Abstract
The sulfur regulatory system of Neurospora crassa consists of a group of sulfur-regulated structural genes (e.g., arylsulfatase) that are under coordinate control of the CYS3 positive regulator and sulfur controller (SCON) negative regulators. Here we report on the cloning of scon-3+, which encodes a polypeptide of 171 amino acids and is a Skp1 family homolog. Repeat-induced point mutation of scon-3+ resulted in a phenotype of constitutive expression of arylsulfatase, a phenotype consistent with other sulfur controller mutants. Northern analysis indicated that, unlike other members of the sulfur regulatory system, expression of scon-3+ is not under the direct control of the CYS3 transcriptional activator. In particular, scon-3+ mRNA was detectable under sulfur repressing or derepressing conditions in a Δcys-3 mutant. In yeast, Skp1p and an F-box protein binding partner are core constituents of a class of E3 ubiquitin ligases known as SCF complexes. The N. crassa negative regulator SCON2 contains an F-box motif essential for the operation of the sulfur regulatory system and suggests a role for an SCF complex in the N. crassa sulfur regulatory system. A crucial set of experiments, by using a yeast two-hybrid approach with confirming coimmunoprecipitation assays, demonstrated that SCON3 interacts with SCON2 in a manner dependent upon the F-box motif of SCON2. The protein-protein interaction detected between SCON2 and SCON3 represents the initial demonstration in a filamentous fungus of functional interaction between putative core components of a SCF complex.
The sulfur regulatory system of Neurospora crassa is composed of a set of trans-acting regulatory genes and a group of structural genes that encode enzymes used in the uptake and assimilation of a variety of sulfur compounds (20, 27). When N. crassa cultures are grown under conditions of sulfur limitation (i.e., derepressing conditions), then the entire group of sulfur-related genes is coordinately expressed (20). The structural genes involved encode for arylsulfatase, choline sulfatase, choline sulfate permease, methionine permease, sulfate permeases I and II, and an extracellular protease (20, 27). Essentially, the sulfur regulatory system monitors the cellular sulfur status and expresses the sulfur structural genes as needed to ensure an adequate internal supply of sulfur, while subjecting the sulfur-related genes to repression under conditions of sulfur sufficiency.
The positive regulator of sulfur-controlled gene expression in N. crassa is CYS3, a basic region-leucine zipper protein that functions as a DNA-binding transcriptional activator (20, 24, 25). cys-3+ gene expression is controlled in part by a positive feedback loop involving the CYS3 protein and in part by the negative regulatory sulfur controller genes, scon-1+ and scon-2+ (25, 26). scon-1 and scon-2 mutants show constitutive expression of cys-3+ and the sulfur structural genes (26). scon-2+ has been cloned and found to be expressed only under conditions of sulfur limitation (16, 26). The SCON2 protein contains two domains implicated in protein-protein interactions: (i) a region of six WD40 (or β-transducin) repeats and (ii) a motif that we originally termed the “N-terminal domain” (16). The N-terminal domain is now referred to as the F-box motif (1, 12).
F-box motifs are found in a large number regulatory proteins (12, 14, 28). In Saccharomyces cerevisiae, F-box proteins have been shown to assemble with Skp1p,Cdc53p, and Rbx1p to form a complex known as the SCF (for Skp1p/Cdc53p/F-box) (28, 38). SCF complexes act as E3 ubiquitin ligases to target proteins for ubiquitin-mediated proteolysis. SCF complexes have been implicated in diverse processes, including cell cycle progression, glucose sensing, and developmental processes (7, 10, 12, 14, 28, 43). The F-box protein component is responsible for SCF complex target specificity, with the F-box motif thought to be responsible for the interaction with Skp1p (1, 29, 38). In yeast, Met30p is the F-box protein that is homologous to SCON2 (41).
Previous work has demonstrated that the F-box domain of SCON2 is required for SCON2's role as a regulator within the N. crassa sulfur control system (17). The ability of SCON2 to function as a negative regulator of sulfur-related gene expression may be due to the ability of SCON2 to form a functional SCF complex in N. crassa via protein-protein interactions between the F-box motif of SCON2 and the N. crassa homolog of yeast Skp1p. Skp1 homologs, both designated sconC, have been found in Aspergillus nidulans (31) and Microsporum canis (GenBank AF408428) but have not been examined for possible molecular interactions with other putative SCF complex constituents.
We report here the isolation and analysis of scon-3+, a new member of the N. crassa sulfur regulatory system. scon-3+ encodes a protein showing strong homology to the Skp1p family of proteins. Repeat-induced point mutation (RIP) of the scon-3+ locus resulted in the production of mutants having a phenotype of constitutive expression of arylsulfatase, confirming the putative role of scon-3+ in sulfur gene regulation. Importantly, we found that SCON3 interacted with SCON2 in vivo as detected by two-hybrid assays and in vitro by using coimmunoprecipitation. Further, the interaction between SCON3 and SCON2 was found to be dependent upon the F-box motif of SCON2. The data presented supports a role for an SCF complex partially comprised of SCON2 and SCON3 as an crucial component required for the regulated expression of sulfur-related genes in N. crassa.
MATERIALS AND METHODS
Strains and culture conditions.
The N. crassa strains Δcys-3 (18-4) and scon-2 (PSD272) have been described previously (25, 26). 74OR23-1a was used as the wild-type strain for these studies. N. crassa cultures were grown at 25°C on minimal Vogel medium (6) with supplements as required. Cells were grown under sulfur-repressing and sulfur-derepressing conditions by using Vogel-minus-sulfur medium supplemented with 5.0 mM methionine and 0.25 mM methionine, respectively (25). The assay of arylsulfatase activity, which is normally derepressed under sulfur-limiting growth conditions (27), was used to confirm the presence of sulfur derepression or repression. Arylsulfatase assays were performed by monitoring p-nitrophenol liberation at 405 nm from p-nitrophenyl sulfate according to standard methods (21, 27).
Crosses were carried out with cornmeal agar or Westergard-Mitchell medium (6). Homokaryons were isolated by growth on Westergard-Mitchell medium with 1 mM iodoacetate, harvesting of the microconidia, and filtration through 5-μm (pore-size) Millex filters (8).
Plasmid constructs and gene cloning.
