Abstract
The Na/K-ATPase was discovered as an energy transducing ion pump. A major difference between the Na/K-ATPase and other P-type ATPases is its ability to bind a group of chemicals called cardiotonic steroids (CTS). The plant-derived CTS such as digoxin are valuable drugs for the management of cardiac diseases, whereas ouabain and marinobufagenin (MBG) have been identified as a new class of endogenous hormones. Recent studies have demonstrated that the endogenous CTS are important regulators of renal Na+ excretion and blood pressure. The Na/K-ATPase is not only an ion pump, but also an important receptor that can transduce the ligand-like effect of CTS on intracellular protein kinases and Ca2+ signaling. Significantly, these CTS-provoked signaling events are capable of reducing the surface expression of apical NHE3 (Na/H exchanger isoform 3) and basolateral Na/K-ATPase in renal proximal tubular cells. These findings suggest that endogenous CTS may play an important role in regulation of tubular Na+ excretion under physiological conditions; conversely, a defect at either the receptor level (Na/K-ATPase) or receptor-effector coupling would reduce the ability of renal proximal tubular cells to excrete Na+, thus culminating/resulting in salt-sensitive hypertension.
Renal adaptation to high dietary salt intake involves a graded decrease in proximal tubule (PT) sodium reabsorption. Abnormalities in such renal adaptation may contribute to the etiology of salt-sensitive hypertension. The topics of the Na/K-ATPase, CTS, blood pressure and sodium homeostasis have been extensively reviewed [1–5]. As an active ion transporter, the central role of the Na/K-ATPase is to maintain intracellular Na+ and K+ balance as well as to keep an inwardly directed Na+ gradient at the expense of ATP. Early studies indicate that the Na/K-ATPase is engaged in interaction with other membrane and soluble proteins such as ankyrin [6]. Recent studies demonstrate that the Na/K-ATPase also functions as a classical receptor, capable of converting CTS binding into activation of various protein kinase cascades. Moreover, the CTS-activated Na/K-ATPase signaling depends on the formation of specific signaling microdomains that couple the receptor Na/K-ATPase to its down-stream effectors. Functionally, activation of Na/K-ATPase-mediated signaling by CTS plays an important role in the regulation of surface expression of basolateral Na/K-ATPase and apical NHE3 in renal proximal tubular cells and consequently transcellular Na+ transport. In this review, we will focus on these new findings, addressing the role of newly appreciated Na/K-ATPase signaling function in regulation of blood pressure and sodium homeostasis.
1. The Na/K-ATPase
The Na/K-ATPase (EC 3.6.3.9), known as the sodium pump, was discovered by Skou in 1957 [7]. It belongs to the family of P-type ATPases and consists of two non-covalently linked α and β subunits [8–10]. Several α subunits (the "catalytic subunit" containing ATP, CTS, and other ligand binding sites) and β subunits (essential for the assembly of a functional enzyme) have been identified and functionally characterized. The α1 subunit, along with β1, is the predominant “housekeeping” enzyme of most cells. The renal proximal tubule exclusively expresses the α1 subunit on the basolateral membrane. The other subunits (α2, α3, α4, β2, and β3) are expressed in a tissue-specific manner. A γ subunit (a member of the FXYD-containing polypeptides, that are not an integral part of the enzyme and are also expressed in a tissue specific manner) may modulate the Na/K-ATPase enzymatic activity [11]. Based on the crystal structures of SERCA (the calcium ATPase of skeletal muscle sarcoplasmic reticulum) [12], Sweadner and Donnet [13] first predicted three distinct functional domains in the Na/K-ATPase α1 subunit, which has been largely confirmed by the crystal structure of the Na/K-ATPase [14]. These domains contains different functional binding motifs that are involved in the interaction with other membrane and structural proteins, receptors, and signaling molecules including Src, PLC-γ, PI3K, IP3R, ankyrin, adducin, and caveolin-1 [3, 6, 15–20]. Binding to these proteins not only regulates the distribution and ion pumping function of the Na/K-ATPase, but also makes it possible for the receptor Na/K-ATPase to convert CTS binding to the activation of protein kinase cascades and the generation of second messengers [3, 21–23].
2. The Na/K-ATPase/Src receptor complex and CTS-induced activation of protein kinase cascades
The concept that the Na/K-ATPase functions as a signal transducer in response to CTS stimulation was first demonstrated in cultured cardiac myocytes and renal epithelial cells [24–30]. Up to date, the Na/K-ATPase has been identified as an ion transporter, a signal transducing receptor for both endogenous and exogenous CTS, and an important scaffolding protein, capable of organizing cell-specific signaling microdomains by interacting with different partners [3, 8, 9, 17, 26–29, 31–45]. For example, stimuli other than CTS (e.g., reactive oxygen species (ROS) and those activating G-protein-coupled receptors (GPCRs)), are able to increase the interaction between the Na/K-ATPase and signaling proteins such as PI3K, PKC and arrestins, which leads to a cell-specific endo/exocytosis of the Na/K-ATPase and a change in Na/K-ATPase activity [18, 46–48]. On the other hand, we have reported that binding of CTS to the Na/K-ATPase activates Na/K-ATPase-associated Src. Subsequently, the activated Src transactivates receptor tyrosine kinases (RTKs) such as the EGF receptor (EGFR), which ultimately converts CTS binding to the activation of serine/threonine kinases, lipid kinases, and lipases. Several important structural bases of this signaling mechanism have been recently revealed [17, 22, 39, 41, 42, 49]. These actions of CTS can occur in the absence of changes in intracellular ion concentrations [28, 50]. Like other receptors, activation of Na/K-ATPase signaling by CTS induces the endocytosis of the Na/K-ATPase, thus terminating or propagating the signaling or targeting it to specific intracellular compartments [50–52]. Moreover, accumulated data have revealed the complexity and diversity of CTS-activated Na/K-ATPase signaling, its regulatory mechanisms, and its significance in human health and diseases (e.g., Refs. [15, 32, 34–36, 53–64]). For example, ouabain can stimulate protein kinase cascades and regulate cell growth in cardiac myocytes, renal epithelial cells, vascular smooth and endothelial cells, as well as skeletal muscle cells [32, 35, 54–56, 65]. Both “signaling” and “ion pumping” functions of the Na/K-ATPase could work in concert in regulation of cellular functions, and the overall effect may be dependent on cell type, expression of different α subunit, and interaction with other receptors.
2.1. Src and CTS-induced protein tyrosine phosphorylation
The status of protein tyrosine phosphorylation depends on the overall balance of protein tyrosine kinases and tyrosine phosphatases. Like cytokine receptors and GPCRs [66, 67], the Na/K-ATPase has no intrinsic tyrosine kinase activity, thus requiring non-receptor tyrosine kinases to render the Na/K-ATPase capability of protein tyrosine phosphorylation. In CTS-activated Na/K-ATPase signaling, the most proximal step is the activation of nonreceptor tyrosine kinase Src. Src family kinases (e.g., Src, Hck and Lck) are 52–62 kDa membrane-associated non-receptor tyrosine kinases [68–71]. They are important regulators of various signal transduction pathways. Src contains several functional domains. The acetylated N-terminus mediates the association of the kinase with the membrane. It also consists of a SH3 domain, a SH2 domain, a linker region that can bind the Src SH3 domain, a kinase domain and a C-terminal regulatory domain. Two important and highly conserved tyrosine residues are a key to the regulation of Src kinase activity. Phosphorylation of Y529 leads to an intramolecular interaction between the SH2 domain and pY529, which facilitates binding of the SH3 domain to the linker region polyproline type II helix. This SH3-mediated interaction, in turn, inhibits formation of a salt bridge in the N lobe of the kinase domain involving E310 and K295, and thus keeping Src in an inactive state. In accordance with the above model, Src can be activated when the interaction between the SH2 domain and pY529 is disrupted due to the competitive binding of the SH2 domain to the phosphotyrosine in other proteins such as EGFR. In addition, binding of Src SH3 to a protein ligand could also keep Src in an open conformation, leading to the formation of the salt bridge and activation of Src. Finally, autophosphorylation of Y418 further stabilizes the Src in the open conformation and this stimulates Src activity.
Ouabain stimulates dose- and time-dependent tyrosine phosphorylation of multiple proteins in a Src-dependent manner. Ouabain-stimulated Src activation was initially observed in cardiac myocytes, LLC-PK1 and A7r5 cells. In these cells, ouabain not only stimulated Src kinase activity, but also increased tyrosine phosphorylation of EGFR, resulting in formation of the Na/K-ATPase/Src/EGFR signaling complex [26, 27]. Moreover, ouabain increased phosphorylation of Src at Y418, but had no effect on Y529 phosphorylation. Pretreatment with Src or Src family kinase inhibitors (PP2 and herbimycin A) abolished ouabain-induced Src activation and formation of the active Na/K-ATPase/Src/EGFR complex. Moreover, ouabain-stimulated tyrosine phosphorylation of EGFR was observed in the SYF + c-Src cells, but not in SYF cells (The SYF cells are derived from mouse embryos harboring functional null mutations in both alleles of the Src family kinases Src, Yes, and Fyn. The SYF + c-Src cells are the SYF cells that are rescued by c-Src) [26, 27].
2.2. The formation of the Na/K-ATPase/Src complex
By association with JAK and/or Src kinases, cytokine receptors and GPCRs are capable of stimulating tyrosine phosphorylation of other proteins. Similarly, besides providing the binding site for the ligand (CTS), we found that the Na/K-ATPase-associated Src functions as a signal transducer, amplifying and converting the binding signal to protein tyrosine phosphorylation [22]. Specifically, the Na/K-ATPase α1 subunit directly interacts with Src to form a functional receptor complex within the caveolar microdomain [17, 41, 42, 72]. The formation of this receptor complex was demonstrated by immunofluorescence imaging analysis showing co-localization of these two proteins in the plasma membrane, co-immunoprecipitation showing the formation of a signaling protein complex, fluorescence resonance energy transfer (FRET) analysis indicating the close proximity, as well as in vitro GST pull-down assay indicating direct interactions between the α1 subunit of the Na/K-ATPase and Src. Multiple domains of both proteins are involved in this direct interaction. In a resting state, the Src SH2 domain binds to the A domain of α1 subunit (i.e., the second cytosolic loop (CD2) connecting transmembrane helices 2 and 3), whereas the Src kinase domain binds to the N domain (nucleotide binding domain) of α1 subunit. Moreover, the association of the Src kinase domain to the N domain keeps Src in an inactive state. Interestingly, while the binding of the Src SH2 domain to the α1 CD2 exists in a “constitutive” manner, the interaction between the Src kinase domain and the α1 N domain is regulated by ouabain. Specifically, binding of ouabain to the Na/K-ATPase/Src complex releases the Src kinase domain from the α1 N domain, and consequently activates the Na/K-ATPase-associated Src. It is important to point out that this mode of regulation and signaling is unique to the Na/K-ATPase/Src complex [22].
The formation of a functional Na/K-ATPase receptor complex depends on the integrity of caveolae. Caveolae were first identified as flask-shaped vesicular invaginations of plasma membrane enriched in cholesterol, glycosphingolipids, and sphingomyelin. Caveolins are 21–24 kDa membrane-associated scaffolding proteins that serve as a protein marker of caveolae [73]. Three caveolin genes have been identified and the expression of the different isoforms is tissue-specific. Caveolins directly interact with many signaling proteins via the scaffolding domain’s binding to CBM (caveolin-binding motif) of target proteins. The mammalian Na/K–ATPase α1 subunit contains two potential CBMs [41]: one locates in the cytosolic N-terminal domain near the first transmembrane helix (M1) and the other resides at the extracellular side of M10. The appearance of the N-terminal CBM correlates well with the occurrence of the domain for ouabain binding [3]. The Na/K-ATPase was co-localized with caveolin and concentrated in caveolae in many different cells including cardiac myocytes, smooth muscle and renal epithelial cells [41, 72, 74]. Functionally, ouabain regulated this interaction and stimulated the formation of the Na/K-ATPase/Src/caveolin signaling complex. Disruption of caveolar structure by depletion of either cholesterol by methyl β-cyclodextrin (MβCD) or caveolin-1 by siRNA re-distributed the Na/K-ATPase and Src from the caveolae to other compartments, resulting in the inhibition of CTS-induced activation of protein kinases [41].
