Abstract
Recombinant human mitochondrial transcription factor A protein (rhTFAM) was evaluated for its acute effects on cultured cells and chronic effects in mice. Fibroblasts incubated with rhTFAM acutely increased respiration in a chloramphenicol-sensitive manner. SH-SY5Y cells showed rhTFAM concentration-dependent reduction of methylpyridinium (MPP+)-induced oxidative stress and increases in lowered ATP levels and viability. Mice treated with weekly i.v. rhTFAM showed increased mitochondrial gene copy number, complex I protein levels and ATP production rates; oxidative damage to proteins was decreased ~50%. rhTFAM treatment improved motor recovery rate after treatment with MPTP and dose-dependently improved survival in the lipopolysaccharide model of endotoxin sepsis.
Keywords: recombinant TFAM, mitochondrial biogenesis, ATP synthesis, MPTP, sepsis
1. Introduction
Aging can be defined as a condition in which the organism becomes less successful over time in adapting to internal and external stresses and eventually dies (Figueiredo et al., 2009; Fukui and Moraes, 2009; Gredilla et al., 2008; Hayashi et al., 2008; Huang and Hood, 2009; Jang and Remmen, 2009; Long et al., 2009; Mattson et al., 2008; Petrosillo et al., 2008; Reeve et al., 2008; Smigrodzki and Khan, 2005; Van Remmen and Jones, 2009; Wallace, 2005; Zhou et al., 2009). In aerobic organisms dependent on mitochondrial oxidative phosphorylation, aging is frequently associated with decline of bioenergetic efficiency leading to depressed ATP generation capacity (Figueiredo et al., 2009; Huang and Hood, 2009; Jeng et al., 2008; Kadenbach et al., 2009; Mattson et al., 2008; Shadel, 2008; Swerdlow and Khan, 2009; Zhou et al., 2008) and increased leakage of electrons from the electron transport chain yielding reactive oxygen species (ROS) (Ma et al., 2009; Mattson et al., 2008; Miyazawa et al., 2009; Petrosillo et al., 2008). ROS-driven oxidative stress damage to mitochondrial nucleic acids, protein and lipids further impairs ATP production, leading to a downward bioenergetic spiral (Figueiredo et al., 2009; Huang and Hood, 2009). However, this “free radical theory of aging” has its critics (Jang and Remmen, 2009; Van Remmen and Jones, 2009)
In mammals, these aging-related impairments particularly affect high-energy, post-mitotic tissues such as brain, retina, cardiac and skeletal muscle that are rich in mitochondria. In human substantia nigra neurons (Bender et al., 2006; Bender et al., 2008; Kraytsberg et al., 2006) and skeletal muscle (Huang and Hood, 2009) (and references therein), individual neurons or muscle fibers can show aging-related, major losses of mitochondrial respiratory capacity associated with increased proportions of ~5 kbase or larger deletions of mitochondrial DNA (mtDNA). Such mtDNA deletions may accumulate from defective repair of oxidatively or damaged mtDNA (Krishnan et al., 2008), appear to have a replicative advantage (Fukui and Moraes, 2009) and when present in high abundance, can result in severely deficient mitochondrial respiratory chain function (Bender et al., 2006; Kraytsberg et al., 2006). Oxidative stress damage to mtDNA may induce its degradation (Shokolenko et al., 2009), and enzyme systems for repair of oxidatively damaged mtDNA decline in brain with aging (de Souza-Pinto et al., 2008; Gredilla et al., 2008). Aging-related sarcopenia (muscle loss) and neurodegeneration may thus derive in part from loss of intact mtDNA due to age-related, increasing oxidative damage, decreased repair, increased destruction, increased deletions and replicative advantages of deleted mtDNA's. Although life span is presently finite for all living things including humans, reversal or prevention of aging-related mitochondrial bioenergetic decline is likely to improve the quality of life during the aging process.
Sepsis is a serious, frequently fatal acute illness that arises as a result of exposure to bacterial wall components that result initially in impaired cardiovascular integrity followed by multiple organ failure. Tissues from septic animals and humans exhibit “cytopathic hypoxia”, a condition of impaired mitochondrial respiration occurring in spite of the presence of adequate oxygen and metabolic substrates (Crouser, 2004). Possible causes of cytopathic hypoxia include post-translational modifications of respiratory proteins (Samavati et al., 2008) that may arise in part from increased production of nitric oxide (Brealey et al., 2002; Crouser, 2004). Such a relationship between increased nitric oxide and oxidative stress, reduced mitochondrial complex I activity and ATP levels, associated with reduced survival has been demonstrated in muscle tissues from humans experiencing sepsis (Brealey et al., 2002). If morbidity and mortality in sepsis derive in part from this acute mitochondrial failure, then strategies to improve respiration may improve outcome from sepsis.
We have developed a novel approach to increasing mitochondrial bioenergetic function and stimulating mitochondrial biogenesis in vitro and in vivo. Our technology is based on use of recombinant human mitochondrial transcription factor A (rhTFAM), engineered with a protein transduction domain (PTD) to cross plasma membranes easily, and a SOD2 mitochondrial localization sequence (MLS) to target the mitochondrial matrix. TFAM is a transcription factor produced in the cytoplasm and imported into mitochondria where it directly controls the expression of mitochondrial DNA-encoded genes and mtDNA replication (Ekstrand et al., 2004; Fisher and Clayton, 1988; Scarpulla, 2006, 2008; Shadel, 2008). TFAM is a dual high mobility group box (HMGB) protein that can bind site-specifically to mtDNA and facilitate the recruiting of mitochondrial RNA polymerase and mitochondrial transcription factors B1/B2 (Gangelhoff et al., 2009). Aside from its essential function as a transcription factor, TFAM also non-specifically binds mtDNA irrespective of DNA sequence specificity. It can coat mtDNA at a ratio up to about 35-50 TFAM molecules per mitochondrial genome, supporting robust transcription but not providing complete mtDNA coating (Cotney et al., 2007; Maniura-Weber et al., 2004). TFAM also coordinates the incorporation of multiple mtDNA molecules into multi-protein spheroid bodies known as nucleoids that are physically located along the inner membrane and are the fundamental units of mtDNA segregation (Kaufman et al., 2007).
We have shown that our engineered rhTFAM with its “mitochondrial transduction domain” (PTD+MLS) rapidly localizes to mitochondria in vitro (Iyer et al., 2009). rhTFAM is also capable of binding mtDNA in vitro and may be used to deliver mtDNA to mitochondria (Keeney et al., 2009). A single exposure to rhTFAM of SH-SY5Y neural cybrid cells containing near-homoplasmic abundance of mtDNA with the G11778A mutation of Leber's optic neuropathy (LHON) reversibly increased respiration and mitochondrial gene expression, suggesting stimulation of mitochondrial biogenesis (Iyer et al., 2009). In a subsequent study we treated Parkinson's disease cybrid cells with rhTFAM alone or complexed with human mtDNA (Keeney et al., 2009). Respiration could be restored in nearly anaerobic cells, and stimulation of mitochondrial biogenesis was again observed, including increases in expression of TFAM and the mitochondrial biogenesis transcription factor PGC-1 alpha (Diaz and Moraes, 2008; Handschin and Spiegelman, 2006; Lin et al., 2005).
Our initial study using i.v. treatment of normal mice with rhTFAM showed improved motor function and increased mitochondrial respiration (Iyer et al., 2009). In the present study we have expanded those findings by treating an additional group of mice with rhTFAM and examining mitochondrial physiology in more detail. We provide evidence in vitro and in vivo for stimulation of mtDNA replication and ETC protein translation as well as increased ATP synthesis rates associated with reduced oxidative stress damage. Further, we demonstrate potential efficacy of rhTFAM in improving recovery of motor function in mice with experimental parkinsonism from MPTP treatment and increasing survival of mice with experimental sepsis. In combination, these findings support the viability of future studies of rhTFAM as a therapeutic that can enhance mitochondrial function in vivo.
