Abstract
Women experience a decline in estrogen and androgen levels after natural or surgically induced menopause, effects that are associated with a loss of sexual desire and bone mineral density. Studies in our laboratories have shown the beneficial effects of selective androgen receptor modulators (SARMs) in the treatment of osteoporosis and muscle wasting in animal models. A series of S-3-(phenoxy)-2-hydroxy-2-methyl-N-(4-cyano-3-trifluoromethyl-phenyl)-propionamide analogs was synthesized to evaluate the effects of B-ring substitutions on in vitro and in vivo pharmacologic activity, especially female sexual motivation. The androgen receptor (AR) relative binding affinities ranged from 0.1 to 26.5% (relative to dihydrotestosterone) and demonstrated a range of agonist activity at 100 nM. In vivo pharmacologic activity was first assessed by using male rats. Structural modifications to the B-ring significantly affected the selectivity of the SARMs, demonstrating that single-atom substitutions can dramatically and unexpectedly influence activity in androgenic (i.e., prostate) and anabolic (i.e., muscle) tissues. (S)-N-(4-cyano-3-trifluoromethyl-phenyl)-3-(3-fluoro,4-chlorophenoxy)-2-hydroxy-2-methyl-propanamide (S-23) displayed full agonist activity in androgenic and anabolic tissues; however, the remaining SARMs were more prostate-sparing, selectively maintaining the size of the levator ani muscle in castrated rats. The partner-preference paradigm was used to evaluate the effects of SARMs on female sexual motivation. With the exception of two four-halo substituted analogs, the SARMs increased sexual motivation in ovariectomized rats, with potency and efficacy comparable with testosterone propionate. These results indicate that the AR is important in regulating female libido given the nonaromatizable nature of SARMs and it could be a superior alternative to steroidal testosterone preparations in the treatment of hypoactive sexual desire disorder.
Androgens are involved in a number of physiological roles in women, including bone mineral density, muscle mass and strength, libido, and sexual response (Sherwin and Gelfand, 1987; Hiort, 2002; Davis et al., 2008b). Hypoactive sexual desire disorder (HSDD) is a disorder that afflicts naturally postmenopausal women (Dennerstein et al., 2001; Shifren et al., 2006) and, to a greater extent, those who have undergone bilateral oophorectomy (Zussman et al., 1981; Nathorst-Böös and von Schoultz, 1992). The disorder is characterized by chronic or recurrent loss or decrease in interest in sexual activity, causing interpersonal distress (Schmidt, 1994) and severely affecting a woman's quality of life. It is believed that the decrease in libido, sexual receptivity, and responsiveness is associated with free testosterone levels that decline after surgically induced or natural menopause.
Clinical trials in premenopausal women revealed that sexual desire and arousal may be enhanced when administered a once-daily spray of a 90-μg metered-dose transdermal testosterone preparation for 16 weeks compared with placebo (Davis et al., 2008a). Likewise, when postmenopausal women received a biweekly 300 μg/day testosterone patch for 24 weeks the frequency of satisfying sexual events, including desire, arousal, orgasm, pleasure, and responsiveness, as well as body image, increased compared with placebo (Shifren et al., 2006). In addition, clinical trials of a 300 μg/day testosterone patch improved sexual functioning, desire, fantasies, and well being in bilaterally oophorectomized women receiving concomitant estrogen therapy after 12 weeks of treatment (Buster et al., 2005) or 16 weeks of treatment (Simon et al., 2005). The most common side effects associated with transdermal testosterone treatment in women include hirsutism and acne and rare occurrences of alopecia, breast pain, weight gain, and voice deepening. However, clinical use of transdermal testosterone administration for HSDD has been problematic because of the lack of long-term safety data on breast cancer and cardiovascular risks (i.e., increased blood triglycerides) associated with exogenous testosterone administration.
Sexual behavior in rats is characterized by consummatory and appetitive components. Consummatory aspects describe the receptive behavior, including the lordosis reflex to allow copulation by a male. The appetitive components include the soliciting or proceptive behaviors to attract and arouse sexual interest. Blasberg et al. (1998) reported that nonaromatizable anabolic steroids inhibit sexual receptivity in hormonally primed ovariectomized (OVX) rats; however, the effects of anabolic steroids on proceptive behaviors have not been determined.
The development and potential clinical use of tissue-selective androgen receptor modulators (SARMs) have advanced tremendously over the past few years. A variety of chemical scaffolds that modulate the androgen receptor (AR) in a tissue-selective manner have been identified. We were the first to report the structure–activity relationships, both in vitro and in vivo, for anabolic and androgenic activities and tissue selectivity of these nonsteroidal AR ligands (Dalton et al., 1998; He et al., 2002; Yin et al., 2003b). Of these SARMs, we have reported a few with potential use in androgen-related conditions, including male hormonal contraception (Chen et al., 2005a; Jones et al., 2009) and muscle strength and benign prostate hyperplasia (Gao et al., 2005) in male animal models. In female rats, Kearbey et al. (2007) reported the effects of an aryl-propionamide SARM on bone in an ovariectomized model of postmenopausal osteoporosis. The SARM inhibited ovariectomy-induced loss of whole-body bone mass density, improved bone strength, decreased fat mass, and increased lean body mass (Kearbey et al., 2007).
In the current studies, we examined the effect of different B-ring substitutions of aryl-propionamide SARMs on AR binding affinity and AR- and ER-mediated transcriptional activation, virilizing potential and pharmacologic activity in orchidectomized (ORX) male and ovariectomized rats. Furthermore, we examined the effects of these SARMs on the sexual motivation and solicitation of progesterone-primed ovariectomized rats in a partner-preference model to evaluate the preference of the female for a sexually active intact male or a sexually inactive orchidectomized male and to help clarify whether the androgen receptor plays a role in the sexual behavior of females. A SARM structurally similar to (S)-N-(4-cyano-3-trifluoromethyl-phenyl)-3-(3-fluoro,4-chlorophenoxy)-2-hydroxy-2-methyl-propanamide (S-23) (Jones et al., 2009) is able to maintain mating behavior in sexually experienced male rats after long-term castration (unpublished data). The dose-dependent effect of S-23 on female sexual motivation and a series of SARMs with structural modifications on the B-ring was evaluated at a single, high dose in the partner-preference paradigm, and their effects on the growth of the endometrial and myometrial layers of the uterus were studied. Testosterone propionate (TP) and a few of the SARMs were also evaluated for their ability to inhibit ovariectomy-induced elevations in luteinizing hormone (LH) and follicle-stimulating hormone (FSH). The results of these studies provide the first evidence of structurally diverse SARMs in this animal model and provide definitive proof of the importance of AR function to female sexual motivation.
Materials and Methods
In Vitro AR Binding Affinity.
