Abstract
Aquaporins and Rh proteins can function as gas (CO2 and NH3) channels. The present study explores the urea, H2O, CO2, and NH3 permeability of the human urea transporter B (UT-B) (SLC14A1), expressed in Xenopus oocytes. We monitored urea uptake using [14C]urea and measured osmotic water permeability (Pf) using video microscopy. To obtain a semiquantitative measure of gas permeability, we used microelectrodes to record the maximum transient change in surface pH (ΔpHS) caused by exposing oocytes to 5% CO2/33 mM HCO3− (pHS increase) or 0.5 mM NH3/NH4+ (pHS decrease). UT-B expression increased oocyte permeability to urea by >20-fold, and Pf by 8-fold vs. H2O-injected control oocytes. UT-B expression had no effect on the CO2-induced ΔpHS but doubled the NH3-induced ΔpHS. Phloretin reduced UT-B-dependent urea uptake () by 45%, by 50%, and ()NH3 by 70%. p-Chloromercuribenzene sulfonate reduced by 25%, by 30%, and ()NH3 by 100%. Molecular dynamics (MD) simulations of membrane-embedded models of UT-B identified the monomeric UT-B pores as the main conduction pathway for both H2O and NH3 and characterized the energetics associated with permeation of these species through the channel. Mutating each of two conserved threonines lining the monomeric urea pores reduced H2O and NH3 permeability. Our data confirm that UT-B has significant H2O permeability and for the first time demonstrate significant NH3 permeability. Thus the UTs become the third family of gas channels. Inhibitor and mutagenesis studies and results of MD simulations suggest that NH3 and H2O pass through the three monomeric urea channels in UT-B.
Keywords: carbon dioxide transport, ammonia transport, water transport, urea transport, membrane protein
gases had been thought to cross biological membranes simply by dissolving in and then diffusing through the lipid phase of the membrane. Since the discovery that aquaporin 1 (AQP1) and RhAG, proteins highly expressed in the membrane of red blood cells (RBCs), are capable of transporting both carbon dioxide (CO2; Refs. 4, 7, 8, 10, 35, 37) and ammonia (NH3; refs. 33, 35, 38, 45), the field of gas channels has been evolving rapidly (2). Together, AQP1 and RhAG account for ∼90% of the CO2 traffic across human RBC membranes (7, 8). AQP1 can also transport another gas relevant to RBCs, namely, nitric oxide (NO; Refs. 15, 16). Based on these observations, it is of interest to determine if the urea transporter B (UT-B), a significant membrane protein in RBCs, might also function as a gas channel and transport CO2 or NH3.
Urea transporters (UTs) belong to the SLC14 family of solute carriers and are responsible for the facilitated diffusion of urea across the plasma membranes. Humans have two UT genes: SLC14A1, which encodes UT-B, and SLC14A2, which generates splice variants UT-A1 to UT-A3. UT-A1 and UT-A3 are primarily localized in the inner medullary collecting duct (IMCD; Refs. 48, 51), while UT-A2 is expressed in the thin descending limb of the loop of Henle (61). These transporters recycle and thereby concentrate urea in the renal medulla and as a result indirectly promote the generation of a concentrated urine (via effects on NaCl gradients) and also allow the excretion of urea (the main nitrogenous waste) with a minimal volume of water (12). Facilitated urea transport in both UT-A and UT-B can be inhibited by phloretin. However, only in the case of UT-B is urea transport also blocked by Hg2+ or p-chloromercuribenzene sulfonate (pCMBS).
UT-B is present in the descending vasa recta of the kidney (40, 69) and also has a broad distribution throughout the body including liver (27) and brain (predominantly astrocytes; Refs. 1, 27). A major site of UT-B expression is the RBC membrane (39, 57), where UT-B is the basis of the Kidd blood-group antigen (39, 69).
Yang and Verkman found that rat UT-B, when expressed at high levels in Xenopus oocytes, not only transports urea but also contributes significantly to osmotic water permeability (Pf), and this contribution is reduced by phloretin and pCMBS (71). Sidoux-Walter et al. (47), working with human UT-B in Xenopus oocytes, replicated the Yang-Verkman result at high levels of UT-B expression but showed that, at progressively lower levels, the Pf fell off substantially before the [14C]urea uptake, concluding that UT-B is permeable to H2O only at artificially high surface densities in the plasma membrane. However, an alternative explanation is that, at higher levels of expression, the apparent influx of the highly permeant [14C]urea saturates rapidly due to depletion of [14C]urea from the unstirred layer around the cell and buildup of [14C]urea at the inner surface of the cell membrane. Compared with their wild-type counterparts, UT-B knockout (KO) mice have increased plasma urea, reduced urinary urea, decreased urine osmolality and urinary concentrating ability, polyuria, reduced urea and H2O transport in RBCs, depression, and (in mice >52-wk-old) heart blockade (26, 70). The UT-B/AQP1 double KO (72) shows a more pronounced phenotype, with poor survival [50% die by postnatal day 10 (P10), 100% by P14], 30% reduction in body weight by P11, a decrease in the Pf of RBCs that is greater than for either knockout alone, and an increased hematocrit (Hct).
The crystal structures of the bacterial homolog Desulfovibrio vulgaris UT (dvUT; Ref. 25) and bovine UT-B (bUT-B; Ref. 24) show that the transporters are homotrimers, reminiscent of the homotrimeric structure of AmtB (a bacterial Rh homolog; Refs. 20, 21, 73) and RhCG (a kidney-specific homolog of RhAG; Ref. 13) and the homotetrameric structure of aquaporins (52, 53, 55, 63, 64). Each UT monomer consists of a front-half protomer (6 helices) and a homologous back-half protomer (6 helices) that form a monomeric, hydrophilic urea channel. In the middle of the three monomers is a hydrophobic central cavity, somewhat analogous to the central pores of Rh proteins and aquaporins. Presumably, gas-channel specificities rely on four major criteria: the chemical and physical properties of the gas and the chemical and physical properties of the pathway(s) formed by the protein. At the outset of this study, we hypothesized that H2O, CO2, and NH3 could pass through the hydrophilic, monomeric urea channel of UT-B.
The present study, performed on human UT-B (hUT-B) expressed in Xenopus oocytes, confirms that UT-B is permeable to urea and H2O, and demonstrates for the first time that hUT-B is permeable to NH3, although not to CO2. Because phloretin, pCMBS, and point mutations all inhibit urea and H2O and NH3 transport through hUT-B, we suspected that a common pathway is involved in the transport of these molecular species. Molecular dynamics (MD) simulations performed on membrane-bound bUT-B, the only structurally known UT-B, characterize the mechanism and reveal low free-energy barriers against H2O and NH3 permeation through the monomeric pores. This analysis thus supports the conclusion that H2O and NH3 most likely traverse the membrane through the monomeric urea pores of hUT-B. This NH3 permeability could enhance the ability of RBCs to take up NH3 in various tissues and then to off-load it in the liver for detoxification. The NH3 permeability of hUT-B makes UTs the third family of membrane channels with demonstrated gas permeability.
MATERIALS AND METHODS
Expression in Xenopus Oocytes
cRNA synthesis.
The full-length clone for hUT-B was purchased from Origene (product no. SC114602), and the DNA sequence confirmed. We used the restriction enzyme SmaI to obtain linearized hUT-B cDNA, which we purified using the QIAquick PCR purification kit (Qiagen, Valencia, CA). Transcribed capped cRNA was synthesized using the T7 mMessage mMachine Kit (Ambion, Austin, TX), followed by cRNA purification and concentration using the RNeasy MinElute RNA Cleanup Kit (Qiagen). The site directed mutants T177V and T339V were generated using the QuikChange Site-Directed Mutagenesis Kit (catalog no. 200518; Stratagene, Cedar Creek, TX) according to the manufacturer's protocol.
Isolation of Xenopus oocytes.
Ovaries were surgically removed from anesthetized frogs, and oocytes were separated using a collagenase treatment (46, 56). Stage V-VI oocytes were selected and stored until use at 18°C in OR3 medium, which was supplemented with penicillin (300 U/ml) and streptomycin (300 μg/ml). The Institutional Animal Care and Use Committee (IACUC) at Case Western Reserve University approved the protocols for housing and handling of Xenopus laevis.
Microinjection of cRNAs.
