Abstract
The differentiation and patterning of tendon fibroblasts is at present a poorly understood aspect of musculoskeletal development in the vertebrate limb. Precursors of tendon fibroblasts originate in the somatic mesoderm adjacent to the early limb bud and gradually become incorporated into the limb mesenchyme as development proceeds. It is unclear whether these progenitor cells are committed to the tendon lineage at this early stage, or whether cells become committed only as they are incorporated into a developing tendon. Following a review of our current knowledge of early tendon development, we present recent results from our preliminary studies looking at tendon cell commitment. Using a lacZ encoding replication-deficient retrovirus, we have mapped regions of the early limb bud that contain presumptive tendon progenitor cells, and later used these sites for implanting labelled fetal tendon fibroblasts into developing limbs. Following implantation, we found that these cells successfully re-incorporated into developing proximal and distal tendons, but also surprisingly contributed to other tissue lineages within the limb. Our results suggest that fetal tendon fibroblasts may not be irreversibly committed to a tendon cell fate in the limb and may be somewhat plastic in their ability to integrate into other tissue lineages during development.
Keywords: cell commitment, chick, lacZ gene, morphogenesis, musculoskeletal
Introduction
The functional integrity of the musculoskeletal system relies on the co-ordinated development of each of its constituent members, namely muscle, tendon and cartilage (see earlier papers in this volume by Buckingham et al., Francis-West et al., Sanz-Ezquerro and Tickle). Much is known of the development and differentiation of muscle and cartilage; however, the genesis and patterning of tendons has evoked relatively little research in the past. This is surprising since tendons are of equal functional importance to their counterparts within this system. Tendons are dynamic and exhibit a number of important specialized regions along their length designed to allow force transfer from muscle to bone in the most effective manner contributing to the overall integrity of the musculoskeletal system.
The majority of studies on tendon morphogenesis have focused on the distal autopod tendons within the limb (reviewed in Benjamin & Ralphs, 2000). One of the earliest stages of limb tendon development is characterized by the appearance of the mesenchymal lamina, a scaffold for the subsequent condensation of pretendinous mesenchymal cells (Hurle et al. 1989, 1990). This structure has never been detected within proximal dorsal and ventral tendon-forming regions (Kardon, 1998) and demonstrates one of a number of differences between proximal and distal tendons seen during development. These differences are highlighted further when the relationship between these tendons and developing muscles and skeletal elements are considered. In the absence of muscles, for example, proximal tendons fail to segregate and subsequently degenerate, whereas distal tendons continue to form, although require attachment to the muscle bellies for their maturation (Shellswell & Wolpert, 1977; Christ et al. 1979; Kieny & Chevallier, 1979; Brand et al. 1985; Kardon, 1998). It appears therefore that proximal tendon development is tightly co-ordinated with the development of the muscles and that distal tendon morphogenesis is, in part, associated with muscle development but is mainly coupled with the development of the forming skeletal elements (Kardon, 1998). These differences are certainly related to the fact that distal tendons develop in spatial isolation from some of their muscle bellies whilst the patterning and development of the proximal tendons occurs in close proximity to muscle precursor populations (Kardon, 1998).
Although some advances have recently been made to understand the development of tendons on both a cellular and molecular level, many important aspects of tendon morphogenesis have yet to be elucidated. We are beginning to understand a number of the detailed events that occur during the later stages of tendon development in the limb (reviewed in Benjamin & Ralphs, 2000); however, the initial stages of tendon development are still relatively unclear. To be able to unravel these early steps, a marker that specifically labels early tendon primordia as well as differentiated tendons has been a necessity. A number of tendon-specific genetic markers have been described, although their expression appears to be restricted to distal tendons and do not mark early events in tendon development. These markers are often expressed in broad domains that later change their expression patterns to become restricted to specific regions within the tendons. Follistatin, for example, is detected from stage 23 in the developing chick hind limb bud and is expressed sequentially in a proximo-distal fashion near the tips of the digits before becoming restricted to the periphery of the tendons later in development (D’Souza & Patel, 1999).
In contrast, Eph-A4, although initially detected in dorsal and ventral subectodermal mesoderm overlying digital cartilage where tendons develop, is later detected within the core of the tendons (D’Souza & Patel, 1999). Additionally, Eya 1 and Eya 2, two mouse homologues of Drosophila eya, are initially expressed in the connective tissue precursor cells in the hind limb buds before becoming associated with developing tendon primordia (Xu et al. 1997). Interestingly, Eya 1 expression eventually becomes localized within the dorsal flexor tendons whereas Eya 2 expression is restricted to ventral extensor tendons, suggesting that these genes may be differentially involved in the spatial patterning of ventral verses dorsal limb connective tissues. Two murine homeobox-containing genes, six 1 and six 2, are also expressed within developing tendons with expression patterns complementary to those of Eya 1 and Eya 2, respectively (Oliver et al. 1995; Xu et al. 1997).
Several growth factors have also been shown to be associated with tendon morphogenesis (Merino et al. 1998; Edom-Vovard et al. 2001). In conjunction with roles in initiation, outgrowth and patterning of vertebrate limbs, Fgf8 has been implicated in tendon development, with Fgf8 transcripts identified in all tendons of the limb, being associated with the parts of the tendon near the muscle extremities (Edom-Vovard et al. 2001). This growth factor, however, is only expressed later in development once muscle–tendon attachments have formed. Another growth factor, Tgfβ2, has also been described, with patterns of expression restricted to digit tendons (Merino et al. 1998).
