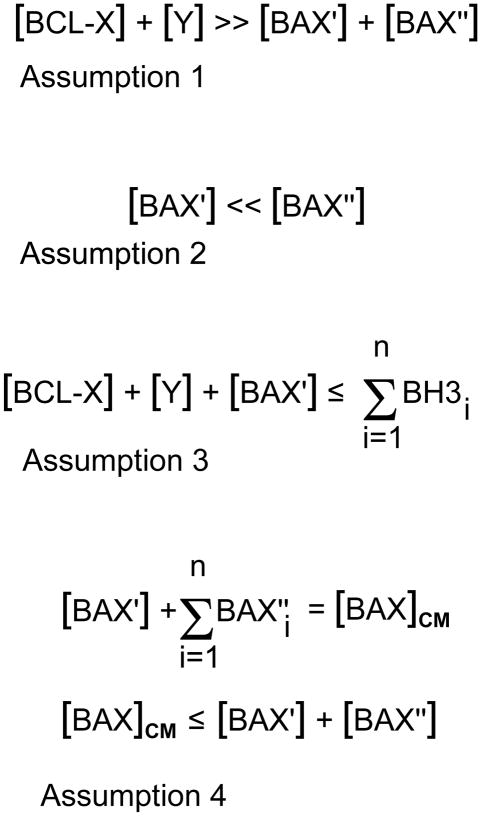Abstract
Studies of the functions of members of the Bcl2 gene family suggested that apoptosis was controlled by a rheostat in which anti-apoptotic proteins like BCL2 bound and sequestered pro-apoptotic proteins like BAX. Our current understanding of these proteins suggests that this is a simplistic model. The new rheostat model predicts that BH3-only peptides act as neutralizing ligands for the anti-apoptotic proteins, thus allowing molecules like BAX to become activated and initiate mitochondrial dysfunction - a critical step in the intrinsic apoptotic program. Studies of retinal ganglion cell apoptosis indicate that a threshold of BAX expression is required for its successful activation, which is independent of the overall concentration of anti-apoptotic proteins in these cells.
Keywords: retinal ganglion cells, BAX, Bcl2 gene family, rheostat model, apoptosis
1. Introduction – Bcl2 gene family function and retinal ganglion cell soma death
The Bcl2 gene family is a group of genes that encode proteins with similar structural domains. The prototype gene, BCL2, was found as the gene overexpressed in B-Cell lymphomas as the result of a t(14:18) chromosomal translocation, where the BCL2 coding region was placed under the control of an immunoglobulin enhancer element (Bakhshi et al., 1985; Tsujimoto et al., 1985; Cleary et al., 1986; McDonnell and Korsmeyer 1991). BCL2, itself, was found to block cell death when overexpressed in a variety of cell types. Importantly, mice overexpressing Bcl2 in neurons, by virtue of being directed by the promoter for neuron-specific enolase (Nse), exhibited increased numbers of retinal ganglion cell somas, both after developmental pruning and after optic nerve axotomy (Bonfanti et al., 1996; Cenni et al., 1996). These studies clearly demonstrated that ganglion cells were responsive to manipulation of the expression of Bcl2 gene family proteins.
After the discovery of BCL2, a variety of other genes with similar features were identified. Genes from this family are now classified by the presence of at least 1 of 4 BCL2 Homology (BH) amino acid domains found in BCL2. Of these 4, perhaps the BH3 domain is the most critical for the complex series of interactions exhibited by these proteins. Surprisingly, although genes with BH domains all appear to play a role in regulating apoptosis, they can have either an anti-apoptotic effect, such as Bcl2 or the neuronal homolog BclX, or a pro-apoptotic effect, such as the related genes Bax and Bak. In addition to genes with multiple BH domains, the Bcl2 gene family contains a select subset of small proteins that carry only the BH3 domain (BH3-only peptides). Invariably, the BH3-only proteins have pro-apoptotic functions, although by themselves they are not sufficient to cause cell death (Zong et al., 2001). The finding of BH3-only proteins underscores the significance of the BH3 domain in the functions of these proteins. Mutation of the BH3 domain in BAX, for example, destroys its ability to execute the cell death program (Wang et al., 1998). Similarly, several groups found that the BH3 domain was critical for both hetero- and homo-oligomerization of members of this family of proteins (Zha et al., 1996). X-ray crystallography of BCL-X and BAK showed that these proteins were able to form globular structures with a hydrophobic cleft, allowing other proteins with BH3 domains to bind to them (Muchmore et al., 1997; Sattler et al., 1997). More complete reviews of the Bcl2 gene family members can be found elsewhere (Adams and Cory 1998; Adams and Cory 2007), including a summary of members that are active in retinal ganglion cells (Nickells et al., 2008).
Over the last decade, several laboratories have investigated the functions of members of the Bcl2 gene family in the process of retinal ganglion cell death in both acute and chronic models of optic nerve lesion. As noted above, Bcl2 overexpression in transgenic mice can block ganglion cell soma death. Bcl2, itself, however, is not predominantly expressed in retinal neurons, including ganglion cells. Instead, the homolog BclX appears to be the dominant anti-apoptotic gene expressed in the retina (Levin et al., 1997), similar to a majority of neurons in mammals (González-García et al., 1995). Of the two major pro-apoptotic genes, Bax and Bak, only Bax is essential for ganglion cell death. This is in contradiction to many other cell types, which exhibit reliance on both BAX and BAK proteins to undergo apoptosis (Lindsten et al., 2000; Wei et al., 2001), and, conversely, require both genes to be knocked out in order to block the process. Ganglion cells, like many neurons, however, are completely resistant to developmental pruning and optic nerve lesions (both acute, such as crush, or chronic, such as in glaucoma), when just the Bax gene is mutated in mice (Mosinger Ogilvie et al., 1998; Li et al., 2000; Libby et al., 2005). The reason for this selective requirement for Bax is unclear, but may stem from a neuronal-specific processing mechanism of Bak transcripts that converts this mRNA to yield a BH3-only peptide (Uo et al., 2005).
Ganglion cells also express a variety of BH3-only genes, including Bim (Näpänkangas et al., 2003; McKernan and Cotter 2007), Bad (Rickman et al., 1999; Huang et al., 2005a; Yang et al., 2008; Levkovitch-Verbin et al., 2009), and Bid (Huang et al., 2005b; Das et al., 2006). The roles of the BH3-only peptides are not known, but recent models suggest that they act as sentinels of various stages, or aspects, of the progress of the apoptotic program. It is believed that they principally function by preferentially binding to anti-apoptotic Bcl2 family proteins and antagonize their function, thus indirectly allowing pro-apoptotic proteins, like BAX, to function (the indirect activation model) (Adams and Cory 2007). When BH3-only peptides become activated, is a function of how and when cell death signals are received by cells. Bim expression, for example, can be transcriptionally activated, and then the peptide is post-translationally modified, during a period of crisis after neurotrophin deprivation (Putcha et al., 2001; Putcha et al., 2003). BAD is a latent protein that becomes released from the 14-3-3 inhibitor by dephosphorylation, during periods of high intracellular Ca2+ signaling (Wang et al., 1999). BID is proteolytically processed to tBID, by activated caspase 8, during activation of the extrinsic pathway of apoptosis (Li et al., 1998). Other BH3-only peptides are activated by separate events. Importantly, any given dying cell may activate, and accumulate, multiple different BH3-only peptides, that are likely to co-operate in the ultimate activation of the apoptotic program. The reasoning for this apparent “overkill” in the activation of BH3-only peptides will be explored in more detail in section 4 of this review.
