Abstract
Cell swelling activates or upregulates a number of anion channels. Of the volume-activated or -regulated anion channels (VAACs or VRACs), the volume-sensitive outwardly rectifying anion channel (VSOR) is most prominently activated and ubiquitously expressed. This channel is known to be involved in a variety of physiological processes including cell volume regulation, cell proliferation, differentiation and cell migration as well as cell turnover involving apoptosis. Recent studies have shown that VSOR activity is also involved in a number of pathophysiological processes including the acquisition of cisplatin resistance by cancer cells, ischaemia–reperfusion-induced death of cardiomyocytes and hippocampal neurons, glial necrosis under lactacidosis as well as neuronal necrosis under excitotoxicity. Moreover, VSOR serves as the pathway for glutamate release from astrocytes under ischaemic conditions and when stimulated by bradykinin, an initial mediator of inflammation. So far, many signalling molecules including the EGF receptor, PI3K, Src, PLCγ and Rho/Rho kinase have been implicated in the regulation of VSOR activity. However, our pharmacological studies suggest that these signals are not essential components of the swelling-induced VSOR activation mechanism even though some of these signals may play permissive or modulatory roles. Molecular identification of VSOR is required to address the question of how cells sense volume expansion and activate VSOR.
Changes in cell volume are associated with a variety of physiological and pathophysiological processes including the proliferation and migration of cells as well as the cellular response to ischaemia and the induction of cell death (see Nilius et al. 1997; Okada, 1997; Lang et al. 1998; d’Anglemont de Tassigny et al. 2003; Okada et al. 2004). In most cases, cells regulate their volume after cell volume fluctuations. Regulatory volume decrease (RVD) is accomplished mainly by releasing K+ and Cl− as well as osmotically obligated water from the intracellular compartment of swollen cells. Since the K+ conductance is much larger than the Cl− conductance in most cell types (except red blood cells and muscle cells), a marked increase in Cl− conductance is more important for swollen cells when regulating their volume. Osmotic cell swelling activates or upregulates a number of anion channels including the maxi-anion channel, ClC-2 and dBest1 (see Okada et al. 2009). Of these volume-activated or -regulated anion channels (VAACs or VRACs), the most prominently activated and widely expressed is the volume-sensitive outwardly rectifying Cl− channel (VSOR), named in such a way to distinguish it from other VAACs or VRACs including inwardly rectifying ClC-2 anion channels, which belongs to the CLC family, ohmic maxi-anion channels and dBest1, which belongs to the bestrophin family and exhibits a sigmoidal current–voltage (I–V) relationship (Okada, 1997; Okada et al. 2009). The VSOR current is phenotypically characterized not only by its volume sensitivity and mild outward rectification but also by its intermediate single-channel conductance, low-field strength anion selectivity, broad-spectrum sensitivity to anion channel blockers, intracellular ATP dependence, and open channel block by extracellular ATP (Strange et al. 1996; Nilius et al. 1997; Okada, 1997, 2006). Despite its well-characterized properties, the molecular identity of VSOR is not known (see Okada et al. 2009). This review describes the roles of VSOR in pathophysiological conditions including ischaemia–reperfusion, excitotoxicity, lactacidosis, cisplatin resistance and inflammation. It also highlights puzzles about the activation mechanisms of VSOR.
Roles of VSOR in apoptosis, ischaemia–reperfusion-induced cell death and cisplatin resistance
After osmotic cell swelling, VSOR is activated, and through the RVD process, prevents the cell from undergoing sustained cell swelling that may result in necrotic cell death. In contrast to its role in rescuing the cell, VSOR activation may also induce necrotic neuronal cell death under excitotoxic conditions (see below) as well as induce programmed cell death after stimulation with apoptosis inducers or after ischaemia–reperfusion stress.
Apoptosis induction requires persistent cell shrinkage (see Shimizu et al. 2007) which is called apoptotic volume decrease (AVD) (Maeno et al. 2000; Okada et al. 2001). As shown in Fig. 1 (upper scheme), the AVD process, which is accomplished by exit of KCl and osmotically obligated water from the cell (Okada et al. 2001), involves activation of VSOR when cells are stimulated by a mitochondrion-mediated apoptosis inducer (such as staurosporine) or a death receptor-mediated apoptosis inducer (such as Fas ligand or TNFα) (see Okada et al. 2004, 2006). By virtue of the hyperpolarization produced by activation of K+ channels, VSOR activity induces Cl− efflux, leading to the reduction of cell volume.
Figure 1. Roles of VSOR activity in apoptotic and necrotic cell death.
