Abstract
The cytokine TNF is a critical mediator of immune and inflammatory responses. The TNF gene is an immediate early gene, rapidly transcribed in a variety of cell types following exposure to a broad range of pathogens and signals of inflammation and stress. Regulation of TNF gene expression at the transcriptional level is cell type- and stimulus-specific, involving the recruitment of distinct sets of transcription factors to a compact and modular promoter region. In this review, we describe our current understanding of the mechanisms through which TNF transcription is specifically activated by a variety of extracellular stimuli in multiple cell types, including T cells, B cells, macrophages, mast cells, dendritic cells, and fibroblasts. We discuss the role of nuclear factor of activated T cells and other transcription factors and coactivators in enhanceosome formation, as well as the contradictory evidence for a role for nuclear factor κB as a classical activator of the TNF gene. We describe the impact of evolutionarily conserved cis-regulatory DNA motifs in the TNF locus upon TNF gene transcription, in contrast to the neutral effect of single nucleotide polymorphisms. We also assess the regulatory role of chromatin organization, epigenetic modifications, and long-range chromosomal interactions at the TNF locus.
TNF plays a critical role in the innate and adaptive immune response and in the normal function of lymphocytes, monocytes, macrophages, neutrophils, and dendritic cells [1, 2]. Although TNF was initially described as a product of macrophages [3], later studies demonstrated that the TNF gene is in fact expressed in a wide range of cell types, including T cells, B cells, NK cells, mast cells, dendritic cells, and fibroblasts [4–11]. Although the secretion of TNF as a mature protein is regulated at the transcriptional, posttranscriptional, translational, and posttranslational levels, this review will examine our current understanding of the mechanisms that control activation of TNF gene expression at the level of transcription, the first step in TNF production.
At the level of transcription, the TNF gene is activated in response to a diversity of specific stimuli that are characteristic of cellular activation, inflammation, infection, and stress. Among these stimuli are calcium signaling, such as calcium influx triggered by ionophores; pathogens, such as bacteria and viruses; mitogens, such as phorbol esters; chemical stress, such as osmotic stress, and radiation, such as UV light (table 1). Inducers of TNF gene transcription also include ligands for several classes of receptors, including antigen receptors, such as the T cell receptor; pattern recognition receptors, such as Toll-like receptors [12], and receptors for cytokines, including the two cognate receptors for TNF itself (table 1).
Table 1.
Inducers of TNF transcription. Certain stimuli (asterisk) require a costimulus in some cell types.
| Stimuli | Reference | |
|---|---|---|
| PRR ligands | ||
|
| ||
| TLR2 | Peptidoglycan (Gram-positive bacteria) | [214] |
| Atypical LPS (P. gingivalis) | [215] | |
|
| ||
| TLR2/TLR6 | Lipoteichoic acid (Gram-positive bacteria) | [216] |
| Diacylated lipoproteins, e.g. MALP-2 | [217] | |
| Zymosan | [218] | |
|
| ||
| TLR3 | Double-stranded RNA, e.g. poly (I:C) | [219] |
|
| ||
| TLR4 | LPS (Gram-negative bacteria) | [220, 221] |
| Synthetic lipid A | [222] | |
| Taxol | [223] | |
|
| ||
| TLR7 | Loxoribine | [224] |
|
| ||
| TLR7/TLR8 | Single-stranded RNA, e.g. poly I, poly C | [225] |
| Imidazoquinoline compounds, e.g. imiquimod | [226] | |
|
| ||
| TLR9 | Bacterial CpG-DNA | [225] |
|
| ||
| NOD2 | Muramyl dipeptide | [227] |
|
| ||
| Antigen receptor ligands | ||
|
| ||
| T cell receptor | Anti-CD3 | [15] |
| PHA | [4] | |
|
| ||
| B cell receptor | Anti-IgG | [13] |
|
| ||
| Fc receptor ligands | ||
|
| ||
| Mast cell receptor (FcεRI) | IgE + antigen | [10] |
|
| ||
| NK cell receptor (FcγRIIIA/CD16a) | Anti-CD16, immune complexes | [228] |
|
| ||
| Other stimuli | ||
|
| ||
| Cytokines | Interleukin-1 | [221] |
| Interleukin-2 | [229] | |
| IFN-γ* | [230] | |
| Granulocyte-macrophage colony stimulating factor (GM-CSF) | [231] | |
| TNF | [232] | |
|
| ||
| Mitogens | Concanavalin A | [233] |
| PMA* | [221] | |
|
| ||
| Superantigens | Staphylococcal toxic shock syndrome toxin-1 | [234] |
| Staphylococcal enterotoxin B | [234] | |
|
| ||
| Phosphatase inhibitors | Okadaic acid | [235, 236] |
| Calyculin A | [235] | |
|
| ||
| Calcium ionophore | Ionomycin* | [15] |
|
| ||
| Radiation | UV light | [237] |
| X-rays | [238] | |
|
| ||
| Osmotic stress | Raffinose | [45] |
|
| ||
| High glucose | [239] | |
|
| ||
| Silica particles | [240] | |
|
| ||
| Bacteria | Listeria monocytogenes | [241, 242] |
| Staphylococcus aureus | [7] | |
| Mycobacterium tuberculosis | [243] | |
| Salmonella typhimurium | [242] | |
| Escherichia coli | [244] | |
|
| ||
| Viruses | Sendai virus | [245] |
| Human cytomegalovirus | [246] | |
| Vesicular stomatitis virus | [219] | |
| Herpes simplex virus type II | [219] | |
|
| ||
| Protozoans | Plasmodium falciparum | [247] |
| Trypanosoma cruzi | [248] | |
| Schistosoma mansoni | [249] | |
Notably, induction of TNF gene transcription after exposure to certain stimuli in specific cell types is paradigmatic of an immediate early gene. For example, after T and B cell activation or after lipopolysaccharide (LPS) stimulation of monocytes, TNF mRNA is transcribed within minutes and is independent of de novo protein synthesis [13–15]. In T cells in particular, TNF is one of the first genes expressed after cellular activation and is one of the few genes that can be induced by signaling through the T cell receptor in the absence of protein synthesis [15] and a CD28 costumulatory signal [15, 16]. Furthermore, the calcium influx component of T cell activation alone can induce TNF transcription [15].
Tight control of TNF expression in specific cell types and after specific stimuli is essential for cellular homeostasis and normal physiology in humans, as evidenced by the finding that dysregulated TNF levels are associated with multiple disease states, including asthma, rheumatoid arthritis, cardiovascular diseases, Crohn’s disease, type II diabetes, eczema, multiple sclerosis, psoriasis, systemic lupus erythematosus, septic shock, and several different forms of cancer [17, 18]. Dysregulation of TNF expression has also been linked to differential susceptibility to several major infectious diseases including tuberculosis and cerebral malaria, when too little or too much TNF is produced, respectively [19, 20]. Thus, the study of TNF gene regulation not only provides an outstanding model system for the study of cell type- and stimulus-specific eukaryotic gene regulation, but also has direct translational implications for understanding a variety of human diseases. The understanding of basic regulatory pathways and identification of mediators leading to TNF gene expression in particular cell types and tissues can provide targets for the design and development of clinically important therapeutic agents that modulate its expression.
Cell Type- and Stimulus-Specific Regulation of TNF Gene Transcription
TNF gene transcription is regulated by nucleoprotein complexes known as enhanceosomes [21–24]. Enhanceosomes consist of sets of transcription factors and coactivators that associate in a higher-order structure with enhancer or promoter regions of a gene and then function in synergy to drive transcription [25, 26]. Notably, studies of TNF gene regulation expanded our understanding of the role of enhanceosomes in transcriptional regulation in general with the novel demonstration that TNF enhanceosome assembly is cell type- and stimulus-specific, involving distinct sets of transcription factors and coactivators [21–24, 27].
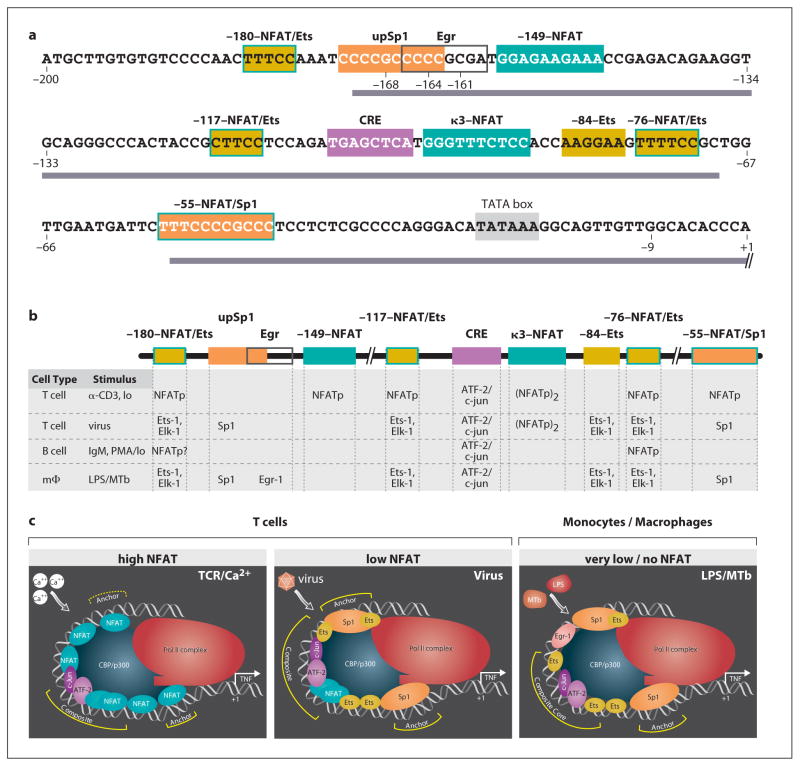
The early observation that TNF was produced by multiple cell types led to experiments to define the sequences involved in transcriptional regulation of the gene in several cell types in response to different stimuli. The 5′ UTR of the TNF gene contains a proximal promoter region of approximately 200 nucleotides (nt) upstream of the mRNA cap site (fig. 1a) that is very highly conserved in mammals [28–31] and almost totally conserved in higher primates [32, 33]. The −200 nt proximal TNF promoter in both humans and mice is sufficient to drive transcription in response to multiple stimuli, including T cell and B cell activation [15, 34–36], calcium ionophore [15], bacterial LPS [22, 37–42], virus infection [37], TNF [43, 44], Mycobacterium tuberculosis (MTb) [24], and osmotic stress [45]. In some cell types, an even smaller core region of the TNF proximal promoter suffices for induction, such as by the mitogen phorbol 12-myristate 13-acetate (PMA) [14, 43, 46, 47].
Fig. 1.
Cell type- and stimulus-specific enhanceosome formation at the proximal TNF promoter. a Sequence of human proximal TNF promoter showing the positions of transcription factor binding sites (boxes), regions of highest sequence conservation in the primate lineage (bar) [33], and positions of fixed genetic differences in nonhuman primates (−9 G/T) and in orangutan and gibbon species (−168 G/A, −164 C/T, −161 C/T) [32, 33]. Adapted from Baena et al. [33]. b Transcription factors that bind and function at sites in the indicated cell types in response to the indicated stimuli [15, 21–24, 34, 35]. c Model of the role of ambient NFAT levels upon TNF enhanceosome formation, with proteins bound at the CRE/κ3/Ets composite site interacting with ‘anchor’ complexes to stabilize interactions with CBP/p300 and the RNA Pol II complex [23, 24]. Adapted from Barthel et al. [24].
The TNF promoter is both compact and modular in its organization. The relative spacing of DNA motifs in the promoter is critical for TNF gene transcription [23, 24], consistent with the specific architectural requirements of distinct sets of factors sitting on the same face of the DNA helix for enhanceosome formation [25, 26]. Moreover, a number of TNF activator binding motifs can be recognized by more than one class of transcription factor depending on the cell type and stimulus and the ambient concentration of different factors in the nucleus [21–24, 34, 35] (fig. 1b, c). This allows the TNF promoter a remarkable degree of flexibility to respond to a variety of stimuli in a specific manner through a short cis-regulatory region.
The proximal TNF promoter (fig. 1a) contains a TATA box and multiple cis-acting elements that are binding motifs for transcription factors. Six nuclear factor of activated T cells (NFAT) binding sites have been identified [15, 23, 35, 48], along with four Ets/Elk binding sites [22, 23, 49] and two Sp1 binding sites [14, 23, 50]. Three of the NFAT sites overlap Ets/Elk sites, while one NFAT site overlaps the downstream Sp1 site [22, 23]. There is also an Egr binding site [50], adjacent to the upstream Sp1 site [22], and a cyclic AMP response element (CRE) [46, 51].
This CRE site, which binds a heterodimer of the basic region-leucine zipper (bZIP) proteins ATF-2 and c-jun [21, 22, 24, 34, 35, 44, 45, 52–57], is a critical regulatory element of the TNF gene in all cell types and under all conditions so far tested [21–24, 34, 35, 39, 41, 42, 44, 45, 49, 51, 54–56]. The TNF CRE functions as part of a potent composite element together with the adjacent κ3-NFAT site when fused to a minimal TNF promoter or to a heterologous promoter [21, 22, 24, 34, 35, 39, 52]. Although the binding of ATF-2/c-jun and NFATp to the CRE is not cooperative [35], the proteins function in a synergistic fashion to activate TNF gene transcription. This was shown in experiments where overexpression of ATF-2, c-jun, and NFATp in Drosophila Schneider-2 cells, which are devoid of these factors, resulted in synergistic activation of a TNF reporter gene [21].
The formation of cell type- and stimulus-specific enhanceosomes at the TNF promoter involves mutually exclusive binding of NFAT, Sp1, and Ets/Elk transcription factors to specific DNA motifs (fig. 1b). Experimental evidence indicates that if there is sufficient nuclear localization of NFAT, then NFAT will outcompete Ets/Elk or Sp1 binding at these sequences. For example, the amount of NFATp that translocates to the nucleus in T cells upon ionomycin stimulation is higher than upon virus infection, consistent with higher levels of calcium influx induced by ionomycin relative to virus [21]. After ionomycin stimulation, all six NFAT sites are occupied by NFATp [21, 23, 34]. But after virus induction, with lower levels of nuclear NFATp, Sp1 can successfully compete for binding at both Sp1 sites in the proximal promoter [21, 23]. Similarly, after LPS stimulation of monocytes where no inducible translocation of NFATp can be detected, Sp1 and Ets/Elk proteins can successfully bind to sites that are occupied by NFATp in T cells [22, 23] (fig. 1b).
Cell type- and stimulus-specific assembly of TNF enhanceosomes is thus typified by differential occupancy of overlapping DNA motifs in the TNF promoter. These studies have led to a model in which the ambient level of NFAT in the nucleus determines the composition of the TNF enhanceosome, where a combination of core ‘composite’ complexes of ATF-2/c-jun-NFAT or ATF-2/c-jun-NFAT-Ets/Elk proteins, centered at the CRE/κ3-NFAT site, interact with other ‘anchor’ complexes to stabilize interactions with coactivators, such as CBP/p300, and the general transcription machinery (fig. 1c). This flexible configuration is consistent with activation of the TNF gene in response to different transcription factors at different concentrations in the nucleus after a particular stimulus.
Role of Nuclear Factor of Activated T Cells in TNF Gene Regulation
Of all of the transcription factors involved in the regulation of TNF gene expression, NFAT has the clearest role. The NFAT family of proteins consists of five distinct transcription factors in vertebrates: NFATp (also known as NFATc2 or NFAT1), NFATc (NFATc1 or NFAT2), NFAT3 (NFATc4), NFAT4 (NFATc3 or NFATx), and NFAT5 (TonEBP or OREBP). NFATp, NFATc, NFAT3, and NFAT4 reside in the cytoplasm in a hyperphosphorylated state; upon activation they are dephosphorylated by the calcium-dependent phosphatase calcineurin and undergo rapid nuclear translocation, and are thus sensitive to the calcineurin inhibitors cyclosporin A (CsA) and FK506. Unlike the four calcineurin-dependent factors, NFAT5 is responsive to hypertonic stress and integrin activation in addition to T cell stimulation and is constitutively nuclear, save for a small proportion that translocates to the nucleus under hypertonic conditions. NFATp, NFATc, NFAT4, and NFAT5 are expressed in a wide range of cell types and tissues, while NFAT3 is restricted to nonlymphoid cells. NFAT5 binds DNA as an obligate dimer and is dimeric in solution, while the calcineurin-dependent NFAT proteins bind DNA as monomers or dimers; NFAT monomers bind cooperatively with other transcription factors at NFAT/AP-1 elements and other composite DNA motifs [58–61].
The first evidence that NFAT was involved in regulation of the TNF gene came from studies showing that TNF transcription in T cells in response to engagement of the T cell receptor with anti-CD3 or treatment with ionomycin was CsA-sensitive and dependent upon the κ3 site in the proximal promoter [15]. Expression of TNF mRNA was found to be independent of de novo protein synthesis, and a constitutive anti-CD3− and ionomycin-inducible factor that was blocked from the nucleus by CsA was found to bind the κ3 site [15]. This factor thus clearly exhibited the properties assigned to NFAT [62] prior to the cloning of the first NFAT family member, NFATp [63]. This was further supported by the observation that induction of TNF gene transcription was specifically dependent upon the phosphatase activity of calcineurin, both in anti-CD3− or ionomycin-stimulated T cells and in anti-IgG-stimulated B cells [64]. Later experiments confirmed that the observed CsA- and FK506-sensitive factor was NFATp, which bound to κ3 as a dimer [48]. NFATp was also later detected in the FK506-sensitive complex bound to κ3 following PMA and ionomycin treatment of a murine mast cell line [65].