The ligand binding domain (LBD) of the AR fused with glutathione S-transferase (GST) was expressed as a recombinant protein (AR GST-LBD) as described previously (Bohl et al., 2005). The equilibrium binding constant (Ki) of each of the S-(4-cyano-3-trifluoromethyl)-propionamide analogs was determined in a radiolabeled competitive binding assay (Mukherjee et al., 1996). S-3-(4-cyanophenoxy)-2-hydroxy-2-methyl-N-(4-cyano-3-trifluromethylphenyl)-propionamide (S-22), S-23, (S)-N-(4-cyano-3-trifluoromethyl-phenyl)-3-(4-fluorophenoxy)-2-hydroxy-2-methyl-propanamide (S-24), (S)-N-(4-cyano-3-trifluoromethyl-phenyl)-3-(4-chlorophenoxy)-2-hydroxy-2-methyl-propanamide (S-25), S-3-(4-bromophenoxy)-2-hydroxy-2-methyl-N-(4-cyano-3-trifluoromethyl-phenyl)-propionamide (S-26), S-3-(4-iodophenoxy)-2-hydroxy-2-methyl-N-(4-cyano-3-trifluoromethyl-phenyl)-propionamide (S-27), S-3-(3,4-difluorophenoxy)-2-hydroxy-2-methyl-N-(4-cyano-3-trifluoromethyl-phenyl)-propionamide (S-28), S-3-(3,4-dichloro-phenoxy)-2-hydroxy-2-methyl-N-(4-cyano-3-trifluoromethyl-phenyl)-propionamide (S-29), S-3-(4-acetamidophenoxy)-2-hydroxy-2-methyl-N-(4-cyano-3-trifluoromethyl-phenyl)-propionamide (S-30), and (R)-N-(4-cyano-3-trifluoromethyl-phenyl)-3-(3-fluoro, 4-chlorophenoxy)-2-hydroxy-2-methyl-propanamide (R-23) all were synthesized in our laboratories as described previously (Marhefka et al., 2004). Chemical purities were confirmed by mass spectroscopy and nuclear magnetic resonance and determined to be more than 99%. Increasing concentrations (3–104 nM) of each compound of interest were incubated with 4 nM [3H]MIB (PerkinElmer Life and Analytical Sciences, Waltham, MA) and AR GST-LBD at 4°C for 18 h. Protein was also incubated with and without a high concentration (10−6 M) of unlabeled MIB (PerkinElmer Life and Analytical Sciences) to determine total and nonspecific binding, respectively. The plates were harvested with GF/B filters on the Unifilter-96 Harvester (PerkinElmer Life and Analytical Sciences) and washed three times with ice-cold buffer. The filter plates were dried at room temperature, Microscint-O cocktail (PerkinElmer Life and Analytical Sciences) was added to each well and sealed, and radioactivity was counted in a TopCount NXT Microplate Scintillation Counter (PerkinElmer Life and Analytical Sciences). The specific binding of [3H]MIB at each concentration of the compound of interest was determined by subtracting the nonspecific binding of [3H]MIB and expressed as a percentage of the specific binding in the absence of competitor. The concentration of the compound of interest that reduced the specific binding of [3H]MIB by 50% (IC50) was determined by nonlinear regression with SigmaPlot (Systat Software Inc., San Jose, CA) using the standard four-parameter logistic curve. The equilibrium binding constant (Ki) was then calculated by: Ki = Kd × IC50/(Kd + L), where Kd is the equilibrium dissociation constant of [3H]MIB, and L is the concentration of [3H]MIB (4 nM). The binding affinities of the compounds of interest are expressed relative to dihydrotestosterone (DHT; Sigma-Aldrich, St. Louis, MO).
In Vitro AR- and Estrogen Receptor-Mediated Transcriptional Activation.
The in vitro functional activity of the compounds of interest were determined by the ability of each ligand to induce AR-mediated or estrogen receptor (ER)-mediated transcriptional activation in a cotransfection system (Yin et al., 2003c). At ∼90% confluence, CV-1 cells (AR) or human embryonic kidney 293 cells (ERα) were transiently transfected in 15-cm dishes in serum-free Dulbecco's minimal essential medium (HyClone Laboratories, Logan, UT), using Lipofectamine (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Cells in each dish were transfected with 45 μg of GRELuc, 1 μg of CMVLuc (Renilla luciferase), and 2.5 μg of CMVhAR, CMVhERα, or CMVhERβ expression vector. Cells were allowed to recover for 12 h and were then seeded into 24-well plates (8 × 104 per well) in Dulbecco's minimal essential medium containing 2% charcoal-stripped fetal bovine serum (HyClone Laboratories) and allowed to recover for an additional 8 h before drug treatment. Previous experiments in our laboratories determined that the lowest concentration of DHT that maximally induced AR-mediated transcriptional activation was 1 nM (Dalton et al., 1998). Likewise, 1 nM estradiol (E2; Sigma-Aldrich) was required to maximally induce ERα and ERβ-mediated transcriptional activation (data not shown). Therefore, in the present study, the transcriptional activation induced by 1 nM of DHT or E2 was set as 100% and used as the reference for quantifying the agonist activity of the test compounds. Twenty-four hours after drug treatment, cells were washed with Dulbecco's phosphate-buffered saline (PBS) (HyClone Laboratories) and lysed with 50 μl/well of passive lysis buffer for 30 min at room temperature. An aliquot (25 μl) of cell lysate was used for luciferase assays (Dual-Luciferase Reporter Assay System; Promega, Madison, WI). Transcriptional activity in each well was calculated as the ratio of luciferase activity to Renilla luciferase activity to avoid variations in transfection efficiency and cell number. Transcriptional activity induced by each compound of interest was expressed as the percentage of that induced by 1 nM DHT (AR) or E2 (ERα and ERβ).
In Vitro Amino Terminus–Carboxyl Terminus Interaction.
The amino terminus–carboxyl terminus (N-C) interaction of the AR can be identified by a mammalian two-hybrid assay. CV-1 cells were maintained and transfected as stated above. Cells in each dish were transfected with 45 μg of pG5Luc, 2.5 μg of pACT AR-N-terminal domain, 2.5 μg of pBind AR-LBD, and 1 μg of CMVLuc. If the ligand/receptor complex facilitates interaction between the N-terminal domain and the LBD, the close proximity of the activating domain to the DNA binding domain results in an increase in luciferase activity. Cells were subsequently plated and treated as described above. Experiments in our laboratories determined that 10 nM DHT was the lowest concentration that maximally induced the N-C interaction (data not shown). Therefore, in the present study, the interaction induced by 10 nM DHT was set as 100% and used as the reference for quantifying the activity of the test compounds. Luciferase assays were performed as described above, and the N-C interaction induced by each compound of interest was expressed as the percentage of that induced by 10 nM DHT.
Animals.
Male and female Sprague-Dawley rats were purchased from Harlan (Indianapolis, IN). The animals were maintained on a 12-h light, 12-h dark cycle with food and water available ad libitum. All animal studies were conducted under the auspices of animal protocols approved by the Institutional Laboratory Animal Care and Use Committee at the University of Tennessee (male pharmacologic studies) or Ohio State University (female behavior studies and uterine effects).
In Vivo Pharmacologic Activity.
The in vivo pharmacologic activity of each AR ligand was first determined in castrated male rats. Animals weighing approximately 200 g were randomly distributed into groups (n = 5/group). Animals were orchidectomized via scrotal incision under ketamine/xylazine anesthesia 24 h before drug treatment and received daily subcutaneous injections of the test compound, at a dose rate of 1 mg/day, for 14 days. Each AR ligand was freshly dissolved in vehicle containing dimethyl sulfoxide (DMSO; Sigma-Aldrich)/polyethylene glycol 300 (PEG300; Sigma-Aldrich) [10/90 (v/v)] before daily administration. An additional two groups of animals (n = 5/group) with or without castration received vehicle only and served as castrate or intact control groups, respectively. Animals were weighed, anesthetized, and sacrificed within 24 h after the last dose. The ventral prostate, seminal vesicles, and levator ani muscle were removed, cleared of extraneous tissue, and weighed. All organ weights were normalized to total body weight and compared. The weights of the prostate and seminal vesicles were used to evaluate androgenic activity, and the levator ani muscle weight was used as a measure of anabolic activity. Percentage changes were determined by comparison with intact animals.
Effects of Treatment on Sexual Motivation in Female Rats.
