Abstract
Sprouty2 is a feedback regulator that controls the Ras-Raf-MEK-ERK mitogen-activated protein kinase (MAPK) pathway at multiple levels, one way being through direct interaction with Raf kinases. Consistent with a role as a tumor suppressor, Sprouty2 expression is often down-regulated in human cancers. However, Sprouty2 is up-regulated in some cancers, suggesting the existence of post-transcriptional mechanisms that permit evasion of Sprouty2-mediated anti-tumorigenic properties. We report that MAPK activation induces Sprouty2 phosphorylation on six serine residues, which reduced Sprouty2 association with wild-type B-Raf. Mutation of these six serines to non-phosphorylatable alanines increased the ability of Sprouty2 to inhibit growth factor-induced MAPK activation. Oncogenic B-Raf mutants such as B-Raf V600E did not associate with Sprouty2, but this resistance to Sprouty2 binding was not due to phosphorylation. Instead, the active kinase conformation induced by oncogenic mutation prevents Sprouty2 binding. These results reveal a dual mechanism that affects the Sprouty2/B-Raf interaction: Sprouty phosphorylation and B-Raf conformation.
Keywords: Sprouty, B-Raf, MAPK, signal transduction, ERK
Introduction
Sprouty was identified in a Drosophila screen for modifiers of airway branching induced by fibroblast growth factor (FGF) signaling (1). Four mammalian Sprouty orthologues (Sprouty 1-4; Sprouty2 being the closest to Drosophila Sprouty (1-4)) comprise a family of receptor tyrosine-kinase (RTK) feedback-regulators that modulate the Ras-Raf-MEK-ERK mitogen-activated protein kinase (MAPK) pathway (5). As a consequence, they regulate processes such as proliferation, survival and motility in response to RTK activation and influence the development of many tissues (6).
Clinical and experimental evidence is consistent with Sprouty proteins being tumor suppressors. When overexpressed, Sprouty proteins suppressed proliferation of cultured tumor cells (7-9), and urethane-induced lung cancer in mice (10). Conversely, siRNA-mediated Sprouty2 knockdown induced melanocyte proliferation (11), while Sprouty2 deletion enhanced K-Ras-induced lung cancer in mice (12). If Sprouty genes were tumor suppressors, expression would be expected to be repressed or lost in cancers, which has been observed in a variety of tumors (13-19). However, Sprouty levels are elevated in melanoma cells expressing oncogenic B-Raf (11, 20, 21) or N-Ras (20) and in gastrointestinal stromal tumors (GIST) expressing activated c-Kit (22, 23), suggesting that there may be post-transcriptional mechanisms to evade its tumor-suppressive effects.
Sprouty proteins are comprised of a variable N-terminus and a highly-conserved cysteine-rich C-terminus (24), which regulate MAPK signaling at multiple levels depending on cell context and growth factor receptor activated. One way they inhibit MAPK activation is by directly interacting with and inhibiting activation of Raf kinases (11, 25-28). We previously reported that Sprouty2 did not associate with oncogenic B-Raf mutants, which allows for increased active ERK levels in melanoma cell lines expressing mutant B-Raf despite elevated Sprouty2 (11). In this study, we investigated the Sprouty2/B-Raf interaction and propose two factors that influence this association: Sprouty2 phosphorylation at specific sites, and the B-Raf kinase domain conformation.
Materials and Methods
Plasmids and Proteins
pEF Myc-B-Raf constructs and pcDNA3 FLAG-Sprouty2 were described previously (11). pcDNA3 HA B-Raf was from W. Kolch, Beatson Institute, UK. pEXV MEK:EE was from C. J. Marshall, Institute for Cancer Research, UK. For site directed mutagenesis, FLAG-Sprouty2 was subcloned into the Gateway vector system (Invitrogen) and point mutations were introduced using the QuickChange kit (Stratagene) according to manufacturer's protocol.
Recombinant human wild-type and V600E glutathione-S-transferase (GST)-B-Raf catalytic domains were from Cell Signaling Technology or Millipore. FLAG-Sprouty2 was cloned into pGEX-4T3 (GE Healthcare) and expressed and purified using standard methods (29).
Alternatively, FLAG-Sprouty2 was subcloned into pGEX-6P3 (GE Healthcare), expressed and purified as described above. Flag Sprouty2 was cleaved from GST by incubation with 80 U Prescission Protease (GE Healthcare) in cleavage buffer (50 mM Tris pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM DTT) for 4 h at 4°C.
In vitro transcribed and translated (IVTT) [35S]-Methionine-labeled HA B-Raf was generated from pcDNA3 HA B-Raf using the TNT T7 Quick Coupled Transcription Translation kit (Promega) according to the manufacturer's protocol.
Cell Culture and Transfections
HEK293 cells were cultured in Dulbeco's modified Eagle's medium (DMEM) supplemented with 10% (v/v) fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin (Invitrogen) at 37°C and 5% CO2. Cells were transfected with FuGENE6 (Roche) according to manufacturer's protocol and harvested 24 h later. Amounts of wild-type and phosphorylation site mutant Sprouty2 plasmids transfected were adjusted to equilibrate protein levels.
Parental NIH3T3 cells were maintained in DMEM supplemented with 10% (v/v) Donor Calf Serum (DCS; Invitrogen). Details of generation of TETOFF Sprouty2 cell lines are in Supplemental Methods. Mouse embryo fibroblasts were maintained in DMEM with 10% (v/v) fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin at 37°C and 10% CO2, and cell lysates prepared, as described in (30)
Cell Extraction and Immunoblotting
Whole cell lysates were prepared and western blotted as described previously (11). Primary antibodies used were as follows: murine anti-FLAG (Sigma-Aldrich); rabbit anti-Myc 9B11 (Cell Signaling Technology); murine anti-pERK1/2 (Cell Signaling Technology); rabbit anti-ERK1/2 (Millipore); rabbit anti-GST (Cell Signaling Technology); rabbit anti-Sprouty (Upstate Biotechnology) and rabbit anti-MEK (Cell Signaling Technology). Alexa-Fluor680 (Molecular Probes) or IRDye800 (Rockland)-conjugated secondary antibodies were detected by infra-red imaging (Li-Cor Odyssey).
Immunoprecipitations
Myc-B-Raf complexes were immunoprecipitated from HEK293 WCL and analyzed for associated FLAG-Sprouty2 as described previously (11). Detailed methods can be found in the Supplemental Methods section.
In Vivo Cell Labeling
HEK293 cells transiently expressing FLAG-Sprouty2 and Myc-B-Raf V600E were cultured overnight in phosphate-free DMEM supplemented with 0.2 mCi/ml [32P] (Amersham Biosciences). Cells were lysed in Tris lysis buffer (TLB; 50 mM Tris pH 7.5, 150 mM NaCl, 1% Triton 1 mM, Na3VO4, 50 mM NaF, 5 mM β-glycerophosphate, 2 mM EDTA, 1 mM DTT, 1 mM PMSF and 1× Complete protease inhibitor cocktail (Roche)), FLAG-Sprouty2 was immunoprecipitated from WCL and analyzed by SDS-PAGE followed by Coomassie staining with Simply Blue SafeStain (Invitrogen) and autoradiography.
