Abstract
Background
Depression affects approximately one‐quarter of people treated with dialysis and is considered an important research uncertainty by patients and health professionals. Treatment for depression in dialysis patients may have different benefits and harms compared to the general population due to different clearances of antidepressant medication and the severity of somatic symptoms associated with end‐stage kidney disease (ESKD). Guidelines suggest treatment of depression in dialysis patients with pharmacological therapy, preferably a selective serotonin reuptake inhibitor. This is an update of a review first published in 2005.
Objectives
To evaluate the benefit and harms of antidepressants for treating depression in adults with ESKD treated with dialysis.
Search methods
We searched Cochrane Kidney and Transplant's Specialised Register to 20 January 2016 through contact with the Information Specialist using search terms relevant to this review.
Selection criteria
Randomised controlled trials (RCTs) comparing antidepressant treatment with placebo or no treatment, or compared to another antidepressant medication or psychological intervention in adults with ESKD (estimated glomerular filtration rate < 15 mL/min/1.73 m2).
Data collection and analysis
Data were abstracted by two authors independently onto a standard form and subsequently entered into Review Manager. Risk ratios (RR) for dichotomous data and mean differences (MD) for continuous data were calculated with 95% confidence intervals (95% CI).
Main results
Four studies in 170 participants compared antidepressant therapy (fluoxetine, sertraline, citalopram or escitalopram) versus placebo or psychological training for 8 to 12 weeks. In generally very low or ungradeable evidence, compared to placebo, antidepressant therapy had no evidence of benefit on quality of life, had uncertain effects on increasing the risk of hypotension (3 studies, 144 participants: RR 1.72, 95% CI 0.75 to 3.92), headache (2 studies 56 participants: RR 2.91, 95% CI 0.73 to 11.57), and sexual dysfunction (2 studies, 101 participants: RR 3.83, 95% CI 0.63 to 23.34), and increased nausea (3 studies, 114 participants: RR 2.67, 95% CI 1.26 to 5.68). There were few or no data for hospitalisation, suicide or all‐cause mortality resulting in inconclusive evidence. Antidepressant therapy may reduce depression scores during treatment compared to placebo (1 study, 43 participants: MD ‐7.50, 95% CI ‐11.94 to ‐3.06). Antidepressant therapy was not statistically different from group psychological therapy for effects on depression scores or withdrawal from treatment and a range of other outcomes were not measured.
Authors' conclusions
Despite the high prevalence of depression in dialysis patients and the relative priority that patients place on effective treatments, evidence for antidepressant medication in the dialysis setting is sparse and data are generally inconclusive. The relative benefits and harms of antidepressant therapy in dialysis patients are poorly known and large randomised studies of antidepressants versus placebo are required.
Keywords: Adult; Humans; Antidepressive Agents; Antidepressive Agents/therapeutic use; Citalopram; Citalopram/therapeutic use; Depression; Depression/therapy; Fluoxetine; Fluoxetine/therapeutic use; Kidney Failure, Chronic; Kidney Failure, Chronic/psychology; Kidney Failure, Chronic/therapy; Psychotherapy; Quality of Life; Randomized Controlled Trials as Topic; Renal Dialysis; Renal Dialysis/psychology; Sertraline; Sertraline/therapeutic use
Plain language summary
Antidepressants for treating depression in adults with end‐stage kidney disease treated with dialysis
Background
People treated with dialysis frequently experience depression and anxiety. Depression in this situation is linked to poor quality of life and increased complications, such as needing to be admitted to hospital, or stopping dialysis treatment. Patients, their families, and health care workers agree that caring for depression symptoms appropriately and finding effective treatments is really important. Antidepressant drugs may not be removed from the body as quickly for people with kidney disease and so may cause more side effects. Despite depression being very common and treatment having potentially different side‐effects compared with people without kidney disease, a previous version of this review in 2005 found only a single research study. It is unknown whether antidepressant treatment works and is safe for people with kidney failure.
A summary of whether antidepressant therapy works and is safe in people with kidney failure would be relevant to patients and their families, health care workers, and policy makers to generate patient‐centred treatment policies.
This review looks at whether we know whether drug treatment works to improve symptoms of depression in adults treated with dialysis without causing common and severe side effects.
Study characteristics
We included all studies which have looked at drug treatment against placebo (sugar pill) or other kinds of mental health support. People included in the studies had an equal chance of receiving either treatment.
Key results
Unfortunately, even though depression is very common and finding good treatments for depression are highly valued by patients on dialysis, there are only a few small studies to tell us about whether drug treatments are both safe and reduce symptoms. Based on this information, we still don't know whether depression treatment works well for people treated with dialysis and is safe (doesn't cause excess and serious side effects).
Quality of the evidence
The question of whether drugs can reduce symptoms of depression and improve quality of life for people on dialysis is still important. We need a big study that involves dialysis patients and assesses a commonly‐used antidepressant drug with a placebo and measures the treatment effects based on what patients and their families value most.
Summary of findings
Summary of findings for the main comparison. Antidepressant therapy versus placebo for depression in dialysis patients.
| Antidepressant therapy versus placebo for depression in dialysis patients | ||||||
|
Patient or population: dialysis patients with depression Settings: dialysis patients Intervention: selective serotonin reuptake inhibitor (SSRI) antidepressant (8 to 12 weeks) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk per 1000 patients treated for 8 to 12 weeks | Corresponding risk per 1000 patients treated for 8 to 12 weeks | |||||
| Placebo | SSRI | |||||
| Dizziness or hypotension | 135 | 97 more (34 fewer to 394 more) | 1.72 (0.75 to 3.92) | 114 (3) | ⊕1,2,3 very low | Downgraded as follows: 1Study limitations (studies generally at unclear or high risk of bias for many domains) 2Severe imprecision (2 grades): risk estimate includes null effect and estimate consistent with both appreciable benefit and harm 3Directness: data derived from small number of studies in specific settings which may not be generalisable |
| Nausea | 118 | 197 more (30 more to 473 more) | 2.67 (1.26 to 5.68) |
114 (3) | ⊕⊕⊕1,2 moderate | Downgraded as follows: 1Study limitations (studies generally at unclear or high risk of bias for many domains) 2Directness: data derived from small number of studies in specific settings which may not be generalisable |
| Sexual dysfunction | 19 | 54 more (7 fewer to 423 more) | 3.83 (0.63 to 23.34) |
101 (2) | ⊕1,2,3 very low | Downgraded as follows: 1Study limitations (studies generally at unclear or high risk of bias for many domains) 2Severe imprecision (2 grades): risk estimate includes null effect and estimate consistent with both appreciable benefit and harm 3Directness: data derived from small number of studies in specific settings which may not be generalisable |
| All‐cause mortality | 20 | No difference (18 fewer to 280 more) | 1.00 (0.07 to 15.12) |
50 (1) | ⊕1,2,3 very low | Downgraded as follows: 1Study limitations (studies generally at unclear or high risk of bias for many domains) 2Severe imprecision (2 grades): risk estimate includes null effect and estimate consistent with both appreciable benefit and harm 3Directness: data derived from small number of studies in specific settings which may not be generalisable |
| *The basis for the assumed risk (e.g. the median control group risk across studies) calculated from data in the meta‐analyses. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Relative risk | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Background
Description of the condition
Adults who have end‐stage kidney disease (ESKD) ‐ defined as estimated glomerular filtration rate (eGFR) < 15 mL/min/1.73 m2 ‐ commonly experience a heavy burden of symptoms that includes fatigue/tiredness, depression and anxiety (Murtagh 2007). In a recent systematic review of observational studies (Palmer 2013a), approximately 25% of adults with chronic kidney disease (CKD) fulfilled diagnostic criteria for major depression based on a clinical interview, a prevalence that may exceed that of other settings including primary care, cancer, heart failure and diabetes (Anderson 2001; Mitchell 2011; O'Connor 2009). Depression associated with kidney disease is linked to poorer health outcomes including increased mortality (Palmer 2013b), worse quality of life (Lopes 2002; Tsay 2002), lower adherence to recommended dietary and fluid restrictions (Sensky 1996), increased utilisation of health care (Abbas Tavallaii 2009), and hospitalisation (Hedayati 2005). Health‐related quality of life may correlate more strongly with depression than with measures of dialysis adequacy (Martin 2000; Steele 1996). Depressed patients treated with peritoneal dialysis have higher complication rates from peritonitis (Juergenson 1996), while dialysis patients are more likely to commit suicide than the general population (Abram 1971; Haenel 1980). Among older dialysis patients, rates of hospitalisation for depression as a primary diagnosis are significantly greater than among patients with ischaemic heart disease, cerebrovascular disease and peptic ulcer disease (Kimmel 1998).
In addition to prevalence and severity of depressed mood in dialysis patients, adult patients on or nearing dialysis, their caregivers and health care professionals collectively cite depression among their top 10 current research uncertainties in kidney disease (Manns 2014). Similarly, patients consider the psychosocial aspects of living with CKD and reducing symptoms of kidney disease as priorities to be considered within current research agendas (Tong 2008), suggesting the optimal management depression is an important and unanswered question in the care of patients with ESKD.
