanted.
Late engraftment ranges from 12 to 24 weeks.
Animal succumbed to isoflurane toxicity.
Peripheral Blood Analysis of Transplant Recipients.
Peripheral blood analysis was performed as previously described (7). At various time points after transplantation, 150 μl of peripheral blood was collected from the retroorbital plexus of anesthetized transplant recipients. In control experiments, peripheral blood for controls was taken from untransplanted mice. Nucleated cells were stained with anti-CD45.1-biotin (clone A20), rat-IgG2a-FITC (R35–95), rat-IgG2b-FITC (A95–1), B220-FITC (RA3–6B2), Thy-1-FITC (30-H12), Gr-1-FITC (RB6–8C5), and Mac-1-FITC (M1/70) (all from PharMingen). CD45.1-biotin was detected by subsequent staining with streptavidin–phycoerythrin (Molecular Probes). Stained blood samples were then analyzed by flow cytometry by using a two-laser instrument, FACScan (Becton Dickinson).
Muscle Injury Assays.
Muscle cells were isolated from C57BL/6 ROSA26 mice, which express β-galactosidase systemically and constitutively (17), or C57/MlacZ mice, which express β-galactosidase under the control of the myosin light chain 3F promoter (18). Cells were fractionated on the basis of Sca-1 and CD45 expression by FACS or magnetic enrichment followed by FACS to achieve high purity. Fractionated cells were suspended in 20 μl of HBSS at various concentrations (Table 2) and injected into the tibialis anterior (TA) muscle of C57BL/6 animals that do not express β-galactosidase. The TA muscles of the recipient animals were injected with 20 μl of the cardiotoxin Naja mossambica mossambica (Sigma) 24 h before injection of fractionated cells. Two to three weeks after injection, the TA muscles were carefully dissected from transplanted animals, frozen in 2-methylbutane cooled in liquid nitrogen, and stored at −80°C. Muscles were cryosectioned at 12 μm by using a cryotome (Shandon, Pittsburgh) and stained overnight with 5-bromo-4-chloro-3-indolyl β-d-galactoside (X-Gal; Sigma) at 37°C. Slides were rinsed with water, counterstained with nuclear fast red (Vector Laboratories), and visualized by using a Zeiss axioplane 2 epifluorescent microscope (charge-coupled device; Photometrics, Tucson, AZ). Differential interference contrast microscopy images were acquired at ×40.
Table 2.
Summary of in vivo muscle regeneration experiments
| Injected sample | No. of injected cells × 103 | No. of injected muscles | Engraftment*
|
|
|---|---|---|---|---|
| Moderate† | Extensive‡ | |||
| Unsorted | 43–100 | 5 | 3 | 2 |
| Sca-1+ | 44–84 | 6 | 2 | 1 |
| Sca-1− | 80–140 | 6 | 0 | 5 |
| Sca-1+/CD45+ | 17–78 | 9 | 1 | 2 |
| Sca-1+/CD45− | 3.4–12 | 9 | 1 | 3 |
| C57/mlacZ CD45+ | 124 | 4 | 3 | 0 |
| 74 | 2 | 1 | 0 | |
| 40 | 4 | 4 | 0 | |
| C57/MlacZ CD45− | 74 | 2 | 0 | 2 |
| 15 | 4 | 2 | 1 | |
| PB | 18–195 | 3 | 0 | 0 |
All experiments were performed with Rosa26-derived muscle cells except those noted as C57/MlacZ.
Engraftment is defined as a fiber staining either solid or punctate blue throughout and identifiable in multiple serial sections of TA muscle.
Moderate engraftment is defined as faint β-galactosidase staining in one to two blue fibers identified or unorganized aligned blue cells.
Extensive engraftment is defined as more than three blue fibers identified or dark blue nuclei clearly incorporated into a regenerating fiber.
Results
In Vitro Hematopoietic Progenitor Activity of Fractionated Muscle-Derived Cells.
