Abstract
The immunogenicity of an antigen can be dramatically increased by displaying it in a dense, multivalent context, such as on the surface of a virus or virus-like particle (VLP). Here we describe a highly versatile VLP platform for peptide display based on VLPs of the RNA bacteriophage PP7. We show that this platform can be used for the engineered display of specific peptide sequences as well as for the construction of random peptide libraries. Peptides representing the FLAG epitope, the V3 loop of HIV gp120, and a broadly cross-type neutralizing epitope from L2, the minor capsid protein of Human Papillomavirus type 16 (HPV16), were inserted into an exposed surface loop of a form of PP7 coat protein in which the two identical polypeptides of coat were fused together to form a single-chain dimer. The recombinant proteins assembled into VLPs, displayed these peptides on their surfaces, and induced high titer antibody responses. The single-chain dimer was also highly tolerant of random 6-, 8-, and 10-amino acid insertions. PP7 VLPs displaying the HPV16 L2 epitope generated robust anti-HPV16 L2 serum antibodies after intramuscular injection that protected mice from genital infection with HPV16 pseudovirus as well as a heterologous HPV pseudovirus type, HPV45. Thus, PP7 VLPs are well-suited for the display of a wide diversity of peptides in a highly immunogenic format.
Keywords: VLP, HPV vaccine, bacteriophage
1. Introduction
Many viral structural proteins have the intrinsic ability to spontaneously self-assemble into virus-like particles (VLPs), which structurally resemble infectious virions, but lack viral nucleic acid. VLPs elicit high-titer and long-lived antibody responses upon vaccination and make excellent vaccines against the virus from which they were derived [1, 2]. Two VLP-based vaccines (for Hepatitis B Virus and Human Papillomavirus [HPV]) have been clinically approved [3, 4], and others, such as for Norwalk and Influenza viruses, are in clinical development [5, 6]. VLPs induce strong immune responses for several reasons. First, particulate antigens such as VLPs are preferentially taken up and presented by antigen presenting cells, such as dendritic cells [7–9]. Second, the highly multivalent structure of VLPs is highly stimulatory to B cells. Multivalent antigens such as VLPs engage multiple B cell receptors, promote crosslinking of these receptors, and elicit quantitatively stronger antibody responses than antigens with low valency [10–12].
Because of their potent immunogenicity, VLPs have also been exploited as platforms to increase the antibody responses to antigens that are normally poorly immunogenic. VLP display can increase the immunogenicity of a variety of different antigens, including short peptides, full-length proteins, and other molecules [1, 13, 14]. VLP display can even be utilized to induce antibody responses against self-antigens, which are normally subject to the mechanisms of B cell tolerance [10, 15–18]. VLP-based vaccines targeting nicotine [19], angiotensin [20], and amyloid-beta [21], to name a few, are currently in clinical trials.
Previously we described the use of VLPs of the RNA bacteriophage MS2 for peptide display [22]. By genetically fusing two copies of the MS2 coat protein, we created a single-chain dimmer with increased thermodynamic stability and vastly improved tolerance of insertions in its AB-loop. The MS2 coat protein dimer was widely tolerant of genetic insertion of defined peptide sequences as well as random peptide insertions. Recombinant MS2 VLPs elicited high titer IgG antibodies against the inserted sequences. Moreover, MS2 coat protein single-chain dimers produced correctly assembled VLPs that specifically encapsidated the mRNA encoding their synthesis, raising the possibility that they could be used in affinity selections protocols analogous to filamentous phage display. But, MS2 is only one member of a large family of bacteriophages whose individual members share similar molecular biology. We suspected that, following similar design principles, other phage VLPs could be adapted to this same purpose. Here we describe the engineering of VLPs of PP7, a bacteriophage of Pseudomonas aeruginosa, for the purposes of peptide display.
PP7 VLPs offer several potential advantages and improvements over MS2 VLPs. First, the particles are more stable thermodynamically, because of the presence of stabilizing inter-subunit disulfide bonds [23]. For many practical applications, including vaccines, increased stability is a desirable trait. Second, PP7 VLPs are not cross-reactive immunologically with those of MS2 [24]. This could be important in applications where serial administration of VLPs may be necessary. Third, we anticipated that the correct folding and assembly of the PP7 VLP might be more resistant to the destabilizing effects of peptide insertion, or that it might at least show tolerance of some peptides not tolerated in MS2 VLPs.
In this paper we show that the single-chain dimer of PP7 coat protein is broadly tolerant of random and specific peptide insertions, is highly immunogenic, and packages the RNA that directs its synthesis. One specific PP7 VLP vaccine successfully induced antibodies against a peptide derived from the V3 loop of HIV that is the target of neutralizing antibodies. A second vaccine induced antibodies against a broadly cross-neutralizing epitope from the minor capsid protein L2 of HPV 16.
Although some cross-reactivity has been observed between closely related HPV genotypes [25], the protection provided upon vaccination with HPV VLPs comprised of the major capsid protein, L1, is largely HPV type-specific. For example, women who are vaccinated with HPV16 L1-VLPs are completely protected against HPV16-related disease (cervical intraepithelial neoplasia; CIN), but not against disease caused by other high-risk HPV types [26, 27]. Unlike L1-specific neutralizing antibodies, neutralizing antibodies that target the minor capsid protein, L2, are broadly cross-neutralizing [28], suggesting that neutralizing epitopes on L2 are conserved across HPV types. For example, antisera raised against a peptide representing amino acids 1–88 from bovine papillomavirus type 1 (BPV-1) L2 can cross-neutralize a diverse panel of mucosal and cutaneous HPVs [29]. Similarly, vaccination with HPV16 and BPV-1 L2 peptides protects rabbits against challenge with two different rabbit papillomaviruses [30]. In principle, L2 vaccines may be able to overcome the type-specific limitations of HPV vaccines based on L1 VLPs. Unfortunately, however, the neutralizing titers produced upon L2 vaccination are considerably lower than for L1 VLP vaccines, particularly against heterologous HPV types [28]. Therefore, it is likely that an L2 vaccine will only be effective if its immunogenicity is enhanced, such as by display on VLPs. Here, we show that the PP7 VLP vaccine displaying an L2 epitope induces antibody responses that protect mice from genital challenge with homologous (HPV16) and heterologous (HPV45) pseudovirus infection.
2. Materials and Methods
2.1. Construction of plasmids
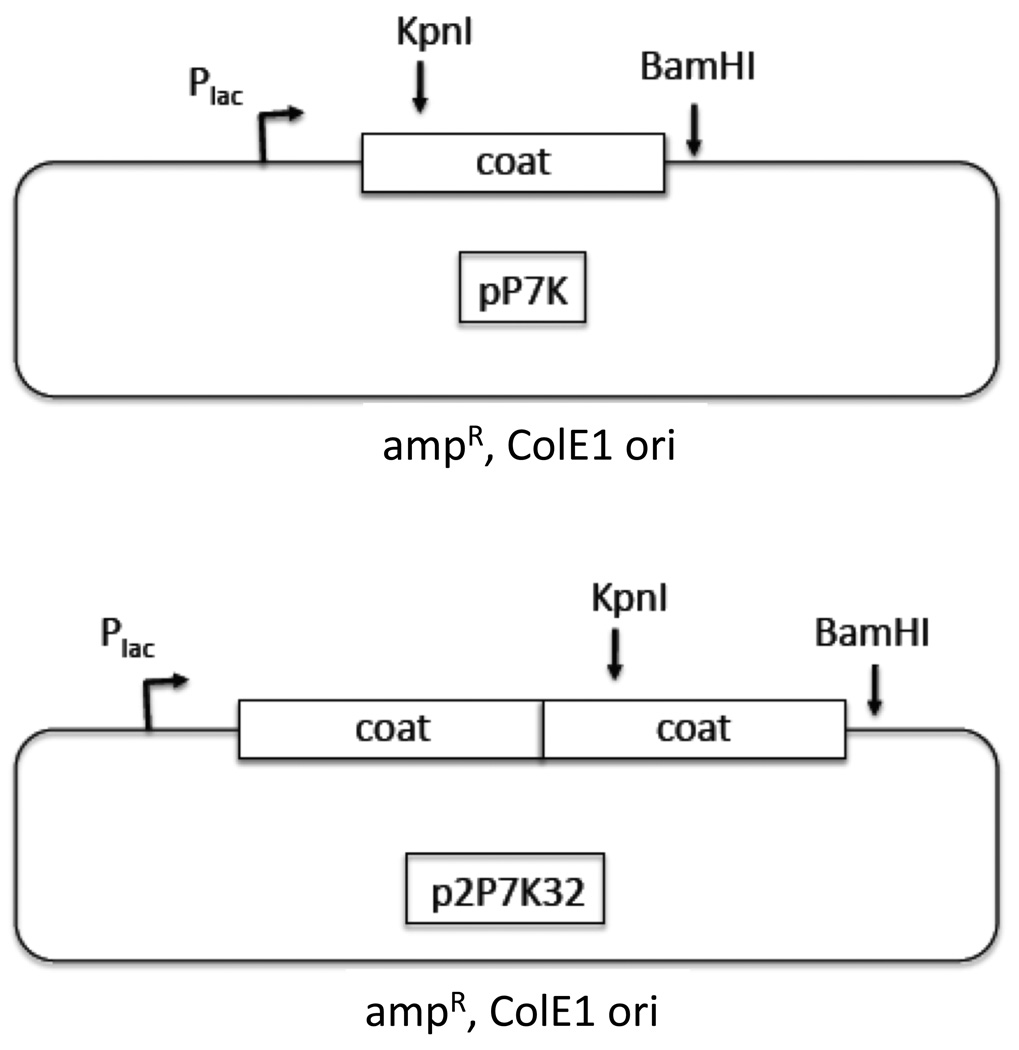
A PCR overlap extension method was used to mutate the PP7 coat gene sequence to introduce a unique KpnI restriction site, thus facilitating the insertion of foreign sequences into the plasmid we call pP7K (shown in Figure 1). This modification resulted in an amino acid substitution (E11T) shown in Figure 2. This substitution was well tolerated, since the mutant coat protein assembles correctly into a VLP (not shown). We also modified a plasmid called p2P7, described previously [23], which codes for the expression of a version of coat protein in which two copies of coat protein are genetically fused into a “single-chain” dimer. This modified plasmid, which we call p2P7K32, contains the unique KpnI site in the downstream copy of coat (Figure 1).