2. The rheostat model of apoptosis
The finding that pro- and anti-apoptotic proteins could form heterodimers provided an attractive hypothesis of how members of the Bcl2 family could interact and create a life-or-death switch for cells. This hypothesis was introduced by the late Stanley Korsmeyer in the early 1990’s and was coined the rheostat model (Korsmeyer et al., 1993; Korsmeyer 1999). The principal basis for the model came from early studies examining the crude stoichiometry of BCL2 and BAX in different cell lines, and the susceptibility of these lines to cytokine deprivation. Cells with a high BCL2/BAX ratio (generally 2.0 or greater) were “roughly” more resistant, while cells with ratios less than 1.0 were “roughly” more susceptible (Oltvai et al., 1993). Similarly, the sensitivity of different melanoma cell lines to ceramide toxicity was associated with varying ratios of these two proteins (Raisova et al., 2001), where cells with apparently higher ratios of BAX to BCL2 were generally more susceptible. Thus, it appeared that the basic function of anti-apoptotic proteins was to bind up and sequester free BAX molecules. Consistent with this model, experiments in which BCL2 levels were artificially increased, showed that cells could become more resistant to apoptotic stimuli. Similarly, several laboratories showed that p53-dependent apoptosis was associated with the transcriptional activation of the human BAX gene (Miyashita and Reed 1995; Xiang et al., 1998). Presumably, the p53-driven transcription of BAX altered the ratio between BAX and BCL2, favoring apoptosis.
The discovery of BH3-only peptides also fit the model. BH3-only peptides were found to function upstream of BCL2 and BAX, and had higher binding affinities for anti-apoptotic Bcl2 family proteins, suggesting that their principal function was to bind to BCL2 (or BCL-X):BAX heterodimers, causing the displacement of BAX (Yang et al., 1995). Once free of its anti-apoptotic antagonist, BAX could then activate apoptosis.
This classical model, although elegant, has now been challenged (Shacka and Roth 2006). First, studies in Richard Youle’s laboratory demonstrated that although Bcl2 family member proteins may be able to form homooligomers, they were not able to heterodimerize under normal physiological conditions (Hsu et al., 1997; Hsu and Youle 1997; Hsu and Youle 1998). Second, studies in retinal ganglion cells have questioned the importance of BAX stoichiometry as it relates to BCL-X levels (Semaan et al., 2010). In these experiments, it was discovered that two different strains of mice, which express different levels of BAX, but similar levels of BCL-X, both exhibit the same kinetics of ganglion cell loss after optic nerve crush (Figure 1). Additionally, retinal ganglion cells typically express much higher levels of BclX (BCLX) than Bax (BAX). At the transcript level, for example, BclX mRNA can be 5–10 fold more abundant than Bax mRNA (Kim et al., 2006; Semaan et al., 2010). Gene dosage experiments in mice expressing a mutant Bax allele, do reveal that the level of BAX is important for the activation of apoptosis in retinal ganglion cells, however (Libby et al., 2005; Semaan et al., 2005; Semaan and Nickells 2008; Semaan et al., 2010). Bax+/− mice express only 50% of the level of mRNA and protein, and, in many strains of mice, this is sufficient to dramatically increase the resistance of ganglion cells to acute and chronic injury to the optic nerve (Nickells et al., 2008). Conversely, increasing Bax expression over normal wild type levels, does not increase apoptotic susceptibility. Transgenic mice that dramatically overexpress Bax in neurons, for example, show no evidence of increased developmental or injury induced apoptosis (Bernard et al., 1998). These experiments appear to indicate that a specific threshold of Bax expression is required, but as I will argue below, this is more likely altering the ability of BAX proteins to become activated after all the anti-apoptotic family members have been neutralized. Third, the development of the rheostat model was made before the discovery that multiple proteins in the Bcl2 gene family existed, and could be expressed in the same cell type. Thus, the simplistic view of measuring, for example, the concentration of BAX and BCL-X, precluded involvement of other proteins that could be partners in the rheostat effect. This does not necessarily disprove the rheostat model, but does caution the urge to over-interpret data gathered from assaying only a subset of the players that may be involved. Fourth, and finally, as of yet, there has only been a rudimentary attempt to adequately quantify protein levels of Bcl2 family members being expressed in cells. All of this data has been collected from western blot analyses, and do not take into account variables associated with blotting technology. For example, it is not reasonable to compare protein levels using both monoclonal and polyclonal antibodies. Similarly, different proteins have different binding affinities for solid surface substrates, such as nitrocellulose. Simplistically, adequate protein quantification can be achieved, preferably using immunoabsorbant assays (ELISA), where protein levels are measured against a standard curve of purified antigen. These experiments have not been conducted for quantification of Bcl2 family proteins in cells, leaving all data collected that support the rheostat model questionable as to their accuracy.
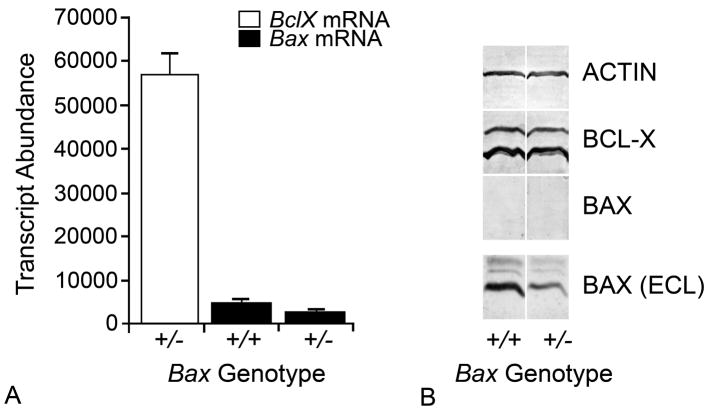
Fig. 1.
Quantitative analysis of BclX and Bax expression in the mouse retina. (A) Quantitative PCR of BclX and Bax transcripts in the retinas of Bax+/+ and Bax+/− mice. BclX mRNA was quantified from Bax+/− animals, but similar levels are detected in animals of all three possible Bax genotypes. In each case, transcript abundance was calculated against a standard curve for each PCR product that was done in concert with the qPCR of the retinal cDNA. Loading of sample template was controlled by the separate quantification of mRNA for the S16 small ribosomal subunit protein. Bax+/− mice express approximately 50% of the amount of Bax mRNA as wild type mice. Alternatively, the same samples exhibit approximately 10 fold higher levels of BclX mRNA. (B) Western blots of Bax+/+ and Bax+/− mice for ACTIN, BCL-X, and BAX protein. Development of the blots was maximized for BCL-X levels, at which point BAX protein is barely, if at all, detectable. In a separate blot, BAX levels were visible using a more sensitive chemiluminescent ECF detection system. Although western blotting technology is limited in its ability to compare quantities of different proteins in the same sample, results like this corroborate quantitative analyses showing much greater levels of BclX mRNA in the retina.
