Summation
Antibodies are highly versatile proteins with the ability to be used to target diverse compounds, such as radionuclides for imaging and therapy, or drugs and toxins for therapy, but also can be used unconjugated to elicit therapeutically beneficial responses, usually with minimal toxicity. This update describes a new procedure for forming multivalent and/or multispecific proteins, known as the dock-and-lock (DNL) technique. Developed as a procedure for preparing bispecific antibodies capable of binding divalently to a tumor antigen and monovalently to a radiolabeled hapten-peptide for pretargeted imaging and therapy, this methodology has the flexibility to create a number of other biologic agents of therapeutic interest. A variety of constructs, based on anti-CD20 and CD22 antibodies, have been made, with results showing that multispecific antibodies have very different properties from the respective parental monospecific antibodies. The technique is not restricted to antibody combination, but other biologics, such as interferon-α2b, have been prepared. These types of constructs not only allow small biologics to be sustained in the blood longer, but also to be selectively targeted. Thus, DNL technology is a highly flexible platform that can be used to prepare many different types of agents that could further improve cancer detection and therapy.
Key words: recombinant antibodies, bispecific antibodies, non-Hodgkin's lymphoma, pretargeting, molecular imaging, antibody therapy, radioimmunotherapy
Introduction
Antibodies might not be the proverbial “magic bullet” as initially hoped, but they undoubtedly have played a very important role in the detection and treatment of cancer, helping pave the way for a new era of targeted therapy. As is often the case when new discoveries are made, our visions of what they can be are ahead of our ability to put them into practice. For example, studies first performed in the early 1950s established the feasibility of specifically targeting radionuclides to tumors in animal models, but at the time, there were not any suitable targets known to be associated with human tumors.1–3 Many other technical hurdles, such as better-defined antibodies and improved ways to radiolabel proteins, would not become available for 20–30 years. Even in the 1970s, when the localization of a radiolabeled antibody to a human tumor-associated antigen was finally demonstrated, still, affinity-purified polyclonal antibodies were the state of the art, and computer-assisted subtraction technology was required to visualize uptake.4–8 By the 1980s, new chemistries for radiolabeling proteins were being developed that greatly expanded the possibilities for a variety of imaging and therapeutic radionuclides, and, importantly, murine monoclonal antibodies became available.9–19 Hybridoma technology ultimately ushered in a new age of molecular engineering that has revolutionized the applications of antibodies and biologics alike. Murine monoclonal antibodies were chimerized and then humanized to reduce immunogenicity. These techniques became increasingly refined, being able to isolate the Fv region of the heavy and light chains of an antibody. By tethering these two chains together with a small peptide linker, a whole new fragment form, the single-chain Fv (scFv), was born.20–24 Antibodies have been revised perhaps more than any other biologic in an effort to enhance effector functions, change pharmacokinetic properties, and alter the number of binding sites, leading to a plethora of many antibody forms.25 In addition to the constructs based on human IgG, there are now nanobodies, which are 15-kD antibody structures derived from other animal species (e.g., camelid, shark),26–30 but there are also an increasing number and varieties of structures that mimic the binding found in an antibody, such as different types of affibodies, aptamers, and SHAL.31–49 In this overview, we summarize our progress in the development of a variety of molecular constructs prepared by a new modular method, termed the dock-and-lock (DNL) procedure.
Directly Radiolabeled Conjugates
Our group has been active in the development and testing of radioimmunoconjugates, starting with the initial testing of affinity-purified polyclonal antibodies, to the development of murine monoclonal antibodies and then their humanization, focusing primarily on 99mTc-labeled Fab’ fragments for detection and 131I- or 90Y-labeled IgG for therapy.50–58 Over time, the deficiencies of a directly radiolabeled Fab’ fragment were revealed when new positron-emission tomography (PET)-imaging systems, together with a simple radiolabeled sugar analog (18F-deoxyglucose; 18F-FDG), provided substantial improvements in uptake and image contrast (i.e., sensitivity). In many cancers, glycolytic differences between normal tissues and some malignancies also provide a high level of specificity.59 18F-FDG-PET imaging ultimately displaced most of the directly radiolabeled antibodies that were U.S. Food and Drug Administration (FDA)-approved, including the development of antibody fragments for imaging.
Directly radiolabeled IgG has been the leading form of antibody used in therapeutic applications, primarily because animal models have shown that the IgG form has the highest uptake in tumor. The higher concentration is derived primarily from a long circulation half-life that allows the possibility of multiple passes before being eliminated. However, by lingering in the circulation, the amount of radioactivity that can be administered is limited because of the continued exposure of the red marrow that causes hematologic toxicity. While antibody fragments and other smaller constructs that clear more rapidly from the blood have been reported, on occasion, to improve responses,60–62 often the improvements are marginal because antibody fragments also have reduced uptake in the tumor. While higher administered activity is possible because of faster blood clearance, often the lower amount of radioactivity delivered to the tumor does not appreciably enhance the therapeutic index. A further impediment to using antibody fragments is that their use is generally restricted to radioiodine, since radioiodine is readily eliminated from the tissues. When radiometals are bound directly to fragments <∼60 kD, they become entrapped in the kidneys, resulting in a substantially higher fraction of the injected activity being deposited in the kidneys than in the tumor (this may be 2- to 10-fold higher). Thus, even by reducing hematologic toxicity due to a faster blood clearance, the tumor still would receive a lower radiation dose than that tolerated by the kidneys, which would lead to inadequate tumor dosing. For example, fractionated external beam radiation to the kidneys is limited to 2300 cGy,63,64 yet solid tumors are irradiated to levels nearly twice this level. Clinical experience with radiolabeled peptides, such as 90Y-DOTATOC, have suggested that the radiation dose tolerance of the kidneys can be increased to 2700 cGy for an internally administered radionuclide, and efforts to refine the techniques for calculating renal dosimetry will likely lead to better correlations between toxicity and radiation-absorbed dose.65–67 However, if a directly radiolabeled antibody fragment cannot at least provide a 2-fold improvement in tumor/kidney dose delivery, objective responses will be difficult to achieve in solid tumors. Indeed, because radioimmunotherapy of advanced solid tumors has been so poor, many investigators have turned to compartmental applications, rather than systemic use.68 Hematologic tumors, such as non-Hodgkin's lymphoma (NHL), are much more sensitive to radiation, and thus even when limited by hematological toxicity, significant objective responses are possible with directly radiolabeled IgG.69
IgG is eliminated through the liver, but even with radiometal-labeled IgG, hematologic toxicity is dose limiting, and therefore, hepatic doses do not reach levels where hepatic toxicity is a concern. Some of the newer constructs, such as scFv-Fc, that were specifically modified to enhance blood clearance,70,71 but whose molecular size would preclude renal elimination, also are eliminated in the liver. Consistent with many of the directly radiolabeled antibody fragments, Kenanova et al. found that radioiodinated scFv-Fc would be the best choice for therapy.72 Even though specific linkage chemistries have been developed that would reduce the hepatic uptake of chelated radiometals bound to an antibody,73–75 and there are procedures to reduce renal uptake of chelated radiometals,76–79 none of these approaches has improved the prospects for directly radiolabeled antibodies to be used therapeutically in solid tumors with anything other than radioiodine. Clearing IgG from the blood with an anti-antibody is, likewise, limited to use with radioiodinated products,80 while removing radioconjugates through extracorporeal means can be applied irrespective of the radionuclide.81,82 However, extracorporeal removal is not initiated until the conjugate has had sufficient time to localize in the tumor, and thus there is still considerable radiation exposure over the first few days before the radioactivity is removed.
