Abstract
More than 40 years after the discovery of vasoactive intestinal peptide (VIP), its transcriptome in the immune system has still not been completely elucidated. In an attempt to understand the biological role of this neuropeptide in immunity, we chose CD4 T cells as a cellular system. Agilent Mouse Whole Genome microarrays were hybridized with fluorescently labeled total RNA isolated from resting CD4 T cells cultured −/+ 10−7 M VIP for five hours or PMA/ionomycin activated CD4 T cells cultured −/+ 10−7 M VIP for five hours . These VIP-regulated transcriptomes were analyzed by Significance Analysis of Microarrays (SAM) and Ingenuity Pathway Analysis (IPA) software to identify relevant signaling pathways modulated by VIP in the absence and presence of T cell activation. In resting CD4 T cells, VIP modulated 368 genes, ranging from 3.49 to − 4.78 fold. In the PMA/ionomycin activated CD4 T cells, 326 gene expression levels were changed by VIP, ranging from 2.94 to −1.66 fold. IPA analysis revealed that VIP exposure alters cellular function through EGFR signaling in resting CD4 T cells, and modulates immediate early genes, Fos and CREM/ICER, in activated CD4 T cells. These gene expression changes are suggested to explain at a molecular level how VIP can regulate T cell homing to the gut and induce regulatory T cell generation.
Keywords: Vasoactive Intestinal Peptide, Vasoactive Intestinal Peptide Receptor 1, Microarray, EGFR, Fos, T cell homing, regulatory T cells
1. Introduction
Vasoactive intestinal peptide (VIP) is a secreted, 28 amino acid protein that modulates numerous cell functions, such as migration and proliferation (Newman et al., 2005; Ottaway, 1987). Isolated in 1970 by Drs. Said and Mutt from porcine intestine (Said and Mutt, 1970), this peptide has a diverse tissue expression profile consistent with its pleiotropic activities. In the central nervous system, VIP acts as a neurotransmitter and potent neurotropic agent, and is also delivered to primary and secondary immune organs where it modulates immune function through a paracrine mechanism. VIP secretion by activated CD4 Th2 cells is a secondary autocrine/paracrine source that can modulate immune function, and VIP has been suggested to function as a Th2 cytokine with clinical potential in the treatment of certain autoimmune diseases (Ben-Horin and Chowers, 2008; Li et al., 2006). For example, VIP is protective against collagen induced arthritis (Delgado et al., 2001), inflammatory bowel disease (Abad et al., 2003), uveoretinitis (Keino et al., 2004) and experimental autoimmune encephalomyelitis(Gonzalez-Rey et al., 2006). The mechanism for this immunosuppressive effect by VIP is now thought to be due to the generation of antigen-specific regulatory T cells (Pozo et al., 2009). Unfortunately, more than 40 years after the identification of VIP, knowledge of its transcriptome in the CNS and the immune system has still not been well addressed. In the absence of such data, it will remain extremely difficult to fully understand the biological role of this neuropeptide.
A major impediment to obtaining a true VIP receptor-regulated transcriptome is the expression of several potential VIP-binding receptors in different combinations depending on the cell type. Thus, signal transduction elicited by these receptors would make it difficult to distinguish which receptor-mediated signaling pathway was responsible for gene expression changes. For example, vasoactive intestinal peptide/pituitary adenylate cyclase activating polypeptide receptor - 1 (VPAC1) and VPAC2 are expressed in the CNS and in the immune system, and bind VIP with a sub-nM Kd. These receptors belong to the secretin class IIB, G-protein coupled receptor (GPCR) superfamily, and elicit to varying extents, cAMP/PKA, PLD and PLC/Ca2+ activation (Delgado et al., 2004). A third receptor called pituitary adenylate cyclase activating polypeptide receptor 1 (PAC1), also expressed in the CNS and the immune system, binds VIP with a µM Kd (Dautzenberg et al., 1999). Lastly, formyl peptide receptor-like 1 (FPRL1) exhibits weak affinity for VIP, and is widely expressed, including immune cells (El Zein et al., 2008). Therefore, in an attempt to continue the process of ascertaining the biological role of VIP in the immune system, as well as, satisfying the requirement for the high likelihood of a single VIP receptor (VPAC1) being predominately expressed, we chose CD4 T cells as a cellular system for determining a VIP-regulated transcriptome.
In support of identifying the VIP-regulated transcriptome using CD4 T cells, we offer the following rationale. First, we and others have demonstrated high, constitutive levels of VPAC1 with little, if any, detectable VPAC2 expression in both mouse and human resting CD4 T cells (Delgado et al., 1996; Lara-Marquez et al., 2001; Vomhof-DeKrey and Dorsam, 2008). Second, we have also shown that receptor reversal takes place during T cell activation with VPAC1 becoming downregulated and the inducible VPAC2 becoming upregulated, however, such changes peak between 12–96 hours post activation (Voice et al., 2004; Vomhof-DeKrey et al., 2008). Lastly, there has been no evidence that PAC-1 is expressed on T lymphocytes, with only weak expression of FPRL-1 (El Zein et al., 2008). Moreover, the FPRL-1 protein may not be presented on the plasma membrane of leukocytes until after cellular activation. For these reasons, CD4 T cells were chosen to identify the VIP-regulated transcriptome.
In this study, murine CD4 T cells were isolated and used to identify global changes in gene expression in the absence (resting) and presence of PMA/ionomycin (activated) for five hours with and without 10−7 M VIP. Whole mouse genome arrays were utilized from six independent biological experiments with a 10% false discovery rate (FDR) as determined by the Significance Analysis of Microarrays (SAM). This analysis identified 103 and 58 unique genes differentially regulated ≥1.5 fold by VIP in resting versus activated CD4 T cells, of which 12 genes were commonly regulated. Ingenuity Pathway Analysis (IPA) identified a VIP interactome centered on the expression of epidermal growth factor receptor (EGFR) in resting CD4 T cells. In the presence of PMA/ionomycin, the VIP interactome supports a modest, but fundamental change in the repertoire of AP-1 and the inducible cAMP response element (ICER), which are transcriptionally competent nuclear master regulators. Collectively, these data encompass the mouse VIP-regulated transcriptome and interactome, and may provide important new knowledge regarding the molecular pathways controlled by VIP in T cell trafficking, while regulating Treg effector cell differentiation in activated cells.
2. Materials and Methods
2.1 Mice
Wild type C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME) and bred in ventilated cabinets (VWR) at North Dakota State University as previously described (Benton et al., 2009). Spleens from 6–32 week old males and females were harvested by standard dissection techniques in a sterile hood. All mouse protocols were approved by the NDSU IACUC board and met all federal guidelines.
2.2 Murine CD4 T cell Isolation
Mouse splenic CD4 T cells were purified as previously described (Vomhof-DeKrey and Dorsam, 2008). Briefly, each independent experiment (six biological replicates) used five to ten harvested spleens forced through a wire mesh in PBS at RT, and passed through a 70 µm sieve. Erythrocytes were lysed, passed through a 70 µm sieve a second time, and centrifuged as above. Cells were resuspended in 93 µl of PBS/0.5% BSA with 7 µl of anti-mouse CD4 magnetic beads/1 × 107 cells (Miltenyi Biotech; Auburn, CA) at 4–8°C for 20 minutes, and purified on a Miltenyi Auto-MACS instrument using the Possel program (Miltenyi Biotech; Auburn, CA). Some samples did not undergo erythrocyte lysis.
2.3 Antibody staining
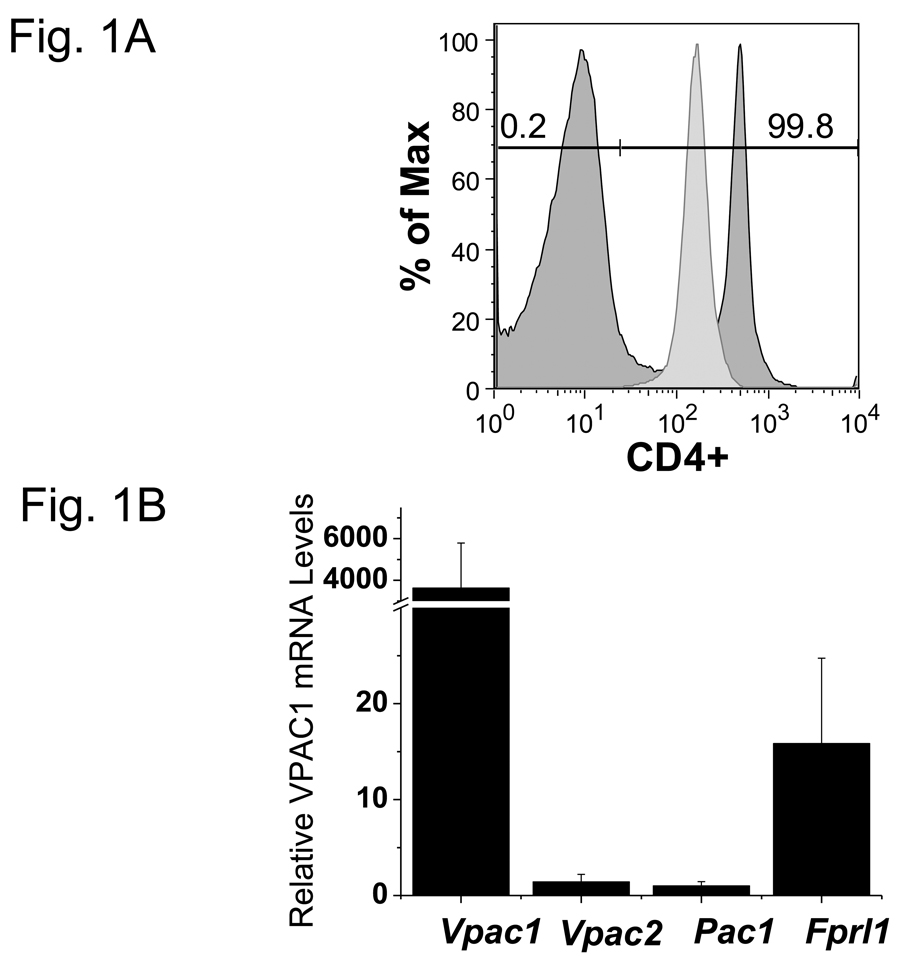
To determine percent purity, 0.5 × 106 total splenocytes (pre-separation) and enriched CD4 T cells (post-separation) were resuspended in 100 µl PBS/0.5% BSA, and incubated with 1:200 FITC conjugated rat anti-mouse CD4 antibody (clone RM4-4, Biolegend, San Diego, CA) for 30 min at 4°C in the dark as previously published (Benton et al., 2009). Cells were washed with 1 ml PBS/0.5% BSA, centrifuged for 5 min at 500 × g, and resuspended in 300µl PBS/0.5% BSA. Rabbit anti-mouse CD25 (IL-2 receptor alpha chain; PC61.5 clone) and anti-mouse CD62L (L-selectin; MEL-14 clone) antibodies purchased from Biolegend were used to stain isolated cells. Flow cytometry was performed on a Becton Dickinson FacsCalibur (Franklin Lakes, NJ) or an Accuri flow cytometer (Ann Arbor, MI). Note that the presence of anti-mouse CD4 magnetic beads did reduce the decade shift slightly for the FITC conjugated rat anti-mouse CD4 antibody (clone RM4-4), but still shows successful enrichment (Fig. 1A).
Figure 1.
