Summary
The CCA sequence is conserved at the 3' end of all mature tRNA molecules to function as the site of amino acid attachment. This sequence is acquired and maintained by stepwise nucleotide addition by the ubiquitous CCA enzyme, which is an unusual RNA polymerase that does not use a nucleic acid template for nucleotide addition. Crystal structural work has divided CCA enzymes into two structurally distinct classes, which differ in the mechanism of template-independent nucleotide selection. Recent kinetic work of the class II E. coli CCA enzyme has demonstrated a rapid and uniform rate constant for the chemistry of nucleotide addition at each step of CCA synthesis, although the enzyme uses different determinants to control the rate of each step. Importantly, the kinetic work reveals that, at each step of CCA synthesis, E. coli CCA enzyme has an innate ability to discriminate against tRNA backbone damage. This discrimination suggests the possibility of a previously unrecognized quality control mechanism that would prevent damaged tRNA from CCA maturation and from entering the ribosome machinery of protein synthesis. This quality control is relevant to cellular stress conditions that damage tRNA backbone and predicts a role of CCA addition in stress response.
Keywords: Transfer RNA maturation, minihelix domain, anticodon-stem-loop domain, stress response
Introduction
The survivability of cells depends on tRNAs to perform the essential function of decoding genetic information into protein sequences. Each tRNA is charged with a specific amino acid that matches with the anticodon triplet, which in turn matches with a codon in genomic DNA or RNA. The acylation with an amino acid is to the terminal ribose of the CCA sequence, which is conserved at the 3' end in all three domains of life and occupies positions 74–76 in the tRNA sequence framework (Figure 1). In the tRNA cloverleaf secondary structure, the CCA sequence protrudes from the acceptor stem helical structure as a single-stranded motif. Upon folding of tRNA into an L shaped tertiary structure, the CCA sequence is placed at the end of the coaxially stacked acceptor stem and T stem. The acceptor-T helical arm is also known as the minihelix domain and is presumably an ancient part of the tRNA molecule [1]. The other long helical arm of the L is formed by coaxially stacking the dihydrouridine (D) stem with the anticodon stem to place the anticodon triplet at positions 34–36 opposite from the CCA end. The L is stabilized in the elbow region by a tertiary core, which is made up of nucleotides in the D loop, variable loop, and T loop. Upon entering the ribosome to a codon position complementary to the anticodon, a tRNA presents the amino acid attached to the CCA sequence for peptide bond synthesis, thus decoding the nucleic acid codon into a building block of proteins.
Figure 1.
Canonical tRNA structure. (a) Cloverleaf secondary structure of E. coli tRNACys. (b) The L shaped tertiary structure of E. coli tRNACys [72]. The minihelix domain encompassing the acceptor stem, T stem, and T loop is shown by brackets.
Possession of an intact CCA is a fundamental requirement for tRNAs to participate in the decoding process. It is through this sequence that a tRNA can be aminoacylated and brought to the ribosome. Inside the ribosome, the CCA sequence is in constant demand, making dynamic interactions with a changing ensemble of ribosomal proteins and RNA elements as the tRNA moves through the ribosome [2]. In each of these interactions, the precise CCA sequence is required for specific recognition by its binding partners. Many of the reaction steps for peptide bond formation are energy-dependent. For example, the aminoacylation reaction consumes an ATP, the entry of an aminoacyl-tRNA to the ribosome consumes a GTP, and the translocation of a tRNA through the ribosome after peptide bond formation consumes another GTP. Thus, the possession of an intact CCA sequence can be thought of as the quality tag for a tRNA's entry to the energy-consuming translational process. The importance of the CCA sequence to the energy charge of the cell also suggests a role of the sequence in the well being of mitochondria, the main provider of cellular energy. Indeed, defective CCA addition to human mitochondrial tRNAs is linked to cardiovascular diseases and early infant death syndromes [3–5]. Thus, studies of CCA addition to tRNAs may also be of relevance to some human health issues.
The CCA enzymes, ubiquitous in all living organisms, synthesize and maintain the CCA sequence by stepwise nucleotide addition. Although the CCA enzymes were discovered nearly 50 years ago [6], they have been studied only with respect to their molecular function in tRNA maturation, without paying much attention to their biological context. Importantly, recent kinetic analysis of E. coli CCA enzyme has uncovered an ability to discriminate against backbone-damaged tRNA [7], such that while intact tRNAs are rapidly equipped with the CCA tag, damaged tRNAs are delayed for addition of the tag. This finding suggests the possibility of a previously unrecognized quality control by the CCA enzyme to reject damaged tRNAs from maturation and from protein synthesis. The concept of quality control is likely applicable to a broad range of bacterial and eukaryotic CCA enzymes based on their shared sequence and structural homology [8, 9] and should be highly relevant under cellular stress conditions that damage tRNA. This review puts emphasis on kinetic analysis of CCA addition to tRNA, from which the concept of tRNA quality control by CCA addition was derived.
CCA addition in tRNA maturation
The addition of CCA is an important step in the tRNA maturation process, which varies among different organisms (reviewed in [10, 11]). In E. coli and related bacteria, the CCA sequence is encoded in all tRNA genes and is produced during transcription of precursor tRNA transcripts. Processing of precursor transcripts begins with an endonucleolytic cleavage on the 3' side by the enzyme RNase E [11]. Precursor tRNAs with short 3' trailers are then processed at the 5' end by the endonuclease RNase P, followed by trimming of the 3' trailers by exonucleases RNase PH and RNase T, whereas those with long trailers are first processed by exonucleases PNPase or RNase II to shorter sequences, followed by processing at the 5' end by RNase P and removal of the remaining 3' trailer by RNase PH and RNase T [10]. The appearance of the CCA sequence in a short 3' trailer is an important determinant for processing at the 5' end by RNase P [12]. In the Gram-positive Bacillus subtilis and related bacteria, some tRNA genes encode the CCA sequence. For those that doe not encode the CCA sequence, processing of RNase E-cleaved precursor transcripts can begin with RNase P in the presence of a long 5' leader, followed by processing on the 3' end by the endonuclease RNase Z [13] to cleave off the 3' trailer sequences after position 73 of the discriminator nucleotide [14]. The CCA sequence is then added onto position 73 by the CCA enzyme. In other Gram-positive bacteria, eukaryotes (in both cytoplasm and mitochondria), and archaea, the CCA sequence is not encoded in tRNA genes and must be added post-transcriptionally in a mechanism similar to that of CCA-minus transcripts in B. subtilis.
