Abstract
Although heme insertion into proteins enables their function in bioenergetics, metabolism, and signaling, the mechanisms and regulation of this process is not fully understood. We developed a means to study cellular heme insertion into apo-protein targets over a 3 h time period, and then investigated how nitric oxide (NO) released from a chemical donor (NOC-18) might influence heme (protoporphyrin IX) insertion into seven targets that present a range of protein structure, heme ligation, and function (three NO synthases, two cytochrome P450’s, catalase, and hemoglobin). NO blocked cellular heme insertion into all seven apo-protein targets. The inhibition occurred at relatively low (nM/min) fluxes of NO, was reversible, and did not involve changes in intracellular heme level, activation of guanylate cyclase, or inhibition of mitochondrial ATP production. These aspects and the range of protein targets suggest that NO can act as a global inhibitor of heme insertion, possibly by inhibiting a common step in the process.
Keywords: Cytochrome P450, Gene expression, Heat shock protein, Heme, Hemoglobin
Introduction
Heme proteins perform widespread and essential roles throughout biology, including electron transfer, redox transformations, small molecule transport, metabolism, catabolism, and signal transduction (1–3). To perform these roles, heme proteins have evolved into a number of structural classes. The most common group utilizes protoporphyrin IX bound within the proteins. Members of this group include globins like hemoglobin and myoglobin, heme thiolate enzymes like cytochrome P450’s (CYP) and NO synthases (NOS), peroxidases, and PAS-type signaling proteins (4–7).
The steps of heme biosynthesis and how heme functions in proteins are well-studied (8–10). However, with the exception of cytochrome c biogenesis (11) and some aspects of heme acquisition and catabolism (12, 13), relatively little is known about how heme is transported within eukaryotic cells and becomes inserted into soluble protein targets, or how these processes might be regulated. These steps are critical, given that free heme is bioactive and potentially cytotoxic (14) and is normally kept at low intracellular levels (15). A number of cytosolic heme-binding proteins have been described, which include liver fatty acid binding protein (16), glutathione S-transferase (17), heme binding protein HBP23 (peroxiredoxin 1) (18), heme binding protein HBP22 (19) and PAS-domain proteins (20, 21). However, their possible roles in heme transport or insertion reactions have not been tested. Heme insertion into catalase was shown to possibly occur within the peroxisome (22), and myeloperoxidase trafficking in cells was found to depend on its heme content (23). Hsp90 is required for heme insertion into neuronal NOS (24), implying that there is an active cellular mechanism that involves multiple proteins. Regarding regulation, a general deficiency in iron or heme can down-regulate heme protein biosynthesis at the level of gene transcription or translation (25–27), which is upstream from the heme insertion process.
We previously reported that nitric oxide (NO) could block heme insertion into inducible NO synthase (iNOS) (29). In that study, iNOS protein expression was induced in the RAW264.7 macrophage cell line by cytokine immune activation, and thus the effect involved NO that was generated by iNOS within the cells. After some of the early-expressed iNOS protein had formed an active dimer, the NO it produced was able to progressively antagonize heme insertion into any subsequently-generated iNOS protein, such that by 8 h post induction, heme insertion into any new iNOS protein was essentially blocked and the iNOS accumulated as heme-free monomers (apo-iNOS) within the cells. A variety of experiments established that the NO did not impact heme availability within the cells but was rather acting to prevent heme insertion (29). Following this study, the possibility that NO has a broader role in controlling cellular heme insertion was not pursued and is still unclear.
To address this issue we sought to examine the effect of NO on heme insertion into several target proteins. We first developed a cell culture method that can test the ability of NO (generated in a controlled manner by a chemical NO donor) to inhibit cellular heme insertion into any soluble protein target. We utilized our method to test if NO inhibits heme insertion into iNOS and six other heme proteins that utilize protpoporphyrin IX and present a diversity of protein structure and function, namely, two constitutively-expressed mammalian NOS enzymes (endothelial NOS, eNOS; neuronal NOS, nNOS), two cytochrome P450 enzymes (CYP3A4 and 2D6), catalase, and hemoglobin. We found that NO blocked cellular heme insertion into all of these target proteins, and therefore appears capable of global effects on cellular heme insertion. The possible mechanisms and biological impacts are discussed.
Experimental Procedures
Materials
Chemicals were purchased from Sigma (St. Louis, MO) or Fischer chemicals (New Jersey). Molecular mass markers were purchased from Biorad. Enzymes were purchased from New England Bioloabs (Beverly, MA). PIF was provided by Dr. John Parkinson of Berlex Biosciences. Hemin was purchased from Sigma. Stock solutions of hemin were freshly prepared as described elsewhere (30). Mouse catalase cDNA (31) was a gift of Dr. Serpil Ezurum (Lerner Research Institute, Cleveland Clinic). cDNAs for soluble forms of cytochrome P450 2D6 and 3A4 (32, 33) were a gift from Dr. F. Peter Guengerich (Vanderbilt University). HEK293T cells that stably express either eNOS (34) or nNOS (35) were gifts from Drs. Bill Sessa (Yale University) and Solomon Snyder (Johns Hopkins University), respectively. Human erythroid leukemia (HEL) cell line K562 were purchased from ATCC. Mouse IFN-γ was purchased from Genentech (South San Francisco, CA).
Antibodies
Rabbit polyclonal antibody specific to hemoglobin was purchased from Santa Cruz biotechnology. Mouse monoclonal antibody specific to Flag was purchased from Sigma. A monoclonal antibody specific to catalase was obtained from Santa Cruz biotechnology. Anti-eNOS, nNOS and iNOS antibodies were obtained from BD Transduction Laboratories. These antibodies were used in western blots and in immunoprecipitation assays following procedures outlined by the manufacturers.
Cell culture methods, heme depletion, and preparation of centrifuged cell lysates
All cell lines were grown in 100 mm tissue culture dishes. Mouse macrophage RAW 264.7 cells and human embryonic kidney (HEK293T) cells were cultured in DMEM containing 10% FBS and 5000 units of penicillin-streptomycin. HEK293T cells that were stably-transfected to express eNOS or nNOS were cultured in a 1:1 mixture of DMEM and HAM’s F-12 containing L-glutamine and pyruvate, 4.5 g/L glucose, 5000 units/L of Pen-strep, 10% FBS, and 250 μg/ml of Geneticin (G418). Human erythroid leukemia (HEL) K562 cells were grown in RPMI 1640 medium containing 10% FBS and 5000 units/L penicillin-streptomycin.
To inhibit heme biosynthesis and deplete stores of intracellular heme the cell lines were cultured with 250 μM succinyl acetone (SA) for 48 to 72 h prior to use (36). Afterward, the RAW 264.7 cells were given fresh media + SA and induced to express iNOS with Escherichia coli LPS (50 μg/ml) and mouse IFN-γ (10 ng/ml) in the presence of 3 mM L-NAME (a NOS NO synthesis inhibitor) as described previously (29). After 16 h of induction, the cells were given fresh media that also contained the protein synthesis inhibitor cycloheximide (10 μg/ml), incubated for 30 minutes, and then further incubated with the indicated concentrations of hemin for variable times as described in the text. In some cases, the NO donor NOC-18 alone or with hemin was also added. In other cases following iNOS induction, RAW 264.7 cells were given Antimycin A (10 μM) or 8-Bromo-cGMP (1 mM) for 30 min prior to hemin addition. At the point of cell harvest, the monolayers were washed twice with 4 ml cold PBS containing 1mg/ml of glucose, and cells on each plate were collected by scraping in presence of 500μl of lysis buffer (40 mM EPPS buffer pH 7.6, 10 % Glycerol, 3mM DTT, 150 mM NaCl and 1% NP40). The collected cells were lysed by 3 cycles of freeze-thawing (in liquid nitrogen and at 37 °C, respectively). The lysates were centrifuged for 30 min at 4 °C and the supernatants were collected and stored at −70 °C. Total protein contents of the supernatants was determined using the Bio-Rad protein assay kit. Similar procedures of culture with SA, cycloheximide, hemin, NOC-18, and cell supernatant production and storage were utilized for the K562 (HEL), HEK293T, and stable-transfected HEK293T cells.
Transient transfection of cells
Cultures (50–60% confluent) of SA-treated RAW 264.7 or HEK293T cells were transfected with pTRE plasmid containing cDNA of CYP 2D6 and CYP 3A4, or pS3 plasmid containing cDNA for human catalase, using Lipofectamine and following protocols as described by the manufacturer (Invitrogen). The transfected cells were cultured for up to 42 h in the presence of SA to allow for expression of the apo-proteins. Afterward, the culture medium was replaced with fresh medium containing cycloheximide, NOC-18, L-NAME (when needed), and/or hemin, and the cells were further processed as required in each experiment. The transfection experiments were carried out in duplicate or triplicate plates and the experiments were repeated at least three times.
Confocal Microscopy
SA-pretreated Raw 264.7 cells were cultured on glass coverslips. Cells were cytokine induced for 16h in the continued presence of SA. PIF (1 μM) and as indicated hemin (5 M) and NOC-18 (125 M) were added to the induced cells for 3h at 37°C. Cells on coverslips were washed 3 times with PBS and distilled water and fixed with 4% formaldehyde. Coverslips were mounted on the glass microscope slides with Vectashield mounting medium containing DAPI (Vector labs) to visualize nuclei. Confocal images were taken under 63X objective lens (zoom 3) of a Leica TCS-SP_AOSB laser confocal microscope and processed using Leica confocal software version 2.5.