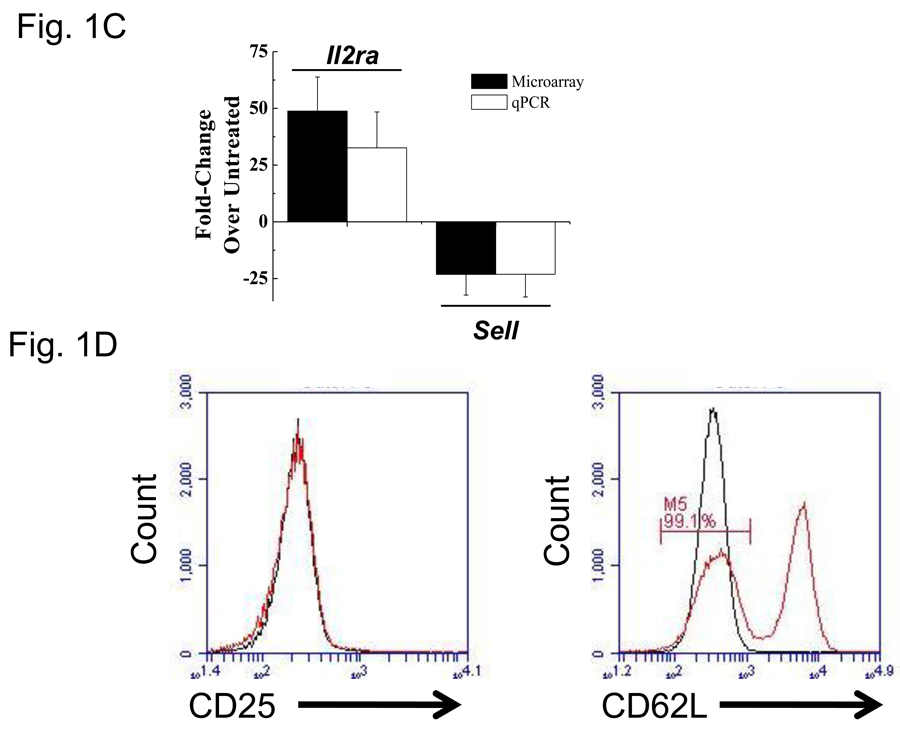
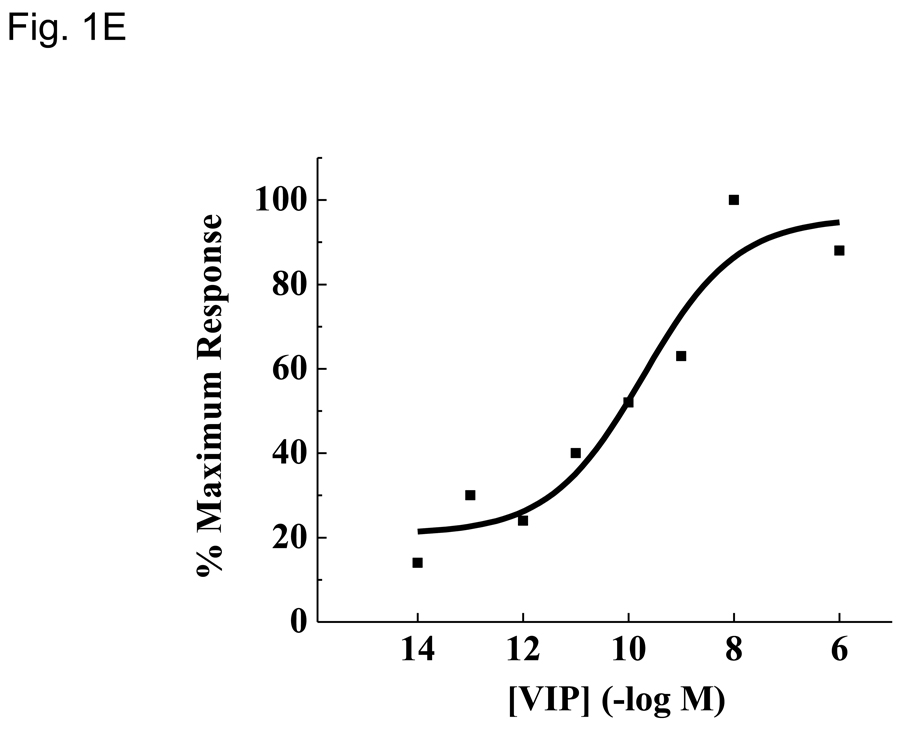
VIP receptor expression levels and phenotypic changes due to PMA/ionomycin treatment. Data is presented as means +/− SEM from three independent experiments calculated by Origin software unless otherwise noted. A. Isolation of highly enriched murine CD4 T cells. Pre- and post anti-CD4 magnetic bead (proprietary clone, Miltenyi) samples were incubated with FITC conjugated anti-mouse CD4 antibody (RM4-4) and analyzed by a FacsCalibur (Materials and Methods). Histogram plots were generated by FloJo software and percent purity is indicated. This data is representative of six independent biological experiments. B. Vpac1 is the predominant VIP-binding receptor expressed by CD4 T cells. MACS purified CD4 T cells were used to measure the relative expression levels of four known VIP receptors, Vpac1, Vpac2, Pac1 and Fprl1 by qRT-PCR. Data was normalized to Actb, and fold changes calculated by the ΔΔCt method, with Pac1 levels arbitrarily set to 1. The y-axis has a break from 30 to 3,000 as indicated by two slashes (//). C. Expected mRNA expression changes of Il2ra and Sell induced by PMA/ionomycin treatment. Purified CD4 T cells were cultured in the presence or absence of 10 ng/ml PMA and 1 µM ionomycin for 5 hours. Real-time PCR analysis was performed as described in 1B. Microarray averages were calculated from normalized pixel intensity values calculated from six (microarray) or three (qRT-PCR) independent biological experiments. Black bars represent microarray values and open bars represent qRT-PCR values. D. Expected phenotypic surface expression levels of CD25 (Il2ra) and CD62L (Sell) before and after PMA/ionomycin treatment. Purified CD4 T cells were sequentially incubated with or without PMA/ionomycin for 5 hours followed by staining with anti-CD25 (left panel) or anti-CD62 (right panel), and analyzed by an Accuri flow cytometer (Ann Arbor, MI). The red line represents cells incubated in media alone, and the black line represents cells incubated with PMA/ionomycin. These data show expected changes as there is complete shedding of CD62L with PMA/ionomycin. E. Use of biologically active VIP ligand. Chinese Hamster Ovary (CHO-K1) cells were stably transfected with human Vpac1 cDNA. Cells were incubated with varying concentrations of VIP ranging from 10−14 to 10−6 M (Materials and Methods). After fifteen minutes, cells were lysed and [cAMP]i were measured by a competitive ELISA. Curve fitting was performed in Origin graphical software.
2.4 Quantitative RT- PCR
Total RNA was isolated using RNeasy mini columns (Qiagen, Valencia, CA) with on-column DNAse I treatment performed as previously published (Vomhof-DeKrey and Dorsam, 2008). For cDNA synthesis, 0.5 microgram of total RNA was reverse transcribed using 250 ng of random hexamers and M-MLV reverse transcriptase according to the manufacturer’s protocol (Promega; Madison, WI). Samples were incubated at 37°C for 60 minutes and used immediately or stored at −20°C until assayed. Five microliters of cDNA was used as template with 400 nM specific primers (IDT DNA technologies, Coralville, IA; Supplemental Table 1) and 7.5 µl of 2× Fast SYBR Green Mastermix. Samples were amplified in an ABI7500 instrument (Applied Biosystems, Foster City, CA), and relative changes were calculated by the ΔΔCt method using β-actin (Actb) as a reference control gene.
2.5 cAMP quantitation
Intracellular cAMP concentration was measured by a competitive ELISA (Assay Designs; Ann Arbor, MI) as previously published (Vomhof-DeKrey et al., 2008). Briefly, stable CHO-K1 clones overexpressing human VPAC1 were seeded in 96 well tissue culture plates (Corning) in 100 ul at 5 ×106 cells/ml and incubated for 24 hours in culture media and G418 at 1 ug/ml. Cells were washed twice with 1× PBS and pretreated with the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IMBX) in HBSS, 0.5% BSA (binding buffer) for 45 minutes at 37°C to limit cAMP catabolism. Cells were washed as above and incubated with binding buffer and increasing concentrations of exogenously added VIP ligand (10−14 to 10−6 M) for 15 minutes at 37°C. Cells were then washed with ice-cold PBS and lysed with 0.1 M HCl. The plate was incubated at RT with shaking for 30 minutes and immediately used for measurements in the cAMP competitive ELISA or stored at −20°C until assayed. Each ELISA measurement was accompanied with a cAMP standard curve (0.78 – 200 pmol/ml) and a positive (10−6M forskolin) and negative control (water), and optical density (450 nm) was measured using a Multiskan Spectrum spectrophotometer (Thermo Scientific; Waltham, MA).
2.6 Murine CD4 T cell in vitro culture conditions
Cells were cultured at 2 × 106 cells/ml in RPMI supplemented with 10% defined fetal bovine serum, L-glutamine, 1× penicillin/streptomycin at 37°C in 5% CO2. PMA (phorbol 12- myristate 13- acetate) and ionomycin were purchased from Sigma Aldrich (St. Louis, MO), and VIP was purchased from American Peptide Company (Sunnyvale, CA). Resting cells received water (vehicle control) or 10−7M VIP for 5 hours, and DMSO vehicle control for PMA/ionomycin. For T cell activation conditions, cells were treated with 10 ng/ml PMA and 1 µM ionomycin in the presence or absence of 10−7M VIP for 5 hours.
2.7 Global expression profiling
All microarray experiments and statistical analyses were performed by the Marshall University Genomics Core Facility (http://musom.marshall.edu/genomics/). To determine the effect of VIP on resting and PMA/ionomycin activated mouse CD4 T lymphocyte gene expression, we performed expression profiling using Agilent Mouse Whole Genome Arrays (Agilent, Santa Clara, CA) in a balanced block design. RNAs from individual VIP resting (CD4 T cells −/+VIP) and activated (PMA/ionomycin −/+ VIP) were compared in a single array so that there were six biologically independent sets of array data for each comparison. Total RNA quality was determined on an Agilent 2100 Bioanalyzer. All samples had RNA integrity numbers (RIN) greater than 8. Total RNA (250 ng) was used for labeling using the QuickAmp labeling kit (Agilent) and cyanine (Cy)-3-CTP and Cy5-CTP (Perkin Elmer, Waltham, MA). Three of the six arrays contained control cRNA labeled with Cy5 and VIP-treated cRNA labeled with Cy3 (Perkin Elmer, Waltham, MA). The remaining three arrays were flip-labeled to minimize the effect of bias in dye incorporation. Following purification of labeled cRNAs, 825 ng of Cy3- and Cy5-labeled cRNAs were combined and hybridized for 17 hours at 65°C in an Agilent hybridization oven. Microarrays were then washed and scanned using Agilent DNA Microarray Scanner.
2.8 Statistical Analysis of Microarray Data
Feature intensities were extracted in lowess-normalized form from the scanned image using Agilent Feature Extraction software. All extracted data were exported to Microsoft Excel (Microsoft Corporation, Redmond, WA) as tab delimited files. For each feature, a value was considered present if at least one channel had a signal well above background according to Feature Extraction. The log base 2 of the expression ratios of resting with VIP ligand (sample) to resting without VIP ligand (control) or the log base 2 of the expression ratios of activated with VIP ligand (sample) to activated without VIP ligand (control) were computed, and assembled into a single tab-delimited file for each comparison. Features for which less than 50% of the arrays produced a present value were removed from future analyses. Each file was then imported into the Multiple Experiment Viewer (MeV) v4.3 for statistical analysis (Saeed et al., 2006).
Calculated log ratios were compared for significant deviation from zero using one-class Significance Analysis of Microarrays (SAM) (Tusher et al., 2001). SAM was performed with the maximum number of unique permutations available, and delta values were chosen to give a median False Discovery Rate (FDR) of 10%. All other parameters were set to the MeV defaults. The resulting list of statistically significant probes was exported to tab-delimited format and opened using Microsoft Excel. For each feature, the mean log base 2 ratio across all arrays was computed and used to calculate the average fold change between samples and controls. For genes which were represented by multiple features on the array, the feature with the largest fold change was reported. The log base 2 ratios were used to calculate a 95% confidence interval for fold change. Features for which the confidence interval includes 1 were removed from the results. This initial expression profile (“no threshold” dataset) was subsequently filtered for a minimum fold change greater than 1.5 (threshold dataset). The “no threshold” data set was used in the IPA analysis of VIP-regulated interactome (Fig. 4–Fig 6). Microarray data may be accessed at the NCBI Gene Expression Omnibus (GEO) database (GSE18526).
Figure 4.
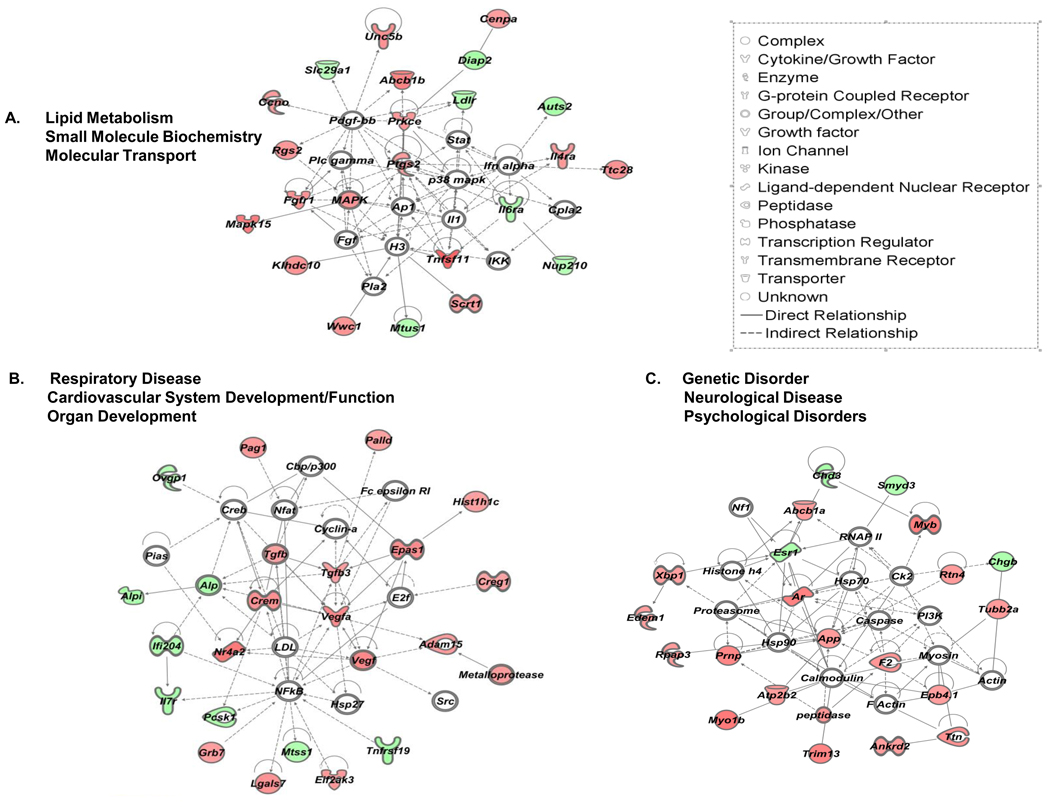
IPA generated networks of VIP modulated genes and their corresponding networks in resting CD4 T cells. No user-defined cutoffs for expression values were used for network generation. Networks scores were generated by Ingenuity’s Network Generation Algorithm, and were assigned the following scores, A. 36, B. 31 and C. 31, respectively. Open symbols are intermediate molecules, “non-focus genes,” placed in the network by the Ingenuity software, but are not regulated by VIP. “Focus genes” are regulated by VIP and are denoted by red symbols for upregulated genes, and green symbols indicate downregulated genes. Symbols representing functional categories of the molecules are listed in the legend.
