Abstract
Chemotherapy is the primary established systemic treatment for patients with triple-negative breast cancer (TNBC) in both the early and advanced-stages of the disease. The lack of targeted therapies and the poor prognosis of patients with TNBC have fostered a major effort to discover actionable molecular targets to treat patients with these tumours. Massively parallel sequencing and other ‘omics’ technologies have revealed an unexpected level of heterogeneity of TNBCs and have led to the identification of potentially actionable molecular features in some TNBCs, such as germline BRCA1/2 mutations or ‘BRCAness’, the presence of the androgen receptor, and several rare genomic alterations. Whether these alterations are molecular ‘drivers’, however, has not been clearly established. A subgroup of TNBCs shows a high degree of tumour-infiltrating lymphocytes that also correlates with a lower risk of disease relapse and a higher likelihood of benefit from chemotherapy. Proof-of-principle studies with immune-checkpoint inhibitors in advanced-stage TNBC have yielded promising results, indicating the potential benefit of immunotherapy for patients with TNBC. In this Review, we discuss the most relevant molecular findings in TNBC from the past decade and the most promising therapeutic opportunities derived from these data.
The clinical and molecular heterogeneity of breast cancer is well known. The advancement and widespread application of ‘omics’ technologies (genomics, epigenomics, transcriptomics or proteomics, among others) has provided unprecedented insights and novel understanding of the molecular complexity of this disease1–5. In spite of this complexity, clinical decisions still rely primarily on the assessment of three markers: the expression of the endocrine receptors for oestrogen and progesterone (ER and PgR, respectively) and the aberrant expression of HER2. The definition of triple-negative breast cancer (TNBC) applies to all tumours that lack the expression of ER, PgR and HER2, all of which are molecular targets of therapeutic agents. Nevertheless, chemotherapy is still the primary established treatment option for patients with early-stage and those with advanced-stage TNBC6.
Patients with TNBC typically have a relatively poorer outcome compared with those with other breast cancer subtypes owing to an inherently aggressive clinical behaviour and a lack of recognized molecular targets for therapy7. Herein, we summarize the current understanding of the molecular landscape of TNBC and describe the molecular and biological features that are emerging as possible actionable targets for the treatment of this disease.
Immunohistochemical definition of TNBC
The diagnosis of TNBC depends on the accurate assessment of ER and PgR protein expression levels by immunohistochemistry (IHC), and of HER2 by IHC and/or fluorescence in situ hybridization (FISH). The accuracy of this assessment is crucial to avoid the risk of a false diagnosis of ER-negative and/or HER2-negative disease in patients that would potentially benefit from endocrine therapy and/or HER2-targeted drugs. Many efforts have been made to optimize and standardize the methods for measuring the status of ER, PgR and HER2 (REFS 8,9). The assessment of these markers, however, is still subject to significant pre-analytical, analytical and post-analytical variability, as illustrated by the persistent discrepancy of the results from central and local laboratory assessments10,11. Data from gene expression studies12,13 have confirmed that a conservative cut-off point of <1% of ER/PgR-positive tumour cells (assessed using IHC) should be adopted — as suggested by current guidelines8 — to reduce the number of breast tumours inappropriately defined as TNBC.
Key points.
The routine diagnosis of triple-negative breast cancer (TNBC) depends on the accurate assessment of the status of the oestrogen receptor (ER), progesterone receptor (PgR) and HER2
Chemotherapy remains the standard therapeutic approach for TNBC at all stages, with platinum compounds having a relevant role, especially in patients harbouring BRCA1/2 mutations or ‘BRCAness’
‘Omics’ technologies have provided unprecedented insights into the molecular complexity and heterogeneous clinical behaviour of TNBC but, to date, none of the newly developed molecular classifications has demonstrated clinical utility
Several potentially actionable molecular alterations, frequently affecting PI3K/mTOR or RAS/RAF/MEK, have been found in TNBC, but none have been confirmed as a ‘driver alteration’, nor have any TNBC subsets been shown to be ‘addicted’ to them
Targeted agents currently under clinical investigation in TNBC include PARP inhibitors, PI3K inhibitors, MEK inhibitors, anti-androgen therapies, heat shock protein 90 inhibitors, histone deacetylase inhibitors, and their combinations
TNBC is remarkably heterogeneous in terms of the tumour microenvironment; tumour lymphocyte infiltration is associated with good prognosis and a response to chemotherapy, which provides a strong rationale for testing immunotherapies in TNBC
The molecular landscape of TNBC
Beyond the need for an accurate diagnosis of TNBC, this type of tumour has a heterogeneous clinical behaviour, and patients with this disease frequently have a poor prognosis. Thus, a concerted effort has been undertaken to understand the molecular basis of this heterogeneity and to discover actionable molecular targets for TNBC. Many of these efforts have focused on assigning TNBC molecular characteristics into subgroups with specific courses of disease and homogeneous patterns of sensitivity to chemotherapy or new therapies.
Interpatient heterogeneity
TNBC is considered a single clinical entity and uniformly treated with chemotherapy, but molecular profiling with massively parallel sequencing and other ‘omics’ technologies has revealed an unexpectedly high level of heterogeneity as well as some common features1,4. A balanced approach between the simple pragmatism of the current clinical therapeutic guidelines and the molecular complexity of TNBC would be the desirable approach to the management of patients with this disease. Thus, much research has focused on identifying practical TNBC subtypes bearing uniformly actionable molecular features.
Histological classification
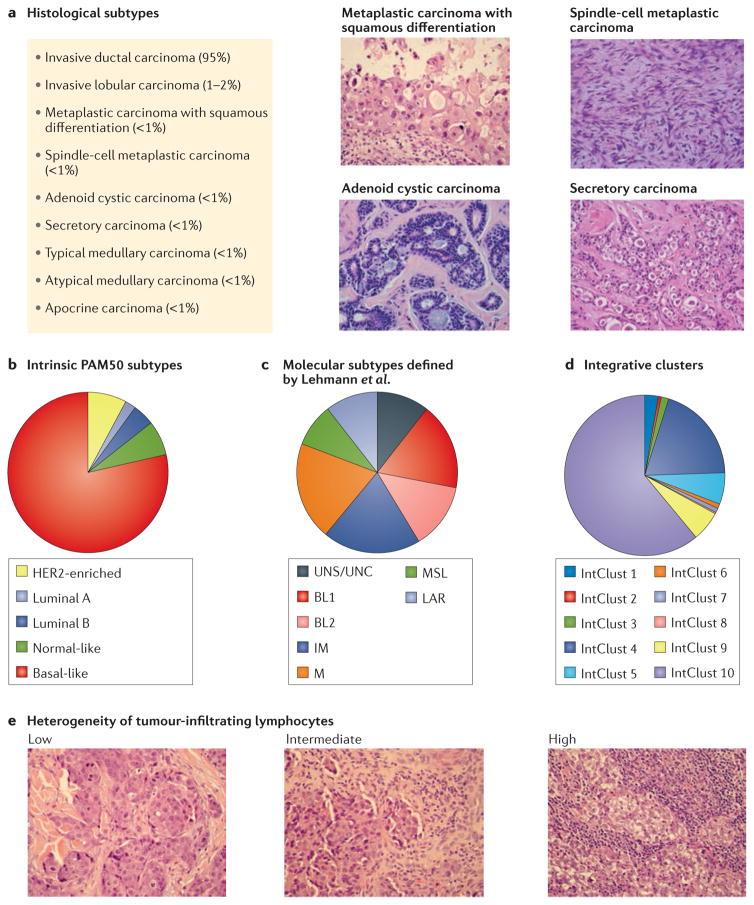
The majority of TNBCs (95%) are classified histologically as invasive mammary carcinomas of no specific type (or, alternatively, invasive ductal carcinomas) and lack distinctive histological characteristics, but other different subtypes have been described14 (FIG. 1). The classically described medullary carcinoma, shown by gene-expression profiling to be a subgroup within TNBC15, is rare (0.4–1%) and characterized by high lymphoplasmacytic infiltration and good outcome compared to other subtypes16. The reproducibility of this histological definition, however, has not been confirmed and whether improved outcomes can be achieved by adapting adjuvant treatment selection for patients within this subgroup is unclear. Other subtypes with unique phenotypes — including adenoid cystic carcinoma14,17, adenosquamous carcinoma and fibromatosis-like spindle-cell metaplastic carcinomas — are rare (<1%) and less aggressive, and typically only capable of local recurrence, a feature that should be considered when planning adjuvant treatment17. Interestingly, adenoid cystic carcinoma constitutes a genomically-distinct subgroup characterized by a low frequency of copy-number aberrations and a characteristic chromosomal translocation t(6;9) (q22–23;p23–24), which leads to the MYB–NFIB fusion gene, present in ~90% of cases of this TNBC subtype17.
Figure 1. The heterogenous landscape of triple-negative breast cancer.
Different approaches to dissect the complex molecular landscape of TNBCs are presented. a | Histological subtypes. Some rare but relevant subtypes are shown for illustrative purposes. b | Gene-expression-based subtypes of triple-negative breast cancer (TNBC) according to PAM5023. c | Gene-expression-based subtypes defined by Lehmann et al.29. d | Integrative clusters (IntClust) of genomic and transcriptomic data applied to basal-like breast cancer (BLBC) defined by gene-expression5. e | Heterogeneity of tumour-infiltrating lymphocytes. Tumours with low, intermediate and high lymphocyte infiltration are shown for illustrative purposes. BL1, basal-like 1; BL2, basal-like 2; IM, immunomodulatory; LAR, luminal androgen receptor; M, mesenchymal; MSL, mesenchymal stem-like; UNC, unclassified; UNS, unstable.
Molecular classification
Several attempts have been made to establish a molecular classification of TNBC based on the results of both transcriptomic and genomic studies. In this section, we do not aim to present all the existing classification systems for TNBC but rather, to discuss those that we consider potentially more relevant from the perspective of clinical benefit or more illustrative of the true degree of molecular heterogeneity, despite attempts to simplify classification. Pivotal studies of gene expression have identified different ‘intrinsic’ subtypes of breast cancer (basal-like, HER2-enriched, luminal A and B and normal-like; FIG. 1)18–20, which largely correspond, but do not overlap, with groups defined by hormone receptors and HER2-status. Notably, the definition of intrinsic subtypes is consistent with a systematic study in which 3,527 specimens from 12 different types of cancer (including breast cancer) were classified into 11 major molecular subtypes mainly related to their ‘tissue-of-origin’ rather than ‘within-a-tissue’ features21. In this study, breast cancer represents one of the exceptions, confirming that basal-like breast cancer (BLBC) is a unique disease entity, despite having the same tissue of origin as luminal/ER-positive tumours18–20. ‘Basal-like’ was the term chosen to define this subgroup because tumour cells express genes characteristic of normal basal/myoepithelial cells, such as KRT5, KRT14 and KRT17 (cytokeratins 5, 14 and 17, respectively), and EGFR22. More than 90% of BLBCs are TNBCs12. Conversely, BLBC represents the most frequent subtype of TNBC (55–81%)23,24 (FIG. 1). Within TNBC, none of the intrinsic subtypes — including BLBC — differ significantly in terms of the rate of pathological complete response (pCR) or survival after neoadjuvant chemotherapy24, and all derive similar benefit from platinum compounds25–27. Basal-like tumours have distinct molecular features compared with other TNBC subtypes23,28, but they also are markedly heterogeneous.
To better dissect TNBC-specific tumour heterogeneity, Lehmann and colleagues29 defined six new TNBC subtypes (TNBCtype) on the basis of gene-expression profiles: two basal-like-related subgroups (basal-like 1 (BL1) and 2 (BL2)), two mesenchymal-related subgroups (mesenchymal (M) and mesenchymal stem-like (MSL)), one immunomodulatory subgroup (IM) and one luminal androgen receptor group (LAR)29 (FIG. 1C). Tumours within the LAR subtype can be defined as TNBCs by IHC but histologically and genetically resemble luminal-like ER-positive breast cancer (FIG. 1). The LAR subtype of TNBC is characterized by expression of the androgen receptor (AR) in the presence of a luminal-like expression signature and thus, might be treated with agents that target AR, as is the case of prostate cancer29. Interestingly, some of the TNBCtype subtypes are closely linked to histological types: IM tumours overlap with medullary breast cancer, M and MSL tumours with metaplastic breast cancer, and LAR tumours with apocrine tumours29.
A direct comparison of 374 TNBC samples extracted from 14 datasets was performed to determine the relationship between the TNBCtype and intrinsic molecular (PAM50) subtypes30. Most TNBCs were classified as basal-like (80.6%), followed by HER2-enriched (10.2%), normal-like (4.7%), luminal B (3.5%) and luminal A (1.1%)30 using PAM50 subtyping. Comparison of the overlap between the PAM50 classification and the TNBCtype showed that, with the exception of MSL and LAR, the majority of the other TNBCtype subtypes were classified as basal-like tumours by PAM50 analysis (BL1(99%), BL2 (95%), IM (84%) and M (97%)). About 50% of MSL TNBCs were classified as basal-like, 28% as normal-like and 14% as luminal B tumours. Finally, tumours within the LAR subtype were mainly classified as HER2-enriched (74%) or luminal B (14%)30.
