Abstract
TREK (TWIK-RElated K+ channels) and TRAAK (TWIK-Related Arachidonic acid Activated K+ channels) were expressed in COS-7 cells, and the channel activities were recorded from inside-out membrane patches using holding potential of -40 mV in symmetrical 150 mM K+ solution. Intracellular application of an oxidizing agent, 5,5'-dithio-bis (2-nitrobenzoic acid) (DTNB), markedly decreased the activity of the TREK2, and the activity was partially reversed by the reducing agent, dithiothreitol (DTT). In order to examine the possibility that the target sites for the oxidizing agents might be located in the C-terminus of TREK2, two chimeras were constructed: TREK2 (1-383)/TASK3C and TREK2 (1-353)/TASK3C. The channel activity in the TREK2 (1-383)/TASK3C chimera was still inhibited by DTNB, but not in the TREK2 (1-353)/TASK3C chimera. These results indicate that TREK2 is inhibited by oxidation, and that the target site for oxidation is located between the amino acid residues 353 and 383 in the C-terminus of the TREK2 protein.
Keywords: TREK2; Oxidizing agent; 5,5'-dithio-bis(2-nitrobenzoic acid) (DTNB); dithiothreitol (DTT); C-terminus; Two-pore domain K+ channel; K2P
INTRODUCTION
Reactive oxygen species (ROS) cause cell damage by reacting with various cellular constituents, including membrane lipids, proteins, and DNA. This damage may be initiated by alterations in cellular homeostasis by changing the levels of several physiological ions. Changes in the gating of ion channels can initiate the redox signaling system. Regulation of channel activity by ROS has been reported for several ion channels, such as Ca2+-activated K+ channel (DiChiara and Reinhart, 1997; Tang et al, 2001), N-methyl-D-aspartate receptor, NR1 (Sullivan et al, 1994), voltage-gated K+ channel Kv1.4 (Ruppersberg et al, 1991), ryanodine receptor (Haarmann et al, 1999), inwardly rectifying K+ channel IRK1 (Ruppersberg and Fakler, 1996), G protein-coupled inward rectifying K+ channel GIRK (Zeidner et al, 2001), and two-pore domain K+ (K2P) channel, particularly in TREK2 (Kim et al, 2007).
The K2P channels are known to be constitutively active at membrane potentials across the physiological range and are likely to help set the resting membrane potential. K2P channels comprise two pore-forming region and four transmembrane- spanning domains, and the K2P channel family consists of TWIK, TASK, TALK, TRAAK/TREK, THIK, TRESK and KCNK7 sub-families (Honore, 2007; Kim, 2005).
Among these sub-families, TASK1, TASK3 and TREK1 have previously been reported as candidates for oxygen-sensing channels, although the effect of hypoxia on TREK1 still remains controversial (Buckler and Honore, 2005; Caley et al, 2005; Miller et al, 2003; Miller et al, 2005; Miller et al, 2004). Inhibition of TREK1 by hypoxia requires the C-terminus of the channel (Miller et al, 2005), and structural modification of the channel can also lead to hypoxic effects. Indeed, the C-terminal domains of TREK1 and TREK2 may modulate electrophysiological properties that are critical for channel activation, resulting from membrane stretching, temperature changes and intracellular acidosis (Honore et al, 2002; Kim et al, 2001b; Maingret et al, 2000; Maingret et al, 1999; Patel et al, 1998).
We postulated that the C-terminus of TREK2 may be involved in redox-sensing mechanisms in a manner similar to TREK1 and other K2P channels, and that TREK2 can be modulated by DTNB. We, therefore, performed a chimeric analysis to identify the target sites for the action of DTNB in TREK2.
METHODS
Cell culture and transfection
COS-7 cells were plated on 35 mm culture dishes one day before transfection. Rat TREK2 and its mutant forms were cloned into the pcDNA3.1 vector. The plasmids were then co-transfected with green fluorescent protein (GFP) into COS-7 cells using the LipoFectamine reagent (Invitrogen, Calsbad, CA). GFP expression in the transfected cells was detected using a Nikon microscope equipped with a mercury lamp light source. Cells were examined 2-3 days after transfection.
Solutions
For single channel recording, the pipette and the bath solution contained (mM) 150 KCl, 1 MgCl2, 10 HEPES and 5 EGTA titrated to pH 7.2 with HCl. All chemicals were from Sigma. To ensure a rapid turnover of the solution, the rate of perfusion was kept above 5 ml min-1, which corresponded to 50 times the bath volume (100 µl) per minute. The reference electrode was a 3M KCl-agar bridge electrode.