3. BAX activation and function
A critical component to understanding the interplay of Bcl2 family proteins is knowledge of how BAX is activated in cells. In most cells, BAX is a latent protein present in the cytoplasm (Figure 2). It is inactive, and exists as a monomer folded into a globular conformation (Lalier et al., 2007; Green and Chipuk 2008). The globular BAX exhibits a hydrophobic cleft that will allow access to the BH3 domain of other proteins, but other important domains, such as the C-terminal region, which encodes for a putative mitochondrial targeting sequence, remain hidden (Nechushtan et al., 1999; Valentijn et al., 2008). During activation of the apoptotic process, BAX becomes unfolded. The critical data showing this is the exposure of an epitope in the N-terminus that can be recognized by the 6A7 monoclonal antibody (Hsu and Youle 1998; Lalier et al., 2007). As part of this process, BAX also translocates from the cytosol to the mitochondrial outer membrane (MOM) (Hsu et al., 1997; Wolter et al., 1997), where it reportedly forms large oligomers with itself (Pastorino et al., 1998; Antonsson et al., 2000; Eskes et al., 2000; Antonsson et al., 2001; Nechushtan et al., 2001; Kuwana et al., 2002; Dejean et al., 2005). Although BAX can form oligomers in any lipid bilayer, this process is favored in membranes containing high levels of cardiolipins, making mitochondrial membranes a preferred target (Kuwana et al., 2002; Lucken-Ardjomande et al., 2008). The formation of oligomers in the membrane leads to the initial stages of mitochondrial dysfunction that is the hallmark of intrinsic apoptosis. In this process, there is depolarization of the mitochondrial outer membrane, and the release of cytochrome c into the cytosol. Cytochrome c becomes a messenger to activate the apoptosome, essentially an initiator complex that leads to the activation of caspase 9 and eventually the caspase cascade (Liu et al., 1996; Kluck et al., 1997; Reed 1997; Zou et al., 1997; Slee et al., 1999). How BAX oligomers are able to perform this feat is still in doubt. The most popular theory suggests that these oligomers can form pore complexes in the membrane, large enough for cytochrome c to escape. Other models suggest that BAX simply destabilizes the MOM, leading to leakage of cytochrome c (Basanez et al., 1999). Yet another model suggests that BAX interacts with an existing channel in the MOM called the mitochondrial apoptosis-inducing channel (MAC) (Dejean et al., 2005).
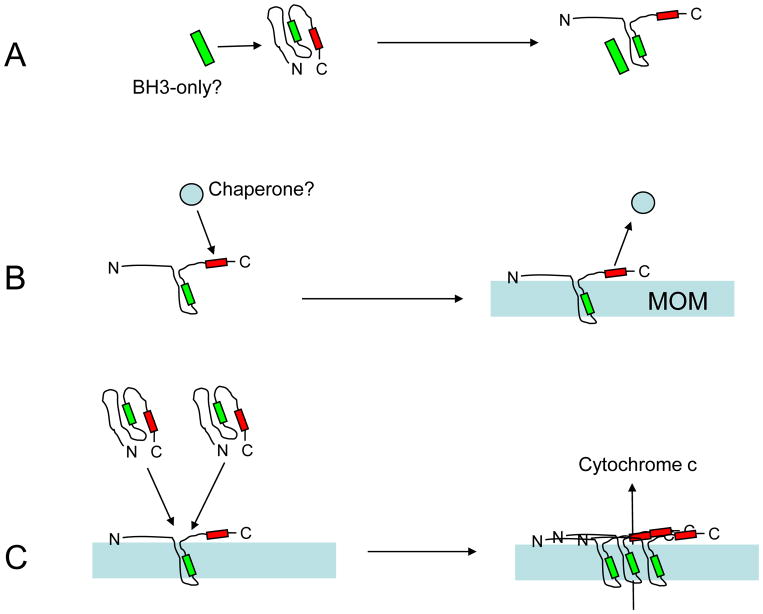
Fig. 2.
Summary diagram of the proposed mechanism of BAX activation and function. (A) In most cells, BAX exists as a latent globular protein already present in the cytosol. The folded structure hides a hydrophobic cleft that is partially composed of the BH3 homology domain (green box), and a putative C-terminal domain, which has structural similarity to a mitochondrial targeting sequence (red box). The process of BAX activation begins with a change in protein conformation, which exposes the hydrophobic cleft, the N-terminus (containing the antigenic site for the 6A7 monoclonal antibody), and the mitochondrial targeting sequence. Recent studies indicate that BAX unfolding is initiated by separate peptides similar to the BIM BH3 domain, although this interaction is not directly with the BAX BH3 domain. (B) Either in concert with the unfolding step, or as a consequence of it, BAX becomes integrated into the mitochondrial outer membrane (MOM). Studies on other proteins containing mitochondrial targeting sequences, suggest that this domain interacts with some chaperone that helps direct these proteins to the appropriate organelles. Currently, there is no direct evidence that BAX interacts with a cellular chaperone, but BAX constructs missing this domain are not able to translocate to mitochondria in the absence of wild type BAX proteins. (C) Once some BAX proteins become inserted into the MOM, they appear to act as a sink for other BAX molecules. In this secondary recruitment step, truncated BAX proteins lacking the translocation domain are able to insert into the MOM, suggesting that they are activated by direct interaction with exposed BH3 domains of BAX molecules already in the membrane. As BAX molecules aggregate in the MOM, they potentially create pore structures that facilitate the release of cytochrome c and downstream events of the apoptotic cascade.
The activation of latent BAX molecules is still a question of considerable debate, but recent studies suggest that the unfolding of the globular form of this protein is mediated by interaction with a BH3-only peptide (Gavathiotis et al., 2008). The data supporting this is that synthetic peptides that mimic the BH3 domain of BIM are able to interact with globular BAX, surprisingly through an interaction on the BAX molecule that is distinct from BAX’s BH3 domain. Similarly surprising, is the evidence that BH3-only peptides preferentially bind to anti-apoptotic proteins, although at high enough concentrations, it is conceivable that peptides like BIM could also interact with inactive BAX. This interaction is still in question, but it is clear that something must interact with latent, globular, BAX molecules to force them to unfold and expose domains like the mitochondrial targeting sequence.