2.3. Signaling through protein kinase cascades
Recent studies from many laboratories have revealed that CTS, at concentrations that do not cause significant inhibition of cellular pumping function, are capable of activating the Na/K-ATPase/Src receptor complex. Functionally, activation of this receptor complex by CTS results in stimulation of the protein kinase cascades and generation of second messengers. For example, binding of ouabain to this receptor complex recruits and trans-activates the EGFR in a Src-dependent manner by phosphorylating EGFR at sites other than the major autophosphorylation site Y1173 [26, 27, 35]. The trans-activated EGFR further recruits adaptor protein Shc to the complex, resulting in activation of the Ras/Raf/MEK/ERK1/2 cascade [27, 35]. On the other hand, Src activation also stimulates the PLC-γ/PKC cascade through tyrosine phosphorylation of PLC-γ at Y783, which increases the production of DAG (1,2-diacylglycerol) and IP3 from hydrolysis of PIP2, resulting in the activation of PKCε and an increase in Ca2+ release from the ER [39]. It is important to recognize that these ouabain-induced signaling events occur in a cell-specific manner. For example, ouabain simulates the Src-dependent activation and translocation of several PKC isoforms in cardiac myocytes, a step that is required for ouabain-induced ERK1/2 activation by cross-talking to the Ras/Raf/ERK1/2 cascade [75]. Moreover, ouabain is also able to induce phosphorylation of Akt (protein kinase B) by activation of Src and PI3K in cardiac myocytes [76]. The concerted effects of Akt, ERK1/2 and calcium signaling ultimately produce hypertrophic growth in cardiac myocytes, stimulate proliferation in renal epithelial cells [25, 76, 77], but cause growth inhibition in some cancer cells [78]. This type of signal transduction, in general, has now been confirmed in different types of cultured cells and in intact animals [26, 35, 38, 40, 55, 72, 79]. Clearly, identification of this new cellular signaling mechanism has provided new insight into the molecular action of CTS. It has also established a new target for developing novel agonists and antagonists of this receptor complex. To this end, recent studies have shown that some derivatives of ouabain, such as rostafuroxin, can antagonize the effect of ouabain on Src, but have less effect on Na/K-ATPase activity [38]. Moreover, we have recently developed a peptide Src inhibitor (pNaKtide) from the mapping studies of the interaction between the Na/K-ATPase and Src. As expected, pNaKtide functions as a specific ouabain antagonist in cultured cardiac myocytes [49].
2.4. The α1 subunit knock-down and Src activation
Further probing of the functionality of the Na/K-ATPase/Src receptor complex is achieved by characterization of several α1 knock-down cell lines (e.g., LLC-PK1-derived PY-17 and TCN23-19 cells) [42]. We found that a graded α1 knock-down with a vector carrying α1-specific siRNA increases the basal Src activity in these cells. Consequently, α1 knockdown stimulates the activities of known Src effectors such as FAK and ERK1/2 in these cells. More importantly, it also abolishes ouabain-induced activation of Src and ERK1/2. When PY17 or TCN23-19 cells, expressing less than 10% of α1, are rescued by rat α1, the expression of rat α1 is able to restore basal Src activity. In addition, ouabain regains the ability to stimulate Src and ERK1/2 in the rescued cells at a much higher concentration, which is consistent with the established differences in ouabain sensitivity between pig and rat α1. Moreover, when Src activity was analyzed in heterozygous α1 knockout (α1+/−) mice, a 30% decrease in α1 expression resulted in more than a 2-fold increase in cellular basal Src activity in the liver [80]. Furthermore, expression of a pumping-null rat α1 mutant (which can interact with Src directly) in the knockdown cells is also able to restore α1-knock-down induced changes in Src and FAK activity [42], suggesting that the basal Src activity could be regulated by the expression level (or availability) of the α1 subunit independent of the pumping function of the Na/K-ATPase.
In short, the newly indentified Na/K-ATPase/Src interaction plays an important signaling role. First, it keeps Src in an inactive state through which it regulates the cellular protein kinase cascades. Second, formation of the Na/K-ATPase/Src complex provides a functional receptor for CTS to stimulate protein kinase cascades and generate second messengers. Clearly, the physiological significance of these modes of regulation remains to be tested in vivo.
3. The Na/K-ATPase and intracellular Ca2+ signaling
As a secondary messenger, intracellular Ca2+ has a broad and versatile effect in regulating cellular functions such as contraction, secretion, proliferation and apoptosis. Many mechanisms have been identified in regulation of intracellular Ca2+ signaling. Recent studies have demonstrated a role of the Na/K-ATPase in formation of cell-specific Ca2+-signaling microdomains.
3.1. The Na/K-ATPase interacts with NCX
Digitalis drugs regulate intracellular Ca2+ in a cell-specific manner. In cardiac and smooth muscle myocytes, this regulation appears to depend on the coupling between the Na/K-ATPase and NCX [81–84]. Blaustein and colleagues [19, 85] have recently demonstrated that the N-termini of the Na/K-ATPase α2 and α3 subunits interacts with NCX to form a specific Ca2+-signaling microdomain that makes it possible for low concentrations of ouabain to provoke calcium signaling in smooth muscle cells and astrocytes. The amino acids Leu-27 and Ala-35 of the α2/α3 N-termini are essential for targeting/tethering α2/α3 to the plasma membrane microdomain where the SR/endoplasmic reticulum (ER) is present in a “junctional” subplasmalemmal space (plasmERosome). A local increase of Na+ concentration near or within the Ca2+-signaling microdomain by the Na/K-ATPase inhibition stimulates a local increase of Ca2+ concentration, which could result in a greater amount of Ca2+ release from SR/ER [86–90]. Structurally, ankyrin-B is important for formation of this signaling microdomain because it can link the ER IP3R to the plasma membrane Na/K-ATPase and NCX [91]. Interestingly, recent studies also indicate that the α1 isoform is equally capable of interacting with NCX to regulate intracellular calcium in cardiac myocytes [92].
3.2. The Na/K-ATPase interacts with IP3R
Recent studies have identified another important calcium signaling microdomain involving the interaction between the Na/K-ATPase and ER IP3R in renal epithelial cells. IP3R is a family of Ca2+ release channels predominately localized in the ER membrane, and mainly regulated by IP3 generation and changes in cytoplasmic Ca2+. The IP3R can interact and regulate ion channels, protein kinase/phosphatases, and structural proteins. Activation of GPCRs or RTKs can activate/recruit PLC-β or PLC-γ to the plasma membrane, resulting in an increase in IP3 production and the opening of IP3R [93, 94].
Cellular Ca2+ signaling can be categorized into calcium oscillations with different frequency (in a temporal aspect) and calcium transients at specific microdomains (in a spatial aspect) [95]. Aperia’s laboratory first reported direct interaction of the Na/K-ATPase α1 subunit with IP3R in the ER membrane [34]. This interaction, which requires intracellular scaffolding protein ankyrin-B [96], is essential for ouabain-induced low-frequency calcium oscillations in renal epithelial cells. The IP3R C-terminus contains a binding site for binding of ankyrin, and ankyrin has been shown to bind the residues 142–166 of the α1 subunit A domain [97]. This interaction may play a role in stabilization of the plasma membrane pool of the Na/K-ATPase [98]. Moreover, ankyrin modulates IP3R–dependent Ca2+ release by recruiting IP3R to lipid rafts [99, 100]. Thus, formation of this Na/K-ATPase/ankyrin/IP3R signaling complex could provide a cell-specific and robust receptor for CTS to provoke a calcium signaling cascade in renal epithelial cells. Functionally, ouabain-induced calcium oscillations protect renal epithelial cells from serum starvation-induced apoptosis through activating the NF-κB pathway [34]. Mechanistically, ouabain-simulated calcium oscillations are independent of intracellular Na+ concentration, PLC activation and IP3 generation in renal epithelial cells [53], suggesting that ouabain-induced changes in the interaction between the Na/K-ATPase and IP3R may be sufficient to stimulate calcium oscillations. This mode of ouabain-induced calcium oscillations is also observed in hippocampal astrocytes [101]. A three-amino acid sequence (LKK) at the α1 N-terminus is essential for binding to IP3R and ouabain-induced calcium oscillations [20]. GST pull-down assays showed that the 1–604 segment of the IP3R N-terminus binds to the α1 subunit. Interestingly, the amino acids L and A that flank the LKK sequence, are important for targeting the α2 and the α3 to the NCX signaling microdomain in astrocytes [19].
We have also reported that the Na/K-ATPase binds directly with IP3R to form a calcium-regulatory microdomain and facilitates ouabain-activated signal transduction in LLC-PK1 cells [39]. Ouabain can stimulate calcium transients in the absence or presence of extracellular Ca2+. The Na/K-ATPase/Src receptor complex plays a critical role in formation and regulation of this calcium-signaling microdomain. In response to ouabain, the Na/K-ATPase interacts with PLC-γ and IP3R to form a calcium-regulatory microdomain to generate ouabain-activated calcium signaling. Binding of ouabain to the Na/K-ATPase/Src complex stimulates tyrosine phosphorylation of IP3R and the generation of IP3 and DAG, leading to the opening of IP3R and an increase in intracellular calcium [39]. Increases in DAG generation also activate PKCε. Structurally, we found that PLC-γ binds to the third cytosolic domain of the α1 subunit whereas IP3R interacts with the N-terminus of the α1 subunit [39], indicating that the α1 subunit contains two different domains that have the capability of tethering PLC-γ and its effector IP3R together to form a signaling complex. Both PLC-γ and IP3R co-immunoprecipitated with the Na/K-ATPase/Src complex, and ouabain regulates the formation of this signaling microdomain in a Src-dependent manner. Interestingly, the formation of this Na/K-ATPase/Src/PLC-γ/IP3R complex is also involved in Ca2+ signaling induced by stimuli other than CTS in LLC-PK1 [102]. A graded knock-down of the Na/K-ATPase α1 subunit resulted in parallel attenuation of ATP-induced ER Ca2+ release, which can be restored by knock-in of a rat α1 subunit. The α1 knock-down also reduced both angiotensin II and EGF-induced ER Ca2+ release. The effect of α1 expression on ATP-induced ER Ca2+ release was not because of a defect in P2Y receptor expression and function, or the ER Ca2+ content. Instead, the α1 knock-down redistributed the IP3R away from the plasma membrane proximity. Consistently, we found that expression of the N-terminus (Ala1-Ser160) segment of the rat α1 can function as a dominant negative mutant, disrupting formation of the Na/K-ATPase/PLC-γ/IP3R complex and attenuating the ATP-induced ER Ca2+ release.
In short, the Na/K-ATPase plays an important role in the formation of functional calcium signaling microdomains in a cell-specific manner. Functionally, formation of these microdomains allows physiological concentrations of CTS to regulate intracellular calcium signaling. It also enables other stimuli to provoke efficient calcium signaling. In relation to the topics of this review, it is of interest to note that calcium signaling plays an important role in regulation of intracellular trafficking of ion transporters in renal epithelial cells (see discussion in the next section).
4. Endogenous CTS as natriuretic hormones
CTS include plant-derived digitalis drugs such as digoxin and ouabain, and vertebrate-derived aglycones such as bufalin and MBG [31, 44]. Both ouabain and MBG have been identified as endogenous steroids whose production and secretion are regulated by multiple stimuli including angiotensin II and adrenocorticotropic hormone (ACTH) [2, 4, 103]. The concentrations of CTS were markedly increased under clinical conditions of high salt loading, chronic renal failure, and congestive heart failure [37, 104–110]. Moreover, recent studies have also revealed many extra-cardiac actions of these chemicals [45, 64, 111–114]. In addition, low doses of CTS not only induced hypertension in rats, but also caused significant cardiovascular remodeling independent of their effect on blood pressure [38, 40, 60, 62].