Top, roles of VSOR activity in the apoptotic volume decrease (AVD) in response to apoptotic stimuli, ischaemia–reperfusion and acquired cisplatin resistance of cancer cells. Bottom, roles of VSOR activity in the necrotic volume increase (NVI) in response to lactacidosis or excitotoxicity. For details, see the text. Abbreviations: STS, staurosporine; FasL, Fas ligand; TNFα, tumor necrosis factor α; ROS, reactive oxygen species; GluR, glutamate receptor; NHE, Na+/H+ exchanger; MCT, monocarboxylate transporter; RVI, regulatory volume increase; RVD, regulatory volume decrease.
The platinum-based drug cisplatin is an anticancer drug widely used in cancer therapy. Cisplatin-induced apoptotic death of cancer cells is also associated with activation of VSOR (see Shimizu et al. 2008) (Fig. 1: upper scheme). For the following reasons, VSOR is an essential part of the mechanism of cisplatin-induced apoptosis in human epidermoid cancer KB cells: first, pretreatment of KB cells with cisplatin enhanced VSOR activity (Ise et al. 2005). Second, pharmacological blockade of VSOR reduced the sensitivity of KB cells to cisplatin (Ise et al. 2005). Third, cisplatin-resistant KCP-4 cells, a subclone derived from KB cells, were found to have no VSOR activity (Lee et al. 2007). Fourth, KCP-4 cells acquired sensitivity to cisplatin when VSOR activity was partially restored by pretreatment with the histone deacetylase (HDAC) inhibitors trichostatin A (TSA) and apicidin (Lee et al. 2007; Shimizu et al. 2008). In light of the latter two observations, it appears that the acquisition of cisplatin resistance by human epidermoid cancer cells (KB cells) involves impaired VSOR activity.
VSOR activity is also involved in the apoptotic death of cardiomyocytes and brain neurons subjected to ischaemia–reperfusion (Fig. 1: upper scheme). Mouse ventricular myocytes in primary culture underwent apoptosis following a profound increase in intracellular reactive oxygen species (ROS), in response to ischaemia–reperfusion (Wang et al. 2005). The VSOR blockers 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS), 5-nitro-2-(3-phenylpropylamino)benzoic acid (NPPB) and phloretin rescued cells from apoptotic death when applied during reperfusion, but not in the period of ischaemia (Wang et al. 2005). Hippocampal neurons are known to undergo delayed neuronal death (DND), which occurs largely by apoptosis, several days after transient forebrain ischaemia in vivo. Inoue et al. (2007) demonstrated that DND induction is strongly abrogated by in vivo administration of DIDS or genistein, which effectively blocks VSOR activity in mouse hippocampal neurons.
To bring about apoptotic cell death, AVD must persist by the sustained activation of VSOR under iso-osmotic conditions, without triggering a regulatory volume increase (RVI) (Fig. 1: upper scheme). In fact, in HeLa cells undergoing apoptosis, the RVI mechanism was demonstrated to be impaired (Maeno et al. 2006; Numata et al. 2008).
Roles of VSOR in necrotic cell death under lactacidosis and excitotoxicity
Necrotic cell death occurs in parallel with normotonic cell swelling, called necrotic volume increase (NVI) (Okada et al. 2001), which persists until the cell membrane ruptures. Figure 1 (lower scheme) depicts the NVI process under lactacidosis and excitotoxicity.
Cerebral ischaemia, hypoxia and trauma are frequently associated with lactacidosis, in which acid and lactate are accumulated through augmented glycolysis-fermentation reactions. Lactacidosis results in the swelling of neuronal and glial cells not only because of facilitated uptake of lactate and H+ via proton-coupled monocarboxylate transporters (MCTs) and of Na+ via Na+/H+ exchangers (NHEs), but also because of dysfunction of RVD, due to inhibition of VSOR activity by protons overaccumulated within the cells (Nabekura et al. 2003) (Fig. 1 lower scheme). Following swelling, glial cells undergo necrotic cell death; however, such lactacidosis-induced necrosis is attenuated when lactacidosis-resistant anion channels are introduced exogenously into glial cells (Okada et al. 2004). In conditions of lactacidosis, it therefore appears that inhibition of VSOR activity induces necrotic cell death.
Neurotoxicity caused by exposure to excessive glutamate released from glial cells is called excitotoxicity and is associated with stroke, cerebral ischaemia, brain trauma and a number of neurodegenerative disorders. During excitotoxic insults, brain neurons undergo somatic swelling and develop dendritic focal swellings called varicosities. Initiation of such excitotoxic neuronal swelling is induced by water inflow driven by Na+ inflow via glutamate receptor cation channels (GluRs) and Cl− inflow via GABAA receptor anion channels (GABAARs); this swelling activates VSOR. Thus, once activated VSOR serves as a pathway, not for volume-regulatory Cl− efflux, but for swelling-aggravated Cl− influx during the prominent depolarization induced by GluR activation (Fig. 1: lower scheme). When neurons repolarize after washout of glutamate, VSOR participates in RVD rescuing the cell from excitotoxicity (Inoue & Okada, 2007). However, when excitotoxic insults are prolonged, neuronal swelling persists not only because of continued inflow of Na+, Cl− and osmotically obliged water, but also because of RVD dysfunction caused by the ‘reversed’ operation of VSOR; this leads to necrotic neuronal death (Inoue & Okada, 2007). Therefore, it appears that VSOR activation exerts dual, reciprocal actions on neuronal excitotoxic injury, serving as (i) a volume-regulatory anion exit pathway after washout of glutamate, which rescues the cell and (ii) as an NVI-inducing anion entry pathway under prolonged excitotoxic insults, which kills the cell.