Tenascin, an extracellular protein, is an excellent marker for tendon development and has been used extensively to detect early tendon primordia and is also found within differentiated tendons (Chiquet & Fambrough, 1984; Kardon, 1998). Both the mesenchymal lamina within distal regions of the limbs and newly formed proximal tendon blastemas are rich in tenascin. This protein, however, is not found exclusively within tendons and is present within tissues such as nerve and cartilage, thus complicating its use as a specific marker for tendon development and differentiation (Chiquet & Fambrough, 1984; Kardon, 1998).
More recently, a novel tissue-specific marker, scleraxis, has been detected within connective tissues that mediate the attachment of muscles to their respective skeletal elements (tendons) and the attachment of bone to bone (ligaments) in the chick limb (Schweitzer et al. 2001). Expression of this gene can be detected within early tendon progenitor populations and also identifies differentiating and individually anatomically distinct tendons. Four specific phases of scleraxis expression have been described within tendons of the limb buds (Schweitzer et al. 2001) beginning with the localization of scleraxis-expressing cells in the proximomedial domain of limb buds from stage 21, where expression is superficial within both the dorsal and ventral regions of the limb. This early detection of tendon precursor populations precedes the stage when these cells can be recognized using tenascin as a marker (Kardon, 1998). The second phase of scleraxis expression can be detected between stage 25 and 27 where the pattern of expression begins to become more dynamic and complex. Scleraxis-expressing cells coalesce to form limb-specific patterns with the beginnings of the first fibrous elements of the tendons. This expression proceeds in a proximal–distal fashion with the first true tendon fibres evident at stage 28, a stage that denotes the beginning of the third phase. During this penultimate phase, further fibres form, which subsequently begin to elongate to produce more mature tendons. By stage 31 the final phase of scleraxis expression is reached with scleraxis marking all the limb tendons. Thus, the elaborate pattern of tendon formation and the subsequent attachments to their respective muscles and skeletal elements is clearly revealed by the expression of this gene. The early expression patterns of scleraxis appear to overlap the domains of early migrating myoblast precursor populations (Schweitzer et al. 2001); however, scleraxis expression does not appear to be reliant on the presence of myoblasts within the limb, as similar patterns of scleraxis expression are evident in splotch mice, where mice are devoid of limb muscles due to a mutation in the Pax3 gene. Since muscle and tendon have different mesodermal origins (Chevallier et al. 1977; Christ et al. 1977; Kieny & Chevallier, 1979; Ordahl & Le Douarin, 1992), and tendons initially form in the absence of a muscle population (Kardon, 1998), the studies by Schweitzer et al. (2001) appear to show that the scleraxis gene specifies early tendon progenitor pools. It is unclear at present how the scleraxis gene is regulated during tendon morphogenesis; however, recent studies implicate Fgf4 as a possible player (Edom-Vovard et al. 2002). Fgf4 transcripts are normally located at muscle extremities near the prospective myotendinous junction, where future tendons will attach to their associated muscle (Edom-Vovard et al. 2001). If Fgf4 is absent as it is in the case in aneural or muscleless limbs, scleraxis and other tendon markers are down-regulated (Edom-Vovard et al. 2002). In contrast, if Fgf4 is misexpressed, ectopic expression of scleraxis results. Together these findings suggest that Fgf4 is necessary for the maintenance of scleraxis expression in developing tendons.
The majority of the cells present throughout a tendon are fibroblasts. These cells run in short longitudinal rows along the length of a tendon separated by its extensive extracellular matrix (reviewed in Benjamin & Ralphs, 2000). The precursors of tendon fibroblasts originate in the somatic mesoderm adjacent to the early limb bud and gradually become incorporated into the limb mesenchyme as development proceeds (Kieny & Chevallier, 1979; Ros et al. 1995; Kardon, 1998). Signals from the overlying ectoderm appear to induce and stimulate the proliferation of presumptive tendon fibroblast progenitors (Schweitzer et al. 2001); however, it is still unclear at present whether these progenitor cells are committed to the tendon lineage at this early stage, or whether cells only become committed as they are incorporated into a developing tendon.
It has been shown that signals from the limb mesenchyme (e.g. BMPs) appear to repress areas of tendon formation, assuring that only cells in specific domains take on a tendon fate (Schweitzer et al. 2001). To look at the question of tendon cell commitment further, we have recently begun to evaluate whether tendon fibroblasts already recruited into developing tendons are irreversibly committed to the tendon cell fate.
In the first phase of our studies, we attempted to locate the site of tendon progenitor cells within the early limb bud by injecting a lacZ encoding replication-deficient retrovirus into specific regions of stage 18–24 chick limbs. Results demonstrated that we were able to label cells in specific locations destined to be incorporated into developing tendons and these injected locations appeared to largely match those sites positive for scleraxis expression (Schweitzer et al. 2001).
We subsequently used these loci for the main part of our investigations, in which we injected fetal tendon fibroblasts, previously transfected with the lacZ gene, into early limb buds so that we could determine whether these previously ‘committed’ cells could reincorporate into developing tendons. Our data clearly show that these fetal tendon cells are indeed able to reincorporate into both proximal and distal tendons and become arranged in a typical longitudinal orientation. More surprisingly, however, labelled cells, thought to be derived from the fetal tendon fibroblasts, were also incorporated into a number of other tissue lineages within the developing limb, despite injections being made into presumptive tendon regions. The results achieved were similar whether fibroblasts were cultured for short or more prolonged periods prior to injection into the limb. Overall, these findings demonstrate that fetal tendon fibroblasts can reincorporate into developing tendons, but are not restricted to a tendon cell fate. The results indicate that cells in the fetal tendon lineage are probably quite plastic.
