Abstract
Stress-promoted mutations that occur in nondividing cells (adaptive mutations) have been implicated strongly in causing genetic variability as well as in species survival and evolutionary processes. Oxidative stress-induced DNA damage has been associated with generation of adaptive His+ and Met+ but not Leu+ revertants in strain Bacillus subtilis YB955 (hisC952 metB5 leuC427). Here we report that an interplay between MutY and MutSL (mismatch repair system [MMR]) plays a pivotal role in the production of adaptive Leu+ revertants. Essentially, the genetic disruption of MutY dramatically reduced the reversion frequency to the leu allele in this model system. Moreover, the increased rate of adaptive Leu+ revertants produced by a MutSL knockout strain was significantly diminished following mutY disruption. Interestingly, although the expression of mutY took place during growth and stationary phase and was not under the control of RecA, PerR, or σB, a null mutation in the mutSL operon increased the expression of mutY several times. Thus, in starved cells, saturation of the MMR system may induce the expression of mutY, disturbing the balance between MutY and MMR proteins and aiding in the production of types of mutations detected by reversion to leucine prototrophy. In conclusion, our results support the idea that MMR regulation of the mutagenic/antimutagenic properties of MutY promotes stationary-phase mutagenesis in B. subtilis cells.
Adaptive or stationary-phase mutagenesis is a process that allows stressed populations of cells to acquire mutations that favor growth after the application of nonlethal selective pressure (8, 42). Although this biological phenomenon was first described for Escherichia coli, further studies demonstrated its occurrence in other prokaryotes as well as in eukaryotic organisms (9, 16, 21). Independent of the organism employed, this type of mutagenesis has proven to be important to explain biological processes such as survival, speciation, pathogenesis, antibiotic resistance, and evolution. Specific to this report, the existence of stationary-phase mutagenesis has been demonstrated with amino acid-starved Bacillus subtilis strain YB955, which carries three amino acid auxotrophies, at the hisC952 (amber), metB5 (ochre), and leuC427 (missense) alleles (39, 49). Studies using this model revealed that this process is not dependent on σB, the sigma factor that controls the general stress regulon (56). In addition, this process occurs in the absence of a functional RecA protein (49) and is developmentally regulated by the competence-associated transcription factors ComA and ComK (12, 49, 61). The involvement of YqjH, a member of the Y superfamily of DNA polymerases, and of the mismatch repair system (MMR; encoded by the mutSL operon) in this mutagenic process suggested the existence of a transient hypermutability state or subpopulation in nondividing starved B. subtilis cells which can subsequently generate advantageous mutations (41, 53). Transcription-associated mutagenesis stimulated by the transcription repair-coupling factor Mfd has been suggested as a mechanism to produce stationary-phase-associated mutations in this microorganism (43). In a recent work, Pybus et al. (43) demonstrated that transcriptional derepression directly correlates with stationary-phase-associated mutations under selective pressure. Interestingly, the Mfd protein was shown to play a significant role in decreasing damage-induced mutagenesis in actively growing cells while enhancing or permitting mutagenesis in stationary-phase cells.
Endogenous and environmental DNA-damaging agents constantly threaten the chemical integrity of cellular nucleic acids. Oxidative stress-promoted lesions like those induced by the hydroxyl radical (·OH) are among the most prominent DNA modifications within cells (14). This factor oxidizes DNA, either directly or indirectly, generating 8-oxo-G, which induces GC→TA and AT→CG transversions (52). The DNA glycosylases MutM and MutY prevent the mutagenic effects of 8-oxo-G: MutM eliminates 8-oxo-G from 8-oxo-G:C pairs, and MutY releases adenines from 8-oxo-G:A mismatches (30, 52). The deoxynucleoside triphosphate (dNTP) and NTP pools may also be targets for oxidative modifications, generating 8-oxo-dGTP and 8-oxo-GTP. The first of these is potentially mutagenic due to its incorporation opposite adenine residues by the replicative machinery (27). On the other hand, incorporation of 8-oxo-GTP into mRNAs may promote transcriptional mutagenesis (51). B. subtilis relies on the glycosylases MutM and MutY to contend with the potential mutagenic effects of the 8-oxo-G:A mispairing, as well as on the NUDIX proteins YtkD and MutT to prevent the potentially cytotoxic and genotoxic effects of oxidized precursors during DNA and RNA synthesis (10, 38, 45).
A recent study revealed that adaptive mutagenesis is strongly potentiated in starved B. subtilis cells lacking a functional GO system (loss of the YtkD, MutM, and MutY proteins) and suggested that oxidative stress is an important component in the generation of genetic diversity during stationary phase (55). Significantly, the results of that study demonstrate that the types of DNA mispairings promoted by oxidative stress in starved cells are processed in a cooperative manner by both the MMR and GO systems (55). The full extent of this cooperation for mutation suppression versus mutation generation by these systems remains to be elucidated completely. For instance, results obtained with B. subtilis strain YB9555 indicated that oxidative DNA damage is associated with production of adaptive His+ and Met+ revertants, while reversion of the specific leu allele used appeared to depend on other types of DNA lesions recognized by MMR but was not associated with oxidative stress-induced mutagenesis (55). Here we advance our studies on the production of genetic diversity, as affected by factors that prevent oxidative damage, and report that adaptive reversion at the leuC427 allele occurs through a mechanism that involves MMR modulation of the mutagenic/antimutagenic activity of MutY.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains used in this study are listed in Table 1. B. subtilis YB955 is a prophage-“cured” strain that contains the hisC952, metB5, and leuC427 alleles (48, 60, 61). Procedures for transformation and isolation of chromosomal and plasmid DNAs were as described previously (4, 11, 44). B. subtilis strains were maintained on tryptic blood agar base (TBAB) (Acumedia Manufacturers, Inc., Lansing, MI). Liquid cultures of B. subtilis strains were routinely grown in Penassay broth (PAB) (antibiotic A3 medium; Difco Laboratories, Sparks, MD). When required, neomycin (Neo; 10 μg/ml) tetracycline (Tet; 10 μg/ml), spectinomycin (Sp; 100 μg/ml), chloramphenicol (Cm; 5 μg/ml), erythromycin (Er; 3 μg/ml), or rifampin (Rif; 10 μg/ml) was added to media. E. coli cultures were grown in Luria-Bertani (LB) medium (31) supplemented with ampicillin (Amp) to a final concentration of 100 μg/ml.
TABLE 1.
