Abstract
Protein kinase A (PKA)-dependent phosphorylation of the cardiac Ca2+ release channel/ryanodine receptor (RyR2) is believed to directly dissociate FKBP12.6 from the channel, causing abnormal channel activation and Ca2+ release. To gain insight into the structural basis of the regulation of RyR2 by PKA, we determined the three-dimensional location of the PKA site S2030. Green fluorescent protein (GFP) was inserted into the wild type (wt) RyR2 and RyR2 mutant, A4860G, after T2023. The resultant GFP-RyR2 fusion proteins, RyR2T2023-GFP and RyR2(A4860G)T2023-GFP, were expressed in HEK293 cells and functionally characterized. Ca2+ release assays revealed that both GFP-RyR2 fusion proteins formed caffeine- and ryanodine-sensitive Ca2+ release channels. Further analyses using [3H]ryanodine binding demonstrated that the insertion of GFP into RyR2 wt after T2023 reduced the sensitivity of the channel to activation by Ca2+ or caffeine. RyR2(A4860G)T2023-GFP was found to be structurally more stable than RyR2T2023-GFP and was subsequently used as a basis for three-dimensional reconstruction. Cryo-electron microscopy and single particle image processing of the purified RyR2(A4860G)T2023-GFP protein revealed the location of the inserted GFP, and hence the S2030 PKA site in domain 4, a region that may be involved in signal transduction between the transmembrane and cytoplasmic domains. Like the S2808 PKA site reported previously, the S2030 site is not located close to the FKBP12.6 binding site mapped previously, indicating that neither of these PKA sites is directly involved in FKBP12.6 binding. Based on the three-dimensional localizations of a number of residues or regions, a model for the subunit organization in the structure of RyR2 is proposed.
Keywords: RyR2, FKBP12.6, Subunit organisation, GFP, Cryo-electron microscopy, PKA
INTRODUCTION
Heart failure (HF) is the leading cause of mortality. A significant proportion of HF patients suffer sudden cardiac death (SCD), which results from aberrant electrical signalling within the heart, leading to arrhythmias and ventricular tachycardia (VT)[1, 2]. In many cases, the cause of SCD can be attributed directly to structural abnormalities caused by HF or other factors. There remain, however, a proportion of individuals who suffer SCD and yet whose hearts present no structural defects [3]. In these patients, VT is believed to be caused by spontaneous Ca2+ release from the sarcoplasmic reticulum (SR), which leads to membrane depolarization or so-called delayed afterdepolarizations (DADs) [1, 2, 4].
In addition to normal depolarization-stimulated Ca2+ release during excitation-contraction coupling, it is known that when the SR Ca2+ content reaches a critical level, spontaneous SR Ca2+ release in the form of Ca2+ waves or Ca2+ oscillations occurs in cardiac cells in the absence of membrane depolarization. Considering its dependence on the SR Ca2+ store, we refer to this depolarization-independent Ca2+ overload-induced SR Ca2+ release as store-overload-induced Ca2+ release (SOICR) [5, 6]. A number of mutations have been identified in RyR2, in familial groups that lower the threshold for SOICR and ultimately cause VT [7]. These mutations give rise to at least two genetic forms of VT: catecholaminergic polymorphic ventricular tachycardia (CPVT) and arrhythmogenic right ventricular dysplasia type 2 (ARVD2) [7]. Patients carrying these mutations often display no symptoms at rest, but under stress or conditions of increased β-adrenergic stimulation, they experience arrhythmias of varying severity. The phosphorylation of RyR2 by protein kinase A (PKA) under stressful conditions via the activation of the β-adrenergic receptor signalling pathway is widely accepted to be an important mediator of these arrhythmias, as well as playing an important role in regulating the channel in non-pathological conditions [8, 9]. PKA has multiple effects on Ca2+ handling within a myocyte, it phosphorylates a host of proteins crucial to the regulation of contraction and relaxation, including L-type Ca2+ channels and phospholamban, in addition to RyR2. The net result of PKA activation is to increase the SR Ca2+ load, SR Ca2+ release, relaxation rate, and ultimately cardiac output [10]. The molecular effects of PKA phosphorylation on the majority of Ca2+ handling proteins have long been established. However, the impact of PKA phosphorylation of RyR2 has remained elusive. We have recently found that the phosphorylation of RyR2 by PKA increases the propensity for SOICR by sensitizing the channel to SR luminal Ca2+ [11]. This effectively reduces the threshold at which Ca2+ is released, leading to SOICR at a lower SR [Ca2+]. Which PKA phosphorylation site on RyR2 is responsible for this effect, however, remains controversial.
Marks and his colleagues have shown that RyR2 is phosphorylated exclusively at Ser-2808 [12]. They have also shown that the mutation S2808A abolishes the effect of PKA on the channel and the S2808D mutation mimics PKA phosphorylated RyR2. They also used S2808A-knock-in mice to illustrate that PKA cannot phosphorylate any additional sites in RyR2 [13]. In contrast, two major PKA phosphorylation sites have been identified in RyR2 by other groups: S2030 and S2808. S2808 was the first site to be characterised, originally as a Ca2+/calmodulin dependent protein kinase II (CaMKII) site by Jones’ group [14, 15]. More recently, S2030 has also been identified as a PKA phosphorylation site [16]. Studies comparing the two sites suggest that S2030 is likely the more important one in terms of regulation of the activity of the RyR2 channel [17]. These studies show that S2808 is heavily phosphorylated at rest and therefore undergoes only a modest change in phosphorylation in response to PKA activation. In contrast, S2030 displays little basal phosphorylation and undergoes robust phosphorylation under PKA stimulation both in vitro and in vivo. Additionally, mutation of S2030 to phosphomimetic glutamic acid increases the activity of the channel, whereas the equivalent mutation of S2808 has little effect on the activity of the channel [11, 18]. Furthermore, a recent study by Valdivia’s group shows that the abolition of the S2808 phosphorylation site in mice does not markedly alter the response of the heart to β-adrenergic stimulation or the single channel properties of RyR2 isolated from these mice [19]. The reasons for these discrepancies regarding PKA phosphorylation of RyR2 are unclear. One possible explanation may be differences in experimental conditions.
To gain structural insight into the mechanism of how the phosphorylation of S2030 by PKA increases the activity of the channel, we determined the three-dimensional location of S2030 in RyR2. We inserted a green fluorescent protein (GFP) tag into the RyR2 molecular sequence after T2023, adjacent to the S2030 PKA phosphorylation site. The GFP-fusion protein was then purified and subjected to cryo-electron microscopy (cryo-EM) and single particle image processing. A three-dimensional reconstruction from the particle images shows that the inserted GFP, and hence the S2030 PKA site, is situated in domain 4, a region that may be involved in signal transduction between the transmembrane and cytoplasmic domains.
MATERIALS AND METHODS
Materials
Restriction endonucleases and DNA modifying enzymes were purchased from New England BioLabs Inc (Ipswich, MA). The anti-RyR and anti-GFP antibodies were obtained from Affinity BioReagents (Golden, CO). Soybean phosphatidylcholine was obtained from Avanti Polar Lipids, Inc (Alabaster, AL). [(3-Cholamidopropyl)-dimethylammonio]-1-propane sulfonate (CHAPS) and other reagents were purchased from Sigma (St. Louis, MO).
