Abstract
The ubiquitin-proteasome pathway (UPP) is the primary cytosolic proteolytic machinery for the selective degradation of various forms of damaged proteins. Thus, the UPP is an important protein quality control mechanism. In the canonical UPP, both ubiquitin and the 26S proteasome are involved. Substrate proteins of the canonical UPP are first tagged by multiple ubiquitin molecules and then degraded by the 26S proteasome. However, in non-canonical UPP, proteins can be degraded by the 26S or the 20S proteasome without being ubiquitinated. It is clear that a proteasome is responsible for selective degradation of oxidized proteins, but the extent to which ubiquitination is involved in this process remains a subject of debate. While many publications suggest that the 20S proteasome degrades oxidized proteins independent of ubiquitin, there is also solid evidence indicating that ubiquitin and ubiquitination are involved in degradation of some forms of oxidized proteins. A fully functional UPP is required for cells to cope with oxidative stress and the activity of the UPP is also modulated by cellular redox status. Mild or transient oxidative stress up-regulates the ubiquitination system and proteasome activity in cells and tissues and transiently enhances intracellular proteolysis. Severe or sustained oxidative stress impairs the function of the UPP and decreases intracellular proteolysis. Both the ubiquitin conjugation enzymes and the proteasome can be inactivated by sustained oxidative stress, especially the 26S proteasome. Differential susceptibilities of the ubiquitin conjugation enzymes and the 26S proteasome to oxidative damage lead to an accumulation of ubiquitin conjugates in cells in response to mild oxidative stress. Thus, increased levels of ubiquitin conjugates in cells appear to be an indicator of mild oxidative stress.
INTRODUCTION
There are two major intracellular proteolytic pathways in the cells: the lysosomal pathway and the ubiquitin-proteasome pathway (UPP) [1–3]. Whereas the lysosomal pathway plays an important role in degradation of long-lived bulk proteins, particularly membrane-bound proteins, the UPP is the primary cytosolic protein degradation pathway [4–6]. In this article we will review roles for the UPP in response to oxidative stress and the effects of oxidative stress on function of the UPP. In its simplest form, the UPP involves two discrete steps: (1) covalent attachment of multiple ubiquitin molecules to the protein substrate, and (2) degradation of the ubiquitin-tagged protein by the 26S proteasome with the release of free and reusable ubiquitin. In some cases, ubiquitin is degraded together with the tagged substrates by the proteasome [7].
Ubiquitin is a highly conserved 76–amino acid polypeptide and its most widely understood function is to tag intracellular proteins for proteasomal degradation. Covalent attachment of ubiquitin to the protein substrate proceeds via a three-step cascade mechanism. Initially, the ubiquitin-activating enzyme, E1, activates the C-terminal glycine residue of ubiquitin via formation of a high-energy thiol ester with an internal E1 cysteine residue. One of dozens of ubiquitin-conjugating enzymes, E2s, transfers the activated ubiquitin, also via an E2~ubiquitin thiol ester intermediate, to the substrate that is specifically bound to a member of the ubiquitin-protein ligase family, E3s. In some cases, an E3~ubiquitin high-energy thiol ester intermediate is formed before the ubiquitin is transferred to the E3 bound substrate. The E3 catalyzes the formation of a peptide/isopeptide bond between a carboxyl group at the C-terminus of ubiquitin and an amine group of the substrate. There are two genes in the human genome that encode different isoforms of E1 and each form has a distinct preference for E2s [8–11]. There are at least 37 genes in the human genome that encode distinctive E2s [12]. The number of the genes encoding E3s is over one thousand [13, 14]. The diversity of E2s and E3s and the combinatorial possibilities of various E2 and E3 in various cellular contexts provide the molecular basis for the stringent substrate specificity of the UPP.
In most cases, multiple ubiquitins are conjugated to the initial ubiquitin moiety to form polyubiquitin chains. A chain of 4 or more ubiquitin moieties is often required for substrate recognition by the 26S proteasome complex [15–17]. For most substrates, the first ubiquitin is often linked to the ε-amino group of an internal lysine residue of the target protein. However, for some protein substrates, such as MyoD and p16INK4a, the first ubiquitin is fused to the free and exposed N-terminal residue of the substrate to generate a linear peptide bond [18]. In successive reactions, a polyubiquitin chain is synthesized by the progressive transfer of additional activated ubiquitin moieties to an internal lysine residue or the N-terminus of the previously conjugated ubiquitin molecule.
Ubiquitin has 7 internal lysine residues. Together with the amine group at the N-terminus there are 8 possible positions for the second ubiquitin to attach. For the third ubiquitin, there are 15 possible positions of attachment. Thus, the structures of polyubiquitin chains in cells can be highly diverse [19–22]. The synthesis of particular polyubiquitin chain linkages appears to be catalyzed by specific E2–E3 combinations [23–29], and different topologies and lengths of the ubiquitin chains have different functional outcomes [30]. For example, ubiquitin chains conjugated through the lysine at position 48 of ubiquitin (Lys 48) lead to proteasomal degradation of the modified substrate, whereas chains linked at Lys 63 are instead implicated in signalling or trafficking events [31–33].
The 26S proteasome is a 2.4 MDa complex composed of two multisubunit complexes: a catalytic core, also called the 20S proteasome, and a regulatory complex, also called the 19S regulatory particle or PA700. The 20S proteasome is a 700 kDa complex composed of two copies of 14 different gene products arranged in four axially stacked heptameric rings (α1–7, β1–7, β1–7, α1–7). The α-subunits form the gates of cylindrical structure of the 20S proteasome and the β-subunits carry the catalytic sites which line the central lumen of the proteolytic chamber. Substrates reach this proteolytic chamber via 13 Å pores formed by the α-subunit rings at either end of the cylinder [34]. However, these pores are normally obstructed by the amino termini of α-subunits. The topological arrangement of the 20S proteasome precludes access of native proteins to the catalytic sites and partially contributes to the selectivity of the proteasome [34].
The 19S regulatory particle (PA700) is a multi-subunit complex that binds to either or both ends of the 20S cylinder, thereby positioning the PA700 as a gatekeeper for substrate entry to the 20S proteasome. PA700 includes six distinct AAA-family ATPases (Rpt1–Rpt6) arranged in a hexameric ring that extends axially from the outer α rings of the 20S proteasome [35]. The ATP-dependent interaction between PA700 and the catalytic core promotes opening of the pores of the 20S proteasome and provides an access portal for substrates to the catalytic core. The ATPase subunits of PA700 also contribute to the unfolding of the protein substrates and delivery of the unfolded protein substrates into the proteolytic chamber through the narrow pores of the 20S proteasome [36–39]. Some of the non-ATPase subunits of PA700 display deubiquitinating activity (also called isopeptidase activity), while others serve as ubiquitin interaction subunits to recruit polyubiquitinated substrates to the proteasome [40]. The overall process of 26S proteasome-catalyzed proteolysis depends on ATP hydrolysis. The exact energy-consuming steps in proteolysis remain unclear but are likely linked to substrate unfolding, translocation, and deubiquitination [41].
There are many additional proteins that reversibly associate with the proteasome and play a role in its regulatory functions. These proteasome-associated proteins include ubiquitin ligases, deubiquitinating enzymes (enzymes which release ubiquitin moieties from ubiquitin conjugates) and polyubiquitin chain-binding proteins [42–45].
The modular and dynamic composition of the proteasome and its multiple regulators allows the formation of different isoforms of the proteasome to fulfill a wide array of physiological functions. In cells, the total number of proteasomes as well as the subunit composition of proteasomes can be altered in response to physiological demands [46, 47]. The 20S proteasome exists in two distinct forms that differ in their catalytic subunits. Mammals contain two genes for each of the three catalytic subunits. Two of these genes (β1i and β5i) are encoded in the major histocompatibility locus. Under certain physiological states such as enhanced immune function, β1i and β5i, together with a third gene (β2i), are conditionally expressed and selectively incorporated into newly synthesized proteasome instead of their constitutive counterparts (β1, β5 and β2, respectively) [34]. This specific class of proteasome which contains inducible catalytic subunits is called immuno-proteasome and participates in antigen presentation by enhancing production of certain class I peptides [48]. Animals lacking genes for the inducible catalytic subunits cannot produce the antigenic peptides, whereas over-expression of these inducible proteasome subunits enhances antigen presentation.
In addition to the 19S regulatory complex (PA700), there are several other regulatory proteins or protein complexes, such as PA200 and PA28 that bind directly to the outer α-rings of 20S proteasomes [49]. Unlike the PA700, these alternative regulators are not ATPases and do not bind polyubiquitin chains, suggesting that they may direct the proteasome in ubiquitin-independent proteolytic functions. Furthermore, two proline-rich proteins, PI31 and Pr39, also bind and inhibit proteasome function via directly blocking 20S proteasome activity or attenuating binding of proteasome activators [50, 51]. The dynamic states and diversity of the regulatory complex of the proteasome suggest that the proteasome may function in both ubiquitin-dependent and ubiquitin-independent manners.
Consistent with multiple configurations of the proteasome in cells, proteasome-mediated degradation also occurs in different manners. In the canonical UPP, proteins are degraded by the 26S proteasome in an ATP-dependent and ubiquitin-dependent manner. However, the 20S proteasome also exists in free form and some proteins, including oxidized proteins, are degraded by the 20S proteasome in an ATP-independent and ubiquitin-independent manner [52–58]. In rare instances proteins are degraded by the 26S proteasome in an ATP-dependent but ubiquitination-independent process [39, 59–62]. The ubiquitin-independent proteasomal degradation is considered as a non-canonical UPP function. The substrate specificity of different forms of the proteasome has been cleverly exploited to determine relative susceptibility of the different components of the UPP to oxidative stress [63].
The UPP in protein quality control
Protein quality control is a post-translational process which involves folding of newly synthesized proteins and either refolding or degradation of proteins that fail to attain or maintain a native structure. Protein quality control is crucial because it prevents the accumulation of potentially toxic unfolded or misfolded proteins. A growing body of evidence indicates that accumulation of damaged or abnormal proteins is associated with various age-related diseases such as Parkinson's disease, Huntington's disease, Alzheimer's disease, cataract and age-related macular degeneration [6, 64–71]
The accumulation of damaged or abnormal proteins may arise from their enhanced generation or deficiency in repair or removal. The generation of abnormal proteins may be due to genetic mutation, failure to properly fold, or denaturation induced by various environmental stresses such as heat, oxidation, heavy metals, and ultraviolet light exposure. Unfolded or partially unfolded proteins are structurally unstable, and they tend to aggregate and precipitate. Accumulation of aggregation-prone proteins interferes with normal cellular functions [72, 73]. To avoid disruption of cellular function by unfolded proteins, organisms have evolved multiple levels of protein quality control systems which recognize proteins with abnormal structures. These protein quality control mechanisms either refold them to normal conformation or target them for degradation. Molecular chaperones and proteases are central players in the protein quality control process. Whereas molecular chaperones play central roles in the refolding of misfolded or unfolded proteins, intracellular proteolysis is responsible for the removal of unrepaired proteins.
The majority of intracellular proteins are either degraded by the UPP or by the lysosomal pathway. These two proteolytic pathways have different substrate preferences [74]. In general, short lived regulatory proteins, including damaged proteins or otherwise partially unfolded proteins are degraded by the UPP. Proteins degraded by the UPP normally contain intrinsic degradation signals (also called degrons). These signals are highly diverse, ranging from unique peptide sequences to specific domains or single amino acid residues at the N-terminus of the proteins [75]. Certain modifications of internal residues such as methionine oxidation and protein carbonyl formation also increase the susceptibility of certain proteins to UPP-mediated degradation [76–79]. The majority of membrane-bound or organelle-associated proteins are degraded by the lysosomal pathway, either through endocytosis or autophagy mechanisms. Recent studies indicate that the UPP and lysosomal degradation pathways work closely in a coordinated manner [74, 80]. For example, oligo/monoubiquitination of receptor and other membrane proteins is required for efficient endocytosis [81, 82].
The selective degradation of abnormal proteins has been known for about 30 years [83, 84], and the role of the UPP in this process has been proposed since the discovery of this pathway [6, 76, 77, 85–90]. For example, various forms of abnormal proteins, such as mutant [91–95], truncated [96], deamidated [97], denatured [98, 99] and oxidized proteins are degraded in a proteasome-dependent manner [52, 76–79, 100–104]. However, the mechanism by which the UPP distinguishes abnormal from native proteins remains an unsolved mystery. Although the 19S regulatory complex of the 26S proteasome interacts directly with denatured proteins [36–38, 105], most proteins degraded by the proteasome are first tagged by a polyubiquitin chain. It is believed that the ubiquitin conjugating enzymes (E2s) and ubiquitin ligases (E3s) are jointly responsible for substrate recognition and ubiquitination [4, 106]. Thus, these enzymes are essential in selecting proteins for degradation. Mutations in specific E2 proteins lead to defects in degradation of proteins with different classes of artificial hydrophobic degradation signals, suggesting that the UPP recognizes exposed hydrophobic regions in some proteins [107].
