Abstract
Posttranslational modifications, along with isoform splicing, of titin determine the passive tension development of stretched sarcomeres. It was recently shown that PKCα phosphorylates two highly-conserved residues (S26 and S170) of the PEVK region in cardiac titin, resulting in passive tension increase. To determine how each phosphorylated residue affects myocardial stiffness, we generated three recombinant mutant PEVK fragments (S26A, S170A and S170A/S26A), each flanked by Ig domains. Single-molecule force spectroscopy shows that PKCα decreases the PEVK persistence length (from 0.99 nm to 0.68 nm); the majority of this decrease is attributable to phosphorylation of S26. Before PKCα, all three mutant PEVK fragments showed at least 40% decrease in persistence length compared to wildtype. Furthermore, Ig domain unfolding force measurements indicate that PEVK’s flanking Ig domains are relatively unstable compared to other titin Ig domains. We conclude that phosphorylation of S26 is the primary mechanism through which PKCα modulates cardiac stiffness.
Introduction
Passive tension development in the sarcomere plays a crucial role in proper diastolic function. The giant protein titin, also called connectin, spans the half-sarcomere from Z-disk to M-line [1], and is responsible for the development of passive tension within the sarcomere [2]. Titin contains an extensible I-band region that comprises three distinct regions: serially-linked immunoglobulin (Ig)-like domains, the N2B element, and the PEVK region, which primarily contains proline (P), glutamate (E), valine (V), and lysine (K) residues [3]. These three spring-like elements develop force when extended and, along with collagen, determine myocardial passive stiffness.
Isoform splicing is a long-term pathway that alters myocardial passive stiffness. Cardiac titin is composed of two isoforms: the small (2.97 kDa) N2B titin and larger N2BA titin isoform (3.2 – 3.4 MDa). Exon splicing pathways determine the differential expression of titin’s I-band element, and result in N2BA titin containing a larger PEVK domain than the N2B element, in addition to a variable number of Ig domains [4]. The 188-residue PEVK sequence examined in this investigation is the entire PEVK domain of the cardiac N2B isoform, the main isoform in the left ventricle, but it is also present in the N2BA isoform [5]. At a given extension, the cardiac N2B isoform develops more force than the N2BA isoform because of its shorter end-to-end length. This shorter isoform length results in a greater fractional extension of the elastic elements in the I-band, which leads to greater force development [6].
The critical importance of relative fractional extension in determining passive tension dynamics of the I-band is evident in spring element KO studies. Increased passive tension was measured in PEVK-KO mice and was attributed to the extensional increase in the spring-like N2B element [7]. Increase in passive tension was also measured in N2B-KO mice, and it was determined that the PEVK region extended most in response to the loss of N2B [8]. The deletion of one of the spring elements in the I-band necessitates increased stretch of the other two spring elements to compensate for the loss of extension that the deleted element previously contributed.
Posttranslational modifications represent a short-term mechanism for altering the force-extension dynamics of titin’s extensible I-band. Kinase phosphorylation of various I-band elements is one of the most extensively studied aspects of myocardial modulation. Protein kinase A, C, and G (PKA, PKC, and PKG) have all been shown to phosphorylate either the N2B or PEVK element of titin’s I-band [9-11]. The N2B and PEVK domains are the primary sources of titin-based passive force development [12], and kinase phosphorylation alters myocardial passive stiffness by changing the force-extension relationships of these domains. Phosphorylation of the N2B element accompanied PKA-dependent passive tension reduction in isolated myocytes [9], and site-directed mutagenesis showed that N2B is a PKG substrate that, when phosphorylated, reduces N2B resistance to stretch [10]. Furthermore, hypophosphorylation of titin has been associated with high levels of passive tension in patients with heart failure (HF) [13].
Recently, it was shown that PKCα, a key member of the PKC serine/threonine kinase family, phosphorylates titin [11]. In vitro kinase assays determined that titin’s extensible PEVK domain is a PKCα substrate, and the combination of mass spectrometry and site-directed mutagenesis pinpointed two PEVK residues as the primary PKCα phosphorylation sites: serine 26 and serine 170. Muscle mechanics experiments from the same study found that PKCα treatment on skinned left ventricle (LV) myocardium increased passive tension. In order to measure how the individual serine residues affect passive tension development, both pre- and post-PKCα phosphorylation, we performed single molecule force spectroscopy on WT PEVK and three mutant PEVK: serine 26 mutated to alanine (S26A), serine 170 mutated to alanine (S170A), and a S170A/S26A double mutant. The purpose of this study was to determine the relative contributions of both PKCα phosphorylation sites to changes in PEVK extensibility.
Materials and Methods
The recombinant PEVK (N2B cardiac isoform) molecules were expressed in E. coli as previously described [14] with site-directed mutagenesis as in Labeit et al [15]. We constructed three PEVK mutants (two single mutants – S26A and S170A – and one double mutant – S170A/S26A) in addition to WT PEVK molecules. The purified PEVK recombinant fragments were phosphorylated via an in vitro kinase assay as described [11], with minor modifications. Briefly, the purified PEVK recombinant fragments (~0.3 μg/μl) were incubated with recombinant PKCα human (Enzo Life Sciences) (0.066 U/μl) in activating solution (in mM: BES 6.7 pH 7.0, CaCO3-EGTA 1.7, MgCl2 2.5, Na2-ATP 11.0, DTT 0.25, K-propionate 7.6, creatine phosphate 2.5, NaCl 38.3, glycerol 5.3%), lipid activator (Upstate) (phosphatidylserine 0.17 mg/ml, diacylglycerol 0.02 mg/ml, triton X-100 0.05%), phosphatase inhibitors (NaF 8.3 mM, Na3VO4 1.7 mM), and protease inhibitors (leupeptin 20 ug/ml, E-64 10 uM, and PMSF 0.2 mM). The samples were incubated for 6h at 30°C, aliquoted, quick frozen in liquid nitrogen, and stored at −80°C. Phosphorylation levels were determined by a radioactive kinase assay using [γ-32P]ATP and autoradiography: see Results in Hidalgo et al [11]. Measured phosphorylation levels varied depending on incubation and relative kinase concentration. Incubation time and PKCα concentration were selected such that maximum phosphorylation levels were realized. This systematic approach gave us confidence that the vast majority of PEVK were phosphorylated using this optimized protocol.