Cell culture and DNA Transfection
HEK293 cells were maintained in Dulbecco’s modified Eagle’s medium as described previously [20]. HEK293 cells grown on 100-mm tissue culture dishes for 18–20 hours after subculture were transfected with 12 µg of wild type RyR2 (RyR2 wt), RyR2T2023-GFP, RyR2(A4860G), or RyR2(A4860G)T2023-GFP cDNA using Ca2+ phosphate precipitation [21].
Construction of RyR2T2023-GFP and RyR2(A4860G)T2023-GFP
The cloning and construction of the 15-kb full-length cDNA encoding the mouse cardiac RyR2 sequence has been described previously [22]. The cDNA encoding GFP flanked by Gly-rich spacers and AscI sites was obtained by PCR as described previously [23]. The Asc I site was introduced into the sequence of the RyR2 wt or RyR2(A4860G) construct after T2023 by overlap-extension using the polymerase chain reaction (PCR). The GFP cDNA flanked by AscI sites were inserted into the full-length RyR2 cDNA. The sequences of all PCR fragments and the orientation of the inserted GFP cDNA were verified by DNA sequencing analysis.
Ca2+ release and [3H]ryanodine binding assays
Measurements of free cytosolic Ca2+ concentrations in the transfected HEK293 cells using fluorescent Ca2+ indicator dye fluo-3, and of equilibrium [3H]ryanodine binding to cell lysate were performed as described previously [21].
GST-FKBP12.6 pull-down, immuno-precipitation and Immunoblotting
GST-FKBP12.6 pull-down, immunoprecipitation, and immunoblotting were carried out as described previously [24].
Expression and purification of RyR2(A4860G) and RyR2(A4860G)T2023-GFP
HEK293 cells grown for 24–26 hours after transfection were washed three times with PBS plus 2.5 mM EDTA and harvested in the same solution by centrifugation for 8 min at 700× g in an IEC Centra-CL2 centrifuge. The cell pellets were solubilized in a lysis buffer containing 25 mM Na-PIPES (pH 7.2), 140 mM NaCl, 5 mM EGTA, 2.5 mM dithiothreitol, 1% CHAPS, 0.5% phosphatidylcholine, and a protease inhibitor mixture (2 mM benzamidine, 4 µg/ml leupeptin, 2 µg/ml pepstatin A, 4 µg/ml aprotinin, and 0.75 mM phenylmethylsulfonyl fluoride) and incubated on ice for one hour. Insoluble material was removed by centrifugation at 16,000× g twice in a microcentrifuge at 4°C for 30 min and the cell lysates were collected. Sucrose (400 mM final concentration) and NaCl (400 mM final concentration) were added to the cell lysates. Glutathione-Sepharose beads (100 µl) containing 400 µg of bound glutathione S-transferase (GST)-FKBP12.6 fusion protein were added to the cell lysates and incubated at 4°C with rotation for 2 days. The beads were collected by centrifugation and washed four times with lysis buffer containing 0.5% CHAPS and 0.25% phosphatidylcholine. The RyR2 proteins were eluted from the beads by incubation with 100 mM glutathione in 25 mM Na-PIPES (pH 7.2), 400 mM NaCl, 400 mM sucrose, 1 mM EGTA, 0.2 mM CaCl2, 2.5 mM dithiothreitol, 0.4% CHAPS, 0.16% phosphatidylcholine, 200 mM Tris/HEPES (pH 7.4), and a protease inhibitor mixture, for 15 min. The eluate was aliquoted, frozen in liquid nitrogen, and stored at −80°C.
Fluorescent imaging
To detect the green fluorescence of RyR2T2023-GFP and RyR2(A4860G)T2023-GFP, HEK293 cells were grown on glass coverslips placed in a 100-mm tissue culture dish, and transfected with either RyR2(A4860G)T2023-GFP or RyR2T2023-GFP cDNA as described above. 24 hours after transfection, the coverslips were washed three times with PBS, mounted on a glass slide, and visualized with a Zeiss Axioskop2 fluorescent microscope equipped with a FITC filter set (Chroma) using a 40× Neofluar objective (Zeiss).
Cryo-electron microscopy and image processing
The expression and purification of RyR2(A4860G)T2023-GFP were carried out as described previously [23]. The purified RyR2(A4860G)T2023-GFP was diluted 5–10 fold with EM dilution buffer (20 mM Na.PIPES, pH 7.2, 400 mM KCl, 3 mM EGTA, 0.5% CHAPS, 2 mM DTT, and 2 µg/ml leupeptin). Grids were prepared for cryo-EM according to standard methods [25]. Cryo-EM data collection and image processing were performed as described previously [26].
RESULTS
Insertion of GFP into RyR2 after T2023
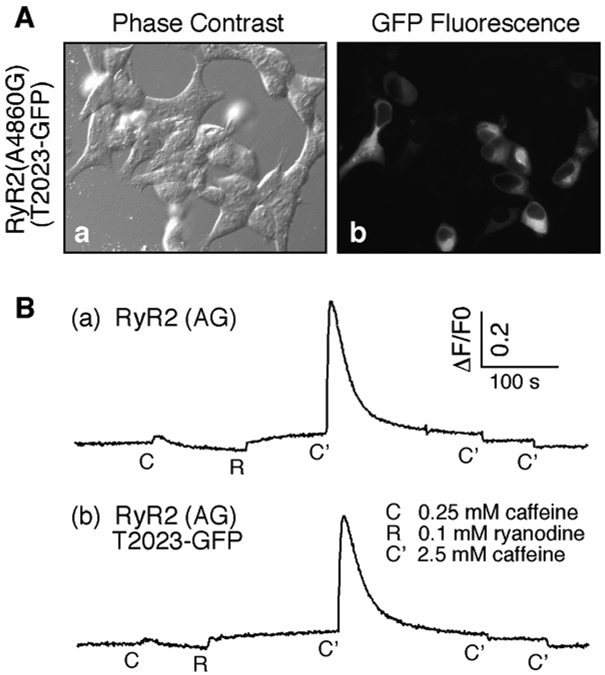
To explore the structural basis of the regulation of RyR2 by PKA-dependent phosphorylation, we used green fluorescent protein (GFP) as a structural marker, to map the three-dimensional location of a major PKA site, S2030. GFP, flanked by glycine linkers so as to minimize potential structural hindrances [27, 28], was inserted into the primary amino acid sequence of RyR2 after residue T2023 (Fig. 1A), creating the fusion protein RyR2T2023-GFP. We have previously shown that, barring any major structural changes, the insertion of GFP close to the region of interest is an effective method for mapping the site in three dimensions [23, 24, 29–32]. To confirm the expression of RyR2T2023-GFP, HEK293 cells were transfected with RyR2T2023-GFP cDNA and imaged using fluorescent microscopy. Fig. 1B shows that RyR2T2023-GFP was readily expressed in HEK293 cells. The presence of the GFP fluorescence also indicates that the protein was folded correctly, as incorrect folding of GFP eliminates the protein’s fluorescence [28, 33].
Fig. 1. Construction and characterization of RyR2T2023-GFP.
(A) Schematic illustration of construction of RyR2T2023-GFP. The open bar represents the RyR’s ~5,000 amino acid sequence. Mutations in the RyR2 sequence implicated in sudden cardiac death are largely clustered in three regions (light gray boxes). The PKA phosphorylation sites, Ser-2030, Ser-2808, and Ser-2814, are indicated as circles (PO4), as is the calmodulin binding site (CaM). GFP flanked by two glycine rich spacers was inserted after Thr-2023 (dark gray band). (B) Bright field (left) and green fluorescence images (right) of HEK293 cells expressing RyR2T2023-GFP. (C) The measurement of Ca2+ release of RyR2 wt and RyR2T2023-GFP following the addition of caffeine and ryanodine. Traces shown are representative of 3 separate experiments.