Dahl, Knudsen and Iwai were the first to propose the existence of a hormonal natriuretic factor that might cause a sustained increase in blood pressure in salt-sensitive hypertensive rats [115]. Subsequently,de Wardener and others came to the conclusion that this hormonal natriuretic factor inhibits the Na/K-ATPase and Blaustein described how an increase in endogenous Na/K-ATPase inhibitors might cause vascular contraction and then a rise in blood pressure [116–118]. In 1980, de Wardener and MacGregor summarized the state of research at the time, proposed an insightful scheme explaining how Na/K-ATPase inhibitor works as a natriuretic hormone [119]. In essence, they contended that the Na/K-ATPase inhibitor (endogenous CTS) will rise in response to either a defect in renal Na+ excretion or high salt intake. This increase, while returning Na+ balance toward normal by increasing renal Na+ excretion, might also cause hypertension through acting on the vascular Na/K-ATPase. Over the last thirty years, much has been learned, largely due to the effort of Blaustein’s laboratory (reviewed in [5]), about how increases in endogenous CTS change vascular contraction. However, the pathophysiological significance of endogenous CTS (e.g., as natriuretic hormone) has been a subject of debate since it was first proposed [2, 105, 120] until Lingrel’s laboratory reported their gene replacement studies, which unequivocally demonstrated that endogenous CTS play an important role in regulation of renal Na+ excretion and blood pressure through the Na/K-ATPase [121, 122]. Specifically, they have generated several lines of mice in which the endogenous α subunit is replaced by a mutant that alters the ouabain-sensitivity of the Na/K-ATPase. For example, they generated a line of “humanized” α1S/S mice where the endogenous ouabain-insensitive α1 is replaced by an ouabain-sensitive (human like) α1 mutant, and used these mice to explore the role of endogenous CTS in regulation of renal function and blood pressure. Should endogenous CTS be important for these regulations, increased CTS sensitivity in α1S/S mice would make these mice more sensitive to conditions that raise circulating CTS. Indeed, when ACTH was administered to raise endogenous CTS, it caused much severe hypertension in α1S/S mice in comparison to their control littermates. Moreover, they demonstrated that expression of the CTS-sensitive α1 mutant significantly increased renal Na+ excretion, confirming the natriuretic function of endogenous CTS as proposed by the pioneers of the field [115–119, 123]. Furthermore, while endogenous ouabain levels were reduced either by administration of anti-ouabain antibody or by active immunization with ouabain-albumin conjugate, a reduction in natriuresis was observed, suggesting that endogenous ouabain is a natriuretic hormone and has a physiological role in controlling sodium homeostasis in normal rats [124].
5. Regulation of renal sodium handling by CTS through the activation of Na/K-ATPase-mediated signal transduction
5.1. Coordinated regulation of NHE3 and the Na/K-ATPase
5.1.1 NHE3 regulation
NHE3 (SLC9A3) belongs to a family of electroneutral mammalian Na+/H+ exchangers (please see reviews [125–127]). In the renal proximal tubules, NHE3 resides in the apical membrane, mediating Na+, HCO3−, and fluid reabsorption [128, 129]. Moreover, vesicular NHE3 activity regulates endosomal pH, and consequently affects receptor-mediated endocytosis as well as endocytic vesicle fusion [130, 131]. Consistent with its cellular function, up-regulation of NHE3 activity and expression in the proximal tubule is associated with the development of hypertension [132–135]. Conversely, reduction of NHE3 surface expression or NHE3 activity occurs during pressure natriuresis in rats [136–139]. As expected, NHE3-deficient mice are hypotensive and develop acidosis [140–142] because of reduced Na+ reabsorption and increased Na+ excretion in the proximal tubule. These observations put renal Na+ reabsorption through NHE3 in a central position in the development and control of salt-loading- and volume expansion-mediated hypertension. Structurally, NHE3 has a predicted N-terminal hydrophobic iontranslocating domain, and a variable C-terminal hydrophilic domain which contains regulatory sequences [143]. The NHE3 activity is regulated at various levels through different mechanisms, mainly via phosphorylation, trafficking and transcriptional regulation [126, 127, 144]. The surface expression of NHE3 is mainly regulated by changes in endocytosis/exocytosis, and is considered to be the primary regulatory mechanism of NHE3 activity. NHE3 has been found to traffic between the plasma membrane and early/recycling endosomes (EE/RE) via a clathrin- and PI3K-dependent pathway [130, 145–152]. The NHE3 activity can be stimulated by exocytosis [152–154] or inhibited by endocytosis [136, 155, 156]. Activation of Src, PKA, PKC and increases in intracellular Ca2+ are involved in the regulation of NHE3 trafficking.
5.1.2. Coordinated Na+ regulation
In the kidney, the proximal tubule mediates over 60% of the filtered Na+ reabsorption. The Na+ reabsorption in the proximal tubule involves apical Na+ entry via NHE3 (and other co-/counter-transporters) and basolateral Na+ extrusion primarily occurring through the Na/K-ATPase. Coordinated regulation of NHE3 and the Na/K-ATPase is critical in maintaining intracellular Na+ homeostasis and extracellular fluid volume. It is believed that the apical Na+ entry through NHE3 is the rate limiting step because the functional reserve of the Na/K-ATPase in the nephron is more than sufficient even under some pathological conditions [157]. Although the mechanisms are still being elucidated, accumulating evidence supports the notion that the expression and activity of the basolateral Na/K-ATPase and apical NHE3 in renal proximal tubule cells are coordinated and coupled. During pressure natriuresis, for instance, McDonough’s laboratory has shown that the surface expression and activities of both NHE3 and the Na/K-ATPase are simultaneously down-regulated [136, 138, 158]. Similarly, salt loading also activates this regulatory mechanism to remove Na+ from the body [159]. During the development of hypertension in SHR (spontaneous hypertensive rat), the expression and activity of both the Na/K-ATPase and NHE3 are elevated in comparison to the normotensive control rats [132, 160–163]. One of the best studied paradigms of hormonal natriuresis is the renal dopamine system [164–166]. The natriuretic properties of dopamine have been recognized for more than 40 years [167]. Renal dopamine release increases in response to high salt intake or volume expansion. Activation of apical and basolateral D1-like dopamine receptors stimulates PLC-β and cAMP-PKA pathways and increases intracellular Ca2+. These pathways work in concert and produce the coordinated down-regulation of NHE3 and the Na/K-ATPase, and consequently natriuresis [164–166, 168, 169]. While Aperia’s laboratory first revealed the pathways involved in dopamine-induced regulation of Na/K-ATPase activity [170–172], which is related to endocytosis of the Na/K-ATPase [173], Moe and others have mapped the pathway of regulation of NHE3 phosphorylation and trafficking [144, 156, 174].
5.2. Coordinated regulation of the Na/K-ATPase and NHE3 by CTS
Interestingly, our recent studies have demonstrated that CTS, like dopamine [156, 173, 174], are also capable of stimulating a coordinated down-regulation of apical NHE3 and basolateral Na/K-ATPase in cultured proximal tubular cells [50–52, 175, 176]. Functionally, this coordinated regulation inhibits active transepithelial Na+ transport (from apical to basolateral side). Mechanistically, this regulation by ouabain depends on activation of the receptor function, but not the inhibition of the Na/K-ATPase because it requires the activation of Src, PI3K and increases in intracellular Ca2+. Moreover, we have observed that MBG-infusion also induced endocytosis of renal tubular Na/K-ATPase in rats, which could be prevented by antibody-mediated neutralization of infused MBG [176].
5.2.1. Low concentrations of ouabain inhibit transepithelial 22Na+ transport
In LLC-PK1 monolayers grown on Transwell® membrane support, exposure to ouabain (10–100 nM) on the basolateral aspect, but not the apical aspect, caused significant inhibition of active transepithelial 22Na+ flux. Similar findings were observed when LLC-PK1 cells were treated with the deproteinated extract of serum derived from patients with chronic renal failure [177]. The inhibitory effect of ouabain in LLC-PK1 cells is due to ouabain-induced inhibition of both the Na/K-ATPase and NHE3, leading to decreases in their overall ion exchange activities (86Rb+ uptake and 22Na+ uptake). Inhibition of c-Src or PI3K prevents this inhibitory effect on transepithelial flux. Ouabain has no discernable effects on cell morphology, viability, transepithelial electrical resistance, tight junction integrity, and intracellular [Na+]. The effects of ouabain are fully reversible (after ouabain wash-out) in terms of cellular “pumping” activity and transepithelial 22Na+ flux.
5.2.2. Ouabain induces endocytosis of the Na/K-ATPase
Ouabain-induced redistribution of the Na/K-ATPase was first observed by Cook and Lamb in their early studies [178, 179]. In LLC-PK1 cells, ouabain and MBG cause decreases in membrane-bound Na/K-ATPase (thus overall ion “pumping” activity) in the absence of a detectable change in intracellular [Na+]. First, ouabain stimulates clathrin-dependent endocytosis of the Na/K-ATPase α1 subunit as evidenced by a decrease in surface biotinylated α1 and a concomitant accumulation of α1 in EE/RE fractions. This regulation requires caveolin-1 (caveolae and caveolin-1 are present in LLC-PK1 cells and human proximal tubule [180, 181]) and the activation of c-Src and PI3K. Inhibition of either c-Src or PI3K prevents ouabain-induced endocytosis of the Na/K-ATPase. The role of c-Src in this regulation is further supported by the observation that ouabain can induce endocytosis of the Na/K-ATPase in SYF + c-Src cells, but not in SYF cells. Second, the endocytosed [3H]ouabain/Na/K-ATPase/Src/EGFR complex can be detected in both early and late endosomes. Third, ouabain enhances protein-protein interactions among the α1 subunit, clathrin heavy chain, the adaptor protein AP-2 α subunit, and PI3K [51, 52]. In short, ouabain stimulates endocytosis of the Na/K-ATPase by activating the receptor Na/K-ATPase/Src complex.
5.2.3. Ouabain regulates apical NHE3: a process mediated by ouabain-activated Na/K-ATPase signaling
Ouabain regulates NHE3 through two different pathways in LLC-PK1 cells, i.e., transcriptional regulation in the long-term [175] and trafficking regulation in the short-term [50]. In the long-term (over 12 h), ouabain reduces NHE3 promoter activity as well as NHE3 protein and mRNA expression [175]. These effects are abolished by inhibition of either c-Src or PI3K. Promoter mapping identified that ouabain-response elements reside in a region between −450 and −1194 nt and that ouabain reduces the binding of transcriptional factor Sp1 to its cognate cis-element. In the short-term (30 min to a few hours), ouabain regulates NHE3 trafficking [50]. Ouabain inhibits NHE3 activity (22Na+ uptake) and transepithelial 22Na+ flux, which is due to ouabain-induced down-regulation of apical NHE3. Only administration of ouabain in the basolateral, but not in the apical aspect, reduces surface NHE3. Concomitantly, ouabain accumulates NHE3 into EE/RE fractions, as in the case of the Na/K-ATPase α1 subunit. Acute ouabain treatment has no effect on total NHE3 protein and mRNA expression. Inhibition of either c-Src or PI3K or disruption of caveolae/lipid rafts structure (by cholesterol depletion with Mβ-CD) is sufficient to block ouabain-induced down-regulation of surface NHE3.
In short, ouabain down-regulates surface NHE3 expression by activating the basolateral receptor Na/K-ATPase/Src complex in renal epithelial cells. However, it remains to be established whether ouabain-activated short-term regulation involves stimulation of endocytosis or inhibition of exocytosis/recycling of NHE3 or both. Moreover, because ouabain still binds to endocytosed Na/K-ATPase, it would be of interest to test whether ouabain-induced regulation of NHE3 trafficking comes from the endocytosed Na/K-ATPase/Src complex or directly from the plasma membrane.