Figure 6.
Identification of the direct VIP interactome for resting and activated datasets. In IPA software, the identical datasets used in Figure 4 and 5 for resting or activated samples, were selected to Build a network using the Connect function in IPA for A. resting and B. activated datasets. The symbol legend is located in Figure 4.
2.9 Network analysis
Excel files containing gene names and fold change values from SAM analyses using a 10% FDR were uploaded onto the Ingenuity Pathway Analysis (IPA) website (version 7.5) (Ingenuity Systems, Inc, Mountain View, CA). Analysis settings included the reference set of the Ingenuity Knowledge Base (Genes only), which is a manually curated database of the scientific literature that documents the direct and indirect relationships between genes and gene products, such as protein-protein or protein-DNA interactions, activation/inhibition, localization, translocation or expression (Calvano et al., 2005). Summary of Analysis reports were generated for resting and activated datasets individually, and included the top five most significant Networks created by the proprietary IPA Network Generation Algorithm. Networks are constructed to contain up to 35 Focus Genes (VIP modulated gene targets) and are optimized for high degrees of interconnectivity among them. They present the interactions between “Focus Genes” and intermediate “non-Focus Genes” based on the premise that highly interconnected molecules may be more likely to characterize a biological function. IPA reports also contained the top five most significant Biological Functions (Diseases and Disorders, Molecular and Cellular Functions, Physiological System Development and Functions), Canonical Pathways and Toxicological Lists. Network Scores and p values were assigned by IPA software. The Core Analysis prepares a Summary of Analysis report including Networks of genes categorized by functional relationships. Networks are graphical displays that describe the relationships between a subset of genes and their neighboring genes. For example, if two molecules have a relationship, such as a protein-protein interaction or one activates the expression of the other, they are connected by a line. If they are related to a third molecule and a fourth, then additional lines are drawn, and a network is formed. According to the degree of interconnectedness among the molecules observed, a higher or lower IPA network score is assigned. The top three Networks of each dataset were imported into the Path Designer to assign symbols to the molecules to designate their functions.
3. Results
3.1 Assessment of CD4 T cell purity, VIP receptor expression profile and phenotypic changes of activated cells
As this study aimed to identify VIP gene targets in primary CD4 T cells, isolation by magnetic bead technology was performed to obtain a high purity (≥95%) of this cell population from total splenocytes as determined by flow cytometry (Fig.1A). To evaluate the predominant VIP receptor expressed on CD4 T cells, the mRNA expression levels of four known VIP receptor genes, Vpac1, Vpac2, Pac1 and Fprl1 were measured by qRT-PCR. Vpac1 was the predominately expressed VIP receptor, showing 208 fold higher levels than Fprl1 and 3327 fold higher levels than Vpac2 or Pac1 (Fig. 1B). Figure 1C demonstrates that these cells were successfully activated by PMA/ionomycin treatment (Truneh et al., 1985) based on measured mRNA levels by qRT-PCR and microarray analysis that revealed an upregulation of IL-2 receptor alpha chain (Il2ra) transcript and a downregulation of L-selectin (Sell) transcript (Altman et al., 1992; Buhrer et al., 1990). To assess surface protein levels, flow cytometry was utilized, and as expected, after five hours of stimulation, the cells exhibited CD62L (Sell) shedding, but no changes in CD25 (Il2ra) protein expression could be detected at this time point. These data confirmed an early activation CD4 T cell phenotype in the cells assessed at 5 hours (Fig. 1D). Lastly, Figure 1E demonstrates that the synthetic VIP used in these studies was biologically active, inducing readily detectable intracellular cAMP ([cAMP]i) concentration in human VPAC1 overexpressing CHO-K1 clones (Fig. 1E). These data validated that we were able to obtain a highly purified, resting, splenic CD4 T cell population expressing high levels of VPAC1, which resulted in an early activated phenotype after PMA/ionomycin treatment (5 hours) that were assessed for gene expression changes using a functionally active VIP ligand.
3.2 Microarray analysis identifies a VIP transcriptome in resting and PMA/ionomycin activated mouse CD4 T cells
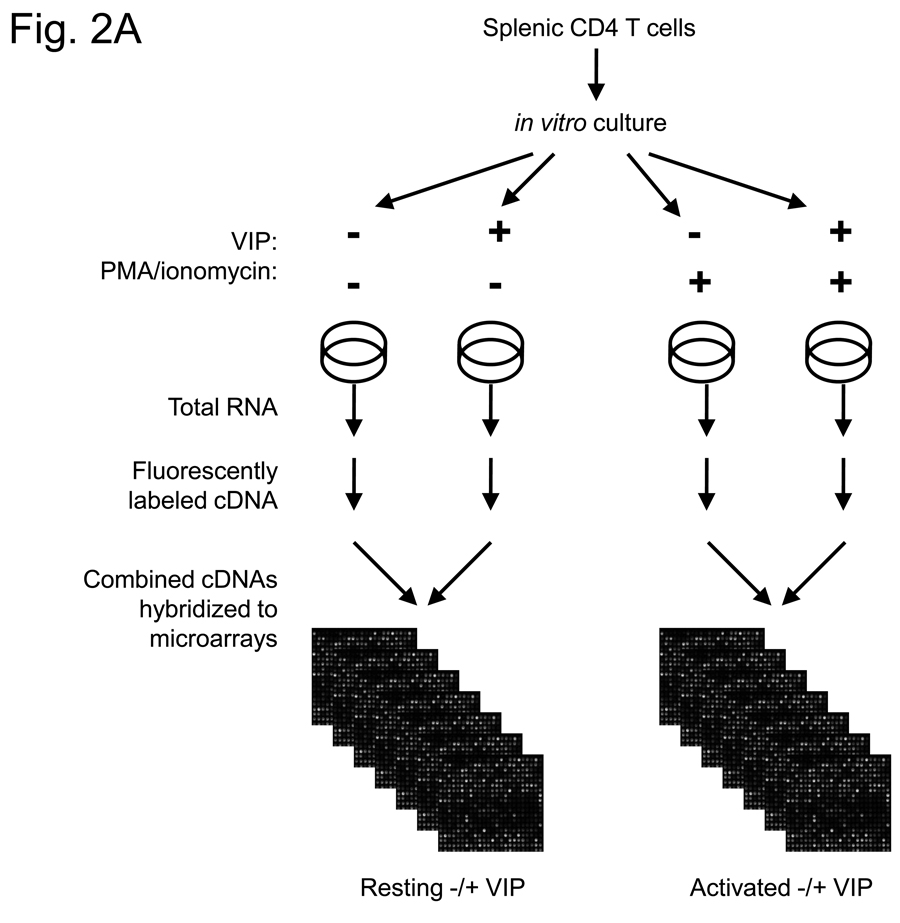
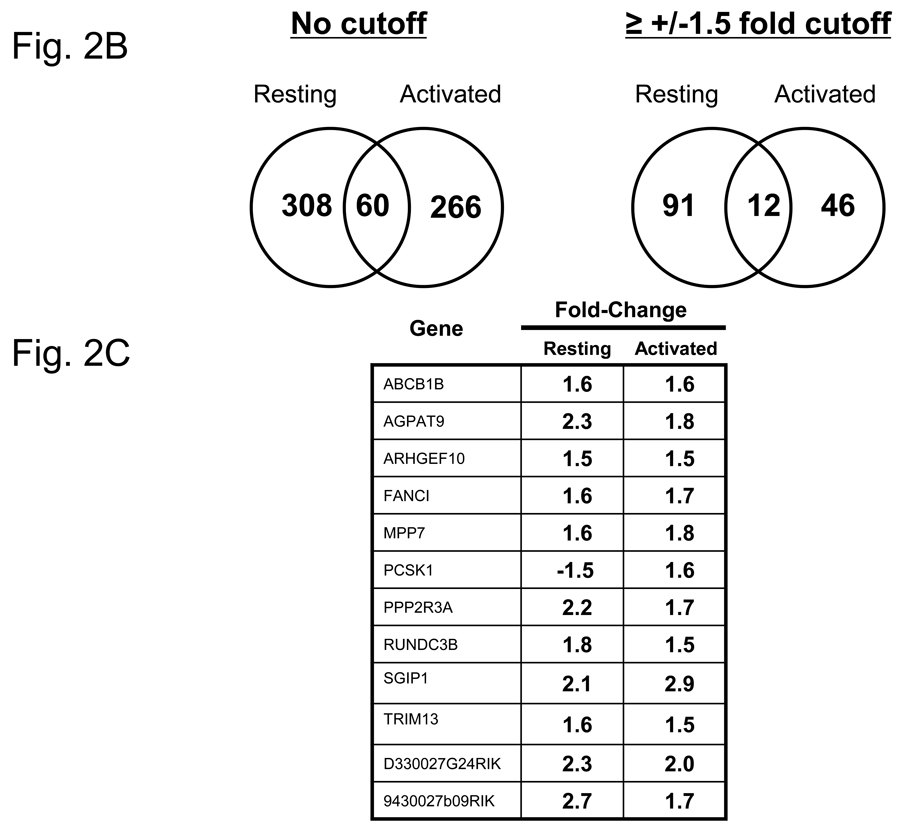
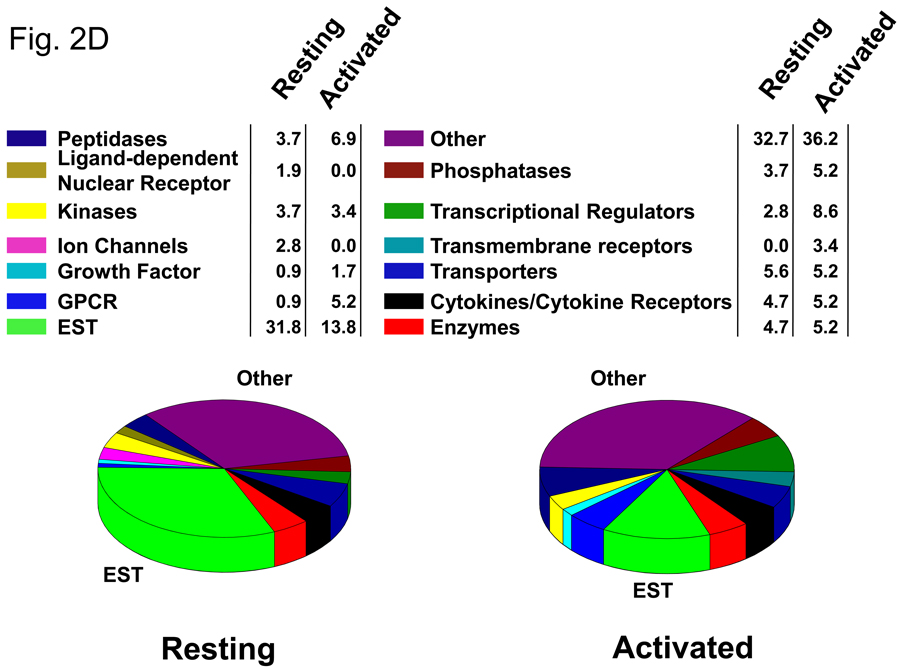
In order to identify the early genes modulated by VIP in CD4 T cells, we used DNA microarray expression profiling. Purified CD4 T cells were incubated in vitro in the absence and presence of PMA/ionomycin (resting and activated), in which each group was cultured without and with 10−7M VIP for five hours. While this concentration is higher than that found physiologically, it is still well within the acceptable range generally used in the VIP field. Fluorescently-labeled cRNAs were prepared from total RNA and hybridized to Agilent Whole Mouse Genome Arrays containing 45,220 features which represent approximately 41,000 unique mouse genes and transcripts (Figure 2A). Microarray expression ratios were analyzed by the SAM program. Using a 10% FDR, 368 genes were identified as modulated by VIP in the resting cells as shown in the Venn diagram in Figure 2B. This dataset included 286 upregulated genes and 82 downregulated genes, ranging from 3.49 fold upregulated to 4.78 fold downregulated. Using the same FDR, 326 genes were identified to be modulated by VIP in the presence of PMA/ionomycin. These data identified 312 upregulated genes and 14 downregulated genes ranging from 2.94 fold upregulated to 1.66 fold downregulated. Using a 1.5 fold cutoff and deleting genes for which the confidence intervals included 1, two smaller datasets were generated and shown in Fig. 2B (right side). There were 12 transcripts in common between resting and activated cells, which are listed in Figure 2C. Both resting and activated datasets with a 1.5 fold cutoff were uploaded into the Ingenuity Pathway Analysis search engine (http://www.ingenuity.com/). IPA grouped these transcripts into functional categories and defined their cellular locations. Graphical representation of functional group percentages and a list of fold changes with their cellular locations are shown in Figure 2D and Table 1. A complete list of SAM-filtered genes identified for resting and activated samples, including ESTs and accession numbers, are provided in Supplemental Tables 2 and 3, respectively. These data represent the early gene targets in the VIP transcriptome of murine CD4 T cells. We conclude that CD4 T cells interpret VIP, presumably induced by VPAC1 receptor signaling, by regulating two nearly unique transcriptomes (resting and activated) with ≥81% uniquely modulated genes. It would therefore follow that cellular changes by VIP would be dictated primarily by the activation status of CD4 T cells.
