Abstract
Neuronal plasticity that develops in the cortex during learning is assumed to represent memory content, but the functions of such plasticity are actually unknown. The shift in spectral tuning in primary auditory cortex (A1) to the frequency of a tone signal is a compelling candidate for a substrate of memory because it has all of the cardinal attributes of associative memory: associativity, specificity, rapid induction, consolidation, and long-term retention. Tuning shifts increase the representational area of the signal in A1, as an increasing function of performance level, suggesting that area encodes the magnitude of acquired stimulus significance. The present study addresses the question of the specific function of learning-induced associative representational plasticity. We tested the hypothesis that specific increases in A1 representational area for an auditory signal serve the mnemonic function of enhancing memory strength for that signal. Rats were trained to bar-press for reward contingent on the presence of a signal tone (5.0 kHz), and assessed for memory strength during extinction. The amount of representational area gain for the signal frequency band was significantly positively correlated with resistance to extinction to the signal frequency in two studies that spanned the range of task difficulty. These findings indicate that specific gain in cortical representational area underlies the strength of the behaviorally-relevant contents of memory. Thus, mnemonic functions of cortical plasticity are determinable.
Keywords: associative learning, extinction, neurophysiology, plasticity, primary auditory cortex
A central tenet of neuroscience is that neuronal plasticity underlies learning and memory. Memories have specific content, i.e., they are stored representations of experiences, whether biographical (“episodic”), general knowledge (“semantic”), or other forms. Much research has focused on the detection of neuronal plasticity using a wide range of recording and other approaches across the levels at which brains are organized: molecular, cellular, circuit, systems, and larger scale. Neuronal plasticity that develops during learning is generally assumed to constitute memory content. However, although the function of plasticity in a general sense is assumed to underlie memory and support adaptive behavior, the detection of plasticity in a given structure under a given learning situation does not actually reveal its particular function. For example, although learning-related plasticity could represent the detailed content of acquired experience itself, there are several alternatives. These include a change in neuronal state that enables storage, modulatory processes that influence the strength of memory, and modifications of the interactions of different memory systems. Moreover, the amount of plasticity could subserve different functions. Thus, a greater number of cells that develop plasticity within a structure might increase future detectability of a signal stimulus in a noisy environment, serve to increase the amount of stored detail about the relevant experience, facilitate future switching of attention to a particular feature of that experience, etc.
Learning-related plasticity becomes a good candidate to represent memory content if it can be shown to have the major attributes of memory. Learning-induced changes in frequency tuning within the primary auditory cortex (A1) meet this criterion. Thus, learning that a tone signals reinforcement (classical conditioning) or the opportunity to obtain a reward or avoid a punishment (instrumental conditioning) is accompanied by shifts in frequency receptive fields from the original best frequency (BF) (i.e., peak of the tuning curve) toward or to the frequency of the tonal signal (1, 2). Such tuning shifts are not only associative (i.e., require that the tone predict reinforcers), but have other cardinal attributes of associative memory. Tuning shifts are highly specific (shifts either to or within fractions of an octave of the signal’s frequency) (3), are rapidly formed (in as few as five trials) (4, 5), consolidate over hours and days (become larger over time without additional training) (6, 7), and endure for months (tracked to at least 8 weeks) (8). Moreover, specific tuning shifts develop across different types of learning (classical and instrumental conditioning) (1, 2, 9), different types of motivation (appetitive and aversive) (1, 10, 11), and tasks (simple and two-tone discrimination training) (7). Moreover, they develop similarly across species (e.g., bat, cat, gerbil, guinea pig, monkey, and rat) (2, 4, 12 –16), including humans (17, 18). Local tuning shifts extended across A1 produce a specific increase in the area of frequency representation of the signal frequency band within the tonotopic organization of A1 (11, 14, 15, 19). These specific receptive field shifts and map expansions are examples of “high order (cortical) associative representational plasticity” (HARP) because they constitute orderly changes in the processing of environmental stimuli within the representation of a sensory dimension.
The establishment of HARP provides an opportunity to determine the functions of learning-related cortical plasticity. A cardinal feature of all memories is the strength of their content, whether fragile or robust. Thus, one function of plasticity might be to provide for memory strength. Rutkowski and Weinberger (15) found that the greater the behavioral importance of a tone, the greater the amount of expansion of its representation in the tonotopic map of A1. Memories that have increased behavioral significance might reasonably be expected to have greater strength. If so, then a learning-induced increase in the amount of representational area in A1 might confer greater memory strength. We tested this hypothesis by determining the relationship between the amount of representational area and resistance to experimental extinction.
Results
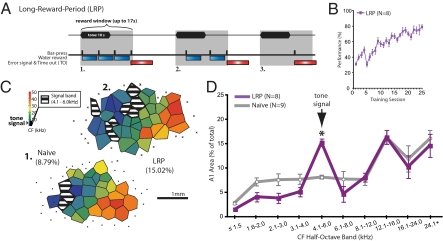
Adult male Sprague–Dawley rats (n = 8) were trained to bar-press (BP) for water reward beginning at the onset of a 10-s signal tone (5.0 kHz) and a 7-s period of silence after tone offset (17-s reward period) (Fig. 1A). BPs during subsequent silent intertrial intervals (ITI) were discouraged by eliciting a flashing light error signal and time-out on 50% of occurrences. Failure to respond at least once during the tone precluded the opportunity to BP for reward during the 7 s after tone offset (Fig. 1A, example 3). This long-reward-period (LRP) protocol was designed to promote a behavioral pattern in which subjects BP starting at tone onset but ignore tone offset. The use of this learning strategy produces reliable expanded representation of the tone signal frequency band in A1 (19).
