Abstract
The p53 family (p53, p73, p63) plays an instrumental role in the cellular stress response, including induction of cell cycle arrest and apoptosis in response to DNA damage (1, 2). In addition to p63 and p73 isotypes capable of transactivating downstream target gene expression (TA isotypes), both genes also express dominant negative inhibitory isoforms, such as ΔNp63α. ΔNp63α is degraded in response to DNA damaging agents, thereby enabling an effective cellular response to genotoxic agents. Here, we identify a key molecular mechanism underlying the regulation of ΔNp63α expression in response to extrinsic stimuli, such as chemotherapeutic agents or TNF-α. We show that ΔNp63α interacts with IκB kinase (IKK), a multisubunit protein kinase that consists of two catalytic subunits, IKKα and IKKβ, and a regulatory subunit, IKKγ (NEMO-NF-κB essential modifier). We find that IKKβ kinase promotes ubiquitin-mediated proteasomal degradation of ΔNp63α, whereas a kinase-deficient mutant IKKβ-K44A fails to do so. Cytokine- or chemotherapy-induced stimulation of IKKβ leads to degradation of ΔNp63α and augments trans-activation of p53 family-induced genes involved in the cellular response to DNA damage. Conversely, inhibition of IKKβ with a NEMO-binding peptide or siRNA-mediated silencing IKKβ expression attenuates cytokine- or chemotherapy induced degradation of ΔNp63α. These data demonstrate that IKKβ plays an essential role in regulating ΔNp63α in response to extrinsic stimuli. Our findings suggest that the activation of IKK may be a mechanism by which levels of ΔNp63α are reduced, thereby rendering the cells susceptible to cell death in the face of cellular stress or DNA damage.
Keywords: ΔNp63α, IKKβ, Ubiquitination, cisplatin, TNF-α
INTRODUCTION
The p53 family includes the product encoded by the p53 tumor suppressor gene and its homologs, p63 and p73. p53 plays an instrumental role in the cellular stress response, including induction of cell cycle arrest and apoptosis in response to DNA damage. Whereas p53 is frequently lost in human cancers, p63 is located in a region on chromosome 3q27-ter that is amplified in various cancers (3, 4). Both p63 and p53 genes encode proteins that possess transactivation, DNA binding and oligomerization domains. However, unlike p53, the p63 gene contains two separate promoters that direct expression of two fundamentally different classes of proteins (5). One, denoted TAp63, is marked by an acidic N terminus with homology to the transactivation domain of p53. A second promoter, located within an intron and over 30 kb downstream, gives rise to N-terminally truncated (ΔN) products that lack the TA domain (5–7). ΔNp63 can readily compete with p53 (or transactivating p63 or p73 isoforms) for DNA target sites, and also form transactivation-incompetent heterocomplexes between ΔN proteins and p53, TAp73 or TAp63 (4, 5, 8). Since ΔNp63 acts as a dominant negative that counteracts the function of transactivating p53 family members, this dynamic balance is a crucial determinant of cellular responses to genotoxic chemotherapeutic agents(5, 9, 10)..
p63 is constitutively expressed in the stem cell compartment of many epithelial tissues (5). The high level of p63 in epithelial stem cells is made up almost entirely of ΔNp63 isoforms, indicating a role for dominant–negative or repressive versions of p63 in epithelial stem cell identity. p63-null mice lack limbs and a wide range of epithelial structures including skin, prostate, breast and urothelia (11, 12). The inability of p63-deficient embryos to develop and maintain multi-layered, regenerative epithelia highlight the essential role of p63 in the survival of epithelial stem cells (11). Whereas p53 and p73 are stabilized and up-regulated in response to genotoxic insults (UV radiation, chemotherapeutic agents), ΔNp63α is degraded in response to these agents (13–15). This degradation of ΔNp63α could augment the function of transactivating p53 family members and enable an effective cellular response to genotoxic agents (16).
In this study, we identify a key molecular mechanism underlying the regulation of ΔNp63α expression in response to extrinsic stimuli, such as chemotherapeutic agents or TNF-α. We show that ΔNp63α interacts with IκB kinase (IKK), a multisubunit protein kinase that consists of two catalytic subunits, IKKα and IKKβ, and a regulatory subunit, IKKγ (NEMO-NF-κB essential modifier) (17, 18). We find that IKKβ kinase promotes ubiquitin-mediated degradation of ΔNp63α (19). Cytokine- or chemotherapy-induced stimulation of IKK leads to degradation of ΔNp63α and augments trans-activation of p53 family-induced genes involved in the cellular response to DNA damage. Conversely, inhibition of IKKβ with a NEMO-binding peptide or siRNA-mediated silencing IKKβ expression attenuates cytokine- or chemotherapy induced degradation of ΔNp63α. These data demonstrate that IKKβ plays an essential role in regulating ΔNp63α in response to extrinsic stimuli. Our findings suggest that the activation of IKK may be a mechanism by which levels of ΔNp63α are reduced, thereby rendering the cells susceptible to cell death in the face of cellular stress or DNA damage.
MATERIALS AND METHODS
Plasmids
ΔNp63α-Flag was amplified by PCR amplification of ΔNp63α cDNA and cloned into the BamHI and NotI sites of pCDNA3.1/hygro (Invitrogen, Carlsbad, CA). Flag-IKKβ and IKKα were provided by Dr. Karin (UCSD, CA). HA-IKKβ and HA-IKKα were provided by Dr. Zandi (Univ. of South California, LA, CA). Mutant HA-IKKα and Flag-IKKβ were provided by Dr. Green (UCSF, CA). Plasmid HA-Ub construct was provided by Dr. Bohmann (University of Rochester, NY, USA). Bax-Luciferase expression vector was provided by Dr. John Reed (Burnham Institute for Medical Research, CA, USA) and p21WAF1-Luciferase construct was a gift from Dr. Bert Vogelstein (Johns Hopkins University, Baltimore, USA). All clones were sequenced to rule out any mutation.
Cell Cultures and Transfection
The human head and neck cancer cell line JHU 022 and the human lung carcinoma cell line (H1299) were maintained in RPMI supplemented with 10% heat-inactivated fetal bovine serum (FBS; Invitrogen, Carlsbad, CA, USA), 100 U/ml penicillin and 100μg/ml streptomycin. Cells were maintained in a humidified atmosphere containing 5% CO2 at 37°C. H1299 and JHU 022 cells were transiently transfected with the indicated expression plasmids using FuGENE HD transfection reagent (Roche Molecular Biochemicals, Indianapolis, IN) in accordance with the manufacturer’s specifications. IKKα and IKKβ siRNA were obtained from Imgenex (San Diego, CA). The transfected cells were treated with or without 75μM cisplatin or 20 ng/ml TNF-α before harvesting them for cellular lysates.
Antibodies and Immunoblot Analysis
Monoclonal anti-p63 (4A4), monoclonal anti-HA, monoclonal anti-IKKβ, and polyclonal anti-IKKα antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Monoclonal anti-FLAG (M2) and anti-β-Actin antibodies were obtained from Sigma Chemicals (St. Louis, MO). Western blot was performed as described in (13). Nuclear and cytoplasmic fractions were prepared as described in (20).