Pretargeting
Pretargeting is an innovative alternative concept for localizing radionuclides, based on the selectivity of an antibody, and was first proposed by Reardon et al.83 This group developed antibodies to metal-binding chelates and envisioned a system where an unlabeled specialized antibody protein, a bispecific antibody (bsMAb) that had one arm to bind to a tumor-associated antigen and another to a chelate, would be given separately from the targeting of a radiolabeled chelate. Radiolabeled chelates were known to clear very quickly from the body, minimizing residence time in the blood and retention in normal tissues, and thus this concept had the potential to alleviate the long residence time of an IgG and allow radiometals to be used without concern for excessive uptake in normal tissues. This concept was examined as a method for improved imaging of colorectal cancer at a time when 111In-labeled IgGs were unable to detect hepatic metastases, because of elevated 111In-IgG concentrations in normal liver.84–86 In contrast, liver metastases were visualized as hot lesions in patients given a pretargeting procedure, consisting of a chemically conjugated bsMAb composed of a Fab’ fragment to carcinoembryonic antigen (CEA) and a Fab’ fragment to a chelate, which was followed 5 days later with the 111In-labeled chelate.87 The 5-day interval allowed the bsMAb time to localize in the tumor and, importantly, clear from the blood and tissues. It was later discovered that tumor uptake of the radiolabeled chelate could be improved significantly, if the chelate was presented in a divalent form. By joining two haptens through a short peptide linker (two amino acids), the divalent hapten-peptide would still clear quickly and efficiently from blood and tissues, but within the tumor, where the concentration of the antihapten antibody was higher than in the blood, an “affinity enhancement” was achieved.88
Although pretargeting procedures had improved the visualization of hepatic lesions compared to 111In-labeled IgG, 99mTc-labeled Fab’ fragments provided similar improvements, but could be read within 2–24 hours,89 and so, interest in pretargeting for imaging waned. However, pretargeting as a therapeutic approach continued to hold interest, and data to support this role were reported by a number of groups.90–98 One of the more significant findings was reported by Axworthy et al.,99 who found that despite being cleared exceptionally fast from the blood, radiolabeled biotin could be pretargeted to a streptavidin-antibody conjugate in a tumor at a level comparable to that of the directly radiolabeled IgG. Our group initially evaluated a streptavidin-based pretargeting approach, using an anti-CEACAM5 (CEA) antibody coupled to streptavidin,100 but because we had also humanized this antibody, it seemed counterproductive to then conjugate an immunogenic protein such as streptavidin to it. Thus, in collaboration with French investigators, who had been using a chemically conjugated murine anti-CEA bsMAb, we began evaluating a bsMAb-pretargeting procedure. Dosimetry estimates from initial studies, using a 99mTc and 188Re-labeled di-DTPA-peptide, suggested that this approach had favorable radiation doses to tumors.101 A variety of chemically conjugated bsMAb were prepared, including anti-CEA IgG, F(ab′)2, and Fab’ coupled to a single Fab’ of an anti-(In)DTPA antibody to assess which form of bsMAb would produce the most favorable pretargeting results.102 The F(ab′)2 × Fab’ bsMAb was preferred, because tumor uptake of the radiolabeled di-DTPA-peptide was the highest when tumors were pretargeted with this form, but it gave comparable tumor: nontumor ratios as the Fab’ × Fab’, whose faster blood clearance produced the lowest blood and tissue levels. The IgG × Fab’ conjugate failed, because by the time it cleared sufficiently from the blood to minimize interaction with the hapten-peptide, the amount in the tumor had decreased, reducing hapten-peptide localization in the tumor, as compared to the F(ab′)2 × Fab’ conjugate. These studies suggested that the optimal construct for therapy would be a bsMAb with divalent binding to the tumor and monovalent binding to the hapten, but it was important that it be cleared efficiently. Again, monovalent binding of the antihapten arm of the bsMAb is also important, because even with a divalent hapten, when the concentration is low in the blood, the association of the hapten-peptide with the bsMAb is reversible and short-lived, so this will only temporarily delay the clearance of the hapten-peptide. This is quite different from streptavidin-based pretargeting, where the ultrahigh affinity of streptavidin for the radiolabeled biotin creates irreversible binding, and hence some type of clearing/blocking step is an essential component of this method.
The last piece of the puzzle was the nature of the hapten-binding system. We started with the indium-loaded cyclic, DTPA (diethylene triamine pentaacetic acid), as the hapten, since this system had been examined extensively.92 131I had been used in preclinical and clinical studies for therapy, and in our initial testing, we found rhenium isotopes could be used by coupling a specific binding ligand to the peptide core of a diDTPA-peptide. However, the antihapten antibody in this system was very specific for indium-loaded DTPA, and while 90Y would certainly bind to the DTPA, it was not very stable. If we placed a DOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid) chelate on the peptide core, we were concerned that even if we preloaded the DTPA with indium, this process might compromise 90Y-binding to DOTA, but a greater concern was that the 90Y might bind the DTPA, leading to a more unstable conjugate. Another binding system based on the hapten HSG (histamine-succinyl-glycine) had been reported.103,104 Because HSG was not involved in binding radionuclides, di-HSG-peptides could be developed with different ligands optimized to bind a given radionuclide, opening the possibility for a more universal binding system. Peptides were prepared with DOTA and the same 99mTc-binding ligand as used with the (In)DTPA-hapten system, and studies showed two different chemically conjugated bsMAb from using the anti-HSG hapten antibody produced highly favorable tumor localization.105 Having these encouraging data, we initiated a program to engineer humanized bispecific antibodies.
We chose to develop recombinant bsMAbs for pretargeting applications, using the binding domains of the humanized anti-CEACAM5 MAb, hMN-14, and the anti-HSG MAb, 679.106,107 Our initial endeavors involved the creation of scFv-based bispecific diabodies. A number of iterations were produced initially in prokaryotes. Our ultimate diabody construct, BS1.5H, comprised a 53.5-kDa heterodimer formed from the noncovalent association of two coexpressed heterologous polypeptides.108 The first polypeptide had the amino-terminal end of hMN-14VK coupled to the carboxyl terminal end of h679VH by a five-amino-acid-long peptide linker. The second polypeptide had the amino-terminal end of h679VK coupled to the carboxyl terminal end of hMN-14VH by a similar peptide linker. BS1.5H was shown to bind CEA and HSG simultaneously and with similar binding affinity as the parental antibodies. The diabodies cleared very rapidly from the blood and normal tissues of mice (99% was eliminated in less than 8 hours), but was an effective pretargeting agent for human colorectal tumor xenografts, allowing a similar tumor uptake of 111In-HSG peptide, but with superior tumor-to-nontumor ratios, compared to a chemically conjugated (Fab x Fab) bsMAb.108
We were encouraged by the pretargeting results from using bispecific diabodies, but because diabodies are bound monovalently, they dissociate relatively rapidly from the tumor. We hypothesized that the ideal bsMAb for pretargeting might be a trivalent construct with a molecular mass >70 kDa that binds bivalently to the tumor antigen and monovalently to the hapten. A number of variations of novel scFv-based constructs, which were extensions of the bispecific diabody design, were produced. The most promising, hBS14, comprised an ∼80-kDa heterodimer of two complementary polypeptides, each having three variable domains connected by short peptide linkers.109 Indeed, the elimination rate of hBS14 was slower and pretargeting with hBS14 resulted in enhanced tumor uptake and retention, compared to the results obtained from using the hBS1.5 diabody.
Having settled on the desired properties of a pretargeting bsMAb, we next commenced to develop constructs that could be produced with high yield, preferably in myeloma cell culture. We reasoned that high levels of productivity of hBS14 were challenging, because the molecule was too unnatural for expression in mammalian cell culture and too complex for microbial fermentation. Typically, even simple scFv constructs cannot be expressed at high levels in mammalian cells; however, we have had considerable success in producing IgG or Fab fragments with high productivity in myeloma cultures. Since the low yield of hBS14 was likely due to its lack of the CH1 domain, which is critical for the folding and transporting of IgG, we decided to develop an alternative type of trivalent bsMAb, based on the “tribody” design,110 where two scFvs derived from the variable domains of hMN-14 are fused to h679 Fab. Once again, several varieties were generated by having an hMN-14 scFv fused to the carboxyl terminal end of both the Fd and light chain of an h679 Fab fragment. The resulting tribody structure, which has a molecular mass of ∼105 kDa comprising an anti-HSG Fab and two anti-CEA scFvs, was, indeed, expressed at high levels in myeloma cells and performed similarly to hBS14 in pretargeting experiments. Although this approach was promising, all of the tribody variants, which used varying linker peptides and scFv arrangements, produced heterogeneous products with considerable proportions of dimeric and multimeric forms. The dimerization/oligomerization was likely a result of the inherent domain swapping property of the scFvs. At this point, we decided to make trivalent structures based solely on Fab fragments. The result was the invention of the dock-and-lock method.111
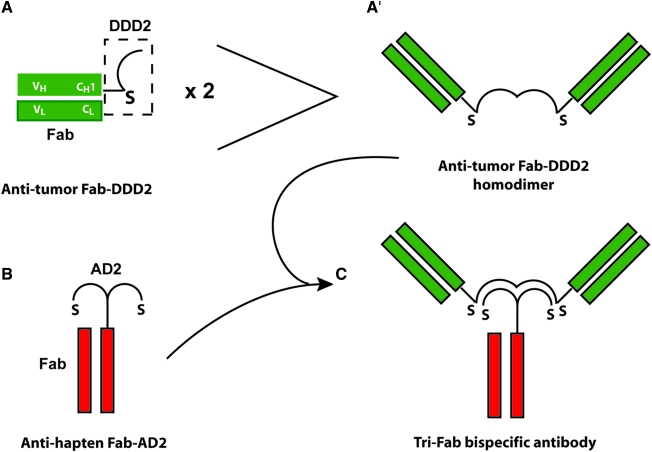
The DNL method utilizes the natural binding interaction of peptides derived from two human proteins. One of the peptides, called the dimerization and docking domain (or DDD), consists of a 44-amino-acid sequence derived from the regulatory subunit of human type II A-kinase.112 The DDD spontaneously forms a very stable homodimer and provides a docking site for the second peptide, called the anchor domain (or AD), which consists of a 17-amino-acid sequence derived from an interactive human A-kinase anchor protein.113 Once docked, the three peptides (two DDD and one AD) are further locked by the formation of disulfide bonds via cysteine residues that were engineered into each domain. DNL modules (DDD- or AD-modules) comprise these peptides fused to a biomolecule, such as a Fab. DNL products are generated by the combination of a monomeric AD-module with a dimeric DDD-module, which initially associate noncovalently (dock) and are subsequently locked into a completely covalent complex under mild redox conditions (Fig. 1). DNL products are well suited for in vivo applications, since they are highly stable in vivo and both peptides are derived from human proteins, thus minimizing immunogenicity. The first application of the DNL method was the production of a bispecific tri-Fab (TF) called TF2.111 By fusing the DDD2 peptide to the carboxyl terminal end of the CH1 domain, a recombinant hMN-14 Fab-DDD2 module was produced at high levels in myeloma culture. The Fab-DDD spontaneously forms a very stable homodimer, and the dimerized DDD2 peptide forms a docking site for an AD peptide. A recombinant h679 Fab-AD2 module, in which the AD2 peptide is fused to the carboxyl terminal end of the CH1 domain, was also produced at high levels in a separate myeloma culture. To generate TF2, hMN-14 Fab-DDD2 was combined with the h679 Fab-AD2 under mild redox conditions, resulting in the nearly quantitative formation of a bispecific tri-Fab (TF2) having two binding arms for CEA and a third for HSG. A single HSG-based affinity chromatography resulted in the purification of a homogeneous product of defined composition.