The effects of SARMs on female sexual motivation were determined in OVX rats. Female animals weighing approximately 150 g were randomly distributed into groups (n = 6/group). Animals were ovariectomized via dorsal incision under ketamine/xylazine anesthesia 24 h before drug treatment and received daily subcutaneous injections of the test compound. An additional two groups of animals (n = 6/group) with or without ovariectomization received vehicle only and served as gonadectomized or intact control groups, respectively. Compounds of interest were freshly dissolved in vehicle containing DMSO/PEG300 [5/95 (v/v)] and administered at a daily dose of 3 mg/kg/day for 14 days. Doses ranging from 0.05 to 0.75 mg/day were also administered for S-23, a SARM previously shown to be useful in regulating male fertility (Jones et al., 2009). TP (Sigma-Aldrich) was used as a positive control, and TP coadministered with R-bicalutamide (an antiandrogen) was used to confirm the importance of AR activation to the observed effects.
Behavioral testing was performed in a three-compartment chamber with wood shavings covering the floor and conducted during the early portion of the dark cycle under dim red light within 12 h after the last dose. Sexually experienced female rats were acclimated to the chamber during three separate 15-min periods, two the week before behavioral testing and another immediately before testing. During this time, the female rats explored the testing chamber in the absence of stimulus male rats. Four hours before behavioral testing, female rats received a subcutaneous injection of 0.1 mg of progesterone (5% DMSO in PEG300; Sigma-Aldrich) to facilitate sexual behavior. After the final 15-min acclimation period, individual females were restricted to the central compartment, while the stimulus male rats, one intact and one castrated (castrated via scrotal incision under ketamine/xylazine anesthesia 14 days before behavioral testing) weighing more than 400 g, were sequestered individually in the lateral compartments. Six different pairs of males (one intact and one castrated) were used for each treatment group, with differing intact and castrated males being used for each individual female. The rats underwent a 5-min habituation period with opaque partitions in place. After this period, the opaque partitions were removed and the female rat was able to move freely throughout the three chambers for the 30-min behavioral testing period. The larger size of the male rats prevented movement between compartments. The duration of time spent in each compartment by the OVX female was recorded. Compartment entries (intact compartment, central compartment, or castrated compartment) were scored when all four paws of the female rat passed through the tube into a compartment. The amount of time (minutes) the female spent with the intact male and castrate are reported. Animals were weighed, anesthetized, and sacrificed within 24 h after the last dose. The uterus was removed, cleared of extraneous tissue, and weighed. Uterine weights were normalized to body weight and compared. Percentage changes were determined by comparison to intact animals. In a separate study, uteri from animals treated with 3 mg/kg/day (14 days) of TP, and the most active (S-23 and S-26) and least active (S-25) SARMs were fixed in 4% paraformaldehyde in PBS for histology. The uteri were dehydrated and stained with hematoxalylin and eosin and analyzed.
Hormone Assays.
Serum LH and FSH were measured by using the MILLIPLEX MAP rat pituitary kit, according to the manufacturer's instructions (Millipore, Billerica, MA). The lower limits of quantitation for LH and FSH were 0.0049 and 0.477 ng/ml, respectively.
Statistical Analyses.
All statistical analyses were performed by using single-factor ANOVA followed by Dunnett's multiple comparison test. Differences in which p < 0.05 were considered statistically significant.
Results
In Vitro Activity.
An in vitro radiolabeled competitive binding assay was used to determine the relative AR binding affinity of each SARM, which was expressed as a percentage relative to DHT (relative binding affinity; RBA). The series of aryl propionamide analogs showed RBAs ranging from 3 to 27% (Table 1). The R-isomer of compound S-23 (R-23) bound the AR very weakly with an RBA of 0.1% of DHT, confirming the enantioselective binding of this series of compounds. The para-monosubstituted halogen derivatives bound the AR with affinity directly varying with the electronegativity of the substituent. The disubstituted derivatives (S-28 and S-29) bound with slightly less affinity than their monosubstituted counterparts (S-24 and S-25). S-22 containing the cyano group at the para-position bound the AR with an RBA of 16.8%. However, the meta-F, para-Cl derivative (S-23) displayed the highest binding affinity with an RBA of 26.5%.
TABLE 1.
Chemical structures, AR binding affinity, and in vitro activity of nonsteroidal AR ligands
AR relative binding affinities were determined by using a radiolabeled competitive assay and presented as a percentage of DHT. The ability of the compound of interest to induce AR- or ER-mediated transcription was determined by using a cotransfection assay. The transcriptional activity induced by each compound at a concentration of 100 or 1000 nM was reported as the percentage of that observed for 1 nM DHT or E2 for the AR and ER, respectively. Data are presented as mean ± S.D.
 | ||||||
|---|---|---|---|---|---|---|
| Compound | R1 | R2 | RBA | AR In Vitro Activity | ERα In Vitro Activity | ERβ In Vitro Activity |
| S-22 | CN | H | 16.8 ± 1.3a | 94 ± 27a | 11.4 ± 1.6 | 17.7 ± 0.9 |
| S-23 | Cl | F | 26.5 ± 2.3b | 101.2 ± 11.3 | 25.6 ± 3.5 | 28.1 ± 0.7 |
| S-24 | F | H | 12.1 ± 0.3c | 82.4 ± 9.4 | 19.6 ± 2.4 | 18.5 ± 0.6 |
| S-25 | Cl | H | 11.8 ± 0.3c | 82.5 ± 4.6 | 24.1 ± 5.5 | 21.3 ± 0.4 |
| S-26 | Br | H | 4.6 ± 3.3d | 87.6 ± 24.0 | 8.4 ± 0.5 | 18.9 ± 0.3 |
| S-27 | I | H | 3.2 ± 0.3d | 64.8 ± 14.9 | 11.0 ± 2.3 | 20.1 ± 0.6 |
| S-28 | F | F | 9.7 ± 1.4e | 86 ± 7e | 8.9 ± 0.9 | 20.0 ± 0.3 |
| S-29 | Cl | Cl | 8 ± 2e | 92 ± 2e | 8.8 ± 4.9 | 24.9 ± 0.3 |
| S-30 | NHC(O)CH3 | H | 4.8 ± 5.1 | 80.5 ± 9.6 | 4.7 ± 1.9 | 21.0 ± 0.5 |
| R-23f | Cl | F | 0.1 ± 0.06 | 0.19 ± 0.06 | 6.1 ± 0.7 | 20.8 ± 0.5 |
Reported by Kim et al., 2005.
Reported by Jones et al., 2009.
Reported by Fisher, 2004.
Reported by Kim, 2006.
Reported by Chen et al., 2005b.
Opposite stereochemical conformation to structure shown above.
The ability of these SARMs to induce AR-, ERα-, and ERβ-mediated transcriptional activation was determined in an in vitro cotransfection assay. At 100 nM, S-23 displayed the most activity by stimulating AR-mediated transcriptional activation to 101% of that observed for 1 nM DHT (Table 1). The cyano-containing compound S-22 and dichloro compound S-29 stimulated the AR similarly to 94 and 92% of DHT, respectively. The remaining SARMs stimulated AR-mediated transcription to a lesser extent, ranging from 64.8 to 87.6%. The ability of the SARMs to induce ERα- and ERβ-mediated transcriptional activation was also determined. S-23, S-24, and S-25 displayed weak ERα partial agonist activity, by stimulating ERα-mediated transcription to 25, 19, and 24% of that observed for 1 nM E2, respectively (Table 1). S-23 was the only SARM tested that exhibited minimal ERβ-mediated transcription (28.1%) compared with that of the vehicle control (19.3%; Table 1).
