Abstract
Neoplastic transformation results in a wide variety of cellular alterations that impact the growth, survival, and general behavior of affected tissue. Although genetic alterations underpin the development of neoplastic disease, epigenetic changes can exert an equally significant effect on neoplastic transformation. Among neoplasia-associated epigenetic alterations, changes in cellular glycosylation have recently received attention as a key component of neoplastic progression. Alterations in glycosylation appear to not only directly impact cell growth and survival but also facilitate tumor-induced immunomodulation and eventual metastasis. Many of these changes may support neoplastic progression, and unique alterations in tumor-associated glycosylation may also serve as a distinct feature of cancer cells and therefore provide novel diagnostic and even therapeutic targets.
Keywords: glycoprotein, glycosylation, glycans, oligosaccharides, cancer, transformation, biomarkers, immunohistochemistry
INTRODUCTION
As neoplastic disease continues to be one of the most formidable challenges in modern medicine, accurate diagnosis and treatment of neoplasia remain a fundamental focus of biomedical research. Although early studies recognized that cells can accumulate abundant morphological changes following neoplastic transformation, it was not until the genetic basis for neoplastic disease became apparent that the molecular mechanisms of neoplastic progression began to be known (1). As a variety of mutations appear to drive the progression of neoplasia in different types of tissue, understanding the impact of DNA mutations continues to be a central goal in the study of neoplastic disease (1). Although these studies continue to provide significant insight into the genetic parameters that may govern neoplastic progression, epigenetic alterations may exert an equally powerful effect on the outcome of neoplastic disease (2). Indeed, some of the earliest studies seeking to understand potential differences in neoplastic and normal cells recognized that a variety of metabolic and other changes commonly occur within neoplastic lesions (3). Although many of these alterations likely reflect downstream consequences of genetic mutations, epigenetic changes in and of themselves may not be readily recognized as the outcome of a single genetic mutation but instead may reflect the integrated consequence of a variety of genetic and nongenetic changes that facilitate the development of neoplastic transformation.
One of the classic examples of epigenetic changes that occur during neoplastic transformation is posttranslational glycosylation (2). Glycosylation reflects the coordinated effort of a complex array of enzymes, organelles, and other factors that are needed to successfully generate carbohydrate-associated posttranslational modifications. These modifications of proteins by covalent addition of organic and inorganic moieties can occur on virtually all known proteins in mammalian cells, and they represent an orchestrated and fascinating mechanism to expand the linear information of DNA into a grand panorama of three-dimensional structures. More specifically, glycosylation represents a unique set of protein modifications that follow enzymatic additions of sugar and may involve the linkage of monosaccharides, or even whole oligosaccharides (glycans), in a preformed fashion to specific amino acids within glycoproteins. At least 9 of the 20 amino acids can be modified by a variety of carbohydrates, ranging from a single monosaccharide to glycan chains containing hundreds of monosaccharides. In mammals the major glycans comprise 10 monosaccharide building blocks—glucose (Glc), galactose (Gal), N-acetylglucosamine (GlcNAc), N-acetylgalactosamine (GalNAc), fucose (Fuc), mannose (Man), xylose (Xyl), glucuronic acid (GlcA), iduronic acid (IdoA), and 5-N-acetylneuraminic acid (Neu5Ac, or sialic acid)—all derivable from glucose in every cell (Figure 1) (4); thus, glycosylation is comparable to phosphorylation in terms of the range of modified sites (5) and certainly exceeds phosphorylation in terms of the complexity of modifications. Note that glycosylation is different from glycation, the nonenzymatic modification of lysine residues on proteins with glucose or other monosaccharides—a prominent problem in diabetes (6, 7).
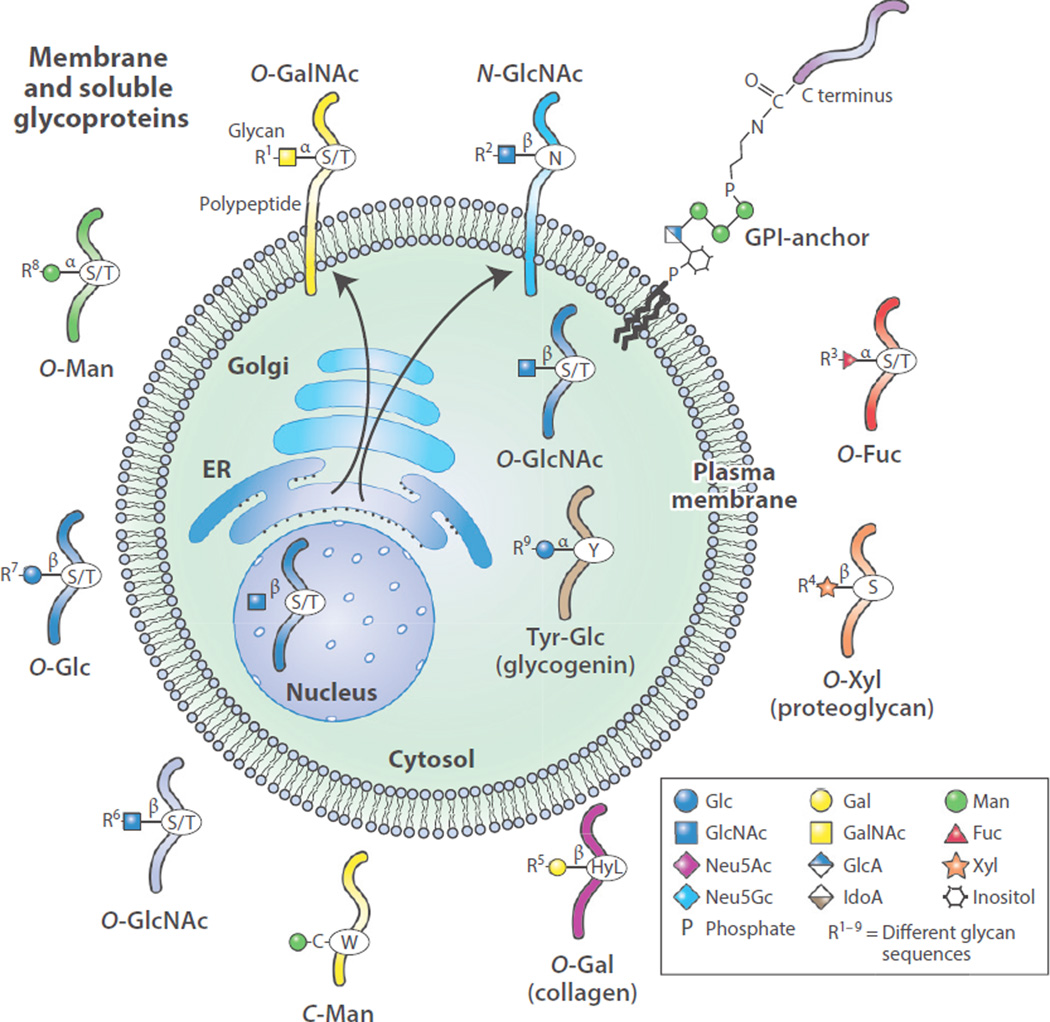
Figure 1.
Animal cells synthesize a wide assortment of glycoproteins in which different amino acids may be modified to contain specific glycan structures. Biosynthesis of such glycoproteins is initiated in the secretory pathway comprising the endoplasmic reticulum and Golgi apparatus of cells and can lead to membrane localization and secretion of glycoproteins. In addition, O-GlcNAc may be added to glycoproteins in the cytoplasm, nucleus, and mitochondria. This single GlcNAc residue is not extended but can be reversibly added and removed. The multiple other types of glycan linkages to proteins can be extended (R groups indicated) with many additional sugar molecules to form oligosaccharides or polysaccharides, all termed glycans. The 10 common monosaccharides that make up animal glycans are indicated: N-acetylglucosamine (GlcNAc), N-acetylgalactosamine (GalNAc), 5-N-acetylneuraminic acid (Neu5Ac, or sialic acid), fucose (Fuc), galactose (Gal), glucose (Glc), glucuronic acid (GlcA); iduronic acid (IdoA); mannose (Man), and xylose (Xyl). 5-N-Glycolylneuraminic acid (Neu5Gc), a non-natural sugar, is also shown. Other abbreviations: ER, endoplasmic reticulum; GPI, glycosylphosphatidylinositol; HyL, hydroxylated lysine.
Given the complexity of protein glycosylation and its fundamental impact on a diverse range of biological processes (8, 9), it is not surprising that seemingly minor alterations in carbohydrate structure can significantly impact the biology of a cell. As changes in cellular behavior and alterations in protein glycosylation accompany neoplastic transformation (10), understanding the mechanisms and consequences of variations in glycosylation associated with neoplastic disease will provide important insight into neoplastic progression (2). This review focuses on changes in glycoprotein glycans accompanying neoplastic transformation and the implications of these alterations in the progression of neoplastic disease. In addition, we examine potential opportunities to utilize these changes as diagnostic markers and novel therapeutic targets.
GENERAL ASPECTS OF PROTEIN GLYCOSYLATION
Before launching into biosynthetic mechanisms of protein glycosylation, we first briefly consider the history and major advances through the years that have brought protein glycosylation and glycosciences in general into the forefront of modern biomedical research. The key developments in this area began with the finding by Landsteiner that blood group antigens are distinguishing features of blood cells and could be used to accurately predict the outcome of a blood transfusion (11, 12), followed by the subsequent structural identification of these antigens as carbohydrates (Table 1) (13). Parallel developments in chemistry and biology led to the realization that glycans and glycoconjugates not only serve as key barriers to transfusion and transplantation but also perform fundamental roles in a wide variety of biological processes. Cell surface glycans constitute the major portion of the membrane (glycocalyx) and of secreted molecules from all cells (14). Not only do changes in the expression and structures of glycans accompany development and differentiation, but factors that regulate glycosylation and glycoconjugate metabolism also play a central role in the pathogenesis of a host of lysosomal storage diseases (Tay–Sachs, Fabry, etc.) and many other congenital disorders of glycosylation (15–17). Efforts to understand the role of glycosylation in each of these diseases also uncovered a multitude of glycan-binding proteins (GBPs) with the ability to regulate a wide variety of biological processes, ranging from leukocyte trafficking to innate immunity (18, 19). As a result, a variety of studies within glycoscience demonstrate that carbohydrate-based posttranslational modifications and the GBPs that recognize them can significantly impact fundamental biological processes (19). As neoplastic progression typically reflects alterations not only in neoplastic cell survival and growth but also in cellular migration, metastasis, and host antitumor immunity, it is not surprising that alterations in glycosylation impact a wide spectrum of key biological processes needed for the development and progression of neoplastic disease (Table 1) (2, 4, 10, 20, 21).
Table 1.
Milestones in studies on glycosylation and cancer
| Year(s) | Milestone | Reference(s) |
|---|---|---|
| General functions of cell surface glycans | ||
| 1950s–1960s | Structural definition of carbohydrate-based ABO(H) blood group antigens | 13, 227–229 |
| 1960s–1970s | Discovery that plant lectins (PHA, ConA) are mitogenic for peripheral lymphocytes and recognize specific glycopeptides | 230, 231 |
| 1964 | Finding that glycosidase treatment of cells alters cellular interactions in vivo | 232 |
| 1966–1974 | Identification of a hepatic receptor for asialoglycoproteins | 233–235 |
| Surface glycans in cancer | ||
| 1949 | Demonstration of decreased expression of human blood group A antigen in gastric cancer | 236 |
| 1962 | Finding that ascites tumor cells express unusual sialylated glycans | 237, 238 |
| 1963–1969 | Description of altered agglutination (by WGA and ConA) of virally transformed cells | 239–246 |
| 1968 | Demonstration of altered glycolipids in virally transformed cells | 247 |
| 1969–1971 | Demonstration of altered glycopeptide sizes upon cellular transformation | 248–251 |
| Monoclonal antibodies identifying specific glycan-based biomarkers of cancer | ||
| 1968 | Demonstration of antigenicity of glycoprotein glycans | 252 |
| 1970s–1980s | Discovery of T, Tn, and sialyl Tn (STn) antigens as tumor biomarkers | 253–256 |
| 1977 | Discovery of Forssman antigen in human gastrointestinal tumors | 257 |
| 1978 | Discovery of stage-specific embryonic antigen and oncofetal antigen SSEA-1 | 258, 259 |
| 1979–1983 | Identification of CA 19-9 as a circulating cancer-associated antigen, sialyl Lewis a (SLea) | 105, 260–262 |
| 1983 | Demonstrated expression of CA 125 (MUC16) in epithelial cancer and serum | 263, 264 |
| 1988 | Development of monoclonal antibodies to Tn antigen | 265 |
| 1988 | Altered fucosylation of serum α-fetoprotein (AFP) used as biomarker for hepatocellular carcinoma | 266 |
| 1981–1992 | Development of monoclonal antibody to the STn antigen | 267–269 |
Protein glycosylation ultimately results in the modification of many different protein products within a given cell. Indeed, membrane and secreted proteins are nearly all glycosylated, with only rare exceptions of nonglycosylated proteins in the secretome, such as small peptide hormones, insulin, glucagon, and human serum albumin (22–24). The elaboration of complex glycans on glycoproteins and glycolipids is a major function of the Golgi apparatus (Figure 1). However, the initiation of many types of protein glycosylation, such as N-glycosylation, O-mannosylation, and glycosylphosphatidylinositol (GPI) anchor addition, occurs in the endoplasmic reticulum (ER). Such protein modifications are typically posttranslational but occasionally cotranslational and are irreversible, with one known exception, O-GlcNAc, which occurs on proteins in the cytoplasm/nucleus as discussed below. The glycosylation of secreted and membrane glycoproteins requires the specific actions of an assortment of glycosyltransferases; over 200 gene-encoded enzymes are known (4). The molecular mechanisms of protein substrate recognition and modification are very poorly understood, especially in regard to the organization and topology of the Golgi apparatus. The residence time of glycoconjugates in the Golgi apparatus is likely very short, seconds to minutes, yet the efficiency of glycosylation in this tiny gatekeeping organelle is astonishingly high. ER/Golgi transport of glycoproteins and localization of glycosylating enzymes in the secretory pathway are required for normal protein glycosylation, and these processes may be altered, as discussed below, in tumor cells.
A common misconception is that protein glycosylation is relatively random and characterized by high degrees of heterogeneity. In reality, glycosylation is typically site specific, and specific classes of glycans are found on restricted subsets of glycoproteins (25). Indeed, at any single site on a protein, a relatively limited number of major structures (microheterogeneity) or even a single major structure (homogeneity) is typically present. For example, polysialic acid, linear sequences of Neu5Ac in α2–8 linkage, occurs primarily on NCAM (26) and a few other glycoproteins (27); the polysialic acid on NCAM is restricted to N-glycans in specific immunoglobulin domains (28), and that on neuropilin-2 occurs on O-glycans within a single cluster of Ser/Thr residues (29). O-Mannosylation in mammalian cells is relatively restricted to α-dystroglycan (30), but certainly not exclusively, and extended O-mannose-containing glycans on α-dystroglycan can also contain the novel mannose 6-phosphate linker (31) to which repeating units of xylose and glucuronate are added (32), again in a protein-specific manner. The most famous protein-specific glycosylation occurs on lysosomal hydrolases, where high-mannose-type N-glycans are modified by a unique pathway involving a protein-specific GlcNAc 1-phosphate transferase that adds GlcNAc 1-phosphate to the C-6 of mannose, and the mannose 6-phosphate signal is subsequently exposed by removal of GlcNAc, all within the Golgi (33).
Thus, the primary and secondary structures of proteins generally define the types of posttranslational glycosylation to which they are subjected. N-Glycosylation requires –Asn-Xaa-Ser/Thr– and rarely utilizes –Asn-Xaa-Cys–(where Xaa ≠ Pro) (34); O-xylosylation generally occurs on Ser within –Ser-Gly-Xaa-Gly–(where Xaa ≠ Pro) (35); and C-mannosylation (36) requires the C-terminal –Trp-Ser-Xaa-Trp or internal motif –Trp-Ser-Xaa-Trp-Ser–(37). GPI anchor addition within the ER, such as to the cellular prion protein (38)—which, like many other membrane glycoproteins, including acetylcholinesterase, also has N-glycans (39)—requires a unique propeptide, a cleavable C-terminal sequence (40). Such GPI anchor sites can now be more readily predicted using the database tool PredGPI (38). By contrast, we know little about the protein sequence or structure dictating initiation for most of the other glycosylation pathways, many of which occur in the Golgi (or in the cytoplasm/nucleus, as for O-GlcNAc on many different glycoproteins and O-Glc to initiate glycogen). For example, O-mannosylation occurs in the ER either co- or post-translationally using dolichol-phosphomannose as the donor and appears to prefer Ser/Thr-rich regions within specific types of structural determinants (41). Addition of O-Fuc and O-Glc occurs on Ser/Thr residues within specific consensus sequences of epidermal growth factor (EGF) repeats, as in Notch (42) and a few other glycoproteins; these repeats are small, cysteine-rich motifs with six conserved cysteines and three disulfide bonds and are found in many different secreted and cell surface glycoproteins (43, 44). Predictions of O-GalNAc addition to specific Ser/Thr residues can be made on the basis of known databases and primary and secondary structures of proteins using the tool NetOGlyc 4.0 (http://www.cbs.dtu.dk/services/NetOGlyc/) (24). Recent studies on O-GalNAc addition indicate that >85% of all proteins with a signal sequence in the secretory pathway have a least one Ser/Thr site that is modified with O-GalNAc-type O-glycans (24). Collagen and collagen-like molecules containing hydroxylated Lys (HyK) within the Gly-Xaa-HyK glycosylation motifs (45) may acquire the disaccharide Glcα1-2Galβ1 (46) through the action of two collagen β-galactosyltransferases that specifically recognize HyK residues (47).
A fascinating modification of intracellular cytoplasmic, nuclear, and mitochondrial proteins was discovered in 1984: the enzymatic addition of a single sugar GlcNAc (O-β-GlcNAc) donated by UDP-GlcNAc to specific Ser/Thr residues of proteins by the enzyme O-GlcNAc transferase (OGT) (48). This modification occurs on hundreds of nuclear and cytoplasmic proteins and represents the only normally reversible glycosylation in animals, as the O-β-GlcNAc may be removed by an O-GlcNAcase in these compartments and then re-added by the OGT. This modification can be competitive with phosphorylation but can also have its own unique sites. O-β-GlcNAc additions are probably at least as common as Ser/Thr phosphorylation and represent a method of regulating protein functions that is independent from phosphorylation (49). A form of OGT occurs in the mitochondria (50), and a number of key proteins in oxidative phosphorylation and the tricarboxylic acid cycle are modified (51). The site specificity and function of the single enzyme OGT are regulated by its association with a large number of accessory proteins that direct its interactions with specific protein sites and substrates. Interestingly, a novel OGT termed EOGT occurs in the lumen of the ER and can add O-GlcNAc to specific EGF-like domains (52). Evidence is accumulating that O-GlcNAcylation may contribute to altered signaling and gene expression in tumor cells (53). In vertebrates, the only other glycosylation demonstrated in the cytoplasm is glycogen, which contains Glcα1–4(Glcα1–4/6)nGlcα1–Tyr, in which the initiating Tyr residue within glycogen is self-glucosylated (54) and then further modified by glycogen synthase using UDP-Glc as the donor (55). Overall, in addition to the protein substrates themselves, there are many other factors that can regulate glycosylation in cells and contribute to biosynthesis of the cellular glycome and glycoproteome (see sidebar, Some Major Factors That Affect Protein Glycosylation in Tumor Cells).
It should be noted that the addition of glycans can result in significant alterations to the overall three-dimensional structure of a modified protein. Unlike the primary amino acid sequence of a protein, which dictates its tertiary structure and often results in many amino acids being buried within the protein’s core, glycan modifications reside on the surface, often extending as large molecular masses away from the attached protein. In this way, heavily glycosylated proteins may be viewed as a tree, with glycan branches extending away from a core protein trunk but often having intramolecular associations with the trunk or other branches. Given the significant role glycans play in the molecular composition of a glycoprotein, alterations in glycosylation can significantly impact overall glycoprotein charge and conformation and therefore readily alter its biological activity (4, 17). Furthermore, as each branch may be modified by a particular glycosyltransferase and a given glycosyltransferase may be responsible for impacting the glycosylation of many different glycoproteins, relatively simple changes in one glycosyltransferase can impact the biology of many different proteins in meaningful ways, thus enabling rather subtle genetic changes to induce highly pleiotropic effects on cancer cell survival and overall progression (2, 4).