Recent studies indicate that molecular chaperones, in particular Hsp90/Hsp70, are utilized by the UPP to recognize abnormal proteins [98, 99, 108]. As indicated in Figure 1, various modifications may alter protein conformation, resulting in the exposure of hydrophobic regions which are normally buried in properly folded proteins. The exposure of hydrophobic regions may provide a signal for the discrimination of denatured or unfolded proteins from native proteins by Hsp90 or other chaperones [109]. For example, the C-terminus of Hsc70-interacting protein (CHIP), a co-chaperone with ubiquitin ligase activity, can target chaperone-bound substrates for degradation. CHIP contains a C-terminal U-box domain and three N-terminal tetratricopeptide repeat (TPR) domains. The C-terminal U-box domain is responsible for the ubiquitin ligase activity and the N-terminal TPR domains are responsible for the interaction with Hsp70 and Hsp90. By interacting with molecular chaperones (Hsp90 or Hsp70) and ubiquitin conjugating enzymes (Ubc4/5), CHIP catalyzes the ubiquitination of chaperone-bound proteins. Thus, CHIP functions as a bridge or molecular switch between the refolding and degradation arms of the protein quality control system. In addition to CHIP, other chaperone-interacting ubiquitin ligases, such as parkin, also participate in ubiquitinating misfolded or unfolded proteins [110].
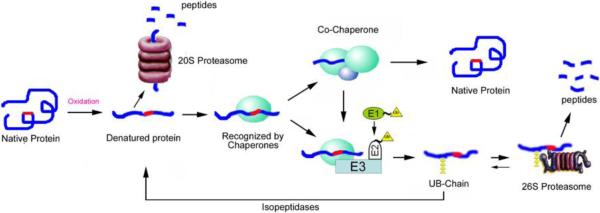
Fig.1. Recognition and degradation of oxidatively modified proteins by the UPP.
This model predicts that most, if not all, proteins have intrinsic signals for interaction with molecular chaperones or the ubiquitination system. These signals (red) are hidden in properly folded native proteins and they are not recognized by the protein quality control systems. Upon environmental stress, such as oxidation, proteins could be unfolded with exposure of the recognition signals, such as hydrophobic patches. Some of the oxidized (unfolded) proteins can be recognized and degraded by the 20S proteasome directly whereas others are recognized by molecular chaperones. With the help of other chaperones or co-factors, molecular chaperones are capable of refolding the denatured proteins in an ATP-dependent manner. If the denatured proteins cannot be refolded rapidly, the chaperone bound substrates are ubiquitinated (yellow triangles) by chaperone-interacting ubiquitin-ligases, such as CHIP. The ubiquitinated substrates are recognized and degraded by the 26S proteasome. If the ubiquitinated proteins were deubiquitinated by isopeptidases, the denatured proteins would have a second chance to be refolded by molecular chaperones. The parallel/competitive functional relationship between the UPP and molecular chaperones assures the efficiency of the protein quality control systems to get rid of abnormal proteins.
A common goal of the UPP and molecular chaperones is to prevent the accumulation of abnormal proteins. Both pathways do so by recognizing proteins with abnormal structures. However, to date it is not possible to predict why some denatured proteins are refolded by chaperones whereas others are degraded by the UPP. It appears that cellular protein quality control systems have a triage mechanism. The first level of triage must be identification of the proteins that are damaged and require repair or removal. The quality control system must be able to distinguish between native (properly folded, and assembled) proteins and everything else that might be considered non-native or abnormal, including partially unfolded, misfolded, incorrectly modified, unassembled subunits of complexes, or proteins in incorrect cellular compartments. Once damaged proteins have been identified, a second decision must be made: Is the protein repairable? In principle, chaperones should have the first opportunity to fix damaged proteins. The proteins that are damaged beyond repair could then be degraded by the UPP.
How is the decision made to refold or to degrade a protein? The ability of both the UPP and chaperones to interact with the same damaged or misfolded protein allows these pathways to operate in a parallel or competitive manner to either refold or degrade a given target protein (Fig. 1). We and others propose that the fate of the damaged protein depends on the kinetics and affinity of interaction between the damaged proteins and molecular chaperones or the chaperone component of the UPP as well as upon the availability of the proteolytic machinery [99, 111, 112]. If the chaperone-bound nonnative protein is efficiently refolded with the assistance of other cochaperones, such Hsp40 and p23, it is removed from the triage system and returned to cellular function. However, if the chaperone-bound nonnative protein is not efficiently refolded by chaperones, the co-chaperone CHIP, which interacts with both chaperones and Ubc4/5 or other E2s, brings the chaperone-bound proteins to the ubiquitination machinery. If the ubiquitinated proteins are deubiquitinated by isopeptidases, including those associated with the 26S proteasome, the denatured proteins would have a second chance to be refolded by chaperones. Thus, the relative activities of ubiquitination and deubiquitination also participate in determining the fate of denatured proteins.
The parallel or competitive mode of action between the UPP and chaperones in the protein quality control process appears to be energy inefficient, resulting in the undesirable destruction of some repairable proteins. This may explain why ~30% of nascent proteins are degraded before they are folded properly, sometimes with dire consequences [113]. For example, premature degradation of inefficiently folded mutant cystic fibrosis transmembrane conductance regulator (CFTR) (ΔF508) causes cystic fibrosis [91]. CHIP is one of the ubiquitin ligases that target the mutant CFTR for ubiquitination and degradation [114, 115]. Blockage of the ubiquitination and degradation of CFTR by inactivation of CHIP allows a pool of CFTRΔF508 to be correctly folded and partially restores cellular function [116]. Similarly, blocking the premature degradation of mutant glucocerebrosidase (N370S or L444P) by proteasome inhibitors enhances its correct folding and partially corrects the loss of function phenotypes of mutant glucocerebrosidase [68]. From a teleological perspective, the cost of destruction of partially functional and functional proteins can be justified by efficient elimination of gain of function toxic mutants or damaged proteins. Maintenance of the proteome is also achieved by the parallel or competitive modes of action between the UPP and chaperones in the protein quality control process. If one system fails, the other system would serve as the backup. For instance, it has been shown that inhibition of the UPP results in up-regulation of heat shock proteins [117–120]. Conversely, inhibition of chaperone activity also promotes the degradation of Hsp90 client proteins by the proteasome [121].
The role of UPP in degradation of oxidatively damaged proteins and responses to oxidative stress
Oxidative stress, damage caused by excessive reactive oxygen species (ROS) or their derivatives, is one of the common insults encountered by cells and organisms. The overall effects of the oxidants depend on local concentrations, subcellular localization and the proximity of the oxidant to target molecules. Excessive oxidants can damage almost all cellular components, including carbohydrate, protein, DNA, lipids and cause dysfunction of cells, tissues and organisms. To cope with oxidative stress, organisms have developed multiple defence mechanisms to alleviate oxidative damage. These defence mechanisms include antioxidants and antioxidant enzymes, molecular chaperones, and proteolytic systems. Whereas antioxidants and antioxidant enzymes quench or metabolize ROS, molecular chaperones help to repair oxidatively damaged proteins. Proteolytic systems selectively degrade the oxidatively damaged proteins. It has been shown in various types of cells or tissues that oxidatively modified proteins are selectively degraded [52, 53, 57, 58, 78, 90, 100, 101, 104, 122–130].
An increasing body of literature indicates that the proteasome is the major cytoplasmic proteolytic machinery for preferential degradation of damaged versus normal proteins. It has been demonstrated that various forms of oxidized proteins are degraded at faster rates than their native counterparts and inhibition of the proteasome stabilizes oxidized proteins in intact cells or in cell-free systems [77–79, 89, 90, 101, 102, 127, 131–135]. While there is consensus that some form of the proteasome plays a key role in selective degradation of oxidized proteins [57, 58, 78, 79, 101, 102, 128–135], the extent to which of ubiquitination is involved in the degradation of oxidized proteins remains controversial. There are hundreds of papers implicating some form of the proteasome in the degradation of oxidized proteins and the vast majority of those publications suggest that oxidized proteins are degraded by the 20S proteasome independent of ubiquitin [52, 55, 56, 100–104, 124, 125, 129, 136, 137]. However, there is also solid evidence that supports the involvement of ubiquitination in degradation of some oxidized proteins [76–79, 127, 138, 139].
Both substrate ubiquitination and degradation of ubiquinated substrates by the 26S proteasome require ATP. Assembly of the 26S proteasome is also an ATP-dependent process. In contrast, the 20S proteasome degrades proteins in an ATP-independent manner. Therefore, early studies used ATP-dependence to distinguish ubiquitin-dependent degradation from ubiquitin-independent degradation [52, 103, 127, 140, 141].
A number of studies demonstrated that ATP has no stimulating effect on the degradation of oxidized proteins in cell lysates [52, 56, 103, 140–142]. In some cases, addition of ATP to cell lysate reduced the degradation of oxidized proteins in cell-free lysates. Accordingly, Davies and colleagues concluded that oxidized proteins are degraded by the 20S proteasome independent of the 19S regulator or ubiquitin [52, 55, 56, 100, 101, 103, 143]. In support of that conclusion, Davies and colleagues demonstrated that disruption of the ubiquitination system did not impair the degradation of oxidized proteins [56].
The evidence that support the involvement of ubiquitin in degradation of oxidized proteins includes the preferential ubiquitination of certain oxidized proteins [76–79, 138] and accumulation of ubiquitinated species of oxidized proteins upon proteasome inhibition [77, 78]. The stabilization of ubiquitinated species of oxidized proteins by proteasome inhibitors indicates that these ubiquitin conjugates are en route to proteasomal degradation. The correlation between transient increases in levels of ubiquitinated proteins and increased intracellular proteolysis during recovery from mild oxidative stress [89, 90] also implies that ubiquitin is involved in restoring protein homeostasis during recovery from oxidative stress. We also demonstrated that addition of Ubc4/5 to cell lysates promotes the degradation of a specific form of oxidized (glutathiolated) proteins and that addition of a dominant negative ubiquitin to cell lysates also inhibits the degradation of glutathiolated proteins [128]. A recent study provided direct evidence for the involvement of ubiquitination in proteasomal degradation of oxidized proteins [139]. In that study, the authors demonstrated that USP14, a proteasome-associated deubiquitinating enzyme, is a negative regulator of UPP by its trimming of the ubiquitin chain of substrates. They further demonstrated that inhibition of USP14 not only promoted the degradation of classic substrates of the UPP, but also promoted the clearance oxidized proteins and enhanced the resistance to oxidative stress [139], indicating that ubiquitination is involved in targeting oxidized proteins for degradation.
Collectively, the available data indicate that the proteasome plays an important role in selectively degrading oxidatively modified proteins, either in a ubiquitin-independent manner or ubiquitin-dependent manner. However, how the proteasome distinguishes oxidized proteins from native proteins remain to be elucidated.
The selective oxidation of several amino acids and their resulting products have been evaluated as possible determinants for recognition by the proteasome or by the ubiquitination machinery. Levine and colleagues demonstrated an increase in the proteolysis of glutamine synthetase after oxidizing a threshold level of methionine residues [144] and Lasch et al. found a correlation between tyrosine oxidation and proteasomal degradation of RNase A [145]. However, it should be kept in mind that oxidation of specific residues is often associated with changes in secondary, tertiary, and even quaternary structures of the substrate proteins. Therefore, it is plausible that oxidatively modified proteins are recognized in a manner that is, ultimately, similar to that of other forms of damaged or abnormal proteins [146].
Several studies indicate that oxidized proteins are partially unfolded due to the loss of regular secondary and tertiary structures within the domain of the oxidative impact [103, 128., 136, 145]. A consequence of the oxidation-induced unfolding is the exposure of hydrophobic patches from the interior of the protein globule to the surface. The exposure of hydrophobic patches may be a molecular basis for recognition of oxidized proteins by chaperones or by the proteasome. Thus, molecular chaperones may compete with the proteasome for oxidatively modified proteins [99], and ATP-dependent refolding of oxidatively modified proteins by molecular chaperones may explain why addition of ATP to cell lysate reduces the degradation of some oxidatively modified proteins [55, 100, 142].
UPP activity is governed by cellular redox status
Similar to other protective mechanisms, the capacity of the UPP to degrade proteins in cells or tissues is altered in response to environmental insults, including oxidative stress. It has been demonstrated in several cell types that exposure to various forms of oxidative stress results in a transient increase in the intracellular degradation of both short-lived and long-lived cellular proteins. An increase in substrate availability is one the reasons for the increased intracellular protein degradation in response to oxidative stress. Enhancement of ubiquitination and degradation capacities in response to oxidative stress is another reason for the increased intracellular protein degradation [89, 90, 100, 124, 143, 147, 148]. We demonstrated that both E1 and E2 activities of the ubiquitin conjugation system increased in response to mild oxidative stress [77, 89, 149] and Pickering et al demonstrated that the levels and activity of the proteasome, including immunoproteasome, increased upon adaption to mild oxidative stress [143].