The day following isolation, oocytes were injected either with cRNA encoding hUT-B (25 ng given as 25 nl of a 1 ng/nl cRNA solution), the T177V or T339V mutants, or, in the case of control (i.e., “H2O”) oocytes, with sterile water (25 nl; Ambion). After injection, the oocytes were stored, at 18°C in OR3 medium supplemented with 500 U of penicillin and 500 U of streptomycin and were used in experiments 4 days after injection. We verified surface expression of hUT-B using surface biotinylation of oocytes (see below).
Solutions.
The ND96 solution contained the following: 96 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, and 5 mM HEPES, pH 7.50, osmolality 195 mosmol/kgH2O. For Pf assays, we used a hypotonic ND96 variant (100 mosmol/kgH2O) that contained only 43 mM NaCl. The CO2/HCO3− solution was identical to ND96 except that 33 mM NaHCO3 replaced 33 mM NaCl, and the solution was bubbled with 5% CO2/balanced O2. The NH3/NH4+ solution was a variant of ND96 in which we replaced 0.5 mM NaCl with 0.5 mM NH4Cl.
Surface Expression Measurements
Biotinylation.
Surface biotinylation was performed using the EZ-Link Sulfo-NHS-Biotinylation Kit (part no. 21425; Thermo Fisher Scientific, Rockford, IL) according to the manufacturer's recommendations with some modification. Before surface tagging, the PBS (part no. 28372; Thermo Fisher Scientific) and TBS (part no. 28376; Thermo Fisher Scientific) were diluted by one-third to reduce the osmolality of the solutions to match that of our other oocyte solutions, so that the oocytes would not be exposed to a hypoosmotic condition. Groups of 30 oocytes, expressing hUT-B or the T177V or T339V mutants, or injected with H2O, were incubated for 1 h with 5 ml of PBS plus 0.24 mg/ml EZ-link-sulfo-NHS-biotin. The reactions were quenched by addition of 250 μl of the supplied Quenching Solution. The oocytes were then washed in 0.67× TBS for 5 min. Cell lysis was performed by adding 300 μl of lysis buffer (0.67× TBS, 1% TX-100, and cOmplete Mini EDTA-free protease inhibitor tablet; part no. 04693124001; Roche, Indianapolis, IN) to the oocytes and breaking the cells by pipetting the cells up and down 30 times, using a P200 tip. The lysate was centrifuged at 3,000 g for 10 min to remove unsolubilized debris. After this step, a small aliquot of the supernatant was removed (30–50 μl) and mixed in a 1:1 ratio with 2× sample buffer; this sample represents the total solubilized expression fraction. The remainder of the supernatant was mixed with 200 μl of the NeutrAvidin Gel (part no. 29201; Thermo Fisher Scientific) and transferred to a Spin X column (part no. 8163; Corning, Pittston, PA). The samples were continuously mixed on a rocker platform for 1 h at room temperature. The spin columns were washed three times with lysis buffer to remove unbound contaminants, and then the biotinylated membrane proteins were eluted by applying 300 μl of 2× sample buffer plus 50 mM DTT. We then incubated for 1 h at room temperature on a rocker platform and then centrifuged at 1,000 g for 2 min. This final elution step represents the surface fraction.
Western blot analysis.
Total and surface biotinylation samples (see above) from oocytes expressing hUT-B or the T177V or T339V mutants or injected with H2O were separated by SDS-PAGE on 12% Tris-glycine gels (part no. EC60052BOX; Invitrogen, Grand Island, NY) at 125 V. The samples were transferred to PVDF membranes using the iBlot (part no. IB1001; Invitrogen) apparatus for 7–8 min. The membranes were washed briefly with TBST (TBS + Tween 20) and then transferred to TBST plus 5% powdered milk. The membranes were probed with a polyclonal UT-B antibody (part no. SC-134144; Santa Cruz Biotechnology, Santa Cruz, CA), and detected using ECL plus Western Blotting Detection Reagents (GE Healthcare Biosciences, Pittsburgh, PA).
Physiological Measurements
Measurement of oocyte urea uptake.
Oocyte urea transport activity was measured by monitoring [14C]urea uptake (39). Groups of 5–10 oocytes were incubated in ND96 plus 5 μCi of [14C]urea and 1 mM unlabeled urea for 0, 2, 5, and 10 min. The oocytes were then washed four times in ND96 plus 1 mM unlabeled urea. For the inhibition experiments, the oocytes were preincubated in 0.5 mM phloretin (catalog no. AC307651000; Arcos Organics, Fair Lawn, NJ) ×20 min or 1.0 mM pCMBS (catalog no. C367750; Toronto Research Chemicals, North York, ON, Canada) for 10 min and then washed three times in ND96 before being exposed to the isotope. After the isotope exposure and quenching, individual oocytes were placed in 100 μl of 5% SDS and lysed by pipetting up and down through a P200 pipette tip. The entire lysate was then transferred to 5 ml of scintillation fluid and analyzed for [14C]urea.
Measurement of osmotic water permeability of oocytes.
We determined Pf using a volumetric assay (3, 43, 60). Briefly, we dropped a group of up to about 12 oocytes into a Petri dish that contained the aforementioned 100 mosmol/kgH2O solution, acquiring video images every 1–2 s and obtaining the time course of the projection area of the oocyte. In computing Pf (35), we assumed the oocyte to be a sphere with a true surface area (S) eightfold greater than that of the idealized sphere (3). For the inhibition experiments, the oocytes were preincubated in 0.5 mM phloretin or 1.0 mM pCMBS as noted above.
Measurement of surface pH.
We used microelectrodes were used to monitor surface pH (pHS; Ref. 35). Briefly, the pH electrode, with a tip diameter of 15 μm, was filled at its tip with H+ ionophore mixture B (catalog no. 95293; Fluka Chemical, Ronkonkoma, NY) and connected to a model FD223 electrometer (World Precision Instruments, Sarasota, FL). As an extracellular reference electrode, we used a glass micropipette filled with 3 M KCl and connected, via a calomel half-cell, to a model 750 electrometer (World Precision Instruments). The electrometers were connected to electronic subtraction circuitry (made in-house), which in turn was connected to the inputs of an analog-digital converter (sampling rate was 1 per 500 ms) inside a Windows-based computer. The extracellular solution flowed at 3 ml/min. Using a MPC-200 system micromanipulator (Sutter Instrument, Novato, CA), we positioned the pHS electrode tip either in the bulk extracellular fluid or caused the tip to dimple ∼40 μm into the oocyte surface. The pHS electrode contacted the oocyte just inside the “shadow” of the flowing extracellular fluid. We continuously monitored membrane potential (Vm) using microelectrodes filled with 3 M KCl; all oocytes had initial Vm values at least as negative as −40 mV. For experiments with inhibitors, we preincubated oocytes in 0.5 mM phloretin or 1 mM pCMBS as described above.
Before applying a solution containing CO2/HCO3− or NH3/NH4+, we calibrated the pHS electrode with its tip in the bulk phase of the ND96 solution (pH 7.50). After returning the tip to the oocyte surface, applying the solution containing CO2 or NH3, and then allowing pHS to stabilize, we again withdrew the pHS electrode tip to the bulk phase of the CO2 or NH3 solution (also pH 7.50). The maximum pHS excursion (ΔpHS) during the CO2 or NH3 exposure is the maximum pHS after addition of CO2 (or the minimum pHS after addition of NH3) minus the pHS in ND96 prevailing just before the solution change.
Statistics for physiology experiments.
We present data are presented as means ± SE. In comparing the difference between two means, we performed Student's t-tests (two tails). When comparing more than two means, we performed a one-way ANOVA. P < 0.05 was considered significant.
MD Simulation Protocols
All the simulations were performed using NAMD 2.9 (42) with CHARMM27 force field (31) with Φ/Ψ cross-term map (CMAP) corrections (32) for proteins and CHARMM36 all-atom additive parameters for lipids (22). Water was modeled as TIP3P (19). All the simulations were maintained at 1.0 atm pressure using the Nosé-Hoover Langevin piston method (9) and at 310°K temperature using Langevin dynamics with a damping coefficient of 0.5 ps−1 applied to all nonhydrogen atoms. Short-range interactions were cut off at 12 Å with a smoothing function applied after 10 Å, and long-range electrostatic forces were calculated using the particle mesh Ewald (PME) method (6) at a grid density of >1 Å−3. Bonded, nonbonded, and PME calculations were performed at 2-, 2-, and 4-fs intervals, respectively.
Potential of mean force calculations.