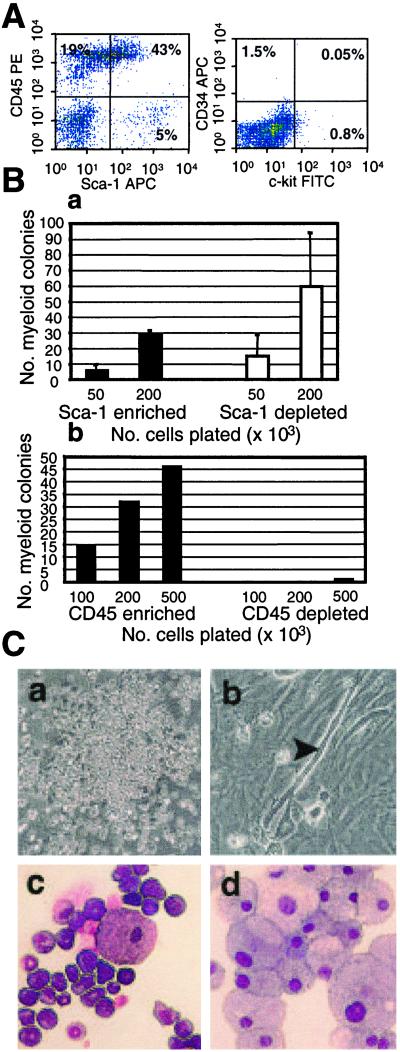
Muscle-derived cells were analyzed for expression of hematopoietic markers, including Sca-1, CD45, c-kit, and CD34, by antibody staining and FACS analysis. Fig. 1A shows that the muscle-derived cells had a large proportion of Sca-1- and CD45-positive cells: in this representative analysis, 43% of the cells were Sca-1+/CD45+. A significant population of Sca-1+/CD45− cells (5%) appeared to express Sca-1 at a higher level than the Sca-1+/CD45+ cells. Notably, there was no detectable expression of either CD34 or c-kit in this freshly prepared suspension of muscle-derived cells (Fig. 1A).
Figure 1.
Sca-1-positive and -negative and CD45-positive muscle cells display in vitro hematopoietic progenitor activity. (A) Freshly isolated muscle-derived cells were stained with monoclonal antibodies recognizing CD45 and Sca-1 analyzed by flow cytometry. (B) Sca-1-positive and -negative muscle-derived cells were plated into methylcellulose and assessed for colony number 9 days after plating (a). CD45-positive and -negative cells were also plated into methylcellulose and assessed for colony number 9 days after plating (b). (C) Methylcellulose cultures of CD45-positive cells (a). Cultures of CD45-negative cells (b). The arrowhead indicates an actively twitching myofiber. Unfractionated muscle-derived colonies were picked, cytospun, and stained with Wright–Giemsa (c and d).
To evaluate in vitro hematopoietic progenitor activity, muscle cell fractions were separated on the basis of Sca-1 and CD45 expression by using a magnetic cell sorter. Fresh cell preparations initially contained around 54% Sca-1-expressing cells. Sca-1-magnetic enrichments typically yielded a Sca-1-positive fraction that was 95% pure and a Sca-1-negative fraction that was 74% pure (Fig. 4, which is published as supporting information on the PNAS web site, www.pnas.org). Cells were plated at various concentrations in semisolid methycellulose medium supplemented with cytokines that promote myeloid differentiation. Both the Sca-1-negative and -positive fractions of muscle-derived cells generated a significant number of hematopoietic colonies (Fig. 1Ba).
Fresh muscle cell preparations contained an average of around 53% CD45-expressing cells (Fig. 4). After magnetic enrichment, the CD45-positive fraction was typically 96% pure, whereas the CD45-negative fraction was 99% pure. Only the CD45-positive fraction generated hematopoietic colonies (Fig. 1Bb). CD45-negative cell cultures were composed of a confluent layer of fibroblastic looking cells with actively twitching myofibers present throughout (Fig. 1Cb). No myofibers were seen in the CD45-positive cultures, but numerous normal hematopoietic colonies were observed (Fig. 1Ca). Unfractionated muscle also generated substantial numbers of hematopoietic colonies in vitro, although at a lower frequency than WBM (Fig. 5, which is published as supporting information on the PNAS web site). The colonies generated by unfractionated muscle-derived cells were typical myeloid colonies (Fig. 1 C c and d).
