Abstract
The replication proteins Rep and Rep′ of porcine circovirus type 1 (PCV1) are both capable of introducing and resealing strand discontinuities at the viral origin of DNA replication in vitro underlying genome amplification by rolling-circle replication. The PCV1 origin of replication encompasses the minimal binding site (MBS) of the Rep and Rep′ proteins and an inverted repeat with the potential to form a stem-loop. In this study, both elements of the PCV1 origin were demonstrated to be essential for viral replication in transfected cells. Furthermore, investigation of conserved amino acid motifs within Rep and Rep′ proteins revealed that the mutation of motifs I, II, and III and of the GKS box interfered with viral replication. In vitro studies demonstrated that motifs I to III were essential for origin cleavage, while the GKS box was dispensable for the initiation of viral replication. A covalent link between Rep/Rep′ and the DNA after origin cleavage was demonstrated, providing a mechanism for energy conservation for the termination of replication.
The family Circoviridae comprises several viruses with single-stranded, covalently closed circular DNA genomes, i.e., Porcine circovirus (PCV) (9, 22), Beak and feather disease virus (1), Goose circovirus (30), Canary circovirus (25), and Pigeon circovirus (18). Two genotypes of PCV have been identified. PCV type 1 (PCV1) (1,759 nucleotides [nt]) is widespread in swine, but until now, the virus has not been linked to any animal disease. In contrast, PCV type 2 (PCV2) (1,768 nt) is associated with postweaning multisystemic wasting syndrome (PMWS) (6). Animals affected by PMWS show fever, dyspnea, weight retardation, and lymphadenopathy (4). The outcome of experimental PCV2 infections was widely variable and ranged from the occurrence of microscopic lesions to full-blown PMWS, indicating that PMWS is a multifactorial disease (13).
PCV particles are icosahedral and nonenveloped, with a diameter of 16 to 18 nm. The genomes of PCV1 and PCV2 are ambisense organized and display a homology of 68 to 76%. The largest open reading frame (ORF) (3) encodes two replication proteins, Rep and Rep′, which are both indispensable for viral replication (20). Rep represses the promoter of the rep gene (19), and Rep and Rep′ bind to the double-stranded origin of replication (28) and introduce and reseal strand discontinuities within the single-stranded origin of replication (27). The second-largest ORF comprises the capsid protein Cap. Recently, the expression of a third ORF inducing apoptosis has been reported for PCV2 (16), which may be involved in the distinct pathogenesis of PCV1 and PCV2. Based upon sequence comparisons, three conserved amino acid motifs (motifs I, II, and III) as well as a GKS box (or P loop) have been identified within the Rep protein (Fig. 1) (22). Mutations in these four elements, which are common in enzymes initiating rolling-circle replication (RCR) (7), interfered with PCV1 replication in cell culture (20). Motif II is involved in the coordination of bivalent cations (8, 12, 15, 31), which were demonstrated to be indispensable for the cleavage of the PCV1 origin in vitro (27). In the case of the geminiviruses Tomato yellow leaf curl virus and Faba bean necrotic yellows virus, a tyrosine within motif III (Tyr-103 and Tyr-79, respectively) has been identified as being the catalytic center for the initiation of unidirectional leading-strand synthesis (5, 11).
FIG. 1.
(A) A linear map of the circular genome of PCV1 is depicted. ORFs are indicated by open bars, and transcripts of Rep and Rep′ with splice sites are indicated by horizontal arrows. Positions and sequences of conserved amino acid motifs I to III and the GKS box are given. (B) Conserved sequence elements within the origin of PCV1 are shown at the top of the figure. Oligonucleotides used for in vitro studies and origin plasmids tested for replication in the reporter gene assay are indicated below. Only sequences deviating from the wt origin sequence are denoted.
The intergenic region of PCV1 comprises the origin of replication containing characteristic sequence elements: an inverted repeat, 5′-AAGTGCGCTG-3′, forms a putative stem-loop structure with the conserved nonanucleotide 5′-T/AAGTATTAC-3′ in its apex, which is also present in all avian circoviruses. The inverted repeat and the nonamer are essential for cleavage and ligation of origin fragments in vitro (27), and cruciform extrusion from the double-stranded replicative form is assumed to provide the single-stranded DNA (ssDNA) conformation necessary for cleavage in vivo. Hexamer repeats (5′-CGG/TCAG-3′) are found adjacent to the stem-loop as a part of the minimal binding site (MBS) responsible for the recruitment of the replication proteins Rep and Rep′ (28). Similar features are also found in the Geminiviridae and Nanoviridae, ssDNA plant viruses replicating via RCR. Replication of PCV has been analyzed, and the initial point of viral-strand DNA synthesis has been mapped: Rep and Rep′ bind to and cleave the viral origin in a strand-specific manner between nucleotides 7 and 8 of the nonamer, thereby generating the 3′-hydroxyl group for priming unidirectional leading-strand synthesis. Replication is terminated by Rep/Rep′ in a second cleavage reaction after the regeneration of the origin, thereby releasing unit-length monomers in a Rep/Rep′-catalyzed nucleotidyltransfer reaction (27).
