Abstract
Gal4-mediated activation of GAL gene transcription in Saccharomyces cerevisiae requires the interaction of Gal3 with Gal80, the Gal4 inhibitor protein. While it is known that galactose and ATP activates Gal3 interaction with Gal80, neither the mechanism of activation nor the surface that binds to Gal80 is known. We addressed this through intragenic suppression of GAL3C alleles that cause galactose-independent Gal3–Gal80 interaction. We created a new allele, GAL3SOC, and showed that it suppressed a new GAL3C allele. We tested the effect of GAL3SOC on several newly isolated and existing GAL3C alleles that map throughout the gene. All except one GAL3C allele, D368V, were suppressible by GAL3SOC. GAL3SOC and all GAL3C alleles were localized on a Gal3 homology model that is based on the structure of the highly related Gal1 protein. These results provide evidence for allosterism in the galactose- and ATP-activation of Gal3 binding to Gal80. In addition, because D368V and residues corresponding to Gal80-nonbinder mutations colocalized to a domain that is absent in homologous proteins that do not bind to Gal80, we suggest that D368 is a part of the Gal80-binding surface.
REGULATION of gene expression is a predominant way for cells to react and adapt to environmental signals. This is typically carried out by genetic switches that either activate or repress the transcription of a specific subset of genes. One example is the well-studied GAL gene switch that is used by Saccharomyces cerevisiae (Sc) and Kluyveromyces lactis (Kl) and related yeasts to adapt to the presence of galactose, melibiose, and lactose by activating the expression of the GAL genes that encode enzymes for metabolizing these carbon sources (Johnston 1987; Lohr et al. 1995; Zenke et al. 1996; Rubio-Texeira 2005; Sellick and Reece 2005).
The S. cerevisiae GAL gene switch operates through the activities of three regulatory proteins: a transcriptional activator ScGal4, its inhibitor ScGal80, and the galactose sensor Gal3. ScGal4 binds to a consensus DNA sequence (UASGAL) within the GAL gene promoters independently of galactose. In the presence of galactose the ScGal4 transcription activation domain activates transcription, but in the absence of galactose the ScGal4 activation domain is inhibited through its binding to ScGal80 (Torchia et al. 1984; Yocum and Johnston 1984; Bram and Kornberg 1985; Ma and Ptashne 1987; Selleck and Majors 1987; Leuther and Johnston 1992). Relief of this inhibition occurs when galactose- and ATP-activated Gal3 binds to ScGal80 (Bajwa et al. 1988; Bhat and Hopper 1992; Suzuki-Fujimoto et al. 1996; Blank et al. 1997; Yano and Fukasawa 1997; Platt and Reece 1998; Peng and Hopper 2002; Pilauri et al. 2005). The S. cerevisiae GAL gene switch acts rapidly, with galactose-induced GAL gene mRNAs appearing within minutes of exposure to galactose (Yarger et al. 1984; Torchia and Hopper 1986; Bryant and Ptashne 2003).
Gal3 is paralogous with the galactokinase enzyme ScGal1 in S. cerevisiae (Wolfe and Shields 1997) and shares 72% amino acid identity with it. ScGal1 is a bifunctional protein that serves as a galactokinase and is activated by galactose and ATP to bind to ScGal80 (Bhat and Hopper 1991, 1992; Platt and Reece 1998; Timson et al. 2002). However, ScGal1 does not contribute to the normally rapid GAL gene switch in response to galactose since in contrast to GAL3, the ScGAL1 gene is not expressed at detectable levels in the absence of galactose (St. John and Davis 1981). The capacity of galactose- and ATP-activated ScGal1 to bind to ScGal80 and relieve inhibition of ScGal4 is independent of its galactokinase activity (Bhat and Hopper 1992). Moreover, Gal3 does not possess galactokinase activity (Bhat et al. 1990), but can acquire such activity with the insertion of a serine and alanine at a highly conserved P-loop motif (Platt et al. 2000). On the basis of all of the above, it is likely therefore that Gal3 lost its galactokinase activity in the process of evolving to become a more specialized component of the GAL gene switch in S. cerevisiae.
The GAL gene switches of S. cerevisiae and K. lactis are overall similar but are not interchangeable due to several differences. K. lactis lacks a GAL3 gene, and the K. lactis GAL gene switch activity corresponding to that of Gal3 is carried out by the bifunctional KlGal1 protein, which serves as a galactokinase and a galactose- and ATP-dependent KlGal80-binding protein (Meyer et al. 1991; Zenke et al. 1996; Vollenbroich et al. 1999; Menezes et al. 2003). Unlike ScGAL1, KlGAL1 is expressed at appreciable levels in the absence of galactose, providing ample levels of the KlGal1 protein (Dickson and Riley 1989). Although ScGal3 is 57% identical to KlGal1, it binds only poorly to KlGal80 and supports only low levels of KlGal4 activation (Zenke et al. 1996). Another difference between the S. cerevisiae and K. lactis GAL gene switches is that KlGAL4, but not ScGAL4, is subjected to autoregulation due the presence of a UASGAL in its promoter (Czyz et al. 1993).
Genetic studies of Gal3 and KlGal1 have yielded mutants that affect their interactions with ScGal80 and KlGal80, respectively. Constitutive mutants have been identified and characterized for both GAL3 (GAL3C alleles) and KlGAL1. They cause galactose-independent interaction with ScGal80/KlGal80 and galactose-independent activation of the switch (Blank et al. 1997; Vollenbroich et al. 1999; Menezes et al. 2003). Mutants of gal3 and Klgal1 have been isolated and shown to cause defects in interactions with ScGal80 and KlGal80, respectively (Suzuki-Fujimoto et al. 1996; Vollenbroich et al. 1999; Menezes et al. 2003). However, the amino acid changes in the gal3 mutants are not known.
On the basis of sequence homologies, the Gal3, ScGal1, and KlGal1 proteins belong to the GHMP superfamily of small molecule kinases that includes galactokinase, homoserine kinase, mevalonate kinase, and phosphomevalonate kinase (Bork et al. 1993). The structures of several members of the superfamily have been determined, including homoserine kinase, mevalonate kinase, phosphomevalonate kinase, and four galactokinases. The galactokinases are those from Lactococcus lactis (Thoden and Holden 2003), Pyrococcus furiosus (Hartley et al. 2004), human (Thoden et al. 2005b), and Saccharomyces cerevisiae (Thoden et al. 2005a).
KlGAL1 constitutive and Klgal1 interaction-defective mutants have been mapped to a KlGal1 homology model based on the structure of the mevalonate kinase (MVK) from Methanococcus jannaschii, a protein that shares only 13% amino acid identity with KlGal1. The KlGAL1 constitutive mutants were shown to localize at a putative “hinge region” of the model, whereas the Klgal1 interaction-defective mutants localized predominantly within the so-called “upper-” and “lower-” lip domains of the model. On the basis of the localization of the various mutations on the KlGal1 homology model, it has been proposed that galactose and ATP induce a conformational change within KlGal1 that brings the two separated upper- and lower-lip domains together to form the common binding surface for KlGal80 (Menezes et al. 2003). However, the predictive value of the KlGal1 homology model is limited, as it does not account for a third of the amino acid sequence of Gal3 and KlGal1, precluding reliable mapping of a subset of mutants in the model.
