Abstract
Cell-signaling cascades are convergent targets for developmental neurotoxicity of otherwise unrelated agents. We compared organophosphates (chlorpyrifos, diazinon), an organochlorine (dieldrin) and a metal (Ni2+) for their effects on neuronotypic PC12 cells, assessing gene transcription involved in the cyclic AMP pathway. Each agent was introduced during neurodifferentiation at a concentration of 30 μM for 24 or 72 hr and we assessed 69 genes encoding adenylyl cyclase isoforms and regulators, G-protein α- and β,γ-subunits, protein kinase A subtypes and the phosphodiesterase family. We found strong concordance among the four agents across all the gene families, with the strongest relationships for the G-proteins, followed by adenylyl cyclase, and lesser concordance for protein kinase A and phosphodiesterase. Superimposed on this pattern, chlorpyrifos and diazinon were surprisingly the least alike, whereas there was strong concordance of dieldrin and Ni2+ with each other and with each individual organophosphate. Further, the effects of chlorpyrifos differed substantially depending on whether cells were undifferentiated or differentiating. To resolve the disparities between chlorpyrifos and diazinon, we performed analyses in rat brain regions after in vivo neonatal exposures; unlike the in vitro results, there was strong concordance. Our results show that unrelated developmental neurotoxicants can nevertheless produce similar outcomes by targeting cell signaling pathways involved in neurodifferentiation during a critical developmental period of vulnerability. Nevertheless, a full evaluation of concordance between different toxicants requires evaluations of in vitro systems that detect direct effects, as well as in vivo systems that allow for more complex interactions that converge on the same pathway.
Keywords: Adenylyl Cyclase, Chlorpyrifos, Cyclic AMP, Diazinon, Dieldrin, Gene transcription patterns, G-Proteins, Microarrays, Nickel, Organochlorine insecticides, Organophosphate insecticides, PC12 cells, Phosphodiesterase, Protein Kinase A
INTRODUCTION
Organophosphate pesticides (OPs) all share a common mechanism for systemic toxicity, namely their ability to inhibit cholinesterase (Mileson et al., 1998; Pope, 1999). Nevertheless, the developmental neurotoxicity of the OPs reflects additional mechanisms that can operate at exposures devoid of the signs of cholinergic hyperstimulation, which typically requires >70% inhibition of cholinesterase (Clegg and van Gemert, 1999), and even below the threshold for any detectable loss of cholinesterase activity (Gupta, 2004; Slotkin, 1999, 2004; Slotkin et al., 2005). Cell signaling cascades that control neural cell replication and differentiation appear to be among the most sensitive targets for OP developmental neurotoxicity and in particular, the pathway regulating cyclic AMP (cAMP) levels appears to be particularly important in mediating cholinesterase-independent mechanisms (Curtin et al., 2006; Meyer et al., 2003, 2004, 2005; Schuh et al., 2002; Song et al., 1997; Ward and Mundy, 1996; Yanai et al., 2002). Importantly, exposure of developing neurons to OP pesticides result in lasting changes in the expression and/or function of the key signaling proteins in the pathway, including the number and activity of G-protein-coupled receptors, the concentration and function of the stimulatory (Gs) and inhibitory (Gi) G-proteins, and subtype expression and catalytic efficiency of adenylyl cyclase (AC) itself (Adigun et al., 2009; Meyer et al., 2003, 2004, 2005; Song et al., 1997). Indeed, disparities in the targeting of cell signaling pathways likely accounts for divergent neurochemical and behavioral outcomes after exposure to different members of the OP class (Adigun et al., 2009; Aldridge et al., 2004, 2005; Levin et al., 2001; Roegge et al., 2008; Slotkin et al., 2001, 2008a, c; Slotkin and Seidler, 2005; Timofeeva et al., 2008).
Because cell signaling pathways are shared by diverse neurotransmitter and hormonal systems, they may serve as points of convergence for the effects of otherwise unrelated developmental neurotoxicants. For example, we recently described surprising similarities in the neurodevelopmental outcomes of exposure to OPs, organochlorines, heavy metals, polyaromatic hydrocarbons, glucocorticoid steroids (Kreider et al., 2005a, b, 2006) and perfluorinated alkyls (Powers et al., 2009; Slotkin et al., 2007b, 2008b, 2009; Slotkin and Seidler, 2009c, d); the likely points of crosstalk lie in the integration of trophic signals that mediate common events in neurodifferentiation, notably the inputs converging on protein kinases, including protein kinase A, the primary downstream target of cAMP (Kreider et al., 2005a, 2006; Meyer et al., 2003, 2004, 2005; Schuh et al., 2002; Slotkin and Seidler, 2007; Song et al., 1997; Yanai et al., 2002). In the current study, we set out to determine the extent to which related and unrelated developmental neurotoxicants alter cAMP signaling through direct effects on activation or repression of genes encoding the key elements of the pathway: G-proteins, AC isoforms and regulatory proteins, protein kinase A subtypes and the various members of the phosphodiesterase family. We focused on two OPs, chlorpyrifos (CPF) and diazinon (DZN), an organochlorine pesticide (dieldrin) and a heavy metal (Ni2+), all of which have been well-characterized for effects on neural cell replication and differentiation (Slotkin et al., 2007b, 2009; Slotkin and Seidler, 2008, 2009a, b, d). These agents also have intrinsic interest because of environmental concerns about human exposure and safety. Dieldrin is known to produce developmental neurotoxicity (Brannen et al., 1998; Kitazawa et al., 2001, 2003; Liu et al., 1998; Slotkin et al., 2007b; Uzoukwu and Sleight, 1972); nickel shows fetal accumulation similar to that of lead (Casey and Robinson, 1978; Jacobsen et al., 1978) and shares neurotoxic actions with lead and cadmium (Benters et al., 1996).
For our evaluations, we used PC12 cells, a classical in vitro model for neuronal development (Teng and Greene, 1994) that has already been validated to reproduce the mechanisms and outcomes found after in vivo OP exposures (Bagchi et al., 1995, 1996; Crumpton et al., 2000a, b; Das and Barone, 1999; Flaskos et al., 1994; Jameson et al., 2006, 2007; Li and Casida, 1998; Nagata et al., 1997; Qiao et al., 2001, 2005; Slotkin et al., 2007a, b, 2008d, 2009; Song et al., 1998; Tuler et al., 1989). With the addition of nerve growth factor, PC12 cells begin to differentiate, forming neuritic projections and acquiring electrical excitability and neuronal phenotypes (Fujita et al., 1989; Song et al., 1998; Teng and Greene, 1994). We evaluated gene expression patterns with cDNA microarrays, using a planned comparisons approach that focused on the relevant gene families, rather than performing a blanket evaluation of the entire transcriptome (Slotkin and Seidler, 2007, 2009d; Slotkin et al., 2007c, 2008d, 2009); we then assessed concordance of the effects of the various agents across gene classes within the cAMP pathway, rather than relying on changes in the expression of individual genes (Slotkin and Seidler, 2009a, b; Slotkin et al., 2009). First, we determined the extent to which CPF, DZN, dieldrin and Ni2+ show convergent or divergent actions on gene expression in differentiating cells. Second, we assessed whether these changes involve a critical period by comparing the effects of CPF on undifferentiated PC12 cells versus cells undergoing neurodifferentiation. Finally, we contrasted the concordance of CPF and DZN in vitro to the in vivo effects in the developing rat brain after neonatal exposure to the same agents, so as to determine the relative contributions of direct neurotoxicant effects on gene expression in isolated cells as compared to those that depend on more complex processes involving the intact brain.
RESULTS
CPF, DZN, dieldrin and Ni2+ in differentiating PC12 cells
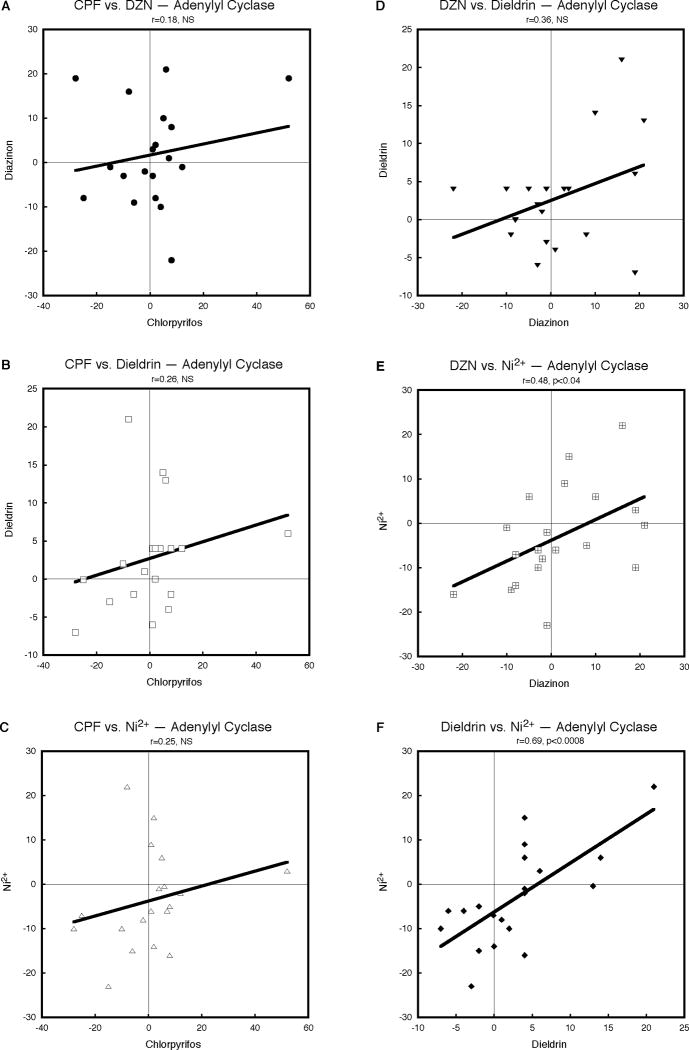
Despite the fact that CPF and DZN are both OPs, there was only slight, nonsignificant concordance in their effects on AC gene expression in differentiating cells (Fig. 1A). Similarly, the effects of CPF showed small, positive correlations with those of dieldrin (Fig. 1B) and Ni2+ (Fig. 1C), as did that for DZN and dieldrin (Fig. 1D), with none of the individual effects of sufficient magnitude to achieve statistical significance. However, the relationship between DZN and Ni2+ was stronger (Fig. 1E), and that of dieldrin and Ni2+ strongest (Fig. 1F). It was notable that, even for the nonsignificant regression relationships, all the correlations were positive. Accordingly, besides the pairwise comparisons, we established the overall significance of the concordance across all four agents using the χ2 test for combining p-values; the global relationship for effects on AC gene expression was highly significant (p < 0.002, Table 1).