Fig. 1.
Bar-pressing for water rewards to tones in the long-reward-window (LRP) group. Behavior and representational area in A1. (A) The LRP protocol required animals to BP for rewards during a 17-s reward window that began at tone onset. Up to two water rewards (blue bars) could be delivered during the tone with the possibility of one more reward in the remaining 17-s window (examples 1 and 2) for a maximum of three rewards per trial. If no BPs were made during the tone, then BPs after the tone were not rewarded (example 3). BPs during silent intertrial-intervals were signaled as errors with a flashing light during a time-out period (red bars) 50% of the time (i.e., 3–7 s extension of time until the next trial). (B) LRP group performance improved across sessions (asymptote = 76.2 ± 2.3%; days 1–17, n = 8; day 18–19, n = 7; day 20–21, n = 6; day 22–25, n = 5). (C) Exemplar maps of characteristic frequency (CF) from naïve (1) and an LRP subjects (2) show a gain of signal area in the trained animal. Striped polygons show the area of representation of the signal-frequency within a half-octave band and values show the percent of total area for the signal band. (D) The relative amount (percent) of representational area occupied by half octave bands across the tonotopic map of A1 in the LRP and naïve groups. Note that the only significant gain in area was at the signal-frequency band in the LRP group (5.0 kHz ±0.25 octaves; 4.1–6.0 kHz) (asterisk).
Group performance improved across training sessions [F (24,199) = 27.37; P < 0.0001], to an asymptotic level of 76.2% after 24.8 sessions (Fig. 1B). On the day after individual attainment of asymptote, subjects underwent an extinction-test session. All subjects exhibited a decrement of BP responses during extinction, enabling a comparison of the strength of memory with changes in cortical areas of frequency representation.
The LRP group displayed a significant increase in representational area. This proved to be highly specific to the signal frequency (e.g., exemplar LRP subject; Fig. 1C). The spread of representational signal-specific area ranged from 7.89% to 21.70% of the total area of A1, permitting a within-group correlational analysis of area gain and the strength of memory (below).
The mean area for the signal frequency of 5.0 kHz (±0.25 octaves, “signal band” = 4.1–6.0 kHz) was 15.00% (±1.61) of the total area of A1. This was a >2-fold increase in area compared to a group of naïve (untrained, n = 9) animals for which the identical frequency range constituted only 7.67% (±1.62) of A1 [t (15) = 4.05, P < 0.001] (Fig. 1D). Gain in area was limited to the signal frequency. No other frequency bands developed a significant gain in representational area (all P > 0.05) (Table S1). The representational gain in area for the signal appeared to be largely at the expense of frequency representation on the low-side of the signal band, because the 2.1–4.0 kHz band alone developed a significant loss of area [t (15) = –2.07, P < 0.05] (Table S2).
Memory strength was assessed using the standard method of determining behavioral resistance to extinction. Extinction [loss of response after discontinuation of reinforcement (20)] is a form of new learning that opposes the original behavior based on the prior signal–reward association. Thus, the rate of extinction traditionally has been used to index the strength of a prior association (21 –23). Extinction was conducted in a single session before neurophysiological recording. In addition to the signal-frequency, five nonsignal test frequencies were intermixed randomly, to permit determination of the frequency specificity of extinction (i.e., signal vs. nonsignal frequencies). Because the number of responses in extinction is a sensitive measure of memory strength (24), we compared the relative number of responses to the signal tone vs. responses to the nonsignal test tones during extinction. Animals with the greatest memory strength for the signal tone should be most resistant to extinguish responses to the signal, indicated by a greater proportion of BPs to the 5.0-kHz signal frequency relative to nonsignal frequencies (25).
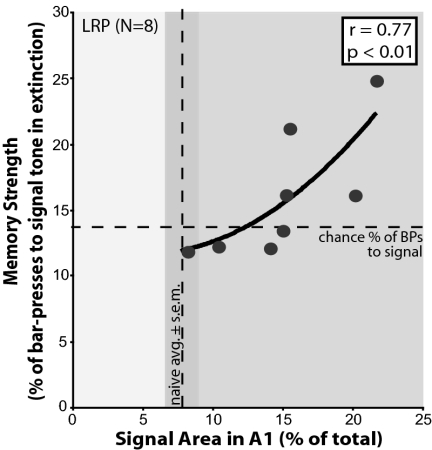
Fig. 2 shows the relationship between the percent of responses to the signal frequency compared with responses to all other frequencies and the representational area of the signal frequency band Fig. 2. The amount of signal-specific area gain in A1 induced by learning was positively correlated with the strength of memory for the signal tone (r = 0.77, P < 0.01). This curvilinear relationship became positive only when the percent of responses to the signal frequency became greater than chance. Thus, the greater the representational area above the naïve level, the greater the resistance to extinction—i.e., the greater the increased strength of memory for the signal. Furthermore, this relationship only existed for the signal frequency; resistance to extinguish responses to nonsignal frequencies was not positively correlated with the amount of their areal representation in A1 (Table S3). These findings strongly suggest that a function of learning-induced signal-specific gain in cortical representation is to confer increased memory strength.
Fig. 2.