UV-visible spectroscopy
UV-visible wavelength scans were recorded at room temperature between 350–700 nm on a Cary or Shimadzu spectrophotometer. To measure heme incorporation into NOS or CYP proteins the supernatants were diluted 1 to 3 fold in EPPS buffer (40 mM EPPS buffer pH 7.6, 10% glycerol and 150 mM NaCl) in a cuvette, bubbled with CO, and then reduced by adding a small amount of dithionite. Spectra of each sample were recorded before the CO-bubbling and after dithionite addition to obtain a calculated difference spectrum. The concentrations of heme-containing NOS were determined from the Soret absorbance peak at 444 nm using the extinction coefficient 74 mM−1cm−1 (37). The heme content for hemoglobin was determined from the Soret absorption peak at 414, using the extinction coefficient of 342.5 mM−1cm−1 for the tetramer. The heme content of CYP enzymes in cell supernatants was estimated from the absorbance difference at 450 nm of the ferrous CO-bound and ferrous enzyme forms, using an extinction coefficient of 91 mM−1cm−1 (38).
NO release from NOC-18
The NO-mediated conversion of oxy-hemoglobin to methemoglobin was used to determine the rate of NO release from NOC-18 at 37 °C. Various concentrations of NOC-18 were added to cuvettes that contained DMEM, 10% FBS, and 10 μM oxy-hemoglobin. The absorbance gain at 401nm was recorded over a 3.5 h period. The rate of NO release was calculated using the difference extinction coefficient of 38 mM−1cm−1 (39).
NOC-18 Dose curve
RAW264.7 cells with SA pre-treatment were induced to express iNOS with LPS and γ-interferon. Cycloheximide was added 30 min prior to addition of 7.5 μM hemin and various concentrations of NOC-18 (0, 5, 10, 25, 50, 75, 100, 125, and 150 μM). The cells were further incubated for 3 hours. Cells were harvested, the supernatants prepared, and the concentration of heme-containing iNOS was determined as described above.
SDS-PAGE, Western blotting, and Heme Stain analysis
In general, 40 μg of total protein from each cell supernatant was loaded onto 8% or 12% denaturing SDS-PAGE gels. Resolved proteins were electro-blotted onto PVDF membranes, probed with the respective antibodies, and visualized using an ECL kit (GE-Amersham). Heme staining of SDS-PAGE gels was performed according to Klatt et al (40). Supernatant samples to run on these gels were prepared in 6x Laemmli loading buffer without beta-mercaptoethanol. SDS-PAGE was performed in a cold room. Gels were washed in a methanol-sodium acetate solution (3:7 v/v; pH 5, 0.25 M) for 10 min and incubated in a freshly-prepared solution of methanol-sodium acetate buffer containing 6 mM o-dianisidine for 20 min. Gels were then developed for 1 h by adding 60 mM hydrogen peroxide. After developing, the gels were washed for 30 min for destaining in a solution of water, acetic acid, and methanol (8:1:1 v/v) and then photographed.
Measurement of cellular NO Synthesis
NO synthesis by macrophage cells RAW 264.7 was estimated using a colorimetric assay for nitrite as previously described (41).
Pyridine hemechromogen assay
Total heme in cell supernantants was measured through the formation of a pyridine heme chromogen (29). Briefly, 60μl of the cell supernatant was mixed with 240 μl heme chromogen reagent (40:60 pyridine:H2O, 200 mM NaOH), and the heme iron was reduced by adding a few grains of sodium dithionite. Heme chromogen formation was detected at 556 nm and the concentration was calculated using a molar extinction coefficient of 34.6 mM−1 cm−1.
Partial purification of hemoglobin from K562 cells
Hemoglobin was partially purified from K562 cell supernatants following a procedure derived from earlier techniques (42). The K562 cell supernatants were loaded onto DEAE-cellulose (Whatman DE-52) columns equilibrated with 0.2 M glycine buffer (pH 7.8). The bound proteins were washed extensively with 0.2 M glycine buffer containing 5 mM NaCl (pH 7.8). Hemoglobin was eluted with 0.2 M glycine buffer containing 100 mM NaCl (pH 7.8).
ATP measurement
Cellular ATP concentrations were estimated in a 96 well luminescent plate reader, using the ATPlite assay kit (Perkin Elmer). The ATPLite assay system is based on the production of light caused by the reaction of ATP with added luciferase and D-luciferin.
Catalase activity assay
Catalase activity in cell supernatants was measured in a 96 well plate reader, using a catalase activity assay kit (Cayman chemicals). The assay involves the catalase-mediated conversion of methanol to formaldehyde in the presence of added hydrogen peroxide. The formaldehyde is detected spectrophotometrically after its reaction with 4-amino-3-hydrazino-5-mercapto-1,2,4-triazole (Purplad) (43).
Results
Method to study heme insertion into cellular proteins
Fig. 1 diagrams the general method we developed to study heme insertion into target proteins expressed in intact cells. Cell cultures were made heme-deficient by growing them for 2 or 3 days in the presence of SA (36), which blocks heme biosynthesis by inhibiting d-aminolevulinic acid dehydratase, the second enzyme of the heme biosynthetic pathway. The target protein of interest was then induced or expressed in its heme-free (apo) form in the cells over a 16–42 h period. Hemin and cycloheximide were then added to the cell culture medium and the cells were cultured for an additional 2 to 3 hours in the presence or absence of hemin and other additives, followed by cell lysis and assessment of target protein expression and heme incorporation by various methods. Advantages of this method include a relatively short time window for monitoring heme insertion into the target protein (0 to 3 h) and the use of cycloheximide to prevent the synthesis of new proteins (target proteins or otherwise) during the heme incorporation period. These aspects minimize or eliminate confounding effects that might be caused by gene induction or changes in target protein levels in the cells in response to added heme or NO (44–47), because these effects typically require new protein synthesis and/or cellular processes that only manifest after longer periods of exposure.
Figure 1. General method to study heme insertion into heme-free protein targets in cells, and the effect of NO.
See text for details.
Heme incorporation into iNOS
Fig. 2 and Fig. S1(supplemental data) contain data from representative experiments that examined how varying the concentration of added hemin affects cellular heme incorporation into apo-iNOS expressed in RAW264.7 cells. We measured heme incorporation after 3 h of hemin exposure by generating soluble cell supernatants and forming the ferrous heme-CO complex, which absorbs at 444 nm and specifically detects only heme-containing iNOS because no other heme-thiolate protein is expressed at detectable levels in the RAW264.7 cells. Fig. S1 contains the UV-visible spectral traces recorded for the cell supernatants, while Fig. 2A shows the hemin dose-response curve that was derived from this data after normalizing the concentrations of heme-replete iNOS to the total protein content of each cell supernatant. Over the 3 h incubation period we observed a maximal heme incorporation with 7.5μM added heme, and usually observed less heme incorporation at higher concentrations as shown (10 μM). An optimal heme concentration range of 4 to 8 μM was observed in replica experiments (data not shown). Fig. 2B shows that apo-iNOS accumulated in the SA-treated cells, consistent with previous results (29) and showing that the 3 h hemin treatment did not effect total iNOS protein expression in the cells. Heme incorporation into iNOS as determined by the P450 spectroscopic measure was confirmed by in-gel heme staining of the iNOS protein band (Fig. 2C).
Figure 2. Cellular heme incorporation into iNOS as a function of added hemin concentration.
Raw 264.7 cells were treated with SA for 48 hours and then induced for 16 hours to express apo-iNOS. The cells were then incubated for 3 h with the indicated concentrations of hemin, and soluble cell supernatants were prepared for analysis. Panel A, content of heme-replete iNOS in the cell supernatant samples as determined by difference spectroscopy of the dithionite-reduced, CO-treated samples. Panel B, Western analysis of total iNOS protein expression in the cell supernatant samples that contained equal total protein. Panel C, SDS-PAGE of supernatant samples (equal total protein) followed by in-gel heme staining of the iNOS protein band. Data are representative of 3 separate experiments.
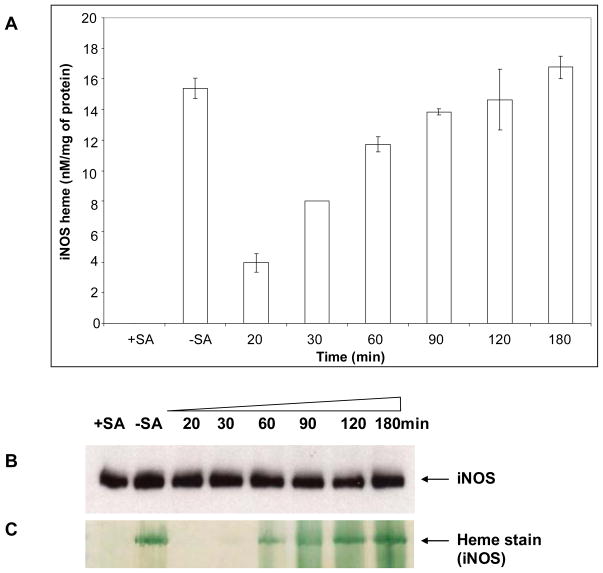
We next performed similar experiments to determine the kinetics of heme incorporation into apo-iNOS expressed in the SA-treated RAW 264.7 cells. Fig. 3A shows the time course for heme incorporation into apo-iNOS, which was detectable after 20 min of cell culture and approached a plateau after 90 to 120 min. By this point, the cells had restored an amount of heme-replete iNOS that was equivalent to the amount of heme-containing iNOS in control cell cultures that had not been made heme-deficient with SA before or during the induction of iNOS protein expression (-SA, Fig. 3A). Equivalent iNOS protein was present in the cell cultures at each harvest time (Fig. 3B), and the time course of iNOS heme insertion as determined spectroscopically was confirmed by in-gel heme staining (Fig. 3C). The data show that cellular heme incorporation into a target protein can be studied using exogenously added hemin over a relatively short time frame.
Figure 3. Time course of cellular heme insertion into iNOS.
RAW 264.7 cells were cultured and induced to express apo-iNOS as explained in the legend of Fig. 1. Supernatants were made from cell cultures harvested at the indicated times following 7.5 μM hemin addition. Panel A, Buildup of iNOS heme content (determined by the spectroscopic method) versus time, with values normalized per mg total protein. Panel B, Western analysis of total iNOS protein expression in the cell supernatant samples that contained equal total protein. Panel C, In-gel heme staining of the iNOS protein band. Data are representative of 3 separate experiments.