PCR with the primers 5′-AAGAATTCTTYATGCARGTYGAYCARGARATGCTNTTY-3′ and 5′-ACGGAATTCRTCNGCCCARTCYTTRTC(C/G)CG(C/G)CG-3′ (where R is purine and Y is pyrimidine) and wild-type N. crassa chromosomal DNA as a template was used to amplify a 300-bp portion of the scon-3+ gene. The primers were designed by using highly conserved regions determined from alignments of available Skp1 family protein sequences (i.e., S. cerevisiae, A. nidulans, and Homo sapiens), as well as incorporation of established N. crassa codon preferences. The PCR product was digested with EcoRI and inserted into pSPORT1 to generate pSC3. pSC3 was used to probe a λJ1 genomic library (obtained from the Fungal Genetics Stock Center, Kansas City, Kans.) by plaque hybridization and a hybridizing clone, designated λscon3, was isolated. Subsequently, λscon3 was sequenced by the dideoxy method (both strands). A 3-kb ApoI fragment containing scon-3+ was subcloned from λscon3 into pCB1004 to make pSCON3, which was used for primer extension and RIP-related experiments. pSCON3 has been deposited at the Fungal Genetics Stock Center (Kansas City, Kans.).
The following constructs were used to determine the cDNA sequence of scon-3+ and generate fusion constructs for the analysis of interactions between SCON3 and SCON2. The oligonucleotide primers 5′-CGGAATTCATGGCGGAGAACGACGAACGTGCC-3′ and 5′-GTCTTGGTCGACTAACGGTCTTCCGCCCATTCGTTCTCG-3′ were used to amplify scon-3+ cDNA by PCR with an N. crassa λgt10 cDNA library (constructed previously in this laboratory) (16) as a template. The PCR product generated was cloned into the yeast two-hybrid binding domain (BD) vector pBD-GAL4 Cam to create pBD-scon3. The oligonucleotides 5′-CCTTGGGAATTCATGTCGTCCGTCCTCAT-3′ and 5′-AACCCCGTCGACCCTCACTGCCGACAGGGC-3′ were used to amplify scon-2+ by PCR with the plasmid pSC2-C3MAL as a template. pSC2-C3MAL contains the full-length N. crassa scon-2+ cDNA sequence, as previously described (15). The PCR fragment produced was cloned into the yeast two-hybrid activation domain (AD) vector pGAD424 to create pAD-scon2. The oligonucleotide primer pair 5′-CCTTGGGAATTCATGTCGTCCGTCCTCAT-3′ and 5′-CCTTGTGTCGACTCACTGCCGACAGGGCTT-3′ were used to amplify codons 1 to 205 of scon-2+ from an N. crassa λgt10 cDNA library template. The resulting PCR product was cloned into the yeast two-hybrid AD vector pGAD424 to create pAD-scon2ΔWD40. The oligonucleotide primers 5′-CCGAATTCAGCCGATACAAGTTGTCGGTT-3′ and 5′-CCTTGTGTCGACTCACTGCCGACAGGGCTT-3′ were used to amplify codons 265 to 650 of scon-2+ by PCR with an N. crassa λgt10 cDNA library as a template. The PCR-amplified fragment was then cloned into the yeast two-hybrid AD vector pGAD424 to create pAD-scon2ΔFbox. All constructs were sequenced by the dideoxy method.
RIP of scon-3+.
N. crassa wild-type 74OR23-1a was transformed by the scon-3+ gene on a 3-kb fragment carried in the pSCON3 construct by using spheroplasts prepared by the Novozyme 234 method (42). pSCON3 also contains the A. nidulans trpC promoter coupled to the hygromycin B phosphotransferase coding sequence. Hygromycin-resistant homokaryotic transformants were crossed to wild-type 74-OR23 (4, 36), and the progeny analyzed for sulfur regulatory or other potential defects. Of the RIP progeny isolated, a phenotypically representative isolate 12b-1 was studied more extensively. The specificity of the RIP events to the scon-3+ locus was confirmed by restoration of a wild-type phenotype by transformation with the wild-type scon-3+ gene. The RIP allele 12b-1 was subsequently cloned by PCR and sequenced by the dideoxy method.
mRNA isolation and Northern blot analysis.
Total RNA was isolated by the phenol extraction procedure of Reinert et al. (32) as modified by Paietta (26). Briefly, mycelial samples were harvested, frozen in liquid nitrogen, and homogenized in a 1:1 mixture of phenol-chloroform-isoamyl alcohol (49:49:2) and Sarkosyl extraction buffer. After phenol-chloroform and chloroform extractions, precipitation, and sodium acetate washes, the poly(A)+ mRNA was isolated by oligo(dT)-cellulose chromatography. Northern blot and hybridization conditions were as described previously (25). 32P-labeled probes were prepared by random priming (9). Northern blots were probed with the the consitutively expressed am+ gene (11) of N. crassa to confirm that comparable amounts of poly(A)+ mRNA were loaded for each sample. In addition, the scon-2+ gene was used as a control to demonstrate the typical sulfur-regulated expression of genes in the sulfur control system. Quantitation of Northern blots was accomplished by using a Molecular Dynamics PhosphorImager.
Transcription start site determination.
The 5′ end of the scon-3+ transcript was mapped by primer extension and S1 nuclease analysis. For primer extension, the end-labeled oligonucleotide 5′-TCGTTGCTCTGGAGAGATACCTTCTGGAGGGCACG-3′, complementary to nucleotides 19 to 53 of scon-3+, was hybridized to 50 μg of N. crassa poly(A)+ mRNA. The primer was extended for 1 h at 42°C with 200 U of murine Moloney leukemia virus RNase H− reverse transcriptase (16). Primer extension products were sized by comparison to a dideoxy sequence ladder generated from pSCON3 by using the same primer as used for the primer extension reaction. For S1 nuclease analysis, the end-labeled oligonucleotide 5′-GATTTGGCCATCGTTGCTCTGGAG-3′ (complementary to nucleotides 40 to 63), was hybridized to M13mp18 containing the scon-3+ gene under previously described conditions (16). The resulting hybrid was extended by use of the Klenow fragment of Escherichia coli DNA polymerase I, and the extended primer was cut with ApoI. The resulting 243-bp probe was hybridized to 20 μg of N. crassa poly(A)+ mRNA. The probe-mRNA hybrids were then digested with 100 U of S1 nuclease at 30°C as described previously (16).
Yeast two-hybrid analysis.
S. cerevisiae YRG-2 (2) was initially transformed with pBD-Gal4 Cam or the bait construct pBD-scon3. After initial selection for expression of TRP1, these tranformants were then subjected to a second transformation by either pGAD424, pAD-scon2, pAD-scon2ΔWD40, or pAD-scon2ΔFbox. The resulting double transformants were initially grown on minimal media (SD-Leu-Trp) to select for expression of TRP1 and LEU2 (2). Double transformants were then isolated and screened for induction of HIS3 expression, as measured by growth on minimal media (SD-Leu-Trp-His) containing 20 mM 3-amino-1,2,4-triazole. Double transformants were also assayed for expression of lacZ by growth on minimal medium (SD-Leu-Trp) containing X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) with sucrose as the sole carbon source.