Fig. 1.
The pP7K and p2P7K32 plasmids.
Fig. 2.
Nucleotide and amino acid sequences of wild-type and recombinant PP7 coat proteins near the AB-loop. (A) and (B) show the N-terminal sequence of wild-type PP7 coat protein and the mutant coat protein encoded by pP7K and p2P7K32. (C) and (D) show the specific primers used to insert the FLAG, V3, HPV16 L2, and random sequences into the downstream AB-loop of the PP7 single-chain dimer. All sequences are shown 5’ to 3’; the KpnI restriction site is shown in italics and the peptide insertion is shown in bold text. All of these primers yield an insertion which is flanked by a thr residue on the N-terminal side and a Glu (the wild-type position 11 amino acid) on the C-terminal side.
We used PCR to insert target sequences into the PP7 coat. Synthetic 5’-primers were designed to encode the KpnI restriction site, the inserted peptide sequence, and then a sequence derived from PP7 coat (shown in Figure 2). In each PCR reaction we used a reverse primer that anneals to a vector sequence downstream of a unique BamHI restriction site in pP7K and p2P7K32. The PCR product was cloned between KpnI and BamHI in the expression vectors described above and the resulting recombinants were verified by sequence analysis.
2.2. Protein expression, purification and functional assays
To test the recombinant proteins for translational repressor activity, each plasmid was introduced into E. coli strain CSH41F– containing the translational repression reporter plasmid pRZP7 [31] and then plated on LB agar containing the β-galactosidase chromogenic substrate, 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-Gal). Properly folded coat proteins repress translation of β-galactosidase and yield white colonies.
To determine the expression levels of recombinant proteins, cell pellets from 1 ml overnight cultures were resuspended in 250 µl of buffer (10 mM Tris-HCl, pH7.4, 100 mM NaCl, 0.1 mM MgSO4, 0.01 mM EDTA) and then lysed by sonication for 10 seconds. The soluble fraction was collected by centrifugation, and then bacterial DNA was digested by treatment with 2 µg/ml DNase with 2 mM MgCl2. Rapid assessment of a recombinant protein’s ability to assemble into a VLP was performed by electrophoresis of sonicated cell lysates on a 1% agarose, 50mM potassium phosphate, pH 7.5 gel. Gels were stained with ethidium bromide to reveal the presence of VLPs, which contain host RNAs. The identity of the VLPs was then confirmed by transferring the contents of the gel to nitrocellulose and probing with mouse anti-PP7 serum (at a 1:2000 dilution) followed by an alkaline phosphatase-conjugated goat anti-mouse IgG (at a 1:5000 dilution).
VLPs were purified from 100 ml bacterial cultures grown overnight in E. Coli strain CSH41F– in LB media. Pellets were suspended in 10 ml of lysozyme solution (50 mM Tris-HCl, pH8.5, 100 mM NaCl, 10 mM EDTA, 10 mM DTT) along with 0.1 g of hen egg lysozyme and incubated on ice. After 1 h deoxycholate was added (to a final concentration of 0.05%), samples were incubated on ice for another 30 minutes, and then the samples were sonicated. After sonication samples were treated with DNase and RNaseA (both at 2 µg/ml) for 2 hours at 37°C. After centrifugation the soluble fraction was collected and proteins were precipitated by addition of ammonium sulfate to 80% saturation. Following centrifugation, pellets were solubilized in sepharose column buffer (SCB; 10 mM Tris-HCl, pH7.4, 100 mM NaCl, 0.1 mM MgSO4, 0.01 mM EDTA) and applied to a sepharose CL-4B column. Fractions containing VLPs were identified by agarose gel electrophoresis, pooled, and then quantitated by Bradford assay.
2.3. Libraries of random sequence peptides
Libraries of random sequence peptides inserted in the AB-loop of PP7 coat protein were created using the primers shown in Figure 2 and the general strategy described previously [22]. Different 5’ primers were designed to insert 6, 8, or 10 codons of the sequence NNY (where N is A, C, G, or T, and Y is T or C). The resulting PCR products were digested with KpnI and BamHI and ligated to similarly digested vector fragments of p2P7K32. These ligation mixtures were used to transform the strain CSH41F containing plasmid pRZP7, and plated on LB medium containing X-Gal. After overnight incubation at 37°C the relative numbers of blue and white colonies were determined. From each library, 24 white colonies were picked and grown overnight in LB at 37°C. Bacteria were lysed by sonication, subjected to agarose gel electrophoresis, and then visualized by staining with ethidium bromide and by Western blot, as described above.
2.4. Packaging of coat-specific RNAs
The unit length PP7 coat and the single-chain dimer were cloned into the vector pET3d, a plasmid that contains the T7 promoter and transcription terminator, producing the plasmids called pETP7K and pET2P7K32. These plasmids were transformed into the bacterial strain BL21(DE3)pLysS and coat protein expression was induced using IPTG. VLPs were extracted and purified as described previously [32]. RNAs were extracted from VLPs using phenol/chloroform and applied to duplicate 1.5% agarose gels containing formaldehyde. One gel was stained with ethidium bromide and the other was blotted to nitrocellulose and probed with a PP7 coat-specific synthetic oligonucleotide (5’-AGGCGCAGCCGACCCACCAC-3’) complementary to the sense-strand and labeled at its 5’ end with biotin. The RNA was visualized by chemiluminescence using the Brightstar system from Ambion. Control RNAs were produced by transcription of pETP7K and pET2P7K32 in vitro using T7 RNA polymerase.
2.5. Immunological characterization of recombinant VLPs
To ensure that antibodies specific for the inserted epitope bound VLPs, recombinant VLPs were immobilized overnight at 4°C onto an ELISA plate (Immulon 2) at 500 ng per well. The wells were then blocked with PBS and 0.5% non-fat dry milk for two hours at room temperature. Dilutions of an anti-FLAG monoclonal (M2, Sigma) or an anti-L2 antibody (RG-1) [33] were added to the wells, and incubated at room temperature for two hours. The reactivity of either monoclonal antibody to the VLPs was determined by incubating a horseradish peroxidase (HRP)-labeled goat anti-mouse IgG (Jackson Immunoresearch, West Grove, PA) at a dilution of 1:5000 in blocking buffer in the wells for 1 h at room temperature. The plate was developed with 2,2'-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) (ABTS) and the OD 405 was measured using an OpSys MR plate reader (Thermo Labsystems, Waltham, MA).
2.6. Immunization and characterization of antisera
To assess whether the recombinant VLPs elicited antibody responses against the target peptides, C57Bl/6 and B10 mice were inoculated with VLPs. Groups of six mice (4 C57Bl/6 mice and 2 B10 mice) were immunized intramuscularly with 10 µg of VLPs plus incomplete Freund’s Adjuvant (IFA) in a total volume of 100 µl. All mice were boosted with the same amount of VLPs two weeks later. Sera was collected before each inoculation and weekly for three to four weeks after the boost. All animal care was in accordance with the National Institutes of Health and with the University of New Mexico guidelines.
Antibody titers against target antigens were determined by coating plates with 500 ng of target peptide (either a synthetic HPV16 L2 peptide representing L2 amino acids 14–40 [SATQLYKTCKQAGTCPPDIIPKVEGKT] conjugated to streptavidin or a synthetic HIV V3 peptide conjugated to KLH) in a total volume of 50 µl overnight at 4°C. Plates were blocked with blocking buffer for two hours at room temperature. Antisera was serially diluted in blocking buffer and then added to the wells for two hours. Reactivity to antigen was determined using an HRP-labelled goat anti-mouse IgG secondary antibody (1:4000) for one hour. Upon development with ABTS, absorbance at 405 nm was measured.
