Abstract
Sex-specific differences in dispersal, survival, reproductive success, and natural selection differentially affect the effective population size (Ne) of genomic regions with different modes of inheritance such as sex chromosomes and mitochondrial DNA. In papionin monkeys (macaques, baboons, geladas, mandrills, drills, and mangabeys), for example, these factors are expected to reduce Ne of paternally inherited portions of the genome compared to maternally inherited portions. To explore this further, we quantified relative Ne of autosomal DNA, X and Y chromosomes, and mitochondrial DNA using molecular polymorphism and divergence information from pigtail macaque monkeys (Macaca nemestrina). Consistent with demographic expectations, we found that Ne of the Y is lower than expected from a Wright–Fisher idealized population with an equal proportion of males and females, whereas Ne of mitochondrial DNA is higher. However, Ne of 11 loci on the X chromosome was lower than expected, a finding that could be explained by pervasive hitchhiking effects on this chromosome. We evaluated the fit of these data to various models involving natural selection or sex-biased demography. Significant support was recovered for natural selection acting on the Y chromosome. A demographic model with a skewed sex ratio was more likely than one with sex-biased migration and explained the data about as well as an ideal model without sex-biased demography. We then incorporated these results into an evaluation of macaque divergence and migration on Borneo and Sulawesi islands. One X-linked locus was not monophyletic on Sulawesi, but multilocus data analyzed in a coalescent framework failed to reject a model without migration between these islands after both were colonized.
THE effective size of a population (Ne) determines the relative impact of genetic drift and natural selection on mutations with mild effects on fitness (Charlesworth 2009). Differences in Ne are hypothesized to affect virtually every aspect of genome evolution, including rates of molecular evolution, abundance of introns and transposable elements, and persistence of duplicate genes, and this has important implications for the evolution of complexity via both adaptive and degenerative processes (Lynch 2007). Of relevance are not only the number of different individuals in a population, but also the number of copies of a gene within each individual. In diploid species with separate sexes, sex chromosomes and mitochondrial DNA (mtDNA) differ in copy number from autosomal DNA (aDNA): both sexes have two alleles at autosomal loci whereas in species with male heterogamy, males have one X and one Y chromosome, females have two Xs, and a female/male pair has effectively only one copy of mtDNA due to maternal inheritance. Sex-specific differences in demographic parameters such as migration, adult sex ratio, and variance in reproductive success also affect relative copy number and associated levels of neutral polymorphism at mtDNA, aDNA, the X chromosome (xDNA), and the Y chromosome (yDNA) (Hedrick 2007).
The effective population size is the number of individuals in a Wright–Fisher idealized population (Fisher 1930; Wright 1931) that have the same magnitude of genetic drift as an observed population, where ideal individuals are diploid, and have discrete (nonoverlapping) generations, constant population size, and random mating. Ne can be quantified in terms of variance in allele frequency over generations (variance Ne) or variance in inbreeding over time (inbreeding Ne). If population size is constant with random mating, these approaches for quantifying Ne produce identical results (Kimura and Crow 1963; Whitlock and Barton 1997). At mutation–drift equilibrium with an equal number of males and females and a Poisson distributed number of offspring with a mean of two offspring per individual, Ne-aDNA and Ne-xDNA are expected to be four and three times as large, respectively, as Ne-yDNA and Ne-mtDNA; we refer to this as the “ideal expectation with an equal proportion of males and females.”
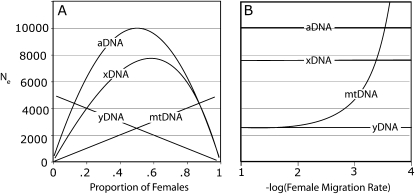
Demography can alter relationships between Ne of different parts of the genome. For example, extreme skew in adult sex ratio can cause Ne of uniparentally inherited portions of the genome to exceed Ne of biparentally inherited portions (Figure 1A; Nunney 1993; Caballero 1994; Hoelzer 1997; Hedrick 2007). With a skewed sex ratio, the more common sex has a higher variance in reproductive success than the rare one, and this causes the overall variance in reproductive success to increase as the sex-ratio bias increases (Nunney 1993). Sex-biased dispersal such as female philopatry also alters relationships between Ne-aDNA, Ne-xDNA, Ne-yDNA, and Ne-mtDNA (Figure 1B), causing Ne of portions of the genome that disperse less to increase (Nei and Takahata 1993; Hoelzer 1997; Wang and Caballero 1999).
Figure 1.—
Ne of aDNA, xDNA, mtDNA, and yDNA as a function of (A) sex ratio skew and (B) the probability of female dispersal. In B, a finite island model of subdivided populations of constant size is assumed with a population size of 10,000 individuals, 10 subpopulations, and a male probability of migration equal to 0.1.
At least five factors related to natural selection also can cause the relative Ne of aDNA, xDNA, yDNA, and mtDNA to depart from expectations: (1) very low or absent recombination in mtDNA and a portion of yDNA, (2) haploidy of mtDNA and yDNA, (3) hemizygosity of xDNA in males, (4) sexual selection and differences in gene content, and (5) differences in the rate and variance of mutation. “Selective sweeps” in which an advantageous mutation is fixed by natural selection, reduces Ne of linked sites (Maynard Smith and Haigh 1974) and this can affect the entire mitochondrial genome and nonrecombining portion of the Y chromosome. Nonrecombining portions of yDNA and mtDNA are also affected by stochastic loss of alleles containing the fewest deleterious mutations (“Muller's ratchet”; Muller 1964; Felsenstein 1974), which results in a gradual decline of fitness of these chromosomes over time. Ne of nonrecombining DNA is further reduced by elimination of variation linked to substantially deleterious mutations (“background selection”; Charlesworth et al. 1993), by interference between linked polymorphisms that impedes fixation of advantageous alleles and extinction of deleterious ones (the “Hill–Robertson effect”; Hill and Robertson 1966; McVean and Charlesworth 2000), and by increased frequency of deleterious mutations linked to advantageous ones during a selective sweep (“genetic hitchhiking”; Rice 1987). Hemizygous X-linked and haploid Y-linked loci in males and mtDNA loci in both sexes are more vulnerable to recessive deleterious mutations because they are not masked by a second allele (Otto and Goldstein 1992). Hemizygosity on the X chromosome can also increase the rate of selective sweeps when advantageous mutations are recessive (Charlesworth et al. 1987). Similarly, these loci are also susceptible to recessive species incompatibilities—a factor that at least partially accounts for Haldane's rule for hybrid sterility (Haldane 1922; Orr 1997). Sexual selection differentially influences the probability of fixation of mutations depending on mode of inheritance (Wade and Shuster 2004), especially mutations with antagonistic fitness effects between the sexes (Gibson et al. 2002). Additionally, the rate of evolution of animal mtDNA is much higher than aDNA, xDNA, and yDNA (Haag-Liautard et al. 2008) and this presumably contributes to variation in the frequency of nonneutral mutations in different parts of the genome.
Differences among Ne of mtDNA, yDNA, xDNA, and aDNA are thought to be particularly pronounced in papionin monkeys (macaques, baboons, geladas, mandrills, drills, and mangabeys). These monkeys have a highly sex-biased adult demography; females form stable philopatric groups of close relatives, whereas males generally change social groups and disperse more widely (Dittus 1975). Often adult sex ratio of papionins is female biased (Dittus 1975; Melnick and Pearl 1987; O'Brien and Kinnard 1997; Okamoto and Matsumura 2001), and males have higher variance in reproductive success than females (Dittus 1975; de Ruiter et al. 1992; Keane et al. 1997; Van Noordwijk and Van Schaik 2002; Widdig et al. 2004). These sex differences predict strong population subdivision of mtDNA with little or no subdivision of aDNA, deep mtDNA coalescence times, and frequent mtDNA paraphyly among species, and discordant genealogical relationships between mtDNA and yDNA—and this has been observed in multiple studies (Melnick and Pearl 1987; Melnick 1988; Melnick and Hoelzer 1992; Melnick et al. 1993; Hoelzer et al. 1994; Evans et al. 1999, 2001, 2003; Tosi et al. 2000, 2002, 2003; Newman et al. 2004). Female philopatry and obligate male migration is a common social system in mammals (Greenwood 1980; Dobson 1982; Johnson 1986), though less so in humans (Seielstad et al. 1998), and molecular variation provides an effective tool for exploring the impact of natural selection and demography on aDNA, the sex chromosomes, and mtDNA (Nachman 1997; Bachtrog and Charlesworth 2002; Stone et al. 2002; Berlin and Ellegren 2004; Hellborg and Ellegren 2004; Wilder et al. 2004; Hammer et al. 2008).
We explored the genetic effects of demography and linked selection in structuring sequence polymorphism of a papionin monkey—the macaques—at two levels. We first tested whether levels of polymorphism in aDNA, xDNA, yDNA, and mtDNA in a Bornean population of the pigtail macaque, Macaca nemestrina, match expectations under scenarios involving natural selection and also whether the data might be explained by simple demographic models with sex-specific dispersal or a biased sex ratio. We then explored demography on a larger, inter-island scale by estimating the time of divergence between macaques on Borneo and Sulawesi islands and by testing for evidence of ongoing migration between these islands.
MATERIALS AND METHODS
Molecular data:
We sequenced sections of 30 unlinked regions of aDNA, xDNA, yDNA, and mtDNA from M. nemestrina and all Sulawesi macaque species except M. brunnescens (Tables 1 and 2). All the loci are or are tightly linked to coding regions. After excluding loci with no divergence compared to an outgroup, the autosomal data included up to 8765 bp per individual after gaps were removed from 14 loci, with a mean of 473 silent sites and 15 chromosomes sequenced per locus. X-chromosome data included up to 10,273 bp per individual after gaps were removed from 14 loci, with a mean of 420 silent sites and 13 chromosomes sequenced per locus. Y-chromosome data included 3725 bp after gaps were removed and 1721 silent sites from eight distinct portions of the nonrecombining region of the Y chromosome of eight individuals. Mitochondrial data included 1735 bp and spanned the 3′ region of the cytochrome b gene, the tRNAThr and tRNAPro, and 484 bp of the control region. For analyses of the impact of genetic drift on different parts of the macaque genome (i.e., the Ne of these genomic regions) of M. nemestrina, portions of mtDNA data were excluded due to ambiguous homology with the outgroups we considered (see below), resulting in a reduced data set of 1478 base pairs spanning 1104 bp of the cytochrome b gene and 374 bp of the control region, for a total of 652 silent sites under the assumption that the control region evolves neutrally (an assumption we discuss below). To minimize the impact of population structure, in this analysis we analyzed samples of M. nemestrina from Borneo Island only. Each data type (aDNA, xDNA, yDNA, and mtDNA) was collected from an overlapping but not identical panel of individuals. Primers and additional information on genetic samples are available in supporting information, File S1, and Evans et al. (2003), and data are deposited in GenBank (accession numbers in Tables 1 and 2 and text below).
TABLE 1.
