Abstract
Cellular regulatory networks are organized such that many proteins have few interactions, whereas a few proteins have many. These densely connected protein “hubs” are critical for the system-wide behavior of cells, and the capability of selectively perturbing a subset of interactions at these hubs is invaluable in deciphering and manipulating regulatory mechanisms. SELEX-generated RNA aptamers are proving to be highly effective reagents for inhibiting targeted proteins, but conventional methods generate one or several aptamer clones that usually bind to a single target site most preferred by a nucleic acid ligand. We advance a generalized scheme for isolating aptamers to multiple sites on a target molecule by reducing the ability of the preferred site to select its cognate aptamer. We demonstrate the use of this scheme by generating aptamers directed to discrete functional surfaces of the yeast TATA-binding protein (TBP). Previously we selected “class 1” RNA aptamers that interfere with the TBP's binding to TATA-DNA. By masking TBP with TATA-DNA or an unamplifiable class 1 aptamer, we isolated a new aptamer class, “class 2,” that can bind a TBP·DNA complex and is in competition with binding another general transcription factor, TFIIA. Moreover, we show that both of these aptamers inhibit RNA polymerase II-dependent transcription, but analysis of template-bound factors shows they do so in mechanistically distinct and unexpected ways that can be attributed to binding either the DNA or TFIIA recognition sites. These results should spur innovative approaches to modulating other highly connected regulatory proteins.
Keywords: hubs, SELEX, TATA-binding protein, transcription factor TFIIA
Proteins communicate with each other through stable or transient interactions to create regulatory networks that modulate cell function. The architecture of these networks possess a highly heterogeneous scale-free topology, that is, most proteins connect to only one or few other proteins, whereas a few proteins connect to many and serve as densely connected “hubs” (1). This feature carries important implications concerning experimental and therapeutic control of cellular phenotypes; the consequence of a perturbation is affected to a large extent by the topologic position of the perturbed protein in the network. Cellular control systems may be effectively manipulated if the protein hubs can be selectively modulated in vivo. Promising candidates for the implementation of such modulation are RNA aptamers (2, 3). Ideally, a set of aptamers could provide a means of selectively perturbing a subset of connections of a “hub” protein. This requires that aptamers against different sites on a single protein be identified. Some important regulatory hubs such as the oncoprotein Ras GTPase (4, 5) and the TATA-binding protein (TBP) (6, 7) have a single conserved structural domain bearing multiple discrete sites recognized by other molecules. With conventional in vitro evolution methods such as SELEX, it is difficult, if not impossible, to isolate multiple aptamers binding to different sites on this type of target.
Conventional SELEX was developed by emulating the natural evolution of organisms (8–10), which requires three conditions: replication, variation, and competition (differential fitness). In an in vitro evolution experiment, replication is realized by enzymatic polymerization of nucleic acids. Variation is primarily embodied in the complexity of the initial combinatorial sequence pool, which appears to be comprehensive at 1012 to 1015 variants (11); however, additional complexity may also be introduced by error-prone PCR (12). Differential fitness is minimized during replication (amplification) and defined primarily by the condition of the “selection” step; an individual is selected because its “phenotype” is fit to perform a task such as binding to a target and rewarded to replicate its “genome.” The difficulty encountered when one attempts to isolate multiple aptamers that bind a complex target set can be understood in the context of evolutionary dynamics. Conventionally, a single set of selective conditions is maintained throughout the course of the experiments defining a fixed rank of fitness for each individual candidate in the pool. Although this ensures that candidates with the highest fitness, or affinity, for a particular site are isolated, aptamers for some other target sites may have very low fitness and are not isolated under this fixed set of selection conditions.
TBP is a general transcription factor and a core component of promoters used by all three types of eukaryotic RNA polymerases. TBP binds DNA with moderate affinity and interacts with other core promotor factors, positive and negative regulatory factors, and coactivators. Therefore, it functions as a hub of gene activity and regulation (7). In this study, we devised experimental procedures for the exhaustive isolation of aptamers by altering the relative growth rate of candidate aptamer clones as they evolve. In particular, we isolated aptamers against two discrete sites that are not physically separable on TBP. Moreover, we demonstrate that the aptamers binding these discrete sites are both effective in inhibiting TBP function and they do so by distinct mechanisms.