Figure 2.
Identification of the early VIP transcriptome in resting and activated CD4 T cells. A. Five to ten C57BL/6J spleens were harvested for each independent experiment (n=6), and CD4 T cells were isolated by magnetic bead technology. Cells were cultured in the absence (resting) or presence of PMA/ionomycin (activated), and cultured with or without 10−7 M VIP for five hours (four groups). Total RNA was harvested from resting and activated samples, fluorescently labeled, and hybridized to Agilent Whole Genome Microarrays. These data were then analyzed by SAM and Ingenuity Pathway Analysis software (Materials and Methods). B. Venn diagrams of the number of transcripts uniquely or commonly (overlapping) modulated by VIP in resting and PMA/ionomycin activated CD4 T cells (left side) as identified by SAM analysis with a 10% FDR. The left diagram shows all VIP-modulated genes (no threshold applied), while the right panel shows the number of VIP modulated genes after a ≥ −/+ 1.5 fold cutoff. C. Gene names that are commonly regulated by VIP (after a ≥ −/+ 1.5 fold cutoff) with fold change values indicated. D. Pie charts showing the percentage changes of genes in fourteen functional categories identified by IPA for the resting and activated datasets.
Table 1.
VIP-modulated genes in resting and PMA/ionomycin activated CD4 T cells grouped by function
| Symbol | Entrez Gene Name | Resting Fold Change |
Activated Fold Change |
|---|---|---|---|
| Cytokines/Cytokine Receptors | |||
| Cxcl10 | chemokine (C-X-C motif) ligand 10 (IP-10) | 1.806 | |
| Il5 | interleukin 5 (colony-stimulating factor, eosinophil) | 1.697 | |
| Il5ra | interleukin 5 receptor, alpha | 1.523 | |
| Il7r | interleukin 7 receptor | −1.53 | |
| Il17rc | interleukin 17 receptor C | 1.782 | |
| Tnfsf11 a | tumor necrosis factor (ligand) superfamily, member 11 (RANKL) | 2.03 | |
| Tnfrsf19 | tumor necrosis factor receptor superfamily, member 19 | −1.568 | |
| Tslp | thymic stromal lymphopoietin | 1.512 | |
| Enzymes | |||
| Agpat9 a | 1-acylglycerol-3-phosphate O-acyltransferase 9 | 2.346 | 1.825 |
| Chd3 | chromodomain helicase DNA binding protein 3 | −1.53 | |
| Chst2 | carbohydrate (N-acetylglucosamine-6-O) sulfotransferase 2 | 1.502 | |
| Far2 | fatty acyl CoA reductase 2 | 1.638 | |
| Galc | galactosylceramidase | 1.588 | |
| Gnat1 | guanine nucleotide binding protein (G protein), alpha transducing activity polypeptide 1 | 2.161 | |
| Hsd3b4 | hydroxy-delta-5-steroid dehydrogenase, 3 beta- and steroid delta-isomerase 4 | 1.71 | |
| G-protein coupled receptors | |||
| Ccr7 | chemokine (C-C motif) receptor 7 | 1.756 | |
| Ccr8 | chemokine (C-C motif) receptor 8 | 1.825 | |
| Lpar4 | lysophosphatidic acid receptor 4 | 1.598 | |
| Lrrc8d | leucine rich repeat containing 8 family, member D | 1.576 | |
| Growth factors | |||
| Pdgfb | platelet-derived growth factor beta polypeptide | −1.584 | |
| Vegfa a | vascular endothelial growth factor A | 1.57 | |
| Ion channels | |||
| Cnga1 | cyclic nucleotide gated channel alpha 1 | −1.672 | |
| Gria3 | glutamate receptor, ionotrophic, AMPA 3 | −1.846 | |
| Scn3b | sodium channel, voltage-gated, type III, beta | 1.871 | |
| Kinases | |||
| Ephb2 | EPH receptor B2 | 1.667 | |
| Mapk15 | mitogen-activated protein kinase 15 | 1.715 | |
| Mos | v-mos Moloney murine sarcoma viral oncogene homolog | 1.543 | |
| Pak1 a | p21 protein (Cdc42/Rac)-activated kinase 1 | 1.624 | |
| Prkca | protein kinase C, alpha | 1.878 | |
| Yes1 | v-yes-1 Yamaguchi sarcoma viral oncogene homolog 1 | 1.519 | |
| Ligand-dependent nuclear receptors | |||
| Ar | androgen receptor | 1.649 | |
| Nr4a2 | nuclear receptor subfamily 4, group A, member 2 | 1.746 | |
| Peptidases | |||
| Adamts15 | ADAM metallopeptidase with thrombospondin type 1 motif, 15 | −4.011 | |
| Arhgef10 | Rho guanine nucleotide exchange factor (GEF) 10 | 1.502 | 1.51 |
| Mmp2 | matrix metallopeptidase 12 (macrophage elastase) | 1.54 | |
| Pcsk1 | proprotein convertase subtilisin/kexin type 1 | −1.545 | 1.588 |
| Plg | plasminogen | −2.426 | |
| Usp2 | ubiquitin specific peptidase 2 | 1.635 | |
| Phosphatases | |||
| Alpi | alkaline phosphatase, intestinal | −1.505 | |
| Dusp10 | dual specificity phosphatase 10 | 1.617 | |
| Minpp1 | multiple inositol polyphosphate histidine phosphatase, 1 | 1.79 | |
| Nudt4 | nudix (nucleoside diphosphate linked moiety X)-type motif 4 | 1.674 | |
| Ppp2r3a | protein phosphatase 2 (formerly 2A), regulatory subunit B'', alpha | 2.242 | 1.716 |
| Ptpn4 | protein tyrosine phosphatase, non-receptor type 4 (megakaryocyte) | 1.569 | |
| Transcription regulators | |||
| Crem | cAMP responsive element modulator (ICER) | 1.562 | |
| Egr3 | early growth response 3 | −1.874 | |
| Epas1 a | endothelial PAS domain protein 1 | 1.808 | |
| Fos | v-fos FBJ murine osteosarcoma viral oncogene homolog | 1.668 | |
| Mitf | microphthalmia-associated transcription factor | −1.593 | |
| Myb a | v-myb myeloblastosis viral oncogene homolog (avian) | 1.68 | |
| Tceal1 | transcription elongation factor A (SII)-like 1 (p21) | 1.872 | |
| Tsc22d3 | TSC22 domain family, member 3 | 1.501 | |
| Transmembrane receptors | |||
| Cd14 | CD14 molecule | 1.67 | |
| Plaur a | plasminogen activator, urokinase receptor (uPAR) | 1.636 | |
| Transporters | |||
| Abcb1b a | ATP-binding cassette, sub-family B (MDR/TAP), member 1B | 1.617 | 1.602 |
| Abcb4 | ATP-binding cassette, sub-family B (MDR/TAP), member 4 | 2.104 | |
| Azgp1 | alpha-2-glycoprotein 1, zinc-binding | 1.625 | |
| Exoc4 | exocyst complex component 4 | 1.566 | |
| Slc27a2 | solute carrier family 27 (fatty acid transporter), member 2 | 1.634 | 1.541 |
| Syt13 | synaptotagmin XIII | 3.492 | |
| Tmed3 | transmembrane emp24 protein transport domain containing 3 | 1.515 | |
| Others | |||
| Aim1 | absent in melanoma 1 | 1.554 | |
| Arhgap15 | Rho GTPase activating protein 15 | −1.78 | |
| Arhgap18 | Rho GTPase activating protein 18 | 1.727 | |
| Arhgap29 a | Rho GTPase activating protein 29 | −1.517 | |
| Arrdc3 | arrestin domain containing 3 | 1.586 | |
| Auts2 | autism susceptibility candidate 2 | −1.53 | |
| Cilp2 | cartilage intermediate layer protein 2 | −1.669 | |
| Cldn5 | claudin 5 | 1.876 | |
| Coch | coagulation factor C homolog, cochlin (Limulus polyphemus) | 1.687 | |
| Col11a2 | collagen, type XI, alpha 2 | −1.535 | |
| Ctla2a | cytotoxic T lymphocyte-associated protein 2 alpha | 1.648 | |
| Cytip a | cytohesin 1 interacting protein (Pscdbp) | 2.035 | |
| Dfna5 | deafness, autosomal dominant 5 | 1.652 | |
| Diaph2 | diaphanous homolog 2 (Drosophila) | −1.558 | |
| Dnah17 | dynein, axonemal, heavy chain 17 | 1.857 | |
| Emba | embigin homolog (mouse) | 1.688 | |
| Eml1 | echinoderm microtubule associated protein like 1 | 1.775 | |
| Epb41 | erythrocyte membrane protein band 4.1 (elliptocytosis 1, RH-linked) | 1.56 | |
| Fanci a | Fanconi anemia, complementation group I | 1.642 | 1.744 |
| Fndc5 | fibronectin type III domain containing 5 | 1.93 | |
| Frmpd1 | FERM and PDZ domain containing 1 | 1.529 | |
| Gckr | glucokinase (hexokinase 4) regulator | 1.54 | |
| Grap2 | GRB2-related adaptor protein 2 | 1.59 | |
| Hist1h1c | histone cluster 1, H1c | 1.545 | |
| Igsf3 | immunoglobulin superfamily, member 3 | −1.512 | |
| Impg2 | interphotoreceptor matrix proteoglycan 2 | −1.787 | |
| Khdrbs3 | KH domain containing, RNA binding, signal transduction associated 3 | 1.751 | |
