Abstract
All cells must traffic proteins into and across their membranes. In bacteria, several pathways have evolved to enable protein transfer across the inner membrane, the periplasm, and the outer membrane. The major route of protein translocation in and across the cytoplasmic membrane is the general secretion pathway (Sec-pathway). The biogenesis of membrane proteins not only requires protein translocation but also coordinated targeting to the membrane beforehand and folding and assembly into their protein complexes afterwards to function properly in the cell. All these processes are responsible for the biogenesis of membrane proteins that mediate essential functions of the cell such as selective transport, energy conversion, cell division, extracellular signal sensing, and motility. This review will highlight the most recent developments on the structure and function of bacterial membrane proteins, focusing on the journey that integral membrane proteins take to find their final destination in the inner membrane.
Keywords: Membrane protein biogenesis, Membrane protein structure, Membrane targeting, Sec complex, Signal recognition particle, Topology, YidC insertase
Introduction
Gram-negative bacteria are surrounded by two distinct membranes: the inner (cytoplasmic) membrane, and outer (periplasmic) membrane. The two membranes, which are separated by a peptidoglycan-containing periplasm, are composed of phospholipids but differ considerably in their structure and composition. The bacterial inner membrane is composed of a symmetrical phospholipid bilayer. The bacterial outer membrane, in contrast, is an asymmetrical bilayer containing mostly phospholipids and lipopolysaccharides in the inner leaflet and in the outer leaflet, respectively. Both membranes contain numerous membrane proteins that are critical for many cellular functions. These proteins play key roles in energy transduction and are involved in the sensing and signal transduction of environmental stimuli as well as the uptake and efflux of substances. The biogenesis of these proteins requires coordinated targeting to the membrane, insertion into or translocation across the membrane, and subsequent assembly into multi-protein complexes. Membrane proteins have to reach their destination either in the inner or the outer membrane and have to be folded correctly in order to function in the cell. To facilitate their targeting, folding, and assembly, a range of molecular chaperones, translocation, and insertion machineries are required. The information of how these membrane proteins reach their final destination is contained within the amino-acid sequence of the protein. In general, proteins with α-helical membrane spanning segments are found in the inner membrane, whereas amphipathic β-barrel proteins (called outer-membrane proteins or Omps) are located in the outer membrane. In E. coli approximately 20–30% of all encoded proteins are inner-membrane proteins and approximately 2% are outer-membrane proteins.
Synthesis of membrane proteins
All proteins are synthesized at ribosomes in the cytosol. Since membrane proteins are composed of predominantly hydrophobic amino acids, special attention has to be taken after the polypeptide chain leaves the ribosomal tunnel. To prevent aggregation of the hydrophobic parts of the protein in the cytoplasm, chaperone proteins are localized at the exit site of the ribosome, ready to interact with the emerging polypeptide chain. One such chaperone is the trigger factor (TF) that binds to the ribosomal protein L23 [1] (Fig. 1). TF interacts with virtually all emerging nascent chains, especially hydrophobic nascent polypeptides emerging at the ribosomal exit [2, 3]. The chaperone TF is able to prevent premature folding by accommodating newly synthesized polypeptides within its substrate-binding cavity [4]. Subsequently, nascent polypeptides not destined to the inner membrane can interact with the ATP-dependent DnaK and GroEL chaperone systems for further folding of the proteins [5–7]. While E. coli can tolerate the deletion of either TF or DnaK, a combined deletion is lethal at temperatures above 30°C because it causes misfolding and aggregation of newly synthesized proteins [8]. In contrast, TF does not generally assist the export of secretory proteins [9]. Presecretory proteins that contain a cleavable signal sequence are assisted by the cytosolic chaperone protein SecB (Fig. 1). SecB binds to unfolded polypeptides after dissociation of TF and delivers them in a translocation-competent conformation to the peripheral membrane component of the protein-secretion apparatus, SecA. It has also been shown that DnaK and GroEL promote the export of several proteins [10, 11]. E. coli strains lacking SecB respond by up-regulating DnaK and GroEL and vice versa [12, 13].
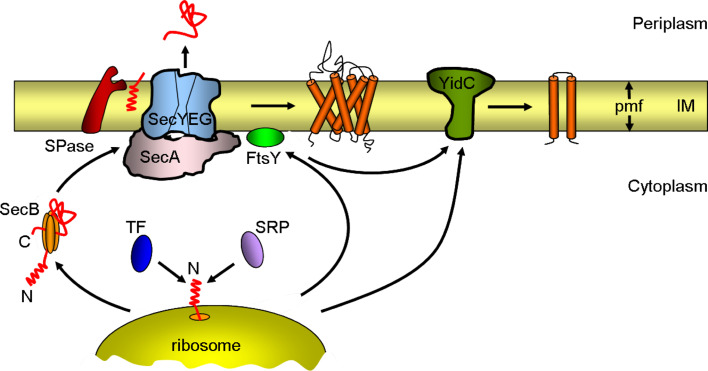
Fig. 1.
Schematic overview of bacterial inner-membrane protein biogenesis. Newly synthesized proteins are targeted to the Sec complex either by the signal recognition particle (SRP) as soon as they emerge from the ribosome tunnel (co-translational translocation, mainly inner-membrane proteins) or by the tetrameric SecB chaperone after translation (post-translational translocation, mainly secretory and outer-membrane proteins). Trigger factor (TF) competes with SRP for the binding of the nascent protein. Proteins destined for the inner (IM) or outer membrane are transported into or across the inner membrane through the Sec complex. The complex consists of the SecYEG protein-conducting channel and the ATPase motor SecA. The signal peptidase (SPase) cleaves the signal sequence from preproteins at the outer face of the inner membrane. A few membrane proteins insert into the inner membrane via YidC. For simplicity, SecYEG is shown without its accessory components (SecDFYajC and YidC). pmf proton motive force
Also competing for the same pool of newly synthesized polypeptides is the signal recognition particle (SRP). Likewise, SRP also binds to the ribosomal protein L23 and screens the emerging nascent chain for a hydrophobic signal sequence, such as a transmembrane helix of inner-membrane proteins [14, 15]. If such a sequence emerges, the SRP binds firmly and targets the ribosome-nascent chain (RNC) complex to the Sec complex via an interaction with FtsY, the membrane-associated SRP receptor. In vitro binding and cross-linking studies suggest that TF and SRP are able to bind simultaneously to L23 [16, 17]. It is proposed that TF and SRP sample nascent chains on the ribosome in a nonexclusive fashion, and that binding of FtsY to the ribosome-bound SRP complex excludes TF from the ribosome thereby enabling the docking of the ribosome to the Sec complex [16].
Membrane targeting of proteins
SecB
Most secreted proteins (i.e., proteins that reside in the periplasm or the outer membrane of Gram-negative bacteria) are synthesized as preproteins with a cleavable signal peptide at their N-terminus. These proteins are targeted to the cytoplasmic membrane post-translationally by the molecular chaperone SecB (Fig. 1). The homotetrameric SecB chaperone probably binds to preproteins by recognizing exposed hydrophobic surfaces [18]. While SecB binds to the mature part of a preprotein, the signal sequence is exposed for downstream interactions. The SecB-preprotein complex is then targeted to the Sec complex composed of a peripheral protein, SecA, and the membrane-bound Sec translocase [19]. This interaction is enhanced by binding of the signal sequence to SecA [20]. SecB interacts with the C-terminal domain of SecA (see [21] and references therein). After SecA recognizes the SecB-delivered preproteins, SecB is released from the preprotein [22]. This allows SecB to re-bind to the next newly synthesized nascent secretory protein. The core of the Sec translocase consists of the integral membrane proteins SecY, SecE, and SecG, which constitute a heterotrimeric protein complex. SecA promotes the translocation of the preproteins through the pore by ATP hydrolysis (see [23, 24] for a review). After translocation, the signal peptide is removed proteolytically by the bacterial signal peptidase generating the mature protein. Bacterial signal peptides that target proteins to the secretory pathway are usually characterized by a positively charged amino-terminal (n-) region, a central hydrophobic apolar (h-) region of 7–12 residues and a more polar carboxy-terminal (c-) region that serves as a recognition site for the signal peptidase enzyme [25].
SRP and its receptor
In contrast, most integral membrane proteins do not contain a cleavable signal peptide. The information for integration into the membrane is contained within their hydrophobic transmembrane segments (TMSs). In general, the N-terminal TMS serves as the internal targeting signal or uncleaved signal sequence. The majority of inner-membrane proteins and a few secretory proteins are targeted in a co-translational manner to the SecYEG complex by the SRP and its receptor [26]. The interaction of SRP with the signal peptide is dependent on the hydrophobicity of the h-region [27]. Cross-linking studies have shown that the efficiency of cross-linking to SRP is correlated with the hydrophobicity of the signal sequence [28, 29]. E. coli presecretory proteins can be re-routed into the SRP pathway by increasing the hydrophobicity of their signal sequences [30]. In addition to the hydrophobicity of the TMS, basic amino acids are also suggested to promote binding of the signal peptide with SRP through electrostatic interactions [31]. For integral membrane proteins that have a large hydrophilic cytoplasmic domain at their N-terminus, as in the case of the sensor protein KdpD, the proteins are recognized at an early stage for co-translational targeting. Recent studies have shown that a short amphiphilic sequence at the beginning of the N-terminal cytoplasmic region of KdpD serves as a signal sequence for SRP and is capable of binding to SRP [32]. Most of the analyzed membrane proteins thus far have demonstrated a requirement of SRP for their targeting, including the leader peptidase Lep [33], FtsQ [34], the mannitol permease MtlA [35], SecY [36], and MalF [37].
SRP is conserved in all three kingdoms of life (see [38, 39] for a review). The composition of SRP varies in the different organisms, but the SRP core is a cytosolic ribonucleoprotein particle consisting of the protein SRP54 (Ffh for “fifty-four-homologue” in Bacteria) bound to SRP RNA (helix 8 in Eukarya and Archaea, domain IV in Bacteria) [39, 40]. The bacterial SRP receptor consists of only one protein, FtsY (filamentous temperature sensitive Y), the homologue of the α-subunit of the SRP receptor (SRα) in Eukarya. In Bacteria, there is no known homologue of the eukaryotic β-subunit of the SRP receptor (SRβ), an integral membrane protein that serves to anchor SRα to the membrane. All the bacterial SRP components are essential for cell growth.
SRP binds to the hydrophobic signal sequence of a nascent chain as it emerges from the translating ribosome. The resulting RNC-SRP complex is then targeted to the receptor, FtsY, at the membrane. There an interaction involving two guanosine triphosphatases (GTPases) between SRP and its receptor catalyzes the release of the nascent chain from SRP [41]. The nascent chain, freed from SRP, enters the Sec translocase for insertion into the membrane [38, 42]. After release, SRP can enter the next cycle of protein targeting. The activation of GTP hydrolysis in the targeting complex is essential for protein translocation [43, 44]. In E. coli, only a fraction of FtsY is found stably associated with the cytoplasmic membrane [45]. The interaction of FtsY with the cytoplasmic membrane is required for the release of nascent proteins from the SRP-FtsY complex [29, 46]. Although FtsY in E. coli lacks a TMS, it binds to membranes by interacting with phospholipids [47–50]. At least two lipid-binding sites have been suggested to exist within FtsY (Fig. 2) [47, 51]. One binding site is located at the N-terminus of FtsY, which probably forms an α-helical conformation (helix 1) [50, 52]. A second binding site is located at the interface between the A- and the N- domain of FtsY, which has been shown to form an amphipathic helix (helix 2) [49]. Although both helices have been shown to interact with anionic phospholipids, it is the cooperative action of the two lipid-binding helices that allows a very stable membrane contact with FtsY [50]. Only helix 2 is essential for FtsY function [49]. Removal of helix 1 reduces the stability of the FtsY membrane contact but only slightly affects the FtsY function [50, 52]. Besides the interaction with membrane lipids, FtsY also interacts directly with the Sec complex most likely via its conserved NG-domains [53, 54].
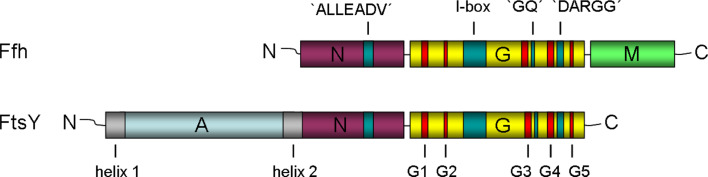
Fig. 2.
Schematic representation of the three-domain structure of Ffh and FtsY of E. coli. The domains are indicated with different colors: N domain purple, G domain yellow, M domain green, and A domain light blue. The five consensus elements responsible for GTP binding and hydrolysis (G1–G5) are highlighted in red, whereas the I-box and the inter-domain communication motifs (ALLEADV, DARGG, GQ) are indicated in blue. The two lipid-binding sites of FtsY are shown in grey
Both Ffh and the receptor, FtsY, are multi-domain proteins (Fig. 2). Ffh is composed of three domains, an amino-terminal N domain, a central GTPase G domain, and a methionine-rich carboxy-terminal M domain that binds both SRP RNA and signal peptides [39]. FtsY also comprises three domains, an acidic amino-terminal A domain, a central N domain and a carboxy-terminal GTPase G domain. The NG domains of Ffh and FtsY exhibit high sequence similarity to each other, and the crystal structures of the NG domains of Ffh from Thermus aquaticus and of FtsY from E. coli revealed a high degree of structural similarity [55, 56]. In Ffh and FtsY, the N domain comprises a four-helix bundle and is closely associated with the adjacent G domain. The G domain shows the typical Ras-like GTPase fold with five conserved elements (G1–G5) for nucleotide binding and hydrolysis and an additional α-β-α insertion (I-box or IBD) motif that is unique to SRP GTPases [55]. Inter-domain communication between the N and G domain in Ffh and FtsY involves the ALLEADV motif in the N domain, and the GQ and the DARGG motifs in the G domain (see [40] for a review). The M domain of Ffh is involved in binding the signal sequence of a substrate protein [57] and accommodating it in its hydrophobic groove [58]. The crystal structure of the E. coli Ffh M domain bound to the fragment of the SRP RNA showed that a part of the conserved IV domain of the 4.5S RNA lies adjacent to the hydrophobic groove in the M domain and appears to create an extended signal peptide-binding pocket [58]. This suggests that this binding site composed of both protein and RNA would accommodate a signal peptide through a combination of hydrophobic interactions and electrostatic contacts. The presence of a signal sequence in the M domain could trigger the release of the translocating protein into the translocation pore of SecYEG [59].
During the last few years, a number of structures of SRP with its interaction partners, the ribosome [60] and FtsY [61, 62], have been solved using X-ray and cryo-electron microscopy. The crystal structure of the Ffh-FtsY complex from Thermus aquaticus revealed that the two GTPases align almost parallel in a symmetrical arrangement that brings their two active sites together to form a shared active site at the interface between them [61, 62]. Although significant progress has been made, the atomic structure of the SRP-RNA complex with a bound signal peptide is required to put the pieces together to show how Ffh and its receptor guide a nascent chain into the translocation pore.
Membrane insertion of proteins
The secretory pathway
The general secretory (Sec) pathway is engaged in translocating both secretory proteins across the membrane as well as integrating proteins into the cytoplasmic membrane (Fig. 1). Depending on the substrate, the Sec complex has to switch between two operational modes: a transversal opening for allowing secretory proteins across to the periplasm and a lateral opening for the insertion of TMSs. The SecYEG complex is highly conserved and homologues have been found in Eukaryotes (Sec61p, comprising α, γ and β-subunits) and Archaea (SecYEβ) [63]. The peripheral SecA protein is the motor subunit of the SecYEG complex. It is required for the translocation of secretory proteins and insertion of inner-membrane proteins with large (≥60 amino acids) hydrophilic periplasmic domains [64, 65]. SecA like FtsY also binds to membrane phospholipids [66], and to the Sec complex via a direct interaction with SecY [67]. SecA appears to bind to anionic lipids with its C-terminal domain [68].