Potential of mean force (PMF) for water permeation (the free energy profile of water moving along the pore axis) was generated using the equilibrium MD simulations for wild-type (apo-bUT-B) and double-mutant (T172V/T334V) systems, described elsewhere (24), as well as for two single-mutant (T172V and T334V) systems prepared from the apo-bUT-B system by mutating the respective residues. The single-mutant systems were subjected to minimization for 1,000 steps followed by ∼0.3 ns of simulation in which all the protein heavy atoms except those of the mutated residues were harmonically restrained (k = 5 kcal/mol/Å2). The single-mutant systems were later simulated for 10 ns each under isothermal-isobaric (NPT) conditions. Histograms of z-coordinate positions of water within the cylindrical region of (x − x0)2 + (y − y0)2 < r2, where r = 10 Å and x0 and y0 are the x and y components of the center of mass (COM) of the Cα atoms of channel-lining residues (Si, Sm, and So) of each monomer, were constructed at 0.25 Å bin width from the last 8 ns of the simulations. The PMF profiles were calculated from the histograms and were normalized with respect to the PMF at Si or So sites.
The PMF profile for ammonia (NH3) was calculated using umbrella sampling (US), initiated from the 50-ns equilibrated structure of the apo-bUT-B simulation. In each US set, 53 umbrella windows at 0.5-Å intervals were defined along the channel axis, covering a range from z = −13 to +13 Å with the origin (z = 0) at the COM of the Cα atoms of channel-lining residues (from the Si, Sm, and So sites) of each monomer. The water molecule closest to the center of each window in each monomeric pore was replaced by the ligand (NH3) to generate the starting configurations. Each window was initially minimized for 1,000 steps using the conjugated gradient algorithm. Then, a 2-ns US simulation was carried out with the position of the nitrogen atom of NH3 being restrained only along the z-axis by a harmonic potential,
where k = 3 kcal/mol/Å2 and zi is the center of the respective window. Half-harmonic restraints, in the form of
where kc = 10 kcal/mol/Å2, r = 5 Å, and x0 and y0 are the x and y components of COM of the Cα atoms of the residues of the Si, Sm, and So sites of each monomeric pore and were also applied to confine the sampling to a cylindrical region encompassing each monomeric pore. The z-coordinates of the nitrogen atom of NH3 in each monomeric pore were recorded at 0.1-ps intervals. Including only the last 1.5 ns of the US simulations (15,000 data points for each monomer, each window, and each set), the weighted histogram analysis method (WHAM; Ref. 50) was used to reconstruct the PMF profiles at 0.25 Å bin width, which were later normalized with respect to the average PMF at Si and So sites.
RESULTS
Surface Expression
The Xenopus oocyte system has been used previously to characterize the urea and H2O transport properties of UTs (47, 72). Before functionally characterizing hUT-B, we confirmed that the membrane protein is not only synthesized but also inserted into the plasma membrane. Using the membrane-impermeable reagent EZ-link-sulfo-NHS-biotin, it is possible to selectively modify and thus isolate proteins displaying lysine residues on the surface of the oocyte. The Western blot in Fig. 1 shows that oocytes injected with H2O have no detectable signal (lanes 1 and 2), whereas oocytes injected with cRNA encoding hUT-B express the hUT-B protein at the surface (lanes 3 and 4). We detected the immunoreactive hUT-B bands at 120–130 kDa, which presumably represents a biotinylated trimeric species (monomeric MW of hUT-B, ∼42 kDa) and is thus consistent with the biological assembly in the X-ray crystal structure of the bacterial (25) and bovine proteins (24). Studying whole tissue samples of UT-B from rat and chimpanzee, Timmer et al. (54) observed signals at 40–50 and ∼100 kDa, the relative amounts of which varied considerably among tissues (54).
Fig. 1.

Western blot of human urea transporter B (hUT-B) extracted from Xenopus oocyte plasma membranes. Left: molecular-mass (MW) markers are displayed. Middle and right: representative Western blots of detergent-solubilized, biotinylated protein extracts from oocyte lysates, isolated using NeutrAvidin Resin. Thus the bands in H2O and UT-B represent hUT-B resident in the plasma membrane. Lanes 1 and 2 represent, respectively, the equivalent of 1 and 2 H2O-injected oocytes. Lanes 3 and 4 represent, respectively, the equivalent of 1 and 2 oocytes expressing hUT-B.
Urea and Water Permeability
Our next goal was to determine the extent to which the surface expression of hUT-B increased the urea and water permeability of oocytes.
[14C]urea uptake.
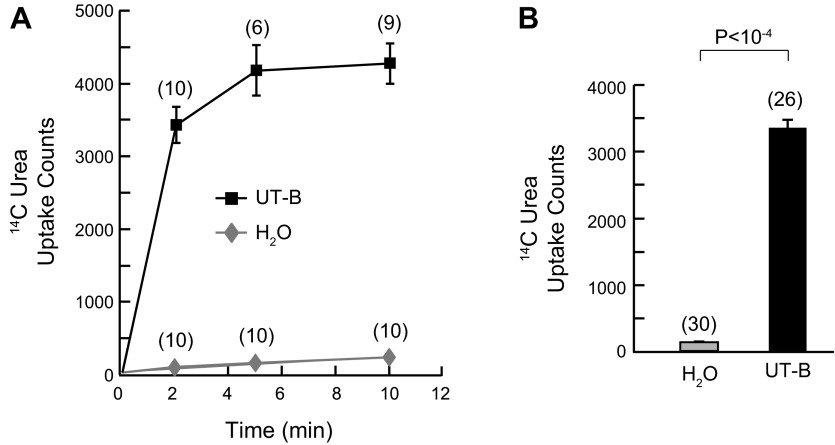
Figure 2A shows a time course, from 0 to 10 min, of [14C]urea uptake by oocytes expressing hUT-B or injected with H2O. The oocytes expressing hUT-B display rapid uptake of [14C]urea. After only 2 min the oocytes have taken up nearly 80% of the steady-state amount. The H2O-injected controls exhibit a much smaller [14C]urea uptake that rises linearly with time. At the 5-min time point, the oocytes expressing hUT-B have a [14C]urea uptake that is indistinguishable from that at 10 min. Figure 2B summarizes [14C]urea uptake data at 5 min for a larger number of experiments. These data demonstrate that oocytes expressing hUT-B have a urea uptake that is >20-fold greater than H2O-injected oocytes, consistent with the studies of other laboratories (71).
Fig. 2.
Uptake of [14C]urea by oocytes injected with H2O or cRNA encoding hUT-B. A: time course of [14C]urea uptake. Oocytes were incubated in 200 μl of ND96 containing 5 μCi of [14C]urea (1 mM total urea) and then removed after 0, 2, 5, and 10 min. Values represent individual oocytes from 1 batch of oocytes. B: uptake data at the 5-min time point. Values represent the means ± SE for individual oocytes from 3 different batches of oocytes studied on 3 different days as in A. Values represent the means and ± SE for individual oocytes. The number of oocytes for each time point are shown in parentheses. Error bars are omitted when smaller than the symbol. For statistical analysis, we performed a Student's t-test (two tailed).
Urea traverses the plasma membrane mainly via monomeric urea channels-formed by the six transmembrane α-helices of the UT protein (24, 25). It is well known that UT-mediated urea transport is inhibited by phloretin (47, 71) and pCMBS (71). Phloretin has been used extensively as an inhibitor of urea and glucose transport (30). pCMBS is an organic mercurial compound that covalently modifies the sulfhydryl group of cysteine residues and inhibits a wide range of transporter proteins. Figure 3A summarizes the effect of the inhibitors on [14C]urea uptake by oocytes either injected with H2O or with cRNA encoding hUT-B. In Fig. 3B, we summarize the component of [14C]urea uptake that depends on the presence of hUT-B, obtained by subtracting day-matched flux data of H2O-injected oocytes from data of oocytes expressing hUT-B. On average, phloretin inhibits hUT-B-dependent [14C]urea uptake by 45% and pCMBS by 25%.
Fig. 3.
Effect of inhibitors on [14C]urea uptake by oocytes injected with H2O or cRNA encoding hUT-B. A: raw uptake data at the 5-min time point (see Fig. 2A). B: portion of uptake dependent on hUT-B. From the uptake of each hUT-B oocyte, we subtracted the mean uptake of day-matched H2O controls. Data are means ± SE, with number of observations in parentheses. We performed a one-way ANOVA, followed by a Student-Newman-Keuls (SNK) analysis (P shown for individual comparisons are show). All data were obtained on the same day, from the same batch of oocytes, exposed to urea and the mean H2O data for 1 day was subtracted from everything else. Cont, control (H2O-injected) oocytes; Phlor, exposed to phloretin; pCMBS, p-chloromercuribenzene sulfonate.