Memory strength correlates with the amount of signal-specific area gains in A1 in the LRP group. The x axis shows the amount of relative area in A1 for the signal-frequency (half-octave band). The y axis shows the strength of memory for the signal tone (5.0 kHz) determined by its resistance to extinction relative to five other nonsignal frequencies (2.8, 7.5, 12.9, 15.8, and 21.7 kHz). LRP animals showed increases in area relative to the naïve group (vertical dashed line, s.e.m. marked by shaded area). The increase of memory strength was defined as the proportion of bar-pressing to the signal tone greater above chance (estimated as the percentage of responses to the signal tone if behavior were equal across all six test frequencies: [100%/(6 possible tone responses + 1 for possibility of no response) = 14.29%; see Methods] (horizontal dashed line). Increased memory strength for the signal tone is significantly positively correlated with the amount of area gain in A1 (best fit regression, curvilinear: r = 0.77, P < 0.01).
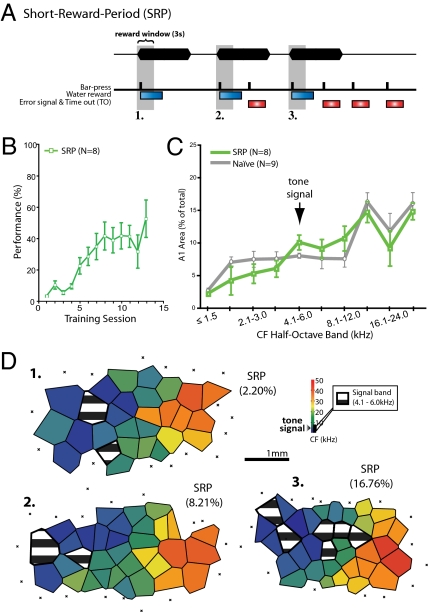
If specific representational gain of area serves a general function as a substrate of the strength of memory, then it should be found in a wide range of circumstances, including different levels of task difficulty. In this initial study of the neural substrates of memory strength, we used a fairly easy task; the LRP group attained asymptotic performance of 76.2% correct. Therefore, we devised a second, much more difficult version of the auditory-contingent task of bar-pressing for water reward. Subjects had to BP within 3 s of tone onset, but also stop responding before offset of the continuing tone (8, 9, or 10 s pseudorandomly intermixed; Methods) (Fig. 3A, example 1). This short-reward-period (SRP) group (n = 8) was punished for responses after 3 s. All other BPs during the signal tone were signaled as errors by a flashing light and time-out period (Fig. 3A, example 2) as were BPs after tone offset and (Fig. 3A, example 3).
Fig. 3.
Bar-pressing for water rewards to tones in the SRP group. Behavior and representational area in A1. (A) The SRP protocol required animals to BP for rewards during a brief 3-s reward window that began at tone onset. Only one water reward could be delivered per trial. All BPs during the tone after 3 s and during ITI were signaled as errors with a flashing light during a time-out period. Example 1 shows an optimal pattern of behavior: the BP is limited to the 3-s reward window after tone onset. Example 2 shows a correct response followed by an erroneous BP later during the tone. Example 3 shows a correct BP followed by errors later during the tone and during the silent part of the ITI. (B) Group performance improved across sessions (asymptote = 42.6 ± 8.1%; days 1–11, n = 8; day 12, n = 3; day 13, n = 2). (C) Group CF distributions (half-octave bands) across A1 compared to a naïve group did not reveal a significant gain in area at any signal-frequency band. (D) Exemplar maps for three subjects (1–3) show variability in the amount of signal-band representation within the SRP group. Striped polygons show the area of representation of the signal-frequency. Percentages indicate the relative amount of A1 area occupied by the signal-frequency (±0.25 octaves). Examples show cases of smaller (#1, 2.20%), approximately equal (#2, 8.21%), or larger (#3, 16.76%) signal-band areas than naïve (7.67 ± 1.62%; see Fig. 1D).
Performance of the SRP group improved across training [F (12,103) = 13.98; P < 0.0001] and reached an asymptote of 42.6% after 11.6 sessions (Fig. 3B). Although this group attained asymptote more rapidly than did the LRP group, its level of performance was significantly worse [t (6) = 7.29, P < 0.0001] with four out of eight subjects performing between merely 11.4% and 28.7% at asymptote.
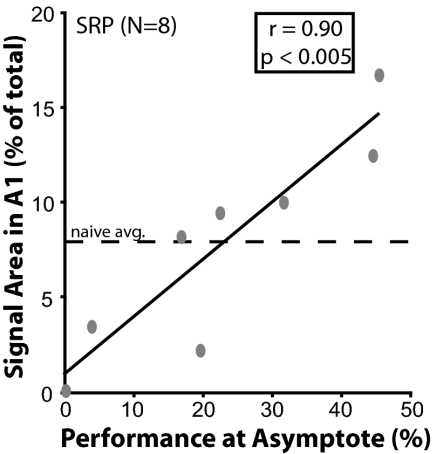
The SRP group failed to exhibit any significant representational gains in area (Fig. 3C; all half-octave CF bands, P > 0.05; see Table S4). This is not surprising given the group’s poor average performance. The absence of a significant gain of area reflects a large range of representational area of the signal band (Fig. 3D). Despite the high variability in cortical area, it was not randomly related to performance. Asymptotic performance was significantly positively correlated with the amount of signal-specific area in A1 (r = 0.90, P < 0.005; Fig. 4). This finding replicates and extends that of Rutkowski and Weinberger (15), who used a different variant of the bar-pressing task.
