Abstract
The heat shock protein Hsp90 has been the focus of many studies since it was suggested that it acts to mediate the buffering of phenotypic variation. Hsp90-mediated buffering may result in the accumulation of cryptic genetic variation that, when released either as a consequence of environmental or genetic stress, increases the evolvability of a population. Recent studies using laboratory-induced mutations of Hsp90 and/or chemical inhibition to disrupt Hsp90 function confirm that Hsp90 can buffer cryptic genetic variation. We have previously identified a naturally occurring variant in the charged linker region of the Hsp90 gene, and now examine whether this variant is associated with altered levels of trait variability. The variant is associated with the release of cryptic genetic variation for canalized morphological (bristle) traits, but not for uncanalized morphological (wing and bristle) traits, and the effect on canalized traits depends on culture temperature. This suggests that natural genetic variation in Hsp90 may mediate the evolution of canalized morphological traits even if it does not influence the expression of variation for uncanalized traits.
Keywords: Hsp90, canalization, cryptic genetic variation, natural variation
1. Introduction
The origin and maintenance of phenotypic variation is a central theme in evolutionary biology, because adaptive phenotypic evolution depends on heritable variation in phenotypes. One of the more intriguing observations is that wild-type phenotypes are robust to much environmental and genetic variation (Sangster et al. 2008a; McGuigan & Sgrò 2009). Waddington (1942) coined the term canalization to describe this phenotypic robustness; individuals with different genotypes have the same phenotype (or a limited set of phenotypes), which is expressed despite variation in the environment. This contrasts with uncanalized traits that vary in a continuous manner. A key consequence of canalization is the build up of cryptic genetic variation that exists in the genome but is not expressed phenotypically. Yet, in spite of this, organisms show remarkable levels of phenotypic variation. Waddington was among the first researchers to articulate a link between environmental stress, the expression of novel phenotypes and evolvability, the ability of a population to respond to selection. Stressful or novel environments are predicted to disrupt canalization, resulting in the release of cryptic phenotypic and genetic variation, thereby increasing the evolvability of populations. Interest in the evolutionary significance of canalization and cryptic genetic variation increased dramatically following the identification of a possible mechanistic link between canalization and environmental stress through the heat shock protein Hsp90 (Rutherford & Lindquist 1998).
Several groups have found that a reduction of Hsp90 function releases cryptic genetic variation in a range of morphological traits in diverse taxa. Studies in Drosophila have demonstrated release of cryptic genetic variation for canalized traits, but not necessarily for uncanalized traits (Rutherford & Lindquist 1998; Milton et al. 2006; Kellermann et al. 2007) although there are exceptions (Debat et al. 2006). These studies suggest differences in the control of genetic variation for canalized versus (some) uncanalized traits, at least in Drosophila (McGuigan & Sgrò 2009). In addition, manipulation of Hsp90 in Arabidopsis and Danio has been shown to release cryptic genetic variation in uncanalized as well as canalized traits, although effects are dependent on genetic background (Yeyati et al. 2007; Sangster et al. 2008b). Taken together, results from a diverse literature suggest a complex interplay between Hsp90 function, genetic background, environmental conditions and the release of cryptic genetic variation particularly for continuously varying quantitative traits likely to contribute to fitness (McGuigan & Sgrò 2009).
Laboratory studies on Hsp90 impairment provide important insight into the mechanisms that underlie the regulation of phenotypic variability, but do not directly address whether Hsp90 mediates the regulation of phenotypic variability in nature. Mutations derived by laboratory mutagenesis are expected to have more dramatic phenotypic effects than naturally segregating variation (van der Straten et al. 1997), and RNAi disruption of Hsp90 (Sangster et al. 2008a) likewise may have effects on Hsp90 expression and functioning much larger than those found in nature. It is not known if natural variants in Hsp90 or genes involved in its regulation might exert effects on phenotypic variability in wild populations. Several studies have detected variation in levels of gene expression of Hsp90 in response to changes in environmental conditions in natural populations (e.g. Cara et al. 2005; Chen et al. 2005; Hayward et al. 2005), but these studies have not considered correlated patterns of phenotypic variability, nor considered Hsp90 function. Kohler et al. (2009) detected changes in Hsp90 protein levels in response to thermal stress in terrestrial snails, but found no association between morphological variability and Hsp90 protein levels.
Hsp90 exhibits a high level of sequence conservation across animal and plant taxa (Gupta 1995; Pepin et al. 2001), and few studies have so far focused on intra-specific variation. In a recent screen of the Hsp90 gene in natural populations of Drosophila melanogaster from eastern Australia, we (Sgrò et al. 2008) found two nonsynonymous changes in the coding region of Hsp90, both of which are deletions of a lysine residue. One lysine residue deletion, at amino acid position 234, has previously been reported in multiple lines of D. melanogaster (Hasson & Eanes 1996), while the second lysine residue deletion has only been found in one previously published sequence (Shapiro et al. 2007). The deletion at amino acid position 234 is in complete linkage disequilibrium with the cosmopolitan inversion 3(L)Payne, but the second deletion at amino acid position 254 is not (Sgrò et al. 2008). The deletion at amino acid position 234 shows a strong linear latitudinal cline, reflecting its linkage with In3(L)P, which also displays clinal changes in frequency along the eastern coast of Australia. The second deletion at amino acid position 254 does not exhibit clinal variation (Sgrò et al. 2008).
