Abstract
The epithelial sodium channel (ENaC) is responsible for Na+ and fluid absorption across colon, kidney, and airway epithelia. We have previously identified SPLUNC1 as an autocrine inhibitor of ENaC. We have now located the ENaC inhibitory domain of SPLUNC1 to SPLUNC1's N terminus, and a peptide corresponding to this domain, G22-A39, inhibited ENaC activity to a similar degree as full-length SPLUNC1 (∼2.5 fold). However, G22-A39 had no effect on the structurally related acid-sensing ion channels, indicating specificity for ENaC. G22-A39 preferentially bound to the β-ENaC subunit in a glycosylation-dependent manner. ENaC hyperactivity is contributory to cystic fibrosis (CF) lung disease. Addition of G22-A39 to CF human bronchial epithelial cultures (HBECs) resulted in an increase in airway surface liquid height from 4.2 ± 0.6 to 7.9 ± 0.6 μm, comparable to heights seen in normal HBECs, even in the presence of neutrophil elastase. Our data also indicate that the ENaC inhibitory domain of SPLUNC1 may be cleaved away from the main molecule by neutrophil elastase, which suggests that it may still be active during inflammation or neutrophilia. Furthermore, the robust inhibition of ENaC by the G22-A39 peptide suggests that this peptide may be suitable for treating CF lung disease.—Hobbs, C. A., Blanchard, M. G., Kellenberger, S., Bencharit, S., Cao, R., Kesimer, M., Walton, W. G., Redinbo, M. R., Stutts, M. J., Tarran, R. Identification of SPLUNC1's ENaC-inhibitory domain yields novel strategies to treat sodium hyperabsorption in cystic fibrosis airways.
Keywords: glycosylation, BPIFA1, PLUNC, LUNX, neutrophil elastase
The epithelial sodium channel (ENaC) is the apical conduit for Na+ absorption across a wide range of epithelia, including renal, gastrointestinal, and airway (1). The airway surface liquid (ASL) is composed of a mucus layer, which is responsible for trapping both inhaled particles and pathogens such as bacteria, and a periciliary layer that facilitates ciliary beating and serves as a cell surface lubricant (2, 3). Airway epithelia can both absorb Na+ through ENaC and secrete Cl− through the cystic fibrosis transmembrane conductance regulator (CFTR). A precise balance of Cl− secretion and Na+ absorption across the airway is critical for regulating ASL volume. Normal (NL) human bronchial epithelial cultures (HBECs) regulate ASL height to an optimal depth of ∼7 μm, while cystic fibrosis (CF) HBECs consistently show a much lower depth of ∼3 μm (2). ENaC is negatively regulated by CFTR (4), and in patients with CF, CFTR's absence results in ENaC hyperactivity, which leads to uncontrolled absorption of Na+ and ASL volume depletion that slows or abolishes mucus transport. This lack of mucus transport leads to a failure to physically clear the lungs of accumulated mucus and inhaled pathogens and causes chronic lung infections, which eventually leads to their destruction (5).
ENaC is made up of 3 subunits, α, β, and γ, which share ∼30% sequence homology (6). Structurally, each subunit is made up of 2 transmembrane domains, short N- and C-terminal cytoplasmic tails, and a large extracellular loop that contains numerous sites for N-linked glycosylation (7, 8). Activation of this channel occurs through proteolytic cleavage of the extracellular loops of the α- and γ-ENaC subunits by furin-type convertases (9, 10), membrane-bound channel activating proteases (CAPs), such as prostasin (CAP1) and TMPRSS4 (CAP2), and/or soluble proteases, including the serine proteases trypsin and neutrophil elastase (NE) (11). When these proteases are blocked by specific protease inhibitors, such as aprotinin for trypsin-like proteases, ENaC activation is attenuated (12). Alternatively, the cleaved segments of α- and γ-ENaC may bind back into the channel and serve as inhibitory peptides (13, 14). Little is known about the physiological regulation of these key ENaC proteolytic processes. However, we recently hypothesized that a soluble modulator of ENaC existed in the ASL and designed a proteomic screen to identify it (15, 16). Our data indicated that the short palate, lung and nasal epithelial clone 1 (SPLUNC1) was the soluble modulator of ENaC activity and knockdown of SPLUNC1 in NL HBECs abolished ENaC regulation and led to CF-like ASL volume depletion (16). SPLUNC1 is endogenously secreted into the ASL, and we hypothesize that it functions as an ASL volume sensor: As ASL volume increases, SPLUNC1 becomes diluted, removing the inhibition of ENaC and signaling for absorption to begin; conversely, when ASL volume is low, SPLUNC1 is concentrated, causing less ENaC activity.
SPLUNC1 is a 256-aa protein that belongs to the bactericidal permeability-increasing (BPI)-fold containing family A and is also known as BPIFA1, LUNX, PLUNC, and SPURT. SPLUNC1 is expressed in the upper airways and nasopharyngeal regions and may also be expressed in Na+-absorbing tissues, including the colon and kidney (16). Based on sequence similarity with BPI-like proteins, SPLUNC1 was hypothesized to be an innate defense protein, and indeed, SPLUNC1 has been shown to be both antimicrobial and to reduce surface tension (17–20). More recently, SPLUNC1 has been proposed to be a multifunctional defense protein, since its knockdown in vivo has been shown to decrease mucus clearance (21) as well as to increase Mycoplasma pneumoniae infection (17). Due to the wide variety of functions assigned to SPLUNC1, we set out to identify its ENaC inhibitory domain to better understand how this protein functions and how it interacts with ENaC.
MATERIALS AND METHODS
cDNA and cRNA
Full-length SPLUNC1 cDNA was kindly provided by Dr. Colin Bingle (University of Sheffield Medical School, Sheffield, UK). This construct was used to create the SPLUNC1 truncant cDNA. Truncants were made with 60, 30, 15, 11.25, and 7.5% of SPLUNC1 remaining, each ending at amino acid residue 173, 83, 43, 29, and 24, respectively. Complementary RNAs of rat αβγ ENaC subunits, full-length, and truncants of SPLUNC1 were made as described previously (16).
Oocyte studies
Xenopus laevis oocytes were harvested and injected as described previously (22). Oocytes were studied 24 h postinjection using the 2-electrode voltage-clamp technique as described previously (16). Where appropriate, oocytes were incubated with G22-A39 or a control peptide, ADG, (described below) for 1 h prior to recording. In some experiments β-ENaCS518C was used, which forms ENaCs that are locked into a fully open state with an open probability near 1.0 by exposure to the sulfhydral reactive reagent, [2-(trimethyl-ammonium)ethyl]methanethiosulfonate bromide (MTSET). MTSET was added at a concentration of 1 mM to the oocyte bath, as described previously (23).
Peptides
Peptides were synthesized and purified by the University of North Carolina (UNC) Microprotein Sequencing and Peptide Synthesis Facility. The sequence of the G22-A39 peptide was GGLPVPLDQTLPLNVNPA. A control peptide of G22-A39, ADG, was made by alphabetizing the sequence. The sequence of ADG was ADGGLLLLNNPPPPQTVV. Both peptides were used with either a free or biotinylated N terminus as needed. Biotinylation had no effect on G22-A39's ability to inhibit ENaC (n=6).
Electrophysiological measurements of acid-sensing ion channels (ASICs)
Previously described cell lines expressing human ASIC1a, human ASIC2a, and rat ASIC3 were used in these experiments (24). Electrophysiological measurements were carried out with an EPC10 patch-clamp amplifier (Heka Electronics, Lambrecht, Germany) as described previously (25).
Cell culture
HEK293T cells were cultured in DMEM/F12 medium containing 10% FBS, 1× penicillin/streptomycin, 0.2 μg/ml puromycin, and 0.1 mM hygromycin at 37°C/5% CO2 on 6-well plastic plates. Cells were transfected according to the manufacturer's instructions using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). Cells were transfected when 90–95% confluent with 0.5 μg of plasmid DNA per construct per well and incubated at 37°C/5% CO2 overnight. CHO cell lines stably expressing human ASIC1a, human ASIC2a, and rat ASIC3 were used in the electrophysiological measurements of ASICs (24).
Human excess donor lungs and excised recipient lungs were obtained at the time of lung transplantation, and cells were harvested by enzymatic digestion, as described previously, under a protocol approved by the UNC Institutional Review Board (15). HBECs were maintained at an air–liquid interface in a modified bronchial epithelial growth medium (BEGM) with 5% CO2 at 37°C and used 2-5 wk after seeding on 12-mm T-clear inserts (Corning-Costar, Corning, NY, USA) (26).