2. Methods
Ethics statement
All experiments with mice were conducted according to NIH guidelines under the auspices of approved animal protocols. Experiments with mice used to generate data for Figures 5-10 were conducted under the auspices of a protocol approved by the University of Virginia Animal Care Committee. Experiments with mice used to generate data for Figures 3, 4 and 11 were conducted by a contractor (Invitek, Inc.; www.invitekinc.com) under the auspices of their own animal protocols. rhTFAM production
Figure 5.
ATP synthesis in mitochondrial preparations from brains (a), hearts (b) and skeletal muscles (c) of mice exercise tested and treated with rhTFAM (“exercise-TFAM”, n=3) or vehicle CTL (“exercise-CTL”, n=3) compared to no exercise, no treatment (“CTL”, n=4). Shown are mean +/- SEM ATP levels following addition of ADP to mitochondrial preparations in buffer containing glutamate/malate/succinate/cytochrome C. Statistical analysis by ANOVA showed: (a) p=0.044 comparing TFAM-exercise to CTL-exercise; (c) interaction of time with exercise, p=0.0005 for TFAM and p=0.0087 for CTL.
Figure 10.
Total skeletal muscle Complex I protein levels (normalized to beta actin) are increased in rhTFAM treated mice. Levels are expressed as percentage of mean CTL values. Shown are the results for individual Complex I subunits, and at the top, a composite of all four Complex I subunits.
Figure 3.
rhTFAM enters brain mitochondria after I.V. injection. Normal adult male C57BL/6 mice were injected via tail vein with rhTFAM (200uL, n=6/timepoint), followed by sacrifice and brain removal at various timepoints. Mitochondrial fractions were prepared; 100 ug of mouse brain mitochondria were lysed in SDS-PAGE loading buffer and run on a 10%Bis-Tris gel. Gels were transferred to PVDF and probed with an anti-HA tag antibody (Invitrogen) and cytochrome c loading control antibody (Santa Cruz). Pierce ECL Femto Chemilumenscence Kit was used to detect secondary antibody signal. Optical density units were calculated and normalized to the highest value representing 100%.
Figure 4.
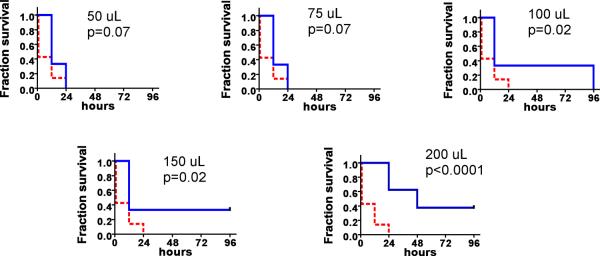
rhTFAM improves motor function after MPTP treatment. Normal adult male C57BL/6 mice were injected via tail vein with varying doses of rhTFAM (or saline vehicle) every four days (n=6 mice/dose). After the third rhTFAM injection treatment with MPTP was begun (30 mg/kg I.P. qd X5). After the sixth rhTFAM treatment (12 days after starting MPTP), animals were tested over three consecutive days for duration on a slowly accelerating rotarod (from 4 rpm to 40 rpm over 6 minutes). The slopes of improvement in duration were calculated across the three test days for each animal. Slopes of improvement in mice treated with rhTFAM doses of 50 uL (p=0.019) and 75 uL (p=0.027) but not with the other rhTFAM doses were significantly different from saline vehicle.
Figure 11.
rhTFAM treatment increases survival of mice given LPS endotoxin (25 mg/kg). Shown are survival curves for vehicle (n=14) and rhTFAM treated (n=6-8) mice. Vehicle-treated survival is shown in red dashed lines, rhTFAM survival in blue solid lines. P values were determined by the Cox-Mantel test.
Recombinant human TFAM (rhTFAM) with an N-terminal, 11-arginine protein transduction domain (PTD) and SOD2 mitochondrial localization sequence (MLS) was produced and purified as described (Iyer et al., 2009). The final protein was stored in 50% glycerol at -20 °C. Binding of DNA by rhTFAM was analyzed with EMSA. One unit (U) of rhTFAM is that amount of solution that binds and retards in the EMSA one microgram of DNA.
Whole cell oxygen consumption
30,000 mouse and human fibroblast cells/well were cultured in BD OxygenBiosensor™ Plates with Cytodex-3 beads to enable attachment in normal DMEM with 10% FBS. Two hours before treatment with rhTFAM (1U/well), a subset of wells were treated with chloramphenicol (150ug/ml) to block mitochondrial translation of mtDNA. Control cells were treated with vehicle (1uL/well of a 50% glycerol and PBS solution). Over the next 60 minutes, fluorescence was assessed every minute according to manufacturer's protocol in a Tecan M200 plate reader. Data are expressed as normalized fluorescence units according to the manufacturer's protocol. Cells were obtained from American Type Culture Collection (www.atcc.org).
Exposure of SH-SY5Y neuroblastoma to rhTFAM and MPP+ or rotenone
Human SH-SY5Y neuroblastoma (www.atcc.org) were incubated with 0-3 U/well of rhTFAM and the complex I inhibitors 1 μM methylpyridinium (MPP+) or 2 μM rotenone for 24 hours. Cells were assayed for viability (MTT), ROS (DCF) and ATP levels.
Penetration of i.v. rhTFAM into brain mitochondria
Normal adult male C57BL/6 mice were injected via tail vein with rhTFAM (200uL, n=6/timepoint). At various time points, mice were sacrificed and brains removed. Brain tissue was Dounce homogenized and mitochondrial fractions were prepared. 100 ug of mouse brain mitochondria were lysed in SDS-PAGE loading buffer and run on a 10%Bis-Tris gel. Gels were transferred to PVDF and probed with an anti-HA tag antibody (Invitrogen) and cytochrome c loading control antibody (Santa Cruz). Pierce ECL Femto Chemilumenscence Kit was used to detect secondary antibody signal.
Treatment of mice with rhTFAM and MPTP
Normal adult male C57BL/6 mice (n=6/group) received I.V. tail vein injections of four different doses of the same batch of rhTFAM (50uL, 75 uL, 100 uL, 150 uL), or saline vehicle, every four days for 24 days (6 doses). After the third dose, the mice were treated with MPTP 30 mg/kg I.P. qd × 5 doses to induce nigrostriatal degeneration and parkinsonism (Jackson-Lewis and Przedborski, 2007). At the end of the rhTFAM dosing (12 days after starting MPTP treatment) the mice were tested on a rotarod for duration on an accelerating rotarod (from 4 rpm to 40 rpm over 6 minutes), or on a continuous rotarod (8 rpm for 6 minutes). The mice had three test sessions/day for three consecutive days. (These experiments were carried out by a subcontractor- Invitek, Corporation, Hayward, CA)
Mitochondrial ATP synthesis rate assay
A group of young adult male mice were treated with weekly tail vein injections of rhTFAM (n=4) or equal volume vehicle CTL (n=4) over the course of 4 weeks of rotarod exercise testing, with tissue homogenates and mitochondrial pellets prepared from brain, heart and skeletal muscle as previously described (Iyer et al., 2009). This group of mice represents a second, independent group from that described in our initial work (Iyer et al., 2009)
0.6 ml of the P2 pellet suspension of rhTFAM or vehicle CTL treated tissue dissolved in “MiRO5” buffer was added to the respirometer chamber of Oroboros Oxygraph-2K followed by the substrates for complex I to IV (glutamate-10mM, malate-2mM; succinate-10 mM; cytochrome C-10 uM). ATP synthesis was initiated by adding 5mM ADP. 100 ul from each sample were then removed at different time points (0, 2, 4, 6, 8, 10, 12, 15, 20 minutes) and added to 100 uL of extraction buffer. ATP was measured with the ‘CellTiter-Glo Luminescent Cell Viability Assay' (Promega).