Materials and methods
Viral preparation
CXL lacZ encoding replication-deficient retrovirus was obtained from D17 packaging cells, a cell line transfected with pCXL DNA (Mikawa et al. 1991). Virus was harvested from Dulbecco's modified Eagle's medium (DMEM) plus 7% fetal calf serum (FCS) and 1% penicillin/streptomycin (S/P) conditioned overnight by near confluent monolayers of D17 cells. Following clarification to remove any cellular debris, 100 µg mL−1 of polybrene (hexadimethrine bromide – Sigma) was added to the supernatant prior to viral injections or infections of cultures of tendon fibroblasts. Culturing and harvesting procedures were based on those described by Mikawa et al. (1991). The concentration of CXL virus was found to be approximately 106 CFU mL−1 as measured on primary cultures of embryonic chick limb mesenchyme cells. Similar cultures were used to continually detect the replication incompetency of the virus and at no stage were helper viruses detected.
Preparation of retrovirally labelled fetal tendon cells
An illustrative summary of this procedure is shown in Fig. 1. Briefly, tendons were carefully dissected from 8-day-old chick hind limbs and cut into small fragments. Care was taken to ensure that only tendons, and therefore tendon fibroblasts, were harvested during the dissection, although very minor contamination from other cell types can never be absolutely ruled out. The fragments were digested in a solution of 0.05% trypsin/EDTA for 30 min at 37 °C. Following trypsin inactivation, the fetal tendon cells were resuspended in CXL viral supernatant and cultured overnight. The viral supernatant was replaced with fresh growth media (DMEM plus 10% FCS) the following day and all cultures were then maintained until use (either up to 48 h or from 72 h to 60 days). For embryo injections, the fetal tendon cells were trypsinized and resuspended in injection medium (DMEM plus 10% FCS) at a cell concentration of 1 × 107 cells mL−1.
Fig. 1.
Schematic diagram summarizing the steps performed in preparing fetal tendon fibroblasts for subsequent injection into early hind limb buds. Tendon was dissected from 8-day chick hind limbs (step 1) and cut into small fragments to aid cell dissociation (step 2). The resultant fetal tendon cells were resuspended and cultured in the CXL viral supernatant for 24 h (step 3). Following addition of fresh growth medium cells were cultured for specific periods of time before being resuspended in fresh growth medium at a cell concentration of 1 × 107 cells mL−1 (step 4) and injected into tendon-forming regions of the hind limb (step 5).
Avian embryo injections
Fertilized White Leghorn chicken eggs (Poyndon Farm, Herts., UK) were incubated at 38 °C in a humidified chamber until stages 18–24 (Hamburger & Hamilton, 1951). Following the removal of 1 mL of thin albumen, eggs were windowed and younger embryos stained with 0.5% neutral red (BDH)/Hanks balanced salt solution to aid in visualization. Prior to the injection of retrovirus or virally labelled fetal tendon fibroblasts, each embryo was staged according to the criteria of Hamburger & Hamilton (1951). Using a surgically sharp, flamed tungsten needle, a small incision was made into the vitelline membrane overlying the proposed injection site. Previously pulled injection quality glass micropipettes (Clark Instruments) were back filled with retroviral supernatant or virally labelled fetal tendon fibroblast cell suspension and connected to a PV820 pneumatic picopump (World Precision Instruments) set at 10 psi. The micropipette was lowered into specific regions of the hind limb (superficial dorsal and ventral regions were the focus of this study) and a volume of retroviral supernatant or fetal tendon fibroblast cell suspension was delivered. Detailed records were made of each injection, before sealing each egg with adhesive tape and returning to the incubator. For each set of injections, several embryos received sham injections, which contained only the injection medium and no virus/cells.
Histochemical detection of β-galactosidase
Embryos were incubated until stages 36–37 and subsequently stained for β-galactosidase activity. Each embryo was quickly decapitated, staged and fixed in 2% paraformaldehyde (BDH). Following an extensive period of washing in PBS (0.01 m phosphate-buffered saline, pH 7.2), embryos were transferred to a minimal volume of X-gal staining solution containing 20 mm K3Fe(CN)6, 20 mm K4Fe(CN)63H2O, 2 mm MgCl2 and 1 mg mL−1 X-gal (5-bromo-4-chloro-3-indolyl β-galactopyranoside – Molecular Probes) in PBS. All embryos were incubated in the dark overnight at 37 °C to allow development of the blue precipitate. Following re-fixing in 2% paraformaldehyde, embryos were washed in PBS before transferring to 70% ethanol. Embryos were first analysed as whole mounts for the obvious appearance of the blue reaction product before carefully dissecting to reveal any deeper labelling. All labelling was fully recorded and photographs taken at each stage of the dissection process on Kodak 64T film. Images were scanned with a Canoscan 2700F (Canon) and formatted using Adobe Photoshop (version 5.5).
Histological processing
In cases where identification of a labelled structure or the precise location of labelled cells was difficult, the tissue of interest was processed for routine histology. Briefly, dissected regions containing the labelled structures were dehydrated through a graded series of alcohols and xylene, and embedded in paraffin wax. Sections were cut at 8 µm and mounted onto glycerine albumen-coated slides. Sections were lightly counterstained with eosin before being coverslipped and viewed using a Leica DMRB microscope. Micrographs were captured using a 3CCD video camera (JVC) and formatted using Adobe Photoshop (version 5.5).
Results
Location of tendon precursors in the early limb bud
The first phase of our investigations was designed to locate the site of tendon progenitor cells within the early limb bud by injecting a lacZ encoding replication-deficient retrovirus into specific regions of stage 18–24 chick limbs. Once identified, these locations would be used in the second part of our study as the sites for injecting fetal tendon fibroblasts into the developing limb. We aimed our injections largely into superficial dorsal and ventral proximo-medial domains of the limb, regions that have recently been implicated as containing tendon progenitor cells based on the expression of the scleraxis gene from as early as stage 21 of chick limb development (Schweitzer et al. 2001).