It should be noted that induction of necrotic cell death involves activation and inhibition of VSOR during brain injury due to excitotoxicity and lactacidosis, respectively.
Roles of VSOR in glutamate release from glial cells under ischaemic or inflammatory conditions
The major excitatory neurotransmitter in the brain, glutamate, is released by neurons at excitatory synapses as well as by glial cells, serving to transfer information between neurons and/or glial cells (Fellin et al. 2006; Ni et al. 2007; Yang et al. 2008). Glutamate release from glial cells plays essential roles not only in excitotoxic neuronal injury in cerebral ischaemia/hypoxia and trauma (Kimelberg, 2005), but also in glia-to-neuron signal transduction under inflammatory conditions (Bezzi et al. 2001). So far, six mechanisms or pathways have been proposed to be involved in glutamate release from glial cells (for review, see: Malarkey & Parpura, 2008): (1) vesicular exocytosis, (2) reversed operation of glutamate transporters, (3) cystine/glutamate antiporters, (4) connexin hemi-channels, (5) P2X7 receptor cation channels, and (6) volume-activated anion channels. Currently, it is not clear whether glutamate is released through specific mechanisms under particular conditions.
By monitoring the release of preloaded [3H]-l-glutamate or [3H]-d-aspartate, Kimelberg and his collaborators have provided indirect evidence for the involvement of volume-activated or -regulated anion channels (VAACs or VRACs) in swelling- and ischaemia-induced release of excitatory amino acids from rat astrocytes (see Kimelberg, 2005). Recently, we have provided more direct evidence by measuring released glutamate and anionic channel currents in mouse astrocytes (Liu et al. 2006). Osmotic swelling and chemical ischaemia activated two types of VAACs or VRACs, VSOR and the maxi-anion channel, which both served as pathways for glutamate release. Also, it is noted that in pre-swollen rat microglia, stimulation with zymosan activates VSOR via NOX4-mediated H2O2 production and enhanced release of [3H]-d-aspartate (Harrigan et al. 2008).
Bradykinin is an initial mediator of inflammation known to be released after brain injury or stroke (Gröger et al. 2005). In response to stimulation with bradykinin, rat astrocytes release glutamate, causing an NMDA receptor-mediated Ca2+ response in neighboring neurons (Parpura et al. 1994). Our recent study (Liu et al. 2009) shows that VSOR is activated by ROS produced when bradykinin stimulates the B2 receptor (B2R) of mouse astrocytes and that VSOR serves as the glutamate release pathway in these cells. Since mouse astrocytes stimulated with bradykinin did not exhibit noticeable cell swelling (Liu et al. 2009), it is likely that cell swelling is not required for ROS-induced VSOR activation in astrocytes, just as is the case for ROS-induced VSOR activation in human epithelial HeLa cells stimulated with the apoptotic inducer staurosporine (Shimizu et al. 2004).
Since astrocyte swelling is known to occur not only upon a hypotonic challenge, but also upon cerebral ischaemia (Kimelberg, 2005), it is possible that ischaemia-induced glutamate release is caused by swelling of astrocytes. Thus, hypotonicity- and ischaemia-induced glutamate release is mediated by the swelling-induced activation of two types of VAACs or VRACs (VSOR and the maxi-anion channel), whereas bradykinin-induced glutamate release is mediated by the ROS-induced activation of VSOR alone in astrocytes (Fig. 2).
Figure 2. Roles of VSOR activity in glutamate release from astrocytes in response to ischaemia- or hypotonicity-induced cell swelling and to bradykinin-induced ROS production.
For details, see the text. Abbreviations: B2R, B2 bradykinin receptor; ROS, reactive oxygen species.
Puzzles about the activation mechanisms of VSOR
A large number of studies have been performed to elucidate the activation mechanism of VSOR, and many signalling molecules including components of the MAP kinase cascade have been shown to regulate VSOR activity (for review, see Nilius et al. 1997; Okada, 1997; d’Anglemont de Tassigny et al. 2003; Nilius & Droogmans, 2003; Stutzin & Hoffmann, 2006). Figure 3 illustrates the network of intracellular signalling mechanisms activated during osmotic cell swelling or plasma membrane expansion (for citations, see the legend of Fig. 3).
