Abstract
Introduction
Protein profiles of endoscopically collected pancreatic juice from normal, chronic pancreatitis patients and pancreatic cancer patients were compared to identify diagnostic biomarkers of pancreatic cancer.
Methods
Secretin was injected intravenously and pancreatic juice was collected via selective cannulation of the pancreatic duct during endoscopic retrograde cholangiopancreatography. Pancreatic juices consisting of three pooled samples for normal control, chronic pancreatitis, and pancreatic cancer patients were compared using two-dimensional gel electrophoresis, and the proteins were subsequently identified using MALDI-TOF–MS.
Results
Thirty-five protein spots were up-regulated twofold in pancreatic cancer compared with the levels in the normal controls, and 85 protein spots were present in pancreatic cancer samples but not in normal controls. After excluding spots that were also expressed in chronic pancreatitis, 26 protein spots that were up-regulated or only expressed in pancreatic cancer samples were identified. Among the identified proteins, we confirmed the expressions of BIG2, PRDX6, and REG1α in pancreatic cancer tissue using immunohistochemistry. ELISA showed that the serum level of REG1α was significantly higher in patients with pancreatic cancer than it was in the normal controls (P = 0.023). With the best cut-off value, the sensitivity and specificity of REG1α to differentiate normal and pancreatic cancer were 82.6 and 81.8%, compared with 69.6 and 100% for CA19-9.
Conclusions
We have shown that pancreatic juice is a good source of pancreatic cancer tumor markers. Further studies are needed to determine the clinical implications of REG1α and other markers.
Keywords: Pancreatic cancer, Pancreatic juice, Biomarker
Introduction
Pancreatic cancer is a lethal cancer because it is usually diagnosed at an advanced stage and resistant to treatment. Over the years, a tremendous amount of effort has been put into improving the survival outcome of pancreatic cancer patients, and we now know more about the process of pancreatic carcinogenesis. However, the identification of clinically applicable potential targets for diagnosis and treatment among multiple accumulated genetic and molecular changes remains to be carried out (Shen et al. 2004).
A high-throughput search using proteomic analysis has been suggested as a promising tool for the identification of such targets (Yuan et al. 2002). Three types of specimens—pancreatic tissues, pancreatic juice, and serum—are available for such analysis (Chen et al. 2005). Pancreatic cancer tissue contains biomarkers in the highest concentration, and several proteins such as S100A6, annexin A4, cyclophilin A, cathepsin D, galectin-1, 14-3-3ζ α-enolase, peroxiredoxin I, TM2, and S100A8 are found to be upregulated in pancreatic cancer tissue (Shen et al. 2004). However, pancreatic cancer tissue is difficult to procure, and these proteins cannot be used as markers in the absence of additional preparation. Serum has the best accessibility and is an ideal source of biomarkers. However, due to abundant proteins such as albumin and immunoglobulins, low abundant biomarkers are difficult to identify. For these reasons, pancreatic juice may be the optimal research specimen for pancreatic cancer because it probably has a higher concentration of cancer-associated proteins than does serum along with a better accessibility than pancreatic cancer tissue (Gronborg et al. 2004; Thongboonkerd 2007). Previous studies already reported some biomarkers in the pancreatic juice of pancreatic cancer such as hepatocarcinoma-intestine-pancreas/pancreatitis-associated protein and lipocalin 2 (Gronborg et al. 2004).
There are several ways to collect pancreatic juice. One method is the collection of pancreatic juice during surgery (Gronborg et al. 2004). However, this method negated the advantage of using pancreatic juice and was not much different from conducting proteomic analysis with pancreatic cancer tissue. Recently, few studies have reported the collection of pancreatic juice via endoscopy, which is less invasive than surgery. Collecting pancreatic juice also enables collecting pancreatic juice from normal and pancreatitis patients. However, pancreatic juice collected during endoscopy can be contaminated with blood or bile, which may affect the protein profiles (Zhou et al. 2007; Tian et al. 2008). Moreover, our previous experience indicated that the contrast medium, which was used during endoscopy to identify the pancreatic duct and insert catheter to collect pancreatic juice, could interfere with electrophoresis.
In this study, we collected pancreatic juice, without the use of contrast medium, by simply inserting a catheter into the main pancreatic duct using a guidewire. In addition, secretin was used to gush out proteins that had collected in the main pancreatic duct and its branches (Zhou et al. 2007). This method allowed for the collection of a large amount of non-contaminated pancreatic juice. By comparing the protein profiles of pancreatic juices from normal, chronic pancreatitis, and pancreatic cancer patients, we aimed to identify diagnostic biomarkers of pancreatic cancer.
Patients and methods
Pancreatic juice was collected during endoscopic retrograde cholangiopancreatography (ERCP). After positioning an endoscope at the second portion of the duodenum, a guidewire was inserted into the main pancreatic duct under fluoroscopy. A catheter was gently inserted along the guidewire and placed deeply in the neck portion of the pancreas and over the stricture to avoid contamination with bile and body fluid. After placing the catheter within the main pancreatic duct, secretin (1U/Kg; ChiRhoClin, Inc, MD, USA) was slowly injected, and pancreatic juice was gently aspirated with the catheter. The initial 2 ml of pancreatic juice was immediately treated with 5 μg/ml protease inhibitor (Complete Protease Inhibitor Tablets, Roche, IN, USA) and 30 μg/ml soybean trypsin inhibitor (Roche, IN, USA). Cell debris was removed via centrifugation at 12,000 rpm for 10 min at 4°C, and the supernatant was stored at −80°C until analysis.
Pancreatic juice was collected from five patients with pancreatic cancer, three patients with chronic pancreatitis, and three normal patients. Normal patients underwent ERCP for recurred ampullar adenoma or remnant cholelithiasis surveillance. The diagnosis of pancreatic cancer was confirmed pathologically, and chronic pancreatitis and normal control were confirmed clinically. Written informed consent was obtained from all subjects for the use of their pancreatic juice samples.