In this work, we address the mechanism by which the binding of galactose and ATP activates Gal3 binding to ScGal80. We identified a suppressor (GAL3SOC) of a new GAL3C mutation and determined its in vivo and in vitro suppression activities for several new and existing GAL3C alleles. GAL3SOC suppressed all but one of the GAL3C alleles. We localized the GAL3 and KlGAL1 mutations on a Gal3 homology model based on a crystal structure of the ScGal1 protein that provided a full template for Gal3, including a 100 amino acid domain that was not accounted for in the KlGal1 homology model. The GAL3SOC allele and the suppressible GAL3C alleles colocalized to one region of the model whereas the single nonsuppressible allele mapped to another region along with Klgal1 interaction-defective mutations. We suggest that the first region is involved in allosterism, while the second region is involved in binding to ScGal80.
MATERIALS AND METHODS
Media and growth conditions:
Yeast were cultured at 30° on either standard nonselective YEP medium or selective SC medium (Sherman 1991). For culture under uninduced conditions the carbon source used was 0.05% (final concentration) glucose, 3% glycerol, and 2% lactic acid (pH adjusted to 5.7 with potassium hydroxide). For culture under inducing conditions the above media was supplemented with 2% (final concentration) galactose. Bacteria were cultured at 37° in LB broth or agar plates supplemented with 50 mg/liter of ampicillin.
Yeast and bacteria strains:
The yeast strain AH109 (MATa trp1-901 leu2-3,112 ura3-52 his3-200 gal4Δ gal80Δ LYS2∷GAL1UAS-GAL1TATA-HIS3 GAL2UAS-GAL2TATA-ADE2 URA3∷MEL1UAS-MEL1TATA-lacZ) was used for the yeast two-hybrid selection (CLONTECH). The yeast strains Sc781 (MATa ade1 ile leu2-3,112 ura3-52 trp1-HIII his3-Δ1 MEL1 LYS2∷GAL1UAS-GAL1TATA-HIS3 gal3Δ gal1Δ) and Sc787 (MATa ade1 ile leu2-3,112 ura3-52 trp1-HIII his3-Δ1 MEL1 LYS2∷GAL1UAS-GAL1TATA-HIS3 gal3Δ gal80Δ gal1Δ) were derived from strain SJ21R (Johnston and Hopper 1982), as previously described (Blank et al. 1997). Both Sc781 and Sc787 strains carry the chromosomal MEL1 gene that has a single Gal4-binding site within its promoter and an integrated HIS3 gene (expressed from a GAL promoter bearing four Gal4-binding sites (PGALHIS3) (Flick and Johnston 1990). The bacteria Escherichia coli MG7-α was used to propagate plasmids (Griffith and Gietz 2003).
PCR mutagenesis of GAL3, gap-repair, and yeast two-hybrid selection:
Mutagenesis of the GAL3 gene was performed using Taq DNA polymerase (Sigma, St. Louis) and the manganese (Mn)-dITP error-prone PCR method (Xu et al. 1999; Fenton et al. 2002). The plasmid pAKS46 carrying the GAL3 gene fused to the DNA-binding domain of ScGAL4 was used as the template for the PCR reactions. The GAL3 gene was divided into four regions by using four sets of primers: region-I, DIEP42 (5′-GCGACATCATCATCGGAAGAGAGTAG-3′) and DIEP43 (5′-GCAGATGAGAGTCCACCACCAGTAGG-3′); region-II, DIEP44 (5′-GACGAAAAAAATCCATCCATTACCTTAAC-3′) and DIEP45 (5′-CAGACGTTGCTTGATCCATACCACC-3′); region-III, DIEP46 (5′-GCTCCGGAAAGATTTAATAATACACCC-3′) and DIEP47 (5′-GCATCTTGAGTAAACGTTCAATACCAG-3′); and region-IV, DIEP48 (5′-GAGTAATAGAGGTAACAGTTGCTG-3′) and DIEP49 (5′-CTTTCGCGCCACTTCCTTGCCGT-3′). A PCR product pool was generated for each region separately and was used with the cognate-gapped GAL3 gene in pAKS46 to reconstitute the complete plasmid, using a homologous recombination-based gap-repair method (Muhlrad et al. 1992) as described previously (Blank et al. 1997). The four cognate-gapped plasmids were created by the following restriction digestions: gap-I, EcoRI/BglII; gap-II, BlpI; gap-III, BglII/XcmI; gap-IV, XcmI/NcoI. Each PCR product pool and cognate-gapped plasmid were combined and used to transform the yeast strain AH109 carrying pVP16GAL80. We used ∼1 × 105 cells for each gap-repair transformation, resulting in ∼800 primary Trp+ Leu+ prototrophs for each gap. The transformants were then replica-plated to select for Trp+ Leu+ Ade+ prototrophs in the absence of galactose. For each gap, ∼80 Trp+ Leu+ Ade+ prototrophs arose. Plasmid was isolated from the prototrophs and retransformed into AH109 carrying pVP16GAL80 to retest for the two-hybrid interaction. Candidates that repeated the two-hybrid interaction in the absence of galactose were sequenced and subjected to further analysis.
Plasmid constructions:
The plasmid pAKS46 (DBD–GAL3) was constructed as follows. The GAL3 gene was amplified by PCR using the primers G1 (5′-CGGATCCGCATGAATACAAACGTTCCAATA-3′) and BLNK06 (5′-AGCTGGCGAAAGGGGGATGTG-3′) from the template pTEB16 (Blank et al. 1997). The ∼2.1-kb product was digested with BamHI and then ligated to pGBT9 (CLONTECH) digested with BamHI. The plasmids pVP16GAL80 (Sil et al. 1999), pTEB16 (GAL3 under its native promoter), pMPW60 (GSTGAL3 under the ADH2 promoter), pMPW61 (GST under the ADH2 promoter), and pMPW82 (ScGAL80 under the ADH2 promoter) were constructed as previously described (Blank et al. 1997). Plasmids pGP160 and pGP161 were derived from pTEB16 (Blank et al. 1997) by site-directed mutagenesis. All other plasmids were generated by standard restriction digestion and ligation.
Spot assay for cell growth:
Sc781 transformants were grown to an OD600 of ∼1.0 in selective media. Each culture was adjusted to the same number of cells, and serial 10-fold dilutions were made with dH2O. A total of 6 μl of a 10−1, 10−2, 10−3, and 10−4 dilution of each cell culture was spotted onto tryptophan and histidine dropout agar plates supplemented with 10 mm 3-AT (3-amino-1,2,4-triazole). The growth of cells was evaluated on plates incubated for 3–6 days.