PEVK molecules, flanked by their native Ig domains (Ig27 and Ig84), were stretched at a constant speed of 1000 nm/s using an atomic force microscope (AFM) (MFP3D; Asylum Research, Santa Barbara, CA) as previously described [16]. Briefly, 10 μg/ml of protein in assay buffer (AB; 25mM BES, 2.5mM EGTA, 1.5mM MgCl2, 1.25mM NaATP, 165mM KCl, 5mM DTT, pH 7.0) was spotted on a cleaned glass microscope slide. To allow for maximal adsorption, the spotted slides were incubated overnight at 4°C. Unbound molecules were removed from the slide through multiple washes with 200 μl AB. Surface protein density was kept low to minimize nonspecific protein-protein interactions and the probability of multiple molecules attaching to the AFM tip. Adsorbed Ig27-PEVK-Ig84 molecules were stretched in AB by contacting the non-conductive silicon nitride cantilever tip (MLCT; Veeco Probes) with the protein-coated surface and retracting the cantilever at 1000 nm/s. Tip extension-retraction cycles tethered our Ig-PEVK-Ig fragment about 1% of the time. When a molecule attached to the cantilever tip, a force versus displacement curve was generated as the tip was retracted from the surface. The displacement of the cantilever base was determined via an integrated linear voltage differential transformer. To determine the longitudinal force exerted on the molecule as the tip is pulled away from the surface, Hooke’s Law F = -kx was used, where F(x) is the force needed to extend the molecule a given distance x and k is the spring constant of the cantilever. Cantilever stiffness was established by measuring its mean thermally driven vertical displacement (x) and then applying the equipartition theorem: k<x2> = kBT, where kB is Boltzmann’s constant and T is absolute temperature. Typical cantilever stiffness was ~25-30 pN/nm, and the root-mean-square (rms) force noise was ~15 pN for an unloaded cantilever (no tethered molecules). This rms force noise value reveals the fluctuations in our force readings due to thermal energy (as well as system imperfections). To accurately measure the force-extension relationship of PEVK, the end-to-end length (z) of the tethered molecule (the distance between the glass surface and the cantilever tip) was calculated by correcting the cantilever base displacement (ξ) for cantilever bending: z = ξ – F/k.
We analyzed the force-extension traces generated by stretching the PEVK molecule by fitting the force versus displacement data with the wormlike chain (WLC) equation [17]:
F is force needed to extend the molecule (pN), PL is the persistence length of the molecule (nm) (a quantification of the molecule’s bending rigidity), z is the end-to-end length of the molecule (nm), CL is the molecule’s contour length (nm), kB is Boltzmann’s constant, and T is absolute temperature. Note that both PL and CL are inversely proportional to force. During repetitive extension-retraction cycles, if the cantilever tip tethers a molecule when it contacts the protein-coated surface, a force-extension curve is generated as the tip pulls away from the surface. Because the molecular end-to-end distance is constrained by the vertical displacement of the cantilever tip, the molecule is stretched during tip retraction (Figure 1B). As the tip retracts, the molecule resists extension. This restoring force is entropic in nature and is described by the WLC equation. As the molecule is stretched, the entropic force increases and the cantilever tip is displaced farther from equilibrium. On our force-extension traces, this relationship is seen as a slow increase in force at short extensions that nonlinearly increases as the cantilever tip is pulled farther away from the surface (Figure 1C). When sufficient force develops, the less stable Ig domain undergoes an abrupt unfolding transition that lengthens the entire molecule; this well-defined unfolding event results in an unfolding force peak. This process is repeated until the second Ig domain unfolds and again when the molecule breaks away from either the surface or tip. The unfolding of an Ig domain should increase the CL of the molecule from ~ 4 nm to ~ 26 nm (folded Ig ~ 4 nm, fullyextended Ig = 68 residues × 0.38 nm/res ~ 26 nm). This sudden increase in CL results in a steep drop in force, as evident in the force-extension curves. We analyzed traces with three regularly spaced force peaks, which ensured that the PEVK domain was fullyextended, and fit the trace leading up to the first peak with the WLC equation to determine the PL and CL of PEVK.
Figure 1. Single molecule force spectroscopy of PEVK.
A) The I-band of the cardiac titin N2B isoform. Single molecule force spectroscopy was performed on recombinant protein fragments containing the PEVK domain and flanking Ig domains. B) Schematic of the AFM experiment. When the cantilever tip tethers our recombinant protein, force develops in the molecule (which bends the tip) as the tip retracts from the surface. Initially, the PEVK element extends (event 1), which generates the force trace leading up to the first force peak. When sufficient force develops, an Ig domain unfolds (event 2), and a distinct force peak develops. This unfolding event increases the CL of the molecule, which reduces the force in the system immediately after Ig domain unfolding. The second force peak is due to unfolding of the other Ig domain, and the third force peak is due to molecular displacement from either the tip or slide. C) We fit the trace leading up to the first force peak with the WLC equation to determine PEVK resistance to stretch. The force peak at low extensions (< 25 nm) is due to the adhesive force between the cantilever tip and slide surface and is excluded from our WLC fits.
We also fit the traces leading up to the second and third force peaks to determine the CL increase due to the unfolding of the Ig domains. By fitting all three force peaks and comparing the CL differences between each peak we can determine if the trace was generated by a single Ig-PEVK-Ig molecule tethered between the glass surface and cantilever tip. If a trace showed only three force peaks with peak spacing consistent with the CL increase subsequent to a single Ig domain unfolding event, we had high confidence that the trace was generated by a single tethered molecule. If the tip had tethered more than one molecule, we would see more than three force peaks. In addition, these force peaks would show unequal spacing due to the independence of Ig domain unfolding between different tethered molecules. The effect of changes in PEVK PL after point-mutations and PKCα phosphorylation at the sarcomere level were calculated with a serially-linked inverted WLC model [4, 18].
