Abstract
Obesity is a major risk factor for cardiovascular diseases, but the mechanisms for increased cardiovascular risk in obesity are still unclear. Inflammation and increased oxidative stress are two potential mechanisms proposed to play a major role in the morbidity associated with obesity. Studies that investigate these mechanisms rely on biomarkers, but validated biomarkers for obesity-related cardiovascular outcomes are lacking. By finding optimal biomarkers, diagnostic criteria for cardiovascular diseases can be refined in the obese beyond “traditional” risk factors to identify early pathologic processes. The objective of this review is to identify potential early biomarkers resulting from obesity and associated with cardiovascular disease. Studies were initially identified through the search engine PubMed by using the keywords “obesity” and “biomarker.” Subsequently, combinations of the keywords “obesity,” “biomarker,” “cardiovascular risk,” “adipose tissue,” “adipokine,” “adipocytokine,” and “oxidative stress” were used. The SOURCE database and Online Mendelian Inheritance in Man (OMIM) were used to obtain more information on the biomarkers. Results of the searches yielded a large number of potential biomarkers that occur in obesity and which either correlate with traditional cardiovascular risk factors or predict subsequent cardiovascular events. Several biomarkers are promising regarding their biologic properties, but they require further validation in humans.
Keywords: adipose tissue, cardiovascular diseases, inflammation, obesity, oxidative stress
The prevalence of obesity, defined as a body mass index (weight (kg)/height (m)2) of ≥30 kg/m2, has been rising for decades. It is estimated that 32 percent of US adults were obese and 17 percent of children aged 2–19 years were overweight (gender- and age-specific body mass index per-centile ≥95) in 2003–2004 (1). On the basis of mortality data from the National Health and Nutrition Examination Survey, the excess number of deaths in 2000 attributed to obesity was 112,000 compared with a healthy body mass index of 18.5–25 kg/m2 (2). The estimated years of life lost among Black men and White men aged 20 years with a body mass index of >45 kg/m2 is 20 and 13, respectively; the corresponding figures for Black women and White women are 5 years and 8 years (3). Increased mortality attributed to obesity results partly from the propensity for increased risk of chronic diseases such as cardiovascular disease (CVD), especially if obesity began at an early age.
In adolescents, obesity is significantly associated with increased risk of CVD, with an odds ratio of 19.2 for high cumulative cardiovascular risk (4). Increased risk of CVD in obesity strongly correlates with traditional risk factors such as diabetes, hypertension, and hyperlipidemia (5, 6). Such traditional risk factors are validated in many populations (7–10) for the diagnosis and management of CVD. The underlying mechanisms for the association of obesity and traditional risk factors with CVD are still not fully known. One opportunity for elucidating these mechanisms most likely involves identifying other biomarkers that result from obesity and that independently enhance future susceptibility for CVD. Such nontraditional biomarkers will further refine risk assessment and aid in CVD prevention. Ultimately, nontraditional biomarkers may prove useful in translational medicine for improving the prediction of CVD in the obese.
Examples of emerging risk factors proposed to predict atherosclerosis, a pathologic process associated with CVD, include lipoprotein(a), increased homocysteine, increased inflammatory markers, and prothrombotic factors (6, 11). Their predictive value in the population and in comparison with standard lipid screening is still debatable. Of these risk factors, only a few, such as C-reactive protein, an inflammatory marker, are extensively studied and shown to be variably associated with obesity or cardiovascular endpoints (12–14). There is consistent awareness of the need for other biomarkers that occur after the development of obesity and that may predict later CVD onset. Such biomarkers for early detection have yet to be identified.
Different mechanisms are implicated in the link between obesity and CVD. For example, the fetal origin of metabolic risk (15), epigenetic gene regulation (16), and the “pup in a cup” model (17) are potential causes of increased CVD risk in obesity. This review focuses on two other mechanisms of current interest: inflammation and increased oxidative stress. Both can play a role in promoting CVD, such as increased endothelial dysfunction, an early predictor of cardiovascular injury in obese individuals (18). Notably, both mechanisms are associated with the accumulation of fat that occurs in obesity.
Abnormal fat accumulation is associated with inflammatory changes, including recruitment of macrophages (19) and activation of endothelial cells (19), which promotes vascular disease (20). Indeed, several molecules produced by adipocytes (adipocytokines) are associated with increased cardiovascular risk (21–25) partly through increased expression of inflammatory genes (26) and induction of systemic inflammation (27). Increased secretion of inflammatory molecules can be modified by weight loss (28), thus indicating the dynamic influence of adipose tissue in inflammation. Accumulation of adipose tissue also leads to increased oxidative stress (29) partly via the oxidant effects of free fatty acids (29). Leukocytes derived from obese individuals and healthy individuals infused with free fatty acids suffer from increased oxidative stress (30, 31). Oxidative stress in turn may promote metabolic complications such as insulin resistance and induces a proinflammatory state via deregulation of adipocytokines (29). Thus, oxidative stress is both induced by and adversely impacts adipose tissue function. Consequently, biomarkers for oxidative stress may play a role in obesity-induced CVD.
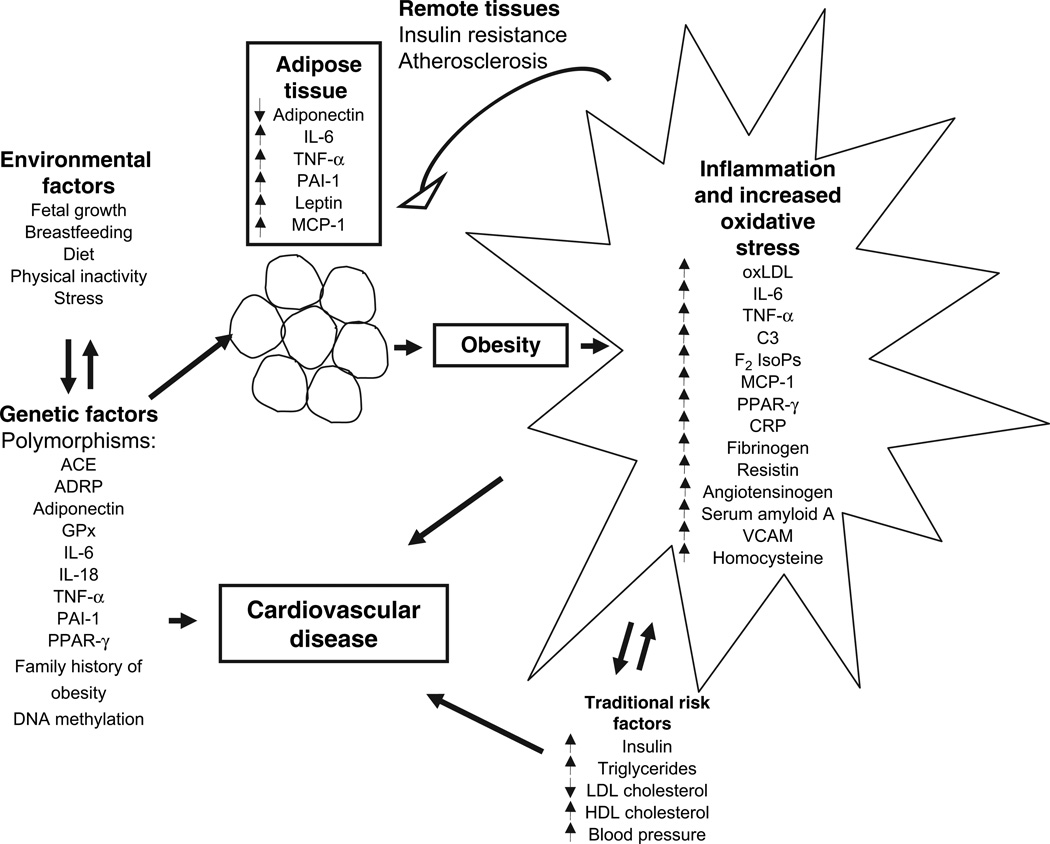
Figure 1 is a schematic representation of potential biomarkers that result from obesity.
FIGURE 1.
A proposed schematic for the development of biomarkers that result early in the course of obesity and that may act as risk factors for cardiovascular outcomes. Genetic and environmental factors may interact to alter expression of biomarkers from adipose tissue. Fat deposition is accompanied by increased biomarkers for inflammation and oxidative stress, which can in turn impact adipose tissue function. The biomarkers interact with traditional risk factors that may develop later, leading to cardiovascular disease manifestations. ACE, angiotensin-converting enzyme; ADRP, adipocyte differentiation-related protein; CRP, C-reactive protein; C3, complement factor 3; F2 IsoPs, F2 isoprostanes; GPx, glutathione peroxidase; HDL, high density lipoprotein; IL-6, interleukin 6; IL-18, interleukin 18; LDL, low density lipoprotein; MCP-1, monocyte chemoattractant protein 1; oxLDL, oxidized low density lipoprotein; PAI-1, plasminogen activator inhibitor 1; PPAR-γ, peroxisome proliferator-activated receptor gamma; TNF-α, tumor necrosis factor alpha; VCAM, vascular cell adhesion molecule.
The purpose of this review is to identify novel biomarkers for inflammation and oxidative stress that can be used for early detection of obesity-related CVD risk. Our aim is to assess which biomarkers warrant further investigation and eventual clinical use for predicting CVD. Nontraditional biomarkers have the potential to aid in understanding the mechanisms for the effect of obesity on cardiovascular outcomes independent of traditional risk factors for CVD (7–10). We performed a literature review of biomarkers associated with obesity and their potential role in cardiovascular risk.
MATERIALS AND METHODS
Articles eligible for inclusion met the following criteria: 1) they discussed nontraditional biomarkers associated with obesity, and 2) they studied biomarkers associated with CVD and CVD risk factors. Since the focus of this review is on nontraditional biomarkers in obesity, the following biomarkers were excluded: total cholesterol, fasting blood glucose, blood pressure, high density lipoprotein cholesterol, triglycerides, and smoking. CVD outcomes comprised cerebrovascular disease (cerebral embolism, thrombosis, and hemorrhage), peripheral arterial disease, coronary heart disease or coronary artery disease, and myocardial infarction. Research papers in the English language published as of mid-2006 were reviewed. Studies were initially identified through the search engine PubMed (32) by using the keywords “obesity” and “biomarker.” Subsequently, combinations of the keywords “obesity,” “biomarker,” “cardiovascular risk,” “adipose tissue,” “adipokine,” “adipocytokine,” and “oxidative stress” were used. Emphasis was placed on biomarkers for inflammation and oxidative stress. The SOURCE database (33) was assessed to gain more insight into the adipocytokines. Online Mendelian Inheritance in Man (34) was used to obtain more information on various genes and phenotypes of interest. Further information was obtained by examining reference lists of relevant articles.
RESULTS
Adipocytokines and inflammatory biomarkers
In addition to energy storage and regulation of endocrine function, adipocytokines can alter inflammatory responses, and they can promote endothelial injury and dysfunction, all of which predispose to atherosclerosis (35). Abdominal (visceral) distribution of adipose tissue is thought to have more detrimental effects than generalized obesity. In fact, visceral obesity is significantly associated with abnormal cytokine secretion and adverse metabolic risk factors (13, 36, 37). Adipocytokines can play a role in the observed link between obesity and its associated morbidities, such as coronary artery disease and insulin resistance (21, 38, 39); this possibility is still under debate (39). This section of the review briefly describes major factors synthesized by adipose tissues that may play a role in obesity-related CVD.
Adipocyte differentiation-related protein
In vitro studies
Adipocyte differentiation-related protein, also referred to as adipophilin, is an adipose-tissue-specific membrane protein. Its mRNA levels are markedly augmented during early adipocyte differentiation in mice (40). The significance of adipocyte differentiation-related protein in obesity lies in its ability to promote the storage of triglycerides (41) and, consequently, fatty liver in humans and mice (42).
Interpretation
There is a deficiency of human and animal studies that investigate the association of this protein with obesity-related morbidity in humans.
Adiponutrin
In vitro studies
This membrane-bound protein functions in triacylglycerol hydrolysis. It is mainly expressed in adipose tissue, and it may play a role in energy homeostasis (33) and membrane trafficking of other adipose proteins (43). As of completion of this review, no Online Mendelian Inheritance in Man information had been published.
In vivo studies
Adiponutrin mRNA expression is increased during early stages of adipocyte differentiation, in mice subjected to starvation and then fed a high-carbohydrate diet, and in genetically obese rats (43).
Human studies
Average adiponutrin expression levels in subcutaneous adipose tissue of humans were not statistically different between obese and nonobese individuals (44). Expression levels, however, were extremely responsive to changes in acute and 21-day energy intake. Adiponutrin did not correlate with adiponectin or leptin mRNA levels, and it showed a negative correlation with fasting glucose and a positive association with insulin sensitivity, while no correlation was observed with triglycerides or other clinical parameters (44). Although these results suggest a role in glucose homeostasis, this latter study was restricted to Caucasian females with a small sample size; thus, further studies in humans are needed.
Interpretation
Given the fact that adiponutrin is exclusive to adipose tissue and is modulated by nutrient intake, it may be beneficial for use in studies of energy homeostasis. Adiponutrin is responsive to obesity in mice (43), implying a possible role in adipogenesis and/or maintenance of a differentiated state. Adiponutrin does not appear to be a measure of adiposity, and studies are lacking regarding its effect on long-term disease risk in humans. Lack of secretion into the plasma does not make it suitable for epidemiologic studies because it may not be easy to measure in the field.
Adiponectin
In vitro studies
The adiponectin gene, or ACDC, ACRP 30, ADIPOQ, and APM1, is exclusively expressed in adipose tissue and encodes a protein similar to complement factor C1q and some collagen molecules. It is highly expressed during adipocyte differentiation (45). Adiponectin levels may be influenced by increased oxidative stress, as demonstrated by inhibition of adiponectin expression and secretion in adipocytes in response to glucose oxidase exposure (46).
In vivo studies
The protective roles of adiponectin include reduction of tissue triglyceride content and inhibition of insulin resistance in diabetic and obese mouse models (47). Adiponectin itself was reduced in obese rodents (48). Given its protective role on the vascular endothelium (49), adiponectin’s reduction in obesity may play a part in obesity-related vascular damage.
Human studies
Plasma levels of adiponectin were inversely associated with body mass index, percentage of body fat, and fasting plasma insulin in different ethnic groups (50–52), and they were increased with a 21 percent reduction in mean body mass index (53). Oxidative stress may play a role in altering adiponectin levels in obesity, since plasma adiponectin was inversely associated with increased oxidative stress as measured by urinary isoprostanes (54). Some studies signified the role of adiponectin in regulating vascular function.