Peptide pulldown assay and Western blotting
HEK293T cells were transfected with double-tagged human ENaC subunits with HA and V5 epitopes at the N and C termini, respectively, in combination with wild-type untagged subunit cDNA. When all three ENaC subunits were expressed, 0.5 μg of each subunit was transfected per well. When expressed individually, 0.75 μg of the subunit was transfected per well. The transfected cells were lysed 24 h later using Nonidet P-40 buffer with 1× complete EDTA-free protease inhibitor (Roche, Basel, Switzerland). The lysate was centrifuged at 16,300 g for 15 min at 4°C, and the supernatant was collected. Protein concentration was determined using the BCA assay, and 500 μg of protein plus 0.25 mg peptide and 100 μl of neutravidin were added to a spin column and rotated end-over-end at 4°C for 24 h (all ThermoFisher Scientific, Rockford, IL, USA). Flow-through was collected by centrifugation at 1000 g for 30 s. The beads were then washed 5 times with Nonidet P-40 buffer. Bound protein was eluted by boiling at 95°C for 10 min in 75 μl of 2× LDS NuPAGE sample buffer with 1× sample reducing agent, followed by centrifugation at 16,300 g for 2 min. Samples were resolved on 4–12% Bis-Tris gels in MES and transferred to a nitrocellulose membrane using iBlot, setting P3 for 8 min (Invitrogen, Carlsbad, CA, USA). The membrane was probed using 1:1000 anti-V5 antibody (Invitrogen) overnight at 4°C in 3% fish gelatin in TBS-T. The blot was then incubated for 1 h at room temperature with an ECL sheep anti-mouse IgG secondary antibody and detected by ECL reagent (ThermoFisher Scientific, Waltham, MA, USA) or by incubation with a goat anti-mouse IRDye secondary antibody and analyzed by an Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE, USA).
Deglycosylation
Peptide pulldown assays were performed as described above. Samples were eluted by the addition of 100 μl of 0.1 M sodium citrate (pH 5.5) and 0.1% SDS to the beads and incubating at 100°C for 2 min, followed by centrifugation at 16,300 g for 2 min. The samples were divided equally, and half was treated with 1 μl of endoglycosidase H (EndoH) and incubated at 37°C for 2 min. After incubation, all samples were lyophilized and then reconstituted in 30 μl LDS NuPAGE sample buffer with 1× sample reducing agent (Invitrogen). Western blots were completed as described above. A concentration of 5 μg/ml tunicamycin (Sigma-Aldrich, St Louis, MO, USA) was added to the cell transfection medium, and the cells were incubated overnight at 37°C/5% CO2. The following day, the protocol for the peptide pulldown assay was performed as described above.
ASL height measurement
To label the ASL, 20 μl PBS containing 10 kDa rhodamine dextran (0.2–2 mg/ml; Invitrogen) was added to HBEC mucosal surfaces, as described previously (27). When added, peptides with or without 100 nM NE, 1 U/ml aprotinin, activated neutrophil supernatant (ANS; ref. 28), or 10 μM sivelestat (Sigma-Aldrich) were added to the mucosal surface along with the rhodamine dextran. Bumetanide (100 μM) was added to the serosal solution. The HBEC mucosal surfaces were washed with 500 μl PBS for 30 min prior to experimentation to remove accumulated endogenous SPLUNC1. SPLUNC1 recovery into the ASL was measured over time by lavaging with 200 μl PBS at timed intervals after the initial wash/volume load. To detect SPLUNC1, 20% by volume of the lavage was transferred onto a nitrocellulose membrane using a slot-blot apparatus (GE Healthcare, Pittsburgh, PA, USA). The blot was processed as described above using an anti-hPLUNC primary antibody (R&D Systems, Minneapolis, MN, USA).
Transepithelial potential difference (Vt) studies
A single-barreled Vt-sensing electrode was positioned in the ASL by micromanipulator and used in conjunction with a macroelectrode in the serosal solution to measure Vt using a voltmeter (World Precision Instruments, Sarasota, FL, USA), as described previously (16).
Circular dichroism (CD)
G22-A39 (100 μM) was dissolved in a buffer containing 10 mM sodium phosphate (pH 7.4) in a 1 mm cuvette. Using a Chirascan-plus instrument (Applied Photophysics Ltd., Leatherhead, UK), 5 individual spectra from 185 to 280 nm were recorded at 25 ± 1.0°C and averaged. All measurements were corrected for buffer signal.
Expression and purification of human SPLUNC1
The SPLUNC1-Δ19 construct was created by cloning amino acid residues 20–256 out of the SPLUNC1 cDNA described above for entry into pMCSG7 for protein expression. BL21-CodonPlus competent cells (Agilent Technologies, Santa Clara, CA, USA) were transformed with the expression plasmid and cultured in the presence of antifoam (50 μl), ampicillin (100 μg/ml), and chloramphenicol (34 μg/ml) in Luria broth medium with vigorous shaking at 37°C until an OD600 of 0.6 was attained. Expression was induced with the addition of isopropyl-1-thio-d-galactopyranoside (IPTG). Bacteria were collected by centrifugation at 4500 g for 20 min at 4°C. Cell pellets were resuspended in buffer A (20 mM potassium phosphate, pH 7.4; 50 mM imidazole; 500 mM NaCl; and 0.02% NaAzide) along with lysozyme, DNase1, and protease inhibitor tablets. Cells were sonicated, and cell lysate was separated into soluble and insoluble fractions using high-speed centrifugation. The soluble fraction was filtered, then flowed over a Ni-NTA His-Trap gravity column and washed with buffer A. The bound protein was eluted with buffer B (20 mM potassium phosphate, pH 7.4; 500 mM imidazole; 500 mM NaCl; and 0.02% NaAzide) and separated using an S200 gel filtration column on an ÄKTAxpress (GE Healthcare). The histidine tag was removed with Tev protease, leaving amino acid residues serine, asparagine, and alanine on the N terminus of the protein. Another round of nickel and HPLC purification removed the tag from the purified SPLUNC1-Δ19. We confirmed that SPLUNC1-Δ19 purified in this fashion inhibited ENaC HBECs (n=6).
NE cleavage of SPLUNC1-Δ19 and analysis by liquid chromatography-electrospray ionization tandem mass spectrometry (LC-ESI/MS/MS)
NE was added to SPLUNC1-Δ19 at a ratio of 1:1000 (enzyme:protein) followed by incubation for 5, 15, and 60 min at 37°C. Digested samples (5 μl) were introduced to a Q-Tof micro mass spectrometer (Waters, Manchester, UK) via a nanoAcquity UPLC system (Waters, Milford MA, USA), as described previously (29). Briefly, the analytical system was configured with a PepMap C18 (LC packing, 300 μm ID × 5 mm; ThermoFisher Scientific, Waltham, MA, USA) preconcentration column in series with an Atlantis (Waters) dC18 NanoEase (75 m × 150 mm) nanoscale analytical column. Peptides were separated on the column with a gradient of 5% acetonitrile in 0.1% formic acid to 60% acetonitrile in 0.1% formic acid over 60 min. All data were acquired using MassLynx 4.1 software (Waters). The raw data acquired were processed using the ProteinLynx module of MassLynx 4.1 to produce *.pkl (peaklist) files, which are suitable for the MS/MS ion database search via search engines. The data processed were searched against the Uniprot protein database (release 2011_09; http://www.uniprot.org) using an in-house MASCOT 2.2 search engine (Matrix Science, London, UK) MASCOT probability-based Mowse individual ion scores > 40 were accepted as indicating identity or extensive homology (P<0.05).
SPLUNC1 peptides in patients with CF and donor sputum
Human sputum samples were obtained as described previously (30). The study protocol was approved by the UNC Committee on the Protection of Rights of Human Subjects, and informed consent was obtained. Sputum from 4 patients with CF and 4 healthy donors was pooled. Pooled sputum (1 ml) from each group was diluted in 4 ml PBS. The samples were filtered through a 0.22-μm membrane (Millipore, Bedford, MA, USA). The resulting filtrates were injected onto an Ettan LC chromatographic system (Amersham Pharmacia Biotech, Piscataway, NJ, USA) with a Superdex 200 HR 10/30 chromatography column. The large proteins were separated from the low-molecular-weight peptides with PBS elution at a flow rate of 0.3 ml/min. The peptide pool was dried down 10× by volume using a vacuum concentrator and then mixed 1:1 with 1% formic acid and subjected to nano-LC-ESI/MS/MS and analyzed using the above parameters.
Statistical analyses
Unless otherwise noted, all data are presented as means ± se for n experiments. Differences between means were tested for statistical significance using paired or unpaired t tests when the variances were homogeneously distributed, or in the case of nonhomogeneity of variance, the Wilcoxon rank-sum or Mann-Whitney U tests were used as appropriate. From such comparisons, differences yielding values of P < 0.05 were judged to be significant. HBECs derived from ≥3 donors were used per experiment, and experiments using cell lines were repeated on 3 separate occasions. All analyses were conducted using Instat software (GraphPad, San Diego, CA, USA).