Statistics
Data are presented as mean±SE. Significance was determined using two-way ANOVA, using mutation and PKCα as factors. Post hoc comparisons were made using Tukey HSD. Probability values <0.05 were taken as significant.
Results
It was recently found that PKCα phosphorylates cardiac N2B PEVK at two highly-conserved serine residues. In order to deduce the individual contributions of the two residues to the dynamics of passive tension in the sarcomere, we probed four PEVK constructs—wildtype, serine 170 mutated to alanine (S170A), serine 26 mutated to alanine (S26A), and a S170A/S26A double mutant—using single molecule force spectroscopy. The WT and all three mutant PEVK are flanked by an Ig domain on either side, which creates a characteristic saw-tooth pattern when the molecule is stretched and unfolded (Figure 1C). The force peak below 25 nm extension is due to adhesion between the cantilever tip and slide surface and is excluded from our WLC fits. The force-extension trace up to the first unfolding peak (event 1 in Figure 1B/C) describes the extension of PEVK, and by fitting this trace with the WLC equation, we quantified the two parameters that determine molecular force-extension dynamics: PL and CL. Figure 2 shows typical results for all four PEVK constructs in the absence of PKCα; each trace shows a similar characteristic “fingerprint” representing two Ig domain unfolding events followed by displacement of the tethered molecule from one or both of its anchoring points.
Figure 2. AFM force-extension curves.
Sample force-extension curves for all four PEVK fragments pre-PKCα phosphorylation: A) WT; B) S170A/S26A; C) S170A; D) S26A. The trace leading up to the first force peak is fit with the WLC equation to extract the PL and CL of PEVK. The force peaks at short extension are due to adhesion between slide surface and cantilever tip, and are excluded from our fits. Note that all peaks have the three-peak “fingerprint” characteristic of an extension-retraction cycle stretching only one Ig-PEVK-Ig molecule.
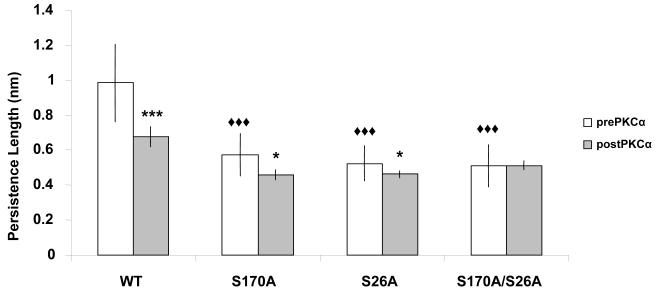
To gain insight regarding PEVK’s structural mechanics, we examined the effects of point mutations on the PL of PEVK prior to PKCα treatment. By fitting the force trace leading up to the first force peak with the WLC equation, we can extract the PL of PEVK. Figure 3 shows that all three mutant PEVK have significantly decreased PL values compared to WT (data summary in Table 1). There is no significant difference among mutants. This result suggests that both phosphorylation sites on the PEVK domain are important for maintaining native structure, and that disrupting this structure increases titin-based passive tension. The WT PEVK PL value presented here is slightly lower than our previous study (~ 1.0 nm vs. ~ 1.2 nm) [11], but this difference, as well as the PL difference post-PKCα, is not significant. However, PKCα phosphorylation does show a greater effect in our previous study, although the determinant of this difference is currently unclear. The structure of PEVK is not known (it was initially believed to be a random coil), but it has more recently been suggested that PEVK may contain polyproline II helix motifs [19-20], although the high concentration of proline residues and charge clusters is believed to likely hinder the formation of stable structures [3]. The data presented here supports the idea that PEVK is not simply a random-coil: a point mutation would not change the PL of a random coil to a measurable degree. Mutation of serine 26 decreased PEVK PL by 47%, while mutation of serine 170 decreased PEVK PL by 42%. The reduction in PL following the double mutation (48%) is comparable to each of the single mutants. This somewhat surprising result suggests that, while each single mutation disrupts the local structure of the PEVK to reduce overall PL, concurrent mutation of both serine residues allows new interactions to develop among regions of the PEVK that were altered by a single mutation. The specific peptide-peptide interactions unique to PEVK fragments with both point mutations—with each mutation affecting the structure associated with that particular residue—require further study.
Figure 3. PEVK PL measurements.
Average PL for WT, S170A, S26A, and S170A/S26A fragments, pre- and post-PKCα phosphorylation. Each mutated PEVK shows a significant decrease in PL compared to WT, although there is no significance among mutants. Post-PKCα, all fragments except the double mutant have decreased PL. This shows that S26 and S170 are the only two residues that influence PEVK mechanics following PKCα phosphorylation. This also supports the notion that there are only two PKCα substrates in the PEVK region. (◆: significant vs. pre-PKCα WT; *: significant vs. pre-PKCα for given PEVK group; three symbols, p < 0.001; one symbol, p < 0.05).
Table 1.
