Abstract
The process of parturition involves the complex interplay of factors that change the excitability and contractile activity of the uterus. We have compared the relative gene expression profile of myometrium from rats before parturition (21 days pregnant) and during delivery, using high-density DNA microarray. Of 8,740 sequences available in the array, a total of 3,782 were detected as present. From the sequences that were significantly altered, 59 genes were upregulated and 82 genes were downregulated. We were able to detect changes in genes described to have altered expression level at term, including connexin 43 and 26, cyclooxygenase 2, and oxytocin receptor, as well as novel genes that have been not previously associated with parturition. Quantitative real-time PCR on selected genes further confirmed the microarray data. Here we report for the first time that aquaporin5 (AQP5), a member of the aquaporin water channel family, was dramatically downregulated during parturition (∼100-fold by microarray and ∼50-fold by real-time PCR). The emerging profile highlights biochemical cascades occurring in a period of ∼36 h that trigger parturition and the initiation of myometrium reverse remodeling postpartum. The microarray analysis uncovered genes that were previously suspected to play a role in parturition. This regulation involves genes from immune/inflammatory response, steroid/lipid metabolism, calcium homeostasis, cell volume regulation, cell signaling, cell division, and tissue remodeling, suggesting the presence of multiple and redundant mechanisms altered in the process of birth.
Keywords: smooth muscle, microarray, labor, aquaporin, gene regulation
the uterus maintains a state of relative quiescence throughout most of pregnancy, but during labor myometrium smooth muscle contracts in a synchronized manner to expel the fetus. In this period, the myometrium becomes insensitive to relaxant signals and responsive to contractile stimuli. Several hormonal, chemical, and mechanical signals may play a role in the transition of the quiescent uterus to the contracting uterus. Challis and Lye (14) proposed a unifying hypothesis that as term approaches, a group of genes encoding for key “contraction-associated proteins” (CAPs) is activated. CAPs are necessary to augment the response to uterotonins such as oxytocin and prostaglandins, initiate excitation, and increase the frequency and amplitude of contractions (15). The oxytocin receptor (49), corticotropin-releasing hormone receptors (71), the gap junction connexin 43 (Cx43) (57), and the cyclooxygenase (COX) enzyme (23) are the best-characterized examples of CAPs. To better understand the complex process of normally timed labor, several studies have used microarray technology to identify novel genes that are differentially expressed in uterus during labor, both in humans (5, 9, 10, 16, 31, 43) and in rodents (5, 38, 75). Although each study identified up to 50 genes that are significantly up- or downregulated during labor, only a few of the detected genes are common to these studies. These differences could be largely due to the selected time point of pregnancy, the tissue composition, and/or species dependence. In fact, a recent comprehensive review on microarray studies concluded that the onset of labor remains without a clear characterization, possibly because the changes in gene expression take place in a very short time that is difficult to record with this methodology (9). We decided to set a strict time point by comparing in rats the pattern of gene expression at late pregnancy (day 21 of pregnancy) with that found during labor after the expulsion of the second pup, a period of 36 h in which most of the changes associated with labor/delivery occur. Furthermore, because uterine samples of late-pregnant animals could be contaminated with endometrium and placental tissue, in these studies we used only myometrium. Using Affymetrix high-density DNA microarray, we have confirmed increased expressions of so-called CAPS genes (i.e., Cx43, oxytocin receptor, COX2) as well uncovering genes that were not previously known to be involved in the parturition process in rat myometrium. Microarray data of selected genes with large (50- to 100-fold) and small (∼2- to 4-fold) expression changes were validated by quantitative real-time PCR (QRT-PCR).
MATERIALS AND METHODS
Total RNA Preparation
Animal protocols were approved by the UCLA School of Medicine Animal Committee. Uteri were dissected from late-pregnant (LP, 21 days) rats and rats during labor (DL) after the expulsion of the second pup. After dissection uteri were placed immediately in cold Ca2+-free PBS buffer, and peripheral large blood vessels, external surrounding connective tissue, and placenta were removed. The endometrium superficial layer was separated by gentle scraping. The myometrium was weighed, finely minced, and homogenized with a Polytron (Polytron PT3000, Brinkmann). To extract total RNA the Ambion Totally RNA kit (Ambion, Austin, TX) was used. RNA concentration was determined with a spectrophotometer at 260-nm optical density (OD). The RNA purity was assessed by the OD ratio 260 nm/280 nm and the RNA integrity by the comparison of the 28S and 18S bands from 1 μg of total RNA in 0.8% agarose gels in Tris-borate-EDTA buffer with 0.5 μg/ml ethidium bromide.
cRNA Preparation and Microarray Analysis
RNA samples were processed according to the protocol indicated by Affymetrix (Santa Clara, CA) and performed in the DNA Microarray Core Facility of the Department of Human Genetics, UCLA School of Medicine. cDNA was synthesized with the SuperScript Choice System (Life Technologies, Grand Island, NY) with an oligo(dT) primer containing the sequence of the T7 promoter region (Genset, La Jolla, CA). The cDNA was then used to perform in vitro transcription incorporating biotin-labeled nucleotides with the Enzo BioArray kit (Enzo Biochem). Biotin-labeled cRNA was fragmented and hybridized to the Affymetrix Rat RG U34-A GeneChip containing 8,740 probe sets targeting different sequences. The arrays were washed, stained, and scanned with Fluidics Station 400 (Affymetrix) and an Agilent Scanner (Hewlett-Packard).
To determine presence or absence for each sequence in the experimental cRNA, microarray data were initially processed with Affymetrix Microarray suite 4.0. Since microarrays may differ in their hybridization patterns, to quantify cRNA sequence expression levels the values of each cell intensity were calculated with the Li and Wong statistical model applied to all the microarrays (dChip software) (51, 52). Since scanned images may have different overall brightness, a normalization procedure is needed to adjust the brightness of the arrays to a comparable level. dChip software performs “invariant set normalization,” which automatically chooses a subset of genes with small within-subset rank difference among arrays (the invariant set), to serve as the basis for fitting a normalization curve. Once the normalization procedure was completed, the mean expression index (EI) of each gene was calculated according to the Li and Wong statistical model (52). Determinations were performed on individual RNA samples from three animals in each condition (21 days late pregnant and during labor); in one pair of data the determinations were validated by triplicate determinations. Ten microarrays were analyzed, five LP (arrays 1, 2, and 3 and 2 replicates of array 1) and five DL (arrays 1, 2, and 3 and 2 replicates of array 1). Fold changes are the average of three arrays from LP and DL animals. P values ≤0.05 were considered statistically significant with the two-tailed t-test for independent samples. The frequency distribution of fold changes for genes with P < 0.05 showed a peak at 1.8- and −1.8-fold for up- and downregulation, respectively. This peak abruptly decayed as a function of smaller absolute changes. In agreement with this, we selected a cutoff threshold of 1.79-fold change that covered all the biologically significant genes that were statistically significant by several statistical methods (17). GenBank accession numbers are given in parentheses. All data are stored in GEO with MIAME (Minimum Information About a Microarray Experiment)-compliant microarray database (accession no. GSE12799).