Adiponectin correlated with endothelial-dependent vaso-dilatation and was found to have potent antiinflammatory effects on the cellular components of blood vessel walls (55, 56). Adiponectin negatively correlated with increased carotid intimamedia thickness in obese children (57). Potential mechanisms for the protective role of adiponectin on endothelial cells include inhibiting tumor necrosis factor alpha (TNF-α)–induced expression of cell adhesion molecules (56) and inducing inhibitors of metalloproteinases via interleukin (IL)-10 (58). Implications of adiponectin’s protective role were demonstrated in several studies.
Adiponectin was significantly reduced in patients with coronary artery disease compared with controls matched on age and body mass index (56) after adjustment for other risk factors such as diabetes mellitus, smoking, dyslipidemia, and hypertension (59). It was also associated with a decreased risk of myocardial infarction after adjustment for traditional risk factors, but the risk was attenuated when adjusted for high density lipoprotein and low density lipoprotein cholesterol (60). This latter study was performed among only males employed in health care, which may have introduced some bias. Overall, the above results suggest a link between adiponectin regulation with oxidative stress and increased CVD risk.
Interpretation
Because adiponectin is both a marker and possibly a mediator of CVD, it has a good potential to predict adverse cardiovascular events. The precise target of adiponectin is unknown, and the nature of gender differences in its expression, as reported by some studies (52), needs to be elucidated. Methods commonly used to assess the role of adiponectin in endothelial dysfunction, such as response to reactive hyperemia, may not be specific and require further validation. Since the design of several human studies performed so far is cross-sectional or case-control, there is still a need for prospective assessment of adiponectin’s clinical utility and predictive power.
Resistin
In vitro studies
Resistin is expressed in adipose tissues and is regulated by nutritional status (61, 62). It is reduced during adipogenic differentiation of human-derived adipocytes (63).
In vivo studies
Although circulating resistin was reported to be increased in obesity, its expression in adipose tissue was unchanged (64) in some murine models of obesity compared with lean animals. In contrast, circulating resistin levels were higher in obese mice compared with lean controls (64).
Human studies
Serum resistin correlated with obesity in some adult studies (65, 66); in others, it did not (67). Serum resistin did not differ significantly between obese and nonobese children (68, 69) nor did it change with body mass index over time (70). There is controversy regarding the contribution of resistin to insulin resistance in obesity because some studies find an association while others do not (61, 63, 71). Plasma resistin was associated with inflammatory mediators as well as coronary calcification, an indicator of the degree of atherosclerosis (72). This study was cross-sectional, however, and the temporality of the findings could not be assessed.
Interpretation
Additional studies are needed to elucidate resistin’s biologic function and contribution, if any, to CVD.
IL-6
In vitro studies
IL-6 is an inflammatory marker induced by TNF-α in cultured subcutaneous adipose cells (23). IL-6 regulates lipid metabolism and C-reactive protein production, both of which are known risk factors for CVD (73). IL-6 may also promote insulin resistance. One mechanism by which IL-6 antagonizes insulin action is via inhibition of insulin-stimulated glucose transport (23).
In vivo studies
IL-6-deficient mice develop obesity that is associated with altered carbohydrate and lipid metabolism as well as leptin resistance, and which is partially reversed by IL-6 treatment (74). Intracerebroventricular injection of IL-6 increases energy expenditure in these mice (74) and decreases body weight by 8.4 percent (75), thereby demonstrating that IL-6 can act centrally in regulating energy homeostasis. IL-6 also plays a role in insulin resistance, as demonstrated by increased insulin sensitivity in IL-6-depleted mice (76).
Human studies
IL-6 is increased in obesity and correlates with insulin resistance (73, 77, 78). IL-6 levels are also responsive to weight loss (79); for example, a 30 percent reduction in fat mass is associated with a 25–30 percent decrease in IL-6 (80). Genetic studies show an association of IL-6 with body mass index and fat mass (81) as well as with insulin levels (82). Prospective studies of IL-6 in relation to CVD risk are deficient, thus hindering proper assessment of the role of IL-6 in CVD.
Interpretation
The effects of aberrant IL-6 production in obesity are not fully known but may involve alterations in inflammatory signals or lipid homeostasis.
IL-18
IL-18 is produced by a variety of hemopoietic and nonhemopoietic cells. It induces production of reactive oxygen species, as well as T lymphocytes (Th1 and Th2), natural killer cells, neutrophils, and intracellular adhesion molecule 1 and vascular cell adhesion molecule 1 expression on endothelial cells. IL-18 was found in human atheroma tissues, and it induced the expression of several inflammatory molecules in vascular smooth muscle cells, endothelial cells, and macrophages. Information on regulation of IL-18 synthesis, cytokine release, and mediation of its activities is still not fully known (83).
In vivo studies
IL-18-deficient mice developed obesity because of increased food intake (84). These mice also developed insulin resistance at the level of the liver, muscle, and adipose tissue because of increased glucose production. Replacement of IL-18 in the brain reduced food intake and reversed the hyperglycemia (84).
Human studies
Although IL-18 levels were significantly increased in adipocytes derived from obese individuals (85), levels were significantly reduced following weight loss in individuals with regular (86) and morbid (87) obesity. IL-18 was independently associated with the metabolic syndrome, a known risk factor for CVD, after adjustment for age, gender, body mass index, and insulin (88). In a prospective study, IL-18 was found to be associated with coronary events in males, independent of age, body mass index, inflammatory biomarkers, and classic lipid predictors (89). These findings demonstrate the effect of inflammation and immune modulation on CVD via the actions of IL-18.
Interpretation
Although IL-18 shows promise in predicting CVD, further prospective studies are required. Population-based cutoff values need to be determined.
Leptin
In vitro studies
Leptin regulates the production of cytokines such as IL-6 and TNF-α (90). Leptin also causes hypertrophy of human (91) and rat (92) smooth muscle cells. It signals via the generation of reactive oxygen species (91) as well as stimulation of p38 mitogen activated protein kinase and signal transducers and activators of transcription 3 (93).
In vivo studies
Leptin is produced by adipose tissue as well as the heart (94). By regulating energy expenditure, leptin can regulate lymphocyte survival (95, 96). Its effects on vascular function are demonstrated by its ability to inhibit acetylcholine-induced vasodilatation in animals (97). Animal models further corroborate the association of leptin with cardiovascular abnormalities such as hypertrophy of vascular smooth muscle cells (98), formation of occlusive thrombus (99), and altered myocardial contractility (100).
Human studies
Leptin is also produced by the brain, which is involved in the regulation of body weight (101). It is closely associated with obesity and risk factors for CVD such as increased systolic blood pressure (102). One mechanism by which leptin can increase CVD risk is via increased production of inflammatory markers (27, 101, 103, 104), and it plays a role in the early induction of vascular dysfunction. The latter is suggested by its association with impaired cross-sectional compliance of arteries from diabetic children (105) and impaired arterial distension in adolescents (106). A link with CVD was directly demonstrated in its independent association with hemorrhagic stroke (107) and acute myocardial infarction (108) in prospective studies. Leptin was also positively associated with future development of other CVD outcomes including death from CVD and coronary revascularization after adjustment for age, gender, smoking, prior myocardial infarction, unstable angina, blood pressure, C-reactive protein, ratio of low density lipoprotein to high density lipoprotein cholesterol, insulin resistance, fibrinogen, and number of coronary vessels with significant stenosis (109).
Interpretation
Further work is needed to elucidate the effect of leptin on endothelial cells, and more prospective studies are required. Increasing evidence for its role in modulating immune and vascular function, as well as its association with discrete CVD outcomes in humans, adds support to leptin’s ability to provide significant risk prediction with good clinical utility.
Plasminogen activator inhibitor 1 (PAI-1)
In vitro studies
Cultured adipocytes from PAI-1-deficient mice show enhanced adipocyte differentiation and glucose uptake, whereas PAI-1 overexpression causes inhibition of adipocyte differentiation (110).
In vivo studies
The role of PAI-1 in obesity is reflected by animal models. Evidence from obese mice shows that TNF-α (22) and immobilization-induced stress (111) stimulate the production of PAI-1 in adipose tissues and the heart. Mice that lack PAI-1 are protected from obesity (112), whereas the inhibition of PAI-1 is associated with enhanced cardiac recovery following myocardial infarction (113) and reduced aortic wall thickening as promoted by angiotensin II and a high-salt diet (114) in mice. Paradoxically, PAI-1 inhibition was also associated with increased growth of atherosclerotic plaques in mice predisposed to atherosclerosis (115).
Human studies
Blood levels of PAI-1 correlate with body mass index and increased waist circumference (37, 116, 117) and decrease following weight loss (118, 119). In some studies, PAI-1 increased risk of recurrent myocardial infarction (120), atrial fibrillation (121), and progression of coronary atherosclerosis (122) following myocardial infarction; in other studies, it was not associated with subsequent coronary events (123). One possible mechanism for the association of PAI-1 with CVD could be through enhanced expression and release of PAI-1 from visceral adipose tissue, which is highly associated with adverse metabolic risk factors (124, 125). In fact, PAI-1 is associated with several metabolic risk factors including increased cortisol (126), high triglycerides (127), TNF-α production (21), insulin resistance (128, 129), glucose intolerance (130), low adiponectin (131), and increased oxidative stress (132). PAI-1 was also highly expressed in human atherosclerotic lesions (133). Genetic polymorphisms in PAI-1 have been linked with obesity, insulin resistance, and increased triglycerides in some studies (134, 135) but not in others (136).
Interpretation
The adverse effect of PAI-1 on CVD independent of traditional risk factors remains to be confirmed in longitudinal studies.
TNF-α
In vitro studies
TNF-α is expressed in adipocytes and is associated with obesity (137), adipocyte cell volume (138), and inhibition of glucose uptake in adipocytes from lean individuals (137). The roles of TNF-α in immune regulation are varied and include stimulation of growth factor production from adipose progenitor cells and mesenchymal stem cells (139), regulation of apoptosis (140), and regulation of cytokine expression in adipocytes (141).
In vivo studies
In vivo evidence for the role of TNF-α in immune regulation is demonstrated by its close association with the development of bronchial-associated lymphoid tissue in rats (142) as well as its role in suppressing T-cell proliferation (143). Adipose tissue production of TNF-α was increased in genetically obese mice (144, 145). TNF-α was also found to be increased in muscle (146), but not serum (146) or macrophages (147), from mice with diet-induced obesity. TNF-α was found to mediate insulin resistance in animal models of obesity (148, 149), a finding thought to occur through inhibition of insulin receptor signaling (150). It was also associated with increased visceral fat deposition and insulin resistance following partial removal of subcutaneous adipose tissue from mice (151). Notably, this insulin resistance was reversed by neutralization of TNF-α using a specific antibody (151). Studies in mice deficient for the TNF-α receptor gave conflicting results (152), which showed that TNF-α was not associated with insulin resistance.
Human studies
In some studies (36, 153), secretion of TNF-α was increased in relation to obesity and was reduced following a minimum 10 percent (154) but not 2 percent (30) weight loss. Its release into the circulation was not associated with obesity in other studies (24, 73). This discrepancy may be partly due to decreased cleavage of the membrane form of TNF-α despite increased production in mature adipocytes (145). Although some studies did not find an association between TNF-α and increased insulin resistance (155), other studies found that TNF-α was associated with glucose uptake and insulin resistance (156), partly through increased expression of IL-18 in muscle (157). In addition, TNF-α was found to be increased in nonobese Mexican Americans, who, compared with non-Hispanic Whites, are known to be more predisposed to obesity and subclinical inflammation. These differences were present independent of total adiposity and abdominal fat (158). TNF-α was also found to be associated with von Willebrand factor, a risk factor for CVD, in obese individuals (159) and with stroke severity (160).
Interpretation
Mechnisms for TNF-α’s link with CVD in obesity are still unclear.
Biomarkers for oxidative stress
As shown in the previous section of this review, abnormal regulation of adipocytokines in obesity plays a role in promoting CVD. One mechanism for this abnormal regulation may be through increased oxidative stress. Pharmacologic inhibition of oxidative stress increased plasma adiponectin and decreased adipose tissue expression of other adipocytokines such as PAI-1 and TNF-α in obese mice (144). Therefore, abrogating the effect of increased oxidative stress ameliorated the abnormal regulation of adipocytokines that occurs in obesity. Mice genetically predisposed to atherosclerosis exhibited larger atherosclerotic lesions when rendered vitamin E deficient, thus implying a role for increased oxidative stress in modifying risk of atherosclerosis in susceptible organisms (161). In contrast, mice that overexpressed antioxidant enzymes exhibited fewer atherosclerotic changes (162), demonstrating the beneficial effect of reduced oxidative stress. In addition, increased oxidative stress was associated with increased atherosclerosis in hypercholesterolemic rabbits that received advanced oxidation protein products, compared with controls (163). Human studies further demonstrate the role of oxidative stress in obesity.
In addition to promoting abnormal adipocytokine expression with consequent inflammation, oxidative stress itself may result from the inflammatory changes that occur in obesity, as demonstrated in other chronic inflammatory states including asthma and rheumatic diseases (164, 165). Therefore, a vicious cycle that provokes increased oxidative stress in obesity may exist. Reactive oxygen species that lead to increased oxidative stress can be generated in adipocytes (29) and in other cell types such as leukocytes (30), all of which can be a source of increased oxidative stress in obese humans.
Increased oxidative stress is independently associated with obesity measures including body mass index and waist-hip ratio (20, 166) and improves upon weight loss of at least 2 percent (30). It is also associated with several CVD risk factors including smoking, blood glucose, and hyperlipidemia (20, 166–168). Oxidative stress may also promote endothelial dysfunction, atherogenesis (169–172), and coronary heart disease independent of traditional risk factors (173). Antioxidant supplementation was found to be beneficial in reducing CVD risk in some studies but not others (174–176). Controversial findings may be due to lack of a strong effect of oxidative stress on atherosclerosis itself (177), choice of antioxidant therapy (178), confounding by other dietary and nondietary factors (178), or potentially inadequate evidence for an antioxidant effect of the therapy (179). Moreover, the mechanism for antioxidant action and the applicability of findings in specific population groups is still unclear. This evidence underscores the need to define reliable and noninvasive biomarkers of oxidative stress and to understand their relation with CVD risk factors in order to clarify the role of oxidative stress in development of adverse cardiovascular events.