Overall the results from our injections demonstrated that we were able to label cells destined to be incorporated into developing tendons from as early as stage 21 and these injected loci appeared to largely match those sites that are positive for scleraxis expression (Schweitzer et al. 2001). Analysis of the resulting embryos in whole mount showed that injections gave rise to labelled cells within a number of developing tendons including those of gastrocnemius externus and gastrocnemius internus, which resulted from injections made into the superficial dorsal proximo-medial limb domain at stage 21 (Fig. 2A–C). In each case studied, cells were clearly arranged in a longitudinal manner within the tissue, reproducing the orientation of cells found in the adult tendon.
Fig. 2.

Typical labelling observed in hind limbs following injection of lacZ encoding retrovirus into presumptive tendon-forming regions of early limb buds. (A) Labelled cells present within tendons along the dorsal surface of the shank (S) (white arrow) and also in the dermis underlying the dorsal surface of the limb in the region of the shank-foot junction (black arrow). (B) Closer inspection shows that labelled cells run in longitudinal rows along the tendon. (C) Histological analysis reveals that labelled cells are present within the common tendon of gastrocnemius (t. GE/GI) at the distal region of the shank. Other tissue lineages were labelled as a result of the viral injections, including skeletal muscles, dermis and other connective tissues. Histological sections illustrated here reveal labelled cells within the muscle of flexor perforatus 4 (FP4) in the shank (D), dermis of the foot (E), periosteum of the developing tibiotarsus bone (TT) within the shank (F) and within connective tissue layers of a branch of the peroneus communicans nerve within the ventral shank (G). (H) Specimen showing a small discrete cluster of individual labelled chondrocytes within the tibiotarsus (TT) of the shank. (I) Labelled cells revealed within feather bud dermis on the dorsal surface of the shank. Scale bar = 100 µm.
In addition to labelling of tendon progenitor cells, injections of the lacZ encoding retrovirus resulted in the infection of precursors of other tissue lineages including skeletal muscle, dermis, cartilage and other connective tissues. In many cases, labelled cells were identified in a variety of tissues within an individual specimen and were not restricted in their proximo-distal destination, suggesting that the precursors of these tissues may reside in similar locations within the early limb bud. In the case of muscle and various connective tissues such as those of the bone and nerves, labelled cells were often isolated or widely distributed throughout the tissue (Fig. 2D–G). In contrast, in the case of cartilage and feather bud dermis, labelled cells were typically arranged in small discrete clusters (Fig. 2H,I). It was not always possible to identify the specific tissue type labelled by the injections. For example, in the case of skeletal muscle, labelled cells were not always clearly within developing myotubes and could have been incorporated into the intimately arranged muscle connective tissues. At no stage were β-gal-positive cells present within processed hind limbs that had received sham injections.
Commitment of fetal tendon fibroblasts to the tendon cell fate
In the main part of our study we used the sites identified from the viral injections as locations for implanting fetal tendon fibroblasts into developing limb buds to ascertain whether these cells are able to re-incorporate into nascent tendons and whether they are irreversibly committed to the tendon lineage. Superficial dorsal and ventral proximo-medial domains of the limb were injected with fetal tendon fibroblasts, previously transfected with the lacZ gene via the CXL retroviral vector. In some cases the fetal tendon fibroblasts were cultured for a minimal time period, up to approximately 48 h, whilst in other cases cells were maintained in culture from 72 h to 60 days, prior to implanting back into developing limbs. In this way we could check to see whether a prolonged culture period affected the capacity of the cells to re-incorporate into the developing tendons and whether major phenotypic changes had occurred in vitro.
Results clearly showed that fetal tendon fibroblasts maintained in culture are able to re-incorporate into developing tendons following injection into primitive tendon-forming regions. Labelled cells were identified within both proximal and distal tendons and were in all cases arranged in a typical longitudinal orientation. In one case, for example, where cells grown in culture for a prolonged period were injected into the superficial ventral proximo-medial region of the hind limb at stage 19+, a region of tendon cell incorporation was subsequently evident running longitudinally along the ventral side of the distal hind limb in close proximity to the developing tarsometatarsus bone of the foot (Fig. 3A).
Fig. 3.

Fetal tendon fibroblasts transfected with the lacZ gene and maintained in culture are able to re-incorporate into developing tendons following injection of these cells into early tendon-forming regions. (A) Labelled cells running in a longitudinal orientation along the proximal portion of the ventral foot (arrow) below the tarsometatarsus (TM). (B) Histological analysis of A reveals that cells are incorporated within tendon on the ventral side of the foot (*). Cells are also revealed within the connective tissue layers of surrounding muscles (arrows). (C) Labelled cells running longitudinally within the common tendon of gastrocnemius (black arrow) of the dorsal shank of another specimen and also cells incorporated into the connective tissues of the gastrocnemius muscle (white arrow). Scale bar = 100 µm.
On histological analysis, incorporation of the fetal tendon fibroblasts within the tendon was clearly observed and found close to the myotendinous junction (Fig. 3B). Tendon cell incorporation was also observed within the proximal hind limb and in the specimen illustrated in Fig. 3(C) an area of β-gal-positive cells is apparent running longitudinally along the dorsal shank within the common tendon of gastocnemius externus and internus. In this case the fetal tendon cells were again grown in culture for a prolonged period and injected superficially into the dorsal region of the centre of the hind limb bud at stage 23−.