FIG. 1.
Example of a tri-Fab bispecific antibody prepared by the dock-and-lock procedure. The CH1 of an antitumor Fab is modified with a short linker that attaches a DDD2 unit to the Fab (A). This structure will spontaneously form a homodimer consisting of two Fab units (A′). Another cell line expressing the antihapten, Fab-AD2 (B), is prepared, and when mixed with the antitumor, Fab-DDD2, a tri-Fab structure is formed (C). The strategically placed cysteine (S) residues in both the DDD2 and AD2 peptides allowing the structure to be covalently linked for greater stability.
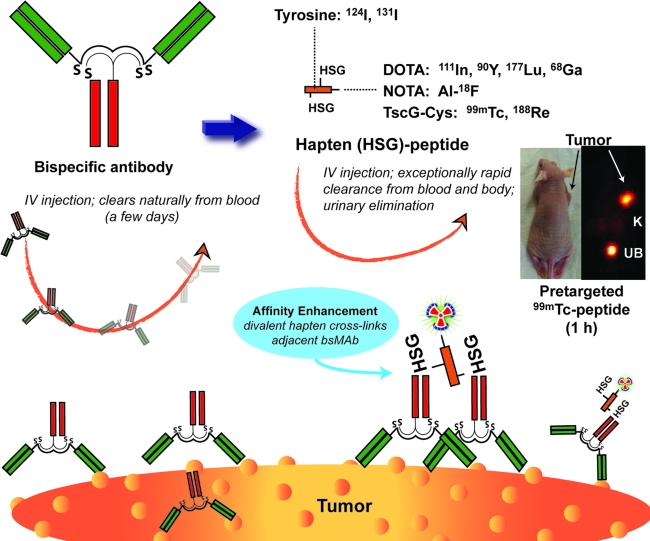
Initial in vivo studies focused on the targeting capability of the bsMAb, particularly whether this 157-kD protein, which was the same size as an IgG, would clear like an IgG and have its stability. In mice, TF2 cleared unexpectedly fast, with <1% injected dose/g (ID/g) in the blood within 16 hours (Table 1).111 Tumor uptake peaked within 4 hours and had decreased to 5% ID/g at 16 hours. It appeared that the spleen was likely involved in the rapid clearance. A 99mTc-labeled hapten-peptide was administered 16 hours after the TF2 injection. Within 1 hour, tumor uptake was 30 3.1% ± 13.7% ID/g, while the levels in blood and normal tissues, except kidney, were less than 1% ID/g. At 24 hours, tumor uptake was 16.3 ± 2.9% ID/g, with tumor: blood, liver, and kidney ratios of 400:1, 100:1, and 14:1. Figure 2 schematically illustrates the bsMAb pretargeting procedure, using the tri-Fab bsMAb with a 99mTc-labeled di-HSG-peptide. The TF2 bsMAb also has been used in several imaging studies, first demonstrating the sensitivity afforded by the pretargeting method, using a 124I-labeled hapten-peptide to disclose micrometastatic lesions (≤0.3 mm) in the lungs of nude mice injected intravenously with a human colon cancer cell line; these tumors were not detected with 18F-FDG.114 Additional imaging studies, using a 18F-labeled di-HSG-NOTA-peptide, have been reported,115 and more recently, we have shown that TF2-pretargeting, using a 68Ga-labeled hapten-peptide is more specific for tumor targeting than 18F-FDG.116 Thus, the TF2 construct is an excellent candidate for pretargeting, and clinical pretargeting studies are now underway.
Table 1.
Biodistribution of 131I-TF2 in LS174 Human Colonic Tumor-Bearing Nude Mice
| |
Percent injected dose per gram |
|||
|---|---|---|---|---|
| 0.5 hour | 2 hours | 4 hours | 16 hours | |
| LS-174T | 4.4 ± 1.1 | 9.2 ± 1.2 | 10.3 ± 2.1 | 5.3 ± 1.1 |
| Liver | 11.7 ± 2.2 | 8.4 ± 0.9 | 4.2 ± 0.1 | 0.32 ± 0.02 |
| Spleen | 22.0 ± 6.0 | 24.9 ± 8.2 | 15.4 ± 1.4 | 0.73 ± 0.14 |
| Kidney | 13.45 ± 0.6 | 6.3 ± 0.5 | 3.9 ± 0.2 | 0.31 ± 0.04 |
| Lungs | 9.0 ± 1.4 | 5.0 ± 0.6 | 3.9 ± 0.1 | 0.33 ± 0.06 |
| Blood | 36.2 ± 3.5 | 15.5 ± 2.4 | 9.1 ± 0.9 | 0.68 ± 0.07 |
| Stomach | 3.0 ± 0.5 | 26.0 ± 5.6 | 50.8 ± 10.8 | 0.85 ± 0.10 |
| Small intestine | 2.2 ± 0.2 | 3.1 ± 0.5 | 2.1 ± 0.1 | 0.19 ± 0.03 |
| Large intestine | 0.8 ± 0.0 | 1.4 ± 0.1 | 1.6 ± 0.1 | 0.21 ± 0.04 |
FIG. 2.
Pretargeting using a tri-Fab bispecific antibody and a radiolabeled di-HSG-peptide. The tri-Fab bispecific antibody is given intravenously, and over a few days, it has localized in the tumor and cleared sufficiently from the blood so that the radiolabeled di-HSG-peptide can be given. This peptide can be prepared in a manner for radiolabeling with a number of different radionuclides. The divalent HSG residues enhance avidity and permit crosslinking of adjacent bsMAb on the tumor surface. The small peptide very quickly distributes throughout the fluidic volume of the body, being rapidly eliminated in the urine. The mouse image illustrates the targeting of a subcutaneous human colon tumor by an anti-CEA bsMAb used to pretarget a 99mTc-labeled di-HSG-peptide. Within 1 hour, the tumor is clearly seen, with the kidneys (K) being the only major normal tissue seen in the background, and the urinary bladder (UB) filled with the eliminated 99mTc-peptide.
We have since prepared a number of other “TF” bsMAb constructs, using different antitumor antibodies paired with the anti-HSG Fab. Two of these pretargeting systems have been reported, one using an anti-CD20 and another using an antipancreatic mucin antibody for targeting NHL and pancreatic cancer, respectively.95,96,117 All of these systems have performed well, with evidence of improved imaging and therapy.
DNL Versatility
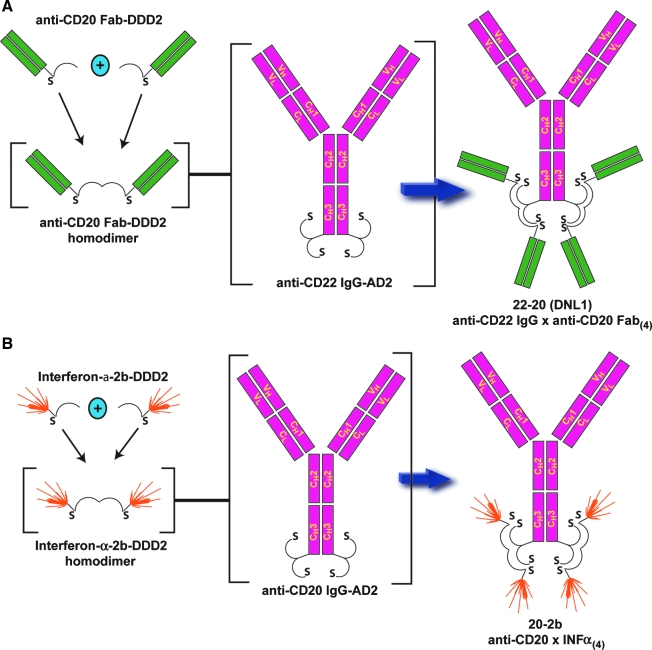
Although developed as a technology to prepare bsMAb for pretargeting, it soon became apparent that the DNL technology was a highly flexible platform for building multivalent, multispecific constructs for a number of purposes.118,119 For example, the CH3 terminus of the IgG heavy chain can be fused with the AD2 component, so that when the IgG is assembled, two AD2 sites will be present, each capable of binding another protein fused with the DDD2. Since the DDD2 forms homodimers, each AD2 site can be occupied by a construct that itself is divalent. Rossi et al.120 reported the ability to form trimeric, tetrameric, and hexameric constructs of the humanized anti-CD20 monoclonal antibody, veltuzumab121 (also known as hA20), using the DNL procedure. Additionally, AD2 constructs of veltuzumab IgG and the humanized anti-CD22 IgG, epratuzumab,122,123 were prepared, along with the DDD2 constructs of their respective Fab fragments (Fig. 3A). The Fab-DDD2 constructs naturally formed homodimers, so that when they were added to the IgG-AD2 form, three distinct recombinant proteins were formed: veltuzumab IgG with four epratuzumab Fabs (designated DNL2 or 20-22), epratuzumab IgG with four veltuzumab Fabs (designated DNL1 or 22-20), and a hexavalent veltuzumab IgG with four veltuzumab Fabs (Hex-hA20 or 20-20).124
FIG. 3.