Osmotic water permeability.
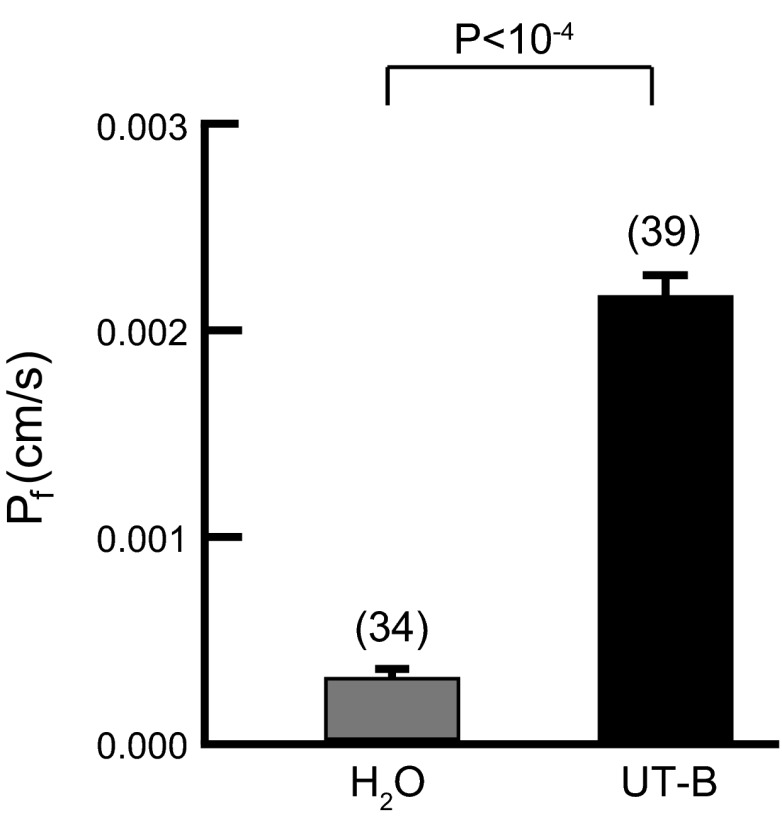
There is some debate as to whether UT-B is capable of conducting water. While increasing extracellular osmolality, we used video microscopy to monitor the rate of oocyte swelling and thereby compute Pf. The results summarized in Fig. 4 confirm that hUT-B significantly increases the Pf of oocytes, raising the rate of swelling by ∼10-fold over the value of H2O-injected oocytes. Figure 5A summarizes the effects of inhibitors on Pf for control oocytes and those expressing hUT-B and shows that preincubation of oocytes with phloretin or pCMBS has no significant effect on H2O-injected oocytes but significantly reduces the Pf of oocytes expressing hUT-B. Figure 5B summarizes the inhibitor data in terms of the component of Pf that requires hUT-B. This analysis shows that phloretin reduces UT-B-mediated H2O permeability by ∼50% and pCMBS by ∼30%.
Fig. 4.
Osmotic water permeability (Pf) measurements from oocytes injected with H2O or cRNA encoding hUT-B. Here we summarize data from 4 batches of oocytes, examined on 4 different days. Each day, we studied similar numbers of day-matched H2O and hUT-B oocytes that we exposed to 100 mosmol/kgH2O ND96 as we observed swelling. Values represent the means ± SE; the number of oocytes are in parentheses. We performed Student's t-test (two tailed) for statistical comparison.
Fig. 5.

Effect of inhibitors on the Pf of oocytes injected with H2O or cRNA encoding hUT-B. A: we preincubated oocytes injected with H2O or expressing hUT-B in a ND96 sham solution (Cont) or in ND96 containing 0.5 mM phloretin (Phlor) for 20 min or 1.0 mM pCMBS for 10 min. After washing the oocytes, we measured Pf as described in Fig. 4. B: bar graphs are corrected for the Pf of H2O-injected oocytes and thus represents the UT-B dependent Pf. Each day we determined a mean Pf for H2O-injected oocytes and subtracted this background value from the Pf of each UT-B oocyte studied on that day. We performed a one-way ANOVA, followed by a SNK analysis (P shown for individual comparisons).
CO2 and NH3 Permeability
We used microelectrodes positioned at the surface of the oocytes to monitor surface pH changes as we exposed oocytes to a solution containing either 5% CO2/33 mM HCO3− or 0.5 mM NH3/NH4+. If a protein increases the influx of CO2, then pHS will transiently alkalinize (8, 34–36). The maximum height of the pHS spike (ΔpHS) is a semiquantitative index of the CO2 influx (49). Conversely, if a protein increases the influx of NH3, then pHS will transiently acidify. Previously, this laboratory has demonstrated that AQP1 as well as RhAG and AmtB conduct both CO2 and NH3 (35). Here we find that, as we introduce CO2/HCO3−, the (ΔpHS)CO2 is no larger in oocytes expressing hUT-B than in those injected with H2O (Fig. 6A, left, and Fig. 6B). In contrast, exposing oocytes expressing hUT-B to 0.5 mM NH3/NH4+ causes a negatively shifting (ΔpHS)NH3, the magnitude of which is nearly twice as great as for oocytes injected with H2O (Fig. 6A, right, and 6C). Thus hUT-B conducts NH3 but not CO2.
Fig. 6.
Surface pH (pHS) measurements in oocytes exposed to CO2/HCO3− or NH3/NH4+. A, bottom: describe the basis for the observed changes in surface pH (pHS) changes upon exposure to solutions containing 5% CO2/33 mM/HCO3− or 0.5 mM NH3/NH4+. In each experiment, we exposed the oocyte to CO2/HCO3− for 5–8 min (enough time for pHS to spike upwards and then decay to a stable value), then washed out the CO2/HCO3− for 10–15 min (enough time for pHS to spike downwards and then decay to a stable values), and then finally exposed the oocyte to NH3/NH4+. Traces in A, top, are representative pHS transients from oocytes injected with H2O or expressing hUT-B. B: bars summarize the results of a larger number of experiments, like those shown in A. We performed Student's t-test (two tailed) for statistical comparison.
To elucidate the molecular mechanism of NH3 transport through hUT-B, we determined (ΔpHS)NH3 in the presence of the same inhibitors that we used in the [14C]urea and Pf assays. Again, we preincubated oocytes in either phloretin or pCMBS, washed, and then monitored pHS during an NH3/NH4+ exposure. As summarized in Fig. 7A, neither phloretin nor pCMBS significantly affect (ΔpHS)NH3 in H2O-injected oocytes. However, in oocytes expressing hUT-B, each drug substantially reduces (ΔpHS)NH3. As summarized in Fig. 7B, phloretin reduces the UT-B-dependent component of (ΔpHS)NH3 by 70%, and pCMBS reduces this component by virtually 100%. These fractional inhibitions are larger than in either the [14C]urea or Pf assays.
Fig. 7.

Effect of inhibitors on ΔpHS in oocytes injected with H2O or cRNA encoding hUT-B. A: we preincubated oocytes injected with H2O or expressing UT-B in a ND96 sham solution (Cont) or in ND96 containing 0.5 mM phloretin (Phlor) for 20 min or 1.0 mM pCMBS for 10 min. After washing the oocytes, we assayed ΔpHS during NH3/NH4+ exposures, as described in Fig. 6. B: bar graphs are corrected for the ΔpHS of H2O-injected oocytes, and thus represents the UT-B-dependent maximal ΔpHS. Each day we determined a mean ΔpHS for H2O-injected oocytes and subtracted this background value from the ΔpHS of each UT-B oocyte studied on that day. We performed a one-way ANOVA, followed by a SNK analysis (P shown for individual comparisons).
Structural Basis and Energetics of NH3 and H2O Conduction Through UT-B
To investigate the involvement of the monomeric pores in the conduction of H2O and NH3 molecules as indicated by our physiological experiments and to characterize the pathway, mechanism, and energetics associated with such conduction events, we performed MD simulations on a membrane-embedded model of bUT-B, for which a high-resolution structure has been solved (24). Here we report free-energy (PMF) profiles associated with permeation of H2O and NH3 through the monomeric pores (Fig. 8). By way of comparison, we also refer to a previous study in which we calculated the PMF associated with the permeation of urea through the monomeric pores of bUT-B trimer using US calculations (24). That study identified two major barrier regions against urea permeation around the Sm site (located between T172 and T334 of bUT-B, corresponding to T177 and T339 of hUT-B, respectively; see Fig. 8A), with heights of ∼5 kcal/mol at T172, and ∼4 kcal/mol at T334 (24), which would allow for urea transport through the pores at room temperature.
