Abstract
Background and objectives
We undertook a systematic review and meta-analysis of published cohort studies and case-control studies to estimate (1) the risk of pregnancy complications among patients with CKD versus those without CKD and (2) the risk of CKD progression among pregnant patients versus nonpregnant controls with CKD.
Design, setting, participants, & measurements
We searched electronic databases for studies published between 1946 and 2014, and we reviewed articles using validity criteria. Random-effects analytical methods were used.
Results
Twenty-three studies (14 with data for adverse pregnancy outcomes and 9 for renal outcomes) with 506,340 pregnancies were included. Pregnancy with CKD had greater odds of preeclampsia (odds ratio [OR], 10.36; 95% confidence interval [95% CI], 6.28 to 17.09), premature delivery (OR, 5.72; 95% CI, 3.26 to 10.03), small for gestational age/low birth weight (OR, 4.85; 95% CI, 3.03 to 7.76), cesarean section (OR, 2.67; 95% CI, 2.01 to 3.54), and failure of pregnancy (OR, 1.80; 95% CI, 1.03 to 3.13). Subgroup analysis showed that odds of preeclampsia (P<0.01) and premature delivery (P<0.01) were higher in women with nondiabetic nephropathy compared with diabetic nephropathy, and the odds of preeclampsia (P=0.01) and premature delivery (P<0.01) were higher in women with macroproteinuria compared with microproteinuria. The median for follow-up time for renal events was 5 years (interquartile range, 5–14.7 years). There were no significant differences in the occurrence of renal events between CKD pregnant women and those without pregnancy (OR, 0.96; 95% CI, 0.69 to 1.35). Subgroup analysis showed that publication year, sample size, follow-up years, type of primary disease, CKD classification, level of serum creatinine at baseline, proteinuria, and level of systolic BP did not modify the renal outcomes.
Conclusions
The risks of adverse maternal and fetal outcomes in pregnancy are higher for women with CKD versus pregnant women without CKD. However, pregnancy was not a risk factor for progression of renal disease in women with CKD before pregnancy.
Keywords: chronic kidney disease, renal function, pregnancy, cesarean section, follow-up studies, gestational age, humans, infant, low birth weight, pre-eclampsia, premature birth
Introduction
The prevalence of CKD in women of childbearing age ranges from 0.1% to 3% (1–8). Furthermore, pregnancy is generally considered to be a risk factor for the progression of CKD (4–8). Previous systematic reviews (9,10) indicated that the risk of adverse maternal and fetal outcomes was higher in patients with CKD compared with those without CKD, but the influence of pregnancy on renal outcomes was undetermined (9,10). Thus, estimating the odds of pregnancy complications in CKD versus non-CKD and the odds of progression of CKD among pregnant patients versus nonpregnant controls with CKD is important from a clinical and public health perspective. Here, we conducted a systematic review and meta-analysis of published cohort studies and case-control studies to obtain an overall estimate of the potential effect of CKD and pregnancy on each other.
Materials and Methods
We conducted and reported this systematic review according to published guidelines, using a prespecified protocol (11,12).
Eligibility Criteria
We included cohort studies and case-control studies that reported maternal or fetal outcomes in pregnant women with CKD and without CKD as a comparator group who may or may not have had other comorbidities (e.g., diabetes mellitus) or studies that reported renal outcomes in pregnant women with CKD and nonpregnant women with CKD as a comparator group. Primary studies defined CKD as any of the following: abnormal serum creatinine (SCr)/abnormal GFR and/or proteinuria with a specific primary or secondary kidney disease. CKD is classified into five stages. Adverse maternal outcomes were defined by the primary study authors and included preeclampsia, eclampsia, and maternal mortality. Adverse fetal outcomes included premature births, small for gestational age (SGA), low birth weight, neonatal mortality, and stillbirths. Renal outcomes included incidence of ESRD requiring RRT, doubling of SCr, and 50% decrement of eGFR/creatinine clearance rate (CCr). We included full-text articles and abstracts published in any language that reported at least one outcome of interest. We excluded studies of women with a history of autoimmune diseases (SLE, ANCA-associated systemic vasculitis, or Sjögren syndrome), hereditary kidney disease, kidney transplant, or maintenance dialysis, as well as studies of women with AKI or a single kidney.
Search Strategy
We searched the following electronic databases from the date of inception up to November 2014: MEDLINE (Ovid, 1946–2014) and Embase (1988–2014). Relevant studies were identified using relevant text words and medical subject headings that included all spellings of “pregnancy” and “pregnancy complications” combined with all spellings of “chronic renal insufficiency,” “glomerulonephritis,” “kidney disease,” and “diabetic nephropathies.” The search was limited to cohort or case-control studies, without language restrictions (Supplemental Appendix 1). References from identified studies were manually scanned to identify other relevant studies.
Study Selection
Full-text articles considered potentially relevant were retrieved. To ensure accuracy, the articles were independently screened the full-text articles for inclusion. Disagreement over eligibility was resolved by discussion or with the help of another reviewer.
Study Quality
The evidential level of each outcome of the studies was determined in accordance with the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system and was conducted with GRADEprofiler 3.6 (14). Publication bias was assessed by creating and examining funnel plots. A sensitivity analysis was performed by omitting studies with the smallest number of participants and investigating the influence on the overall meta-analysis estimate.
Statistical Analyses
The data was independently abstracted in duplicate to increase accuracy and reduce measurement bias (15). Disagreements were resolved with the help of another reviewer. The following data were extracted: title, author, year, journal, period of study, country, type of study, number of cases, number of pregnancies, mean age, type of study population, preeclampsia, gestational age, birth weight, preterm delivery, cesarean section, other maternal complications, stillbirth/neonatal death, SGA, other neonatal complications, the number of adverse renal outcome events, baseline and follow-up BP, proteinuria, eGFR, and SCr. We also calculated stillbirth, fetal death, and neonatal death together as pregnancy failure because these outcomes were so serious that they led to failure of pregnancy.