In summary, both the Sca-1-positive and -negative populations from muscle contained in vitro myeloid progenitor potential. In contrast, the CD45-positive fraction contains exclusively hematopoietic progenitors, whereas the CD45-negative fraction contains myogenic progenitors.
In Vivo Hematopoietic Potential of Fractionated Muscle-Derived Cells.
The in vivo hematopoietic potential of fractionated Sca-1 and CD45 muscle-derived cells was assessed by using a competitive transplantation assay in which the test population was transplanted into lethally irradiated recipients together with WBM from distinguishable mouse strains. The competitor WBM assists rescue of the irradiated animals and permits semiquantitative analysis of the hematopoietic activity of the test population relative to the competitor WBM, because the numbers of transplanted cells are known. The test population and competitor WBM are isolated from congenic mouse strains with CD45 alleles that differ by a few amino acids. The relative contribution of the test and competitor populations to peripheral blood of recipients are distinguished with monoclonal antibodies specific for the two alleles.
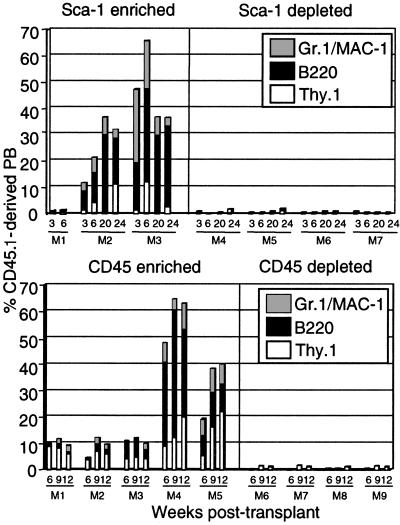
Fractionated muscle-derived cells were isolated from C57BL/6-CD45.1 mice and transplanted into lethally irradiated C57BL/6-CD45.2 recipients along with 2 × 105 C57BL/6-CD45.2 WBM competitor cells. At different time points after transplant, PB of recipient animals was assayed for muscle-derived reconstitution by FACS analysis. Animals transplanted with Sca-1-positive cells showed significant long-term in vivo engraftment (Fig. 2). High levels of engraftment were maintained up to 24 weeks after transplant, although there was high interexperiment variability in overall activity (Table 1). Hematopoietic engraftment was multilineage, as evidenced by the coexpression of several lineage specific markers with CD45.1: Gr.1 and Mac-1 (myeloid lineage), Thy-1 (T cells), and B220 (B cells) (Fig. 2). None of the animals transplanted with Sca-1-negative cells showed any evidence of muscle-derived hematopoietic engraftment (Table 1 and Fig. 2). Similarly, when animals were transplanted with either CD45-positive or -negative muscle-derived cells, only animals receiving CD45-positive cells showed high-level multilineage engraftment (Table 1 and Fig. 2). This engraftment was detectable by FACS up to at least 20 weeks after transplant.
Figure 2.
In vivo hematopoietic activity of Sca-1 and CD45-sorted populations of muscle-derived cells. Muscle was isolated from C57BL/6-CD45.1 mice and magnetically enriched for Sca-1 or CD45 expression. Positive and negative populations were transplanted into lethally irradiated C57BL/6-CD45.2 recipients along with 2 × 105 C57BL/6-CD45.2 WBM cells. PB of recipients was analyzed via FACS for CD45.1 and hematopoietic lineage markers at the indicated time points after transplantation. In the representative Sca-1 transplant shown, Thy.1 staining was not performed at 20 weeks after transplant and therefore does not appear.
Animals were also transplanted with FACS-sorted Sca-1+/CD45+ and Sca-1+/CD45− cells in a competitive transplantation assay. Multiple experiments confirmed our previous findings: only animals transplanted with Sca-1+/CD45+ cells showed multilineage long-term engraftment, whereas animals transplanted with Sca-1+/CD45− cells failed to yield in vivo hematopoietic engraftment (data not shown). Animals transplanted with up to 12 × 106 PB cells also did not display in vivo hematopoietic engraftment (data not shown), indicating that the muscle-derived hematopoietic engraftment is not due to PB contamination.