The SecA ATPase is a multi-domain protein that contains two nucleotide-binding domains (NBD1 and NBD2), a polypeptide crosslinking domain (PPXD), an α-helical wing domain (HWD), and an α-helical scaffold domain (HSD) [69]. The crystal structure of SecA bound to the SecY complex from Thermotoga maritima revealed a “two-helix finger” formed by two helices of SecA’s HSD inside the cytoplasmic funnel of the SecY channel [70]. It is suggested that a polypeptide chain moves from the tip of the two-helix finger of SecA into the SecY pore by movements inside the cytoplasmic funnel during the ATP hydrolysis cycle [71] (Fig. 3). SecA binds in its ATP-bound state to the polypeptide chain, pushes it into the SecY pore, and then releases it in its ADP-bound state [72, 73]. Backsliding of the chain is reduced by its interactions with the SecY pore. Repeated cycles of ATP binding and hydrolysis, as well as polypeptide binding and release, result in the stepwise translocation of the polypeptide across the membrane. Each additional ATP hydrolysis cycle is accompanied by the translocation of 20–30 residues of polypeptide [74]. After completion of translocation, SecA can dissociate from the SecYEG complex. Once polypeptide translocation has been initiated, the proton motive force (pmf) can further drive the reaction in the absence of ATP when SecA is not bound with the translocating polypeptide at SecYEG [75]. Although it is generally accepted that SecA is dimeric in the cytosol, there is controversy concerning the oligomeric state of SecA during active protein translocation. Likewise, the stoichiometry of the SecYEG heterotrimers within the active translocase complex is still a topic of debate. Numerous biochemical [76–78] and structural [79–81] studies demonstrate that the SecYEG complex (and the Sec61p complex) forms higher-order oligomers, predominantly dimers and tetramers. Electron microscopic images of purified Sec complexes from different species revealed ring-like structures containing between two and four copies of the Sec complex [79, 80]. Similar to the bacterial system, multimeric ring-link structures were also observed for the Sec61p complex [82]. Further biochemical analysis by blue native gel electrophoresis, fluorescence resonance energy transfer, and chemical cross-linking revealed that the SecYEG complex exists as a monomer or dimer [76, 83, 84]. A recent cross-linking study of the E. coli SecA-SecYEG system proposes that in a SecYEG dimer one copy of SecYEG acts as a docking station for SecA, whereas the other copy is used as the translocation pore [85]. If a single copy of the Sec complex can form a translocation pore for exiting polypeptides, what is the role of oligomerization? The answer is not yet known, but one possibility is that the formation of monomeric, dimeric, and tetrameric states of the SecYEG complex (and the Sec61p complex) might be influenced by its interaction with ligands, like SecA or the ribosome [80–82].
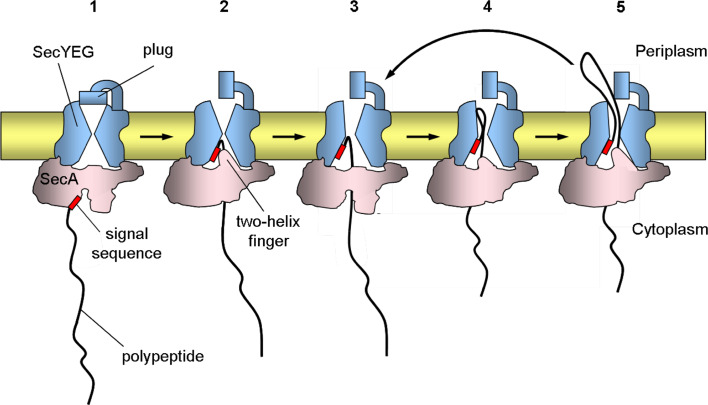
Fig. 3.
SecA-mediated protein translocation through the SecY pore. The scheme shows a model for the different steps in translocation. 1 SecA binds to a polypeptide substrate bearing an N-terminal signal sequence. 2 SecA opens toward SecY and transfers the signal sequence and early mature region of the polypeptide into SecY with the help of the two-helix finger. The polypeptide inserts as a loop structure. This step requires ATP hydrolysis. The insertion of the signal sequence of the polypeptide into SecY causes the plug to move away from the pore, which leads to a fully activated channel. 3 For further translocation, SecA releases the polypeptide into the pore and the helix finger is pulled back and reset. 4 SecA binds the next section of the polypeptide and moves toward the translocationally active SecY. 5 SecA then pushes the next section of the polypeptide into the pore by the helix finger. The signal sequence remains stationary, while the mature part of the polypeptide passes through the pore. These steps are coupled to ATP hydrolysis cycles and steps 3–5 are repeated until the polypeptide is fully translocated (not shown). For simplicity, SecA and SecYEG are shown as monomers and the initial stages of the process with SecB are not shown. The thick black line represents the polypeptide and the red rectangle represents the N-terminal signal sequence
SecY, the pore-forming component of the complex, consists of ten transmembrane (TM) helices (TM1–TM10), whereas SecE has three transmembrane helices and SecG only two transmembrane helices in E. coli [86]. SecY and SecE are essential for viability in E. coli, whereas SecG is not required for cell viability at 37°C. SecY forms a stable complex with SecE that protects it from degradation by the membrane-bound protease FtsH [87, 88]. The crystal structure of the SecYEβ complex from the Archaeon Methanococcus jannaschii in a closed state revealed an hourglass-shaped channel with aqueous funnels and a ring of hydrophobic amino acids at its constriction near the center of the membrane within the SecY subunit [89]. The external funnel is gated by a plug formed by a short helix (helix 2a) (Fig. 3). This plug hinders the passage of small molecules during translocation. It was proposed that when the channel opens for polypeptide translocation, helix 2a swings outward, opening the extracellular channel. The TM2b, TM3, TM7, and TM8 regions of SecY were proposed to comprise the lateral gate, for the exit of hydrophobic segments of newly synthesized proteins. Cross-linking data revealed that the translocating chain mainly contacts residues in the narrowest part of the hourglass-shaped channel [90]. Recently, another crystal structure of the SecYE complex from Thermus thermophilus was resolved [91]. This crystal structure in a “pre-open” state identified a SecA-SecYE interface that comprises SecA contacting the SecYE protein.
SecD, SecF, and YajC are additional translocase subunits that can interact with the SecYEG core [92]. Both SecD and SecF consist each of six TMSs in E. coli [93]. The single-spanning protein YajC, which has no known function in the Sec complex, has been found associated also with AcrB, which is a component of the multidrug efflux complex AcrB:AcrA:TolC [94]. Both SecD and SecF facilitate membrane insertion and export of several proteins [95, 96].
YidC
Not all inner-membrane proteins use SecYEG for insertion, a small subset of integral membrane proteins are targeted to YidC where they are inserted into the membrane in a Sec-independent manner (Fig. 1). This alternative Sec-independent insertion pathway is used by a number of small inner-membrane proteins, including the phage coat proteins M13 [97] and Pf3 [98], the endogenous inner-membrane proteins Fo c (subunit c of the F1Fo-ATP synthase) [99], and MscL (mechanosensitive channel of large conductance) [100]. A common feature of these membrane proteins is their small size and the presence of short hydrophilic periplasmic domains. YidC can also function together with the Sec complex to promote membrane insertion of larger proteins. YidC has been shown to interact with the Sec complex via the SecDFYajC complex [101, 102]. In the Sec-dependent pathway, YidC most likely acts downstream of the Sec channel in the lateral transfer of TMSs from the Sec pore into the lipid bilayer [103–105]. Photo-cross-linking studies using ribosome-bound nascent chains of the single-spanning membrane protein FtsQ revealed that FtsQ first binds to SecY and at a later stage moves towards YidC [105]. The in vivo depletion of YidC shows only a minor effect on the Sec-dependent proteins requiring YidC such as Lep [97], FtsQ [105], and MtlA [104]. Recent studies on the biogenesis of the endogenous E. coli inner-membrane protein CyoA (subunit II of the cytochrome bo 3 ubiquinol oxidase complex) have shown that YidC can also function upstream of the Sec complex. The N-terminal region of CyoA was shown to require YidC, whereas the large C-terminal periplasmic domain was Sec-dependent [106, 107].
YidC was first identified in bacteria based on the sequence homology with Oxa1 in mitochondria (see [108] for a review). In addition to bacteria, YidC homologues are also found in mitochondria (Oxa1 and Cox18/Oxa2) [109, 110] and chloroplasts (Alb3 and Alb4) [111, 112], where they all are thought to promote membrane insertion. The YidC homologues Oxa1, Cox18, Albino 3, and Albino 4 have been shown to complement YidC insertase activity when expressed in E. coli [113–116]. Unlike the eukaryotic YidC homologues, which span the membrane five times, E. coli YidC spans the membrane six times, and has a large periplasmic loop (P1) between its first two TMSs. Multiple sequence alignments reveal that the second, third, and fifth TMSs of YidC contain conserved residues in the YidC/Oxa1/Alb3 family [117]. Site-specific cross-linking revealed that the conserved third TMS of YidC contacts the first TMS of both nascent Sec-dependent and Sec-independent substrates [118]. In addition, the TMS of the coat protein of bacteriophage Pf3 can be cross-linked in vivo to the first and third TMS of YidC shortly after synthesis [119]. The membrane insertion of Pf3 coat protein can also be reconstituted with proteoliposomes containing only YidC. This demonstrates that YidC functions catalytically as a membrane insertase [120].
YidC seems to also play a role as a membrane chaperone for more complex membrane proteins by assisting in the release of transmembrane helices from the Sec complex, in helix packing, and in the proper folding of polytopic membrane proteins [121]. An in vitro study using lactose permease (LacY) suggests that YidC functions in the co-translational folding of this inner-membrane protein [122]. In the absence of YidC, it appears that LacY cannot achieve its final tertiary structure because the newly synthesized protein is not correctly inserted in the bilayer to fold properly. In addition, YidC is also required for the folding and assembly of the inner-membrane protein MalF, which is part of the maltose transport complex (MalFGK2) in E. coli [37]. The importance of YidC in membrane protein folding and assembly is also suggested by the observation that YidC depletion leads to the induction of the Cpx stress response pathway, which senses protein misfolding of membrane proteins [123]. In addition, both the phage shock protein family and the cold shock protein family genes, especially pspA and cspA, are highly up-regulated following YidC depletion [124]. The depletion of YidC also affects the pmf by hindering the functional assembly of F1Fo-ATPase and the cytochrome o oxidase [125].
The integration of YidC itself into the inner membrane of E. coli requires SecYEG and the coordinated activity of both SRP and SecA [126]. Although SecA does not contribute to the SRP-targeting pathway, when long, hydrophilic periplasmic domains are encountered, as in the case of the large periplasmic P1 domain of YidC, SecA is required to catalyze their translocation across the membrane.
Folding and assembly of membrane proteins
Less understood is the folding and assembly of polytopic membrane proteins into their final tertiary conformation and, thus helix orientation and packing arrangements. Many integral membrane proteins function in multi-protein complexes including the Sec complex, the F1Fo ATP synthase complex, the ABC-transporters and the respiratory chain complexes. After membrane insertion, the subunits of these membrane protein complexes must locate each other and assemble correctly to function properly in the cell. If a polypeptide is unstable and fails to assemble correctly, the proteins in the complex are often degraded (see [127] for a review). Protein misfolding triggers specific responses such as the expression of chaperones, proteases, and other folding catalysts to counteract protein misassembly. Protein misfolding and aggregation lead to a significant reorganization of the membrane and lipid composition in the cell [128]. The folding of the outer membrane protein (OmpA) is strongly dependent on the biophysical properties of the membrane [129]. Mechanosensitive channels such as the widely studied MscL, whose function is to respond to mechanical stress applied to the cell membrane, have altered activities in different bilayers [130, 131].
In E. coli, FtsH is a cytoplasmic membrane protein and ATP-dependent zinc metalloprotease involved in the degradation of unstable membrane proteins. FtsH is known to play a central role in membrane protein quality control. It degrades the SecY subunit of the protein-conducting channel [88] and subunit a of F1Fo ATP synthase [132] when they exist in unassembled states in the membrane. Another membrane protein degraded by FtsH is YccA, which spans the membrane seven times [133]. FtsH can initiate processive proteolysis of membrane protein substrates by recognizing their ends when they protrude sufficiently into the cytosol [134, 135]. The ATP-dependent protease starts proteolysis from a specific initiation site on the substrate protein and then continues to degrade the entire molecule of the substrate [127].
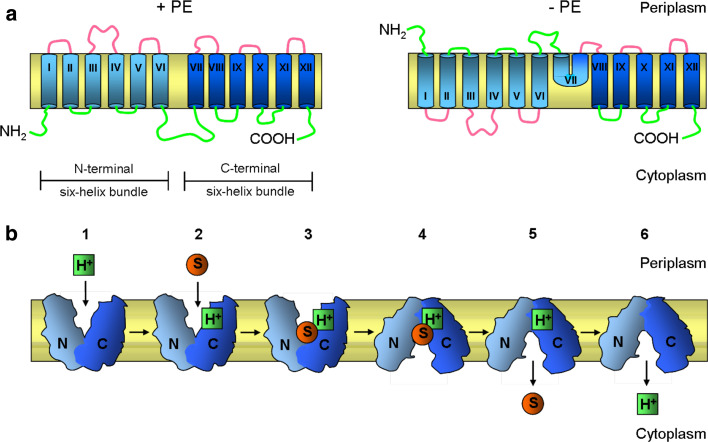
Phospholipids and the composition of the lipid bilayer play an important role in the regulation of integral membrane protein folding and function. The natural lipid bilayer environment consists of a hydrophobic interior region and a polar or charged headgroup region with aqueous solvent on either side of the bilayer. Integral membrane protein must interact with all these different solvents. At the same time, the lipids form a tight seal around the membrane proteins so that proton and chemical gradients are maintained across the membrane. The cytoplasmic membrane of E. coli contains ~70–75 mol% phosphatidylethanolamine (PE) lipids, with the rest being dominated by anionic phosphatidylglycerol (PG, 20%), and to a lesser extent cardiolipin (CL, 5%). Certain lipids such as PE are required for correct topology of integral membrane proteins in E. coli [136–139]. In the absence of PE, defects occur in the function and topological organization of LacY [136] (Fig. 4a). LacY consists of 12 transmembrane helices with both the N- and C-termini exposed to the cytoplasm. LacY inserts into the membrane in the absence of PE in an inverted orientation of the first six transmembrane helices and associated extramembrane domains with respect to the membrane bilayer [136, 139]. This LacY protein is able to carry out facilitated, but not active, transport [140]. A similar requirement for PE was observed with phenylalanine permease (PheP) [137] and γ-aminobutyric acid permease (GabP) [138]. For both proteins, assembly in the absence of PE resulted in the inversion of their N-terminal TMSs and their adjoining cytoplasmic and periplasmic domains.
Fig. 4.
Membrane topology and function of lactose permease (LacY) of E. coli. a Topological organization of LacY in E. coli membranes with (+PE) and without (−PE) phosphatidylethanolamine (PE). LacY consists of 12 transmembrane helices divided into two six-helix bundles. In the absence of PE (−PE), the N-terminal six-helix bundle with their associated extramembrane domains adopts an inverted topology with respect to the membrane bilayer, whereas the C-terminal six-helix bundle, except for TM VII, retains its native topology. The topological organization of TM VII in −PE cells remains unknown. The transmembrane helices are labeled in roman numerals consecutively from the N-(NH2) to C-(COOH) terminus. The cytoplasmic (green) and periplasmic (pink) extramembrane domains are indicated. b Schematic representation of conformational changes in LacY upon sugar binding. LacY uses a symport mechanism to couple the transport of lactose and H+ across the membrane. The proposed mechanism of transport alternates between the outward-facing conformation (exposed to the periplasmic side) shown in steps 1–3 and the inward-facing conformation (exposed to the cytoplasm) shown in steps 4–6. Starting from the outward-facing conformation 1 LacY is protonated 2 prior to substrate binding. 3 The binding of substrate to the binding site probably induces a conformation change in the two halves of the protein (N- and C-terminal six-helix bundles) that results in the inward-facing conformation. 4 The substrate is then released into the cytoplasm 5 followed by release of the H+. 6 After releasing the H+ inside, LacY returns to the outward-facing conformation 1. The N- and C-terminal six-helix bundles of LacY are colored light and dark blue, respectively. Substrate and H+ are represented by orange circles and green squares, respectively
Changes in lipid composition also affect the lateral pressure profile of the membrane. An increase in PE, which affects the chain lateral pressure within the bilayer, lowers the folding yield of bacteriorhodopsin [141] and the multidrug-proton antiporter EmrE [142]. The increased lateral chain pressure caused by PE lipids has been found to decrease the rate of insertion of a transmembrane helix or protein across a bilayer. Furthermore, anionic lipids were found to be important in the membrane interactions of SecA [66, 143, 144].