Point mutation and chimeric constructs
PCR was used to generate deletions and chimeras in TREK2 and TASK3, as described previously (Kim et al, 2001b). The point mutation was performed using a site-directed mutation kit (Stratagene, La Jolla, CA). All constructs were sequenced to confirm the correct reading frames and amino acid sequences present, including the C-terminus of TREK2.
Electrophysiological studies (voltage clamp recording and analysis)
Single channel currents were recorded from single COS-7 cells in the outside-out patches or inside-out patches configuration. Voltage clamping was performed using an Axopatch-200 (Axon Instruments, Union city, CA). All recordings were performed at room temperature (22~24℃). The recorded signal was filtered at 1 kHz using an 8-pole bessel filter (-3dB: Frequency Devices) and transferred to a computer using the digidata 1,200 interface (Axon Instruments, Union City, CA) at a sampling rate of 5 kHz. Single-channel currents were analyzed with the pClamp program (Version 6.03, Axon Instruments, Union City, CA). The current tracings, shown in the figures, were filtered at 1 kHz. When using patches that contained many channel openings (>5 channel levels), currents were integrated over time to determine the relative channel activity. n represented the number of channels in the patch, and Po represented the probability of a channel being open.
Statistics
The data are shown as means±SEM. n represents the number of cells tested. The significance of the differences between the means was established using a Student's t-test. p<0.05 was regarded as significant. All statistical analyses were conducted with the Origin program (Microcal, Northampton, MA).
RESULTS
The effect of chemical oxidizing agents on K2P channels
To confirm whether two-pore domain K+ channels can be regulated by chemical oxidizing agents, we tested the effect of DTNB on TREK1, TRAAK, and TREK2. Representative single-channel openings were recorded in a symmetrical 150 mM KCl solution before and after intracellular application of DTNB, following the formation of inside-out patches (Fig. 1). DTNB solution (2 mM) markedly inhibited the activity of TREK2, in the excised inside-out patch configurations compared to the bath solution (89±5%, n=30). However, the activities of TREK1 and TRAAK channels were not decreased or even slightly increased by the addition of DTNB (Fig. 1). These DTNB-based effects on TREK1 and TRAAK were different from those of TREK2, and therefore, we focused our further efforts on TREK2. TASK1 and TASK3 were also unaffected by DTNB (data not shown). Extensive washout of DTNB for at least 5 min did not reverse its inhibitory effects on TREK2, and the channels remained as inactive as they were in the presence of the oxidizing agent. The inhibition of TREK2 by DTNB was partially reversed by the addition of reducing agent, dithiothreitol (DTT), in most of the patches tested (Fig. 1). The inhibition of TREK2 by DTNB was dose-dependent, and the half inhibitory concentration (IC50) was 650.2±12.0 µM, with a Hill coefficient of 0.94±0.32 (Fig. 1C, n=8). To test the extracellular response of the membrane impermeable DTNB on TREK2, we analyzed upon outside-out patch configurations in TREK2-expressing COS-7 cells (Fig. 2, n=5). DTNB had little effect on TREK2 when it was present on the extracellular side.
Fig. 1.
The effects of oxidizing chemical agent, DTNB, on TRAAK, TREK1, and TREK2 in inside-out patch configurations. (A) The left panel shows the general single channel activity without DTNB, and the right panel shows channel activity with DTNB (2 mM). DTNB was applied to the bath solution, and the holding potential was held at -40 mV. "Close" represents the closed-channel level, and "open" represents the open-channel level. (B) The relative channel activity with DTNB on each channel. (C) Dose response curve of DTNB on TREK2. The pipette and bath solution contained 150 mM KCl, 5 mM EGTA, 10 mM HEPES, and 1 mM MgCl2. The asterisk indicates a significant difference from the respective control (p<0.05).
Fig. 2.
The effect of extracellular application of DTNB (2 mM) and DTT (5 mM) on TREK2 using outside-out patch configurations. The holding potential was held at -40 mV. (A) The channel activity was not changed by DTNB and DTT. (B) Expanded scale trace of channel activity from A.
These results suggest that the active site of TREK2, which is modulated by redox agents, is located on the intracellular side and is membrane-delimited. However, the reversible effects of DTNB may depend on intracellular factor(s) that may be absent in the inside-out patch configuration.