TYPES OF CHANGES IN PROTEIN GLYCOSYLATION IN CANCER
There have been many excellent recent reviews on the overall nature of changes in glycosylation and glycan-mediated processes in tumor cell biology as well as other disease processes (2, 17, 56–60). Here we focus on protein glycosylation as a posttranslational modification involved in cancer biology, both as a biomarker and as a contributor to the development and progression of cancer. The major types of changes in protein glycosylation associated with cellular transformation include changes in O-glycans (GalNAc-Ser/Thr) and N-glycans (Figure 2), which may occur both early and late in cancer progression and metastasis (Figure 3). These changes in glycosylation can be characterized by specific changes in O- and N-glycan core structures. In addition, alterations in glycosyltransferase expression, defined as production of transcripts and enzyme activity, may not only significantly impact the generation of different core glycans but also govern the degree of core glycan branching (61, 62), which in turn can alter overall glycan structure and function.
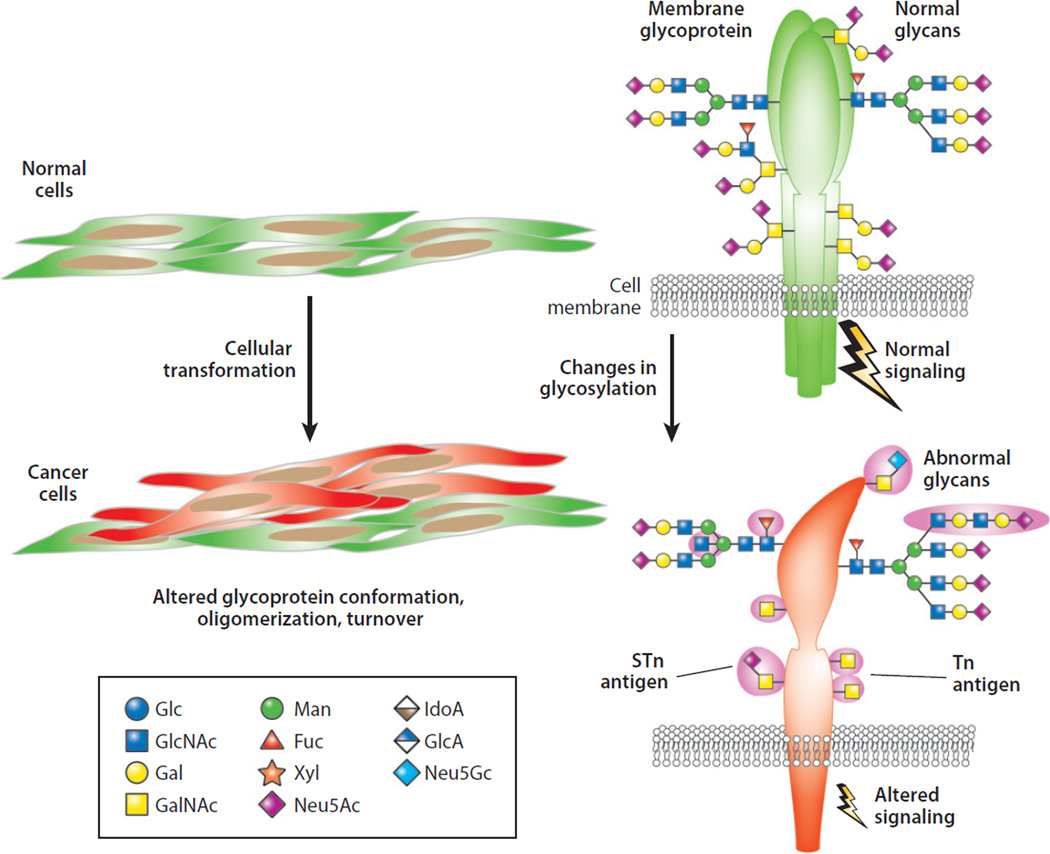
Figure 2.
Cellular transformation is typically accompanied by changes in protein glycosylation on multiple types of glycans; the most commonly studied are N-glycans and O-glycans. Changes in protein glycosylation can result in altered glycoprotein conformation, oligomerization, and turnover and can also be associated with altered cell signaling pathways. Frequently observed altered O-glycans include the Tn and STn antigens. Abbreviations: Fuc, fucose; Gal, galactose; GalNAc, N-acetylgalactosamine; Glc, glucose; GlcA, glucuronic acid; GlcNAc, N-acetylglucosamine; IdoA, iduronic acid; Man, mannose; Neu5Ac, 5-N-acetylneuraminic acid (sialic acid); Neu5Gc, 5-N-glycolylneuraminic acid; STn, sialyl Tn; Xyl, xylose.
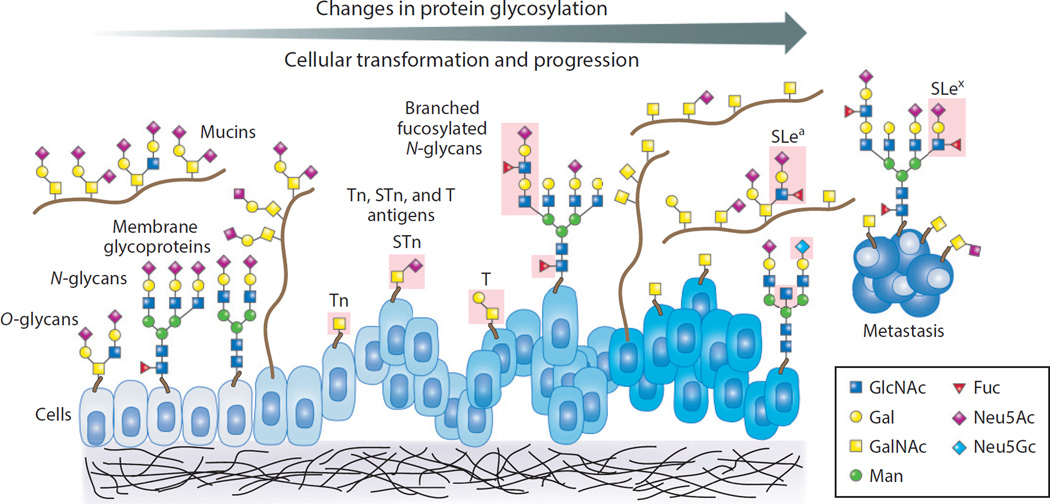
Figure 3.
During cellular transformation, changes in protein glycosylation on membrane and soluble glycoproteins, such as mucins, are typical and may occur early and/or late in cancer progression, but this phenomenon is not well understood. Different types of changes are shown in the pink-boxed areas, highlighting changes in O-glycans (T, Tn, and STn antigens) and altered expression of branched and fucosylated N- and O-glycans, including changes in Lewis antigens (SLex and SLea). Abbreviations: Fuc, fucose; Gal, galactose; GalNAc, N-acetylgalactosamine; GlcNAc, N-acetylglucosamine; Man, mannose; Neu5Ac, 5-N-acetylneuraminic acid (sialic acid); Neu5Gc, 5-N-glycolylneuraminic acid; SLe, sialyl Lewis; STn, sialyl Tn.
In addition to alterations in core glycans, each of these carbohydrates can be further modified to generate unique terminal glycan motifs that may also undergo specific changes following neoplastic transformation. For example, highly fucosylated glycans, such as Lewis antigens [Lewisa/b (Lea/b) and Lewisx/y (Lex/y)], can become enriched on the cell surface following neoplastic transformation (63–65). Similarly, sialylation, a common terminal glycan modification, can also undergo significant changes during neoplastic progression (66). Similar changes in the expression of long polymers of lactosamine (polyLacNAc) can also become enriched on neoplastic cells (Figure 4) (67). In each of these situations, the types of cell surface glycans present on a given glycoprotein are dictated in part by the expression, localization, and activity of the glycosyltransferases in a given cell. As a result, slight alterations in glycosyltransferase expression or function can significantly impact the types of glycan modifications in a variety of ways, each with the potential to impact the biological activity of a cell (4). With modern methods and expansions in our understanding of the vast repertoire of glycan classes in cells, changes in other types of protein glycosylation are increasingly observed.
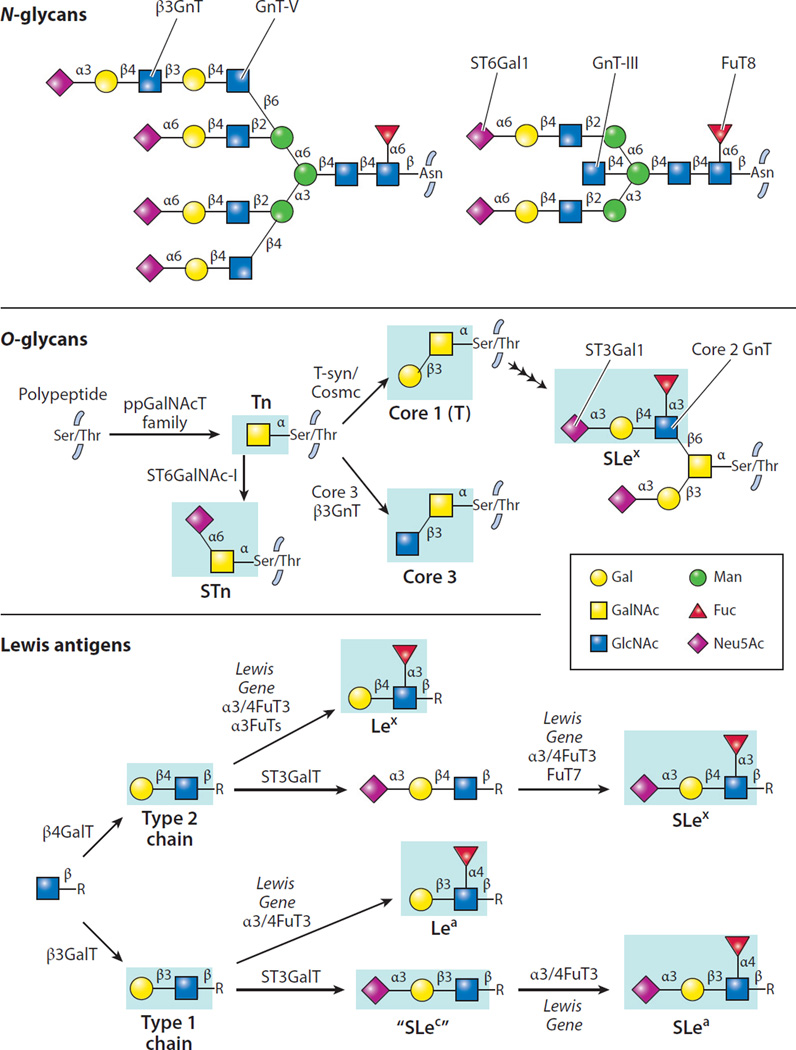
Figure 4.
Specific structures of N- and O-glycans and Lewis antigens along with enzymes responsible for addition of specific sugar residues. Each glycosyltransferase indicated requires a nucleotide sugar donor and acts to add a sugar in a specific anomeric linkage (α or β) to a specific acceptor glycan. Antigens indicated in blue boxes represent the major determinants recognized by monoclonal antibodies. Abbreviations: Fuc, fucose; Gal, galactose; GalNAc, N-acetylgalactosamine; Glc, glucose; GlcA, glucuronic acid; GlcNAc, N-acetylglucosamine; IdoA, iduronic acid; Le, Lewis; Man, mannose; Neu5Ac, 5-N-acetylneuraminic acid (sialic acid); Neu5Gc, 5-N-glycolylneuraminic acid; SLe, sialyl Lewis; STn, sialyl Tn; Xyl, xylose. The Lewis gene encodes the fucosyltransferase responsible for Lewis antigen synthesis.
DETECTION OF CHANGES IN PROTEIN GLYCOSYLATION: CHALLENGES AND FUTURE DIRECTIONS
In an effort to understand the impact of changes in glycosylation following neoplastic progression, numerous studies have sought to characterize and catalog the types of alterations in carbohydrate modifications that occur following neoplastic transformation. Some of the earliest histochemical stains were used to highlight distinct molecular features of cancer-targeted glycosylation (68). These early studies strongly suggested that tissue transformation into neoplastic lesions resulted in significant changes in glycosylation (69), but the potential impact of these changes and the specific nature of the alterations within neoplastic lesions remained unknown. Indeed, it was not until the advent of monoclonal antibodies that more definitive studies could be done to describe the expression and localization of any antigenic determinant, including glycans (69, 70). As a result, it is not surprising that the vast majority of studies that have sought to examine protein glycosylation in neoplastic lesions have employed monoclonal antibodies, followed by less specific polyclonal antibodies and even GBPs such as plant lectins. These studies have provided significant insight into changes that may occur following neoplastic transformation and suggest that alterations in glycan epitopes nearly always accompany neoplastic transformation.
Although antibody-based approaches to examining posttranslational modifications continue to provide important insight into changes in glycosylation following neoplastic transformation, glycans pose unique challenges. As most epitopes recognized by monoclonal antibodies reflect amino acid sequences, these distinct epitopes can often be mapped out with reasonable certainty, allowing the specificity of a given antibody to be accurately determined. In contrast, as glycosylation often reflects posttranslational modifications of many different proteins, defining the exact carbohydrate epitope recognized by a particular antibody can prove difficult (14). As a result, although many studies continue to attempt to describe changes in glycosylation following neoplastic transformation, using these data to define the expression of an individual epitope within tissue sections using traditional immunohistochemical approaches can be challenging.
Although genomic approaches can lend themselves to detection of changes in neoplastic lesions and could be considered when examining alterations in glycosyltransferase expression, the end product of glycosylation reflects the coordinated effort of many different enzymes, whose expression, localization, and posttranslational modifications can significantly impact enzyme activity and therefore affect the final glycosylation product on the cell surface (14). As a result, very specific and defined reagents that directly interact with the glycan product itself are needed in order to use potential changes in glycosylation as an effective diagnostic, prognostic, and even therapeutic target in routine clinical practice. In an effort to overcome challenges associated with using inadequately defined monoclonal antibodies or other reagents to define the expression and localization of specific glycan epitopes, several investigators developed novel tools that completely revolutionized the process of defining the carbohydrate specificity of a particular detection reagent (71). These tools comprise a series of glycan microarrays, characterized by the use of a variety of highly defined glycans obtained from chemoenzymatic synthesis, natural sources, or a combination of both, to generate robust and diverse libraries of the carbohydrate determinants that are needed to accurately define the binding specificity of a carbohydrate-reactive testing agent (72–77). Although most of the anti-carbohydrate antibodies used previously to examine tissue expression of distinct carbohydrate epitopes have not been examined on glycan microarrays, these types of studies are ongoing. Such studies will likely provide significant insight into the glycan epitopes that a particular anti-carbohydrate reagent recognizes and thus will significantly aid in the interpretation of past and future studies seeking to explore glycophenotypes of neoplastic lesions using traditional immunohistochemical approaches.
In addition to the advent of novel tools to define the specificity of previously developed anti-carbohydrate detecting agents, unique approaches to generating highly specific anti-carbohydrate antibodies may also significantly aid in the development of the repertoire of highly specific yet diverse reagents—capable of recognizing a variety of glycan epitopes—that is needed to accurately define potential changes in glycosylation within a neoplastic lesion. As some anti-carbohydrate antibodies are IgM (78), such reagents tend to be low affinity, which directly impacts their ability to specifically engage a particular glycan epitope. Furthermore, as the mammals that often serve as the vehicle to develop monoclonal reagents share common features of human glycosylation, immunological tolerance to glycan epitopes may further limit the immune responses needed to develop reagents with high affinity and specificity toward carbohydrate antigens. Recent studies suggest that jawless vertebrates, such as lampreys, can make highly specific antibodies through a completely different recombination program secondary to the unique evolution of their adaptive immune system. Given the evolutionary distance between humans and jawless vertebrates (79), recent studies have attempted to make highly specific reagents against carbohydrate antigens in these model systems (80). These studies demonstrated that very specific antibodies against blood group antigens could be developed (81), strongly suggesting that a similar approach may be used to generate additional anti-carbohydrate antibodies relevant to examining tumor-associated changes in glycosylation.
It should be noted that although the use of antibodies or similar reagents provides a practical approach that could be incorporated into the workflow of many immunohistochemistry laboratories, the accessibility of individual carbohydrate epitopes can be heavily influenced by the glycoprotein to which they are attached. Similarly, other unique structural features within the glycocalyx and beyond might sterically impede interactions. Thus, lack of detection of a particular glycan epitope should be interpreted with caution, even when standard approaches, such as positive controls, are used in parallel. Mass spectrometry may overcome these and other limitations. Indeed, technological breakthroughs in mass spectrometric analysis of intact tissue for specific glycan epitopes will likely provide a more objective approach to examining potential changes in neoplastic glycosylation (82). Similar to genomic approaches, successful mass spectrometric analysis will likely require the neoplastic cells within the analyzed sample to display a sufficient degree of alteration in glycosylation, as mixtures of commonly occurring glycosylation patterns associated with normal cells in the sample may obfuscate real tumor-associated signals. Thus, although the examination of changes in glycosylation in cancer lesions poses unique challenges, recent developments in glycomics offer promising solutions and may reveal specific associations between altered glycosylation and neoplastic progression.
Despite current limitations in the accurate detection of specific tumor-associated alterations in glycosylation, early studies using partially defined reagents provide some intriguing suggestions about the expression and consequences of a variety glycan epitopes within neoplastic lesions. For example, several studies using reagents thought to be specific to Tn and STn antigens indicated that these antigens appear to be preferentially expressed in poorly differentiated adenocarcinoma of the colon (83), hinting at a potential role for these antigens in the pathophysiology of colon cancer. Additional studies suggested a similar association in individuals with gastric cancer: Increased expression of STn appears to be associated with a significantly poorer prognosis (84, 85). A similarly reduced prognosis was reported in patients with increased T antigen expression in bladder cancer (86). Changes in terminal glycan epitopes also appear to convey poor prognosis in individuals with neoplastic disease. For example, increased expression of the glycan epitope sialyl Lewisa antigen (SLea) appears to correlate with increased metastatic disease and poorer overall survival in patients with colon cancer (Figure 4) (87). Similar results were obtained from analysis of a related glycan antigen, SLex, in colorectal, prostate, and breast cancer and a combination of SLea, SLex, and Ley antigens in patients with non–small cell lung cancer (88–91). Although these results may simply reflect correlative changes, studies examining the biological consequences of these antigens strongly suggest that these altered glycoforms directly impact the metastatic potential of neoplastic disease (92–98).
Whereas several glycan signatures appear to convey a poorer prognosis, the expression of some other glycan epitopes may be associated with a more favorable outcome. In blood group–positive individuals, for example, retention rather than loss of blood group A expression on non–small cell lung cancer correlates with a fairer prognosis (99). Similar studies demonstrated that bladder or oral cancer patients who lost blood group antigen expression exhibited poorer survival (100, 101). Although blood group antigen expression itself may reduce cellular proliferation and metastasis, the truncated and/or altered glycosylation resulting from the loss of blood group antigen expression, such as the development of STn or Tn antigens (84, 85), may actually be responsible, at least in part, for the altered biology observed in these individuals (102).
SERUM GLYCOPROTEIN BIOMARKERS FOR HUMAN CANCER
Many currently employed antibodies that recognize commonly occurring alterations in neoplastic cell antigens actually target glycoproteins or directly recognize altered tumor-associated carbohydrate antigens. Importantly, these antigenic targets were often identified after isolation of monoclonal antibodies developed following exposure of animals to neoplastic cells, reinforcing the unique nature of some of these carbohydrate alterations. Several of these glycoproteins and glycan-related biomarkers (glycobiomarkers) are currently being used for screening, diagnosis, and/or management of human cancer (Table 2; see 103 for a recent review). A few of the more commonly employed antigens are outlined below.
Table 2.
Carbohydrate tumor markers
| Markera | Cancer type | Reference(s) |
|---|---|---|
| AFP | Hepatocellular | 270 |
| β-hCG | Testicular, ovarian | 271, 272 |
| CA 15-3 | Breast, lung, prostate | 273, 274 |
| CA 19-9 | Gastrointestinal (pancreatic) | 275–277 |
| CA 27.29 | Breast, lung, prostate | 278 |
| CA 125 | Ovarian | 279, 280 |
| CA 549 | Ovarian | 281 |
| CEA | Colorectal | 282, 283 |
| CEACAMs | Colorectal, pancreatic | 284, 285 |
| HER2 | Breast | 286, 287 |
| onfFN | Thyroid | 288 |
| PLAP | Testicular, muscle | 289 |
| PSA | Prostate | 290–293 |
| sTn antigen | Colon, other | 83, 294 |
| TAG-72 | Ovarian, other | 295 |
| TG | Thyroid | 288 |
| Tn antigen | Colon, breast, cervical, other | 83, 132, 296 |
Common abbreviated marker names are shown: AFP, α-fetoprotein; β-HCG, β human chorionic gonadotropin; CA, cancer antigen; CEA, carcinoembryonic antigen; CEACAMs, carcinoembryonic antigen–related cell adhesion molecules; onfFN, oncofetal fibronectin; PLAP, placental alkaline phosphatase; PSA, prostate-specific antigen; STn antigen, sialyl Tn antigen; TAG-72, tumor-associated glycoprotein 72; TG, thyroglobulin.