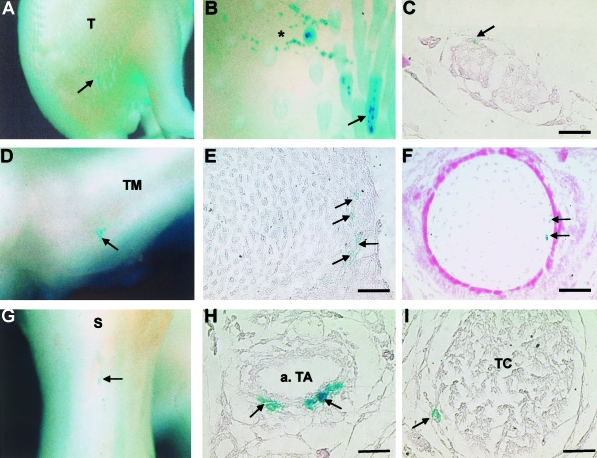
In addition to the incorporation of labelled fetal tendon fibroblasts into developing tendons, β-gal-positive cells were also surprisingly revealed in a number of other tissue lineages within the developing limb. Fetal tendon cells that had been maintained in culture prior to implantation and their progeny were found within feather buds (Fig. 4A,B), dermis (Fig. 4A,B), connective tissue layers of nerves (Fig. 4C), periosteum (Fig. 4D,E), cartilage (Fig. 4D,F), blood vessels (Fig. 4G,H) and skeletal muscle (Fig. 4G,I). In each case the patterns of cellular labelling were similar to those resulting from the viral injections in the first part of this study, with cells either isolated and widely distributed throughout the tissue or alternatively arranged in small discrete clusters, as in the case of the cartilage labelling, for example. Labelling within skeletal muscles appeared to be associated only with the connective tissue layers, the endo-, peri- and epimysium that surround and encase the muscle compartments and was not evident within the nascent or developing myotubes.
Fig. 4.
Fetal tendon fibroblasts transfected with the lacZ gene and maintained in culture are also found within a number of other tissue lineages following injection into early tendon-forming regions of developing limbs. (A) Embryo showing labelled cells incorporated in the dorsal thigh region. (B) Higher magnification of A reveals the labelled cells are within the dermis (*) and the mesenchyme of the feather buds (arrow). (C) Histological section showing labelled cells within the connective tissue layers of a superficial ventral nerve in the distal limb. (D) Labelled cells within the ridges of the tarsometatarsus (TM) of the foot (arrow). Histological analysis reveals that labelled cells are present within the periosteum (E – arrows) and also within the cartilage (F – arrows). (G) Labelled cells running longitudinally along the anterior aspect of the shank (S) (arrow). Histological analysis of G reveals that labelled cells are present within the fibrocollagenous layers of the tibialis anterior artery (a. TA) (H – arrows) and within the connective tissue layers of the tibialis cranalis (TC) muscle (I – arrow). Scale bar = 100 µm.
Where labelled fetal tendon fibroblasts had only been cultured for short periods of time (≤ 48 h), prior to implantation into developing limb buds, results showed that these cells were also able to contribute to a number of different tissue lineages and were not restricted to incorporating into just tendon. Labelled cells were often revealed in a number of tissues within the same hind limb including dermis and the connective tissue layers of nerves and blood vessels. In addition, injected cells did not appear to be restricted to incorporating into specific regions within the limb and in many cases β-gal-positive cells were observed in both proximal and more distal locations.
In the case illustrated in Fig. 5, for example, labelled cells were manifest in both the thigh and shank regions. Labelling in the shank was confined to small clusters of cells within the dermis, whilst within the thigh, labelling was evident deep within the main neurovascular bundle.
Fig. 5.
Fetal tendon fibroblasts, transfected with the lacZ gene and cultured for minimal periods of time are found in a number of different tissue types following injection into early tendon-forming regions of developing limbs. (A) Labelled cells incorporated deep within the thigh (T) just below the developing femur (F) within the main neurovascular bundle (black arrow). Labelling also present within the dermis on the dorsal surface of the shank (S) (white arrow). (B) Higher magnification of A reveals that the labelled cells are present along the length of the nerves within this region, the main ischiadicus nerve (n. I), and its branches, the peroneus communicans nerve (n. PC) and the tibialis nerve (n. T). Histological analysis reveals that the cells are incorporated into the connective tissue layers of these nerves. (C) Labelled cells within the tibialis nerve (arrow). (D) Labelled cells within the peroneus communicans nerve (arrow). (E) Labelled cells are also revealed within the fibrocollagenous layers of the iliaca artery (a. I) (arrow), which is also a constituent of this neurovascular bundle. (F) Labelled cells are present along the length of the artery and following its bifurcation in the distal region of the thigh. Scale bar = 100 µm.
Incorporated fetal tendon cells and their progeny were observed in small clusters or as individual cells running longitudinally along the ischiadicus nerve and within its branches, peroneus communicans and tibialis nerves (Fig. 5B). Although confined to the thigh, β-gal-positive cells were observed along the length of the nerves, and using histological analysis were confirmed as being incorporated within the connective tissue layers of the nerves (Fig. 5C,D). Histology also revealed the presence of labelled fetal tendon fibroblasts within the iliaca artery, which is also a constituent element of the same neurovascular bundle described here. Incorporation of these cells was noted within the fibrocollagenous layers of this structure and labelled cells appeared to span the length of the artery with cells incorporated following the bifurcation at the distal portion of the thigh (Fig. 5F).