For organisms that do not encode the CCA sequence in tRNA genes, the CCA enzyme assumes the essential function of synthesizing the CCA sequence. In the case of the eukaryotic Saccharomyces cerevisiae, the CCA enzyme is essential for growth [15]. For organisms that do encode the CCA sequence in tRNA genes, the CCA enzyme performs the repair function to regenerate the CCA sequence from damaged 3' ends arising from non-specific nuclease digestion or from end-turnover of tRNA, usually by the action of RNase T [16]. Although the repair function is not essential for cell viability, due to the existence of de novo synthesis of CCA-plus tRNA transcripts, it is nonetheless required for normal cell growth [16], suggesting that accumulation of damaged or incomplete 3' ends of tRNA is deleterious to cells. Depending on the nature of the tRNA 3' ends, the CCA enzyme catalyzes stepwise nucleotide addition of C74, C75, and A76, using CTP and ATP as the respective nucleotide donors. However, while acting as an RNA polymerase, all known CCA enzymes are distinguished in that they do not need a nucleic acid template to direct nucleotide addition [6]. This template-independent but precise CCA synthesis is exceptional when compared to the lack of specificity of the other template-independent nucleic acid polymerases, polyA polymerase and terminal deoxylnucleotidyl transferase, which synthesize homopolymers of variable lengths.
Structural studies of CCA enzymes
Intense efforts have been dedicated to solve crystal structures of CCA enzymes in order to understand the structural basis for their template-independent specificity. An early sequence alignment has categorized archaeal CCA enzymes as members of the class I nucleotidyl transferase family, whereas bacterial and eukaryotic enzymes are members of the class II [17]. At present, crystal structures of the class I Archaeoglobus fulgidus CCA enzyme in binary complexes with CTP or ATP [18, 19] or with a full-length tRNA [20], and in ternary complexes with bound nucleotides together with minihelices of different maturation stages of the 3' end [21, 22] are available. Crystal structures of class II enzymes include the apo form of the human enzyme [8], binary complexes of the Bacillus stearothermophilus enzyme with CTP or ATP [9], and ternary complexes of the Thermotoga maritime enzyme with bound nucleotides and their respective minihelices [23]. Notably, some deep-rooted and slow-evolving bacteria (Aquifex aeolicus, Deinococcus radiodurans, Synechocystis sp.) split their class II CCA enzyme into a C-adding enzyme for addition of C74 and C75, and an A-adding enzyme for addition of A76 [24–27]. The structure of the ternary complex of the A-adding enzyme of A. aeolicus bound with an ATP analog and a tRNA substrate is available [28]. These structures show that although the two classes of enzymes both consist of the head, neck, body, and tail domains, they are fundamentally different in the arrangement of secondary structures of individual domains and share little amino acid sequence homology between them (Figure 2). In addition, while class I enzymes dimerize through tail-to-tail interaction of the monomers, class II enzymes dimerize by head-to-head interaction. The only recognizable structural homology between the two classes is in the head domain, which houses the active site for catalysis of nucleotide addition.
Figure 2.
Crystal structures of two classes of CCA enzymes. (a) The structure of the class I Archaeoglobus fulgidus enzyme (Protein Data Bank (PDB) code 1R8A). (b) The structure of the class II Bacillus stearothermophilus enzyme (PDB code 1M1W). Both enzymes consist of the head, neck, body, and tail domains, with different orientation of α helices and β sheets.
The two classes differ in the mechanism of nucleotide selection. Class I enzymes utilize an enzyme-RNA co-templating mechanism [18, 20], in which the active site collaborates with the RNA primers and uses the dynamics of the enzyme-RNA conformational changes to determine the nucleotide specificity. For example, upon CTP binding to the class I A. fulgidus enzyme for addition of C74 and C75, the head domain moves toward the neck domain, while shifting the catalytic cleft from an open to a close conformation, where reactive groups are juxtaposed and aligned for catalysis. The open-to-close transition is induced only by the correct CTP substrate. After C75 addition, the active site stays in the closed conformation and proceeds with ATP binding for addition of A76 [22]. By contrast, class II enzymes make use of a protein-templating mechanism [9], in which the catalytic head domain and the NTP-binding neck domain play a major role in determining the number and specificity of nucleotide addition [23]. Although the details of the active site movements for each nucleotide addition are not yet clearly defined for class II enzymes, an induced-fit rearrangement of the active site by binding of the correct NTP binding is envisioned to establish the nucleotide specificity.
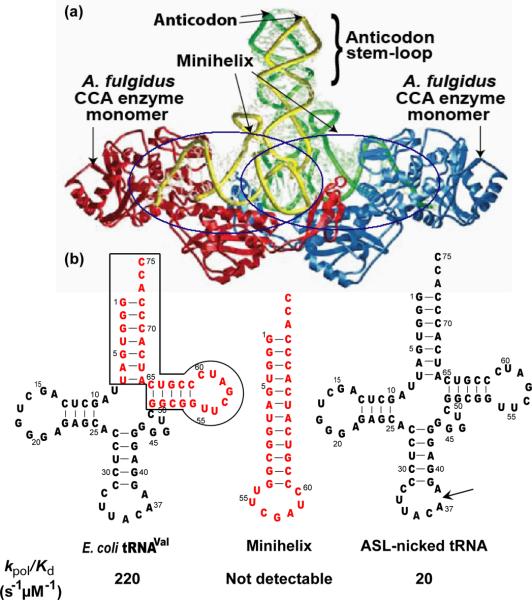
An interesting feature of the tRNA-bound crystal structures of both classes of CCA enzymes [20, 28] is that these enzymes form contacts exclusively with the minihelix domain of tRNA (Figure 3a). Specifically, the head and neck domains are expanded to accommodate the acceptor-T stem loop of the tRNA substrate and to help to insert the 3' end directly into the active site of the head domain. The body domain stabilizes the acceptor end of the tRNA, while the tail domain anchors the T loop in the tertiary core of the L shape to prevent the 3' end from being dislodged. In the class I structure of the A. fulgidus enzyme, no enzyme contact is observed with the anticodon stem-loop (ASL), which is completely projected away from the active site [20] (Figure 3a). The stereochemistry of the enzyme wrapping around the minihelix domain is fixed whether the enzyme is co-crystallized with a full-length tRNA or with minihelices [20]. This provides the basis to propose the minihelix recognition model, which is consistent with footprint analysis [29] and puts forward the notion that the determinants for CCA addition reside only in the enzyme's interaction with the minihelix domain. Although the ASL region is not visible in the tRNA-bound class II crystal structure of the A. aeolicus A-adding enzyme [28], structural modeling supports the minihelix model for class II enzymes as well [20, 23].