| Moblk2b a | MOB1, Mps One Binder kinase activator-like 2B (yeast) | 1.852 | |
| Mpp7 | membrane protein, palmitoylated 7 (MAGUK p55 subfamily member 7) | 1.59 | 1.822 |
| Myo1b a | myosin IB | 1.751 | |
| Nrip3 | nuclear receptor interacting protein 3 | 1.704 | |
| Parp8 | poly (ADP-ribose) polymerase family, member 8 | 2.142 | |
| Pdlim4 | PDZ and LIM domain 4 | −1.608 | |
| Podxl2 | podocalyxin-like 2 | 1.726 | |
| Prnp | prion protein | 1.571 | |
| Pvrl3 | poliovirus receptor-related 3 | 1.523 | |
| Ramp3 | receptor (G protein-coupled) activity modifying protein 3 | 1.755 | |
| Rapgef6 | Rap guanine nucleotide exchange factor (GEF) 6 | −1.783 | |
| Rgs2 | regulator of G-protein signaling 2, 24kDa | 1.731 | |
| Rundc3b | RUN domain containing 3B | 1.762 | 1.546 |
| Sgip1 a | SH3-domain GRB2-like (endophilin) interacting protein 1 | 2.08 | 2.941 |
| Sgtb | small glutamine-rich tetratricopeptide repeat (TPR)-containing, beta | 1.517 | |
| Sh3bgrl2 | SH3 domain binding glutamic acid-rich protein like 2 | 1.842 | |
| Sspn | sarcospan (Kras oncogene-associated gene) | 1.557 | |
| Tctex1d1 | Tctex1 domain containing 1 | 2.371 | |
| Tex11 | testis expressed 11 | 1.753 | |
| Trim13 a | tripartite motif-containing 13 | 1.604 | 1.519 |
| Vps37b | vacuolar protein sorting 37 homolog B (S. cerevisiae) | 1.562 | |
| Ypel4 | yippee-like 4 (Drosophila) | 1.608 | |
| Zbtb20 a | zinc finger and BTB domain containing 20 | −1.664 | |
| Zcchc12 | zinc finger, CCHC domain containing 12 | 1.633 | |
Transcripts which were represented by more than one microarray feature
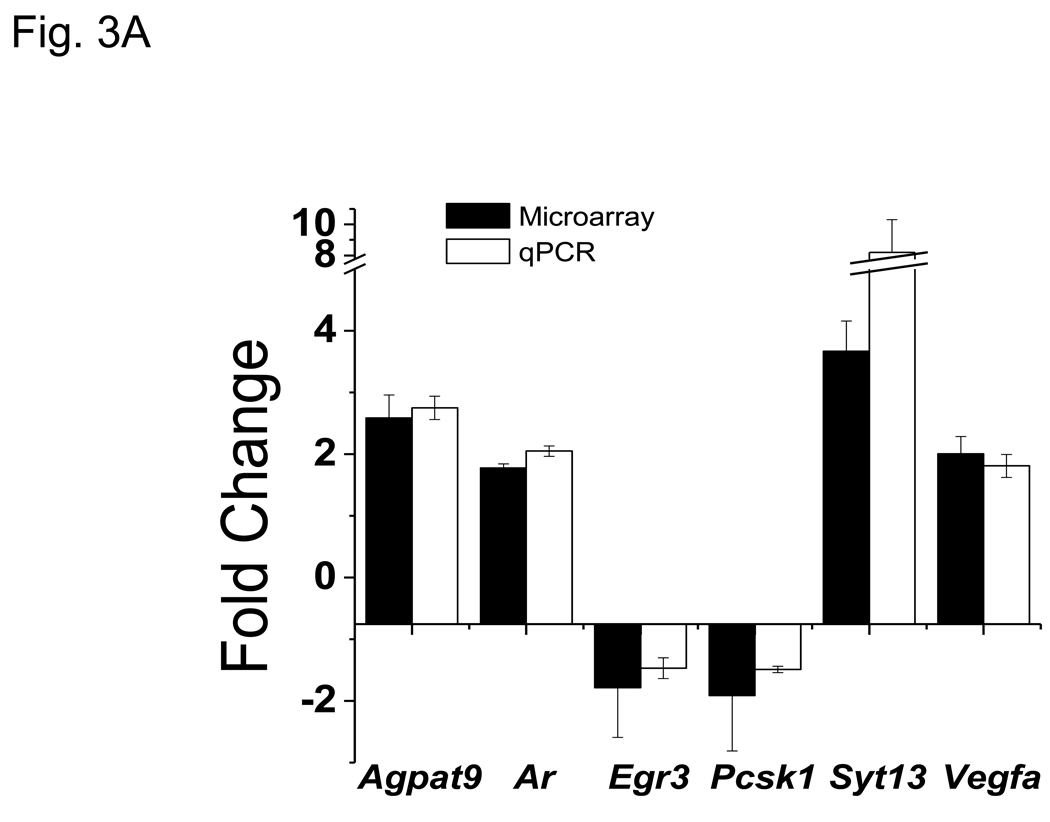
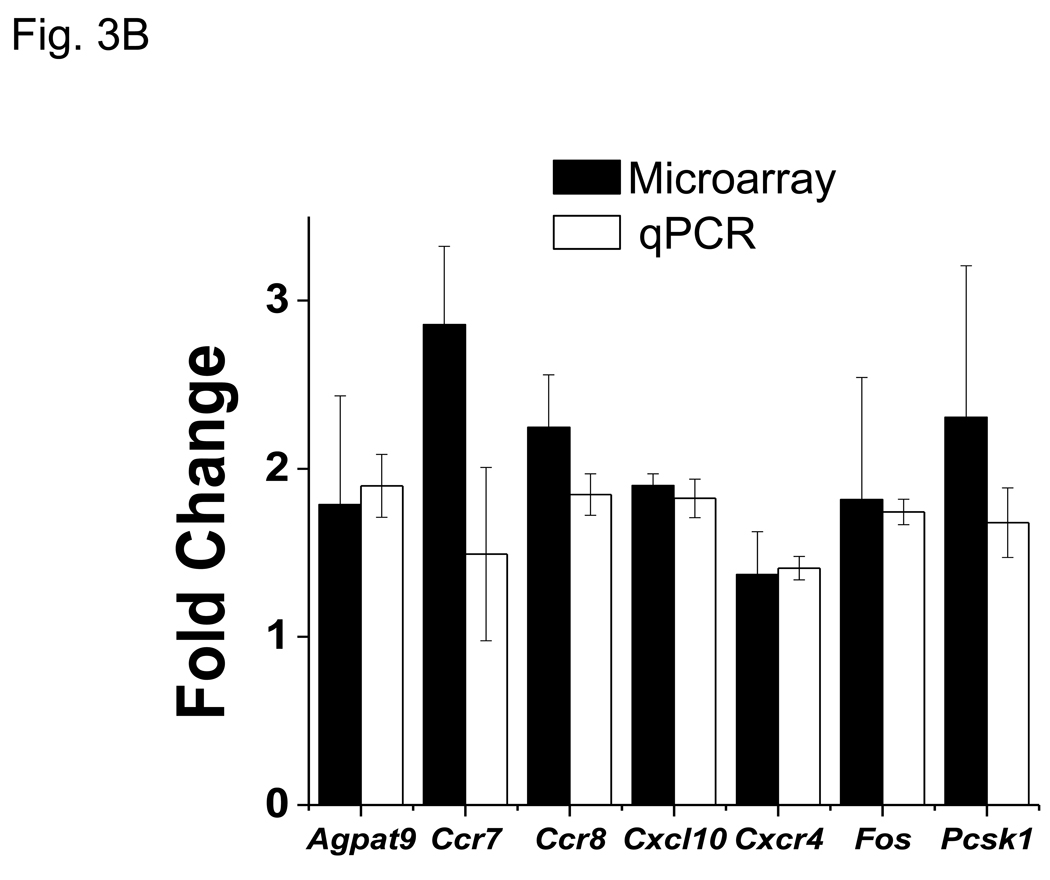
3.3 Verification of VIP-regulated microarray gene expression changes by qRT-PCR
Microarray ratios obtained for several VIP-modulated transcripts were independently corroborated by qRT-PCR analysis using primary CD4 T cells. All 13 genes analyzed by qRT-PCR showed a change in expression by VIP treatment similar to fold changes obtained by microarray analyses (Figs. 3A and 3B). Collectively, we conclude that this VIP transcriptome represents an accurate and precise dataset of VIP regulated gene expression changes.
Figure 3.
qPCR corroboration of VIP modulated gene targets identified by microarray analysis. RT-PCR was utilized to measure transcript level changes in response to VIP treatment. Gene of interest values were normalized to Actb and data represents means +/− SEM calculated by the ΔΔCt method from six (microarray) or three (qPCR) independent experiments as indicated using A. resting, and B. activated samples. Black bars represent microarray values and open bars represent qRT-PCR values.
3.4 Network analyses of the VIP transcriptome in CD4 T cells
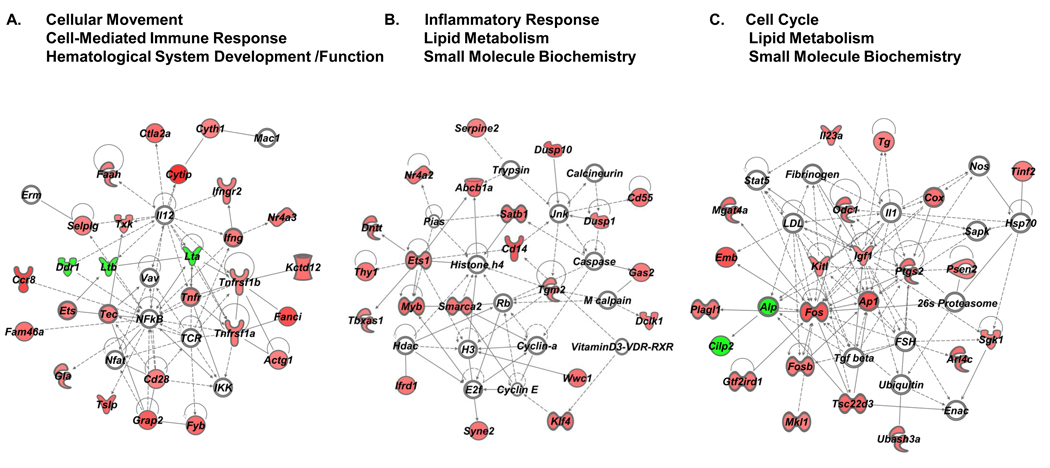
To interpret our data in the context of biological processes, pathways and networks, IPA Core Analyses were conducted using the 10% FDR SAM dataset without a threshold cutoff, thus increasing the probability of identifying biologically relevant signaling pathways modulated by VIP that might normally be ignored based on a purely statistical basis (i.e. using a 1.5 fold filter). The top three IPA generated gene networks for the resting and PMA/ionomycin activated datasets are shown in Figure 4 and Figure 5, with Table 2 listing the top five categories of functions and related diseases modulated by VIP. Consistent with the observation that VIP signaling promotes nearly unique transcriptomes dependent on PMA/ionomycin exposure, the IPA network analysis showed significantly different changes at the molecular and functional levels (60% and 40%, respectively), and completely different disease states (Table 2). The complete lists of genes in each functional category with p values assigned by the IPA software are provided in Supplemental Tables 4 and 5. Examples include lists of genes involved in cellular movement, homing and chemotaxis for the resting dataset (APP, CHST2, EGFR, EPHB2, F2, FGFR1, IL5, PAK1, UPAR/PLAUR, PLG, PRKCA, PRNP, COX2/PTGS2, RNASE2, TNFSF11, VEGFA), and genes involved in T cell proliferation the activated dataset (CD14, CD28, CXCR4, DUSP10, F2RL1, FYB, GRAP2, IGF1, IL23A, KITLG, MYB, PDE4D, PTGS2/COX2, RGS2, SATB1, TCF7, TG, THY1, TNFRSF1A, TNFRSF1B, TSC22D3, TSLP, TXK, UBASH3A). However, significant functions were also shared between the resting and activated datasets demonstrating that VIP signaling may control cell cycle checkpoints and apoptosis, as well as, regulate hematopoietic developmental programs through the small, but common gene set identified in Figure 2B and 2C. Taken together, VIP signaling affects similar and different CD4 T cell functions, which may translate to vastly different physiological states, dependent on the activation status of T cells.
Figure 5.
IPA generated networks of VIP modulated genes and their corresponding networks in activated CD4 T cells. IPA analysis was conducted as described in Fig. 4. Network scores were A. 39, B. 35 and C. 32, respectively. The functional classifications denoted by the symbols are found in the legend for Figure 4.
Table 2.
Most significant biological functions as determined by IPA
| Resting CD4 T cells −/+ VIP | PMA/ionomycin Activated CD4 T cells −/+ VIP | ||
|---|---|---|---|
| Molecular and Cellular Functions | |||
| Name | # of Molecules | Name | # of Molecules |
| Cell Death | 68 | Cellular Movement | 60 |
| Cellular Compromise | 22 | Cellular Growth and Proliferation | 79 |
| Cell Cycle | 39 | Cell Cycle | 40 |
| Carbohydrate Metabolism | 14 | Cellular Development | 70 |
| Lipid Metabolism | 27 | Cell Death | 78 |
| Physiological System Development and Functions | |||
| Name | # of Molecules | Name | # of Molecules |
| Skeletal and Muscular System Development/Function | 31 | Cell-Mediated Immune Response | 71 |
| Cardiovascular System Development/Function | 28 | Hematological System Development/Function | 58 |
| Tissue Morphology | 38 | Tissue Morphology | 49 |
| Hematological System Development/Function | 31 | Skeletal and Muscular System Development/Function | 28 |
| Organismal Development | 42 | Hematopoiesis | 42 |
| Diseases and Disorders | |||
| Name | # of Molecules | Name | # of Molecules |
| Neurological Disease | 100 | Immunological Disease | 67 |
| Developmental Disorder | 32 | Dermatological Diseases/Conditions | 29 |
| Endocrine System Disorder | 64 | Inflammatory Disease | 69 |
| Reproductive System Disease | 37 | Cancer | 101 |
| Genetic Disorder | 161 | Inflammatory Response | 46 |
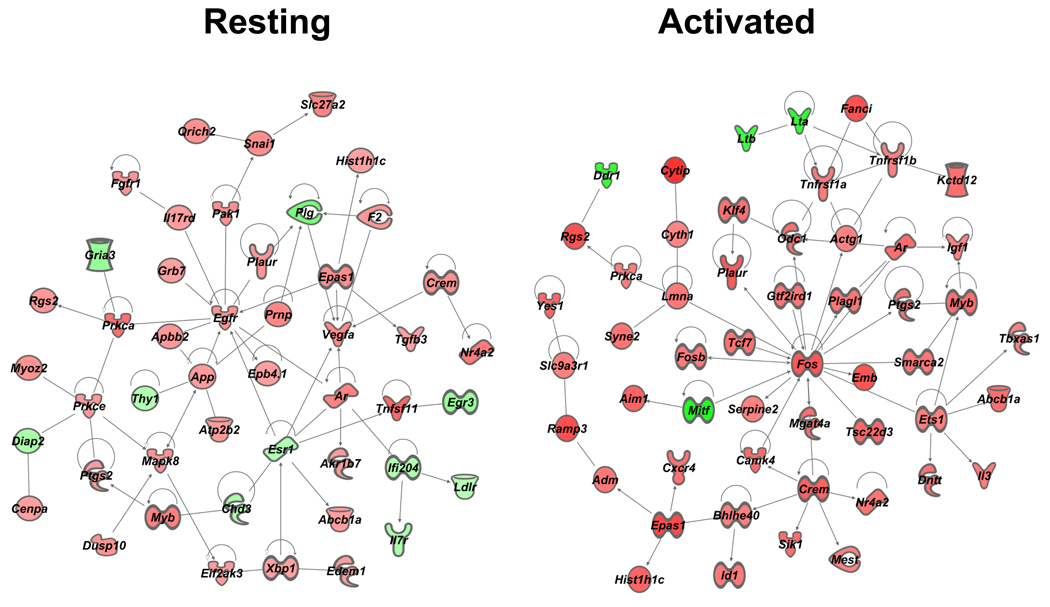
We also investigated relationships between molecules without a bias towards functional categorization. To this end, we queried the same two resting and activated “no threshold” datasets by using IPA’s Build and Connect functions to reveal networks of only direct interactions (the indirect option was deselected). One large network, termed “VIP interactome,” for each dataset emerged, and these are shown in Figure 6. A major hub of connectivity for the resting sample network was Egfr with 11 edges (direct connections), while the activated sample network showed Fos as a hub of connectivity, as it appears to play quite a significant role possessing 19 direct relationships. We conclude that VIP signaling may be altering cellular function through EGFR signaling in resting CD4 T cells, while altering immediate early genes (Fos, FosB and CREM/ICER) in activated CD4 T cells.