The association between TNBCtype and response to neoadjuvant chemotherapy was evaluated in a retrospective analysis of 130 patients with TNBC treated with neoadjuvant taxane and anthracycline-based regimens31. Subtype-specific responses differed across groups, with patients with BL1 tumours achieving the highest pCR rate (52%) and patients with tumours classified as BL2, LAR and MSL having the lowest response rates (0%, 10% and 23%, respectively). Overall TNBC subtype was shown to be an independent predictor of pCR status (P = 0.022) using a likelihood ratio test31. However, the sample size of this study31 was small and these findings require additional validation to determine whether TNBC subtypes are useful for predicting the risk of relapse and likelihood of benefit from chemotherapy.
In a different study5, 10 integrative clusters (IntClust) of breast cancer were defined on the basis of combined genomic and transcriptomic data. BLBCs were heterogeneously distributed among different groups (FIG. 1D), with IntClust 4 and 10 accounting for 80% of them5. Breast cancer heterogeneity has clinical relevance, as illustrated by the fact that tumours classified as IntClust 4 have extensive lymphocytic infiltration with a strong immune and inflammatory signature and few copy-number aberrations (‘CNA-devoid’ subgroup) and patients within this subgroup had a favourable outcome. Conversely, BLBCs within the IntClust 10 subtype have high genomic instability and frequently display major chromosomal aberrations, such as chromosome 5 loss, 8q gain, 10p gain or 12p gain5.
Novel druggable molecular alterations
On average, TNBCs carry 1.68 somatic mutations per Mb of coding regions (~60 somatic mutations in each tumour). The mutation burden is not uniform across TNBC, and some tumours have a high mutation burden (more than 4.68 somatic mutations per Mb)1,3, and a frequent occurrence of multiple copy-number aberrations involving genes that lead to multiple pathway alterations1,4,5,32 (BOX 1).
Box 1. Potentially actionable pathways in TNBC.
DNA repair pathway
BRCA1/2 mut/del
PI3K/mTOR pathway
PIK3CA mut/amp, AKT3 amp/mut, PTEN del/mut, TSC1 del/mut, INPP4B del, TSC1
RAS/RAF/MEK pathway
FGFR1 amp, EGFR amp, IGF1R amp, ERBB2 mut, ERBB3 mut, ERBB4 mut, BRAF amp/mut, KRAS amp/mut, HRAS mut, DUSP4 del
Cell-cycle checkpoints
RB1 del, CDK6 amp, CCND1 amp, CCND2 amp
JAK/STAT pathway
JAK2 amp
Other pathways
Androgen receptor pathway
Notch pathway
JNK/AP-1 pathway
HIF1-α/ARNT network
amp, gene amplification; del, gene deletion; mut, gene mutation; TNBC, triple-negative breast cancer.
Among the most frequent mutations in TNBC, only TP53 mutations are found at a high frequency (60–70% of mutations), and are more common in basal-like (62–80%) than in non-basal TNBC (43%)1,4. PIK3CA is the next most commonly mutated gene in TNBC (~10% globally)4,33, although mutations in this gene are significantly more frequent in LAR TNBCs (46.2%) than in the other subtypes (average 4.5%)33. All other mutations occur at a low (1–5%) to very low frequency (<1%) in TNBC, and some of them are actionable (for example, ERBB2)34 or are a target of existing therapies (for example, BRAFV600E)4. The low frequency of TNBC driven by rare aberrations represents a challenge for ad hoc drug development, and requires the implementation of new trial designs and widespread adoption of genomic screening in clinical practice35,36. Potentially actionable deletions (such as PTEN or INPP4B) and amplifications (such as PIK3CA, KRAS, BRAF, EGFR, FGFR1, FGFR2, IGFR1, KIT or MET) have been reported in TNBCs at variable frequencies (1–40%)1,4,5, but their actionability has not been proven yet. Multiple aberrations can affect the same pathway at different levels (BOX 1), which can lead, for example, to a frequent PI3K/AKT pathway activation, meaning that considering targeting PIK3CA alterations alone could be a clinical oversight1,2,4,37. Importantly, the results of these genomic studies have also provided evidence of the substantial clonality and intratumour heterogeneity of TNBC, which can have important clinical implications, such as for the development of resistance to therapies or the lack of responses to targeted therapies4,38–40.
BRCA mutations and ‘BRCAness’
The BRCA1 and BRCA2 genes encode proteins critical for maintaining DNA integrity and genomic stability41,42. BRCA1 and BRCA2 are tumour-suppressor proteins essential for cell division, DNA replication error control, DNA repair and apoptosis. In breast cancer, the presence of germline mutations in BRCA1 or BRCA2 is characterized by a basal-like phenotype (in tumours with BRCA1 mutations but not in those with BRCA2 mutations), ER-negativity, EGFR overexpression, MYC amplification, TP53 mutations, loss of RAD51-dependent focus formation, extreme genomic instability and sensitivity to DNA-crosslinking agents43. The tight association between BRCA1 mutations, a basal-like phenotype and TNBC will be explored in this section.
In addition to BRCA1 and BRCA2, ATM and TP53 are other critical genes in the DNA-damage response signalling pathway, which might have a role in breast tumorigenesis. BRCA1 or BRCA2 are required and critical for the repair of DNA double-strand breaks by homologous recombination44, contribute to DNA repair and transcriptional regulation in response to DNA damage, and regulate other genes involved in DNA repair, the cell cycle and apoptosis45. Increasing evidence supports a role for BRCA1 in double-strand DNA break repair, in part through its interaction with RAD51 and proteins from the Fanconi anaemia group46,47. Cell lines deficient in BRCA1 or other components of the Fanconi anaemia/BRCA1 pathway are more sensitive to X-ray-induced DNA damage and DNA-crosslinking agents, such as cisplatin46,48.
BRCA1 mutations are rare (<5%) in sporadic tumours, but pathological high-grade breast cancers more frequently display loss of heterozygosity (LOH) and/or abnormal expression of ATM, BRCA1 and TP53 (REF. 49). The presence of germline mutations in BRCA1/2 increases the lifetime risk of breast cancer to 60–70%50 and occurs in ~10% of patients with TNBC1,4,51. Promoter methylation52, somatic BRCA1/2 mutation, and gene deletion have been described as alternative mechanisms to impair BRCA1/2 function that likely contribute to ‘BRCAness’ genotypes1,4 — which are associated with a biological and clinical phenotype similar to that of tumours harbouring mutations in BRCA1/2, but without these alterations — but, whether these mechanisms confer the same functional deficiency as germline BRCA1/2 mutations is currently unclear. In BRCA1/2-proficient TNBC, the overexpression of other genes, such as ID4 (REF. 53) or HORMAD1 (REF. 54), can be a potential driver of genomic instability and BRCAness.
Breast tumours arising in patients who carry BRCA1 mutations have many molecular features of basal-like sporadic breast tumours, including a greater likelihood of being high-grade, ER/PgR-negative, HER2-negative, and a high frequency of TP53 mutations55. The existence of a tight association between BRCA1 mutations, basal-like breast cancer and TNBC56 has raised the question as to whether BRCA1 loss of function through other mechanisms participates in the pathogenesis of sporadic BLBC and TNBC; such an association could be exploited therapeutically with rational clinical trials exploring the role of chemotherapy and biological agents targeting defective DNA-repair pathways.
Assays to evaluate BRCAness and HR deficiency
The role of BRCA mutations in homologous recombination-deficiency (HRD) has been well-established, but other diverse molecular alterations can also drive HRD, such as mutations in PALB2, BARD1, RAD51D, BRIP1 (REF. 57) and RAD51C58, and HORMAD1 protein over-expression54, which suppresses RAD51-dependent homologous repair. A number of specific, quantitative biochemical assays have been developed with the aim of establishing a clinical biomarker to define HRD in tumours, such as the HRD–loss of heterozygosity (HRD-LOH) assay59, HRD–telomeric allelic imbalance (HRD-TAI) assay60, and the HRD-large-scale state transition (HRD-LST) assay61. Although promising, the clinical utility of these assays remains to be proven.
Therapies for TNBC
Chemotherapy for TNBC
Cytotoxic chemotherapy remains the mainstay of treatment for TNBC, with data from many studies over the past two decades showing significant benefit of chemotherapy in the neoadjuvant, adjuvant and metastatic setting62,63. Despite the lack of known targetable bio-markers, and an overall poor prognosis, patients with TNBC have a higher response to chemotherapy than patients with other breast cancer types, which is sometimes referred to as the TNBC paradox because of its high risk of recurrence without any treatment but also higher likelihood of benefit from treatment64. Studies of neoadjuvant chemotherapy have consistently reported higher response rates in patients with TNBC than those with non-TNBC65,66, and approximately 30–40% of patients with early-stage TNBC treated with standard neoadjuvant anthracycline and taxane-based chemotherapy regimens achieve a pCR after treatment67. A preferred chemotherapy regimen in the neoadjuvant or adjuvant setting has not yet been determined, but dose-dense and high-dose regimens seem to be more effective in patients with TNBC68–70. Taxanes and anthracyclines are effective treatments of TNBC and remain important agents in this setting71–73.
Despite optimal systemic chemotherapy, fewer than 30% of women with metastatic breast cancer survive 5 years after diagnosis, and virtually all women with metastatic TNBC will ultimately die of their disease74. Many combination chemotherapy regimens have been studied in an effort to improve the outcomes of these patients and, although combination therapies have resulted in improved response rates compared with single agents, this advance has been made at the expense of increased toxicity and with no benefits in patient survival75. For patients with metastatic disease, the ESMO76 and ASCO77 guidelines recommend the use of sequential single-agent chemotherapy, except in the presence of visceral crisis or rapidly progressing disease76,77. No specific chemotherapy agents are considered preferred agents in the metastatic setting; however, capecitabine might not be as effective for patients with TNBC as it is for patients with hormone receptor-positive breast cancer78.
Clinical trials with platinum agents in TNBC
Platinum salts, including carboplatin and cisplatin, lead to DNA crosslink strand breaks that result in apoptosis specifically in cells unable to efficiently repair these lesions. Clinical and preclinical evidence of enhanced sensitivity to DNA-damaging agents in TNBC compared with other subtypes was established over a decade ago79. The intrinsic genomic instability of a subset of TNBCs with defects in DNA repair has generated a growing body of preclinical and clinical data examining the role of platinum-based chemotherapy for TNBC.
In a single arm, multicentre phase II study evaluating platinum monotherapy in 86 patients with metastatic TNBC (mTNBC)26 overall response rates (ORR) of 32% and 19% for cisplatin and carboplatin, were reported, respectively. Patients who carried mutations in BRCA1/2 (n = 11) had a higher ORR (54.5%) than those with wild type BRCA1/2 (n = 66; ORR: 19.7%). Neither basal-like subtype, presence of TP53 mutations, PIK3CA mutations, or p63 and/or p73 status were correlated with durable responses or longer progression-free, or overall survival26. Higher HRD scores were detected in patients with mutations in BRCA1/2 than in those with wild-type BRCA1/2, and were associated with platinum sensitivity. Subsequently, in the Triple-Negative Breast Cancer Trial (TNT)27 investigators randomly allocated 376 patients with mTNBC to receive first-line treatment with either carboplatin or docetaxel. This trial provided no evidence that unselected patients with advanced-stage TNBC are more likely to respond to carboplatin than to docetaxel (ORR: 31.4% and 35.6%, respectively; P=0.44)27. Patients with BRCA1/2 mutations, however, had improved response rates to carboplatin than docetaxel (ORR: 68.0% and 33.3%, respectively; P= 0.03)27. Interestingly, in this study the definition of a dichotomized HRD score did not enable selectivity for sensitivity to carboplatin over docetaxel. Furthermore, a similar performance was observed for treatment with carboplatin or docetaxel in patients with tumours defined as basal-like by PAM50-based Prosigna™ (Nanostring Technologies, Seattle, USA), but docetaxel outperformed carboplatin in patients with non-basal-like tumours27. In summary, the overall clinical efficacy of platinum-based agents in patients with mTNBC has been modest, and molecular assays to identify those patients who will have a better response have not been convincingly better than determining the BRCA1/2 mutation status alone.