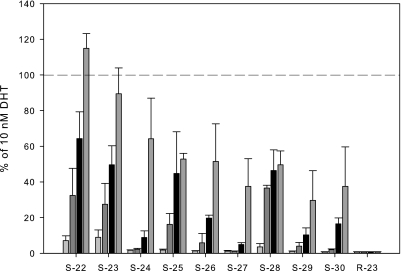
The ability of these SARMs to induce interaction between the amino terminus and the carboxyl terminus of the AR was determined in a two-hybrid assay. The SARMs displayed a dose-dependent increase in interaction, with S-22 and S-23 maximally inducing the interaction to 115 and 90% of 10 nM DHT, respectively (Fig. 1). The remaining SARMs only partially induced the N-C interaction, with minimal activity seen up to 100 nM. However, S-22, S-23, S-25, and S-28 induced activity to 32, 27, 16, and 36% of DHT at 10 nM, respectively.
Fig. 1.
In vitro N-C interaction. The ability of each SARM to induce interaction between the amino terminus and the carboxyl terminus of the AR was determined in a two-hybrid assay. The activity induced by each compound at 1, 10, 100, or 1000 nM is reported as a percentage of that observed for 10 nM DHT. Dashed line indicates 100% (i.e., 10 nM DHT). Data are presented as mean ± S.D.
Pharmacologic Activity in Orchidectomized and Ovariectomized Rats.
The in vivo androgenic and anabolic activity of the series of SARMs was first determined in castrated male rats after 14 days of drug administration. After castration and subsequent depletion of endogenous testosterone, the prostate and levator ani muscle significantly decreased in size to 9 and 39.7% of that observed for intact control, respectively. Previous studies in our laboratories displayed the nonselective nature of TP in castrated male rats, producing an equipotent and equi-efficacious dose-dependent increase in both androgenic and anabolic tissues. The weights of the prostate and levator ani muscle were restored to 121 and 70% of intact control at 0.75 mg/day, respectively (Yin et al., 2003a). Likewise, S-23 displayed only moderate tissue selectivity at 1 mg/day, maximally maintaining the size of the prostate at 110% and the levator ani muscle at 136% of intact control (Table 2). S-22 and S-30 displayed the greatest selectivity for anabolic tissues, increasing the size of the levator ani muscle to 136 and 128%, while maintaining the prostate at only 51.1 and 41.4% of that observed in intact control, respectively. S-24 was the least active SARM in this series, maintaining the prostate at only 8% and the levator ani muscle at 70% of intact control. The remaining SARMs displayed tissue selectivity for anabolic tissues, maintaining the levator ani muscle close to that of intact control (ranging from 82% for S-29 to 111% for S-25) and the prostate around 50% (ranging from 33% for S-29 to 60% for S-25). In addition, the inactive isomer of S-23 (R-23), displayed no in vivo activity with tissue weights similar to castrate control, with the prostate and levator ani muscle reducing to 7 and 34% of that observed for intact control, respectively.
TABLE 2.
Pharmacologic activity of SARMs in castrated male rats
Castrated male rats were treated with SARMs (1 mg/d) for 14 days. Vehicle-treated intact and castrate groups were also included as controls. Prostate and levator ani muscle weights were measured at the end of the treatment period to determine androgenic and anabolic activity of each SARM, respectively. All organ weights were normalized to body weight and presented as a percentage of the vehicle-treated, intact control group. Data are presented as mean ± S.D.
| Compound |
Emax |
|
|---|---|---|
| Prostate | Levator Ani | |
| % of intact control | ||
| S-22 | 51.1 ± 4.2a | 136 ± 3.5a |
| S-23 | 110 ± 25.6b | 136 ± 8.8b |
| S-24 | 7.7 ± 1.7c | 69.9 ± 9.0c |
| S-25 | 60.3 ± 1.0c | 111 ± 1.8c |
| S-26 | 45.4 ± 7.2 | 97.1 ± 9.8 |
| S-27 | 41.8 ± 8.9 | 94.2 ± 8.1 |
| S-28 | 39.2 ± 6.3 | 98.7 ± 4.4 |
| S-29 | 33.4 ± 7.0 | 82.2 ± 6.2 |
| S-30 | 41.4 ± 8.8 | 128 ± 12.7 |
| R-23 | 6.7 ± 0.3 | 34.4 ± 4.5 |
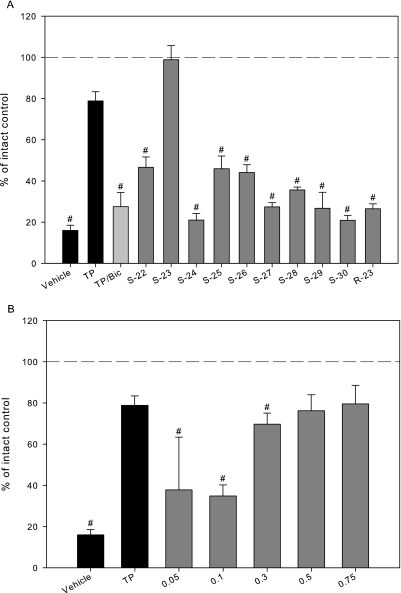
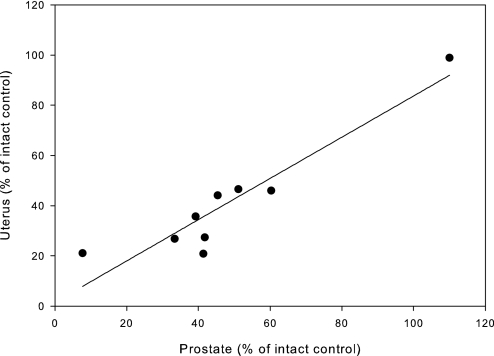
The in vivo pharmacologic activity of these SARMs in OVX female rats was assessed by uterine weight. Upon excision of ovaries, the uterus reduces to approximately 16% of that observed in intact control. Administration of 3 mg/kg/day TP maintains the uterus at 79%; however, the ability of TP to maintain the size of the uterus was inhibited by cotreatment with bicalutamide, which caused the uterus to reduce to 28% of intact control (Fig. 2A). S-23 displayed the most androgenic activity in ORX males and also showed the greatest uterine activity in OVX females, maintaining the uterus at 98.9% of intact control at 3 mg/kg/day. In addition, the uterus responded in a dose-dependent manner to S-23, increasing in size from 37.8% at 0.05 mg/day to 79.5% at 0.75 mg/day compared with intact control (Fig. 2B). With the exception of S-23, all of the SARMs demonstrated weaker partial agonist activity in the uterus than TP, with the uteri ranging in size from 21% for S-30 to 47% for S-22 at 3 mg/kg/day. R-23 maintained the size of the uterus at 27% of that observed in intact control. Additionally, the in vivo pharmacologic activity of these SARMs in male (prostate) and female (uterus) rats is highly correlated, with a correlation coefficient of 0.94 (Fig. 3).
Fig. 2.
Pharmacologic activity of SARMs in OVX female rats. A, pharmacologic activity of a series of SARMs in OVX female rats. B, dose-response of S-23 in OVX rats. OVX rats were treated with TP, TP/bicalutamide (TP/Bic), or a SARM (3 mg/kg/day) for 14 days. Vehicle-treated intact and OVX groups were also included as controls. Uterine weights were measured at the end of the treatment period, normalized to body weight, and presented as a percentage of the vehicle-treated, intact control group. Dashed lines indicate 100% (i.e., intact control). Data are presented as mean ± S.D. ∗, p < 0.05 compared with the vehicle-treated, intact control group.
Fig. 3.