The respiratory chain complexes
A prominent family of membrane protein complexes is the members of the respiratory chain that enable cells to oxidize various chemical compounds and collect the energy in a transmembrane potential. In eukaryotic cells, these complexes are exclusively localized in the mitochondrial membrane, which pinpoints to their bacterial origin. Bacteria have evolved a variety of different respiratory chains that vary from sulfate to nitrate, fumarate, and oxygen as their terminal electron acceptors. Each system, expressed in certain bacterial strains, provides the metabolic basis for their existence in distinct ecological niches.
The respiratory chain complexes are localized in the inner membrane. Depending on the availability of oxygen, E. coli expresses two different NADH dehydrogenases, NDH-1 and NDH-2, and two different ubiquinol oxidase complexes, cytochrome bo 3 complex (contains heme b and heme o) and cytochrome bd complex (contains heme b and heme d). The bo 3 ubiquinol oxidase from E. coli contains four subunits, CyoABCD (or subunits II, I, III, IV, respectively), and is the predominant oxidase when the oxygen levels are high, whereas under low-oxygen conditions, the bd ubiquinol oxidase is more abundant.
When oxygen is limiting, the proton-coupled dehydrogenase NDH-1 is expressed that consists of seven transmembrane and six peripheral subunit proteins designated as NuoA to NuoN, where NuoC and NuoD are fused proteins in E. coli [145]. NDH-1 is one of the largest protein complexes in the bacterial membrane with a total mass of ~550 kDa. Electron microscopy has shown that the protein complex has an L-shaped structure, with the hydrophobic arm embedded in the membrane and the hydrophilic peripheral arm protruding into the cytoplasm [146, 147]. Recently, the crystal structure of the hydrophilic peripheral arm of NDH-1 from Thermus thermophilus has been solved [148]. The crystal structure revealed that this subcomplex consists of eight subunits and contains all the redox centers of the enzyme, including nine iron-sulfur clusters. The assembly of the NDH-1 complex is impaired in YidC-depleted cells [149], suggesting that YidC is involved in the insertion or assembly process. In contrast, NDH-2 consists of a single protein, NdhA, of 434 amino acids and is not capable of translocating protons across the membrane. It is presumably membrane-anchored at the C-terminus [150]. So far, no tertiary structure is available for NDH-2. Both enzymes oxidize NADH and reduce ubiquinone to ubiquinol.
The bo 3 ubiquinol oxidase is a terminal oxidase in E. coli that is closely related to the cytochrome oxidase of mitochondria. The structure of the complex has been solved at 3.5 Å resolution, which allows to follow the path of the electron from the bound ubiquinol to the oxygen [151]. It involves a heme b, a heme o 3 and a copper center (CuB) in the CyoB subunit (subunit I) that is a 15-spanning membrane protein. The ubiquinol binding site is localized in CyoA (subunit II) that has two transmembrane helices and an N-terminal lipid moiety. In the bo 3 ubiquinol oxidase, the subunits CyoC (subunit III) and CyoD (subunit IV) span the membrane five and three times, respectively. The membrane insertion of CyoA has been extensively studied and involves YidC to translocate the first periplasmic loop and SecYEG to translocate the C-terminal region [106, 107]. The protein is synthesized with a signal sequence that is cleaved by signal peptidase II after its acylation [152]. Depletion of YidC also revealed a reduction in the level of CyoB in the inner membrane of E. coli [125]. Recently, the assembly pathway of bo 3 ubiquinol oxidase of E. coli was proposed to occur in a preferred order, in which subunits CyoC and CyoD assemble first, followed by CyoB and CyoA [153].
In E. coli, anaerobic respiration occurs with various systems, all involving a NADH dehydrogenase and ubiquinol [154]. Ubiquinol is oxidized by the fumarate reductase, FrdABCD, whereby the C and D subunits are three-spanning integral membrane proteins that have their N-termini located in the cytoplasm and their C-termini in the periplasm [155]. The remaining two subunits, the flavoprotein FrdA and the iron protein FrdB, comprise the cytoplasmic part of the complex. Since in YidC-depleted cells fumarate reduction is inhibited and the assembly of FrdABCD is strongly reduced, the insertion of subunits C and D might require YidC [149]. Likewise, the nitrate reduction is hampered in the absence of YidC. In E. coli, the nitrate reductase is composed of three subunits, NarGHI, where NarI spans the membrane five times and harbors two b-type cytochromes that transport the electrons to the subunits NarH and NarG at the cytoplasmic face of the membrane [156].
The photosynthesis complexes
Since photosynthesis is an evolutionary old process that converts solar energy into chemical energy, a number of bacteria have relatively simple systems when compared to plants. In particular, the photosynthetic systems in purple bacteria, such as Rhodopseudomonas viridis and Rhodobacter sphaeroides have been extensively investigated. The photosynthetic reaction center was one of the first membrane protein complexes to be crystallized and structurally characterized [157, 158]. It consists of three protein subunits, the M, L and H proteins. Two of these protein subunits, M and L, span the membrane five times and contain carotenoid and bacteriochlorophylls. Only the L-linked bacteriochlorophylls transfer the electrons to the bound ubiquinone.
Light-harvesting complexes (LHC) are present in photosynthetic membranes to increase the efficiency of light energy harvesting. In Rhodospirillum rubrum, the LHC surrounds the reaction center as a ring structure composed of two small proteins, the α and β subunits. Both are single-spanning proteins that assemble into ring structures of 16 heterodimers [159]. Bacteriochlorophylls and carotinoids are bound to the heterodimers that absorb the light and transfer the excitation energy to the reaction center.
The evolution of the photosynthetic system in cyanobacteria to use water as a source of electrons was made possible by combining two reaction centers in tandem, the photosystem II and I. A wealth of structural data demonstrate the complexity of these systems in Synechococcus (see [160] for a review). Recent studies have shown that the cpSRP (SRP in chloroplasts) and the YidC homologue (Alb3) are important for the assembly of several light-harvesting chlorophyll-binding proteins into thylakoid membranes [111, 161]. In the cyanobacterium Synechocystis sp. PCC6803, a deletion of the YidC homologue causes severe defects in the assembly of the photosystems [162].
The ATP synthase complex
The F1Fo ATP synthase of E. coli is comprised of two subcomplexes, the membrane-integral Fo complex (a 1 b 2 c 10) and the peripheral F1 complex (α 3 β 3 γδε) (Fig. 5). The ATP synthase plays a key role in biological energy metabolism. The F1Fo ATP synthase converts the energy stored in a transmembrane electrochemical gradient of protons or Na+ ions into ATP or generates a transmembrane ion gradient at the expense of ATP hydrolysis under conditions of low driving force. While the peripheral membrane F1 complex synthesizes or hydrolyzes ATP, the integral membrane Fo complex serves as a proton pump that is sensitive to the antibiotic oligomycin. The crystal structure of the mitochondrial F1 complex has been solved at high resolution [163]. Recently, two crystal structures of the rotor ring from a Na+-translocating bacterium, Ilyobacter tartaricus [164] and from a proton-translocating cyanobacterium, Spirulina platensis [165] have been solved. The complete structure of the ATP synthase complex of E. coli has been modeled [166].
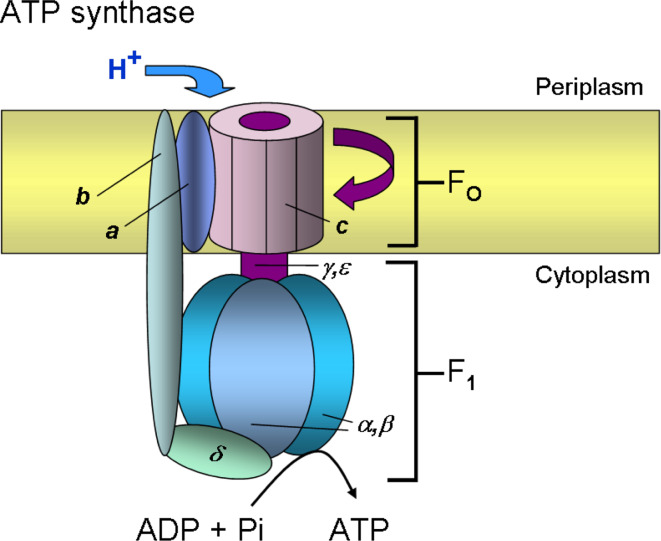
Fig. 5.
Model of E. coli F1Fo-ATPase. The ATP synthase consists of two subcomplexes with eight different proteins, the membrane-integral Fo complex (a 1 b 2 c 10) and the peripheral F1 complex (α 3 β 3 γδε). The c subunit of Fo is linked to the γ and ε subunits to form the central rotor (purple). Subunits b and δ form a stator which ensures that subunits a in Fo and the α 3 β 3 hexamer of F1 do not rotate with the central rotor (γεc ring). The proton pathway lies between the a and c subunits. Either H+ translocation through Fo or ATP hydrolysis in F1 leads to the rotary movement of the central rotor element (γεc ring)
Subunit a (Fo a) of the Fo complex is a polytopic membrane protein with five TMSs with a N-out/C-in orientation. Subunit b (Fo b) is a single-spanning membrane protein with a short N-terminal region located in the periplasm and a longer C-terminus in the cytoplasm. Subunit c (Fo c) consists of two transmembrane helices that form a helical hairpin. The short N- (eight residues) and C-termini (three residues) are located in the periplasm. The subunit c forms an oligomeric ring in the membrane that rotates in response to the pmf. In the center of the c ring, the γε proteins bind and extend the rotation into the peripheral F1 complex. Two b subunits and one a subunit form the membrane-embedded stator that binds to the peripheral α 3 β 3 complex via the δ protein.
Recent studies have shown that the membrane insertion of Fo c is strictly dependent on YidC [99, 167, 168], whereas Fo a and Fo b of the F1Fo ATP synthase require both YidC and SecYEG for membrane insertion [169, 170]. The membrane insertion of Fo a and Fo b is also dependent on SRP [169, 170]. The translocation of the N-terminal tail of Fo a requires the pmf, whereas the translocation of the second periplasmic loop is pmf-independent [169]. Previous studies showed that Fo a is unstable in the absence of the partner molecules, Fo b and Fo c, and is readily degraded by the ATP-dependent membrane protease FtsH [132, 171].
The positive-charged residues in the cytoplasmic loop (M-region) of Fo c appear to be important determinants for YidC binding and subsequent membrane insertion [172]. Removal of these M-region charges affects the binding of Fo c to YidC and membrane insertion. Previously, it had been demonstrated that proteoliposomes containing only YidC support both the stable membrane insertion and oligomerization of Fo c [99].
The permeases
Transmembrane ion gradients are used to transport nutrients and other solutes into the interior of the bacterial cell. In contrast to primary active transporters, which utilize a primary energy source (i.e., ATP hydrolysis, photon absorption or electron flow) to catalyze the transport of substrates, secondary active transporters utilize the free energy stored in the electrochemical ion gradient to drive the transport of substrates. These secondary transporters include the permeases which are operating either as symporters or antiporters, respective of their transport direction and ion movement. The most prominent permease is the lactose permease of E. coli that transports β-galactosides with protons. A crystal structure of LacY [173] combined with a wealth of biochemical data (see [174] for a review) has led to an alternating access model for lactose/H+ symport (Fig. 4b). The N-terminal and C-terminal half of LacY fold into two pseudosymmetrical six-helix bundles. The interface of these N- and C-terminal six-helix bundles forms an internal hydrophilic cavity accessible to one side of the membrane [173]. The crystal structure shows the sugar-binding sites in detail in the center of the hydrophilic cavity. A possible mechanism of transport is suggested by first binding a proton, then binding of lactose followed by a conformational change where the N- and C-terminal six-helix bundles move to close the periplasmic opening and open a cytoplasmic cavity. The substrate and the proton are then released and the conformation may return to the outward-facing cavity. Proton translocation involves residues primarily in the C-terminal six-helix bundle. Intriguingly, the presence of the substrate on the periplasmic side controls the opening of the outward cavity [175]. The basic structure and transport mechanism observed for the lactose permease is likely to be valid for other permeases and members of the major facilitator superfamily (MFS) of membrane transporters [176]. These include the multidrug transporters AcrB and EmrD [177, 178], the melibiose transporter MelB [179], and GlpT [180], a glycerol phosphate antiporter.
LacY is targeted to the membrane co-translationally by the SRP pathway and requires the SecYEG complex for insertion into the inner membrane (see [122] and references therein). The Sec complex anchors the protein in the membrane as verified by its resistance to urea extraction. YidC does not appear to be required for the insertion of LacY into the membrane, but is important in the folding of LacY into its final tertiary conformation in the membrane [122]. In vitro experiments with monoclonal antibodies, directed against conformational epitopes on LacY, revealed that YidC is required for exposing the antigenic epitopes of LacY in the correct conformation.
Other secondary transporters that support the alternating access model for membrane transport are the Na+-coupled symporters LeuT (Na+/leucine transporter) [181], BetP (Na+/betaine transporter) [182], vSGLT (Na+/galactose transporter) [183], and Mhp1 (benzyl-hydantoin transporter) [184]. Crystal structures of the different Na+ symporters revealed a similar overall architecture in the structures, which is not reflected in sequence similarity. Although their number of transmembrane helices differ from 12 (LeuT, BetP, Mhp1) to 14 (vSGLT), they all share a core structure of inverted repeats of five transmembrane helices each. The structures of these different Na+ symporters indicate an alternating mechanism of transport involving at least three states: open to outside, occluded, and open to inside. The different conformations interconvert to bring about membrane transport. To date, nothing is known about the synthesis, insertion, and assembly of these transporters. Most likely, they are operating as monomers in the membrane. However, BetP is organized as trimers and it has been shown that the C-terminal region and helix seven of one monomer make contact to the next monomer [182].
The ABC transporters
ATP-binding cassette (ABC) transporters are a large family of membrane protein complexes that use the binding and hydrolysis of ATP to power the transport of solutes across the membrane [185, 186]. The ATP-driven transporters are three component systems, involving a periplasmic substrate-binding protein, a transmembrane transporter unit, and a cytoplasmic ATP-binding component. These primary transporters are mainly involved in sugar and amino-acid uptake, but some are known to export lipo-oligosaccharides, lipidA, and teichoic acids [187]. In bacteria, the majority of ABC transporters are importers. The best-studied ABC transporter is the maltose importer MalFGK2 (Fig. 6).
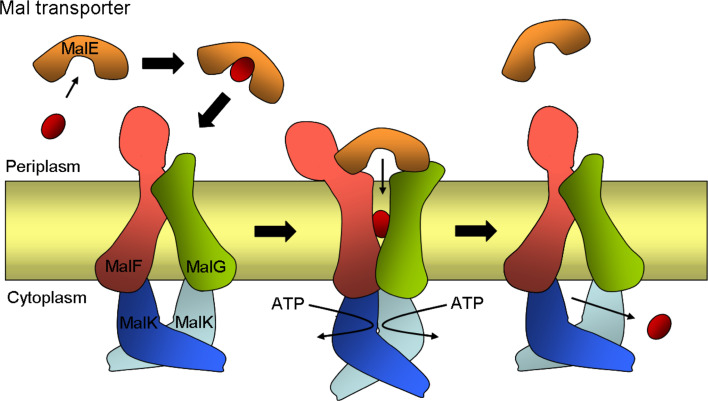
Fig. 6.