5.2.4. Ouabain-induced regulation of α1 and NHE3 is independent of intracellular [Na+]
High concentrations of ouabain are known to increase intracellular [Na+], depolarize the proximal tubule, and affect tight junction of epithelial cells. In LLC-PK1 cells, ouabain (up to 100 nM) has no effect on intracellular [Na+], transepithelial electrical resistance and tight junction integrity, suggesting that ouabain is not likely to increase passive Na+ transport by depolarizing LLC-PK1 monolayers. To further define whether effects of ouabain on the Na/K-ATPase and NHE3 are independent of intracellular [Na+], we have assessed the change in intracellular transporters after the equilibrium of intracellular [Na+] with extracellular [Na+] is achieved by using conventional “Na+-clamping” methods [182]. LLC-PK1 cells (both control and ouabain-treated) are pretreated either with 20 µM monensin or with 10 µM monensin plus 5 µM gramicidins for 30 min. Both “clamping” methods raise basal levels of α1 and NHE3 in EE/RE (monensin is known to accumulate proteins in intracellular compartments). However, ouabain is still able to further accumulate more α1 and NHE3 in EE/RE. These observations indicate that ouabain-induced trafficking of α1 and NHE3 can be independent of intracellular [Na+] [50].
It is well established that high salt intake or volume expansion increases both dopamine and CTS. Interestingly, it has been shown that dopamine-induced regulation of the Na/K-ATPase in proximal tubules of Dahl S rats was defective because of apparent decoupling between the binding of dopamine to its D1 receptor and activation of GPCRs [183–187]. In response to salt-loading, Dahl S rats have a similar diuretic, but much less CTS-related natriuretic response than that of Dahl R rats [188]. Both dopamine and CTS are capable of regulating activity and trafficking of the Na/K-ATPase and NHE3 in the renal proximal tubule. Even though the initiating steps and signaling pathways are different, they share some signaling steps such as the activation of PLC/PKC and calcium signaling. It will be of great interest to further assess the role of CTS-induced regulation of the Na/K-ATPase and NHE3 in renal handling of Na+. Moreover, it would be equally important to test whether there is a cross-talk between CTS- and dopamine-activated signaling pathways in regulation of renal Na+ handling.
6. Perspective
As pointed out by Guyton many years ago [189], the kidney is the most important organ in the regulation of Na+ handling and thus blood pressure, which has now been well documented (for review see [190, 191]). Although relationships amongst CTS, renal Na+ handling and hypertension were proposed many years ago, there has been an explosion of recent data coming from a number of laboratories which support this idea [103–105, 108–110, 121, 122, 192–197]. As discussed, recent reports from Lingrel’s laboratory clearly demonstrated a specific role of the α isoforms of the Na/K-ATPase and its interaction with endogenous CTS in the regulation of Na+ excretion and blood pressure in intact animals [121, 122, 195]. Working from the ligand perspective, studies have demonstrated that CTS are present in measurable amounts under normal physiological conditions, and that a number of disease states are associated with elevations in the circulating levels of CTS. We and others, during last ten years, have developed a new concept that the Na/K-ATPase has an ion pumping-independent receptor function that can confer the agonist-like effects of CTS on intracellular signal transduction. Moreover, recent studies have demonstrated that this newly discovered cellular signaling mechanism operates in intact animals in response to CTS stimulation. Thus, it is of critical importance to test the role of Na/K-ATPase-mediated signal transduction in renal Na+ handling in intact animals. Needless to say, it would be equally important to assess whether there is any regulatory defect in this signaling mechanism occurring in salt-sensitive hypertensive animals.
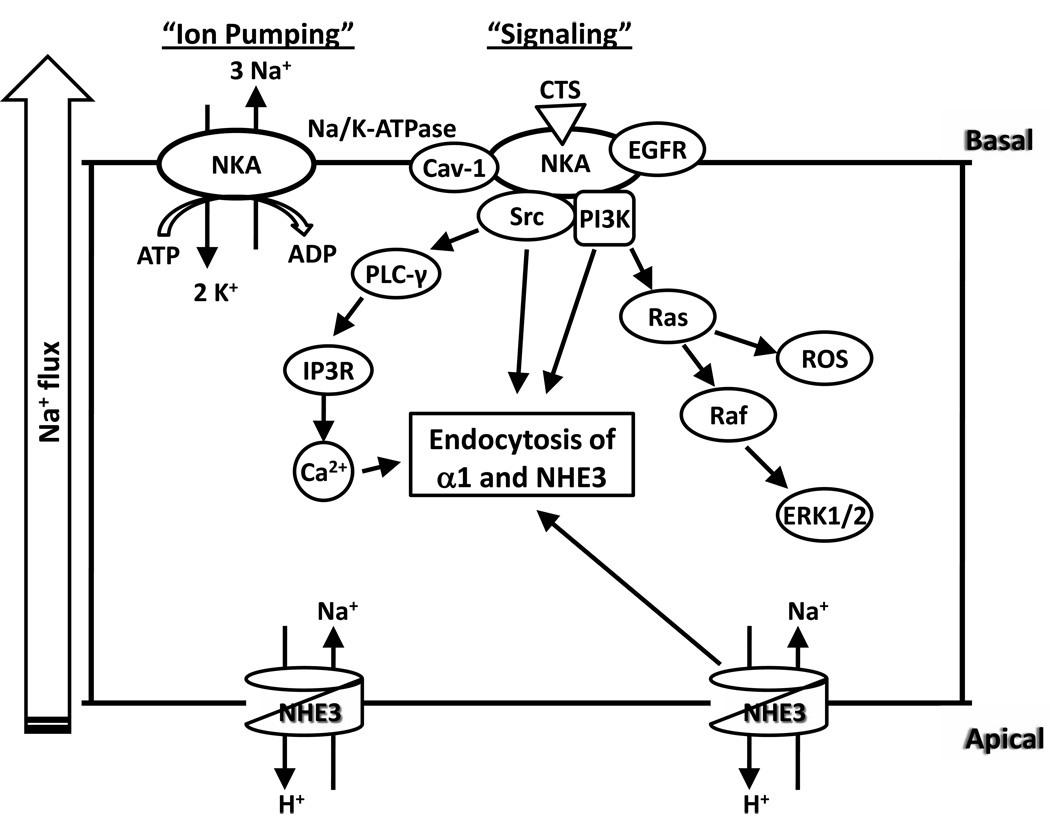
Figure 1.
Schematic presentation of ouabain-induced regulation of basolateral Na/K-ATPase and apical NHE3 in renal proximal tubule cells. NKA, Na/K-ATPase.
Acknowledgement
We thank Ms Martha Heck for editing the manuscript. The work was supported by NIH grants 5R01GM078565 and 2P01HL036573.
The abbreviations used are
- CTS
cardiotonic steroids
- EE fractions
Rab5- and EEA-1-positive early endosome fractions
- EGFR
epidermal growth factor receptor
- IP3R
IP3 receptor
- MBG
marinobufagenin
- NHE3
Na+/H+ exchanger isoform 3
- PI3K
phosphatidylinositol 3-kinase
- PKC
protein kinase C
- PLC
phospholipase C
- ROS
reactive oxygen species.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- 1.Feraille E, Doucet A. Sodium-potassium-adenosinetriphosphatase-dependent sodium transport in the kidney: hormonal control. Physiol Rev. 2001;81(1):345–418. doi: 10.1152/physrev.2001.81.1.345. [DOI] [PubMed] [Google Scholar]
- 2.Schoner W, Scheiner-Bobis G. Endogenous and exogenous cardiac glycosides: their roles in hypertension, salt metabolism, and cell growth. Am J Physiol Cell Physiol. 2007;293(2):C509–C536. doi: 10.1152/ajpcell.00098.2007. [DOI] [PubMed] [Google Scholar]
- 3.Xie Z, Cai T. Na+-K+--ATPase-mediated signal transduction: from protein interaction to cellular function. Mol Interv. 2003;3(3):157–168. doi: 10.1124/mi.3.3.157. [DOI] [PubMed] [Google Scholar]
- 4.Bagrov AY, Shapiro JI, Fedorova OV. Endogenous cardiotonic steroids: physiology, pharmacology, and novel therapeutic targets. Pharmacol Rev. 2009;61(1):9–38. doi: 10.1124/pr.108.000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blaustein MP, et al. The pump, the exchanger, and endogenous ouabain: signaling mechanisms that link salt retention to hypertension. Hypertension. 2009;53(2):291–298. doi: 10.1161/HYPERTENSIONAHA.108.119974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson WJ, Veshnock PJ. Ankyrin binding to (Na+ + K+)ATPase and implications for the organization of membrane domains in polarized cells. Nature. 1987;328(6130):533–536. doi: 10.1038/328533a0. [DOI] [PubMed] [Google Scholar]
- 7.Skou JC. The influence of some cations on an adenosine triphosphatase from peripheral nerves. Biochim Biophys Acta. 1957;23(2):394–401. doi: 10.1016/0006-3002(57)90343-8. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan JH. Biochemistry of Na,K-ATPase. Annu Rev Biochem. 2002;71:511–535. doi: 10.1146/annurev.biochem.71.102201.141218. [DOI] [PubMed] [Google Scholar]
- 9.Blanco G, Mercer RW. Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am J Physiol. 1998;275(5 Pt 2):F633–F650. doi: 10.1152/ajprenal.1998.275.5.F633. [DOI] [PubMed] [Google Scholar]
- 10.Lingrel JB, Kuntzweiler T. Na+,K(+)-ATPase. J Biol Chem. 1994;269(31):19659–19662. [PubMed] [Google Scholar]
- 11.Geering K. Function of FXYD proteins, regulators of Na, K-ATPase. J Bioenerg Biomembr. 2005;37(6):387–392. doi: 10.1007/s10863-005-9476-x. [DOI] [PubMed] [Google Scholar]
- 12.Toyoshima C, et al. Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 A resolution. Nature. 2000;405(6787):647–655. doi: 10.1038/35015017. [DOI] [PubMed] [Google Scholar]
- 13.Sweadner KJ, Donnet C. Structural similarities of Na,K-ATPase and SERCA, the Ca(2+)-ATPase of the sarcoplasmic reticulum. Biochem J. 2001;356(Pt 3):685–704. doi: 10.1042/0264-6021:3560685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morth JP, et al. Crystal structure of the sodium-potassium pump. Nature. 2007;450(7172):1043–1049. doi: 10.1038/nature06419. [DOI] [PubMed] [Google Scholar]
- 15.Barwe SP, et al. Novel role for Na,K-ATPase in phosphatidylinositol 3-kinase signaling and suppression of cell motility. Mol Biol Cell. 2005;16(3):1082–1094. doi: 10.1091/mbc.E04-05-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jordan C, et al. Identification of a binding motif for ankyrin on the alpha-subunit of Na+,K(+)-ATPase. J Biol Chem. 1995;270(50):29971–29975. doi: 10.1074/jbc.270.50.29971. [DOI] [PubMed] [Google Scholar]
- 17.Tian J, et al. Binding of Src to Na+/K+-ATPase forms a functional signaling complex. Mol Biol Cell. 2006;17(1):317–326. doi: 10.1091/mbc.E05-08-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yudowski GA, et al. Phosphoinositide-3 kinase binds to a proline-rich motif in the Na+, K+-ATPase alpha subunit and regulates its trafficking. Proc Natl Acad Sci U S A. 2000;97(12):6556–6561. doi: 10.1073/pnas.100128297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song H, et al. An N-terminal sequence targets and tethers Na+ pump alpha2 subunits to specialized plasma membrane microdomains. J Biol Chem. 2006;281(18):12929–12940. doi: 10.1074/jbc.M507450200. [DOI] [PubMed] [Google Scholar]
- 20.Zhang S, et al. Distinct role of the N-terminal tail of the Na,K-ATPase catalytic subunit as a signal transducer. J Biol Chem. 2006;281(31):21954–21962. doi: 10.1074/jbc.M601578200. [DOI] [PubMed] [Google Scholar]
- 21.Xie Z, Askari A. Na(+)/K(+)-ATPase as a signal transducer. Eur J Biochem. 2002;269(10):2434–2439. doi: 10.1046/j.1432-1033.2002.02910.x. [DOI] [PubMed] [Google Scholar]
- 22.Li Z, Xie Z. The Na/K-ATPase/Src complex and cardiotonic steroid-activated protein kinase cascades. Pflugers Arch. 2009;457(3):635–644. doi: 10.1007/s00424-008-0470-0. [DOI] [PubMed] [Google Scholar]
- 23.Tian J, Xie ZJ. The Na-K-ATPase and calcium-signaling microdomains. Physiology (Bethesda) 2008;23:205–211. doi: 10.1152/physiol.00008.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peng M, et al. Partial inhibition of Na+/K+-ATPase by ouabain induces the Ca2+-dependent expressions of early-response genes in cardiac myocytes. J Biol Chem. 1996;271(17):10372–10378. doi: 10.1074/jbc.271.17.10372. [DOI] [PubMed] [Google Scholar]