Three pancreatic juice samples composed of three pooled samples for the normal control, chronic pancreatitis, and pancreatic cancer groups were prepared, each containing a total of 1.5 mg protein. Desalting was achieved through TCA/acetone precipitation. Trichloroacetic acid (TCA, 50% (w/v); Sigma, T9159) was added to a final TCA concentration of 5–8%. The samples were mixed gently by inverting the tube 5–6 times and were incubated on ice for 2 h. Samples were centrifuged at 14,000×g for 15 min, and the supernatant was discarded. The pellet was resuspended in 200 μl cold acetone, incubated on ice for 15 min, and centrifuged at 14,000×g for 20 min. After discarding the acetone, and the pellet was air dried and then dissolved in 200 μl sample buffer and stored at −80°C until use in 2-DE analysis (Na et al. 2009).
For electrophoresis, 1.0 mg protein was diluted with sample buffer and carrier ampholyte was added. Next, 1.0 mg protein was rehydrated and isoelectrically focused on an 18-cm Immobiline Drystrip pH 3-10NL (GE Healthcare). The IPG strips were reduced and alkylated in equilibration buffer and two-dimensionally separated using SDS–PAGE (9–17%). The gels were stained with CBB solution overnight, and scanned using a GS710 scanning densitometer (Bio-Rad, Hemel Hempstead, UK). The gel images were analyzed using Image Master Platimun 5 (GE Healthcare). A spot was accepted as statistically significant if the P value was <0.05, and a cut-off ratio greater than 2.0-fold was established for differentially expressed spots.
Two-dimensional electrophoresis (2-DE) spots selected for analysis with matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF–MS) were excised manually from CBB-stained preparative gel, destained, and then digested with trypsin (Promega, Southampton, UK). Tryptic peptides were desalted and purified using a mixture of Poros R2 and Oligo R3, as described previously (Na et al. 2009). The MS spectra of peptides were generated via spectrometric analysis using a 4800 MALDI-TOF/TOF analyzer (Applied Biosystems, Foster City, CA, USA) in the reflectron/delayed extraction mode with an accelerating voltage of 20 kV, with data summed from 500 laser pulses. The spectrum was calibrated against the tryptic auto-digested peaks (m/z 842.5090 and 2211.1046), and monoisotopic peptide masses were obtained with Data Explorer 3.5 (PerSeptive Biosystems). A mass range of m/z 800–4,000 was used with 1,000 shots per spectrum. For MALDI-TOF–MS, GPS 3.1 software (Applied Biosystems) was used for peak generation. MASCOT (Matrix Science, Boston, MA) was used to identify peptide sequences present in the protein sequence database (NCBInr) (Na et al. 2009).
Immunostaining was performed on human pancreatic tissue using standard procedures with antibody to brefeldin A-inhibited guanine nucleotide-exchange protein 2 (BIG2, Abcam Plc, MA, USA), regenerating islet-derived 1 alpha (REG1α, Abcam Plc, MA, USA), and peroxiredoxin 6 (PRDX6, Santa Cruz Biotechnology, Inc, CA, USA). Antibodies to BIG2 and REG1α were rabbit polyclonal antibodies used at dilutions of 1:1,000 and 1:500, respectively, and the antibody to PRDX6 was a mouse monoclonal antibody used at a dilution of 1:1,000. The slides were deparaffinized in xylene and rehydrated in graded alcohol. Endogenous peroxidase activity was blocked with methanol containing 0.3% hydrogen peroxide at room temperature for 10 min. Microwave antigen retrieval was performed in citrate buffer (0.01 M, pH 6.0) for 3 min. Slides were then incubated with 10% normal donkey serum for 1 h to reduce background non-specific staining. Blocked sections were incubated in primary antibody at 4°C overnight. The sections were incubated with EnVision/HPR, Rabbit/Mouse (DakoCytomation, CA, USA) and diaminobenzidine (DAB+) chromogen. The sections were counterstained with hematoxylin, dehydrated, and then mounted.
Serum levels of REG1 were measured using standard sandwich ELISA with mouse monoclonal antibody and rabbit polyclonal antibody to REG1α (AbBcam, MA, USA). A 96-well immuno-module microplate (MAXISORP, Nalgen Nunc International, Rochester, NY, USA) was precoated with monoclonal antibody (mAb) to REG1α (clone 1A4; Abcam) through incubation overnight at 4°C, followed by blocking for 2 h at room temperature. A 1/20 dilution of serum was added to the precoated assay plate. After incubation for 3 h, a polyclonal antibody to REG1α was added. Finally, a 5000-fold dilution of horseradish peroxidase (HRP)-labeled anti-rabbit IgG (Santacruz) was added, and the reaction was allowed to proceed for 1 h. After five washes, TMB substrate solution (KPL, Gaithersburg, Maryland, USA) was added to the wells and allowed to react within 15 min. The reaction was measured using a photometer at a wavelength of 450 nm and a reference wavelength of 620 nm. For the calibration curve, we used recombinant REG1α (GenWay Biotech, CA, USA), which was serially diluted from 4,000 to 10 ng/ml in a 96-well microplate and assayed using ELISA (Takayama et al. 2010).
Serum REG1α levels in normal and pancreatic cancer patients were measured using ELISA as described above. Written informed consent was obtained from all subjects and/or guardians for the use of their blood samples. Chronic pancreatitis and pancreatic cancer were diagnosed clinically and pathologically, respectively. The serum lipase level at the time of sampling was less than two times the upper normal limit. For comparison, serum CA19-9 level was measured with a commercial immunochemiluminescence kit (VITROS® ECiQ Immunodiagnostic System, Ortho Clinical Diagnostics) that can measure concentrations as great as 20,000 U/ml. The normal range of CA19-9 was 0.0–37.0 U/ml.