Quantitative Real-Time PCR
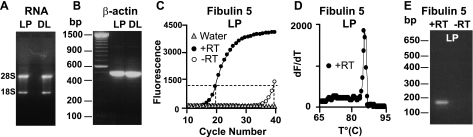
Microarray data were independently validated in a set of genes with small, intermediate, and large changes (∼2- to ∼100-fold) using QRT-PCR performed in triplicate with 3–5 RNA-independent isolations in each LP and DL group. Mean values were normalized to LP. RNA samples from different animals were used for QRT-PCR and microarray determinations. Total RNA was isolated by the TRIzol method (Invitrogen), and the quality of total RNA was evaluated by visual assessment of 28S-to-18S ribosomal RNA ratio on agarose gels. Figure 1A shows an example of total RNA isolated from myometrium of LP and DL rats. The quality of total RNA was further evaluated with primers spanning a 200-bp intron within the rat β-actin sequence to serve as a control for genomic DNA contamination (forward primer 5′-GGCTACAGCTTCACCACCAC-3′ and reverse primer 5′-TACTCCTGCTTGCTGATCCAC-3′) (V01217). The presence of only one single band of the expected molecular mass at ∼500 bp with the absence of the predicted 700-bp band that would include 200 bp from the intron (Fig. 1B) demonstrates the lack of genomic DNA contamination in both LP and DL total RNA. High-quality total RNA (2 μg) was then reverse transcribed to single-stranded cDNA by priming with gene-specific primers and the Ominiscript kit (Qiagen) in the absence and presence of reverse transcriptase (RT). Gene-specific primers and their corresponding accession numbers are given in Table 1. QRT-PCR was performed with an iCycler iQ (Bio-Rad) with Platinum PCR Supermix-UDG (GIBCO BRL) and SYBR Green I (27). Specific products were detected as a clear single peak at their melting temperature in the first derivative of fluorescence (dF/dt) vs. temperature plot (melting curve) and as a single band at the expected molecular mass in agarose gel electrophoresis at the end of the experiment (Fig. 1, D and E). The transcript level of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal reference gene (forward primer 5′-TCCTGCACCACCAACTGCTTAG-3′ and reverse primer 5′-GATGACCTTGCCCACAGCCTTG-3′) (AF106860). The reaction conditions were 5 min at 95°C followed by 40 cycles at 95°C for 45 s, 60°C for 45 s, and 72°C for 45 s. An example of the QRT-PCR reaction (fluorescence vs. cycle number plot) for Fibulin 5 gene is shown in Fig. 1C. Samples were total RNA of LP myometrium converted to DNA in the presence of RT, of the reaction mixture without RT (negative control), and of water instead of RNA (Fig. 1C). In the presence of RT the threshold cycle was ∼20, while in the absence of RT the threshold cycle increased to ∼39. This threshold difference (19 cycles) indicates 524,288 (or 219)-fold lower transcript levels in the absence of RT, which is an estimate of the degree of genomic DNA contamination. Confirming the purity of the RNA and the quality of the primers, the melting curve showed a single sharp peak only in the presence of RT (Fig. 1D) and the QRT-PCR products run at the end of the experiment showed a single band of the expected molecular size only in the presence of RT (Fig. 1E). No products were detected when total RNA was converted to DNA in the absence of RT (Fig. 1E).
Fig. 1.
Assessment of total RNA quality and criteria for quantitative real-time PCR (QRT-PCR) quantification. A: agarose gel showing typical high 28S-to-18S ratio (∼3.5) indicating good RNA quality from late-pregnancy (LP) and during-labor (DL) myometrium. B: single PCR product of ∼500 nt obtained with primers spanning a 200-bp intronic region of β-actin gene, indicating no detectable genomic DNA contamination in cDNAs from both LP and DL myometrium. C: fluorescence intensity vs. cycle number plots of QRT-PCR reactions for Fibulin 5 in LP in the presence (+RT) and absence (−RT) of reverse transcriptase and when water was used instead of RNA. D: melting curve [first derivative of fluorescence (dF/dt) vs. temperature (T)] of QRT-PCR product showing a single peak in the presence of RT. E: single QRT-PCR product of the expected molecular mass of ∼180 nt was obtained only in the presence of RT at the end of the QRT-PCR experiment. No product was detected without RT.
Table 1.
Comparison of LP/DL fold change in expression by microarray and QRT-PCR
| Gene |
Fold Change (LP/DL) |
Primer | Accession No. | |
|---|---|---|---|---|
| Microarray | QRT-PCR | |||
| Aquaporin5 (AQP5) | −106.2 | −45.2 | F: 5′-GGCCCTGCGGTGGTCATGAA-3′ | U16245 |
| R: 5′-GTCAGCTCGATGGTCTTCTTCC-3′ | ||||
| P-glycoprotein/multidrug resistance1 (MDR) | −77.9 | −122 | F: 5′-GTCCAGGAAGCGCTGGACAAA-3′ | M81855 |
| R: 5′-TCATGAGCGCTTTGCTCCAGC-3′ | ||||
| Sodium-myo-inositol cotransporter (SMIT) Signal peptidase | −4.3 | −3.8 | F: 5′-GCAGCCAAAAACATTGCTCATG-3′ | AJ001290 |
| R: 5′-GAGCTGCAATCATCACTGCCAT-3′ | ||||
| −2.3 | −1.3 | F: 5′-AAGGACAACACTGGCTGGAGAA-3′ | L11319 | |
| R: 5′-TGGACCAGCACAAATAAACCCA-3′ | ||||
| Deiodinase iodothyronine type III | −1.9 | −5 | F: 5′-AAGGACAACACTGGCTGGAGAA-3′ | L11319 |
| R: 5′-TGGACCAGCACAAATAAACCCA-3′ | ||||
| Fc-γ | −1.8 | −2.0 | F: 5′-CATCACTGTCCAAGAGCCCAAA-3′ | X73371 |
| R: 5′-AATGGCAGCTACAGCAATTC-3′ | ||||
| Transforming growth factor-β3 (TGF-β3) | +2.9 | +7.4 | F: 5′-CGAACCTAAGGGTTACTATGCC-3′ | U03491 |
| R: 5′-TCAGCTGCACTTACACGACTT C-3′ | ||||
| Retinaldehyde | +2.3 | +2.11 | F: 5′-CGTTACAGATGACATGCGGATC-3′ | U60063 |
| Dehydrogenase | R: 5′-AGGACACCATGAGAGCCTTGTT-3′ | |||
| Fibulin 5 | +1.9 | +1.6 | F: 5′-TTGGGGATAACCGCTGTATGTG-3′ | NM-019153 |
| R: 5′-CCTGTTTGCCGCATGTAGAACT-3′ | ||||
Comparison of change in gene expression [late pregnancy (LP)/during labor (DL)] with microarray and quantitative real-time PCR (QRT-PCR): −, downregulation; +, upregulation. Forward (F) and reverse (F) primers used for QRT-PCR are given with GenBank accession numbers.