Figure 3.
The minihelix recognition model. (a) Crystal structure of the class I Archaeoglobus fulgidus CCA enzyme (PDB code 1SZ1), in which one enzyme monomer (blue) binds to the minihelix domain of one tRNA (green), while the other monomer (red) binds the minihelix domain of the other tRNA (yellow). Both monomers project the ASL domain of the tRNA anticodon stem-loop away from the enzyme. (b). Kinetic evaluation of the minihelix recognition model, using the sequence framework of E. coli tRNAVal as the substrate. While E. coli CCA enzyme exhibits a high value of kpol/Kd of 200 μM−1s−1 for addition of A76 to the full-length tRNA-C75 substrate, it discriminates against the minihelix domain with no measurable value of kpol/Kd, and it exhibits only a kpol/Kd value of 20 μM−1s−1 with a damaged tRNA harboring a backbone break in the ASL between positions 37 and 38 (indicated by an arrow). Data are from reference [7].
Kinetic analysis of CCA addition
Early steady-state kinetic analysis showed that minihelices were efficient substrates for both classes of CCA enzymes [30–32], which provided initial support for the minihelix recognition model. However, a recent study revealed that steady-state kinetics as measured in multiple enzyme turnovers is dominated by the release of the nucleic acid product from one turnover to the next, and thus it does not report on the chemical step of nucleotide addition [33]. The development of new assays to measure single-turnover kinetics of CCA addition was recently accomplished [7], focusing on the class II E. coli CCA enzyme as a model, which has been previously characterized by steady-state analysis [31, 32]. Single turnover assays examine the enzyme in excess of the tRNA substrate such that in average each enzyme can perform no more than one turnover. Single turnover kinetics provides information on the rate and equilibrium constants for one nucleotide addition. These kinetic parameters are appropriate for appraisal of crystal structures, which usually report the active site either before or during nucleotide addition. Equally important, because both the enzyme and tRNA are tested in single turnover kinetics in μM concentrations similar to those in vivo, such assays provide insight into the enzyme's cellular activity.
The single turnover assays have revealed that E. coli CCA enzyme rapidly catalyzes nucleotide addition to full-length tRNA with a maximum rate constant of ~170 s−1, which is the same for all three steps of CCA addition [7]. The maximum rate constant is obtained from a series of measurements of single turnover rate as a function of enzyme concentration and fitting the data to a hyperbolic function. The ability to reach a maximum rate constant as the enzyme concentration increases means that the rate of nucleotide addition is not limited by the enzyme binding to substrates, but rather by the events associated with nucleotidyl transfer. The maximum rate constant is thus is defined as kpot, which is the rate constant of polymerization of one nucleotide onto the nucleic acid substrate and is comparable to the kpol of the template-dependent T7 RNA polymerase [34], consistent with the notion that the active site of all nucleic acid polymerases is structurally and catalytically homologous [35]. Because kpol is measured in rapid binding equilibria, the concentration of the enzyme that yields half of kpol defines the apparent equilibrium binding constant Kd of the enzyme for the tRNA substrate. If the formation of enzyme-substrate complexes immediately proceeds to the chemistry of nucleotidyl transfer, this apparent Kd would be the true equilibrium binding constant. However, crystal structural analysis of both classes of CCA enzymes has identified conformational transition of enzyme-substrate complexes before each nucleotidyl transfer as an important step to ensure fidelity [21–23]. In all three steps of CCA addition, the apparent Kd values for the tRNA substrate are in the range of 1–3 μM, which is close to the physiological concentration of tRNA available to the CCA enzyme in vivo [17]
As with all nucleic acid polymerases, the kpol term of CCA addition encompasses the event of nucleotide binding-induced rearrangement of the active site and the event of bond-breaking and bond-forming steps of nucleotidyl transfer. However, unlike template-dependent polymerases, which control kpol by relying on constant determinants throughout the course of polymerization, E. coli CCA enzyme employ different determinants for the C-adding reactions relative to the A-adding reaction. Specifically, the enzyme controls kpol of C74 and C75 addition at the level of the chemical step of nucleotidyl transfer, whereas the conformational transition of the active site determines kpol in the case of A76 addition [33]. The diversity of the rate-determining strategy for individual steps of CCA addition may be a fundamental feature of template-independent nucleic acid polymerization.
The rate kpol is significantly faster than the previous estimates of kcat, which confirms that steady-state analysis does not report on the events associated with nucleotide addition by the CCA enzyme. The nature of kcat is elucidated by a pre-steady-state analysis [33], in which the kinetics of multiple turnovers of nucleotide addition is monitored with high concentrations of the enzyme and tRNA (both in μM) under rapid equilibrium binding condition. For all three steps of CCA addition, the kinetics exhibits a rapid burst of nucleotide addition in the first turnover followed by a slower and linear rate in steady state. The appearance of burst kinetics indicates that the release of the nucleic acid product after each addition is slow, and that the rate of each release as determined from the steady-state phase is <3 s−1, 9.5 s−1, and 15.5 s−1 after addition of C74, C75, and A76, respectively [33]. The significantly higher kpol than each of the kcat values implies processivity of the enzyme, which means that although the enzyme has the option to release the product after each addition, it prefers the option of staying bound with the product and proceeding with the next addition. This processivity can be demonstrated if the docking of each product by the enzyme for the next round of addition is fast compared to the rate of the product release. Future experiments are necessary to provide a quantitative evaluation of the processivity. Notably, while the enzyme progressively accelerates product release as it approaches the completion of CCA synthesis, the final product release remains slow relative to kpol, suggesting that additional protein factors may assist in the release. A steady-state study suggests that the Hfq protein, initially identified as the host factor required for replication of phage Qβ RNA [36] and recently shown to have the ability to regulate the function of non-coding RNAs [37, 38], may play a role in product release from the E. coli CCA enzyme [39].