The intensities of the immunohistochemical reactions of BIG2, REG1, and PRDX6 were compared between normal and cancerous tissue samples using the Chi-square test. Serum REG1α and CA19-9 levels were compared between normal and pancreatic cancer patients using student’s t test. Receiver operating characteristic (ROC) curves were used to assess the diagnostic abilities of REG1 and CA19-9. Values of P < 0.05 were conserved significant. All statistical analyses were performed using SPSS for Windows version 12.0 (SPSS, IL, USA).
Results
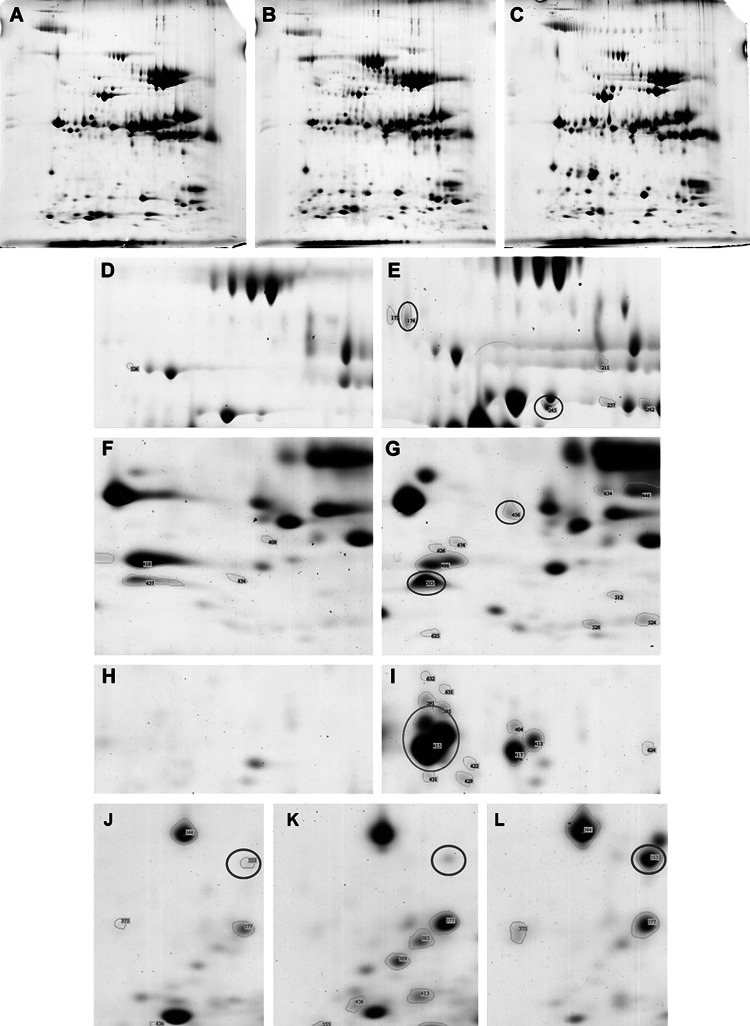
A total of 202, 251, and 210 protein spots were noted in the pooled pancreatic juice samples from normal control, chronic pancreatitis, and pancreatic cancer patients, respectively. In pancreatic cancer samples, 35 protein spots were up-regulated and 21 were down-regulated twofold compared with those of the normal controls. Thirty-eight protein spots were noted in pancreatic cancer but not in the controls. Among spots that were up-regulated or expressed only in pancreatic cancer, we excluded protein spots that were also up-regulated or only expressed in chronic pancreatitis compared with those of the normal control. This resulted in 17 protein spots that were present only in the pancreatic juice of pancreatic cancer patients (Table 1), and nine protein spots that showed stronger intensity in the pancreatic juice of pancreatic cancer patients compared with those in chronic pancreatitis and normal control patients (Table 2). Thus, a total of 26 protein spots were identified as pancreatic cancer-associated proteins (Fig. 1). Selected protein spots were further identified with MALDI-TOF–MS. Among the identified proteins were well-known pancreatic juice proteins such as REG1α (also known as lithostatine and pancreatic stone protein) and procarboxypeptidase as well as newly identified proteins such as BIG2 and PRDX6.
Table 1.
Protein spots present only in pancreatic juice from pancreatic cancer patients
| Spot number | Accession | Protein | % vol | Protein score |
|---|---|---|---|---|
| 174 | gi|5052123 | Brefeldin A-inhibited guanine nucleotide-exchange protein 2 | 0.079 | 62 |
| 245 | gi|157830140 | Chain A, human procarboxypeptidase A2 | 0.051 | 93 |
| 343 | gi|809185 | Annexin V | 0.050 | 159 |
| 357 | gi|119610226 | Fructosamine 3 kinase, isoform CRA_b | 0.046 | 61 |
| 391 | gi|38014595 | RRBP1 protein | 0.073 | 67 |
| 395 | gi|38648808 | RNF20 protein | 0.075 | 63 |
| 402 | gi|169204274 | PREDICTED: hypothetical protein | 0.221 | 66 |
| 404 | gi|181402 | Epidermal cytokeratin 2 | 0.044 | 82 |
| 413 | gi|15637419 | Anti-pneumococcal capsular polysaccharide immunoglobulin heavy chain variable region | 0.243 | 73 |
| 415 | gi|119619983 | Regenerating islet-derived 1 alpha | 3.606 | 55 |
| 417 | gi|119599697 | hCG20001, isoform CRA_c | 0.462 | 69 |
| 456 | gi|41322912 | Plectin isoform 1f | 0.062 | 71 |
| 468 | gi|16552261 | Unnamed protein product | 0.079 | 68 |
| 486 | gi|7021040 | Unnamed protein product | 0.561 | 51 |
| 503 | gi|4758638 | Peroxiredoxin 6 | 0.444 | 88 |
| 524 | gi|55669910 | Chain A, crystal structure of the Ga module complexed with human serum albumin | 0.069 | 121 |
| 634 | gi|17986258 | Myosin, light chain 6, alkali, smooth muscle and non-muscle isoform 1 | 0.086 | 73 |
Table 2.