Schematic drawing of inward- and outward-facing structures of the maltose transporter, MalFGK2, of E. coli. The maltose transporter complex is composed of a periplasmic maltose-binding protein (MalE), two integral membrane proteins, MalF and MalG, and two copies of the cytoplasmic ATP-binding cassette subunit MalK. In the transport cycle, MalE binds and delivers maltose to the resting state transporter in the closed conformation (shown on the left). Interactions with MalE in the presence of maltose stimulate ATPase activity of the transporter by triggering major conformational changes in which the two cytosolic MalK subunits close and the transmembrane helices of MalF and MalG reorient to receive the substrate from MalE (shown in the middle). After ATP hydrolysis, the outward-facing conformation is no longer stable and the transporter returns to the inward-facing resting state releasing maltose (shown on the right). Maltose is indicated by a red sphere
The maltose transporter MalFGK2 of E. coli is composed of two integral membrane proteins, MalF and MalG, which form the translocation pathway, and two copies of the ATP-binding cassette subunit MalK [188]. A periplasmic maltose-binding protein (MBP), MalE acts as a maltose receptor in delivering maltose to MalFGK2. The MBP also stimulates the ATPase activity of MalFGK2 [189]. The crystal structure of the intact transporter MalFGK2 with the MBP was determined to high resolution in an outward-facing conformation (open to the periplasm) [190] and recently, another structure of this complex has been resolved in an inward-facing conformation (open to the cytoplasm) where both ATP-binding sites of the MalK dimer are closed with ATP [191]. Comparison of the two structures of the transporter reveals details of the transport mechanism for ABC importers, which involves global re-arrangements of the integral membrane proteins MalF and MalG with the closure and opening of the ATP-binding sites of the MalK dimer.
The integral membrane subunit MalF consists of eight TMSs with both N- and C-termini located in the cytoplasm [192]. MalF includes a large periplasmic domain (MalF-P2) between the third and the fourth TMS. In the crystal structure, MalF-P2 is in contact with MalE [190]. For its biogenesis, MalF is targeted to the inner membrane via the SRP pathway and insertion is SecAYE-dependent [37]. YidC is required for the folding stability of MalF before it is incorporated into the MalFGK2 complex and contacting the six-spanning MalG subunit [37].
PTS transporters
In bacteria, the transport of some sugars across the membrane is mediated by the phosphoenolpyruvate dependent phosphotransferase system (PTS) (see [193] for a review). PTS is a bacterial signal-transduction pathway that involves phosphoryl transfer from phosphoenolpyruvate to enzyme I, HPr, enzyme II to the membrane-bound sugar. Enzyme II consists of an integral membrane component, termed enzyme IIC or IICD, and two associated components in the cytoplasm, enzyme IIA and enzyme IIB, that transfer the energy-rich phosphate to the substrate, which is bound to IIC [194]. Enzyme II is sugar-specific and of modular architecture. Depending on the sugar, enzyme II exists as a single protein (mannitol PTS), as two proteins IIA and IICB (glucose PTS), or as three proteins IIAB, IIC, and IID (mannose PTS), where IIC and IID are localized in the membrane. The glucose enzyme IICB spans the membrane eight times, whereas the mannose IIC is a six-spanning and IID a single-spanning inner-membrane protein [195]. To date, structural data exist only for the soluble PTS components showing details of the phosphoryl transfer sites (see [196] and references therein). A 5-Å two-dimensional projection map is available for the membrane-embedded C-domain of the mannitol-specific enzyme II-Mtl [197]. EIIC functions as a dimer that has one binding site for mannitol [198]. The biosynthesis and membrane insertion of EIIC has not been studied in detail. However, circularly permuted variants of the E. coli EIICB-Glc have been constructed and analyzed for activity [199]. One of them, CL3, has the TMSs 7 and 8 at the N-terminus and the TMSs 1–6 at the C-terminus. Although the order of the transmembrane regions is changed, the activity of the protein reached 70% of the wild-type, suggesting that the initiation of membrane insertion is not restricted to TM1 at the N-terminus.
Aquaporins and mechanosensitive channels
Bacteria are often exposed to osmolarity changes in their natural habitats. Aquaporins (AQPs) are a large family of membrane channels that are highly specific for water (AQPs) or small uncharged hydrophilic solutes such as glycerol or urea (aquaglyceroporins) [200, 201]. These channel proteins do not allow the permeation of small or charged solutes or even protons, thereby preserving proton gradients. Sequence analysis suggested that the members of the AQP family arose by tandem gene duplication [202] where the N-terminal segment has ~20% conservation with the C-terminal segment [203]. The structures of several AQPs in diverse life forms have been determined to date (see [204] and references therein). The crystal structures of AQPs revealed a common molecular architecture containing four monomeric subunits, each consisting of six transmembrane spanning α-helices, assembled into a homotetrameric structure. The six TMSs from each subunit form an independent aqueous pore, whereby helices 1–3 and helices 4–6 sit in the membrane in opposite orientations. A seventh transmembrane region is created by two unusually long loops, which form half helices in the channel from opposite sides of the membrane, creating a constitutively open, narrow aqueous pathway. Both ends of these half helices contain the highly conserved Asn-Pro-Ala signature motif near its center. Studies have suggested that the two asparagines in the Asn-Pro-Ala motif form hydrogen bonds with water molecules and mediate the exchange of waters between the two halves of the channel by flipping the water molecule into a different orientation (see [205] and references therein). How AQP Z of E. coli is inserted into the membrane is presently unknown. The protein has been synthesized in vitro and was inserted into liposomes, suggesting that it might do this without an insertase or translocase [206]. The addition of purified FtsY increased the binding of the protein to the liposomes but not its insertion.
Bacteria also have the ability to sense physical stress within its environment with the help of mechanosensitive ion channels. For example, the mechanosensitive channels, MscL and MscS, play a primary role in protecting cells exposed to osmotic downshock [207]. Determination of the crystal structure of a mechanosensitive channel of large conductance, MscL, from Mycobacterium tuberculosis revealed a homopentameric channel in an apparently closed state [208]. Each subunit consists of two transmembrane α-helices, TM1 and TM2, and a cytoplasmic N- and C-terminal domain. The closed pore is lined by the five tightly packed TM1 segments, whereas the five TM2 segments form an external surrounding ring. The pore of the closed channel is approximately 18 Å in diameter at the periplasmic side but narrows to approximately 2 Å at the cytoplasmic side. The constriction is believed to be the channel gate, which is formed by a group of amino acids (five Val and five Ile) at the cytoplasmic end of TM1. The diameter of the protein is found to increase by 16 Å upon channel activation. In contrast, the crystal structure of MscL from Staphylococcus aureus revealed a tetrameric channel in an expanded intermediate state [209]. It is not uncommon in different species that certain multimeric membrane proteins form distinct oligomers. In E. coli, MscL requires only SRP and YidC for membrane targeting and insertion [100].
The structure of a mechanosensitive channel of small conductance, MscS, revealed a homoheptameric channel [210]. Each homoheptameric subunit consists of three transmembrane helices (TM1–TM2–TM3) with the N-terminus in the periplasm and the C-terminus in the cytoplasm. In this channel, the TM3 helices line the channel pore, whereas the TM1 and TM2 helices are in a direct contact with the surrounding lipids.
The sensor proteins
Various signals, particularly the presence of nutrients such as amino acids and sugars, are detected at the periplasmic surface of bacteria by integral sensor proteins. In E. coli, five different membrane-bound chemotactic sensor proteins are known: Tsr, Tar, Tap, Trg, and Aer that sense serine, aspartate and maltose, dipeptides, ribose and galactose, and oxygen, respectively. A number of other bacteria have more transmembrane chemoreceptors, also known as methyl-accepting chemotaxis proteins, e.g., Vibrio cholerae has 45 different chemotactic sensor proteins [211]. The chemotactic sensor proteins form arrays at the cell poles (see [212] for a review). Each subunit of the transmembrane chemoreceptor dimer spans the inner membrane twice creating a membrane-spanning 4-α-helix bundle. The homodimers assemble into trimers of dimers and associate with CheW and CheA in the cytoplasm. CheW is a linker protein and CheA is a histidine kinase. The ligand binds to the periplasmic domain of the receptor [213, 214]. Depending on the external stimuli (attractant or repellent), the receptor signaling decreases or increases the autophosphorylation activity of CheA. In the case of autophosphorylation, the phosphoryl group is transferred to a response regulator, CheY, which in turn controls the rotational direction of the flagellar motor. It has been reported that the serine chemoreceptor Tsr of E. coli, which consists of a large periplasmic domain of about 150 amino acids, requires SecA for membrane insertion [215]. Three-dimensional images of Tar-GFP have shown that the aspartate chemoreceptor Tar colocalizes transiently with the Sec machinery that forms a helical structure in the cell [216].
The KdpD protein of E. coli is a membrane component of the KdpD/E two-component signal transduction system involved in maintaining the intracellular osmolarity. The integral sensor protein KdpD is unusual, because it consists of a large cytoplasmic N-terminal domain, four closely spaced transmembrane regions, and an extended cytoplasmic C-terminal domain. The membrane insertion of KdpD occurs independent of the Sec complex and YidC, but requires the pmf [217] and SRP [32].
The flagellar motor complex
Motile bacteria express one or many flagella that rotate clockwise or counter-clockwise depending on their regulation by chemotactic signaling proteins. The flagellar motor component, which represents the basal body, is located in the inner membrane and structured as an oligomeric ring structure (Fig. 7). The central component of the rotor is the two-spanning inner-membrane protein FliF that assembles into a 26mer to form the ring structure with a diameter of about 24 nm [218]. To this ring, three soluble proteins, FliGMN, bind at the cytoplasmic side. In the center of the ring, the flagellar export complex is situated composed of six membrane proteins, FlhAB and FliOPQR and a soluble part composed of FliHIJ in the cytoplasm. On the periplasmic side, FlgBCFG, and a rod adapter protein (FliE), bind to the rotor forming the rod that connects to the flagellar filament. The stator surrounding the ring structure is composed of only two inner-membrane proteins, the four-spanning MotA and the single-spanning MotB protein that is bound to the peptidoglycan at the C-terminus. 4MotA/2MotB form hetero-hexameric complexes and provide the proton pathway. In E. coli, an aspartic acid residue (Asp32) in the transmembrane region of MotB is functionally critical and might be protonated [219]. About a dozen of these complexes couple the proton movement with torque generation of one ring. To do this, specific electrostatic contacts between MotA and FliG occur [219]. However, the detailed mechanism of how the ring turns relative to the stator is presently unknown.
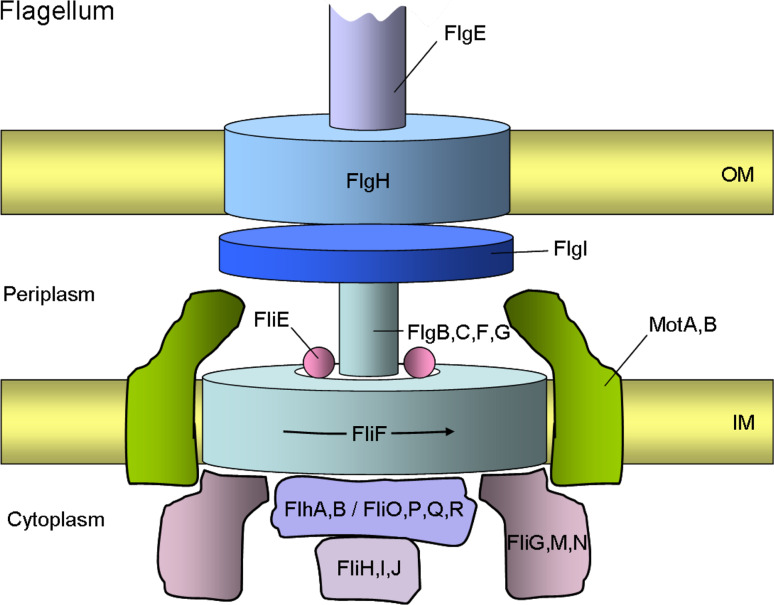
Fig. 7.
A schematic representation of a bacterial flagellum basal body. The flagellum, which works as a rotary motor, consists of a motor component called the basal body, which spans from the cytoplasm to the outer membrane (OM), a flexible hook, and a filament. The basal body is composed of a rotor, a rod, a bushing, and a switch regulator. The core of the rotor is made up of FliF. The rod components are composed of four proteins, FlgB, FlgC, FlgF, and FlgG, and a rod adapter protein, FliE. The outer ring structures assembled around the rod consists of FlgI and FlgH and are thought to act as a molecular bushing of the rotary axial structure of the flagellum. The direction of rotation is controlled by the switch proteins, FliG, FliM and FliN. The stator part of the motor is composed of MotA and MotB, which span the inner membrane (IM). MotB is anchored to the peptidoglycan layer with its C-terminal periplasmic domain. Together both proteins form the proton pathway that powers the flagellum motor. The flagellar secretion apparatus (T3SS) consists of six integral membrane proteins FlhA, FlhB, FliO, FliP, FliQ, and FliR, and three soluble components, FliH, FliI, and FliJ, at the cytoplasmic face of the flagellar basal body. These proteins facilitate the export of flagellar substrates and are dependent on ATP hydrolysis by the ATPase FliI protein. The basal body of the flagellum is linked to a flexible hook consisting of FlgE and to a helical filament (FliC). The flagellum can rotate in either a clockwise or a counter-clockwise (arrow) direction
The assembly of the flagellar motor complex has been extensively studied for the later steps that occur from the inside to the outside. First, the FliF ring is assembled in the inner membrane to which the flagellar export complex, the rod, hook, and filament is added on [220]. The assembly of the flagellum involves YidC and the Sec complex and no flagella are observed after depletion of YidC or SecE [124, 221]. The L and P ring is composed of FlgH and FlgI, respectively. These proteins are exported by the Sec complex whereas the other components of the late assembly steps are translocated by the flagellar export complex [222]. The flagellar export complex is related to the type III secretion system and the FliI component harbors the ATP-binding site that might drive the export of the individual flagellar components [223]. ATP hydrolysis is controlled by FliH and it interacts with the FliI hexamer. FlhA and FlhB interact with each other and could build the transmembrane export pore since they are located in the membrane and interact with a substrate [224]. FlhA and FliR are eight-spanning membrane proteins; FlhB is a five-spanning and FliP a four-spanning membrane protein with a cleavable signal sequence.
Conclusions
Investigating the biogenesis of membrane proteins has clearly shown that there is not a uniform pathway for these proteins to insert into the membrane bilayer. Different targeting and integrating factors such as SRP, SecB, Sec(A)YEG, and YidC can be envisioned as modules. Various combinations of these modules result in the different biogenesis pathways of membrane proteins. In the last two decades, major progress has been made in understanding the membrane transport and function of membrane proteins due to recent high-resolution structures, revealing a more detailed insight into overall domain organization and the function of possible conformational changes. In particular, the new structures of ABC transporters and permeases show how these fascinating proteins may function. This research is now providing a first glimpse into how membrane proteins move to transport ions, water, and sugar across the bilayer. Although our knowledge has improved significantly, many questions still remain unanswered about membrane proteins, for example how they tightly fold and assemble into multi-subunit complexes, but these are challenges for the future.
Acknowledgments
We apologize to the authors whose work we were unable to cite because of space limitations. We thank the Deutsche Forschungsgemeinschaft (DFG) for the long-standing support of our research.