Figure 3. Regulation of VSOR activity by signalling molecule cascades.
To date, the following intracellular signalling cascades have been observed to be activated upon osmotic cell swelling and have been reported to be associated with activation or augmentation of VSOR activity: the Ras–Raf–MEK–ERK, PI3K–NOX–H2O2, Src–PLCγ–Ca2+ and Rho–ROK–MLCK pathways. The earliest reports for these signals are as follows: angiotensin II type 1 (AT1R) (Browe & Baumgarten, 2004), epidermal growth factor receptor (EGFR) (Tilly et al. 1993), mitogen-activated protein kinase kinase (MEK) (Crepel et al. 1998), extracellular signal-regulated kinase (ERK) (Tilly et al. 1993), phosphatidylinositol 3-kinase (PI3K) (Feranchak et al. 1998), NADPH oxidase (NOX) and H2O2 (Varela et al. 2004), tyrosine kinase Src (Lepple-Wienhues et al. 1998), phospholipase Cγ (PLCγ) (Varela et al. 2007), Ca2+ (Basavappa et al. 1995), low molecular weight GTPase Rho (Tilly et al. 1996), Rho kinase (ROK) (Nilius et al. 1999), and myosin light chain kinase (MLCK) (Nilius et al. 2000).
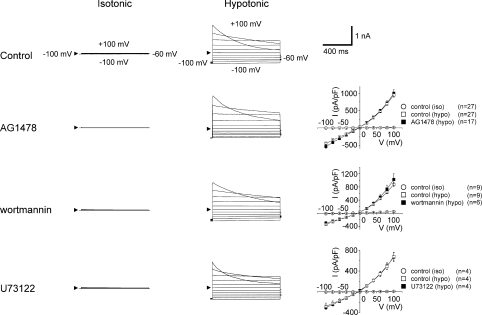
Using pharmacological tools, we recently examined the roles of different intracellular signalling pathways in osmotically swollen human epithelial HeLa cells; the data are illustrated in Fig. 4 and summarized in Table 1. VSOR current was partially suppressed, but not abolished, by losartan, an antagonist of the angiotensin II type 1 receptor (AT1R). However, blocking the downstream kinase, epidermal growth factor receptor (EGFR), with AG1478 failed to suppress VSOR activity (Fig. 4: second row). Downstream of EGFR, there exist three signalling pathways (Fig. 3): the Ras–Raf–MEK–ERK (where MEK and ERK represent mitogen-activated protein kinase kinase and extracellular signal-regulated kinase, respectively), PI3K–NOX–H2O2 (where PI3K and NOX represent phosphatidylinositol 3-kinase and some isoform of NADPH oxidase, respectively), and Src–PLCγ–Ca2+ pathways. Antagonists of MEK (U0126) and downstream ERK (FR180204) significantly suppressed, but failed to abolish, VSOR current. By contrast, VSOR activity was insensitive to wortmannin, an antagonist of PI3K (Fig. 4: third row). Although PP2, a blocker of Src partially suppressed VSOR activity, U73122, an antagonist of downstream PLCγ, failed to affect VSOR currents (Fig. 4: bottom row). Therefore, it appears that EGFR, PI3K and PLCγ are not essential components in the swelling-induced activation mechanisms, but AT1R, MEK, ERK and PP2 may play modulatory or permissive roles in VSOR activity in HeLa cells. Consistent with the conclusions of (Nilius & Droogmans, 2003), two antagonists of Rho kinase (ROK), RhoK inhibitor and RhoK inhibitor-2, both suppressed, but did not abolish, VSOR currents, again suggesting a permissive role of the Rho–ROK–MLCK pathway in the modulation (but not activation per se) of VSOR.
Figure 4. Pharmacological characterization of VSOR whole-cell currents.
Whole-cell recordings were performed in the absence (Control) or presence of the indicated agents, as described in the legend for Table 1, before (Isotonic: iso) and during hypotonic stimulation (Hypotonic: hypo). Arrowheads represent the zero current level. Each symbol in the I–V curves represents the mean value of instantaneous current density (measured 5 ms after the onset of the test pulse) ±s.e.m. (vertical bar).
Table 1.