- 25.Huang L, Kometiani P, Xie Z. Differential regulation of Na/K-ATPase alpha-subunit isoform gene expressions in cardiac myocytes by ouabain and other hypertrophic stimuli. J Mol Cell Cardiol. 1997;29(11):3157–3167. doi: 10.1006/jmcc.1997.0546. [DOI] [PubMed] [Google Scholar]
- 26.Haas M, Askari A, Xie Z. Involvement of Src and epidermal growth factor receptor in the signal-transducing function of Na+/K+-ATPase. J Biol Chem. 2000;275(36):27832–27837. doi: 10.1074/jbc.M002951200. [DOI] [PubMed] [Google Scholar]
- 27.Haas M, et al. Src-mediated inter-receptor cross-talk between the Na+/K+-ATPase and the epidermal growth factor receptor relays the signal from ouabain to mitogen-activated protein kinases. J Biol Chem. 2002;277(21):18694–18702. doi: 10.1074/jbc.M111357200. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, et al. Ouabain interaction with cardiac Na+/K+-ATPase initiates signal cascades independent of changes in intracellular Na+ and Ca2+ concentrations. J Biol Chem. 2000;275(36):27838–27844. doi: 10.1074/jbc.M002950200. [DOI] [PubMed] [Google Scholar]
- 29.Tian J, Gong X, Xie Z. Signal-transducing function of Na+-K+-ATPase is essential for ouabain's effect on [Ca2+]i in rat cardiac myocytes. Am J Physiol Heart Circ Physiol. 2001;281(5):H1899–H1907. doi: 10.1152/ajpheart.2001.281.5.H1899. [DOI] [PubMed] [Google Scholar]
- 30.Huang L, Li H, Xie Z. Ouabain-induced hypertrophy in cultured cardiac myocytes is accompanied by changes in expression of several late response genes. J Mol Cell Cardiol. 1997;29(2):429–437. doi: 10.1006/jmcc.1996.0320. [DOI] [PubMed] [Google Scholar]
- 31.Akera T, Brody TM. Inotropic action of digitalis and ion transport. Life Sci. 1976;18(2):135–144. doi: 10.1016/0024-3205(76)90017-5. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen AN, Wallace DP, Blanco G. Ouabain binds with high affinity to the Na,K-ATPase in human polycystic kidney cells and induces extracellular signal-regulated kinase activation and cell proliferation. J Am Soc Nephrol. 2007;18(1):46–57. doi: 10.1681/ASN.2006010086. [DOI] [PubMed] [Google Scholar]
- 33.Liu L, et al. Involvement of Na+/K+-ATPase in hydrogen peroxide-induced hypertrophy in cardiac myocytes. Free Radic Biol Med. 2006;41(10):1548–1556. doi: 10.1016/j.freeradbiomed.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 34.Aizman O, et al. Quabain, a steroid hormone that signals with slow calcium oscillations. Proc Natl Acad Sci U S A. 2001;98(23):13420–13424. doi: 10.1073/pnas.221315298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aydemir-Koksoy A, Abramowitz J, Allen JC. Ouabain-induced signaling and vascular smooth muscle cell proliferation. J Biol Chem. 2001;276(49):46605–46611. doi: 10.1074/jbc.M106178200. [DOI] [PubMed] [Google Scholar]
- 36.Dmitrieva RI, Doris PA. Ouabain is a potent promoter of growth and activator of ERK1/2 in ouabain-resistant rat renal epithelial cells. J Biol Chem. 2003;278(30):28160–28166. doi: 10.1074/jbc.M303768200. [DOI] [PubMed] [Google Scholar]
- 37.Fedorova OV, et al. Endogenous ligand of alpha(1) sodium pump, marinobufagenin, is a novel mediator of sodium chloride--dependent hypertension. Circulation. 2002;105(9):1122–1127. doi: 10.1161/hc0902.104710. [DOI] [PubMed] [Google Scholar]
- 38.Ferrandi M, et al. Organ hypertrophic signaling within caveolae membrane subdomains triggered by ouabain and antagonized by PST 2238. J Biol Chem. 2004;279(32):33306–33314. doi: 10.1074/jbc.M402187200. [DOI] [PubMed] [Google Scholar]
- 39.Yuan Z, et al. Na/K-ATPase tethers phospholipase C and IP3 receptor into a calcium-regulatory complex. Mol Biol Cell. 2005;16(9):4034–4045. doi: 10.1091/mbc.E05-04-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kennedy DJ, et al. Central role for the cardiotonic steroid marinobufagenin in the pathogenesis of experimental uremic cardiomyopathy. Hypertension. 2006;47(3):488–495. doi: 10.1161/01.HYP.0000202594.82271.92. [DOI] [PubMed] [Google Scholar]
- 41.Wang H, et al. Ouabain assembles signaling cascades through the caveolar Na+/K+-ATPase. J Biol Chem. 2004;279(17):17250–17259. doi: 10.1074/jbc.M313239200. [DOI] [PubMed] [Google Scholar]
- 42.Liang M, et al. Functional Characterization of Src-interacting Na/K-ATPase Using RNA Interference Assay. J Biol Chem. 2006;281(28):19709–19719. doi: 10.1074/jbc.M512240200. [DOI] [PubMed] [Google Scholar]
- 43.Liang M, et al. Identification of a pool of non-pumping Na/K-ATPase. J Biol Chem. 2007;282(14):10585–10593. doi: 10.1074/jbc.M609181200. [DOI] [PubMed] [Google Scholar]
- 44.Schoner W, Scheiner-Bobis G. Endogenous and exogenous cardiac glycosides and their mechanisms of action. Am J Cardiovasc Drugs. 2007;7(3):173–189. doi: 10.2165/00129784-200707030-00004. [DOI] [PubMed] [Google Scholar]
- 45.Kaunitz JD. Membrane transport proteins: not just for transport anymore. Am J Physiol Renal Physiol. 2006;290(5):F995–F996. doi: 10.1152/ajprenal.00515.2005. [DOI] [PubMed] [Google Scholar]
- 46.Comellas AP, et al. Hypoxia-mediated degradation of Na,K-ATPase via mitochondrial reactive oxygen species and the ubiquitin-conjugating system. Circ Res. 2006;98(10):1314–1322. doi: 10.1161/01.RES.0000222418.99976.1d. [DOI] [PubMed] [Google Scholar]
- 47.Bertorello AM, Sznajder JI. The dopamine paradox in lung and kidney epithelia: sharing the same target but operating different signaling networks. Am J Respir Cell Mol Biol. 2005;33(5):432–437. doi: 10.1165/rcmb.2005-0297TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dada LA, et al. Role of the small GTPase RhoA in the hypoxia-induced decrease of plasma membrane Na,K-ATPase in A549 cells. J Cell Sci. 2007;120(Pt 13):2214–2222. doi: 10.1242/jcs.003038. [DOI] [PubMed] [Google Scholar]
- 49.Li Z, et al. NaKtide, a Na/K-ATPase-derived Peptide Src Inhibitor, Antagonizes Ouabain-activated Signal Transduction in Cultured Cells. J Biol Chem. 2009;284(31):21066–21076. doi: 10.1074/jbc.M109.013821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cai H, Wu L, Qu W, Malhotra D, Xie Z, Shapiro JI, Liu J. Regulation of Apical NHE3 Trafficking by Ouabain-Induced Activation of Basolateral Na/K-ATPase Receptor Complex. Am J Physiol Cell Physiol. 2008;294(2):C555–C563. doi: 10.1152/ajpcell.00475.2007. [DOI] [PubMed] [Google Scholar]
- 51.Liu J, et al. Ouabain induces endocytosis of plasmalemmal Na/K-ATPase in LLC-PK1 cells by a clathrin-dependent mechanism. Kidney Int. 2004;66(1):227–241. doi: 10.1111/j.1523-1755.2004.00723.x. [DOI] [PubMed] [Google Scholar]
- 52.Liu J, et al. Ouabain-induced endocytosis of the plasmalemmal Na/K-ATPase in LLC-PK1 cells requires caveolin-1. Kidney Int. 2005;67(5):1844–1854. doi: 10.1111/j.1523-1755.2005.00283.x. [DOI] [PubMed] [Google Scholar]
- 53.Miyakawa-Naito A, et al. Cell signaling microdomain with Na,K-ATPase and inositol 1,4,5-trisphosphate receptor generates calcium oscillations. J Biol Chem. 2003;278(50):50355–50361. doi: 10.1074/jbc.M305378200. [DOI] [PubMed] [Google Scholar]
- 54.Saunders R, Scheiner-Bobis G. Ouabain stimulates endothelin release and expression in human endothelial cells without inhibiting the sodium pump. Eur J Biochem. 2004;271(5):1054–1062. doi: 10.1111/j.1432-1033.2004.04012.x. [DOI] [PubMed] [Google Scholar]
- 55.Kotova O, et al. Cardiotonic steroids stimulate glycogen synthesis in human skeletal muscle cells via a Src- and ERK1/2-dependent mechanism. J Biol Chem. 2006;281(29):20085–20094. doi: 10.1074/jbc.M601577200. [DOI] [PubMed] [Google Scholar]
- 56.Khundmiri SJ, et al. Ouabain induces Cell Proliferation through Calcium Dependent Phosphorylation of Akt (Protein Kinase B) in Opossum Kidney Proximal Tubule Cells. Am J Physiol Cell Physiol. 2006;291(6):C1247–C1257. doi: 10.1152/ajpcell.00593.2005. [DOI] [PubMed] [Google Scholar]
- 57.Trevisi L, et al. Antiapoptotic effect of ouabain on human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2004;321(3):716–721. doi: 10.1016/j.bbrc.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 58.Ferrari P, et al. Targeting Ouabain- and Adducin-dependent mechanisms of hypertension and cardiovascular remodeling as a novel pharmacological approach. Med Hypotheses. 2007;68(6):1307–1314. doi: 10.1016/j.mehy.2006.07.058. [DOI] [PubMed] [Google Scholar]
- 59.Jung J, et al. Molecular mechanism of cofilin dephosphorylation by ouabain. Cell Signal. 2006;18(11):2033–2040. doi: 10.1016/j.cellsig.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 60.Jiang X, Ren YP, Lv ZR. Ouabain induces cardiac remodeling in rats independent of blood pressure. Acta Pharmacol Sin. 2007;28(3):344–352. doi: 10.1111/j.1745-7254.2007.00496.x. [DOI] [PubMed] [Google Scholar]
- 61.Blasiole B, et al. Separate Na,K-ATPase genes are required for otolith formation and semicircular canal development in zebrafish. Dev Biol. 2006;294(1):148–160. doi: 10.1016/j.ydbio.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 62.Skoumal R, et al. Involvement of endogenous ouabain-like compound in the cardiac hypertrophic process in vivo. Life Sci. 2007;80(14):1303–1310. doi: 10.1016/j.lfs.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 63.Thundathil JC, Anzar M, Buhr MM. Na+/K+ATPase as a signaling molecule during bovine sperm capacitation. Biol Reprod. 2006;75(3):308–317. doi: 10.1095/biolreprod.105.047852. [DOI] [PubMed] [Google Scholar]
- 64.Larre I, et al. Contacts and cooperation between cells depend on the hormone ouabain. Proc Natl Acad Sci U S A. 2006;103(29):10911–10916. doi: 10.1073/pnas.0604496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Elkareh J, et al. Marinobufagenin Stimulates Fibroblast Collagen Production and Causes Fibrosis in Experimental Uremic Cardiomyopathy. Hypertension. 2006 doi: 10.1161/01.HYP.0000252409.36927.05. [DOI] [PubMed] [Google Scholar]
- 66.Ihle JN, Kerr IM. Jaks and Stats in signaling by the cytokine receptor superfamily. Trends Genet. 1995;11(2):69–74. doi: 10.1016/s0168-9525(00)89000-9. [DOI] [PubMed] [Google Scholar]