Descriptive statistics of data from Borneo M. nemestrina
| Locus | Location | bp | n | SSites | S | π | D | Divergence/site | Accession |
|---|---|---|---|---|---|---|---|---|---|
| AFP | aDNA | 828 | 16 | 777 | 10 | 0.0031 | −0.797 | 0.013 | HM071638–HM071645 |
| APOE | aDNA | 632 | 18 | 583 | 2 | 0.0010 | −0.376 | 0.014 | HM071624–HM071632 |
| B2M | aDNA | 830 | 18 | 644 | 9 | 0.0036 | −0.413 | 0.008 | HM071595–HM071603 |
| BETA | aDNA | 616 | 24 | 375 | 5 | 0.0040 | 0.332 | 0.021 | HM071574–HM071587 |
| CCL2 | aDNA | 862 | 16 | 862 | 22 | 0.0075 | −0.085 | 0.012 | HM071557–HM071564 |
| NRAMP | aDNA | 433 | 18 | 421 | 2 | 0.0017 | −0.564 | 0.002 | HM071492–HM071500 |
| TTR | aDNA | 858 | 8 | 858 | 13 | 0.0050 | −0.714 | 0.021 | HM071454–HM071456 |
| ASIP | aDNA | 439 | 18 | 439 | 3 | 0.0016 | −0.568 | 0.025 | HM071834–HM071842 |
| ATXN10 | aDNA | 644 | 12 | 644 | 5 | 0.0030 | 0.644 | 0.017 | HM071613–HM071618 |
| GPR15 | aDNA | 575 | 16 | 138 | 2 | 0.0026 | −1.030 | 0.014 | HM071541–HM071548 |
| IRBP | aDNA | 452 | 10 | 452 | 4 | 0.0041 | −0.134 | 0.011 | HM071527–HM071530 |
| KFL10 | aDNA | 477 | 16 | 133 | 3 | 0.0037 | −1.313 | 0.023 | HM071510–HM071517 |
| PDYN | aDNA | 488 | 18 | 113 | 2 | 0.0020 | −1.511* | 0.009 | HM071474–HM071482 |
| TRIM22 | aDNA | 631 | 8 | 177 | 0 | 0.0000 | – | 0.006 | HM071461–HM071464 |
| AMLEX | xDNA | 809 | 15 | 454 | 0 | 0.0000 | – | 0.000 | HM071147–HM071158 |
| CXORF15 | xDNA | 572 | 11 | 506 | 1 | 0.0004 | −1.12608 | 0.004 | HM071212–HM071219 |
| DBX | xDNA | 461 | 15 | 461 | 2 | 0.0017 | 0.628 | 0.015 | HM071172–HM071183 |
| EFiAX | xDNA | 500 | 12 | 457 | 0 | 0.0000 | – | 0.011 | HM071191–HM071199 |
| NLGN4X | xDNA | 699 | 15 | 215 | 5 | 0.0046 | −1.200 | 0.014 | HM071434–HM071445 |
| PRX | xDNA | 505 | 15 | 299 | 10 | 0.0089 | −0.376 | 0.023 | HM071409–HM071420 |
| RPMX | xDNA | 570 | 11 | 495 | 0 | 0.0000 | – | 0.016 | HM071388–HM071395 |
| SMCX | xDNA | 825 | 12 | 384 | 1 | 0.0004 | −1.148* | 0.005 | HM071366–HM071374 |
| SOX3 | xDNA | 940 | 11 | 249 | 0 | 0.0000 | – | 0.000 | HM071346–HM071353 |
| TBL1X | xDNA | 1605 | 12 | 1359 | 16 | 0.0032 | −0.823 | 0.013 | HM071327–HM071335 |
| TMSB4X | xDNA | 564 | 11 | 422 | 1 | 0.0004 | −1.128 | 0.014 | HM071306–HM071313 |
| USP9X | xDNA | 783 | 14 | 158 | 1 | 0.0008 | −1.155 | 0.019 | HM071282–HM071292 |
| UTX | xDNA | 753 | 15 | 186 | 0 | 0.0000 | – | 0.000 | HM071257–HM071268 |
| ZFX | xDNA | 826 | 13 | 188 | 0 | 0.0000 | – | 0.011 | HM071233–HM071243 |
| mtdna | mtdna | 1735 | 10 | 652 | 133 | 0.0674 | −0.608 | 0.658 | HM071125–HM071134 |
| yDNA | yDNA | 3725 | 8 | 1721 | 1 | 0.0002 | −0.991 | 0.019 | HM071713–HM071717, |
| HM071719–HM071721, | |||||||||
| HM071733–HM071737, | |||||||||
| HM071739–HM071741, | |||||||||
| HM071755–HM071762, | |||||||||
| HM071776–HM071780, | |||||||||
| HM071782–HM071784, | |||||||||
| HM071798–HM071802, | |||||||||
| HM071804–HM071806, | |||||||||
| HM071817–HM071824 |
Locus name (locus), genomic location (location), base pairs sequenced (bp), number of individuals sequenced (n), number of silent sites analyzed (SSites), number of silent polymorphisms (S), average pairwise diversity of silent sites with Jukes–Cantor correction for multiple substitutions (π), Tajima's D statistic based on silent sites (D), silent divergence per silent site from baboons (divergence/site), and accession nos. (accession). Undefined values are indicated by a dash and Tajima's D values with individually significant difference from zero are indicated with an asterisk; mtDNA sequences were used in mlHKA and phylogenetic analysis but not MIMAR analysis.
TABLE 2.
Descriptive statistics of data from the Sulawesi macaques
| Locus | Location | bp | n | SSites | S | π | D | Accession |
|---|---|---|---|---|---|---|---|---|
| AFP | aDNA | 828 | 10/4 | 777 | 8/4 | 0.00377/0.00267 | −/−0.758 | HM071633–HM071637 |
| APOE | aDNA | 632 | 10/4 | 583 | 7/4 | 0.00329/0.00344 | −0.952/−0.756 | HM071619–HM071623 |
| B2M | aDNA | 830 | 14/4 | 644 | 1/0 | 0.00041/0 | −0.339/– | HM071588–HM071594 |
| BETA | aDNA | 616 | 18/4 | 375 | 3/0 | 0.00115/0 | −1.398/– | HM071565–HM071573 |
| CCL2 | aDNA | 862 | 16/4 | 862 | 16/6 | 0.00391/0.00349 | −1.187/−0.784 | HM071549–HM071556 |
| NRAMP | aDNA | 433 | 18/6 | 421 | 7/3 | 0.00421/0.00334 | −0.438/0.363 | HM071483–HM071491 |
| TTR | aDNA | 858 | 8/2 | 858 | 9/3 | 0.00284/0.0035 | −1.460/– | HM071449–HM071453 |
| ASIP | aDNA | 439 | 16/6 | 439 | 6/1 | 0.00291/0.00076 | −1.005/−0.930 | HM071826–HM071833 |
| ATXN10 | aDNA | 423 | 18/6 | 423 | 6/3 | 0.00201/0.00284 | −1.688/−0.442 | HM071604–HM071612 |
| GPR15 | aDNA | 575 | 20/6 | 138 | 2/0 | 0.00211/0 | −1.134/– | HM071531–HM071540 |
| IRBP | aDNA | 452 | 18/6 | 452 | 7/4 | 0.00335/0.00429 | −0.869/0.579 | HM071518–HM071526 |
| KLF10 | aDNA | 477 | 18/6 | 133 | 4/1 | 0.00413/0.00253 | −1.582/−0.905 | HM071501–HM071509 |
| PDYN | aDNA | 488 | 18/6 | 113 | 0/0 | 0.0000/0.0000 | −/− | HM071465–HM071473 |
| TRIM22 | aDNA | 631 | 8/– | 178 | 3/– | 0.00525/– | −0.791/– | HM071457–HM071460 |
| AMLEX | xDNA | 809 | 10/3 | 454 | 1/0 | 0.00078/0 | 0.005– | HM071137–HM071159 |
| CXORF15 | xDNA | 572 | 9/2 | 506 | 1/0 | 0.00044/0 | −1.39*/– | HM071202–HM071211 |
| DBX | xDNA | 466 | 10/3 | 462 | 5/1 | 0.00217/0.00108 | −2.28*/– | HM071162–HM071171 |
| EFiAX | xDNA | 500 | 4/– | 457 | 2/– | 0.00214/– | −0.71*/– | HM071186–HM071190 |
| NLGN4X | xDNA | 699 | 10/3 | 215 | 1/1 | 0.00253/0.00312 | 4.61*/– | HM071424–HM071433 |
| PRX | xDNA | 605 | 10/3 | 314 | 4/1 | 0.00192/0 | 3.69*/– | HM071399–HM071408 |
| RPMX | xDNA | 570 | 10/3 | 496 | 3/1 | 0.00121/0.00101 | −1.56*/– | HM071378–HM071387 |
| SMCX | xDNA | 830 | 9/2 | 385 | 0/0 | 0/0 | −/− | HM071357–HM071365 |
| SOX3 | xDNA | 940 | 7/3 | 249 | 0/0 | 0/0 | −/− | HM071339–HM071345 |
| TBL1X | xDNA | 1605 | 10/3 | 1359 | 13/4 | 0.00251/0.00221 | −0.30/– | HM071317–HM071326 |
| TMSB4X | xDNA | 564 | 10/3 | 422 | 0/0 | 0/0 | −/− | HM071296–HM071305 |
| USP9X | xDNA | 783 | 10/3 | 187 | 1/0 | 0.00298/0.00268 | −1.16*/– | HM071272–HM071281 |
| UTX | xDNA | 753 | 10/3 | 186 | 0/0 | 0/0 | −/− | HM071247–HM071256 |
| ZFX | xDNA | 826 | 10/3 | 188 | 0/0 | 0/0 | −/− | HM071223–HM071232 |
| yDNA | yDNA | 2626 | 10/3 | 1073 | 15/2 | 0.0042/0.00124 | −4.17*/– | HM071683–HM071692, |
| HM071745–HM071754, | ||||||||
| HM071766–HM071775, | ||||||||
| HM071788–HM071797 |
Abbreviations follow Table 1. Numbers before slashes refer to statistics for sequences from all Sulawesi individuals; numbers after slashes refer to statistics for only individuals from the western population of M. tonkeana. Tajima's D is indicated by a dash when sample size was <4 or when undefined and individually significant departure from zero is indicated with an asterisk. Accession nos. for Sulawesi mtDNA sequences are listed in the text and were not included in the MIMAR analysis.
MtDNA vs. numts:
Mitochondrial DNA sequences are frequently transferred into the nuclear genome, giving rise to nuclear sequences of mitochondrial origin (numts) (Bensasson et al. 2001). We took multiple steps to test whether we actually did sequence real mtDNA sequences rather than numts. These steps included (a) manual inspection of sequence chromatograms for multiple peaks which, barring heteroplasmy, should not be present, (b) translation of the coding region of cytochrome b to check for stop codons or frameshift mutations, (c) using BLAST (Altschul et al. 1997) to test whether the top hit of our putative mtDNA sequences was M. mulatta mtDNA rather than M. mulatta nuclear DNA using Build 1.1 of the complete genome sequence, (d) independent sequencing of three overlapping regions of putative mtDNA to permit checks for consistency, and (e) estimation of evolutionary relationships among our putative mtDNA sequences and mtDNA sequences of other primates and numt sequences from M. mulatta. In (e) we also included unpublished numts sequences from M. nemestrina and Sulawesi macaques. We retained in our analysis only those sequences that appeared to be mtDNA sequences by all of these criteria.