2.7. Genital HPV challenge
Groups of five Balb/c mice were immunized intramuscularly with 10 µg wild-type PP7 VLPs, PP7–16L2 VLPs, or HPV16 L1-VLPs (with incomplete Freund’s adjuvant, IFA) twice at a two-week interval. As an additional control, a group of mice was immunized with IFA alone. Three weeks after the second vaccination, mice were genitally challenged with ~108 IU HPV16 or HPV45 pseudovirions encapsidating a luciferase reporter plasmid (pCLucf), as described previously [34–36]. At 48 h following the introduction of pseudovirus, the mice were each given an intravaginal instillation of 20 µl of XenoLight D-Luciferin Potassium Salt (0.3 mg; Caliper Life Sciences) and imaged with a Xenogen IVIS 100 (Caliper Life Sciences). Images were taken at 3 min post-installation of luciferin at medium binning with a 30-s exposure. Images were then analyzed by drawing an equally sized region of interest for each mouse and measuring average radiance (photons/second/cm2/sr) within this region.
3. Results
3.1. PP7 coat protein folding and assembly tolerate most random 6-mer, 8-mer, and 10-mer peptide insertions
Previously, we demonstrated that a single-chain dimer version of the MS2 coat protein was widely tolerant of diverse peptide insertions into the surface-exposed AB-loop. The three-dimensional structure of the PP7 capsid shows that it is comprised of a coat protein whose tertiary structure closely mimics that of MS2, even though the amino acid sequences of the two proteins show only about 12% sequence identity [37]. The PP7 protein also possesses an AB-loop into which peptides may be inserted following a scheme similar to the one we described previously for MS2 [22]. As in the MS2 case, we began by mutating the PP7 coat sequence to contain a site for the restriction endonuclease KpnI, thus facilitating insertion of foreign sequences in the plasmid we call pP7K (Figure 1). This modification resulted in the amino acid substitution (E11T) shown in Figure 2b, which was apparently well tolerated, since the mutant coat protein represses translation (this assay is explained below) and coat protein assembles correctly into a VLP (data not shown). Again following the MS2 example, we suspected that the folding of a single chain dimer version of PP7 coat protein would be more resistant to AB-loop insertions than the conventional dimer. Its construction was described previously [23], but here we report its use for peptide display for the first time. We modified the single-chain dimer to contain a KpnI site only in the downstream copy of the coding sequence, producing p2P7K32 (Figure 1), allowing the insertion of sequences at amino acid 11 of the downstream copy of coat.
To test the general tolerance of PP7 coat protein to AB-loop insertions, we created libraries of random sequence peptides inserted into the AB-loop of pP7K or into the second AB-loop of p2P7K32 (shown schematically in Figure 2c). The random sequences consisted of six, eight, or ten copies of the sequence NNY (where N is any nucleotide and Y is a pyrimidine). These libraries code for substantial diversity (15 of the 20 possible amino acids will be represented) but avoid the possibility of introducing a stop codon, which would complicate analysis of recombinants.
The functionality of coat proteins containing random insertions was tested by two assays. First, we assessed the translational repression activity of recombinant PP7 coat proteins. PP7 coat normally functions as a translational repressor, shutting off synthesis of the viral replicase by binding to a specific RNA hairpin structure containing its ribosome-binding site (the translational operator). We described previously the construction of pRZP7, a plasmid that fuses the PP7 translational operator to the E. coli lacZ gene, thus placing β-galactosidase synthesis under control of coat protein’s translational repressor activity [31]. Because it confers resistance to a different antibiotic (chloramphenicol), and because it comes from a different incompatibility group (i.e. it uses the p15A replication origin), it can easily be maintained in the same E. coli strain as pP7K or p2P7K32, both of which confer resistance to ampicillin and use a colE1 origin. Both of these plasmids are derived from pUC119 and express coat protein at relatively low levels from the lac promoter. The expression of PP7 coat protein from pP7K or p2P7K32 represses translation of β-galactosidase expressed from pRZP7. This makes it easy to determine whether a given peptide insertion has interfered with the ability of coat protein to correctly fold, since defective coat proteins give blue colonies on plates containing the β-galactosidase chromogenic substrate known as X-gal, whereas a properly functioning coat protein yields white colonies.
Second, we assessed the presence of VLPs in lysates of cells expressing a peptide-coat protein recombinant by electrophoresis on agarose gel of cells lysed by sonication. Ethidium bromide staining detects the RNA-containing VLPs, whose presence can be confirmed by western blot analysis using anti-PP7 serum. Electrophoresis of the VLPs in an agarose gel shows that each construct contains RNA (it stains with ethidium bromide). Often recombinant VLPs exhibit altered electrophoretic mobility relative to wild-type VLPs due to charge differences conferred by inserted peptides
These libraries were introduced into strain CSH41F-/pRZP7 and plated on X-Gal plates to test the ability of recombinant protein to inhibit translation. The vast majority of transformants were bona fide recombinants, since control ligations without insert fragment gave 1000x fewer colonies, a result subsequently verified by restriction analysis of plasmids extracted from 24 clones from each of the libraries.
When we cloned random sequences into pP7K 100% of the transformants were blue. We tested only a small number of the pP7K recombinants, but found that all were defective for protein folding and failed to make VLPs (not shown). This confirms our expectation from prior experiments with MS2 that the conventional PP7 dimer is normally destabilized by peptide insertions in the AB-loop [22]. In the single-chain dimer of p2P7K32, however, virtually 100% of peptide insertions were compatible with the translational repressor function of coat protein. In other words, they produced sufficient properly folded coat protein to repress translation like the wild-type protein. This result was obtained with all the NNY libraries; 6-mer, 8-mer and 10-mer. Twenty-four white colonies from each of the libraries were transferred to duplicate 1ml cultures. From one culture set, crude cell lysates were prepared for agarose gel analysis of VLPs. From the other set, plasmids were isolated and subjected to restriction enzyme digestion and gel electrophoresis, verifying that all contained an insertion of the expected length. We also isolated plasmids from the few blue colonies we encountered. Sequence analysis indicated that all of them contained plasmids resulting from aberrant ligation events, and generally did not contain an intact coat sequence (not shown).
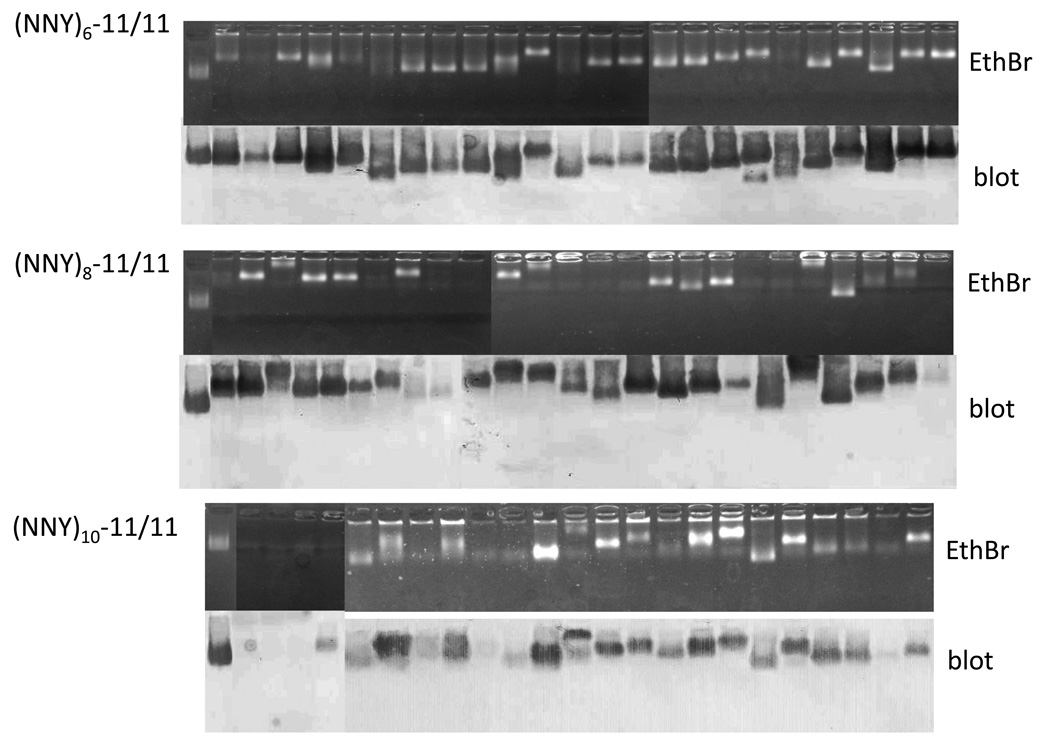
To test directly for the presence of VLPs, crude cell lysates were prepared from cultures derived from the twenty-four randomly chosen transformants and then subjected to agarose gel electrophoresis. Gels were stained with ethidium bromide and a duplicate was blotted to nitrocellulose and probed with mouse anti-PP7 VLP serum and a horseradish peroxidase labeled second antibody. A clone was regarded as positive for VLP synthesis when it contained a band in both the stained gel and western blot. The results (Figure 3) demonstrate that nearly all of the 6-mer clones produced VLPs at some level, although a few show reduced yields. The vast majority of 8-mer clones also produced readily identifiable VLPs. The efficiency dropped somewhat as insertion length increased to 10-mers, but still a clear majority of the clones (19/24) produced VLPs. Note that the mobilities of the individual particles were variable, consistent with the expectation that some peptides alter the surface charge of the VLP by incorporating charged amino acids. Thus, the AB-loop of the PP7 single-chain is broadly tolerant of 6-, 8-, and 10-mer insertions.
