Abstract
The HIV protein Nef is thought to mediate immune evasion and promote viral persistence in part by down-regulating major histocompatibility complex class I protein (MHC-I or HLA-I) from the cell surface. Two different models have been proposed to explain this phenomenon as follows: 1) stimulation of MHC-I retrograde trafficking from and aberrant recycling to the plasma membrane, and 2) inhibition of anterograde trafficking of newly synthesized HLA-I from the endoplasmic reticulum to the plasma membrane. We show here that Nef simultaneously uses both mechanisms to down-regulate HLA-I in peripheral blood mononuclear cells or HeLa cells. Consistent with this, we found by using fluorescence correlation spectroscopy that a third of diffusing HLA-I at the endoplasmic reticulum, Golgi/trans-Golgi network, and the plasma membrane (PM) was associated with Nef. The binding of Nef was similarly avid for native HLA-I and recombinant HLA-I A2 at the PM. Nef binding to HLA-I at the PM was sensitive to specific inhibition of endocytosis. It was also attenuated by cyclodextrin disruption of PM lipid micro-domain architecture, a change that also retarded lateral diffusion and induced large clusters of HLA-I. In all, our data support a model for Nef down-regulation of HLA-I that involves both major trafficking itineraries and persistent protein-protein interactions throughout the cell.
Keywords: Adapter Proteins, Cell Surface Receptor, Endocytosis, Histocompatibility, Human Immunodeficiency Virus, FCS, FRAP, Protein Sorting
Introduction
Viral persistence is a fundamental feature of HIV infection; it is due to an ineffective immune response induced directly by the virus. Multiple mechanisms have been proposed to explain this, including depletion of and immune evasion by HIV-infected CD4+ T cells. The HIV-encoded membrane protein Nef is thought to be an important mediator of immune evasion (1). The underlying mechanisms of Nef immunomodulation have not been fully delineated but appear to involve Nef-induced down-regulation of major immune cell receptors from the plasma membrane, including CD4, MHC-I and -II, CD28, and DC-SIGN (2–5). Nef-dependent down-regulation of MHC-I may be particularly important because this may impede clearance of HIV-infected cells by MHC-restricted CD8+ cytotoxic T lymphocytes (1, 6 and references therein). Animal viruses have evolved different strategies to subvert nearly every step in the assembly, maturation, trafficking, and antigen presentation by MHC-I (7, 8). The study of how Nef perturbs trafficking of MHC-I and other immune receptors is important because it may provide new insights into both basic mechanisms regulating HIV persistence and general mechanisms of antigen presentation, as well as offer new strategies for vaccine development. Knowledge of Nef mechanisms may help guide therapeutic schemes based on the decryption of persistently infected clones.
Genetic and biochemical studies have shown that at least three distinct subdomains of Nef are required for down-regulating HLA-I as follows: an N-terminal α-helix with a conserved Met at position 20 (Met-20), an SH3-binding polyproline motif 72PXXP75, and an acidic domain, 62EEEE65 (9–12). However, a consensus has not yet been established at either the molecular or cellular level for how Nef down-regulates HLA-1. Data supporting defective retrograde receptor trafficking, also known as recycling, by internalization of the receptor from the plasma membrane and/or return of internalized receptors to the plasma membrane have been published (3, 10, 13). In contrast, there is evidence for defective anterograde trafficking in which the newly synthesized MHC-1 complex is misrouted in an AP1-dependent manner from the TGN3 to endolysosomes for premature degradation (14–16).
The two models envision different roles for the three functional motifs of Nef with regard to HLA-I down-regulation. In the retrograde model, the three motifs of Nef are required in a hierarchical manner as follows: 1) for binding to PACS-2 in the TGN through the 62EEEE65 motif; 2) for binding and activation of a TGN-localized Src family kinase through the 72PXXP75 motif; and 3) for inhibiting HLA-I recycling through the Met-20 motif (10). In contrast, the anterograde mechanism postulates that the same three Nef motifs are required for facilitating and stabilizing a ternary complex between Nef, the cytoplasmic tail of HLA-I and AP1 adapter (17, 18). Importantly, the proponents of this model affirm that Nef does not bind to MHC-I at the cell surface (14) and that Nef mutants that lack an effect on MHC-I do not bind the receptor (19). A significant limitation in studies supporting both viewpoints is that the binding analyses did not involve live cell conditions to establish subcellular distribution but rather steady-state interactions in cell lysates.
These models are not mutually exclusive, and they have not been evaluated simultaneously in the same cell systems. Aberrant MHC-I trafficking as proposed by each model may have a different immunological outcome. If Nef were to exclusively disrupt the anterograde transport of nascent MHC-I, no HIV-I antigens will be presented for developing a cytotoxic T lymphocyte repertoire. If, however, the defect lies in the retrograde transport, the reduced levels of HIV antigen loaded MHC-I at the cell surface may compromise cytotoxic T lymphocyte surveillance and killing of infected cells. In this work, we have addressed the gaps in the knowledge on how Nef may impact MHC-I traffic through a combined biochemical, biophysical, and cell biological study of Nef influence on native and recombinant HLA-I trafficking in human PBMCs, the human T cell line Jurkat, and the epithelial cell line HeLa.
EXPERIMENTAL PROCEDURES
Cells and Recombinant DNA Constructs
Nef alleles and selected Nef mutant cDNAs were PCR-amplified from the respective HIV and simian immunodeficiency proviruses or other recombinant plasmids and cloned into a pCG vector with an HA tag at the 3′ end. NL4-3 Nef and a null mutant, NX (20), were also cloned in a bicistronic pIRES vector (Clontech) upstream of GFP ORF. Rous sarcoma virus LTR-linked HLA-I A2 (RSV2 Neo backbone) was a gift from Eric Long, NIAID, National Institutes of Health. The A2 ORF was PCR-amplified and subsequently cloned into a CMV promoter-like plasmid. GFP/YFP-tagged dominant-negative and constitutively active mutants of endocytic adapters and effectors have been described (21) Expression plasmids for Cerulean or Venus fluorescent proteins fused to the C terminus of Nef (Nef-CerFP), HLA-I A2 (A2-VenusFP), WT, or L413A/L414A mutant CD4 were constructed by inserting the PCR-amplified Nef, HLA-I A2, WT, or L413A/L414A CD4 ORFs between the BglII and HindIII sites of p(eCFP), p(eYFP), pCerulean A206K-N1, or pVenus A206K-N1 plasmids.
Chemicals and Enzymes
Ikarugamycin was from AXXORA LLC, San Diego. Methyl-β-cyclodextrin was from Cyclodextrin Corp. Endoglycosidases were from New England Biolabs, Beverly, MA.
Antibodies
The following reagents were obtained from commercial sources: murine mAbs against γ-, δ-, and ϵ-adaptins, CD63, CD71 (transferrin receptor); early endosomal antigen-1 (EEA1); FITC-conjugated anti-clathrin heavy chain (CHC) (BD Immunocytometry, San Diego); unconjugated Alexa 488, phycoerythrin, or allophycocyanin-conjugated CD4 and CD8, anti-GOLGIN-97 (Invitrogen); unconjugated or biotinylated polytropic anti-HLA-I mAbs B9.12.1 (Beckman Coulter, CA); W/632 or anti-HLA-I A2 mAb, BB7.2 (Serotec, New York); against CHC, α- and γ-adaptin, Na+/K+-ATPase, and mannose 6-phosphate receptor (Affinity Bioreagents); against Arf6 and ARNO-GEF (Abcam); against LAMP1 (H4A3) and LAMP2 (H4B4) (Developmental Studies Hybridoma Bank, University of Iowa); rabbit polyclonal antibodies against β-COP, EEA1, furin (Affinity Bioreagents); CHC, γ-adaptin and PACS-1 (Abcam); CD71 (TfnR) (Zymed Laboratories Inc.); goat anti-actin (Santa Cruz Biotechnology) and sheep anti-TGN46 (Serotec). Murine mAb against AP3 σ3 subunit (22) was from Juan Bonifacino of NICHD, National Institutes of Health, and purified rabbit antibody against AP1 μ1 chain was from Linton Traub of the University of Pittsburgh. Rabbit anti-PACS1 antisera 18193 and 17703 were from Gary Thomas of Vollum Institute, Portland, OR. Secondary antibodies to mouse, rabbit, goat, sheep, and human IgG conjugated to various Alexa dyes or Texas Red and dye-conjugated streptavidins or neutravidins were purchased from Invitrogen and HRP-conjugated goat anti-murine, -rabbit, or -human IgG and donkey anti-goat IgG were from Pierce.
Cell Culture and Transfections
HeLa cells were transfected as described previously (21). Peripheral blood lymphocytes isolated from lymphocyte-rich leukopaks were provided by the Department of Transfusion Medicine at the Clinical Center, National Institutes of Health, as described previously. Peripheral blood lymphocytes and T cell lines were transfected using an Amaxa Biosystems nucleofector with the recommended reagents (20, 21).
Flow Cytometry
General protocols of flow cytometry using HeLa cell transfectants and primary T cells and lines have been described before (20, 21).
Drug Treatments
Ikarugamycin treatment was at 4, 5, or 7 μm for 2 h at 37 °C followed by washing in growth medium just before harvesting. The cells were at room temperature for TPTCFCCS experiments no longer than 30 min. The potency of ikarugamycin to inhibit classical clathrin-dependent endocytic processes was carefully titered in different cell types as described under the “Results” and in figure legends. For MβCD treatment, cells were treated at room temperature for 20–30 min with MβCD (10% in Hanks' balanced salt solution) and washed twice with RPMI 1640 medium. Removal of cholesterol was corroborated with filipin staining as described before (21). TPTCCS and FRAP analyses were done before and after treatment. Cells were incubated in Alexa 647 mAb (W632 and BB7.2) diluted 1:50 for 30 min at room temperature and washed three times with serum containing BSA. The cells were at room temperature no longer than 1 h.
Immunofluorescence Microscopy
Twenty four hours after transfection, HeLa cells grown on glass coverslips were stained for immunofluorescence microscopy. Jurkat cells, grown in suspension, were attached to fibronectin-coated coverslips. Cells were processed for microscopy as described previously (20).
RNA-mediated Interference
RNA-mediated interference of clathrin, PACS-1, or vps35 was performed using small interfering RNA duplexes (siRNAs) of the following sequence: CHC, GCUGGGAAAACUCUUCAGA or UAAUCCAAUUCGAAGACCAAU; AP1 μ chain, GGCAUCAAGUAUCGGAAGA; AP2 μ chain, GUGGAUGCCUUUCGGGUCA; AP2α chain, GAGCAUGUGCACGCUGGCCA; AP3 μ chain, GGAGAACAGUUCUUGCGGC; AP3 δ chain, GGACGAGGCAAAAUACAUA; PACS-1, CUCAGUGGUCAUCGCUGUGAA, GAUCGUCCUUCCAGCUAGU, or GACGAAGAUCUCCGGAAAG; and vps35, GGUCCAGUCAUUCCAAAUG. The AP1 γ subunit, Arf6, and ARNO-GEF were knocked down using the respective on-target Smart Pool siRNAs from Dharmacon. HeLa cells were transfected twice at 72-h intervals with 200 nm of the siRNAs, using Oligofectamine (Invitrogen). After 144 h of siRNA treatment, the cells were transfected as described above for expression of Nef, CD4, and CD8. Human T cell lines and peripheral blood lymphocytes were nucleofected once with 100 nm siRNA using the Amaxa system. The cells were rested for 24–36 h before nucleofecting with an additional 100 nm siRNA and expression plasmids as indicated in the relevant figure legends.
TPTCFCCS
For TPTCFCCS experiments, low level expression was achieved by using 1/4th the regular amount of plasmid DNAs and limiting expression to 4–6 h. For visualizing ER, Golgi, or the plasma membrane, transfectants were stained at 25 °C for 30 min with ER-Tracker Red, BODIPY Texas Red-ceramide, or wheat germ agglutinin conjugates (Invitrogen) and used within the next 30–45 min.
Two-photon imaging and TPTCFCCS measurements were carried out using a modified Alba II system (ISS Inc.) in the Ultrafast Laser Microscopy facility of NHLBI, National Institutes of Health. The excitation source was a 100-fs pulsed tunable titanium sapphire laser (MaiTai®, Spectra-Physics®, Newport). The wavelength was set to 920 nm for the HLA-I A2-Venus/eYFP (HLA-I A2-V) and Nef-Cerulean (Nef-CerFP) experiments. The excitation power was set to <6 milliwatts at the microscope entrance. The microscope was a Zeiss Axiovert 135 M using an E750SP 2P dichroic filter (Chroma Inc.) to eliminate the IR exciting light. The objective was a ×100 Plan-NeoFluar oil objective (Zeiss Inc.) with NA 1.3. A piezo scanner (100 × 100 μm travel, Mad City Labs, Inc.) and a z-scanning PIFOC (100 μm, Mad City Labs, Inc.) controlled by the ISS software was used to image HeLa cells at a resolution of 0.2 or 1.0 ms/pixel.
For the HLA-I A2-Venus and Nef-CerFP experiments, a 515-nm dichroic mirror was used to split the detected light onto two channels. Additional 580 ± 30 nm bandpass emission filters were placed before channel 1 (eYFP/Venus channel) to minimize the contribution of the Cerulean signal. For Cerulean detection, a 470 ± 40-nm filter was used in channel 2 (Nef-CerFP channel). For excitation of the organelle-specific dyes at 850 nm, a 630 LP filter was used in channel 1 instead of the 580 nm bandpass used for eYFP detection.
Confocal volume was calibrated by measuring the diffusion of rhodamine 110 and Alexa 488 after excitation at 920 nm. The beam waist, wo, was found to be 0.3 μm, whereas the axial waist zo was 1.4 μm. A diffusion coefficient for Cerulean of 14 ± 4 μm2/s was obtained in the cytoplasm at room temperature. Using these filters and 920 nm excitation (favors eYFP and Venus over Cerulean and eCFP), there was less than 5% bleed through with little to no cross-correlation in the eYFP/Venus channel arising from eCFP/Cerulean. The diffusion of Nef-CerFP was found to be 8.7 ± 2.8 μm2/s in the cytoplasm. Other controls included measuring the diffusion coefficient of cells co-transfected with Cerulean- and Venus-tagged LL/AA CD4 mutant (0.90 ± 0.7 μm2/s). This value can be compared with diffusion coefficient of Venus-tagged CD4LA alone, 1.05 ± 0.37 μm2/s. Other controls included Nef-CerFP by itself at the PM, 3.4 ± 2.1 μm2/s. Also, we measured a two-component diffusion of HA2Y at the plasma membrane, 6.0 ± 0.8 and 0.21 ± 0.06 μm2/s.