Bacillus subtilis strains and plasmids used in this study
| Strain or plasmid | Relevant genotyped | Reference or transformation in this study |
|---|---|---|
| YB955 | hisC952 metB5 leuC427 xin-1 SpβSENS | 49 |
| HB2078 | perR::kan Kanr | 15 |
| YB9102 | ΔsigB::Cmr | 49 |
| PERM598 | 168 ΔmutY::SpSpr | 55 |
| PERM668c | hisC952 metB5 leuC427 xin-1 SpβSENS pMUTIN4::mutY Emr | pPERM666→YB955b |
| PERM704c | ΔmutY::SpSpr | PERM598→YB955a |
| PERM739c | ΔmutSL::NeoNeor | 36 |
| PERM828c | ΔmutSL::NeoΔmutY::SpNeor Spr | PERM739→PERM704a |
| PERM896 | ΔmutSL::Neo pMUTIN4::mutY Emr Neor | pPERM666→PERM739b |
| PERM897 | perR::kan pMUTIN4::mutY Emr Kanr | pPERM666→HB2078b |
| PERM898c | ΔsigB::Cm pMUTIN4::mutY Emr Cmr | pPERM666→YB102b |
| PERM899 | ΔmutY::Sp mutant with a Phs-mutY construct inserted into the amyE locus; Spr | pPERM852→PERM704b |
| PERM795c | hisC952 metB5 leuC427 xin-1 SpβSENS pMUTINFLAG::mutY Emr | pPERM790→YB955b |
| PERM947 | ΔmutSL::Neo pMUTINFLAG::mutY Emr Neor | pPERM790→PERM739b |
| pPERM626 | pCR-BluntII-TOPO with a 319-bp BamHI/HindIII PCR product containing a mutY fragment; Kanr | This study |
| pdr-111-amyE-Phyperspank | bla-and spec-carrying Phyperspank promoter | 37 |
| pPERM707 | pCR-BluntII-TOPO with a 1,107-bp HindIII/KpnI PCR product containing mutY; Kanr | This study |
| pPERM735 | pCR-BluntII-TOPO with a 1,157-bp HindIII/SalI PCR product containing mutY; Kanr | This study |
| pPERM790 | pMUTIN-FLAG carrying a 1,107-bp HindIII/KpnI fragment from pPERM707; Emr | This study |
| pPERM852 | pdr-111-amyE-Phyperspank carrying a 1,157-bp HindIII/SalI fragment from pPERM735; Spr | This study |
| pPERM666 | pMUTIN4 carrying a 319-bp BamHI/HindIII DNA fragment encompassing 208 bp upstream and 111 bp downstream of the mutY translational start codon; Emr | This study |
Chromosomal DNA from the strain to the left of the arrow was used to transform the strain to the right of the arrow.
Plasmid DNA from the strain to the left of the arrow was used to transform the strain to the right of the arrow.
The background for this strain is YB955.
Cm, chloramphenicol; Em, erythromycin; Kan, kanamycin; Neo, neomycin; Sp, spectinomycin.
Construction of mutant strains and design of a construct to overexpress mutY.
Interruption of mutY from B. subtilis YB955 was achieved by transforming this strain with genomic DNA isolated from B. subtilis PERM598 (mutY::Spr), generating strain B. subtilis PERM704 (ΔmutY) (Table 1). To overexpress mutY, the following strategy was implemented. The open reading frame (ORF) of mutY was PCR amplified using chromosomal DNA from B. subtilis 168 and the oligonucleotide primers 5′-CGAAAGCTTGGGGAGAAACACAT-3′ (forward) and 5′-GAAGTCGACTACAGCCACGG-3′ (reverse). The primers were designed to insert HindIII and SalI sites, respectively (underlined). Amplification was performed with Vent DNA polymerase (New England Biolabs, Beverly, MA), and the PCR product (1,157 bp) was purified from a low-melting-point agarose gel and cloned into pCR-Bunt-II-TOPO vector (Invitrogen Life Technologies, Carlsbad, CA) to obtain the construct pPERM735 (Table 1). Plasmid pPERM735 was treated with HindIII/SalI enzymes to release a 1,157-bp fragment of the mutY open reading frame, which was purified from a low-melting-point agarose gel and ligated into the HindIII/SalI sites of pdr-111-amyE-pHyperSpank, immediately downstream of the isopropyl-beta-d-thiogalactopyranoside (IPTG)-inducible pHyperspank promoter (Phs) (a gift from David Rudner). The resulting plasmid (pPERM852) was amplified in E. coli XL10-GOLD Kanr (Stratagene, Cedar Creek, TX) and was introduced by transformation into B. subtilis PERM704 (mutY mutant) to generate the strain B. subtilis PERM899 (Table 1). A mutSL mutY mutant was constructed by the transformation of a B. subtilis mutSL mutant (36) with genomic DNA from PERM704, generating the strain PERM828 (Table 1). The double-crossover events leading to the inactivation of the appropriate genes or to insertion of the construct to overexpress mutY into the amyE locus were confirmed by PCR, using specific oligonucleotide primers (data not shown).
Construction and integration of mutY-lacZ and mutY-flag fusions.
Construction of a transcriptional fusion between mutY and the lacZ gene was performed in the integrative plasmid pMUTIN4 (54) by inserting a 319-bp BamHI/HindIII fragment from the plasmid pPERM626 (Table 1) into pMUTIN4, previously digested with BamHI and HindIII. The resulting construct containing the mutY-lacZ fusion was designated pPERM666 and was propagated in E. coli DH5α. Plasmid pPERM666 was introduced by transformation into competent cells of strains B. subtilis YB955 and B. subtilis PERM739 (ΔmutSL) (36) to obtain the strains B. subtilis PERM668 (Err) and PERM896 (Neor Err), respectively (Table 1).
An in-frame translational fusion between mutY and the FLAG epitope was constructed in the vector pMUTIN-FLAG (20). To this end, a DNA fragment encompassing 23 bp upstream (including the Shine-Dalgarno sequence) of the translational start codon to the last codon of the mutY ORF was amplified by PCR, utilizing Vent DNA polymerase (New England Biolabs, Beverly, MA) and the oligonucleotide primers 5′-CGAAGCTTGCGGGAAAAGGAGGTATGGGG-3′ (forward) and 5′-GCGGTACCCTTGCCCAGTCTTCTTTTCAA-3′ (reverse), which inserted HindIII and KpnI sites (underlined) into the cloned DNA. The PCR-amplified DNA fragment (1,107 bp) was first ligated into pCR-BluntII-TOPO (Invitrogen, Carlsbad, CA) and then replicated in E. coli XL10-GOLD Kanr (Stratagene, Cedar Creek, TX). The resulting construct (pPERM707) was treated with HindIII and KpnI, the 1,107-bp mutY insert was ligated into HindIII/KpnI-treated pMUTIN-FLAG, and the ligation products were introduced by transformation into competent cells of E. coli XL10-GOLD Kanr (Stratagene, Cedar Creek, TX). This strategy generated plasmid pPERM790, which was used to transform B. subtilis YB955 as well as the ΔmutSL mutant strain (PERM739), generating strains B. subtilis PERM795 and PERM947 (Table 1), respectively. The crossover events leading to insertion of the mutY-lacZ and mutY-flag fusions into the corresponding loci were confirmed by PCR with specific oligonucleotide primers (data not shown).
Stationary-phase mutagenesis assays.
Essentially, cultures were grown in flasks containing antibiotic A3 medium with aeration (250 rpm) at 37°C to 90 min after the cessation of exponential growth (designated T90). Growth was monitored with a spectrophotometer measuring the optical density at 600 nm (OD600). The stationary-phase mutagenesis assays were performed as previously described (36, 49), using solid Spizizen minimal medium (SMM; 1× Spizizen salts supplemented with 0.5% glucose and either 50 μg or 200 ng of the required amino acid/ml and 50 μg each of isoleucine and glutamic acid/ml). The concentration of the amino acid used depended on the reversion tested. For instance, in selecting His+ revertants, 50 μg (each) of methionine and leucine/ml was added to the medium and 200 ng of histidine/ml was added. Isoleucine and glutamic acid were added as described previously (48) in order to protect the viability of the cells. The number of revertants was scored daily. The initial number of bacteria plated for each experiment was estimated by serial dilution of the bacterial cultures and then plating of the cells on LB medium. The experiments were repeated at least three times. The survival rates of the bacteria plated on the minimal selective medium were determined as follows. Three agar plugs were removed from each selection plate daily. The plugs were removed with sterile Pasteur pipettes and taken from areas of the plates where no growth of revertant colonies was observed. The plugs were suspended in 400 μl of 1× Spizizen salts, mixed, diluted, and plated on SMM containing all essential amino acids (50 μg/ml). Again, the number of colonies was determined following 48 h of growth at 37°C.
Analysis of mutation rates.