The NFATp dimer was then shown to function in a cooperative fashion with ATF-2/c-jun at the CRE/κ3-NFAT composite element, also in a CsA-sensitive manner [34]. Unlike the canonical NFAT/AP-1 composite element of the interleukin-2 gene promoter, in which binding of NFAT monomer is required for recruitment of its bZIP partner [58, 59, 61], binding of NFATp dimer and ATF-2/c-jun to CRE/κ3-NFAT was shown to be noncooperative [35]. In addition to κ3-NFAT, which is located at −97 to −88, the five other NFAT binding sites identified in the human and murine TNF promoters through DNase footprinting with recombinant NFATp are designated by their positions at −180, −149, −117, −76, and −55 relative to the mRNA cap site (fig. 1a) [23, 35, 36]. NFATc, NFAT3, and NFAT4 bind to these same NFAT sites with slightly varying affinities [45].
A role for cell type-specific function of NFATp in activated T versus B cells was also demonstrated. For example, while binding of NFATp to the κ3-NFAT was shown to be dispensable for activation of the proximal TNF promoter in B cells (in response to ionomycin or PMA and ionomycin), the high-affinity −76-NFAT site was strictly required for TNF gene activation in T and B cells [34, 35]. All of the NFAT sites seem to play a role in T cell activation of TNF in that mutations in the −180, −149, −117, and −55-NFAT sites all reduce inducible levels of activation of the proximal TNF promoter in T cells [23, 34, 35]. Thus, DNA motifs with different affinities for NFAT and other factors, such as Ets/Elk and Sp1 proteins, are required for the regulation of TNF transcription and are involved in the precise modulation of gene expression in T cells and other cells.
Chromatin immunoprecipitation (ChIP) assays later confirmed that NFATp was indeed inducibly recruited to the TNF promoter in its native chromatin context upon treatment of T cells with ionophore or virus and upon treatment of B cells with PMA and ionomycin [21]. Similarly, NFATc was also shown by ChIP to be recruited to the TNF promoter in L929 fibroblasts, which lack NFATp, upon treatment with virus [21]. In ChIP assays with bone marrow-derived murine mast cells, both NFATp and NFATc were shown to be recruited to the TNF promoter upon treatment with ionomycin [66]. Taken together, these studies provided evidence that NFAT binding elements and NFAT proteins play a critical role in TNF gene regulation.
Detailed analysis of NFATp dimer binding at the CRE/κ3-NFAT composite element revealed that dimerization of NFATp is required for transcriptional activation mediated by the CRE/κ3-NFAT site. This was independent of the orientation of the κ3-NFAT site relative to the CRE site, consistent with the lack of physical interaction between ATF-2/c-jun and NFATp [52]. However, ATF-2/c-jun and NFATp function in a synergistic fashion at the CRE/κ3-NFAT element despite their lack of cooperative binding. This functional synergy suggested that the activation domains of NFATp played a role in TNF transcription. Indeed, two subdomains in the N- and C-terminal activation domains of NFATp are required for interaction with CBP and activation of transcription mediated by the CRE/κ3-NFAT site and the TNF promoter [52]. This is in agreement with a study in which the C-terminal activation domain of NFATp was shown to be required for TNF transcription and to function in isolation as a dominant-negative inhibitor of TNF transcription in the Jurkat human T cell line [67]. These studies demonstrated that in the context of the TNF promoter, the activation domains of NFATp specifically contribute to the activation of gene transcription.
NFAT3 has also been implicated in TNF gene regulation in cells of a nonlymphoid lineage. Activation of a TNF gene reporter and expression of endogenous TNF mRNA in response to UV radiation in murine embryonic fibroblasts is blocked by RNA interference (RNAi) of NFAT3; furthermore, in response to UV radiation, activation of the TNF reporter is strongly inhibited by mutation of the −180 and −76-NFAT sites, and NFAT3 is recruited to the endogenous TNF promoter [68]. RNAi of NFAT3 also inhibited TNF transcription in a murine epidermal cell line in response to silica or the carcinogen (+/−)-benzo[a]pyrene-7,8-diol-9,10-epoxide [69, 70]. A specific role for NFAT4 in the regulation of TNF gene transcription has not been characterized, although as noted above, recombinant NFAT4 can bind to all six NFAT binding sites in the TNF promoter [45].
By contrast, while recombinant NFATp, NFATc, NFAT3, and NFAT4 bind to the same set of sites in the TNF promoter in DNase footprinting assays, recombinant NFAT5 only binds to two of the NFAT sites in the TNF promoter: κ3-NFAT and −76-NFAT [45]. ChIP assays showed that NFAT5 associates with the TNF promoter in response to osmotic stress in Jurkat cells [71], while inhibition of NFAT5 by RNAi, transfection of a dominant-negative form of NFAT5, or mutation of the κ3-NFAT and −76-NFAT sites inhibits activation of the proximal TNF gene promoter in response to osmotic stress in L929 cells [45]. Thus, NFAT5 also plays a key role in TNF gene regulation in certain cell types and under certain conditions.
The critical role of NFAT in TNF gene regulation shown by these biochemical and cell-based assays has been supported by multiple studies using transgenic mice. In particular, these in vivo studies have provided evidence that NFATp is the NFAT protein primarily responsible for TNF gene regulation in T cells. NFATp-deficient mice display defects in T cell-derived TNF expression in response to anti-CD3 (or anti-CD3 and anti-CD28) antibodies and to superantigen [36, 72, 73]. This is in contrast to findings in NFATc-deficient murine T cells, which produce wild-type levels of TNF [74, 75]. Furthermore, TNF mRNA expression induced by treatment with ionomycin or IgE crosslinking in bone marrow-derived mast cells is inhibited by deficiency of NFATp but not NFAT4 [66], and TNF expression from murine T cells lacking both NFATp and NFAT4 is diminished at levels similar to those observed in NFATp-deficient mice [72, 76].
Strikingly, in vivo evidence for a stimulus-specific role for NFATp in TNF gene regulation was demonstrated in a murine model of TNF-mediated superantigen-induced lethal shock. NFATp-deficient mice were resistant to T cell-dependent septic shock as compared to wild-type control mice, but were susceptible to macrophage-driven LPS-induced septic shock [36]. These experiments thus provided definitive in vivo evidence that TNF gene expression is dependent upon distinct factors in T cells and in monocytic cells.
Residual TNF expression in NFATp-deficient cells appears to be driven by NFATc, as deletion of both NFATp and NFATc from murine lymphoid cells eliminates any detectable TNF expression in T cells upon primary or secondary stimulation under Th1 and Th2 conditions [77]. Similarly, in bone marrow-derived mast cells, ionomycin-induced TNF mRNA expression is inhibited by RNAi of either NFATp or NFATc [66]. RNAi of NFATc in NFATp-deficient mice further inhibits ionomycin-induced TNF mRNA expression, and ectopic expression of NFATc rescues this expression [66]. Furthermore, transgenic mice expressing a constitutively nuclear form of NFATc display enhanced TNF transcription in unstimulated and anti-CD3-stimulated peripheral T lymphocytes [78], although expression of constitutively nuclear NFAT4 in transgenic mice also greatly enhances TNF expression in Th1 cells induced by PMA or PMA and ionomycin [79].
Notably, a parallel to these results from transgenic mice is found in studies of human T cells in which NFAT signaling is impaired. T cells from severe combined immune deficiency patients, which had nearly undetectable levels of NFAT binding to DNA in EMSAs, were unable to produce TNF [80]. Similarly, in Wiskott-Aldrich syndrome patients, TNF secretion was abrogated or strongly reduced in CD4+ and CD8+ T cells, which correlated with a reduction in nuclear localization of both NFATp and NFATc [81].
Nuclear Factor-κB-Independent TNF Gene Transcription
The nuclear factor-κB (NF-κB) proteins are a major family of transcription factors involved in control of the immune response. This family includes p50, p65 (RelA), c-Rel, p52, and RelB, which bind DNA as obligate homo- or heterodimers [82–84]. TNF is often described in the literature as one of the classical NF-κB-dependent proinflammatory cytokines, largely based upon early studies that concluded that there was a major role for NF-κB in TNF gene expression in response to LPS in cells of the monocyte/macrophage lineage. However, a direct role for NF-κB in the activation of TNF transcription was not clear from these initial studies.
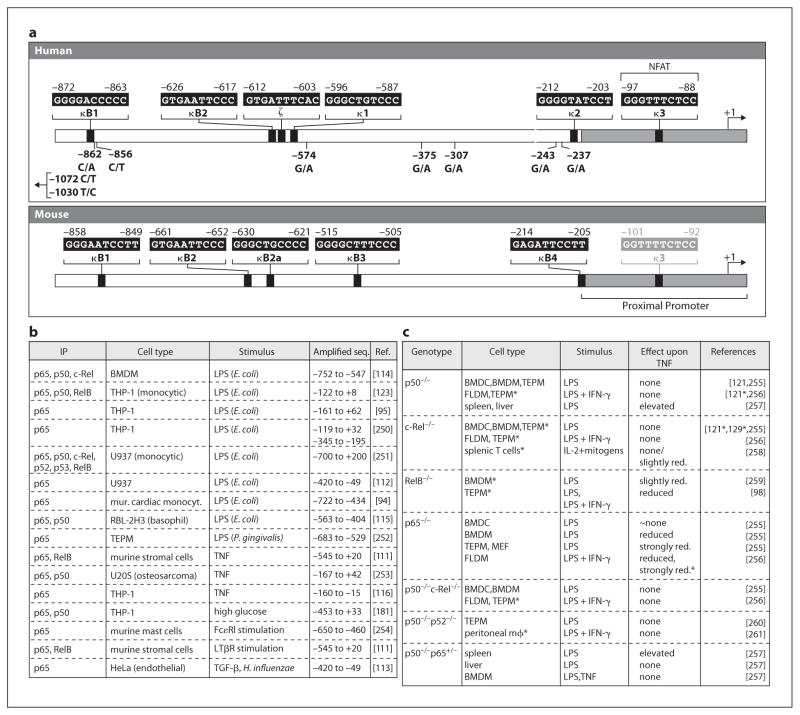
Based on their similarity to known NF-κB binding sites, sequences in the 5′ flanking region of both the human and the murine TNF genes were initially designated as κB motifs [28, 37, 85, 86]. In the human TNF promoter, these sites were named κ1, κ2, and κ3 [37]. Later, three additional NF-κB-like sites were identified: κB1, κB2, and ζ [29, 87]. In the murine TNF promoter, these sites were initially called κB1, κB2, κB2b, κB3, and κB4 [28, 85, 86]; κB2b was later renamed κB2a [29]. Notably, all of these sites in the human and mouse genes, except the human κ3 site, lie outside of the proximal TNF promoter shown to be sufficient for induction of the gene by multiple stimuli and in diverse cell types (fig. 2a).
Fig. 2.
NF-κB and TNF gene regulation. a Putative NF-κB and NF-κB-like sites in the human and murine TNF promoters, along with the positions of SNPs in the human TNF promoter. b Stimulus-dependent association of NF-κB proteins (in at least one condition or time point studied) with the TNF promoter in ChIP assays. c Impact of deletions of NF-κB proteins in mice upon TNF gene transcription (asterisk denotes studies and cell types where only protein expression was assayed) in response to various stimuli). TEPM = Thioglycollate-elicited peritoneal macrophages; FLDM = fetal liver-derived macrophages; BMDM = bone marrow-derived macrophages; BMDC = bone marrow-derived dendritic cells; MEF = murine embryonic fibroblasts.
A role for NF-κB in LPS-mediated murine TNF gene expression was originally postulated based on the binding of inducible factors to sites in the distal murine TNF promoter, particularly κB3 (fig. 2a), a murine-specific κB site which exhibited the highest affinity for a factor with the properties of NF-κB and conferred LPS inducibility upon a heterologous promoter when present in two or three copies [28, 85, 86]. This view that NF-κB was involved in TNF gene regulation in monocytes was reinforced by indirect evidence from studies where NF-κB was inhibited by dexamethasone and pyrrolidine dithiocarbamate, and the correlation of these data with experiments showing that the same compounds also inhibited TNF mRNA expression in monocytes or macrophages [85, 88]. Subsequent studies reported that inhibition of NF-κB activity by dominant-negative versions of IκB-α and IκB kinase was also correlated with inhibition of LPS-induced TNF transcription in monocytic cell lines, murine dendritic cells, and primary human macrophages [29, 89–95].
However, deletion or site-directed mutation of κ1 or κ2 did not affect LPS induction of the human TNF promoter in gene reporter assays, and multiple copies of κ1, κ2, and κ3 did not confer LPS or virus induciblity on a heterologous minimal promoter [21, 22, 37, 39], as would be expected for a bona fide NF-κB binding site such as PRDII in the interferon-β promoter [96]. These observations led to the conclusion, even in 1990, that these sites were not typical NF-κB sites [37]. Although κB1 (fig. 2a) was shown to have the highest affinity for NF-κB in vitro of all of the distal human TNF promoter sites, its mutation also had little or no effect on TNF transcription [29, 87]. The human κ2 site has consistently been shown to have little or no affinity for NF-κB and to play no role in TNF gene transcription [29, 37–39, 87, 88, 97]. Furthermore, the κ2 and κB1 sites are not present in the murine promoter [28, 29], while the murine κB3 site, which has the highest affinity for NF-κB among the murine κB-like sites [29, 85, 86, 98], is not present in the TNF promoter in humans and other primates [28, 29, 32, 33]. In conclusion, all of these data argue against an important conserved regulatory role for these sites in murine or human TNF gene regulation.
Three NF-κB sites in the distal human TNF promoter, κB2, ζ, and κ1 (fig. 2a), are clustered in a region of higher evolutionary sequence conservation relative to the rest of the distal promoter, but only κB2 is completely conserved between mice and primates [29, 32, 33]. Although mutations in these low-affinity NF-κB sites were reported to reduce LPS inducibility of the human TNF gene by 40–50% in reporter assays [29, 87], these reports were hard to reconcile with studies showing that mutation of κ1 [37] and even deletion of the entire distal promoter [22, 37–39, 41] did not impact LPS induction of a human TNF reporter gene. Similarly, in gene reporter assays with the murine TNF promoter, deletion of the distal promoter region [42] and mutation of κB1 (and in some cases, κB3, κB2, and κB2a) had little to no effect on LPS-induced TNF transcription [29, 99]. Thus, there has been no clear functional role demonstrated for these NF-κB motifs in the activation of murine or human TNF gene transcription.
A number of studies have reported, using antibody supershift assays, the binding of NF-κB p50 and p65 to the most proximal of the human κB-like sites, κ3 [38, 39, 55, 97, 100–102]. However, the κ3 site (later renamed κ3-NFAT since it was shown to be an important functional NFAT site as described above) has little or no affinity for NF-κB, particularly in studies comparing the relative affinities of NF-κB (or NF-κB-like) sites in EMSAs and quantitative DNase I footprinting assays [29, 87, 88, 103]. In fact, replacement of the κ3-NFAT site (5′-GGGTTTCTCC-3′) with the well-characterized functional NF-κB site from the interferon-β gene promoter (PRDII, 5′-GGGAAATTCC-3′) confers virus inducibility on the TNF proximal promoter in HeLa cells, a cell type in which the wild-type TNF proximal promoter is not virus inducible [103]. This is consistent with the DNA-binding properties of NF-κB, given that the κ3-NFAT site (5′-GGGTTTCTCC-3′) diverges from the canonical NF-κB (5′-GGGRNTTYCC-3′ or 5′-GGGRNNTYCC-3′) and p65 (5′-GGGRNTTTCC-3′ and 5′-NGGRNTTYCC-3′) consensus motifs at two key positions (underlined) and bears little resemblance to the noncanonical NF-κB consensus motif (5′-RGGAGATTG-3′) which preferentially binds RelB/p52 [95, 104–107]. Furthermore, the corresponding κ3 sequence in the murine proximal TNF promoter (5′-GGTTTTCTCC-3′) is an NFAT binding site [36] that has never been implicated as an NF-κB binding site. Notably, the G to T change at position three in mouse as compared to human (5′-GGGTTTCTCC-3′) would not favor NF-κB binding, but would be expected to make it an even stronger NFAT binding site.
Several studies employed the ChIP assay to provide evidence for the binding of NF-κB, typically p65, to the TNF promoter in response to a variety of stimuli in different cell types (fig. 2b). The region of the TNF promoter amplified in these ChIP assays varied considerably, with some encompassing the distal promoter and others the proximal (fig. 2b). The standard ChIP assay has a level of resolution of ~300 bp [108] and may disrupt native chromatin configuration [109]. In fact, global ChIP studies have identified multiple regions that are retained by immunoprecipitation of p50 or p65 but contain no NF-κB binding sites [95, 110]. Thus, it is important to correlate ChIP assays with other protein-DNA interaction assays and with functional data such as gene reporter assays before concluding that a protein detected by ChIP is functioning via a specific DNA site. While some of these ChIP-based studies employed EMSAs with consensus NF-κB motifs or NF-κB motifs from the distal TNF promoter or other gene promoters [111–116], none examined direct binding of NF-κB to the κ3-NFAT site, or to any other site in the proximal TNF promoter, and correlated such data with a functional role for the site.
Furthermore, in transgenic mice, deletion of the genes encoding the NF-κB proteins p50, c-Rel, p52, and RelB had little or no effect upon expression of TNF mRNA or protein, although in macrophages and embryonic fibroblasts lacking p65, TNF mRNA levels were strongly reduced (fig. 2c). Inhibition of NF-κB activity by genetic deletion of the upstream regulator NEMO/IκB kinase-γ or by the proteasome inhibitor lactacystin revealed that production of wild-type levels of TNF mRNA at late time points after treatment with LPS or virus was NF-κB-dependent, but induction of TNF transcription was not. This indicated a postinduction role for NF-κB, perhaps through control of signal transduction pathways or chromatin remodeling, rather than classical activation through NF-κB binding to the proximal promoter [103]. While the mechanistic details remain to be determined, this may at least partially reconcile the seemingly contradictory evidence regarding the role of NF-κB in TNF gene regulation in cells of the monocyte/macrophage lineage and explain how NF-κB inhibition could potentially inhibit TNF expression through a secondary effect.