Response of RyR2T2023-GFP to caffeine, ryanodine, and Ca2+
To confirm that the insertion of GFP at T2023 did not cause any major functional or structural changes, we compared the responses of RyR2T2023-GFP to caffeine and ryanodine to that of RyR2 wt. The response to caffeine was determined by measuring Ca2+ release in HEK293 cells transfected with RyR2T2023-GFP using the Ca2+ indicator dye fluo-3. As shown in Fig. 1C, the insertion of GFP did not markedly alter the response of RyR2T2023-GFP to caffeine. Caffeine stimulation elicited an increase in fluorescence corresponding to the openings of the channels and a release of Ca2+ from intracellular stores. The addition of ryanodine produced a slow increase in fluorescence and diminished the response to repeated caffeine stimulations. This pattern of response is most likely due to binding of ryanodine to the open channel and holding the channel in the open state. The ryanodine-modified “open” channel therefore cannot be further stimulated by caffeine. Alternatively, the application of ryanodine in the presence of caffeine may cause complete store depletion, preventing any response from subsequent additions of caffeine.
To further evaluate the properties of RyR2T2023-GFP, we carried out [3H]ryanodine binding assays. Ryanodine binding reflects the open probability of the channel, since ryanodine can only bind to the channel in the open conformation. As shown in Fig. 2A, RyR2T2023-GFP exhibited a reduced sensitivity to Ca2+ activation relative to RyR2 wt, with an EC50 of 0.32 ± 0.01 µM (mean ± SEM, n=4), significantly higher than the EC50 of the wt (0.18 ± 0.02 µM, n=6)(P < 0.0001). In a similar assay where various concentrations of caffeine were applied (Fig. 2B), RyR2T2023-GFP again displayed a reduced sensitivity to activation by caffeine. The EC50 values for caffeine activation of [3H]ryanodine binding were 7.85 ± 0.44 mM (mean ± SEM, n=3) for RyR2T2023-GFP and 3.44 ± 0.18 mM (n=6) for RyR2 wt (P < 0.0001). We have previously shown that insertions of GFP into other sites (including the other PKA phosphorylation site, S2808) did not significantly alter the sensitivity of the channel to activation by Ca2+ or caffeine [23, 26, 29–32]. The apparent reduction in the sensitivity to both Ca2+ and caffeine activation of RyR2T2023-GFP indicates that the S2030 PKA site lies in a structurally important region of RyR2.
Fig. 2. Ca2+ - and caffeine-dependent activation of RyR2T2023-GFP.
(A) [3H]ryanodine binding to cell lysates prepared from HEK293 cells transfected with RyR2 wt (filled circles) or RyR2T2023-GFP (unfilled circles) was carried out at various concentrations of Ca2+ in the presence of 5 nM [3H]ryanodine. (B) [3H]ryanodine binding to cell lysates prepared from HEK293 cells transfected with RyR2 wt (filled circles) or RyR2T2023-GFP (unfilled circles) was carried out at various concentrations of caffeine in the presence of ~43 nM (pCa = 7.37) Ca2+ in the presence of 5 nM [3H]ryanodine. Concentrations of caffeine are in millimolar. Data shown are mean ± SEM (n = 3–6).
Generation of RyR2(A4860G)T2023-GFP for three-dimensional reconstruction
We have previously shown that the A4860G mutation, located in the inner pore helix of RyR2, results in more structurally homogeneous receptors in cryo-EM than does RyR2 wt, and so this mutant is superior for three-dimensional mapping than RyR2 wt [26, 34]. The RyR2(A4860G) mutant displays a markedly reduced basal activity and response to luminal Ca2+ and Ca2+ overload, suggesting that A4860G stabilizes the closed state of the channel (Jiang et al., in press). We therefore inserted GFP after T2023 in the RyR2(A4860G) background, resulting in a GFP-tagged RyR2(A4860G), RyR2(A4860G)T2023-GFP. Fig. 3A shows that like RyR2T2023-GFP, RyR2(A4860G)T2023-GFP is readily expressed in HEK293 cells. Fig. 3B shows that RyR2(A4860G)T2023-GFP displayed the same response to caffeine and ryanodine as does RyR2(A4860G), suggesting that the insertion of GFP into the RyR2(A4860G) background at T2023 does not grossly affect the function of the channel. Fig. 3B also illustrates the impact of the A4860G mutation on the activity of the channel. The response to low concentrations of caffeine was markedly reduced, compared to that of RyR2 wt (Fig. 1C), as reported previously [34]. As with RyR2T2023-GFP, we further characterized RyR2(A4860G)T2023-GFP by measuring the Ca2+ and caffeine dependence of [3H]ryanodine binding. Fig. 4A shows that the insertion of GFP after T2023 in the RyR2(A4860G) background slightly increased the EC50 value for Ca2+ dependent activation of [3H]ryanodine binding from 0.41 µM ± 0.05 (RyR2(A4860G), mean ± SEM, n=6) to 0.69 ± 0.09 µM (RyR2(A4860G)T2023-GFP, n=3) (P < 0.05). There is also a small but non-significant difference (P > 0.1) in the caffeine dependent activation of [3H]ryanodine binding with EC50 values of 13.1 mM ± 1.13 (n=3) and 17.3 mM ± 4.01 (n=3) for RyR2(A4860G) and RyR2(A4860G)T2023-GFP, respectively (Fig. 4B). The smaller shift in both the Ca2+ and caffeine dependence of [3H]ryanodine binding due to the insertion of GFP in the RyR2(A4860G) background as compared to that in the RyR2 wt background (Fig. 2) likely resulted from the much reduced response of the RyR2(A4860G) mutant to Ca2+ and caffeine.
Fig. 3. Expression and functional characterization of RyR2(A4860G)T2023-GFP.
(A) Bright field (left) and green fluorescence images (right) of HEK293 cells expressing RyR2T2023-GFP. (B) The measurement of Ca2+ release of RyR2(A4860G) and RyR2(A4860G)T2023-GFP following the addition of caffeine and ryanodine. Traces shown are representative of 3 separate experiments.
Fig. 4. Ca2+ - and caffeine-dependent activation of RyR2(A4860G)T2023-GFP.
(A) [3H]ryanodine binding to cell lysates prepared from HEK293 cells transfected with RyR2(A4860G) (filled circles) or RyR2(A4860G)T2023-GFP (unfilled circles) was carried out at various concentrations of Ca2+ in the presence of 5 nM [3H]ryanodine. (B) [3H]ryanodine binding to cell lysates prepared from HEK293 cells transfected with RyR2(A4860G) (filled circles) or RyR2(A4860G)T2023-GFP (unfilled circles) was carried out at various concentrations of caffeine in the presence of ~43 nM (pCa = 7.37) Ca2+ in the presence of 5 nM [3H]ryanodine. Concentrations of caffeine are in millimolar. Data shown are mean ± SEM (n = 3–6).