In vitro Transcription and Translation.
PCR was used to produce templates for transcription that carried a T7 promoter and fused coding region specifying an epitope for later use in coimmunoprecipitation experiments. The oligonucleotide primers 5′-AAATTGTAATACGACTCACTATAGGGCGAGCCGCCACCATGGAGGAGCAGAAGCTGATCTCAGAGGAGGACCTGGGTCAAAGACAGTTGACTGTATCG-3′ and 5′-GTCTTGGTCGACTAACGGTCTTCCGCCCATTCGTTCTCG-3′ were used, with pBD-scon3 as a template, in a PCR amplification to introduce a T7 promoter sequence and c-Myc epitope tag upstream of scon-3+. The oligonucleotide primers 5′-AAATTGTAATACGACTCACTATAGGGCGAGCCGCCACCATGTACCCATACGACGTTCCAGATTACGCTCCACCAAACCCAAAAAAAGAG-3′ and 5′-ACTTGCGGGGTTTTTCAGTATCTACGAT-3′ were used, with pAD-scon2, pAD-scon2ΔWD40, or pAD-scon2ΔFbox as a template in a PCR amplification to introduce a T7 promoter sequence and hemagglutinin (HA) epitope tag upstream of scon-2+, scon2ΔWD40, and scon2ΔFbox, respectively. The resulting PCR products were then used as substrates for coupled in vitro transcription and translation with a T7 RNA polymerase and rabbit reticulocyte lysate mixture (Promega) with [35S]methionine as a radiolabel.
In vitro coimmunoprecipitation of SCON2 and SCON3.
Duplicate samples containing 10 μl of in vitro-translated c-Myc-SCON3, HA-SCON2, HA-SCON2ΔWD40, or HA-SCON2ΔFbox either alone or 10 μl of c-Myc-SCON3 plus 10 μl of HA-SCON2, HA-SCON2ΔWD40, or HA-SCON2ΔFbox were incubated at 25°C. After 1 h, 1 μg of c-Myc monoclonal antibody or HA-tag polyclonal antibody was then added to one tube of each sample pair, and the samples were incubated for a further hour at 25°C. The samples were then precipitated, washed, electrophoresed, and fixed according to the manufacturer's specification (Clontech).
Nucleotide sequence accession number.
The genomic DNA sequence of scon-3+ has been deposited in GenBank (AF402682). The nucleotide sequence of the RIP-generated 12b-1 allele of scon-3+ has also been deposited as AY135642.
RESULTS
Sequence and organization of the scon-3+ gene.
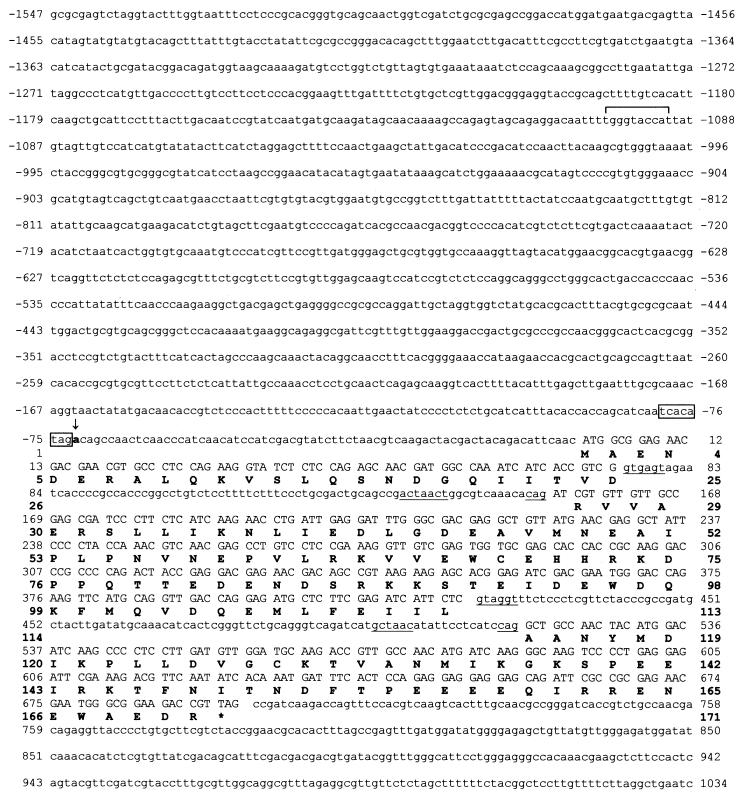
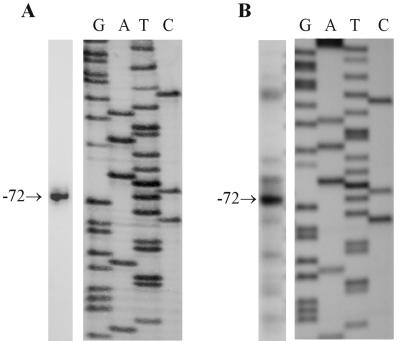
The scon-3+ gene was isolated by using mixed oligonucleotide primed PCR amplification to generate a partial gene segment, followed by hybridization screening of a λJ1 genomic library to identify a clone containing the entire gene. The complete nucleotide sequence of the scon-3+ gene and flanking 5′ region contained within a 2,581-bp genomic segment is presented in Fig. 1. The scon-3+ gene encodes a polypeptide of 171 amino acids with a molecular mass of 19.9 kDa. Isolation and sequencing of scon-3+ cDNA clones revealed that the coding region is interrupted by two introns of 80 and 98 bp. Nucleotide sequences at the exon-intron boundaries, as well as the internal lariat sequence conform to established N. crassa consensus sequences (3). A single, strong transcriptional start site at nucleotide −72 was identified by both primer extension and S1 nuclease transcript analysis (Fig. 2). The initiation site is found immediately adjacent to the sequence TCACATAG, which closely matches the transcription initiation start site consensus determined for N. crassa (3). The promoter region does not contain any sequences exactly matching the established consensus binding site for the sulfur regulatory system transcription activator, CYS3 (19, 37). A single site, at −1100, has a two-base mismatch versus the consensus sequence (ATGRYRYCAT) and represents, based on our prior binding site studies (37), a weak potential site for CYS3. In comparison, the N. crassa scon-2+ regulatory gene, which is clearly under CYS3 control based on Northern blot analysis, has four putative CYS3 binding sites (17).
FIG. 1.