Fig. 3.
Agarose gel electrophoresis of whole cell lysates of 24 clones from each of the various libraries described in the text. Each of these yielded a white colony on X-Gal plates. The top half of each set is the ethidium bromide stained gel, and the bottom half is a western blot of a duplicate gel. The left-most lane in each set is the p2P7K32 control. Variations in electrophoretic mobility reflect charge differences conferred by the inserted peptides.
3.2. PP7 VLPs encapsidate coat-specific mRNA
The compatibility of the PP7 coat single-chain dimer with diverse peptide insertions suggests the possibility that this platform could be used to create large VLP libraries in which the individual members display different peptides on their surfaces. Such a library could then be screened by affinity selection to identify peptides with specific binding activities, in a process similar to filamentous phage display. One of the essential features of phage display is a linkage of phenotype to genotype. Identified phage must encapsidate the nucleic acid encoding its synthesis, allowing recovery of affinity-selected sequences. We showed previously that MS2 VLPs efficiently package their mRNAs, even in the absence of the presumed packaging signal from viral RNA [22], and that the sequences they contain could be recovered from VLPs by reverse transcription and PCR. Thus, we wanted to determine whether PP7 VLPs also encapsidated their mRNAs.
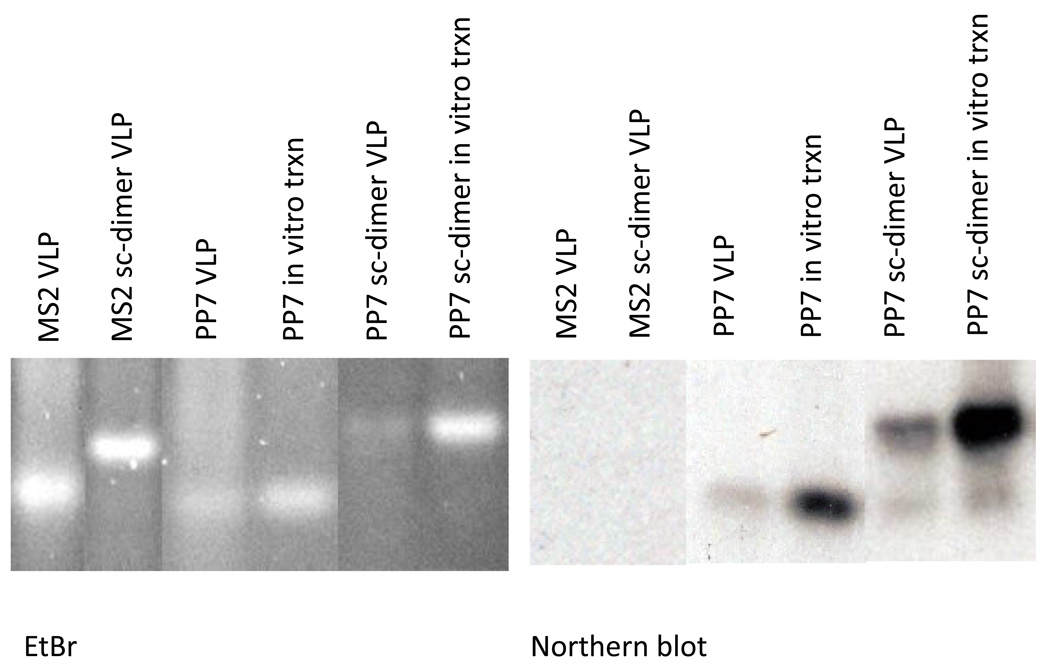
For high-level expression and RNA encapsidation tests, the PP7 coat sequences of pP7K and p2P7K32 were cloned into a plasmid containing the T7 promoter and transcription terminator, producing the plasmids called pETP7K and pET2P7K32. These plasmids, when introduced into E. coli strain BL21(DE3), synthesized large amounts of coat protein (not shown). The resulting VLPs were purified by chromatography on Sepharose CL4B as described previously [31, 32]. RNA was purified from VLPs by phenol/chloroform extraction and subjected to electrophoresis in duplicate agarose gels containing formaldehyde. One gel was stained with ethidium bromide and the other was blotted to nitrocellulose (Northern blot) where PP7 sequences were detected using a labeled synthetic oligonucleotide specific for the coat sense-strand. RNAs produced by transcription in vitro of pETP7K and pET2P7K32 with T7 RNA polymerase were utilized as standards. As shown in Figure 4, each PP7 VLP contains a predominant species whose mobility is identical to that of the in vitro transcription product, and which hybridizes specifically with the PP7 coat-specific probe. We conclude that PP7 VLPs encapsidate their mRNAs, establishing the genotype-phenotype linkage necessary for affinity selection.
Fig. 4.
Electrophoresis on formaldehyde/agarose gel of RNAs extracted from VLPs. The left panel shows the ethidium bromide-stained gel and a Northern blot probed with a labeled PP7 coat-specific oligonucleotide is shown in the right panel. MS2 VLP and PP7 VLP refer to the RNAs extracted from conventional VLPs from MS2 and PP7 coat protein, respectively. Sc-dimer refers to RNAs extracted from single-chain dimer VLPs. Some lanes contain RNAs produced by in vitro transcription (txpn) of MS2 and PP7 coat protein expression plasmids, as indicated.
3.3. Insertion of specific peptide sequences into the AB-loop of PP7 coat
To create the VLPs that display heterologous peptides we designed PCR primers that allowed us to clone three defined sequences into the AB-loop of PP7 coat. These sequences (shown in Figure 2d) encoded the following peptides; the 8 amino acid FLAG epitope (FLAG; DYKDDDDK), a 10 amino acid peptide derived from the V3 loop of gp120 from the Human Immunodeficiency Virus LAI (HIVLAI) V3 loop (V3; IQRGPGRAPV) and is the target of HIV neutralizing antibodies [38], and a 15 amino acid epitope corresponding to amino acids 17–31 from the HPV16 L2 minor capsid protein (16L2; QLYKTCKQAGTCPPD) that represents a broadly cross-neutralizing HPV epitope. Previously, we successfully inserted the FLAG and V3 peptides into the AB-loop of the single-chain dimer version of MS2 coat [22, 39]. However, insertion of the 16L2 peptide into MS2 coat caused a defect in protein folding and VLP assembly (unpublished data).
The functionality of coat protein encoded by the resulting plasmids (p2P7-Flag, p2P7-V3, and p2P7–16L2) was tested for translational repression activity and mobility on an agarose gel. All three recombinant coat proteins produced white colonies, indicating that coat protein was functional. Electrophoresis of the recombinant VLPs in an agarose gel showed that each construct contains RNA (it stains with ethidium bromide) and analysis by Western blot using polyclonal anti-PP7 serum confirmed that these bands contain PP7 VLPs (not shown). When applied to a Sepharose 4B column, all three recombinant proteins eluted at the same fractions as wild-type PP7 VLPs (not shown). Thus, all three recombinant single-chain dimer coat proteins formed VLPs.
3.4. Peptides displayed on PP7 VLPs are displayed to the immune system and are immunogenic
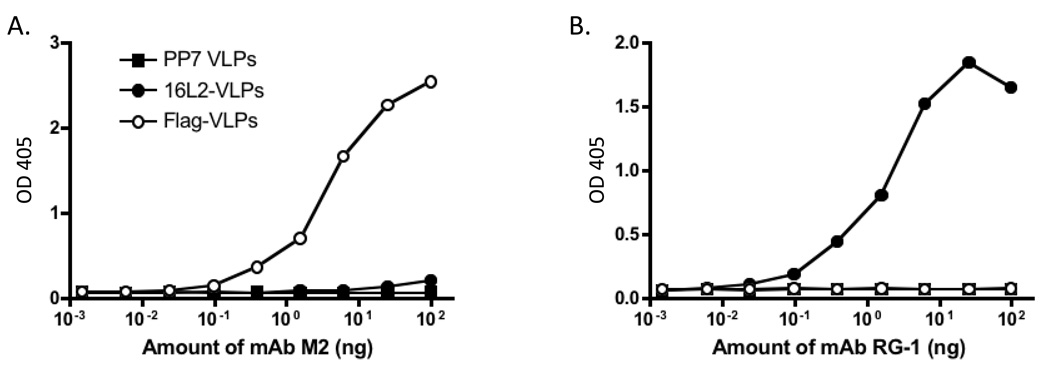
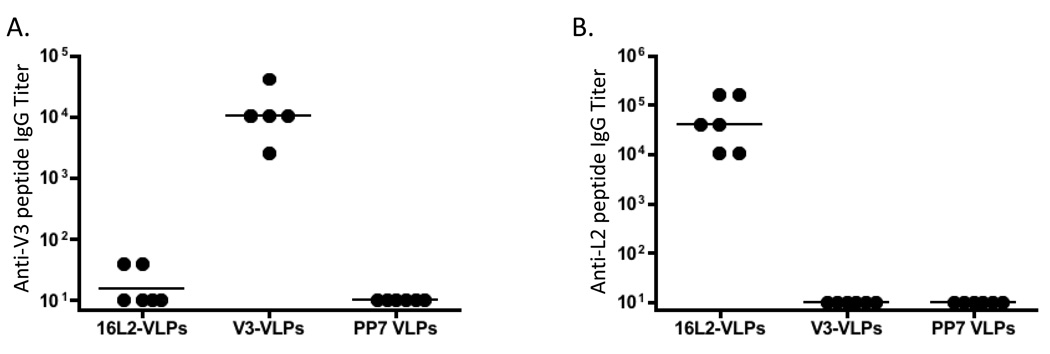
To demonstrate that peptides inserted into the PP7 AB-loop were indeed displayed on the surface of VLPs, we assessed the ability of a monoclonal antibody (mAb) specific for the FLAG epitope and a mAb (RG-1; [33]) specific for the HPV16 L2 sequence to bind to recombinant PP7 VLPs by ELISA. As shown in Figure 5, the anti-FLAG mAb bound strongly to FLAG-VLPs, but not L2 VLPs, and mAb RG-1 bound to 16L2-VLPs, but not FLAG-VLPs.