However, all components of the UPP (E1, E2s, some E3s, proteasome and deubiquitinating enzymes) are also impaired by extensive oxidative insults [149–157]. The susceptibility of the UPP to oxidative stress may have been anticipated, since E1, E2s, some E3s and deubiquitinating enzymes have a cysteine residue in their active sites. Exposure to oxidative stress rapidly depletes reduced glutathione (GSH) and elevates the levels of oxidized glutathione (GSSG). GSSG reacts with the cysteine residues in the active site of E1 and E2, forming mixed disulfide bonds, and blocking their binding to ubiquitin [150]. In addition, other types of modifications, such as S-nitrosylation, can inactivate these enzymes [152]. When oxidants are removed from the medium and cells are allowed to recover, the GSH level is restored, and the levels of ubiquitin conjugates, together with the ability to form the conjugates are also restored [90, 150, 151]. After several hours of recovery from oxidation, there is hyperactivation of E1 and increased ubiquitination and proteolysis relative to levels found in untreated cells [89, 90, 158]. Thus, intracellular proteolysis can show a biphasic response to oxidative stress. As indicated in Figure 2, mild to moderate oxidative stress increases susceptibility of proteins to degradation and enhances the proteolytic capacity, therefore, promoting intracellular protein degradation. In contrast, extensive but non lethal oxidative stress impairs the functions of the proteolytic system, reducing intracellular protein degradation [89, 90, 100, 124, 150, 151, 159] and inducing intracellular accumulation and aggregation of damaged or abnormal proteins.
Figure 2. The response of UPP to oxidative stress.
The UPP is an important protein quality control mechanism for selective degradation of various damaged proteins, including oxidized proteins. However, the components of the UPP are also targets of oxidative insults. The differential susceptibility of different components of the UPP dictates the response of the UPP to different levels of oxidative stress. Mild oxidative stress increases substrate availability and up-regulates the ubiquitin conjugation systems, therefore, enhancing intracellular protein degradation. The timely degradation of oxidatively modified proteins prevents their accumulation and aggregation. Sustained oxidative stress inactivates the proteasome without inhibiting the ubiquitination system and results in the accumulation of ubiquitin conjugates in the cells. Age- and stress-related inactivation of the proteasome may be related to the accumulation of ubiquitin-containing inclusion bodies in cells of various age-related diseases. Extensive oxidative stress not only inactivates the proteasome, but also inhibits the ubiquitination system and results in a decrease in levels of newly formed ubiquitin conjugates and intracellular degradation. Oxidative inactivation of the UPP will accelerate the accumulation of oxidative damaged proteins and reduce cell viability.
The proteasome is also a target of oxidative stress. Reactive oxygen species and reactive lipid peroxidation products, such as 4-hydroxynonenal (HNE), impair the proteasome [104, 153–156, 160]. Based on ATP-stimulated and ATP-independent degradation of fluorogenic peptide suc-LLVY-MCA, Reinheckel et al. showed that the 26S proteasome was more susceptible than the 20S proteasome to oxidative inactivation in K562 cells [161]. While the ATP-independent degradation of the fluorogenic peptide suc-LLVY-MCA was not affected by H2O2 concentrations of up to 5 mM, the ATP-stimulated degradation of suc-LLVY-MCA by the 26S proteasome began to decline at 400 μM and was completely abolished at 1 mM oxidant treatment. Extensive oxidative insults may result in non-degradable cross-linked protein aggregates and these may impair the functions of the proteasome indirectly in intact cells [162, 163] or in cell-free systems [160, 164–167].
The relative susceptibility of the ubiquitination system and the proteasome to oxidative stress was recently compared in retinal pigment epithelial cells [149]. It was demonstrated that in the retinal cells the proteasome is more susceptible to oxidative inactivation than the ubiquitination enzymes [149]. Sustained exposure to as low as 50 μM H2O2 resulted in 30–50% inactivation of the proteasome, whereas these levels of oxidative stress had no detectable effect on ubiquitin conjugation enzymes and ubiquitination activity. Given the fact that the 26S proteasome is more susceptible to oxidative stress than the 20S proteasome [161], the loss of the peptidase activity of the proteasome upon oxidative stress is most like the due to inactivation of the 26S proteasome. Oxidative inactivation of the 26S proteasome prior to inactivation of the ubiquitination system may explain why levels of endogenous ubiquitin conjugates increase in response to oxidative stress in various types of cells [77, 78, 89, 90, 158, 168–173]. Age-related inactivation or inhibition of the proteasome [137, 174] may also contribute to the increased levels of ubiquitin conjugates in old tissues [168, 169, 175].
Interaction between the UPP and cellular antioxidant systems
Cellular antioxidant systems provide the primary defence mechanisms for cells to cope with oxidative stress via directly quenching reactive oxygen species. The UPP provides a secondary defence mechanism via degradation of abnormal proteins. Just as the UPP is affected by cellular redox status, the UPP also regulates cellular redox status. Regulation of the intracellular redox status by the UPP is mediated by degradation of nuclear factor-E2-related factor 2 (Nrf2), an important transcription factor involved in the transcriptional activation of antioxidant enzymes [176, 177]. Nrf2 binds to the antioxidant-response element (ARE) and regulates ARE-mediated expression of antioxidant enzymes, such as catalase, superoxide dismutase, NAD(P)H:quinone oxidoreductase, certain forms of glutathione-S-transferases, and γ-glutamate cysteine ligase regulatory subunit [178–180]. As with many other transcription factors, the abundance of Nrf2 is regulated by UPP-mediated degradation [177]. The Kelch-like ECH-associating protein (Keap1) is a cytosolic inhibitor of Nrf2 [181, 182]. The main function of Keap1 is to serve as an adapter for the cullin 3-dependent ubiquitin ligase [183, 184]. Keap1 binds to Cul3 via its N-terminal BTB/POZ domain and binds to Nrf2 via its C-terminal Kelch domain [185], leading to the ubiquitination and degradation of Nrf2 through the 26S proteasome [176, 182, 186, 187]. Under normal cellular conditions, Nrf2 is constantly ubiquitinated by the cytosolic Keap1/Cul3/Rbx1 complex and degraded by the proteasome. When a cell is exposed to oxidative stress, Nrf2 dissociates from the Keap1/Cul3 complex and translocates into the nucleus, leading to activation of ARE-mediated gene expression [188]. A recent study indicates that a specific ubiquitin conjugating enzyme, UbcM2, acts as a redox sensor and a regulator of Nrf2 activation [189]. When the function of the UPP was impaired or inhibited, Nrf2 accumulated in cells and activated the expression of many antioxidant enzymes [186, 190–192]. The stabilization of Nrf2 and subsequently up-regulation of antioxidant enzymes explains why partial inhibition of the UPP results in cytoprotection under certain conditions [186, 190–195]. Nrf2 also controls the expression of subunits of the proteasome and Nrf2 activation enhances the expression of proteasome subunits and increases proteasome activity [196]. The activation of Nrf2 may explain why pre-treatment of cultured neocortical neurons with low doses of proteasome inhibitors led to increased, rather than decreased, proteasome activity [197]. The interrelationship between the UPP and antioxidant system appears to very complicated and warrants further investigation.
Elevated levels of ubiquitin conjugates as an indicator of chronic mild oxidative stress
The different sensitivities of the 26S proteasome and ubiquitin conjugation machinery to oxidative stress may account for the accumulation of ubiquitin conjugates under conditions of mild oxidative stress, and such accumulation may be a useful indicator of cellular oxidative stress. The steady state levels of endogenous ubiquitin conjugates are the net balance between the rate of ubiquitin conjugation and the rate of degradation and/or deubiquitination. The rate of ubiquitination depends on availability of substrates, the activity of ubiquitin conjugating enzymes, such as E1, E2s and E3s and ATP levels. The rate of turnover of ubiquitin conjugates in cells or tissues is determined by the activity of the 26S proteasome and deubiquitinating enzymes. Whereas the 26S proteasome degrades the ubiquitinated substrates with the release of free ubiquitin, deubiquitinating enzymes remove ubiquitin from the substrates and spare the substrates from degradation. Although all of these enzymes could be inactivated by oxidative stress, the susceptibilities of these enzymes to oxidative stress differ. For example, mild oxidative stress up-regulates ubiquitin conjugating activity and promotes formation of ubiquitin conjugates [77, 89, 149]. Since oxidized proteins are preferred substrates for ubiquitination [77, 78], mild oxidative stress would likely increase the substrate availability for ubiquitination [89]. In comparison, the proteasome, particular the 26S proteasome, is more susceptible to oxidative stress than ubiquitin conjugation enzymes. Physiologically relevant oxidative stress could reduce proteasome activity in many cell types [149, 153–157]. As shown in Figure 3, levels of endogenous ubiquitin conjugates in the cells or tissues increase in response to mild oxidative stress or other cellular insults in vivo and in vitro [77, 78, 89, 90, 158, 168–173]. Thus, accumulation of ubiquitin conjugates in cells or tissues may indicate mild oxidative stress. Importantly, the increased levels of ubiquitin conjugates can be detected prior to changes in GSSG/GSH ratio or other oxidative markers, such as protein carbonyls [198], making the accumulation of ubiquitin conjugates among the most sensitive indicators of cellular oxidative stress. In contrast, extensive oxidative stress may reduce the levels of ubiquitin conjugates in cells due to inactivation of the ubiquitin conjugating enzymes [150, 151]. Since the formation of ubiquitin conjugates is ATP-dependent, depletion of ATP by severe oxidative stress also contributes to the decline in levels of ubiquitin conjugates [90]. Thus, whereas accumulation of ubiquitin conjugates in general indicates mild oxidative stress, a dramatic depletion of ubiquitin conjugates may also result from severe oxidative stress. When a decline in levels of ubiquitin conjugates is observed in cells or tissue, other markers of oxidative stress should be measured to ascertain that the decline in ubiquitin conjugates is related to severe oxidative stress.
Figure 3. Ubiquitination responses to various types of oxidative stress.
Panel A. Lymphoblastoid cells (L-40) from a normal human at a concentration of 0.5 ×106 cells/ml were treated with 80 ng/ml of neocarzinostatin (NCS) for 0 – 4h. Equal amounts of soluble protein (50 μg) from each exposure group were separated by SDS-PAGE and transferred to nitrocellulose. Endogenous ubiquitin conjugates were detected by Western blotting analysis of cellular extracts using an anti-ubiquitin antiserum. Panel B. 23 month old Emory mice were injected i.p.with 20 mg/kg paraquat dissolved in 0.9% saline, 24 h prior to sacrifice. Endogenous ubiquitin conjugates in the soluble fraction were detected in 50 μg protein from each exposure group as in panel A. Panel C. Rabbit lens epithelial (RLE) cells were treated with a single bolus of 500 μM H2O2 for 1h and then were allowed to recover in normal medium for the times indicated, in the absence (upper panel) or presence (lower panel) of proteasome inhibitor (lactacystin). The cells were lysed and endogenous ubiquitin conjugates were determined as in panel A. Panel D. RLE cells were treated with a constant level of H2O2 for 6h. De novo ubiquitin conjugation assays were performed using endogenous conjugating enzymes and substrates along with exogenous125I-labeled ubiquitin. Panel E. Lenses from 3 or 29 month old rats were treated with a single bolus of 500 μM H2O2 for 30 min. Proteins were extracted from the cortex of these lenses and levels of endogenous ubiquitin conjugates were determined as described in panel A. Panel F. Human retinal pigment epithelial (RPE) cells were treated with the indicated levels of H2O2 for 5 min with (REC) or without additional 10 min recovery in PBS. Levels of endogenous ubiquitin conjugates were detected as described in panel A. Panel G. Bovine retinas were treated with the indicated concentrations of H2O2 for 30 min. Levels of endogenous ubiquitin conjugates were detected as described in panel A. Panel H. Human RPE cells were preloaded with lipofuscin and then exposed to blue light for the time indicated. Levels of endogenous ubiquitin conjugates were detected. Panel I. Human RPE cells were treated with 250 μM diamide for the time indicated with or without recovery. De novo ubiquitin conjugates were formed using endogenous conjugating enzymes and endogenous substrates with exogenous125I-labeled ubiquitin.
Physiologic significance of the UPP-related response to oxidative stress
As mentioned above, a common response to mild oxidative stress is an increase in levels of ubiquitin conjugates [149, 153–157] with a concomitant transient increase in intracellular proteolysis [89, 90, 100, 124, 129, 148, 199–201]. This is consistent with UPP playing a role in the timely removal oxidized or other forms of damaged proteins caused by oxidative stress. Enhanced degradation of some regulatory proteins, such as I-κB, in response to mild oxidative stress may also play a role in coping with oxidative stress [159, 202, 203]. Failure to mount a UPP-related stress response may have severe consequences. For example, both ubiquitin and proteasome activity are required for cells to cope with various stresses, including heavy metals, amino acid analogs [204, 205], and oxidative stress [78, 147]. Impairment of the UPP either by chemical inhibition of the proteasome or by over expression of dominant negative ubiquitin sensitizes cells to oxidative stress [78, 147]. Age-related decline in proteasome activity is also associated with increased levels of oxidized proteins [129, 206–209] and cellular senescence [104, 196]. Similarly, inactivation of the proteasome by sustained oxidative stress can also impair cell signalling pathways and render cells more susceptible to stresses [159].