MEL1 α-galactosidase enzyme activity assay:
To determine the expression level of the MEL1 α-galactosidase gene, Sc781 cells harboring the indicated plasmids were grown to an OD600 of ∼0.5 in 5 ml of selective media (−galactose, uninduced cultures). To induce the cultures, galactose was added to the uninduced cultures to a final concentration of 2%. Both the uninduced and the induced cultures were incubated for an additional 3 hr. A total of 1 ml of each culture was centrifuged and the supernatant was assayed for α-galactosidase activity as follows. A total of 120 μl of the supernatant was mixed with 360 μl of assay buffer [2 volumes of 0.5 m sodium acetate, pH 4.5, and 1 volume of 100 mm p-nitrophenyl α-d-galactopyranoside (PNP-α-Gal, Sigma)]. The reaction was incubated at 30° for 5 hr and terminated by adding 520 μl of stop buffer (1 m sodium carbonate), and the OD410 was recorded (Hao et al. 2004). The selective media served as the blank. Three independent transformants for each plasmid were assayed and the average and standard deviation were reported. The amount of PNP-α-Gal hydrolyzed can be determined as previously described (Post-Beittenmiller et al. 1984) by calculating the concentration of p-nitrophenol using its molar extinction coefficient (18.3 × 103).
Pull-down assay for GSTGal3 and ScGal80 interaction:
Sc787 cells cotransformed with plasmids carrying GSTGAL3 and ScGAL80 were grown to mid-log phase for preparation of yeast whole-cell extracts. The pull-down binding assay utilizing GSTGal3 fusion was carried out as previously described with the following modifications. Whole-cell extracts containing GSTGal3 and ScGal80 were prepared as described (Blank et al. 1997) using a modified lysis buffer (20 mm HEPES, pH 7.4, 0.5% Triton X-100, 200 mm NaCl, 0.5 mm EDTA, 2 mm DTT, and 5 mm MgCl2). Protease inhibitor cocktails (PIC-D: 88 mg/ml PMSF and 1 mg/ml pepstatin A in DMSO; PIC-W: 157 mg/ml benzamidine, 0.5 mg leupeptin, and 0.5 mg bestatin in water) were added to all lysis buffer and all subsequent solutions at 1:1000 dilutions. The whole-cell extract (1–8 mg) was brought up to a volume of 400 μl with lysis buffer containing 2 mm ATP (−galactose) or lysis buffer containing 2 mm ATP and 25 mm galactose (+galactose). Glutathione sepharose beads (Amersham Biosciences, Piscataway, NJ) were equilibrated with the lysis buffer and resuspended in the lysis buffer as a 50% slurry. The whole-cell extracts were then incubated with 50 μl of 50% glutathione sepharose beads on a rotator at 4° for 4 hr. The beads were pelleted and washed three times with 500 μl of either lysis buffer containing 2 mm ATP or lysis buffer containing 2 mm ATP and 25 mm galactose. The beads were then boiled for 5 min in 40 μl of 1× SDS–PAGE sample-loading buffer (62.5 mm Tris-HCl, pH 6.8, 10% glycerol, 2% SDS, 100 mm DTT, and 0.003% Pyronin Y) before analysis by standard SDS–PAGE and Western blot. Antibodies for GST and ScGal80 were used together at a 1:200 dilution.
Multiple sequence alignment:
The alignment was generated by the T-Coffee server (3DCoffee, regular mode) (Poirot et al. 2004). The results were saved as ALN and MSF files and edited further using the GeneDoc program (http://www.psc.edu/biomed/genedoc/), CLUSTAL_X (Thompson et al. 1997), and Microsoft Word.
Homology modeling:
The Gal3 homology model was based on the structure of ScGal1 (Thoden et al. 2005a), which shares 72% protein-sequence identity with Gal3. The homology model was obtained using the threading path as implemented in Prime v1.2. Energy minimization of main chain and side chain interactions was performed as standard in this protocol. The backbone angles of the resulting structure fell within the allowed regions of a Ramachandran map, indicating that the energy minimization had not perturbed the geometry of the structure. Residues 290–297 were not modeled because this region was disordered in the ScGal1 structure. This precluded the mapping of the Gal3-equivalent positions of the KlGAL1 constitutive mutants E285K and G286F in Figure 5. The model was rendered using the programs GRASP (Nicholls et al. 1991) and MOLSCRIPT (Kraulis 1991).
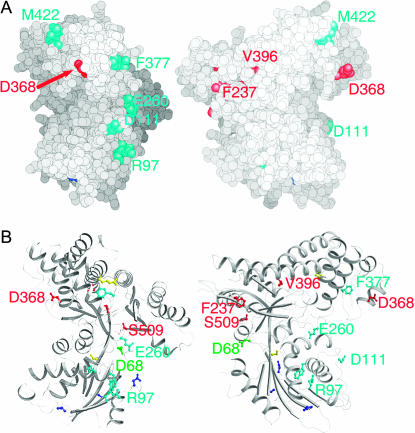
Figure 5.
Locations of residues corresponding to mutations affecting binding to Sc/KlGal80 ona Gal3 homology model.(A) Location of residues shown in space-filling representation. Residues colored cyan are the Gal3 equivalent residues of Klgal1 reg−kin+ mutations. The positions of GAL3C mutations included in the suppression analyses are red. The two views are approximately orthogonal, due to a rotation around the y-axis in a counterclockwise direction. (B) Ribbon diagram in the same orientations as in A. In addition to the coloring adopted in A, residues in yellow are the Gal3-equivalent positions of KlGal1 constitutive mutations, residues in blue are the Gal3-equivalent positions of Klgal1 reg−kin− mutations, and the position of GAL3SOC is green. Residues that are described in detail in the text have been labeled. For clarity, the labeling of other residues has been omitted.
RESULTS
Isolation of new GAL3C alleles:
A previously conducted extensive selection for GAL3C mutations that cause galactose-independent expression of a PGALHIS3 reporter yielded five mutations that occurred at only four discrete positions throughout the GAL3 gene (Blank et al. 1997). We sought to isolate additional GAL3C mutations representing, if possible, other locations within the gene. To isolate new GAL3C alleles, we carried out a yeast two-hybrid selection for GAL3 mutations that cause Gal3 to bind to ScGal80 independently of galactose. The wild-type Gal3 protein interacts with ScGal80 in the two-hybrid assay only in the presence of galactose. We identified six novel GAL3 missense variants that exhibited a two-hybrid interaction with ScGal80 in the absence of galactose (data not shown). Their locations are given in Table 1.
TABLE 1.
GAL3 and KlGAL1 mutations
| Mutation | Reference | |
|---|---|---|
| GAL3SOC allele | ||
| D68S | This study | |
| GAL3C alleles | ||
| L50P | This study | |
| V69E | This study | |
| V69E/D70V | Blank et al. (1997) | |
| V203I | This study | |
| F237Y | Blank et al. (1997) | |
| D368V | Blank et al. (1997) | |
| V396A | This study | |
| F414L | This study | |
| S509D | This study | |
| S509P/L | Blank et al. (1997) | |
| K510E | This study | |
| KlGAL1 constitutives | Equivalent Gal3 position | Reference |
| S40F | S44 | Menezes et al. (2003) |
| E285K | H291 | Vollenbroich et al. (1999) |
| G286F | S295 | Menezes et al. (2003) |
| L385F | L394 | Menezes et al. (2003) |
| KlGAL1 reg− kin+ | ||
| R94C | R97 | Vollenbroich et al. (1999) |
| D108A | D111 | Menezes et al. (2003) |
| W114R | W117 | Menezes et al. (2003) |
| E254C | E260 | Vollenbroich et al. (1999) |
| F368S/M409S | F377/M422 | Menezes et al. (2003) |
| KlGAL1 reg− kin− | ||
| T74P/F91L | L78/F94 | Menezes et al. (2003) |
| V146F | C152 | Vollenbroich et al. (1999) |
Each GAL3 mutation was recreated as a GSTGAL3 fusion and evaluated for its effect for binding to ScGal80 in an in vitro pull-down assay. Wild-type GSTGal3 interacts with ScGal80 only in the presence of galactose. In contrast, all six mutants had a galactose-independent interaction with ScGal80 (Figure 1A). The GST-fusions of all of the variants except K510E were present in cells at levels equivalent to that of the wild-type GSTGal3. The K510E variant was present at very low levels. The remaining mutants, except L50P, retained full galactose-responsiveness, showing ScGal80-binding levels similar to wild-type GSTGal3 in the presence of galactose.
