Abstract
Our previous study has shown that activating peripheral μ-receptors is necessary for switching the bronchopulmonary C-fibers (PCFs)-mediated rapid shallow breathing (RSB) into an apnea by systemic administration of fentanyl. The brainstem nuclei, such as the medial nucleus tractus solitarius (mNTS) and the Pre-Botzinger Complex (PBC), are required for completing the PCF-mediated respiratory reflexes. Moreover, these areas contain abundant μ-receptors and their activation prolongs expiratory duration (TE). Thus, we asked if central μ-receptors, especially those in the mNTS and PBC, are involved in fully expressing this RSB-apnea switch by fentanyl. In anesthetized rats, the cardiorespiratory responses to right atrial injection of phenylbiguanide (PBG, 3–6 μg/kg) were repeated after: 1) fentanyl (iv), a μ-receptor agonist, alone (8 μg/kg, iv); 2) fentanyl following microinjection of naloxone methiodide (NXM, an opioid receptor antagonist) into the cisterna magna (10 μg/4 μl); 3) the bilateral mNTS (10 mM, 20 nl); or 4) PBC (10 mM, 20 nl). Our results showed that PBG shortened TE by 37 ± 6 % (RSB, from 0.41 ± 0.05 to 0.26 ± 0.03 s, P < 0.01), but it markedly prolonged TE by 5.8-fold (an apnea, from 0.50 ± 0.04 s to 2.9 ± 0.57 s, P < 0.01) after fentanyl (iv). Pretreatment with NXM injected into the cisterna magna or the PBC, but not the mNTS, prevented the fentanyl-induced switch. This study, along with our previous results mentioned above, suggests that although peripheral μ-receptors are essential for triggering the fentanyl-induced switch, central μ-receptors, especially those in the PBC, are required to fully exhibit such switch.
Keywords: Fentanyl, bronchopulmonary C-fibers, the medial nucleus tractus solitarius, the pre-Botzinger Complex, respiratory rhythm
1. Introduction
Rapid shallow breathing (RSB) can be produced by stimulating bronchopulmonary C-fibers (PCFs) which constitute 75–90% of the sensory fibers in the pulmonary branches of the vagus nerve (Agostoni et al., 1957; Jammes et al., 1982; Lee et al., 2003; Mei et al., 1980). PCFs are critical in modulating respiratory frequency, and its excitatory or inhibitory impacts on respiratory frequency are dependent on the stimulating intensity on these sensory nerve endings. For example, to stimulate PCF, right atrial bolus injection of phenylbiguanide (PBG) at a low dose evokes RSB, but it at a high dose induces an apnea (Coleridge and Coleridge, 1994; Dutta and Deshpande, 2010; Moreira et al., 2007). Because μ-receptor agonists could sensitize/activate PCFs (Willette and Sapru, 1982), we previously tested whether systemic administration of μ-receptor agonist fentanyl would switch the PCFs-mediated RSB into an apnea via sensitizing PCFs (Zhang et al., 2010). As the result, fentanyl switched the PCFs-mediated RSB into an apnea, at least, by acting peripheral opioid receptors, especially those in nodose ganglia. These data favor a peripheral mechanism by which fentanyl could directly facilitate PCFs’ activity to significantly augment the PBG-induced PCF excitation, and consequently, turn the PBG-evoked RSB into an apnea.
Respiratory reflexes triggered by PCF activation are centrally regulated. There are, at least, two medullary regions essential for completing the PCF-mediated respiratory reflexes (Kubin et al., 2006). One is the medial nucleus tractus solitarius (mNTS) (Kubin et al., 2006) that receives PCFs’ inputs, and another one is the pre-Botzinger complex (PBC) (Paton, 1997; Wilson and Bonham, 1997), a presumed respiratory rhythm pacemaker (Feldman and Del Negro, 2006; Smith et al., 1991). Because opioid μ-receptors extensively exist in the brainstem including the mNTS and PBC (Ding et al., 1996; Ding et al., 1998; Haji et al., 2003; Krajnik et al., 2010) and fentanyl has an easy access to the central nervous system (Bellet et al., 1980), we tested whether the central μ-receptors, especially those in the mNTS and PBC, would be involved in fully expressing the fentanyl-induced switch of RSB-apnea.
2. Results
2.1. The fentanyl-induced VE inhibition is attenuated by blocking central μ-receptors
As presented in Table 1, central administration of NXM does not markedly change cardiorespiratory variables except that microinjection of NXM into the PBC decreased f and consequently increased TE. Table 2 summarizes the cardiorespiratory changes induced by fentanyl alone and coupled with different central NXM pretreatments. As illustrated, intravenous administration of fentanyl alone significantly inhibited baseline VE by 40% due to the depression of f and VT with an increased TE. Respiratory frequency was gradually decreased by slow injection of fentanyl (iv) and became stable and regular 5 min later. The fentanyl-induced VE inhibition (−40 ± 2%) was significantly attenuated by microinjecting NXM into the cisterna magna (−23 ± 2%, P < 0.01), the mNTS (−26 ± 2%, P < 0.01), and the PBC (−19 ± 2%, P < 0.01). With respect to baseline cardiovascular variables, fentanyl alone increased MBP, but this fentanyl-induced pressor effect was eliminated after NXM microinjected into the cisterna magna, mNTS or PBC. Fentanyl alone or coupled with central NXM pretreatments failed to significantly alter HR although there was a tendency of HR decrease.
Table 1.
Comparison of baseline cardiorespiratory variables before and after local microinjection of naloxone methiodide and vehicle.