Results
In Vitro Evolution on Two Fitness Landscapes.
From an operational point of view, different target sites on a single domain are by definition present at concentrations that are fixed relative to one another. A means of effectively altering this fixed ratio should also alter the enrichment dynamics of the aptamers during the course of evolution. If a ligand already exists for a site on the protein, including it in the binding mixture would mask its target site and decrease the availability of the site to the candidate aptamers being selected, which would in turn increase the relative growth rate of aptamers for other sites. In the case of nucleic acid-binding proteins, many of which are important regulatory hubs, the chemical features of their natural nucleic acid-binding sites seem to be more inviting to RNA aptamers than other sites on the proteins. The presence of a ligand to such a preferred site during the process of selection should divert aptamer selection to other surfaces of the protein.
The conserved C-terminal core domain of TBP is <30 kDa in size and folds into a pseudosymmetric saddle shape. Multiple functional sites that interact with general transcription factors have been mapped on TBP as small clusters of amino acids (13, 14). As shown in Fig. 1A, the concave surface of TBP interacts mainly with the TATA element and induces a sharp bend in the DNA (15). TBP's convex side is recognized by many general transcription factors, activators, and repressors (7, 16). Based on these features, we anticipated that a conventional SELEX would yield “winner” aptamers for the concave, DNA-binding side of the molecule in the final, highly competitive rounds of selection but that different aptamers, which bind these other factor binding surfaces of TBP, may also exist in earlier rounds of selection.
Fig. 1.
Isolation of aptamers for discrete functional sites on the surface of TATA-binding protein (TBP). (A) A structural model representing the DNA·TBP·TFIIA·TFIIB quaternary complex (taken from ref. 19). TBP in black, and flanking TFIIB, the two subunits of TFIIA in gray are shown as ribbon models; the DNA is represented using a space-filling model. Human TFIIB was modeled with the crystal structure of the yeast tertiary complex. (B) Predicted secondary structure of AptTBP-12 and AptTBP-101, respectively, using mfold developed by Zuker (18). Capital letters represent randomized region, and lowercase letters signify the constant regions. The “true” aptamer moiety defined by mutagenesis is enclosed in the box.
We started with an RNA pool that was estimated to contain >1015 individuals and carried it through 12 cycles of selection and amplification with yeast TBP. A sample of 32 individuals yielded eight clones of aptamers belonging to a single class; they all recognize the DNA-binding side of the molecule. As documented elsewhere (17), these aptamers (designated class 1) acted in distinct modes and served as useful probes to yield rich information about TBP interactions during transcription initiation and reinitiation. To divert aptamers to other sites, we took a pool of an early generation selected by yeast TBP under conventional conditions, the third generation (G3) in the previous experiment (17), and performed selection and amplification with TATA-DNA, the natural ligand of TBP, included in the binding mixture. After four generations, we found that a single clone later named AptTBP-101 (class 2) dominated the selected pool (12 members of 13 individuals sequenced), and this clone was not isolated in the selection without TATA-DNA (17). To further demonstrate the use of this method in cases in which no natural ligand is available, we also performed in parallel an evolution from G3 with a previously isolated class 1 aptamer included in place of the TATA-DNA. We used a reduced form of AptTBP-12 (formerly no. 12) that fully retained its binding capability; however, it lacked the constant regions and could not be amplified along with the candidates during this stage of evolution. Four generations later, we isolated multiple individuals of the same class 2 clone, AptTBP-101.
Aptamers Binding to Discrete Sites on TBP.