Oxidized low density lipoproteins (oxLDLs)
In vitro studies
Low density lipoproteins can be oxidized by endothelial cells and macrophages into oxLDL, which is cytotoxic and immunogenic and which may alter coagulation processes as well as gene expression in arterial walls. OxLDL is more readily taken up by macrophages that accumulate on arterial walls; this accumulation strongly contributes to the development of atherosclerotic lesions (180).
In vivo studies
Formation of oxLDL was significantly decreased in aortic segments and smooth muscle cells from mice overexpressing antioxidants. The reverse was found in mice deficient for antioxidants (181). Furthermore, these mice altered the ability of oxLDL to induce apoptosis in aortic smooth muscle cells, a cytotoxic mechanism implicated in atherosclerosis (181). OxLDL also induced cellular damage and irregular electrical activity in ventricular myocytes from guinea pigs (182). These studies demonstrate the potential role of oxLDL in promoting CVD.
Human studies
No significant difference in serum oxLDL was found between obese and nonobese humans (103), but oxLDL was associated with increased waist circumference (183) and increased body mass index (184). A relation between increased oxLDL and insulin resistance was found (184), but this association could be confounded by plasma triglycerides (185). In addition to accumulation on blood vessel walls, another potential mechanism for the role of oxLDL in atherosclerosis is via leptin, since oxLDL was closely associated with plasma leptin levels (184). The role of increased oxLDL in cardiovascular outcomes was demonstrated by its association with ischemic damage in cortical lesions (186) as well as coronary heart disease (187–189). Other studies contradicted these findings, whereby antibodies to oxLDL were not associated with subsequent coronary heart disease (190).
Interpretation
The effect of oxLDL on atherosclerosis is still unclear. To our knowledge, clinical trials aimed at measuring the effectiveness of inhibition of low density lipoprotein oxidation on CVD risk are currently lacking.
F2 isoprostanes
F2 isoprostanes are prostaglandin-like compounds formed from the oxidation of arachidonic acid, a fatty acid found in cell membranes. Although not primary products of lipid oxidation, F2 isoprostanes possess good biomarker properties that make them suitable for use in human and animal studies, such as stability and specificity (191).
In vitro studies
F2 isoprostanes induced cell proliferation and collagen synthesis in rat hepatic cells (192). A direct role for the involvement of oxidative stress in inflammation was shown by the effect of F2 isoprostanes on human macrophages, in which F2 isoprostanes induced the expression of IL-8 and intracellular inflammatory signals (193).
In vivo studies
F2 isoprostanes are used as biomarkers for increased oxidative stress in various animal models including models of allergic lung inflammation (194), increased susceptibility to atherosclerosis (161), and obesity (29).
Human studies
The utility of F2 isoprostanes as biomarkers for increased oxidative stress extends to humans. F2 isoprostanes are significantly increased in obesity (20, 29, 172, 195) and following the infusion of free fatty acids, particularly in obese humans (196). Importantly, F2 isoprostanes are localized to atherosclerotic arteries (197) and show an association with CVD (195, 198, 199). In one study, an F2 isoprostane concentration of ≥ 131 pmol/mmol was positively associated with coronary heart disease in univariate analysis (odds ratio = 27.3, 95 percent confidence interval: 10.4, 71.4) and in two multiple logistic regression models (odds ratio = 19.3, 95 percent confidence interval: 5.4, 68.8 and odds ratio = 30.8, 95 percent confidence interval: 7.7, 124) (200).
Interpretation
Although not the sole by-product of increased oxidation stress, lipid oxidation may be vital in the pathogenesis of CVD. Hence, the search for biomarkers that reflect this process may be beneficial in predicting CVD. F2 isoprostanes are widely used to assess lipid oxidation and have demonstrated their ability to mediate adverse biologic changes in animals and humans. The specificity and sensitivity of F2 isoprostanes in identifying CVD independent of traditional risk factors still needs to be determined. More studies should be performed to validate its use and compare it with other biomarkers in the presence of disease.
Glutathione peroxidase
Glutathione peroxidase and its substrate glutathione are a major antioxidant defense system against increased oxidative stress.
In vitro studies
Glutathione peroxidase is expressed following adipocyte differentiation in bovine and human cells (201) and may be regulated by sex hormones (202). The cellular activity of glutathione peroxidase correlates with resistance to oxidative stress as measured by percentage of cell survival in response to oxidant damage (203).
In vivo studies
Serum glutathione peroxidase is increased in animal models of obesity (204, 205). In support of its role in preventing vascular injury mediated by oxidative stress, mice deficient for cellular glutathione peroxidase exhibit abnormal vascular and myocardial histology including endothelial dysfunction (206, 207).
Human studies
Glutathione peroxidase is present in most mammalian cells including the endothelium, and its expression is affected by smoking and gender (208). Glutathione peroxidase activity is low or undetectable in atherosclerotic plaques in humans (209). Importantly, in prospective studies (210, 211), risk of cardiovascular events decreased with increasing glutathione peroxidase activity independent of other risk factors.
Interpretation
Glutathione peroxidase demonstrates strong antioxidant and antiatherosclerotic properties and shows promise in its ability to protect the vascular endothelium from oxidative stress. Its validity as a predictive biomarker, however, requires further validation.
Angotensin-converting enzyme
In vivo studies
Other biomarkers
Angiotensin-converting enzyme synthesizes angiotensin II, a potent vasoconstrictor. Both increase following exposure to high glucose levels and may play a role in decreasing cellular proliferation, as demonstrated in human mesothelial cells (212).
In vitro studies
An example of the role of angiotensin-converting enzyme in inducing metabolic risk is evident in obese mice. Inhibition of angiotensin-converting enzyme in obese mice reduces PAI-1 levels in the blood and heart and inhibits perivascular fibrosis of coronary arteries (213).
Human studies
Although plasma measurements of angiotensin-converting enzyme are reproducible in humans, they are difficult to interpret because of large interindividual variation (214). Angiotensin-converting enzyme genotype was associated with body mass index in some studies (215) but not others (216). Interestingly, the relation between body mass index and coronary heart disease was strengthened after adjustment for angiotensin-converting enzyme genotype in one study (age- and gender-adjusted odds ratio = 2.63, 95 percent confidence interval: 1.19, 5.82 from odds ratio = 2.22, 95 percent confidence interval: 1.4, 3.6) (215). An insertion/deletion polymorphism was found to influence circulating levels of angiotensin-converting enzyme (214); such polymorphisms may play a role in influencing susceptibility to CVD. Angiotensin-converting enzyme insertion/deletion polymorphisms in different populations were associated with CVD such as coronary heart disease and stroke in some studies (215, 217, 218) but not others (219).
Interpretation
Although there is some evidence for the ability of angiotensin-converting enzyme genotype to influence coronary heart disease risk, more longitudinal studies are required to assess its importance compared with other risk factors for coronary heart disease.
Peroxisome proliferator-activated receptor gamma (PPAR-γ)
In vitro studies
PPAR-γ has adipogenic and insulin-sensitizing actions, as demonstrated by the in vivo actions of thiazolidinediones, which are PPAR-γ agonists that enhance differentiation of pluripotent stem cells to adipocytes in culture (220). PPAR-γ is also expressed in vascular cells and protects the vascular wall by inhibiting migration of both endothelial cells (221) and vascular smooth muscle cells (222).
In vivo studies
One of the first tissues to express PPAR-γ during embryogenesis is adipose tissue. The importance of PPAR-γ in cellular function is evident in mice that lack PPAR-γ activity, which die early before birth (223). Deficiency of PPAR-γ also interferes with cardiac formation and adipose tissue development (223). Because PPAR-γ is involved in adipose tissue development, it may play a role in promoting obesity under certain circumstances. In fact, PPAR-γ antagonists prevent obesity induced by a high-fat diet in mice (224). Furthermore, PPAR-γ inhibition by gene targeting causes reduced fat mass in mice (225).
Human studies
PPAR-γ is activated by fatty acids, lipoprotein-derived products, and lipid-lowering agents. It has antiatherosclerotic and antiinflammatory properties reflected by the actions of PPAR agonists commonly used to correct lipid levels and thus lower cardiovascular risk (226). Genetic polymorphisms in PPAR-γ are associated with body weight in some studies (227–229) but not others (230). PPAR-γ is also positively associated with atherosclerosis (231) and CVD (232) and is inversely associated with serum insulin (104) in obesity.
Interpretation
PPAR-γ has great potential to modulate lipid metabolism and immune responses in blood vessels, but its contribution to cardiovascular risk is not fully elucidated. Human studies on PPAR-γ need to be replicated to ascertain its impact on CVD risk prediction. Thus, it is still early to establish PPAR-γ polymorphisms as biomarkers for CVD in obese individuals.
Complement factor 3
In vitro studies
Complement factor 3 is a component of the complement system that functions in host defense mechanisms such as bactericidal activity, chemotaxis, and increased phagocytosis (233, 234). It is induced during acute inflammation by several factors such as TNF-α (235) and is regulated by proteolytic cleavage (236).
In vivo studies
The role of complement factor 3 in immune regulation is evident in complement factor 3–deficient mice, which develop decreased airway hyper-responsiveness and IL-4 production compared with controls in a mouse model of pulmonary allergy (237). Interestingly, inhibition of IL-6 in mice impairs the production of complement factor 3 as well as the development of complement factor 3–induced autoimmune myocarditis and inflammatory T-cell responses following immunization with a peptide from cardiac tissue (238). Inhibition of complement factor 3 action in mice also reduces the influx of leukocytes to venous grafts (239). Complement factor 3 is strongly associated with obesity in obese rat models (240) partly because of the actions of acylation-stimulating protein, a product of complement factor 3 cleavage derived from adipocytes. Acylation-stimulating protein stimulates glucose uptake and storage of fatty acids (241). The obesity-promoting actions of complement factor 3–derived acylation-stimulating protein may explain why complement factor 3 knockout mice develop reduced body fat and oxygen consumption because of lack of acylation-stimulating protein synthesis (241). Likewise, acylation-stimulating protein knockout mice accumulate less fat and are more sensitive to insulin action (242).
Human studies
Complement factor 3 is associated with obesity in children in the presence of normal lipid profiles (243). The role of complement factor 3 in fat deposition and immune regulation is also demonstrated in complement factor 3–deficient humans, who develop partial lipodystrophy (fat loss) in the upper body (244) as well as immunologic abnormalities (245). Complement factor 3 was significantly correlated with mean body mass index in a cross-sectional study of coronary heart disease patients (246) as well as with metabolic risk factors for CVD such as insulin levels and triglycerides (247, 248). In addition, complement factor 3 was a significant predictor of coronary heart disease after adjustment for age and gender alone (odds ratio = 3.13, 95 percent confidence interval: 1.35, 7.3); after adjustment for age, gender, smoking, total cholesterol, and use of statins (odds ratio = 3.5, 95 percent confidence interval: 1.27, 9.62); and after adjustment for age, log C-reactive protein, and fibrinogen (odds ratio = 2.74, 95 percent confidence interval: 1.03, 7.26). Levels of complement factor 3 of ≥1.6 g/liter were associated with coronary heart disease after adjustment for age, gender, use of statins, systolic blood pressure, total cholesterol, and smoking (odds ratio = 1.89, 95 percent confidence interval: 1.01, 3.58) (246). Complement factor 3 was also better than C-reactive protein in predicting risk of atrial fibrillation (249) and coronary artery disease (250).
Interpretation
Evidence from animal models and humans indicates that complement factor 3 can promote fat deposition and is associated with common risk factors for CVD. Its utility in predicting CVD is growing stronger and in some cases supersedes C-reactive protein. Further population-based studies are required to determine its sensitivity and specificity in predicting CVD in obese individuals.
Monocyte chemoattractant protein 1 (MCP-1)
In vitro studies
MCP-1 plays a role in the recruitment of monocytes to sites of injury and infection (251). Metabolic and inflammatory mediators such as insulin and TNF-α induce MCP-1 expression in murine adipocytes (252).
In vivo studies
MCP-1 is increased in adipose tissue and plasma of obese rodents (252, 253) and can modify expression of adipose genes involved in glucose and fat metabolism (252). Mice that overexpress MCP-1 in adipose tissue develop insulin resistance and glucose intolerance, whereas MCP-1 knockout mice fed a high-fat diet are resistant to these conditions compared with wild-type mice fed the same diet (253). The role of MCP-1 in inflammation and atherosclerosis is demonstrated in some studies. MCP-1 is expressed by vascular endothelial cells and infiltrating macrophages during the early regenerative stages following ischemic injury (254). It localizes to sites of arterial damage in experimental atherosclerosis (255). Deletion of MCP-1 in mice susceptible to atherosclerosis results in a substantial reduction in the formation of atherosclerotic lesions (256).
Human studies
MCP-1 is present in macrophages and endothelial cells of atherosclerotic lesions (257) and is positively associated with obesity and coronary artery disease (258) in humans.
Clinical implications
Studies point to the potential association of MCP-1 with atherosclerosis, fat accumulation, and adverse cardiovascular risk, but prospective evidence for the role of MCP-1 in predicting CVD is still lacking.
CONCLUSIONS
Biomarkers associated with obesity may prove useful for early identification of susceptible individuals, and they may add more value to the attributable risk of developing overt CVD (259, 260). Biomarkers produced from adipose tissue, and those with roles in inflammation and oxidative stress, are increasingly being studied in humans. While some are investigated more than others, the majority lack consistency in their associations with both obesity and CVD.
The actions of biomarkers such as IL-18, angiotensin-converting enzyme genotype, F2 isoprostanes, oxLDL, and glutathione peroxidase are promising. Rigorous prospective evaluation and comparisons with traditional risk factors are required to enable us to determine their predictive power in the population. In addition, population-based cutoff values need to be addressed. Adiponectin is both a biomarker and possibly a mediator of CVD. The precise target of adiponectin is unknown, however, and the reason for gender differences in its expression as reported by some studies (52) needs to be elucidated. Since several human studies performed so far are cross-sectional or case-control, there is still a need for prospective assessment of adoponectin’s clinical utility and predictive power. Measurement methods for TNF-α, as well as site of adipose tissue biopsy, create variability in results across different studies. PAI-1 is an interesting biomarker in obesity because it is related to thrombosis, which can lead to vascular ischemia, but the active form of PAI-1 is unstable and has a half-life of 30 minutes (261). Despite its significance as an independent risk factor in many studies, its predictive power can be surpassed by insulin resistance and possibly other major risk factors. Evidence is increasing for a role of leptin and PPAR-γ in modulating immune responses and lipid metabolism; they may provide significant risk prediction with good clinical utility, but their contribution to cardiovascular risk has not been fully elucidated.