Effect of NO on heme incorporation into iNOS
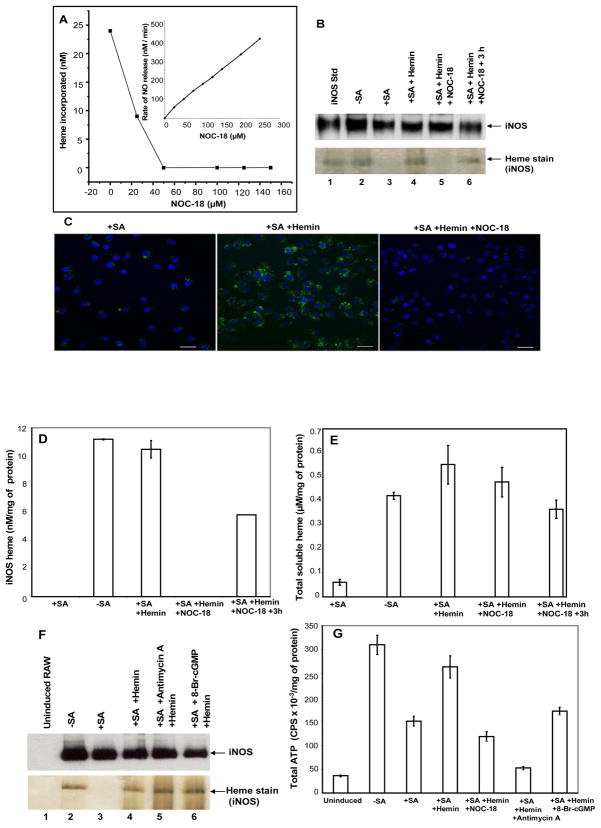
In order to study how NO affects heme incorporation in a controlled manner, we chose to use a well-characterized NO releasing chemical agent (NOC-18) (48). We first determined the rate of NO release from NOC-18 under our cell culture conditions using the oxyhemoglobin spectroscopic assay for NO. Fig. 4A (inset) shows that NO release from NOC-18 was linear and concentration-dependent. We next determined what concentration of NOC-18 would inhibit heme incorporation into apo-iNOS in our cell culture model. SA-treated, cytokine-induced RAW 264.7 cells that contained apo-iNOS were given various concentrations of NOC-18 (0-150 μM) at the point of hemin and cycloheximide addition, were cultured 3 h further, and then had heme incorporation into iNOS assessed. Fig. 4A shows that NOC-18 inhibited heme incorporation into iNOS in a concentration-dependent manner, with complete inhibition requiring NOC-18 concentrations above 50 μM. On this basis, we chose to utilize 125 μM NOC-18 in our subsequent cell culture studies. At 125 μM NOC-18 the measured rate of NO release under our culture conditions was 210 nM NO per min (Fig. 4A inset).
Figure 4. Effect of NO on cellular heme incorporation into iNOS and potential importance of cellular heme uptake, ATP level, or activation of soluble guanylate cyclase.
RAW 264.7 cultures with or without 48 h SA pretreatment were induced to express iNOS. Cultures were then treated with 7.5 μM hemin in the absence or presence of the indicated NOC-18 concentrations for 3 h, the cells were harvested and cell supernatants prepared. In some cases the cultures were washed to remove NOC-18 and heme after 3 h and then were cultured an additional 3 h before harvest. In other cases following iNOS induction, RAW 264.7 cells were pretreated for 30 min with Antimycin A (10 μM) or 8-Bromo-cGMP (1mM) prior to hemin addition. Panel A, heme incorporation into iNOS (determined spectroscopically) as a function of NOC-18 concentration. Inset, rates of NO release by NOC-18 under the culture conditions. Panel B, heme incorporation into iNOS under the indicated culture conditions and using 125 μM NOC-18. Panel C, Cellular heme incorporation into iNOS as determined by PIF staining and confocal fluorescence microscopy. SA-pretreated Raw 264.7 cells cultured on glass coverslips were induced to express apo-iNOS and the effect of NOC-18 on heme incorporation was assessed as described above. Confocal images were taken under different conditions as indicated. Panel D, equal total protein contents from each cell supernatant underwent SDS-PAGE analysis and were either heme-stained (the iNOS protein bands are shown) or subject to Western analysis using an anti-iNOS antibody. Panel E, cell supernatants were analyzed for total soluble heme content using the pyridine hemechromogen method. Panel F, equal total protein amounts from each cell supernatant were either Western blotted with iNOS antibody or heme-stained. Panel G, total ATP in the cell supernatants was measured using the ATPlite assay kit, and normalized with respect to the total protein concentrations. Results are representative of two or three trials each.
Results from a typical cell culture experiment that used 125 μM NOC-18 to block heme insertion into apo-iNOS are shown in Fig. 4B. We also tested for reversibility of the NO effect by removing the NOC-18 from a few of the cell cultures after the 3 h incubation with hemin, and then culturing these cells for an additional 3 h in fresh media without NOC-18 or hemin (but with cycloheximide) before lysing the cells. The data confirm that 125 μM NOC-18 blocked cellular heme incorporation into apo-iNOS over the 3 h period, and also show that the block was significantly reversed during a subsequent 3 h culture period after removing the NOC-18. The NO inhibition of iNOS heme incorporation was also shown by fluorescent microscopy using PIF (Fig. 4C), an iNOS-specific heme-binding compound (36), and by in-gel heme staining (Fig. 4D). Thus, NO released from NOC-18 can inhibit cellular heme incorporation into iNOS, much as does NO that is naturally-generated from iNOS (29), and the NO effect on heme incorporation is reversed within a few hours after removing the NOC-18.
Potential importance of NO on cellular heme uptake, ATP level, and activation of soluble guanylate cyclase
We tested potential mechanisms by which NO might block heme incorporation. We measured the heme content of the cell supernatants by the pyridine heme chromogen method (29) to judge whether NO inhibited hemin uptake by the cells (Fig. 4E). The induced, SA-treated RAW264.7 cells had very low intracellular levels of heme compared to induced cells that were not treated with SA, confirming the effectiveness of the SA treatment. NOC-18 did not prevent the cells from internalizing the exogenously added hemin. Given that heme incorporation is likely to be an energy-requiring process, and NO can inhibit mitochondrial energy production, we checked whether NO may act by lowering the cellular ATP content. However, the data in Fig. 4F and 4G show that heme incorporation into apo-iNOS was insensitive to the mitochondrial poison antimycin A, despite its lowering the cellular ATP level below what was present in the NOC-18 treated cells. We also found that 8-bromo cGMP did not inhibit heme insertion into apo-iNOS (Fig. 4F), indicating that the NO effect on heme insertion is independent of its activating soluble guanylate cyclase.
Effect of NO on heme incorporation into other proteins
We adopted our cell culture and analysis procedures described above to study how NO might impact cellular heme insertion into the two constitutive NOS, two CYP enzymes, a peroxidase, and a globin.
eNOS and nNOS
In these cases, we utilized stabily-transfected HEK293T cells that constitutively express either eNOS or nNOS (34, 35). The cells were pre-cultured for 2 or 3 days with or without SA to deplete intracellular heme and to allow build up apo-eNOS or apo-nNOS. The continued expression of eNOS and nNOS under the SA condition was verified by Western analysis (Figs. 5 and 6 panels B). The cells were then subject to culture for 3 h with cycloheximide and with or without hemin and NOC-18. Figs. 5 and 6, panels A and Fig. S2 (supplemental data) contain representative data demonstrating that NO released from NOC-18 inhibited heme insertion into both eNOS and nNOS, and that the NO inhibition was partly reversed during a subsequent 3 h culture after removing the NOC-18. Equivalent eNOS or nNOS protein expression was observed under all treatment conditions, and the heme incorporation results were confirmed by in-gel heme staining of the cell supernatants (Figs. 5 and 6, panels B). Thus, NO inhibited cellular heme incorporation into eNOS and nNOS.
Figure 5. Effect of NO on cellular heme incorporation in nNOS.
HEK293T cell lines stably-expressing nNOS were treated with SA for 48 h followed by 7.5 μM hemin treatment for 3 h in the absence and presence of 125 μM NOC-18. Cells were harvested and cell supernatants prepared. Some cultures treated with hemin and NOC-18 were washed to remove NOC-18 and heme and then cultured for 3 h longer prior to harvesting. Panel A, Heme incorporation into nNOS under the indicated conditions (determined by the spectroscopic method) with values normalized per mg total protein. Panel B, nNOS protein expression in aliquots of equal total protein content for each supernatant sample, and in-gel heme-staining of the nNOS protein bands. Results are representative of three trials.
Figure 6. Effect of NO on cellular heme incorporation in eNOS.
Similar experiments were performed with HEK293T cells stably expressing eNOS as described in the legend of Figure 5. Panel A, Heme incorporation into eNOS under the indicated conditions (determined by the spectroscopic method) with values normalized per mg total protein. Panel B, eNOS protein expression in aliquots of equal total protein content for each supernatant sample, and in-gel heme-staining of the eNOS protein bands. Results are representative of three trials.
CYP 2D6 and 3A4
We next tested if NO would effect heme insertion into cytochrome P450 enzymes, which are five-coordinate heme-thiolate enzymes like NOS but otherwise share no primary, secondary, or tertiary protein structural features (49, 50). HEK293T cells that had been made heme-deficient by a few days culture with SA were transiently-transfected to express either of two CYP proteins (2D6 and 3A4) that had been genetically modified at their N-termini to allow their expression in a more soluble form (31, 32). Heme incorporation was then examined after a subsequent 3 h culture period with hemin in the presence or absence of NOC-18. Figs. 7B and C show that the cells expressed similar levels of CYP proteins independent of the SA treatment. Heme incorporation into the apo-CYP proteins was detectable by either UV-visible spectroscopy (Fig. 7A, and Fig. S3, in the supplemental data) or by in-gel heme staining of the cell supernatants (Fig. 7B and C). The SA treatment caused the cells to primarily express the apo form of either CYP enzyme, and heme was incorporated into the apo-forms during incubation of the cells with hemin. NOC-18 blocked heme incorporation into CYP 2D6 and 3A4 enzymes, and heme incorporation resumed in both cases during cell culture after removal of the NOC-18.