The class 2 aptamer, AptTBP-101, which was selected after masking the DNA-binding domain of TBP, has a high affinity for TBP similar to that of the conventionally selected class 1 aptamers. The affinity of AptTBP-101 to TBP was measured by a filter binding assay to be 2 nM comparable to that of the AptTBP-12·TBP complex (17). Based on the predicted secondary structures using the computer software mfold (18), we made a series of several dozen linear deletion, stem deletion, circular permutation, and base paring covariation derivatives of both AptTBP-12 and AptTBP-101 to confirm these structures and to map the “true” aptamer moieties (full details are available on request). The results are depicted in Fig. 1B. The sequences and structures enclosed in the rectangular boxes are necessary and sufficient for TBP binding. The functional moiety of AptTBP-12 has a predicted hairpin structure with an apical loop and an internal loop. AptTBP-101 has a three-way junction, an internal loop, and an apical loop.
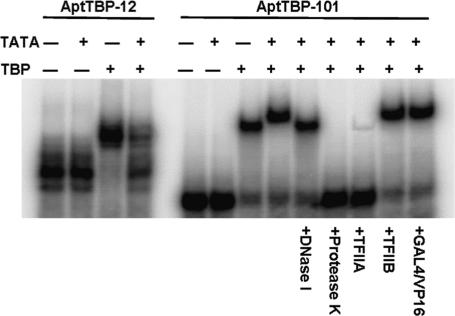
The first indication that AptTBP-101 indeed bound to a surface other than the DNA-binding site, and thus belonged to a different class, came from an electrophoretic mobility shift assay (EMSA). All class 1 aptamers bound to purified TBP to generate shifted bands with identical mobility. In contrast, AptTBP-101 bound TBP to produce a shifted complex with a different mobility as shown in Fig. 2. Moreover, in contrast to AptTBP-12, which competed with TATA-DNA in binding to TBP, AptTBP-101 was able to generate a “supershift” in the presence of the TATA-DNA, indicating the formation of a triple complex with both TBP and TATA-DNA. The identity of this RNA·DNA·protein complex was verified by its sensitivity to DNase I and proteinase K, which clearly demonstrated that this supershifted aptamer complex contained both TATA-DNA and TBP. Moreover, the cobinding of both aptamer RNA and TATA-DNA to TBP demonstrated that the AptTBP-101-binding site did not overlap with the DNA-binding site.
Fig. 2.
AptTBP-12 and AptTBP-101 recognize the DNA site and the TFIIA site, respectively. Shown is an EMSA result using labeled RNA probes with indicated proteins, DNA, and treatments.
To further pinpoint the binding site of AptTBP-101, we tested its ability to compete with other proteins that recognize TBP in the TBP·TATA complex. As shown, TFIIA, but not TFIIB or Gal4/VP16, was able to block the binding of AptTBP-101 when present in excess. Therefore, the binding site of AptTBP-101 appears to overlap with the site recognized by TFIIA. Referring to the model in Fig. 1A, AptTBP-101 binds to a surface of TBP that is distinct from both the TFIIB and the TATA-DNA-binding surfaces (19).
Mechanistically Distinct Inhibition of TBP Function by Aptamers.
We expected that RNA aptamers that bind tightly to critical surfaces of TBP would inhibit TBP function. Several class 1 aptamers were shown previously to inhibit RNA polymerase II (Pol II)-dependent transcription efficiently (17). We tested the effects of the class 2 aptamer AptTBP-101 in a similar in vitro assay. When a DNA template containing an adenovirus major late promoter was used with yeast whole-cell extracts, as shown in Fig. 3A, AptTBP-101 inhibited Pol II-dependent transcription as potently as AptTBP-12. They both inhibited single as well as multiple rounds of transcription when added at the time of preinitiation complex (PIC) formation. Both aptamers also inhibited transcription in the presence of exogenous Gal4-VP16 activator (Fig. 3B). The aptamer effects were specific to TBP because this inhibition of transcription could be completely reversed by adding an excess of purified TBP (20) (Fig. 3B). When added after PIC formation, both aptamers were unable to inhibit the first single round of transcription but blocked reinitiation (17) (and data not shown). Because AptTBP-101 had little effect on TBP·TATA interaction and mainly interfered with interaction between TBP and TFIIA, this result suggests that the TBP·TFIIA interaction is important for basal transcription at least in the in vitro transcription system used here.