Summary estimates of the odds ratio (ORs) and 95% confidence intervals (95% CIs) or mean difference and 95% CIs for continuous variables were obtained using a random-effects model. Heterogeneity across the included studies was analyzed using the I2 statistic (16). We performed additional subgroup analyses by pooling estimates for subgroups defined by the median, and the level of adjustment when these were reported. The definition of macroproteinuria was PCR >500 mg/g or urinary albumin-to-creatinine ratio >300 mg/g according to the Kidney Disease Improving Global Outcomes 2012 CKD guideline, and the definition of microproteinuria was the level of proteinuria below macroproteinuria. All analyses were performed using Stata (release 11.0) or RevMan (release 5.2) software. A standard level (P<0.05) of statistical significance was used in all analyses.
Results
Study Selection and General Information
We screened and evaluated 6372 citations, of which 143 were reviewed in full text. Twenty-two were selected as relevant studies for our analysis (17–37), and one study that was published as an American Society of Nephrology 2014 meeting abstract was also added. A flow chart for the identification and selection of studies is shown in Figure 1.
Figure 1.
Flow chart showing the number of citations retrieved by individual searches and number of trials included in the systematic review.
Among 23 retrieved studies, 12 (17–22,24,25,30,32,33) were prospective studies, 7 (24–29,31,35,37) were retrospective studies, and 4 (23,25,34,36) did not mention whether they were retrospective or prospective. Twelve studies were from European countries, five were from the United States, four were from Asia, and two were from Brazil.
Of the 23 studies (n=505,759 pregnancies), 14 (17–22,27,28,30,32,33,35,37) of the reported adverse maternal or fetal outcomes in pregnant women with CKD. The definition of CKD varied across the studies. Among 14 studies, 8 (17–22,30,32) enrolled patients diagnosed with diabetic nephropathy (DN) with proteinuria, 3 (33,37) defined CKD according to the National Kidney Foundation Kidney Disease Outcomes Quality Initiative definition, 1 defined CKD according to by medical coding (27), and 1 defined CKD by biopsy (35). One study (28) enrolled pregnant women with low kidney function defined as SCr ≥1.5 mg/dl. Six studies (27,28,33,35,37) compared the maternal or fetal outcomes in pregnant women with CKD with healthy pregnant women. The remaining eight studies involving patients with DN had a control group with comorbidities of diabetes with normal proteinuria and kidney function. Four (19,20,27,33) of 14 studies accounted for potential confounding factors such as proteinuria, hypertension, systolic BP, and so forth (Tables 1 and 2).
Table 1.
Characteristics of included studies reporting the association of CKD with adverse pregnancy outcomes
| Reference | Country | Study Design | Period | Definition of CKD | Definition of Control Group | Pregnancy Outcomes Involved | Level of Adjustment |
|---|---|---|---|---|---|---|---|
| Farwell et al., 2013 (37) | USA | Retrospective | 2008 | CKD, defined according to the KDOQI guidelines | General population in the 2008 California Birth Registry | Preeclampsia, preterm delivery, neonatal death, intrauterine fetal demise, SGA, low birth weight, and mode of delivery stratified for nulliparous and multiparous patients | None |
| Young et al., 2011 (22) | Brazil | Prospective | 2010–2011 | DN defined as preconceptional persistent macroproteinuria (>0.5g/24 h) in the absence of urinary tract infection and other renal diseases | Type 1 women with diabetes with normoalbuminuria | Premature birth; pregnant hypertension; preeclampsia; polyhydramnios; urinary tract infection; low birth weight; fetal macrosomia; neonatal hypoglycemia | None |
| Jensen et al., 2010 (20) | Denmark | Prospective | 1993–1999 | Type 1 diabetes with microalbuminuria before conception and/or during the first trimester (UAER between 30 and 300 mg/24 h or between 20 and 200 μg/min) | Type 1 women with diabetes with normoalbuminuria | Preeclampsia; SGA; birth weight | Age; BMI; duration of diabetes nulliparity; prepregnancy insulin dose; BP ≥140/90 mmHg at first visit; proliferative retinopathy; first-trimester A1C; third-trimester A1C |
| Piccoli et al., 2010 (33) | Italy | Prospective | 1999–2007 | CKD, defined according to the KDOQI guidelines | Physiologic low-risk pregnancies | Cesarean section; gestational age; SGA; admission to NICU | CKD stage; age; nulliparous; Caucasian; referral; proteinuria; hypertension |
| Vasario, 2010 | Italy | Prospective | 1999–2007 | CKD stage 1, defined according to the KDOQI guidelines | Physiologic low-risk pregnancies | Preterm delivery; admission to the NICU; cesarean section | None |
| Nielsen et al., 2009 (21) | Denmark | Prospective | 2004–2006 | Type 1 diabetes with microalbuminuria (30–299 mg/24 h), or DN (UAER ≥300 mg/24 h) accordingly, before 14 completed gestational wk | Type 1 diabetes with normoalbuminuria (UAER <30 mg/24 h) before 14 completed gestational wk | Preeclampsia; gestational age; SGA; LGA; perinatal mortality | None |
| Trevisan et al., 2004 (28) | Brazil | Retrospective | 1989–1999 | LKF was established whenever serum creatinine was ≥1.5 mg/dl | Pregnant women with normal renal function | Abortion; caesarian; dead fetus; preeclampsia; urinary tract infection; BP | None |