Although promising, the clinical utility of nontraditional biomarkers for obesity-related CVD is still limited mainly by lack of replication of findings and temporal associations. Obesity is an established inflammatory condition. More evidence is needed to demonstrate that oxidative stress is indeed a clinical correlate of obesity and just as strong as inflammation in promoting CVD. Part of the answer to this question lies in the choice of biomarker as well as in teasing out the reasons for lack of effect of antioxidant supplementation in intervention studies. It may be too early for clinicians to utilize some of the above biomarkers, but efforts must continue to validate their use in human populations in order to refine disease classification and personalize treatment modalities. A good example of an emerging biomarker is C-reactive protein, which is now widely used in predicting progression of chronic inflammatory conditions in clinical settings. However, C-reactive protein is not specific to obesity. There is a growing clinical need for biomarkers that are specific to obesity and that predict CVD risk. Studies that determine the therapeutic implications of these biomarkers beyond the prediction of metabolic risk factors or arterial damage are needed. Such nontraditional biomarkers may prove useful in predicting CVD risk, particularly in susceptible subgroups.
ACKNOWLEDGMENTS
This work was funded by National Institute of Environmental Health Sciences institutional grant 5 T32 ES10957. Conflict of interest: none declared.
Abbreviations
- CVD
cardiovascular disease
- IL
interleukin
- MCP-1
monocyte chemoattractant protein 1
- oxLDL
oxidized low density lipoprotein
- PAI-1
plasminogen activator inhibitor 1
- PPAR-γ
peroxisome proliferator-activated receptor gamma
- TNF-α
tumor necrosis factor alpha
REFERENCES
- 1.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM, Graubard BI, Williamson DF, et al. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293:1861–1867. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- 3.Fontaine KR, Redden DT, Wang C, et al. Years of life lost due to obesity. JAMA. 2003;289:187–193. doi: 10.1001/jama.289.2.187. [DOI] [PubMed] [Google Scholar]
- 4.Goodman E, Dolan LM, Morrison JA, et al. Factor analysis of clustered cardiovascular risks in adolescence: obesity is the predominant correlate of risk among youth. Circulation. 2005;111:1970–1977. doi: 10.1161/01.CIR.0000161957.34198.2B. [DOI] [PubMed] [Google Scholar]
- 5.Fung TT, Rimm EB, Spiegelman D, et al. Association between dietary patterns and plasma biomarkers of obesity and cardiovascular disease risk. Am J Clin Nutr. 2001;73:61–67. doi: 10.1093/ajcn/73.1.61. [DOI] [PubMed] [Google Scholar]
- 6.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 7.Wilson PW, D’Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 8.Nag SS, Pearson TA, Ma L, et al. Estimating cholesterol treatment rates among individuals with multiple risk factors and without coronary heart disease. Am J Cardiol. 2005;95:862–864. doi: 10.1016/j.amjcard.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 9.Oser CS, Harwell TS, Strasheim C, et al. Increasing prevalence of cardiovascular risk factors among American Indians in Montana. Am J Prev Med. 2005;28:295–297. doi: 10.1016/j.amepre.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 10.Morabia A, Costanza MC. The obesity epidemic as harbinger of a metabolic disorder epidemic: trends in overweight, hypercholesterolemia, and diabetes treatment in Geneva, Switzerland, 1993–2003. Am J Public Health. 2005;95:632–635. doi: 10.2105/2004.047877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: a comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA. 2001;285:2481–2485. doi: 10.1001/jama.285.19.2481. [DOI] [PubMed] [Google Scholar]
- 12.Warnberg J, Moreno LA, Mesana MI, et al. Inflammatory mediators in overweight and obese Spanish adolescents. The AVENA Study. Int J Obes Relat Metab Disord. 2004;28(suppl 3):S59–S63. doi: 10.1038/sj.ijo.0802809. [DOI] [PubMed] [Google Scholar]
- 13.Lemieux I, Pascot A, Prud’homme D, et al. Elevated C-reactive protein: another component of the atherothrombotic profile of abdominal obesity. Arterioscler Thromb Vasc Biol. 2001;21:961–967. doi: 10.1161/01.atv.21.6.961. [DOI] [PubMed] [Google Scholar]
- 14.Rogowski O, Shapira I, Toker S, et al. Obesity-related correlation between C-reactive protein and the calculated 10-y Framingham Coronary Heart Disease Risk Score. Int J Obes (Lond) 2005;29:772–777. doi: 10.1038/sj.ijo.0802939. [DOI] [PubMed] [Google Scholar]
- 15.Barker DJ, Eriksson JG, Forsen T, et al. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol. 2002;31:1235–1239. doi: 10.1093/ije/31.6.1235. [DOI] [PubMed] [Google Scholar]
- 16.Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003;23:5293–5300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel MS, Srinivasan M. Metabolic programming: causes and consequences. J Biol Chem. 2002;277:1629–1632. doi: 10.1074/jbc.R100017200. [DOI] [PubMed] [Google Scholar]
- 18.Licata G, Scaglione R, Dominguez LJ. Early markers of cardiovascular damage in obese subjects. Nutr Metab Cardiovasc Dis. 1999;9:78–86. [PubMed] [Google Scholar]
- 19.Curat CA, Miranville A, Sengenes C, et al. From blood monocytes to adipose tissue-resident macrophages: induction of diapedesis by human mature adipocytes. Diabetes. 2004;53:1285–1292. doi: 10.2337/diabetes.53.5.1285. [DOI] [PubMed] [Google Scholar]
- 20.Keaney JF, Jr, Larson MG, Vasan RS, et al. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23:434–439. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 21.You T, Yang R, Lyles MF, et al. Abdominal adipose tissue cytokine gene expression: relationship to obesity and metabolic risk factors. Am J Physiol Endocrinol Metab. 2005;288:E741–E747. doi: 10.1152/ajpendo.00419.2004. [DOI] [PubMed] [Google Scholar]
- 22.Samad F, Uysal KT, Wiesbrock SM, et al. Tumor necrosis factor alpha is a key component in the obesity-linked elevation of plasminogen activator inhibitor 1. Proc Natl Acad Sci U S A. 1999;96:6902–6907. doi: 10.1073/pnas.96.12.6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rotter V, Nagaev I, Smith U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J Biol Chem. 2003;278:45777–45784. doi: 10.1074/jbc.M301977200. [DOI] [PubMed] [Google Scholar]
- 24.Hotamisligil GS, Arner P, Caro JF, et al. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stentz FB, Umpierrez GE, Cuervo R, et al. Proinflammatory cytokines, markers of cardiovascular risks, oxidative stress, and lipid peroxidation in patients with hyperglycemic crises. Diabetes. 2004;53:2079–2086. doi: 10.2337/diabetes.53.8.2079. [DOI] [PubMed] [Google Scholar]
- 26.Lee YH, Nair S, Rousseau E, et al. Microarray profiling of isolated abdominal subcutaneous adipocytes from obese vs non-obese Pima Indians: increased expression of inflammation-related genes. Diabetologia. 2005;48:1776–1783. doi: 10.1007/s00125-005-1867-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maachi M, Pieroni L, Bruckert E, et al. Systemic low-grade inflammation is related to both circulating and adipose tissue TNFalpha, leptin and IL-6 levels in obese women. Int J Obes Relat Metab Disord. 2004;28:993–997. doi: 10.1038/sj.ijo.0802718. [DOI] [PubMed] [Google Scholar]
- 28.Clement K, Viguerie N, Poitou C, et al. Weight loss regulates inflammation-related genes in white adipose tissue of obese subjects. FASEB J. 2004;18:1657–1669. doi: 10.1096/fj.04-2204com. [DOI] [PubMed] [Google Scholar]
- 29.Furukawa S, Fujita T, Shimabukuro M, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dandona P, Mohanty P, Ghanim H, et al. The suppressive effect of dietary restriction and weight loss in the obese on the generation of reactive oxygen species by leukocytes, lipid peroxidation, and protein carbonylation. J Clin Endocrinol Metab. 2001;86:355–362. doi: 10.1210/jcem.86.1.7150. [DOI] [PubMed] [Google Scholar]
- 31.Tripathy D, Mohanty P, Dhindsa S, et al. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes. 2003;52:2882–2887. doi: 10.2337/diabetes.52.12.2882. [DOI] [PubMed] [Google Scholar]
- 32.PubMed. National Library of Medicine and the National Institutes of Health. Bethesda, Maryland: 1993. ( www.pubmed.gov) [Google Scholar]
- 33.SOURCE. Genetics Department. Stanford University; Palo Alto, California: 2000. ( http://source.stanford.edu) [Google Scholar]
- 34.OMIM: Online Mendelian Inheritance in Man. Center for Medical Genetics, Johns Hopkins University, Baltimore, Maryland, and National Center for Biotechnology Information, National Library of Medicine. Bethesda, Maryland: 1999. ( http://www.ncbi.nlm.nih.gov/omim/) [Google Scholar]
- 35.Lau DC, Dhillon B, Yan H, et al. Adipokines: molecular links between obesity and atherosclerosis. Am J Physiol Heart Circ Physiol. 2005;288:H2031–H2041. doi: 10.1152/ajpheart.01058.2004. [DOI] [PubMed] [Google Scholar]
- 36.Park HS, Park JY, Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-alpha and IL-6. Diabetes Res Clin Pract. 2005;69:29–35. doi: 10.1016/j.diabres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 37.Mertens I, Van Gaal LF. Visceral fat as a determinant of fibrinolysis and hemostasis. Semin Vasc Med. 2005;5:48–55. doi: 10.1055/s-2005-871741. [DOI] [PubMed] [Google Scholar]
- 38.Bujalska IJ, Kumar S, Stewart PM. Does central obesity reflect “Cushing’s disease of the omentum”? Lancet. 1997;349:1210–1213. doi: 10.1016/S0140-6736(96)11222-8. [DOI] [PubMed] [Google Scholar]
- 39.Pi-Sunyer FX. Comorbidities of overweight and obesity: current evidence and research issues. Med Sci Sports Exerc. 1999;31:S602–S608. doi: 10.1097/00005768-199911001-00019. [DOI] [PubMed] [Google Scholar]
- 40.Eisinger DP, Serrero G. Structure of the gene encoding mouse adipose differentiation-related protein (ADRP) Genomics. 1993;16:638–644. doi: 10.1006/geno.1993.1241. [DOI] [PubMed] [Google Scholar]
- 41.Magnusson B, Asp L, Bostrom P, et al. Adipocyte differentiation-related protein promotes fatty acid storage in cytosolic triglycerides and inhibits secretion of very low-density lipoproteins. Arterioscler Thromb Vasc Biol. 2006;26:1566–1571. doi: 10.1161/01.ATV.0000223345.11820.da. [DOI] [PubMed] [Google Scholar]
- 42.Motomura W, Inoue M, Ohtake T, et al. Up-regulation of ADRP in fatty liver in human and liver steatosis in mice fed with high fat diet. Biochem Biophys Res Commun. 2006;340:1111–1118. doi: 10.1016/j.bbrc.2005.12.121. [DOI] [PubMed] [Google Scholar]
- 43.Baulande S, Lasnier F, Lucas M, et al. Adiponutrin, a transmembrane protein corresponding to a novel dietary- and obesity-linked mRNA specifically expressed in the adipose lineage. J Biol Chem. 2001;276:33336–33344. doi: 10.1074/jbc.M105193200. [DOI] [PubMed] [Google Scholar]
- 44.Liu YM, Moldes M, Bastard JP, et al. Adiponutrin: a new gene regulated by energy balance in human adipose tissue. J Clin Endocrinol Metab. 2004;89:2684–2689. doi: 10.1210/jc.2003-031978. [DOI] [PubMed] [Google Scholar]
- 45.Scherer PE, Williams S, Fogliano M, et al. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 46.Soares AF, Guichardant M, Cozzone D, et al. Effects of oxidative stress on adiponectin secretion and lactate production in 3T3-L1 adipocytes. Free Radic Biol Med. 2005;38:882–889. doi: 10.1016/j.freeradbiomed.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 47.Yamauchi T, Kamon J, Waki H, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 48.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 49.Matsuda M, Shimomura I, Sata M, et al. Role of adiponectin in preventing vascular stenosis. The missing link of adipo-vascular axis. J Biol Chem. 2002;277:37487–37491. doi: 10.1074/jbc.M206083200. [DOI] [PubMed] [Google Scholar]
- 50.Weyer C, Funahashi T, Tanaka S, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 51.Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 52.Kern PA, Di Gregorio GB, Lu T, et al. Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes. 2003;52:1779–1785. doi: 10.2337/diabetes.52.7.1779. [DOI] [PubMed] [Google Scholar]
- 53.Yang WS, Lee WJ, Funahashi T, et al. Weight reduction increases plasma levels of an adipose-derived anti-inflammatory protein, adiponectin. J Clin Endocrinol Metab. 2001;86:3815–3819. doi: 10.1210/jcem.86.8.7741. [DOI] [PubMed] [Google Scholar]
- 54.Nakanishi S, Yamane K, Kamei N, et al. A protective effect of adiponectin against oxidative stress in Japanese Americans: the association between adiponectin or leptin and urinary isoprostane. Metabolism. 2005;54:194–199. doi: 10.1016/j.metabol.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 55.Ouchi N, Kihara S, Funahashi T, et al. Obesity, adiponectin and vascular inflammatory disease. Curr Opin Lipidol. 2003;14:561–566. doi: 10.1097/00041433-200312000-00003. [DOI] [PubMed] [Google Scholar]