The deletion at amino acid position 254 occurs within the charged linker region of the Hsp90 protein. This charged region is important for peptide binding to the N-terminal of Hsp90 (Scheibel et al. 1999) and regulation by co-chaperones (Hainzl et al. 2009), although its disruption can be rescued by partial sequences (Hainzl et al. 2009). Small deletions in the region might therefore have subtle effects on Hsp90 function. We test this hypothesis using both canalized and uncanalized traits in lines derived from a natural D. melanogaster population.
2. Material and methods
(a). Field collections
One thousand field-inseminated females were collected from Wandin (approx. 60 km northeast of Melbourne) in May 2006. They were returned to the laboratory, where each female was placed individually into a vial and allowed to lay for 4 days, at which time she was transferred to a fresh vial. Once larval development was observed within each of these vials, the females were placed at −80°C until DNA was extracted for genotyping (see below).
(b). Screening for lysine deletions
DNA was extracted from the field-collected females following the Chelex extraction protocol, and all females were genotyped for both lysine deletions. The forward and reverse primers for the deletion at amino acid position 234 were 5′-TATCGGTATG-3′ and 5′-CAAAATCGAGGATG-3′, respectively, while the forward and reverse primers for the deletion at amino acid position 254 were 5′-ATGAGGATGCCGACAAG-3′ and 5′-GGACCTCATTCCAGAGTAC-3′, respectively. The forward primers were labelled with radioisotope P33, and the PCR product run on a 5 per cent polyacrylamide gel at 65 W for 3–4 h.
One hundred of the original 1000 field females were heterozygous for the deletion at amino acid position 254. None of these females contained the lysine deletion at amino acid position 234. Only one of the 1000 field females was found to be homozygous for the deletion at amino acid position 254, suggesting that this mutation does have fitness consequences in nature.
(c). Generation of experimental lines
The F1 progeny of the 100 isofemale lines heterozygous for the lysine deletion allele at amino acid position 254 (referred to as the ‘deletion allele’ throughout the remainder of the text, as opposed to the ‘non-deletion’ allele) were used to initiate pair-wise matings between lines. Multiple females from each of the heterozygous lines were individually crossed to a male from a different heterozygous line. Female/male pairs were placed in vials until larval development was observed. The female/male pair was then placed at −80°C for a day after which time their DNA was extracted using the Chelex extraction protocol.
Flies were genotyped for the presence of the deletion as described above. The offspring of any pair matings that involved heterozygous or homozygous females/males for the deletion were retained and used in a second round of pair-wise matings (as described above), and again lines were selected whose parents were heterozygous and homozygous for the deletion. By selecting lines based on the genotype of parents, we were able to isolate lines that were homozygous for the deletion and lines that were homozygous for the non-deletion allele. By carefully noting the pedigree of these pair matings, we were able to ensure that each of the resulting homozygous deletion and non-deletion lines were independent of each other (i.e. were not related). Thus, although the lines were derived from the same natural outbred population, they represent independent replicates of the two genotypes with respect to the Hsp90 locus. Importantly, since all resulting homozygous non-deletion and deletion lines were subject to exactly the same crossing scheme, and were derived from the same outbred population, any differences evident between these lines (i.e.genotypes) can be attributed to associations with the two allele classes (non-deletion or deletion) rather than differences in residual genetic variation between them.
In total, 28 deletion homozygous lines and 28 non-deletion homozygous lines were isolated. Finally, heterozygous individuals (with different genetic backgrounds) were generated by performing single crosses between each homozygous deletion and one other homozygous non-deletion line, resulting in a total of 56 lines because these crosses were done reciprocally. We call these heterozygous lines because we only tested individuals at the F1 when they were all heterozygous for the deletion.
(d). Linkage disequilibrium between the lysine deletion and additional markers
To check that any phenotypic effects of the deletion were not due to linkage with another gene, we genotyped all homozygous deletion and non-deletion lines for an additional five markers in close proximity to the Hsp90 locus (see the electronic supplementary material, table S1). Five females from each line were screened for each of these markers, and the extent of linkage disequilibrium analysed. Initial attempts to estimate linkage disequilibrium between the markers and Hsp90 calculating D and Hedrick's D′ (D/Dmax) resulted in unrealistically high estimate of D′. This was because of low allele frequencies and few possible haplotypes (Umina et al. 2005; Sgrò et al. 2008) in addition to unknown gametic phase. The D values are therefore not displayed. To test for linkage disequilibrium between Hsp90 and the additional markers we therefore performed a Pearson's ρ correlation analysis between all markers on arcsine square root transformed allele frequencies. The most common allele for each marker was used in this analysis (Umina et al. 2005).
(e). Hsp90 gene expression levels
We sequenced the promoter region of Hsp90 of the parental homozygous deletion and non-deletion lines and found that none of them were polymorphic. These results indicated that there was no reason to expect the lines used in the experiments described to differ in Hsp90 gene expression, and by extension Hsp90 protein levels. However, to test for any differences in gene expression we also performed quantitative real-time PCR on 3rd instar larvae from the deletion and non-deletion homozygous lines reared at both 25°C and 29°C. To obtain larvae for this assay, females from each line were allowed to oviposit for 2 h on plastic spoons filled with a yeast–treacle–semolina medium covered with yeast paste to stimulate oviposition. Eggs were then transferred into six replicate vials, 100 eggs per vial, for each line. One set of three replicate vials was placed at 25°C and the other set at 29°C and 60 staged 3rd instar larvae from each replicate vial were snap frozen using liquid nitrogen, and then stored at −80°C until RNA extraction.