| Biesenbach et al., 2000 (17) | Austria | Prospective | 1985–1993 | Long-standing type 1 diabetes with preconceptional persistent macroproteinuria (>0.5g/24 h) in the absence of urinary tract infection and other renal diseases | Women with type 1 diabetes without nephropathy (urinary albumin <30 mg/24 h) | Caesarian section; premature delivery; birth weight; SGA; LGA; polyhydramnios; preeclampsia/eclampsia; nephrotic syndrome; respiratory distress syndrome; hyperbilirubinemia; congenital anomaly | None |
| Ekbom et al., 2001 (19) | Denmark | prospective | 1996–1999 | Type 1 diabetes with microalbuminuria was defined as urinary albumin excretion of 30–300 mg/24 h, and DN was defined as urinary albumin excretion >300 mg/24 h | Women with type 1 diabetes with normal urinary albumin excretion <30 mg/24 h | Preeclampsia; pregnancy-induced hypertension; proteinuria >3 g/24 h; gestational age; perinatal mortality; SGA; birth weight; congenital malformations | Baseline variables of urinary albumin excretion, systolic BP, HbA1c, white classification, age, BMI, parity, smoking |
| Fink et al., 1998 (27) | USA | Retrospective | 1987–1993 | ICD-9 codes | Pregnant women whose linked hospitalization record did not indicated a diagnoses of renal disease | Polyhydramnios; oligohydramnios; first-trimester bleeding; amniocentesis; preeclampsia; prolonged labor; preterm labor; dysfunctional labor; cesarean section; SGA; 5-min Apgar; fetal distress | Maternal age, trimester of first prenatal visit, parity, and smoking; year of delivery |
| Miodovnik et al., 1996 (32) | USA | Prospective | 1978–1991 | Insulin-dependent diabetes mellitus with nephropathy was defined as a total protein excretion rate ≥0.5 g/24 h, or persistent positive albuminuria (dipstick ≥+2) in the absence of bacteriuria before 16 wk of gestation | Insulin-dependent diabetes mellitus without nephropathy | Infant birth weight; stillbirth/neonatal death; major malformation; gestational age; IUGR; LGA; cesarean section; PIH; preeclampsia; RDS | None |
| Kimmerle et al., 1995 (30) | Germany | Prospective | 1982–1992 | Pregnancies of pregestational insulin-dependent diabetic women with persistent macroproteinuria (>0.4 g/24 h) or ≥1+dipstick regardless of CCr; or in the absence of macroproteinuria but on the basis of hypertension and a CCr <80 mg/ml in the first trimester in the absence of urinary tract infection and other causes of renal disease | Pregnancies of pregestational insulin-dependent diabetic women without nephropathy | Preeclampsia-like syndrome; intrauterine/perinatal deaths; gestational age; cesarean section; birth weight; SGA; respiratory distress syndrome; Hospital days of newborns; congenital malformations | None |
| Combs et al., 1993 (18) | USA | Prospective | 1982–1991 | Diabetes with proteinuria (199–499 mg/24 h, or ≥0.5 g/24 h), before 20 completed gestational wk in the absence of urinary tract infection, and CCr ≥10 ml/kg per day | Diabetes with proteinuria (<0.199 g/24 h) before 20 completed gestational wk | Gestational hypertension; preeclampsia; preterm; birth weight | None |
| Leppert et al., 1979 (35) | USA | Retrospective | 1974–1976 | Renal disease was certified by biopsy; excluding systemic disease | Female siblings of renal disease patients | Spontaneous abortion; stillbirths; preterm; SGA | None |
KDOQI, Kidney Disease Outcomes Quality Initiative; DN, diabetic nephropathy; UAER, urinary albumin excretion rate; LKF, low kidney function; ICD-9, International Classification of Diseases, Ninth Revision; CCr, creatinine clearance rate; SGA, small for gestational age; NICU, neonatal intensive care unit; LGA, large for gestational age; IUGR, intrauterine growth restriction; PIH, pregnancy-induced hypertension; RDS, respiratory distress syndrome of newborn; BMI, body mass index; HbA1C, hemoglobin A1C.
Table 2.
Summary of the baseline characteristics of pregnancies in patients with or without CKD
| Reference | With CKD | Without CKD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Pregnancies | Mean Age (yr) | Baseline eGFR/CCr (ml/min per 1.73 m2 [ml/min]) | Baseline SCr (mg/dl) | Baseline SBP (mmHg) | Baseline Proteinuria (A/T) | No. of Pregnancies | Mean Age (yr) | Baseline eGFR/CCr (ml/min per 1.73 m2 [ml/min]) | Baseline SCr (mg/dl) | Baseline SBP (mmHg) | Baseline Proteinuria (A/T) | |
| Farwell et al., 2013 (37) | 125 | — | — | — | — | — | 502,061 | — | — | — | — | — |
| Young et al., 2011 (22) | 11 | 28 | 81 | 0.8 | — | 119 (A) | 32 | 25 | 98 | 0.6 | — | 7.78 (A) |
| Jensen et al., 2010 (20) | 84 | 27 | — | — | — | 30–300 (A) | 762 | 28 | — | — | — | — |
| Piccoli et al., 2010 (33) | 91 | 31 | 129/75/ 48/23 | 0.64/0.89/ 1.45/2.82 | — | 0.1/0.15/ 0.7/0.56 (T) | 267 | 29 | — | — | — | — |
| Vasario, 2010 | 61 | 31 | ≥90 | Normal | — | — | 260 | 29 | — | Normal | — | — |
| Nielsen et al., 2009 (21) | 17 | 30/31 | — | 0.58/0.64 | — | 91/690 (A) | 125 | 31 | 51 | — | — | 7 (A) |
| Trevisan et al., 2004 (28) | 25 | 29 | — | ≥1.5 | 154 | — | 50 | 29 | — | — | 118 | — |
| Biesenbach et al., 2000 (17) | 10 | 22 | — | — | — | ≥0.5 (T) | 30 | 23 | — | — | — | <30 (A) |
| Ekbom et al., 2001(19) | 37 | 29/30 | — | 1.03 | 123 | 69/1120 (A) | 203 | 30 | — | — | 115 | — |
| Fink et al., 1998 (27) | 169 | — | — | — | — | — | 506 | — | — | — | — | — |
| Miodovnik et al., 1996 (32) | 46 | 35 | 94 | — | — | ≥0.5 (T) | 136 | 37 | — | — | — | — |
| Kimmerle et al., 1995 (30) | 36 | — | — | — | — | 110 | — | — | — | — | — | |
| Combs et al., 1993 (18) | 107 | 28/27 | 117/93 | 0.79/0.90 | — | 0.199–0.499/ ≥ 0.5 (T) | 204 | 28 | 117 | 0.75 | — | — |
| Leppert et al., 1979 (35) | 114 | — | — | — | — | — | 80 | — | — | — | — | — |
Dash indicates that no relevant data was provided. CCr, creatinine clearance rate; SCr, serum creatinine; SBP, systolic BP; A, albuminuria (mg/24 h); T, total proteinuria (g/24 h).
