Abstract
1. Prion diseases are a group of rare, fatal neurodegenerative diseases, also known as transmissible spongiform encephalopathies (TSEs), that affect both animals and humans and include bovine spongiform encephalopathy (BSE) in cattle, scrapie in sheep, chronic wasting disease (CWD) in deer and elk, and Creutzfeldt–Jakob disease (CJD) in humans. TSEs are usually rapidly progressive and clinical symptoms comprise dementia and loss of movement coordination due to the accumulation of an abnormal isoform (PrPSc) of the host-encoded prion protein (PrPc).
2. This article reviews the current knowledge on PrPc and PrPSc, prion replication mechanisms, interaction partners of prions, and their cell surface receptors. Several strategies, summarized in this article, have been investigated for an effective antiprion treatment including development of a vaccination therapy and screening for potent chemical compounds. Currently, no effective treatment for prion diseases is available.
3. The identification of the 37 kDa/67 kDa laminin receptor (LRP/LR) and heparan sulfate as cell surface receptors for prions, however, opens new avenues for the development of alternative TSE therapies.
KEY WORDS: bovine spongiform encephalopathy, Creutzfeldt–Jakob disease, heparan sulfate, 37 kDa/67 kDa laminin receptor, LRP/LR, prion, PrP therapy, transmissible spongiform encephalopathy
INTRODUCTION
Prion diseases or transmissible spongiform encephalopathies are incurable neurodegenerative disorders, which occur both in humans and animals. Human TSEs include Kuru (Gajdusek and Zigas, 1957), Gerstmann–Sträussler–Scheinker syndrome (GSS) (Gerstmann et al., 1936), fatal familial insomnia (FFI) (Lugaresi et al., 1986), and Creutzfeldt–Jakob disease (CJD) (Creutzfeldt, 1920), which is the most prominent prion disease in humans. However, all of them (as summarized in Table I) are a group of rapidly progressive disorders characterized by a defined spectrum of clinical abnormalities. They share a spongiform degeneration of the brain and a variable amyloid plaque formation and can appear as sporadic, inherited, or iatrogenic disorders.
Table I.
Summary of the Initial Description of Human TSEs
| TSE | Initially described | Reference |
|---|---|---|
| Creutzfeldt–Jakob disease (CJD) | 1920 | (Creutzfeldt, 1920) |
| Sporadic Creutzfeldt–Jakob disease (sCJD) | 1921 | (Jakob, 1921) |
| Familial Ceutzfeldt–Jakob disease (fCJD) | 1924 | (Kirschbaum, 1924) |
| Iatrogenic Creutzfeldt–Jakob disease (iCJD) | 1974 | (Duffy et al., 1974) |
| New variant Creutzfeldt–Jakob disease (vCJD) | 1996 | (Will et al., 1996) |
| Gerstmann–Sträussler–Scheinker syndrome (GSS) | 1928 | (Gerstmann et al., 1936) |
| Kuru | 1957 | (Gajdusek and Zigas, 1957) |
| Fatal familial insomnia (FFI) | 1986 | (Lugaresi et al., 1986) |
| Sporadic fatal insomnia (sFI) | 1999 | (Mastrianni et al., 1999; Parchi et al., 1999) |
Transmissible spongiform encephalopathies (TSEs) also frequently occur in different animal species. Scrapie in sheep and goats (McGowan, 1922), feline spongiform encephalopathy (FSE) in cats (Wyatt, 1990), transmissible mink encephalopathy (TME) (Burger and Hartsough, 1965), chronic wasting disease in wild ruminants (CWD) (Williams and Young, 1980), bovine spongiform encephalopathy (BSE) in cattle (Wells et al., 1987), and encephalopathies of a number of zoo animals (exotic ungulate encephalopathy, EUE) (Jeffrey and Wells, 1988; Kirkwood et al., 1990) have been described. A hallmark of all prion diseases is the accumulation of an abnormal, partially proteinase-resistant isoform of the cellular prion protein (PrPc), which represents a cell-surface glycosylphosphatidyl inositol (GPI) anchored protein (Stahl and Prusiner, 1991). PrPc is highly conserved among mammals (Schätzl et al., 1995) and is expressed in many tissues with notably high levels in the brain of animals and humans (Kretzschmar et al., 1986; Moudjou et al., 2001). The conversion of the host-encoded PrPc into the abnormal disease-inducing isoform (PrPSc) involves a conformational change and is important in the pathogenesis of these diseases (Prusiner, 1994). Several hypotheses about the nature of the infectious agent have been proposed. Initially, the agent was thought to be a slow virus (Sigurdsson, 1954; Thormar, 1971). In 1967, J. S. Griffith postulated the hypothesis that the causative agent might be a protein (Griffith, 1967). The theory of a self-propagating proteinaceous agent (Bolton et al., 1982) was proposed after the isolation of a protease-resistant sialoglycoprotein specifically associated with infectivity, designated the prion protein (PrP) (Bolton et al., 1982). The term prion, which was devised by Stanley Prusiner, is the abbreviation for “proteinaceous infectious particle” and was defined as “small proteinaceous infectious particle that resists inactivation by procedures which modify nucleic acids” (Bolton et al., 1982; Prusiner, 1982).
THE CELLULAR PRION PROTEIN PrPc
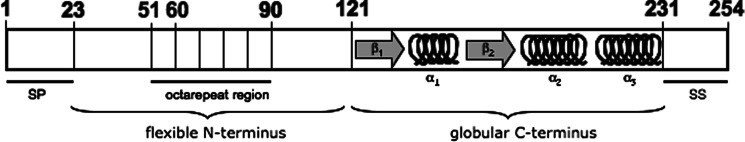
The prion protein (PrPc) is a normal cellular glycosylphosphatidyl inositol (GPI) anchored protein and is highly conserved among mammalian species (Schätzl et al., 1995; Wopfner et al., 1999). It has been identified in various animals including birds (Harris et al., 1993), pisces (Gibbs and Bolis, 1997), and marsupials (Windl et al., 1995), and may be present in all vertebrates. PrP mRNA is constitutively expressed in the brains of adult animals with a high expression in neurons (Kretzschmar et al., 1986). Substantial amounts have also been found in heart (Brown et al., 1990), skeletal muscle (Brown et al., 1998; Bosque et al., 2002), lymphoid tissue and leukocytes (Liu et al., 2001; Paltrinieri et al., 2004), intestinal tissues (Morel et al., 2004), and uterus and testis (Tanji et al., 1995). In 1986, the human PrP gene (Prn-p) has been mapped to 20p12-pter (Liao et al., 1986; Robakis et al., 1986; Sparkes et al., 1986). The conformation of the cellular isoform of murine PrP was first determined by nuclear magnetic resonance (NMR) studies (Riek et al., 1996). Since then, NMR measurements on the prion protein from various species were performed and revealed that they all have global architecture similarities. The prion protein has a flexible, unstructured N-terminal region and a well-ordered C-terminal globular domain, which includes three α-helices and two antiparallel β-sheet structures (Riek et al., 1996). The N-terminal region contains a segment of several octapeptide-repeat regions that preferentially bind copper (Hornshaw et al., 1995) (Fig. 1). Infrared spectroscopy and circular dichroism demonstrated that the secondary structure of PrPc is mainly composed of α-helices (42%), whereas PrPSc consists mainly of β-sheets (Table II) (Cohen et al., 1994). In Syrian hamster and mice, PrPc is synthesized as a precursor of 254 amino acids while the human Prn-p encodes a prion protein of 253 amino acids in length. During trafficking through the secretory pathway of the cell, the N-terminal signal peptide is cleaved off in the endoplasmatic reticulum (Hope et al., 1986) and 23 C-terminal residues demerge upon addition of the GPI anchor at serine (Ser) 231 (Stahl et al., 1987). Cell culture studies revealed that PrPc constitutively cycles between the cell surface and an endocytic compartment with a transit time of approx. 60 min and more than 95% of the internalized protein is recycled back to the cell surface (Shyng et al., 1993).
Fig. 1.
Schematic view of structural elements of the murine cellular prion protein. The depicted murine cellular PrP (PrPc) is a GPI-anchored protein of 254 amino acid residues. During PrPc processing, a 22 amino acid N-terminal signal peptide (SP) is removed and 23 carboxy-terminal amino acid residues (signal sequence (SS)) are cleaved upon the addition of the glycosylphosphatidyl inositol anchor to Ser-231. The N-terminal region contains a series of four octapeptide repeats that have been implicated in the binding of metal ions (Whittal et al., 2000). The first repeat represents a nonarepeat due to an additional glycin residue. The repeat has a histidine residue substituted by a glutamine and, therefore, fails to bind copper (Leliveld et al., 2006). The globular C-terminus of the molecule folds into three α-helices and an antiparallel ß-sheet, whereas the N-terminal part of the protein is flexible as determined in solution. The structure of the murine PrP 121-231 was initially solved by Riek and colleagues (Riek et al., 1996), and that of hamster PrP 29-231 by Donne and colleagues (Donne et al., 1997).
Table II.
Comparison of Biochemical Features of PrPc, PrPSc, and PrP27-30
| PrP isoform | PrPc | PrPSc | PrP27-30 |
|---|---|---|---|
| Infectivity | Noninfectious | Infectious | Infectious |
| Protease status | Sensitive | Partially resistant | Resistant |
| Solubility | Soluble | Insoluble | Insoluble |
| Aggregation status | Monomer/dimer/oligomer | Aggregates | Amyloid fibrils |
| Secondary structure | α-helices (42%), | α-helices (30%), | α-helices (21%), |
| β-sheets (3%) | β-sheets (43%) | β-sheets (54%) |
THE PATHOGENIC ISOFORM PrPSc AND REPLICATION MECHANISMS
In TSEs, the cellular prion protein PrPc can be converted into a pathogenic isoform referred to as PrPSc that shows great resistance to radiation and nucleases (Alper et al., 1967). The high proportion of β-sheets in PrPSc renders it insoluble and markedly resistant to proteases (Table II) (Cohen and Prusiner, 1998). Digestion with proteinuse K results in a 27–30 kDa fragment, termed PrPres (Bolton et al., 1982). PrP27-30 is unusually stable at high temperatures and can only be inactivated by protein denaturants that modify the structure of PrP27-30 (Prusiner et al., 1993). After detergent and protease treatment, PrP27-30 was found to accumulate into rod-shaped polymers that are insoluble in aqueous and organic solvents as well as nonionic detergents. In contrast, PrPSc (the full-length infectious conformer of PrPc) has a tendency to form aggregates but not amyloid fibrils (McKinley et al., 1991) (Table II).
Given the same primary sequence of PrPc and PrPSc (Basler et al., 1986), the different properties of PrPc and PrPSc seemed likely to involve posttranslational modifications. Extensive biochemical investigations have failed to reveal any covalent differences between PrPc and PrPSc (Stahl et al., 1993). By contrast, spectroscopic studies demonstrated a conformational difference between PrPc and PrPSc. PrPc has a high α-helical content of approx. 42%, with little or no β-sheets (approx. 3%), whereas PrPSc contains approx. 30% α-helices and approx. 45% β-sheets (Pan et al., 1993) (Table II). PrPSc formation is supposed to occur via the interaction between PrPc and PrPSc, which is able to convert the host protein into a likeness of itself (Griffith 1967; Bolton et al., 1982). The mechanism by which PrPSc triggers further PrPSc production is unknown, although two major models have been proposed. The catalytic model (Prusiner, 1991) proposes that the presence of PrPSc catalyzes the conversion of PrPc to PrPSc. Alternatively, it has been proposed that the formation of PrPSc is a nucleation-dependent process (Lansbury and Caughey, 1995). The cellfree in vitro conversion process was shown to be consistent with the nucleation-dependent polymerization mechanism of PrPSc formation and inconsistent with the heterodimer mechanism (Caughey et al., 1995). Regardless of the underlying mechanisms, there is more and more evidence supporting the initial idea of self-replicating prions consisting of protein-only, which has in fact been long debated. Although compelling evidence supports this hypothesis, generation of infectious prion particles in vitro has not been convincingly demonstrated for a long period of time. Thus, it was shown in 2004, by Legname and colleagues, that recombinant murine prion protein (including amino acid residues 89–230) produced in E. coli can be converted into an infectious PrP form being able to cause a prion disease-like phenotype in transgenic (PrP89-230 expressing) and, in the second round, in wild-type mice (Legname et al., 2004). In addition, Castilla and colleagues showed that PrP conversion can be mimicked in vitro by protein misfolding cyclic amplification (PMCA), resulting in indefinite amplification of infectious PrPres as shown by bioassays in hamsters (Castilla et al., 2005).