Description of the intervention
Potential treatments for depression in adults with ESKD include psychosocial and psychological interventions (e.g. cognitive behavioural therapy (CBT), psychodynamic psychotherapy, interpersonal psychotherapy, non‐directive or supportive therapy and counselling). In addition, antidepressant medication may be used to treat depression including selective serotonin reuptake inhibitors (SSRI), serotonin‐norepinephrine (noradrenaline reuptake inhibitors (SNRI), tricyclic antidepressants (TCA), and monoamine oxidase inhibitors (MAOI). Clinical guidelines suggest treatment with antidepressant medication (with SSRIs as first‐line) in adults with CKD who meet diagnostic criteria for depression, based on observational studies and limited evidence of harm (ERBP 2012). Drug clearance of many antidepressants or their active metabolites is substantially reduced by kidney failure (selegiline, amitripylinoxide, venlafaxine, desvenlafaxine, milnacipran, bupropion, reboxetine) and may be altered by dialysis treatment (ERBP 2012). Accordingly the balance of harm and benefit of antidepressant therapy in adults with kidney disease may be different from that of the general population.
How the intervention might work
Associations between depression and treatment adherence, hospitalisation, mortality and quality of life in the setting of kidney disease suggest adequate treatment of depressed mood has a potential role to play in improved outcomes in this clinical setting. Existing studies have shown that depression is linked to impaired nutrition in adults with advanced kidney disease and that antidepressant therapy together with psychotherapy might improve nutritional parameters in depressed patients (Friend 1997; Koo 2003; Koo 2005). In addition, depression is associated with inflammation in dialysis patients which has been suggested as a putative contributor to cardiovascular disease in the setting of other non‐communicable diseases (Emerging Risk Factors Collaboration 2010; Vaccarino 2007). Depression is also linked to reduced social support, elevated social conflict and a greater likelihood of withdrawing from dialysis treatment (Lacson 2012). Reduced adherence with medical treatment may be caused by depression and is in turn linked to poorer outcomes, suggesting an additional causal mechanism between depression and health care utilisation that might be ameliorated by effective treatment for depression (DiMatteo 2000).
Why it is important to do this review
The high prevalence of depressive symptoms in adults treated with dialysis, associations with poor health outcomes, the markedly altered pharmacology of drug interventions for depression caused by kidney failure, together with the prioritisation of depression by patients as a key contemporary uncertainty in the care of people with CKD mandates the need for robust evidence synthesis of available treatments. In an earlier version of this review, current to February 2005, a single short‐term study comparing fluoxetine with placebo was identified. This review aims to update the current evidence for antidepressant agents to treat depression in adults with ESKD treated with dialysis and to evaluate our confidence in the evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (Guyatt 2011).
Objectives
To evaluate the benefit and harms of antidepressants for treating depression in adults with ESKD treated with dialysis.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs and quasi‐RCTs (in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth, or other predictable methods) measuring the effect of dietary interventions in adults with ESKD treated with dialysis. We also included cross‐over studies but used only data from the first randomisation period in the review. In the event that outcome data were absent, we excluded the study from meta‐analysis but included the study in the narrative systematic review.
Types of participants
Inclusion criteria
Patients aged 18 years and over with ESKD (eGFR < 15 mL/min/1.73 m2) treated with dialysis (either haemodialysis or peritoneal dialysis) were included. Depressive disorder was considered present when investigators assessed participants using any diagnostic tool including interview or depression scale.
Exclusion criteria
We excluded studies including patients with bipolar affective disorder.
Types of interventions
Inclusion criteria
We included studies comparing antidepressants to placebo, no intervention or psychological interventions. The following drugs were considered.
TCA and related antidepressant drugs (amitriptyline hydrochloride, amoxapine, clomipramine hydrochloride, dosulepin/dothiepin hydrochloride, doxepin, imipramine hydrochloride, nortriptyline, lofepramine, maprotiline, trazodone hydrochloride)
SSRI (citalopram, escitalopram, fluoxetine, fluvoxamine maleate, paroxetine, sertraline)
MAOI (moclobemide, phenelzine, isocarboxazid, tranylcypromine)
Others (mirtazapine, nefazodone, reboxetine, venlafaxine).
Studies where there was equivalent supportive or other psychotherapy in the two arms were included (i.e. antidepressant plus psychotherapy compared with placebo plus psychotherapy). Studies with electroconvulsive therapy (ECT) in combination with antidepressants versus antidepressants alone were not included. The dosages of the drugs given and the duration of treatment were recorded.
Exclusion criteria
Studies of euphoriants (e.g. amphetamines) and adjuvants (tryptophan, lithium, carbamazepine) were excluded.
Types of outcome measures
Primary outcomes
Health‐related quality of life
Adverse events.
Secondary outcomes
Patient centred outcomes
Hospitalisation
All‐cause mortality
Suicides or suicide attempts
Withdrawal from study intervention
Withdrawal from dialysis.
Surrogate outcomes
Depression score.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant's Specialised Register to 20 January 2016 through contact with the Information Specialist using search terms relevant to this review. The Specialised Register contains studies identified from the following sources.
Monthly searches of the Cochrane Central Register of Controlled Trials CENTRAL
Weekly searches of MEDLINE OVID SP
Handsearching of renal‐related journals and the proceedings of major renal conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected renal journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of these strategies as well as a list of handsearched journals, conference proceedings and current awareness alerts are available in the Specialised Register section of information about the Cochrane Kidney and Transplant.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of clinical practice guidelines, review articles and relevant studies
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies
For the original review the American College of Physicians database and PsycINFO were also searched.
Data collection and analysis
Selection of studies
For this update review (2016), study titles and abstracts were reviewed by two authors. Full text articles of studies considered relevant were obtained and reviewed for eligibility by both authors.
Data extraction and management
For this update, data extraction and assessment of risk of bias was performed by two authors using a previously prepared standard data extraction form which were piloted before use. We assessed and extracted characteristics regarding the study design, participants and methods, intervention and outcome details, summary statistics and associated commentaries. We resolved any disagreements through consultation with review author.
We extracted the following information.
Characteristics of participants: number of participants randomised, age, sex, multi‐morbidity (diabetes, prior myocardial infarction, prior stroke, hypertension]) depression score, tool used to assess depression, medication, type of dialysis, time on dialysis treatment
Characteristics of interventions: description of intervention(s), route, daily dose, duration of treatment
Study design: setting, year of publication, crossover or parallel study, primary outcome, months of follow up, risks of bias
Outcome measures: primary and secondary outcome measures, summary statistics of continuous data (mean, standard deviation (SD)) and dichotomous data (number who experienced endpoint and number at risk).
Studies reported in non‐English language journals were translated before assessment. Where more than one publication of one study existed, reports were grouped together and the publication with the most complete data was used in the analyses. Where relevant outcomes were only published in earlier versions, these data were used. Any discrepancy between published versions was highlighted.
Assessment of risk of bias in included studies
The following items were assessed independently by two authors using the risk of bias assessment tool (Higgins 2011) (seeAppendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
We resolved disagreements regarding the risk of bias adjudications by consultation with a third review author.
Measures of treatment effect
Dichotomous data
For dichotomous outcomes (hospitalisation, mortality, suicide or suicide attempts, withdrawal from study treatment, withdrawal from dialysis, adverse events), results were expressed as risk ratio (RR) with 95% confidence intervals (CI).
Continuous data
Where continuous scales of measurement were used to assess the effects of treatment (health‐related quality of life, depression score), the mean difference (MD) was used, or the standardised mean difference (SMD) if different scales had been used.
Unit of analysis issues
Studies with multiple treatment groups
In studies comparing the efficacy of more than two interventions we considered the following:
If the different antidepressant interventions were of the same class (e.g. SSRI), we summarised the different experimental conditions into a single group that was compared with the control group for dichotomous outcomes (we summed the sample sizes and the number of people with events across the treatment groups). For continuous data, we entered the means and standard deviations of a single intervention group (usually the highest dose) for comparison with the control group. Where appropriate, we considered sensitivity analyses, testing the impact of including the alternative intervention group in analyses.
If the different interventions were of different classes (e.g. SSRI or TCA versus placebo), we included each treatment group in separate meta‐analyses, ensuring we did not include outcome data for the control group participants more than once in a single meta‐analysis
Cross‐over studies
We included cross‐over studies in meta‐analyses only if it was possible to extract data for the treatment and control groups from the first treatment period.
Dealing with missing data
Any further information required from the original author was requested by written correspondence and any relevant information obtained was to be included in the review. Evaluation of important numerical data such as screened, randomised patients as well as intention‐to‐treat, as‐treated and per‐protocol population were carefully performed. Attrition rates, for example drop‐outs, losses to follow‐up and withdrawals were investigated. Issues of missing data and imputation methods (for example, last‐observation‐carried‐forward) were critically appraised (Higgins 2011).
Assessment of heterogeneity
We first assessed for statistical heterogeneity visually by inspecting forest plots of standardised mean effect sizes and of RR. Furthermore, we applied a Chi2 test to assess heterogeneity. The test has low power in general but especially when the sample size of the included studies is low or there are only a few included studies. Therefore, we used a P value of 0.10 to determine statistical significance. In addition, we used the I2 statistic. The I2 statistic describes the percentage of variability in effect estimates that is due to heterogeneity rather than sampling error. We used conventions of interpretation that were defined by Higgins (Higgins 2003). In the case of substantial levels (50% to 90%) and considerable levels (75% to 100%) of heterogeneity, we examined data by subgroup and sensitivity analyses (see Subgroup analysis and investigation of heterogeneity; Sensitivity analysis) for different aspects of clinical and methodological heterogeneity.