Immunoprecipitation Analysis
Cells were transfected with various constructs, washed with PBS, and lysed using TRITON-X lysis buffer (50mM Tris-HCl, pH 7.4; 150mM NaCl, 1mM EDTA; 1% TRITON-X100) containing protease and phosphatase inhibitor cocktails (Sigma Chemical Co). Lysates were pre-cleaned with protein A-sepharose beads and then incubated for 2h at 4°C with primary antibody or affinity matrix. The immune complexes were precipitated with protein A-Sepharose beads for 4 h at 4°C, and the nonspecific bound proteins were removed by washing the beads with the NP-40 lysis buffer three times at 4°C. The beads were loaded in Laemmli sample buffer directly and analyzed by immunoblotting with the indicated antibodies.
Ubiquitination Assay
JHU 022 cells were transiently co-transfected with the expression plasmid for HA-tagged ubiquitin and Flag-ΔNp63α, together with or without increasing amounts of either the IKKβ, IKKβ-K44A, IKKα, or IKKα-K44M expression plasmid, as indicated. Total amounts of transfected DNA per transfection were kept constant by pcDNA3 (Invitrogen). 48 h after transfection, cells were treated with 25μM MG-132 and collected in lysis buffer (50mM Tris-HCl, pH 7.4; 150mM NaCl, 1mM EDTA; 1% TRITON-X100) containing protease and phosphatase inhibitors (Sigma Chemical Co). Ubiquitinated proteins were recovered by HA resin (Sigma) and analyzed by immunoblotting with the anti-Flag or anti-p63 antibody.
Measurement of p63 half-life
Cycloheximide (100 μg/ml) (Sigma) was added to JHU 022 cells 24 h after transfection with the indicated combination of plasmids. Protein levels were determined by collecting cells at the indicated time points, and immunoblotting was performed as described above. The membrane was re-probed with β-Actin to confirm equal loading.
Promoter Luciferase Activity Assays
JHU 022 cells were transiently transfected with either the Bax or p21WAF1 reporter plasmids along with 500 ng of either ΔNp63α or TA- p63α expression plasmid (where indicated) in the presence or absence of 1.0μg of either the IKKβ expression plasmid or the empty pGL3-Basic vector using FuGENE HD according to the manufacturer’s protocol (Roche, Indianapolis IN). DNA concentration in each transfection was kept constant using pCDNA3.1 plasmid (Invitrogen, Carlsbad CA). The transfection efficiency was determined by the Renilla luciferase gene-containing pRL-CMV plasmid (Promega, Madison, WI). 48 h after transfection, cells were washed twice with phosphate-buffered saline (PBS) and lysed in a lysis buffer (5x PLBR, Promega, Madison, WI) with gentle shaking at room temperature for 20 min. Cell lysates were centrifuged at 13,000 rpm on a table top centrifuge for 2 min to pellet the cell debris. The supernatants were transferred to a fresh tube, and the dual luciferase activity in cell extracts was determined according to the manufacturer’s protocol (Promega). Briefly, each assay mixture contained 2 μl (2 μg) of the cell lysates and 10 μl of a firefly luciferase-measuring buffer (LARll R, Promega, Madison, WI). The firefly luciferase activity was measured by a luminometer (the luminometer was programmed to perform a 2-s pre-measurement delay, followed by a 10-s measurement period for each reporter assay). After measuring the promoter luciferase activity (Stop & GloR, Promega, Madison, WI), a Renilla luciferase-measuring buffer was added, and the Renilla luciferase activity was then measured. Each transfection was performed in duplicate, and all experiments were repeated at least three times.
Apoptosis Assays
JHU-022 cells were transfected with 500 ng of either ΔNp63α or TAp63α expression plasmid (where indicated) in the presence or absence of 1.0μg of either the IKKβ expression plasmid or the empty pcDNA3.1 vector as indicated, and analyzed for apoptosis using Annexin V and PI staining. Percentage apoptosis was determined by analyzing the cells on the FAC Star flow cytometer (Becton Dickinson, Franklin Lakes, NJ). Data analysis was done using the software “Cell Quest”.
RESULTS
IKK interacts with and regulates ΔNp63α in response to Cisplatin or TNF-α
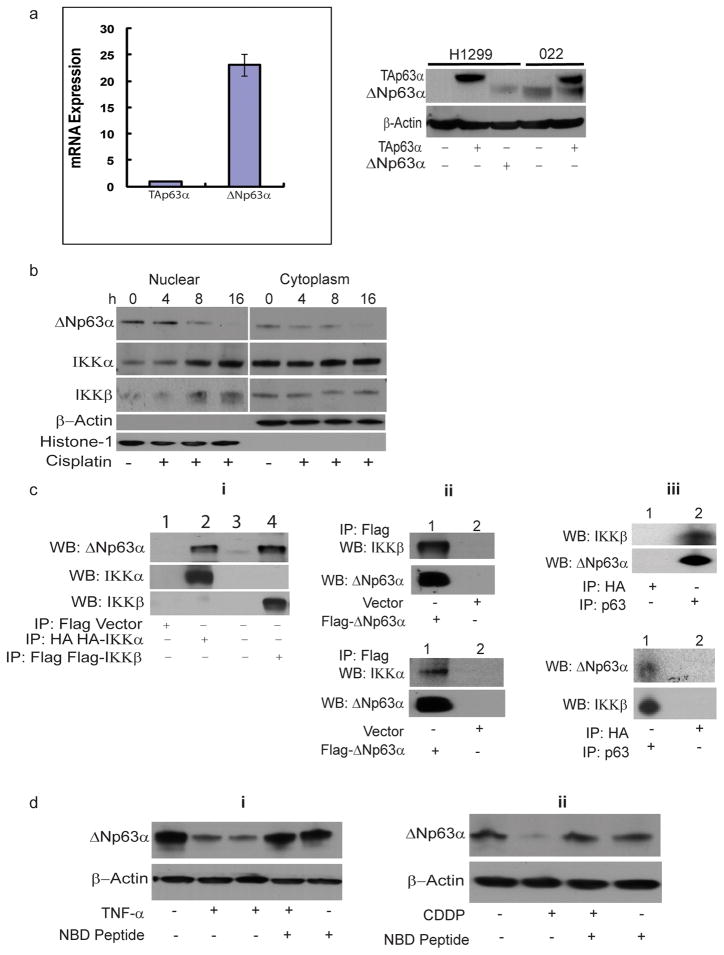
We performed isoform-specific real-time quantitative RT-PCR to detect isoforms containing or lacking the N-terminal transactivation domain (TAp63α and ΔNp63α, respectively) in JHU-022 HNSCC cells. Whereas ΔNp63α mRNA is highly expressed in JHU-022 cells, these cells exhibit very low expression of TAp63α mRNA (Expression of ΔNp63α mRNA is more than 23-fold more abundant than that of TAp63α mRNA) (Fig. 1a). In accordance with the differential expression of ΔNp63α and TAp63α mRNA, Western blot analysis confirmed that JHU-022 cells display high levels of ΔNp63α protein but undetectable levels of the TAp63α isoform (Fig. 1a).
Figure 1. IKK interacts with and regulates ΔNp63α in response to Cisplatin or TNF-α.