Testing for impacts of natural selection or demography–mlHKA analysis:
The relative effective sizes of aDNA, xDNA, yDNA, and mtDNA for M. nemestrina were evaluated using a maximum likelihood version of the Hudson, Kreitman, Aguade test (HKA test; Hudson et al. 1987) as implemented by mlHKA version 2 (Wright and Charlesworth 2004). Because mlHKA assumes no recombination within loci and free recombination between loci, sequenced regions from the Y chromosome were pooled to represent a single locus. Sequence data from mtDNA were also treated as one locus. We first used mlHKA to evaluate the likelihood of an idealized model, where the relative relationship Ne-aDNA:Ne-xDNA:Ne-yDNA:Ne-mtDNA is 1:0.75:0.25:0.25. This model was compared to models with natural selection on yDNA, mtDNA, or xDNA and also with demographic models with a skewed sex ratio or sex-biased dispersal that allowed Ne-mtDNA, Ne-yDNA, or Ne-xDNA to vary with respect to Ne-aDNA according to the relationships and assumptions discussed in File S1. MlHKA runs were performed on the SHARCNET computer network (www.sharcnet.ca). Only silent sites were considered; at least 100,000 generations were performed for each analysis or set of parameter values. Sites with insertion/deletion polymorphisms, missing data, and the tRNAThr and tRNAPro genes of mtDNA were excluded from analysis. Demographic models were compared using the Akaike Information Criterion (AIC) as detailed in Wagenmakers and Farrell (2004).
Polymorphism statistics were calculated using the program DNAsp version 4.0 (Rozas et al. 2003) or manually, and Jody Hey's (Rutgers University) HKA program was used to perform simulations to assess departures from expected levels of polymorphism and divergence across loci.
Multiple substitutions:
A crucial aspect of the HKA framework is quantification of silent divergence from an outgroup to control for locus-specific differences in mutation rate. For aDNA, xDNA, and yDNA, we applied a Jukes–Cantor correction for silent divergence between a randomly selected M. nemestrina individual and an outgroup sequence, using DNAsp. As an outgroup, we tested the feasibility of using another macaque monkey species (M. fascicularius or M. mulatta), a baboon (Papio species), and an ape, Homo sapiens. For the baboon, sequences from P. hamadryas were obtained from the Baylor College of Medicine and yDNA sequences were obtained from a male P. anubis individual from the Toronto Zoo.
A macaque outgroup was deemed not useful because many loci had zero divergence. A human outgroup was dismissed because of the challenge of accurately estimating divergence in mtDNA. With the baboon outgroup, zero divergence was detected at 3 xDNA loci (Table 1) and these were therefore discarded from the analysis of Ne on Borneo M. nemestrina, leaving 27 loci total (14 aDNA, 11 xDNA, 1 yDNA, and 1 mtDNA). In the analysis of macaque divergence and migration between Borneo and Sulawesi discussed below, mtDNA was excluded to avoid having to determine the derived state at sites that had experienced recurrent substitutions, leaving 29 loci for the analysis involving all Sulawesi species. A total of 27 loci were used for the analysis involving only the western population of M. tonkeana because 2 loci (EFiAX and TRIM22) had insufficient data from this Sulawesi population.
Because mtDNA has a much higher rate of evolution than nuclear DNA in mammals, recurrent substitutions could lead to an underestimation of divergence, even after Jukes–Cantor correction. We addressed this problem by estimating silent divergence between baboons and M. nemestrina with maximum likelihood using PAML version 4.2 (Yang 1997). We included sequences from other primates to break up the long branch between the ingroup (M. nemestrina from Borneo) and the outgroup (baboons, accession no. Y18001), including M. sylvanus, M. tibetana, M. mulatta, M. hecki, M. maura, and M. nemestrina from Sumatra and Thailand (accession nos. AJ309865, EU294187, AY612638, HM071114, and HM071118) and also more distantly related taxa including members of the following genera: Pan, Pongo, Hylobates, Nasalis, and Trachypithecus (accession nos. AP008920, X93335, X97707, X99256, EU004476, and EU004477). Transfer RNA and insertion/deletion polymorphisms were removed from the analysis, as were the first 63 bp of the control region due to ambiguous alignment.
The baseml and codeml programs of PAML were used to separately estimate the number of substitutions in the mtDNA control region and cytochrome b. For the control region, the GTR model was significantly preferred over the HKY85 model [P = 0.0161, degrees of freedom (d.f.) = 4] and a divergence of 134 substitutions was estimated. For cytochrome b, we compared a codon model with one nonsynonymous to synonymous substitutions per site rate ratio (ω) for the entire phylogeny to a model with two ω's: one for branches between the baboon and a randomly selected M. nemestrina individual and another for other branches of the phylogeny. The more parameterized model was significantly more likely (P = 0.0130, d.f. = 1) and a divergence of 295 substitutions was estimated. Thus, a total divergence of 429 silent substitutions between baboon and macaques was estimated on the basis of preferred maximum likelihood models.
Testing for migration between Sulawesi and Borneo—MIMAR analysis:
Different species of macaques are capable of producing fertile hybrid offspring (Bernstein and Gordon 1980). We used a coalescent approach to test for evidence of gene flow and to estimate time of divergence between macaque species on Borneo and Sulawesi islands. We considered a demographic model with no migration after both islands were colonized and a model with asymmetric migration between Borneo and Sulawesi using the March 3, 2009 version of MIMAR (Becquet and Przeworski 2007). For each of these models, we analyzed data from Borneo M. nemestrina and all of the Sulawesi macaque species (except M. brunnescens) and also a smaller data set including Borneo M. nemestrina and the western population of M. tonkeana. An advantage of considering all Sulawesi macaques is that macaque migration between Borneo and Sulawesi could have involved multiple allopatric species on Sulawesi. An advantage of considering only the western population of M. tonkeana is that the migration model, which assumes no population structure in the descendant (or ancestral) lineages, is violated to a lesser extent. Intralocus recombination was accommodated using recombination scalars for each type of genomic location (i.e., aDNA had a scalar of 1, xDNA had a scalar of 0.5, and mtDNA and yDNA had scalars equal to 0).
MIMAR analysis uses polymorphism summary statistics to estimate model parameters on the basis of coalescent simulations. Polarization of polymorphisms as ancestral or derived was achieved using PAML with M. mulatta and H. sapiens as outgroups and the ingroup fit to a star phylogeny (Foxe et al. 2009). Maximum likelihood inheritance scalars estimated from our most likely demographic model of M. nemestrina from Borneo were used (i.e., we used 0.18 instead of the neutral expectation of 0.25 for the inheritance scalar of yDNA and 0.87 instead of the neutral expectation of 0.75 for xDNA, see below), and a mutation rate scalar was estimated on the basis of divergence from humans because multiple studies were available with estimated divergence times between papionins and humans. Assuming a generation time of 5 years (Dittus 1975; Lindburg and Harvey 1996) and a divergence time between humans and macaques of 37 million years (Yoder and Yang 2004), we estimated a mutation rate (μ) of 4.44 × 10−9 substitutions/base pair/generation. We used this rate in the MIMAR analysis because it was faster than a rate estimated on the basis of a divergence time between M. mulatta and M. nemestrina of 5 million years ago (Delson 1980; Köhler et al. 2000; Tosi et al. 2003), but slower than a rate calculated on the basis of a more recent time of divergence between humans and macaques of 23 million years (Glazko and Nei 2003). These other estimates were 2.61 × 10−9 and 6.70 × 10−9 substitutions/base pair/generation, respectively.
Results from each model were evaluated with goodness-of-fit tests (Becquet and Przeworski 2007) in which simulations were performed with recombination using parameters estimated by each model, and fit assessed on the basis of how often summary statistics from these simulations match the observed data. The summary statistics include the polymorphism information that was used in the MIMAR analysis, and also statistics not used in the analysis including Tajima's D (Tajima 1989), FST (calculated following Hudson et al. 1992), and nucleotide diversity (π) (Nei and Li 1979). We performed goodness-of-fit tests either by drawing from the posterior distributions of the parameter values or by using the mean of the posterior distribution of each parameter as a point estimate for simulations. As an additional test of model fit we used a χ2 test to compare maximum likelihood values obtained from three independent MIMAR runs per model using a different random seed for each run.
Genealogical estimation:
As an additional step toward better understanding macaque migration between Borneo and Sulawesi, we performed phylogenetic analysis on mtDNA and yDNA using MrBayes version 3.1.2 (Huelsenbeck and Ronquist 2001) and a model of evolution selected with MrModeltest version 2 (Nylander 2004). Though bifurcating phylogenies may incorrectly represent evolutionary relationships among recombining X-linked or autosomal genes, we also performed phylogenetic analysis on one X-linked locus (TBL1X) because variation at this locus appears paraphyletic on Sulawesi. Allelic phase of TBL1X polymorphism was inferred using the method of Clark (1990). All MrBayes runs were performed for 2,000,000 generations and with a burn-in of 1,000,000 generations, selected based on inspection of the likelihood of the posterior distribution of trees. For phylogenetic analysis of yDNA, additional sequences were included (accession nos. HM071646–HM071663, HM071665–HM071681, HM071682–HM071701, HM071703–HM071722, HM071724–HM071742, HM071744–HM071763, HM071765–HM071785, HM071787–HM071807, and HM071809–HM071825) that were not used in HKA tests due to incomplete data for M. nemestrina or Papio, bringing the total base pairs of yDNA up to a maximum of 6155 bp per individual. Phylogenetic analysis of mtDNA included only macaques so alignment of the full 1735 bp was straightforward; data from M. sylvanus, M. tibetana, and M. mulatta (accession nos. AJ309865, EU294187, and AY612638) were used as outgroups and new sequences were obtained from M. maura, M. nigrescens, M. nigra, M. hecki, M. tonkeana, M. ochreata, and M. nemestrina from Borneo, Sumatra, and Thailand (Table 1; accession nos. HM071115–HM071136).
RESULTS
Molecular polymorphism in Borneo pigtail macaques—explanations related to natural selection:
Table 1 shows summaries of nucleotide polymorphism for all loci surveyed in M. nemestrina from Borneo. Thirteen of 14 autosomal loci were polymorphic at silent sites, while 8 of 14 X-linked loci showed silent polymorphism. We observed only one segregating site out of 1721 silent sites on the Y chromosome, while the mtDNA showed much higher levels of polymorphism.
