Abstract
Traumatic brain injury (TBI) is an international health concern often resulting in chronic neurological abnormalities, including cognitive deficits, emotional disturbances, and motor impairments. An anti-CD11d monoclonal antibody that blocks the CD11d/CD18 integrin and vascular cell adhesion molecule (VCAM)-1 interaction following experimental spinal cord injury improves functional recovery, while reducing the intraspinal number of neutrophils and macrophages, oxidative activity, and tissue damage. Since the mechanisms of secondary injury in the brain and spinal cord are similar, we designed a study to evaluate fully the effects of anti-CD11d treatment after a moderate lateral fluid percussion TBI in the rat. Rats were treated at 2 h after TBI with either the anti-CD11d antibody or an isotype-matched control antibody 1B7, and both short (24- to 72-h) and long (4-week) recovery periods were examined. The anti-CD11d integrin treatment reduced neutrophil and macrophage levels in the injured brain, with concomitant reductions in lipid peroxidation, astrocyte activation, amyloid precursor protein accumulation, and neuronal loss. The reduced neuroinflammation seen in anti-CD11d-treated rats correlated with improved performance on a number of behavioral tests. At 24 h, the anti-CD11d group performed significantly better than the 1B7 controls on several water maze measures of spatial cognition. At 4 weeks post-injury the anti-CD11d-treated rats had better sensorimotor function as assessed by the beam task, and reduced anxiety-like behaviors, as evidenced by elevated-plus maze testing, compared to 1B7 controls. These findings suggest that neuroinflammation is associated with behavioral deficits after TBI, and that anti-CD11d antibody treatment is a viable strategy to improve neurological outcomes after TBI.
Keywords: adult brain injury, behavioral assessments, inflammation, oxidative stress, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is an international health concern, often resulting in chronic neurological abnormalities including cognitive deficits, emotional disturbances, and motor impairments (Maas et al., 2000; Marshall, 2000; Rao and Lyketsos, 2000). The development of effective pharmacological interventions for TBI has had only limited success (Doppenberg et al., 2004; Thompson et al., 2005). This lack of effective treatment is likely due to the complex pathological processes of primary and secondary injury that follow TBI (Atif et al., 2009; Morganti-Kossmann et al., 2002; Schmidt et al., 2005). Primary injury consists of damage induced by the mechanical forces applied to the brain at the time of impact (Graham et al., 2000; Marshall, 2000). Secondary injury results from processes that are initiated by the primary insult, such as activation of inflammatory processes that can induce secondary damage by apoptosis, lipid peroxidation, oxidative stress, or free radical formation (Bao et al., 2004a, 2004b; Farkas et al., 1998; Hall, 1995; Juurlink and Paterson, 1998; Morganti-Kossmann et al., 2001; Schmidt et al., 2005; Taoka and Okajima, 1998). The neuroinflammatory response is characterized by the activation of microglia and astrocytes, the release of proinflammatory cytokines and chemokines, and the migration of leukocytes across the blood–brain barrier (Ghirnikar et al., 1998; Holmin et al., 1998; Kadhim et al., 2008; Laird et al., 2008; Morganti-Kossmann et al., 2007). These infiltrating leukocytes can further exacerbate the inflammatory response and worsen secondary damage through the production of proinflammatory mediators and free radicals (Juurlink and Paterson, 1998; Schoettle et al., 1990; Taoka and Okajima, 1998; Zhuang et al., 1993).
The infiltration of leukocytes into the central nervous system (CNS) is mediated in part by CD11/CD18 integrins, a family of membrane-bound glycoproteins. The CD11/CD18 heterodimer is composed of a common CD18 (β2) subunit, and one of four CD11 alpha subunits (a–d). The CD11d/CD18 integrin is expressed on monocytes/macrophages and neutrophils, and binds to adhesion molecules in both rats (vascular adhesion molecule-1; VCAM-1), and humans (VCAM-1 and intercellular adhesion molecule; ICAM; Bevilacqua, 1993; Hogg and Leitinger, 2001). In previous work in our laboratory, we used an anti-CD11d monoclonal antibody (mAb) to block the CD11d/CD18 and VCAM-1 interaction following experimental spinal cord injury (SCI) in rats (Bao et al., 2004a, 2004b, 2005; Gris et al., 2004; Oatway et al., 2005; Saville et al., 2004). These studies revealed that short-lasting treatment with a CD11d mAb for up to 48 h after SCI improves functional recovery, while reducing the number of neutrophils and macrophages, the formation of reactive free radicals, lipid peroxidation, protein nitration, and DNA damage (Bao et al., 2004a, 2004b; Gris et al., 2004; Saville et al., 2004). As these mechanisms also contribute to secondary injury following TBI, we set out to evaluate the therapeutic potential of the anti-CD11d treatment following TBI in rats. Herein we report that the anti-CD11d treatment reduces neutrophil and macrophage infiltration into the injured brain, and consequently reduces lipid peroxidation, free radical formation, astrocyte activation, amyloid precursor protein (APP) expression, and neuronal loss. The neuroprotective effect of the CD11d mAb treatment correlates with reduced impairment in tests of spatial cognition, anxiety, and sensorimotor function.
Methods
Subjects
All procedures and behavioral tests were performed in accordance with the guidelines of the Canadian Council on Animal Care, and were approved by the University of Western Ontario Animal Use Subcommittee. The subjects were 114 adult male Long-Evans hooded rats obtained from Charles River Laboratories (Quebec, Canada). Prior to surgery the rats weighed between 250 and 300 g, and were naïve to all experimental procedures. After surgery the rats were housed individually for the remainder of the study under a 12-h light/dark cycle and were allowed access to food and water ad libitum. Long-Evans rats were chosen for these studies because they exhibit superior cognitive ability and prolonged functional impairments following TBI, relative to other rat strains (Tan et al., 2009). Cognitive deficits and chronic neurological impairments are common in the clinical TBI setting. The numbers of rats used for each part of the study are outlined in Table 1.
Table 1.
Numbers of Rats Used in Each Part of the Study
| MPO and MDA assays | 24 h | 72 h | 4 weeks |
|---|---|---|---|
| Sham-injured | 6 | 7 | 5 |
| TBI 1B7 control | 7 | 8 | 6 |
| TBI anti-CD11d | 7 | 7 | 5 |
|
| |||
| Western blotsa | 24 h | 72 h | 4 weeks |
|
| |||
| Sham-injured | 5 | 5 | 5 |
| TBI 1B7 control | 5 | 5 | 5 |
| TBI anti-CD11d | 5 | 5 | 5 |
Neutrophil, macrophage (ED-1), GFAP, APP, and NeuN blots.
Tissues used in the assays were sampled from 6 sham, 7 1B7, and 7 anti-CD11d rats at 24 h, from 7 sham, 8 1B7, and 7 anti-CD11d rats at 72 h, and from 5 sham, 6 1B7, and 5 anti-CD11d rats at 4 weeks.
GFAP, glial fibrillary acidic protein; APP, amyloid precursor protein; NeuN, neuronal nuclear antigen; TBI, traumatic brain injury; MPO myeloperoxidase; MDA, malondialdehyde.
Treatment groups
Rats were randomly assigned to one of three treatment conditions: sham-injury with saline injection (sham), treatment of TBI rats with an isotype-matched control mAb designated 1B7 or with an anti-CD11d mAb. In previous studies using anti-integrin mAb, we have shown that animals treated with saline have similar outcomes to those treated with an isotype-matched antibody (Fleming et al., 2008; Gris et al., 2004; Oatway et al., 2005). Therefore, we elected to include only a saline-treated sham-injury group. The rats received their assigned treatment (saline, 1B7, or CD11d mAb) at 2 h post-injury via tail vein injection (1.0 mg/kg). The antibody treatments were in vials marked A or B, and the code for their identity was established by an investigator who did not participate in the experiments. Groups of rats undergoing fluid percussion injury (FPI) surgery on a particular day contained those treated with 1B7 or with the CD11d mAb. Sham-injured rats were prepared on the same days as TBI rats and had the same survival periods. The rats were tested on behavioral tasks of spatial cognition, sensorimotor ability, and anxiety-like behavior. As TBI is associated with acute and chronic changes, both short recovery (SR, 24 h) and long recovery (LR, 4 week) periods were used. The following groups were tested: anti-CD11d-SR, n = 25; anti-CD11d-LR, n = 14; 1B7 control-SR, n = 24; 1B7 control-LR, n = 14; sham-SR, n = 24; and sham-LR, n = 14. To provide brain tissue for histological analysis of leukocyte infiltration after injury (Carlos et al., 1997; Clark et al., 1996; Donnelly and Popovich, 2008; Utagawa et al., 2008), approximately half of the SR rats were perfused 24 h post-injury after the completion of elevated-plus maze testing. All other rats were perfused immediately after the completion of behavioral testing approximately 72 h or 30 days post-injury.
Fluid percussion brain injury model
TBI was induced using a standardized fluid percussion injury method as previously described (Thompson et al., 2005). The fluid percussion force (2.5–3.0 atm) was chosen based on force values used in previous studies. The rats were placed in a sealed acrylic glass box into which 4% isoflurane and 2 L/min oxygen flow was introduced for anesthesia. Under aseptic conditions the rats underwent a craniotomy. All craniotomies were circular windows (3 mm diameter) centered over the following coordinates with reference to the bregma: anterior/posterior −3.0 mm; medial/lateral 6.0 mm (Paxinos and Watson, 1986). A hollow plastic injury cap was sealed over the craniotomy with silicone adhesive and cyanoacrylate. Three small stainless steel screws were inserted into the skull surrounding the injury cap to provide anchors for dental acrylic, which attached the injury cap to the skull. After the dental acrylic hardened the scalp was sutured, the injury cap was filled with sterile saline, and the rat was attached to the FPI device. At the first response of hindlimb withdrawal to a toe pinch, the rats in the anti-CD11d or 1B7 control groups received a single fluid percussion pulse of 2.5–3.0 atm. Sham-injured rats had the same craniotomy and injury cap but were removed from the FPI device without receiving fluid percussion (Thompson et al., 2005).