Assessment of reporting biases
In order to minimise publication bias, we made every attempt to include unpublished studies (e.g. by searching online trial registries). In order to assess for publication bias, we implemented funnel plots (effect versus standard error of the effect size) when a sufficient number of studies was available (according to recommendations of the Higgins 2011 section 10 Addressing reporting biases). For the analysis and the interpretation of the funnel plots, other reasons for asymmetry besides publication bias were considered (e.g. differences in methodological quality; true heterogeneity in intervention effects).
Data synthesis
Data were summarised using the random‐effects model and the fixed‐effect model was also used to ensure robustness of the model chosen and susceptibility to outliers.
Subgroup analysis and investigation of heterogeneity
Although subgroup analyses have to be treated with caution, as they are hypothesis‐forming rather than hypothesis‐testing, we considered a priori defined analyses in order to explore whether methodological and clinical differences between the studies may have systematically influenced the differences that were observed in the treatment outcomes. However, insufficient data were available to conduct subgroup analyses for the primary outcomes.
Sensitivity analysis
We considered sensitivity analyses to explore the influence of the following factors on effect size, however insufficient data were available.
Repeating the analysis excluding unpublished studies
Repeating the analysis taking account of risk of bias, as specified
Repeating the analysis excluding any very long or large studies to establish how much they dominate the results
Repeating the analysis excluding studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), and country.
'Summary of findings' tables
We have presented the main results of the review in a 'Summary of findings' table. This table presents key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schunemann 2011a). The 'Summary of findings' table also include an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (GRADE 2008). The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schunemann 2011b). We have presented the following outcomes.
Dizziness or hypotension
Nausea
Sexual dysfunction
All‐cause mortality
Results
Description of studies
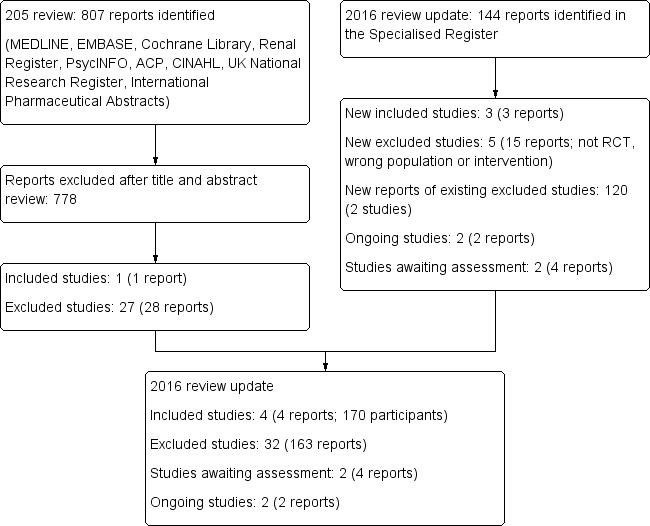
Results of the search
2005 review
A total of 807 titles were identified in our initial search (MEDLINE (125), PSYCHINFO (570), EMBASE (234), ACP (3), The Cochrane Library (The Cochrane Database of Systematic Reviews (34), DARE (2), CENTRAL (23)), CINAHL (7), UK National Research Register (1), International Pharmaceutical Abstracts (8)). After screening, 29 full papers were retrieved for assessment for inclusion. Twenty seven studies (28 papers) were excluded as they were not RCTs (10 studies), the interventions were not relevant to this review (14), or the populations were not ones being assessed by this review (3). One study (Blumenfield 1997) with a total of 12 randomised patients was eligible for inclusion. This study compared fluoxetine with placebo in a subset of depressed dialysis patients. The selected patients were derived from a dialysis population who were either referred for psychiatric consultation, or were patients during one dialysis shift who were assessed for major depressive disorder by a psychiatrist. The study did not give standard deviations for the results but we were able to calculate it from the other data provided (mean and exact P values) in the paper. The study duration was eight weeks.
2016 review update
A search was conducted in January 2016 to update the review (Figure 1). This new search identified 144 new reports. Of these, 15 reports were of six new excluded studies (CAST Study 2013; Ciarambino 2012; dos Rios Santos 2013; SMILE Study 2010; Solak 2012; Turk 2010), 120 reports were of two existing excluded studies (ADEMEX Study 2002; HEMO Study 1997) and three reports were of three new included studies (Hosseini 2012; Taraz 2013; Yazici 2012). One study was translated to English before assessment and data extraction (Yazici 2012).
1.
Study selection flow diagram
Prior to publication four reports of two new studies (Gharekhani 2014; Solak 2010) and two ongoing studies (NCT02358343; NCT02407821) were identified. These will be assessed in a future update of this review.
Included studies
See: Characteristics of included studies
Overall, a total of four studies were included in the present review (involving 170 participants) (Blumenfield 1997; Hosseini 2012; Taraz 2013; Yazici 2012).
Design
Three of the four studies were reported as double‐blind and placebo‐controlled (Blumenfield 1997; Taraz 2013; Yazici 2012). One study reported psychological training as the control intervention (Hosseini 2012). Studies were all of short duration. Participants were followed up for eight weeks in two studies (Blumenfield 1997; Yazici 2012) and 12 weeks in two studies (Hosseini 2012; Taraz 2013). The studies included 14 (Blumenfield 1997), 44 (Hosseini 2012), 50 (Taraz 2013) and 62 (Yazici 2012) participants.
Settings
Participants were all treated with haemodialysis. One study was conducted in the USA (Blumenfield 1997), two were conducted in Iran (Hosseini 2012; Taraz 2013) and one was conducted in Turkey (Yazici 2012).
Participants
The diagnosis of major depression as a criterion for participant inclusion varied among the studies. Blumenfield 1997 diagnosed major depression if participants fulfilled 16 of the first 17 items of the Hamilton Depression Scale. Hosseini 2012 included participants who had a Hospital Anxiety and Depression Score of 8 or above. Taraz 2013 identified major depression using a Beck Depression Inventory (BDI‐II) score of 16 or above. Yazici 2012 included participants meeting DSM‐IV (Diagnostic and Statistical Manual of Mental Disorders, 4th Edition) criteria during an interview. The ages of participants were as follows.
Blumenfield 1997: unclear
Hosseini 2012: mean ages were 49.1 ± 14.5 years and 52.3 ± 15.6 years for the treatment and control groups, respectively
Taraz 2013: median ages were 60 and 65 years (IQR 22 to 24.5)
Yazici 2012: mean ages were 49.3 ± 10.3 and 52.8 ± 11.8 years.
The proportion of men ranged between 43% (Hosseini 2012) and 58% (Taraz 2013).
Intervention and comparators
The antidepressant medications prescribed in the studies together with the suggested treatment doses in ESKD (Hedayati 2012) are shown in Table 2. Prescribed doses in two included studies were comparatively low given the suggested dose range (Blumenfield 1997; Yazici 2012), one study used an antidepressant not recommended for use in people with an eGFR < 20 mL/min (citalopram) (Hosseini 2012) and in one study the dose of escitalopram (caution with use in the dialysis setting) was unclear (Taraz 2013).
1. Comparison of drug doses prescribed in studies against recommended dosing for people with end‐stage kidney disease (ESKD).
| Study | Antidepressant | Dose prescribed in study | Dose suggested in ESKD | Dose level in study compared to recommendation1 |
| Blumenfield 1997 | Fluoxetine | 20 mg/d | 20 to 80 mg/d; no dose adjustment recommended but long half‐life; use with caution | Low |
| Hosseini 2012 | Citalopram | 20 mg/d | Initial dose 10 mg/d; active metabolite; not recommended for eGFR < 20 mL/min | High |
| Taraz 2013 | Escitalopram | Uncertain | Use with caution in severe renal impairment | High |
| Yazici 2012 | Sertraline | 50 to 100 mg/d | 50 to 200 mg/d; no dose adjustment recommended but active metabolite is renally excreted. | Low‐moderate |
1The reference doses of antidepressant medication for treatment of depression in patients with ESKD are derived from Hedayati 2012.
In three studies, an antidepressant was compared with matching placebo involving 126 participants (Blumenfield 1997; Taraz 2013; Yazici 2012) and in one study an antidepressant was compared with psychological training in 44 participants (Hosseini 2012). All studies evaluated an SSRI antidepressant including fluoxetine 20 mg daily (Blumenfield 1997), citalopram 20 mg/d (Hosseini 2012), escitalopram uncertain dose daily (Yazici 2012) and sertraline 50 mg daily for 2 weeks then 100 mg daily (Taraz 2013). In Hosseini 2012 the comparator treatment included group participation in six sessions of one hour teamwork training every other day including explaining the anatomy of the kidney, pathophysiology and causes of kidney failure, treatment modalities with advantages and disadvantages, the mechanism of haemodialysis, the required care in haemodialysis, the stages of adaptation, and techniques of problem‐solving, stress management and muscle relaxation. The participants who received citalopram did not attend these sessions.
Outcomes
All studies were included in the meta‐analysis. None of the studies reported data for health‐related quality of life, while all studies reported some adverse events related to treatment although this was not systematic. No study reported hospitalisation, suicide or attempted suicide, withdrawal from dialysis, or adherence to the recommended dialysis treatment as study endpoints. One study reported deaths during follow up (Taraz 2013), all reported withdrawal from study treatment and all four reported effects of treatment on depression scores (either change in score during follow up and/or end of treatment values) (Blumenfield 1997; Hosseini 2012; Taraz 2013; Yazici 2012). Data for change in depression scores for an antidepressant versus placebo was only possible for one study (Taraz 2013).