(a) Isoform-specific real-time quantitative RT-PCR to detect isoforms TAp63α and ΔNp63α in JHU-022 cells (left panel). Western blot analysis of cellular lysates from either the p53/p63 deficient cell line H1299 or JHU-022 cells (right panel) that were untransfected or transfected with either TAp63α or ΔNp63α expression plasmids, as indicated.
(b) Loss of ΔNp63α and nuclear accumulation of IKKβ and IKKα in JHU-022 cells in response to treatment with cisplatin. At the indicated time periods after treatment with cisplatin, JHU-022 cells were fractionated into nuclear and cytoplasmic fractions and analyzed by immunobloting with the indicated antibodies.
(c) Complex formation between ΔNp63α with either IKKβ or IKKα. Panel i: JHU-022 cells were transfected with either HA-IKKα or Flag-IKKβ or empty Flag-vector. Whole cell lysates were immunoprecipitated with either anti-flag or anti-HA matrix, as indicated, and subjected to western blot analysis using either anti-p63 or anti-IKKα or anti-IKKβ antibodies, as indicated. Panel ii: J H U-022 cells were transfected with Flag-ΔNp63α or empty Flag–vector. Immunoprecipitation was performed using anti-flag matrix and the membrane was blotted with either anti-IKKβ, anti-IKKα, or anti-p63 antibody, as indicated. Panel iii: Immunoprecipitates of JHU-022 cell lysates using either anti-p63 or anti-IKKβ antibody were subjected to immunoblot analysis using either anti-IKKβ or anti-p63 antibody, as indicated.
(d) IKK is required for TNF-α– or cisplatin-mediated reduction of ΔNp63α in JHU-022 cells. Panel i: JHU-022 cells were pre-treated with or without 100μM NEMO inhibitory peptide or vehicle control for 2h followed by treatment with or without 20 ng/ml TNF-α for 10h. Whole cell lysates were collected and subjected to Western blot using anti-p63 antibody. (lane 1 – untreated cells, lane 2 – TNF-α for 10h, lane 3 – TNF-α for 16h, lane 4 – NBD+TNF-α, lane 5 – NBD alone). Panel ii: JHU-022 cells were pre-treated with or without 100μM NEMO inhibitory peptide or vehicle control for 2h followed by treatment with or without cisplatin for 8h. Cells were harvested and subjected to Western blot using anti-p63 antibody.
We examined the effect of cisplatin on expression of ΔNp63α in JHU-022 HNSCC cells. Treatment of cells with cisplatin resulted in decreased levels of ΔNp63α within 8h of exposure (Fig. 1b). The loss of ΔNp63α in response to cisplatin was associated with concomitant accumulation of the IKK subunits, IKKα and IKKβ, in the nuclear fractions of treated cells (Fig. 1b). This inverse relationship raised the possibility that activation of IKK may play a role in the reduction of ΔNp63α in response to cisplatin treatment. To explore this notion, we investigated whether ΔNp63α physically interacts with either IKKα or IKKβ in JHU-022 cells transfected with expression vectors encoding either HA-IKKα or Flag-IKKβ Immunoblot analysis with an anti-p63 antibody demonstrated the presence of endogenous ΔNp63α in anti-flag or anti-HA precipitated immune complexes from transfected cell lysates (Fig. 1c, Panel i). Conversely, immunoblot analysis with either an anti-IKKα– or an anti-IKKβ– specific antibody revealed the presence of both IKK subunits in anti-flag immunoprecipitates derived from lysates of JHU-022 cells transfected with Flag-ΔNp63α (Fig. 1c, Panel ii). The interaction between endogenous ΔNp63α and IKKβ was established by immunoblot analysis of anti-p63 immunoprecipates with an anti-IKKβ antibody, and further confirmed by immunoblot analysis of anti-IKKβ immunoprecipates with an anti-p63 antibody (Fig. 1c, Panel iii). These results confirmed the interaction between ΔNp63α and the catalytic subunits of the IKK complex.
To determine whether the levels of ΔNp63α are influenced by IKK, JHU-022 cells were exposed to TNF-α, a cytokine that activates the IKK complex. Treatment of cells with TNF-α resulted in the loss of ΔNp63α within 10h of exposure (Fig. 1d–i). To investigate the role of IKK activity in TNF-α-mediated reduction of ΔNp63α, JHU-022 cells were exposed to TNF-α in the presence of a cell-permeable NEMO (IKKγ) binding domain (NBD) peptide which prevents the activation of the IKK complex. Pre-treatment of cells with the NBD peptide prevented TNF-α-mediated reduction of ΔNp63α, thereby confirming the requirement of IKK activity in cytokine-induced loss of ΔNp63α (Fig. 1d–i). To determine whether IKK activity mediates an analogous loss of ΔNp63α in response to genotoxic chemotherapeutic agents, JHU-022 cells were treated with cisplatin, with or without pre-treatment with the NBD peptide. Cisplatin mediated reduction of ΔNp63α was attenuated in the presence of the NBD peptide (Fig. 1d–ii).
IKKβ kinase activity mediates the reduction of ΔNp63α levels by cisplatin or TNF-α
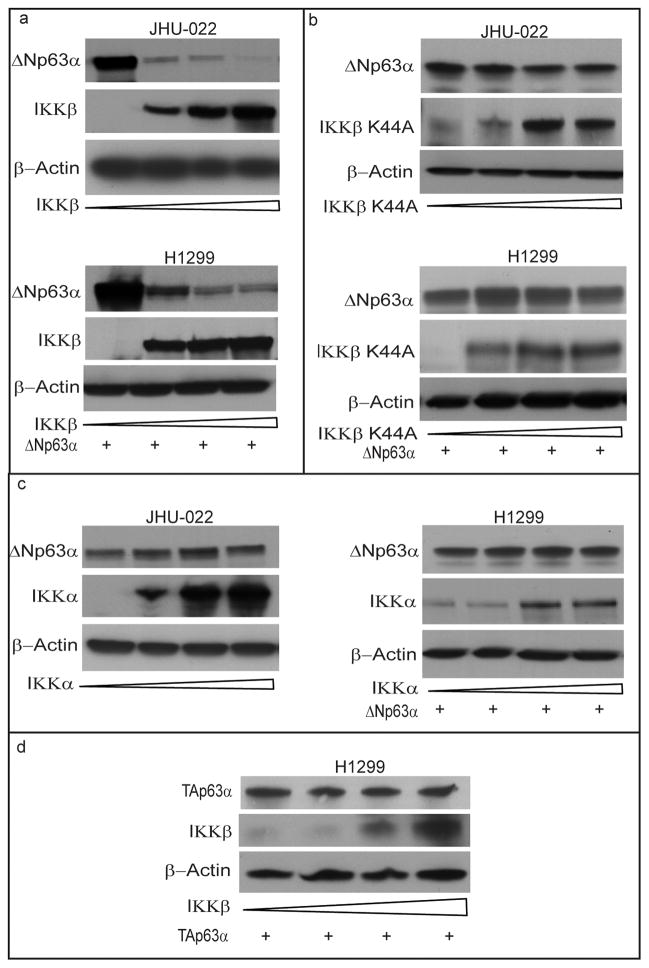
IKK is a multisubunit protein kinase that consists of two catalytic subunits, IKKα and IKKβ. To determine the identity of the specific IKK subunit responsible for decreasing ΔNp63α, JHU-022 cells were transfected with expression vectors encoding either wild-type IKKβ or IKKα, or their respective kinase-deficient mutants (IKKβ-K44A, IKKα-K44M). Forced overexpression of IKKβ significantly decreased the steady state levels of ΔNp63α in JHU- 022 cells (Fig. 2a). In contrast, overexpression of the kinase-deficient IKKβ-K44A in JHU-022 cells did not result in a corresponding reduction of ΔNp63α (Fig. 2b). Unlike IKKβ, overexpression of either IKKα (Fig. 2c) or its kinase-deficient mutant IKKα-K44M (data not shown) did not effect any significant change in the steady state level of ΔNp63α.