Cancer antigen (CA) 19-9 corresponds to a carbohydrate structure initially found in glycolipids and now known to also occur on glycoproteins, such as mucins, that contain the SLea antigen (104). It is expressed primarily in pancreatic and biliary tract cancers but may also be present in patients with other malignancies, such as ovarian cancer and pancreatitis. CA 19-9 was first characterized by a monoclonal antibody, 1116-NS19-9, generated by immunizing mice with a human colorectal cancer cell line (105). Its sensitivity and specificity as a serum biomarker for pancreatic cancer are 79–81% and 82–90%, respectively (106).
The CA 125 antigen was discovered in 1981 with the mouse monoclonal antibody OC125.1, generated by immunizing mice with an ovarian cancer cell line (107). The CA 125 antigen is on MUC16 (108), which is a highly N- and O-glycosylated type I membrane glycoprotein with a long extracellular domain. The extracellular domain of MUC16 is prominently detected in serum, yet the molecular mechanism(s) of its release from the cell surface is unknown. Elevated serum CA 125 antigen is often used as a diagnostic biomarker for ∼85% of patients with ovarian cancer; however, this marker exhibits a high false-positive rate because many different conditions can cause an increase in CA 125, including other cancers, such as endometrial and peritoneal, in addition to other non-neoplastic conditions, including uterine fibroids, endometriosis, pelvic inflammatory disease, cirrhosis, and pregnancy (109).
Carcinoembryonic antigen (CEA) is a group of GPI-anchored glycoproteins involved in cell adhesion (110). CEA is normally produced in intestinal tissue during fetal development, and its levels drop significantly just before birth. Therefore, it is normally present at very low levels in the blood of healthy adults. In colorectal carcinoma, CEA expression resumes its high levels and CEA levels in the blood are consequently elevated. CEA measurement is mainly used as a tumor marker to stage malignancy, monitor colorectal carcinoma treatment, and identify recurrences after surgical resection. Serial CEA measurements can detect recurrent colorectal cancer with a sensitivity of ∼80% and a specificity of ∼70% (111). Notably, CEA levels may also be raised in other carcinomas, including gastric, pancreatic, lung, breast, and medullary, as well as in some non-neoplastic conditions.
Prostate-specific antigen (PSA) is a glycoprotein of the kallikrein protease family (specifically, human kallikrein 3) primarily produced by the prostate. Its level in the serum of healthy men is very low; however, it increases in association with different pathological states of the prostate, such as benign tumor, prostatitis, and prostate cancer. PSA has been employed extensively for prostate cancer screening and is one of the most widely used tumor markers (112). However, not all prostate tumors cause increased levels of serum PSA. Indeed, studies using PSA as a biomarker for prostate cancer have shown dramatic variation in its specificity and sensitivity (113). However, recent studies have indicated that altered PSA glycosylation patterns may provide an additional discriminatory marker and thus provide a more specific method to differentiate significant and insignificant increases in PSA levels when screening for prostate cancer (114).
α-Fetoprotein (AFP) was one of the first so-called oncofetal antigens to be described (115). Normally secreted by fetal liver and present in fetal serum, AFP is associated with hepatocellular carcinoma and nonseminomatous germ cell tumors when present in adult serum. Similar to PSA, unique glycoforms of AFP, such as forms with altered sialylation, may be used to distinguish elevated AFP levels associated with hepatocarcinoma from the general increases in serum AFP that can result from other benign liver diseases. The core-fucosylated form of AFP was reported to be more specific to hepatocellular carcinoma (116), but another study showed that high levels of AFP and fucosylated AFP (AFP-L3) could be found in patients with pancreatic acinar cell carcinoma (117).
HER2 (also known as ErbB2 or HER2/neu) is a member of the EGF receptor (EGFR) family and is a tyrosine kinase. HER2 is an N-glycosylated glycoprotein (118) that is overexpressed in several malignancies, especially breast cancer. It is the therapeutic target of the monoclonal antibody trastuzumab as well as a series of inhibitors designed to target its tyrosine kinase activity (119).
Human chorionic gonadotropin (hCG) belongs to a group of glycoprotein hormones that includes luteinizing hormone, follicle-stimulating hormone, and thyroid-stimulating hormone. All of these hormones are composed of two subunits: an α subunit common to all three and a β subunit specific to each. hCG is normally produced by the placenta, and serum levels of hCG are commonly used to monitor pregnancy and pregnancy disorders. Recent studies have shown that the synthesis of hCG is a characteristic feature of a wide variety of malignant and nonmalignant tumors. High levels of hCG are associated with trophoblastic disease and nonseminomatous germ tumors. Elevated levels of hyperglycosylated hCG that contains more complex glycan structures appear to be more specific to patients with malignancies (120).
CA 15-3 and CA 27–29 are different epitopes on the same protein, mucin 1 (MUC1) (121, 122). Upregulated MUC1 expression is associated with breast cancer, although the CA 27–29 epitope appears to have enhanced sensitivity and specificity when compared with CA 15-3. For example, CA 27–29 is elevated in 30% of patients with low-stage disease and in 60–70% of patients with advanced-stage breast cancer. However, when combined with CEA, CA 15-3 raises the specificity for breast cancer up to 95%. MUC1 may also be elevated in patients with other tumors and diseases.
POTENTIAL GLYCOBIOMARKERS FOR HUMAN CANCER
Many different glycoproteins and tumor-associated carbohydrate antigens continue to be used in the clinical management of patients with neoplastic disease, and several recent studies using highly defined carbohydrate recognition reagents suggest that additional glycan markers may provide highly specific tags for recognizing and potentially targeting neoplastic lesions. For example, recent results suggest that the Tn and STn carbohydrate antigens may be the most commonly altered O-glycansongly on glycoproteins (Figure 2),such as mucins, within neoplastic lesions.Tnand/or STn are highly expressed by many types of tumors, such as colon, breast, pancreatic, lung, cervix, and ovarian. Studies suggest their expression is associated with tumor progression and metastasis. Therefore, Tn and STn may serve not only as a prognostic marker but also as a therapeutic target, as recently outlined in several reviews (e.g., 123, 124). Furthermore, combination of Tn or STn with other glycobiomarkers, especially mucin biomarkers, may improve the diagnostic specificity of cancer.
Human tumor-associated glycoprotein 72 (TAG-72) is a carcinoma mucin expressed in colon, breast, pancreatic, ovarian, lung, and gastric cancers. Epitopes of TAG-72 recognized by the monoclonal antibodies B72.3 and CC49 are the STn and sialyl T [ST; Galβ1–3(NeuAcα2–6)GalNAcα1–Ser/Thr] antigens, respectively (125, 126). A humanized version of CC49 has been generated and is under clinical trial for use in radioimmunoguided surgery (127). Because STn antigen recognized by B72.3 is expressed strictly on tumors, whereas T and ST antigens are normal O-glycans seen in hematopoietic cells, STn would be predicted to be a more attractive target for human cancer. Efforts have been made to make STn-KLH and other forms of STn antigens as vaccine immunotherapeutics for breast cancer.
GPI-anchored glycoproteins contain a GPI anchor rather than a transmembrane domain. The GPI anchor is a glycan and lipid posttranslational modification added to proteins in the ER (Figure 1). GPI-anchored glycoproteins were recently found to be elevated in the plasma of patients with many types of cancers, including breast, ovarian, kidney, liver, lung, colon, and brain (128, 129). Its potential diagnostic and screening value is under evaluation.
N-Glycolylneuraminic acid (Neu5Gc)-containing glycans have recently been identified in human tissues. Human cells normally can synthesize Neu5Ac but not Neu5Gc due to an evolutionary inactivation the CMP-Neu5Ac hydroxylase that converts CMP-Neu5Ac to CMP-Neu5Gc in lower species such as bovines, chickens, and rodents (130). Human carcinomas can metabolically incorporate and present the dietary nonhuman sialic acid, Neu5Gc, which differs from the human sialic acid Neu5Ac by one oxygen atom. Normally, there are low levels of anti-Neu5Gc antibodies in circulation. In the presence of carcinomas, however, these antibody levels are elevated. As a result, increases in polyclonal anti-Neu5Gc antibodies may also serve as a potential cancer biomarker (131).
It is worth noting that many of these glycobiomarkers were established by generating monoclonal antibodies through immunization of mice with tumor cells. Many of the tumor-specific antibodies developed using this approach were later discovered to recognize glycan or glycoprotein epitopes. As a result, this strategy—combined with the use of jawless vertebrates as hosts for antibody production and glycan microarrays to define specificity—may continue to prove useful in the discovery of additional carbohydrate-based biomarkers for diagnosing and potentially treating neoplastic disease.
GENETIC AND BIOCHEMICAL MECHANISMS FOR CHANGES IN PROTEIN GLYCOSYLATION
Protein glycosylation is regulated by complex mechanisms, including the expression and localization of glycosyltransferases and the ratio of glycosyltransferase activity to donor substrate availability. In transformed cells, altered glycosylation often results from altered expression, altered activity, or mislocalization of glycosyltransferases and related proteins, such as chaperones, that regulate their activity.
Genetic Mutations
Tumor cells carry many gene mutations, yet documented mutations of genes encoding glycosyltransferases are relatively uncommon in tumor cells. However, there are a few exceptions. Expression of Tn and STn antigens on O-glycoproteins and mucins is associated with pathological situations such as Tn syndrome and human tumors such as cervical cancer (132). Studies have shown that mutation or deletion of the X-linked Cosmc gene is one of the major mechanisms by which the key enzyme T-synthase is inactivated; without the assistance of the functional chaperone Cosmc, T-synthase is misfolded, leaving it unable to modify the Tn antigen on glycoproteins (123). Additionally, in colon cancer, a mutation in ppGalNAcT12 was identified (133), but its consequences remain elusive.
Misregulated Expression of Glycosyltransferase and Chaperone Genes
Like that of other genes, the expression of genes encoding glycosyltransferases is regulated through both transcription factors and epigenetic mechanisms. As a result, each tissue or cell type has a unique set of glycosyltransferases that generate specific types of glycan structures on their mucins and other glycoproteins, both secreted and membrane bound. In transformed cells, the expression of glycosyltransferases is often misregulated. In normal mammary gland, for example, GnT-V is expressed either not at all or at very low levels (Figure 4). However, in breast cancer, it is upregulated by the transcription factor Ets through the HER2 pathway, resulting in highly branched N-glycan structures on tumor cells (134). Higher expression levels of FuT8 in tumor cells compared with the surrounding normal hepatocytes results in elevated core-fucosylated AFP, which can thus serve as a relatively specific biomarker for hepatocellular carcinoma (135). Examples of such upregulated expression also include enzymes responsible for the synthesis of Lex, SLex, Lea, and SLea antigens in many types of tumors (63–65). Upregulation of β3GnT8 results in increased levels of polylactosamine structures in colorectal carcinoma (67), and similar alterations in GnT-III increase bisected N-glycans in liver cancer (61, 62).
Polypeptide GalNAc transferases (ppGalNAcTs) are the enzymes responsible for the initiation of mucin-type O-glycosylation. Overexpression of the ppGalNAcT gene GALNT6 was detected in breast cancer and may contribute to mammary carcinogenesis through aberrant glycosylation and stabilization of MUC1 (136). Its expression has also been observed in gastric cancer and is associated with the presence of venous invasion (137). In addition, ppGalNAcT-14 is overexpressed in colorectal carcinoma and pancreatic cancer and is associated with altered sensitivity to TRAIL-induced apoptosis through modulation of the O-glycosylation of death receptors on these tumor cells (138). ST6GalNAc-I, whose product is primarily responsible for the sialylation of the Tn antigen to form the STn antigen on O-glycoproteins and mucins, is not normally expressed in any human tissues. In many types of human tumors, however, the STn antigen is detected at high levels, presumably due to the upregulation of ST6GalNAc-I in tumor cells, such as in breast cancer (139). However, how these glycosyltransferases are upregulated in tumors is not fully understood.
Expression of the Tn antigen in human metastatic pancreatic cancers has been associated with epigenetic silencing of the Cosmc gene by hypermethylation, as determined by exome sequencing of many glycosyltransferase genes and the Cosmc gene in primary and metastatic specimens (140). Furthermore, directed deletion of Cosmc in cell lines induces oncogenic features including altered cell growth and invasion (140). This is also interesting in light of prior studies showing that hypermethylation of the Cosmc gene occurs in Tn4 cells, an immortalized B cell line from a male patient with a Tn-syndrome-like phenotype (141). In that case, hypermethylation is associated with expression of Tn antigen and loss of T-synthase in a reversible fashion as treatment of cells with 5-aza-2′-deoxycytidine, which reverses methylation, causes restoration of Cosmc transcripts.
Conversely, overexpression of Cosmc and/or T-synthase and expression of the T antigen has been associated with malignant behavior of cells (142–144). The T-synthase (C1GALT1) has been reported to be overexpressed in hepatocellular carcinoma and expression is associated with advanced disease and survival (145). Mechanistically, it was demonstrated that overexpression altered β1 integrin, leading to changes in signaling and adhesion. Interestingly, overexpression of T-synthase led to enhanced metastasis of hepatocellular carcinoma in NOD/SCID mice, whereas knockdown of T-synthase led to decreased metastasis. Although it is not clear how or whether overexpression of Cosmc and/or T-synthase actually leads to general enhanced T antigen expression and altered protein glycosylation in many types of tumors, such studies suggest that altered expression of these proteins may somehow be associated with malignant phenotypes.
ABO blood group glycosyltransferases are the enzymes responsible for the synthesis of ABO blood groups, mainly on erythrocytes but also on squamous and gastrointestinal tract epithelial cells (146). However, they are silenced in some oral squamous cell carcinomas due to hypermethylation (147). Deletion or reduction of A and B epitopes in other solid tumors, such as gastric and bladder carcinoma, has also been observed, yet the mechanisms for altered expression of A and B glycosyltransferases are not fully understood (146). There are also examples of glycosyltransferase expression that is decreased or suppressed in tumor cells compared with their normal counterparts, including loss of expression of C3GnT (148) and low expression of β4GalNAcT2 (149) in colorectal carcinoma and decreased expression of αGnT in gastric carcinomas (150). However, the mechanisms by which the expression of these genes is decreased also remain unclear.
Mislocalization of Glycosyltransferases
Glycan structures are built in a sequential fashion by a set of glycosyltransferases localized in the ER and Golgi apparatus (Figures 1 and 4). Even within the Golgi apparatus, glycosyltransferases are not evenly distributed, but rather specifically reside in the cis, medial, and trans Golgi cisternae and the trans Golgi network through complicated and not fully characterized mechanisms (151). Correct localization of glycosyltransferases also relies on the integrity of Golgi structures. Furthermore, the structures of the Golgi are dynamic rather than in a steady state. Therefore, it is easy to imagine that altered glycan structures may arise from the mislocalization of glycosyltransferases and altered Golgi architecture. For example, highly active Src kinase can relocate the normally cis-Golgi enzyme ppGalNAcT2 back to the ER through a COP-I-dependent pathway, resulting in the expression of Tn antigen on glycoproteins in the ER, which in turn probably alters sites of O-glycosylation (152). The Golgi structure is often altered and its pH often increased in tumor cells, which may also contribute to the mislocalization of glycosyltransferases and/or their enzyme activities in tumor cells.
In summary, the detailed mechanisms that produce all the altered glycan structures in tumor cells await full investigation. Much more work needs to be done to elucidate the mechanisms responsible for regulating the expression and localization of glycosyltransferases and to forge a comprehensive understanding of the detailed dynamics of Golgi structures.
BIOLOGICAL ROLES OF PROTEIN GLYCOSYLATION IN NORMAL AND CANCER CELLS
Given their location on numerous cell surface glycoproteins, glycans can impact a wide variety of cellular functions, ranging from glycoprotein trafficking to cellular signaling. As a result, alterations in glycosylation can significantly impact the localization and stability of cell surface receptors and their sensitivity to a broad range of signaling molecules, with obvious implications in cellular division, differentiation, and localization within tissue. Thus, not only do alterations in glycosylation appear to correlate with changes in neoplastic cell behavior, with implications for an individual patient’s prognosis, but these changes likely reflect fundamental alterations in the biology of the neoplastic cell that may be critical in the spread of disease.
Although cell surface receptors signal through a variety of mechanisms following ligand engagement, ligand-induced oligomerization of cell surface receptors reflects a common theme among receptors involved in regulating cell growth (153). Recent studies demonstrate that alterations in glycosylation may significantly impact the intrinsic ability of cell surface receptors to undergo appropriate oligomerization, thereby directly influencing the sensitivity of these receptor systems to stimulation. For example, inhibition of complex O-glycan formation, a common feature of neoplastic cells, results in impaired sensitivity of DR4 and DR5, apoptosis-inducing receptors that relay signaling by TRAIL (138). As TRAIL normally induces death in neoplastic cells through engagement of DR4 and DR5 (154), cancer-associated alterations in O-glycosylation of these receptors likely reduces the cells’ sensitivity to this antineoplastic pathway in vivo, thereby conferring a selective advantage with regard to the TRAIL surveillance program (154). Additional complex O-glycan formation, including core 2 O-glycan generation, appears to directly correlate with cancer invasion (155). Similarly, expression of STn antigen appears to inhibit cell adhesion, likewise increasing cell spread and the potential for metastasis (156).
Just as alterations in complex O-glycosylation appear to influence a variety of signaling pathways that may be directly or indirectly related to cancer (138, 157), alterations in N-glycans also likely impact the survival and progression of neoplastic cells. For example, increased expression of GnT-V, which can be driven by ras oncogenes and generates a β1–6 GlcNAc N-glycan branch commonly found in tetraantennary N-glycans (158, 159), results in impaired epithelial contact inhibition and significantly increased cellular motility, key features of cells undergoing neoplastic transformation (Figure 4) (160). Similarly, increased expression of GnT-V-dependent N-glycan modifications appears to enhance the invasiveness of glioma, colon cancer, and gastric cancer cell lines (161, 162), possibly through loss of inhibition of collagenase activity, which in turn enhances the ability of cells to percolate through normal extracellular matrix barriers (162, 163). In contrast, increased expression of GnT-V may actually enhance cellular sensitivity to apoptosis through unknown mechanisms (164), once again demonstrating the pleiotropic and occasionally opposing activities of altered glycosylation on neoplastic cell survival. Similarly, overexpression of GnT-III, which adds a β1–4 bisecting branch to N-glycans, appears to inhibit EGFR sensitivity to EGF (Figure 4) (165), thereby reducing cellular sensitivity to the proliferative effects of EGF on sustained cell growth. Additional studies demonstrate that critical N-glycans serve to inhibit autodimerization and therefore autonomous activation of other growth receptors, such as ErbB3 (166), providing another pivotal checkpoint whereby N-glycans may regulate key cellular processes involved in cell proliferation and potential progression to neoplastic transformation.
In addition to alterations in core O- or N-glycans, changes in terminal glycan structures may likewise induce changes in cellular behavior that may enhance the growth and spread of neoplastic disease. Whereas decreases in sialylation may enhance integrin-mediated cellular adhesion (167), increases in sialylation appear to inhibit integrin interactions with extracellular constituents, such as fibronectin (168, 169), thereby potentially facilitating cancer spread and eventual metastasis. In contrast, enhancing the sialylation and fucosylation of N-glycans on the EGFR of various lung cancer cell lines appears to inhibit EGFR dimerization (170), thus inhibiting this important process in the continued survival and progression of some types of lung cancer (171). Consistent with this, transfection of cells with the sialidase gene decreases EGFR sialylation and activity in vitro (172). Furthermore, altered expression of α2–6 sialylation appears to enhance growth of various glioblastoma cell lines through a glycoprotein-independent mechanism (173), suggesting that alterations in the sialylation of various cell surface molecules can significantly impact cellular viability. Alterations in cellular fucosylation through upregulation of FuT8 also appear to regulate cellular proliferation, as knockdown of FuT8 in cell lines derived from non–small cell lung cancer results in a significant reduction in cellular proliferation rate in vitro (174).
In addition to intrinsically altering the location of cell surface receptors and their sensitivity to key ligands required for cell growth and invasion, alterations in glycosylation also appear to affect the ability of neoplastic cells to engage and differentially impact the activity of infiltrating immune cells normally responsible for immunosurveillance. For example, truncated O-glycans in the form of O-GalNAc can interact with macrophage galactose-type lectin (MGL) (175); this engagement may enhance the uptake of unique tumor-specific glycoproteins (176). This lectin normally plays an important role in responses to helminthes; thus, neoplastic cell engagement of MGL appears to deviate the immune response away from a potentially productive Th1 cell–mediated immune response toward a more tolerogenic phenotype that likely plays a critical role in the establishment of immunological tolerance to a neoplastic lesion (177). Similar engagement of natural killer (NK) cells by STn may inhibit NK cell–mediated antitumor immunity (178). The utilization of dendritic cell C-type lectins to escape or otherwise favorably influence immunity does not appear to be limited to MGL; several other lectins that likely engage other commonly occurring cancer-associated glycan signatures, such as Lea and Leb, may also inadvertently play a role in facilitating the type of immunoprivileged environment that favors neoplastic cell survival in vivo (179).