- 67.McGarrigle D, Huang XY. GPCRs signaling directly through Src-family kinases. Sci STKE. 2007;2007(392):pe35. doi: 10.1126/stke.3922007pe35. [DOI] [PubMed] [Google Scholar]
- 68.Xu W, Harrison SC, Eck MJ. Three-dimensional structure of the tyrosine kinase c-Src. Nature. 1997;385(6617):595–602. doi: 10.1038/385595a0. [DOI] [PubMed] [Google Scholar]
- 69.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 70.Moarefi I, et al. Activation of the Src-family tyrosine kinase Hck by SH3 domain displacement. Nature. 1997;385(6617):650–653. doi: 10.1038/385650a0. [DOI] [PubMed] [Google Scholar]
- 71.Sicheri F, Moarefi I, Kuriyan J. Crystal structure of the Src family tyrosine kinase Hck. Nature. 1997;385(6617):602–609. doi: 10.1038/385602a0. [DOI] [PubMed] [Google Scholar]
- 72.Liu L, et al. Role of caveolae in signal-transducing function of cardiac Na+/K+-ATPase. Am J Physiol Cell Physiol. 2003;284(6):C1550–C1560. doi: 10.1152/ajpcell.00555.2002. [DOI] [PubMed] [Google Scholar]
- 73.Razani B, Woodman SE, Lisanti MP. Caveolae: from cell biology to animal physiology. Pharmacol Rev. 2002;54(3):431–467. doi: 10.1124/pr.54.3.431. [DOI] [PubMed] [Google Scholar]
- 74.Hardin CD, Vallejo J. Caveolins in vascular smooth muscle: form organizing function. Cardiovasc Res. 2006;69(4):808–815. doi: 10.1016/j.cardiores.2005.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mohammadi K, et al. Role of protein kinase C in the signal pathways that link Na+/K+-ATPase to ERK1/2. J Biol Chem. 2001;276(45):42050–42056. doi: 10.1074/jbc.M107892200. [DOI] [PubMed] [Google Scholar]
- 76.Liu L, et al. Association of PI3K-Akt signaling pathway with digitalis-induced hypertrophy of cardiac myocytes. Am J Physiol Cell Physiol. 2007;293(5):C1489–C1497. doi: 10.1152/ajpcell.00158.2007. [DOI] [PubMed] [Google Scholar]
- 77.Khundmiri SJ, et al. Ouabain stimulates protein kinase B (Akt) phosphorylation in opossum kidney proximal tubule cells through an ERK-dependent pathway. Am J Physiol Cell Physiol. 2007;293(3):C1171–C1180. doi: 10.1152/ajpcell.00535.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tian J, et al. Changes in sodium pump expression dictate the effects of ouabain on cell growth. J Biol Chem. 2009;284(22):14921–14929. doi: 10.1074/jbc.M808355200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kometiani P, Liu L, Askari A. Digitalis-induced signaling by Na+/K+-ATPase in human breast cancer cells. Mol Pharmacol. 2005;67(3):929–936. doi: 10.1124/mol.104.007302. [DOI] [PubMed] [Google Scholar]
- 80.Chen Y, et al. Regulation of Intracellular Cholesterol Distribution by Na/K-ATPase. J Biol Chem. 2009;284(22):14881–14890. doi: 10.1074/jbc.M109.003574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Akera T, Larsen FS, Brody TM. Correlation of cardiac sodium- and potassium-activated adenosine triphosphatase activity with ouabain-induced inotropic stimulation. J Pharmacol Exp Ther. 1970;173(1):145–151. [PubMed] [Google Scholar]
- 82.Blaustein MP, Juhaszova M, Golovina VA. The cellular mechanism of action of cardiotonic steroids: a new hypothesis. Clin Exp Hypertens. 1998;20(5–6):691–703. doi: 10.3109/10641969809053247. [DOI] [PubMed] [Google Scholar]
- 83.Langer GA. Effects of digitalis on myocardial ionic exchange. Circulation. 1972;46(1):180–187. doi: 10.1161/01.cir.46.1.180. [DOI] [PubMed] [Google Scholar]
- 84.Moore ED, et al. Coupling of the Na+/Ca2+ exchanger, Na+/K+ pump and sarcoplasmic reticulum in smooth muscle. Nature. 1993;365(6447):657–660. doi: 10.1038/365657a0. [DOI] [PubMed] [Google Scholar]
- 85.Juhaszova M, Blaustein MP. Na+ pump low and high ouabain affinity alpha subunit isoforms are differently distributed in cells. Proc Natl Acad Sci U S A. 1997;94(5):1800–1805. doi: 10.1073/pnas.94.5.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Arnon A, Hamlyn JM, Blaustein MP. Ouabain augments Ca2+ transients in arterial smooth muscle without raising cytosolic Na+ Am J Physiol Heart Circ Physiol. 2000;279(2):H679–H691. doi: 10.1152/ajpheart.2000.279.2.H679. [DOI] [PubMed] [Google Scholar]
- 87.Arnon A, Hamlyn JM, Blaustein MP. Na(+) entry via store-operated channels modulates Ca(2+) signaling in arterial myocytes. Am J Physiol Cell Physiol. 2000;278(1):C163–C173. doi: 10.1152/ajpcell.2000.278.1.C163. [DOI] [PubMed] [Google Scholar]
- 88.Lee MY, et al. Local subplasma membrane Ca2+ signals detected by a tethered Ca2+ sensor. Proc Natl Acad Sci U S A. 2006;103(35):13232–13237. doi: 10.1073/pnas.0605757103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hilgemann DW, et al. Steady-state and dynamic properties of cardiac sodium-calcium exchange. Sodium-dependent inactivation. J Gen Physiol. 1992;100(6):905–932. doi: 10.1085/jgp.100.6.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mak DO, McBride S, Foskett JK. Inositol 1,4,5-trisphosphate [correction of tris-phosphate] activation of inositol trisphosphate [correction of tris-phosphate] receptor Ca2+ channel by ligand tuning of Ca2+ inhibition. Proc Natl Acad Sci U S A. 1998;95(26):15821–15825. doi: 10.1073/pnas.95.26.15821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tuvia S, et al. Ankyrin-B is required for intracellular sorting of structurally diverse Ca2+ homeostasis proteins. J Cell Biol. 1999;147(5):995–1008. doi: 10.1083/jcb.147.5.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dostanic I, et al. The alpha 1 isoform of Na,K-ATPase regulates cardiac contractility and functionally interacts and co-localizes with the Na/Ca exchanger in heart. J Biol Chem. 2004;279(52):54053–54061. doi: 10.1074/jbc.M410737200. [DOI] [PubMed] [Google Scholar]
- 93.Patterson RL, Boehning D, Snyder SH. Inositol 1,4,5-trisphosphate receptors as signal integrators. Annu Rev Biochem. 2004;73:437–465. doi: 10.1146/annurev.biochem.73.071403.161303. [DOI] [PubMed] [Google Scholar]
- 94.Rebecchi MJ, Pentyala SN. Pentyala, Structure, function, and control of phosphoinositide-specific phospholipase C. Physiol Rev. 2000;80(4):1291–1335. doi: 10.1152/physrev.2000.80.4.1291. [DOI] [PubMed] [Google Scholar]
- 95.Thomas AP, et al. Spatial and temporal aspects of cellular calcium signaling. FASEB J. 1996;10(13):1505–1517. [PubMed] [Google Scholar]
- 96.Liu X, et al. Ankyrin B modulates the function of Na,K-ATPase/inositol 1,4,5-trisphosphate receptor signaling microdomain. J Biol Chem. 2008;283(17):11461–11468. doi: 10.1074/jbc.M706942200. [DOI] [PubMed] [Google Scholar]
- 97.Zhang Z, et al. Structure of the ankyrin-binding domain of alpha-Na,K-ATPase. J Biol Chem. 1998;273(30):18681–18684. doi: 10.1074/jbc.273.30.18681. [DOI] [PubMed] [Google Scholar]
- 98.Devarajan P, et al. Na,K-ATPase transport from endoplasmic reticulum to Golgi requires the Golgi spectrin-ankyrin G119 skeleton in Madin Darby canine kidney cells. Proc Natl Acad Sci U S A. 1997;94(20):10711–10716. doi: 10.1073/pnas.94.20.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bourguignon LY, Iida N, Jin H. The involvement of the cytoskeleton in regulating IP3 receptor-mediated internal Ca2+ release in human blood platelets. Cell Biol Int. 1993;17(8):751–758. doi: 10.1006/cbir.1993.1136. [DOI] [PubMed] [Google Scholar]
- 100.Singleton PA, Bourguignon LY. CD44 interaction with ankyrin and IP3 receptor in lipid rafts promotes hyaluronan-mediated Ca2+ signaling leading to nitric oxide production and endothelial cell adhesion and proliferation. Exp Cell Res. 2004;295(1):102–118. doi: 10.1016/j.yexcr.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 101.Liu XL, et al. Na,K-ATPase generates calcium oscillations in hippocampal astrocytes. Neuroreport. 2007;18(6):597–600. doi: 10.1097/WNR.0b013e3280b07bc9. [DOI] [PubMed] [Google Scholar]
- 102.Chen Y, et al. Regulation of inositol 1,4,5-trisphosphate receptor-mediated calcium release by the Na/K-ATPase in cultured renal epithelial cells. J Biol Chem. 2008;283(2):1128–1136. doi: 10.1074/jbc.M708025200. [DOI] [PubMed] [Google Scholar]
- 103.Schoner W. Endogenous cardiac glycosides, a new class of steroid hormones. Eur J Biochem. 2002;269(10):2440–2448. doi: 10.1046/j.1432-1033.2002.02911.x. [DOI] [PubMed] [Google Scholar]
- 104.Komiyama Y, et al. A novel endogenous digitalis, telocinobufagin, exhibits elevated plasma levels in patients with terminal renal failure. Clin Biochem. 2005;38(1):36–45. doi: 10.1016/j.clinbiochem.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 105.Manunta P, Hamilton BP, Hamlyn JM. Salt intake and depletion increase circulating levels of endogenous ouabain in normal men. Am J Physiol Regul Integr Comp Physiol. 2006;290(3):R553–R559. doi: 10.1152/ajpregu.00648.2005. [DOI] [PubMed] [Google Scholar]
- 106.Gottlieb SS, et al. Elevated concentrations of endogenous ouabain in patients with congestive heart failure. Circulation. 1992;86(2):420–425. doi: 10.1161/01.cir.86.2.420. [DOI] [PubMed] [Google Scholar]
- 107.Manunta P, et al. Left ventricular mass, stroke volume, and ouabain-like factor in essential hypertension. Hypertension. 1999;34(3):450–456. doi: 10.1161/01.hyp.34.3.450. [DOI] [PubMed] [Google Scholar]
- 108.Fedorova OV, Doris PA, Bagrov AY. Endogenous marinobufagenin-like factor in acute plasma volume expansion. Clin Exp Hypertens. 1998;20(5–6):581–591. doi: 10.3109/10641969809053236. [DOI] [PubMed] [Google Scholar]
- 109.Hamlyn JM, et al. Identification and characterization of a ouabain-like compound from human plasma. Proc Natl Acad Sci U S A. 1991;88(14):6259–6263. doi: 10.1073/pnas.88.14.6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fedorova OV, et al. Marinobufagenin, an endogenous alpha-1 sodium pump ligand, in hypertensive Dahl salt-sensitive rats. Hypertension. 2001;37(2 Part 2):462–466. doi: 10.1161/01.hyp.37.2.462. [DOI] [PubMed] [Google Scholar]