Fig. 3.
Mechanistically distinct inhibitory effects by AptTBP-12 and AptTBP-101 on Pol II-dependent in vitro transcription. (A) Both AptTBP-12 and AptTBP-101 inhibit transcription equally effectively. The TATA-binding protein (TBP) concentration in the whole-cell extract is ≈20 nM. The concentration indicated is that of the aptamers. (B) The inhibitory effects of aptamers are reversed by excess TBP. Transcription in whole-cell yeast extracts by using pG5MLT (adenovirus major late promoter with 5X Gal4-binding sites upstream) in the presence of Gal4-VP16. Aptamer (50 nM) was added with template; TBP (500 nM) was added to extract before aptamer and template.
To assess whether the different aptamers affected PIC assembly in mechanistically distinct ways, we used the immobilized promoter template assay to monitor protein levels at the promoter (21). In this assay, a biotinylated template DNA fragment containing the HIS4 core promoter and an upstream Gal4-binding site was immobilized onto streptavidin-coated magnetic beads. PICs were assembled on this template in the presence of increasing concentrations of aptamer RNA in yeast whole-cell extract. The proteins were then released by restriction digestion of the DNA, run on a 12% polyacrylamide gel, and subjected to Western analysis using specific antibodies. The effect of concentration of AptTBP-101 on the inhibition of TFIIA association with the template (Fig. 4A) parallels its inhibitory effects on transcription (Fig. 3A). Although higher concentrations of AptTBP-12 are required to inhibit the association of TBP with template, significant inhibition is seen at submicromolar concentrations (Fig. 4B). The different RNA aptamers have clearly distinct effects in this assay. As shown in Fig. 4, AptTBP-12 caused a reduction in the levels of the general transcription factors TBP, TFIIA, TFIIB, and TFIIE on the promoter DNA. In contrast, AptTBP-101 did not affect promoter occupancy of TBP and TFIIE throughout the range of RNA concentrations explored, but it did reduce dramatically the occupancy of TFIIA and TFIIB. In this assay, the effects of aptamer on both active and inactive PICs, or otherwise bound general transcription factors, are measured. Because active PICs only consist of 5% to 10% of the total PIC (21) and other bound complexes containing these factors might be less accessible to inhibitors, more aptamers may be required to displace these factors from the immobilized template assay than to inhibit in vitro transcription.
Fig. 4.
Aptamers in different classes affect preinitiation complex assembly in mechanistically distinct ways. Factors bound to the immobilized template were quantified after washing by Western blot analysis with antibodies to TATA-binding protein (TBP), TFIIA, TFIIB, and TFIIE, and a representative blot is shown beneath each graph. (A) The effects of low concentrations of AptTBP-12 and AptTBP-101 on TFIIA and TFIIB association with template. (B) Effects of aptamers on the four general transcription factors at aptamer concentrations that produce saturating or near saturating inhibitory effects. Graphs shown are representative of three independent experiments, and standard errors are shown. Each data set has been normalized to the no-aptamer data point.
Discussion
We describe a strategy for selecting RNA aptamers that bind and inhibit distinct macromolecular interaction surfaces of highly connected regulatory proteins. High-affinity inhibitors of particular protein–protein and protein–nucleic acid interactions are extremely valuable in both therapeutic applications and in basic biologic investigations. Nonetheless, it has been particularly hard to identify multiple drugs that target different interacting surfaces of proteins that serve as key regulatory hubs in macromolecular interaction networks. By masking the preferred interacting surface of TBP with its natural ligand or an unamplifiable aptamer, we selected a new RNA aptamer that binds with high affinity and interferes with a second distinct protein interaction site on TBP. These aptamer RNAs are effective inhibitors of TBP function and each acts by distinct mechanism both in simple binding assays and in the more complex milieu of a whole-cell extract. Although the two aptamer classes bind equally well to TBP, our selection without TATA DNA or the unamplifiable AptTBP-12 competitors produced only class 1 aptamers. We assume this phenomenon is a consequence of the more complex structure of the class 2 aptamer that make it rarer in the population than multiple simpler structures that produce class I aptamers to the DNA-binding surface of TBP. In our limited survey of the selected pools, we found eight families of class 1 aptamer and only one in class 2. Additionally, this masking selection scheme should be equally viable when the selection is a performed against a mixture of molecules or when the aptamer candidates are DNA instead of RNA.