- 56.Ouchi N, Kihara S, Arita Y, et al. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473–2476. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- 57.Pilz S, Horejsi R, Moller R, et al. Early atherosclerosis in obese juveniles is associated with low serum levels of adiponectin. J Clin Endocrinol Metab. 2005;90:4792–4796. doi: 10.1210/jc.2005-0167. [DOI] [PubMed] [Google Scholar]
- 58.Kumada M, Kihara S, Ouchi N, et al. Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation. 2004;109:2046–2049. doi: 10.1161/01.CIR.0000127953.98131.ED. [DOI] [PubMed] [Google Scholar]
- 59.Kumada M, Kihara S, Sumitsuji S, et al. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23:85–89. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 60.Pischon T, Girman CJ, Hotamisligil GS, et al. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 61.Steppan CM, Bailey ST, Bhat S, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 62.McTernan PG, McTernan CL, Chetty R, et al. Increased resistin gene and protein expression in human abdominal adipose tissue. J Clin Endocrinol Metab. 2002;87:2407–2410. doi: 10.1210/jcem.87.5.8627. [DOI] [PubMed] [Google Scholar]
- 63.Janke J, Engeli S, Gorzelniak K, et al. Resistin gene expression in human adipocytes is not related to insulin resistance. Obes Res. 2002;10:1–5. doi: 10.1038/oby.2002.1. [DOI] [PubMed] [Google Scholar]
- 64.Lee JH, Bullen JW, Jr, Stoyneva VL, et al. Circulating resistin in lean, obese, and insulin-resistant mouse models: lack of association with insulinemia and glycemia. Am J Physiol Endocrinol Metab. 2005;288:E625–E632. doi: 10.1152/ajpendo.00184.2004. [DOI] [PubMed] [Google Scholar]
- 65.Azuma K, Katsukawa F, Oguchi S, et al. Correlation between serum resistin level and adiposity in obese individuals. Obes Res. 2003;11:997–1001. doi: 10.1038/oby.2003.137. [DOI] [PubMed] [Google Scholar]
- 66.Degawa-Yamauchi M, Bovenkerk JE, Juliar BE, et al. Serum resistin (FIZZ3) protein is increased in obese humans. J Clin Endocrinol Metab. 2003;88:5452–5455. doi: 10.1210/jc.2002-021808. [DOI] [PubMed] [Google Scholar]
- 67.Silha JV, Krsek M, Skrha JV, et al. Plasma resistin, adiponectin and leptin levels in lean and obese subjects: correlations with insulin resistance. Eur J Endocrinol. 2003;149:331–335. doi: 10.1530/eje.0.1490331. [DOI] [PubMed] [Google Scholar]
- 68.Gerber M, Boettner A, Seidel B, et al. Serum resistin levels of obese and lean children and adolescents: biochemical analysis and clinical relevance. J Clin Endocrinol Metab. 2005;90:4503–4509. doi: 10.1210/jc.2005-0437. [DOI] [PubMed] [Google Scholar]
- 69.Zou CC, Liang L, Hong F, et al. Serum adiponectin, resistin levels and non-alcoholic fatty liver disease in obese children. EndocrJ. 2005;52:519–524. doi: 10.1507/endocrj.52.519. [DOI] [PubMed] [Google Scholar]
- 70.Reinehr T, Roth CL, Menke T, et al. Resistin concentrations before and after weight loss in obese children. Int J Obes (Lond) 2006;30:297–301. doi: 10.1038/sj.ijo.0803116. [DOI] [PubMed] [Google Scholar]
- 71.Sentinelli F, Romeo S, Arca M, et al. Human resistin gene, obesity, and type 2 diabetes: mutation analysis and population study. Diabetes. 2002;51:860–862. doi: 10.2337/diabetes.51.3.860. [DOI] [PubMed] [Google Scholar]
- 72.Reilly MR, Lehrke M, Wolfe ML, et al. Resistin is an inflammatory marker of atherosclerosis in humans. Circulation. 2005;111:932–939. doi: 10.1161/01.CIR.0000155620.10387.43. [DOI] [PubMed] [Google Scholar]
- 73.Mohamed-Ali V, Goodrick S, Rawesh A, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82:4196–4200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- 74.Wallenius V, Wallenius K, Ahren B, et al. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med. 2002;8:75–79. doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]
- 75.Wallenius K, Wallenius V, Sunter D, et al. Intracerebroventricular interleukin-6 treatment decreases body fat in rats. Biochem Biophys Res Commun. 2002;293:560–565. doi: 10.1016/S0006-291X(02)00230-9. [DOI] [PubMed] [Google Scholar]
- 76.Klover PJ, Clementi AH, Mooney RA. Interleukin-6 depletion selectively improves hepatic insulin action in obesity. Endocrinology. 2005;146:3417–3427. doi: 10.1210/en.2004-1468. [DOI] [PubMed] [Google Scholar]
- 77.Bastard JP, Jardel C, Bruckert E, et al. Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. J Clin Endocrinol Metab. 2000;85:3338–3342. doi: 10.1210/jcem.85.9.6839. [DOI] [PubMed] [Google Scholar]
- 78.Vozarova B, Weyer C, Hanson K, et al. Circulating interleukin-6 in relation to adiposity, insulin action, and insulin secretion. Obes Res. 2001;9:414–417. doi: 10.1038/oby.2001.54. [DOI] [PubMed] [Google Scholar]
- 79.Bougoulia M, Triantos A, Koliakos G. Plasma interleukin-6 levels, glutathione peroxidase and isoprostane in obese women before and after weight loss. Association with cardiovascular risk factors. Hormones (Athens) 2006;5:192–199. doi: 10.14310/horm.2002.11182. [DOI] [PubMed] [Google Scholar]
- 80.Bruun JM, Verdich C, Toubro S, et al. Association between measures of insulin sensitivity and circulating levels of interleukin-8, interleukin-6 and tumor necrosis factor-alpha. Effect of weight loss in obese men. Eur J Endocrinol. 2003;148:535–542. doi: 10.1530/eje.0.1480535. [DOI] [PubMed] [Google Scholar]
- 81.Huang QY, Shen H, Deng HY, et al. Linkage and association of the CA repeat polymorphism of the IL6 gene, obesity-related phenotypes, and bone mineral density (BMD) in two independent Caucasian populations. J Hum Genet. 2003;48:430–437. doi: 10.1007/s10038-003-0053-z. [DOI] [PubMed] [Google Scholar]
- 82.Berthier MT, Paradis AM, Tchernof A, et al. The interleukin 6-174G/C polymorphism is associated with indices of obesity in men. J Hum Genet. 2003;48:14–19. doi: 10.1007/s100380300002. [DOI] [PubMed] [Google Scholar]
- 83.Gracie JA, Robertson SE, McInnes IB. Interleukin-18. J Leukoc Biol. 2003;73:213–224. doi: 10.1189/jlb.0602313. [DOI] [PubMed] [Google Scholar]
- 84.Netea MG, Joosten LA, Lewis E, et al. Deficiency of interleukin-18 in mice leads to hyperphagia, obesity and insulin resistance. Nat Med. 2006;12:650–656. doi: 10.1038/nm1415. [DOI] [PubMed] [Google Scholar]
- 85.Skurk T, Kolb H, Muller-Scholze S, et al. The proatherogenic cytokine interleukin-18 is secreted by human adipocytes. Eur J Endocrinol. 2005;152:863–868. doi: 10.1530/eje.1.01897. [DOI] [PubMed] [Google Scholar]
- 86.Esposito K, Pontillo A, Ciotola M, et al. Weight loss reduces interleukin-18 levels in obese women. J Clin Endocrinol Metab. 2002;87:3864–3866. doi: 10.1210/jcem.87.8.8781. [DOI] [PubMed] [Google Scholar]
- 87.Schernthaner GH, Kopp HP, Kriwanek S, et al. Effect of massive weight loss induced by bariatric surgery on serum levels of interleukin-18 and monocyte-chemoattractant-protein-1 in morbid obesity. Obes Surg. 2006;16:709–715. doi: 10.1381/096089206777346763. [DOI] [PubMed] [Google Scholar]
- 88.Hung J, McQuillan BM, Chapman CM, et al. Elevated interleukin-18 levels are associated with the metabolic syndrome independent of obesity and insulin resistance. Arterioscler Thromb Vasc Biol. 2005;25:1268–1273. doi: 10.1161/01.ATV.0000163843.70369.12. [DOI] [PubMed] [Google Scholar]
- 89.Blankenberg S, Luc G, Ducimetiere P, et al. Interleukin-18 and the risk of coronary heart disease in European men: the Prospective Epidemiological Study of Myocardial Infarction (PRIME) Circulation. 2003;108:2453–2459. doi: 10.1161/01.CIR.0000099509.76044.A2. [DOI] [PubMed] [Google Scholar]
- 90.Mattioli B, Straface E, Quaranta MG, et al. Leptin promotes differentiation and survival of human dendritic cells and licenses them for Th1 priming. J Immunol. 2005;174:6820–6828. doi: 10.4049/jimmunol.174.11.6820. [DOI] [PubMed] [Google Scholar]
- 91.Li L, Mamputu JC, Wiernsperger N, et al. Signaling pathways involved in human vascular smooth muscle cell proliferation and matrix metalloproteinase-2 expression induced by leptin: inhibitory effect of metformin. Diabetes. 2005;54:2227–2234. doi: 10.2337/diabetes.54.7.2227. [DOI] [PubMed] [Google Scholar]
- 92.Zeidan A, Purdham DM, Rajapurohitam V, et al. Leptin induces vascular smooth muscle cell hypertrophy through angiotensin II- and endothelin-1-dependent mechanisms and mediates stretch-induced hypertrophy. J Pharmacol Exp Ther. 2005;315:1075–1084. doi: 10.1124/jpet.105.091561. [DOI] [PubMed] [Google Scholar]
- 93.Shin HJ, Oh J, Kang SM, et al. Leptin induces hypertrophy via p38 mitogen-activated protein kinase in rat vascular smooth muscle cells. Biochem Biophys Res Commun. 2005;329:18–24. doi: 10.1016/j.bbrc.2004.12.195. [DOI] [PubMed] [Google Scholar]
- 94.Purdham DM, Zou MX, Rajapurohitam V, et al. Rat heart is a site of leptin production and action. Am J Physiol Heart Circ Physiol. 2004;287:H2877–H2884. doi: 10.1152/ajpheart.00499.2004. [DOI] [PubMed] [Google Scholar]
- 95.Papathanassoglou E, El-Haschimi K, Li XC, et al. Leptin receptor expression and signaling in lymphocytes: kinetics during lymphocyte activation, role in lymphocyte survival, and response to high fat diet in mice. J Immunol. 2006;176:7745–7752. doi: 10.4049/jimmunol.176.12.7745. [DOI] [PubMed] [Google Scholar]
- 96.Demas GE, Sakaria S. Leptin regulates energetic tradeoffs between body fat and humoural immunity. Proc Biol Sci. 2005;272:1845–1850. doi: 10.1098/rspb.2005.3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Knudson JD, Dincer UD, Zhang C, et al. Leptin receptors are expressed in coronary arteries, and hyperleptinemia causes significant coronary endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2005;289:H48–H56. doi: 10.1152/ajpheart.01159.2004. [DOI] [PubMed] [Google Scholar]
- 98.Rajapurohitam V, Gan XT, Kirshenbaum LA, et al. The obesity-associated peptide leptin induces hypertrophy in neonatal rat ventricular myocytes. Circ Res. 2003;93:277–279. doi: 10.1161/01.RES.0000089255.37804.72. [DOI] [PubMed] [Google Scholar]
- 99.Bodary PF, Westrick RJ, Wickenheiser KJ, et al. Effect of leptin on arterial thrombosis following vascular injury in mice. JAMA. 2002;287:1706–1709. doi: 10.1001/jama.287.13.1706. [DOI] [PubMed] [Google Scholar]
- 100.Nickola MW, Wold LE, Colligan PB, et al. Leptin attenuates cardiac contraction in rat ventricular myocytes. Role of NO. Hypertension. 2000;36:501–505. doi: 10.1161/01.hyp.36.4.501. [DOI] [PubMed] [Google Scholar]
- 101.Eikelis N, Lambert G, Wiesner G, et al. Extra-adipocyte leptin release in human obesity and its relation to sympathoadrenal function. Am J Physiol Endocrinol Metab. 2004;286:E744–E752. doi: 10.1152/ajpendo.00489.2003. [DOI] [PubMed] [Google Scholar]
- 102.Schutte R, Huisman HW, Schutte AE, et al. Leptin is independently associated with systolic blood pressure, pulse pressure and arterial compliance in hypertensive African women with increased adiposity: the POWIRS study. J Hum Hypertens. 2005;19:535–541. doi: 10.1038/sj.jhh.1001856. [DOI] [PubMed] [Google Scholar]
- 103.Suzuki K, Ito Y, Ochiai J, et al. Relationship between obesity and serum markers of oxidative stress and inflammation in Japanese. Asian Pac J Cancer Prev. 2003;4:259–266. [PubMed] [Google Scholar]
- 104.Krempler F, Breban D, Oberkofler H, et al. Leptin, peroxisome proliferator-activated receptor-gamma, and CCAAT/ enhancer binding protein-alpha mRNA expression in adipose tissue of humans and their relation to cardiovascular risk factors. Arterioscler Thromb Vasc Biol. 2000;20:443–449. doi: 10.1161/01.atv.20.2.443. [DOI] [PubMed] [Google Scholar]
- 105.Atabek ME, Kurtoglu S, Demir F, et al. Relation of serum leptin and insulin-like growth factor-1 levels to intima-media thickness and functions of common carotid artery in children and adolescents with type 1 diabetes. Acta Paediatr. 2004;93:1052–1057. doi: 10.1111/j.1651-2227.2004.tb02717.x. [DOI] [PubMed] [Google Scholar]
- 106.Singhal A, Farooqi IS, Cole TJ, et al. Influence of leptin on arterial distensibility: a novel link between obesity and cardiovascular disease? Circulation. 2002;106:1919–1924. doi: 10.1161/01.cir.0000033219.24717.52. [DOI] [PubMed] [Google Scholar]
- 107.Soderberg S, Ahren B, Stegmayr B, et al. Leptin is a risk marker for first-ever hemorrhagic stroke in a population-based cohort. Stroke. 1999;30:328–337. doi: 10.1161/01.str.30.2.328. [DOI] [PubMed] [Google Scholar]
- 108.Soderberg S, Ahren B, Jansson JH, et al. Leptin is associated with increased risk of myocardial infarction. J Intern Med. 1999;246:409–418. doi: 10.1046/j.1365-2796.1999.00571.x. [DOI] [PubMed] [Google Scholar]