RNA was extracted from all samples and 2 µg converted to cDNA using random primers (Invitrogen) and Bioscript RT (Bioline). Quantitative real-time PCR runs were performed on the Rotor-Gene 6000 (Corbett Lifescience) using Sensi-mix reaction mixture (Quantace) and gene-specific primers for Hsp90 (F-5′-CAT ACA AGA TGC CAG AAG AAG C-3′, R-5′-TGG GGT CAG TAA GGG ACT CA-3′) and the internal normalizer gene cyclin k (F-5′-GAG CAT CCTTAC ACC TTT CTC CT-3′, R-5′-TAA TCT CCG GCT CCC ACT G-3′). Levels of Hsp90 transcript relative to cyclin k were determined in all lines and expressed linearly with the mean value across the lines equal to zero.
(f). Traits scored
Two canalized and two uncanalized traits were examined. Scutellar and thoracic bristles are canalized (largely invariant) in D. melanogaster (Milton et al. 2003), while sternopleural bristles and wing area are uncanalized in this species, displaying continuous variation. These traits have previously been used to examine the effects of laboratory-derived Hsp90 mutations and chemical inhibitors of Hsp90 function on phenotypic buffering in Drosophila (Milton et al. 2003, 2005; Kellermann et al. 2007). We therefore chose these traits as a means of assessing the extent to which the deletion at amino acid position 254 similarly affected the expression of phenotypic variation of these traits. The canalized number of scutellar and thoracic bristles is 4 and 22, respectively. The number of bristles was scored for both the left and right sides of each fly and then combined. We were interested specifically in examining the effect of the deletion on the phenotypic variance among individuals, not within individuals, so we did not examine any of the traits for asymmetry. This is because phenotypic evolution occurs at the level of the population and depends on heritable phenotypic variation among individuals of a population. In addition, previous work (Milton et al. 2003) has shown that Hsp90 has little effect on the asymmetry of these traits. To score variation in body size, the left wing was removed with fine forceps and mounted on glass slides with double-sided tape, and protected with a coverslip. Wing images were captured with a Wild M3 dissector microscope attached to a digital camera. Wings were landmarked for the eight junctions of the longitudinal veins with the wing margins or cross-veins, and their x and y coordinates were recorded using the program TPSDIG v. 1.31 (written by F. J. Rohlf, Department of Ecology and Evolution, Stony Brook, NY, USA). Body size was measured as wing centroid size, calculated as the square root of the sum of squared inter-landmark distances (Hoffmann & Shirriffs 2002).
(g). Experimental conditions
Flies were reared at two experimental temperatures, constant 25°C and 29°C, since early work by Rutherford & Lindquist (1998) demonstrated an effect of temperature stress (30°C) on Hsp90 mediated buffering. We chose to use 29°C since this temperature reflects the upper limit at which populations can be continuously cultured (David et al. 1983). Females from each line were allowed to oviposit for 12 h on spoons filled with a yeast–treacle–semolina medium covered with yeast paste to stimulate oviposition. Eggs were then transferred into six replicate vials, 100 eggs per vial, for each line. One set of three replicate vials were placed at 25°C and the other set at 29°C to complete development. All flies emerging from these vials were frozen and scored for the traits described above. From each replicate vial, 20 females and 20 males were selected at random and scored for all traits.
(h). Statistical analyses
Preliminary analyses indicated that the reciprocal heterozygote crosses did not differ from each other (see the electronic supplementary material, table S2), so they were combined for all analyses described below. Different analyses were used for the canalized and uncanalized traits.
(i). Uncanalized traits
For these traits (sternopleural bristles and wing centroid size), the effects of genotype (deletion homozygous, non-deletion homozygous or heterozygous) and line within each genotype on the mean phenotype were tested using a nested one-way analysis of variance with genotype as a fixed factor, and line nested within genotype as a random variable. Separate ANOVAs were performed for the 25°C and 29°C treatments, and sexes were analysed separately.
To test for effects of temperature and genotype on the phenotypic variability of these traits, we performed Levene's test on log-transformed data following Dworkin (2005).
(ii). Canalized traits
For the canalized bristle traits (scutellar and thoracic bristles), each genotype was divided into two groups: the number of flies that exhibited the canalized phenotype and the number of flies that had less than the canalized phenotype (Milton et al. 2005). This was done for flies reared at 25°C and 29°C separately. To determine the effect of genotype on the proportion of flies exhibiting the common canalized phenotype or uncommon decanalized phenotype, chi-square contingency tests (Milton et al. 2005) were performed comparing the number of flies in the different classes across the three genotypes (deletion homozygous, non-deletion homozygous and heterozygous). Chi-square contingency tests were also performed to determine the extent to which any effects were background/strain-dependent by comparing the number of individuals in these two classes across all lines within each genotype. Low numbers of individuals in cells for this part of the analysis meant that the probabilities based on the chi-square distribution were not valid; significance levels were therefore determined by permutation using SPSS (Milton et al. 2005).