Purification, cryo-EM, and three-dimensional reconstruction of RyR2(A4860G)T2023-GFP
To obtain sufficient purified protein for cryo-EM, a large number of HEK293 cells (80 plates, 10 cm diameter) were transfected with the RyR2(A4860G)T2023-GFP cDNA. Cell lysates were prepared from which RyR2(A4860G)T2023-GFP proteins were purified by affinity chromatography, with GST-FKBP12.6 used as the affinity ligand [24]. The purified protein was analyzed by SDS-PAGE and Western blotting (Fig. 5A). A single high molecular mass band was detected in the purified sample of RyR2(A4860G)T2023-GFP. Compared with RyR2(A4860G), the RyR2(A4860G)T2023-GFP band migrated more slowly, as expected, due to the additional mass of GFP. The RyR2(A4860G)T2023-GFP band was recognized by both the anti-RyR and anti-GFP antibodies (Fig. 5Aa, 5Ab). On the other hand, the purified RyR2(A4860G) protein was recognized only by the anti-RyR antibody (Fig. 5Aa, 5Ab).
Fig. 5. Immunoblotting and cryo-electron microscopy of purified RyR2(A4860G)T2023-GFP.
(A) RyR2(A4860G)T2023-GFP protein was purified from cell lysate by affinity chromatography using GST-FKBP12.6 as the affinity ligand. The purified RyR2(A4860G)T2023-GFP and RyR2(A4860G) proteins were solubilized, separated in 6% SDS-PAGE, and transferred to nitrocellulose membranes. The membrane was probed either with an anti-RyR antibody or an anti-GFP antibody for Western blotting. (B) A portion of a cryo-EM micrograph of the purified RyR2(A4860G)T2023-GFP proteins embedded in a thin layer of vitreous ice is shown. Several individual RyR2(A4860G)T2023-GFP particles are marked with white circles. The scale bar represents 500 Å.
The purified RyR2(A4860G)T2023-GFP proteins were diluted in a lipid-and sucrose-free buffer. This was applied to carbon coated EM grids, which were rapidly frozen in liquid ethane. Images were then recorded using cryo-EM. Fig. 5B show a typical electron micrograph of frozen hydrated RyR2(A4860G)T2023-GFP. Individual apparently intact channels, as compared to previous studies using channels from natural sources, were observed [35, 36]. Through selection of particles with their 4-fold symmetry axes oriented perpendicular to the carbon support film, and alignment of the images by cross-correlation, two-dimensional images were formed (Fig. 6). Overall, the two dimensional image of RyR2(A4860G)T2023-GFP correlated closely with that of RyR2(A4860G) reported previously [26], apart from the presence of additional small regions of density. To aid in identifying the subtle differences between RyR2(A4860G)T2023-GFP and RyR2(A4860G), a difference map was created (Fig. 6C) by subtraction of the structure of RyR2(A4860G) from that of RyR2(A4860G)T2023-GFP. The brightest areas shown in Fig. 6D represent protein mass present in RyR2(A4860G)T2023-GFP, but not in RyR2(A4860G). These areas of extra mass were located in regions that likely correspond to domain 3 or domain 4 in the three-dimensional structure of RyR2. Statistical analysis revealed that these regions showed significant differences at the 99.9% confidence level. These differences almost certainly correspond to the additional mass of GFP, an interpretation confirmed (see below) by more precise mapping of the location of GFP within the three-dimensional structure.
Fig. 6. Two-dimensional averages of RyR2(A4860G)T2023-GFP and RyR2(A4860G).
(A) Two-dimensional average of RyR2(A4860G)T2023-GFP (n = 245 particle images) "top" view; (B) Top view of the two-dimensional average of RyR2(A4860G) (n = 266 particle images). (C) Difference map obtained by subtraction of B from A. The top view represents the projection of the channel as seen from the cytoplasmic side. The largest differences shown in (C) corresponding to the additional masses due to the insertion of GFP, are seen as bright white areas, one of which is circled. (D) Map of statistically significant regions of difference obtained by t test; map is displayed at >99.9% confidence level. The width of each frame is 544 Å.
Three-dimensional reconstructions were obtained by a projection matching procedure [37]. The final three-dimensional reconstructions of RyR2(A4860G) and RyR2(A4860G)T2023-GFP were computed from 7,591 and 9,203 particles, respectively. Four-fold symmetry was enforced in both reconstructions. The final resolution was estimated to be 27Å by Fourier shell correlation (with a cut-off of 0.5) for both RyR2(A4860G) and RyR2(A4860G)T2023-GFP [38]. The difference map was calculated by subtraction of the three-dimensional volume of RyR2(A4860G) from that of RyR2(A4860G)T2023-GFP.
Figures 7A and B show surface representations of the three-dimensional reconstructions of RyR2(A4860G) and RyR2(A4860G)T2023-GFP. The reconstructions reveal the distinctive mushroom shape of RyR2, consisting of a large cytoplasmic assembly with at least 10 distinct domains and the smaller transmembrane domain assembly (labelled 1–10 and TA, respectively) [35]. As expected from the two-dimensional data, the two reconstructions are highly comparable; however, closer inspection reveals small, significant differences. As an aid to better enable visualization of these differences Fig. 7C displays the threshold difference map obtained by subtraction of the three-dimension reconstruction of RyR2(A4860G) from that of RyR2(A4860G)T2023-GFP and superimposing the differences onto RyR2(A4860G). The differences, shown in green, are present on each of the four identical monomers. The difference map shows that the inserted GFP is located in domain 4. We are confident that the these differences maxima correspond to GFP, since they were the only significant disparities between the structures obtained for RyR2(A4860G)T2023-GFP and RyR2(A4860G). Moreover, the volume of these additions corresponds to a molecular mass of 28 kDa (assuming a protein density of 1.37 g/cm3), [35] a value that agrees well with the molecular mass of GFP. Furthermore, the locations of the GFP in the three-dimensional reconstruction are consistent with those in the two-dimensional projections (Fig. 6). Based on these results, we conclude that the S2030 PKA phosphorylation site is located in domain 4 in the cytoplasmic assembly of the RyR2 structure.
Fig. 7. Three-dimensional surface representations and difference maps of RyR2(A4860G)T2023-GFP and RyR2(A4860G).
The three-dimensional reconstruction of RyR2(A4860G) is shown in blue and RyR2(A4860G)T2023-GFP is shown in green. The difference map [RyR2(A4860G)T2023-GFP minus RyR2(A4860G)] shown in green is superimposed on the three-dimensional reconstruction of RyR2(A4860G) (blue). The three-dimensional reconstructions are shown in three views: left, top view from the cytoplasmic surface, which in situ would face the transverse tubule; middle, side view; right, view toward the bottom of the channel (i.e. as it would appear if viewed from the lumen of the sarcoplasmic reticulum). The numbers on the cytoplasmic assembly indicate the distinguishable domains.
DISCUSSION
We have recently demonstrated that RyR2 is phosphorylated by PKA at two major sites, S2030 and S2808 [16], and that S2030, rather than S2808, is the major site responding to acute β-adrenergic stimulation [17]. In line with these observations, mutation of the S2808 site did not prevent activation of the channel by PKA [18]. On the other hand, mutation of the S2030 site diminished the effect of PKA [11]. Furthermore, recent in vivo studies have revealed that abolition of the S2808 phosphorylation site in mice has no significant impact on the response of RyR2 to PKA phosphorylation or β-adrenergic stimulation at the single channel, cardiac myocytes, and intact heart levels [19]. More recently, we have demonstrated that the phosphorylation of RyR2 at S2030 by PKA sensitizes the channel to activation by luminal Ca2+ and increases the propensity for SOICR [11]. Given the strong correlation between SOICR and triggered arrhythmias, it is likely that the phosphorylation of RyR2 by PKA plays an important role in stress-induced arrhythmias. Taken together, these observations suggest that S2030 is the major site in RyR2 that mediates the effect of PKA-dependent phosphorylation. However, the molecular mechanism by which PKA phosphorylation at S2030 enhances luminal Ca2+ activation and SOICR is unknown. The objective of the current study was to map the location of S2030 onto the three-dimensional structure of RyR2, in an attempt to gain a better understanding of how the phosphorylation of this site affects the activity of the channel.