Quality control of tRNA by CCA addition
An important finding of the single turnover kinetics is that while the E. coli CCA enzyme efficiently recognizes the full-length tRNA for nucleotide addition, it does not recognize minihelices efficiently [7] (Figure 3b). For each step of CCA addition, while the rate for a single round of nucleotide addition to minihelices increases with enzyme concentration, the increase is linear and the kinetics do not reach saturation, even with exceedingly high concentrations of the enzyme. This indicates that the rate is limited by the inability of the enzyme to form rapid equilibrium binding with minihelices. As such, the kpol value of nucleotide addition and the Kd value of the enzyme for the minihelix substrate in each step of nucleotide addition cannot be determined. The inaccessibility of these kinetic constants indicates that minihelices are kinetically discriminated by the CCA enzyme from nucleotide addition under rapid equilibrium binding conditions. This is supported by the slow association rate of the enzyme with minihelices (~104 M−1s−1) relative to the diffusion-controlled association (107 M−1s−1) [7]. Thus, although minihelices provide the primary contact with CCA enzymes in crystal structures, the minihelix recognition model is insufficient to account for the kinetics of nucleotide addition under single turnover conditions.
A second important finding is that, even in the context of a full-length tRNA, the E. coli CCA enzyme has an innate ability to discriminate against backbone damage. For example, a tRNA harboring a backbone nick in the T loop is unable to function as a substrate for the kinetics of nucleotide addition to reach saturation, similar to the kinetics of minihelices [7]. Because the nick in the T loop is localized in the minihelix domain, this example demonstrates the ability of the enzyme to scrutinize the integrity of the domain for rapid equilibrium binding. An unexpected finding, however, is that the enzyme can also detect damage far away from the minihelix domain, such as in the anticodon loop [7]. For example, a tRNA harboring a backbone nick in the anticodon loop exhibits defective kinetics. While this nicked tRNA is able to function as a substrate for saturation kinetics of nucleotide addition, and while it can reach a kpol similar to that of the intact tRNA, the nicked tRNA has a ~10 fold-reduced affinity for the enzyme [7] (Figure 3b). This indicates a kinetic defect of the nicked tRNA at the binding step. Based on the kinetic specificity factor of kpol/Kd (where kpol is the rate of nucleotide addition in one round of nucleotide addition, and Kd is the equilibrium binding constant of the tRNA substrate for the enzyme), the damage in the anticodon-stem-loop decreases the specificity factor by 6-fold for C74 and C75 addition and by 10-fold for A76 addition. It appears that the enzyme scrutinizes the integrity of the tRNA substrate before each nucleotide addition, with the strongest scrutiny at the A76 addition step. This suggests that the enzyme uses its contact with the minihelix domain to mediate a long-range communication with the rest of the tRNA molecule. Alternatively, the backbone break in the anticodon loop leads to tRNA mis-folding either before or during enzyme interaction with the minihelix domain, leading to signaling of the damage to the minihelix domain and in turn to the enzyme active site. It will be of interest to determine if less drastic damage (such as mis-folding of the tRNA L shape) than the damage of a backbone break might also activate the enzyme signaling mechanism from a distal site to the minihelix domain to exert the quality control.
A different kind of quality control has been recently proposed for both the E. coli and human CCA enzymes [40]. It was shown that, even if these enzymes harbor a specific mutation in the active site that abolishes the selectivity of the correct nucleotide for each step of addition, both enzymes have a back-up mechanism to synthesize CCA to high levels. This back-up mechanism is detectable when these enzymes are presented with all four natural NTPs but is suppressed when only one of the four nucletoides is present. The requirement of all four natural NTPs for the manifestation of the back-up mechanism suggests that it would serve as an emergency means to ensure cell survival. The molecular basis for the back-up mechanism and how it arises is unknown. Insight into this mechanism must await further single turnover kinetic analysis of both native and mutant enzymes.
Implication for tRNA quality control in vivo
The kinetic results of single turnover analysis raise the hypothesis that the E. coli CCA enzyme, and by inference the closely related human CCA enzyme [8], would inspect the tRNA backbone and perform nucleotide addition only to backbone-intact species. Figure 4 outlines the concept of this quality control, in which backbone-intact tRNAs would be rapidly tagged with CCA to become eligible for entry to the ribosome, where they would be protected by components of the ribosome machinery, such as aminoacyl-tRNA synthetases, elongation factors and ribosomal proteins and RNA. In contrast, backbone-damaged tRNAs would be delayed for CCA addition and, without the protection of binding partners, would be rapidly degraded by RNA surveillance mechanisms that exist in all cells. According to this hypothesis, although the CCA enzyme does not actually remove the damage as an active quality control, it distinguishes between the normal and damaged tRNA, which is the first and most important step in successful quality control.
Figure 4.
The two phases in the lifetime of a tRNA in E. coli. (a) In the maturation phase, the precursor tRNA is processed at the 3' end by 3' to 5' exonucleases, and by endonucleolytic cleavage at the 5' end. The matured tRNA is challenged by RNase T in E. coli for 3' end turnover and relies on the CCA enzyme for restoration of A76. It is during this restoration stage that the CCA quality control inspects the tRNA integrity and discriminates against stress-induced damage. The CCA sequence is rapidly repaired on intact tRNA and is aminoacylated by an aminoacyl-tRNA synthetase (aaRS), whereas the damaged tRNA is delayed for CCA addition and is degraded by 3' to 5' RNases as part of RNA surveillance machineries. (b) In the protein synthesis phase, the tRNA moiety of the intact aminoacyl-tRNA is shown in orange while the aminoacyl moiety is shown as a red dot. This intact aminoacyl-tRNA is escorted by elongation factor EF-Tu in complex with GTP to the ribosome A site, where it is adjacent to a peptidyl tRNA on the P site (in yellow) and a discharged tRNA on the E site (in green). After GTP hydrolysis and release of EF-Tu, the polypeptide chain of the P site tRNA is transferred to the aminoacyl moiety of the A-site tRNA. With the assistance of GTP hydrolysis, the elongation factor EF-G promotes translocation of the ribosome-tRNA-mRNA complex, moving the A site and P site tRNAs to the P site and E site, respectively, while releasing the E site tRNA from the ribosome. The ribosome with the vacant A site then awaits the entry of the next intact aminoacyl-tRNA.