The effect of point-mutations and PKCα phosphorylation on the PL of PEVK
| PrePKCα PL (nm) | PostPKCα PL (nm) | Percent Change | |
|---|---|---|---|
| WT | 0.986 ± .0649 | 0.676 ± .0557 *** | −31.4% |
| S170A | 0.575 ± .0420 ◆◆◆ | 0.459 ± .0281 * | −20.17% |
| S26A | 0.525 ± .0215 ◆◆◆ | 0.463 ± .0189 * | −11.81% |
| S170A/S26A | 0.510 ± .0368 ◆◆◆ | 0.513 ± .0267 | +0.59% |
Significant compared to pre-PKCα
Significant compared to pre-PKCα WT
One symbol: p < 0.05
Three symbols: p < 0.001
The extensibility of PEVK was also studied after PKCα phosphorylation. Figure 3 shows that phosphorylation decreased the PL of all PEVK constructs except the S170A/S26A mutant. The most significant decrease was observed in WT PEVK, which demonstrated a 31% drop in bending rigidity. Phosphorylation of serine 26 resulted in a 20% PL decrease, and phosphorylation of serine 170 induced a 12% decrease (Table 1). PKCα had no effect on the double mutant, which supports the hypothesis that cardiac N2B PEVK has only two critical phosphorylation sites. It is interesting to note that the PL reduction of the individual serine residues combine to account for the entire reduction of PL in WT PEVK. It has been shown that serine 26 is the primary PKCα substrate [11], and the PL data presented herein trends in support of that observation.
We also investigated the effects of point mutations and PKCα phosphorylation on the CL of PEVK. In the absence of PKCα, there is no significant difference between any PEVK populations, and the average PEVK CL is 59.6 ± 1.5 nm. If PEVK was a random-coil structure, one would expect that the CL would be ~ 71 nm ((188 aa)*(0.38nm/aa)). We previously reported WT PEVK CL to be ~ 71 nm [11], but upon removal of outliers (more than 2 SD away from mean), the previous data and current data are not significantly different. The shorter than expected CL shown in this study therefore suggests that PEVK contains structural motifs, formed from amino acid interactions, which effectively shorten PEVK’s CL compared to a random-coil polypeptide. Although there is no significant CL differences between PEVK groups pre-PKCα, there is significant change within a group after PKCα phosphorylation. Figure 4 shows that the PEVK CL, which is determined by fitting the force-extension trace up to the first unfolding peak with the WLC equation, is significantly reduced in both the WT and S170A mutants. WT CL was reduced from 62.5 ± 3.5 nm to 54.6 ± 1.5 nm (P<0.05) and S170A CL was reduced from 63.3 ± 2.9 nm to 53.2 ± 1.7 nm (P<0.01). This small CL reduction following PKCα is not present in previous work [11]; a possible explanation is that the previous study was more restrictive in analyzing force-extension traces that showed contour lengths less than expected.
Figure 4. PEVK CL measurements.
PEVK CL is determined from fitting the trace up to the first force peak with the WLC equation. Prior to PKCα, there is no significant difference between PEVK fragments. After phosphorylation, WT and S170A PEVK exhibit significant decrease in CL. The addition of PO42− to S26 and S170 likely reduces CL via attractive interactions between phosphoserine and positively-charged residues/PEVK repeat motifs. A mild competitive effect from phosphorylated S170 may explain why a slightly larger CL decrease is measured in S170A compared to WT. (*: significant between pre- and post-PKCα sets of a given PEVK group; two symbols, p < 0.01; one symbol, p < 0.05. There are no significant differences due to mutations alone).
We also determined whether or not the point mutations or PKCα activity altered Ig dynamics. To investigate this, we analyzed the difference in CL between successive force peaks in our force-extension curves. The first two force peaks represent the transition of an Ig domain from a compact, globular structure to an unfolded, polypeptide chain. Assuming that the PEVK domain is fully extended (except for the PEVK structures with high stability) before the first Ig domain unfolds, the increase in CL between consecutive force peaks is solely attributed to an Ig unfolding event. Figure 5 plots CL versus peak number for all PEVK fragments, both pre- and post-PKCα phosphorylation. The slope of the line fit to CL vs. peak number represents the average CL gain per unfolding event. The average CL gain pre-PKCα treatment for WT, S170A, S26A, and S170A/S26A were 23.4, 23.9, 24.4, and 23.5 nm, respectively, with no significance between PEVK groups. Following PKCα treatment, the average CL gain for WT, S170A, S26A, and S170A/S26A were 24.3, 26.9, 28.2, and 25.3 nm, respectively, again with no significance between groups. Average CL gain slightly increases following PKCα phosphorylation, but none of these increases are significant within a group, i.e. pre-PKCα WT vs. post-PKCα WT.
Figure 5. CL gain following Ig unfolding.
To determine the average CL gain following Ig domain unfolding, we plot mean CL vs. force peak number. After both Ig domains unfold, force still develops as the cantilever is retracted because the molecule is still anchored to the slide surface and cantilever tip. The third force peak in our force-extension traces is due to the molecule de-adsorbing from either or both of its anchoring contact points, which is a stochastic process. Therefore, a well-defined force peak may not always develop due to premature displacement of the molecule from slide or tip. To accurately measure the CL gain between peaks 2 and 3, we disregarded force-extension curves that did not contain a third force peak that could be well-fit with the WLC equation. A) Pre-PKCα, the mean CL gain due to Ig unfolding for WT, S170A, S26A, and S170A/S26A is 23.4, 23.9, 24.6, and 23.5 nm, respectively, with no significance between groups. B) Post-PKCα, the mean CL gain for WT, S170A, S26A, and S170A/S26A is 24.3, 26.9, 28.2, and 25.3, respectively, again with no significance between groups. In addition, there is no significance pre- and post-PKCα for a given group.
The unfolding force (UF) of the Ig peaks was also analyzed. There was no significant change in UF following PKCα phosphorylation of PEVK, an effect which is expected because PKCα presumably does not phosphorylate the Ig domains of cardiac titin’s I-band. The mean UF of the Ig domains is presented in Table 2. Although the UF of peak 1 is lower in WT PEVK compared to the mutants, there is only significant difference between WT and S26A prior to PKCα phosphorylation. For a comparison with UF of other Ig domains, see below.
Table 2.