Multivalent, multispecific antibodies or multispecific antibody-interferon constructs formed by the dock-and-lock method. (A) The CH3 terminus of an anti-CD22 IgG heavy chain is modified with the AD2 peptide sequence, forming an IgG with two AD2 moieties. Fab-DDD2 of an anti-CD20 antibody form homodimers that will bind and lock on the IgG-AD2 to form an IgG structure with four anti-CD20 Fab-binding arms. (B) The same IgG-AD2 structure can also be used to bind to different cytokines, in this example alpha-interferon-2b, with the interferon modified with the AD2 sequence so that the final structure is an IgG capable of targeting four interferons.
Rossi et al.124 showed that the constituents in the respective structures retained their ability to engage their cognate antigen on the target cell surface, but these new bispecific constructs had different properties from their parental IgG. For example, veltuzumab does not readily internalize, and epratuzumab does, yet the 22-20 behaved more like veltuzumab, with slow internalization, while 50% of 20-22 internalized within 1 hour. Thus, it appears that the constituent with the most binding arms controlled internalization. Growth inhibition studies in three cell lines found 22-20 to be more potent in vitro, which is consistent with other studies that had shown multivalent structures (e.g., trivalent, tetravalent, or hexavalent) were more potent than the divalent IgG form.120 They also found that a trivalent form (Tri-hA20; three Fabs) can potently inhibit the proliferation of CD20-positive cells in vitro without crosslinking, suggesting that a minimum valency of 3 is required to induce growth inhibition effectively for an anti-CD20 antibody in the absence of second antibody crosslinking. The IgG-AD2 construct retains CDC and ADCC activity, but when the Fab-DDD2s were bound, the constructs lost CDC activity, but retained ADCC.124 Interestingly, the 20-22 and 22-20 constructs were less toxic to normal human B-cells than veltuzumab (epratuzumab was not effective in these in vitro assays); however, both of these DNL constructs were as effective as veltuzumab in killing either Daudi or Raji tumor cells, suggesting that the constructs might, preferentially, kill B-lymphoma cells.
The evaluation of the hexavalent veltuzumab (Hex-hA20) revealed that it had biologic properties attributable to different types of anti-CD20 IgG.124 For example, Hex-hA20 was negative for CDC and calcium mobilization, but positive for antiproliferation, apoptosis, and homotypical adhesion, all properties associated with type II anti-CD20 IgG. However, it also was positive for trafficking to lipid rafts, a characteristic unique to type I anti-CD20 IgG. Hex-hA20 also appears to induce caspase-dependent and -independent apoptosis. Thus, multivalent antibodies can have different behavior, as compared to their bivalent parental antibodies, including evidence of increased antigen-binding and enhanced antiproliferative potency.
The 22-20 and 20-22 constructs were shown to be stable in human and mouse sera in vitro, yet when radioiodinated and injected in mice, these ∼360-kD constructs cleared at a faster rate than the corresponding IgG forms. While no evidence for the presence of radiolabeled F(ab′)2 or Fab forms was found in serum samples isolated from these mice, increasing amounts of the IgG-AD2 form were seen. We believe this appearance of “instability” might be related to the internalization of these constructs, perhaps involving the same processes active in IgG catabolism, but during this process, the molecule dissociates, and while the F(ab′)2 and Fab structures clear from the blood rapidly, the IgG remains in detectable amounts.
In tumor-bearing animals, the 20-22 construct resulted in a longer median survival than the 22-20 with the Daudi tumor cell line. This activity was significantly reduced when mice were pretreated with agents to deplete natural killer cells and neutrophils, illustrating that the mechanism of action was related to effector cell activity. We found previously that epratuzumab IgG is not active in NHL models, while veltuzumab is. However, clinically, epratuzumab is active,122 and thus, the improved efficacy of 20-22 versus 22-20 in mice very likely reflects the lack of activity of epratuzumab IgG in mice.
The DNL technology can create many other types of fusion proteins. Rossi et al. recently reported the development of a construct composed of an anti-CD20 IgG-AD2 with human interferon α2b-DDD2, creating a 255-kD 20-2b molecule that can target four moles of IFN-α2b to CD20-expressing tumors (Fig. 3B).125 Based on cell-based reporter assay for quantifying interferon (IFN), 20-2b had a specific activity of 5300 IU/pmol, which when tested in the same assay, was greater than both PEGASYS (170 IU/pmol) and PEG-Intron (3400 IU/pmol), two commercially available pegylated alpha-IFNs that each contain a single mole of IFN (∼43 and 60 kD, respectively). The construct retained activity, as determined in an antiviral assay in vivo, with comparable activity to PEG-Intron, but 10-fold higher than PEGASYS. Against Daudi cells, 20-2b had an EC50 of 0.25 pM, which was 8 times better than PEGASYS (pegylated IFN) and ∼30-fold better than a nontargeting IgG-IFN-α2b, illustrating that a targeted IFN can be more potent than a nontargeted one. Further, 20-2b had enhanced ADCC activity, compared to veltuzumab, but like the other IgG-Fab constructs, lost its CDC. At least in lymphoma, it appears that antitumor activity from the 20-2b construct is derived from both the IFN-α2b and the anti-CD20 IgG. Another advantage of this type of construct is its pharmacokinetics. IFN-α2b has a very short mean residence time, and this is why pegylated and albumin-fusion proteins have essentially replaced IFN-α2b in clinical use. In mice, 20-2b had a significantly longer mean residence time (47.6 hour) than either PEGASYS (33.5 hour) or PEG-Intron (17.1 hour). Testing the efficacy of the IFN-α2b construct in mice is compromised because mouse effector cells have a low sensitivity to IFN-α2b. Nevertheless, the 20-2b construct was superior to veltuzumab and other control IFN-α2b immunocytokines in the highly IFN-sensitive Daudi cell line, but also in Raji, which is less sensitive to veltuzumab and 1000-fold less sensitive to IFN-α2b as Daudi, as well as in Nemalwa, which expresses less CD20 and is considered insensitive to anti-CD20 therapy.
Conclusions
The versatile DNL technology has provided many opportunities for antibody-based targeted compounds and has opened the possibilities for creating a host of new multivalent and even bispecific structures that could have enhanced therapeutic potential. Tri-Fab forms of bispecific antibodies have been used successfully for pretargeting radionuclides for improved imaging and therapy. Multivalent forms, with as many as six binding sites, can increase avidity, but more importantly, may improve the activation of signaling pathways that can enhance cell killing. Multispecific forms can work in a similar manner, but through the binding of different antigens. Finally, the DNL platform provides a convenient mechanism for producing a variety of fusion proteins, such as immunocytokines, in a modular manner that can be used to change the pharmacokinetic profile of a cytokine and/or endow a specific targeting functionality.
Acknowledgments
This work was supported, in part, by USPHS grants P01 CA10395 and R01 CA 115755. The authors thank Drs. William J. McBride, Habibe Karacay, and Thomas Cardillo for their collaboration.
Disclosure Statement
Drs. Rossi, Chang, and Goldenberg are employed or have a financial interest in Immunomedics, Inc. Dr. Sharkey has no financial conflicts.
About the Authors

Robert M. Sharkey, is the Director of Clinical Research at the Garden State Cancer Center, Belleville, NJ. Dr. Sharkey received his Ph.D. in 1982 at the University of Kentucky, and has been involved in preclinical and clinical research, focusing on radiolabeled antibodies, and more recently with bispecific antibody pretargeting.

Chien-Hsing (Ken) Chang joined Immunomedics, Inc., in 1989 and currently serves as Vice President of Research & Development at both Immunomedics, Inc., and IBC Pharmaceuticals, Inc., a fully owned subsidiary of Immunomedics, Inc. Dr. Chang started his career in the biopharmaceutical industry as a Senior Scientist (1984–1985) and a Staff Scientist (1985–1986) at Hybritech, where he developed the prototype bispecific antibodies for pretargeting by chemically crosslinking two Fab' fragments. Prior to his tenure with Immunomedics, Dr. Chang held the highest technical position as a Research Fellow at Centocor (1987–1988). Dr. Chang received his Ph.D. in Bioinorganic Chemistry from the Johns Hopkins University in 1976. His academic experience also included post-doctoral training in protein chemistry at The Johns Hopkins University (1976–1977), nucleic acid chemistry at The Ohio State University (1977–1979), and bioconjugate chemistry at the University of California, Davis (1980–1984). Dr. Chang has been a member of the advisory editorial board of Bioconjugate Chemistry since that journal's inauguration in 1990.

Edmund A. Rossi received his Ph.D. in Molecular Cell Biology from the University of Miami School of Medicine in 1996. After completing his post-doctoral research in the laboratory of Dr. Charles Rubin at the Albert Einstein College of Medicine in 2000, Dr. Rossi joined Immunomedics, Inc. and its wholly owned subsidiary, IBC Pharmaceuticals Inc., where he developed a number of novel bispecific antibody designs. He is currently the Director of Protein Engineering at Immunomedics/IBC, and has been instrumental in the development of the Dock-N-Lock (DNL) method, which he has applied to create a variety of novel bioconjugates, including multivalent bispecific antibodies, immunocytokines, and immunotoxins.