Figure 1.
Pairwise correlations of the effects of CPF, DZN, dieldrin and Ni2+ on expression of AC genes, calculated from Supplemental Tables 1 and 2 as the percent change from corresponding control values. Linear correlation coefficients are shown at the top of each panel and the line represents the least-squares fit of the data. NS, not significant.
Table 1.
Compound comparisons of gene expression effects across gene classes and treatments
| AC | G-protein α-subunits | G-protein β,γ-subunits | Protein kinase A | Phospho-diesterase | Row χ2, p-value | |
|---|---|---|---|---|---|---|
| Differentiating cells | ||||||
| CPF vs. DZN | 0.70 | 0.014 | 0.80 | 0.35 | 0.89 | 12, NS |
| CPF vs. dieldrin | 0.30 | 0.0001 | 0.0001 | 0.08 | 0.26 | 47, <0.0001 |
| CPF vs. Ni2+ | 0.18 | 0.11 | 0.004 | 0.03 | 0.41 | 31, <0.001 |
| DZN vs. dieldrin | 0.11 | 0.0002 | 0.81 | 0.03 | 0.08 | 34, < 0.001 |
| DZN versus Ni2+ | 0.04 | 0.0003 | 0.10 | 0.58 | 0.0004 | 44, < 0.0001 |
| dieldrin vs. Ni2+ | 0.0008 | 0.002 | 0.02 | 0.15 | 0.33 | 41, < 0.0001 |
| Column χ2, p-values | 32, < 0.002 | 77, <0.0001 | 43, <0.0001 | 26, <0.02 | 24, <0.02 | 205, <0.0001 |
| CPF in undifferentiated vs. differentiating cells | 0.03 | 0.41 | 0.88 | 0.14 | 0.09 | 18, NS |
| CPF vs. DZN in vivo | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 110, <0.0001 |
Values shown are the individual p-values for each comparison, along with the corresponding χ2 test evaluating the effects for each row, and for differentiating cells, each column. NS, not significant.
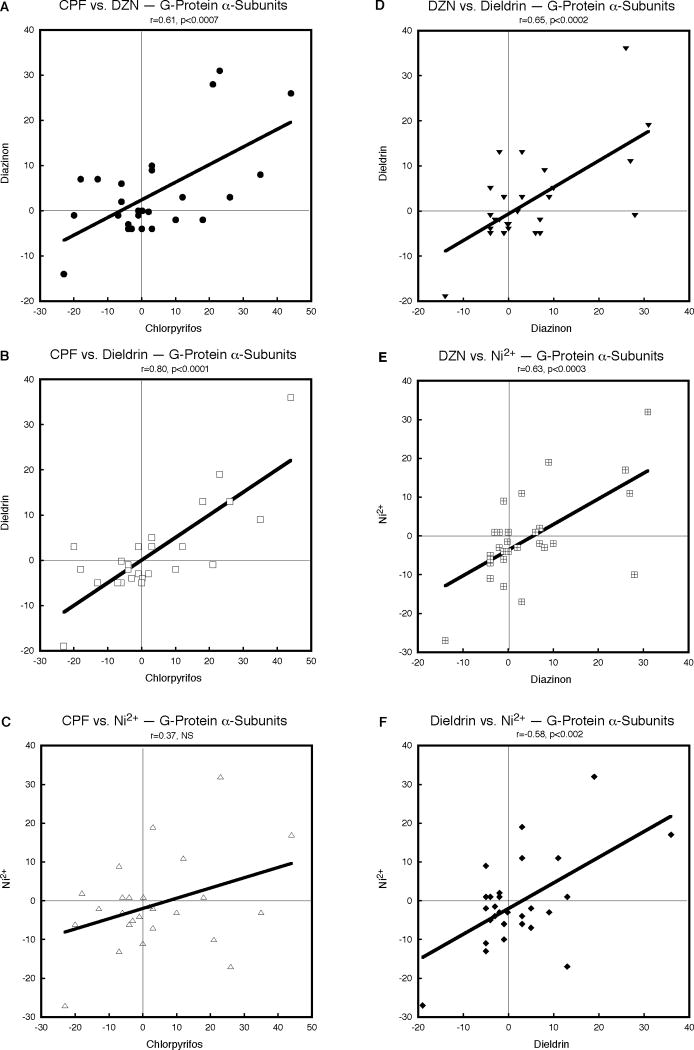
In general, the concordance for effects of CPF, DZN, dieldrin and Ni2+ on G-protein αsubunit genes was much higher than for AC. CPF and DZN showed a strong, statistically significant correlation (Fig. 2A) and the relationship between CPF and dieldrin was even stronger (Fig. 2B). CPF also showed a positive correlation with Ni2+ (Fig. 2C) but the effect was smaller and nonsignificant. On the other hand, all the remaining pairwise combinations were robust and significant: DZN versus dieldrin (Fig. 2D), DZN versus Ni2+ (Fig. 2E) and dieldrin versus Ni2+ (Fig. 2F). Reflecting the positive correlations, the overall concordance for effects of the four agents on G-protein α-subunit gene expression was highly significant (p < 0.0001, Table 1).
Figure 2.
Pairwise correlations of the effects of CPF, DZN, dieldrin and Ni2+ on expression of G-protein α-subunit genes, calculated from Supplemental Tables 1 and 2 as the percent change from corresponding control values. Linear correlation coefficients are shown at the top of each panel and the line represents the least-squares fit of the data. NS, not significant.
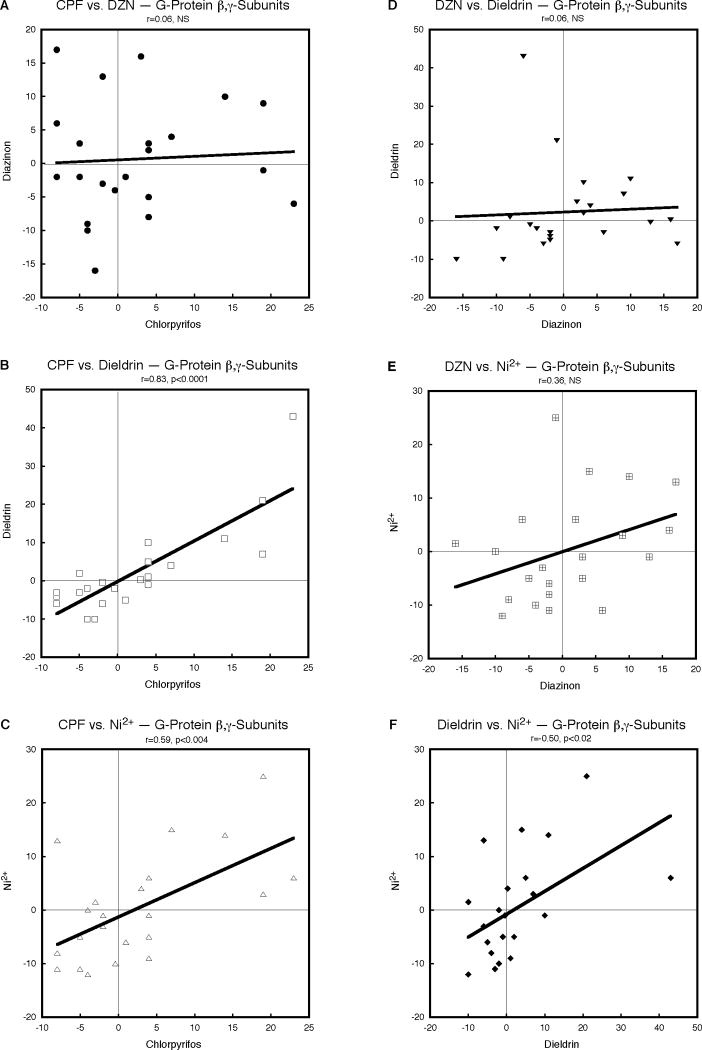
The interrelationships of neurotoxicant effects on the genes encoding G-protein β,γ-subunits were still significant but less uniform than for the α-subunits. CPF and DZN did not show any apparent concordance (Fig. 3A) but CPF was highly concordant with dieldrin (Fig. 3B) and Ni2+ (Fig. 3C). DZN and dieldrin had unrelated effects (Fig. 3D); DZN and Ni2+ showed a stronger relationship that still did not achieve statistical significance (Fig. 3E). On the other hand, the effects of dieldrin and Ni2+ were positively and significantly correlated (Fig. 3F). Taken together, the fact that all correlations were in the same direction (positive) reinforced an overall common pattern as established by the χ2 test (p < 0.0001, Table 1) but this clearly reflected the dominating effect of the three individual correlations that were significant.