The precise subcellular localization of PrPSc propagation remains controversial. There is evidence, however, that either late-endosome-like organelles or lysosomes are involved (Mayer et al., 1992; Arnold et al., 1995). A role for lipid rafts in the formation of PrPSc is deduced from the finding that both PrPc and PrPSc are present in rafts isolated from infected cells (Baron and Caughey, 2003; Botto et al., 2004). It was also shown that PrPc lacking the GPI anchor is converted into PrPSc (Chesebro et al., 2005)
THE FUNCTION OF PrP
The exact physiological role of the cellular prion protein PrPc still remains obscure, although some possible biological functions have been described. The proposed functions include a neuroprotective function due to antiapoptotic activity (Bounhar et al., 2001; Diarra-Mehrpour et al., 2004), a functional role in copper metabolism due to its copper-binding capacity (Brown et al., 1997), involvement in signal transduction (Koch et al., 1991; Mouillet-Richard et al., 2000), memory formation (Collinge et al., 1994), and neuritogenesis (Graner et al., 2000). Mice lacking PrP (Prnp0/0) showed no obvious phenotype (Bueler et al., 1992), although they have abnormalities in synaptic physiology (Collinge and Palmer, 1994) and in circadian rhythm and sleep (Tobler et al., 1996). Prnp0/0 mice were shown to be completely resistant to prion disease (Bueler et al., 1993). Several lines of PrP knockout mice have been generated to unveil the function of PrPc (Weissmann and Flechsig, 2003).
The finding that lymphocytes express PrPc on the cell surface implicates a role in lymphocyte activation (Cashman et al., 1990). Anti-PrPc antibodies cause partial inhibition of mitogen driven T-cell proliferation giving evidence for a role in modulating T-cell responses (Bainbridge and Walker, 2005). The fact that PrPc is abundantly expressed in the lymphoid tissue and acts as a signaling molecule on T-cells implicates a role in the development and normal function of the immune system (Mazzoni et al., 2005; Ballerini et al., 2006).
The molecular mechanism of PrP protection against oxidative stress is still unclear, but PrP may reduce copper-mediated oxidative stress due to its copper-binding activity (Vassallo and Herms, 2003). Since PrP knockout mice exhibit approx. 50% lower copper concentration in synaptosomal fractions than wild-type mice, it was suggested that PrPc might regulate the copper concentration in the synaptic region and may play a role in the reuptake of copper into the presynapse (Kretzschmar et al., 2000).
Furthermore, it has been shown that PrPc harbors a copper/zinc-dependent superoxide-dismutase (SOD) that provides PrPc with antioxidant activity. By deletion of the octapeptide repeat region involved in copper binding, the SOD activity was abolished (Brown et al., 1999). In vivo experiments revealed that protein and lipid oxidation is increased in skeletal muscle, heart, and liver in Prnp0/0 mice suggesting a PrPc function related to cellular antioxidant defenses (Klamt et al., 2001).
PrPc is known to be attached to the plasma membrane through a glycosylphosphatidyl inositol (GPI) anchor and may act as a cell-surface receptor mediating cell-surface signaling or cell adhesion. Recently, a coupling of PrPc to the nonreceptor tyrosine kinase Fyn was observed (Mouillet-Richard et al., 2000). Furthermore, PrPc has been described to regulate serotonergic receptor signaling and, thus, acting as a protagonist for the homeostasis of serotonergic neurons (Mouillet-Richard et al., 2005).
In addition, several experimental findings suggest a major role for PrPc in cell survival or cell death. In a yeast two-hybrid system, PrPc was demonstrated to interact selectively with the Bcl-2 protein (Kurschner and Morgan, 1995), that is a suppressor of the programmed cell death. Recently, the antiapoptotic activity of PrP has been shown in a human breast carcinoma cell line (Diarra-Mehrpour et al., 2004). Cross linking of PrPc using monoclonal antibodies resulted in rapid and extensive apoptosis in hippocampal and cerebellar neurons suggesting that PrPc acts in the control of neuronal survival (Solforosi et al., 2004). Expression of PrPc in gastric cancer cell line led to an upregulation of Bcl-2 whereas p53 and Bax were downregulated (Liang et al., 2006). However, coaggregation of cytosolic PrP with Bcl-2 leads to the induction of apoptosis (Rambold et al., 2006).
Despite all knowledge, there is some controversy on the protective function of PrPc. It has been demonstrated that in some cell lines, the overexpression of PrPc increases the susceptibility of these cells to staurosporine-induced apoptosis (Paitel et al., 2002, 2003). In addition, it was proposed that endogenous cellular prion protein sensitizes neurons to apoptotic stimuli through a p53-dependent caspase 3 mediated activation (Paitel et al., 2003, 2004).
INTERACTION PARTNERS OF THE CELLULAR PRION PROTEIN
More than 10 years ago, the existence of a cellular receptor for prions was proposed. It was reasoned that the cellular prion protein PrPc would require a transmembrane protein to trigger intracellular events (Shyng et al., 1994). Different proteins (Table III) have been shown to interact with the cellular prion protein including laminin (Graner et al., 2000), which is an extracellular matrix protein, N-CAM (Schmitt-Ulms et al., 2001), a cell surface component with an important role in neuronal aggregation, and tyrosin kinase Fyn implicating a role of PrP in cell signaling (Mouillet-Richard et al., 2000).
Table III.
Summary of Major Binding Partners for the Cellular Prion Protein, Their Proposed Function, and Subcellular Localization
| PrPc binding molecules | Proposed function | Subcellular localization | Reference |
|---|---|---|---|
| Synapsin 1b | Signal transduction | Intracellular vesicles | (Spielhaupter and Schätzl, 2001) |
| Grb2 | Signal transduction | Intracellular vesicles | (Spielhaupter and Schätzl, 2001) |
| Pint 1 | Unknown | Unknown | (Spielhaupter and Schätzl, 2001) |
| Tyrosine kinase Fyn | Binding/internalization | Plasma membrane | (Mouillet-Richard et al., 2000) |
| Caveolin-1 | Binding/internalization | Caveolae/rafts | (Mouillet-Richard et al., 2000) |
| Clathrin | Binding/internalization | Clathrin-coated pits | (Mouillet-Richard et al., 2000) |
| CK2 | Binding/internalization | Caveolae/rafts | (Meggio et al., 2000) |
| STI 1 | Neuroprotection, neuronal outgrowth | Cell surface | (Zanata et al., 2002) |
| Bcl-2 | Antiapoptotic/proapoptotic function | Cytoplasm | (Kurschner and Morgan, 1995; Sakudo et al., 2003; Rambold et al., 2006) |
| p75 | Binding/internalization | Caveolae/rafts | (Della-Bianca et al., 2001) |
| Laminin | Cell differentiation/cell growth/movement ECM formation | Cell surface | (Graner et al., 2000) |
| GAGs | Proposed role in prion pathogenesis/receptor for prions | Cell surface | (Pan et al., 2002; Hijazi et al., 2005) |
| HSPGs/HS | Cofactor for PrPSc synthesis/receptor for prions | Cell surface | (Gabizon et al., 1993) |
| (Horonchik et al., 2005) | |||
| Co-receptor for PrPc | (Hundt et al., 2001) | ||
| N-CAM | Caveolae-like domain | Caveolae-like domain | (Schmitt-Ulms et al., 2001) |
| Hsp60 | Might influence PrP conversion | Mitochondria (main) (ER, Golgi, secretory granules, membrane fractions) | (Edenhofer et al., 1996) |
| Nrf2 | Unknown | Unknown | (Yehiely et al., 1997) |
| Aplp1 | Unknown | Cell surface | (Yehiely et al., 1997) |
| 37 kDa/67 kDa Laminin Receptor (LRP/LR) | PrPc binding and internalization (prion protein receptor)/PrPSc binding and internalization (PrPSc receptor) | Cell surface | (Rieger et al., 1997; Gauczynski et al., 2001) |
| (Morel et al., 2005; Gauczynski et al., 2006) | |||
| NRAGE | Neuronal viability | Cytosol | (Bragason and Palsdottir, 2005) |
| Tubulin | Intracellular trafficking | Microtubular network cytoskeleton | (Nieznanski et al., 2005) |
| ZAP-70 | T-cell activation | Glycosphingolipid-enriched microdomains | (Mattei et al., 2004) |
Employing complementary hydropathy, a 66 kDa membrane protein that binds PrPc both in vitro and in vivo was found (Martins et al., 1997) and it was reasoned that this protein might act as a cellular prion protein receptor. However, the same group identified this 66 kDa protein as stress-inducible-protein1 (STI1), playing a role in neurite outgrowth and neuroprotection (Zanata et al., 2002). Parallel to this study, we identified in a yeast two-hybrid screen, the 37 kDa laminin receptor precursor (LRP) as an interaction partner for the prion protein (Rieger et al., 1997).
37 kDa LRP/67 kDa LR AND HEPARAN SULFATE PROTEOGLYCANES AS RECEPTORS/CORECEPTORS FOR PrPc AND PrPSc
Further in vitro studies on neuronal and nonneuronal cells validated that both forms of the laminin receptor, the 37 kDa LRP and the 67 kDa high affinity laminin receptor act as the receptor for the cellular prion protein. Corresponding binding domains on LRP/LR as well as on PrP were identified by yeast-two hybrid technology (Hundt et al., 2001), revealing both a direct and an indirect, HSPG-dependent binding site.
The 37 kDa LRP is thought to be the precursor of the 67 kDa LR, which was first isolated from melanoma cells due to its high binding capacity to laminin (Rao et al., 1983). Although LRP consists of a transmembrane domain (amino acid residue 86–101, (Castronovo et al., 1991)), it is abundantly localized in the cytoplasm (Romanov et al., 1994). In mammalian cells, it has been demonstrated that both the 37 kDa LRP and the 67 kDa LR are present in plasma membrane fractions (Gauczynski et al., 2001). The exact mechanism by which the 37 kDa precursor forms the mature 67 kDa isoform is up to now speculative. Data from the yeast two-hybrid analysis showed that LRP failed to interact with itself (Hundt et al., 2001), which would argue against homodimerization. Analysis of the membrane-bound 67 kDa LR indicated that acylation of LRP is involved in the processing of the receptor (Landowski et al., 1995) and the authors suggest that the 67 kDa form consists of a homodimer of the LRP polypeptide modified by fatty acid chains. On the contrary, a later study postulated that the 67 kDa LR is a heterodimer stabilized by fatty acid-mediated interactions (Buto et al., 1998). Interestingly, mammalian genomes contain multiple copies of the LRP gene, particularly 6 copies in murine and 26 copies in the human genome (Jackers et al., 1996). Sequencing revealed that over 50% of the 37 kDa LRP gene copies were pseudogenes most probably generated by retropositional events suggesting that the accumulation of several copies of this gene might have given a survival advantage to the cell in the course of evolution (Jackers et al., 1996).