Effects of antagonists of signalling molecules on whole-cell VSOR currents in swollen HeLa cells
| Drug concentration (μm) | Relative current density (at −100 mV) | Number of observations | |
|---|---|---|---|
| Control | — | 1 | 98 |
| Losartan | 500 | 0.80 ± 0.04* | 11 |
| AG1478 | 1 | 1.21 ± 0.11 | 17 |
| U0126† | 5 | 0.59 ± 0.06* | 11 |
| FR180204† | 10 | 0.76 ± 0.12* | 11 |
| Wortmannin | 10 | 1.05 ± 0.01 | 6 |
| Wortmannin† | 10 | 0.91 ± 0.16 | 10 |
| PP2† | 10 | 0.81 ± 0.05* | 26 |
| U73122† | 10 | 1.01 ± 0.12 | 4 |
| RhoK inhibitor† | 1 | 0.61 ± 0.07* | 8 |
| RhoK inhibitor-2† | 20 | 0.62 ± 0.08* | 12 |
Whole-cell currents were recorded at −100 mV in the absence (Control) or presence of each drug for 20–40 min using isotonic pipette solution containing (mm) 110 CsCl, 2 MgSO4, 1 EGTA, 10 Hepes, 2 Na2ATP, 0.3 Na3GTP and 50 mannitol (pH 7.3) and hypotonic bath solution containing (mm) 110 CsCl, 5 MgSO4, 10 Hepes and 45 mannitol (pH 7.5). †After the following pretreatments: U0126, 10–60 min; FR18024, 15 h; wortmannin, 10–60 min; PP2, 10–360 min, U73122, 10–60 min; RhoK inhibitor, 10–60 min; RhoK inhibitor-2, 10–60 min.
P < 0.05.
VSOR current is known to be activated only above a threshold level of cell swelling (relative cell surface area, ∼1.25; relative cell volume, ∼1.4) with current magnitude increasing linearly as cell surface areas expand over a certain range above the threshold (Okada, 1997) (Fig. 5, continuous line). The volume expansion sensitivity of VSOR is augmented by a number of factors (such as microfilament disruption and P-glycoprotein overexpression) which decrease the threshold for VSOR activation or increase the sensitivity of VSOR to volume expansion (Miwa et al. 1997; Okada, 1997). Therefore, it is possible that permissive signals and their modulators affect VSOR sensitivity to volume expansion in two ways: (i) by inducing a leftward shift (Fig. 5: broken line and filled arrow) or (ii) by increasing the slope (Fig. 5: dotted line and open arrow) of the relationship between VSOR current density and relative outer cell surface area; these changes might occur along with or without a change in the threshold level of cell swelling for VSOR activation.
Figure 5. Volume expansion sensitivity of VSOR activity.
The relationship between relative cell surface area and VSOR current density is shown. Arrowhead indicates the threshold cell volume for VSOR activation in response to cell swelling. Filled and open arrows represent augmentation of the volume expansion sensitivity of VSOR by decreasing the threshold without changing the slope (broken line) and by increasing the slope without changing the threshold (dotted line). For details, see the text.
The VSOR current can be activated under isotonic conditions by intracellular dialysis with GTPγS or by reduction of intracellular ionic strength (for review, see Okada, 1997; Nilius & Droogmans, 2003; Stutzin & Hoffmann, 2006). GTPγS may affect many signalling molecules, especially G-protein-coupled receptors (such as AT1R) and small G-proteins (such as Ras), involved in VSOR regulation (Fig. 3). Reduced ionic strength may affect the activity of signalling protein molecules, which ordinarily have multi-valent negative charges, by changing their surface potential. In the whole-cell mode of the patch-clamp technique, isotonic conditions do not necessarily mean symmetrical osmolarity conditions between extracellular and intracellular solutions, because of the existence of poorly diffusible macromolecular (colloidal) osmolytes within the cytosol (which usually give an effective osmolality reaching 30–40 mosmol (kg H2O)−1). When the effective intracellular osmolarity (πi) composed of the pipette solution (πp) plus the cytosolic colloidal osmolarity (πc) exceeds the osmolarity of the extracellular solution (πo), the cell swells. Therefore, it is possible that imperceptible cell swelling is involved in apparently isovolumetric activation of VSOR if its threshold of volume expansion sensitivity is lowered by GTPγS or reduced ionic strength.
Apart from the mechanisms of VSOR modulation, the mechanism of VSOR activation per se is poorly understood. It is possible that ROS, such as H2O2, are capable of acting as activators of VSOR (Browe & Baumgarten, 2004; Shimizu et al. 2004; Varela et al. 2004). However, ROS-induced VSOR activation takes place without volume expansion (Shimizu et al. 2004). How does the cell sense changes in volume expansion and activate VSOR? To answer this question precisely, we await the molecular identification of VSOR.
In conclusion, VSOR plays essential roles not only in a variety of physiological processes including cell volume regulation, cell proliferation, cell migration and cell turnover involving apoptosis, but also in a number of pathophysiological processes occurring under ischaemia, ischaemia–reperfusion, lactacidosis, excitotoxicity and inflammation as well as in cisplatin resistance of cancer cells. Thus, this anion channel may provide a new target for drugs or gene manipulation that can counter these pathological insults. Molecular identification of VSOR is an urgent task that should greatly promote elucidation of its activation and volume sensing mechanisms, and lead to the development of new therapeutic strategies for alleviating various pathological conditions.