(iii). Response to selection
To determine whether any decanalization associated with the lysine deletion was due to the release of cryptic genetic variation, we performed selection on the decanalized scutellar and thoracic bristle phenotypes in non-deletion homozygous and lysine deletion homozygous populations. Selection was initiated by first combining deletion homozygous lines to form a mass-bred population homozygous for the deletion. A mass-bred population homozygous for the common (non-deletion) allele was also initiated by combining replicate homozygous lines. From these mass-bred populations, we initiated six replicate deletion homozygous and six replicate non-deletion homozygous selection lines. Owing to logistical requirements of performing such a selection experiment, selection was only performed at 29°C. For the first generation of selection, 2000 females and 2000 males from each line were screened for both decanalized scutellar and thoracic bristle phenotypes (again due to the logistical requirements associated with an experiment of this size). The minimum number of individuals with the decanalized phenotypes was 28 in one of the selection lines homozygous for the common allele, so all replicate lines were subsequently initiated with 28 individuals (14 females and 14 males). Every generation thereafter, 2000 females and 2000 males were screened for the decanalized scutellar and thoracic bristle phenotypes, and those displaying decanalized phenotypes were chosen for the next generation. The decanalized phenotypes in several non-deletion and deletion replicate lines were lost during the selection process, reflecting the very low frequency of occurrence of these bristle phenotypes perhaps in combination with environmental changes affecting the expression of these phenotypes across generations. Selection response was regressed against generation for all replicate selection lines, and combined probability tests (Sokal & Rohlf 1981) were performed to test for a significant response to selection in the deletion versus non-deletion lines.
3. Results
(a). Canalized traits
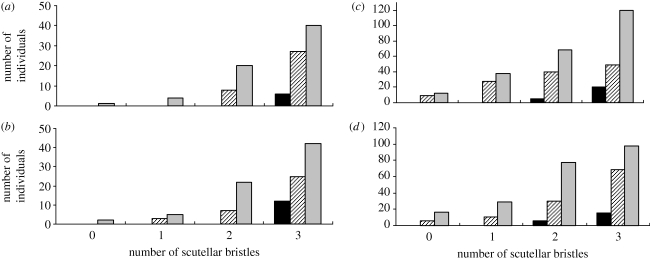
The number of flies within each genotype displaying the decanalized scutellar bristle phenotypes at 25°C and 29°C is shown in figure 1. The total number of flies scored for all genotypes was equal, so we have only shown the proportion of individuals within each decanalized class for simplicity. Genotype had a significant effect on the number of flies displaying the decanalized scutellar bristle phenotype; the deletion homozygous lines had significantly more flies displaying this phenotype at 25°C (females: χ2 = 13.56, d.f. = 6, p = 0.036; males: χ2 = 13.60, d.f. = 6, p = 0.034) and 29°C (females: χ2 = 22.00, d.f. = 6, p = 0.001; males: χ2 = 16.04, d.f. = 6, p = 0.014). Exposure to the higher temperature of 29°C significantly increased the proportion of flies displaying the decanalized scutellar phenotype in both females and males across all genotypes (figure 1), which corresponds with the earlier results by Rutherford & Lindquist (1998).
Figure 1.
Number of flies within each genotype displaying the decanalized scutellar bristle phenotypes at 25°C ((a) females, (b) males) and 29°C ((c) females, (d) males). Deletion homozygous genotype has significantly more flies displaying the decanalized phenotype at 25°C and 29°C in both sexes (black bar, non-deletion homozygous; striped bar, heterozygous; grey bar, deletion homozygous).
Within each genotype, the number of individuals displaying the decanalized scutellar phenotype varied among the heterozygous lines and deletion homozygous lines at 25°C (see the electronic supplementary material, table S3). At 29°C, the number of individuals displaying the decanalized scutellar phenotype varied among the non-deletion lines in females only and also among the deletion homozygous lines (see the electronic supplementary material, table S3).
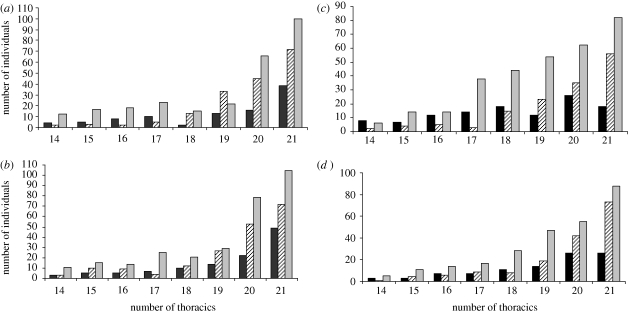
The number of flies within each genotype displaying the decanalized thoracic bristle phenotypes at 25°C and 29°C is shown in figure 2. The total number of flies scored for all genotypes was equal, so we have only shown the proportion of individuals within each decanalized class for simplicity. Genotype had a significant effect on the number of flies displaying the decanalized thoracic bristle phenotype; the deletion homozygotes had significantly more flies displaying the decanalized thoracic bristle phenotype in females (χ2 = 83.68, d.f. = 14, p < 0.001) and males (χ2 = 62.04, d.f. = 14, p < 0.001) at 25°C, but only for females at 29°C (χ2 = 54.57, d.f. = 14, p = 0.0001). In contrast to scutellar bristles, the proportion of flies exhibiting the decanalized thoracic phenotype was marginally lower at 29°C compared with 25°C (figure 2).
Figure 2.
Number of flies within each genotype displaying the decanalized thoracic bristle phenotypes at 25°C ((a) females, (b) males) and 29°C ((c) females, (d) males). Deletion homozygous genotype has significantly more flies displaying the decanalized phenotype at 25°C (females and males) and at 29°C (females only). Black bar, non-deletion homozygous; striped bar, heterozygous; grey bar, deletion homozygous.
Within each genotype, the number of individuals displaying the decanalized thoracic phenotype only varied among the deletion homozygous lines at 25°C (see the electronic supplementary material, table S3). At 29°C, there was a significant difference among the non-deletion homozygous lines for males but not females, and also among the deletion homozygous line females (see the electronic supplementary material, table S3).