Close inspection of the contribution of muscle-derived cells to myeloid, T, and B lineages suggested that myeloid engraftment may be low relative to the WBM-derived reconstitution, whereas the lymphoid engraftment correlated with the WBM. Statistical analysis of animals that had been transplanted with FACS-purified Sca-1+/CD45+ muscle-derived cells supported this suspicion (P = 0.01; Fig. 6, which is published as supporting information on the PNAS web site), but this may be due to the overall low levels of engraftment.
In Vivo Muscle Regenerative Activity of Fractionated Muscle Cells.
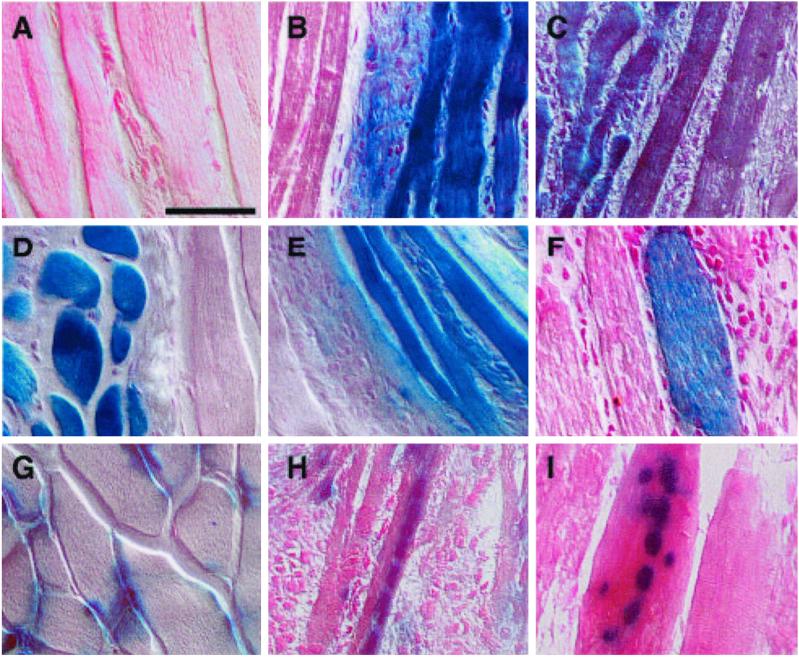
We sought to qualitatively evaluate the in vivo myogenic potential of the enriched populations of muscle-derived cells. To accomplish this goal, muscle cells were isolated from C57BL/6-ROSA26 mice that express β-galactosidase systemically and constitutively (17) and fractionated on the basis of Sca-1 expression via magnetic enrichment or sorted for Sca-1 and CD45 expression via FACS. The TA muscle of recipient C57BL/6 mice was injected with cardiotoxin followed by the test population 24 h later. Cardiotoxin induces death of differentiated myofibers and subsequent muscle regeneration. Injured muscle injected with HBSS and stained with X-Gal did not result in blue staining (Fig. 3A), indicating the absence of nonspecific X-Gal staining. Unsorted muscle-derived cells were able to incorporate readily into regenerating muscle fibers (Fig. 3B and Table 2). Both Sca-1-positive (Fig. 3C) and Sca-1-negative (Fig. 3D) populations of muscle-derived cells were able to incorporate into regenerating muscle fibers of preinjured animals (see also Table 2). This incorporation is evidenced by the extensive staining of multiple whole non-ROSA26 regenerating muscle fibers (Fig. 3 C and D).
Figure 3.
In vivo myogenic potential of muscle-derived cells. Muscle-derived cells were purified from C57BL/6-ROSA26 (B–F) or C57/MlacZ (H, I) mice by flow cytometry or magnetically followed by flow cytometry. Test populations were injected into the TA muscles of non-ROSA26 animals that had been injected with cardiotoxin 24 h earlier. The TA muscles were removed from recipients 2–3 weeks later, cryosectioned, and stained with X-Gal for β-galactosidase expression. (Bar = 50 μM.) (A) Injured muscle injected with HBSS. (B) Muscle injected with unfractionated muscle-derived cells. The large blue tracts are fibers regenerated from Rosa26-derived cells. (C) Incorporation of Sca-1-positive muscle cells. (D) Incorporation of Sca-1-negative muscle cells. (E) Incorporation of Sca-1+/CD45+ cells. (F) Incorporation of Sca-1+/CD45− cells. (G) Section of unmanipulated C57/MlacZ muscle. Note the nuclear localization of lacZ at the edges of the fibers shown in cross section. (H) CD45-positive C57/MlacZ-derived cells injected into regenerating muscle and sectioned longitudinally. (I) CD45-negative C57/MlacZ incorporation.