Figure 1.
Newly isolated GAL3 mutations constitutively activate the GAL gene switch.(A) Galactose-independent interaction between GSTGal3 and Gal80 in vitro, as determined by a pull-down assay. Yeast whole-cell extracts containing GSTGal3 and Gal80 were incubated at 4° for 4 hr with glutathione sepharose, washed three times, and then boiled and separated on SDS–PAGE and analyzed by Western blot. GSTGAL3 and GAL80 were expressed under the ADH2 promoter in pMPW60 and pMPW82, respectively. (B) Constitutive in vivo expression of the integrated PGALHIS3 reporter gene determined by a colony-growth assay. Strain Sc781 carrying the indicated plasmid was grown in liquid culture to late log phase and adjusted to the same number of cells. Dilutions (10-fold) were spotted on selective agar plates and incubated for 3–6 days. Wild-type and GAL3 mutants were expressed under their native promoter in pTEB16. (C) Constitutive expression of the endogenous MEL1 gene determined by an α-galactosidase enzymatic-activity assay. Strain Sc781 carrying the indicated plasmid was grown in liquid culture to an OD600 of 0.5, and the cell-free culture media was removed and assayed for α-galactosidase activity. Three independent transformants were assayed, and the average and standard deviation were reported. Wild-type and GAL3 mutants were expressed under their native promoter in pTEB16.
Since the new GAL3 mutants exhibited galactose-independent interaction with ScGal80, we expected that they would cause constitutive activation of ScGal4 in vivo. To confirm this, we first established each mutation within an otherwise wild-type GAL3 gene under its native promoter in the yeast strain Sc781. Each GAL3 mutation was then evaluated for its effect on the GAL gene switch by carrying out a PGALHIS3 expression-dependent colony-growth assay. Wild-type GAL3 activated the GAL gene expression only in the presence of galactose. Each of the six GAL3 mutations caused constitutive, galactose-independent activation of the GAL switch, as determined by colony growth in the absence of histidine and galactose (Figure 1B), indicating that they are new GAL3C alleles. These new GAL3C alleles, with the exception of L50P mutant, retained galactose-responsiveness and fully activated the GAL switch in the presence of galactose. The somewhat weaker galactose-responsiveness of the L50P that was observed might be due to its somewhat weaker interaction with ScGal80 in the presence of galactose (Figure 1A).
To quantify the constitutivity of these new GAL3C alleles, expression levels of an endogenous, galactose-regulated gene, MEL1 (Post-Beittenmiller et al. 1984) was determined using an enzymatic-activity assay for its encoded α-galactosidase. Wild-type GAL3 supported appreciable α-galactosidase activity only in the presence of galactose, whereas the L50P, V69E, and V396A alleles supported appreciable α-galactosidase activity independently of galactose. The remaining three GAL3C alleles supported much lower levels of activity (Figure 1C). The L50P variant exhibited a weak response to galactose compared to wild-type GAL3 and the other variants. The results in Figure 1 confirm that the mutations identified from the two-hybrid selection are indeed GAL3C alleles.
Five of the six GAL3C alleles isolated here were found to contain new mutations that are different from those of pre-existing GAL3C alleles (Blank et al. 1997). The V69E mutation was the only one previously identified as a double mutation V69E/D70V, but until now was not known to be the mutation conferring constitutivity. Clearly, the V69E alone is sufficient to cause the constitutive phenotype. The new GAL3C alleles did not reveal any strong clustering within the GAL3 sequence either as a group or when considered with the previously isolated GAL3C alleles. The widespread distribution of GAL3C throughout the gene might explain why previous attempts to isolate a single peptide of Gal3 or KlGal1 able to interact with ScGal80 or KlGal80, respectively, were unsuccessful (Diep and Hopper, unpublished data; Vollenbroich et al. 1999).
GAL3-D68S suppressed the constitutive phenotype conferred by GAL3-S509D:
In comparing the structures of homoserine kinase (HSK, PDB code 1H72) and the L. lactis galactokinase (PDB code 1PIE), we noticed a conserved interaction. In HSK, E31 formed a hydrogen bond with T291. In the L. lactis galactokinase, the positions of these residues were reciprocally exchanged to T57 and Q388 such that they are equivalent to T291 and E31, respectively, of HSK. This type of exchange is known as “concerted substitution.” Curiously, we have isolated GAL3C mutations that closely correspond to the positions described above. The V69E mutation is next to D68, which corresponds to E31 of HSK in a sequence alignment, while the S509P/L mutations correspond to T291 of HSK (alignment not shown). We wondered if a concerted substitution could also occur in Gal3 by a reciprocal exchange of D68 and S509, as observed for the L. lactis galactokinase.
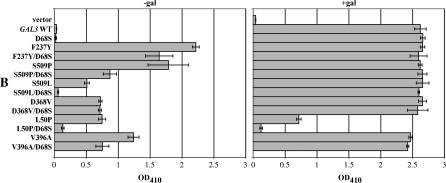
To investigate this further, we created two new mutations to produce a reciprocal exchange in side chain characteristics: D68S and S509D. The GAL gene switch phenotype of cells carrying the single and double mutations was determined by evaluating PGALHIS3-dependent cell growth of Sc781. Cells carrying the D68S mutation had a phenotype similar to wild-type GAL3 and grew on histidine-deficient selective agar only in the presence of galactose (see below in Figure 3A), whereas cells with the S509D mutation grew in the absence of galactose (Figure 2A). Therefore, the GAL3–S509D is a new GAL3C allele. In contrast to the robust growth of cells carrying GAL3–S509D, cells carrying GAL3-S509D/D68S grew poorly in the absence of galactose (Figure 2A). In the presence of galactose, the growth of cells carrying the GAL3–D68S, the GAL3–S509D, or the GAL3–S509D/D68S was similar to that of wild-type GAL3, indicating that the suppressed growth of the double mutant was not due to a loss of Gal3 function.
Figure 3.
Specificity in suppression of GAL3C alleles.(A) Expression of the integrated PGALHIS3 reporter gene in the yeast strain Sc781 determined by a colony-growth assay. (B) Expression of the endogenous MEL1 gene in the yeast strain Sc781 determined by an α-galactosidase enzymatic-activity assay. (C) Interaction between Gal3 and Gal80 in vitro as determined by a pull-down assay. (D) Protein levels of the Gal3-V396A and Gal3-V396A/D68S mutant proteins not tagged with GST, as determined by a Western blot.