RESULTS AND DISCUSSION
Changes in Global Pattern of Gene Activity Between Late Pregnancy and Parturition
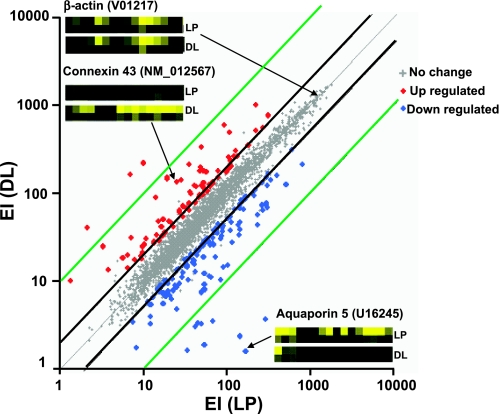
To analyze changes in gene expression in rat myometrium between late pregnancy and the initial stage of parturition during labor we used high-density oligonucleotide arrays with probe sets for 8,740 sequences (6,400 gene and 2,340 expressed sequence tag sequences). Figure 2 shows the average EI plot of present genes in DL vs. LP (3 independent samples per stage). The analysis shows that 3,782 sequences (43.3% of available sequences) were detected and that 59 genes were upregulated (1.6%) while 82 genes were downregulated (2.2%). The global pattern of expression favors upregulation of CAPs and downregulation of relaxing- and hypertrophy-associated proteins. The insets in Fig. 2 show examples of probe pair sets for β-actin, whose transcripts do not change, for Cx43, whose expression increases (5.3-fold) and for AQP5, whose expression decreases (−106-fold) in LP compared with DL.
Fig. 2.
Scatter plot of gene expression in rat uterine smooth muscle from LP vs. DL. Expression index (EI) data are the average of 3 microarrays per condition. Central thin gray diagonal line shows identical expression; parallel thick black and blue lines represent 2-fold and 10-fold deviation, respectively. Small gray dots represent sequences with no significant changes, red dots sequences significantly upregulated sequences, and blue dots downregulated sequences (P < 0.05). Insets: probe pair sets from LP and DL rats for β-actin (V01217), CX43 (NM_012567), and aquaporin-5 (AQP5; U16245) in rat myometrium. Intensity level of each cell reflects the degree of hybridization, which correlates with the amount of the corresponding transcripts in the original mRNA.
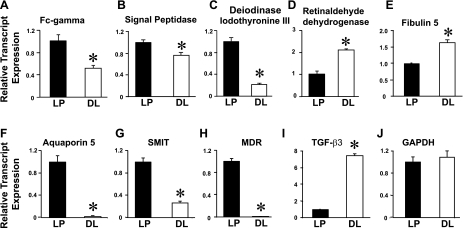
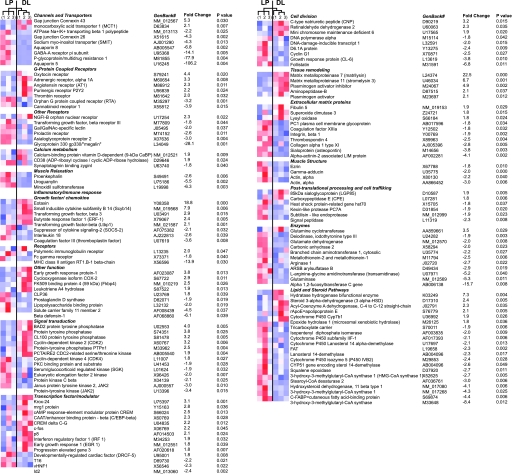
We have performed hierarchical cluster analysis between the samples with the average linkage method (28, 52). This analysis groups a set of genes with a similar expression pattern regardless of their mRNA origin. In all cases, the hierarchical cluster analysis grouped the samples in two main clusters corresponding to LP and DL, which provides an indication of the validity of the observed differences. Figure 4 illustrates the cluster analysis in each gene (blue: low expression; red: high expression), the GenBank accession number, fold change, and P values (LP 1, 2, 3 and DL 1, 2, 3). Because the microarray analysis showed that the majority of the genes were only down- or upregulated by -2-fold between LP and DL, we randomly selected five genes that were either up- or downregulated between 1.8- and 2.4-fold for QRT-PCR analysis. Figure 3 shows relative transcript expression of FC-γ, Signal peptidase, Deiodinase iodothyronine type III, Retinaldehyde dehydrogenase, and Fibulin 5 normalized to LP. Consistent with microarray determinations, QRT-PCR experiments showed that myometrium FC-γ (−2-fold), Signal peptidase (−1.3-fold), and Deiodinase iodothyronine type III (−5-fold) were downregulated, whereas Retinaldehyde dehydrogenase (+2.1-fold) and Fibulin 5 (+1.6-fold) were upregulated at almost similar fold change as in microarray data (Table 1, Fig. 3, A–E). We also selected several other genes that were up- or downregulated between 3- and 100-fold by microarray analysis in DL compared with LP: AQP5, −45.2-fold; Sodium-myo-inositol cotransporter (SMIT), −3.8-fold; P-glycoprotein/multidrug resistance1 (MDR), −122-fold; and transforming growth factor-β3 (TGF-β3), +7.2-fold (Fig. 3, F–I). GAPDH was used as the housekeeping gene, and its transcript levels were similar in LP and DL (Fig. 3J). The melting curves showed a single sharp peak for all genes as described in Fig. 1, indicating the presence of a single PCR product. In summary, QRT-PCR on selected genes with relatively large and small changes in expression between LP and DP further validated the microarray data.
Fig. 3.
Validation of microarray data with QRT-PCR. Relative transcript levels were measured in the linear range of the fluorescence vs. cycle curve as in Fig. 1C. A: Fc-γ. B: Signal peptidase. C: Deiodinase iodothyronine type III. D: Retinaldehyde dehydrogenase. E: Fibulin 5. F: AQP5. G: Sodium-myo-inositol cotransporter (SMIT). H: P-glycoprotein/multidrug resistance1 (MDR). I: transforming growth factor-β3 (TGF-β3). J: glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Mean values were normalized to LP from 3–5 independent RNA isolations in each LP and DL group. Values were FC-γ 1 ± 0.11 (n = 5, LP), 0.49 ± 0.04 (n = 5, DL); Signal peptidase 1 ± 0.04 (LP), 0.75 ± 0.05 (DL); Deiodinase iodothyronine type III 1 ± 0.23 (n = 5, LP), 0.33 ± 0.06 (n = 5, DL); Retinaldehyde dehydrogenase 1 ± 0.14 (n = 5, LP), 2.11 ± 0.05 (n = 5, DL); Fibulin 5 1 ± 0.03 (n = 5, LP), 1.6 ± 0.08 (n = 5, DL); AQP5, 1 ± 0.10 (n = 3, LP), 0.022 ± 0.008 (n = 3, DL); SMIT 1 ± 0.06 (n = 3, LP), 0.26 ± 0.03 (n = 3, DL); MDR 1 ± 0.05 (n = 3, LP), 0.008 ± 0.003 (n = 3, DL); TGF-βIII 1 ± 0.08 (n = 3, LP), 7.43 ± 0.16 (n = 3, DL). GAPDH transcripts were practically identical in LP (1 ± 0.10, n = 8) and DL (1.04 ± 0.09, n = 8) rats.