Interestingly, interaction of NF-κB with the distal TNF promoter appears to have a role in another aspect of TNF transcription: LPS tolerance, the persistent state of repressed transcription following prolonged exposure to LPS [117]. In the murine TNF promoter, the κB3 site mediates this repression [118], and binding of p50 homodimer to the murine κB3 site and binding of p50 or p52 homodimer to the human κ1 site are upregulated during LPS tolerance [97, 118–121]. Moreover, abrogation or inhibition of p50 and RelB expression in primary macrophages or macrophage cell lines counteracts the repression of TNF transcription during LPS tolerance [121–123].
Other Transcription Factors Involved in Activating TNF Gene Transcription
A number of studies have implicated other transcription factors in TNF gene regulation, including proteins of the bZIP, signal transducer and activator of transcription (STAT), and interferon regulatory factor (IRF) families. Some of these results are relatively preliminary in nature. For example, a role for the bZIP protein C/EBPβ (also known as NF-IL6) in the regulation of TNF gene transcription in cells of the monocyte/macrophage lineage was initially postulated primarily from results with ectopic expression of full-length and dominant-negative forms of the protein and EMSAs with motifs overlapping the κ3-NFAT and −180-NFAT/Ets sites [124–126]. However, later studies contradicted a specific role for C/EBPβ in LPS-induced TNF gene transcription in macrophages [97, 127–129]. Another bZIP protein, Nrf1, has only been detected by EMSA in a complex that binds CRE/κ3-NFAT in stimulated mast cells [130, 131]. Similarly, ChIP assays showed phosphorylated STAT1 binding to the TNF promoter region upon IFN-γ stimulation of murine macrophages [132], although gene knockouts of STAT1 and STAT4 and mutation of a putative STAT3 binding site in the distal murine TNF promoter had only modest effects upon LPS-induced TNF expression and transcription [133, 134].
Another transcription factor implicated in TNF gene regulation is LPS-induced TNF-α factor (LITAF), also known as p53-induced gene 7 (PIG7) [135, 136]. LITAF binds a motif in the distal human TNF promoter (5′-CTCCC-3′, at −515 to −511) and forms a functional complex with STAT6(B) [137, 138]. Reduction in LITAF expression levels resulted in partial inhibition of endogenous TNF mRNA and protein expression [135, 136, 139], and ectopic expression of LITAF, or LITAF and STAT6(B), increased transcription mediated by the human TNF promoter and secretion of TNF protein [137, 138]. However, deletion of the LITAF binding site in the context of the human TNF promoter (including −991 nt) had no effect upon LPS-induced transcription in reporter assays [137]. Thus, it is possible that, like NF-κB, binding of LITAF to the distal TNF promoter plays a postinduction role in the maintenance of mRNA expression. Indeed, it has been suggested that the two factors act in an additive fashion via independent signal transduction pathways [139].
Recent studies have suggested a role for IRF proteins in TNF transcription. ChIP assays detected binding to the TNF promoter by IRF-1 and IRF-8 in response to IFN-γ stimulation of a murine macrophage cell line [140] and by IRF-3 in response to LPS and PMA under conditions of chronic ethanol exposure in a human macrophage cell line [141]. Furthermore, RNAi of IRF-3 in mouse embryonic fibroblasts inhibited LPS-induced TNF mRNA expression [142]. However, while some studies described putative IRF binding sites in the TNF promoter [140, 141], and IRF-3 in particular was implicated in a late-phase autocrine loop of TNF gene activation by LPS [142, 143], the binding of IRF proteins to a specific DNA site was not tested. Thus, a direct role of IRF proteins in TNF gene transcription remains to be elucidated.
Single Nucleotide Polymorphisms, Fixed Genetic Differences, and TNF Transcriptional Regulation
The TNF gene resides between the class I human leukocyte antigen (HLA)-B and class II HLA-DR loci in the most polymorphic region of the human genome: the major histocompatibility complex, or human histocompatibility locus (HLA), located on human chromosome 6 [144, 145]. Several single nucleotide polymorphisms (SNPs) have been identified in the vicinity of the human TNF locus, particularly in the distal TNF promoter, and several have been suggested to influence disease outcome through a direct impact upon the transcriptional regulation of TNF [146–150].
We note that initial studies incorrectly numbered the positions of SNPs relative to the TNF mRNA cap site, which should have proceeded 5′ from the adenine at the +1 position [151]. While the ‘traditional’ numbering system (e.g. −308 instead of −307) persists in some papers in the literature, the corrected numbering has been used in several studies and in the Cytokine Gene Polymorphism in Human Disease database [152] and is used throughout this review (fig. 2a). TNF promoter SNPs have been associated with a wide variety of disease conditions [146–150, 152]. Intriguingly, multiple SNPs in the TNF promoter (−1030 T/C, −862 C/A, −856 C/T, −307 G/A, and −243 G/A) are in linkage with HLA molecules in extended haplotypes [151, 153–155], which are conserved blocks of DNA sequences between HLA-B and HLA-DR [156, 157]. They in fact serve as markers of extended haplotypes [146–149, 152].
Notably, a number of TNF promoter SNPs, including several purported to impact TNF gene regulation, in fact serve as markers of ancestry in human populations (−1030 T/C, −862 C/A, −856 C/T, −574 G/A, −375 G/A, −307 G/A, −243 G/A, −237 G/A, +69 C/G, and +70 +C) and correspond to ancestral alleles in the primate lineage (−1072 C/T, −1030 T/C, −862 C/A, −375 G/A, −307 G/A, and −237 G/A) [30, 32, 33, 155]. For example, the −856 SNP was relatively common (13–14%) in individuals of Caucasian and Cambodian descent and unusually prevalent (30–45%) in Amerindian (Quechua and Paez) populations but was absent in Malawians [155]. Thus, TNF promoter SNPs are likely to be in linkage with other genes in the major histocompatibility complex locus, which may in turn impact resistance and susceptibility to, and severity of, disease independent of their effect, or lack of effect, on TNF gene expression. Consistent with this hypothesis that SNPs are markers of divergence, and not recently selected traits impacting disease susceptibility, is the finding that the rare allele of the −307 human SNP is present in Old World monkey species, indicating that the adenine at that position was fixed in Old World monkeys when they diverged from apes roughly 25 million years ago [32, 33].
Notably, all SNPs that have been detected upstream of the TNF gene in more than one individual lie outside of the proximal promoter (fig. 2a). Moreover, sequence analysis of the TNF promoter region (up to 1.2 kb upstream of the start site of transcription) in primates revealed areas of high conservation within the ~200 bp proximal promoter. Comparative sequence analysis led to the identification of ‘phylogenetic footprints’ between positions −131 to −63 and −53 to −45 [32]. Regions of very low accumulated sequence variation were also revealed by phylogenetic shadowing from −171 to −70 and from −54 to +29 [33]. Strikingly, these sequences overlap motifs essential for TNF gene regulation and for enhanceosome formation (fig. 1a).
Intriguingly, in great apes and in species representing the four gibbon genera, the proximal TNF promoter sequence is identical to that found in humans, except for a G to T transversion at position −9 common to all non-human primates and certain fixed genetic differences within or flanking the upstream Sp1 site at −172 to −163 [32, 33] (fig. 1a). In humans, African great apes, and Old World monkeys, this sequence is 5′-CCCCGCCCCCGCG-3′, while the sequence is 5′-CCCCACCCCCGTG-3′ in orangutans (Pongo pygmaeus and Pongo abelii) and 5′-CCCCACCCTCGCG-3′ in the northern white-cheeked gibbon (Nomascus leucogenys leucogenys). The G to A transition at position −168 strongly inhibited the binding of Sp1 and Sp3 to the site in EMSAs. Strikingly, the orangutan and gibbon TNF promoters with this −168 G/A transition displayed decreased levels of transcriptional activation in response to LPS and MTb in monocytic cells but not in response to ionomycin in T cells [33]. This observation is consistent with the cell type- and inducer-specific role of Sp1 in the regulation of the TNF gene [21–24], and suggests that a distinct set of infectious disease pressures were involved in the evolution of the innate immune response and TNF in Asian versus African apes, which impacted the Sp1 site [33].
Despite the associations between TNF promoter SNPs and disease, a direct impact of TNF promoter polymorphisms upon TNF transcription has not been conclusively demonstrated [146–150]. For example, in the case of the most commonly studied TNF promoter SNP, −307 G/A, some reports concluded that the −307 A allele yielded an increase in transcription in reporter assays [158–162]. In some of these studies, the effect was only observed when the 3′ UTR was included in the reporter construct, and with only a small subset of cell types and stimuli [160, 162]. Other studies reported no differences between the alleles in comparable reporter assay systems [151, 163–165]. Contrasting results were obtained in EMSAs with nuclear extracts and probes spanning the −307 region of the TNF promoter [160, 161]. Furthermore, ChIP assays in human lymphoblastoid cell lines heterozygous at the −307 G/A SNP showed that there was no allele-specific difference in recruitment of RNA polymerase II to the TNF promoter [166]. Thus, taken together the data indicate that the −307 SNP, which appears as a fixed difference from humans in Old World monkeys, has no clear transcriptional role.
A number of studies have also reported that binding of the transcription factor Oct-1 to sites in the human TNF promoter is specific for the variant alleles of the −375 G/A, −856 C/T, and −862 C/A SNPs [167–170]. As was the case for the −307 G/A SNP, some groups reported a change in activity mediated by some or all of these rare alleles [154, 167, 171], while others reported no difference in activity [151, 165, 169, 172]. Since the binding of NF-κB p50 homodimer to κB1 (fig. 2a) is inhibited by the presence of an adenine at −862 [170, 171], the −862 C/A SNP might have an impact on LPS-mediated tolerance at the level of transcription; however, in reporter assays in human monocytes, the presence of the −862 A allele only resulted in an increase in LPS-induced transcription when the 3′ UTR was present [171]. Finally, like the −307 SNP and other SNPs, the −375 SNP falls outside of the evolutionarily conserved regions of the TNF promoter, consistent with the lack of a direct impact of the variant alleles upon activation of TNF gene transcription.
Epigenetic Regulation of TNF Transcription
Gene transcription involves not only the assembly of transcription factors and coactivators at gene promoter and enhancer regions, but also the regulation of accessibility to DNA in the context of chromatin. Transcriptionally active genes are marked by a number of covalent modifications of both histones and DNA [173–177]. For example, histone acetyltransferases, including general transcription factors, coactivators, and sequence-specific transcription factors, promote the formation of ‘open’ areas of chromatin by altering the net charge of nucleosomes through acetylation of lysine residues in the N-terminal tails of histones H3 and H4 [178–180]. TNF transcription is associated with multiple histone acetyltransferases, including ATF-2 [21, 22, 24, 34, 35, 44, 53–57], CBP/p300 [22, 24, 27, 181–183], p/CAF [181, 184], and GCN5 [184]. CBP, in particular, is specifically required for TNF gene transcription in response to T cell activation [27, 182]. Furthermore, a component of the SWI/SNF chromatin remodeling complex that interacts with acetylated histones, BRG1, is associated with the TNF promoter in unstimulated J774 monocytic cells, consistent with a poised preinduction open chromatin conformation [185].
In multiple studies, acetylation of histone H3 and histone H4 at the TNF promoter region has been correlated with TNF transcription in primary cells and cell lines of the monocyte/macrophage and T cell lineage. Increased histone H3 and H4 acetylation at the TNF promoter region (and, in some cases, at the third intron of TNF) in primary human monocytes or human monocytic cell lines (e.g. THP-1) has been associated with induction of TNF transcription by LPS [186, 187] and high glucose concentrations [181], maturation of monocytes to macrophages [188], diabetes [181], and systemic lupus erythematosus [189]. Moreover, IFN-γ treatment of primary human monocytes led to increased, persistent H4 acetylation along with recruitment of ATF-2 and RNA Pol II, yielding a ‘poised’ pretranscription state and, subsequently, elevated LPS-induced levels of both TNF transcription and histone H3 and H4 acetylation at the TNF promoter [187]. In Jurkat T cells, phytohemagglutinin (PHA)/PMA stimulation led to acetylation of histone H3 at the TNF promoter, correlating with the recruitment of both p/CAF and GCN5, while histone H4 was constitutively acetylated [184]. Notably, a transactivator of transcription (Tat) protein from HIV-1 subtype E (HIV-1TH64 Tat), which specifically inhibited TNF transcription, also inhibited p/CAF recruitment to the TNF promoter and decreased levels of histone H3 acetylation and GCN5 recruitment [184]. Enrichment of acetylated histone H3 and H4 was also reported in the vicinity of the TNF promoter in PMA/ionomycin-stimulated Jurkat cells [190].
The complex transcriptional regulatory code of histones includes not only acetylation, but also methylation, phosphorylation, and ubiquitination [174–177]. Histone modifications that are typically associated with gene derepression and transcription have been detected at the TNF promoter. Mono-, di-, and trimethylation of lysine 4 of histone H3 (H3K4) has been observed at the TNF promoter following LPS or TNF stimulation of THP-1 cells and PMA/ionomycin stimulation of Jurkat cells [116, 186, 190]. Phosphorylation of serine 10 of histone H3 (H3S10) has been observed at the TNF promoter in THP-1 cells, but not primary human dendritic cells, following LPS stimulation [123, 191]. Furthermore, levels of dimethylated H3K4, which often marks genes that are competent for transcription, have been shown to decrease at the TNF promoter following LPS induction, replaced by a peak (at the TNF promoter and, in some cases, TNF intron 3) of trimethylated H3K4, which typically marks active transcription [186]. By contrast, several kinds of non-TNF-expressing cells, including LPS-tolerant cells, display relatively lower levels of methylation at H3K4 and phosphorylation at H3S10 and higher levels of di- or trimethylation of H3K9, which is a marker of repressed genes that recruits heterochromatin protein 1 (HP1), which in turn promotes gene silencing [123, 186, 192]. The functional role of these modifications was indicated by the observation that inhibition of H3K4 methylation through RNAi of the histone methyltransferase SET7/9 or of components of the mixed-lineage leukemia histone methyltransferase complex reduced TNF transcription [116, 186], while inhibition of H3K9 methylation through RNAi of the histone methyltransferase G9a in LPS-tolerant cells decreased HP1 binding and restored TNF transcription [192]. Thus, while the specific modifications involved vary with cell type and stimulus, histone methylation and phosphorylation influences TNF gene transcription.
In eukaryotic genomes, methylation of DNA at cytosine residues, typically at CpG or CpNpG sequences, generally represses gene transcription [174–176]. Initial studies demonstrated that in various primary cell types, including monocytes and lymphocytes, the TNF gene and proximal TNF promoter were unmethylated, while in non-TNF-expressing HeLa cells, they were highly methylated [193]. Moreover, fusion of murine 3T3 fibroblasts to RAW 264.7 macrophages resulted in hybrid cells which, like the 3T3 cells, had a highly methylated TNF locus and did not express TNF [194]. The TNF proximal promoter and first exon were also shown to be highly methylated in K562 cells and THP-1 clones that did not express TNF, but unmethylated in TNF-expressing THP-1 clones and HL60 promyelocytic leukemia cells. Similarly, demethylation of TNF locus correlates with both differentiation and competence to express TNF: the TNF proximal promoter and first exon were highly methylated in human embryonic stem cells and embryoid bodies, exon 1 was demethylated in hematopoietic stem cells and liver cells, and both the TNF proximal promoter and exon 1 were demethylated in primary monocytes and macrophages [186]. Inhibition of DNA methylation at the TNF locus can, in turn, enhance transcription of the TNF gene: 5-azacytidine enhanced LPS-mediated TNF production in THP-1 cells [186], while in LPS-tolerant THP-1 cells, RNAi of G9a inhibited recruitment of the DNA methyltransferase Dnmt3a/b, restoring TNF transcription [192]. Thus, DNA methylation is another epigenetic gene-regulatory mechanism that impacts TNF transcription.
