Abstract
For many years, tonsillectomy has been used routinely in children to treat chronic or recurrent acute tonsillitis. Palatine tonsils are secondary lymphoid organs and the major barrier protecting the digestive and respiratory tracts from potential invasive microorganisms. They have been used as sources of lymphoid tissue; however, despite the hundreds of papers published on tonsillectomy, no studies addressing the functionality of the CD4+ and CD8+ T cells from chronically infected tonsils have yet been published. The aim of this study was to analyse the functionality of the CD4+ and CD8+ T cells with respect to tonsillar tissue. We used an affordable approach to measure the frequency of antigen-specific CD4+ T cells, the direct ex-vivo cytotoxicity of CD8+ T cells, memory T cell phenotype, cytokine profile and DC phenotype. Our results demonstrate that CD4+ and CD8+ T cells from tonsillar tissue are totally functional, as shown by their ability to produce cytokines, to degranulate and to differentiate into effector-memory T cells.
Keywords: CD154, cytotoxicity, DC, polyfunctional T cells, T cells
Introduction
A host's ability to defend itself against viral and microbial pathogens requires an effective innate immune response capable of rapidly recognizing and responding to those pathogens. The adaptive response involves T and B cells that recognize foreign antigens, and activate with great specificity in response to them. Most viral infections in the upper airway induce a strong T helper type 1 (Th1) immune response, which is required for efficient eradication of the organisms; however, some respiratory viruses have been associated with a deficient Th1 response and increased allergic inflammation [1],[2]. CD19+ B cells also play a role by producing neutralizing antibodies against viruses [3], while macrophages and dendritic cells (DC) contribute to the innate and adaptive immune response by producing cytokines, anti-bacterial peptides, eliminating bacteria via phagocytosis or presenting antigens to T cells [4],[5].
Currently, polychromatic flow cytometry is one of the most powerful tools for the study of the immune system, and allows us to explore specialized populations of naive, memory or central memory T cells, as well as B cells [6]. Moreover, we can identify the heterogeneous expression of the cell surface markers on DC. This characterization can be achieved only through flow cytometry, as this procedure makes it possible to analyse multiple cell surface and intracellular markers at the single cell level.
Palatine tonsils are secondary lymphoid organs and the major barrier protecting the digestive and respiratory tracts from potential invasive microorganisms. They have also been used as sources of lymphoid tissue [7]. Histologically, tonsils have a surface of stratified squamous epithelium and follicular germinal centres with a mantle zone and an interfollicular area [8]. T cells are located mainly in the extra-follicular spaces, while DC can be identified in the germinal centres as well as in the follicular area [9]. Tonsils are lymphoid organs in which T cells complete their maturation, but also identify cells that harbour pathogens.
For many years, tonsillectomy has been used routinely in children to treat chronic or recurrent acute tonsillitis [10],[11]. However, despite the hundreds of papers published on tonsillectomy, no studies have yet been published addressing the functionality of the CD4+ and CD8+ T cells from chronically infected tonsils.
The aim of this study, therefore, was to analyse CD4+ and CD8+ T cell functionality with respect to tonsillar tissue. We used an affordable approach to measure the frequency of antigen-specific CD4+ T cells, the direct ex-vivo cytotoxicity of CD8+ T cells, memory T cell phenotype, cytokine profile and DC phenotype. Our results demonstrate clearly that CD4+ and CD8+ T cells from tonsillar tissue are totally functional, as shown by their ability to produce cytokines, to degranulate and to differentiate into effector-memory T cells. Interdigitating DCs (iDC) and plasmocytoid DCs (pDC) were also identified in tonsillar tissue.
Materials and methods
Patients
After obtaining approval from the Ethics Committee and appropriate informed consent from the participants, a consecutive series of children undergoing tonsillectomy as treatment for tonsillar hypertrophy were enrolled into this study.