Protein spots more intensely expressed in pancreatic juice from pancreatic cancer patients
| Spot number | Accession | Protein | Cancer (% vol) | Normal (% vol) | Fold increase (cancer/normal) | Protein score |
|---|---|---|---|---|---|---|
| 191 | gi|157830140 | Chain A, human procarboxypeptidase A2 | 0.114 | 0.021 | 5.43 | 106 |
| 194 | gi|21465928 | Chain A, human procarboxypeptidase B | 2.436 | 0.228 | 10.68 | 75 |
| 204 | gi|21465928 | Chain A, human procarboxypeptidase B | 0.154 | 0.022 | 7 | 121 |
| 253 | gi|5931600 | Pyrophosphatase | 0.819 | 0.257 | 3.19 | 65 |
| 263 | gi|9955255 | Chain A, crystal structure of the complex between the leech carboxypeptidase inhibitor | 0.508 | 0.167 | 3.04 | 67 |
| 353 | gi|4416404 | Nebulin | 0.358 | 0.019 | 18.84 | 66 |
| 363 | gi|56204475 | Glyceronephosphate O-acyltransferase 0.249 | 0.661 | 0.319 | 2.07 | 45 |
| 372 | gi|1346343 | Keratin, type II cytoskeletal 1 | 0.249 | 0.058 | 4.29 | 90 |
| 546 | gi|12407383 | Tripartite motif protein TRIM5 isoform beta | 0.872 | 0.127 | 6.87 | 68 |
Fig. 1.
Representative two-dimensional electrophoresis gel images of pancreatic juice proteins from normal control (a), chronic pancreatitis (b), and pancreatic cancer (c) patients. Protein spots for BIG2 (blue circle) and PCPA2 (red circle) in normal control (d) and pancreatic cancer (e) samples. Protein spots for plectin (blue circle) and PRDX6 (red circle) in normal control (f) and pancreatic cancer (g) samples. Protein spots for REG1α (red circle) in normal control (h) and pancreatic cancer (i) samples. Protein spots for nebulin (red circle) in normal control (j), chronic pancreatitis (k), and pancreatic cancer (l) samples
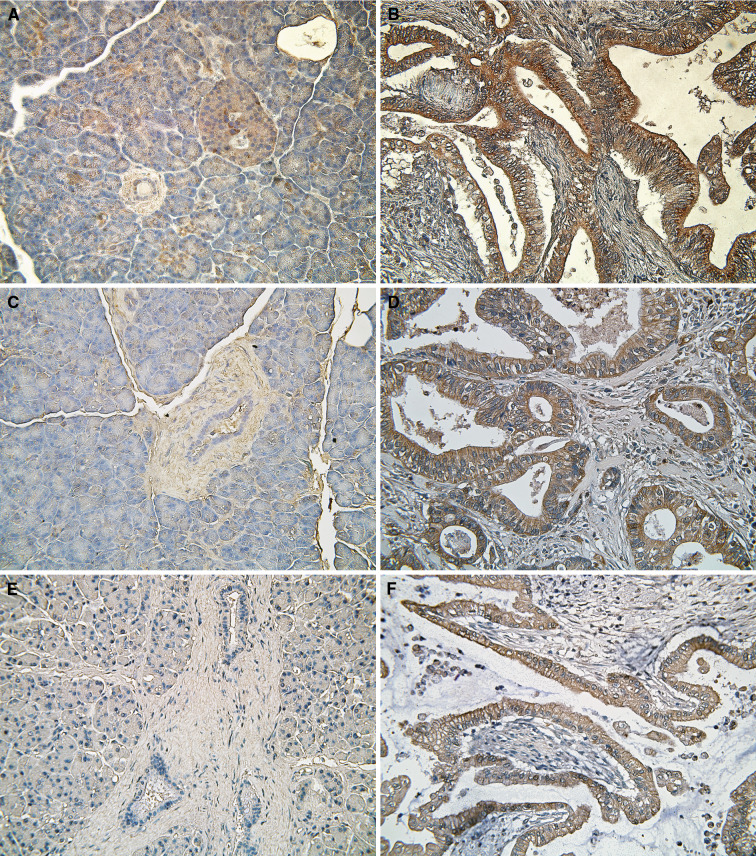
Immunohistochemistry was performed on 22 samples of pancreatic cancer tissue and 11 normal pancreatic samples. All of the tissues were confirmed as cancer or normal prior to the immunohistochemistry. Staining for BIG2 was weak in normal islet cells, heterogeneously weak in normal acinar cells, and focally weak in normal ductal cells. However, cancer cells showed very strong BIG2 staining (
Fig. 2a, b), especially in the luminal cytoplasm where the cells were ductal shaped. Fifteen (83.3%) of 18 cancer tissues showed strong BIG2 expression compared with three of nine (33.3%) normal tissues. PRDX6 was almost undetectable in normal pancreatic cells. In six (66.7%) of nine normal pancreas tissues, there was no PRDX6 expression in normal islet, acinar, or ductal cells. In contrast, only three (20.0%) of 15 cancer tissues had no PRDX6 expression. In cancer cells, expression of PRDX6 was particularly strong along the membrane (Fig. 2c, d). REG1α expression was either not detected or was weak in normal pancreas acinar, ductal, and islet cells. However, REG1α showed strong expression in most of the cancer cells; 13 (68.4%) of 19 cancer tissues showed strong REG1α expression, especially in the cytoplasm and along the cancer cell membrane (Fig. 2e, f). Expressions of BIG2, PRDX6, and REG1α were both stronger and more frequently observed in cancer cells than in normal pancreas cells (Table 3, P = 0.018, 0.039, and 0.001, respectively).
Fig. 2.