Ig domain mean unfolding force (pN)
| WT−/− | S170A−/− | S26A−/− | S170A/S26A−/− | |
|---|---|---|---|---|
| Peak 1 | 116 ± 8 | 156 ± 11 | 163 ± 9* | 156 ± 14 |
| Peak 2 | 185 ± 27 | 178 ± 16 | 185 ± 17 | 185 ± 32 |
|
|
||||
| WT+/+ | S170A+/+ | S26A+/+ | S170A/S26A+/+ | |
|
|
||||
| Peak 1 | 132 ± 10 | 166 ± 15 | 165 ± 12 | 155 ± 12 |
| Peak 2 | 158 ± 13 | 166 ± 26 | 184 ± 23 | 187 ± 20 |
Significant compared to WT−/− , p < 0.05
DISCUSSION
Diastolic pressure results from the development of passive force, which is largely determined intracellularly by titin’s extensible I-band region and extracellularly by collagen networks. Recent work reveals that both long-term and short-term pathways affect the passive tension dynamics in the myocardium, which result in a variety of similar and disparate effects. The major long-term pathway for modulating myocardial stiffness is titin isoform splicing, a process that occurs on the time frame of weeks [21].
Numerous direct short-term responses also influence the passive force response to sarcomere stretch, notably the regulation of various protein kinases via upstream adrenergic pathways. PKA and PKG phosphorylation of the elastic N2B element of titin have been associated with passive tension reduction [9-10], and our group recently showed that PKC phosphorylates the cardiac titin N2B isoform on two serine residues located within the 188-residue PEVK element, S26 and S170 [11]. Unlike PKA/PKG phosphorylation of titin’s I-band, PKC phosphorylation corresponded with an increase in passive tension. PKC, which is activated by α1-adrenergic pathways, has been implicated in various cardiovascular diseases, including ischemic heart disease, cardiac hypertrophy, hypertension, and atherosclerosis (for a recent review see Murphy and Frishman [23]). It has been shown that PKCα, the predominant isoform expressed in mouse, human, and rabbit heart [24-26], is upregulated in patients with dilated cardiomyopathy and ischemic cardiomyopathy [27], and in rats with end-stage HF [28]. Mice lacking the gene for PKCα show enhanced cardiac contractility and reduced susceptibility to HF [29], which suggests that PKC inhibitors could be viable candidates for HF treatment.
In order to determine the relative contributions of each phosphorylated serine residue to the measured increase in passive tension following PKCα treatment, we generated four recombinant PEVK fragments: WT, S26A, S170A, and S170A/S26A. Using single molecule force spectroscopy, we investigated the effect that point mutations have on the force-extension relationship of PEVK, both pre- and post-PKCα treatment. Because the force-extension response of the elastic spring elements of titin’s I-band can be quantified using the WLC equation [12, 14, 16, 30-33], we were able to fit our single molecule force spectroscopy data with the WLC equation to extract the relevant physical parameters (PL and CL) of all PEVK fragments (both +/- PKCα). From these parameters, we are able to model the physiological effect that individual residues have on the sarcomeric resistance to stretch.
On the molecular level, a change in CL or PL is associated with structural transitions or changes in the energy landscape. For example, the formation or destruction of stable secondary (and tertiary) structures may change the effective length of a molecule, and the presence of energetic potentials, i.e. electrostatic, may change the effective stiffness of a molecule [34-35]. Recognition of the dynamics of physical changes in a spring-like molecule, such as PEVK, gives insight into that molecule’s structural composition. The complete sequence of human cardiac titin shows that the PEVK sequence is primarily composed of ~ 28-residue repeats and polyE motifs [36]. In cardiac N2B PEVK, all the negatively-charged polyE motifs and 55 of the 60 (as found in soleus titin) PEVK repeats are spliced out [37]. The PEVK structure is not well-defined, but the existence of structural motifs has been proposed. Although high proline concentration precludes the formation of sizeable α-helices and β-sheet structures [38-39], NMR and circular dichroism (CD) experiments suggest that polyproline helix-coil motifs are fundamental structural features of PEVK [19-20]. In addition, the PEVK sequence, especially around S26 and S170, is highly conserved in a wide variety of species, which also suggests that PEVK is structured, at least in part [11]. If PEVK was simply a molecular random-coil, its elastic spring properties would not be significantly altered by sequence drift and would not be evolutionarily conserved.
We found that WT PEVK has PL ~ 1 nm in the absence of PKCα, which is consistent with published values [14, 30, 40]. Serine-to-alanine mutations significantly reduced PL in both single mutants and the double mutant (Figure 3); there was no significance difference in PL among the three mutant populations. The significant decrease of PEVK PL following both serine-to-alanine point mutations suggest that both S26 and S170 are important in maintaining structural integrity. Physically, a reduction in PL of an amino acid polypeptide can be attributed to the dissolution of stabilizing interactions, i.e. hydrogen bonds, between domains, which increases the translational and rotational degrees of freedom of the peptide sequences that were previously constrained by the stabilizing interaction. The “release” of these degrees of freedom increases the number of configurations that the molecule can occupy. This enlargement of phase-space volume that the molecule can populate increases the relative entropy associated with the molecule’s configuration at a given end-to-end length. An increase in molecular end-to- end length reduces the entropy of the system and is therefore statistically improbable. This resistance to a lower entropic system, or entropic force, favors a compact protein conformation, and is the restoring force that opposes molecular extension in AFM experiments. The WLC equation shows that the force needed to extend an entropic spring is inversely proportional to the molecule’s PL, implying that a measured decrease in PL corresponds to an increase in molecular entropy. Therefore, it is proposed that the S26A and S170A mutations disrupt the stabilizing interactions involving the native serine residues, which increases the conformational entropy of PEVK and results in a lower effective PL.
We focused our investigation on the effect that PKCα phosphorylation has on the sarcomeric force-extension relationship by quantifying the phosphorylation effect at the single molecule level. Comparing the PL differences post-PKCα treatment suggests that S26 plays a bigger role than S170 in the modulation of myocardial stiffness. The 31% reduction in PL of WT PEVK post-PKCα is well accounted for by the 20% and 12% PL reduction measured in S170A and S26A, respectively. The larger effect measured in the S170A mutant could be due to several factors. One factor is that S26 has been shown to be more readily phosphorylated by PKCα compared to S170 [11]. Another factor could be that S26 is more actively involved in determining the structure of PEVK compared to S170, and that phosphorylation disturbs these interactions. However, the PL values of S26A and S170A pre-PKCα are not significantly different, which is not consistent with the latter explanation. It is more likely that PKCα phosphorylation has a larger effect on the PL of S170A because S26 is the primary site of PKCα phosphorylation.