Gene expression can be regulated by sex hormones (26). Thus it is likely that some of the reported changes could be related to the hormonal changes taking place between LP and DL animals. In fact, in the studied short window of 36 h after day 21 of pregnancy in rats there is a dramatic rise of estrogen from ∼20 to ∼60 pg/ml and a reduction of progesterone from ∼20 to 1.1 ng/ml (57). Similarly, in rodents during midpregnancy placental lactogen is very low (5 ng/ml), peaking before parturition (∼25 ng/ml).
To have a general picture of the changes in gene expression together with their functional consequences we grouped genes with significant changes in expression according to their main biological function (Fig. 4). We identified several functionally related clusters of genes that account for different processes occurring during the transition between the noncontractile LP myometrium and delivery.
Fig. 4.
Genes differentially expressed in LP and DL. Ten microarrays were analyzed: 5 LP (arrays 1, 2, 3 and 2 replicates of array 1) and 5 DL (arrays 1, 2, 3 and 2 replicates of array 1) (NIH GEO Database accession no. GSE12799). Genes are grouped by their biological function. Color columns show hierarchical cluster analysis of LP and DL in 3 single arrays for each. Color indicates expression level relative to the mean (white): low is blue, and high is red. Top: relationship between the arrays. Each row displays individual genes, with their gene description, GenBank accession no., fold change, and significance (P value > 0.05). Fold changes are the average of 3 arrays from LP and DL animals.
Contraction Regulation
Channels and transporters.
Gap junctions are highly regulated at the onset of labor and have an essential role in the synchronization of myometrium contraction. Cx43 and Cx26 are the two major gap junctions in myometrium. In the rat they have a characteristic pattern of expression: Cx26 has its peak expression 2 days before labor, while Cx43 has its peak expression during delivery (62). We have observed the same pattern of expression, 5.3-fold upregulation of Cx43 and −4.3-fold downregulation of Cx26, at delivery (Figs. 2 and 4). AQP proteins are a family of channels expressed in numerous mammalian tissues, where they play a fundamental role in regulating water transport across cell membranes (8). Specific AQP isoforms are expressed in both rat and mouse uterus (47, 65). Furthermore, the expression and distribution of some AQPs are altered during the preimplantaion period in mouse and rat uterus (53–55, 65). Consistent with a high degree of AQP remodeling in the uterus, here we report significant downregulation of two members of the AQP family during delivery, AQP5 by about −45- to −100-fold (determined by QRT-PCR and microarray, respectively) and AQP8 by −7-fold during delivery, underscoring their potential role in parturition.
Recent studies in rat uterus showed that both AQP5 and AQP8 were upregulated during the first 20 days of pregnancy by 20- and 6-fold, respectively. Consistent with our studies, both proteins were downregulated from day 20 to day 23 of pregnancy (−4-fold for AQP5 and −2-fold for AQP8) (38). However, there was no change in the expression of AQP5 when comparing term versus labor, whereas AQP8 was further downregulated in labor (38). We have observed similar downregulation of AQP8 in rat myometrium from day 21 to labor. However, the dramatic downregulation of AQP5 (−100-fold in microarray data and −45-fold in QRT-PCR) between LP and DP in our work is not in agreement with the previous study (38). This discrepancy could be due to slight differences in the selected time points or to the differences in the tissue composition of uterus that was contaminated with endometrium (38).
We observed the downregulation of SMIT (−4.3-fold), which may diminish entrance of the osmolyte myo-inositol, as a mechanism of cell volume homeostasis in a hyperosmotic environment (74). An associated mechanism leading to reduced ion transport during delivery is the downregulation of the β1-subunit of the Na+-K+-ATPase, which regulates the level of expression of the α-subunit of the Na+-K+-ATPase (3, 36).
GABAA receptor has been identified in uterus, is composed of five subunits that form a ligand-gated Cl− channel, and seems to be related to the relaxed state (44). The π-subunit increases receptor sensitivity to progesterone derivatives like allopregnanolone, whose concentration peaks near the end of pregnancy (day 19), returning to control values immediately before delivery (day 21) (20). In fact, we observed a significant downregulation (−14.1-fold) in the mRNA expression of GABAA receptor π-subunit during delivery, in agreement with reports by others favoring a more contractile myometrium during delivery (34).
A very significantly downregulated gene was P-glycoprotein (−77.9-fold microarray data, −122-fold QRT-PCR), an ATP-dependent transmembrane pump capable of transporting numerous compounds including toxic metabolites out of the cell. This protein is considered to regulate the transfer of several substances from mother to fetus through the placenta, and to protect the fetus from toxic substances (72). Its downregulation at term may be associated with the interruption of metabolic traffic between the placenta and the uterine wall for expulsion of the fetus.
G protein-coupled receptors.
Activation of G protein-coupled receptors (GPCRs) targeting the activation of phospholipase C (PLC) leading to inositol 1,4,5-trisphosphate release of intracellular Ca2+ and stimulation of Ca2+ influx is the predominant contracting pathway in myometrium (4, 68). Several seven-transmembrane domain GPCRs targeting PLC, including oxytocin, endothelin, prostaglandins (FP, EP1, and EP3 receptors), angiotensin, and bradykinin, have been described in myometrium (15, 33). Oxytocin and prostaglandins have been proposed as parturition triggers (49, 50). In fact, the data in Fig. 4 confirm the upregulation of the oxytocin receptor (4.3-fold) and other GPCRs via Gαq/11 such as the α1A-adrenergic receptor (3.3-fold) (25), the AT1 angiotensin receptor (2.3-fold) (21), the P2y2 purinergic receptor (2.2-fold) (40), and the thrombin receptor (2.0-fold) (69). The redundancy in the upregulation of this set of GPCRs may explain the paradox that in oxytocin receptor-knockout mice parturition remained unaffected (61).
Cannabinoid receptor 1 was the most significantly downregulated seven-transmembrane receptor (−3.9-fold). This receptor is coupled to Gαi/o, inhibiting adenylyl cyclase and decreasing calcium conductance through voltage-activated calcium channels (18). Its downregulation may facilitate calcium influx.
Other receptors.
The prolactin receptor, which belongs to the family of cytokine receptors, was downregulated (−2.6-fold) during delivery. This receptor signals via associated kinases (Jak/Stat, Ras-Raf-MAPK, and Src tyrosine kinases) (39). Since prolactin seems to potentiate progesterone action that favors a myometrium quiescent state, the downregulation of the prolactin receptor may enhance myometrium contractility (22, 59).
TGF-β receptor types I, II, and III belong to the serine/threonine kinase receptor family. The activity of these receptors phosphorylates and activates transcription factors (SMAD proteins), which carry the signal to the nucleus (6, 58). The TGF-βIII receptor is downregulated (−1.8-fold) during labor. Previous work has shown that TGF-βI and II receptors are upregulated in human myometrium during late pregnancy and are downregulated during labor (42).
Glycoprotein 330 (megalin) is a member of the LDL receptor family that binds lipoprotein (a), mediating its cellular uptake and degradation (60). The dramatic reduction of glycoprotein 330 during delivery (−28.1-fold) could be related to the return to normal levels after parturition of the general increase during pregnancy of total cholesterol, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol (56).
Calcium metabolism.