Figure 7. Effect of NO on cellular heme incorporation into CYP 2D6 and 3A4.
HEK293T cells were treated with SA for 48 h followed by transient transfection to initiate CYP 2D6 or 3A4 protein expression. At 24 h post-transfection the cells were treated with 7.5 μM hemin alone or with 125 μM NOC-18 for 3 h before being harvested and supernatants prepared. Some cell cultures were washed to remove NOC-18 and hemin and were then cultured for an additional 3 h prior to harvesting. Panel A, Heme incorporation into the CYP apo-enzymes under the indicated conditions (determined by the spectroscopic method) with values normalized per mg total protein. Panels B and C, Western analysis of CYP protein expression levels in aliquots of equal total protein content for the supernatant samples, and corresponding in-gel heme staining of the CYP protein bands. Results are representative of three trials.
Catalase
Catalase is a heme-containing peroxidase that catalyzes the dismutation of H2O2 to water and O2. The enzyme is a tetramer and each subunit contains a catalytically-essential, five-coordinate heme whose iron is ligated to the protein via a tyrosine oxygen (51). We transiently transfected SA-treated HEK293T cells with an expression vector for human catalase (31), and then examined heme incorporation into the expressed apo-enzyme after subsequently culturing the cells 3 h with hemin in the presence or absence of NOC-18. Fig. 8, panel A contains representative data we obtained with cell supernatants generated under each experimental condition. Catalase protein expression was increased in the SA-treated transfected cells, and the level of catalase protein expression was unaffected by the 3 h NOC-18 or hemin treatments. Corresponding in-gel heme staining of the cell supernatant samples shows that there was a very low heme content in the catalase expressed in the SA-treated cells. However, the apo-catalase was able to incorporate added hemin. The heme incorporation was inhibited by NOC-18, but could be restored upon removal of the NOC-18 and further culture for 3 h. Fig. 8, panel B shows the corresponding catalase activity levels we measured in the cell supernatant samples. Despite an increase in total catalase protein expression, the cellular catalase activity in the catalase-transfected, SA-treated cells did not increase above the background level (vector), consistent with the cells expressing apo-catalase under this condition. The subsequent culture with hemin greatly increased the cellular catalase activity, and the increase in activity was much less in cells receiving coincident NOC-18 treatment. The catalase activity in the NOC-18 treated cells did increase after the NOC-18 was removed and the cells were cultured an additional 3 h. These catalase activity measurements are consistent with the heme-staining results in Panel A, and together establish that NO blocked cellular heme incorportation into catalase in a relatively reversible manner.
Figure 8. Effect of NO on cellular heme incorporation into catalase.
HEK293T cells were treated with SA for 48 h followed by transient transfection of vector for catalase expression. At 24 h post-transfection the cells were treated with 7.5 μM hemin alone or plus 125 μM NOC-18 and were incubated for 3 h before being harvested. Some of the cultures had their media replaced to study hemin incorporation for 3 h after NOC-18 removal. (A) Western analysis of catalase expression in aliquots of 250μg total protein from each indicated supernatant sample, and corresponding in-gel heme staining of the catalase bands. (B) Catalase activity determined for aliquots of equal total protein from the indicated supernatant samples. Results are representative of three trials.
Hemoglobin
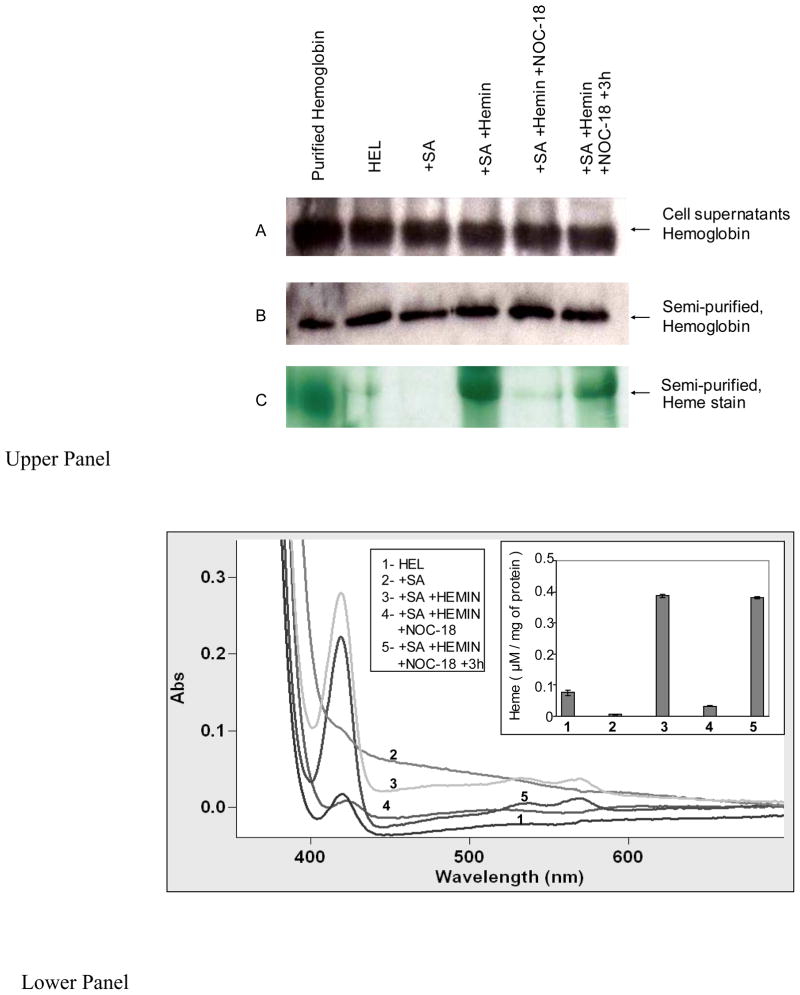
We next examined if NO could block cellular heme insertion into hemoglobin by utilizing the human erythroid leukemia (HEL) cell line K562, which is known to express hemoglobin (52). K562 cells that had been pre-cultured with SA were given hemin for 3 h in the absence or presence of NOC-18, and the cell supernatants were collected and processed to determine hemoglobin content and heme insertion. Some of the cell cultures that received hemin and NOC-18 were washed to remove the NOC-18 and were then cultured an additional 3 h to test for reversibility of the NO effect. Cell cultures that were only SA-treated or were not SA-treated served as controls. Because the spectrum of hemoglobin is fairly similar to other cellular heme proteins or non-specifically bound heme, we performed DEAE-cellulose column chromatography on identical total protein amounts from each cell supernatant to semi-purify the hemoglobin. This assured that we could quantify its heme content. Eluted hemoglobin samples from each cell supernantant were scanned in a UV-visible spectrophotometer and also resolved on SDS-PAGE to undergo Western analysis for total hemoglobin protein content and in-gel heme staining. Aliquots of samples also underwent Western analysis for their hemoglobin content prior to and following the semi-purification step.
The results of Western analysis (Fig. 9, upper panel, A and B) show that the various cell treatments did not significantly alter expression of hemoglobin in the K562 cells, and also show that equivalent amounts of hemoglobin protein were present in the eluted semi-purified samples following DEAE-cellulose chromatography. The absorption spectra of the semi-purified hemoglobin samples are shown in Fig. 9, lower panel. The data confirm that the K562 cells contained hemoglobin and show that culturing the cells with SA significantly reduced the heme content of their hemoglobin. Heme addition to the SA-treated cells enabled heme incorporation into the apo-hemoglobin, and NOC-18 significantly inhibited this heme incorporation. Culturing the NOC-18 treated cells for an additional 3 h after NOC-18 removal resulted in heme incorporation into the apo-hemoglobin. The bar graph in the lower panel inset quantifies the spectral results based on the Soret absorbance we obtained for the semi-purified hemoglobin samples at 414 nm. The spectroscopic results were confirmed by in-gel heme staining of the eluted samples (Fig. 9, upper panel, C). We conclude that cellular heme insertion into hemoglobin was inhibited by NO, but recovered after the NO donor was removed.
Figure 9. Effect of NO on cellular heme insertion into hemoglobin.
Human erythroid leukemia (HEL) cell line K562 were treated with SA for 72 h and then the cells were given 7.5 μM hemin alone or plus 125 μM NOC-18 and were incubated for 3 h before being harvested. Some of the cultures had their media replaced to study hemin incorporation for 3 h after NOC-18 removal. Upper panels- (A) Western analyses of hemoglobin expression in equal total protein amounts of each cell supernatant as indicated or (B) hemoglobin levels in the semi-purified supernatant samples, (C) the corresponding in-gel heme stain of the semi-purified hemoglobin protein bands. Lower panel- UV-visible spectra of semi-purified hemoglobin fractions from the various supernatants as indicated, depicting the Soret absorption peak at 414 nm. Inset shows a bar graph depicting the heme content of the semi-purified hemoglobin fractions based on the Soret abrobance at 414 nm. Results are representative of three trials.
Discussion
We found that NO inhibits cellular heme insertion into a variety of protein targets. The different structures, heme-binding folds, and heme iron ligating residues (tyrosine, histidine, cysteine) of the target proteins imply a global nature for the NO inhibition. NO was effective at relatively low flux rates (nM NO/min), the inhibition took hold quickly, and was relieved fairly quickly when the NO donor was removed. The inhibition did not involve NO effects on soluble guanylate cyclase, gene induction or protein expression, cellular ATP content, or heme uptake. Together, the results are consistent with NO acting on a common step during cellular heme insertion.