(b). Uncanalized traits
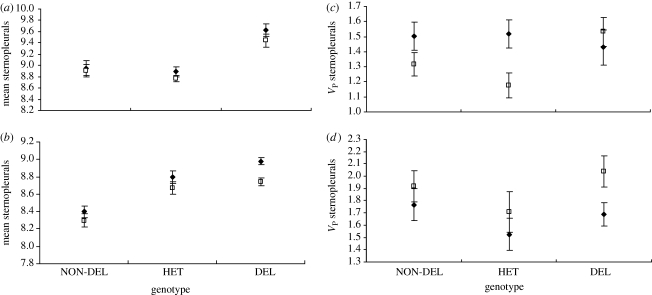
At 25°C, genotype and replicate line within genotype had a significant effect on the mean number of sternopleural bristles in both females (genotype: F2,75 = 4.319, p = 0.0168; line (genotype): F75,6720 = 6.291, p < 0.0001) and males (genotype: F2,74 = 5.044, p = 0.009; line (genotype): F74,6720 = 6.823, p = 0.0001), with the deletion homozygotes having a higher mean number of sternopleural bristles than the non-deletion homozygotes or heterozygotes (figure 3a). At 29°C, genotype and replicate line within genotype had a significant effect on the mean number of sternopleural bristles in females (genotype: F2,75 = 4.785, p = 0.011; line (genotype): F75,6700 = 4.029, p < 0.0001) while in males genotype had no effect on mean sternopleural bristle number but line within genotype did (genotype: F2,75 = 1.068 = 0.349, line (genotype): F75,6720 = 3.121, p < 0.0001; figure 3b). This suggests that Hsp90 genotype affects trait means under some conditions, but also that genetic effects on these traits unrelated to the Hsp90 genotypes are present.
Figure 3.
Sternopleural bristle means and phenotypic variances (VP) at (a,c) 25°C and (b,d) 29°C for non-deletion homozygous (NON-DEL), heterozygous (HET) and deletion homozygous (DEL) genotypes. Means and VP averaged across all lines within each genotype. Error bars represent one standard error. Closed diamonds, females; open squares, males.
Levene's test on log-transformed sternopleural bristle number shows that genotype had no significant effect on the phenotypic variance of the uncanalized traits in either females or males at 25°C (females: F2,6720 = 2.253, p = 0.105; males: F2,6720 = 1.996, p = 0.136) or at 29°C (females: F2,6700 = 1.771, p = 0.181; males: F2,6700 = 2.235, p = 0.107; figure 3c,d).
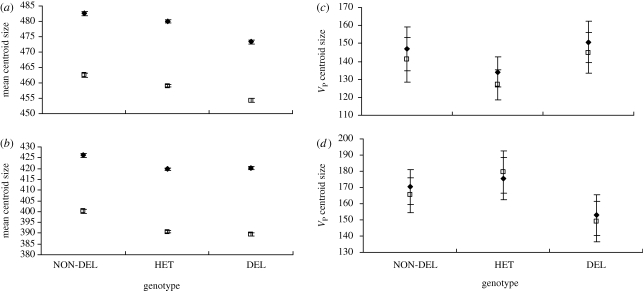
Genotype and line within genotype also had a significant effect on the mean centroid size at 25°C (females: genotype, F2,76 = 8.2634, p = 0.0006; line (genotype), F67,6715 = 5.842, p < 0.0001; males: genotype, F2,76 = 7.352, p = 0.0012; line (genotype), F76,6715 = 6.675, p < 0.0001) and 29°C (females: genotype, F2,76 = 5.007, p = 0.0089; line (genotype), F76,6715 = 3.414, p < 0.0001; males: genotype, F2,76 = 5.975, p = 0.0039; line (genotype), F76,6715 = 4.775, p < 0.0001; figure 4a,b). The non-deletion homozygotes were significantly larger than the heterozygotes and deletion homozygotes at both temperatures, while the heterozygotes were significantly larger than the deletion homozygotes at 25°C but not 29°C (figure 4a,b).
Figure 4.
Centroid size means and phenotypic variances (VP) at (a,c) 25°C and (b,d) 29°C for non-deletion homozygous (NON-DEL), heterozygous (HET) and deletion homozygous (DEL) genotypes. Means and VP averaged across all lines within each genotype. Error bars represent one standard error. Closed diamonds, females; open squares, males.
Levene's test on log-transformed centroid size also shows that genotype had no effect on phenotypic variance for wing centroid size in either females or males at 25°C (females: F2,6715 = 0.893, p = 0.409; males: F2,6715 = 0.986, p = 0.373) or 29°C (females: F2,6715 = 1.875, p = 0.157; males: F2,6715 = 1.476, p = 0.229; figure 4c,d).
(c). Linkage disequilibrium between Hsp90 and additional markers
There was little evidence for linkage disequilibrium between the Hsp90 locus and the closest neighbouring markers after correcting for multiple comparisons. Hsp90 was in strong and significant linkage disequilibrium with microsatellite 3L262 750gt (Pearson's ρ = 0.575, α = 0.002 after correcting for multiple comparisons), but there was no significant linkage between the remaining markers (not shown). The most common allele at these other loci was not associated with the incidence of decanalized phenotypes in the lines for any of the loci (see the electronic supplementary material, table S4) suggesting that the effects on canalization were likely to reflect the effects of the Hsp90 deletion allele rather than those of nearby loci. The significant correlations between Hsp90 and marker 3L262 750gt and the frequency of decanalized phenotypes reflect the significant linkage disequilibrium between the two loci.
(d). Hsp90 gene expression levels
Genotype had no significant effect on Hsp90 transcript levels at either 25°C (genotype: F1,55 = 1.944, p > 0.05; line (genotype): F55,168 = 1.012, p > 0.05) or 29°C (genotype: F1,55 = 0.123, p>0.05; line (genotype): F55,168 = 1.113, p > 0.05) indicating that differences in mean and phenotypic variance described above cannot be accounted for by differing levels of Hsp90 transcript.