Damage to tRNA can occur both before and after maturation. Recognition of tRNA damage before maturation is by processing enzymes [41]. After maturation, tRNA is challenged by 3' end turnover that removes the terminal A76, which is the action of RNase T in E. coli. For example, an estimated 15% of E. coli tRNA is missing A76 due to the 3' end turnover when the CCA enzyme is inactivated [16]. This fraction is not insignificant, because alteration of tRNA levels by 10–20% can have a profound effect on the rate of protein synthesis so as to arrest cell growth [42] or to drive cell proliferation and oncogenic transformation [43]. Thus, it is during A76 addition to the tRNA molecules damaged by the 3' end turnover that the CCA quality control would have the most profound impact. Notably, in organisms such as humans that require the CCA enzyme to synthesize the CCA sequence onto tRNA, the CCA quality control would be relevant already during tRNA maturation. Damage to tRNA can arise from mis-incorporation of nucleotides, incorrect processing and folding, and incomplete post-transcriptional modifications [44], leading to aberrant structures prone to nuclease cleavage. Also, some bacterial and eukaryotic tRNAs carry introns in the anticodon loop that need to be removed. Splicing intermediates without properly re-ligated backbones have been found in cells [45]. Most importantly, damage to the anticodon loop frequently occurs under cellular stress conditions. For example, during phage T4 infection, E. coli activates a defense mechanism by using nuclease PrrC to cleave a specific anticodon [46]. Under SOS or nutrient-depletion stress, many natural E. coli strains secrete anticodon-specific nucleases as toxins (colicins D and E5) to attack competing bacteria [47, 48]. Similar toxins are secreted by yeast [49]. Under developmental stress associated with cell differentiation, Gram-positive bacteria (Streptomyces coelicolor [50]), lower eukaryotes (Aspergillus fumigatus [51], Giardia lamblia [52]), and the fruit fly Drosophila [53] respond by inducing cleavage of the tRNA anticodon loop. Under oxidative stress, eukaryotic cells respond by cleaving the anticodon loop region [54, 55]. Half molecules of tRNAs are also found in plants during stress related to perturbation of nutrient transport [56]. Obviously, cleavage near the anticodon generates non-productive tRNA, the discrimination of which by the CCA enzyme would be an efficient strategy and logical checkpoint to protect cells from the costly disturbance of the protein synthesis machinery.
Cleavage of the anticodon loop, except in the case of anticodon toxins, is catalyzed by the stress-activated endonuclease Rny1p in yeast [57] and angiogenin in humans [55]. Both enzymes are normally sequestered within the cell membrane but are released into the cytosol during stress to serve as markers for cell damage. The homolog of Rny1p in E. coli is the endonuclease RNase I, a periplasmic enzyme that is released into the cytosol during stress induced by chemicals or antibiotics [58]. The parallel between Rny1p and RNase I in response to stress suggests a role of RNase I in stressed-induced anticodon cleavage in bacteria.
Removal of damaged tRNA is the responsibility of cellular RNA surveillance mechanisms. Nicked tRNAs are damaging to the ribosome because they induce incorrect assembly of the small and large ribosomal subunits and prevent proper communication between the two subunits during the ribosome accommodation of a new aminoacyl-tRNA to the decoding site. Nicked tRNAs have been found to cause cell death in E. coli [58, 59], while half tRNA fragments have been found to affect degradation or repression of specific mRNA, leading to inhibition of translation [60]. Active removal of damage constitutes the second step of quality control. However, the identities of the RNases that follow the CCA discrimination are unknown. Notably, E. coli has seven exo RNases that degrade damaged RNA in the 3' to 5' direction [11], many of which have overlapping activities in multiple surveillance pathways. For example, polynucleotide phosphorylase (PNPase) is one of the seven enzymes, which acts with RNase R on tRNA decay and separately with RNase II on mRNA decay [61, 62]. RNase T is another example, which is involved in 3' maturation of both tRNA and rRNA [63]. Additionally, cellular stresses can change the levels and relationships among RNases. For example, in response to cold shock, RNase R and PNPase are increased in quantities [64, 65]. Further, accessory proteins may facilitate the action of RNases to remove damaged tRNA after the CCA discrimination. For example, many RNases require a 3' overhang to serve as a binding site from which degradation is initiated [66–68]. This overhang is typically synthesized by polyA polymerase. The Hfq protein has also been shown to stimulate the activity of both E. coli CCA enzyme and polyA polymerase [39, 69, 70], suggesting a role in tRNA metabolism. Thus, the cellular networks for surveillance of damaged tRNA appear to be complex.
Perspective and future studies
Elucidation of the role of the CCA enzyme in the cellular network of tRNA quality control and the identities of the RNases accompanying the CCA enzyme constitute new questions that warrant active investigation. It is important to note that, while the quality control by CCA addition was discovered from kinetic analysis under conditions similar to those in vivo, this does not imply a straightforward extrapolation of the concept to cells. First, the complexity of the cellular environments makes it difficult to predict what kind of damage would most likely elicit the CCA quality control (mis-folding of tRNA or backbone nicks), and whether the CCA quality control would dominate over other tRNA quality control mechanisms in vivo. Second, the effective cellular concentrations of tRNA, CCA enzyme, and nucleotide substrates for CCA addition are difficult to establish with precision, and there is little information at present on the kinetics and rate-limiting steps of any CCA enzyme in vivo. These uncertainties call for experimental testing and evaluation of the CCA quality control concept in vivo. Should this quality control prove significant in vivo, then it would suggest a mechanism to prevent damaged tRNAs from entering the ribosome. By preventing cell death due to accumulation of damaged tRNAs [58, 59] and by giving cells an opportunity to mount defense strategies in response to stress, this quality control can potentially impact therapies against diseases. Notably, while both bacteria and human hosts respond to stress by inflicting tRNA damage, the outcome of the stress response appears to favor bacterial virulence. For example, while stresses induce bacteria to express virulence genes, the human hosts harboring these bacteria usually respond by releasing catecholamine hormones (e.g. nor-epinephrine) that further stimulate bacterial growth and virulence [71], thus giving bacteria the upper hand. Also, stress-induced tRNA cleavage has been strongly implicated to associate with cancers and other diseases [60]. An investigation of the CCA quality control may lead to new ways to target this mechanism in pathogenic bacterial or in cancer cells with potential clinical significance.
Acknowledgements
The author thanks Lena Ma, Cuiping Liu, and Georges Lahoud for making figures and thanks NIH GM068561 for supporting the work.