Potential mechanisms of action
NO could impact cellular heme insertion by direct or indirect means. One mechanism could involve NO binding directly to the heme iron during its transfer or insertion. NO displays sufficiently good affinity toward both liganded and unliganded heme (53, 54). Indeed, chemical agents that coordinate strongly to heme in cells, like the substituted imidazoles clotrimazole and miconazole (55), are known to block cellular heme insertion into iNOS (56, 57). However, these bulky imidazoles are likely to act through a steric effect, while NO is a small ligand that can be accommodated within the heme pocket of most heme proteins. When NO binds to the heme, it typically reduces the strength of the axial bond formed between the heme iron and a coordinating protein residue, and can weaken the bond or can even cause it to break (53). These effects have been demonstrated when NO binds several heme proteins including NOS (58, 59), CYP (60–62), soluble gualylate cyclase (63, 64), and hemoglobin (65, 66), some of which are target proteins in our current study. Axial ligation is only a partial energetic contributor to heme binding in proteins, which also involves ionic and hydrophobic or van der Walls contributions between the protein and the porphyrin ring (67–70). However, axial ligation can be coupled to associated protein conformational changes (71–73). Thus, if NO binding to heme iron weakens or prevents axial ligand formation and any associated protein conformational changes, this could potentially interfere with stable heme binding to a heme carrier or target protein, or disrupt heme transfer between them. Indeed, metalloporphyrins that do not form stable axial ligand bonds are sometimes not inserted well into target proteins in cellular reconstitution systems (71). NO was also proposed to release heme from CYP enzymes when it was studied at the cell and microsomal membrane level2 (62). However, NO binding does not typically cause heme to be released from purified heme proteins, and the literature shows that NO binding does not release heme from any of the purified protein targets that we studied here. In sum, NO could act by directly binding to the heme iron, which by altering the characteristics of the protein-heme axial bond could interfere with productive heme transport or stable heme insertion into the target apo-protein. This mechanism would be consistent with our observing a rapid onset of the inhibition and its relatively quick relief upon removal of the NO donor. We are further investigating this possibility.
NO might also inhibit cellular heme insertion by causing covalent modifications in heme transfer proteins or in the apo-protein targets themselves. Common NO-based protein modifications include nitrosation of cysteine residues or nitration of tyrosine or tryptophan residues (74, 75). These protein modifications require that NO be transformed into chemically distinct nitrosating and nitrating species, respectively, which are processes that occur naturally in most cellular systems where NO is generated by NOS or is released from a chemical NO donor (74). Evidence indicates that protein nitrosation or nitration reactions can occur very soon after cells become exposed to NO (76, 77), and the repair or loss of these protein modifications can also be sufficiently rapid (78, 79) to potentially match what we observed regarding the onset and relief of the NO inhibition of cellular heme insertion. Proteomic studies show that NO-driven S-nitrosation or nitration in cells has preferred protein targets (80–83), including hsp90 (84, 85), which has been implicated in heme insertion into neuronal NOS (24). Denitrosation of cysteine residues can occur and involve reactions that are catalyzed by cellular enzymes including thioredoxin, glutathione SNO reductase, and protein disulfide isomerase (78, 81, 86 and 87). Such enzymes might control the sensitivity of cellular heme insertion to NO or the capacity of cells to recover heme insertion. Bound heme may also facilitate protein nitration in some cases (88–90). Our current work provides a platform to investigate these aspects and determine whether nitrosative or nitrative protein modification impact cellular heme insertion.
Possible Biological Implications
The proteins we identified whose heme insertion is sensitive to NO perform critical biological functions, including oxidant removal (catalase), O2 transport (hemoglobin), cell signaling and immuno-protection (NOS), oxidation of xenobiotics and arachidonic acid metabolism (CYP). All of these functions depend on heme being incorporated into the proteins. Although this lends significance to our findings, what is the likelihood that NO generated naturally by NOS enzymes would inhibit cellular heme insertion as we observed here using NOC-18? As noted previously, we purposely utilized NOC-18 instead of a cellular NOS enzyme in order to achieve a standard and reproducible means to study how NO effects cellular heme insertion into different target proteins. One issue we can consider is the NO flux (released from NOC-18) that was required to inhibit cellular heme insertion, and whether this flux is achievable in cells expressing NOS enzymes. A NOC-18 concentration of 50 to 100 μM completely inhibited heme insertion in our cell cultures. This produced measured NO fluxes of between 50 and 100 nM/min in our culture condition, and based on measurements done in similar cell culture experiments (92), would be expected to create a steady-state concentration of about 50 to 100 nM NO in the cultures. This range is comparable to the NO flux and NO concentration that is generated in cultures of primary cells that express iNOS naturally or do so following infections of the host animal or treatment with cytokines or bacterial lipopolysaccharide (Kuppfer cells, tissue macrophages, lung epithelial cells) (91–93) and in several cell lines that express iNOS (RAW 264.7 and J774 macrophage cells; CaCo and A549 epithelial cells) (94–96, 106). On the other hand, cells that express eNOS or nNOS tend to generate a much smaller NO flux that might be too low to significantly inhibit cellular heme insertion, all else being equal. These considerations suggest that NO is probably able to broadly inhibit heme insertion in cells or tissues that express iNOS. Indeed, evidence in the literature already indicates or implies that iNOS-derived NO can inhibit cellular heme insertion into four of the protein targets we studied here, namely iNOS, hemoglobin, catalase, and CYP. This is most clearly shown for iNOS: Inducing iNOS expression in a macrophage cell line led to NO generation that was sufficient to inhibit heme insertion into iNOS protein that was subsequently expressed in the cells (29). Similar data is available for hemoglobin. Malech and colleagues (52) showed that inducing iNOS expression in the hemoglobin-containing K562 melanoma cells (also used here) led to much less hemoglobin spectral signal in the cells, which they attributed to a lower protein expression and to buildup of apo-hemoglobin. Catalase activity is also down-regulated when iNOS is expressed in a variety of cells, without causing a significant drop in the catalase protein expression level (97). CYP enzymes deserve a special consideration due to the many reports that show iNOS expression diminishes the activity or heme content of CYP in a variety of settings, including whole-animal, hepatocytes, microsomes, and transfected cell lines (98–102). In these cases, inducing iNOS expression with cytokines or through inflammation typically meant that relatively long exposures to NO were involved (several hours or days), which makes it difficult to rule out multiple mechanisms of action in causing their observed changes in CYP activity or heme content. Nevertheless, in some studies the effect on CYP activity was correlated with a diminished CYP heme spectral signal, and a significant proportion of the lost activity was recoverable upon blocking the NO source and providing heme (62). Interestingly, the levels of total CYP protein were often not changed much in these studies, despite NO diminishing the CYP heme spectroscopic signals and their enzymatic activities. This is consistent with iNOS-derived NO blocking heme insertion into the CYP proteins and causing them to accumulate in an apo form, similar to what we observed in our SA-treated cell cultures. Thus, our current findings highlight a new mechanism by which iNOS induction and consequent NO release may diminish CYP and catalase activities and limit formation of functional NOS or hemoglobin; namely, NO can broadly inhibit heme insertion into apo-enzymes. This acute effect on heme insertion would perhaps complement and overlap with other cellular mechanisms of NO that arise during a longer or chronic NO exposure (i.e., changes in gene induction, protein expression and degradation, or heme biosynthesis), or perhaps that involve NO causing loss of previously-inserted heme (as suggested only for the CYP enzymes). On a broader scale, the NO inhibition of cellular heme insertion might help explain how anemia or altered drug metabolism can develop in subjects with chronic inflammation that is associated with parasitic infections (103), alcoholism (104), or advanced age (105). These possibilities can now be investigated.
Supplementary Material
Acknowledgments
We thank Drs. F. P. Guengerich, W. Sessa, S. Snyder, H. Malech, and S. Erzurum for providing cell lines or cDNAs to study the various heme proteins, and thank Dr. Doug Thomas for helpful discussions.
This work is supported by National Institute of Health Grants CA53914, GM51491 and HL076491 (to D.J.S.).