Protein extraction and Western Blotting
Total protein was extracted from the tissue homogenates of both the first (Iyer et al., 2009) and the second groups of rhTFAM/CTL treated mice using RIPA buffer (1:5 ratio) and 100mM PMSF (1:100 ratio) after adding protease inhibitor cocktail I (CalBioChem) Proteins were assayed with the Bio-Rad protein assay kit. Equal amounts (50 ug to 125 ug) of rhTFAM or buffer CTL total proteins from paired samples of different tissues were denatured at 100°C for 5 minutes in sample buffer and electrophoresed through a 12.0% Bis-Tris Criterion™ precast gel (BioRad). The resolved proteins were transferred to nitrocellulose membrane using iBlot Dry Blotting system (Invitrogen). The membrane was then incubated with either primary antibodies (MitoSciences) for different subunits of Complex I (MS 105 for NDUFB8, MS 107 for NDUFB4, MS 109 for a 8 KDa subunit, MS 110 for NDUFS3, MS 111 for NDUFA9), or with complex I-V (MitoProfile® Total OXPHOS Human Antibody cocktail directed against 5 subunits), or with loading controls-mouse anti β-actin (Abcam ab8224) and mouse anti-SOD2 (Abcam ab16956). After washing, all blots were incubated with secondary antibodies IRDye® anti-mouse 800 and anti-rabbit 680 (Li-cor) and imaged by Odyssey infrared imaging system (Li-cor). Individual band intensities were normalized against β-actin band intensity.
Protein oxidation measurement
Carbonyl groups on oxidatively modified proteins were detected by (OxyBlotTM Protein oxidation Detection Kit by Chemicon International) derivatization with 2,4-dinitrophenylhydrazine (DNPH) followed by electrophoresis and immunoblotting for DNPH. About 42 ∝g of each rhTFAM or vehicle CTL total protein was derivatized and loaded on a 12.0% Bis-Tris CriterionTM precast gel (BioRad). For each sample a paired negative control was included with derivatization-control solution. The proteins after separation were transferred to a nitrocellulose membrane and incubated with rabbit primary anti-DNP antibody along with loading controls-mouse anti β-actin (Abcam ab8224) and mouse anti SOD2 (Abcam ab16956). IRDye® anti-mouse 800 and anti-rabbit 680 (Li-cor) were used as secondary antibodies. The membranes were imaged and the intensity of the entire anti-DNPH lane was scanned and quantified with an Odyssey infrared imaging system (Li-cor).
Nucleic acid extraction and RT-qPCR
Total genomic DNA and total RNA were extracted from the tissue homogenates using the AllPrep DNA/RNA Mini kit (Qiagen) and quantified with Quant-IT DNA and Quant-IT Ribo Green RNA assays (Invitrogen). iScript (BioRad) was used to generate complimentary DNA (cDNA) from total RNA (~1 ug). 10 ng of gDNA and cDNA derived from 50 ng of total RNA were used for qPCR assays that were carried out in an iQ5 cycler (BioRad). Primers and TaqMan probes were designed using Beacon Designer software. For mtDNA genes, an external standard of circular mtDNA generated by exonuclease treatment of rat liver genomic DNA was used. For nuclear genes a mouse brain cDNA was generated and dilutions used as an external standard. Human Roche genomic DNA served as external standard for 18S rRNA determinations. cDNA's were diluted an additional 100-fold for 18S rRNA assays. The sequences for primers and probes used are available from the corresponding author (JPB).
Treatment of mice with rhTFAM and lipopolysaccharide (LPS) endotoxin
Male C57BL/6J mice at seven weeks of age were co-administrated LPS (Escherichia coli 0111:B4; Sigma), 25 mg/kg and vehicle (n=14) or rhTFAM at varying doses (n=6-8). After twenty-four hours of endotoxin challenge, a second dose of rhTFAM was given followed by third dose after forty-eight hours. After ninety-six hours of endotoxin challenge, surviving animals were sacrificed. Survival statistics were monitored throughout the study.
Statistical analysis
Parametric or non-parametric paired t-tests were applied to the data, based on whether the data distributed normally or not. In some data sets, one-way ANOVA's were used to search for significance. Survival curves were analyzed by the log-rank (Mantel-Cox) test. InStat and Prism softwares for Mac (GraphPad) were used for these analyses.
3. Results
rhTFAM acutely increases cell respiration
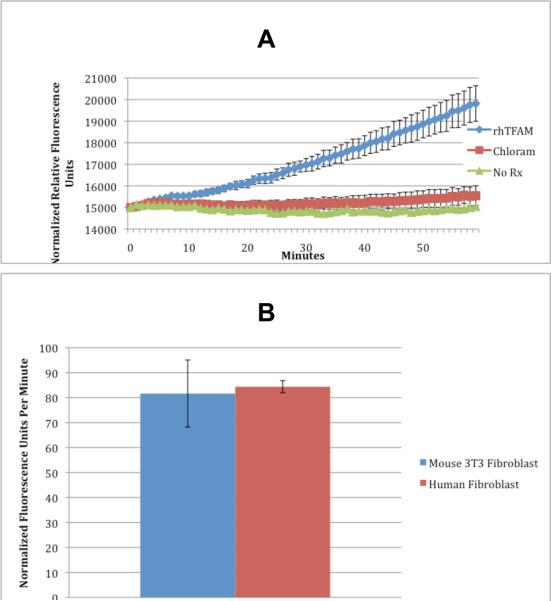
To establish mechanism of action of rhTFAM in vitro, we conducted oxygen consumption experiments on mouse 3T3 fibroblast cells using the BD OxygenBiosensor™ Assay. Mouse fibroblasts were pretreated with chloramphenicol sufficient to inhibit translation of mtDNA derived gene products. Cells were treated with rhTFAM sufficient to bind 1ug of DNA (1U) or vehicle control and immediately assayed for changes in oxygen consumption. We found acute increases in oxygen consumption in rhTFAM treated mouse fibroblasts beginning within minutes that were blocked by chloramphenicol (Figure 1A). We also compared rhTFAM stimulated acute (first hour) oxygen consumption in human and mouse fibroblasts. rhTFAM treatment produced a similar acute increase in oxygen consumption rate in both cell types (Figure 1B).
Figure 1.
rhTFAM rapidly increases respiration of cells in culture. A. Intact mouse 3T3 fibroblast cells treated with rhTFAM (at 0 minutes) consume significantly more oxygen than controls (p<.001) within minutes expressed as normalized relative fluorescence units. RhTFAM stimulated oxygen consumption can be blocked by using chloramphenicol, an inhibitor of translation of mtDNA encoded proteins of the electron transport chain, suggesting rhTFAM is increasing oxygen consumption by stimulating mtDNA. The results are the combination of 3 separate experiments of 12 wells per condition for an n=36 per group. Error bars are standard error from the mean. B. rhTFAM treatment causes a similar increase in oxygen consumption in both mouse and human fibroblasts.
RhTFAM protects SH-SY5Y neuroblastoma against complex I inhibitor neurotoxins
SH-SY5Y cells were co-treated with rhTFAM at increasing amounts and MPP+ at 1uM. Twenty-four hours later, cells were assayed for ROS, ATP and cell viability. Untreated SH-SY5Y cells were used to normalize values and data was expressed as percent of untreated cells. MPP+ treatment caused a 20% increase in ROS (p<.01) which was abrogated with rhTFAM. ATP levels were increased by rhTFAM (p<.01) compared to MPP+ alone. Cell viability was reduced by 50% at 24 hours from MPP+ treatment (p<.01). The reduction in cell viability was rescued by rhTFAM at all doses (p<.01) (Figure 2A).