Excluded studies
See: Characteristics of excluded studies.
Thirty two studies did not meet our inclusion criteria and were excluded because of one of the following reasons: not assessing antidepressant medication, not including participants treated with dialysis, not including participants meeting criteria for depression, and were not RCTs.
Risk of bias in included studies
See Characteristics of included studies and Figure 2 and Figure 3. The risk of bias for many domains was generally unclear or high.
2.
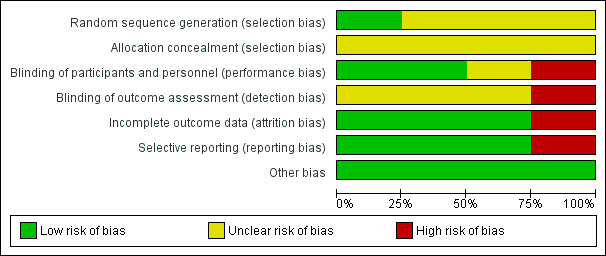
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
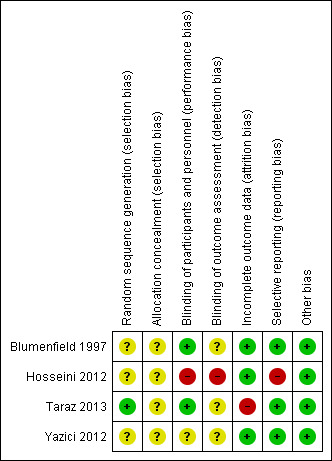
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Only one of the four studies reported the methods used to generate the random sequence for allocation (Taraz 2013).
Allocation concealment
All studies failed to report details of allocation concealment and were classified as unclear risk.
Blinding
Two studies reported that both participants and investigators were blinded to treatment allocation (Blumenfield 1997; Taraz 2013), one did not report blinding (Yazici 2012) and one used a comparator intervention (group‐based counselling) that made it unlikely that treatment allocation was blinded (Hosseini 2012). None of the studies specifically reported blinded outcome assessment for any outcome.
Incomplete outcome data
Three studies reported loss to follow‐up < 10% of participants, and were classified as low risk (Blumenfield 1997; Hosseini 2012; Yazici 2012) while Taraz 2013 was classified as high risk.
Selective reporting
Three studies provided detailed descriptions of adverse events and depression scores and were adjudicated as low risk (Blumenfield 1997; Taraz 2013; Yazici 2012). Hosseini 2012 indicated that adverse events were not different between groups but provided no additional specific details and was classified high risk.
Other potential sources of bias
None of the studies appeared to have other sources of bias.
Effects of interventions
See: Table 1
Antidepressant versus placebo
Primary outcomes
Quality of life
This outcome was not reported in the available studies.
Adverse events attributable to treatment or control intervention
The number of participants experiencing adverse events in each study is reported in Table 3, Table 4, and Table 5. In meta‐analysis, antidepressant therapy had uncertain effects on increasing the risk of on dizziness or hypotension (Analysis 1.1.1 (3 studies, 114 participants): RR 1.72, 95% CI 0.75 to 3.92; I2 = 0%), increased nausea (Analysis 1.1.2 (3 studies, 114 participants): RR 2.67, 95% CI 1.26 to 5.68; I2 = 0%), and had uncertain risks of sexual dysfunction (Analysis 1.1.3 (2 studies, 101 participants): RR 3.83, 95% CI 0.63 to 23.3; I2 = 0%) and headache (Analysis 1.1.4 (2 studies, 56 participants): RR 2.91, 95% CI 0.73 to 11.57; I2 = 0%).
2. Number of patients experiencing adverse events (Blumenfield 1997).
| System | Event | Fluoxetine (6) | Placebo (7) |
| Autonomic | Dry mouth | 0 | 1 |
| Cardiovascular | Hypotension | 4 | 1 |
| Gastrointestinal | Abdominal pain | 1 | 2 |
| Constipation | 0 | 1 | |
| Diarrhoea | 1 | 1 | |
| Gastroenteritis | 0 | 2 | |
| Nausea | 5 | 2 | |
| Vomiting | 3 | 3 | |
| Musculoskeletal | Myalgia | 1 | 1 |
| Neurological | Dizziness | 1 | 0 |
| Headache | 3 | 0 | |
| Insomnia | 2 | 1 | |
| Sensation disturbance | 1 | 0 | |
| Tremors | 1 | 0 | |
| Psychiatric | Abnormal thought | 1 | 0 |
| Anxiety | 0 | 1 | |
| Nervousness | 1 | 1 | |
| Respiratory | Bronchitis | 1 | 0 |
| Cough | 0 | 2 | |
| Dyspnoea | 1 | 0 | |
| Pharyngitis | 1 | 0 | |
| Rhinitis | 1 | 0 | |
| Upper respiratory tract infection | 1 | 0 | |
| Skin | Furunculosis | 1 | 0 |
| Pruritis | 1 | 0 | |
| Skin ulcer | 0 | 1 | |
| Other | Dehydration | 0 | 1 |
| Oedema | 0 | 1 | |
| Flu syndrome | 1 | 0 | |
| Tooth infection | 1 | 0 |
3. Number of participants experiencing adverse events (Taraz 2013).
| System | Event | Sertraline (21) | Placebo (22) |
| Autonomic | Sexual dysfunction | 2 | 1 |
| Cardiovascular | Dizziness | 5 | 3 |
| Gastrointestinal | Dyspepsia | 6 | 4 |
| Anorexia | 2 | 4 | |
| Nausea | 7 | 3 | |
| Other | Headache | 4 | 2 |
| Hair loss | 1 | 1 |
4. Number of patients experiencing adverse events (Yazici 2012).
| System | Event | Escitalopram (28) | Placebo (30) |
| Autonomic | Ejaculation disorder or impotence | 4 | 0 |
| Cardiovascular | Feeling of dizziness | 4 | 4 |
| Gastrointestinal | Nausea | 5 | 2 |
| Diarrhoea | 2 | 1 | |
| Psychiatric | Insomnia | 4 | 2 |
| Somnolence | 2 | 2 | |
| Other | Flu‐like symptoms | 1 | 2 |
1.1. Analysis.
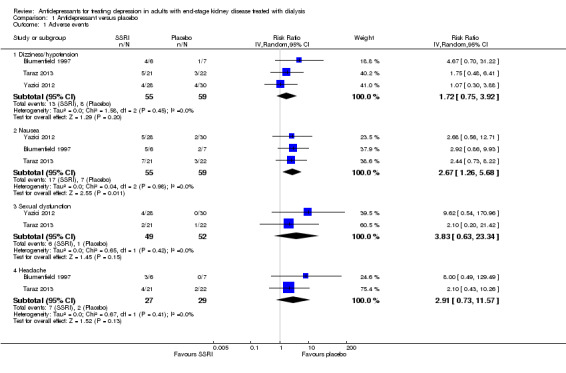
Comparison 1 Antidepressant versus placebo, Outcome 1 Adverse events.
Secondary outcomes
All‐cause mortality
Taraz 2013 reported antidepressant therapy had uncertain effects on all‐cause mortality with few events (Analysis 1.2 (50 participants): RR 1.00, 95% CI 0.07 to 15.12).
1.2. Analysis.
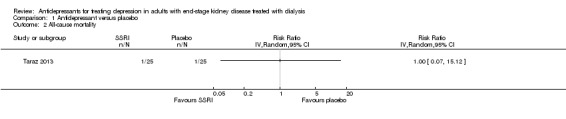
Comparison 1 Antidepressant versus placebo, Outcome 2 All‐cause mortality.
Withdrawal from antidepressant medication
There was no difference in withdrawal from study treatment between antidepressant therapy and placebo, although the confidence interval was wide (Analysis 1.3 (3 studies, 118 participants): RR 1.39, 95% CI 0.44 to 4.47; I2 = 0%).
1.3. Analysis.
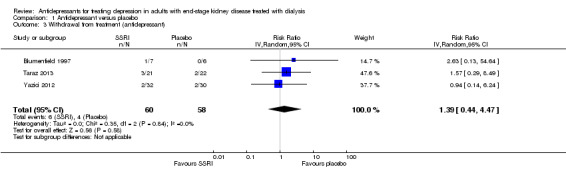
Comparison 1 Antidepressant versus placebo, Outcome 3 Withdrawal from treatment (antidepressant).
Depression score
Only Taraz 2013 reported this outcome was reported in an extractable form. Sertraline therapy reduced the Beck Depression Inventory score significantly compared to placebo (Analysis 1.4 (43 participants): MD ‐7.50, 95% CI ‐11.94 to ‐3.06).
1.4. Analysis.

Comparison 1 Antidepressant versus placebo, Outcome 4 End of treatment depression score.
In narrative results, Blumenfield 1997 reported that depression scores were not significantly different between treatment groups at the end of the study (including the Beck Depression Inventory, the Brief Symptom Inventory, the Hamilton Depression Inventory, the Montgomery Asberg Depression Scale, and the self‐evaluating depression score assessed using an electronic visual analogue instrument). Yazici 2012 reported a significantly lower Hamilton Rating Scale for Depression score with antidepressant treatment at end of follow up (10.5, minimum 4, maximum 35 with escitalopram versus 28, minimum 7, maximum 35) (P = 0.001 for difference).