Figure 2.
The suppression of constitutivity of GAL3-S509D by GAL3-D68S.(A) Expression of the integrated PGALHIS3 reporter gene in the yeast strain Sc781 determined by a colony-growth assay. (B) Expression of the endogenous MEL1 gene in the yeast strain Sc781 determined by an α-galactosidase enzymatic-activity assay. (C) Interaction between Gal3 and Gal80 in vitro as determined by a pull-down assay.
We next quantified the suppression using the α-galactosidase enzymatic-activity assay for MEL1 gene expression. Cells with the S509D mutation expressed MEL1 in the absence of galactose (Figure 2B), confirming that it is a constitutive allele. In contrast, cells with the S509D/D68S mutation expressed MEL1 at a much lower level, verifying the suppression observed above. Cells with either the S509D or S509D/D68S mutations showed fully induced MEL1 expression in response to galactose similar to wild-type GAL3.
The suppression that was evident from the PGALHIS3 and MEL1 reporter assays was most likely due to suppression of Gal3C binding to Gal80. To confirm this we performed GSTGAL3 pull-down assays. The GSTGal3–S509D protein showed galactose-independent interaction with ScGal80 (Figure 2C). The GSTGal3–S509D/D68S protein showed notably less interaction with ScGal80 in the absence of galactose. However, in the presence of galactose, both proteins exhibited levels of binding to ScGal80 equivalent to the wild-type GSTGal3.
The results of all the above assays taken together firmly establish D68S as an intragenic suppressor of S509D, a mutation that we show confers to Gal3 the capacity to bind to ScGal80 independently of the normally required ligand, galactose. We call the D68S allele GAL3SOC (suppressor of constitutivity). These results demonstrate for the first time that a single amino acid substitution within the N-terminal domain of Gal3 can alter a new property of the protein that is conferred by a single amino acid substitution within its C terminus.
Suppression of constitutivity by GAL3SOC is GAL3C allele specific:
The discovery of the GAL3SOC allele led us to test its ability to suppress other GAL3C mutations that showed appreciable constitutivity. These were L50P, F237Y, D368V, V396A, S509P, and S509L. Mutations V203I, F414L, and K510E were not included in the suppression analysis because their levels of constitutivity in the MEL1 (α-galactosidase) and the pull-down assays were too low for reliable suppression testing.
Each mutation was combined with D68S to produce a double mutation allele. Suppression of each was evaluated using the following assays: PGALHIS3 reporter-based colony growth (Figure 3A), MEL1-expressed α-galactosidase activity (Figure 3B), and the GSTGal3-ScGal80 pull-down assay (Figure 3C). As mentioned above, the GAL3–D68S allele yielded similar results to those of wild-type GAL3 in all three assays. All double mutants except L50P/D68S retained full galactose-responsive induction as indicated by all three assays. D68S suppression of constitutivity was evident in all three assays for the S509P and S509L alleles. The F237Y allele was not suppressed in the PGALHIS3-based growth assay, weakly suppressed in the MEL1 α-galactosidase assay, and moderately suppressed in the pull-down assay. V396A was moderately suppressed by D68S in both the PGALHIS3 and MEL1 assays. In repeated attempts, the GSTGal3–V396A/D68S protein was not detectable. However, the untagged protein was detectable (Figure 3D), consistent with its ability to fully induce the GAL switch in the presence of galactose in the PGALHIS3 and MEL1 assays. In contrast to the four suppressible alleles described above, D368V/D68S showed no suppression in all three assays.
We note that the magnitude of constitutivity determined for some GAL3C alleles was different, depending on the assay used. For example, the S509P allele showed moderate GAL gene expression in vivo, but only weak interaction with ScGal80 in the pull-down assay. We do not know the reason for this, but perhaps it reflects mechanistic aspects of the Gal3–ScGal80 interplay that are not well understood. For example, recent evidence points to ScGal80 nucleocytoplasmic shuttling, monomer–dimer equilibrium, and monomer vs. dimer preferences for binding Gal3 and ScGal4 as key features of the GAL gene switch (Pilauri et al. 2005).
Despite the discrepancies between the in vivo and in vitro assays, the results presented here show that the GAL3SOC allele suppressed the GAL3C alleles at positions 237, 396, and 509, but not at position 368.
Homology model of Gal3:
Gal3 and ScGal1 belong to the GHMP kinase superfamily, but they contain an ∼100 amino acid insertion domain relative to other members (Figure 4). All family members contain conserved motifs for the binding of ATP and specific substrates. The structures of several members have been determined and found to have a shared core fold (Zhou et al. 2000; Bonanno et al. 2001; Romanowski et al. 2002; Yang et al. 2002; Luz et al. 2003; Thoden and Holden 2003). In general, the polypeptide chain folds into two domains of approximately equal size. The active site is located at a cleft between the N- and C-terminal domains.
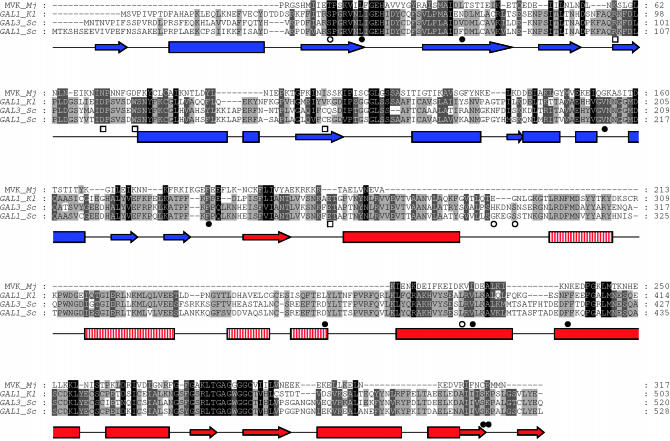
Figure 4.
Multiple sequence alignment of Gal3 and related proteins and the predicted secondary structure of the Gal3 homology model. The multiple sequence alignment was generated using the T-Coffee server. The predicted secondary structure of the Gal3 homology model is represented by arrows (β-sheets) and rectangles (α-helices). The N-terminal domain is blue while the C-terminal domain is red. The four-helical insertion domain is represented by vertical red lines. (•) GAL3C alleles, (○) KlGAL1 constitutive mutants, and (□) Klgal1 interaction-defective mutants. MVK_Mj is mevalonate kinase of Methanococcus jannaschii, GAL1_Kl is GAL1 of Kluyveromyces lactis, GAL3_Sc and GAL1_Sc are GAL3 and GAL1, respectively, of Saccharomyces cerevisiae.
To understand the allele-specific suppression data in a structural context, we calculated a homology model for Gal3 (Figure 5) on the basis of the recently determined ligand-bound structure of ScGal1 (Thoden et al. 2005a), which shares 72% amino acid identity with Gal3. Analysis of the surface charge showed a negatively charged channel that housed the galactose and ANP. In general, the remainder of the surface was uncharged with the exception of the C terminus, which was more negatively charged. In this model, residues 285–381 compose the insertion domain. It forms a four-helix motif and folds on the edge of the C-terminal domain (Figures 4 and 5).