Localization of the PKA phosphorylation site S2030 to domain 4 and its functional implication
To map the three-dimensional location of the S2030 site, we inserted GFP into the RyR2 molecular sequence after T2023. The GFP-tagged RyR2 protein was expressed, purified, and imaged using cryo-EM, after which its structure was reconstructed using single particle imaging analysis. The inserted GFP, and hence the S2030 site, was found to lie in domain 4 within the cytoplasmic assembly of the RyR2 structure. Little is known about the corresponding amino acid sequence and functional role of domain 4, since no sites have previously been mapped within this region. Based on the RyR1 structure reported recently at ~10 Å resolution [39, 40], domain 4 is situated between domain 4a, which forms a column extending into the transmembrane assembly (channel-gating region), and domain 3, which forms the “handle” structure that extends into the “clamp” structure at the corner of the cytoplasmic assembly. Interestingly, the binding sites of previously mapped RyR modulators (e.g. FKBP and CaM), and disease-linked RyR2 mutation sites were located in the clamp and handle structures, suggesting that these structures are important for channel regulation and gating. It has been shown that both the transmembrane and clamp regions undergo large conformational changes between the open and closed states of the channel [41]. It is possible that domain 4 forms part of the signalling pathway, conveying signals to and from the channel-regulating (i.e. the clamp region) and channel-gating (i.e. the transmembrane assembly) domains. Accordingly, PKA-dependent phosphorylation of S2030 located in domain 4 may affect the gating of the channel by modulating such communication between the channel-gating and -regulating domains. In this regard, it is of interest to note that the binding site for CaM was localized to a region between domains 3 and 4 [42], suggesting that the interaction between domains 3 and 4 may be another critical area of modulation. In support of this view, the sites of imperatoxin A binding [43] and divergent region I [23] were also mapped to this region.
The functional significance of domain 4 was further indicated by the observation that the insertion of GFP into this domain altered the response of the RyR2 channel to Ca2+ and caffeine. We have previously inserted GFP into multiple regions within the primary sequence of RyR2 [23, 24, 26, 29–32]. None of these insertions has resulted in any significant alterations in the channel’s function. The insertion of GFP after T2023, however, resulted in a channel with a reduced sensitivity to both Ca2+ and caffeine activation, despite the fact that GFP was flanked with glycine spacers, in order to ease the accommodation of the moiety, and to minimise potential perturbations to the structure. These data suggest that T2023, and therefore the S2030 site, must lie within a structurally confined and functionally important region of RyR2. This implies that domain 4 and its interactions with neighbouring domains are important in the gating of the channel or channel sensitivity to stimuli. Further functional and structural characterizations of additional GFP insertions near T2023 should provide more definitive information about the functional significance and sequence composition of domain 4.
The S2030 PKA site is not close to the FKBP12.6 binding site
Marks’ group showed that phosphorylation of RyR2 by PKA induced dissociation of FKBP12.6 from the channel [44]. They proposed that the phosphorylation of S2808 by PKA was directly responsible for dissociation, through an electrostatic repulsion between the phosphorylated S2808 and a negatively charged residue in the FKBP12.6 molecule. However, we have recently obtained direct structural evidence indicating that this is not the case [26]. We found that the S2808 PKA site was located in domain 6, far away from the previously mapped FKBP12.6 binding site in domain 9 near its junction with domain 3, indicating that a direct interaction between FKBP12.6 and S2808 is unlikely. In the present study, we have localized the other PKA site in RyR2, S2030, to domain 4, which is also not in close proximity to the FKBP12.6 binding site. Again and as for S2808, a direct interaction between S2030 and FKBP12.6 is unlikely, which is consistent with our previous observation that stoichiometric phosphorylation of recombinant or native RyR2 by PKA does not dissociate FKBP12.6 [45].
A proposed model for RyR2 subunit organization
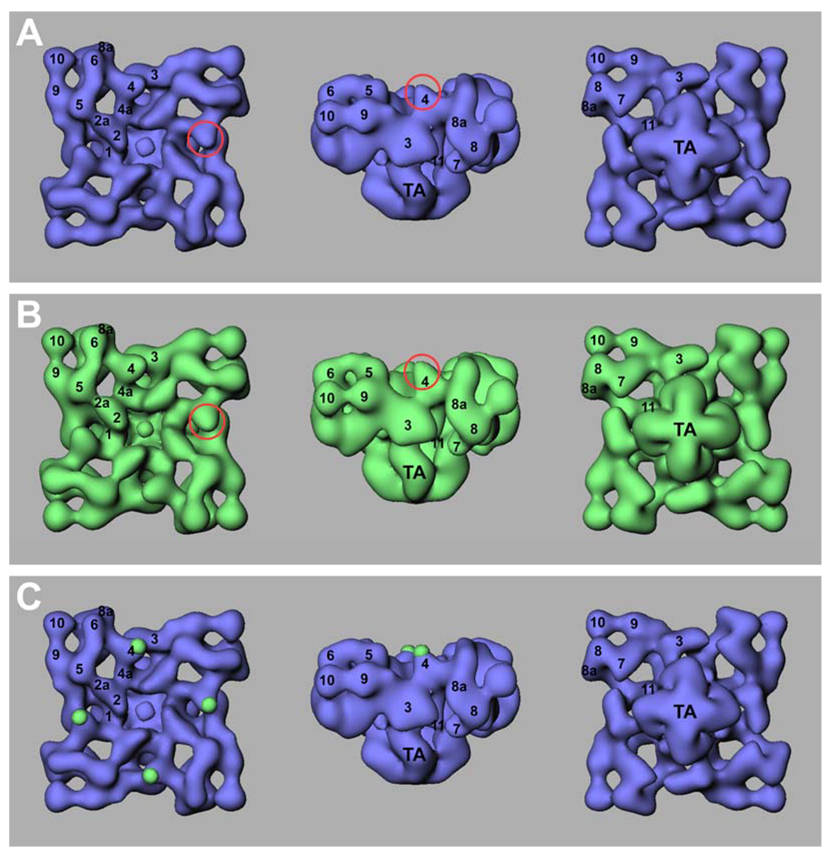
RyRs are tetrameric structures composed of four identical subunits. Three-dimensional reconstruction of RyRs has provided increasingly detailed information about the organization of the internal cytoplasmic domains and the transmembrane assembly [39, 40]. However, the best resolution to date (~10 Å) is still too low to unambiguously reveal the organization and boundaries of the RyR subunits. Alternatively, we may be able to gain some clues to the organization of its subunits by mapping specific residues or sequences that are distributed along the linear sequence of RyR2 onto the three-dimensional structure. To this end, a number of residues or sequences have been mapped in the three-dimensional structure of RyR (Fig. 8). These include (1) the N-terminus, mapped near to the center of the clamp in a region surrounded by domains 5, 6, 9, and 10 [32]; (2) residue S437, located within the N-terminal MH/CPVT region, mapped between domains 5 and 9 [29]; (3) residue Y846, also located in the N-terminal MH/CPVT region, mapped within domain 9 [46]; (4) residue T1366, located within the divergent region II (DRII), mapped to domain 8a [24]; (5) the FKBP binding site (residues 1815–1855), mapped to domain 9; (6) residue T1874, located in DRIII, mapped to domain 9 [31]; (7) residue T2023, mapped to domain 4 (this study); (8) residue S2367, within the central MH/CPVT region, mapped between domains 5 and 6 [30]; (9) an anti-RyR antibody (34c) epitope (residues 2722–2769), mapped between domains 6 and 8a; (10) residue Y2801, corresponding to the S2808 phosphorylation site, mapped to domain 6 [26]; (11) the CaM binding site (residues 3614–3643), mapped to domain 3 near domain 4 [42]; (12) residue D4365, within DRI, mapped to domain 3 [23]; and (13) an anti-RyR antibody epitope (residues 4425–4554), mapped to domain 3 [47]. Based on the distribution of these mapped residues or sequences, the linear amino acid sequence of RyR can be divided into three segments (Fig. 8): (I) residues 1–1874, encompassing green circles 1–6; (II) residues 2023–2801, including green circles 7–10, and (III) residue 3614–4554, encompassing green circles 11–13. As seen in Fig. 8A, the N-terminal segment (residues 1–1874, green circles 1–6) is located at one side of the clamp structure that forms each of the corners of the cytoplasmic assembly, whereas the central segment (residues 2023–2801, green circles 7–10) is located in domains 4 and 6, which forms the other side of the clamp structure. The C-terminal segment (3614–4554, green circles 11–13) is located in domain 3, which forms the side of the cytoplasmic assembly. The mapped residues or sequences within each segment are closely grouped, suggesting that these segments are likely to form multiple interconnected domains. However, how these three segments are linked together to form one subunit in the three-dimensional structure of the tetrameric RyR2 is unclear.