Immunohistochemical analysis of the expressions of BIG2, PRDX6, and REG1α in normal pancreas and pancreatic cancer tissues. BIG2, PRDX6, and REG1α are not expressed or only weakly expressed in normal pancreatic tissues (a, c, and e, respectively) but are strongly expressed in pancreatic cancer tissues (b, d, and f, respectively)
Table 3.
Immunohistochemistry
| Staining intensity | P value* | ||||
|---|---|---|---|---|---|
| None | Weak | Strong | |||
| BIG2 | Normal | 2 (22.2%) | 4 (44.4%) | 3 (33.3%) | 0.018 |
| Cancer | 0 (0%) | 3 (16.7%) | 15 (83.3%) | ||
| PRDX6 | Normal | 6 (66.7%) | 3 (33.3%) | 0 (0%) | 0.039 |
| Cancer | 3 (20.0%) | 7 (46.7%) | 5 (33.3%) | ||
| REG1α | Normal | 5 (55.6%) | 4 (44.4%) | 0 (0%) | 0.001 |
| Cancer | 1 (5.3%) | 5 (26.3%) | 13 (68.4%) | ||
* Chi-square test
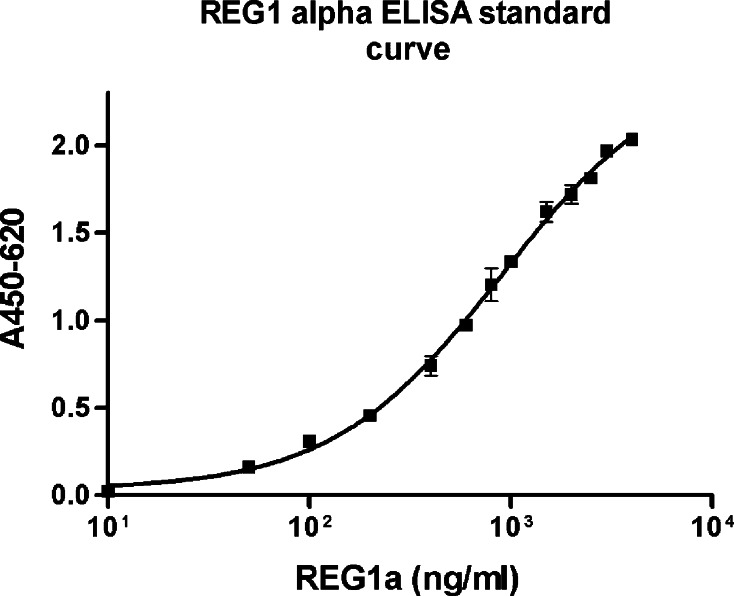
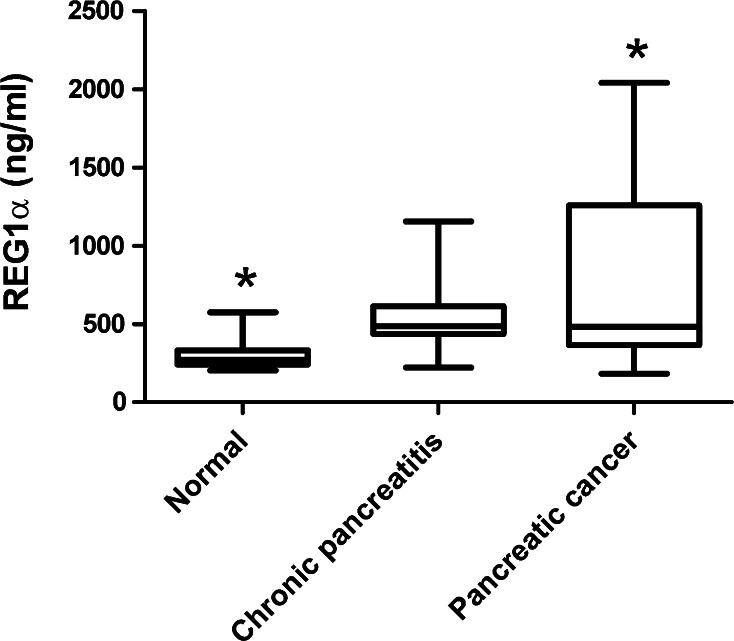
The detection limit of ELISA was 4000 ng/ml. Linear range was 0.68–2904.02 ng/ml (Fig. 3). R 2 was 0.9799. Serum samples from nine normal subjects, eight patients with chronic pancreatitis, and 23 patients with pancreatic cancer were used for ELISA. Median serum REG1α levels in normal, chronic pancreatitis, and pancreatic cancer were 272.38 (range: 203.41–574.66 ng/ml), 470.47 (range: 222.15–1155.47 ng/ml), and 481.73 (181.86–2040.48 ng/ml), respectively. An ANOVA test revealed that the difference between serum REG1α levels in normal and pancreatic cancer patients was significant (P = 0.023) (Fig. 4). Median serum CA19-9 levels in normal, chronic pancreatitis, and pancreatic cancer were 6.50 (range: 3.40–15.40 ng/ml), 17.40 (range: 2.60–113.00 ng/ml), and 1,440.00 (range: 4.20–20,000 ng/ml), respectively, with serum CA19-9 levels in normal and pancreatic cancer patients tending toward a significant difference (P = 0.066). The area under the curve was calculated using a ROC curve to evaluate the differentiation of serum REG1α and CA19-9 levels between non-cancer (normal and chronic pancreatitis) and pancreatic cancer samples. For REG1α, the area under the curve was 0.669 (95% confidence interval: 0.503–0.835), and that of CA19-9 was 0.819 (95% confidence interval: 0.689–0.949) (Fig. 5a). With the best cut-off value of 327.97 ng/ml, the sensitivity and specificity of REG1α to differentiate pancreatic cancer from other groups were 82.6 and 47.4%. For CA19-9, the sensitivity and specificity were 69.6 and 89.5% at a cut-off value of 37 U/ml. When we excluded chronic pancreatitis, the areas under the curve for serum REG1α and CA19-9 were 0.771 (95% confidence interval: 0.606–0.936) and 0.885 (95% confidence interval: 0.774–0.997), respectively (Fig. 5b). With a best cut-off value of 335.12 ng/ml, the sensitivity and specificity for differentiating normal and pancreatic cancer were 82.6 and 81.8% for REG1α and 69.6 and 100% for CA19-9, respectively.