In contrast to the benefit of transient increase in ubiquitin conjugating activity in response to mild oxidative stress [89, 90, 158, 168, 169], sustained activation of the conjugating activity would result in accumulation of high mass ubiquitin conjugates, a common feature associated with aging and age-related disorders [168, 169, 175, 210, 211]. The ubiquitin pool in cells is highly dynamic since conjugation and de-conjugation are continuously ongoing processes. Although ubiquitin is a relatively abundant protein, the pool of free ubiquitin is surprisingly small [212]. Constant high levels of polyubiquitin conjugates would diminish the levels of free ubiquitin in cells [44, 212]. Indeed, it has been noted that accumulation of polyubiquitin conjugates in response to stress coincides with a reduction in the level of free ubiquitin and monoubiquitylated histone H2A [213, 214]. Monoubiquitination of histone H2A is an important epigenetic marker for chromatin condensation and transcriptional regulation [215]. Alterations in levels of monoubiquitinated H2A may have significant impact on gene expression, development and health [216]. The depletion of free ubiquitin would also affect other ubiquitin-dependent cellular processes, such as endocytosis [217] transcription [218] and DNA repair [219]. Given the limited availability of free ubiquitin and the high demands during cellular stress, increased biosynthesis of ubiquitin may be important for maintaining the pool of free ubiquitin during and after stress [220]. In addition to transcriptional activation, the levels of free ubiquitin can also be increased by accelerating the disassembly of ubiquitin chains [45, 221]. For example, the induction of Ubp6, a proteasome associated deubiquitinating enzyme, may be among the mechanisms for replenishing the free ubiquitin pool in response to stresses [45]. Consistent with the idea that prolonged depletion of free ubiquitin would result in cellular malfunctions, mice that carry recessive mutations in Usp14, a deubiquitinating enzyme, develop neurological disorders [222].
Summary
The UPP plays critical roles in a myriad of cellular functions, including protein quality control, regulation of proliferation, DNA repair, and signal transduction [6]. A functional UPP is also required for the cells to cope with various types of stress, including exposure to heavy metals [204, 205], amino acid analogs, and oxidation [78, 147]. The level of ubiquitin-protein conjugates in cells reflects the balance between the rate of formation and the rates of degradation and deubiquitination. Mild oxidative stress increases the rate of ubiquitin conjugation via increasing substrate availability and enhancing activities of ubiquitin conjugating enzymes [78, 89, 223], thus increases the rate of ubiquitination. Sustained physiologically relevant levels of oxidative stress may also impair proteasome activity and reduce the degradation of ubiquitinated substrates, contributing to the accumulation of ubiquitin conjugates in cells or tissues [149, 152–157, 224, 225]. Thus, whereas increased level of endogenous ubiquitin conjugates in general is a sensitive indicator of mild oxidative stress, a dramatic decline in levels of ubiquitin conjugates may indicate severe oxidative stress, because extensive oxidative stress can reduce the levels of ubiquitin conjugates in the cells via inactivating ubiquitin conjugation enzymes [90, 150, 151]. Transient UPP-related response to oxidative stress may be essential for cells to restore homeostasis during recovery from oxidative stress. Dysregulation of UPP-related responses to oxidative stress may be related to age-related accumulation of damaged proteins and its associated disorders.
ACKNOWLEDGEMENTS
This work is supported partially by NIH grants EY11717 (to FS), EY13250 (to AT), USDA CRIS 1950-51000-060-01A, grants from the American Health Assistance Foundation and a Johnson and Johnson Focused Giving Award to AT. The authors also thank Dr. Elizabeth Whitcomb and Ms. Karen Weikel for their critical reading and editing of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895–899. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- [2].Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ciechanover A. Intracellular protein degradation from a vague idea through the lysosome and the ubiquitin-proteasome system and on to human diseases and drug targeting: Nobel Lecture, December 8, 2004. Ann N Y Acad Sci. 2007;1116:1–28. doi: 10.1196/annals.1402.078. [DOI] [PubMed] [Google Scholar]
- [4].Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- [5].Ciechanover A. The ubiquitin proteolytic system and pathogenesis of human diseases: a novel platform for mechanism-based drug targeting. Biochem Soc Trans. 2003;31:474–481. doi: 10.1042/bst0310474. [DOI] [PubMed] [Google Scholar]
- [6].Shang F, Taylor A. Function of the ubiquitin proteolytic pathway in the eye. Exp Eye Res. 2004;78:1–14. doi: 10.1016/j.exer.2003.10.003. [DOI] [PubMed] [Google Scholar]
- [7].Shabek N, Herman-Bachinsky Y, Ciechanover A. Ubiquitin degradation with its substrate provides clues to proteasome regulation. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0905746106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Handley-Gearhart PM, Stephen AG, Trausch-Azar JS, Ciechanover A, Schwartz AL. Human ubiquitin-activating enzyme, E1. Indication of potential nuclear and cytoplasmic subpopulations using epitope-tagged cDNA constructs. J. Biol. Chem. 1994;269:33171–33178. [PubMed] [Google Scholar]
- [9].Handley-Gearhart PM, Trausch-Azar JS, Ciechanover A, Schwartz AL. Rescue of complex temperature-sensitive phenotype of chinese hamster ovary E36ts20 cells by expression of human ubiquitin activating enzyme cDNA. Biochem. J. 1994;304:1015–1020. doi: 10.1042/bj3041015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Shang F, Deng G, Obin M, Wu CC, Gong X, Smith D, Laursen RA, Andley UP, Reddan JR, Taylor A. Ubiquitin-activating enzyme (E1) isoforms in lens epithelial cells: origin of translation, E2 specificity and cellular localization determined with novel site-specific antibodies. Exp Eye Res. 2001;73:827–836. doi: 10.1006/exer.2001.1091. [DOI] [PubMed] [Google Scholar]
- [11].Jin J, Li X, Gygi SP, Harper JW. Dual E1 activation systems for ubiquitin differentially regulate E2 enzyme charging. Nature. 2007;447:1135–1138. doi: 10.1038/nature05902. [DOI] [PubMed] [Google Scholar]
- [12].Michelle C, Vourc'h P, Mignon L, Andres CR. What was the set of ubiquitin and ubiquitin-like conjugating enzymes in the eukaryote common ancestor? J Mol Evol. 2009;68:616–628. doi: 10.1007/s00239-009-9225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pickart CM, Eddins MJ. Ubiquitin: structures, functions, mechanisms. Biochim Biophys Acta. 2004;1695:55–72. doi: 10.1016/j.bbamcr.2004.09.019. [DOI] [PubMed] [Google Scholar]
- [14].Deshaies RJ, Joazeiro CA. RING domain E3 ubiquitin ligases. Annu Rev Biochem. 2009;78:399–434. doi: 10.1146/annurev.biochem.78.101807.093809. [DOI] [PubMed] [Google Scholar]
- [15].Beal R, Deveraux Q, Xia G, Rechsteiner M, Pickart C. Surface hydrophobic residues of multiubiquitin chains essential for proteolytic targeting. Proc. Natl. Acad. Sci. USA. 1996;93:861–866. doi: 10.1073/pnas.93.2.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pickart CM. Targeting of substrates to the 26S proteasome. Faseb J. 1997;11:1055–1066. doi: 10.1096/fasebj.11.13.9367341. [DOI] [PubMed] [Google Scholar]
- [17].Lam YA, Lawson TG, Velayutham M, Zweier JL, Pickart CM. A proteasomal ATPase subunit recognizes the polyubiquitin degradation signal. Nature. 2002;416:763–767. doi: 10.1038/416763a. [DOI] [PubMed] [Google Scholar]
- [18].Ciechanover A, Ben-Saadon R. N-terminal ubiquitination: more protein substrates join in. Trends Cell Biol. 2004;14:103–106. doi: 10.1016/j.tcb.2004.01.004. [DOI] [PubMed] [Google Scholar]
- [19].Pickart CM, Fushman D. Polyubiquitin chains: polymeric protein signals. Curr Opin Chem Biol. 2004;8:610–616. doi: 10.1016/j.cbpa.2004.09.009. [DOI] [PubMed] [Google Scholar]
- [20].Peng J, Schwartz D, Elias JE, Thoreen CC, Cheng D, Marsischky G, Roelofs J, Finley D, Gygi SP. A proteomics approach to understanding protein ubiquitination. Nat Biotechnol. 2003;21:921–926. doi: 10.1038/nbt849. [DOI] [PubMed] [Google Scholar]
- [21].Xu P, Duong DM, Seyfried NT, Cheng D, Xie Y, Robert J, Rush J, Hochstrasser M, Finley D, Peng J. Quantitative proteomics reveals the function of unconventional ubiquitin chains in proteasomal degradation. Cell. 2009;137:133–145. doi: 10.1016/j.cell.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Komander D, Reyes-Turcu F, Licchesi JD, Odenwaelder P, Wilkinson KD, Barford D. Molecular discrimination of structurally equivalent Lys 63-linked and linear polyubiquitin chains. EMBO Rep. 2009;10:466–473. doi: 10.1038/embor.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kirisako T, Kamei K, Murata S, Kato M, Fukumoto H, Kanie M, Sano S, Tokunaga F, Tanaka K, Iwai K. A ubiquitin ligase complex assembles linear polyubiquitin chains. Embo J. 2006;25:4877–4887. doi: 10.1038/sj.emboj.7601360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jin L, Williamson A, Banerjee S, Philipp I, Rape M. Mechanism of ubiquitin-chain formation by the human anaphase-promoting complex. Cell. 2008;133:653–665. doi: 10.1016/j.cell.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rahighi S, Ikeda F, Kawasaki M, Akutsu M, Suzuki N, Kato R, Kensche T, Uejima T, Bloor S, Komander D, Randow F, Wakatsuki S, Dikic I. Specific recognition of linear ubiquitin chains by NEMO is important for NF-kappaB activation. Cell. 2009;136:1098–1109. doi: 10.1016/j.cell.2009.03.007. [DOI] [PubMed] [Google Scholar]
- [26].Wang T, Yin L, Cooper EM, Lai MY, Dickey S, Pickart CM, Fushman D, Wilkinson KD, Cohen RE, Wolberger C. Evidence for bidentate substrate binding as the basis for the K48 linkage specificity of otubain 1. J Mol Biol. 2009;386:1011–1023. doi: 10.1016/j.jmb.2008.12.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Iwai K, Tokunaga F. Linear polyubiquitination: a new regulator of NF-kappaB activation. EMBO Rep. 2009;10:706–713. doi: 10.1038/embor.2009.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Williamson A, Wickliffe KE, Mellone BG, Song L, Karpen GH, Rape M. Identification of a physiological E2 module for the human anaphase-promoting complex. Proc Natl Acad Sci U S A. 2009;106:18213–18218. doi: 10.1073/pnas.0907887106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ye Y, Rape M. Building ubiquitin chains: E2 enzymes at work. Nat Rev Mol Cell Biol. 2009;10:755–764. doi: 10.1038/nrm2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ikeda F, Dikic I. Atypical ubiquitin chains: new molecular signals. 'Protein Modifications: Beyond the Usual Suspects' review series. EMBO Rep. 2008;9:536–542. doi: 10.1038/embor.2008.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chen ZJ. Ubiquitin signalling in the NF-kappaB pathway. Nat Cell Biol. 2005;7:758–765. doi: 10.1038/ncb0805-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Barriere H, Nemes C, Du K, Lukacs GL. Plasticity of polyubiquitin recognition as lysosomal targeting signals by the endosomal sorting machinery. Mol Biol Cell. 2007;18:3952–3965. doi: 10.1091/mbc.E07-07-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lauwers E, Jacob C, Andre B. K63-linked ubiquitin chains as a specific signal for protein sorting into the multivesicular body pathway. J Cell Biol. 2009;185:493–502. doi: 10.1083/jcb.200810114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Baumeister W, Walz J, Zuhl F, Seemuller E. The proteasome: paradigm of a self-compartmentalizing protease. Cell. 1998;92:367–380. doi: 10.1016/s0092-8674(00)80929-0. [DOI] [PubMed] [Google Scholar]