Several clinical studies have examined the role of platinum-based agents in the neoadjuvant treatment of patients with TNBC. These studies utilize the pCR rate as an end point because the general assumption is that patients who achieve a pCR exhibit improved long-term outcomes66, whereas a high residual disease burden at post-therapy surgery is correlated with a higher risk of disease recurrence and death80,81. Approximately 30% of women with TNBC treated with anthracycline and taxane-based neoadjuvant chemotherapy will achieve a pCR after treatment63,67. Consistently, patients with TNBC who achieved a pCR after neoadjuvant chemotherapy had improved disease-free survival rates than those patients who did not achieve a pCR (hazard ratio (HR) 0.24; 95% CI 0.18–0.33; P<0.001) 63,67. By contrast, Byrski et al.82 reported in a separate study that 50 out of 82 (61%) patients with stage I–III TNBC harbouring BRCA1 mutations achieved a pCR after neoadjuvant therapy with cisplatin. In another small single-arm study in 28 women with stage II or III TNBC treated with four cycles of neoadjuvant single-agent cisplatin, the pCR rate was 22% (including the only two patients with BRCA1 germline mutations), with four (14.2%) complete responses and 14 (50%) partial responses (overall clinical response rate 64%)83. Finally, in a trial with 51 patients with TNBC treated with neoadjuvant cisplatin and the anti-VEGF antibody bevacizumab84, the pCR was 15%. These results suggest that platinum-based agents are effective as single-agent regimens (and/or bevacizumab) in patients with early-stage TNBC, and support the evaluation of addition of platinum-based agents to standard taxane–anthracycline-based neoadjuvant regimens.
Two large randomized trials further explored the effect of platinum-based agents on TNBC in the neo-adjuvant setting. The CALGB40603 trial85 evaluated the effect of adding carboplatin and/or bevacizumab to paclitaxel followed by dose-dense doxorubicin and cyclophosphamide in 443 patients with stage II or III non-inflammatory TNBC. The addition of carboplatin increased the pCR rate in the breast from 46% to 60% (P = 0.0018), and in breast/axilla from 41% to 54% (P = 0.0029)85. The GeparSixto trial86 included 315 patients with stage II or III that were randomly assigned to receive a regimen containing anthracycline, taxane and bevacizumab, with or without carboplatin. The pCR rate in breast/axilla increased from 36.9% to 53.2% (P = 0.005) for those patients who received carboplatin86. In both trials, however, a substantially increased toxicity was observed, and many patients (48%86 and 20%85) were unable to complete therapy as planned. The addition of cisplatin or carboplatin to neoadjuvant treatment of TNBC is clearly associated with significantly higher rates of pCR, which suggests that the absence of ER/PgR/HER2 could be a potential therapeutic target for these drugs in patients with early-stage disease.
In the PrECOG 0105 single-arm neoadjuvant trial52, 80 patients with TNBC were treated with gemcitabine, carboplatin and the poly(ADP-ribose) polymerase (PARP) inhibitor iniparib52. Patients with mutations in BRCA1/2 had the highest response rate (75%, with residual cancer burden (RCB)87 of 0 or 1). The HRD-LOH assay was used to identify patients without BRCA1/2 mutation and a high HRD-LOH score (>10), who achieved a remarkable response rate (59%). By contrast, in the group of patients without mutations in BRCA1/2 and also a low HRD-LOH score (<10), the favourable responses (RCB 0 or 1) were only 8%52. This result is concordant with the findings of the already mentioned TBCRC009 trial26, but it is in contrast with the results of the TNT study, in which the HRD-LOH assay did not define higher sensitivity to carboplatin over docetaxel27. One possible explanation is that the test might be more efficient at capturing high generic chemosensitivity than platinum-specific sensitivity and, therefore, the clinical utility of this assay remains to be defined.
Despite the added toxicity, a reasonable option is to consider the addition of a platinum-based agent in the neoadjuvant setting for patients with high-risk BRCA-mutated TNBC, and for patients with TNBC, for whom an increased clinical response to systemic treatment could improve locoregional control (for example, patients with inflammatory TNBC or with inoperable TNBC at the time of diagnosis). The clinical utility of the addition of platinum-based agents to standard neoadjuvant and adjuvant chemotherapy regimens, however, remains controversial mainly because of the added toxicities, but also because of the unclear correlation between an improved pCR rate and improved event-free survival (EFS) in the neoadjuvant setting. This lack of correlation was illustrated by the CtNEO meta-analysis across different molecular subtypes63. This study was performed to evaluate whether the improvement of the pCR rate in a neoadjuvant trial can be a reliable predictor (surrogate end point) of an improvement in EFS. The GeparSixto86 and CALGB40603 (REF. 85) phase II trials clearly demonstrated the benefit of adding a platinum-based agent to the systemic treatment of patients with TNBC in the neoadjuvant setting, but these trials were underpowered to address improvements in disease-free survival and overall survival, and were non-informative on these crucial clinical end points. For the time being, the achievement of pCR has good prognostic value for a positive individual clinical outcome but not for predicting the survival benefit that would be derived from a new intervention63.
Targeted therapies
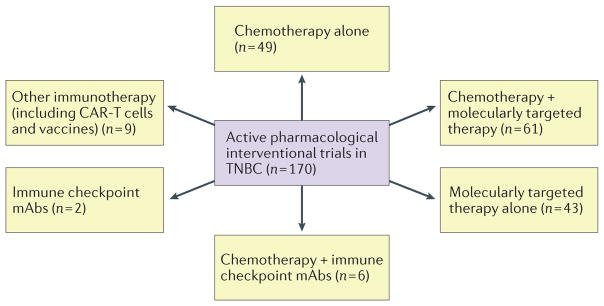
Given the heterogeneity of TNBC, personalized treatment strategies targeting molecular tumour-specific alterations would be the most appropriate to effectively treat the 60–70% of patients with TNBC who do not fully respond to chemotherapy. A number of studies have focused on the identification of molecular targets that might be effectively targeted in TNBC as single agents, or that might circumvent intrinsic resistance to chemotherapy33,88–90. As many as 90% of TNBCs that persist after chemotherapy contain alterations in pathways that can be targeted with agents currently under clinical investigation91, such as PARP inhibitors, PI3K inhibitors, MEK inhibitors, heat shock protein 90 (HSP 90) inhibitors and histone deacetylase (HDAC) inhibitors (FIG. 2).
Figure 2. Active pharmacological interventional trials in TNBC.
An overview of the clinical trials for triple-negative breast cancer (TNBC) active as of July 2015 is depicted (trials including immunotherapies were updated in November 2015). Targets of molecularly targeted therapies included angiogenesis (VEGF, VEGFR, integrins), homologous repair (poly(ADP-ribose) polymerase (PARP)), serine/threonine-protein kinase (Chk1/2), PI3K/mTOR, growth factor receptors (EGFR, MET), hormone receptors (androgen receptor (AR)), Ras/MAPK, and Notch/γ-secretase, among others. Immune-checkpoint inhibitors included anti-programmed cell death 1 (PD-1), anti-programmed cell death 1 ligand 1 (PD-L1) and anti-cytotoxic T-lymphocyte protein 4 (CTLA 4) monoclonal antibodies (mAbs).
PARP inhibitors
PARP is an abundant, constitutively expressed nuclear enzyme that catalyses the transfer of ADP-ribose from NAD+ to target proteins and facilitates DNA repair, cellular proliferation and signalling to other critical cell-cycle proteins and oncogenes through this mechanism92. At sites of DNA damage, PARP activates intracellular signalling pathways that modulate DNA repair and cell survival through poly(ADP)-ribosylation of several nuclear proteins involved in chromatin architecture and DNA metabolism. Immediate catalytic activation of PARP in response to DNA single-strand and double-strand breaks has been reported at levels up to 500-fold of basal levels93,94.
PARP inhibition results in double-strand breaks in replicating cells95. In cells with wild-type BRCA1/2, double-strand breaks are repaired via homologous recombination, but in BRCA1/2-deficient cells, homologous recombination is impaired and thus, DNA strand breaks rely on PARP1 functionality for repair95,96. Therefore, inhibition of PARP1 by RNA interference or with chemical inhibitors leads to severe, highly selective toxicity in BRCA1 and BRCA2-defective cells97, so-called ‘synthetic lethality’ (REF. 98). Importantly, sensitivity to PARP inhibition depends on HRD, which can be caused by mechanisms other than inherited BRCA1 or BRCA2-deficiency95. PARP inhibitors have at least three roles in cancer treatment: sensitization to chemotherapy and radiotherapy, synthetic lethality in tumours from patients with hereditary mutations in BRCA1/2 genes, and leveraging of putative ‘BRCA-like’ defects and defects in DNA repair, such as those identified in several TNBC subtypes95,99.
Significant single-agent activity was reported with the PARP inhibitor olaparib in patients with BRCA-deficient metastatic breast cancer. Overall responses ranged from 22% (100 mg twice per day) to 41% (400 mg twice per day) with minimal toxicity100. A phase II study evaluating olaparib as a single agent for patients with either recurrent high-grade serous or poorly differentiated ovarian carcinoma, or with TNBC101 did not report any responses in patients with TNBC harbouring sporadic mutations in BRCA1/2 (BRCA-proficient), even though the target lesions were reduced in size by >30% in five out of 10 (50%) patients with breast cancer who had BRCA1 or BRCA2 mutations, but were not confirmed objective responders101. Several phase III trials investigating the use of olaparib in the metastatic (NCT02000622) and neoadjuvant (NCT02032823) setting, for patients with mutations in BRCA, are ongoing. Importantly, there are several additional PARP inhibitors currently being tested in clinical trials, such as veliparib (phase III; NCT02163694), talazoparib (phase III; NCT01945775), niraparib (phase III; NCT01905592) and rucaparib (phase I/II; NCT01074970). Iniparib, a putative PARP inhibitor previously investigated in patients with breast cancer102, generated substantial excitement before failing phase III clinical trials (NCT00938652), however, subsequent laboratory investigations proved that this drug was not an effective inhibitor of PARP102.
Mature results from the adaptive neoadjuvant I-SPY2 biomarker trial103 demonstrated that the addition of veliparib and carboplatin to standard chemotherapy regimens for patients with stage II or III TNBC improved pCR rates from 26% to 52%103. However, whether the addition of veliparib to carboplatin contributed in any way to the increase in pCR rate is unclear because the results obtained with combination therapy were quite similar to those observed with the use of carboplatin only in the GeparSixto86 and CALGB40603 (REF. 85) neoadjuvant trials.
Finally, a randomized phase II trial of cisplatin with or without rucaparib104 was initiated for patients with TNBC and/or BRCA mutations who had a residual tumour burden in the breast or axilla after being treated with neoadjuvant chemotherapy consisting of an anthracycline plus a taxane104. Preliminary data show that the disease-free survival at 1 year is similar (~76%) for both treatment groups, and rucaparib did not add substantial toxicity to the cisplatin regimen104. Thus, although PARP inhibitors clearly demonstrate benefit when administered as single agents, their benefit when used in addition to platinum agents is unclear in HRD TNBC.
Anti-androgen therapy
The LAR subtype of TNBC shows sensitivity to AR antagonists in vitro and in vivo 29 (FIG. 3). A phase II trial of the androgen-blocking agent bicalutamide in patients with AR-positive mTNBC was reported105. AR was evaluated using IHC in 424 patients with ER and PgR-negative breast cancer, 12% of whom showed >10% nuclear staining. Patients with AR-positive tumours received single-agent bicalutamide, and 19% of patients had a clinical benefit (defined as a complete or partial response, or stable disease) at 6 months of follow-up, and the median progression-free survival was short (12 weeks)105. Nonetheless, the study provided evidence of the potential efficacy of hormone-directed therapy in AR-positive TNBC105. Subsequently, the results of another phase II study with enzalutamide106, a more-potent AR inhibitor, in advanced-stage AR-positive TNBC were reported. AR prevalence was higher than previously reported, with 55% of patients having ≥10% AR by IHC. In those 75 evaluable patients (AR ≥10%), two complete responses and five partial responses were observed106. Interestingly, an androgen-driven gene signature denoted Dx+, which seemed to predict a superior clinical outcome, was defined by gene profiling106. The proposed signature is of potential clinical relevance, but requires formal validation in independent series of patients.
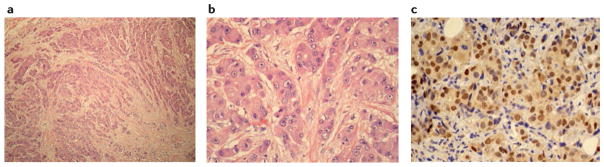
Figure 3. Histological characteristics of luminal androgen receptor (LAR) triple-negative breast cancer.
a | On low magnification, infiltrative nests and cords of eosinophilic tumour cells surrounded by a desmoplastic tumour stroma devoid of tumour-infiltrating lymphocytes can be observed. b | Tumour cells contain large epithelioid nuclei with prominent nucleoli and abundant granular eosinophilic cytoplasm. As depicted, rates of mitosis are frequently low in LAR. c | Nuclear expression of androgen receptors, detectable by immunohistochemistry, is a hallmark of LAR.
LAR-subtype cell lines, which are also enriched with PIK3CA activating mutations, exhibit strong sensitivity to PI3K inhibitors, as well as to androgen blockers29,107. The co-evolution of PIK3CA mutations with AR-dependency is similar to that observed for ER-positive breast cancers, which frequently contain PIK3CA mutations107,108. Thus, the utility of targeting both PI3K and AR is being explored in a phase I/II study of the combination of an androgen blocker (enzalutamide) with a PI3K inhibitor (taselisib) for patients with AR-positive TNBC (NCT02457910). The additive efficacy and toxicity of PI3K inhibition in this patient population will be compared with the available data on enzalutamide monotherapy in TNBC106.