Fig. 3.
The standard curve of ELISA for REG1α
Fig. 4.
Serum REG1α levels in normal control and pancreatic cancer samples. Serum REG1α level was significantly higher in patients with pancreatic cancer than in normal control samples. *Student’s t test, P = 0.014
Fig. 5.
Receiver operating characteristic curves of REG1α and CA19-9. Dotted and straight lines represent CA19-9 and REG1α, respectively. a Pancreatic cancer versus normal control and chronic pancreatitis samples. The areas under the curve for REG1 and CA19-9 were 0.669 and 0.819, respectively. b Pancreatic cancer versus normal control samples. The areas under the curve for REG1 and CA19-9 were 0.771 and 0.885, respectively. Dotted and straight lines represent CA19-9 and REG1α, respectively
Discussion
Since the first report by Gronborg et al. (2004), there have been several attempts to find useful biomarkers in pancreatic juice (Gronborg et al. 2004; Rosty et al. 2002; Zhou et al. 2007). A high concentration of cancer-specific proteins are present in pancreatic juice since pancreatic cancer usually originates from the pancreatic duct, from which pancreatic juice can be collected. Pancreatic juice levels of CA19-9 and CEA, only proven to be useful marker in pancreatic cancer, are much higher than in serum (Kawai et al. 2004). Hepatocarcinoma-intestine-pancreas/pancreatitis-associated protein I, lipocalin 2, tumor rejection antigen (pg96), azurocidin, serine proteinase-2 (PRSS2) preproprotein, and pancreatic lipase-related protein-1 (PLRP1) are some of proteins identified in the pancreatic juice of pancreatic cancer (Gronborg et al. 2004; Gao et al. 2010). Our study also demonstrated that pancreatic juice is a good source of biomarkers for pancreatic cancer.
Even though pancreatic juice is theoretically an ideal source of cancer biomarkers; however, this fluid in reality is not easily collected. A few studies have attempted to collect pancreatic juice endoscopically, but the method was crude and technically difficult (Berthelemy et al. 1995; Zhou et al. 2007; Tian et al. 2008). Avoiding contamination with blood and bile and collecting a sufficient amount of juice have been key issues. We aimed to achieve better results by improving several aspects of the collection procedure. First, the catheter was placed deep within the pancreatic duct above the stricture, if one was present. This allowed for collection of pancreatic juice that had been contaminated minimally with bile or body fluid. Second, the catheter was inserted at the outset of the procedure to avoid contamination via inflamed, damaged pancreatic and bile ducts. Third, the catheter was placed in the absence of contrast medium, which contains a very high concentration of salt and interferes with electrophoresis. Instead, we used a guidewire to direct the catheter, the use of which also minimized pancreatic duct injury and juice contamination. Finally, secretin was used to gush out proteins accumulated within the pancreatic duct. This method was very successful in collecting a large amount of pancreatic juice in a short period of time (Zhou et al. 2007). As a result, if a catheter was able to be placed at the pancreatic duct, good-quality pancreatic juice could be collected in more than 70% of the cases.
The pancreatic juice analyzed in this study contained various isoforms of procarboxypeptidase, which were activated by trypsin to carboxypeptidase. Previous studies have investigated procarboxypeptidase as a biomarker for pancreatic cancer and found that the serum level of procarboxypeptidase was significantly higher in cancer patients than in normal controls, possibly due to injury to the acinar cells. Paradoxically, in more advanced pancreatic cancer, the serum level of procarboxypeptidase decreased. Since procarboxypeptidase is secreted by acinar cells and not by cancer cells, the level of this enzyme in pancreatic juice is affected by various confounding factors, complicating the interpretation of its significance (Shamamian et al. 2006). Nebulin, a very large protein (600–800 kDa) and a component of myofibrillar protein, was also strongly expressed in the pancreatic juice of pancreatic cancer patients (Stedman et al. 1988). Plectin was another large protein identified in pancreatic juice and was recently detected in various cancers (Lee et al. 2004; Bausch et al. 2009). Both of these proteins were detected in the low-molecular-weight range, suggesting that the tested pancreatic juice probably contained fragments of these proteins. Interestingly, many proteins with unidentified function were also noted in the pancreatic juice of pancreatic cancer, and more researches are needed to identify their implications.
Among many proteins identified by proteomic analysis, we confirmed that BIG2, PRDX6, and REG1α were expressed in pancreatic cancer tissue using immunohistochemistry. BIG2 has a molecular weight of 190 KDa and plays a role in membrane trafficking (Yamaji et al. 2000). It promotes that activation of ADP-ribosylation factor, which might be involved in the invasion and metastasis of cancer cells (Boulay et al. 2008). PRDX6 is a member of the perodiredoxin family of proteins that degrade hydrogen peroxide and alkyl hydroperoxides. Several recent studies have reported that this family of protein is found in various cancers (Karihtala et al. 2003; Lee et al. 2002; Lehtonen et al. 2004). PRDX6 seems to play a role in cancer invasion and chemoresistance (Lee et al. 2009; Pak et al. 2010).