Monoclonal antibodies (mAbs) used for flow cytometry
For the flow cytometry panel and the lineage-specific panels, the following monoclonal antibodies were used: CD1c fluorescein isothiocyanate (FITC) (clone L161), CD3 FITC (clone HIT3a), CD3 phycoerythrin cyanin 5 (PECy5) (clone HIT3a), CD4 allophycocyanin (APC)/Cy7 (clone RPA-T4), CD8 PECy7 (clone HIT8a), CD11c FITC (clone 3·9), CD14 PECy7 (clone HCD14), CD16 FITC (clone 3G8), CD19 PECy7 (clone HIB19), CD19 FITC (clone HIB19), CD33 PE (clone WM53), CCR7 APC (clone TG8/CCR7), CD38 APC (clone HIT2), CD40 PE (clone G28·5), CD45 RA FITC (clone HI100), CD56 (NCAM) FITC (clone HCD56), CD56 (NCAM) PECy7 (clone HCD56), CD62L APC (clone DREG-56), CD107a (LAMP-1) FITC (clone 1D4B), CD123 PECy5 (clone 6H6), CD154 APC (clone 24–31) (from Biolegend, San Diego, CA, USA) CD1a PE (clone HI149), CD11c PE (clone S-HCL-3), CD19 PE (clone HIB19), CD107b FITC (clone H4B4) and IgD FITC (clone IA6-2) (from BD Bioscience, San José, CA, USA).
Cell preparation and organ culture model
Tonsils obtained by tonsillectomy were cut manually into small pieces and placed in complete RPMI-1640 medium supplemented with 10% fetal bovine serum (Gibco, Grand Island, NY, USA), 100 U/ml penicillin and 100 mg/ml streptomycin (Sigma-Aldrich, Steinheim, Germany). Next, the cells were passed through a cell strainer (40 µm; BD Falcon, Franklin Lakes, NJ, USA), and tonsillar mononuclear cells (TMCs) were isolated by the gradient centrifugation method using LymphoprepTM (Accurate Chemical-Scientific, Westbury, NY, USA). After centrifugation, TMCs were removed from the interface and cells were washed three times with sterile phosphate-buffered saline (PBS), resuspended in complete RPMI-1640 medium (Gibco), counted and adjusted at 1 × 106/ml concentration. Using trypan blue exclusion, TMC viability was 95–98%. TMCs were plated in a 24-well plate in complete RPMI-1640 and incubated at 37°C in 5% CO2 for a period of 24 h before every experiment.
CD4+ antigen-specific T cell identification protocol
Under the standard in-vitro cultured conditions described above, TMCs (1 × 106/ml) were plated and stimulated in a 24-well plate for 16 h with Staphylococcal enterotoxin B (SEB) (5 µg/ml; Sigma-Aldrich, St Louis, MO, USA). CD154-allophycocyanin (APC) (10 ul/1 × 106/ml; Biolegend) was added to the cell culture prior to stimulation. Monensin (5 µg/ml; Biolegend) was added to the cell culture during the last 2 h. Optimal stimulation conditions were determined based on the expression of CD154 after stimulation with different concentrations of SEB (2·5–120 µg/ml) and after different stimulation times (4–24 h).
Direct ex-vivo cytotoxicity assay for CD8+ T cells
The TMCs (1 × 106) were incubated with SEB (5 µg/ml; Sigma-Aldrich) to activate the cells. Conjugated antibodies to the granular membrane proteins CD107a and CD107b were added to the cells prior to stimulation. In each experiment, a negative control (unstimulated cells) and isotype controls were included to control for the spontaneous expression of CD107a/b. The cultures were incubated for 4 h and brefeldin A (5 µg/ml; Biolegend) was added to the cell culture during the last 2 h. To determine the intracellular expression of perforin, cells were fixed (fixation buffer; Biolegend) and permeabilized (Perm/Wash buffer; BD Pharmingen, San José, CA, USA). After permeabilization, cells were washed twice and then stained with anti-human perforin mAb. Cells were washed and resuspended in 1% paraformaldehyde. Data were collected with a fluorescence activated cell sorter (FACS) Aria flow cytometer (V.6·1; Becton Dickinson, San José, CA, USA) and analysed with FlowJo software (version 9·1, TreeStar, Ashland, OR, USA). Typically, 500 000 events were acquired.
Short-term in-vitro stimulation
The TMCs (1 × 106/ml) were stimulated with anti-CD3 (1 µg/ml final concentration; Biolegend) and anti-CD28 (1 µg/ml final concentration; Biolegend) for 16 h and incubated at 37°C in 5% CO2. Unstimulated TMCs were used to assess non-specific/background cytokine production. Brefeldin A (Biolegend; 5 µg/ml final concentration) was added during the last 2 h. Following stimulation, the TMCs were harvested, washed with PBS, and then stained with anti-CD3, anti-CD4 and anti-CD8 mAbs (Biolegend) for 30 min at 4°C. Subsequently, the TMCs cells were fixed (fixation buffer; Biolegend) and permeabilized (Perm/Wash buffer; BD Pharmingen). After permeabilization the cells were washed twice, and then stained with anti-human interleukin (IL-2), interferon (IFN)-γ and tumour necrosis factor (TNF)-α mAbs. Cells were washed, resuspended in 1% paraformaldehyde and analysed by flow cytometry.