| VE (ml/min) | f (breaths/min) | VT (ml) | TE (s) | MBP (mmHg) | HR (beats/min) | ||
|---|---|---|---|---|---|---|---|
| NXM (ic) (n = 6) | Before | 264 ± 32 | 100 ± 6 | 2.5 ± 0.2 | 0.38 ± 0.03 | 94 ± 5 | 383 ± 16 |
| After | 267 ± 38 | 97 ± 6 | 2.7 ± 0.4 | 0.39 ± 0.04 | 91 ± 7 | 380 ± 17 | |
| NXM (mNTS) (n = 6) | Before | 253 ± 20 | 104 ± 7 | 2.5 ± 0.3 | 0.36 ± 0.03 | 87 ± 4 | 358 ± 21 |
| After | 251 ± 22 | 98 ± 8 | 2.6 ± 0.3 | 0.38 ± 0.05 | 86 ± 6 | 364 ± 23 | |
| NXM (PBC) (n = 6) | Before | 274 ± 12 | 98 ± 9 | 3.2 ± 0.4 | 0.39 ± 0.03 | 98 ± 5 | 361 ± 12 |
| After | 262 ± 11 | 75 ± 7* | 3.7 ± 0.5 | 0.52 ± 0.05* | 97 ± 10 | 358± 15 | |
| Vehicle (ic) (n = 6) | Before | 270 ± 13 | 94 ± 6 | 2.9 ± 0.1 | 0.42 ± 0.04 | 97 ± 5 | 374 ± 14 |
| After | 273 ± 14 | 96 ± 7 | 2.9 ± 0.2 | 0.40 ± 0.03 | 96 ± 5 | 376 ± 24 | |
| Vehicle (PBC) (n = 6) | Before | 259 ± 15 | 89 ± 6 | 2.6 ± 0.2 | 0.43 ± 0.04 | 88 ± 8 | 353 ± 19 |
| After | 253 ± 24 | 92 ± 8 | 2.5 ± 0.3 | 0.41 ± 0.03 | 93 ± 9 | 367 ± 28 |
Data are shown as mean ± SE;
P < 0.05, compared with “before”. ic, intracisternal microinjection; f, respiratory frequency; HR, heart rate; MBP, mean arterial blood pressure; mNTS, local microinjection into the medial nucleus tractus solitarius; NXM, naloxone methiodide; PBC, local microinjection into pre-Botzinger complex; TE, expiratory duration; VE, minute ventilation; VT, tidal volume.
Table 2.
Effect of intravenous administration of fentanyl alone or coupled with a given pretreatment on baseline cardiorespiratory variables
| VE (ml/min) | f (breaths/min) | VT (ml) | TE (s) | MBP (mmHg) | HR (beats/min) | ||
|---|---|---|---|---|---|---|---|
| FEN (iv) (n = 6) | Before | 253 ± 21 | 102 ± 5 | 2.5 ± 0.2 | 0.37 ± 0.03 | 92 ± 5 | 347 ± 21 |
| After | 151± 20 ** | 76 ± 6 ** | 2.1± 0.2 ** | 0.52±0.06** | 108±7 ** | 329 ± 28 | |
| NXM (ic)+FEN (iv) (n = 6) | Before | 265 ± 24 | 96 ± 6 | 3.0 ± 0.3 | 0.39 ± 0.03 | 86 ± 7 | 381 ± 26 |
| After | 204 ± 28 * | 85 ± 2 * | 2.6 ± 0.3 ** | 0.45 ± 0.04* | 95 ± 9 | 352 ± 28 | |
| NXM (mNTS)+FEN (iv) (n = 6) | Before | 252 ± 30 | 98 ± 7 | 2.6 ± 0.3 | 0.38 ± 0.03 | 82 ± 6 | 364 ± 27 |
| After | 178 ± 21 * | 85 ± 6 * | 2.1 ± 0.3* | 0.46 ± 0.04* | 87 ± 7 | 326 ± 36 | |
| NXM (PBC)+FEN (iv) (n = 6) | Before | 259 ± 9 | 108 ± 8 | 2.4 ± 0.2 | 0.35 ± 0.03 | 98 ± 8 | 384 ± 12 |
| After | 213 ± 8 ** | 91 ± 7 * | 2.3 ± 0.3 | 0.41 ± 0.04* | 97 ± 9 | 358 ± 25 | |
| Vehicle (ic)+FEN (iv) (n = 6) | Before | 271 ± 11 | 97 ± 5 | 2.8 ± 0.1 | 0.38 ± 0.02 | 94 ± 7 | 368 ± 26 |
| After | 170 ± 12 ** | 75 ± 5 ** | 2.1 ± 0.1 ** | 0.52 ± 0.04** | 112 ± 9 * | 341 ± 28 | |
| Vehicle (PBC)+FEN (iv) (n = 6) | Before | 257 ± 14 | 96 ± 5 | 2.6 ± 0.2 | 0.38 ± 0.03 | 102 ± 5 | 379 ± 10 |
| After | 162 ± 13** | 73 ± 6 * | 2.0 ± 0.1** | 0.53 ± 0.05* | 126 ± 6* | 354 ± 25 |
Data are shown as mean ± SE;
P < 0.05
P < 0.01 compared with “before”. ic, intracisternal microinjection; f, respiratory frequency; HR, heart rate; MBP, mean arterial blood pressure; mNTS, local microinjection into the medial nucleus tractus solitarius; NXM, naloxone methiodide; PBC, local microinjection into pre-Botzinger complex; TE, expiratory duration; VE, minute ventilation; VT, tidal volume.
2.2. Intracisternal infusion of NXM prevents the fentanyl-induced switch
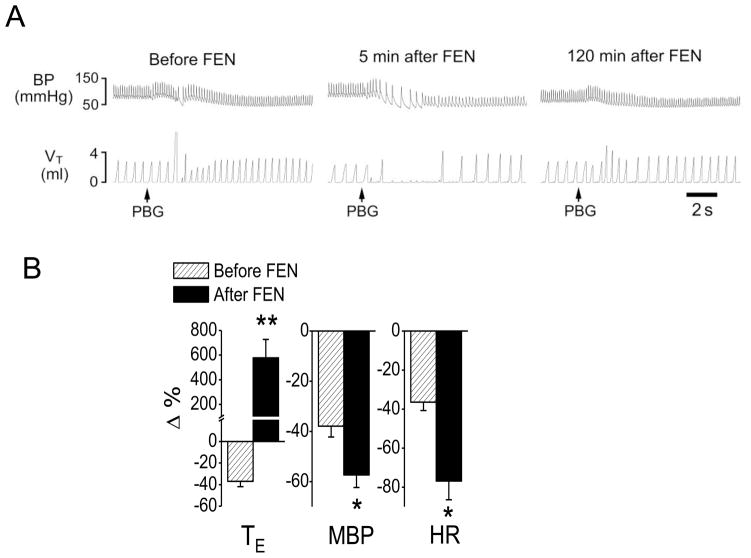
We first confirmed that systemically activating μ receptors by fentanyl (iv) switched the PCF-mediated RSB into an apnea. As shown in Fig. 1, bolus injection of PBG (3–6 μg/kg) into the right atrium produced RSB by shortening TE (−37%, from 0.41 ± 0.05 to 0.26 ± 0.03 s, P < 0.01) accompanied with hypotension (from 94 ± 6 to 61 ± 6 mmHg, P < 0.01) and bradycardia (from 347 ± 21 to 209 ± 17 beats/min, P < 0.05). After systemic fentanyl administration, the same dose of PBG produced a long-lasting apnea with TE 5.8-fold longer than the control (increasing from 0.50 ± 0.04 s to 2.9 ± 0.57 s, P < 0.01). Furthermore, the PBG-induced hypotension and bradycardia were markedly aggravated after fentanyl, i.e., MBP changed from −37% to −57% and HR altered from −36% to −76%. All of these results were similar to our previous report (Zhang et al., 2010).