(e). Response to selection
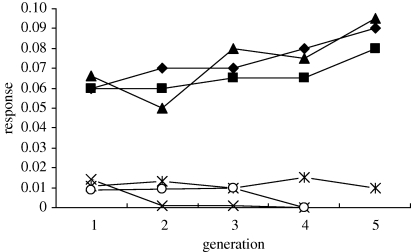
This experiment tested whether there was a genetic basis to variation in the canalized bristle traits evident in the deletion homozygous lines. Of the six replicate lines of each genotype used to initiate the selection experiment, the decanalized scutellar and thoracic bristle phenotypes were lost from three deletion homozygous and non-deletion homozygous lines within the first two generations. The decanalized scutellar and thoracic bristle phenotypes were lost from an additional two non-deletion homozygous lines during selection (figure 5). Nonetheless, a significant response to selection was observed in the lysine deletion homozygous lines compared with the non-deletion homozygous lines (figure 5, combined probability test: deletion lines χ2 = 20.293, d.f. = 6, p = 0.002; non-deletion lines χ2 = 0.584, d.f. = 6, p = 0.439), indicating that the observed increase in phenotypic variance for the canalized scutellar and thoracic bristles in the deletion homozygous lines could be selected across generations and did have a genetic basis. Epigenetic factors may have contributed to the lack of a significant selection response in some of the lines.
Figure 5.
Response to selection on decanalized scutellar and thoracic bristles at 29°C. Filled diamond, deletion homozygous; filled triangle, deletion homozygous; filled square, deletion homozygous; asterisk, non-deletion homozygous; cross; non-deletion homozygous; open circle, non-deletion homozygous.
4. Discussion
We have found evidence for a naturally occurring deletion variant of Hsp90 associated with decanalization. The effect of the variant on phenotypic variability was restricted to canalized bristle traits; no effect was evident for the uncanalized bristle or wing traits. The expression of increased variability also depended on temperature. Our results are consistent with previous work in D. melanogaster using laboratory-generated mutants showing that Hsp90 mutations buffer phenotypic variation for canalized bristle traits but not uncanalized traits (Milton et al. 2005). However, the decanalizing effect associated with the lysine deletion differed from the effects of the Hsp90 mutations examined by Milton et al. (2005). Of the seven mutant alleles they examined, only one affected the thoracic bristle phenotype, and none had any effect on the scutellar bristles. In contrast, the naturally occurring lysine deletion in Hsp90 examined here was associated with effects on both the canalized scutellar and thoracic bristle traits. Milton et al. (2005) only scored females, so we are unable to compare our sex-specific effect for thoracic bristles to their study.
We have also found that the decanalizing effects of the lysine deletion were strain specific; replicates within the deletion homozygous lines differed in the extent to which individuals displayed the decanalized scutellar and thoracic bristle phenotypes. The strain-specific nature of Hsp90 effects was previously noted by Rutherford & Lindquist (1998). Strain-specific effects may arise because of epigenetic effects on decanalization, whereby some strains have a genetic background that makes the expression of cryptic variation more probable (Sollars et al. 2003).
Because decanalized scutellar and thoracic bristle phenotypes were observed at a very low frequency in the lines homozygous for the non-deletion allele, there is still variation for canalized traits segregating in the source population, irrespective of Hsp90 variation. We can measure the extent to which the lysine deletion contributed to decanalized phenotypes compared with this residual variation by investigating the total number of decanalized phenotypes in the population. On average, the frequency of decanalized phenotypes was 10.54 per cent (708/6720) in the deletion versus 3.42 per cent (230/6720) in the non-deletion lines at 25°C and 15.45 per cent (1038/6720) in the deletion versus 3.84 per cent (258/6720) in the non-deletion lines at 29°C. These frequencies are higher than those reported by Rutherford & Lindquist (1998) for their heterozygote mutant lines and controls, suggesting that the source population from which we collected our experimental lines harbours substantial amounts of variation (Hsp90-buffered and Hsp90-independent) for these traits.
Previous studies (Milton et al. 2003, 2005) have demonstrated an effect of Hsp90 mutations on the mean of uncanalized traits. Consistent with these data, the means of both uncanalized traits were significantly affected by genotype in this study, but the direction of the effect was inconsistent across traits. The lysine deletion significantly reduced mean wing size compared with the non-deletion homozygous lines at both 25°C and 29°C. Similar effects were reported by Milton et al. (2005) in their study of laboratory-derived Hsp90 mutant alleles. We also found that the mean number of sternopleural bristles was significantly higher in the deletion homozygous lines compared with the non-deletion homozygous lines at 25°C and 29°C (females only). In contrast, two of the Hsp90 mutant alleles examined by Milton et al. (2005) significantly reduced the mean number of sternopleural bristles. The extent to which shifts in mean phenotype may affect the evolutionary trajectory of a population will depend on the availability of genetic variation in the direction of selection for the traits in question (McGuigan & Sgrò 2009). The changes in body size and sternopleural bristles observed in this study were not associated with any increases in phenotypic variance, indicating that adaptive shifts in these traits are unlikely to be affected by the Hsp90 variants.