References
- 1.Kramer G, Rauch T, Rist W, Vorderwülbecke S, Patzelt H, Schulze-Specking A, Ban N, Deuerling E, Bukau B. L23 protein functions as a chaperone docking site on the ribosome. Nature. 2002;419:171–174. doi: 10.1038/nature01047. [DOI] [PubMed] [Google Scholar]
- 2.Kaiser CM, Chang HC, Agashe VR, Lakshmipathy SK, Etchells SA, Hayer-Hartl M, Hartl FU, Barral JM. Real-time observation of trigger factor function on translating ribosomes. Nature. 2006;444:455–460. doi: 10.1038/nature05225. [DOI] [PubMed] [Google Scholar]
- 3.Rutkowska A, Mayer MP, Hoffmann A, Merz F, Zachmann-Brand B, Schaffitzel C, Ban N, Deuerling E, Bukau B. Dynamics of trigger factor interaction with translating ribosomes. J Biol Chem. 2008;283:4124–4132. doi: 10.1074/jbc.M708294200. [DOI] [PubMed] [Google Scholar]
- 4.Ferbitz L, Maier T, Patzelt H, Bukau B, Deuerling E, Ban N. Trigger factor in complex with the ribosome forms a molecular cradle for nascent proteins. Nature. 2004;431:590–596. doi: 10.1038/nature02899. [DOI] [PubMed] [Google Scholar]
- 5.Bukau B, Deuerling E, Pfund C, Craig EA. Getting newly synthesized proteins into shape. Cell. 2000;101:119–122. doi: 10.1016/S0092-8674(00)80806-5. [DOI] [PubMed] [Google Scholar]
- 6.Frydman J. Folding of newly translated proteins in vivo: the role of molecular chaperones. Annu Rev Biochem. 2001;70:603–647. doi: 10.1146/annurev.biochem.70.1.603. [DOI] [PubMed] [Google Scholar]
- 7.Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- 8.Vorderwülbecke S, Kramer G, Merz F, Kurz TA, Rauch T, Zachmann-Brand B, Bukau B, Deuerling E. Low temperature or GroEL/ES overproduction permits growth of Escherichia coli cells lacking trigger factor and DnaK. FEBS Lett. 2004;559:181–187. doi: 10.1016/S0014-5793(04)00052-3. [DOI] [PubMed] [Google Scholar]
- 9.Lee HC, Bernstein HD. Trigger factor retards protein export in Escherichia coli . J Biol Chem. 2002;277:43527–43535. doi: 10.1074/jbc.M205950200. [DOI] [PubMed] [Google Scholar]
- 10.Wild J, Altman E, Yura T, Gross CA. DnaK and DnaJ heat shock proteins participate in protein export in Escherichia coli . Genes Dev. 1992;6:1165–1172. doi: 10.1101/gad.6.7.1165. [DOI] [PubMed] [Google Scholar]
- 11.Wild J, Rossmeissl P, Walter WA, Gross CA. Involvement of the DnaK–DnaJ–GrpE chaperone team in protein secretion in Escherichia coli . J Bacteriol. 1996;178:3608–3613. doi: 10.1128/jb.178.12.3608-3613.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wild J, Walter WA, Gross CA, Altman E. Accumulation of secretory protein precursors in Escherichia coli induces the heat shock response. J Bacteriol. 1993;175:3992–3997. doi: 10.1128/jb.175.13.3992-3997.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Müller JP. Influence of impaired chaperone or secretion function on SecB production in Escherichia coli . J Bacteriol. 1996;178:6097–6104. doi: 10.1128/jb.178.21.6097-6104.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu SQ, Peske F, Wieden HJ, Rodnina MV, Wintermeyer W. The signal recognition particle binds to protein L23 at the peptide exit of the Escherichia coli ribosome. RNA. 2003;9:566–573. doi: 10.1261/rna.2196403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ullers RS, Houben EN, Raine A, ten Hagen-Jongman CM, Ehrenberg M, Brunner J, Oudega B, Harms N, Luirink J. Interplay of signal recognition particle and trigger factor at L23 near the nascent chain exit site on the Escherichia coli ribosome. J Cell Biol. 2003;161:679–684. doi: 10.1083/jcb.200302130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buskiewicz I, Deuerling E, Gu SQ, Jöckel J, Rodnina MV, Bukau B, Wintermeyer W. Trigger factor binds to ribosome-signal-recognition particle (SRP) complexes and is excluded by binding of the SRP receptor. Proc Natl Acad Sci USA. 2004;101:7902–7906. doi: 10.1073/pnas.0402231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raine A, Ivanova N, Wikberg JE, Ehrenberg M. Simultaneous binding of trigger factor and signal recognition particle to the E. coli ribosome. Biochimie. 2004;86:495–500. doi: 10.1016/j.biochi.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Randall LL, Hardy SJ, Topping TB, Smith VF, Bruce JE, Smith RD. The interaction between the chaperone SecB and its ligands: evidence for multiple subsites for binding. Protein Sci. 1998;7:2384–2390. doi: 10.1002/pro.5560071115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Driessen AJ, Manting EH, van der Does C. The structural basis of protein targeting and translocation in bacteria. Nat Struct Biol. 2001;8:492–498. doi: 10.1038/88549. [DOI] [PubMed] [Google Scholar]
- 20.Fekkes P, de Wit JG, van der Wolk JP, Kimsey HH, Kumamoto CA, Driessen AJ. Preprotein transfer to the Escherichia coli translocase requires the co-operative binding of SecB and the signal sequence to SecA. Mol Microbiol. 1998;29:1179–1190. doi: 10.1046/j.1365-2958.1998.00997.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhou J, Xu Z. Structural determinants of SecB recognition by SecA in bacterial protein translocation. Nat Struct Biol. 2003;10:942–947. doi: 10.1038/nsb980. [DOI] [PubMed] [Google Scholar]
- 22.Fekkes P, van der Does C, Driessen AJM. The molecular chaperone SecB is released from the carboxy-terminus of SecA during initiation of precursor protein translocation. EMBO J. 1997;16:6105–6113. doi: 10.1093/emboj/16.20.6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vrontou E, Economou A. Structure and function of SecA, the preprotein translocase nanomotor. Biochim Biophys Acta. 2004;1694:67–80. doi: 10.1016/j.bbamcr.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Papanikou E, Karamanou S, Economou A. Bacterial protein secretion through the translocase nanomachine. Nat Rev Microbiol. 2007;5:839–851. doi: 10.1038/nrmicro1771. [DOI] [PubMed] [Google Scholar]
- 25.von Heijne G. Protein targeting signals. Curr Opin Cell Biol. 1990;2:604–608. doi: 10.1016/0955-0674(90)90100-s. [DOI] [PubMed] [Google Scholar]
- 26.Herskovits AA, Bochkareva ES, Bibi E. New prospects in studying the bacterial signal recognition particle pathway. Mol Microbiol. 2000;38:927–939. doi: 10.1046/j.1365-2958.2000.02198.x. [DOI] [PubMed] [Google Scholar]
- 27.Valent QA, Kendall DA, High S, Kusters R, Oudega B, Luirink J. Early events in preprotein recognition in E. coli: interaction of SRP and trigger factor with nascent polypeptides. EMBO J. 1995;14:5494–5505. doi: 10.1002/j.1460-2075.1995.tb00236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valent QA, de Gier J-WL, von Heijne G, Kendall DA, ten Hagen-Jongman CM, Oudega B, Luirink J. Nascent membrane and presecretory proteins synthesized in Escherichia coli associate with signal recognition particle and trigger factor. Mol Microbiol. 1997;25:53–64. doi: 10.1046/j.1365-2958.1997.4431808.x. [DOI] [PubMed] [Google Scholar]
- 29.Valent QA, Scotti PA, High S, de Gier J-WL, von Heijne G, Lentzen G, Wintermeyer W, Oudega B, Luirink J. The Escherichia coli SRP and SecB targeting pathways converge at the translocon. EMBO J. 1998;17:2504–2512. doi: 10.1093/emboj/17.9.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee HC, Bernstein HD. The targeting pathway of Escherichia coli presecretory and integral membrane proteins is specified by the hydrophobicity of the targeting signal. Proc Natl Acad Sci USA. 2001;98:3471–3476. doi: 10.1073/pnas.051484198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peterson JH, Woolhead CA, Bernstein HD. Basic amino acids in a distinct subset of signal peptides promote interaction with the signal recognition particle. J Biol Chem. 2003;278:46155–46162. doi: 10.1074/jbc.M309082200. [DOI] [PubMed] [Google Scholar]
- 32.Maier KS, Hubich S, Liebhart H, Krauss S, Kuhn A, Facey SJ. An amphiphilic region in the cytoplasmic domain of KdpD is recognized by the signal recognition particle and targeted to the Escherichia coli membrane. Mol Microbiol. 2008;68:1471–1484. doi: 10.1111/j.1365-2958.2008.06246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Gier J-WL, Mansournia P, Valent QA, Phillips GJ, Luirink J, von Heijne G. Assembly of a cytoplasmic membrane protein in Escherichia coli is dependent on the signal recognition particle. FEBS Lett. 1996;399:307–309. doi: 10.1016/s0014-5793(96)01354-3. [DOI] [PubMed] [Google Scholar]
- 34.van der Laan M, Nouwen N, Driessen AJM. SecYEG proteoliposomes catalyze the Δψ-dependent membrane insertion of FtsQ. J Biol Chem. 2004;279:1659–1664. doi: 10.1074/jbc.M306527200. [DOI] [PubMed] [Google Scholar]
- 35.Koch HG, Hengelage T, Neumann-Haefelin C, MacFarlane J, Hoffschulte HK, Schimz KL, Mechler B, Müller M. In vitro studies with purified components reveal signal recognition particle (SRP) and SecA/SecB as constituents of two independent protein-targeting pathways of Escherichia coli . Mol Biol Cell. 1999;10:2163–2173. doi: 10.1091/mbc.10.7.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seluanov A, Bibi E. FtsY, the prokaryotic signal recognition particle receptor homologue, is essential for biogenesis of membrane proteins. J Biol Chem. 1997;272:2053–2055. doi: 10.1074/jbc.272.4.2053. [DOI] [PubMed] [Google Scholar]
- 37.Wagner S, Pop O, Haan GJ, Baars L, Koningstein G, Klepsch MM, Genevaux P, Luirink J, de Gier J-W. Biogenesis of MalF and the MalFGK2 maltose transport complex in Escherichia coli requires YidC. J Biol Chem. 2008;283:17881–17890. doi: 10.1074/jbc.M801481200. [DOI] [PubMed] [Google Scholar]
- 38.Keenan RJ, Freymann DM, Stroud RM, Walter P. The signal recognition particle. Annu Rev Biochem. 2001;70:755–775. doi: 10.1146/annurev.biochem.70.1.755. [DOI] [PubMed] [Google Scholar]
- 39.Luirink J, Sinning I. SRP-mediated protein targeting: structure and function revisited. Biochim Biophys Acta. 2004;1694:17–35. doi: 10.1016/j.bbamcr.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 40.Wild K, Rosendal KR, Sinning I. A structural step into the SRP cycle. Mol Microbiol. 2004;53:357–363. doi: 10.1111/j.1365-2958.2004.04139.x. [DOI] [PubMed] [Google Scholar]
- 41.Connolly T, Rapiejko P, Gilmore R. Requirement of GTP hydrolysis for dissociation of the signal recognition particle from its receptor. Science. 1991;252:1171–1173. doi: 10.1126/science.252.5009.1171. [DOI] [PubMed] [Google Scholar]
- 42.Koch HG, Moser M, Müller M. Signal recognition particle-dependent protein targeting, universal to all kingdoms of life. Rev Physiol Biochem Pharmacol. 2003;146:55–94. doi: 10.1007/s10254-002-0002-9. [DOI] [PubMed] [Google Scholar]
- 43.Bange G, Wild K, Sinning I. Protein translocation: checkpoint role for SRP GTPase activation. Curr Biol. 2007;17:R980–R982. doi: 10.1016/j.cub.2007.09.041. [DOI] [PubMed] [Google Scholar]
- 44.Shan SO, Chandrasekar S, Walter P. Conformational changes in the GTPase modules of the signal reception particle and its receptor drive initiation of protein translocation. J Cell Biol. 2007;178:611–620. doi: 10.1083/jcb.200702018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luirink J, ten Hagen-Jongman CM, van der Weijden CC, Oudega B, High S, Dobberstein B, Kusters R. An alternative protein targeting pathway in Escherichia coli: studies on the role of FtsY. EMBO J. 1994;13:2289–2296. doi: 10.1002/j.1460-2075.1994.tb06511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bahari L, Parlitz R, Eitan A, Stjepanovic G, Bochkareva ES, Sinning I, Bibi E. Membrane targeting of ribosomes and their release require distinct and separable functions of FtsY. J Biol Chem. 2007;282:32168–32175. doi: 10.1074/jbc.M705429200. [DOI] [PubMed] [Google Scholar]
- 47.de Leeuw E, te Kaat K, Moser C, Menestrina G, Demel R, de Kruijff B, Oudega B, Luirink J, Sinning I. Anionic phospholipids are involved in membrane association of FtsY and stimulate its GTPase activity. EMBO J. 2000;19:531–541. doi: 10.1093/emboj/19.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Millman JS, Qi H-Y, Vulcu F, Bernstein HD, Andrews DW. FtsY binds to the Escherichia coli inner membrane via interactions with phosphatidylethanolamine and membrane proteins. J Biol Chem. 2001;276:25982–25989. doi: 10.1074/jbc.M011331200. [DOI] [PubMed] [Google Scholar]
- 49.Parlitz R, Eitan A, Stjepanovic G, Bahari L, Bange G, Bibi E, Sinning I. Escherichia coli signal recognition particle receptor FtsY contains an essential and autonomous membrane-binding amphipathic helix. J Biol Chem. 2007;282:32176–32184. doi: 10.1074/jbc.M705430200. [DOI] [PubMed] [Google Scholar]
- 50.Braig D, Bär C, Thumfart J-O, Koch H-G. Two cooperating helices constitute the lipid-binding domain of the bacterial SRP receptor. J Mol Biol. 2009;390:401–413. doi: 10.1016/j.jmb.2009.04.061. [DOI] [PubMed] [Google Scholar]
- 51.de Leeuw E, Poland D, Mol O, Sinning I, ten Hagen-Jongman CM, Oudega B, Luirink J. Membrane association of FtsY, the E. coli SRP receptor. FEBS Lett. 1997;416:225–229. doi: 10.1016/s0014-5793(97)01238-6. [DOI] [PubMed] [Google Scholar]
- 52.Weiche B, Bürk J, Angelini S, Schiltz E, Thumfart JO, Koch H-G. A cleavable N-terminal membrane anchor is involved in membrane binding of the Escherichia coli SRP receptor. J Mol Biol. 2008;377:761–773. doi: 10.1016/j.jmb.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 53.Angelini S, Deitermann S, Koch HG. FtsY, the bacterial signal-recognition particle receptor, interacts functionally and physically with the SecYEG translocon. EMBO Rep. 2005;6:476–481. doi: 10.1038/sj.embor.7400385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Angelini S, Boy D, Schiltz E, Koch HG. Membrane binding of the bacterial signal recognition particle receptor involves two distinct binding sites. J Cell Biol. 2006;174:715–724. doi: 10.1083/jcb.200606093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Freymann DM, Keenan RJ, Stroud RM, Walter P. Structure of the conserved GTPase domain of the signal recognition particle. Nature. 1997;385:361–364. doi: 10.1038/385361a0. [DOI] [PubMed] [Google Scholar]
- 56.Montoya G, Svensson C, Luirink J, Sinning I. Crystal structure of the NG domain from the signal-recognition particle receptor FtsY. Nature. 1997;385:365–368. doi: 10.1038/385365a0. [DOI] [PubMed] [Google Scholar]
- 57.Zopf D, Bernstein HD, Johnson AE, Walter P. The methionine-rich domain of the 54-kD protein subunit of the signal recognition particle contains an RNA-binding site and can be crosslinked to a signal sequence. EMBO J. 1990;9:4511–4517. doi: 10.1002/j.1460-2075.1990.tb07902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Batey RT, Rambo RP, Lucast L, Rha B, Doudna JA. Crystal structure of the ribonucleoprotein core of the signal recognition particle. Science. 2000;287:1232–1239. doi: 10.1126/science.287.5456.1232. [DOI] [PubMed] [Google Scholar]
- 59.Grudnik P, Bange G, Sinning I. Protein targeting by the signal recognition particle. J Biol Chem. 2009;390:775–782. doi: 10.1515/BC.2009.102. [DOI] [PubMed] [Google Scholar]
- 60.Halic M, Becker T, Pool MR, Spahn CM, Grassucci RA, Frank J, Beckmann R. Structure of the signal recognition particle interacting with the elongation-arrested ribosome. Nature. 2004;427:808–814. doi: 10.1038/nature02342. [DOI] [PubMed] [Google Scholar]
- 61.Egea PF, Shan SO, Napetschnig J, Savage DF, Walter P, Stroud RM. Substrate twinning activates the signal recognition particle and its receptor. Nature. 2004;427:215–221. doi: 10.1038/nature02250. [DOI] [PubMed] [Google Scholar]
- 62.Focia PJ, Shepotinovskaya IV, Seidler JA, Freymann DM. Heterodimeric GTPase core of the SRP targeting complex. Science. 2004;303:373–377. doi: 10.1126/science.1090827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pohlschröder M, Prinz WA, Hartmann E, Beckwith J. Protein translocation in the three domains of life: variations on a theme. Cell. 1997;91:563–566. doi: 10.1016/s0092-8674(00)80443-2. [DOI] [PubMed] [Google Scholar]
- 64.Kuhn A. Alterations in the extracellular domain of M13 procoat protein make its membrane insertion dependent on secA and secY . Eur J Biochem. 1988;177:267–271. doi: 10.1111/j.1432-1033.1988.tb14372.x. [DOI] [PubMed] [Google Scholar]
- 65.Andersson H, von Heijne G. Sec dependent and sec independent assembly of E. coli inner-membrane proteins: the topological rules depend on chain length. EMBO J. 1993;12:683–691. doi: 10.1002/j.1460-2075.1993.tb05702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Breukink E, Demel RA, de Korte-Kool G, de Kruijff B. SecA insertion into phospholipids is stimulated by negatively charged lipids and inhibited by ATP: a monolayer study. Biochemistry. 1992;31:1119–1124. doi: 10.1021/bi00119a021. [DOI] [PubMed] [Google Scholar]
- 67.Karamanou S, Bariami V, Papanikou E, Kalodimos CG, Economou A. Assembly of the translocase motor onto the preprotein-conducting channel. Mol Microbiol. 2008;70:311–322. doi: 10.1111/j.1365-2958.2008.06402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Breukink E, Nouwen N, van Raalte A, Mizushima S, Tommassen J, de Kruijff B. The C terminus of SecA is involved in both lipid binding and SecB binding. J Biol Chem. 1995;270:7902–7907. doi: 10.1074/jbc.270.14.7902. [DOI] [PubMed] [Google Scholar]
- 69.Hunt JF, Weinkauf S, Henry L, Fak JJ, McNicholas P, Oliver DB, Deisenhofer J. Nucleotide control of interdomain interactions in the conformational reaction cycle of SecA. Science. 2002;297:2018–2026. doi: 10.1126/science.1074424. [DOI] [PubMed] [Google Scholar]
- 70.Zimmer J, Nam Y, Rapoport TA. Structure of a complex of the ATPase SecA and the protein-translocation channel. Nature. 2008;455:936–943. doi: 10.1038/nature07335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Erlandson KJ, Miller SBM, Nam Y, Osborne AR, Zimmer J, Rapoport TA. A role for the two-helix finger of the SecA ATPase in protein translocation. Nature. 2008;455:984–987. doi: 10.1038/nature07439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Economou A, Wickner W. SecA promotes preprotein translocation by undergoing ATP-driven cycles of membrane insertion and deinsertion. Cell. 1994;78:835–843. doi: 10.1016/s0092-8674(94)90582-7. [DOI] [PubMed] [Google Scholar]
- 73.Erlandson KJ, Or E, Osborne AR, Rapoport TA. Analysis of polypeptide movement in the SecY channel during SecA-mediated protein translocation. J Biol Chem. 2008;283:15709–15715. doi: 10.1074/jbc.M710356200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van der Wolk JP, de Wit JG, Driessen AJ. The catalytic cycle of the Escherichia coli SecA ATPase comprises two distinct preprotein translocation events. EMBO J. 1997;16:7297–7304. doi: 10.1093/emboj/16.24.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
-
75.Schiebel E, Driessen AJ, Hartl FU, Wickner W.