Apnea, tune of unconsciousness, and self-righting reflex were monitored immediately following injury. Apnea times were determined as the time from injury to the return of spontaneous breathing. Time of unconsciousness was determined by the return of hindlimb withdrawal in response to toe pinch. Self-righting was determined as the time from injury to return to an upright position from lying on the side. As shown in Table 2, CD11d- and 1B7-mAb-treated TBI rats displayed significantly longer periods of apnea, unconsciousness, and self-righting reflex times than sham-injured rats. Four rats died as a result of FPI and one rat lost its injury cap prior to the start of behavioral testing and was removed from the study. After these immediate post-injury tests were completed all rats received a subcutaneous injection of analgesic (ketoprofen, 5 mg/kg). Two hours post-injury, the rats received tail vein injections of their assigned treatment or saline. Behavioral testing began after each group’s recovery time was complete.
Table 2.
Neurological and Immunohistochemical Results
| Immune cell counts | Neutrophil | Macrophage | Macrophage |
|---|---|---|---|
| 24 h | 72 h | 4 weeks | |
| Sham-injured | 4 | 4 | 7 |
| TBI 1B7 control | 4 | 4 | 6 |
| TBI anti-CD11d | 4 | 4 | 6 |
|
| |||
| Histochemical and immunohistochemical staining | 24 h | 72 h | 4 weeks |
|
| |||
| Sham-injured | 5 | 5 | 9 |
| TBI 1B7 control | 5 | 5 | 9 |
| TBI anti-CD11d | 5 | 5 | 9 |
|
| |||
| Hippocampal neuron counts | 24 h | 72 h | 4 weeks |
|
| |||
| Sham-injured | 4 | 5 | 9 |
| TBI 1B7 control | 4 | 5 | 8 |
| TBI anti-CD11d | 4 | 5 | 7 |
| Elevated-plus maze task | Short recovery (24 h)a | Long recovery (4 weeks)b |
|---|---|---|
| Sham-injured | 24 | 14 |
| TBI 1B7 control | 24 | 14 |
| TBI anti-CD11d | 25 | 14 |
| Water maze and beam task | Short recovery (24 h) | Long recovery (4 weeks) |
|---|---|---|
| Sham-injured | 12 | 7 |
| TBI 1B7 control | 12 | 7 |
| TBI anti-CD11d | 12 | 7 |
| Treatment group | Neurological measures
|
||
|---|---|---|---|
| Apnea (sec) | Unconsciousness (sec) | Self-righting (sec) | |
| CD11d | 7.94 ± 0.84a | 98.05 ± 12.54a | 685.77 ± 202.03a |
| 1B7 control | 7.64 ± 0.54a | 98.24 ± 12.45a | 694.84 ± 183.27a |
| Sham-injured | 0 ± 0 | 0 ± 0 | 272.84 ± 17.64 |
Tissues used in these analyses were sampled from 5 rats per group at 24 h and at 72 h after injury and from 9 rats per group at 4 weeks after injury.
12 of these rats were not tested further as they were perfused at 24 h or 72 h after injury and used for assays and counts.
7 rats were not tested further; these 7 rats and the 7 rats that underwent further testing were perfused for assays, cell counting, and histochemistry at 4 weeks.
Different horn sham–self-righting (p < 0.001).
The CD11d- and 1B7 mAb-treated groups displayed significantly longer apnea, unconsciousness, and self-righting reflex times than the sham-injured group.
TBI, traumatic brain injury.
Tissue preparation for histochemical and biochemical analyses
For histological examination at 24 h, 72 h, and 4 weeks after injury, the animals were anesthetized (2.5 g/kg urethane), and perfused transcardially with saline, followed by 4% paraformaldehyde in phosphate-buffered saline (PBS; pH 7.2–7.4). The brains were removed, post-fixed for 24 h at 4°C, cryoprotected in increasing concentrations of sucrose, and sectioned into 35-μm sections for immunohistochemical staining. The entire brain was sectioned coronally and floating sections containing a visible hippocampus (approximately 3 mm caudal to the bregma) were saved for immunohistochemical staining. Approximately 10 of these sections were mounted on slides for hematoxylin and eosin staining. For biochemical assays and Western blotting, animals at 24 h, 72 h, and 4 weeks post-injury were perfused with cold 0.9% NaCl and their were brains removed. The brainstem and cerebellum were removed and the injured half of the remaining brain was bisected and subsequently homogenized and stored at −80°C.
Immunohistochemistry and histology
Monoclonal mouse antibodies: anti-ED1 (1:500; Serotec, Raleigh, NC), anti-NeuN (1:500, Chemicon, Temecula, CA), anti-glial fibrillary acidic protein (GFAP, 1:500; Sigma-Aldrich, St. Louis, MO), and polyclonal rabbit anti-rat antibodies against amyloid precursor protein (APP, 1:200; Sigma-Aidrich) and against a 56kDa neutrophil protein (1:20,000, a gift of Dr. Daniel Anthony, Oxford University, Oxford, U.K.) were used for immunohistochemical staining. Representative sections (at least 3 per animal) of the injured area from each animal were processed free-floating for staining as described previously (Bao et al., 2005). Immunoreactivity was revealed with a glucose-diaminobenzidine-nickel solution. Fields of view were captured digitally by a Retiga 1300 videocamera (Quantitative Imaging Corporation, Burnaby, BC), and ImagePro Plus Software (Media Cybernetics, Silver Spring, MD). For quantification of the inflammatory infiltrates, photomicrographs were captured from coronal sections deemed by inspection of the tissue structure to be close to the level of injury (approximately −3.0 mm to the bregma). Using Image Pro Plus software the color threshold was adjusted to detect immunostained inflammatory cells, and the number of colored objects was counted within a 0.2-mm2 area of interest centered on the lesion epicenter at a depth that included the area of greatest immunoreactivity. Neurons immunoreactive for neuronal nuclear antigen (NeuN) were counted in the hippocampus at 4 weeks post-injury as an indication of neuronal survival levels (D’Avila et al., 2012). For this analysis, a 20× field of view (0.33 mm2 in area) was sampled using the hippocampal shape as the landmark. The selected field included the end of CA2 and beginning of CA3, ~3 mm caudal to the bregma. Cells were counted visually and an average number over three sections was calculated for each animal. Because sections were sampled from a group of floating sections, stereological methods for counting were not used.
Western blotting analysis
Tissue sample preparation and protein determination were performed as described previously (Bao et al., 2004b). Primary antibodies used included: anti-ED1 (1:1000; Serotec), anti-GFAP (1:5000; Sigma-Aldrich), anti-App (1:2000; Sigma-Aldrich) anti-neutrophil (1:20,000, a gift of Dr. Daniel Anthony, Oxford University, Oxford, U.K.), anti-NeuN (1:5000; Chemicon), and anti-β-actin (1:10,000; Sigma-Aldrich). Signal detection was facilitated with enhanced chemiluminescence (ECL kit; Amersham, Piscataway, NJ). Immunoreactive bands were scanned by an imaging densitometer (BioRad GS-700 Imaging Densitometer), and the results were quantified using Multi-Analyst software (Bio-Rad). Densitometric values were normalized for protein loading using β-actin as a loading control.
MPO assay
MPO enzymatic activity is derived mostly from neutrophils and to a lesser extent from macrophages. This activity, an estimate of the extent of neutrophil infiltration/activation and macrophage activation, was measured as described previously (Bao et al., 2004b). MPO activity was calculated using a standard curve prepared with purified human MPO (Sigma-Aldrich). Results are expressed as units per milligram of protein.
Measurement of lipid peroxidation
The relative levels of aldehydes, including malondialdehyde (MDA), indicators of lipid peroxidation, were measured using the thiobarbituric acid reactive substances (TBARS) assay, as previously described (Ohkawa et al., 1979). MDA bis (dimethyl acetal; Sigma-Aldrich) was used as a standard, and the level of lipid peroxide was expressed as nanomoles of TBARS/mg protein.
Behavioral tests day 1
Elevated-plus maze. Behavioral testing was performed as previously described (Shultz et al., 2011a). Day 1 testing began 24 h after TBI for SR rats, or 28 days after TBI for LR rats. First, anxiety-associated behavior was assessed using the elevated-plus maze, consisting of two closed arms (arms enclosed by 18-inch-high walls), and two open arms (arms not enclosed by walls). The rats were allowed to explore the maze freely for 5 min. Following testing, a videotape of the trial was scored by a person blind to treatment group, and the number of entries into, and the amount of time spent in, each arm were recorded. Time spent in the open arm was decreased in rats with more anxiety-related behaviors. Accordingly, a percentage score was calculated for the time spent in the open arm: time in open arm/[time in open arm + time in closed arm] (Saucier et al., 2008; Shultz et al., 2011b; Steimer and Driscoll, 2003; Zhu et al., 2006). The number of entries into the closed arm of the maze was also calculated as a measure of locomotion. Half of the SR rats were sacrificed following the elevated-plus maze testing for analysis at 24 h post-injury.
Water maze
Next, spatial cognition was assessed using a circular water maze. A clear acrylic glass escape platform was hidden 2 cm below the water’s surface in the center of the southeast quadrant of the pool. Polypropylene beads floating on the water prevented the rats from seeing the hidden platform (Cain and Boughey, 1993). Acquisition training began immediately following elevated-plus maze testing and consisted of 10 training trials. A trial began by placing the rat in the pool adjacent to and facing the pool wall, and ended when the rat stood on the hidden platform. Each trial began at one of four pool wall start locations (north, south, east, or west) according to a pseudo-random schedule of start locations that prevented repeated sequential starts from the same location. As the four start locations varied in distance from the hidden platform, the time to reach the platform was averaged for every block of two trials [e.g., block 1 = (trial 1 + trial 2)/2]. Behavior was analyzed by a tracking system that digitized each swim trial (Poly-Track; San Diego Instruments, San Diego, CA). The tracking system also permitted analysis of the percentage of time the rats swam directly toward the platform or swam around it. Search time and direct and circle swims were used as measures of spatial place memory (Morris, 1989; Whishaw and Jarrard, 1995). Swim speed was used as a measure of motor ability and was objectively calculated in centimeters per second by the Poly-Track system.