RESULTS
Identification of SPLUNC1's ENaC inhibitory domain
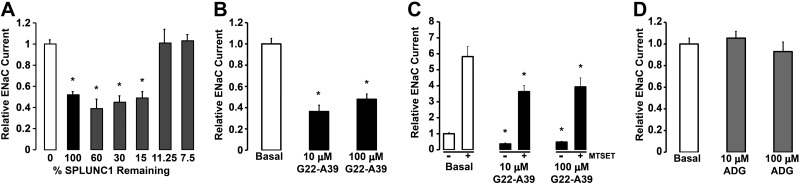
To identify the region of SPLUNC1 responsible for this inhibition, 5 C-terminal SPLUNC1-deletion mutants (truncants) were made, and their effect on the amiloride sensitive ENaC current was measured after coinjection with αβγ-ENaC (Fig. 1A). Deletion of up to 85% of SPLUNC1 resulted in similar inhibition of ENaC as seen with full-length SPLUNC1. However, truncants lacking ∼89 and 93% of SPLUNC1 could not inhibit ENaC (Fig. 1A). Since the first ∼20 residues are predicted to be an N-terminal signaling sequence, this narrowed the ENaC inhibitory domain of SPLUNC1 to residues 22–39. We then synthesized a peptide that corresponds to this region (22GGLPVPLDQTLPLNVNPA39), named G22-A39. When oocytes coinjected with α-, βS518C-, γ-ENaC subunits were incubated with 10 or 100 μM G22-A39 for 1 h, an ∼2.5-fold decrease in INA was observed (P<0.05), indicating that we had identified the ENaC inhibitory domain of SPLUNC1 (Fig. 1B). To further explore the effects of G22-A39, MTSET was added during recording to lock the channel into a fully open position and give an approximation of the number of active channels in the plasma membrane (23, 31). MTSET significantly increased ENaC activity ∼6-fold over basal current levels (Fig. 1C). In the presence of G22-A39, MTSET still raised INA above basal levels. However, the increase was significantly less than under control conditions, suggesting that exposure to G22-A39 resulted in a decrease in surface ENaC levels (Fig. 1C). In contrast, when incubated with the control peptide, ADG, no difference in the INA was observed (Fig. 1D).
Figure 1.
Identification of the ENaC inhibitory domain of SPLUNC1. A) Effect of C-terminal SPLUNC1 deletions on the amiloride-sensitive ENaC current. αβγ-ENaC subunits were coinjected into Xenopus oocytes with the corresponding SPLUNC1 truncants, and the relative ENaC current was measured by the 2-electrode voltage-clamp method (n=10–14). B–D) Oocytes were coinjected with α-, βS518C-, γ-ENaC subunits and incubated for 1 h with G22-A39 or the control peptide, ADG (all n=11). B) Effect of 10 and 100 μM G22-A39 on the amiloride-sensitive ENaC current. C) Effect of MTSET on the amiloride-sensitive ENaC current in the presence or absence of 10 or 100 μM G22-A39. D) Effect of 10 and 100 μM ADG on the amiloride-sensitive ENaC current. *P < 0.05 vs. basal or nontransfected current.
Structurally related ASICs are not affected by G22-A39
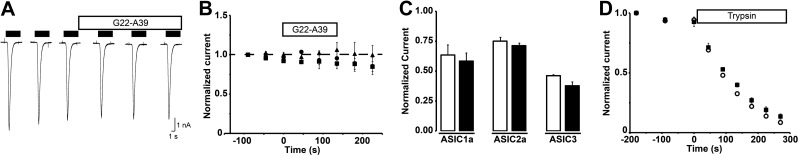
We tested whether SPLUNC1 affected the function of the related ENaC/degenerin family members, the ASICs. To assess possible effects of G22-A39 on the pH dependence of ASIC activation and on current amplitude, channels were activated by a change from pH 7.4 to a pH corresponding to the steep phase of their activation curve, pH 6.6, for ASIC1a and ASIC3, and 4.0 for ASIC2a. At these stimulation pH values, a change in current expression or pH dependence would be readily detected as a change in the measured current amplitude. Stimulations of 5 s duration were performed every 45 s to allow recovery from inactivation between stimulations. Three control values were obtained before switching to a pH 7.4 solution containing 10 μM G22-A39. The G22-A39 peptide was then present during 3 stimulation rounds in both the conditioning (pH 7.4) and the acidic stimulation solution before it was washed out. Figure 2A illustrates a typical experiment with an ASIC1a-expressing cell. Average current amplitudes were normalized to the first control value and plotted as a function of time (Fig. 2B). The peptide had no apparent effect on the activation properties of the different ASICs tested (n=3 by channel type, P>0.05). No changes in current kinetics were observed (not shown). ASICs inactivate directly from the closed state when exposed to pH <7.4 but insufficiently acidic to activate the channel. This process is known as steady-state inactivation (SSI). Its pH dependence has been determined and has its midpoint at pH 7.2 for ASIC1a, pH 7.1 for ASIC3, and pH 5.6 for ASIC2a (25). To detect changes in the pH dependence of SSI due to the presence of the peptide, cells were incubated 40 s in a conditioning solution with a pH close to the midpoint of SSI, in the presence or absence of the peptide (7.1 for ASIC1a and ASIC3; pH 5.6 for ASIC2a). The conditioning period was followed by activation with an acidic stimulus (pH 6.0 for ASIC1a and ASIC3; pH 4 for ASIC2a). Figure 2C plots the average values of the current after the conditioning period normalized to the maximal current obtained with a conditioning pH of 7.4. The peptide does not modify the pH dependence of steady-state inactivation of the tested ASICs.
Figure 2.
G22-A39 peptide does not affect the function of ASIC1a, ASIC2a, and ASIC3 channels. Whole-cell currents were measured from CHO cells stably expressing ASIC subunits, voltage clamped to −60 mV. Stimulations lasted 5 s and were performed every 45 s. A) Typical experiment with an ASIC1a-expressing cell. Cells were stimulated 3 times with pH 6.6 (ASIC1a and ASIC3) or pH 4 (ASIC2a). Between stimulations, cells were returned to a pH 7.4 conditioning solution for 40 s to allow recovery from inactivation. The conditioning solution was then switched to a pH 7.4 solution containing 10 μM G22-A39. Three stimulations in the presence of 10 μM G22-A39 were performed before washing off the peptide. B) Current amplitudes of the above described experiments were normalized to the first control value and plotted as a function of time. G22-A39 was added at t = 0 s. Squares, ASIC1a; circles, ASIC2a; triangles, ASIC3; all n = 3. C) Cells were incubated for 40 s in a pH 7.1 (ASIC1a and ASIC3) or 5.6 (ASIC2a) conditioning solution, then activated using an acidic stimulus (pH 5 for ASIC1a and ASIC3; pH 4 for ASIC2a). Experiments were performed with or without 10 μM G22-A39 in the conditioning solution. Current amplitudes measured during the acidic stimulus were normalized to the control amplitude obtained with a pH 7.4 conditioning solution. Open bars, control; solid bars, G22-A39; all n = 3–4. D) Cells expressing ASIC1a were stimulated 3 times with a pH 6.6 stimulus before 40 μg/ml trypsin was added to the pH 7.4 solution. Stimulations were performed every 45 s. Protocol was performed with or without 10 μM G22-A39 in all solutions. Average current is plotted as a function of time. Trypsin was added at t = 0. Open circles, control; solid squares, G22-A39; all n = 3–5.
G22-A39 peptide does not prevent the cleavage of ASIC1a by trypsin
Cleavage of ASIC1a channels by trypsin leads to a shift in the pH dependence of activation to more acidic values (24). ASIC1a was first activated 3 times by pH 6.6, every 45 s. The conditioning solution was then switched to a pH 7.4 solution containing 40 μg/ml trypsin with or without 10 μM G22-A39, and currents were measured every 45 s at pH 6.6. Due to the trypsin-elicited time-dependent shift in the pH dependence of activation, we observed a gradual reduction in pH 6.6-evoked current in the presence of trypsin, as illustrated in Fig. 2D. This current decrease was not prevented by G22-A39.