Abbreviations
- tRNA
transfer RNA
- ASL
anticodon-stem-loop domain of tRNA
- D nucleotides
dihydrouridine nucleotides
- T-loop
the TΨC loop of tRNA
- aaRS
aminoacyl-tRNA synthetase
References
- 1.Tamura K, Schimmel P. Oligonucleotide-directed peptide synthesis in a ribosome- and ribozyme-free system. Proc Natl Acad Sci U S A. 2001;98:1393–1397. doi: 10.1073/pnas.98.4.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korostelev A, Trakhanov S, Laurberg M, Noller HF. Crystal structure of a 70S ribosome-tRNA complex reveals functional interactions and rearrangements. Cell. 2006;126:1065–1077. doi: 10.1016/j.cell.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 3.Tomari Y, Hino N, Nagaike T, Suzuki T, Ueda T. Decreased CCA-addition in human mitochondrial tRNAs bearing a pathogenic A4317G or A10044G mutation. J Biol Chem. 2003;278:16828–16833. doi: 10.1074/jbc.M213216200. [DOI] [PubMed] [Google Scholar]
- 4.Levinger L, Oestreich I, Florentz C, Morl M. A pathogenesis-associated mutation in human mitochondrial tRNALeu(UUR) leads to reduced 3'-end processing and CCA addition. J Mol Biol. 2004;337:535–544. doi: 10.1016/j.jmb.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Levinger L, Morl M, Florentz C. Mitochondrial tRNA 3' end metabolism and human disease. Nucleic Acids Res. 2004;32:5430–5441. doi: 10.1093/nar/gkh884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deutscher MP. tRNA nucleotidyltransferase. Enzymes. 1982;15:183–215. [Google Scholar]
- 7.Dupasquier M, Kim S, Halkidis K, Gamper H, Hou YM. tRNA integrity is a prerequisite for rapid CCA addition: implication for quality control. J Mol Biol. 2008;379:579–588. doi: 10.1016/j.jmb.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Augustin MA, Reichert AS, Betat H, Huber R, Morl M, Steegborn C. Crystal Structure of the Human CCA-adding Enzyme: Insights into Template-independent Polymerization. J Mol Biol. 2003;328:985–994. doi: 10.1016/s0022-2836(03)00381-4. [DOI] [PubMed] [Google Scholar]
- 9.Li F, Xiong Y, Wang J, Cho HD, Tomita K, Weiner AM, Steitz TA. Crystal structures of the Bacillus stearothermophilus CCA-adding enzyme and its complexes with ATP or CTP. Cell. 2002;111:815–824. doi: 10.1016/s0092-8674(02)01115-7. [DOI] [PubMed] [Google Scholar]
- 10.Hartmann RK, Gossringer M, Spath B, Fischer S, Marchfelder A. The making of tRNAs and more - RNase P and tRNase Z. Progress in molecular biology and translational science. 2009;85:319–368. doi: 10.1016/S0079-6603(08)00808-8. [DOI] [PubMed] [Google Scholar]
- 11.Deutscher MP. Degradation of RNA in bacteria: comparison of mRNA and stable RNA. Nucleic Acids Res. 2006;34:659–666. doi: 10.1093/nar/gkj472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wegscheid B, Hartmann RK. The precursor tRNA 3'-CCA interaction with Escherichia coli RNase P RNA is essential for catalysis by RNase P in vivo. RNA. 2006;12:2135–2148. doi: 10.1261/rna.188306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dutta T, Deutscher MP. Catalytic properties of RNase BN/RNase Z from Escherichia coli: RNase BN is both an exo- and endoribonuclease. J Biol Chem. 2009;284:15425–15431. doi: 10.1074/jbc.M109.005462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wegscheid B, Hartmann RK. In vivo and in vitro investigation of bacterial type B RNase P interaction with tRNA 3'-CCA. Nucleic Acids Res. 2007;35:2060–2073. doi: 10.1093/nar/gkm005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aebi M, Kirchner G, Chen JY, Vijayraghavan U, Jacobson A, Martin NC, Abelson J. Isolation of a temperature-sensitive mutant with an altered tRNA nucleotidyltransferase and cloning of the gene encoding tRNA nucleotidyltransferase in the yeast Saccharomyces cerevisiae. J Biol Chem. 1990;265:16216–16220. [PubMed] [Google Scholar]
- 16.Zhu L, Deutscher MP. tRNA nucleotidyltransferase is not essential for Escherichia coli viability. EMBO J. 1987;6:2473–2477. doi: 10.1002/j.1460-2075.1987.tb02528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yue D, Maizels N, Weiner AM. CCA-adding enzymes and poly(A) polymerases are all members of the same nucleotidyltransferase superfamily: characterization of the CCA-adding enzyme from the archaeal hyperthermophile Sulfolobus shibatae. RNA. 1996;2:895–908. [PMC free article] [PubMed] [Google Scholar]
- 18.Xiong Y, Li F, Wang J, Weiner AM, Steitz TA. Crystal structures of an archaeal class I CCA-adding enzyme and its nucleotide complexes. Mol Cell. 2003;12:1165–1172. doi: 10.1016/s1097-2765(03)00440-4. [DOI] [PubMed] [Google Scholar]
- 19.Okabe M, Tomita K, Ishitani R, Ishii R, Takeuchi N, Arisaka F, Nureki O, Yokoyama S. Divergent evolutions of trinucleotide polymerization revealed by an archaeal CCA-adding enzyme structure. EMBO J. 2003;22:5918–5927. doi: 10.1093/emboj/cdg563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiong Y, Steitz TA. Mechanism of transfer RNA maturation by CCA-adding enzyme without using an oligonucleotide template. Nature. 2004;430:640–645. doi: 10.1038/nature02711. [DOI] [PubMed] [Google Scholar]
- 21.Tomita K, Ishitani R, Fukai S, Nureki O. Complete crystallographic analysis of the dynamics of CCA sequence addition. Nature. 2006;443:956–960. doi: 10.1038/nature05204. [DOI] [PubMed] [Google Scholar]
- 22.Toh Y, Numata T, Watanabe K, Takeshita D, Nureki O, Tomita K. Molecular basis for maintenance of fidelity during the CCA-adding reaction by a CCA-adding enzyme. EMBO J. 2008;27:1944–1952. doi: 10.1038/emboj.2008.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toh Y, Takeshita D, Numata T, Fukai S, Nureki O, Tomita K. Mechanism for the definition of elongation and termination by the class II CCA-adding enzyme. EMBO J. 2009;28:3353–3365. doi: 10.1038/emboj.2009.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomita K, Weiner AM. Collaboration between CC- and A-adding enzymes to build and repair the 3'-terminal CCA of tRNA in Aquifex aeolicus. Science. 2001;294:1334–1336. doi: 10.1126/science.1063816. [DOI] [PubMed] [Google Scholar]