Multi-parametric flow cytometry
The surface phenotype of fresh TMCs was determined by flow cytometry. mAbs conjugated with FITC, PE, peridinin chlorophyll (PerCP), APC, APC-Cy7 and PE-Cy7 were used. All antibodies were titrated previously to determine optimal concentrations. A description of the different antibodies is provided in Table 1. Cells were incubated using a premixed antibody cocktail for 30 min at 4°C, washed with PBS containing 2% FBS and fixed with 1% paraformaldehyde. Data were analysed using flow cytometry.
Table 1.
Frequency of tonsillar lymphocytes.
| Population | Frequency (%) | CCR7 (MFI) |
|---|---|---|
| Lymphocyte T* | 18 ± 2·49 | |
| Lymphocyte T CD4+ | 73·9 ± 8·3 | |
| TCM* | 30·3 (29·2–54·6) | 1130 (896–1829) |
| Naive | 4·8 (2·7–8·2) | 3481 (2623–4043) |
| TEMRA | 5·7 (2·7–9) | 3295 (2786–4144) |
| TEM | 56·4 (38·9–62·4) | 1234 (916–1521) |
| Lymphocyte T CD8+ | 14·7 ± 5·2 | |
| TCM | 16·9 (6–25·3) | 1921 (1132–2740) |
| Naive | 20·0 (15·9–36·9) | 4527 (2929–5553) |
| TEMRA | 33·6 (27·3–51) | 4488 (2129–4847) |
| TEM | 33·5 (10·5–51·6) | 705 (503–826) |
| Lymphocyte B | 32·9 ± 11·3 | |
| CD19+CD38+ | 80·3 ± 3·2 | 5983 (5227–6950) |
Mean ± standard deviation
median (min–max). TN: naive T cells; TCM: central memory T cells; TEM: effector memory T cells; TEMRA: terminal effector T cells; MFI: mean fluorescence intensity.
Statistical analysis
Data were expressed as median and interquartile range (IQR) or mean ± standard deviation (s.d.). Comparisons of data among experimental conditions were performed using the non-parametric Mann–Whitney U-test and the Kruskal–Wallis test (95% confidence interval) with Dunnett's post-test comparison. Values of P < 0·05 were considered to be statistically significant. Analysis was performed using Prism version 5·0 software (GraphPad Software, Inc., San Diego, CA, USA).
Results
Study population
Sixteen children were enrolled into this study. The average age was 6·07 years (range 4–9 years). The study group comprised 12 girls and four boys.
Identification of T cells and memory T cell subsets
TMCs from 16 children were stained with multiple fluorescently conjugated monoclonal antibodies directed to CD3, CD4, CD8, CD45RA, CD62L and CCR7, in order to identify the frequency of total T cells and their state of differentiation. As shown in Table 1, the percentage of total CD3+ T cells in the tonsillar tissue was 18·0% ± 2·4 with a CD4 : CD8 ratio of 5·02. Next, based on the surface expression of the memory markers, the CD4+ and CD8+ T cells were classified as: naive T cells (TN) (CD3+ CD45RA+ CD62L+), central memory T cells (TCM) (CD3+ CD45RA-CD62L+), effector memory T cells (TEM) (CD3+ CD45RA-CD62L-) and terminal effector T cells (TEMRA) (CD3+ CD45RA+CD62L-). As shown in Fig. 1, we identified naive T cells and three memory subsets in the tonsillar tissue. The memory profile was slightly different between the CD4+ and the CD8+ T cells (Table 1). TCM and TEM were more frequent in the CD4+ T cell compartment (P = 0·005), while TN and TEMRA were more frequent in the CD8+ T cell compartment (P = 0·002). We found that many, but not all, naive CD4+ and CD8+ T cells as well as CD4+ and CD8+ TEMRA T cells expressed the homing receptor CCR7 (Table 1). These results suggest that naive and memory T cells can recirculate through T and B cell zones of tonsils where they can encounter antigens.
Fig. 1.