The purpose of this study was to assess the impact of the conserved sequence elements within the origin of replication as well as the conserved amino acid motifs of Rep/Rep′ and to dissect their role with respect to distinct steps of PCV replication. Our results showed that the stem-loop and hexamers H1 and H2 are essential for replication and that the cleavage of the origin depends on motifs I, II, and III. Tyr-93 within motif III of PCV1 Rep/Rep′ confers cleavage activity in vitro. Although Rep exhibits ATPase activity, ATP hydrolysis is not required for the initiation and termination of viral replication in vitro, whereas GKS mutants did not replicate in a cell culture-based replication assay. Energy for DNA ligation is conserved by a covalent link between Rep/Rep′ and the DNA substrate after cleavage.
MATERIALS AND METHODS
Construction of plasmids.
PCR fragments were generated with the High Fidelity PCR system (Roche Diagnostics, Mannheim, Germany). Primers and oligonucleotide sequences are given in Table 1. Plasmids pORF4A, pRep-mutI, pRep-mutII, and pRep-mutP have been described previously (19). Mutant rep genes were subcloned into vector pTriEx6HN (27) or pGEX-6P-1 (Amersham Biosciences, Freiburg, Germany). pTriEx6HN-repmutY93 was generated by amplification from template pORF4A using primer pairs F660/B274A and F245/B661. Primer B661 introduced an amino acid exchange from Tyr-93 to Phe-93 in the Rep protein. PCR fragments were restricted with PstI and AscI (F660/B274A) or BamHI (F245/B661) and cloned into AscI- and BamHI-restricted pTriEx6HN. For the generation of pTriEx6HN-rep′mutI, the SacI fragment (322 nt) from pTriEx6HN-repmutI (positions 2374 to 2695) was inserted into SacI-restricted pTriEx6HN-rep′. PCR fragments from plasmid template pRep-mutI using primers F245 and B226 were restricted with EcoRI and BamHI and cloned into EcoRI- and BamHI-restricted vector pTriEx6HN for the generation of pTriEx6HN-repmutI. Compared to the wild-type (wt) Rep protein, RepmutI carries an amino acid exchange from Phe-16 to Leu-16 and from Asn-19 to Lys-19. RepmutII is characterized by an amino acid exchange from Gln-56 to Pro-56. RepmutP displays an amino acid exchange from Lys-177 to Arg-177 and from Ser-178 to Ile-178. For the generation of plasmids pGEX-repmutII and pGEX-repmutP, PCR fragments were generated from plasmid templates pRep-mutII and pRep-mutP using primers F225 and B226. PCR fragments were restricted with BamHI and EcoRI and cloned into the BamHI- and EcoRI-restricted vector pGEX-6P-1. Plasmid pRL16 and its variants, pRL16-1, pRL16-2, pRL16-3, pRL16-4, pRL16-12, and pRL16-34, are based on pGL3-p (Promega, Mannheim, Germany) and carry the origin fragment of PCV1 (positions 647 to 819) (19). pRL17-1, pRL17-2, and pRL17-3 were constructed via hybridization of synthetic oligonucleotides (F467/B468 and F469/B470 [pRL17-1], F471/B472 and F473/B474 [pRL17-2], and F475/B476 and F477/B470 [pRL17-3]). Products were subsequently ligated into the MscI- and BglII-restricted vector pRL16-34. Sequence alterations with respect to the wt are given in Fig. 1. Inserts are reversely oriented to rule out influence on reporter gene expression by the promoter of the rep gene.
TABLE 1.
Oligonucleotides and primersa
| Oligonucleotide or primer | Sequence |
|---|---|
| Synthetic oligonucleotides | |
| F467 | 5′-CCACGTCATCCTATAAAAGTGAAAGAAGTG-3′ |
| B468 | 5′-P-CTGCAGGAGTAATACTACAGCAGCGCACTTCTTTCACTTTTATAGGATGACGTGG-3′ |
| F469 | 5′-P-CGCTGCTGTAGTATTACTCCTGCAGCACGGCAGCGGCAGCACCTCCCGGGA-3′ |
| B470 | 5′-GATCTCCCGGGAGGTGCTGCCGCTGCCGTG-3′ |
| F471 | 5′-CCACGTCATCCTATAAAAGTGAAAGGGCTG-3′ |
| B472 | 5′-P-GTGCGCTGGTAATACTACAGATCTGCAGCCCTTTCACTTTTATAGGATGACGTGG-3′ |
| F473 | 5′-P-CAGATCTGTAGTATTACCAGCGCACTTCGGCAGCGGCAGCACCTCCCGGGA-3′ |
| B474 | 5′-GATCTCCCGGGAGGTGCTGCCGCTGCCGAA-3′ |
| F475 | 5′-CCACGTCATCCTATAAAAGTGAAAGTGCTG-3′ |
| B476 | 5′-P-CTGCAGGAGTAATACTACAGTCCTGCAGCACTTTCACTTTTATAGGATGACGTGG-3′ |
| F477 | 5′-P-CAGGACTGTAGTATTACTCCTGCAGCACGGCAGCGGCAGCACCTCCCGGGA-3′ |
| Primers | |
| F660 | 5′-AACTGCAGTAAAGAAGGCCACAT-3′ |
| B274A | 5′-GGCGCGCCCGATGTGATAACAAAAAAGACTCAGT-3′ |
| F245 | 5′-CGGGATCCAAGCAAGAAAAGCGGC-3′ |
| B661 | 5′-AACTGCAGAATTCTTTATTCTGCT-3′ |
| B226 | 5′-GGAATTCGATGTGATAACAAAAAAGACTCAGT-3′ |
| F225 | 5′-CGGGATCCCCAAGAAGAAAAGCGGCCCG-3′ |
Restriction sites are underlined.
Expression and purification of PCV1 replication proteins Rep and Rep′.