Nucleotide and predicted amino acid sequence of the scon-3+ gene. The sequence is shown from 1,547 nucleotides upstream of the translation start codon to 339 nucleotides downstream of the stop codon (indicated by an asterisk). The nucleotides are numbered relative to the initiator ATG codon. The predicted polypeptide sequence is given below the nucleotide sequence in single-letter code. The 5′ splice sites, the 3′ splice sites, and the internal lariat sequences within the introns are underlined. The sequence corresponding to the N. crassa transcriptional initiation site consensus is boxed. A vertical arrow indicates the major transcriptional initiation site at −72. A potential CYS3 binding site at −1100 within the scon-3+ promoter region is bracketed.
FIG. 2.
Determination of the transcription start site for scon-3+. (A) S1 nuclease analysis. The size of the S1 nuclease-protected fragment was determined by comparison with a DNA sequencing reaction generated with the same primer and template used to produce the S1 probe. An arrow indicates the protected fragment corresponding to a start site at nucleotide −72 (Fig. 1). (B) Primer extension. Primer extension products were sized by comparison against a dideoxy-sequencing reaction generated with the same primer and with pSCON3 as a template. An arrow indicates the major primer extension product corresponding to a transcriptional initiation site at −72.
Sequence analysis of SCON3.
Database searches with SCON3 revealed highly significant matches to the members of the Skp1 protein family. An alignment of SCON3 with the M. canis and A. nidulans SCONC proteins, as well as other Skp1 family homologs, is shown in Fig. 3. SCON3 shows the highest homology (87%) to SCONC of M. canis (GenBank no. AF408428). The conservation of residue identity relative to N. crassa for the Skp1 family proteins presented in Fig. 3 ranges from 70 to 87%, excluding S. cerevisiae. S. cervisiae Skp1p differs from the others in the grouping by having an interior 32-amino-acid segment that is not observed in the other proteins. As with all of the proteins shown in Fig. 3, SCON3 contains a PEST sequence (33), which would allow for targeting of the protein for degradation and rapid turnover. An ATP/GTP-binding motif, termed the P-loop (35), is also present in SCON3 and other members of the Skp 1 protein family (Fig. 3).
FIG. 3.
SCON3 sequence homologies. The deduced amino acid sequences of N. crassa (Nc) SCON3 (AF402682), M. canis (Mc) SCONC (AF40848), A. nidulans (An) SCONC (AAB18274), and the Skp1 proteins from H. sapiens (Hs) (AAH25673), A. thaliana (At) (NP_565123), D. melanogaster (Dm) (NP_477340), S. cereviseae (Sc) (AAC49492), and S. pombe (Sp) (NP_595455) were optimally aligned by using CLUSTALW. The resulting alignment was shaded by using the BOXSHADE program. Sequences, other than SCON3, were obtained from BLASTP searches. Identical residues are shown as white on black, whereas similar residues are shown as black on gray. Brackets indicate PEST and P-loop sequences. Invariant residues are represented in the consensus line as capital letters, whereas conserved residues are represented as lowercase letters.
Induction of scon-3 mutants by RIP.
RIP can be used to efficiently induce targeted mutations in N. crassa (4, 6, 36). Multiple point mutations in a target sequence can be obtained by inserting into a wild-type strain an extra copy of the gene for which a functional disruption is required and then crossing the transformant to the wild type (36). A RIP experiment was conducted for the scon-3 locus by introducing one or more copies of scon-3+ into the wild type by transformation and then crossing the transformant with the wild type. The progeny were then analyzed for any phenotypic alterations compared to the wild type. To ensure that no general class of sulfur regulatory mutants were overlooked, methionine supplementation was used for the germination and culturing of cross-progeny. Screening of the progeny revealed no sulfur auxotrophs but did reveal progeny showing a phenotype of constitutive expression of arylsulfatase (Table 1).
TABLE 1.
Arylsulfatase activity of wild-type, sulfur mutant, and scon-3RIP strains
| Strain | Arylsulfatase sp acta on:
|
|
|---|---|---|
| High-sulfur mediumb | Low-sulfur mediumc | |
| Wild type | <0.05 | 8.9 |
| cys-3(P22) | <0.05 | <0.05 |
| scon-1(36-18) | 9.4 | 9.9 |
| scon-2(PSD272) | 10.2 | 11.7 |
| scon-3RIP(12b-1) | 8.1 | 8.3 |
| scon-3RIP(12b-1) scon-3+d | <0.05 | 8.5 |
Expressed as nanomoles per minute per milligram of total protein.
High-sulfur medium with 5.0 mM methionine.
Low-sulfur medium with 0.25 mM methionine.
Isolate of scon-3RIP (12b-1) transformed with scon-3+.
Constitutive expression of sulfur-related genes (e.g., arylsulfatase) is the typical phenotype of mutants given the sulfur controller designation (see Table 1). The RIP progeny were examined for any variations from wild-type in terms of (i) growth or morphology (i.e., variations in colony size or shape and variations in hyphal or conidial morphology) and (ii) fertility in crosses, but no mutant phenotypes were observed. The observed RIP phenotype is therefore similar to that seen in N. crassa scon-1(36-18), scon-2 (PSD272) (see Table 1), and A. nidulans sconC mutants (31). Only conditional yeast mutants (skp1-11 and skp1-12) have been found that show defective sulfur-related regulation of MET25 (homocysteine synthase) (29) but have an pleiotropic range of effects (e.g., cell cycle arrest) (1, 5, 29). Finally, when the scon-3RIP mutants were transformed with the wild-type scon-3+ gene normal sulfur gene regulation was restored (Table 1), demonstrating the specificity of the RIP effect on the scon-3 gene.
To further analyze the RIP-generated mutant, the molecular nature of the 12b-1 allele was determined by cloning and subsequent nucleotide sequencing. The nucleotide sequence of 12b-1 (GenBank no. AY135642) demonstrates clear evidence of RIP-induced mutations, which include a Gln78Stop mutation within the coding region, as well as Gln40Lys, Gly43Ser, and Arg63Gln mutations occurring prior to the introduced stop codon. The RIP-introduced stop codon results in a predicted truncated protein of 77 amino acids compared to the full length of 171 amino acids for SCON3. Although the mutations severely alter the protein and specifically generate the sulfur-related phenotype, we cannot rule out the possibility of partial functionality, particularly since there is scon-3RIP transcript detectable by Northern blot analysis (data not shown).
Analysis of scon-3+ gene expression.