Figure 3.
Pairwise correlations of the effects of CPF, DZN, dieldrin and Ni2+ on expression of G-protein β,γ-subunit genes, calculated from Supplemental Tables 1 and 2 as the percent change from corresponding control values. Linear correlation coefficients are shown at the top of each panel and the line represents the least-squares fit of the data. NS, not significant.
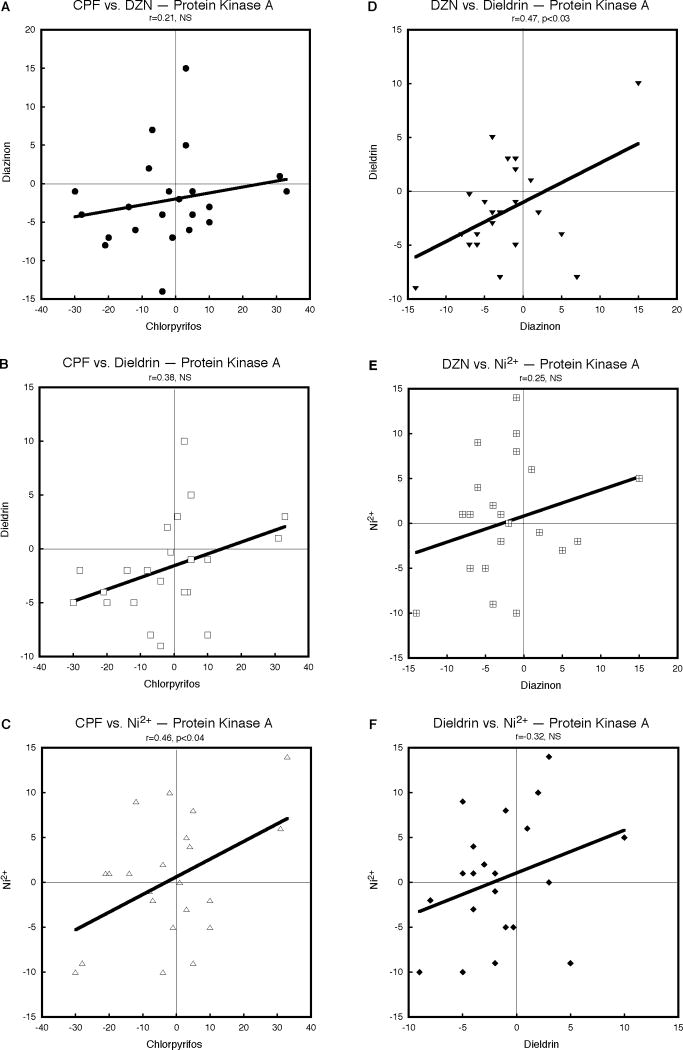
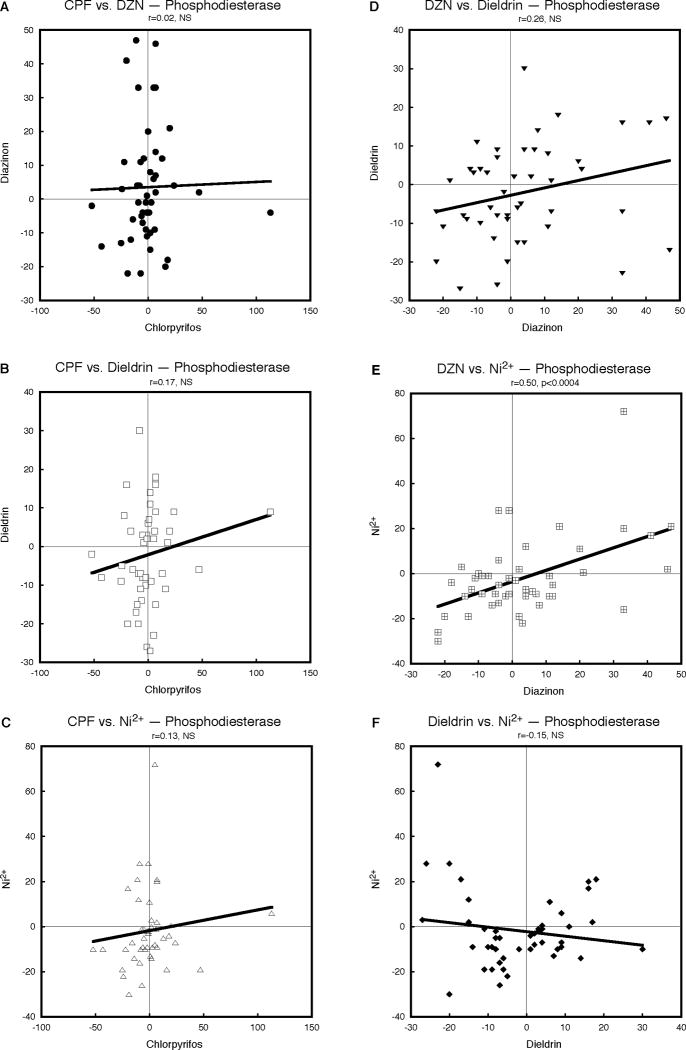
For the protein kinase A gene family, CPF and DZN again showed a slightly positive but nonsignificant relationship (Fig. 4A); the correlation between CPF and dieldrin was better (Fig. 4B) and that between CPF and Ni2+ even stronger, reaching statistical significance (Fig. 4C). A similar, significant relationship was seen for DZN and dieldrin (Fig. 4D), whereas the correlations for DZN versus Ni2+ (Fig. 4E) and dieldrin versus Ni2+ (Fig. 4F) were positive but not sufficiently large to cross the threshold for statistical significance. The weaker relationship for this class of genes was evident in a less robust outcome for the combined χ2 test (p < 0.02, Table 1). Likewise, the results for the phosphodiesterase gene family showed significant but weaker overall relationships among the neurotoxicants, with positive but nonsignificant correlations for CPF versus DZN (Fig. 5A), CPF versus dieldrin (Fig. 5B), CPF versus Ni2+ (Fig. 5C) and DZN versus dieldrin (Fig. 5D). There was more robust, significant concordance between DZN and Ni2+ (Fig. 5E) but the relationship between dieldrin and Ni2+ was slightly discordant (i.e. nonsignificant, negative correlation). Nevertheless, the positive direction for five of the six pairwise comparisons resulted in a statistically significant overall concordance (p < 0.02, Table 1).
Figure 4.
Pairwise correlations of the effects of CPF, DZN, dieldrin and Ni2+ on expression of protein kinase A genes, calculated from Supplemental Tables 1 and 2 as the percent change from corresponding control values. Linear correlation coefficients are shown at the top of each panel and the line represents the least-squares fit of the data. NS, not significant.
Figure 5.
Pairwise correlations of the effects of CPF, DZN, dieldrin and Ni2+ on expression of phosphodiesterase genes, calculated from Supplemental Tables 1 and 2 as the percent change from corresponding control values. Linear correlation coefficients are shown at the top of each panel and the line represents the least-squares fit of the data. NS, not significant.
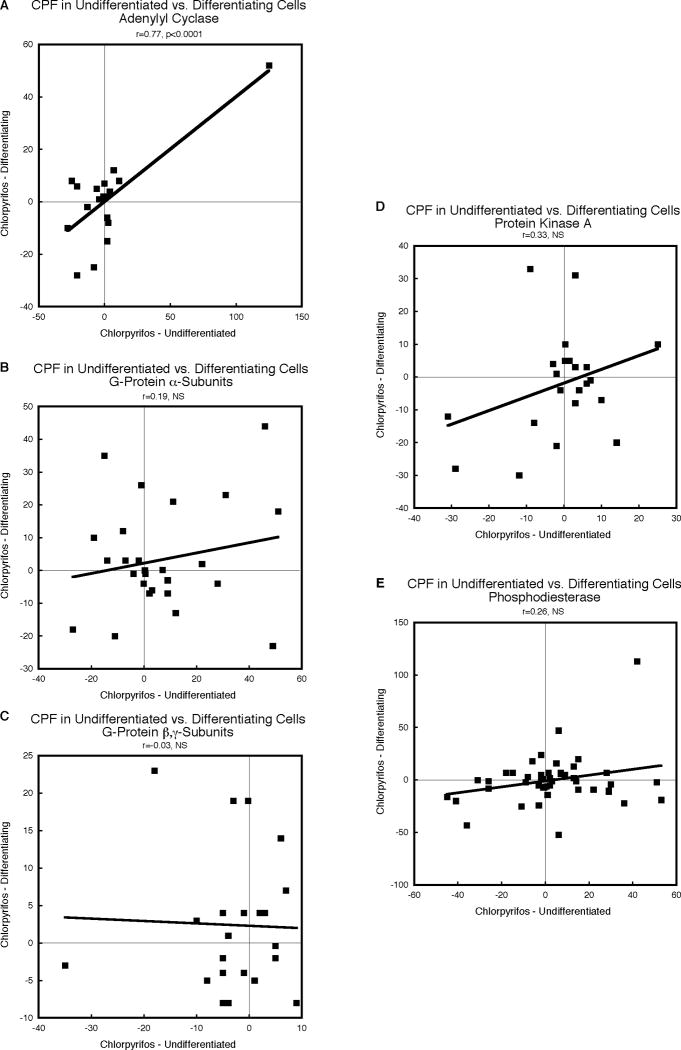
CPF in undifferentiated versus differentiating PC12 cells
The effects of CPF on AC genes correlated significantly between undifferentiated and differentiating cells but the relationship depended completely on a single value (gnat3 at 1d of exposure), without which there would have been no significant concordance (Fig. 6A). Similarly, the effects of CPF on G-protein αsubunits (Fig. 6B) and β,γ-subunits (Fig. 6C) showed virtually no correlation between undifferentiated and differentiating cells; although the relationships for protein kinase A (Fig. 6D) and phosphodiesterase (Fig. 6E) were stronger, none of these achieved statistical significance. The overall weakness of the correlation was confirmed by the lack of statistical significance for compound comparisons of all gene families using the χ2 test (Table 1).