Escherichia coli RosettaBlue(DE3)pLacI competent cells (Merck Biosciences, Bad Soden, Germany) were transformed with pTriEx6HN-rep, pTriEx6HN-rep′, pTriEx6HN-rep′mutI, and pTriEx6HN-repmutY93 (PCV1). Expression and purification of recombinant proteins were performed as described previously (27). Glutathione S-transferase (GST)-RepmutII and GST-RepmutP were expressed in E. coli BL21 cells (Amersham Biosciences) after induction with IPTG (isopropyl-β-d-thiogalactopyranoside) (0.2 mM) at 30°C. GST was expressed from plasmid pGEX-6P-1 as a negative control. GST fusion proteins and GST were purified with glutathione-Sepharose (Amersham Biosciences) after cell lysis using BugBuster protein extraction reagent in combination with Benzonase nuclease and rLysozyme solution (Merck Biosciences). Purified Rep and Rep′ recombinant proteins and GST were concentrated and rebuffered in Tris-EDTA buffer (pH 7.5) using Microcon centrifugal filter devices (Millipore Corporation, Bedford, MA). Aliquots of the purified proteins were fractionated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and visualized by staining with Coomassie brilliant blue dye.
In vitro cleavage reaction and detection of covalent linkage of Rep to DNA.
DNA cleavage reactions and covalent linkage were performed with oligonucleotides corresponding to the PCV1 plus-strand origin (Fig. 1). A total of 0.5 pmol of 5′-Cy5-labeled F301 was incubated with 500 ng of protein in cleavage buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 10 mM MgCl2, 5 mM dithiothreitol, 1 mM EDTA) in a total volume of 30 μl for 90 min at 37°C. Cleavage products were analyzed in nondenaturing 5.5% polyacrylamide gels and visualized using the FLA-2000 Fluorescent Image Analyzer (FUJIFILM). Covalent linkage of Rep to the DNA was investigated with oligonucleotide F1165 labeled with biotin at the 3′ end (Fig. 1). Five hundred nanograms of His-Rep protein was incubated with 1, 10, 50, or 100 pmol of F1165. After the addition of SDS loading buffer (0.25 mM Tris-HCl [pH 6.8], 50 mM dithiothreitol, 1% SDS, 10% glycerol, 0.1% bromphenol blue) and heating for 5 min at 95°C, cleavage products were separated in denaturing 10% polyacrylamide gels. Following immobilization on a polyvinylidene difluoride membrane, protein-DNA complexes were visualized using either monoclonal anti-biotin peroxidase conjugate (1.5 μg/ml; Sigma) or monoclonal anti-His tag antibody (3 μg/ml; Dianova) and monoclonal anti-mouse peroxidase conjugate (80 ng/ml; Sigma).
ATPase assay.
Five hundred nanograms of protein was incubated in a solution containing 50 mM Tris-HCl (pH 7.5), 100 mM NaCl, 10 mM MgCl2, 100 μM ATP, and 2 μCi [γ-32P]ATP in a total volume of 32 μl for 45 min at 37°C. The reaction was stopped by the addition of 200 μl 7.5% charcoal activated in 50 mM HCl-5 mM H3PO4. ATP was precipitated after 5 min at room temperature (13,000 rpm, 10 min; Heraeus). The radioactivity in the supernatant was determined by liquid scintillation counting.
Replication assay.
The activity of mutant Rep proteins was investigated in a replication assay based upon luciferase (luc) reporter gene expression (21). PK15 cells were cotransfected with plasmid pRL16 carrying the origin of PCV1 and the luc gene as well as a plasmid expressing the wt or mutant Rep protein. Conversely, the activities of origin mutants were determined after cotransfection of pORF4A expressing wt Rep/Rep′ and a plasmid carrying the mutated origin of replication of PCV1 as well as the luc gene. A rise in the copy number of the plasmid via replication is reflected in the increasing reporter gene activity. One hundred nanograms of the origin plasmid plus 100 ng of the replicase plasmid were cotransfected with 50 ng of Rous sarcoma virus-β-galactosidase into PK15 cells, and the latter was used to standardize for transfection efficiency. The preparation of cell lysates and determination of luciferase and galactosidase activity were described previously (21).
RESULTS
Generation and expression of mutant PCV1 replication proteins.
The capability of Rep and Rep′ to cleave and reseal the viral ssDNA was demonstrated recently (27). The present study investigates the impact of amino acid motifs that are conserved in the Rep and Rep′ proteins upon the initiation and termination of viral replication. The contribution of each of the four conserved motifs was analyzed with the mutant proteins Rep′mutI (F16TLNN changed to L16TLKN [boldface type indicates altered amino acids]), RepmutII (H54LQGF to H54LPGF), RepmutY93 (Y93CSK to F93CSK), and RepmutP (G176KS to G176RI) (20). PCV1 Rep/Rep′ and mutant proteins RepmutY93 and Rep′mutI were expressed as N-terminal His-tagged proteins in E. coli, while RepmutII and RepmutP were expressed as GST fusion proteins. The apparent molecular mass of His-Rep and His-RepmutY93 corresponded to 36 kDa (Fig. 2A, lanes 2 and 4), that of His-Rep′ and His-Rep′mutI corresponded to 20 kDa (Fig. 2A, lanes 3 and 5), and that of GST-Rep, GST-RepmutII, and GST-RepmutP corresponded to 60 kDa (Fig. 2B, lanes 1, 2, and 3).
FIG. 2.