Fig. 1.
Fentanyl-induced changes in cardiorespiratory responses to phenylbiguanide (PBG). A: A representative recording showing that fentanyl (FEN, 8 μg/kg, iv) converted the PBG-induced RSB (left) into a long-lasting apnea (middle) and this converting effect declined 2 h later (right). The traces from the top to bottom are arterial blood pressure (BP) and tidal volume (VT). B: Group data of comparing the cardiorespiratory responses to PBG before and after FEN. N = 6; mean ± SE. Note: in B, all the cardiorespiratory responses to PBG were significant (P < 0.01). * P < 0.05 and ** P < 0.01 compared with before fentanyl. TE, expiratory duration; MBP, mean arterial blood pressure; HR, heart rate.
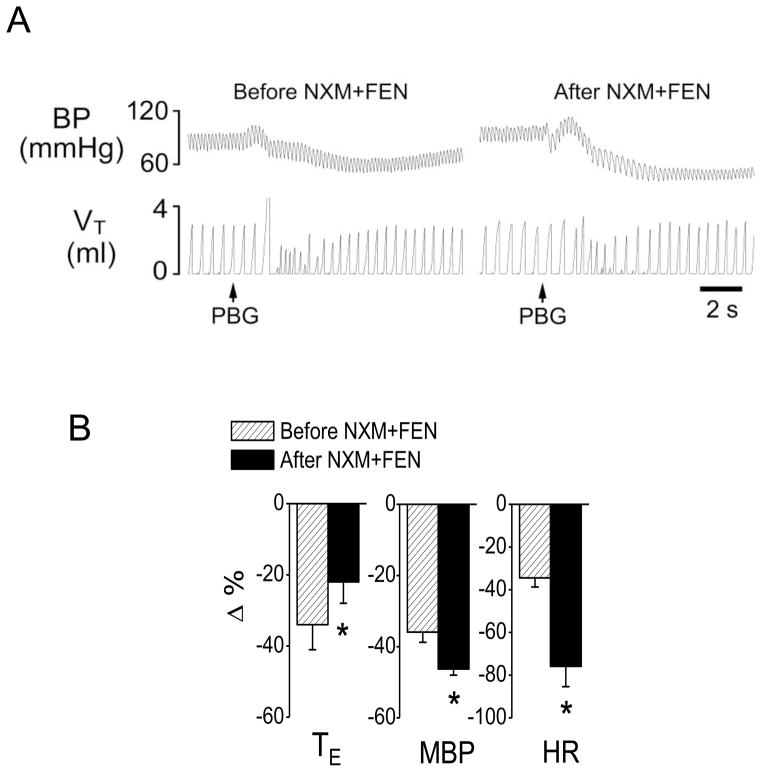
To test whether activating central μ-receptors was involved in the fentanyl-induced switch, intracisternal infusion of NXM was performed (Ang et al., 1999) before systemic injection of fentanyl. As shown in Fig. 2, intracisternal infusion of NXM prevented the fentanyl-induced switch with a prolonged TE of the PBG-induced RSB, and failed to alter the fentanyl-induced aggravation of the PBG-induced hypotension and bradycardia. Intracisternal infusion of vehicle did not significantly change the fentanyl-induced switch (TE prolonged by 651 ± 134% and 580 ± 148% after fentanyl with or without intracisternal infusion of vehicle, respectively, P > 0.05). The next approach was designed to test whether the fentanyl-induced switching effect could be reversed by NXM. After fentanyl turned the PBG-induced RSB into an apnea (TE = 3.5 ± 0.8 s) in two rats, PBG was applied again 15 min after intracisternal injection of NXM. We found that following intracisternal injection of NXM, right atrial injection of PBG only slightly prolonged TE (from 0.52 ± 0.11 s to 0.8 ± 0.2 s), but failed to cause the apnea. In other words, blocking central μ-receptors reversed the fentanyl switching effect.
Fig. 2.
Intracisternal injection of naloxone methiodide (NXM) prevents fentanyl (FEN)-induced switch. A: A representative recording showing the cardiorespiratory response to PBG before (right) and after (left) FEN following intracisternal injection of NXM. The traces from the top to bottom are arterial blood pressure (BP) and tidal volume (VT). B: Group data showing the effects of FEN on the cardiorespiratory responses to PBG. N = 6; mean ± SE. Note: in B, all the cardiorespiratory responses to PBG were significant (P < 0.01). * P < 0.05 compared with before fentanyl. TE, expiratory duration; MBP, mean arterial blood pressure; HR, heart rate.
2.3. Intra-mNTS microinjection of NXM fails to prevent the fentanyl-induced switch
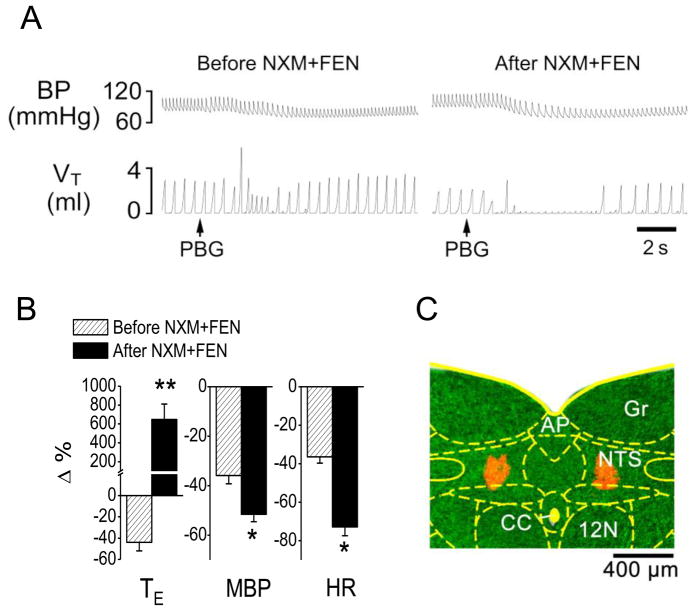
This experiment was undertaken to testing whether blocking the μ-receptors in the mNTS could prevent the fentanyl-produced switch. After intra-mNTS administration of NXM, fentanyl still switched the PBG-induced RSB into an apnea with the PBG-induced hypotension and bradycardia response aggravated (Fig. 3).