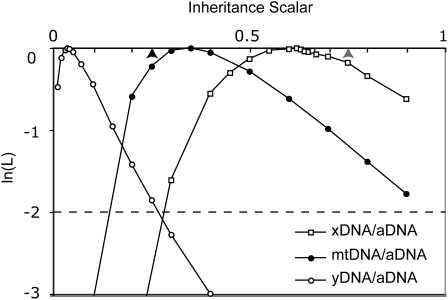
To explore whether different amounts of genetic hitchhiking on different chromosomes are driving differences in Ne, we estimated the Ne of each chromosome relative to aDNA and tested whether it departed significantly from the ideal expectation with an equal proportion of males and females. The maximum likelihood value of Ne-mtDNA/Ne-aDNA is 0.35, Ne-yDNA/Ne-aDNA is 0.035, and Ne-xDNA/Ne-aDNA is 0.62. These values indicate that, compared to the ideal expectation with an equal number of males and females, 40% more polymorphism was observed at mtDNA than expected, whereas only 14% of the expected polymorphism was observed at yDNA and only 83% of the expected polymorphism was observed at the X loci. However, compared to aDNA, departure from the ideal expectation with an equal proportion of males and females is not significant for any of these comparisons [P = 0.506 (mtDNA), 0.157 (yDNA), and 0.550 (xDNA), d.f. = 1]. For each locus the 95% confidence intervals of these ratios as indicated by a departure of two log units from the maximum likelihood value include the ideal expectation with an equal proportion of males and females (Figure 2).
Figure 2.—
Likelihood surfaces of Ne-xDNA/Ne-aDNA, Ne-yDNA/Ne-aDNA, and Ne-mtDNA/Ne-aDNA, ratios. A solid arrowhead indicates the ideal expectation with an equal number of males and females for Ne-yDNA/Ne-aDNA, and Ne-mtDNA/Ne-aDNA (inheritance = 0.25) and a shaded arrowhead indicates the expectation for Ne-xDNA/Ne-aDNA (inheritance = 0.75). Note that the most likely Ne-xDNA/Ne-aDNA ratio is lower than the ideal expectation with an equal number of females and males and also lower than the predictions from the demographic models we examined. However, 95% confidence limits, which are the parameter values with <2 ln(L) units lower than the maximum likelihood value (i.e. above the dashed line), include both of these expectations.
We then tested for departures from the expected Ne-mtDNA, Ne-yDNA, and Ne-xDNA independently while all of the other loci were fixed at the ideal expectation with an equal proportion of males and females. A model allowing free estimation of Ne-yDNA (−lnL = 113.680) was almost significantly better than the ideal expectation with an equal proportion of males and females according to a likelihood ratio test (P = 0.0550, d.f. = 1) and comparison using AIC weights suggests that the model with natural selection on yDNA is better than the other models and is ∼2.5 times better than the neutral model (Table 3). A model that allows for a higher than expected Ne-mtDNA (−lnL = 115.004) is not significantly better than the ideal expectation with an equal proportion of males and females based on a likelihoods ratio test (−lnL = 115.521, P = 0.3092, d.f. = 1), nor is a model allowing a lower than expected Ne-xDNA (−lnL = 115.399, P = 0.489, d.f. = 1).
TABLE 3.
Model comparison using the Akaike Information Criterion (AIC)
| lnL | d.f. | AIC | wAIC | |
|---|---|---|---|---|
| Analysis: all | ||||
| Ideal | −115.5210 | 0 | 231.042 | 0.191 |
| Skew | −114.6370 | 1 | 231.274 | 0.170 |
| Migration/mtDNA selection | −115.0040 | 1 | 232.008 | 0.118 |
| yDNA selection | −113.6800 | 1 | 229.360 | 0.422 |
| xDNA selection | −115.3990 | 1 | 232.798 | 0.079 |
| Analysis: no extremes | ||||
| Ideal | −89.7016 | 0 | 179.403 | 0.177 |
| Skew | −88.7143 | 1 | 179.429 | 0.175 |
| Migration/mtDNA selection | −89.5035 | 1 | 181.007 | 0.079 |
| yDNA selection | −87.6583 | 1 | 177.317 | 0.503 |
| xDNA selection | −89.7016 | 1 | 181.403 | 0.065 |
Comparisons were performed using all of the data with nonzero divergence (analysis: all) and after excluding five loci with extreme χ2 deviances (analysis: no extremes). The models considered include the ideal expectation with an equal proportion of males and females (ideal), a model with a skewed sex ratio (skew), and a model with a sex-biased migration (migration) or balancing selection on mtDNA (mtDNA selection), selection on yDNA (yDNA selection), or selection on xDNA (xDNA selection). The natural logarithm (lnL), degrees of freedom (d.f.), AIC, and weighted AIC (wAIC), which reflects the relative support for each model, are listed.
One concern is that natural selection on the control region of the mtDNA might affect polymorphism and divergence. To test this possibility, we analyzed synonymous sites in the cytochrome b gene and sites in the control region separately to test whether the inheritance scalars for each region were significantly different when inheritance scalars of the other loci were fixed at the ideal expectation with an equal proportion of males and females. The maximum likelihood inheritance scalars for the coding and noncoding regions were 0.37 and 0.60, respectively. Neither scalar departed significantly from the neutral expectation and, on the basis of the likelihood surfaces for each region, neither departed significantly from the maximum likelihood estimate of the other. For these reasons we considered the entire mtDNA data set in our analyses.
While these results provide little evidence suggesting chromosome-wide effects of selective sweeps on xDNA, localized hitchhiking on the X and/or autosomes could also be affecting our results. However, a multilocus HKA test does not recover significant departure from the neutral expectation when all loci were analyzed (sum of deviations = 26.1272, which is <0.228 of the simulated values). When this test was repeated with only X-linked loci included, the results again were not significant (sum of deviations = 12.7853, which was <0.085 of the simulated values). When this test was repeated with only aDNA loci included, the results also were not significant (sum of deviations = 10.6069, which was <0.391 of the simulated values). We also compared a model in which each xDNA locus was under selection to the neutral model, but the selection model was not significantly more likely (−lnL= −110.125, P = 0.461, d.f. = 11). Thus, with the caveat that our conclusions are based on available data, these results do not suggest pervasive hitchhiking on xDNA or aDNA is required to account for polymorphism in Borneo M. nemestrina.
Explanations related to demography:
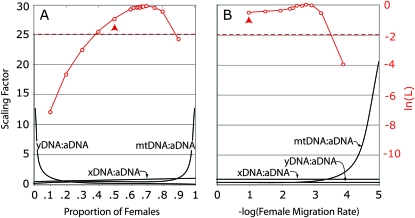
Sex-specific demography could also influence polymorphism of Ne-aDNA, Ne-xDNA, Ne-mtDNA, and Ne-yDNA in Borneo pigtail macaques. To test whether the combined data depart significantly from the ideal expectation with an equal number of males and females, we evaluated the likelihood of the M. nemestrina polymorphism and divergence data under two demographic models using mlHKA. The first included a sex-ratio skew and the second included different levels of sex-biased migration. The inheritance scalars for the migration model are identical to the model discussed above with balancing selection on mtDNA (i.e., 1 for aDNA, 0.75 for xDNA, 0.25 for yDNA, and a maximum likelihood estimate for the scalar for mtDNA (Figure 1B). Model comparison using the AIC did not recover significant support for either demographic model when all of the data were analyzed (Table 3). The demographic model with a female-biased sex ratio is more likely than the model with sex-biased dispersal largely because it better accommodates the low level of polymorphism that was observed in yDNA (Figure 1). AIC weights suggest that the model with a skewed sex ratio is about as good as the ideal model (Table 3). The most likely proportion of females was 70% (Figure 3A) and the most likely female migration rate is 0.0015 individuals per generation, which is 66 times less than the male migration rate which was fixed at 0.1 individuals per generation (Figure 3B).
Figure 3.—
Scaling factors for Ne-xDNA:Ne-aDNA, Ne-yDNA:Ne-aDNA, and Ne-mtDNA:Ne-aDNA (black lines; left y-axis) under a demographic model with (A) skewed sex ratio and (B) sex-biased dispersal, and likelihood (red lines; right y-axis) of M. nemestrina polymorphism and divergence data under each model given these scaling factors. The scaling factors were calculated according to model expectations described in File S1. Model B assumes male migration is 0.1 and a population size of 10,000 individuals divided into 10 equally sized subpopulations. Red arrowheads indicate the likelihood of ideal expectation with no sex ratio skew in A and no sex-bias in dispersal in B. A dotted red line indicates the 95% confidence interval 2lnL units below the maximum likelihood value.
One concern is that this analysis could be affected by natural selection affecting individual loci that we lack statistical power to detect, especially given that all of the loci are or are linked to protein coding regions. To explore this possibility, we used Jody Hey's HKA program to evaluate the χ2 deviations of polymorphism and divergence with respect to the neutral expectation at each type of locus (aDNA or xDNA), and then excluded three aDNA loci (AFP, APOE, and CCL2) and two xDNA loci (RPMX and EFiAX) that had the highest deviations from neutral expectations. While no χ2 deviation departed significantly from neutrality, CCL2 has particularly high polymorphism given its divergence compared to other aDNA loci whereas the other four loci have lower polymorphism compared to other loci in the same category (aDNA or xDNA).
When the models with natural selection and demography were compared again using this reduced data set, the results were similar (Table 3). The model with a selective sweep of yDNA is again preferred and the AIC suggests that the model with a skewed sex ratio is about as good as the ideal expectation with an equal proportion of males and females. With the reduced data set the most likely female proportion was 0.69 and the most likely female migration rate was 0.003 per generation, which is 33 times less than the male migration rate.
Macaque migration between Borneo and Sulawesi?
To estimate the time of divergence and explore demographic scenarios for macaques on Borneo and Sulawesi, we compared the fit of multilocus data to two “inter-island” demographic models. In the first model, an ancestral population splits into two descendant populations with no subsequent migration. This model has four parameters: divergence time (T), the mutation parameter of the ancestral population (θA = 4Neμ), and mutation parameters of each current population (Borneo and Sulawesi, θB and θS, respectively). In the second model, asymmetric migration occurs from Borneo to Sulawesi at rate mBS and from Sulawesi to Borneo at rate mSB, where mBS/4Ne-Sulawesi is the fraction of individuals in each generation on Sulawesi that have migrated from Borneo (and vice versa for mSB/4Ne-Borneo). Using the program MIMAR, we managed to obtain good mixing using parameter values and uniform priors detailed in File S1.
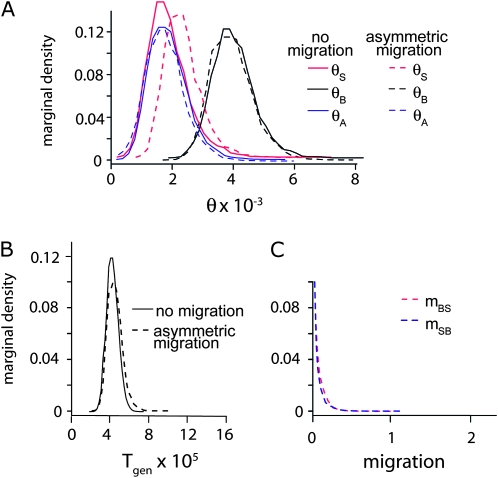
When data from multiple Sulawesi macaque species are analyzed with Borneo M. nemestrina, θS is higher than θB (Figure S1). Because population structure on Sulawesi undoubtedly influenced this result, we also performed a separate analysis that considered a subset of the Sulawesi sequences from the western population of M. tonkeana and the Borneo M. nemestrina (Figure 4). The western population of M. tonkeana is genetically distinct from the eastern population (Evans et al. 2001). In this analysis, estimates of θS and θB were more similar (Figure 4).