Correlation of male and female pharmacologic activity. OVX female rats and ORX male rats were treated with a SARM, 3 mg/kg/day or 1 mg/day, respectively, for 14 days. Uterine and prostate weights were measured at the end of the treatment period, normalized to body weight, and presented as a percentage of the vehicle-treated, intact control group. Uterine and prostatic activity of each SARM were compared, and nonlinear regression was performed, with a correlation of 0.94.
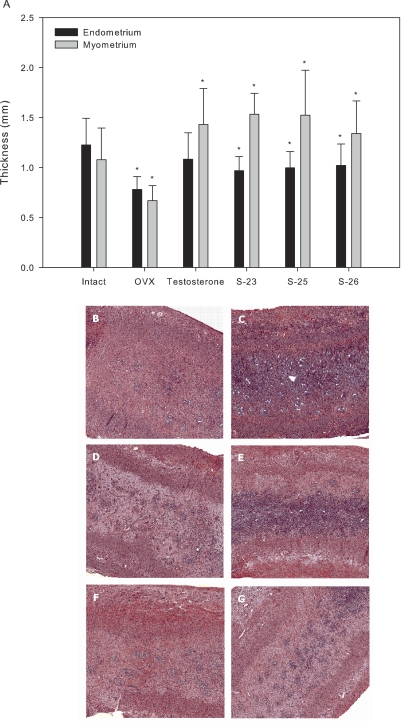
The effects of TP and SARMs on endometrial and myometrial thickness were determined by histological analysis. The average thickness of the endometrium and myometrium in the intact control group was 1.23 and 1.08 mm, respectively (Fig. 4A). Ovariectomy reduced these layers of the uterine wall to 0.78 and 0.67 mm, respectively. TP and SARMs selectively increased the myometrial layer, increasing the thicknesses to larger than that observed for intact control, ranging from 1.34 mm (S-26) to 1.53 mm (S-23). TP and SARMs maintained the thickness of the endometrium slightly below that of intact control.
Fig. 4.
Effects of TP and SARMs on endometrial and myometrial thickness. OVX female rats were treated with TP or a SARM (3 mg/kg/day) for 14 days. Vehicle-treated intact and OVX groups were also included as controls. Uteri were fixed in 4% paraformaldehyde in PBS for histology. The uteri were dehydrated, stained with hematoxalylin and eosin, and analyzed. A, graphical representation of endometrial and myomtrial thicknesses. Data are presented as mean ± S.D. ∗, p < 0.05 compared with the vehicle-treated, intact control group. B–G, magnified views (×2.5) of the endometrial and myometrial layers in intact, OVX, TP-, S-23-, S-25-, and S-26-treated animals, respectively.
Effects of Treatment on Female Sexual Motivation in Ovariectomized Rats.
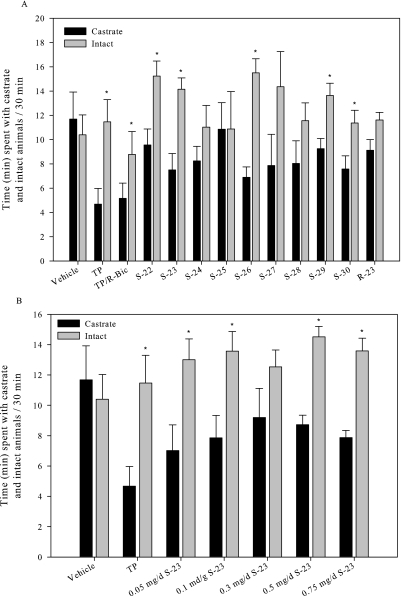
The influence of SARMs on female sexual motivation and solicitation in OVX female rats was analyzed in a partner-preference paradigm. As expected in this model, the progesterone-primed, vehicle-treated OVX female rat displayed no preference for the sexually active intact male or sexually inactive castrated male, spending on average only 2% more time with the castrated male (Fig. 5A). On the contrary, a significant increase in sexual motivation was observed in TP-treated females, which displayed a preference for the intact male of 22.6%. When TP was coadministered with bicalutamide, sexual preference was reduced. Six of the nine SARMs caused a significant increase in sexual motivation, displaying a preference for the intact males over castrate. Although S-26 was the most active of the SARMs tested, the other monosubstituted, para-halogen derivatives (i.e., S-24, S-25, and S-27) showed less or no ability to enhance sexual motivation. With the unexplained exception of the group receiving doses of 0.3 mg/day, all females that received S-23, primed with progesterone, demonstrated a significant increase in sexual motivation, with similar increases seen at doses ranging from 0.05 mg/day to 3 mg/kg/day (Fig. 5B). It should be noted that although S-30 displayed a significant increase in sexual motivation it showed the least pharmacologic activity in the uterus. It is noteworthy that treatment with R-23 failed to elicit a significant increase in sexual motivation, indicating the stereoselective and AR-dependent nature of these pharmacologic effects.
Fig. 5.
Effects of SARMs on sexual motivation in OVX female rats. A, effects of TP and SARMs in OVX rats. B, dose-response of S-23 in OVX rats. OVX rats were treated with TP, TP/bicalutamide (TP/R-Bic), or a SARM (3 mg/kg/day) for 14 days. S-23 was also analyzed at 0.5, 0.1, 0.3, 0.5, and 0.75 mg/day for 14 days. A vehicle-treated OVX group was also included as control. Behavioral testing was performed in a three-compartment chamber for 30 min. The duration of time spent in each compartment by the female was recorded. The total time spent with the castrate and intact males is reported. Data are presented as mean ± S.D. ∗, a significant difference (p < 0.05) between the time spent with the intact animal compared with the castrate for each group.
Effects of Treatment on Serum LH and FSH.
Serum LH and FSH in intact female rats measured 0.8 and 8.2 ng/ml, respectively (Table 3). Ovariectomy led to a significant elevation in serum LH (16.9 ng/ml) and FSH (158 ng/ml). At this dose, TP and all SARMs inhibited elevation of LH and measured below that of intact control. TP and S-23 were below the limit of quantitation. Ovariectomy-induced increases in FSH were also inhibited by TP and all SARMs, with the greatest reduction again observed with TP and S-23.
TABLE 3.
Effect of treatment on serum LH and FSH
Ovariectomized rats were treated with TP or a SARM (3 mg/kg/day) for 14 days. Vehicle-treated intact and OVX groups were also included as controls. Serum was collected at necropsy and analyzed for LH and FSH levels. I and O indicate a significant difference between the group and the intact control group or OVX group, respectively, as analyzed by single-factor ANOVA with p < 0.05. Data presented as mean ± S.D. (n = 5/group).
| Group | LH | FSH |
|---|---|---|
| ng/ml | ||
| Intact | 0.8 ± 0.2O | 8.2 ± 2.7O |
| ORX | 16.9 ± 2.4I | 158.4 ± 34.2I |
| Testosterone | <0.0049I,O | 27.8 ± 7.2I,O |
| S-23 | <0.0049I,O | 24.7 ± 5.1I,O |
| S-25 | 0.38 ± 0.2I,O | 34.9 ± 9.9I,O |
| S-26 | 0.43 ± 0.08I,O | 40.2 ± 10.4I,O |
Discussion
Several clinical trials have displayed the beneficial effects of transdermal testosterone formulations for female sexual dysfunction (FSD) in women with low serum testosterone levels. Women in these trials reported improvements in sexual desire, pleasure, and orgasms (Buster et al., 2005; Simon et al., 2005; Shifren et al., 2006; Davis et al., 2008a). However, untoward effects associated with steroidal preparations plague the widespread use of testosterone for many indications, including HSDD. The recent developmental breakthroughs of nonsteroidal SARMs provide an alternative to testosterone for clinical use. SARMs have favorable pharmacokinetic profiles and tissue selectivity, thereby maintaining the anabolic actions of endogenous androgens without the virilizing consequences associated with traditional steroidal androgen therapies.