Purified PCV1 Rep and Rep′ recombinant fusion proteins. His-tagged proteins His-Rep, His-Rep′, His-RepmutY93, and His-Rep′mutI (A, lanes 2, 3, 4, and 5, respectively) were expressed in E. coli and subsequently purified by affinity chromatography using Ni-nitrilotriacetic acid agarose beads. The GST fusion proteins GST-Rep, GST-RepmutII, and GST-RepmutP (B, lanes 1, 2, and 3, respectively) were purified using glutathione-Sepharose after expression in E. coli. Rebuffered and concentrated proteins were fractionated by SDS-polyacrylamide gel electrophoresis and visualized by staining with Coomassie brilliant blue dye. The apparent molecular mass was determined by comparison with a protein standard with the indicated molecular masses (A, lane 1; B lane 4).
Analysis of Rep/Rep′ mutants with respect to cleavage activity.
The dependence of PCV1 Rep/Rep′ cleavage activity on motifs I to III and the GKS box was investigated in vitro. His-tagged or GST-fused Rep/Rep′ protein was incubated with the 5′-Cy5-labeled oligonucleotide F301 (Fig. 1) comprising the inverted repeat with the conserved nonamer (indicated as 10-12-10) (Fig. 3) and four adjacent hexamers (indicated as 6-6-5-6-6) corresponding to the viral plus strand of PCV1. A signal representing the 5′-terminal cleavage product was indicative of cleavage activity (Fig. 3, lanes 2, 3, 7, and 10). Since the expression of RepmutI in E. coli did not result in sufficient amounts of soluble protein, the impact of motif I on origin cleavage was analyzed using His-Rep′mutI instead of His-RepmutI. Incubation of F301 with His-Rep′mutI (Fig. 3, lane 5) and GST-RepmutII (Fig. 3, lane 9) did not result in cleavage of the substrate. Since DNA cleavage is commonly accomplished via a nucleophilic attack by a hydroxyl group of either a conserved tyrosine or serine residue of the Rep protein (14, 23, 26, 29), we assumed that this function is exerted by Tyr-93 in motif III of PCV1 Rep/Rep′. Consequently, Tyr-93 was replaced by Phe in His-RepmutY93. The activity of this mutant was completely abolished (Fig. 3, lane 4), suggesting that Tyr-93 of PCV1 Rep is crucial for the initiation of viral replication. In contrast, GST-RepmutP was active in the in vitro cleavage assay (Fig. 3, lane 10), corroborating the observation that ATP hydrolysis is dispensable for origin cleavage in vitro (27). Motifs I to III are essential for Rep and Rep′ to cleave the origin of replication, while the GKS box is not.
FIG. 3.
Impact of conserved amino acid motifs within PCV1 Rep/Rep′ on origin cleavage. Purified fusion protein (500 ng) was incubated with 0.5 pmol oligonucleotide in the cleavage reaction in vitro. Cy5-labeled oligonucleotide F301 representing the conserved sequences of the PCV1 origin of replication was incubated with His-Rep/Rep′ (lanes 2 and 3), His-RepmutY93 (lane 4), His-Rep′mutI (lane 5), GST-Rep (lane 7), GST-RepmutII (lane 9), or GST-RepmutP (lane 10). As a negative control (n.c.), purified GST protein was used (lane 8). Samples were resolved on native polyacrylamide gels and compared to oligonucleotides of defined sizes (lanes 1 and 6). Positions and sizes of oligonucleotides are marked, and cleavage products are highlighted by arrowheads.
Covalent linkage of PCV1 Rep to the 5′ end after cleavage.
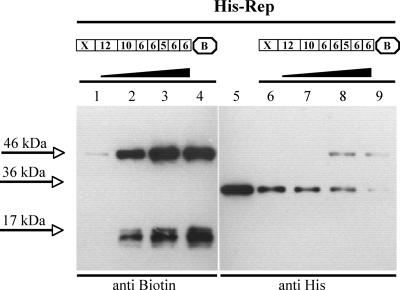
A round of RCR is terminated by the cleavage and ligation of the newly synthesized ssDNA strand. This step is usually independent of ATP hydrolysis, and energy is conserved by a covalent link of the replication protein to the DNA (10, 14, 27). To determine whether Rep is transferred to the 5′ end of the cleavage product, His-Rep was incubated with increasing amounts of 3′ biotin-labeled oligonucleotide F1165 (Fig. 1). F1165 comprises sequence alterations upstream of the inverted repeat, which inhibited reassociation after cleavage (21). In the Western blot, an anti-biotin antibody detected the DNA in the complex, while the protein was visualized with an anti-His tag antibody. With both reagents, retardation of the Rep-DNA complex was observed (Fig. 4, lanes 1 to 4 and 6 to 9), and its molecular mass corresponded to approximately 46 kDa. Comparable results were observed when the PCV1 His-Rep′ protein was studied (T. Steinfeldt, unpublished data), showing that Rep and Rep′ are covalently linked to DNA after cleavage.
FIG. 4.
PCV1 Rep is covalently attached to the DNA after cleavage. The purified His-Rep fusion protein (500 ng) was incubated with 1 (lane 1 and 6), 10 (lane 2 and 7), 50 (lane 3 and 8), or 100 (lane 4 and 9) pmol of oligonucleotide F1165 comprising sequence alterations upstream of the inverted repeat and labeled with biotin at the 3′ end in the cleavage reaction in vitro. As a control, purified His-Rep was incubated without an oligonucleotide under the same conditions (lane 5). Samples were resolved on denaturing 10% polyacrylamide gels after the addition of SDS loading buffer and heating to 95°C for 5 min. Immunoblots were detected with anti-biotin antibody (lanes 1 to 4) or anti-His tag antibody (lanes 5 to 9). The molecular masses of free substrate, free protein, and the protein-DNA complex were determined by comparison with a protein standard.