Both AptTBP-12 and AptTBP-101 inhibit Pol II-dependent transcription in mechanistically distinct manners, which is consistent with observations made by EMSA using purified components that indicated that they interact with distinct regions on the surface of TBP. AptTBP-12 is thought to interact with TBP on its concave underside, the region essential for DNA binding. In the presence of AptTBP-12, TBP is unable to bind to the promoter as efficiently and this in turn precludes the assembly of other general transcription factors into the PIC (Fig. 4B). This reduction of all of the general transcription factors at the promoter can account for the decreased level of RNA synthesis. Consistent with the EMSA-binding assay (Fig. 2), we found AptTBP-101 reduces the presence of TFIIA but not TBP on the immobilized template. Although TFIIB does not appear to compete with AptTBP-101 for binding to TBP in our EMSA assay, TFIIB levels are also reduced by AptTBP-101 on the immobilized template. This reduction in the context of a yeast extract may be attributable to the decreased stability of PICs that do not contain TFIIA, which has been shown to act at an early step of PIC formation (22).
It is also interesting to note that AptTBP-101 affected the binding of TFIIA and TFIIB but not TFIIE. Although the addition of TFIIE to a PIC in vitro is generally thought to require the prior addition of TBP, TFIIB, TFIIF, and Pol II (14, 23), TFIIE can maintain its DNA template association in extracts in the absence of TFIIB, TFIIF, and Pol II as a component of the stable reinitiation “scaffold” (21). Moreover, TFIIE maintains genome contacts in vivo in the apparent absence of Pol II and some of the other general transcription factors (24). The effects of AptTBP-101 on the different equilibrium distributions of promoter-associated and free TFIIB and TFIIE could be a consequence of assembly of a transient preinitiation complex in which TFIIE is recruited and stably maintained even after TFIIB leaves the complex. Additionally, TFIIE has been shown to interact directly with DNA and stimulate TBP binding to the promoter in the absence of other basal factors (25), and such a cooperative interaction could itself account for differential associations of template seen in the presence of AptTBP-101 (Fig. 4B). Finally, TFIIE could be recruited to DNA through its known interactions with transcription activators (26, 27). Further investigations will be required to fully define mechanistically the precise effects of these aptamers on promoter structure and function. Nonetheless, the studies presented here not only demonstrate that the two aptamers inhibit transcription by distinct mechanisms that are a consequence of their ability to target correspondingly distinct surfaces of TBP, but they also provide intriguing and unexpected insights into the interplay of general transcription factor associations with a promoter in the complex milieu of a yeast whole-cell extract.
Aptamers identified using this method should spur innovative approaches to modulating other “hubs” of cellular regulatory networks. The availability of aptamers to multiple sites on a “hub” will enable selective blockage or connection of the “hub” with other nodes in the network in vivo with spatial and temporal precision. We have established general methods to regulate the production of aptamers in vivo (2), which can be coupled with the control of aptamer activity through allosteric transitions (28). By using these reagents and methods, “plug-and-play” molecular devices may be constructed to test the effects of perturbing functions of many proteins, especially those that are “hubs,” in different ways and in different combinations. The general strategies described should prove effective in identifying inhibitors of particular surfaces of cell signaling proteins that act as critical hubs in controlling cell cycle and cancer progression.
Materials and Methods
Protein Preparation.
Recombinant yeast TBP and yeast TFIIA were prepared as described (17). Recombinant yeast TFIIB was a generous gift from J. Fu and M. H. Suh (Cornell University, Ithaca). Antibodies against transcription factors were generous gifts of S. Buratowski (TBP and TFIIE), M. Hampsey (TFIIB), and S. Hahn (TFIIA).