- [35].Smith DM, Benaroudj N, Goldberg A. Proteasomes and their associated ATPases: a destructive combination. J Struct Biol. 2006;156:72–83. doi: 10.1016/j.jsb.2006.04.012. [DOI] [PubMed] [Google Scholar]
- [36].Strickland E, Hakala K, Thomas PJ, DeMartino GN. Recognition of misfolding proteins by PA700, the regulatory subcomplex of the 26 S proteasome. J Biol Chem. 2000;275:5565–5572. doi: 10.1074/jbc.275.8.5565. [DOI] [PubMed] [Google Scholar]
- [37].Braun BC, Glickman M, Kraft R, Dahlmann B, Kloetzel PM, Finley D, Schmidt M. The base of the proteasome regulatory particle exhibits chaperone-like activity. Nat Cell Biol. 1999;1:221–226. doi: 10.1038/12043. [DOI] [PubMed] [Google Scholar]
- [38].Benaroudj N, Goldberg AL. PAN, the proteasome-activating nucleotidase from archaebacteria, is a protein-unfolding molecular chaperone. Nat Cell Biol. 2000;2:833–839. doi: 10.1038/35041081. [DOI] [PubMed] [Google Scholar]
- [39].Benaroudj N, Tarcsa E, Cascio P, Goldberg AL. The unfolding of substrates and ubiquitin-independent protein degradation by proteasomes. Biochimie. 2001;83:311–318. doi: 10.1016/s0300-9084(01)01244-5. [DOI] [PubMed] [Google Scholar]
- [40].Husnjak K, Elsasser S, Zhang N, Chen X, Randles L, Shi Y, Hofmann K, Walters KJ, Finley D, Dikic I. Proteasome subunit Rpn13 is a novel ubiquitin receptor. Nature. 2008;453:481–488. doi: 10.1038/nature06926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Pickart CM, Cohen RE. Proteasomes and their kin: proteases in the machine age. Nat Rev Mol Cell Biol. 2004;5:177–187. doi: 10.1038/nrm1336. [DOI] [PubMed] [Google Scholar]
- [42].Schmidt M, Hanna J, Elsasser S, Finley D. Proteasome-associated proteins: regulation of a proteolytic machine. Biol Chem. 2005;386:725–737. doi: 10.1515/BC.2005.085. [DOI] [PubMed] [Google Scholar]
- [43].Hanna J, Hathaway NA, Tone Y, Crosas B, Elsasser S, Kirkpatrick DS, Leggett DS, Gygi SP, King RW, Finley D. Deubiquitinating enzyme Ubp6 functions noncatalytically to delay proteasomal degradation. Cell. 2006;127:99–111. doi: 10.1016/j.cell.2006.07.038. [DOI] [PubMed] [Google Scholar]
- [44].Hanna J, Meides A, Zhang DP, Finley D. A ubiquitin stress response induces altered proteasome composition. Cell. 2007;129:747–759. doi: 10.1016/j.cell.2007.03.042. [DOI] [PubMed] [Google Scholar]
- [45].Crosas B, Hanna J, Kirkpatrick DS, Zhang DP, Tone Y, Hathaway NA, Buecker C, Leggett DS, Schmidt M, King RW, Gygi SP, Finley D. Ubiquitin chains are remodeled at the proteasome by opposing ubiquitin ligase and deubiquitinating activities. Cell. 2006;127:1401–1413. doi: 10.1016/j.cell.2006.09.051. [DOI] [PubMed] [Google Scholar]
- [46].Glickman MH, Raveh D. Proteasome plasticity. FEBS Lett. 2005;579:3214–3223. doi: 10.1016/j.febslet.2005.04.048. [DOI] [PubMed] [Google Scholar]
- [47].Lecker SH, Goldberg AL, Mitch WE. Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. J Am Soc Nephrol. 2006;17:1807–1819. doi: 10.1681/ASN.2006010083. [DOI] [PubMed] [Google Scholar]
- [48].Goldberg AL, Cascio P, Saric T, Rock KL. The importance of the proteasome and subsequent proteolytic steps in the generation of antigenic peptides. Mol Immunol. 2002;39:147–164. doi: 10.1016/s0161-5890(02)00098-6. [DOI] [PubMed] [Google Scholar]
- [49].Rechsteiner M, Hill CP. Mobilizing the proteolytic machine: cell biological roles of proteasome activators and inhibitors. Trends Cell Biol. 2005;15:27–33. doi: 10.1016/j.tcb.2004.11.003. [DOI] [PubMed] [Google Scholar]
- [50].McCutchen-Maloney SL, Matsuda K, Shimbara N, Binns DD, Tanaka K, Slaughter CA, DeMartino GN. cDNA cloning, expression, and functional characterization of PI31, a proline-rich inhibitor of the proteasome. J Biol Chem. 2000;275:18557–18565. doi: 10.1074/jbc.M001697200. [DOI] [PubMed] [Google Scholar]
- [51].Anbanandam A, Albarado DC, Tirziu DC, Simons M, Veeraraghavan S. Molecular basis for proline- and arginine-rich peptide inhibition of proteasome. J Mol Biol. 2008;384:219–227. doi: 10.1016/j.jmb.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Pacifici RE, Salo DC, Davies KJA. Macroxyproteinase (M.O.P.): a 670 kDa proteinase complex that degrades oxidatively denatured proteins in red blood cells. Free Rad. Biol. Med. 1989;7:521–536. doi: 10.1016/0891-5849(89)90028-2. [DOI] [PubMed] [Google Scholar]
- [53].Salo DC, Pacifici RE, Lin SW, Giulivi C, Davies KJ. Superoxide dismutase undergoes proteolysis and fragmentation following oxidative modification and inactivation. J Biol Chem. 1990;265:11919–11927. [PubMed] [Google Scholar]
- [54].Dean RT, Geiseg S, Davies MJ. Reactive species and their accumulation on radical-damaged proteins. TIBS. 1993:437–441. doi: 10.1016/0968-0004(93)90145-d. [DOI] [PubMed] [Google Scholar]
- [55].Shringarpure R, Grune T, Davies KJ. Protein oxidation and 20S proteasome-dependent proteolysis in mammalian cells. Cell Mol Life Sci. 2001;58:1442–1450. doi: 10.1007/PL00000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Shringarpure R, Grune T, Mehlhase J, Davies KJ. Ubiquitin conjugation is not required for the degradation of oxidized proteins by proteasome. J Biol Chem. 2003;278:311–318. doi: 10.1074/jbc.M206279200. [DOI] [PubMed] [Google Scholar]
- [57].Ferrington DA, Sun H, Murray KK, Costa J, Williams TD, Bigelow DJ, Squier TC. Selective degradation of oxidized calmodulin by the 20 S proteasome. J Biol Chem. 2001;276:937–943. doi: 10.1074/jbc.M005356200. [DOI] [PubMed] [Google Scholar]
- [58].Balog EM, Lockamy EL, Thomas DD, Ferrington DA. Site-specific methionine oxidation initiates calmodulin degradation by the 20S proteasome. Biochemistry. 2009;48:3005–3016. doi: 10.1021/bi802117k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Murakami Y, Matsufuji S, Kameji T, Hayashi S-I, Igarashi K, Tamura T, Tanaka K, Ichihara A. Ornithine decarboxylase is degraded by the 26S proteosome without ubiquitin. Nature. 1992;360:597–599. doi: 10.1038/360597a0. [DOI] [PubMed] [Google Scholar]
- [60].Tarcsa E, Szymanska G, Lecker S, O'Connor CM, Goldberg AL. Ca2+-free calmodulin and calmodulin damaged by in vitro aging are selectively degraded by 26 S proteasomes without ubiquitination. J Biol Chem. 2000;275:20295–20301. doi: 10.1074/jbc.M001555200. [DOI] [PubMed] [Google Scholar]
- [61].Zhang M, Pickart CM, Coffino P. Determinants of proteasome recognition of ornithine decarboxylase, a ubiquitin-independent substrate. Embo J. 2003;22:1488–1496. doi: 10.1093/emboj/cdg158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Matsuzawa S, Cuddy M, Fukushima T, Reed JC. Method for targeting protein destruction by using a ubiquitin-independent, proteasome-mediated degradation pathway. Proc Natl Acad Sci U S A. 2005;102:14982–14987. doi: 10.1073/pnas.0507512102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kelly SM, Vanslyke JK, Musil LS. Regulation of ubiquitin-proteasome system mediated degradation by cytosolic stress. Mol Biol Cell. 2007;18:4279–4291. doi: 10.1091/mbc.E07-05-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Kopito RR, Ron D. Conformational disease. Nat Cell Biol. 2000;2:E207–209. doi: 10.1038/35041139. [DOI] [PubMed] [Google Scholar]
- [65].Julien JP. Amyotrophic lateral sclerosis. unfolding the toxicity of the misfolded. Cell. 2001;104:581–591. doi: 10.1016/s0092-8674(01)00244-6. [DOI] [PubMed] [Google Scholar]
- [66].Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. 2004;10(Suppl):S10–17. doi: 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]
- [67].Morimoto RI. Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes Dev. 2008;22:1427–1438. doi: 10.1101/gad.1657108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Mu TW, Ong DS, Wang YJ, Balch WE, Yates JR, 3rd, Segatori L, Kelly JW. Chemical and biological approaches synergize to ameliorate protein-folding diseases. Cell. 2008;134:769–781. doi: 10.1016/j.cell.2008.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- [70].Cohen E, Dillin A. The insulin paradox: aging, proteotoxicity and neurodegeneration. Nat Rev Neurosci. 2008;9:759–767. doi: 10.1038/nrn2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Rattner A, Nathans J. Macular degeneration: recent advances and therapeutic opportunities. Nat Rev Neurosci. 2006;7:860–872. doi: 10.1038/nrn2007. [DOI] [PubMed] [Google Scholar]
- [72].Glabe CG. Structural classification of toxic amyloid oligomers. J Biol Chem. 2008;283:29639–29643. doi: 10.1074/jbc.R800016200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Ono K, Condron MM, Teplow DB. Structure-neurotoxicity relationships of amyloid beta-protein oligomers. Proc Natl Acad Sci U S A. 2009;106:14745–14750. doi: 10.1073/pnas.0905127106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Korolchuk VI, Menzies FM, Rubinsztein DC. A novel link between autophagy and the ubiquitin-proteasome system. Autophagy. 2009;5:862–863. doi: 10.4161/auto.8840. [DOI] [PubMed] [Google Scholar]
- [75].Ravid T, Hochstrasser M. Diversity of degradation signals in the ubiquitin-proteasome system. Nat Rev Mol Cell Biol. 2008;9:679–690. doi: 10.1038/nrm2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Hershko A, Heller H, Eytan E, Reiss Y. The protein substrate binding site of the ubiquitin-protein ligase system. J. Biol. Chem. 1986;261:11992–11999. [PubMed] [Google Scholar]
- [77].Shang F, Nowell TR, Jr., Taylor A. Removal of oxidatively damaged proteins from lens cells by the ubiquitin-proteasome pathway. Exp Eye Res. 2001;73:229–238. doi: 10.1006/exer.2001.1029. [DOI] [PubMed] [Google Scholar]
- [78].Dudek EJ, Shang F, Valverde P, Liu Q, Hobbs M, Taylor A. Selectivity of the ubiquitin pathway for oxidatively modified proteins: relevance to protein precipitation diseases. Faseb J. 2005;19:1707–1709. doi: 10.1096/fj.05-4049fje. [DOI] [PubMed] [Google Scholar]
- [79].Iwai K, Drake SK, Wehr NB, Weissman AM, LaVaute T, Minato N, Klausner RD, Levine RL, Rouault TA. Iron-dependent oxidation, ubiquitination, and degradation of iron regulatory protein 2: implications for degradation of oxidized proteins. Proc. Natl. Acad. Sci. USA. 1998;95:4924–4928. doi: 10.1073/pnas.95.9.4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Korolchuk VI, Mansilla A, Menzies FM, Rubinsztein DC. Autophagy inhibition compromises degradation of ubiquitin-proteasome pathway substrates. Mol Cell. 2009;33:517–527. doi: 10.1016/j.molcel.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Hicke L. A new ticket for entry into budding vesicles-ubiquitin. Cell. 2001;106:527–530. doi: 10.1016/s0092-8674(01)00485-8. [DOI] [PubMed] [Google Scholar]
- [82].Saksena S, Sun J, Chu T, Emr SD. ESCRTing proteins in the endocytic pathway. Trends Biochem Sci. 2007;32:561–573. doi: 10.1016/j.tibs.2007.09.010. [DOI] [PubMed] [Google Scholar]
- [83].Goldberg AL. Degradation of abnormal proteins in Escherichia coli (protein breakdown-protein structure-mistranslation-amino acid analogs-puromycin) Proc Natl Acad Sci U S A. 1972;69:422–426. doi: 10.1073/pnas.69.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Etlinger JD, Goldberg AL. A soluble ATP-dependent proteolytic system responsible for the degradation of abnormal proteins in reticulocytes. Proc Natl Acad Sci U S A. 1977;74:54–58. doi: 10.1073/pnas.74.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Hershko A, Eytan E, Ciechanover A. Immunochemical analysis of the turnover of ubiquitin-protein conjugates in intact cells. J. Biol. Chem. 1982;257:13964–13970. [PubMed] [Google Scholar]