Spontaneous mutation frequencies to rifampin resistance in growing cells were determined as previously described (55). Essentially, the appropriate strains were grown for 12 h at 37°C in antibiotic A3 medium with proper antibiotics. Mutation frequencies were determined by plating aliquots on six LB plates containing 10 μg/ml rifampin, and the rifampin-resistant (Rifr) colonies were counted after 1 day of incubation at 37°C. The number of cells used to calculate the frequency of mutation to Rifr was determined by plating aliquots of appropriate dilutions on LB plates without rifampin and incubating the plates for 24 to 48 h at 37°C. These experiments were repeated at least three times (36, 40). The growth-dependent reversion rates for the Leu+ phenotype were measured by fluctuation tests with the Lea-Coulson formula, r/m − ln(m) = 1.24 (23, 40), as previously described (46, 49, 50).
Beta-galactosidase assays.
B. subtilis strains PERM668 and PERM896, containing a transcriptional mutY′-lacZ fusion, were propagated in liquid A3 medium. Aliquots of 1.5 ml were collected from cultures in exponential growth phase or stationary phase. Cells were washed with 0.1 M Tris-HCl (pH 7.5), pelleted by centrifugation, and stored at −20°C until determination of β-galactosidase activity (35). Briefly, washed cell samples were first disrupted with lysozyme and subjected to centrifugation; the β-galactosidase activity present in the supernatant was then determined as previously described, using ortho-nitro-phenyl-β-d-galactopyranoside (ONPG) as a substrate (35).
Induction experiments.
Experiments were performed to analyze whether H2O2, NaCl, ethanol, or mitomycin C induced the expression of the transcriptional mutY-lacZ fusion in the strain B. subtilis PERM668. Each compound was tested independently as described below. Cells were grown in A3 medium to an OD600 of 0.5. The cultures were divided into two subcultures of equal volume, and each of the compounds described above was added to one subculture, to the following final concentrations: H2O2, 0.1 mM; NaCl, 4%; ethanol, 4%; and mitomycin C, 0.5 μg/ml. The second subculture served as a control. Cells were harvested after 2 h of induction and assayed for β-galactosidase activity.
RT-PCR experiments.
Total RNA from exponentially growing or stationary-phase B. subtilis YB955 cells grown in A3 medium was isolated by using Tri reagent (Molecular Research Center, Inc.). Reverse transcription-PCRs (RT-PCRs) were performed with the RNA samples and a Master AMP RT-PCR kit (Epicentre Technologies, Madison, WI) according to the manufacturer's instructions. The primers used for RT-PCR were 5′-AAGGGCTCGGCTATTATTCGC-3′ (forward) and 5′-AAGCAGGCATGAGGGTGATTT-3′ (reverse), which generated a 371-bp RT-PCR product extending from 259 bp downstream from the start codon of mutY to 630 bp downstream of this point. As a control, in each experiment, the absence of chromosomal DNA in the RNA samples was assessed by PCRs carried out with Vent DNA polymerase (New England Biolabs, Beverly, MA) and the set of primers described above. The size of the RT-PCR product was determined by utilizing the 1-kb-Plus DNA ladder (Life Technologies, Rockville, MD) during agarose gel electrophoresis.
Western blot assay.
B. subtilis strain YB955 was cultivated with shaking in liquid antibiotic A3 medium at 37°C. Aliquots of 1.5 ml were collected from cultures in exponential growth phase and stationary phase. Cells were collected by centrifugation (21,300 × g; 1 min), washed twice with 0.1 M Tris-HCl (pH 7.5) buffer, and stored at −20°C. Bacterial pellets were resuspended in 0.2 ml of the same buffer supplemented with a protease inhibitor cocktail (Roche, Mannheim, Germany) and were disrupted by sonication with a VCX130-PB Vibra Cell apparatus (Sonics and Materials Inc., Newton, CT). The cell lysate was subjected to centrifugation (27,200 × g; 20 min; 4°C) to eliminate undisrupted cells and cell debris. The supernatant was separated, and its protein concentration was determined with a Coomassie (Bradford) protein assay kit (Pierce, Rockford, IL). Protein aliquots (100 μg) were separated in SDS-12% polyacrylamide gels and then electrotransferred to polyvinylidene difluoride (PVDF) membranes. Western blot analyses were performed with a FLAG monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) diluted 10,000-fold and then processed with an ECL (enhanced chemiluminescence) Western blotting system (Amersham Pharmacia, Buckinghamshire, United Kingdom).
DNA sequencing.
Leu+ revertant colonies of the mutSL and mutSL mutY strains collected from plates from adaptive mutagenesis assays during days 5, 6, and 7 were independently propagated in liquid antibiotic A3 medium, and chromosomal DNA was isolated from each sample (11). A 303-bp fragment encompassing nucleotides 353 to 655 of the leuC ORF was amplified by PCR with Vent DNA polymerase (New England Biolabs, Beverly, MA) and the oligonucleotide primers 5′-CGCTTCCAGGGAAAACGATTG-3′ (forward) and 5′-CGATGGATGAACGAATGACTG-3′ (reverse). Sequencing was performed by Functional Biosciences, Inc. (Madison, WI), on a Prism 3730 DNA analyzer, using an ABI BigDye Terminator v1.1 cycle sequencing kit (Applied Biosystems).
RESULTS
Loss of MutY has different effects on prototrophic reversion rates.
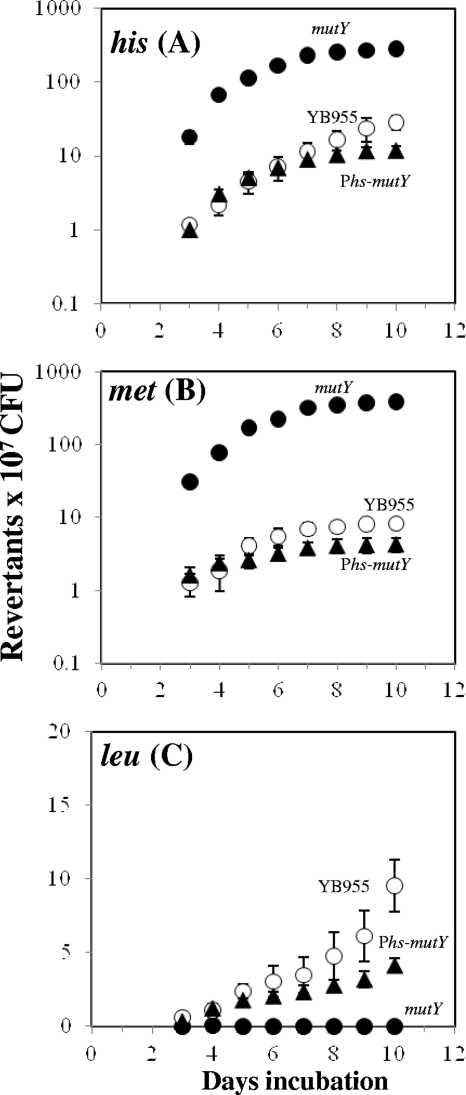
As noted above, the reversion assay system used in this work measures the reversion of nonsense mutations in the metB and hisC alleles and a missense mutation at the leuC allele in stationary-phase cells of B. subtilis strain YB955 (49). Using this system, we investigated the effects of genetic inactivation of mutY on the production of His+, Met+, and Leu+ revertants, specifically during stationary phase (i.e., adaptive mutagenesis). The results showed that in comparison with the parental strain, the numbers of His+ and Met+ colonies increased around 4 and 5 times, respectively, for the ΔmutY strain (Fig. 1 A and B). In contrast, the absence of mutY completely abolished the production of Leu+ revertants (Fig. 1C), suggesting that during stationary phase MutY may play a role in generating mutations at certain sites or DNA sequences. The results shown in Fig. 1C support this suggestion, since overexpression of mutY from the IPTG-inducible Phs promoter restored the ability of the ΔmutY mutant to generate Leu+ revertants. Moreover, compatible with the idea that MutY also has an antimutagenic role during the adaptive mutagenesis process, our results showed that the IPTG induction of the Phs-mutY cassette decreased the production of His+ and Met+ colonies to the levels produced by the parental strain (Fig. 1A and B).