Fig. 5.
Binding of monoclonal antibodies to recombinant PP7 VLPs. 500 ng of wild-type PP7 VLPs, L2-VLPs, or FLAG-VLPs were bound to wells of an ELISA plate and then were reacted with dilutions of an anti-FLAG mAb (M2, panel A) or an anti-L2 mAb (RG-1, panel B). Binding was detected using a horseradish peroxidase-labeled goat anti-mouse IgG secondary followed by development with ABTS. Reactivity was determined by measurement of the absorbance at 405 nm (OD 405).
To test the immunogenicity of the VLPs, mice were immunized with V3-VLP or 16L2-VLPs by intramuscular injection according to the regimen described in Materials and Methods. Sera from the mice were tested, by end-point dilution ELISA, for IgG antibodies specific for either the V3 or the 16L2 peptide (Figure 6). Mice immunized with V3-VLPs or 16L2-VLPs generated high-titer (geometric mean titer > 104) IgG responses against the corresponding peptide, but not against the heterologous peptide. Thus, peptides displayed on the surface of PP7 single-chain dimer VLPs display the high immunogenicity that is characteristic of other VLP-displayed antigens.
Fig. 6.
IgG antibody responses in groups of mice immunized with wild-type PP7 VLPs, V3-VLPs, or 16L2-VLPs. End-point dilution ELISA titers against (A) a peptide representing a portion of the V3 loop from HIVLAI conjugated to KLH or (B) a peptide representing amino acids 14–40 from HPV16 L2 conjugated to streptavidin. Results are from sera obtained three to four weeks after the second vaccination. Each datum point represents the antibody titer from an individual mouse. Lines represent the geometric mean titer for each group.
3.5. PP7 VLPs displaying a HPV16 L2 peptide can induce neutralizing antibodies that protect mice from homologous and heterologous genital HPV pseudovirus challenge
Current HPV vaccines are based on VLPs comprised of the HPV major capsid protein L1. Although HPV L1-VLP vaccines are highly effective, they are type-restricted, meaning that they only provide protection against the types specifically targeted by the vaccines and a few other, very closely related, types. In contrast, the HPV minor capsid protein, L2, contains broadly cross-type neutralizing epitopes [28, 29]. Therefore, a monovalent vaccine targeting L2 might provide protection against many more HPV types than the current multivalent L1 VLP vaccines.
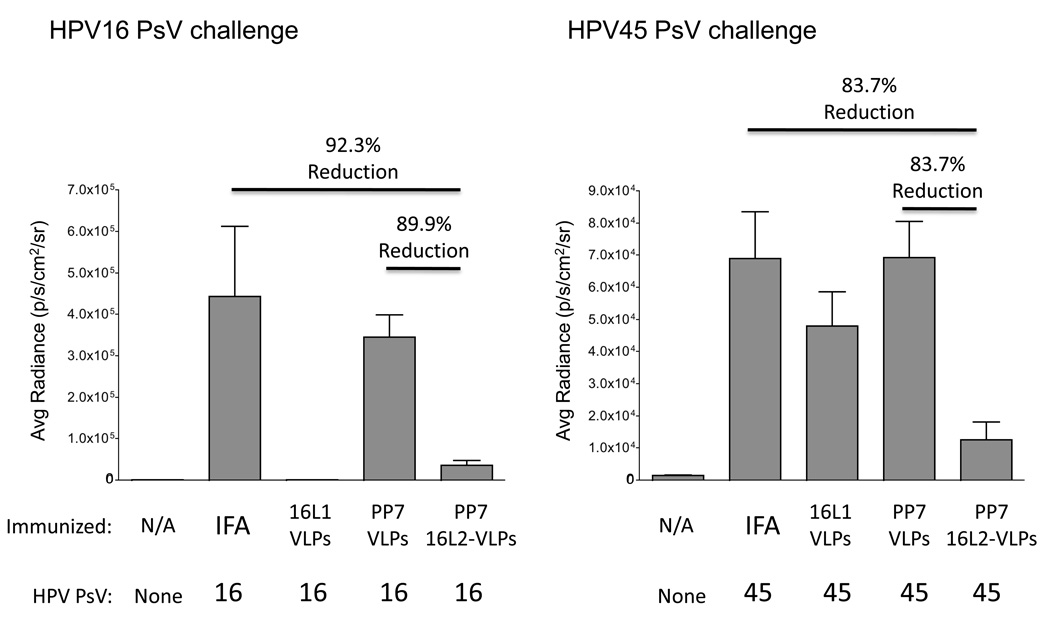
The 16L2-VLP vaccine we designed contains amino acids 17–31 from HPV16 L2, a region shown to contain one or more highly cross-reactive neutralizing epitopes [33, 40], suggesting that the 16L2 VLPs could potentially protect against HPV challenge. We assessed whether 16L2-VLPs could protect mice from HPV challenge using a HPV pseudovirus/mouse genital challenge model, first reported by Roberts and colleagues [34]. Groups of five Balb/c mice were given two intramuscular injections of HPV16 L1-VLPs, wild type PP7 VLPs, or 16L2-VLPs, or adjuvant (IFA) alone, and then, three weeks after the boost, challenged intravaginally with a high dose (~108 IU) of HPV pseudovirus carrying a luciferase reporter. As a negative control, mice were mock-challenged with PBS. Infection was detected as a bioluminescent signal two days after the administration of pseudovirions, immediately after intravaginal instillation of the challenged mice with the reporter substrate, luciferin.
As shown in Figure 7, mice immunized with 16L2-VLPs were strongly (~90%) protected from infection with the homologous pseudovirus, HPV16, whereas mice immunized with wild-type PP7 VLPs were not protected. We also tested whether vaccination with 16L2-VLPs could protect mice from genital infection with a heterologous HPV type. We chose HPV45 pseudovirus because it is not closely related to HPV16 and because its L2(17–31) sequence varies from the HPV16 sequence at three of the fifteen amino acid positions. Immunization with HPV16 L1 VLPs did not protect mice from HPV45 challenge. However, 16L2-VLP-immunized mice were protected (~83%) from genital infection with HPV45 pseudovirus. Thus, 16L2-VLPs have potential as a pan-HPV vaccine.
Fig. 7.
Mice immunized with PP7 16L2-VLPs are protected from vaginal challenge with HPV16 or HPV45 pseudovirions. Groups of five mice were immunized two times with 10 µg 16L2-VLPs, wild-type PP7 VLPs, or HPV16 L1-VLPs formulated in incomplete Freund’s adjuvant (IFA). As an additional control, mice were immunized with IFA alone. Three weeks after the second immunization mice were intravaginally challenged with 108 IU of HPV16 pseudovirus (left panel) or HPV45 pseudovirus (right panel) containing a luciferase reporter. As a control, a group of five mice were not infected. Luciferase activity was quanititated 48 hours after infection by taking images 3 min post-installation of luciferin at medium binning with a 30-s exposure. Images were then analyzed by drawing an equally sized region of interest for each mouse and measuring average radiance (photons/second/cm2/sr) within this region. Results shown are the mean average radiance for each group of five mice. Error bars represent the standard error of the mean. Lines above pairs of data indicate the percent reduction of signal in mice immunized with 16L2-VLPs relative to wild-type PP7 VLPs or the IFA control. All comparisons shown here are statistically significant (p<0.01) as calculated by T-test.
4. Discussion
The genetic insertion of target epitopes into viral structural proteins to generate chimeric VLPs can be an effective method for constructing immunogens capable of eliciting high titer antibody responses. Successful construction of recombinant VLPs guarantees regular display of target epitopes at high density and in the same conformation on the surface of the VLP, enhancing immunogenicity. Recombinant VLPs might also have certain manufacturing advantages compared to other methods for arraying target molecules on the surface of VLPs, such as chemical conjugation of peptides. However, the ability to display peptides on VLPs is often limited by structural features of the virus platform. Target peptides must be exposed on the surface of the VLP and should not interfere with protein folding and assembly of VLPs. Several VLP platforms have been adapted for display of a wide range of peptides, including Hepatitis B virus core antigen and its animal hepadnavirus relatives [14], HPV [41], tobacco mosaic virus [42], and human rhinovirus [43]. However, it is likely that most existing display platforms have limitations in their ability to display diverse peptides without extensive insertion-specific manipulations (as exemplified by the hepadnavirus system [44]). Certainly the capacity of any of these platforms to display random peptides has not been explicitly tested. We show here that a single-chain dimer version of the RNA bacteriophage PP7, like its structural cousin MS2, tolerates both specific insertions as well as an impressive percentage of random insertions. In addition, PP7 VLPs encapsidate the RNA that encodes their synthesis, raising the possibility that libraries of PP7 VLPs displaying random peptides could be used in affinity selection applications analogous to filamentous phage display.