PKCα phosphorylation also reduced CL in WT and S170A PEVK. Prior to PKCα, PEVK CL is ~ 60 nm, which is less than expected for an unfolded 188-residue polypeptide. However, the substantial reduction in PL following point mutations and PKCα phosphorylation necessarily implies that PEVK contains structured domains, and our CL measurements are consistent with this. The small CL reduction post-PKCα can be explained by electrostatic considerations The addition of a negatively-charged phosphate molecule to the polar side chain of S26 or S170, perhaps more pronounced in S26, effectively changes the serine into a polar residue with a negatively charged side chain. At neutral pH, phosphoserine typically carries a −2 net charge [41] due to phosphoric acid’s pKa2 value of ~ 6.1. Cardiac N2B PEVK comprises five ~ 28-residue PEVK repeats, which carry a net positive charge and have isoelectric points between 9-10 (AFM experiments executed at pH 7). In addition, there are 43 combined arginine and lysine residues (which contain positively-charged side chains) in the 188-residue PEVK sequence. The negative charge of phosphoserine may attractively interact with either positively-charged peptides in the PEVK repeats or with specific arginine/lysine residues. This attractive potential between segments of the PEVK that would normally be repulsive (or have no interaction potential) could explain the reduced CL after PKCα phosphorylation.
The CL gain between successive force peaks is attributed to the unfolding of an Ig domain. The average CL gain between force peaks for all samples is ~ 25 nm, which is slightly higher than expected assuming the absence of all secondary structure following Ig unfolding. Ig27 and Ig84 contain 68 and 67 residues, respectively, as determined by Simple Modular Architecture Research Tool (SMART) software [42-43], and we therefore expect to see a ~ 22 nm CL increase following an Ig domain unfolding event (~ 26 nm for fully unfolded Ig minus the diameter of a folded Ig domain (~ 4 nm)). The slightly higher than expected CL gain can be explained by the unfolding of structural motifs in PEVK that were stable up to the first force peak.
We also analyzed the UF of Ig27 and Ig84, and found that PKCα does not affect Ig UF, as expected. An unexplained finding that requires further study is that UF is lower for WT PEVK than for the mutants. However, a comparison with previous results of Ig domains from titin’s proximal and distal tandem Ig segments shows that Ig27 and Ig84 are relatively unstable. Considering all PEVK groups (both pre- and post-PKCα phosphorylation), the average UF of peak 1 and peak 2 is 152 ± 4 pN and 178 ± 7 pN, respectively. At the same pulling speed (1000 nm/s), it has been shown that the average UF of tandem Ig 1-8 and Ig 8-15 is ~ 221 pN and ~ 232 pN, respectively [16]. At 500 nm/s stretch speed, the mean unfolding force of tandem Ig 91-98 domains was reported to be ~230 pN [14]. Closer to the UF measured in this study, AFM experiments that stretched the flexible N2B element of the I-band, along with the 3 Ig domains (Ig 24-26) that flank the N2B element (see Figure 1A), showed that the average UF of these flanking Ig domains, from least stable to most stable, are ~ 147, ~ 177, and ~ 191 pN. Combined with the UF results of Ig 24-26, our results of Ig27 and Ig84 suggest that the Ig domains that immediately flank the elastic N2B and PEVK regions of cardiac titin’s N2B isoform are less mechanically stable than the proximal and distal tandem Ig domains that define the N and C termini of the I-band, respectively. SMART software determined that all but one of the Ig-like domains from the Ig 1-15 and Ig 91-98 set contain between 80-90 residues and belong to the general Ig class of proteins. However, Ig25, Ig26, Ig27, and Ig84, the four domains that immediately flank N2B and PEVK, respectively, all belong to the IgC2 family of immunoglobulin and contain between 65-70 residues. This structural difference between the Ig domains that flank the two dynamic spring elements in the I-band and the tandem Ig domains in titin may help explain the significant differences in UF between the two groups.
We used a serially-linked WLC model [4, 18] to determine the effect that PEVK PL and CL changes have on the titin-based tension that develops at SLs greater than slack length. Figure 6 shows that, following PKCα phosphorylation, passive tension increases, an effect attributed to PL and CL reduction following PKCα phosphorylation. Reduced PL is responsible for the majority of force increase at shorter SL, and reduced CL results in higher force increases at longer SLs. The SL-dependent force contribution of reduced CL stems from the nonlinear dependence of CL on entropic force, as described by the WLC equation. At a SL ~ 2.2 μm, the post-PKCα force increase (~ 15%) derives equally from PL and CL decreases. In skinned mouse myocardium (which primarily expresses the N2B isoform [44]), it was shown that PKCα phosphorylation increases titin-based passive tension by ~ 25% at a SL of 2.2μm. Our data suggests that the majority of this passive tension increase derives from direct phosphorylation of S26 and S170 in the PEVK element. Upon phosphorylation of these residues in cardiac tissue, the PEVK PL decreases, and more external work is needed to increase sarcomere length and expand the heart.
Figure 6. The effect of PKCα on titin-based passive tension in the sarcomere.
To determine how changes in PEVK PL and CL alter the resistance to stretch of an entire titin molecule, we use an inverted WLC equation to sum the relative extensions of all three I-band elements at a given force. For a single titin molecule (cardiac N2B isoform), PKCα phosphorylation increases the force needed to extend titin at a given length. At SL ~ 2.2 μm, we calculate that PKCα phosphorylation increases passive force by 15%.