The decanalizing effect associated with the lysine deletion is not due to differences in Hsp90 gene expression, since transcript levels did not differ between genotypes. This also makes it unlikely that protein levels differ between genotypes. This result is expected since no polymorphisms were observed in the promoter region of either non-deletion or homozygous deletion lines. In addition, it is unlikely that the results can be explained by linkage disequilibrium between the Hsp90 locus and some other gene, since linkage weakened the further away from the Hsp90 locus a marker was located and there were no associations between alleles at mirosatellite loci and the incidence of decanalized phenotypes. Previous work (Sgrò et al. 2008) has indicated that linkage disequilibrium falls quickly away from the lysine deletion polymorphism and that there is no linkage disequilibrium between the deletion and the only other nonsynonomous change in Hsp90 that is in complete linkage disequilibrium with one of the cosmopolitan inversions. Finally, both the non-deletion and deletion homozygous lines were subjected to the same number of pair-wise matings among outbred individuals, so it is unlikely that inbreeding or differences in overall levels of genetic variation between the genotypes independent of the Hsp90 locus contributed to the results described here.
The data therefore suggest that the lysine deletion, which is a structural mutation occurring in the highly charged linker region of the Hsp90 gene, is associated with a change in Hsp90 protein function which in turn results in decanalization of usually invariant bristle traits. Previous work suggests that this charged region may be important for appropriate peptide binding to the N-terminal of the Hsp90 protein (Scheibel et al. 1999) and loss of function of Hsp90 through altered regulation by co-chaperones, although partial reconstruction of this region can help rescue function (Hainzl et al. 2009). Presumably a small deletion of a single amino acid in the charged region is likely to have quite subtle effects on Hsp90 function. Additional experiments where alleles are manipulated in controlled backgrounds are needed to isolate these allele-specific effects.
The small but significant response to selection observed in the deletion homozygous lines indicates that the increase in phenotypic variation for scutellar and thoracic bristles in the lysine deletion lines has a genetic basis, although epigenetic factors may also play a role and help explain why selection responses were not observed in all lines. The lysine deletion was associated with decanalization resulting in the release of cryptic genetic variation for previously canalized traits. These results are consistent with the notion that disruption of Hsp90 function resulted in the release of cryptic variation that can be selected (Rutherford & Lindquist 1998). The lysine deletion examined in this current study did not cause the range of morphological abnormalities initially described by Rutherford & Lindquist (1998). This suggests that effects associated with the lysine deletion examined in this study are likely to be quite subtle when compared with the loss of Hsp90 function as in EMS derived mutants. In nature, mutations in Hsp90 with severe phenotypic consequences are likely to be quickly lost due to selection. Although the homozygote form of the deletion allele examined in the current study was observed at very low frequencies (1/1000) in the natural population sampled suggesting some deleterious effects of this allele, the frequency of the deletion allele varies from 0 to 15 per cent in populations of D. melanogaster from eastern Australia (Sgrò et al. 2008). Thus, our results clearly demonstrate the potential for genetic canalization to contribute to phenotypic buffering. The importance of genetic versus environmental canalization needs to be resolved empirically.
In conclusion, we have provided evidence that a naturally occurring variant in the Hsp90 gene is associated with buffering of phenotypic variation for canalized morphological traits in an outbred population of D. melanogaster recently derived from nature. The adaptive significance of this Hsp90 mediated buffering remains equivocal; the release of cryptic variation for scutellar or thoracic bristles may increase the evolvability of these morphological characters, but we have not yet made direct connections to fitness. Whether this naturally occurring variant of Hsp90 is associated with similar effects for ecologically important traits such as stress resistance in natural populations under a range of environmental conditions remains to be determined.
Acknowledgements
We thank Jodie Taylor, Alen Rako, Christina Schmuki and Vanessa Kellermann for technical assistance and the Australian Research Council for financial support via their Fellowship (to both CMS, AAH) and Discovery grant schemes.
References
- Cara J., Aluru N., Moyano F., Vijayan M.2005Food-deprivation induces Hsp70 and Hsp90 protein expression in larval gilthead sea bream and rainbow trout. Comp. Biochem. Physiol. 142, 426–431 [DOI] [PubMed] [Google Scholar]
- Chen B., Kayukawa T., Monteiro A., Ishikawa Y.2005The expression of the Hsp90 gene in response to winter and summer diapauses and thermal-stress in the onion maggot, Deila antiqua. Insect Mol. Biol. 14, 697–702 (doi:10.1111/j.1365-2583.2005.00602.x) [DOI] [PubMed] [Google Scholar]
- David J. R., Allemand R., Van Herrewege J., Cohet Y.1983Ecophysiology: abiotic factors. In The genetics and biology of Drosophila, pp. 105–170 London, UK: Academic Press [Google Scholar]
- Debat V., Milton C., Rutherford S., Klingenberg C., Hoffmann A. A.2006Hsp90 and the quantitiave variation of wing shape in Drosophila melanoagster. Evolution 60, 2529–2538 [PubMed] [Google Scholar]