RepmutI, RepmutII, RepmutY93, and RepmutP do not support replication of PCV1 in cultured cells.
The impact of the four conserved amino acid motifs on DNA replication was investigated in cell cultures by using a luc-based reporter gene assay (32). Mutant Rep proteins were tested for catalytic activity with respect to the PCV1 wt origin. For this purpose, PK15 cells were cotransfected with pRL16 carrying the wt origin plus the luc gene and plasmids carrying the mutated rep gene of PCV1. Plasmid pRL16 cotransfected with vector pTriEx6HN or pSVL-SV40 as well as plasmid pTriEx6HN-rep or pORF4A plus vector pGL3-p served as negative controls (Fig. 5, bars 1 and 7 and 2 and 8), while plasmid pORF4A or pTriEx6HN-rep in combination with pRL16 (Fig. 5, bars 3 and 9) was a positive control. Replication activity was seen with neither Rep-mutI, Rep-mutII, or RepmutY93 nor with Rep-mutP (Fig. 5, bars 4, 5, 6, and 10), demonstrating that not only motifs I to III but also the GKS box plays a pivotal role for replication of PCV1 in cell culture.
FIG. 5.
Impact of conserved amino acid motifs within PCV1 Rep/Rep′ on viral replication. Replication proteins Rep and Rep′ mutated in the conserved amino acid motifs I, II, and III or the GKS box were tested for replication activity. Plasmids encoding the recombinant protein were cotransfected with pRL16 carrying the wt PCV1 origin of replication (bars 4, 5, 6, and 10) and compared to the replication activity of wt Rep/Rep′ (pORF4A and pTriEx6HN-rep [bars 3 and 9]). In the negative controls, the replication activity of wt Rep/Rep′ was determined under repression of replication (pGL3-p [bars 2 and 8]), as was the replication activity of pRL16 in the absence of trans-active replication factors Rep and Rep′ (pSVL-SV40 and pTriEx6HN [bars 1 and 7]).
PCV1 Rep hydrolyzes ATP.
The G176KS sequence is conserved in proteins mediating ATP binding and hydrolysis (28). It is present in the Rep protein of PCV1 but not in the Rep′ protein. His-Rep or His-Rep′ was incubated with [γ-32P]ATP, and ATPase activity was determined. As expected, His-Rep hydrolyzed ATP, while ATPase activity could not be detected for His-Rep′ (Fig. 6, bars 2 and 4). ATP hydrolysis was strictly dependent on the presence of Mg2+ (Fig. 6, bar 3), while incubation with ssDNA and double-stranded DNA (dsDNA) did not influence the catalytic activity (Steinfeldt, unpublished). A negative control that omitted His-Rep verified that the catalytic activity was Rep specific (Fig. 6, bar 1). ATP hydrolysis was slightly reduced with His-RepmutY93 (Fig. 6, bar 6) and diminished about 50% with GST-RepmutII (Fig. 6, bar 5). When GST-RepmutP was tested in the ATPase assay, ATP hydrolysis was completely abolished (Fig. 6, bar 7), demonstrating that this motif confers ATPase activity.
FIG. 6.
ATPase assay. PCV1 replication proteins His-Rep and His-Rep′ (bars 2, 3, and 4), and mutated PCV1 proteins GST-RepmutII, His-RepmutY93, and GST-RepmutP (bars 5, 6, and 7, respectively) were incubated in the presence of [γ-32P]ATP in vitro. After precipitation of residual [γ-32P]ATP, ATPase activity was determined by measuring radioactive free phosphate in the supernatant and compared with that of the negative control (n.c.). Unless otherwise noted, the reaction mixture was supplemented with Mg2+.
Conserved sequences within the origin of PCV1 are essential for viral replication.
The MBS of Rep and Rep′ comprises the right part of the stem-loop and the two adjacent hexamers and recruits the proteins to the double-stranded origin of replication (27). Remarkably, we showed recently that the cleavage of origin fragments in vitro does not depend on the MBS (27).
To investigate the impact of the conserved DNA sequence elements on replication in cell culture, mutant origin versions were constructed (Fig. 1). PK15 cells were cotransfected with pORF4A encoding the rep gene of PCV1 and luc plasmids carrying the wt or mutated origin. Negative controls combined either the PCV1 Rep/Rep′ proteins with the origin-deprived luc plasmid pGL3-p or vector pSVL-SV40 with the PCV1 wt origin of replication (Fig. 7A, bars 1 and 2). The latter serves as the benchmark for luciferase expression from a nonreplicating plasmid. In the positive control, plasmid pRL16 carrying the PCV1 wt origin comprising the putative stem-loop and the four hexamers H1 to H4 was combined with wt Rep/Rep′, and a fourfold increase of luciferase activity was seen (Fig. 7A, bar 3). In plasmids pRL16-1, pRL16-2, pRL16-3, and pRL16-4, the indicated hexamer was mutated from 5′-CG(G/T)CAG-3′ to 5′-CCCGGG-3′, while in plasmids pRL16-12 and pRL16-34, two adjacent hexamers, either H1/H2 or H3/H4, were modified. When plasmids pRL16-1, pRL16-2, and pRL16-12 were cotransfected with pORF4A (Fig. 7A, bars 5, 7, and 9), the replication activity of pRL16-2 was strongly reduced, and no activity was seen for pRL16-1 and pRL16-12. In contrast, plasmids pRL16-3, pRL16-4, and pRL16-34 revealed no decrease in replication activity (Fig. 7A, bars 11, 13, and 15).