Of the 23 studies, 9 (23,25,26,29,31,34,36,38) (n=1342) described the renal outcomes of pregnant women with CKD. Mean follow-up was 8.6 years (range, 3–18 years) and median follow-up was 5 years (interquartile range, 5–14.7 years). Five studies (23,25,26,38) enrolled pregnant women with IgA nephropathy, one study (26) dealt with DN, and three studies (29,31,34) analyzed primary GN with various diagnoses. The patients in the control groups were all nonpregnant women with matched age, type of disease, and renal function. Six of nine studies (29,31,34,36,38) accounted for confounding factors such as proteinuria, mean arterial pressure, eGFR, and so forth (Tables 3 and 4).
Table 3.
Characteristics of included studies reporting the association of pregnancy with renal outcomes
| Reference | Country | Study Design | Follow-Up (yr) | Definition of CKD | LKF (%) | Level of Adjustment |
|---|---|---|---|---|---|---|
| Su et al., 2014 (13) | China | Retrospective | 4 | IgAN was certified by biopsy | 11 (10.6) | Proteinuria; MAP; eGFR |
| Shimizu et al., 2010 (26) | Japan | Prospective | 3 | IgAN was performed by analyzing laboratory data, histology, and prognosis | 8 (28) | None |
| Limardo et al., 2010 (24) | Italy | Prospective | 5/10 | IgAN certified by biopsy; SCr >1.2 mg/dl or ≤1.2 mg/dl | 10 (100) | Angiotensin-converting enzyme inhibitor; angiotensin receptor blockers; steroids; immunosuppressant agents; baseline proteinuria; hypertensive status |
| Rossing et al., 2002 (36) | Denmark | — | 16 | DN was defined as persistent albuminuria ≥300 mg/d, presence of diabetic retinopathy, and the absence of any laboratory or clinical evidence other kidney or renal tract disease | 3 (12) | Arterial BP; albuminuria; HbA1C |
| Hemmelder et al., 1995 (29) | Netherlands | Retrospective | 18 | Primary GN was certified by biopsy, including FSGS, IgAN, MPGN, MN, MCD | 0 | Age; proteinuria; constant |
| Jungers et al., 1995 (31) | France | Retrospective | 14.7 | Primary GN was certified by biopsy, including FSGS, IgAN, MPGN, MN, MCD, with SCr ≤1.24 mg/dl | 0 | Histologic form of GN; hypertension; proteinuria |
| Abe, 1994 (25) | Japan | — | 5 | IgAN was certified by biopsy | — | None |
| Abe, 1991 (23) | Japan | — | 5 | IgAN was certified by biopsy | 0 | None |
| Barceló et al., 1986 (34) | Spain | — | 5 | Primary GN was certified by biopsy, including IgAN, glomerulosclerosis, MPGN, MN, focal GN | 5 (10) | Histologic form of GN; hypertension; proteinuria |
Dash indicates that no relevant data was provided. IgAN, Ig A nephropathy; SCr, serum creatinine; DN, diabetic nephropathy; MPGN, membranous proliferative GN; MN, membranous nephropathy; MCD, minimal change disease; LKF, low kidney function; MAP, mean arterial BP; HbA1C, hemoglobin A1C.
Table 4.