The effect of DTNB on the TREK2 C-terminus
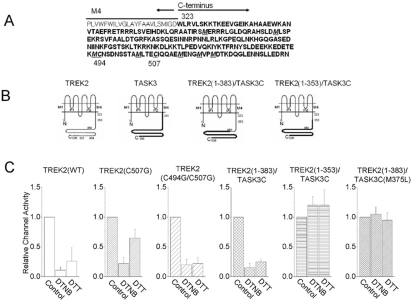
Amongst the amino acid residues, methionine and cysteine are most easily oxidized and susceptible to oxidation by OH-. It is, therefore, highly possible that the two cysteine residues (Cys494 and Cys507) and the seven methionine residues (Met375, Met391, Met493, Met503, Met514, Met518, and Met521) in the C-terminus of TREK2 may be possible targets for oxidation (Fig. 3 and Fig. 5). To address this possibility, the two cysteine residues at positions 494 and 507 were mutated to test the inhibitory actions of DTNB. These mutants were still inhibited by DTNB, even in mutants with double mutations (Cys494Gly and Cys507Gly, 80±9%, n=7, Fig. 5). Furthermore, we constructed a deletion series of the TREK2 C-terminus, and we also replaced a portion of the TREK2 C-terminus with the TASK3 C-terminus (Fig. 3).
Fig. 3.
The intracellular C-terminus of TREK2 modulates DTNB effects upon channel activity. (A) The first and second panels show the effect of DTNB (2 mM) on wild type TREK2 (1-538) and TREK2 chimera [TREK2 (1383)/TASK3C], respectively. The third panel shows the effect of DTNB on TREK2 chimera [TREK2 (1-353)/TASK3C]. The fourth panel shows the effect of DTNB on mutant TREK2 (1-383, M375L)/TASK3C (methionine-to-leucine substitution at position 375). (B) Expanded scale trace of channel activity from the A.
Fig. 5.
Summary of the effects of DTNB on wild type TREK2 and mutants. (A) The amino acid sequence of the C-terminus of TREK2. (B) Membrane topologies of TREK2 and mutants show two pore-forming domains and four transmembrane segments (The membrane topology has been modified from Kim et al, 2001b). The amino acid positions indicate where the C-terminal replacements were made. The portion of the TREK2 C-terminus that was replaced with the C-terminus of TASK3. (C) The bar graph shows a summary of the effects of DTNB on wild type TREK2 and mutants.
We observed a decreased effect of DTNB in the chimera that had 155 amino acids of the TREK2 C-terminal end replaced with the C-terminus of TASK3 [TREK2(1-383)/TASK3C, 85±7%, n=11] (second panel of Fig. 3). The other chimera, where the 185 amino acids of the TREK2 C-terminal end were replaced with the C-terminus of TASK3 [TREK2(1-353)/TASK3C], was not inhibited by the oxidizing agent (n=7) (third panel of Fig. 3). These results suggest that the target site of DTNB may be located between the amino acids between positions 358 and 383 in the TREK2 C-terminus. Based on these results, we assumed that the methionine residue at position 375 is likely to be a redox regulatory target, and therefore, we tested mutant TREK2 (1-383, M375L)/TASK3C (methionine- to-leucine substitution at position 375). The channel activity was not inhibited by the oxidizing agent (n=3, fourth panel of Fig. 3). However, it was not entirely clear whether the methionine residue at position 375 was, in fact, a redox regulatory target of TREK2, because we did not test the mutant of the wild typeTREK2.
The cytoplasmic C-terminus of TREK2 (215 C-terminal amino acids long, out of a total of the 538 amino acids in TREK2) is longer than that of the other mechanosensitive K2P channels (TREK1 and TRAAK). It has earlier been suggested that truncation of the TREK2 C-terminal end can reduce mechanosensitivity, arachidonic acid sensitivity, and intracellular hydrogen sensitivity (Bang et al, 2000; Kim et al, 2001b). Therefore, we compared the effects of intracellular acidic pH on wild type TREK2 and TREK2 (1-353)/TASK3C chimera in the presence or absence of DTNB (Fig. 4). As expected, wild type TREK2 and TREK2 (1-353)/TASK3C chimera were activated by acidic pH in the absence of DTNB. The activation of wild type TREK2 by acidic pH was blocked by DTNB. However, the activation of TREK2 (1-353)/TASK3C chimera by acidic pH was not affected by DTNB (n=6). These results confirmed that the C-terminus of TREK2 was involved in the DTNB-mediated inhibition, and suggested that the redox regulatory sites of TREK2 that interact with oxidizing agents are located between amino acids 353 and 383.
Fig. 4.