- Dworkin I.2005Canalization, cryptic variation and developmental buffering: a critical examination and analytical perspective. In Variation (eds Hallgrimsson B., Hall B.). Amsterdam, The Netherlands: Elsevier Academic Press [Google Scholar]
- Gupta S.1995Phylogenetic analysis of the 90 Kd heat-shock protein sequences and an examination of the relationship among animals, plants and fungi species. Mol. Biol. Evol. 12, 1063–1073 [DOI] [PubMed] [Google Scholar]
- Hainzl O., Lapina M., Buchner J., Richter K.2009The charged linker region is an important regulator of Hsp90 function. J. Biol. Chem. 284, 22 559–22 567 (doi:10.1074/jbc.M109.031658) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson E., Eanes W. F.1996Contrasting histories of three gene regions associated with In(3L)Payne of Drosophila melanogaster. Genetics 144, 1565–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward S., Pavlides S., Tammariello S., Rinehart J., Denlinger D. L.2005Temporal expression patterns of diapause-associated genes in flesh fly pupae from the onset of diapause through to post-diapause quiescence. J. Insect Physiol. 51, 631–640 (doi:10.1016/j.jinsphys.2004.11.009) [DOI] [PubMed] [Google Scholar]
- Hoffmann A. A., Shirriffs J.2002Geographic variation for wing shape in Drosophila serrata. Evolution 56, 1068–1073 [DOI] [PubMed] [Google Scholar]
- Kellermann V., Hoffman A. A., Sgrò C. M.2007Hsp90 inhibition and the expression of phenotypic variability in the rainforest species Drosophila birchii. Biol. J. Linnean Soc. 92, 457–465 (doi:10.1111/j.1095-8312.2007.00875.x) [Google Scholar]
- Kohler H., Lazzara R., Dittbrenner N., Caopowiez Y., Mazzia C., Triebskorn R.2009Snail phenotypic variation and stress proteins: do different heat response strategies contribute to Waddington's widget in field populations? J. Exp. Zool. (Mol. Dev. Evol.) 312B, 136–147 (doi:10.1002/jez.b.21253) [DOI] [PubMed] [Google Scholar]
- McGuigan K., Sgrò C. M.2009Evolutionary consequences of cryptic genetic variation. Trends Ecol. Evol. 24, 305–311 [DOI] [PubMed] [Google Scholar]
- Milton C., Huynh B., Batterham P., Rutherford S., Hoffmann A. A.2003Quantitative trait symmetry independent of Hsp90 buffering: distinct modes of genetic canalisation and developmental stability. Proc. Natl Acad. Sci. USA 11, 13 396–13 401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton C., Batterham P., McKenzie J., Hoffmann A. A.2005Effect of E(sev) and Su(Raf) Hsp83 mutants and Trans-heterozygotes on bristle trait means and variation in Drosophila melanogaster. Genetics 171, 119–130 (doi:10.1534/genetics.104.038463) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton C., Ulane C. M., Rutherford S.2006Control of canalization and evolvability by Hsp90. PLoS ONE 1, 1–12 (doi:10.1371/journal.pone.0000075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepin K., Momose N., Ishida N., Nagata K.2001Molecular cloning of the horse Hsp90 cDNA and its cimparative analysis with other vertebrate Hsp90 sequences. J. Vet. Med. Sci. 63, 115–124 (doi:10.1292/jvms.63.115) [DOI] [PubMed] [Google Scholar]
- Rutherford S., Lindquist S.1998Hsp90 as a capacitor for morphological evolution. Nature 396, 336–342 (doi:10.1038/24550) [DOI] [PubMed] [Google Scholar]
- Sangster T., Salathia N., Udurrage S., Milo R., Schellenberg K., Lindquist S., Queitsch C.2008aHsp90 affects the expression of genetic variation and developmental stability in quantiative traits. Proc. Natl Acad. Sci. USA 105, 2963–2968 (doi:10.1073/pnas.0712200105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangster T., et al. 2008bHsp90-buffered genetic variation is common in Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 105, 2969–2974 (doi:10.1073/pnas.0712210105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibel T., Siegmund H., Jeanicke R., Ganz P., Lilie H., Buchner J.1999The charged region of Hsp90 modulates the function of the N-terminal domain. Proc. Natl Acad. Sci. USA 96, 1297–1302 (doi:10.1073/pnas.96.4.1297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgrò C. M., Milton C. C., Jensen L. T., Frydenberg J., Loeschcke V., Batterham P., Hoffmann A. A.2008Nucleotide diversity in the Hsp90 gene in natural populations of Drosophila melanogaster from Australia. Insect Mol. Biol. 17, 685–697 (doi:10.1111/j.1365-2583.2008.00843.x) [DOI] [PubMed] [Google Scholar]
- Shapiro J., et al. 2007Adaptive genic evolution in the Drosophila genomes. Proc. Natl Acad. Sci. USA 104, 2271–2276 (doi:10.1073/pnas.0610385104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal R., Rohlf F.1981Biometry New York, NY: WH Freeman and Company [Google Scholar]
- Sollars V., Lu X., Xiao L., Wang X., Garfinkel M. D., Ruden D. M.2003Evidence for an epigenetic mechanism by which Hsp90 acts as a capacitor for morphological evolution. Nat. Genet. 33, 70–74 (doi:10.1038/ng1067) [DOI] [PubMed] [Google Scholar]
- Umina P., Weeks A. R., Kearney M. R., McKechnie S. W., Hoffmann A. A.2005A rapid shift in a classic clinal pattern in Drosophila reflecting climate change. Science 308, 691–693 (doi:10.1126/science.1109523) [DOI] [PubMed] [Google Scholar]
- van der Straten A., Rommel C., Dickson B., Hafen E.1997The heat shock protein 83 (Hsp83) is required for Raf-mediated signalling in Drosophila. EMBO J. 16, 1961–1969 (doi:10.1093/emboj/16.8.1961) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington C.1942Canalization of development and the inheritance of acquired characters. Nature 150, 563–565 (doi:10.1038/150563a0) [DOI] [PubMed] [Google Scholar]
- Yeyati P., Bancewicz R., Maule J., van Heyningen V.2007Hsp90 selectively modulates phenotype in vertebrate development. PLOS Genet. 3, 431–447 [DOI] [PMC free article] [PubMed] [Google Scholar]