Regarding the function of LRP/LR, the 37 kDa LRP appears to be a multifunctional protein (Fig. 2) involved in the translational machinery (Auth and Brawerman, 1992) and has also been identified as p40 ribosome-associated protein (Makrides et al., 1988). LRP has also been found in the nucleus, where it is tightly associated with nuclear structures (Sato et al., 1996) and binds to DNA through associations with histones H2A, H2B, and H4 (Kinoshita et al., 1998). The 37 kDa/67 kDa LRP/LR has been described to act as a receptor for laminin, elastin, and carbohydrates (Ardini et al., 1998), as well as a receptor for Venezuelean equine encephalitis virus (VEE) (Ludwig et al., 1996), Sindbis virus (Wang et al., 1992), and Dengue virus (Tio et al., 2005) (Table III and Fig. 2). Very recently, LRP/LR has been identified as a receptor for Adeno-associated Virus (AAV) serotypes 8, 2, 3, and 9 (Akache et al., 2006). Due to the colocalization of LRP/LR and PrP on the surface of mammalian cells, a possible role of LRP/LR for PrP binding and internalization was assumed. Cell-binding assays revealed, that the PrPc internalization process represents an active LRP/LR-mediated event (Gauczynski et al., 2001). Due to the identification of various LRP/LR isoforms, additional studies have been performed to detect the isoforms that are present in the central nervous system and bind PrP. Several LRP/LR isoforms corresponding to different maturation states of the receptor were identified, including a 44 kDa, 60 kDa, 67 kDa, and a 220 kDa form. All of these isoforms were able to bind PrP, supporting a physiological role for the laminin receptor/PrP interaction in the brain (Simoneau et al., 2003). A closer insight into the fine cellular distribution of LRP/LR in the central nervous system was obtained by using immunohistochemistry in adult rat brain (Baloui et al., 2004). It has been shown that the 67 kDa LR is the major receptor form, which is expressed within the cytoplasm and at the plasma membrane in most neurons and in a subset of glia cells. In contrast, the 37 kDa LRP is much less abundant in adult than in postnatal central nervous system and its expression is restricted to a subclass of cortical interneurons known to be particularly sensitive to abnormal prion accumulation and rapidly degenerate during early stages of CJD (Belichenko et al., 1999). In addition, recent studies showed that LRP/LR is not only involved in the PrPc metabolism, but has also a crucial role in prion propagation. Using antisense LRP RNA and small interfering (si) RNAs specific for LRP mRNA, PrPSc levels in scrapie-infected neuronal cells were reduced demonstrating the necessity for the laminin receptor LRP/LR for PrPSc propagation in cultured cells (Leucht et al., 2003). Moreover, in a recent study, it has been shown that bovine PrPSc is internalized by human enterocytes via LRP/LR-mediated endocytosis (Morel et al., 2005) using the Caco-2/TC7 cell model system. Analysis of the presence of PrPSc after supply of prion-contaminated brain homogenate from different sources in Caco-2/TC7 enterocytes revealed that BSE prions were specifically internalized and accumulate in human enterocytes, whereas murine-adapted scrapie-prions were not endocytosed. PrPBSE-containing vesicles visualized in these cells colocalized with LRP/LR in the subapical compartment. PrPBSE internalization was blocked by the anti-LRP antibody W3 approving that prion endocytosis in human enterocytes is mediated by the 37 kDa/67 kDa laminin receptor LRP/LR. Even more recently, the specificity of prion binding in dependency of the 37 kDa/67 kDa LRP/LR has been shown on BHK cells overexpressing LRP (Gauczynski et al., 2006). This effect can be inhibited efficiently by the LRP-specific polyclonal antibody W3 as well as by polysulfated glycans such as pentosan polysulfate and heparan mimetics (Gauczynski et al., 2006). Moreover, it has been shown that GAGs (Hijazi et al., 2005), especially heparan sulfate (Horonchik et al., 2005) act also as receptors for PrPSc. Taken together, prions like other infectious agents such as viruses may use LRP/LR and heparan sulfate as receptors, presumably in synergy as suggested also in case of PrPc (Gauczynski et al., 2001; Hundt et al., 2001), which also employs LRP/LR and HSPGs as receptors and coreceptors, respectively (Fig. 2).
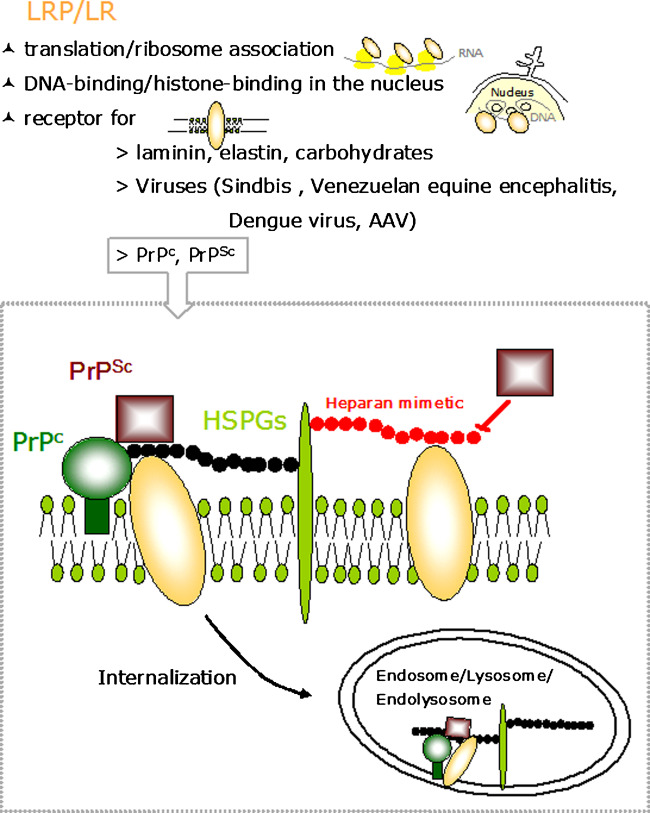
Fig. 2.
Cellular functions of LRP/LR and model of the LRP/LR-/HSPG-dependent PrPc and PrPSc binding and internalization. Upper panel: LRP/LR is associated to ribosomes and is involved in the translation machinery (i), binds to nuclear DNA through its association with histones contributing to the maintenance of nuclear structures (ii), and acts as a receptor for laminin, elastin, and carbohydrates, as well as viruses and PrPc and PrPSc. Lower panel: PrPc (green circle) anchored by glycosylphosphatidyl inositol (GPI) (green bar) becomes internalized by LRP/LR (yellow ovals) (Gauczynski et al., 2001) utilizing HSPGs (light green bars and black chain) as cofactors/coreceptors (Hundt et al., 2001). PrPSc (purple squares) binds to the cell surface in a LRP/LR-(Gauczynski et al., 2006), and heparan sulfate (black chain)/ HSPG- (Horonchik et al., 2005) dependent manner. The PrP/LRP-LR/HSPG complex becomes internalized into endo-/lysosomes. LRP/LR (Morel et al., 2005; Gauczynski et al., 2006) and heparan sulfates (Horonchik et al., 2005) mediate presumably in synergy PrPSc internalization. Polysulfated glycans such as the heparan mimetics HM 2602 and HM 5004 (red chain) block PrPSc binding to the cells by competing with the binding to heparan sulfate and LRP/LR (Gauczynski et al., 2006). Pentosan polysulfate (SP-54) and phycarin sulfate may have similar effects as the HMs (adopted from (Gauczynski et al., 2006)). AAV denotes Adeno-Associated Virus.
THERAPEUTIC STRATEGIES FOR THE TREATMENT OF PRION DISEASES
Since variant Creutzfeldt–Jakob disease appeared, numerous strategies and targets have been proposed for a therapy of prion diseases, including:
stabilization of the structure of PrPc to prevent the transconformation from PrPc to PrPSc
interference of the binding of PrPSc to PrPc
inhibition of the formation of the abnormal form of PrP
prevention of PrP synthesis
destruction of PrPSc aggregates
inhibition of the prion protein receptor(s)
The inhibition of the PrPSc accumulation, however, is the most studied target. There are a number of compounds that have been shown to efficiently interfere with the PrPSc accumulation, such as Congo red (Ingrosso et al., 1995) and analogs (Demaimay et al., 1997), certain cyclic tetrapyrrols such as porphyrins and phtalocyanines (Priola et al., 2000), sulfated polyanions such as dextran sulfate 500 (Farquhar and Dickinson, 1986), pentosan polysulfate (Caughey and Raymond, 1993) and suramin (Gilch et al., 2001), as well as polyene antibiotics such as AmB and its derivative MS 8209 (Adjou et al., 1995) (Table IV). Many other compounds have been identified to have an effect on the formation of pathological PrPSc in vitro and in vivo, but only flupirtine, an analgetic, is possibly beneficial in humans (Otto et al., 2004). To identify novel substances regarding a therapeutic potency, assays for the screening of large compound libraries, e.g., a high-throughput assay for the identification of drugs, which interfere with the PrPc/PrPSc interaction, were developed (Bertsch et al., 2005). Although several substances have been identified to date, which inhibit PrPSc formation, unfortunately most of them show only significant effects when administered long before the clinical onset. At present, there is no effective therapy for clinically affected TSE patients available, so that TSEs usually culminate in death.
Table IV.
Summary of Major Components Exhibiting Therapeutic Antiprion Effects
| Class of compounds | Example | Reference |
|---|---|---|
| Polysulfonated, polyanionic substances | Dextran sulfate, | (Farquhar and Dickinson, 1986; Diringer and Ehlers, 1991) |
| suramin, | (Gilch et al., 2001) | |
| pentosan polysulfate, | (Caughey and Raymond, 1993; Farquhar et al., 1999; Gauczynski et al., 2006) | |
| heparan sulfate mimetics | (Adjou et al., 2003; Gauczynski et al., 2006) | |
| Amyloidotropic intercalators | Congo red | (Caughey and Race, 1992; Poli et al., 2004) |
| Polyene antibiotics | Amphotericin B (AmB), | (Pocchiari et al., 1987) |
| MS 8209 | (Adjou et al., 1995) | |
| Filipin | (Marella et al., 2002) | |
| Cyclic tetrapyrrols | Porphyrines, phtalocyanines | (Caughey et al., 1998; Priola et al., 2000) |
| Polyamines | DOSPA, | (Winklhofer and Tatzelt, 2000) |
| SuperFect, polyethyleneimine | (Supattapone et al., 2001) | |
| Anthracyclines | IDX | (Tagliavini et al., 1997) |
| Phenothiazines | Chlorpromazine | (Achour 2002; Benito-Leon 2004) |
| Acridines/bis-acridines | Quinacrine | (Korth et al., 2001) |
| Designer peptides | β-sheet breaker | (Reilly 2000; Oishi et al., 2003) |
| RNA aptamers | RNA aptamer Ap1/2/3 (antiprion effect not proven) | (Weiss et al., 1997) |
| RNA aptamer DP7 | (Proske et al., 2002) | |
| RNA aptamer 60-3 (antiprion effect not proven) | (Sekiya et al., 2006) |
Another strategy was based on the finding that PrP-specific antibodies antagonize prion propagation both in vitro and in vivo (for a review, see (Buchholz et al., 2006)). It was proven, that chronically scrapie-infected neuroblastoma cells have been cured by a monoclonal antiprion protein (PrP) antibody (Enari et al., 2001). In a murine model, treatment using this monoclonal antibody has delayed the development of prion disease (White et al., 2003). Application of monoclonal antibodies raised against recombinant PrP resulted also in a reduction of PrPSc level in mouse neuroblastoma cells (Pankiewicz et al., 2006).