G22-A39 interacts through the β-ENaC subunit
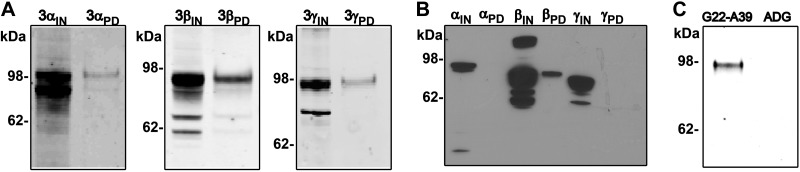
Using coimmunoprecipitation, we have previously demonstrated that SPLUNC1 binds to αβγ-ENaC (16). A pulldown assay was performed using biotin-labeled G22-A39 to determine whether the peptide could also interact with ENaC in a similar fashion. Three different transfections were performed with one of the three subunits HA/V5 tagged and the other two untagged. The cell lysates were then incubated with biotinylated G22-A39 bound to neutravidin beads. As observed with full-length SPLUNC1, G22-A39 was found to pull down all three ENaC subunits (Fig. 3A). To further characterize this interaction, each subunit was expressed individually, and the pulldown assay was repeated. In this case, only β-ENaC was detected in the elution, indicating that G22-A39 binds exclusively to the β-ENaC subunit and not to the α or γ subunits (Fig. 3B). When the pulldown assay of the β-ENaC subunit was performed with ADG, β-ENaC was not detected in the elution, confirming that G22-A39 is specifically binding to the β-ENaC subunit (Fig. 3C).
Figure 3.
G22-A39 interacts specifically with the β-ENaC subunit. A) Typical Western blot of the triple-transfected αβγ-ENaC peptide pulldown assay. The pulldown assay was performed with 1 V5-tagged subunit and 2 untagged subunits as designated. B) Typical Western blot of the G22-A39 peptide pulldown assay of individually expressed ENaC subunits. IN, input; PD, pulldown elution. C) Typical Western blot showing the pulldown assay performed with G22-A39 or ADG. No β-ENaC was observed in the elution with the ADG peptide, confirming that the observed β-ENaC is from specific interaction with the G22-A39; n = 3.
β-ENaC glycosylation is required for the β-ENaC/G22-A39 interaction
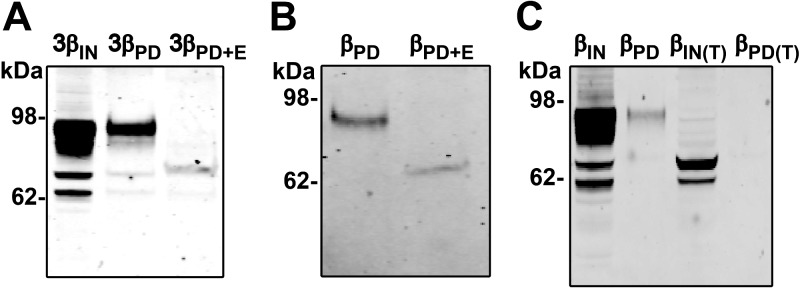
The predicted molecular mass of a nonglycosylated β-ENaC subunit is ∼73 kDa. However, the extracellular loop of β-ENaC contains 12 possible sites for N-linked glycosylation, and β-ENaC is typically observed at 94–96 kDa due to extensive N-linked glycosylation (10). As seen in Fig. 3, the molecular mass of the β-ENaC subunit, which predominantly interacted with the G22-A39 peptide, was ∼94 kDa. To confirm that this was glycosylated β-ENaC, the elutions of the G22-A39/ α(V5β)γ ENaC pulldowns were deglycosylated with EndoH (Fig. 4). On treatment with EndoH, the 94-kDa β-ENaC band shifted to ∼73 kDa, consistent with the deglycosylation of β-ENaC (Fig. 4A). This experiment was repeated with the β-ENaC subunit expressed alone. On EndoH treatment, this band shifted to ∼73 kDa (Fig. 4B). EndoH treatment could only be performed once the pulldown assay had been completed; therefore, to test whether G22-A39 could interact with nonglycosylated β-ENaC, we exposed cells to tunicamycin, an inhibitor of N-linked glycosylation (32). The pulldown assay was then performed on the tunicamycin-treated cell lysate (Fig. 4C). As seen in the input lane, treatment with tunicamycin reduced the molecular mass of the β-ENaC subunit to ∼73 kDa, confirming that the protein was deglycosylated. No β-ENaC was observed in the elution of the tunicamycin-treated pulldown. Combined with the EndoH data, this indicates that G22-A39 is interacting with a specific, glycosylated form of β-ENaC.
Figure 4.
β-ENaC/G22-A39 interaction is glycosylation dependent. A) Typical Western blot of the αβγ-ENaC peptide pulldown assay with the β-ENaC subunit V5-tagged and untagged α- and γ-ENaC subunits. Pulldown assay was performed, and the elution was treated with EndoH. B) Typical Western blot of the β-ENaC-only peptide pulldown assay with the β-ENaC subunit V5 tagged. Pulldown assay was performed, and the elution was treated with EndoH. PD, pulldown elution; PD+E, pulldown elution with EndoH treatment. C) Typical Western blot of the tunicamycin-treated β-ENaC-only pulldown assay. IN(T), input of tunicamycin-treated sample; PD (T), pulldown elution of tunicamycin-treated sample; n = 3.
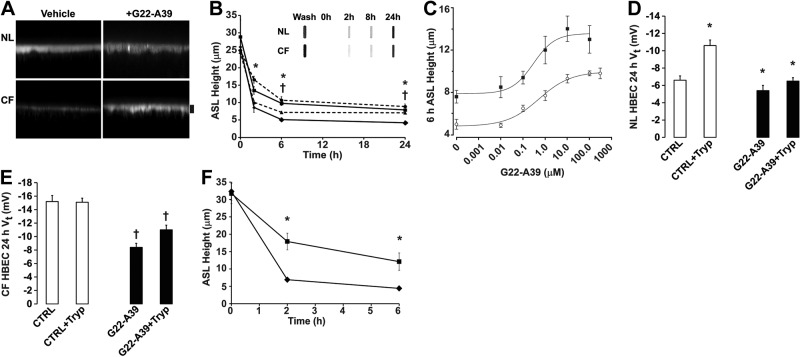
G22-A39 attenuates ASL hyperabsorption in CF HBECs through ENaC inhibition
To determine whether G22-A39 could inhibit ENaC-dependent ASL absorption in native airway epithelia, we measured ASL height over time in both NL and CF HBECs after treatment with G22-A39 (Fig. 5A, B). SPLUNC1 is endogenously secreted by both NL and CF HBECs, which could affect ASL volume regulation, especially in NL HBECs (16). Thus, we standardized the mucosal washing/volume-loading protocol accordingly so that every culture had endogenous SPLUNC1 removed prior to t = 0. Each culture was left with undisturbed ASL for 24 h. They were then incubated for 30 min with 500 μl PBS, followed by 2 quick successive washes with 500 μl PBS. Then, 20 μl of PBS containing rhodamine-dextran was added as a volume challenge with or without peptide. Slot-blot analysis revealed that it took 24 h for SPLUNC1 levels in the ASL to recover to baseline values (Fig. 5B, inset). Vehicle-treated CF HBECs had a significantly lower ASL height than NL controls. However, the ASL height in CF G22-A39 treated samples, 7.9 ± 0.6 μm, was significantly higher than the CF controls, 4.2 ± 0.6 μm. This increase in ASL height in G22-A39 exposed CF HBECs was comparable to that observed in NL HBECs (∼8 μm). Next, a full dose response was completed in order to calculate the IC50 by measuring the ASL height of both NL and CF HBECs 6 h after addition of G22-A39 (Fig. 5C). The IC50 was not significantly different between NL and CF HBECs, 0.29 ± 0.19 and 0.52 ± 0.23 μM, respectively.
Figure 5.
G22-A39 inhibits CF ASL hyperabsorption. A) Confocal micrographs of NL and CF ASL height 24 h after exposure to G22-A39 or vehicle (control). Scale bar = 7 μm. B) Mean ASL height over time in NL and CF HBECs with or without addition of G22-A39; dashed lines indicate NL HBECs (triangles, control; circles, G22-A39), nondashed lines indicate CF HBECs (diamonds, control; squares, G22-A39), n = 6. *P < 0.05 for NL vs. basal; †P < 0.05 for CF vs. basal. Inset: typical slot blot for SPLUNC1 lavaged from the mucosal surfaces of NL and CF HBECs at timed intervals. Mucosal surfaces were left undisturbed for 24 h, washed immediately prior to initiating ASL height experiments, and in parallel cultures, subsequent SPLUNC1 recovery was determined. C) Change in ASL height with increasing concentration of G22-A39 in NL (solid squares) and CF (open circles) HBECs. D) Thin-film transepithelial PD for NL HBECs. Open bars, control; solid bars, G22-A39; n = 10. *P < 0.05 vs. corresponding control. E) Thin-film transepithelial PD for CF HBECs. Open bars, control; solid bars, G22-A39; n = 6. †P < 0.05 vs. corresponding control. F) ASL height over time in NL HBECs in the presence of 100 μM bumetanide with (squares) or without G22-A39 (diamonds), n = 6. †P < 0.05 vs. no G22-A39.