The structure–activity relationships that define the interaction between novel nonsteroidal ligands and the AR have been extensively studied in our laboratories (Dalton et al., 1998; He et al., 2002; Yin et al., 2003b; Marhefka et al., 2004; Chen et al., 2005b; Kim et al., 2005; Bohl et al., 2008) and many others (Long et al., 2008; Zhao et al., 2008; Zhou et al., 2008; Nirschl et al., 2009). In our quest to develop novel SARMs, innumerable structural modifications were made to the aryl propionamide backbone to optimize in vitro and in vivo activity. Key structural elements include an electron-deficient aromatic A-ring, a methyl group linked to the chiral carbon (S-isomer), an ether linkage, and electronegative substituents in the meta- and/or para-positions of the aromatic B-ring (Table 1). A series of nonsteroidal AR ligands was developed to study the influence of multiple B-ring substitutions on binding affinity and pharmacologic activity.
In the current study, in vitro and in vivo assays were used to assess the potential efficacy of SARMs for FSD. Previous studies revealed the importance of hydrogen bond acceptor groups in the meta- and para-positions of the A-ring (Yin et al., 2003b; Bohl et al., 2004). In addition, previous reports of structure–activity relationships demonstrated hydrophilic electron-withdrawing groups were favorable on the para-position of the B-ring, whereas halogens could be placed at any position (Bohl et al., 2004; Chen et al., 2005b). Therefore, in the current study, structural modifications were made only to the B-ring because it is critical for agonist activity. The inactive isomer of S-23 (R-23) did not bind the AR or induce AR-, ERα-, or ERβ-mediated transcriptional activity, revealing the stereoselective nature of the AR for these ligands. The para-monosubstituted halogen derivatives bound the AR with RBAs ranging from 3.2% for the para-iodo derivative to 12.1% for the para-fluoro derivative, demonstrating that affinity increases with electronegativity (F > Cl > Br > I). Affinity was hindered when the same halogen was added to the meta-position of the B-ring (compare S-24 with S-28 and S-25 with S-29). Binding affinity was negatively influenced by adding extra bulk at the para-position, as evidenced by the acetamide on S-30 and the decreasing affinity as bulk increases on the monosubstituted halogen derivatives (I > Br > Cl > F). Modifying the meta- and para-substituents had a small influence on the ability of each SARM to stimulate AR-mediated transcriptional activation, with maximal transactivation abilities ranging between 80 and 101% for all but one SARM (S-27). In addition, the SARMs displayed minimal cross-reactivity with the ERα and ERβ, which was observed only at high concentrations (i.e., 1 μM). As a whole, in vitro measures of binding affinity and transactivation ability did little to distinguish one compound from another.
The potency of each SARM in androgenic and anabolic tissues is dramatically influenced by modifying B-ring substituents. With the exception of S-23, halogen-substituted SARMs demonstrated partial agonist activity in androgenic tissues and partial (S-24 and S-29) to full agonist activity in anabolic tissues in castrated male rats. S-23 bound the AR with the highest affinity and was the least tissue-selective SARM, displaying full agonist activity in androgenic and anabolic tissues in castrated rats with the dose-response curve in the prostate shifted slightly toward higher doses. The electronegative cyano group of S-22 also displayed high AR binding affinity; however, the monosubstitution offered more tissue selectivity than the disubstituted S-23. The bulky para-acetamide of A-30 significantly reduced AR binding affinity, but was highly tissue-selective and exhibited potent anabolic activity.
A separate study showed that a SARM structurally similar to S-23 when combined with estradiol benzoate is able to restore mating behavior in long-term castrated rats (Jones et al., 2009). Likewise, Miner et al., (2007) reported a bicyclic quinolinone derivative that is able to prevent loss of sexual behavior that occurs after castration. The ability of the nonaromatizable SARMs to support male sexual function speaks to the importance of the AR in this gender. However, the role of androgens in female sexual behavior remains questionable.
The partner-preference paradigm is frequently used to evaluate the proceptive behavior, or soliciting actions, of female rats. The test animal is given the opportunity to choose between a sexually active intact male and either a receptive female or a sexually inactive ORX male (stimulus animals). Edwards and Pfeifle (1983) showed that when OVX rats were primed with estradiol benzoate and progesterone they displayed high levels of proceptive behavior in the presence of sexually active males, but this activity significantly decreased when a sexually inactive male or female was present. Estrogen was administered to elicit sexual behavior; however progesterone is required to facilitate both proceptive and receptive behaviors (Erskine, 1989). Allan et al. (2007) were the first to report the effectiveness of SARMs in female rats, showing that a pyrazoline-derived SARM was able to enhance a female's preference for sexually active males.
As women age, androgen production declines, as does sexual desire and activity. Suppressed circulating free testosterone levels may also result from oral estrogen therapy (Tazuke et al., 1992), glucocorticosteroid administration (Abraham, 1974), chemotherapy, or irradiation. The most accepted indication for transdermal testosterone therapy in women with low circulating testosterone is FSD. The current study was designed to investigate the effects of TP and SARMs on female sexual motivation in the partner-preference paradigm. Reduction of endogenous estrogen and testosterone levels after ovariectomy decreased the female's sexual activity, showing no preference for the intact or castrated male. However, progesterone-primed, SARM-treated rats preferred the company of the intact male, with S-22, S-23 (dose-response), and S-26 being comparable with TP-treated rats, without adversely affecting uterine growth. Females administered low doses (0.05 and 0.1 mg/day) of S-23 displayed a preference for the intact male while maintaining the uterus at less than 40% of intact control. The extent of N-C interaction induced by a ligand seemed to be a reliable predictor of the pharmacologic activity of the ligand in the uterus and the prostate. S-22, S-23, S-25, S-26, and S-27 were the most efficacious in vitro by inducing the N-C interaction at 10 and 100 nM. The same SARMs increased the weight of the uterus and prostate to the greatest extent. It is noteworthy that growth observed after TP and SARM administration seems to be caused by growth of the myometrium, the smooth muscle of the uterus, and not the endometrium. In addition, in vivo pharmacologic activity in male and female rats is highly correlated, with a correlation coefficient of 0.94 (p = 0.0002) for uterine and prostatic activity.
S-25 and S-26 similarly maintained the weight of the uterus in females and prostate and levator ani muscle in male rats. However, S-26 maintained sexual motivation, whereas S-25 did not. Serum LH and FSH levels were measured to determine whether this discrepancy could be caused by their ability (or inability) to penetrate the central nervous system. However, S-25 and S-26 inhibited OVX-induced elevations in LH and FSH to a similar extent; therefore, lack of central nervous system penetration does not explain the inability of S-25 to maintain sexual motivation.
There does not seem to be a correlation between B-ring substitutions and the ability of the SARMs to support female sexual motivation. The cyano-substituted SARM (S-22) and the mixed halogen-substituted SARM (S-23) bind the AR with high affinity, possess potent anabolic activity, and enhance sexual motivation in female rats. In addition, monosubstituted halogen-containing SARMs bind the AR with varying affinities and display a wide range of in vivo activities. However, the ability of S-23 and the inability of S-24 to enhance sexual motivation, coupled with their activity in castrated male rats, suggest that the ability of SARMs to enhance sexual motivation may be associated with their androgenic and anabolic pharmacologic properties. Most SARMs improved sexual motivation, with potency and efficacy comparable with that of TP. Estrogen is an active metabolite of testosterone, but it is not clear whether the actions of testosterone in women are predominantly mediated via the AR or after aromatization via the ER. However, SARMs are nonaromatizable and do not cross-react with the ER, indicating that the AR plays an important role in female libido. Coadministration of TP with an antiandrogen-reduced sexual preference of the female for an intact male also implies that the AR is involved in female sexual behavior. These results suggest that SARMs could be an effective therapy for FSD.