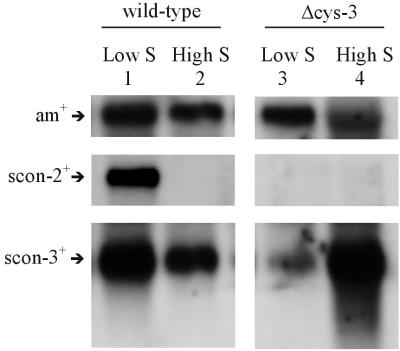
scon-3+ transcript levels were initially assayed in wild-type N. crassa grown on high and low levels of sulfur (i.e., repressing and derepressing conditions, respectively). Northern blots were prepared and probed with the cloned scon-3+ gene. In the blot shown in Fig. 4 (lanes 1 and 2), a 1.1-kb message showed hybridization to the scon-3+ probe and was clearly detectable under both sulfur repressing and derepressing conditions. The steady-state level of scon-3+ transcript, however, showed a substantially higher level under sulfur derepressing conditions (approximately twofold more compared to the sulfur repressing condition based on phosphorimager quantitation of the blot). Typically, we have found tightly regulated expression for the other components of the sulfur regulatory system that have been examined (e.g., ars-1+, cys-3+, and scon-2+) (26). The scon-2+ gene included as a control and used to reprobe the blot in Fig. 4 demonstrates the expected high expression under low-sulfur conditions and no expression under high-sulfur conditions. A further experiment examined the level of scon-3+ transcript in the Δcys-3 mutant grown under sulfur derepressing and repressing conditions. The scon-3+ transcript was detectable under derepressing conditions in Δcys-3 (as lane 3 in Fig. 4 demonstrates), but the level of scon-3+ was twofold lower under derepressing conditions compared to wild-type based on the phosphorimager data. In sharp contrast, the level of scon-3+ transcript was highly elevated in Δcys-3 grown under repressing conditions compared to wild-type (an approximate twofold increase). The unusual pattern of expression for scon-3+ transcript levels in Δcys-3 was consistently observed between mRNA preparations, and the high sulfur response is unlike that observed for other known genes in the N. crassa sulfur regulatory system (i.e., Δcys-3 mutants show highly reduced transcript levels of sulfur-related genes under both derepressing and repressing conditions). scon-2+, again acting as a control, demonstrates no expression in Δcys-3 as expected (Fig. 4). All of the Northern blot experiments conducted used the constitutively expressed am+ gene as a control probe for the Northern blots to ensure that the bulk mRNA levels in the samples were comparable.
FIG. 4.
Northern blot analysis of scon-3+ expression. (Left panel) Northern analysis of poly(A)+ RNA isolated from wild-type N. crassa grown under derepressing (lane 1) and repressing conditions (lane 2). Northern blots were prepared and probed with 32P-labeled scon-2+, scon-3+, and am+ DNA. scon-2+ is included to demonstrate the expression pattern typically seen for genes within the N. crassa sulfur control system. The am+ gene served as a control to ensure comparability between samples. (Right panel) Northern analysis of poly(A)+ RNA isolated from the Δcys-3 strain of N. crassa grown for 24 h under derepressing (lane 3) and repressing (lane 4) conditions. Northern blots were prepared as described above and probed with 32P-labeled scon-2+, scon-3+, and am+ DNA. Note that the scon-2+ transcript was not observed in Δcys-3. The am+ gene again served as a control to ensure comparability between samples.
Protein-protein interaction between SCON2 and SCON3.
The SCON2 and SCON3 proteins represent major constituents of a hypothetical SCF complex involved in sulfur-related gene regulation in N. crassa. Initial screening for in vivo interactions between SCON2 and SCON3 were carried out by using the yeast two-hybrid system with BD and AD derived from the GAL4 system (2, 30). Full-length SCON3 and full-length SCON2, as well as truncated versions of SCON2 with either a deletion of the F-box domain or WD-40 domain, were assayed for interactions. A vector, pBDscon3, encoding the wild-type SCON3 protein was tested against pAD vectors containing three versions of SCON2(wild-type, ΔF-box, and ΔWD-40). Two significant interactions, detectable by the expression of lacZ activity, were observed: (i) pBDscon3 (wild type) showed interaction with pADscon2 (wild type), and (ii) pBDscon3 (wild type) showed interaction with pADscon2ΔWD40 (Fig. 5). In demonstration of the importance of the F-box domain for SCON2-SCON3 interactions, the experiments showed no interaction present between pBDscon3(wild-type) and pADscon2ΔF-box. Although deletion of the WD-40 domain in SCON2 had no detectable effect on the interaction present, the deletion of the SCON2 F-box eliminated the interaction between SCON2 and SCON3. The result is consistent with the putative role of the F-box to interact with Skp1 homologs, whereas the WD-40 domain is thought to be involved in the protein-protein interaction with the particular target protein of the SCF complex. The entire range of control combinations tested were negative in these experiments (Fig. 5).
FIG. 5.
In vivo association of SCON2 and SCON3. The Gal4 AD, an AD fusion with full-length SCON2 (residues 1 to 650), an AD fusion with the N-terminal domain of SCON2 (residues 1 to 250), or an AD fusion with the C-terminal domain of SCON2 (residues 265 to 650) were tested for their ability to interact with a Gal4 BD fusion with SCON3 in the yeast two-hybrid assay. Each patch represents an independent transformation of the yeast YRG-2 host strain expressing the indicated proteins. The various pAD-scon2 constructs or the pAD control were cotransformed with pBD-scon3 into the YRG-2 yeast host strain. Interaction between fusion proteins was assayed by their ability to induce expression of β-galactosidase on SD-Leu-Trp media augmented with X-Gal and having sucrose as the sole carbon source. Note that YRG-2 cotransformed with pBD-scon3 and either pAD-scon2 or pAD-scon2Δwd40 tested positive for expression of lacZ, indicating the presence of protein-protein interaction. In contrast, yeast cotransformed with pBD-scon3 and pAD-scon2Δfbox tested negative for induction of lacZ expression. Controls, including pAD with pBD (not shown), were all negative for interactions in the assays.