PI3K inhibitors
Mutations affecting ‘hotspot’ regions of the PIK3CA gene, which encodes the PI3K catalytic subunit α, are the most frequent activating mutations in this gene in TNBC (10.2%)1. Additionally, loss of PTEN, a negative regulator of the PI3K pathway, occurs in 9.6% of TNBCs4 and, in general, is mutually exclusive with PIK3CA hotspot mutations.
Preclinical studies of the combination of a DNA damaging agent with PI3K inhibitors have provided a rationale for using PI3K inhibitors in non-LAR tumours by demonstrating that, in addition to regulating cell growth, metabolism, and survival, PI3K also stabilizes double-strand breaks by interacting with the homologous recombination complex and creating a BRCA1/2-deficient-like state109. PI3K blockade promotes HRD by downregulating BRCA1/2, creating a BRCA-mutant-like tumour state and thus, sensitizing BRCA1/2-proficient tumours to PARP inhibition. Consistent with this observation, the concomitant PTEN loss observed in tumours with mutant BRCA1 can reverse HRD110. A phase I study of the pan-PI3K inhibitor buparlisib (BKM120, Novartis, Switzerland) in combination with olaparib for patients with solid tumours that include mTNBC is under way (NCT01623349).
MEK inhibitors
A large number of cell lines derived from TNBC and BLBC are sensitive to MEK inhibition in vitro; BLBC cell lines have broader sensitivity to MEK inhibitors than to PI3K inhibitors111. Several TNBC cell lines that are sensitive to MEK inhibitors harbour mutations that affect the Ras/MAPK pathway, including activating mutations in BRAF, HRAS, or KRAS, which are extremely rare in patients with TNBC112. Nonetheless, many TNBC cell lines show upregulation of the Ras/MAPK pathway without any oncogenic mutation identified in components from the Ras/MAPK pathway113. In those situations, aberrant activation of the Ras/MAPK pathway can be attributed to activation or overexpression of growth factor receptors (such as EGFR, IGF1R, FGFR1 or VEGFR, among others), or to gene copy-number alterations (gains and amplifications) in key Ras/MAPK constituents such as BRAF and KRAS, which have been observed at moderate frequencies in BLBC (30 and 33%, respectively), and result in increased gene expression1,114,115, although the contribution of the latter to pathway activation has not been determined experimentally. Another proposed mechanism of activation of the Ras/MAPK pathway in TNBC is the genetic and/or epigenetic loss of DUSP4, a negative regulator of ERK1/2 and JNK1/2, which is associated with BLBC Ras–ERK activation90,116. In preclinical studies, loss or downregulation of DUSP4 enhances chemotherapeutic resistance in TNBC, and contributes to the maintenance of the tumour-initiating cancer cell population, which can be overcome with inhibitors of the Ras/MAPK pathway, and possibly the JNK/AP1 pathway28,51.
MEK activation can support the stabilization of c-Myc, the product of an important oncogene amplified in ~30% of patients with TNBC or BLBC114,117. Duncan et al.118 demonstrated that, although single-agent MEK inhibition can induce c-Myc degradation in TNBC, this effect simultaneously induces the expression and activation of receptor tyrosine kinases that can overcome MEK inhibition and cause resistance to therapy118. These data suggest that combinations of MEK inhibitors with small molecules or monoclonal antibodies targeting receptor-tyrosine kinases might be effective therapies, but the efficacy of such combinations has not been validated clinically118. The use of MEK inhibitors in combination with chemotherapy or other targeted agents to treat TNBC and BLBC is currently under clinical investigation; however, appropriate biomarkers that might enable optimal patient selection have not been defined. Little data on the efficacy of MEK inhibitors in TNBC have been published, but, importantly, in a phase Ib trial in patients with solid tumours (n= 31) treated with gemcitabine and trametinib (an orally available potent inhibitor of MEK1/2), the only complete response to therapy occurred in a patient with mTNBC119.
Inhibitors of the cancer stem-cell population
Breast cancer stem cells, also known as tumour-initiating cells, represent a dynamic subpopulation of tumour cells that retain the properties of mammary stem cells and, most notably, the ability to repopulate a heterogeneous tumour (with both luminal and basal cytokeratin compartments) from a single cell120. Breast cancer stem cells have slower proliferation rates and higher degrees of resistance to chemotherapy compared with non-stem cancer cells121, and often demonstrate changes in phenotype similar to those associated with cells undergoing epithelial-to- mesenchymal transition122,123. The stem cell population typically represents a small fraction of the overall tumour cell population and can be identified or enriched for in breast cancers through marker-based methods such as measurements of aldehyde dehydrogenase activity (ALDEFLUOR assay)120,124,125, assessing the expression of integrin receptors, or the ability to exclude ABC transporter substrates126,127. Initial findings suggested that this population of cells might represent the subset of tumour cells that, spared by chemotherapy, contribute to resistance and accelerated recurrence after traditional therapies121, and thus, would constitute an ideal target for novel therapeutic discovery in combination with chemotherapy. Among TNBC, BLBCs and some specific subtypes defined by independent groups128,129 are enriched for cancer stem cells. However, while the existence of phenotypic breast cancer stem cells is supported by a large body of preclinical and clinical evidence, the pathways that contribute to the maintenance of this cell population are not clearly defined. Data from independent studies have shown that the Ras/MAPK116, JAK/STAT130, Wnt131, TGF-β132, Hedgehog133 and Notch134 pathways can all contribute to the maintenance of breast cancer stem cells, depending on the particular model or cell line used. As such, no clearly defined ‘inhibitors’ of breast cancer stem cells have been established.
Other molecular targets
Several alterations in molecules and pathways are, or have been under investigation as therapeutic targets to treat metastatic TNBC. EGFR is amplified in about 2% of breast cancers1, and is over-expressed more frequently in BLBC than in non-BLBC. Given this finding, and the long-standing availability of clinically approved inhibitors of EGFR (such as erlotinib, gefitinib, lapatinib, cetuximab and panitumumab), several trials have tested the efficacy of these agents in patients with TNBC. Two phase II trials have reported non-statistically significant improvements in patient outcomes when cetuximab was combined with platinum agents135,136. No patient selection criteria were included in these trials and, therefore, it remains possible that benefit could be derived if patients that harbour EGFR amplification were selected a priori for future trials.
Clinically approved HDAC inhibitors are also under investigation in combination with cisplatin for patients with mTNBC (NCT02393794), or as monotherapy (NCT02623751). Additional areas under investigation include targeting the hypoxia-inducible factor 1-α (HIF1-α) pathway137, c-Met (NCT01738438)138 and integrins (such as integrin β-5) involved in angiogenesis and other tumorigenic phenotypes139. Finally, rare activating mutations in ERBB2 are also found in TNBC, and a clinical trial with neratinib, an irreversible inhibitor of EGFR, is ongoing (NCT01953926)34.
The immune system and TNBC
The key role of the immune system in cancer has been recognized for decades140 but, only in the past few years, immunotherapy has been established as a major cancer therapy141–143, with remarkable activity and curative potential in patients with a broad range of tumours144,145. Our understanding of the complex interplay between cancer and the immune system has improved substantially by moving from the concept of ‘immune surveillance’ (REF. 146) to that of ‘immunoediting’ (REF. 147), a term that well describes the dual host-protecting and tumour-sculpting actions of the immune system, and has three phases: elimination, equilibrium and escape. The elimination phase in the immunoediting hypothesis corresponds with the immune surveillance function. In the equilibrium phase, although the immune system has failed in eliminating all clinically detected tumours, it can be actively and effectively engaged to keep the tumour under control, for instance reducing the risk of metastatic spread148. During the equilibrium phase, the immune system can select cancer cells with a particular genotype and phenotype through evolutionary pressure favouring the development of immunological anergy, tolerance or indifference (escape phase)147. An example of tumour immunoediting in TNBC is provided by the presence of CASP8 mutations37, which can abrogate the death induced by cytolytic CD8+ T cells, and has been described as a common mechanism of immune escape in many solid tumours149.
The original idea that cancer arises as a failure of ‘immune surveillance’ has been the basis for immuno-therapy for many years, in which the main focus of this therapeutic modality was to attempt to induce more effective and specific tumour recognition through vaccines. The results from clinical trials have consistently been frustrating and the only clinical benefits reported have been anecdotical. The current understanding that unique mechanisms of immune regulation and escape are actively engaged during immunoediting to dampen host immunity is the basis for a different therapeutic strategy, consisting of targeting the immune checkpoints to elicit, reinvigorate and potentially expand the magnitude of pre-existing anticancer immune responses150,151.
The higher genomic instability and mutational burden of TNBC results in a higher propensity to generate neoantigens, which can be recognized as ‘non-self’ by the adaptive immune system152. Accordingly, TNBCs have a higher amount of tumour-infiltrating lymphocytes (TILs)153 and higher programmed cell death 1 ligand 1 (PD-L1) protein154,155 or mRNA156,157 expression compared with other breast cancer subtypes. The ligand PD-L1 and its receptor, programmed cell death protein 1 (PD-1), are involved in the regulation of immune tolerance. PD-L1 expression is significantly associated with the presence of TILs154–156, which suggests that the most common mechanism of regulation of PD-L1 expression in TNBC is regulatory feedback (acquired resistance) to immune engagement. An extremely heterogeneous pattern of immune infiltration, however, has been described among TNBC subtypes158 (FIG. 1E). A significant association has also been found between PD-L1 mRNA expression and the presence of PD-L1 copy-number alterations, with BLBC having the highest frequency of PD-L1 gains/amplifications (17%)157. In addition, the loss of PTEN expression in TNBCs is associated with PD-L1 overexpression156, confirming an association between increased PI3K signalling and the presence of PD-L1 (REF. 159). These findings suggest that, in addition to acquired resistance mechanisms, PD-L1 expression can also be regulated by molecular alterations and oncogenic pathways (intrinsic resistance), linking molecular and immune heterogeneity.
The immune system as a biomarker
Prognostic relevance of immune infiltration
High TIL infiltration has been associated with a lower risk of relapse in patients with breast cancer for over 50 years160–161; now these findings can be explored in the context of molecular subtypes. Moreover, to understand the role and contribution of the immune system to the clinical behaviour of breast cancer, it is important to discriminate between ‘pure’ prognostic value (recurrence risk in the absence of any treatment) and predictive value, the latter indicating the effect on recurrence risk that is caused by benefit from systemic treatment. The association between immune system activation and risk of recurrence can be assessed in patients treated with adjuvant or neoadjuvant therapy, but in this patient population the risk is affected both by natural history and treatment benefit, and therefore it is not possible to discriminate between prognostic and predictive biomarkers.
The majority of TNBCs are treated with adjuvant or neoadjuvant chemotherapy and thus, few studies have investigated the prognostic value of assessing the immune system (TABLE 1). In patients with untreated ER-negative breast tumours, the high expression of B-cell and immunoglobulin-based metagenes is associated with a low risk of developing distant metastases162,163, whereas in patients with untreated TNBC, elevated expression of the STAT1-related and T-cell-related metagenes is associated with a low risk of distant-metastasis164,165. A similar trend has been described for a follicular helper T (TFH) cell signature166. Collectively, these studies162–166 suggest that effective engagement of the immune system, although insufficient to eliminate the tumour, can help to reduce the risk of tumour spreading, or maintain tumour dormancy148. This observation supports the existence and relevance of the equilibrium phase described in the cancer immunoediting hypothesis150, however, the mechanistic contribution of the immune system to improved patient outcomes has not been demonstrated yet.
Table 1.