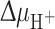 and ATP function at different steps of the catalytic cycle of preprotein translocase. Cell. 1991;64:927–939. doi: 10.1016/0092-8674(91)90317-r. [DOI] [PubMed] [Google Scholar]
and ATP function at different steps of the catalytic cycle of preprotein translocase. Cell. 1991;64:927–939. doi: 10.1016/0092-8674(91)90317-r. [DOI] [PubMed] [Google Scholar] - 76.Bessonneau P, Besson V, Collinson I, Duong F. The SecYEG preprotein translocation channel is a conformationally dynamic and dimeric structure. EMBO J. 2002;21:995–1003. doi: 10.1093/emboj/21.5.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Duong F. Binding, activation and dissociation of the dimeric SecA ATPase at the dimeric SecYEG translocase. EMBO J. 2003;22:4375–4384. doi: 10.1093/emboj/cdg418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tziatzios C, Schubert D, Lotz M, Gundogan D, Betz H, Schägger H, Haase W, Duong F, Collinson I. The bacterial protein-translocation complex: SecYEG dimers associate with one or two SecA molecules. J Mol Biol. 2004;340:513–524. doi: 10.1016/j.jmb.2004.04.076. [DOI] [PubMed] [Google Scholar]
- 79.Meyer TH, Ménétret JF, Breitling R, Miller KR, Akey CW, Rapoport TA. The bacterial SecY/E translocation complex forms channel-like structures similar to those of the eukaryotic Sec61p complex. J Mol Biol. 1999;285:1789–1800. doi: 10.1006/jmbi.1998.2413. [DOI] [PubMed] [Google Scholar]
- 80.Manting EH, van der Does C, Remigy H, Engel A, Driessen AJ. SecYEG assembles into a tetramer to form the active protein translocation channel. EMBO J. 2000;19:852–861. doi: 10.1093/emboj/19.5.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Scheuring J, Braun N, Nothdurft L, Stumpf M, Veenendaal AK, Kol S, van der Does C, Driessen AJ, Weinkauf S. The oligomeric distribution of SecYEG is altered by SecA and translocation ligands. J Mol Biol. 2005;354:258–271. doi: 10.1016/j.jmb.2005.09.058. [DOI] [PubMed] [Google Scholar]
- 82.Hanein D, Matlack KE, Jungnickel B, Plath K, Kalies KU, Miller KR, Rapoport TA, Akey CW. Oligomeric rings of the Sec61p complex induced by ligands required for protein translocation. Cell. 1996;87:721–732. doi: 10.1016/s0092-8674(00)81391-4. [DOI] [PubMed] [Google Scholar]
- 83.Yahr TL, Wickner WT. Evaluating the oligomeric state of SecYEG in preprotein translocase. EMBO J. 2000;19:4393–4401. doi: 10.1093/emboj/19.16.4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mori H, Tsukazaki T, Masui R, Kuramitsu S, Yokoyama S, Johnson AE, Kimura Y, Akiyama Y, Ito K. Fluorescence resonance energy transfer analysis of protein translocase. SecYE from Thermus thermophilus HB8 forms a constitutive oligomer in membranes. J Biol Chem. 2003;278:14257–14264. doi: 10.1074/jbc.M300230200. [DOI] [PubMed] [Google Scholar]
- 85.Osborne AR, Rapoport TA. Protein translocation is mediated by oligomers of the SecY complex with one SecY copy forming the channel. Cell. 2007;129:97–110. doi: 10.1016/j.cell.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 86.Veenendaal AKJ, van der Does C, Driessen AJM. The protein-conducting channel SecYEG. Biochim Biophys Acta. 2004;1694:81–95. doi: 10.1016/j.bbamcr.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 87.Joly JC, Leonard MR, Wickner WT. Subunit dynamics in Escherichia coli preprotein translocase. Proc Natl Acad Sci USA. 1994;91:4703–4707. doi: 10.1073/pnas.91.11.4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kihara A, Akiyama Y, Ito K. FtsH is required for proteolytic elimination of uncomplexed forms of SecY, an essential protein translocase subunit. Proc Natl Acad Sci USA. 1995;92:4532–4536. doi: 10.1073/pnas.92.10.4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van den Berg B, Clemons WM, Jr, Collinson I, Modis Y, Hartmann E, Harrison SC, Rapoport TA. X-ray structure of a protein-conducting channel. Nature. 2004;427:36–44. doi: 10.1038/nature02218. [DOI] [PubMed] [Google Scholar]
- 90.Cannon KS, Or E, Clemons WM, Jr, Shibata Y, Rapoport TA. Disulfide bridge formation between SecY and a translocating polypeptide localizes the translocation pore to the center of SecY. J Cell Biol. 2005;169:219–225. doi: 10.1083/jcb.200412019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tsukazaki T, Mori H, Fukai S, Ishitani R, Mori T, Dohmae N, Perederina A, Sugita Y, Vassylyev DG, Ito K, Nureki O. Conformational transition of Sec machinery inferred from bacterial SecYE structures. Nature. 2008;455:988–991. doi: 10.1038/nature07421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Duong F, Wickner W. Distinct catalytic roles of the SecYE, SecG and SecDFyajC subunits of preprotein translocase holoenzyme. EMBO J. 1997;16:2756–2768. doi: 10.1093/emboj/16.10.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gardel C, Johnson K, Jacq A, Beckwith J. The secD locus of E. coli codes for two membrane proteins required for protein export. EMBO J. 1990;9:3209–3216. doi: 10.1002/j.1460-2075.1990.tb07519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Törnroth-Horsefield S, Gourdon P, Horsefield R, Brive L, Yamamoto N, Mori H, Snijder A, Neutze R. Crystal structure of AcrB in complex with a single transmembrane subunit reveals another twist. Structure. 2007;15:1663–1673. doi: 10.1016/j.str.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 95.Pogliano JA, Beckwith J. SecD and SecF facilitate protein export in Escherichia coli . EMBO J. 1994;13:554–561. doi: 10.1002/j.1460-2075.1994.tb06293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen M, Xie K, Yuan J, Yi L, Facey SJ, Pradel N, Wu L-F, Kuhn A, Dalbey RE. Involvement of SecDF and YidC in the membrane insertion of M13 procoat mutants. Biochemistry. 2005;44:10741–10749. doi: 10.1021/bi047418k. [DOI] [PubMed] [Google Scholar]
- 97.Samuelson JC, Chen M, Jiang F, Möller I, Wiedmann M, Kuhn A, Phillips GJ, Dalbey RE. YidC mediates membrane protein insertion in bacteria. Nature. 2000;406:637–641. doi: 10.1038/35020586. [DOI] [PubMed] [Google Scholar]
- 98.Chen M, Samuelson JC, Jiang F, Müller M, Kuhn A, Dalbey RE. Direct interaction of YidC with the Sec-independent Pf3 coat protein during its membrane protein insertion. J Biol Chem. 2002;277:7670–7675. doi: 10.1074/jbc.M110644200. [DOI] [PubMed] [Google Scholar]
- 99.van der Laan M, Bechtluft P, Kol S, Nouwen N, Driessen AJM. F1Fo ATP synthase subunit c is a substrate of the novel YidC pathway for membrane protein biogenesis. J Cell Biol. 2004;165:213–222. doi: 10.1083/jcb.200402100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Facey SJ, Neugebauer SA, Krauss S, Kuhn A. The mechanosensitive channel protein MscL is targeted by the SRP to the novel YidC membrane insertion pathway of Escherichia coli . J Mol Biol. 2007;365:995–1004. doi: 10.1016/j.jmb.2006.10.083. [DOI] [PubMed] [Google Scholar]
- 101.Nouwen N, Driessen AJ. SecDFyajC forms a heterotetrameric complex with YidC. Mol Microbiol. 2002;44:1397–1405. doi: 10.1046/j.1365-2958.2002.02972.x. [DOI] [PubMed] [Google Scholar]
- 102.Xie K, Kiefer D, Nagler G, Dalbey RE, Kuhn A. Different regions of the nonconserved large periplasmic domain of Escherichia coli YidC are involved in the SecF interaction and membrane insertase activity. Biochemistry. 2006;45:13401–13408. doi: 10.1021/bi060826z. [DOI] [PubMed] [Google Scholar]
- 103.Scotti PA, Urbanus ML, Brunner J, de Gier JW, von Heijne G, van der Does C, Driessen AJM, Oudega B, Luirink J. YidC, the Escherichia coli homologue of mitochondrial Oxa1p, is a component of the Sec translocase. EMBO J. 2000;19:542–549. doi: 10.1093/emboj/19.4.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Beck K, Eisner G, Trescher D, Dalbey RE, Brunner J, Müller M. YidC, an assembly site for polytopic Escherichia coli membrane proteins located in immediate proximity to the SecYE translocon and lipids. EMBO Rep. 2001;2:709–714. doi: 10.1093/embo-reports/kve154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Urbanus ML, Scotti PA, Fröderberg L, Sääf A, de Gier J-WL, Brunner J, Samuelson JC, Dalbey RE, Oudega B, Luirink J. Sec-dependent membrane protein insertion: sequential interaction of nascent FtsQ with SecY and YidC. EMBO Rep. 2001;2:524–529. doi: 10.1093/embo-reports/kve108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Celebi N, Yi L, Facey SJ, Kuhn A, Dalbey RE. Membrane biogenesis of subunit II of cytochrome bo oxidase: contrasting requirements for insertion of the N-terminal and C-terminal domains. J Mol Biol. 2006;357:1428–1436. doi: 10.1016/j.jmb.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 107.van Bloois E, Haan GJ, de Gier JW, Oudega B, Luirink J. Distinct requirements for translocation of the N-tail and C-tail of the Escherichia coli inner membrane protein CyoA. J Biol Chem. 2006;281:10002–10009. doi: 10.1074/jbc.M511357200. [DOI] [PubMed] [Google Scholar]
- 108.Dalbey RE, Kuhn A. Evolutionarily related insertion pathways of bacterial, mitochondrial, and thylakoid membrane proteins. Annu Rev Cell Dev Biol. 2000;16:51–87. doi: 10.1146/annurev.cellbio.16.1.51. [DOI] [PubMed] [Google Scholar]
- 109.Bonnefoy N, Chalvet F, Hamel P, Slonimski PP, Dujardin G. OXA1, a Saccharomyces cerevisiae nuclear gene whose sequence is conserved from prokaryotes to eukaryotes controls cytochrome oxidase biogenesis. J Mol Biol. 1994;239:201–212. doi: 10.1006/jmbi.1994.1363. [DOI] [PubMed] [Google Scholar]
- 110.Funes S, Nargang FE, Neupert W, Herrmann JM. The Oxa2 protein of Neurospora crassa plays a critical role in the biogenesis of cytochrome oxidase and defines a ubiquitous subbranch of the Oxa1/YidC/Alb3 protein family. Mol Biol Cell. 2004;15:1853–1861. doi: 10.1091/mbc.E03-11-0789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Moore M, Harrison MS, Peterson EC, Henry R. Chloroplast Oxa1p homolog albino3 is required for post-translational integration of the light harvesting chlorophyll-binding protein into thylakoid membranes. J Biol Chem. 2000;275:1529–1532. doi: 10.1074/jbc.275.3.1529. [DOI] [PubMed] [Google Scholar]
- 112.Gerdes L, Bals T, Klostermann E, Karl M, Philippar K, Hünken M, Soll J, Schünemann D. A second thylakoid membrane-localized Alb3/Oxa1/YidC homologue is involved in proper chloroplast biogenesis in Arabidopsis thaliana . J Biol Chem. 2006;281:16632–16642. doi: 10.1074/jbc.M513623200. [DOI] [PubMed] [Google Scholar]
- 113.Jiang F, Yi L, Moore M, Chen M, Rohl T, van Wijk K-J, de Gier J-WL, Henry R, Dalbey RE. Chloroplast YidC homolog Albino3 can functionally complement the bacterial YidC depletion strain and promote membrane insertion of both bacterial and chloroplast thylakoid proteins. J Biol Chem. 2002;277:19281–19288. doi: 10.1074/jbc.M110857200. [DOI] [PubMed] [Google Scholar]
- 114.van Bloois E, Nagamori S, Koningstein G, Ullers RS, Preuss M, Oudega B, Harms N, Kaback HR, Herrmann JM, Luirink J. The Sec-independent function of Escherichia coli YidC is evolutionary-conserved and essential. J Biol Chem. 2005;280:12996–13003. doi: 10.1074/jbc.M414094200. [DOI] [PubMed] [Google Scholar]
- 115.van Bloois E, Koningstein G, Bauerschmitt H, Herrmann JM, Luirink J. Saccharomyces cerevisiae Cox18 complements the essential Sec-independent function of Escherichia coli YidC. FEBS J. 2007;274:5704–5713. doi: 10.1111/j.1742-4658.2007.06094.x. [DOI] [PubMed] [Google Scholar]
- 116.Benz M, Bals T, Gügel IL, Piotrowski M, Kuhn A, Schünemann D, Soll J, Ankele E. Alb4 of Arabidopsis promotes assembly and stabilization of a non chlorophyll-binding photosynthetic complex, the CF1CFo-ATP synthase. Mol Plant. 2009;2:1410–1424. doi: 10.1093/mp/ssp095. [DOI] [PubMed] [Google Scholar]
- 117.Yen MR, Harley KT, Tseng YH, Saier MH., Jr Phylogenetic and structural analyses of the oxa1 family of protein translocases. FEMS Microbiol Lett. 2001;204:223–231. doi: 10.1111/j.1574-6968.2001.tb10889.x. [DOI] [PubMed] [Google Scholar]
- 118.Yu Z, Koningstein G, Pop A, Luirink J. The conserved third transmembrane segment of YidC contacts nascent Escherichia coli inner-membrane proteins. J Biol Chem. 2008;283:34635–34642. doi: 10.1074/jbc.M804344200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Klenner C, Yuan J, Dalbey RE, Kuhn A. The Pf3 coat protein contacts TM1 and TM3 of YidC during membrane biogenesis. FEBS Lett. 2008;582:3967–3972. doi: 10.1016/j.febslet.2008.10.044. [DOI] [PubMed] [Google Scholar]