Figure 2. IKKβ kinase mediates the reduction of ΔNp63α levels by cisplatin or TNF-α.
(a,b and c) JHU-022 and p53/p63 deficient H1299 cells were transfected with increasing concentrations (0, 0.5, 1 and 1.5μg) of expression plasmids encoding either IKKβ (a), IKKβK44A (b), or IKKα (c) as indicated; Western blot was performed using the indicated antibodies. p53/p63 deficient H1299 cells were also transfected with 1μg of ΔNp63α expression plasmid. (d) p53/p63 deficient H1299 cells were transfected with 1μg of TAp63α expression plasmid with increasing concentrations (0, 0.5, 1 and 1.5μg) of expression plasmids encoding IKKβ and subjected to Western blotting with the indicated antibodies.
To determine whether IKKβ directly affects the stability of ΔNp63α, we examined the effect of co-transfecting a p63- and p53-deficient lung cancer cell line (H1299) with an expression vector encoding ΔNp63α and an expression vector encoding either wild-type or kinase-deficient IKK subunits. Forced expression of IKKβ, but not it’s kinase deficient mutant resulted in a dose-dependent decrease in levels of expression of exogenous ΔNp63α (Fig. 2a,b). In contrast, forced expression of IKKα did not affect the levels of expression of exogenous ΔNp63α (Fig. 2c). Whereas IKKβ decreased the level of exogenous ΔNp63α, forced expression of IKKβ did not affect the levels of exogenous TAp63α in H1299 cells (Fig. 2d). These data indicate that IKKβ directly reduces the stability and/or promotes the degradation of ΔNp63α.
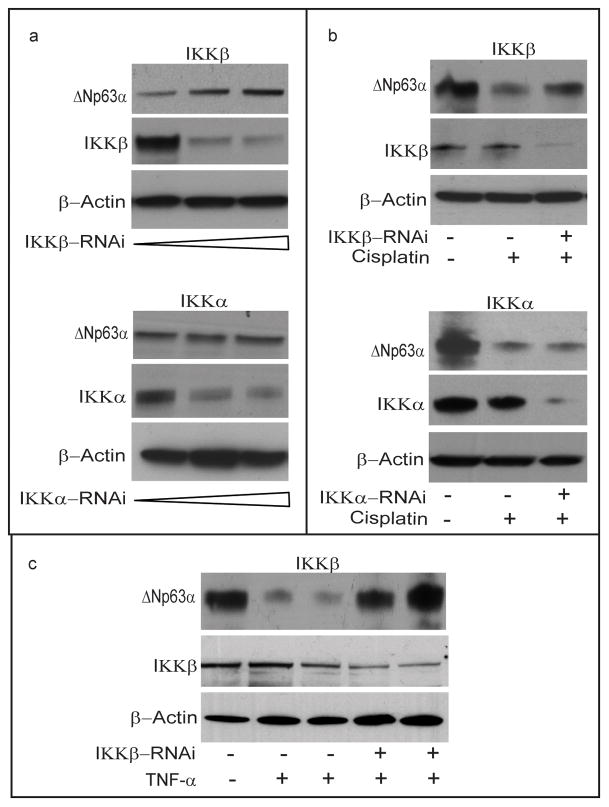
To determine whether activation of IKK is required for the reduction of endogeous ΔNp63α in response to cisplatin, we examined the effect of silencing the expression of each IKK subunit with expression vectors encoding either IKKβ RNAi or IKKα RNAi. As previously noted, JHU-022 cells that were transfected with IKKβ expressed very low levels of endogenous ΔNp63α; co-transfection of these cells with the plasmid encoding IKKβ RNAi resulted in restoration of basal levels of ΔNp63α (Fig. 3a). In contrast to IKKβ siRNA, no significant change in the stability of ΔNp63α was observed in response to transfection with the IKKα RNAi plasmid (Figure 3a). To determine whether IKK is required for the degradation ofΔNp63α in response to cisplatin, endogenous IKKβ or IKKα was knocked down with transfection of the respective IKK RNAi plasmid. Inhibition of IKKβ expression by RNAi attenuated the degradation of ΔNp63α in response to cisplatin (Fig. 3b). In contrast, RNAi mediated knockdown of IKKα did not result in any significant impairment in the degradation ofΔNp63α in response to cisplatin (Fig 3b). To determine whether IKK is also required for the degradation of ΔNp63α in response to TNF-α, endogenous IKKβ was knocked down with transfection of the IKKβ RNAi plasmid. Inhibition of IKKβ expression by RNAi attenuated the degradation of ΔNp63α in response to TNF-α (Fig. 3c).
Figure 3. IKKβ is required for the reduction of endogeous ΔNp63α in response to cisplatin or TNF-α.
(a) JHU-022 cells were transfected with increasing concentrations of either IKKβ RNAi plasmid or IKKα RNAi plasmid, and subjected to Western blot analysis using anti-p63 antibodies to assess the endogenous levels of p63. (b) JHU-022 cells were transfected with or without IKKβ RNAi plasmid or IKKα RNAi plasmid; 24h after transfection, the cells were treated with or without 75μM cisplatin for 8h, and cell lysates were subjected to Western blot using anti-p63 antibodies. (c) JHU-022 cells were transfected with or without IKKβ RNAi plasmid; 24h after transfection, the cells treated with or without TNF-α. Cells were harvested and lysates were subjected to Western blot using anti-63 antibody. (lane1 - untreated cells, lane 2 - TNF-α for 10h, lane 3 - TNF-α for 16h, lane 4 - IKKβ RNAi + TNF-α for 10h, lane 5 - IKKβ RNAi + TNF-α for 16h).