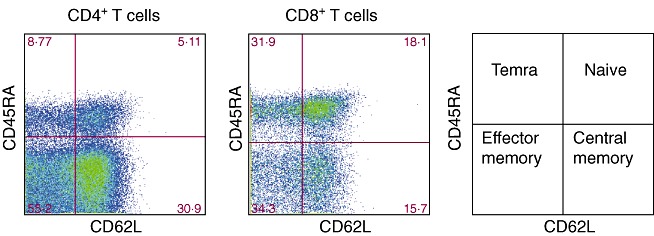
Phenotypical profile of memory CD41 and CD81 T cell subsets using multi-colour flow cytometry. Tonsillar mononuclear cells were stained with directly conjugated monoclonal antibodies against CD3, CD4, CD8, CD45RA, CD62L and CCR7 in a six-colour experiment. Cells were first identified based on side-scatter and CD3 properties, followed by gating on CD4+ or CD8+ T cells. Differential gating on CD4+ or CD8+ T cells based on CD62L and CD45RA basal expression revealed four populations: naive T cells (CD45RA+CD62L+), central memory T cells (CD45RA-CD62L+), effector memory T cells (CD45RA-CD62L-) and terminally differentiated effector memory T cells (TEMRA) (CD45RA+CD62L-). At least 105 events were analysed.
Antigen-specific CD4+ T cells in tonsillar tissue
In order to evaluate whether antigen-specific T cells can be identified in tonsillar tissue, the expression of CD154 (CD40L) on the cell surface of CD4+ T cells following stimulation with SEB was analysed. Total TMCs from 10 children undergoing tonsillectomy were stimulated with SEB and incubated in the presence of the secretion inhibitor monensin during the last 2 h. After stimulation, the cells were stained with antibodies to CD3 and CD4. When the TMCs were cultured in the medium alone, 0·69% of the CD4+ T cells expressed CD154. The percentage of expression increased when brefeldin A was added to the cell culture (5·3%), while the maximum percentage of CD154 expression was measured when both SEB and the secretion inhibitors were added (9·3%). Isotype control did not induce CD154 expression (Fig. 2).
Fig. 2.
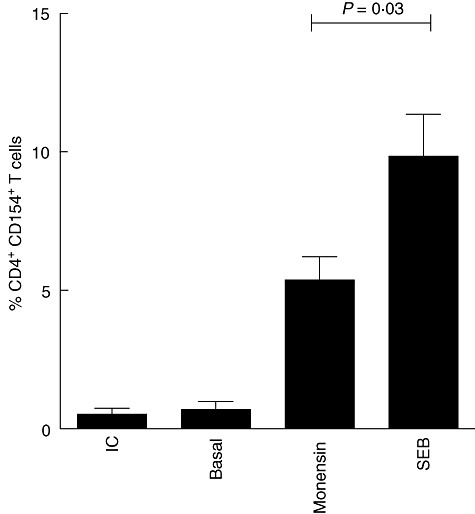
Assessment of antigen-specific CD4+ T cells according to intracellular antigen-induced CD154 expression. Comparison of CD154 expression in CD4+ T cells cultured for 16 h in the presence of medium alone, monensin alone or Staphylococcal enterotoxin B (SEB) plus monensin. The percentage of CD154-expressing CD4+ T cells is shown. The bars show mean ± standard deviation (n = 10). Analysis of variance (anova) with Bonferroni's post-test.
CD107a and b are expressed on the surface of tonsillar CD8+ T cells and are associated with the loss of intracellular perforin
To analyse the cytotoxic functionality of activated tonsillar CD8+ T cells, we examined the expression of CD107a and b on the cell surface of CD8+ T cells following activation with SEB. Total TMCs from 10 children undergoing tonsillectomy were cultured with CD107a/b mAbs, stimulated with SEB and incubated in the presence of the secretion inhibitor brefeldin A and monensin during the last 2 h. A total of 39·6% of the CD8+ T cells expressed surface CD107a/b after 4 h of stimulation (Fig. 3), indicating that degranulation had probably occurred within the responding CD8+ T cells. This initial result appeared promising, given the fact that no previous studies have shown that tonsillar CD8+ T cells have the capacity to degranulate. Perforin is released from cytotoxic granules during the degranulation process; therefore, acquisition of the cell surface markers CD107a and b on CD8+ T cells after stimulation can be associated with a loss of intracellular perforin. To address this issue, we stimulated the TMCs with SEB and performed a time–course to compare the levels of intracellular perforin with the cell surface expression of CD107a and b. As shown in Fig. 3, CD107a and b were expressed rapidly after stimulation (0·5 h), concomitant with a loss of perforin expression. After 4 h of stimulation, practically every perforin-expressing CD8+ T cell had degranulated, indicating that the expression of CD107a/b occurs as a result of degranulation, rather than de-novo production.
Fig. 3.