For the MβCD and ikarugamycin experiments, a 570 LP filter was used instead of the 560 ± 30 nm bandpass filter to capture as much as possible of the VenusFP signal. For the Alexa 647-labeled antibodies for HLA experiments, a 660 dichroic filter (Semrock Inc.) was used to separate the Cerulean and Alexa 647 channels. The excitation wavelength was 850 nm for experiments involving Alexa 647-labeled antibodies against HLA-I-ABC (HA) or HLA-I-A2 (HA2) and Nef-CerFP. A 692 ± 40 nm filter (Semrock Inc.) was used in channel 1 to detect the Alexa 647 signal in this case. The same filter mentioned above was used for Cerulean detection in these experiments. The calibration at 850 nm yielded a focal volume with waists wo = 0.25 μm and zo = 1.6 μm. The molecular weight of the monomeric antibody was about 150 kDa with a diffusion coefficient in water of 56 ± 16 μm2/s.
Data were acquired at a sampling rate of 100 kHz with the ISS Alba II system. The cells were imaged at 0.2 or 1.0 ms/pixel and up to three locations per cell, and data from at least 5 or 6 cells from each of 3–6 independent transfections were selected for TPTCFCCS acquisition. Each point was observed sequentially five times lasting 15–20 s. The cells were analyzed 4–5 h after transfection, when protein levels are low and only weak fluorescent protein images are possible. The experiments were repeated 5–6 times on different days. The correlation function is calculated from the intensity trace F(t) as shown in Equation 1 (23),
 |
where δFi(t) = Fi(t) −〈 Fi(t)〉 and the 〈 〉 denote a time average. The autocorrelation function is obtained when i = j and the cross-correlation when i ≠ j. The resulting autocorrelation function traces were averaged and fit as described below. The fitting of the ACFs was done using the Vista software (ISS Inc.) with a three-dimensional diffusion model for the TGN and the ER with one diffusing species. For the PM experiments, topology required a two-dimensional diffusion model with one or two components. Equation 2 used for the three-dimensional fitting is shown below (24),
 |
Veff is the effective volume; C is the concentration; Di is the diffusion coefficient of the ith species; and ωo and zo are the radial and axial 1/e2 fitting parameters. At τ = 0, the Go is inversely proportional to the number of particles present in the volume. For two-dimensional diffusing species, the equation reduces to Equation 3 (24),
 |
where ρ is the particle number per area, and ∑i=1n fi = 1 with fi denotes the fraction of the ith population. The percent bound was calculated from the Go ratio of the limiting concentration of the autocorrelation function and that obtained for the cross-correlation function.
Fluorescence Recovery after Photobleaching (FRAP) Assay
HeLa cell transfectants in 2-well Lab-Tek chambers (Nunc Corp.) were imaged on a Leica TCS SP5 confocal microscope with an Olympus Plan-Apochromat 63×oil immersion objective. Transfections were at 37 °C for 12 h using 0.5 μg each of HLA-A2 or CD4 and Nef fusion plasmids per 105 cells on each of 2-well Titer-Tek culture chambers. For the FRAP assay, ER and Golgi were visualized by co-transfecting 0.5 μg of pDsRed-ER or pDsRed-Golgi monomer. Plasma membrane was stained with Alexa 647-conjugated wheat germ agglutinin. Cerulean was excited at 405 nm by a UV laser with the emission detected at a 475-nm detector; Venus, DsRed, and Alexa 647 were excited at 514, 567, or 633 nm of an argon laser with the respective emissions detected at a 528-, 605-, or 647-nm detector. Prior to imaging, the cultures were put aside at 25 °C for 1 h, and the cells were imaged up to 30–45 min after the addition of organelle-specific dyes.
FRAP data were acquired in resonance scan mode using the FRAP Wizard (Leica Instruments, Malvern, PA) software. Scan frequency was set at 8000 Hz, and the argon laser power was adjusted to 40% with the confocal pinhole set at 2 μm. Imaging was acquired in a 512 × 80 pixel format with low laser intensity excitation. Fly mode was selected for binning the FRAP image series. Photobleaching of Venus-FP in a typical region of interest (ROI) of 1 μm (h) × 1 μm (w) was performed at 514 nm by 1 scan at 0.078 s at full laser power. 10 scans at 0.078-s intervals were acquired before bleaching and post-bleaching image acquisition was set for 200 scans at a 0.078-s interval. Two ROI were set in the ER, Golgi, or plasma membrane of each cell, one ROI for image acquisition post-photobleaching, and the other ROI was set as nonbleached control. In each cell, fluorescence recovery data collected in bleached ROI were normalized against values collected in nonbleached ROI and averaged for values from 5 to 10 cells corresponding to 3–5 independent experiments. The data were also normalized to the loss of signal due to the bleach pulse.
The Origin Pro software (version 7, OriginLabs Inc.) was used to generate the fitted curve and t-half (t½) from the normalized fluorescence recoveries. FRAP data for HLA-I A2-Venus recovery were fit to a two component exponential decay given by Equation 4,
where t1 and t2 are the diffusion times; A1 and A2 are the pre-exponential factors, and y0 is F, the immobile fraction. The diffusion coefficients were calculated according to Axelrod et al. (25) given by τD = ω2γ/4D (26).
Statistical Analysis
Biochemical and immunological experiments were done in duplicate. Quantitative microscopic experiments were done in duplicate three times or more as indicated under the appropriate sections. Results are expressed as mean ± S.E. Significance levels of differences in means were determined by unpaired two-tailed Student's t test using Prism5 or Microsoft EXCEL.
RESULTS
Nef Down-regulates MHC-I with Variable Efficacy in Different Cell Types
Recombinant Nef was able to down-regulate native MHC-I and recombinant HLA-I A2 and other HLA-I alleles in epithelial and hematopoietic cells but with variable efficacy. In particular, Nef was less effective in HeLa cells than in the T cell line Jurkat or in PBMCs. Differences in efficacy for down-regulation of native versus recombinant HLA-I in T cells and epithelial cells were observed among many different Nef alleles derived from clinical isolates of HIV-1 and simian immunodeficiency virus. The variability appears to be due to cellular factors, because in all cases all Nef alleles tested had the same efficacy in any given cell type (supplemental Results, supplemental Table 1, and supplemental Figs. 1 and 2).
HIV-1 Nef Down-regulation of Native HLA-I Relies on Multiple Molecular Mediators of Endocytosis
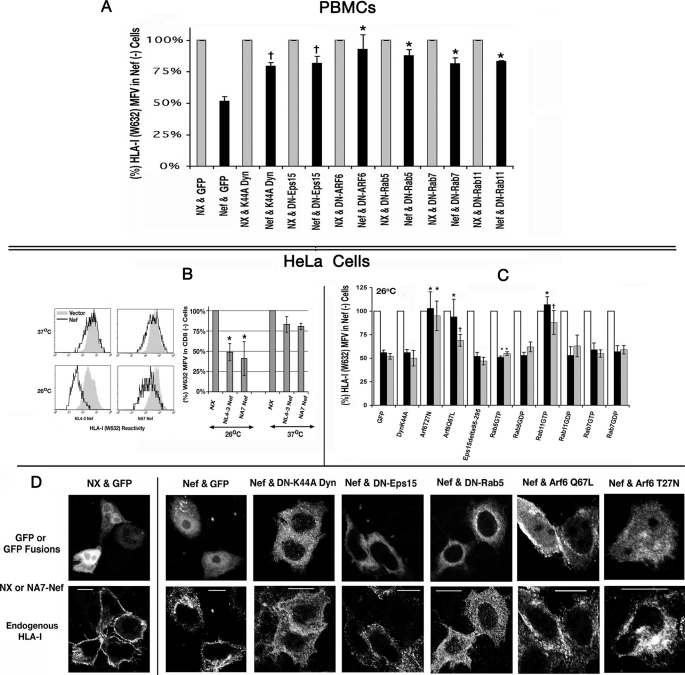
We evaluated the relative contribution of endocytosis in Nef-induced HLA-I down-regulation in PBMCs using two types of genetic inhibitors of endocytic trafficking as follows: 1) GFP- or YFP-tagged dominant-negative inhibitor forms of vesicular GTPases (GDP-bound) or constitutively active mutants (GTP-bound) of the GTPases dynamin, Eps15, Rab5, Rab11, Rab7, and Arf6. In quiescent PBMCs, surface expression of HLA-I was reduced to 50 ± 3% of control by Nef in the absence of these endocytic inhibitors but only to 76 ± 4, 75 ± 3, 86 ± 6, 81 ± 5, 76 ± 4, and 77 ± 4% of control in the presence of the inhibitors Eps15 deletion mutant, K44A dynamin mutant, Rab5-S34N, Rab7-T22N, Rab11-S25N, and Arf6-T27N mutants, respectively (Fig. 1A).
FIGURE 1.
A, Nef-mediated clearance of innate HLA-I from the plasma membrane of quiescent PBMCs was reversed by genetic inhibitors of endocytosis. Cells were co-transfected with NL-3 Nef or an NX at a 2-fold molar excess over GFP or GFP/YFP-tagged dominant-negative inhibitors of endocytosis. HLA-I expression for G/YFP-gated cells was measured using Alexa 647-conjugated W/632 mAb. FACS histogram profile of HLA-I for Nef (black) cells is overlaid with the corresponding one for non-Nef (gray) transfectants. HLA-I MFVs of GFP (or YFP)-gated populations for the null and the Nef transfectants averaged from four experiments are plotted as histograms (with error bars) in sets of two, with the value for null Nef arbitrarily set to 100. †, n = 3, p < 0.008; *, n = 4, p < 0.008. B, innate HLA-I on HeLa cells was down-regulated by HIV-1 Nef better at 26 than at 37 °C, and this effect was reversed by certain genetic inhibitors of endocytosis. FACS histogram profiles of native HLA-I in HeLa cells co-transfected with WT Nef (NL4-3 or NA7 allele) or NX and CD8 expression plasmid. At 6 h post-transfection, cells were downshifted to 26 °C or continued at 37 °C for another 12–16 h. HLA-I profiles of vector (gray) and Nef (black) transfectants gated for CD8 are overlaid in each panel. HLA-I MFVs of CD8-gated populations in the vector and Nef transfectants at 26 or 37 °C are plotted as histograms to the right, with the vector value arbitrarily set to 100. Averaged results from four experiments are plotted with S.E. C, effect of genetic inhibitors of endocytosis. HeLa cells were co-transfected with NL-3 (black) or NA7 Nef (gray) or a null mutant (white) at a 2-fold molar excess over GFP or GFP/YFP-tagged dominant-negative inhibitors of endocytosis. After shifting the transfectants to 26 °C for 18 h, HLA-I expression for GFP/YFP-gated cells was measured using Alexa 647-conjugated W/632 mAb. HLA-I MFVs of GFP (or YFP)-gated populations for the null and the two Nef transfectants averaged from four experiments are plotted as histograms (with error bars) in sets of three, with the value for null Nef arbitrarily set to 100. †, n = 4, p < 0.008; *, n = 5, p < 0.008. D, subcellular distribution of native HLA-I in HeLa cells co-expressing Nef or NX and GFP or GFP (or YFP)-tagged effectors or inhibitors of endocytosis. HeLa cells were transfected with a 2-fold molar excess of HIV-1 Nef over GFP or GFP/YFP fusion protein plasmids. At these ratios, Nef expression was present in all the GFP/YFP + cells. At 18 h after transfection, cells were downshifted to room temperature (25 °C) for 4–6 h, rinsed, fixed in 4% paraformaldehyde, permeabilized in 0.1% Triton X-100, and stained with the Alexa 647-conjugated polytropic HLA-I mAb, W632, before processing for microscopy. Individual channels corresponding to GFP and W632 fluorescence extracted from the RGB images are shown.
To further define the biochemical mechanisms regulating the Nef effect, we used HeLa cells, a frequently used cell system for isolating Nef mechanisms. It is important to recall, however, that HeLa cells are epithelial cells and therefore not a target of HIV-1. We first inquired whether the Nef effect on HLA-I is enhanced at lower temperatures where one may expect reduced intracellular HLA-I transport rates (14, 27). As shown in Fig. 1B, Nef induced only an ∼20% loss of HLA-I at 37 °C versus a significant (2–3-fold) response (to 50 ± 6 or 39 ± 12% of control by NL4-3 or NA7 allele) upon a 12-h downshift to 26 °C (Fig. 1B). Downshifting to 26 °C did not, however, alter the Nef effect either in T cell lines or in quiescent PBMCs (data not shown).
We measured the density of native HLA-I at the PM of HeLa cells co-expressing Nef or a null mutant (NX) with GFP or GFP/YFP fusion proteins of various vesicular GTPases. At 26 °C, Nef down-regulated HLA-I to 48 ± 4% of control, NX. In a pairwise comparison of HLA-I MFVs in the Nef(+) versus Nef(− population from the same transfection, or separate WT versus NX transfections, the dominant-negative inhibitors of Arf6 (T27N) and the constitutively active mutants of Arf6 (Q67L) and Rab11 induced significant reversal of NA7 and NL4-3 Nef effects from 48 ± 4 to 110 ± 8 or 91 ± 8% for Arf6T27N; to 84 ± 8 or 69 ± 6% for Arf6Q67L; and 111 ± 10 or 87 ± 7% for Rab11GTP (Fig. 1C).
The above results were corroborated by immunofluorescence microscopy. Steady-state distribution of HLA-I was examined for Nef and GFP or the GFP/YFP fusion proteins described above. In the absence of functional Nef expression, HLA-I normally resided at the PM, whereas in the Nef-expressing GFP(+) cells substantial HLA-I was present in perinuclear vesicles with the remaining receptor distributed in a speckled pattern near the PM (Fig. 1D, compare panels NX & GFP and Nef & GFP). In cells expressing GFP- or YFP-tagged K44A dynamin, Eps15 deletion, and Rab5-GDP mutant, HLA-I trapping in the perinuclear vesicles was markedly reduced with most of the receptor now redistributed to more peripheral punctate vesicles resembling clathrin-coated vesicles (CCVs) at the plasma membrane. Although Arf6 G-proteins substantially reversed the Nef effect at the PM (Fig. 1C, right), HLA-I was still found in some large vesicular and tubular structures (Fig. 1D) that have been termed Arf6 endosomes (28).