Discussion
The genesis and patterning of tendon fibroblasts during limb formation is probably the least understood aspect of musculoskeletal development. Recent studies have shown that signals from the overlying ectoderm appear to induce and stimulate the proliferation of presumptive tendon fibroblast progenitors (Schweitzer et al. 2001); however, it is not clear whether these progenitor cells are committed to the tendon lineage at this early stage, or whether cells only become committed as they are incorporated into a developing tendon. In the present study we have begun to examine the question of tendon cell commitment in the early limb bud. We utilized the lacZ reporter gene, encoding for β-galactosidase, firstly to identify sites of tendon progenitor populations in vivo and secondly to label fetal tendon fibroblasts in vitro before implanting these cells into developing limb buds at sites known to contain tendon precursor populations.
Location of tendon precursors in the early limb bud
Using the lacZ gene as a marker, we were able to infect progenitor cells within the developing limb and therefore map the regions of the early limb bud that contain tendon precursor cells. In the majority of cases we found that injections made superficially into either dorsal or ventral proximo-medial domains of the limb subsequently gave rise to labelled cells within nascent tendons, suggesting that these locations are presumptive tendon-forming regions. These injected sites appear largely to match those regions that are positive for the expression of scleraxis, a gene that has recently been implicated in specifying tendon progenitor pools (Schweitzer et al. 2001). These results suggest that the cells in these regions contribute to tendon formation, although the cells may not necessarily have an innate commitment to this lineage at this stage.
Injections of lacZ encoding retrovirus into early limb buds also resulted in labelling within a number of other tissues lineages including skeletal muscle, dermis, cartilage and other connective tissues. In the majority of cases labelled cells were found in several different tissue types within an individual specimen. Previous studies in the lab have shown that the virus does not appear to spread widely after injection (D. J. R. Evans, unpubl. data) and therefore these results indicate that the progenitor cell populations of the various tissues infected by the virus are probably contiguous with each other in the early limb bud and not spatially restricted at this time. This is not particularly surprising as the progenitors of all these cell types are present within the mesenchyme of the limb by this stage and are not clearly delineated into different tissue primordia. Myogenic precursors, for example, migrate into the limb bud mesenchyme from adjacent somites starting at stage 17 (Jacob et al. 1979; Hayashi & Ozawa, 1991), and enter a phase of rapid proliferation, a period when the myogenic cells come into contact with other precursor populations, before subsequently aggregating into dorsal and ventral muscle masses (Chevallier, 1978; Schroeter & Tosney, 1991; Kardon, 1998; Oldfield et al. 2001).
It is essential for a normal functional limb that the various tissue elements develop in a co-ordinated fashion during embryogenesis and therefore precursor cell populations must interact with one another. Muscle and tendon progenitor cells have been shown to have a distinct relationship during early limb development with both populations spatially associated with one another during this period (Kardon, 1998; Schweitzer et al. 2001).
The pattern of labelling within each tissue type appeared to fall into one of two types. In the case of muscle and various connective tissues such as those of the bone and nerves, labelled cells were often isolated or widely distributed throughout the tissue, whereas in cartilage and feather bud dermis, labelled cells were typically arranged in small discrete clusters. These two distinct patterns of cellular labelling may reflect either proliferation or migration characteristics of the different progenitor populations. The appearance of clusters of labelled cells, for example, suggests that infected cells have proliferated in situ and have not subsequently migrated. In contrast, where labelled cells are widely distributed throughout a tissue, it is fair to say that infected cells have probably migrated extensively.
Commitment of fetal tendon fibroblasts to the tendon cell fate
We used the sites identified from the viral injections as locations for implanting fetal tendon fibroblasts into developing limb buds to demonstrate the commitment of these cells to the tendon cell fate. Our results demonstrated that fetal tendon fibroblasts, maintained for a prolonged period, were able to re-incorporate into nascent tendons. Labelled cells were identified within both proximal and distal tendons and were arranged in a typical longitudinal orientation. These cells had the capacity to incorporate along the whole length of the developing tendons including within the specialized regions of the tissue, such as at the developing muscle–tendon interface, where the myotendinous junction forms. In each of the cases where labelled cells were incorporated into tendon, injections had been made superficially into either dorsal or ventral proximo-medial domains of the limb, regions previously identified as tendon-forming regions.
It is possible that the implanted fetal tendon cells are not committed to a particular lineage, and were therefore able to respond to signals within these regions. One such signal may have specified cells to the tendon cell fate, e.g. scleraxis (Schweitzer et al. 2001) and therefore the cells were subsequently recruited into developing tendons. Alternatively, the labelled cells remained committed to the tendon lineage even after implantation and were therefore only able to become re-incorporated into tendon tissue. Initially both of these possibilities seem plausible; however, they are complicated by other data from these experiments, which surprisingly show fetal tendon fibroblasts, previously maintained in culture for either short or long periods, incorporating into a number of different tissues within the limb.
Overall we identified transplanted tendon fibroblasts within the dermis of the skin and feather buds, cartilage and also connective tissues of nerves, skeletal muscles and blood vessels. Each of these different tissue lineages is derived from progenitor cells within the limb mesenchyme, which originate from the somatic mesoderm of the lateral plate (Dhouailly & Kieny, 1972; Chevallier et al. 1977; Christ et al. 1977) and are all largely fibroblastic in nature. The results of our study could therefore suggest that the tendon cells are never fully committed to the tendon lineage, although may show some bias, and are able to incorporate into any somatic mesoderm-derived tissues when confronted by the appropriate signals. We cannot rule out the possibility, however, that the phenotype of the tendon cells may have changed whilst in culture so that cells take on an ‘uncommitted’ fibroblast phenotype, which allows them to integrate into a number of different tissue types when transplanted back into a developing limb bud. This seems less likely as cells that were maintained for either short or prolonged periods before implanting produced similar results. Whichever is the case, however, implanted fetal tendon fibroblasts appear to fully take on the characteristics of the tissue they incorporate into, as demonstrated by the patterns of cell labelling identified in each tissue.