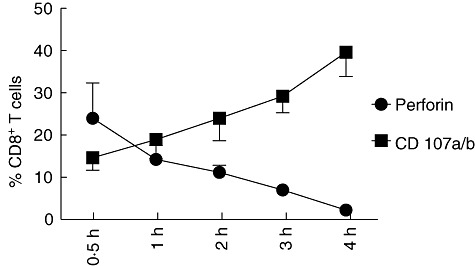
Acquisition of cell surface CD107 is correlated with a loss of intracellular perforin in CD8+ T cells. Tonsillar mononuclear cells were stimulated with Staphylococcal enterotoxin B (SEB) and incubated for up to 4 h in the presence of (a) anti-CD107 and (b) fluorescein isothiocyanate (FITC) and brefeldin. At the time-points designated on the figure, cells were removed, washed and permeabilized, followed by CD3, CD8 and perforin staining. Accumulative data of 10 independent experiments are shown. Dots are mean ± standard deviation (n = 10).
Cytokine production by polyfunctional CD4+ and CD8+ T cells
CD4+ and CD8+ T cells play a crucial role in adaptive immune responses, and a recent and widely accepted concept suggests that ‘polyfunctional’ T cells, in particular those simultaneously producing IL-2, TNF-α and IFN-γ upon antigen challenge, are a key factor in controlling viral and bacterial infections [12]–[14]. To assess whether CD4+ and CD8+ T cells from tonsillar tissue have the ability to secrete multiple cytokines simultaneously, we used a short-term stimulation assay. TMCs were stimulated for 16 h and subjected to a five-colour flow cytometric assay, as described in the Methods section. The negative control [unstimulated peripheral blood mononuclear cells (PBMCs) were stained with the same panel of mAbs] was used to identify the location of positive gates. Background < 1·0% was achieved in every staining and was subtracted from the positive results in each sample (Fig. 4a,b). We found that T cells producing more than one cytokine can be identified simultaneously within tonsillar tissue. Polyfunctional (TNF-α+, IL-2+, IFN-γ+) CD8+ T cells were significantly higher than polyfunctional CD4+ T cells (Fig. 4c). No significant differences between CD4+ and CD8+ T cells were observed for double producers (TNF-α+/IL-2+, TNF-α+/IFN-γ+, IL-2+/IFN-γ+) or single producers (TNF-α+, IL-2+ or IFN-γ+).
Fig. 4.
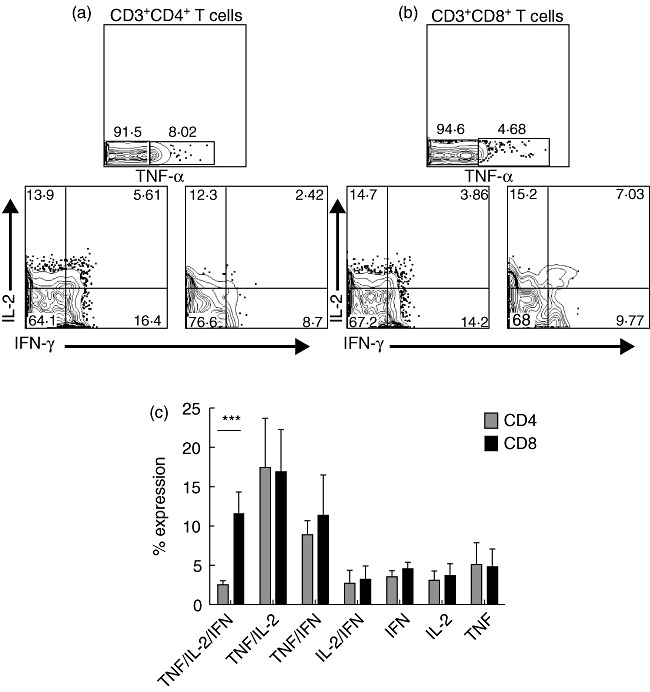
Polyfunctional cytokine-producing CD4+ and CD8+ T cells within tonsillar tissue after in-vitro stimulation. Simultaneous analysis of the functional profile of CD4+ and CD8+ T cells on the basis of interferon (IFN)-γ, interleukin (IL)-2 or tumour necrosis factor (TNF)-α production (a) and (b). Qualitative analysis of CD3+CD4+ and CD3+CD8+ T cell responses by polychromatic flow cytometry. Shown are representative flow cytometry analyses of the functional profile of CD4+ and CD8+ T cell response. Profiles are gated on live CD3+CD4+ or CD3+CD8+ T cells, and the various combinations of IFN-γ, IL-2 and TNF-α are shown following stimulation with CD3 and CD28 monoclonal antibodies (mAbs) (c). All the possible combinations of the various functions are shown on the x-axis, whereas the percentages of the distinct cytokine-producing cell subsets within CD4+ and CD8+ T cells are shown on the y-axis. Cells were gated on CD3+ lymphocytes, followed by gating on CD4+ or CD8+ cells and then analysed for cytokine production. The bars show mean ± standard deviation (n = 10). ***P < 0·0001, as calculated by the t-test.