Fig. 4.
Relationship between performance and the representational area for the signal frequency of 5.0 kHz (±0.25 octaves). Vertical dashed line indicates the mean representational area in the naïve group (7.67%). The level of asymptotic performance in the SRP group is significantly positively correlated with the amount of representational area gain (r = 0.90, P < 0.005).
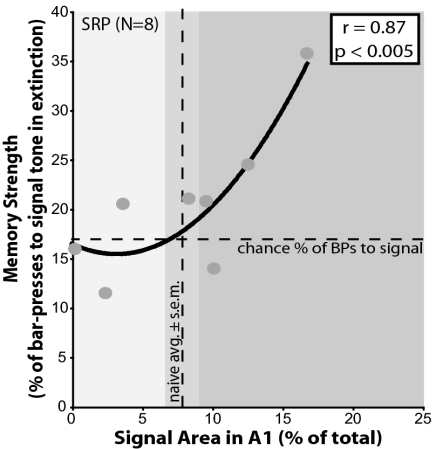
Even without an overall group change in area, it was possible to determine the extent to which the representation of the signal frequency might be related to memory strength in the difficult task. The amount of signal-specific area in A1 was highly correlated with resistance to extinction for the signal tone (r = 0.87, P < 0.005). As in the case of the LRP group in the easy task, this relationship was curvilinear. Also like the findings for the easy task, this relationship became positive only when resistance to extinction for the signal tone was above chance; this applied only to SRP subjects that developed gains in A1 signal-area (Fig. 5). Those subjects lacking area gains did not exhibit increased memory strength. As for the LRP group, this relationship only existed for the signal frequency; resistance to extinguish responses to nonsignal frequencies was not positively correlated with the amount of their frequency representation in A1 (Table S5). Thus, the enhancement of memory strength was directly related to the amount of area gain in A1 for the signal frequency. These findings support the generality of the principle that area gain confers increased memory strength by showing that it obtains across levels of task difficulty.
Fig. 5.
Memory strength is correlated with the amount of signal-specific area in A1 in the SRP group. The x axis shows the amount of relative area in A1 for the signal-frequency (±0.25 octaves). Vertical dashed line indicates the mean area of representation in the naïve group (±SEM marked by shaded area). The y axis shows the strength of memory for the signal tone (5.0 kHz) determined by its resistance to extinction relative to four other nonsignal frequencies (1.1, 2.4, 10.6, and 22.4 kHz). Increase of memory strength was defined as the proportion of bar-pressing to the signal tone greater than chance. Chance level was estimated as the percentage of responses to the signal tone if behavior were equal across all five test frequencies ([100%/(5 possible tone responses + 1 for possibility of no response) = 16.67%]; see Methods) (horizontal dashed line). Memory strength is significantly positively correlated with the amount of signal-representational area in A1 (best fit regression, curvilinear: r = 0.87, P < 0.005).
Discussion
These experiments tested the hypothesis that a gain of representational area in the primary auditory cortex would lead to increased strength of specific memory. This “area gain/memory strength” hypothesis emphasizes a distinction between the causes of learning-induced plasticity, such as the formation of an association, and a neglected aspect of inquiry—namely, the functions of such plasticity. Two groups of rats were trained to learn a tone-contingent instrumental response to obtain water rewards, in relatively easy (group LRP) and highly difficult (groups SRP) tasks. We found a significant positive relationship between signal-specific gains of representational area in A1 and resistance to extinction for the signal frequency band in both groups, despite the wide range of task difficulties and corresponding asymptotic performance level (76.2% vs. 42.6%, respectively). No other frequencies developed significant gains in representational area. Moreover, the shape of the function relating area gain to the extinction-based measure of memory strength was the same in both groups: curvilinear. In both cases, the gain in area developed only in subjects that showed memory strength for the signal frequency that was greater than that of a naïve control group—i.e., above chance (Figs. 2 and 5). The specificity of the findings for the signal frequency band (5.0 kHz ± 0.25 octaves) demonstrates that the findings reflect memory for the signal frequency rather than sound per se. In addition, the relationship only exists between memory strength for the signal tone and its gain in representational area induced by learning; no such relationship existed between the resistance to extinguish responses to nonsignal tones and their area in A1. Thus, the area gain-memory strength correlations in the LRP and SRP groups reflect signal-specific associative learning, as opposed to any putative nonspecific or nonassociative processes. The results support the hypothesis that learning-induced gains in representational area underlie increased strength of memory.
Of course, the current findings are correlational and cannot themselves demonstrate that area gain is a “mechanism” for memory strength. Interventional approaches, such as directly manipulating the area of representation and predicting the strength of memory, would permit tests of mechanism.
How might gains in area confer increased memory strength? Many factors can weaken memories. For example, memories are subject to degradation by processes of forgetting and to suppression by extinction and interference. All of these assailants may be counteracted by increasing the number of neurons that represent the content of memory. On the assumption that memories involve networks of functionally linked neurons, the greater the number of participatory cells, the more extensive the network. Forgetting would have to degrade enlarged networks, and new learning in the form of extinction would have to suppress more extensive networks. If weakly formed, as in the case of relatively unimportant experiences, enlarged representations of memories would not be established, making them more susceptible. In contrast, memories of greater behavioral significance are more resistant to weakening. A particularly unfortunate example is posttraumatic stress disorder (PTSD), in which traumatic memories are intrusive (26, 27). These considerations find experimental support in the finding that the greater the importance of a sound, the greater the area of its representation in the primary auditory cortex (15).