Beam task
Finally, training was done on a beam task that assesses sensorimotor function (Kolb and Whishaw, 1985). One edge of the beam was 4 cm wide and the other edge was 2 cm wide. For acclimation to the beam task, the rats were given a training session consisting of five trials to traverse the beam with the 4cm edge facing up, and another five trials with the 2-cm edge of the beam facing up.
Behavioral tests day 2
Testing on day 2 began 48 h after TBI for SR rats or 29 days after TBI for LR rats. The beam test session began approximately 24 h after the beam training session and consisted of 10 trials. A trial was begun by placing a rat at one end of the beam and ended when the animal successfully reached the goal platform. A maximum of 60 sec was allowed for each trial. Traverse time and the number of slips and falls were used as measures of sensorimotor function.
Immediately following beam testing, the rats underwent a second water maze session for reversal training. The reversal test is a more challenging cognitive test, as it assesses the capacity of the rats to disregard what they had learned in the previous trial and to learn a new location for the escape platform. The procedures for the reversal session were identical to acquisition except that the hidden platform was now located in the opposite quadrant of the pool relative to the location during acquisition.
Statistical analysis
Histochemical and biochemical results were analyzed using a completely randomized one-way analysis of variance (ANOVA). Differences between means were determined by the post-hoc Student Neuman Keuls test. These analyses were done using SigmaStat (Systat Software, San Jose, CA). Water maze search time and beam traverse time were analyzed using repeated-measures ANOVA with injury as the between-subjects factor and trial as the within-subjects factor. One-way ANOVA, with injury as the between-subjects factor, was used to analyze the percent of time in the open arm, closed arm entries, direct and circle swims, swim speed, and slips and falls. Fisher’s LSD post-hoc pair-wise comparisons were carried out when appropriate. These analyses were done using SPSS 17.0 (IBM, Armonk, NY). Mean values in all comparisons are expressed ± standard error (SE). Significance in all analyses was accepted at p ≤ 0.05. Values of p presented in the results refer to the post-hoc tests. Details of the ANOVA are presented in the figure legends, with the exception of water maze search time and beam traverse time, which are given in the results section.
Results
Histochemical and biochemical findings at 24 h, 72 h, and 4 weeks after TBI
Sections from sham-injured, 1B7-, and CD11d mAb-treated rats were stained with hematoxylin and eosin 24 h after injury or sham surgery to examine their acute pathological changes. Sections from the sham-injured rats revealed normal brain parenchyma both at the cortical surface and in the deeper layers including the hippocampal gyrus (Fig. 1A and D). In contrast, sections from 1B7-treated controls (Fig. 1B and E), and from CD11d-treated rats (Fig. 1C and F), demonstrated increased cellularity in the cortex, and marked hemorrhage and tissue disruption in the deeper layers immediately above the hippocampus. Cells with morphology typical of neutrophils could be detected adjacent to the areas of hemorrhage.
FIG. 1.
Histological sections of injured brain stained with hematoxylin and eosin 24 h after fluid percussion injury (FPI). The section is at the location of the FPI and includes the hippocampus. Areas boxed in low-power photomicrographs (A–C) are shown at high-power in panels D–F. Sections from the 1B7 control rat and the anti-CD11d-treated rat show areas of tissue disruption and hemorrhage immediately above and including the hippocampus (arrowheads; scale bar in A–C = 200 μm; scale bar in D–F = 100 μm).
Leukocyte infiltration
We assessed the effect of the anti-CD11d treatment on neutrophil infiltration into the injured brain at 24 h post-FPI by immunostaining brain sections with an antibody to a 56-kDa protein expressed by rat neutrophils. Whereas sections from the sham-injured brains demonstrated only a few neutrophils (confined to blood vessels) at 24 h after TBI (Fig. 2A, panels 1 and 4), many neutrophils were detected near the TBI injury site in the parenchyma of the cortex in 1B7 control rats (Fig. 2A, panels 2 and 5). After anti-CD11d treatment, the density of these cells appeared to be reduced (Fig. 2A, panels 3 and 6). Counts of immunostained neutrophils in a 0.2-mm2 area of interest centered on the lesion epicenter in sections from sham, 1B7, and CD11d mAb-treated rats 24 h after injury demonstrated significant increases in the numbers of neutrophils in 1B7 control FPI rats compared to the sham-injured group (Fig. 2B; p < 0.01), no significant increase in the anti-CD11d group, and significantly fewer neutrophils in the anti-CD11d group than in the 1B7 group (p < 0.05). The presence of neutrophils in the brain was also quantified using the anti-rat neutrophil antibody in a Western blot analysis. Neutrophil protein (56 kDa) expression was low in homogenates from sham-injured rats at 24 and 72 h after TBI (Fig. 2C), but was increased significantly at 24 h (sevenfold, p < 0.001), and 72 h (~ fivefold, p < 0.001) after injury. Treatment with the anti-CD11d antibody significantly reduced neutrophil protein levels by 51% (p < 0.01) at 24 h, and by 43% (p < 0.01) at 72 h after injury. At 4 weeks after TBI, the 56-kDa neutrophil protein expression did not differ among the three groups.
FIG. 2.
Neutrophil infiltration into the injured brain is reduced by the anti-CD11d treatment. (A) Photomicrographs of the cortex centered on the lesion epicenter (or a comparable position in sham-injured rats), immunostained by an anti-neutrophil antibody. The sections from sham-injured, 1B7 control, and anti-CD11d-treated rats are shown at low power in panels 1–3, respectively, and the boxed areas are shown at high power in panels 4–6 (scale bar in A1–3 = 100 μm, and in A4–6 = 100 μm). (B) Left panel shows counts of neutrophils within a 0.2-mm2 area of interest centered on the epicenter in the injured brain in the sham, 1B7 control, and anti-CD11d-treated groups (n = 4/group) at 24 h after injury (*significantly different from sham animals, and #significantly different from the 1B7 control group, p ≤ 0.05 after the Student Neuman Keuls test and one-way analysis of variance [ANOVA]; ANOVA for neutrophil count: F2,9 = 8.6, p = 0.008). (B) Right panels show myeloperoxidase (MPO) activity in brain homogenates after traumatic brain injury (TBI) at 24 h in sham-injured animals (n = 6), 1B7 controls, and anti-CD11d animals (n = 7/group); at 72 h in these groups (n = 7, 8, and 7, respectively); and at 4 weeks (n = 5, 6, and 5, respectively; ANOVA: 24 h, F2,17 = 13.63, p < 0.001; 72 h, F2,19 = 11.75, p < 0.001; 4 weeks, F2,13 = 2.74, p = 0.102). (C) Neutrophil protein identified by Western blotting in brain homogenates expressed as means ± standard error with a representative autoradiogram shown above each set of histograms. Neutrophil protein is shown at 24 h, 72 h, and 4 weeks in sham-injured, 1B7 controls, and anti-CD11d groups (n = 5/group; ANOVA: 24 h, F2,12 = 20.52, p < 0.001; 72 h, F2,12 = 20.70, p < 0.001; 4 weeks, F2,12 = 2.92, p = 0.09; A.U., arbitrary units). Color image is available online at www.liebertonline.com/neu
The effect of the anti-CD11d treatment was also assessed on MPO enzymatic activity, a measure of neutrophil infiltration/activation, and to a lesser degree of macrophage activation. MPO was minimal in the brains of sham-injured rats at 24 h, 72 h, and 4 weeks after TBI (Fig. 2B, right). At 24 and 72 h after TBI, MPO activity in the brain homogenates of the 1B7 controls increased significantly (by eightfold at 24 h, by threefold at 72 h; p < 0.001) compared to the sham-injured rats. The anti-CD11d treatment reduced MPO activity by 54% and 42%, respectively, from that in the 1B7-treated controls (p < 0.01 for both time points).
The effect of the anti-CD11d treatment on monocyte/microglia/macrophage populations in the brain lesions was assessed by quantifying ED-1 (CD11b) immunoreactivity at 72 h and 4 weeks after TBI. In the brains of sham-injured rats, only a few ED-1 immunoreactive (Ir) cells were present (Fig. 3A, panels 1 and 4). At 72 h and 4 weeks after injury, the density of ED-1-Ir cells was clearly increased in the 1B7 control rats (Fig. 3A, panels 2 and 5). These cells had the rounded morphology typical of macrophages and activated microglia, and they were distributed from the superficial cortical layers through to the deeper layers. After CD11d antibody treatment, the density of the ED-1-Ir macrophages was clearly reduced (Fig. 3A, panels 3 and 6). The number of ED-1-Ir macrophages was counted in each of the three groups at 72 h and 4 weeks after TBI (Fig. 3C). The number of macrophages in the 1B7 controls were increased compared to the number in sham-injured rats by 6.6-fold (p < 0.001) at 72 h, and 15-fold at 4 weeks (p < 0.01) post-injury. Anti-CD11d treatment reduced these increases by 65% and 54%, respectively (p < 0.001; p = 0.05).
FIG. 3.