Figure 4.—
Posterior distributions of parameters of (A) 4Neμ of macaque monkey populations on Borneo (θB), the Sulawesi species M. tonkeana (θS), the ancestor of these populations before divergence (θA), (B) divergence time (T), and for the asymmetric migration model, (C) migration from Borneo to Sulawesi (mBS), and from Sulawesi to Borneo (mSB).
Goodness-of-fit comparisons between a model without migration and a model with migration produced similar results for the comparison between Bornean M. nemestrina and the western population of M. tonkeana and the comparison between Bornean M. nemestrina and all Sulawesi macaques. For both analyses the addition of migration parameters did not substantially improve the fit of the model to the data (Table 4). For both analyses, neither model fit the observed mean Tajima's D for the M. nemestrina population from Borneo, which was more negative than the simulations (Table 4). In comparisons between Borneo M. nemestrina and all Sulawesi macaques, neither model fit the observed value of FST, πS, DS, or DB (Table 4). This is best explained by population structure on Sulawesi, which is not incorporated into the models we considered, but causes sequence divergence between species to be treated as polymorphism. When only the western population of M. tonkeana was compared to M. nemestrina, simulations matched the observed data for these statistics (Table 4). Likelihood ratio tests also did not reject the model with no migration for either analysis (Table 5).
TABLE 4.
Predictive posterior probabilities from simulations generated by sampling the posterior distribution of model parameters and from point estimates of model parameters
| S1 | S2 | Ss | Sf | SST | πS | πB | DS | DB | |
|---|---|---|---|---|---|---|---|---|---|
| M. tonkeana vs. Borneo (posterior) | |||||||||
| No migration | 0.433 | 0.584 | 0.177 | 0.590 | 0.184 | 0.352 | 0.200 | 0.267 | 0.024 |
| Asymmetric migration | 0.411 | 0.594 | 0.131 | 0.615 | 0.195 | 0.305 | 0.190 | 0.176 | 0.023 |
| M. tonkeana vs. Borneo (point estimate) | |||||||||
| No migration | 0.344 | 0.572 | 0.095 | 0.634 | 0.108 | 0.253 | 0.126 | 0.000 | 0.016 |
| Asymmetric migration | 0.322 | 0.581 | 0.054 | 0.658 | 0.117 | 0.212 | 0.125 | 0.000 | 0.016 |
| Sulawesi vs. Borneo (posterior) | |||||||||
| No migration | 0.422 | 0.651 | 0.243 | 0.524 | 0.003 | 0.026 | 0.314 | 0.000 | 0.008 |
| Asymmetric migration | 0.442 | 0.653 | 0.192 | 0.552 | 0.002 | 0.024 | 0.295 | 0.000 | 0.008 |
| Sulawesi vs. Borneo (point estimate) | |||||||||
| No migration | 0.353 | 0.665 | 0.143 | 0.545 | 0.001 | 0.003 | 0.239 | 0.000 | 0.002 |
| Asymmetric migration | 0.382 | 0.682 | 0.115 | 0.575 | 0.000 | 0.003 | 0.233 | 0.000 | 0.005 |
| Observed values | |||||||||
| Borneo M. nemestrina and M. tonkeana | 35 | 113 | 4 | 26 | 0.391 | 0.805 | 1.216 | −0.454 | −0.529 |
| Borneo M. nemestrina and Sulawesi macaques | 118 | 111 | 7 | 24 | 0.294 | 1.076 | 1.132 | −0.733 | −0.529 |
S1, S2, Ss, and Sf are segregating sites statistics used in the MIMAR analysis, with 1 and 2 referring to Sulawesi and Borneo, respectively (see MIMAR documentation for details). FST, πS, πB, Ds, and DB reflect population structure, nucleotide polymorphism, and Tajima's D statistic of Sulawesi(S) and Borneo(B). Observed values are indicated below the predictive posterior probabilities; values for segregating sites are the sum over all loci whereas other statistics are the means over all loci.
TABLE 5.
Maximum likelihood estimates (−lnL) of demographic models and model comparison
| Model | No migration | Migration | d.f. | P value |
|---|---|---|---|---|
| Sulawesi vs. Borneo | 160.77121 | 160.1456 | 2 | 0.5349 |
| M. tonkeana vs. Borneo | 125.09111 | 125.00007 | 2 | 0.913 |
The estimated time of divergence was similar whether or not the migration parameter was included. Divergence time in the model with no migration has a 95% confidence interval of 413,743–648,785 generations (comparison with all Sulawesi macaques) or 317,050–557,463 generations (comparison with western population of M. tonkeana), which translates to ∼2.1–3.2 or 1.6–2.8 million years ago, respectively, assuming a generation time of 5 years. The models with migration parameters have similar estimates for divergence (95% confidence interval of 424,870–677,694 generations or 2.1–3.4 million years ago for the comparison with all Sulawesi macaques and 324,084–622,045 or 1.6–3.1 million years ago for the comparison with M. tonkeana).
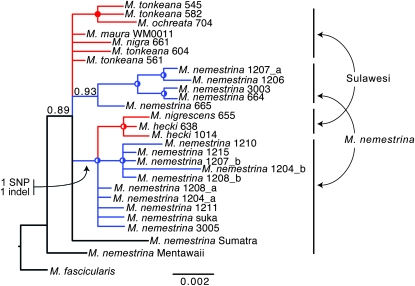
Use of summary statistics in MIMAR does not take advantage of genealogical information that is available in the data, and phylogenetic analysis therefore can provide additional insights. MtDNA and yDNA are monophyletic in Sulawesi macaques with respect to M. nemestrina (Figure S2). Similar to results of MIMAR analysis, these phylogenies do not support substantial migration between Borneo and Sulawesi macaques. Other information suggests more recent gene flow between island populations of M. nemestrina on Sumatra, Borneo, and Mentawai islands than between any of these populations and the Sulawesi macaques. For example, yDNA is monophyletic in M. nemestrina from Sumatra, Mentawai, and Borneo islands with respect to Sulawesi macaques (Figure S2), and a deletion in locus CXorf15 was found in M. nemestrina from Sumatra, Borneo, and Mentawai islands, but not in Sulawesi macaques or an outgroup (M. mulatta). Interestingly, mtDNA from Borneo M. nemestrina is more closely related to the Sulawesi macaques than to conspecifics on Sumatra or mainland Asia (Figure S2; Tosi et al. 2003). Additionally, two distinct lineages at an X-linked locus, TBL1X are shared across the Makassar Strait between Borneo and Sulawesi (Figure 5). This is best explained by TBL1X polymorphism in the ancestral individual or population that initially dispersed to Sulawesi.
Figure 5.—
TBL1X is not monophyletic in Sulawesi macaques. A well-supported clade with a synapomorphic SNP and a synapomorphic insertion/deletion (indel) occurs on Sulawesi and on Borneo. One TBLIX lineage was sampled on northwest Sulawesi in M. hecki and M. nigrescens and in M. nemestrina from northeast, west, and south Borneo. The other lineage was sampled in several other Sulawesi macaque species and in M. nemestrina from west and south Borneo. Posterior probabilities ≥100 or 95% are indicated by circles on nodes that are filled or half filled, respectively. The posterior probability of Sulawesi paraphyly, which does not take into account additional support of the insertion/deletion, is 99%. This indicates that the chance that these linked mutations arose independently on either side of the Makassar Strait is remote. For female samples, each allele is indicated with an “a” or a “b” following the name. Scale bars indicate the number of substitutions per site; sample information is listed in File S1.
Takenaka et al. (1987) proposed multiple macaque dispersals to Sulawesi to account for amino acid sequence variation at positions 9 and 13 of beta hemoglobin of macaques from Borneo and Sulawesi. We sequenced genomic DNA that encodes this amino acid variation and did not recover derived polymorphisms that were shared among macaques on both sides of the Makassar Strait. Twenty-four M. nemestrina individuals from Borneo were not variable at any of the codon positions for either of these amino acid positions (all had AAT at amino acid position 9 and ACC at amino acid position 13). A sequence from M. mulatta suggests that this is the ancestral state. Variation in Sulawesi macaques was observed and included the ancestral codon at each position plus an AAA and a GAT codon at position 9, and an AAC and an ATC at position 13. At each position, derived codons differ from the ancestral codons by one nucleotide substitution. But because individuals homozygous for both ancestral codons were found on Sulawesi (in M. maura and the west population of M. tonkeana), multiple dispersal events to Sulawesi are not required to account for beta hemoglobin polymorphism on Sulawesi.
Recombination in macaque mtDNA?:
A signal of recombination was detected in mtDNA of papionin monkeys (Piganeau et al. 2004; Tsaousis et al. 2005), including M. nemestrina (R. Setyadji, B. Suryobroto, T. Watanabe, and O. Takenaka, unpublished data; Evans et al. 1999), Sulawesi macaques (Evans et al. 1999), baboons (Newman et al. 2004), and mandrills (Telfer et al. 2003). After correction for multiple tests, one of four indirect tests for recombination used by Piganeau et al. (2004) support recombination in the mtDNA data in this study from Borneo M. nemestrina (probability of the null hypothesis of no recombination (Pno recomb) = 0.216, 0.015, 0.768, and >0.001 according to LDr2, LDD′, geneconv, and Max χ2 statistic tests). Two of these tests detect recombination in mtDNA of Sulawesi macaques (Pno recomb = 0.027, 0.106, <0.001, and <0.001 according to LDr2, LDD′, geneconv, and Max χ2 statistic tests). However, we suspect that factors other than recombination could explain these results. For example, mutational “cold spots” in a subset of sequences can cause false positives (Sawyer 1989). Additionally, the “Max χ2 statistic” (Maynard Smith 1992) and the “neighbor similarity score”/“reticulate” method (Jakobsen and Easteal 1996) used by Piganeau et al. (2004) and Tsaousis et al. (2005) have elevated false positives when heterogeneous substitution rates are autocorrelated (Bruen et al. 2006). Recurrent substitution or mutational hotspots could also lead to incorrect inferences of recombination (Galtier et al. 2006; Wright et al. 2008). For these reasons, we also suspect that paraphyly of Sulawesi macaque mtDNA reported by Evans et al. (1999) based on data from another region of the mtDNA is better attributed to homoplasy, as has been suggested (Deinard and Glen Smith 2001), than to recombination.
DISCUSSION
Polymorphism in pigtail macaques:
The ratio of neutral polymorphism to divergence is expected to be equivalent in mtDNA and yDNA and lower in xDNA compared to aDNA after accounting for differences in mutation rate and the number of chromosomes sequenced (Hudson et al. 1987). But in pigtail macaques (M. nemestrina) from Borneo, levels of polymorphism were lower than expected in yDNA and xDNA and higher than expected in mtDNA. By focusing on silent sites in the mlHKA and MIMAR analyses we minimized the impact of direct selection on the level of polymorphism we assayed, but natural selection on linked variation could also play a significant role. To explore this possibility, we considered models where polymorphism of yDNA, mtDNA, or xDNA was influenced by natural selection. A model with natural selection on yDNA was favored over other models, including the neutral model (Table 3). One polymorphic site was observed in the M. nemestrina yDNA data with a derived mutation present in one individual. This observation is suggestive of background selection as opposed to a selective sweep although the data clearly are too limited to make strong conclusions with respect to the nature of natural selection. Models with natural selection on xDNA and mtDNA were not significantly preferred over the neutral model.