Fig. 8.
Structure of the selectivity filter and potential of mean force (PMF) profiles of NH3 and H2O permeation through the monomeric pore of bUT-B. A: key channel-lining residues around the selectivity filter forming the major substrate binding sites (Si, Sm, and So) are shown in licorice. Residue numbers refer to bUT-B. B: PMF profile for H2O calculated from unbiased simulations of wild-type bUT-B, double mutant (T172V/T334V), and single mutants (T172V and T334V). C: PMF profiles calculated using umbrella sampling simulations for permeation of NH3 through monomeric pores, as describes in materials and methods.
We used a similar approach here, based on MD simulations and US calculations to characterize the mechanism and energetics of permeation of H2O and NH3 through the monomeric pores. We calculated the PMF for H2O permeation using the distribution of H2O molecules obtained from unbiased MD simulations and calculated the PMF for NH3 permeation using US calculations. The free-energy profiles associated with the permeation of these small molecular species through the monomeric pores of bUT-B are shown in Fig. 8. In both cases, the Sm region, which constitutes the narrowest portion of the pore, seems to present the main energy barrier, although the barrier is rather small and easily surmountable at room temperature, allowing permeation of these species through the monomeric pores. Our simulations reveal that the bUT-B monomeric pores are permeable to H2O, a phenomenon that was suggested to play a role in the mechanism of urea transport (24). The PMF profile for H2O permeation exhibits a barrier of only ∼2 kcal/mol at the Sm site (Fig. 8B). The PMF profile for NH3 permeation reveals a maximum free-energy barrier of only ∼2.2 kcal/mol at the Sm site (Fig. 8C), clearly indicating that NH3 can permeate the channel very efficiently. Three local minima at the Sm site qualitatively match between the PMF profiles of H2O and NH3. The simulation predicts that the T172V/T334V double mutant results in a significant decrease in H2O permeation through the Sm site (Fig. 8B) by constricting the pore at that region (24). On the other hand, the single mutants raise the barrier for H2O permeation around the position of the mutation, still preserving some of the structure at the Sm site (Fig. 8B). Small changes of the pore size by hydrophobic residue mutations might be sufficient also for attenuating the influx of polar urea and NH3.
Structural analysis of the central (trimeric) cavity, together with our observation that no water penetrated into this region during the simulations, strongly suggest that the central cavity is an unlikely pathway for ammonia conduction. We attribute this observation to the hydrophobicity and physical occlusion of this cavity to both sides of the membrane.
Effect of Mutations in the NH3 Pore
Others previously showed that, in bovine UT-B, the mutation T334V, in the selectivity filter of the monomeric urea pore, substantially reduces the transport of [14C]urea (24). We generated the analogous mutation T339V in hUT-B and measured [14C]urea uptake, H2O permeability, and NH3 uptake. We also mutated a s conserved threonine (T177V) in the selectivity filter. Figure 9 summarizes the results. Figure 9A, like Fig. 2B, demonstrates that hUT-B expression greatly enhances the uptake of [14C]urea into the oocytes. Although the T177V mutation has no effect on urea uptake, T339V reduces urea uptake to a level only slightly greater than in H2O-injected oocytes. Both T177V and T339V substantially reduce channel-dependent Pf (Fig. 9B), T339V taking to virtually zero. Similarly, both T177V and T339V markedly reduce channel-dependent (ΔpHS)NH3 (Fig. 9C), T339V again being the more effective. These data show that both threonine residues are crucial for the movement of H2O and NH3 through UT-B. However, T177 appears not to be critical for urea. It is likely that the T339V reduces the diameter of the monomeric urea channel such that it blocks the passage of urea, H2O, and NH3.
Fig. 9.

Effect of point mutants T177V and T339V on [14C]urea, H2O, and NH3 influx. A: UT-B-dependent [14C]urea uptake data for oocytes injected with H2O or expressing hUT-B, T177V, or T339V at the 5-min time point (similar to Fig. 2B). B: UT-B-dependent Pf values, as described in materials and methods, we exposed oocytes to 100 mosmol/kgH2O ND96 as we observed swelling. C: UT-B-dependent changes in surface pH (pHS) elicited by exposing oocytes to 0.5 mM NH3/NH4+. For each oocyte expressing hUT-B, T177V, or T339V, we obtained the raw parameter value {[14C]urea uptake, Pf, or (ΔpHS)NH3} and from that subtracted the corresponding parameter value of day-matched H2O-injected controls; the result is the UT-B-dependent parameter value. In A–C, the values represent means ± SE for individual oocytes, from 3 different batches of oocytes studied on 3 different days. Number of oocytes is shown in parentheses. We performed a one-way ANOVA, followed by a SNK analysis (P shown for individual comparisons).
DISCUSSION
The two major observations in the present study are 1) the confirmation of the conclusion of Yang and Verkman (71, 72) that hUT-B conducts H2O; and 2) the novel insight that hUT-B also conducts NH3.
The classical view had been that all transport of gases across biological membranes occurs as the gas simply dissolves in the lipid phase of the cell membrane and then moves by simple diffusion through the lipid. This idea has been challenged since the discovery of the first gas-impermeable membrane (62) and the observation that AQP1 conducts CO2 (5, 37), making AQP1 the first identified gas channel. Subsequent experimental work also showed that AQP1 can conduct NH3 (38) and NO (16), and simulation studies provided a structural basis and putative protein pathways for conduction of these gas species through aquaporins (65–67).
The second family of gas channels is the Rhesus proteins, which conduct NH3 (20, 44, 45). In human RBCs, RhAG and the rest of the Rh complex are responsible for about half the CO2 permeability, with AQP1 being responsible for the other half (7). Thus both AQP1 and RhAG conduct both CO2 and NH3. Perhaps even more interesting is the study by Musa-Aziz et al. (35), which demonstrated for the first time that, like ion channels, gas channels (i.e., AQPs and Rh proteins) exhibit selectivity for one gas over another (i.e., NH3 vs. CO2).
The demonstration in the present study that hUT-B conducts NH3 makes the UTs the third family of gas channels, along with the AQPs and Rhs. Interestingly, Endeward et al. (7) demonstrated that JKnull human RBCs, which lack UT-B, show no deficiency in CO2 transport-consistent with our conclusion that hUT-B lacks appreciable CO2 conductance (Fig. 6B). The ability of hUT-B to conduct H2O and NH3 but not CO2 is reminiscent of three mammalian AQP: 3, 7, and 8 (11). Other AQPs can conduct H2O and CO2 but not NH3 (AQP0, AQP4-M23, and AQP5); H2O but not either CO2 or NH3 (AQP2 and AQP4-M1); CO2 and NH3 but not H2O (AQP6); or H2O, CO2, and NH3 (AQP1 and AQP9; Ref. 11). Thus it is possible that other UTs exist with permeability profiles distinct from that of hUT-B.
Insights from UT Structures
The three-dimensional structure of bacterial UT homolog dvUT, as well as the mammalian bUT-B, have been solved using X-ray crystallography (24, 25). Both proteins are homotrimers. Moreover, purified hUT-B is thought to be a trimer as well, based on size-exclusion chromatography (24).
At the threefold axis of symmetry of bUT-B is a large, hydrophobic central cavity, reminiscent of the central pores of AQPs (14, 17, 18, 63, 64) and rhesus proteins (13, 20, 21, 29). However, it appears that this central cavity is poorly accessible to the extra- and intracellular aqueous phases (24). We used the program MOLE (41) to determine if bUT-B has a continuous pore that could serve as a conduit for gas transport. The program detects the three monomeric urea channels of bUT-B. It also detects the large central cavity and a narrow tunnel (∼1.5 Å in diameter) that connects the central cavity to the extracellular fluid. However, MOLE does not detect a tunnel between the central cavity and the intracellular fluid. For dvUT (25), MOLE does not even detect either a central cavity or any tunnels. The occlusion of this central cavity is also evident from our simulations in which no transmembrane water permeation through this cavity was observed. We cannot rule out the possibility that breathing of the UT-B molecule might provide transient tunnels, broad enough to allow permeation by a gas. Nevertheless, if such transient pathways exist, they do not conduct appreciable amounts of CO2 under the conditions of our experiments.