Chromatin Remodeling at the TNF/Lymphotoxin Locus
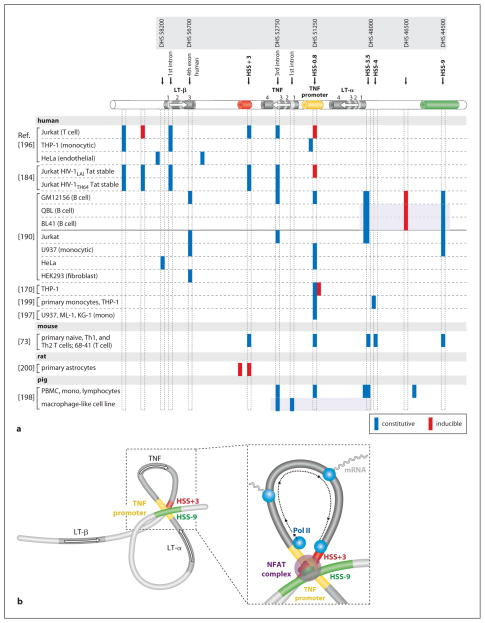
‘Open’ regions in chromatin accessible to DNA-binding proteins yield DNase-hypersensitive sites (HSSs); many cytokine loci have been examined using DNase I hypersensitivity assays [173]. The TNF gene resides in a locus with the lymphotoxin-α and -β (LT-α and LT-β) genes, and numerous cell type-specific HSSs have been identified (fig. 3a). The first HSS identified in the TNF/LT locus was the proximal TNF promoter itself, consistent with its interaction with multiple transcription factors in the context of chromatin, as shown by in vivo genomic footprinting [195, 196]. The TNF promoter HSS was present in unstimulated and PMA-induced TNF-expressing human myelomonocytic cell lines (ML-1, U937, and KG-1), but not a non-TNF-expressing human erythroid cell line (HEL) [197]. In porcine peripheral blood mono-nuclear cells, constitutive HSSs at the TNF and LT-α promoter regions and the third intron of TNF were observed at the TNF/LT locus, while a macrophage-like porcine cell line, in which LT-α was not transcribed, had HSSs at the first and third introns of TNF (fig. 3a); HSSs were not observed in porcine fibroblasts or a porcine kidney cell line, in which TNF was not expressed [198]. Constitutive HSSs were detected in both the TNF and LT-α promoters in THP-1 cells and human monocytes stimulated by superantigen and LPS, respectively [199]. Two TNF-inducible HSSs were identified ~3 kb downstream of the start site of TNF transcription in rat astrocytes; the stronger HSS bound NF-κB p50/p65 and was linked to TNF-induced TNF gene activation [200] (fig. 3a). In Jurkat T cells, a constitutive HSS in TNF intron 3 was functionally linked to T cell-specific TNF transcription in response to PMA/ionomycin [196]. Activation-dependent HSSs were also found in the TNF promoter and ~8 kb downstream of the start site of TNF transcription in Jurkat cells, with constitutive HSSs in the TNF 3′ UTR, the first intron of LT-β, and upstream of LT-β, while in unstimulated and MTb- or LPS-stimulated THP-1 cells, HSSs were present near the start site of TNF transcription, at the first LT-β intron, and upstream of LT-β (fig. 3a). By contrast, in non-TNF-expressing HeLa cells, constitutive HSSs were only found at the LT-β 5′ and 3′ UTRs (fig. 3a) [196]. In THP-1 cells, constitutive and LPS-inducible HSSs were also reported within the proximal and distal TNF promoter, respectively [170] (fig. 3a). Thus, cell type and stimulus appear to influence accessibility of the TNF locus to transcription factors and the general transcription machinery.
Fig. 3.
Cell type- and stimulus-specific chromatin organization of the TNF/LT locus. a Diagram of the genes (modeled after the murine locus, but note that human LT-β has one more exon than murine LT-β) and approximate positions of constitutive and inducible HSSs identified in the TNF/LT locus, with nomenclature from Tsytsykova et al. [73] (HSS) and Taylor et al. [190] (DHS) noted above. Shaded boxes indicate subsets of sites assayed within one study. b Model of the higher-order conformation of the TNF/LT locus in activated T cells. Activation-dependent intrachromosomal interactions would place NFAT-containing promoter/enhancer complexes into close proximity (TNF promoter-HSS+3, TNF promoter-HSS−9, and HSS+3-HSS−9) and circularize the TNF gene (TNF promoter-HSS+3) to facilitate reinitiation by the general transcription machinery. Adapted from Tsytsykova et al. [73].
Another approach to epigenetic regulation at the TNF locus involves viral protein products. For example, the TNF promoter HSS was present upon PMA/PHA induction in Jurkat T cells stably expressing Tat protein derived from an HIV-1 subtype that promoted TNF transcription (HIV-1LAI Tat) but not in cells expressing a Tat protein from a different HIV-1 subtype that inhibited p/CAF recruitment to the TNF promoter and suppressed TNF transcription (HIV-1TH64 Tat). The latter cells instead displayed increased cleavage at the HSS in the TNF 3′ UTR [184]. Thus, coinfection of T cells with HIV-1 and subsequent production of Tat proteins from different HIV subtypes results in epigenetic modification of the TNF chromatin environment, consistent with the important role chromatin remodeling has in TNF gene expression.
In primary naïve, Th1, and Th2 murine T cells and the murine T cell line 68-41, constitutive HSSs were observed at the TNF promoter (HSS−0.8), ~3 kb downstream of the TNF start site of transcription near the 3′ UTR (HSS+3), the LT-α promoter (HSS−3.5 and HSS−4), and ~9 kb upstream of the TNF gene and ~5 kb upstream of the LT-α gene (HSS−9; fig. 3a). HSS+3 and HSS−9 bind NFATp and exhibit increased acetylation of associated histone H3 and H4 upon stimulation with anti-CD3/CD28, and function as NFAT-dependent enhancers of TNF transcription [73]. Most recently, a comprehensive DNase hypersensitivity profile of the TNF/LT locus was performed in a panel of human cell lines: the B cell line GM12156, Jurkat, U937, HeLa, and the embryonic kidney cell line HEK293T [190]. This confirmed the existence of HSS−9 and HSSs at the TNF promoter, LT-α promoter, and TNF third intron and identified a number of novel HSSs (fig. 3a), mainly in the region 10–12 kb upstream of LT-α [190]. In summary, consistent with a major impact upon regulation of TNF gene transcription, chromatin remodeling across the TNF/LT locus exposes highly conserved enhancer and promoter regions to transcription factors and other regulatory proteins in a cell type- and stimulus-specific fashion.
Higher-Order Intrachromosomal Interactions at the TNF/Lymphotoxin Locus
Yet another level of regulation that can impact gene transcription at native loci is provided by long-range intra- and interchromosomal interactions [173, 201–203]. Higher-order chromatin configurations that result from these interactions can bring widely separated gene regulatory regions into close proximity with gene promoters and physically organize gene loci into regions of high local concentrations of transcription factors, or ‘transcription factories’ [173, 202, 203]. These long-range interactions can be examined using chromatin conformation capture (3C) assays, which combine ChIP with ligation of DNA fragments that lie in close proximity in the native chromatin context [202, 203]. Using 3C assays, it was shown that upon activation of T cells, intrachromosomal interactions occur between the TNF promoter and HSS+3, between the TNF promoter and HSS−9, and between HSS+3 and HSS−9 [73]. These interactions would thus bring together distal enhancers and the TNF promoter, increasing the local concentration of enhancer complexes containing NFATp (fig. 3b). Interactions between the TNF promoter and HSS+3 would also circularize the TNF gene and thus facilitate the recycling of the general transcription machinery (fig. 3b), consistent with the rapid and early expression of high levels of TNF mRNA in T cells. This promotion of transcription through looping was previously proposed for yeast genes [204], but TNF is the first mammalian gene found to undergo activation-dependent looping. Given that certain transcription factors have been shown to be required for establishing higher-order intrachromosomal interactions [205–210], NFATp may be required for the interactions between the TNF promoter and distal enhancers at the TNF/LT locus [73, 211].
Higher-order configuration of gene loci can sequester certain genes into inactive chromatin regions [209, 212]. Thus, in addition to promoting transcription of TNF, intrachromosomal interactions at the TNF/LT locus may contribute to the distinct regulation of TNF, LT-α, and LT-β. For example, interactions between the TNF promoter and HSS−9 would place the LT-α gene in a loop that would sequester the transcriptional start site of the gene from enhancer complexes clustered at the TNF promoter (fig. 3b). Notably, the arrangement and orientation of the three genes has been preserved in mammalian evolution and is conserved between placentals and marsupials [31, 213]. It remains to be determined whether the TNF gene moves into an area where RNA polymerase II and actively transcribing genes are colocalized, that is, a bona fide transcription factory [202, 203], although it is interesting to note that both LPS stimulation and monocyte differentiation drives the TNF locus from heterochromatin to euchromatin, which correlates with the cells’ ability to produce TNF [186]. Given the cell type-specific nature of HSSs in the TNF/LT locus, it will be fascinating to determine whether higher-order intra- and interchromosomal interactions involving the TNF/LT locus are also cell type- and stimulus-specific.
Conclusion
A multitude of potential input signals in the cell lead to the specific output of initiation of TNF gene transcription. The TNF gene thus presents a paradigm of cell type- and stimulus-specific gene regulation. This is reflected in the assembly of distinct enhanceosomes at the proximal TNF promoter and in the pattern of chromosomal organization of the TNF/LT locus. The functional importance of the TNF promoter and enhancer regions within the locus is underscored by the evolutionary conservation of sequences associated with transcription factor binding, epigenetic modifications, and higher-order chromosomal interactions. We have described the critical roles of NFAT, enhanceosome formation, coactivator recruitment, epigenetic modifications, chromatin remodeling, and intrachromosomal interactions in TNF gene regulation. Further characterization of the factors and elements involved in cell type- and stimulus-specific regulation of TNF gene transcription will continue to elucidate basic issues in eukaryotic gene regulation while defining potential therapeutic targets for precise manipulation of TNF expression in disease states.
Acknowledgments
We thank Renate Hellmiss for graphic artwork. Supported by NIH grant R01GM076685 to A.E.G.
References
- 1.Aggarwal BB, Samanta A, Feldmann M. TNFα. In: Oppenheim J, Feldmann M, editors. Cytokine Reference. Orlando: Academic Press; 2000. pp. 413–434. [Google Scholar]
- 2.Grivennikov SI, Kuprash DV, Liu ZG, Nedospasov SA. Intracellular signals and events activated by cytokines of the tumor necrosis factor superfamily: from simple paradigms to complex mechanisms. Int Rev Cytol. 2006;252:129–161. doi: 10.1016/S0074-7696(06)52002-9. [DOI] [PubMed] [Google Scholar]
- 3.Beutler B, Cerami A. Cachectin and tumor necrosis factor as two sides of the same biological coin. Nature. 1986;320:584. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- 4.Cuturi MC, Murphy M, Costa-Giomi MP, Weinmann R, Perussia B, Trinchieri G. Independent regulation of tumor necrosis factor and lymphotoxin production by human peripheral blood lymphocytes. J Exp Med. 1987;165:1581–1594. doi: 10.1084/jem.165.6.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steffen M, Ottmann OG, Moore MA. Simultaneous production of tumor necrosis factor-α and lymphotoxin by normal T cells after induction with IL-2 and anti-T3. J Immunol. 1988;140:2621–2624. [PubMed] [Google Scholar]
- 6.Sung SS, Bjorndahl JM, Wang CY, Kao HT, Fu SM. Production of tumor necrosis factor/cachectin by human T cell lines and peripheral blood T lymphocytes stimulated by phorbol myristate acetate and anti-CD3 antibody. J Exp Med. 1988;167:937–953. doi: 10.1084/jem.167.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sung SS, Jung LK, Walters JA, Chen W, Wang CY, Fu SM. Production of tumor necrosis factor/cachectin by human B cell lines and tonsillar B cells. J Exp Med. 1988;168:1539–1551. doi: 10.1084/jem.168.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niitsu Y, Watanabe N, Neda H, Yamauchi N, Maeda M, Sone H, Kuriyama H. Induction of synthesis of tumor necrosis factor in human and murine cell lines by exogenous recombinant human tumor necrosis factor. Cancer Res. 1988;48:5407–5410. [PubMed] [Google Scholar]
- 9.Goldfeld AE, Maniatis T. Coordinate viral induction of tumor necrosis factor α and interferon β in human B cells and monocytes. Proc Natl Acad Sci USA. 1989;86:1490–1494. doi: 10.1073/pnas.86.5.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon JR, Galli SJ. Mast cells as a source of both preformed and immunologically inducible TNF-α/cachectin. Nature. 1990;346:274–276. doi: 10.1038/346274a0. [DOI] [PubMed] [Google Scholar]
- 11.Caux C, Massacrier C, Vanbervliet B, Dubois B, Van Kooten C, Durand I, Banchereau J. Activation of human dendritic cells through CD40 cross-linking. J Exp Med. 1994;180:1263–1272. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 13.Goldfeld AE, Flemington EK, Boussiotis VA, Theodos CM, Titus RG, Strominger JL, Speck SH. Transcription of the tumor necrosis factor α gene is rapidly induced by anti-immunoglobulin and blocked by cyclosporin A and FK506 in human B cells. Proc Natl Acad Sci USA. 1992;89:12198–12201. doi: 10.1073/pnas.89.24.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldfeld AE, Strominger JL, Doyle C. Human tumor necrosis factor α gene regulation in phorbol ester stimulated T and B cell lines. J Exp Med. 1991;174:73–81. doi: 10.1084/jem.174.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldfeld AE, McCaffrey PG, Strominger JL, Rao A. Identification of a novel cyclosporin-sensitive element in the human tumor necrosis factor α gene promoter. J Exp Med. 1993;178:1365–1379. doi: 10.1084/jem.178.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diehn M, Alizadeh AA, Rando OJ, Liu CL, Stankunas K, Botstein D, Crabtree GR, Brown PO. Genomic expression programs and the integration of the CD28 costimulatory signal in T cell activation. Proc Natl Acad Sci USA. 2002;99:11796–11801. doi: 10.1073/pnas.092284399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aggarwal BB, Shishodia S, Takada Y, Jackson-Bernitsas D, Ahn KS, Sethi G, Ichikawa H. TNF blockade: an inflammatory issue. Ernst Schering Res Found Workshop. 2006:161–186. doi: 10.1007/3-540-37673-9_10. [DOI] [PubMed] [Google Scholar]
- 18.Sethi G, Sung B, Aggarwal BB. TNF: A master switch for inflammation to cancer. Front Biosci. 2008;13:5094–5107. doi: 10.2741/3066. [DOI] [PubMed] [Google Scholar]
- 19.Lou J, Lucas R, Grau GE. Pathogenesis of cerebral malaria: recent experimental data and possible applications for humans. Clin Microbiol Rev. 2001;14:810–820. doi: 10.1128/CMR.14.4.810-820.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobs M, Samarina A, Grivennikov S, Botha T, Allie N, Fremond C, Togbe D, Vasseur V, Rose S, Erard F, Monteiro A, Quesniaux V, Ryffel B. Reactivation of tuberculosis by tumor necrosis factor neutralization. Eur Cytokine Netw. 2007;18:5–13. doi: 10.1684/ecn.2007.0083. [DOI] [PubMed] [Google Scholar]
- 21.Falvo JV, Uglialoro AM, Brinkman BM, Merika M, Parekh BS, Tsai EY, King HC, Morielli AD, Peralta EG, Maniatis T, Thanos D, Goldfeld AE. Stimulus-specific assembly of enhancer complexes on the tumor necrosis factor alpha gene promoter. Mol Cell Biol. 2000;20:2239–2247. doi: 10.1128/mcb.20.6.2239-2247.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsai EY, Falvo JV, Tsytsykova AV, Barczak AK, Reimold AM, Glimcher LH, Fenton MJ, Gordon DC, Dunn IF, Goldfeld AE. A lipopolysaccharide-specific enhancer complex involving Ets, Elk-1, Sp1, and CREB binding protein and p300 is recruited to the tumor necrosis factor alpha promoter in vivo. Mol Cell Biol. 2000;20:6084–6094. doi: 10.1128/mcb.20.16.6084-6094.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsytsykova AV, Goldfeld AE. Inducer-specific enhanceosome formation controls tumor necrosis factor alpha gene expression in T lymphocytes. Mol Cell Biol. 2002;22:2620–2631. doi: 10.1128/MCB.22.8.2620-2631.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barthel R, Tsytsykova AV, Barczak AK, Tsai EY, Dascher CC, Brenner MB, Goldfeld AE. Regulation of tumor necrosis factor alpha gene expression by mycobacteria involves the assembly of a unique enhanceosome dependent on the coactivator proteins CBP/p300. Mol Cell Biol. 2003;23:526–533. doi: 10.1128/MCB.23.2.526-533.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carey M. The enhanceosome and transcriptional synergy. Cell. 1998;92:5–8. doi: 10.1016/s0092-8674(00)80893-4. [DOI] [PubMed] [Google Scholar]