Since active immunization suffers from high costs, researchers have developed passive immunization strategies although this issue deals with the problem of autotolerance. Recently, it became obvious that the induction of a native PrPc-specific antibody response (in contrast to a response against recombinant PrPc produced in bacteria) may help to overcome tolerance. Novel strategies have been developed to obtain such responses by passive immunization (Goni et al., 2005; Nikles et al., 2005). Alternatively, single chain antibodies, which usually can be produced easier and faster than full-length antibodies, revealed an antiprion effect in neuro-blastoma cells (Donofrio et al., 2005).
For the treatment of neurodegenerative diseases such as Alzheimer's disease, AAV-mediated gene delivery has already been established (Zhang et al., 2003; Feng et al., 2004; Hara et al., 2004; Sanftner et al., 2005).
THERAPEUTIC APPROACHES TARGETING LRP/LR
Polysulfated Glycanes
Polysulfated glycanes such as heparan mimetics (HMs) or pentosan polysulfate block PrPSc binding to target cells by interfering with the PrPSc-LRP/LR-HSPG binding and internalization complex (Gauczynski et al., 2006) and prolong the survival times of scrapie-infected mice (Farquhar et al., 1999; Adjou et al., 2003). Therefore, these substances are promising tools for the treatment of TSEs.
Transdominant Negative LRP Mutants
Recently, it has been shown that a LRP mutant encompassing only the extracellular domain of LRP/LR (LRP102-295::FLAG) might act in a transdominant negative manner as a decoy by trapping PrP molecules (Vana and Weiss, 2006). In vitro studies revealed that the LRP mutant is able to reduce the PrPSc accumulation in scrapie-infected neuronal cells (Vana and Weiss, 2006) and, thus, might have potential for the development of a TSE therapy.
Antibodies
The PrP binding capacity of LRP offers strategies in therapeutic approaches against prion diseases. Antibodies directed against LRP/LR such as W3 are able to block PrPSc propagation in cultured cells (Leucht et al., 2003), prohibit PrPBSE internalization by human enterocytes (Morel et al., 2005), and interfere with PrP27-30 binding to mammalian cells (Gauczynski et al., 2006). Therefore, antibodies directed against the receptor might be powerful tools in the treatment of prion diseases.
However, this antibody format might not be suitable for a therapy in animals or humans. Single chain antibodies are a promising alternative, which are already in use for cancer treatments such as Herceptin® (De Lorenzo et al., 2004) for the treatment of breast cancer (Zhou and Zhong, 2004). Although they consist of a lower molecular weight (30 kDa) compared to the complete immunoglobulins, they reveal a better tissue penetration and higher binding affinity. For therapeutic application, scFv directed against 37 kDa/67 kDa LRP/LR might be passively delivered by intracerebral injection directly into brain regions where massive PrPSc propagation takes place. In addition, permanent expression and secretion of scFvs might be achieved by gene therapeutic strategies employing lentiviral or AAV-based vector systems.
RNA Interference and Antisense RNA
A further strategy to interfere with PrPSc propagation is the knockdown of LRP/LR by siRNA and antisense RNA technology. Successfull knockdown has already been shown for PrP using Prn-p-specific sequences. Thus, the transfection of siRNA duplices corresponding to the murine Prn-p triggered specific Prn-p gene silencing in scrapie-infected neuroblastoma cells and caused a rapid loss of their PrPres content (Daude et al., 2003). Accordingly, it was shown in scrapie-infected neuronal cells that transfection of either LRP antisense RNA or LRP-specific siRNAs ablated LRP/LR expression and prevented PrPSc propagation in scrapie-infected neuronal cells (Leucht et al., 2003), confirming a requirement of LRP/LR for PrPSc propagation in cultured cells.
However, a permanent effect using RNAi may be achieved by lentivirus-mediated gene transfer to specifically knockdown disease-relevant genes (Ralph et al., 2005; Raoul et al., 2005). Therefore, a lentivirus-based RNAi gene therapy strategy using HIV-derived vectors expressing LRP-specific siRNAs represents an innovative approach in TSE treatment.
CONCLUSIONS
Prion diseases are rare diseases and the protein-only hypothesis (Bolton et al., 1982; Prusiner, 1982) is an approved theory to explain the characteristics of TSEs. However, the natural function of the cellular prion protein (PrPc) remains enigmatic. A series of strategies for TSE treatment are currently under consideration. The majority of these therapeutics target the prion protein itself, destabilize the PrPSc structure, or interfere with the binding of PrPc to PrPSc. Nevertheless, no treatment has been shown to prevent the appearance of clinical symptoms and death in animal models or in CJD patients. In June 2004, the PRION-1 clinical trial (3 years) was started to assess the activity and safety of quinacrine in human prion disease since there are no other drugs available that are considered suitable for human evaluation. Thus, further work is essential to establish treatments that efficiently medicate prion diseases.
The discovery of the 37 kDa/67 kDa laminin receptor (LRP/LR) as the cell surface receptor for the cellular (PrPc) (Gauczynski et al., 2001) and a receptor for infectious prions (Morel et al., 2005; Gauczynski et al., 2006) as well as GAGs (Hijazi et al., 2005)/heparan sulfate (Horonchik et al., 2005) as receptors for PrPSc and HSPGs as cofactors/coreceptors for PrPc (Hundt et al., 2001) opens new avenues for the development of alternative antiprion therapies. Antibodies directed against LRP/LR (Leucht et al., 2003), small interfering RNAs directed against LRP mRNA (Leucht et al., 2003), LRP mutants (Vana and Weiss, 2006), and polysulfated glycanes interfering with the PrPSc/LRP/LR interaction process (Gauczynski et al., 2006) represent alternative promising tools for the treatment of prion diseases.
ACKNOWLEDGMENTS
The authors thank the Bundesministerium für Bildung und Forschung (Grants 01-KO-0106 and KO-01-0514), the Bavarian Prion Research Foundation (Grant LMU 4), and the European Commission (Grants QLRT-2000-02085 and NoE NeuroPrion FOOD-CT-2004-506579) for financial support.
REFERENCES
- Achour, A. (2002). Phenothiazines and prion diseases: A potential mechanism of action towards oxidative stress. Int. J. Antimicrob. Agents20:305–306. [DOI] [PubMed] [Google Scholar]
- Adjou, K. T., Demaimay, R., Lasmezas, C., Deslys, J. P., Seman, M., and Dormont, D. (1995). MS-8209, a new amphotericin B derivative, provides enhanced efficacy in delaying hamster scrapie. Antimicrob. Agents Chemother.39(12):2810–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adjou, K. T., Simoneau, S., Sales, N., Lamoury, F., Dormont, D., Papy-Garcia, D., Barritault, D., Deslys, J. P., and Lasmezas, C. I. (2003). A novel generation of heparan sulfate mimetics for the treatment of prion diseases. J. Gen. Virol.84(Pt. 9):2595–2603. [DOI] [PubMed] [Google Scholar]
- Akache, B., Grimm, D., Pandey, K., Yant, S. R., Xu, H., and Kay, M. A. (2006). The 37/67-kilodalton Laminin Receptor is a receptor for Adeno-associated-Virus Serotypes 8, 2, 3, and 9. J. Virol.80(19):9831–9836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper, T., Cramp, W. A., Haig, D. A., and Clarke, M. C. (1967). Does the agent of scrapie replicate without nucleic acid? Nature214(90):764–766. [DOI] [PubMed] [Google Scholar]
- Ardini, E., Pesole, G., Tagliabue, E., Magnifico, A., Castronovo, V., Sobel, M. E., Colnaghi, M. I., and Menard, S. (1998). The 67-kDa laminin receptor originated from a ribosomal protein that acquired a dual function during evolution. Mol. Biol. Evol.15(8):1017–1025. [DOI] [PubMed] [Google Scholar]
- Arnold, J. E., Tipler, C., Laszlo, L., Hope, J., Landon, M., and Mayer, R. J. (1995). The abnormal isoform of the prion protein accumulates in late-endosome-like organelles in scrapie-infected mouse brain. J. Pathol.176(4):403–411. [DOI] [PubMed] [Google Scholar]
- Auth, D., and Brawerman, G. (1992). A 33-kDa polypeptide with homology to the laminin receptor: Component of translation machinery. Proc. Natl. Acad. Sci. U. S. A.89(10):4368–4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge, J., and Walker, K. B. (2005). The normal cellular form of prion protein modulates T cell responses. Immunol. Lett.96(1):147–150. [DOI] [PubMed] [Google Scholar]
- Ballerini, C., Gourdain, P., Bachy, V., Blanchard, N., Levavasseur, E., Gregoire, S., Fontes, P., Aucouturier, P., Hivroz, C., and Carnaud, C. (2006). Functional implication of cellular prion protein in antigen-driven interactions between T cells and dendritic cells. J. Immunol.176(12):7254–7262. [DOI] [PubMed] [Google Scholar]
- Baloui, H., von Boxberg, Y., Vinh, J., Weiss, S., Rossier, J., Nothias, F., and Stettler, O. (2004). Cellular prion protein/laminin receptor: Distribution in adult central nervous system and characterization of an isoform associated with a subtype of cortical neurons. Eur. J. Neurosci.20(10):2605–2616. [DOI] [PubMed] [Google Scholar]
- Baron, G. S., and Caughey, B. (2003). Effect of glycosylphosphatidylinositol anchor-dependent and -independent prion protein association with model raft membranes on conversion to the protease-resistant isoform. J. Biol. Chem.278(17):14883–14892. [DOI] [PubMed] [Google Scholar]
- Basler, K., Oesch, B., Scott, M., Westaway, D., Walchli, M., Groth, D. F., McKinley, M. P., Prusiner, S. B., and Weissmann, C. (1986). Scrapie and cellular PrP isoforms are encoded by the same chromosomal gene. Cell46(3):417–428. [DOI] [PubMed] [Google Scholar]
- Belichenko, P. V., Miklossy, J., Belser, B., Budka, H., and Celio, M. R. (1999). Early destruction of the extracellular matrix around parvalbumin-immunoreactive interneurons in Creutzfeldt–Jakob disease. Neurobiol. Dis.6(4):269–279. [DOI] [PubMed] [Google Scholar]
- Benito-Leon, J. (2004). Combined quinacrine and chlorpromazine therapy in fatal familial insomnia. Clin. Neuropharmacol.27(4):201–203. [DOI] [PubMed] [Google Scholar]