David M. Goldenberg, (Sc.D., M.D.), is President and Founder of the Center for Molecular Medicine and Immunology, and its Garden State Cancer Center unit, in Belleville, NJ. Beginning in 1972, when he located to the University of Kentucky Medical Center, Dr. Goldenberg began developing models and preclinical targeting studies with radiolabeled antibodies to carcinoembryonic antigen (CEA), culminating in the first clinical studies on radioimmunodetection of cancer, and then later radioimmunotherapy, two terms he coined for antibody-based scintigraphy and targeted radiation therapy. His group has continued advancing these fields both preclinically and clinically, using a number of cancer targets, such as AFP, PAP, HCG, CSAp, CD22, CD20, CD74, HLA-DR, PAM4 antigen, and EGP-1 (or TROP-2), also studying naked antibody effects and mechanisms of action, as well as toxin- and drug-conjugates. The method of pretargeting has been a major focus of this group, particularly advanced by the authors of this review who developed and advanced the dock-and-lock (DNL) method of producing recombinant, multivalent, bispecific antibodies used in pretargeted radioimmunodetection and radioimmunotherapy. Dr. Goldenberg has been honored for the contributions of this team by The Society of Nuclear Medicine, the Swedish Societies of Oncology and Radiology, the British Radiology Institute, the Indian Association of Nuclear Medicine, The Society for Oncodevelopmental Biology, and the University of Tel Aviv. He is also the founder of Immunomedics, Inc., and IBC Pharmaceuticals, Inc.
References
- 1.Pressman D. The zone of localization of antibodies: The specific localization of antibodies to rat kidney. Cancer. 1949;2:697. doi: 10.1002/1097-0142(194907)2:4<697::aid-cncr2820020416>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 2.Pressman D. Korngold L. The in vivo localization of anti–Wagner-osteogenic-sarcoma antibodies. Cancer. 1953;6:619. doi: 10.1002/1097-0142(195305)6:3<619::aid-cncr2820060319>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 3.Bale WF. Spar IL. Goodland RL. Experimental radiation therapy of tumors with I-131-carrying antibodies to fibrin. Cancer Res. 1960;20:1488. [PubMed] [Google Scholar]
- 4.Goldenberg DM. Hansen HJ. Carcinoembryonic antigen present in human colonic neoplasms serially propagated in hamsters. Science. 1972;175:1117. [PubMed] [Google Scholar]
- 5.Primus FJ. Wang RH. Goldenberg DM, et al. Localization of human GW-39 tumors in hamsters by radiolabeled heterospecific antibody to carcinoembryonic antigen. Cancer Res. 1973;33:2977. [PubMed] [Google Scholar]
- 6.Goldenberg DM. Preston DF. Primus FJ, et al. Photoscan localization of GW-39 tumors in hamsters using radiolabeled anticarcinoembryonic antigen immunoglobulin G. Cancer Res. 1974;34:1. [PubMed] [Google Scholar]
- 7.Primus FJ. Macdonald R. Goldenberg DM, et al. Localization of GW-39 human tumors in hamsters by affinity-purified antibody to carcinoembryonic antigen. Cancer Res. 1977;37:1544. [PubMed] [Google Scholar]
- 8.Goldenberg DM. DeLand F. Kim E, et al. Use of radiolabeled antibodies to carcinoembryonic antigen for the detection and localization of diverse cancers by external photoscanning. NEJM. 1978;298:1384. doi: 10.1056/NEJM197806222982503. [DOI] [PubMed] [Google Scholar]
- 9.Pettit WA. DeLand FH. Bennett SJ, et al. Radiolabeling of affinity-purified goat anti-carcinoembryonic antigen immunoglobulin G with technetium-99m. Cancer Res. 1980;40:3043. [PubMed] [Google Scholar]
- 10.Eckelman WC. Paik CH. Reba RC. Radiolabeling of antibodies. Cancer Res. 1980;40:3036. [PubMed] [Google Scholar]
- 11.Epenetos AA. Nimmon CC. Arklie J, et al. Detection of human cancer in an animal model using radio-labelled tumour-associated monoclonal antibodies. Br J Cancer. 1982;46:1. doi: 10.1038/bjc.1982.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baldwin RW. Pimm MV. Antitumor monoclonal antibodies for radioimmunodetection of tumors and drug targeting. Cancer Metastasis Rev. 1983;2:89. doi: 10.1007/BF00046907. [DOI] [PubMed] [Google Scholar]
- 13.Hnatowich DJ. Layne WW. Childs RL, et al. Radioactive labeling of antibody: A simple and efficient method. Science. 1983;220:613. doi: 10.1126/science.6836304. [DOI] [PubMed] [Google Scholar]
- 14.Goodwin DA. Meares CF. McCall MJ, et al. Chelate conjugates of monoclonal antibodies for imaging lymphoid structures in the mouse. J Nucl Med. 1985;26:493. [PubMed] [Google Scholar]
- 15.Kozak RW. Raubitschek A. Mirzadeh S, et al. Nature of the bifunctional chelating agent used for radioimmunotherapy with yttrium-90 monoclonal antibodies: Critical factors in determining in vivo survival and organ toxicity. Cancer Res. 1989;49:2639. [PubMed] [Google Scholar]
- 16.Hnatowich DJ. Recent developments in the radiolabeling of antibodies with iodine, indium, and technetium. Semin Nucl Med. 1990;20:80. doi: 10.1016/s0001-2998(05)80178-3. [DOI] [PubMed] [Google Scholar]
- 17.Gansow OA. Newer approaches to the radiolabeling of monoclonal antibodies by use of metal chelates. Int J Rad Appl Instrum B. 1991;18:369. doi: 10.1016/0883-2897(91)90063-q. [DOI] [PubMed] [Google Scholar]
- 18.Griffiths GL. Goldenberg DM. Jones AL, et al. Radiolabeling of monoclonal antibodies and fragments with technetium and rhenium. Bioconjug Chem. 1992;3:91. doi: 10.1021/bc00014a001. [DOI] [PubMed] [Google Scholar]
- 19.Deshpande SV. DeNardo SJ. Kukis DL, et al. Yttrium-90-labeled monoclonal antibody for therapy: Labeling by a new macrocyclic bifunctional chelating agent. J Nucl Med. 1990;31:473. [PubMed] [Google Scholar]
- 20.Huston JS. Levinson D. Mudgett-Hunter M, et al. Protein engineering of antibody binding sites: Recovery of specific activity in an anti-digoxin single-chain Fv analogue produced in Escherichia coli. Proc Natl Acad Sci U S A. 1988;85:5879. doi: 10.1073/pnas.85.16.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson S. Bird RE. Construction of single-chain Fv derivatives monoclonal antibodies and their production in Escherichia coli. Meth Enzymol. 1991;203:88. doi: 10.1016/0076-6879(91)03006-3. [DOI] [PubMed] [Google Scholar]
- 22.Pack P. Pluckthun A. Miniantibodies: Use of amphipathic helices to produce functional, flexibly linked dimeric Fv fragments with high avidity in Escherichia coli. Biochemistry. 1992;31:1579. doi: 10.1021/bi00121a001. [DOI] [PubMed] [Google Scholar]
- 23.Yokota T. Milenic DE. Whitlow M, et al. Rapid tumor penetration of a single-chain Fv and comparison with other immunoglobulin forms. Cancer Res. 1992;52:3402. [PubMed] [Google Scholar]
- 24.Pack P. Kujau M. Schroeckh V, et al. Improved bivalent miniantibodies, with identical avidity as whole antibodies, produced by high cell density fermentation of Escherichia coli. Biotechnology (NY) 1993;11:1271. doi: 10.1038/nbt1193-1271. [DOI] [PubMed] [Google Scholar]
- 25.Sharkey RM. Goldenberg DM. Novel radioimmunopharmaceuticals for cancer imaging and therapy. Curr Opin Invest Drugs. 2008;9:1302. [PubMed] [Google Scholar]
- 26.Cortez-Retamozo V. Backmann N. Senter PD, et al. Efficient cancer therapy with a nanobody-based conjugate. Cancer Res. 2004;64:2853. doi: 10.1158/0008-5472.can-03-3935. [DOI] [PubMed] [Google Scholar]
- 27.Revets H. De Baetselier P. Muyldermans S. Nanobodies as novel agents for cancer therapy. Exp Opin Biol Ther. 2005;5:111. doi: 10.1517/14712598.5.1.111. [DOI] [PubMed] [Google Scholar]
- 28.Tijink BM. Laeremans T. Budde M, et al. Improved tumor targeting of anti-epidermal growth factor receptor nanobodies through albumin binding: Taking advantage of modular nanobody technology. Mol Cancer Ther. 2008;7:2288. doi: 10.1158/1535-7163.MCT-07-2384. [DOI] [PubMed] [Google Scholar]
- 29.Vincke C. Loris R. Saerens D, et al. General strategy to humanize a camelid single-domain antibody and identification of a universal humanized nanobody scaffold. J Biol Chem. 2009;284:3273. doi: 10.1074/jbc.M806889200. [DOI] [PubMed] [Google Scholar]