The PP7 display system described here has a number of similarities with our previously reported MS2 VLP display system. In both cases the ability to display heterologous peptides is predicated on a single-chain dimer of coat protein, in which both subunits of the coat dimer are translated as a single polypeptide. Both MS2 and PP7 VLPs can accommodate a wide range of specific and random peptide sequences and recombinant VLPs generate high titer antibody responses. Furthermore, both VLPs package the RNA that codes for the recombinant coat protein, which is a necessary component if libraries of recombinant VLPs will be used to identify VLPs with specific binding characteristics by affinity selection.
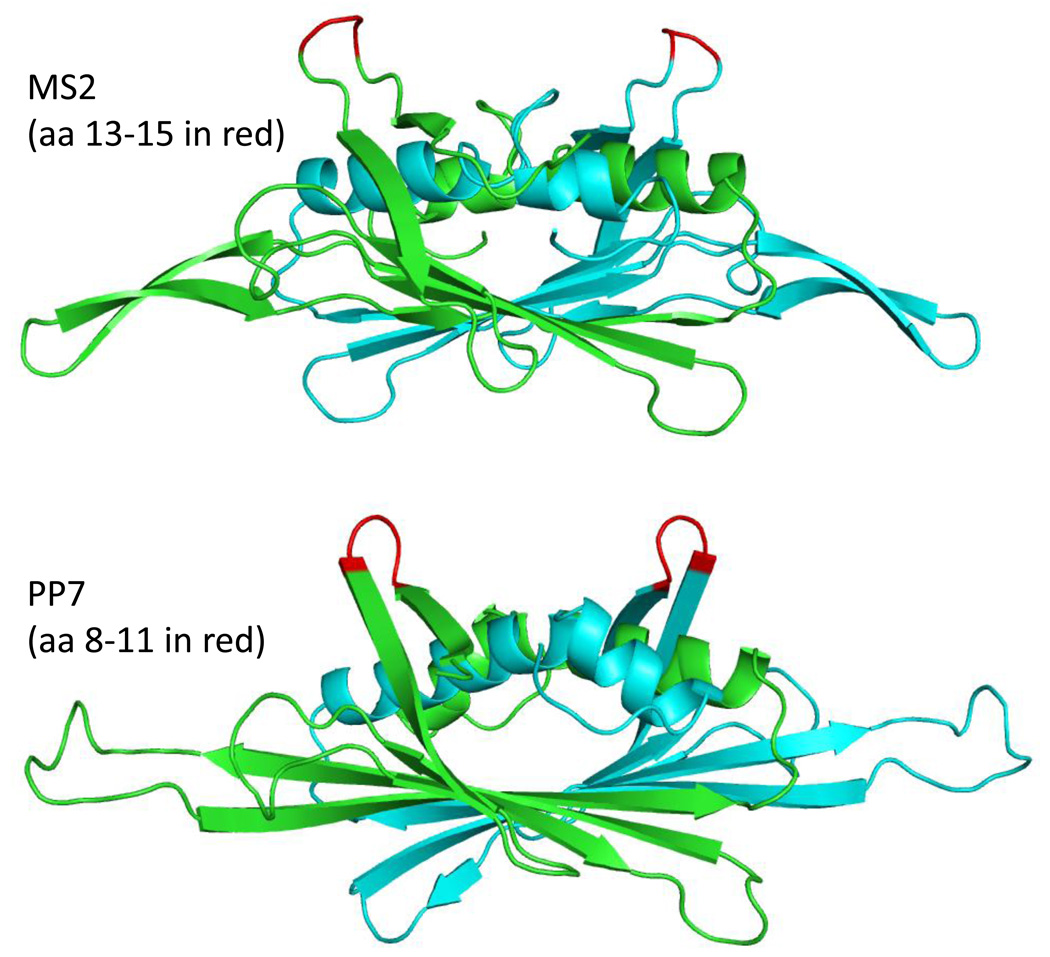
However, PP7 and MS2 differ in several respects that might potentially be exploited. Although the tertiary structures of the two proteins are similar, and the arrangement of the subunits in capsids is similar, detailed structural differences do exist. For example, the conformations of the AB-loops of the two proteins show significant differences (Figure 8). Moreover, their local structural environments are also distinct, raising the possibility that inserted peptides may experience different structural constraints. Thus, one platform could be better suited than the other for display of any given peptide, both with respect to its tolerance of the insertion, and in terms of its ability to stabilize a desirable conformation. Although these differences do not appear to affect the immunogenicity of an epitope inserted into either AB-loop, they could influence a peptide’s ability to mimic the epitope's native structure and thereby subtly influence the quality of antibodies generated. The presence of intersubunit disulfide bonds (lacking in MS2 capsids) confers high stability to PP7 VLPs; we have shown that PP7 VLPs only begin to denature at temperatures approaching 90°C [23]. Although FLAG-displaying PP7 VLPs are about 10°C less stable than wild type VLPs, they remain significantly more resistant to denaturation than many other VLPs (e.g. those of MS2). One might expect that the higher stability of PP7 VLPs could also confer an increased tolerance of peptide insertions. This has not been explicitly tested, but we think it unlikely, since the inserted peptide probably exerts its effect on folding of the coat protein precursors to capsids, before the VLP-stabilizing disulfides are formed. Although the ability of PP7 and MS2 to accept 6-, 8-, and 10-mer insertions is similar, our results with designed peptides (e.g. the 15 amino acid 16L2 sequence) suggest that PP7 is able to accept some peptides that are incompatible with display on MS2 VLPs. Additional experiments are necessary to determine whether PP7 is generally more tolerant of peptide insertions, or whether each VLP is simply better suited than the other to the display of certain specific peptides. Finally, the antibodies induced by PP7 VLPs do not cross-react with MS2 [24]. This could be important in developing vaccines to induce T cell responses or in targeted drug delivery applications where serial administration of VLPs may be necessary and the presence of pre-existing antibodies against the platform could be detrimental. Thus, we envision peptide display on PP7 VLPs as a complementary technology to that based on MS2.
Fig. 8.
Structures of the MS2 and PP7 coat protein dimers seen edge-on, with their polypeptide chains in blue and green, and the AB-loops shown in red. These structures were created using the program Polyview 3D (http://polyview.cchmc.org/polyview3d.html).
Genetic display of peptides on PP7 VLPs is well suited for the precise targeting of specific B-cell epitopes known to be the target of neutralizing antibodies. For many pathogens, including influenza [45, 46], Hepatitis C virus [47], and HIV [48], the target epitopes of broadly neutralizing antibodies are poorly immunogenic, meaning that full-length proteins are inadequate for the induction of antibody responses by vaccination. On the other hand, the use of peptide epitopes as vaccines is limited because of their poor immunogenicity unless coupled to carrier proteins. Thus, the VLP platform allows for targeted introduction of specific peptide epitopes in a highly immunogenic context. As an example, we targeted a broadly neutralizing epitope near the N-terminus of the HPV16 L2 protein. This epitope is the target of an HPV neutralizing monoclonal antibody [33], and it was already shown that the corresponding synthetic peptide, when linked to a universal T helper epitope, can elicit cross-neutralizing antibodies against HPV [40]. PP7 VLPs displaying the L2 epitope induced high-titer peptide-specific antibodies, and the antibodies were of high enough titer to protect mice from high-dose genital challenge with homologous (HPV16) as well as heterologous (HPV45) pseudovirus. Thus, PP7 VLPs show utility for the targeted induction of antibodies against specific epitopes.
In general, the utility of vaccines targeting HPV L2 has been limited by the poor immune responses elicited by L2. As we and others (see [49, 50]) have demonstrated, one method for increasing the immunogenicity of L2 is through multivalent display of L2 peptides on VLPs. However, a disadvantage of vaccination with a single HPV L2 peptide is that protection is greater for the homologous virus than for heterologous HPV. Jagu and colleagues showed that a concatenated multitype L2 fusion protein elicited more broadly neutralizing antibodies than recombinant L2 derived from a single HPV type [51]. The flexibility of the PP7 VLP display technology should make it possible to rapidly develop PP7 VLPs that display L2 peptides from a variety of different HPV types that could then be used as a combination vaccine.
In this study we show that parenteral administration of 16L2-VLPs is effective at preventing genital infection. Presumably, this protection is mediated by systemic IgG, which can migrate into the genital tract, likely through transudation to the cervical epithelium [25]. For example, a clinical study of the HPV vaccine indicated that HPV-specific IgG titers in the cervicovaginal mucus after intramuscular VLP vaccination are generally about 10-fold lower than in the serum and drop approximately 10-fold more around the time of ovulation in women with a normal menstrual cycle [52]. As an alternative, induction of local antibody responses in the genital mucosa might lead to stronger protection from genital infection. Our laboratory has shown that immunization with VLPs using an intramuscular prime-pulmonary boost protocol results in high-titer systemic antibody responses and local mucosal responses in the respiratory and genital mucosa [53]. Thus, we hypothesize that the induction of mucosal IgA by pulmonary vaccination or, perhaps, intravaginal immunization would lead to more sustained antibody levels in the cervicovaginal tract.