In Vitro Evolution.
The template-primer system of the starting pool was described previously (17). The length of randomized region is 50 bases. The procedure of selection and amplification were almost identical to that described previously (17, 29). To mask the DNA-binding surface of TBP, a “mini” version of AptTBP-12 (m12) or the TATA-DNA was included in the binding reaction in excess to TBP. They were incubated with TBP for 30 minutes before adding the RNA pool to the mix. The m12 was chemically synthesized by Dharmacon Research (LaFayette, CO) and has the following sequence: 5′-GGCGCCGUGCCCGGUUUGGAUAGGCACAUAAGACGCC-3′.
EMSA.
All RNA-protein-binding assays were performed in 20-μl reaction volumes. Binding with TBP was performed as previously described (17). Binding reactions with TBP contained 0.5 nM labeled aptamer and 25 nM yeast TBP. Where indicated, TATA-DNA was included at 50 nM, other recombinant proteins at 200 nM, DNaseI at 0.25 units/μl, and proteinase K at 1 μg/μl. The running buffer for TBP·aptamer complexes was 0.5× TG with 0.5 mM magnesium acetate.
In Vitro Transcription.
Transcription was performed according to a previously described protocol (17).
Immobilized Template Assay.
The protocol was adapted from (21). The template used for PIC assembly is a 594 bp biotinylated fragment amplified from pSH515 (plasmid obtained from Steve Hahn) containing one Gal4-binding site upstream of a modified HIS4 core promoter (−141 to +3 with respect to translation start site). The template was immobilized on streptavidin-coated magnetic beads (Dynabeads; Promega) and PICs were then assembled in the presence of yeast whole cell extract (prepared as described in ref. 30) and indicated aptamers for 30 minutes. The complexes were then washed, bound factors were released by restriction digestion of the template DNA with PstI, separated on a 12% SDS/PAGE gel, and transferred to a nitrocellulose membrane (Protran, BA85; Whatman). Proteins were then detected by immunoblotting with ECL kits (Amersham Bio-sciences, Piscataway, NJ). Band intensities were determined by using National Institutes of Health Image software.
Acknowledgments
We thank Michael Kotlikoff, Li Niu, and David Shub for helpful comments on the manuscript. This work was supported by National Institutes of Health Grant GM 40918 (to J.T.L.) and by startup funds from the College of Arts and Sciences, University at Albany, State University of New York (Albany, NY) (to H.S.).
Abbreviations
- EMSA
electrophoretic mobility shift assay
- PIC
preinitiation complex
- Pol II
RNA polymerase II
- TBP
TATA-binding protein.
Footnotes
Conflict of interest statement: H.S. and J.T.L. have a patent and patent submissions on RNA aptamers and aptamer-related methods.
This article is a PNAS direct submission.