Nef-induced Clearance of Recombinant HLA-I A2 from the PM Was Partially Reversed by Some Genetic Inhibitors of Endocytosis
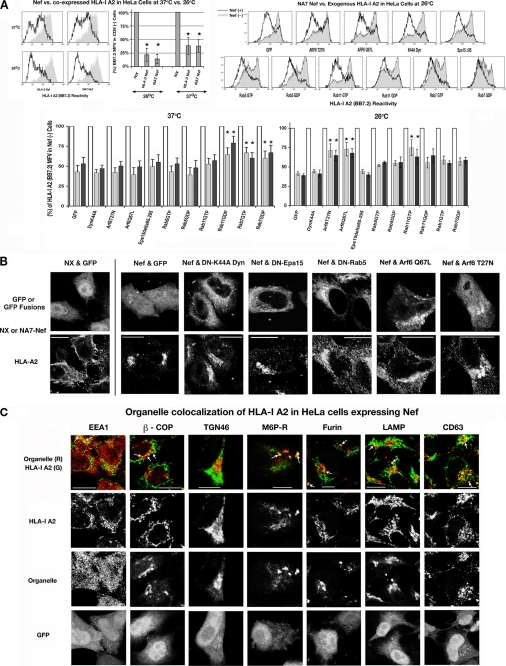
We inquired whether the Nef effect on recombinant HLA-I A2 would mimic that on native HLA-I. In HeLa cells, NA7 and NL4-3 Nefs were more effective on the A2 allele than native, reducing the MFV of A2 to 33 ± 6 or 34 ± 5% at 37 °C and 22 ± 4 or 14 ± 3% at 26 °C (Fig. 2A, top left). Furthermore, genetic inhibitors of endocytosis were not as effective in reversing the Nef effect on A2. At 26 °C, there was a modest reversal of both the NA7 and NL4-3 Nef effects as follows: from 41 ± 3 and 39 ± 3% to 72 ± 8 and 64 ± 6% for ARF6T27N; to 73 ± 7 and 68 ± 6% for ARF6Q67L, and to 71 ± 7 and 66 ± 6% for Rab11-Q67L (Fig. 2A, top and bottom right). Minor reversals of Nef effect were also observed with other Rab5, Rab7, and Rab11 mutants. At 37 °C, only the dominant-negative Rab11-S25N induced a more modest reversal of the NL4-3 and NA7 effects, to 54 ± 6 or 59 ± 5%, respectively (Fig. 2A, bottom left). The genetic inhibitors were ineffective in Jurkat cells (data not shown).
FIGURE 2.
A, in HeLa cells HIV-1 Nef down-regulated recombinant HLA-I A2 only slightly better at 26 than 37 °C, and this effect was reversed slightly by certain genetic inhibitors of endocytosis. FACS profiles (top left) of HLA-I A2 expression in HeLa cells co-transfected with plasmids expressing WT Nef (NL4-3 or NA7 allele) or vector, HLA-I A2, and CD8 (left). Conditions are as in Fig. 1B. Effect of genetic inhibitors of endocytosis. HeLa cells were co-transfected with a plasmid expressing HLA-I A2 and NL-3 (black), NA7 Nef (gray), or a null mutant (white) plasmid at a 2-fold molar excess over GFP or GFP/YFP-tagged dominant-negative inhibitors of endocytosis. Average A2 MFVs of null and Nef transfectants at 37 and 26 °C are plotted as histograms with error bars as in B. *, n = 4, p < 0.01. B, subcellular distribution of HLA-I A2 in HeLa cells co-expressing Nef and GFP or GFP/YFP-tagged effectors or inhibitors of endocytosis. Conditions are as described for Fig. 1D except for the use of HLA-I A2-specific BB7.2 mAb. C, co-localization of HLA-I A2 and Nef with various subcellular organelles in HeLa cells. HeLa cells were transfected with an HLA-I A2 IRES GFP bicistronic vector. HLA-I A2 was stained with Alexa 647-conjugated BB7.2 mAb. β-COP, EEA-1, clathrin, furin, and mannose 6-phosphate receptor (M6P-R) were detected with the respective rabbit antisera, TGN with sheep anti-TGN 46, and LAMP and CD63 with the respective murine mAbs followed by secondary staining with Alexa 568-conjugated anti-rabbit, -sheep, or -mouse IgGs, respectively. Individual channels corresponding to HLA-I A2, the respective organelle, and GFP fluorescence extracted from the RGB images are shown below composite pictographs of HLA-I A2 in green and organelle in red. Scale bars, 10 μm.
The stronger Nef effect on recombinant HLA-I A2 versus innate HLA-I was supported by immunofluorescence microscopy (which also added important spatial information). Absent Nef, almost all the HLA-I A2 was observed at the PM; in the presence of Nef, HLA-I A2 was trapped into perinuclear vesicles (Fig. 2B, NX and GFP versus Nef and GFP). The dominant-negative mutants of Rab5 and ARF6 and the constitutively active form of ARF6 induced some redistribution of HLA-I A2 from perinuclear vesicles to punctate vesicles at the periphery (Fig. 2B), whereas neither the K44A dynamin mutant nor the Eps15 deletion mutant had any effect.
Nef Sequesters Recombinant HLA-I A2 in Post-Golgi and Endolysosomal Vesicles
We stained HeLa cells co-transfected with plasmids expressing HLA-I A2, and a Nef IRES GFP bicistronic plasmid encoding Nef and GFP was prepared and then stained with a mixture of antibodies against component(s) of subcellular organelles and against HLA-I A2 (Fig. 2C). HLA-I A2 was sequestered in a few early endosomal vesicles. There was more extensive co-localization of HLA-I A2 with CD63 and LAMP-1 markers for late endosomes and lysosomes. There was also modest co-localization with mannose 6-phosphate receptor, which transports the TGN resident proteins from the Golgi complex. HLA-I A2 was not co-localized with TGN46. Given rapid decay of CD4 in Nef(+) cells, we inquired whether CD4 would appear in the same transport vesicles. In Nef-expressing cells, CD4 was redistributed in a pattern similar to that of HLA-I A2. There was considerable co-localization of CD4 with CD63, mannose 6-phosphate receptor, and TGN46 positive vesicles and less co-staining with LAMP-1-positive vesicles and some co-localization with early endosomal antigen (supplemental Fig. 3). Both HLA-I A2 and CD4 co-localized with β-COP vesicles, more so for CD4. It appears that notwithstanding the modus operandi, Nef causes both the recombinant HLA-I A2 and CD4 to be sequestered in post-Golgi and endolysosomal vesicles. This may presage a β-COP-dependent pathway as proposed previously (16).
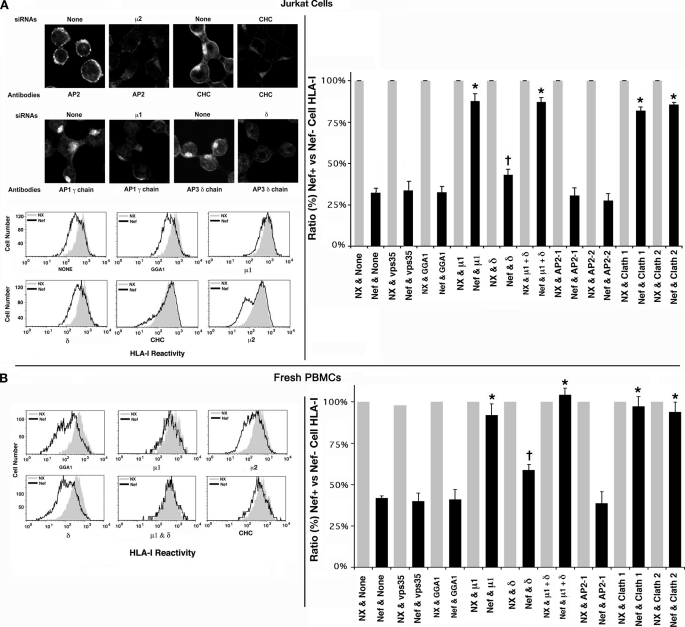
Nef Down-regulation of Native HLA-I in PBMCs and T Cell Lines Was Reversed by siRNA Knockdown of AP1 Subunits or Clathrin Heavy Chain
Nef has been proposed to induce TGN retention and eventual lysosomal degradation of HLA-I by recruiting and stabilizing AP1-HLA-I complexes (15, 16). Subsequently, it was shown by siRNA knockdown that clathrin was also crucial to HLA-I diversion (27). Treatment of Jurkat cells with siRNAs against the μ2 or α (data not shown) chain of AP2, μ1 or γ (data not shown) chain of AP1, δ chain of AP3, or CHC induced a marked depletion of AP2, AP1, AP3, and clathrin-positive vesicles, respectively, as shown by immunofluorescence (Fig. 3A, top left). Knockdown of the AP1 μ1 or γ chains substantially reversed the Nef effect from 31 ± 2 or 38 ± 2% to 86 ± 6 or 88 ± 8% (n = 4, p < 0.01) of control levels for Jurkat cells and quiescent PBMCs, respectively (Fig. 3, A and B, histograms on the right, data for γ chain not shown). Clathrin knockdown fully restored the PM HLA-I levels. Ablation of AP2 μ or α (data not shown) chain resulted in a mild but statistically significant enhancement of Nef-induced HLA-I down-regulation from 31 ± 2 or 38 ± 2% to 26 ± 2 or 32 ± 3% in Jurkat cells and PBMCs, respectively (n = 5, p < 0.05). AP3 δ chain knockdown also led to slight yet significant reversal of Nef effect (from 31 ± 2 or 38 ± 2% to 40 ± 3 or 55 ± 4% in Jurkat cells and PBMCs; n = 5, p < 0.05) (Fig. 3, A and B, histograms on the right).
FIGURE 3.
A, Nef-induced down-regulation of native HLA-I was reversed by siRNA knockdown of AP1 subunits or CHC in Jurkat cells and quiescent PBMCs. A, selected subsets (Mock, AP1 μ1a, AP2 μ2, AP3 δ, or CHC siRNA-transfected) of Jurkat cells were analyzed by immunofluorescence microscopy using antibodies against AP1 γ chain, AP2 α chain, AP3 δ chain, or CHC (top left). Following siRNA knockdown, cells were transfected with bicistronic plasmids encoding GFP and Nef or NX mutant. Relative HLA-I MFVs for GFP-gated cells are plotted pairwise for Nef(+) and Nef(−) cells, with the latter values arbitrarily assigned 100% in each pair. FACS histogram profiles of HLA-I for selected siRNA transfectants. MFVs for NL4-3 Nef (black) expressers are overlaid with the corresponding one for the non-Nef (gray) cells (bottom left). HLA-I MFVs of CD8-gated populations for the null (gray) and the Nef expressers (in the context of siRNA knockdown) averaged from four experiments are plotted pairwise as histograms (with error bars) in sets of three, with the value for null Nef arbitrarily set to 100 (right). B, effect of siRNA knockdown on Nef-induced HLA-I down-regulation in PBMCs. FACS histogram profiles of HLA-I for selected siRNA transfectants are gated for GFP expression (left). Average HLA-I MFVs plotted pairwise (with error bars) for null and Nef expressers in the context siRNA induced knockdown. †, n = 3, p < 0.03, and *, n = 3, p < 0.005 for the indicated siRNAs versus none in Nef(+) cells.
Differential Effects of siRNA Knockdown of PACS-1, ARF6, and ARNO on Nef Effects on HLA-I
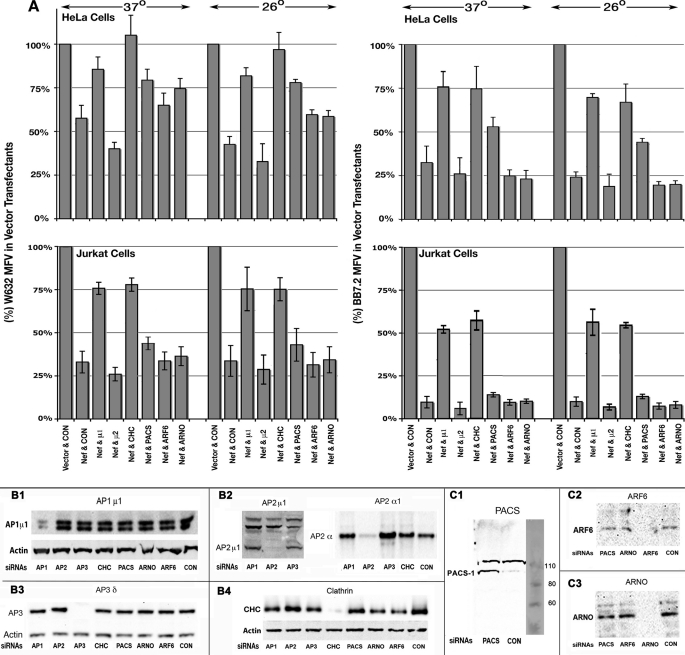
First we confirmed that Nef clearing of the recombinant HLA-I A2 was also dependent on the same adapter subunits and clathrin as native HLA-I (supplemental “Results” and supplemental Fig. 4). Nef effect differs at 26 versus 37 °C in HeLa cells versus T cells lines; to see if this reflected different intracellular itineraries, we undertook a more extensive siRNA knockdown analysis of Nef effect on HLA-I and CD4. Following siRNA knockdown, we co-transfected Nef or the inactive NX with CD8 and allowed the cells to grow for 18–24 h at 37 and 26 °C after plasmid transfection. We quantified PM density of native or recombinant HLA-I and CD4 in CD8 (either NA7 or NL4-3 allele)-gated populations. Cell aliquots were saved for immunoblot quantitation of the respective siRNA target. As shown in Fig. 4, B1–B4 and C1–C3, the various siRNAs knocked down the cognate proteins to almost undetectable levels. Nef induced a slight loss (to 58 ± 5% of control MFV) of native HLA-I in HeLa cells at 37 °C. There was a significant improvement in the Nef effect at 26 °C, with the PM HLA-I reduced to 38 ± 3% of control. AP1 μ1 (Fig. 4A, top left) or γ (data not shown) chain knockdown led to a significant reversal of Nef-mediated HLA-I down-regulation in HeLa cells to MFVs approaching 84 ± 6.8 or 79 ± 5.8% at 37 or 26 °C, respectively. CHC knockdown also led to restoration of HLA-I in Nef expressers to 106 ± 9 or 94 ± 8% at 37 or 26 °C, as was also shown for Jurkat cells and PBMCs (Fig. 3) at 37 °C. siRNA knockdown of AP2 μ chain enhanced HLA-I down-regulation slightly, from 58 ± 5 or 38 ± 3 to 38 ± 2 or 31 ± 3% at 37 and 26 °C, respectively (Fig. 4A, top left). Similar results were obtained in two experiments with AP2 α chain knockdown (data not shown). Knockdown of AP1 and clathrin in Jurkat cells resulted in a marked reversal of Nef effect on native HLA-I at 37 and 26 °C, notwithstanding that Nef was much more potent in Jurkat cells (29 ± 3 or 28 ± 3% versus 58 ± 5 or 38 ± 3% in HeLa cells at 37 or 26 °C) (Fig. 4A, bottom left). AP2 ablation resulted in a mild enhancement (<20%) of the Nef effect in both cells.
FIGURE 4.