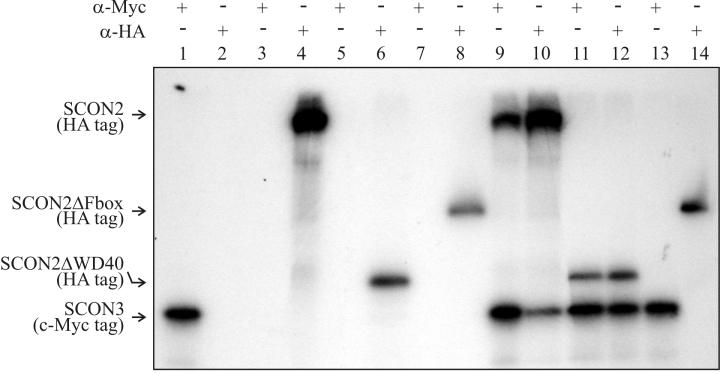
A second set of assays was done by using in vitro coimmunoprecipitation (30) to provide confirmation of the interactions observed between SCON2 and SCON3 in the yeast two-hybrid system. For these experiments, a T7 promoter and tag sequence encoding an epitope (either Myc or HA) were added to the SCON2 and SCON3 coding sequence by PCR with appropriately designed primers. The PCRs produced the templates used for coupled transcription and translation. SCON3 was generated in its full-length with a HA tag, whereas SCON2 was generated in full-length, ΔF-box, and ΔWD-40 forms all with a Myc tag. In each case, proteins of the expected sizes were produced with the transcripts translated separately and examined by polyacrylamide gel electrophoresis. After transcription and translation, the SCON2 and SCON3 products were incubated in various combinations, and antibodies to the HA and Myc epitopes were used to assay whether the proteins were interacting and could be coimmunoprecipitated or not. In the experiments shown in Fig. 6, coimmunoprecipitation was seen only in the following combinations: (i) full-length SCON3 plus full-length SCON2 and (ii) full-length SCON3 plus SCON2 with WD-40 region deleted. No coimmunoprecipitation was seen when the F-box was deleted from SCON2 and tested against SCON3. In addition, all control combinations were negative for coimmunoprecipitation demonstrating the specificity of the the assay. The protein-protein interactions detected by the coimmunoprecipitation assays agreed exactly with the yeast two-hybrid assays.
FIG. 6.
Coimmunoprecipitation of SCON2 and SCON3. The proteins were transcribed and translated in vitro from PCR products. Prior to transcription and translation, c-Myc epitope and T7 promoter sequences were added upstream of the scon-3 coding region via PCR, whereas an HA epitope tag and T7 promoter region were added upstream of the scon-2 constructs via PCR. [35S]methionine was included in the translation mixture to generate the radiolabeled products: SCON3-Myc, SCON2-HA, SCON2ΔWD40-HA, and SCON2ΔFbox-HA. After translation, coimmunoprecipitation was carried out as described in Materials and Methods. After elution from the protein A-beads, 10 μl of the immunoprecipitate was loaded onto a sodium dodecyl sulfate-15% polyacrylamide gel. Lane 1, SCON3 plus c-Myc antibody; lane 2, SCON3 plus HA antibody; lane 3, SCON2 plus c-Myc antibody; lane 4, SCON2 plus HA antibody; lane 5, SCON2ΔWD40 plus c-Myc antibody; lane 6, SCON2ΔWD40 plus HA antibody; lane 7, SCON2ΔFbox plus c-Myc antibody; lane 8, SCONΔFbox plus HA antibody; lane 9, SCON3 plus SCON2 plus c-Myc antibody (note that both proteins were precipitated); lane 10, SCON3 plus SCON2 plus HA antibody (note that both proteins were precipitated); lane 11, SCON3 plus SCON2ΔWD40 plus c-Myc antibody (note that both proteins were precipitated); lane 12, SCON3 plus SCON2ΔWD40 plus HA antibody, note that both proteins were precipitated; lane 13, SCON3 plus SCON2ΔFbox plus c-Myc antibody (note that only the SCON3 band is present); lane 14, SCON3 plus SCON2ΔFbox plus HA antibody (note that only the SCON2ΔF-box band is present). Lanes 9 to 12 demonstrate coimmunoprecipitation and protein-protein interactions between SCON3 and SCON2, whereas lanes 13 and 14 demonstrate the necessity of an F-box in SCON2 for the interaction with SCON3 to occur.
DISCUSSION
We report here on the cloning and characterization of sulfur controller-3, which encodes a protein that acts as a negative regulator in the N. crassa sulfur regulatory system and belongs to the Skp1 gene family. The Skp1 grouping, including SCON3, exhibits a high degree of protein sequence conservation (Fig. 3). SCON3 is most similar to two fungal homologs: SconC in the dermatophyte M. canis (87%; GenBank no. AF408428) and SCONC in A. nidulans (31) (85%). Members of the Skp1 family have been shown to be essential in various cellular functions ranging from glucose sensing to cell cycle progression (12, 13, 18, 34, 44). In S. cerevisiae, only conditional alleles of Skp1 are known and result in cell cycle arrest in G1 phase, whereas other alleles result in cell cycle arrest in G2 phase (1, 5, 29). The conditional yeast Skp1 mutants, skp1-11 and skp1-12, show a range of pleiotropic effects, including defective sulfur gene regulation (e.g., MET25) (29). Within the Skp1 family, however, the only currently known mutations that result solely in a phenotype of altered expression of sulfur-related genes, as represented here by arylsulfatase activity levels, are at the sconC locus of A. nidulans (31) and the N. crassa RIP-generated mutants of scon-3 that we report here. The RIP-induced scon-3 mutant phenotype (which may not represent the null phenotype) is similar to the observed phenotype of other sulfur controller mutants (e.g., scon-1 and scon-2) (Table 1). The lack of pleiotropic effects may indicate an evolutionary divergence of function, but we must include a caveat that other more severe or lethal phenotypes may not have been recoverable from the screenings. In this regard, many eukaryotes have multiple versions of skp1-like proteins (e.g., Caenorhabditis elegans has 21 skp1-like proteins) (23). Extensive searches of the recently available N. crassa genome (release 3; http://www-genome.wi.mit.edu) have revealed, however, only the presence of the Skp1 homolog reported in the present study.
In A. nidulans, sconC transcript levels are not significantly affected by sulfur concentration in the wild type (31). A. nidulans metR1 mutants show an increased level of sconC transcript under sulfur derepressing conditions (31). Based upon these data it has been suggested that, in A. nidulans, METR may act as a negative regulator of sconC expression (31). Given the fact that the sulfur regulatory systems of N. crassa and A. nidulans show many similarities (22), including homology between the positive regulators CYS3 and METR, we investigated the possibility that CYS3 might influence the expression of SCON3 in N. crassa. We observed detectable expression of scon-3+ under repressing and derepressing conditions in both wild-type and Δcys-3 strains. In wild-type N. crassa the steady-state level of scon-3+ transcript was substantially higher under sulfur derepressing conditions, an expression pattern similar to what has been observed for other components of the N. crassa sulfur regulatory system and perhaps suggesting positive transcriptional control of scon-3+ by CYS3. However, in repeated experiments, the Δcys-3 strain demonstrated an unusual pattern of scon-3+ expression. Under low-sulfur conditions, expression was severely reduced, whereas under high-sulfur conditions expression was elevated. As a control, reprobing the blots with scon-2+ (Fig. 4) demonstrated the expected regulated expression for that gene. The Δcys-3 data are not in agreement with the expected pattern of expression for a gene under control of CYS3, nor do they agree with a model of regulation where CYS3 acts as a negative regulator of scon-3+ expression. The unusual expression pattern suggests several possibilities, where scon-3+ may (i) encode a more stable component of the SCF complex; (ii) be subject to posttranscriptional controls; or (iii) be involved in other, as-yet-undefined, cellular functions.