Identification of Sprouty2 phosphorylation sites by Mass Spectrometry
FLAG-Sprouty2 was immunoprecipitated from HEK293 cells, eluted with Laemmli buffer, separated by SDS-PAGE and detected by staining with Brilliant Blue G Colloidal Coomassie (Sigma). Sprouty2 bands were excised, destained with successive ammonium bicarbonate and acetonitrile washes and digested with trypsin as described previously (31). Extracted tryptic peptides were analyzed by LC-MS with precursor of 79 scanning as described previously (32). Detailed methods can be found in the Supplemental Methods.
Phosphorylation of recombinant Sprouty2 in B-Raf V600E cell lysate
2 μg GST-FLAG-Sprouty2 immobilized on glutathione beads was incubated with 0.74 MBq γ-32P-ATP, 200 μM ATP, 10 μg/ml creatine kinase, and 5 mM creatine phosphate in 2.5 mg WCL from HEK293 cells transiently expressing Myc-B-Raf V600E at 30°C for 2 h. Beads were washed 5× in Tris-NP40 buffer (50 mM Tris pH 8, 150 mM NaCl, 1.0% (v/v) NP40, 1 mM PMSF, 1 mM DTT, and Complete protease inhibitor cocktail) and GST-FLAG-Sprouty2 was eluted in Laemmli buffer, separated by SDS-PAGE and analyzed by Coomassie staining and autoradiography.
Peptide Arrays
23-mer peptides offset by 2 amino acids that covered the mouse Sprouty2 sequence were arrayed onto membranes (Cancer Research UK, London, UK). Arrays were blocked in 5% (w/v) bovine serum albumin/Tris-buffered saline (BSA/TBS) for 1 h at room temperature prior to incubation with 50 μl [35S]-Methionine labeled IVTT HA B-Raf in 5% (w/v) BSA/TBS for 1 h at room temperature. Membranes were washed three times in TBS/0.1% (v/v) Tween, allowed to dry and exposed to film.
Pull-down of FLAG-Sprouty2 with GST-B-Raf
0.5 μg recombinant soluble GST or GST-B-Raf catalytic domain was incubated with 1 μg recombinant FLAG-Sprouty2 in 50 μl Tris-NP40 buffer at 30°C for 30 min. The reaction was diluted with 250 μl Tris-NP40 buffer and GST-B-Raf complexes were affinity purified on Glutathione Sepharose 4B beads at 4°C for 2 h. Beads were washed 5× in Tris-NP40 buffer and complexes were eluted in Laemmli buffer and analyzed by SDS-PAGE as described above.
B-Raf siRNA
NIH3T3 cells were transfected with siRNA against B-Raf (siRNA1 Invitrogen RSS300183; siRNA4 Dharmacon L-094802-00) or mock transfected without RNA duplex. Further details can be found in Supplemental Methods section.
Results
Sprouty2 phosphorylation regulates the association with B-Raf
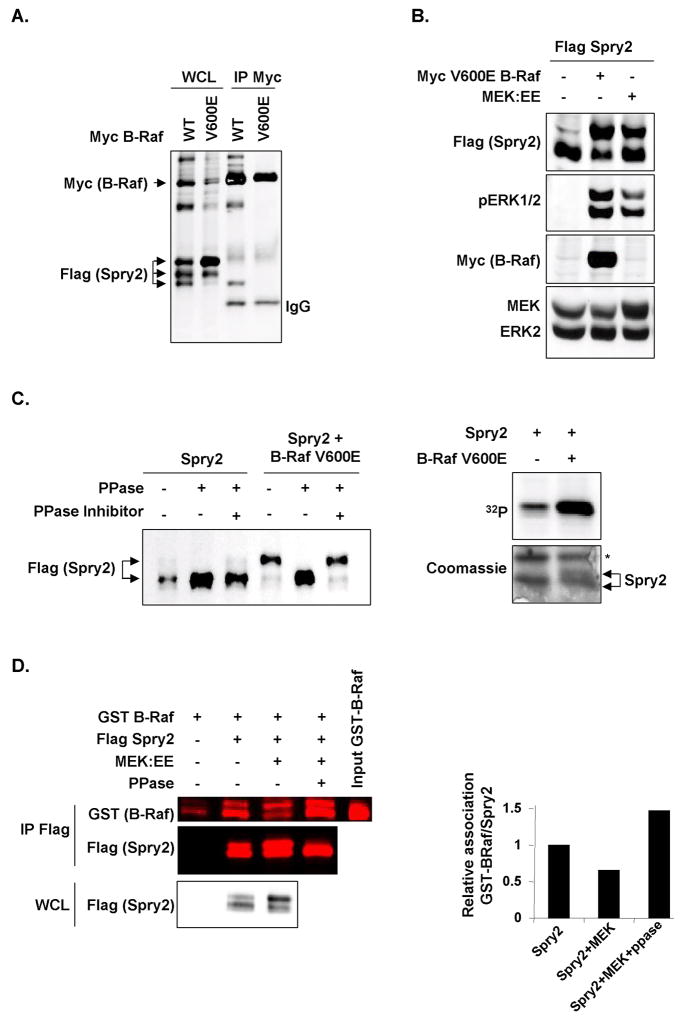
When whole cell lysates (WCL) from HEK293 cells transfected with FLAG-tagged Sprouty2 (Spry2) and either wild-type (WT) or an activated oncogenic form of myc-tagged B-Raf (V600E) were run on SDS-PAGE beside anti-Myc immunoprecipitations, only the fastest migrating Sprouty2 electrophoretic variant co-purified with wild-type B-Raf (Fig. 1A). While the distribution of Sprouty2 electrophoretic forms was relatively equal when co-transfected with wild-type B-Raf, there was a shift towards the slower forms in cells expressing B-Raf V600E, with markedly less of the fastest migrating form that had associated with wild-type B-Raf (Figure 1A). The shift from fast to slow electrophoretic variants was observed when Sprouty2 was co-transfected with B-Raf V600E or constitutively-active MEK1 (MEK:EE) (33), accompanied by increased ERK1 and ERK2 phosphorylation (pERK1/2), suggesting that the mobility shift resulted from MAPK activation (Fig. 1B). It should be noted that typically only 2 electrophoretic forms are consistently well-separated on SDS-PAGE (e.g. Fig. 1B); separation into 3 forms is variable likely due to variations in electrophoresis conditions. Consistent with previous results (34), the slower migrating Sprouty2 could be shifted to the faster form when immunoprecipitated protein was incubated with calf intestinal phosphatase (PPase; Fig. 1C left). Furthermore, in cells metabolically-labeled with [32P]-orthophosphate, co-expression of B-Raf V600E resulted in increased Sprouty2 phosphorylation (Fig. 1C right, upper panel). The [32P]-labeled Sprouty2 band co-migrated with the slower-migrating Coomassie-stained band, consistent with the mobility shift resulting from phosphorylation (Fig. 1C right, lower panel). These results indicate that B-Raf preferentially associates with faster-mobility hypophosphorylated Sprouty2, and suggest that MAPK activation leads to Sprouty2 phosphorylation and reduced B-Raf association. In support of this conclusion, we determined using quantitative direct scanning of western blots and near-infrared fluorophore conjugated secondary antibodies that binding of recombinant GST-B-Raf catalytic domain was reduced by 40% if immunoprecipitated Sprouty2 had been co-expressed with active MEK:EE, which was reversed by phosphatase pretreatment of the immunoprecipitated Sprouty2 (Fig. 1D). The reduced B-Raf binding was associated with a MEK1-induced Sprouty2 mobility shift, which also was reversed by phosphatase (Fig. 1D), consistent with Sprouty2 phosphorylation negatively-regulating B-Raf binding. Interestingly, B-Raf association was higher (150%) and the proportion of fast-migrating Sprouty2 greater following phosphatase treatment than for Spouty2 immunoprecipitated from cells not expressing MEK:EE, suggesting that basal Sprouty2 phosphorylation influenced B-Raf binding and electrophoretic mobility (Fig. 1D). As a result, there was a greater than 2-fold difference in B-Raf binding of Sprouty2 co-expressed with active MEK without or with phosphatase treatment, indicating the contribution of phosphorylation in modifying this interaction. These results indicate that MAPK activation by oncogenic B-Raf results in a higher proportion and/or stoichiometry of Sprouty2 phosphorylation that antagonizes B-Raf binding.