- Bertsch, U., Winklhofer, K. F., Hirschberger, T., Bieschke, J., Weber, P., Hartl, F. U., Tavan, P., Tatzelt, J., Kretzschmar, H. A., and Giese, A. (2005). Systematic identification of antiprion drugs by high-throughput screening based on scanning for intensely fluorescent targets. J. Virol.79(12):7785–7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton, D. C., McKinley, M. P., and Prusiner, S. B. (1982). Identification of a protein that purifies with the scrapie prion. Science218(4579):1309–1311. [DOI] [PubMed] [Google Scholar]
- Bosque, P. J., Ryou, C., Telling, G., Peretz, D., Legname, G., DeArmond, S. J., and Prusiner, S. B. (2002). Prions in skeletal muscle. Proc. Natl. Acad. Sci. U. S. A.99(6):3812–3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botto, L., Masserini, M., Cassetti, A., and Palestini, P. (2004). Immunoseparation of prion protein-enriched domains from other detergent-resistant membrane fractions, isolated from neuronal cells. FEBS Lett.557(1–3):143–147. [DOI] [PubMed] [Google Scholar]
- Bounhar, Y., Zhang, Y., Goodyer, C. G., and LeBlanc, A. (2001). Prion protein protects human neurons against Bax-mediated apoptosis. J. Biol. Chem.276(42):39145–39149. [DOI] [PubMed] [Google Scholar]
- Bragason, B. T., and Palsdottir, A. (2005). Interaction of PrP with NRAGE, a protein involved in neuronal apoptosis. Mol. Cell. Neurosci.29(2):232–244. [DOI] [PubMed] [Google Scholar]
- Brown, D. R., Qin, K., Herms, J. W., Madlung, A., Manson, J., Strome, R., Fraser, P. E., Kruck, T., von Bohlen, A., Schulz-Schaeffer, W., Giese, A., Westaway, D., and Kretzschmar, H. (1997). The cellular prion protein binds copper in vivo. Nature390(6661):684–687. [DOI] [PubMed] [Google Scholar]
- Brown, D. R., Schmidt, B., Groschup, M. H., and Kretzschmar, H. A. (1998). Prion protein expression in muscle cells and toxicity of a prion protein fragment. Eur. J. Cell Biol.75(1):29–37. [DOI] [PubMed] [Google Scholar]
- Brown, D. R., Wong, B. S., Hafiz, F., Clive, C., Haswell, S. J., and Jones, I. M. (1999). Normal prion protein has an activity like that of superoxide dismutase. Biochem. J.344(Pt. 1):1–5. [PMC free article] [PubMed] [Google Scholar]
- Brown, H. R., Goller, N. L., Rudelli, R. D., Merz, G. S., Wolfe, G. C., Wisniewski, H. N., and Robakis, N. K. (1990). The mRNA encoding the scrapie agent protein is present in a variety of non-neuronal cells. Acta Neuropathol. (Berl.)80(1):1–6. [DOI] [PubMed] [Google Scholar]
- Buchholz, C. J., Bach, P., Nikles, D., and Kalinke, U. (2006). Prion protein-specific antibodies for therapeutic intervention of transmissible spongiform encephalopathies. Expert Opin. Biol. Ther.6:293–300. [DOI] [PubMed] [Google Scholar]
- Bueler, H., Aguzzi, A., Sailer, A., Greiner, R. A., Autenried, P., Aguet, M., and Weissmann, C. (1993). Mice devoid of PrP are resistant to scrapie. Cell73(7):1339–1347. [DOI] [PubMed] [Google Scholar]
- Bueler, H., Fischer, M., Lang, Y., Bluethmann, H., Lipp, H. P., DeArmond, S. J., Prusiner, S. B., Aguet, M., and Weissmann, C. (1992). Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature356(6370):577–582. [DOI] [PubMed] [Google Scholar]
- Burger, D., and Hartsough, G. R. (1965). Encephalopathy of mink. II. Experimental and natural transmission. J. Infect. Dis.115(4):393–399. [DOI] [PubMed] [Google Scholar]
- Buto, S., Tagliabue, E., Ardini, E., Magnifico, A., Ghirelli, C., Van Den Brule, F., Castronovo, V., Colnaghi, M. I., Sobel, M. E., and Menard, S. (1998). Formation of the 67-kDa laminin receptor by acylation of the precursor. J. Cell. Biochem.69(3):244–251. [DOI] [PubMed] [Google Scholar]
- Cashman, N. R., Loertscher, R., Nalbantoglu, J., Shaw, I., Kascsak, R. J., Bolton, D. C., and Bendheim, P. E. (1990). Cellular isoform of the scrapie agent protein participates in lymphocyte activation. Cell61(1):185–192. [DOI] [PubMed] [Google Scholar]
- Castilla, J., Saa, P., Hetz, C., and Soto, C. (2005). In vitro generation of infectious scrapie prions. Cell121(2):195–206. [DOI] [PubMed] [Google Scholar]
- Castronovo, V., Taraboletti, G., and Sobel, M. E. (1991). Functional domains of the 67-kDa laminin receptor precursor. J. Biol. Chem.266(30):20440–20446. [PubMed] [Google Scholar]
- Caughey, B., Kocisko, D. A., Raymond, G. J., and Lansbury, P. T. Jr. (1995). Aggregates of scrapie-associated prion protein induce the cell-free conversion of protease-sensitive prion protein to the protease-resistant state. Chem. Biol.2(12):807–817. [DOI] [PubMed] [Google Scholar]
- Caughey, B., and Race, R. E. (1992). Potent inhibition of scrapie-associated PrP accumulation by Congo red. J. Neurochem.59(2):768–771. [DOI] [PubMed] [Google Scholar]
- Caughey, B., and Raymond, G. J. (1993). Sulfated polyanion inhibition of scrapie-associated PrP accumulation in cultured cells. J. Virol.67(2):643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughey, W. S., Raymond, L. D., Horiuchi, M., and Caughey, B. (1998). Inhibition of protease-resistant prion protein formation by porphyrins and phthalocyanines. Proc. Natl. Acad. Sci. U. S. A.95(21):12117–12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro, B., Trifilo, M., Race, R., Meade-White, K., Teng, C., LaCasse, R., Raymond, L., Favara, C., Baron, G., Priola, S., Caughey, B., Masliah, E., and Oldstone, M. (2005). Anchorless prion protein results in infectious amyloid disease without clinical scrapie. Science308(5727):1435–1439. [DOI] [PubMed] [Google Scholar]
- Cohen, F. E., Pan, K. M., Huang, Z., Baldwin, M., Fletterick, R. J., and Prusiner, S. B. (1994). Structural clues to prion replication. Science264(5158):530–531. [DOI] [PubMed] [Google Scholar]
- Cohen, F. E., and Prusiner, S. B. (1998). Pathologic conformations of prion proteins. Annu. Rev. Biochem.67:793–819. [DOI] [PubMed] [Google Scholar]
- Collinge, J., and Palmer, M. S. (1994). Molecular genetics of human prion diseases. Philos. Trans. R. Soc. Lond. B. Biol. Sci.343(1306):371–378. [DOI] [PubMed] [Google Scholar]
- Collinge, J., Whittington, M. A., Sidle, K. C., Smith, C. J., Palmer, M. S., Clarke, A. R., and Jefferys, J. G. (1994). Prion protein is necessary for normal synaptic function. Nature370(6487):295–297. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt, H. G. (1920). Über eine eigenartige Erkrankung des Zentralnervensystems. Vorläufige Mitteilung. Z. f. d. ges. Neurol. und Psych., 1–18.
- Daude, N., Marella, M., and Chabry, J. (2003). Specific inhibition of pathological prion protein accumulation by small interfering RNAs. J. Cell Sci.116(Pt. 13):2775–2779. [DOI] [PubMed] [Google Scholar]
- Della-Bianca, V., Rossi, F., Armato, U., Dal-Pra, I., Costantini, C., Perini, G., Politi, V., and Della Valle, G. (2001). Neurotrophin p75 receptor is involved in neuronal damage by prion peptide-(106–126). J. Biol. Chem.276(42):38929–38933. [DOI] [PubMed] [Google Scholar]
- De Lorenzo, C., Tedesco, A., Terrazzano, G., Cozzolino, R., Laccetti, P., Piccoli, R., and D'Alessio, G. (2004). A human, compact, fully functional anti-ErbB2 antibody as a novel antitumour agent. Br. J. Cancer91:1200–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaimay, R., Adjou, K. T., Beringue, V., Demart, S., Lasmezas, C. I., Deslys, J. P., Seman, M., and Dormont, D. (1997). Late treatment with polyene antibiotics can prolong the survival time of scrapie-infected animals. J. Virol.71(12):9685–9689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diarra-Mehrpour, M., Arrabal, S., Jalil, A., Pinson, X., Gaudin, C., Pietu, G., Pitaval, A., Ripoche, H., Eloit, M., Dormont, D., and Chouaib, S. (2004). Prion protein prevents human breast carcinoma cell line from tumor necrosis factor alpha-induced cell death. Cancer Res.64(2):719–727. [DOI] [PubMed] [Google Scholar]
- Diringer, H., and Ehlers, B. (1991). Chemoprophylaxis of scrapie in mice. J. Gen. Virol.72(Pt. 2):457–460. [DOI] [PubMed] [Google Scholar]
- Donne, D. G., Viles, J. H., Groth, D., Mehlhorn, I., James, T. L., Cohen, F. E., Prusiner, S. B., Wright, P. E., and Dyson, H. J. (1997). Structure of the recombinant full-length hamster prion protein PrP(29-231): the N terminus is highly flexible. Proc. Natl. Acad. Sci. U. S. A.94(25):13452–13457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donofrio, G., Heppner, F. L., Polymenidou, M., Musahl, C., and Aguzzi, A. (2005). Paracrine inhibition of prion propagation by anti-PrP single-chain Fv miniantibodies. J. Virol.79(13):8330–8338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy, P., Wolf, J., Collins, G., DeVoe, A. G., Streeten, B., and Cowen, D. (1974). Letter: Possible person-to-person transmission of Creutzfeldt–Jakob disease. N. Engl. J. Med.290(12):692–693. [PubMed] [Google Scholar]
- Edenhofer, F., Rieger, R., Famulok, M., Wendler, W., Weiss, S., and Winnacker, E. L. (1996). Prion protein PrPc interacts with molecular chaperones of the Hsp60 family. J. Virol.70(7):4724–4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enari, M., Flechsig, E., and Weissmann, C. (2001). Scrapie prion protein accumulation by scrapie-infected neuroblastoma cells abrogated by exposure to a prion protein antibody. Proc. Natl. Acad. Sci. U. S. A.98(16):9295–9299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar, C. F., Dickinson, A., and Bruce, M. (1999). Prophylactic potential of pentosan polysulphate in spongiform transmissible encephalopathies. Lancet353:117. [DOI] [PubMed] [Google Scholar]
- Farquhar, C. F., and Dickinson, A. G. (1986). Prolongation of scrapie incubation period by an injection of dextran sulphate 500 within the month before or after infection. J. Gen. Virol.67(Pt. 3):463–473. [DOI] [PubMed] [Google Scholar]
- Feng, X., Eide, F. F., Jiang, H., and Reder, A. T. (2004). Adeno-associated viral vector-mediated ApoE expression in Alzheimer's disease mice: low CNS immune response, long-term expression, and astrocyte specificity. Front. Biosci.9:1540–1546. [DOI] [PubMed] [Google Scholar]
- Gabizon, R., Meiner, Z., Halimi, M., and Ben-Sasson, S. A. (1993). Heparin-like molecules bind differentially to prion-proteins and change their intracellular metabolic fate. J. Cell. Physiol.157:319–325. [DOI] [PubMed] [Google Scholar]
- Gajdusek, D. C., and Zigas, V. (1957). Degenerative disease of the central nervous system in New Guinea; the endemic occurrence of Kuru in the native population. N. Engl. J. Med.257(20):974–978. [DOI] [PubMed] [Google Scholar]