Each monomer contains 10 transmembrane helices, which assemble to form the urea channel. In the middle of each monomeric urea channel is a selectivity filter, with three regions: Si, Sm, and, So. This filter is postulated to exclude charged species, such as, e.g., H+ and NH4+, but to allow the passage of urea and H2O, as demonstrated by previous studies (24, 71), and confirmed by the present study.
Inhibitor Sensitivity of Urea, H2O, and NH3 Transport Through hUT-B
Figure 3B shows that we can block ∼45% of the urea transport through hUT-B with phloretin and ∼25% with pCMBS. Similarly, Fig. 5B shows that we can block ∼50% of the H2O conductance with phloretin and 30% with pCMBS. The similar inhibitor profiles in Fig. 3B and Fig. 5B are consistent with the hypothesis that urea and H2O both move through the monomeric pores of hUT-B. Given the chemical similarities between H2O and NH3, one might predict that NH3 also would move through the monomeric pores. However, it is interesting that the inhibition of NH3 transport by phloretin (∼70%) and by pCMBS (∼100%) was substantially greater than of either urea or H2O transport. One explanation is that our readout of urea transport is saturated, which may lead to an underestimation of the inhibition. However, this line of reasoning does not account for the differences in inhibitor profile between NH3 and H2O.
Another explanation is that phloretin and pCMBS, at least in part, reduce NH3 transport through hUT-B by a mechanism unique for NH3. The molecular mechanism for NH3 transport through rhesus proteins is thought to involve the recruitment and deprotonation of NH4+ (to yield H+ and NH3), just above the entrance to the pore (20, 21). After NH3 passes through the pore, the NH3 is reprotonated, and NH4+ diffuses away on the opposite side. In the case of RhCG, Gruswitz et al. (13) have postulated that the recruitment and deprotonation require acidic amino-acid residues on the extracellular side (E166) and intracellular side (D218, D278, and E329) of the pore. Sequence alignments of bUT-B and hUT-B reveal three conserved aspartate residues (human D138, D216, and D237) and one conserved glutamate residue (E279) near the extracellular mouth of the monomeric ammonia pore. Similarly, we identify five conserved aspartate residues (human D41, D51, D60, D113, and D172) and three conserved glutamate residues (E44, E371, and E372) near the intracellular mouth of the pore. Thus it is possible that phloretin and pCMBS, in addition to any direct blockade of the monomeric pore, interfere with the chemistry of NH4+ recruitment and deprotonation, accounting for the greater inhibition of NH3 transport vs. urea or H2O transport.
Mutants and Permeation Pathway Energetics
Our inhibitor profiles (Figs. 3, 5, and 7) and MD simulations (Fig. 9) suggest that urea, H2O, and NH3 all traverse the membrane utilizing the same pathway, presumably the monomeric urea pores. To characterize the pathway of permeation further, we mutated two conserved threonine residues located in the urea pore (T177V and T339V). In bUT-B, the mutation analogous to T339V in hUT-B results in a loss of urea transport activity (24). Indeed, in hUT-B, we obtain the same result with not only urea transport but also H2O and NH3 transport. On the other hand, we find that the T177V mutation in hUT-B (analogous to T172V in bUT-B) has no effect on urea influx but substantially attenuates H2O and NH3 transport. Understanding why the T177V mutation has no effect on urea permeability will require further investigation.
Physiological Significance
The average RBC contains ∼14,000 copies of UT-B (28), a major function of which is to allow the intracellular fluid of the RBC to participate in the carriage of urea from the site of urea synthesis (the liver) to the site of urea excretion (the kidney).
In mice, UT-B has about the same single-channel H2O permeability as AQP1 (72). However, due to its low abundance, compared with that of AQP1 (∼200,000 copies per cell; Ref. 28), UT-B contributes only ∼8% of the overall H2O permeability of the RBC.
As a gas channel, UT-B likely plays a role, along with AQP1 and the RhAG, in allowing the intracellular fluid of the RBC to contribute to the carriage of NH3 from the sites of its synthesis (most cells in the body) to the sites of its detoxification (the liver). To our knowledge, the effect of the knockout of UT-B on plasma NH3 levels has yet to be tested. We would not be surprised to see a small elevation in plasma NH3 concentration for two reasons. First, the build-up of urea in the plasma could reduce the hepatic consumption of NH3 in the production of urea. Second, the effective reduction in the volume of distribution for NH3 in blood, to the extent that UT-B contributes NH3 carriage by RBCs, could lead to higher plasma NH3 levels. However, plasma NH3 levels clearly do not rise to those necessary to produce hepatic encephalopathy.
In addition to its potential role in NH3 carriage by RBCs, UT-B in the RBC membrane would reduce the reflection coefficient for urea and (along with AQP1 and RhAG) would reduce the reflection coefficient for NH3. In the renal medulla, these effects would reduce the effective osmolality contributed by urea and NH3, and thereby reduce cell-volume changes as RBCs move into and out of the renal medulla.
Hepatocytes express UT-A protein (23), at least UT-B mRNA (54), AQP9 (58, 59), RhCG, and RhBG (68). AQP9 and UT proteins could contribute to hepatic urea efflux, and all five proteins, which have family members capable of NH3 transport, could in principle contribute to hepatic NH3 uptake. Thus it will be important to characterize the NH3 permeability of these proteins.
Conclusion
In the present study, we have examined four transport modes of hUT-B, as heterologously expressed in Xenopus oocytes. Besides confirming that hUT-B functions as a phloretin- and pCMBS-sensitive urea channel, we provide evidence supporting the earlier contested conclusion that hUT-B functions as a phloretin- and pCMBS-sensitive H2O channel. In addition, we rule out hUT-B as a significant CO2 channel but show for the first time that hUT-B is a phloretin- and pCMBS-sensitive NH3 channel, the first evidence that a UT can function as a gas channel. The most straightforward interpretation of the inhibitor profiles, mutational studies, and MD is that urea, H2O, and NH3 all pass through the same monomeric pores of hUT-B.
GRANTS
R. R. Geyer was supported by Postdoctoral Fellowship N00014-09-1-0246 from the Office of Naval Research. R. Musa-Aziz was supported by Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP No. 08/128663). This work was supported by Office of Naval Research Grant N00014-11-1-0889 and NIH grant DK81567 (to W. F. Boron) and National Institute of General Medical Sciences Grants R01-GM-086749, R01-GM-101048, U54-GM-087519, and P41-GM-104601 (to E. Tajkhorshid). All simulations have been performed at XSEDE resources (Grant No. MCA06N060).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.R.G., R.M.-A., and E.T. conception and design of research; R.R.G., R.M.-A., G.E., and P.M. performed experiments; R.R.G., R.M.-A., G.E., P.M., and E.T. analyzed data; R.R.G., R.M.-A., G.E., P.M., and E.T. interpreted results of experiments; R.R.G., R.M.-A., G.E., P.M., and E.T. prepared figures; R.R.G., R.M.-A., G.E., P.M., E.T., and W.F.B. drafted manuscript; R.R.G., R.M.-A., G.E., P.M., E.T., and W.F.B. edited and revised manuscript; R.R.G., R.M.-A., G.E., P.M., E.T., and W.F.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dale Huffman for computer support and Mark Parker for assistance with the [14C]urea experiments.