Gene-expression studies on the prognostic and predictive value of immune-related functions in TNBC
| Study | TNBC definition | Immune-related signature | Association with outcome |
|---|---|---|---|
| Prognostic value before adjuvant treatment | |||
| Desmedt et al. (2008)164 | ER-negative/HER2-negative | 95-gene STAT1-related immune metagene | Lower risk of distant metastasis in ‘high expression’ group |
| Callari et al. (2016)165 | ER-negative/HER2-negative | 6-consensus T-cell-related metagene | Lower risk of distant metastasis in ‘high expression’ group |
| Gu-Trantien et al. (2013)166 | ER-negative/HER2-negative | 8-gene follicular helper T (TFH)-cell signature | Marginal significant lower risk of distant metastasis in ‘high expression’ group |
| Predictive value of benefit from neoadjuvant chemotherapy | |||
| Sikov et al. (2015)25 | TNBC | IGG, T-cell, CD8 and B-cell signatures | Higher pCR rate in ‘high expression’ group |
| Sabatier et al. (2015)157 | BLBC | PD-L1 expression (mRNA) | Higher pCR rate in ‘high expression’ group |
| Callari et al. (2016)165 | ER-negative/HER2-negative | 6-consensus T-cell-related metagene | Higher pCR rate in ‘high expression’ group |
| Gu-Trantien et al. (2013)166 | ER-negative/HER2-negative | 8-gene follicular helper T (TFH)-cell signature, TH1-cell signature and CXCL13 | Higher pCR rate in ‘high expression’ group |
| Desmedt et al. (2011)167 | ER-negative/HER2-negative | 95-gene STAT1-related immune metagene | Higher pCR rate in ‘high expression’ group |
| West et al. (2011)168 | ER-negative and TNBC | 8-gene lymphocyte expression signature | Higher pCR rate in ‘high expression’ group |
| Ignatiadis et al. (2012)169 | ER-negative/HER2-negative | 95-gene STAT1 related immune metagene and 7-gene IR module | Higher pCR rate in ‘high expression’ group |
| Denkert et al. (2015)170 | TNBC | Several immune-related genes (CXCL9, PDCD1, CCL5, CXCL13, CD279, CTLA4, IDO1) | Higher pCR rate in ‘high expression’ group |
| Mixed prognostic/predictive value in mixed setting (untreated and systemic CT) | |||
| Sabatier et al. (2015)157 | Basal-like | PD-L1 expression (mRNA) | Lower risk of relapse and death in ‘high expression’ group |
| Teschendorff et al. (2007)178 | ER-negative/HER2-negative | 7-gene prognostic immune-related module | Lower risk of distant metastasis in ‘high expression’ group |
| Rody et al. (2009)179 | ER-negative and ER-negative/HER2-negative | Lymphocyte-specific kinase metagene | Lower risk of relapse in ‘high expression’ group |
| Staaf et al. (2010)180 | BLBC | HER2-derived prognostic predictor-enriched in immune genes | Lower risk of distant metastasis in ‘high expression’ group |
| Sabatier et al. (2011)181 | BLBC | 28-kinase metagene associated with immune response | Superior disease-free survival in ‘high expression’ group |
| Rody et al. (2011)182 | ER-negative/HER2-negative | High B-cell metagene/low IL-8-metagene subgroup | Lower risk of relapse in this group |
| Nagalla et al. (2013)183 | BLBC | B-cell/plasma cell, monocyte/dendritic-cell and T-cell/NK metagenes | Lower risk of relapse in ‘high expression’ group |
BLBC, basal-like breast cancer; CT, chemotherapy; ER, oestrogen receptor; IR, immune response; NK, natural killer cells; pCR, pathological complete response; TNBC, triple negative breast cancer.
Predictive value of the immune system
In TNBC, the association between the presence of TILs or expression of immune markers and the likelihood of achieving a pCR after neoadjuvant chemotherapy is consistent and strong (TABLES 1,2). A high level of expression of immune markers is associated with different immune cell types and functions and has been associated with benefit from chemotherapy in TNBCs25,157,165–171. This association has also been confirmed by evaluation of the TIL density170,171. Overall, these data suggest that the immune system collaborates with the action of chemotherapy in TNBCs, as suggested by data from preclinical studies172,173. However, whether drug-specific immunomodulation properties173, such as inducing immunogenic tumour cell death (postulated for anthracyclines) or engaging different immune effector mechanisms, are associated with different clinical outcomes is unknown and is currently an active area of investigation. Interestingly, assessments of the immune milieu modulation after neoadjuvant chemotherapy in patients with TNBC with residual disease has shown that the immune microenvironment can be turned from ‘cold’ (containing few TILs) to ‘hot’ (higher TIL presence) in some patients174,175. Tumours that remain or become ‘cold’ after chemotherapy have a higher risk of relapse compared with tumours that remain or become ‘hot’ (REFS 176,177). These data also support the concept of chemotherapeutic agents having immunomodulatory activity, and thus, acting as an immunological adjuvant in the tumour microenvironment to stimulate vaccination-induced antitumour immunity174. Whether the immune system has different prognostic and predictive roles in different molecular subtypes of TNBCs has not been defined yet.
Table 2.
Prognostic and predictive value of TILs in TNBC
| Study | TNBC definition | Systemic treatment | TIL assessment*‡ | Association with outcome |
|---|---|---|---|---|
| Predictive value of chemotherapy benefit | ||||
| Denkert et al. (2015)170 | TNBC | Neoadjuvant CT | sTILs as continuous and categorical marker to define LPBC versus non-LPBC | Higher pCR rate in LPBC group |
| Issa-Nummer et al. (2013)171 | ER-negative/HER2-negative | Neoadjuvant CT | TILs as categorical marker to define LPBC versus non-LPBC | No significant association |
| Mixed prognostic/predictive value (baseline TIL assessment) | ||||
| Loi et al. (2013)153 | ER-negative/HER2-negative | Adjuvant CT | sTILs and iTILs as continuous and categorical marker to define LPBC versus non-LPBC | Better DFS and OS in LPBC group |
| Dieci et al. (2015)175 | ER-negative/HER2-negative | Untreated and adjuvant CT | sTILs and iTILs as continuous marker to define LPBC versus non-LPBC | Lower risk of death in LPBC group |
| Loi et al. (2014)184 | TNBC | Adjuvant CT | sTILs as continous marker to define LPBC versus non-LPBC | Lower risk of distant relapse in LPBC group |
| Adams et al. (2014)185 | TNBC | Adjuvant CT | sTILs as continuous and categorical marker to define LPBC versus non-LPBC | Lower risk of distant relapse and death in LPBC group |
| Mixed prognostic/predictive value (post-treatment TIL assessment) | ||||
| Loi et al. (2016)176 | TNBC | Neoadjuvant CT | sTILs assessed in residual disease as continuous and categorical marker to define three groups with high, intermediate and low sTIL content | Lower risk of recurrence and death in high sTIL group in residual disease |
| Dieci et al. (2014)177 | TNBC | Neoadjuvant CT (some also post-surgery CT) | sTILs and iTILs assessed in the residual disease as continuous and categorical marker to define LPBC versus non-LPBC | Lower risk of distant relapse and death in LPBC group |
In continuous evaluation, unit increases in the marker (sTILs or iTILs) — without any cut off points — are evaluated. In categorical evaluation, the marker is evaluated by applying cut-off points to define high and low groups; cut-off points can vary across different studies.
The cut off for lymphocyte predominant breast cancer (LPBC) definition was ≥50% or >50% according to different studies.
CT, chemotherapy; DFS, disease free survival; ER, oestrogen receptor; iTIL, intratumoural TIL; OS, overall survival; pCR, pathological complete response; PgR, progesterone receptor; sTIL, stromal TIL; TIL, tumour-inflitrating lymphocyte; TNBC, triple-negative breast cancer.
Overall, considering that, in TNBCs, a ‘hot’ immune microenvironment is associated with a better prognosis and a higher likelihood of benefit from chemotherapy, it should not be surprising that many investigators have identified a strong association between high levels of immune markers or TILs and a low risk of relapse and/or death in patients with early-stage TNBC treated with systemic chemotherapy153,157,165,175,178–185 (TABLES 1,2). These results suggest that, in TNBC, the risk of recurrence in the early disease setting can be effectively defined by adopting the appropriate immune markers for risk stratification. These results also distinguish a subgroup of patients with TNBC characterized by a ‘cold’ immune microenvironment that has a high risk of relapse, despite treatment, and a low likelihood of benefit from cytotoxic chemotherapy165. This subgroup is a high priority in the list of unmet needs in TNBCs165. Evidence for the clinical utility of TIL evaluation, however, is still scarce, in part because TIL assessment lacks sufficient standardization; however, efforts to improve consistency and reproducibility are under way186,187.
The new age of immunotherapy for TNBC
The findings that a population of TNBC is immunogenic and actively engaged by the immune system provides a strong rationale for testing immunotherapies. Two phase I trials with immune-checkpoint inhibitors in patients with advanced-stage TNBC have been reported188,189. In one of them188, patients with advanced-stage TNBC positive for PD-L1 expression were treated with the anti-PD-1 monoclonal antibody pembrolizumab with a response rate of 18.5% (five out of 27 patients). Seven additional patients had stable-disease188. Of the screened patients, ≥1% PD-L1 expression was detected using IHC labelling of stromal or tumour cells in archival specimens from 58% of patients with the 22C3 antibody188. In another trial with the anti-PD-L1 antibody MPDL3280A189, PD-L1-positive disease (defined as PD-L1 expression in ≥5% TILs as determined by IHC with a proprietary Genentech/Roche antibody), the ORR in the 21 evaluable patients was similar (19%)189. Despite patient selection criteria based on PD-L1 expression, patients with PD-L1-negative disease in three different solid malignancies (melanoma191, squamous-cell non-small-cell lung cancer92 and renal-cell carcinoma93) have already derived substantial survival benefit from the PD-1-targeting antibody nivolumab, thus challenging the validity of PD-L1 as a selection marker. Furthermore, the two breast-cancer trials188,189 have brought into focus the remarkable problem of assay heterogeneity and the scoring criteria adopted, making standardization and harmonization of PD-L1-testing a major issue and an urgent goal. Different trials are ongoing to establish the role of immune-checkpoint inhibitors alone or in combination, and of other immunotherapies in TNBCs (TABLE 3). In light of the promising preliminary results obtained with immune-checkpoint inhibitors, their expected curative potential145 and their beneficial safety profile, these agents are already being assessed for the treatment of patients with early-stage TNBC. Three trials in patients with stage I–III TNBC are currently ongoing to evaluate the potential activity of immune-checkpoint inhibitors in combination with chemotherapy in the neoadjuvant setting. In the phase III trial NeoTRIPaPDL1 (NCT02620280), patients with locally-advanced TNBC will be randomly assigned to receive nab-paclitaxel and carboplatin with or without a PD-L1-inhibitor (atezolizumab). Notably, the primary end point will be event-free survival. A phase II trial will evaluate atezolizumab in combination with nab-paclitaxel (NCT02530489). Finally, a phase I/II trial will test the safety and efficacy of durvalumab, another anti-PD-L1 antibody, in combination with weekly nab-paclitaxel followed by dose-dense chemotherapy containing cyclophosphamide and doxorubicin (NCT02489448).
Table 3.
Clinical trials testing immunotherapies in patients with breast cancer
| Disease setting | Phase | Clinical trial reference number | Breast cancer | Immunotherapies (alone or in combination) | Control arm treatment |
|---|---|---|---|---|---|
| Trials including only patients with TNBC | |||||
| Metastatic | I/II | NCT02513472 | TNBC | Pembrolizumab*/eribulin mesylate | NA |
| II | NCT02499367 | TNBC | Nivolumab*/doxorubicin (low dose) or cyclophosphamide metronomic or radiation therapy or cisplatin | NA | |
| NCT02447003 | TNBC | Pembrolizumab | NA | ||
| III | NCT02555657 | TNBC | Pembrolizumab | Single-agent CT (capecitabine, eribulin, gemcitabine or vinorelbine) | |
| NCT02425891 | TNBC | Atezolizumab‡/nab-paclitaxel | Nab-paclitaxel | ||
| Adjuvant | II | NCT02539017 | TNBC | Vaccine (DC-CIK)/EC followed by docetaxel | EC followed by docetaxel |
| Neoadjuvant | I/II | NCT02489448 | TNBC | Durvalumab‡/nab-paclitaxel followed by ddAC | NA |
| II | NCT02530489 | TNBC | Atezolizumab/nab-paclitaxel | NA | |
| III | NCT02620280 | TNBC (LABC only) | Atezolizumab/nab-paclitaxel/carboplatin | Nab-paclitaxel/carboplatin | |
| Trials including patients with breast cancer | |||||
| Metastatic | I | NCT02303366 | All | Pembrolizumab/stereotactic ablative body radiosurgery | NA |
| I/II | NCT00003432 | All (CEA- positive only) | Vaccine (CEA RNA-pulsed DC) | NA | |
| NCT01421017 | All (with skin metastasis) | Imiquimod (TLR7 agonist)/radiotherapy or cyclophosphamide | NA | ||
| II | NCT02536794 | HER2- negative | Durvalumab/tremelimumab§ | NA | |
| NCT02411656 | HER2- negative | Pembrolizumab | NA | ||
| NCT02563925 | All (with brain metastasis) | Tremelimumab/brain radiotherapy or stereotactic | NA | ||
| NCT00083278 | All | Ipilimumab§ | NA | ||
| NCT01792050 | HER2- negative | Indoximod (IDO inhibitor)/paclitaxel or docetaxel | Paclitaxel or docetaxel | ||
| NCT02491697 | All | Vaccine (DC-CIK)/capecitabine | Capecitabine | ||
BC, breast cancer; CEA, carcinoembryonic antigen; CT, chemotherapy; DC, dendritic cells; DC-CIK, dendritic cells co-cultured with cytokine-induced killer cells; ddAC, dose-dense doxorubicin and cyclophosphamide; EC, epirubicin and cyclophosphamide; LABC, locally advanced breast cancer; TLR7, tool like receptor 7; NA, not applicable; TNBC, triple negative breast cancer.
Anti-programmed cell death protein 1 (PD-1) monoclonal antibodies (mAbs): nivolumab and pembrolizumab (anti-PD1).