In the case of skeletal muscle, we were not always clear whether labelled cells were incorporated into developing muscle fibres or the surrounding connective tissues. We have assumed that most of the labelled cells were integrated into the endo-, peri- or epi-mysium of the muscle; however, it is possible that the fetal tendon fibroblasts made a conversion to the myogenic lineage (usually thought to be exclusively derived from the paraxial mesoderm; reviewed in Wigmore & Evans, 2002) and fused with other myogenic cells to form nascent myotubes. The ability of fibroblasts to convert to the myogenic lineage has been demonstrated previously, with dermal fibroblasts shown to take on a myogenic phenotype in both mdx mice (the genetic homologue of Duchenne muscular dystrophy) and regenerating normal muscle (Gibson et al. 1995; Pye & Watt, 2001).
During each phase of this study transplanted cells were not restricted along the proximo-distal axis of the developing limb. In many cases cells were recruited into tissues in both the thigh and the shank regions, despite implanting the labelled cells in proximo-medial locations within the limb bud, suggesting that these cells are able to fully integrate into tissues along the length of the limb. Most tissue elements within the limb bud are specified to differentiate along the long axis of the limb in a proximal to distal sequence, with proximal structures forming first, followed by distal structures (reviewed in Wolpert, 1999). The observation that implanted cells in our experiments can contribute to structures within both the thigh and the shank regions indicates that the cells are not positionally specified at this early stage and are able to incorporate into structures located distal to the original injection site.
In conclusion, we have been able to identify regions within the developing chick limb bud that contain presumptive tendon progenitor cells from as early as stage 21, loci that appear to largely match those sites positive for scleraxis expression (Schweitzer et al. 2001). In addition, when fetal tendon fibroblasts are subsequently implanted into these locations we found that these cells successfully re-incorporated into the developing tendons, but also contributed to a number of other tissue lineages within the limb. These findings suggest that fetal tendon fibroblasts may not be irreversibly committed to a tendon cell fate during the early stages of limb morphogenesis and may be quite plastic in their ability to contribute to different tissue lineages within the limb.
Acknowledgments
We are grateful to Dr Takashi Mikawa (Cornell University Medical College) for the generous gift of the CXL packaging cell line. We also thank Dr Elaine Rees for her help with the retroviral packaging cell line and Mr Derek Scarborough for his assistance with some of the histological preparations carried out during this study. We are also grateful to Drs Jim Ralphs and Michael Benjamin for useful discussions. This work was funded by the Wellcome Trust and Cardiff University.
References
- Benjamin M, Ralphs J. The cell and developmental biology of tendons and ligaments. Int. Rev. Cytol. 2000;196:85–130. doi: 10.1016/s0074-7696(00)96003-0. [DOI] [PubMed] [Google Scholar]
- Brand B, Christ B, Jacob HJ. An experimental analysis of the developmental capabilities of distal parts of avian leg buds. Am. J. Anat. 1985;173:321–340. doi: 10.1002/aja.1001730408. [DOI] [PubMed] [Google Scholar]
- Chevallier A, Kieny M, Mauger A. Limb-somite relationship: Origin of the limb musculature. J. Embryol. Exp. Morph. 1977;41:245–258. [PubMed] [Google Scholar]
- Chevallier A. Etude de la migration des cellules somitiques dans le mésoderme somatopleural de l’ébauche de l’aile. Roux's Arch. Dev. Biol. 1978;184:57–73. doi: 10.1007/BF00848669. [DOI] [PubMed] [Google Scholar]
- Chiquet M, Fambrough DM. Chick myotendinous antigen. I. A monoclonal antibody as a marker for tendon and muscle morphogenesis. J. Cell Biol. 1984;98:1926–1936. doi: 10.1083/jcb.98.6.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ B, Jacob HJ, Jacob M. Experimental analysis of the origin of wing musculature in avian embryos. Anat. Embryol. 1977;150:171–186. doi: 10.1007/BF00316649. [DOI] [PubMed] [Google Scholar]
- Christ B, Jacob HJ, Jacob M. Differentiating abilities of avian somatopleural mesoderm. Experientia. 1979;35:1376–1378. doi: 10.1007/BF01964018. [DOI] [PubMed] [Google Scholar]
- D’Souza D, Patel K. Involvement of long- and short-range signalling during early tendon development. Anat. Embryol. 1999;200:367–375. doi: 10.1007/s004290050286. [DOI] [PubMed] [Google Scholar]
- Dhouailly D, Kieny M. The capacity of the flank somatic mesoderm of early bird embryos to participate in limb development. Dev. Biol. 1972;28:162–175. doi: 10.1016/0012-1606(72)90134-0. [DOI] [PubMed] [Google Scholar]
- Edom-Vovard F, Bonnin MA, Duprez D. Fgf8 transcripts are located in tendons during embryonic chick limb development. Mech. Dev. 2001;108:203–206. doi: 10.1016/s0925-4773(01)00483-x. [DOI] [PubMed] [Google Scholar]