- 120.Serek J, Bauer-Manz G, Struhalla G, van den Berg L, Kiefer D, Dalbey RE, Kuhn A. Escherichia coli YidC is a membrane insertase for Sec-independent proteins. EMBO J. 2004;23:294–301. doi: 10.1038/sj.emboj.7600063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dalbey RE, Kuhn A. YidC family members are involved in the membrane insertion, lateral integration, folding, and assembly of membrane proteins. J Cell Biol. 2004;166:769–774. doi: 10.1083/jcb.200405161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nagamori S, Smirnova IN, Kaback HR. Role of YidC in folding polytopic membrane proteins. J Cell Biol. 2004;165:53–62. doi: 10.1083/jcb.200402067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shimohata N, Nagamori S, Akiyama Y, Kaback HR, Ito K. SecY alterations that impair membrane protein folding and generate a membrane stress. J Cell Biol. 2007;176:307–317. doi: 10.1083/jcb.200611121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang P, Kuhn A, Dalbey RE (2010) Global change of gene regulation and cell physiology in YidC-depleted E. coli. J Bacteriol. doi:10.1128/JB.00484-09 [DOI] [PMC free article] [PubMed]
- 125.van der Laan M, Urbanus ML, ten Hagen-Jongman CM, Nouwen N, Oudega B, Harms N, Driessen AJ, Luirink J. A conserved function of YidC in the biogenesis of respiratory chain complexes. Proc Natl Acad Sci USA. 2003;100:5801–5806. doi: 10.1073/pnas.0636761100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Koch HG, Moser M, Schimz KL, Müller M. The integration of YidC into the cytoplasmic membrane of Escherichia coli requires the signal recognition particle, SecA and SecYEG. J Biol Chem. 2002;277:5715–5718. doi: 10.1074/jbc.C100683200. [DOI] [PubMed] [Google Scholar]
- 127.Ito K, Akiyama Y. Cellular functions, mechanism of action, and regulation of FtsH protease. Annu Rev Microbiol. 2005;59:211–231. doi: 10.1146/annurev.micro.59.030804.121316. [DOI] [PubMed] [Google Scholar]
- 128.Ami D, Natalello A, Schultz T, Gatti-Lafranconi P, Lotti M, Doglia SM, de Marco A. Effects of recombinant protein misfolding and aggregation on bacterial membranes. Biochim Biophys Acta. 2009;1794:263–269. doi: 10.1016/j.bbapap.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 129.Hong H, Tamm LK. Elastic coupling of integral membrane protein stability to lipid bilayer forces. Proc Natl Acad Sci USA. 2004;101:4065–4070. doi: 10.1073/pnas.0400358101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Perozo E, Cortes DM, Sompornpisut P, Kloda A, Martinac B. Open channel structure of MscL and the gating mechanism of mechanosensitive channels. Nature. 2002;418:942–948. doi: 10.1038/nature00992. [DOI] [PubMed] [Google Scholar]
- 131.Moe P, Blount P. Assessment of potential stimuli for mechano-dependent gating of MscL: effects of pressure, tension, and lipid headgroups. Biochemistry. 2005;44:12239–12244. doi: 10.1021/bi0509649. [DOI] [PubMed] [Google Scholar]
- 132.Akiyama Y, Kihara A, Ito K. Subunit a of proton ATPase Fo sector is a substrate of the FtsH protease in Escherichia coli . FEBS Lett. 1996;399:26–28. doi: 10.1016/s0014-5793(96)01283-5. [DOI] [PubMed] [Google Scholar]
- 133.Kihara A, Akiyama Y, Ito K. Different pathways for protein degradation by the FtsH/HflKC membrane-embedded protease complex: an implication from the interference by a mutant form of a new substrate protein YccA. J Mol Biol. 1998;279:175–188. doi: 10.1006/jmbi.1998.1781. [DOI] [PubMed] [Google Scholar]
- 134.Chiba S, Akiyama Y, Mori H, Matsuo E, Ito K. Length recognition at the N-terminal tail for the initiation of FtsH-mediated proteolysis. EMBO Rep. 2000;1:47–52. doi: 10.1093/embo-reports/kvd005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chiba S, Akiyama Y, Ito K. Membrane protein degradation by FtsH can be initiated from either end. J Bacteriol. 2002;184:4775–4782. doi: 10.1128/JB.184.17.4775-4782.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bogdanov M, Heacock PN, Dowhan W. A polytopic membrane protein displays a reversible topology dependent on membrane lipid composition. EMBO J. 2002;21:2107–2116. doi: 10.1093/emboj/21.9.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang W, Bogdanov M, Pi J, Pittard AJ, Dowhan W. Reversible topological organization within a polytopic membrane protein is governed by a change in membrane phospholipids composition. J Biol Chem. 2003;278:50128–50135. doi: 10.1074/jbc.M309840200. [DOI] [PubMed] [Google Scholar]
- 138.Zhang W, Campbell HA, King SC, Dowhan W. Phospholipids as determinants of membrane protein topology. Phosphatidylethanolamine is required for the proper topological organization of the γ-aminobutyric acid permease (GabP) of Escherichia coli . J Biol Chem. 2005;280:26032–26038. doi: 10.1074/jbc.M504929200. [DOI] [PubMed] [Google Scholar]
- 139.Xie J, Bogdanov M, Heacock P, Dowhan W. Phosphatidylethanolamine and monoglucosyldiacylglycerol are interchangeable in supporting topogenesis and function of the polytopic membrane protein lactose permease. J Biol Chem. 2006;281:19172–19178. doi: 10.1074/jbc.M602565200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bogdanov M, Dowhan W. Phosphatidylethanolamine is required for in vivo function of the membrane-associated lactose permease of Escherichia coli . J Biol Chem. 1995;270:732–739. doi: 10.1074/jbc.270.2.732. [DOI] [PubMed] [Google Scholar]
- 141.Allen SJ, Curran AR, Templer RH, Meijberg W, Booth PJ. Controlling the folding efficiency of an integral membrane protein. J Mol Biol. 2004;342:1293–1304. doi: 10.1016/j.jmb.2004.07.041. [DOI] [PubMed] [Google Scholar]
- 142.Miller D, Charalambous K, Rotem D, Schuldiner S, Curnow P, Booth PJ. In vitro unfolding and refolding of the small multidrug transporter EmrE. J Mol Biol. 2009;393:815–832. doi: 10.1016/j.jmb.2009.08.039. [DOI] [PubMed] [Google Scholar]
- 143.Lill R, Dowhan W, Wickner W. The ATPase activity of SecA is regulated by acidic phospholipids, SecY, and the leader and mature domains of precursor proteins. Cell. 1990;60:271–280. doi: 10.1016/0092-8674(90)90742-w. [DOI] [PubMed] [Google Scholar]
- 144.Hendrick JP, Wickner W. SecA protein needs both acidic phospholipids and SecY/E protein for functional high-affinity binding to the Escherichia coli plasma membrane. J Biol Chem. 1991;266:24596–24600. [PubMed] [Google Scholar]
- 145.Weidner U, Geier S, Ptock A, Friedrich T, Leif H, Weiss H. The gene locus of the proton-translocating NADH: ubiquinone oxidoreductase in Escherichia coli. Organization of the 14 genes and relationship between the derived proteins and subunits of mitochondrial complex I. J Mol Biol. 1993;233:109–122. doi: 10.1006/jmbi.1993.1488. [DOI] [PubMed] [Google Scholar]
- 146.Guénebaut V, Schlitt A, Weiss H, Leonard K, Friedrich T. Consistent structure between bacterial and mitochondrial NADH:ubiquinone oxidoreductase (complex I) J Mol Biol. 1998;276:105–112. doi: 10.1006/jmbi.1997.1518. [DOI] [PubMed] [Google Scholar]
- 147.Peng G, Fritzsch G, Zickermann V, Schägger H, Mentele R, Lottspeich F, Bostina M, Radermacher M, Huber R, Stetter KO, Michel H. Isolation, characterization and electron microscopic single particle analysis of the NADH:ubiquinone oxidoreductase (complex I) from the hyperthermophilic eubacterium Aquifex aeolicus . Biochemistry. 2003;42:3032–3039. doi: 10.1021/bi026876v. [DOI] [PubMed] [Google Scholar]
- 148.Sazanov LA, Hinchliffe P. Structure of the hydrophilic domain of respiratory complex I from Thermus thermophilus . Science. 2006;311:1430–1436. doi: 10.1126/science.1123809. [DOI] [PubMed] [Google Scholar]
- 149.Price CE, Driessen AJ. YidC is involved in the biogenesis of anaerobic respiratory complexes in the inner membrane of Escherichia coli . J Biol Chem. 2008;283:26921–26927. doi: 10.1074/jbc.M804490200. [DOI] [PubMed] [Google Scholar]
- 150.Rapisarda VA, Chehín RN, De Las Rivas J, Rodríguez-Montelongo L, Farías RN, Massa EM. Evidence for Cu(I)-thiolate ligation and prediction of a putative copper-binding site in the Escherichia coli NADH dehydrogenase-2. Arch Biochem Biophys. 2002;405:87–94. doi: 10.1016/s0003-9861(02)00277-1. [DOI] [PubMed] [Google Scholar]
- 151.Abramson J, Riistama S, Larsson G, Jasaitis A, Svensson-Ek M, Laakkonen L, Puustinen A, Iwata S, Wikstöm M. The structure of the ubiquinol oxidase from Escherichia coli and its ubiquinone binding site. Nat Struct Biol. 2000;7:910–917. doi: 10.1038/82824. [DOI] [PubMed] [Google Scholar]
- 152.Ma J, Katsonouri A, Gennis RB. Subunit II of the cytochrome bo 3 ubiquinol oxidase from Escherichia coli is a lipoprotein. Biochemistry. 1997;36:11298–11303. doi: 10.1021/bi9709710. [DOI] [PubMed] [Google Scholar]
- 153.Stenberg F, von Heijne G, Daley DO. Assembly of the cytochrome bo 3 complex. J Mol Biol. 2007;371:765–773. doi: 10.1016/j.jmb.2007.05.045. [DOI] [PubMed] [Google Scholar]
- 154.Tran QH, Bongaerts J, Vlad D, Unden G. Requirement for the proton-pumping NADH dehydrogenase I of Escherichia coli in respiration of NADH to fumarate and its bioenergetic implications. Eur J Biochem. 1997;244:155–160. doi: 10.1111/j.1432-1033.1997.00155.x. [DOI] [PubMed] [Google Scholar]
- 155.Iverson TM, Luna-Chavez C, Cecchini G, Rees DC. Structure of the Escherichia coli fumarate reductase respiratory complex. Science. 1999;284:1961–1966. doi: 10.1126/science.284.5422.1961. [DOI] [PubMed] [Google Scholar]
- 156.Bertero MG, Rothery RA, Palak M, Hou C, Lim D, Blasco F, Weiner JH, Strynadka NC. Insights into the respiratory electron transfer pathway from the structure of nitrate reductase A. Nat Struct Biol. 2003;10:681–687. doi: 10.1038/nsb969. [DOI] [PubMed] [Google Scholar]
- 157.Deisenhofer J, Michel H. The photosynthetic reaction centre from the purple bacterium Rhodopseudomonas viridis . Science. 1989;245:1463–1473. doi: 10.1126/science.245.4925.1463. [DOI] [PubMed] [Google Scholar]
- 158.Ermler U, Fritzsch G, Buchanan SK, Michel H. Structure of the photosynthetic reaction centre from Rhodobacter sphaeroides at 2.65 Å resolution: cofactors and protein-cofactor interactions. Structure. 1994;2:925–936. doi: 10.1016/s0969-2126(94)00094-8. [DOI] [PubMed] [Google Scholar]
- 159.Fotiadis D, Qian P, Philippsen A, Bullough PA, Engel A, Hunter CN. Structural analysis of the reaction center light-harvesting complex I photosynthetic core complex of Rhodospirillum rubrum using atomic force microscopy. J Biol Chem. 2004;279:2063–2068. doi: 10.1074/jbc.M310382200. [DOI] [PubMed] [Google Scholar]
- 160.Nelson N, Yocum CF. Structure and function of photosystems I and II. Annu Rev Plant Biol. 2006;57:521–565. doi: 10.1146/annurev.arplant.57.032905.105350. [DOI] [PubMed] [Google Scholar]
- 161.Woolhead CA, Thompson SJ, Moore M, Tissier C, Mant A, Rodger A, Henry R, Robinson C. Distinct Albino3-dependent and -independent pathways for thylakoid membrane protein insertion. J Biol Chem. 2001;276:40841–40846. doi: 10.1074/jbc.M106523200. [DOI] [PubMed] [Google Scholar]
- 162.Spence E, Bailey S, Nenninger A, Møller SG, Robinson C. A homolog of Albino3/OxaI is essential for thylakoid biogenesis in the cyanobacterium Synechocystis sp. PCC6803. J Biol Chem. 2004;279:55792–55800. doi: 10.1074/jbc.M411041200. [DOI] [PubMed] [Google Scholar]
- 163.Abrahams JP, Leslie AGW, Lutter R, Walker JE. Structure at 2.8 Å resolution of F1-ATPase from bovine heart mitochondria. Nature. 1994;370:621–628. doi: 10.1038/370621a0. [DOI] [PubMed] [Google Scholar]
- 164.Meier T, Polzer P, Diederichs K, Welte W, Dimroth P. Structure of the rotor ring of F-type Na+-ATPase from Ilyobacter tartaricus . Science. 2005;308:659–662. doi: 10.1126/science.1111199. [DOI] [PubMed] [Google Scholar]
- 165.Pogoryelov D, Yildiz O, Faraldo-Gómez JD, Meier T. High-resolution structure of the rotor ring of a proton-dependent ATP synthase. Nat Struct Mol Biol. 2009;16:1068–1073. doi: 10.1038/nsmb.1678. [DOI] [PubMed] [Google Scholar]
- 166.Junge W, Sielaff H, Engelbrecht S. Torque generation and elastic power transmission in the rotary FoF1-ATPase. Nature. 2009;459:364–370. doi: 10.1038/nature08145. [DOI] [PubMed] [Google Scholar]
- 167.Yi L, Jiang F, Chen M, Cain B, Bolhuis A, Dalbey RE. YidC is strictly required for membrane insertion of subunits a and c of the F1Fo ATP synthase and SecE of the SecYEG translocase. Biochemistry. 2003;42:10537–10544. doi: 10.1021/bi034309h. [DOI] [PubMed] [Google Scholar]
- 168.Kol S, Turrell BR, de Keyzer J, van der Laan M, Nouwen N, Driessen AJM. YidC-mediated membrane insertion of assembly mutants of subunit c of the F1Fo ATPase. J Biol Chem. 2006;281:29762–29768. doi: 10.1074/jbc.M605317200. [DOI] [PubMed] [Google Scholar]
- 169.Yi L, Celebi N, Chen M, Dalbey RE. Sec/SRP requirements and energetics of membrane insertion of subunits a, b, and c of the Escherichia coli F1Fo ATP synthase. J Biol Chem. 2004;279:39260–39267. doi: 10.1074/jbc.M405490200. [DOI] [PubMed] [Google Scholar]
- 170.Kol S, Majczak W, Heerlien R, van der Berg JP, Nouwen N, Driessen AJM. Subunit a of the F1Fo ATP synthase requires YidC and SecYEG for membrane insertion. J Mol Biol. 2009;390:893–901. doi: 10.1016/j.jmb.2009.05.074. [DOI] [PubMed] [Google Scholar]