Outcomes not reported
Hospitalisation, suicide or attempted suicide, and withdrawal from dialysis therapy were not reported by any of the included studies.
Antidepressant versus psychological counselling
Primary outcomes
Quality of life
This outcome was not reported in one available study (Hosseini 2012).
Adverse events attributable to treatment or control intervention
Hosseini 2012 reported that antidepressant treatment "was well tolerated by all of the patients and no severe adverse effects were reported".
Secondary outcomes
Withdrawal from antidepressant medication
Hosseini 2012 reported no difference in withdrawal from study treatment between antidepressant therapy and psychological intervention, although the confidence interval was wide (Analysis 2.1 (44 participants): RR 3.00, 95% CI 0.34 to 26.6).
2.1. Analysis.
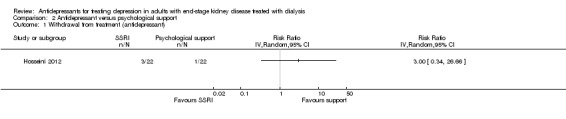
Comparison 2 Antidepressant versus psychological support, Outcome 1 Withdrawal from treatment (antidepressant).
Depression score
Hosseini 2012 reported the depression score (Hospital Anxiety and Depression Scale) was similar at the end of follow up for the antidepressant treatment and psychological support groups (Analysis 2.2 (40 participants): MD ‐1.07, 95% CI ‐3.85 to 1.71).
2.2. Analysis.
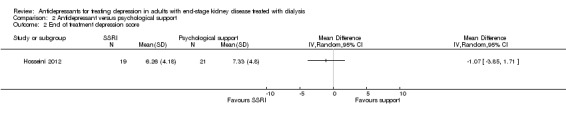
Comparison 2 Antidepressant versus psychological support, Outcome 2 End of treatment depression score.
Outcomes not reported
All‐cause mortality, hospitalisation, suicide or attempted suicide, and withdrawal from dialysis therapy were not reported by any of the included studies.
Subgroup analysis
The paucity of studies precluded planned subgroup analyses.
Assessment of heterogeneity
For all outcomes in which two or more studies could be included in meta‐analysis, there was no evidence of statistical heterogeneity in any analyses.
Assessment of publication bias
Insufficient data precluded assessment of funnel plots to evaluate for evidence of small study effects.
Discussion
Summary of main results
In this systematic review and meta‐analysis, evidence for the effectiveness and safety of antidepressant medication in adults with ESKD treated with dialysis is very sparse. Three studies have compared an SSRI with placebo involving 126 participants and a single study has compared SSRI therapy with psychological support in 44 participants. All studies were short, limited to 12 weeks or fewer and meta‐analyses for all outcomes included 118 participants or fewer. We generally had very low confidence in the estimated treatment effects or effects were not estimable due to limitations in study reporting, substantial imprecision in treatment effects and small numbers of studies, limiting the applicability of the findings to the general dialysis population.
Compared to placebo, short‐term SSRI therapy may reduce clinical depression scores in ungradeable evidence, at the expense of incurring nausea. Effects on all‐cause mortality, suicide, other adverse events such as hypotension or dizziness, headache and sexual dysfunction, withdrawal from dialysis, and hospitalisation were not estimable due to insufficient data. Compared to group psychological training, SSRI therapy had no statistical effect on depression scores, and withdrawal from treatment was difficult to ascertain due to few events in the available study.
Overall, despite the potential prevalence of major depression in adults treated with dialysis, estimated to affect up to one‐quarter of patients, and the priority patients and caregivers place on management of depression as an important clinical uncertainty, available study evidence for the safety and efficacy of antidepressant therapy is currently lacking.
Overall completeness and applicability of evidence
While our review was based on a highly sensitive electronic search strategy and included studies from the Cochrane Kidney and Transplant's Specialised Register of studies, which includes results from handsearching and journal alerts, the paucity of existing studies evaluating pharmacological treatment of depression in the setting of dialysis patients means there are considerable evidence gaps. First, studies evaluated depression using heterogeneous methods including depression scales and interview techniques. Due to the heavy symptom burden of ESKD, depression scales in the general population may lack validity in the setting of kidney failure and falsely detect the presence of depression due to somatic symptoms associated with kidney disease, such as fatigue and anorexia. Few studies exist to validate depression tools in the context of ESKD. Our recent meta‐analysis (although not specifically designed to assess diagnostic test accuracy for depression scales) suggested that depression scales are likely to over‐estimate depression prevalence in this population (Palmer 2013a).
Studies were short in duration (12 weeks or fewer). The relative efficacy and safety of longer term antidepressant treatment is unknown. Outcome data were sparse and confidence intervals were frequently very wide, including for important adverse events including hypotension. The development of a core set of patient‐prioritised outcomes for depression in adults with CKD, which are required to be reported in clinical studies would assist with building evidence for these drugs in dialysis patients as studies cumulate. Additionally, as clearance of antidepressant drugs or their metabolites is commonly impaired in ESKD, safety data for antidepressant medication are required. Notably, in this review only SSRI were studied. The effectiveness and safety of other agents, including TCA remains unknown. Information was limited to small studies conducted in the USA, Turkey and Iran and results may not be generalisable to other national settings.
Quality of the evidence
We graded our confidence in the evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (Guyatt 2011), which considers study limitations, imprecision, indirectness, inconsistency and publication bias. Overall, most studies had high or unclear risks of bias for most domains of study reporting assessed. Estimated effects on efficacy and safety outcomes were frequently imprecise with confidence intervals that were consistent with both considerable benefit or harm. The generalisability (directness) of the evidence was limited by a gross paucity of available studies and publication bias (the effects of small studies on treatment effects) could not be assessed. While treatment estimates showed no evidence of statistical heterogeneity, the small number of studies limited the power of statistical testing and important inconsistency between studies could not excluded. Overall, based on important limitations, we have generally very low confidence in the evidence for the benefits and harms of antidepressant therapy in dialysis patients and any estimations of effect are very uncertain.
Potential biases in the review process
While this review was conducted according to a prespecified protocol and is reported using Cochrane methods, the review has limitations which should be considered when interpreting the results. First, relatively few data were available resulting in inconclusive evidence for many outcomes including mortality, suicide, hospitalisation, depression scores and adverse events. Second, while the included studies appeared similar in their treatment approaches to depression, there was insufficient power in analyses to detect any heterogeneity between differing studies. Third, adverse event data were collated ad hoc in the included studies and important adverse events may not have been captured. Finally, outcome data for patient‐important outcomes were not available in most studies, which reduced our confidence in the reliability of these treatment effects. This was particularly the case for reporting of quality of life domains.
Agreements and disagreements with other studies or reviews
In 2012, a meta‐analysis of antidepressant therapy for adults with stage 3 to 5 CKD (eGFR < 60 mL/min/1.73 m2) included both randomised and non‐randomised studies (ERBP 2012). In that meta‐analysis, three RCTs were identified including one ongoing study in adults not treated with dialysis (CAST Study 2013) and two studies in dialysis patients (Blumenfield 1997; Yazici 2012). In that review, insufficient outcome data were available for Yazici 2012 which was an abstract only publication at the time of publication, and, similar to this review, Blumenfield 1997 was adjudicated to show similar effects of fluoxetine on depression scores as placebo, with numerically higher numbers of adverse events with fluoxetine including hypotension. Based on evidence from nine additional non‐randomised studies, the review authors concluded that there was evidence suggesting benefit for antidepressant therapy with commonly‐experienced but mild side‐effects. The review concluded that evidence for treatment for depression is currently insufficient to guide treatment and that a well‐designed RCT is greatly needed. Guidelines also suggest early re‐assessment of therapy to assess effectiveness and to avoid prolonged use (ERBP 2012). Based on this updated Cochrane review, our findings are consistent with the need to evaluate antidepressant treatment in dialysis patients within the setting of new RCTs.
We note also that a relative lack of evidence for efficacy of antidepressant treatment in the setting of CKD is consistent with a broader concern about the efficacy of antidepressant treatment in the general population (Turner 2008). Given that the effects of antidepressants are modest even for people with normal kidney function, it is quite plausible that even small increases in treatment‐related harm in dialysis patients might offset this small benefit, even if the efficacy of treatment is similar for dialysis patients compared to the general population.
Authors' conclusions
Implications for practice.
Our main finding was that there is insufficient evidence to support prescribing of antidepressant medications in adults treated with dialysis outside of participation in RCTs. The potential for increased harm with antidepressant medication (due to impaired clearance of drugs and their metabolites) is also insufficiently evaluated by current evidence and application of research information derived from the general population may not be appropriate. Data from a few studies in this review suggests nausea may be caused by antidepressant therapy. Identification of effective treatments for depression is highly valued by patients mandating the need for further placebo‐controlled RCTs evaluating both drug effectiveness and safety based on study endpoints prioritised by patients and clinicians.
Implications for research.