Acknowledgments
The authors would like to thank Dr Ravshan Z. Sabirov for reading the manuscript and pertinent discussion, Dr Elbert L. Lee for editing the English and Ms Tomomi Okayasu for secretarial assistance. This work was supported by Grant-in-Aid for Scientific Research (A) from the Japan Society for the Promotion of Science (JSPS) and that for Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT).
Author's present address
T. Numata: Department of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering, Kyoto University, Kyoto 615-8510, Japan.
References
- Basavappa S, Chartouni V, Kirk K, Prpic V, Ellory JC, Mangel AW. Swelling-induced chloride currents in neuroblastoma cells are calcium dependent. J Neurosci. 1995;15:3662–3666. doi: 10.1523/JNEUROSCI.15-05-03662.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, Vescovi A, Bagetta G, Kollias G, Meldolesi J, Volterra A. CXCR4-activated astrocyte glutamate release via TNFα: amplification by microglia triggers neurotoxicity. Nat Neurosci. 2001;4:702–710. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- Browe DM, Baumgarten CM. Angiotensin II (AT1) receptors and NADPH oxidase regulate Cl− current elicited by β1 integrin stretch in rabbit ventricular myocytes. J Gen Physiol. 2004;124:273–287. doi: 10.1085/jgp.200409040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepel V, Panenka W, Kelly MEM, MacVicar BA. Mitogen-activated protein and tyrosine kinases in the activation of astrocyte volume-activated chloride current. J Neurosci. 1998;18:1196–1206. doi: 10.1523/JNEUROSCI.18-04-01196.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Anglemont de Tassigny A, Souktani R, Ghaleh B, Henry P, Berdeaux A. Structure and pharmacology of swelling-sensitive chloride channels, ICl,swell. Fundam Clin Pharmacol. 2003;17:539–553. doi: 10.1046/j.1472-8206.2003.00197.x. [DOI] [PubMed] [Google Scholar]
- Fellin T, Sul JY, D’Ascenzo M, Takano H, Pascual O, Haydon PG. Bidirectional astrocyte-neuron commnunication: the many roles of glutamate and ATP. Novartis Found Symp. 2006;276:233–237. doi: 10.1002/9780470032244.ch16. [DOI] [PubMed] [Google Scholar]
- Feranchak AP, Roman RM, Schwiebert EM, Fitz JG. Phosphatidylinositol 3-kinase contributes to cell volume regulation through effects on ATP release. J Biol Chem. 1998;273:14906–14911. doi: 10.1074/jbc.273.24.14906. [DOI] [PubMed] [Google Scholar]
- Gröger M, Lebesgue D, Pruneau D, Relton J, Kim SW, Nussberger J, Plesnila N. Release of bradykinin and expression of kinin B2 receptors in the brain: role for cell death and brain edema formation after focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2005;25:978–989. doi: 10.1038/sj.jcbfm.9600096. [DOI] [PubMed] [Google Scholar]
- Harrigan TJ, Abdullaev IF, Jourd’heuil D, Mongin AA. Activation of microglia with zymosan promotes excitatory amino acid release via volume-regulated anion channels: the role of NADPH oxidases. J Neurochem. 2008;106:2449–2462. doi: 10.1111/j.1471-4159.2008.05553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H, Ohtaki H, Nakamachi T, Shioda S, Okada Y. Anion channel blockers attenuate delayed neuronal cell death induced by transient forebrain ischemia. J Neurosci Res. 2007;85:1427–1435. doi: 10.1002/jnr.21279. [DOI] [PubMed] [Google Scholar]
- Inoue H, Okada Y. Roles of volume-sensitive chloride channel in excitotoxic neuronal injury. J Neurosci. 2007;27:1445–1455. doi: 10.1523/JNEUROSCI.4694-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ise T, Shimizu T, Lee EL, Inoue H, Kohno K, Okada Y. Roles of volume-sensitive Cl− channel in cisplatin-induced apoptosis in human epidermoid cancer cells. J Membr Biol. 2005;205:139–145. doi: 10.1007/s00232-005-0779-y. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK. Astrocytic swelling in cerebral ischemia as a possible cause of injury and target for therapy. Glia. 2005;50:389–397. doi: 10.1002/glia.20174. [DOI] [PubMed] [Google Scholar]