A mitochondrial targeting sequence is typically defined by a hydrophobic domain flanked on either side by positively charged amino acids (Herrmann and Neupert 2000). Generally, proteins with these domains appear to be recognized by chaperones that facilitate movement to of the protein to the MOM (Herrmann and Neupert 2000). Anti-apoptotic proteins, like BCL-X, contain classic mitochondrial targeting domains, whereas BAX contains a region that is similar (George et al., 2010) at its C-terminus. Nevertheless, BAX proteins lacking this region are not able to translocate to mitochondria after an apoptotic stimulus, and cells exclusively expressing truncated proteins are resistant to cell death signals (Valentijn et al., 2008). In addition to this pattern of unfolding, translocation, and MOM insertion, activated BAX appears to have the ability to recruit and activate other BAX molecules without the requirements of interactions with separate BH3-only peptides, or a chaperone to direct translocation (Tan et al., 2006). Data for this is supported by experiments showing that BAX proteins lacking a mitochondrial targeting sequence are also able to insert into the MOM, but only when wild type BAX proteins are present at the same time (Cartron et al., 2008; Valentijn et al., 2008). Thus, the sequence of activating steps for BAX appears to be (i) globular BAX interacts with an activating BH3-containing peptide and unfolds, exposing its own BH3 domain and mitochondrial targeting sequence, (ii) the targeting sequence is recognized by an unidentified chaperone, and the BAX protein is translocated to the mitochondria, (iii) the activated BAX molecule inserts into the MOM, and (iv) activates other globular BAX proteins, in a process of secondary activation. A critical point in this sequence is understanding how secondary activation works. It is possible that MOM-bound BAX molecules attract globular proteins, but this is unlikely. More probable is that MOM-bound BAX acts like a sink for globular BAX molecules that are closely associated with the membrane. It is conceivable that oligomerization of BAX is also a cooperative event, with BAX binding affinity to the growing oligomer increasing with the recruitment of additional monomers. As of yet, this phenomenon has not been described for BAX oligomerization, but with the advent of high resolution confocal microscopy and BAX fusion proteins containing fluorescent markers, the recruitment and sequential binding of BAX monomers may be quantifiable in the near future.
How does the study of retinal ganglion cells impact the theoretical analyses of BAX function? Retinal ganglion cells are like many neurons in that they are dependent only on BAX function to execute the intrinsic apoptotic program (Mosinger Ogilvie et al., 1998; Li et al., 2000; Libby et al., 2005). Interestingly, the dependence on BAX expression in mice can be titrated in retinal ganglion cells, depending on the genetic background of the cells carrying a mutant allele of this gene. The original knock-out allele for Bax was generated in embryonic stem cells originating from 129/Sv mice. These cells were implanted into embryos from C57BL/6 females and lines were generated that were essentially a genetic mix of both lines (129B6). These lines exhibited complete neuronal resistance to apoptotic stimuli if they were Bax−/−, but showed normal cell death responses as Bax+/− cells (Knudson et al., 1995; Deckwerth et al., 1996; Deckwerth et al., 1998; Mosinger Ogilvie et al., 1998; Sun and Oppenheim 2003; Semaan et al., 2010). The movement of the BaxKO allele onto DBA/2J mice, however, revealed that on this background, Bax+/− retinal ganglion cells exhibited complete resistance to optic nerve crush, similar to the knock-out condition (Libby et al., 2005; Semaan et al., 2010). Part of the underlying mechanism for this different phenotype appears to be differential transcription rates from the remaining wild type Bax allele in these mice, associated with a single nucleotide polymorphism in the promoters of the Bax gene. This polymorphism can effectively double the transcription rate of Bax in in vitro experiments, and this translates into nearly double the concentration of Bax mRNA and protein in neurons of 129B6 mice (Semaan et al., 2010). Consequently, 129B6Bax+/− mice have approximately the same concentration of BAX as wild type DBA/2J animals, which is sufficient for successful completion of the apoptotic program. Combined with a previous study showing that overexpression of Bax in transgenic mice does not cause an increase in the rate of neuronal apoptosis (Bernard et al., 1998), we developed a threshold model for BAX function (Figure 3). In this model, we speculated that a specific concentration of BAX protein was required in cells in order for the normal activation and function of this protein. Cells with levels of BAX below this threshold would be resistant to cell death, while cells with any level of BAX above this threshold, would exhibit a maximum cell death response. Subsequent experiments have been conducted to test the threshold hypothesis. In these studies, we titrated the expression of an exogenous Bax gene present in HCT116BAX−/− tissue culture cells. These BAX-deficient cells are normally resistant to non-steroidal anti-inflammatory drugs (Zhang et al., 2000), but maximum cell death could be achieved when the titrated level of exogenous protein reached a specific threshold. More interestingly, subthreshold levels of BAX exhibited an inability to translocate to the MOM, suggesting that low levels of BAX impair some part of the early initiating events in the activation step (Semaan and Nickells, manuscript submitted).
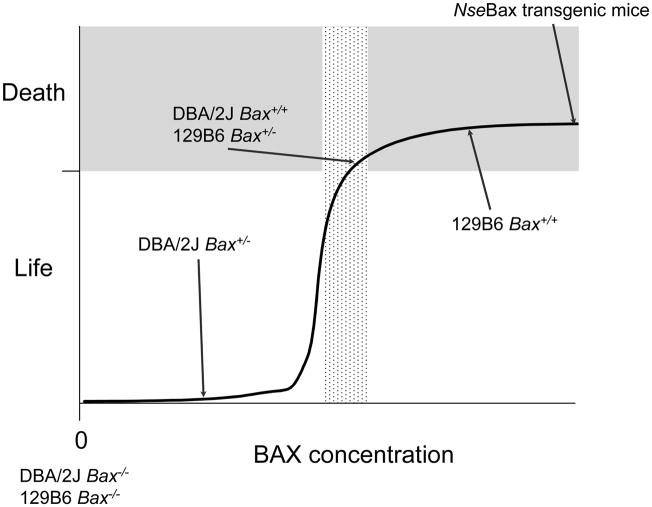
Fig. 3.
Summary of the relationship between retinal ganglion cell apoptosis and Bax expression in two lines of mice. Original data for this model is given in Semaan et al (Semaan et al., 2010). Experiments were conducted on two strains (129B6 and DBA/2J), which carried a mutant allele of Bax. Left eyes of mice were subjected to optic nerve crush (Li et al., 1999), and cells in the retinal ganglion cell layer were counted in both eyes, two weeks after the crush surgery. Bax−/− ganglion cells in both lines exhibited complete resistance to optic nerve crush (white zone), while wild type mice from both lines exhibited comparable levels of cell loss (grey zone). DBA/2JBax+/− mice, exhibited complete resistance, similar to knock-out mice. Conversely, 126B6Bax+/− mice exhibited normal cell loss. Quantitative evaluation of both transcripts and protein in these revealed that DBA/2J animals carried approximately half of the BAX present in 129B6 mice. Comparatively, transgenic mice overexpressing Bax under the control of the Neuron specific enolase promoter (NseBax) show normal kinetics of neuronal apoptosis (Bernard et al., 1998). These data suggest that the commitment to cell death is an all-or-none phenomenon, and that BAX levels (stippled box) must be at a certain threshold to reach this committed step.
4. Variations of the rheostat model
For the most part, a rheostat still exists for proteins in the Bcl2 gene family. It is generally accepted that anti-apoptotic proteins function, in part, to antagonize the activity of pro-apoptotic proteins like BAX and BAK. The mechanism of this antagonism is still unknown, but the localization of proteins like BCL-X to organellar membranes suggests they may be preventing wayward BAX molecules from accidentally inserting and beginning the chain reaction of oligomer formation. Early data, for example, demonstrated that BCL2 was able to inhibit the channel-forming ability of BAX in vitro (Antonsson et al., 1997). Thus, it is reasonable that a cell interested in survival, would generate a large excess of these protective proteins in order to prevent accidents.