- 25.Tomita K, Weiner AM. Closely related CC- and A-adding enzymes collaborate to construct and repair the 3'-terminal CCA of tRNA in Synechocystis sp. and Deinococcus radiodurans. J Biol Chem. 2002;277:48192–48198. doi: 10.1074/jbc.M207527200. [DOI] [PubMed] [Google Scholar]
- 26.Bralley P, Chang SA, Jones GH. A phylogeny of bacterial RNA nucleotidyltransferases: Bacillus halodurans contains two tRNA nucleotidyltransferases. J Bacteriol. 2005;187:5927–5936. doi: 10.1128/JB.187.17.5927-5936.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neuenfeldt A, Just A, Betat H, Morl M. Evolution of tRNA nucleotidyltransferases: a small deletion generated CC-adding enzymes. Proc Natl Acad Sci U S A. 2008;105:7953–7958. doi: 10.1073/pnas.0801971105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomita K, Fukai S, Ishitani R, Ueda T, Takeuchi N, Vassylyev DG, Nureki O. Structural basis for template-independent RNA polymerization. Nature. 2004;430:700–704. doi: 10.1038/nature02712. [DOI] [PubMed] [Google Scholar]
- 29.Shi PY, Maizels N, Weiner AM. CCA addition by tRNA nucleotidyltransferase: polymerization without translocation? EMBO J. 1998;17:3197–3206. doi: 10.1093/emboj/17.11.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi PY, Weiner AM, Maizels N. A top-half tDNA minihelix is a good substrate for the eubacterial CCA- adding enzyme. RNA. 1998;4:276–284. [PMC free article] [PubMed] [Google Scholar]
- 31.Seth M, Thurlow DL, Hou YM. Poly(C) synthesis by class I and class II CCA-adding enzymes. Biochemistry. 2002;41:4521–4532. doi: 10.1021/bi0120953. [DOI] [PubMed] [Google Scholar]
- 32.Hou YM, Gu SQ, Zhou H, Ingerman L. Metal-Ion-Dependent Catalysis and Specificity of CCA-Adding Enzymes: A Comparison of Two Classes. Biochemistry. 2005;44:12849–12859. doi: 10.1021/bi0509402. [DOI] [PubMed] [Google Scholar]
- 33.Kim S, Liu C, Halkidis K, Gamper HB, Hou YM. Distinct kinetic determinants for the stepwise CCA addition to tRNA. RNA. 2009;15:1827–1836. doi: 10.1261/rna.1669109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anand VS, Patel SS. Transient state kinetics of transcription elongation by T7 RNA polymerase. J Biol Chem. 2006;281:35677–35685. doi: 10.1074/jbc.M608180200. [DOI] [PubMed] [Google Scholar]
- 35.Steitz TA. A mechanism for all polymerases. Nature. 1998;391:231–232. doi: 10.1038/34542. [DOI] [PubMed] [Google Scholar]
- 36.Franze de Fernandez MT, Hayward WS, August JT. Bacterial proteins required for replication of phage Q ribonucleic acid. Pruification and properties of host factor I, a ribonucleic acid-binding protein. J Biol Chem. 1972;247:824–831. [PubMed] [Google Scholar]
- 37.Gottesman S. The small RNA regulators of Escherichia coli: roles and mechanisms*. Annu Rev Microbiol. 2004;58:303–328. doi: 10.1146/annurev.micro.58.030603.123841. [DOI] [PubMed] [Google Scholar]
- 38.Storz G, Opdyke JA, Zhang A. Controlling mRNA stability and translation with small, noncoding RNAs. Curr Opin Microbiol. 2004;7:140–144. doi: 10.1016/j.mib.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 39.Scheibe M, Bonin S, Hajnsdorf E, Betat H, Morl M. Hfq stimulates the activity of the CCA-adding enzyme. BMC Mol Biol. 2007;8:92. doi: 10.1186/1471-2199-8-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lizano E, Scheibe M, Rammelt C, Betat H, Morl M. A comparative analysis of CCA-adding enzymes from human and E. coli: differences in CCA addition and tRNA 3'-end repair. Biochimie. 2008;90:762–772. doi: 10.1016/j.biochi.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 41.Li Z, Reimers S, Pandit S, Deutscher MP. RNA quality control: degradation of defective transfer RNA. EMBO J. 2002;21:1132–1138. doi: 10.1093/emboj/21.5.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cruz-Vera LR, Hernandez-Ramon E, Perez-Zamorano B, Guarneros G. The rate of peptidyl-tRNA dissociation from the ribosome during minigene expression depends on the nature of the last decoding interaction. J Biol Chem. 2003;278:26065–26070. doi: 10.1074/jbc.M301129200. [DOI] [PubMed] [Google Scholar]
- 43.Marshall L, Kenneth NS, White RJ. Elevated tRNA(iMet) synthesis can drive cell proliferation and oncogenic transformation. Cell. 2008;133:78–89. doi: 10.1016/j.cell.2008.02.035. [DOI] [PubMed] [Google Scholar]
- 44.Alexandrov A, Chernyakov I, Gu W, Hiley SL, Hughes TR, Grayhack EJ, Phizicky EM. Rapid tRNA decay can result from lack of nonessential modifications. Mol Cell. 2006;21:87–96. doi: 10.1016/j.molcel.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 45.Ho CK, Rauhut R, Vijayraghavan U, Abelson J. Accumulation of pre-tRNA splicing '2/3' intermediates in a Saccharomyces cerevisiae mutant. EMBO J. 1990;9:1245–1252. doi: 10.1002/j.1460-2075.1990.tb08232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levitz R, Chapman D, Amitsur M, Green R, Snyder L, Kaufmann G. The optional E. coli prr locus encodes a latent form of phage T4-induced anticodon nuclease. EMBO J. 1990;9:1383–1389. doi: 10.1002/j.1460-2075.1990.tb08253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ogawa T, Tomita K, Ueda T, Watanabe K, Uozumi T, Masaki H. A cytotoxic ribonuclease targeting specific transfer RNA anticodons. Science. 1999;283:2097–2100. doi: 10.1126/science.283.5410.2097. [DOI] [PubMed] [Google Scholar]