- [86].Finley D, Ozkaynak E, Varshavsky A. The yeast polyubiquitin gene is essential for resistance to high temperature, starvation, and other stresses. Cell. 1987;48:1035–1046. doi: 10.1016/0092-8674(87)90711-2. [DOI] [PubMed] [Google Scholar]
- [87].Jahngen JH, Haas AL, Ciechanover A, Blondin J, Eisenhauer D, Taylor A. The eye lens has an active ubiquitin-protein conjugation system. J. Biol. Chem. 1986;261:13760–13767. [PubMed] [Google Scholar]
- [88].Taylor A, Davies KJA. Protein oxidation and loss of protease activity may lead to cataract formation in the aged lens. Free Rad. Biol. Med. 1987;3:371–377. doi: 10.1016/0891-5849(87)90015-3. [DOI] [PubMed] [Google Scholar]
- [89].Shang F, Gong X, Taylor A. Activity of ubiquitin dependent pathway in response to oxidative stress: Ubiquitin activating enzyme (E1) is transiently upregulated. J. Biol. Chem. 1997;272:23086–23093. doi: 10.1074/jbc.272.37.23086. [DOI] [PubMed] [Google Scholar]
- [90].Shang F, Taylor A. Oxidative stress and recovery from oxidative stress are associated with altered ubiquitin conjugating and proteolytic activities in bovine lens epithelial cells. Biochem. J. 1995;307:297–303. doi: 10.1042/bj3070297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Ward CL, Omura S, Kopito RR. Degradation of CFTR by the ubiquitin-proteasome pathway. Cell. 1995;83:121–127. doi: 10.1016/0092-8674(95)90240-6. [DOI] [PubMed] [Google Scholar]
- [92].Benharouga M, Haardt M, Kartner N, Lukacs GL. COOH-terminal truncations promote proteasome-dependent degradation of mature cystic fibrosis transmembrane conductance regulator from post-Golgi compartments. J Cell Biol. 2001;153:957–970. doi: 10.1083/jcb.153.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Gelman MS, Kannegaard ES, Kopito RR. A principal role for the proteasome in endoplasmic reticulum-associated degradation of misfolded intracellular cystic fibrosis transmembrane conductance regulator. J Biol Chem. 2002;277:11709–11714. doi: 10.1074/jbc.M111958200. [DOI] [PubMed] [Google Scholar]
- [94].Hyun DH, Lee M, Halliwell B, Jenner P. Proteasomal inhibition causes the formation of protein aggregates containing a wide range of proteins, including nitrated proteins. J Neurochem. 2003;86:363–373. doi: 10.1046/j.1471-4159.2003.01841.x. [DOI] [PubMed] [Google Scholar]
- [95].Hoffman EK, Wilcox HM, Scott RW, Siman R. Proteasome inhibition enhances the stability of mouse Cu/Zn superoxide dismutase with mutations linked to familial amyotrophic lateral sclerosis. J Neurol Sci. 1996;139:15–20. [PubMed] [Google Scholar]
- [96].Zhang X, Dudek EJ, Liu B, Ding L, Fernandes AF, Liang JJ, Horwitz J, Taylor A, Shang F. Degradation of C-terminal truncated alpha A-crystallins by the ubiquitin-proteasome pathway. Invest Ophthalmol Vis Sci. 2007;48:4200–4208. doi: 10.1167/iovs.07-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Dudek EJ, Lampi KJ, Lampi JA, Shang F, King J, Wang Y, Taylor A. Ubiquitin proteasome pathway-mediated degradation of proteins: effects due to site-specific substrate deamidation. Invest Ophthalmol Vis Sci. 2010;51:4164–4173. doi: 10.1167/iovs.09-4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Murata S, Minami Y, Minami M, Chiba T, Tanaka K. CHIP is a chaperone-dependent E3 ligase that ubiquitylates unfolded protein. EMBO Rep. 2001;2:1133–1138. doi: 10.1093/embo-reports/kve246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Marques C, Guo W, Pereira P, Taylor A, Patterson C, Evans PC, Shang F. The triage of damaged proteins: degradation by the ubiquitin-proteasome pathway or repair by molecular chaperones. Faseb J. 2006;20:741–743. doi: 10.1096/fj.05-5080fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Grune T, Reinheckel T, Davies KJA. Degradation of oxidized proteins in K562 human hematopoietic cells by proteasome. J. Biol. Chem. 1996;271:15504–15509. doi: 10.1074/jbc.271.26.15504. [DOI] [PubMed] [Google Scholar]
- [101].Grune T, Reinheckel T, Davies KJ. Degradation of oxidized proteins in mammalian cells. FASEB Journal. 1997;11:526–534. [PubMed] [Google Scholar]
- [102].Grune T, Davies KJ. Breakdown of oxidized proteins as a part of secondary antioxidant defenses in mammalian cells. Biofactors. 1997;6:165–172. doi: 10.1002/biof.5520060210. [DOI] [PubMed] [Google Scholar]
- [103].Pacifici RE, Kono Y, Davies KJA. Hydrophobicity as the signal for selective degradation of hydroxyl radical modified hemoglobin by the multicatalytic protease complex, proteasome. J. Biol. Chem. 1993;268:15405–15411. [PubMed] [Google Scholar]
- [104].Grune T, Merker K, Jung T, Sitte N, Davies KJ. Protein oxidation and degradation during postmitotic senescence. Free Radic Biol Med. 2005;39:1208–1215. doi: 10.1016/j.freeradbiomed.2005.06.009. [DOI] [PubMed] [Google Scholar]
- [105].Liu CW, Millen L, Roman TB, Xiong H, Gilbert HF, Noiva R, DeMartino GN, Thomas PJ. Conformational remodeling of proteasomal substrates by PA700, the 19 S regulatory complex of the 26 S proteasome. J Biol Chem. 2002;277:26815–26820. doi: 10.1074/jbc.M201782200. [DOI] [PubMed] [Google Scholar]
- [106].Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- [107].Sadis S, Atienza C, Jr., Finley D. Synthetic signals for ubiquitin-dependent proteolysis. Mol Cell Biol. 1995;15:4086–4094. doi: 10.1128/mcb.15.8.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Meacham GC, Patterson C, Zhang W, Younger JM, Cyr DM. The Hsc70 co-chaperone CHIP targets immature CFTR for proteasomal degradation. Nat Cell Biol. 2001;3:100–105. doi: 10.1038/35050509. [DOI] [PubMed] [Google Scholar]
- [109].Feldman DE, Frydman J. Protein folding in vivo: the importance of molecular chaperones. Curr Opin Struct Biol. 2000;10:26–33. doi: 10.1016/s0959-440x(99)00044-5. [DOI] [PubMed] [Google Scholar]
- [110].Morishima Y, Wang AM, Yu Z, Pratt WB, Osawa Y, Lieberman AP. CHIP deletion reveals functional redundancy of E3 ligases in promoting degradation of both signaling proteins and expanded glutamine proteins. Hum Mol Genet. 2008;17:3942–3952. doi: 10.1093/hmg/ddn296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].McDonough H, Patterson C. CHIP: a link between the chaperone and proteasome systems. Cell Stress Chaperones. 2003;8:303–308. doi: 10.1379/1466-1268(2003)008<0303:calbtc>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Cyr DM, Hohfeld J, Patterson C. Protein quality control: U-box-containing E3 ubiquitin ligases join the fold. Trends Biochem Sci. 2002;27:368–375. doi: 10.1016/s0968-0004(02)02125-4. [DOI] [PubMed] [Google Scholar]
- [113].Schubert U, Anton LC, Gibbs J, Norbury CC, Yewdell JW, Bennink JR. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature. 2000;404:770–774. doi: 10.1038/35008096. [DOI] [PubMed] [Google Scholar]
- [114].Younger JM, Chen L, Ren HY, Rosser MF, Turnbull EL, Fan CY, Patterson C, Cyr DM. Sequential quality-control checkpoints triage misfolded cystic fibrosis transmembrane conductance regulator. Cell. 2006;126:571–582. doi: 10.1016/j.cell.2006.06.041. [DOI] [PubMed] [Google Scholar]
- [115].Younger JM, Ren HY, Chen L, Fan CY, Fields A, Patterson C, Cyr DM. A foldable CFTR{Delta}F508 biogenic intermediate accumulates upon inhibition of the Hsc70-CHIP E3 ubiquitin ligase. J Cell Biol. 2004;167:1075–1085. doi: 10.1083/jcb.200410065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Grove DE, Rosser MF, Ren HY, Naren AP, Cyr DM. Mechanisms for Rescue of Correctable Folding Defects in CFTR{Delta}F508. Mol Biol Cell. 2009;20:4059–4069. doi: 10.1091/mbc.E08-09-0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Mathew A, Mathur SK, Morimoto RI. Heat shock response and protein degradation: regulation of HSF2 by the ubiquitin-proteasome pathway. Mol Cell Biol. 1998;18:5091–5098. doi: 10.1128/mcb.18.9.5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Bush KT, Goldberg AL, Nigam SK. Proteasome inhibition leads to a heat shock response, induction of endoplasmic reticulum chaperones and thermotolerance. J. Biol. Chem. 1997;272:9086–9092. doi: 10.1074/jbc.272.14.9086. [DOI] [PubMed] [Google Scholar]
- [119].Lee DH, Goldberg AL. Proteasome inhibitors cause induction of heat shock proteins and trehalose, which together confer thermotolerance in Saccharomyces cerevisiae. Mol Cell Biol. 1998;18:30–38. doi: 10.1128/mcb.18.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Bian Q, Fernandes AF, Taylor A, Wu M, Pereira P, Shang F. Expression of K6W-ubiquitin in lens epithelial cells leads to upregulation of a broad spectrum of molecular chaperones. Mol Vis. 2008;14:403–412. [PMC free article] [PubMed] [Google Scholar]
- [121].Mimnaugh EG, Xu W, Vos M, Yuan X, Isaacs JS, Bisht KS, Gius D, Neckers L. Simultaneous inhibition of hsp 90 and the proteasome promotes protein ubiquitination, causes endoplasmic reticulum-derived cytosolic vacuolization, and enhances antitumor activity. Mol Cancer Ther. 2004;3:551–566. [PubMed] [Google Scholar]
- [122].Rivett AJ. Preferential degradation of the oxidatively modified forms of glutamine synthetase by intracellular mammalian proteases. J. Biol. Chem. 1985;260:300–305. [PubMed] [Google Scholar]
- [123].Rivett AJ. Purification of a liver alkaline protease which degrades oxidatively modified glutamine synthetase. Characterization as a high molecular weight cysteine proteinase. J Biol Chem. 1985;260:12600–12606. [PubMed] [Google Scholar]
- [124].Grune T, Reinheckel T, Joshi M, Davies KJA. Proteolysis in cultured liver epithelial cells during oxidative stress. Role of multicatalytic proteinase complex, proteasome. J. Biol. Chem. 1995;270:2344–2351. doi: 10.1074/jbc.270.5.2344. [DOI] [PubMed] [Google Scholar]
- [125].Grune T, Blasig IE, Sitte N, Roloff B, Haseloff R, Davies KJ. Peroxynitrite increases the degradation of aconitase and other cellular proteins by proteasome. J Biol Chem. 1998;273:10857–10862. doi: 10.1074/jbc.273.18.10857. [DOI] [PubMed] [Google Scholar]
- [126].Gieche J, Mehlhase J, Licht A, Zacke T, Sitte N, Grune T. Protein oxidation and proteolysis in RAW264.7 macrophages: effects of PMA activation. Biochim Biophys Acta. 2001;1538:321–328. doi: 10.1016/s0167-4889(01)00083-0. [DOI] [PubMed] [Google Scholar]
- [127].Huang LL, Shang F, Nowell TR, Jr., Taylor A. Degradation of differentially oxidized α-crystallins in bovine lens epithelial cells. Exp. Eye Res. 1995;61:45–54. doi: 10.1016/s0014-4835(95)80057-3. [DOI] [PubMed] [Google Scholar]
- [128].Zetterberg M, Zhang X, Taylor A, Liu B, Liang JJ, Shang F. Glutathiolation Enhances the Degradation of {gamma}C-crystallin in Lens and Reticulocyte Lysates, Partially via the Ubiquitin-Proteasome Pathway. Invest Ophthalmol Vis Sci. 2006;47:3467–3473. doi: 10.1167/iovs.05-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Sitte N, Merker K, Von Zglinicki T, Grune T, Davies KJ. Protein oxidation and degradation during cellular senescence of human BJ fibroblasts: part I--effects of proliferative senescence. Faseb J. 2000;14:2495–2502. doi: 10.1096/fj.00-0209com. [DOI] [PubMed] [Google Scholar]
- [130].Sitte N, Merker K, von Zglinicki T, Grune T. Protein oxidation and degradation during proliferative senescence of human MRC-5 fibroblasts. Free Radic Biol Med. 2000;28:701–708. doi: 10.1016/s0891-5849(99)00279-8. [DOI] [PubMed] [Google Scholar]
- [131].Blazquez H, Fominaya JM, Hofsteenge J. Oxidation of sulfhydryl group of ribonuclease inhibitor in epithelial cells is sufficient for its intracellular degradation. J. Biol. Chem. 1996;271:18638–18642. doi: 10.1074/jbc.271.31.18638. [DOI] [PubMed] [Google Scholar]
- [132].Shang F, Taylor A. Oxidation and protein degradation in the lens. Mol. Aspects Med. 1997;18:339–351. [Google Scholar]