To initiate apoptosis, however, anti-apoptotic proteins must be neutralized. This provides an explanation for many of the characteristics of the BH3-only peptides. First, they bind preferentially to anti-apoptotic proteins, such as BCL-X (Chen et al., 2005; Willis et al., 2007). Second, they cannot activate cell death without functional BAX proteins present, indicating that they function upstream of BAX (Putcha et al., 2003; Adams and Cory 2007). Third, in order for BAX to function, all anti-apoptotic proteins must be neutralized (Fletcher et al., 2008), thus providing a true sense of a stoichiometric interaction, not between BAX and anti-apoptotic proteins, but between the latter and BH3-only peptides. To activate cell death, it is in the best interest of the cells to “stack the deck” in favor of the BH3-only side of this equation, and this may be why cells, including retinal ganglion cells, have demonstrated the expression of multiple different members of the BH3-only family.
In addition to neutralizing anti-apoptotic proteins, BH3-only peptides also appear to be required for initially activating BAX. Again, the overexpression of multiple members of the BH3-only family favors this event, especially since the interaction with BAX is not as favorable as it is to molecules like BCL-X. Thus, overexpression of BH3-only proteins is required to both neutralize anti-apoptotic proteins, while leaving enough of an excess of unbound peptides to overcome the unfavorable binding equilibrium with BAX targets.
Finally, although the activation of BAX itself appears to be only peripherally related to the apoptotic rheostat switch, this process is also concentration dependent. In this case, some facet of the activation pathway of BAX requires a certain concentration of BAX protein to be present. Our studies have not yet elucidated what part of this interaction is critically involved. It is possible that low concentrations of BAX severely impair an already unfavorable interaction with activating BH3-only peptides. This is probably not the case, since we predict that the BH3-only peptides would be in large excess anyway. Instead, low concentrations of BAX are more likely to impair secondary recruitment to the MOM after the initial activation step has occurred. Essentially, secondary recruitment would predict that BAX molecules are sequestered only when they are close enough to interact with activated BAX in the MOM. If movement of BAX molecules to this spatially restricted region is passive, then the speed at which a critical mass of BAX in the MOM could be obtained may be dramatically attenuated. In this scenario, cells with subthreshold levels of BAX would be predicted eventually reach a point where they could activate the apoptotic program, and in fact we do see this in retinal ganglion cells of crush resistant Bax+/− mice. Here, wild type mice exhibit nearly complete ganglion cell loss by 3 weeks, while Bax+/− mice only just begin to show evidence of cell loss by 8 weeks. Conversely, Bax−/− mice exhibit resistance indefinitely (Semaan et al., 2010).
The interplay of the Bcl2 family proteins can be summarized mathematically (Figure 4), including requirements for life, neutralization of anti-apoptotic members by BH3-only peptides, BAX activation steps, and the BAX threshold.
Fig. 4.
Mathematical assumptions governing variations in the rheostat model. In this series of equations, important aspects of the different levels of members of the Bcl2 gene family proteins are considered for their roles in blocking apoptosis and/or in the activation of the intrinsic cell death program. Variables are given as either cellular concentrations (written in square brackets) or as absolute numbers of molecules, in which each variable is written in summation form. The variables are:
BCL-X = the principal anti-apoptotic protein expressed in retinal ganglion cells, Y = other molecules of the Bcl2 gene family with anti-apoptotic properties. This may include, but not be limited to, molecules such as BCL2, MCL1, BCL-W, etc.
BAX′ = molecules of BAX that are initially converted from a globular soluble structure to become inserted in the mitochondrial outer membrane. For the purposes of this argument, this is considered the primary BAX activating event, and probably requires interaction with one or more members of the BH3-only peptide family.
BAX″ = all other latent BAX molecules that are present in the cytosol, but are not activated by BH3-only peptide interactions. Instead, these molecules become the pool of BAX proteins that are secondarily activated after the unfolding and insertion of BAX′ molecules.
BAXCM = the critical mass of BAX molecules that must be assembled as oligomers in the mitochondrial membrane. This is a hypothetical variable and makes the assumption that functional destabilization of the mitochondrial membrane requires a specific density of BAX proteins to assemble.
BH3 = BH3-only peptides that become activated during the course of apoptosis of ganglion cell somas.
Assumption 1. This equation summarizes the probable association of BAX molecules and anti-apoptotic molecules present in living, healthy, ganglion cells. Semi-quantitative western blots to measure protein levels, and quantification of transcripts using real-time PCR suggest that just BCL-X alone is several fold more abundant than BAX levels in ganglion cells. Although BAX must become activated in order to execute the cell death program, the excess of antagonistic BCL-X likely prevents “accidental” activation events, which may be one reason why BCL-X is already localized to the surface of organellar membranes.
Assumption 2. This equation summarizes the hypothetical assumption that BAX activation occurs in two stages. The first stage requires only minimal initial activation of BAX (BAX′) by interaction of globular BAX with a BH3-only protein. Once unfolded, BAX′ is able to both insert into the mitochondrial outer membrane, and act as a sink to activate other molecules of globular BAX in the cytosol (BAX″). This event, by itself, is probably not sufficient to elicit cell death, but is likely sufficient to initiate recruitment of other BAX proteins.
Assumption 3. This equation summarizes the critical event leading to the activation of BAX′ molecules, and is essentially the core element of the modern rheostat model. In this model, enough BH3-only proteins must be recruited to inactivate all anti-apoptotic proteins present in a living cell ([BCL-X] + [Y]) as well as still be available to activate BAX′. Equilibrium effects, in which the binding of BH3-only proteins is favored for some Bcl2 family proteins over others, probably requires and excess of BH3-only peptides (n) in order to form partners with all the relevant variables in this equation.
Assumption 4. These two equations summarize the threshold requirements for BAX in order to form functional oligomers in the mitochondrial outer membrane. First, a critical mass of activated BAX is required for oligomer formation. The critical mass is composed of BAX′ molecules, and some portion (n) of BAX″ molecules. It is likely, however, that cells generally contain enough latent BAX molecules to achieve BAXCM, and cells with large excesses of BAX do not exhibit a greater cell death response once critical mass is established. Cells with BAX content below the critical mass threshold are resistant to apoptosis, however. In this model, I assume that [BAX′] ≪ [BAXCM], therefore, subthreshold levels of BAX probably affect the efficiency of secondary recruitment of BAX″ molecules to the mitochondria, thus delaying the formation of BAXCM. Evidence for this is the very slow activation of apoptosis over several months in Bax+/− retinal ganglion cells that are initially resistant to optic nerve crush (Semaan et al., 2010).
5. Conclusion
Understanding the functions of the Bcl2 family proteins is a critical element to understanding the molecular control of apoptosis. The revised rheostat model indicates that neutralizing BCL2 and associated proteins is a critical step in promoting apoptosis. Normally, this step is accomplished by the action of BH3-only peptides. In cancer-related studies, this facet of the control mechanism has launched the development of new BH3 mimetics designed to overcome BCL2 function in chemotherapy resistant tumors (Lessene et al., 2008). In chronic neurodegeneration, however, preventing the apoptotic program is paramount, and studies of the rheostat function suggest that there are two viable targets for this, including increasing the numbers of anti-apoptotic proteins in neurons to overcome the rheostat switch, or reducing BAX concentration to independently cripple the ability of this protein to become activated.