- 109.Wolk R, Berger P, Lennon RJ, et al. Plasma leptin and prognosis in patients with established coronary atherosclerosis. J Am Coll Cardiol. 2004;44:1819–1824. doi: 10.1016/j.jacc.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 110.Liang X, Kanjanabuch T, Mao SL, et al. Plasminogen activator inhibitor-1 modulates adipocyte differentiation. Am J Physiol Endocrinol Metab. 2006;290:E103–E113. doi: 10.1152/ajpendo.00605.2004. [DOI] [PubMed] [Google Scholar]
- 111.Yamamoto K, Kojima T, Adachi T, et al. Obesity enhances the induction of plasminogen activator inhibitor-1 by restraint stress: a possible mechanism of stress-induced renal fibrin deposition in obese mice. J Thromb Haemost. 2005;3:1495–1502. doi: 10.1111/j.1538-7836.2005.01399.x. [DOI] [PubMed] [Google Scholar]
- 112.Ma LJ, Mao SL, Taylor KL, et al. Prevention of obesity and insulin resistance in mice lacking plasminogen activator inhibitor 1. Diabetes. 2004;53:336–346. doi: 10.2337/diabetes.53.2.336. [DOI] [PubMed] [Google Scholar]
- 113.Xiang G, Schuster MD, Seki T, et al. Down-regulation of plasminogen activator inhibitor 1 expression promotes myocardial neovascularization by bone marrow progenitors. J Exp Med. 2004;200:1657–1666. doi: 10.1084/jem.20040221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Weisberg AD, Albornoz F, Griffin JP, et al. Pharmacological inhibition and genetic deficiency of plasminogen activator inhibitor-1 attenuates angiotensin II/salt-induced aortic remodeling. Arterioscler Thromb Vasc Biol. 2005;25:365–371. doi: 10.1161/01.ATV.0000152356.85791.52. [DOI] [PubMed] [Google Scholar]
- 115.Luttun A, Lupu F, Storkebaum E, et al. Lack of plasminogen activator inhibitor-1 promotes growth and abnormal matrix remodeling of advanced atherosclerotic plaques in apolipo-protein E-deficient mice. Arterioscler Thromb Vasc Biol. 2002;22:499–505. doi: 10.1161/hq0302.104529. [DOI] [PubMed] [Google Scholar]
- 116.Mertens I, Van der Planken M, Corthouts B, et al. Is visceral adipose tissue a determinant of von Willebrand factor in overweight and obese premenopausal women? Metabolism. 2006;55:650–655. doi: 10.1016/j.metabol.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 117.Appel SJ, Harrell JS, Davenport ML. Central obesity, the metabolic syndrome, and plasminogen activator inhibitor-1 in young adults. J Am Acad Nurse Pract. 2005;17:535–541. doi: 10.1111/j.1745-7599.2005.00083.x. [DOI] [PubMed] [Google Scholar]
- 118.Kockx M, Leenen R, Seidell J, et al. Relationship between visceral fat and PAI-1 in overweight men and women before and after weight loss. Thromb Haemost. 1999;82:1490–1496. [PubMed] [Google Scholar]
- 119.Jellema A, Plat J, Mensink RP. Weight reduction, but not a moderate intake of fish oil, lowers concentrations of inflammatory markers and PAI-1 antigen in obese men during the fasting and postprandial state. Eur J Clin Invest. 2004;34:766–773. doi: 10.1111/j.1365-2362.2004.01414.x. [DOI] [PubMed] [Google Scholar]
- 120.Hamsten A, de Faire U, Walldius G, et al. Plasminogen activator inhibitor in plasma: risk factor for recurrent myo-cardial infarction. Lancet. 1987;2:3–9. doi: 10.1016/s0140-6736(87)93050-9. [DOI] [PubMed] [Google Scholar]
- 121.Sinkovic A. Plasminogen activator inhibitor-1 in patients with atrial arrhythmias during acute myocardial infarction, treated with streptokinase. Blood Coagul Fibrinolysis. 2002;13:741–747. doi: 10.1097/00001721-200212000-00011. [DOI] [PubMed] [Google Scholar]
- 122.Bavenholm P, de Faire U, Landou C, et al. Progression of coronary artery disease in young male post-infarction patients is linked to disturbances of carbohydrate and lipopro-tein metabolism and to impaired fibrinolytic function. Eur Heart J. 1998;19:402–410. doi: 10.1053/euhj.1997.0752. [DOI] [PubMed] [Google Scholar]
- 123.Thompson SG, Kienast J, Pyke SD, et al. Hemostatic factors and the risk of myocardial infarction or sudden death in patients with angina pectoris. European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group. N Engl J Med. 1995;332:635–641. doi: 10.1056/NEJM199503093321003. [DOI] [PubMed] [Google Scholar]
- 124.Fain IN, Madan AK, Hiler ML, et al. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145:2273–2282. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- 125.Alessi MC, Peiretti F, Morange P, et al. Production of plasminogen activator inhibitor 1 by human adipose tissue: possible link between visceral fat accumulation and vascular disease. Diabetes. 1997;46:860–867. doi: 10.2337/diab.46.5.860. [DOI] [PubMed] [Google Scholar]
- 126.Ayachi SE, Paulmyer-Lacroix O, Verdier M, et al. llbeta-Hydroxysteroid dehydrogenase type 1-driven cortisone reactivation regulates plasminogen activator inhibitor type 1 in adipose tissue of obese women. J Thromb Haemost. 2006;4:621–627. doi: 10.1111/j.1538-7836.2006.01811.x. [DOI] [PubMed] [Google Scholar]
- 127.Mehta J, Mehta P, Lawson D, et al. Plasma tissue plasminogen activator inhibitor levels in coronary artery disease: correlation with age and serum triglyceride concentrations. J Am Coll Cardiol. 1987;9:263–268. doi: 10.1016/s0735-1097(87)80373-x. [DOI] [PubMed] [Google Scholar]
- 128.Romano M, Guagnano MT, Pacini G, et al. Association of inflammation markers with impaired insulin sensitivity and coagulative activation in obese healthy women. J Clin Endo-crinol Metab. 2003;88:5321–5326. doi: 10.1210/jc.2003-030508. [DOI] [PubMed] [Google Scholar]
- 129.Dandona P, Aljada A, Chaudhuri A, et al. Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation. 2005;111:1448–1454. doi: 10.1161/01.CIR.0000158483.13093.9D. [DOI] [PubMed] [Google Scholar]
- 130.Pannacciulli N, De Mitrio V, Marino R, et al. Effect of glucose tolerance status on PAI-1 plasma levels in overweight and obese subjects. Obes Res. 2002;10:717–725. doi: 10.1038/oby.2002.98. [DOI] [PubMed] [Google Scholar]
- 131.Mertens I, Ballaux D, Funahashi T, et al. Inverse relationship between plasminogen activator inhibitor-I activity and adi-ponectin in overweight and obese women. Interrelationship with visceral adipose tissue, insulin resistance, HDL-chol and inflammation. Thromb Haemost. 2005;94:1190–1195. [PubMed] [Google Scholar]
- 132.Ferroni P, Guagnano MT, Manigrasso MR, et al. Increased plasminogen activator inhibitor-1 levels in android obesity: correlation with oxidative stress. J Thromb Haemost. 2005;3:1086–1087. doi: 10.1111/j.1538-7836.2005.01395.x. [DOI] [PubMed] [Google Scholar]
- 133.Schneiderman J, Sawdey MS, Keeton MR, et al. Increased type 1 plasminogen activator inhibitor gene expression in atherosclerotic human arteries. Proc Natl Acad Sci USA. 1992;89:6998–7002. doi: 10.1073/pnas.89.15.6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bensen JT, Hsu FC, Brown WM, et al. Association analysis of the plasminogen activator inhibitor-1 4G/5G polymorphism in Hispanics and African Americans: the IRAS family study. Hum Hered. 2004;57:128–137. doi: 10.1159/000079243. [DOI] [PubMed] [Google Scholar]
- 135.Lopes C, Dina C, Durand E, et al. PAI-1 polymorphisms modulate phenotypes associated with the metabolic syndrome in obese and diabetic Caucasian population. Diabetologia. 2003;46:1284–1290. doi: 10.1007/s00125-003-1170-0. [DOI] [PubMed] [Google Scholar]
- 136.Kinik ST, Atac FB, Verdi H, et al. The effect of plasminogen activator inhibitor-1 gene 4G/5G polymorphism on glucose and lipid metabolisms in Turkish obese children. Clin Endo-crinol (Oxf) 2005;62:607–610. doi: 10.1111/j.1365-2265.2005.02268.x. [DOI] [PubMed] [Google Scholar]
- 137.Good M, Newell FM, Haupt LM, et al. TNF and TNF receptor expression and insulin sensitivity in human omental and subcutaneous adipose tissue—influence of BMI and adipose distribution. Diab Vasc Dis Res. 2006;3:26–33. doi: 10.3132/dvdr.2006.003. [DOI] [PubMed] [Google Scholar]
- 138.Winkler G, Kiss S, Keszthelyi L, et al. Expression of tumor necrosis factor (TNF)-alpha protein in the subcutaneous and visceral adipose tissue in correlation with adipocyte cell volume, serum TNF-alpha, soluble serum TNF-receptor-2 concentrations and C-peptide level. Eur J Endocrinol. 2003;149:129–135. doi: 10.1530/eje.0.1490129. [DOI] [PubMed] [Google Scholar]
- 139.Wang M, Crisostomo PR, Herring C, et al. Human progenitor cells from bone marrow or adipose tissue produce VEGF, HGF, and IGF-I in response to TNF by a p38 MAPK-dependent mechanism. Am J Physiol Regul Integr Comp Physiol. 2006;291:R880–R884. doi: 10.1152/ajpregu.00280.2006. [DOI] [PubMed] [Google Scholar]
- 140.Chen JY, Chi CW, Chen HL, et al. TNF-alpha renders human peritoneal mesothelial cells sensitive to anti-Fas antibody-induced apoptosis. Nephrol Dial Transplant. 2003;18:1741–1747. doi: 10.1093/ndt/gfg275. [DOI] [PubMed] [Google Scholar]
- 141.Ruan H, Hacohen N, Golub TR, et al. Tumor necrosis factor-alpha suppresses adipocyte-specific genes and activates expression of preadipocyte genes in 3T3-L1 adipocytes: nuclear factor-kappaB activation by TNF-alpha is obligatory. Diabetes. 2002;51:1319–1336. doi: 10.2337/diabetes.51.5.1319. [DOI] [PubMed] [Google Scholar]
- 142.Delettieres D, Frecha CA, Roux ME. Early appearance of TNF-alpha and other cytokines in bronchus associated lymphoid tissues (BALT) from growing Wistar rats. What is the role of TNF-alpha? Clin Dev Immunol. 2004;11:253–259. doi: 10.1080/17402520400001686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zganiacz A, Santosuosso M, Wang J, et al. TNF-alpha is a critical negative regulator of type 1 immune activation during intracellular bacterial infection. J Clin Invest. 2004;113:401–413. doi: 10.1172/JCI18991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kurata A, Nishizawa H, Kihara S, et al. Blockade of angio-tensin II type-1 receptor reduces oxidative stress in adipose tissue and ameliorates adipocytokine dysregulation. Kidney Int. 2006;70:1717–1724. doi: 10.1038/sj.ki.5001810. [DOI] [PubMed] [Google Scholar]
- 145.Xu H, Uysal KT, Becherer JD, et al. Altered tumor necrosis factor-alpha (TNF-alpha) processing in adipocytes and increased expression of transmembrane TNF-alpha in obesity. Diabetes. 2002;51:1876–1883. doi: 10.2337/diabetes.51.6.1876. [DOI] [PubMed] [Google Scholar]
- 146.Borst SE, Conover CF. High-fat diet induces increased tissue expression of TNF-alpha. Life Sci. 2005;77:2156–2165. doi: 10.1016/j.lfs.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 147.Bedoui S, Velkoska E, Bozinovski S, et al. Unaltered TNF-alpha production by macrophages and monocytes in diet-induced obesity in the rat. J Inflamm (Lond) 2005;2:2. doi: 10.1186/1476-9255-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cheung AT, Ree D, Kolls JK, et al. An in vivo model for elucidation of the mechanism of tumor necrosis factor-alpha (TNF-alpha)-induced insulin resistance: evidence for differential regulation of insulin signaling by TNF-alpha. Endocrinology. 1998;139:4928–4935. doi: 10.1210/endo.139.12.6336. [DOI] [PubMed] [Google Scholar]
- 149.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 150.Hotamisligil GS, Peraldi P, Budavari A, et al. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271:665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- 151.Ishikawa K, Takahashi K, Bujo H, et al. Subcutaneous fat modulates insulin sensitivity in mice by regulating TNF-alpha expression in visceral fat. Horm Metab Res. 2006;38:631–638. doi: 10.1055/s-2006-954580. [DOI] [PubMed] [Google Scholar]
- 152.Schreyer SA, Chua SC, Jr, LeBoeuf RC. Obesity and diabetes in TNF-alpha receptor-deficient mice. J Clin Invest. 1998;102:402–411. doi: 10.1172/JCI2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Olszanecka-Glinianowicz M, Zahorska-Markiewicz B, Janowska J, et al. Serum concentrations of nitric oxide, tumor necrosis factor (TNF)-alpha and TNF soluble receptors in women with overweight and obesity. Metabolism. 2004;53:1268–1273. doi: 10.1016/j.metabol.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 154.Zahorska-Markiewicz B, Janowska J, Olszanecka-Glinianowicz M, et al. Serum concentrations of TNF-alpha and soluble TNF-alpha receptors in obesity. Int J Obes Relat Metab Disord. 2000;24:1392–1395. doi: 10.1038/sj.ijo.0801398. [DOI] [PubMed] [Google Scholar]
- 155.Koistinen HA, Bastard JP, Dusserre E, et al. Subcutaneous adipose tissue expression of tumour necrosis factor-alpha is not associated with whole body insulin resistance in obese nondiabetic or in type-2 diabetic subjects. Eur J Clin Invest. 2000;30:302–310. doi: 10.1046/j.1365-2362.2000.00625.x. [DOI] [PubMed] [Google Scholar]
- 156.Wybranska I, Malczewska-Malec M, Niedbal S, et al. The TNF-alpha gene Ncol polymorphism at position −308 of the promoter influences insulin resistance, and increases serum triglycerides after postprandial lipaemia in familiar obesity. Clin Chem Lab Med. 2003;41:501–510. doi: 10.1515/CCLM.2003.076. [DOI] [PubMed] [Google Scholar]
- 157.Krogh-Madsen R, Plomgaard P, Moller K, et al. Influence of TNF-alpha and IL-6 infusions on insulin sensitivity and expression of IL-18 in humans. Am J Physiol Endocrinol Metab. 2006;291:E108–E114. doi: 10.1152/ajpendo.00471.2005. [DOI] [PubMed] [Google Scholar]