- 30.Wesolowski J. Alzogaray V. Reyelt J, et al. Single domain antibodies: Promising experimental and therapeutic tools in infection and immunity. Med Microbiol Immunol. 2009;198:157. doi: 10.1007/s00430-009-0116-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nord K. Gunneriusson E. Ringdahl J, et al. Binding proteins selected from combinatorial libraries of an alpha-helical bacterial receptor domain. Nat Biotechnol. 1997;15:772. doi: 10.1038/nbt0897-772. [DOI] [PubMed] [Google Scholar]
- 32.Skerra A. “Anticalins”: a new class of engineered ligand-binding proteins with antibody-like properties. J Biotechnol. 2001;74:257. doi: 10.1016/s1389-0352(01)00020-4. [DOI] [PubMed] [Google Scholar]
- 33.Gebauer M. Skerra A. Engineered protein scaffolds as next-generation antibody therapeutics. Curr Opin Chem Biol. 2009;13:245. doi: 10.1016/j.cbpa.2009.04.627. [DOI] [PubMed] [Google Scholar]
- 34.Stumpp MT. Binz HK. Amstutz P. DARPins: A new generation of protein therapeutics. Drug Discov Today. 2008;13:695. doi: 10.1016/j.drudis.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 35.Skerra A. Alternative binding proteins: Anticalins—harnessing the structural plasticity of the lipocalin ligand pocket to engineer novel binding activities. FEBS J. 2008;275:2677. doi: 10.1111/j.1742-4658.2008.06439.x. [DOI] [PubMed] [Google Scholar]
- 36.Nygren PA. Skerra A. Binding proteins from alternative scaffolds. J Immunol Meth. 2004;290:3. doi: 10.1016/j.jim.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 37.Xu L. Aha P. Gu K, et al. Directed evolution of high-affinity antibody mimics using mRNA display. Chem Biol. 2002;9:933. doi: 10.1016/s1074-5521(02)00187-4. [DOI] [PubMed] [Google Scholar]
- 38.Ronnmark J. Hansson M. Nguyen T, et al. Construction and characterization of affibody-Fc chimeras produced in Escherichia coli. J Immunol Meth. 2002;261:199. doi: 10.1016/s0022-1759(01)00563-4. [DOI] [PubMed] [Google Scholar]
- 39.Binz HK. Amstutz P. Kohl A, et al. High-affinity binders selected from designed ankyrin repeat protein libraries. Nat Biotechnol. 2004;22:575. doi: 10.1038/nbt962. [DOI] [PubMed] [Google Scholar]
- 40.Tran TA. Rosik D. Abrahmsén L, et al. Design, synthesis, and biological evaluation of a multifunctional HER2-specific affibody molecule for molecular imaging. Eur J Nucl Med Mol Imaging. 2009;36:1864. doi: 10.1007/s00259-009-1176-z. [DOI] [PubMed] [Google Scholar]
- 41.Tolmachev V. Orlova A. Nilsson FY, et al. Affibody molecules: Potential for in vivo imaging of molecular targets for cancer therapy. Exp Opin Biol Ther. 2007;7:555. doi: 10.1517/14712598.7.4.555. [DOI] [PubMed] [Google Scholar]
- 42.Orlova A. Magnusson M. Eriksson TL, et al. Tumor imaging using a picomolar affinity HER2-binding affibody molecule. Cancer Res. 2006;66:4339. doi: 10.1158/0008-5472.CAN-05-3521. [DOI] [PubMed] [Google Scholar]
- 43.Yan AC. Levy M. Aptamers and aptamer-targeted delivery. RNA Biol. 2009;6:316. doi: 10.4161/rna.6.3.8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tavitian B. Duconge F. Boisgard R, et al. In vivo imaging of oligonucleotidic aptamers. Meth Mol Biol. 2009;535:241. doi: 10.1007/978-1-59745-557-2_15. [DOI] [PubMed] [Google Scholar]
- 45.Bardou C. Borie C. Bickle M, et al. Peptide aptamers for small-molecule drug discovery. Meth Mol Biol. 2009;535:373. doi: 10.1007/978-1-59745-557-2_21. [DOI] [PubMed] [Google Scholar]
- 46.Balhorn R. Hok S. Burke PA, et al. Selective high-affinity ligand antibody mimics for cancer diagnosis and therapy: Initial application to lymphoma/leukemia. Clin Cancer Res. 2007;13:5621s. doi: 10.1158/1078-0432.CCR-07-1128. [DOI] [PubMed] [Google Scholar]
- 47.DeNardo GL. Hok S. Van Natarajan A, et al. Characteristics of dimeric (bis) bidentate selective high-affinity ligands as HLA-DR10 beta antibody mimics targeting non-Hodgkin's lymphoma. Int J Oncol. 2007;31:729. [PubMed] [Google Scholar]
- 48.DeNardo GL. Natarajan A. Hok S, et al. Pharmacokinetic characterization in xenografted mice of a series of first-generation mimics for HLA-DR antibody, Lym-1, as carrier molecules to image and treat lymphoma. J Nucl Med. 2007;48:1338. doi: 10.2967/jnumed.107.041095. [DOI] [PubMed] [Google Scholar]
- 49.DeNardo GL. Mirick GR. Hok S, et al. Molecular specific and cell selective cytotoxicity induced by a novel synthetic HLA-DR antibody mimic for lymphoma and leukemia. Int J Oncol. 2009;34:511. [PubMed] [Google Scholar]
- 50.Goldenberg DM. Gaffar SA. Bennett SJ, et al. Experimental radioimmunotherapy of a xenografted human colonic tumor (GW-39) producing carcinoembryonic antigen. Cancer Res. 1981;41:4354. [PubMed] [Google Scholar]
- 51.Sharkey RM. Pykett MJ. Siegel JA, et al. Radioimmunotherapy of the GW-39 human colonic tumor xenograft with 131I-labeled murine monoclonal antibody to carcinoembryonic antigen. Cancer Res. 1987;47:5672. [PubMed] [Google Scholar]
- 52.Sharkey RM. Motta-Hennessy C. Gansow OA, et al. Selection of a DTPA chelate conjugate for monoclonal antibody targeting to a human colonic tumor in nude mice. Int J Cancer. 1990;46:79. doi: 10.1002/ijc.2910460116. [DOI] [PubMed] [Google Scholar]
- 53.Goldenberg DM. Goldenberg H. Sharkey RM, et al. Clinical studies of cancer radioimmunodetection with carcinoembryonic antigen monoclonal antibody fragments labeled with 123I or 99mTc. Cancer Res. 1990;50:909s. [PubMed] [Google Scholar]
- 54.Griffiths GL. Goldenberg DM. Diril H, et al. Technetium-99m, rhenium-186, and rhenium-188 direct-labeled antibodies. Cancer. 1994;73:761. doi: 10.1002/1097-0142(19940201)73:3+<761::aid-cncr2820731303>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 55.Sharkey RM. Behr TM. Mattes MJ, et al. Advantage of residualizing radiolabels for an internalizing antibody against the B-cell lymphoma antigen, CD22. Cancer Immunol Immunother. 1997;44:179. doi: 10.1007/s002620050371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Govindan SV. Shih LB. Goldenberg DM, et al. 90Yttrium-labeled complementarity-determining-region-grafted monoclonal antibodies for radioimmunotherapy: Radiolabeling and animal biodistribution studies. Bioconjug Chem. 1998;9:773. doi: 10.1021/bc980040g. [DOI] [PubMed] [Google Scholar]
- 57.Juweid M. Sharkey RM. Swayne LC, et al. Pharmacokinetics, dosimetry, and toxicity of rhenium-188-labeled anti-carcinoembryonic antigen monoclonal antibody, MN-14, in gastrointestinal cancer. J Nucl Med. 1998;39:34. [PubMed] [Google Scholar]
- 58.Juweid ME. Hajjar G. Swayne LC, et al. Phase I/II trial of 131I-MN-14F(ab)2 anti-carcinoembryonic antigen monoclonal antibody in the treatment of patients with metastatic medullary thyroid carcinoma. Cancer. 1999;85:1828. doi: 10.1002/(sici)1097-0142(19990415)85:8<1828::aid-cncr25>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 59.Fletcher JW. Djulbegovic B. Soares HP, et al. Recommendations on the use of 18F-FDG PET in oncology. J Nucl Med. 2008;49:480. doi: 10.2967/jnumed.107.047787. [DOI] [PubMed] [Google Scholar]
- 60.Behr TM. Behe M. Stabin MG, et al. High-linear energy transfer (LET) alpha versus low-LET beta emitters in radioimmunotherapy of solid tumors: Therapeutic efficacy and dose-limiting toxicity of 213Bi- versus 90Y-labeled CO17-1A Fab′ fragments in a human colonic cancer model. Cancer Res. 1999;59:2635. [PubMed] [Google Scholar]
- 61.Behr TM. Memtsoudis S. Sharkey RM, et al. Experimental studies on the role of antibody fragments in cancer radioimmunotherapy: Influence of radiation dose and dose rate on toxicity and anti-tumor efficacy. Int J Cancer. 1998;77:787. doi: 10.1002/(sici)1097-0215(19980831)77:5<787::aid-ijc19>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 62.Buchegger F. Pelegrin A. Delaloye B, et al. Iodine-131-labeled MAb F(ab′)2 fragments are more efficient and less toxic than intact anti-CEA antibodies in radioimmunotherapy of large human colon carcinoma grafted in nude mice. J Nucl Med. 1990;31:1035. [PubMed] [Google Scholar]