- 111.Mijatovic T, et al. The cardenolide UNBS1450 is able to deactivate nuclear factor kappaB-mediated cytoprotective effects in human non-small cell lung cancer cells. Mol Cancer Ther. 2006;5(2):391–399. doi: 10.1158/1535-7163.MCT-05-0367. [DOI] [PubMed] [Google Scholar]
- 112.Newman RA, et al. Oleandrin-mediated oxidative stress in human melanoma cells. J Exp Ther Oncol. 2006;5(3):167–181. [PubMed] [Google Scholar]
- 113.Kaplan JH. A moving new role for the sodium pump in epithelial cells and carcinomas. Sci STKE. 2005;2005(289):pe31. doi: 10.1126/stke.2892005pe31. [DOI] [PubMed] [Google Scholar]
- 114.Ferrari P, et al. Rostafuroxin: an ouabain antagonist that corrects renal and vascular Na+-K+-ATPase alterations in ouabain and adducin-dependent hypertension. Am J Physiol Regul Integr Comp Physiol. 2006;290(3):R529–R535. doi: 10.1152/ajpregu.00518.2005. [DOI] [PubMed] [Google Scholar]
- 115.Dahl LK, Knudsen KD, Iwai J. Humoral transmission of hypertension: evidence from parabiosis. Circ Res. 1969;24(5) Suppl:21–33. [PubMed] [Google Scholar]
- 116.De Wardener HE. Natriuretic hormone. Clin Sci Mol Med. 1977;53(1):1–8. doi: 10.1042/cs0530001. [DOI] [PubMed] [Google Scholar]
- 117.Haddy FJ, Overbeck HW. The role of humoral agents in volume expanded hypertension. Life Sci. 1976;19(7):935–947. doi: 10.1016/0024-3205(76)90284-8. [DOI] [PubMed] [Google Scholar]
- 118.Blaustein MP. Sodium ions, calcium ions, blood pressure regulation, and hypertension: a reassessment and a hypothesis. Am J Physiol. 1977;232(5):C165–C173. doi: 10.1152/ajpcell.1977.232.5.C165. [DOI] [PubMed] [Google Scholar]
- 119.de Wardener HE, MacGregor GA. Dahl's hypothesis that a saluretic substance may be responsible for a sustained rise in arterial pressure: its possible role in essential hypertension. Kidney Int. 1980;18(1):1–9. doi: 10.1038/ki.1980.104. [DOI] [PubMed] [Google Scholar]
- 120.Kelly RA, Smith TW. The search for the endogenous digitalis: an alternative hypothesis. Am J Physiol. 1989;256(5 Pt 1):C937–C950. doi: 10.1152/ajpcell.1989.256.5.C937. [DOI] [PubMed] [Google Scholar]
- 121.Dostanic-Larson I, et al. The highly conserved cardiac glycoside binding site of Na,K-ATPase plays a role in blood pressure regulation. Proc Natl Acad Sci U S A. 2005;102(44):15845–15850. doi: 10.1073/pnas.0507358102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Loreaux EL, et al. Ouabain-Sensitive alpha1 Na,K-ATPase enhances natriuretic response to saline load. J Am Soc Nephrol. 2008;19(10):1947–1954. doi: 10.1681/ASN.2008020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bricker NS. The control of sodium excretion with normal and reduced nephron populations. The preeminence of third factor. Am J Med. 1967;43(3):313–321. doi: 10.1016/0002-9343(67)90188-x. [DOI] [PubMed] [Google Scholar]
- 124.Nesher M, et al. Physiological roles of endogenous ouabain in normal rats. Am J Physiol Heart Circ Physiol. 2009;297(6):H2026–H2034. doi: 10.1152/ajpheart.00734.2009. [DOI] [PubMed] [Google Scholar]
- 125.Donowitz M, Li X. Regulatory Binding Partners and Complexes of NHE3. Physiol Rev. 2007;87(3):825–872. doi: 10.1152/physrev.00030.2006. [DOI] [PubMed] [Google Scholar]
- 126.Alexander RT, Grinstein S. Tethering, recycling and activation of the epithelial sodium-proton exchanger, NHE3. J Exp Biol. 2009;212(Pt 11):1630–1637. doi: 10.1242/jeb.027375. [DOI] [PubMed] [Google Scholar]
- 127.Bobulescu IA, Moe OW. Luminal Na(+)/H (+) exchange in the proximal tubule. Pflugers Arch. 2009;458(1):5–21. doi: 10.1007/s00424-008-0595-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Amemiya M, et al. Expression of NHE-3 in the apical membrane of rat renal proximal tubule and thick ascending limb. Kidney Int. 1995;48(4):1206–1215. doi: 10.1038/ki.1995.404. [DOI] [PubMed] [Google Scholar]
- 129.Biemesderfer D, et al. NHE3: a Na+/H+ exchanger isoform of renal brush border. Am J Physiol. 1993;265(5 Pt 2):F736–F742. doi: 10.1152/ajprenal.1993.265.5.F736. [DOI] [PubMed] [Google Scholar]
- 130.D'Souza S, et al. The epithelial sodium-hydrogen antiporter Na+/H+ exchanger 3 accumulates and is functional in recycling endosomes. J Biol Chem. 1998;273(4):2035–2043. doi: 10.1074/jbc.273.4.2035. [DOI] [PubMed] [Google Scholar]
- 131.Gekle M, Freudinger R, Mildenberger S. Inhibition of Na+-H+ exchanger-3 interferes with apical receptor-mediated endocytosis via vesicle fusion. J Physiol. 2001;531(Pt 3):619–629. doi: 10.1111/j.1469-7793.2001.0619h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.LaPointe MS, et al. Na+/H+ exchange activity and NHE-3 expression in renal tubules from the spontaneously hypertensive rat. Kidney Int. 2002;62(1):157–165. doi: 10.1046/j.1523-1755.2002.00406.x. [DOI] [PubMed] [Google Scholar]
- 133.Li XX, et al. D(1) dopamine receptor regulation of NHE3 during development in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2001;280(6):R1650–R1656. doi: 10.1152/ajpregu.2001.280.6.R1650. [DOI] [PubMed] [Google Scholar]
- 134.Kelly MP, et al. Activity and Expression of Na+-H+ Exchanger Isoforms 1 and 3 in Kidney Proximal Tubules of Hypertensive Rats. Circ Res. 1997;80(6):853–860. doi: 10.1161/01.res.80.6.853. [DOI] [PubMed] [Google Scholar]
- 135.Hayashi M, et al. Na+/H+-exchanger 3 activity and its gene in the spontaneously hypertensive rat kidney. J Hypertens. 1997;15(1):43–48. [PubMed] [Google Scholar]
- 136.Zhang Y, et al. Rapid redistribution and inhibition of renal sodium transporters during acute pressure natriuresis. Am J Physiol. 1996;270(6 Pt 2):F1004–F1014. doi: 10.1152/ajprenal.1996.270.6.F1004. [DOI] [PubMed] [Google Scholar]
- 137.McDonough AA, Leong PK, Yang LE. Mechanisms of pressure natriuresis: how blood pressure regulates renal sodium transport. Ann N Y Acad Sci. 2003;986:669–677. doi: 10.1111/j.1749-6632.2003.tb07281.x. [DOI] [PubMed] [Google Scholar]
- 138.Yang L, et al. Acute hypertension provokes internalization of proximal tubule NHE3 without inhibition of transport activity. Am J Physiol Renal Physiol. 2002;282(4):F730–F740. doi: 10.1152/ajprenal.00298.2001. [DOI] [PubMed] [Google Scholar]
- 139.Yang LE, et al. Differential traffic of proximal tubule Na+ transporters during hypertension or PTH: NHE3 to base of microvilli vs. NaPi2 to endosomes. Am J Physiol Renal Physiol. 2004;287(5):F896–F906. doi: 10.1152/ajprenal.00160.2004. [DOI] [PubMed] [Google Scholar]
- 140.Schultheis PJ, et al. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet. 1998;19(3):282–285. doi: 10.1038/969. [DOI] [PubMed] [Google Scholar]
- 141.Lorenz JN, et al. Micropuncture analysis of single-nephron function in NHE3-deficient mice. Am J Physiol. 1999;277(3 Pt 2):F447–F453. doi: 10.1152/ajprenal.1999.277.3.F447. [DOI] [PubMed] [Google Scholar]
- 142.Ledoussal C, et al. Renal salt wasting in mice lacking NHE3 Na+/H+ exchanger but not in mice lacking NHE2. Am J Physiol Renal Physiol. 2001;281(4):F718–F727. doi: 10.1152/ajprenal.2001.281.4.F718. [DOI] [PubMed] [Google Scholar]
- 143.Orlowski J, Grinstein S. Diversity of the mammalian sodium/proton exchanger SLC9 gene family. Pflugers Arch. 2004;447(5):549–565. doi: 10.1007/s00424-003-1110-3. [DOI] [PubMed] [Google Scholar]
- 144.Moe OW. Acute regulation of proximal tubule apical membrane Na/H exchanger NHE-3: role of phosphorylation, protein trafficking, and regulatory factors. J Am Soc Nephrol. 1999;10(11):2412–2425. doi: 10.1681/ASN.V10112412. [DOI] [PubMed] [Google Scholar]
- 145.Chow C-W, et al. The Epithelial Na+/H+ Exchanger, NHE3, Is Internalized through a Clathrin-mediated Pathway. J. Biol. Chem. 1999;274(53):37551–37558. doi: 10.1074/jbc.274.53.37551. [DOI] [PubMed] [Google Scholar]
- 146.Li X, et al. Na+-H+ exchanger 3 (NHE3) is present in lipid rafts in the rabbit ileal brush border: a role for rafts in trafficking and rapid stimulation of NHE3. J Physiol. 2001;537(Pt 2):537–552. doi: 10.1111/j.1469-7793.2001.00537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Donowitz M, et al. Short-term regulation of NHE3 by EGF and protein kinase C but not protein kinase A involves vesicle trafficking in epithelial cells and fibroblasts. Ann N Y Acad Sci. 2000;915:30–42. doi: 10.1111/j.1749-6632.2000.tb05221.x. [DOI] [PubMed] [Google Scholar]
- 148.Fan L, et al. Dual mechanisms of regulation of Na/H exchanger NHE-3 by parathyroid hormone in rat kidney. J Biol Chem. 1999;274(16):11289–11295. doi: 10.1074/jbc.274.16.11289. [DOI] [PubMed] [Google Scholar]
- 149.Kurashima K, et al. Endosomal recycling of the Na+/H+ exchanger NHE3 isoform is regulated by the phosphatidylinositol 3-kinase pathway. J Biol Chem. 1998;273(33):20828–20836. doi: 10.1074/jbc.273.33.20828. [DOI] [PubMed] [Google Scholar]
- 150.Li X, et al. Carbachol regulation of rabbit ileal brush border Na+-H+ exchanger 3 (NHE3) occurs through changes in NHE3 trafficking and complex formation and is Src dependent. J Physiol. 2004;556(Pt 3):791–804. doi: 10.1113/jphysiol.2004.060921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tsuganezawa H, Preisig PA, Alpern RJ. Dominant negative c-Src inhibits angiotensin II induced activation of NHE3 in OKP cells. Kidney Int. 1998;54(2):394–398. doi: 10.1046/j.1523-1755.1998.00029.x. [DOI] [PubMed] [Google Scholar]
- 152.Janecki AJ, et al. Basic fibroblast growth factor stimulates surface expression and activity of Na(+)/H(+) exchanger NHE3 via mechanism involving phosphatidylinositol 3-kinase. J Biol Chem. 2000;275(11):8133–8142. doi: 10.1074/jbc.275.11.8133. [DOI] [PubMed] [Google Scholar]
- 153.Yang X, et al. Acid incubation causes exocytic insertion of NHE3 in OKP cells. Am J Physiol Cell Physiol. 2000;279(2):C410–C419. doi: 10.1152/ajpcell.2000.279.2.C410. [DOI] [PubMed] [Google Scholar]
- 154.Lee-Kwon W, et al. Constitutively active phosphatidylinositol 3-kinase and AKT are sufficient to stimulate the epithelial Na+/H+ exchanger 3. J Biol Chem. 2001;276(33):31296–31304. doi: 10.1074/jbc.M103900200. [DOI] [PubMed] [Google Scholar]