Increased area of representation may serve mnemonic processes other than memory strength per se. For example, memory that is encoded and stored in a larger network might be more readily recalled in cued retrieval because cues might be more likely to encounter a neuronal entry-point into the memory network. Similarly, nonexternally cued recall could be facilitated because the internal search mechanism is more likely to encounter a member of an extended network than one of a more restricted network. Thus, area gains could also serve to increase the accuracy of memory retrieval by increasing the likelihood that information from the cortically expanded representation will come into attention (28). Indeed, human imaging studies have shown that cortical areas that are activated during acquisition are the same areas engaged during retrieval (29), suggesting that areas modified by learning participate in the retrieval of specific memories.
Increased numbers of neurons that become best responsive to the same incoming stimulus have several implications. For example, they could amplify the downstream influence of behaviorally important stimuli relative to nonsignal stimuli. This amplified transmission of information could be due to an increase in the overall magnitude of activity for the signal, in the coordination of signal-evoked activity when neurons respond similarly to the same cortical inputs, or both. For example, learning-induced facilitation of temporal processing, including decreased variability in spike latencies, may increase the synchrony of ensemble firing (30). Thus, downstream sensory, motor, and other targets could be more heavily impacted by the presence of the signal in the environment. This could lead to signal-specific facilitation of attention, general cognition, decision-making, motor-planning, and the execution of relevant behaviors. In support of this type of schema, local amplification of signal-specific cortical activity by focal microstimulation produces shorter response latencies and the biasing of choices toward the feature that is processed by the involved neurons, both in auditory (31) and visual cortices (32).
Proposed mechanisms of HARP, included expanded representation, have implicated neuromodulatory systems such as dopamine (33), norepinephrine (34), and serotonin (35). Cholinergic modulation of auditory cortical responses by the nucleus basalis (NB) has been shown to have a primary role for the induction of HARP in A1 (36, 37). Stimulation of the NB paired with a specific pure tone induces local tuning shifts toward the tonal frequency (38 –40) and gains in representational area across A1 (41). Moreover, tone/NB pairing also induces actual specific behavioral memory (42 –44). Tone paired with stimulation of the basolateral amygdala (BLA) also can produce signal-directed tuning shifts in A1 (45, 46) and is likely to do so through an interaction with the NB (47, 48). Furthermore, the effects of NB and BLA involvement in representational plasticity in A1 are likely to include the participation of top-down influences from the prefrontal cortex (PFC) (18, 49). Extensive lines of behavioral research have shown that posttraining manipulations of the BLA can strengthen memories, including those of emotionally arousing experiences (50 –53). Together with the current findings, this research suggests that a likely target for the strengthening of a memory through the BLA–NB–PFC triumvirate, or other modulatory pathway, is to increase the cortical representation for the behaviorally relevant features of an experience that comprise the content of memory.
Although the current findings concern area gains in the primary auditory cortex, we suggest that the relationship between representational area and memory strength applies to the cerebral cortex in toto. Auditory learning using pure tones has been at the core of inquiry concerning HARP because of its experimental advantages. Chief among these is the existence of a tonotopic map in A1 that permits relatively accessible detection of a learning-based increase in signal representation. However, there is no basis for assuming that the relationship between representational gain and memory strength is limited to the primary auditory cortex. Indeed, insofar as a primary sensory cortex has mnemonic functions, it is likely that traditional areas assumed to underlie memory, such as “association” fields, also support memory strength. Future research will need to expand studies of the neural bases of memory strength and also initiate inquiry into other potential functions of learning-related plasticity in the auditory cortex and elsewhere.
Methods
Subjects.
The subjects were 25 male Sprague–Dawley rats (300-325 g; Charles River Laboratories), housed in individual cages in a temperature-controlled (22 °C) vivarium, and maintained on a 12/12 h light/dark cycle (lights on at 7:00 AM) with ad libitum access to food and water before the onset of training. They were handled daily and retained in the vivarium for a minimum of 1 week before any treatments. Once training began, they were water-deprived and given water supplements only to maintain body weight ∼15% below nondeprived littermates (83.9% ± 1.1 body weight of ad libitum). All procedures were performed in accordance with the University of California at Irvine Animal Research Committee and the National Institutes of Health Animal Welfare guidelines.
Training Apparatus and Tone Generation.
The details of initial training and the training apparatus were described previously (54) and are available in SI Methods.
Training Protocols.
After bar-press shaping, all animals were trained to respond to 5.0-kHz tones for water rewards during 45-min daily sessions until asymptotic levels of performance were reached (stable levels of performance across ≥4 days, CV ≤ 0.10). BPs during reward periods resulted in the delivery of water. BPs during the ITI between reward periods were either inconsequential or resulted in the delivery of an error-signaled time-out period (i.e., lengthening of time in addition to the programmed ITI duration until the next tone trial). Both LRP and SRP protocols were designed to encourage the use of tone onsets to solve the problem of obtaining water rewards to tones. The details of how this was achieved are outlined below for each protocol.
Long-Reward-Window Protocol.
LRP subjects could BP for reward during a 17-s window that began at tone onset. Tone duration was 10 s. Thus, a correct response made the water cup available for 5 s so that two rewards were possible during the tone, and one reward after the tone’s offset. BPs that occurred outside of the reward window resulted in a flashing-light error signal (200 ms on/off) and a 3-s (for first 4 days when ITI = 4–12 s) or 7-s (thereafter when ITI = 5–25 s) time-out for 50% of the errors, randomly programmed. If subjects did not BP during the tone, then BPs after tone offset during the remaining reward window were not rewarded.