Macrophages in the injured brain are reduced by the anti-CD11d treatment. (A and B) Photomicrographs 72 h or 4 weeks post-injury of the cortex centered on the lesion epicenter (or a comparable position in sham-injured rats), immunostained by an anti-ED-1 antibody. The sections from sham-injured, 1B7 control, and anti-CD11d-treated rats are shown at low power in panels 1–3, respectively, and the boxed areas are shown at high power in panels 4–6 (*significantly different from sham animals, and #significantly different from the 1B7 control group, p ≤ 0.05 after the Student Neuman Keuls test and one-way analysis of variance; scale bar in A and B1–3 = 100 μm, and in A and B4–6 = 100 μm). Arrows indicate positions of blood vessels, and the arrowhead indicates a typical ED-1-expressing macrophage. (C) Left panel shows counts of macrophages in a 0.2-mm2 area within the injured brain in the sham, 1B7 control, and anti-CD11d-treated groups at 72 h (n = 4/group; analysis of variance [ANOVA]: F2,9 = 61.34, p < 0.001). Right panel shows counts of macrophages at 4 weeks after traumatic brain injury (TBI) (n = 7, 6, and 6, respectively; ANOVA: F2,16 = 7.04, p = 0.006). (D) Macrophage protein identified by Western blotting in cord homogenates expressed as mean values ± standard error, with a representative autoradiogram shown above each set of histograms. Macrophage protein is shown at 24 h, 72 h, and 4 weeks in the sham-injured, 1B7 control, and anti-CD11d groups (n = 5/group; ANOVA: 24 h, F2,12 = 7.15, p = 0.009; 72 h, F2,12 = 17.44, p < 0.001; 4 weeks, F2,12 = 15.95, p < 0.001; A.U., arbitrary units). Color image is available online at www.liebertonline.com/neu
Macrophage levels were also quantified using Western blot analysis for ED-1 (Fig 3C and D). ED-1 expression (110-kDa band) was low in the brain homogenates of sham-injured rats, but was increased significantly after TBI at 24 h (3.5-fold, p < 0.01), 72 h (5.3-fold, p < 0.001), and 4 weeks (4-fold, p < 0.001). Treatment with the anti-CD11d antibody significantly reduced these increases by 46%, 39%, and 30%, respectively (p < 0.05 for all time points).
Lipid peroxidation
An indication of lipid peroxidation that could be due to free radical tissue damage and/or to activation of the arachidonic acid cascade was evaluated by a TBARS assay that measures the relative levels of MDA and other aldehydes in tissue homogenates (Fig. 4). After injury in the 1B7 controls, TBARS concentrations were increased significantly compared to sham-injured rats, by 59%, 72%, and 56%, respectively (p < 0.05 for all time points). After the anti-CD11d treatment, TBARS in the groups at 24 h, 72 h, and 4 weeks was significantly reduced compared to the 1B7 controls, by 28%, 27%, and 26%, respectively (p < 0.05 for all time points), and these values were no different from those in sham-injured rats.
FIG. 4.
Lipid peroxidation is reduced by the anti-CD11d treatment. TBARS concentrations at 24 h after TBI in the sham-injured (n = 6), 1B7 control, and anti-CD11d (n = 7 each) groups; at 72 h in these groups (n = 7, 7, and 8, respectively), and at 4 weeks (n = 5, 6, and 5, respectively; analysis of variance [ANOVA]: 24 h, F2,17 = 4.68, p = 0.024; 72 h, F2,19 = 5.10, p = 0.017; 4 weeks, F2,13 = 5.3, p = 0.021; ANOVA, analysis of variance; TBI, traumatic brain injury; TBARS, thiobarbituric acid reactive substances; *significantly different from sham animals, and #significantly different from the 1B7 control group, p ≤ 0.05 after the Student Neuman Keuls test and one-way ANOVA).
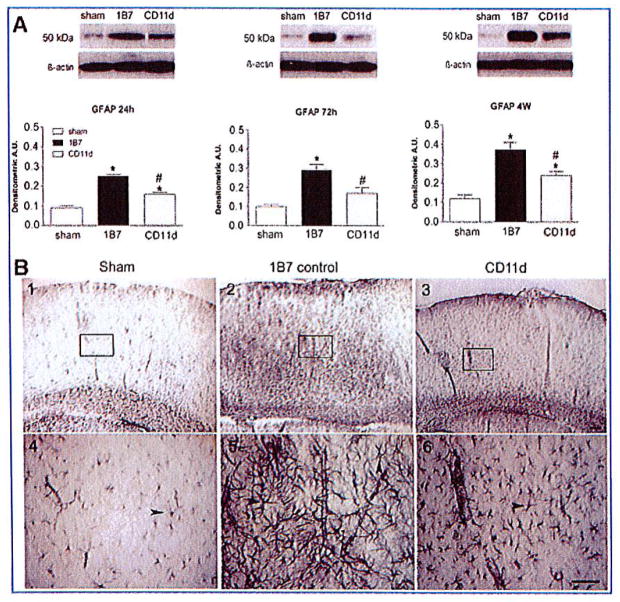
Glial fibrillary acidic protein
The effect of the anti-CD11d treatment on astrocyte activation in the brain lesions of sham-injured rats, 1B7 mAb-treated controls, and CD11d mAb-treated rats was assessed by examining GFAP levels by Western blot analysis (Fig. 5A). GFAP (~50 kDa) expression increased significantly, by ~3-fold in the 1B7 control rats at 24 h, 72 h and 4 weeks after TBI compared to sham-injured rats (p < 0.001 for all time points). The anti-CD11d treatment significantly dampened the injury-induced increase in GFAP expression (seen in the 1B7 controls) by 37% at 24 h (p < 0.001), by 41% at 72 h (p < 0.01), and by 37% at 4 weeks (p ≤ 0.01) after TBI. Immunohistochemistry using the GFAP antibody on sections from injured brains 4 weeks post-injury demonstrated increased astrogliosis, with densely-stained astrocytes bearing long, overlapping hypertrophic processes in the brains of the 1B7 mAb-treated controls (Fig. 5B, panels 2 and 5), compared to similar sections from CD11d mAb-treated or sham-injured control rats (Fig. 5B, panels 1, 3, 4, and 6). The same pattern of glial activation and treatment effect of the anti-CD11d antibody was observed in brain sections from rats examined at 24 h and 72 h after TBI (data not shown).
FIG. 5.
Astrogliosis assessed by the expression of glial fibrillary acidic protein (GFAP) in the injured brain is reduced by the anti-CD11d treatment. (A) GFAP, identified by Western blotting in cord homogenates, is shown at 24 h, 72 h, and 4 weeks, in the sham-injured, 1B7 control, and anti-CD11d groups (n = 5/group; analysis of variance [ANOVA]: 24 h, F2,12 = 35.75, p < 0.001; 72 h, F2,12 = 15.38, p < 0.001; 4 weeks, F2,12 = 22.27, p < 0.001). (B) Shown are photomicrographs of brain sections at the location of the fluid percussion injury immunostained by an antibody to GFAP. Boxed areas in panels 1–3 are shown at higher power in panels 4–6 (scale bars = 100 μm in A1–3 and in A4–6). Arrowheads indicate examples of reactive astrocytes. Note the increased intensity of anti-GFAP staining and the presence of overlapping, hypertrophic GFAP-positive processes in the sections from 1B7 controls (panels 2 and 5), compared to anti-CD11d treated rats (panels 3 and 6; *significantly different from sham animals, and #significantly different from the 1B7 control group, p ≤ 0.05 after the Student Neuman Keuls test and one-way ANOVA; A.U., arbitrary units). Color image is available online at www.liebertonline.com/neu
Amyloid precursor protein (APP)
APP expression has been shown to increase after neuronal injury due to TBI (Itoh et al., 2009; Murakami et al., 1998; Otsuka et al., 1991). The effect of the anti-CD11d treatment on neuronal damage was assessed in homogenates of the rat brains using Western blot analysis (Fig. 6A). Expression of APP (90 kDa) was evident in the brains of all sham-injured rats. This expression increased significantly after TBI in the 1B7 control rats at 24 h (by 3-fold, p < 0.01), at 72 h (by 3.4-fold, p < 0.001), and 4 weeks (by 4.4-fold, p < 0.01). The anti-CD11d treatment significantly reduced the injury-induced increase in APP expression (from the 1B7 control values), by 32% at 24 h (p < 0.05), by 31% at 72 h (p < 0.05), and by 39% at 4 weeks (p = 0.050) after TBI. Immunohistochemistry demonstrated low levels of APP expression in small punctate areas of the cortex in the sham-injured rats at 4 weeks after injury (Fig. 6B, panels 1 and 4). In contrast, large dark patches of APP immunoreactivity were present in the cortical areas adjacent to the lesions in 1B7 control rats at 4 weeks after the injury (Fig. 6B, panels 2 and 5). APP expression in the brains of anti-CD11d-treated rats at this time appeared less intense, and the patches of immunoreactivity were smaller than in the 1B7 controls (Fig. 6B, panels 3 and 6). A similar pattern of APP expression and effect in sham-injured, 1B7, and anti-CD11d-treated rats was observed at 24 h and 72 h after TBI (data not shown).
FIG. 6.
The increase in amyloid precursor protein (APP) expression in the injured brain is reduced by the anti-CD11d treatment. (A) APP, identified by Western blotting in brain homogenates, is shown at 24 h, 72 h, and 4 weeks, in the sham-injured, 1B7 control, and anti-CD11d groups (n = 5/group; analysis of variance [ANOVA]: 24 h, F2,12 = 10.46, p = 0.002; 72 h, F2,12 = 22.11, p < 0.001; 4 weeks, F2,12 = 9.04, p = 0.004). (B) Photomicrographs of the cortex centered on the lesion epicenter (or a comparable position in sham-injured rats) immunostained by an antibody to APP. Boxed areas in panels 1–3 are shown at high power in panels 4–6 (scale bars = 100 μm in A1–3 and A4–6). Arrowheads indicate APP-positive aggregates (*significantly different from sham animals, and #significantly different from the 1B7 control group, p ≤ 0.05 after the Student Neuman Keuls test and one-way ANOVA; A.U., arbitrary units). Color image is available online at www.liebertonline.com/neu
Neuronal survival
To provide a general estimate of neuronal survival after TBI, Western blotting for the neuronal marker NeuN was done using the brain homogenates. Robust expression of this protein was evident in all of the brains sampled (Fig. 7). Densitometry demonstrated that compared to sham-injured rats, expression of the NeuN protein decreased significantly in 1B7 control rats at 24 h (by 47%, p < 0.01), at 72 h (by 61%, p < 0.001), and at 4 weeks (by 67%, p < 0.001). The decreases in NeuN expression (from the sham-injured group) were smaller after anti-CD11d treatment (by 23%, 29%, and 38%, respectively), and all were significantly different from those in the 1B7 control group (p < 0.05, p < 0.01, and p = 0.05, respectively).