Figure 6.
Correlations of the effects of CPF on undifferentiated versus differentiating cells for each the five classes of genes, calculated from Supplemental Tables 1 and 2 as the percent change from corresponding control values: AC (A), G-protein α-subunits (B), G-protein β,γsubunits (C), protein kinase A (D) and phosphodiesterase (E). Linear correlation coefficients are shown at the top of each panel and the line represents the least-squares fit of the data. NS, not significant.
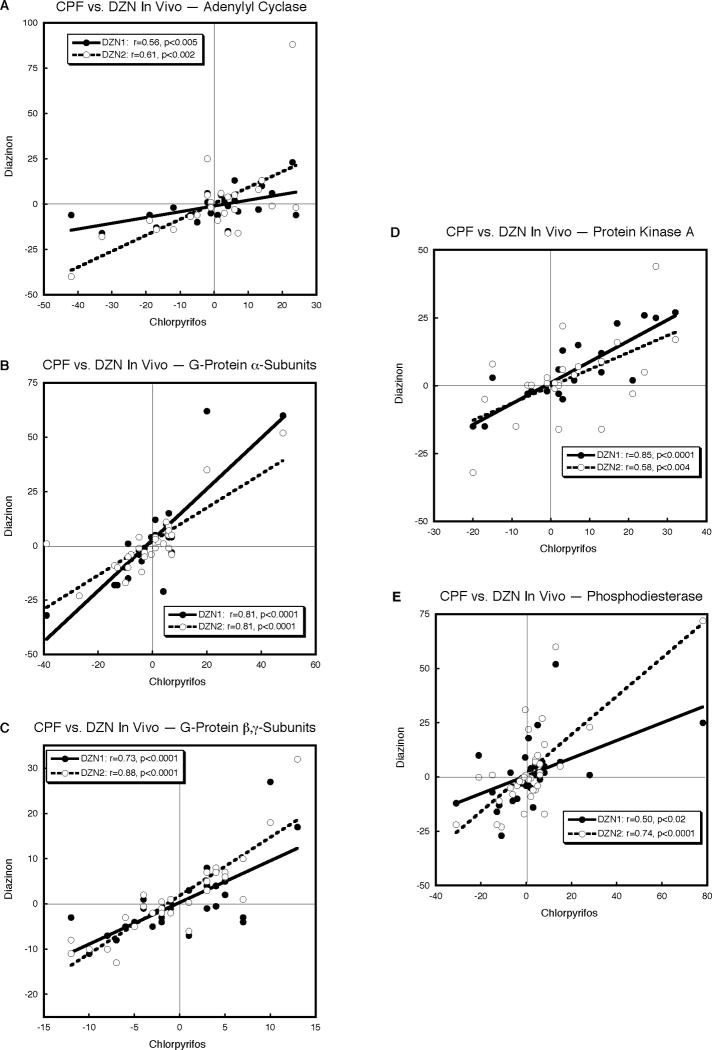
CPF and DZN in vivo
CPF and DZN administration to neonatal rats elicited significantly concordant transcriptional effects for all five gene families in forebrain and brainstem, resulting in a highly-significant overall relationship (p < 0.0001, Table 1). Regardless of whether the comparison involved AC (Fig. 7A), G-protein α-subunits (Fig. 7B), G-protein β,γ-subunits (Fig. 7C), protein kinase A (Fig. 7D), or phosphodiesterase (Fig. 7E), the correlations of CPF with either dose of DZN were strongly positive and statistically significant.
Figure 7.
Correlations of the effects of CPF (1 mg/kg) and DZN (1 or 2 mg/kg) on gene expression in vivo, evaluated on postnatal day 5 in forebrain and brainstem from neonatal rats given each agent daily from postnatal days 1–4: AC (A), G-protein α-subunits (B), G-protein β,γ-subunits (C), protein kinase A (D) and phosphodiesterase (E). Linear correlation coefficients are shown within each panel and the line represents the least-squares fit of the data.
DISCUSSION
Our results indicate that otherwise unrelated developmental neurotoxicants nevertheless converge on cell signaling involving cAMP, while at the same time, there are clear dichotomies between OPs, CPF and DZN, that might be expected to be the most similar. Considering the four compounds together, we found strong concordance for combining all the potential pairwise comparisons (χ2=205, p < 0.0001, Table 1), a reflection of the fact that, of the 30 evaluations, 29 showed a positive correlation and only one a negative correlation, a highly, non-random outcome (p < 0.0001 compared to a random expectation of 15 positive correlations and 15 negative correlations). Further, these similarities were observed in isolated cells, pointing to direct effects of the agents on transcription of genes involved in the control of cAMP. Embedded within the overall interrelationships, the concordance was strongest for effects on G-proteins, followed by the AC genes, and least for the genes involved in downstream effectors and regulators, protein kinase A and phosphodiesterase (see column χ2 values, Table 1). Thus, effects on the steps required for the generation of cAMP provide the predominant points of similarity; consistent with this conclusion, for CPF and DZN, we found that in vivo exposures produce alterations in cAMP generation in response to stimulants acting at the level of G-proteins and AC itself (Adigun et al., 2009; Meyer et al., 2004; Song et al., 1997). The implication is thus inescapable that OPs, organochlorines and metals may all lead to similar neurodevelopmental outcomes despite the underlying differences in their chemical properties and classification. In turn, the in vitro findings can thus guide future in vivo examinations to test that prediction.
Superimposed on the general concordance of effects among CPF, DZN, dieldrin and Ni2+, there were important dichotomies that provide further mechanistic information and predictions about in vivo effects. First, the effects of CPF were highly dependent on the differentiation state of the cells, with little relationship evident between gene transcription in undifferentiated cells and those undergoing neurodifferentiation. This is consistent with the existence of critical periods of vulnerability of developing neurons to OPs. Thus, although OP effects can be seen at neurodevelopmental stages ranging from cell replication through the final stages of neurodifferentiation, the period surrounding the transition from replication to differentiation appears to be one of the most sensitive phases (Jameson et al., 2006; Slotkin, 1999, 2004, 2005; Slotkin et al., 2007b, 2008d, 2009; Slotkin and Seidler, 2008; Song et al., 1998). In the current study, our evaluations in differentiating cells specifically occupied the initial phases of that transition, highlighting the dissimilarities to the effects on gene expression in the undifferentiated state. The outcome is thus critically dependent upon the stage of neurodifferentiation at which exposure occurs, even for the same agent. Given the important role of cAMP in neurodifferentiation (Mark and Storm, 1997; McManus et al., 1999; Stachowiak et al., 2003; Williams et al., 1998), our findings again reinforce the mechanistic relationships between stage-specific effects of neurotoxicants on gene expression controlling cAMP levels, and their ultimate disruption of neurodevelopment.