IKKβ promotes ubiquitin-mediated proteasomal degradation of ΔNp63α
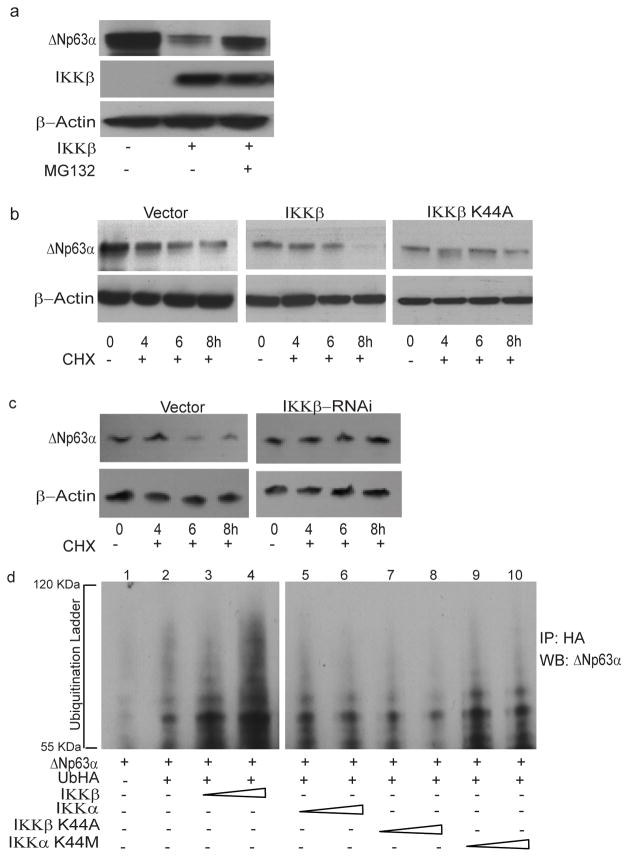
Phosphorylation of substrates, such as IκB proteins, by the IKK complex targets them for ubiquitin-mediated proteasomal degradation. To determine whether IKKβ promotes proteasomal degradation of ΔNp63α, JHU-022 cells were transfected with an expression vector encoding IKKβ and then either treated with the proteasome inhibitor, MG132, or left untreated. Whereas expression of IKKβ via transfection resulted in loss of endogenous ΔNp63α, treatment of transfected cells with MG132 significantly attenuated IKKβ-mediated degradation of ΔNp63α (Fig. 4a). These data suggested that the reduction of ΔNp63α by IKKβ may involve proteasome-mediated degradation. To directly assess whether IKKβ reduces the stability of ΔNp63α protein, we analyzed the effect of expressing either IKKβ or its kinase-deficient mutant (IKKβ K44A) on the half-life of ΔNp63α in JHU-022 cells. Cells were co-transfected with a plasmid encoding ΔNp63α and an expression vector encoding either IKKβ or IKKβ K44A, and then exposed to cycloheximide at 24h following transfection. Immunoblot analysis of ΔNp63α at serial time intervals showed that expression of IKKβ decreased the half-life of ΔNp63α (Fig. 4b); however, expression of the kinase-deficient mutant, IKKβ K44A, did not exhibit a corresponding effect on the stability ofΔNp63α (Fig. 4b). To confirm the effect of IKKβ on the half life of ΔNp63α, JHU-022 cells were transfected with a control vector or IKKβ RNAi vector, and exposed to cycloheximide 24h following transfection. Immunoblot analysis of ΔNp63α at serial time points showed that in the IKKβ silencing by RNAi prolongs the half-life of ΔNp63α (Fig. 4c).
Figure 4. IKKβ promotes ubiquitin-mediated proteasomal degradation of ΔNp63α.
(a) JHU-022 cells were transfected with or without an expression plasmid encoding IKKβ; 24h after transfection, cells were treated with 10μM MG132 for 10h. Whole cell lysates were subjected to Western Blot analysis using anti-IKKβ or anti-p63 antibodies.
(b) JHU-022 cells were transfected with constant amount of ΔNp63α expression plasmid with or without expression plasmids encoding either IKKβ, IKKβ K44A, or empty vector, as indicated. 24h after transfection, the cells were treated with 100μg/ml cycloheximide. At the indicated time points, whole cell lysates were analyzed for ΔNp63α by immunoblotting. Actin was used for loading control.
(c) JHU-022 cells were transfected with constant amount of IKKβ RNAi expression plasmid. 24h after transfection, the cells were treated with 100μg/ml cycloheximide. At the indicated time points, whole cell lysates were analyzed for endogenous ΔNp63α by immunoblotting. Actin was used for loading control.
(d) JHU-022 cells were co-transfected with ΔNp63α and Ub-HA expression plasmids, with or without increasing concentrations of an expression vector encoding either IKKβ, IKKβK44A, IKKα or IKKαK44M. At 36h following transfection, cells were treated with MG132 for 10h. Cell lysates were immunoprecipitated with anti-HA-matrix and subjected to western blot analysis with an antibody that recognizes ΔNp63α to assess the ubiquitination levels of ΔNp63α.
Since IKKβ interacts with ΔNp63α and promotes its kinase-dependent degradation, we next investigated whether the ubiquitin–proteasome pathway is involved in this process. JHU-022 cells were co-transfected with Flag-ΔNp63α and Ub-HA expression plasmids, with or without increasing concentrations of an expression vector encoding either Flag-IKKβ, FlagIKKβK44A, Flag-IKKα or Flag-IKKαK44M. At 36h following transfection, cells were treated with MG132 for 10h. Cell lysates were immunoprecipitated with anti-HA-matrix and subjected to western blot analysis with an antibody that recognizes ΔNp63α. Expression of IKKβ resulted in a dose-dependent elevation of the ubiquitination of ΔNp63α protein (Fig. 4d, compare lane 2 to lanes 3 and 4). In contrast, no significant change in the ubiquitination levels of ΔNp63α was observed in response to expression of either IKKβK44A, IKKα or IKKαK44M (Fig. 4d). These data demonstrate that IKKβ promotes kinase-dependent ubiquitin-mediated degradation of ΔNp63α.
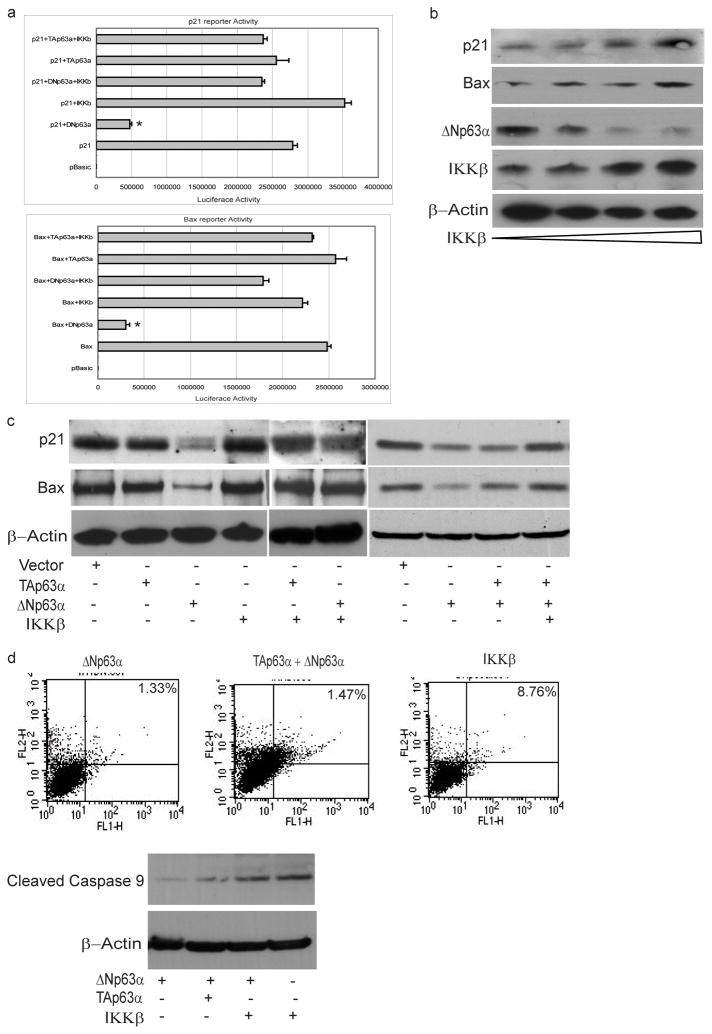
IKKβ counteracts ΔNp63α-mediated repression of p53-dependent transactivation
ΔNp63 proteins compete with p53, TAp73, or TAp63 for DNA target sites, and form transactivation-incompetent heterocomplexes with them (5, 21). As such, ΔNp63 acts as a dominant negative that counteracts the function of transactivating p53 family members (22). Since IKKβ promoted the ubiquitin-mediated degradation of ΔNp63α, this raised the possibility that IKK β may enhance p53-dependent transactivation of genes. We investigated the effect of expressing ΔNp63α on the transactivation of two established p53-inducible target genes, p21(WAF1/CIP1) and BAX. Forced expression of ΔNp63α via introduction of the ΔNp63α expression plasmid resulted in a significant reduction of the activity of Bax and p21WAF1 reporter plasmids in JHU-022 cells (Fig. 5a). However, co-transfection of an expression vector encoding IKKβ counteracted the repression of either promoter by ΔNp63α (Fig. 5a). In contrast, forced expression of TAp63α, alone or in combination with IKKβ, did not affect the activity of either the Bax or the p21WAF1 reporter plasmids (Fig. 5a).