In conclusion, the current studies examined the binding affinity and activity of a series of nonsteroidal SARMs. Structural modifications to the meta- and para-positions of the B-ring resulted in a range of in vitro AR binding affinities and functional activities. With the exception of S-23, all of the SARMs tested were prostate-sparing, selectively maintaining the size of the levator ani muscle in castrated male rats. Most nonsteroidal SARMs improved sexual motivation of OVX female rats in a partner-preference paradigm, comparable with TP, while maintaining the uterine weights at less than 50% of intact control. These studies indicate that it is possible to develop a SARM that enhances sexual motivation and is selective but does not adversely effect proliferation of the endometrium (e.g., S-30). SARMs, with many advantages over traditional steroidal androgen preparations, could be beneficial in the clinical treatment of hypoactive sexual desire disorder.
Acknowledgments
We thank Terry Costello, Stacey Barnett, and Katie Kail for help with the animal studies and Dr. Anand Kulkarni of the University of Tennessee Health Science Center for help with the histology of uterine samples.
This work was supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases [Grant 1 RO1 DK59800-08].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.168880.
- SARM
- selective androgen receptor modulator
- AR
- androgen receptor
- ER
- estrogen receptor
- FSH
- follicle-stimulating hormone
- LH
- luteinizing hormone
- DHT
- dihydrotestosterone
- MIB
- mibolerone
- CMV
- cytomegalovirus
- TP
- testosterone propionate
- E2
- estradiol
- PEG300
- polyethylene glycol 300
- HSDD
- hypoactive sexual desire disorder
- OVX
- ovariectomized
- ORX
- orchidectomized
- ANOVA
- analysis of variation
- N-C
- amino terminus–carboxyl terminus
- DMSO
- dimethyl sulfoxide
- LBD
- ligand binding domain
- GST
- glutathione S-transferase
- PBS
- phosphate-buffered saline
- FSD
- female sexual dysfunction
- RBA
- relative binding affinity
- S-22
- S-3-(4-cyanophenoxy)-2-hydroxy-2-methyl-N-(4-cyano-3-trifluromethylphenyl)-propionamide
- S-23
- (S)-N-(4-cyano-3-trifluoromethyl-phenyl)-3-(3-fluoro, 4-chlorophenoxy)-2-hydroxy-2-methyl-propanamide
- S-24
- (S)-N-(4-cyano-3-trifluoromethyl-phenyl)-3-(4-fluorophenoxy)-2-hydroxy-2-methyl-propanamide
- S-25
- (S)-N-(4-cyano-3-trifluoromethyl-phenyl)-3-(4-chlorophenoxy)-2-hydroxy-2-methyl-propanamide
- S-26
- S-3-(4-bromophenoxy)-2-hydroxy-2-methyl-N-(4-cyano-3-trifluoromethyl-phenyl)-propionamide
- S-27
- S-3-(4-iodophenoxy)-2-hydroxy-2-methyl-N-(4-cyano-3-trifluoromethyl-phenyl)-propionamide
- S-28
- S-3-(3,4-difluorophenoxy)-2-hydroxy-2-methyl-N-(4-cyano-3-trifluoromethyl-phenyl)-propionamide
- S-29
- S-3-(3,4-dichloro-phenoxy)-2-hydroxy-2-methyl-N-(4-cyano-3-trifluoromethyl-phenyl)-propionamide
- S-30
- S-3-(4-acetamidophenoxy)-2-hydroxy-2-methyl-N-(4-cyano-3-trifluoromethyl-phenyl)-propionamide
- R-23
- (R)-N-(4-cyano-3-trifluoromethyl-phenyl)-3-(3-fluoro,4-chlorophenoxy)-2-hydroxy-2-methyl-propanamide.
References
- Abraham GE. (1974) Ovarian and adrenal contribution to peripheral androgens during the menstrual cycle. J Clin Endocrinol Metab 39:340–346 [DOI] [PubMed] [Google Scholar]
- Allan GF, Tannenbaum P, Sbriscia T, Linton O, Lai MT, Haynes-Johnson D, Bhattacharjee S, Zhang X, Sui Z, Lundeen SG. (2007) A selective androgen receptor modulator with minimal prostate hypertrophic activity enhances lean body mass in male rats and stimulates sexual behavior in female rats. Endocrine 32:41–51 [DOI] [PubMed] [Google Scholar]
- Blasberg ME, Robinson S, Henderson LP, Clark AS. (1998) Inhibition of estrogen-induced sexual receptivity by androgens: role of the androgen receptor. Horm Behav 34:283–293 [DOI] [PubMed] [Google Scholar]
- Bohl CE, Chang C, Mohler ML, Chen J, Miller DD, Swaan PW, Dalton JT. (2004) A ligand-based approach to identify quantitative structure–activity relationships for the androgen receptor. J Med Chem 47:3765–3776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohl CE, Gao W, Miller DD, Bell CE, Dalton JT. (2005) Structural basis for antagonism and resistance of bicalutamide in prostate cancer. Proc Natl Acad Sci USA 102:6201–6206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohl CE, Wu Z, Chen J, Mohler ML, Yang J, Hwang DJ, Mustafa S, Miller DD, Bell CE, Dalton JT. (2008) Effect of B-ring substitution pattern on binding mode of propionamide selective androgen receptor modulators. Bioorg Med Chem Lett 18:5567–5570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buster JE, Kingsberg SA, Aguirre O, Brown C, Breaux JG, Buch A, Rodenberg CA, Wekselman K, Casson P. (2005) Testosterone patch for low sexual desire in surgically menopausal women: a randomized trial. Obstet Gynecol 105:944–952 [DOI] [PubMed] [Google Scholar]
- Chen J, Hwang DJ, Bohl CE, Miller DD, Dalton JT. (2005a) A selective androgen receptor modulator for hormonal male contraception. J Pharmacol Exp Ther 312:546–553 [DOI] [PubMed] [Google Scholar]
- Chen J, Hwang DJ, Chung K, Bohl CE, Fisher SJ, Miller DD, Dalton JT. (2005b) In vitro and in vivo structure–activity relationships of novel androgen receptor ligands with multiple substituents in the B-ring. Endocrinology 146:5444–5454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton JT, Mukherjee A, Zhu Z, Kirkovsky L, Miller DD. (1998) Discovery of nonsteroidal androgens. Biochem Biophys Res Commun 244:1–4 [DOI] [PubMed] [Google Scholar]
- Davis S, Papalia MA, Norman RJ, O'Neill S, Redelman M, Williamson M, Stuckey BG, Wlodarczyk J, Gardner K, Humberstone A. (2008a) Safety and efficacy of a testosterone metered-dose transdermal spray for treating decreased sexual satisfaction in premenopausal women: a randomized trial. Ann Intern Med 148:569–577 [DOI] [PubMed] [Google Scholar]
- Davis SR, McCloud P, Strauss BJ, Burger H. (2008b) Testosterone enhances estradiol's effects on postmenopausal bone density and sexuality. Maturitas 61:17–26 [DOI] [PubMed] [Google Scholar]
- Dennerstein L, Dudley E, Burger H. (2001) Are changes in sexual functioning during midlife due to aging or menopause? Fertil Steril 76:456–460 [DOI] [PubMed] [Google Scholar]
- Edwards DA, Pfeifle JK. (1983) Hormonal control of receptivity, proceptivity, and sexual motivation. Physiol Behav 30:437–443 [DOI] [PubMed] [Google Scholar]