There is a great need for undertaking further RCTs that assess the effectiveness of antidepressant treatment in patients treated with dialysis. New studies are needed to improve our confidence about the effectiveness and safety of drug treatments for depression. Studies evaluating drug therapy should be sufficiently powered to detect adverse events and patient‐centred outcomes. Patient and clinician priorities for clinical endpoints should be considered in the design of future RCTs. Standardisation of study design and outcomes would facilitate prospective and collaborative meta‐analysis of treatment effects. Ideally, new studies would include participants meeting diagnostic criteria for depression based on interview methods and evaluate longer term treatment and in patients with a range of depression severity including severe forms of depression. Greater understanding of the barriers to treatment implementation and the sustainability of interventions and follow up would be helpful.
What's new
| Date | Event | Description |
|---|---|---|
| 28 February 2016 | New search has been performed | Changed authorship and updated review |
| 28 February 2016 | New citation required and conclusions have changed | Three new studies added, no change to conclusion. Adverse event data reported |
History
Protocol first published: Issue 4, 2003 Review first published: Issue 2, 2005
| Date | Event | Description |
|---|---|---|
| 14 October 2008 | Amended | Converted to new review format. |
Acknowledgements
The 2005 version of this systematic review was funded by the National Kidney Research Fund (UK). We would like to thank Dr Michael Blumenfield for responding to our request for details regarding his study.
The 2016 version of this systematic review received no specific funding.
We would like to thank the Cochrane Kidney and Transplant editorial team for their support, information and advice. We would also like to thank the referees who provided invaluable advice during its preparation.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimisation (minimisation may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. sub‐scales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Antidepressant versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse events | 3 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Dizziness/hypotension | 3 | 114 | Risk Ratio (IV, Random, 95% CI) | 1.72 [0.75, 3.92] |
| 1.2 Nausea | 3 | 114 | Risk Ratio (IV, Random, 95% CI) | 2.67 [1.26, 5.68] |
| 1.3 Sexual dysfunction | 2 | 101 | Risk Ratio (IV, Random, 95% CI) | 3.83 [0.63, 23.34] |
| 1.4 Headache | 2 | 56 | Risk Ratio (IV, Random, 95% CI) | 2.91 [0.73, 11.57] |
| 2 All‐cause mortality | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 3 Withdrawal from treatment (antidepressant) | 3 | 118 | Risk Ratio (IV, Random, 95% CI) | 1.39 [0.44, 4.47] |
| 4 End of treatment depression score | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 2. Antidepressant versus psychological support.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Withdrawal from treatment (antidepressant) | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 2 End of treatment depression score | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Blumenfield 1997.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
*Not included in meta‐analyses |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation was not mentioned in the original paper. However the author informed through personal communication that patients were allotted to their respective groups with randomisation done by pharmacy. Method unknown |
| Allocation concealment (selection bias) | Unclear risk | The random assignment was done by the hospital pharmacy |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Psychiatrists (i.e. both investigators and outcome assessors) and patients were blind, Placebo was similar in appearance to fluoxetine capsules; double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up: 1/14 (7.1%) |
| Selective reporting (reporting bias) | Low risk | No study protocol was available. The study investigators described adverse events associated with treatment and treatment effects on depression scores. Other patient‐prioritised outcomes were not reported such as quality of life |
| Other bias | Low risk | None observed |
Hosseini 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unlikely to be blinded as active comparison including psychological training |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not described for any outcome and unlikely to be blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up: 4/44 (9.1%) |
| Selective reporting (reporting bias) | High risk | No study protocol was available. The study investigators described adverse events associated with treatment, although actual numbers of events and a detailed description were not provided. Treatment effects on depression scores were detailed. Other patient‐prioritised outcomes were not reported such as quality of life |
| Other bias | Low risk | None observed |
Taraz 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation list |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded. Placebo tablets were visibly identical to sertraline tablets |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described for any outcome |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 7/50 participants were not included in final analysis (14.0%) |
| Selective reporting (reporting bias) | Low risk | No study protocol was available. The study investigators described adverse events associated with treatment and treatment effects on depression scores. Other patient‐prioritised outcomes were not reported such as quality of life. The study reported mortality during follow up |
| Other bias | Low risk | None observed |
Yazici 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described for any outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up: 4/62 (6.5%) |
| Selective reporting (reporting bias) | Low risk | No study protocol was available. The study investigators described adverse events associated with treatment and treatment effects on depression scores. Other patient‐prioritised outcomes were not reported such as quality of life |
| Other bias | Low risk | None observed |
AV ‐ arteriovenous; CKD ‐ chronic kidney disease; DM ‐ diabetes mellitus; ESKD ‐ end‐stage kidney disease; HD ‐ haemodialysis; IQR ‐ interquartile range; M/F ‐ male/female; RCT ‐ randomised controlled trial; SD ‐ standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ADEMEX Study 2002 | RCT: intervention CAPD vs CAPD + CrCl target; not assessing treatment of depression |
| Ancarani 1993 | The active drug (S‐adenosyl‐L‐methionine) is not one of the groups of drugs considered for inclusion in this review |
| Briggs 2004 | RCT; psychosocial intervention |
| CAST Study 2013 | Not dialysis population |
| Chang 2004a | RCT; intervention not relevant to the review |
| Cho 2004 | RCT; psychosocial intervention |
| Ciarambino 2012 | Not CKD population |
| De Felice 1996 | Not RCT (survey) |
| Deniston 1990 | Not RCT; outcomes not relevant to this review |
| dos Rios Santos 2013 | Not evaluating antidepressants |
| HEMO Study 1997 | All dialysis patients; quality of life a secondary outcome |
| Kennedy 1989 | Not RCT |
| Koo 2005 | Not RCT |
| Kurella 2005 | Not RCT |
| Levy 1996 | Not RCT |
| Lieh 2004 | Not RCT; mixed population |
| Miller 2005 | Mixed population; psychosocial interventions |
| Moug 2004 | Pilot RCT; psychosocial intervention |
| Pearlman 1988 | Not RCT (case report) |
| Seabolt 2001 | Not RCT (case report) |
| Sharp 2005 | RCT; assessing interventions for fluid restriction compliance |
| SMILE Study 2010 | Not RCT; not physical measures |
| Solak 2012 | Not evaluating antidepressant |
| Tsay 2004a | Not evaluating antidepressant |
| Tsay 2004b | Not evaluating antidepressant |
| Tsay 2004c | Not evaluating antidepressant |
| Tsay 2005 | RCT; psychosocial intervention |
| Turk 2010 | Not evaluating antidepressant |
| van Vilsteren 2005 | RCT; psychosocial intervention |
| Wuerth 2001 | Not RCT |
| Zetin 1980 | The active drug (zinc) used in this study is not one of the group of drugs considered eligible for inclusion |
| Zetin 1982 | Study population consisted of all dialysis patients, not just depressed dialysis patients |
CAPD ‐ continuous ambulatory peritoneal dialysis; CKD ‐ chronic kidney disease; CrCl ‐ creatinine clearance; RCT ‐ randomised controlled trial
Differences between protocol and review
'Summary of findings' table has been incorporated.
Contributions of authors
2016 review update
SP and PN undertook searches
PN, MR, VS, and KSR identified studies and helped with data extraction
SP and PN drafted the review
All authors provided intellectual review of the drafts
SP and PN revised the review in response to comments
SP and PN generated the figures
GS provided input in case of any disagreement during screening process
All authors read and approved the review before submission
2005 review
Allison MacLeod, Conal Daly and Paul Roderick conceived the review with input from the Renal Association (UK), Standards and Audit sub‐committee.
AMM helped with resolving differences in study inclusion and data extraction.
KSR and Sheila Wallace undertook searches.
KSR and CD screened search results, assessed retrieved articles, assessed quality of papers, extracted data and wrote the review.
PR gave advice on outcome measures, study inclusion criteria and helped with resolving differences in study inclusion and data extraction.
Janet Butler gave advice on the background for the review, types of intervention and outcome measures and wrote the review.