In summary, AFM studies indicate that both PKCα substrates in the extensible I-band of cardiac N2B titin, S26 and S170 of the PEVK element, are important for the mechanical properties of the myocardium. The force-extension dynamics of PEVK are important in determining the development of passive tension in stretched myocardium, and mutation of S26 and S170 significantly changes PEVK response to stretch. PKCα phosphorylation of S26 and S170 also increases the force needed to extend PEVK in a residue-specific manner, and we propose that this is a novel mechanism for modulating myocardial passive stiffness.
Acknowledgements
We thank Carlos Hidalgo for preparation of phosphorylated samples. Brian Anderson received support from NIH training grant GM084905. Supported by NIH HL062881 to H.G. and by the DFG (La668/13-1) and the NAR Initiative University of Heidelberg to S.L.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Furst DO, et al. The organization of titin filaments in the half-sarcomere revealed by monoclonal antibodies in immunoelectron microscopy: a map of ten nonrepetitive epitopes starting at the Z line extends close to the M line. J Cell Biol. 1988;106(5):1563–72. doi: 10.1083/jcb.106.5.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horowits R, et al. A physiological role for titin and nebulin in skeletal muscle. Nature. 1986;323(6084):160–4. doi: 10.1038/323160a0. [DOI] [PubMed] [Google Scholar]
- 3.Labeit S, Kolmerer B. Titins: giant proteins in charge of muscle ultrastructure and elasticity. Science. 1995;270(5234):293–6. doi: 10.1126/science.270.5234.293. [DOI] [PubMed] [Google Scholar]
- 4.Granzier HL, Labeit S. Titin and its associated proteins: the third myofilament system of the sarcomere. Adv Protein Chem. 2005;71:89–119. doi: 10.1016/S0065-3233(04)71003-7. [DOI] [PubMed] [Google Scholar]
- 5.Freiburg A, et al. Series of exon-skipping events in the elastic spring region of titin as the structural basis for myofibrillar elastic diversity. Circ Res. 2000;86(11):1114–21. doi: 10.1161/01.res.86.11.1114. [DOI] [PubMed] [Google Scholar]
- 6.Trombitas K, et al. Extensibility of isoforms of cardiac titin: variation in contour length of molecular subsegments provides a basis for cellular passive stiffness diversity. Biophys J. 2000;79(6):3226–34. doi: 10.1016/S0006-3495(00)76555-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Granzier HL, et al. Truncation of titin’s elastic PEVK region leads to cardiomyopathy with diastolic dysfunction. Circ Res. 2009;105(6):557–64. doi: 10.1161/CIRCRESAHA.109.200964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Radke MH, et al. Targeted deletion of titin N2B region leads to diastolic dysfunction and cardiac atrophy. Proc Natl Acad Sci U S A. 2007;104(9):3444–9. doi: 10.1073/pnas.0608543104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamasaki R, et al. Protein kinase A phosphorylates titin’s cardiac-specific N2B domain and reduces passive tension in rat cardiac myocytes. Circ Res. 2002;90(11):1181–8. doi: 10.1161/01.res.0000021115.24712.99. [DOI] [PubMed] [Google Scholar]
- 10.Kruger M, et al. Protein kinase G modulates human myocardial passive stiffness by phosphorylation of the titin springs. Circ Res. 2009;104(1):87–94. doi: 10.1161/CIRCRESAHA.108.184408. [DOI] [PubMed] [Google Scholar]
- 11.Hidalgo C, et al. PKC phosphorylation of titin’s PEVK element: a novel and conserved pathway for modulating myocardial stiffness. Circ Res. 2009;105(7):631–8. doi: 10.1161/CIRCRESAHA.109.198465. 17 p following 638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Granzier H, Labeit S. Structure-function relations of the giant elastic protein titin in striated and smooth muscle cells. Muscle Nerve. 2007;36(6):740–55. doi: 10.1002/mus.20886. [DOI] [PubMed] [Google Scholar]
- 13.Borbely A, et al. Hypophosphorylation of the Stiff N2B Titin Isoform Raises Cardiomyocyte Resting Tension in Failing Human Myocardium. Circ Res. 2009 doi: 10.1161/CIRCRESAHA.108.193326. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe K, et al. Molecular mechanics of cardiac titin’s PEVK and N2B spring elements. J Biol Chem. 2002;277(13):11549–58. doi: 10.1074/jbc.M200356200. [DOI] [PubMed] [Google Scholar]
- 15.Labeit D, et al. Calcium-dependent molecular spring elements in the giant protein titin. Proc Natl Acad Sci U S A. 2003;100(23):13716–21. doi: 10.1073/pnas.2235652100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu Y, et al. Single molecule force spectroscopy of cardiac titin’s N2B element-effects of the molecular chaperone alpha B-crystallin with disease causing mutations. J Biol Chem. 2009 doi: 10.1074/jbc.M809743200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bustamante C, et al. Entropic elasticity of lambda-phage DNA. Science. 1994;265(5178):1599–600. doi: 10.1126/science.8079175. [DOI] [PubMed] [Google Scholar]
- 18.Moroz JD, Nelson P. Torsional directed walks, entropic elasticity, and DNA twist stiffness. Proc Natl Acad Sci U S A. 1997;94:14418–22. doi: 10.1073/pnas.94.26.14418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma K, Kan L, Wang K. Polyproline II helix is a key structural motif of the elastic PEVK segment of titin. Biochemistry. 2001;40(12):3427–38. doi: 10.1021/bi0022792. [DOI] [PubMed] [Google Scholar]
- 20.Gutierrez-Cruz G, Van Heerden AH, Wang K. Modular motif, structural folds and affinity profiles of the PEVK segment of human fetal skeletal muscle titin. J Biol Chem. 2001;276(10):7442–9. doi: 10.1074/jbc.M008851200. [DOI] [PubMed] [Google Scholar]