- 63.Emami B. Lyman J. Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 64.O'Donoghue J. Relevance of external beam dose-response relationships to kidney toxicity associated with radionuclide therapy. Cancer Biother Radiopharm. 2004;19:378. doi: 10.1089/1084978041425025. [DOI] [PubMed] [Google Scholar]
- 65.Barone R. Borson-Chazot F. Valkema R, et al. Patient-specific dosimetry in predicting renal toxicity with 90Y-DOTATOC: Relevance of kidney volume and dose rate in finding a dose-effect relationship. J Nucl Med. 2005;46(Suppl 1):99S. [PubMed] [Google Scholar]
- 66.Valkema R. Pauwels SA. Kvols LK, et al. Long-term follow-up of renal function after peptide receptor radiation therapy with 90Y-DOTA0,Tyr3-octreotide and 177Lu-DOTA0, Tyr3-octreotate. J Nucl Med. 2005;46(Suppl 1):83S. [PubMed] [Google Scholar]
- 67.Bodei L. Cremonesi M. Ferrari M, et al. Long-term evaluation of renal toxicity after peptide receptor radionuclide therapy with 90Y-DOTATOC and 177Lu-DOTATATE: The role of associated risk factors. Eur J Nucl Med Mol Imaging. 2008;35:1847. doi: 10.1007/s00259-008-0778-1. [DOI] [PubMed] [Google Scholar]
- 68.Sharkey RM. Goldenberg DM. Use of antibodies and immunoconjugates for the therapy of more accessible cancers. Adv Drug Deliv Rev. 2008;60:1407. doi: 10.1016/j.addr.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Buchegger F. Press OW. Delaloye AB, et al. Radiolabeled and native antibodies and the prospect of cure of follicular lymphoma. Oncologist. 2008;13:657. doi: 10.1634/theoncologist.2008-0020. [DOI] [PubMed] [Google Scholar]
- 70.Kenanova V. Olafsen T. Crow DM, et al. Tailoring the pharmacokinetics and positron emission tomography imaging properties of anti-carcinoembryonic antigen single-chain Fv-Fc antibody fragments. Cancer Res. 2005;65:622. [PMC free article] [PubMed] [Google Scholar]
- 71.Olafsen T. Kenanova VE. Wu AM. Tunable pharmacokinetics: Modifying the in vivo half-life of antibodies by directed mutagenesis of the Fc fragment. Nat Protoc. 2006;1:2048. doi: 10.1038/nprot.2006.322. [DOI] [PubMed] [Google Scholar]
- 72.Kenanova V. Olafsen T. Williams LE, et al. Radioiodinated versus radiometal-labeled anti-carcinoembryonic antigen single-chain Fv-Fc antibody fragments: Optimal pharmacokinetics for therapy. Cancer Res. 2007;67:718. doi: 10.1158/0008-5472.CAN-06-0454. [DOI] [PubMed] [Google Scholar]
- 73.DeNardo GL. Kroger LA. Meares CF, et al. Comparison of 1,4,7,10-tetraazacyclododecane-N,N′,N″,N‴-tetraacetic acid (DOTA)-peptide-ChL6, a novel immunoconjugate with catabolizable linker, to 2-iminothiolane-2-[p-(bromoacetamido)benzyl]-DOTA-ChL6 in breast cancer xenografts. Clin Cancer Res. 1998;4:2483. [PubMed] [Google Scholar]
- 74.Peterson JJ. Meares CF. Cathepsin substrates as cleavable peptide linkers in bioconjugates, selected from a fluorescence quench combinatorial library. Bioconjug Chem. 1998;9:618. doi: 10.1021/bc980059j. [DOI] [PubMed] [Google Scholar]
- 75.Peterson JJ. Meares CF. Enzymatic cleavage of peptide-linked radiolabels from immunoconjugates. Bioconjug Chem. 1999;10:553. doi: 10.1021/bc990010t. [DOI] [PubMed] [Google Scholar]
- 76.Behr TM. Goldenberg DM. Becker W. Reducing the renal uptake of radiolabeled antibody fragments and peptides for diagnosis and therapy: Present status, future prospects, and limitations. Eur J Nucl Med. 1998;25:201. doi: 10.1007/s002590050216. [DOI] [PubMed] [Google Scholar]
- 77.van Eerd JE. Vegt E. Wetzels JF, et al. Gelatin-based plasma expander effectively reduces renal uptake of 111In-octreotide in mice and rats. J Nucl Med. 2006;47:528. [PubMed] [Google Scholar]
- 78.Vegt E. van Eerd JE. Eek A, et al. Reducing renal uptake of radiolabeled peptides using albumin fragments. J Nucl Med. 2008;49:1506. doi: 10.2967/jnumed.108.053249. [DOI] [PubMed] [Google Scholar]
- 79.Stahl AR. Wagner B. Poethko T, et al. Renal accumulation of 111In-DOTATOC in rats: Influence of inhibitors of the organic ion transport and diuretics. Eur J Nucl Med Mol Imaging. 2007;34:2129. doi: 10.1007/s00259-007-0519-x. [DOI] [PubMed] [Google Scholar]
- 80.Goldenberg DM. Sharkey RM. Ford E. Anti-antibody enhancement of iodine-131 anti-CEA radioimmunodetection in experimental and clinical studies. J Nucl Med. 1987;28:1604. [PubMed] [Google Scholar]
- 81.Chen JQ. Strand SE. Tennvall J, et al. Extracorporeal immunoadsorption compared to avidin chase: Enhancement of tumor-to-normal tissue ratio for biotinylated rhenium-188-chimeric BR96. J Nucl Med. 1997;38:1934. [PubMed] [Google Scholar]
- 82.Linden O. Kurkus J. Garkavij M, et al. A novel platform for radioimmunotherapy: Extracorporeal depletion of biotinylated and 90Y-labeled rituximab in patients with refractory B-cell lymphoma. Cancer Biother Radiopharm. 2005;20:457. doi: 10.1089/cbr.2005.20.457. [DOI] [PubMed] [Google Scholar]
- 83.Reardon DT. Meares CF. Goodwin DA, et al. Antibodies against metal chelates. Nature. 1985;316:265. doi: 10.1038/316265a0. [DOI] [PubMed] [Google Scholar]
- 84.Abdel-Nabi HH. Schwartz AN. Goldfogel G, et al. Colorectal tumors: Scintigraphy with In-111 anti-CEA monoclonal antibody and correlation with surgical, histopathologic, and immunohistochemical findings. Radiology. 1988;166:747. doi: 10.1148/radiology.166.3.3277244. [DOI] [PubMed] [Google Scholar]
- 85.Abdel-Nabi HH. Schwartz AN. Higano CS, et al. Colorectal carcinoma: Detection with indium-111 anticarcinoembryonic-antigen monoclonal antibody ZCE-025. Radiology. 1987;164:617. doi: 10.1148/radiology.164.3.3303117. [DOI] [PubMed] [Google Scholar]
- 86.Halpern SE. Haindl W. Beauregard J, et al. Scintigraphy with In-111-labeled monoclonal antitumor antibodies: Kinetics, biodistribution, and tumor detection. Radiology. 1988;168:529. doi: 10.1148/radiology.168.2.3393677. [DOI] [PubMed] [Google Scholar]
- 87.Stickney DR. Anderson LD. Slater JB, et al. Bifunctional antibody: A binary radiopharmaceutical delivery system for imaging colorectal carcinoma. Cancer Res. 1991;51:6650. [PubMed] [Google Scholar]
- 88.Le Doussal JM. Martin M. Gautherot E, et al. In vitro and in vivo targeting of radiolabeled monovalent and divalent haptens with dual specificity monoclonal antibody conjugates: Enhanced divalent hapten affinity for cell-bound antibody conjugate. J Nucl Med. 1989;30:1358. [PubMed] [Google Scholar]
- 89.Moffat FL., Jr. Pinsky CM. Hammershaimb L, et al. Clinical utility of external immunoscintigraphy with the IMMU-4 technetium-99m Fab′ antibody fragment in patients undergoing surgery for carcinoma of the colon and rectum: Results of a pivotal, phase III trial. The Immunomedics Study Group. J Clin Oncol. 1996;14:2295. doi: 10.1200/JCO.1996.14.8.2295. [DOI] [PubMed] [Google Scholar]
- 90.Karacay H. Brard PY. Sharkey RM, et al. Therapeutic advantage of pretargeted radioimmunotherapy using a recombinant bispecific antibody in a human colon cancer xenograft. Clin Cancer Res. 2005;11:7879. doi: 10.1158/1078-0432.CCR-05-1246. [DOI] [PubMed] [Google Scholar]
- 91.Axworthy DB. Reno JM. Hylarides MD, et al. Cure of human carcinoma xenografts by a single dose of pretargeted yttrium-90 with negligible toxicity. Proc Natl Acad Sci U S A. 2000;97:1802. doi: 10.1073/pnas.97.4.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Barbet J. Kraeber-Bodere F. Vuillez JP, et al. Pretargeting with the affinity enhancement system for radioimmunotherapy. Cancer Biother Radiopharm. 1999;14:153. doi: 10.1089/cbr.1999.14.153. [DOI] [PubMed] [Google Scholar]
- 93.Gautherot E. Bouhou J. Le Doussal JM, et al. Therapy for colon carcinoma xenografts with bispecific antibody-targeted, iodine-131-labeled bivalent hapten. Cancer. 1997;80:2618. doi: 10.1002/(sici)1097-0142(19971215)80:12+<2618::aid-cncr37>3.3.co;2-p. [DOI] [PubMed] [Google Scholar]
- 94.Gautherot E. Rouvier E. Daniel L, et al. Pretargeted radioimmunotherapy of human colorectal xenografts with bispecific antibody and 131I-labeled bivalent hapten. J Nucl Med. 2000;41:480. [PubMed] [Google Scholar]