The activity of ADP ribose cyclase (CD38) stimulates cyclic ADP ribose synthesis that triggers Ca2+ release from intracellular stores through ryanodine receptor/channel. CD38 transcripts were increased (1.9-fold) during delivery. This increase can facilitate Ca2+ release from intracellular stores, inducing more forceful contraction during labor (19).
Muscle relaxation.
Genes promoting muscle relaxation were downregulated: the opioid receptor agonist precursor proenkephalin (76), uroguanylin, which stimulates the cGMP synthesis and nitric oxide production favoring smooth muscle relaxation (11, 12), and minoxidil sulfotransferase, which catalyzes the synthesis of minoxidil sulfate, a KATP potassium channel opener (1).
Immune response, receptors, and other functions.
Normal pregnancy requires the suppression of the inflammatory response in the reproductive tissue in order to tolerate the developing fetus (70). However, at the time of labor, activation of the inflammatory response could facilitate delivery. Here we found that early growth response protein-1 (EGR-1), which is associated with an inflammatory-like reaction in the ovary (30), is significantly upregulated (+3.8-fold) in DL.
Prostaglandins seem to accentuate the effect of other mediators and leukocyte recruiters in inducing the cascade of events necessary for birth (48). In accord with this hypothesis, we have observed a significant upregulation of COX2 (a gene related to prostaglandin synthesis and inflammatory response), which is in agreement with the upregulation of COX2 detected in human myometrium during labor (45). However, in another report no change of COX2 mRNA was found in human myometrium (5). We have also observed a significant upregulation of the chemokines eotaxin (+18.8-fold) and Scyb14 (+7.9-fold), involved in the recruitment of leukocytes that can be involved in the inflammatory response (13, 41). Other cytokines upregulated are TGF-β1 (+2.1-fold) and -β3 (+2.9-fold), whose function still remains unclear (42). ERF-1, another inflammatory response gene, was also upregulated (+2.4-fold). Following this pattern, the suppressor of cytokine signaling SOCS-2 was downregulated (−2.1-fold), contributing to the cytokine cascade. Moreover, we were able to observe the downregulation of prostaglandin D synthase (−1.9-fold), which has been associated with the anti-inflammatory response (46). Also, we have observed the downregulation of interleukin 18 (−2.6-fold), coagulation factor III (−3.6-fold), β-defensin-1 (−6.1-fold), solute carrier 11-2 (−4.5-fold), Fc-γ receptor (−1.8-fold), and lipopolysaccharide binding protein (−2.0-fold), whose function still remains to be elucidated. In summary, during delivery there is upregulation of proinflammatory and downregulation of anti-inflammatory genes.
Signal Transduction and Control of Gene Expression
Signal transduction.
Signal transduction cascades showed upregulation of phosphatases (BAD2, protein tyrosine phosphatase, CL100 protein tyrosine phosphatase, and protein tyrosine phosphatase PTPn1) and downregulation of kinases (PKC binding protein and substrate, serum/glucocorticoid-regulated kinase, eukaryotic elongation factor 2 kinase, PKC-β, and janus protein tyrosine kinase 2). The downregulation of kinase activity could be related to a diminished gene expression and cell proliferation to stop uterine growth. Reduction of phosphorylation also inhibits relaxant pathways and stimulates contraction (68). The cyclin kinases (CDC2, CDK2, and CDK4) were upregulated. They form part of the cyclin-dependent kinase complex involved in the regulation of the cell cycle having an important role in tissue repair and regeneration (63).
Transcription factor/modulator.
We have observed the upregulation of several transcription factors related to tissue proliferation and cancer including MRG1 (+2.8-fold), C/EBP-β (+2.3-fold), c-fos (+2.2-fold) p8 (+2.1-fold), progression-elevated gene 3 (+1.8-fold), and DRCF-5 (+1.8-fold), suggesting the presence of proliferation and tissue remodeling. Similar c-fos upregulation (+2.2-fold) has been reported in mouse uterus from day 13 to day 19 and in human myometrium in term labor compared with preterm not in labor (5, 37, 66). In agreement with observations by others (2), we have observed the upregulation of cAMP response element modulator protein (CREM, +2.5-fold) and truncated CREM delta C-G alternating spliced CREM-lacking intron (c-G) (+2.2-fold).
Cell division.
We also observed the downregulation of genes associated with differentiation and cell growth: T-16 (−2.2-fold), vHNF-1 (−2.3-fold), and Id-2 (−2.4-fold). We also observed the increase in expression of genes that inhibit smooth muscle proliferation like CNP (+3.2-fold) (35) and Retinaldehyde dehydrogenase 2 (+2.3-fold microarray and +2.1-fold QRT-PCR) (7). Also, genes related to cell division, proliferation, and growth induction were downregulated, including DNA polymerase α (−1.8-fold), D6.1A protein (−2.4-fold), cyclin G1 (−2.5-fold), CL6 (−3.8-fold), and follistatin (−6.8-fold), supporting the end of the hyperplasic phase.
Structural Remodeling
Tissue remodeling.
Complementary to the downregulation of the anchoring proteins, shredding enzymes are necessary to allow the physical separation of the placenta and uterine wall. Afterwards, the uterus undergoes a substantial remodeling and size reduction called postpartum uterine involution. Both processes involve digestion of extracellular matrix proteins by specific proteases (32). The process involves several different proteinases in a redundant mechanism (67). We observed significant upregulation of three metalloproteinases, matrilysin (22.5-fold), stromelysin-3 (6.7-fold), and plasminogen activator (2.1-fold), and aminopeptidase B (2.1-fold). Upregulation of stromelysin-3 by eightfold and matrilysin (tissue remodeling gene) by sixfold between days 13.5 and 19 of gestation was previously reported in mouse uterus (5). In human myometrium matrilysin and stromelysin have been upregulated 7- and 3.5-fold, respectively, in preterm labor compared with preterm not in labor (5). Paradoxically, the inhibitor of the plasminogen activator (+4.9) was also upregulated, suggesting a tight regulation of this proteolytic response that can be locally affected.
Extracellular matrix proteins.
Structural components of the extracellular matrix like thrombospondin 4 (−2.5-fold) and collagen α1 (−2.8-fold) are downregulated, in agreement with the idea of the end of uterine growth. However, we have detected a significant upregulation of fibulin-5 (+1.9-fold microarray data and +1.6-fold QRT-PCR), an elastic fiber component of the extracellular matrix whose role may be related to sustaining tissue elasticity (24).
Muscle structure.
During pregnancy there is an increase in the number (hyperplasia) and size (hypertrophy) of smooth muscle cells, being predominantly hyperplasia at the first stages of gestation and hypertrophy at the final stage, both stopping at the end of gestation (29). We have observed that genes related to cell structure and contractile machinery did not change or were downregulated (ezrin −1.8-fold, γ-adductin −2.0-fold, α-actin −3.0-fold), consistent with the phase of myometrium involution after parturition.
Posttranslational processing and cell trafficking.
Lysosomes are the site of degradation of obsolete intracellular material during autophagy and of extracellular macromolecules after endocytosis and phagocytosis. We have determined the downregulation of several intracellular proteases, responsible for protein processing. They are PC7A (−1.9-fold), subtilisin-like endoprotease (−1.9-fold), and signal peptidase (−2.3-fold), which are responsible for protein maturation. The role of this regulation may be related to modification in the targeting of protein necessary for sustaining relaxant machinery.