- 21.Wu Y, et al. Changes in titin isoform expression in pacing-induced cardiac failure give rise to increased passive muscle stiffness. Circulation. 2002;106(11):1384–9. doi: 10.1161/01.cir.0000029804.61510.02. [DOI] [PubMed] [Google Scholar]
- 22.Nagueh SF, et al. Altered titin expression, myocardial stiffness, and left ventricular function in patients with dilated cardiomyopathy. Circulation. 2004;110(2):155–62. doi: 10.1161/01.CIR.0000135591.37759.AF. [DOI] [PubMed] [Google Scholar]
- 23.Murphy S, Frishman WH. Protein kinase C in cardiac disease and as a potential therapeutic target. Cardiol Rev. 2005;13(1):3–12. doi: 10.1097/01.crd.0000124914.59755.8d. [DOI] [PubMed] [Google Scholar]
- 24.Hambleton M, et al. Pharmacological- and gene therapy-based inhibition of protein kinase Calpha/beta enhances cardiac contractility and attenuates heart failure. Circulation. 2006;114(6):574–82. doi: 10.1161/CIRCULATIONAHA.105.592550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pass JM, et al. Enhanced PKC beta II translocation and PKC beta II-RACK1 interactions in PKC epsilon-induced heart failure: a role for RACK1. Am J Physiol Heart Circ Physiol. 2001;281(6):H2500–10. doi: 10.1152/ajpheart.2001.281.6.H2500. [DOI] [PubMed] [Google Scholar]
- 26.Ping P, et al. Ischemic preconditioning induces selective translocation of protein kinase C isoforms epsilon and eta in the heart of conscious rabbits without subcellular redistribution of total protein kinase C activity. Circ Res. 1997;81(3):404–14. doi: 10.1161/01.res.81.3.404. [DOI] [PubMed] [Google Scholar]
- 27.Bowling N, et al. Increased protein kinase C activity and expression of Ca2+-sensitive isoforms in the failing human heart. Circulation. 1999;99(3):384–91. doi: 10.1161/01.cir.99.3.384. [DOI] [PubMed] [Google Scholar]
- 28.Belin RJ, et al. Augmented protein kinase C-alpha-induced myofilament protein phosphorylation contributes to myofilament dysfunction in experimental congestive heart failure. Circ Res. 2007;101(2):195–204. doi: 10.1161/CIRCRESAHA.107.148288. [DOI] [PubMed] [Google Scholar]
- 29.Liu Q, et al. Protein kinase C{alpha}, but not PKC{beta} or PKC{gamma}, regulates contractility and heart failure susceptibility: implications for ruboxistaurin as a novel therapeutic approach. Circ Res. 2009;105(2):194–200. doi: 10.1161/CIRCRESAHA.109.195313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li H, et al. Multiple conformations of PEVK proteins detected by single-molecule techniques. Proc Natl Acad Sci U S A. 2001;98(19):10682–6. doi: 10.1073/pnas.191189098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kellermayer MS, et al. Mechanical fatigue in repetitively stretched single molecules of titin. Biophys J. 2001;80(2):852–63. doi: 10.1016/S0006-3495(01)76064-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kellermayer MS, et al. Folding-unfolding transitions in single titin molecules characterized with laser tweezers. Science. 1997;276(5315):1112–6. doi: 10.1126/science.276.5315.1112. [DOI] [PubMed] [Google Scholar]
- 33.Rief M, et al. Reversible unfolding of individual titin immunoglobulin domains by AFM. Science. 1997;276(5315):1109–12. doi: 10.1126/science.276.5315.1109. [DOI] [PubMed] [Google Scholar]
- 34.Baumann CG, et al. Ionic effects on the elasticity of single DNA molecules. Proc Natl Acad Sci U S A. 1997;94(12):6185–90. doi: 10.1073/pnas.94.12.6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forbes JG, et al. Titin PEVK segment: charge-driven elasticity of the open and flexible polyampholyte. J Muscle Res Cell Motil. 2005;26(6-8):291–301. doi: 10.1007/s10974-005-9035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bang ML, et al. The complete gene sequence of titin, expression of an unusual approximately 700-kDa titin isoform, and its interaction with obscurin identify a novel Z-line to I-band linking system. Circ Res. 2001;89(11):1065–72. doi: 10.1161/hh2301.100981. [DOI] [PubMed] [Google Scholar]
- 37.Greaser M. Identification of new repeating motifs in titin. Proteins. 2001;43(2):145–9. doi: 10.1002/1097-0134(20010501)43:2<145::aid-prot1026>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 38.Williamson MP. The structure and function of proline-rich regions in proteins. Biochem J. 1994;297(Pt 2):249–60. doi: 10.1042/bj2970249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.MacArthur MW, Thornton JM. Influence of proline residues on protein conformation. J Mol Biol. 1991;218(2):397–412. doi: 10.1016/0022-2836(91)90721-h. [DOI] [PubMed] [Google Scholar]
- 40.Linke WA, et al. PEVK domain of titin: an entropic spring with actin-binding properties. J Struct Biol. 2002;137(1-2):194–205. doi: 10.1006/jsbi.2002.4468. [DOI] [PubMed] [Google Scholar]
- 41.Andrew CD, et al. Effect of phosphorylation on alpha-helix stability as a function of position. Biochemistry. 2002;41(6):1897–905. doi: 10.1021/bi0113216. [DOI] [PubMed] [Google Scholar]
- 42.Letunic I, Doerks T, Bork P. SMART 6: recent updates and new developments. Nucleic Acids Res. 2009;37:D229–32. doi: 10.1093/nar/gkn808. Database issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schultz J, et al. SMART, a simple modular architecture research tool: identification of signaling domains. Proc Natl Acad Sci U S A. 1998;95(11):5857–64. doi: 10.1073/pnas.95.11.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lahmers S, et al. Developmental control of titin isoform expression and passive stiffness in fetal and neonatal myocardium. Circ Res. 2004;94(4):505–13. doi: 10.1161/01.RES.0000115522.52554.86. [DOI] [PubMed] [Google Scholar]