4. Discussion
To date, only small scale studies have been performed on the VIP transcriptome in Th2 CD4 T cells, lung, colon and synovial fibroblasts (Abad et al., 2005; Arranz et al., 2008; Hamidi et al., 2008; Sharma et al., 2006). To our knowledge, the present study represents the largest microarray survey of a VIP transcriptome in any cell type, and thus presents a comprehensive set of early VIP gene targets in a T lymphocyte, most of which are new to the neuroimmunology field. However, several VIP targets identified in this study have also been previously published, and support the present study, though the direction of change observed was not always the same. For example, VIP was shown to inhibit Cxcl10 (IP-10) expression in LPS-stimulated total splenocytes and purified CD11b macrophages (Jiang et al., 2002). In contrast, we show Cxcl10 expression to be upregulated in PMA/ionomycin activated T cells. Another example is the transcription factor, Egr3, that has been shown to be downregulated by VIP in an anti-CD3 activated T cell hybridoma (Delgado and Ganea, 2001), while we did not show this gene being modulated by VIP in PMA/ionomycin activated CD4 T cells, but rather in resting cells. These types of discrepancies may be due to “real” differences like cell types, VIP receptor expression combinations and/or activating conditions, or “manipulative” differences through cell culture conditions. Nonetheless, the agreement between these VIP target changes in gene expression lends credence to both the current study and those previously reported in the literature.
It is now well-accepted that VIP-binding sites observed on mouse and human resting CD4 T cells are due to VPAC1 receptor expression (Johnson et al., 1996). Our data supports this observation as our quantitative analysis of enriched CD4 T cells clearly revealed an enormous skewing towards VPAC1 mRNA expression dwarfing that of other known VIP binding receptors: VPAC2, PAC1 and FPRL-1, respectively (Fig. 1B). Our experimental setup was purposefully short (5 hours) to limit potential changes in VIP receptor expression levels (Vomhof-DeKrey and Dorsam, 2008; Vomhof-DeKrey et al., 2008). For these reasons, we propose that the VIP transcriptome identified by this study is indicative of the VIP/VPAC1 receptor signaling axis in both resting and activated CD4 T cells. However, we cannot rule out the contribution of alternatively spliced variants of VPAC1 (Bokaei et al., 2006), and/or unknown VIP receptors expressed by resting CD4 T cells participating in the observed VIP-regulated transcriptome.
In the late 1980’s, Ottaway et al. showed strong evidence that the presence of VIP binding sites was crucial for proper T cell homing to lymphatic compartments, most notably to mesenteric lymph nodes and Peyer’s patches (PP) (Ottaway, 1984). VIP-induced chemotaxis was shown to be sensitive to tyrosine inhibition, pertussis and cholera toxins, but insensitive to PKC inhibition. Several groups subsequently showed that purified mouse and human T cells elicited a significantly repressed chemotactic response to VIP due to T cell receptor signaling (e.g. activation) (Johnston et al., 1994). As activated CD4 T cells show significant VPAC1 mRNA downregulation (Lara-Marquez et al., 2001; Vomhof-DeKrey et al., 2008), reduced VIP-induced chemotaxis of activated T cells supports a VPAC1 signaling dependent mechanism (Johnston et al., 1994). Importantly, there exists strong anatomical evidence for a VIP/VPAC1-induced chemotactic recruitment of naïve T cells to gut lymph and PP. VIPergic nerves have been identified in proximity with specialized endothelial cells leading to postcapillary venules of PP, thus providing a neuronally-supplied, paracrine signal for blood CD4 T cells to migrate into PP (Ottaway et al., 1987). These data imply that in vivo homing of resting, unactivated CD4 T cells, in sharp contrast to activated CD4 T cells, positively respond to the VIP/VPAC1 signaling axis for proper trafficking to lymph compartments in the gut tract.
One major contribution of our IPA evaluated microarray study provides a possible molecular mechanism mediating VIP-induced homing of resting CD4 T cells. The VIP interactome in the absence of PMA/Ionomycin showed epidermal growth factor receptor (EGFR) as the most predominant hub of connectivity with 11 edges (Fig. 5). VIP addition induced mRNA expression of Egfr by 1.4 fold (range 1.3–1.6 fold; n=6) after 5 hours. This modest upregulation of Egfr mRNA coincided with the upregulation (1.3 fold; range 1.1–1.6; n=6) of Adam15, a disintegrin and metalloprotease 15 enzyme, a membrane-anchored glycoprotein that is responsible for proteolytic detachment of membrane-associated soluble EGFR ligands, and increased adhesion to integrins through its RGD domain (Charrier-Hisamuddin et al., 2008; Tanaka et al., 2004). The upregulation of Egfr and Adam15 may represent a coordinated response to VIP resulting in the elevation of EGFR signaling. A future goal will be to determine whether different VIP exposure intervals, and different VIP concentrations will result in greater, fewer or different gene expression changes. Furthermore, there is evidence for a rapid and direct increase in EGFR signaling by VIP in human breast cancer cells, as VPAC1 signaling has been shown to transactivate EGFR (HER1) and HER2 proteins within minutes of VIP addition through a cAMP/PKA/Src dependent pathway (Valdehita et al., 2009). We conclude that rapid (min) and slow (hours) elevation of EGFR signaling may mediate CD4 T cell directed movement into PP induced by neuronally supplied VIP ligand.
Additional evidence supporting VIP-regulated trafficking of resting CD4 T cells is the identification of additional signaling molecules known to be involved in EGFR signaling that are also involved in regulating cell migration. First, growth factor receptor bound (Grb) 7, a cytoplasmic adaptor molecule containing a src homology (SH) 2 domain, has been shown to be recruited to tyrosine phosphorylated EGFR dimers to enhance cell migration (Holt and Daly, 2005; Margolis et al., 1992). In fact, Grb7 has a conserved region encompassing a Ras-association (RA) and a pleckstrin homology (PH) domain that has high homology with the cell migration Mig10 protein in C. elegans (Manser et al., 1997). Second, p21 activated serine/threonine kinase 1 (Pak1), is part of the Rac/cdc42 GTPase signaling pathway, and essential for cell migration (Guo et al., 2007). The EGFR kinase inhibitor, ZD1839, blocked Pak1 activation and EGF-induced cytoskeletal rearrangements and tumor invasion in human head and neck cancer cells (Yang et al., 2004). Moreover, PAK1 also plays an essential role in neuronal guidance and cell migration in the CNS, where VIP acts as a neurotransmitter (Nikolic, 2008). Third, is the zinc finger transcriptional repressor, Snail1, whose protein expression is upregulated by EGFR activation mediated by several signaling molecules, including PAK1 (Hipp et al., 2008). PAK1 activity has been shown to alter the subcellular localization of Snail1 through phosphorylation (Yang et al., 2005). Snail1 is one of the most potent transcriptional repressors of E-Cadherin, a suppressor of tumor invasion, and the loss of this protein is a hallmark of epithelial-mesenchymal transition that assists in tumor metastasis (Birchmeier and Behrens, 1994). Fourth, the type I transmembrane amyloid precursor protein (APP), which causes neuronal plaques in Alzheimer’s disease, plays a role in cell adhesion and migration in neurons and keratinocytes, and its structure would suggest a growth factor like molecule (Sabo et al., 2001; Sheng et al., 2009). Fifth, the urokinase-plasminogen system (uPA) has been demonstrated to act at the leading edge of polarized, migrating cells and is a tumorigenic marker (Kook et al., 1994; Nusrat and Chapman, 1991). Two uPA genes were differentially modulated by VIP. The urokinase-type plasminogen activator receptor (uPAR/Plaur) was upregulated, whereas plasminogen (Plg) was downregulated. Plaur can physically bind and therefore illicit enhanced EGFR signaling (Mazzieri et al., 2006). Lastly, there were a number of other genes that encode for proteins related to cellular migration, adhesion and cytoskeletal reorganization. They were: Scrt1 (Nakakura et al., 2001) , Wwc1 (Duning et al., 2008), Unc5B (Wang et al., 2009), Palld (Goicoechea et al., 2009), Lgals (Gendronneau et al., 2008), Tubb2a (Valenzuela-Fernandez et al., 2008) and Myo1b (Tang and Ostap, 2001). In toto, we conclude that VIP induces a resting CD4 T cell program that promotes directed T cell migration (e.g. homing) through an EGFR centrally regulated signaling pathway. We further speculate that neuronally relevant genes (e.g. APP and PAK1) have been usurped by nature to regulate T cell biology by VIP/VPAC1 signaling, a well-established neuronal signaling axis. Future research will be essential to determine the regulatory controls and cellular context mediating this molecular mechanism in vivo.
VIP has been demonstrated to affect lipid metabolism by shifting the balance from adipogenesis towards lipolysis (Richter et al., 1989). Interestingly, this metabolic response may be consistent with a VIP-regulated migratory program in resting CD4 T cells. Support for this supposition is an elegant study that showed the need for eicosanoid production (PGD2) as a necessary second signal for activated neutrophils to complete their transmigration from the vasculature to the interstitial stroma (Tull et al., 2009). It would therefore be enticing to speculate that the lipid metabolism effects by VIP, as determined by our IPA analysis (Fig 4 and Fig 5), is also needed for resting T cell transmigration during its normal trafficking route from the vasculature to lymph. Elevated PGD2 production is dependent on the availability of its arachidonate (AA) precursor and enzymatic activity of prostaglandin synthetase I (COX1) and the inducible COX2 (Ptsg2) (Sandig et al., 2007). Our VIP interactome indicates an increase in COX2/Ptsg2 message that might provide the necessary elevated enzyme activity needed for diverting AA release towards production of prostaglandins, a necessary signal for transmigration of activated neutrophils. A plausible explanation for how VIP might be shifting the balance towards lipid peroxidation and prostaglandin production is through vascular endothelial growth factor (Vegfa), previously shown to be upregulated by VIP and supported by this study, which binds to the VEGF receptor, and upregulates COX2 expression in certain cell types (Soltau and Drevs, 2009) (http://www.genome.jp/kegg/pathway/hsa/hsa04370.html). In contrast, VIP has been shown to downregulate COX2 expression in myeloid cells (macrophages), microglial cells and dendritic cells (Gonzalez-Rey and Delgado, 2008). Currently, these differences in VIP-mediated modulation of COX2 are unexplained, but may be due to cell-context dependent regulation of COX2 by the VIP signaling axis.
Treatment of CD4 T cells with PMA/ionomycin mimics TCR signal transduction that leads to an elevation of Il- 2 and a proliferative, “activated” cellular program (Grier and Mastro, 1986). Significant alterations in the transcriptional profile occur during T cell activation, including increases in the transcriptional rate of certain Jun and Fos proteins that dimerize to form the immediate early transcription factor called AP-1 (Crabtree and Clipstone, 1994). These early changes in gene expression are necessary to induce the proliferative phenotype observed during TCR signaling. However, IL-2 expression and subsequent T cell proliferation can be blocked by an antagonistic signal transduction pathway. This opposing pathway elevates the intracellular second messenger, cAMP ([cAMP]i), resulting in protein kinase A (PKA) activation, and leading to a “reshuffling” of the AP-1 composition due to subtle changes in Jun and Fos expression (Tamir et al., 1996). It would therefore follow that VIP treatment of CD4 T cells would lead to the activation of the cAMP/PKA pathway as observed for other cAMP inducing agonists. To support this supposition, our data shows increased Fos expression, coupled with a 30% increase in FosB expression. These data are supported by a study showing cell-permeable cAMP (dbcAMP) elevating Fos and FosB expression over that observed by activating conditions alone (Tamir et al., 1996). In contrast, these studies also showed differential changes in Jun (decrease) and JunB (increase) due to co-treatment with dbcAMP and TPA/ionomycin, but were measured at 1–3 hour versus our 5 hour time point. Such early changes in Jun and Fos expression by VIP have been reported by Wang et al. that demonstrated similar changes between 1–3 hours using primary CD4 T cells. However, this same group did not measure a change in FosB expression due to VIP treatment (Wang et al., 2000). Such discrepancies in Jun and Fos expression due to cAMP inducing agents (VIP or dbcAMP) are most likely a result of temporal differences and/or the use of T cell lines versus primary CD4 T cells isolated from different mouse strains (C57Bl/6 or Balb/c). Nonetheless, modest changes of 30% in Fos and FosB by VIP during T cell activation can indeed cause drastic downstream phenotypic changes in proliferation and differentiation. The precedent for this suggestion is supported by subtle changes in Sox2 gene expression (e.g. 50%), which can cause embryonic stem cells to proliferate and differentiate into linage restricted phenotypes, whereas greater than 2 fold changes in Sox2 induces apoptosis (Kopp et al., 2008). This phenomenon of small molar mass changes bringing about significant cellular responses is well known in the transcription field and has been coined squelching (Cahill et al., 1994).