We also considered demographic models with a skewed sex ratio and with sex-biased dispersal, which also differentially affect polymorphism of different genomic regions depending on the mode of inheritance. For example, in the model with a skewed sex ratio, if the proportion of females is 70%, the inheritance scalar of xDNA would be 0.87, of yDNA would be 0.18, and of mtDNA would be 0.42 compared to an inheritance scalar of 1 for aDNA (Figures 1A and 3A). Likewise, if the female migration rate were only 0.0015 individual/generation but males were essentially panmictic, then the inheritance scalar of mtDNA would be 0.38 while the scalars for xDNA, yDNA, and aDNA would be equal to the ideal expectation with an equal proportion of males and females (0.75, 0.25, and 1, respectively; Figures 1B and 3B). While none of these demographic models provided a significant improvement from the ideal expectation with an equal proportion of males and females according to likelihood ratio tests, model comparison using the AIC suggests that a demographic model with a female-biased sex ratio is about as good at explaining the data as the ideal expectation with an equal number of male and females. Similar results were recovered when three aDNA and two xDNA loci are excluded on the basis of their χ2 deviation from neutral expectations. Interestingly, simulations of savanna baboons based on empirically estimated age-specific fertility and survival for both sexes did not recover a departure from expected values on the basis of a Poisson-distributed variation in reproductive success in aDNA and sex-linked loci (Storz et al. 2001). The AIC model comparison of our genetic data, however, suggests that a female-biased sex ratio provides a reasonable alternative to the ideal explanation, at least in the Borneo population of M. nemestrina.
Sex-biased dispersal and female-biased adult sex ratios are well known in papionins, particularly macaque monkeys (Dittus 1975; Melnick and Pearl 1987; Van Noordwijk and Van Schaik 2002). Multiple examples have been described where mtDNA diversity is high within closely related populations or where yDNA diversity across species is low (Melnick and Hoelzer 1992, 1994; Melnick et al. 1993; Evans et al. 2001, 2003; Tosi et al. 2002, 2003), implicating demographic factors in addition to natural selection as the cause of the patterns of molecular polymorphism of macaque mtDNA and yDNA. So we were surprised to not recover a more pronounced improvement of the “non-ideal” demographic models, particularly the model with a skewed sex ratio. This could be explained by a lack of statistical power due to insufficient data or to undetected hitchhiking effects on some loci, particularly on the X chromosome. As discussed earlier, natural selection has increased efficacy on recessive mutations on the X chromosome in hemizygous males. Natural selection associated with dosage compensation or with genes that have sex-specific effects could also impact evolution of X-linked loci (Marin et al. 2000; Sturgill et al. 2007). Another possibility is that our mlHKA approach is overly conservative because intralocus recombination is not incorporated. Because intralocus recombination decreases the variance in polymorphism patterns among alleles, we would expect narrower confidence intervals around the maximum likelihood estimate if recombination were included. A third possibility is that this result was biased because polymorphic loci are overrepresented, especially in aDNA. Autosomal data were selected for sequencing on the basis of putatively single-copy M. nemestrina sequences in GenBank. We also sequenced the beta hemoglobin gene because this locus was used in a previous study of macaques on Borneo and Sulawesi (Takenaka et al. 1987).
Theoretical models have been developed that accommodate realistic aspects of papionin demography such as overlapping generations, variation in population size, different mating systems, age-specific fecundity, age-dependent survivorship, and sex-specific variance in reproductive success (Nunney 1991, 1993; Caballero 1994; Chesser and Baker 1996; Charlesworth 2001; Laporte and Charlesworth 2002; Nomura 2002; Engen et al. 2007). Demographic models we examined make unrealistic assumptions about these variables, but have the advantage that they avoid approximations of life history parameter values that are poorly characterized for M. nemestrina and difficult to estimate in general (Charlesworth 2001). For example, the life stage of male migration varies among species of papionin monkey (Abernethy et al. 2002; Bergman et al. 2008) and behavioral estimates of demographic parameters such as variance in male reproductive success can be inaccurate (Slade et al. 1998).
Other demographic simplifications relate to the nature of population structure, temporal consistency of population size, and their impact on Ne. When migration is high (as it presumably is in aDNA, xDNA, and yDNA of the Borneo population of M. nemestrina), differences among these models are not important because the population is essentially panmictic in these parts of the genome (Hoelzer et al. 1998; Wang and Caballero 1999). So the model of population structure matters most in this study for mtDNA. As migration decreases, Ne and coalescence time approach infinity (Nei and Takahata 1993) and the impact of decreased migration becomes evident in a two-dimensional stepping stone model before it does in the standard island model (Hoelzer et al. 1998). Real populations do not have population structures that correspond to the finite island model of subdivided populations, and a stepping stone (Kimura 1953) or neighborhood model (Wright 1943) might be a better approximation of population structure in macaque mtDNA. Also, if the assumption of constant subpopulation size is relaxed—such as in a metapopulation with deme extinction and recolonization—Ne of a subdivided population could actually be much smaller than the census size (Maruyama and Kimura 1980; Nei and Takahata 1993; Whitlock and Barton 1997; Wang and Caballero 1999). This is because variation in subpopulation size, or increased variance in reproductive success among subpopulations, affects genetic drift. In any case, the true nature of population subdivision may be difficult to translate into expected levels of polymorphism (Laporte and Charlesworth 2002).
Another assumption of the demographic models we considered is that all loci are in mutation–drift equilibrium. Changes in population size such as a bottleneck or population growth, can differentially affect aDNA, xDNA, yDNA, and mtDNA due to variation in Ne (Fay and Wu 1999). Given the number of segregating sites and individuals sampled in the Borneo population of M. nemestrina, Tajima's D varied significantly from the neutral expectation for two loci (Table 1) and simulations indicate that the mean Tajima's D across loci is significantly lower than the equilibrium expectation (P = 0.001). This suggests that in addition to sex-specific macaque demography, changes in population size could have influenced levels of polymorphism in M. nemestrina from Borneo.
Inter-island demography:
Sulawesi and Borneo probably were never connected by dry land (Hall 2001). At one extreme, Sulawesi could have been colonized once by a pregnant female from a highly inbred (homozygous) population. At the other, this island could have been colonized by a large polymorphic population, followed by ongoing genetic exchange. Nonmonophyly of TBL1X on Sulawesi allows us to rule out the first scenario.
Perhaps not surprisingly, MIMAR analysis argues against ongoing migration from Sulawesi to Borneo in that estimated migration rates from Sulawesi to Borneo are at or near the limit of zero, and goodness-of-fit tests and likelihood ratio tests indicate that including migration parameters does not substantially improve the fit of the model to the observed data. We therefore conclude that we cannot reject a model without migration for macaques on Borneo and Sulawesi.
Time of divergence between M. tonkeana and Borneo M. nemestrina is estimated to be in the late Pliocene or Early Pleistocene. Sea level changed extensively during this time (Hall 2001) and the initial dispersal of macaques from Borneo to Sulawesi across the Makassar Strait, which presumably occurred by rafting, conceivably could have been facilitated by periodic sea level drops, which made the strait more narrow. Because we used a point estimate of the rate of mutation, the posterior distribution of divergence times does not incorporate uncertainty in this parameter. Additionally, inaccuracies in the mutation rate parameter could stem from (1) variation in mutation rate over time, (2) inaccurate estimates of divergence between humans and macaques, and (3) inaccurate estimates of the macaque generation time. Our confidence intervals on divergence time estimates are therefore underestimates.
The models we tested with MIMAR make simplifying assumptions, including no population structure in the ancestral or descendant populations, constant population size of each population, and a constant rate of migration. All of these assumptions are undoubtedly violated to some degree, and in particular, none of the models we tested accommodate macaque population structure on Sulawesi (Evans et al. 2001, 2003), as in, for example, Evans et al. (2008). Although violation of some model assumptions can lead to biased parameter estimates (Becquet and Przeworski 2009), the estimated divergence time from Borneo M. nemestrina recovered from the analysis using all Sulawesi macaques is similar to that recovered using only the western population of M. tonkeana.
Conclusions:
Within a genome, mtDNA, yDNA, xDNA, and aDNA have distinct evolutionary histories marked by differences in recombination, ploidy, gene content, mode of inheritance, natural selection, and demography. These variables led to degeneration of the nonrecombining portion of the Y chromosome (Charlesworth and Charlesworth 2000; Gurbich and Bachtrog 2008; Koerich et al. 2008; Vibranovski et al. 2009), a streamlined mtDNA genome that generally lacks persistent gene duplicates (Ballard and Rand 2005), and a “large X” effect on speciation—the X chromosome tends to be rich in genes that reduce fertility in hybrids compared to autosomal chromosomes (Coyne and Orr 1989; Coyne 1992; Masly and Presgraves 2007). In theory, extreme differences in survival and age-specific fertility between males and females would be required to produce substantial departures in the relative Ne-aDNA, Ne-xDNA, Ne-yDNA, and Ne-mtDNA from the Poisson expectation for an equal adult sex ratio (Charlesworth 2001). In the papionin monkey M. nemestrina, departure from ideal expectations based on an equal proportion of males and females is suggested by yDNA and mtDNA, but not xDNA, and these can be explained by sex-specific demography, natural selection, or both. Going forward, further insights about the relative contributions of these processes could be gained from additional data from genomic regions that are less susceptible to hitchhiking, for example by targeting regions with high rates of recombination that are far from genic regions (Singh et al. 2007).
Acknowledgments
We thank N. Andayani and J. Supriatna for facilitating fieldwork; C. Becquet, J. Dushoff, B. Golding, C. Langley, T. Slotte, J. Wang, and two anonymous reviewers for advice and helpful comments; A. Eyre-Walker for providing software for testing recombination; the Baylor College of Medicine for providing sequences from P. hamadryas; and the Toronto Zoo for providing a tissue sample from P. anubis. This research was supported by grants to B.J.E. from the Canadian Foundation for Innovation (no. 10715), National Science and Engineering Research Council (no. RGPIN-283102-07), and by a grant to J. McGuire, R. Brown, and B.J.E. from the National Science Foundation (no. 0640967).
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.110.116228/DC1.
Sequence data from this article have been deposited with the EMBL/GenBank Data Libraries under accession nos. HM071114–HM071842.