A, differential HLA-I response to Nef in Jurkat versus HeLa cells in the context of siRNA knockdown of vesicular adapter proteins, clathrin, PACS-1, Arf6, and ARNO. A, histograms showing average MFVs (with error bars) of native HLA-I (left) or plasmid-expressed A2 allele (right) in HeLa (top) or Jurkat (bottom) cells expressing Nef in the context of siRNA knockdown of the indicated proteins. Jurkat or HeLa cells were transfected for 36 h with the respective siRNAs twice before co-transfection with Nef or NX (vector) plasmid with one expressing CD8, CD4, and/or HLA-I A2 expression plasmid(s) and incubated at 37 or 26 °C for 18–24 h. ‡, n = 4, p < 0.04; †, n = 4, p < 0.02; *, n = 4, p < 0.008 for the indicated siRNA knockdowns versus no siRNA (CON) in Nef(+) cells. B1–C3, approximately 2 × 106 (HeLa or Jurkat) cells were disrupted in 0.5 ml of lysis buffer, and initially 50 μl of 15,000 × g supernatants were immunoblotted for actin. After adjusting the volume to constant actin values, the indicated vesicular or adapter proteins were detected by immunoblotting extracts of cells treated with the siRNAs listed below the respective blots, using rabbit antibody against AP1 μ1 chain (B1) and AP2 μ and murine mAbs against AP2 α chain (B2), AP3 δ chain (B3), clathrin heavy chain (B4), rabbit antiserum against PACS-I (C1), and murine mAbs against Arf6 (C2) and ARNO (C3).
Nef induced robust down-regulation of recombinant HLA-A2 in HeLa cells to 30 ± 3 or 24.2 ± 1% of control at 37 or 26 °C. AP1 μ1 or γ (data not shown) chain or CHC siRNA knockdown induced a substantial reversal of Nef effect to 75 ± 6 or 74.4 ± 8% at 37 °C and to 72 ± 4 or 69 ± 6% at 26 °C for AP1 or CHC, respectively (Fig. 4A, top right). In Jurkat cells, Nef induced a marked loss (to 8 ± 0.5% of control) of recombinant HLA-I A2 at the PM at 37 or 26 °C. AP1 or CHC knockdowns led to moderation of this strong Nef effect, 51 ± 3 or 54 ± 5% for AP1 and 55 ± 5 or 52 ± 2% for CHC knockdown at 37 and 26 °C, respectively (Fig. 4A, bottom right). In this background, AP2 knockdown still induced a slight enhancement of Nef-mediated HLA-I A2 down-regulation (to 5.5 ± 0.3% of control). AP3 δ chain knockdown did not significantly reverse Nef-induced down-regulation of native or recombinant MHC-I in HeLa or Jurkat cells at either temperature (data not shown).
Ablation of PACS-1 led to significant reversal of Nef effect on native HLA-I in HeLa cells at 37 (from 58 ± 5 to 79 ± 7%) and 26 °C (from 38 ± 3 to 77 ± 4.8%) (Fig. 4A, top left). Similar results were obtained using two other siRNAs targeting PACS-1 (data not shown). However, PACS-1 depletion induced only moderation of the Nef effect on native HLA-I in Jurkat cells, from 29 ± 3 to 42 ± 2% and from 27 ± 3 to 39 ± 3% at 37 or 26 °C respectively (Fig. 4A, bottom left). Although PACS-1 depletion modestly reversed the Nef effect on recombinant HLA-I A2 in HeLa cells (31 ± 3 to 52 ± 4% and from 24.2 ± 1 to 44 ± 3% at 37 or 26 °C, Fig. 4A, top right), HLA-I A2 down-regulation was unaffected in Nef-expressing Jurkat cells after PACS-1 knockdown (Fig. 4A, bottom right).
Above, we showed that both Arf6Q67L and Arf6T27N mutants partially rectified the Nef-mediated down-modulation of native HLA-I in HeLa cells and PBMCs. To confirm these observations, we evaluated the effects of siRNA knockdown of Arf6 and ARNO GEF. Ablation of Arf6 using a mixture of three different siRNAs led to a weak reversal of the Nef effect on native HLA-I in HeLa cells at 37 °C (from 58 ± 5 to 66 ± 5% of control MFV) and more at 26 °C (from 38 ± 3 to 57 ± 4%). Likewise, ARNO knockdown with a pool of three siRNAs in HeLa cells induced a modest reversal of Nef effect (from 58 ± 5 to 75 ± 6% at 37 °C and from 38 ± 3 to 56 ± 4% at 26 °C) (Fig. 4A, top left). These findings were validated using single species of siRNAs against Arf6 and ARNO (data not shown). Knockdown of either Arf6 or ARNO had no effect of Nef-mediated down-modulation of recombinant HLA-I A2 in HeLa cells or of native or recombinant HLA-I in Jurkat lymphocytes (Fig. 4A). We considered bystander (off-site) effects of siRNA knockdown strategy by examining the effect of siRNA knockdown on native CD4 in Nef(+) Jurkat and HeLa cells. As illustrated in supplemental Fig. 5, only AP2 and clathrin depletion resulted in a significant reversal of Nef-induced CD4 down-regulation in Jurkat lymphocytes and HeLa cells. Thus, our portfolio of cofactor manipulation findings indicated that Nef subverts both antero- and retrograde trafficking of MHC-I alleles more predominantly in the anterograde trafficking of recombinant HLA-I A2 in T cells than in HeLa cells while impairing the endocytic traffic of native HLA-I in quiescent PBMCs and HeLa cells.
Live Imaging and Dynamics by TPTCFCCS and FRAP
The biochemical methods described above quantify the net effects of Nef, integrated over various long incubation times. We inquired whether the Nef effects on innate or recombinant HLA-I (and on the trafficking of CD4 versus HLA-I) would be visible in real time observations of complex formation between Nef and these receptors in the subcellular organelles. We acquired snapshots (∼2 min) for the interactions of Nef-Cerulean fluorescent protein (Nef-CerFP) with YFP or Venus-tagged HLA-A2, WT CD4, or LL/AA CD4 mutant proteins in the TGN, ER, and PM in HeLa cells at 25 °C using TPTCFCCS. The labeling was innocuous as shown by the supplemental Fig. 6. Nef-CFP fusion protein down-regulated CD4 and HLA-I A2 as well as unmodified Nef, and conversely HLA-I A2-Venus fusion was down-regulated by Nef as well as untagged HLA-I A2.
TPTCFCCS records the fluctuations in fluorescence intensity caused by the entry to and exit of labeled molecules from a very small open focal volume. This femtoliter excitation zone is optically defined by the instrument confocal pinhole (for a one-photon FCS instrument) or, in our case, by the shape of the two-photon excitation probability (29). The fluctuations track instantaneous local changes in the chromophore concentration. Both the concentration and diffusion coefficients of the fluorescent molecules are thus determined (30, 31). The diffusion times τD recovered are functions of both the size of the diffusing complex and the effective viscosity and topology of the local milieu. Cytoplasmic “free” diffusion rates can be benchmarked and calibrated with known proteins; hence a measured τD (see “Experimental Procedures”) provides the molecular weight. On a two-dimensional surface like a smooth membrane, the overall complex size becomes less important than the girth of the portion that is stuck in a viscid membrane; hence, the cross-section of the membrane-spanning element could instead be deduced. In convoluted vesicular surfaces, however, anomalous diffusion (proteins that must follow winding roads or wander into their dead ends rather than cross them) yields τD that is more difficult to link to a particular complex size. Worse, fluorescent proteins trapped in submicron vesicles may even bleach during FCS, excluding them from the average.4 Nevertheless, τD is often a useful measure of relative hydrodynamic radii of the complex(es).
Two-photon, Two-color Cross-correlation
Simultaneous excitation and detection of two fluorophores with different emission maxima allow cross-correlation of fluorescence intensity versus time. Cross-correlation is simply a measure of how often the two colors blink together, signaling they are transiting the focal spot together.
If two fluorescent species do not diffuse together (i.e. are noninteracting), the fluctuations in (cross) intensity will be entirely uncorrelated. If there is 100% complex formation, the fluctuations will be 100% cross-correlated in time.
We often refer to the cross-correlation as a measure of co-mobility, i.e. two different colored proteins need not directly bind each other, but if they bind any common partner(s), they will diffuse together, and the cross-correlated blinking will occur. This permits us to examine such binding at very low particle concentrations. Because the ROI examined by FCS are in the 0.1 μm2 range, the number of fluctuations are significant, as average occupancy of only a few molecules is common (provided that fluorescent protein expression is maintained at modest submicromolar levels). With correlation in time, TPTCFCCS measurements reflect solely the mobility characteristics of the target(s) in the various subcellular locales. By analyzing the ACF and CCFs from ROI with different intensities (i.e. concentrations, Ci), the concentrations of free (CC and CV) and bound (CCV) Cerulean- and Venus-tagged proteins can in principle be calculated. FCS has been used to evaluate in “real” time (minutes) the avidity of macromolecular interactions at the plasma membrane (32–34) and complex dynamics inside organelles such as mitochondria (35) and the Golgi network (36). Although many of these studies have elucidated parameters relating to macromolecular transport and assembly, etc., few, if any, have used two-photon excitation with its facile cross-correlation.
Cross-correlation of Nef and HLA-I A2 in Subcellular Organelles
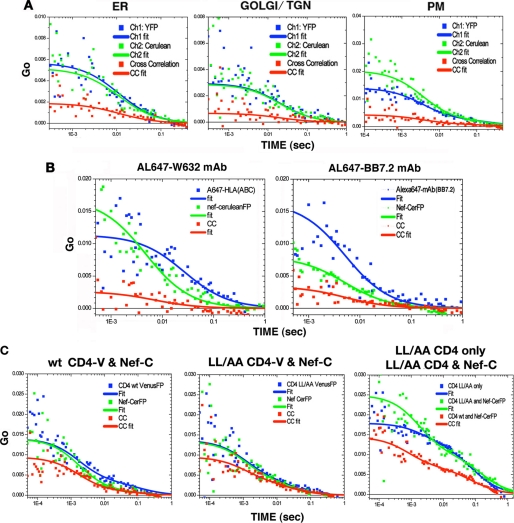
HeLa transfectants expressing HLA-I A2 fused with fluorescent protein (eYFP, Venus, or Cerulean) and Nef fused with CFP or Cerulean were used to obtain TPTCFCCS data by single focal point acquisitions of emitted photon count traces. To find and target the ER, Golgi/TGN, and the PM, the organelles were counterstained with BODIPY Texas Red-conjugated glibencamide, BODIPY Texas Red-ceramide, and Texas Red-conjugated wheat germ agglutinin, respectively. The organelle-specific dyes were two-photon excited at 840 nm. HLA-I A2-eY/Venus and Nef-CerFP were then detected by excitation at 920 nm (avoiding the counterstain). ACF profiles for HLA-I A2-Venus in blue, Nef-CerFP in green, and cross-correlation in red are shown for representative data in Fig. 5. There was little or no cross-correlation between controls Nef-CerFP and Venus-FP or between controls HLA-I A2-Venus and Cerulean-FP (data not shown).
FIGURE 5.
A, single point FCCS data acquisition from ER, Golgi/TGN, and PM loci. Transfections were done at 26 °C for 4 h using 0.5 μg each of HLA-A2 or CD4 and Nef fusion plasmids per 105 cells on each of 2-well Titer-Tek culture chambers. ER was visualized by staining with BODIPY Texas Red-conjugated glibencamide, Golgi/TGN with BODIPY Texas Red-conjugated ceramide, and PM with Texas Red-conjugated wheat germ agglutinin, all of which were excited at 840 nm, although HLA-I A2-V and Nef-C were detected by excitation at 920 nm. ACF profiles for Nef-C in green, A2-V in blue, and cross-correlation (CC) in red are shown on the right panels of each row (100 × 100 μm). B, auto- and cross-correlation plots of Alexa 647-conjugated W632 (left) or BB7.2 (right) mAb specific for native HLA-I or the plasmid expressed A2 allele, respectively, in the context of Nef-C expression in the respective cells. C, ACF (CD4 in blue and Nef in green) and CCF (red) profiles in cells co-expressing WT or LL/AA CD4-V and Nef-C are shown in the left and middle panels, respectively. Changes in diffusion rates of WT or LL/AA CD4 in the presence of Nef were deduced by comparing the CD4 ACF profile of LL/AA CD4 only (blue), LL/AA CD4 and Nef (green), and WT CD4 and Nef (red), right panel. Note that the diffusion coefficient changes leftward from something very large to somewhat smaller.
Because the analysis was limited to the Nef-bound fraction of HLA-I A2, the ACF data were fit to a simple diffusion model without any constraints. The cross-correlation profile followed both the Nef and HLA-I A2 ACFs throughout the time range (Fig. 5A). Thus, in these organelles, the cross-correlating (co-mobile) species have similar transport properties to the average (both free + bound) proteins.
The degree of binding in a simple ACF/CCF plot is directly extracted by comparing the intercept (Go, G(O)) of the CCF fit to the lowest Go of the two ACFs, i.e. the CCF is compared with the most concentrated of the two ACF partners. The CCF Go cannot exceed that ACF Go value, and Go(CCF)/Go(ACF) determines the fraction bound (i.e. the portion of the more abundant species that is tied up in co-mobile complexes). Thus, one can deduce that about one-third of the more abundant fluorescent species (which in each case happened to be A2-Venus) was complexed with Nef (37% in the ER, 23% in the Golgi/TGN network, and 30% at the PM). As an aside, attempted FCS of Nef and HLA-I A2 within endolysosomal vesicles was unsuccessful, because of the small vesicular volumes (as mentioned above) and high nonrandom mobility, which resulted in widespread intensity heterogeneities causing “stepped” intensity time traces. In the environs where many labeled proteins are confined to actively moving vesicles, FCS data reduction can be disrupted by flow artifacts and “spiking” (i.e. the passage of an enormous fluorescent aggregate through the focal volume); the correction of these is beyond the scope of this study.
Comparison of Co-mobility of Nef with the Native Versus Recombinant HLA-I at the PM
We inquired whether the native HLA-I would also exhibit ∼30% binding for Nef at the PM. Cells expressing Nef-CerFP alone or with HLA-I A2 were stained with Alexa 647-labeled W632 or BB7.2 mAbs against native HLA-I or the A2 allele (Fig. 5B). The fraction (∼35%) of native HLA-I (via W632 mAb binding) found complexed with Nef-CerFP at the plasma membrane was comparable with the ∼30% HLA-I A2-Venus binding level with Nef. A slightly larger fraction (∼40%) of BB7.2 mAb (specific for HLA-I A2) was complexed with Nef, suggesting this bivalent antibody might engage a bit more Nef (perhaps through receptor cross-linking).