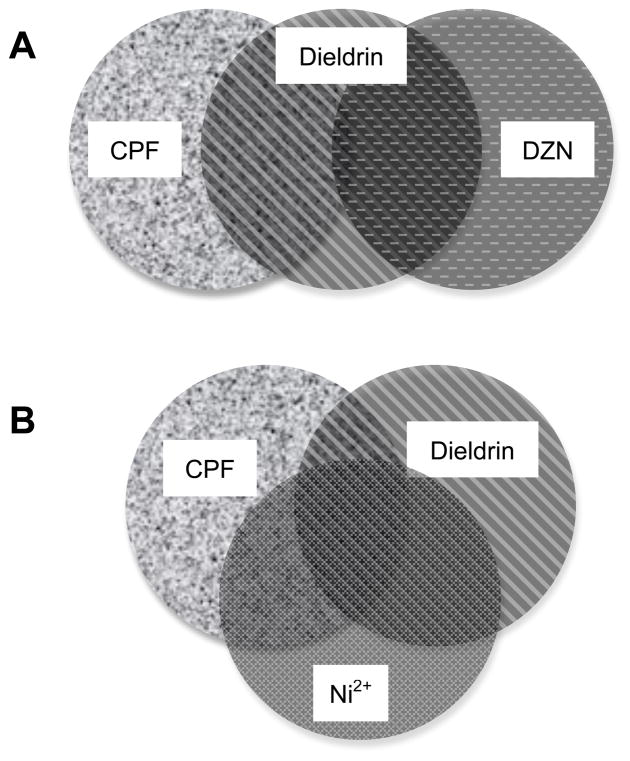
The second dichotomy appeared in the pairwise comparisons of individual agents, where there was a surprising divergence between CPF and DZN, the two toxicants that might be expected to be the most related, since they are both OPs. In fact, of the six pairings, CPF and DZN were the least alike (row χ2 comparisons, Table 1). The effects of these two OPs on gene expression involved in the cAMP signaling pathway clearly do not depend on their shared property as cholinesterase inhibitors, the mechanism that instead contributes to their similarities for systemic toxicity. Indeed, studies of downstream cAMP phosphorylation targets confirm that CPF produces its effects at concentrations well below those required for cholinesterase inhibition (Schuh et al., 2002). Our findings reinforce earlier conclusions showing that CPF has unique and/or more sensitive actions on signaling and neurotrophic pathways as compared to other OPs (Slotkin et al., 2007c, 2008d, 2009; Slotkin and Seidler, 2009a); in contrast, DZN, even when given in the same developmental period as chlorpyrifos and in pharmacodynamically-equivalent doses, elicits lesser long-term disruption of synaptic function and behavior (Aldridge et al., 2004, 2005; Levin et al., 2001; Roegge et al., 2008; Slotkin et al., 2001, 2008a, c; Slotkin and Seidler, 2005; Timofeeva et al., 2008). Despite their dissimilarities, however, both CPF and DZN had strong concordance with the effects of the other two agents (row χ2 comparisons, Table 1). The interrelationships can best be illustrated with schematic Venn diagrams. Although both CPF and DZN have significant overlap with the effects of dieldrin, the two OPs have little concordance with each other (Fig. 8A), so that the common relationship to dieldrin involves different genes for each OP; however, CPF, dieldrin and Ni2+ all have significant mutual overlap (Fig. 8B) and similar relationships pertain to DZN and the other two agents (not shown). The close resemblance of the transcriptional responses to DZN, dieldrin and Ni2+ are entirely consistent with their shared effects on neurotrophic responses (Slotkin et al., 2009) and downstream neurotoxic endpoints (Slotkin et al., 2007b; Slotkin and Seidler, 2008). The implication is inescapable: these results predict that OPs such as CPF and DZN, dieldrin (an organochlorine) and Ni2+ (a metal) may in fact produce convergent developmental neurotoxicant outcomes, even if the OPs themselves may diverge in part.
Figure 8.
Venn diagram illustrating the relationships among CPF, DZN, dieldrin and Ni2+ for their global effects on the five classes of genes as calculated in Table 1. (A) CPF and dieldrin have strong concordance, as do DZN and dieldrin, but the effects of CPF and DZN are only weakly related because the gene effects shared between CPF and dieldrin differ from those involved in the correlation of DZN and dieldrin. (B) CPF, dieldrin and Ni2+ all share common patterns of effects on gene expression.
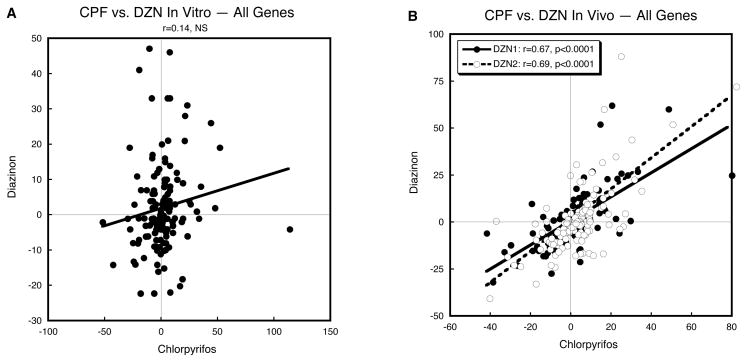
Although in vitro test systems enable the detection of direct effects of neurotoxicants on gene transcription and differentiation endpoints, they are inherently limited in that they cannot evaluate effects that depend on interactions between neurons and glia, architectural assembly of the brain, or any of the myriad, more complex interactions that occur in the intact organism. Here, we found strong dichotomies between CPF and DZN in their effects on gene transcription related to the cAMP signaling pathway in PC12 cells and we have already highlighted how those differences are reflected in divergent outcomes. Nevertheless, CPF and DZN also have a number of similarities in their developmental neurotoxicant effects, particularly as they pertain to targeting of specific brain regions and neurotransmitter systems and their associated behaviors, as well as shared actions on formation of neurites and on proliferation of glial cells (Qiao et al., 2001; Roegge et al., 2008; Slotkin, 1999, 2004, 2005; Slotkin et al., 2006b, 2007b, 2008a, c; Slotkin and Seidler, 2009d; Timofeeva et al., 2008). Accordingly, in addition to the in vitro studies conducted here, we performed a comparable concordance evaluation using gene expression data from our earlier work on the effects of CPF and DZN in vivo, in neonatal rats; we identified much stronger concordance involving highly significant correlations for each class of genes and across all classes together (Table 1). The strong in vivo relationship can be seen by comparing concordance of all the cAMP pathway genes in a single correlation: whereas there was no significant overall correlation for the in vitro evaluations (Fig. 9A), the same comparisons were highly concordant for in vivo exposures (Fig. 9B). Thus, in the intact brain, CPF and DZN do actually produce similar effects on the cAMP-related genes but the common outcome reflects indirect actions requiring the intact brain, rather than depending upon direct effects on differentiating neural cells, as seen with the PC12 cell model. Clearly, although in vitro models permit identification of direct effects of neurotoxicants on neurodifferentiation, a full picture of similarities and differences of neurotoxicant effects directed toward a given pathway requires additional examinations of the effects in the intact organism. It would thus be worthwhile to pursue similar examinations of the effects of dieldrin and Ni2+. Similarly, a key limitation of the microarray approach is that the measured effects occur at the mRNA level, which does not necessarily entail a corresponding change at the level of protein expression or cell function. We have studies currently underway to evaluate the extent to which functional changes correspond to the effects of each toxicant on gene expression.
Figure 9.
Comparison of in vitro (A) and in vivo (B) correlations of gene expression values combined from all five classes. Linear correlation coefficients are shown at the top of each panel and the line represents the least-squares fit of the data. NS, not significant.
In this study, we used planned comparisons of specific pathways targeted by the neurotoxicants and analyzed the data through the determination of shared properties (i.e. a standard “principal components” approach); the rationale for this has appeared previously (Slotkin and Seidler, 2007; Slotkin et al., 2007c, 2008d) but is worth repeating here. Planned comparisons and pathway analysis are distinct from the use of microarrays to find a handful of genes that are affected the most, within the global examination of the tens of thousands of genes present on the microarrays. Planned comparisons are based instead on testing a specific hypothesis that centers around a defined set of genes, and rests on known, validating outcomes from prior work, in this case for the organophosphates. With examination of the entire genome, verification via RT-PCR and other techniques is required because the enormous number of comparisons generates many false positive findings (e.g. the >2000 genes that would be false positives if we had considered all 42,000 probes on the array). For our study, we compared only 69 genes that would generate only 3–4 false positives, and for interpretation, we relied on the overall pattern of multiple gene changes for each agent, as well as effects that were repeated across different treatments and/or different times, rather than changes in any one gene. The odds of all those genes being false positives is astronomically small. However, even for individual genes, there were multiple probes and multiple spots on a given array (see Experimental Procedures), so the changes cannot be “chance.” Unlike many array studies, where a single mRNA set combined from multiple samples might be evaluated, we evaluated separate samples for each treatment condition, so again it is inconceivable that one could statistically produce these outcomes by accident. Indeed, one of the key points of this study is to demonstrate that a planned comparisons approach may provide a superior strategy for the use of microarray data, provided that the relevant target pathways are known in advance.
In conclusion, our findings bolster the increasing evidence that the various OPs differ in their underlying mechanisms of developmental neurotoxicity, over and above their shared property as cholinesterase inhibitors, in this case involving distinct outcomes at the levels of the genes encoding the critical proteins of the cAMP signaling pathway. In addition, we found unexpected concordance in the effects of unrelated neurotoxicants, dieldrin and Ni2+, on the same gene families, indicating that different classes of compounds can nonetheless converge on common final pathways. Finally, the results obtained here illustrate how a combined use of a cell culture system, an animal model, and microarrays can guide future studies toward specific endpoints that can distinguish similarities and disparities in the effects of diverse developmental neurotoxicants.