- Gauczynski, S., Nikles, D., El-Gogo, S., Papy-Garcia, D., Rey, C., Alban, S., Barritault, D., Lasmezas, C. I., and Weiss, S. (2006). The 37-kDa/67-kDa laminin receptor acts as a receptor for infectious prions and is inhibited by polysulfated glycanes. J. Infect. Dis.194(5):702–709. [DOI] [PubMed] [Google Scholar]
- Gauczynski, S., Peyrin, J. M., Haik, S., Leucht, C., Hundt, C., Rieger, R., Krasemann, S., Deslys, J. P., Dormont, D., Lasmezas, C. I., and Weiss, S. (2001). The 37-kDa/67-kDa laminin receptor acts as the cell-surface receptor for the cellular prion protein. EMBO J.20(21):5863–5875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstmann, J., Straussler, E., and Scheinker, I. (1936). Über eine eigenartige hereditär-familiäre Erkrankung des Zentralnervensystems. Zugleich ein Beitrag zur Frage des vorzeitigen lokalen Alterns. Zeitschrift für die gesamte Neurologie und Psychiatrie154:736–762. [Google Scholar]
- Gibbs, C. J. Jr., and Bolis, C. L. (1997). Normal isoform of amyloid protein (PrP) in brains of spawning salmon. Mol. Psychiatry2(2):146–147. [DOI] [PubMed] [Google Scholar]
- Gilch, S., Winklhofer, K. F., Groschup, M. H., Nunziante, M., Lucassen, R., Spielhaupter, C., Muranyi, W., Riesner, D., Tatzelt, J., and Schatzl, H. M. (2001). Intracellular re-routing of prion protein prevents propagation of PrP(Sc) and delays onset of prion disease. EMBO J.20(15):3957–3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goni, F., Knudsen, E., Schreiber, F., Scholtzova, H., Pankiewicz, J., Carp, R., Meeker, H. C., Rubenstein, R., Brown, D. R., Sy, M. S., Chabalgoity, J. A., Sigurdsson, E. M., and Wisniewski, T. (2005). Mucosal vaccination delays or prevents prion infection via an oral route. Neuroscience133(2):413–421. [DOI] [PubMed] [Google Scholar]
- Graner, E., Mercadante, A. F., Zanata, S. M., Forlenza, O. V., Cabral, A. L., Veiga, S. S., Juliano, M. A., Roesler, R., Walz, R., Minetti, A., Izquierdo, I., Martins, V. R., and Brentani, R. R. (2000). Cellular prion protein binds laminin and mediates neuritogenesis. Brain Res. Mol. Brain Res.76(1):85–92. [DOI] [PubMed] [Google Scholar]
- Griffith, J. S. (1967). Self-replication and scrapie. Nature215(105):1043–1044. [DOI] [PubMed] [Google Scholar]
- Hara, H., Monsonego, A., Yuasa, K., Adachi, K., Xiao, X., Takeda, S., Takahashi, K., Weiner, H. L., and Tabira, T. (2004). Development of a safe oral Abeta vaccine using recombinant adeno-associated virus vector for Alzheimer's disease. J. Alzheimers Dis.6:483–488. [DOI] [PubMed] [Google Scholar]
- Harris, D. A., Lele, P., and Snider, W. D. (1993). Localization of the mRNA for a chicken prion protein by in situ hybridization. Proc. Natl. Acad. Sci. U. S. A.90(9):4309–4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijazi, N., Kariv-Inbal, Z., Gasset, M., and Gabizon, R. (2005). PrPSc incorporation to cells requires endogenous glycosaminoglycan expression. J. Biol. Chem.280:17057–17061. [DOI] [PubMed] [Google Scholar]
- Hope, J., Morton, L. J., Farquhar, C. F., Multhaup, G., Beyreuther, K., and Kimberlin, R. H. (1986). The major polypeptide of scrapie-associated fibrils (SAF) has the same size, charge distribution and N-terminal protein sequence as predicted for the normal brain protein (PrP). EMBO J.5(10):2591–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornshaw, M. P., McDermott, J. R., and Candy, J. M. (1995). Copper binding to the N-terminal tandem repeat regions of mammalian and avian prion protein. Biochem. Biophys. Res. Commun.207(2):621–629. [DOI] [PubMed] [Google Scholar]
- Horonchik, L., Tzaban, S., Ben-Zaken, O., Yedidia, Y., Rouvinski, A., Papy-Garcia, D., Barritault, D., Vlodavsky, I., and Taraboulos, A. (2005). Heparan sulfate is a cellular receptor for purified infectious prions. J. Biol. Chem.280:17062–17067. [DOI] [PubMed] [Google Scholar]
- Hundt, C., Peyrin, J. M., Haik, S., Gauczynski, S., Leucht, C., Rieger, R., Riley, M. L., Deslys, J. P., Dormont, D., Lasmezas, C. I., and Weiss, S. (2001). Identification of interaction domains of the prion protein with its 37-kDa/67-kDa laminin receptor. EMBO J.20(21):5876–5886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingrosso, L., Ladogana, A., and Pocchiari, M. (1995). Congo red prolongs the incubation period in scrapie-infected hamsters. J. Virol.69(1):506–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackers, P., Clausse, N., Fernandez, M., Berti, A., Princen, F., Wewer, U., Sobel, M. E., and Castronovo, V. (1996). Seventeen copies of the human 37 kDa laminin receptor precursor/p40 ribosome-associated protein gene are processed pseudogenes arisen from retropositional events. Biochim. Biophys. Acta1305(1–2):98–104. [DOI] [PubMed] [Google Scholar]
- Jakob, A. (1921). Über eigenartige Erkrankungen des Zentralnervensystems mit bemerkenswerten anatomischen Befunden (spastische Pseudosklerose-Encephalomyelopathie mit dissemierten Degenerationsherden). Vorläufige Mitteilung. Z. Ges. Neurol. Psychiatr64:147–228. [Google Scholar]
- Jeffrey, M., and Wells, G. A. (1988). Spongiform encephalopathy in a nyala (Tragelaphus angasi). Vet. Pathol.25(5):398–399. [DOI] [PubMed] [Google Scholar]
- Kinoshita, K., Kaneda, Y., Sato, M., Saeki, Y., Wataya-Kaneda, M., and Hoffmann, A. (1998). LBP-p40 binds DNA tightly through associations with histones H2A, H2B, and H4. Biochem. Biophys. Res. Commun.253:277–282. [DOI] [PubMed] [Google Scholar]
- Kirkwood, J. K., Wells, G. A., Wilesmith, J. W., Cunningham, A. A., and Jackson, S. I. (1990). Spongiform encephalopathy in an arabian oryx (Oryx leucoryx) and a greater kudu (Tragelaphus strepsiceros). Vet. Rec.127(17):418–420. [PubMed] [Google Scholar]
- Kirschbaum, W. (1924). Zwei eigenartige Erkrankungen des Zentralnervensystems nach Art der spastischen Pseudosklerose (Jakob). Zeitschrift für die gesamte Neurologie und Psychiatrie92:175–220. [Google Scholar]
- Klamt, F., Dal-Pizzol, F., Conte da Frota, Ml Jr, Walz, R., Andrades, M. E., da Silva, E. G., Brentani, R. R., Izquierdo, I., and Fonseca Moreira, J. C. (2001). Imbalance of antioxidant defense in mice lacking cellular prion protein. Free Radic. Biol. Med.30(10):1137–1144. [DOI] [PubMed] [Google Scholar]
- Koch, C. A., Anderson, D., Moran, M. F., Ellis, C., and Pawson, T. (1991). SH2 and SH3 domains: Elements that control interactions of cytoplasmic signaling proteins. Science252(5006):668–674. [DOI] [PubMed] [Google Scholar]
- Korth, C., May, B. C., Cohen, F. E., and Prusiner, S. B. (2001). Acridine and phenothiazine derivatives as pharmacotherapeutics for prion disease. Proc. Natl. Acad. Sci. U. S. A.98(17):9836–9841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzschmar, H. A., Prusiner, S. B., Stowring, L. E., and DeArmond, S. J. (1986). Scrapie prion proteins are synthesized in neurons. Am. J. Pathol.122(1):1–5. [PMC free article] [PubMed] [Google Scholar]
- Kretzschmar, H. A., Tings, T., Madlung, A., Giese, A., and Herms, J. (2000). Function of PrP(C) as a copper-binding protein at the synapse. Arch. Virol.16(Suppl.):239–249. [DOI] [PubMed] [Google Scholar]
- Kurschner, C., and Morgan, J. I. (1995). The cellular prion protein (PrP) selectively binds to Bcl-2 in the yeast two-hybrid system. Brain Res. Mol. Brain Res.30(1):165–168. [DOI] [PubMed] [Google Scholar]
- Landowski, T. H., Dratz, E. A., and Starkey, J. R. (1995). Studies of the structure of the metastasis-associated 67 kDa laminin binding protein: Fatty acid acylation and evidence supporting dimerization of the 32 kDa gene product to form the mature protein. Biochemistry34(35):11276–11287. [DOI] [PubMed] [Google Scholar]
- Lansbury, P. T. Jr., and Caughey, B. (1995). The chemistry of scrapie infection: Implications of the “ice 9” metaphor. Chem. Biol.2(1):1–5. [DOI] [PubMed] [Google Scholar]
- Legname, G., Baskakov, I. V., Nguyen, H. O., Riesner, D., Cohen, F. E., DeArmond, S. J., and Prusiner, S. B. (2004). Synthetic mammalian prions. Science305(5684):673–676. [DOI] [PubMed] [Google Scholar]
- Leliveld, S. R., Dame, R. T., Wuite, G. J., Stitz, L., and Korth, C. (2006). The expanded octarepeat domain selectively binds prions and disrupts homomeric prion protein interactions. J. Biol. Chem.281:3268–3275. [DOI] [PubMed] [Google Scholar]
- Leucht, C., Simoneau, S., Rey, C., Vana, K., Rieger, R., Lasmezas, C. I., and Weiss, S. (2003). The 37 kDa/67 kDa laminin receptor is required for PrP(Sc) propagation in scrapie-infected neuronal cells. EMBO Rep.4(3):290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, J., Pan, Y. L., Ning, X. X., Sun, L. J., Lan, M., Hong, L., Du, J. P., Liu, N., Liu, C. J., Qiao, T. D., and Fan, D. M. (2006). Overexpression of PrP and its antiapoptosis function in gastric cancer. Tumor Biol.27:84–91. [DOI] [PubMed] [Google Scholar]
- Liao, Y. C., Lebo, R. V., Clawson, G. A., and Smuckler, E. A. (1986). Human prion protein cDNA: molecular cloning, chromosomal mapping, and biological implications. Science233(4761):364–367. [DOI] [PubMed] [Google Scholar]
- Liu, T., Li, R., Wong, B. S., Liu, D., Pan, T., Petersen, R. B., Gambetti, P., and Sy, M. S. (2001). Normal cellular prion protein is preferentially expressed on subpopulations of murine hemopoietic cells. J. Immunol.166(6):3733–3742. [DOI] [PubMed] [Google Scholar]
- Ludwig, G. V., Kondig, J. P., and Smith, J. F. (1996). A putative receptor for Venezuelan equine encephalitis virus from mosquito cells. J. Virol.70(8):5592–5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugaresi, E., Medori, R., Montagna, P., Baruzzi, A., Cortelli, P., Lugaresi, A., Tinuper, P., Zucconi, M., and Gambetti, P. (1986). Fatal familial insomnia and dysautonomia with selective degeneration of thalamic nuclei. N. Engl. J. Med.315(16):997–1003. [DOI] [PubMed] [Google Scholar]
- Makrides, S., Chitpatima, S. T., Bandyopadhyay, R., and Brawerman, G. (1988). Nucleotide sequence for a major messenger RNA for a 40 kilodalton polypeptide that is under translational control in mouse tumor cells. Nucleic Acids Res.16(5):2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marella, M., Lehmann, S., Grassi, J., and Chabry, J. (2002). Filipin prevents pathological prion protein accumulation by reducing endocytosis and inducing cellular PrP release. J. Biol. Chem.277(28):25457–25464. [DOI] [PubMed] [Google Scholar]