Figure 2.
rhTFAM improves viability and ATP levels and reduces ROS production in SH-SY5Y neuroblastoma cells exposed to MPP+ or rotenone. A. SH-SY5Y cells were co-treated with rhTFAM at increasing amounts and MPP+ at 1uM. Twenty-four hours later, cells were assayed for ROS, ATP and cell viability. Untreated SH-SY5Y cells were used to normalize values and data was expressed as percent of untreated SH-SY5Y. MPP+ treatment caused a 20% increase in ROS (p<.01) which was abrogated with rhTFAM. ATP levels were increased by rhTFAM (p<.01) compared to MPP+ alone. Cell viability was reduced by 50% at 24 hours from MPP+ treatment (p<.01). The reduction in cell viability was rescued by rhTFAM at all doses (p<.01).
B. Similar experiments were carried out using the Complex I inhibitor, rotenone (2uM). Rotenone treatment caused a 40% increase in ROS (p<.01) which was abrogated with rhTFAM. ATP levels were normalized by rhTFAM (p<.01) compared to rotenone alone. Cell viability was reduced by 60% at 24 hours from rotenone treatment (p<.01). The reduction in cell viability was completely rescued by rhTFAM at the intermediate and highest dose (p<.01).
Similar experiments were carried out using the Complex I inhibitor, rotenone (2uM). SH-SY5Y cells were co- treated with rhTFAM at increasing amounts and rotenone at 2uM. Twenty-four hours later, cells were assayed for ROS, ATP and cell viability. Untreated SH-SY5Y cells were used to normalize values and data was expressed as percent of untreated SH-SY5Y. Rotenone treatment caused a 40% increase in ROS (p<.01) which was abrogated with rhTFAM. ATP levels were normalized by rhTFAM (p<.01) compared to rotenone alone. Cell viability was reduced by 60% at 24 hours from rotenone treatment (p<.01). The reduction in cell viability was completely rescued by rhTFAM at the intermediate and highest dose (p<.01) (Figure 2B)
I.V. rhTFAM rapidly enters brain mitochondria
The concentration of rhTFAM in mouse brain mitochondria following I.V. injection was followed on Western blot using an antibody against the HA epitope and normalizing to cytochrome C content as a marker for mitochondrial mass. Figure 3 shows that levels of brain mitochondrial rhTFAM peaked at 12 hours and then declined with a half-life of ~32 hours.
I.V. rhTFAM improves motor function after MPTP treatment
We assessed whether ongoing treatment with rhTFAM improved motor outcomes after treatment with MPTP. MPTP is a parkinsonian pro-neurotoxin that kills substantia nigra dopamine neurons after being oxidized by MAO-B to methylpyridinium ion (MPP+) that then accumulates inside dopaminergic neurons through the dopamine transporter. MPP+ is then additionally concentrated into mitochondria where it inhibits complex I activity. We treated C57BL/6 mice with q4d I.V. rhTFAM for 3 doses and then began a subacute MPTP regimen (30 mg/kg/day X 5 doses) that produces mainly an apoptotic nigral neuronal death (Jackson-Lewis and Przedborski, 2007). After an additional 3 doses of rhTFAM, motor function was assessed on an accelerating rotarod that tests both motor endurance and motor learning. By calculating the slopes of increases in time on the rotarod across the three testing days, we found that rhTFAM treatment at 50uL/dose and 75uL/dose, but not that at 100 uL/dose or 150 uL/dose, improved motor performance (Figure 4).
I.V. rhTFAM treatment increases mitochondrial ATP synthesis and mitochondrial biogenesis while reducing oxidative stress damage
We evaluated ATP synthesis rates in mitochondrial preparations, where ATP synthesis was driven by both complex I and II substrates added together, and exogenous cytochrome c was provided to be sure that it was not rate limiting. We compared with repeated measures ANOVA the “rhTFAM” (mice with repeated exercise testing treated with rhTFAM) and “vehicle CTL” (mice with repeated exercise testing treated with vehicle CTL) groups and used regular ANOVA to compare these to the “CTL” (no exercise, no treatment) group.
Figure 5A shows that for brain mitochondria, there was a significant effect on mitochondrial ATP synthesis rate from rhTFAM treatment compared to vehicle CTL treatment. There was a highly significant interaction between rhTFAM treatment and time. There was a trend towards a positive result, but no statistically significant effect of exercise alone on brain mitochondrial ATP synthesis rate.
For heart mitochondria there was no significant effect of rhTFAM treatment (Figure 5B). Overall rhTFAM treatment tended to depress ATP synthesis, but the kinetics were biphasic in contrast to those observed in brain and skeletal muscle.
In skeletal muscle mitochondria, there was a highly significant interaction between exercise and time on ATP synthesis in both rhTFAM and vehicle CTL groups (Figure 5C). There was not a significant effect of rhTFAM on ATP synthesis.
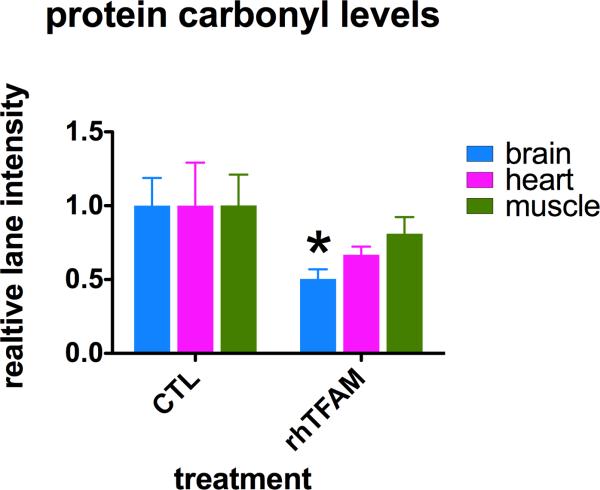
We then used Western blot to measure levels of protein carbonyls in total tissue homogenates as a marker for oxidative stress damage. Figure 6 shows that relative to vehicle CTL treated animals, protein carbonyl levels in all tissues from rhTFAM treated mice appeared reduced and were reduced to the greatest degree (~50%) and significantly only in brain. Thus, in spite of increased maximal brain mitochondrial respiration capacity, there was no evidence for increased oxidative damage, and in fact, oxidative damage was lowered by half.
Figure 6.
Protein carbonyl levels as indicators of oxidative stress damage in homogenates of brains, heart and muscles of mice treated with rhTFAM (n=6) or buffer CTL (n=6). Shown are mean +/- SEM values of total blot lane intensities after DNPH derivatization, electrophoresis, nitrocellulose blotting and DNPH immunostaining, normalized to mean CTL values for each tissue. *p<0.05.
We then sought to delineate at the molecular genetic and proteomic levels how brain mitochondrial respiratory capacity was increased and simultaneously oxidative stress damage was reduced by rhTFAM treatment. We used qPCR to assay copy number of mtDNA genes and RT-qPCR to assay gene transcription (at the mRNA level) for mtDNA genes and genes for uncoupling proteins (UCP-2, UCP-3), anti-oxidant enzymes (catalase, glutathione peroxidase, glutathione reductase) and mitochondrial biogenesis (PGC-1 alpha). All RT-qPCR results were normalized to levels of 18S rRNA in either the genomic mtDNA or cDNA samples. For proteomic analysis we used Western blot determinations of mitochondrial ETC proteins, a marker for mitochondrial mass (porin), a marker for mitochondrial matrix (SOD2) and beta actin loading control. Western blots were normalized to total lane protein determined by SyproRose staining and imaging of the blots prior to incubation with antibodies.
Figure 7 shows that with multiplex qPCR measurements in the same sample levels of mouse mitochondrial genes 16S rRNA, CO1, CO3 and CytB, we found a significant ~50% elevation in mtDNA gene copy number (Fig 7A) but were unable to detect a change in mtDNA gene transcription (Fig 7B).
Figure 7.