Table 1 summarizes all the results for HLA-I A2-Venus and Nef-CerFP in the ER and Golgi membrane network and the plasma membrane. Results obtained from studies using fluorescent antibodies targeting either the native HLA-I or recombinant HLA-I A2 are also included. The calculated diffusion coefficients were from fits to a single diffusing complex undergoing three-dimensional diffusion for studies within the ER and TGN; in contrast, two-dimensional diffusion was assumed in the PM. Nef expression induced minor decreases in the diffusion coefficient and a 10% increase in the amount of the faster HLA-I species at the PM (Table 4). The antibody-labeled HLA-I-Nef complexes are large (Table 4), although not quite as large as the largest covalent complexes. On occasion, a small amount of fast antibody component (presumed free) was visible.
TABLE 1.
Binding potential and diffusion parameters of HLA-I, WT, or mutant CD4 for Nef in subcellular organelles
Auto- and cross-correlation (GNef or GHLA/Gcc) data were fit to one diffusing component for the Golgi and ER, and a two-component two-dimensional diffusion model was used to analyze PM data. Effect of HIV-1 Nef on the diffusion rates of HLA-I-A2 in different subcellular organelles was deduced from the FCS assay. Dt,fast or Dt,slow and % fast or slow represent diffusion rates of the fast or slow components, and their relative abundances (in %) are given.
| Organelle | Percent bound | Diffusion coefficient (μm2/s) |
|---|---|---|
| A2-Venus + Nef-CerFP | ||
| Golgi | 23 ± 7 | 0.5 ± 0.1 |
| ER | 37 ± 6 | 0.8 ± 0.1 |
| PM | 30 ± 4 | 4.9 ± 1.1; 0.23 ± 0.14 |
| A2 + Nef-CerFP | ||
| PM (A647 W632 mAb) | 35 ± 20 | 0.72 ± 0.16 |
| A2 + Nef-CerFP | ||
| PM (A647 BB7.2 mAb) | 42 ± 19 | 0.39 ± 0.16 |
| WT CD4-Venus + Nef-CerFP | ||
| PM | 70 ± 20 | 7.8 ± 0.7; 0.37 ± 0.16 |
| LL/AA CD4-Venus + Nef-CerFP | ||
| PM | 90 ± 30 | 6.6 ± 0.8; 0.32 ± 0.11 |
TABLE 4.
Summary of results for the molecular radii calculated from the respective diffusion coefficients using the Saffman and Delbruck Equation 5 (D <2 μm2/s)
The Stokes Einstein equation was applied for coefficients above this value using a η = 6 centipoise.
| Protein(s) at the PM with fast/slow component | Diffusion coefficients | Radius (nm) | Fraction (%) |
|---|---|---|---|
| A2-eYFP | |||
| Fast | 6.0 | 5.96 | 50 |
| Slow | 0.21 | 39.8 | 50 |
| A2-Venus + Nef-CerFP | |||
| Fast | 4.9 | 7.29 | 60 |
| Slow | 0.23 | 38.2 | 40 |
| W632–647 + Nef-CerFP | 0.72 | 14 | 100 |
| B7.2–647 + Nef-CerFP | 0.39 | 28 | 100 |
| MβCD treatment | |||
| A2-Venus + Nef-CerFP | |||
| Fast | 1.76 | 2 | 54 |
| Slow | 0.46 | 24 | 46 |
| IKA treatment | |||
| A2-Venus + Nef-CerFP | |||
| Fast | 2.3 | 15 | 72 |
| Slow | 0.21 | 40 | 28 |
| LL/AA CD4-Venus only | 0.9 | 10 | 100 |
| LL/AA CD4-Venus + Nef-CerFP | |||
| Fast | 6.6 | 5.42 | 55 |
| Slow | 0.32 | 32 | 45 |
| WT CD4-Venus + Nef-CerFP | |||
| Fast | 7.8 | 4 | 31 |
| Slow | 0.37 | 29 | 69 |
| MβCD treatment | |||
| LL/AA CD4-Venus + Nef-CerFP | |||
| Fast | 3.7 | 10 | 50 |
| Slow | 0.21 | 40 | 50 |
| IKA treatment | |||
| LL/AA CD4-eYFP + Nef-CerFP | |||
| Fast | 2.3 | 15 | 68 |
| Slow | 0.09 | 51 | 32 |
Both WT and LL/AA Mutant CD4 at the Plasma Membrane Were Found Bound to Nef
Physical interaction of HIV-1 Nef and CD4 has been demonstrated in vitro (37, 38) and in vivo in insect and mammalian cell expression systems expressing both proteins (39). Stable complexes between Nef-resistant LL/AA CD4 mutant and Nef have been demonstrated suggesting that the CD4 di-leucine may not be critical for Nef binding but may instead be needed to interact with the adapter complex (40). We examined the distribution of WT or LL/AA CD4 on live and permeabilized cells expressing WT Nef-GFP (supplemental Fig. 7A1). In live cells expressing Nef-GFP, WT CD4 was markedly reduced at the PM. In contrast, Nef-resistant LL/AA CD4 was abundant at the PM. In permeabilized cells, both WT and LL/AA CD4 were visualized in intracellular vesicles in Nef-GFP cells. Although the intracellular CD4 represented most if not all of WT CD4, only minor amounts of LL/AA CD4 were sequestered intracellularly (supplemental Fig. 7A1, compare patterns of EC CD4 with IC CD4). This is consistent with the FACS analysis, showing marked loss of WT but not the LL/AA CD4 at the PM in cells expressing Nef or Nef-CerFP (supplemental Fig. 7, A2 and B). We (20) and others (41) have shown that LL/AA CD4 does not undergo constitutive endocytosis.
By TPTCFCCS, we found almost all CD4 is sequestered in co-mobile complexes with Nef as follows: 70 ± 20 or 90 ± 30% of WT or LL/AA CD4 mutant moved in Nef-bound complexes (Table 1). From the shape of the CCF, one can even infer that Nef recruited both CD4 proteins from some sort of larger, slower complex.
ACFs of LA/AA CD4 alone, WT CD4 with Nef, and LL/AA CD4 with Nef were plotted together. Differences were apparent in the shape of the curves (Fig. 5C). The diffusion coefficient for LL/AA CD4 alone was apparently monodisperse at 0.9 ± 0.7 μm2/s, but in the presence of Nef there were two discernable components with diffusion coefficients of 6.6 ± 2 and 0.32 ± 0.17 μm2/s, in approximately equal amounts. For WT CD4 in the presence of Nef, the diffusion parameters were 7.8 ± 4 μm2/s for a 31 ± 12% fraction and 0.37 ± 0.18 μm2/s for a 69 ± 22% fraction (Table 4). This confirms that Nef mobilizes a portion of CD4 into protein (or lipoprotein) complexes that are smaller than those found absent Nef.
Unfortunately, attempts to evaluate the cross-correlation potential between Nef and WT or LL/AA CD4 in intracellular organelles were unsuccessful because of the following: 1) there was a paucity of LL/AA CD4 in the intracellular organelles relative to Nef, and 2) some intracellular WT CD4 was sequestered in small and highly mobile endolysosomal vesicles, which are photobleached readily.
In all, the TPTCFCCS minute by minute snapshots revealed that during the down-regulating process, Nef is co-mobile with about a third of HLA-I (in several cellular locations). Avidity of Nef for CD4 is even stronger in real time, and nearly all the CD4 is co-mobile with Nef at the PM, breaking away from large native complexes to wander the surface more quickly with Nef.
FRAP Analysis of Nef Effect on the Lateral Mobility of HLA-I A2
FRAP, unlike FCS, measures the diffusion of proteins over distances comparable with the sizes of organelles. We measured the two-dimensional diffusion coefficients (Dt) of various mobile fraction(s) (Mf) of HLA-I A2-Venus in the ER, Golgi/TGN, and PM in the presence or absence of Nef by monitoring the FRAP. Three fractions were identified in our study, an immobile fraction and two others with a difference of 10–20 times in diffusion times. The corresponding diffusion coefficients (Dt) were simply labeled “slow FRAP” and “very slow FRAP.” We chose these terms to acknowledge the relative time scale sensitivity of FRAP versus FCS. Fast FCS and Slow FCS categories for rates correspond approximately to tens of μm2/s versus units of μm2/s, respectively (e.g. Fast from about 5–50 μm2/s and Slow from about 0.2 to 2 μm2/s). Thus, slow FCS rates are comparable with the fastest FRAP rates seen. The FRAP very slow and immobile terms would be lost to bleaching in FCS. The percent immobile fraction in FRAP was also measured and is included in Tables 2 and 3.
TABLE 2.
Effect of HIV-1 Nef on HLA-I-A2 diffusion rates in the ER and the Golgi deduced by FRAP
| Golgi |
ER |
|||
|---|---|---|---|---|
| A2-Cer and A2-V | Nef-Cer and A2-V | A2-Cer and A2-Vs | Nef-Cer and A2-V | |
| t½ (s) | 0.38 | 0.89 | 0.34 | 0.38 |
| tfast | 0.18 ± 0.05 | 0.28 ± 0.08 | 0.18 ± 0.05 | 0.22 ± 0.06 |
| Dfast (μm2/s) | 0.34 | 0.22 | 0.34 | 0.28 |
| % fast | 30 ± 3.3 | 28 ± 3 | 44.2 ± 3 | 50 ± 5.0 |
| tslow | 3.4 ± 0.5 | 6 ± 1.3 | 3.8 ± 0.90 | 3.9 ± 1.0 |
| Dslow (μm2/s) | 0.018 | 0.011 | 0.0164 | 0.0158 |
| % slow | 29 ± 3 | 34 ± 2 | 34 ± 3 | 34 ± 3 |
| % immobile | 41 ± 1 | 38.2 ± 2 | 22.1 ± 2 | 16 ± 2 |
TABLE 3.
Effect of HIV-1 Nef on the diffusion rates of HLA-I A2 and LL/AA CD4 at the PM deduced by FRAP
Effect of HIV-1 Nef on the diffusion rates of HLA-I A2 in different subcellular organelles deduced from the FRAP assay. Dt,fast or Dt,slow and % fast or slow represent diffusion rates of the fast or slow components and their relative abundance (in %).
| A2-Cer and A2-Venus | Nef-Cer and A2-Venus | LL/AA CD4-Cer and LL/AA CD4-Venus | Nef-CerFP and LL/AA CD4-Venus | |
|---|---|---|---|---|
| t½ (s) | 0.50 | 1.09 | 2.09 | 1.60 |
| tfast | 0.23 ± 0.04 | 0.27 ± 0.11 | 0.86 ± 0.25 | 0.68 ± 0.35 |
| Dfast (μm2/s) | 0.27 | 0.23 | 0.07 | 0.09 |
| % fast | 40 ± 3 | 25 ± 4 | 32 ± 5 | 27 ± 7 |
| tslow | 4.1 ± 0.5 | 4.5 ± 0.7 | 8.6 ± 4.2 | 5.0 ± 2.0 |
| Dslow (μm2/s) | 0.02 | 0.01 | 0.01 | 0.01 |
| % slow | 40 ± 2 | 43 ± 2 | 50 ± 4 | 48 ± 9 |
| % immobile | 20 ± 1 | 31 ± 2 | 19 ± 8 | 25 ± 3 |
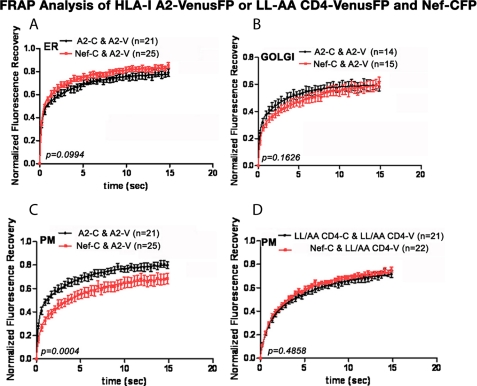
In the ER, 44.2% of HLA-I A2-Venus had a Dt of 0.34 μm2/s, and another 34% had a Dt of 0.0164 μm2/s, and these rates were not significantly altered by Nef expression with the corresponding diffusion rates 0.3 μm2/s for the 50% slow fraction and 0.02 μm2/s for the 34% very slow fraction. Nef slightly reduced the amount of the immobile fraction (22 versus 16%) (Fig. 6A and Table 2). At 0.34 and 0.02 μm2/s, the diffusion rates of the two mobile HLA-I A2 fractions in the Golgi network were remarkably similar to those in ER. However, there was a substantial doubling of the immobile fraction at the expense of the fast moving fraction in the Golgi. Nef induced a slight and statistically insignificant change to 0.22 and 0.01 μm2/s (Fig. 6B and Table 2).
FIGURE 6.
Effect of Nef on the FRAP recoveries of HLA-I A2 in the ER (A), the Golgi (B), and the plasma membrane (C) are shown. The corresponding curve fits of FRAP data for the LL/AA CD4 mutant at the plasma membrane are shown in D. Experimental condition as are detailed under “Experimental Procedures.”
Nef did not significantly change the diffusion rates of either the slow or the very slow moving HLA-I at the plasma membrane, but it significantly decreased the quantity of “slow” diffusing molecules from 40 to 25% leaving the “very slow” diffusing quantity almost unchanged (from 40 ± 2 to 44 ± 2%). This necessarily increased the immobile fraction at the PM from 20 ± 1 to 31 ± 2% (Fig. 6C and Table 3).
FRAP Analysis of Nef Effects on the Lateral Mobility of WT and LL/AA Mutant CD4 at the PM
Our efforts at FRAP analysis of WT CD4-Venus in HeLa cells co-expressing Nef-CerFP were unsuccessful, because most if not all the tagged CD4 protein exhibited rapid constitutive endocytosis with rapid diffusion in and out of tiny fast moving vesicles much less than the typical 1 × 1 μm ROI for FRAP analysis. Therefore, we used an L413A/L414A CD4 mutant that does not undergo constitutive endocytosis and is resistant to Nef.
Nef did not alter the relative distributions of the mobile and immobile fractions or the diffusion rates of either the slow or the very slow moving fraction of LL/AA CD4 (Fig. 6D and Table 3). LL/AA CD4, which lacks constitutive endocytosis, had a Dt of 0.073 μm2/s for the faster fraction, almost four time slower than the 0.266 μm2/s rate for the slow HLA-I A2 at the plasma membrane.
Nef Effects on Two-dimensional Diffusion at the PM
Two diffusion components were observed for CD4 by FCCS analysis, with most of the binding between CD4 and Nef occurring in the more mobile fraction. It is important to remember that FCCS cannot quantify the “immobile and the “slowest” fractions of FRAP (due to bleaching). Most of the Nef at the plasma membrane was found to bind CD4 dileucine mutant. In contrast, only about 30% of HLA-I-A2 at the plasma membrane bound to Nef.