- [133].Sitte N, Merker K, Grune T. Proteasome-dependent degradation of oxidized proteins in MRC-5 fibroblasts. FEBS Lett. 1998;440:399–402. doi: 10.1016/s0014-5793(98)01495-1. [DOI] [PubMed] [Google Scholar]
- [134].Ullrich O, Grune T. Proteasomal degradation of oxidatively damaged endogenous histones in K562 human leukemic cells. Free Radic Biol Med. 2001;31:887–893. doi: 10.1016/s0891-5849(01)00672-4. [DOI] [PubMed] [Google Scholar]
- [135].Friguet B. Oxidized protein degradation and repair in ageing and oxidative stress. FEBS Lett. 2006;580:2910–2916. doi: 10.1016/j.febslet.2006.03.028. [DOI] [PubMed] [Google Scholar]
- [136].Giulivi C, Pacifici RE, Davies KJ. Exposure of hydrophobic moieties promotes the selective degradation of hydrogen peroxide-modified hemoglobin by the multicatalytic proteinase complex, proteasome. Arch Biochem Biophys. 1994;311:329–341. doi: 10.1006/abbi.1994.1245. [DOI] [PubMed] [Google Scholar]
- [137].Davies KJ, Shringarpure R. Preferential degradation of oxidized proteins by the 20S proteasome may be inhibited in aging and in inflammatory neuromuscular diseases. Neurology. 2006;66:S93–96. doi: 10.1212/01.wnl.0000192308.43151.63. [DOI] [PubMed] [Google Scholar]
- [138].Yamanaka K, Ishikawa H, Megumi Y, Tokunaga F, Kanie M, Rouault TA, Morishima I, Minato N, Ishimori K, Iwai K. Identification of the ubiquitin-protein ligase that recognizes oxidized IRP2. Nat Cell Biol. 2003;5:336–340. doi: 10.1038/ncb952. [DOI] [PubMed] [Google Scholar]
- [139].Lee BH, Lee MJ, Park S, Oh DC, Elsasser S, Chen PC, Gartner C, Dimova N, Hanna J, Gygi SP, Wilson SM, King RW, Finley D. Enhancement of proteasome activity by a small-molecule inhibitor of USP14. Nature. 2010;467:179–184. doi: 10.1038/nature09299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Davies KJ, Goldberg AL. Proteins damaged by oxygen radicals are rapidly degraded in extracts of red blood cells. J Biol Chem. 1987;262:8227–8234. [PubMed] [Google Scholar]
- [141].Fagan JM, Waxman L. The ATP-independent pathway in red blood cells that degrades oxidant-damaged hemoglobin. J. Biol. Chem. 1992;267:23015–23022. [PubMed] [Google Scholar]
- [142].Shang F, Huang L, Taylor A. Degradation of native and oxidized beta- and gamma-crystallins using bovine lens epithelial cell and rabbit reticulocyte extracts. Curr. Eye Res. 1994;13:423–431. doi: 10.3109/02713689408999870. [DOI] [PubMed] [Google Scholar]
- [143].Pickering AM, Koop AL, Teoh CY, Ermak G, Grune T, Davies KJ. The immunoproteasome, the 20S proteasome and the PA28alphabeta proteasome regulator are oxidative-stress-adaptive proteolytic complexes. Biochem J. 2010;432:585–594. doi: 10.1042/BJ20100878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Levine RL, Mosoni L, Berlett BS, Stadtman ER. Methionine residues as endogenous antioxidants in proteins. Proceedings of the National Academy of Sciences USA. 1996;93:15036–15040. doi: 10.1073/pnas.93.26.15036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Lasch P, Petras T, Ullrich O, Backmann J, Naumann D, Grune T. Hydrogen peroxide-induced structural alterations of RNAse A. J Biol Chem. 2001;276:9492–9502. doi: 10.1074/jbc.M008528200. [DOI] [PubMed] [Google Scholar]
- [146].Dunten RL, Cohen RE. Recognition of modified forms of ribonuclease A by the ubiquitin system. J Biol Chem. 1989;264:16739–16747. [PubMed] [Google Scholar]
- [147].Shang F, Deng G, Liu Q, Guo W, Haas AL, Crosas B, Finley D, Taylor A. Lys6-modified ubiquitin inhibits ubiquitin-dependent protein degradation. J Biol Chem. 2005;280:20365–20374. doi: 10.1074/jbc.M414356200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Pirlich M, Muller C, Sandig G, Jakstadt M, Sitte N, Lochs H, Grune T. Increased proteolysis after single-dose exposure with hepatotoxins in HepG2 cells. Free Radic Biol Med. 2002;33:283–291. doi: 10.1016/s0891-5849(02)00880-8. [DOI] [PubMed] [Google Scholar]
- [149].Zhang X, Zhou J, Fernandes AF, Sparrow JR, Pereira P, Taylor A, Shang F. The proteasome: a target of oxidative damage in cultured human retina pigment epithelial cells. Invest Ophthalmol Vis Sci. 2008;49:3622–3630. doi: 10.1167/iovs.07-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Jahngen-Hodge J, Obin M, Gong X, Shang F, Nowell T, Gong J, Abasi H, Blumberg J, Taylor A. Regulation of ubiquitin conjugating enzymes by glutathione following oxidative stress. J. Biol. Chem. 1997;272:28218–28226. doi: 10.1074/jbc.272.45.28218. [DOI] [PubMed] [Google Scholar]
- [151].Obin M, Shang F, Gong X, Handelman G, Blumberg J, Taylor A. Redox regulation of ubiquitin-conjugating enzymes: mechanistic insights using the thiol-specific oxidant diamide. FASEB Journal. 1998;12:561–569. doi: 10.1096/fasebj.12.7.561. [DOI] [PubMed] [Google Scholar]
- [152].Yao D, Gu Z, Nakamura T, Shi ZQ, Ma Y, Gaston B, Palmer LA, Rockenstein EM, Zhang Z, Masliah E, Uehara T, Lipton SA. Nitrosative stress linked to sporadic Parkinson's disease: S-nitrosylation of parkin regulates its E3 ubiquitin ligase activity. Proc Natl Acad Sci U S A. 2004;101:10810–10814. doi: 10.1073/pnas.0404161101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Ishii T, Sakurai T, Usami H, Uchida K. Oxidative modification of proteasome: identification of an oxidation-sensitive subunit in 26 s proteasome. Biochemistry. 2005;44:13893–13901. doi: 10.1021/bi051336u. [DOI] [PubMed] [Google Scholar]
- [154].Caballero M, Liton PB, Epstein DL, Gonzalez P. Proteasome inhibition by chronic oxidative stress in human trabecular meshwork cells. Biochem Biophys Res Commun. 2003;308:346–352. doi: 10.1016/s0006-291x(03)01385-8. [DOI] [PubMed] [Google Scholar]
- [155].Okada K, Wangpoengtrakul C, Osawa T, Toyokuni S, Tanaka K, Uchida K. 4-Hydroxy-2-nonenal-mediated impairment of intracellular proteolysis during oxidative stress. Identification of proteasomes as target molecules. J Biol Chem. 1999;274:23787–23793. doi: 10.1074/jbc.274.34.23787. [DOI] [PubMed] [Google Scholar]
- [156].Conconi M, Petropoulos I, Emod I, Turlin E, Biville F, Friguet B. Protection from oxidative inactivation of the 20S proteasome by heat-shock protein 90. Biochem J. 1998;333(Pt 2):407–415. doi: 10.1042/bj3330407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Ding Q, Keller JN. Proteasome inhibition in oxidative stress neurotoxicity: implications for heat shock proteins. J Neurochem. 2001;77:1010–1017. doi: 10.1046/j.1471-4159.2001.00302.x. [DOI] [PubMed] [Google Scholar]
- [158].Shang F, Gong X, Palmer HJ, Nowell TR, Taylor A. Age-related decline in ubiquitin conjugation in response to oxidative stress in the lens. Exp. Eye Res. 1997;64:21–30. doi: 10.1006/exer.1996.0176. [DOI] [PubMed] [Google Scholar]
- [159].Wu M, Bian Q, Liu Y, Fernandes AF, Taylor A, Pereira P, Shang F. Sustained oxidative stress inhibits NF-kappaB activation partially via inactivating the proteasome. Free Radic Biol Med. 2009;46:62–69. doi: 10.1016/j.freeradbiomed.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Shringarpure R, Grune T, Sitte N, Davies KJ. 4-Hydroxynonenal-modified amyloid-beta peptide inhibits the proteasome: possible importance in Alzheimer's disease. Cell Mol Life Sci. 2000;57:1802–1809. doi: 10.1007/PL00000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Reinheckel T, Ullrich O, Sitte N, Grune T. Differential impairment of 20S and 26S proteasome activities in human hematopoietic K562 cells during oxidative stress. Arch Biochem Biophys. 2000;377:65–68. doi: 10.1006/abbi.2000.1717. [DOI] [PubMed] [Google Scholar]
- [162].Bence NF, Sampat RM, Kopito RR. Impairment of the ubiquitin-proteasome system by protein aggregation. Science. 2001;292:1552–1555. doi: 10.1126/science.292.5521.1552. [DOI] [PubMed] [Google Scholar]
- [163].Bennett EJ, Bence NF, Jayakumar R, Kopito RR. Global impairment of the ubiquitin-proteasome system by nuclear or cytoplasmic protein aggregates precedes inclusion body formation. Mol Cell. 2005;17:351–365. doi: 10.1016/j.molcel.2004.12.021. [DOI] [PubMed] [Google Scholar]
- [164].Friguet B, Szweda LI. Inhibition of the multicatalytic proteinase (proteasome) by 4-hydroxy-2-nonenal cross-linked protein. FEBS Lett. 1997;405:21–25. doi: 10.1016/s0014-5793(97)00148-8. [DOI] [PubMed] [Google Scholar]
- [165].Bulteau AL, Moreau M, Nizard C, Friguet B. Impairment of proteasome function upon UVA- and UVB-irradiation of human keratinocytes. Free Radic Biol Med. 2002;32:1157–1170. doi: 10.1016/s0891-5849(02)00816-x. [DOI] [PubMed] [Google Scholar]
- [166].Grune T, Jung T, Merker K, Davies KJ. Decreased proteolysis caused by protein aggregates, inclusion bodies, plaques, lipofuscin, ceroid, and 'aggresomes' during oxidative stress, aging, and disease. Int J Biochem Cell Biol. 2004;36:2519–2530. doi: 10.1016/j.biocel.2004.04.020. [DOI] [PubMed] [Google Scholar]
- [167].Breusing N, Grune T. Regulation of proteasome-mediated protein degradation during oxidative stress and aging. Biol Chem. 2008;389:203–209. doi: 10.1515/BC.2008.029. [DOI] [PubMed] [Google Scholar]
- [168].Scrofano MM, Shang F, Nowell TR, Jr., Gong X, Smith DE, Kelliher M, Dunning J, Mura CV, Taylor A. Calorie restriction, stress and the ubiquitin-dependent pathway in mouse livers. Mech Ageing Dev. 1998;105:273–290. doi: 10.1016/s0047-6374(98)00097-9. [DOI] [PubMed] [Google Scholar]
- [169].Scrofano MM, Shang F, Nowell TR, Jr., Gong X, Smith DE, Kelliher M, Dunning J, Mura CV, Taylor A. Aging, calorie restriction and ubiquitin-dependent proteolysis in the livers of Emory mice. Mech Ageing Dev. 1998;101:277–296. doi: 10.1016/s0047-6374(97)00178-4. [DOI] [PubMed] [Google Scholar]
- [170].Adamo AM, Pasquini LA, Moreno MB, Oteiza PI, Soto EF, Pasquini JM. Effect of oxidant systems on the ubiquitylation of proteins in the central nervous system. J Neurosci Res. 1999;55:523–531. doi: 10.1002/(SICI)1097-4547(19990215)55:4<523::AID-JNR12>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- [171].Ramanathan M, Hassanain M, Levitt M, Seth A, Tolman JS, Fried VA, Ingoglia NA. Oxidative stress increases ubiquitin--protein conjugates in synaptosomes. Neuroreport. 1999;10:3797–3802. doi: 10.1097/00001756-199912160-00014. [DOI] [PubMed] [Google Scholar]
- [172].Figueiredo-Pereira ME, Yakushin S, Cohen G. Accumulation of ubiquitinated proteins in mouse neuronal cells induced by oxidative stress. Mol. Biol. Reports. 1997;24:25–38. doi: 10.1023/a:1006848405975. [DOI] [PubMed] [Google Scholar]
- [173].Shang F, Dudek E, Liu Q, Boulton ME, Taylor A. Protein quality control by the ubiquitin proteolytic pathway: Roles in resistance to oxidative stress and disease. Israel Journal of Chemistry. 2006;46:145–158. [Google Scholar]
- [174].Vernace VA, Arnaud L, Schmidt-Glenewinkel T, Figueiredo-Pereira ME. Aging perturbs 26S proteasome assembly in Drosophila melanogaster. Faseb J. 2007;21:2672–2682. doi: 10.1096/fj.06-6751com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Ohtsuka H, Takahashi R, Goto S. Age-related accumulation of high-molecular-weight ubiquitin protein conjugates in mouse brains. J Gerontol A Biol Sci Med Sci. 1995;50:B277–281. doi: 10.1093/gerona/50a.5.b277. [DOI] [PubMed] [Google Scholar]
- [176].Villeneuve NF, Lau A, Zhang DD. Regulation of the Nrf2-Keap1 Antioxidant Response by the Ubiquitin Proteasome System: An Insight into Cullin-Ring Ubiquitin Ligases. Antioxid Redox Signal. 2010 doi: 10.1089/ars.2010.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Nguyen T, Sherratt PJ, Huang HC, Yang CS, Pickett CB. Increased protein stability as a mechanism that enhances Nrf2-mediated transcriptional activation of the antioxidant response element. Degradation of Nrf2 by the 26 S proteasome. J Biol Chem. 2003;278:4536–4541. doi: 10.1074/jbc.M207293200. [DOI] [PubMed] [Google Scholar]