Other Functions
Enzymes.
We have detected changes in several genes related to general metabolism of amino acids (glutamate dehydrogenase, −2.0-fold; glutaminase, −5.0-fold; branched-chain aminotransferase, −2.2-fold) and glycolipids (ARSB, −2.9-fold; fucosyltransferase C, −15.7-fold), oxidative stress response (carbonic anhydrase, −2.0-fold; metallothionein, −2.5-fold), and l-arginine:glycine amidinotransferase, which catalyzes the rate-limiting step in creatine biosynthesis (−5.2-fold); most of these showed significant downregulation. The role of the regulation of these genes remains elusive.
Lipid and steroid pathways.
We have determined the upregulation of genes involved in fatty acid β-oxidation, a mechanism related to energy generation. The hydratase hydrogenase bifunctional enzyme (+7.3-fold) and MCAD (+2.3-fold) are upregulated at birth, suggesting the existence of molecular machinery for energy generation using fatty acids as substrate. In relationship to steroid metabolism, we detected the upregulation of 3α-HSD (+2.4-fold), a molecular switch with the role of regulating the accessibility of steroid hormone that can bind to the receptor (64). Also, we determined the upregulation of cytochrome P-450 Cyp7b1 (+1.9-fold), which is associated with steroid synthesis from C21 to C19 (73).
The main observation points to a systematic downregulation of the full pathway of cholesterol synthesis: HMG-CoA synthase (−8.4-fold, −4.3-fold, and −2.7-fold), IPP isomerase (−2.0-fold), squalene epoxidase (−2.7-fold), and lanosterol 14-demethylase (−2.6-fold, −2.3-fold, and −2.1-fold). These enzymes account for four of the five main steps in cholesterol biosynthesis. We also determined the downregulation of two other steroid metabolism enzymes, cytochrome P-450 IIF-1 (−2.1-fold) and cytochrome P-450 IVB2 (−2.4-fold). This observation suggests the existence of the molecular elements for an autocrine steroid system in the uterus that may account for potential differences in local steroid concentration during pregnancy and parturition.
Conclusions
In recent times, global analysis of gene expression through microarrays has become a very important and powerful tool for better understanding of molecular mechanisms of physiological processes and pathogenesis. Since the molecular changes experienced by the myometrium during parturition are highly complex, high-throughput technology is required to understand these events. Here we showed global changes in gene expression in rat myometrium during labor. We have determined the changes in expression of genes involved in myometrium contractility, calcium homeostasis, cell volume, immune/inflammatory response, signal transduction, steroid/lipid pathways, transcription factors, and tissue remodeling. Among these, the −100-fold downregulation of water channel protein AQP5 was the most striking. The clusters of genes showed a trend that favors a more contractile and less quiescent status in myometrium within a period of 36 h between late pregnancy and delivery, giving clues to the possible role of genes not previously described in this system that may lead to a better understanding of the labor event.
GRANTS
This work was supported by National Institutes of Health Grants HD-046510 (E. Stefani) and HL-077705 (L. Toro) and American Heart Association National Center Grant 0435116N (M. Eghbali).
Acknowledgments
Present addresses: G. Helguera, Div. of Surgical Oncology, Dept. of Surgery, David Geffen School of Medicine at University of California Los Angeles, Los Angeles, CA 90095-1778; D. Sforza, Dept. of Computational and Data Sciences, George Mason University, Fairfax, VA 22030.
Address for reprint requests and other correspondence: E. Stefani, David Geffen School of Medicine at UCLA, Dept. of Anesthesiology, BH-520A CHS, Box 957115, Los Angeles, CA 90095-7115 (e-mail: estefani@ucla.edu).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Anderson RJ, Kudlacek PE, Clemens DL. Sulfation of minoxidil by multiple human cytosolic sulfotransferases. Chem Biol Interact 109: 53–67, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Bailey J, Sparey C, Phillips RJ, Gilmore K, Robson SC, Dunlop W, Europe-Finner GN. Expression of the cyclic AMP-dependent transcription factors, CREB, CREM and ATF2, in the human myometrium during pregnancy and labour. Mol Hum Reprod 6: 648–660, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Beggah AT, Beguin P, Bamberg K, Sachs G, Geering K. Beta-subunit assembly is essential for the correct packing and the stable membrane insertion of the H,K-ATPase alpha-subunit. J Biol Chem 274: 8217–8223, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Berridge MJ Capacitative calcium entry. Biochem J 312: 1–11, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bethin KE, Nagai Y, Sladek R, Asada M, Sadovsky Y, Hudson TJ, Muglia LJ. Microarray analysis of uterine gene expression in mouse and human pregnancy. Mol Endocrinol 17: 1454–1469, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Blobe GC, Schiemann WP, Pepin MC, Beauchemin M, Moustakas A, Lodish HF, O'Connor-McCourt MD. Functional roles for the cytoplasmic domain of the type III transforming growth factor beta receptor in regulating transforming growth factor beta signaling. J Biol Chem 276: 24627–24637, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Boettger-Tong HL, Stancel GM. Retinoic acid inhibits estrogen-induced uterine stromal and myometrial cell proliferation. Endocrinology 136: 2975–2983, 1995. [DOI] [PubMed] [Google Scholar]
- 8.Borgnia M, Nielsen S, Engel A, Agre P. Cellular and molecular biology of the aquaporin water channels. Annu Rev Biochem 68: 425–458, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Breuiller-Fouche M, Charpigny G, Germain G. Functional genomics of the pregnant uterus: from expectations to reality, a compilation of studies in the myometrium. BMC Pregnancy Childbirth 7, Suppl 1: S4, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breuiller-Fouche M, Germain G. Gene and protein expression in the myometrium in pregnancy and labor. Reproduction 131: 837–850, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Buhimschi IA, Martin-Clark O, Aguan K, Thompson LP, Weiner CP. Differential alterations in responsiveness in particulate and soluble guanylate cyclases in pregnant guinea pig myometrium. Am J Obstet Gynecol 183: 1512–1519, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Buhimschi IA, Yallampalli C, Buhimschi CS, Saade GR, Garfield RE. Distinct regulation of nitric oxide and cyclic guanosine monophosphate production by steroid hormones in the rat uterus. Mol Hum Reprod 6: 404–414, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Cao X, Zhang W, Wan T, He L, Chen T, Yuan Z, Ma S, Yu Y, Chen G. Molecular cloning and characterization of a novel CXC chemokine macrophage inflammatory protein-2 gamma chemoattractant for human neutrophils and dendritic cells. J Immunol 165: 2588–2595, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Challis JRG, Lye SJ. Parturition. In: The Physiology of Reproduction, edited by Knobil E, Neill JD. New York: Raven, 1994, p. 985–1031.