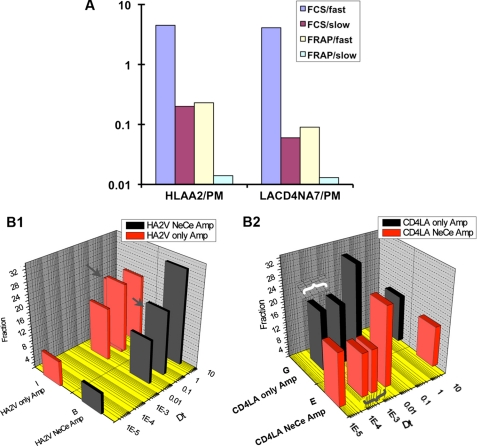
We compared slow diffusion parameters obtained for HLA-I A2- and LL/AA CD4 at the plasma membrane by TPTCFCCS with those obtained from FRAP experiments. As illustrated by Fig. 7, the slow diffusion coefficient obtained by FRAP is comparable with the slow component observed in FCCS. However, it must be noted that although the in vivo dynamics of proteins or membranes and physical interaction(s) of proteins can be evaluated by FCCS or FRAP, the smallest ROI evaluated by FRAP are in the μm2 range, enough to encompass whole subcellular organelles, vesicles, and cytoskeletal elements. As a result, the relative Dt measured by FRAP can reflect complex binding and “sieving” interactions of the fluorescent species with its environment or sequestration within ≤1-μm structures.
FIGURE 7.
A, comparison of parameters obtained by FCCS versus FRAP for the PM interactions of HLA-I A2-Venus or LL/AA CD4-eY/Venus with Nef-CerFP. The slow component from FCCS was comparable with the fast component seen in FRAP. Diffusion coefficients were obtained from FCCS and FRAP analysis of HLA-I A2-eY/V (B1) and LL/AA CD4-eY. B2, consolidation of FCCS and FRAP data. The FRAP data were normalized to the slow component of the FCCS data. If only one component was identified in the FCCS analysis, then FRAP data were normalized to this Dt. Immobile fraction has been plotted with a Dt of 1e−5 due to the log scale constraint. Arrow (B1) and braces (B2) denote the FRAP/FCCS coefficient(s) that were normalized and have the same “height.” They could be thought as a single component.
Thus, FCCS is best at quantifying “neighborhood “ transport, dominated by diffusion, whereas FRAP extends over wider areas (“citywide”) more dependent on traffic patterns and jams. Conversely, FCCS is hampered in studying slower diffusion by the possibility that long residence times in the FCS spot link to photobleaching. FCS is also less tolerant of nondiffusive transport that may be visible but averaged on the extended time scale of FRAP. Nevertheless, the Nef effect was quite pronounced in FCS experiments and had only subtle effects on FRAP curves.
Inhibition of Clathrin-dependent Endocytosis Partially Reversed Nef-induced Loss of HLA-I, Retarded Lateral Mobility of HLA-I and CD4, and Decreased Their Binding to Nef
HLA-I is endocytosed both by clathrin-dependent and -independent pathways (42–44). Nef is presumed to act as a connector between CD4 and endocytic machinery (45, 46) through components of CCV. This suggests that an inhibitor of clathrin-mediated endocytosis could reverse Nef-mediated CD4 down-regulation. The macrolide antibiotic, ikarugamycin (IKA) from Streptomyces phaeochromogenes subsp. ikaruganensis, that inhibits the uptake of clathrin-dependent PM receptors without affecting their internal trafficking (47) was in fact shown to block CD4 down-regulation in response to phorbol 12-myristate 13-acetate or Nef (48).
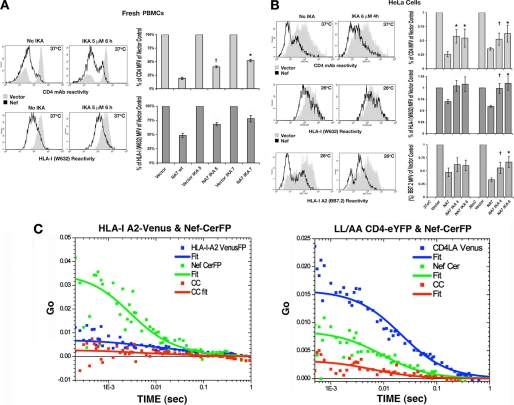
After confirming that IKA was a specific inhibitor of clathrin-dependent endocytosis (supplemental Fig. 8), we tested the effects of IKA treatment on the PM levels of CD4, native HLA-I, or HLA-I A2 in Nef(+) versus Nef(−) HeLa and Jurkat cells or quiescent PBMCs. In HeLa cells, Nef reduced the PM levels of CD4 to 24 ± 3 or 31 ± 2% of control at 37 or 26 °C. Treatment with 4 or 6 μm IKA for 4 h partially reversed this CD4 effect of Nef, with the PM CD4 levels increasing to 59 ± 7 and 53 ± 8 with 4 μm or 51 ± 6 and 63 ± 7% of control levels with 6 μm IKA at 37 and 26 °C, respectively (Fig. 8A). Under the same conditions, the relatively modest down-regulation of native HLA-I (68 ± 5 and 54 ± 3.9% at 37 and 26 °C) was completely reversed, with the HLA-I levels after 4 or 6 μm IKA treatment increasing to 98 ± 10 and 103 ± 11% or 91 ± 8 and 101 ± 9% at 37 and 26 °C, respectively (Fig. 8A). IKA treatment was less effective in reversing the Nef-induced down-regulation of recombinant HLA-I A2 at 37 °C, with PM HLA-I A2 levels increasing from 43 ± 4 to 63 ± 6 or 61 ± 5% at 4 or 6 μm IKA, respectively (Fig. 8A). Although IKA treatment of Nef(+) Jurkat cells partially restored CD4 levels from 22 ± 1 to 54 ± 6% of control at 37 °C (Fig. 8B), it did not restore plasmid-expressed HLA-I A2 levels and had an intermediate effect on the loss of native HLA-I, resulting in an increase in the HLA-I MFV from 31 ± 2 to 50 ± 5% at 37 °C. IKA treatment also partially reversed Nef-induced down-regulation of CD4 and HLA-I on quiescent PBMCs. CD4 MFV of Nef(+) cells increased from 19 ± 1 to 39 ± 2 or 50 ± 4% after 4 h at 5 or 7 μm, respectively (Fig. 8C). Native HLA-I MFVs in Nef expressing PBMCs increased from 48 ± 3 to 64 ± 4.3 or 77 ± 6% after 5 or 7 mm IKA treatment (Fig. 8C).
FIGURE 8.
Inhibition of clathrin-dependent endocytosis partially reversed Nef-induced down-regulation of native HLA-I. A, FACS histogram profiles of CD4, innate HLA-I, and recombinant HLA-I A2 in HeLa cells co-transfected with plasmids expressing WT Nef (NL4-3 or NA7 allele) or NX (vector), HLA-I A2, and CD8 with (right) or without (left) IKA treatment at 6 μm for 4 h. Receptor MFV profiles of vector (gray) and Nef (black) transfectants gated for CD8 are overlaid in each panel. CD4 results were at 37 °C and HLA-I results were at 26 °C. Histograms on the right show average (n = 4 or 5) expression profiles (with error bars) of native CD4 and HLA-I or recombinant HLA-I A2 in Jurkat cells transfected with vector or Nef plasmid with or without 5 μm IKA treatment for 4 h. B, FACS histogram profiles of CD4 (top) or HLA-I (bottom) in quiescent PBMCs expressing Nef or no Nef (vector), treated with (right) or without (left) 5 μm IKA for 4 h at 37 °C. MFV profiles of vector (gray) and Nef (black) transfectants gated for CD8 are overlaid in each panel. The statistical histograms (with error bars) to the right of each set of FACS profiles represent average (n = 4) MFV results at 37 and 26 °C with 4 and 6 μm IKA for HeLa cells or and 7 μm IKA for PBMCs. †, n = 4, p < 0.03, and *, n = 4, p < 0.01, for IKA-treated versus untreated Nef(+) cells. C, auto- and cross-correlation profiles of IKA (5 μm for 4 h at 26 °C)-treated cells. ACFs were determined at single points on the membrane.
Using FCS, we evaluated the effect of IKA on the diffusion of HLA-I A2 or LL/AA CD4 at PM in the context of Nef expression (Fig. 8D, left). Diffusion coefficients for HLA-I A2 markedly decreased after IKA treatment of HLA-I A2-Venus and Nef-CerFP co-transfectants (Fig. 8D, left, and Table 4). The recovered values for a fit to a two-component diffusion model undergoing two-dimensional diffusion were 2.3 ± 0.8 and 0.2 ± 0.1 μm2/s. The faster component had a considerably reduced Dt compared with the 4.9 μm2/s Dt for untreated cells (Table 4). Perhaps coincidentally, this change correlated with the appearance of larger complexes of HLA-I A2 at the PM unable to be endocytosed (Fig. 8D, left). The slower component did not change significantly in IKA-treated cells. IKA marginally reduced the cross-correlation (co-mobility) between Nef and HLA-I A2, resulting in a calculated 19 ± 10% co-mobility, to be contrasted with the 30 ± 4% found in the untreated cells.
IKA affected the Dt for plasma membrane-associated LL/AA CD4 more profoundly with the rate of the faster component dropping from 6.6 to 2.3 ± 0.8 μm2/s (Table 4). This change was accompanied by a drop in the percent bound from 70 ± 20 to 40 ± 10% in the treated cells. Even the Dt of the slower component was reduced by >3-fold (Fig. 8D, right and Table 4). IKA treatment has been shown to increase the residence time of CD4 at the plasma membrane (48). In Nef-expressing cells, CD4 is cleared from the plasma membrane within 2 h. IKA treatment prolonged the CD4 presence at the plasma membrane by 60%. Although the overall bound fraction of Nef was reduced (Fig. 8D, right, and Table 4), the considerable decrease in the diffusion rates probably resulted from an increase in the large complexes formed at the plasma membrane between Nef, unknown cellular partners, and HLA-I A2 or LL/AA CD4 when clathrin function was persistently impaired.
Multimolecular Interactions between Nef and HLA-I A2 or LL/AA CD4 at the PM Were Disrupted by Cholesterol Depletion
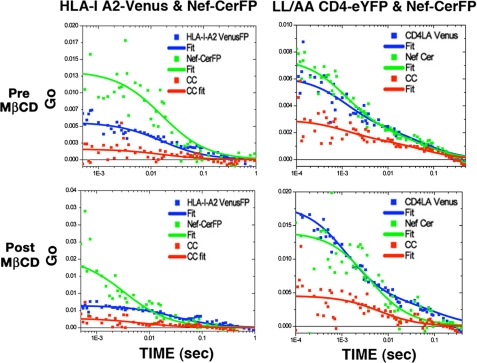
MHC-I and MHC-II proteins associate with detergent-resistant membranes and co-distribute with different raft markers upon antibody clustering or during immunological synapse formation (49–52). In accordance, endocytic trafficking of MHC molecules was dependent on such lipid raft integrity (44, 50, 53). Although we are unaware of the role(s) of the “raft” concept in our measurement, cholesterol depletion inhibited both the constitutive and Nef-enhanced internalization of HLA-I but not of CD4 (supplemental Results and supplemental Fig. 9).
MβCD extraction altered the HLA-I A2 and Nef ACF and CCF profiles significantly. The ACFs obtained at representative points on the PM of cells before and after MβCD extraction are shown on Fig. 9, left. The diffusion was fit to a two-dimensional model for two mobile species. The diffusion coefficients obtained for untreated cells were composed of a faster component of 4.9 ± 1.1 μm2/s and a slower one at 0.23 ± 0.14 μm2/s. The average diffusion coefficients for cross-correlation curves post-treatment were 1.76 ± 0.28 and 0.46 ± 0.15 μm2/s (Table 4). These values are graphically represented by the histograms in Fig. 10A. In this case, we have a small increase of the slow diffusion coefficient and a halving of the fast diffusion coefficient. Thus cholesterol depletion induced significant HLA-I clustering and disrupted its interaction with Nef, and this pattern implied that Nef preferred the faster smaller version of these two species.
FIGURE 9.
Cholesterol depletion disrupts the association of Nef with HLA-I A2 (left) or LL/AA CD4 (right) at the PM. The ACFs (receptor in blue and Nef in red) and CCFs (red) data points were obtained at single points on the membrane before or after MβCD extraction.
FIGURE 10.
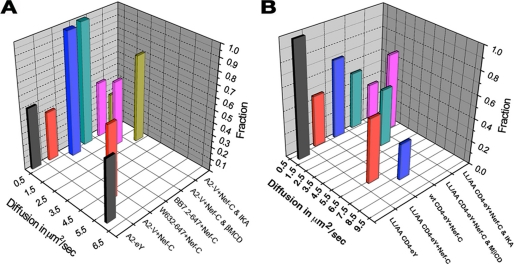
A, diffusion coefficients and relative abundance of HLA-I Nef complexes. A2-V refers to HLA-I-A2-Venus fusion protein; BB7.2-647 denotes Alexa 647-conjugated BB7.2 mAb used to detect recombinant HLA-I-A2 at the PM, and W632-647 refers to Alexa 647-conjugated W632 mAb used to detect native HLA-I at the PM. B, diffusion coefficients and relative abundance of each fraction are plotted for CD4 (WT or L413A/L414A mutant) FCCS measurements.
ACF data for LL/AA CD4-eY and Nef-CerFP obtained before and after treatment with MβCD of double-transfected cells are shown in Fig. 9, right. The average diffusion coefficients obtained for the pretreated cells were 6.6 ± 2.4 and 0.32 ± 0.17 μm2/s for the cross-correlation. The post-treatment gave values of 3.7 ± 2.2 and 0.21 ± 0.07 μm2/s (Table 4). Averaged Dt values from many determinations are denoted by the histograms in Fig. 10B. We found some cells in which the treatment completely abolished the fast component and other cells in which there was little effect on the diffusion coefficient.
For diffusing membrane-spanning lipoprotein complexes, the Dt should depend strongly on membrane viscosity and the size of the (presumed cylindrical) spanning segment; hence, transmembrane segment radii can be calculated from the corresponding diffusion coefficient(s). Presuming lateral diffusion is the only process and modeling our complex as a cylindrical or ellipsoidal structure, we applied the Saffman and Delbruck Equation 5 (54, 55):
 |
Where a is the radius; DL is the lateral diffusion; h is the thickness of the membrane; ηw is the viscosity of the surrounding phase; η is the viscosity of the membrane; T is temperature, and γ is the Euler constant. We obtained a value of 6 centipoise for ηw from measuring the diffusion of Cerulean in the cytoplasm of HeLa cells. Viscosity at the PM has been determined to be 1.3 poise (55, 56). Using this value in the formula, we obtained for a diffusion coefficient of 0.2 μm2/s for a membrane-spanning protein (5 nm) and a viscosity of ∼0.13 Pa·s (1.3 poise) a radius of ∼40 nm. Fig. 10 is a graphic illustration depicting the diffusion coefficients of the various mobile fractions together with their relative abundances determined for the different experimental conditions. The numerical values in Table 4 include in addition the putative cylinder radii of various mobile complexes of HLA-I and CD4s at the plasma membrane. As water is much less viscous than membrane, all these calculations pertain only to the membrane-spanning portion of these species.