FIG. 7.
Conserved origin sequences exert different impacts on viral replication. Plasmids carrying wt or mutated origin fragments were tested for replication in PK15 cells in the presence or absence of the replication proteins Rep and Rep′. The replication activity of wt (pRL16) or mutated origin plasmids (pRL16 and pRL17 derivatives) was determined by cotransfection with pORF4A (PCV1 Rep/Rep′) and compared to the replication rate of the same replicon in the absence of replication proteins (pSVL-SV40) (A, bars 2 to 15; B, bars 2 to 9). Plasmid pGL3-p encompasses the luc gene but misses the cis-active origin and represents PCV1 Rep/Rep′ replication activity under the repression of replication (A and B, bar 1).
Another set of plasmids carried origin mutants disabled in the formation of the putative stem-loop. In plasmid pRL17-1, the upstream sequence 5′-AAGTGCGCTG-3′ of the nonamer was altered to 5′-GGCTGCAGAT-3′, and in pRL17-2, the downstream sequence 5′-CAGCGCACTT-3′ of the nonamer was altered to 5′-TCCTGCAGCA-3′. In pRL17-3, the original sequence was replaced by another inverted repeat. Modification resulted in a loss of replication activity for pRL17-1 and pRL17-3, while replication activity was reduced for pRL17-2 (Fig. 7B, bars 5, 7, and 9). Taken together, our results indicate that hexamers H1 and H2 are essential for the efficient replication of PCV1 and that H3/H4 cannot replace H1/H2. Moreover, sequence alteration in the stem-loop impairs the replication of PCV1, but mutations downstream of the nonamer can be compensated to some extent.
DISCUSSION
Influence of conserved motifs I to III and GKS on initiation of viral replication.
Rep and Rep′ of PCV1 act as RCR initiator proteins in vitro (8, 12, 15). F16TLNN (motif I), H54LQGF (motif II), Y93CSKE (motif III), and G176KS are conserved amino acid motifs, which are common among other RCR initiator proteins, e.g., geminiviral Rep proteins.
These motifs have been tested in a preliminary study in a DpnI assay, which revealed their significance for the replication activity of PCV1 in cell culture. The present continuative study used a quantitative and more sensitive reporter gene assay and moreover dissected the contribution of the motifs for origin cleavage in vitro. No cleavage activity was observed for His-Rep′mutI and GST-RepmutII; these mutants also did not support replication in cell culture, but they were able to hydrolyze ATP. The role of motif I in the catalytic activity of Rep/Rep′ is unknown, while motif II is assumed to mediate the coordination of bivalent cations in analogy to metalloenzymes (27, 31). Bivalent cations have recently been shown to be indispensable for Rep/Rep′-mediated cleavage of origin fragments in vitro (14).
Among RCR-mediating enzymes, the cleavage of the phosphodiester bond usually occurs by a nucleophilic attack of a conserved tyrosine or serine residue: Tomato yellow leaf curl virus Tyr-103 and Faba bean necrotic yellows virus Tyr-79 Rep proteins have been identified as being mediators of origin cleavage, which subsequently become attached to the 5′ end of the 3′ cleavage product via a tyrosylester (31). The mutation of Tyr-93 in motif III of PCV1 Rep to Phe did not influence the ability of RepmutY93 to bind to dsDNA (Steinfeldt, unpublished) or hydrolyze ATP, but cleavage activity in vitro as well as replication in cell culture were completely abolished. These results proved not only that His-RepY93 is functional but moreover that binding to dsDNA and cleavage of ssDNA are distinct events during the initiation of replication. We demonstrated for the first time that PCV1 Rep is covalently linked to the DNA after cleavage, a finding that is corroborated by another recent study of PCV2 (24). Since the mutagenesis of Tyr-93 in motif III of PCV1 Rep (5′-YCSKE-3′) inhibited cleavage, we suppose that Tyr-93 executes this reaction, and a second cleavage necessary for the termination of replication is promoted by an additional Tyr-93 in an oligomer composed of at least two replication proteins. This assumption is supported by the detection of homo- and heterocomplexes of PCV1 Rep and Rep′ in vitro and in vivo (T. Finsterbusch, unpublished data).
Rep hydrolyzes ATP, while Rep′ does not exhibit this capability. Mutagenesis of motifs II and III did not compromise ATPase activity, which was strictly dependent on the integrity of the GKS box and the presence of bivalent cations, while the addition of ssDNA and dsDNA did not enhance ATPase activity. The lack of ATPase activity of RepmutP coincides with the loss of replication activity in cell culture, suggesting that ATP hydrolysis by PCV1 Rep is indispensable for its function in viral replication. The role of ATP hydrolysis during the viral replication cycle remains to be determined: although ATP is not essential for cleaving the phosphodiester bond in vitro, the addition of ATP increased the cleavage activity of PCV1 Rep (Steinfeldt, unpublished). Rep binding may mediate the partial unwinding of the origin and thereby may lead to cruciform extrusion and the generation of an ssDNA region, which is recognized and cleaved by Rep and Rep′. A similar mechanism for the replication initiator of plasmid pT181 has been described previously (17). A putative helicase activity of the PCV Rep protein associated with ATP hydrolysis could lead to a further unwinding of DNA strands as a prerequisite for DNA synthesis. This hypothesis is endorsed by the identification of a putative helicase domain within the C terminus of the Rep proteins of PCV1 and PCV2 (12), similar to other viral helicases (28).