List of Abbreviations
- NO
nitric oxide
- NOS
nitric oxide synthase
- EPPS
4-(2-hydroxyethyl)-1-piperazinepropanesulfonic acid
- SA
succinyl acetone
- CYP
cytochromes
- PIF
pyrimidine imidazole FITC
- LPS
Escherichia coli bacterial lipopolysaccharide
- IFN-γ
interferon gamma
- L-NAME
Nω-nitro-L-arginine methyl ester
- HEL
human erythroid leukemia
Footnotes
The ability of NO to cause heme loss from CYP was not tested in purified enzyme systems.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Raven E, Ortiz de Montellano PR. Editorial: The chemistry and biochemistry of heme proteins. Nat Prod Rep. 2007;24:499. [Google Scholar]
- 2.Paoli M, Marles-Wright J, Smith A. Structure-function relationships in heme-proteins. DNA Cell Biol. 2002;21:271–280. doi: 10.1089/104454902753759690. [DOI] [PubMed] [Google Scholar]
- 3.Milo R, Hou JH, Springer M, Brenner MP, Kirschner MW. The relationship between evolutionary and physiological variation in hemoglobin. Proc Natl Acad Sci U S A. 2007;104:16998–7003. doi: 10.1073/pnas.0707673104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poulos TL. Structural biology of heme monooxygenases. Biochem Biophys Res Commun. 2005;338:337–345. doi: 10.1016/j.bbrc.2005.07.204. [DOI] [PubMed] [Google Scholar]
- 5.Zhu Y, Silverman RB. Revisiting heme mechanisms. A perspective on the mechanisms of nitric oxide synthase (NOS), Heme oxygenase (HO), and cytochrome P450s (CYP450s) Biochemistry. 2008;47:2231–2243. doi: 10.1021/bi7023817. [DOI] [PubMed] [Google Scholar]
- 6.Davies MJ, Hawkins CL, Pattison DI, Rees MD. Mammalian heme peroxidases: from molecular mechanisms to health implications. Antioxid Redox Signal. 2008;10:1199–1234. doi: 10.1089/ars.2007.1927. [DOI] [PubMed] [Google Scholar]
- 7.Chan MK. Recent advances in heme-protein sensors. Curr Opin Chem Biol. 2001;5:216–222. doi: 10.1016/s1367-5931(00)00193-9. [DOI] [PubMed] [Google Scholar]
- 8.Heinemann IU, Jahn M, Jahn D. The biochemistry of heme biosynthesis. Arch Biochem Biophys. 2008;474:238–251. doi: 10.1016/j.abb.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Ponka P. Cell biology of heme. Am J Med Sci. 1999;318:241–256. doi: 10.1097/00000441-199910000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Poulos TL. The Janus nature of heme. Nat Prod Rep. 2007;24:504–510. doi: 10.1039/b604195g. [DOI] [PubMed] [Google Scholar]
- 11.Richard-Fogal CL, Frawley ER, Bonner ER, Zhu H, San Francisco B, Kranz RG. A conserved haem redox and trafficking pathway for cofactor attachment. EMBO J. 2009;28:2349–2359. doi: 10.1038/emboj.2009.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.West AR, Oates PS. Mechanisms of heme iron absorption: current questions and controversies. World J Gastroenterol. 2008;14:4101–4110. doi: 10.3748/wjg.14.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yi L, Jenkins PM, Leichert LI, Jakob U, Martens JR, Ragsdale SW. Heme regulatory motifs in heme oxygenase-2 form a thiol/disulfide redox switch that responds to the cellular redox state. J Biol Chem. 2009;284:20556–20561. doi: 10.1074/jbc.M109.015651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsiftsoglou AS, Tsamadou AI, Papadopoulou LC. Heme as key regulator of major mammalian cellular functions: molecular, cellular, and pharmacological aspects. Pharmacol Ther. 2006;111:327–345. doi: 10.1016/j.pharmthera.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 15.Wijayanti N, Katz N, Immenschuh S. Biology of heme in health and disease. Curr Med Chem. 2004;11:981–986. doi: 10.2174/0929867043455521. [DOI] [PubMed] [Google Scholar]
- 16.Vincent SH, Muller-Eberhard U. A protein of the Z class of liver cytosolic proteins in the rat that preferentially binds heme. J Biol Chem. 1985;260:14521–14528. [PubMed] [Google Scholar]
- 17.Harvey JW, Beutler E. Binding of heme by glutathione S-transferase: a possible role of the erythrocyte enzyme. Blood. 1982;60:1227–1230. [PubMed] [Google Scholar]
- 18.Iwahara SI, Satoh H, Song DX, Webb J, Burlingame AL, Nagae Y, Muller-Eberhard U. Purification, characterization, and cloning of a heme-binding protein (23 kDa) in rat liver cytosol. Biochemistry. 1995;34:13398–13406. doi: 10.1021/bi00041a017. [DOI] [PubMed] [Google Scholar]
- 19.Dias JS, Macedo AL, Ferreira GC, Peterson FC, Volkman BF, Goodfellow BJ. The first structure from the SOUL/HBP family of heme-binding proteins, murine P22HBP. J Biol Chem. 2006;281:31553–31561. doi: 10.1074/jbc.M605988200. [DOI] [PubMed] [Google Scholar]
- 20.Kitanishi K, Igarashi J, Hayasaka K, Hikage N, Saiful I, Yamauchi S, Uchida T, Ishimori K, Shimizu T. Heme-binding characteristics of the isolated PAS-A domain of mouse Per2, a transcriptional regulatory factor associated with circadian rhythms. Biochemistry. 2008;47:6157–6168. doi: 10.1021/bi7023892. [DOI] [PubMed] [Google Scholar]
- 21.Taketani S. Aquisition, Mobilization and Utilization of Cellular Iron and Heme: Endless Findings and Growing Evidence of Tight Regulation. Tohoku J Exp Med. 2005;205:297–318. doi: 10.1620/tjem.205.297. [DOI] [PubMed] [Google Scholar]
- 22.Lazarow PB, de Duve C. The synthesis and turnover of rat liver peroxisomes. V. Intracellular pathway of catalase synthesis. J Cell Biol. 1973;59:507–524. doi: 10.1083/jcb.59.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nauseef WM, McCormick S, Yi H. Roles of heme insertion and the mannose-6-phosphate receptor in processing of the human myeloid lysosomal enzyme, myeloperoxidase. Blood. 1992;80:2622–2633. [PubMed] [Google Scholar]
- 24.Billecke SS, Draganov DI, Morishima Y, Murphy PJM, Dunbar AY, Pratt WB, Osawa Y. The role of hsp90 in heme-dependent activation of apo-neuronal nitric-oxide synthase. J Biol Chem. 2004;279:30252–30258. doi: 10.1074/jbc.M403864200. [DOI] [PubMed] [Google Scholar]
- 25.Jane-Jane C. Regulation of protein synthesis by the heme-regulated eIF2 kinase: relevance to anemias. Blood. 2007;109:2693–2699. doi: 10.1182/blood-2006-08-041830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klausner RD, Rouault TA. A double life: cytosolic aconitase as a regulatory RNA binding protein. Mol Biol Cell. 1993;4:1–5. doi: 10.1091/mbc.4.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bogdan C. Nitric oxide and the regulation of gene expression. Trends Cell Biol. 2001;11:66–75. doi: 10.1016/s0962-8924(00)01900-0. [DOI] [PubMed] [Google Scholar]
- 28.Ponka P. Tissue-specific regulation of iron metabolism and heme synthesis: distinct control mechanisms in erythroid cells. Blood. 1997;89:1–25. [PubMed] [Google Scholar]
- 29.Albakri QA, Stuehr DJ. Intracellular assembly of inducible NO synthase is limited by nitric oxide-mediated changes in heme insertion and availability. J Biol Chem. 1996;271:5414–5421. doi: 10.1074/jbc.271.10.5414. [DOI] [PubMed] [Google Scholar]
- 30.Okabe T, Fujisawa M, Takaku F. Long-term cultivation and differentiation of human erythroleukemia cells in a protein-free chemically defined medium. Proc Natl Acad Sci U S A. 1984;81:453–455. doi: 10.1073/pnas.81.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erzurum SC, Lemarchand P, Rosenfeld MA, Yoo JH, Crystal RG. Protection of human endothelial cells from oxidant injury by adenovirus-mediated transfer of the human catalase cDNA. Nucleic Acids Res. 1993;21:1607–1612. doi: 10.1093/nar/21.7.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanna IH, Kim MS, Guengerich FP. Heterologous expression of cytochrome P450 2D6 mutants, electron transfer, and catalysis of bufuralol hydroxylation: the role of aspartate 301 in structural integrity. Arch Biochem Biophys. 2001;393:255–261. doi: 10.1006/abbi.2001.2510. [DOI] [PubMed] [Google Scholar]
- 33.Hosea NA, Miller GP, Guengerich FP. Elucidation of distinct ligand binding sites for cytochrome P450 3A4. Biochemistry. 2000;39:5929–5939. doi: 10.1021/bi992765t. [DOI] [PubMed] [Google Scholar]
- 34.Presta A, Liu J, Sessa WC, Stuehr DJ. Substrate binding and calmodulin binding to endothelial nitric oxide synthase coregulate its enzymatic activity. Nitric Oxide. 1997;1:74–87. doi: 10.1006/niox.1996.0110. [DOI] [PubMed] [Google Scholar]
- 35.McMillan K, Bredt DS, Hirsch DJ, Snyder SH, Clark JE, Masters BS. Cloned, expressed rat cerebellar nitric oxide synthase contains stoichiometric amounts of heme, which binds carbon monoxide. Proc Natl Acad Sci U S A. 1992;89:11141–11145. doi: 10.1073/pnas.89.23.11141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Panda K, Chawla-Sarkar M, Santos C, Koeck T, Erzurum SC, Parkinson JF, Stuehr DJ. Visualizing inducible nitric-oxide synthase in living cells with a heme-binding fluorescent inhibitor. Proc Natl Acad Sci U S A. 2005;102:10117–10122. doi: 10.1073/pnas.0408972102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stuehr DJ, Ikeda-Saito M. Spectral characterization of brain and macrophage nitric oxide synthases. Cytochrome P-450-like hemeproteins that contain a flavin semiquinone radical. J Biol Chem. 1992;267:20547–20550. [PubMed] [Google Scholar]