To test whether G22-A39 specifically affected ENaC in NL and CF HBECs, the 24 h transepithelial potential difference Vt was measured (Fig. 5D, E). In the NL HBECs, a basal Vt of −6.6 ± 0.5 mV was observed, and this decreased to −10.6 ± 0.7 mV following a brief exposure to trypsin, which is indicative of ENaC activation (15). G22-A39 had little additional effect on the 24 h Vt in NL HBECs. Consistent with our previous observation that CF ENaC remains fully active and nonresponsive to trypsin (15), the CF vehicle-exposed HBECs had an elevated Vt of −15.2 ± 0.9 mV, and trypsin had no further effect. In contrast, after 24 h exposure to G22-A39, the CF HBEC Vt was significantly lowered to −8.4 ± 0.9 mV, and a 30-min exposure to trypsin changed the Vt to −11 ± 0.7 mV, suggesting that G22-A39 works through ENaC in CF HBECs. In contrast, ADG had no effect on NL or CF HBEC Vt (both n=6). To further confirm that G22-A39 functions by inhibiting ENaC hyperabsorption, the ASL height of NL HBECs was measured over time in the presence of bumetanide with or without G22-A39 (Fig. 5F). Serosal bumetanide significantly decreased NL ASL height toward CF levels (i.e., <5 μm). In contrast G22-A39 could maintain significantly greater ASL height in the presence of bumetanide, indicating that G22-A39 increases ASL height by inhibiting absorption not secretion.
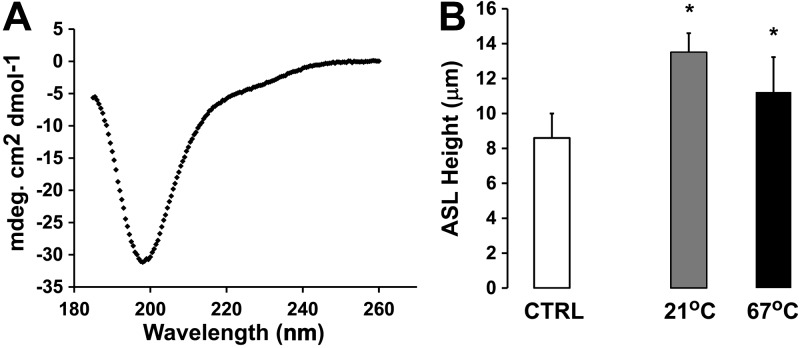
G22-A39 has no intrinsic structure
To better understand how G22-A39 may interact with ENaC, we next looked for intrinsic structure in this peptide. We first used the program PSIPRED to predict its structure (33, 34). Consistent with a lack of predicted structure with PSIPRED, we also failed to detect any secondary structure by circular dichroism (Fig. 6A). To functionally test this hypothesis, we heat denatured G22-A39 by incubating it at 67°C for 30 min and then added it to the mucosal surface of CF HBECs. The ASL height of the CF HBECs was measured 2 h later. The heat-denatured G22-A39 could prevent ASL hyperabsorption to the same extent as the non-heat-denatured peptide (Fig. 6B), suggesting that G22-A39 performs its function with no intrinsic secondary structure.
Figure 6.
G22-A39 maintains ASL height of CF HBECs with no intrinsic structure. A) Far-UV CD spectra of G22-A39 at 25°C. B) ASL height of CF HBECs at 2 h. Open bar, control; shaded bar, G22-A39 at 21°C; solid bar, G22-A39 heated to 67°C then added to culture. *P < 0.05 vs. control.
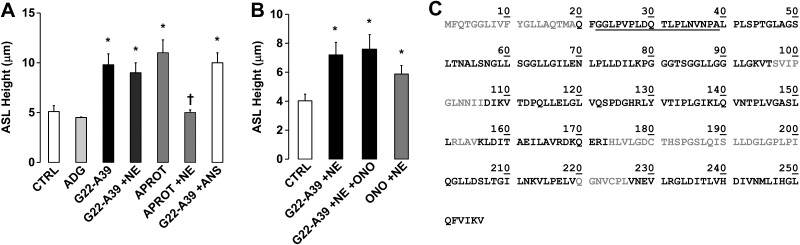
G22-A39 prevents CF ASL hyperabsorption in the presence of NE
CF airways are characterized by chronic neutrophilia and display a significantly higher level of NE activity than NL airways (35). Since NE has previously been shown to activate ENaC by cleaving the γ subunit (11), we tested whether G22-A39 was still capable of inhibiting CF ASL hyperabsorption in the presence of this protease (Fig. 7A). Mucosal addition of G22-A39, but not ADG, to CF HBECs resulted in a significant increase in ASL height. Similarly, aprotinin, which blocks trypsin-like proteases, could also maintain ASL height to a similar degree as G22-A39. Interestingly, G22-A39 maintained its ability to prevent CF ASL hyperabsorption in the presence of NE, while aprotinin could not prevent NE-induced ASL height depletion. ANS, which is derived from human neutrophils, contains high levels of NE activity (28) but did not diminish G22-A39's ability to prevent CF ASL volume hyperabsorption. When the NE inhibitor sivelestat, also known as ONO-5046, was added to the ASL along with G22-A39 and NE, no further effect on ASL height was observed (Fig. 7B). Together, these data suggest that G22-A39 may be able to prevent ASL hyperabsorption, even in the presence of the high proteolytic activity that is typically present in diseased CF lungs.
Figure 7.
G22-A39 prevents ASL hyperabsorption in the presence of NE. A, B) Bar graphs of ASL height at 8 h in CF HBECs. A) Open bars, control; solid bars, 100 μM G22-A39; dark shaded bars, 10 μM aprotinin. APROT, aprotinin. NE was added at 100 nM. ANS was diluted 1:1 with PBS. All n = 6. *P < 0.05 vs. control, †P < 0.05 vs. aprotinin. B) Open bar, control; solid bars, 100 μM G22-A39 with 100 nM NE with or without 10 μM ONO; shaded bar, 100 nM NE with 10 μM ONO. All n = 6. *P < 0.05 vs. control. C) Sequence of SPLUNC1 obtained by mass spectrometry, with the observed residues after 5 min exposure to NE in solid black. G22-A39 peptide is underscored.
Cleavage of SPLUNC1 by NE
To determine whether peptides corresponding to the N-terminal ENaC inhibitory domain of SPLUNC1 may be released from the main molecule by NE, the SPLUNC1-Δ19 recombinant protein, which lacks the N-terminal signaling sequence but contains the ENaC inhibitory domain, was exposed to NE for 5, 15, and 60 min, and the resulting cleavage products were determined by mass spectrometry. Similar sequence coverage was observed at all three time points ∼80% (Fig. 7C and Table 1). Three peptides were observed by mass spectrometry that spanned the ENaC inhibitory domain (i.e., residues 23–45) at all three time points: 2 ions corresponding to peptide 23GLPVPLDQTLPLNVNPALPLSPT45 with a m/z of 1183.742+ and 789.493+ were observed; 2 ions corresponding to peptide 27PLDQTLPLNVNPALPLSPT45 with a m/z of 1000.592+ and 667.393+ were observed; and 1 ion corresponding to peptide 32LPLNVNPALPLSPT45 with a m/z of 723.412+ was observed. In addition to this, in the 15- and 60-min samples, an ion corresponding to peptide 20QFGGLPVPLDQTLPL34 with a m/z of 797.992+ was observed. Several other peptides spanning this region were observed in the 60-min sample only, including an ion corresponding to peptide 23GLPVPLDQTLPLNVNPA39 with a m/z of 879.532+. All peptides observed in the 5-min sample are listed in Table 1.
Table 1.