Impact of conserved DNA elements within the origin on viral replication.
Previous studies revealed that the MBS, the right part of the putative stem-loop structure plus the two adjacent hexamer sequences H1/H2, recruits Rep/Rep′ to double-stranded origin fragments. The observation that viral replication in cell culture is highly dependent on the presence of the two inner hexamers H1 and H2 confirms the binding of Rep/Rep′ to the double-stranded origin of replication. H3/H4 cannot replace inactivated H1/H2. This is corroborated by the finding that a mutation engineered into H1/H2 induced the deletion of the mutated sequences and replacement by the downstream H3/H4 (2). Moreover, the stem-loop was found to influence replication: mutagenesis of the downstream sequence of the nonamer reduced replication, while an alteration of the upstream or both sequences abolished the activity completely. This cannot be due to the secondary element per se, since in 17-3, the original stem-loop was replaced by another inverted repeat.
The present study has expanded the current understanding of the replication of PCV1: after the conversion of the ssDNA into a dsDNA replicative intermediate, Rep and Rep′ are recruited to the origin of replication via binding to the MBS including hexamers H1/H2. Cruciform extrusion induces the exposure of the nonamer sequence as ssDNA, which is subsequently recognized and cleaved by Rep/Rep′. Cleavage generates a free 3′-hydroxyl group, which serves as a primer for DNA synthesis, while the replication proteins become covalently attached to the 5′ end after cleavage. RCR motifs I to III are essential for in vitro cleavage of the DNA as well as for the replication of PCV1 in cell culture, while the GKS box is indispensable for replication in cell culture but not for cleavage.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (Ma 2126/2-1) and the European Union (fifth FP, QLK2-CT-1999-00307; sixth FP, 513928, PCVD).
Footnotes
Published ahead of print on 14 March 2007.
REFERENCES
- 1.Bassami, M. R., D. Berryman, G. E. Wilcox, and S. R. Raidal. 1998. Psittacine beak and feather disease virus nucleotide sequence analysis and its relationship to porcine circovirus, plant circoviruses, and chicken anaemia virus. Virology 249:453-459. [DOI] [PubMed] [Google Scholar]
- 2.Cheung, A. K. 2005. Mutational analysis of the direct tandem repeat sequences at the origin of DNA replication of porcine circovirus type 1. Virology 339:192-199. [DOI] [PubMed] [Google Scholar]
- 3.Danen-Van Oorschot, A. A., D. F. Fischer, J. M. Grimbergen, B. Klein, S. Zhuang, J. H. Falkenburg, C. Backendorf, P. H. Quax, A. J. Van der Eb, and M. H. Noteborn. 1997. Apoptin induces apoptosis in human transformed and malignant cells but not in normal cells. Proc. Natl. Acad. Sci. USA 94:5843-5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darwich, L., J. Segales, and E. Mateu. 2004. Pathogenesis of postweaning multisystemic wasting syndrome caused by porcine circovirus 2: an immune riddle. Arch. Virol. 149:857-874. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 5.del Solar, G., R. Giraldo, M. J. Ruiz-Echevarria, M. Espinosa, and R. Diaz-Orejas. 1998. Replication and control of circular bacterial plasmids. Microbiol. Mol. Biol. Rev. 62:434-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellis, J., S. Krakowka, M. Lairmore, D. Haines, A. Bratanich, E. Clark, G. Allan, C. Konoby, L. Hassard, B. Meehan, K. Martin, J. Harding, S. Kennedy, and F. McNeilly. 1999. Reproduction of lesions of postweaning multisystemic wasting syndrome in gnotobiotic piglets. J. Vet. Diagn. Investig. 11:3-14. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert, W., and D. Dressler. 1968. DNA replication: the rolling circle model. Cold Spring Harb. Symp. Quant. Biol. 33:473-484. [DOI] [PubMed] [Google Scholar]
- 8.Hafner, G. J., M. R. Stafford, L. C. Wolter, R. M. Harding, and J. L. Dale. 1997. Nicking and joining activity of banana bunchy top virus replication protein in vitro. J. Gen. Virol. 78:1795-1799. [DOI] [PubMed] [Google Scholar]
- 9.Hamel, A. L., L. L. Lin, and G. P. Nayar. 1998. Nucleotide sequence of porcine circovirus associated with postweaning multisystemic wasting syndrome in pigs. J. Virol. 72:5262-5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heyraud-Nitschke, F., S. Schumacher, J. Laufs, S. Schaefer, J. Schell, and B. Gronenborn. 1995. Determination of the origin cleavage and joining domain of geminivirus Rep proteins. Nucleic Acids Res. 23:910-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ilyina, T. V., and E. V. Koonin. 1992. Conserved sequence motifs in the initiator proteins for rolling circle DNA replication encoded by diverse replicons from eubacteria, eucaryotes and archaebacteria. Nucleic Acids Res. 20:3279-3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koonin, E. V., and T. V. Ilyina. 1992. Geminivirus replication proteins are related to prokaryotic plasmid rolling circle DNA replication initiator proteins. J. Gen. Virol. 73:2763-2766. [DOI] [PubMed] [Google Scholar]