FRAP Analyses of Cholesterol Depletion Effects Are in Agreement with FCS Findings
By FRAP analysis, we observed that cholesterol depletion increased the size of the immobile fraction of HLA-I A2 at the PM almost 2-fold (from 20 to 36%) and correspondingly decreased the size of the fast moving fraction (Table 5). Cholesterol extraction induced similar changes in the slow and immobile fractions LL/AA CD4 (Table 6). Nef expression did not modify these changes induced by cholesterol depletion. Thus, the results of FRAP analysis agreed in general with the conclusions deduced from the ACF curves.
TABLE 5.
FRAP analysis of diffusion rates of HLA-I-A2 at the plasma membrane in the context Nef expression after cholesterol depletion
| PM A2C-A2V | PM NA7C-A2V | MβCD/PM A2C and A2V | MβCD/PM NA7C and A2V | |
|---|---|---|---|---|
| t½ (s) | 0.5 | 1.1 | 1 | 1.6 |
| t(fast) | 0.23 ± 0.04 | 0.27 ± 0.11 | 0.07 | 0.167 |
| Dffast (μm2/s) | 0.27 | 0.23 | 0.90 | 0.38 |
| % fast | 40 ± 3 | 25 ± 4 | 19 | 25 |
| t(slow) | 4 ± 0.5 | 4 ± 0.7 | 3 | 6.3 |
| Dfslow (μm2/s) | 0.015 | 0.014 | 0.022 | 0.01 |
| % slow | 40 ± 2 | 43 ± 2 | 45 | 46 |
| % immobile | 20 ± 1 | 31 ± 2 | 36 | 29 |
TABLE 6.
FRAP analysis of diffusion rates of LL/AA CD4 at the plasma membrane in the context of Nef expression after cholesterol depletion
| LL/AA CD4C-C and V | Nef-C and LL/AA CD4 V | MβCD/LL/AA CD4 C and V | MβCD/Nef-C and LL/AA CD4 V | |
|---|---|---|---|---|
| t½ (s) | 2.1 | 1.6 | 1.5 | 2.4 |
| t(fast) | 0.86 ± 0.26 | 0.68 ± 0.36 | 0.09 | 0.16 |
| Dffast (μm2/s) | 0.07 | 0.09 | 0.70 | 0.39 |
| % fast | 32 ± 6 | 27 ± 7 | 18 | 18 |
| t(slow) | 8.6 ± 4 | 4.9 ± 1.8 | 4.4 | 6.4 |
| Dfslow (μm2/s) | 0.01 | 0.01 | 0.01 | 0.01 |
| % slow | 50 ± 4 | 48 ± 9 | 43 | 49 |
| % immobile | 19 ± 8 | 25 ± 3 | 39 | 33 |
For FCS, the diffusion coefficients obtained for many of the complexes are similar to those reported for Golgi resident proteins (57) and MHC class I proteins in the ER (58) and the PM (59). The values obtained for three-dimensional diffusion inside of the organelles yield apparent molecular weights in the millions assuming a cytoplasmic viscosity of 6 centipoise (60), calculated from the presumed free diffusion of Cerulean in the cytoplasm of HeLa cells at room temperature. These unlikely sizes suggested that simple free diffusion models could not be applied to the large multimolecular complexes observed within convoluted or vesicular structures. The degree to which they remain membrane-bound on convoluted surfaces versus diffusing in some nonideal media is unclear; we simply wish to avoid leading readers to numbers for “complex sizes” when effective viscosity and free path is unknown here.
DISCUSSION
This study establishes that HIV Nef down-regulation of MHC-I may involve both proposed mechanisms in human PBMCs as follows: 1) accelerated receptor endocytosis from and aberrant recycling to the plasma membrane, and 2) misrouting of newly synthesized MHC-I from TGN to endolysosomes for degradation. These results support the relevance of both the anterograde and retrograde trafficking pathways for Nef-induced MHC-I down-regulation established by previous authors (3, 10, 13–15) and extend them in the following four ways: 1) by defining their relative importance in primary HIV target cells; 2) by identifying differential utilization in different cell types; 3) by establishing their relative importance for native versus recombinant HLA-I; and 4) by delineating the differential molecular requirements for the Nef effect in multiple cell types. Our work also provides the first biophysical association measurement between Nef and MHC-I in living cells and quantifies (minute by minute) the extent of interaction at the subcellular level. Consistent with the functional effects of Nef on MHC-I down-regulation through effects on both anterograde and retrograde trafficking itineraries, we found that Nef associates with the target molecule(s) throughout the cell.
Our data provide an expanded list of specific factors that mediate the Nef effect on native HLA-I in PBMCs, including clathrin, AP1 adapter, the GTPases Arf6, Rab7, and Rab11, as well as dynamin and Eps15. Eps15 is tightly linked to clathrin-dependent endocytosis (61), and dynamin is critical for both clathrin-dependent and -independent endocytosis barring those pathways regulated by Arf6, Cdc42, or RhoA (62–65). AP1 adapter regulates intervesicular transport, and Arf6 regulates clathrin-independent endocytosis, whereas Rab11 and Rab7 regulate recycling endosomes and endolysosomal fusion, respectively.
As in PBMCs, the mechanism for Nef down-regulation of native HLA-I in HeLa cells involved Arf6, Rab7, and Rab11 GTPases, clathrin, and AP1 adapter(s), but in addition it was also dependent on PACS-I and ARNO. These observations confirm previous reports and extend knowledge of regulation Nef effect to Rab7 and Rab11.
With regard to recombinant HLA-I A2, we confirmed distinct molecular regulation of the Nef effect in different cell contexts. Thus, in Jurkat cells Nef-induced down-regulation of recombinant HLA-I A2 was only reversed by siRNA knockdown of AP1 adapter(s) and clathrin, whereas in HeLa cells knockdown of PACS-1 adapters and genetic inhibitors of Arf6 and Rab GTPases could also reverse the Nef effect. The reason for these differences is not yet known.
Previous in vitro and in vivo investigations using tagged proteins (including Nef-MHC-I hybrids) and co-precipitations identified physical interactions between Nef and HLA-I. In particular, Nef has been reported to bind hypophosphorylated MHC-I early in the secretory pathway but not mature MHC-I at the cell surface (14). Consistent with this, Nef mutants lacking the ability to down-regulate HLA-I failed to co-precipitate with the receptor (19). Structural determinants of Nef binding to cellular partners include Nef residues 62EEEE65 (standardize nomenclature) and to a lesser extent 72PXXP75 for direct formation of a ternary complex with the μ1 subunit of AP1 and the tyrosine-containing cytoplasmic domain of HLA-I (17, 18, 66).
A limitation of this type of approach is that the results report only steady-state measurements without spatiotemporal information. Minute by minute FCCS analysis overcomes these limitations while still providing quantitative information. Using this technique, we found that nearly a third of HLA-I A2-Venus fusion protein at the plasma membrane and in intracellular organelles (ER and Golgi/TGN) was present in Nef-bound complexes. Consistent with this, Nef also bound equivalent fractions of fluorescent antibody-tagged native (and recombinant) HLA-I at the cell surface. If only the AP1 subunit was critical for stabilizing Nef and HLA-I interaction as has been reported previously (17, 18, 66), then FCS should have identified more extensive interactions in the Golgi/TGN vesicles.
Under steady-state conditions, it has been previously reported that MHC-I molecules at the cell surface are nearly evenly divided into two populations, with mean half-lives of 1 and 20 h; the short lived fast trafficking population corresponds to receptor that has not bound peptide or has bound low affinity peptide (67). It remains to be determined whether the subpopulation of MHC-I we found associated with Nef by FCCS corresponds to one or the other of these kinetically distinct MHC-I species. MHC-I loaded with fluorescent peptides of different affinities (and colors) could be employed to evaluate binding of peptide-loaded MHC-I to Nef, but this is beyond the scope of this study.
Using FCCS, we also found that both wild type CD4 and the endocytosis-resistant LL/AA mutant of CD4 had strong affinity for Nef, with 70 ± 20% of WT or 90 ± 30% of the mutant at the PM associated with Nef. The greater affinity (during the clearance process) of Nef for CD4 versus HLA-I is in agreement with the end point observations that Nef is much more potent in down-regulating CD4 versus HLA-I from the cell surface.
To cross-validate the molecular dynamics of MHC-I down-regulation by Nef, we used FRAP imaging. Analysis of steady-state recombinant HLA-I A2 in HeLa cells lacking Nef identified an immobile fraction and two mobile fractions that had a 10–20-fold difference in diffusion coefficients. The relative distribution of these components was similar to the previous reports of murine H2L in L cells (59) and human MHC-I on HeLa cells (68), and the diffusion coefficient of the very slow species matched the comparable species identified by single particle tracking studies (69). Although Nef did not change the diffusion coefficients measured by FRAP of either the slow or the very slow diffusing fraction of HLA-I at the plasma membrane significantly, there was a significant decrease in the amount of the slow diffusing fraction and a reciprocal increase in the amount of the immobile fraction. These data suggest that Nef induces clustering of surviving HLA-I and reduces the average lateral mobility of HLA-I. In contrast, Nef did not alter the relative distributions of the mobile and immobile fractions or the diffusion rates of either the slow or the very slow moving fraction of LL/AA CD4, which is not susceptible to endocytosis whether Nef is present or not.
IKA has previously been shown (48) to block Nef-mediated down-regulation of CD4 in various cell types. Consistent with our clathrin knockdown data, we found that this agent could partially reverse Nef-induced down-regulation of native HLA-I in both HeLa cells and resting PBMCs. Moreover, our FCCS analysis indicated that IKA treatment significantly reduced the Nef-bound fraction of both HLA-I and CD4 (by ∼50%) and decreased their lateral mobility (by >60%), while facilitating receptor sequestering near or on the cell surface. This analysis in living cells is consistent with previously published observations using hypertonic saline or dominant-negative dynamin expression to inhibit clathrin-coated vesicle traffic (43). In a recent report using total internal reflection fluorescence microscopy (70), Nef at the plasma membrane was exclusively associated with clathrin-coated pits and vesicles (CCPs and CCVs). We too observed that WT and three different Nef mutants extensively co-localized with CCVs (supplemental Fig. 10). If all Nef-positive spots at the PM were associated with CCP(V)s, then Nef binding fractions of CD4 and MHC-I might represent receptors being recruited into the CCVs, and if Nef association with clathrin were critical for binding to CD4 and MHC-I, then clathrin depletion or inactivation would eliminate Nef binding to either receptor. This was what was observed in IKA-treated cells.
Our data from acute cholesterol depletion experiments further suggested that Nef binding to HLA-I in complexes at the plasma membrane is an important determinant of the Nef effect. Cholesterol depletion inhibited Nef down-regulation of HLA-I and changed the auto- and cross-correlation profiles significantly. There was a marked decrease in the diffusion coefficient of the faster species of HLA-I in the cross-correlation profile, suggesting significant disruption in the normal Nef-HLA-I interaction. Cholesterol depletion has been shown previously to reduce lateral mobility and increase clustering of HLA-I, resulting from redistribution of plasma membrane phosphatidylinositol 4,5-bisphosphate and reorganization of the cytoskeleton (71, 72). The marked decrease in the diffusion of faster HLA-I species in the auto-correlation profile suggests unusual HLA-I clustering, as does the large increase in the immobile fraction of HLA-I seen in the FRAP analysis. In HeLa cells, cholesterol depletion reduced constitutive and Nef-enhanced internalization of native HLA-I more than recombinant HLA-I A2, but the reason for this is unknown (supplemental Fig. 9, C versus D).
It is important to note that our endocytosis assay values are the net results of internalization and recycling. Recycling rates of HLA-I are difficult to quantify because both β2-microglobulin and peptide dissociate from internalized HLA-I, and about half of the internalized receptor is degraded under physiological conditions (53, 73). Nevertheless, treatments that inhibited “net” endocytosis increased HLA-I clustering and inhibited the Nef binding potential.
If Nef enhances endocytosis of native HLA-I, and depletion of clathrin and AP1 can reverse the Nef effect, then why did depletion of AP2 not also reverse the Nef effect? Clathrin-coated vesicles are involved in endocytosis and intracellular vesicle traffic between TGN and late lysosomes (74). AP2 facilitates clathrin-driven endocytosis, whereas AP1 facilitates clathrin-mediated vesicle trafficking between the TGN and endosomes (although there is still some question about directionality) (75). In the absence of AP1, both endocytosed HLA-I and newly synthesized HLA-I in transit to the cell surface would have recycled or followed the default pathway to the plasma membrane, rather than being diverted to the TGN and to MVBs/lysosomes. In contrast, depletion of AP2 might divert HLA-I to clathrin-independent endocytic pathways (76), such as through Arf6 that may in turn be subverted by Nef.
It was of interest that mutations at the three Nef motifs deemed critical for the HLA-I effect only induced a partial reversal of the Nef effect on both the innate and recombinant HLA-I levels at the plasma membrane in T cell lines. In HeLa cells and quiescent PBMCs, mutation at the Glu-62–65 or the M20A motif of Nef completely restored the native but not the plasmid-expressed HLA-I levels (supplemental Fig. 11). Whether the EEEE at position 62 is a PACS-binding motif (10, 12) or whether residues Glu-62–65 and to a less extent, prolines at positions 72 and 78 in Nef are required for direct interaction with the AP1 μ1 subunit and HLA-I (18, 66), the incomplete rescue with the Nef mutants suggests that these critical motifs may be players in both antero- and retrograde trafficking defects.
A limitation of FCCS and other live cell imaging analyses is the uncertainty whether two associated proteins actually bind directly and/or interact functionally versus whether the correlation is indirect and facilitated by unidentified adapters. In this regard, we are currently attempting to define ternary Nef interactions with HLA-I and candidate vesicle adapters, including clathrin. A related limitation in live cell imaging is artifacts induced by protein overexpression. We have tried to avoid these by limiting recombinant protein expression to modest promotion levels and for short times, and we have not noted any vesicular changes (77, 78); of course, we cannot yet rule out overexpression artifacts. We should note that our FCS measurements are effectively done at low submicromolar concentration levels, and thus these studies have been conducted under minimal perturbation conditions. Given the HeLa cell volume of ∼2 pl, our data have been collected at Nef copy levels well under 105 per cell, which is within a range compatible with the Nef levels anticipated in early infection.