- 26.Merika M, Thanos D. Enhanceosomes. Curr Opin Genet Dev. 2001;11:205–208. doi: 10.1016/s0959-437x(00)00180-5. [DOI] [PubMed] [Google Scholar]
- 27.Falvo JV, Brinkman BMN, Tsytsykova AV, Tsai EY, Yao T-P, Kung AL, Goldfeld AE. A stimulus-specific role for CREB-binding protein (CBP) in T cell receptor-activated tumor necrosis factor α gene expression. Proc Natl Acad Sci USA. 2000;97:3925–3929. doi: 10.1073/pnas.97.8.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shakhov AN, Collart MA, Vassalli P, Nedospasov SA, Jongeneel CV. κB-type enhancers are involved in lipopolysaccharide-mediated transcriptional activation of the tumor necrosis factor α gene in primary macrophages. J Exp Med. 1990;171:35–47. doi: 10.1084/jem.171.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuprash DV, Udalova IA, Turetskaya RL, Kwiatkowski D, Rice NR, Nedospasov SA. Similarities and differences between human and murine TNF promoters in their response to lipopolysaccharide. J Immunol. 1999;162:4045–4052. [PubMed] [Google Scholar]
- 30.Goldfeld AE, Leung JY, Sawyer SA, Hartl DL. Post-genomics and the neutral theory: variation and conservation in the tumor necrosis factor-α promoter. Gene. 2000;261:19–25. doi: 10.1016/s0378-1119(00)00477-7. [DOI] [PubMed] [Google Scholar]
- 31.Cross JG, Harrison GA, Coggill P, Sims S, Beck S, Deakin JE, Graves JA. Analysis of the genomic region containing the tammar wallaby (Macropus eugenii) orthologues of MHC class III genes. Cytogenet Genome Res. 2005;111:110–117. doi: 10.1159/000086379. [DOI] [PubMed] [Google Scholar]
- 32.Leung JY, McKenzie FE, Uglialoro AM, Flores-Villanueva PO, Sorkin BC, Yunis EJ, Hartl DL, Goldfeld AE. Identification of phylogenetic footprints in primate tumor necrosis factor-α promoters. Proc Natl Acad Sci USA. 2000;97:6614–6618. doi: 10.1073/pnas.97.12.6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baena A, Mootnick AR, Falvo JV, Tsytskova AV, Ligeiro F, Diop OM, Brieva C, Gagneux P, O’Brien SJ, Ryder OA, Goldfeld AE. Primate TNF promoters reveal markers of phylogeny and evolution of innate immunity. PLoS One. 2007;2:e621. doi: 10.1371/journal.pone.0000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsai EY, Jain J, Pesavento PA, Rao A, Goldfeld AE. Tumor necrosis factor alpha gene regulation in activated T cells involves ATF-2/Jun and NFATp. Mol Cell Biol. 1996;16:459–467. doi: 10.1128/mcb.16.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsai EY, Yie J, Thanos D, Goldfeld AE. Cell-type-specific regulation of the human tumor necrosis factor alpha gene in B cells and T cells by NFATp and ATF-2/JUN. Mol Cell Biol. 1996;16:5232–5244. doi: 10.1128/mcb.16.10.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsytsykova AV, Goldfeld AE. Nuclear factor of activated T cells transcription factor NFATp controls superantigen-induced lethal shock. J Exp Med. 2000;192:581–586. doi: 10.1084/jem.192.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldfeld AE, Doyle C, Maniatis T. Human tumor necrosis factor α gene regulation by virus and lipopolysaccharide. Proc Natl Acad Sci USA. 1990;87:9769–9773. doi: 10.1073/pnas.87.24.9769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trede NS, Tsytsykova AV, Chatila T, Goldfeld AE, Geha RS. Transcriptional activation of the human TNF-α promoter by superantigen in human monocytic cells: role of NF-κB. J Immunol. 1995;155:902–908. [PubMed] [Google Scholar]
- 39.Yao J, Mackman N, Edgington TS, Fan ST. Lipopolysaccharide induction of the tumor necrosis factor-α promoter in human monocytic cells. Regulation by Egr-1, c-Jun, and NF-κB transcription factors. J Biol Chem. 1997;272:17795–17801. doi: 10.1074/jbc.272.28.17795. [DOI] [PubMed] [Google Scholar]
- 40.Haudek SB, Natmeßnig BE, Redl H, Schlag G, Giroir BP. Genetic sequences and transcriptional regulation of the TNFA promoter: comparison of human and baboon. Immunogenetics. 1998;48:202–207. doi: 10.1007/s002510050424. [DOI] [PubMed] [Google Scholar]
- 41.Means TK, Pavlovich RP, Roca D, Vermeulen MW, Fenton MJ. Activation of TNF-α transcription utilizes distinct MAP kinase pathways in different macrophagepopulations. J Leukoc Biol. 2000;67:885–893. doi: 10.1002/jlb.67.6.885. [DOI] [PubMed] [Google Scholar]
- 42.O’Donnell PM, Taffet SM. The proximal promoter region is essential for lipopolysaccharide induction and cyclic AMP inhibition of mouse tumor necrosis factor-α. J Interferon Cytokine Res. 2002;22:539–548. doi: 10.1089/10799900252982016. [DOI] [PubMed] [Google Scholar]
- 43.Leitman DC, Mackow ER, Williams T, Baxter JD, West BL. The core promoter region of the tumor necrosis factor α gene confers phorbol ester responsiveness to upstream transcriptional activators. Mol Cell Biol. 1992;12:1352–1356. doi: 10.1128/mcb.12.3.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brinkman BM, Telliez JB, Schievella AR, Lin LL, Goldfeld AE. Engagement of tumor necrosis factor (TNF) receptor 1 leads to ATF-2- and p38 mitogen-activated protein kinase-dependent TNF-α gene expression. J Biol Chem. 1999;274:30882–30886. doi: 10.1074/jbc.274.43.30882. [DOI] [PubMed] [Google Scholar]
- 45.Esensten JH, Tsytsykova AV, Lopez-Rodriguez C, Ligeiro FA, Rao A, Goldfeld AE. NFAT5 binds to the TNF promoter distinctly from NFATp, c, 3 and 4, and activates TNF transcription during hypertonic stress alone. Nucleic Acids Res. 2005;33:3845–3854. doi: 10.1093/nar/gki701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Economou JS, Rhoades K, Essner R, McBride WH, Gasson JC, Morton DL. Genetic analysis of the human tumor necrosis factor α/cachectin promoter region in a macrophage cell line. J Exp Med. 1989;170:321–326. doi: 10.1084/jem.170.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rhoades KL, Golub SH, Economou JS. The regulation of the human tumor necrosis factor α promoter region in macrophage, T cell, and B cell lines. J Biol Chem. 1992;267:22102–22107. [PubMed] [Google Scholar]
- 48.McCaffrey PG, Goldfeld AE, Rao A. The role of NFATp in cyclosporin A-sensitive tumor necrosis factor-α gene transcription. J Biol Chem. 1994;269:30445–30450. [PubMed] [Google Scholar]
- 49.Krämer B, Wiegmann K, Krönke M. Regulation of the human TNF promoter by the transcription factor Ets. J Biol Chem. 1995;270:6577–6583. doi: 10.1074/jbc.270.12.6577. [DOI] [PubMed] [Google Scholar]
- 50.Krämer B, Meichle A, Hensel G, Charnay P, Krönke M. Characterization of an Krox-24/Egr-1-responsive element in the human tumor necrosis factor promoter. Biochim Biophys Acta. 1994;1219:413–421. doi: 10.1016/0167-4781(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 51.Leitman DC, Ribeiro RC, Mackow ER, Baxter JD, West BL. Identification of a tumor necrosis factor-responsive element in the tumor necrosis factor α gene. J Biol Chem. 1991;266:9343–9346. [PubMed] [Google Scholar]
- 52.Falvo JV, Lin CH, Tsytsykova AV, Hwang PK, Thanos D, Goldfeld AE, Maniatis T. A dimer-specific function of the transcription factor NFATp. Proc Natl Acad Sci USA. 2008 doi: 10.1073/pnas.0810648105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Newell CL, Deisseroth AB, Lopez-Berestein G. Interaction of nuclear proteins with an AP-1/CRE-like promoter sequence in the human TNF-α gene. J Leukoc Biol. 1994;56:27–35. doi: 10.1002/jlb.56.1.27. [DOI] [PubMed] [Google Scholar]
- 54.Diaz B, Lopez-Berestein G. A distinct element involved in lipopolysaccharide activation of the tumor necrosis factor-α promoter in monocytes. J Interferon Cytokine Res. 2000;20:741–748. doi: 10.1089/10799900050116453. [DOI] [PubMed] [Google Scholar]
- 55.Steer JH, Kroeger KM, Abraham LJ, Joyce DA. Glucocorticoids suppress tumor necrosis factor-α expression by human monocytic THP-1 cells by suppressing transactivation through adjacent NF-κB and c-Jun-activating transcription factor-2 binding sites in the promoter. J Biol Chem. 2000;275:18432–18440. doi: 10.1074/jbc.M906304199. [DOI] [PubMed] [Google Scholar]
- 56.Sato H, Watanabe A, Tanaka T, Koitabashi N, Arai M, Kurabayashi M, Yokoyama T. Regulation of the human tumor necrosis factor-α promoter by angiotensin II and lipopolysaccharide in cardiac fibroblasts: different cis-acting promoter sequences and transcriptional factors. J Mol Cell Cardiol. 2003;35:1197–1205. doi: 10.1016/s0022-2828(03)00210-4. [DOI] [PubMed] [Google Scholar]
- 57.Hayakawa J, Mittal S, Wang Y, Korkmaz KS, Adamson E, English C, Ohmichi M, McClelland M, Mercola D. Identification of promoters bound by c-Jun/ATF2 during rapid large-scale gene activation following genotoxic stress. Mol Cell. 2004;16:521–535. doi: 10.1016/j.molcel.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 58.Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17:2205–2232. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- 59.Macian F. NFAT proteins: Key regulators of T-cell development and function. Nat Rev Immunol. 2005;5:472–484. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- 60.Aramburu J, Drews-Elger K, Estrada-Gelonch A, Minguillón J, Morancho B, Santiago V, López-Rodríguez C. Regulation of the hypertonic stress response and other cellular functions by the Rel-like transcription factor NFAT5. Biochem Pharmacol. 2006;72:1597–1604. doi: 10.1016/j.bcp.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 61.Wu H, Peisley A, Graef IA, Crabtree GR. NFAT signaling and the invention of vertebrates. Trends Cell Biol. 2007;17:251–260. doi: 10.1016/j.tcb.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 62.Ullman KS, Northrop JP, Verweij CL, Crabtree GR. Transmission of signals from the T lymphocyte antigen receptor to the genes responsible for cell proliferation and immune function: the missing link. Annu Rev Immunol. 1990;8:421–452. doi: 10.1146/annurev.iy.08.040190.002225. [DOI] [PubMed] [Google Scholar]
- 63.McCaffrey PG, Luo C, Kerppola TK, Jain J, Badalian TM, Ho AM, Burgeon E, Lane WS, Lambert JN, Curran T, Verdine GL, Rao A, Hogan PG. Isolation of the cyclosporin-sensitive T cell transcription factor NFATp. Science. 1993;262:750–754. doi: 10.1126/science.8235597. [DOI] [PubMed] [Google Scholar]
- 64.Goldfeld AE, Tsai E, Kincaid R, Belshaw PJ, Schreiber SL, Strominger JL, Rao A. Calcineurin mediates human tumor necrosis factor α gene induction in stimulated T and B cells. J Exp Med. 1994;180:763–768. doi: 10.1084/jem.180.2.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Prieschl EE, Gouilleux-Gruart V, Walker C, Harrer NE, Baumruker T. A nuclear factor of activated T cell-like transcription factor in mast cells is involved in IL-5 gene regulation after IgE plus antigen stimulation. J Immunol. 1995;154:6112–6119. [PubMed] [Google Scholar]
- 66.Klein M, Klein-Hessling S, Palmetshofer A, Serfling E, Tertilt C, Bopp T, Heib V, Becker M, Taube C, Schild H, Schmitt E, Stassen M. Specific and redundant roles for NFAT transcription factors in the expression of mast cell-derived cytokines. J Immunol. 2006;177:6667–6674. doi: 10.4049/jimmunol.177.10.6667. [DOI] [PubMed] [Google Scholar]
- 67.Kaminuma O, Kitamura F, Kitamura N, Hiroi T, Miyoshi H, Miyawaki A, Miyatake S. Differential contribution of NFATc2 and NFATc1 to TNF-α gene expression in T cells. J Immunol. 2008;180:319–326. doi: 10.4049/jimmunol.180.1.319. [DOI] [PubMed] [Google Scholar]
- 68.Song L, Li J, Ye J, Yu G, Ding J, Zhang D, Ouyang W, Dong Z, Kim SO, Huang C. p85α acts as a novel signal transducer for mediation of cellular apoptotic response to UV radiation. Mol Cell Biol. 2007;27:2713–2731. doi: 10.1128/MCB.00657-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ke Q, Li J, Ding J, Ding M, Wang L, Liu B, Costa M, Huang C. Essential role of ROS-mediated NFAT activation in TNF-α induction by crystalline silica exposure. Am J Physiol Lung Cell Mol Physiol. 2006;291:L257–264. doi: 10.1152/ajplung.00007.2006. [DOI] [PubMed] [Google Scholar]
- 70.Ouyang W, Hu Y, Li J, Ding M, Lu Y, Zhang D, Yan Y, Song L, Qu Q, Desai D, Amin S, Huang C. Direct evidence for the critical role of NFAT3 in benzo[a] pyrene diol-epoxide-induced cell transformation through mediation of inflammatory cytokine TNF induction in mouse epidermal Cl41 cells. Carcinogenesis. 2007;28:2218–2226. doi: 10.1093/carcin/bgm115. [DOI] [PubMed] [Google Scholar]
- 71.López-Rodriguez C, Aramburu J, Jin L, Rakeman AS, Michino M, Rao A. Bridging the NFAT and NF-κB families: NFAT5 dimerization regulates cytokine gene transcription in response to osmotic stress. Immunity. 2001;15:47–58. doi: 10.1016/s1074-7613(01)00165-0. [DOI] [PubMed] [Google Scholar]
- 72.Hodge MR, Ranger AM, Charles de la Brousse F, Hoey T, Grusby MJ, Glimcher LH. Hyperproliferation and dysregulation of IL-4 expression in NF-ATp-deficient mice. Immunity. 1996;4:397–405. doi: 10.1016/s1074-7613(00)80253-8. [DOI] [PubMed] [Google Scholar]
- 73.Tsytsykova AV, Rajsbaum R, Falvo JV, Ligeiro F, Neely SR, Goldfeld AE. Activation-dependent intrachromosomal interactions formed by the TNF gene promoter and two distal enhancers. Proc Natl Acad Sci USA. 2007;104:16850–16855. doi: 10.1073/pnas.0708210104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ranger AM, Hodge MR, Gravallese EM, Oukka M, Davidson L, Alt FW, de la Brousse FC, Hoey T, Grusby M, Glimcher LH. Delayed lymphoid repopulation with defects in IL-4-driven responses produced by inactivation of NF-ATc. Immunity. 1998;8:125–134. doi: 10.1016/s1074-7613(00)80465-3. [DOI] [PubMed] [Google Scholar]
- 75.Yoshida H, Nishina H, Takimoto H, Marengere LE, Wakeham AC, Bouchard D, Kong YY, Ohteki T, Shahinian A, Bachmann M, Ohashi PS, Penninger JM, Crabtree GR, Mak TW. The transcription factor NF-ATc1 regulates lymphocyte proliferation and Th2 cytokine production. Immunity. 1998;8:115–124. doi: 10.1016/s1074-7613(00)80464-1. [DOI] [PubMed] [Google Scholar]
- 76.Ranger AM, Oukka M, Rengarajan J, Glimcher LH. Inhibitory function of two NFAT family members in lymphoid homeostasis and Th2 development. Immunity. 1998;9:627–635. doi: 10.1016/s1074-7613(00)80660-3. [DOI] [PubMed] [Google Scholar]
- 77.Peng SL, Gerth AJ, Ranger AM, Glimcher LH. NFATc1 and NFATc2 together control both T and B cell activation and differentiation. Immunity. 2001;14:13–20. doi: 10.1016/s1074-7613(01)00085-1. [DOI] [PubMed] [Google Scholar]
- 78.Pan M, Winslow MM, Chen L, Kuo A, Felsher D, Crabtree GR. Enhanced NFATc1 nuclear occupancy causes T cell activation independent of CD28 costimulation. J Immunol. 2007;178:4315–4321. doi: 10.4049/jimmunol.178.7.4315. [DOI] [PubMed] [Google Scholar]
- 79.Chen J, Amasaki Y, Kamogawa Y, Nagoya M, Arai N, Arai K, Miyatake S. Role of NFATx (NFAT4/NFATc3) in expression of immunoregulatory genes in murine peripheral CD4+ T cells. J Immunol. 2003;170:3109–3117. doi: 10.4049/jimmunol.170.6.3109. [DOI] [PubMed] [Google Scholar]
- 80.Feske S, Muller JM, Graf D, Kroczek RA, Drager R, Niemeyer C, Baeuerle PA, Peter HH, Schlesier M. Severe combined immunodeficiency due to defective binding of the nuclear factor of activated T cells in T lymphocytes of two male siblings. Eur J Immunol. 1996;26:2119–2126. doi: 10.1002/eji.1830260924. [DOI] [PubMed] [Google Scholar]
- 81.Trifari S, Sitia G, Aiuti A, Scaramuzza S, Marangoni F, Guidotti LG, Martino S, Saracco P, Notarangelo LD, Roncarolo MG, Dupre L. Defective Th1 cytokine gene transcription in CD4+ and CD8+ T cells from Wiskott-Aldrich syndrome patients. J Immunol. 2006;177:7451–7461. doi: 10.4049/jimmunol.177.10.7451. [DOI] [PubMed] [Google Scholar]