Fig. 3.
Microinjection of naloxone methiodide (NXM) into the medial nucleus tractus solitarius (mNTS) does not prevent the fentanyl (FEN)-induced the switching of phenylbiguanide (PBG)-induced RSB into an apnea. A: A representative recording showing the cardiorespiratory response to PBG before (right) and after (left) FEN following intra-mNTS injection of NXM. The traces from the top to bottom are arterial blood pressure (BP) and tidal volume (VT). B: Group data showing FEN impact on the cardiorespiratory responses to PBG before and after intra-mNTS injection of NXM. N = 6; mean ± SE. Note: in B, all the cardiorespiratory responses to PBG were significant (P < 0.01). * P < 0.05 and ** P < 0.01 compared with before fentanyl. TE, expiratory duration; MBP, mean arterial blood pressure; HR, heart rate. C: A representative slice containing the mNTS, in which the injection locations are stained by fluorescence microbeads (red). AP, area postrema; CC, central canal; NTS, the nucleus of the solitary tract; Gr, gracile nucleus; 12N, hypoglossal nucleus.
2.4. Intra-PBC microinjection of NXM prevents the fentanyl-induced switch
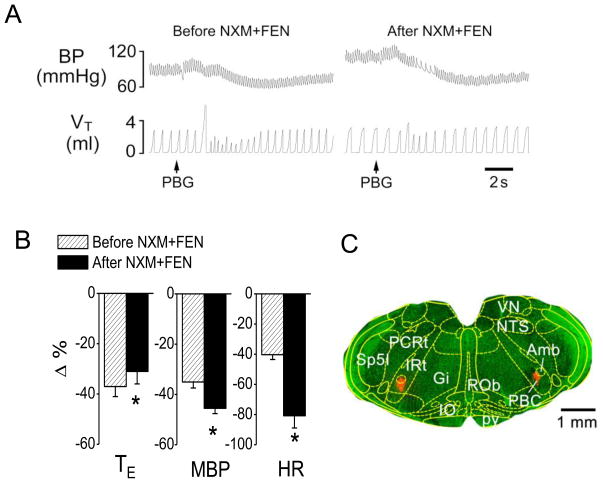
To determine whether PBC μ-receptors were involved in the switch, we blocked μ-receptors in the PBC before systemic administration of fentanyl. We found that intra-PBC administration of NXM eliminated the fentanyl-induced switch with a prolonged TE of the PBG-induced RSB, and failed to alter the fentanyl-induced aggravation of the PBG-induced hypotension and bradycardia (Fig. 4). In contrast, intra-PBC microinjection of vehicle did not significantly change the fentanyl-induced switch (TE prolonged by 621 ± 164% and 580 ± 148% after fentanyl with or without intra-PBC microinjection of vehicle, respectively, P > 0.05). To test the unique role of PBC μ-receptors in modulation of the switching effect of fentanyl, we microinjected NXM into the region outside the PBC in two rats and found that, after this NXM microinjection, fentanyl still switched PBG-induced RSB into an apnea (TE changed from 0.53 ± 0.12 s to 3.1 ± 0.68 s) accompanied with the PBG-induced hypotension (from −32% to −51%) and bradycardia response (from −34% to −72%) aggravated.
Fig. 4.
Microinjection of naloxone methiodide (NXM) into pre-Botzinger (PBC) prevents the fentanyl (FEN)-induced switch. A: A representative recording showing the cardiorespiratory response to PBG before (right) and after (left) FEN following intra-PBC injection of NXM. The traces from the top to bottom are arterial blood pressure (BP) and tidal volume (VT). B: Group data showing FEN impact on the cardiorespiratory responses to PBG before and after intra-PBC injection of NXM. N = 6; mean ± SE. Note: in B, all the cardiorespiratory responses to PBG were significant (P < 0.01). * P < 0.05 compared with before fentanyl. TE, expiratory duration; MBP, mean arterial blood pressure; HR, heart rate. C: A representative slice containing the PBC, in which the injection locations are stained by fluorescence microbeads (red). Amb, ambiguus nucleus; Gi, gigantocellular reticular nucleus; IO, inferior olive nucleus; IRt, intermediate reticular nucleus; NTS, nucleus of the solitary tract; PBC, pre-Botzinger complex; PCRt, parvicellular reticular nucleus; pv, pyramidal tract; ROb, raphe obscurus nucleus; VN, vestibular nucleus; Sp5I, spinal 5 nucleus, interpolar part.
3. Discussion
The most important finding in this study is that activating central μ-receptors, particularly those in the PBC rather than the mNTS, is required for accomplishing the RSB-apnea switch induced by systemic fentanyl administration. In this study, intravenous administration of fentanyl switched the PBG-induced RSB into an apnea, similar to our previous study (Zhang et al., 2010). Following blockade of central or PBC μ-receptors, we found that fentanyl no longer made such switching effect, suggesting an involvement of central μ-receptors, particularly those in the PBC. This finding is consistent with previous reports showing that PCF activation excites expiratory neurons and suppresses the firing of inspiratory neurons in the ventral respiratory group, leading to a change in respiratory rhythm (Wilson and Bonham, 1997), and the activation of PBC μ-receptors caused breathing depression or even arrest in the anesthetized rats (Montandon et al., 2011). In contrast, although the mNTS receives PCFs’ inputs (Kubin et al., 2006) and is likely involved in RSB (Bonham and Joad, 1991), the blockade of mNTS μ-receptors failed to alter the fentanyl-induced switch, suggesting a limited involvement of them.
The pathway involved in modulating respiratory rhythm by activation of PCFs has not been established. However, studies have shown that after relayed in the NTS (Kubin et al., 2006; Moreira et al., 2007), signals emanating from the PCFs further project to the retrotrapezoid nucleus (RTN) to inhibit RTN chemoreception (Moreira et al., 2007). Based on the reciprocal projection between the RTN and PBC (Feldman and Del Negro, 2006), this modulatory effect on RTN chemoreception by PCFs activation may subsequently change RTN excitatory drive to the PBC, leading to alteration of respiratory rhythm (Moreira et al., 2007). The further studies are required to determine the role of this pathway (PCF-RTN-PBC) in the fentanyl-induced switch.