Peptides observed in the neutrophil elastase 5-min cleavage assay of SPLUNC1-Δ19
| Residue | Sequence | Residue | Sequence |
|---|---|---|---|
| 23–45 | GLPVPLDQTLPLNVNPALPLSPT | 122–131 | QSPDGHRLYV |
| 27–45 | PLDQTLPLNVNPALPLSPT | 123–131 | SPDGHRLYV |
| 32–45 | LPLNVNPALPLSPT | 124–131 | PDGHRLYV |
| 46–61 | GLAGSLTNALSNGLLS | 125–131 | DGHRLYV |
| 46–67 | GLAGSLTNALSNGLLSGGLLGI | 132–141 | TIPLGIKLQV |
| 46–86 | GLAGSLTNALSNGLLSGGLLGILENLPLLDILKPGGGTSGG | 132–148 | TIPLGIKLQVNTPLVGA |
| 53–86 | NALSNGLLSGGLLGILENLPLLDILKPGGGTSGG | 132–155 | TIPLGIKLQVNTPLVGASLLRLAV |
| 53–95 | NALSNGLLSGGLLGILENLPLLDILKPGGGTSGGLLGGLLGKV | 138–148 | KLQVNTPLVGA |
| 53–96 | NALSNGLLSGGLLGILENLPLLDILKPGGGTSGGLLGGLLGKVT | 138–149 | KLQVNTPLVGAS |
| 54–95 | ALSNGLLSGGLLGILENLPLLDILKPGGGTSGGLLGGLLGKV | 138–151 | KLQVNTPLVGASLL |
| 62–95 | GGLLGILENLPLLDILKPGGGTSGGLLGGLLGKV | 142–155 | NTPLVGASLLRLAV |
| 62–96 | GGLLGILENLPLLDILKPGGGTSGGLLGGLLGKVT | 149–155 | SLLRLAV |
| 67–95 | ILENLPLLDILKPGGGTSGGLLGGLLGKV | 156–166 | KLDITAEILAV |
| 68–95 | LENLPLLDILKPGGGTSGGLLGGLLGKV | 156–173 | KLDITAEILAVRDKQERI |
| 74–86 | LDILKPGGGTSGG | 160–173 | TAEILAVRDKQERI |
| 77–95 | LKPGGGTSGGLLGGLLGKV | 164–173 | LAVRDKQERI |
| 77–96 | LKPGGGTSGGLLGGLLGKVT | 165–172 | AVRDKQER |
| 84–95 | SGGLLGGLLGKV | 165–173 | AVRDKQERI |
| 84–96 | SGGLLGGLLGKVT | 166–173 | VRDKQERI |
| 87–95 | LLGGLLGKV | 201–219 | QGLLDSLTGILNKVLPELV |
| 87–96 | LLGGLLGKVT | 231–236 | LRGLDI |
| 106–131 | IDIKVTDPQLLELGLVQSPDGHRLYV | 231–239 | LRGLDITLV |
| 107–118 | DIKVTDPQLLEL | 231–243 | LRGLDITLVHDIV |
| 107–121 | DIKVTDPQLLELGLV | 231–247 | LRGLDITLVHDIVNMLI |
| 107–131 | DIKVTDPQLLELGLVQSPDGHRLYV | 237–247 | TLVHDIVNMLI |
| 109–118 | KVTDPQLLEL | 240–247 | HDIVNMLI |
| 109–121 | KVTDPQLLELGLV | 240–256 | HDIVNMLIHGLQFVIKV |
| 109–131 | KVTDPQLLELGLVQSPDGHRLYV | 248–253 | HGLQFV |
| 111–131 | TDPQLLELGLVQSPDGHRLYV | 248–254 | HGLQFVI |
| 119–131 | GLVQSPDGHRLYV | 248–256 | HGLQFVIKV |
| 121–131 | VQSPDGHRLYV |
Next, we determined which, if any, SPLUNC1 peptides were present in human sputum. In sputum obtained from healthy subjects, 4 peptides were observed that spanned the ENaC inhibitory domain: an ion corresponding to peptide 28LDQTLPLNVNPALPLSPT45 with a m/z of 952.032+, an ion corresponding to peptide 29DQTLPLNVNPALPLSPT45 with a m/z of 895.492+, an ion corresponding to peptide 32LPLNVNPALPLSPT45 with a m/z of 723.412+, and an ion corresponding to peptide 35NVNPALPLSPT45 with a m/z of 561.812+ were observed. Surprisingly, no peptides were observed in CF sputum before residue 46, suggesting that SPLUNC1's regulation of ENaC may be defective in CF airways.
DISCUSSION
We found that a peptide reprising the ENaC inhibitory domain of SPLUNC1, G22-A39, inhibited ENaC activity to the same extent as full-length SPLUNC1 (Fig. 1A, B). In the presence of MTSET, G22-A39 caused a significant decrease in ENaC activity, suggesting that G22-A39 exposure results in a decrease in N, consistent with previous data demonstrating that SPLUNC1 lowers ENaC surface densities (31). In addition, the ratio of the MTSET current divided by basal current was significantly greater in the presence of G22-A39 than in its absence (9-fold vs. 5.7-fold). This ratio has previously been taken as an indicator of channel open probability (Po), and the higher fold increase may indicate that ENaC resides in a lower Po in the presence of G22-A39 (Fig. 1C) (36). Therefore, while it is likely that G22-A39 decreases N, we cannot exclude the possibility that G22-A39 may also have an additional effect on Po.
Despite being structurally related to ENaC, our experiments do not show any effect of G22-A39 on ASIC activity. ASICs are the target of peptide toxins and of short peptides and several small, charged peptides have been shown to acutely modulate ASIC function (37, 38). Currently, no evidence indicates endogenous regulation of ASICs by proteases, although it is known that ASIC1 pH dependence is changed by exposure to serine proteases (24). In the absence of an endogenous regulation of ASICs by proteases in the cell system used (24), a long-term exposure to G22-A39 is not expected to have an effect. We therefore tested the acute effect of G22-A39 by recording ASIC currents. The inhibitory toxins and the inactivation-modifying peptides all contain Arg and/or Lys residues that appear to be important for their function (38, 39), while the G22-A39 peptide does not contain Arg or Lys residues. As a caveat, since the experiments were performed with the G22-A39 peptide, we cannot exclude the possibility that other parts of SPLUNC1 might still interact with ASICs.
We found that G22-A39 can pull down all three subunits when they are coexpressed (Fig. 3A). However, when each subunit was expressed individually, only the β-ENaC subunit was pulled down (Fig. 3B, C). These data suggest that a complex may form between the α-, β-, and γ-ENaC subunits rather than binding of the α- or γ-ENaC subunits to G22-A39. Alternatively, it may be possible that when coexpressed, parts of the α- and γ-ENaC extracellular domains may fold into a different conformation that is conducive to α- and γ-ENaC binding to G22-A39 when β-ENaC is present.
The predominant band observed in the peptide pulldown assay for β-ENaC, both when all three subunits were coexpressed and when only the β-ENaC subunit was expressed, was ∼94 kDa (Fig. 3A, B), which corresponds to glycosylated β-ENaC (10). In contrast, the expected molecular mass for nonglycosylated β-ENaC is ∼73 kDa. When we pulled down β-ENaC and exposed the elution to EndoH, the 94-kDa β-ENaC band pulled down with G22-A39 was shifted to ∼73 kDa, indicating that the form of β-ENaC binding to the G22-A39 peptide is glycosylated with noncomplex, high-mannose N-glycans (Fig. 4A, B). To confirm this interaction, cells were grown and transfected in the presence of tunicamycin, which prevents N-linked glycosylation. Under these conditions, G22-A39 could not pull down the nonglycosylated (i.e., tunicamycin-sensitive) form of β-ENaC (Fig. 4C). Taken together, our data lead us to conclude that the G22-A39/ β-ENaC interaction is strongly dependent on the glycosylation state of β-ENaC.
In addition to inhibiting ENaC in oocytes, G22-A39, but not the control, peptide ADG, could also reduce ASL absorption, and over a period of 24 h, G22-A39 restored ASL height in CF HBECs to ∼8 μm, which is comparable to that observed in NL HBECs (Figs. 5A, B and 7A). Vt was measured in these same cultures to confirm that G22-A39 was in fact functioning by inhibiting ENaC (Fig. 5D, E). The thin-film Vt reflects a bumetanide-sensitive component (Cl− secretion through CFTR) and an amiloride/trypsin-sensitive component (Na+ absorption through ENaC) (15). In NL HBECs, the bumetanide-sensitive Vt is stable with time, while the trypsin-sensitive component changes with time as ENaC is inactivated by SPLUNC1 (15, 16). In contrast, CF HBECs have no bumetanide-sensitive Vt due to the lack of CFTR, and these cultures are also insensitive to trypsin, unless pretreated with a protease inhibitor like aprotinin, since ENaC is not spontaneously regulated (15). However, in both NL and CF HBECs, G22-A39 caused a decrease in the 24-h Vt and in CF HBECs' induced trypsin sensitivity, giving further evidence that G22-A39 is acting through ENaC. Together, these data indicate that G22-A39 can serve to restore the regulation of ENaC that is lacking in CF HBECs.
G22-A39 is located at the N terminus of SPLUNC1 in a region that is predicted to be intrinsically disordered. Using CD, we demonstrated that the synthesized G22-A39 peptide also displayed no secondary structure (Fig. 6A). This lack of secondary structure was further confirmed by the observation that heat-denatured G22-A39 still prevented ASL hyperabsorption in CF HBECs (Fig. 6B). Other investigators have recently shown that unstructured regions of proteins play an important role in protein-protein interactions and that their flexibility is essential to their function (40). It is also possible that the several proline residues followed by a hydrophobic residue in the G22-A39 sequence are important for its function. Proline-rich motifs have been recognized to play an important role in protein-protein interactions (41), and the roles of the prolines, as well as the other residues, in G22-A39 are currently being explored to further characterize and optimize their inhibitory function.