References
- Abernethy, K. A., L. J. T. White and E. J. Wickings, 2002. Hordes of mandrills (Mandrillus sphinx): extreme group size and seasonal male presence. J. Zool. 258 131–137. [Google Scholar]
- Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang et al., 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtrog, D., and B. Charlesworth, 2002. Reduced adaptation of a non-recombining neo-Y chromosome. Nature 416 323–326. [DOI] [PubMed] [Google Scholar]
- Ballard, J. W. O., and D. M. Rand, 2005. The population biology of mitochondrial DNA and its phylogenetic implications. Annu. Rev. Ecol. Syst. 36 621–642. [Google Scholar]
- Becquet, C., and M. Przeworski, 2007. A new approach to estimate parameters of speciation models with application to apes. Genome Res. 17 1505–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becquet, C., and M. Przeworski, 2009. Learning about modes of speciation by computational approaches. Evolution 63 2547–2562. [DOI] [PubMed] [Google Scholar]
- Bensasson, D., D. Zhang and D. L. Hartl, 2001. Mitochondrial pseudogenes: evolution's misplaced witnesses. Trends Ecol. Evol. 16 314–321. [DOI] [PubMed] [Google Scholar]
- Bergman, T. J., J. E. Phillips-Conroy and C. J. Jolly, 2008. Behavioral variation and reproductive success of male baboons (Papio anubis × Papio hamadryas) in a hybrid social group. Am. J. Primatol. 70 136–147. [DOI] [PubMed] [Google Scholar]
- Berlin, S., and H. Ellegren, 2004. Chicken W: a genetically uniform chromosome in a highly variable genome. Proc. Natl. Acad. Sci. 101 15967–15969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein, I., and T. Gordon, 1980. Mixed taxa introductions, hybrids, and macaque systematics, pp. 125–147 in The Macaques: Studies in Behavior, Ecology, and Evolution., edited by D. G. Lindburg. Van Nostrand Reinhold Co., New York.
- Bruen, T. C., H. Philippe and D. Bryant, 2006. A simple and robust statistical test for detecting the presence of recombination. Genetics 172 2665–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero, A., 1994. Developments in the prediction of effective population size. Heredity 73 657–679. [DOI] [PubMed] [Google Scholar]
- Charlesworth, B., 2001. The effect of life-history and mode of inheritance on neutral genetic variability. Gen. Res. 77 153–166. [DOI] [PubMed] [Google Scholar]
- Charlesworth, B., 2009. Effective population size and patterns of molecular evolution and variation. Nat. Rev. Genet. 10 195–205. [DOI] [PubMed] [Google Scholar]
- Charlesworth, B., and D. Charlesworth, 2000. The degeneration of Y chromosomes. Phil. Trans. R. Soc. Lond. Ser. B 355 1563–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth, B., J. A. Coyne and N. H. Barton, 1987. The relative rates of evolution of sex chromosomes and autosomes. Am. Nat. 130 113–146. [Google Scholar]
- Charlesworth, B., M. T. Morgan and D. Charlesworth, 1993. The effect of deleterious mutations on neutral molecular variation. Genetics 134 1289–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesser, R. K., and R. J. Baker, 1996. Effective sizes and dynamics of uniparentally and diparentally inherited genes. Genetics 144 1225–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, A. G., 1990. Inference of haplotype from PCR-amplified samples of diploid populations. Mol. Biol. Evol. 7 111–122. [DOI] [PubMed] [Google Scholar]
- Coyne, J. A., 1992. Genetics and speciation. Nature 355 511–515. [DOI] [PubMed] [Google Scholar]
- Coyne, J. A., and H. A. Orr, 1989. Two rules of speciation, pp. 180–207 in Speciation and Its Consequences, edited by J. Otte and J. A. Endler. Sinauer, Sunderland, MA.
- de Ruiter, J. R., W. Scheffrahn, G. J. J. M. Trommelen, A. G. Uitterlinden, R. D. Martin et al., 1992. Male social rank and reproductive success in wild long-tailed macaques., pp. 175–191 in Paternity in Primates: Genetic Tests and Theories, edited by R. D. Martin, A. F. Dixon and E. J. Wickings. Karger, Basel, Switzerland.
- Deinard, A., and D. Glen Smith, 2001. Phylogenetic relationships among the macaques: evidence from the nuclear locus NRAMP1. J. Hum. Evol. 41 45–59. [DOI] [PubMed] [Google Scholar]
- Delson, E., 1980. Fossil macaques, phyletic relationships and a scenario of development, pp. 10–30 in The Macaques: Studies in Ecology, Behavior, and Evolution, edited by D. G. Lindburg. Van Nostrand Reinhold, New York.
- Dittus, W., 1975. Population dynamics of the toque macaque, Macaca sinica, pp. 125–152 in Socioecology and Psychology of Primates, edited by R. H. Tuttle, Mounton, The Hague.
- Dobson, F. S., 1982. Competition for mates and predominant juvenile male dispersal in mammals. Anim. Behav. 30 1183–1192. [Google Scholar]
- Engen, S., T. H. Ringsby, B. Saether, R. Lande, H. Jensen et al., 2007. Effective size of fluctuating populations with two sexes and overlapping generations. Evolution 61 1873–1885. [DOI] [PubMed] [Google Scholar]
- Evans, B. J., J. A. McGuire, R. M. Brown, N. Andayani and J. Supriatna, 2008. A coalescent framework for comparing alternative models of population structure with genetic data: evolution of Celebes toads. Biol. Lett. 4 430–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, B. J., J. C. Morales, J. Supriatna and D. J. Melnick, 1999. Origin of the Sulawesi macaques (Cercopithecidae, Macaca) as inferred from a mitochondrial DNA phylogeny. Biol. J. Linn. Soc. 66 539–560. [Google Scholar]
- Evans, B. J., J. Supriatna, N. Andayani and D. J. Melnick, 2003. Diversification of Sulawesi macaque monkeys: decoupled evolution of mitochondrial and autosomal DNA. Evolution 57 1931–1946. [DOI] [PubMed] [Google Scholar]
- Evans, B. J., J. Supriatna and D. J. Melnick, 2001. Hybridization and population genetics of two macaque species in Sulawesi, Indonesia. Evolution 55 1685–1702. [DOI] [PubMed] [Google Scholar]
- Fay, J. C., and C.-I. Wu, 1999. A human population bottleneck can account for the discordance between patterns of mitochondrial versus nuclear DNA variation. Mol. Biol. Evol. 16 1003–1005. [DOI] [PubMed] [Google Scholar]
- Felsenstein, J., 1974. The evolutionary advantage of recombination. Genetics 78 737–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher, R. A., 1930. The Genetical Theory of Natural Selection. Clarendon, Oxford.
- Foxe, J. P., T. Slotte, E. A. Stahl, B. Neuffer, H. Hurka et al., 2009. Recent speciation associated with the evolution of selfing in Capsella. Proc. Natl. Acad. Sci. USA 106 5241–5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galtier, N., D. Enard, Y. Radondy, E. Basin and K. Belkhir, 2006. Mutation hotspots in mammalian mitochondrial DNA. Genome Res. 16 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson, J. R., A. K. Chippendale and W. R. Rice, 2002. The X chromosome is a hot spot for sexually antagonistic fitness variation. Proc. Biol. Soc. 269 499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazko, G. V., and M. Nei, 2003. Estimation of divergence times for major lineages of primate species. Mol. Biol. Evol. 20 424–434. [DOI] [PubMed] [Google Scholar]
- Greenwood, P. J., 1980. Mating systems, philopatry and dispersal in birds and mammals. Anim. Behav. 28 1140–1162. [Google Scholar]
- Gurbich, T. A., and D. Bachtrog, 2008. Gene content evolution on the X chromosome. Curr. Opin. Genet. Dev. 18 493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haag-Liautard, C., N. Coffey, D. Houle, M. Lynch, B. Charlesworth et al., 2008. Direct estimation of the mitochondrial DNA mutation rate in Drosophila melanogaster. PLoS Biol. 6 e204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldane, J. B. S., 1922. Sex-ratio and unisexual sterility in hybrid animals. J. Genet. 12 101–109. [Google Scholar]
- Hall, R., 2001. Cenozoic reconstructions of SE Asia and the SW Pacific: changing patterns of land and sea, pp. 35–56 in Faunal and Floral Migrations and Evolution in SE Asia-Australia, edited by I. Metcalfe, J. Smith, M. Morwood and I. Davidson. Swets and Zeitlinger Publishers, Lisse.
- Hammer, M. F., F. L. Mendez, M. P. Cox, A. E. Woerner and J. D. Wall, 2008. Sex-biased evolutionary forces shape genomic patterns of human diversity. PLoS Genet. 4 e1000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick, P. W., 2007. Sex: differences in mutation, recombination, selection, gene flow, and genetic drift. Evolution 61 2750–2771. [DOI] [PubMed] [Google Scholar]
- Hellborg, L., and H. Ellegren, 2004. Low levels of nucleotide diversity in mammalian Y chromosomes. Mol. Biol. Evol. 21 158–163. [DOI] [PubMed] [Google Scholar]
- Hill, W. G., and A. Robertson, 1966. The effect of linkage on limits to artificial selection. Gen. Res. 8 269–294. [PubMed] [Google Scholar]
- Hoelzer, G. A., 1997. Inferring phylogenies from mtDNA variation: mitochondrial-gene trees versus nuclear-gene trees revisited. Evolution 51 622–626. [DOI] [PubMed] [Google Scholar]
- Hoelzer, G. A., W. Dittus, M. V. Ashley and D. J. Melnick, 1994. The local distribution of highly divergent mitochondrial DNA haplotypes in toque macaques (Macaca sinica) at Polonnaruwa, Sri Lanka. Mol. Ecol. 3 451–458. [DOI] [PubMed] [Google Scholar]
- Hoelzer, G. A., J. Wallman and D. J. Melnick, 1998. The effects of social structure, geographical structure, and population size on the evolution of mitochondrial DNA: II. Molecular clocks and the lineage sorting period. J. Mol. Evol. 47 21–31. [DOI] [PubMed] [Google Scholar]
- Hudson, R. R., M. Kreitman and M. Aguadé, 1987. A test of neutral molecular evolution based on nucleotide data. Genetics 160 765–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson, R. R., M. Slatkin and W. P. Maddison, 1992. Estimation of levels of gene flow from DNA sequence data. Genetics 132 583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsenbeck, J. P., and F. Ronquist, 2001. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics 17 754–755. [DOI] [PubMed] [Google Scholar]
- Jakobsen, I. B., and S. Easteal, 1996. A program for calculating and displaying compatibility matrices as an aid in determining reticulate evolution in molecular sequences. Comp. Appl. Biosci. 12 1640–1643. [DOI] [PubMed] [Google Scholar]
- Johnson, C. N., 1986. Sex-biased philopatry and dispersal in mammals. Oecologia (Berlin) 69 626–627. [DOI] [PubMed] [Google Scholar]
- Keane, B., W. P. Dittus and D. J. Melnick, 1997. Paternity assessment in wild groups of toque macaques Macaca sinica at Polonnaruwa, Sri Lanka using molecular markers. Mol. Ecol. 6 267–282. [DOI] [PubMed] [Google Scholar]
- Kimura, M., 1953. ‘Stepping-stone’ model of populations. Ann. Rep. Natl. Inst. Genet. Japan 3 62–63. [Google Scholar]
- Kimura, M., and J. F. Crow, 1963. The measurement of effective population number. Evolution 17 279–288. [Google Scholar]
- Koerich, L. B., X. Wang, A. G. Clark and A. B. Carvalho, 2008. Low conservation of gene content in the Drosophila Y chromosome. Nature 456 949–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler, M., S. Moyà-Solà and D. M. Alba, 2000. Macaca (Primates, Cercopithecidae) from the Late Miocene of Spain. J. Hum. Evol. 38 447–452. [DOI] [PubMed] [Google Scholar]
- Laporte, V., and B. Charlesworth, 2002. Effective population size and population subdivision in demographically structured populations. Genetics 162 501–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindburg, D. G., and N. C. Harvey, 1996. Reproductive biology of captive lion-tailed macaques, pp. 318–341 in Evolution and Ecology of Macaque Societies, edited by J. Fa and D. G. Lindburg. Cambridge University Press, Cambridge, UK.