Alterations in glycosylation can clearly impact cellular survival and engagement of immune effector cells through a variety of immune cell GBPs. In addition, cancer cells appear to possess the capacity to secrete their own GBPs, which can likewise impact neoplastic growth and survival. Galectins, which are soluble GBPs overexpressed in many types of cancer, represent a classic and well-studied example (20). Galectins can affect cell proliferation and survival through intercellular interactions with key players in cell growth and viability (20, 21, 180), but similar to C-type lectins, they likely evolved as innate immune proteins with the capacity to significantly impact immune cell viability and function (181–183). As a result, it seems that the secretion of various galectin family members by neoplastic cells can significantly modulate immune function. For example, galectin-1, the first family member described, likely engages dendritic cells and T cells, inducing alterations in cytokine production that ultimately prevent the effective elimination of neoplastic cells, thus providing a mechanism whereby these GBPs might enhance neoplastic cell survival (184, 185). Several studies suggest that, in addition to regulating immune function (186–188), galectins may directly facilitate neoplastic cell adhesion to the extracellular matrix and enhance endothelial cell–mediated extravasation (189). Given that these proteins also appear to directly regulate angiogenesis, they may influence neoplastic cell survival in a variety of ways (190–192).
LESSONS FROM ANIMAL MODELS OF CANCER
Whereas in vitro studies provide compelling insights into the potential impacts of altered glycosylation on cell-intrinsic behavior, including cell growth and viability, examination of cancer cells in vivo can illuminate the potential consequences of altered glycosylation for the interaction of neoplastic cells with environmental factors that may be critical for their survival, growth, and metastasis. Consistent with the ability of GnT-V overexpression to enhance neoplastic cell survival in culture, early studies suggested that simple inhibition of total N-glycans could significantly reduce neoplastic cell growth in vivo (Figure 4) (193). Increased GnT-V activity secondary to overexpression of H-ras likewise resulted in increased metastatic potential in several cell lines in vivo (159). More recent studies also suggested a central role for GnT-V in this process, as GnT-V-knockout mice display significantly reduced polyomavirus-induced tumor formation, which appears to reflect, in part, altered phosphatidylinositol 3-kinase–dependent cell proliferation (194). Variations in GnT-V expression may not only impact cell growth and survival but also facilitate metastasis. Tumors in GnT-V-knockout mice appear to display not only decreased growth but also impaired metastasis (194); the latter may simply be indicative of the former, but it also may reflect direct inhibition of metastatic spread. In contrast, expression of GnT-III (Figure 4) appears to inhibit metastatic spread of melanoma (195), providing an additional mechanism whereby GnT-III may negatively regulate cancer cell survival and progression. Similarly, enhanced α2–6 sialylation secondary to overexpression of an α2–6 sialyltransferase (ST6Gal1) (Figure 4) also inhibits cell growth and metastasis, suggesting that terminal glycan modifications can likewise impact cell growth and survival in vivo (173, 196).
Although altered glycosylation likely influences a variety of cellular features that contribute to an enhanced or reduced propensity for metastatic spread, early studies in leukocyte biology demonstrated that cell surface carbohydrates can play a key role in leukocyte extravasation. As neoplastic cells may utilize similar pathways during hematogenous spread, studies on neoplastic cell metastasis began to examine whether cancer cells may co-opt a similar pathway during intravascular dissemination. Indeed, neoplastic cell expression of SLex, a potential ligand for the E- and P-selectin vascular adhesion molecules, portends a poor prognosis for individuals with a variety of different neoplastic diseases. Metastatic tumors express higher levels of SLea or SLex compared with primary tumors (88, 197), once again suggesting that expression of these ligands may directly convey metastatic potential. Subsequent studies demonstrated that inhibition of complex O-glycans bearing selectin ligands reduces attachment to endothelial cells (198). Similarly, increased expression of SLea and SLex secondary to altered glycosyltransferase expression enhances neoplastic cell binding to P- and E-selectins (92–96). Tumor carbohydrate-mediated interactions with vascular adhesion molecules not only facilitate endothelial attachment but also appear to directly mediate vascular spread of cancer cells (97, 98). Whereas some of these altered glycoforms may facilitate metastasis, the same changes may serve as targets for NK cell–mediated immunosurveillance (199), suggesting that evolution may have selected these changes as potentially poor prognosticators in tumor growth.
Several studies suggest that, in addition to selectin-mediated extravasation, other endothelial lectins may also participate in cancer cell metastasis. For example, in addition to reports documenting a role for galectins in leukocyte extravasation (200), a variety of papers suggest that cancer cells may express galectin ligands (201), which in turn facilitate cancer cell metastasis. In contrast to selectin-mediated cancer cell extravasation, galectin-mediated homotypic interactions appear to facilitate the formation of tumor emboli that may directly enhance tumor lodging in distal capillaries, which may in turn enhance subsequent extravasation and metastasis (202). Consistent with this, galectin-3 expression in various cell lines correlates with metastatic potential in a murine model, and overexpression of galectin-3 in galectin-3-negative cell lines results in the acquisition of a more aggressive phenotype in vivo. Addition of factors thought to inhibit galectin binding in vivo, such as modified citrus pectin, likewise inhibits tumor growth and metastatic progression in vivo (203), strongly suggesting that galectin-3 or related galectin family members may play a key role in this process. Although the ability of galectin family members to modulate cell-cell and cell-matrix interactions likely accounts for a significant share of their impact, galectins also possess intracellular roles that can directly impact cell growth and survival (204). Consistent with the role galectins may play in favorably modulating immunity in tumor microenvironments, cancer cells expressing galectin-1 can inhibit T cell function and induce a tolerogenic phenotype in surrounding dendritic cells (184). Furthermore, galectin-1 also appears to facilitate tumor angiogenesis (186, 205), possibly through a mechanism that conveys resistance to vascular endothelial growth factor inhibition (186). As a result, just as alterations in glycosylation can potentially affect the function of cancer cells and the progression of cancer, GBPs not only decode these complex carbohydrates but in doing so regulate a wide variety of biological processes relevant to neoplastic cell growth and survival.
Besides their direct impact on cancer progression, GBP-carbohydrate interactions can also influence many of the sequelae associated with metastatic disease. For example, patients with Trousseau syndrome develop migratory microthrombi that are characteristically resistant to warfarin anticoagulation, suggesting a thrombin-independent mechanism of generation. In a recent model of Trousseau syndrome, mucin isolated from glycoproteins could induce significant platelet-rich microthrombi that remained sensitive to heparin-induced inhibition, similar to what occurs clinically (206). Consistent with previous reports suggesting that heparin may engage P- and L-selectin, heparin failed to inhibit microthrombus formation in P- and L-selectin-deficient recipients (206). Furthermore, P- and L-selectin-knockout recipients displayed significantly attenuated microthrombus formation following mucin challenge, strongly suggesting that these carbohydrate-binding proteins may be directly involved in this process (206). Indeed, subsequent studies demonstrated that neutrophil selectin, PSGL-1, and platelet P-selectin work in concert to facilitate microthrombus development following engagement of adenocarcinoma-derived mucin (207). Thus, GBP-carbohydrate interactions appear to directly engage a variety of biological phenomena that impact direct and indirect consequences of neoplastic disease.
Whereas neoplasia-associated alterations appear to impact progression and disease, cell surface carbohydrates on non-neoplastic cells may also inadvertently facilitate the development of neoplastic lesions. Although humans can generate de novo many of the monosaccharide substrates required for complex glycan formation, some monosaccharides found in nature, but not synthesized by our own cells, can be absorbed and utilized by the human glycoprotein synthesis machinery. A classic example of this is the “nonhuman” sialic acid, Neu5Gc, which, as stated above, can be incorporated into human glycoconjugates through dietary sources such as red meat. As this alternative form of sialic acid is not present normally, immunological tolerance fails to develop. As a result, when Neu5Gc incorporation into glycoconjugates occurs, “autoantibodies” to this sugar develop, which have been proposed to enhance inflammatory pathways associated with cancer initiation (208). In a similar manner, exposure to microbes that express carbohydrate antigens that look like us not only may enhance the probability of autoimmunity but also may likewise facilitate neoplastic progression secondary to autoimmune-mediated inflammatory changes that set the stage for neoplastic transformation (209).
TARGETED THERAPIES BASED ON CHANGES IN PROTEIN GLYCOSYLATION
Current cancer-specific therapies target either the unique products of chromosomal rearrangements that typically result in the generation of specific chimeric gain-of-function protein products, as occurs in chronic myelogenous leukemia (210), or the overexpression of a particular target, as occurs in HER2-positive breast cancer (211). Although these alterations represent fundamental changes critical in the progression of the corresponding types of neoplastic disease, each of these mutations occurs in only a select number of cancers (210, 211), thus limiting the therapeutic potential of targeting these alterations to a relatively narrow spectrum of neoplastic disease. In contrast, as changes in posttranslational modifications, such as glycosylation, may occur on a wide variety of cell types, specific changes in neoplasia-related glycosylation may be found on multiple types of cancer, providing potentially novel, specific, and therefore unique therapeutic targets for a broad range of neoplastic lesions (69).
In addition to their potential to impact a wide variety of cells, the very nature of posttranslational modifications allows a single genetic defect in an enzyme to exert a fundamental impact on the phenotype of a cell (14). For example, as a single glycosyltransferase can modify many different glycoproteins, and may even modify an individual glycoprotein at multiple locations, alterations in the expression or function of a particular glycosyltransferase can alter the glycosylation of a variety of glycoproteins at multiple sites per glycoprotein, ultimately inducing significant, dense, and specific changes in the carbohydrate profile of a cell (14, 69). In this way, these types of posttranslational modifications provide a unique opportunity to translate a single genetic lesion or alteration in the expression of a glycosyltransferase to a highly amplified and unique marker that may be used to specifically target neoplastic cells (69).
In addition to being present at multiple copies per glycoprotein and on multiple glycoproteins per cell, complex carbohydrate modifications occur overwhelmingly on proteins that reside on the cell surface, where antibody-based therapeutics can directly engage them and thereby direct endogenous immune effector cells to neoplastic lesions (14). Similarly, antibody-based therapeutics designed to specifically target neoplasia-associated changes in glycosylation would enable significant increases in the effective concentration of conjugated toxic antineoplastic therapeutics and therefore enhance the specificity of chemotherapeutic approaches.In addition, vaccine approaches using neoplasia-specific carbohydrates possess the same capacity to induce anti-carbohydrate antibodies that may enhance immunological memory against commonly occurring neoplasia-specific glycosylation and therefore enhance immunological surveillance (212). Consistent with this, several vaccine strategies appear to induce specific neoplastic anti-carbohydrate antibodies that show significant promise in the prevention of neoplastic disease (213, 214). However, it should be noted that, although vaccination approaches might indeed induce significant immunity to a cancer-related carbohydrate antigen, such immunity may produce selective pressures that enable neoplastic lesions to develop independent of a particular carbohydrate antigen (215). As a result, development of therapeutics that target existing cancer carbohydrate antigens may provide an equally compelling approach to treat neoplastic disease.
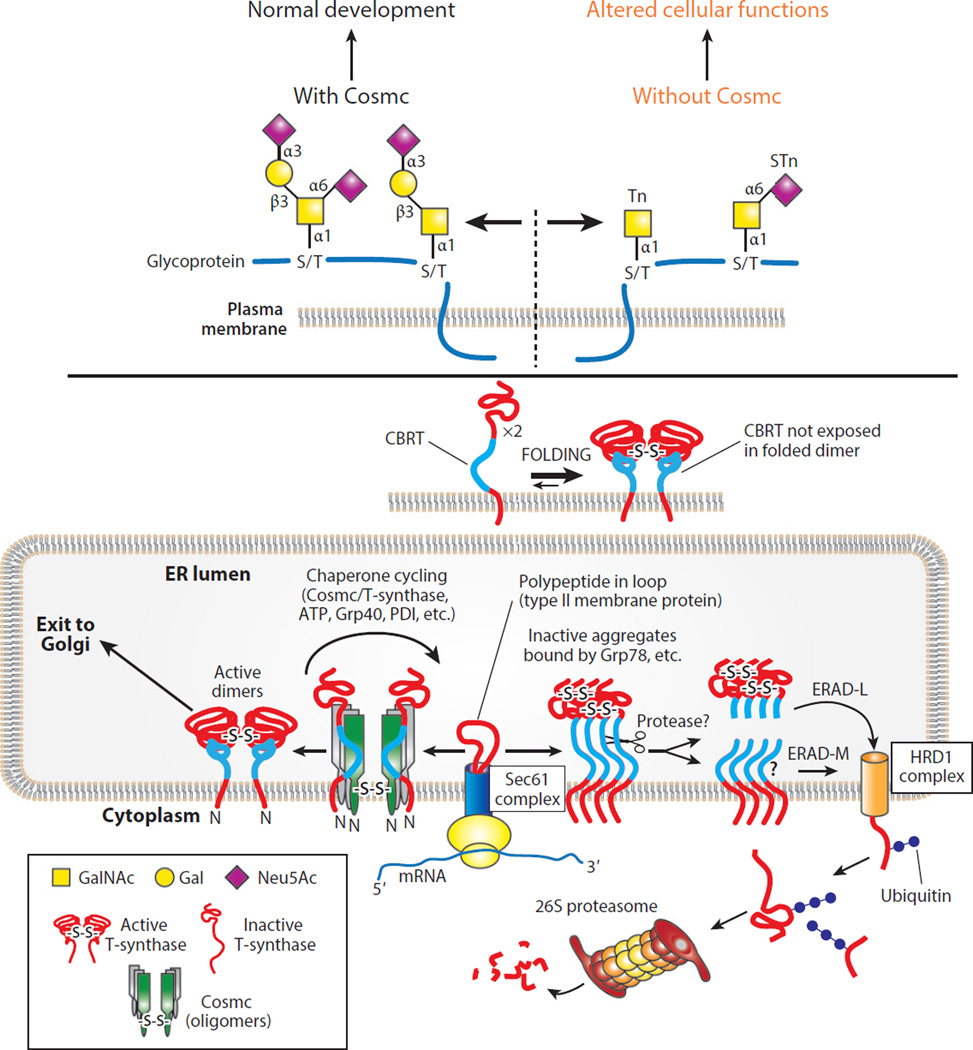
A classic example of a genetic change yielding a useful target cancer neoantigen is the formation of the Tn and STn antigens, which, as stated above, reflects the loss of the X-linked chaperone Cosmc and thus results in the loss of T-synthase activity, the rate-limiting enzyme necessary for complex O-glycan formation (Figure 5) (216). Whereas ppGalNAcTs do not need T-synthase for their enzymatic activity, T-synthase requires the product of ppGalNAcTs, which is a GalNAcα1–Ser/Thr or the Tn antigen, to form a core 1 O-glycan (otherwise known as the T antigen) (Figure 4). As a result, ppGalNAcTs continue to generate significant Tn antigen in the absence of T-synthase (132, 216). As O-glycans can occur on over 80% of cell surface glycans, this results in significant accumulation of the Tn antigen on the cell (217). The Tn antigen is normally completely modified to the T antigen by T-synthase; thus, accumulation of Tn antigen on many different glycoproteins on the cell surface results in the formation of antigenic determinants not normally present on the cell (102) and can therefore alter the conformation and function of glycoproteins (Figure 2) (157). As a result, the Tn antigen provides an unprecedented example of a truly neoplasia-specific antigen that does not appreciably occur normally but can be expressed on numerous targets on the cell surface following the loss of a single enzyme (102). Although the extent of Tn expression within neoplastic lesions, as detected using highly defined agents, remains to be fully elucidated, preliminary studies using very specific reagents suggest that this antigen may be present on nearly 50% of all neoplastic lesions from diverse sources of tissue, including breast, colon, ovary, lung, and endometrium (S.R. Stowell, T. Ju & R.D. Cummings, unpublished results).
Figure 5.
The expression of the Tn and/or STn antigens can occur in cells lacking the molecular chaperone Cosmc. Cosmc is present in the ER of animal cells and has a single client protein, the T-synthase, to which it binds cotranslationally in the ER to prevent oligomerization and destruction in the proteasome. Successful binding of Cosmc to the T-synthase requires the presence of a novel CBRT (297), which is exposed in non-native T-synthase but becomes buried and inaccessible in the folded T-synthase. Once folded properly, the T-synthase moves to the Golgi apparatus, where it acts quantitatively on the products of the ppGalNAcTs to generate normal O-glycans (102). Abbreviations: CBRT, Cosmc-binding region within T-synthase; ER, endoplasmic reticulum; Gal, galactose; GalNAc, N-acetylgalactosamine; Neu5Ac, 5-N-acetylneuraminic acid (sialic acid); ppGalNAcT, polypeptide GalNAc transferase; STn, sialyl Tn.
In addition to the loss of glycosyltransferase function, aberrant expression of a particular enzyme may inadvertently truncate or otherwise modify a cell surface glycoprotein and therefore likewise generate a unique cancer-associated glycan antigen. For example, whereas expression of the STn antigen likely occurs in neoplastic cells as a result of the loss of Cosmc function and subsequent generation of a substrate for the α2–6 sialyltransferase capable of modifying the Tn antigen to form STn (102), several studies suggest that overexpression of the α2–6 sialyltransferase may outcompete the T-synthase and therefore generate the STn antigen directly (218, 219), effectively preventing T-synthase from generating normally occurring core 1 O-glycans. Although overexpression of α2–6 sialyltransferase would be predicted to generate an aberrantly truncated and therefore potentially specific neoplastic alteration, some neoplastic lesions appear to exhibit glycosylation that significantly deviates from the tissue of origin but that may exist in similar structural form in other tissues. For example, other alterations in glycosyltrans-ferase function may result in significant increases in the formation of the T antigen, Lea, Leb, Ley, Lex, GM2, GD3, and others (69). Although some of these antigens exhibit expression on other cells, their overexpression on neoplastic cells may provide enhanced targets for neoplasia-related drugs, allowing increased specificity for cells bearing enriched levels of these antigens compared with normal tissue (69).
In addition to changes in glycosylation associated with genetic or even epigenetic changes, altered expression of glycosyltransferases and baseline changes in metabolism (220, 221) may cause neoplastic cells to preferentially incorporate sugar analogs that can be used to metabolically tag a neoplastic cell, thereby providing another unique cancer target. For example, early work demonstrated that sugar analogs can be equipped with unique chemical modifications that do not appear to affect incorporation into a cell’s surface carbohydrate repertoire but that can, owing to their unique engineered chemistry, be readily detected and therefore targeted to specifically engage a modified cell with an antibody- or chemical-based probe (59, 221–224). Incorporation of antineoplastic agents into these probes could offer another strategy to specifically target neoplastic lesions that may preferentially incorporate a particular carbohydrate analog (59, 224).
Whereas specifically targeting neoplasia-associated changes in glycosylation provides an attractive opportunity for novel therapeutic approaches, the expression of altered glycosylation also offers an opportunity to inhibit fundamental biological processes that neoplastic cells rely on for progression. For example, as altered expression of cell surface glycans may directly facilitate neoplastic cell spreading and eventual metastasis, inhibition of selectin- or galectin-mediated cancer cell extravasation may also inhibit the pathological sequelae of neoplastic disease by directly blocking the functional consequences of altered glycosylation (20, 185, 225, 226). Similarly, as overexpression or altered expression of GBPs, such as galectins, may directly promote the development of an immunoprivileged environment or facilitate nutrient delivery by favorably altering vascularization, inhibiting these GBPs may also inhibit neoplastic disease progression through critical glycosylation-dependent pathways required for overall neoplastic cell survival (20, 185, 205). As a result, cancer glycans and the proteins that recognize them may not only serve as unique targets for direct toxic compounds designed to specifically eliminate neoplastic lesions; they may also enable the manipulation of key biological pathways critical for the progression of neoplastic disease.
CONCLUSIONS
Modern studies have now shown that glycoproteins are found on all animal cells and that their glycan structures are commonly altered upon cellular transformation. Such changes in glycosylation provide new directions for understanding the molecular nature of cancer and cellular transformation and offer new opportunities for identifying biomarkers of disease and developing interventional strategies for treatment. Because glycans are abundant on the surface of tumor cells, and because specific glycan structures, such as the Tn and STn antigens, may be uniquely expressed on many types of tumors, these may serve as novel targets for diagnosing disease, characterizing prognosis, and delivering drugs. Increasingly, biochemical, molecular, and genetic studies are showing that alterations in protein glycosylation can be a major contributor to the transformation process and that alterations in the glycocalyx of cells can change the tumor microenvironment and metastatic process with significant effects on the progression of neoplastic disease. Future studies will undoubtedly uncover previously unrecognized roles for glycosylation in the pathogenesis of neoplasia and provide important and novel targets in the treatment of this formidable disease.