The GAL3SOC and GAL3C alleles that were included in the suppression analysis (F237Y, D368V, V396A, and S509P/L) were mapped on the Gal3 homology model, along with the Gal3 equivalent positions of KlGAL1 mutants shown in Table 1. The mutants were found to localize predominantly to two regions. One region consists of the GAL3SOC alleles (green in Figure 5) and the suppressible GAL3C alleles (F237Y, V396A, and S509P/L, red in Figure 5). This region is localized around a previously designated “hinge region” (Menezes et al. 2003). The second region is remote from the hinge region and consists of the single nonsuppressible GAL3C allele (D368V, red) along with Klgal1 interaction-defective mutants (cyan in Figure 5). Also in the model, the KlGAL1-constitutive mutants are yellow and the Klgal1 mutants defective in both regulation and kinase activity are blue. In light of the suppression analysis, the significance of these two regions is discussed below.
DISCUSSION
In this study, we have provided evidence for the involvement of allosterism in the activation of Gal3. Our evidence comes from intragenic suppression analyses of newly and previously isolated GAL3C alleles and the localization of these alleles on a Gal3 homology model. We created a new allele GAL3SOC and showed that it suppressed four of five GAL3C alleles. The four suppressible alleles colocalize with GAL3SOC to a region in the Gal3 homology model that correspond to a putative ligand-regulatable hinge region in a KlGal1 homology model that was thought to modulate remote KlGal80-binding determinants. The single nonsuppressible GAL3C allele (GAL3C–D368V) localized to a different region that also includes residues corresponding to Klgal1 mutants that cause defects in binding to KlGal80.
GAL3C–D368V localized to the so-called insertion domain. There is indirect evidence that the insertion domain plays an essential role in binding of Gal3 to ScGal80. The E. coli galactokinase is unable to complement the gal3 deletion, and in common with bacterial galactokinases, lacks the insertion domain (Bhat et al. 1990). By contrast, the yeast galactokinase gene, ScGAL1, does encode the insertion domain and can complement the gal3 deletion (Bhat et al. 1990; Bhat and Hopper 1991). The fact that the GAL3C–D368V allele is located within the insertion domain strengthens the notion that this domain is involved in the Gal3–ScGal80 interaction. The Gal3C–D368V protein is unique because in the absence of galactose it binds ScGal80 as well as wild-type Gal3 in the presence of galactose (Blank et al. 1997). However, the other Gal3C proteins (V69E/D70V, F237Y, S509P, and S509L) that all localized to the putative ligand-regulated hinge region retained dependence on galactose and ATP for wild-type levels of binding to ScGal80. We surmise that the constitutivity of these alleles is due to an allosteric effect that causes a local conformational change at the putative hinge region of Gal3 that partially mimics the galactose- and ATP-bound state. In contrast, we suggest that the constitutivity of the Gal3C–D368V protein is most likely caused by a change either directly at the surface for ScGal80 binding or at an intermediate site within the allosterism pathway between the hinge region and the ScGal80 docking site(s). Strikingly, Gal3 residues corresponding to Klgal1 mutants with defective KlGal80 interaction colocalize along a surface that is proximal to D368 in the Gal3 homology model. This surface is largely hydrophobic in nature and consists of several noncontiguous peptides. If this is the ScGal80-binding surface, it could perhaps explain why attempts to isolate a single Gal3 peptide showing ScGal80 binding have been unsuccessful.
Allosteric activation of Gal3 binding to ScGal80 caused by galactose and ATP may share a similar mechanistic feature with human glucokinase, an enzyme that displays a “hinge-regulated” activity. The binding of glucose and ATP to the active site of the glucokinase forms the ligand-bound active enzyme (Kamata et al. 2004). Missense mutations that occur at positions distributed throughout the sequence but colocalize to a hinge region cause the protein to partially adopt the ligand-bound state with high enzymatic activities (Glaser et al. 1998; Christesen et al. 2002; Gloyn 2003; Cuesta-Munoz et al. 2004; Pedelini et al. 2005). The binding of a small molecule at this hinge region also activated the enzyme (Grimsby et al. 2003), further supporting the idea of allosterism via a hinge-regulated activity. We envision a somewhat similar scenario for Gal3 in accordance with the classic definition of allosterism as the binding of ligand(s) at one site controls the binding of ligand(s) at a second site. The GAL3C alleles located at the hinge region could promote the active ligand-bound state (normally achieved by galactose and ATP) that allosterically effects presentation of the binding surface for the ligand, ScGal80. Introducing a second compensatory mutation (GAL3SOC) within the hinge region could alter the local structure and partially reverse the constitutive effect. This scenario would not apply to the GAL3C allele at position D368 that is unaffected by the GAL3SOC allele, presumably due to its remote position from the hinge region.
In summary, the data presented here provide evidence for the involvement of allosterism in the mechanism for galactose and ATP regulation of Gal3. The results of the intragenic suppression analyses together with the locations of suppressible and nonsuppressible alleles on a Gal3 homology model, implicate the putative hinge region as the key ligand-responsive component for modulating the presentation of residues at the ScGal80-binding surface(s). This notion is similar to the overall mechanistic picture that has been suggested for KlGal1, but is further supported by the juxtaposition of the GAL3C–D368V allele and the Klgal1 mutants defective in interaction with KlGal80. Just how similar Gal3 and KlGal1 are in their mechanisms for galactose- and ATP-regulated binding to ScGal80/KlGal80 remains to be seen.
Acknowledgments
We thank Anita K. Hopper, Sergei Grigoryev, and John Flanagan for critical evaluation of the manuscript. We also thank Hazel M. Holden and James B. Thoden for the ScGal1 coordinates and their Gal3 homology model coordinates prior to their publication. This work was supported by the National Institute of Health grant GM-27975 to J.E.H.