FIG. 7.
Expression of neuronal nuclear antigen (NeuN) in the injured brain. NeuN, identified by Western blotting in brain homogenates, is shown at 24 h, 72 h, and 4 weeks in the sham-injured, 1B7 control, and anti-CD11d groups (n = 5/group; analysis of variance [ANOVA]: 24 h, F2,12 = 10.42, p = 0.002; 72 h, F2,12 = 28.96, p < 0.001; 4 weeks, F2,12 = 12.52, p = 0.001; *significantly different from sham animals, and #significantly different from the 1B7 control group, p ≤ 0.05 after the Student Neuman Keuls test and one-way ANOVA; A.U., arbitrary units).
Because of the apparent tissue damage seen near the hippocampus in the hematoxylin and eosin-stained sections at 24 h after TBI (Fig. 1), and evidence from others that the hippocampus is particularly vulnerable to injury following lateral FPI (Grady et al., 2003), we evaluated the hippocampus as a site in which changes in neuron numbers might be detected by immunohistochemistry after TBI. At 4 weeks after TBI, the density of neurons distributed in the CA2-CA3 region of the hippocampus appeared decreased in the tissue sections of 1B7 control rats compared to the sham-injured and CD11d-mAb-treated rats (Fig. 8A). Although differences between the 1B7 group and the anti-CD11d group sometimes were not obvious from casual inspection, counts of the hippocampal neurons at 4 weeks after TBI revealed a significant increase (~ 20%) in the number of NeuN-expressing cells within a defined area of interest in the CA2-CA3 region in anti-CD11d-treated rats compared to the 1B7 control group (Fig. 8B, p<0.05). No differences in hippocampal neuronal counts were detected among the groups at 24 h or 72 h after TBI.
FIG. 8.
Counts of neurons in the hippocampus identified by neuronal nuclear antigen (NeuN). (A) Photomicrographs of sections of the hippocampus within the region of the FPI immunostained by an antibody to NeuN. The sections from the sham-injured, 1B7 control, and anti-CD11d groups are shown at low power in panels 1–3, and boxed areas are shown at high power in panels 4–6 (scale bars = 100 μm in A1-3 and A4-6). (B) Counts of neurons in a 0.33-mm2 area of interest (boxed areas in the images) within the hippocampus. Neuron counts are shown at 24h in the sham-injured, 1B7 control, and anti-CD11d groups (n = 4/group) at 24h, 72h (n = 5/group), and 4 weeks (n=9, 8, and 7, respectively; analysis of variance [ANOVA]: 24h, F2,9 = 0.06, p = 0.943; 72h, F2,12 = 0.15, p = 0.865; 4 weeks, F2,21=4.35, p = 0.026; *Significantly different from sham animals, and #significantly different from the 1B7 control group, p≤0.05 after the Student Neuman Keuls test and one-way ANVOVA). Color image is available online at www.liebertonline.com/neu
Behavioral findings: Water maze
To assess the effects of the anti-CD11d treatment on spatial cognition we analyzed acquisition time and the percentage of direct and circle swims in sham, 1B7, and CD11d mAb-treated rats. At 24 h post-injury, acquisition search time decreased in all groups as testing progressed, as indicated by a significant effect of trial (Fig. 9A; F9,306=5.473, p<0.001). However, search time decreased less in the 1B7 control and anti-CD11d groups than in the sham-injured group, as indicated by a significant effect of treatment (F2,34 = 7.655, p<0.01; 1B7 control and anti-CD11d >sham, p<0.05). In addition, a non-significant trend was noted for 1B7 control rats to display longer search times than anti-CD11d rats (p<0.10). The direct and circle swim data were consistent with the search time data in revealing fewer direct and circle swims in the 1B7 control group during acquisition compared to sham-injured rats (Fig. 9B, p<0.01). Anti-CD11d rats tended to display fewer direct and circle swims than sham-injured rats (p<0.10). Swim speed did not differ among the three groups (Fig. 9C).
FIG. 9.
The anti-CD11d treatment improved spatial cognition in the short recovery (SR) group as assessed by water maze testing. (A) Graph of search times for the acquisition task in the SR analysis for sham-injured rats (SHAM-SR, open triangles), 1B7-treated rats (1B7-SR, gray squares), and anti-CD11d-treated rats (CD11d-SR, solid black circles). Each block plotted on the x-axis represents the average of results of 2 of the 10 trials (n = 12 rats/group; *anti-CD11d-SR and 1B7-SR groups had significantly longer search times than the sham-SR group). (B) Percent of direct and circle swims in the acquisition task in the SR analysis for the three groups of rats. Histogram bars represent means of data during 10 water maze trials (*1B7-SR rats had significantly fewer direct and circle swims than the sham-SR rats; analysis of variance [ANOVA]: F2,36 = 5.616, p<0.01). (C) Swim speeds during these trials of acquisition testing did not differ between the three groups. (D) Search times for the reversal task in the SR analysis of reversal training (format and number of rats as in A; #the anti CD11d-SR group had significantly shorter search times compared to the 1B7-SR group, but did not differ from the sham-SR group; *the 1B7-SR group had significantly longer search times than the sham-SR group). (E) Direct and circle swims in the reversal task for the three groups of SR rats (#significantly more direct and circle swims than the 1B7-SR group; *different from sham-SR; ANOVA: F2,36 = 4.223, p<0.05). The anti-CD11d-SR group did not differ from the sham-SR group. (F) Swim speeds during these trials of acquisition training did not differ between the three groups.
During reversal training 48 h post-injury, search time decreased in all groups as testing progressed, as indicated by a significant effect of trial (Fig. 9D; F9,306=4.330, p<0.001). However, search times decreased more in the anti-CD11d and sham-injured groups than in the 1B7 control group, as indicated by a significant effect of treatment (F2,34 = 7.912, p<0.001; 1B7 control > sham-injured and anti-CD11d rats; p < 0.01). The direct and circle swim data were consistent with the search time data in revealing more direct and circle swims in the anti-CD11d and sham-injured groups than in the 1B7 control group (Fig. 9E; p<0.05). Again swim speed did not differ among the treatment groups (Fig. 9F).
At 4 weeks post-injury search times during acquisition training did not differ between groups (Fig. 10A). However, whereas the anti-CD11d treated rats did not differ significantly from sham-injured rats, the 1B7 control rats displayed fewer direct and circle swims than sham-injured rats (Fig. 10B; p <0.01). In addition, the 1B7 control rats tended to display fewer direct and circle swims than anti-CD11d rats (p <0.10). Swim speed did not differ among groups during the 4-week acquisition training (Fig. 10C). Furthermore, the groups demonstrated no significant differences in search time, direct and circle swims, or swim speed during reversal training.
FIG. 10.
Anti-CD11d treatment improved spatial cognition in the long recovery (LR) water maze. (A and D) No differences in search time in the acquisition or reversal tasks were found among the sham-injured rats (SHAM-SR, open triangles), 1B7-treated rats (1B7-SR, gray squares), and anti-CD11d-treated rats (CD11d-SR, solid black circles; n = 7 rats/group) tested 4 weeks post-injury. (B and E) Percentages of direct and circle swims in the study groups during acquisition and reversal training. Histogram bars represent means of data collected during the 10 water maze trials (*significantly different from sham-LR rats; F2,41 =4.346, p<0.05). The CD11d-LR group was no different from the sham-LR group. (C and F) Swim speeds during acquisition and reversal training were no different between the groups.
Behavioral findings: Beam task
Sensorimotor testing on the beam task at 48 h after injury demonstrated that the 1B7 control group executed more slips and falls than the sham-injured group (Fig. 11A; p<0.01). In contrast, the anti-CD11d treatment group did not differ from the sham-injured group. No significant group differences in beam traverse times were detected (p > 0.05; data not shown). During beam testing 4 weeks post-injury the anti-CD11d and sham-injured groups displayed fewer slips and falls than the 1B7 control group (Fig. 11B; p<0.05). No significant group differences in beam traverse times were found (p>0.05; data not shown).
FIG. 11.
The anti-CD11d treatment improved long recovery (LR) beam task performance. (A) Number of slips and falls during the beam task in the sham-short recovery (SHAM-SR), 1B7-SR, and CD11d-SR groups of rats (n = 12/group). Histogram bars represent means of data collected during the 10 beam task trials (*significantly different from the sham-SR group; F2,36=3.748, p<0.05). The CD11d-SR group did not differ from sham-SR group. (B) Number of slips and falls in the LR groups (n =7/group; #significantly different from the 1B7-LR group; *different from the sham-LR group; F2,41 = 4.526, p<0.05). The CD11d-LR group did not differ from the sham-LR group.
Behavioral findings: Elevated-plus maze
At 24 h post-injury, no differences were found in the percent of time spent in the open arm or number of closed arm entries (Fig. 12A and B; p>0.05). However, testing at 4 weeks post-injury demonstrated that the anti-CD11d and sham-injured groups spent a greater proportion of time in the open arms compared to the 1B7 control group (Fig. 12C; p<0.05). The number of closed arm entries did not differ between groups (Fig. 12D).
FIG. 12.
Anti-CD11d treatment reduces anxiety in long recovery (LR) testing. Time spent in the open arm of the maze (reciprocally related to anxiety; A), and number of entries into the closed arm (B), by the short recovery (SR) sham-injured (SHAM-SR, n =24), 1B7 (1B7-SR, n = 24), and anti-CD11d (CD11d-SR, n = 25) groups of rats. Histogram bars represent time or entries accrued during 5 min of free exploration. No significant differences were detected among the groups at this time. Time spent in the open arm of the maze (C), and number of closed arm entries (D), by the LR groups of rats (n = 14 rats/group; #significantly different from the 1B7-LR group; *significantly different from the sham-LR group; F2,41 =3.925, p<0.05). The CD11d-LR group did not differ from the sham-LR group in time spent in the open arm. The three LR groups did not differ from each other in the number of entries into the closed arm (SHAM-LR, sham-long recovery; 1B7-LR, 1B7-long recovery; CD11d-LR, CD11d-long recovery).