FIG. 1.
Stationary-phase-induced reversions of the his (A), met (B), and leu (C) mutant alleles of the YB955 (○), PERM704 (mutY) (•), and PERM899 (mutY amyE::Phs-mutY) (▴) B. subtilis strains are described in Materials and Methods. The results are average numbers of accumulated revertants for six different selection plates. This experiment was performed at least three times.
As shown in Fig. 2, the ΔmutY and ΔmutY-amyE::Phs-mutY strains exhibited similar survival rates to that of the parental strain YB955 during the time course of the experiments. Thus, no net increase or decrease in viable cell count was observed for the two strains. In conclusion, the increases in the numbers of His+ and Met+ revertant colonies and the decrease in the number of Leu+ revertant colonies observed in this mutant strain were not the results of differential growth or survival with respect to the parental strain.
FIG. 2.
Abilities of strains PERM704 (mutY) (A) and PERM899 (mutY amyE::Phs-mutY) (B) to survive histidine (black bars), methionine (gray bars), and leucine (white bars) starvation. Three plugs of bacteria containing agar were taken from selection plates each day for testing of viability of bacteria (see Materials and Methods for details). The experiments were repeated at least twice.
Spontaneous mutation rates in cells lacking MutY.
Due to an anticipated antimutagenic role of the MutY protein in preventing the damage caused by oxidation of DNA (45), we determined the mutation frequency to a Rifr phenotype of a mutY-deficient strain. The results revealed that loss of the mutY gene function increased the spontaneous mutation rate to Rifr 9-fold compared to that for an isogenic strain that produced a functional MutY protein (Table 2). Surprisingly, overexpression of this gene in the strain carrying the mutY mutation at the original locus increased the mutation frequency to rifampin resistance around 80 times with respect to the parental strain YB955 (Table 2). Moreover, in the ΔmutY strain, as detailed in Fig. 3 A, the growth-dependent fluctuation rates of the Leu+ revertants decreased compared with those of the YB955 parental strain, suggesting that MutY plays a promutagenic role in the production of colonies with a Leu+ phenotype. In agreement with this hypothesis, the production of Leu+ colonies by the MutY-deficient strain was restored to the level of the parental strain (YB955) following expression of mutY from the IPTG-inducible Phs promoter (Fig. 3A).
TABLE 2.
Spontaneous mutation frequencies of mutSL- and mutY-deficient strainsa
| Strain | Mean Rifr mutation frequency ± SD (10−9)b | Relative frequency |
|---|---|---|
| YB955 | 1.6 ± 0.92 | 1 |
| mutYmutant | 13.74 ± 0.217 | 8.58 |
| mutY::amyE-Phs-mutY mutant | 129.145 ± 18.52 | 80.71 |
| mutSL mutant | 106.67 ± 2.003 | 66.66 |
| mutY mutSL mutant | 74.53 ± 1.94 | 46.58 |
Cells were grown for 12 h at 37°C in antibiotic A3 medium (Difco) supplemented with the proper antibiotics, and aliquots were plated on six LB plates containing 10 μg/ml rifampin. Rifr colonies were counted after 1 day of incubation at 37°C.
Values shown are averages for three independent experiments ± standard deviations.
FIG. 3.
(A) Analysis of mutation rates. B. subtilis strains YB955 (parental strain), PERM704 (mutY), PERM899 (mutY amyE::Phs-mutY), PERM739 (mutSL), and PERM828 (mutY mutSL) were tested for the ability to produce Leu+ revertants during exponential growth as described in Materials and Methods. The mutation rates were calculated as previously described, with the formula m/2Nt. Results presented are average mutation rates for three individual fluctuation tests. Error bars represent 1 standard error. (B) Stationary-phase-induced reversion to Leu+ of the YB955 (○), PERM739 (mutSL) (•), and PERM828 (mutY mutSL) (▴) B. subtilis strains were determined as described in Materials and Methods. Results are average numbers of accumulated revertants for six different selection plates. This experiment was performed at least three times.
MutSL and MutY modulate stationary-phase-associated mutagenesis in B. subtilis.
Previous results revealed that a mutSL knockout of B. subtilis YB955 showed an increase in the number of adaptive Leu+ revertants (36, 49). On the other hand, the overproduction of the MutSL proteins during stationary phase significantly reduced the number of adaptive mutations (36, 49). These results suggest that during stationary phase, saturation of the MMR system may contribute to generating a hypermutagenic population responsible for generating adaptive mutations (36, 49). Our results revealed that generation of stationary-phase-associated Leu+ revertants is fully dependent on the presence of MutY (Fig. 1C). Considering these observations, we investigated whether the increased reversion frequency to Leu+ observed for the mutSL mutant (36) was dependent (at least in part) on the mutagenic action of mutY. To this end, we constructed a strain in which the mutSL and mutY genes were all inactivated and found that the reversion frequency of the leu allele in this strain was statistically indistinguishable from that found in YB955, the parental strain (Fig. 3B). These results suggest that during stationary phase, under conditions that saturate or inactivate the MMR system, MutY promotes the generation of mutations that increase the frequency of leu reversion. A similar result was observed in growing B. subtilis cells, since the loss of MutY diminished the Leu+ reversion frequency of the mutSL strain to the levels exhibited by the parental strain (Fig. 3A).
YfhQ promotes the formation of intragenic suppressors.
To determine the types of mutations that conferred leucine prototrophy, several independent adaptive Leu+ revertants generated over 5, 6, or 7 days by the mutSL or mutSL mutY strain were sequenced for the leuC allele to determine the type of mutation that generated the reversion. The results of this analysis showed that although most of the Leu+ revertants produced by the ΔmutSL MutY-replete strain contained A→G transitions at codon 427 (true reversions), some G→A transitions and C→A transversions were also detected (Table 3). Importantly, several of the Leu+ revertants of the ΔmutSL mutY strain contained only A→G transitions (Table 4).
TABLE 3.
Base changes in leu revertants of the ΔmutSL straina
| Revertant allele (day of expt) | Revertant sequenced | Position of mutation | Type of mutation | DNA change (result of mutation) |
|---|---|---|---|---|
| leuC (5) | 1 | 346 | Transition | G→A (Gly→Ser) |
| 2 | 359 | Transversion | C→A (Pro→Glu) | |
| 427 | Transition | A→G (Arg→Gly) | ||
| 3 | 427 | Transition | A→G (Arg→Gly) | |
| leuC (6) | 4 | 427 | Transition | A→G (Arg→Gly) |
| 5 | ? | ? | ? | |
| 6 | 427 | Transition | A→G (Arg→Gly) | |
| 7 | 363 | Transition | G→A (Gly→Glu) | |
| 8 | 427 | Transition | A→G (Arg→Gly) | |
| leuC (7) | 9 | 427 | Transition | A→G (Arg→Gly) |
| 10 | 427 | Transition | A→G (Arg→Gly) |
Chromosomal DNA was isolated (11) from Leu+ revertant colonies of the mutSL strain collected from plates of adaptive mutagenesis assays during day 5, 6 or 7. A 303-bp fragment encompassing nucleotides 353 to 655 of the leuC ORF was amplified by PCR and sequenced as described in Materials and Methods. ?, mutations were not found in the sequenced leuC gene.