References
- Bajwa, W., T. E. Torchia and J. E. Hopper, 1988. Yeast regulatory gene GAL3: carbon regulation; UASGal elements in common with GAL1, GAL2, GAL7, GAL10, GAL80, and MEL1; encoded protein strikingly similar to yeast and Escherichia coli galactokinases. Mol. Cell. Biol. 8: 3439–3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat, P. J., and J. E. Hopper, 1991. The mechanism of inducer formation in gal3 mutants of the yeast galactose system is independent of normal galactose metabolism and mitochondrial respiratory function. Genetics 128: 233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat, P. J., and J. E. Hopper, 1992. Overproduction of the GAL1 or GAL3 protein causes galactose-independent activation of the GAL4 protein: evidence for a new model of induction for the yeast GAL/MEL regulon. Mol. Cell. Biol. 12: 2701–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat, P. J., D. Oh and J. E. Hopper, 1990. Analysis of the GAL3 dignal transduction pathway activating GAL4 protein-dependent transcription in Saccharomyces cerevisiae. Genetics 125: 281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank, T. E., M. P. Woods, C. M. Lebo, P. Xin and J. E. Hopper, 1997. Novel Gal3 proteins showing altered Gal80p binding cause constitutive transcription of Gal4p-activated genes in Saccharomyces cerevisiae. Mol. Cell. Biol. 17: 2566–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonanno, J. B., C. Edo, N. Eswar, U. Pieper, M. J. Romanowski et al., 2001. Structural genomics of enzymes involved in sterol/isoprenoid biosynthesis. Proc. Natl. Acad. Sci. USA 98: 12896–12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bork, P., C. Sander and A. Valencia, 1993. Convergent evolution of similar enzymatic function on different protein folds: the hexokinase, ribokinase, and galactokinase families of sugar kinases. Protein Sci. 2: 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bram, R. J., and R. D. Kornberg, 1985. Specific protein binding to far upstream activating sequences in polymerase II promoters. Proc. Natl. Acad. Sci. USA 82: 43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant, G. O., and M. Ptashne, 2003. Independent recruitment in vivo by Gal4 of two complexes required for transcription. Mol. Cell. Biochem. 11: 1301–1309. [DOI] [PubMed] [Google Scholar]
- Christesen, H. B., B. B. Jacobsen, S. Odili, C. Buettger, A. Cuesta-Munoz et al., 2002. The second activating glucokinase mutation (A456V): implications for glucose homeostasis and diabetes therapy. Diabetes 51: 1240–1246. [DOI] [PubMed] [Google Scholar]
- Cuesta-Munoz, A. L., H. Huopio, T. Otonkoski, J. M. Gomez-Zumaquero, K. Nanto-Salonen et al., 2004. Severe persistent hyperinsulinemic hypoglycemia due to a de novo glucokinase mutation. Diabetes 53: 2164–2168. [DOI] [PubMed] [Google Scholar]
- Czyz, M., M. M. Nagiec and R. C. Dickson, 1993. Autoregulation of GAL4 transcription is essential for rapid growth of Kluyveromyces lactis on lactose and galactose. Nucleic Acids Res. 21: 4378–4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson, R. C., and M. I. Riley, 1989. The lactose-galactose regulon of Kluyveromyces lactis. Biotechnology 13: 19–40. [PubMed] [Google Scholar]
- Fenton, C., H. Xu, E. I. Petersen, S. B. Petersen and M. R. el-Gewely, 2002. Random mutagenesis for protein breeding. Methods Mol. Biol. 182: 231–241. [DOI] [PubMed] [Google Scholar]
- Flick, J. S., and M. Johnston, 1990. Two systems of glucose repression of the GAL1 promoter in Saccharomyces cerevisiae. Mol. Cell. Biol. 10: 4757–4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser, B., P. Kesavan, M. Heyman, E. Davis, A. Cuesta et al., 1998. Familial hyperinsulinism caused by an activating glucokinase mutation. N. Engl. J. Med. 338: 226–230. [DOI] [PubMed] [Google Scholar]
- Gloyn, A. L., 2003. Glucokinase (GCK) mutations in hyper- and hypoglycemia: Maturity-onset diabetes of the young, permanent neonatal diabetes, and hyperinsulinemia of infancy. Hum. Mutat. 22: 353–362. [DOI] [PubMed] [Google Scholar]
- Griffith, M., and R. D. Gietz, 2003. Escherichia coli endA deletion strain for use in two-hybrid shuttle vector selection. Biotechniques 35: 272–274, 276, 278. [DOI] [PubMed] [Google Scholar]
- Grimsby, J., R. Sarabu, W. L. Corbett, N.-E. Haynes, F. T. Bizzarro et al., 2003. Allosteric activators of glucokinase: potential role in diabetes yherapy. Science 301: 370–373. [DOI] [PubMed] [Google Scholar]
- Hao, Z., K. N. Jha, Y.-H. Kim, S. Vemuganti, V. A. Westbrook et al., 2004. Expression analysis of the human testis-specific serine/threonine kinase (TSSK) homologues. A TSSK member is present in the equatorial segment of human sperm. Mol. Hum. Reprod. 10: 433–444. [DOI] [PubMed] [Google Scholar]
- Hartley, A., S. E. Glynn, V. Barynin, P. J. Baker, S. E. Sedelnikova et al., 2004. Substrate specificity and mechanism from the structure of Pyrococcus furiosus galactokinase. J. Mol. Biol. 337: 387–398. [DOI] [PubMed] [Google Scholar]
- Johnston, M., 1987. A model fungal gene regulatory mechanism: the GAL genes of Saccharomyces cerevisiae. Microbiol. Rev. 51: 458–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston, S. A., and J. E. Hopper, 1982. Isolation of the yeast regulatory gene GAL4 and analysis of its dosage effects on the galactose/melibiose regulon. Proc. Natl. Acad. Sci. USA 79: 6971–6975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata, K., M. Mitsuya, T. Nishimura, J.-i. Eiki and Y. Nagata, 2004. Structural basis for allosteric regulation of the monomeric allosteric enzyme human glucokinase. Structure 12: 429–438. [DOI] [PubMed] [Google Scholar]
- Kraulis, P. J., 1991. MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallog. 24: 946–950. [Google Scholar]
- Leuther, K. K., and S. A. Johnston, 1992. Nondissociation of GAL4 and GAL80 in vivo after galactose induction. Science 256: 1333–1335. [DOI] [PubMed] [Google Scholar]
- Lohr, D., P. Venkov and J. Zlatanova, 1995. Transcriptional regulation in the yeast GAL gene family: a complex genetic network. FASEB J 9: 777–787. [DOI] [PubMed] [Google Scholar]
- Luz, J. G., C. A. Hassig, C. Pickle, A. Godzik, B. J. Meyer et al., 2003. XOL-1, primary determinant of sexual fate in C. elegans, is a GHMP kinase family member and a structural prototype for a class of developmental regulators. Genes Dev. 17: 977–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, J., and M. Ptashne, 1987. The carboxy-terminal 30 amino acids of GAL4 are recognized by GAL80. Cell 50: 137–142. [DOI] [PubMed] [Google Scholar]
- Menezes, R. A., C. Amuel, R. Engels, U. Gengenbacher, J. Labahn et al., 2003. Sites for interaction between Gal80p and Gal1p in Kluyveromyces lactis: structural model of galactokinase based on homology to the GHMP protein family. J. Mol. Biol. 333: 479–492. [DOI] [PubMed] [Google Scholar]
- Meyer, J., A. Walker-Jonah and C. P. Hollenberg, 1991. Galactokinase encoded by GAL1 is a bifunctional protein required for induction of the GAL genes in Kluyveromyces lactis and is able to suppress the gal3 phenotype in Saccharomyces cerevisiae. Mol. Cell. Biol. 11: 5454–5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlrad, D., R. Hunter and R. Parker, 1992. A rapid method for localized mutagenesis of yeast genes. Yeast 8: 79–82. [DOI] [PubMed] [Google Scholar]