Declarations of interest
Suetonia C Palmer: none known
Patrizia Natale: none known
Marinella Ruospo: none known
Valeria Saglimbene: none known
Kannaiyan S Rabindranath: none known
Jonathan C Craig: none known
Giovanni FM Strippoli: none known
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Blumenfield 1997 {published data only}
- Blumenfield M, Levy NB, Spinowitz B, Charytan C, Beasley CM Jr, Dubey AK, et al. Fluoxetine in depressed patients on dialysis. International Journal of Psychiatry in Medicine 1997;27(1):71‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hosseini 2012 {published data only}
- Hosseini SH, Espahbodi F, Mirzadeh Goudarzi SM. Citalopram versus psychological training for depression and anxiety symptoms in hemodialysis patients. Iranian Journal of Kidney Diseases 2012;6(6):446‐51. [MEDLINE: ] [PubMed] [Google Scholar]
Taraz 2013 {published data only}
- Taraz M, Khatami MR, Dashti‐Khavidaki S, Akhonzadeh S, Noorbala AA, Ghaeli P, et al. Sertraline decreases serum level of interleukin‐6 (IL‐6) in hemodialysis patients with depression: results of a randomized double‐blind, placebo‐controlled clinical trial. International Immunopharmacology 2013;17(3):917‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yazici 2012 {published data only}
- Yazici AE, Erdem P, Erdem A, Yazici K, Acar ST, Basterzi AD, et al. Efficacy and tolerability of escitalopram in depressed patients with end stage renal disease: an open placebo‐controlled study [Depresyonu olan son donem bobrek yetmezligi hastalarinda essitalopramin etkinligi ve tolerabilitesi: Bir acik plasebo kontrollu calisma]. Klinik Psikofarmakoloji Bulteni 2012;22(1):23‐30. [EMBASE: 2012129390] [Google Scholar]
References to studies excluded from this review
ADEMEX Study 2002 {published data only}
- Paniagua R, Amato D, Mujais S, Vonesh E, Ramos A, Correa‐Rotter R, et al. Predictive value of brain natriuretic peptides in patients on peritoneal dialysis: results from the ADEMEX trial. Clinical Journal of The American Society of Nephrology: CJASN 2008;3(2):407‐15. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paniagua R, Amato D, Ramos A, Vonesh EF, Mujais SK, the Mexican Nephrology Collaborative Study Group. Summary results of the Mexican adequacy (ADEMEX) clinical trial on mortality and morbidity in peritoneal dialysis [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):235A. [CENTRAL: CN‐00447090] [Google Scholar]
- Paniagua R, Amato D, Vonesh E, Correa‐Rotter R, Ramos A, Moran J, et al. Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. Journal of the American Society of Nephrology 2002;13(5):1307‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Paniagua R, Amato D, Vonesh E, Guo A, Mujais S. Effects of increased peritoneal clearances on patient quality of life: results from the ADEMEX trial [abstract no: SU‐PO933]. Journal of the American Society of Nephrology 2003;14(Nov):741A. [CENTRAL: CN‐00550541] [Google Scholar]
- Paniagua R, Amato D, Vonesh E, Guo A, Mujais S, Mexican Nephrology Collaborative Study Group. Health‐related quality of life predicts outcomes but is not affected by peritoneal clearance: The ADEMEX trial. Kidney international 2005;67(3):1093‐104. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Paniagua R, Amato D, Vonesh E, Mujais S. Effects of increasing dialysis prescription on residual renal function: results from the ADEMEX trial. [abstract no: SU‐PO931]. Journal of the American Society of Nephrology 2003;14(Nov):740A. [CENTRAL: CN‐00550558] [Google Scholar]
- Sloand JA, Leypoldt JK, Culleton BF, Gellens ME, Paniagua R, Amato D, et al. Assessing creatinine clearance from modification of diet in renal disease study equations in the ADEMEX cohort: limitations and potential applications. Clinical Journal of The American Society of Nephrology: CJASN 2011;6(3):598‐604. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ancarani 1993 {published data only}
- Ancarani E, Biondi B, Bolletta A, Cestra D, Bella E, Nirchi MA, et al. Major depression complicating hemodialysis in patients with chronic renal failure: A multicenter, double‐blind, controlled clinical trial of S‐ adenosyl‐L‐methionine versus placebo. Current Therapeutic Research ‐ Clinical & Experimental 1993;54(6):680‐6. [EMBASE: 1994039415] [Google Scholar]
Briggs 2004 {published data only}
- Briggs LA, Kirchhoff KT, Hammes BJ, Song MK, Colvin ER. Patient‐centered advance care planning in special patient populations: a pilot study. Journal of Professional Nursing 2004;20(1):47‐58. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
CAST Study 2013 {published data only}
- Jain N, Trivedi MH, Rush AJ, Carmody T, Kurian B, Toto RD, et al. Rationale and design of the Chronic Kidney Disease Antidepressant Sertraline Trial (CAST). Contemporary Clinical Trials 2013;34(1):136‐44. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2004a {published data only}
- Chang ST, Chen CL, Chen CC, Hung KC. Clinical events occurrence and the changes of quality of life in chronic haemodialysis patients with dry weight determined by echocardiographic method. International Journal of Clinical Practice 2004;58(12):1101‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Chang ST, Chen CL, Chen CC, Lin FC, Wu D. Enhancement of quality of life with adjustment of dry weight by echocardiographic measurement of inferior vena cava diameter in patients undergoing chronic hemodialysis. Nephron Clinical Practice 2004;97(3):C90‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cho 2004 {published data only}
- Cho YC, Tsay SL. The effect of acupressure with massage on fatigue and depression in patients with end‐stage renal disease. Journal of Nursing Research 2004;12(1):51‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ciarambino 2012 {published data only}
- Ciarambino T, Ferrara N, Castellino P, Paolisso G, Coppola L, Giordano M. Effects of six days a week low protein dietary intervention on depressive symptoms in elderly diabetic subjects [Effetti della dieta ipoproteica 6 giorni la settimana sui sintomi depressivi in anziani diabetici tipo 2]. Giornale di Gerontologia 2012;60(1):8‐13. [EMBASE: 2012317209] [Google Scholar]
De Felice 1996 {published data only}
- Felice SL, Lyons MD, Gaffar MC, Sheridan MJ. U.S. ‐ Italy L‐carnitine hemodialysis utilization survey. Dialysis & Transplantation 1996;25(6):368‐73. [EMBASE: 1996178046] [Google Scholar]
Deniston 1990 {published data only}
- Deniston OL, Luscombe FA, Buesching DP, Richner RE, Spinowitz BS. Effect of long‐term epoetin beta therapy on the quality of life of hemodialysis patients. ASAIO Transactions 1990;36(3):157‐60. [MEDLINE: ] [PubMed] [Google Scholar]
dos Rios Santos 2013 {published data only}
- dos Reis Santos I, Danaga AR, Carvalho Aguiar I, Oliveira EF, Dias IS, Urbano JJ, et al. Cardiovascular risk and mortality in end‐stage renal disease patients undergoing dialysis: sleep study, pulmonary function, respiratory mechanics, upper airway collapsibility, autonomic nervous activity, depression, anxiety, stress and quality of life: a prospective, double blind, randomized controlled clinical trial. BMC Nephrology 2013;14(1):215. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
HEMO Study 1997 {published data only}
- Agar BU, Culleton BF, Fluck R, Leypoldt JK. Potassium kinetics during hemodialysis. Hemodialysis International 2015;19(1):23‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Allen K, Dwyer JT, Frydrych A, Leung J, Paranandi L, Poole D, et al. Predictors of functional ability at baseline in the hemodialysis (HEMO) study [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):152‐3A. [CENTRAL: CN‐00520312] [Google Scholar]
- Allon M, Depner TA, HEMO Study Group. Effect of hemodialysis dose and membrane on infectious outcomes: results from the HEMO study [abstract no: SA‐P0766]. Journal of the American Society of Nephrology 2002;13(Program & Abstracts):421A. [CENTRAL: CN‐00444149] [Google Scholar]
- Allon M, Ornt DB, Schwab SJ, Delmez J, Kusek JA, Martin AA, et al. Factors determining the prevalence of A‐V fistulas (AVF) in hemodialysis (HD) patients in the HEMO study [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):200A. [CENTRAL: CN‐00550466] [Google Scholar]
- Allon M, Radeva M, Bailey J, Beddhu S, Butterly D, Coyne DW, et al. Association of serum albumin and disease category with severity of infection: data from the HEMO study [abstract no: SU‐PO840]. Journal of the American Society of Nephrology 2003;14(Nov):720A. [CENTRAL: CN‐00550473] [DOI] [PubMed] [Google Scholar]
- Argyropoulos C, Larive B, Miskulin D, Greene T, Beddhu S, Rocco M, et al. Predictors of mortality and hospitalization among elderly hemodialysis patients over a three year period [abstract no: SU‐PO595]. Journal of the American Society of Nephrology 2007;18(Abstracts Issue):715A. [CENTRAL: CN‐00747279] [Google Scholar]
- Argyropoulos C, Roumelioti ME, Cheung A, Kellum JA, Weissfeld L, Unruh M. Dialyzer reuse and patient outcomes in the HEMO Study [abstract no: M676]. World Congress of Nephrology; 2009 May 22‐26; Milan, Italy. 2009.