The effect of intracellular acidic pH on wild type TREK2 and a chimera [TREK2 (1-353)/TASK3C] in the presence of DTNB. (A) An intracellular acidic pH activated wild type TREK2 in the absence of DTNB, but intracellular acidic pH did not activate wild type TREK2 in the presence of DTNB. (B) An intracellular acidic pH activated the chimera [TREK2 (1-353)/TASK3C] regardless of the presence of DTNB.
DISCUSSION
In this study, we found that TREK2 channel activity in a TREK2 (1-383)/TASK3C chimera was modulated by oxidizing agents, but not in another chimera, TREK2 (1-353)/TASK3C. However, TREK1 and TRAAK activities were not decreased by oxidizing agents, suggesting that the C-terminus of TREK2 contains a crucial regulatory site.
The C-terminal domain has been known to be critical for TREK1 and TREK2 activation, resulting from membrane stretching, temperature and intracellular acidosis (Honore et al, 2002; Kim et al, 2001b; Maingret et al, 2000; Maingret et al, 1999; Patel et al, 1998). On the other hand, the C-terminus of TRAAK is not important for mediating fatty acid sensitivity (Kim et al, 2001a). The differential effect of redox agents on K2P channels such as TRAAK and TREKs may explain various degrees of redox modulation, due to different reactivities of the sulfhydryl or sulfur group of cysteine or methionine residues on the channel protein itself or the presence of distinct redox regulation proteins. Activation of wild type TREK2 by acidic pH was blocked by DTNB, however, activation of the TREK2 (1-353)/TASK3C chimera by acidic pH was not affected by DTNB, suggesting that the target sites for channel activation by acidic pHs or oxidizing agents may be located in different positions.
Although we confirmed that the effects of DTNB are targeted towards C-terminal cysteine residues, we could not exclude the possibility that a cysteine residue at position 28 in the N-terminus is a redox target because we did not test the effect of DTNB on N-terminal TREK2 mutants. In addition, we could not exclude the possibility that the oxidizing agent interacted with other transmembrane regions in addition to the C-terminus. Therefore, further studies are needed to identify the interactions between oxidizing agents and other amino acid residues, such as Cys494, Cys507, Met375, Met391, Met493, Met503, Met514, Met518, and Met521 in TREK2.
In conclusion, TREK2 channels expressed in COS-7 cells can be modulated by intracellular oxidants, and possible target site of the oxidant is located between the amino acid residues 353-383 in the C-terminus of the TREK2 channel protein.
ACKNOWLEDGEMENTS
This work was supported by grants from the Korea Research Foundation (KRF-2002-015-EP0016 and KRF-2003-015-E00019) and the research grant of the Chungbuk National University in 2006.
ABBREVIATIONS
- TREK
TWIK-related K+ channel
- TRAAK
TWIK-related arachidonic acid activated K+ channel
- TASK
TWIK-related acid sensitive K+ channel
- DTNB
5,5'-dithio-bis (2-nitrobenzoic acid
- DTT
dithiothreitol
- K2P channel
Two-pore domain channel
References
- 1.Bang H, Kim Y, Kim D. TREK-2, a new member of the mechanosensitive tandem-pore K+ channel family. J Biol Chem. 2000;275:17412–17419. doi: 10.1074/jbc.M000445200. [DOI] [PubMed] [Google Scholar]
- 2.Buckler KJ, Honore E. The lipid-activated two-pore domain K+ channel TREK-1 is resistant to hypoxia: implication for ischaemic neuroprotection. J Physiol. 2005;562(Pt 1):213–222. doi: 10.1113/jphysiol.2004.077503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caley AJ, Gruss M, Franks NP. The effects of hypoxia on the modulation of human TREK-1 potassium channels. J Physiol. 2005;562(Pt 1):205–212. doi: 10.1113/jphysiol.2004.076240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DiChiara TJ, Reinhart PH. Redox modulation of hslo Ca2+-activated K+ channels. J Neurosci. 1997;17:4942–4955. doi: 10.1523/JNEUROSCI.17-13-04942.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haarmann CS, Fink RH, Dulhunty AF. Oxidation and reductionof pig skeletal muscle ryanodine receptors. Biophys J. 1999;77:3010–3022. doi: 10.1016/S0006-3495(99)77132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Honore E. The neuronal background K2P channels: focus on TREK1. Nat Rev Neurosci. 2007;8:251–261. doi: 10.1038/nrn2117. [DOI] [PubMed] [Google Scholar]