Figure 1.
Sprouty2 phosphorylation regulates association with B-Raf. A, Cells were transfected with FLAG-Sprouty2 and Myc-B-Raf WT or V600E. WCL and Myc immunoprecipitates (IP Myc) were western blotted. B, Western blots of cells co-expresssing FLAG-Sprouty2 and Myc-B-Raf V600E or MEK1:EE. C, Left panel, FLAG-Sprouty2 transfected alone or with Myc-B-Raf V600E was immunoprecipitated and treated with CIP phosphatase (PPase) and phosphatase inhibitor as indicated. Right panel, Metabolic [32P] labeling of cells transfected with FLAG-Sprouty2 alone or with Myc-B-Raf V600E as indicated. Immunoprecipitated Sprouty2 was analyzed by autoradiography (upper panel) and Coomassie staining (lower panel). Asterisk=non-specific protein. D, FLAG-Sprouty2 transfected alone or with MEK1:EE was immunoprecipitated, the sample co-expressed with MEK1:EE was divided in two and treated with or without phosphatase as indicated. Immunoprecipitated Sprouty2 was then incubated with GST-B-Raf catalytic domain and recovered by anti-FLAG immunoprecipitation. Anti-FLAG IP (IP FLAG), WCL and input GST-B-Raf were western blotted for GST and/or FLAG.
Sprouty2 is phosphorylated on multiple serine residues
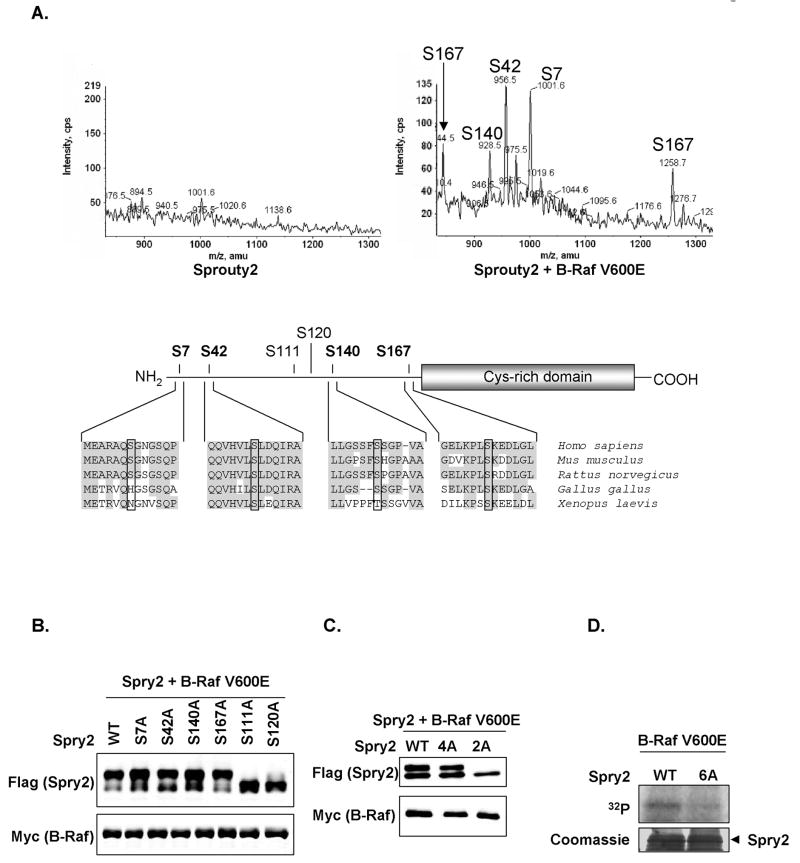
To identify B-Raf-induced Sprouty2 phosphorylation sites, we purified Sprouty2 expressed with or without B-Raf V600E and analyzed tryptic fragments by mass spectrometry (MS) using a precursor ion scan of m/z −79 on a triple quadrupole instrument in negative ion mode followed by an ion trap high resolution scan, which produces a mass spectrum that contains only the molecular ions of the phosphopeptides present in the sample (32). While no basal Sprouty2 phosphorylation was detected (Fig. 2A, left), four sites were identified (Ser7, Ser42, Ser140 and Ser167; numbering = mouse Sprouty2) when co-expressed with B-Raf V600E (Fig. 2A, right). All lie within the Sprouty2 N-terminal half and are highly conserved in vertebrate species (Fig. 2A. lower panel).
Figure 2.
B-Raf induces Sprouty2 phosphorylation on multiple serine residues. A, LC-MS analysis of Sprouty2 tryptic peptides with precursor 79 scanning on a 4000 Q-Trap mass spectrometer. Extracted ion chromatograms for the phosphopeptides detected from immunoprecipitated Sprouty2 without (left) or with (right) co-expressed B-Raf V600E, identities of each phosphorylation site shown. Serine 167 phosphopeptide ion was detected as the M-2H (1256.7) and M-3H (844.5) ions whereas the other three phosphopeptides were detected as M-2H ions. Schematic representation showing Sprouty2 phosphorylation sites identified by mass spectrometry in bold. Sequence alignment compares Sprouty2 homology surrounding these sites (highlighted by boxes) in selected species. Conserved Sprouty2 cysteine-rich domain and previously-identified sites Ser111 and Ser120 are shown. B, and C, Wild-type or phosphorylation site mutant FLAG-Sprouty2 co-expressed with B-Raf V600E were western blotted for FLAG and Myc. D, Recombinant GST-FLAG-Sprouty2 wild-type or 6A were incubated with WCL expressing Myc-B-Raf V600E in the presence of γ–[32P]-ATP and analyzed for [32P] incorporation by autoradiography (upper panel) and Coomassie staining (lower panel).
Individual mutations to non-phosphorylatable alanines (S7A, S42A, S140A, S167A) did not significantly affect the B-Raf V600E-induced Sprouty2 mobility shift (Fig. 2B). It was previously reported that EGF-induced phosphorylation of two sites on human Sprouty2 corresponding to Ser111 and Ser120 resulted in slower Sprouty2 electrophoretic mobility (35). We found that mutation of either site to alanine (S111A, S120A) reduced but did not completely reverse the B-Raf V600E-induced mobility shift (Fig. 2B), suggesting that these sites were also modified downstream of B-Raf. Mutation of the four phosphorylation sites identified by MS (4A) did not affect Sprouty2 mobility (Fig. 2C) but did result in a loss in Sprouty2 phosphorylation detectable by MS (Supplemental Fig. 1B). Mutation of both S111 and S120 to alanines (2A) completely abolished the B-Raf V600E-induced mobility shift (Fig. 2C). Furthermore, 32P incorporation on E. coli-expressed GST-Sprouty2 induced by incubation with WCL from B-Raf V600E-expressing HEK293 cells was markedly diminished in the 6A mutant compared to wild-type Sprouty2 (Fig. 2D). Based on the direct and indirect evidence: identification by MS, electrophoretic mobility shift and 32P incorporation, we have identified six serine residues that appear to be the principal phosphorylation sites modified in response to MAPK activation, with the caveat that there may be additional minor phosphorylation sites not detected by MS or that may not influence Sprouty2 electrophoretic mobility.