Summary of the baseline characteristics of patients with CKD with or without pregnancy
| Reference | With Pregnancy | Without Pregnancy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases (W/P) | Mean Age (yr) | Baseline eGFR/CCr (ml/min) | Baseline SCr (mg/dl) | Baseline SBP (mmHg) | Baseline Proteinuria (A/T) | Cases | Mean Age (yr) | Baseline eGFR/CCr (ml/min) | Baseline SCr (mg/dl) | Baseline SBP (mmHg) | Baseline Proteinuria (A/T) | |
| Su et al., 2014 (13) | 62 (W)/69 (P) | 28 | 97.6 (24.9) | 0.85 (0.27) | 109 (13.5) | 0.79 (T) | 65 | 28 | 99.7 (22.6) | 0.83 (0.26) | 111.8 (10.4) | 0.80 (T) |
| Shimizu et al., 2010 (26) | 29 (W) | 31 | 72.6 (7.8) | 0.76 (0.12) | 110.1 (9.1) | 0.46 (T) | 45 | 31 | 70.9 (20.7) | 0.90 (0.15) | 110.5 (13) | 0.85 (T) |
| Limardo et al., 2010 (24) | 10/136 (W) | 29 | 92 (170)/47.2 (14.7) | 0.87 (0.15)/1.65 (0.39) | — | 1.75/1.0 (T) | 12/87 | 28 | 89 (18)/49.2 (8.4) | 0.86 (0.16)/1.6 (0.19) | — | 1.9/0.5 (T) |
| Rossing et al., 2002 (36) | 26 (W)/31 (P) | 24 | — | 0.89 (0.26) | 128 (11) | 534 (279.7) (A) | 67 | 27 | — | 0.89 (0.26) | 133 (14) | 597 (659.8) (A) |
| Hemmelder et al.,1995 (29) | 19 (W) | — | — | 0.9 | 130 | 3 (T) | 31 | — | — | 0.9 | 130 | 3 (T) |
| Jungers et al., 1995 (31) | 143 (W) | — | — | ≤1.24 | — | — | 107 | — | — | ≤1.24 | — | — |
| Abe et al., 1994 (25) | 36 (W)/39 (P) | 25 | 83 (13) | 0.79 (0.20) | 119 (10) | 0.6 (0.4) (T) | 35 | 25 | 87 (15) | 0.7 (0.20) | 121 (17) | 0.9 (0.6) (T) |
| Abe et al., 1991 (23) | 30 (W) | — | 81 (13) | — | — | — | 32 | — | 88 (16) | — | — | — |
| Barceló et al., 1986 (34) | 48 (W)/66 (P) | 29 | — | 0.83 (0.36) | — | 1.49 (0.53) (T) | 36 | 27 | — | 1.01 (0.47) | — | 1.03 (1.49) (T) |
Dash indicates that no relevant data was provided. P, number of pregnancies; W, number of women; CCr, creatinine clearance rate; SCr, serum creatinine; A, albuminuria (mg/24 h); T, total proteinuria (g/24 h).
Effect of the Kidney Disease on Pregnancy
Pregnancy outcomes in kidney disease varied among the studies. We analyzed preeclampsia, premature birth, SGA/low birth weight, cesarean section, and failure of pregnancy (including stillbirth, fetal death, and neonatal death) in pregnancies with CKD, according to former studies.
Nine studies (18–22,27,28,32,37) evaluated the occurrence of preeclampsia. Overall, there were 14,993 events in 504,700 pregnancies. The overall preeclampsia OR was 10.36 (95% CI, 6.28 to 17.09; P<0.01; Figure 2) in women with CKD compared with women without CKD. Ten studies (2,17,19–21,27,28,32,33,35) reported 25,273 failures of pregnancy among 505,038 pregnancies. Compared with the controls, patients with CKD with pregnancy had a significantly higher rate of pregnancy failure (OR, 1.80; 95% CI, 1.03 to 3.13; P=0.04; Figure 3). The odds of premature birth, cesarean section, and SGA/low birth weight were also higher in women with CKD, with ORs of 5.72 (95% CI, 3.26 to 10.03) (Supplemental Figure 1), 4.85 (95% CI, 3.03 to 7.76) (Supplemental Figure 2), and 2.67 (95% CI, 2.01 to 3.54) (Supplemental Figure 3), respectively. The details are shown in the Supplemental Material.
Figure 2.
Overall odds ratios of the association of CKD and preeclampsia. 95% CI, 95% confidence interval.
Figure 3.
Overall odds ratios of the association of CKD and failure of pregnancy (including stillbirth, fetal death, and neonatal death). 95% CI, 95% confidence interval.
Subgroup analysis showed that odds of preeclampsia (P=0.002), and premature birth (P<0.01) were higher in women with nondiabetic nephropathy than those with DN (Figure 4), and the odds of preeclampsia (P=0.01) and premature delivery (P<0.01) were higher in women with macroproteinuria compared with those with microproteinuria (Figure 4). The identified between-study heterogeneity in failure of pregnancy (P=0.03) was also caused by differences in sample size (Figure 4).
Figure 4.
Odds ratios of CKD on pregnancy complications according to subgroups of publication year, sample size, type of study population, and proteinuria. Microproteinuria indicates albuminuria 30–300 mg/24 h or total proteinuria 150–500 mg/24 h, whereas macroproteinuria indicates albuminuria ≥300 mg/24 h or total proteinuria ≥500 mg/24 h. 95% CI, 95% confidence interval; DN, diabetic nephropathy; SGA, small for gestational age.
Most studies did not provide the full baseline data, such as BP, eGFR/CCr, and SCr, which did not allow us to analyze whether these factors affected the heterogeneity of pregnancy outcomes.
Effect of Pregnancy on Kidney Disease
Eight studies (23,25,29,31,34,36,38) reported 216 renal outcomes in 1268 participants. Renal outcomes were defined as doubling of SCr levels, 50% decrement of eGFR/CCr, or ESRD. Compared with the control group, there was no difference in renal outcomes of pregnant women with CKD (OR, 0.96; 95% CI, 0.69 to 1.35; P=0.83) (Figure 5). Only part of one study (36) enrolled pregnant women with low kidney function (SCr >1.2 mg/dl) before pregnancy. There was also no significant difference in renal outcomes compared with the control group (OR, 0.93; 95% CI, 0.17 to 5.15; Figure 5). Subgroup analysis indicated that there was no significant difference according to type of participants, CKD stage, baseline systolic BP, baseline proteinuria, and level of SCr, publication year, sample size, and follow-up year (Figure 6).
Figure 5.
Overall odds ratios of the association of pregnancy and renal events (including doubling of serum creatinine levels, 50% decrement of eGFR/CCr, and ESRD). 95% CI, 95% confidence interval; CCr, creatinine clearance rate.