TABLE 4.
Base changes in leu revertants of the ΔmutSL mutY straina
| Revertant allele (day of expt) | Revertant sequenced | Position of mutation | Type of mutation | DNA change (result of mutation) |
|---|---|---|---|---|
| leuC (5) | 1 | ? | ? | ? |
| 2 | 427 | Transition | A→G (Arg→Gly) | |
| leuC (6) | 3 | 427 | Transition | A→G (Arg→Gly) |
| 4 | ? | ? | ? | |
| 5 | 427 | Transition | A→G (Arg→Gly) | |
| 6 | 427 | Transition | A→G (Arg→Gly) | |
| 7 | 427 | Transition | A→G (Arg→Gly) | |
| 8 | 427 | Transition | A→G (Arg→Gly) | |
| leuC (7) | 9 | 427 | Transition | A→G (Arg→Gly) |
| 10 | ? | ? | ? |
Chromosomal DNA was isolated (11) from Leu+ revertant colonies of the mutSL mutY strain collected from plates from adaptive mutagenesis assays during day 5, 6, or 7. A 303-bp fragment encompassing nucleotides 353 to 655 of the leuC ORF was amplified by PCR and sequenced as described in Materials and Methods. ?, mutations were not found in the sequenced leuC gene.
Analysis of mutY expression during the growth cycle of B. subtilis.
As noted above, our results suggested that levels of MutY and MMR may be interconnected to modulate the production of mutations such as those in the Leu+ revertants during growth and stationary phase. In support of this hypothesis, our results demonstrated that the levels of expression of a transcriptional mutY-lacZ fusion increased around 5 times in a mutSL-deficient genetic background (Fig. 4 A). The implications of this result prompted us to analyze in more detail the temporal pattern of mutY expression and to determine the levels of its encoded product during the life cycle of B. subtilis. Expression of mutY was first analyzed by utilizing the strain B. subtilis PERM668, which harbors a single copy of a mutY-lacZ transcriptional fusion. To avoid sporulation, the strain was propagated in liquid antibiotic A3 medium; under these conditions, mutY-directed β-galactosidase activity was detected in growing B. subtilis cells, and the maximum level of β-galactosidase was reached during the transition phase of growth (from exponential to stationary phase). This activity was still observed 4 h after the onset of the stationary phase (Fig. 4B). This temporal pattern of mutY expression was further investigated in YB955 by RT-PCR. RNA samples were collected during exponential growth as well as during the transition and stationary phases of growth. RT-PCR experiments confirmed the presence of mutY mRNAs during the three developmental phases analyzed (Fig. 5 A). We also analyzed the levels of MutY during the growth cycle of a B. subtilis strain harboring an in-frame translational mutY-flag fusion. Results of Western blot analysis using an anti-FLAG antibody demonstrated the presence of similar amounts of MutY in cells that were either actively growing or in the early or late stationary phase of growth (Fig. 5B).
FIG. 4.
(A) Levels of β-galactosidase produced by B. subtilis strains PERM668 (YB955) (• and ○) and PERM896 (ΔmutSL) (▪ and □). Strains were grown in liquid antibiotic (A3) medium. Cell samples were collected at the indicated times and treated with lysozyme, and the extracts were assayed for β-galactosidase as described in Materials and Methods. The data shown are average values for triplicate independent experiments ± standard deviations (SD) for β-galactosidase specific activity in strains PERM668 (○) and PERM896 (□) and for A600 values for strains PERM668 (•) and PERM896 (▪). (B) Levels of β-galactosidase in a mutY-lacZ transcriptional fusion during vegetative and stationary phases of growth. B. subtilis strain PERM668 was grown in liquid antibiotic (A3) medium. Cell samples were collected at the indicated times and treated with lysozyme, and the extracts were assayed for β-galactosidase as described in Materials and Methods. Data shown are average values for triplicate independent experiments ± SD for β-galactosidase specific activity (▴) and for A600 values (•).
FIG. 5.
(A) RT-PCR analysis of mutY transcription during vegetative and stationary phases of growth. RNA samples (∼1 μg) isolated from a B. subtilis YB955 A3 culture, at the steps indicated, were processed for RT-PCR analysis as described in Materials and Methods. The arrow shows the size of the expected RT-PCR product. 16S and 23S rRNA bands are shown in the lower panel. (B) Western blot analysis of MutY-FLAG synthesis during vegetative and stationary phases of growth. B. subtilis strain YB955 was grown in liquid A3 medium. Cell extract samples (100 μg of protein; see Materials and Methods), harvested at the steps indicated, were separated by SDS-PAGE and transferred to PVDF membranes (Bio-Rad, Hercules, CA). The blots were stained with Ponceau red (top), probed with a FLAG monoclonal antibody diluted 10,000-fold, and then processed with an ECL Western blot system (bottom). The positions of molecular size markers are indicated to the left of the stained membrane. T0 is the time point in the culture when the slopes of the logarithmic and stationary phases of growth intercepted. T90, T180, and T270 indicate the time in minutes after T0. Veg., vegetative growth.
Levels of a mutY-lacZ fusion are not increased by different types of stress inducers.
The B. subtilis strain PERM668, containing a mutY-lacZ fusion integrated at mutY, was used to investigate whether the mutY gene is induced as part of an oxidative stress regulon (15). Accordingly, B. subtilis strain PERM668 was grown to the mid-exponential phase and treated for 4 h with hydrogen peroxide (200 μM). The results in Fig. 6 A reveal that no transcription induction occurred following treatment of the strain with this oxidative stress-inducing chemical. These results suggest that mutY does not belong to the PerR regulon; in support of this, we found that in a PerR-deficient strain, the expression pattern of the mutY-lacZ fusion was indistinguishable from that observed in the parental strain PERM668 (Fig. 6B).
FIG. 6.
(A) Lack of induction of a mutY-lacZ fusion by mitomycin C (M-C), hydrogen peroxide, and sodium chloride. B. subtilis PERM668 was propagated in A3 medium, in three independent flasks, to an OD600 of 0.5. At this point, each culture was divided equally into 2 flasks; one set of flasks was left untreated (a, d, and f), and the other set was treated with either mitomycin C (0.5 μg/ml) (b), H2O2 (200 μM (e), or NaCl (4% [wt/vol]) (g). (c) B. subtilis YB3001 containing a recA′-lacZ fusion was treated with mitomycin C (0.5 μg/ml). Cells harvested after 1 h of induction were assayed for β-galactosidase activity as described in Materials and Methods. Values represent averages for triplicate independent experiments ± SD. (B) Expression of a mutY-lacZ fusion in PerR and σB genetic backgrounds. Levels of β-galactosidase produced by B. subtilis strains PERM668 (YB955) (• and ○), PERM897 (perR) (▪ and □), and PERM898 (sigB) (▴ and ▵) are shown. Strains were grown in liquid antibiotic (A3) medium. Cell samples were collected at the indicated times and treated with lysozyme, and the extracts were assayed for β-galactosidase as described in Materials and Methods. The data shown are average values for triplicate independent experiments ± SD for β-galactosidase specific activity in strains PERM668 (○), PER897 (□), and PERM898 (▴) and for A600 values for strains PERM668 (•), PERM897 (▪), and PER898 (▴).