- Martins, V. R., Graner, E., Garcia-Abreu, J., de Souza, S. J., Mercadante, A. F., Veiga, S. S., Zanata, S. M., Neto, V. M., and Brentani, R. R. (1997). Complementary hydropathy identifies a cellular prion protein receptor. Nat. Med.3(12):1376–1382. [DOI] [PubMed] [Google Scholar]
- Mastrianni, J. A., Nixon, R., Layzer, R., Telling, G. C., Han, D., DeArmond, S. J., and Prusiner, S. B. (1999). Prion protein conformation in a patient with sporadic fatal insomnia. N. Engl. J. Med.340(21):1630–1638. [DOI] [PubMed] [Google Scholar]
- Mattei, V., Garofalo, T., Misasi, R., Circella, A., Manganelli, V., Lucania, G., Pavan, A., and Sorice, M. (2004). Prion protein is a component of the multimolecular signaling complex involved in T cell activation. FEBS Lett.560:14–18. [DOI] [PubMed] [Google Scholar]
- Mayer, R. J., Landon, M., Laszlo, L., Lennox, G., and Lowe, J. (1992). Protein processing in lysosomes: The new therapeutic target in neurodegenerative disease. Lancet340(8812):156–159. [DOI] [PubMed] [Google Scholar]
- Mazzoni, I. E., Ledebur, H. C. Jr., Paramithiotis, E., and Cashman, N. (2005). Lymphoid signal transduction mechanisms linked to cellular prion protein. Biochem. Cell Biol.83(5):644–653. [DOI] [PubMed] [Google Scholar]
- McGowan, J. P. (1922). Scrapie in sheep. Scott. J. Agric.5:365–375. [Google Scholar]
- McKinley, M. P., Meyer, R. K., Kenaga, L., Rahbar, F., Cotter, R., Serban, A., and Prusiner, S. B. (1991). Scrapie prion rod formation in vitro requires both detergent extraction and limited proteolysis. J. Virol.65(3):1340–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meggio, F., Negro, A., Sarno, S., Ruzzene, M., Bertoli, A., Sorgato, M. C., and Pinna, L. A. (2000). Bovine prion protein as a modulator of protein kinase CK2. Biochem. J.352(Pt. 1):191–196. [PMC free article] [PubMed] [Google Scholar]
- Morel, E., Andrieu, T., Casagrande, F., Gauczynski, S., Weiss, S., Grassi, J., Rousset, M., Dormont, D., and Chambaz, J. (2005). Bovine Prion Is Endocytosed by Human Enterocytes via the 37 kDa/67 kDa Laminin Receptor. Am. J. Pathol.167(4):1033–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel, E., Fouquet, S., Chateau, D., Yvernault, L., Frobert, Y., Pincon-Raymond, M., Chambaz, J., Pillot, T., and Rousset, M. (2004). The cellular prion protein PrPc is expressed in human enterocytes in cell-cell junctional domains. J. Biol. Chem.279(2):1499–1505. [DOI] [PubMed] [Google Scholar]
- Moudjou, M., Frobert, Y., Grassi, J., and La Bonnardiere, C. (2001). Cellular prion protein status in sheep: tissue-specific biochemical signatures. J. Gen. Virol.82(Pt. 8):2017–2024. [DOI] [PubMed] [Google Scholar]
- Mouillet-Richard, S., Ermonval, M., Chebassier, C., Laplanche, J. L., Lehmann, S., Launay, J. M., and Kellermann, O. (2000). Signal transduction through prion protein. Science289(5486):1925–1928. [DOI] [PubMed] [Google Scholar]
- Mouillet-Richard, S., Pietri, M., Schneider, B., Vidal, C., Mutel, V., Launay, J. M., and Kellermann, O. (2005). Modulation of serotonergic receptor signaling and cross-talk by prion protein. J. Biol. Chem.280:4592–4601. [DOI] [PubMed] [Google Scholar]
- Nieznanski, K., Nieznanska, H., Skowronek, K. J., Osiecka, K. M., and Stepkowski, D. (2005). Direct interaction between prion protein and tubulin. Biochem. Biophys. Res. Commun.334:403–411. [DOI] [PubMed] [Google Scholar]
- Nikles, D., Bach, P., Boller, K., Merten, C. A., Montrasio, F., Heppner, F. L., Aguzzi, A., Cichutek, K., Kalinke, U., and Buchholz, C. J. (2005). Circumventing tolerance to the prion protein (PrP): Vaccination with PrP-displaying retrovirus particles induces humoral immune responses against the native form of cellular PrP. J. Virol.79(7):4033–4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi, T., Hagiwara, K., Kinumi, T., Yamakawa, Y., Nishijima, M., Nakamura, K., and Arimoto, H. (2003). Effects of beta-sheet breaker peptide polymers on scrapie-infected mouse neuroblastoma cells and their affinities to prion protein fragment PrP(81-145). Org. Biomol. Chem.1:2626–2629. [DOI] [PubMed] [Google Scholar]
- Otto, M., Cepek, L., Ratzka, P., Doehlinger, S., Boekhoff, I., Wiltfang, J., Irle, E., Pergande, G., Ellers-Lenz, B., Windl, O., Kretzschmar, H. A., Poser, S., and Prange, H. (2004). Efficacy of flupirtine on cognitive function in patients with CJD: A double-blind study. Neurology62(5):714–718. [DOI] [PubMed] [Google Scholar]
- Paitel, E., Alves da Costa, C., Vilette, D., Grassi, J., and Checler, F. (2002). Overexpression of PrPc triggers caspase 3 activation: Potentiation by proteasome inhibitors and blockade by anti-PrP antibodies. J. Neurochem.83(5):1208–1214. [DOI] [PubMed] [Google Scholar]
- Paitel, E., Fahraeus, R., and Checler, F. (2003). Cellular prion protein sensitizes neurons to apoptotic stimuli through Mdm2-regulated and p53-dependent caspase 3-like activation. J. Biol. Chem.278(12):10061–10066. [DOI] [PubMed] [Google Scholar]
- Paitel, E., Sunyach, C., Alves da Costa, C., Bourdon, J. C., Vincent, B., and Checler, F. (2004). Primary cultured neurons devoid of cellular prion display lower responsiveness to staurosporine through the control of p53 at both transcriptional and post-transcriptional levels. J. Biol. Chem.279(1):612–618. [DOI] [PubMed] [Google Scholar]
- Paltrinieri, S., Comazzi, S., Spagnolo, V., Rondena, M., Ponti, W., and Ceciliani, F. (2004). Bovine Doppel (Dpl) and prion protein (PrP) expression on lymphoid tissue and circulating leukocytes. J. Histochem. Cytochem.52(12):1639–1645. [DOI] [PubMed] [Google Scholar]
- Pan, K. M., Baldwin, M., Nguyen, J., Gasset, M., Serban, A., Groth, D., Mehlhorn, I., Huang, Z., Fletterick, R. J., Cohen, F. E., et al. (1993). Conversion of alpha-helices into beta-sheets features in the formation of the scrapie prion proteins. Proc. Natl. Acad. Sci. U. S. A.90(23):10962–10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, T., Wong, B. S., Liu, T., Li, R., Petersen, R. B., and Sy, M. S. (2002). Cell-surface prion protein interacts with glycosaminoglycans. Biochem. J.368(Pt. 1):81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankiewicz, J., Prelli, F., Sy, M. S., Kascsak, R. J., Kascsak, R. B., Spinner, D. S., Carp, R. I., Meeker, H. C., Sadowski, M., and Wisniewski, T. (2006). Clearance and prevention of prion infection in cell culture by anti-PrP antibodies. Eur. J. Neurosci.23(10):2635–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parchi, P., Capellari, S., Chin, S., Schwarz, H. B., Schecter, N. P., Butts, J. D., Hudkins, P., Burns, D. K., Powers, J. M., and Gambetti, P. (1999). A subtype of sporadic prion disease mimicking fatal familial insomnia. Neurology52:1757–1763. [DOI] [PubMed] [Google Scholar]
- Pocchiari, M., Schmittinger, S., and Masullo, C. (1987). Amphotericin B delays the incubation period of scrapie in intracerebrally inoculated hamsters. J. Gen. Virol.68(Pt. 1):219–223. [DOI] [PubMed] [Google Scholar]
- Poli, G., Martino, P. A., Villa, S., Carcassola, G., Giannino, M. L., Dall'Ara, P., Pollera, C., Iussich, S., Tranquillo, V. M., Bareggi, S., Mantegazza, P., and Ponti, W. (2004). Evaluation of anti-prion activity of congo red and its derivatives in experimentally infected hamsters. Arzneimittelforschung54:406–415. [DOI] [PubMed] [Google Scholar]
- Priola, S. A., Raines, A., and Caughey, W. S. (2000). Porphyrin and phthalocyanine antiscrapie compounds. Science287(5457):1503–1506. [DOI] [PubMed] [Google Scholar]
- Proske, D., Gilch, S., Wopfner, F., Schätzl, H. M., Winnacker, E. L., and Famulok, M. (2002). Prion-protein-specific aptamer reduces PrPSc formation. Chembiochem3:717–725. [DOI] [PubMed] [Google Scholar]
- Prusiner, S. B. (1982). Novel proteinaceous infectious particles cause scrapie. Science216(4542):136–144. [DOI] [PubMed] [Google Scholar]
- Prusiner, S B. (1991). Molecular biology of prion diseases. Science252(5012):1515–1522. [DOI] [PubMed] [Google Scholar]
- Prusiner, S. B. (1994). Molecular biology and genetics of prion diseases. Philos. Trans. R. Soc. Lond. B. Biol. Sci.343(1306):447–463. [DOI] [PubMed] [Google Scholar]
- Prusiner, S. B., Groth, D., Serban, A., Stahl, N., and Gabizon, R. (1993). Attempts to restore scrapie prion infectivity after exposure to protein denaturants. Proc. Natl. Acad. Sci. U. S. A.90(7):2793–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph, G. S., Radcliffe, P. A., Day, D. M., Carthy, J. M., Leroux, M. A., Lee, D. C., Wong, L. F., Bilsland, L. G., Greensmith, L., Kingsman, S. M., Mitrophanous, K. A., Mazarakis, N. D., and Azzouz, M. (2005). Silencing mutant SOD1 using RNAi protects against neurodegeneration and extends survival in an ALS model. Nat. Med.11:429–433. [DOI] [PubMed] [Google Scholar]
- Rambold, A. S., Miesbauer, M., Rapaport, D., Bartke, T., Baier, M., Winklhofer, K. F., and Tatzelt, J. (2006). Association of Bcl-2 with misfolded prion protein is linked to the toxic potential of cytosolic PrP. Mol. Biol. Cell [DOI] [PMC free article] [PubMed]
- Rao, N. C., Barsky, S. H., Terranova, V. P., and Liotta, L. A. (1983). Isolation of a tumor cell laminin receptor. Biochem. Biophys. Res. Commun.111(3):804–808. [DOI] [PubMed] [Google Scholar]
- Raoul, C., Abbas-Terki, T., Bensadoun, J. C., Guillot, S., Haase, G., Szulc, J., Henderson, C. E., and Aebischer, P. (2005). Lentiviral-mediated silencing of SOD1 through RNA interference retards disease onset and progression in a mouse model of ALS. Nat. Med.11:423–428. [DOI] [PubMed] [Google Scholar]
- Reilly, C. E. (2000). Beta-sheet breaker peptides reverse conformation of pathogenic prion proteins. J. Neurol.247:319–320. [DOI] [PubMed] [Google Scholar]
- Rieger, R., Edenhofer, F., Lasmezas, C. I., and Weiss, S. (1997). The human 37-kDa laminin receptor precursor interacts with the prion protein in eukaryotic cells. Nat. Med.3(12):1383–1388. [DOI] [PubMed] [Google Scholar]
- Riek, R., Hornemann, S., Wider, G., Billeter, M., Glockshuber, R., and Wuthrich, K. (1996). NMR structure of the mouse prion protein domain PrP(121–321). Nature382(6587):180–182. [DOI] [PubMed] [Google Scholar]
- Robakis, N. K., Devine-Gage, E. A., Jenkins, E. C., Kascsak, R. J., Brown, W. T., Krawczun, M. S., and Silverman, W. P. (1986). Localization of a human gene homologous to the PrP gene on the p arm of chromosome 20 and detection of PrP-related antigens in normal human brain. Biochem. Biophys. Res. Commun.140(2):758–765. [DOI] [PubMed] [Google Scholar]