Of these proteins, we measured the serum level of REG1α with ELISA. REG protein is also known as protein stone protein and lithostathine. The reg gene family is a multigene family grouped into four subclasses, types I, II, III, and IV (Legoffic et al. 2009; Okamoto 1999). Among them, REG1α plays a role in cellular differentiation of the pancreas and is mitogenic to ductal and beta cells (Sanchez et al. 2009). It also has been detected in cholangiocarcinoma, gastric cancer, and pancreatic cancers (Harada et al. 2001; Zhou et al. 2010; Sekikawa et al. 2005). In the pancreas, REG1α was known to stimulate the growth of pancreatic β cell, but recently it was also found that REG1α secreted from cancer cell could accelerate tumor progression (Zhou et al. 2010). Gronborg et al. reported that pancreatitis-associated protein 2 was increased in the pancreatic juice of pancreatic cancer, and pancreatitis-associated protein 2 had sequence similar to REG1α (Gronborg et al. 2004). We showed the serum level of REG1α was higher in pancreatic cancer patients than in normal control and compared with CA19-9, and the overall performance of REG1α was similar, although its sensitivity was higher. In two cases with an initial diagnosis of chronic pancreatitis that was later changed to pancreatic cancer, the CA19-9 level was normal, but the REG1α level was >1,000 ng/ml. There is a report that the expression of REG1α was associated with tumor size and diabetes mellitus (Zhou et al. 2010), but in our study, the serum REG1α level was not associated with both factors. We could not include a lot of samples to confirm actual clinical usefulness of REG1α. Since various conditions such as previous ERCP and pancreatitis might affect the serum level of REG1α (Hayakawa et al. 1993; Bluth et al. 2006), the measurement of serum REG1α in a large population consisting of various pancreatic diseases and conditions is warranted for further study.
Through proteomic analysis, we have shown that pancreatic juice may be a good source of pancreatic cancer tumor markers. Proteomic analysis of pancreatic juice may identify markers for the early diagnosis of pancreatic cancer and possibly provide new therapeutic targets. Further studies are needed to determine the clinical implications of REG1α and other markers and find additional biomarkers in pancreatic juice.
Acknowledgments
This research was supported by the Intelligent Microsystem Center, one of the 21st Century’s Frontier R&D Projects sponsored by the Korea Ministry of Knowledge Economy. This study was also supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea (A030003).
Conflict of interest
We declare that we have no conflict of interest.
References
- Bausch D, Mino-Kenudson M, Castillo CF-D, Warshaw AL, Kelly KA, Thayer SP (2009) Plectin-1 is a biomarker of malignant pancreatic intraductal papillary mucinous neoplasms. J Gastrointest Surg 13(11):1948–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthelemy P, Bouisson M, Escourrou J, Vaysse N, Rumeau JL, Pradayrol L (1995) Identification of K-ras mutations in pancreatic juice in the early diagnosis of pancreatic cancer. Ann Intern Med 123(3):188–191 [DOI] [PubMed] [Google Scholar]
- Bluth MH, Patel SA, Dieckgraefe BK, Okamoto H, Zenilman ME (2006) Pancreatic regenerating protein (reg I) and reg I receptor mRNA are upregulated in rat pancreas after induction of acute pancreatitis. World J Gastroenterol 12(28):4511–4516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulay PL, Cotton M, Melancon P, Claing A (2008) ADP-ribosylation factor 1 controls the activation of the phosphatidylinositol 3-kinase pathway to regulate epidermal growth factor-dependent growth and migration of breast cancer cells. J Biol Chem 283(52):36425–36434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Pan S, Brentnall TA, Aebersold R (2005) Proteomic profiling of pancreatic cancer for biomarker discovery. Mol Cell Proteomics 4(4):523–533 [DOI] [PubMed] [Google Scholar]
- Gao J, Zhu F, Lv S, Li Z, Ling Z, Gong Y, Jie C, Ma L (2010) Identification of pancreatic juice proteins as biomarkers of pancreatic cancer. Oncol Rep 23(6):1683–1692 [DOI] [PubMed] [Google Scholar]
- Gronborg M, Bunkenborg J, Kristiansen TZ, Jensen ON, Yeo CJ, Hruban RH, Maitra A, Goggins MG, Pandey A (2004) Comprehensive proteomic analysis of human pancreatic juice. J Proteome Res 3(5):1042–1055 [DOI] [PubMed] [Google Scholar]
- Harada K, Zen Y, Kanemori Y, Chen TC, Chen MF, Yeh TS, Jan YY, Masuda S, Nimura Y, Takasawa S, Okamoto H, Nakanuma Y (2001) Human REG I gene is up-regulated in intrahepatic cholangiocarcinoma and its precursor lesions. Hepatology 33(5):1036–1042 [DOI] [PubMed] [Google Scholar]
- Hayakawa T, Kondo T, Shibata T, Kitagawa M, Sakai Y, Sobajima H, Tanikawa M, Nakae Y, Hayakawa S, Katsuzaki T et al (1993) Serum pancreatic stone protein in pancreatic diseases. Int J Pancreatol 13(2):97–103 [DOI] [PubMed] [Google Scholar]
- Karihtala P, Mantyniemi A, Kang SW, Kinnula VL, Soini Y (2003) Peroxiredoxins in breast carcinoma. Clin Cancer Res 9(9):3418–3424 [PubMed] [Google Scholar]
- Kawai M, Uchiyama K, Tani M, Onishi H, Kinoshita H, Ueno M, Hama T, Yamaue H (2004) Clinicopathological features of malignant intraductal papillary mucinous tumors of the pancreas: the differential diagnosis from benign entities. Arch Surg 139(2):188–192 [DOI] [PubMed] [Google Scholar]