The PP7 VLP platform potentially enables the production of vaccine candidates by two different approaches. First, using a rational design approach, we can engineer the display of a specific peptide epitope (such as the L2 epitope) already identified by other means. Alternatively, this system potentially allows us to employ a more empirical approach to vaccine identification in which epitopes are affinity-selected from random or semi-random sequence peptide libraries using an appropriate agent, such as a neutralizing antibody against a pathogen. Because each individual member of the VLP library encapsidates the RNA that codes for its expression, selectants could be recovered by RT-PCR, cloned back into the PP7 expression vector, and then subjected to additional rounds of selection, in a process that is analogous to filamentous phage display. However, one major difference is that peptides displayed on filamentous phage are of low copy number and poorly immunogenic. In order to be used as vaccines, peptide epitopes identified by filamentous phage display must be produced synthetically and then linked to a carrier protein that often displays the epitope in a structural context unrelated to one in which it was identified. As a consequence, peptides identified by filamentous phage display frequently lose the ability to induce antibodies whose characteristics mimic those of the selecting antibody. In contrast, display of peptides on PP7 VLPs should combine into a single platform the means to both identify epitopes by affinity selection, and to present them to the immune system in a highly immunogenic context. This coupling of capabilities, affinity selection and epitope presentation, could facilitate epitope discovery and peptide vaccine development, especially for those epitopes where monoclonal antibodies are available but generation or presentation of their epitopes in a useful vaccine format has met with difficulty.
Acknowledgements
We thank Richard Roden for providing us with the mAb RG-1. In addition, we thank Zoe Hunter, Paul Durfee, and Susana Pang for technical assistance. This study was supported by the NIH (U19 AI084081 to B.C. and R01 GM042901 to D.S.P.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chackerian B. Virus-like particles: flexible platforms for vaccine development. Expert Rev Vaccines. 2007 Jun;6(3):381–390. doi: 10.1586/14760584.6.3.381. [DOI] [PubMed] [Google Scholar]
- 2.Spohn G, Bachmann MF. Exploiting viral properties for the rational design of modern vaccines. Expert Rev Vaccines. 2008 Feb;7(1):43–54. doi: 10.1586/14760584.7.1.43. [DOI] [PubMed] [Google Scholar]
- 3.Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, et al. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children.Taiwan Childhood Hepatoma Study Group. N Engl J Med. 1997 Jun 26;336(26):1855–1859. doi: 10.1056/NEJM199706263362602. [DOI] [PubMed] [Google Scholar]
- 4.Romanowski B, de Borba PC, Naud PS, Roteli-Martins CM, De Carvalho NS, Teixeira JC, et al. Sustained efficacy and immunogenicity of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6.4 years. Lancet. 2009 Dec 12;374(9706):1975–1985. doi: 10.1016/S0140-6736(09)61567-1. [DOI] [PubMed] [Google Scholar]
- 5.Herbst-Kralovetz M, Mason HS, Chen Q. Norwalk virus-like particles as vaccines. Expert Rev Vaccines. 2010 Mar;9(3):299–307. doi: 10.1586/erv.09.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quan FS, Vunnava A, Compans RW, Kang SM. Virus-like particle vaccine protects against 2009 H1N1 pandemic influenza virus in mice. PLoS ONE. 2010;5(2):e9161. doi: 10.1371/journal.pone.0009161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bachmann MF, Lutz MB, Layton GT, Harris SJ, Fehr T, Rescigno M, et al. Dendritic cells process exogenous viral proteins and virus-like particles for class I presentation to CD8+ cytotoxic T lymphocytes. Eur J Immunol. 1996 Nov;26(11):2595–2600. doi: 10.1002/eji.1830261109. [DOI] [PubMed] [Google Scholar]
- 8.Fifis T, Gamvrellis A, Crimeen-Irwin B, Pietersz GA, Li J, Mottram PL, et al. Size-dependent immunogenicity: therapeutic and protective properties of nano-vaccines against tumors. J Immunol. 2004 Sep 1;173(5):3148–3154. doi: 10.4049/jimmunol.173.5.3148. [DOI] [PubMed] [Google Scholar]
- 9.Moron G, Rueda P, Casal I, Leclerc C. CD8alpha- CD11b+ dendritic cells present exogenous virus-like particles to CD8+ T cells and subsequently express CD8alpha and CD205 molecules. J Exp Med. 2002;195(10):1233–1245. doi: 10.1084/jem.20011930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chackerian B, Durfee MR, Schiller JT. Virus-like display of a neo-self antigen reverses B cell anergy in a B cell receptor transgenic mouse model. J Immunol. 2008 May 1;180(9):5816–5825. doi: 10.4049/jimmunol.180.9.5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fehr T, Bachmann MF, Bucher E, Kalinke U, Di Padova FE, Lang AB, et al. Role of repetitive antigen patterns for induction of antibodies against antibodies. J Exp Med. 1997;185(10):1785–1792. doi: 10.1084/jem.185.10.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Milich DR, Chen M, Schodel F, Peterson DL, Jones JE, Hughes JL. Role of B cells in antigen presentation of the hepatitis B core. Proc Natl Acad Sci U S A. 1997 Dec 23;94(26):14648–14653. doi: 10.1073/pnas.94.26.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jennings GT, Bachmann MF. Immunodrugs: therapeutic VLP-based vaccines for chronic diseases. Annu Rev Pharmacol Toxicol. 2009;49:303–326. doi: 10.1146/annurev-pharmtox-061008-103129. [DOI] [PubMed] [Google Scholar]
- 14.Whitacre DC, Lee BO, Milich DR. Use of hepadnavirus core proteins as vaccine platforms. Expert Rev Vaccines. 2009 Nov;8(11):1565–1573. doi: 10.1586/erv.09.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bachmann MF, Rohrer UH, Kundig TM, Burki K, Hengartner H, Zinkernagel RM. The influence of antigen organization on B cell responsiveness. Science. 1993;262(5138):1448–1451. doi: 10.1126/science.8248784. [DOI] [PubMed] [Google Scholar]
- 16.Chackerian B, Lenz P, Lowy DR, Schiller JT. Determinants of autoantibody induction by conjugated papillomavirus virus-like particles. J Immunol. 2002;169(11):6120–6126. doi: 10.4049/jimmunol.169.11.6120. [DOI] [PubMed] [Google Scholar]
- 17.Chackerian B, Lowy DR, Schiller JT. Induction of autoantibodies to mouse CCR5 with recombinant papillomavirus particles. Proc Natl Acad Sci USA. 1999;96:2373–2378. doi: 10.1073/pnas.96.5.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chackerian B, Lowy DR, Schiller JT. Conjugation of a self-antigen to papillomavirus-like particles allows for efficient induction of protective autoantibodies. J Clin Invest. 2001 Aug;108(3):415–423. doi: 10.1172/JCI11849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maurer P, Jennings GT, Willers J, Rohner F, Lindman Y, Roubicek K, et al. A therapeutic vaccine for nicotine dependence: preclinical efficacy, and Phase I safety and immunogenicity. Eur J Immunol. 2005 Jul;35(7):2031–2040. doi: 10.1002/eji.200526285. [DOI] [PubMed] [Google Scholar]
- 20.Ambuhl PM, Tissot AC, Fulurija A, Maurer P, Nussberger J, Sabat R, et al. A vaccine for hypertension based on virus-like particles: preclinical efficacy and phase I safety and immunogenicity. J Hypertens. 2007 Jan;25(1):63–72. doi: 10.1097/HJH.0b013e32800ff5d6. [DOI] [PubMed] [Google Scholar]
- 21.Li Q, Cao C, Chackerian B, Schiller J, Gordon M, Ugen KE, et al. Overcoming antigen masking of anti-amyloidbeta antibodies reveals breaking of B cell tolerance by virus-like particles in amyloidbeta immunized amyloid precursor protein transgenic mice. BMC Neurosci. 2004 Jun 8;5(1):21. doi: 10.1186/1471-2202-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peabody DS, Manifold-Wheeler B, Medford A, Jordan SK, do Carmo Caldeira J, Chackerian B. Immunogenic display of diverse peptides on virus-like particles of RNA phage MS2. J Mol Biol. 2008 Jun 27;380(1):252–263. doi: 10.1016/j.jmb.2008.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caldeira JC, Peabody DS. Stability and assembly in vitro of bacteriophage PP7 virus-like particles. J Nanobiotechnology. 2007;5:10. doi: 10.1186/1477-3155-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olsthoorn RC, Garde G, Dayhuff T, Atkins JF, Van Duin J. Nucleotide sequence of a single-stranded RNA phage from Pseudomonas aeruginosa: kinship to coliphages and conservation of regulatory RNA structures. Virology. 1995 Jan 10;206(1):611–625. doi: 10.1016/s0042-6822(95)80078-6. [DOI] [PubMed] [Google Scholar]