In addition to Fos and FosB, VIP co-treatment with PMA/ionomycin changed the expression levels of an additional 13 transcription factors with most participating in direct interactions with Fos (Fig. 6). One of these genes is called cAMP responsive element modulator (CREM/ICER), is upregulated by VIP irrespective of the presence of T cell activation, and further supports VIP induction of gene expression changes due to the activation of the cAMP/PKA pathway. Interestingly, VIP upregulates two additional Crem isoforms during T cell activation. These Crem isoforms, measured only during T cell activation, are due to an alternative promoter induced by increases in [cAMP]i that produces shorter Crem transcripts called inducible cAMP early repressor (ICER). ICER potently blocks IL-2 expression and T cell proliferation by competing with basic helix loop helix transcription factors (AP-1 and CREB) at TRE responsive elements in the IL-2 promoter (and other mitogenic promoters), thus uncoupling crucial stimulatory transcriptional cassettes such as NFAT, NF-kB and CBP/p300 from physical contact with RNA Polymerase II (Masquilier and Sassone-Corsi, 1992). Ganea’s group has also observed an elevation in ICER with VIP treatment using activated CD4 T cells, and thus supports our present finding (unpublished data from (Wang et al., 2000)).
Over the past decade, VIP has been clearly demonstrated to protect against several animal disease models that show pathological similarities to human inflammatory and autoimmune disease, including collagen induced arthritis (Delgado et al., 2001), inflammatory bowel disease (Abad et al., 2003), uveoretinitis (Keino et al., 2004) and experimental autoimmune encephalomyelitis(Gonzalez-Rey et al., 2006). This protective effect appears to be due to a shift from a Th1 to a Th2 immune response regulated by VIP, which has been substantiated by several laboratories through both in vitro and in vivo studies. One major cause for VIP-regulated protection is the tremendous induction of CD4+/CD25+/FoxP3+ regulatory T cells (Treg) from peripheral CD4+/CD25−/FoxP3− T cells, which dampens both IL-2 production and Th1 cell mediated immunity (Chen et al., 2008) . In 2007, Bodor et al. showed that the transcriptional repressor ICER is pivotal in suppression of T cell proliferation and activation by silencing IL-2 expression (Bodor et al., 2007). The molecular mechanism of VIP immune protection and Treg differentiation may be due to its ability to alter early mitogenic transcriptional programs (ICER, c-Fos and FosB) capable of blocking cell-mediated immunity, while promoting a Th2 polarization in certain settings. TGF-β induces Treg generation and our data shows TGF- β3 being upregulated in resting, but not activated T cells (Shah and Qiao, 2008). This may present an intriguing possibility that the effect on T cells by VIP is dependent on previous temporal exposures. Thus, CD4 T cells in lamina propria, for example, that are bathed in high levels of VIP (Said and Mutt, 1970), may elevate their threshold for T cell activation, and exhibit tolerance to self antigens, through their ability to induce differentiation into ICER expressing Treg cells.
In this study, we have identified two, nearly unique, VIP-mediated transcriptomes in resting versus activated CD4 T cells. These data strongly support the conclusion that CD4 T cells respond differently to VIP based on their activation status. We propose that resting CD4 T cells mediate directed cell movement orchestrated by EGFR signaling. During T cell activation, an immediate early transcriptional program appears to be initiated by VIP, a cAMP-inducing agent, involving a subtle change in AP-1 composition and ICER expression, among others, that is a driving force for immune protection against certain inflammatory disorders. These gene expression changes are also suggested to explain at a molecular level how VIP, presumably acting through VPAC1, can allow for naïve cell homing to the gut, while inducing an altered differentiation program during T cell activation of Treg effector cells. Additional studies will be crucial to validate these phenotypic cellular changes in vivo, which is a major future goal of our research effort.
Supplementary Material
Acknowledgements
This study, and the use of the Core Biology Facility, was made possible by a K01 Career Award DK064828 to GPD, and NIH grant 2P20 RR015566 from the National Center for Research Resources. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. We wish to thank the Marshall University Genomics Core for completion of microarray experiments. The Genomics Core is supported by NCRR COBRE grant (5P20RR020180) and WV-INBRE grant (5P20RR016477).
Abbreviations
- GPCR
G-protein coupled receptor
- VIP
Vasoactive Intestinal Peptide
- VPAC1
Vasoactive Intestinal Peptide Receptor 1
- CNS
central nervous system
- PP
Peyer’s Patches
- SAM
Significance Analysis of Microarrays
- IPA
Ingenuity Pathway Analysis
- Treg
regulatory T cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abad C, Juarranz Y, Martinez C, Arranz A, Rosignoli F, Garcia-Gomez M, Leceta J, Gomariz RP. cDNA array analysis of cytokines, chemokines, and receptors involved in the development of TNBS-induced colitis: homeostatic role of VIP. Inflamm Bowel Dis. 2005;11:674–684. doi: 10.1097/01.mib.0000171872.70738.58. [DOI] [PubMed] [Google Scholar]
- Abad C, Martinez C, Juarranz MG, Arranz A, Leceta J, Delgado M, Gomariz RP. Therapeutic effects of vasoactive intestinal peptide in the trinitrobenzene sulfonic acid mice model of Crohn's disease. Gastroenterology. 2003;124:961–971. doi: 10.1053/gast.2003.50141. [DOI] [PubMed] [Google Scholar]
- Altman A, Mally MI, Isakov N. Phorbol ester synergizes with Ca2+ ionophore in activation of protein kinase C (PKC)alpha and PKC beta isoenzymes in human T cells and in induction of related cellular functions. Immunology. 1992;76:465–471. [PMC free article] [PubMed] [Google Scholar]
- Arranz A, Gutierrez-Canas I, Carrion M, Juarranz Y, Pablos JL, Martinez C, Gomariz RP. VIP reverses the expression profiling of TLR4-stimulated signaling pathway in rheumatoid arthritis synovial fibroblasts. Mol Immunol. 2008;45:3065–3073. doi: 10.1016/j.molimm.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Ben-Horin S, Chowers Y. Neuroimmunology of the gut: physiology, pathology, and pharmacology. Curr Opin Pharmacol. 2008;8:490–495. doi: 10.1016/j.coph.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Benton KD, Hermann RJ, Vomhof-Dekrey EE, Haring JS, Van der Steen T, Smith J, Dovat S, Dorsam GP. A transcriptionally permissive epigenetic landscape at the vasoactive intestinal peptide receptor-1 promoter suggests a euchromatin nuclear position in murine CD4 T cells. Regul Pept. 2009 doi: 10.1016/j.regpep.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchmeier W, Behrens J. Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta. 1994;1198:11–26. doi: 10.1016/0304-419x(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Bodor J, Fehervari Z, Diamond B, Sakaguchi S. ICER/CREM-mediated transcriptional attenuation of IL-2 and its role in suppression by regulatory T cells. Eur J Immunol. 2007;37:884–895. doi: 10.1002/eji.200636510. [DOI] [PubMed] [Google Scholar]
- Bokaei PB, Ma XZ, Byczynski B, Keller J, Sakac D, Fahim S, Branch DR. Identification and characterization of five-transmembrane isoforms of human vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide receptors. Genomics. 2006;88:791–800. doi: 10.1016/j.ygeno.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Buhrer C, Berlin C, Thiele HG, Hamann A. Lymphocyte activation and expression of the human leucocyte-endothelial cell adhesion molecule 1 (Leu-8/TQ1 antigen) Immunology. 1990;71:442–448. [PMC free article] [PubMed] [Google Scholar]
- Cahill MA, Ernst WH, Janknecht R, Nordheim A. Regulatory squelching. FEBS Lett. 1994;344:105–108. doi: 10.1016/0014-5793(94)00320-3. [DOI] [PubMed] [Google Scholar]
- Calvano SE, Xiao W, Richards DR, Felciano RM, Baker HV, Cho RJ, Chen RO, Brownstein BH, Cobb JP, Tschoeke SK, Miller-Graziano C, Moldawer LL, Mindrinos MN, Davis RW, Tompkins RG, Lowry SF. A network-based analysis of systemic inflammation in humans. Nature. 2005;437:1032–1037. doi: 10.1038/nature03985. [DOI] [PubMed] [Google Scholar]
- Charrier-Hisamuddin L, Laboisse CL, Merlin D. ADAM-15: a metalloprotease that mediates inflammation. FASEB J. 2008;22:641–653. doi: 10.1096/fj.07-8876rev. [DOI] [PubMed] [Google Scholar]
- Chen G, Hao J, Xi Y, Wang W, Wang Z, Li N, Li W. The therapeutic effect of vasoactive intestinal peptide on experimental arthritis is associated with CD4+CD25+ T regulatory cells. Scand J Immunol. 2008;68:572–578. doi: 10.1111/j.1365-3083.2008.02178.x. [DOI] [PubMed] [Google Scholar]
- Crabtree GR, Clipstone NA. Signal transmission between the plasma membrane and nucleus of T lymphocytes. Annu Rev Biochem. 1994;63:1045–1083. doi: 10.1146/annurev.bi.63.070194.005145. [DOI] [PubMed] [Google Scholar]
- Dautzenberg FM, Mevenkamp G, Wille S, Hauger RL. N-terminal splice variants of the type I PACAP receptor: isolation, characterization and ligand binding/selectivity determinants. J Neuroendocrinol. 1999;11:941–949. doi: 10.1046/j.1365-2826.1999.00411.x. [DOI] [PubMed] [Google Scholar]
- Delgado M, Abad C, Martinez C, Leceta J, Gomariz RP. Vasoactive intestinal peptide prevents experimental arthritis by downregulating both autoimmune and inflammatory components of the disease. Nat Med. 2001;7:563–568. doi: 10.1038/87887. [DOI] [PubMed] [Google Scholar]
- Delgado M, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit expression of Fas ligand in activated T lymphocytes by regulating c-Myc, NF-kappa B, NF-AT, and early growth factors 2/3. J Immunol. 2001;166:1028–1040. doi: 10.4049/jimmunol.166.2.1028. [DOI] [PubMed] [Google Scholar]
- Delgado M, Martinez C, Johnson MC, Gomariz RP, Ganea D. Differential expression of vasoactive intestinal peptide receptors 1 and 2 (VIP-R1 and VIP-R2) mRNA in murine lymphocytes. J Neuroimmunol. 1996;68:27–38. doi: 10.1016/0165-5728(96)00063-x. [DOI] [PubMed] [Google Scholar]
- Delgado M, Pozo D, Ganea D. The significance of vasoactive intestinal peptide in immunomodulation. Pharmacol Rev. 2004;56:249–290. doi: 10.1124/pr.56.2.7. [DOI] [PubMed] [Google Scholar]
- Duning K, Schurek EM, Schluter M, Bayer M, Reinhardt HC, Schwab A, Schaefer L, Benzing T, Schermer B, Saleem MA, Huber TB, Bachmann S, Kremerskothen J, Weide T, Pavenstadt H. KIBRA modulates directional migration of podocytes. J Am Soc Nephrol. 2008;19:1891–1903. doi: 10.1681/ASN.2007080916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Zein N, Badran B, Sariban E. VIP differentially activates beta2 integrins, CR1, and matrix metalloproteinase-9 in human monocytes through cAMP/PKA, EPAC, and PI-3K signaling pathways via VIP receptor type 1 and FPRL1. J Leukoc Biol. 2008;83:972–981. doi: 10.1189/jlb.0507327. [DOI] [PubMed] [Google Scholar]
- Gendronneau G, Sidhu SS, Delacour D, Dang T, Calonne C, Houzelstein D, Magnaldo T, Poirier F. Galectin-7 in the control of epidermal homeostasis after injury. Mol Biol Cell. 2008;19:5541–5549. doi: 10.1091/mbc.E08-02-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goicoechea SM, Bednarski B, Garcia-Mata R, Prentice-Dunn H, Kim HJ, Otey CA. Palladin contributes to invasive motility in human breast cancer cells. Oncogene. 2009;28:587–598. doi: 10.1038/onc.2008.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Delgado M. Vasoactive intestinal peptide inhibits cyclooxygenase-2 expression in activated macrophages, microglia, and dendritic cells. Brain Behav Immun. 2008;22:35–41. doi: 10.1016/j.bbi.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Fernandez-Martin A, Chorny A, Martin J, Pozo D, Ganea D, Delgado M. Therapeutic effect of vasoactive intestinal peptide on experimental autoimmune encephalomyelitis: down-regulation of inflammatory and autoimmune responses. Am J Pathol. 2006;168:1179–1188. doi: 10.2353/ajpath.2006.051081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grier CE, 3rd, Mastro AM. Phorbol ester circumvents the need for macrophages as well as for mitogenic lectins in the stimulation of lymphocytes with wheat germ agglutinin or the calcium ionophores A23187 or ionomycin. J Leukoc Biol. 1986;40:511–523. doi: 10.1002/jlb.40.5.511. [DOI] [PubMed] [Google Scholar]