Fig. 8. Three-dimensional surface representations illustrating all previously mapped locations and proposed models of subunit organization.
Panel A shows the three-dimensional structure of RyR with top view (T-tubule face) (a), side view (b), and bottom view (SR face) (c) [39], and all of the previously mapped sites indicated in green circles. These mapped sites are (1) the N-terminus, (2) residue S437, (3) residue Y846, (4) residue T1366, (5) the FKBP binding site (residues 1815–1855), (6) residue T1874, (7) residue T2023, (8) residue S2367, (9) an anti-RyR antibody (34c) epitope (residues 2722–2769), (10) residue Y2801, (11) the CaM binding site (residues 3614–3643), (12) residue D4365, and (13) an anti-RyR antibody epitope (residues 4425–4554). The numbers in blue or red indicate subdomains of RyR2. Panel B shows three possible models (a, b, and c) of subunit organization. The top view of the three-dimensional structure of RyR is shown. Roman numerals I, II, and III depict the locations of segments I (residues 1–1874), II (2023–2801), and III (3614–4554) of RyR2, respectively. Dash-lines indicate possible subunit boundaries.
Figure 8B shows three possible models for how the three segments are packed together. In the first model (Fig. 8Ba), segment III is situated between segment I and II. Hence, some intra-subunit interactions between the C-terminal (III) and N-terminal (I) or central (II) segments may exist. Inter-subunit interactions between the N-terminal (I) segment of one subunit and the central (II) segment of the neighbouring subunit may also be expected. In the second model (Fig. 8Bb), segment I is located between segments II and III. This model predicts that intra-subunit interactions would exist between the N-terminal (I) and central (II) or C-terminal (III) segments, and that inter-subunit interactions would exist between the central (II) segment of one subunit and the C-terminal (III) segment of the neighbouring subunit. In the third model (Fig. 8Bc), segment II is packed between segments I and III. Likewise, intra-subunit interactions are predicted between the central (II) and N-terminal (I) or C-terminal (III) segments, and inter-subunit interactions between the N-terminal (I) segment of one subunit and the C-terminal (III) segment of the neighbouring subunit. At present, the results of our three-dimensional mapping studies do not unambiguously distinguish between these three models of subunit organization, further three-dimensional mapping studies are needed. For instance, three-dimensional localization of residues between 1874 (green circle 6 in domain 9) and 2023 (green circle 7 in domain 4) (Fig. 8Ab) should provide critical information on whether domain 9 is connected to domain 4 via domain 3, as in the first model (Fig. 8Ba), or via domains 5 and 6, as in the second and third models (Fig. 8Bb and Fig 8Bc). In other words, three-dimensional mapping of residues 1874–2023 should suffice to separate the first model from the second and third models. Similarly, three-dimensional localization of residues between 2801 (green circle 10 in domain 6) and 3614 (green circle 11 in domain 3) should provide clues to whether domain 6 is connected to domain 3 via domain 5, as in the second model (Fig. 8Bb), or via domain 4, as in the third model (Fig. 7Bc). Hence, three-dimensional mapping of residues 2801–3614 should allow us to choose between the second and third models, if the 1874–2034 mapping ruled out the first model.
Recently, as noted earlier, a three-dimensional RyR structure has been reconstructed at ~10 Å [39]. Analysis of the density distribution of multiple cross-sections of the RyR structure revealed extensive interconnectivity among domains 2, 2a, 3, 4, 4a, and 5 within the structural section outlined by the blue dash-line (Fig. 8Ba). The analysis also revealed the existence of some clear separations of mass along the blue dash-line (Fig. 8Ba). This pattern of density distribution suggests that the domains outlined by the blue dash-line are likely interconnected. Based on these putative internal mass arrangements and on our three-dimensional localizations of various residues, we propose that each structural section outlined by the blue dash-line in the Fig. 8Ba, represents one subunit in the tetrameric structure of RyR2. Clearly, more investigations are required to test this working hypothesis on subunit organization, and to unravel the intra- and inter-subunit interactions within the tetrameric structure of RyR2.
In summary, in the present study, we have localized the S2030 PKA phosphorylation site onto domain 4 in the three-dimensional structure of RyR2. The three-dimensional mapping of the S2030 PKA site not only reveals new insight into the mechanism of RyR2 regulation by phosphorylation, but also provides a framework for future investigations of the RyR2 subunit organization and inter-domain and inter-subunit interactions.
Acknowledgments
This work was supported by research grants from the Canadian Institutes of Health Research and the Heart and Stroke Foundation of Canada, Alberta to S.R.W.C., the American Heart Association grant 0430076N to Z.L., and the National Institutes of Health grant AR40615 to T.W. We would like to thank the Wadsworth Center’s DNA Sequencing Core and Electron Microscopy Core Facilities, and the Resource for Visualization of Biological Complexity (NIH Biotechnological Resource Grant RR01219).