Identification of the tonsillar DC composition
A common feature of all types of DCs is their high expression level of the human leucocyte antigen D-related (HLA-DR) molecule, including DCs in peripheral blood or DCs in the tonsillar tissue. Exclusion of Lin+ cells (CD3, CD14, CD16, CD19 and CD56) from the cytometric analysis resulted in a highly enriched fraction of DCs, identified by HLA-DR expression (73·7% ± 6·5 s.d., n = 16). Lin-HLA-DR+DCs were heterogeneous in their expression of other markers, such as CD1a, CD1c, CD11c, CD33 and CD40 (Fig. 5). Lin-HLA-DR+ DCs also showed a low expression of the co-stimulatory markers CD80 and CD86, suggesting that they were not fully mature. We also look for the total frequency of two subsets of DC that are found within tonsillar tissue. Plasmacytoid DCs (HLA-DR+CD123+) (0·345 ± 0·12%) and interdigitating DCs (HLA-DR+CD40+) (64·3 ± 3·9%) was enumerated based on the surface expression of characteristic markers (n = 5).
Fig. 5.
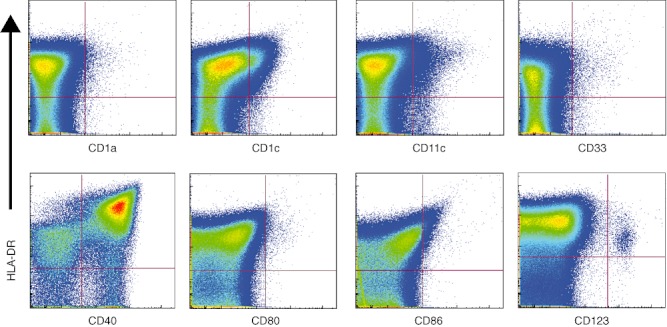
Expression of dendritic cell (DC) markers on tonsillar mononuclear cells (TMCs). The DC fraction from TMCs was analysed for expression of the DC and activation markers: CD1a, CD1c, CD11c, CD33, CD40, CD80, CD86 and CD123. The pseudo-colour density plots show the electronic gates used to identify double-positive subsets based on human leucocyte D-related (HLA-DR) expression.
Discussion
The goal of this study was to investigate the functional capacity of CD4+ and CD8+ T cells, and iDC and pDC frequency in tonsillar tissue. Thus, we established an organ culture model to provide scientific evidence that tonsillar T cells are indeed functional, and that chronically hypertrophic tonsils can be used for immunological assays as a source of cells from secondary lymphoid tissues.
Tonsils are key secondary lymphoid organs of effective immunity where naive T cells recognize cognate peptide/major histocompatibility complex (MHC) molecules presented by iDCs and pDCs, and undergo clonal expansion and differentiation into effector and memory T cells to control local infections [9]. The main function of the tonsils is to recognize and eliminate pathogens, thus leading to the onset of local adaptive immunity [15]. Therefore, in order to recognize antigens, activate and proliferate, CD4+ and CD8+ T cells must be in close contact with the DC subsets that form part of the squamous epithelium in the tonsils. In tonsils, chronic exposure to bacterial and viral antigens induces immunological memory, which generates changes in the number and distribution of CD4+ and CD8+ T cells. With the aid of iDCs and pDC, extensive proliferation of antigen-specific T cells will ensue, resulting in the production of effector T cells that limit the infection. While most healthy adults display well-recognized memory T cell subsets in peripheral blood, little is known about the memory compartmentalization of T cells within tonsillar tissue from children. In this study, we ascertained that tonsillar T cells can be classified clearly into four subsets: TN (CD3+CD45RA+CD62L+), TCM (CD3+CD45RA-CD62L+), TEM (CD3+CD45RA-CD62L-) and TEMRA (CD3+CD45RA+CD62L-). We also found that TCM and TEM were more frequent in the CD4+ T cell compartment, whereas TN and TEMRA were more frequent in the CD8+ T cell compartment. Tonsil naive and memory T cells showed a heterogeneous expression of the lymphoid homing marker CCR7. The nature of the expression of CCR7 by naive T cells is related to their ability to recirculate through the paracortical T cell zone in the tonsil, while expression of CCR7 by memory T cells describes a subset that lack immediate effector functions, but efficiently stimulate DCs and differentiate into CCR7-effector cells upon secondary stimulation [16]. Memory-effector T cell subsets can be accumulated in the tonsils or other lymphoid tissues such that upon the re-entry of a pathogen, these onsite effector T cells will rapidly produce cytokines and show direct ex-vivo cytotoxic activity in the case of CD8+ T cells, even without requiring proliferation [16],[17].