RT-qPCR assay of mtDNA gene copy number (a) and gene expression (b) in brain genomic DNA's (a) or cDNA's (b) isolated/prepared from mice treated with rhTFAM or vehicle CTL. 16S rRNA, CO1, CO3 and CytB were simultaneously assayed in a multiplex qPCR assay using an external standard of circular mouse mtDNA made from liver genomic DNA treated with ATP-dependent exonuclease. Values for individual mtDNA genes are normalized to 18S rRNA content in each sample, did not differ from each other and are combined. * p<0.05
We then searched for evidence of changes in PGC-1 alpha expression, a major transcriptional regulator of mitochondrial biogenesis. We found ~ linear relationships between mtDNA gene copy numbers and PGC-1a expression and mtDNA gene transcription and PGC-1a expression in vehicle CTL treated brain samples (Figure 8, left graphs). In rhTFAM treated brain samples there was no evidence to support a relationship between mtDNA copy number and PGC-1a expression, whereas there was a modest correlation between mtDNA gene transcription and PGC-1a expression (Figure 8, right graphs). It is of interest that in both CTL and TFAM groups, where all mice were from the same strain/breeder and were treated identically, we observed such heterogeneity of expression of PGC-1 alpha and mtDNA genes, with each varying 2 to 3-fold across individuals.
Figure 8.
Relationships in vehicle CTL-treated brain genomic DNA (a) and cDNA (b) samples between 18S rRNA normalized expression in cDNA of PGC-1alpha and levels of mtDNA genes (a) or gene expression (b). Shown are mean +/- SEM 18S rRNA normalized levels of mtDNA genes 16S rRNA, CO1, CO3, CytB.
In addition to analyzing ETC activity at the RNA level we directly assayed the levels of several ETC proteins in brain, heart and skeletal muscle. In homogenates from brain (Figure 9) and skeletal muscle (Figure 10), but not in heart (not shown), we observed an increase after rhTFAM treatment in the total levels of four Complex I ETC proteins. In both brain and skeletal muscle the effect size was small (~50% elevation) but observed consistently across all Complex I subunits. The elevation in all combined complex I proteins reached significance in brain (p=0.012) and almost reached significance in skeletal muscle (p=0.067), but did not reach significance for individual complex I subunits. We did not find significant changes in single proteins from Complexes II, III, or V (not shown).
Figure 9.
Total brain Complex I protein levels (normalized to beta actin) are increased in rhTFAM treated mice. Levels are expressed as percentage of mean CTL values. Shown are the results for individual Complex I subunits, and at the top, a composite of all four Complex I subunits.
We used qPCR to assess expression of mitochondrial uncoupling proteins 2 and 3 but found no detectable differences (Supplemental File 2). With qPCR we searched for changes in expression of several major antioxidant enzymes (catalase, glutathione peroxidase, glutathione reductase, SOD2) and could not find differences (not shown).
rhTFAM treatment increases survival after LPS endotoxin treatment
Normal seven week-old male mice pretreated with vehicle rapidly died after treatment with LPS endotoxin (Figure 11), with no survivors after 24 hours. Pretreatment/treatment with rhTFAM dose-dependently increased survival (Figure 11). The increase in survival was significant for the 100 uL, 150 uL and 200 uL rhTFAM doses.
4. Discussion
In this study we describe results of several in vitro and in vivo experiments that indicate it is possible to improve mitochondrial physiology using rhTFAM, to improve function in cells exposed to MPP+ and rotenone in vitro, and rescue motor performance in mice exposed to MPTP. In earlier studies we showed with cells in culture that rhTFAM alone or complexed with mtDNA rapidly localized to mitochondria in less than an hour (Iyer et al., 2009; Keeney et al., 2009). In the present study we found that mouse fibroblasts incubated with rhTFAM rapidly increased respiration that was sensitive to chloramphenicol, implying a dependence on mitochondrial translation.
This surprising finding supporting rapid rhTFAM-induced mtDNA gene mRNA translation raises questions about the likely mechanism, since other inducers of mitochondrial biogenesis may take longer to act. For example, Ghosh et al observed increases in several markers of mitochondrial biogenesis in NT2 cells two weeks after exposure to pioglitazone, a PPAR-γ agonist (Ghosh et al., 2007). Early effects of TFAM overexpression have been observed in HeLa cells, where levels of some mitochondrial RNAs increased within 3 hours of transfection with a TFAM-expressing plasmid, but mitochondrial DNA copy number was not increased (Maniura-Weber et al., 2004).
Our use of a PTD-linked transcription factor rather than a protein-expressing plasmid may be responsible for the faster response we observed: rhTFAM is delivered to mitochondria almost immediately without the need for activation of cytoplasmic transcription mechanisms (Iyer et al., 2009). Nevertheless, the question remains how can the expression of a small number of mitochondrial genome-encoded genes result in a rapid increase in oxygen consumption which would require assembly of ETC complexes containing dozens of subunits encoded by the nuclear genome.
An explanation of this phenomenon may be provided by the observation that some of the mitochondrially-encoded ETC proteins (which form a part of complexes I, III, IV and V) are known to act as a nidus for the formation of fully active ETC complexes (Lazarou et al., 2007). Though Lazarou, et al found that integration of mtDNA encoded protein subunits into active OXPHOS complexes required hours, we found rapid activation of oxygen consumption within minutes. We hypothesize that adding rhTFAM stimulates the transcription of such subunits in multiple mitochondria, independently of nuclear gene transcription.
Our data suggest that mitochondria are capable of ramping up transcription and translation very quickly, within minutes, with the proper stimulus. The mitochondrial ETC subunits produced in mitochondria could then interact with a pool of nuclear-encoded ETC subunits already present, initiating formation of full ETC complexes (Lazarou et al., 2007; McKenzie et al., 2007). If comparable processes occur in vivo, then mitochondrial respiration may increase within hours after systemic rhTFAM injection. We have yet to perform a pharmacodynamic study to explore that possibility.
Eukaryotes were formed by the fusion of a proto-eukaryote, the predecessor of the nucleus and cytoplasm, with an alpha-proteobacterial symbiont, the precursor of mitochondria (Vellai and Vida, 1999). During early eukaryotic evolution most of the genes present in the mitochondrial symbiont were transferred to the nucleus, yet the transfer process stalled at least 600 million years ago (prior to evolution of e.g. vertebrates which all maintain the same complement of mtDNA genes), indicating that independently functioning mitochondrial genomes may be an indispensable feature of the eukaryotic cell. A mechanistic explanation for this state of affairs may be provided by the observation that eukaryotic cells are orders of magnitude larger than prokaryotes and thus need local mechanisms of controlling metabolism in various compartments of the cell. It has been postulated that mitochondrial DNA may be a part of this local metabolic control, and that each mitochondrial genome is be capable of integrating signals from its neighborhood to precisely titrate the number of active ETC complexes to the local energetic needs, as proposed by John Allen (Allen, 1993). Our observation that activation of mtDNA-encoded genes produces extremely rapid increases in ETC activity, not achievable with purely nuclear control mechanisms, supports this explanation for the existence of mtDNA.
If this interpretation is true, TFAM would be a part of the local rapid response network for energetic needs. The overall cellular level of TFAM would act as a potentiometer allowing the nucleus to set a general level of responsiveness of mitochondria to local needs. The exact nature of the mechanisms involved in transducing local energy demand into increased expression of mtDNA-encoded genes is unclear, although post-translational modifications of the TFAM protein could play a role. It is known that TFAM undergoes acetylation (Dinardo et al., 2003) and glycosylation (Suarez et al., 2008). Further research on rhTFAM may shed light on this issue.
Our observation of decrease in ROS damage despite increases in oxidative metabolism (ATP synthesis) is not surprising in light of recent studies. For example, exercise induces increases in oxidative metabolism and reduces ROS damage through multiple mechanisms, including increases in antioxidant enzyme expression [46-48]. Additionally, complex I is the major site of free-radical production in the ETC. The sustained increase in respiration brought about by uncoupling, similar in magnitude to rhTFAM, actually reduces ROS production by complex I [49]. It is not complex I activity per se that is responsible for ROS production, but rather electron back flow through complex I (Kushnareva et al., 2002). Improving complex I electron flow prevents back flow and thus reduces ROS production.