In response to a nongrowing or starvation state, B. subtilis exhibits a cellular response regulated by the RNA polymerase σB factor (17). It has been demonstrated that transcription of σB-dependent genes is strongly induced by heat, salt, acid, or ethanol as well as by energy depletion (2, 3, 5, 57). Our results showed that treatment of exponentially growing B. subtilis PERM668 cells with sodium chloride (4%) for 15 min to 2 h did not induce the expression of the mutY-lacZ fusion, suggesting that mutY is not part of the σB stress response (Fig. 6A). To better support this conclusion, we recombined the mutY-lacZ fusion in the mutY locus of a σB-deficient strain and found that the levels of β-galactosidase expressed in this genetic background did not differ significantly from those observed in the B. subtilis strain PERM668 (Fig. 6B).
Since MutY is involved in DNA repair, we investigated whether the transcription of its encoding gene is under the control of the global SOS response (47). This gene circuitry, which controls the expression of several DNA repair genes (including recA), is induced in B. subtilis by certain DNA-damaging agents as well as during the development of competence (24, 25). Our results showed that addition of the compound mitomycin C (0.5 μg/ml) to exponentially growing cells of the strain PERM668 did not increase the expression levels of the mutY-lacZ fusion above those produced by the untreated control (Fig. 6A). As a positive control, we found that the amount of mitomycin C used in these experiments, 0.5 μg/ml, increased the level of β-galactosidase in a recA′-lacZ fusion-containing strain roughly 4 times with respect to that in the untreated control (Fig. 6A). Taken together, these results indicate that the transcriptional machinery of B. subtilis keeps active expression of mutY during the exponential growth and stationary phases. However, despite its role in maintaining the genomic integrity of B. subtilis, transcription of mutY is not part of the major or well-characterized gene expression circuitries that respond to the main stresses encountered by this microorganism.
DISCUSSION
Oxidative stress-induced nucleic acid damage and the potential mutations that arise from such damage have confronted living systems throughout the evolutionary process (7, 14). It is not surprising that multiple systems have evolved to reduce the mutagenic and lethal potentials of this type of damage (7, 14, 46, 55, 58). For the purpose of this report, it has been established that oxidative stress-induced DNA damage is an important factor that promotes stationary-phase mutagenesis in B. subtilis (55) as well as in other organisms (6, 7, 46). A recent report revealed that in the strain B. subtilis YB955, the genetic disruption of the GO system potentiated the generation of stationary-phase-associated His+ and Met+ revertants. Although this effect was not observed for the leuC allele, overexpression of the mutSL operon did significantly reduce the production of Leu+ revertants in the GO-deficient strain (55). These results suggested that reversion of the leu allele does not involve oxidative stress lesions but another type(s) of damage recognized by the MMR system. However, another possibility, not tested in those experiments, is that an interaction between the proteins involved in the MMR system and in prevention of oxidative damage promotes mutagenic events.
To begin to distinguish between the two possibilities mentioned above, we investigated the generation of mutations in strains lacking one or both of these DNA repair mechanisms. Our results showed that loss of MutY completely abolished the production of adaptive Leu+ revertants; in contrast, the rates of His+ and Met+ colonies increased in this mutant strain (Fig. 1). These results indicate that MutY plays completely different roles in the generation of adaptive mutants in B. subtilis and that the actual effect may be dependent upon the DNA sequence. As noted above, the his and met alleles in strain YB955 correspond to nonsense mutations, which in a triple mutY mutM ytkD mutant are subject mostly to reversion by suppressor mutations (55). Thus, in the mutY-deficient strain, accumulation of damaged bases generated by direct attack of hydroxyl radicals or following incorporation of oxidized precursors from the dNTP pools is likely to increase the number of amber and ochre suppressor mutations that produce adaptive His+ and Met+ revertants. In contrast, previous reports indicated that suppressor mutations are not involved in reversion of the leu allele (43, 49).
In growing B. subtilis cells with a functional MMR system, disruption of mutY increased the mutation frequency to Rifr; intriguingly, overexpression of mutY increased the generation of these types of mutations even more than did the inactivation of the relevant genes (Table 2). Thus, during growth, MutY seems to play both antimutagenic and promutagenic roles. In the first case, MutY may prevent the occurrence of G→T transversions derived from misreplication of the 8-oxoguanine-containing template DNA (28, 29, 33). The second situation seems to be a consequence of the ability of the enzyme to compete for a fraction of the base mispairs that are processed by MutSL. This suggestion is supported by experiments showing that the mutation rates to Rifr of an MMR-deficient strain are notoriously diminished following disruption of mutY (Table 2). A mechanism in which MutS and MutY compete for processing of A-C mismatches has also been postulated to operate in growing E. coli cells (1, 22). Interestingly, it has been shown that 43% of the spontaneous Rifr mutations that occur in vegetative cells correspond to A→G transitions (34), i.e., the type of transition that results after MutY processing of A-C mispairs. Additionally, is tempting to speculate that during growth, B. subtilis cells maintain a balance in the levels of MutY and MutSL; such an equilibrium would be important to allow MutSL to coordinate the proper action of MutY when acting over A-GO, A-C, and/or A-G mismatches.
As noted above, other types of base modifications, but not those promoted by oxidative stress, seem to be associated with reversion of the specific leu allele utilized in these studies (55). Thus, under physiological conditions that saturate the MMR system, such as those postulated to exist in a population of stressed cells (36), MutY could escape from MMR control to freely operate over A-C, A-G, and/or C-A mismatches (26), removing adenine and introducing (through the BER pathway) mutations that revert the Leu− phenotype. In support of this mechanism, our results revealed that production of the majority of the Leu+ revertants generated by a MutSL-deficient strain is dependent on the existence of a functional MutY protein (Fig. 3B). Furthermore, our results of sequence analysis tentatively support this mechanism, as they revealed that the Leu+ revertants produced by the ΔmutSL MutY-replete strain showed the occurrence of G→A, C→A, and A→G mutations. Importantly, only A→G transitions were detected in the Leu+ revertants following disruption of the mutY gene in the mismatch repair-deficient strain (Tables 3 and 4).
Although saturation of the MMR system seems to be a common factor involved in the reversion of the leuC allele in both phases of growth, this process may potentially occur more frequently in stressed (nondividing) cells, as they have a natural tendency to accumulate DNA damages that are substrates for MMR (36, 62). In contrast, in growing cells, this situation is more controlled, not only by the proofreading activity of the replicative machinery but also by the base excision repair pathway (14).
Our results from the expression analysis and protein detection assays revealed that mutY mRNA and its encoded product are present during exponential growth and stationary phase. A careful analysis of the mutY region in the B. subtilis genome revealed that this gene is part of the mutY-fabL-sspE cluster (59). Although it can be argued that disruption of mutY can potentially exert a polar effect over fabL and sspE, there is compelling evidence showing that (i) sspE can be transcribed monocistronically during late sporulation by EσG (59) and (ii) an fabL-sspE bicistronic mRNA is generated from two putative promoters located immediately downstream of mutY (59). These observations indicate that the phenotypic effect exhibited by the mutY strain is not the result of polar effects. Our results corroborated this contention, since the effects on the reversion rates observed with the mutY strain were alleviated by integrating into the amyE locus a copy of mutY whose transcription was controlled by the IPTG-inducible Phs promoter of the integrative pHyperspank vector (Fig. 1). Importantly, these effects were not observed when the MutY-deficient strain carried a single copy of the pHyperspank vector inserted into its genome (data not shown). Altogether, these results confirmed that the increase in the production of His+ and Met+ revertants and the abolishment of colonies with Leu+ reversions depended exclusively on the loss of mutY.