Sprouty2 phosphorylations cooperate to regulate B-Raf binding
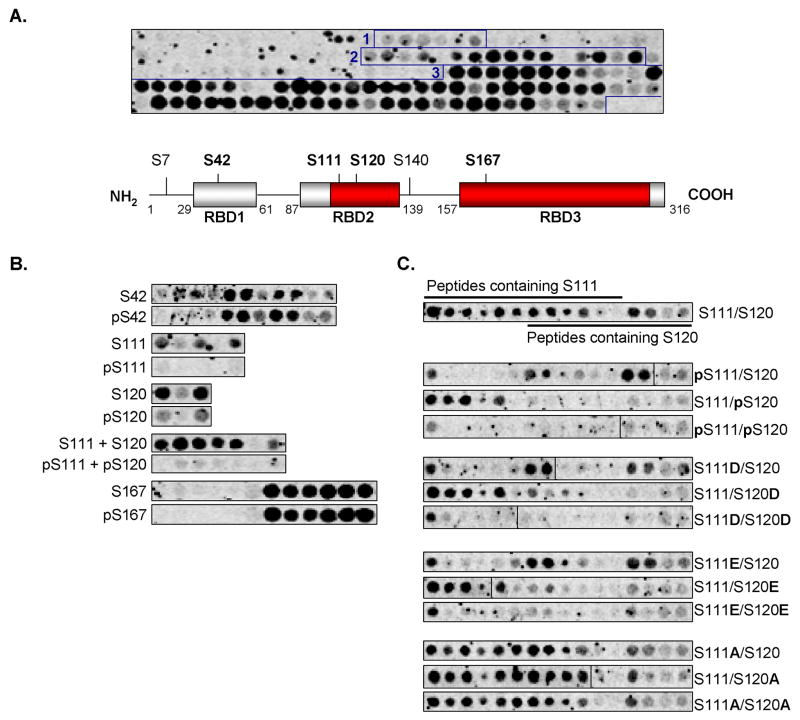
Having identified B-Raf-induced Sprouty2 phosphorylations, we next determined where B-Raf bound to Sprouty2 and whether specific phosphorylations affected B-Raf binding. A peptide array consisting of 23-mers, each consecutively shifted by two residues and spanning the entire Sprouty2 protein, was incubated with [35S]-methionine-labeled wild-type B-Raf produced by IVTT. The pattern of [35S]-labeling revealed direct interactions between B-Raf and three distinct Sprouty2 regions that we named Raf Binding Domains 1-3 (RBD1-3: Fig. 3A). Interaction with RBD1 was relatively weak while regions of strong binding were detected within RBD2 and RBD3 (Fig. 3A; indicated in red). Four of the six Sprouty2 phosphorylation sites modified in the presence of B-Raf (S42, S111, S120, S167) lie within these binding domains (Fig. 3A). B-Raf binding to peptides with corresponding phosphoserine residues was reduced for pS111 and pS120, but not for pS42 or pS167 (Fig. 3B). Given that in the initial phosphopeptide array many peptides containing pS111 also contained pS120, we examined whether phosphorylation of either site individually would inhibit B-Raf binding. Individual substitution of either S111 or S120 with phosphoserine inhibited B-Raf binding and no binding was observed when both sites were substituted (Fig. 3C). Similarly, phosphomimetic aspartate or glutamate substitutions at either site inhibited B-Raf binding. Finally, substitution of S111 or S120 with alanine had no effect on B-Raf association, indicating that alanines were equivalent to non-phosphorylated serines (Fig. 3C; summarized in Supplementary Table 1). Collectively, these results indicate that B-Raf directly binds Sprouty2 at 3 domains and that phosphorylation of Ser111 or Ser120 within RBD2 inhibits this interaction. A similar pattern of phosphorylation-regulated interaction was observed with Raf-1, indicating that it is likely a general phenomenon for Raf kinases (data not shown).
Figure 3.
Sprouty2 phosphorylation within Raf Binding Domain 2 inhibits B-Raf binding. A, Upper panel, binding of [35S]-Methionine labeled in vitro transcribed/translated (IVTT) HA-tagged B-Raf to a Sprouty2 peptide array, comprised of 23-mer peptides each shifted by 2 residues. Three Raf-binding domains (RBD 1-3) were detected (boxed peptide spots). Lower panel, schematic representation of mouse Sprouty2 protein indicating RBD 1-3 (grey boxes) positions. Higher affinity B-Raf binding highlighted in red. Sprouty2 phosphorylation sites in bold. B, [35S]-Methionine labeled HA-tagged B-Raf binding to arrayed Sprouty2 peptides with serine or phosphoserine residues as indicated. C, [35S]-Methionine labeled HA-tagged B-Raf binding to arrayed Sprouty2 peptides with aspartate, glutamate or alanine substitutions at Ser111 and/or Ser120 as indicated.
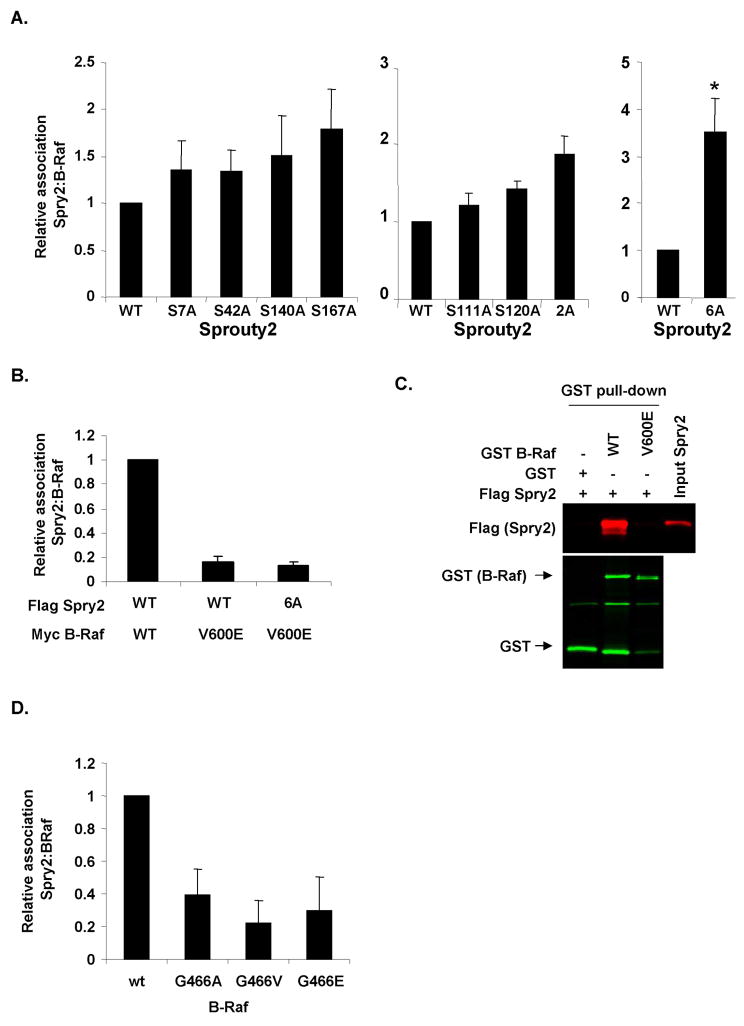
We next investigated how Sprouty2 phosphorylation affected B-Raf binding in cells. Individual mutation of S7, S42, S140 or S167 to alanines had no effect on Sprouty2 binding to wild-type B-Raf (Fig. 4A left panel and Supplemental Fig. 2A). Although individual mutation to S111A or S120A had no significant effect on B-Raf binding, mutation of both increased binding almost 2-fold (Fig. 4A middle panel and Supplemental Fig.2B). Intriguingly, when all six sites were mutated to alanines there was a significant ∼3.5-fold increase in B-Raf binding (Fig. 4A right panel and Supplemental Fig.2C). Therefore, although mutation of S7, S42, S140 or S167 individually had little effect on B-Raf association, collective phosphorylation of these sites likely co-operates with S111 and S120 phosphorylation within RBD2 to inhibit B-Raf binding.