SOME MAJOR FACTORS THAT AFFECT PROTEIN GLYCOSYLATION IN TUMOR CELLS.
-
■
Levels of expression of specific glycosyltransferases
-
■
Localization of glycosyltransferases in the secretory compartments and other cellular compartments, such as the nucleus and mitochondria
-
■
Expression of specific molecular chaperones that regulate protein folding and quality control of glycoproteins and glycosyltransferases
-
■
Site- and protein-specific nature of glycosyltransferases
-
■
Levels of expression of specific glycosidases in the processing pathway
-
■
Levels of expression of lysosomal hydrolases in lysosomes and cellular secretions
-
■
Availability of protein substrates
-
■
Availability and levels of nucleotide sugars and 3′-phosphoadenosine-5′-phosphosulfate (PAPS)
-
■
Activity of nucleotide sugar and monosaccharide transporters
-
■
Glycoprotein turnover kinetics
-
■
pH of the ER and Golgi
-
■
Competition reactions between glycosyltransferases for similar glycan acceptors
SUMMARY POINTS.
The membranes and secretions of animal and human cells contain glycoproteins, including membrane receptors, transporters, hormones, and signaling molecules, that incorporate an assortment of carbohydrate structures linked to specific amino acids.
Common linkages to membrane and secreted glycoproteins include Asn-linked oligosac-charides (N-glycans) and Ser/Thr-linked oligosaccharides (O-glycans), and changes in these glycans are commonly found in tumor cells.
Changes in glycosylation arise from altered expression of glycosyltransferases, enzymes that modify glycans in the ER and Golgi apparatus during their biosynthesis.
Alterations in protein glycosylation can perturb the structure and function of glycoproteins by changing their oligomerization, turnover, conformation, and interactions with other molecules.
Changes in protein glycosylation can contribute directly to tumor progression and metastasis.
Altered glycan structures can alter the adhesion of tumor cells and their interactions with the microenvironment.
Novel glycan structures on tumor cells, such as the Tn and STn antigens, represent new targets for the diagnosis and treatment of neoplastic disease.
Much mechanistic work remains to be done to define the specific roles of protein glycans as drivers or passengers in neoplastic transformation.
ACKNOWLEDGMENTS
This work was supported in part by the National Blood Foundation, Hemophilia of Georgia, the Burroughs Wellcome Trust Career Award for Medical Scientists, and the National Institutes of Health (NIH) grant DP5OD019892 to S.R.S.; NIH grants P41GM103694, R24GM 098791, and P01HL085607 to R.D.C.; and NIH grant U01CA168930 to T.J. and R.D.C.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Sean R. Stowell, Email: srstowe@emory.edu.
Richard D. Cummings, Email: rdcummi@emory.edu.
LITERATURE CITED
- 1.Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643–649. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- 2.Fuster MM, Esko JD. The sweet and sour of cancer: glycans as novel therapeutic targets. Nat. Rev. Cancer. 2005;5:526–542. doi: 10.1038/nrc1649. [DOI] [PubMed] [Google Scholar]
- 3.Jones PA, Laird PW. Cancer epigenetics comes of age. Nat. Genet. 1999;21:163–167. doi: 10.1038/5947. [DOI] [PubMed] [Google Scholar]
- 4.Cummings RD, Pierce JM. The challenge and promise of glycomics. Chem. Biol. 2014;21:1–15. doi: 10.1016/j.chembiol.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunter T. Why nature chose phosphate to modify proteins. Philos. Trans. R. Soc. B. 2012;367:2513–2516. doi: 10.1098/rstb.2012.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bucala R, Cerami A. Advanced glycosylation: chemistry, biology, and implications for diabetes and aging. Adv. Pharmacol. 1992;23:1–34. doi: 10.1016/s1054-3589(08)60961-8. [DOI] [PubMed] [Google Scholar]
- 7.Wautier JL, Schmidt AM. Protein glycation: a firm link to endothelial cell dysfunction. Circ. Res. 2004;95:233–238. doi: 10.1161/01.RES.0000137876.28454.64. [DOI] [PubMed] [Google Scholar]
- 8.Marth JD, Grewal PK. Mammalian glycosylation in immunity. Nat. Rev. Immunol. 2008;8:874–887. doi: 10.1038/nri2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moremen KW, Tiemeyer M, Nairn AV. Vertebrate protein glycosylation: diversity, synthesis and function. Nat. Rev. Mol. Cell Biol. 2012;13:448–462. doi: 10.1038/nrm3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorelik E, Galili U, Raz A. On the role of cell surface carbohydrates and their binding proteins (lectins) in tumor metastasis. Cancer Metastasis Rev. 2001;20:245–277. doi: 10.1023/a:1015535427597. [DOI] [PubMed] [Google Scholar]
- 11.Schwarz HP, Dorner F. Karl Landsteiner and his major contributions to haematology. Br. J. Haematol. 2003;121:556–565. doi: 10.1046/j.1365-2141.2003.04295.x. [DOI] [PubMed] [Google Scholar]
- 12.Stowell SR, Winkler AM, Maier CL, Arthur CM, Smith NH, et al. Initiation and regulation of complement during hemolytic transfusion reactions. Clin. Dev. Immunol. 2012;2012:307093. doi: 10.1155/2012/307093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morgan WT, Watkins WM. The inhibition of the haemagglutinins in plant seeds by human blood group substances and simple sugars. Br. J. Exp. Pathol. 1953;34:94–103. [PMC free article] [PubMed] [Google Scholar]
- 14.Cummings RD. The repertoire of glycan determinants in the human glycome. Mol. Biosyst. 2009;5:1087–1104. doi: 10.1039/b907931a. [DOI] [PubMed] [Google Scholar]
- 15.Freeze HH, Aebi M. Altered glycan structures: the molecular basis of congenital disorders of glycosylation. Curr. Opin. Struct. Biol. 2005;15:490–498. doi: 10.1016/j.sbi.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Neufeld EF. Lysosomal storage diseases. Annu. Rev. Biochem. 1991;60:257–280. doi: 10.1146/annurev.bi.60.070191.001353. [DOI] [PubMed] [Google Scholar]
- 17.Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855–867. doi: 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 18.de Waard A, Hickman S, Kornfeld S. Isolation and properties of β-galactoside binding lectins of calf heart and lung. J. Biol. Chem. 1976;251:7581–7587. [PubMed] [Google Scholar]
- 19.Raman R, Raguram S, Venkataraman G, Paulson JC, Sasisekharan R. Glycomics: an integrated systems approach to structure-function relationships of glycans. Nat. Methods. 2005;2:817–824. doi: 10.1038/nmeth807. [DOI] [PubMed] [Google Scholar]
- 20.Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat. Rev. Cancer. 2005;5:29–41. doi: 10.1038/nrc1527. [DOI] [PubMed] [Google Scholar]
- 21.Arthur CM, Baruffi MD, Cummings RD, Stowell SR. Evolving mechanistic insights into galectin functions. Methods Mol. Biol. 2015;1207:1–35. doi: 10.1007/978-1-4939-1396-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boersema PJ, Geiger T, Wisniewski JR, Mann M. Quantification of the N-glycosylated secretome by super-SILAC during breast cancer progression and in human blood samples. Mol. Cell. Proteomics. 2013;12:158–171. doi: 10.1074/mcp.M112.023614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slade PG, Hajivandi M, Bartel CM, Gorfien SF. Identifying the CHO secretome using mucin-type O-linked glycosylation and click-chemistry. J. Proteome Res. 2012;11:6175–6186. doi: 10.1021/pr300810f. [DOI] [PubMed] [Google Scholar]
- 24.Steentoft C, Vakhrushev SY, Joshi HJ, Kong Y, Vester-Christensen MB, et al. Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. EMBO J. 2013;32:1478–1488. doi: 10.1038/emboj.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanisch FG, Breloy I. Protein-specific glycosylation: signal patches and cis-controlling peptidic elements. Biol. Chem. 2009;390:619–626. doi: 10.1515/BC.2009.043. [DOI] [PubMed] [Google Scholar]
- 26.Finne J, Finne U, Deagostini-Bazin H, Goridis C. Occurrence of α2–8 linked polysialosyl units in a neural cell adhesion molecule. Biochem. Biophys. Res. Commun. 1983;112:482–487. doi: 10.1016/0006-291x(83)91490-0. [DOI] [PubMed] [Google Scholar]
- 27.Muhlenhoff M, Rollenhagen M, Werneburg S, Gerardy-Schahn R, Hildebrandt H. Polysialic acid: versatile modification of NCAM, SynCAM 1 and neuropilin-2. Neurochem. Res. 2013;38:1134–1143. doi: 10.1007/s11064-013-0979-2. [DOI] [PubMed] [Google Scholar]
- 28.Foley DA, Swartzentruber KG, Lavie A, Colley KJ. Structure and mutagenesis of neural cell adhesion molecule domains: evidence for flexibility in the placement of polysialic acid attachment sites. J. Biol. Chem. 2010;285:27360–27371. doi: 10.1074/jbc.M110.140038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rollenhagen M, Buettner FF, Reismann M, Jirmo AC, Grove M, et al. Polysialic acid on neuropilin-2 is exclusively synthesized by the polysialyltransferase ST8SiaIV and attached to mucin-type O-glycans located between the b2 and c domain. J. Biol. Chem. 2013;288:22880–22892. doi: 10.1074/jbc.M113.463927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Endo T. Structure, function and pathology of O-mannosyl glycans. Glycoconj. J. 2004;21:3–7. doi: 10.1023/B:GLYC.0000043740.26062.2c. [DOI] [PubMed] [Google Scholar]
- 31.Yoshida-Moriguchi T, Willer T, Anderson ME, Venzke D, Whyte T, et al. SGK196 is a glycosylation-specific O-mannose kinase required for dystroglycan function. Science. 2013;341:896–899. doi: 10.1126/science.1239951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inamori K, Yoshida-Moriguchi T, Hara Y, Anderson ME, Yu L, Campbell KP. Dystroglycan function requires xylosyl- and glucuronyltransferase activities of LARGE. Science. 2012;335:93–96. doi: 10.1126/science.1214115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kornfeld S, Mellman I. The biogenesis of lysosomes. Annu. Rev. Cell Biol. 1989;5:483–525. doi: 10.1146/annurev.cb.05.110189.002411. [DOI] [PubMed] [Google Scholar]
- 34.Dell A, Galadari A, Sastre F, Hitchen P. Similarities and differences in the glycosylation mechanisms in prokaryotes and eukaryotes. Int. J. Microbiol. 2010;2010:148178. doi: 10.1155/2010/148178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bourdon MA, Krusius T, Campbell S, Schwartz NB, Ruoslahti E. Identification and synthesis of a recognition signal for the attachment of glycosaminoglycans to proteins. PNAS. 1987;84:3194–3198. doi: 10.1073/pnas.84.10.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doucey MA, Hess D, Cacan R, Hofsteenge J. Protein C-mannosylation is enzyme-catalysed and uses dolichyl-phosphate-mannose as a precursor. Mol. Biol. Cell. 1998;9:291–300. doi: 10.1091/mbc.9.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buettner FF, Ashikov A, Tiemann B, Lehle L, Bakker H. C. elegans DPY-19 is a C-mannosyltransferase glycosylating thrombospondin repeats. Mol. Cell. 2013;50:295–302. doi: 10.1016/j.molcel.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Puig B, Altmeppen H, Glatzel M. The GPI-anchoring of PrP: implications in sorting and pathogenesis. Prion. 2014;8:11–18. doi: 10.4161/pri.27892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsai YH, Liu X, Seeberger PH. Chemical biology of glycosylphosphatidylinositol anchors. Angew. Chem. Int. Ed. Engl. 2012;51:11438–11456. doi: 10.1002/anie.201203912. [DOI] [PubMed] [Google Scholar]
- 40.Orlean P, Menon AK. Thematic review series: lipid posttranslational modifications. GPI anchoring of protein in yeast and mammalian cells, or: how we learned to stop worrying and love glycophospholipids. J. Lipid Res. 2007;48:993–1011. doi: 10.1194/jlr.R700002-JLR200. [DOI] [PubMed] [Google Scholar]
- 41.Lommel M, Strahl S. Protein O-mannosylation: conserved from bacteria to humans. Glycobiology. 2009;19:816–828. doi: 10.1093/glycob/cwp066. [DOI] [PubMed] [Google Scholar]
- 42.Stanley P, Okajima T. Roles of glycosylation in Notch signaling. Curr. Top. Dev. Biol. 2010;92:131–164. doi: 10.1016/S0070-2153(10)92004-8. [DOI] [PubMed] [Google Scholar]
- 43.Leonhard-Melief C, Haltiwanger RS. O-Fucosylation of thrombospondin type 1 repeats. Methods Enzymol. 2010;480:401–416. doi: 10.1016/S0076-6879(10)80018-7. [DOI] [PubMed] [Google Scholar]
- 44.Takeuchi H, Haltiwanger RS. Enzymatic analysis of the protein O-glycosyltransferase, Rumi, acting toward epidermal growth factor-like (EGF) repeats. Methods Mol. Biol. 2013;1022:119–128. doi: 10.1007/978-1-62703-465-4_10. [DOI] [PubMed] [Google Scholar]
- 45.Song E, Mechref Y. LC-MS/MS identification of the O-glycosylation and hydroxylation of amino acid residues of collagen α-1 (II) chain from bovine cartilage. J. Proteome Res. 2013;12:3599–3609. doi: 10.1021/pr400101t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spiro RG. Characterization and quantitative determination of the hydroxylysine-linked carbohydrate units of several collagens. J. Biol. Chem. 1969;244:602–612. [PubMed] [Google Scholar]
- 47.Schegg B, Hulsmeier AJ, Rutschmann C, Maag C, Hennet T. Core glycosylation of collagen is initiated by two β(1-O)galactosyltransferases. Mol. Cell. Biol. 2009;29:943–952. doi: 10.1128/MCB.02085-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O. Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease. Annu. Rev. Biochem. 2011;80:825–858. doi: 10.1146/annurev-biochem-060608-102511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bond MR, Hanover JA. O-GlcNAc cycling: a link between metabolism and chronic disease. Annu. Rev. Nutr. 2013;33:205–229. doi: 10.1146/annurev-nutr-071812-161240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Love DC, Kochan J, Cathey RL, Shin SH, Hanover JA. Mitochondrial and nucleocytoplasmic targeting of O-linked GlcNAc transferase. J. Cell Sci. 2003;116:647–654. doi: 10.1242/jcs.00246. [DOI] [PubMed] [Google Scholar]
- 51.Cao W, Cao J, Huang J, Yao J, Yan G, et al. Discovery and confirmation of O-GlcNAcylated proteins in rat liver mitochondria by combination of mass spectrometry and immunological methods. PLOS ONE. 2013;8:e76399. doi: 10.1371/journal.pone.0076399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sakaidani Y, Nomura T, Matsuura A, Ito M, Suzuki E, et al. O-Linked-N-acetylglucosamine on extracellular protein domains mediates epithelial cell-matrix interactions. Nat. Commun. 2011;2:583. doi: 10.1038/ncomms1591. [DOI] [PubMed] [Google Scholar]
- 53.Slawson C, Hart GW. O-GlcNAc signalling: implications for cancer cell biology. Nat. Rev. Cancer. 2011;11:678–684. doi: 10.1038/nrc3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lomako J, Lomako WM, Whelan WJ. A self-glucosylating protein is the primer for rabbit muscle glycogen biosynthesis. FASEB J. 1988;2:3097–3103. doi: 10.1096/fasebj.2.15.2973423. [DOI] [PubMed] [Google Scholar]
- 55.Roach PJ, Depaoli-Roach AA, Hurley TD, Tagliabracci VS. Glycogen and its metabolism: some new developments and old themes. Biochem. J. 2012;441:763–787. doi: 10.1042/BJ20111416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hauselmann I, Borsig L. Altered tumor-cell glycosylation promotes metastasis. Front. Oncol. 2014;4:28. doi: 10.3389/fonc.2014.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lau KS, Dennis JW. N-Glycans in cancer progression. Glycobiology. 2008;18:750–760. doi: 10.1093/glycob/cwn071. [DOI] [PubMed] [Google Scholar]
- 58.Christiansen MN, Chik J, Lee L, Anugraham M, Abrahams JL, Packer NH. Cell surface protein glycosylation in cancer. Proteomics. 2014;14:525–546. doi: 10.1002/pmic.201300387. [DOI] [PubMed] [Google Scholar]
- 59.Dube DH, Bertozzi CR. Glycans in cancer and inflammation—potential for therapeutics and diagnostics. Nat. Rev. Drug Discov. 2005;4:477–488. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- 60.Brooks SA, Carter TM, Royle L, Harvey DJ, Fry SA, et al. Altered glycosylation of proteins in cancer: What is the potential for new anti-tumour strategies. Anti-Cancer Agents Med. Chem. 2008;8:2–21. doi: 10.2174/187152008783330860. [DOI] [PubMed] [Google Scholar]
- 61.Song EY, Kang SK, Lee YC, Park YG, Chung TH, et al. Expression of bisecting N-acetylglucosaminyltransferase-III in human hepatocarcinoma tissues, fetal liver tissues, and hepatoma cell lines of Hep3B and HepG2. Cancer Investig. 2001;19:799–807. doi: 10.1081/cnv-100107741. [DOI] [PubMed] [Google Scholar]
- 62.Mori S, Aoyagi Y, Yanagi M, Suzuki Y, Asakura H. Serum N-acetylglucosaminyltransferase III activities in hepatocellular carcinoma. J. Gastroenterol. Hepatol. 1998;13:610–619. doi: 10.1111/j.1440-1746.1998.tb00699.x. [DOI] [PubMed] [Google Scholar]
- 63.Ogawa J, Inoue H, Koide S. Expression of α-1,3-fucosyltransferase type IV and VII genes is related to poor prognosis in lung cancer. Cancer Res. 1996;56:325–329. [PubMed] [Google Scholar]
- 64.Togayachi A, Kudo T, Ikehara Y, Iwasaki H, Nishihara S, et al. Up-regulation of Lewis enzyme (Fuc-TIII) and plasma-type α1,3fucosyltransferase (Fuc-TVI) expression determines the augmented expression of sialyl Lewis x antigen in non-small cell lung cancer. Int. J. Cancer. 1999;83:70–79. doi: 10.1002/(sici)1097-0215(19990924)83:1<70::aid-ijc14>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 65.Julien S, Ivetic A, Grigoriadis A, QiZe D, Burford B, et al. Selectin ligand sialyl-Lewis x antigen drives metastasis of hormone-dependent breast cancers. Cancer Res. 2011;71:7683–7693. doi: 10.1158/0008-5472.CAN-11-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bresalier RS, Ho SB, Schoeppner HL, Kim YS, Sleisenger MH, et al. Enhanced sialylation of mucin-associated carbohydrate structures in human colon cancer metastasis. Gastroenterology. 1996;110:1354–1367. doi: 10.1053/gast.1996.v110.pm8613039. [DOI] [PubMed] [Google Scholar]
- 67.Ishida H, Togayachi A, Sakai T, Iwai T, Hiruma T, et al. A novel β1,3-N-acetylglucosaminyltransferase (β3Gn-T8), which synthesizes poly-N-acetyllactosamine, is dramatically upregulated in colon cancer. FEBS Lett. 2005;579:71–78. doi: 10.1016/j.febslet.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 68.McManus JF. Histological and histochemical uses of periodic acid. Stain Technol. 1948;23:99–108. doi: 10.3109/10520294809106232. [DOI] [PubMed] [Google Scholar]
- 69.Hakomori S. Aberrant glycosylation in tumors and tumor-associated carbohydrate antigens. Adv. Cancer Res. 1989;52:257–331. doi: 10.1016/s0065-230x(08)60215-8. [DOI] [PubMed] [Google Scholar]
- 70.Warnock ML, Stoloff A, Thor A. Differentiation of adenocarcinoma of the lung from mesothelioma. Periodic acid-Schiff, monoclonal antibodies B72.3, and Leu M1. Am. J. Pathol. 1988;133:30–38. [PMC free article] [PubMed] [Google Scholar]
- 71.Bochner BS, Alvarez RA, Mehta P, Bovin NV, Blixt O, et al. Glycan array screening reveals a candidate ligand for Siglec-8. J. Biol. Chem. 2005;280:4307–4312. doi: 10.1074/jbc.M412378200. [DOI] [PubMed] [Google Scholar]