Sca-1+/CD45+ and Sca-1+/CD45− muscle-derived cells were also injected into regenerating muscle and became incorporated into regenerating fibers (Fig. 3 E and F; Table 2). Postsort purity checks reveal that the Sca-1+/CD45+ sorted population was routinely greater than 99.2% pure with no observed contamination from the CD45-negative population; the minor contamination observed is typically derived from the Sca-1−/CD45+ gate. To verify that CD45-positive muscle-derived cells were functionally incorporating into regenerating muscle fibers, cells isolated from C57/MlacZ mice, which express nuclear localized β-galactosidase under the control of the muscle-specific myosin light chain 3F promoter (ref. 18; Fig. 3G) were fractionated on the basis of CD45 expression via magnetic enrichment followed by FACS and injected into cardiotoxin-injured TA muscles of C57BL/6 animals. The CD45+ population was found by postsort purity check to be greater than 99.5% pure. Because this transgene is expressed only in muscle tissue (19), muscle-derived cells will stain with X-Gal only if they differentiate into muscle. At 2 weeks after cell injection, C57/MlacZ-derived CD45-positive cells occasionally became incorporated into differentiated muscle fibers as shown in Fig. 3H (Table 2). CD45-negative cells became incorporated into muscle fibers with high frequency (Fig. 3I; Table 2), as evidenced by aligned blue nuclei in fibers of regenerating tissue.
A qualitative difference between the nature of the myogenic engraftment of the CD45-positive and -negative muscle-derived cells was also observed in multiple experiments: CD45-negative muscle-derived cells were routinely found to express very high levels of the transgene, as evidenced by dark blue staining when exposed to X-Gal, whereas CD45-positive muscle-derived cells appeared to express lower levels of the transgene, evidenced by less vivid staining in the presence of X-Gal. Furthermore, engrafted CD45-negative cells were always observed as clear dark blue aligned nuclei positioned centrally in a regenerated whole muscle fiber. In contrast, CD45-positive cells were very rarely observed as nuclei clearly incorporated into a whole regenerated muscle fiber. Rather, these cells were most often observed as single cells in an unorganized state, a significant fraction aligned in single file poised to begin the fusion process.
Because the muscle injury assay used in these experiments allows only a qualitative assessment of the myogenic potential of the CD45-positive and -negative populations, it is not possible to draw quantitative conclusions from these experiments. However, these data demonstrate that the CD45-positive muscle-derived cells contain only limited myogenic potential relative to CD45-negative cells.
Discussion
Muscle-Derived Hematopoietic Cells Express Sca-1 and CD45.
We have shown here that a Sca-1+/CD45+ population is responsible for the majority of in vivo hematopoietic activity derived from murine muscle. Sca-1+/CD45+ cells were found to reconstitute the major lineages of the peripheral blood when transplanted into lethally irradiated recipients, whereas Sca-1+/CD45− cells showed no in vivo hematopoietic activity. The muscle-derived reconstitution could not be accounted for by peripheral blood contamination of muscle preparations. Interestingly, the Sca-1+/CD45+ muscle cells do not express c-kit, which has been shown to be expressed by murine HSCs. Therefore, the phenotype of the ms-HSC does not seem to correlate perfectly with that of the WBM HSCs, although it is possible that the enzymatic digestion used to purify the muscle-derived cells destroys the c-kit epitope. Furthermore, the hematopoietic enrichment of the CD45+ muscle-derived cells reported here was significantly lower than previous reports (7). We believe this discrepancy is due to differences in our isolation procedure and in the use of a culture period in our previous study.