- 171.Hermolin J, Fillingame RH. Assembly of Fo sector of Escherichia coli H+ ATP synthase. Interdependence of subunit insertion into the membrane. J Biol Chem. 1995;270:2815–2817. doi: 10.1074/jbc.270.6.2815. [DOI] [PubMed] [Google Scholar]
- 172.Kol S, Nouwen N, Driessen AJM. The charge distribution in the cytoplasmic loop of subunit c of the F1Fo ATPase is a determinant for YidC targeting. J Biol Chem. 2008;283:9871–9877. doi: 10.1074/jbc.M709408200. [DOI] [PubMed] [Google Scholar]
- 173.Abramson J, Smirnova I, Kasho V, Verner G, Kaback HR, Iwata S. Structure and mechanism of the lactose permease of Escherichia coli . Science. 2003;301:610–615. doi: 10.1126/science.1088196. [DOI] [PubMed] [Google Scholar]
- 174.Guan L, Kaback HR. Lessons from lactose permease. Annu Rev Biophys Biomol Struct. 2006;35:67–91. doi: 10.1146/annurev.biophys.35.040405.102005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Smirnova I, Kasho V, Choe JY, Altenbach C, Hubbell WL, Kaback HR. Sugar binding induces an outward facing conformation of LacY. Proc Natl Acad Sci USA. 2007;104:16504–16509. doi: 10.1073/pnas.0708258104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Saier MH., Jr Families of transmembrane sugar transport proteins. Mol Microbiol. 2000;35:699–710. doi: 10.1046/j.1365-2958.2000.01759.x. [DOI] [PubMed] [Google Scholar]
- 177.Murakami S, Nakashima R, Yamashita E, Yamaguchi A. Crystal structure of bacterial multidrug efflux transporter AcrB. Nature. 2002;419:587–593. doi: 10.1038/nature01050. [DOI] [PubMed] [Google Scholar]
- 178.Yin Y, He X, Szewczyk P, Nguyen T, Chang G. Structure of the multidrug transporter EmrD from Escherichia coli . Science. 2006;312:741–744. doi: 10.1126/science.1125629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yousef MS, Guan L. A 3D structure model of the melibiose permease of Escherichia coli represents a distinctive fold for Na+ symporters. Proc Natl Acad Sci USA. 2009;106:15291–15296. doi: 10.1073/pnas.0905516106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Huang Y, Lemieux MJ, Song J, Auer M, Wang DN. Structure and mechanism of the glycerol-3-phosphate transporter from Escherichia coli . Science. 2003;301:616–620. doi: 10.1126/science.1087619. [DOI] [PubMed] [Google Scholar]
- 181.Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl−dependent neurotransmitter transporters. Nature. 2005;437:215–223. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
- 182.Ressl S, Terwisscha van Scheltinga AC, Vonrhein C, Ott V, Ziegler C. Molecular basis of transport and regulation in the Na+/betaine symporter BetP. Nature. 2009;458:47–52. doi: 10.1038/nature07819. [DOI] [PubMed] [Google Scholar]
- 183.Faham S, Watanabe A, Besserer GM, Cascio D, Specht A, Hirayama BA, Wright EM, Abramson J. The crystal structure of a sodium galactose transporter reveals mechanistic insights into Na+/sugar symport. Science. 2008;321:810–814. doi: 10.1126/science.1160406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Weyand S, Shimamura T, Yajima S, Suzuki S, Mirza O, Krusong K, Carpenter EP, Rutherford NG, Hadden JM, O’Reilly J, Ma P, Saidijam M, Patching SG, Hope RJ, Norbertczak HT, Roach PC, Iwata S, Henderson PJ, Cameron AD. Structure and molecular mechanism of a nucleobase-cation-symport-1 family transporter. Science. 2008;322:709–713. doi: 10.1126/science.1164440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Higgens CF. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 186.Davidson AL, Maloney PC. ABC transporters: how small machines do a big job. Trends Microbiol. 2007;15:448–455. doi: 10.1016/j.tim.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 187.Paulsen IT, Beness AM, Saier MH., Jr Computer-based analyses of the protein constituents of transport systems catalyzing export of complex carbohydrates in bacteria. Microbiology. 1997;143:2685–2699. doi: 10.1099/00221287-143-8-2685. [DOI] [PubMed] [Google Scholar]
- 188.Davidson AL, Nikaido H. Purification and characterization of the membrane-associated components of the maltose transport system from Escherichia coli . J Biol Chem. 1991;266:8946–8951. [PubMed] [Google Scholar]
- 189.Davidson AL, Shuman HA, Nikaido H. Mechanism of maltose transport in Escherichia coli: transmembrane signalling by periplasmic binding proteins. Proc Natl Acad Sci USA. 1992;89:2360–2364. doi: 10.1073/pnas.89.6.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Oldham ML, Khare D, Quiocho FA, Davidson AL, Chen J. Crystal structure of a catalytic intermediate of the maltose transporter. Nature. 2007;450:515–521. doi: 10.1038/nature06264. [DOI] [PubMed] [Google Scholar]
- 191.Khare D, Oldham ML, Orelle C, Davidson AL, Chen J. Alternating access in maltose transporter mediated by rigid-body rotations. Mol Cell. 2009;33:528–536. doi: 10.1016/j.molcel.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Froshauer S, Green GN, Boyd D, McGovern K, Beckwith J. Genetic analysis of the membrane insertion and topology of MalF, a cytoplasmic membrane protein of Escherichia coli . J Mol Biol. 1988;200:501–511. doi: 10.1016/0022-2836(88)90539-6. [DOI] [PubMed] [Google Scholar]
- 193.Deutscher J, Francke C, Postma PW. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol Mol Biol Rev. 2006;70:939–1031. doi: 10.1128/MMBR.00024-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Cai M, Williams DC, Jr, Wang G, Lee BR, Peterkofsky A, Clore GM. Solution structure of the phosphoryl transfer complex between the signal-transducing protein IIAGlucose and the cytoplasmic domain of the glucose transporter IICBGlucose of the Escherichia coli glucose phosphotransferase system. J Biol Chem. 2003;278:25191–25206. doi: 10.1074/jbc.M302677200. [DOI] [PubMed] [Google Scholar]
- 195.Huber F, Erni B. Membrane topology of the mannose transporter of Escherichia coli K12. Eur J Biochem. 1996;239:810–817. doi: 10.1111/j.1432-1033.1996.0810u.x. [DOI] [PubMed] [Google Scholar]
- 196.Oberholzer AE, Schneider P, Siebold C, Baumann U, Erni B. Crystal structure of enzyme I of the phosphoenolpyruvate: sugar phosphotransferase system in the dephosphorylated state. J Biol Chem. 2009;284:33169–33176. doi: 10.1074/jbc.M109.057612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Koning RI, Keegstra W, Oostergetel GT, Schuurman-Wolters G, Robillard GT, Brisson A. The 5 Å projection structure of the transmembrane domain of the mannitol transporter enzyme II. J Mol Biol. 1999;287:845–851. doi: 10.1006/jmbi.1999.2650. [DOI] [PubMed] [Google Scholar]
- 198.Veldhuis G, Broos J, Poolman B, Scheek RM. Stoichiometry and substrate affinity of the mannitol transporter, EnzymeIImtl, from Escherichia coli . Biophys J. 2005;89:201–210. doi: 10.1529/biophysj.105.062877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Beutler R, Ruggiero F, Erni B. Folding and activity of circularly permuted forms of a polytopic membrane protein. Proc Natl Acad Sci USA. 2000;97:1477–1482. doi: 10.1073/pnas.0305463397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Agre P. The aquaporin water channels. Proc Am Thorac Soc. 2006;3:5–13. doi: 10.1513/pats.200510-109JH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hedfalk K, Törnroth-Horsefield S, Nyblom M, Johanson U, Kjellbom P, Neutze R. Aquaporin gating. Curr Opin Struct Biol. 2006;16:447–456. doi: 10.1016/j.sbi.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 202.Pao GM, Wu LF, Johnson KD, Höfte H, Chrispeels MJ, Sweet G, Sandal NN, Saier MH., Jr Evolution of the MIP family of integral membrane transport proteins. Mol Microbiol. 1991;5:33–37. doi: 10.1111/j.1365-2958.1991.tb01823.x. [DOI] [PubMed] [Google Scholar]
- 203.Wistow GJ, Pisano MM, Chepelinsky AB. Tandem sequence repeats in transmembrane channel proteins. Trends Biochem Sci. 1991;16:170–171. doi: 10.1016/0968-0004(91)90065-4. [DOI] [PubMed] [Google Scholar]
- 204.Viadiu H, Gonen T, Walz T. Projection map of aquaporin-9 at 7 Å resolution. J Mol Biol. 2007;367:80–88. doi: 10.1016/j.jmb.2006.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Smolin N, Li B, Beck DA, Daggett V. Side-chain dynamics are critical for water permeation through aquaporin-1. Biophys J. 2008;95:1089–1098. doi: 10.1529/biophysj.107.125187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hovijitra NT, Wuu JJ, Peaker B, Swartz JR. Cell-free synthesis of functional aquaporin Z in synthetic liposomes. Biotechnol Bioeng. 2009;104:40–49. doi: 10.1002/bit.22385. [DOI] [PubMed] [Google Scholar]
- 207.Booth IR, Louis P. Managing hypoosmotic stress: aquaporins and mechanosensitive channels in Escherichia coli . Curr Opin Microbiol. 1999;2:166–169. doi: 10.1016/s1369-5274(99)80029-0. [DOI] [PubMed] [Google Scholar]
- 208.Chang G, Spencer RH, Lee AT, Barclay MT, Rees DC. Structure of the MscL homolog from Mycobacterium tuberculosis: a gated mechanosensitive ion channel. Science. 1998;282:2220–2226. doi: 10.1126/science.282.5397.2220. [DOI] [PubMed] [Google Scholar]
- 209.Liu Z, Gandhi CS, Rees DC. Structure of a tetrameric MscL in an expanded intermediate state. Nature. 2009;461:120–124. doi: 10.1038/nature08277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Bass RB, Strop P, Barclay M, Rees DC. Crystal structure of Escherichia coli MscS, a voltage-modulated and mechanosensitive channel. Science. 2002;298:1582–1587. doi: 10.1126/science.1077945. [DOI] [PubMed] [Google Scholar]
- 211.Szurmant H, Ordal GW. Diversity in chemotaxis mechanisms among the Bacteria and Archaea. Microbiol Mol Biol Rev. 2004;68:301–319. doi: 10.1128/MMBR.68.2.301-319.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Hazelbauer GL, Falke JJ, Parkinson JS. Bacterial chemoreceptors: high-performance signaling in networked arrays. Trends Biochem Sci. 2008;33:9–19. doi: 10.1016/j.tibs.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Yeh JI, Biemann HP, Privé GG, Pandit J, Koshland DE, Jr, Kim SH. High-resolution structures of the ligand binding domain of the wild-type bacterial aspartate receptor. J Mol Biol. 1996;262:186–201. doi: 10.1006/jmbi.1996.0507. [DOI] [PubMed] [Google Scholar]
- 214.Kim KK, Yokota H, Kim SH. Four-helical-bundle structure of the cytoplasmic domain of a serine chemotaxis receptor. Nature. 1999;400:787–792. doi: 10.1038/23512. [DOI] [PubMed] [Google Scholar]
- 215.Gebert JF, Overhoff B, Manson MD, Boos W. The Tsr chemosensory transducer of Escherichia coli assembles into the cytoplasmic membrane via a SecA-dependent process. J Biol Chem. 1988;263:16652–16660. [PubMed] [Google Scholar]
- 216.Shiomi D, Yoshimoto M, Homma M, Kawagishi I. Helical distribution of the bacterial chemoreceptor via colocalization with the Sec protein translocation machinery. Mol Microbiol. 2006;60:894–906. doi: 10.1111/j.1365-2958.2006.05145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Facey SJ, Kuhn A. The sensor protein KdpD inserts into the Escherichia coli membrane independent of the Sec translocase and YidC. Eur J Biochem. 2003;270:1724–1734. doi: 10.1046/j.1432-1033.2003.03531.x. [DOI] [PubMed] [Google Scholar]
- 218.Suzuki H, Yonekura K, Namba K. Structure of the rotor of the bacterial flagellar motor revealed by electron cryomicroscopy and single-particle image analysis. J Mol Biol. 2004;337:105–113. doi: 10.1016/j.jmb.2004.01.034. [DOI] [PubMed] [Google Scholar]
- 219.Zhou J, Sharp LL, Tang HL, Lloyd SA, Billings S, Braun TF, Blair DF. Function of protonatable residues in the flagellar motor of Escherichia coli: a critical role for Asp 32 of MotB. J Bacteriol. 1998;180:2729–2735. doi: 10.1128/jb.180.10.2729-2735.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kubori T, Shimamoto N, Yamaguchi S, Namba K, Aizawa S. Morphological pathway of flagellar assembly in Salmonella typhimurium . J Mol Biol. 1992;226:433–446. doi: 10.1016/0022-2836(92)90958-m. [DOI] [PubMed] [Google Scholar]
- 221.Pradel N, Decorps A, Ye C, Santini CL, Wu LF. YidC-dependent translocation of green fluorescence protein fused to the FliP cleavable signal peptide. Biochimie. 2005;87:191–196. doi: 10.1016/j.biochi.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 222.Macnab RM. Type III flagellar protein export and flagellar assembly. Biochim Biophys Acta. 2004;1694:207–217. doi: 10.1016/j.bbamcr.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 223.Fan F, Macnab RM. Enzymatic characterization of FliI. An ATPase involved in flagellar assembly in Salmonella typhimurium . J Biol Chem. 1996;271:31981–31988. doi: 10.1074/jbc.271.50.31981. [DOI] [PubMed] [Google Scholar]
- 224.Minamino T, Macnab RM. Interactions among components of the Salmonella flagellar export apparatus and its substrates. Mol Microbiol. 2000;35:1052–1064. doi: 10.1046/j.1365-2958.2000.01771.x. [DOI] [PubMed] [Google Scholar]