- Lang F, Busch GL, Ritter M, Volkl H, Waldegger S, Gulbins E, Haussinger D. Functional significance of cell volume regulatory mechanisms. Physiol Rev. 1998;78:247–306. doi: 10.1152/physrev.1998.78.1.247. [DOI] [PubMed] [Google Scholar]
- Lee EL, Shimizu T, Ise T, Numata T, Kohno K, Okada Y. Impaired activity of volume-sensitive Cl− channel is involved in cisplatin resistance of cancer cells. J Cell Physiol. 2007;211:513–521. doi: 10.1002/jcp.20961. [DOI] [PubMed] [Google Scholar]
- Lepple-Wienhues A, Szabo I, Laun T, Kaba NK, Gulbins E, Lang F. The tyrosine kinase p56lck mediates activation of swelling-induced chloride channels in lymphocytes. J Cell Biol. 1998;141:281–286. doi: 10.1083/jcb.141.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H-T, Akita T, Shimizu T, Sabirov RZ, Okada Y. Bradykinin-induced astrocyte-neuron signaling: glutamate release is mediated by ROS-activated volume-sensitive outwardly rectifying anion channels. J Physiol. 2009 doi: 10.1113/jphysiol.2008.165084. doi 10.1113/jphysiol.2008.165084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H-T, Tashmukhamedov BA, Inoue H, Okada Y, Sabirov RZ. Roles of two types of anion channels in glutamate release from mouse astrocytes under ischemic or osmotic stress. Glia. 2006;54:343–357. doi: 10.1002/glia.20400. [DOI] [PubMed] [Google Scholar]
- Maeno E, Ishizaki Y, Kanaseki T, Hazama A, Okada Y. Normotonic cell shrinkage because of disordered volume regulation is an early prerequisite to apoptosis. Proc Natl Acad Sci U S A. 2000;97:9487–9492. doi: 10.1073/pnas.140216197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeno E, Takahashi N, Okada Y. Dysfunction of regulatory volume increase is a key component of apoptosis. FEBS Lett. 2006;580:6513–6517. doi: 10.1016/j.febslet.2006.10.074. [DOI] [PubMed] [Google Scholar]
- Malarkey EB, Parpura V. Mechanisms of glutamate release from astrocytes. Neurochem Int. 2008;52:142–154. doi: 10.1016/j.neuint.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabekura T, Morishima S, Cover TL, Mori S-I, Kannan H, Komune S, Okada Y. Recovery from lactacidosis-induced glial cell swelling with the aid of exogenous anion channels. Glia. 2003;41:247–259. doi: 10.1002/glia.10190. [DOI] [PubMed] [Google Scholar]
- Ni Y, Malarkey EB, Parpura V. Vesicular release of glutamate mediates bidirectional signaling between astrocytes and neurons. J Neurochem. 2007;103:1273–1284. doi: 10.1111/j.1471-4159.2007.04864.x. [DOI] [PubMed] [Google Scholar]
- Miwa A, Ueda K, Okada Y. Protein kinase C-independent correlation between P-glycoprotein expression and volume sensitivity of Cl− channel. J Membr Biol. 1997;157:63–69. doi: 10.1007/s002329900216. [DOI] [PubMed] [Google Scholar]
- Nilius B, Droogmans G. Amazing chloride channels: an overview. Acta Physiol Scand. 2003;177:119–147. doi: 10.1046/j.1365-201X.2003.01060.x. [DOI] [PubMed] [Google Scholar]
- Nilius B, Eggermont J, Voets T, Buyse G, Manolopoulos V, Droogmans G. Properties of volume-regulated anion channels in mammalian cells. Prog Biophys Mol Biol. 1997;68:69–119. doi: 10.1016/s0079-6107(97)00021-7. [DOI] [PubMed] [Google Scholar]
- Nilius B, Prenen J, Walsh MP, Carton I, Bollen M, Droogmans G, Eggermont J. Myosin light chain phosphorylation-dependent modulation of volume-regulated anion channels in macrovascular endothelium. FEBS Lett. 2000;466:346–350. doi: 10.1016/s0014-5793(00)01097-8. [DOI] [PubMed] [Google Scholar]
- Nilius B, Voets T, Prenen J, Barth H, Aktories K, Kaibuchi K, Droogmans G, Eggermont J. Role of Rho and Rho kinase in the activation of volume-regulated anion channels in bovine endothelial cells. J Physiol. 1999;516:67–74. doi: 10.1111/j.1469-7793.1999.067aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numata T, Sato K, Okada Y, Wehner F. Hypertonicityinduced cation channels rescue cells from staurosporine-elicited apoptosis. Apoptosis. 2008;13:895–903. doi: 10.1007/s10495-008-0220-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y. Volume expansion-sensing outward-rectifier Cl− channel: fresh start to the molecular identity and volume sensor. Am J Physiol Cell Physiol. 1997;273:C755–789. doi: 10.1152/ajpcell.1997.273.3.C755. [DOI] [PubMed] [Google Scholar]