EXPERIMENTAL PROCEDURE
Cell cultures
Because of the clonal instability of the PC12 cell line (Fujita et al., 1989), the experiments were performed on cells that had undergone fewer than five passages. As described previously (Qiao et al., 2003; Song et al., 1998), PC12 cells (American Type Culture Collection, 1721-CRL, obtained from the Duke Comprehensive Cancer Center, Durham, NC) were seeded onto poly-D-lysine-coated plates in RPMI-1640 medium (Invitrogen, Carlsbad, CA) supplemented with 10% inactivated horse serum (Sigma Chemical Co., St. Louis, MO), 5% inactivated fetal bovine serum (Sigma), and 50 μg/ml penicillin streptomycin (Invitrogen). Incubations were carried out with 7.5% CO2 at 37°C, standard conditions for PC12 cells. To initiate neurodifferentiation (Jameson et al., 2006; Slotkin et al., 2007b; Teng and Greene, 1994) twenty-four hours after seeding, the medium was changed to include 50 ng/ml of 2.5 S murine nerve growth factor (Invitrogen). Along with the nerve growth factor, we added 30 μM of each of the test agents: CPF (Chem Service, West Chester, PA), DZN (Chem Service), dieldrin (Chem Service) or NiCl2 (Sigma). The concentration was chosen from earlier studies that demonstrated adverse effects on differentiation of PC12 cells without outright cytotoxicity (Jameson et al., 2007; Qiao et al., 2001; Slotkin et al., 2007b, 2008d). Because of the limited water solubility of the three insecticides, these agents were dissolved in dimethylsulfoxide (final concentration 0.1%), which was also added to the control cultures and to cultures containing NiCl2; this concentration of dimethylsulfoxide has no effect on PC12 cell growth or differentiation (Qiao et al., 2001, 2003; Song et al., 1998). Cultures were examined 24 and 72 hr after commencing exposure, with 5–8 independent cultures evaluated for each treatment at each time point. Each culture was run on a separate array. We used two time points so as to be able to evaluate changes in gene expression regardless of whether the mRNA for a given gene has a rapid turnover (and hence can rise rapidly) or a slower turnover that would require a longer period to show corresponding increases or decreases. For CPF, we evaluated the effects both on undifferentiated cells (without addition of nerve growth factor) and during differentiation, whereas for the other agents, we studied the effects only during differentiation.
Animal treatments
No additional animal studies were conducted for this work. Instead, we utilized the gene expression data from our previously-published microarray study of the effects of CPF and DZN in neonatal rats (Slotkin and Seidler, 2007), with the new addition of concordance comparisons as required for the present study. Details of the animal treatments have been published (Slotkin and Seidler, 2007) and therefore will be described only briefly. Neonatal rats received daily injections of CPF or DZN in dimethylsulfoxide vehicle on postnatal days 1–4, whereas control animals received equivalent injections of the vehicle. For both agents, we utilized doses below the threshold for growth retardation and the first signs of systemic toxicity (Campbell et al., 1997; Slotkin et al., 2006a; Whitney et al., 1995): 1 mg/kg for CPF and 1 or 2 mg/kg for DZN. The CPF treatment and the higher dose of DZN elicit less than 20% cholinesterase inhibition, well below the 70% threshold necessary for symptoms of cholinergic hyperstimulation (Clegg and van Gemert, 1999), whereas the lower dose of DZN produces no measurable inhibition (Slotkin, 1999, 2004; Slotkin et al., 2006b; Song et al., 1997; Whitney et al., 1995). On postnatal day 5 (24 hr after the last dose), one male pup was selected from each of five litters in each treatment group and separate determinations were made for the forebrain and brainstem from each animal.
Microarray determinations
Our earlier studies detailed all the techniques for mRNA isolation, preparation of cDNA, conversion to cRNA incorporating cyanine-3 (reference RNA) or cyanine-5 (sample RNA), verification of RNA purity and quality, hybridization to the microarrays, washing and scanning (Slotkin and Seidler, 2007; Slotkin et al., 2007c, 2008d). These all involve commercial kits and standardized procedures, and since the current studies were done identically, the techniques will not be described here. The mRNA used for the reference standard was created by pooling aliquots from each of the samples in the study so as to ensure measurable levels of all genes expressed over the background. Array normalizations and error detection were also carried out by standard procedures described previously (Slotkin and Seidler, 2007; Slotkin et al., 2007c, 2008d). We used Agilent Whole Rat Genome Arrays (Agilent Technologies, Palo Alto, CA), type G4131A for the studies of CPF in undifferentiated and differentiating cells and for the in vivo studies, whereas type G4131F was used for the studies of DZN, dieldrin and Ni2+ in differentiating cells. The two chips contain exactly the same gene sequences but the latter has a lower detection threshold; however, all the genes reported here passed the quality control filters with both arrays.
For many of the genes, the arrays contain multiple probes and/or replicates of the same probe in different locations on the chip, and these were used to verify the reliability of values and the validity of the measures on the chip. In these cases, to avoid artificially inflating the number of genes utilized for concordance analysis, we limited each gene to a single set of values, selecting those obtained for the probe showing the smallest intragroup variance. The other values for that gene were used only to corroborate direction and magnitude of change. We also validated the readings on the arrays through the use of duplicate arrays for one sample selected from each treatment group (Slotkin and Seidler, 2007; Slotkin et al., 2007c).
Statistical procedures
Because of the requirement to normalize the data across arrays and within each gene, the absolute values for a given gene are meaningless, so only the relative differences between treatments can be compared. Accordingly, concordance was evaluated using the percentage change from the control values, calculating the linear correlation coefficient between pairs of agents for each class of genes. For reference, the raw values (mean ± SE) for normalized expression for all the genes appear in Supplemental Table 1 for the evaluations of CPF effects in undifferentiated and differentiating cells. Supplemental Table 2 shows the values for the effects of DZN, dieldrin and Ni2+ in differentiating cells and the corresponding values for the in vivo study were published previously (Slotkin and Seidler, 2007). It should be noted that the absolute values cannot be compared for the three sets of determinations because reference mRNAs were constructed from the samples contained in each study, as described above, and thus differed among the studies; nevertheless, the percentage changes from the control value can be compared, since taking the experimental values as a ratio to the corresponding control normalizes the values for the inter-experimental differences. Additional details of the nomenclature and sequence for each gene appeared earlier (Slotkin and Seidler, 2007).
In addition to pairwise concordance comparisons of the various agents, we also evaluated groupings of correlations for each class of genes and across classes, using the χ2 test for combining p-values: χ2 = Σ[−2 ln(pi)], with degrees of freedom corresponding to twice the number of p-values used in the calculation. In the two cases where a correlation coefficient connoted an opposite direction of change from the others, the associated χ2 value for that comparison was subtracted from the sum instead of being added to it. Significance for all tests was assumed at p < 0.05.
Supplementary Material
Acknowledgments
Acknowledgments/disclaimers: Research was supported by NIH ES10356. TAS has provided expert witness testimony in the past three years at the behest of the following law firms: The Calwell Practice (Charleston WV), Frost Brown Todd (Charleston WV), Weltchek Mallahan & Weltchek (Lutherville MD), Finnegan Henderson Farabow Garrett & Dunner (Washington DC), Frommer Lawrence Haug (Washington DC), Carter Law (Peoria IL), Corneille Law (Madison WI), Angelos Law (Baltimore MD), Kopff, Nardelli & Dopf (New York NY), Gutglass Erickson Bonville & Larson (Madison WI) and Pardieck Law (Seymour IN).
Abbreviations
- AC
adenylyl cyclase
- cAMP
cyclic AMP
- CPF
chlorpyrifos
- DZN
diazinon
- OP
organophosphate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adigun AA, Wrench N, Seidler FJ, Slotkin TA. Neonatal organophosphorus pesticide exposure alters the developmental trajectory of cell signaling cascades controlling metabolism: differential effects of diazinon and parathion. Environ Health Perspect. 2009 doi: 10.1289/ehp.0901237. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge JE, Seidler FJ, Slotkin TA. Developmental exposure to chlorpyrifos elicits sex-selective alterations of serotonergic synaptic function in adulthood: critical periods and regional selectivity for effects on the serotonin transporter, receptor subtypes, and cell signaling. Environ Health Perspect. 2004;112:148–155. doi: 10.1289/ehp.6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge JE, Levin ED, Seidler FJ, Slotkin TA. Developmental exposure of rats to chlorpyrifos leads to behavioral alterations in adulthood, involving serotonergic mechanisms and resembling animal models of depression. Environ Health Perspect. 2005;113:527–531. doi: 10.1289/ehp.7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi D, Bagchi M, Hassoun EA, Stohs SJ. In vitro and in vivo generation of reactive oxygen species, DNA damage and lactate dehydrogenase leakage by selected pesticides. Toxicology. 1995;104:129–140. doi: 10.1016/0300-483x(95)03156-a. [DOI] [PubMed] [Google Scholar]