Discussion
The analysis of neutrophils and macrophages in the injured brains revealed a significant reduction in their levels in rats treated with the CD11d mAb. The reduced inflammatory response after TBI was paralleled by decreases in MPO levels, lipid peroxidation, astrogliosis, axonal injury, and neuronal loss in the brains of anti-CD11d-treated rats. The behavioral studies demonstrated that anti-CD11d integrin antibody treatment also reduced cognitive impairment, anxiety-like behaviors, and sensorimotor loss, relative to treatment with a control antibody after TBI. These robust changes were accomplished by a single intravenous treatment with the CD11d mAb. The anti-inflammatory effects of this treatment clearly lasted up to 72 h after the injury. This acute modification of the inflammatory cell population within the injured brain had long-lasting effects on the 4-week behavioral outcomes, as well as normalizing the glial and neuronal population responses at this time. Our data also confirm a preliminary study that demonstrated reduced contusion volume and macrophage infiltration in CD11d mAb-treated rats after TBI (Utagawa et al., 2008). Despite the reduction of the macrophage population in the injured brains in our CD11d mAb-treated animals, they had no infections in the CNS or elsewhere. This likely reflects the fact that the treatment was completed 48h post-injury, thus limiting the window of time during which the animals might be vulnerable to infection due to CD11d antibody-compromised leukocyte trafficking. These results are also similar to the functional improvements and reductions in inflammation demonstrated using acute administration of an ICAM-1 antibody (ICAM-1 is the receptor on endothelial cells that binds CD11d/CD18), and a P-selectin antibody (P-selectin also mediates neutrophil adhesion to endothelial cells) after FPI in rats (Grady et al., 1999; Knoblach and Faden, 2002).
Neuroinflammation and oxidative injury
The reduced neutrophil and macrophage populations in the injured brain after anti-CD11d treatment decreased the neuronal loss and axonal damage caused by the FPI, as the 1B7 control rats had significantly more of this loss than the anti-CD11d-treated group. The neutrophil influx was key to the intensity of the inflammation, as many of the macrophages likely originated from microglial activation initiated in part by the presence of the neutrophils (David and Kroner, 2011). The neurotoxic actions of inflammation within the brain are known to contribute to secondary damage after TBI (Dringen, 2005; Morganti-Kossmann et al., 2001; Schmidt et al., 2005). It follows that neuroinflammation and neuronal injury and loss could have contributed to the behavioral impairments induced by lateral FPI in the current study. The oxidative burst of activated neutrophils and macrophages generates free radicals that can lead to secondary CNS injury (Juurlink and Paterson, 1998; Lewen et al., 2000). Free radicals can induce tissue damage in the form of peroxidation of lipid membranes, protein nitration, and nucleic acid damage (Leski et al., 2001), all of which critically alter cellular function. The enzyme MPO is part of the pathway that generates oxidative stress via formation of hydrogen peroxide and other oxidative molecules. MPO is present in large quantities in granules within neutrophils, and in lesser quantities in macrophages (Barone et al., 1991; Bradley et al., 1982). At 24 h and 72 h post-injury, the anti-CD11d mAb treatment reduced MPO levels in the injured brain. Downstream lipid peroxidation in the injured brain was also significantly reduced by the anti-CD11d treatment.
The inflammatory response leads to a variety of cellular reactions within the injured brain. Astrogliosis was readily detected in rat brains after FPI by the intense increase in expression of GFAP that lasted for at least 4 weeks. Astrogliosis is both a marker of neuropathology and an active participant in neuroinflammation (Hamby and Sofroniew, 2010). Astrocytes respond to a host of cytokines that may be released by activated neutrophils and macrophages, such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and IL-1b, and may in turn release the same as well additional cytokines that regulate nearby microglia, neurons, and other astrocytes (Sofroniew, 2009). Thus the reduced astrogliosis in anti-CD11d-treated rats may be a reflection of, and may help maintain, the reduced neuroinflammation.
Expression of APP has been shown to increase after TBI, and is associated with axonal damage (Itoh et al., 2009; Murakami et al., 1998; Otsuka et al., 1991). Accumulations of APP were found in the injured cortex, and Western blots quantified increased APP expression as early as 24 h after injury. Increased expression of APP was maintained for the 4-week duration of our study. The anti-CD11d treatment reduced the increase in APP expression compared to 1B7 mAb-treated controls, but the APP in CD11d mAb-treated rats remained greater than in sham-injured rats, perhaps indicating that the reduction in inflammation reduced, but did not completely prevent, inflammatory-mediated axonal injury.
The secondary neuronal loss, due in part to inflammation, was monitored by Western blot analysis for NeuN levels, and by counting NeuN-expressing cells in the hippocampus. The Western blot analysis of brain homogenates revealed a significant reduction in NeuN expression at 24 h, 72 h, and 4 weeks after injury in 1B7 controls compared to sham-injured rats. Whereas NeuN expression levels were also lower in CD11d mAb-treated rats compared to sham-injured rats at these time points, NeuN expression was still significantly greater in the anti-CD11d-treated rats compared to the 1B7 controls at all time points tested. Cell counts in the CA2-CA3 regions of the hippocampus revealed a significant reduction (22%) in the number of NeuN-expressing cells in 1B7 controls compared to sham-injured rats at 4 weeks, but not at the earlier time points. The anti-CD11d treatment prevented this loss of hippocampal neurons, a likely mechanism for some of the behavioral recovery associated with the treatment (see further discussion below). The Western blot analysis of NeuN expression showed more differences between groups earlier (at 24 and 72 h) than the cell counts in the hippocampus, likely indicating that neuronal loss is occurring outside of the hippocampus, and/or that neurons may reduce NeuN expression without subsequently dying.
Behavioral outcomes
Previous research from our laboratory and others revealed cognitive deficits in the water maze in the presence of a neuroinflammatory response (Aiguo et al., 2010; Shultz et al., 2009; Wu et al., 2006). Other lateral FPI studies have also found motor, emotional, and cognitive impairments that occurred in the presence of extensive damage to the hippocampus and the frontal, parietal, and temporal cortices (Jones et al., 2008; Wahl et al., 2000). Accordingly, damage to the hippocampus and other areas may have contributed to the behavioral impairments seen in the elevated-plus maze, water maze, and beam task in the 1B7 controls. The CD11d treatment after FPI reduced neuronal loss in the hippocampus and more generally in the injured hemisphere, an effect that likely accounts for the reduced behavioral impairments seen in this group.
Short-term cognitive impairments were present in lateral FPI rats at 24 h after injury, regardless of treatment, as both the anti-CD11d and 1B7 control groups displayed longer search times than sham-injured rats during water maze acquisition. However, during water maze reversal, the anti-CD11d group performed as well as the sham-injured group, spending less time searching for the platform and displaying more direct and circle swims than 1B7 control rats. In addition, by 4 weeks after injury, the anti-CD11d rats displayed no water maze impairments, whereas the 1B7 control rats had cognitive deficits, indicated by significantly fewer direct and circle swims during acquisition than the sham-injured rats. Accordingly, rats treated with anti-CD11d antibody were cognitively impaired only during water maze acquisition at 24 h after the TBI, whereas rats treated with the 1B7 control antibody were cognitively impaired at 24 h and at the late 4-week recovery time point.
The fine motor deficits on the beam task experienced by the 1B7 control rats suggest that locomotor ability may need to be considered when interpreting water maze results. However, swim speed, a direct measure of motor ability in the water maze, did not differ among the groups, and the 1B7 mAb-treated rats did not demonstrate difficulty in swimming or climbing onto the hidden platform. Thus the fine motor impairments experienced by the 1B7 group were unlikely to have accounted directly for the deficits observed in the water maze. At 24 h and 4 weeks post-injury the anti-CD11d-treated rats performed as well as the sham-injured groups on the beam task, and at 4 weeks post-injury the anti-CD11d-treated rats performed significantly better than the 1B7 mAb-treated rats. The 1B7 mAb-treated rats displayed significantly more slips and falls than the sham-injured groups at both 24 h and 4 weeks post-injury. These results suggest that the anti-CD11d antibody treatment preserved motor function following lateral FPI. As the 1B7 mAb-control rats showed no impairments on the measures of beam traverse time, swim speed, or closed arm entries in the elevated-plus maze, we suggest that their beam task impairments were a result of fine motor deficits rather than gross motor impairments (Shultz et al., 2009).
Previous studies have shown that decreased time spent in the open arm of the elevated-plus maze represents an increase in anxiety-like behavior (Jones et al., 2008; Walf and Frye, 2007). The 1B7 control rats spent less time in the open arm of the elevated-plus maze compared to both the sham-injured rats and CD11d mAb-treated rats at 4 weeks after the TBI, suggesting that these rats experienced heightened levels of anxiety, and that the CD11d treatment reduced the anxiogenic effect of lateral FPI. As locomotor impairments might confound elevated-plus maze results, the finding that 1B7 control rats displayed impairments on the beam task must be considered in the interpretation of elevated-plus maze findings. Although 1B7 control rats displayed increased slips and falls on the beam task, they were not impaired on the measures of beam traverse time, the number of closed arm entries in the elevated-plus maze, or swim speed in the water maze. Therefore, 1B7 rats likely experienced fine motor deficits, or possibly impairments of balance mechanisms, but not gross locomotor impairment that would confound elevated-plus maze findings. Although the CD11d mAb-treated rats demonstrated better behavioral recovery than the 1B7 controls, they still performed significantly worse than sham-injured controls on some behavioral measures, and displayed some neuronal loss. Irreversible primary injuries may have contributed to these findings (Graham et al., 2000; Marshall, 2000). Moreover, the anti-CD11d treatment reduced but did not completely prevent the neutrophil and macrophage infiltration of the injured brain, leading to an attenuated inflammatory response that may have led to the impaired behavior. As the current study is only the second to use the CD11d mAb to treat TBI, the treatment regimen applied may not have been optimal, generating a less robust outcome than may otherwise be possible.