Performance (P) across training was calculated as the proportion of rewarded BPs made during the response window, relative to the sum of all rewarded and error-signaled BPs in a session (P = [# rewarded BPs/(# rewarded BPs + # error-signaled BPs)] × 100%). A value of 100% would indicate that all BPs occurred during the reward window; a value of 0% would indicate that none occurred during this period.
Short-Reward-Window Protocol.
SRP subjects could bar-press for reward during a brief 3-s reward window that began at tone onset. A correct response made the water cup available for 4 s; only one reward was possible for each trial. BPs that occurred outside of the reward window, including those that occurred during the remainder of the 10-s tone (see Fig. 3A, examples 2 and 3), resulted in a flashing-light error signal (200 ms on/off) and 3-s time-out (i.e., lengthening of time in addition to the regularly scheduled ITI period, 8–18 s).
Performance (P) across training was calculated as the number of responses during the tone relative to all BPs during a session and was weighted according to the proportion of rewarded BPs made during the reward period, relative to all BPs made during tones (P = [(# tone BPs/Total # BPs) × (# rewarded BPs/# tone BPs)] × 100%).
Extinction Testing.
Extinction sessions began with 10 signal-frequency rewarded trials to ensure that animals remained motivated to bar-press. Extinction trials without rewards were initiated immediately thereafter. The signal tone (5.0 kHz) and five other nonsignal frequencies were presented in the LRP group: 2.8, 7.5, 12.9, 15.8, and 21.7 kHz (150 trials total). The SRP group was tested using the signal tone (5.0 kHz) and a second set of four other nonsignal frequencies: 1.1, 2.4, 10.6, and 22.4 kHz (200 trials total) (SI Methods).
Measuring Memory Strength.
Memory strength was determined for the signal frequency as the proportion of all responses made to presentations of the signal tone during extinction relative to the total number of BPs made during the session. Such a relative measure is useful because it makes possible an index of memory strength for the specific frequency of the signal. We calculated memory strength (MemSt) as [# BPs to the signal frequency/(total # BPs during extinction)] × 100%. A chance level of responding to the signal-tone frequency relative to the other extinction test frequencies was defined as a threshold for memory enhancement. Chance—i.e., memory enhancement threshold—accounted for the possibility of responding equally to each of the test tones (i.e., # extinction frequencies) or not at all (i.e., 1 possibility of an absent response) and was calculated as 100%/(# extinction frequencies + 1 absence of response).
Neurophysiological Mapping and Analysis of Tonotopic A1 Area.
Extracellular multiunit mapping of A1 was performed in anesthetized animals after training and testing. Complete mapping required 60–80 electrode penetrations. The characteristic frequency at each site was determined from the frequency response area (FRA) obtained using pseudorandom presentation of pure tones (0.5–54.0 kHz in quarter-octave steps, 0–80 dB SPL in 10-dB increments). Offline construction of Voronoi tessellations was used to determine the areal distribution of CFs across A1 (SI Methods).
Supplementary Material
Acknowledgments
We thank Jacquie Weinberger, Natalie Gross, and Gabriel K. Hui for technical assistance. This work was supported by National Institutes of Health (National Institute on Deafness and Other Communication Disorders) Grants DC-02938 (to N.M.W.) and DC-009163 (to K.M.B.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/1000159107/DCSupplemental.
References
- 1.Bakin JS, Weinberger NM. Classical conditioning induces CS-specific receptive field plasticity in the auditory cortex of the guinea pig. Brain Res. 1990;536:271–286. doi: 10.1016/0006-8993(90)90035-a. [DOI] [PubMed] [Google Scholar]
- 2.Bakin JS, South DA, Weinberger NM. Induction of receptive field plasticity in the auditory cortex of the guinea pig during instrumental avoidance conditioning. Behav Neurosci. 1996;110:905–913. doi: 10.1037//0735-7044.110.5.905. [DOI] [PubMed] [Google Scholar]
- 3.Weinberger NM. Associative representational plasticity in the auditory cortex: a synthesis of two disciplines. Learn Mem. 2007;14:1–16. doi: 10.1101/lm.421807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diamond DM, Weinberger NM. Classical conditioning rapidly induces specific changes in frequency receptive fields of single neurons in secondary and ventral ectosylvian auditory cortical fields. Brain Res. 1986;372:357–360. doi: 10.1016/0006-8993(86)91144-3. [DOI] [PubMed] [Google Scholar]
- 5.Edeline J-M, Pham P, Weinberger NM. Rapid development of learning-induced receptive field plasticity in the auditory cortex. Behav Neurosci. 1993;107:539–551. doi: 10.1037//0735-7044.107.4.539. [DOI] [PubMed] [Google Scholar]
- 6.Galván VV, Weinberger NM. Long-term consolidation and retention of learning-induced tuning plasticity in the auditory cortex of the guinea pig. Neurobiol Learn Mem. 2002;77:78–108. doi: 10.1006/nlme.2001.4044. [DOI] [PubMed] [Google Scholar]