- 95.Sharkey RM. Karacay H. Johnson CR, et al. Pretargeted versus directly targeted radioimmunotherapy combined with anti-CD20 antibody consolidation therapy of non-Hodgkin's lymphoma. J Nucl Med. 2009;50:444. doi: 10.2967/jnumed.108.058602. [DOI] [PubMed] [Google Scholar]
- 96.Sharkey RM. Karacay H. Litwin S, et al. Improved therapeutic results by pretargeted radioimmunotherapy of non-Hodgkin's lymphoma with a new recombinant, trivalent, anti-CD20, bispecific antibody. Cancer Res. 2008;68:5282. doi: 10.1158/0008-5472.CAN-08-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pagel JM. Hedin N. Drouet L, et al. Eradication of disseminated leukemia in a syngeneic murine leukemia model using pretargeted anti-CD45 radioimmunotherapy. Blood. 2008;111:2261. doi: 10.1182/blood-2007-06-097451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Press OW. Corcoran M. Subbiah K, et al. A comparative evaluation of conventional and pretargeted radioimmunotherapy of CD20-expressing lymphoma xenografts. Blood. 2001;98:2535. doi: 10.1182/blood.v98.8.2535. [DOI] [PubMed] [Google Scholar]
- 99.Axworthy DB. Fritzberg AR. Hylarides MD, et al. Preclinical evaulation of an anti-tumor monoclonal antibody/strep-tavidin conjugate for pretargeted 90Y radioimmunotherapy in a mouse xenograft model. J Immunother. 1994;16:158. [Google Scholar]
- 100.Sharkey RM. Karacay H. Griffiths GL, et al. Development of a streptavidin-anti-carcinoembryonic antigen antibody, radiolabeled biotin pretargeting method for radioimmunotherapy of colorectal cancer. Studies in a human colon cancer xenograft model. Bioconjug Chem. 1997;8:595. doi: 10.1021/bc970101v. [DOI] [PubMed] [Google Scholar]
- 101.Karacay H. McBride WJ. Griffiths GL, et al. Experimental pretargeting studies of cancer with a humanized anti-CEA x murine anti-[In-DTPA] bispecific antibody construct and a 99mTc-/188Re-labeled peptide. Bioconjug Chem. 2000;11:842. doi: 10.1021/bc0000379. [DOI] [PubMed] [Google Scholar]
- 102.Karacay H. Sharkey RM. McBride WJ, et al. Pretargeting for cancer radioimmunotherapy with bispecific antibodies: Role of the bispecific antibody's valency for the tumor target antigen. Bioconjug Chem. 2002;13:1054. doi: 10.1021/bc0200172. [DOI] [PubMed] [Google Scholar]
- 103.Hosono M. Hosono MN. Kraeber-Bodere F, et al. Two-step targeting and dosimetry for small-cell lung cancer xenograft with anti-NCAM/antihistamine bispecific antibody and radioiodinated bivalent hapten. J Nucl Med. 1999;40:1216. [PubMed] [Google Scholar]
- 104.Janevik-Ivanovska E. Gautherot E. Hillairet de Boisferon M, et al. Bivalent hapten-bearing peptides designed for iodine-131 pretargeted radioimmunotherapy. Bioconjug Chem. 1997;8:526. doi: 10.1021/bc970083h. [DOI] [PubMed] [Google Scholar]
- 105.Sharkey RM. McBride WJ. Karacay H, et al. A universal pretargeting system for cancer detection and therapy using bispecific antibody. Cancer Res. 2003;63:354. [PubMed] [Google Scholar]
- 106.Sharkey RM. Juweid M. Shevitz J, et al. Evaluation of a complementarity-determining region-grafted (humanized) anti-carcinoembryonic antigen monoclonal antibody in preclinical and clinical studies. Cancer Res. 1995;55:5935s. [PubMed] [Google Scholar]
- 107.Morel A. Darmon M. Delaage M. Recognition of imidazole and histamine derivatives by monoclonal antibodies. Mol Immunol. 1990;27:995. doi: 10.1016/0161-5890(90)90122-g. [DOI] [PubMed] [Google Scholar]
- 108.Rossi EA. Sharkey RM. McBride W, et al. Development of new multivalent-bispecific agents for pretargeting tumor localization and therapy. Clin Cancer Res. 2003;9:3886S. [PubMed] [Google Scholar]
- 109.Rossi EA. Chang CH. Losman MJ, et al. Pretargeting of carcinoembryonic antigen-expressing cancers with a trivalent bispecific fusion protein produced in myeloma cells. Clin Cancer Res. 2005;11:7122s. doi: 10.1158/1078-0432.CCR-1004-0020. [DOI] [PubMed] [Google Scholar]
- 110.Schoonjans R. Willems A. Schoonooghe S, et al. Fab chains as an efficient heterodimerization scaffold for the production of recombinant bispecific and trispecific antibody derivatives. J Immunol. 2000;165:7050. doi: 10.4049/jimmunol.165.12.7050. [DOI] [PubMed] [Google Scholar]
- 111.Rossi EA. Goldenberg DM. Cardillo TM, et al. Stably tethered multifunctional structures of defined composition made by the dock-and-lock method for use in cancer targeting. Proc Natl Acad Sci U S A. 2006;103:6841. doi: 10.1073/pnas.0600982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Baillie GS. Scott JD. Houslay MD. Compartmentalisation of phosphodiesterases and protein kinase A: Opposites attract. FEBS Lett. 2005;579:3264. doi: 10.1016/j.febslet.2005.03.089. [DOI] [PubMed] [Google Scholar]
- 113.Wong W. Scott JD. AKAP signalling complexes: Focal points in space and time. Nat Rev Mol Cell Biol. 2004;5:959. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- 114.Sharkey RM. Karacay H. Vallabhajosula S, et al. Metastatic human colonic carcinoma: Molecular imaging with pretargeted SPECT and PET in a mouse model. Radiology. 2008;246:497. doi: 10.1148/radiol.2462070229. [DOI] [PubMed] [Google Scholar]
- 115.McBride WJ. Sharkey RM. Karacay H, et al. A novel method of 18F radiolabeling for PET. J Nucl Med. 2009;50:991. doi: 10.2967/jnumed.108.060418. [DOI] [PubMed] [Google Scholar]
- 116.Schoffelen R. Sharkey RM. Goldenberg DM, et al. Pretargeted immunoPET imaging of CEA-expressing tumors with a bispecific antibody and a 68Ga- and 18F-labeled hapten-peptide in mice with human tumor xenografts. Mol Cancer Ther. 2010 doi: 10.1158/1535-7163.MCT-09-0862. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gold DV. Goldenberg DM. Karacay H, et al. A novel bispecific, trivalent antibody construct for targeting pancreatic carcinoma. Cancer Res. 2008;68:4819. doi: 10.1158/0008-5472.CAN-08-0232. [DOI] [PubMed] [Google Scholar]
- 118.Chang CH. Rossi EA. Goldenberg DM. The dock-and-lock method: A novel platform technology for building multivalent, multifunctional structures of defined composition with retained bioactivity. Clin Cancer Res. 2007;13:5586s. doi: 10.1158/1078-0432.CCR-07-1217. [DOI] [PubMed] [Google Scholar]
- 119.Goldenberg DM. Rossi EA. Sharkey RM, et al. Multifunctional antibodies by the dock-and-lock method for improved cancer imaging and therapy by pretargeting. J Nucl Med. 2008;49:158. doi: 10.2967/jnumed.107.046185. [DOI] [PubMed] [Google Scholar]
- 120.Rossi EA. Goldenberg DM. Cardillo TM, et al. Novel designs of multivalent anti-CD20 humanized antibodies as improved lymphoma therapeutics. Cancer Res. 2008;68:8384. doi: 10.1158/0008-5472.CAN-08-2033. [DOI] [PubMed] [Google Scholar]
- 121.Goldenberg DM. Rossi EA. Stein R, et al. Properties and structure-function relationships of veltuzumab (hA20), a humanized anti-CD20 monoclonal antibody. Blood. 2009;113:1062. doi: 10.1182/blood-2008-07-168146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Leonard JP. Goldenberg DM. Preclinical and clinical evaluation of epratuzumab (anti-CD22 IgG) in B-cell malignancies. Oncogene. 2007;26:3704. doi: 10.1038/sj.onc.1210370. [DOI] [PubMed] [Google Scholar]
- 123.Goldenberg DM. Epratuzumab in the therapy of oncological and immunological diseases. Exp Rev Anticancer Ther. 2006;6:1341. doi: 10.1586/14737140.6.10.1341. [DOI] [PubMed] [Google Scholar]
- 124.Rossi EA. Goldenberg DM. Cardillo TM, et al. Hexavalent bispecific antibodies represent a new class of anticancer therapeutics: 1. Properties of anti-CD20/CD22 antibodies in lymphoma. Blood. 2009;113:6161. doi: 10.1182/blood-2008-10-187138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rossi EA. Goldenberg DM. Cardillo TM, et al. CD20-targeted tetrameric interferon-alpha, a novel and potent immunocytokine for the therapy of B-cell lymphomas. Blood. 2009;114:3864. doi: 10.1182/blood-2009-06-228890. [DOI] [PMC free article] [PubMed] [Google Scholar]