Anti programmed cell death 1 ligand 1 (PD L1) mAbs: atezolizumab and durvalumab. § Anti cytotoxic T lymphocyte protein 4 (CTLA 4) mAbs: ipilimumab and tremelimumab.
Conclusions
Massively parallel sequencing technologies have helped to define the molecular landscape of TNBC to an unprecedented resolution. These results have demonstrated that TNBC is comprised of many different disease entities, in sharp contrast with the apparent homogeneity of their nomenclature. The vast heterogeneity of TNBC also extends to the tumour immune microenvironment, which displays a full array of different levels of lymphocyte and monocyte infiltration and activation of inhibitory checkpoints such as PD-1/PD-L1.
The molecular and immune features of TNBC have so far shaped treatment and patient selection. Patients with tumours harbouring BRCA1/2 aberrations might benefit from treatment with platinum compounds and PARP inhibitors; patients with other TNBC types carrying defects in genes related to homologous recombination (‘BRCAness’) could have a similar pattern of drug sensitivity. The majority of actionable genomic alterations are rare in TNBC, but tend to affect more frequently a few molecular pathways (such as PI3K/mTOR or RAS/RAF/MEK). However, the identification of situations in which these are ‘driver alterations’ remains challenging. Several agents targeting these pathways are being investigated but, in light of the low frequency of the target population, the design of new modalities of drug development and novel approaches to genomic breast cancer screening needs to be undertaken.
The variegate nature of the immune microenvironment in TNBC considerably influences the risk of relapse and response to chemotherapy, and has provided the rationale for the application of immunotherapies. The importance of immune-checkpoint inhibitors has already been established in the treatment of many solid tumours, in which they have shown to be game changers. Immune-checkpoint inhibitors, which also have antitumour activity in metastatic TNBC, represent a very promising therapeutic option that is now ready for investigation in the setting of neoadjuvant and adjuvant therapy of early-stage TNBC. All in all, the field is prepared for major breakthroughs in the understanding and treatment of this, so far, therapeutically elusive group of breast cancers. One possible avenue is immuno- molecular therapy, which integrates immune and molecular features to devise novel combinatorial approaches based on targeting intracellular molecular alterations and modulating the immune response176.
Acknowledgments
This report was supported in part by the Associazione Italiana per la Ricerca sul Cancro (AIRC) grant to G.B. (MFGA 13428) and the Fondazione Michelangelo for the advancement of the study and treatment of cancer grant to G.B.
Footnotes
Author contributions
G.B., J.M.B, I.A.M. and L.G. researched data for the article. M.E.S. provided histological pictures. G.B., J.M.B, I.A.M., M.E.S. and L.G. contributed to discussing the article’s content. All authors wrote, reviewed and edited the manuscript before submission.
Competing interests statement
G.B received honorarium from Roche. L.G. had an advisory role for Merck and Roche. J.M.B., I.A.M. and M.E.S. declare no competing interests.
FURTHER INFORMATION
ClinicalTrials.gov: https://www.clinicaltrials.gov
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.The Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banerji S, et al. Sequence analysis of mutations and translocations across breast cancer subtypes. Nature. 2012;486:405–409. doi: 10.1038/nature11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kandoth C, et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502:333–339. doi: 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah SP, et al. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature. 2012;486:395–399. doi: 10.1038/nature10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curtis C, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486:346–352. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network. National Comprehensive Cancer Network Breast Cancer. 2015 https://www.nccn.org/store/login/login.aspx?ReturnURL=http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
- 7.Malorni L, et al. Clinical and biologic features of triple-negative breast cancers in a large cohort of patients with long-term follow-up. Breast Cancer Res Treat. 2012;136:795–804. doi: 10.1007/s10549-012-2315-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hammond ME, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolff AC, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 10.McCullough AE, et al. Central pathology laboratory review of HER2 and ER in early breast cancer: an ALTTO trial [BIG 2-06/NCCTG N063D (Alliance)] ring study. Breast Cancer Res Treat. 2014;143:485–492. doi: 10.1007/s10549-013-2827-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Viale G, et al. High concordance of protein (by IHC), gene (by FISH; HER2 only), and microarray readout (by TargetPrint) of ER, PgR, and HER2: results from the EORTC 10041/BIG 03–04 MINDACT trial. Ann Oncol. 2014;25:816–823. doi: 10.1093/annonc/mdu026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheang MC, et al. Defining breast cancer intrinsic subtypes by quantitative receptor expression. Oncology. 2015;20:474–482. doi: 10.1634/theoncologist.2014-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwamoto T, et al. Estrogen receptor (ER) mRNA and ER-related gene expression in breast cancers that are 1% to 10% ER-positive by immunohistochemistry. J Clin Oncol. 2012;30:729–734. doi: 10.1200/JCO.2011.36.2574. [DOI] [PubMed] [Google Scholar]
- 14.Weigelt B, Reis-Filho JS. Histological and molecular types of breast cancer: is there a unifying taxonomy? Nat Rev Clin Oncol. 2009;6:718–730. doi: 10.1038/nrclinonc.2009.166. [DOI] [PubMed] [Google Scholar]
- 15.Bertucci F, et al. Gene expression profiling shows medullary breast cancer is a subgroup of basal breast cancers. Cancer Res. 2006;66:4636–4644. doi: 10.1158/0008-5472.CAN-06-0031. [DOI] [PubMed] [Google Scholar]
- 16.Huober J, et al. Prognosis of medullary breast cancer: analysis of 13 International Breast Cancer Study Group (IBCSG) trials. Ann Oncol. 2012;23:2843–2851. doi: 10.1093/annonc/mds105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wetterskog D, et al. Adenoid cystic carcinomas constitute a genomically distinct subgroup of triple-negative and basal-like breast cancers. J Pathol. 2012;226:84–96. doi: 10.1002/path.2974. [DOI] [PubMed] [Google Scholar]
- 18.Perou C, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 19.Sorlie T, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parker JS, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27:1160–1167. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoadley KA, et al. Multiplatform analysis of 12 cancer types reveals molecular classification within and across tissues of origin. Cell. 2014;158:929–944. doi: 10.1016/j.cell.2014.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 23.Prat A, et al. Molecular characterization of basal-like and non-basal-like triple-negative breast cancer. Oncology. 2013;18:123–133. doi: 10.1634/theoncologist.2012-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prat A, et al. Predicting response and survival in chemotherapy-treated triple-negative breast cancer. Br J Cancer. 2014;111:1532–1541. doi: 10.1038/bjc.2014.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sikov WM, et al. Abstract S4-05: impact of intrinsic subtype by PAM50 and other gene signatures on pathologic complete response (pCR) rates in triple-negative breast cancer (TNBC) after neoadjuvant chemotherapy (NACT) +/− carboplatin (Cb) or bevacizumab (Bev): CALGB 40603/150709 (Allianc. Cancer Res. 2015;75:S4–05. [Google Scholar]
- 26.Isakoff SJ, et al. TBCRC009: a multicenter phase II clinical trial of platinum monotherapy with biomarker assessment in metastatic triple-negative breast cancer. J Clin Oncol. 2015;33:1902–1909. doi: 10.1200/JCO.2014.57.6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tutt A, et al. Abstract S3-01: the TNT trial: a randomized phase III trial of carboplatin (C) compared with docetaxel (D) for patients with metastatic or recurrent locally advanced triple negative or BRCA1/2 breast cancer (CRUK/07/012) Cancer Res. 2015;75:S30–01. [Google Scholar]
- 28.Carey L, Winer E, Viale G, Cameron D, Gianni L. Triple-negative breast cancer: disease entity or title of convenience? Nat Rev Clin Oncol. 2010;7:683–692. doi: 10.1038/nrclinonc.2010.154. [DOI] [PubMed] [Google Scholar]
- 29.Lehmann BD, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lehmann BD, Pietenpol JA. Identification and use of biomarkers in treatment strategies for triple-negative breast cancer subtypes. J Pathol. 2014;232:142–150. doi: 10.1002/path.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masuda H, et al. Differential response to neoadjuvant chemotherapy among 7 triple-negative breast cancer molecular subtypes. Clin Cancer Res. 2013;19:5533–5540. doi: 10.1158/1078-0432.CCR-13-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ciriello G, et al. Emerging landscape of oncogenic signatures across human cancers. Nat Genet. 2013;45:1127–1133. doi: 10.1038/ng.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lehmann BD, et al. PIK3CA mutations in androgen receptor-positive triple negative breast cancer confer sensitivity to the combination of PI3K and androgen receptor inhibitors. Breast Cancer Res. 2014;16:406. doi: 10.1186/s13058-014-0406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bose R, et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov. 2013;3:224–237. doi: 10.1158/2159-8290.CD-12-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jameson JL, Longo DL. Precision medicine — personalized, problematic, and promising. N Engl J Med. 2015;372:2229–2234. doi: 10.1056/NEJMsb1503104. [DOI] [PubMed] [Google Scholar]
- 36.Redig AJ, Jänne PA. Basket trials and the evolution of clinical trial design in an era of genomic medicine. J Clin Oncol. 2015 doi: 10.1200/JCO.2014.59.8433. http://dx.doi.org/10.1200/JCO.2014.59.8433. [DOI] [PubMed]
- 37.Stephens PJ, et al. The landscape of cancer genes and mutational processes in breast cancer. Nature. 2012;486:400–404. doi: 10.1038/nature11017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ding L, et al. Genome remodelling in a basal-like breast cancer metastasis and xenograft. Nature. 2010;464:999–1005. doi: 10.1038/nature08989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Navin N, et al. Tumour evolution inferred by single-cell sequencing. Nature. 2011;472:90–94. doi: 10.1038/nature09807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y, et al. Clonal evolution in breast cancer revealed by single nucleus genome sequencing. Nature. 2014;512:155–160. doi: 10.1038/nature13600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Venkitaraman AR. Linking the cellular functions of BRCA genes to cancer pathogenesis and treatment. Annu Rev Pathol. 2009;4:461–487. doi: 10.1146/annurev.pathol.3.121806.151422. [DOI] [PubMed] [Google Scholar]
- 42.Atchley DP, et al. Clinical and pathologic characteristics of patients with BRCA-positive and BRCA-negative breast cancer. J Clin Oncol. 2008;26:4282–4288. doi: 10.1200/JCO.2008.16.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turner N, Tutt A, Ashworth A. Hallmarks of ‘BRCAness’ in sporadic cancers. Nat Rev Cancer. 2004;4:814–819. doi: 10.1038/nrc1457. [DOI] [PubMed] [Google Scholar]
- 44.Yun MH, Hiom K. CtIP-BRCA1 modulates the choice of DNA double-strand-break repair pathway throughout the cell cycle. Nature. 2009;459:460–463. doi: 10.1038/nature07955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshida K, Miki Y. Role of BRCA1 and BRCA2 as regulators of DNA repair, transcription, and cell cycle in response to DNA damage. Cancer Sci. 2004;95:866–871. doi: 10.1111/j.1349-7006.2004.tb02195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scully R, et al. Association of BRCA1 with Rad51 in mitotic and meiotic cells. Cell. 1997;88:265–275. doi: 10.1016/s0092-8674(00)81847-4. [DOI] [PubMed] [Google Scholar]
- 47.Taniguchi T, et al. S-phase-specific interaction of the Fanconi anemia protein, FANCD2, with BRCA1 and RAD51. Blood. 2002;100:2414–2420. doi: 10.1182/blood-2002-01-0278. [DOI] [PubMed] [Google Scholar]
- 48.Bhattacharyya A, Ear US, Koller BH, Weichselbaum RR, Bishop DK. The breast cancer susceptibility gene BRCA1 is required for subnuclear assembly of Rad51 and survival following treatment with the DNA cross-linking agent cisplatin. J Biol Chem. 2000;275:23899–23903. doi: 10.1074/jbc.C000276200. [DOI] [PubMed] [Google Scholar]
- 49.Ding SL, et al. Abnormality of the DNA double-strand-break checkpoint/repair genes, ATM, BRCA1 and TP53, in breast cancer is related to tumour grade. Br J Cancer. 2004;90:1995–2001. doi: 10.1038/sj.bjc.6601804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Antoniou A, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Foulkes WD, et al. Germline BRCA1 mutations and a basal epithelial phenotype in breast cancer. J Natl Cancer Inst. 2003;95:1482–1485. doi: 10.1093/jnci/djg050. [DOI] [PubMed] [Google Scholar]
- 52.Telli ML, et al. Phase II study of gemcitabine, carboplatin, and iniparib as neoadjuvant therapy for triple-negative and BRCA1/2 mutation-associated breast cancer with assessment of a tumor-based measure of genomic instability: PrECOG 0105. J Clin Oncol. 2015;33:1895–1901. doi: 10.1200/JCO.2014.57.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Turner NC, et al. BRCA1 dysfunction in sporadic basal-like breast cancer. Oncogene. 2007;26:2126–2132. doi: 10.1038/sj.onc.1210014. [DOI] [PubMed] [Google Scholar]