Figure 6.
Odds ratios of pregnancy on renal outcomes according to subgroups of sample size, publication year, follow-up year, type of study population, CKD classification, baseline serum creatinine, baseline systolic BP, and baseline proteinuria. 95% CI, 95% confidence interval; A, albuminuria (mg/24 h); DN, diabetic nephropathy; SBP, systolic BP; SCr, serum creatinine; T, total proteinuria (g/24 h).
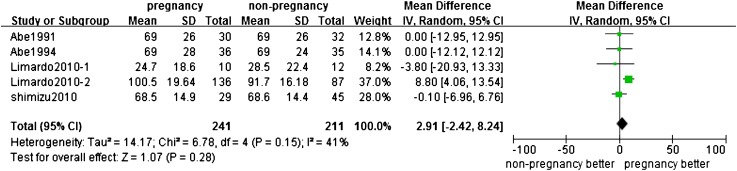
Four studies (23,25,26,38) reported the eGFR/CCr at the end of follow-up point (452 participants). There was no significant difference in eGFR/CCr at the end of studies between pregnant groups and nonpregnant groups (mean difference 2.91 ml/min; 95% CI, −2.42 to 8.24; P=0.28; Figure 7).
Figure 7.
Outcome of eGFR/CCr in women with CKD after pregnancy compared with non-pregnancy. 95% CI, 95% confidence interval; CCr, creatinine clearance rate. IV, method of analysis was inverse variance.
Risk of Bias within Studies
The GRADE evaluation indicated that the outcomes of preeclampsia and premature delivery had high-quality evidence. However, the quality of the evidence on SGA/low birth weight was low, and failure of pregnancy, cesarean section, and renal events were very low (details are provided in the Supplemental Table 1).
Funnel plots suggested no statistical evidence of publication bias by Begg’s test (preeclampsia, P=0.92; failure of pregnancy, P=0.86; renal outcomes, P=0.75). The figures are displayed in the Supplemental Figure 4.
Sensitivity Analyses
We explored the reasons for heterogeneity in pregnancy outcomes. The baseline proteinuria level and type of disease were significant effect modifiers and partly accounted for the heterogeneity in preeclampsia and premature delivery (Figure 4). We also excluded one study that involved the smallest number of participants from all of the analyses: None of the results (including pregnant complications and renal outcomes) were altered.
Discussion
Our meta-analysis of 23 selected studies including 504,826 pregnancies and 1514 pregnancies with CKD investigated the associations between pregnancy and CKD outcomes as well as CKD and fetal/maternal outcomes during pregnancy. The key finding of the systematic review of 216 renal events in 1268 participants was that there was no significant difference in renal outcomes in pregnant women compared with nonpregnant women who had CKD. This association was uniformly consistent across subgroups characterized by type of kidney disease, grade of CKD, systolic BP, and urinary proteins, which are traditionally considered important risk factors for the decline of renal function. We also observed that adverse pregnancy events, including preeclampsia, premature birth, SGA/low birth weight, and cesarean section, were remarkably higher in women with CKD than in women without CKD. In particular, risks of failure of pregnancy, including stillbirth, fetal death, and neonatal death were higher in pregnant women with CKD.
The odds of adverse pregnancy outcomes were different for various renal diseases. The odds of premature delivery and preeclampsia were significantly higher in women with nondiabetic nephropathy compared with those with DN. One reason for the difference might be the different stage of renal function. The definition of DN in the studies retrieved was the detection of proteinuria, and renal function was in the normal range. However, renal function in two studies (28,33) with nondiabetic nephropathy was insufficient. This indicated that renal function status might influence the pregnancy outcomes in patients with CKD. However, further analysis was impossible because of a lack of sufficient baseline data in our study. Some studies indicated that pregnant women with low kidney function delivered preterm because of complications, including worsening renal function, increasing BP, and anemia (9,41). Therefore, renal insufficiency might have an effect on the risk of premature delivery and preeclampsia.
Some pregnant women with CKD in the studies retrieved in our meta-analysis had normal renal function, and the definition of CKD was more likely according to albuminuria (17–22,29,30,32,36). We found that the odds of preeclampsia and premature delivery were higher in women with macroproteinuria compared with women with microproteinuria. Higher rates of successful pregnancy outcomes in women with DN have been reported with treatment with angiotensin-converting enzyme inhibitors combined with strict metabolic control for at least 6 months before gestation (42). However, inhibitors of the renin angiotensin system should not be continued once pregnancy is either planned or detected because of serious risk of fetal malformations. Therefore, measuring proteinuria is important before or early in pregnancy, because proteinuria could predict patients at high risk for complications (43).
Traditionally, it was considered that CKD progressively worsens in pregnant women, especially with serious pathologic changes or renal insufficiency before pregnancy (42–44). Therefore, women with CKD were usually advised to avoid gestation, except for recipients of renal transplants with stable renal function. However, in our meta-analysis, there was no significant difference in renal outcomes between pregnant women with stage 1–3 CKD and those without pregnancy. One possible reason was that women with serious renal insufficiency were unable to experience gestation. In addition, many pregnant women may have chosen to terminate gestation when their renal disease began to deteriorate. The termination of pregnancy would stop or reverse the progression of renal disease, which would make it possible for renal outcomes in such pregnant women to become comparable with nonpregnant women with CKD. However, because most of the studies examining CKD outcomes among pregnant women included patients with IgA nephropathy, these results may not be extrapolated to groups of patients with other underlying causes of renal disease.