We believe that both peripheral and central μ-receptors underlie the fentanyl-induced switch. It is well accepted that completing a chemoreflex requires both peripherally activating the sensory fibers and centrally processing the signal emanating from the sensory fibers. Our previous data have shown that activation of peripheral μ-receptors was necessary for triggering the fentanyl-induced RSB-apnea switch because fentanyl failed to switch the PBG-induced RSB to an apnea after the blockage of peripheral μ-receptors (Zhang et al., 2010). In the present study, we demonstrate that activation of central μ-receptors is also required to exert the fentanyl-induced switch. These results allow us to reason that PCF sensitization by acting their μ-receptors triggers the fentanyl-induced switch, while PBC μ-receptors are involved in centrally modulating the processing of the signal from the PCFs to fully express this switch. The mechanism underlying the involvement of PBC μ-receptors in the fentanyl-induced switch is not clear. One possibility is that activation of PBC μ-receptors depresses the inspiratory neurons activity, which makes an apnea easier to occur when PCFs are stimulated. It was reported that inspiratory neurons received inhibitory inputs from expiratory neurons in lateral medulla in cats (Lindsey et al., 1987) and PCF stimulation increases expiratory neural firing and decreases inspiratory neuronal activity, contributing to the apneic genesis (Wilson and Bonham, 1997). The inhibition of inspiratory neurons could also be observed when μ-receptors agonists were applied in vivo and vitro studies (Lalley, 2003; Takeda et al., 2001). Therefore, it is possible that activation of PBC μ-receptors may facilitate the shifting from inspiration to expiration, which may contribute to switching the PCFs-mediated RSB into the apnea. Considering the extensive presence of μ-receptors in the respiratory-related pontomedullary nuclei (Ding et al., 1996), other regions’ involvement cannot be ruled out.
In this study, systemic fentanyl challenge substantially depressed baseline VE and this inhibitory impact was attenuated after pretreatment with NXM microinjected into the cisterna magna, the mNTS, or the PBC. These data clearly demonstrate that central μ-receptors, the mNTS and PBC μ-receptors in particular, are involved in the fentanyl-induced baseline VE depression, supported by other studies (Lalley, 2003; Montandon et al., 2011; Zhang et al., 2011). For another, our data showed that fentanyl augmented the PCF-mediated hypotension and bradycardia and this gravitation was not affected by central NXM pretreatments. In our previous study (Zhang et al., 2010), we intravenously injected selective peripheral opioid receptor antagonist NXM (Hayashida et al., 2004) before fentanyl administration and found that, after blocking peripheral opioid receptors, fentanyl no longer aggravated the PBG-induced hypotension and bradycardia, suggesting peripheral μ-receptors’ involvement in the cardiovascular modulation by fentanyl. We also found that systemic fentanyl challenge increased MBP and this response disappeared after NXM microinjected into the mNTS or PBC. It was reported that this pressor response to fentanyl resulted from the released endogenous catecholamines (Hoehe and Duka, 1993). Activation of μ-receptors in the mNTS elicit pressor responses (Hassen and Feuerstein, 1987; Zhang et al., 2011) and this effect was mediated by increased sympathetic nerve activity (Hassen and Feuerstein, 1987). In fact, the PBC and its vicinity regions (rostroventrolateral medulla region) have the ability to mediate a variety of circulatory reflexes and sympathetic responses (Sun, 1995; Wang et al., 2002) although the impact of local μ-receptors on ABP is debatable (Lonergan et al., 2003; Miyawaki et al., 2002; Montandon et al., 2011).
There are some concerns in the present study. Although the volume microinjected in the PBC is relatively small (20 nl), we could not rule out the possibility that the injected drug diffused to the neighboring nuclei like the more rostral Botzinger complex and the more medial nucleus retroambigualis. However, the fact that microinjection of NXM into the site 1 mm rostral to the PBC failed to prevent fentanyl-induced switch suggests a limited diffusion. In addition, intracisternal injection of NXM attenuated but did not prevent fentanyl-induced VE inhibition while it blocked fentanyl’s switching effect. This may be due to different mechanisms (central pathways or the threshold for opioids exerting their influence on different respiratory activities) underlying the effects of fentanyl on modulating baseline VE and the PCFs-mediated reflex.
Our new finding is potentially relevant to the clinic. RSB commonly occurs in patients with pulmonary inflammation (infection), congestion, and edema (Churchill and Cope, 1929; Hatridge et al., 1989; Roussos and Koutsoukou, 2003). These patients are more vulnerable to suffering from respiratory depression and even respiratory failure than normal subjects when opioids are clinically administered as analgesics, mainly due to acting on μ-receptors (Gruber and Tschernko, 2003; Horton and Barber, 2009). However, the reason for this vulnerability is not clear. In this study, we found that central μ-receptors, especially those in the PBC, are critical for fentanyl to switch the PCFs-mediated RSB to an apnea. This study, along with our previous results showing an involvement of peripheral μ-receptors, suggests that although peripheral μ-receptors are essential for triggering the fentanyl-induced switch, central μ-receptors, especially those in the PBC, are required to fully express this switch. This novel concept may benefit our understanding why opioids are much more depressant to respiration in those patients with pulmonary disorders.
4. Experimental procedures
4.1 Animals
Forty pathogen-free Sprague-Dawley male rats (400–500 g) were purchased from Charles River Laboratories, Inc. (Wilmington, MA), housed in the animal facility at Lovelace Respiratory Research Institute (LRRI) in filter top cages, and provided with water and food ad libitum. The room was constantly ventilated and the temperature kept at 23°C. The experimental protocols were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and approved by LRRI’s Institutional Animal Care and Use Committee, which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International, USA.