The abbreviations used are
- ARVD2
arrhythmogenic right ventricular dysplasia type 2
- CaM
calmodulin
- CaMKII
calmodulin dependent protein kinase II
- CPVT
catecholaminergic polymorphic ventricular tachycardia
- DAD
delayed afterdepolarization
- EM
electron microscopy
- FKBP12.6
12.6 kDa FK506 binding protein
- GFP
green fluorescent protein
- HF
Heart failure
- PKA
protein kinase A
- RyR
ryanodine receptor
- RyR1 and RyR2
type 1 (skeletal) RyR and type 2 (cardiac) RyR, respectively
- SCD
sudden cardiac death
- SOICR
store-overload-induced Ca2+ release
- VT
ventricular tachycardia
- wt
wild type
REFERENCES
- 1.Marban E. Cardiac channelopathies. Nature. 2002;415:213–218. doi: 10.1038/415213a. [DOI] [PubMed] [Google Scholar]
- 2.Pogwizd S, Bers D. Calcium cycling in heart failure: The arrhythmia connection. J.Cardiovasc.Electrophysiol. 2002;13:88–91. doi: 10.1046/j.1540-8167.2002.00088.x. [DOI] [PubMed] [Google Scholar]
- 3.Tester DJ, Spoon DB, Valdivia HH, Makielski JC, Ackerman MJ. Targeted mutational analysis of the RyR2-encoded cardiac ryanodine receptor in sudden unexplained death: A molecular autopsy of 49 medical examiner/coroner's cases. Mayo Clin.Proc. 2004;79:1380–1384. doi: 10.4065/79.11.1380. [DOI] [PubMed] [Google Scholar]
- 4.Lakatta EG. Functional implications of spontaneous sarcoplasmic reticulum Ca2+ release in the heart. Cardiovasc.Res. 1992;26:193–214. doi: 10.1093/cvr/26.3.193. [DOI] [PubMed] [Google Scholar]
- 5.Jiang D, Xiao B, Yang D, Wang R, Choi P, Zhang L, Cheng H, Chen SRW. RyR2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced Ca2+ release (SOICR) PNAS. 2004;101:13062–13067. doi: 10.1073/pnas.0402388101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang D, Wang R, Xiao B, Kong H, Hunt DJ, Choi P, Zhang L, Chen SRW. Enhanced store overload-induced Ca2+ release and channel sensitivity to luminal Ca2+ activation are common defects of RyR2 mutations linked to ventricular tachycardia and sudden death. Circ.Res. 2005;97:1173–1181. doi: 10.1161/01.RES.0000192146.85173.4b. [DOI] [PubMed] [Google Scholar]
- 7.George CH, Jundi H, Thomas NL, Fry DL, Lai FA. Ryanodine receptors and ventricular arrhythmias: Emerging trends in mutations, mechanisms and therapies. J.Mol.Cell.Cardiol. 2007;42:34–50. doi: 10.1016/j.yjmcc.2006.08.115. [DOI] [PubMed] [Google Scholar]
- 8.Pogwizd SM, Bers DM. Cellular basis of triggered arrhythmias in heart failure. Trends Cardiovasc.Med. 2004;14:61–66. doi: 10.1016/j.tcm.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Priori SG, Napolitano C, Memmi M, Colombi B, Drago F, Gasparini M, DeSimone L, Coltorti F, Bloise R, Keegan R, Cruz Filho FE, Vignati G, Benatar A, DeLogu A. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2002;106:69–74. doi: 10.1161/01.cir.0000020013.73106.d8. [DOI] [PubMed] [Google Scholar]
- 10.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 11.Xiao B, Tian X, Xie W, Jones PP, Cai S, Wang X, Jiang D, Kong H, Zhang L, Chen K, Walsh MP, Cheng H, Chen SR. Functional consequence of PKA-dependent phosphorylation of the cardiac ryanodine receptor: Sensitization of store-overload-induced Ca2+ release (SOICR) J.Biol.Chem. 2007 doi: 10.1074/jbc.M703510200. [DOI] [PubMed] [Google Scholar]
- 12.Wehrens XH, Lehnart SE, Reiken S, Vest JA, Wronska A, Marks AR. Ryanodine receptor/calcium release channel PKA phosphorylation: A critical mediator of heart failure progression. Proc.Natl.Acad.Sci.U.S.A. 2006;103:511–518. doi: 10.1073/pnas.0510113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lehnart SE, Terrenoire C, Reiken S, Wehrens XHT, Song L-, Tillman EJ, Mancarella S, Coromilas J, Lederer WJ, Kass RS, Marks AR. Stabilization of cardiac ryanodine receptor prevents intracellular calcium leak and arrhythmias. PNAS. 2006;103:7906–7910. doi: 10.1073/pnas.0602133103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Witcher DR, Kovacs RJ, Schulman H, Cefali DC, Jones LR. Unique phosphorylation site on the cardiac ryanodine receptor regulates calcium channel activity. J.Biol.Chem. 1991;266:11144–11152. [PubMed] [Google Scholar]
- 15.Witcher DR, Strifler BA, Jones LR. Cardiac-specific phosphorylation site for multifunctional Ca2+/calmodulin-dependent protein kinase is conserved in the brain ryanodine receptor. J.Biol.Chem. 1992;267:4963–4967. [PubMed] [Google Scholar]
- 16.Xiao B, Jiang MT, Zhao M, Yang D, Sutherland C, Lai FA, Walsh MP, Warltier DC, Cheng H, Chen SRW. Characterization of a novel PKA phosphorylation site, serine-2030, reveals no PKA hyperphosphorylation of the cardiac ryanodine receptor in canine heart failure. Circ.Res. 2005;96:847–855. doi: 10.1161/01.RES.0000163276.26083.e8. [DOI] [PubMed] [Google Scholar]
- 17.Xiao B, Zhong G, Obayashi M, Yang D, Chen K, Walsh M, Shimoni Y, Cheng H, Ter Keurs H, Chen S. Ser-2030, but not ser-2808, is the major phosphorylation site in cardiac ryanodine receptors responding to protein kinase A activation upon beta-adrenergic stimulation in normal and failing hearts. Biochem.J. 2006;396:7–16. doi: 10.1042/BJ20060116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stange M, Xu L, Balshaw D, Yamaguchi N, Meissner G. Characterization of recombinant skeletal muscle (ser-2843) and cardiac muscle (ser-2809) ryanodine receptor phosphorylation mutants. J.Biol.Chem. 2003;278:51693–51702. doi: 10.1074/jbc.M310406200. [DOI] [PubMed] [Google Scholar]
- 19.Benkusky NA, Weber CS, Scherman JA, Farrell EF, Hacker TA, John MC, Powers PA, Valdivia HH. Intact {beta}-adrenergic response and unmodified progression toward heart failure in mice with genetic ablation of a major protein kinase A phosphorylation site in the cardiac ryanodine receptor. Circ.Res. 2007 doi: 10.1161/CIRCRESAHA.107.153007. [DOI] [PubMed] [Google Scholar]
- 20.Chen SRW, Li X, Ebisawa K, Zhang L. Functional characterization of the recombinant type 3 Ca2+ release channel (ryanodine receptor) expressed in HEK293 cells. J.Biol.Chem. 1997;272:24234–24246. doi: 10.1074/jbc.272.39.24234. [DOI] [PubMed] [Google Scholar]
- 21.Li P, Chen SR. Molecular basis of ca(2)+ activation of the mouse cardiac ca(2)+ release channel (ryanodine receptor) J.Gen.Physiol. 2001;118:33–44. doi: 10.1085/jgp.118.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao M, Li P, Li X, Zhang L, Winkfein RJ, Chen SR. Molecular identification of the ryanodine receptor pore-forming segment. J.Biol.Chem. 1999;274:25971–25974. doi: 10.1074/jbc.274.37.25971. [DOI] [PubMed] [Google Scholar]
- 23.Liu Z, Zhang J, Li P, Chen SR, Wagenknecht T. Three-dimensional reconstruction of the recombinant type 2 ryanodine receptor and localization of its divergent region 1. J.Biol.Chem. 2002;240:24. doi: 10.1074/jbc.M208124200. [DOI] [PubMed] [Google Scholar]