Figure 5. IKKβ counteracts ΔNp63α-mediated repression of p53-dependent transactivation.
(a) JHU-022 cells were transfected with either p21 or Bax luciferase promoter construct along with Renilla luciferase plasmid, with or without IKKβ and/or ΔNp63α or TAp63α, as indicated. The amount of DNA per transfection was kept constant by using empty pCDNA3.1 vector. At 24 h post-transfection, the luciferase activity was determined. The transfection efficiency was standardized against Renilla luciferase. Results shown are representative of three independent experiments. * indicated p ≤ 0.001.
(b) JHU-022 cells were transfected with increasing concentrations of IKKβ expression plasmid and Western blot analysis was performed with the indicated antibodies to assess the endogenous levels of the indicated proteins.
(c) JHU-022 cells were transfected with empty vector or expression vector encoding either TAp63α, ΔNp63α, or IKKβ (alone or in combination with each other, as indicated), and the endogenous levels of p21 and Bax protein levels were assessed by Western Blotting.
(d) JHU-022 cells were transfected with either ΔNp63α, TAp63α, or IKKβ (alone or in combination with each other, as indicated) and analyzed after 24h for activation of caspase-9 (Western blot assay of caspase-9 cleavage product) and induction of apoptosis (measured using annexin V/PI staining).
As previously noted, JHU-022 cells that were transfected with the IKKβ expression plasmid expressed very low levels of endogenous ΔNp63α; forced over-expression of IKKβ in these cells resulted in the increased expression of endogenous p21 and BAX (Fig. 5b). We further examined the effect of co-transfecting JHU-022 with an expression vector encoding either ΔNp63α or TAp63α in combination with or without the expression vector encoding IKKβ. Consistent with their respective effects on the activity of Bax and p21WAF1 reporter plasmids, forced expression of ΔNp63α, but not TAp63α, led to a decrease in the expression of endogenous p21 and Bax proteins (Fig. 5c). Forced expression of ΔNp63α continued to suppress the expression of endogenous p21 and Bax even in the presence of co-expressed exogenous TAp63α(Fig. 5c). However, co-expression of IKKβ with ΔNp63α restored the endogenous expression of both p21 and Bax (Fig. 5c). Consistent with the ability of ΔNp63α to repress the transactivation of endogenous pro-apoptotic genes by p53 family members, JHU-022 cells transfected with ΔNp63α, with or without co-expression of exogenous TAp63α, did not exhibit any appreciable activation of caspase-9 or induction of cell death after 24h (ΔNp63α =1.33%; ΔNp63α with TAp63α =1.47%)(Fig. 5d). However, transfection of JHU-022 cells with an expression vector encoding IKKβ promoted the activation of caspase-9 and induction of apoptosis (IKKβ = 8.76%) (Fig. 5d). These data confirm the ability of ΔNp63α to repress the transactivating function of p53 family members, and demonstrate that IKKβ–mediated degradation of ΔNp63α can counteract this process and enhance transactivation of genes involved in the cellular response to DNA damage.
DISCUSSION
Inactivation of tumor suppressor genes or activation of protooncogenes that control cell proliferation and survival in response to cellular stress are key determinants of the response of human tumors to commonly used genotoxic anticancer agents. p53 is a prototypic tumor suppressor which plays an instrumental role in the induction of cell cycle arrest and apoptosis in response to DNA damage. These functions ensure the maintenance of genomic integrity in proliferating cell compartments, and prevent the propagation of genetic errors that lead to tumor progression (23). While p53 mutations are common in diverse types of cancer, including Head and Neck Squamous Cell Carcinomas (HNSCC), the other p53 family members, p63 and p73, are also critical determinants of the progression and therapeutic response of various malignancies (1, 2). In addition to p63 and p73 isotypes capable of transactivating downstream target gene expression (TA isotypes), both genes also express dominant negative inhibitory isoforms (ΔN-isotypes) that counteract p53 or TA isotypes (24, 25). ΔNp63α can inhibit the activity of p53, TAp73, or TAp63 by a variety of mechanisms, including competitive interaction with similar cis elements in downstream gene target promoters (26). Amplification and overexpression of ΔNp63α is the most common oncogenic event in primary HNSCC (3). Specific amplification of p63 is also observed in over half of all squamous cell carcinomas of the lung, supporting its frequent involvement in common squamous cell carcinomas (27).
ΔNp63 induces proliferation and growth of tumor cells in vitro and in vivo, and leads to β-catenin-increased accumulation and signaling (28). Conversely, RNAi knockdown of ΔNp63α from malignant cells overexpressing this oncogenic protein results in cell death, accompanied by cleavage of the poly(ADP)-ribosylating enzyme PARP-1 (29). Inhibition of endogenous ΔNp63α by lentivirus RNAi in HNSCC cells induces the expression of pro-apoptotic genes, Puma and Noxa, suggesting that ΔNp63α is required for cell survival (26). Induction of these genes following knockdown of ΔNp63α by RNAi requires transactivation of p73 isoforms, suggesting that the elevation of endogenous ΔNp63α in HNSCC promotes survival through direct repression of p73-dependent transcription of pro-apoptotic genes. As such, ΔNp63α can promote tumorigenesis through direct protein-protein interactions in key proliferative pathways, direct regulation of target genes, or inhibition of the transactivation activity of other members of the p53 family.