- 72.Arthur CM, Rodrigues LC, Baruffi MD, Sullivan HC, Heimburg-Molinaro J, et al. Examining galectin binding specificity using glycan microarrays. Methods Mol. Biol. 2015;1207:115–131. doi: 10.1007/978-1-4939-1396-1_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Song X, Xia B, Stowell SR, Lasanajak Y, Smith DF, Cummings RD. Novel fluorescent glycan microarray strategy reveals ligands for galectins. Chem. Biol. 2009;16:36–47. doi: 10.1016/j.chembiol.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arthur CM, Cummings RD, Stowell SR. Using glycan microarrays to understand immunity. Curr. Opin. Chem. Biol. 2014;18:55–61. doi: 10.1016/j.cbpa.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rillahan CD, Paulson JC. Glycan microarrays for decoding the glycome. Annu. Rev. Biochem. 2011;80:797–823. doi: 10.1146/annurev-biochem-061809-152236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Borgert A, Heimburg-Molinaro J, Song X, Lasanajak Y, Ju T, et al. Deciphering structural elements of mucin glycoprotein recognition. ACS Chem. Biol. 2012;7:1031–1039. doi: 10.1021/cb300076s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pedersen JW, Blixt O, Bennett EP, Tarp MA, Dar I, et al. Seromic profiling of colorectal cancer patients with novel glycopeptide microarray. Int. J. Cancer. 2011;128:1860–1871. doi: 10.1002/ijc.25778. [DOI] [PubMed] [Google Scholar]
- 78.Marcus DM, Perry L, Gilbert S, Preud’homme JL, Kyle R. Human IgM monoclonal proteins that bind 3-fucosyllactosamine, asialo-GM1, and GM1. J. Immunol. 1989;143:2929–2932. [PubMed] [Google Scholar]
- 79.Hong X, Ma MZ, Gildersleeve JC, Chowdhury S, Barchi JJ, Jr, et al. Sugar-binding proteins from fish: selection of high affinity “lambodies” that recognize biomedically relevant glycans. ACS Chem. Biol. 2013;8:152–160. doi: 10.1021/cb300399s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yu C, Ali S, St. Germain J, Liu Y, Yu X, et al. Purification and identification of cell surface antigens using lamprey monoclonal antibodies. J. Immunol. Methods. 2012;386:43–49. doi: 10.1016/j.jim.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Han BW, Herrin BR, Cooper MD, Wilson IA. Antigen recognition by variable lymphocyte receptors. Science. 2008;321:1834–1837. doi: 10.1126/science.1162484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Morelle W, Michalski JC. Analysis of protein glycosylation by mass spectrometry. Nat. Protoc. 2007;2:1585–1602. doi: 10.1038/nprot.2007.227. [DOI] [PubMed] [Google Scholar]
- 83.Itzkowitz SH, Yuan M, Montgomery CK, Kjeldsen T, Takahashi HK, et al. Expression of Tn, sialosyl-Tn, and T antigens in human colon cancer. Cancer Res. 1989;49:197–204. [PubMed] [Google Scholar]
- 84.Werther JL, Rivera-MacMurray S, Bruckner H, Tatematsu M, Itzkowitz SH. Mucin-associated sialosyl-Tn antigen expression in gastric cancer correlates with an adverse outcome. Br. J. Cancer. 1994;69:613–616. doi: 10.1038/bjc.1994.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yamada T, Watanabe A, Yamada Y, Shino Y, Tanase M, et al. Sialosyl Tn antigen expression is associated with the prognosis of patients with advanced gastric cancer. Cancer. 1995;76:1529–1536. doi: 10.1002/1097-0142(19951101)76:9<1529::aid-cncr2820760905>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 86.Coon JS, Weinstein RS, Summers JL. Blood group precursor T-antigen expression in human urinary bladder carcinoma. Am. J. Clin. Pathol. 1982;77:692–699. doi: 10.1093/ajcp/77.6.692. [DOI] [PubMed] [Google Scholar]
- 87.Nakayama T, Watanabe M, Katsumata T, Teramoto T, Kitajima M. Expression of sialyl Lewisa as a new prognostic factor for patients with advanced colorectal carcinoma. Cancer. 1995;75:2051–2056. doi: 10.1002/1097-0142(19950415)75:8<2051::aid-cncr2820750804>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 88.Nakamori S, Kameyama M, Imaoka S, Furukawa H, Ishikawa O, et al. Increased expression of sialyl Lewisx antigen correlates with poor survival in patients with colorectal carcinoma: clinicopathological and immunohistochemical study. Cancer Res. 1993;53:3632–3637. [PubMed] [Google Scholar]
- 89.Ogawa J, Sano A, Inoue H, Koide S. Expression of Lewis-related antigen and prognosis in stage I non–small cell lung cancer. Ann. Thorac. Surg. 1995;59:412–415. doi: 10.1016/0003-4975(94)00866-6. [DOI] [PubMed] [Google Scholar]
- 90.Nakagoe T, Fukushima K, Nanashima A, Sawai T, Tsuji T, et al. Comparison of the expression of ABH/Lewis-related antigens in polypoid and non-polypoid growth types of colorectal carcinoma. J. Gastroenterol. Hepatol. 2001;16:176–183. doi: 10.1046/j.1440-1746.2001.02425.x. [DOI] [PubMed] [Google Scholar]
- 91.Jorgensen T, Berner A, Kaalhus O, Tveter KJ, Danielsen HE, Bryne M. Up-regulation of the oligosaccharide sialyl LewisX: a new prognostic parameter in metastatic prostate cancer. Cancer Res. 1995;55:1817–1819. [PubMed] [Google Scholar]
- 92.Yago K, Zenita K, Ginya H, Sawada M, Ohmori K, et al. Expression of α-(1,3)-fucosyltransferases which synthesize sialyl Lex and sialyl Lea, the carbohydrate ligands for E- and P-selectins, in human malignant cell lines. Cancer Res. 1993;53:5559–5565. [PubMed] [Google Scholar]
- 93.Walz G, Aruffo A, Kolanus W, Bevilacqua M, Seed B. Recognition by ELAM-1 of the sialyl-Lex determinant on myeloid and tumor cells. Science. 1990;250:1132–1135. doi: 10.1126/science.1701275. [DOI] [PubMed] [Google Scholar]
- 94.Phillips ML, Nudelman E, Gaeta FC, Perez M, Singhal AK, et al. ELAM-1 mediates cell adhesion by recognition of a carbohydrate ligand, sialyl-Lex . Science. 1990;250:1130–1132. doi: 10.1126/science.1701274. [DOI] [PubMed] [Google Scholar]
- 95.Lowe JB, Stoolman LM, Nair RP, Larsen RD, Berhend TL, Marks RM. ELAM-1-dependent cell adhesion to vascular endothelium determined by a transfected human fucosyltransferase cDNA. Cell. 1990;63:475–484. doi: 10.1016/0092-8674(90)90444-j. [DOI] [PubMed] [Google Scholar]
- 96.Barthel SR, Wiese GK, Cho J, Opperman MJ, Hays DL, et al. Alpha 1,3 fucosyltransferases are master regulators of prostate cancer cell trafficking. PNAS. 2009;106:19491–19496. doi: 10.1073/pnas.0906074106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Borsig L, Wong R, Hynes RO, Varki NM, Varki A. Synergistic effects of L- and P-selectin in facilitating tumor metastasis can involve non-mucin ligands and implicate leukocytes as enhancers of metastasis. PNAS. 2002;99:2193–2198. doi: 10.1073/pnas.261704098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim YJ, Borsig L, Varki NM, Varki A. P-selectin deficiency attenuates tumor growth and metastasis. PNAS. 1998;95:9325–9330. doi: 10.1073/pnas.95.16.9325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee JS, Ro JY, Sahin AA, Hong WK, Brown BW, et al. Expression of blood-group antigen A—a favorable prognostic factor in non-small-cell lung cancer. N. Engl. J. Med. 1991;324:1084–1090. doi: 10.1056/NEJM199104183241603. [DOI] [PubMed] [Google Scholar]
- 100.Biondi C, Campi C, Escovich L, Garcia Borras S, Racca A, Cotorruelo C. Loss of A, B and H antigens in oral cancer. Immunologia. 2008;27:127–131. [Google Scholar]
- 101.Newman AJ, Jr, Carlton CE, Jr, Johnson S. Cell surface A, B, or O(H) blood group antigens as an indicator of malignant potential in stage A bladder carcinoma. J. Urol. 1980;124:27–29. doi: 10.1016/s0022-5347(17)55275-1. [DOI] [PubMed] [Google Scholar]
- 102.Ju T, Aryal RP, Kudelka MR, Wang Y, Cummings RD. The Cosmc connection to the Tn antigen in cancer. Cancer Biomark. 2014;14:63–81. doi: 10.3233/CBM-130375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Peracaula R, Barrabes S, Sarrats A, Rudd PM, de Llorens R. Altered glycosylation in tumours focused to cancer diagnosis. Dis. Markers. 2008;25:207–218. doi: 10.1155/2008/797629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur. J. Surg. Oncol. 2007;33:266–270. doi: 10.1016/j.ejso.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 105.Koprowski H, Steplewski Z, Mitchell K, Herlyn M, Herlyn D, Fuhrer P. Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet. 1979;5:957–971. doi: 10.1007/BF01542654. [DOI] [PubMed] [Google Scholar]
- 106.Ballehaninna UK, Chamberlain RS. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: an evidence based appraisal. J. Gastrointest. Oncol. 2012;3:105–119. doi: 10.3978/j.issn.2078-6891.2011.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bast RC, Jr, Feeney M, Lazarus H, Nadler LM, Colvin RB, Knapp RC. Reactivity of a monoclonal antibody with human ovarian carcinoma. J. Clin. Investig. 1981;68:1331–1337. doi: 10.1172/JCI110380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yin BW, Lloyd KO. Molecular cloning of the CA125 ovarian cancer antigen: identification as a new mucin, MUC16. J. Biol. Chem. 2001;276:27371–27375. doi: 10.1074/jbc.M103554200. [DOI] [PubMed] [Google Scholar]
- 109.Su Z, Graybill WS, Zhu Y. Detection and monitoring of ovarian cancer. Clin. Chim. Acta. 2013;415:341–345. doi: 10.1016/j.cca.2012.10.058. [DOI] [PubMed] [Google Scholar]
- 110.Hammarstrom S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin. Cancer Biol. 1999;9:67–81. doi: 10.1006/scbi.1998.0119. [DOI] [PubMed] [Google Scholar]
- 111.Duffy MJ. Carcinoembryonic antigen as a marker for colorectal cancer: Is it clinically useful? Clin. Chem. 2001;47:624–630. [PubMed] [Google Scholar]
- 112.Ward AM, Catto JW, Hamdy FC. Prostate specific antigen: biology, biochemistry and available commercial assays. Ann. Clin. Biochem. 2001;38:633–651. doi: 10.1258/0004563011901055. [DOI] [PubMed] [Google Scholar]
- 113.Lilja H, Ulmert D, Vickers AJ. Prostate-specific antigen and prostate cancer: prediction, detection and monitoring. Nat. Rev. Cancer. 2008;8:268–278. doi: 10.1038/nrc2351. [DOI] [PubMed] [Google Scholar]
- 114.Gilgunn S, Conroy PJ, Saldova R, Rudd PM, O’Kennedy RJ. Aberrant PSA glycosylation—a sweet predictor of prostate cancer. Nat. Rev. Urol. 2013;10:99–107. doi: 10.1038/nrurol.2012.258. [DOI] [PubMed] [Google Scholar]
- 115.Terentiev AA, Moldogazieva NT. Alpha-fetoprotein: a renaissance. Tumour Biol. 2013;34:2075–2091. doi: 10.1007/s13277-013-0904-y. [DOI] [PubMed] [Google Scholar]
- 116.Moriya S, Morimoto M, Numata K, Nozaki A, Shimoyama Y, et al. Fucosylated fraction of alpha-fetoprotein as a serological marker of early hepatocellular carcinoma. Anticancer Res. 2013;33:997–1001. [PubMed] [Google Scholar]
- 117.Hiraoka A, Nakahara H, Kawasaki H, Shimizu Y, Hidaka S, et al. Huge pancreatic acinar cell carcinoma with high levels of AFP and fucosylated AFP (AFP-L3) Intern. Med. 2012;51:1341–1349. doi: 10.2169/internalmedicine.51.6536. [DOI] [PubMed] [Google Scholar]
- 118.Contessa JN, Bhojani MS, Freeze HH, Rehemtulla A, Lawrence TS. Inhibition of N-linked glycosylation disrupts receptor tyrosine kinase signaling in tumor cells. Cancer Res. 2008;68:3803–3809. doi: 10.1158/0008-5472.CAN-07-6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kumler I, Tuxen MK, Nielsen DL. A systematic review of dual targeting in HER2-positive breast cancer. Cancer Treat. Rev. 2014;40:259–270. doi: 10.1016/j.ctrv.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 120.Cole LA, Butler SA. Hyperglycosylated human chorionic gonadotropin and human chorionic gonadotropin free β-subunit: tumor markers and tumor promoters. J. Reprod. Med. 2008;53:499–512. [PubMed] [Google Scholar]
- 121.Gendler SJ. MUC1, the renaissance molecule. J. Mamm. Gland Biol. Neoplasia. 2001;6:339–353. doi: 10.1023/a:1011379725811. [DOI] [PubMed] [Google Scholar]
- 122.Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat. Rev. Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 123.Ju T, Otto VI, Cummings RD. The Tn antigen—structural simplicity and biological complexity. Angew. Chem. Int. Ed. 2011;50:1770–1791. doi: 10.1002/anie.201002313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ju T, Wang Y, Aryal RP, Lehoux SD, Ding X, et al. Tn and sialyl-Tn antigens, aberrant O-glycomics as human disease markers. Proteomics Clin. Appl. 2013;7:618–631. doi: 10.1002/prca.201300024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.O’Boyle KP, Markowitz AL, Khorshidi M, Lalezari P, Longenecker BM, et al. Specificity analysis of murine monoclonal antibodies reactive with Tn, sialylated Tn, T, and monosialylated (2→6) T antigens. Hybridoma. 1996;15:401–408. doi: 10.1089/hyb.1996.15.401. [DOI] [PubMed] [Google Scholar]
- 126.Hanisch FG, Uhlenbruck G, Egge H, Peter-Katalinic J. A B72.3 second-generation-monoclonal antibody (CC49) defines the mucin-carried carbohydrate epitope Galβ(1–3) [NeuAcα(2–6)]GalNAc. Biol. Chem. 1989;370:21–26. doi: 10.1515/bchm3.1989.370.1.21. [DOI] [PubMed] [Google Scholar]
- 127.Fang L, Holford NH, Hinkle G, Cao X, Xiao JJ, et al. Population pharmacokinetics of humanized monoclonal antibody HuCC49ΔCH2 and murine antibody CC49 in colorectal cancer patients. J. Clin. Pharmacol. 2007;47:227–237. doi: 10.1177/0091270006293758. [DOI] [PubMed] [Google Scholar]
- 128.Zhao P, Nairn AV, Hester S, Moremen KW, O’Regan RM, et al. Proteomic identification of glycosylphosphatidylinositol anchor-dependent membrane proteins elevated in breast carcinoma. J. Biol. Chem. 2012;287:25230–25240. doi: 10.1074/jbc.M112.339465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dolezal S, Hester S, Kirby PS, Nairn A, Pierce M, Abbott KL. Elevated levels of glycosylphosphatidylinositol (GPI) anchored proteins in plasma from human cancers detected by C. septicum alpha toxin. Cancer Biomark. 2014;14:55–62. doi: 10.3233/CBM-130377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Varki A. Colloquium paper: uniquely human evolution of sialic acid genetics and biology. PNAS. 2010;107(Suppl. 2):8939–8946. doi: 10.1073/pnas.0914634107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Padler-Karavani V, Hurtado-Ziola N, Pu M, Yu H, Huang S, et al. Human xeno-autoantibodies against a non-human sialic acid serve as novel serum biomarkers and immunotherapeutics in cancer. Cancer Res. 2011;71:3352–3363. doi: 10.1158/0008-5472.CAN-10-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ju T, Lanneau GS, Gautam T, Wang Y, Xia B, et al. Human tumor antigens Tn and sialyl Tn arise from mutations in Cosmc . Cancer Res. 2008;68:1636–1646. doi: 10.1158/0008-5472.CAN-07-2345. [DOI] [PubMed] [Google Scholar]
- 133.Guda K, Moinova H, He J, Jamison O, Ravi L, et al. Inactivating germ-line and somatic mutations in polypeptide N-acetylgalactosaminyltransferase 12 in human colon cancers. PNAS. 2009;106:12921–12925. doi: 10.1073/pnas.0901454106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chen L, Zhang W, Fregien N, Pierce M. The her-2/neu oncogene stimulates the transcription of N-acetylglucosaminyltransferase V and expression of its cell surface oligosaccharide products. Oncogene. 1998;17:2087–2093. doi: 10.1038/sj.onc.1202124. [DOI] [PubMed] [Google Scholar]
- 135.Noda K, Miyoshi E, Uozumi N, Yanagidani S, Ikeda Y, et al. Gene expression of α1–6 fucosyltransferase in human hepatoma tissues: a possible implication for increased fucosylation of α-fetoprotein. Hepatology. 1998;28:944–952. doi: 10.1002/hep.510280408. [DOI] [PubMed] [Google Scholar]
- 136.Park JH, Nishidate T, Kijima K, Ohashi T, Takegawa K, et al. Critical roles of mucin 1 glycosylation by transactivated polypeptide N-acetylgalactosaminyltransferase 6 in mammary carcinogenesis. Cancer Res. 2010;70:2759–2769. doi: 10.1158/0008-5472.CAN-09-3911. [DOI] [PubMed] [Google Scholar]
- 137.Gomes J, Marcos NT, Berois N, Osinaga E, Magalhaes A, et al. Expression of UDP-N-acetyl-D-galactosamine: polypeptide N-acetylgalactosaminyltransferase-6 in gastric mucosa, intestinal metaplasia, and gastric carcinoma. J. Histochem. Cytochem. 2009;57:79–86. doi: 10.1369/jhc.2008.952283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wagner KW, Punnoose EA, Januario T, Lawrence DA, Pitti RM, et al. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat. Med. 2007;13:1070–1077. doi: 10.1038/nm1627. [DOI] [PubMed] [Google Scholar]
- 139.Sewell R, Backstrom M, Dalziel M, Gschmeissner S, Karlsson H, et al. The ST6GalNAc-I sialyltransferase localizes throughout the Golgi and is responsible for the synthesis of the tumor-associated sialyl-Tn O-glycan in human breast cancer. J. Biol. Chem. 2006;281:3586–3594. doi: 10.1074/jbc.M511826200. [DOI] [PubMed] [Google Scholar]
- 140.Radhakrishnan P, Dabelsteen S, Madsen FB, Francavilla C, Kopp KL, et al. Immature truncated O-glycophenotype of cancer directly induces oncogenic features. PNAS. 2014;111:E4066–E4075. doi: 10.1073/pnas.1406619111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mi R, Song L, Wang Y, Ding X, Zeng J, et al. Epigenetic silencing of the chaperone Cosmc in human leukocytes expressing Tn antigen. J. Biol. Chem. 2012;287:41523–41533. doi: 10.1074/jbc.M112.371989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Huang J, Che MI, Lin NY, Hung JS, Huang YT, et al. The molecular chaperone Cosmc enhances malignant behaviors of colon cancer cells via activation of Akt and ERK. Mol. Carcinog. 2014;53(Suppl. 1):E62–E71. doi: 10.1002/mc.22011. [DOI] [PubMed] [Google Scholar]
- 143.Hung JS, Huang J, Lin YC, Huang MJ, Lee PH, et al. C1GALT1 overexpression promotes the invasive behavior of colon cancer cells through modifying O-glycosylation of FGFR2. Oncotarget. 2014;5:2096–2106. doi: 10.18632/oncotarget.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wu YM, Liu CH, Huang MJ, Lai HS, Lee PH, et al. C1GALT1 enhances proliferation of hepatocellular carcinoma cells via modulating MET glycosylation and dimerization. Cancer Res. 2013;73:5580–5590. doi: 10.1158/0008-5472.CAN-13-0869. [DOI] [PubMed] [Google Scholar]
- 145.Liu CH, Hu RH, Huang MJ, Lai IR, Chen CH, et al. C1GALT1 promotes invasive phenotypes of hepatocellular carcinoma cells by modulating integrin β1 glycosylation and activity. PLOS ONE. 2014;9:e94995. doi: 10.1371/journal.pone.0094995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hakomori S. Antigen structure and genetic basis of histo-blood groups A, B and O: their changes associated with human cancer. Biochim. Biophys. Acta. 1999;1473:247–266. doi: 10.1016/s0304-4165(99)00183-x. [DOI] [PubMed] [Google Scholar]
- 147.Dabelsteen E, Gao S. ABO blood-group antigens in oral cancer. J. Dent. Res. 2005;84:21–28. doi: 10.1177/154405910508400103. [DOI] [PubMed] [Google Scholar]