References
- 1.Jeong H, Mason SP, Barabasi AL, Oltvai ZN. Nature. 2001;411:41–42. doi: 10.1038/35075138. [DOI] [PubMed] [Google Scholar]
- 2.Shi H, Hoffman BE, Lis JT. Proc Natl Acad Sci USA. 1999;96:10033–10038. doi: 10.1073/pnas.96.18.10033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Famulok M, Blind M, Mayer G. Chem Biol. 2001;8:931–939. doi: 10.1016/s1074-5521(01)00070-9. [DOI] [PubMed] [Google Scholar]
- 4.Kolch W. Biochem J. 2000;351:289–305. [PMC free article] [PubMed] [Google Scholar]
- 5.Corbett KD, Alber T. Trends Biochem Sci. 2001;26:710–716. doi: 10.1016/s0968-0004(01)01974-0. [DOI] [PubMed] [Google Scholar]
- 6.Nikolov DB, Hu SH, Lin J, Gasch A, Hoffmann A, Horikoshi M, Chua NH, Roeder RG, Burley SK. Nature. 1992;360:40–46. doi: 10.1038/360040a0. [DOI] [PubMed] [Google Scholar]
- 7.Pugh BF. Gene. 2000;255:1–14. doi: 10.1016/s0378-1119(00)00288-2. [DOI] [PubMed] [Google Scholar]
- 8.Tuerk C, Gold L. Science. 1990;249:505–510. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- 9.Ellington AD, Szostak JW. Nature. 1990;346:818–822. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- 10.Robertson DL, Joyce GF. Nature. 1990;344:467–468. doi: 10.1038/344467a0. [DOI] [PubMed] [Google Scholar]
- 11.Knight R, Yarus M. RNA. 2003;9:218–230. doi: 10.1261/rna.2138803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartel DP, Szostak JW. Science. 1993;261:1411–1418. doi: 10.1126/science.7690155. [DOI] [PubMed] [Google Scholar]
- 13.Tang H, Sun X, Reinberg D, Ebright RH. Proc Natl Acad Sci USA. 1996;93:1119–1124. doi: 10.1073/pnas.93.3.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bryant GO, Martel LS, Burley SK, Berk AJ. Genes Dev. 1996;10:2491–2504. doi: 10.1101/gad.10.19.2491. [DOI] [PubMed] [Google Scholar]
- 15.Chasman DI, Flaherty KM, Sharp PA, Kornberg RD. Proc Natl Acad Sci USA. 1993;90:8174–8178. doi: 10.1073/pnas.90.17.8174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burley SK, Roeder RG. Annu Rev Biochem. 1996;65:769–799. doi: 10.1146/annurev.bi.65.070196.004005. [DOI] [PubMed] [Google Scholar]
- 17.Fan X, Shi H, Adelman K, Lis JT. Proc Natl Acad Sci USA. 2004;101:6934–6939. doi: 10.1073/pnas.0401523101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zuker M. Science. 1989;244:48–52. doi: 10.1126/science.2468181. [DOI] [PubMed] [Google Scholar]
- 19.Geiger JH, Hahn S, Lee S, Sigler PB. Science. 1996;272:830–836. doi: 10.1126/science.272.5263.830. [DOI] [PubMed] [Google Scholar]
- 20.Fan X, Shi H, Lis JT. Nucleic Acids Res. 2005;33:838–845. doi: 10.1093/nar/gki212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yudkovsky N, Ranish JA, Hahn S. Nature. 2000;408:225–229. doi: 10.1038/35041603. [DOI] [PubMed] [Google Scholar]
- 22.Ranish JA, Yudkovsky N, Hahn S. Genes Dev. 1999;13:49–63. doi: 10.1101/gad.13.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buratowski S, Hahn S, Guarente L, Sharp PA. Cell. 1989;56:549–561. doi: 10.1016/0092-8674(89)90578-3. [DOI] [PubMed] [Google Scholar]
- 24.Zanton SJ, Pugh BF. Genes Dev. 2006;20:2250–2265. doi: 10.1101/gad.1437506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yokomori K, Verrijzer CP, Tjian R. Proc Natl Acad Sci USA. 1998;95:6722–6727. doi: 10.1073/pnas.95.12.6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sauer F, Fondell JD, Ohkuma Y, Roeder RG, Jackle H. Nature. 1995;375:162–164. doi: 10.1038/375162a0. [DOI] [PubMed] [Google Scholar]
- 27.Zhu A, Kuziora MA. J Biol Chem. 1996;271:20993–20996. doi: 10.1074/jbc.271.35.20993. [DOI] [PubMed] [Google Scholar]
- 28.Buskirk AR, Landrigan A, Liu DR. Chem Biol. 2004;11:1157–1163. doi: 10.1016/j.chembiol.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 29.Shi H, Hoffman BE, Lis JT. Mol Cell Biol. 1997;17:1649–1657. doi: 10.1128/mcb.17.5.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woontner M, Wade PA, Bonner J, Jaehning JA. Mol Cell Biol. 1991;11:4555–4560. doi: 10.1128/mcb.11.9.4555. [DOI] [PMC free article] [PubMed] [Google Scholar]