Our review had a number of strengths. We compared not only maternal and fetal outcomes between women with and without CKD but also CKD progression in women with CKD who did or did not become pregnant. A previous systematic review conducted by Nevis et al. (10) assessed the maternal and fetal outcomes only. Another systematic review (9) produced bias in selection on account of excluding IgA nephropathy, which is the most common primary GN.
There were several limitations in our meta-analysis. First, the data recorded in our study were not robust, because most related studies were performed in a single center with limited numbers of participants and overall low methodologic quality. Second, there was insufficient data to assess the degree of risk at various levels of albuminuria and SCr/eGFR on adverse pregnant outcomes. Third, there were only a few studies (23–25) that assessed the association between renal pathology and pregnancy outcome. Finally, we could not evaluate the effect of pregnancy on patients with stage 4 CKD because of a lack of relevant studies. Furthermore, our review did not analyze the subgroup data for important risk factors for renal events (including heavy proteinuria >3g/d or hypertension) because there were no individual patient data.
The risks of adverse maternal and fetal outcomes were significantly higher in women with CKD compared with women without CKD. We recommend that women be assessed for renal disease when they plan to become pregnant. Pregnancy did not lead to a decline in renal function in women with relative normal renal function before pregnancy; however, long-term evaluation of the incidence of CKD progression, or ESRD in women with CKD who terminate pregnancy as renal function worsens, needs further assessment.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank the native English-speaking scientists of Elixigen Company (Huntington Beach, CA) for editing our manuscript.
This work was supported by grants from the National Natural Science Foundation (81100503) and the Natural Science Foundation of China to the Innovation Research Group (81021004).
And we thank the authors J.J.Z and X.X.M for their work in the evaluating studies and abstracting the data.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.09250914/-/DCSupplemental.
References
- 1.Chadban SJ, Briganti EM, Kerr PG, Dunstan DW, Welborn TA, Zimmet PZ, Atkins RC: Prevalence of kidney damage in Australian adults: The AusDiab kidney study. J Am Soc Nephrol 14[Suppl 2]: S131–S138, 2003 [DOI] [PubMed] [Google Scholar]
- 2.US Centers for Disease Control and Prevention: National Health and Nutrition Examination Survey. Available at: http://www.cdc.gov/nchs/nhanes.htm. Accessed September 22 2010
- 3.Zhang L, Wang F, Wang L, Wang W, Liu B, Liu J, Chen M, He Q, Liao Y, Yu X, Chen N, Zhang JE, Hu Z, Liu F, Hong D, Ma L, Liu H, Zhou X, Chen J, Pan L, Chen W, Wang W, Li X, Wang H: Prevalence of chronic kidney disease in China: A cross-sectional survey. Lancet 379: 815–822, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Hou S: Historical perspective of pregnancy in chronic kidney disease. Adv Chronic Kidney Dis 14: 116–118, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Vidaeff AC, Yeomans ER, Ramin SM: Pregnancy in women with renal disease. Part I: General principles. Am J Perinatol 25: 385–397, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Fischer MJ: Chronic kidney disease and pregnancy: Maternal and fetal outcomes. Adv Chronic Kidney Dis 14: 132–145, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Podymow T, August P, Akbari A: Management of renal disease in pregnancy. Obstet Gynecol Clin North Am 37: 195–210, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Williams D, Davison J: Chronic kidney disease in pregnancy. BMJ 336: 211–215, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piccoli GB, Conijn A, Attini R, Biolcati M, Bossotti C, Consiglio V, Deagostini MC, Todros T: Pregnancy in chronic kidney disease: Need for a common language. J Nephrol 24: 282–299, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Nevis IF, Reitsma A, Dominic A, McDonald S, Thabane L, Akl EA, Hladunewich M, Akbari A, Joseph G, Sia W, Iansavichus AV, Garg AX: Pregnancy outcomes in women with chronic kidney disease: A systematic review. Clin J Am Soc Nephrol 6: 2587–2598, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group : Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J Clin Epidemiol 62: 1006–1012, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB: Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283: 2008–2012, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Su X, Lv J, Liu Y, Shi S, Liu L, Zhang H: Pregnancy outcomes in patients with IgA nephropathy. Presented at Kidney Week 2014, Philadelphia, November 11–16, 201418436948 [Google Scholar]
- 14.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ, GRADE Working Group : GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336: 924–926, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buscemi N, Hartling L, Vandermeer B, Tjosvold L, Klassen TP: Single data extraction generated more errors than double data extraction in systematic reviews. J Clin Epidemiol 59: 697–703, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Woodward M: Epidemiology: Study Design and Data Analysis, Boca Raton, FL, Chapman and Hall/CRC, 2005 [Google Scholar]
- 17.Biesenbach G, Grafinger P, Zazgornik J, Stöger H: Perinatal complications and three-year follow up of infants of diabetic mothers with diabetic nephropathy stage IV. Ren Fail 22: 573–580, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Combs CA, Rosenn B, Kitzmiller JL, Khoury JC, Wheeler BC, Miodovnik M: Early-pregnancy proteinuria in diabetes related to preeclampsia. Obstet Gynecol 82: 802–807, 1993 [PubMed] [Google Scholar]