Carattino et al. (9) previously identified a 26-aa peptide that is excised from the α-ENaC subunit during proteolytic processing, residues D206–R231. When these residues are added back to cleaved ENaC, they inhibit ENaC activity (9). This 26-mer was further refined to a region of 8 aa, residues L211–L218 (14). Both the 26-mer and 8-mer α-ENaC peptides caused a reduction in the NPo of ENaC, and they concluded that the reduction largely reflects a change in channel Po (9, 14, 42). Interestingly, the sequence of the 8-mer, LPHPLQRL, shares 50% sequence identity with residues L24–T31 (LPVPLDQT) of the G22-A39 peptide. However, the IC50 of the 8-mer was calculated to be 106 and 76 μM in NL and CF HBECs, respectively, which was much higher than the G22-A39 peptide (0.29 and 0.52 μM in NL and CF HBECs, respectively). The difference in the IC50s could be due to the peptide-ENaC subunit interaction, and the α-ENaC 8-mer is thought to bind to residues within the finger and thumb domains of α-ENaC (43), while our data show that G22-A39 targets the β-ENaC subunit. Further characterization of the G22-A39 sequence will be needed to distinguish which residues play the largest role in the SPLUNC1 ENaC inhibitory domain.
High levels of proteolytic activity are typically present in the diseased CF lung, leading to potential protein degradation by NE. In the case of G22-A39, this could lead to decreased efficacy and/or duration of action. However, a single dose of G22-A39 prevented CF ASL hyperabsorption over a 24-h period and was unaffected by either purified NE or activated neutrophil supernatant (Fig. 7A). The ability of G22-A39 to function in a proteolytically active environment makes G22-A39 a strong therapeutic candidate for restoring ASL height in patients with CF. Since G22-A39 activity was unaffected by NE, we speculated that a region corresponding to this peptide may be released from SPLUNC1 upon proteolysis. Mass spectrometry analysis revealed that cleavage of recombinant SPLUNC1-Δ19 by NE indeed resulted in the formation of peptides corresponding to the G22-A39 region (Fig. 7C and Table 1), suggesting that release of this region may be a mechanism to make the ENaC inhibitory domain of SPLUNC1 more available during times of inflammation; i.e., when both SPLUNC1 and NE levels are increased. This release of a G22-A39-like region may serve to inhibit ENaC, increase hydration of the mucus layer, and increase mucus clearance, which would be beneficial for innate lung defense. Interestingly, similar peptides to G22-A39 were observed in NL human sputum samples but were undetectable in CF sputum. Free NE is absent from the lungs of healthy individuals. However, the peptides that we detected may arise from the normal breakdown of SPLUNC1 by the extracellular proteases that are present in the airways (30). SPLUNC1 is detectable in CF HBEC ASL (Fig. 5B) and may even be up-regulated in CF airways, (44, 45). However, we do not detect autoregulation of ENaC in CF HBECs (15). Since G22-A39 inhibits CF ENaC (Figs. 5 and 7), as does aprotinin (Fig. 7), our data suggest that the level of the dysregulation lies with SPLUNC1 and not with ENaC. Thus, we hypothesize that SPLUNC1 is defective and fails to regulate ENaC in CF airways, which contributes to CF ASL dehydration. However, further experimentation will be required to determine the nature of the putative defect in SPLUNC1 in CF airways.
The long duration of action of G22-A39 (24 h inhibition with a single dose) may be due to the ability of G22-A39 to bind directly to β-ENaC, unlike the small molecule inhibitor amiloride, which is rapidly transported across the epithelia and has a half-life in the ASL of 10 min (27). Furthermore, we speculate that since G22-A39 binds to β-ENaC, it will not be actively transported across the airways and would avoid the off-target effects seen with amiloride and its analogues, such as inhibition of ENaC in the kidneys, which can lead to natriuresis and reduced blood pressure. In summary, G22-A39 is heat stable and protease resistant and inhibits ENaC/ASL hyperabsorption for up to 24 h in CF HBECs, suggesting that it has therapeutic potential for the treatment of CF lung disease.
Acknowledgments
The authors thank Dr. Ashley G. Henderson for making the sputum samples available and Dr. Rebecca P. Hughey for helpful discussions on the glycosylation experiments. The authors gratefully acknowledge the technical assistance of Michael Watson, Hong He, and Yan Dang. The authors also thank the University of North Carolina (UNC) Cystic Fibrosis Center Molecular and Cell Culture Cores, as well as the UNC Macromolecular Interactions Facility.
Funding was provided by the Cystic Fibrosis Foundation Resource Development Program, grant R026 (CFF RDP-R026); U.S. National Institutes of Health grants PPG P01 HL034322, 5 P30 DK 065988-08, R01HL103940, and R01HL108927; and the UNC–Chapel Hill School of Medicine.
Footnotes
- ANS
- activated neutrophil supernatant
- ASIC
- acid-sensing ion channel
- ASL
- airway surface liquid
- CD
- circular dichroism
- CF
- cystic fibrosis
- CFTR
- cystic fibrosis transmembrane conductance regulator
- ENaC
- epithelial sodium channel
- EndoH
- endoglycosidase H
- HBEC
- human bronchial epithelial culture
- LC-ESI/MS/MS
- liquid chromatography-electrospray ionization tandem mass spectrometry
- MTSET
- [2-(trimethyl-ammonium)ethyl]methanethiosulfonate bromide
- NE
- neutrophil elastase
- NL
- normal
- SPLUNC1
- short palate, lung, and nasal epithelial clone 1
REFERENCES
- 1. Garty H., Palmer L. G. (1997) Epithelial sodium channels: function, structure, and regulation. Physiol. Rev. 77, 359–396 [DOI] [PubMed] [Google Scholar]
- 2. Knowles M. R., Boucher R. C. (2002) Mucus clearance as a primary innate defense mechanism for mammalian airways. J. Clin. Invest. 109, 571–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ratjen F. (2006) Restoring airway surface liquid in cystic fibrosis. N. Engl. J. Med. 354, 291–293 [DOI] [PubMed] [Google Scholar]
- 4. Stutts M. J., Canessa C. M., Olsen J. C., Hamrick M., Cohn J. A., Rossier B. C., Boucher R. C. (1995) CFTR as a cAMP-dependent regulator of sodium channels. Science 269, 847–850 [DOI] [PubMed] [Google Scholar]
- 5. Rowe S. M., Miller S., Sorscher E. J. (2005) Cystic fibrosis. N. Engl. J. Med. 352, 1992–2001 [DOI] [PubMed] [Google Scholar]
- 6. Canessa C. M., Schild L., Buell G., Thorens B., Gautschi I., Horisberger J. D., Rossier B. C. (1994) Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature 367, 463–467 [DOI] [PubMed] [Google Scholar]
- 7. Canessa C. M., Merillat A. M., Rossier B. C. (1994) Membrane topology of the epithelial sodium channel in intact cells. Am. J. Physiol. 267, C1682–1690 [DOI] [PubMed] [Google Scholar]
- 8. Renard S., Lingueglia E., Voilley N., Lazdunski M., Barbry P. (1994) Biochemical analysis of the membrane topology of the amiloride-sensitive Na+ channel. J. Biol. Chem. 269, 12981–12986 [PubMed] [Google Scholar]
- 9. Carattino M. D., Sheng S., Bruns J. B., Pilewski J. M., Hughey R. P., Kleyman T. R. (2006) The epithelial Na+ channel is inhibited by a peptide derived from proteolytic processing of its alpha subunit. J. Biol. Chem. 281, 18901–18907 [DOI] [PubMed] [Google Scholar]
- 10. Hughey R. P., Mueller G. M., Bruns J. B., Kinlough C. L., Poland P. A., Harkleroad K. L., Carattino M. D., Kleyman T. R. (2003) Maturation of the epithelial Na+ channel involves proteolytic processing of the alpha- and gamma-subunits. J. Biol. Chem. 278, 37073–37082 [DOI] [PubMed] [Google Scholar]
- 11. Rossier B. C., Stutts M. J. (2009) Activation of the epithelial sodium channel (ENaC) by serine proteases. Annu. Rev. Physiol. 71, 361–379 [DOI] [PubMed] [Google Scholar]
- 12. Bridges R. J. (2002) Transepithelial measurements of bicarbonate secretion in Calu-3 cells. Methods Mol. Med. 70, 111–128 [DOI] [PubMed] [Google Scholar]