The muscle-derived cells were also found to generate hematopoietic colonies in vitro. Both the Sca-1-positive and -negative fractions of muscle-derived cells generated myeloid colonies, although only the Sca-1-positive fraction had in vivo reconstituting activity. This discrepancy could be due to the presence of progenitors in the Sca-1-negative population that are active in vitro but are unable to sustain hematopoiesis in vivo. Alternatively, the in vitro hematopoietic activity could come from contaminating Sca-1-positive cells in the preparation, because cells expressing low levels of Sca-1 could be detected up to 24%. Hence, it appears that the expression of high levels of Sca-1 correlates with in vivo hematopoietic activity in the muscle, as in bone marrow (14).
In contrast to Sca-1, CD45 fractionation was able to segregate the in vitro hematopoietic progenitor potential. CD45-positive cells yielded myeloid colonies, whereas CD45-negative cells generated myogenic cells and differentiated myofibers. This absolute segregation of hematopoietic activity was also observed in long-term in vivo hematopoietic transplantation experiments.
CD45-Negative Muscle-Derived Cells Contain the Majority of Myogenic Activity.
Both in vitro and in vivo, CD45-negative cells generated muscle. Satellite cells are potently myogenic muscle stem cells outside of the hematopoietic lineage and therefore would be expected to be CD45-negative. Our high degree of purity (99%) and myogenic activity suggests that this population contains the majority of satellite cells.
Surprisingly, CD45-positive cells were also found to display myogenic potential, although only to a limited degree and only in vivo. CD45-positive cells derived from Rosa26 transgenic mice became incorporated into muscle fibers on injection into cardiotoxin-injured muscle. In a more rigorous test, when highly purified CD45-positive muscle-derived cells were isolated from C57/MlacZ mice, which should express lacZ in a muscle-specific manner, and were injected into preinjured muscle, they were found to up-regulate the expression of the transgene and very occasionally to become incorporated into muscle. These data may imply that the CD45-positive cells have the potential to respond to a myogenic environment, but that the myogenic program is retarded in these cells relative to the CD45-negative cells. Ultimately, clonal analysis will be required to determine whether CD45-positive cells indeed have myogenic activity.
ms-HSCs Are Ultimately Derived from the Hematopoietic System.
These data have fundamental implications regarding the origin of muscle-derived HSCs. We have found that the cell population responsible for the majority of muscle-derived hematopoietic activity expresses CD45. This antigen is used as a marker of cells of hematopoietic origin and has not yet been described on nonhematopoietic cells (15), strongly suggesting that ms-HSCs are derived from the hematopoietic system rather than the adult muscle progenitor cell population, as originally proposed (1, 7, 20). Therefore, muscle-derived hematopoietic activity is not due to transdifferentiation or stem cell “plasticity.” Furthermore, HSCs appear to share several features of WBM-derived HSCs: ms-HSCs behave as WBM-derived HSCs in in vivo transplantation studies, both express CD45 and Sca-1, and both display limited myogenic activity in vivo (1). Thus, muscle-derived hematopoiesis likely results from bona fide HSCs resident in skeletal muscle, which is further supported by recent transplantation studies (21).
It has previously been reported that the ms-HSC also falls into the Hoechst SP of muscle cells that efflux the fluorescent dye Hoechst 33342 (1). A recent study found that Pax7 null mice have normal numbers of muscle SP cells but lack satellite cells (20), suggesting that muscle SP and satellite cells are two distinct populations. These data are also consistent with our finding that the CD45-positive ms-HSC may be distinct from the satellite cell and main muscle progenitor cell compartment.
Models of the Origin of ms-HSCs.
We propose that the ms-HSCs are ultimately either derived from or intimately related to the hematopoietic system. Thus, ms-HSCs are not the result of stem cell “plasticity” in the sense that muscle stem cells are not being reprogrammed to differentiate into a hematopoietic cell. Instead, ms-HSCs may end up in muscle as a result of specific or nonspecific homing events at some point during development. We envision three possibilities: the ms-HSCs could be primitive mesodermal multipotent stem cells, with the potential to become both blood and muscle, that have been left behind during development (“developmental leftovers”) and remain there in a state of quiescence in the adult animal (such multipotential cells or HSCs could be seeding skeletal muscle from the fetal liver or aorta-gonad-mesonephros region during development), or ms-HSCs could be seeding adult skeletal muscle via the circulation after adult bone marrow hematopoiesis is established. Future studies will be required to distinguish among these possibilities. Regardless of which model of muscle-derived hematopoiesis is shown to be correct, the fact that a hematopoietically derived population with HSC-like activity pools in skeletal muscle is indisputable. Clearly, these cells must occupy a specific niche within the skeletal muscle. Are they found in vessels? Do they penetrate to the interstitum? Future experiments will be required to elucidate the niche of this cell population.