4.2. General Animal Preparation
The rats were anesthetized with urethane [1200 mg/kg, intraperitoneal (ip)]. As needed, supplemental urethane (300 mg/kg, ip) was administered to completely eliminate eye-blink and limb-withdrawal reflex throughout the experiment. The general animal preparation was the same as we previously reported in rats (Zhang et al., 2007). Briefly, the right femoral vein was cannulated for solution infusion and the right femoral artery for monitoring arterial blood pressure (BP) and heart rate (HR). The right jugular vein was also cannulated for the bolus injection of phenylbiguanide (PBG) into the pulmonary circulation with the catheter tip placed just above right atrium. The trachea below the larynx was exposed through a midline incision, tracheotomized, and cannulated. The tracheal cannula was connected to a pneumotachograph to record airflow. Animals were placed into a rigid metal frame with their heads fixed and centered in a stereotaxic apparatus (Model 1404, Kopf, Tujunga, CA). The neck muscles were incised and deflected to expose the atlanto-occipital membrane for intracisternal injection. The animal was exposed to 50% O2 in nitrogen throughout the experiment and the core temperature was monitored with a rectal probe and maintained at 36.5–37.5°C by a water heating pad and radiant heat lamp.
4.3. Microinjection
Loading Agents
Unfilamented glass capillaries (1B100-3, 1/0.58 mm OD/ID, WPI, USA) were pulled in a horizontal pipette puller (DMZ-Universal Puller, Germany) and the long-shank micropipette was broken back to a desired tip size (15 ~ 20 μm, OD). The opioid receptor antagonist naloxone methiodide (NXM) (Hayashida et al., 2004) (Sigma-Aldrich, St. Louis, MO) dissolved in 0.9% saline containing red florescence microbeads (dilutions of 1:1, Lumafluor, Inc., USA) or vehicle were siphoned automatically or drawn through a little negative pressure into the micropipette. The loaded volume was calculated and administered as reported previously (Zhang et al., 2011). We selected NXM as it is more confined, if microinjected into the given area in the medulla, to the local site compared with naloxone (Schroeder et al., 1991).
Microinjection
For intracisternal injection, a glass micropipette with NXM solution (10 μg/4 μl or 50 μg/4 μl) was inserted into the cisterna magna (Mulkey et al., 2004). For local microinjection into the mNTS, the micropipette prefilled with NXM solution (10 mM, 20 nl each side), as seen under an operating microscope (Photo-Zusatz, Germany), was advanced by a micromanipulator into the central site of the mNTS after exposing the calamus scriptorius (obex) (Durakoglugil and Orer, 2008; McKay and Feldman, 2008). The tip of the micropipette was positioned at the site 0.4 mm bilateral to the calamus scriptorius and 0.8 mm deep from the dorsal surface. For microinjection into the PBC, the ventral surface of the brainstem was exposed through a ventral approach as reported before (Xu et al., 1992). The tip of the micropipette loaded with NXM (10 mM, 20 nl each side) was advanced into the central site of the PBC: 2.2 mm left and right to the middleline, 0.4 mm rostral to the first branch of the facial nerve, 1.0 mm deep below the surface of the medulla. In our pilot study, this PBC location was functionally identified by the evoked tachypnea immediately after local microinjection of DL-homocysteic acid (DLH, 10 mM, 20 nl each side, Sigma-Aldrich, St. Louis, MO) as reported before (Mustapic et al., 2010; Wang et al., 2002). The access distance to the PBC (1.0 mm) from the ventral approach, compared to the dorsal approach (> 5.0 mm), is so short that an accurate microinjection can be easily achieved. In some animals, microinjections were purposely performed 1.0 mm rostral to the PBC injection sites to test the unique role of PBC.
Identification of microinjection site
At the end of the experiment the brain was fixed in situ by perfusion of 0.1 M phosphate-buffered saline (PBS) at a pH of 7.4 and then 4% paraformaldehyde in PBS through the left ventricle of the heart. The brainstem was removed and subsequently sectioned at a 50-μm thickness by a microtome (Leica, CM 1850, Microsystems GMbH, Nussioch, Germany). The area marked by fluorescent beads was detected under a fluorescence microscope.
4.4. PCFs’ Stimulation
Right atrial bolus injection of PBG (3–6 μg/kg) was utilized to induce RSB by selectively stimulating PCFs (Wilson and Bonham, 1997). PBG (0.05 ml) was first slowly loaded into the catheter (volume = 0.08 ml) and then quickly flushed into the right atrium with 0.12 ml saline.
4.5. Experiment Protocol
To confirm that fentanyl is able to switch the PBG-induced RSB into an apnea, PBG was injected into the right atrium to evoke the RSB in six rats. Ten min, at least, was allowed for recovery from the RSB. Then the same PBG was injected 5 min after systemic administration of fentanyl (8 μg/kg). This five min was chosen because the respiratory inhibition by fentanyl became stable after this time.
To test whether intracisternal microinjection of NXM could alter the fentanyl-induced switching effect, the protocol was the same as described above with the exception that 15 min prior to intravenous administration of fentanyl, intracisternal infusion of NXM (10 μg/4 μl, n = 6) or vehicle (n = 6) was employed for 2–3 min. This relative long-duration (15 min) was used to allow the thorough spread of infused NXM into the brainstem respiratory-related nuclei (Bellet et al., 1980). We also tested whether the fentanyl-induced switching effect could be reversed by NXM (50 μg/4 μl). After fentanyl turned the PBG-induced RSB into an apnea in two rats, PBG injection was applied again 15 min following intracisternal injection of NXM.
Since our preliminary data showed that intracisternal infusion of NXM could prevent the fentanyl-induced switching effect, the following experiments were designed to estimate the effects of locally blocking μ-receptors within the mNTS or the PBC on the fentanyl-induced switch. The protocols were the same as described above with the exception that NXM, instead of intracisternal infusion, was microinjected bilaterally into the mNTS (n = 6) or the PBC (n = 6) within 4–6 s. Because our preliminary data also showed a positive result after microinjection of NXM into the PBC but not the mNTS, vehicle was microinjected into the PBC in six other rats to serve as an operation control. In two other rats, microinjections of NXM were performed into the regions outside the PBC (1.0 mm rostral to the PBC) to test the unique role of the PBC
4.6. Data Acquisition and Analysis
Raw data of the airflow, arterial blood pressure (MBP), HR, tracheal pressure (Pt), and rectal temperature were digitized, monitored, and recorded using a PowerLab/8sp (model ML 785; AD Instruments Inc., Colorado Springs, CO) connected to a computer employing the PowerLab Chart 5 software. The airflow signals were integrated to generate expiratory duration (TE), tidal volume (VT), respiratory frequency (f), and minute ventilatory volume (VE). TE was measured as the duration from the beginning of expiration to the beginning of next inspiration based on the airflow signals. The baseline cardiorespiratory variables were collected for 1 min immediately before and 5 min after agents administration. These variables were expressed as absolute values. With respect to the responses to PBG, baseline TE and associated cardiovascular values were averaged 1 min before PBG administration as controls, and the values from the PBG-evoked four fast breaths (RSB) or the apneic response were measured as the responses. These responses were presented as percentage changes from the control (3%). A TE value that was three-fold longer than the control was defined as an apnea (Peng et al., 2007). Paired-t test was used to test the effects of a given treatment on the baseline cardiorespiratory variables, while repeated two-way ANOVA was employed to detect significant changes in the evoked cardiorespiratory responses to PBG and significant effect of a given treatment on the responses. If an overall test was significant, Tukey test was used for specific comparisons between individual groups. The software Statistica 6.0 (StatSoft, Inc., Tulsa, OK) was used for statistical analysis. All data are presented as means ± standard error (SE). The difference was considered significant at a P value < 0.05.