- Bagchi D, Bhattacharya G, Stohs SJ. In vitro and in vivo induction of heat shock (stress) protein (Hsp) gene expression by selected pesticides. Toxicology. 1996;112:57–68. doi: 10.1016/0300-483x(96)03350-1. [DOI] [PubMed] [Google Scholar]
- Benters J, Schafer T, Beyersmann D, Hechtenberg S. Agonist-stimulated calcium transients in PC12 cells are affected differentially by cadmium and nickel. Cell Calcium. 1996;20:441–446. doi: 10.1016/s0143-4160(96)90007-x. [DOI] [PubMed] [Google Scholar]
- Brannen KC, Devaud LL, Liu JP, Lauder JM. Prenatal exposure to neurotoxicants dieldrin or lindane alters tert-butylbicyclophosphorothionate binding to GABA(A) receptors in fetal rat brainstem. Dev Neurosci. 1998;20:34–41. doi: 10.1159/000017296. [DOI] [PubMed] [Google Scholar]
- Campbell CG, Seidler FJ, Slotkin TA. Chlorpyrifos interferes with cell development in rat brain regions. Brain Res Bull. 1997;43:179–189. doi: 10.1016/s0361-9230(96)00436-4. [DOI] [PubMed] [Google Scholar]
- Casey CE, Robinson MF. Copper, manganese, zinc, nickel, cadmium and lead in human foetal tissues. Br J Nutrition. 1978;39:639–646. doi: 10.1079/bjn19780079. [DOI] [PubMed] [Google Scholar]
- Clegg DJ, van Gemert M. Determination of the reference dose for chlorpyrifos: proceedings of an expert panel. J Toxicol Environ Health. 1999;2:211–255. doi: 10.1080/109374099281179. [DOI] [PubMed] [Google Scholar]
- Crumpton TL, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos in vivo and in vitro: effects on nuclear transcription factor involved in cell replication and differentiation. Brain Res. 2000a;857:87–98. doi: 10.1016/s0006-8993(99)02357-4. [DOI] [PubMed] [Google Scholar]
- Crumpton TL, Seidler FJ, Slotkin TA. Is oxidative stress involved in the developmental neurotoxicity of chlorpyrifos? Dev Brain Res. 2000b;121:189–195. doi: 10.1016/s0165-3806(00)00045-6. [DOI] [PubMed] [Google Scholar]
- Curtin BF, Pal N, Gordon RK, Nambiar MP. Forskolin, an inducer of cAMP, up-regulates acetylcholinesterase expression and protects against organophosphate exposure in neuro 2A cells. Mol Cell Biochem. 2006;290:23–32. doi: 10.1007/s11010-005-9084-4. [DOI] [PubMed] [Google Scholar]
- Das KP, Barone S. Neuronal differentiation in PC12 cells is inhibited by chlorpyrifos and its metabolites: is acetylcholinesterase inhibition the site of action? Toxicol Appl Pharmacol. 1999;160:217–230. doi: 10.1006/taap.1999.8767. [DOI] [PubMed] [Google Scholar]
- Flaskos J, McLean WG, Hargreaves AJ. The toxicity of organophosphate compounds towards cultured PC12 cells. Toxicol Lett. 1994;70:71–76. doi: 10.1016/0378-4274(94)90146-5. [DOI] [PubMed] [Google Scholar]
- Fujita K, Lazarovici P, Guroff G. Regulation of the differentiation of PC12 pheochromocytoma cells. Environ Health Perspect. 1989;80:127–142. doi: 10.1289/ehp.8980127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RC. Brain regional heterogeneity and toxicological mechanisms of organophosphates and carbamates. Toxicol Mech Meth. 2004;14:103–143. doi: 10.1080/15376520490429175. [DOI] [PubMed] [Google Scholar]
- Jacobsen N, Alfheim I, Jonsen J. Nickel and strontium distribution in some mouse tissues. Passage through placenta and mammary glands. Res Comm Chem Pathol Pharmacol. 1978;20:571–584. [PubMed] [Google Scholar]
- Jameson RR, Seidler FJ, Qiao D, Slotkin TA. Chlorpyrifos affects phenotypic outcomes in a model of mammalian neurodevelopment: critical stages targeting differentiation in PC12 cells. Environ Health Perspect. 2006;114:667–672. doi: 10.1289/ehp.8750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson RR, Seidler FJ, Slotkin TA. Nonenzymatic functions of acetylcholinesterase splice variants in the developmental neurotoxicity of organophosphates: chlorpyrifos, chlorpyrifos oxon and diazinon. Environ Health Perspect. 2007;115:65–70. doi: 10.1289/ehp.9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa M, Anantharam V, Kanthasamy AG. Dieldrin-induced oxidative stress and neurochemical changes contribute to apoptopic cell death in dopaminergic cells. Free Radical Biol Med. 2001;31:1473–1485. doi: 10.1016/s0891-5849(01)00726-2. [DOI] [PubMed] [Google Scholar]
- Kitazawa M, Anantharam V, Kanthasamy AG. Dieldrin induces apoptosis by promoting caspase-3-dependent proteolytic cleavage of protein kinase Cδ in dopaminergic cells: relevance to oxidative stress and dopaminergic degeneration. Neuroscience. 2003;119:945–964. doi: 10.1016/s0306-4522(03)00226-4. [DOI] [PubMed] [Google Scholar]
- Kreider ML, Aldridge JE, Cousins MM, Oliver CA, Seidler FJ, Slotkin TA. Disruption of rat forebrain development by glucocorticoids: critical perinatal periods for effects on neural cell acquisition and on cell signaling cascades mediating noradrenergic and cholinergic neurotransmitter/neurotrophic responses. Neuropsychopharmacology. 2005a;30:1841–1855. doi: 10.1038/sj.npp.1300743. [DOI] [PubMed] [Google Scholar]
- Kreider ML, Levin ED, Seidler FJ, Slotkin TA. Gestational dexamethasone treatment elicits sex-dependent alterations in locomotor activity, reward-based memory and hippocampal cholinergic function in adolescent and adult rats. Neuropsychopharmacology. 2005b;30:1617–1623. doi: 10.1038/sj.npp.1300716. [DOI] [PubMed] [Google Scholar]
- Kreider ML, Tate CA, Cousins MM, Oliver CA, Seidler FJ, Slotkin TA. Lasting effects of developmental dexamethasone treatment on neural cell number and size, synaptic activity and cell signaling: critical periods of vulnerability, dose-effect relationships, regional targets and sex selectivity. Neuropsychopharmacology. 2006;31:12–35. doi: 10.1038/sj.npp.1300783. [DOI] [PubMed] [Google Scholar]
- Levin ED, Addy N, Christopher NC, Seidler FJ, Slotkin TA. Persistent behavioral consequences of neonatal chlorpyrifos exposure in rats. Dev Brain Res. 2001;130:83–89. doi: 10.1016/s0165-3806(01)00215-2. [DOI] [PubMed] [Google Scholar]
- Li WW, Casida JE. Organophosphorus neuropathy target esterase inhibitors selectively block outgrowth of neurite-like and cell processes in cultured cells. Toxicol Lett. 1998;98:139–146. doi: 10.1016/s0378-4274(98)00116-7. [DOI] [PubMed] [Google Scholar]
- Liu JP, Brannen KC, Grayson DR, Morrow AL, Devaud LL, Lauder JM. Prenatal exposure to the pesticide dieldrin or the GABA(A) receptor antagonist bicuculline differentially alters expression of GABA(A) receptor subunit mRNAs in fetal rat brainstem. Dev Neurosci. 1998;20:83–92. doi: 10.1159/000017302. [DOI] [PubMed] [Google Scholar]
- Mark MD, Storm DR. Coupling of epidermal growth factor (EGF) with the antiproliferative activity of cAMP induces neuronal differentiation. J Biol Chem. 1997;272:17238–17244. doi: 10.1074/jbc.272.27.17238. [DOI] [PubMed] [Google Scholar]
- McManus MF, Chen LC, Vallejo I, Vallejo M. Astroglial differentiation of cortical precursor cells triggered by activation of the cAMP-dependent signaling pathway. J Neurosci. 1999;19:9004–9015. doi: 10.1523/JNEUROSCI.19-20-09004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Seidler FJ, Cousins MM, Slotkin TA. Developmental neurotoxicity elicited by gestational exposure to chlorpyrifos: when is adenylyl cyclase a target? Environ Health Perspect. 2003;111:1871–1876. doi: 10.1289/ehp.6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Seidler FJ, Aldridge JE, Tate CA, Cousins MM, Slotkin TA. Critical periods for chlorpyrifos-induced developmental neurotoxicity: alterations in adenylyl cyclase signaling in adult rat brain regions after gestational or neonatal exposure. Environ Health Perspect. 2004;112:295–301. doi: 10.1289/ehp.6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Seidler FJ, Aldridge JE, Slotkin TA. Developmental exposure to terbutaline alters cell signaling in mature rat brain regions and augments the effects of subsequent neonatal exposure to the organophosphorus insecticide, chlorpyrifos. Toxicol Appl Pharmacol. 2005;203:154–166. doi: 10.1016/j.taap.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Mileson BE, Chambers JE, Chen WL, Dettbarn W, Ehrich M, Eldefrawi AT, Gaylor DW, Hamernik K, Hodgson E, Karczmar AG, Padilla S, Pope CN, Richardson RJ, Saunders DR, Sheets LP, Sultatos LG, Wallace KB. Common mechanism of toxicity: a case study of organophosphorus pesticides. Toxicol Sci. 1998;41:8–20. doi: 10.1006/toxs.1997.2431. [DOI] [PubMed] [Google Scholar]
- Nagata K, Huang CS, Song JH, Narahashi T. Direct actions of anticholinesterases on the neuronal nicotinic acetylcholine receptor channels. Brain Res. 1997;769:211–218. doi: 10.1016/s0006-8993(97)00707-5. [DOI] [PubMed] [Google Scholar]
- Pope CN. Organophosphorus pesticides: do they all have the same mechanism of toxicity? J Toxicol Environ Health. 1999;2:161–181. doi: 10.1080/109374099281205. [DOI] [PubMed] [Google Scholar]