- 38.Omura T, Sato R. The carbon monoxide-binding pigment of liver microsomes. II. Solubilization, purification, and properties. J Biol Chem. 1964;239:2379–2385. [PubMed] [Google Scholar]
- 39.Panda K, Haque MM, Garcin-Hosfield ED, Durra D, Getzoff ED, Stuehr DJ. Surface charge interactions of the FMN module govern catalysis by nitric-oxide synthase. J Biol Chem. 2006;281:36819–36827. doi: 10.1074/jbc.M606129200. [DOI] [PubMed] [Google Scholar]
- 40.Klatt P, Pfeiffer S, List BM, Lehner D, Glatter O, Bachinger HP, Werner ER, Schmidt K, Mayer B. Characterization of heme-deficient neuronal nitric-oxide synthase reveals a role for heme in subunit dimerization and binding of the amino acid substrate and tetrahydrobiopterin. J Biol Chem. 1996;271:7336–7342. doi: 10.1074/jbc.271.13.7336. [DOI] [PubMed] [Google Scholar]
- 41.Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5:62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- 42.Riggs A. Preparation of blood hemoglobins of vertebrates. In: Antonini E, Rossi-Bernardi L, Chiancone E, editors. Hemoglobins and myoglobins, Methods in Enzymol. Vol. 76. New York: Academic Press; 1981. pp. 5–28. [DOI] [PubMed] [Google Scholar]
- 43.Johansson LH, Borg LAH. A spectrophotometric method for determination of catalase activity in small tissue samples. Anal Biochem. 1988;174:331–336. doi: 10.1016/0003-2697(88)90554-4. [DOI] [PubMed] [Google Scholar]
- 44.Datta PK, Lianos EA. Nitric oxide induces heme oxygenase-1 gene expression in mesangial cells. Kidney Int. 1999;55:1734–1739. doi: 10.1046/j.1523-1755.1999.00429.x. [DOI] [PubMed] [Google Scholar]
- 45.Balla J, Jacob HS, Balla G, Nath K, Eaton JW, Vercellotti GM. Endothelial-cell heme uptake from heme proteins: induction of sensitization and desensitization to oxidant damage. Proc Natl Acad Sci U S A. 1993;90:9285–9289. doi: 10.1073/pnas.90.20.9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zamora R, Vodovotz Y, Aulak KS, Kim PK, Kane JM, 3rd, Alarcon L, Stuehr DJ, Billiar TR. A DNA microarray study of nitric oxide-induced genes in mouse hepatocytes: implications for hepatic heme oxygenase-1 expression in ischemia/reperfusion. Nitric Oxide. 2002;7:165–186. doi: 10.1016/s1089-8603(02)00104-0. [DOI] [PubMed] [Google Scholar]
- 47.Kanakiriya SK, Croatt AJ, Haggard JJ, Ingelfinger JR, Tang SS, Alam J, Nath KA. Heme: a novel inducer of MCP-1 through HO-dependent and HO-independent mechanisms. Am J Physiol Renal Physiol. 2003;284:F546–F554. doi: 10.1152/ajprenal.00298.2002. [DOI] [PubMed] [Google Scholar]
- 48.Mooradian DL, Hutsell TC, Keefer LK. Nitric oxide (NO) donor molecules: effect of NO release rate on vascular smooth muscle cell proliferation in vitro. J Cardiovasc Pharmacol. 1995;25:674–678. [PubMed] [Google Scholar]
- 49.Scott EE, Halpert JR. Structures of cytochrome P450 3A4. Trends Biochem Sci. 2005;30:5–7. doi: 10.1016/j.tibs.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 50.Rowland P, Blaney FE, Smyth MG, Jones JJ, Leydon VR, Oxbrow AK, Lewis CJ, Tennant MG, Modi S, Eggleston DS, Chenery RJ, Bridges AM. Crystal structure of human cytochrome P450 2D6. J Biol Chem. 2006;281:7614–7622. doi: 10.1074/jbc.M511232200. [DOI] [PubMed] [Google Scholar]
- 51.Reid TJ, 3rd, Murthy MR, Sicignano A, Tanaka N, Musick WD, Rossmann MG. Structure and heme environment of beef liver catalase at 2.5 A resolution. Proc Natl Acad Sci U S A. 1981;78:4767–4771. doi: 10.1073/pnas.78.8.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rafferty SP, Domachowske JB, Malech HL. Inhibition of hemoglobin expression by heterologous production of nitric oxide synthase in the K562 erythroleukemic cell line. Blood. 1996;88:1070–1078. [PubMed] [Google Scholar]
- 53.Cooper CE. Nitric oxide and iron proteins. Biochim Biophys Acta. 1999;1411:290–309. doi: 10.1016/s0005-2728(99)00021-3. [DOI] [PubMed] [Google Scholar]
- 54.Kharitonov VG, Sharma VS, Magde D, Koesling D. Kinetics of nitric oxide dissociation from five- and six-coordinate nitrosyl hemes and heme proteins, including soluble guanylate cyclase. Biochemistry. 1997;36:6814–6818. doi: 10.1021/bi970201o. [DOI] [PubMed] [Google Scholar]
- 55.Helmick RA, Fletcher AE, Gardner AM, Gessner CR, Hvitved AN, Gustin MC, Gardner PR. Imidazole antibiotics inhibit the nitric oxide dioxygenase function of microbial flavohemoglobin. Antimicrob Agents Chemother. 2005;49:1837–1843. doi: 10.1128/AAC.49.5.1837-1843.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sennequier N, Wolan D, Stuehr DJ. Antifungal imidazoles block assembly of inducible NO synthase into an active dimer. J Biol Chem. 1999;274:930–938. doi: 10.1074/jbc.274.2.930. [DOI] [PubMed] [Google Scholar]
- 57.Blasko E, Glaser CB, Devlin JJ, Xia W, Feldman RI, Polokoff MA, Phillips GB, Whitlow M, Auld DS, McMillan K, Ghosh S, Stuehr DJ, Parkinson JF. J Biol Chem. 2002;277:295–302. doi: 10.1074/jbc.M105691200. [DOI] [PubMed] [Google Scholar]
- 58.Huang L, Abu-Soud HM, Hille R, Stuehr DJ. Nitric oxide-generated P420 nitric oxide synthase: characterization and roles for tetrahydrobiopterin and substrate in protecting against or reversing the P420 conversion. Biochemistry. 1999;38:1912–1920. doi: 10.1021/bi981954t. [DOI] [PubMed] [Google Scholar]
- 59.Voegtle HL, Sono M, Adak S, Pond AE, Tomita T, Perera R, Goodin DB, Ikeda-Saito M, Stuehr DJ, Dawson JH. Spectroscopic characterization of five- and six-coordinate ferrous-NO heme complexes. Evidence for heme Fe-proximal cysteinate bond cleavage in the ferrous-NO adducts of the Trp-409Tyr/Phe proximal environment mutants of neuronal nitric oxide synthase. Biochemistry. 2003;42:2475–2484. doi: 10.1021/bi0271502. [DOI] [PubMed] [Google Scholar]
- 60.Ouellet H, Lang J, Couture M, Ortiz de Montellano PR. Reaction of Mycobacterium tuberculosis cytochrome P450 enzymes with nitric oxide. Biochemistry. 2009;48:863–872. doi: 10.1021/bi801595t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shiro Y, Fujii M, Isogai Y, Adachi S, Iizuka T, Obayashi E, Makino R, Nakahara K, Shoun H. Iron-ligand structure and iron redox property of nitric oxide reductase cytochrome P450nor from Fusarium oxysporum: relevance to its NO reduction activity. Biochemistry. 1995;34:9052–9058. doi: 10.1021/bi00028a014. [DOI] [PubMed] [Google Scholar]
- 62.Kim Y-M, Bergonia HA, Muller C, Pitt BR, Watkins WD, Lancaster JR. Nitric oxide and intracellular heme. Adv Pharmacol. 1995;34:277–291. doi: 10.1016/s1054-3589(08)61092-3. [DOI] [PubMed] [Google Scholar]
- 63.Zhao Y, Hoganson C, Babcock GT, Marletta MA. Structural changes in the heme proximal pocket induced by nitric oxide binding to soluble guanylate cyclase. Biochemistry. 1998;37:12458–12464. doi: 10.1021/bi9811563. [DOI] [PubMed] [Google Scholar]
- 64.Makino R, Obayashi E, Homma N, Shiro Y, Hori H. YC-1 facilitates release of the proximal His residue in the NO and CO complexes of soluble guanylate cyclase. J Biol Chem. 2003;278:11130–11137. doi: 10.1074/jbc.M209026200. [DOI] [PubMed] [Google Scholar]
- 65.Adachi S, Nagano S, Ishimori K, Watanabe Y, Morishima I, Egawa T, Kitagawa T, Makino R. Roles of proximal ligand in heme proteins: replacement of proximal histidine of human myoglobin with cysteine and tyrosine by site-directed mutagenesis as models for P-450, chloroperoxidase, and catalase. Biochemistry. 1993;32:241–252. doi: 10.1021/bi00052a031. [DOI] [PubMed] [Google Scholar]
- 66.Yonetani T, Tsuneshige A, Zhou Y, Chen X. Electron paramagnetic resonance and oxygen binding studies of alpha-Nitrosyl hemoglobin. A novel oxygen carrier having no-assisted allosteric functions. J Biol Chem. 1998;273:20323–20333. doi: 10.1074/jbc.273.32.20323. [DOI] [PubMed] [Google Scholar]
- 67.Pond AE, Roach MP, Sono M, Rux AH, Franzen S, Hu R, Thomas MR, Wilks A, Dou Y, Ikeda-Saito M, Ortiz de Montellano PR, Woodruff WH, Boxer SG, Dawson JH. Biochemistry. 1999;38:7601–7608. doi: 10.1021/bi9825448. [DOI] [PubMed] [Google Scholar]
- 68.Vetter SW, Terentis AC, Osborne RL, Dawson JH, Goodin DB. Replacement of the axial histidine heme ligand with cysteine in nitrophorin 1: spectroscopic and crystallographic characterization. J Biol Inorg Chem. 2009;14:179–191. doi: 10.1007/s00775-008-0436-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Medlock A, Swartz L, Dailey TA, Dailey HA, Lanzilotta WN. Substrate interactions with human ferrochelatase. Proc Natl Acad Sci U S A. 2007;104:1789–1793. doi: 10.1073/pnas.0606144104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reedy CJ, Gibney BR. Heme protein assemblies. Chem Rev. 2004;104:617–649. doi: 10.1021/cr0206115. [DOI] [PubMed] [Google Scholar]