- 19.Ekbom P, Damm P, Feldt-Rasmussen B, Feldt-Rasmussen U, Mølvig J, Mathiesen ER: Pregnancy outcome in type 1 diabetic women with microalbuminuria. Diabetes Care 24: 1739–1744, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Jensen DM, Damm P, Ovesen P, Mølsted-Pedersen L, Beck-Nielsen H, Westergaard JG, Moeller M, Mathiesen ER: Microalbuminuria, preeclampsia, and preterm delivery in pregnant women with type 1 diabetes: Results from a nationwide Danish study. Diabetes Care 33: 90–94, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nielsen LR, Damm P, Mathiesen ER: Improved pregnancy outcome in type 1 diabetic women with microalbuminuria or diabetic nephropathy: Effect of intensified antihypertensive therapy? Diabetes Care 32: 38–44, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young EC, Pires MLE, Marques LPJ, de Oliveira JEP, Zajdenverg L: Effects of pregnancy on the onset and progression of diabetic nephropathy and of diabetic nephropathy on pregnancy outcomes. Diabetes Metab Syndr 5: 137–142, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Abe S: Pregnancy in IgA nephropathy. Kidney Int 40: 1098–1102, 1991 [DOI] [PubMed] [Google Scholar]
- 24.Limardo M, Imbasciati E, Ravani P, Surian M, Torres D, Gregorini G, Magistroni R, Casellato D, Gammaro L, Pozzi C, Rene e Gravidanza Collaborative Group of the Italian Society of Nephrology : Pregnancy and progression of IgA nephropathy: Results of an Italian multicenter study. Am J Kidney Dis 56: 506–512, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Abe S: The influence of pregnancy on the long-term renal prognosis of IgA nephropathy. Clin Nephrol 41: 61–64, 1994 [PubMed] [Google Scholar]
- 26.Shimizu A, Takei T, Moriyama T, Itabashi M, Uchida K, Nitta K: Effect of kidney disease stage on pregnancy and delivery outcomes among patients with immunoglobulin A nephropathy. Am J Nephrol 32: 456–461, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Fink JC, Schwartz SM, Benedetti TJ, Stehman-Breen CO: Increased risk of adverse maternal and infant outcomes among women with renal disease. Paediatr Perinat Epidemiol 12: 277–287, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Trevisan G, Ramos JG, Martins-Costa S, Barros EJ: Pregnancy in patients with chronic renal insufficiency at Hospital de Clínicas of Porto Alegre, Brazil. Ren Fail 26: 29–34, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Hemmelder MH, de Zeeuw D, Fidler V, de Jong PE: Proteinuria: A risk factor for pregnancy-related renal function decline in primary glomerular disease? Am J Kidney Dis 26: 187–192, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Kimmerle R, Zass RP, Cupisti S, Somville T, Bender R, Pawlowski B, Berger M: Pregnancies in women with diabetic nephropathy: Long-term outcome for mother and child. Diabetologia 38: 227–235, 1995 [PubMed] [Google Scholar]
- 31.Jungers P, Houillier P, Forget D, Labrunie M, Skhiri H, Giatras I, Descamps-Latscha B: Influence of pregnancy on the course of primary chronic glomerulonephritis. Lancet 346: 1122–1124, 1995 [DOI] [PubMed] [Google Scholar]
- 32.Miodovnik M, Rosenn BM, Khoury JC, Grigsby JL, Siddiqi TA: Does pregnancy increase the risk for development and progression of diabetic nephropathy? Am J Obstet Gynecol 174: 1180–1189, discussion 1189–1191, 1996 [DOI] [PubMed] [Google Scholar]
- 33.Piccoli GB, Attini R, Vasario E, Conijn A, Biolcati M, D’Amico F, Consiglio V, Bontempo S, Todros T: Pregnancy and chronic kidney disease: A challenge in all CKD stages. Clin J Am Soc Nephrol 5: 844–855, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barceló P, López-Lillo J, Cabero L, Del Río G: Successful pregnancy in primary glomerular disease. Kidney Int 30: 914–919, 1986 [DOI] [PubMed] [Google Scholar]
- 35.Leppert P, Tisher CC, Cheng SC, Harlan WR: Antecedent renal disease and the outcome of pregnancy. Ann Intern Med 90: 747–751, 1979 [DOI] [PubMed] [Google Scholar]
- 36.Rossing K, Jacobsen P, Hommel E, Mathiesen E, Svenningsen A, Rossing P, Parving HH: Pregnancy and progression of diabetic nephropathy. Diabetologia 45: 36–41, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Farwell J, Emerson J, Wyatt S, Rueda J, Cheng Y, Caughey A: Outcomes of pregnancies complicated by chronic kidney disease. Am J Obstet Gynecol 208: S153–S154, 2013 [Google Scholar]
- 38.Limardo M: [Pregnancy in women with renal disease: Consolidated evidence and open issues]. G Ital Nefrol 27: 444, 2010 [PubMed] [Google Scholar]
- 39.Masuyama H, Nobumoto E, Okimoto N, Inoue S, Segawa T, Hiramatsu Y: Superimposed preeclampsia in women with chronic kidney disease. Gynecol Obstet Invest 74: 274–281, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Bar J, Chen R, Schoenfeld A, Orvieto R, Yahav J, Ben-Rafael Z, Hod M: Pregnancy outcome in patients with insulin dependent diabetes mellitus and diabetic nephropathy treated with ACE inhibitors before pregnancy. J Pediatr Endocrinol Metab 12: 659–665, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Leguizamon G, Reece EA: Effect of medical therapy on progressive nephropathy: Influence of pregnancy, diabetes and hypertension. J Matern Fetal Med 9: 70–78, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Hou SH, Grossman SD, Madias NE: Pregnancy in women with renal disease and moderate renal insufficiency. Am J Med 78: 185–194, 1985 [DOI] [PubMed] [Google Scholar]
- 43.Packham DK, North RA, Fairley KF, Whitworth JA, Kincaid-Smith P: IgA glomerulonephritis and pregnancy. Clin Nephrol 30: 15–21, 1988 [PubMed] [Google Scholar]
- 44.Jungers P, Chauveau D, Choukroun G, Moynot A, Skhiri H, Houillier P, Forget D, Grünfeld JP: Pregnancy in women with impaired renal function. Clin Nephrol 47: 281–288, 1997 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.