- 148.Iwai T, Kudo T, Kawamoto R, Kubota T, Togayachi A, et al. Core 3 synthase is down-regulated in colon carcinoma and profoundly suppresses the metastatic potential of carcinoma cells. PNAS. 2005;102:4572–4577. doi: 10.1073/pnas.0407983102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Malagolini N, Dall’Olio F, Di Stefano G, Minni F, Marrano D, Serafini-Cessi F. Expression of UDP-GalNAc:NeuAcα2,3Galβ-R β1,4(GalNAc to Gal) N-acetylgalactosaminyltransferase involved in the synthesis of Sda antigen in human large intestine and colorectal carcinomas. Cancer Res. 1989;49:6466–6470. [PubMed] [Google Scholar]
- 150.Karasawa F, Shiota A, Goso Y, Kobayashi M, Sato Y, et al. Essential role of gastric gland mucin in preventing gastric cancer in mice. J. Clin. Investig. 2012;122:923–934. doi: 10.1172/JCI59087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tu L, Banfield DK. Localization of Golgi-resident glycosyltransferases. Cell. Mol. Life Sci. 2010;67:29–41. doi: 10.1007/s00018-009-0126-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gill DJ, Chia J, Senewiratne J, Bard F. Regulation of O-glycosylation through Golgi-to-ER relocation of initiation enzymes. J. Cell Biol. 2010;189:843–858. doi: 10.1083/jcb.201003055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, et al. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene. 2006;366:2–16. doi: 10.1016/j.gene.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 154.Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J. Clin. Investig. 1999;104:155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Shimodaira K, Nakayama J, Nakamura N, Hasebe O, Katsuyama T, Fukuda M. Carcinoma-associated expression of core 2 β-1,6-N-acetylglucosaminyltransferase gene in human colorectal cancer: role of O-glycans in tumor progression. Cancer Res. 1997;57:5201–5206. [PubMed] [Google Scholar]
- 156.Julien S, Lagadec C, Krzewinski-Recchi MA, Courtand G, Le Bourhis X, Delannoy P. Stable expression of sialyl-Tn antigen in T47-D cells induces a decrease of cell adhesion and an increase of cell migration. Breast Cancer Res. Treat. 2005;90:77–84. doi: 10.1007/s10549-004-3137-3. [DOI] [PubMed] [Google Scholar]
- 157.Wang Y, Jobe SM, Ding X, Choo H, Archer DR, et al. Platelet biogenesis and functions require correct protein O-glycosylation. PNAS. 2012;109:16143–16148. doi: 10.1073/pnas.1208253109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lu Y, Chaney W. Induction of N-acetylglucosaminyltransferase V by elevated expression of activated or proto-Ha-ras oncogenes. Mol. Cell. Biochem. 1993;122:85–92. doi: 10.1007/BF00925741. [DOI] [PubMed] [Google Scholar]
- 159.Dennis JW, Kosh K, Bryce DM, Breitman ML. Oncogenes conferring metastatic potential induce increased branching of Asn-linked oligosaccharides in rat2 fibroblasts. Oncogene. 1989;4:853–860. [PubMed] [Google Scholar]
- 160.Demetriou M, Nabi IR, Coppolino M, Dedhar S, Dennis JW. Reduced contact-inhibition and substratum adhesion in epithelial cells expressing GlcNAc-transferase V. J. Cell Biol. 1995;130:383–392. doi: 10.1083/jcb.130.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yamamoto H, Swoger J, Greene S, Saito T, Hurh J, et al. β1,6-N-Acetylglucosamine-bearing N-glycans in human gliomas: implications for a role in regulating invasivity. Cancer Res. 2000;60:134–142. [PubMed] [Google Scholar]
- 162.Kim YS, Ahn YH, Song KJ, Kang JG, Lee JH, et al. Overexpression and β-1,6-N-acetylglucosaminylation-initiated aberrant glycosylation of TIMP-1: a “double whammy” strategy in colon cancer progression. J. Biol. Chem. 2012;287:32467–32478. doi: 10.1074/jbc.M112.370064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Liotta LA, Tryggvason K, Garbisa S, Hart I, Foltz CM, Shafie S. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature. 1980;284:67–68. doi: 10.1038/284067a0. [DOI] [PubMed] [Google Scholar]
- 164.Inamori K, Gu J, Ohira M, Kawasaki A, Nakamura Y, et al. High expression of N-acetylglucosaminyltransferase V in favorable neuroblastomas: involvement of its effect on apoptosis. FEBS Lett. 2006;580:627–632. doi: 10.1016/j.febslet.2005.12.089. [DOI] [PubMed] [Google Scholar]
- 165.Rebbaa A, Yamamoto H, Saito T, Meuillet E, Kim P, et al. Gene transfection-mediated overexpression of β1,4-N-acetylglucosamine bisecting oligosaccharides in glioma cell line U373 MG inhibits epidermal growth factor receptor function. J. Biol. Chem. 1997;272:9275–979. doi: 10.1074/jbc.272.14.9275. [DOI] [PubMed] [Google Scholar]
- 166.Yokoe S, Takahashi M, Asahi M, Lee SH, Li W, et al. The Asn418-linked N-glycan of ErbB3 plays a crucial role in preventing spontaneous heterodimerization and tumor promotion. Cancer Res. 2007;67:1935–1942. doi: 10.1158/0008-5472.CAN-06-3023. [DOI] [PubMed] [Google Scholar]
- 167.Semel AC, Seales EC, Singhal A, Eklund EA, Colley KJ, Bellis SL. Hyposialylation of integrins stimulates the activity of myeloid fibronectin receptors. J. Biol. Chem. 2002;277:32830–32836. doi: 10.1074/jbc.M202493200. [DOI] [PubMed] [Google Scholar]
- 168.Dennis J, Waller C, Timpl R, Schirrmacher V. Surface sialic acid reduces attachment of metastatic tumour cells to collagen type IV and fibronectin. Nature. 1982;300:274–276. doi: 10.1038/300274a0. [DOI] [PubMed] [Google Scholar]
- 169.Nadanaka S, Sato C, Kitajima K, Katagiri K, Irie S, Yamagata T. Occurrence of oligosialic acids on integrin α5 subunit and their involvement in cell adhesion to fibronectin. J. Biol. Chem. 2001;276:33657–33664. doi: 10.1074/jbc.M011100200. [DOI] [PubMed] [Google Scholar]
- 170.Liu YC, Yen HY, Chen CY, Chen CH, Cheng PF, et al. Sialylation and fucosylation of epidermal growth factor receptor suppress its dimerization and activation in lung cancer cells. PNAS. 2011;108:11332–11337. doi: 10.1073/pnas.1107385108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.De Luca A, Carotenuto A, Rachiglio A, Gallo M, Maiello MR, et al. The role of the EGFR signaling in tumor microenvironment. J. Cell. Physiol. 2008;214:559–567. doi: 10.1002/jcp.21260. [DOI] [PubMed] [Google Scholar]
- 172.Meuillet EJ, Kroes R, Yamamoto H, Warner TG, Ferrari J, et al. Sialidase gene transfection enhances epidermal growth factor receptor activity in an epidermoid carcinoma cell line, A431. Cancer Res. 1999;59:234–240. [PubMed] [Google Scholar]
- 173.Kroes RA, He H, Emmett MR, Nilsson CL, Leach FE, III, et al. Overexpression of ST6GalNAcV, a ganglioside-specific α2,6-sialyltransferase, inhibits glioma growth in vivo. PNAS. 2010;107:12646–12651. doi: 10.1073/pnas.0909862107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chen CY, Jan YH, Juan YH, Yang CJ, Huang MS, et al. Fucosyltransferase 8 as a functional regulator of nonsmall cell lung cancer. PNAS. 2013;110:630–635. doi: 10.1073/pnas.1220425110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Saeland E, van Vliet SJ, Backstrom M, van den Berg VC, Geijtenbeek TB, et al. The C-type lectin MGL expressed by dendritic cells detects glycan changes on MUC1 in colon carcinoma. Cancer Immunol. Immunother. 2007;56:1225–1236. doi: 10.1007/s00262-006-0274-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Napoletano C, Rughetti A, Agervig Tarp MP, Coleman J, Bennett EP, et al. Tumor-associated Tn-MUC1 glycoform is internalized through the macrophage galactose-type C-type lectin and delivered to the HLA class I and II compartments in dendritic cells. Cancer Res. 2007;67:8358–8367. doi: 10.1158/0008-5472.CAN-07-1035. [DOI] [PubMed] [Google Scholar]
- 177.van Vliet SJ, Bay S, Vuist IM, Kalay H, Garcia-Vallejo JJ, et al. MGL signaling augments TLR2-mediated responses for enhanced IL-10 and TNF-αsecretion. J. Leukoc. Biol. 2013;94:315–323. doi: 10.1189/jlb.1012520. [DOI] [PubMed] [Google Scholar]
- 178.Ogata S, Maimonis PJ, Itzkowitz SH. Mucins bearing the cancer-associated sialosyl-Tn antigen mediate inhibition of natural killer cell cytotoxicity. Cancer Res. 1992;52:4741–4746. [PubMed] [Google Scholar]
- 179.Engering A, Geijtenbeek TB, van Kooyk Y. Immune escape through C-type lectins on dendritic cells. Trends Immunol. 2002;23:480–485. doi: 10.1016/s1471-4906(02)02296-2. [DOI] [PubMed] [Google Scholar]
- 180.Fukumori T, Takenaka Y, Oka N, Yoshii T, Hogan V, et al. Endogenous galectin-3 determines the routing of CD95 apoptotic signaling pathways. Cancer Res. 2004;64:3376–3379. doi: 10.1158/0008-5472.CAN-04-0336. [DOI] [PubMed] [Google Scholar]
- 181.Stowell SR, Arthur CM, Dias-Baruffi M, Rodrigues LC, Gourdine JP, et al. Innate immune lectins kill bacteria expressing blood group antigen. Nat. Med. 2010;16:295–301. doi: 10.1038/nm.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.van Vliet SJ, den Dunnen J, Gringhuis SI, Geijtenbeek TB, van Kooyk Y. Innate signaling and regulation of dendritic cell immunity. Curr. Opin. Immunol. 2007;19:435–440. doi: 10.1016/j.coi.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 183.Stowell SR, Arthur CM, McBride R, Berger O, Razi N, et al. Microbial glycan microarrays define key features of host-microbial interactions. Nat. Chem. Biol. 2014;10:470–476. doi: 10.1038/nchembio.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Soldati R, Berger E, Zenclussen AC, Jorch G, Lode HN, et al. Neuroblastoma triggers an immunoevasive program involving galectin-1-dependent modulation of T cell and dendritic cell compartments. Int. J. Cancer. 2012;131:1131–1141. doi: 10.1002/ijc.26498. [DOI] [PubMed] [Google Scholar]
- 185.Dalotto-Moreno T, Croci DO, Cerliani JP, Martinez-Allo VC, Dergan-Dylon S, et al. Targeting galectin-1 overcomes breast cancer-associated immunosuppression and prevents metastatic disease. Cancer Res. 2013;73:1107–1117. doi: 10.1158/0008-5472.CAN-12-2418. [DOI] [PubMed] [Google Scholar]
- 186.Croci DO, Cerliani JP, Dalotto-Moreno T, Mendez-Huergo SP, Mascanfroni ID, et al. Glycosylation-dependent lectin-receptor interactions preserve angiogenesis in anti-VEGF refractory tumors. Cell. 2014;156:744–758. doi: 10.1016/j.cell.2014.01.043. [DOI] [PubMed] [Google Scholar]
- 187.Stowell SR, Karmakar S, Stowell CJ, Dias-Baruffi M, McEver RP, Cummings RD. Human galectin-1, −2, and −4 induce surface exposure of phosphatidylserine in activated human neutrophils but not in activated T cells. Blood. 2007;109:219–227. doi: 10.1182/blood-2006-03-007153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Stowell SR, Qian Y, Karmakar S, Koyama NS, Dias-Baruffi M, et al. Differential roles of galectin-1 and galectin-3 in regulating leukocyte viability and cytokine secretion. J. Immunol. 2008;180:3091–3102. doi: 10.4049/jimmunol.180.5.3091. [DOI] [PubMed] [Google Scholar]
- 189.Furtak V, Hatcher F, Ochieng J. Galectin-3 mediates the endocytosis of β-1 integrins by breast carcinoma cells. Biochem. Biophys. Res. Commun. 2001;289:845–850. doi: 10.1006/bbrc.2001.6064. [DOI] [PubMed] [Google Scholar]
- 190.Fukushi J, Makagiansar IT, Stallcup WB. NG2 proteoglycan promotes endothelial cell motility and angiogenesis via engagement of galectin-3 and α3β1 integrin. Mol. Biol. Cell. 2004;15:3580–3590. doi: 10.1091/mbc.E04-03-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.D’Haene N, Sauvage S, Maris C, Adanja I, Le Mercier M, et al. VEGFR1 and VEGFR2 involvement in extracellular galectin-1- and galectin-3-induced angiogenesis. PLOS ONE. 2013;8:e67029. doi: 10.1371/journal.pone.0067029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Nangia-Makker P, Honjo Y, Sarvis R, Akahani S, Hogan V, et al. Galectin-3 induces endothelial cell morphogenesis and angiogenesis. Am. J. Pathol. 2000;156:899–909. doi: 10.1016/S0002-9440(10)64959-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Dennis JW, Koch K, Yousefi S, VanderElst I. Growth inhibition of human melanoma tumor xenografts in athymic nude mice by swainsonine. Cancer Res. 1990;50:1867–1872. [PubMed] [Google Scholar]
- 194.Granovsky M, Fata J, Pawling J, Muller WJ, Khokha R, Dennis JW. Suppression of tumor growth and metastasis in Mgat5-deficient mice. Nat. Med. 2000;6:306–312. doi: 10.1038/73163. [DOI] [PubMed] [Google Scholar]
- 195.Yoshimura M, Nishikawa A, Ihara Y, Taniguchi S, Taniguchi N. Suppression of lung metastasis of B16 mouse melanoma by N-acetylglucosaminyltransferase III gene transfection. PNAS. 1995;92:8754–8758. doi: 10.1073/pnas.92.19.8754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Yamamoto H, Oviedo A, Sweeley C, Saito T, Moskal JR. α2,6-Sialylation of cell-surface N-glycans inhibits glioma formation in vivo . Cancer Res. 2001;61:6822–6829. [PubMed] [Google Scholar]
- 197.Petretti T, Kemmner W, Schulze B, Schlag PM. Altered mRNA expression of glycosyltransferases in human colorectal carcinomas and liver metastases. Gut. 2000;46:359–366. doi: 10.1136/gut.46.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kojima N, Handa K, Newman W, Hakomori S. Inhibition of selectin-dependent tumor cell adhesion to endothelial cells and platelets by blocking O-glycosylation of these cells. Biochem. Biophys. Res. Commun. 1992;182:1288–1295. doi: 10.1016/0006-291x(92)91872-n. [DOI] [PubMed] [Google Scholar]
- 199.Ohyama C, Tsuboi S, Fukuda M. Dual roles of sialyl Lewis X oligosaccharides in tumor metastasis and rejection by natural killer cells. EMBO J. 1999;18:1516–1525. doi: 10.1093/emboj/18.6.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sato S, Ouellet N, Pelletier I, Simard M, Rancourt A, Bergeron MG. Role of galectin-3 as an adhesion molecule for neutrophil extravasation during streptococcal pneumonia. J. Immunol. 2002;168:1813–1822. doi: 10.4049/jimmunol.168.4.1813. [DOI] [PubMed] [Google Scholar]
- 201.Sarafian V, Jadot M, Foidart JM, Letesson JJ, Van den Brule F, et al. Expression of Lamp-1 and Lamp-2 and their interactions with galectin-3 in human tumor cells. Int. J. Cancer. 1998;75:105–111. doi: 10.1002/(sici)1097-0215(19980105)75:1<105::aid-ijc16>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 202.Lotan R, Raz A. Low colony formation in vivo and in culture as exhibited by metastatic melanoma cells selected for reduced homotypic aggregation. Cancer Res. 1983;43:2088–2093. [PubMed] [Google Scholar]
- 203.Takenaka Y, Fukumori T, Raz A. Galectin-3 and metastasis. Glycoconj. J. 2004;19:543–549. doi: 10.1023/B:GLYC.0000014084.01324.15. [DOI] [PubMed] [Google Scholar]
- 204.Liu FT, Patterson RJ, Wang JL. Intracellular functions of galectins. Biochim. Biophys. Acta. 2002;1572:263–273. doi: 10.1016/s0304-4165(02)00313-6. [DOI] [PubMed] [Google Scholar]
- 205.Croci DO, Salatino M, Rubinstein N, Cerliani JP, Cavallin LE, et al. Disrupting galectin-1 interactions with N-glycans suppresses hypoxia-driven angiogenesis and tumorigenesis in Kaposi’s sarcoma. J. Exp. Med. 2012;209:1985–2000. doi: 10.1084/jem.20111665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wahrenbrock M, Borsig L, Le D, Varki N, Varki A. Selectin-mucin interactions as a probable molecular explanation for the association of Trousseau syndrome with mucinous adenocarcinomas. J. Clin. Investig. 2003;112:853–862. doi: 10.1172/JCI18882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Shao B, Wahrenbrock MG, Yao L, David T, Coughlin SR, et al. Carcinoma mucins trigger reciprocal activation of platelets and neutrophils in a murine model of Trousseau syndrome. Blood. 2011;118:4015–4023. doi: 10.1182/blood-2011-07-368514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Samraj AN, Laubli H, Varki N, Varki A. Involvement of a non-human sialic acid in human cancer. Front. Oncol. 2014;4:33. doi: 10.3389/fonc.2014.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Heneghan MA, McCarthy CF, Janulaityte D, Moran AP. Relationship of anti-Lewis x and anti-Lewis y antibodies in serum samples from gastric cancer and chronic gastritis patients to Helicobacter pylori-mediated autoimmunity. Infect. Immun. 2001;69:4774–4781. doi: 10.1128/IAI.69.8.4774-4781.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hunter T. Treatment for chronic myelogenous leukemia: the long road to imatinib. J. Clin. Investig. 2007;117:2036–2043. doi: 10.1172/JCI31691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N. Engl. J. Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 212.Hakomori S. Tumor-associated carbohydrate antigens defining tumor malignancy: basis for development of anti-cancer vaccines. Adv. Exp. Med. Biol. 2001;491:369–402. doi: 10.1007/978-1-4615-1267-7_24. [DOI] [PubMed] [Google Scholar]
- 213.Springer GF, Desai PR, Spencer BD, Tegtmeyer H, Carlstedt SC, Scanlon EF. T/Tn antigen vaccine is effective and safe in preventing recurrence of advanced breast carcinoma. Cancer Detect. Prev. 1995;19:374–380. [PubMed] [Google Scholar]
- 214.Slovin SF, Ragupathi G, Musselli C, Fernandez C, Diani M, et al. Thomsen-Friedenreich (TF) antigen as a target for prostate cancer vaccine: clinical trial results with TF cluster (c)-KLH plus QS21 conjugate vaccine in patients with biochemically relapsed prostate cancer. Cancer Immunol. Immunother. 2005;54:694–702. doi: 10.1007/s00262-004-0598-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Miles D, Roche H, Martin M, Perren TJ, Cameron DA, et al. Phase III multicenter clinical trial of the sialyl-TN (STn)-keyhole limpet hemocyanin (KLH) vaccine for metastatic breast cancer. Oncologist. 2011;16:1092–1100. doi: 10.1634/theoncologist.2010-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ju T, Cummings RD. A unique molecular chaperone Cosmc required for activity of the mammalian core 1 β3-galactosyltransferase. PNAS. 2002;99:16613–16618. doi: 10.1073/pnas.262438199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Schjoldager KT, Vakhrushev SY, Kong Y, Steentoft C, Nudelman AS, et al. Probing isoform-specific functions of polypeptide GalNAc-transferases using zinc finger nuclease glycoengineered SimpleCells. PNAS. 2012;109:9893–9898. doi: 10.1073/pnas.1203563109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ikehara Y, Kojima N, Kurosawa N, Kudo T, Kono M, et al. Cloning and expression of a human gene encoding an N-acetylgalactosamine-α2,6-sialyltransferase (ST6GalNAc I): a candidate for synthesis of cancer-associated sialyl-Tn antigens. Glycobiology. 1999;9:1213–1224. doi: 10.1093/glycob/9.11.1213. [DOI] [PubMed] [Google Scholar]
- 219.Brockhausen I, Yang J, Dickinson N, Ogata S, Itzkowitz SH. Enzymatic basis for sialyl-Tn expression in human colon cancer cells. Glycoconj. J. 1998;15:595–603. doi: 10.1023/a:1006967910803. [DOI] [PubMed] [Google Scholar]