Summary Statement.
Our results suggest that activation of central μ-receptors, especially those in the Pre-Botzinger Complex, is required for switching the pulmonary C-fiber-mediated rapid shallow breathing into an apnea by systemic administration of fentanyl.
Systemic administration of fentanyl switches the pulmonary C-fibers-mediated rapid shallow breathing (RSB) into an apnea.
Intracisternal injection of opioid receptor antagonists can prevent this fentanyl-induced RSB-apnea switch.
Blocking PBC, but not mNTS, μ-receptors also prevents this fentanyl-induced switch.
Acknowledgments
Funding: This study is supported by RO1 HL107462 from the National Heart, Lung, and Blood Institute, Bethesda, MD, and American Lung Association Biomedical Research Grant RG-191095-N, New York, NY
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agostoni E, et al. Functional and histological studies of the vagus nerve and its branches to the heart, lungs and abdominal viscera in the cat. J Physiol. 1957;135:182–205. doi: 10.1113/jphysiol.1957.sp005703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang KK, et al. Activation of spinal opioid receptors contributes to hypotension after hemorrhage in conscious rats. Am J Physiol. 1999;276:H1552–8. doi: 10.1152/ajpheart.1999.276.5.H1552. [DOI] [PubMed] [Google Scholar]
- Bellet M, et al. Central cardiovascular effects of narcotic analgesics and enkephalins in rats. Br J Pharmacol. 1980;71:365–9. doi: 10.1111/j.1476-5381.1980.tb10949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonham AC, Joad JP. Neurones in commissural nucleus tractus solitarii required for full expression of the pulmonary C fibre reflex in rat. J Physiol. 1991;441:95–112. doi: 10.1113/jphysiol.1991.sp018740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill ED, Cope O. The rapid shallow breathing resulting from pulmonary congestion and edema. The Journal of Experimental Medicine. 1929;49:531–537. doi: 10.1084/jem.49.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleridge HM, Coleridge JC. Pulmonary reflexes: neural mechanisms of pulmonary defense. Annu Rev Physiol. 1994;56:69–91. doi: 10.1146/annurev.ph.56.030194.000441. [DOI] [PubMed] [Google Scholar]
- Ding YQ, et al. Immunohistochemical localization of mu-opioid receptors in the central nervous system of the rat. J Comp Neurol. 1996;367:375–402. doi: 10.1002/(SICI)1096-9861(19960408)367:3<375::AID-CNE5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Ding YQ, et al. Co-localization of mu-opioid receptor-like immunoreactivity with substance P-LI, calcitonin gene-related peptide-LI and nitric oxide synthase-LI in vagal and glossopharyngeal afferent neurons of the rat. Brain Res. 1998;792:149–53. doi: 10.1016/s0006-8993(98)00205-4. [DOI] [PubMed] [Google Scholar]
- Durakoglugil MS, Orer HS. Cannabinoid Receptor Activation in the Nucleus Tractus Solitaries Produces Baroreflex-Like Responses in the Rat. Int J Biomed Sci. 2008;4:229–237. [PMC free article] [PubMed] [Google Scholar]
- Dutta A, Deshpande S. Cardio-respiratory reflexes evoked by phenylbiguanide in rats involve vagal afferents which are not sensitive to capsaicin. Acta Physiol (Oxf) 2010;200:87–95. doi: 10.1111/j.1748-1716.2010.02105.x. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci. 2006;7:232–42. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber EM, Tschernko EM. Anaesthesia and postoperative analgesia in older patients with chronic obstructive pulmonary disease: special considerations. Drugs Aging. 2003;20:347–60. doi: 10.2165/00002512-200320050-00004. [DOI] [PubMed] [Google Scholar]
- Haji A, et al. Distribution of mu receptors in the ventral respiratory group neurons; immunohistochemical and pharmacological studies in decerebrate cats. Neurosci Lett. 2003;351:37–40. doi: 10.1016/s0304-3940(03)00951-0. [DOI] [PubMed] [Google Scholar]
- Hassen AH, Feuerstein G. mu-Opioid receptors in NTS elicit pressor responses via sympathetic pathways. Am J Physiol. 1987;252:H156–62. doi: 10.1152/ajpheart.1987.252.1.H156. [DOI] [PubMed] [Google Scholar]
- Hatridge J, et al. Rapid shallow breathing caused by pulmonary vascular congestion in cats. J Appl Physiol. 1989;67:2257–64. doi: 10.1152/jappl.1989.67.6.2257. [DOI] [PubMed] [Google Scholar]
- Hayashida K, et al. Bovine lactoferrin has a nitric oxide-dependent hypotensive effect in rats. Am J Physiol Regul Integr Comp Physiol. 2004;286:R359–65. doi: 10.1152/ajpregu.00214.2003. [DOI] [PubMed] [Google Scholar]
- Hoehe M, Duka T. Opiates increase plasma catecholamines in humans. Psychoneuroendocrinology. 1993;18:141–8. doi: 10.1016/0306-4530(93)90065-s. [DOI] [PubMed] [Google Scholar]
- Horton R, Barber C. Opioid-induced respiratory depression resulting from transdermal fentanyl-clarithromycin drug interaction in a patient with advanced COPD. J Pain Symptom Manage. 2009;37:e2–5. doi: 10.1016/j.jpainsymman.2009.02.230. [DOI] [PubMed] [Google Scholar]
- Jammes Y, et al. Afferent and efferent components of the bronchial vagal branches in cats. J Auton Nerv Syst. 1982;5:165–76. doi: 10.1016/0165-1838(82)90037-6. [DOI] [PubMed] [Google Scholar]
- Krajnik M, et al. Local pulmonary opioid network in patients with lung cancer: a putative modulator of respiratory function. Pharmacol Rep. 2010;62:139–49. doi: 10.1016/s1734-1140(10)70251-6. [DOI] [PubMed] [Google Scholar]
- Kubin L, et al. Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol. 2006;101:618–27. doi: 10.1152/japplphysiol.00252.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalley PM. Mu-opioid receptor agonist effects on medullary respiratory neurons in the cat: evidence for involvement in certain types of ventilatory disturbances. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1287–304. doi: 10.1152/ajpregu.00199.2003. [DOI] [PubMed] [Google Scholar]