- 155.Collazo R, et al. Acute regulation of Na+/H+ exchanger NHE3 by parathyroid hormone via NHE3 phosphorylation and dynamin-dependent endocytosis. J Biol Chem. 2000;275(41):31601–31608. doi: 10.1074/jbc.M000600200. [DOI] [PubMed] [Google Scholar]
- 156.Hu MC, et al. Dopamine acutely stimulates Na+/H+ exchanger (NHE3) endocytosis via clathrin-coated vesicles: dependence on protein kinase A-mediated NHE3 phosphorylation. J Biol Chem. 2001;276(29):26906–26915. doi: 10.1074/jbc.M011338200. [DOI] [PubMed] [Google Scholar]
- 157.El Mernissi G, Doucet A. Quantitation of [3H]ouabain binding and turnover of Na-K-ATPase along the rabbit nephron. Am J Physiol. 1984;247(1 Pt 2):F158–F167. doi: 10.1152/ajprenal.1984.247.1.F158. [DOI] [PubMed] [Google Scholar]
- 158.Zhang Y, et al. Reversible effects of acute hypertension on proximal tubule sodium transporters. Am J Physiol. 1998;274(4 Pt 1):C1090–C1100. doi: 10.1152/ajpcell.1998.274.4.C1090. [DOI] [PubMed] [Google Scholar]
- 159.Yang LE, et al. Effects of dietary salt on renal Na+ transporter subcellular distribution, abundance, and phosphorylation status. Am J Physiol Renal Physiol. 2008;295(4):F1003–F1016. doi: 10.1152/ajprenal.90235.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pinho MJ, et al. Organ specific underexpression renal of Na+-dependent B0AT1 in the SHR correlates positively with overexpression of NHE3 and salt intake. Mol Cell Biochem. 2007;306(1–2):9–18. doi: 10.1007/s11010-007-9548-9. [DOI] [PubMed] [Google Scholar]
- 161.Pedrosa R, et al. Dopamine D3 receptor-mediated inhibition of Na+/H+ exchanger activity in normotensive and spontaneously hypertensive rat proximal tubular epithelial cells. Br J Pharmacol. 2004;142(8):1343–1353. doi: 10.1038/sj.bjp.0705893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Garg LC, Narang N. Sodium-potassium-adenosine triphosphatase in nephron segments of spontaneously hypertensive rats. J Lab Clin Med. 1985;106(1):43–46. [PubMed] [Google Scholar]
- 163.Hinojos CA, Doris PA. Altered subcellular distribution of Na+,K+-ATPase in proximal tubules in young spontaneously hypertensive rats. Hypertension. 2004;44(1):95–100. doi: 10.1161/01.HYP.0000132557.16738.92. [DOI] [PubMed] [Google Scholar]
- 164.Aperia AC. Intrarenal dopamine: a key signal in the interactive regulation of sodium metabolism. Annu Rev Physiol. 2000;62:621–647. doi: 10.1146/annurev.physiol.62.1.621. [DOI] [PubMed] [Google Scholar]
- 165.Zeng C, et al. Functional genomics of the dopaminergic system in hypertension. Physiol Genomics. 2004;19(3):233–246. doi: 10.1152/physiolgenomics.00127.2004. [DOI] [PubMed] [Google Scholar]
- 166.Hussain T, Lokhandwala MF. Renal dopamine receptors and hypertension. Exp Biol Med (Maywood) 2003;228(2):134–142. doi: 10.1177/153537020322800202. [DOI] [PubMed] [Google Scholar]
- 167.McDonald RH, Jr, et al. Effect of Dopamine in Man: Augmentation of Sodium Excretion, Glomerular Filtration Rate, and Renal Plasma Flow. J Clin Invest. 1964;43:1116–1124. doi: 10.1172/JCI104996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jose PA, Eisner GM, Felder RA. Role of dopamine receptors in the kidney in the regulation of blood pressure. Curr Opin Nephrol Hypertens. 2002;11(1):87–92. doi: 10.1097/00041552-200201000-00013. [DOI] [PubMed] [Google Scholar]
- 169.Wang X, et al. Dopamine, kidney, and hypertension: studies in dopamine receptor knockout mice. Pediatr Nephrol. 2008;23(12):2131–2146. doi: 10.1007/s00467-008-0901-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bertorello A, Aperia A. Both DA1 and DA2 receptor agonists are necessary to inhibit NaKATPase activity in proximal tubules from rat kidney. Acta Physiol Scand. 1988;132(3):441–443. doi: 10.1111/j.1748-1716.1988.tb08350.x. [DOI] [PubMed] [Google Scholar]
- 171.Bertorello A, Aperia A. Inhibition of proximal tubule Na(+)-K(+)-ATPase activity requires simultaneous activation of DA1 and DA2 receptors. Am J Physiol. 1990;259(6 Pt 2):F924–F928. doi: 10.1152/ajprenal.1990.259.6.F924. [DOI] [PubMed] [Google Scholar]
- 172.Bertorello A, et al. Proximal tubule Na+-K+-ATPase activity is inhibited during high-salt diet: evidence for DA-mediated effect. Am J Physiol. 1988;254(6 Pt 2):F795–F801. doi: 10.1152/ajprenal.1988.254.6.F795. [DOI] [PubMed] [Google Scholar]
- 173.Chibalin AV, et al. Receptor-mediated inhibition of renal Na(+)-K(+)-ATPase is associated with endocytosis of its alpha- and beta-subunits. Am J Physiol. 1997;273(5 Pt 1):C1458–C1465. doi: 10.1152/ajpcell.1997.273.5.C1458. [DOI] [PubMed] [Google Scholar]
- 174.Bacic D, et al. Dopamine acutely decreases apical membrane Na/H exchanger NHE3 protein in mouse renal proximal tubule. Kidney Int. 2003;64(6):2133–2141. doi: 10.1046/j.1523-1755.2003.00308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Oweis S, et al. Cardiac glycoside downregulates NHE3 activity and expression in LLC-PK1 cells. Am J Physiol Renal Physiol. 2006;290(5):F997–F1008. doi: 10.1152/ajprenal.00322.2005. [DOI] [PubMed] [Google Scholar]
- 176.Periyasamy SM, et al. Salt loading induces redistribution of the plasmalemmal Na/K-ATPase in proximal tubule cells. Kidney Int. 2005;67(5):1868–1877. doi: 10.1111/j.1523-1755.2005.00285.x. [DOI] [PubMed] [Google Scholar]
- 177.Liu J, et al. Effects of cardiac glycosides on sodium pump expression and function in LLC-PK1 and MDCK cells. Kidney Int. 2002;62(6):2118–2125. doi: 10.1046/j.1523-1755.2002.00672.x. [DOI] [PubMed] [Google Scholar]
- 178.Cook JS, Tate EH, Shaffer C. Uptake of [3H]ouabain from the cell surface into the lysosomal compartment of HeLa cells. J Cell Physiol. 1982;110(1):84–92. doi: 10.1002/jcp.1041100114. [DOI] [PubMed] [Google Scholar]
- 179.Algharably N, Owler D, Lamb JF. The rate of uptake of cardiac glycosides into human cultured cells and the effects of chloroquine on it. Biochem Pharmacol. 1986;35(20):3571–3581. doi: 10.1016/0006-2952(86)90628-3. [DOI] [PubMed] [Google Scholar]
- 180.Li H, Nord EP. Functional caveolae are a prerequisite for CD40 signaling in human renal proximal tubule cells. Am J Physiol Renal Physiol. 2004;286(4):F711–F719. doi: 10.1152/ajprenal.00308.2003. [DOI] [PubMed] [Google Scholar]
- 181.Moriyama T, et al. Caveolar endocytosis is critical for BK virus infection of human renal proximal tubular epithelial cells. J Virol. 2007;81(16):8552–8562. doi: 10.1128/JVI.00924-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Harootunian AT, et al. Fluorescence ratio imaging of cytosolic free Na+ in individual fibroblasts and lymphocytes. J Biol Chem. 1989;264(32):19458–19467. [PubMed] [Google Scholar]
- 183.Nishi A, Bertorello AM, Aperia A. High salt diet down-regulates proximal tubule Na+, K(+)-ATPase activity in Dahl salt-resistant but not in Dahl salt-sensitive rats: evidence of defective dopamine regulation. Acta Physiol Scand. 1992;144(3):263–267. doi: 10.1111/j.1748-1716.1992.tb09295.x. [DOI] [PubMed] [Google Scholar]
- 184.Nishi A, et al. Dopamine regulation of renal Na+,K(+)-ATPase activity is lacking in Dahl salt-sensitive rats. Hypertension. 1993;21(6 Pt 1):767–771. doi: 10.1161/01.hyp.21.6.767. [DOI] [PubMed] [Google Scholar]
- 185.Felder RA, Jose PA. Mechanisms of disease: the role of GRK4 in the etiology of essential hypertension and salt sensitivity. Nat Clin Pract Nephrol. 2006;2(11):637–650. doi: 10.1038/ncpneph0301. [DOI] [PubMed] [Google Scholar]
- 186.Hussain T, Lokhandwala MF. Renal dopamine DA1 receptor coupling with G(S) and G(q/11) proteins in spontaneously hypertensive rats. Am J Physiol. 1997;272(3 Pt 2):F339–F346. doi: 10.1152/ajprenal.1997.272.3.F339. [DOI] [PubMed] [Google Scholar]
- 187.Felder RA, et al. G protein-coupled receptor kinase 4 gene variants in human essential hypertension. Proc Natl Acad Sci U S A. 2002;99(6):3872–3877. doi: 10.1073/pnas.062694599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Fedorova OV, Lakatta EG, Bagrov AY. Endogenous Na,K pump ligands are differentially regulated during acute NaCl loading of Dahl rats. Circulation. 2000;102(24):3009–3014. doi: 10.1161/01.cir.102.24.3009. [DOI] [PubMed] [Google Scholar]
- 189.Guyton AC. Blood pressure control--special role of the kidneys and body fluids. Science. 1991;252(5014):1813–1816. doi: 10.1126/science.2063193. [DOI] [PubMed] [Google Scholar]
- 190.Cowley AW., Jr Role of the renal medulla in volume and arterial pressure regulation. Am J Physiol. 1997;273(1 Pt 2):R1–R15. doi: 10.1152/ajpregu.1997.273.1.R1. [DOI] [PubMed] [Google Scholar]
- 191.Johnson RJ, et al. Pathogenesis of essential hypertension: historical paradigms and modern insights. J Hypertens. 2008;26(3):381–391. doi: 10.1097/HJH.0b013e3282f29876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ferrandi M, et al. Ouabain-dependent signaling in caveolae as a novel therapeutic target for hypertension. Cell Mol Biol (Noisy-le-grand) 2006;52(8):15–18. [PubMed] [Google Scholar]
- 193.Lopatin DA, et al. Circulating bufodienolide and cardenolide sodium pump inhibitors in preeclampsia. J Hypertens. 1999;17(8):1179–1187. doi: 10.1097/00004872-199917080-00018. [DOI] [PubMed] [Google Scholar]
- 194.Hamlyn JM, Manunta P. Ouabain, digitalis-like factors and hypertension. J Hypertens Suppl. 1992;10(7):S99–S111. [PubMed] [Google Scholar]
- 195.Dostanic I, et al. The alpha2-isoform of Na-K-ATPase mediates ouabain-induced hypertension in mice and increased vascular contractility in vitro. Am J Physiol Heart Circ Physiol. 2005;288(2):H477–H485. doi: 10.1152/ajpheart.00083.2004. [DOI] [PubMed] [Google Scholar]
- 196.de Wardener HE. Franz Volhard Lecture 1996. Sodium transport inhibitors and hypertension. J Hypertens Suppl. 1996;14(5):S9–S18. [PubMed] [Google Scholar]
- 197.Buckalew VM. Endogenous digitalis-like factors. An historical overview. Front Biosci. 2005;10:2325–2334. doi: 10.2741/1701. [DOI] [PubMed] [Google Scholar]