- 7.Honore E, Maingret F, Lazdunski M, Patel AJ. An intracellular proton sensor commands lipid- and mechano-gating of the K+ channel TREK-1. Embo J. 2002;21:2968–2976. doi: 10.1093/emboj/cdf288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim D. Physiology and pharmacology of two-pore domain potassium channels. Curr Pharm Des. 2005;11:2717–2736. doi: 10.2174/1381612054546824. [DOI] [PubMed] [Google Scholar]
- 9.Kim Y, Bang H, Gnatenco C, Kim D. Synergistic interaction and the role of C-terminus in the activation of TRAAK K+ channelsby pressure, free fatty acids and alkali. Pflugers Arch. 2001a;442:64–72. doi: 10.1007/s004240000496. [DOI] [PubMed] [Google Scholar]
- 10.Kim Y, Gnatenco C, Bang H, Kim D. Localization of TREK-2 K+ channel domains that regulate channel kinetics and sensitivity to pressure, fatty acids and pHi. Pflugers Arch. 2001b;442:952–960. doi: 10.1007/s004240100626. [DOI] [PubMed] [Google Scholar]
- 11.Kim Y, Lee SH, Ho WK. Hydrogen peroxide selectively increases TREK-2 currents via myosin light chain kinases. Front Biosci. 2007;12:1642–1650. doi: 10.2741/2176. [DOI] [PubMed] [Google Scholar]
- 12.Maingret F, Lauritzen I, Patel AJ, Heurteaux C, Reyes R, Lesage F, Lazdunski M, Honore E. TREK-1 is a heat-activated background K+ channel. Embo J. 2000;19:2483–2491. doi: 10.1093/emboj/19.11.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maingret F, Patel AJ, Lesage F, Lazdunski M, Honore E. Mechano- or acid stimulation, two interactive modes of activation of the TREK-1 potassium channel. J Biol Chem. 1999;274:26691–26696. doi: 10.1074/jbc.274.38.26691. [DOI] [PubMed] [Google Scholar]
- 14.Miller P, Kemp PJ, Lewis A, Chapman CG, Meadows HJ, Peers C. Acute hypoxia occludes hTREK-1 modulation: re-evaluation of the potential role of tandem P domain K+ channels in central neuroprotection. J Physiol. 2003;548(Pt 1):31–37. doi: 10.1113/jphysiol.2003.040048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller P, Kemp PJ, Peers C. Structural requirements for O2 sensing by the human tandem-P domain channel, hTREK1. Biochem Biophys Res Commun. 2005;331:1253–1256. doi: 10.1016/j.bbrc.2005.04.042. [DOI] [PubMed] [Google Scholar]
- 16.Miller P, Peers C, Kemp PJ. Polymodal regulation of hTREK1 by pH, arachidonic acid, and hypoxia: physiological impact inacidosis and alkalosis. Am J Physiol Cell Physiol. 2004;286:C272–C282. doi: 10.1152/ajpcell.00334.2003. [DOI] [PubMed] [Google Scholar]
- 17.Patel AJ, Honore E, Maingret F, Lesage F, Fink M, Duprat F, Lazdunski M. A mammalian two pore domain mechano-gated S-like K+ channel. Embo J. 1998;17:4283–4290. doi: 10.1093/emboj/17.15.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruppersberg JP, Fakler B. Complexity of the regulation of Kir2.1 K+ channels. Neuropharmacology. 1996;35:887–893. doi: 10.1016/0028-3908(96)00092-5. [DOI] [PubMed] [Google Scholar]
- 19.Ruppersberg JP, Stocker M, Pongs O, Heinemann SH, Frank R, Koenen M. Regulation of fast inactivation of cloned mammalian IKA channels by cysteine oxidation. Nature. 1991;352:711–714. doi: 10.1038/352711a0. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan JM, Traynelis SF, Chen HS, Escobar W, Heinemann SF, Lipton SA. Identification of two cysteine residues that are required for redox modulation of the NMDA subtype of glutamate receptor. Neuron. 1994;13:929–936. doi: 10.1016/0896-6273(94)90258-5. [DOI] [PubMed] [Google Scholar]
- 21.Tang XD, Daggett H, Hanner M, Garcia ML, McManus OB, Brot N, Weissbach H, Heinemann SH, Hoshi T. Oxidative regulation of large conductance calcium-activated potassium channels. J Gen Physiol. 2001;117:253–274. doi: 10.1085/jgp.117.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeidner G, Sadja R, Reuveny E. Redox-dependent gating of G protein-coupled inwardly rectifying K+ channels. J Biol Chem. 2001;276:35564–35570. doi: 10.1074/jbc.M105189200. [DOI] [PubMed] [Google Scholar]