- Lee LY, et al. Functional morphology and physiological properties of bronchopulmonary C-fiber afferents. Anat Rec A Discov Mol Cell Evol Biol. 2003;270:17–24. doi: 10.1002/ar.a.10005. [DOI] [PubMed] [Google Scholar]
- Lindsey BG, et al. Functional associations among simultaneously monitored lateral medullary respiratory neurons in the cat. II. Evidence for inhibitory actions of expiratory neurons. J Neurophysiol. 1987;57:1101–17. doi: 10.1152/jn.1987.57.4.1101. [DOI] [PubMed] [Google Scholar]
- Lonergan T, et al. Mu opioid receptors in rat ventral medulla: effects of endomorphin-1 on phrenic nerve activity. Respir Physiol Neurobiol. 2003;138:165–78. doi: 10.1016/s1569-9048(03)00173-3. [DOI] [PubMed] [Google Scholar]
- McKay LC, Feldman JL. Unilateral ablation of pre-Botzinger complex disrupts breathing during sleep but not wakefulness. Am J Respir Crit Care Med. 2008;178:89–95. doi: 10.1164/rccm.200712-1901OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei N, et al. The composition of the vagus nerve of the cat. Cell Tissue Res. 1980;209:423–31. doi: 10.1007/BF00234756. [DOI] [PubMed] [Google Scholar]
- Miyawaki T, et al. Activation of mu-opioid receptors in rat ventrolateral medulla selectively blocks baroreceptor reflexes while activation of delta opioid receptors blocks somato-sympathetic reflexes. Neuroscience. 2002;109:133–44. doi: 10.1016/s0306-4522(01)00439-0. [DOI] [PubMed] [Google Scholar]
- Montandon G, et al. PreBotzinger complex neurokinin-1 receptor-expressing neurons mediate opioid-induced respiratory depression. J Neurosci. 2011;31:1292–301. doi: 10.1523/JNEUROSCI.4611-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira TS, et al. Activation of 5-hydroxytryptamine type 3 receptor-expressing C-fiber vagal afferents inhibits retrotrapezoid nucleus chemoreceptors in rats. J Neurophysiol. 2007;98:3627–37. doi: 10.1152/jn.00675.2007. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, et al. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci. 2004;7:1360–9. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Mustapic S, et al. Clinically relevant infusion rates of mu-opioid agonist remifentanil cause bradypnea in decerebrate dogs but not via direct effects in the pre-Bötzinger complex region. J Neurophysiol. 2010;103:409–18. doi: 10.1152/jn.00188.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JF. Rhythmic bursting of pre- and post-inspiratory neurones during central apnoea in mature mice. J Physiol. 1997;502(Pt 3):623–39. doi: 10.1111/j.1469-7793.1997.623bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng W, et al. Respiratory syncytial virus infection in anesthetized weanling rather than adult rats prolongs the apneic responses to right atrial injection of capsaicin. J Appl Physiol. 2007;102:2201–6. doi: 10.1152/japplphysiol.01436.2006. [DOI] [PubMed] [Google Scholar]
- Roussos C, Koutsoukou A. Respiratory failure. Eur Respir J. 2003:3s–14s. doi: 10.1183/09031936.03.00038503. [DOI] [PubMed] [Google Scholar]
- Schroeder RL, et al. Methylnaloxonium diffuses out of the rat brain more slowly than naloxone after direct intracerebral injection. Neurosci Lett. 1991;121:173–7. doi: 10.1016/0304-3940(91)90678-m. [DOI] [PubMed] [Google Scholar]
- Smith JC, et al. Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–9. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun MK. Central neural organization and control of sympathetic nervous system in mammals. Prog Neurobiol. 1995;47:157–233. doi: 10.1016/0301-0082(95)00026-8. [DOI] [PubMed] [Google Scholar]
- Takeda S, et al. Opioid action on respiratory neuron activity of the isolated respiratory network in newborn rats. Anesthesiology. 2001;95:740–9. doi: 10.1097/00000542-200109000-00029. [DOI] [PubMed] [Google Scholar]
- Wang H, et al. Depressor and tachypneic responses to chemical stimulation of the ventral respiratory group are reduced by ablation of neurokinin-1 receptor-expressing neurons. J Neurosci. 2002;22:3755–64. doi: 10.1523/JNEUROSCI.22-09-03755.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willette RN, Sapru HN. Peripheral versus central cardiorespiratory effects of morphine. Neuropharmacology. 1982;21:1019–26. doi: 10.1016/0028-3908(82)90116-2. [DOI] [PubMed] [Google Scholar]
- Wilson CG, Bonham AC. Effect of cardiopulmonary C fibre activation on the firing activity of ventral respiratory group neurones in the rat. J Physiol. 1997;504(Pt 2):453–66. doi: 10.1111/j.1469-7793.1997.453be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, et al. Topography of cat medullary ventral surface hypoxic acidification. J Appl Physiol. 1992;73:2631–7. doi: 10.1152/jappl.1992.73.6.2631. [DOI] [PubMed] [Google Scholar]
- Zhang Z, et al. Activation of opioid mu receptors in caudal medullary raphe region inhibits the ventilatory response to hypercapnia in anesthetized rats. Anesthesiology. 2007;107:288–97. doi: 10.1097/01.anes.0000270760.46821.67. [DOI] [PubMed] [Google Scholar]
- Zhang Z, et al. Fentanyl Turns Pulmonary C-Fiber -Mediated Rapid Shallow Breathing into an Apnea in Anesthetized Rats. FASEB. 2010;24:1026.29. [Google Scholar]
- Zhang Z, et al. Activation of opioid 3-receptors in the commissural subdivision of the nucleus tractus solitarius abolishes the ventilatory response to hypoxia in anesthetized rats. Anesthesiology. 2011;115:353–63. doi: 10.1097/ALN.0b013e318224cc1f. [DOI] [PubMed] [Google Scholar]