Furthermore, although we purposely avoided calculating Kd values for MHC or CD4 in unknown multiprotein complexes, it is clear that Nef must have nanomolar affinity for these unknown complexes, as both the complexes and Nef are seen to be present at nanomolar levels in our cross-correlation experiments.
In conclusion, our data support a model in which Nef may associate with a subpopulation of MHC-I throughout the cell and down-regulate MHC-I from the plasma membrane through effects on both anterograde and retrograde itineraries. We have extended biophysical knowledge of this important HIV immune evasion system by defining diffusion parameters of HLA-I and CD4 at the PM, yielding the apparent size of the molecular complexes. These systems can be used to further evaluate the molecular basis of the Nef effect on MHC-I dynamics, for example in cells lacking various vesicle transport proteins. In vivo kinetics are essential to gain mechanistic insight of cellular processes.
Supplementary Material
Acknowledgments
We thank Yong-Ou Kim, currently at the Center for Biomedical Sciences, Korea National Institute of Health, Seoul, Korea, for some preliminary experiments. We thank Jennifer Lippincott-Schwartz of the NICHD, National Institutes of Health, for Cerulean and Venus expression plasmids. We thank Philip Murphy and Joshua Farber of Laboratory of Molecular Immunology, NIAID, National Institutes of Health, for critical review of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health grant from Division of Intramural Research, NIAID.

The on-line version of this article (available at http://www.jbc.org) contains supplemental “Experimental Procedures,” “Results,” Figs. 1–11, Tables I and II, and additional references.
T. J. Stasevich, F. Mueller, A. Michelman-Ribereiro, T. Rosales, J. R. Knutson, and J. G. McNally, submitted for publication.
- TGN
- trans-Golgi network
- PBMC
- peripheral blood mononuclear cell
- ROI
- region of interest
- ER
- endoplasmic reticulum
- PM
- plasma membrane
- ACF
- autocorrelation function
- CCF
- cross-correlation function
- MβCD
- methyl-β-cyclodextrin
- MFV
- mean fluorescence value
- TPTCFCCS
- two-photon two-color fluorescence cross-correlation spectroscopy
- FCS
- fluorescence cross-correlation spectroscopy
- FCCS
- fluorescence cross-correlation spectroscopy
- FRAP
- fluorescence recovery after photobleaching
- CHC
- clathrin heavy chain
- CCV
- clathrin-coated vesicle
- IKA
- ikarugamycin
- NX
- null mutant
- eYFP
- enhanced YFP
- CFP
- cyan fluorescent protein.
REFERENCES
- 1.Collins K. L., Baltimore D. (1999) Immunol. Rev. 168, 65–74 [DOI] [PubMed] [Google Scholar]
- 2.Garcia J. V., Miller A. D. (1991) Nature 350, 508–511 [DOI] [PubMed] [Google Scholar]
- 3.Schwartz O., Maréchal V., Le Gall S., Lemonnier F., Heard J. M. (1996) Nat. Med. 2, 338–342 [DOI] [PubMed] [Google Scholar]
- 4.Sol-Foulon N., Moris A., Nobile C., Boccaccio C., Engering A., Abastado J. P., Heard J. M., van Kooyk Y., Schwartz O. (2002) Immunity 16, 145–155 [DOI] [PubMed] [Google Scholar]
- 5.Stumptner-Cuvelette P., Morchoisne S., Dugast M., Le Gall S., Raposo G., Schwartz O., Benaroch P. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 12144–12149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ploegh H. L. (1998) Science 280, 248–253 [DOI] [PubMed] [Google Scholar]
- 7.Hansen T. H., Bouvier M. (2009) Nat. Rev. Immunol. 9, 503–513 [DOI] [PubMed] [Google Scholar]
- 8.Lilley B. N., Ploegh H. L. (2005) Immunol. Rev. 207, 126–144 [DOI] [PubMed] [Google Scholar]
- 9.Greenberg M. E., Iafrate A. J., Skowronski J. (1998) EMBO J. 17, 2777–2789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blagoveshchenskaya A. D., Thomas L., Feliciangeli S. F., Hung C. H., Thomas G. (2002) Cell 111, 853–866 [DOI] [PubMed] [Google Scholar]
- 11.Hung C. H., Thomas L., Ruby C. E., Atkins K. M., Morris N. P., Knight Z. A., Scholz I., Barklis E., Weinberg A. D., Shokat K. M., Thomas G. (2007) Cell Host Microbe. 1, 121–133 [DOI] [PubMed] [Google Scholar]
- 12.Piguet V., Wan L., Borel C., Mangasarian A., Demaurex N., Thomas G., Trono D. (2000) Nat. Cell Biol. 2, 163–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le Gall S., Erdtmann L., Benichou S., Berlioz-Torrent C., Liu L., Benarous R., Heard J. M., Schwartz O. (1998) Immunity 8, 483–495 [DOI] [PubMed] [Google Scholar]
- 14.Kasper M. R., Roeth J. F., Williams M., Filzen T. M., Fleis R. I., Collins K. L. (2005) J. Biol. Chem. 280, 12840–12848 [DOI] [PubMed] [Google Scholar]
- 15.Roeth J. F., Williams M., Kasper M. R., Filzen T. M., Collins K. L. (2004) J. Cell Biol. 167, 903–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schaefer M. R., Wonderlich E. R., Roeth J. F., Leonard J. A., Collins K. L. (2008) PLoS Pathog. 4, e1000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wonderlich E. R., Williams M., Collins K. L. (2008) J. Biol. Chem. 283, 3011–3022 [DOI] [PubMed] [Google Scholar]
- 18.Noviello C. M., Benichou S., Guatelli J. C. (2008) J. Virol. 82, 1249–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams M., Roeth J. F., Kasper M. R., Filzen T. M., Collins K. L. (2005) J. Virol. 79, 632–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rose J. J., Janvier K., Chandrasekhar S., Sekaly R. P., Bonifacino J. S., Venkatesan S. (2005) J. Biol. Chem. 280, 7413–7426 [DOI] [PubMed] [Google Scholar]
- 21.Venkatesan S., Rose J. J., Lodge R., Murphy P. M., Foley J. F. (2003) Mol. Biol. Cell 14, 3305–3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dell'Angelica E. C., Ooi C. E., Bonifacino J. S. (1997) J. Biol. Chem. 272, 15078–15084 [DOI] [PubMed] [Google Scholar]
- 23.Magde D., Webb W. W., Elson E. (1972) Phys. Rev. Lett. 29, 705–708 [Google Scholar]
- 24.García-Sáez A. J., Schwille P. (2008) Methods 46, 116–122 [DOI] [PubMed] [Google Scholar]
- 25.Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. (1976) Biophys. J. 16, 1055–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lippincott-Schwartz J., Snapp E., Kenworthy A. (2001) Nat. Rev. Mol. Cell Biol. 2, 444–456 [DOI] [PubMed] [Google Scholar]
- 27.Lubben N. B., Sahlender D. A., Motley A. M., Lehner P. J., Benaroch P., Robinson M. S. (2007) Mol. Biol. Cell 18, 3351–3365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caplan S., Naslavsky N., Hartnell L. M., Lodge R., Polishchuk R. S., Donaldson J. G., Bonifacino J. S. (2002) EMBO J. 21, 2557–2567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwille P., Haupts U., Maiti S., Webb W. W. (1999) Biophys. J. 77, 2251–2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bacia K., Kim S. A., Schwille P. (2006) Nat. Methods 3, 83–89 [DOI] [PubMed] [Google Scholar]
- 31.Bacia K., Schwille P. (2007) Nat. Protoc. 2, 2842–2856 [DOI] [PubMed] [Google Scholar]
- 32.Larson D. R., Gosse J. A., Holowka D. A., Baird B. A., Webb W. W. (2005) J. Cell Biol. 171, 527–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Golebiewska U., Nyako M., Woturski W., Zaitseva I., McLaughlin S. (2008) Mol. Biol. Cell 19, 1663–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bacia K., Scherfeld D., Kahya N., Schwille P. (2004) Biophys. J. 87, 1034–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Willems P. H., Swarts H. G., Hink M. A., Koopman W. J. (2009) Methods Enzymol. 456, 287–302 [DOI] [PubMed] [Google Scholar]
- 36.Weiss M., Hashimoto H., Nilsson T. (2003) Biophys. J. 84, 4043–4052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grzesiek S., Stahl S. J., Wingfield P. T., Bax A. (1996) Biochemistry 35, 10256–10261 [DOI] [PubMed] [Google Scholar]
- 38.Preusser A., Briese L., Willbold D. (2002) Biochem. Biophys. Res. Commun. 292, 734–740 [DOI] [PubMed] [Google Scholar]
- 39.Harris M. P., Neil J. C. (1994) J. Mol. Biol. 241, 136–142 [DOI] [PubMed] [Google Scholar]
- 40.Bentham M., Mazaleyrat S., Harris M. (2003) J. Gen. Virol. 84, 2705–2713 [DOI] [PubMed] [Google Scholar]
- 41.Pitcher C., Höning S., Fingerhut A., Bowers K., Marsh M. (1999) Mol. Biol. Cell 10, 677–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Donaldson J. G., Williams D. B. (2009) Traffic 10, 1745–1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang Q., Edidin M. (2001) Biophys. J. 81, 196–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chaudhry A., Verghese D. A., Das S. R., Jameel S., George A., Bal V., Mayor S., Rath S. (2009) J. Immunol. 183, 2415–2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Greenberg M. E., Bronson S., Lock M., Neumann M., Pavlakis G. N., Skowronski J. (1997) EMBO J. 16, 6964–6976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Piguet V., Chen Y. L., Mangasarian A., Foti M., Carpentier J. L., Trono D. (1998) EMBO J. 17, 2472–2481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hasumi K., Shinohara C., Naganuma S., Endo A. (1992) Eur. J. Biochem. 205, 841–846 [DOI] [PubMed] [Google Scholar]
- 48.Luo T., Fredericksen B. L., Hasumi K., Endo A., Garcia J. V. (2001) J. Virol. 75, 2488–2492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anderson H. A., Hiltbold E. M., Roche P. A. (2000) Nat. Immunol. 1, 156–162 [DOI] [PubMed] [Google Scholar]
- 50.Chaudhry A., Das S. R., Jameel S., George A., Bal V., Mayor S., Rath S. (2007) Cell Host Microbe. 1, 37–49 [DOI] [PubMed] [Google Scholar]
- 51.Hiltbold E. M., Poloso N. J., Roche P. A. (2003) J. Immunol. 170, 1329–1338 [DOI] [PubMed] [Google Scholar]
- 52.Knorr R., Karacsonyi C., Lindner R. (2009) J. Cell Sci. 122, 1584–1594 [DOI] [PubMed] [Google Scholar]
- 53.Naslavsky N., Weigert R., Donaldson J. G. (2004) Mol. Biol. Cell 15, 3542–3552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saffman P. G., Delbrück M. (1975) Proc. Natl. Acad. Sci. U.S.A. 72, 3111–3113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pralle A., Keller P., Florin E. L., Simons K., Hörber J. K. (2000) J. Cell Biol. 148, 997–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peters R., Cherry R. J. (1982) Proc. Natl. Acad. Sci. U.S.A. 79, 4317–4321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cole N. B., Smith C. L., Sciaky N., Terasaki M., Edidin M., Lippincott-Schwartz J. (1996) Science 273, 797–801 [DOI] [PubMed] [Google Scholar]
- 58.Pentcheva T., Edidin M. (2001) J. Immunol. 166, 6625–6632 [DOI] [PubMed] [Google Scholar]
- 59.Marguet D., Spiliotis E. T., Pentcheva T., Lebowitz M., Schneck J., Edidin M. (1999) Immunity 11, 231–240 [DOI] [PubMed] [Google Scholar]
- 60.Swaminathan R., Hoang C. P., Verkman A. S. (1997) Biophys. J. 72, 1900–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Benmerah A., Bayrou M., Cerf-Bensussan N., Dautry-Varsat A. (1999) J. Cell Sci. 112, 1303–1311 [DOI] [PubMed] [Google Scholar]
- 62.Henley J. R., Krueger E. W., Oswald B. J., McNiven M. A. (1998) J. Cell Biol. 141, 85–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lamaze C., Dujeancourt A., Baba T., Lo C. G., Benmerah A., Dautry-Varsat A. (2001) Mol. Cell 7, 661–671 [DOI] [PubMed] [Google Scholar]
- 64.Schmid S. L., McNiven M. A., De Camilli P. (1998) Curr. Opin. Cell Biol. 10, 504–512 [DOI] [PubMed] [Google Scholar]
- 65.Mayor S., Pagano R. E. (2007) Nat. Rev. Mol. Cell Biol. 8, 603–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baugh L. L., Garcia J. V., Foster J. L. (2008) J. Virol. 82, 9657–9667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Su R. C., Miller R. G. (2001) J. Immunol. 167, 4869–4877 [DOI] [PubMed] [Google Scholar]
- 68.Georgiou G., Bahra S. S., Mackie A. R., Wolfe C. A., O'Shea P., Ladha S., Fernandez N., Cherry R. J. (2002) Biophys. J. 82, 1828–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smith P. R., Morrison I. E., Wilson K. M., Fernández N., Cherry R. J. (1999) Biophys. J. 76, 3331–3344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Burtey A., Rappoport J. Z., Bouchet J., Basmaciogullari S., Guatelli J., Simon S. M., Benichou S., Benmerah A. (2007) Traffic 8, 61–76 [DOI] [PubMed] [Google Scholar]
- 71.Kwik J., Boyle S., Fooksman D., Margolis L., Sheetz M. P., Edidin M. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 13964–13969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fooksman D. R., Grönvall G. K., Tang Q., Edidin M. (2006) J. Immunol. 176, 6673–6680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Edidin M., Achilles S., Zeff R., Wei T. (1997) Immunogenetics 46, 41–45 [DOI] [PubMed] [Google Scholar]
- 74.Bonifacino J. S., Lippincott-Schwartz J. (2003) Nat. Rev. Mol. Cell Biol. 4, 409–414 [DOI] [PubMed] [Google Scholar]
- 75.Robinson M. S. (2004) Trends Cell Biol. 14, 167–174 [DOI] [PubMed] [Google Scholar]
- 76.Damke H., Baba T., van der Bliek A. M., Schmid S. L. (1995) J. Cell Biol. 131, 69–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Madrid R., Janvier K., Hitchin D., Day J., Coleman S., Noviello C., Bouchet J., Benmerah A., Guatelli J., Benichou S. (2005) J. Biol. Chem. 280, 5032–5044 [DOI] [PubMed] [Google Scholar]
- 78.Costa L. J., Chen N., Lopes A., Aguiar R. S., Tanuri A., Plemenitas A., Peterlin B. M. (2006) Retrovirology 3, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.