- Lynch, M., 2007. The Origins of Genome Architecture. Sinauer, Sunderland, MA.
- Marin, I., M. I. Siegal and B. S. Baker, 2000. The evolution of dosage-compensation mechanisms. Bioessays 22 1106–1114. [DOI] [PubMed] [Google Scholar]
- Maruyama, T., and M. Kimura, 1980. Genetic variability and effective population size when local extinction and recolonization of subpopulations are frequent. Proc. Natl. Acad. Sci. USA 77 6710–6714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masly, J. P., and D. C. Presgraves, 2007. High-resolution genome-wide dissection of the two rules of speciation in Drosophila. PLoS Biol. 5 e243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard Smith, J., 1992. Analyzing the mosaic structure of genes. J. Mol. Evol. 34 126–129. [DOI] [PubMed] [Google Scholar]
- Maynard Smith, J., and J. Haigh, 1974. The hitch-hiking effect of a favourable gene. Gen. Res. 23 23–25. [PubMed] [Google Scholar]
- McVean, G. A. T., and B. Charlesworth, 2000. The effects of Hill-Robertson interference between weakly selected mutations on patterns of molecular evolution and variation. Genetics 155 929–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick, D. J., 1988. The genetic structure of a primate species: rhesus and other cercopithecine monkeys. Int. J. Primatol. 9 195–231. [Google Scholar]
- Melnick, D. J., and G. A. Hoelzer, 1992. Differences in male and female macaque dispersal lead to contrasting distributions of nuclear and mitochondrial DNA variation. Int. J. Primatol. 13 379–393. [Google Scholar]
- Melnick, D. J., G. A. Hoelzer, R. Absher and M. V. Ashley, 1993. MtDNA diversity in rhesus monkeys reveals overestimates of divergence time and paraphyly with neighboring species. Mol. Biol. Evol. 10 282–295. [DOI] [PubMed] [Google Scholar]
- Melnick, D. J., and M. C. Pearl, 1987. Cercopithecines in multimale groups: genetic diversity and population structure, pp. 121–134 in Primate Societies, edited by B. B. Smuts, D. L. Cheney, R. M. Seyfarth, R. W. Wrangham and T. T. Struhsaker. University of Chicago Press, Chicago.
- Muller, H. J., 1964. The relation of recombination to mutational advance. Mutat. Res. 106 2–9. [DOI] [PubMed] [Google Scholar]
- Nachman, M. W., 1997. Patterns of DNA variability at X-linked loci in Mus domesticus. Genetics 147 1303–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei, M., and W. H. Li, 1979. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc. Natl. Acad. Sci. USA 76 5269–5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei, M., and N. Takahata, 1993. Effective population size, genetic diversity and coalescence time in subdivided populations. J. Mol. Evol. 37 240–244. [DOI] [PubMed] [Google Scholar]
- Newman, T. K., C. J. Jolly and J. Rogers, 2004. Mitochondrial phylogeny and systematics of baboons (Papio). Am. J. Phys. Anthropol. 124 17–27. [DOI] [PubMed] [Google Scholar]
- Nomura, T., 2002. Effective size of populations with unequal sex ratio and variation in mating success. J. Anim. Breed. Genet. 119 297–310. [Google Scholar]
- Nunney, L., 1991. The influence of age structure and fecundity on effective population size. Proc. R. Soc. Lond. Ser. B 246 71–76. [DOI] [PubMed] [Google Scholar]
- Nunney, L., 1993. The influence of mating systems and overlapping generations on effective population size. Evolution 47 1329–1341. [DOI] [PubMed] [Google Scholar]
- Nylander, J. A. A., 2004. MrModeltest v2, pp. Evolutionary Biology Centre, Uppsala University, Sweden.
- O'Brien, T. G., and M. F. Kinnard, 1997. Behavior, diet, and movements of the Sulawesi crested black macaque (Macaca nigra). Int. J. Primatol. 18 321–351. [Google Scholar]
- Okamoto, K., and S. Matsumura, 2001. Group fission in Moor macaques. Int. J. Primatol. 22 481–492. [Google Scholar]
- Orr, H. A., 1997. Haldane's rule. Annu. Rev. Ecol. Syst. 28 195–218. [Google Scholar]
- Otto, S. P., and D. B. Goldstein, 1992. Recombination and the evolution of diploidy. Genetics 131 745–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piganeau, G., M. Gardner and A. Eyre-Walker, 2004. A broad survey of recombination in animal mitochondria. Mol. Biol. Evol. 21 2319–2325. [DOI] [PubMed] [Google Scholar]
- Rice, W. R., 1987. Genetic hitchhiking and the evolution of reduced genetic activity of the Y sex chromosome. Genetics 116 161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozas, J., J. C. Sanchez-DelBarrio, X. Messegyer and R. Rozas, 2003. DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics 19 2496–2497. [DOI] [PubMed] [Google Scholar]
- Sawyer, S., 1989. Statistical tests for detecting gene conversion. Mol. Biol. Evol. 6 526–538. [DOI] [PubMed] [Google Scholar]
- Seielstad, M. T., E. Minch and L. L. Cavalli-Sforza, 1998. Genetic evidence for a higher female migration rate in humans. Nat. Genet. 20 278–280. [DOI] [PubMed] [Google Scholar]
- Singh, N. D., J. M. Macpherson, J. D. H Jensen and D. A. Petrov, 2007. Similar levels of X-linked and autosomal nucleotide variation in African and non-African populations of Drosophila melanogaster. BMC Evol. Biol. 7 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade, R. W., C. Moritz, A. R. Hoelzel and H. R. Burton, 1998. Molecular population genetics of the southern elephant seal Mirounga leonina. Genetics 149 1945–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone, A. C., R. C. Griffiths, S. L. Zegura and M. F. Hammer, 2002. High levels of Y-chromosome nucleotide diversity in the genus Pan. Proc. Natl. Acad. Sci. USA 99 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz, J. F., U. Ramakrishnan and S. C. Alberts, 2001. Determinants of effective population size for loci with different modes of inheritance. J. Hered. 92 497–502. [DOI] [PubMed] [Google Scholar]
- Sturgill, D., Y. Zhang, M. Parisi and B. Oliver, 2007. Demascilinization of X chromosomes in the Drosophila genus. Nature 450 238–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima, F., 1989. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenaka, O., M. Hotta, B. Suryoboto and E. Brotoisworo, 1987. Origin and evolution of the Sulawesi macaques. Complete amino acid sequences of seven B chains of three molecular types. Primates 28 99–109. [Google Scholar]
- Telfer, P. T., S. Souquiere, S. L. Clifford, K. A. Abernethy, M. W. Bruford et al., 2003. Molecular evidence for deep phylogenetic divergence in Mandrillus sphinx. Mol. Ecol. 12 2019–2024. [DOI] [PubMed] [Google Scholar]
- Tosi, A. J., J. C. Morales and D. J. Melnick, 2000. Comparison of Y-chromosome and mtDNA phylogenies leads to unique inferences of macaque evolutionary history. Mol. Phylogenet. Evol. 17 133–144. [DOI] [PubMed] [Google Scholar]
- Tosi, A. J., J. C. Morales and D. J. Melnick, 2002. Macaca fascicularis Y-chromosome and mitochondrial markers indicate introgression with Indochinese M. mulatta and a biogeographic barrier in the Isthmus of Kra. Int. J. Primatol. 23 161–178. [Google Scholar]
- Tosi, A. J., J. C. Morales and D. J. Melnick, 2003. Paternal, maternal, and biparental molecular markers provide unique windows onto the evolutionary history of macaque monkeys. Evolution 57 1419–1435. [DOI] [PubMed] [Google Scholar]
- Tsaousis, A. D., D. P. Martin, D. Ladoukakis, D. Posada and E. Zouros, 2005. Widespread recombination in published animal mtDNA sequences. Mol. Biol. Evol. 22 925–933. [DOI] [PubMed] [Google Scholar]
- Van Noordwijk, M. A., and C. P. Van Schaik, 2002. Career moves: transfer and rank challenge decisions by male long-tailed macaques. Behavior 138 359–395. [Google Scholar]
- Vibranovski, M. D., Y. Zhang and M. Long, 2009. Out of X chromosomal gene movement in the Drosophila genus. Genome Res. 19 897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade, M. J., and S. M. Shuster, 2004. Estimating the strength of sexual selection from Y-chromosome and mitochondrial DNA diversity. Evolution 58 1613–1616. [DOI] [PubMed] [Google Scholar]
- Wagenmakers, E., and S. Farrell, 2004. AIC model selection using Akaike weights. Psychon. Bull. Rev. 11 192–196. [DOI] [PubMed] [Google Scholar]
- Wang, J., and A. Caballero, 1999. Developments in predicting the effective population size of subdivided populations. Heredity 82 212–226. [Google Scholar]
- Whitlock, M. C., and N. H. Barton, 1997. The effective size of a subdivided population. Genetics 147 427–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdig, A., F. B. Bercovich, W. J. Streich, U. Sauermann, P. Nürnberg et al., 2004. A longitudinal analysis of reproductive skew in male rhesus macaques. Proc. R. Soc. Lond. Ser. B 271 819–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder, J. A., Z. Mobasher and M. F. Hammer, 2004. Genetic evidence for unequal effective population sizes of human females and males. Mol. Biol. Evol. 21 2047–2057. [DOI] [PubMed] [Google Scholar]
- Wright, S., 1931. Evolution in Mendelian populations. Genetics 16 97–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, S., 1943. Isolation by distance. Genetics 28 114–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, S. I., and B. Charlesworth, 2004. The HKA test revisited: a maximum-likelihood ratio test of the standard neutral model. Genetics 168 1071–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, S. I., N. Nano, J. P. Foxe and V. N. Dar, 2008. Effective population size and tests of neutrality at cytoplasmic genes in Arabidopsis. Genet. Res. Camb. 90 119–128. [DOI] [PubMed] [Google Scholar]
- Yang, Z., 1997. PAML: a program package for phylogenetic analysis by maximum likelihood. CABIOS 13 555–556. [DOI] [PubMed] [Google Scholar]
- Yoder, A. D., and Z. Yang, 2004. Divergence dates for Malagasy lemurs estimated from multiple gene loci: geological and evolutionary context. Mol. Ecol. 13 757–773. [DOI] [PubMed] [Google Scholar]