- 220.Almaraz RT, Tian Y, Bhattarcharya R, Tan E, Chen SH, et al. Metabolic flux increases glycoprotein sialylation: implications for cell adhesion and cancer metastasis. Mol. Cell. Proteomics. 2012;11 doi: 10.1074/mcp.M112.017558. M112.017558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Almaraz RT, Aich U, Khanna HS, Tan E, Bhattacharya R, et al. Metabolic oligosaccharide engineering with N-acyl functionalized ManNAc analogs: cytotoxicity, metabolic flux, and glycan-display considerations. Biotechnol. Bioeng. 2012;109:992–1006. doi: 10.1002/bit.24363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Bull C, Boltje TJ, Wassink M, de Graaf AM, van Delft FL, et al. Targeting aberrant sialylation in cancer cells using a fluorinated sialic acid analog impairs adhesion, migration, and in vivo tumor growth. Mol. Cancer Ther. 2013;12:1935–1946. doi: 10.1158/1535-7163.MCT-13-0279. [DOI] [PubMed] [Google Scholar]
- 223.Sampathkumar SG, Jones MB, Meledeo MA, Campbell CT, Choi SS, et al. Targeting glycosylation pathways and the cell cycle: sugar-dependent activity of butyrate-carbohydrate cancer prodrugs. Chem. Biol. 2006;13:1265–1275. doi: 10.1016/j.chembiol.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 224.Duan X, Cai L, Lee LA, Chen H, Wang Q. Incorporation of azide sugar analogue decreases tumorigenic potential of breast cancer cells by reducing cancer stem cell population. Sci. China Chem. 2013;56:279–285. [Google Scholar]
- 225.Barthel SR, Gavino JD, Descheny L, Dimitroff CJ. Targeting selectins and selectin ligands in inflammation and cancer. Expert Opin. Ther. Targets. 2007;11:1473–1491. doi: 10.1517/14728222.11.11.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Fukuda MN, Ohyama C, Lowitz K, Matsuo O, Pasqualini R, et al. A peptide mimic of E-selectin ligand inhibits sialyl Lewis X-dependent lung colonization of tumor cells. Cancer Res. 2000;60:450–456. [PubMed] [Google Scholar]
- 227.Watkins WM, Morgan WT. Neutralization of the anti-H agglutinin in eel serum by simple sugars. Nature. 1952;169:825–826. doi: 10.1038/169825a0. [DOI] [PubMed] [Google Scholar]
- 228.Kabat EA, Leskowitz S. Immunochemical studies on blood group. XVII. Structural units involved in blood group A and B specificity. J. Am. Chem. Soc. 1955;77:5159–5164. [Google Scholar]
- 229.Kabat EA, Schiffman G. Immunochemical studies on blood groups. XXVIII. Further studies on the oligosaccharide determinants of blood group B and BP1 specificity. J. Immunol. 1962;88:782–787. [PubMed] [Google Scholar]
- 230.Nowell PC. Phytohemagglutinin: an initiator of mitosis in cultures of normal human leukocytes. Cancer Res. 1960;20:462–466. [PubMed] [Google Scholar]
- 231.Kornfeld S, Kornfeld R. Solubilization and partial characterization of a phytohemagglutinin receptor site from human erythrocytes. PNAS. 1969;63:1439–1446. doi: 10.1073/pnas.63.4.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Gesner BM, Ginsburg V. Effect of glycosidases on the fate of transfused lymphocytes. PNAS. 1964;52:750–755. doi: 10.1073/pnas.52.3.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Pricer WE, Jr, Hudgin RL, Ashwell G, Stockert RJ, Morell AG. A membrane receptor protein for asialoglycoproteins. Methods Enzymol. 1974;34:688–691. doi: 10.1016/s0076-6879(74)34090-6. [DOI] [PubMed] [Google Scholar]
- 234.Hudgin RL, Pricer WE, Jr, Ashwell G, Stockert RJ, Morell AG. The isolation and properties of a rabbit liver binding protein specific for asialoglycoproteins. J. Biol. Chem. 1974;249:5536–5543. [PubMed] [Google Scholar]
- 235.Morell AG, Van den Hamer CJ, Scheinberg IH, Ashwell G. Physical and chemical studies on ceruloplasmin. IV. Preparation of radioactive, sialic acid-free ceruloplasmin labeled with tritium on terminal D-galactose residues. J. Biol. Chem. 1966;241:3745–3749. [PubMed] [Google Scholar]
- 236.Oh-Uti K. Polysaccharides and a glycidamin in the tissue of gastric cancer. Tohoku J. Exp. Med. 1949;51:297–304. [Google Scholar]
- 237.Gasic G, Gasic T. Removal and regeneration of the cell coating in tumour cells. Nature. 1962;196:170. doi: 10.1038/196170a0. [DOI] [PubMed] [Google Scholar]
- 238.Gasic G, Gasic T. Removal of sialic acid from the cell coat in tumor cells and vascular endothelium, and its effects on metastasis. PNAS. 1962;48:1172–1177. doi: 10.1073/pnas.48.7.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Burger MM, Goldberg AR. Identification of a tumor-specific determinant on neoplastic cell surfaces. PNAS. 1967;57:359–3566. doi: 10.1073/pnas.57.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Pollack RE, Burger MM. Surface-specific characteristics of a contact-inhibited cell line containing the SV40 viral genome. PNAS. 1969;62:1074–1076. doi: 10.1073/pnas.62.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Burger MM. Isolation of a receptor complex for a tumor specific agglutinin from the neoplastic cell surface. Nature. 1968;219:499–500. doi: 10.1038/219499a0. [DOI] [PubMed] [Google Scholar]
- 242.Benjamin TL, Burger MM. Absence of a cell membrane alteration function in non-transforming mutants of polyoma virus. PNAS. 1970;67:929–934. doi: 10.1073/pnas.67.2.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Inbar M, Sachs L. Structural difference in sites on the surface membrane of normal and transformed cells. Nature. 1969;223:710–712. doi: 10.1038/223710a0. [DOI] [PubMed] [Google Scholar]
- 244.Inbar M, Sachs L. Interaction of the carbohydrate-binding protein concanavalin A with normal and transformed cells. PNAS. 1969;63:1418–1425. doi: 10.1073/pnas.63.4.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Aub JC, Sanford BH, Wang LH. Reactions of normal and leukemic cell surfaces to a wheat germ agglutinin. PNAS. 1965;54:400–402. doi: 10.1073/pnas.54.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Aub JC, Sanford BH, Cote MN. Studies on reactivity of tumor and normal cells to a wheat germ agglutinin. PNAS. 1965;54:396–399. doi: 10.1073/pnas.54.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Hakomori SI, Murakami WT. Glycolipids of hamster fibroblasts and derived malignant-transformed cell lines. PNAS. 1968;59:254–261. doi: 10.1073/pnas.59.1.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Buck CA, Glick MC, Warren L. Glycopeptides from the surface of control and virus-transformed cells. Science. 1971;172:169–171. doi: 10.1126/science.172.3979.169. [DOI] [PubMed] [Google Scholar]
- 249.Meezan E, Wu HC, Black PH, Robbins PW. Comparative studies on the carbohydrate-containing membrane components of normal and virus-transformed mouse fibroblasts. II. Separation of glycoproteins and glycopeptides by Sephadex chromatography. Biochemistry. 1969;8:2518–2524. doi: 10.1021/bi00834a039. [DOI] [PubMed] [Google Scholar]
- 250.Wu HC, Meezan E, Black PH, Robbins PW. Comparative studies on the carbohydrate-containing membrane components of normal and virus-transformed mouse fibroblasts. I. Glucosamine-labeling patterns in 3T3, spontaneously transformed 3T3, and SV-40-transformed 3T3 cells. Biochemistry. 1969;8:2509–2517. doi: 10.1021/bi00834a038. [DOI] [PubMed] [Google Scholar]
- 251.Warren L, Buck CA, Tuszynski GP. Glycopeptide changes and malignant transformation. A possible role for carbohydrate in malignant behavior. Biochim. Biophys. Acta. 1978;516:97–127. doi: 10.1016/0304-419x(78)90005-7. [DOI] [PubMed] [Google Scholar]
- 252.Clamp JR, Jones JV. The antigenicity of the oligosaccharide units of a soluble glycoprotein. Clin. Chim. Acta. 1968;21:165–169. doi: 10.1016/0009-8981(68)90122-8. [DOI] [PubMed] [Google Scholar]
- 253.Springer GF. T and Tn, general carcinoma autoantigens. Science. 1984;224:1198–1206. doi: 10.1126/science.6729450. [DOI] [PubMed] [Google Scholar]
- 254.Springer GF, Desai PR, Banatwala I. Blood group MN antigens and precursors in normal and malignant human breast glandular tissue. J. Natl. Cancer Inst. 1975;54:335–339. [PubMed] [Google Scholar]
- 255.Howard DR, Taylor CR. An antitumor antibody in normal human serum: reaction of anti-T with breast carcinoma cells. Oncology. 1980;37:142–148. doi: 10.1159/000225423. [DOI] [PubMed] [Google Scholar]
- 256.Springer GF, Desai PR. Cross-reacting carcinoma-associated antigens with blood group and precursor specificities. Transplant. Proc. 1977;9:1105–1111. [PubMed] [Google Scholar]
- 257.Hakomori S, Wang SM, Young WW., Jr Isoantigenic expression of Forssman glycolipid in human gastric and colonic mucosa: its possible identity with “A-like antigen” in human cancer. PNAS. 1977;74:3023–3027. doi: 10.1073/pnas.74.7.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Solter D, Knowles BB. Monoclonal antibody defining a stage-specific mouse embryonic antigen (SSEA-1) PNAS. 1978;75:5565–5569. doi: 10.1073/pnas.75.11.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Knowles BB, Aden DP, Solter D. Monoclonal antibody detecting a stage-specific embryonic antigen (SSEA-1) on preimplantation mouse embryos and teratocarcinoma cells. Curr. Top. Microbiol. Immunol. 1978;81:51–53. doi: 10.1007/978-3-642-67448-8_8. [DOI] [PubMed] [Google Scholar]
- 260.Herlyn M, Sears HF, Steplewski Z, Koprowski H. Monoclonal antibody detection of a circulating tumor-associated antigen. I. Presence of antigen in sera of patients with colorectal, gastric, and pancreatic carcinoma. J. Clin. Immunol. 1982;2:135–140. doi: 10.1007/BF00916897. [DOI] [PubMed] [Google Scholar]
- 261.Magnani JL, Steplewski Z, Koprowski H, Ginsburg V. Identification of the gastrointestinal and pancreatic cancer-associated antigen detected by monoclonal antibody 19-9 in the sera of patients as a mucin. Cancer Res. 1983;43:5489–5492. [PubMed] [Google Scholar]
- 262.Sears HF, Herlyn M, Del Villano B, Steplewski Z, Koprowski H. Monoclonal antibody detection of a circulating tumor-associated antigen. II. A longitudinal evaluation of patients with colorectal cancer. J. Clin. Immunol. 1982;2:141–149. doi: 10.1007/BF00916898. [DOI] [PubMed] [Google Scholar]
- 263.Kabawat SE, Bast RC, Jr, Bhan AK, Welch WR, Knapp RC, Colvin RB. Tissue distribution of a coelomic-epithelium-related antigen recognized by the monoclonal antibody OC125. Int. J. Gynecol. Pathol. 1983;2:275–285. doi: 10.1097/00004347-198303000-00005. [DOI] [PubMed] [Google Scholar]
- 264.Bast RC, Jr, Klug TL, St. John E, Jenison E, Niloff JM, et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N. Engl. J. Med. 1983;309:883–887. doi: 10.1056/NEJM198310133091503. [DOI] [PubMed] [Google Scholar]
- 265.Springer GF, Chandrasekaran EV, Desai PR, Tegtmeyer H. Blood group Tn-active macromolecules from human carcinomas and erythrocytes: characterization of and specific reactivity with mono- and poly-clonal anti-Tn antibodies induced by various immunogens. Carbohydr. Res. 1988;178:271–292. doi: 10.1016/0008-6215(88)80118-6. [DOI] [PubMed] [Google Scholar]
- 266.Aoyagi Y, Suzuki Y, Isemura M, Nomoto M, Sekine C, et al. The fucosylation index of alphafetoprotein and its usefulness in the early diagnosis of hepatocellular carcinoma. Cancer. 1988;61:769–774. doi: 10.1002/1097-0142(19880215)61:4<769::aid-cncr2820610422>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 267.Colcher DM, Milenic DE, Schlom J. Generation and characterization of monoclonal antibody B72.3. Experimental and preclinical studies. Target. Diagn. Ther. 1992;6:23–44. [PubMed] [Google Scholar]
- 268.Nuti M, Teramoto YA, Mariani-Costantini R, Hand PH, Colcher D, Schlom J. A monoclonal antibody (B72.3) defines patterns of distribution of a novel tumor-associated antigen in human mammary carcinoma cell populations. Int. J. Cancer. 1982;29:539–545. doi: 10.1002/ijc.2910290509. [DOI] [PubMed] [Google Scholar]
- 269.Colcher D, Hand PH, Nuti M, Schlom J. A spectrum of monoclonal antibodies reactive with human mammary tumor cells. PNAS. 1981;78:3199–3203. doi: 10.1073/pnas.78.5.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Johnson PJ, Poon TC, Hjelm NM, Ho CS, Blake C, Ho SK. Structures of disease-specific serum alpha-fetoprotein isoforms. Br. J. Cancer. 2000;83:1330–1337. doi: 10.1054/bjoc.2000.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Furness P. βhCG as a prognostic marker in prostatic adenocarcinoma. J. Clin. Pathol. 1996;49:693–694. doi: 10.1136/jcp.49.8.693-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Yoshimoto Y, Wolfsen AR, Odell WD. Glycosylation, a variable in the production of hCG by cancers. Am. J. Med. 1979;67:414–420. doi: 10.1016/0002-9343(79)90787-3. [DOI] [PubMed] [Google Scholar]
- 273.Duffy MJ, Duggan C, Keane R, Hill AD, McDermott E, et al. High preoperative CA 15-3 concentrations predict adverse outcome in node-negative and node-positive breast cancer: study of 600 patients with histologically confirmed breast cancer. Clin. Chem. 2004;50:559–563. doi: 10.1373/clinchem.2003.025288. [DOI] [PubMed] [Google Scholar]
- 274.Duffy MJ, Shering S, Sherry F, McDermott E, O’Higgins N. CA 15-3: a prognostic marker in breast cancer. Int. J. Biol. Markers. 2000;15:330–333. doi: 10.1177/172460080001500410. [DOI] [PubMed] [Google Scholar]
- 275.Parikh DA, Durbin-Johnson B, Urayama S. Utility of serum CA19-9 levels in the diagnosis of pancreatic ductal adenocarcinoma in an endoscopic ultrasound referral population. J. Gastrointest. Cancer. 2014;45:74–79. doi: 10.1007/s12029-013-9563-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Ballehaninna UK, Chamberlain RS. Biomarkers for pancreatic cancer: promising new markers and options beyond CA 19-9. Tumour Biol. 2013;34:3279–3292. doi: 10.1007/s13277-013-1033-3. [DOI] [PubMed] [Google Scholar]
- 277.Narimatsu H, Iwasaki H, Nakayama F, Ikehara Y, Kudo T, et al. Lewis and secretor gene dosages affect CA19-9 and DU-PAN-2 serum levels in normal individuals and colorectal cancer patients. Cancer Res. 1998;58:512–518. [PubMed] [Google Scholar]
- 278.Beveridge RA. Review of clinical studies of CA 27.29 in breast cancer management. Int. J. Biol. Markers. 1999;14:36–39. doi: 10.1177/172460089901400107. [DOI] [PubMed] [Google Scholar]
- 279.Saldova R, Struwe WB, Wynne K, Elia G, Duffy MJ, Rudd PM. Exploring the glycosylation of serum CA125. Int. J. Mol. Sci. 2013;14:15636–15654. doi: 10.3390/ijms140815636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Petignat P, Joris F, Obrist R. How CA 125 is used in routine clinical practice. Eur. J. Cancer. 2000;36:1933–1937. doi: 10.1016/s0959-8049(00)00190-8. [DOI] [PubMed] [Google Scholar]
- 281.Dnistrian AM, Schwartz MK, Greenberg EJ, Smith CA, Dorsa R, Schwartz DC. CA 549 as a marker in breast cancer. Int. J. Biol. Markers. 1991;6:139–143. doi: 10.1177/172460089100600301. [DOI] [PubMed] [Google Scholar]
- 282.Amri R, Bordeianou LG, Sylla P, Berger DL. Preoperative carcinoembryonic antigen as an outcome predictor in colon cancer. J. Surg. Oncol. 2013;108:14–18. doi: 10.1002/jso.23352. [DOI] [PubMed] [Google Scholar]
- 283.Salvini R, Bardoni A, Valli M, Trinchera M. β1,3-Galactosyltransferase β3Gal-T5 acts on the GlcNAcβ1→3Galβ1→4GlcNAcβ1→R sugar chains of carcinoembryonic antigen and other N-linked glycoproteins and is down-regulated in colon adenocarcinomas. J. Biol. Chem. 2001;276:3564–3573. doi: 10.1074/jbc.M006662200. [DOI] [PubMed] [Google Scholar]
- 284.Haab BB, Porter A, Yue T, Li L, Scheiman J, et al. Glycosylation variants of mucins and CEACAMs as candidate biomarkers for the diagnosis of pancreatic cystic neoplasms. Ann. Surg. 2010;251:937–945. doi: 10.1097/SLA.0b013e3181d7738d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Jantscheff P, Terracciano L, Lowy A, Glatz-Krieger K, Grunert F, et al. Expression of CEACAM6 in resectable colorectal cancer: a factor of independent prognostic significance. J. Clin. Oncol. 2003;21:3638–3646. doi: 10.1200/JCO.2003.55.135. [DOI] [PubMed] [Google Scholar]
- 286.Dawood S, Broglio K, Buzdar AU, Hortobagyi GN, Giordano SH. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J. Clin. Oncol. 2010;28:92–98. doi: 10.1200/JCO.2008.19.9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Ross JS, Fletcher JA, Bloom KJ, Linette GP, Stec J, et al. Targeted therapy in breast cancer: the HER-2/neu gene and protein. Mol. Cell. Proteomics. 2004;3:379–398. doi: 10.1074/mcp.R400001-MCP200. [DOI] [PubMed] [Google Scholar]
- 288.Hesse E, Musholt PB, Potter E, Petrich T, Wehmeier M, et al. Oncofoetal fibronectin—a tumour-specific marker in detecting minimal residual disease in differentiated thyroid carcinoma. Br. J. Cancer. 2005;93:565–570. doi: 10.1038/sj.bjc.6602741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Goldsmith JD, Pawel B, Goldblum JR, Pasha TL, Roberts S, et al. Detection and diagnostic utilization of placental alkaline phosphatase in muscular tissue and tumors with myogenic differentiation. Am. J. Surg. Pathol. 2002;26:1627–1633. doi: 10.1097/00000478-200212000-00011. [DOI] [PubMed] [Google Scholar]
- 290.Stamey TA, Yang N, Hay AR, McNeal JE, Freiha FS, Redwine E. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N. Engl. J. Med. 1987;317:909–916. doi: 10.1056/NEJM198710083171501. [DOI] [PubMed] [Google Scholar]
- 291.Hernandez J, Thompson IM. Prostate-specific antigen: a review of the validation of the most commonly used cancer biomarker. Cancer. 2004;101:894–904. doi: 10.1002/cncr.20480. [DOI] [PubMed] [Google Scholar]
- 292.Moyer VA. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2012;157:120–134. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 293.Peracaula R, Tabares G, Royle L, Harvey DJ, Dwek RA, et al. Altered glycosylation pattern allows the distinction between prostate-specific antigen (PSA) from normal and tumor origins. Glycobiology. 2003;13:457–470. doi: 10.1093/glycob/cwg041. [DOI] [PubMed] [Google Scholar]
- 294.Itzkowitz SH, Bloom EJ, Kokal WA, Modin G, Hakomori S, Kim YS. Sialosyl-Tn. A novel mucin antigen associated with prognosis in colorectal cancer patients. Cancer. 1990;66:1960–1966. doi: 10.1002/1097-0142(19901101)66:9<1960::aid-cncr2820660919>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 295.Ponnusamy MP, Venkatraman G, Singh AP, Chauhan SC, Johansson SL, et al. Expression of TAG-72 in ovarian cancer and its correlation with tumor stage and patient prognosis. Cancer Lett. 2007;251:247–257. doi: 10.1016/j.canlet.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 296.Springer GF. Immunoreactive T and Tn epitopes in cancer diagnosis, prognosis, and immunotherapy. J. Mol. Med. 1997;75:594–602. doi: 10.1007/s001090050144. [DOI] [PubMed] [Google Scholar]
- 297.Aryal RP, Ju T, Cummings RD. Identification of a novel protein binding motif within the T-synthase for the molecular chaperone Cosmc. J. Biol. Chem. 2014;289:11630–11641. doi: 10.1074/jbc.M114.555870. [DOI] [PMC free article] [PubMed] [Google Scholar]