Conclusions
A single treatment of anti-CD11d integrin antibody administered 2h after moderate TBI reduced cognitive, emotional, and motor impairments in the Long-Evans rat. As the reduction in impairment occurred in parallel with decreased neuroinflammation, oxidative injury, and gross neuronal loss, the anti-CD11d antibody treatment may have improved outcomes by limiting the infiltration of peripheral leukocytes and decreasing the neurotoxic effects of neuroinflammation. Together, these findings suggest that infiltrating leukocytes increase neuroinflammation, secondary brain damage, and functional impairment following TBI, and that the anti-CD11d antibody holds promise as a novel treatment to limit secondary injury processes and improve recovery from TBI.
Acknowledgments
This work was supported the Canadian Institutes of Health Research (L.C.W. and A.B.), and the Natural Sciences and Engineering Research Council of Canada (D.P.C.).
Footnotes
Author Disclosure Statement
No conflicting financial interests exist.
References
- Aiguo W, Zhe Y, Gomez-Pinilla F. Vitamin E protects against oxidative damage and learning disability after mild traumatic brain injury in rats. Neurorehabil Neural Repair. 2010;24:290–298. doi: 10.1177/1545968309348318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atif F, Sayeed I, Ishrat T, Stein DG. Progesterone with vitamin D affords better neuroprotection against excitotoxicity in cultured cortical neurons than progesterone alone. Molecular Med. 2009;15:328–336. doi: 10.2119/molmed.2009.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao F, Chen Y, Dekaban GA, Weaver LC. An anti-CD11d integrin antibody reduces cyclooxygenase-2 expression and protein and DNA oxidation after spinal cord injury in rats. J Neurochem. 2004a;90:1194–1204. doi: 10.1111/j.1471-4159.2004.02580.x. [DOI] [PubMed] [Google Scholar]
- Bao F, Chen Y, Dekaban GA, Weaver LC. Early anti-inflammatory treatment reduces lipid peroxidation and protein nitration after spinal cord injury in rats. J Neurochem. 2004b;88:1335–1344. doi: 10.1046/j.1471-4159.2003.02240.x. [DOI] [PubMed] [Google Scholar]
- Bao F, Dekaban GA, Weaver LC. Anti-CD11d antibody treatment reduces free radical formation and cell death in the injured spinal cord of rats. J Neurochem. 2005;94:1361–1373. doi: 10.1111/j.1471-4159.2005.03280.x. [DOI] [PubMed] [Google Scholar]
- Barone FC, Hillegass LM, Price WJ, White RF, Lee EV, Feuerstein GZ, Sarau HM, Clark RK, Griswold DE. Polymorphonuclear leukocyte infiltration into cerebral focal ischemic tissue: myeloperoxidase activity assay and histologic verification. J Neurosci, Res. 1991;29:336–345. doi: 10.1002/jnr.490290309. [DOI] [PubMed] [Google Scholar]
- Bevilacqua MP. Endothelial-leukocyte adhesion molecules. Annu Rev Immunol. 1993;11:767–804. doi: 10.1146/annurev.iy.11.040193.004003. [DOI] [PubMed] [Google Scholar]
- Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Investigative Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- Cain P, Boughey J. Open learning. Quantum leap Nursing Times. 1993;89:36–37. [PubMed] [Google Scholar]
- Carlos TM, Clark RS, Franicola-Higgins D, Schiding JK, Kochanek PM. Expression of endothelial adhesion molecules and recruitment of neutrophils after traumatic brain injury in rats. J Leukocyte Biol. 1997;61:279–285. doi: 10.1002/jlb.61.3.279. [DOI] [PubMed] [Google Scholar]
- Clark RS, Carlos TM, Schiding JK, Bree M, Fireman LA, DeKosky ST, Kochanek PM. Antibodies against Mac-1 attenuate neutrophil accumulation after traumatic brain injury in rats. J Neurotrauma. 1996;13:333–341. doi: 10.1089/neu.1996.13.333. [DOI] [PubMed] [Google Scholar]
- David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci. 2011;12:388–399. doi: 10.1038/nrn3053. [DOI] [PubMed] [Google Scholar]
- D’Avila JC, Lam TI, Bingham D, Shi J, Won SJ, Kauppinen TM, Massa S, Liu J, Swanson RA. Microglial activation induced by brain trauma is suppressed by post-injury treatment with a PARP inhibitor. J Neuroinflammation. 2012;9:31. doi: 10.1186/1742-2094-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly DJ, Popovich PG. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exper Neurol. 2008;209:378–388. doi: 10.1016/j.expneurol.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doppenberg EM, Choi SC, Bullock R. Clinical trials in traumatic brain injury: lessons for the future. J Neurosurgical Anesthesiol. 2004;16:87–94. doi: 10.1097/00008506-200401000-00019. [DOI] [PubMed] [Google Scholar]
- Dringen R. Oxidative and antioxidative potential of brain microglial cells. Antioxid Redox Signal. 2005;7:1223–1233. doi: 10.1089/ars.2005.7.1223. [DOI] [PubMed] [Google Scholar]
- Farkas I, Baranyi L, Takahashi M, Fukuda A, Liposits Z, Yamamoto T, Okada H. A neuronal C5a receptor and an associated apoptotic signal transduction pathway. J Physiol. 1998;507(Pt. 3):679–687. doi: 10.1111/j.1469-7793.1998.679bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming JC, Bao F, Chen Y, Hamilton EF, Relton JK, Weaver LC. Alpha4beta1 integrin blockade after spinal cord injury decreases damage and improves neurological function. Exp Neurol. 2008;214:147–159. doi: 10.1016/j.expneurol.2008.04.024. [DOI] [PubMed] [Google Scholar]
- Ghirnikar RS, Lee YL, Eng LF. Inflammation in traumatic brain injury: role of cytokines and chemokines. Neurochem Res. 1998;23:329–340. doi: 10.1023/a:1022453332560. [DOI] [PubMed] [Google Scholar]
- Grady MS, Charleston JS, Maris D, Witgen BM, Lifshitz J. Neuronal and glial cell number in the hippocampus after experimental traumatic brain injury: analysis by stereological estimation. J Neurotrauma. 2003;20:929–941. doi: 10.1089/089771503770195786. [DOI] [PubMed] [Google Scholar]
- Grady MS, Cody RF, Jr, Maris DO, McCall TD, Seckin H, Sharar SR, Winn HR. P-selectin blockade following fluid-percussion injury: behavioral and immunochemical sequelae. J Neurotrauma. 1999;16:13–25. doi: 10.1089/neu.1999.16.13. [DOI] [PubMed] [Google Scholar]
- Graham DI, McIntosh TK, Maxwell WL, Nicoll JA. Recent advances in neurotrauma. J Neuropathol Exper Neurol. 2000;59:641–651. doi: 10.1093/jnen/59.8.641. [DOI] [PubMed] [Google Scholar]
- Gris D, Marsh DR, Oatway MA, Chen Y, Hamilton EF, Dekaban GA, Weaver LC. Transient blockade of the CD11d/CD18 integrin reduces secondary damage after spinal cord injury, improving sensory, autonomic, and motor function. J Neurosci. 2004;24:4043–4051. doi: 10.1523/JNEUROSCI.5343-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall ED. Inhibition of lipid peroxidation in central nervous system trauma and ischemia. J Neurological Sci. 1995;134(Suppl):79–83. doi: 10.1016/0022-510x(95)00211-j. [DOI] [PubMed] [Google Scholar]
- Hamby ME, Sofroniew MV. Reactive astrocytes as therapeutic targets for CNS disorders. Neurotherapeutics. 2010;7:494–506. doi: 10.1016/j.nurt.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg N, Leitinger B. Shape and shift changes related to the function of leukocyte integrins LFA-1 and Mac-1. J Leukocyte Biol. 2001;69:893–898. [PubMed] [Google Scholar]
- Holmin S, Soderlund J, Biberfeld P, Mathiesen T. Intracerebral inflammation after human brain contusion. Neurosurgery. 1998;42:291–298. doi: 10.1097/00006123-199802000-00047. discussion 298–299. [DOI] [PubMed] [Google Scholar]
- Itoh T, Satou T, Nishida S, Tsubaki M, Hashimoto S, Ito H. Expression of amyloid precursor protein after rat traumatic brain injury. Neurological Res. 2009;31:103–109. doi: 10.1179/016164108X323771. [DOI] [PubMed] [Google Scholar]
- Jones NC, Cardamone L, Williams JP, Salzberg MR, Myers D, O’Brien TJ. Experimental traumatic brain injury induces a pervasive hyperanxious phenotype in rats. J Neurotrauma. 2008;25:1367–1374. doi: 10.1089/neu.2008.0641. [DOI] [PubMed] [Google Scholar]
- Juurlink BH, Paterson PG. Review of oxidative stress in brain and spinal cord injury: suggestions for pharmacological and nutritional management strategies. J Spinal Cord Med. 1998;21:309–334. doi: 10.1080/10790268.1998.11719540. [DOI] [PubMed] [Google Scholar]
- Kadhim HJ, Duchateau J, Sebire G. Cytokines and brain injury: invited review. J Intensive Care Med. 2008;23:236–249. doi: 10.1177/0885066608318458. [DOI] [PubMed] [Google Scholar]
- Knoblach SM, Faden AI. Administration of either anti-intercellular adhesion molecule-1 or a nonspecific control antibody improves recovery after traumatic brain injury in the rat. J Neurotrauma. 2002;19:1039–1050. doi: 10.1089/089771502760341956. [DOI] [PubMed] [Google Scholar]
- Kolb B, Whishaw IQ. Earlier is not always better: behavioral dysfunction and abnormal cerebral morphogenesis following neonatal cortical lesions in the rat. Behavioural Brain Res. 1985;17:25–43. doi: 10.1016/0166-4328(85)90005-1. [DOI] [PubMed] [Google Scholar]
- Laird MD, Vender JR, Dhandapani KM. Opposing roles for reactive astrocytes following traumatic brain injury. Neurosignals. 2008;16:154–164. doi: 10.1159/000111560. [DOI] [PubMed] [Google Scholar]