- 25.Stanley M, Lowy DR, Frazer I. Chapter 12: Prophylactic HPV vaccines: underlying mechanisms. Vaccine. 2006 Aug 31;24 Suppl 3:S3/106–S3/113. doi: 10.1016/j.vaccine.2006.05.110. [DOI] [PubMed] [Google Scholar]
- 26.Mao C, Koutsky LA, Ault KA, Wheeler CM, Brown DR, Wiley DJ, et al. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia: a randomized controlled trial. Obstet Gynecol. 2006 Jan;107(1):18–27. doi: 10.1097/01.AOG.0000192397.41191.fb. [DOI] [PubMed] [Google Scholar]
- 27.Koutsky LA, Ault KA, Wheeler CM, Brown DR, Barr E, Alvarez FB, et al. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med. 2002;347(21):1645–1651. doi: 10.1056/NEJMoa020586. [DOI] [PubMed] [Google Scholar]
- 28.Roden RB, Yutzy WI, Fallon R, Inglis S, Lowy DR, Schiller JT. Minor capsid protein of human genital papillomaviruses contains subdominant, cross-neutralizing epitopes. Virology. 2000;270(2):254–257. doi: 10.1006/viro.2000.0272. [DOI] [PubMed] [Google Scholar]
- 29.Pastrana DV, Gambhira R, Buck CB, Pang YY, Thompson CD, Culp TD, et al. Cross-neutralization of cutaneous and mucosal Papillomavirus types with anti-sera to the amino terminus of L2. Virology. 2005 Jul 5;337(2):365–372. doi: 10.1016/j.virol.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 30.Gambhira R, Jagu S, Karanam B, Gravitt PE, Culp TD, Christensen ND, et al. Protection of rabbits against challenge with rabbit papillomaviruses by immunization with the N terminus of human papillomavirus type 16 minor capsid antigen L2. J Virol. 2007 Nov;81(21):11585–11592. doi: 10.1128/JVI.01577-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim F, Downey TP, Peabody DS. Translational repression and specific RNA binding by the coat protein of the Pseudomonas phage PP7. J Biol Chem. 2001 Jun 22;276(25):22507–22513. doi: 10.1074/jbc.M102411200. [DOI] [PubMed] [Google Scholar]
- 32.Peabody DS. Translational repression by bacteriophage MS2 coat protein expressed from a plasmid. A system for genetic analysis of a protein-RNA interaction. J Biol Chem. 1990 Apr 5;265(10):5684–5689. [PubMed] [Google Scholar]
- 33.Gambhira R, Karanam B, Jagu S, Roberts JN, Buck CB, Bossis I, et al. A protective and broadly cross-neutralizing epitope of human papillomavirus L2. J Virol. 2007 Dec;81(24):13927–13931. doi: 10.1128/JVI.00936-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roberts JN, Buck CB, Thompson CD, Kines R, Bernardo M, Choyke PL, et al. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med. 2007 Jul;13(7):857–861. doi: 10.1038/nm1598. [DOI] [PubMed] [Google Scholar]
- 35.Johnson KM, Kines RC, Roberts JN, Lowy DR, Schiller JT, Day PM. Role of heparan sulfate in attachment to and infection of the murine female genital tract by human papillomavirus. J Virol. 2009 Mar;83(5):2067–2074. doi: 10.1128/JVI.02190-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cuburu N, Kweon MN, Hervouet C, Cha HR, Pang YY, Holmgren J, et al. Sublingual immunization with nonreplicating antigens induces antibody-forming cells and cytotoxic T cells in the female genital tract mucosa and protects against genital papillomavirus infection. J Immunol. 2009 Dec 15;183(12):7851–7859. doi: 10.4049/jimmunol.0803740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tars K, Fridborg K, Bundule M, Liljas L. The three-dimensional structure of bacteriophage PP7 from Pseudomonas aeruginosa at 3.7-A resolution. Virology. 2000 Jul 5;272(2):331–337. doi: 10.1006/viro.2000.0373. [DOI] [PubMed] [Google Scholar]
- 38.Laman JD, Schellekens MM, Abacioglu YH, Lewis GK, Tersmette M, Fouchier RA, et al. Variant-specific monoclonal and group-specific polyclonal human immunodeficiency virus type 1 neutralizing antibodies raised with synthetic peptides from the gp120 third variable domain. J Virol. 1992 Mar;66(3):1823–1831. doi: 10.1128/jvi.66.3.1823-1831.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peabody DS. Subunit fusion confers tolerance to peptide insertions in a virus coat protein. Arch Biochem Biophys. 1997 Nov 1;347(1):85–92. doi: 10.1006/abbi.1997.0312. [DOI] [PubMed] [Google Scholar]
- 40.Alphs HH, Gambhira R, Karanam B, Roberts JN, Jagu S, Schiller JT, et al. Protection against heterologous human papillomavirus challenge by a synthetic lipopeptide vaccine containing a broadly cross-neutralizing epitope of L2. Proc Natl Acad Sci U S A. 2008 Apr 15;105(15):5850–5855. doi: 10.1073/pnas.0800868105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Slupetzky K, Shafti-Keramat S, Lenz P, Brandt S, Grassauer A, Sara M, et al. Chimeric papillomavirus-like particles expressing a foreign epitope on capsid surface loops. J Gen Virol. 2001 Nov;82(Pt 11):2799–2804. doi: 10.1099/0022-1317-82-11-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith ML, Lindbo JA, Dillard-Telm S, Brosio PM, Lasnik AB, McCormick AA, et al. Modified tobacco mosaic virus particles as scaffolds for display of protein antigens for vaccine applications. Virology. 2006 May 10;348(2):475–488. doi: 10.1016/j.virol.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 43.Arnold GF, Velasco PK, Holmes AK, Wrin T, Geisler SC, Phung P, et al. Broad neutralization of human immunodeficiency virus type 1 (HIV-1) elicited from human rhinoviruses that display the HIV-1 gp41 ELDKWA epitope. J Virol. 2009 May;83(10):5087–5100. doi: 10.1128/JVI.00184-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Billaud JN, Peterson D, Barr M, Chen A, Sallberg M, Garduno F, et al. Combinatorial approach to hepadnavirus-like particle vaccine design. J Virol. 2005 Nov;79(21):13656–13666. doi: 10.1128/JVI.79.21.13656-13666.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ekiert DC, Bhabha G, Elsliger MA, Friesen RH, Jongeneelen M, Throsby M, et al. Antibody recognition of a highly conserved influenza virus epitope. Science. 2009 Apr 10;324(5924):246–251. doi: 10.1126/science.1171491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sui J, Hwang WC, Perez S, Wei G, Aird D, Chen LM, et al. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat Struct Mol Biol. 2009 Mar;16(3):265–273. doi: 10.1038/nsmb.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Law M, Maruyama T, Lewis J, Giang E, Tarr AW, Stamataki Z, et al. Broadly neutralizing antibodies protect against hepatitis C virus quasispecies challenge. Nat Med. 2008 Jan;14(1):25–27. doi: 10.1038/nm1698. [DOI] [PubMed] [Google Scholar]
- 48.Burton DR, Stanfield RL, Wilson IA. Antibody vs. HIV in a clash of evolutionary titans. Proc Natl Acad Sci U S A. 2005 Oct 18;102(42):14943–14948. doi: 10.1073/pnas.0505126102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Slupetzky K, Gambhira R, Culp TD, Shafti-Keramat S, Schellenbacher C, Christensen ND, et al. A papillomavirus-like particle (VLP) vaccine displaying HPV16 L2 epitopes induces cross-neutralizing antibodies to HPV11. Vaccine. 2007 Mar 1;25(11):2001–2010. doi: 10.1016/j.vaccine.2006.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palmer KE, Benko A, Doucette SA, Cameron TI, Foster T, Hanley KM, et al. Protection of rabbits against cutaneous papillomavirus infection using recombinant tobacco mosaic virus containing L2 capsid epitopes. Vaccine. 2006 Jun 29;24(26):5516–5525. doi: 10.1016/j.vaccine.2006.04.058. [DOI] [PubMed] [Google Scholar]
- 51.Jagu S, Karanam B, Gambhira R, Chivukula SV, Chaganti RJ, Lowy DR, et al. Concatenated multitype L2 fusion proteins as candidate prophylactic pan-human papillomavirus vaccines. J Natl Cancer Inst. 2009 Jun 3;101(11):782–792. doi: 10.1093/jnci/djp106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nardelli-Haefliger D, Lurati F, Wirthner D, Spertini F, Schiller JT, Lowy DR, et al. Immune responses induced by lower airway mucosal immunisation with a human papillomavirus type 16 virus-like particle vaccine. Vaccine. 2005 May 25;23(28):3634–3641. doi: 10.1016/j.vaccine.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 53.Hunter Z, Smyth HD, Durfee P, Chackerian B. Induction of mucosal and systemic antibody responses against the HIV coreceptor CCR5 upon intramuscular immunization and aerosol delivery of a virus-like particle based vaccine. Vaccine. 2009 Dec 11;28(2):403–414. doi: 10.1016/j.vaccine.2009.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]