Head and neck squamous cell carcinomas (HNSCC) are usually treated with platinum-based chemotherapy and/or radiation (30). p53 is stabilized and upregulated in response to DNA damage induced by radiation or chemotherapeutic agents (31). Since p53 is a key determinant of cell fate in response to these agents, the frequent loss or inactivation of p53 in human cancers may contribute to resistance to therapy (32, 33). However, many studies have failed to show a direct correlation between p53 mutational status and favorable patient response to chemotherapy. This has turned attention to the role of the other p53 family members in the regulation of HNSCC cell death following chemotherapy. Whereas posttranslational modifications of TAp73 lead to TAp73 activation following DNA damage (34), ΔNp63α is degraded in response to DNA damaging agents (13–15). This degradation of ΔNp63α could augment p53, TAp73, and TAp63 function, thereby enabling or facilitating an effective cellular response to genotoxic agents (1). Consistent with this scenario, p63 levels have been found to correlate with patient response to cisplatin-based treatment (35). This suggests that tumor cell death following DNA damage involves the upregulation of p53 family members and activation of their transactivating function via degradation of ΔNp63α. For head and neck cancers over-expressing ΔNp63α, down-regulation of ΔNp63α in response to DNA damage may be required for maximal activation of TA competent p53 family proteins and a favorable response of patients to treatment (35–37). Therefore, the critical molecular signal(s) responsible for reduction of ΔNp63α in response to DNA damage, may determine the response of cancers to chemotherapeutic agents.
In this paper, we identify a key molecular mechanism underlying the regulation of ΔNp63α expression in response to extrinsic stimuli, such as chemotherapeutic agents or TNF-α. We show that ΔNp63α interacts with IκB kinase (IKK), a multisubunit protein kinase that consists of two catalytic subunits, IKKα and IKKβ, and a regulatory subunit, IKKγ (NEMO-NF-κB essential modifier) (18). We find that IKKβ kinase promotes ubiquitin-mediated degradation of ΔNp63α. Cytokine- or chemotherapy-induced stimulation of IKK leads to degradation of ΔNp63α and augments trans-activation of p53 family-induced genes involved in the cellular response to DNA damage. Conversely, inhibition of IKKβ with a NEMO-binding peptide or siRNA-mediated silencing IKKβ expression attenuates cytokine- or chemotherapy induced degradation of ΔNp63α. These data demonstrate that IKKβ plays an essential role in down-regulating ΔNp63α in response to extrinsic stimuli, and suggest that it may be a key signal that skews the dynamic balance between ΔNp63α and transactivating p53 family proteins (p53, TAp73, TAp63) in response to genotoxic chemotherapeutic agents.
Our findings may also shed light on how wild-type p53 is differentially regulated in normal stem cells and their proliferating progeny. Whereas the most important attribute of stem cells is their prolonged survival and relative resistance to genotoxic insults, the proliferating cells derived from them must become susceptible to cell cycle arrest or apoptosis in response to DNA damage (38). Recent studies suggest that p63 may play a critical role in modulating p53 or TAp73 function in the stem cell compartment (39, 40). The ΔNp63α isoform is constitutively expressed in the stem cell compartment of many epithelial tissues, and p63-deficient embryos fail to develop and maintain multi-layered, regenerative epithelia (11, 12). The essential role of p63 in the survival of epithelial stem cells may be related to the ability of ΔNp63α to act as a dominant negative that counteracts p53 and TAp73 activity (24, 25, 41). As such, growth factor-or cytokine-mediated differentiation of stem cells into the proliferating cell compartment must be coupled to a reduction of ΔNp63α in order for transactivation competent p53 family members to serve as effective sentinels and guardians of genomic integrity. Our findings suggest that the activation of IKK may be a shared mechanism by which levels of ΔNp63α are reduced during growth factor- or cytokine-mediated recruitment of stem cells into the proliferating cell compartment, thereby rendering them susceptible to p53-mediated cell cycle arrest/death in the face of cellular stress or DNA damage. However, proliferating tumor cells may circumvent the effects of the constitutive activation IKK by either amplification of p63 or inactivating mutations in p53, thereby allowing them to resist elimination by genotoxic chemotherapeutic agents.
Acknowledgments
This research was supported by National Institutes of Health Grant 5-R01 DE 13561, titled “The Role of P40 Squamous Cell Carcinoma”.
ABBREVIATIONS
- His
Histidine
- JHU
Johns Hopkins University
- IKK
IκB kinase
- IKKα
I kappa kinase alpha
- IKKβ
I kappa kinase beta
- IKKγ
I kappa kinase gamma
- NEMO
NF-κB essential modifier
- NBD
NEMO binding domain
- TNF-α
Tumor necrosis factor – alpha
- CDDP
Cisplatin
References
- 1.Muller M, Schleithoff ES, Stremmel W, et al. One, two, three--p53, p63, p73 and chemosensitivity. Drug Resist Updat. 2006;9:288–306. doi: 10.1016/j.drup.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Strano S, Dell’Orso S, Mongiovi AM, et al. Mutant p53 proteins: between loss and gain of function. Head Neck. 2007;29:488–96. doi: 10.1002/hed.20531. [DOI] [PubMed] [Google Scholar]
- 3.Hibi K, Trink B, Patturajan M, et al. AIS is an oncogene amplified in squamous cell carcinoma. Proc Natl Acad Sci U S A. 2000;97:5462–7. doi: 10.1073/pnas.97.10.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaghad M, Bonnet H, Yang A, et al. Monoallelically expressed gene related to p53 at 1p36, a region frequently deleted in neuroblastoma and other human cancers. Cell. 1997;90:809–19. doi: 10.1016/s0092-8674(00)80540-1. [DOI] [PubMed] [Google Scholar]
- 5.Yang A, Kaghad M, Wang Y, et al. p63, a p53 homolog at 3q27–29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell. 1998;2:305–16. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- 6.Ishimoto O, Kawahara C, Enjo K, et al. Possible oncogenic potential of DeltaNp73: a newly identified isoform of human p73. Cancer Res. 2002;62:636–41. [PubMed] [Google Scholar]
- 7.Jost CA, Marin MC, Kaelin WG., Jr p73 is a simian [correction of human] p53-related protein that can induce apoptosis. Nature. 1997;389:191–4. doi: 10.1038/38298. [DOI] [PubMed] [Google Scholar]
- 8.Ratovitski EA, Patturajan M, Hibi K, et al. p53 associates with and targets Delta Np63 into a protein degradation pathway. Proc Natl Acad Sci U S A. 2001;98:1817–22. doi: 10.1073/pnas.98.4.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liefer KM, Koster MI, Wang XJ, et al. Down-regulation of p63 is required for epidermal UV-B-induced apoptosis. Cancer Res. 2000;60:4016–20. [PubMed] [Google Scholar]