Acknowledgments
This work was supported by grant R01 EY12223 and vision CORE grant P30 EY16665 from the National Eye Institute and an unrestricted grant from Research to Prevent Blindness provided to the Department of Ophthalmology and Visual Sciences. The author thanks Dr. Cassandra Schlamp for critical review of the manuscript and help in preparation of the figures, and Dr. Sheila Semaan for discussions about BAX activation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams JM, Cory S. The bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–1337. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonsson B, Conti F, Ciavatta A, Montessuit S, Lewis S, Martinou I, Bernasconi L, Bernard A, Mermod JJ, Mazzei G, Maundrell K, Gambale F, Sadoul R, Martinou JC. Inhibition of Bax channel-forming activity by Bcl-2. Science. 1997;277:370–372. doi: 10.1126/science.277.5324.370. [DOI] [PubMed] [Google Scholar]
- Antonsson B, Montessuit S, Lauper S, Eskes R, Martinou JC. Bax oligomerization is required for channel-forming activity in liposomes and to trigger cytochrome c release from mitochondria. Biochem. 2000;345:271–278. [PMC free article] [PubMed] [Google Scholar]
- Antonsson B, Montessuit S, Sanchez B, Martinou JC. Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J Biol Chem. 2001;276:11615–11623. doi: 10.1074/jbc.M010810200. [DOI] [PubMed] [Google Scholar]
- Bakhshi A, Jensen JP, Goldman P, Wright JJ, McBride OW, Epstein AL, Korsmeyer SJ. Cloning the chromosomal breakpoint of t(14;18) human lymphomas: clustering around JH on chromosome 14 and near a transcriptional unit on 18. Cell. 1985;41:899–906. doi: 10.1016/s0092-8674(85)80070-2. [DOI] [PubMed] [Google Scholar]
- Basanez G, Nechushtan A, Drozhinin O, Chanturiya A, Choe E, Tutt S, Wood KA, Hsu Y, Zimmerberg J, Youle RJ. Bax, but not Bcl-xL, decreases the lifetime of planar phospholipid bilayer membranes at subnanomolar concentrations. Proc Natl Acad Sci USA. 1999;96:5492–5497. doi: 10.1073/pnas.96.10.5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard R, Dieni S, Rees S, Bernard O. Physiological and induced neuronal death are not affected in NSE-bax transgenic mice. J Neurosci Res. 1998;52:247–259. doi: 10.1002/(SICI)1097-4547(19980501)52:3<247::AID-JNR1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Bonfanti L, Strettoi E, Chierzi S, Cenni MC, Liu XH, Martinou JC, Maffei L, Rabacchi SA. Protection of retinal ganglion cells from natural and axotomy-induced cell death in neonatal transgenic mice overexpressing bcl-2. J Neurosci. 1996;16:4186–4194. doi: 10.1523/JNEUROSCI.16-13-04186.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartron PF, Bellot G, Oliver L, Grandier-Vazeille X, Manon S, Vallette FM. Bax inserts into the mitochondrial outer membrane by different mechanisms. FEBS Lett. 2008;582:3045–3051. doi: 10.1016/j.febslet.2008.07.047. [DOI] [PubMed] [Google Scholar]
- Cenni MC, Bonfanti L, Martinou JC, Ratto GM, Strettoi E, Maffei L. Long-term survival of retinal ganglion cells following optic nerve section in adult bcl-2 transgenic mice. Eur J Neurosci. 1996;8:1735–1745. doi: 10.1111/j.1460-9568.1996.tb01317.x. [DOI] [PubMed] [Google Scholar]
- Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DC. Differential targeting of pro-survival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Cleary ML, Smith SD, Sklar J. Cloning and structural analysis of cDNAs for bcl-2 and a hybrid bcl-2/immunoglobulin transcript resulting from the t(14;18) translocation. Cell. 1986;47:19–28. doi: 10.1016/0092-8674(86)90362-4. [DOI] [PubMed] [Google Scholar]
- Das A, Garner DP, Del Re AM, Woodward JJ, Kumar DM, Agarwal N, Banik NL, Ray SK. Calpeptin provides functional neuroprotection to rat retinal ganglion cells following Ca2+ influx. Brain Res. 2006;1084:146–157. doi: 10.1016/j.brainres.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Deckwerth TL, Easton RM, Knudson CM, Korsmeyer SJ, Johnson EM., Jr Placement of the BCL2family member BAX in the death pathway of sympathetic neurons activated by trophic factor deprivation. Exp Neurol. 1998;152:150–162. doi: 10.1006/exnr.1998.6846. [DOI] [PubMed] [Google Scholar]
- Deckwerth TL, Elliot JL, Knudson CM, Johnson EM, Jr, Snider WD, Korsmeyer SJ. BAX is required for neuronal death after trophic factor deprivation and during development. Neuron. 1996;17:401–411. doi: 10.1016/s0896-6273(00)80173-7. [DOI] [PubMed] [Google Scholar]
- Dejean LM, Martinez-Caballero S, Guo L, Hughes C, Teijido O, Ducret T, Ichas F, Korsmeyer SJ, Antonsson B, Jonas EA, Kinnally KW. Oligomeric Bax is a component of the putative cytochrome c release channel MAC, mitochondrial apoptosis-induced channel. Mol Biol Cell. 2005;16:2424–2432. doi: 10.1091/mbc.E04-12-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskes R, Desagher S, Antonsson B, Martinou JC. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol Biol Cell. 2000;20:929–935. doi: 10.1128/mcb.20.3.929-935.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JI, Meusburger S, Hawkins CJ, Riglar DT, Lee EF, Fairlie WD, Huang DCS, Adams JM. Apoptosis is triggered when prosurvival Bcl-2 proteins cannot restrain Bax. Proc Natl Acad Sci USA. 2008;105:18081–18087. doi: 10.1073/pnas.0808691105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavathiotis E, Suzuki M, Davis ML, Pitter K, Bird GH, Katz SG, Tu HC, Kim H, Cheng EHY, Tjandra N, Walensky LD. BAX activation is initiated at a novel interaction site. Nature. 2008;455:1076–1081. doi: 10.1038/nature07396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George NM, Targy N, Evans JJ, Zhang L, Luo X. Bax contains two functional mitochondrial targeting sequences and translocates to mitochondria in a conformational change- and homo-oligomerization. J Biol Chem. 2010;285:1384–1392. doi: 10.1074/jbc.M109.049924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-García M, García I, Ding L, O’Shea S, Boise LH, Thompson CB, Núñez G. bcl-x is expressed in embryonic and postnatal neural tissues and functions to prevent neuronal cell death. Proc Natl Acad Sci USA. 1995;92:4304–4308. doi: 10.1073/pnas.92.10.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DR, Chipuk JE. Apoptosis: Stabbed in the BAX. Nature. 2008;455:1047–1049. doi: 10.1038/4551047a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann JM, Neupert W. Protein transport into mitochondria. Curr Opin Microbiol. 2000;3:210–214. doi: 10.1016/s1369-5274(00)00077-1. [DOI] [PubMed] [Google Scholar]