Figure 4.
Effect of mutating Sprouty2 phosphorylation sites on binding to wild-type or cancer associated B-Raf mutants. A, FLAG-Sprouty2 wild-type or phosphorylation site mutants were transfected with Myc-B-Raf V600E as indicated. Myc immunoprecipitates and WCL were western blotted for FLAG or Myc. Sprouty2 association with B-Raf expressed relative to total Sprouty2. Mean values ± standard error of the mean (SEM), normalized to Sprouty2 WT binding to B-Raf, are shown. For left panel n=7, middle panel n=3, right panel n=3. *=significant difference (P<0.05) from wild-type Sprouty2 binding, determined by Student's t test. B, FLAG-Sprouty2 wild-type or phosphorylation site mutants were transfected with Myc wild-type B-Raf or Myc-B-Raf V600E as indicated. Myc immunoprecipitates and WCL were western blotted for FLAG or Myc. Mean values ± SEM (n=12), normalized to binding of Sprouty2 WT to B-Raf WT, are shown. C, GST, GST-B-Raf wild-type or GST-B-Raf V600E were incubated with recombinant FLAG-Sprouty2, recovered on glutathione Sepharose beads (GST pull-down) and analyzed for B-Raf and associated Sprouty2 by western blotting for GST and FLAG. Input FLAG-Sprouty2 was loaded as a positive control. D, FLAG-Sprouty2 was transiently transfected with cancer-associated Myc-B-Raf G466 P-loop mutants as indicated. Myc immunoprecipitates and WCL were western blotted for FLAG or Myc. Mean values ± SEM (n=4), normalized to Sprouty2 binding to WT B-Raf.
Oncogenic B-Raf mutants do not bind Sprouty2 independent of phosphorylation status
We found previously that Sprouty2 did not bind to B-Raf V600E (11) and postulated that this could be due to B-Raf-induced Sprouty2 phosphorylation (Fig. 1A). Since the Sprouty2 6A mutant displayed enhanced binding to wild-type B-Raf (Fig. 4A and Supplemental Fig. 2C), we examined whether inhibiting phosphorylation on these sites would restore B-Raf V600E binding. Surprisingly, Sprouty2 association with B-Raf V600E was unaffected by the 6A mutations (Fig. 4B and Supplemental Fig. 3A). Similarly, while recombinant non-phosphorylated FLAG-Sprouty2 associated with recombinant GST-B-Raf wild-type catalytic domain in vitro, it did not associate with the V600E mutant (Fig. 4C). These results indicate that although Sprouty2 phosphorylation inhibits wild-type B-Raf binding, the V600E B-Raf mutant resists Sprouty2 binding independent of phosphorylation status. One possible explanation is that B-Raf mutations that induce active kinase conformations prevent Sprouty2 binding. We therefore tested three B-Raf mutants that lie within the glycine-rich P-loop that either modestly increase (G466A) or impair kinase activity (G466E and G466V) but which induce an active kinase conformation in each case (36). Similar to B-Raf V600E, they did not significantly bind Sprouty2 relative to wild-type B-Raf (Fig. 4D and Supplemental Fig. 3B), consistent with our previous observation that the oncogenic activation-loop B-Raf mutants L597V, V600D and K601E did not bind Sprouty2 (11). These results indicate that there are two independent factors influencing the B-Raf/Sprouty2 interaction: Sprouty2 phosphorylation and B-Raf kinase domain conformation.
Sprouty2 phosphorylation influences MAPK activation
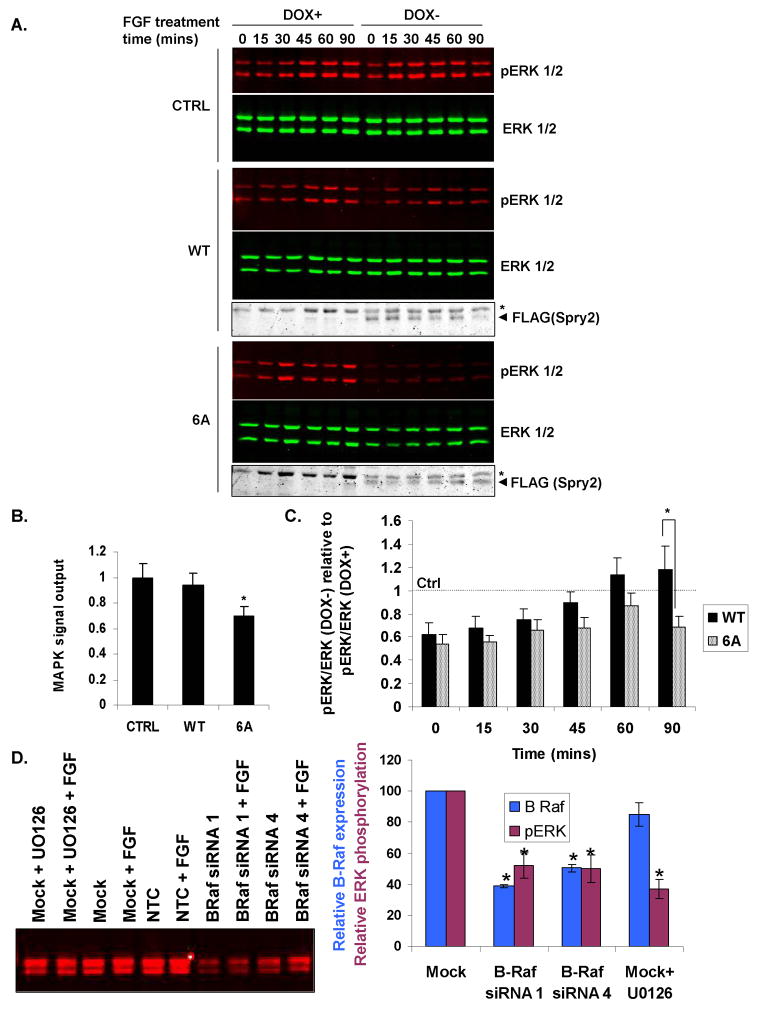
Given that Sprouty2 phosphorylation affects wild-type B-Raf binding, we tested whether the 6A mutant would have an enhanced ability to repress MAPK activation by FGF. We generated NIH3T3 cells expressing tetracycline-regulated (Tet-off) wild-type or 6A mutant Sprouty2. Three wild-type and three 6A Sprouty2 cell lines were selected for their relatively-comparable Sprouty2 expression following doxycycline (DOX) withdrawal (Supplemental Fig. 4). A control non-expressing clone was also established using the empty Tet-responsive vector. FGF-treatment of the control cells resulted in increased phosphorylated ERK1 and ERK2 that were sustained over 90 minutes, which was unaffected by DOX withdrawal (Fig. 5A, upper panel). In contrast, ERK activation was somewhat inhibited when wild-type Sprouty2 was induced following DOX removal, consistent with Sprouty2 being an inhibitor of FGF-induced MAPK activation (37) (Fig. 5A, middle panel). Inhibition of ERK activation was more pronounced in 6A Sprouty2-expressing cells relative to wild-type Sprouty2 (Fig. 5A, lower panel). When the areas under the curve (AUC) for active ERK levels over time were calculated as a measure of signal output (38), 6A Sprouty induction significantly inhibited MAPK signal output relative to wild-type Sprouty2 (Fig. 5B). Although ERK activation in 6A Sprouty2-expressing cells was lower at every timepoint, the differences between wild-type and 6A Sprouty2 did not achieve statistical significance until 90 minutes (Fig. 5C). One interpretation is that wild-type Sprouty2 inhibits FGF-induced MAPK activation, with Sprouty2 phosphorylation at later time points reducing this inhibitory effect. In contrast, the 6A Sprouty2 mutant is not phosphorylated on critical sites and MAPK inhibition presists, decreasing MAPK signal output. Therefore, Sprouty2 phosphorylation may act as an additional regulatory mechanism to control MAPK signaling. Consistent with Sprouty2 inhibition of B-Raf being the cause of reduced MAPK activation, B-Raf knockdown with two independent siRNA duplexes (Figure 5D, left panel) resulted in proportionate and significant reduction in MAPK activation (Figure 5D, right panel).