- Guo D, Tan YC, Wang D, Madhusoodanan KS, Zheng Y, Maack T, Zhang JJ, Huang XY. A Rac-cGMP signaling pathway. Cell. 2007;128:341–355. doi: 10.1016/j.cell.2006.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidi SA, Prabhakar S, Said SI. Enhancement of pulmonary vascular remodelling and inflammatory genes with VIP gene deletion. Eur Respir J. 2008;31:135–139. doi: 10.1183/09031936.00105807. [DOI] [PubMed] [Google Scholar]
- Hipp S, Walch A, Schuster T, Losko S, Laux H, Bolton T, Hofler H, Becker KF. Activation of epidermal growth factor receptor results in Snail protein but not mRNA over-expression in endometrial cancer. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt LJ, Daly RJ. Adapter protein connections: the MRL and Grb7 protein families. Growth Factors. 2005;23:193–201. doi: 10.1080/08977190500196267. [DOI] [PubMed] [Google Scholar]
- Jiang X, Jing H, Ganea D. VIP and PACAP down-regulate CXCL10 (IP-10) and up-regulate CCL22 (MDC) in spleen cells. J Neuroimmunol. 2002;133:81–94. doi: 10.1016/s0165-5728(02)00365-x. [DOI] [PubMed] [Google Scholar]
- Johnson MC, McCormack RJ, Delgado M, Martinez C, Ganea D. Murine T-lymphocytes express vasoactive intestinal peptide receptor 1 (VIP-R1) mRNA. J Neuroimmunol. 1996;68:109–119. doi: 10.1016/0165-5728(96)00085-9. [DOI] [PubMed] [Google Scholar]
- Johnston JA, Taub DD, Lloyd AR, Conlon K, Oppenheim JJ, Kevlin DJ. Human T lymphocyte chemotaxis and adhesion induced by vasoactive intestinal peptide. J Immunol. 1994;153:1762–1768. [PubMed] [Google Scholar]
- Keino H, Kezuka T, Takeuchi M, Yamakawa N, Hattori T, Usui M. Prevention of experimental autoimmune uveoretinitis by vasoactive intestinal peptide. Arch Ophthalmol. 2004;122:1179–1184. doi: 10.1001/archopht.122.8.1179. [DOI] [PubMed] [Google Scholar]
- Kook YH, Adamski J, Zelent A, Ossowski L. The effect of antisense inhibition of urokinase receptor in human squamous cell carcinoma on malignancy. EMBO J. 1994;13:3983–3991. doi: 10.1002/j.1460-2075.1994.tb06714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp JL, Ormsbee BD, Desler M, Rizzino A. Small increases in the level of Sox2 trigger the differentiation of mouse embryonic stem cells. Stem Cells. 2008;26:903–911. doi: 10.1634/stemcells.2007-0951. [DOI] [PubMed] [Google Scholar]
- Lara-Marquez M, O'Dorisio M, O'Dorisio T, Shah M, Karacay B. Selective gene expression and activation-dependent regulation of vasoactive intestinal peptide receptor type 1 and type 2 in human T cells. J Immunol. 2001;166:2522–2530. doi: 10.4049/jimmunol.166.4.2522. [DOI] [PubMed] [Google Scholar]
- Li H, Mei Y, Wang Y, Xu L. Vasoactive intestinal polypeptide suppressed experimental autoimmune encephalomyelitis by inhibiting T helper 1 responses. J Clin Immunol. 2006;26:430–437. doi: 10.1007/s10875-006-9042-2. [DOI] [PubMed] [Google Scholar]
- Manser J, Roonprapunt C, Margolis B. C. elegans cell migration gene mig-10 shares similarities with a family of SH2 domain proteins and acts cell nonautonomously in excretory canal development. Dev Biol. 1997;184:150–164. doi: 10.1006/dbio.1997.8516. [DOI] [PubMed] [Google Scholar]
- Margolis B, Silvennoinen O, Comoglio F, Roonprapunt C, Skolnik E, Ullrich A, Schlessinger J. High-efficiency expression/cloning of epidermal growth factor-receptor-binding proteins with Src homology 2 domains. Proc Natl Acad Sci U S A. 1992;89:8894–8898. doi: 10.1073/pnas.89.19.8894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masquilier D, Sassone-Corsi P. Transcriptional cross-talk: nuclear factors CREM and CREB bind to AP-1 sites and inhibit activation by Jun. J Biol Chem. 1992;267:22460–22466. [PubMed] [Google Scholar]
- Mazzieri R, D'Alessio S, Kenmoe RK, Ossowski L, Blasi F. An uncleavable uPAR mutant allows dissection of signaling pathways in uPA-dependent cell migration. Mol Biol Cell. 2006;17:367–378. doi: 10.1091/mbc.E05-07-0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakakura EK, Watkins DN, Schuebel KE, Sriuranpong V, Borges MW, Nelkin BD, Ball DW. Mammalian Scratch: a neural-specific Snail family transcriptional repressor. Proc Natl Acad Sci U S A. 2001;98:4010–4015. doi: 10.1073/pnas.051014098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman R, Cuan N, Hampartzoumian T, Connor SJ, Lloyd AR, Grimm MC. Vasoactive intestinal peptide impairs leucocyte migration but fails to modify experimental murine colitis. Clin Exp Immunol. 2005;139:411–420. doi: 10.1111/j.1365-2249.2005.02673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolic M. The Pak1 kinase: an important regulator of neuronal morphology and function in the developing forebrain. Mol Neurobiol. 2008;37:187–202. doi: 10.1007/s12035-008-8032-1. [DOI] [PubMed] [Google Scholar]
- Nusrat AR, Chapman HA., Jr An autocrine role for urokinase in phorbol ester-mediated differentiation of myeloid cell lines. J Clin Invest. 1991;87:1091–1097. doi: 10.1172/JCI115070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaway CA. In vitro alteration of receptors for vasoactive intestinal peptide changes the in vivo localization of mouse T cells. J Exp Med. 1984;160:1054–1069. doi: 10.1084/jem.160.4.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaway CA. Selective effects of vasoactive intestinal peptide on the mitogenic response of murine T cells. Immunology. 1987;62:291–297. [PMC free article] [PubMed] [Google Scholar]
- Ottaway CA, Lewis DL, Asa SL. Vasoactive intestinal peptide-containing nerves in Peyer's patches. Brain Behav Immun. 1987;1:148–158. doi: 10.1016/0889-1591(87)90017-1. [DOI] [PubMed] [Google Scholar]
- Pozo D, Anderson P, Gonzalez-Rey E. Induction of Alloantigen-specific human T regulatory Cells by vasoactive intestinal peptide. J. Immunol. 2009;183:4346–4359. doi: 10.4049/jimmunol.0900400. [DOI] [PubMed] [Google Scholar]
- Richter WO, Robl H, Schwandt P. Human glucagon and vasoactive intestinal polypeptide (VIP) stimulate free fatty acid release from human adipose tissue in vitro. Peptides. 1989;10:333–335. doi: 10.1016/0196-9781(89)90039-9. [DOI] [PubMed] [Google Scholar]
- Sabo SL, Ikin AF, Buxbaum JD, Greengard P. The Alzheimer amyloid precursor protein (APP) and FE65, an APP-binding protein, regulate cell movement. J Cell Biol. 2001;153:1403–1414. doi: 10.1083/jcb.153.7.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed AI, Bhagabati NK, Braisted JC, Liang W, Sharov V, Howe EA, Li J, Thiagarajan M, White JA, Quackenbush J. TM4 microarray software suite. Methods Enzymol. 2006;411:134–193. doi: 10.1016/S0076-6879(06)11009-5. [DOI] [PubMed] [Google Scholar]
- Said SI, Mutt V. Polypeptide with broad biological activity: isolation from small intestine. Science. 1970;169:1217–1218. doi: 10.1126/science.169.3951.1217. [DOI] [PubMed] [Google Scholar]
- Sandig H, Pease JE, Sabroe I. Contrary prostaglandins: the opposing roles of PGD2 and its metabolites in leukocyte function. J Leukoc Biol. 2007;81:372–382. doi: 10.1189/jlb.0706424. [DOI] [PubMed] [Google Scholar]
- Shah S, Qiao L. Resting B cells expand a CD4+CD25+Foxp3+ Treg population via TGF-beta3. Eur J Immunol. 2008;38:2488–2498. doi: 10.1002/eji.200838201. [DOI] [PubMed] [Google Scholar]
- Sharma V, Delgado M, Ganea D. Granzyme B, a new player in activation-induced cell death, is down-regulated by vasoactive intestinal peptide in Th2 but not Th1 effectors. J Immunol. 2006;176:97–110. doi: 10.4049/jimmunol.176.1.97. [DOI] [PubMed] [Google Scholar]
- Sheng B, Song B, Zheng Z, Zhou F, Lu G, Zhao N, Zhang X, Gong Y. Abnormal cleavage of APP impairs its functions in cell adhesion and migration. Neurosci Lett. 2009;450:327–331. doi: 10.1016/j.neulet.2008.11.046. [DOI] [PubMed] [Google Scholar]
- Soltau J, Drevs J. Mode of action and clinical impact of VEGF signaling inhibitors. Expert Rev Anticancer Ther. 2009;9:649–662. doi: 10.1586/era.09.19. [DOI] [PubMed] [Google Scholar]
- Tamir A, Granot Y, Isakov N. Inhibition of T lymphocyte activation by cAMP is associated with down-regulation of two parallel mitogen-activated protein kinase pathways, the extracellular signal-related kinase and c-Jun N-terminal kinase. J Immunol. 1996;157:1514–1522. [PubMed] [Google Scholar]
- Tanaka M, Nanba D, Mori S, Shiba F, Ishiguro H, Yoshino K, Matsuura N, Higashiyama S. ADAM binding protein Eve-1 is required for ectodomain shedding of epidermal growth factor receptor ligands. J Biol Chem. 2004;279:41950–41959. doi: 10.1074/jbc.M400086200. [DOI] [PubMed] [Google Scholar]
- Tang N, Ostap EM. Motor domain-dependent localization of myo1b (myr-1) Curr Biol. 2001;11:1131–1135. doi: 10.1016/s0960-9822(01)00320-7. [DOI] [PubMed] [Google Scholar]
- Truneh A, Albert F, Golstein P, Schmitt-Verhulst AM. Early steps of lymphocyte activation bypassed by synergy between calcium ionophores and phorbol ester. Nature. 1985;313:318–320. doi: 10.1038/313318a0. [DOI] [PubMed] [Google Scholar]
- Tull SP, Yates CM, Maskrey BH, O'Donnell VB, Madden J, Grimble RF, Calder PC, Nash GB, Rainger GE. Omega-3 Fatty acids and inflammation: novel interactions reveal a new step in neutrophil recruitment. PLoS Biol. 2009;7:e1000177. doi: 10.1371/journal.pbio.1000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdehita A, Bajo AM, Schally AV, Varga JL, Carmena MJ, Prieto JC. Vasoactive intestinal peptide (VIP) induces transactivation of EGFR and HER2 in human breast cancer cells. Mol Cell Endocrinol. 2009;302:41–48. doi: 10.1016/j.mce.2008.11.024. [DOI] [PubMed] [Google Scholar]
- Valenzuela-Fernandez A, Cabrero JR, Serrador JM, Sanchez-Madrid F. HDAC6: a key regulator of cytoskeleton, cell migration and cell-cell interactions. Trends Cell Biol. 2008;18:291–297. doi: 10.1016/j.tcb.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Voice J, Donnelly S, Dorsam G, Dolganov G, Paul S, Goetzl EJ. c-Maf and JunB mediation of Th2 differentiation induced by the type 2 G protein-coupled receptor (VPAC2) for vasoactive intestinal peptide. J Immunol. 2004;172:7289–7296. doi: 10.4049/jimmunol.172.12.7289. [DOI] [PubMed] [Google Scholar]
- Vomhof-DeKrey EE, Dorsam GP. Stimulatory and suppressive signal transduction regulates vasoactive intestinal peptide receptor-1 (VPAC-1) in primary mouse CD4 T cells. Brain Behav Immun. 2008;22:1024–1031. doi: 10.1016/j.bbi.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vomhof-DeKrey EE, Hermann RJ, Palmer MF, Benton KD, Sandy AR, Dorsam ST, Dorsam GP. TCR signaling and environment affect vasoactive intestinal peptide receptor-1 (VPAC-1) expression in primary mouse CD4 T cells. Brain Behav Immun. 2008;22:1032–1040. doi: 10.1016/j.bbi.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HY, Jiang XM, Ganea D. The neuropeptides VIP and PACAP inhibit IL-2 transcription by decreasing c-Jun and increasing JunB expression in T cells. J Neuroimmunol. 2000;104:68–78. doi: 10.1016/s0165-5728(99)00244-1. [DOI] [PubMed] [Google Scholar]
- Wang W, Reeves WB, Ramesh G. Netrin-1 increases proliferation and migration of renal proximal tubular epithelial cells via the UNC5B receptor. Am J Physiol Renal Physiol. 2009;296:F723–F729. doi: 10.1152/ajprenal.90686.2008. [DOI] [PubMed] [Google Scholar]
- Yang Z, Bagheri-Yarmand R, Wang RA, Adam L, Papadimitrakopoulou VV, Clayman GL, El-Naggar A, Lotan R, Barnes CJ, Hong WK, Kumar R. The epidermal growth factor receptor tyrosine kinase inhibitor ZD1839 (Iressa) suppresses c-Src and Pak1 pathways and invasiveness of human cancer cells. Clin Cancer Res. 2004;10:658–667. doi: 10.1158/1078-0432.ccr-0382-03. [DOI] [PubMed] [Google Scholar]
- Yang Z, Rayala S, Nguyen D, Vadlamudi RK, Chen S, Kumar R. Pak1 phosphorylation of snail, a master regulator of epithelial-to-mesenchyme transition, modulates snail's subcellular localization and functions. Cancer Res. 2005;65:3179–3184. doi: 10.1158/0008-5472.CAN-04-3480. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.