- 7.Edeline J-M, Weinberger NM. Receptive field plasticity in the auditory cortex during frequency discrimination training: selective retuning independent of task difficulty. Behav Neurosci. 1993;107:82–103. doi: 10.1037//0735-7044.107.1.82. [DOI] [PubMed] [Google Scholar]
- 8.Weinberger NM, Javid R, Lepan B. Long-term retention of learning-induced receptive-field plasticity in the auditory cortex. Proc Natl Acad Sci USA. 1993;90:2394–2398. doi: 10.1073/pnas.90.6.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blake DT, Strata F, Churchland AK, Merzenich MM. Neural correlates of instrumental learning in primary auditory cortex. Proc Natl Acad Sci USA. 2002;99:10114–10119. doi: 10.1073/pnas.092278099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kisley MA, Gerstein GL. Daily variation and appetitive conditioning-induced plasticity of auditory cortex receptive fields. Eur J Neurosci. 2001;13:1993–2003. doi: 10.1046/j.0953-816x.2001.01568.x. [DOI] [PubMed] [Google Scholar]
- 11.Hui GK, et al. Conditioned tone control of brain reward behavior produces highly specific representational gain in the primary auditory cortex. Neurobiol Learn Mem. 2009;92:27–34. doi: 10.1016/j.nlm.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao E, Suga N. Experience-dependent corticofugal adjustment of midbrain frequency map in bat auditory system. Proc Natl Acad Sci USA. 1998;95:12663–12670. doi: 10.1073/pnas.95.21.12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheich H, Zuschratter W. Mapping of stimulus features and meaning in gerbil auditory cortex with 2-deoxyglucose and c-Fos antibodies. Behav Brain Res. 1995;66:195–205. doi: 10.1016/0166-4328(94)00140-b. [DOI] [PubMed] [Google Scholar]
- 14.Recanzone GH, Schreiner CE, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci. 1993;13:87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rutkowski RG, Weinberger NM. Encoding of learned importance of sound by magnitude of representational area in primary auditory cortex. Proc Natl Acad Sci USA. 2005;102:13664–13669. doi: 10.1073/pnas.0506838102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez-Lima F, Scheich H. Neural substrates for tone-conditioned bradycardia demonstrated with 2-deoxyglucose. II. Auditory cortex plasticity. Behav Brain Res. 1986;20:281–293. doi: 10.1016/0166-4328(86)90228-7. [DOI] [PubMed] [Google Scholar]
- 17.Molchan SE, Sunderland T, McIntosh AR, Herscovitch P, Schreurs BG. A functional anatomical study of associative learning in humans. Proc Natl Acad Sci USA. 1994;91:8122–8126. doi: 10.1073/pnas.91.17.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris JS, Friston KJ, Dolan RJ. Experience-dependent modulation of tonotopic neural responses in human auditory cortex. Proc R Soc London B. 1998;265:649–657. doi: 10.1098/rspb.1998.0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berlau KM, Gross NM, Weinberger NM. Washington, DC: Society for Neuroscience; 2008. Learning strategies that rely on tone onset during auditory associative learning predict the development of signal-specific plasticity in A1. Program No. 850.23. 2008 Neuroscience Meeting Planner. 2008. Online. [Google Scholar]
- 20.Pavlov IP. Conditioned Reflexes. Mineola, NY: Dover; 1927. [Google Scholar]
- 21.Williams SB. Transfer of extinction effects in the rat as a function of habit strength. J Comp Psychol. 1921;31:263–280. [Google Scholar]
- 22.Kalish HI. Strength of fear as a function of the number of acquisition and extinction trials. J Exp Psychol. 1954;47:1–9. doi: 10.1037/h0053732. [DOI] [PubMed] [Google Scholar]
- 23.Capaldi EJ, Morris MD. Reward schedule effects in extinction: Intertrial interval, memory, and memory retrieval. Learn Motiv. 1974;5:473–483. [Google Scholar]
- 24.Williams SB. Resistance to extinction as a function of the number of reinforcements. J Exp Psychol. 1938;23:506–522. [Google Scholar]
- 25.Hoffeld DR. Primary stimulus generalization and secondary extinction as a function of strength of conditioning. J Comp Physiol Psychol. 1962;55:27–31. doi: 10.1037/h0041977. [DOI] [PubMed] [Google Scholar]
- 26.Elzinga BM, Bremner JD. Are the neural substrates of memory the final common pathway in posttraumatic stress disorder (PTSD)? J Affect Disord. 2002;70:1–17. doi: 10.1016/s0165-0327(01)00351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubin DC, Berntsen D, Bohni MK. A memory-based model of posttraumatic stress disorder: evaluating basic assumptions underlying the PTSD diagnosis. Psychol Rev. 2008;115:985–1011. doi: 10.1037/a0013397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sandeberg A, Lansner A, Peterson KM. Selective enhancement of recall through plasticity modulation in an autoassociative memory. Neurocomputing. 2001;38-40:867–873. [Google Scholar]
- 29.Delazer M, et al. Learning by strategies and learning by drill—evidence from an fMRI study. Neuroimage. 2005;25:838–849. doi: 10.1016/j.neuroimage.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 30.Leon MI, Poytress BS, Weinberger NM. Avoidance learning facilitates temporal processing in the primary auditory cortex. Neurobiol Learn Mem. 2008;90:347–357. doi: 10.1016/j.nlm.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Otto KJ, Rousche PJ, Kipke DR. Cortical microstimulation in auditory cortex of rat elicits best-frequency dependent behaviors. J Neural Eng. 2005;2:42–51. doi: 10.1088/1741-2560/2/2/005. [DOI] [PubMed] [Google Scholar]