- Edom-Vovard F, Schuler B, Bonnin MA, Teillet M, Duprez D. Fgf4 positively regulates scleraxis and tenascin expression in chick limb tendons. Dev. Biol. 2002;247:351–366. doi: 10.1006/dbio.2002.0707. [DOI] [PubMed] [Google Scholar]
- Gibson AJ, Karasinski J, Relvas J, Moss J, Sherratt TG, Strong PN, et al. Dermal fibroblasts convert to a myogenic lineage in mdx mouse muscle. J. Cell Sci. 1995;108:207–214. doi: 10.1242/jcs.108.1.207. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton H. A series of normal stages in the development of the chick embryo. J. Morph. 1951;88:49–92. [PubMed] [Google Scholar]
- Hayashi K, Ozawa E. Vital lablling of somite-derived myogenic cells in the chicken limb bud. Roux's Arch. Dev. Biol. 1991;200:188–192. doi: 10.1007/BF00361336. [DOI] [PubMed] [Google Scholar]
- Hurle JM, Hinchliffe JR, Ros MA, Critchlow MA, Genis-Galvez JM. The extracellular matrix architecture relating to myotendinous pattern formation in the distal part of the developing chick limb: an ultrastructural, histochemical and immunohistochemical analysis. Cell Differ. Dev. 1989;27:103–120. doi: 10.1016/0922-3371(89)90740-5. [DOI] [PubMed] [Google Scholar]
- Hurle JM, Ros Ma Ganan Y, Macias D, Critchlow M, Hinchliffe JR. Experimental analysis of the role of ECM in the patterning of distal tendons of the developing limb bud. Cell Differ. Dev. 1990;30:97–108. doi: 10.1016/0922-3371(90)90078-b. [DOI] [PubMed] [Google Scholar]
- Jacob M, Christ B, Jacob HJ. The migration of myogenic cells from the somites into the leg region of avian embryos. Anat. Embryol. 1979;157:291–309. doi: 10.1007/BF00304995. [DOI] [PubMed] [Google Scholar]
- Kardon G. Muscle and tendon morphogenesis in the avian hind limb. Development. 1998;125:4019–4032. doi: 10.1242/dev.125.20.4019. [DOI] [PubMed] [Google Scholar]
- Kieny M, Chevallier A. Autonomy of tendon development in the embryonic chick wing. J. Embryol. Exp. Morph. 1979;49:153–165. [PubMed] [Google Scholar]
- Merino R, Ganan Y, Macias D, Economides AN, Sampath KT, Hurle JM. Morphogenesis of digits in the avian limb is controlled by FGFs, TGFbetas, and noggin through BMP signalling. Dev. Biol. 1998;200:35–45. doi: 10.1006/dbio.1998.8946. [DOI] [PubMed] [Google Scholar]
- Mikawa T, Fischman DA, Dougherty JP, Brown AMC. In vivo analysis of a new LacZ. retrovirus vector suitable for a cell lineage marking in avian and other species. Exp. Cell Res. 1991;195:516–523. doi: 10.1016/0014-4827(91)90404-i. [DOI] [PubMed] [Google Scholar]
- Oldfield SF, Ralphs JR, Benjamin M, Evans DJR. Morphogenesis of the muscle–tendon interface during avian hindlimb development. J. Anat. 2001;199:222. [Google Scholar]
- Oliver G, Wehr R, Jenkins NA, Copeland NG, Cheyette BN, Hartenstein V, et al. Homeobox genes and connective tissue patterning. Development. 1995;121:693–705. doi: 10.1242/dev.121.3.693. [DOI] [PubMed] [Google Scholar]
- Ordahl CP, Le Douarin NM. Two myogenic lineages within the developing somite. Development. 1992;114:339–353. doi: 10.1242/dev.114.2.339. [DOI] [PubMed] [Google Scholar]
- Pye D, Watt D. Dermal fibroblasts participate in the formation of new muscle fibres when implanted into regenerating normal mouse muscle. J. Anat. 2001;198:163–173. doi: 10.1046/j.1469-7580.2001.19820163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros MA, Rivero FB, Hinchliffe JR, Hurle JM. Immunological and ultrastructural study of the developing tendons of the avian foot. Anat. Embryol. 1995;192:483–496. doi: 10.1007/BF00187179. [DOI] [PubMed] [Google Scholar]
- Schroeter S, Tosney KW. Spatial and temporal patterns of muscle cleavage in the chick thigh and their value as criteria for homology. Am. J. Anat. 1991;191:325–350. doi: 10.1002/aja.1001910402. [DOI] [PubMed] [Google Scholar]
- Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, et al. Analysis of tendon cell fate using scleraxis, a specific marker for tendons and ligaments. Development. 2001;128:3855–3866. doi: 10.1242/dev.128.19.3855. [DOI] [PubMed] [Google Scholar]
- Shellswell GB, Wolpert L. The pattern of muscle and tendon development in the chick wing. In: Ede DA, Hinchliffe JR, Balls M, editors. Vertebrate Limb and Somite Morphogenesis. Cambridge: Cambridge University Press; 1977. pp. 71–86. [Google Scholar]
- Wigmore PM, Evans DJR. Molecular and cellular mechanisms involved in the generation of fiber diversity during myogenesis. Int. Rev. Cytol. 2002;216:175–232. doi: 10.1016/s0074-7696(02)16006-2. [DOI] [PubMed] [Google Scholar]
- Wolpert L. Vertebrate limb development and malformations. Pediatr. Res. 1999;46:247–254. doi: 10.1203/00006450-199909000-00001. [DOI] [PubMed] [Google Scholar]
- Xu PX, Cheng J, Epstein JA, Maas RL. Mouse Eya genes are expressed during limb tendon development and encode a transcriptional activation function. Proc. Natl. Acad. Sci. USA. 1997;94:11974–11979. doi: 10.1073/pnas.94.22.11974. [DOI] [PMC free article] [PubMed] [Google Scholar]