- Romanov, V., Sobel, M. E., pinto da Silva, P., Menard, S., and Castronovo, V. (1994). Cell localization and redistribution of the 67 kD laminin receptor and alpha 6 beta 1 integrin subunits in response to laminin stimulation: an immunogold electron microscopy study. Cell Adhes. Commun.2(3):201–209. [DOI] [PubMed] [Google Scholar]
- Sakudo, A., Lee, D. C., Saeki, K., Matsumoto, Y., Itohara, S., and Onodera, T. (2003). Tumor necrosis factor attenuates prion protein-deficient neuronal cell death by increases in anti-apoptotic Bcl-2 family proteins. Biochem. Biophys. Res. Commun.310:725–729. [DOI] [PubMed] [Google Scholar]
- Sanftner, L. M., Sommer, J. M., Suzuki, B. M., Smith, P. H., Vijay, S., Vargas, J. A., Forsayeth, J. R., Cunningham, J., Bankiewicz, K. S., Kao, H., Bernal, J., Pierce, G. F., and Johnson, K. W. (2005). AAV2-mediated gene delivery to monkey putamen: evaluation of an infusion device and delivery parameters. Exp. Neurol.194:476–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, M., Kinoshita, K., Kaneda, Y., Saeki, Y., Iwamatsu, A., and Tanaka, K. (1996). Analysis of nuclear localization of laminin binding protein precursor p40 (LBP/p40). Biochem. Biophys. Res. Commun.229(3):896–901. [DOI] [PubMed] [Google Scholar]
- Schätzl, H. M., Da Costa, M., Taylor, L., Cohen, F. E., and Prusiner, S. B. (1995). Prion protein gene variation among primates. J. Mol. Biol.245(4):362–374. [DOI] [PubMed] [Google Scholar]
- Schmitt-Ulms, G., Legname, G., Baldwin, M. A., Ball, H. L., Bradon, N., Bosque, P. J., Crossin, K. L., Edelman, G. M., DeArmond, S. J., Cohen, F. E., and Prusiner, S. B. (2001). Binding of neural cell adhesion molecules (N-CAMs) to the cellular prion protein. J. Mol. Biol.314(5):1209–1225. [DOI] [PubMed] [Google Scholar]
- Sekiya, S., Noda, K., Nishikawa, F., Yokoyama, T., Kumar, P. K., and Nishikawa, S. (2006). Characterization and Application of a Novel RNA Aptamer against the Mouse Prion Protein. J. Biochem. (Tokyo)139:383–390. [DOI] [PubMed] [Google Scholar]
- Shyng, S. L., Heuser, J. E., and Harris, D. A. (1994). A glycolipid-anchored prion protein is endocytosed via clathrin-coated pits. J. Cell Biol.125(6):1239–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyng, S. L., Huber, M. T., and Harris, D. A. (1993). A prion protein cycles between the cell surface and an endocytic compartment in cultured neuroblastoma cells. J. Biol. Chem.268(21):15922–15928. [PubMed] [Google Scholar]
- Sigurdsson, B. (1954). Rida-a chronic encephalitis of sheep-with general remarks on infections which develop slowly and some of their special characteristics. Br. Vet. J.110:341–354. [Google Scholar]
- Simoneau, S., Haik, S., Leucht, C., Dormont, D., Deslys, J. P., Weiss, S., and Lasmezas, C. (2003). Different isoforms of the non-integrin laminin receptor are present in mouse brain and bind PrP. Biol. Chem.384(2):243–246. [DOI] [PubMed] [Google Scholar]
- Solforosi, L., Criado, J. R., McGavern, D. B., Wirz, S., Sanchez-Alavez, M., Sugama, S., DeGiorgio, L. A., Volpe, B. T., Wiseman, E., Abalos, G., Masliah, E., Gilden, D., Oldstone, M. B., Conti, B., and Williamson, R. A. (2004). Cross-linking cellular prion protein triggers neuronal apoptosis in vivo. Science303(5663):1514–1516. [DOI] [PubMed] [Google Scholar]
- Sparkes, R. S., Simon, M., Cohn, V. H., Fournier, R. E., Lem, J., Klisak, I., Heinzmann, C., Blatt, C., Lucero, M., Mohandas, T., and et al. (1986). Assignment of the human and mouse prion protein genes to homologous chromosomes. Proc. Natl. Acad. Sci. U. S. A.83(19):7358–7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielhaupter, C., and Schätzl, H. M. (2001). PrPC directly interacts with proteins involved in signaling pathways. J. Biol. Chem.276(48):44604–44612. [DOI] [PubMed] [Google Scholar]
- Stahl, N., Baldwin, M. A., Teplow, D. B., Hood, L., Gibson, B. W., Burlingame, A. L., and Prusiner, S. B. (1993). Structural studies of the scrapie prion protein using mass spectrometry and amino acid sequencing. Biochemistry32(8):1991–2002. [DOI] [PubMed] [Google Scholar]
- Stahl, N., Borchelt, D. R., Hsiao, K., and Prusiner, S. B. (1987). Scrapie prion protein contains a phosphatidylinositol glycolipid. Cell51(2):229–240. [DOI] [PubMed] [Google Scholar]
- Stahl, N., and Prusiner, S. B. (1991). Prions and prion proteins. FASEB J.5(13):2799–2807. [DOI] [PubMed] [Google Scholar]
- Supattapone, S., Wille, H., Uyechi, L., Safar, J., Tremblay, P., Szoka, F. C., Cohen, F. E., Prusiner, S. B., and Scott, M. R. (2001). Branched polyamines cure prion-infected neuroblastoma cells. J. Virol.75(7):3453–3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliavini, F., McArthur, R. A., Canciani, B., Giaccone, G., Porro, M., Bugiani, M., Lievens, P. M., Bugiani, O., Peri, E., Dall'Ara, P., Rocchi, M., Poli, G., Forloni, G., Bandiera, T., Varasi, M., Suarato, A., Cassutti, P., Cervini, M. A., Lansen, J., Salmona, M., and Post, C. (1997). Effectiveness of anthracycline against experimental prion disease in Syrian hamsters. Science276(5315):1119–1122. [DOI] [PubMed] [Google Scholar]
- Tanji, K., Saeki, K., Matsumoto, Y., Takeda, M., Hirasawa, K., Doi, K., and Onodera, T. (1995). Analysis of PrPc mRNA by in situ hybridization in brain, placenta, uterus and testis of rats. Intervirology38(6):309–315. [DOI] [PubMed] [Google Scholar]
- Thormar, H. (1971). Slow infections of the central nervous system II. Z. Neurol.199(3):151–166. [DOI] [PubMed] [Google Scholar]
- Tio, P. H., Jong, W. W., and Cardosa, M. J. (2005). Two dimensional VOPBA reveals laminin receptor (LAMR1) interaction with dengue virus serotypes 1, 2 and 3. Virol. J.2(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler, I., Gaus, S. E., Deboer, T., Achermann, P., Fischer, M., Rulicke, T., Moser, M., Oesch, B., McBride, P. A., and Manson, J. C. (1996). Altered circadian activity rhythms and sleep in mice devoid of prion protein. Nature380(6575):639–642. [DOI] [PubMed] [Google Scholar]
- Vana, K., and Weiss, S. (2006). A trans-dominant negative 37 kDa/67 kDa laminin receptor mutant impairs PrPSc propagation in scrapie-infected neuronal cells. J. Mol. Biol.358(1):57–66. [DOI] [PubMed] [Google Scholar]
- Vassallo, N., and Herms, J. (2003). Cellular prion protein function in copper homeostasis and redox signalling at the synapse. J. Neurochem.86(3):538–344. [DOI] [PubMed] [Google Scholar]
- Wang, K. S., Kuhn, R. J., Strauss, E. G., Ou, S., and Strauss, J. H. (1992). High-affinity laminin receptor is a receptor for Sindbis virus in mammalian cells. J. Virol.66(8):4992–5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss, S., Proske, D., Neumann, M., Groschup, M. H., Kretzschmar, H. A., Famulok, M., and Winnacker, E. L. (1997). RNA aptamers specifically interact with the prion protein PrP. J. Virol.71:8790–8797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann, C., and Flechsig, E. (2003). PrP knock-out and PrP transgenic mice in prion research. Br. Med. Bull.66:43–60. [DOI] [PubMed] [Google Scholar]
- Wells, G. A., Scott, A. C., Johnson, C. T., Gunning, R. F., Hancock, R. D., Jeffrey, M., Dawson, M., and Bradley, R. (1987). A novel progressive spongiform encephalopathy in cattle. Vet. Rec.121(18):419–420. [DOI] [PubMed] [Google Scholar]
- White, A. R., Enever, P., Tayebi, M., Mushens, R., Linehan, J., Brandner, S., Anstee, D., Collinge, J., and Hawke, S. (2003). Monoclonal antibodies inhibit prion replication and delay the development of prion disease. Nature422(6927):80–83. [DOI] [PubMed] [Google Scholar]
- Whittal, R. M., Ball, H. L., Cohen, F. E., Burlingame, A. L., Prusiner, S. B., and Baldwin, M. A. (2000). Copper binding to octarepeat peptides of the prion protein monitored by mass spectrometry. Protein Sci.9:332–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will, R. G., Ironside, J. W., Zeidler, M., Cousens, S. N., Estibeiro, K., Alperovitch, A., Poser, S., Pocchiari, M., Hofman, A., and Smith, P. G. (1996). A new variant of Creutzfeldt-Jakob disease in the UK. Lancet347(9006):921–925. [DOI] [PubMed] [Google Scholar]
- Williams, E. S., and Young, S. (1980). Chronic wasting disease of captive mule deer: a spongiform encephalopathy. J. Wildl. Dis.16(1):89–98. [DOI] [PubMed] [Google Scholar]
- Windl, O., Dempster, M., Estibeiro, P., and Lathe, R. (1995). A candidate marsupial PrP gene reveals two domains conserved in mammalian PrP proteins. Gene159(2):181–186. [DOI] [PubMed] [Google Scholar]
- Winklhofer, K. F., and Tatzelt, J. (2000). Cationic lipopolyamines induce degradation of PrPSc in scrapie-infected mouse neuroblastoma cells. Biol. Chem.381(5–6):463–469. [DOI] [PubMed] [Google Scholar]
- Wopfner, F., Weidenhofer, G., Schneider, R., von Brunn, A., Gilch, S., Schwarz, T. F., Werner, T., and Schätzl, H. M. (1999). Analysis of 27 mammalian and 9 avian PrPs reveals high conservation of flexible regions of the prion protein. J. Mol. Biol.289(5):1163–1178. [DOI] [PubMed] [Google Scholar]
- Wyatt, J. M. (1990). Spongiform encephalopathy in a cat. Vet. Rec.126(20):513. [PubMed] [Google Scholar]
- Yehiely, F., Bamborough, P., Da Costa, M., Perry, B. J., Thinakaran, G., Cohen, F. E., Carlson, G. A., and Prusiner, S. B. (1997). Identification of candidate proteins binding to prion protein. Neurobiol. Dis.3(4):339–355. [DOI] [PubMed] [Google Scholar]
- Zanata, S. M., Lopes, M. H., Mercadante, A. F., Hajj, G. N., Chiarini, L. B., Nomizo, R., Freitas, A. R., Cabral, A. L., Lee, K. S., Juliano, M. A., de Oliveira, E., Jachieri, S. G., Burlingame, A., Huang, L., Linden, R., Brentani, R. R., and Martins, V. R. (2002). Stress-inducible protein 1 is a cell surface ligand for cellular prion that triggers neuroprotection. EMBO J.21(13):3307–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J., Wu, X., Qin, C., Qi, J., Ma, S., Zhang, H., Kong, Q., Chen, D., Ba, D., and He, W. (2003). A novel recombinant adeno-associated virus vaccine reduces behavioral impairment and beta-amyloid plaques in a mouse model of Alzheimer's disease. Neurobiol. Dis.14:365–379. [DOI] [PubMed] [Google Scholar]
- Zhou, J., and Zhong, Y. (2004). Breast cancer immunotherapy. Cell. Mol. Immunol.1:xs247–255. [PubMed] [Google Scholar]