- Lee JB, Yun SJ, Chae HZ, Won YH, Kim YP, Lee SC (2002) Expression of peroxiredoxin and thioredoxin in dermatological disorders. Br J Dermatol 146(4):710–712 [DOI] [PubMed] [Google Scholar]
- Lee KY, Liu YH, Ho CC, Pei RJ, Yeh KT, Cheng CC, Lai YS (2004) An early evaluation of malignant tendency with plectin expression in human colorectal adenoma and adenocarcinoma. J Med 35(1–6):141–149 [PubMed] [Google Scholar]
- Lee SB, Ho JN, Yoon SH, Kang GY, Hwang SG, Um HD (2009) Peroxiredoxin 6 promotes lung cancer cell invasion by inducing urokinase-type plasminogen activator via p38 kinase, phosphoinositide 3-kinase, and Akt. Mol Cells 28(6):583–588 [DOI] [PubMed] [Google Scholar]
- Legoffic A, Calvo E, Cano C, Folch-Puy E, Barthet M, Delpero JR, Ferres-Maso M, Dagorn JC, Closa D, Iovanna J (2009) The reg4 gene, amplified in the early stages of pancreatic cancer development, is a promising therapeutic target. PLoS One 4(10):e7495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtonen ST, Svensk AM, Soini Y, Paakko P, Hirvikoski P, Kang SW, Saily M, Kinnula VL (2004) Peroxiredoxins, a novel protein family in lung cancer. Int J Cancer 111(4):514–521 [DOI] [PubMed] [Google Scholar]
- Na K, Lee EY, Lee HJ, Kim KY, Lee H, Jeong SK, Jeong AS, Cho SY, Kim SA, Song SY, Kim KS, Cho SW, Kim H, Paik YK (2009) Human plasma carboxylesterase 1, a novel serologic biomarker candidate for hepatocellular carcinoma. Proteomics 9(16):3989–3999 [DOI] [PubMed] [Google Scholar]
- Okamoto H (1999) Cyclic ADP-ribose-mediated insulin secretion and Reg, regenerating gene. J Mol Med 77(1):74–78 [DOI] [PubMed] [Google Scholar]
- Pak JH, Choi WH, Lee HM, Joo WD, Kim JH, Kim YT, Kim YM, Nam JH (2010) Peroxiredoxin 6 overexpression attenuates cisplatin-induced apoptosis in human ovarian cancer cells. Cancer Invest 29(1):21–28 [DOI] [PubMed] [Google Scholar]
- Rosty C, Christa L, Kuzdzal S, Baldwin WM, Zahurak ML, Carnot F, Chan DW, Canto M, Lillemoe KD, Cameron JL, Yeo CJ, Hruban RH, Goggins M (2002) Identification of hepatocarcinoma-intestine-pancreas/pancreatitis-associated protein I as a biomarker for pancreatic ductal adenocarcinoma by protein biochip technology. Cancer Res 62(6):1868–1875 [PubMed] [Google Scholar]
- Sanchez D, Mueller CM, Zenilman ME (2009) Pancreatic regenerating gene I and acinar cell differentiation: influence on cellular lineage. Pancreas 38(5):572–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekikawa A, Fukui H, Fujii S, Takeda J, Nanakin A, Hisatsune H, Seno H, Takasawa S, Okamoto H, Fujimori T, Chiba T (2005) REG Ialpha protein may function as a trophic and/or anti-apoptotic factor in the development of gastric cancer. Gastroenterology 128(3):642–653 [DOI] [PubMed] [Google Scholar]
- Shamamian P, Goldberg JD, Ye XY, Stewart JD, White PJ, Gilvarg C (2006) Evaluation of pro-carboxypeptidase A and carboxypeptidase A as serologic markers for adenocarcinoma of the pancreas. HPB (Oxford) 8(6):451–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Person MD, Zhu J, Abbruzzese JL, Li D (2004) Protein expression profiles in pancreatic adenocarcinoma compared with normal pancreatic tissue and tissue affected by pancreatitis as detected by two-dimensional gel electrophoresis and mass spectrometry. Cancer Res 64(24):9018–9026 [DOI] [PubMed] [Google Scholar]
- Stedman H, Browning K, Oliver N, Oronzi-Scott M, Fischbeck K, Sarkar S, Sylvester J, Schmickel R, Wang K (1988) Nebulin cDNAs detect a 25-kilobase transcript in skeletal muscle and localize to human chromosome 2. Genomics 2(1):1–7 [DOI] [PubMed] [Google Scholar]
- Takayama R, Nakagawa H, Sawaki A, Mizuno N, Kawai H, Tajika M, Yatabe Y, Matsuo K, Uehara R, Ono K, Nakamura Y, Yamao K (2010) Serum tumor antigen REG4 as a diagnostic biomarker in pancreatic ductal adenocarcinoma. J Gastroenterol 45(1):52–59 [DOI] [PubMed] [Google Scholar]
- Thongboonkerd V (2007) Proteomics of human body fluids: principles, methods, and applications. Humana Press, Totowa [Google Scholar]
- Tian M, Cui YZ, Song GH, Zong MJ, Zhou XY, Chen Y, Han JX (2008) Proteomic analysis identifies MMP-9, DJ-1 and A1BG as overexpressed proteins in pancreatic juice from pancreatic ductal adenocarcinoma patients. BMC Cancer 8:241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaji R, Adamik R, Takeda K, Togawa A, Pacheco-Rodriguez G, Ferrans VJ, Moss J, Vaughan M (2000) Identification and localization of two brefeldin A-inhibited guanine nucleotide-exchange proteins for ADP-ribosylation factors in a macromolecular complex. Proc Natl Acad Sci USA 97(6):2567–2572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Russell T, Wood G, Desiderio DM (2002) Analysis of the human lumbar cerebrospinal fluid proteome. Electrophoresis 23(7–8):1185–1196 [DOI] [PubMed] [Google Scholar]
- Zhou L, Lu Z, Yang A, Deng R, Mai C, Sang X, Faber KN, Lu X (2007) Comparative proteomic analysis of human pancreatic juice: methodological study. Proteomics 7(8):1345–1355 [DOI] [PubMed] [Google Scholar]
- Zhou L, Zhang R, Wang L, Shen S, Okamoto H, Sugawara A, Xia L, Wang X, Noguchi N, Yoshikawa T, Uruno A, Yao W, Yuan Y (2010) Upregulation of REG Ialpha accelerates tumor progression in pancreatic cancer with diabetes. Int J Cancer 127(8):1795–1803 [DOI] [PubMed] [Google Scholar]