Figure 5.
Inhibition of FGF-stimulated ERK activation by Sprouty2 phosphorylation site mutant. A, NIH3T3 TETOFF FLAG-Sprouty2 control (CTRL), wild-type (WT; clone 2 shown) or 6 alanine (6A; clone 3 shown) cell lines were cultured with or without DOX, stimulated with FGF for times indicated and western blotted for pERK, ERK and FLAG-Sprouty2. Arrowhead=FLAG-Sprouty2. B, FGF-induced MAPK signal output following Sprouty2 induction relative to uninduced condition was determined from areas under the curve (AUC) of time-course experiments. pERK/ERK for DOX- (Sprouty2 induced) conditions expressed relative to pERK/ERK for DOX+ (Sprouty uninduced) conditions were plotted for each timepoint and AUC calculated using the Trapezoidal Rule. Mean values ± SEM, normalized to control clone, from four (control) or seven (WT and 6A) repetitions are shown. *=significant difference (P<0.05) from wild-type determined by Student's t test. C, Ratios of pERK/ERK for DOX- (Sprouty2 induced) conditions expressed relative to pERK/ERK for DOX+ (uninduced) for each timepoint for wild-type or 6A Sprouty2. Mean values ± SEM (n=7), normalized to control clone. *=significant difference (P<0.05) between WT and 6A determined by Student's t test. D, NIH3T3 cells were transfected were mock transfected or transfected with non-targeting control (NTC) or B-Raf siRNAs as indicated. After 48 hours, cells were treated with FGF as above for 45 minutes, either with or without 10 μM U0126. B-Raf expression levels were determined by quantitative Western blotting (left panel), and the relative expression of B-Raf (right, dark blue) and ratio of pERK/ERK (right, burgundy) determined for these conditions. Mean values ± SEM (n=6), normalized to mock transfectant. *=significant difference (P<0.01) from mock transfectant determined by Student's t test.
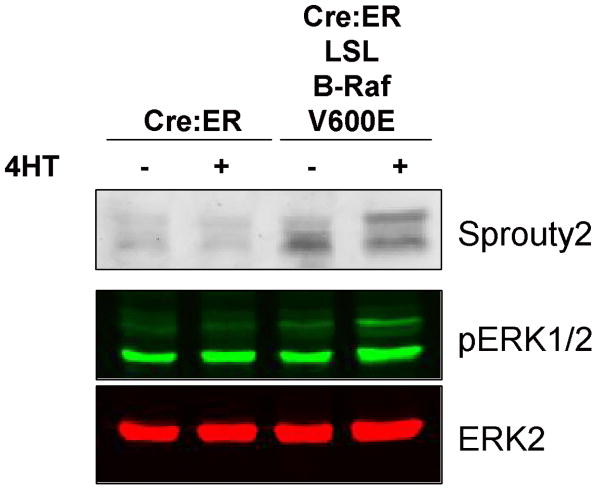
Endogenous Sprouty2 phosphorylation downstream of endogenous V600E B-Raf
We examined how endogenous oncogenic V600E B-Raf affected the mobility of endogenous Sprouty2 in mouse embryonic fibroblasts (MEFs) (30). MEFs from mice that expressed a conditionally activated Cre-estrogen receptor (Cre:ER) alone or in combination with a heterozygous knock-in V600E B-Raf mutation preceded by a Lox-Stop-Lox (LSL) cassette were either left untreated or treated with 50 nM 4-hydroxytamoxifen (4HT) for 96 hours to induce Cre-mediated recombination (39). Sprouty2 levels were low in Cre:ER expressing MEFs either with or without 4HT. In the absence of 4HT, Sprouty2 levels were higher in Cre:ER; LSL B-Raf V600E MEFs, likely due to inherent leakiness of the Cre:ER transgene; however, following 4HT treatment Sprouty2 levels were further increased with a significant proportion shifted to the slower mobility form. Although 4HT did not increase pERK1/2 levels in Cre:ER expressing MEFs, there was an ∼1.8-fold increase in total pERK1/2 staining Cre:ER; LSL B-Raf V600E MEFs following 4HT treatment. These data indicate that endogenous V600E B-Raf is sufficient to induce phosphorylation of endogenous Sprouty2.
Discussion
Sprouty regulation of RTK signaling is complex, since it acts at multiple levels and may have apparently positive and negative actions within the same pathway. That being said, one way Sprouty proteins regulate MAPK signaling is through direct association with Raf kinases (11, 25-28). We observed that B-Raf binding is negatively regulated by Sprouty2 phosphorylation on six serine residues that act in concert; two sites (Ser111 and Ser120) directly affect binding while the remaining four appear to contribute indirectly, possibly by affecting protein conformation. Furthermore, Sprouty2 was unable to associate with B-Raf mutants that adopt active kinase conformations. Therefore, we propose that there are two independent factors that affect the Sprouty/B-Raf interaction: Sprouty2 phosphorylation and B-Raf kinase conformation. These findings have important implications for MAPK signaling in normal and cancer cells.
A significant observation from this study is that activated B-Raf mutants do not bind Sprouty2 regardless of its phosphorylation status. These findings are consistent with, and provide some mechanistic detail for, a recent publication reporting that B-Raf V600E evades a feedback inhibition response that includes elevated Sprouty2 (21).