- 54.Watkins J, et al. Genomic complexity profiling reveals that HORMAD1 overexpression contributes to homologous recombination deficiency in triple-negative breast cancers. Cancer Discov. 2015;5:488–505. doi: 10.1158/2159-8290.CD-14-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matros E, et al. BRCA1 promoter methylation in sporadic breast tumors: relationship to gene expression profiles. Breast Cancer Res Treat. 2005;91:179–186. doi: 10.1007/s10549-004-7603-8. [DOI] [PubMed] [Google Scholar]
- 56.Turner NC, Reis-Filho JS. Basal-like breast cancer and the BRCA1 phenotype. Oncogene. 2006;25:5846–5853. doi: 10.1038/sj.onc.1209876. [DOI] [PubMed] [Google Scholar]
- 57.Couch FJ, et al. Inherited mutations in 17 breast cancer susceptibility genes among a large triple-negative breast cancer cohort unselected for family history of breast cancer. J Clin Oncol. 2015;33:304–311. doi: 10.1200/JCO.2014.57.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schnurbein G, et al. RAD51C deletion screening identifies a recurrent gross deletion in breast cancer and ovarian cancer families. Breast Cancer Res. 2013;15:R120. doi: 10.1186/bcr3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abkevich V, et al. Patterns of genomic loss of heterozygosity predict homologous recombination repair defects in epithelial ovarian cancer. Br J Cancer. 2012;107:1776–1782. doi: 10.1038/bjc.2012.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Birkbak NJ, et al. Telomeric allelic imbalance indicates defective DNA repair and sensitivity to DNA-damaging agents. Cancer Discov. 2012;2:366–375. doi: 10.1158/2159-8290.CD-11-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Popova T, et al. Ploidy and large-scale genomic instability consistently identify basal-like breast carcinomas with BRCA1/2 inactivation. Cancer Res. 2012;72:5454–5462. doi: 10.1158/0008-5472.CAN-12-1470. [DOI] [PubMed] [Google Scholar]
- 62.Early Breast Cancer Trialists’ Collaborative Group. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100 000 women in 123 randomised trials. Lancet. 2012;379:432–444. doi: 10.1016/S0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cortazar P, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 64.Carey LA, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13:2329–2334. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- 65.Darb-Esfahani S, et al. Identification of biology-based breast cancer types with distinct predictive and prognostic features: role of steroid hormone and HER2 receptor expression in patients treated with neoadjuvant anthracycline/taxane-based chemotherapy. Breast Cancer Res. 2009;11:R69. doi: 10.1186/bcr2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liedtke C, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 67.von Minckwitz G, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 68.Citron ML, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21:1431–1439. doi: 10.1200/JCO.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 69.Berry DA, et al. Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA. 2006;295:1658–1667. doi: 10.1001/jama.295.14.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gluz O, et al. Triple-negative high-risk breast cancer derives particular benefit from dose intensification of adjuvant chemotherapy: results of WSG AM-01 trial. Ann Oncol. 2008;19:861–870. doi: 10.1093/annonc/mdm551. [DOI] [PubMed] [Google Scholar]
- 71.Henderson IC, et al. Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21:976–983. doi: 10.1200/JCO.2003.02.063. [DOI] [PubMed] [Google Scholar]
- 72.Mazouni C, et al. Inclusion of taxanes, particularly weekly paclitaxel, in preoperative chemotherapy improves pathologic complete response rate in estrogen receptor-positive breast cancers. Ann Oncol. 2007;18:874–880. doi: 10.1093/annonc/mdm008. [DOI] [PubMed] [Google Scholar]
- 73.Hayes DF, et al. HER2 and response to paclitaxel in node-positive breast cancer. N Engl J Med. 2007;357:1496–1506. doi: 10.1056/NEJMoa071167. [DOI] [PubMed] [Google Scholar]
- 74.Bonotto M, et al. Measures of outcome in metastatic breast cancer: insights from a real-world scenario. Oncologist. 2014;19:608–615. doi: 10.1634/theoncologist.2014-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Conlin AK, Seidman AD. Taxanes in breast cancer: an update. Curr Oncol Rep. 2007;9:22–30. doi: 10.1007/BF02951422. [DOI] [PubMed] [Google Scholar]
- 76.Cardoso F, et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2) Ann Oncol. 2014;25:1871–1888. doi: 10.1093/annonc/mdu385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Partridge AH, et al. Chemotherapy and targeted therapy for women with human epidermal growth factor receptor 2-negative (or unknown) advanced breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2014;32:3307–3329. doi: 10.1200/JCO.2014.56.7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sparano JA, et al. Randomized phase III trial of ixabepilone plus capecitabine versus capecitabine in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2010;28:3256–3263. doi: 10.1200/JCO.2009.24.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kennedy RD, Quinn JE, Mullan PB, Johnston PG, Harkin DP. The role of BRCA1 in the cellular response to chemotherapy. J Natl Cancer Inst. 2004;96:1659–1668. doi: 10.1093/jnci/djh312. [DOI] [PubMed] [Google Scholar]
- 80.Jones RL, et al. The prognostic significance of Ki67 before and after neoadjuvant chemotherapy in breast cancer. Breast Cancer Res Treat. 2009;116:53–68. doi: 10.1007/s10549-008-0081-7. [DOI] [PubMed] [Google Scholar]
- 81.Guarneri V, et al. A prognostic model based on nodal status and Ki-67 predicts the risk of recurrence and death in breast cancer patients with residual disease after preoperative chemotherapy. Ann Oncol. 2009;20:1193–1198. doi: 10.1093/annonc/mdn761. [DOI] [PubMed] [Google Scholar]
- 82.Byrski T, et al. Pathologic complete response to neoadjuvant cisplatin in BRCA1-positive breast cancer patients. Breast Cancer Res Treat. 2014;147:401–405. doi: 10.1007/s10549-014-3100-x. [DOI] [PubMed] [Google Scholar]
- 83.Silver DP, et al. Efficacy of neoadjuvant cisplatin in triple-negative breast cancer. J Clin Oncol. 2010;28:1145–1153. doi: 10.1200/JCO.2009.22.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ryan PD. Neoadjuvant cisplatin and bevacizumab in triple negative breast cancer (TNBC): safety and efficacy [abstract] J Clin Oncol. 2009;27(Suppl):551. [Google Scholar]
- 85.Sikov WM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance) J Clin Oncol. 2015;33:13–21. doi: 10.1200/JCO.2014.57.0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.von Minckwitz G, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol. 2014;15:747–756. doi: 10.1016/S1470-2045(14)70160-3. [DOI] [PubMed] [Google Scholar]
- 87.Symmans WF, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25:4414–4422. doi: 10.1200/JCO.2007.10.6823. [DOI] [PubMed] [Google Scholar]
- 88.Balko J, et al. Profiling of triple-negative breast cancers after neoadjuvant chemotherapy identifies targetable molecular alterations in the treatment-refractory residual disease. Cancer Res. 2012;72:S3–S6. [Google Scholar]
- 89.Bauer JA, et al. RNA interference (RNAi) screening approach identifies agents that enhance paclitaxel activity in breast cancer cells. Breast Cancer Res. 2010;12:R41. doi: 10.1186/bcr2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Balko JM, et al. Profiling of residual breast cancers after neoadjuvant chemotherapy identifies DUSP4 deficiency as a mechanism of drug resistance. Nat Med. 2012;18:1052–1059. doi: 10.1038/nm.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Balko JM, et al. Molecular profiling of the residual disease of triple-negative breast cancers after neoadjuvant chemotherapy identifies actionable therapeutic targets. Cancer Discov. 2014;4:232–245. doi: 10.1158/2159-8290.CD-13-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Audebert M, Salles B, Calsou P. Involvement of poly(ADP-ribose) polymerase-1 and XRCC1/DNA ligase III in an alternative route for DNA double-strand breaks rejoining. J Biol Chem. 2004;279:55117–55126. doi: 10.1074/jbc.M404524200. [DOI] [PubMed] [Google Scholar]
- 93.Shall S, de Murcia G. Poly(ADP-ribose) polymerase-1: what have we learned from the deficient mouse model? Mutat Res. 2000;460:1–15. doi: 10.1016/s0921-8777(00)00016-1. [DOI] [PubMed] [Google Scholar]
- 94.Burkle A. Poly(APD-ribosyl)ation, a DNA damage-driven protein modification and regulator of genomic instability. Cancer Lett. 2001;163:1–5. doi: 10.1016/s0304-3835(00)00694-7. [DOI] [PubMed] [Google Scholar]
- 95.Farmer H, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 96.Bryant HE, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 97.Turner N, Tutt A, Ashworth A. Targeting the DNA repair defect of BRCA tumours. Curr Opin Pharmacol. 2005;5:388–393. doi: 10.1016/j.coph.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 98.Turner NC, et al. A synthetic lethal siRNA screen identifying genes mediating sensitivity to a PARP inhibitor. EMBO J. 2008;27:1368–1377. doi: 10.1038/emboj.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Calabrese CR, et al. Anticancer chemosensitization and radiosensitization by the novel poly(ADP-ribose) polymerase-1 inhibitor AG14361. J Natl Cancer Inst. 2004;96:56–67. doi: 10.1093/jnci/djh005. [DOI] [PubMed] [Google Scholar]
- 100.Tutt A, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376:235–244. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
- 101.Gelmon KA, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 2011;12:852–861. doi: 10.1016/S1470-2045(11)70214-5. [DOI] [PubMed] [Google Scholar]
- 102.Sinha G. Downfall of iniparib: a PARP inhibitor that doesn’t inhibit PARP after all. J Natl Cancer Inst. 2014;106:djt447. doi: 10.1093/jnci/djt447. [DOI] [PubMed] [Google Scholar]
- 103.Rugo HS, et al. Veliparib/carboplatin plus standard neoadjuvant therapy for high-risk breast cancer: first efficacy results from the I-SPY 2 TRIAL [abstract] Cancer Res. 2013;73(Suppl 24):S5–02. [Google Scholar]
- 104.Dwadasi S, et al. Cisplatin with or without rucaparib after preoperative chemotherapy in patients with triple-negative breast cancer (TNBC): Hoosier Oncology Group BRE09-146 [abstract] J Clin Oncol. 2014;32(Suppl):1019. [Google Scholar]
- 105.Gucalp A, et al. Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic breast cancer. Clin Cancer Res. 2013;19:5505–5512. doi: 10.1158/1078-0432.CCR-12-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Traina TA, et al. Results from a phase 2 study of enzalutamide (ENZA), an androgen receptor (AR) inhibitor, in advanced AR+ triple-negative breast cancer (TNBC) [abstract] J Clin Oncol. 2015;33(Suppl):1003. [Google Scholar]
- 107.Gonzalez-Angulo AM, et al. Androgen receptor levels and association with PIK3CA mutations and prognosis in breast cancer. Clin Cancer Res. 2009;15:2472–2478. doi: 10.1158/1078-0432.CCR-08-1763. [DOI] [PubMed] [Google Scholar]
- 108.Stemke-Hale K, et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 2008;68:6084–6091. doi: 10.1158/0008-5472.CAN-07-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ibrahim YH, et al. PI3K inhibition impairs BRCA1/2 expression and sensitizes BRCA-proficient triple-negative breast cancer to PARP inhibition. Cancer Discov. 2012;2:1036–1047. doi: 10.1158/2159-8290.CD-11-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Peng G, et al. Genome-wide transcriptome profiling of homologous recombination DNA repair. Nat Commun. 2014;5:3361. doi: 10.1038/ncomms4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hoeflich KP, et al. In vivo antitumor activity of MEK and phosphatidylinositol 3-kinase inhibitors in basal-like breast cancer models. Clin Cancer Res. 2009;15:4649–4664. doi: 10.1158/1078-0432.CCR-09-0317. [DOI] [PubMed] [Google Scholar]
- 112.Barretina J, et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483:603–607. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Giltnane JM, Balko JM. Rationale for targeting the Ras/MAPK pathway in triple-negative breast cancer. Discov Med. 2014;17:275–283. [PubMed] [Google Scholar]
- 114.Cerami E, et al. The cBio Cancer Genomics Portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Craig DW, et al. Genome and transcriptome sequencing in prospective metastatic triple-negative breast cancer uncovers therapeutic vulnerabilities. Mol Cancer Ther. 2013;12:104–116. doi: 10.1158/1535-7163.MCT-12-0781. [DOI] [PubMed] [Google Scholar]
- 116.Balko JM, et al. Activation of MAPK pathways due to DUSP4 loss promotes cancer stem cell-like phenotypes in basal-like breast cancer. Cancer Res. 2013;73:6346–6358. doi: 10.1158/0008-5472.CAN-13-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Horiuchi D, et al. MYC pathway activation in triple-negative breast cancer is synthetic lethal with CDK inhibition. J Exp Med. 2012;209:679–696. doi: 10.1084/jem.20111512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Duncan JS, et al. Dynamic reprogramming of the kinome in response to targeted MEK inhibition in triple-negative breast cancer. Cell. 2012;149:307–321. doi: 10.1016/j.cell.2012.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Infante JR, et al. A phase 1b study of trametinib, an oral mitogen-activated protein kinase kinase (MEK) inhibitor, in combination with gemcitabine in advanced solid tumours. Eur J Cancer. 2013;49:2077–2085. doi: 10.1016/j.ejca.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 120.Charafe-Jauffret E, et al. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res. 2009;69:1302–1313. doi: 10.1158/0008-5472.CAN-08-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Creighton CJ, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci USA. 2009;106:13820–13825. doi: 10.1073/pnas.0905718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mani SA, et al. The epithelial–mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Creighton CJ, Chang JC, Rosen JM. Epithelial–mesenchymal transition (EMT) in tumor-initiating cells and its clinical implications in breast cancer. J Mammary Gland Biol Neoplasia. 2010;15:253–260. doi: 10.1007/s10911-010-9173-1. [DOI] [PubMed] [Google Scholar]