Supplementary Material
Acknowledgments
We thank members of the laboratory for helpful discussion and comments, Sarah Evans for comments on the manuscript, and Jeff Scott for flow cytometry assistance. This work was funded by a grant from the National Institutes of Health to M.A.G. (DK58192). M.A.G. was an American Society of Hematology Fellow and is a Scholar of the Leukemia and Lymphoma Society. K.A.J. is a fellow of the Leukemia and Lymphoma Society. S.M.F. is supported by National Institutes of Health Training Grant T32 AI07495.
Abbreviations
- WBM
whole bone marrow
- HSC
hematopoietic stem cell
- SP
side population
- ms-HSC
muscle-HSC
- Sca-1
stem cell antigen-1
- PB
peripheral blood
- TA
tibialis anterior
- X-Gal
5-bromo-4-chloro-3-indolyl β-d-galactoside
- FACS
fluorescent activated cell sorting
- HBSS
Hanks' balanced salt solution
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Gussoni E, Soneoka Y, Strickland C D, Buzney E A, Khan M K, Flint A F, Kunkel L M, Mulligan R C. Nature (London) 1999;401:390–394. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- 2.Jackson K A, Majka S M, Wang H, Pocius J, Hartley C J, Majesky M W, Entman M L, Michael L H, Hirschi K K, Goodell M A. J Clin Invest. 2001;107:1395–1402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman I L, Grompe M. Nat Med. 2000;6:1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- 4.Krause D S, Theise N D, Collector M I, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis S J. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 5.Bjornson C R R, Rietze R L, Reynolds B A, Magli M C, Vescovi A L. Science. 1999;283:534–537. doi: 10.1126/science.283.5401.534. [DOI] [PubMed] [Google Scholar]
- 6.Clarke D L, Johansson C B, Wilbertz J, Veress B, Nilsson E, Karlstrom H, Lendahl U, Frisen J. Science. 2000;288:1660–1663. doi: 10.1126/science.288.5471.1660. [DOI] [PubMed] [Google Scholar]
- 7.Jackson K A, Mi T, Goodell M A. Proc Natl Acad Sci USA. 1999;96:14482–14486. doi: 10.1073/pnas.96.25.14482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mauro A. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schultz E, McCormick K M. Rev Physiol Biochem Pharmacol. 1994;123:213–257. doi: 10.1007/BFb0030904. [DOI] [PubMed] [Google Scholar]
- 10.Goodell M A, Brose K, Paradis G, Conner A S, Mulligan R C. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uchida N, Weissman I L. J Exp Med. 1992;175:175–184. doi: 10.1084/jem.175.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morrison S, Weissman I. Immunity. 1994;1:661–673. doi: 10.1016/1074-7613(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 13.Osawa M, Hanada K, Hamada H, Nakauchi H. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 14.Spangrude G J, Heimfeld S, Weissman I L. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 15.Trowbridge I S, Thomas M L. Annu Rev Immunol. 1994;12:85–116. doi: 10.1146/annurev.iy.12.040194.000505. [DOI] [PubMed] [Google Scholar]
- 16.Yablonka-Reuveni Z, Nameroff M. Histochemistry. 1987;87:27–38. doi: 10.1007/BF00518721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zambrowicz B P, Imamoto A, Fiering S, Herzenberg L A, Kerr W G, Soriano P. Proc Natl Acad Sci USA. 1997;94:3789–3794. doi: 10.1073/pnas.94.8.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- 19.Kelly R, Alonso S, Tajbakhsh S, Cossu G, Buckingham M. J Cell Biol. 1995;129:383–396. doi: 10.1083/jcb.129.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seale P, Sabourin L A, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki M A. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 21.Kawada H, Ogawa M. Blood. 2001;98:2008–2013. doi: 10.1182/blood.v98.7.2008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.