- 82.Perkins ND. Integrating cell-signalling pathways with NF-κB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 83.Hayden MS, Ghosh S. Shared principles in NF-κB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 84.Vallabhapurapu S, Karin M. Regulation and function of NF-κB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 85.Collart MA, Baeuerle P, Vassalli P. Regulation of tumor necrosis factor alpha transcription in macrophages: involvement of four κB-like motifs and of constitutive and inducible forms of NF-κB. Mol Cell Biol. 1990;10:1498–1506. doi: 10.1128/mcb.10.4.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Drouet C, Shakhov AN, Jongeneel CV. Enhancers and transcription factors controlling the inducibility of the tumor necrosis factor-α promoter in primary macrophages. J Immunol. 1991;147:1694–1700. [PubMed] [Google Scholar]
- 87.Udalova IA, Knight JC, Vidal V, Nedospasov SA, Kwiatkowski D. Complex NF-κB interactions at the distal tumor necrosis factor promoter region in human monocytes. J Biol Chem. 1998;273:21178–21186. doi: 10.1074/jbc.273.33.21178. [DOI] [PubMed] [Google Scholar]
- 88.Ziegler-Heitbrock HW, Sternsdorf T, Liese J, Belohradsky B, Weber C, Wedel A, Schreck R, Bauerle P, Strobel M. Pyrrolidine dithiocarbamate inhibits NF-κB mobilization and TNF production in human monocytes. J Immunol. 1993;151:6986–6993. [PubMed] [Google Scholar]
- 89.Foxwell B, Browne K, Bondeson J, Clarke C, de Martin R, Brennan F, Feldmann M. Efficient adenoviral infection with IκBα reveals that macrophage tumor necrosis factor α production in rheumatoid arthritis is NF-κB dependent. Proc Natl Acad Sci USA. 1998;95:8211–8215. doi: 10.1073/pnas.95.14.8211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.O’Connell MA, Bennett BL, Mercurio F, Manning AM, Mackman N. Role of IKK1 and IKK2 in lipopolysaccharide signaling in human monocytic cells. J Biol Chem. 1998;273:30410–30404. doi: 10.1074/jbc.273.46.30410. [DOI] [PubMed] [Google Scholar]
- 91.Bondeson J, Browne KA, Brennan FM, Foxwell BMJ, Feldmann M. Selective regulation of cytokine induction by adenoviral gene transfer of IκBα into human macrophages: lipopolysaccharide-induced, but not zymosan-induced proinflammatory cytokines are inhibited, but IL-10 is nuclear factor-κB independent. J Immunol. 1999;162:2939–2945. [PubMed] [Google Scholar]
- 92.Swantek JL, Christerson L, Cobb MH. Lipopolysaccharide-induced tumor necrosis factor-α promoter activity is inhibitor of nuclear factor-κB kinase-dependent. J Biol Chem. 1999;274:11667–11671. doi: 10.1074/jbc.274.17.11667. [DOI] [PubMed] [Google Scholar]
- 93.Moore F, Buonocore S, Aksoy E, Ouled-Haddou N, Goriely S, Lazarova E, Paulart F, Heirman C, Vaeremans E, Thielemans K, Goldman M, Flamand V. An alternative pathway of NF-κB activation results in maturation and T cell priming activity of dendritic cells overexpressing a mutated IκBα. J Immunol. 2007;178:1301–1311. doi: 10.4049/jimmunol.178.3.1301. [DOI] [PubMed] [Google Scholar]
- 94.Hall G, Singh IS, Hester L, Hasday JD, Rogers TB. Inhibitor-κB kinase-β regulates LPS-induced TNF-α production in cardiac myocytes through modulation of NF-κB p65 subunit phosphorylation. Am J Physiol Heart Circ Physiol. 2005;289:H2103–H2111. doi: 10.1152/ajpheart.00393.2005. [DOI] [PubMed] [Google Scholar]
- 95.Lim CA, Yao F, Wong JJ, George J, Xu H, Chiu KP, Sung WK, Lipovich L, Vega VB, Chen J, Shahab A, Zhao XD, Hibberd M, Wei CL, Lim B, Ng HH, Ruan Y, Chin KC. Genome-wide mapping of RELA(p65) binding identifies E2F1 as a transcriptional activator recruited by NF-κB upon TLR4 activation. Mol Cell. 2007;27:622–635. doi: 10.1016/j.molcel.2007.06.038. [DOI] [PubMed] [Google Scholar]
- 96.Maniatis T, Falvo JV, Kim TH, Kim TK, Lin CH, Parekh BS, Wathelet MG. Structure and function of the interferon-β enhanceosome. Cold Spring Harbor Symp Quant Biol. 1998;63:609–620. doi: 10.1101/sqb.1998.63.609. [DOI] [PubMed] [Google Scholar]
- 97.Liu H, Sidiropoulos P, Song G, Pagliari LJ, Birrer MJ, Stein B, Anrather J, Pope RM. TNF-α gene expression in macrophages: regulation by NF-κB is independent of c-Jun or C/EBPβ. J Immunol. 2000;164:4277–4285. doi: 10.4049/jimmunol.164.8.4277. [DOI] [PubMed] [Google Scholar]
- 98.Weih F, Warr G, Yang H, Bravo R. Multifocal defects in immune responses in RelB-deficient mice. J Immunol. 1997;158:5211–5218. [PubMed] [Google Scholar]
- 99.Ye J, Wang L, Zhang X, Tantishaiyakul V, Rojanasakul Y. Inhibition of TNF-α gene expression and bioactivity by site-specific transcription factor-binding oligonucleotides. Am J Physiol Lung Cell Mol Physiol. 2003;284:L386–394. doi: 10.1152/ajplung.00134.2002. [DOI] [PubMed] [Google Scholar]
- 100.Joyce DA, Gimblett G, Steer JH. Targets of glucocorticoid action on TNF-α release by macrophages. Inflamm Res. 2001;50:337–340. doi: 10.1007/PL00012387. [DOI] [PubMed] [Google Scholar]
- 101.Xu Z, Dziarski R, Wang Q, Swartz K, Sakamoto KM, Gupta D. Bacterial peptidoglycan-induced tnf-α transcription is mediated through the transcription factors Egr-1, Elk-1, and NF-κB. J Immunol. 2001;167:6975–6982. doi: 10.4049/jimmunol.167.12.6975. [DOI] [PubMed] [Google Scholar]
- 102.Tzagarakis-Foster C, Geleziunas R, Lomri A, An J, Leitman DC. Estradiol represses human T-cell leukemia virus type 1 Tax activation of tumor necrosis factor-α gene transcription. J Biol Chem. 2002;277:44772–44777. doi: 10.1074/jbc.M205355200. [DOI] [PubMed] [Google Scholar]
- 103.Tsytsykova AV, Falvo JV, Schmidt-Supprian M, Courtois G, Thanos D, Goldfeld AE. Post-induction, stimulus-specific regulation of tumor necrosis factor mRNA expression. J Biol Chem. 2007;282:11629–11638. doi: 10.1074/jbc.M611418200. [DOI] [PubMed] [Google Scholar]
- 104.Kunsch C, Ruben SM, Rosen CA. Selection of optimal κB/Rel DNA-binding motifs: Interaction of both subunits of NF-κB with DNA is required for transcriptional activation. Mol Cell Biol. 1992;12:4412–4421. doi: 10.1128/mcb.12.10.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bonizzi G, Karin M. The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 106.Natoli G, Saccani S, Bosisio D, Marazzi I. Interactions of NF-κB with chromatin: the art of being at the right place at the right time. Nat Immunol. 2005;6:439–445. doi: 10.1038/ni1196. [DOI] [PubMed] [Google Scholar]
- 107.Natoli G. Tuning up inflammation: how DNA sequence and chromatin organization control the induction of inflammatory genes by NF-κB. FEBS Lett. 2006;580:2843–2849. doi: 10.1016/j.febslet.2006.02.072. [DOI] [PubMed] [Google Scholar]
- 108.Orlando V. Mapping chromosomal proteins in vivo by formaldehyde-crosslinked-chromatin immunoprecipitation. Trends Biochem Sci. 2000;25:99–104. doi: 10.1016/s0968-0004(99)01535-2. [DOI] [PubMed] [Google Scholar]
- 109.Litt MD, Simpson M, Gaszner M, Allis CD, Felsenfeld G. Correlation between histone lysine methylation and developmental changes at the chicken β-globin locus. Science. 2001;293:2453–2455. doi: 10.1126/science.1064413. [DOI] [PubMed] [Google Scholar]
- 110.Martone R, Euskirchen G, Bertone P, Hartman S, Royce TE, Luscombe NM, Rinn JL, Nelson FK, Miller P, Gerstein M, Weissman S, Snyder M. Distribution of NF-κB-binding sites across human chromosome 22. Proc Natl Acad Sci USA. 2003;100:12247–12252. doi: 10.1073/pnas.2135255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bonizzi G, Bebien M, Otero DC, Johnson-Vroom KE, Cao Y, Vu D, Jegga AG, Aronow BJ, Ghosh G, Rickert RC, Karin M. Activation of IKKα target genes depends on recognition of specific κB binding sites by RelB:P52 dimers. EMBO J. 2004;23:4202–4210. doi: 10.1038/sj.emboj.7600391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Taggart CC, Cryan SA, Weldon S, Gibbons A, Greene CM, Kelly E, Low TB, O’Neill SJ, McElvaney NG. Secretory leucoprotease inhibitor binds to NF-κB binding sites in monocytes and inhibits p65 binding. J Exp Med. 2005;202:1659–1668. doi: 10.1084/jem.20050768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ishinaga H, Jono H, Lim JH, Kweon SM, Xu H, Ha UH, Xu H, Koga T, Yan C, Feng XH, Chen LF, Li JD. TGF-β induces p65 acetylation to enhance bacteria-induced NF-κB activation. EMBO J. 2007;26:1150–1162. doi: 10.1038/sj.emboj.7601546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Carmody RJ, Ruan Q, Palmer S, Hilliard B, Chen YH. Negative regulation of toll-like receptor signaling by NF-κB p50 ubiquitination blockade. Science. 2007;317:675–678. doi: 10.1126/science.1142953. [DOI] [PubMed] [Google Scholar]
- 115.El Gazzar MA, El Mezayen R, Nicolls MR, Dreskin SC. Thymoquinone attenuates proinflammatory responses in lipopolysaccharide-activated mast cells by modulating NF-kappaB nuclear transactivation. Biochim Biophys Acta. 2007;1770:556–564. doi: 10.1016/j.bbagen.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 116.Li Y, Reddy MA, Miao F, Shanmugam N, Yee JK, Hawkins D, Ren B, Natarajan R. Role of the histone H3 lysine 4 methyltransferase, SET7/9, in the regulation of NF-κB-dependent inflammatory genes. Relevance to diabetes and inflammation. J Biol Chem. 2008;283:26771–26781. doi: 10.1074/jbc.M802800200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Buckley JM, Wang JH, Redmond HP. Cellular reprogramming by Gram-positive bacterial components: a review. J Leukoc Biol. 2006;80:731–741. doi: 10.1189/jlb.0506312. [DOI] [PubMed] [Google Scholar]
- 118.Baer M, Dillner A, Schwartz RC, Sedon C, Nedospasov S, Johnson PF. Tumor necrosis factor alpha transcription in macrophages is attenuated by an autocrine factor that preferentially induces NF-κB p50. Mol Cell Biol. 1998;18:5678–5689. doi: 10.1128/mcb.18.10.5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Frankenberger M, Ziegler-Heitbrock HW. LPS tolerance in monocytes/macrophages: three 3′ cytosins are required in the DNA binding motif for detection of upregulated NF-κB p50 homodimers. Immunobiology. 1997;198:81–90. doi: 10.1016/s0171-2985(97)80029-0. [DOI] [PubMed] [Google Scholar]
- 120.Wedel A, Frankenberger M, Sulski G, Petersmann I, Kuprash D, Nedospasov S, Ziegler-Heitbrock HW. Role of p52 (NF-κB2) in LPS tolerance in a human B cell line. Biol Chem. 1999;380:1193–1199. doi: 10.1515/BC.1999.151. [DOI] [PubMed] [Google Scholar]
- 121.Bohuslav J, Kravchenko VV, Parry GC, Erlich JH, Gerondakis S, Mackman N, Ulevitch RJ. Regulation of an essential innate immune response by the p50 subunit of NF-κB. J Clin Invest. 1998;102:1645–1652. doi: 10.1172/JCI3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wessells J, Baer M, Young HA, Claudio E, Brown K, Siebenlist U, Johnson PF. BCL-3 and NF-κB p50 attenuate lipopolysaccharide-induced inflammatory responses in macrophages. J Biol Chem. 2004;279:49995–50003. doi: 10.1074/jbc.M404246200. [DOI] [PubMed] [Google Scholar]
- 123.El Gazzar M, Yoza BK, Hu JY, Cousart SL, McCall CE. Epigenetic silencing of tumor necrosis factor α during endotoxin tolerance. J Biol Chem. 2007;282:26857–26864. doi: 10.1074/jbc.M704584200. [DOI] [PubMed] [Google Scholar]
- 124.Pope RM, Leutz A, Ness SA. C/EBPβ regulation of the tumor necrosis factor α gene. J Clin Invest. 1994;94:1449–1455. doi: 10.1172/JCI117482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wedel A, Sulski G, Ziegler-Heitbrock HW. CCAAT/enhancer binding protein is involved in the expression of the tumour necrosis factor gene in human monocytes. Cytokine. 1996;8:335–341. doi: 10.1006/cyto.1996.0046. [DOI] [PubMed] [Google Scholar]
- 126.Zagariya A, Mungre S, Lovis R, Birrer M, Ness S, Thimmapaya B, Pope R. Tumor necrosis factor alpha gene regulation: enhancement of C/EBPβ-induced activation by c-Jun. Mol Cell Biol. 1998;18:2815–2824. doi: 10.1128/mcb.18.5.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tanaka T, Akira S, Yoshida K, Umemoto M, Yoneda Y, Shirafuji N, Fujiwara H, Suematsu S, Yoshida N, Kishimoto T. Targeted disruption of the NF-IL6 gene discloses its essential role in bacteria killing and tumor cytotoxicity by macrophages. Cell. 1995;80:353–361. doi: 10.1016/0092-8674(95)90418-2. [DOI] [PubMed] [Google Scholar]
- 128.Gorgoni B, Maritano D, Marthyn P, Righi M, Poli V. C/EBPβ gene inactivation causes both impaired and enhanced gene expression and inverse regulation of IL-12 p40 and p35 mRNAs in macrophages. J Immunol. 2002;168:4055–4062. doi: 10.4049/jimmunol.168.8.4055. [DOI] [PubMed] [Google Scholar]
- 129.Lu YC, Kim I, Lye E, Shen F, Suzuki N, Suzuki S, Gerondakis S, Akira S, Gaffen SL, Yeh WC, Ohashi PS. Differential role for c-Rel and C/EBPβ/δ in TLR-mediated induction of proinflammatory cytokines. J Immunol. 2009;182:7212–7221. doi: 10.4049/jimmunol.0802971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Novotny V, Prieschl EE, Csonga R, Fabjani G, Baumruker T. Nrf1 in a complex with fosB, c-jun, junD and ATF2 forms the AP1 component at the TNFα promoter in stimulated mast cells. Nucleic Acids Res. 1998;26:5480–5485. doi: 10.1093/nar/26.23.5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Prieschl EE, Novotny V, Csonga R, Jaksche D, Elbe-Burger A, Thumb W, Auer M, Stingl G, Baumruker T. A novel splice variant of the transcription factor Nrf1 interacts with the TNFα promoter and stimulates transcription. Nucleic Acids Res. 1998;26:2291–2297. doi: 10.1093/nar/26.10.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Takagi K, Takagi M, Kanangat S, Warrington KJ, Shigemitsu H, Postlethwaite AE. Modulation of TNF-α gene expression by IFN-γ and pamidronate in murine macrophages:regulation by STAT1-dependent pathways. J Immunol. 2005;174:1801–1810. doi: 10.4049/jimmunol.174.4.1801. [DOI] [PubMed] [Google Scholar]
- 133.Chappell VL, Le LX, LaGrone L, Mileski WJ. Stat proteins play a role in tumor necrosis factor ααgene expression. Shock. 2000;14:400–402. doi: 10.1097/00024382-200014030-00027. discussion 402–403. [DOI] [PubMed] [Google Scholar]
- 134.Kamezaki K, Shimoda K, Numata A, Matsuda T, Nakayama K, Harada M. The role of Tyk2, Stat1 and Stat4 in LPS-induced endotoxin signals. Int Immunol. 2004;16:1173–1179. doi: 10.1093/intimm/dxh118. [DOI] [PubMed] [Google Scholar]
- 135.Myokai F, Takashiba S, Lebo R, Amar S. A novel lipopolysaccharide-induced transcription factor regulating tumor necrosis factor α gene expression: molecular cloning, sequencing, characterization, and chromosomal assignment. Proc Natl Acad Sci USA. 1999;96:4518–4523. doi: 10.1073/pnas.96.8.4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bolcato-Bellemin A-L, Mattei M-G, Fenton M, Amar S. Molecular cloning and characterization of mouse LITAF cDNA: role in the regulation of tumor necrosis factor-α (TNF-α) gene expression. J Endotoxin Res. 2004;10:15–23. doi: 10.1179/096805104225003780. [DOI] [PubMed] [Google Scholar]
- 137.Tang X, Fenton MJ, Amar S. Identification and functional characterization of a novel binding site on TNF-α promoter. Proc Natl Acad Sci USA. 2003;100:4096–4101. doi: 10.1073/pnas.0630562100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tang X, Marciano DL, Leeman SE, Amar S. LPS induces the interaction of a transcription factor, LPS-induced TNF-α factor, and STAT6(B) with effects on multiple cytokines. Proc Natl Acad Sci USA. 2005;102:5132–5137. doi: 10.1073/pnas.0501159102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tang X, Metzger D, Leeman S, Amar S. LPS-induced TNF-α factor (LITAF)-deficient mice express reduced LPS-induced cytokine: evidence for LITAF-dependent LPS signaling pathways. Proc Natl Acad Sci USA. 2006;103:13777–13782. doi: 10.1073/pnas.0605988103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vila-del Sol V, Punzón C, Fresno M. IFN-γ-induced TNF-α expression is regulated by interferon regulatory factors 1 and 8 in mouse macrophages. J Immunol. 2008;181:4461–4470. doi: 10.4049/jimmunol.181.7.4461. [DOI] [PubMed] [Google Scholar]