As noted above, during exponential growth, B. subtilis must maintain a harmonious equilibrium in the levels of MutY and MutSL that is required to properly coordinate the processing of the several mismatches recognized by MutY. Such an equilibrium would clearly be compromised in B. subtilis cultures subjected to nutritional stress, in which it has been postulated that there is a hypermutagenic population where the MMR system has a reduced capacity to respond to DNA damage (36, 62). Under such circumstances, the activity of MutY may well escape MMR control, avoiding accurate processing of the several mismatches repaired by this DNA glycosylase. Although the existence of a subpopulation of cells exhibiting transient hypermutagenesis within B. subtilis cultures subjected to nutritional stress has not been tested directly, our results provide support for this concept. In an MMR-deficient strain, the expression levels of a transcriptional mutY-lacZ fusion were upregulated, suggesting that conditions that saturate the MMR capacity of cells induce the expression of mutY. Moreover, although we found that transcription of mutY occurred during both growth and stationary phase, its expression levels were not induced by DNA damage or in PerR- or σB-deficient backgrounds. Hence, it is possible that a mechanism different from the SOS, PerR, or general stress response may exist in a hypermutagenic subpopulation of stressed cells involved in mutY upregulation. These observations are in agreement with experiments showing that the generation of adaptive mutations in B. subtilis is RecA independent (49).
Finally, although an important proportion of the Leu+ revertants can be generated through a competition mechanism between MMR and MutY during processing of A-C mismatches, it is evident that other mutational events give rise to these types of prototrophs. For instance, it has been proposed that derepression of leuC transcription and possibly transcriptional bypass of nondistortive lesions promoted by Mfd are involved in generating this type of revertant. Evidence for the occurrence of this mechanism has been presented previously for the B. subtilis model (43). Experiments aimed to investigate how MutY and/or MMR components are interconnected with Mfd to modulate stationary-phase-associated mutagenesis are presently being performed.
Acknowledgments
This work was supported by the University of Guanajuato and by the Consejo Nacional de Ciencia y Tecnología (CONACYT; grant 84482) of México. R.E.Y. and E.A.R. were supported by the NIH (GM072554 and 2 P20 RR016463) and the NSF (MCB-0843606 and DBI-0649267). B.N.D., L.E.V., R.R., and M.R. were supported by scholarships from CONACYT.
Footnotes
Published ahead of print on 22 October 2010.
REFERENCES
- 1.Bai, H., and A. L. Lu. 2007. Physical and functional interactions between Escherichia coli MutY glycosylase and mismatch repair protein MutS. J. Bacteriol. 189:902-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benson, A. K., and W. G. Haldenwang. 1992. Characterization of a regulatory network that controls sigma B expression in Bacillus subtilis. J. Bacteriol. 174:749-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benson, A. K., and W. G. Haldenwang. 1993. The sigma B-dependent promoter of the Bacillus subtilis sigB operon is induced by heat shock. J. Bacteriol. 175:1929-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boylan, R. J., N. H. Mendelson, D. Brooks, and F. E. Young. 1972. Regulation of the bacterial cell wall: analysis of a mutant of Bacillus subtilis defective in the biosynthesis of teichoic acid. J. Bacteriol. 173:281-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boylan, S. A., A. S. Rutherford, S. M. Thomas, and C. W. Price. 1992. Activation of Bacillus subtilis transcription factor sigma B by a regulatory pathway responsive to stationary phase signals. J. Bacteriol. 174:3695-3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bridges, B. A. 1995. mutY ‘directs’ mutation? Nature 375:741. [DOI] [PubMed] [Google Scholar]
- 7.Bridges, B. A., M. Sekiguchi, and T. Tajiri. 1996. Effect of mutY and mutM/fpg-1 mutations on starvation-associated mutation in Escherichia coli: implications for the role of 7,8-dihydro-8-oxoguanine. Mol. Gen. Genet. 251:352-357. [DOI] [PubMed] [Google Scholar]
- 8.Bridges, B. A. 1998. The role of DNA damage in stationary phase (“adaptive”) mutation. Mutat. Res. 408:1-9. [DOI] [PubMed] [Google Scholar]
- 9.Cairns, J., J. Overbaugh, and S. Miller. 1988. The origin of mutants. Nature 335:142-145. [DOI] [PubMed] [Google Scholar]
- 10.Castellanos-Juárez, F. X., C. Alvarez-Alvarez, R. E. Yasbin, B. Setlow, P. Setlow, and M. Pedraza-Reyes. 2006. YtkD and MutT protect vegetative cells but not spores of Bacillus subtilis from oxidative stress. J. Bacteriol. 188:2285-2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cutting, S. M., and P. B. Vander Horn. 1990. Genetic analysis, p. 27-74. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley and Sons, Sussex, England.
- 12.Dubnau, D. 1991. Genetic competence in Bacillus subtilis. Microbiol. Rev. 55:395-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reference deleted.
- 14.Friedberg, E. C., G. C. Walker, W. Siede, R. D. Wood, R. A. Schultza, and T. Ellenberger. 2006. DNA repair and mutagenesis, 2nd ed. American Society for Microbiology, Washington, DC.
- 15.Fuangthong, M., A. F. Herbig, N. Bsat, and J. D. Helmann. 2002. Regulation of the Bacillus subtilis fur and perR genes by PerR: not all members of the PerR regulon are peroxide inducible. J. Bacteriol. 184:3276-3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halas, A., H. Baranowska, and Z. Policinska. 2002. The influence of the mismatch repair system on stationary-phase mutagenesis in the yeast Saccharomyces cerevisiae. Curr. Genet. 42:140-146. [DOI] [PubMed] [Google Scholar]
- 17.Haldenwang, W. G. 1995. The sigma factors of B. subtilis. Microbiol. Rev. 52:1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reference deleted.
- 19.Reference deleted.
- 20.Kaltwasser, M., T. Wiegert, and W. Schumann. 2002. Construction and application of epitope and green fluorescent protein-tagging integration vectors for Bacillus subtilis. Appl. Environ. Microbiol. 68:2624-2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kasak, L., R. Horak, and M. Kivisaar. 1997. Promoter-creating mutations in Pseudomonas putida: a model system for the study of mutation in starving bacteria. Proc. Natl. Acad. Sci. U. S. A. 94:3134-3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim, M., T. Huang, and J. H. Miller. 2003. Competition between MutY and mismatch repair at AC mispairs in vivo. J. Bacteriol. 185:4626-4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lea, D. E., and C. A. Coulson. 1949. The distribution of the numbers of mutants in bacterial populations. J. Genet. 49:264-285. [DOI] [PubMed] [Google Scholar]
- 24.Love, P. E., and R. E. Yasbin. 1984. Genetic characterization of the inducible SOS-like system of B. subtilis. J. Bacteriol. 160:910-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lovett, C. M., P. E. Love, R. E. Yasbin, and J. W. Robertson. 1988. SOS-like induction in Bacillus subtilis: induction of the RecA protein analog and a damage-inducible operon by DNA damage in Rec+ and DNA repair-deficient strains. J. Bacteriol. 170:1467-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu, A.-L., X. Li, Y. Gu, P. M. Wright, and D.-Y. Chang. 2001. Repair of oxidative DNA damage. Cell Biochem. Biophys. 35:141-170. [DOI] [PubMed] [Google Scholar]
- 27.Maki, H., and M. Sekiguchi. 1992. MutT protein specifically hydrolyses a potent mutagenic substrate for DNA synthesis. Nature 355:273-275. [DOI] [PubMed] [Google Scholar]
- 28.Michaels, M., and J. H. Miller. 1992. The GO system protects organisms from the mutagenic effect of the spontaneous lesion 8-hydroxyguanine (7,8-dihydro-8-oxoguanine). J. Bacteriol. 174:6321-6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michaels, M. L., C. Cruz, A. P. Grollman, and J. H. Miller. 1992. Evidence that MutM and MutY combine to prevent mutations by an oxidatively damaged form of guanine in DNA. Proc. Natl. Acad. Sci. U. S. A. 89:7022-7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michaels, M. L., J. Tchou, A. P. Grollman, and J. H. Miller. 1992. A repair system for 8-oxo-7,8-dihydrodeoxyguanine. Biochemistry 31:10964-10968. [DOI] [PubMed] [Google Scholar]
- 31.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 32.Reference deleted.