REFERENCES
- 1. Berger UV, Tsukaguchi H, Hediger MA. Distribution of mRNA for the facilitated urea transporter UT3 in the rat nervous system. Anat Embryol 197: 405–414, 1998 [DOI] [PubMed] [Google Scholar]
- 2. Boron WF. The Sharpey-Schafer lecture: gas channels. Exp Physiol 95: 1107–1130, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chandy G, Zampighi GA, Kreman M, Hall JE. Comparison of the water transporting properties of MIP and AQP1. J Membr Biol 159: 29–39, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Cooper GJ, Boron WF. The CO2 permeability of the AQP1 water channel, expressed in Xenopus oocytes. J Am Soc Nephrol 8: 16A, 1997 [Google Scholar]
- 5. Cooper GJ, Boron WF. Effect of PCMBS on CO2 permeability of Xenopus oocytes expressing aquaporin 1 or its C189S mutant. Am J Physiol Cell Physiol 275: C1481–C1486, 1998 [DOI] [PubMed] [Google Scholar]
- 6. Darden T, York D, Pedersen L. Particle mesh Ewald–an N.log(N) method for Ewald sums in large systems. J Chem Phys 98: 10089–10092, 1993 [Google Scholar]
- 7. Endeward V, Cartron JP, Ripoche P, Gros G. RhAG protein of the Rhesus complex is a CO2 channel in the human red cell membrane. FASEB J 22: 64–73, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Endeward V, Musa-Aziz R, Cooper GJ, Chen L, Pelletier MF, Virkki LV, Supuran CT, King LS, Boron WF, Gros G. Evidence that quaporin 1 is a major pathway for CO2 transport across the human erythrocyte membrane. FASEB J 20: 1974–1981, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Feller SE, Zhang YH, Pastor RW, Brooks BR. Constant-pressure molecular-dynamics simulation–the Langevin Piston method. J Chem Phys 103: 4613–4621, 1995 [Google Scholar]
- 10. Forster RE, Gros G, Lin L, Ono Y, Wunder M. The effect of 4,4′-diisothiocyanato-stilbene-2,2′-disulfonate on CO2 permeability of the red blood cell membrane. Proc Natl Acad Sci USA 95: 15815–15820, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Giebisch G, Windhager EE. Urine concentration and dilution. In Medical Physiology. A Cellular and Molecular Approach, edited by Boron WF, Boulpaep EL. Philadelphia, PA: Elsevier Saunders, 2009, p. 835–850 [Google Scholar]
- 13. Gruswitz F, Chaudhary S, Ho JD, Schlessinger A, Pezeshki B, Ho CM, Sali A, Westhoff CM, Stroud RM. Function of human Rh based on structure of RhCG at 2.1 A. Proc Natl Acad Sci USA 107: 9638–9643, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harries WE, Akhavan D, Miercke LJ, Khademi S, Stroud RM. The channel architecture of aquaporin 0 at a 2.2-Å resolution. Proc Natl Acad Sci USA 101: 14045–14050, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Herrera M, Garvin JL. Novel role of AQP-1 in NO-dependent vasorelaxation. Am J Physiol Renal Physiol 292: F1443–F1451, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Herrera M, Hong NJ, Garvin JL. Aquaporin-1 transports NO across cell membranes. Hypertension 48: 157–164, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Ho JD, Yeh R, Sandstrom A, Chorny I, Harries WE, Robbins RA, Miercke LJ, Stroud RM. Crystal structure of human aquaporin 4 at 1.8 A and its mechanism of conductance. Proc Natl Acad Sci USA 106: 7437–7442, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Horsefield R, Norden K, Fellert M, Backmark A, Tornroth-Horsefield S, Terwisscha van Scheltinga AC, Kvassman J, Kjellbom P, Johanson U, Neutze R. High-resolution x-ray structure of human aquaporin 5. Proc Natl Acad Sci USA 105: 13327–13332, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML. Comparison of simple potential functions for simulating liquid water. J Chem Phys 79: 926–935, 1983 [Google Scholar]
- 20. Khademi S, O'Connell J, Remis J, Robles-Colmenares Y, Miericke LJW, Stroud RM. Mechanism of ammonia transport by Amt/MEP/Rh: structure of AmtB at 1.35 angstrom. Science 305: 1587–1594, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Khademi S, Stroud RM. The Amt/MEP/Rh family: structure of AmtB and the mechanism of ammonia gas conduction. Physiology (Bethesda) 21: 419–429, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Klauda JB, Venable RM, Freites JA, O'Connor JW, Tobias DJ, Mondragon-Ramirez C, Vorobyov I, MacKerell AD, Pastor RW. Update of the CHARMM all-atom additive force field for lipids: validation on six lipid types. J Phys Chem B 114: 7830–7843, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Klein JD, Timmer RT, Rouillard P, Bailey JL, Sands JM. UT-A urea transporter protein expressed in liver: upregulation by uremia. J Am Soc Nephrol 10: 2076–2083, 1999 [DOI] [PubMed] [Google Scholar]
- 24. Levin EJ, Cao Y, Enkavi G, Quick M, Pan Y, Tajkhorshid E, Zhou M. Structure and permeation mechanism of a mammalian urea transporter. Proc Natl Acad Sci USA 109: 11194–11199, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Levin EJ, Quick M, Zhou M. Crystal structure of a bacterial homologue of the kidney urea transporter. Nature 462: 757–761, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li X, Chen G, Yang B. Urea transporter physiology studied in knockout mice. Front Physiol 3: 217, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lucien N, Bruneval P, Lasbennes F, Belair MF, Mandet C, Cartron JP, Bailly P, Trinh-Trang-Tan MM. UT-B1 urea transporter is expressed along the urinary and gastrointestinal tracts of the mouse. Am J Physiol Regul Integr Comp Physiol 288: R1046–R1056, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Lucien N, Sidoux-Walter F, Roudier N, Ripoche P, Huet M, Trinh-Trang-Tan MM, Cartron JP, Bailly P. Antigenic and functional properties of the human red blood cell urea transporter hUT-B1. J Biol Chem 277: 34101–34108, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Lupo D, Li XD, Durand A, Tomizaki T, Cherif-Zahar B, Matassi G, Merrick M, Winkler FK. The 1.3-A Resolution structure of Nitrosomonas europaea Rh50 and mechanistic implications for NH3 transport by Rhesus family proteins. Proc Natl Acad Sci USA 104: 19303–19308, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Macey RI, Farmer RE. Inhibition of water and solute permeability in human red cells. Biochim Biophys Acta 211: 104–106, 1970 [DOI] [PubMed] [Google Scholar]
- 31. MacKerell AD, Bashford D, Bellott M, Dunbrack RL, Evanseck JD, Field MJ, Fischer S, Gao J, Guo H, Ha S, Joseph-McCarthy D, Kuchnir L, Kuczera K, Lau FT, Mattos C, Michnick S, Ngo T, Nguyen DT, Prodhom B, Reiher WE, Roux B, Schlenkrich M, Smith JC, Stote R, Straub J, Watanabe M, Wiorkiewicz-Kuczera J, Yin D, Karplus M. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J Phys Chem B 102: 3586–3616, 1998 [DOI] [PubMed] [Google Scholar]
- 32. MacKerell AD, Feig M, Brooks CL. Extending the treatment of backbone energetics in protein force fields: limitations of gas-phase quantum mechanics in reproducing protein conformational distributions in molecular dynamics simulations. J Comput Chem 25: 1400–1415, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Marini AM, Matassi G, Raynal V, André B, Cartron JP, Chérif-Zahar B. The human Rhesus-associated RhAG protein and a kidney homologue promote ammonium transport in yeast. Nat Genet 26: 341–344, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Musa-Aziz R, Boron WF, Parker MD. Using fluorometry and ion-sensitive microelectrodes to study the functional expression of heterologously-expressed ion channels and transporters in Xenopus oocytes. Methods 51: 134–145, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Musa-Aziz R, Chen L, Pelletier MF, Boron WF. Relative CO2/NH3 selectivities of AQP1, AQP4, AQP5, AmtB, and RhAG. Proc Natl Acad Sci USA 106: 5406–5411, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Musa-Aziz R, Jiang L, Chen LM, Behar KL, Boron WF. Concentration-dependent effects on intracellular and surface pH of exposing Xenopus oocytes to solutions containing NH3/NH4+. J Membr Biol 228: 15–31, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nakhoul NL, Davis BA, Romero MF, Boron WF. Effect of expressing the water channel aquaporin-1 on the CO2 permeability of Xenopus oocytes. Am J Physiol Cell Physiol 274: C543–C548, 1998 [DOI] [PubMed] [Google Scholar]