Skp1p is a major component in two evolutionarily conserved ubiquitin-conjugating E3 complexes: the anaphase-promoting complex and SCF complexes (14, 14, 28). SCF complexes were first identified in S. cerevisiae and have subsequently been identified in a number of eukaryotic organisms (14). SCF complexes are composed of at least three common subunits: Skp1p, Cdc53p, and Rbx-1p. SCF complexes also contain a modular subunit, an F-box protein, which provides the SCF complex with substrate specificity (12, 28). Each SCF complex is thought to target specific substrates for ubiquitin-mediated proteolysis (38). An SCF complex with Grr1p as the F-box component has been shown to target Cln2p for rapid degradation (18, 39). Another SCF complex, with MET30 as the F-box constituent is involved in the repression of the Met25 gene (29, 40). The yeast Met30 gene (41) is a homolog of the N. crassa scon-2+ gene. Further, the N-terminal F-box domain of SCON2 is required for normal sulfur-mediated gene regulation in N. crassa (17). We have hypothesized that SCON2's ability to function as a repressor of sulfur-regulated gene expression is dependent upon the ability of SCON2 to form a functional SCF complex with CYS3 as the most likely target. To date, a functional test of the predicted molecular interactions in an SCF complex in filamentous fungi had not been carried out. A crucial test of the hypothetical role of SCON3 was in confirming an interaction with SCON2. The two-hybrid experiments presented here show that full-length SCON2 and SCON3 interact with each other. The interaction between SCON2 and SCON3 was clearly dependent on the F-box domain. When the F-box domain was deleted from SCON2, there was no detectable interaction with SCON3. In support of the specificity of the effect was the finding that if only the WD-40 repeats were removed from SCON2, then the interaction with SCON3 could still be detected. Coimmunoprecipitation experiments confirmed these results. These experiments provide the initial functional evidence for interactions between putative SCF components in filamentous fungi and are an important step in defining the N. crassa sulfur control system.
Acknowledgments
This research was supported by a Kettering Medical Innovations Grant to JVP. S.T.S. was the recipient of a fellowship from the Biomedical Sciences Program at Wright State University.
We thank John Turchi for assistance with the figures.
REFERENCES
- 1.Bai, C., P. Sen, K. Hofmann, L. Ma, M. Goebl, J. W. Harper, and S. J. Elledge. 1996. SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell 86:263-274. [DOI] [PubMed] [Google Scholar]
- 2.Bartel, P. L., C.-T. Chien, R. Sternglanz, and S. Fields. 1993. Using the two-hybrid system to detect protein-protein interactions, p. 153-179. In D. A. Hartley (ed.), Cellular interactions in development: a practical approach. Oxford University Press, Oxford, England.
- 3.Bruchez, J. J. P., J. Eberle, and V. E. A. Russo. 1993. Regulatory sequences in the transcription of Neurospora crassa genes: CAAT box, TATA box, introns, poly(A) tail formation sequences. Fung. Genet. Newsl. 40:89-96. [Google Scholar]
- 4.Cambareri, E. B., H. M. Foss, M. R. Rountree, and E. U. Selker. 1989. Repeat induced G-C and A-T mutations in Neurospora. Science 244:1571-1575. [DOI] [PubMed] [Google Scholar]
- 5.Connelly, C., and P. Hieter. 1996. Budding yeast SKP1 encodes an evolutionarily conserved kinetochore protein required for cell cycle progression. Cell 86:275-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis, R. H. 2000. Neurospora: contributions of a model organism. Oxford Press, New York, N.Y.
- 7.Deshaies, R. J., V. Chau, and M. Kirschner. 1995. Ubiquitination of the G1 cyclin Cln2p by a Cdc34p-dependent pathway. EMBO J. 14:303-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebbole, D., and M. S. Sachs. 1990. A rapid and simple method for isolation of Neurospora crassa homokaryons using microconidia. Fung. Genet. Newsl. 37:17-18. [Google Scholar]
- 9.Feinberg, A. P., and B. Vogelstein. 1983. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal. Biochem. 132:6-13. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan, K. B., A. A. Hyman, and P. K. Sorger. 1997. Regulating the yeast kinetochore by ubiquitin-dependent degradation and Skp1p-mediated phosphorylation. Cell 91:491-500. [DOI] [PubMed] [Google Scholar]
- 11.Kinnaird, J. H., M. A. Keighren, J. A. Kinsey, M. Eaton, and J. R. S. Fincham. 1982. Cloning of the am (glutamate dehydrogenase) gene of Neurospora crassa through the use of a synthetic DNA probe. Gene 20:387-396. [DOI] [PubMed] [Google Scholar]
- 12.Kipreos, E. T., and M. Pagano. 2000. The F-box protein family. Genome Biol. 1:3002.1-3002.7. [Online.] http://genomebiology.com. [DOI] [PMC free article] [PubMed]
- 13.Kishi, T., T. Seno, and F. Yamao. 1998. Grr1 functions in the ubiquitin pathway in Saccharomcyes cerevisiae through association with Skp1. Mol. Gen. Genet. 257:143-148. [DOI] [PubMed] [Google Scholar]
- 14.Krek, W. 1998. Proteolysis and the G1-S transition: the SCF connection. Curr. Opin. Genet. Devel. 8:36-42. [DOI] [PubMed] [Google Scholar]
- 15.Kumar, A. 1997. Characterization and functional analysis of the WD-40 and F-box motifs within the Neurospora crassa negative regulator sulfur controller-2. Ph.D. thesis. Wright State University, Dayton, Ohio.