- Okada Y. Cell volume-sensitive chloride channel: Phenotypic properties and molecular identity. In: Lang F, editor. Mechanisms and Significance of Cell Volume Regulation. Basel: Karger; 2006. pp. 9–24. [DOI] [PubMed] [Google Scholar]
- Okada Y, Maeno E, Shimizu T, Dezaki K, Wang J, Morishima S. Receptor-mediated control of regulatory volume decrease (RVD) and apoptotic volume decrease (AVD) J Physiol. 2001;532:3–16. doi: 10.1111/j.1469-7793.2001.0003g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Maeno E, Shimizu T, Manabe K, Mori S, Nabekura T. Dual roles of plasmalemmal chloride channels in induction of cell death. Pflugers Arch. 2004;448:287–295. doi: 10.1007/s00424-004-1276-3. [DOI] [PubMed] [Google Scholar]
- Okada Y, Sato K, Toychiev AH, Suzuki M, Dutta AK, Inoue H, Sabirov RZ. The puzzles of volume-activated anion channels. In: Alvarez-Leefmans FJ, Delpire E, editors. Physiology and Pathology of Chloride Transporters and Channels in the Nervous System : From Molecules to Diseases. San Diego: Elsevier; 2009. in press. [Google Scholar]
- Okada Y, Shimizu T, Maeno E, Tanabe S, Wang X, Takahashi N. Volume-sensitive chloride channels involved in apoptotic volume decrease and cell death. J Membr Biol. 2006;209:21–29. doi: 10.1007/s00232-005-0836-6. [DOI] [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Lee EL, Ise T, Okada Y. Volume-sensitive Cl− channel as a regulator of acquired cisplatin resistance. Anticancer Res. 2008;28:75–83. [PubMed] [Google Scholar]
- Shimizu T, Maeno E, Okada Y. Prerequisite role of persistent cell shrinkage in apoptosis of human epithelial cells. Acta Physiologica Sinica. 2007;59:512–516. [PubMed] [Google Scholar]
- Shimizu T, Numata T, Okada Y. A role of reactive oxygen species in apoptotic activation of volume-sensitive Cl− channel. Proc Natl Acad Sci U S A. 2004;101:6770–6773. doi: 10.1073/pnas.0401604101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange K, Emma F, Jackson PS. Cellular and molecular physiology of volume-sensitive anion channels. Am J Physiol Cell Physiol. 1996;270:C711–730. doi: 10.1152/ajpcell.1996.270.3.C711. [DOI] [PubMed] [Google Scholar]
- Stutzin A, Hoffmann EK. Swelling-activated ion channels: functional regulation in cell-swelling, proliferation and apoptosis. Acta Physiol. 2006;187:27–42. doi: 10.1111/j.1748-1716.2006.01537.x. [DOI] [PubMed] [Google Scholar]
- Tilly BC, Edixhoven MJ, Tertoolen LG, Morii N, Saitoh Y, Narumiya S, de Jonge HR. Activation of the osmo-sensitive chloride conductance involves P21rho and is accompanied by a transient reorganization of the F-actin cytoskeleton. Mol Biol Cell. 1996;7:1419–1427. doi: 10.1091/mbc.7.9.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly BC, Van Den Berghe N, Tertoolen LG, Edixhoven MJ, de Jonge HR. Protein tyrosine phosphorylation is involved in osmoregulation of ionic conductances. J Biol Chem. 1993;268:19919–19922. [PubMed] [Google Scholar]
- Varela D, Simon F, Olivero P, Armisen R, Leiva-Salcedo E, Jorgensen F, Sala F, Stutzin A. Activation of H2O2-induced VSOR Cl− currents in HTC cells require phospholipase Cg1 phosphorylation and Ca2+ mobilisation. Cell Physiol Biochem. 2007;20:773–780. doi: 10.1159/000110437. [DOI] [PubMed] [Google Scholar]
- Varela D, Simon F, Riveros A, Jorgensen F, Stutzin A. NAD(P)H oxidase-derived H2O2 signals chloride channel activation in cell volume regulation and cell proliferation. J Biol Chem. 2004;279:13301–13304. doi: 10.1074/jbc.C400020200. [DOI] [PubMed] [Google Scholar]
- Wang X, Takahashi N, Uramoto H, Okada Y. Chloride channel inhibition prevents ROS-dependent apoptosis induced by ischemia-reperfusion in mouse cardiomyocytes. Cell Physiol Biochem. 2005;16:147–154. doi: 10.1159/000089840. [DOI] [PubMed] [Google Scholar]
- Yang CZ, Zhao R, Dong Y, Chen XQ, Yu AC. Astrocyte and neuron intone through glutamate. Neurochem Res. 2008;33:2480–2486. doi: 10.1007/s11064-008-9758-x. [DOI] [PubMed] [Google Scholar]