- 48.Tomita K, Ogawa T, Uozumi T, Watanabe K, Masaki H. A cytotoxic ribonuclease which specifically cleaves four isoaccepting arginine tRNAs at their anticodon loops. Proc Natl Acad Sci U S A. 2000;97:8278–8283. doi: 10.1073/pnas.140213797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu J, Huang B, Esberg A, Johansson MJ, Bystrom AS. The Kluyveromyces lactis gamma-toxin targets tRNA anticodons. RNA. 2005;11:1648–1654. doi: 10.1261/rna.2172105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haiser HJ, Karginov FV, Hannon GJ, Elliot MA. Developmentally regulated cleavage of tRNAs in the bacterium Streptomyces coelicolor. Nucleic Acids Res. 2008;36:732–741. doi: 10.1093/nar/gkm1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jochl C, Rederstorff M, Hertel J, Stadler PF, Hofacker IL, Schrettl M, Haas H, Huttenhofer A. Small ncRNA transcriptome analysis from Aspergillus fumigatus suggests a novel mechanism for regulation of protein synthesis. Nucleic Acids Res. 2008;36:2677–2689. doi: 10.1093/nar/gkn123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Y, Luo J, Zhou H, Liao JY, Ma LM, Chen YQ, Qu LH. Stress-induced tRNA-derived RNAs: a novel class of small RNAs in the primitive eukaryote Giardia lamblia. Nucleic Acids Res. 2008;36:6048–6055. doi: 10.1093/nar/gkn596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aravin AA, Lagos-Quintana M, Yalcin A, Zavolan M, Marks D, Snyder B, Gaasterland T, Meyer J, Tuschl T. The small RNA profile during Drosophila melanogaster development. Dev Cell. 2003;5:337–350. doi: 10.1016/s1534-5807(03)00228-4. [DOI] [PubMed] [Google Scholar]
- 54.Thompson DM, Lu C, Green PJ, Parker R. tRNA cleavage is a conserved response to oxidative stress in eukaryotes. RNA. 2008;14:2095–2103. doi: 10.1261/rna.1232808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamasaki S, Ivanov P, Hu GF, Anderson P. Angiogenin cleaves tRNA and promotes stress-induced translational repression. J Cell Biol. 2009;185:35–42. doi: 10.1083/jcb.200811106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang S, Sun L, Kragler F. The phloem-delivered RNA pool contains small noncoding RNAs and interferes with translation. Plant Physiol. 2009;150:378–387. doi: 10.1104/pp.108.134767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thompson DM, Parker R. The RNase Rny1p cleaves tRNAs and promotes cell death during oxidative stress in Saccharomyces cerevisiae. J Cell Biol. 2009;185:43–50. doi: 10.1083/jcb.200811119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Deutscher MP. Degradation of stable RNA in bacteria. J Biol Chem. 2003;278:45041–45044. doi: 10.1074/jbc.R300031200. [DOI] [PubMed] [Google Scholar]
- 59.Cheng ZF, Deutscher MP. Quality control of ribosomal RNA mediated by polynucleotide phosphorylase and RNase R. Proc Natl Acad Sci U S A. 2003;100:6388–6393. doi: 10.1073/pnas.1231041100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thompson DM, Parker R. Stressing out over tRNA cleavage. Cell. 2009;138:215–219. doi: 10.1016/j.cell.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 61.Mohanty BK, Kushner SR. Genomic analysis in Escherichia coli demonstrates differential roles for polynucleotide phosphorylase and RNase II in mRNA abundance and decay. Mol Microbiol. 2003;50:645–658. doi: 10.1046/j.1365-2958.2003.03724.x. [DOI] [PubMed] [Google Scholar]
- 62.Deutscher MP, Reuven NB. Enzymatic basis for hydrolytic versus phosphorolytic mRNA degradation in Escherichia coli and Bacillus subtilis. Proc Natl Acad Sci U S A. 1991;88:3277–3280. doi: 10.1073/pnas.88.8.3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li Z, Pandit S, Deutscher MP. 3' exoribonucleolytic trimming is a common feature of the maturation of small, stable RNAs in Escherichia coli. Proc Natl Acad Sci U S A. 1998;95:2856–2861. doi: 10.1073/pnas.95.6.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beran RK, Simons RW. Cold-temperature induction of Escherichia coli polynucleotide phosphorylase occurs by reversal of its autoregulation. Mol Microbiol. 2001;39:112–125. doi: 10.1046/j.1365-2958.2001.02216.x. [DOI] [PubMed] [Google Scholar]
- 65.Cairrao F, Cruz A, Mori H, Arraiano CM. Cold shock induction of RNase R and its role in the maturation of the quality control mediator SsrA/tmRNA. Mol Microbiol. 2003;50:1349–1360. doi: 10.1046/j.1365-2958.2003.03766.x. [DOI] [PubMed] [Google Scholar]
- 66.Cheng ZF, Deutscher MP. An important role for RNase R in mRNA decay. Mol Cell. 2005;17:313–318. doi: 10.1016/j.molcel.2004.11.048. [DOI] [PubMed] [Google Scholar]
- 67.Cheng ZF, Deutscher MP. Purification and characterization of the Escherichia coli exoribonuclease RNase R. Comparison with RNase II. J Biol Chem. 2002;277:21624–21629. doi: 10.1074/jbc.M202942200. [DOI] [PubMed] [Google Scholar]
- 68.Vincent HA, Deutscher MP. Substrate recognition and catalysis by the exoribonuclease RNase R. J Biol Chem. 2006;281:29769–29775. doi: 10.1074/jbc.M606744200. [DOI] [PubMed] [Google Scholar]
- 69.Hajnsdorf E, Regnier P. Host factor Hfq of Escherichia coli stimulates elongation of poly(A) tails by poly(A) polymerase I. Proc Natl Acad Sci U S A. 2000;97:1501–1505. doi: 10.1073/pnas.040549897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mohanty BK, Maples VF, Kushner SR. The Sm-like protein Hfq regulates polyadenylation dependent mRNA decay in Escherichia coli. Mol Microbiol. 2004;54:905–920. doi: 10.1111/j.1365-2958.2004.04337.x. [DOI] [PubMed] [Google Scholar]
- 71.Freestone PP, Sandrini SM, Haigh RD, Lyte M. Microbial endocrinology: how stress influences susceptibility to infection. Trends Microbiol. 2008;16:55–64. doi: 10.1016/j.tim.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 72.Hauenstein S, Zhang CM, Hou YM, Perona JJ. Shape-selective RNA recognition by cysteinyl-tRNA synthetase. Nat Struct Mol Biol. 2004;11:1134–1141. doi: 10.1038/nsmb849. [DOI] [PubMed] [Google Scholar]