- 16.Kumar, A., and J. V. Paietta. 1995. The sulfur controller-2-negative regulatory gene of Neurospora crassa encodes a protein with β-transducin repeats. Proc. Natl. Acad. Sci. USA 92:3343-3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar, A., and J. V. Paietta. 1998. An additional role for the F-box motif: gene regulation within the Neurospora crassa sulfur control network. Proc. Natl. Acad. Sci. USA 95:2417-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lanker, S., M. H. Valdivieso, and C. Wittenberg. 1996. Rapid degradation of the G1 cyclin Cln2 induced by CDK-dependent phosphorylation. Science 271:1597-1601. [DOI] [PubMed] [Google Scholar]
- 19.Li, Q., and G. A. Marzluf. 1996. Determination of the Neurospora crassa CYS3 sulfur regulatory protein consensus DNA-binding site: amino-acid substitutions in the CYS3 bZIP domain that alter DNA-binding specificity. Curr. Genet. 30:298-304. [DOI] [PubMed] [Google Scholar]
- 20.Marzluf, G. A. 1997. Molecular genetics of sulfur assimilation in filamentous fungi and yeast. Annu. Rev. Microbiol. 51:73-96. [DOI] [PubMed] [Google Scholar]
- 21.Metzenberg, R. L., and J. W. Parson. 1966. Altered repression of some enzymes of sulfur utilization in a temperature-conditional lethal mutant of Neurospora. Proc. Natl. Acad. Sci. USA 55:629-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Natorff, R., M. Piotrowska, and A. Paszewski. 1998. The Aspergillus nidulans sulphur regulatory gene sconB encodes a protein with WD40 repeats and an F-box. Mol. Gen. Genet. 257:255-263. [DOI] [PubMed] [Google Scholar]
- 23.Nayak, S., F. E. Santiago, H. Jin, D. Lin, T. Schedl, and E. T. Kipreos. 2002. The Caenorhabditis elegans Skp1-related gene family: diverse functions in cell proliferation, morphogenesis, and meiosis. Curr. Biol. 12:277-287. [DOI] [PubMed] [Google Scholar]
- 24.Paietta, J. V. 1995. Analysis of CYS3 regulatory function in Neurospora crassa by modification of leucine zipper dimerization specificity. Nucleic Acids Res. 23:1044-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paietta, J. V. 1992. Production of the CYS3 regulator, a bZIP DNA-binding protein, is sufficient to iduce sulfur gene expression in Neurospora crassa. Mol. Cell. Biol. 12:1568-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paietta, J. V. 1990. Molecular cloning and analysis of the scon-2 negative regulatory gene of Neurospora crassa. Mol. Cell. Biol. 10:5207-5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paietta, J. V. 1989. Molecular cloning and regulatory analysis of the arylsulfatase structural gene of Neurospora crassa. Mol. Cell. Biol. 9:3630-3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patton, E. E., A. R. Willems, and M. Tyers. 1998. Combinatorial control in ubiquitin-dependent proteolysis: don't Skp the F-box hypothesis. Trends Genet. 14:236-243. [DOI] [PubMed] [Google Scholar]
- 29.Patton, E. E., A. R. Willems, D. Sa, L. Kuras, D. Thomas, K. L. Craig, and M. Tyers. 1998. Cdc53 is a scaffold protein for multiple Cdc34/Skp1/F-box protein complexes that regulate cell division and methionine biosynthesis in yeast. Genes Dev. 12:692-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phizicky, E. M., and S. Fields. 1995. Protein-protein interactions: methods for detection and analysis. Microbiol. Rev. 59:94-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piotrowska, M., R. Natorff, and A. Paszewski. 2000. sconC, a gene involved in the regulation of sulphur metabolism in Aspergillus nidulans, belongs to the SKP1 gene family. Mol. Gen. Genet. 264:276-282. [DOI] [PubMed] [Google Scholar]
- 32.Reinert, W. R., V. B. Patel, and N. H. Giles. 1989. Genetic regulation of the qa gene cluster in Neurospora crassa: induction of qa messenger ribonucleic acid and dependency of qa-1 function. Mol. Cell. Biol. 1:829-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rogers, S. W., R. Wells, and M. Rechsteiner. 1986. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science 234:364-368. [DOI] [PubMed] [Google Scholar]
- 34.Rouillon, A., R. Barbey, E. E. Patton, M. Tyers, and D. Thomas. 2000. Feedback-regulated degradation of the transcriptional activator Met4 is triggered by the SCFMet30 complex. EMBO J. 19:282-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saraste, M., P. R. Sibbald, and A. Wittinghofer. 1990. The P-loop-a common motif in ATP-binding and GTP-binding proteins. Trends Biochem. Sci. 15:430-434. [DOI] [PubMed]
- 36.Selker, E., E. Camareri, P. Garrett, B. Jensen, K. Haack, E. Foss, C. Turpen, M. Singer, and J. Kinsey. 1989. Use of RIP to inactivate genes in Neurospora crassa. Fung. Genet. Newsl. 36:76-77. [Google Scholar]
- 37.Shuler, J. L. 1993. Analysis of the DNA binding of the Neurospora crassa CYS3 sulfur regulatory protein to the ars-1+ and cys-3+ promoters and determination of the consensus binding site sequence. Ph.D. thesis. Wright State University, Dayton, Ohio.
- 38.Skowyra, D., K. L. Craig, M. Tyers, S. J. Elledge, and J. W. Harper. 1997. F-box proteins are receptors that recruit phosphorylated substrates to the SCF ubiquitin-ligase complex. Cell 91:209-219. [DOI] [PubMed] [Google Scholar]
- 39.Skowyra, D., D. M. Koepp, T. Kamura, M. N. Conrad, R. C. Conaway, J. W. Conaway, S. J. Elledge, and J. W. Harper. 1999. Reconstitution of G1 cyclin ubiquitination with complexes containing SCFGrr1 and Rbx1. Science 284:662-665. [DOI] [PubMed] [Google Scholar]
- 40.Smothers, D. B., L. Kozubowski, C. Dixon, M. G. Goebl, and N. Mathias. 2000. The abundance of met30p limits SCFMet30p complex activity and is regulated by methionine availability. Mol. Cell. Biol. 20:7845-7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas, D., L. Kuras, R. Barbey, H. Cherest, P.-L. Blaiseau, and Y. Surdin-Kerjan. 1995. Met30p, a yeast transcriptional inhibitor that responds to S-adenosylmethionine, is an essential protein with WD40 repeats. Mol. Cell. Biol. 15:6526-6534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vollmer, S. J., and C. Yanofsky. 1986. Efficient cloning of genes of Neurospora crassa. Proc. Natl. Acad. Sci. USA 83:4869-4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Willems, A. R., S. Lanker, E. E. Patton, K. L. Craig, T. F. Nason, N. Mathias, R. Kobayashi, C. Wittenberg, and M. Tyers. 1996. Cdc53 targets phosphorylated G1 cyclins for degradation by the ubiquitin proteolytic pathway. Cell 86:453-463. [DOI] [PubMed] [Google Scholar]
- 44.Winston, J. T., P. Strack, P. Beer-Romero, C. Y. Chu, S. J. Elledge, and J. W. Harper. 1999. The SCFβ-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IκBα and β-catenin and stimulates IκBα ubiquitination in vitro. Genes Dev. 13:270-283. [DOI] [PMC free article] [PubMed] [Google Scholar]