Thus, increased ETC activity does not directly and unavoidably cause increases in ROS production. Rather, improving electron flow reduces ROS production while providing bioenergetic benefits, such as improved coupling, ATP production and substrate utilization. It is worth noting that caloric restriction, the most effective methodology extending the lifespans of laboratory animals, is also associated with activation of mitochondrial biogenesis and mitochondrial oxidative capacity (Hepple, 2009), even though the actual substrate fluxes through the ETC are lower than in ad libitum fed animals.
Our in vitro experiments comparing the impact of rhTFAM on mouse (current study) and human cells (Iyer et al., 2009; Keeney et al., 2009) (and current study) show that rhTFAM, a human protein, stimulates mitochondrial gene transcription in both species. This finding is somewhat at odds with the observations of Ekstrand et al. (Ekstrand et al., 2004) who performed cell-free transcription experiments with purified recombinant mouse and human TFAM and found that human TFAM stimulated mitochondrial gene transcription from mouse mtDNA to a lesser degree than mouse TFAM. However, in their in vivo experiments in a mouse knock-in system overexpressing human TFAM, they also found increased expression of the ND6 protein, one of the complex I subunits encoded in mtDNA, in agreement with our finding of increased complex I protein levels in rhTFAM treated mice. Ekstrand et al. did not analyze ATP production in the brain, or ROS production, and did not compare motor performance between wild-type and human TFAM overexpressing mice. Insofar as our assays overlap with the experiments performed by Ekstrand et al., our results are in broad agreement, showing increased mtDNA copy number and increased complex I protein levels. The residual differences between the findings of our studies may be explained by emphasis on different aspects of mitochondrial function and the use of completely different approaches to the delivery of human TFAM to mice, one a genetic overexpression study and another intermittent acute delivery of TFAM protein.
We found that systemic treatment (tail vein injections) with rhTFAM improved motor performance of mice treated with MPTP to create a nigrostriatal deficiency state. In the normal mice treated with rhTFAM, mitochondrial biogenesis and ATP generating capacity increased in brain while oxidative stress damage was reduced. We do not yet understand the mechanisms responsible for this stimulation of mitochondrial biogenesis, which we have also observed in cultured cells (Iyer et al., 2009; Keeney et al., 2009), but this characteristic of systemic rhTFAM treatment may underlie its improvement of motor function after MPTP treatment and prolongation of life in aged mice (Khan, et al, unpublished). rhTFAM treatment also appears to increase mtDNA gene levels in individual mouse nigral neurons isolated by laser capture microdissection (LCM) (Thomas and Bennett, unpublished). If confirmed, the ability of rhTFAM to increase neuronal mitochondrial function offers a novel approach to solving age-related bioenergetic deficiencies of human nigral neurons (Bender et al., 2006; Bender et al., 2008; Kraytsberg et al., 2006) and potentially deficiencies in other tissues.
There are clearly many additional important properties of this novel mitochondrial transcription factor that remain to be determined. We do not yet know if the acute increase in respiration observed in cell culture also occurs in vivo. If it does occur, then rhTFAM therapy might be helpful in conditions such as cerebral ischemia or traumatic brain injury, where improved bioenergetic function may reduce neuronal death.
Our experiments with MPTP treated mice suggest a rhTFAM dose-response with a “U” shaped curve. This type of finding clearly requires confirmation along with an elucidation of more pharmacokinetic and pharmacodynamic data about systemic rhTFAM.
The effects of injected rhTFAM are consistent with TFAM's known mechanisms of action in stimulating mtDNA replication and mtDNA gene expression (Ekstrand et al., 2004; Maniura-Weber et al., 2004; Scarpulla, 2006, 2008), although the effects of human TFAM on mtDNA copy number and gene expression have been reported to be discordant (Ekstrand et al., 2004; Maniura-Weber et al., 2004). Contrarily, TFAM knockdown with siRNA markedly reduced mtDNA gene expression and respiratory capacity (Jeng et al., 2008), and conditional TFAM knockout in heart can cause lethal neonatal cardiomyopathy (Wallace, 2002). These studies place TFAM as an essential transcription factor controlling mitochondrial biogenesis.
These findings agree with the results of two other studies of human TFAM overexpression in mice (Hayashi et al., 2008; Ikeuchi et al., 2005). Mice overexpressing TFAM in the brain showed amelioration of ROS production, and superior maintenance of mitochondrial ETC activities, especially of complexes I and IV, which are most affected in aging. The transgenic mice also showed significant improvement of motor learning (in a rotarod test), working memory (radial water maze performance), as well as hippocampal long-term potentiation. The behavioral improvements were associated with diminished levels of inflammatory markers of aging and diminished levels of mtDNA oxidation products, in parallel with our findings (Hayashi et al., 2008). Mice overexpressing human TFAM in the heart have increased mtDNA copy number (Ikeuchi et al., 2005), causing a significant increase in survival, improved cardiac performance and reduced apoptosis after myocardial infarction (MI). We did not detect increased ETC activities in the hearts of rhTFAM treated, exercised animals, despite robust delivery of rhTFAM to the heart (data not shown). Ikeuchi et al. also failed to detect increased basal complex I and IV activities in the hearts of their mice. Only after MI were significant differences detectable, suggesting increased levels of TFAM can preserve heart mitochondrial function in the face of perturbations such as ischemia.
Our observations of rhTFAM-induced ~50% reductions of oxidative stress protein damage in the setting of increased respiratory capacity are encouraging as a potential therapeutic intervention. We found no evidence of decreased coupling of respiration to ATP synthesis that might account for decreased ROS leakage from the ETC. This is consistent with our qPCR findings of no changes in expression of uncoupling proteins UCP-2 or UCP-3. Our rhTFAM treated mice overall had greater levels of exercise, which can increase heart mitochondrial SOD2 expression (Lawler et al., 2009). At this time, we do not know the origin(s) of the rhTFAM-induced reductions in oxidative stress damage but note that it occurred in all three post-mitotic tissues, including brain.
To summarize, we have developed a mitochondrially-directed therapeutic that increases motor endurance and bioenergetic function in normal, young male mice (Iyer et al., 2009) and improves motor function after MPTP treatment (current study). This treatment appears to be non-toxic in the healthy male mouse, and the reduction of oxidative stress damage makes this approach particularly appealing for further investigation in both mouse models and potentially humans. At this time we are assaying pharmacokinetics and pharmacodynamics of rhTFAM treatment. Our human cell-based studies confirm that rhTFAM stimulates mitochondrial biogenesis and improves bioenergetics, suggesting that systemic rhTFAM treatment of humans would stimulate mitochondrial biogenesis and increase respiratory function. If true, then rhTFAM has the potential to reverse bioenergetic deficiencies of aging, at least in brain and skeletal muscle (Smigrodzki and Khan, 2005). It is worth noting that at least two other interventions increasing mitochondrial activity, PGC1-α overexpression (Wenz et al., 2009), and chronic treatment with rapamycin (Harrison et al., 2009), have increased mouse lifespan. We are currently finalizing a study of chronic rhTFAM treatment of aged mice.
As noted in the introduction, rhTFAM is also a mtDNA-binding protein and can deliver human mtDNA to mitochondria of living cells with improvement of deficient respiration (Keeney et al., 2009). Our initial experiments indicate that rhTFAM can deliver mouse mtDNA in vivo (Khan, et al, unpublished). Subsequent experiments will also focus on the ability of treatments with rhTFAM alone or complexed with mouse mtDNA to improve bioenergetics of aged mouse organs, as a prelude to clinical testing in humans.