- 15.Challis JRG, Matthews SG, Gibb W, Lye SJ. Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev 21: 514–550, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Chan EC, Fraser S, Yin S, Yeo G, Kwek K, Fairclough RJ, Smith R. Human myometrial genes are differentially expressed in labor: a suppression subtractive hybridization study. J Clin Endocrinol Metab 87: 2435–2441, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Chen JJ, Wang SJ, Tsai CA, Lin CJ. Selection of differentially expressed genes in microarray data analysis. Pharmacogenomics J 7: 212–220, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Childers SR, Deadwyler SA. Role of cyclic AMP in the actions of cannabinoid receptors. Biochem Pharmacol 52: 819–827, 1996. [DOI] [PubMed] [Google Scholar]
- 19.Chini EN, de Toledo FG, Thompson MA, Dousa TP. Effect of estrogen upon cyclic ADP ribose metabolism: beta-estradiol stimulates ADP ribosyl cyclase in rat uterus. Proc Natl Acad Sci USA 94: 5872–5876, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Concas A, Follesa P, Barbaccia ML, Purdy RH, Biggio G. Physiological modulation of GABAA receptor plasticity by progesterone metabolites. Eur J Pharmacol 375: 225–235, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Cox BE, Ipson MA, Shaul PW, Kamm KE, Rosenfeld CR. Myometrial angiotensin II receptor subtypes change during ovine pregnancy. J Clin Invest 92: 2240–2248, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daniel JC Jr, Jetton AE, Chilton BS. Prolactin as a factor in the uterine response to progesterone in rabbits. J Reprod Fertil 72: 443–452, 1984. [DOI] [PubMed] [Google Scholar]
- 23.Dong YL, Gangula PR, Fang L, Yallampalli C. Differential expression of cyclooxygenase-1 and -2 proteins in rat uterus and cervix during the estrous cycle, pregnancy, labor and in myometrial cells. Prostaglandins 52: 13–34, 1996. [DOI] [PubMed] [Google Scholar]
- 24.Drewes PG, Yanagisawa H, Starcher B, Hornstra I, Csiszar K, Marinis SI, Keller P, Word RA. Pelvic organ prolapse in fibulin-5 knockout mice: pregnancy-induced changes in elastic fiber homeostasis in mouse vagina. Am J Pathol 170: 578–589, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ducza E, Gaspar R, Marki A, Gyula P, Bottka S, Falkay G. Use of antisense oligonucleotides to verify the role of the alpha1A-adrenergic receptor in the contractility of the rat uterus post partum. Mol Pharmacol 59: 1235–1242, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Edwards DP Regulation of signal transduction pathways by estrogen and progesterone. Annu Rev Physiol 67: 335–376, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Eghbali M, Deva R, Alioua A, Minosyan TY, Ruan H, Wang Y, Toro L, Stefani E. Molecular and functional signature of heart hypertrophy during pregnancy. Circ Res 96: 1208–1216, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA 95: 14863–14868, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Engstrom T, Bratholm P, Vilhardt H, Christensen NJ. Effect of pregnancy on rat myometrial beta2-adrenoceptor mRNA and isoproterenol-induced relaxation of isolated uterine strips. J Endocrinol 153: 393–399, 1997. [DOI] [PubMed] [Google Scholar]
- 30.Espey LL, Ujioka T, Russell DL, Skelsey M, Vladu B, Robker RL, Okamura H, Richards JS. Induction of early growth response protein-1 gene expression in the rat ovary in response to an ovulatory dose of human chorionic gonadotropin. Endocrinology 141: 2385–2391, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Esplin MS, Fausett MB, Peltier MR, Hamblin S, Silver RM, Branch DW, Adashi EY, Whiting D. The use of cDNA microarray to identify differentially expressed labor-associated genes within the human myometrium during labor. Am J Obstet Gynecol 193: 404–413, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Fata JE, Ho AT, Leco KJ, Moorehead RA, Khokha R. Cellular turnover and extracellular matrix remodeling in female reproductive tissues: functions of metalloproteinases and their inhibitors. Cell Mol Life Sci 57: 77–95, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fuchs AR Plasma, membrane receptors regulating myometrial contractility and their hormonal modulation. Semin Perinatol 19: 15–30, 1995. [DOI] [PubMed] [Google Scholar]
- 34.Fujii E, Mellon SH. Regulation of uterine gamma-aminobutyric acid(A) receptor subunit expression throughout pregnancy. Endocrinology 142: 1770–1777, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Fulep E, Vedernikov Y, Saade GR, Garfield RE. Contractility of late pregnant rat myometrium is refractory to activation of soluble but not particulate guanylate cyclase. Am J Obstet Gynecol 185: 158–162, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Geering K The functional role of beta subunits in oligomeric P-type ATPases. J Bioenerg Biomembr 33: 425–438, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Geimonen E, Boylston E, Royek A, Andersen J. Elevated connexin-43 expression in term human myometrium correlates with elevated c-Jun expression and is independent of myometrial estrogen receptors. J Clin Endocrinol Metab 83: 1177–1185, 1998. [DOI] [PubMed] [Google Scholar]
- 38.Girotti M, Zingg HH. Gene expression profiling of rat uterus at different stages of parturition. Endocrinology 144: 2254–2265, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Goffin V, Binart N, Touraine P, Kelly PA. Prolactin: the new biology of an old hormone. Annu Rev Physiol 64: 47–67, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Gorodeski GI, Burfeind P, Gan SU, Pal D, Abdul-Karim FW. Regulation by retinoids of P2Y2 nucleotide receptor mRNA in human uterine cervical cells. Am J Physiol Cell Physiol 275: C758–C765, 1998. [DOI] [PubMed] [Google Scholar]
- 41.Gouon-Evans V, Pollard JW. Eotaxin is required for eosinophil homing into the stroma of the pubertal and cycling uterus. Endocrinology 142: 4515–4521, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Hatthachote P, Morgan J, Dunlop W, Europe-Finner GN, Gillespie JI. Gestational changes in the levels of transforming growth factor-beta1 (TGFbeta1) and TGFbeta receptor types I and II in the human myometrium. J Clin Endocrinol Metab 83: 2987–2992, 1998. [DOI] [PubMed] [Google Scholar]
- 43.Havelock JC, Keller P, Muleba N, Mayhew BA, Casey BM, Rainey WE, Word RA. Human myometrial gene expression before and during parturition. Biol Reprod 72: 707–719, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Hedblom E, Kirkness EF. A novel class of GABAA receptor subunit in tissues of the reproductive system. J Biol Chem 272: 15346–15350, 1997. [DOI] [PubMed] [Google Scholar]