Sprouty gene transcription is regulated by the MAPK pathway (40, 41), consistent with their role as negative feedback regulators, and oncogenic mutations in melanoma and GIST that activate MAPK also lead to elevated Sprouty levels (11, 20-23). At the same time, Sprouty2 phosphorylation is increased in response to MAPK activation, thereby reducing the ability of Sprouty2 to bind B-Raf and to inhibit FGF-activation of MAPK (Fig. 4 and 5). Therefore, it appears that the MAPK pathway both transcriptionally upregulates Sprouty2 and post-transcriptionally attenuates its ability to inhibit MAPK signaling. One possible reason for this arrangement is that Sprouty may be regulated via transcription at a gross level in response to chronic MAPK activation, but once protein levels have been elevated, a further level of acute control may be exerted by varying the phosphorylation-dephosphorylation status. This possibility suggests that Sprouty proteins may not act as simple on/off switches, but might be tunable regulators that influence MAPK signaling kinetics, duration, magnitude and signal output. If phosphorylation inhibits the ability of Sprouty2 to repress B-Raf then it would be predicted that dephosphorylation should increase Sprouty2-mediated MAPK inhibition. Consistent with this possibility, we found that the 6 alanine mutant was significantly better at suppressing FGF-induced MAPK activation at later time points (Fig. 5), while it was recently reported that Xenopus laevis Sprouty2 does not influence acute ERK activation by FGF but does regulate the duration of ERK activity (42).
In conclusion, we have established that the ability of Sprouty2 to act as an inhibitor of B-Raf activity and the MAPK pathway is under dual control by two independent mechanisms: post-translational phosphorylation of Sprouty2 and B-Raf kinase conformation. The phosphorylation status of specific residues on Sprouty2 influences its ability to interact with B-Raf and to modulate MAPK activation in response to growth factors, while evidence suggests that the active B-Raf kinase conformation independently prevents association with Sprouty2. Delineation of the precise signaling pathway leading to Sprouty phosphorylation is an important future goal in light of the important implications these findings have for MAPK signaling in normal and cancer cells.
Supplementary Material
Figure 6.
Mobility shift of endogenous Sprouty2 induced by endogenous V600E B-Raf. Mouse embryo fibroblasts expressing conditionally active Cre:ER alone or in combination with a heterozygous knock-in V600E B-Raf mutation preceded by a Lox-Stop-Lox (LSL) cassette were either left untreated or treated with 50 nM 4-hydroxytamoxifen (4HT) for 96 hours to induce Cre-mediated recombination as indicated. Sprouty2, pERK1/2 and ERK2 levels were determined by quantitative Western blotting
Acknowledgments
Grant Support: This work was supported by grants to MFO from Cancer Research UK and the NIH (CA030721). NM is funded by the Medical Research Council and by the RASOR IRColl grant for Proteomic Technology funded by the BBSRC and EPSRC.
References
- 1.Hacohen N, Kramer S, Sutherland D, Hiromi Y, Krasnow MA. sprouty encodes a novel antagonist of FGF signaling that patterns apical branching of the Drosophila airways. Cell. 1998;92:253–63. doi: 10.1016/s0092-8674(00)80919-8. [DOI] [PubMed] [Google Scholar]
- 2.de Maximy AA, Nakatake Y, Moncada S, Itoh N, Thiery JP, Bellusci S. Cloning and expression pattern of a mouse homologue of drosophila sprouty in the mouse embryo. Mech Dev. 1999;81:213–6. doi: 10.1016/s0925-4773(98)00241-x. [DOI] [PubMed] [Google Scholar]
- 3.Tefft JD, Lee M, Smith S, et al. Conserved function of mSpry-2, a murine homolog of Drosophila sprouty, which negatively modulates respiratory organogenesis. Curr Biol. 1999;9:219–22. doi: 10.1016/s0960-9822(99)80094-3. [DOI] [PubMed] [Google Scholar]
- 4.Minowada G, Jarvis LA, Chi CL, et al. Vertebrate Sprouty genes are induced by FGF signaling and can cause chondrodysplasia when overexpressed. Development. 1999;126:4465–75. doi: 10.1242/dev.126.20.4465. [DOI] [PubMed] [Google Scholar]
- 5.Kim HJ, Bar-Sagi D. Modulation of signalling by Sprouty: a developing story. Nat Rev Mol Cell Biol. 2004;5:441–50. doi: 10.1038/nrm1400. [DOI] [PubMed] [Google Scholar]
- 6.Mason JM, Morrison DJ, Basson MA, Licht JD. Sprouty proteins: multifaceted negative-feedback regulators of receptor tyrosine kinase signaling. Trends Cell Biol. 2006;16:45–54. doi: 10.1016/j.tcb.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Ishida M, Ichihara M, Mii S, et al. Sprouty2 regulates growth and differentiation of human neuroblastoma cells through RET tyrosine kinase. Cancer Sci. 2007;98:815–21. doi: 10.1111/j.1349-7006.2007.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee CC, Putnam AJ, Miranti CK, et al. Overexpression of sprouty 2 inhibits HGF/SF-mediated cell growth, invasion, migration, and cytokinesis. Oncogene. 2004;23:5193–202. doi: 10.1038/sj.onc.1207646. [DOI] [PubMed] [Google Scholar]
- 9.Edwin F, Singh R, Endersby R, Baker SJ, Patel TB. The tumor suppressor PTEN is necessary for human Sprouty 2-mediated inhibition of cell proliferation. J Biol Chem. 2006;281:4816–22. doi: 10.1074/jbc.M508300200. [DOI] [PubMed] [Google Scholar]
- 10.Minowada G, Miller YE. Overexpression of Sprouty 2 in mouse lung epithelium inhibits urethane-induced tumorigenesis. Am J Respir Cell Mol Biol. 2009;40:31–7. doi: 10.1165/rcmb.2008-0147OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsavachidou D, Coleman ML, Athanasiadis G, et al. SPRY2 is an inhibitor of the ras/extracellular signal-regulated kinase pathway in melanocytes and melanoma cells with wild-type BRAF but not with the V599E mutant. Cancer Res. 2004;64:5556–9. doi: 10.1158/0008-5472.CAN-04-1669. [DOI] [PubMed] [Google Scholar]
- 12.Shaw AT, Meissner A, Dowdle JA, et al. Sprouty-2 regulates oncogenic K-ras in lung development and tumorigenesis. Genes Dev. 2007;21:694–707. doi: 10.1101/gad.1526207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SA, Ho C, Roy R, et al. Integration of genomic analysis and in vivo transfection to identify sprouty 2 as a candidate tumor suppressor in liver cancer. Hepatology. 2008;47:1200–10. doi: 10.1002/hep.22169. [DOI] [PubMed] [Google Scholar]
- 14.Fong CW, Chua MS, McKie AB, et al. Sprouty 2, an inhibitor of mitogen-activated protein kinase signaling, is down-regulated in hepatocellular carcinoma. Cancer Res. 2006;66:2048–58. doi: 10.1158/0008-5472.CAN-05-1072. [DOI] [PubMed] [Google Scholar]