- Erskine MS. (1989) Solicitation behavior in the estrous female rat: a review. Horm Behav 23:473–502 [DOI] [PubMed] [Google Scholar]
- Fisher SJ. (2004) Identification of Structure–Activity Relationships of Selective Androgen Receptor Modulators in Preclinical Animal Models. Master's thesis, Ohio State University, Columbus, OH [Google Scholar]
- Gao W, Reiser PJ, Coss CC, Phelps MA, Kearbey JD, Miller DD, Dalton JT. (2005) Selective androgen receptor modulator treatment improves muscle strength and body composition and prevents bone loss in orchidectomized rats. Endocrinology 146:4887–4897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Yin D, Perera M, Kirkovsky L, Stourman N, Li W, Dalton JT, Miller DD. (2002) Novel nonsteroidal ligands with high binding affinity and potent functional activity for the androgen receptor. Eur J Med Chem 37:619–634 [DOI] [PubMed] [Google Scholar]
- Hiort O. (2002) Androgens and puberty. Best Pract Res Clin Endocrinol Metab 16:31–41 [DOI] [PubMed] [Google Scholar]
- Jones A, Chen J, Hwang DJ, Miller DD, Dalton JT. (2009) Preclinical characterization of a (S)-N-(4-cyano-3-trifluoromethyl-phenyl)-3-(3-fluoro,4-chlorophenoxy)-2-hydroxy-2-methyl-propanamide: a selective androgen receptor modulator for hormonal male contraception. Endocrinology 150:385–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearbey JD, Gao W, Narayanan R, Fisher SJ, Wu D, Miller DD, Dalton JT. (2007) Selective androgen receptor modulator (SARM) treatment prevents bone loss and reduces body fat in ovariectomized rats. Pharm Res 24:328–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. (2006) Pharmacokinetics, Metabolism, and Pharmacologic Activities of Nonsteroidal Selective Androgen Receptor Modulators and Their Potential Application to Osteoporosis. Dissertation, Ohio State University, Columbus, OH [Google Scholar]
- Kim J, Wu D, Hwang DJ, Miller DD, Dalton JT. (2005) The para substituent of S-3-(phenoxy)-2-hydroxy-2-methyl-N-(4-nitro-3-trifluoromethyl-phenyl)-propionamides is a major structural determinant of in vivo disposition and activity of selective androgen receptor modulators. J Pharmacol Exp Ther 315:230–239 [DOI] [PubMed] [Google Scholar]
- Long YO, Higuchi RI, Caferro TR, Lau TL, Wu M, Cummings ML, Martinborough EA, Marschke KB, Chang WY, López FJ, et al. (2008) Selective androgen receptor modulators based on a series of 7H-[1,4]oxazino[3,2-g]quinolin-7-ones with improved in vivo activity. Bioorg Med Chem Lett 18:2967–2971 [DOI] [PubMed] [Google Scholar]
- Marhefka CA, Gao W, Chung K, Kim J, He Y, Yin D, Bohl C, Dalton JT, Miller DD. (2004) Design, synthesis, and biological characterization of metabolically stable selective androgen receptor modulators. J Med Chem 47:993–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JN, Chang W, Chapman MS, Finn PD, Hong MH, López FJ, Marschke KB, Rosen J, Schrader W, Turner R, et al. (2007) An orally active selective androgen receptor modulator is efficacious on bone, muscle, and sex function with reduced impact on prostate. Endocrinology 148:363–373 [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Kirkovsky L, Yao XT, Yates RC, Miller DD, Dalton JT. (1996) Enantioselective binding of Casodex to the androgen receptor. Xenobiotica 26:117–122 [DOI] [PubMed] [Google Scholar]
- Nathorst-Böös J, von Schoultz B. (1992) Psychological reactions and sexual life after hysterectomy with and without oophorectomy. Gynecol Obstet Invest 34:97–101 [DOI] [PubMed] [Google Scholar]
- Nirschl AA, Zou Y, Krystek SR, Jr, Sutton JC, Simpkins LM, Lupisella JA, Kuhns JE, Seethala R, Golla R, Sleph PG, et al. (2009) N-aryl-oxazolidin-2-imine muscle selective androgen receptor modulators enhance potency through pharmacophore reorientation. J Med Chem 52:2794–2798 [DOI] [PubMed] [Google Scholar]
- Schmidt C. (1994) Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association, Washington, DC [Google Scholar]
- Sherwin BB, Gelfand MM. (1987) The role of androgen in the maintenance of sexual functioning in oophorectomized women. Psychosom Med 49:397–409 [DOI] [PubMed] [Google Scholar]
- Shifren JL, Davis SR, Moreau M, Waldbaum A, Bouchard C, DeRogatis L, Derzko C, Bearnson P, Kakos N, O'Neill S, et al. (2006) Testosterone patch for the treatment of hypoactive sexual desire disorder in naturally menopausal women: results from the INTIMATE NM1 Study. Menopause 13:770–779 [DOI] [PubMed] [Google Scholar]
- Simon J, Braunstein G, Nachtigall L, Utian W, Katz M, Miller S, Waldbaum A, Bouchard C, Derzko C, Buch A, et al. (2005) Testosterone patch increases sexual activity and desire in surgically menopausal women with hypoactive sexual desire disorder. J Clin Endocrinol Metab 90:5226–5233 [DOI] [PubMed] [Google Scholar]
- Tazuke S, Khaw KT, Barrett-Connor E. (1992) Exogenous estrogen and endogenous sex hormones. Medicine (Baltimore) 71:44–51 [DOI] [PubMed] [Google Scholar]
- Yin D, Gao W, Kearbey JD, Xu H, Chung K, He Y, Marhefka CA, Veverka KA, Miller DD, Dalton JT. (2003a) Pharmacodynamics of selective androgen receptor modulators. J Pharmacol Exp Ther 304:1334–1340 [DOI] [PubMed] [Google Scholar]
- Yin D, He Y, Perera MA, Hong SS, Marhefka C, Stourman N, Kirkovsky L, Miller DD, Dalton JT. (2003b) Key structural features of nonsteroidal ligands for binding and activation of the androgen receptor. Mol Pharmacol 63:211–223 [DOI] [PubMed] [Google Scholar]
- Yin D, Xu H, He Y, Kirkovsky LI, Miller DD, Dalton JT. (2003c) Pharmacology, pharmacokinetics, and metabolism of acetothiolutamide, a novel nonsteroidal agonist for the androgen receptor. J Pharmacol Exp Ther 304:1323–1333 [DOI] [PubMed] [Google Scholar]
- Zhao S, Shen Y, van Oeveren A, Marschke KB, Zhi L. (2008) Discovery of a novel series of nonsteroidal androgen receptor modulators: 5- or 6-oxachrysen-2-ones. Bioorg Med Chem Lett 18:3431–3435 [DOI] [PubMed] [Google Scholar]
- Zhou C, Wu G, Feng Y, Li Q, Su H, Mais DE, Zhu Y, Li N, Deng Y, Yang D, et al. (2008) Discovery and biological characterization of a novel series of androgen receptor modulators. Br J Pharmacol 154:440–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zussman L, Zussman S, Sunley R, Bjornson E. (1981) Sexual response after hysterectomy-oophorectomy: recent studies and reconsideration of psychogenesis. Am J Obstet Gynecol 140:725–729 [DOI] [PubMed] [Google Scholar]