- 45.Huang Y, Ye D, Wu P, Huang Y, Zhang L, Zhou X, Huang Y, Yuan P, Zhang D, Wan J. Expression of cyclooxygenase-2 mRNA and identification of its splice variant in human myometrium obtained from women in labor. J Huazhong Univ Sci Technolog Med Sci 25: 5–7, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Inoue T, Takayanagi K, Morooka S, Uehara Y, Oda H, Seiki K, Nakajima H, Urade Y. Serum prostaglandin D synthase level after coronary angioplasty may predict occurrence of restenosis. Thromb Haemost 85: 165–170, 2001. [PubMed] [Google Scholar]
- 47.Jablonski EM, McConnell NA, Hughes FM Jr, Huet-Hudson YM. Estrogen regulation of aquaporins in the mouse uterus: potential roles in uterine water movement. Biol Reprod 69: 1481–1487, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Kelly RW Pregnancy maintenance and parturition: the role of prostaglandin in manipulating the immune and inflammatory response. Endocr Rev 15: 684–706, 1994. [DOI] [PubMed] [Google Scholar]
- 49.Larcher A, Neculcea J, Breton C, Arslan A, Rozen F, Russo C, Zingg HH. Oxytocin receptor gene expression in the rat uterus during pregnancy and the estrous cycle and in response to gonadal steroid treatment. Endocrinology 136: 5350–5356, 1995. [DOI] [PubMed] [Google Scholar]
- 50.Lefebvre DL, Giaid A, Bennett H, Lariviere R, Zingg HH. Oxytocin gene expression in rat uterus. Science 256: 1553–1555, 1992. [DOI] [PubMed] [Google Scholar]
- 51.Li C, Hung WW. Model-based analysis of oligonucleotide arrays: model validation, design issues and standard error application. Genome Biol 2: 32.1–32.11, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li C, Wong WH. Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc Natl Acad Sci USA 98: 31–36, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lindsay LA, Murphy CR. Aquaporin-1 increases in the rat myometrium during early pregnancy. J Mol Histol 35: 75–79, 2004. [DOI] [PubMed] [Google Scholar]
- 54.Lindsay LA, Murphy CR. Redistribution of aquaporins in uterine epithelial cells at the time of implantation in the rat. Acta Histochem 106: 299–307, 2004. [DOI] [PubMed] [Google Scholar]
- 55.Lindsay LA, Murphy CR. Redistribution of aquaporins 1 and 5 in the rat uterus is dependent on progesterone: a study with light and electron microscopy. Reproduction 131: 369–378, 2006. [DOI] [PubMed] [Google Scholar]
- 56.Lippi G, Albiero A, Montagnana M, Salvagno GL, Scevarolli S, Franchi M, Guidi GC. Lipid and lipoprotein profile in physiological pregnancy. Clin Lab 53: 173–177, 2007. [PubMed] [Google Scholar]
- 57.Lye SJ, Nicholson BJ, Mascarenhas M, MacKenzie L, Petrocelli T. Increased expression of connexin-43 in the rat myometrium during labor is associated with an increase in the plasma estrogen:progesterone ratio. Endocrinology 132: 2380–2386, 1993. [DOI] [PubMed] [Google Scholar]
- 58.Massague J TGF-beta signal transduction. Annu Rev Biochem 67: 753–791, 1998. [DOI] [PubMed] [Google Scholar]
- 59.Mati JK, Mugambi M, Muriuki PB, Thairu K. Effect of prolactin on isolated rabbit myometrium. J Endocrinol 60: 379–380, 1974. [DOI] [PubMed] [Google Scholar]
- 60.Niemeier A, Willnow T, Dieplinger H, Jacobsen C, Meyer N, Hilpert J, Beisiegel U. Identification of megalin/gp330 as a receptor for lipoprotein(a) in vitro. Arterioscler Thromb Vasc Biol 19: 552–561, 1999. [DOI] [PubMed] [Google Scholar]
- 61.Nishimori K, Young LJ, Guo Q, Wang Z, Insel TR, Matzuk MM. Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. Proc Natl Acad Sci USA 93: 11699–11704, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ou CW, Orsino A, Lye SJ. Expression of connexin-43 and connexin-26 in the rat myometrium during pregnancy and labor is differentially regulated by mechanical and hormonal signals. Endocrinology 138: 5398–5407, 1997. [DOI] [PubMed] [Google Scholar]
- 63.Pajalunga D, Mazzola A, Franchitto A, Puggioni E, Crescenzi M. The logic and regulation of cell cycle exit and reentry. Cell Mol Life Sci 65: 8–15, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Penning TM, Pawlowski JE, Schlegel BP, Jez JM, Lin HK, Hoog SS, Bennett MJ, Lewis M. Mammalian 3 alpha-hydroxysteroid dehydrogenases. Steroids 61: 508–523, 1996. [DOI] [PubMed] [Google Scholar]
- 65.Richard C, Gao J, Brown N, Reese J. Aquaporin water channel genes are differentially expressed and regulated by ovarian steroids during the periimplantation period in the mouse. Endocrinology 144: 1533–1541, 2003. [DOI] [PubMed] [Google Scholar]
- 66.Roh CR, Lee BL, Oh WJ, Whang JD, Choi DS, Yoon BK, Lee JH. Induction of c-Jun mRNA without changes of estrogen and progesterone receptor expression in myometrium during human labor. J Korean Med Sci 14: 552–558, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rudolph-Owen LA, Hulboy DL, Wilson CL, Mudgett J, Matrisian LM. Coordinate expression of matrix metalloproteinase family members in the uterus of normal, matrilysin-deficient, and stromelysin-1-deficient mice. Endocrinology 138: 4902–4911, 1997. [DOI] [PubMed] [Google Scholar]
- 68.Sanborn BM Hormones and calcium: mechanisms controlling uterine smooth muscle contractile activity. The Litchfield Lecture. Exp Physiol 86: 223–237, 2001. [DOI] [PubMed] [Google Scholar]
- 69.Shintani Y, Hirano K, Nishimura J, Nakano H, Kanaide H. Enhanced contractile response to thrombin in the pregnant rat myometrium. Br J Pharmacol 131: 1619–1628, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Siiteri PK, Stites DP. Immunologic and endocrine interrelationships in pregnancy. Biol Reprod 26: 1–14, 1982. [DOI] [PubMed] [Google Scholar]
- 71.Stevens MY, Challis JR, Lye SJ. Corticotropin-releasing hormone receptor subtype 1 is significantly up-regulated at the time of labor in the human myometrium. J Clin Endocrinol Metab 83: 4107–4115, 1998. [DOI] [PubMed] [Google Scholar]
- 72.Ushigome F, Takanaga H, Matsuo H, Yanai S, Tsukimori K, Nakano H, Uchiumi T, Nakamura T, Kuwano M, Ohtani H, Sawada Y. Human placental transport of vinblastine, vincristine, digoxin and progesterone: contribution of P-glycoprotein. Eur J Pharmacol 408: 1–10, 2000. [DOI] [PubMed] [Google Scholar]
- 73.Watanabe H, Suzuki A, Mizutani T, Khono S, Lubahn DB, Handa H, Iguchi T. Genome-wide analysis of changes in early gene expression induced by oestrogen. Genes Cells 7: 497–507, 2002. [DOI] [PubMed] [Google Scholar]
- 74.Wiese TJ, Dunlap JA, Conner CE, Grzybowski JA, Lowe WL Jr, Yorek MA. Osmotic regulation of Na-myo-inositol cotransporter mRNA level and activity in endothelial and neural cells. Am J Physiol Cell Physiol 270: C990–C997, 1996. [DOI] [PubMed] [Google Scholar]
- 75.Zhao B, Koon D, Curtis AL, Soper J, Bethin KE. Identification of 9 uterine genes that are regulated during mouse pregnancy and exhibit abnormal levels in the cyclooxygenase-1 knockout mouse. Reprod Biol Endocrinol 5: 28, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhu Y, Pintar JE. Expression of opioid receptors and ligands in pregnant mouse uterus and placenta. Biol Reprod 59: 925–932, 1998. [DOI] [PubMed] [Google Scholar]