- 141.Zhao XJ, Dong Q, Bindas J, Piganelli JD, Magill A, Reiser J, Kolls JK. TRIF and IRF-3 binding to the TNF promoter results in macrophage TNF dysregulation and steatosis induced by chronic ethanol. J Immunol. 2008;181:3049–3056. doi: 10.4049/jimmunol.181.5.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Covert MW, Leung TH, Gaston JE, Baltimore D. Achieving stability of lipopolysaccharide-induced NF-κB activation. Science. 2005;309:1854–1857. doi: 10.1126/science.1112304. [DOI] [PubMed] [Google Scholar]
- 143.Werner SL, Barken D, Hoffmann A. Stimulus specificity of gene expression programs determined by temporal control of IKK activity. Science. 2005;309:1857–1861. doi: 10.1126/science.1113319. [DOI] [PubMed] [Google Scholar]
- 144.Mungall AJ, Palmer SA, Sims SK, et al. The DNA sequence and analysis of human chromosome 6. Nature. 2003;425:805–811. doi: 10.1038/nature02055. [DOI] [PubMed] [Google Scholar]
- 145.Horton R, Wilming L, Rand V, Lovering RC, Bruford EA, Khodiyar VK, Lush MJ, Povey S, Talbot CC, Jr, Wright MW, Wain HM, Trowsdale J, Ziegler A, Beck S. Gene map of the extended human MHC. Nat Rev Genet. 2004;5:889–899. doi: 10.1038/nrg1489. [DOI] [PubMed] [Google Scholar]
- 146.Allen RD. Polymorphism of the human TNF-α promoter – random variation or functional diversity? Mol Immunol. 1999;36:1017–1027. doi: 10.1016/s0161-5890(99)00127-3. [DOI] [PubMed] [Google Scholar]
- 147.Hajeer AH, Hutchinson IV. TNF-α gene polymorphism: clinical and biological implications. Microsc Res Tech. 2000;50:216–228. doi: 10.1002/1097-0029(20000801)50:3<216::AID-JEMT5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 148.Hajeer AH, Hutchinson IV. Influence of TNFα gene polymorphisms on TNFα production and disease. Hum Immunol. 2001;62:1191–1199. doi: 10.1016/s0198-8859(01)00322-6. [DOI] [PubMed] [Google Scholar]
- 149.Bayley J-P, Ottenhoff TH, Verweij CL. Is there a future for TNF promoter polymorphisms? Genes Immun. 2004;5:315–329. doi: 10.1038/sj.gene.6364055. [DOI] [PubMed] [Google Scholar]
- 150.Smith AJ, Humphries SE. Cytokine and cytokine receptor gene polymorphisms and their functionality. Cytokine Growth Factor Rev. 2009;20:43–59. doi: 10.1016/j.cytogfr.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 151.Uglialoro AM, Turbay D, Pesavento PA, Delgado JC, McKenzie FE, Gribben JG, Hartl D, Yunis EJ, Goldfeld AE. Identification of three new single nucleotide polymorphisms in the human tumor necrosis factor-α gene promoter. Tissue Antigens. 1998;52:359–367. doi: 10.1111/j.1399-0039.1998.tb03056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hollegaard MV, Bidwell JL. Cytokine gene polymorphism in human disease: On-line databases, supplement 3. Genes Immun. 2006;7:269–276. doi: 10.1038/sj.gene.6364301. [DOI] [PubMed] [Google Scholar]
- 153.Wilson AG, de Vries N, Pociot F, di Giovine FS, van der Putte LBA, Duff GW. An allelic polymorphism within the human tumor necrosis factor1α promoter region is strongly associated with HLA A1, B8, and DR3 alleles. J Exp Med. 1993;177:557–560. doi: 10.1084/jem.177.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Higuchi T, Seki N, Kamizono S, Yamada A, Kimura A, Kato H, Itoh K. Polymorphism of the 5′-flanking region of the human tumor necrosis factor (TNF)-α gene in Japanese. Tissue Antigens. 1998;51:605–612. doi: 10.1111/j.1399-0039.1998.tb03002.x. [DOI] [PubMed] [Google Scholar]
- 155.Baena A, Leung JY, Sullivan AD, Landires I, Vasquez-Luna N, Quinones-Berrocal J, Fraser PA, Uko GP, Delgado JC, Clavijo OP, Thim S, Meshnick SR, Nyirenda T, Yunis EJ, Goldfeld AE. TNF-α promoter single nucleotide polymorphisms are markers of human ancestry. Genes Immun. 2002;3:482–487. doi: 10.1038/sj.gene.6363898. [DOI] [PubMed] [Google Scholar]
- 156.Alper CA, Awdeh ZL, Raum DD, Yunis EJ. Extended major histocompatibility complex haplotypes in man: role of alleles analogous to murine τ mutants. Clin Immunol Immunopathol. 1982;24:276–285. doi: 10.1016/0090-1229(82)90238-0. [DOI] [PubMed] [Google Scholar]
- 157.Yunis EJ, Larsen CE, Fernandez-Vina M, Awdeh ZL, Romero T, Hansen JA, Alper CA. Inheritable variable sizes of DNA stretches in the human MHC: conserved extended haplotypes and their fragments or blocks. Tissue Antigens. 2003;62:1–20. doi: 10.1034/j.1399-0039.2003.00098.x. [DOI] [PubMed] [Google Scholar]
- 158.Braun N, Michel U, Ernst BP, Metzner R, Bitsch A, Weber F, Rieckmann P. Gene polymorphism at position −308 of the tumor-necrosis-factor-α (TNF-α) in multiple sclerosis and it’s influence on the regulation of TNF-α production. Neurosci Lett. 1996;215:75–78. [PubMed] [Google Scholar]
- 159.Wu WS, McClain KL. DNA polymorphisms and mutations of the tumor necrosis factor-α (TNF-α) promoter in Langerhans cell histiocytosis (LCH) J Interferon Cytokine Res. 1997;17:631–635. doi: 10.1089/jir.1997.17.631. [DOI] [PubMed] [Google Scholar]
- 160.Kroeger KM, Carville KS, Abraham LJ. The −308 tumor necrosis factor-α promoter polymorphism effects transcription. Mol Immunol. 1997;34:391–399. doi: 10.1016/s0161-5890(97)00052-7. [DOI] [PubMed] [Google Scholar]
- 161.Wilson AG, Symons JA, McDowell TL, McDevitt HO, Duff GW. Effects of a polymorphism in the human tumor necrosis factor α promoter on transcriptional activation. Proc Natl Acad Sci USA. 1997;94:3195–3199. doi: 10.1073/pnas.94.7.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kroeger KM, Steer JH, Joyce DA, Abraham LJ. Effects of stimulus and cell type on the expression of the −308 tumour necrosis factor promoter polymorphism. Cytokine. 2000;12:110–119. doi: 10.1006/cyto.1999.0529. [DOI] [PubMed] [Google Scholar]
- 163.Brinkman BM, Zuijdeest D, Kaijzel EL, Breedveld FC, Verweij CL. Relevance of the tumor necrosis factor alpha (TNF α) −308 promoter polymorphism in TNF-α gene regulation. J Inflamm. 1996;46:32–41. [PubMed] [Google Scholar]
- 164.Stuber F, Udalova IA, Book M, Drutskaya LN, Kuprash DV, Turetskaya RL, Schade FU, Nedospasov SA. −308 tumor necrosis factor (TNF) polymorphism is not associated with survival in severe sepsis and is unrelated to lipopolysaccharide inducibility of the human TNF promoter. J Inflamm. 1995;46:42–50. [PubMed] [Google Scholar]
- 165.Bayley J-P, de Rooij H, van den Elsen PJ, Huizinga TW, Verweij CL. Functional analysis of linker-scan mutants spanning the −376, −308, −244, and −238 polymorphic sites of the TNF-α promoter. Cytokine. 2001;14:316–323. doi: 10.1006/cyto.2001.0902. [DOI] [PubMed] [Google Scholar]
- 166.Knight JC, Keating BJ, Rockett KA, Kwiatkowski DP. In vivo characterization of regulatory polymorphisms by allele-specific quantification of RNA polymerase loading. Nat Genet. 2003;33:469–475. doi: 10.1038/ng1124. [DOI] [PubMed] [Google Scholar]
- 167.Knight JC, Udalova I, Hill AV, Greenwood BM, Peshu N, Marsh K, Kwiatkowski D. A polymorphism that affects OCT-1 binding to the TNF promoter region is associated with severe malaria. Nat Genet. 1999;22:145–150. doi: 10.1038/9649. [DOI] [PubMed] [Google Scholar]
- 168.Hohjoh H, Tokunaga K. Allele-specific binding of the ubiquitous transcription factor OCT-1 to the functional single nucleotide polymorphism (SNP) sites in the tumor necrosis factor-alpha gene (TNFA) promoter. Genes Immun. 2001;2:105–109. doi: 10.1038/sj.gene.6363721. [DOI] [PubMed] [Google Scholar]
- 169.van Heel DA, Udalova IA, De Silva AP, McGovern DP, Kinouchi Y, Hull J, Lench NJ, Cardon LR, Carey AH, Jewell DP, Kwiatkowski D. Inflammatory bowel disease is associated with a TNF polymorphism that affects an interaction between the OCT1 and NF-κB transcription factors. Hum Mol Genet. 2002;11:1281–1289. doi: 10.1093/hmg/11.11.1281. [DOI] [PubMed] [Google Scholar]
- 170.Skoog T, Hamsten A, Eriksson P. Allele-specific chromatin remodeling of the tumor necrosis factor-α promoter. Biochem Biophys Res Commun. 2006;351:777–783. doi: 10.1016/j.bbrc.2006.10.114. [DOI] [PubMed] [Google Scholar]
- 171.Udalova IA, Richardson A, Denys A, Smith C, Ackerman H, Foxwell B, Kwiatkowski D. Functional consequences of a polymorphism affecting NF-κB p50-p50 binding to the TNF promoter region. Mol Cell Biol. 2000;20:9113–9119. doi: 10.1128/mcb.20.24.9113-9119.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kaijzel EL, van Krugten MV, Brinkman BM, Huizinga TW, van der Straaten T, Hazes JM, Ziegler-Heitbrock HW, Nedospasov SA, Breedveld FC, Verweij CL. Functional analysis of a human tumor necrosis factor α (TNF-α) promoter polymorphism related to joint damage in rheumatoid arthritis. Mol Med. 1998;4:724–733. [PMC free article] [PubMed] [Google Scholar]
- 173.Lee GR, Kim ST, Spilianakis CG, Fields PE, Flavell RA. T helper cell differentiation: regulation by cis elements and epigenetics. Immunity. 2006;24:369–379. doi: 10.1016/j.immuni.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 174.Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447:407–412. doi: 10.1038/nature05915. [DOI] [PubMed] [Google Scholar]
- 175.Mellor J, Dudek P, Clynes D. A glimpse into the epigenetic landscape of gene regulation. Curr Opin Genet Dev. 2008;18:116–122. doi: 10.1016/j.gde.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 176.Cedar H, Bergman Y. Linking DNA methylation and histone modification: patterns and paradigms. Nat Rev Genet. 2009;10:295–304. doi: 10.1038/nrg2540. [DOI] [PubMed] [Google Scholar]
- 177.Li B, Carey M, Workman JL. The role of chromatin during transcription. Cell. 2007;128:707–719. doi: 10.1016/j.cell.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 178.Marmorstein R, Roth SY. Histone acetyltransferases: function, structure, and catalysis. Curr Opin Genet Dev. 2001;11:155–161. doi: 10.1016/s0959-437x(00)00173-8. [DOI] [PubMed] [Google Scholar]
- 179.Shahbazian MD, Grunstein M. Functions of site-specific histone acetylation and deacetylation. Annu Rev Biochem. 2007;76:75–100. doi: 10.1146/annurev.biochem.76.052705.162114. [DOI] [PubMed] [Google Scholar]
- 180.Lee KK, Workman JL. Histone acetyltransferase complexes: one size doesn’t fit all. Nat Rev Mol Cell Biol. 2007;8:284–295. doi: 10.1038/nrm2145. [DOI] [PubMed] [Google Scholar]
- 181.Miao F, Gonzalo IG, Lanting L, Natarajan R. In vivo chromatin remodeling events leading to inflammatory gene transcription under diabetic conditions. J Biol Chem. 2004;279:18091–18097. doi: 10.1074/jbc.M311786200. [DOI] [PubMed] [Google Scholar]
- 182.Kasper LH, Fukuyama T, Biesen MA, Boussouar F, Tong C, de Pauw A, Murray PJ, van Deursen JM, Brindle PK. Conditional knockout mice reveal distinct functions for the global transcriptional coactivators CBP and p300 in T-cell development. Mol Cell Biol. 2006;26:789–809. doi: 10.1128/MCB.26.3.789-809.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Xu W, Fukuyama T, Ney PA, Wang D, Rehg J, Boyd K, van Deursen JM, Brindle PK. Global transcriptional coactivators CREB-binding protein and p300 are highly essential collectively but not individually in peripheral B cells. Blood. 2006;107:4407–4416. doi: 10.1182/blood-2005-08-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ranjbar S, Rajsbaum R, Goldfeld AE. Transactivator of transcription from HIV type 1 subtype E selectively inhibits TNF gene expression via interference with chromatin remodeling of the TNF locus. J Immunol. 2006;176:4182–4190. doi: 10.4049/jimmunol.176.7.4182. [DOI] [PubMed] [Google Scholar]
- 185.Ramirez-Carrozzi VR, Nazarian AA, Li CC, Gore SL, Sridharan R, Imbalzano AN, Smale ST. Selective and antagonistic functions of SWI/SNF and Mi-2β nucleosome remodeling complexes during an inflammatory response. Genes Dev. 2006;20:282–296. doi: 10.1101/gad.1383206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sullivan KE, Reddy AB, Dietzmann K, Suriano AR, Kocieda VP, Stewart M, Bhatia M. Epigenetic regulation of tumor necrosis factor alpha. Mol Cell Biol. 2007;27:5147–5160. doi: 10.1128/MCB.02429-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Garrett S, Dietzmann-Maurer K, Song L, Sullivan KE. Polarization of primary human monocytes by IFN-γ induces chromatin changes and recruits RNA Pol II to the TNF-α promoter. J Immunol. 2008;180:5257–5266. doi: 10.4049/jimmunol.180.8.5257. [DOI] [PubMed] [Google Scholar]
- 188.Lee JY, Kim NA, Sanford A, Sullivan KE. Histone acetylation and chromatin conformation are regulated separately at the TNF-α promoter in monocytes and macrophages. J Leukoc Biol. 2003;73:862–871. doi: 10.1189/jlb.1202618. [DOI] [PubMed] [Google Scholar]
- 189.Sullivan KE, Suriano A, Dietzmann K, Lin J, Goldman D, Petri MA. The TNFα locus is altered in monocytes from patients with systemic lupus erythematosus. Clin Immunol. 2007;123:74–81. doi: 10.1016/j.clim.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Taylor JM, Wicks K, Vandiedonck C, Knight JC. Chromatin profiling across the human tumour necrosis factor gene locus reveals a complex, cell type-specific landscape with novel regulatory elements. Nucleic Acids Res. 2008;36:4845–4862. doi: 10.1093/nar/gkn444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Saccani S, Pantano S, Natoli G. p38-dependent marking of inflammatory genes for increased NF-κB recruitment. Nat Immunol. 2002;3:69–75. doi: 10.1038/ni748. [DOI] [PubMed] [Google Scholar]
- 192.El Gazzar M, Yoza BK, Chen X, Hu J, Hawkins GA, McCall CE. G9a and HP1 couple histone and DNA methylation to TNFα transcription silencing during endotoxin tolerance. J Biol Chem. 2008;283:32198–32208. doi: 10.1074/jbc.M803446200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kochanek S, Radbruch A, Tesch H, Renz D, Doerfler W. DNA methylation profiles in the human genes for tumor necrosis factors α and β in subpopulations of leukocytes and in leukemias. Proc Natl Acad Sci USA. 1991;88:5759–5763. doi: 10.1073/pnas.88.13.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kruys V, Thompson P, Beutler B. Extinction of the tumor necrosis factor locus, and of genes encoding the lipopolysaccharide signaling pathway. J Exp Med. 1993;177:1383–1390. doi: 10.1084/jem.177.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Becker C, Barbulescu K, Wirtz S, Meyer zum Buschenfelde KH, Pettersson S, Neurath MF. Constitutive and inducible in vivo protein-DNA interactions at the tumor necrosis factor-α promoter in primary human T lymphocytes. Gene Expr. 1999;8:115–127. [PMC free article] [PubMed] [Google Scholar]
- 196.Barthel R, Goldfeld AE. T cell-specific expression of the human TNF-α gene involves a functional and highly conserved chromatin signature in intron 3. J Immunol. 2003;171:3612–3619. doi: 10.4049/jimmunol.171.7.3612. [DOI] [PubMed] [Google Scholar]
- 197.Sung SJ, Walters JA, Hudson J, Gimble JM. Tumor necrosis factor-α mRNA accumulation in human myelomonocytic cell lines. Role of transcriptional regulation by DNA sequence motifs and mRNA stabilization. J Immunol. 1991;147:2047–2054. [PubMed] [Google Scholar]
- 198.Kuhnert P, Peterhans E, Pauli U. Chromatin structure and DNase I hypersensitivity in the transcriptionally active and inactive porcine tumor necrosis factor gene locus. Nucleic Acids Res. 1992;20:1943–1948. doi: 10.1093/nar/20.8.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Espel E, Garcia-Sanz JA, Aubert V, Menoud V, Sperisen P, Fernandez N, Spertini F. Transcriptional and translational control of TNF-α gene expression in human monocytes by major histocompatibility complex class II ligands. Eur J Immunol. 1996;26:2417–2424. doi: 10.1002/eji.1830261023. [DOI] [PubMed] [Google Scholar]
- 200.Kwon J, Lee SJ, Benveniste EN. A 3′ cis-acting element is involved in tumor necrosis factor-α gene expression in astrocytes. J Biol Chem. 1996;271:22383–22390. doi: 10.1074/jbc.271.37.22383. [DOI] [PubMed] [Google Scholar]
- 201.Dekker J. A closer look at long-range chromosomal interactions. Trends Biochem Sci. 2003;28:277–280. doi: 10.1016/S0968-0004(03)00089-6. [DOI] [PubMed] [Google Scholar]