- Powers CM, Wrench N, Ryde IT, Smith AM, Seidler FJ, Slotkin TA. Silver impairs neurodevelopment: studies in PC12 cells. Environ Health Perspect. 2009 doi: 10.1289/ehp.0901149. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos modeled in vitro: comparative effects of metabolites and other cholinesterase inhibitors on DNA synthesis in PC12 and C6 cells. Environ Health Perspect. 2001;109:909–913. doi: 10.1289/ehp.01109909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Violin JD, Slotkin TA. Nicotine is a developmental neurotoxicant and neuroprotectant: stage-selective inhibition of DNA synthesis coincident with shielding from effects of chlorpyrifos. Dev Brain Res. 2003;147:183–190. doi: 10.1016/s0165-3806(03)00222-0. [DOI] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Slotkin TA. Oxidative mechanisms contributing to the developmental neurotoxicity of nicotine and chlorpyrifos. Toxicol Appl Pharmacol. 2005;206:17–26. doi: 10.1016/j.taap.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Roegge CS, Timofeeva OA, Seidler FJ, Slotkin TA, Levin ED. Developmental diazinon neurotoxicity in rats: later effects on emotional response. Brain Res Bull. 2008;75:166–172. doi: 10.1016/j.brainresbull.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuh RA, Lein PJ, Beckles RA, Jett DA. Noncholinesterase mechanisms of chlorpyrifos neurotoxicity: altered phosphorylation of Ca2+/cAMP response element binding protein in cultured neurons. Toxicol Appl Pharmacol. 2002;182:176–185. doi: 10.1006/taap.2002.9445. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Developmental cholinotoxicants: nicotine and chlorpyrifos. Environ Health Perspect. 1999;107(suppl 1):71–80. doi: 10.1289/ehp.99107s171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Cousins MM, Tate CA, Seidler FJ. Persistent cholinergic presynaptic deficits after neonatal chlorpyrifos exposure. Brain Res. 2001;902:229–243. doi: 10.1016/s0006-8993(01)02387-3. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol Appl Pharmacol. 2004;198:132–151. doi: 10.1016/j.taap.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Developmental neurotoxicity of organophosphates: a case study of chlorpyrifos. In: Gupta RC, editor. Toxicity of Organophosphate and Carbamate Pesticides. Elsevier Academic Press; San Diego: 2005. pp. 293–314. [Google Scholar]
- Slotkin TA, Oliver CA, Seidler FJ. Critical periods for the role of oxidative stress in the developmental neurotoxicity of chlorpyrifos and terbutaline, alone or in combination. Dev Brain Res. 2005;157:172–180. doi: 10.1016/j.devbrainres.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. The alterations in CNS serotonergic mechanisms caused by neonatal chlorpyrifos exposure are permanent. Dev Brain Res. 2005;158:115–119. doi: 10.1016/j.devbrainres.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Levin ED, Seidler FJ. Comparative developmental neurotoxicity of organophosphate insecticides: effects on brain development are separable from systemic toxicity. Environ Health Perspect. 2006a;114:746–751. doi: 10.1289/ehp.8828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Tate CA, Ryde IT, Levin ED, Seidler FJ. Organophosphate insecticides target the serotonergic system in developing rat brain regions: disparate effects of diazinon and parathion at doses spanning the threshold for cholinesterase inhibition. Environ Health Perspect. 2006b;114:1542–1546. doi: 10.1289/ehp.9337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, MacKillop EA, Ryde IT, Seidler FJ. Ameliorating the developmental neurotoxicity of chlorpyrifos: a mechanisms-based approach in PC12 cells. Environ Health Perspect. 2007a;115:1306–1313. doi: 10.1289/ehp.10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, MacKillop EA, Ryde IT, Tate CA, Seidler FJ. Screening for developmental neurotoxicity using PC12 cells: comparisons of organophosphates with a carbamate, an organochlorine and divalent nickel. Environ Health Perspect. 2007b;115:93–101. doi: 10.1289/ehp.9527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Comparative developmental neurotoxicity of organophosphates in vivo: transcriptional responses of pathways for brain cell development, cell signaling, cytotoxicity and neurotransmitter systems. Brain Res Bull. 2007;72:232–274. doi: 10.1016/j.brainresbull.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ, Fumagalli F. Exposure to organophosphates reduces the expression of neurotrophic factors in neonatal rat brain regions: similarities and differences in the effects of chlorpyrifos and diazinon on the fibroblast growth factor superfamily. Environ Health Perspect. 2007c;115:909–916. doi: 10.1289/ehp.9901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Bodwell BE, Levin ED, Seidler FJ. Neonatal exposure to low doses of diazinon: long-term effects on neural cell development and acetylcholine systems. Environ Health Perspect. 2008a;116:340–348. doi: 10.1289/ehp.11005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, MacKillop EA, Melnick RL, Thayer KA, Seidler FJ. Developmental neurotoxicity of perfluorinated chemicals modeled in vitro. Environ Health Perspect. 2008b;116:716–722. doi: 10.1289/ehp.11253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Ryde IT, Levin ED, Seidler FJ. Developmental neurotoxicity of low-dose diazinon exposure of neonatal rats: effects on serotonin systems in adolescence and adulthood. Brain Res Bull. 2008c;75:640–647. doi: 10.1016/j.brainresbull.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Developmental neurotoxicants target neurodifferentiation into the serotonin phenotype: chlorpyrifos, diazinon, dieldrin and divalent nickel. Toxicol Appl Pharmacol. 2008;233:211–219. doi: 10.1016/j.taap.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ, Fumagalli F. Targeting of neurotrophic factors, their receptors, and signaling pathways in the developmental neurotoxicity of organophosphates in vivo and in vitro. Brain Res Bull. 2008d;76:424–438. doi: 10.1016/j.brainresbull.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Protein kinase C is a target for diverse developmental neurotoxicants: transcriptional responses to chlorpyrifos, diazinon, dieldrin and divalent nickel in PC12 cells. Brain Res. 2009a;1263:23–32. doi: 10.1016/j.brainres.2009.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Oxidative and excitatory mechanisms of developmental neurotoxicity: transcriptional profiles for chlorpyrifos, diazinon, dieldrin and divalent nickel in PC12 cells. Environ Health Perspect. 2009b;117:587–596. doi: 10.1289/ehp.0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Benzo[a]pyrene impairs neurodifferentiation in PC12 cells. Brain Res Bull. 2009c;80:17–21. doi: 10.1016/j.brainresbull.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Transcriptional profiles reveal similarities and differences in the effects of developmental neurotoxicants on differentiation into neurotransmitter phenotypes in PC12 cells. Brain Res Bull. 2009d;78:211–225. doi: 10.1016/j.brainresbull.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ, Fumagalli F. Unrelated developmental neurotoxicants elicit similar transcriptional profiles for effects on neurotrophic factors and their receptors in an in vitro model. Neurotoxicol Teratol. 2009 doi: 10.1016/j.ntt.2008.11.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Seidler FJ, Saleh JL, Zhang J, Padilla S, Slotkin TA. Cellular mechanisms for developmental toxicity of chlorpyrifos: targeting the adenylyl cyclase signaling cascade. Toxicol Appl Pharmacol. 1997;145:158–174. doi: 10.1006/taap.1997.8171. [DOI] [PubMed] [Google Scholar]
- Song X, Violin JD, Seidler FJ, Slotkin TA. Modeling the developmental neurotoxicity of chlorpyrifos in vitro: macromolecule synthesis in PC12 cells. Toxicol Appl Pharmacol. 1998;151:182–191. doi: 10.1006/taap.1998.8424. [DOI] [PubMed] [Google Scholar]
- Stachowiak EK, Fang X, Myers J, Dunham S, Stachowiak MK. cAMP-Induced differentiation of human neuronal progenitor cells is mediated by nuclear fibroblast growth factor receptor-1 (FGFR1) J Neurochem. 2003;84:1296–1312. doi: 10.1046/j.1471-4159.2003.01624.x. [DOI] [PubMed] [Google Scholar]
- Teng KK, Greene LA. Cultured PC12 cells: a model for neuronal function and differentiation. In: Celis JE, editor. Cell Biology: A Laboratory Handbook. Academic Press; San Diego: 1994. pp. 218–224. [Google Scholar]
- Timofeeva OA, Roegge CS, Seidler FJ, Slotkin TA, Levin ED. Persistent cognitive alterations in rats after early postnatal exposure to low doses of the organophosphate pesticide, diazinon. Neurotoxicol Teratol. 2008;30:38–45. doi: 10.1016/j.ntt.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuler SM, Hazen AA, Bowen JM. Release and metabolism of dopamine in a clonal line of pheochromocytoma (PC12) cells exposed to fenthion. Fund Appl Toxicol. 1989;13:484–492. doi: 10.1016/0272-0590(89)90284-4. [DOI] [PubMed] [Google Scholar]
- Uzoukwu M, Sleight SD. Dieldrin toxicosis: fetotoxicosis, tissue concentrations, and microscopic and ultrastructural changes in guinea pigs. Am J Vet Res. 1972;33:579–583. [PubMed] [Google Scholar]
- Ward TR, Mundy WR. Organophosphorus compounds preferentially affect second messenger systems coupled to M2/M4 receptors in rat frontal cortex. Brain Res Bull. 1996;39:49–55. doi: 10.1016/0361-9230(95)02044-6. [DOI] [PubMed] [Google Scholar]
- Whitney KD, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos: cellular mechanisms. Toxicol Appl Pharmacol. 1995;134:53–62. doi: 10.1006/taap.1995.1168. [DOI] [PubMed] [Google Scholar]
- Williams NG, Zhong HY, Minneman KP. Differential coupling of α1-, α2-, and β-adrenergic receptors to mitogen-activated protein kinase pathways and differentiation in transfected PC12 cells. J Biol Chem. 1998;273:24624–24632. doi: 10.1074/jbc.273.38.24624. [DOI] [PubMed] [Google Scholar]
- Yanai J, Vatury O, Slotkin TA. Cell signaling as a target and underlying mechanism for neurobehavioral teratogenesis. Ann NY Acad Sci. 2002;965:473–478. doi: 10.1111/j.1749-6632.2002.tb04188.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.