- [178].Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci U S A. 1996;93:14960–14965. doi: 10.1073/pnas.93.25.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Wild AC, Moinova HR, Mulcahy RT. Regulation of gamma-glutamylcysteine synthetase subunit gene expression by the transcription factor Nrf2. J Biol Chem. 1999;274:33627–33636. doi: 10.1074/jbc.274.47.33627. [DOI] [PubMed] [Google Scholar]
- [180].Nguyen T, Huang HC, Pickett CB. Transcriptional regulation of the antioxidant response element. Activation by Nrf2 and repression by MafK. J Biol Chem. 2000;275:15466–15473. doi: 10.1074/jbc.M000361200. [DOI] [PubMed] [Google Scholar]
- [181].Dhakshinamoorthy S, Jaiswal AK. Functional characterization and role of INrf2 in antioxidant response element-mediated expression and antioxidant induction of NAD(P)H:quinone oxidoreductase1 gene. Oncogene. 2001;20:3906–3917. doi: 10.1038/sj.onc.1204506. [DOI] [PubMed] [Google Scholar]
- [182].Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Cullinan SB, Gordan JD, Jin J, Harper JW, Diehl JA. The Keap1-BTB protein is an adaptor that bridges Nrf2 to a Cul3-based E3 ligase: oxidative stress sensing by a Cul3-Keap1 ligase. Mol Cell Biol. 2004;24:8477–8486. doi: 10.1128/MCB.24.19.8477-8486.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Zhang DD, Lo SC, Cross JV, Templeton DJ, Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol Cell Biol. 2004;24:10941–10953. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Furukawa M, Xiong Y. BTB protein Keap1 targets antioxidant transcription factor Nrf2 for ubiquitination by the Cullin 3-Roc1 ligase. Mol Cell Biol. 2005;25:162–171. doi: 10.1128/MCB.25.1.162-171.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Sekhar KR, Yan XX, Freeman ML. Nrf2 degradation by the ubiquitin proteasome pathway is inhibited by KIAA0132, the human homolog to INrf2. Oncogene. 2002;21:6829–6834. doi: 10.1038/sj.onc.1205905. [DOI] [PubMed] [Google Scholar]
- [187].Stewart D, Killeen E, Naquin R, Alam S, Alam J. Degradation of transcription factor Nrf2 via the ubiquitin-proteasome pathway and stabilization by cadmium. J Biol Chem. 2003;278:2396–2402. doi: 10.1074/jbc.M209195200. [DOI] [PubMed] [Google Scholar]
- [188].Kaspar JW, Niture SK, Jaiswal AK. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med. 2009;47:1304–1309. doi: 10.1016/j.freeradbiomed.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Plafker KS, Nguyen L, Barneche M, Mirza S, Crawford D, Plafker SM. The ubiquitin-conjugating enzyme UbcM2 can regulate the stability and activity of the antioxidant transcription factor Nrf2. J Biol Chem. 2010;285:23064–23074. doi: 10.1074/jbc.M110.121913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Dreger H, Westphal K, Weller A, Baumann G, Stangl V, Meiners S, Stangl K. Nrf2-dependent upregulation of antioxidative enzymes: a novel pathway for proteasome inhibitor-mediated cardioprotection. Cardiovasc Res. 2009;83:354–361. doi: 10.1093/cvr/cvp107. [DOI] [PubMed] [Google Scholar]
- [191].Dreger H, Westphal K, Wilck N, Baumann G, Stangl V, Stangl K, Meiners S. Protection of vascular cells from oxidative stress by proteasome inhibition depends on Nrf2. Cardiovasc Res. 2010;85:395–403. doi: 10.1093/cvr/cvp279. [DOI] [PubMed] [Google Scholar]
- [192].Westphal K, Stangl V, Fahling M, Dreger H, Weller A, Baumann G, Stangl K, Meiners S. Human-specific induction of glutathione peroxidase-3 by proteasome inhibition in cardiovascular cells. Free Radic Biol Med. 2009;47:1652–1660. doi: 10.1016/j.freeradbiomed.2009.09.017. [DOI] [PubMed] [Google Scholar]
- [193].Chen J, Regan RF. Increasing expression of heme oxygenase-1 by proteasome inhibition protects astrocytes from heme-mediated oxidative injury. Curr Neurovasc Res. 2005;2:189–196. doi: 10.2174/1567202054368344. [DOI] [PubMed] [Google Scholar]
- [194].Meiners S, Ludwig A, Lorenz M, Dreger H, Baumann G, Stangl V, Stangl K. Nontoxic proteasome inhibition activates a protective antioxidant defense response in endothelial cells. Free Radic Biol Med. 2006;40:2232–2241. doi: 10.1016/j.freeradbiomed.2006.03.003. [DOI] [PubMed] [Google Scholar]
- [195].Yamamoto N, Sawada H, Izumi Y, Kume T, Katsuki H, Shimohama S, Akaike A. Proteasome inhibition induces glutathione synthesis and protects cells from oxidative stress: relevance to Parkinson disease. J Biol Chem. 2007;282:4364–4372. doi: 10.1074/jbc.M603712200. [DOI] [PubMed] [Google Scholar]
- [196].Kapeta S, Chondrogianni N, Gonos ES. Nuclear erythroid factor 2-mediated proteasome activation delays senescence in human fibroblasts. J Biol Chem. 2010;285:8171–8184. doi: 10.1074/jbc.M109.031575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Lee CS, Tee LY, Warmke T, Vinjamoori A, Cai A, Fagan AM, Snider BJ. A proteasomal stress response: pre-treatment with proteasome inhibitors increases proteasome activity and reduces neuronal vulnerability to oxidative injury. J Neurochem. 2004;91:996–1006. doi: 10.1111/j.1471-4159.2004.02813.x. [DOI] [PubMed] [Google Scholar]
- [198].Taylor A, Shang F, Nowell T, Galanty Y, Shiloh Y. Ubiquitination capabilities in response to neocarzinostatin and H(2)O(2) stress in cell lines from patients with ataxia-telangiectasia. Oncogene. 2002;21:4363–4373. doi: 10.1038/sj.onc.1205557. [DOI] [PubMed] [Google Scholar]
- [199].Mehlhase J, Grune T. Proteolytic response to oxidative stress in mammalian cells. Biol Chem. 2002;383:559–567. doi: 10.1515/BC.2002.057. [DOI] [PubMed] [Google Scholar]
- [200].Grune T, Reinheckel T, Li R, North JA, Davies KJ. Proteasome-dependent turnover of protein disulfide isomerase in oxidatively stressed cells. Arch Biochem Biophys. 2002;397:407–413. doi: 10.1006/abbi.2001.2719. [DOI] [PubMed] [Google Scholar]
- [201].Grune T, Klotz LO, Gieche J, Rudeck M, Sies H. Protein oxidation and proteolysis by the nonradical oxidants singlet oxygen or peroxynitrite. Free Radic Biol Med. 2001;30:1243–1253. doi: 10.1016/s0891-5849(01)00515-9. [DOI] [PubMed] [Google Scholar]
- [202].Dudek EJ, Shang F, Taylor A. H(2)O(2)-mediated oxidative stress activates NF-kappaB in lens epithelial cells. Free Radic Biol Med. 2001;31:651–658. doi: 10.1016/s0891-5849(01)00634-7. [DOI] [PubMed] [Google Scholar]
- [203].Bowie A, O'Neill LA. Oxidative stress and nuclear factor-kappaB activation: a reassessment of the evidence in the light of recent discoveries. Biochem Pharmacol. 2000;59:13–23. doi: 10.1016/s0006-2952(99)00296-8. [DOI] [PubMed] [Google Scholar]
- [204].Tsirigotis M, Zhang M, Chiu RK, Wouters BG, Gray DA. Sensitivity of mammalian cells expressing mutant ubiquitin to protein damaging agents. J Biol Chem. 2001;11:11. doi: 10.1074/jbc.M109023200. [DOI] [PubMed] [Google Scholar]
- [205].Jungmann J, Reins H-A, Schobert C, Jentsch S. Resistance to cadmium mediated by ubiquitin-dependent proteolysis. Nature. 1993;361:369–371. doi: 10.1038/361369a0. [DOI] [PubMed] [Google Scholar]
- [206].Starke-Reed PE, Oliver CN. Protein oxidation and proteolysis during aging and oxidative stress. Arch. Biochem. Biophys. 1989;275:559–567. doi: 10.1016/0003-9861(89)90402-5. [DOI] [PubMed] [Google Scholar]
- [207].Agarwal S, Sohal RS. Aging and proteolysis of oxidized proteins. Arch. Biochem. Biophys. 1994;309:24–28. doi: 10.1006/abbi.1994.1078. [DOI] [PubMed] [Google Scholar]
- [208].Stadtman ER. Protein oxidation and aging. Science. 1992;257:1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- [209].Breusing N, Arndt J, Voss P, Bresgen N, Wiswedel I, Gardemann A, Siems W, Grune T. Inverse correlation of protein oxidation and proteasome activity in liver and lung. Mech Ageing Dev. 2009;130:748–753. doi: 10.1016/j.mad.2009.09.004. [DOI] [PubMed] [Google Scholar]
- [210].Gelman BB, Schuenke K. Brain aging in acquired immunodeficiency syndrome: increased ubiquitin-protein conjugate is correlated with decreased synaptic protein but not amyloid plaque accumulation. J Neurovirol. 2004;10:98–108. doi: 10.1080/13550280490279816. [DOI] [PubMed] [Google Scholar]
- [211].Clavel S, Coldefy AS, Kurkdjian E, Salles J, Margaritis I, Derijard B. Atrophy-related ubiquitin ligases, atrogin-1 and MuRF1 are up-regulated in aged rat Tibialis Anterior muscle. Mech Ageing Dev. 2006;127:794–801. doi: 10.1016/j.mad.2006.07.005. [DOI] [PubMed] [Google Scholar]
- [212].Dantuma NP, Groothuis TA, Salomons FA, Neefjes J. A dynamic ubiquitin equilibrium couples proteasomal activity to chromatin remodeling. J Cell Biol. 2006;173:19–26. doi: 10.1083/jcb.200510071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Mimnaugh EG, Chen HY, Davie JR, Celis JE, Neckers L. Rapid deubiquitination of nucleosomal histones in human tumor cells caused by proteasome inhibitors and stress response inducers: effects on replication, transcription, translation, and the cellular stress response. Biochemistry. 1997;36:14418–14429. doi: 10.1021/bi970998j. [DOI] [PubMed] [Google Scholar]
- [214].Pan J-X, Short SR, Goff SA, Dice JF. Ubiquitin pools, ubiquitin mRNA levels, and ubiquitin-mediated proteolysis in aging human fibroblasts. Exp. Gerontol. 1993;28:39–49. doi: 10.1016/0531-5565(93)90018-9. [DOI] [PubMed] [Google Scholar]
- [215].Vissers JH, Nicassio F, van Lohuizen M, Di Fiore PP, Citterio E. The many faces of ubiquitinated histone H2A: insights from the DUBs. Cell Div. 2008;3:8. doi: 10.1186/1747-1028-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Sparmann A, van Lohuizen M. Polycomb silencers control cell fate, development and cancer. Nat Rev Cancer. 2006;6:846–856. doi: 10.1038/nrc1991. [DOI] [PubMed] [Google Scholar]
- [217].Mukhopadhyay D, Riezman H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science. 2007;315:201–205. doi: 10.1126/science.1127085. [DOI] [PubMed] [Google Scholar]
- [218].Muratani M, Tansey WP. How the ubiquitin-proteasome system controls transcription. Nat Rev Mol Cell Biol. 2003;4:192–201. doi: 10.1038/nrm1049. [DOI] [PubMed] [Google Scholar]
- [219].Bergink S, Jentsch S. Principles of ubiquitin and SUMO modifications in DNA repair. Nature. 2009;458:461–467. doi: 10.1038/nature07963. [DOI] [PubMed] [Google Scholar]
- [220].Fornace AJ, Alamo I, Hollander MC, Lamoreaux E. Ubiquitin mRNA is a major stress-induced transcript in mammalian cells. Nucleic Acids Res. 1989;17:1215–1230. doi: 10.1093/nar/17.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Kimura Y, Yashiroda H, Kudo T, Koitabashi S, Murata S, Kakizuka A, Tanaka K. An inhibitor of a deubiquitinating enzyme regulates ubiquitin homeostasis. Cell. 2009;137:549–559. doi: 10.1016/j.cell.2009.02.028. [DOI] [PubMed] [Google Scholar]
- [222].Anderson C, Crimmins S, Wilson JA, Korbel GA, Ploegh HL, Wilson SM. Loss of Usp14 results in reduced levels of ubiquitin in ataxia mice. J Neurochem. 2005;95:724–731. doi: 10.1111/j.1471-4159.2005.03409.x. [DOI] [PubMed] [Google Scholar]
- [223].Marques C, Pereira P, Taylor A, Liang JN, Reddy VN, Szweda LI, Shang F. Ubiquitin-dependent lysosomal degradation of the HNE-modified proteins in lens epithelial cells. Faseb J. 2004;18:1424–1426. doi: 10.1096/fj.04-1743fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Cecarini V, Ding Q, Keller JN. Oxidative inactivation of the proteasome in Alzheimer's disease. Free Radic Res. 2007;41:673–680. doi: 10.1080/10715760701286159. [DOI] [PubMed] [Google Scholar]
- [225].Dasuri K, Nguyen A, Zhang L, Sun Fernandez-Kim O, Bruce-Keller AJ, Blalock BA, de Cabo R, Keller JN. Comparison of rat liver and brain proteasomes for oxidative stress-induced inactivation: Influence of ageing and dietary restriction. Free Radic Res. 2008:1–9. doi: 10.1080/10715760802534812. [DOI] [PMC free article] [PubMed] [Google Scholar]