- 158.Ho RC, Davy KP, Hickey MS, et al. Circulating tumor necrosis factor alpha is higher in non-obese, non-diabetic Mexican Americans compared to non-Hispanic white adults. Cytokine. 2005;30:14–21. doi: 10.1016/j.cyto.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 159.Mousa SA. Elevation of plasma von Willebrand factor and tumor necrosis factor-a in obese subjects and their reduction by the low molecular weight heparin tinzaparin. Int Angiol. 2005;24:278–281. [PubMed] [Google Scholar]
- 160.Zaremba J, Losy J. Early TNF-alpha levels correlate with ischaemic stroke severity. Acta Neurol Scand. 2001;104:288–295. doi: 10.1034/j.1600-0404.2001.00053.x. [DOI] [PubMed] [Google Scholar]
- 161.Terasawa Y, Ladha Z, Leonard SW, et al. Increased atherosclerosis in hyperlipidemic mice deficient in alpha-tocopherol transfer protein and vitamin E. Proc Natl Acad Sci USA. 2000;97:13830–13834. doi: 10.1073/pnas.240462697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yang H, Roberts LJ, Shi MJ, et al. Retardation of atherosclerosis by overexpression of catalase or both Cu/Zn-superoxide dismutase and catalase in mice lacking apolipoprotein E. Circ Res. 2004;95:1075–1081. doi: 10.1161/01.RES.0000149564.49410.0d. [DOI] [PubMed] [Google Scholar]
- 163.Liu SX, Hou FF, Guo ZJ, et al. Advanced oxidation protein products accelerate atherosclerosis through promoting oxidative stress and inflammation. Arterioscler Thromb Vasc Biol. 2006;26:1156–1162. doi: 10.1161/01.ATV.0000214960.85469.68. [DOI] [PubMed] [Google Scholar]
- 164.Dworski R, Roberts LJ2nd, Murray JJ, et al. Assessment of oxidant stress in allergic asthma by measurement of the major urinary metabolite of F2-isoprostane, 15-F2t-IsoP (8-iso-PGF2alpha) Clin Exp Allergy. 2001;31:387–390. doi: 10.1046/j.1365-2222.2001.01055.x. [DOI] [PubMed] [Google Scholar]
- 165.Basu S, Whiteman M, Mattey DL, et al. Raised levels of F(2)-isoprostanes and prostaglandin F(2alpha) in different rheumatic diseases. Ann Rheum Dis. 2001;60:627–631. doi: 10.1136/ard.60.6.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Trevisan M, Browne R, Ram M, et al. Correlates of markers of oxidative status in the general population. Am J Epidemiol. 2001;154:348–356. doi: 10.1093/aje/154.4.348. [DOI] [PubMed] [Google Scholar]
- 167.Ferroni P, Basili S, Falco A, et al. Oxidant stress and platelet activation in hypercholesterolemia. Antioxid Redox Signal. 2004;6:747–756. doi: 10.1089/1523086041361587. [DOI] [PubMed] [Google Scholar]
- 168.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 169.Furnkranz A, Schober A, Bochkov VN, et al. Oxidized phospholipids trigger atherogenic inflammation in murine arteries. Arterioscler Thromb Vasc Biol. 2005;25:633–638. doi: 10.1161/01.ATV.0000153106.03644.a0. [DOI] [PubMed] [Google Scholar]
- 170.Kanani PM, Sinkey CA, Browning RL, et al. Role of oxidant stress in endothelial dysfunction produced by experimental hyperhomocyst(e)inemia in humans. Circulation. 1999;100:1161–1168. doi: 10.1161/01.cir.100.11.1161. [DOI] [PubMed] [Google Scholar]
- 171.Khatri JJ, Johnson C, Magid R, et al. Vascular oxidant stress enhances progression and angiogenesis of experimental atheroma. Circulation. 2004;109:520–525. doi: 10.1161/01.CIR.0000109698.70638.2B. [DOI] [PubMed] [Google Scholar]
- 172.Morrow JD. Is oxidant stress a connection between obesity and atherosclerosis? Arterioscler Thromb Vasc Biol. 2003;23:368–370. doi: 10.1161/01.ATV.0000063107.86298.FD. [DOI] [PubMed] [Google Scholar]
- 173.Stephens JW, Gable DR, Hurel SJ, et al. Increased plasma markers of oxidative stress are associated with coronary heart disease in males with diabetes mellitus and with 10-year risk in a prospective sample of males. Clin Chem. 2006;52:446–452. doi: 10.1373/clinchem.2005.060194. [DOI] [PubMed] [Google Scholar]
- 174.Yusuf S, Dagenais G, Pogue J, et al. Vitamin E supplementation and cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:154–160. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- 175.Stephens NG, Parsons A, Schofield PM, et al. Randomised controlled trial of vitamin E in patients with coronary disease: Cambridge Heart Antioxidant Study (CHAOS) Lancet. 1996;347:781–786. doi: 10.1016/s0140-6736(96)90866-1. [DOI] [PubMed] [Google Scholar]
- 176.Alkhenizan AH, Al-Omran MA. The role of vitamin E in the prevention of coronary events and stroke. Meta-analysis of randomized controlled trials. Saudi Med J. 2004;25:1808–1814. [PubMed] [Google Scholar]
- 177.Bleys J, Miller ER3rd, Pastor-Barriuso R, et al. Vitamin-mineral supplementation and the progression of atherosclerosis: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2006;84:880–887. doi: 10.1093/ajcn/84.4.880. quiz 954-5. [DOI] [PubMed] [Google Scholar]
- 178.Knekt P, Ritz J, Pereira MA, et al. Antioxidant vitamins and coronary heart disease risk: a pooled analysis of 9 cohorts. Am J Clin Nutr. 2004;80:1508–1520. doi: 10.1093/ajcn/80.6.1508. [DOI] [PubMed] [Google Scholar]
- 179.Heinecke JW. Is the emperor wearing clothes? Clinical trials of vitamin E and the LDL oxidation hypothesis. Arterioscler Thromb Vasc Biol. 2001;21:1261–1264. doi: 10.1161/hq0801.095084. [DOI] [PubMed] [Google Scholar]
- 180.Witztum JL, Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest. 1991;88:1785–1792. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Guo Z, Van Remmen H, Yang H, et al. Changes in expression of antioxidant enzymes affect cell-mediated LDL oxidation and oxidized LDL-induced apoptosis in mouse aortic cells. Arterioscler Thromb Vasc Biol. 2001;21:1131–1138. doi: 10.1161/hq0701.092092. [DOI] [PubMed] [Google Scholar]
- 182.Zorn-Pauly K, Schaffer P, Pelzmann B, et al. Oxidized LDL induces ventricular myocyte damage and abnormal electrical activity—role of lipid hydroperoxides. Cardiovasc Res. 2005;66:74–83. doi: 10.1016/j.cardiores.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 183.Weinbrenner T, Schroder H, Escurriol V, et al. Circulating oxidized LDL is associated with increased waist circumference independent of body mass index in men and women. Am J Clin Nutr. 2006;83:30–35. doi: 10.1093/ajcn/83.1.30. quiz 181-2. [DOI] [PubMed] [Google Scholar]
- 184.Porreca E, Di Febbo C, Moretta V, et al. Circulating leptin is associated with oxidized LDL in postmenopausal women. Atherosclerosis. 2004;175:139–143. doi: 10.1016/j.atherosclerosis.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 185.Ho RC, Davy K, Davy B, et al. Whole-body insulin sensitivity, low-density lipoprotein (LDL) particle size, and oxidized LDL in overweight, nondiabetic men. Metabolism. 2002;51:1478–1483. doi: 10.1053/meta.2002.35577. [DOI] [PubMed] [Google Scholar]
- 186.Uno M, Harada M, Takimoto O, et al. Elevation of plasma oxidized LDL in acute stroke patients is associated with ischemic lesions depicted by DWI and predictive of infarct enlargement. Neurol Res. 2005;27:94–102. doi: 10.1179/016164105X18395. [DOI] [PubMed] [Google Scholar]
- 187.Toshima S, Hasegawa A, Kurabayashi M, et al. Circulating oxidized low density lipoprotein levels. A biochemical risk marker for coronary heart disease. Arterioscler Thromb Vasc Biol. 2000;20:2243–2247. doi: 10.1161/01.atv.20.10.2243. [DOI] [PubMed] [Google Scholar]
- 188.Nordin Fredrikson G, Hedblad B, Berglund G, et al. Plasma oxidized LDL: a predictor for acute myocardial infarction? J Intern Med. 2003;253:425–429. doi: 10.1046/j.1365-2796.2003.01128.x. [DOI] [PubMed] [Google Scholar]
- 189.Holvoet P, Vanhaecke J, Janssens S, et al. Oxidized LDL and malondialdehyde-modified LDL in patients with acute coronary syndromes and stable coronary artery disease. Circulation. 1998;98:1487–1494. doi: 10.1161/01.cir.98.15.1487. [DOI] [PubMed] [Google Scholar]
- 190.Erkkila AT, Narvanen O, Lehto S, et al. Antibodies against oxidized LDL and cardiolipin and mortality in patients with coronary heart disease. Atherosclerosis. 2005;183:157–162. doi: 10.1016/j.atherosclerosis.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 191.Montuschi P, Barnes PJ, Roberts LJ., 2nd Isoprostanes: markers and mediators of oxidative stress. FASEB J. 2004;18:1791–1800. doi: 10.1096/fj.04-2330rev. [DOI] [PubMed] [Google Scholar]
- 192.Comporti M, Arezzini B, Signorini C, et al. F2-isoprostanes stimulate collagen synthesis in activated hepatic stellate cells: a link with liver fibrosis? Lab Invest. 2005;85:1381–1391. doi: 10.1038/labinvest.3700332. [DOI] [PubMed] [Google Scholar]
- 193.Scholz H, Yndestad A, Damas JK, et al. 8-isoprostane increases expression of interleukin-8 in human macrophages through activation of mitogen-activated protein kinases. Cardiovasc Res. 2003;59:945–954. doi: 10.1016/s0008-6363(03)00538-8. [DOI] [PubMed] [Google Scholar]
- 194.Talati M, Meyrick B, Peebles RS, Jr, et al. Oxidant stress modulates murine allergic airway responses. Free Radic Biol Med. 2006;40:1210–1219. doi: 10.1016/j.freeradbiomed.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 195.Morrow JD. Quantification of isoprostanes as indices of oxidant stress and the risk of atherosclerosis in humans. Arterioscler Thromb Vasc Biol. 2005;25:279–286. doi: 10.1161/01.ATV.0000152605.64964.c0. [DOI] [PubMed] [Google Scholar]
- 196.Stojiljkovic MP, Lopes HF, Zhang D, et al. Increasing plasma fatty acids elevates F2-isoprostanes in humans: implications for the cardiovascular risk factor cluster. J Hypertens. 2002;20:1215–1221. doi: 10.1097/00004872-200206000-00036. [DOI] [PubMed] [Google Scholar]
- 197.Pratico D, Iuliano L, Mauriello A, et al. Localization of distinct F2-isoprostanes in human atherosclerotic lesions. J Clin Invest. 1997;100:2028–2034. doi: 10.1172/JCI119735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Polidori MC, Pratico D, Savino K, et al. Increased F2 isoprostane plasma levels in patients with congestive heart failure are correlated with antioxidant status and disease severity. J Card Fail. 2004;10:334–338. doi: 10.1016/j.cardfail.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 199.Lin CL, Hsu YT, Lin TK, et al. Increased levels of F2-isoprostanes following aneurysmal subarachnoid hemorrhage in humans. Free Radic Biol Med. 2006;40:1466–1473. doi: 10.1016/j.freeradbiomed.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 200.Schwedhelm E, Bartling A, Lenzen H, et al. Urinary 8-iso-prostaglandin F2alpha as a risk marker in patients with coronary heart disease: a matched case-control study. Circulation. 2004;109:843–848. doi: 10.1161/01.CIR.0000116761.93647.30. [DOI] [PubMed] [Google Scholar]
- 201.Yamasaki T, Tahara K, Takano S, et al. Mechanism of plasma glutathione peroxidase production in bovine adipocytes. Cell Tissue Res. 2006;326:139–117. doi: 10.1007/s00441-006-0194-4. [DOI] [PubMed] [Google Scholar]
- 202.Castellon E, Rioseco H, Rojas J, et al. Glutathione peroxidase activity in cell cultures from different regions of human epididymis. Asian J Androl. 2005;7:33–37. doi: 10.1111/j.1745-7262.2005.00017.x. [DOI] [PubMed] [Google Scholar]
- 203.Matsuo M, Ikeda H, Sugihara T, et al. Resistance of cultured human skin fibroblasts from old and young donors to oxidative stress and their glutathione peroxidase activity. Gerontology. 2004;50:193–199. doi: 10.1159/000078347. [DOI] [PubMed] [Google Scholar]
- 204.Asayama K, Nakane T, Dobashi K, et al. Effect of obesity and troglitazone on expression of two glutathione peroxidases: cellular and extracellular types in serum, kidney and adipose tissue. Free Radic Res. 2001;34:337–347. doi: 10.1080/10715760100300291. [DOI] [PubMed] [Google Scholar]
- 205.Beltowski J, Wojcicka G, Gorny D, et al. The effect of dietary-induced obesity on lipid peroxidation, antioxidant enzymes and total plasma antioxidant capacity. J Physiol Pharmacol. 2000;51:883–896. [PubMed] [Google Scholar]
- 206.Forgione MA, Weiss N, Heydrick S, et al. Cellular glutathione peroxidase deficiency and endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2002;282:H1255–H1261. doi: 10.1152/ajpheart.00598.2001. [DOI] [PubMed] [Google Scholar]
- 207.Forgione MA, Cap A, Liao R, et al. Heterozygous cellular glutathione peroxidase deficiency in the mouse: abnormalities in vascular and cardiac function and structure. Circulation. 2002;106:1154–1158. doi: 10.1161/01.cir.0000026820.87824.6a. [DOI] [PubMed] [Google Scholar]
- 208.Raes M, Michiels C, Remacle J. Comparative study of the enzymatic defense systems against oxygen-derived free radicals: the key role of glutathione peroxidase. Free Radic Biol Med. 1987;3:3–7. doi: 10.1016/0891-5849(87)90032-3. [DOI] [PubMed] [Google Scholar]
- 209.Lapenna D, de Gioia S, Ciofani G, et al. Glutathione-related antioxidant defenses in human atherosclerotic plaques. Circulation. 1998;97:1930–1934. doi: 10.1161/01.cir.97.19.1930. [DOI] [PubMed] [Google Scholar]