- 24.Liu Z, Zhang J, Wang R, Wayne Chen SR, Wagenknecht T. Location of divergent region 2 on the three-dimensional structure of cardiac muscle ryanodine receptor/calcium release channel. J.Mol.Biol. 2004;338:533–545. doi: 10.1016/j.jmb.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 25.Wagenknecht T, Frank J, Boublik M, Nurse K, Ofengand J. Direct localization of the tRNA--anticodon interaction site on the escherichia coli 30 S ribosomal subunit by electron microscopy and computerized image averaging. J.Mol.Biol. 1988;203:753–760. doi: 10.1016/0022-2836(88)90207-0. [DOI] [PubMed] [Google Scholar]
- 26.Meng X, Xiao B, Cai S, Huang X, Li F, Bolstad J, Trujillo R, Airey J, Chen SR, Wagenknecht T, Liu Z. Three-dimensional localization of serine-2808, a phosphorylation site in cardiac ryanodine receptor. J.Biol.Chem. 2007 doi: 10.1074/jbc.M704474200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doi N, Yanagawa H. Evolutionary design of generic green fluorescent protein biosensors. Methods Mol.Biol. 2002;183:49–55. doi: 10.1385/1-59259-280-5:049. [DOI] [PubMed] [Google Scholar]
- 28.Niwa H, Inouye S, Hirano T, Matsuno T, Kojima S, Kubota M, Ohashi M, Tsuji FI. Chemical nature of the light emitter of the aequorea green fluorescent protein. Proc.Natl.Acad.Sci.U.S.A. 1996;93:13617–1322. doi: 10.1073/pnas.93.24.13617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang R, Chen W, Cai S, Zhang J, Bolstad J, Wagenknecht T, Liu Z, Chen SR. Localization of an NH(2)-terminal disease-causing mutation hot spot to the "clamp" region in the three-dimensional structure of the cardiac ryanodine receptor. J.Biol.Chem. 2007;282:17785–17793. doi: 10.1074/jbc.M700660200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Z, Wang R, Zhang J, Chen SR, Wagenknecht T. Localization of a disease-associated mutation site in the three-dimensional structure of the cardiac muscle ryanodine receptor. J.Biol.Chem. 2005;280:37941–37947. doi: 10.1074/jbc.M505714200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J, Liu Z, Masumiya H, Wang R, Jiang D, Li F, Wagenknecht T, Chen SRW. Three-dimensional localization of divergent region 3 of the ryanodine receptor to the clamp-shaped structures adjacent to the FKBP binding sites. J.Biol.Chem. 2003;278:14211–14218. doi: 10.1074/jbc.M213164200. [DOI] [PubMed] [Google Scholar]
- 32.Liu Z, Zhang J, Sharma MR, Li P, Chen SR, Wagenknecht T. Three-dimensional reconstruction of the recombinant type 3 ryanodine receptor and localization of its amino terminus. Proc.Natl.Acad.Sci.U.S.A. 2001;98:6104–6619. doi: 10.1073/pnas.111382798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waldo GS, Standish BM, Berendzen J, Terwilliger TC. Rapid protein-folding assay using green fluorescent protein. Nat.Biotechnol. 1999;17:691. doi: 10.1038/10904. 765. [DOI] [PubMed] [Google Scholar]
- 34.Wang R, Bolstad J, Kong H, Zhang L, Brown C, Chen SRW. The predicted TM10 transmembrane sequence of the cardiac Ca2+ release channel (ryanodine receptor) is crucial for channel activation and gating. J.Biol.Chem. 2004;279:3635–3642. doi: 10.1074/jbc.M311367200. [DOI] [PubMed] [Google Scholar]
- 35.Radermacher M, Rao V, Grassucci R, Frank J, Timerman AP, Fleischer S, Wagenknecht T. Cryo-electron microscopy and three-dimensional reconstruction of the calcium release channel/ryanodine receptor from skeletal muscle. J.Cell Biol. 1994;127:411–423. doi: 10.1083/jcb.127.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma MR, Penczek P, Grassucci R, Xin HB, Fleischer S, Wagenknecht T. Cryoelectron microscopy and image analysis of the cardiac ryanodine receptor. J.Biol.Chem. 1998;273:18429–18434. doi: 10.1074/jbc.273.29.18429. [DOI] [PubMed] [Google Scholar]
- 37.Liu Z, Zhang J, Sharma MR, Li P, Chen SR, Wagenknecht T. Three-dimensional reconstruction of the recombinant type 3 ryanodine receptor and localization of its amino terminus. Proc.Natl.Acad.Sci.U.S.A. 2001;98:6104–6619. doi: 10.1073/pnas.111382798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malhotra A, Penczek P, Agrawal RK, Gabashvili IS, Grassucci RA, Junemann R, Burkhardt N, Nierhaus KH, Frank J. Escherichia coli 70 S ribosome at 15 A resolution by cryo-electron microscopy: Localization of fMet-tRNAfMet and fitting of L1 protein. J.Mol.Biol. 1998;280:103–116. doi: 10.1006/jmbi.1998.1859. [DOI] [PubMed] [Google Scholar]
- 39.Samso M, Wagenknecht T, Allen PD. Internal structure and visualization of transmembrane domains of the RyR1 calcium release channel by cryo-EM. Nat.Struct.Mol.Biol. 2005;12:539–544. doi: 10.1038/nsmb938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ludtke SJ, Serysheva II, Hamilton SL, Chiu W. The pore structure of the closed RyR1 channel. Structure. 2005;13:1203–1211. doi: 10.1016/j.str.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orlova EV, Serysheva I, van Heel M, Hamilton SL, Chiu W. Two structural configurations of the skeletal muscle calcium release channel. Nat.Struct.Biol. 1996;3:547–552. doi: 10.1038/nsb0696-547. [DOI] [PubMed] [Google Scholar]
- 42.Wagenknecht T, Radermacher M, Grassucci R, Berkowitz J, Xin HB, Fleischer S. Locations of calmodulin and FK506-binding protein on the three-dimensional architecture of the skeletal muscle ryanodine receptor. J.Biol.Chem. 1997;272:32463–32471. doi: 10.1074/jbc.272.51.32463. [DOI] [PubMed] [Google Scholar]
- 43.Samso M, Trujillo R, Gurrola GB, Valdivia HH, Wagenknecht T. Three-dimensional location of the imperatoxin A binding site on the ryanodine receptor. J.Cell Biol. 1999;146:493–499. doi: 10.1083/jcb.146.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wehrens X, Lehnart S, Huang F, Vest J, Reiken S, Mohler P, Sun J, Guatimosim S, Song L, Rosemblit N, D'Armiento J, Napolitano C, Memmi M, Priori S, Lederer W, Marks A. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell. 2003;113:829–840. doi: 10.1016/s0092-8674(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 45.Xiao B, Sutherland C, Walsh MP, Chen SRW. Protein kinase A phosphorylation at serine-2808 of the cardiac Ca2+-release channel (ryanodine receptor) does not dissociate 12.6-kDa FK506-binding protein (FKBP12.6) Circ.Res. 2004;94:487–495. doi: 10.1161/01.RES.0000115945.89741.22. [DOI] [PubMed] [Google Scholar]
- 46.Liu Z, Wang R, Chen SRW, Wagenknecht T. The amino-terminal and central mutation hotspots are adjacent to each other in the three-dimensional structure of RyR2 as revealed by cryo-electron microscopy. Biophys J. 2006;90:389. [Google Scholar]
- 47.Benacquista BL, Sharma MR, Samso M, Zorzato F, Treves S, Wagenknecht T. Amino acid residues 4425–4621 localized on the three-dimensional structure of the skeletal muscle ryanodine receptor. Biophys.J. 2000;78:1349–1358. doi: 10.1016/S0006-3495(00)76689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]