- 10.Yang A, Walker N, Bronson R, et al. p73-deficient mice have neurological, pheromonal and inflammatory defects but lack spontaneous tumours. Nature. 2000;404:99–103. doi: 10.1038/35003607. [DOI] [PubMed] [Google Scholar]
- 11.Mills AA, Zheng B, Wang XJ, et al. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398:708–13. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- 12.Yang A, Schweitzer R, Sun D, et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398:714–8. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]
- 13.Chatterjee A, Upadhyay S, Chang X, et al. U-box-type ubiquitin E4 ligase, UFD2a attenuates cisplatin mediated degradation of DeltaNp63alpha. Cell Cycle. 2008;7:1231–7. doi: 10.4161/cc.7.9.5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee HO, Lee JH, Kim TY, Lee H. Regulation of DeltaNp63alpha by tumor necrosis factor-alpha in epithelial homeostasis. Febs J. 2007;274:6511–22. doi: 10.1111/j.1742-4658.2007.06168.x. [DOI] [PubMed] [Google Scholar]
- 15.Westfall MD, Joyner AS, Barbieri CE, Livingstone M, Pietenpol JA. Ultraviolet radiation induces phosphorylation and ubiquitin-mediated degradation of DeltaNp63alpha. Cell Cycle. 2005;4:710–6. doi: 10.4161/cc.4.5.1685. [DOI] [PubMed] [Google Scholar]
- 16.Demonacos C, La Thangue NB. Drug discovery and the p53 family. Prog Cell Cycle Res. 2003;5:375–82. [PubMed] [Google Scholar]
- 17.May MJ, D’Acquisto F, Madge LA, et al. Selective inhibition of NF-kappaB activation by a peptide that blocks the interaction of NEMO with the IkappaB kinase complex. Science. 2000;289:1550–4. doi: 10.1126/science.289.5484.1550. [DOI] [PubMed] [Google Scholar]
- 18.Ravi R, Bedi A. NF-kappaB in cancer--a friend turned foe. Drug Resist Updat. 2004;7:53–67. doi: 10.1016/j.drup.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Dejardin E, Droin NM, Delhase M, et al. The lymphotoxin-beta receptor induces different patterns of gene expression via two NF-kappaB pathways. Immunity. 2002;17:525–35. doi: 10.1016/s1074-7613(02)00423-5. [DOI] [PubMed] [Google Scholar]
- 20.Chatterjee A, Mambo E, Osada M, Upadhyay S, Sidransky D. The effect of p53-RNAi and p53 knockout on human 8-oxoguanine DNA glycosylase (hOgg1) activity. Faseb J. 2006;20:112–4. doi: 10.1096/fj.04-3423fje. [DOI] [PubMed] [Google Scholar]
- 21.Shimada A, Kato S, Enjo K, et al. The transcriptional activities of p53 and its homologue p51/p63: similarities and differences. Cancer Res. 1999;59:2781–6. [PubMed] [Google Scholar]
- 22.Rossi M, De Laurenzi V, Munarriz E, et al. The ubiquitin-protein ligase Itch regulates p73 stability. Embo J. 2005;24:836–48. doi: 10.1038/sj.emboj.7600444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen X. The p53 family: same response, different signals? Mol Med Today. 1999;5:387–92. doi: 10.1016/s1357-4310(99)01545-2. [DOI] [PubMed] [Google Scholar]
- 24.Little NA, Jochemsen AG. p63. Int J Biochem Cell Biol. 2002;34:6–9. doi: 10.1016/s1357-2725(01)00086-3. [DOI] [PubMed] [Google Scholar]
- 25.Murray-Zmijewski F, Lane DP, Bourdon JC. p53/p63/p73 isoforms: an orchestra of isoforms to harmonise cell differentiation and response to stress. Cell Death Differ. 2006;13:962–72. doi: 10.1038/sj.cdd.4401914. [DOI] [PubMed] [Google Scholar]
- 26.Rocco JW, Leong CO, Kuperwasser N, DeYoung MP, Ellisen LW. p63 mediates survival in squamous cell carcinoma by suppression of p73-dependent apoptosis. Cancer Cell. 2006;9:45–56. doi: 10.1016/j.ccr.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 27.Tonon G, Brennan C, Protopopov A, et al. Common and contrasting genomic profiles among the major human lung cancer subtypes. Cold Spring Harb Symp Quant Biol. 2005;70:11–24. doi: 10.1101/sqb.2005.70.021. [DOI] [PubMed] [Google Scholar]
- 28.Patturajan M, Nomoto S, Sommer M, et al. DeltaNp63 induces beta-catenin nuclear accumulation and signaling. Cancer Cell. 2002;1:369–79. doi: 10.1016/s1535-6108(02)00057-0. [DOI] [PubMed] [Google Scholar]
- 29.Leong CO, Vidnovic N, DeYoung MP, Sgroi D, Ellisen LW. The p63/p73 network mediates chemosensitivity to cisplatin in a biologically defined subset of primary breast cancers. J Clin Invest. 2007;117:1370–80. doi: 10.1172/JCI30866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forastiere AA, Leong T, Rowinsky E, et al. Phase III comparison of high-dose paclitaxel + cisplatin + granulocyte colony-stimulating factor versus low-dose paclitaxel + cisplatin in advanced head and neck cancer: Eastern Cooperative Oncology Group Study E1393. J Clin Oncol. 2001;19:1088–95. doi: 10.1200/JCO.2001.19.4.1088. [DOI] [PubMed] [Google Scholar]
- 31.Wsierska-Gadek J, Horky M. How the nucleolar sequestration of p53 protein or its interplayers contributes to its (re)-activation. Ann N Y Acad Sci. 2003;1010:266–72. doi: 10.1196/annals.1299.046. [DOI] [PubMed] [Google Scholar]
- 32.Irwin MS. Family feud in chemosensitvity: p73 and mutant p53. Cell Cycle. 2004;3:319–23. [PubMed] [Google Scholar]
- 33.Strano S, Rossi M, Fontemaggi G, et al. From p63 to p53 across p73. FEBS Lett. 2001;490:163–70. doi: 10.1016/s0014-5793(01)02119-6. [DOI] [PubMed] [Google Scholar]
- 34.Strano S, Monti O, Pediconi N, et al. The transcriptional coactivator Yes-associated protein drives p73 gene-target specificity in response to DNA Damage. Mol Cell. 2005;18:447–59. doi: 10.1016/j.molcel.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 35.Zangen R, Ratovitski E, Sidransky D. DeltaNp63alpha levels correlate with clinical tumor response to cisplatin. Cell Cycle. 2005;4:1313–5. doi: 10.4161/cc.4.10.2066. [DOI] [PubMed] [Google Scholar]
- 36.Andrews GA, Xi S, Pomerantz RG, et al. Mutation of p53 in head and neck squamous cell carcinoma correlates with Bcl-2 expression and increased susceptibility to cisplatin-induced apoptosis. Head Neck. 2004;26:870–7. doi: 10.1002/hed.20029. [DOI] [PubMed] [Google Scholar]
- 37.Massion PP, Taflan PM, Jamshedur Rahman SM, et al. Significance of p63 amplification and overexpression in lung cancer development and prognosis. Cancer Res. 2003;63:7113–21. [PubMed] [Google Scholar]
- 38.Eyler CE, Rich JN. Survival of the fittest: cancer stem cells in therapeutic resistance and angiogenesis. J Clin Oncol. 2008;26:2839–45. doi: 10.1200/JCO.2007.15.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaelin WG., Jr The emerging p53 gene family. J Natl Cancer Inst. 1999;91:594–8. doi: 10.1093/jnci/91.7.594. [DOI] [PubMed] [Google Scholar]
- 40.Suliman Y, Opitz OG, Avadhani A, et al. p63 expression is associated with p53 loss in oral-esophageal epithelia of p53-deficient mice. Cancer Res. 2001;61:6467–73. [PubMed] [Google Scholar]
- 41.Finlan LE, Hupp TR. Epidermal stem cells and cancer stem cells: insights into cancer and potential therapeutic strategies. Eur J Cancer. 2006;42:1283–92. doi: 10.1016/j.ejca.2006.01.047. [DOI] [PubMed] [Google Scholar]