- 210.Schnabel R, Lackner KJ, Rupprecht HJ, et al. Glutathione peroxidase-1 and homocysteine for cardiovascular risk prediction: results from the AtheroGene study. J Am Coll Cardiol. 2005;45:1631–1637. doi: 10.1016/j.jacc.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 211.Blankenberg S, Rupprecht HJ, Bickel C, et al. Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. N Engl J Med. 2003;349:1605–1613. doi: 10.1056/NEJMoa030535. [DOI] [PubMed] [Google Scholar]
- 212.Kyuden Y, Ito T, Masaki T, et al. Tgf-betal induced by high glucose is controlled by angiotensin-converting enzyme inhibitor and angiotensin II receptor blocker on cultured human peritoneal mesothelial cells. Perit Dial Int. 2005;25:483–491. [PubMed] [Google Scholar]
- 213.Zaman AK, Fujii S, Sawa H, et al. Angiotensin-converting enzyme inhibition attenuates hypofibrinolysis and reduces cardiac perivascular fibrosis in genetically obese diabetic mice. Circulation. 2001;103:3123–3128. doi: 10.1161/01.cir.103.25.3123. [DOI] [PubMed] [Google Scholar]
- 214.Rigat B, Hubert C, Alhenc-Gelas F, et al. An insertion/ deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest. 1990;86:1343–1346. doi: 10.1172/JCI114844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Riera-Fortuny C, Real JT, Chaves FJ, et al. The relation between obesity, abdominal fat deposit and the angiotensin-converting enzyme gene I/D polymorphism and its association with coronary heart disease. Int J Obes (Lond) 2005;29:78–84. doi: 10.1038/sj.ijo.0802829. [DOI] [PubMed] [Google Scholar]
- 216.Uemura K, Nakura J, Kohara K, et al. Association of ACE I/ D polymorphism with cardiovascular risk factors. Hum Genet. 2000;107:239–242. doi: 10.1007/s004390000358. [DOI] [PubMed] [Google Scholar]
- 217.Slowik A, Turaj W, Dziedzic T, et al. DD genotype of ACE gene is a risk factor for intracerebral hemorrhage. Neurology. 2004;63:359–361. doi: 10.1212/01.wnl.0000130200.12993.0c. [DOI] [PubMed] [Google Scholar]
- 218.Mattu RK, Needham EW, Galton DJ, et al. A DNA variant at the angiotensin-converting enzyme gene locus associates with coronary artery disease in the Caerphilly Heart Study. Circulation. 1995;91:270–274. doi: 10.1161/01.cir.91.2.270. [DOI] [PubMed] [Google Scholar]
- 219.Friedl W, Krempler F, Paulweber B, et al. A deletion polymorphism in the angiotensin converting enzyme gene is not associated with coronary heart disease in an Austrian population. Atherosclerosis. 1995;112:137–143. doi: 10.1016/0021-9150(94)05406-9. [DOI] [PubMed] [Google Scholar]
- 220.Lehmann JM, Moore LB, Smith-Oliver TA, et al. An anti-diabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma) J Biol Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 221.Goetze S, Eilers F, Bungenstock A, et al. PPAR activators inhibit endothelial cell migration by targeting Akt. Biochem Biophys Res Commun. 2002;293:1431–1437. doi: 10.1016/S0006-291X(02)00385-6. [DOI] [PubMed] [Google Scholar]
- 222.Benson S, Wu J, Padmanabhan S, et al. Peroxisome proliferator-activated receptor (PPAR)-gamma expression in human vascular smooth muscle cells: inhibition of growth, migration, and c-fos expression by the peroxisome proliferator-activated receptor (PPAR)-gamma activator troglitazone. Am J Hypertens. 2000;13:74–82. doi: 10.1016/s0895-7061(99)00148-x. [DOI] [PubMed] [Google Scholar]
- 223.Barak Y, Nelson MC, Ong ES, et al. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4:585–595. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- 224.Nakano R, Kurosaki E, Yoshida S, et al. Antagonism of peroxisome proliferator-activated receptor gamma prevents high-fat diet-induced obesity in vivo. Biochem Pharmacol. 2006;72:42–52. doi: 10.1016/j.bcp.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 225.Kubota N, Terauchi Y, Miki H, et al. PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol Cell. 1999;4:597–609. doi: 10.1016/s1097-2765(00)80210-5. [DOI] [PubMed] [Google Scholar]
- 226.Chinetti-Gbaguidi G, Fruchart JC, Staels B. Therapeutical effects of PPAR agonists assessed by biomarker modulation. Biomarkers. 2005;10(suppl l):S30–S36. doi: 10.1080/13547500500216702. [DOI] [PubMed] [Google Scholar]
- 227.Ristow M, Muller-Wieland D, Pfeiffer A, et al. Obesity associated with a mutation in a genetic regulator of adipocyte differentiation. N Engl J Med. 1998;339:953–959. doi: 10.1056/NEJM199810013391403. [DOI] [PubMed] [Google Scholar]
- 228.Cecil JE, Fischer B, Doney AS, et al. The Pro12Ala and C-681G variants of the PPARG locus are associated with opposing growth phenotypes in young schoolchildren. Diabetologia. 2005;48:1496–1502. doi: 10.1007/s00125-005-1817-0. [DOI] [PubMed] [Google Scholar]
- 229.Deeb SS, Fajas L, Nemoto M, et al. A Pro12Ala substitution in PPARgamma2 associated with decreased receptor activity, lower body mass index and improved insulin sensitivity. Nat Genet. 1998;20:284–287. doi: 10.1038/3099. [DOI] [PubMed] [Google Scholar]
- 230.Ghoussaini M, Meyre D, Lobbens S, et al. Implication of the Prol2Ala polymorphism of the PPAR-gamma 2 gene in type 2 diabetes and obesity in the French population. BMC Med Genet. 2005;6:11–18. doi: 10.1186/1471-2350-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Al-Shali KZ, House AA, Hanley AJ, et al. Genetic variation in PPARG encoding peroxisome proliferator-activated receptor gamma associated with carotid atherosclerosis. Stroke. 2004;35:2036–2040. doi: 10.1161/01.STR.0000138784.68159.a5. [DOI] [PubMed] [Google Scholar]
- 232.Pischon T, Pai JK, Manson JE, et al. Peroxisome proliferator-activated receptor-gamma2 P12A polymorphism and risk of coronary heart disease in US men and women. Arterioscler Thromb Vasc Biol. 2005;25:1654–1658. doi: 10.1161/01.ATV.0000171993.78135.7e. [DOI] [PubMed] [Google Scholar]
- 233.Alper CA, Abramson N, Johnston RB, Jr, et al. Studies in vivo and in vitro on an abnormality in the metabolism of C3 in a patient with increased susceptibility to infection. J Clin Invest. 1970;49:1975–1985. doi: 10.1172/JCI106417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Ballow M, Shira JE, Harden L, et al. Complete absence of the third component of complement in man. J Clin Invest. 1975;56:703–710. doi: 10.1172/JCI108141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kawakami Y, Watanabe Y, Yamaguchi M, et al. TNF-alpha stimulates the biosynthesis of complement C3 and factor B by human umbilical cord vein endothelial cells. Cancer Lett. 1997;116:21–26. doi: 10.1016/s0304-3835(97)04737-x. [DOI] [PubMed] [Google Scholar]
- 236.Janssen BJ, Huizinga EG, Raaijmakers HC, et al. Structures of complement component C3 provide insights into the function and evolution of immunity. Nature. 2005;437:505–511. doi: 10.1038/nature04005. [DOI] [PubMed] [Google Scholar]
- 237.Drouin SM, Cony DB, Kildsgaard J, et al. Cutting edge: the absence of C3 demonstrates a role for complement in Th2 effector functions in a murine model of pulmonary allergy. J Immunol. 2001;167:4141–4145. doi: 10.4049/jimmunol.167.8.4141. [DOI] [PubMed] [Google Scholar]
- 238.Eriksson U, Kurrer MO, Schmitz N, et al. Interleukin-6-deficient mice resist development of autoimmune myocarditis associated with impaired upregulation of complement C3. Circulation. 2003;107:320–325. doi: 10.1161/01.cir.0000043802.38699.66. [DOI] [PubMed] [Google Scholar]
- 239.Schepers A, de Vries MR, van Leuven CJ, et al. Inhibition of complement component C3 reduces vein graft atherosclerosis in apolipoprotein E3-Leiden transgenic mice. Circulation. 2006;114:2831–2838. doi: 10.1161/CIRCULATIONAHA.106.619502. [DOI] [PubMed] [Google Scholar]
- 240.Boggs RD, McCumbee WD, Cobbs SL, et al. Increased expression of complement component C3 in the plasma of obese Zucker fa and LA/N fa(f) rats compared with their lean counterparts. Obes Res. 1998;6:361–367. doi: 10.1002/j.1550-8528.1998.tb00364.x. [DOI] [PubMed] [Google Scholar]
- 241.Xia Z, Stanhope KL, Digitale E, et al. Acylation-stimulating protein (ASP)/complement C3adesArg deficiency results in increased energy expenditure in mice. J Biol Chem. 2004;279:4051–4057. doi: 10.1074/jbc.M311319200. [DOI] [PubMed] [Google Scholar]
- 242.Murray I, Sniderman AD, Havel PJ, et al. Acylation stimulating protein (ASP) deficiency alters postprandial and adipose tissue metabolism in male mice. J Biol Chem. 1999;274:36219–36225. doi: 10.1074/jbc.274.51.36219. [DOI] [PubMed] [Google Scholar]
- 243.Cianflone K, Lu H, Smith J, et al. Adiponectin, acylation stimulating protein and complement C3 are altered in obesity in very young children. Clin Endocrinol (Oxf) 2005;62:567–572. doi: 10.1111/j.1365-2265.2005.02260.x. [DOI] [PubMed] [Google Scholar]
- 244.McLean RH, Hoefnagel D. Partial lipodystrophy and familial C3 deficiency. Hum Hered. 1980;30:149–154. doi: 10.1159/000153119. [DOI] [PubMed] [Google Scholar]
- 245.Alper CA, Colten HR, Gear JS, et al. Homozygous human C3 deficiency. The role of C3 in antibody production, C-1s-induced vasopermeability, and cobra venom-induced passive hemolysis. J Clin Invest. 1976;57:222–229. doi: 10.1172/JCI108263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Onat A, Uzunlar B, Hergenc G, et al. Cross-sectional study of complement C3 as a coronary risk factor among men and women. Clin Sci (Lond) 2005;108:129–135. doi: 10.1042/CS20040198. [DOI] [PubMed] [Google Scholar]
- 247.Somani R, Grant PJ, Kain K, et al. Complement C3 and C-reactive protein are elevated in South Asians independent of a family history of stroke. Stroke. 2006;37:2001–2006. doi: 10.1161/01.STR.0000231649.56080.6d. [DOI] [PubMed] [Google Scholar]
- 248.Capuano V, D’Arminio T, La Sala G, et al. The third component of the complement (C3) is a marker of the risk of atherogenesis. Eur J Cardiovasc Prev Rehabil. 2006;13:658–660. doi: 10.1097/01.hjr.0000224485.80349.76. [DOI] [PubMed] [Google Scholar]
- 249.Dernellis J, Panaretou M. Effects of C-reactive protein and the third and fourth components of complement (C3 and C4) on incidence of atrial fibrillation. Am J Cardiol. 2006;97:245–248. doi: 10.1016/j.amjcard.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 250.Ajjan R, Grant PJ, Futers TS, et al. Complement C3 and C-reactive protein levels in patients with stable coronary artery disease. Thromb Haemost. 2005;94:1048–1053. doi: 10.1160/TH05-06-0384. [DOI] [PubMed] [Google Scholar]
- 251.Matsushima K, Larsen CG, DuBois GC, et al. Purification and characterization of a novel monocyte chemotactic and activating factor produced by a human myelomonocytic cell line. J Exp Med. 1989;169:1485–1490. doi: 10.1084/jem.169.4.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci USA. 2003;100:7265–7270. doi: 10.1073/pnas.1133870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Kanda H, Tateya S, Tamori Y, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Shireman PK, Contreras-Shannon V, Reyes-Reyna SM, et al. MCP-1 parallels inflammatory and regenerative responses in ischemic muscle. J Surg Res. 2006;134:145–157. doi: 10.1016/j.jss.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 255.Ohtsuki K, Hayase M, Akashi K, et al. Detection of monocyte chemoattractant protein-1 receptor expression in experimental atherosclerotic lesions: an autoradiographic study. Circulation. 2001;104:203–208. doi: 10.1161/01.cir.104.2.203. [DOI] [PubMed] [Google Scholar]
- 256.Gosling J, Slaymaker S, Gu L, et al. MCP-1 deficiency reduces susceptibility to atherosclerosis in mice that overexpress human apolipoprotein B. J Clin Invest. 1999;103:773–778. doi: 10.1172/JCI5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Takeya M, Yoshimura T, Leonard EJ, et al. Detection of monocyte chemoattractant protein-1 in human atherosclerotic lesions by an anti-monocyte chemoattractant protein-1 monoclonal antibody. Hum Pathol. 1993;24:534–539. doi: 10.1016/0046-8177(93)90166-e. [DOI] [PubMed] [Google Scholar]
- 258.Martinovic I, Abegunewardene N, Seul M, et al. Elevated monocyte chemoattractant protein-1 serum levels in patients at risk for coronary artery disease. Circ J. 2005;69:1484–1489. doi: 10.1253/circj.69.1484. [DOI] [PubMed] [Google Scholar]
- 259.Chambless LE, Folsom AR, Sharrett AR, et al. Coronary heart disease risk prediction in the Atherosclerosis Risk in Communities (ARIC) study. J Clin Epidemiol. 2003;56:880–890. doi: 10.1016/s0895-4356(03)00055-6. [DOI] [PubMed] [Google Scholar]
- 260.Pearson TA. New tools for coronary risk assessment: what are their advantages and limitations? Circulation. 2002;105:886–892. doi: 10.1161/hc0702.103727. [DOI] [PubMed] [Google Scholar]
- 261.Kohler HP, Grant PJ. Plasminogen-activator inhibitor type 1 and coronary artery disease. N Engl J Med. 2000;342:1792–1801. doi: 10.1056/NEJM200006153422406. [DOI] [PubMed] [Google Scholar]