- 202.de Laat W. Long-range DNA contacts: romance in the nucleus? Curr Opin Cell Biol. 2007;19:317–320. doi: 10.1016/j.ceb.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 203.Carter DR, Eskiw C, Cook PR. Transcription factories. Biochem Soc Trans. 2008;36:585–589. doi: 10.1042/BST0360585. [DOI] [PubMed] [Google Scholar]
- 204.O’Sullivan JM, Tan-Wong SM, Morillon A, Lee B, Coles J, Mellor J, Proudfoot NJ. Gene loops juxtapose promoters and terminators in yeast. Nat Genet. 2004;36:1014–1018. doi: 10.1038/ng1411. [DOI] [PubMed] [Google Scholar]
- 205.Drissen R, Palstra RJ, Gillemans N, Splinter E, Grosveld F, Philipsen S, de Laat W. The active spatial organization of the β-globin locus requires the transcription factor EKLF. Genes Dev. 2004;18:2485–2490. doi: 10.1101/gad.317004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Spilianakis CG, Lalioti MD, Town T, Lee GR, Flavell RA. Interchromosomal associations between alternatively expressed loci. Nature. 2005;435:637–645. doi: 10.1038/nature03574. [DOI] [PubMed] [Google Scholar]
- 207.Vakoc CR, Letting DL, Gheldof N, Sawado T, Bender MA, Groudine M, Weiss MJ, Dekker J, Blobel GA. Proximity among distant regulatory elements at the β-globin locus requires GATA-1 and FOG-1. Mol Cell. 2005;17:453–462. doi: 10.1016/j.molcel.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 208.Splinter E, Heath H, Kooren J, Palstra RJ, Klous P, Grosveld F, Galjart N, de Laat W. CTCF mediates long-range chromatin looping and local histone modification in the β-globin locus. Genes Dev. 2006;20:2349–2354. doi: 10.1101/gad.399506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kurukuti S, Tiwari VK, Tavoosidana G, Pugacheva E, Murrell A, Zhao Z, Lobanenkov V, Reik W, Ohlsson R. CTCF binding at the H19 imprinting control region mediates maternally inherited higher-order chromatin conformation to restrict enhancer access to Igf2. Proc Natl Acad Sci USA. 2006;103:10684–10689. doi: 10.1073/pnas.0600326103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ling JQ, Li T, Hu JF, Vu TH, Chen HL, Qiu XW, Cherry AM, Hoffman AR. CTCF mediates interchromosomal colocalization between Igf2/H19 and Wsb1/Nf1. Science. 2006;312:269–272. doi: 10.1126/science.1123191. [DOI] [PubMed] [Google Scholar]
- 211.Cockerill PN. NFAT is well placed to direct both enhancer looping and domain-wide models of enhancer function. Sci Signal. 2008;1:pe15. doi: 10.1126/stke.113pe15. [DOI] [PubMed] [Google Scholar]
- 212.Murrell A, Heeson S, Reik W. Interaction between differentially methylated regions partitions the imprinted genes Igf2 and H19 into parent-specific chromatin loops. Nat Genet. 2004;36:889–893. doi: 10.1038/ng1402. [DOI] [PubMed] [Google Scholar]
- 213.Deakin JE, Papenfuss AT, Belov K, Cross JG, Coggill P, Palmer S, Sims S, Speed TP, Beck S, Graves JA. Evolution and comparative analysis of the MHC class III inflammatory region. BMC Genomics. 2006;7:281. doi: 10.1186/1471-2164-7-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Gupta D, Jin YP, Dziarski R. Peptidoglycan induces transcription and secretion of TNF-α and activation of lyn, extracellular signal-regulated kinase, and rsk signal transduction proteins in mouse macrophages. J Immunol. 1995;155:2620–2630. [PubMed] [Google Scholar]
- 215.Shapira L, Takashiba S, Amar S, Van Dyke TE. Porphyromonas gingivalis lipopolysaccharide stimulation of human monocytes: dependence on serum and CD14 receptor. Oral Microbiol Immunol. 1994;9:112–117. doi: 10.1111/j.1399-302x.1994.tb00044.x. [DOI] [PubMed] [Google Scholar]
- 216.English BK, Patrick CC, Orlicek SL, McCordic R, Shenep JL. Lipoteichoic acid from viridans streptococci induces the production of tumor necrosis factor and nitric oxide by murine macrophages. J Infect Dis. 1996;174:1348–1351. doi: 10.1093/infdis/174.6.1348. [DOI] [PubMed] [Google Scholar]
- 217.Sato S, Nomura F, Kawai T, Takeuchi O, Muhlradt PF, Takeda K, Akira S. Synergy and cross-tolerance between toll-like receptor (TLR) 2- and TLR4-mediated signaling pathways. J Immunol. 2000;165:7096–7101. doi: 10.4049/jimmunol.165.12.7096. [DOI] [PubMed] [Google Scholar]
- 218.Bondeson J, Sundler R. Auranofin inhibits the induction of interleukin 1 beta and tumor necrosis factor α mRNA in macrophages. Biochem Pharmacol. 1995;50:1753–1759. doi: 10.1016/0006-2952(95)02030-6. [DOI] [PubMed] [Google Scholar]
- 219.Wong GH, Goeddel DV. Tumour necrosis factors α and β inhibit virus replication and synergize with interferons. Nature. 1986;323:819–822. doi: 10.1038/323819a0. [DOI] [PubMed] [Google Scholar]
- 220.Beutler B, Krochin N, Milsark IW, Luedke C, Cerami A. Control of cachectin (tumor necrosis factor) synthesis: mechanisms of endotoxin resistance. Science. 1986;232:977–980. doi: 10.1126/science.3754653. [DOI] [PubMed] [Google Scholar]
- 221.Goeddel DV, Aggarwal BB, Gray PW, Leung DW, Nedwin GE, Palladino MA, Patton JS, Pennica D, Shepard HM, Sugarman BJ, Wong GHW. Tumor necrosis factors: gene structure and biological activities. Cold Spring Harbor Symp Quant Biol. 1986;60:597–609. doi: 10.1101/sqb.1986.051.01.072. [DOI] [PubMed] [Google Scholar]
- 222.Perera PY, Manthey CL, Stutz PL, Hildebrandt J, Vogel SN. Induction of early gene expression in murine macrophages by synthetic lipid A analogs with differing endotoxic potentials. Infect Immun. 1993;61:2015–2023. doi: 10.1128/iai.61.5.2015-2023.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Manthey CL, Brandes ME, Perera PY, Vogel SN. Taxol increases steady-state levels of lipopolysaccharide-inducible genes and protein-tyrosine phosphorylation in murine macrophages. J Immunol. 1992;149:2459–2465. [PubMed] [Google Scholar]
- 224.Pope BL, MacIntyre JP, Kimball E, Lee S, Zhou L, Taylor GR, Goodman MG. The immunostimulatory compound 7-allyl-8-oxoguanosine (loxoribine) induces a distinct subset of murine cytokines. Cell Immunol. 1995;162:333–339. doi: 10.1006/cimm.1995.1087. [DOI] [PubMed] [Google Scholar]
- 225.Stacey KJ, Sweet MJ, Hume DA. Macrophages ingest and are activated by bacterial DNA. J Immunol. 1996;157:2116–2122. [PubMed] [Google Scholar]
- 226.Megyeri K, Au WC, Rosztoczy I, Raj NB, Miller RL, Tomai MA, Pitha PM. Stimulation of interferon and cytokine gene expression by imiquimod and stimulation by Sendai virus utilize similar signal transduction pathways. Mol Cell Biol. 1995;15:2207–2218. doi: 10.1128/mcb.15.4.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Le Contel C, Temime N, Charron DJ, Parant MA. Modulation of lipopolysaccharide-induced cytokine gene expression in mouse bone marrow-derived macrophages by muramyl dipeptide. J Immunol. 1993;150:4541–4549. [PubMed] [Google Scholar]
- 228.Anegón I, Cuturi MC, Trinchieri G, Perussia B. Interaction of Fc receptor (CD16) ligands induces transcription of interleukin 2 receptor (CD25) and lymphokine genes and expression of their products in human natural killer cells. J Exp Med. 1988;167:452–472. doi: 10.1084/jem.167.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kovacs EJ, Beckner SK, Longo DL, Varesio L, Young HA. Cytokine gene expression during the generation of human lymphokine-activated killer cells: early induction of interleukin 1β by interleukin 2. Cancer Res. 1989;49:940–944. [PubMed] [Google Scholar]
- 230.Collart MA, Belin D, Vassalli JD, de Kossodo S, Vassalli P. γ-Interferon enhances macrophage transcription of the tumor necrosis factor/cachectin, interleukin 1, and urokinase genes, which are controlled by short-lived repressors. J Exp Med. 1986;164:2113–2118. doi: 10.1084/jem.164.6.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Cannistra SA, Rambaldi A, Spriggs DR, Herrmann F, Kufe D, Griffin JD. Human granulocyte-macrophage colony-stimulating factor induces expression of the tumor necrosis factor gene by the U937 cell line and by normal human monocytes. J Clin Invest. 1987;79:1720–1728. doi: 10.1172/JCI113012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Spriggs D, Imamura K, Rodriguez C, Horiguchi J, Kufe DW. Induction of tumor necrosis factor expression and resistance in a human breast tumor cell line. Proc Natl Acad Sci USA. 1987;84:6563–6566. doi: 10.1073/pnas.84.18.6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Collart MA, Belin D, Vassalli JD, Vassalli P. Modulations of functional activity in differentiated macrophages are accompanied by early and transient increase or decrease in c-fos gene transcription. J Immunol. 1987;139:949–955. [PubMed] [Google Scholar]
- 234.Trede NS, Geha RS, Chatila T. Transcriptional activation of IL-1β and tumor necrosis factor-α genes by MHC class II ligands. J Immunol. 1991;146:2310–2315. [PubMed] [Google Scholar]
- 235.Sung SJ, Walters JA, Fu SM. Stimulation of tumor necrosis factor-α production in human monocytes by inhibitors of protein phosphatase 1 and 2A. J Exp Med. 1992;176:897–901. doi: 10.1084/jem.176.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Rieckmann P, Thevenin C, Kehrl JH. Okadaic acid is a potent inducer of AP-1, NF-κB, and tumor necrosis factor-α in human B lymphocytes. Biochem Biophys Res Commun. 1992;187:51–57. doi: 10.1016/s0006-291x(05)81457-3. [DOI] [PubMed] [Google Scholar]
- 237.Kock A, Schwarz T, Kirnbauer R, Urbanski A, Perry P, Ansel JC, Luger TA. Human keratinocytes are a source for tumor necrosis factor a: evidence for synthesis and release upon stimulation with endotoxin or ultraviolet light. J Exp Med. 1990;172:1609–1614. doi: 10.1084/jem.172.6.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Hallahan DE, Spriggs DR, Beckett MA, Kufe DW, Weichselbaum RR. Increased tumor necrosis factor-α mRNA after cellular exposure to ionizing radiation. Proc Natl Acad Sci USA. 1989;86:10104–10107. doi: 10.1073/pnas.86.24.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Guha M, Bai W, Nadler JL, Natarajan R. Molecular mechanisms of tumor necrosis factor α gene expression in monocytic cells via hyperglycemia-induced oxidant stress-dependent and -independent pathways. J Biol Chem. 2000;275:17728–17739. doi: 10.1074/jbc.275.23.17728. [DOI] [PubMed] [Google Scholar]
- 240.Piguet PF, Collart MA, Grau GE, Sappino AP, Vassalli P. Requirement of tumour necrosis factor for development of silica-induced pulmonary fibrosis. Nature. 1990;344:245–247. doi: 10.1038/344245a0. [DOI] [PubMed] [Google Scholar]
- 241.Ehlers S, Mielke MEA, Blankenstein T, Hahn H. Kinetic analysis of cytokine gene expression in the livers of naive and immune mice infected with Listeria monocytogenes. J Immunol. 1992;149:3016–3022. [PubMed] [Google Scholar]
- 242.Poston RM, Kurlander RJ. Cytokine expression in vivo during murine listeriosis. Infection with live, virulent bacteria is required for monokine and lymphokine messenger RNA accumulation in the spleen. J Immunol. 1992;149:3040–3044. [PubMed] [Google Scholar]
- 243.Barnes PF, Fong SJ, Brennan PJ, Twomey PE, Mazumder A, Modlin RL. Local production of tumor necrosis factor and IFN-γ in tuberculous pleuritis. J Immunol. 1990;145:149–154. [PubMed] [Google Scholar]
- 244.Rugo HS, O’Hanley P, Bishop AG, Pearce MK, Abrams JS, Howard M, O’Garra A. Local cytokine production in a murine model of Escherichia coli pyelonephritis. J Clin Invest. 1992;89:1032–1039. doi: 10.1172/JCI115644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Berent SL, Torczynski RM, Bollon AP. Sendai virus induces high levels of tumor necrosis factor mRNA in human peripheral blood leukocytes. Nucleic Acids Res. 1986;14:8997–9015. doi: 10.1093/nar/14.22.8997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Dudding L, Haskill S, Clark BD, Auron PE, Sporn S, Huang ES. Cytomegalovirus infection stimulates expression of monocyte-associated mediator genes. J Immunol. 1989;143:3343–3352. [PubMed] [Google Scholar]
- 247.Goodier MR, Lundqvist C, Hammarstrom ML, Troye-Blomberg M, Langhorne J. Cytokine profiles for human Vγ9+ T cells stimulated by Plasmodium falciparum. Parasite Immunol. 1995;17:413–423. doi: 10.1111/j.1365-3024.1995.tb00909.x. [DOI] [PubMed] [Google Scholar]
- 248.Van Voorhis WC. Coculture of human peripheral blood mononuclear cells with Trypanosoma cruzi leads to proliferation of lymphocytes and cytokine production. J Immunol. 1992;148:239–248. [PubMed] [Google Scholar]
- 249.Wynn TA, Oswald IP, Eltoum IA, Caspar P, Lowenstein CJ, Lewis FA, James SL, Sher A. Elevated expression of Th1 cytokines and nitric oxide synthase in the lungs of vaccinated mice after challenge infection with Schistosoma mansoni. J Immunol. 1994;153:5200–5209. [PubMed] [Google Scholar]
- 250.Suriano AR, Sanford AN, Kim N, Oh M, Kennedy S, Henderson MJ, Dietzmann K, Sullivan KE. GCF2/LRRFIP1 represses tumor necrosis factor alpha expression. Mol Cell Biol. 2005;25:9073–9081. doi: 10.1128/MCB.25.20.9073-9081.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Schreiber J, Jenner RG, Murray HL, Gerber GK, Gifford DK, Young RA. Coordinated binding of NF-κB family members in the response of human cells to lipopolysaccharide. Proc Natl Acad Sci USA. 2006;103:5899–5904. doi: 10.1073/pnas.0510996103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Amar S, Zhou Q, Shaik-Dasthagirisaheb Y, Leeman S. Diet-induced obesity in mice causes changes in immune responses and bone loss manifested by bacterial challenge. Proc Natl Acad Sci USA. 2007;104:20466–20471. doi: 10.1073/pnas.0710335105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Cvoro A, Tzagarakis-Foster C, Tatomer D, Paruthiyil S, Fox MS, Leitman DC. Distinct roles of unliganded and liganded estrogen receptors in transcriptional repression. Mol Cell. 2006;21:555–564. doi: 10.1016/j.molcel.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 254.Yang YJ, Chen W, Carrigan SO, Chen WM, Roth K, Akiyama T, Inoue J, Marshall JS, Berman JN, Lin TJ. TRAF6 specifically contributes to FcεRI-mediated cytokine production but not mast cell degranulation. J Biol Chem. 2008;283:32110–32118. doi: 10.1074/jbc.M802610200. [DOI] [PubMed] [Google Scholar]
- 255.Wang J, Wang X, Hussain S, Zheng Y, Sanjabi S, Ouaaz F, Beg AA. Distinct roles of different NF-κB subunits in regulating inflammatory and T cell stimulatory gene expression in dendritic cells. J Immunol. 2007;178:6777–6788. doi: 10.4049/jimmunol.178.11.6777. [DOI] [PubMed] [Google Scholar]
- 256.Sanjabi S, Hoffmann A, Liou HC, Baltimore D, Smale ST. Selective requirement for c-Rel during IL-12 P40 gene induction in macrophages. Proc Natl Acad Sci USA. 2000;97:12705–12710. doi: 10.1073/pnas.230436397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Gadjeva M, Tomczak MF, Zhang M, Wang YY, Dull K, Rogers AB, Erdman SE, Fox JG, Carroll M, Horwitz BH. A role for NF-κB subunits p50 and p65 in the inhibition of lipopolysaccharide-induced shock. J Immunol. 2004;173:5786–5793. doi: 10.4049/jimmunol.173.9.5786. [DOI] [PubMed] [Google Scholar]
- 258.Gerondakis S, Strasser A, Metcalf D, Grigoriadis G, Scheerlinck JY, Grumont RJ. Rel-deficient T cells exhibit defects in production of interleukin 3 and granulocyte-macrophage colony-stimulating factor. Proc Natl Acad Sci USA. 1996;93:3405–3409. doi: 10.1073/pnas.93.8.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Caamaño J, Alexander J, Craig L, Bravo R, Hunter CA. The NF-κB family member RelB is required for innate and adaptive immunity to Toxoplasma gondii. J Immunol. 1999;163:4453–4461. [PubMed] [Google Scholar]
- 260.Franzoso G, Carlson L, Xing L, Poljak L, Shores EW, Brown KD, Leonardi A, Tran T, Boyce BF, Siebenlist U. Requirement for NF-κB in osteoclast and B-cell development. Genes Dev. 1997;11:3482–3496. doi: 10.1101/gad.11.24.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Iotsova V, Caamaño J, Loy J, Yang Y, Lewin A, Bravo R. Osteopetrosis in mice lacking NF-κB1 and NF-κB2. Nat Med. 1997;3:1285–1289. doi: 10.1038/nm1197-1285. [DOI] [PubMed] [Google Scholar]