- Hsu YT, Wolter KG, Youle RJ. Cytosol-to-membrane redistribution of Bax and Bcl-XL during apoptosis. Proc Natl Acad Sci USA. 1997;94:3668–3672. doi: 10.1073/pnas.94.8.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YT, Youle RJ. Nonionic detergents induce dimerization among members of the Bcl-2 family. J Biol Chem. 1997;272:13829–13834. doi: 10.1074/jbc.272.21.13829. [DOI] [PubMed] [Google Scholar]
- Hsu YT, Youle RJ. Bax in murine thymus is a soluble monomeric protein that displays differential detergent-induced conformations. J Biol Chem. 1998;273:10777–10783. doi: 10.1074/jbc.273.17.10777. [DOI] [PubMed] [Google Scholar]
- Huang W, Dobberfuhl A, Filippopoulos T, Ingelsson M, Fileta JB, Poulin NR, Grosskreutz CL. Transcriptional up-regulation and activation of initiating caspases in experimental glaucoma. Am J Pathol. 2005b;167:673–681. doi: 10.1016/S0002-9440(10)62042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Fileta JB, Dobberfuhl A, Filippopoulos T, Guo Y, Kwon G, Grosskreutz CL. Calcineurin cleavage is triggered by elevated intraocular pressure, and calcineurin inhibition blocks retinal ganglion cell death in experimental glaucoma. Proc Natl Acad Sci USA. 2005a;102:12242–12247. doi: 10.1073/pnas.0505138102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CY, Kuehn MH, Clark AF, Kwon YH. Gene expression profile of the adult human retinal ganglion cell layer. Mol Vis. 2006;12:1640–1648. [PubMed] [Google Scholar]
- Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1135. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- Knudson CM, Tung KSK, Toutellotte WG, Brown GAJ, Korsmeyer SJ. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science. 1995;270:96–99. doi: 10.1126/science.270.5233.96. [DOI] [PubMed] [Google Scholar]
- Korsmeyer SJ. BCL-2 gene family and the regulation of programmed cell death. Can Res. 1999;59(7 Suppl):1693s–17002. [PubMed] [Google Scholar]
- Korsmeyer SJ, Shutter JR, Veis DJW, Merry DE, Oltvai ZN. Bcl-2/Bax: a rheostat that regulates an anti-oxidant pathway and cell death. Semin Can Biol. 1993;4:327–332. [PubMed] [Google Scholar]
- Kuwana T, Mackey MR, Perkins G, Ellisman MH, Latterich M, Schneiter R, Green DR, Newmeyer DD. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111:331–342. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- Lalier L, Cartron PF, Juin P, Nedelkina S, Manon S, Bechinger B, Vallette FM. Bax activation and mitochondrial insertion during apoptosis. Apoptosis. 2007;12:887–896. doi: 10.1007/s10495-007-0749-1. [DOI] [PubMed] [Google Scholar]
- Lessene G, Czabotar PE, Colman PM. BCL-2 family antagonists for cancer therapy. Nat Rev Drug Discov. 2008;7:989–1000. doi: 10.1038/nrd2658. [DOI] [PubMed] [Google Scholar]
- Levin LA, Schlamp CL, Spieldoch RL, Geszvain KM, Nickells RW. Identification of bcl-2 family genes in the rat retina. Invest Ophthalmol Vis Sci. 1997;38:2545–2553. [PubMed] [Google Scholar]
- Levkovitch-Verbin H, Dardik R, Vander S, Melamed S. Mechanism of retinal ganglion cell death in secondary degeneration of the optic nerve. Exp Eye Res. 2009 doi: 10.1016/j.exer.2009.11.014. e-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- Li Y, Schlamp CL, Nickells RW. Experimental induction of retinal ganglion cell death in adult mice. Invest Ophthalmol Vis Sci. 1999;40:1004–1008. [PubMed] [Google Scholar]
- Li Y, Schlamp CL, Poulsen KP, Nickells RW. Bax-dependent and independent pathways of retinal ganglion cell death induced by different damaging stimuli. Exp Eye Res. 2000;71:209–213. doi: 10.1006/exer.2000.0873. [DOI] [PubMed] [Google Scholar]
- Libby RT, Li Y, Savinova OV, Barter J, Smith RS, Nickells RW, John SWM. Susceptibility to neurodegeneration in glaucoma is modified by Bax gene dosage. PLoS Genet. 2005;1:17–26. doi: 10.1371/journal.pgen.0010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsten T, Ross AJ, King A, Zong W, Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K, Chen Y, Wei M, Eng VM, Adelman DM, Simon MC, Ma A, Golden JA, Evan G, Korsmeyer SJ, MacGregor GR, Thompson CB. The combined functions of proapoptotic Bcl-2 family members Bak and Bax are essential for normal development of multiple tissues. Mol Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- Lucken-Ardjomande S, Montessuit S, Martinou JC. Contributions to Bax insertion and oligomerization of lipids of the mitochondrial outer membrane. Cell Death Differ. 2008;15:929–937. doi: 10.1038/cdd.2008.9. [DOI] [PubMed] [Google Scholar]
- McDonnell TJ, Korsmeyer SJ. Progression from lymphoid hyperplasia to high grade malignant lymphoma im mice transgenic for the t(14;18) Nature. 1991;349:254–256. doi: 10.1038/349254a0. [DOI] [PubMed] [Google Scholar]
- McKernan DP, Cotter TG. A critical role for Bim in retinal ganglion cell death. J Neurochem. 2007;102:922–930. doi: 10.1111/j.1471-4159.2007.04573.x. [DOI] [PubMed] [Google Scholar]
- Miyashita T, Reed JC. Tumor supressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80:293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- Mosinger Ogilvie J, Deckwerth TL, Knudson CM, Korsmeyer SJ. Suppression of developmental retinal cell death but not photoreceptor degeneration in Bax-deficient mice. Invest Ophthalmol Vis Sci. 1998;39:1713–1720. [PubMed] [Google Scholar]
- Muchmore SW, Sattler M, Liang H, Meadows RP, Harlan JE, Yoon HS, Nettesheim D, Chang BS, Thompson CB, et al. X-ray and NMR structure of han Bcl-XL, an inhibitor of programmed cell death. Nature. 1997;381:335–341. doi: 10.1038/381335a0. [DOI] [PubMed] [Google Scholar]
- Näpänkangas U, Lindqvist N, Lindholm D, Hallböök F. Rat retinal ganglion cells upregulate the pro-apoptotic BH3-only protein Bim after optic nerve transection. Mol Brain Res. 2003;120:30–37. doi: 10.1016/j.molbrainres.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Nechushtan A, Smith CL, Hsu YT, Youle RJ. Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J. 1999;18:2330–2341. doi: 10.1093/emboj/18.9.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nechushtan A, Smith CL, Lamensdorf I, Yoon SH, Youle RJ. Bax and Bak coalesce into novel mitochondrial-associated clusters during apoptosis. J Cell Biol. 2001;153:1265–1276. doi: 10.1083/jcb.153.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickells RW, Semaan SJ, Schlamp CL. Involvement of the Bcl2 gene family in the signaling and control of retinal ganglion cell death. Prog Brain Res. 2008;173:423–435. doi: 10.1016/S0079-6123(08)01129-1. [DOI] [PubMed] [Google Scholar]