- 32.Salzman CD, Murasugi CM, Britten KH, Newsome WT. Microstimulation in visual area MT: effects on direction discrimination performance. J Neurosci. 1992;12:2331–2355. doi: 10.1523/JNEUROSCI.12-06-02331.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bao S, Chan VT, Merzenich MM. Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature. 2001;412:79–83. doi: 10.1038/35083586. [DOI] [PubMed] [Google Scholar]
- 34.Manunta Y, Edeline JM. Noradrenergic induction of selective plasticity in the frequency tuning of auditory cortex neurons. J Neurophysiol. 2004;92:1445–1463. doi: 10.1152/jn.00079.2004. [DOI] [PubMed] [Google Scholar]
- 35.Ji W, Suga N. Serotonergic modulation of plasticity of the auditory cortex elicited by fear conditioning. J Neurosci. 2007;27:4910–4918. doi: 10.1523/JNEUROSCI.5528-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Metherate R, Weinberger NM. Cholinergic modulation of responses to single tones produces tone-specific receptive field alterations in cat auditory cortex. Synapse. 1990;6:133–145. doi: 10.1002/syn.890060204. [DOI] [PubMed] [Google Scholar]
- 37.Weinberger NM. Specific long-term memory traces in primary auditory cortex. Nat Rev Neurosci. 2004;5:279–290. doi: 10.1038/nrn1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bakin JS, Weinberger NM. Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis. Proc Natl Acad Sci USA. 1996;93:11219–11224. doi: 10.1073/pnas.93.20.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bjordahl TS, Dimyan MA, Weinberger NM. Induction of long-term receptive field plasticity in the auditory cortex of the waking guinea pig by stimulation of the nucleus basalis. Behav Neurosci. 1998;112:467–479. doi: 10.1037//0735-7044.112.3.467. [DOI] [PubMed] [Google Scholar]
- 40.Dimyan MA, Weinberger NM. Basal forebrain stimulation induces discriminative receptive field plasticity in the auditory cortex. Behav Neurosci. 1999;113:691–702. doi: 10.1037//0735-7044.113.4.691. [DOI] [PubMed] [Google Scholar]
- 41.Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279:1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- 42.McLin DE, 3rd, Miasnikov AA, Weinberger NM. Induction of behavioral associative memory by stimulation of the nucleus basalis. Proc Natl Acad Sci USA. 2002;99:4002–4007. doi: 10.1073/pnas.062057099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miasnikov AA, Chen JC, Weinberger NM. Rapid induction of specific associative behavioral memory by stimulation of the nucleus basalis in the rat. Neurobiol Learn Mem. 2006;86:47–65. doi: 10.1016/j.nlm.2005.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weinberger NM, Miasnikov AA, Chen JC. The level of cholinergic nucleus basalis activation controls the specificity of auditory associative memory. Neurobiol Learn Mem. 2006;86:270–285. doi: 10.1016/j.nlm.2006.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chavez CM, McGaugh JL, Weinberger NM. The basolateral amygdala modulates specific sensory memory representations in the cerebral cortex. Neurobiol Learn Mem. 2009;91:382–392. doi: 10.1016/j.nlm.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chavez CM, McGaugh JL, Weinberger NM. Chicago, IL: Society for Neuroscience; 2009. Stimulation of the basolateral amygdala specifically modifies memory-trace representations in the waking rat. Program No. 163.3. 2009 Neuroscience Meeting Planner. 2009. Online. [Google Scholar]
- 47.Dringenberg HC, Vanderwolf CH. Cholinergic activation of the electrocorticogram: an amygdaloid activating system. Exp Brain Res. 1996;108:285–296. doi: 10.1007/BF00228101. [DOI] [PubMed] [Google Scholar]
- 48.Power AE, Thal LJ, McGaugh JL. Lesions of the nucleus basalis magnocellularis induced by 192 IgG-saporin block memory enhancement with posttraining norepinephrine in the basolateral amygdala. Proc Natl Acad Sci USA. 2002;99:2315–2319. doi: 10.1073/pnas.022627799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang WW, Zhou XM, Zhang JP, Sun XD. Modulation of frequency receptive field plasticity in rat auditory cortical neurons by electrical stimulation of medial prefrontal cortex. Acta Physiol Sinica. 2007;59:784–790. [PubMed] [Google Scholar]
- 50.McGaugh JL, Cahill L, Roozendaal B. Involvement of the amygdala in memory storage: interaction with other brain systems. Proc Natl Acad Sci USA. 1996;93:13508–13514. doi: 10.1073/pnas.93.24.13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- 52.Nathan SV, Griffith QK, McReynolds JR, Hahn EL, Roozendaal B. Basolateral amygdala interacts with other brain regions in regulating glucocorticoid effects on different memory functions. Ann NY Acad Sci. 2004;1032:179–182. doi: 10.1196/annals.1314.015. [DOI] [PubMed] [Google Scholar]
- 53.McGaugh JL, Roozendaal B. Drug enhancement of memory consolidation: historical perspective and neurobiological implications. Psychopharmacology. 2009;202:3–14. doi: 10.1007/s00213-008-1285-6. [DOI] [PubMed] [Google Scholar]
- 54.Berlau KM, Weinberger NM. Learning strategy determines auditory cortical plasticity. Neurobiol Learn Mem. 2008;89:153–166. doi: 10.1016/j.nlm.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.