- 15.Sutterluty H, Mayer CE, Setinek U, et al. Down-regulation of Sprouty2 in non-small cell lung cancer contributes to tumor malignancy via extracellular signal-regulated kinase pathway-dependent and - independent mechanisms. Mol Cancer Res. 2007;5:509–20. doi: 10.1158/1541-7786.MCR-06-0273. [DOI] [PubMed] [Google Scholar]
- 16.Fritzsche S, Kenzelmann M, Hoffmann MJ, et al. Concomitant down-regulation of SPRY1 and SPRY2 in prostate carcinoma. Endocr Relat Cancer. 2006;13:839–49. doi: 10.1677/erc.1.01190. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Thompson B, Ren C, Ittmann M, Kwabi-Addo B. Sprouty4, a suppressor of tumor cell motility, is down regulated by DNA methylation in human prostate cancer. Prostate. 2006;66:613–24. doi: 10.1002/pros.20353. [DOI] [PubMed] [Google Scholar]
- 18.McKie AB, Douglas DA, Olijslagers S, et al. Epigenetic inactivation of the human sprouty2 (hSPRY2) homologue in prostate cancer. Oncogene. 2005;24:2166–74. doi: 10.1038/sj.onc.1208371. [DOI] [PubMed] [Google Scholar]
- 19.Lo TL, Fong CW, Yusoff P, et al. Sprouty and cancer: the first terms report. Cancer Lett. 2006;242:141–50. doi: 10.1016/j.canlet.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 20.Bloethner S, Chen B, Hemminki K, et al. Effect of common B-RAF and N-RAS mutations on global gene expression in melanoma cell lines. Carcinogenesis. 2005;26:1224–32. doi: 10.1093/carcin/bgi066. [DOI] [PubMed] [Google Scholar]
- 21.Pratilas CA, Taylor BS, Ye Q, et al. V600EBRAF is associated with disabled feedback inhibition of RAF-MEK signaling and elevated transcriptional output of the pathway. Proc Natl Acad Sci U S A. 2009;106:4519–24. doi: 10.1073/pnas.0900780106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nielsen TO, West RB, Linn SC, et al. Molecular characterisation of soft tissue tumours: a gene expression study. Lancet. 2002;359:1301–7. doi: 10.1016/S0140-6736(02)08270-3. [DOI] [PubMed] [Google Scholar]
- 23.Frolov A, Chahwan S, Ochs M, et al. Response markers and the molecular mechanisms of action of Gleevec in gastrointestinal stromal tumors. Mol Cancer Ther. 2003;2:699–709. [PubMed] [Google Scholar]
- 24.Cabrita MA, Christofori G. Sprouty proteins, masterminds of receptor tyrosine kinase signaling. Angiogenesis. 2008;11:53–62. doi: 10.1007/s10456-008-9089-1. [DOI] [PubMed] [Google Scholar]
- 25.Reich A, Sapir A, Shilo B. Sprouty is a general inhibitor of receptor tyrosine kinase signaling. Development. 1999;126:4139–47. doi: 10.1242/dev.126.18.4139. [DOI] [PubMed] [Google Scholar]
- 26.Sasaki A, Taketomi T, Kato R, et al. Mammalian Sprouty4 suppresses Ras-independent ERK activation by binding to Raf1. Nat Cell Biol. 2003;5:427–32. doi: 10.1038/ncb978. [DOI] [PubMed] [Google Scholar]
- 27.Yusoff P, Lao DH, Ong SH, et al. Sprouty2 inhibits the Ras/MAP kinase pathway by inhibiting the activation of Raf. J Biol Chem. 2002;277:3195–201. doi: 10.1074/jbc.M108368200. [DOI] [PubMed] [Google Scholar]
- 28.Aranda S, Alvarez M, Turro S, Laguna A, de la Luna S. Sprouty2-mediated inhibition of fibroblast growth factor signaling is modulated by the protein kinase DYRK1A. Mol Cell Biol. 2008;28:5899–911. doi: 10.1128/MCB.00394-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sahai E, Olson MF. Purification of TAT-C3 exoenzyme. Methods Enzymol. 2006;406:128–40. doi: 10.1016/S0076-6879(06)06011-3. [DOI] [PubMed] [Google Scholar]
- 30.Mercer K, Giblett S, Green S, et al. Expression of endogenous oncogenic V600EB-raf induces proliferation and developmental defects in mice and transformation of primary fibroblasts. Cancer Res. 2005;65:11493–500. doi: 10.1158/0008-5472.CAN-05-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campbell D, Morrice N. Identification of protein phosphorylation sites by a combination of mass spectrometry and solid phase Edman sequencing. J Biomol Tech. 2002;13:119–30. [PMC free article] [PubMed] [Google Scholar]
- 32.Williamson BL, Marchese J, Morrice NA. Automated Identification and Quantification of Protein Phosphorylation Sites by LC/MS on a Hybrid Triple Quadrupole Linear Ion Trap Mass Spectrometer. Mol Cell Proteomics. 2006;5:337–46. doi: 10.1074/mcp.M500210-MCP200. [DOI] [PubMed] [Google Scholar]
- 33.Alessi DR, Saito Y, Campbell DG, et al. Identification of the sites in MAP kinase kinase-1 phosphorylated by p74raf-1. Embo J. 1994;13:1610–9. doi: 10.1002/j.1460-2075.1994.tb06424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Impagnatiello MA, Weitzer S, Gannon G, Compagni A, Cotten M, Christofori G. Mammalian sprouty-1 and -2 are membrane-anchored phosphoprotein inhibitors of growth factor signaling in endothelial cells. J Cell Biol. 2001;152:1087–98. doi: 10.1083/jcb.152.5.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DaSilva J, Xu L, Kim HJ, Miller WT, Bar-Sagi D. Regulation of sprouty stability by Mnk1-dependent phosphorylation. Mol Cell Biol. 2006;26:1898–907. doi: 10.1128/MCB.26.5.1898-1907.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wan PT, Garnett MJ, Roe SM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116:855–67. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 37.Hanafusa H, Torii S, Yasunaga T, Nishida E. Sprouty1 and Sprouty2 provide a control mechanism for the Ras/MAPK signalling pathway. Nat Cell Biol. 2002;4:850–8. doi: 10.1038/ncb867. [DOI] [PubMed] [Google Scholar]
- 38.Mayawala K, Gelmi CA, Edwards JS. MAPK cascade possesses decoupled controllability of signal amplification and duration. Biophys J. 2004;87:L01–2. doi: 10.1529/biophysj.104.051888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wickenden JA, Jin H, Johnson M, et al. Colorectal cancer cells with the BRAF(V600E) mutation are addicted to the ERK1/2 pathway for growth factor-independent survival and repression of BIM. Oncogene. 2008;27:7150–61. doi: 10.1038/onc.2008.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ozaki K, Kadomoto R, Asato K, Tanimura S, Itoh N, Kohno M. ERK pathway positively regulates the expression of Sprouty genes. Biochem Biophys Res Commun. 2001;285:1084–8. doi: 10.1006/bbrc.2001.5295. [DOI] [PubMed] [Google Scholar]
- 41.Sasaki A, Taketomi T, Wakioka T, Kato R, Yoshimura A. Identification of a dominant negative mutant of Sprouty that potentiates fibroblast growth factor- but not epidermal growth factor-induced ERK activation. J Biol Chem. 2001;276:36804–8. doi: 10.1074/jbc.C100386200. [DOI] [PubMed] [Google Scholar]
- 42.Hanafusa H, Matsumoto K, Nishida E. Regulation of ERK activity duration by Sprouty contributes to dorsoventral patterning. Nat Cell Biol. 2009;11:106–9. doi: 10.1038/ncb1820. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.