- Leski ML, Bao F, Wu L, Qian H, Sun D, Liu D. Protein and DNA oxidation in spinal injury: neurofilaments—an oxidation target. Free Radical Biol Med. 2001;30:613–624. doi: 10.1016/s0891-5849(00)00500-1. [DOI] [PubMed] [Google Scholar]
- Lewen A, Matz P, Chan PH. Free radical pathways in CNS injury. J Neurotrauma. 2000;17:871–890. doi: 10.1089/neu.2000.17.871. [DOI] [PubMed] [Google Scholar]
- Maas AI, Dearden M, Servadei F, Stocchetti N, Unterberg A. Current recommendations for neurotrauma. Curr Opin Crit Care. 2000;6:281–292. [PubMed] [Google Scholar]
- Marshall LF. Head injury: recent past, present, and future. Neurosurgery. 2000;47:546–561. doi: 10.1097/00006123-200009000-00002. [DOI] [PubMed] [Google Scholar]
- Morganti-Kossmann MC, Rancan M, Otto VI, Stahel PF, Kossmann T. Role of cerebral inflammation after traumatic brain injury: a revisited concept. Shock. 2001;16:165–177. doi: 10.1097/00024382-200116030-00001. [DOI] [PubMed] [Google Scholar]
- Morganti-Kossmann MC, Rancan M, Stahel PF, Kossmann T. Inflammatory response in acute traumatic brain injury: a double-edged sword. Curr Opin Crit Care. 2002;8:101–105. doi: 10.1097/00075198-200204000-00002. [DOI] [PubMed] [Google Scholar]
- Morganti-Kossmann MC, Satgunaseelan L, Bye N, Kossmann T. Modulation of immune response by head injury. Injury. 2007;38:1392–1400. doi: 10.1016/j.injury.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Morris RG. Synaptic plasticity and learning: selective impairment of learning rats and blockade of long-term potentiation in vivo by the N-methyl-D-aspartate receptor antagonist AP5. J Neurosci. 1989;9:3040–3057. doi: 10.1523/JNEUROSCI.09-09-03040.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami N, Yamaki T, Iwamoto Y, Sakakibara T, Kobori N, Fushiki S, Ueda S. Experimental brain injury induces expression of amyloid precursor protein, which may be related to neuronal loss in the hippocampus. J Neurotrauma. 1998;15:993–1003. doi: 10.1089/neu.1998.15.993. [DOI] [PubMed] [Google Scholar]
- Oatway MA, Chen Y, Bruce JC, Dekaban GA, Weaver LC. Anti-CD11d integrin antibody treatment restores normal serotonergic projections to the dorsal, intermediate, and ventral horns of the injured spinal cord. J Neurosci. 2005;25:637–647. doi: 10.1523/JNEUROSCI.3960-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analyt Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Otsuka N, Tomonaga M, Ikeda K. Rapid appearance of beta-amyloid precursor protein immunoreactivity in damaged axons and reactive glial cells in rat brain following needle stab injury. Brain Res. 1991;568:335–338. doi: 10.1016/0006-8993(91)91422-w. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; Sydney: 1986. [Google Scholar]
- Rao V, Lyketsos C. Neuropsychiatric sequelae of traumatic brain injury. Psychosomatics. 2000;41:95–103. doi: 10.1176/appi.psy.41.2.95. [DOI] [PubMed] [Google Scholar]
- Saucier DM, Shultz SR, Keller AJ, Cook CM, Binsted G. Sex differences in object location memory and spatial navigation in Long-Evans rats. Anim Cogn. 2008;11:129–137. doi: 10.1007/s10071-007-0096-1. [DOI] [PubMed] [Google Scholar]
- Saville LR, Pospisil CH, Mawhinney LA, Bao F, Simedrea FC, Peters AA, O’Connell PJ, Weaver LC, Dekaban GA. A monoclonal antibody to CD11d reduces the inflammatory infiltrate into the injured spinal cord: a potential neuroprotective treatment. J Neuroimmunol. 2004;156:42–57. doi: 10.1016/j.jneuroim.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Schmidt OI, Heyde CE, Ertel W, Stahel PF. Closed head injury—an inflammatory disease? Brain Res Brain Res Rev. 2005;48:388–399. doi: 10.1016/j.brainresrev.2004.12.028. [DOI] [PubMed] [Google Scholar]
- Schoettle RJ, Kochanek PM, Magargee MJ, Uhl MW, Nemoto EM. Early polymorphonuclear leukocyte accumulation correlates with the development of posttraumatic cerebral edema in rats. J Neurotrauma. 1990;7:207–217. doi: 10.1089/neu.1990.7.207. [DOI] [PubMed] [Google Scholar]
- Shultz SR, Bao F, Omana V, Chiu C, Brown A, Cain DP. Repeated mild lateral fluid percussion brain injury in the rat causes cumulative long-term behavioral impairments, neuroinflammation, and cortical loss in an animal model of repeated concussion. J Neurotrauma. 2011a;29:281–294. doi: 10.1089/neu.2011.2123. [DOI] [PubMed] [Google Scholar]
- Shultz SR, MacFabe DF, Foley KA, Taylor R, Cain DP. A single mild fluid percussion injury induces short-term behavioral and neuropathological changes in the Long-Evans rat: support for an animal model of concussion. Behavioural Brain Res. 2011b;224:326–335. doi: 10.1016/j.bbr.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Shultz SR, Macfabe DF, Martin S, Jackson J, Taylor R, Boon F, Ossenkopp KP, Cain DP. Intracerebroventricular injections of the enteric bacterial metabolic product propionic acid impair cognition and sensorimotor ability in the Long-Evans rat: further development of a rodent model of autism. Behavioural Brain Res. 2009;200:33–41. doi: 10.1016/j.bbr.2008.12.023. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steimer T, Driscoll P. Divergent stress responses and coping styles in psychogenetically selected Roman high-(RHA) and low-(RLA) avoidance rats: behavioural, neuroendocrine and developmental aspects. Stress. 2003;6:87–100. doi: 10.1080/1025389031000111320. [DOI] [PubMed] [Google Scholar]
- Tan AA, Quigley A, Smith DC, Hoane MR. Strain differences in response to traumatic brain injury in Long-Evans compared to Sprague-Dawley rats. J Neurotrauma. 2009;26:539–548. doi: 10.1089/neu.2008.0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taoka Y, Okajima K. Spinal cord injury in the rat. Prog Neurobiol. 1998;56:341–358. doi: 10.1016/s0301-0082(98)00049-5. [DOI] [PubMed] [Google Scholar]
- Thompson HJ, Lifshitz J, Marklund N, Grady MS, Graham DI, Hovda DA, McIntosh TK. Lateral fluid percussion brain injury: a 15-year review and evaluation. J Neurotrauma. 2005;22:42–75. doi: 10.1089/neu.2005.22.42. [DOI] [PubMed] [Google Scholar]
- Utagawa A, Bramlett HM, Daniels L, Lotocki G, Dekaban GA, Weaver LC, Dietrich WD. Transient blockage of the CD11d/CD18 integrin reduces contusion volume and macrophage infiltration after traumatic brain injury in rats. Brain Res. 2008;1207:155–163. doi: 10.1016/j.brainres.2008.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl S, Barth H, Ciossek T, Aktories K, Mueller BK. Ephrin-A5 induces collapse of growth cones by activating Rho and Rho kinase. J Cell Biol. 2000;149:263–270. doi: 10.1083/jcb.149.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2:322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw IQ, Jarrard LE. Similarities vs. differences in place learning and circadian activity in rats after fimbria-fornix section or ibotenate removal of hippocampal cells. Hippocampus. 1995;5:595–604. doi: 10.1002/hipo.450050610. [DOI] [PubMed] [Google Scholar]
- Wu A, Ying Z, Gomez-Pinilla F. Dietary curcumin counteracts the outcome of traumatic brain injury on oxidative stress, synaptic plasticity, and cognition. Exper Neurol. 2006;197:309–317. doi: 10.1016/j.expneurol.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Zhuang J, Shackford SR, Schmoker JD, Anderson ML. The association of leukocytes with secondary brain injury. J Trauma. 1993;35:415–422. doi: 10.1097/00005373-199309000-00014. [DOI] [PubMed] [Google Scholar]
- Zhu SW, Yee BK, Nyffeler M, Winblad B, Feldon J, Mohammed AH. Influence of differential housing on emotional behaviour and neurotrophin levels in mice. Behavioural Brain Res. 2006;169:10–20. doi: 10.1016/j.bbr.2005.11.024. [DOI] [PubMed] [Google Scholar]
This article has been cited by
- 1.Zhang B, Gensel JC. Is neuroinflammation in the injured spinal cord different than in the brain? Examining intrinsic differences between the brain and spinal cord. Experimental Neurology. 2014;258:112–120. doi: 10.1016/j.expneurol.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Shultz Sandy R, TanXin L, Wright David K, Liu Shijie J, Semple Bridgette D, Leigh Johnston, Jones Nigel C, Cook Andrew D, Hamilton John A, O’Brien Terence J. Granulocyte-Macrophage Colony-Stimulating Factor Is Neuroprotective in Experimental Traumatic Brain Injury. Journal of Neurotrauma. 2014;31:10. doi: 10.1089/neu.2013.3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.John C. GenselSpinal Cord Injury. :339–361. [Google Scholar]
- 4.Gold Eric M, Su Diane, López-Velázquez Luci, Haus Daniel L, Perez Harvey, Lacuesta George A, Anderson Aileen J, Cummings Brian J. Functional assessment of long-term deficits in rodent models of traumatic brain injury. Regenerative Medicine. 2013;8:4. doi: 10.2217/rme.13.41. [DOI] [PubMed] [Google Scholar]
- 5.Shultz Sandy R, Bao Feng, Weaver Lynne C, Cain Donald P, Brown Arthur. Treatment with an anti-CD11d integrin antibody reduces neuroinflammation and improves outcome in a rat model of repeated concussion. Journal of Neuroinflammation. 2013;10:1. doi: 10.1186/1742-2094-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]