- 13. Bruns J. B., Carattino M. D., Sheng S., Maarouf A. B., Weisz O. A., Pilewski J. M., Hughey R. P., Kleyman T. R. (2007) Epithelial Na+ channels are fully activated by furin- and prostasin-dependent release of an inhibitory peptide from the gamma-subunit. J. Biol. Chem. 282, 6153–6160 [DOI] [PubMed] [Google Scholar]
- 14. Carattino M. D., Passero C. J., Steren C. A., Maarouf A. B., Pilewski J. M., Myerburg M. M., Hughey R. P., Kleyman T. R. (2008) Defining an inhibitory domain in the alpha-subunit of the epithelial sodium channel. Am. J. Physiol. Renal Physiol. 294, F47–F52 [DOI] [PubMed] [Google Scholar]
- 15. Tarran R., Trout L., Donaldson S. H., Boucher R. C. (2006) Soluble mediators, not cilia, determine airway surface liquid volume in normal and cystic fibrosis superficial airway epithelia. J. Gen. Physiol. 127, 591–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garcia-Caballero A., Rasmussen J. E., Gaillard E., Watson M. J., Olsen J. C., Donaldson S. H., Stutts M. J., Tarran R. (2009) SPLUNC1 regulates airway surface liquid volume by protecting ENaC from proteolytic cleavage. Proc. Natl. Acad. Sci. U. S. A. 106, 11412–11417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gally F., Di Y. P., Smith S. K., Minor M. N., Liu Y., Bratton D. L., Frasch S. C., Michels N. M., Case S. R., Chu H. W. SPLUNC1 promotes lung innate defense against Mycoplasma pneumoniae infection in mice. Am. J. Pathol. 178, 2159–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chu H. W., Thaikoottathil J., Rino J. G., Zhang G., Wu Q., Moss T., Refaeli Y., Bowler R., Wenzel S. E., Chen Z., Zdunek J., Breed R., Young R., Allaire E., Martin R. J. (2007) Function and regulation of SPLUNC1 protein in Mycoplasma infection and allergic inflammation. J. Immunol. 179, 3995–4002 [DOI] [PubMed] [Google Scholar]
- 19. Zhou H. D., Li X. L., Li G. Y., Zhou M., Liu H. Y., Yang Y. X., Deng T., Ma J., Sheng S. R. (2008) Effect of SPLUNC1 protein on the Pseudomonas aeruginosa and Epstein-Barr virus. Mol. Cell. Biochem. 309, 191–197 [DOI] [PubMed] [Google Scholar]
- 20. Bartlett J. A., Gakhar L., Penterman J., Singh P. K., Mallampalli R. K., Porter E., McCray P. B., Jr. PLUNC: a multifunctional surfactant of the airways. Biochem. Soc. Trans. 39, 1012–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McGillivary G., Bakaletz L. O. The multifunctional host defense peptide SPLUNC1 is critical for homeostasis of the mammalian upper airway. PLoS One 5, e13224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Donaldson S. H., Hirsh A., Li D. C., Holloway G., Chao J., Boucher R. C., Gabriel S. E. (2002) Regulation of the epithelial sodium channel by serine proteases in human airways. J. Biol. Chem. 277, 8338–8345 [DOI] [PubMed] [Google Scholar]
- 23. Snyder P. M. (2000) Liddle's syndrome mutations disrupt cAMP-mediated translocation of the epithelial Na(+) channel to the cell surface. J. Clin. Invest. 105, 45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Poirot O., Vukicevic M., Boesch A., Kellenberger S. (2004) Selective regulation of acid-sensing ion channel 1 by serine proteases. J. Biol. Chem. 279, 38448–38457 [DOI] [PubMed] [Google Scholar]
- 25. Blanchard M. G., Kellenberger S. (2011) Effect of a temperature increase in the non-noxious range on proton-evoked ASIC and TRPV1 activity. Pflügers Arch. 461, 123–139 [DOI] [PubMed] [Google Scholar]
- 26. Randell S. H., Fulcher M. L., O'Neal W., Olsen J. C. Primary epithelial cell models for cystic fibrosis research. Methods Mol. Biol. 742, 285–310 [DOI] [PubMed] [Google Scholar]
- 27. Tarran R., Grubb B. R., Parsons D., Picher M., Hirsh A. J., Davis C. W., Boucher R. C. (2001) The CF salt controversy: in vivo observations and therapeutic approaches. Mol. Cell 8, 149–158 [DOI] [PubMed] [Google Scholar]
- 28. Haynes J., Jr., Obiako B., Hester R. B., Baliga B. S., Stevens T. (2008) Hydroxyurea attenuates activated neutrophil-mediated sickle erythrocyte membrane phosphatidylserine exposure and adhesion to pulmonary vascular endothelium. Am. J. Physiol. Heart Circ. Physiol. 294, H379–H385 [DOI] [PubMed] [Google Scholar]
- 29. Kesimer M., Sheehan J. K. Mass spectrometric analysis of mucin core proteins. Methods Mol. Biol. 842, 67–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kesimer M., Kirkham S., Pickles R. J., Henderson A. G., Alexis N. E., Demaria G., Knight D., Thornton D. J., Sheehan J. K. (2009) Tracheobronchial air-liquid interface cell culture: a model for innate mucosal defense of the upper airways? Am. J. Physiol. Lung Cell. Mol. Physiol. 296, L92–L100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rollins B. M., Garcia-Caballero A., Stutts M. J., Tarran R. SPLUNC1 expression reduces surface levels of the epithelial sodium channel (ENaC) in Xenopus laevis oocytes. Channels (Austin) 4, 255–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yan K., Khoshnoodi J., Ruotsalainen V., Tryggvason K. (2002) N-linked glycosylation is critical for the plasma membrane localization of nephrin. J. Am. Soc. Nephrol. 13, 1385–1389 [DOI] [PubMed] [Google Scholar]
- 33. Jones D. T. (1999) Protein secondary structure prediction based on position-specific scoring matrices. J. Mol. Biol. 292, 195–202 [DOI] [PubMed] [Google Scholar]
- 34. Buchan D. W., Ward S. M., Lobley A. E., Nugent T. C., Bryson K., Jones D. T. Protein annotation and modelling servers at University College London. Nucleic Acids Res. 38, W563–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Voynow J. A., Fischer B. M., Zheng S. (2008) Proteases and cystic fibrosis. Int. J. Biochem. Cell Biol. 40, 1238–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Anantharam A., Tian Y., Palmer L. G. (2006) Open probability of the epithelial sodium channel is regulated by intracellular sodium. J. Physiol. 574, 333–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sherwood T. W., Askwith C. C. (2009) Dynorphin opioid peptides enhance acid-sensing ion channel 1a activity and acidosis-induced neuronal death. J. Neurosci. 29, 14371–14380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Askwith C. C., Cheng C., Ikuma M., Benson C., Price M. P., Welsh M. J. (2000) Neuropeptide FF and FMRFamide potentiate acid-evoked currents from sensory neurons and proton-gated DEG/ENaC channels. Neuron 26, 133–141 [DOI] [PubMed] [Google Scholar]
- 39. Diochot S., Baron A., Rash L. D., Deval E., Escoubas P., Scarzello S., Salinas M., Lazdunski M. (2004) A new sea anemone peptide, APETx2, inhibits ASIC3, a major acid-sensitive channel in sensory neurons. EMBO J. 23, 1516–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bray D. (2005) Flexible peptides and cytoplasmic gels. Genome Biol. 6, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kay B. K., Williamson M. P., Sudol M. (2000) The importance of being proline: the interaction of proline-rich motifs in signaling proteins with their cognate domains. FASEB J. 14, 231–241 [PubMed] [Google Scholar]
- 42. Kashlan O. B., Adelman J. L., Okumura S., Blobner B. M., Zuzek Z., Hughey R. P., Kleyman T. R., Grabe M. Constraint-based, homology model of the extracellular domain of the epithelial Na+ channel alpha subunit reveals a mechanism of channel activation by proteases. J. Biol. Chem. 286, 649–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kashlan O. B., Boyd C. R., Argyropoulos C., Okumura S., Hughey R. P., Grabe M., Kleyman T. R. Allosteric inhibition of the epithelial Na+ channel through peptide binding at peripheral finger and thumb domains. J. Biol. Chem. 285, 35216–35223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bingle L., Barnes F. A., Cross S. S., Rassl D., Wallace W. A., Campos M. A., Bingle C. D. (2007) Differential epithelial expression of the putative innate immune molecule SPLUNC1 in cystic fibrosis. Respir. Res. 8, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Roxo-Rosa M., da Costa G., Luider T. M., Scholte B. J., Coelho A. V., Amaral M. D., Penque D. (2006) Proteomic analysis of nasal cells from cystic fibrosis patients and non-cystic fibrosis control individuals: search for novel biomarkers of cystic fibrosis lung disease. Proteomics 6, 2314–2325 [DOI] [PubMed] [Google Scholar]