Antigen-specific CD4+ T cells in secondary lymphoid organs are usually primed by local DCs and then migrate towards the B cell follicles. After interacting with B cells, antigen-specific CD4+ T cells will differentiate into follicular helper T (TFH) cells that express the ligand for the co-stimulatory molecule CD40 (CD154). This interaction then stimulates the clonal expansion and maturation of memory B cells [CD19+CD20+CD38+immunoglobulin (Ig)D+] to contribute to adaptive immunity [18]. We found that 80·3% of the B cells express CD38 and IgD (markers consistent with memory in B cells) within tonsillar tissue. This high frequency of memory B cells may represent a highly effective mechanism that ensures swift protection against prevalent infections in the tonsils [19],[20].
CD8+ T cells also participate in adaptive immune responses to viral pathogens in the upper airway [21]. CD8+ effector T cells (cytotoxic lymphocytes) have secretory granules containing mediators of cell death, including perforin, granzymes and granulysin [22],[23]. Upon contact between a CD8+ T cell and its target cell, the content of the granules is released to perform cytolysis [24]. Perforin-dependent cytotoxicity is one of the mechanisms used by CD8+ T cells to induce cell death, but CD8+ T cells can also kill in a perforin-independent manner utilizing the FAS ligand, TNF or the TNF-related apoptosis-inducing ligand (TRAIL) [25],[26]. In our in-vitro assay, we identified a relationship between CD107a/b surface expression and the cytotoxic activity of tonsillar CD8+ T cells upon stimulation. These data suggest that CD8+ T cells in the tonsil can mediate the direct killing of a wide range of viral and bacterial pathogens. In addition, we demonstrated that CD8+ T cells had a polyfunctional IFN-γ(+)IL-2(+)TNF-α(+) cytokine response after in-vitro stimulation. The polyfunctional response in CD4+ T cells was less evident. CD4+ and CD8+ double producers (TNF-α+/IL-2+, TNF-α+/IFN-γ+, IL-2+/IFN-γ+) or single producers (TNF-α+, IL-2+ or IFN-γ+) were similar.
In conclusion, we have demonstrated in this study that tonsillar CD4+ and CD8+ T cells are functional, based on their ability to respond to stimulation, activate and then differentiate into antigen-specific and effector T cells; evidence that supports the fact that tonsils can be used as a source of immune cells for specific assays. Our results also challenge the belief that hypertrophic tonsils must be removed because they are no longer functional.
Acknowledgments
We thank Paul Kersey, who kindly provided language corrections. This study was funded by a grant to ISO from CONACYT (104003).
Disclosure
The authors have nothing to disclose.