- 124.Charafe-Jauffret E, et al. Aldehyde dehydrogenase 1-positive cancer stem cells mediate metastasis and poor clinical outcome in inflammatory breast cancer. Clin Cancer Res. 2010;16:45–55. doi: 10.1158/1078-0432.CCR-09-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Huang EH, et al. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res. 2009;69:3382–3389. doi: 10.1158/0008-5472.CAN-08-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pontier SM, Muller WJ. Integrins in mammary-stem-cell biology and breast-cancer progression — a role in cancer stem cells? J Cell Sci. 2009;122:207–214. doi: 10.1242/jcs.040394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Britton KM, et al. Breast cancer, side population cells and ABCG2 expression. Cancer Lett. 2012;323:97–105. doi: 10.1016/j.canlet.2012.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Herschkowitz JI, et al. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol. 2007;8:R76. doi: 10.1186/gb-2007-8-5-r76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Neve RM, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–527. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Marotta LL, et al. The JAK2/STAT3 signaling pathway is required for growth of CD44+CD24– stem cell-like breast cancer cells in human tumors. J Clin Invest. 2011;121:2723–2735. doi: 10.1172/JCI44745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.DiMeo TA, et al. A novel lung metastasis signature links Wnt signaling with cancer cell self-renewal and epithelial–mesenchymal transition in basal-like breast cancer. Cancer Res. 2009;69:5364–5373. doi: 10.1158/0008-5472.CAN-08-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bhola NE, et al. TGF-β inhibition enhances chemotherapy action against triple-negative breast cancer. J Clin Invest. 2013;123:1348–1358. doi: 10.1172/JCI65416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu S, et al. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66:6063–6071. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Harrison H, et al. Regulation of breast cancer stem cell activity by signaling through the Notch4 receptor. Cancer Res. 2010;70:709–718. doi: 10.1158/0008-5472.CAN-09-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Carey LA, et al. TBCRC 001: randomized phase II study of cetuximab in combination with carboplatin in stage IV triple-negative breast cancer. J Clin Oncol. 2012;30:2615–2623. doi: 10.1200/JCO.2010.34.5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Baselga J, et al. Randomized phase II study of the anti-epidermal growth factor receptor monoclonal antibody cetuximab with cisplatin versus cisplatin alone in patients with metastatic triple-negative breast cancer. J Clin Oncol. 2013;31:2586–2592. doi: 10.1200/JCO.2012.46.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hu Y, Liu J, Huang H. Recent agents targeting HIF-1α for cancer therapy. J Cell Biochem. 2013;114:498–509. doi: 10.1002/jcb.24390. [DOI] [PubMed] [Google Scholar]
- 138.Sameni M, et al. Cabozantinib (XL184) inhibits growth and invasion of preclinical TNBC models. Clin Cancer Res. 2016;22:923–934. doi: 10.1158/1078-0432.CCR-15-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bianchi-Smiraglia A, Paesante S, Bakin AV. Integrin β5 contributes to the tumorigenic potential of breast cancer cells through the Src–FAK and MEK–ERK signaling pathways. Oncogene. 2013;32:3049–3058. doi: 10.1038/onc.2012.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ehrlich P. Üeber den jetzigen Stand der Karzinomforschung. Ned Tijdschr Geneeskd. 1909;5:273–290. [Google Scholar]
- 141.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Topalian SL, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Larkin J, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rosenberg SA. Raising the bar: the curative potential of human cancer immunotherapy. Sci Transl Med. 2012;4:127ps8. doi: 10.1126/scitranslmed.3003634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Schadendorf D, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015 doi: 10.1200/JCO.2014.56.2736. http://dx.doi.org/10.1200/JCO.2014.56.2736. [DOI] [PMC free article] [PubMed]
- 146.Burnet FM. The concept of immunological surveillance. Prog Exp Tumor Res. 1970;13:1–27. doi: 10.1159/000386035. [DOI] [PubMed] [Google Scholar]
- 147.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 148.Pusztai L, Karn T, Safonov A, Abu-Khalaf MM, Bianchini G. New strategies in breast cancer: immunotherapy. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-15-1315. http://dx.doi.org/10.1158/1078-0432.CCR-15-1315. [DOI] [PMC free article] [PubMed]
- 149.Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160:48–61. doi: 10.1016/j.cell.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Brown SD. Neo-antigens predicted by tumor genome meta-analysis correlate with increased patient survival. Genome Res. 2014;24:743–750. doi: 10.1101/gr.165985.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Loi S, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02–98. J Clin Oncol. 2013;31:860–867. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
- 154.Wimberly H, et al. PD-L1 expression correlates with tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy in breast cancer. Cancer Immunol Res. 2015;3:326–332. doi: 10.1158/2326-6066.CIR-14-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ali HR, et al. PD-L1 protein expression in breast cancer is rare, enriched in basal-like tumours and associated with infiltrating lymphocytes. Ann Oncol. 2015;26:1488–1493. doi: 10.1093/annonc/mdv192. [DOI] [PubMed] [Google Scholar]
- 156.Mittendorf EA, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2:361–370. doi: 10.1158/2326-6066.CIR-13-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sabatier R, et al. Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget. 2015;6:5449–5464. doi: 10.18632/oncotarget.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Spranger S, et al. Up-regulation of PD-L1, IDO, and Tregs in the melanoma tumor microenvironment is driven by CD8+ T cells. Sci Transl Med. 2013;5:200ra116. doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Crane CA, et al. PI(3) kinase is associated with a mechanism of immunoresistance in breast and prostate cancer. Oncogene. 2008;28:306–312. doi: 10.1038/onc.2008.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Aaltomaa S, et al. Lymphocyte infiltrates as a prognostic variable in female breast cancer. Eur J Cancer. 1992;28:859–864. doi: 10.1016/0959-8049(92)90134-n. [DOI] [PubMed] [Google Scholar]
- 161.Black MM, Speer FD, Opler SR. Structural representations of tumor–host relationships in mammary carcinoma; biologic and prognostic significance. Am J Clin Pathol. 1956;26:250–265. doi: 10.1093/ajcp/26.3.250. [DOI] [PubMed] [Google Scholar]
- 162.Schmidt M, et al. The humoral immune system has a key prognostic impact in node-negative breast cancer. Cancer Res. 2008;68:5405–5413. doi: 10.1158/0008-5472.CAN-07-5206. [DOI] [PubMed] [Google Scholar]
- 163.Bianchini G, et al. Molecular anatomy of breast cancer stroma and its prognostic value in estrogen receptor-positive and -negative cancers. J Clin Oncol. 2010;28:4316–4323. doi: 10.1200/JCO.2009.27.2419. [DOI] [PubMed] [Google Scholar]
- 164.Desmedt C, et al. Biological processes associated with breast cancer clinical outcome depend on the molecular subtypes. Clin Cancer Res. 2008;14:5158–5165. doi: 10.1158/1078-0432.CCR-07-4756. [DOI] [PubMed] [Google Scholar]
- 165.Callari M, et al. Subtype specific metagene-based prediction of outcome after neoadjuvant and adjuvant treatment in breast cancer. Clin Cancer Res. 2016;22:337–345. doi: 10.1158/1078-0432.CCR-15-0757. [DOI] [PubMed] [Google Scholar]
- 166.Gu-Trantien C, et al. CD4+ follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest. 2013;123:2873–2892. doi: 10.1172/JCI67428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Desmedt C, et al. Multifactorial approach to predicting resistance to anthracyclines. J Clin Oncol. 2011;29:1578–1586. doi: 10.1200/JCO.2010.31.2231. [DOI] [PubMed] [Google Scholar]
- 168.West NR, et al. Tumor-infiltrating lymphocytes predict response to anthracycline-based chemotherapy in estrogen receptor-negative breast cancer. Breast Cancer Res. 2011;13:R126. doi: 10.1186/bcr3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ignatiadis M, et al. Gene modules and response to neoadjuvant chemotherapy in breast cancer subtypes: a pooled analysis. J Clin Oncol. 2012;30:1996–2004. doi: 10.1200/JCO.2011.39.5624. [DOI] [PubMed] [Google Scholar]
- 170.Denkert C, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol. 2015;33:983–991. doi: 10.1200/JCO.2014.58.1967. [DOI] [PubMed] [Google Scholar]
- 171.Issa-Nummer Y, et al. Prospective validation of immunological infiltrate for prediction of response to neoadjuvant chemotherapy in HER2-negative breast cancer — a substudy of the neoadjuvant GeparQuinto trial. PLoS ONE. 2013;8:e79775. doi: 10.1371/journal.pone.0079775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- 173.Galluzzi L, Senovilla L, Zitvogel L, Kroemer G. The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov. 2012;11:215–233. doi: 10.1038/nrd3626. [DOI] [PubMed] [Google Scholar]
- 174.Kang TH, et al. Chemotherapy acts as an adjuvant to convert the tumor microenvironment into a highly permissive state for vaccination-induced antitumor immunity. Cancer Res. 2013;73:2493–2504. doi: 10.1158/0008-5472.CAN-12-4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Dieci MV, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in two phase III randomized adjuvant breast cancer trials. Ann Oncol. 2015;26:1698–1704. doi: 10.1093/annonc/mdv239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Loi S, et al. RAS/MAPK activation is associated with reduced tumor-infiltrating lymphocytes in triple-negative breast cancer: therapeutic cooperation between MEK and PD-1/PD-L1 immune checkpoint inhibitors. Clin Cancer Res. 2016;22:1499–1509. doi: 10.1158/1078-0432.CCR-15-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Dieci MV, et al. Prognostic value of tumor-infiltrating lymphocytes on residual disease after primary chemotherapy for triple-negative breast cancer: a retrospective multicenter study. Ann Oncol. 2014;25:611–618. doi: 10.1093/annonc/mdt556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Teschendorff AE, Miremadi A, Pinder SE, Ellis IO, Caldas C. An immune response gene expression module identifies a good prognosis subtype in estrogen receptor negative breast cancer. Genome Biol. 2007;8:R157. doi: 10.1186/gb-2007-8-8-r157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rody A, et al. T-cell metagene predicts a favorable prognosis in estrogen receptor-negative and HER2-positive breast cancers. Breast Cancer Res. 2009;11:R15. doi: 10.1186/bcr2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Staaf J, et al. Identification of subtypes in human epidermal growth factor receptor 2-positive breast cancer reveals a gene signature prognostic of outcome. J Clin Oncol. 2010;28:1813–1820. doi: 10.1200/JCO.2009.22.8775. [DOI] [PubMed] [Google Scholar]
- 181.Sabatier R, et al. A gene expression signature identifies two prognostic subgroups of basal breast cancer. Breast Cancer Res Treat. 2011;126:407–420. doi: 10.1007/s10549-010-0897-9. [DOI] [PubMed] [Google Scholar]
- 182.Rody A, et al. A clinically relevant gene signature in triple negative and basal-like breast cancer. Breast Cancer Res. 2011;13:R97. doi: 10.1186/bcr3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nagalla S, et al. Interactions between immunity, proliferation and molecular subtype in breast cancer prognosis. Genome Biol. 2013;14:R34. doi: 10.1186/gb-2013-14-4-r34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Loi S, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. 2014;25:1544–1550. doi: 10.1093/annonc/mdu112. [DOI] [PubMed] [Google Scholar]
- 185.Adams S, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32:2959–2966. doi: 10.1200/JCO.2013.55.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Salgado R, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26:259–271. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Savas P, et al. Clinical relevance of host immunity in breast cancer: from TILs to the clinic. Nat Rev Clin Oncol. 2016;13:228–241. doi: 10.1038/nrclinonc.2015.215. [DOI] [PubMed] [Google Scholar]
- 188.Nanda R, et al. A phase Ib study of pembrolizumab (MK-3475) in patients with advanced triple-negative breast cancer [abstract] Cancer Res. 2015;75:S1–09. [Google Scholar]
- 189.Emens L, et al. Inhibition of PD-L1 by MPDL3280A leads to clinical activity in patients with metastatic triple-negative breast cancer (TNBC) [abstract] Cancer Res. 2015;75:2859. [Google Scholar]