- 38. Nakhoul NL, Hering-Smith KS, Abdulnour-Nakhoul SM, Hamm LL. Transport of NH3/NH4+ in oocytes expressing aquaporin-1. Am J Physiol Renal Physiol 281: F255–F263, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Olives B, Neau P, Bailly P, Hediger MA, Rousselet G, Cartron JP, Ripoche P. Cloning and functional expression of a urea transporter from human bone marrow cells. J Biol Chem 269: 31649–31652, 1994 [PubMed] [Google Scholar]
- 40. Pallone TL. Characterization of the urea transporter in outer medullary descending vasa recta. Am J Physiol Regul Integr Comp Physiol 267: R260–R267, 1994 [DOI] [PubMed] [Google Scholar]
- 41. Petrek M, Kosinova P, Koca J, Otyepka M. MOLE: a Voronoi diagram-based explorer of molecular channels, pores, and tunnels. Structure 15: 1357–1363, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kale L, Schulten K. Scalable molecular dynamics with NAMD. J Comput Chem 26: 1781–1802, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Preston GM, Jung JS, Guggino WB, Agre P. The mercury-sensitive residue at cysteine 189 in the CHIP28 water channel. J Biol Chem 268: 17–20, 1993 [PubMed] [Google Scholar]
- 44. Ripoche P, Bertrand O, Gane P, Birkenmeier C, Colin Y, Cartron JP. Human Rhesus-associated glycoprotein mediates facilitated transport of NH3 into red blood cells. Proc Natl Acad Sci USA 101: 17222–17227, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ripoche P, Goossens D, Devuyst O, Gane P, Colin Y, Verkman AS, Cartron JP. Role of RhAG and AQP1 in NH3 and CO2 gas transport in red cell ghosts: a stopped-flow analysis. Transfus Clin Biol 13: 117–122, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Romero MF, Fong P, Berger UV, Hediger MA, Boron WF. Cloning and functional expression of rNBC, an electrogenic Na+-HCO3− cotransporter from rat kidney. Am J Physiol Renal Physiol 274: F425–F432, 1998 [DOI] [PubMed] [Google Scholar]
- 47. Sidoux-Walter F, Lucien N, Olives B, Gobin R, Rousselet G, Kamsteeg EJ, Ripoche P, Deen PM, Cartron JP, Bailly P. At physiological expression levels the Kidd blood group/urea transporter protein is not a water channel. J Biol Chem 274: 30228–30235, 1999 [DOI] [PubMed] [Google Scholar]
- 48. Smith CP. Mammalian urea transporters. Exp Physiol 94: 180–185, 2009 [DOI] [PubMed] [Google Scholar]
- 49. Somersalo E, Occhipinti R, Boron WF, Calvetti D. A reaction-diffusion model of CO2 influx into an oocyte. J Theor Biol 309: 185–203, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Souaille M, Roux B. Extension to the weighted histogram analysis method: combining umbrella sampling with free energy calculations. Computer Phys Commun 135: 40–57, 2001 [Google Scholar]
- 51. Stewart GS, Fenton RA, Wang W, Kwon TH, White SJ, Collins VM, Cooper G, Nielsen S, Smith CP. The basolateral expression of mUT-A3 in the mouse kidney. Am J Physiol Renal Physiol 286: F979–F987, 2004 [DOI] [PubMed] [Google Scholar]
- 52. Sui H, Han BG, Lee JK, Walian P, Jap BK. Structural basis of water-specific transport through the AQP1 water channel. Nature 414: 872–878, 2001 [DOI] [PubMed] [Google Scholar]
- 53. Tajkhorshid E, Nollert P, Jensen MO, Miercke LJ, O'Connell J, Stroud RM, Schulten K. Control of the selectivity of the aquaporin water channel family by global orientational tuning. Science 296: 525–530, 2002 [DOI] [PubMed] [Google Scholar]
- 54. Timmer RT, Klein JD, Bagnasco SM, Doran JJ, Verlander JW, Gunn RB, Sands JM. Localization of the urea transporter UT-B protein in human and rat erythrocytes and tissues. Am J Physiol Cell Physiol 281: C1318–C1325, 2001 [DOI] [PubMed] [Google Scholar]
- 55. Tornroth-Horsefield S, Wang Y, Hedfalk K, Johanson U, Karlsson M, Tajkhorshid E, Neutze R, Kjellbom P. Structural mechanism of plant aquaporin gating. Nature 439: 688–694, 2006 [DOI] [PubMed] [Google Scholar]
- 56. Toye AM, Parker MD, Daly CM, Lu J, Virkki LV, Pelletier MF, Boron WF. The human NBCe1-A mutant R881C, associated with proximal renal tubular acidosis, retains function but is mistargeted in polarized renal epithelia. Am J Physiol Cell Physiol 291: C788–C801, 2006 [DOI] [PubMed] [Google Scholar]
- 57. Trinh-Trang-Tan MM, Lasbennes F, Gane P, Roudier N, Ripoche P, Cartron JP, Bailly P. UT-B1 proteins in rat: tissue distribution and regulation by antidiuretic hormone in kidney. Am J Physiol Renal Physiol 283: F912–F922, 2002 [DOI] [PubMed] [Google Scholar]
- 58. Tsukaguchi H, Shayakul C, Berger UV, Mackenzie B, Devidas S, Guggino WB, Van Hoek AN, Hediger MA. Molecular characterization of a broad selectivity neutral solute channel. J Biol Chem 273: 24737–24743, 1998 [DOI] [PubMed] [Google Scholar]
- 59. Tsukaguchi H, Weremowicz S, Morton CC, Hediger MA. Functional and molecular characterization of the human neutral solute channel aquaporin-9. Am J Physiol Renal Physiol 277: F685–F696, 1999 [DOI] [PubMed] [Google Scholar]
- 60. Virkki LV, Franke C, Somieski P, Boron WF. Cloning and functional characterization of a novel aquaporin from Xenopus laevis oocytes. J Biol Chem 277: 40610–40616, 2002 [DOI] [PubMed] [Google Scholar]
- 61. Wade JB, Lee AJ, Liu J, Ecelbarger CA, Mitchell C, Bradford AD, Terris J, Kim GH, Knepper MA. UT-A2: a 55-kDa urea transporter in thin descending limb whose abundance is regulated by vasopressin. Am J Physiol Renal Physiol 278: F52–F62, 2000 [DOI] [PubMed] [Google Scholar]
- 62. Waisbren SJ, Geibel JP, Modlin IM, Boron WF. Unusual permeability properties of gastric gland cells. Nature 368: 332–335, 1994 [DOI] [PubMed] [Google Scholar]
- 63. Walz T, Hirai T, Murata K, Heymann JB, Mitsuoka K, Fujiyoshi Y, Smith BL, Agre P, Engel A. The three-dimensional structure of aquaporin-1. Nature 387: 624–627, 1997 [DOI] [PubMed] [Google Scholar]
- 64. Walz T, Smith BL, Agre P, Engel A. The three-dimensional structure of human erythrocyte aquaporin CHIP. EMBO J 13: 2985–2993, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wang Y, Cohen J, Boron WF, Schulten K, Tajkhorshid E. Exploring gas permeability of cellular membranes and membrane channels with molecular dynamics. J Struct Biol 157: 534–544, 2007 [DOI] [PubMed] [Google Scholar]
- 66. Wang Y, Ohkubo YZ, Tajkhorshid E. Gas conduction of lipid bilayers and membrane channels. Comput Model Membrane Bilayers 60: 343–367, 2008 [Google Scholar]
- 67. Wang Y, Tajkhorshid E. Nitric oxide conduction by the brain aquaporin AQP4. Proteins 78: 661–670, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Weiner ID, Miller RT, Verlander JW. Localization of the ammonium transporters, RhB glycoprotein and RhC glycoprotein, in the mouse liver. Gastroenterology 124: 1432–1440, 2003 [DOI] [PubMed] [Google Scholar]
- 69. Xu Y, Olives B, Bailly P, Fischer E, Ripoche P, Ronco P, Cartron JP, Rondeau E. Endothelial cells of the kidney vasa recta express the urea transporter HUT11. Kidney Int 51: 138–146, 1997 [DOI] [PubMed] [Google Scholar]
- 70. Yang B, Bankir L, Gillespie A, Epstein CJ, Verkman AS. Urea-selective concentrating defect in transgenic mice lacking urea transporter UT-B. J Biol Chem 277: 10633–10637, 2002 [DOI] [PubMed] [Google Scholar]
- 71. Yang B, Verkman AS. Urea transporter UT3 functions as an efficient water channel. Direct evidence for a common water/urea pathway. J Biol Chem 273: 9369–9372, 1998 [DOI] [PubMed] [Google Scholar]
- 72. Yang B, Verkman AS. Analysis of double knockout mice lacking aquaporin-1 and urea transporter UT-B. Evidence for UT-B-facilitated water transport in erythrocytes. J Biol Chem 277: 36782–36786, 2002 [DOI] [PubMed] [Google Scholar]
- 73. Zheng L, Kostrewa D, Berneche S, Winkler FK, Li XD. The mechanism of ammonia transport based on the crystal structure of AmtB of Escherichia coli. Proc Natl Acad Sci USA 101: 17090–17095, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]