References
- 1.Message SD, Laza-Stanca V, Mallia P, et al. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc Natl Acad Sci USA. 2008;105:13562–7. doi: 10.1073/pnas.0804181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lieberman J. The ABCs of granule-mediated cytotoxicity: new weapons in the arsenal. Nat Rev Immunol. 2003;3:361–70. doi: 10.1038/nri1083. [DOI] [PubMed] [Google Scholar]
- 3.Dorner T, Radbruch A. Antibodies and B cell memory in viral immunity. Immunity. 2007;27:384–92. doi: 10.1016/j.immuni.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 5.Itano AA, McSorley SJ, Reinhardt RL, et al. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 2003;19:47–57. doi: 10.1016/s1074-7613(03)00175-4. [DOI] [PubMed] [Google Scholar]
- 6.Maecker HT. Multiparameter flow cytometry monitoring of T cell responses. Methods Mol Biol. 2009;485:375–91. doi: 10.1007/978-1-59745-170-3_25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korsrud FR, Brandtzaeg P. Immune systems of human nasopharyngeal and palatine tonsils: histomorphometry of lymphoid components and quantification of immunoglobulin-producing cells in health and disease. Clin Exp Immunol. 1980;39:361–70. [PMC free article] [PubMed] [Google Scholar]
- 8.van Kempen MJ, Rijkers GT, Van Cauwenberge PB. The immune response in adenoids and tonsils. Int Arch Allergy Immunol. 2000;122:8–19. doi: 10.1159/000024354. [DOI] [PubMed] [Google Scholar]
- 9.Nave H, Gebert A, Pabst R. Morphology and immunology of the human palatine tonsil. Anat Embryol (Berl) 2001;204:367–73. doi: 10.1007/s004290100210. [DOI] [PubMed] [Google Scholar]
- 10.Paradise JL, Bluestone CD, Colborn DK, Bernard BS, Rockette HE, Kurs-Lasky M. Tonsillectomy and adenotonsillectomy for recurrent throat infection in moderately affected children. Pediatrics. 2002;110:7–15. doi: 10.1542/peds.110.1.7. [DOI] [PubMed] [Google Scholar]
- 11.van Staaij BK, van den Akker EH, Rovers MM, Hordijk GJ, Hoes AW, Schilder AG. Effectiveness of adenotonsillectomy in children with mild symptoms of throat infections or adenotonsillar hypertrophy: open, randomised controlled trial. BMJ. 2004;329:651–6. doi: 10.1136/bmj.38210.827917.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Almeida JR, Sauce D, Price DA, et al. Antigen sensitivity is a major determinant of CD8+ T-cell polyfunctionality and HIV-suppressive activity. Blood. 2009;113:6351–60. doi: 10.1182/blood-2009-02-206557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Appay V, van Lier RA, Sallusto F, Roederer M. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry A. 2008;73:975–83. doi: 10.1002/cyto.a.20643. [DOI] [PubMed] [Google Scholar]
- 14.Ciuffreda D, Comte D, Cavassini M, et al. Polyfunctional HCV-specific T-cell responses are associated with effective control of HCV replication. Eur J Immunol. 2008;38:2665–77. doi: 10.1002/eji.200838336. [DOI] [PubMed] [Google Scholar]
- 15.Canessa C, Vierucci S, Azzari C, Vierucci A. The immunity of upper airways. Int J Immunopathol Pharmacol. 2010;23:8–12. [PubMed] [Google Scholar]
- 16.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 17.Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–5. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- 18.Nutt SL, Tarlinton DM. Germinal center B and follicular helper T cells: siblings, cousins or just good friends? Nat Immunol. 2011;12:472–7. doi: 10.1038/ni.2019. [DOI] [PubMed] [Google Scholar]
- 19.Ochsenbein AF, Pinschewer DD, Sierro S, Horvath E, Hengartner H, Zinkernagel RM. Protective long-term antibody memory by antigen-driven and T help-dependent differentiation of long-lived memory B cells to short-lived plasma cells independent of secondary lymphoid organs. Proc Natl Acad Sci USA. 2000;97:13263–8. doi: 10.1073/pnas.230417497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanz I, Wei C, Lee FE, Anolik J. Phenotypic and functional heterogeneity of human memory B cells. Semin Immunol. 2008;20:67–82. doi: 10.1016/j.smim.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hufford MM, Kim TS, Sun J, Braciale TJ. Antiviral CD8+ T cell effector activities in situ are regulated by target cell type. J Exp Med. 2011;208:167–80. doi: 10.1084/jem.20101850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou F. Perforin: more than just a pore-forming protein. Int Rev Immunol. 2010;29:56–76. doi: 10.3109/08830180903349644. [DOI] [PubMed] [Google Scholar]
- 23.Piet B, de Bree GJ, Smids-Dierdorp BS, et al. CD8 T cells with an intraepithelial phenotype upregulate cytotoxic function upon influenza infection in human lung. J Clin Invest. 2011;121:2254–63. doi: 10.1172/JCI44675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oykhman P, Mody CH. Direct microbicidal activity of cytotoxic T-lymphocytes. J Biomed Biotechnol. 2010;2010:249482. doi: 10.1155/2010/249482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voskoboinik I, Dunstone MA, Baran K, Whisstock JC, Trapani JA. Perforin: structure, function, and role in human immunopathology. Immunol Rev. 2010;235:35–54. doi: 10.1111/j.0105-2896.2010.00896.x. [DOI] [PubMed] [Google Scholar]
- 26.Stenger S, Hanson DA, Teitelbaum R, et al. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science. 1998;282:121–5. doi: 10.1126/science.282.5386.121. [DOI] [PubMed] [Google Scholar]