- Nicholls, A., K. A. Sharp and B. Honig, 1991. Protein folding and association: insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins 11: 281–296. [DOI] [PubMed] [Google Scholar]
- Pedelini, L., M. A. Garcia-Gimeno, A. Marina, J. M. Gomez-Zumaquero, P. Rodriguez-Bada et al., 2005. Structure–function analysis of the {alpha}5 and the {alpha}13 helices of human glucokinase: description of two novel activating mutations. Protein Sci. 14: 2080–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, G., and J. E. Hopper, 2002. Gene activation by interaction of an inhibitor with a cytoplasmic signaling protein. Proc. Natl. Acad. Sci. USA 99: 8548–8553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilauri, V., M. Bewley, C. Diep and J. Hopper, 2005. Gal80 dimerization and the yeast GAL gene switch. Genetics 169: 1903–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt, A., and R. J. Reece, 1998. The yeast galactose genetic switch is mediated by the formation of a Gal4p-Gal80p-Gal3p complex. EMBO J. 17: 4086–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt, A., H. C. Ross, S. Hankin and R. J. Reece, 2000. The insertion of two amino acids into a transcriptional inducer converts it into a galactokinase. Proc. Natl. Acad. Sci. USA 97: 3154–3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirot, O., K. Suhre, C. Abergel, E. O'Toole and C. Notredame, 2004. 3DCoffee@igs: a web server for combining sequences and structures into a multiple sequence alignment. Nucleic Acids Res. 32: W37–W40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post-Beittenmiller, M. A., R. W. Hamilton and J. E. Hopper, 1984. Regulation of basal and induced levels of the MEL1 transcript in Saccharomyces cerevisiae. Mol. Cell. Biol. 4: 1238–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanowski, M. J., J. B. Bonanno and S. K. Burley, 2002. Crystal structure of the Streptococcus pneumoniae phosphomevalonate kinase, a member of the GHMP kinase superfamily. Proteins 47: 568–571. [DOI] [PubMed] [Google Scholar]
- Rubio-Texeira, M., 2005. A comparative analysis of the GAL genetic switch between not-so-distant cousins: Saccharomyces cerevisiae versus Kluyveromyces lactis. FEMS Yeast Res. 5: 1115–1128. [DOI] [PubMed] [Google Scholar]
- Selleck, S. B., and J. E. Majors, 1987. In vivo DNA-binding properties of a yeast transcription activator protein. Mol. Cell. Biol. 7: 3260–3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellick, C. A., and R. J. Reece, 2005. Eukaryotic transcription factors as direct nutrient sensors. Trends Biochem. Sci. 30: 405–412. [DOI] [PubMed] [Google Scholar]
- Sherman, F., 1991. Getting started with yeast. Methods Enzymol. 194: 3–21. [DOI] [PubMed] [Google Scholar]
- Sil, A. K., S. Alam, P. Xin, L. Ma, M. Morgan et al., 1999. The Gal3p-Gal80p-Gal4p transcription switch of yeast: Gal3p destabilizes the Gal80p-Gal4p complex in response to galactose and ATP. Mol. Cell. Biol. 19: 7828–7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. John, T. P., and R. W. Davis, 1981. The organization and transcription of the galactose gene cluster of Saccharomyces. J. Mol. Biol. 152: 285–315. [DOI] [PubMed] [Google Scholar]
- Suzuki-Fujimoto, T., M. Fukuma, K. I. Yano, H. Sakurai, A. Vonika et al., 1996. Analysis of the galactose signal transduction pathway in Saccharomyces cerevisiae: interaction between Gal3p and Gal80p. Mol. Cell. Biol. 16: 2504–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoden, J. B., and H. M. Holden, 2003. Molecular structure of galactokinase. J. Biol. Chem. 278: 33305–33311. [DOI] [PubMed] [Google Scholar]
- Thoden, J. B., C. A. Sellick, D. J. Timson, R. J. Reece and H. M. Holden, 2005. a Molecular structure of Saccharomyces cerevisiae Gal1p, a bifunctional galactokinase and transcriptional inducer. J. Biol. Chem. 280: 36905–36911. [DOI] [PubMed] [Google Scholar]
- Thoden, J. B., D. J. Timson, R. J. Reece and H. M. Holden, 2005. b Molecular structure of human galactokinase: implications for type II galactosemia. J. Biol. Chem. 280: 9662–9670. [DOI] [PubMed] [Google Scholar]
- Thompson, J., T. Gibson, F. Plewniak, F. Jeanmougin and D. Higgins, 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25: 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timson, D. J., H. C. Ross and R. J. Reece, 2002. Gal3p and Gal1p interact with the transcriptional repressor Gal80p to form a complex of 1:1 stoichiometry. Biochem. J. 363: 515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torchia, T. E., and J. E. Hopper, 1986. Genetic and molecular analysis of the GAL3 gene in the expression of the galactose/melibiose regulation of Saccharomyces cerevisiae. Genetics 113: 229–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torchia, T. E., R. W. Hamilton, C. L. Cano and J. E. Hopper, 1984. Disruption of regulatory gene GAL80 in Saccharomyces cerevisiae: effects on carbon-controlled regulation of the galactose/melibiose pathway genes. Mol. Cell. Biol. 4: 1521–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenbroich, V., J. Meyer, R. Engels, G. Cardinali, R. A. Menezes et al., 1999. Galactose induction in yeast involves association of Gal80p with Gal1p or Gal3p. Mol. Gen. Genet. 261: 495–507. [DOI] [PubMed] [Google Scholar]
- Wolfe, K. H., and D. C. Shields, 1997. Molecular evidence for an ancient duplication of the entire yeast genome. Nature 387: 708–713. [DOI] [PubMed] [Google Scholar]
- Xu, H., E. I. Petersen, S. B. Petersen and M. R. el-Gewely, 1999. Random mutagenesis libraries: optimization and simplification by PCR. Biotechniques 27: 1102–1104, 1106, 1108. [DOI] [PubMed] [Google Scholar]
- Yang, D., L. W. Shipman, C. A. Roessner, A. I. Scott and J. C. Sacchettini, 2002. Structure of the methanococcus jannaschii mevalonate kinase, a member of the GHMP kinase superfamily. J. Biol. Chem. 277: 9462–9467. [DOI] [PubMed] [Google Scholar]
- Yano, K., and T. Fukasawa, 1997. Galactose-dependent reversible interaction of Gal3p with Gal80p in the induction pathway of Gal4p-activated genes of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 94: 1721–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarger, J. G., H. O. Halvorson and J. E. Hopper, 1984. Regulation of galactokinase (GAL1) enzyme accumulation in Saccharomyces cerevisiae. Mol. Cell. Biochem. 61: 173–182. [DOI] [PubMed] [Google Scholar]
- Yocum, R. R., and M. Johnston, 1984. Molecular cloning of the GAL80 gene from Saccharomyces cerevisiae and characterization of a gal80 deletion. Gene 32: 75–82. [DOI] [PubMed] [Google Scholar]
- Zenke, F. T., R. Engles, V. Vollenbroich, J. Meyer, C. P. Hollenberg et al., 1996. Activation of Gal4p by galactose-dependent interaction of galactokinase and Gal80p. Science 272: 1662–1665. [DOI] [PubMed] [Google Scholar]
- Zhou, T., M. Daugherty, N. V. Grishin, A. L. Osterman and H. Zhang, 2000. Structure and mechanism of homoserine kinase: prototype for the GHMP kinase superfamily. Structure Fold. Des. 8: 1247–1257. [DOI] [PubMed] [Google Scholar]