- Oltvai Z, Milliman C, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- Pastorino JG, Chen ST, Tafani M, Snyder JW, Farber JL. The overexpression of bax produces cell death upon induction of the mitochondrial permeability transition. J Biol Chem. 1998;273:7770–7775. doi: 10.1074/jbc.273.13.7770. [DOI] [PubMed] [Google Scholar]
- Putcha GV, Le S, Frank S, Besirli CG, Clark K, Chu B, Alix S, Youle RJ, LaMarche A, Maroney AC, Johnson EM., Jr JNK-mediated BIM phosphorylation potentiates BAX-dependent apoptosis. Neuron. 2003;38:899–914. doi: 10.1016/s0896-6273(03)00355-6. [DOI] [PubMed] [Google Scholar]
- Putcha GV, Moulder KL, Golden JP, Bouillet P, Adams JA, Strasser A, Johnson EM., Jr Induction of BIM, a proapoptotic BH3-only BCL-2 family member, is critical for neuronal apoptosis. Neuron. 2001;29:615–628. doi: 10.1016/s0896-6273(01)00238-0. [DOI] [PubMed] [Google Scholar]
- Raisova M, Hossini AM, Eberle J, Riebeling C, Wieder T, Sturm I, Daniel PT, Orfanos CE, Geilen CC. The Bax/Bcl-2 ratio determines the susceptibility of human melanoma cells to CD95/Fas-mediated apoptosis. J Invest Dermatol. 2001;117:333–340. doi: 10.1046/j.0022-202x.2001.01409.x. [DOI] [PubMed] [Google Scholar]
- Reed JC. Cytochrome c: can’t live with it--can’t live without it. Cell. 1997;91:559–562. doi: 10.1016/s0092-8674(00)80442-0. [DOI] [PubMed] [Google Scholar]
- Rickman DW, Nacke RE, Bowes Rickman C. Characterization of the cell death promoter, Bad, in the developing rat retina and forebrain. Brain Res Dev Brain Res. 1999;115:41–47. doi: 10.1016/s0165-3806(99)00046-2. [DOI] [PubMed] [Google Scholar]
- Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M, Yoon HS, Shuker SB, Chang BS, JMA, Thompson CB, Fesik SW. Structure of Bcl-XL-Bak peptide complex: recognition between regulators of apoptosis. Science. 1997;275:983–986. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- Semaan SJ, Li Y, Babcic V, John SWM, Nickells RW. Differential regulation of the pro-apoptotic gene Bax in DBA/2J and 129B6 inbred mice affects retinal ganglion cell death after optic nerve crush. Invest Ophthalmol Vis Sci. 2005;46 E-Abstract 1279. [Google Scholar]
- Semaan SJ, Li Y, Nickells RW. A single nucleotide polymorphism in the Bax gene promoter affects transcription and influences retinal ganglion cell death. ASN Neuro. 2010 doi: 10.1042/AN20100003. (manuscript submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semaan SJ, Nickells RW. An all-or-none swtich for apoptosis is linked to a threshold level of BAX. Mol Biol Cell. 2008;19(suppl):132. (abstract 452) [Google Scholar]
- Shacka JJ, Roth JA. Bcl-2 family and the central nervous system: from rheostat to real complex. Cell Death Differ. 2006;13:1299–1304. doi: 10.1038/sj.cdd.4401974. [DOI] [PubMed] [Google Scholar]
- Slee EA, Harte MT, Kluck RM, Wolf BB, Casiano CA, Newmeyer DD, Wang HG, Reed JC, Nicholson DW, Alnemri ES, Green DR, Martin SJ. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J Cell Biol. 1999;144:281–292. doi: 10.1083/jcb.144.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Oppenheim RW. Response of motoneurons to neonatal sciatic nerve axotomy in Bax-knockout mice. Mol Cell Neurosci. 2003;24:875–886. doi: 10.1016/s1044-7431(03)00219-7. [DOI] [PubMed] [Google Scholar]
- Tan C, Dlugosz PJ, Peng J, Zhang ZH, Lapolla SM, Plafker SM, Andrews DW, Lin J. Auto-activation of the apoptosis protein Bax increases mitochondrial membrane permeability and is inhibited by Bcl-2. J Biol Chem. 2006;281:14764–14775. doi: 10.1074/jbc.M602374200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto Y, Gorham J, Crossman J, Jaffe E, Croce CM. The t(14;18) chromosome translocations involved in B-cell neoplasms result from mistakes in VDJ joining. Science. 1985;229:1390–1393. doi: 10.1126/science.3929382. [DOI] [PubMed] [Google Scholar]
- Uo T, Kinoshita Y, Morrison RS. Neurons exclusively express N-Bak, a BH3 domain-only Bak isoform that promotes neuronal apoptosis. J Biol Chem. 2005;280:9065–9073. doi: 10.1074/jbc.M413030200. [DOI] [PubMed] [Google Scholar]
- Valentijn AJ, Upton JP, Bates N, Gilmore AP. Bax targeting to mitochondria occurrs via both tail anchor-dependent and -independent mechanisms. Cell Death Differ. 2008;15:1243–1254. doi: 10.1038/cdd.2008.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HG, Pathan N, Ethell IM, Krajewski S, Yamaguchi Y, Shibasaki F, McKeon F, Bobo T, Franke TF, Reed JC. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science. 1999;284:339–343. doi: 10.1126/science.284.5412.339. [DOI] [PubMed] [Google Scholar]
- Wang K, Gross A, Waksman G, Korsmeyer SJ. Mutagenesis of the BH3 domain of BAX identifies residues critical for dimerization and killing. Mol Cell Biol. 1998;18:6083–6089. doi: 10.1128/mcb.18.10.6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei MC, Zong WX, Cheng EHY, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: A requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen LK, Czabotar PE, Ierino H, Lee EF, Fairlie WD, Bouillet P, Strasser A, Kluck RM, Adams JM, Huang DC. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ. Movement of bax from the cytosol to mitochondria during apoptosis. J Cell Biol. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang H, Kinoshital Y, Knudson CM, Korsmeyer SJ, Schwartzkroin PA, Morrison RS. Bax involvement in p53-mediated cell death. J Neurosci. 1998;18:1363–1373. doi: 10.1523/JNEUROSCI.18-04-01363.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ. Bad, a heterodimeric partner for Bcl-xL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995;80:285–291. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- Yang X, Luo C, Cai J, Pierce WM, Tezel G. Phosphorylation-dependent interaction with 14-3-3 in the regulation of bad trafficking in retinal ganglion cells. Invest Ophthalmol Vis Sci. 2008;49:2483–2494. doi: 10.1167/iovs.07-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha H, Aime-Sempe C, Sato T, Reed JC. Proapoptotic protein Bax heterodimerizes with Bcl-2 and homodimerizes with Bax via a novel domain (BH30 distinct from BH1 and BH2. J Biol Chem. 1996;271:7440–7444. doi: 10.1074/jbc.271.13.7440. [DOI] [PubMed] [Google Scholar]
- Zhang L, Yu J, Park BH, Kinzler KW, Vogelstein B. Role of BAX in the apoptotic response to anticancer agents. Science. 2000;290:989–995. doi: 10.1126/science.290.5493.989. [DOI] [PubMed] [Google Scholar]
- Zong WX, Lindsten T, Ross AJ, MacGregor GR, Thompson CB. BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev. 2001;15:1481–1486. doi: 10.1101/gad.897601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H, Henzel WJ, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]