- 13.Krakowka, S., J. Ellis, F. McNeilly, C. Waldner, and G. Allan. 2005. Features of porcine circovirus-2 disease: correlations between lesions, amount and distribution of virus, and clinical outcome. J. Vet. Diagn. Investig. 17:213-222. [DOI] [PubMed] [Google Scholar]
- 14.Laufs, J., S. Schumacher, N. Geisler, I. Jupin, and B. Gronenborn. 1995. Identification of the nicking tyrosine of geminivirus Rep protein. FEBS Lett. 377:258-262. [DOI] [PubMed] [Google Scholar]
- 15.Laufs, J., W. Traut, F. Heyraud, V. Matzeit, S. G. Rogers, J. Schell, and B. Gronenborn. 1995. In vitro cleavage and joining at the viral origin of replication by the replication initiator protein of tomato yellow leaf curl virus. Proc. Natl. Acad. Sci. USA 92:3879-3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu, J., I. Chen, and J. Kwang. 2005. Characterization of a previously unidentified viral protein in porcine circovirus type 2-infected cells and its role in virus-induced apoptosis. J. Virol. 79:8262-8274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mankertz, A., R. Caliskan, K. Hattermann, B. Hillenbrand, P. Kurzendoerfer, B. Mueller, C. Schmitt, T. Steinfeldt, and T. Finsterbusch. 2004. Molecular biology of porcine circovirus: analyses of gene expression and viral replication. Vet. Microbiol. 98:81-88. [DOI] [PubMed] [Google Scholar]
- 18.Mankertz, A., K. Hattermann, B. Ehlers, and D. Soike. 2000. Cloning and sequencing of columbid circovirus (CoCV), a new circovirus from pigeons. Arch. Virol. 145:2469-2480. [DOI] [PubMed] [Google Scholar]
- 19.Mankertz, A., and B. Hillenbrand. 2002. Analysis of transcription of porcine circovirus type 1. J. Gen. Virol. 83:2743-2751. [DOI] [PubMed] [Google Scholar]
- 20.Mankertz, A., and B. Hillenbrand. 2001. Replication of porcine circovirus type 1 requires two proteins encoded by the viral rep gene. Virology 279:429-438. [DOI] [PubMed] [Google Scholar]
- 21.Mankertz, A., B. Mueller, T. Steinfeldt, C. Schmitt, and T. Finsterbusch. 2003. New reporter gene-based replication assay reveals exchangeability of replication factors of porcine circovirus types 1 and 2. J. Virol. 77:9885-9893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mankertz, A., F. Persson, J. Mankertz, G. Blaess, and H. J. Buhk. 1997. Mapping and characterization of the origin of DNA replication of porcine circovirus. J. Virol. 71:2562-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newman, B. J., and N. D. Grindley. 1984. Mutants of the gamma delta resolvase: a genetic analysis of the recombination function. Cell 38:463-469. [DOI] [PubMed] [Google Scholar]
- 24.Noirot, P., J. Bargonetti, and R. P. Novick. 1990. Initiation of rolling-circle replication in pT181 plasmid: initiator protein enhances cruciform extrusion at the origin. Proc. Natl. Acad. Sci. USA 87:8560-8564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phenix, K. V., J. H. Weston, I. Ypelaar, A. Lavazza, J. A. Smyth, D. Todd, G. E. Wilcox, and S. R. Raidal. 2001. Nucleotide sequence analysis of a novel circovirus of canaries and its relationship to other members of the genus Circovirus of the family Circoviridae. J. Gen. Virol. 82:2805-2809. [DOI] [PubMed] [Google Scholar]
- 26.Reed, R. R., and C. D. Moser. 1984. Resolvase-mediated recombination intermediates contain a serine residue covalently linked to DNA. Cold Spring Harb. Symp. Quant. Biol. 49:245-249. [DOI] [PubMed] [Google Scholar]
- 27.Steinfeldt, T., T. Finsterbusch, and A. Mankertz. 2006. Demonstration of nicking/joining activity at the origin of DNA replication associated with the Rep and Rep′ proteins of porcine circovirus type 1. J. Virol. 80:6225-6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steinfeldt, T., T. Finsterbusch, and A. Mankertz. 2001. Rep and Rep′ protein of porcine circovirus type 1 bind to the origin of replication in vitro. Virology 291:152-160. [DOI] [PubMed] [Google Scholar]
- 29.Timchenko, T., F. de Kouchkovsky, L. Katul, C. David, H. J. Vetten, and B. Gronenborn. 1999. A single Rep protein initiates replication of multiple genome components of faba bean necrotic yellows virus, a single-stranded DNA virus of plants. J. Virol. 73:10173-10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Todd, D., J. H. Weston, D. Soike, and J. A. Smyth. 2001. Genome sequence determinations and analyses of novel circoviruses from goose and pigeon. Virology 286:354-362. [DOI] [PubMed] [Google Scholar]
- 31.Vega-Rocha, S., I. J. Byeon, B. Gronenborn, A. M. Gronenborn, and R. Campos-Olivas. 2007. Solution structure, divalent metal and DNA binding of the endonuclease domain from the replication initiation protein from porcine circovirus 2. J. Mol. Biol. 9:9. [DOI] [PubMed] [Google Scholar]
- 32.Walker, J. E., M. Saraste, M. J. Runswick, and N. J. Gay. 1982. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1:945-951. [DOI] [PMC free article] [PubMed] [Google Scholar]