Finally, our initial experiments with rhTFAM treatment of mice exposed to LPS endotoxin support the concept of stimulating mitochondrial respiration as an effective treatment for humans with sepsis. Future studies will focus on whether rhTFAM treatment can reverse the “cytopathic hypoxia” observed in mitochondria from septic tissues (Crouser, 2004), and if so, how this occurs.
Supplementary Material
Supplemental Figure 1. Distribution of mean +/- SEM weights of mice treated with rhTFAM or vehicle CTL across duration of treatment. BL=baseline.
Supplemental Figure 2. Expression levels of mitochondrial uncoupling proteins UCP-2 and UCP-3 in brain cDNA samples assayed by RT-qPCR and normalized to 18S rRNA content.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. References cited
- Allen JF. Control of gene expression by redox potential and the requirement for chloroplast and mitochondrial genomes. J Theor Biol. 1993;165:609–631. doi: 10.1006/jtbi.1993.1210. [DOI] [PubMed] [Google Scholar]
- Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, Jaros E, Hersheson JS, Betts J, Klopstock T, Taylor RW, Turnbull DM. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet. 2006;38:515–517. doi: 10.1038/ng1769. [DOI] [PubMed] [Google Scholar]
- Bender A, Schwarzkopf RM, McMillan A, Krishnan KJ, Rieder G, Neumann M, Elstner M, Turnbull DM, Klopstock T. Dopaminergic midbrain neurons are the prime target for mitochondrial DNA deletions. Journal of neurology. 2008 doi: 10.1007/s00415-008-0892-9. [DOI] [PubMed] [Google Scholar]
- Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, Davies NA, Cooper CE, Singer M. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360:219–223. doi: 10.1016/S0140-6736(02)09459-X. [DOI] [PubMed] [Google Scholar]
- Cotney J, Wang Z, Shadel GS. Relative abundance of the human mitochondrial transcription system and distinct roles for h-mtTFB1 and h-mtTFB2 in mitochondrial biogenesis and gene expression. Nucleic acids research. 2007;35:4042–4054. doi: 10.1093/nar/gkm424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouser ED. Mitochondrial dysfunction in septic shock and multiple organ dysfunction syndrome. Mitochondrion. 2004;4:729–741. doi: 10.1016/j.mito.2004.07.023. [DOI] [PubMed] [Google Scholar]
- de Souza-Pinto NC, Wilson DM, 3rd, Stevnsner TV, Bohr VA. Mitochondrial DNA, base excision repair and neurodegeneration. DNA Repair (Amst) 2008;7:1098–1109. doi: 10.1016/j.dnarep.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz F, Moraes CT. Mitochondrial biogenesis and turnover. Cell Calcium. 2008;44:24–35. doi: 10.1016/j.ceca.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinardo MM, Musicco C, Fracasso F, Milella F, Gadaleta MN, Gadaleta G, Cantatore P. Acetylation and level of mitochondrial transcription factor A in several organs of young and old rats. Biochem Biophys Res Commun. 2003;301:187–191. doi: 10.1016/s0006-291x(02)03008-5. [DOI] [PubMed] [Google Scholar]
- Ekstrand MI, Falkenberg M, Rantanen A, Park CB, Gaspari M, Hultenby K, Rustin P, Gustafsson CM, Larsson NG. Mitochondrial transcription factor A regulates mtDNA copy number in mammals. Hum Mol Genet. 2004;13:935–944. doi: 10.1093/hmg/ddh109. [DOI] [PubMed] [Google Scholar]
- Figueiredo PA, Powers SK, Ferreira RM, Appell HJ, Duarte JA. Aging impairs skeletal muscle mitochondrial bioenergetic function. J Gerontol A Biol Sci Med Sci. 2009;64:21–33. doi: 10.1093/gerona/gln048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RP, Clayton DA. Purification and characterization of human mitochondrial transcription factor 1. Molecular and cellular biology. 1988;8:3496–3509. doi: 10.1128/mcb.8.8.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui H, Moraes CT. Mechanisms of formation and accumulation of mitochondrial DNA deletions in aging neurons. Hum Mol Genet. 2009;18:1028–1036. doi: 10.1093/hmg/ddn437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangelhoff TA, Mungalachetty PS, Nix JC, Churchill ME. Structural analysis and DNA binding of the HMG domains of the human mitochondrial transcription factor A. Nucleic acids research. 2009;37:3153–3164. doi: 10.1093/nar/gkp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Patel N, Rahn D, McAllister J, Sadeghi S, Horwitz G, Berry D, Wang KX, Swerdlow RH. The thiazolidinedione pioglitazone alters mitochondrial function in human neuron-like cells. Mol Pharmacol. 2007;71:1695–1702. doi: 10.1124/mol.106.033845. [DOI] [PubMed] [Google Scholar]
- Gredilla R, Garm C, Holm R, Bohr VA, Stevnsner T. Differential age-related changes in mitochondrial DNA repair activities in mouse brain regions. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev. 2006;27:728–735. doi: 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Yoshida M, Yamato M, Ide T, Wu Z, Ochi-Shindou M, Kanki T, Kang D, Sunagawa K, Tsutsui H, Nakanishi H. Reverse of age-dependent memory impairment and mitochondrial DNA damage in microglia by an overexpression of human mitochondrial transcription factor a in mice. J Neurosci. 2008;28:8624–8634. doi: 10.1523/JNEUROSCI.1957-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepple RT. Why eating less keeps mitochondria working in aged skeletal muscle. Exerc Sport Sci Rev. 2009;37:23–28. doi: 10.1097/JES.0b013e3181877dc5. [DOI] [PubMed] [Google Scholar]
- Huang JH, Hood DA. Age-associated mitochondrial dysfunction in skeletal muscle: Contributing factors and suggestions for long-term interventions. IUBMB Life. 2009;61:201–214. doi: 10.1002/iub.164. [DOI] [PubMed] [Google Scholar]
- Ikeuchi M, Matsusaka H, Kang D, Matsushima S, Ide T, Kubota T, Fujiwara T, Hamasaki N, Takeshita A, Sunagawa K, Tsutsui H. Overexpression of mitochondrial transcription factor a ameliorates mitochondrial deficiencies and cardiac failure after myocardial infarction. Circulation. 2005;112:683–690. doi: 10.1161/CIRCULATIONAHA.104.524835. [DOI] [PubMed] [Google Scholar]
- Iyer S, Thomas RR, Portell FR, Dunham LD, Quigley CK, Bennett JP., Jr Recombinant Mitochondrial Transcription Factor A with N-terminal Mitochondrial Transduction Domain Increases Respiration and Mitochondrial Gene Expression. Mitochondrion. 2009;9:196–203. doi: 10.1016/j.mito.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson-Lewis V, Przedborski S. Protocol for the MPTP mouse model of Parkinson's disease. Nature protocols. 2007;2:141–151. doi: 10.1038/nprot.2006.342. [DOI] [PubMed] [Google Scholar]
- Jang YC, Remmen VH. The mitochondrial theory of aging: insight from transgenic and knockout mouse models. Exp Gerontol. 2009;44:256–260. doi: 10.1016/j.exger.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Jeng JY, Yeh TS, Lee JW, Lin SH, Fong TH, Hsieh RH. Maintenance of mitochondrial DNA copy number and expression are essential for preservation of mitochondrial function and cell growth. Journal of cellular biochemistry. 2008;103:347–357. doi: 10.1002/jcb.21625. [DOI] [PubMed] [Google Scholar]
- Kadenbach B, Ramzan R, Vogt S. Degenerative diseases, oxidative stress and cytochrome c oxidase function. Trends Mol Med. 2009;15:139–147. doi: 10.1016/j.molmed.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Kaufman BA, Durisic N, Mativetsky JM, Costantino S, Hancock MA, Grutter P, Shoubridge EA. The mitochondrial transcription factor TFAM coordinates the assembly of multiple DNA molecules into nucleoid-like structures. Molecular biology of the cell. 2007;18:3225–3236. doi: 10.1091/mbc.E07-05-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney PM, Quigley CK, Dunham LD, Papageorge CM, Iyer S, Thomas RR, Schwarz KM, Trimmer PA, Khan SM, Portell FR, Bergquist KE, Bennett JP. Mitochondrial Gene Therapy Augments Mitochondrial Physiology in a Parkinson's Disease Cell Model. Hum Gene Ther. 2009 doi: 10.1089/hum.2009.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraytsberg Y, Kudryavtseva E, McKee AC, Geula C, Kowall NW, Khrapko K. Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nat Genet. 2006;38:518–520. doi: 10.1038/ng1778. [DOI] [PubMed] [Google Scholar]