- 71.Hemmens B, Gorren AC, Schmidt K, Werner ER, Mayer B. Haem insertion, dimerization and reactivation of haem-free rat neuronal nitric oxide synthase. Biochem J. 1998;332:337–342. doi: 10.1042/bj3320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ma X, Sayed N, Beuve A, van den Akker F. NO and CO differentially activate soluble guanylyl cyclase via a heme pivot-bend mechanism. EMBO J. 2007;26:578–588. doi: 10.1038/sj.emboj.7601521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Landfried DA, Vuletich DA, Pond MP, Lecomte JT. Structural and thermodynamic consequences of b heme binding for monomeric apoglobins and other apoproteins. Gene. 2007;398:12–28. doi: 10.1016/j.gene.2007.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Foster MW, Hess DT, Stamler JS. Protein S-nitrosylation in health and disease: a current perspective. Trends Mol Med. 2009;15:391–404. doi: 10.1016/j.molmed.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Peluffo G, Radi R. Biochemistry of protein tyrosine nitration in cardiovascular pathology. Cardiovasc Res. 2007;75:291–302. doi: 10.1016/j.cardiores.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 76.Padgett CM, Whorton AR. Glutathione redox cycle regulates nitric oxide-mediated glyceraldehyde-3-phosphate dehydrogenase inhibition. Am J Physiol. 1997;272:C99–108. doi: 10.1152/ajpcell.1997.272.1.C99. [DOI] [PubMed] [Google Scholar]
- 77.Erwin PA, Lin AJ, Golan DE, Michel T. Receptor-regulated dynamic S-nitrosylation of endothelial nitric-oxide synthase in vascular endothelial cells. J Biol Chem. 2005;280:19888–19894. doi: 10.1074/jbc.M413058200. [DOI] [PubMed] [Google Scholar]
- 78.Benhar M, Forrester MT, Stamler JS. Protein denitrosylation: enzymatic mechanisms and cellular functions. Nat Rev Mol Cell Biol. 2009;10:721–732. doi: 10.1038/nrm2764. [DOI] [PubMed] [Google Scholar]
- 79.Koeck T, Fu X, Hazen SL, Crabb JW, Stuehr DJ, Aulak KS. Rapid and selective oxygen-regulated protein tyrosine denitration and nitration in mitochondria. J Biol Chem. 2004;279:27257–27262. doi: 10.1074/jbc.M401586200. [DOI] [PubMed] [Google Scholar]
- 80.Koeck T, Willard B, Crabb JW, Kinter M, Stuehr DJ, Aulak KS. Glucose-mediated tyrosine nitration in adipocytes: targets and consequences. Free Radic Biol Med. 2009;46:884–892. doi: 10.1016/j.freeradbiomed.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mitchell DA, Morton SU, Fernhoff NB, Marletta MA. Thioredoxin is required for S-nitrosation of procaspase-3 and the inhibition of apoptosis in Jurkat cells. Proc Natl Acad Sci U S A. 2007;104:11609–11614. doi: 10.1073/pnas.0704898104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang B, Liao CL, Lin YP, Chen SC, Wang DL. S-nitrosoproteome in Endothelial Cells Revealed by a Modified Biotin Switch Approach Coupled with Western Blot-Based Two-Dimensional Gel Electrophoresis. J Proteome Res. 2009;8:4835–4843. doi: 10.1021/pr9005662. [DOI] [PubMed] [Google Scholar]
- 83.López-Sánchez LM, Muntané J, de la Mata M, Rodríguez-Ariza A. Unraveling the S-nitrosoproteome: tools and strategies. Proteomics. 2009;9:808–818. doi: 10.1002/pmic.200800546. [DOI] [PubMed] [Google Scholar]
- 84.Martínez-Ruiz A, Villanueva L, González de Orduña C, López-Ferrer D, Higueras MA, Tarín C, Rodríguez-Crespo I, Vázquez J, Lamas S. S-nitrosylation of Hsp90 promotes the inhibition of its ATPase and endothelial nitric oxide synthase regulatory activities. Proc Natl Acad Sci U S A. 2005;102:8525–8530. doi: 10.1073/pnas.0407294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pinzar E, Wang T, Garrido MR, Xu W, Levy P, Bottari SP. Angiotensin II induces tyrosine nitration and activation of ERK1/2 in vascular smooth muscle cells. FEBS Lett. 2005;579:5100–5104. doi: 10.1016/j.febslet.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 86.Zhang LM, StCroix C, Cao R, Wasserloos K, Watkins SC, Stevens T, Li S, Tyurin V, Kagan VE, Pitt BR. Cell-surface protein disulfide isomerase is required for transnitrosation of metallothionein by S-nitroso-albumin in intact rat pulmonary vascular endothelial cells. Exp Biol Med. 2006;231:1507–1515. doi: 10.1177/153537020623100909. [DOI] [PubMed] [Google Scholar]
- 87.Sliskovic I, Raturi A, Mutus B. Characterization of the S-denitrosation activity of protein disulfide isomerase. J Biol Chem. 2005;280:8733–8741. doi: 10.1074/jbc.M408080200. [DOI] [PubMed] [Google Scholar]
- 88.Quaroni LG, Seward HE, McLean KJ, Girvan HM, Ost TW, Noble MA, Kelly SM, Price NC, Cheesman MR, Smith WE, Munro AW. Interaction of nitric oxide with cytochrome P450 BM3. Biochemistry. 2004;43:16416–16431. doi: 10.1021/bi049163g. [DOI] [PubMed] [Google Scholar]
- 89.Daiber A, Schöneich C, Schmidt P, Jung C, Ullrich V. Autocatalytic nitration of P450CAM by peroxynitrite. J Inorg Biochem. 2000;81:213–220. doi: 10.1016/s0162-0134(00)00110-0. [DOI] [PubMed] [Google Scholar]
- 90.Maréchal A, Mattioli TA, Stuehr DJ, Santolini J. Activation of peroxynitrite by inducible nitric-oxide synthase: a direct source of nitrative stress. J Biol Chem. 2007;282:14101–14112. doi: 10.1074/jbc.M609237200. [DOI] [PubMed] [Google Scholar]
- 91.Guo FH, De Raeve HR, Rice TW, Stuehr DJ, Thunnissen FB, Erzurum SC. Continuous nitric oxide synthesis by inducible nitric oxide synthase in normal human airway epithelium in vivo. Proc Natl Acad Sci U S A. 1995;92:7809–7813. doi: 10.1073/pnas.92.17.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thomas DD, Espey MG, Ridnour LA, Hofseth LJ, Mancardi D, Harris CC, Wink DA. Hypoxic inducible factor 1alpha, extracellular signal-regulated kinase, and p53 are regulated by distinct threshold concentrations of nitric oxide. Proc Natl Acad Sci U S A. 2004;101:8894–8899. doi: 10.1073/pnas.0400453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Billiar TR, Curran RD, Ferrari FK, Williams DL, Simmons RL. Kupffer cell:hepatocyte cocultures release nitric oxide in response to bacterial endotoxin. J Surg Res. 1990;48:349–353. doi: 10.1016/0022-4804(90)90073-b. [DOI] [PubMed] [Google Scholar]
- 94.Cavicchi M, Whittle BJ. Regulation of induction of nitric oxide synthase and the inhibitory actions of dexamethasone in the human intestinal epithelial cell line, Caco-2: influence of cell differentiation. Br J Pharmacol. 1999;128:705–715. doi: 10.1038/sj.bjp.0702827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Laskin DL, Heck DE, Gardner CR, Feder LS, Laskin JD. Distinct patterns of nitric oxide production in hepatic macrophages and endothelial cells following acute exposure of rats to endotoxin. J Leukoc Biol. 1994;56:751–758. doi: 10.1002/jlb.56.6.751. [DOI] [PubMed] [Google Scholar]
- 96.Robinson MA, Baumgardner JE, Good VP, Otto CM. Physiological and hypoxic O2 tensions rapidly regulate NO production by stimulated macrophages. Am J Physiol Cell Physiol. 2008;294:C1079–1087. doi: 10.1152/ajpcell.00469.2007. [DOI] [PubMed] [Google Scholar]
- 97.Sigfrid LA, Cunningham JM, Beeharry N, Lortz S, Tiedge M, Lenzen S, Carlsson C, Green IC. Cytokines and nitric oxide inhibit the enzyme activity of catalase but not its protein or mRNA expression in insulin-producing cells. J Mol Endocrinol. 2003;31:509–518. doi: 10.1677/jme.0.0310509. [DOI] [PubMed] [Google Scholar]
- 98.Morgan ET. Regulation of cytochromes P450 during inflammation and infection. Drug Metab Rev. 1997;29:1129–1188. doi: 10.3109/03602539709002246. [DOI] [PubMed] [Google Scholar]
- 99.Vuppugalla R, Mehvar R. Short-term inhibitory effects of nitric oxide on cytochrome P450-mediated drug metabolism: time dependency and reversibility profiles in isolated perfused rat livers. Drug Metab Dispos. 2004;32:1446–1454. doi: 10.1124/dmd.104.001487. [DOI] [PubMed] [Google Scholar]
- 100.Wink DA, Osawa Y, Darbyshire JF, Jones CR, Eshenaur SC, Nims RW. Inhibition of cytochromes P450 by nitric oxide and a nitric oxide-releasing agent. Arch Biochem Biophys. 1993;300:115–123. doi: 10.1006/abbi.1993.1016. [DOI] [PubMed] [Google Scholar]
- 101.Stadler J, Schmalix WA, Doehmer J. Inhibition of cytochrome P450 enzymes by nitric oxide. Adv Exp Med Biol. 1996;387:187–193. doi: 10.1007/978-1-4757-9480-9_25. [DOI] [PubMed] [Google Scholar]
- 102.Minamiyama Y, Takemura S, Imaoka S, Funae Y, Tanimoto Y, Inoue M. Irreversible inhibition of cytochrome P450 by nitric oxide. J Pharmacol Exp Ther. 1997;283:1479–1485. [PubMed] [Google Scholar]
- 103.Matthias J, Hoernig S, Doehner W, Okonko DD, Witt C, Anker SD. Anemia and inflammation in COPD. Chest. 2005;127:825–829. doi: 10.1378/chest.127.3.825. [DOI] [PubMed] [Google Scholar]
- 104.McKim SE, Gäbele E, Isayama F, Lambert JC, Tucker LM, Wheeler MD, Connor HD, Mason RP, Doll MA, Hein DW, Artee GA. Inducible nitric oxide synthase is required in alcohol-induced liver injury: studies with knockout mice. Gastroenterology. 2003;125:1834–1844. doi: 10.1053/j.gastro.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 105.Chung HY, Sung B, Jung KJ, Zou Y, Yu BP. The molecular inflammatory process in aging. Antioxid Redox Signal. 2006;8:572–581. doi: 10.1089/ars.2006.8.572. [DOI] [PubMed] [Google Scholar]
- 106.Nalwaya N, Deen WM. Nitric oxide, oxygen, and superoxide formation and consumption in macrophage cultures. Chem Res Toxicol. 2005;18:486–493. doi: 10.1021/tx049879c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.