- 33.Nghiem, Y., M. Cabrera, C. G. Cupples, and J. H. Miller. 1988. The mutY gene: a mutator locus in Escherichia coli that generates G:C to T:A transversions. Proc. Natl. Acad. Sci. U. S. A. 85:2709-2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicholson, W. L., and H. Maughan. 2002. The spectrum of spontaneous rifampin resistance mutations in the rpoB gene of Bacillus subtilis 168 spores differs from that of vegetative cells and resembles that of Mycobacterium tuberculosis. J. Bacteriol. 184:4936-4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nicholson, W. L., and P. Setlow. 1990. Sporulation, germination and outgrowth, p. 391-450. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley and Sons, Sussex, United Kingdom.
- 36.Pedraza-Reyes, M., and R. E. Yasbin. 2004. Contribution of the mismatch DNA repair system to the generation of stationary-phase-induced mutants of Bacillus subtilis. J. Bacteriol. 186:6485-6491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pybus, C., M. Pedraza-Reyes, C. A. Ross, H. Martin, K. Ona, R. E. Yasbin, and E. Robleto. 2010. Transcription-associated mutation in Bacillus subtilis cells under stress. J. Bacteriol. 192:3321-3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramírez, M. I., F. X. Castellanos-Juárez, R. E. Yasbin, and M. Pedraza-Reyes. 2004. The ytkD (mutTA) gene of Bacillus subtilis encodes a functional antimutator 8-oxo-(dGTP/GTP)ase and is under dual control of sigma A and sigma F RNA polymerases. J. Bacteriol. 186:1050-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robleto, E. A., C. Ross, R. E. Yasbin, and M. Pedraza-Reyes. 2007. Stationary phase mutagenesis in Bacillus subtilis: a paradigm to study genetic diversity programs in cells under stress. Crit. Rev. Biochem. Mol. Biol. 42:327-339. [DOI] [PubMed] [Google Scholar]
- 40.Rosche, W. A., and P. L. Foster. 2000. Determining mutation rates in bacterial populations. Methods 20:4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosche, W. A., and P. L. Foster. 1999. The role of transient hypermutators in adaptive mutation in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 96:6862-6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenberg, S. M., C. Thulin, and R. S. Harris. 1998. Transient and heritable mutators in adaptive evolution in the lab and in nature. Genetics 148:1559-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ross, C., C. Pybus, M. Pedraza-Reyes, H.-M. Sung, R. E. Yasbin, and E. Robleto. 2006. Novel role of mfd: effects on stationary-phase mutagenesis in Bacillus subtilis. J. Bacteriol. 188:7512-7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 45.Sasaki, M., Y. Yonemura, and Y. Kurusu. 2000. Genetic analysis of Bacillus subtilis mutator genes. J. Gen. Appl. Microbiol. 46:183-187. [DOI] [PubMed] [Google Scholar]
- 46.Saumaa, S., A. Tover, M. Tark, R. Tegova, and M. Kivisaar. 2007. Oxidative DNA damage defense system in avoidance of stationary-phase mutagenesis in Pseudomonas putida. J. Bacteriol. 189:5504-5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sonenshein, A. L., J. A. Hoch, and R. Losick (ed.). 1993. Bacillus subtilis and other gram-positive bacteria: physiology, biochemistry, and molecular genetics. American Society for Microbiology, Washington, DC.
- 48.Sung, H.-M., and R. E. Yasbin. 2000. Transient growth requirement in Bacillus subtilis following the cessation of exponential growth. Appl. Environ. Microbiol. 66:1220-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sung, H.-M., and R. E. Yasbin. 2002. Adaptive, or stationary-phase, mutagenesis, a component of bacterial differentiation in Bacillus subtilis. J. Bacteriol. 184:5641-5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sung, H.-M., G. Yeamans, C. A. Ross, and R. E. Yasbin. 2003. Roles of YqjH and YqjW, homologs of the Escherichia coli UmuC/DinB or Y superfamily of DNA polymerases, in stationary-phase mutagenesis and UV-induced mutagenesis of Bacillus subtilis. J. Bacteriol. 185:2153-2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taddei, F., H. Hayakawa, M. F. Bouton, A. M. Cirinesi, L. Matic, M. Sekiguchi, and M. Radman. 1997. Counteraction by MutT protein of transcriptional errors caused by oxidative damage. Science 278:128-130. [DOI] [PubMed] [Google Scholar]
- 52.Tajiri, T., H. Maki, and M. Sekiguchi. 1995. Functional cooperation of MutT, MutM and MutY proteins in preventing mutations caused by spontaneous oxidation of guanine nucleotide in Escherichia coli. Mutat. Res. 336:257-267. [DOI] [PubMed] [Google Scholar]
- 53.Tompkins, J. D., J. L. Nelson, J. C. Hazel, S. L. Leugers, J. D. Stumpf, and P. L. Foster. 2003. Error-prone polymerase, DNA polymerase IV, is responsible for transient hypermutation during adaptive mutation in Escherichia coli. J. Bacteriol. 185:3469-3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vagner, V., E. Dervyn, and S. D. Ehrlich. 1998. A vector for systematic gene inactivation in Bacillus subtilis. Microbiology (United Kingdom) 144:3097-3104. [DOI] [PubMed] [Google Scholar]
- 55.Vidales, L. E., L. C. Cardenas, E. Robleto, R. E. Yasbin, and M. Pedraza-Reyes. 2009. Defects in the error prevention oxidized guanine system potentiate stationary-phase mutagenesis in Bacillus subtilis. J. Bacteriol. 191:506-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Völker, U., B. Maul, and M. Hecker. 1999. Expression of the σB-dependent general stress regulon confers multiple stress resistance in Bacillus subtilis. J. Bacteriol. 181:3942-3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Völker, U., S. Engelmann, B. Maul, S. Riethdorf, A. Völker, R. Schmid, H. Mach, and M. Hecker. 1994. Characterization and analysis of the induction of general stress proteins of Bacillus subtilis. Microbiology 140:741-752. [DOI] [PubMed] [Google Scholar]
- 58.Wyrzykowski, J., and M. R. Volkert. 2003. The Escherichia coli methyl-directed mismatch repair system repairs base pairs containing oxidative lesions. J. Bacteriol. 185:1701-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamamoto, H., M. Mori, and J. Sekiguchi. 1999. Transcriptions of genes near the sspE locus of the Bacillus subtilis genome. Microbiology 145:2171-2180. [DOI] [PubMed] [Google Scholar]
- 60.Yasbin, R. E., P. I. Fields, and B. J. Andersen. 1980. Properties of Bacillus subtilis 168 derivatives freed of their natural prophages. Gene 12:155-159. [DOI] [PubMed] [Google Scholar]
- 61.Yasbin, R. E., R. Miehl-Lester, and P. E. Love. 1987. Mutagenesis in Bacillus subtilis, p. 73-84. In M. Alacevic, D. Hranueli, and Z. Tomen (ed.), Genetics of industrial microorganisms. GIM-86, Split, Yugoslavia.
- 62.Yasbin, R. E., and M. Pedraza-Reyes. 2004. Stationary phase-induced mutagenesis: is directed mutagenesis alive and well within neo-Darwinian theory, p. 181-191. In R. Miller (ed.), Microbial evolution: gene establishment, survival, and exchange. ASM Press, Washington, DC.