- Krishnan KJ, Reeve AK, Samuels DC, Chinnery PF, Blackwood JK, Taylor RW, Wanrooij S, Spelbrink JN, Lightowlers RN, Turnbull DM. What causes mitochondrial DNA deletions in human cells? Nat Genet. 2008;40:275–279. doi: 10.1038/ng.f.94. [DOI] [PubMed] [Google Scholar]
- Kushnareva Y, Murphy AN, Andreyev A. Complex I-mediated reactive oxygen species generation: modulation by cytochrome c and NAD(P)+ oxidation-reduction state. Biochem J. 2002;368:545–553. doi: 10.1042/BJ20021121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler JM, Kwak HB, Kim JH, Suk MH. Exercise training inducibility of MnSOD protein expression and activity is retained while reducing prooxidant signaling in the heart of senescent rats. American journal of physiology. 2009;296:R1496–1502. doi: 10.1152/ajpregu.90314.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarou M, McKenzie M, Ohtake A, Thorburn DR, Ryan MT. Analysis of the assembly profiles for mitochondrial- and nuclear-DNA-encoded subunits into complex I. Molecular and cellular biology. 2007;27:4228–4237. doi: 10.1128/MCB.00074-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1:361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Long J, Gao F, Tong L, Cotman CW, Ames BN, Liu J. Mitochondrial decay in the brains of old rats: ameliorating effect of alpha-lipoic acid and acetyl-L-carnitine. Neurochem Res. 2009;34:755–763. doi: 10.1007/s11064-008-9850-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YS, Wu SB, Lee WY, Cheng JS, Wei YH. Response to the increase of oxidative stress and mutation of mitochondrial DNA in aging. Biochim Biophys Acta. 2009 doi: 10.1016/j.bbagen.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Maniura-Weber K, Goffart S, Garstka HL, Montoya J, Wiesner RJ. Transient overexpression of mitochondrial transcription factor A (TFAM) is sufficient to stimulate mitochondrial DNA transcription, but not sufficient to increase mtDNA copy number in cultured cells. Nucleic acids research. 2004;32:6015–6027. doi: 10.1093/nar/gkh921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Gleichmann M, Cheng A. Mitochondria in neuroplasticity and neurological disorders. Neuron. 2008;60:748–766. doi: 10.1016/j.neuron.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie M, Lazarou M, Thorburn DR, Ryan MT. Analysis of mitochondrial subunit assembly into respiratory chain complexes using Blue Native polyacrylamide gel electrophoresis. Anal Biochem. 2007;364:128–137. doi: 10.1016/j.ab.2007.02.022. [DOI] [PubMed] [Google Scholar]
- Miyazawa M, Ishii T, Yasuda K, Noda S, Onouchi H, Hartman PS, Ishii N. The role of mitochondrial superoxide anion (O2(-)) on physiological aging in C57BL/6J mice. J Radiat Res (Tokyo) 2009;50:73–83. doi: 10.1269/jrr.08097. [DOI] [PubMed] [Google Scholar]
- Petrosillo G, Matera M, Casanova G, Ruggiero FM, Paradies G. Mitochondrial dysfunction in rat brain with aging Involvement of complex I, reactive oxygen species and cardiolipin. Neurochem Int. 2008;53:126–131. doi: 10.1016/j.neuint.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Reeve AK, Krishnan KJ, Turnbull DM. Age related mitochondrial degenerative disorders in humans. Biotechnol J. 2008;3:750–756. doi: 10.1002/biot.200800066. [DOI] [PubMed] [Google Scholar]
- Samavati L, Lee I, Mathes I, Lottspeich F, Huttemann M. Tumor necrosis factor alpha inhibits oxidative phosphorylation through tyrosine phosphorylation at subunit I of cytochrome c oxidase. J Biol Chem. 2008;283:21134–21144. doi: 10.1074/jbc.M801954200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpulla RC. Nuclear control of respiratory gene expression in mammalian cells. Journal of cellular biochemistry. 2006;97:673–683. doi: 10.1002/jcb.20743. [DOI] [PubMed] [Google Scholar]
- Scarpulla RC. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiological reviews. 2008;88:611–638. doi: 10.1152/physrev.00025.2007. [DOI] [PubMed] [Google Scholar]
- Shadel GS. Expression and maintenance of mitochondrial DNA: new insights into human disease pathology. Am J Pathol. 2008;172:1445–1456. doi: 10.2353/ajpath.2008.071163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokolenko I, Venediktova N, Bochkareva A, Wilson GL, Alexeyev MF. Oxidative stress induces degradation of mitochondrial DNA. Nucleic acids research. 2009;37:2539–2548. doi: 10.1093/nar/gkp100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smigrodzki RM, Khan SM. Mitochondrial microheteroplasmy and a theory of aging and age-related disease. Rejuvenation research. 2005;8:172–198. doi: 10.1089/rej.2005.8.172. [DOI] [PubMed] [Google Scholar]
- Suarez J, Hu Y, Makino A, Fricovsky E, Wang H, Dillmann WH. Alterations in mitochondrial function and cytosolic calcium induced by hyperglycemia are restored by mitochondrial transcription factor A in cardiomyocytes. American journal of physiology. 2008;295:C1561–1568. doi: 10.1152/ajpcell.00076.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH, Khan SM. The Alzheimer's disease mitochondrial cascade hypothesis: An update. Exp Neurol. 2009 doi: 10.1016/j.expneurol.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Remmen H, Jones DP. Current thoughts on the role of mitochondria and free radicals in the biology of aging. J Gerontol A Biol Sci Med Sci. 2009;64:171–174. doi: 10.1093/gerona/gln058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellai T, Vida G. The origin of eukaryotes: the difference between prokaryotic and eukaryotic cells. Proc Biol Sci. 1999;266:1571–1577. doi: 10.1098/rspb.1999.0817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC. Animal models for mitochondrial disease. Methods in molecular biology (Clifton, N.J. 2002;197:3–54. doi: 10.1385/1-59259-284-8:003. [DOI] [PubMed] [Google Scholar]
- Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annual review of genetics. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenz T, Rossi SG, Rotundo RL, Spiegelman BM, Moraes CT. Increased muscle PGC-1alpha expression protects from sarcopenia and metabolic disease during aging. Proc Natl Acad Sci U S A. 2009;106:20405–20410. doi: 10.1073/pnas.0911570106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhou Q, Lam PY, Han D, Cadenas E. c-Jun N-terminal kinase regulates mitochondrial bioenergetics by modulating pyruvate dehydrogenase activity in primary cortical neurons. J Neurochem. 2008;104:325–335. doi: 10.1111/j.1471-4159.2007.04957.x. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Lam PY, Han D, Cadenas E. Activation of c-Jun-N-terminal kinase and decline of mitochondrial pyruvate dehydrogenase activity during brain aging. FEBS Lett. 2009;583:1132–1140. doi: 10.1016/j.febslet.2009.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Distribution of mean +/- SEM weights of mice treated with rhTFAM or vehicle CTL across duration of treatment. BL=baseline.
Supplemental Figure 2. Expression levels of mitochondrial uncoupling proteins UCP-2 and UCP-3 in brain cDNA samples assayed by RT-qPCR and normalized to 18S rRNA content.