- Athienites NV, Meyer KB, Martin A, Gassman JJ, Levey AS, Hemodialysis (HEMO) Study. Baseline health status and comorbidity in the HEMO pilot study [abstract]. Journal of the American Society of Nephrology 1995;6(3):518. [CENTRAL: CN‐00483061] [Google Scholar]
- Balakrishnan VS, Guo D, Perianayagam MC, Jaber BL, Pereira BJ, Rao M, et al. Plasma IL‐6 predicts clinical outcomes in prevalent hemodialysis patients [abstract]. Journal of the American Society of Nephrology 2003;14(Nov):3A. [CENTRAL: CN‐00550425] [Google Scholar]
- Beddhu S, Baird B, Ma X, Cheung AK, Greene T. Serum alkaline phosphatase and mortality in hemodialysis patients. Clinical Nephrology 2010;74(2):91‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Beddhu S, Kaysen GA, Yan G, Sarnak M, Agodoa L, Ornt D, et al. Association of serum albumin and atherosclerosis in chronic hemodialysis patients. American Journal of Kidney Diseases 2002;40(4):721‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Beddhu S, Yan G, Agodoa L, Beck G, Milford E, Miskulin D, et al. The impact of race and comorbidity on kidney transplantation (KTx) in the HEMO study [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):877A. [CENTRAL: CN‐00520319] [Google Scholar]
- Benz RL, Pressman MR, Brown J. Does membrane flux (F) or dialysis eKT/V dose (K) affect ESRD‐related sleep disorders in hemodialysis (HD) patients (Pts)? An ancillary study of the HEMO trial [abstract no: F‐FC095]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):20A. [CENTRAL: CN‐00444399] [Google Scholar]
- Briggs JP. Evidence‐based medicine in the dialysis unit: a few lessons from the USRDS and the NCDS and HEMO trials. Seminars in Dialysis 2004;17(2):136‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Burrowes JD, Cockram DB, Dwyer JT, Larive B, Paranandi L, Bergen C, et al. Cross‐sectional relationship between dietary protein and energy intake, nutritional status, functional status, and comorbidity in older versus younger hemodialysis patients. Journal of Renal Nutrition 2002;12(2):87‐95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Burrowes JD, Larive B, Chertow GM, Cockram DB, Dwyer JT, Greene T, et al. Self‐reported appetite, hospitalization and death in haemodialysis patients: findings from the Hemodialysis (HEMO) Study. Nephrology Dialysis Transplantation 2005;20(12):2765‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Burrowes JD, Larive B, Cockram DB, Dwyer J, Kusek JW, McLeroy S, et al. Effects of dietary intake, appetite, and eating habits on dialysis and non‐dialysis treatment days in hemodialysis patients: cross‐sectional results from the HEMO study. Journal of Renal Nutrition 2003;13(3):191‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Burrowes JD, Russell GB, Unruh M, Rocco MV. Is nutritional status associated with self‐reported sleep quality in the HEMO study cohort?. Journal of Renal Nutrition 2012;22(5):461‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Chang TI, Flythe JE, Brunelli SM, Muntner P, Greene T, Cheung AK, et al. Visit‐to‐visit systolic blood pressure variability and outcomes in hemodialysis. Journal of Human Hypertension 2014;28(1):18‐24. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang TI, Friedman GD, Cheung AK, Greene T, Desai M, Chertow GM. Systolic blood pressure and mortality in prevalent haemodialysis patients in the HEMO study. Journal of Human Hypertension 2011;25(2):98‐105. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang TI, Paik J, Greene T, Desai M, Bech F, Cheung AK, et al. Intradialytic hypotension and vascular access thrombosis. Journal of the American Society of Nephrology 2011;22(8):1526‐33. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang TI, Paik J, Greene T, Miskulin DC, Chertow GM. Updated comorbidity assessments and outcomes in prevalent hemodialysis patients. Hemodialysis International 2010;14(4):478‐85. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang TI, Shilane D, Brunelli SM, Cheung AK, Chertow GM, Winkelmayer WC. Angiotensin‐converting enzyme inhibitors and cardiovascular outcomes in patients on maintenance hemodialysis. American Heart Journal 2011;162(2):324‐30. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelamcharla M, Yan G, Rocco M, Schulman G, Teehan B, Toto R, et al. Effect of hemodialysis dose and flux on lipoproteins (LP) in the HEMO study [abstract no: W‐PO40022]. Nephrology 2005;10(Suppl 1):A285. [CENTRAL: CN‐00782977] [Google Scholar]
- Cheung AK, Agodoa L, Daugirdas JT, Greene T, Levey AS, Milford E, Ornt DB, et al. Predictive value of blood pressure (BP) for mortality in chronic hemodialysis (HD) patients changes with duration of follow‐up [abstract]. Journal of the American Society of Nephrology 2003;14(Nov):2A. [CENTRAL: CN‐00583167] [Google Scholar]
- Cheung AK, Agodoa LY, Daugirdas JT, Depner TA, Gotch FA, Greene T, et al. Effects of hemodialyzer reuse on clearances of urea and beta2‐microglobulin. The Hemodialysis (HEMO) Study Group. Journal of the American Society of Nephrology 1999;10(1):117‐27. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cheung AK, Greene T, Leypoldt JK, Yan G, Allon M, Delmez J, et al. Association between serum 2‐microglobulin level and infectious mortality in hemodialysis patients. Clinical Journal of the American Society of Nephrology: CJASN 2008;3(1):69‐77. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AK, Levey AS, HEMO Study Group. Effect of dialysis dose and membrane on cardiac outcomes: results from the HEMO Study [abstract]. Journal of the American Society of Nephrology 2002;13(Program & Abstracts):421A. [CENTRAL: CN‐00444794] [Google Scholar]
- Cheung AK, Levin NW, Greene T, Agodoa L, Bailey J, Beck G, et al. Effects of high‐flux hemodialysis on clinical outcomes: results of the HEMO study. Journal of the American Society of Nephrology 2003;14(12):3251‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cheung AK, Levin NW, HEMO Study Group. Effect of high flux (HF) hemodialysis (HD) membranes on clinical outcomes: results from the HEMO study [abstract]. Journal of the American Society of Nephrology 2002;13(Program & Abstracts):432A. [CENTRAL: CN‐00444795] [Google Scholar]
- Cheung AK, Rocco MV, Yan G, Leypoldt JK, Levin NW, Greene T, et al. Serum beta‐2 microglobulin levels predict mortality in dialysis patients: results of the HEMO study. Journal of the American Society of Nephrology 2006;17(2):546‐55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cheung AK, Sarnak MJ, Yan G, Berkoben M, Heyka R, Kaufman A, et al. Cardiac diseases in maintenance hemodialysis patients: results of the HEMO Study. Kidney International 2004;65(6):2380‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Chonchol M, Greene T, Zhang Y, Hoofnagle AN, Cheung AK. Low vitamin D and high fibroblast growth factor 23 serum levels associate with infectious and cardiac deaths in the HEMO study. Journal of the American Society of Nephrology 2016;27(1):227‐37. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumlea WC, Bergen C, Dwyer J, McGhee HA, McLeroy S, Paranandi L. Anthropometric data for hemodialysis patients: an interim report from the HEMO study [abstract]. Journal of the American Society of Nephrology 1997;8(Program & Abstracts):189A. [CENTRAL: CN‐00444834] [Google Scholar]
- Cockram D, Bergen C, Burrowes J, Larive B, Leung J, Poole D, et al. Oral enteral supplement use during baseline in the HEMO trial [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):353A. [CENTRAL: CN‐00444865] [Google Scholar]
- Daugirdas J, Depner T, Gotch F, Greene T, Levin N, Schulman G, et al. Predictors of urea rebound [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):296A. [CENTRAL: CN‐00444990] [Google Scholar]
- Daugirdas J, Greene T, Levin N, Maroni B, Schulman G, Star R. Modeled/anthropometric volume ratios: effect of age, race, and different formulas [abstract no: A1305]. Journal of the American Society of Nephrology 1996;7(9):1510. [CENTRAL: CN‐00583704] [Google Scholar]
- Daugirdas J, Levin N, Bailey J, Beck G, Cheung A, Greene T, et al. Occurrence of intradialytic hypotension (IH) as a risk factor for mortality in the HEMO study [abstract no: SU‐PO803]. Journal of the American Society of Nephrology 2003;14(Nov):711A. [CENTRAL: CN‐00550388] [Google Scholar]
- Daugirdas JT, Depner TA, Gotch FA, Greene T, Keshaviah P, Levin NW, et al. Comparison of methods to predict equilibrated Kt/V in the HEMO Pilot Study. Kidney International 1997;52(5):1395‐405. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Daugirdas JT, Depner TA, HEMO Study Group. Association of achieved eKt/V with mortality: an example of dose targeting bias [abstract]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):613‐4A. [CENTRAL: CN‐00444992] [Google Scholar]
- Daugirdas JT, Greene T, Chertow GM, Depner TA. Can rescaling dose of dialysis to body surface area in the HEMO study explain the different responses to dose in women versus men?. Clinical Journal of the American Society of Nephrology: CJASN 2010;5(9):1628‐36. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugirdas JT, Greene T, Cheung AK, Beck GJ, Levin NW, Kusek JW, et al. Effect of membrane flux intervention in HEMO study patients when grouped by months on dialysis at randomization, serum albumin, diabetes, and b2‐M clearance [abstract no: SA‐PO2611]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):698A. [CENTRAL: CN‐00782996] [Google Scholar]
- Daugirdas JT, Greene T, Depner TA, Levin NW, Chertow GM. Modeled urea distribution volume and mortality in the HEMO Study. Clinical Journal of the American Society of Nephrology: CJASN 2011;6(5):1129‐38. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugirdas JT, Greene T, Depner TA, Leypoldt J, Gotch F, Schulman G, et al. Factors that affect postdialysis rebound in serum urea concentration, including the rate of dialysis: results from the HEMO Study. Journal of the American Society of Nephrology 2004;15(1):194‐203. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Delmez J, Beck G, Beddhu S, Levey A, Sarnak M, Yan G, et al. Effect of high flux hemodialysis (HD) on cerebrovascular (CBV) deaths: results from the HEMO study [abstract no: SU‐PO345]. Journal of the American Society of Nephrology 2004;15(Oct):609A. [CENTRAL: CN‐00550767] [Google Scholar]
- Delmez JA, Yan G, Bailey J, Beck GJ, Beddhu S, Cheung AK, et al. Cerebrovascular disease in maintenance hemodialysis patients: results of the HEMO Study. American Journal of Kidney Diseases 2006;47(1):131‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Depner T, Daugirdas J, Greene T, Allon M, Beck G, Chumlea C, et al. Dialysis dose and the effect of gender and body size on outcome in the HEMO Study. Kidney International 2004;65(4):1386‐94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Depner TA, Daugirdas JT, HEMO Study Group. Does gender influence the effect of dialysis dose on mortality? Results of the HEMO study [abstract no: SA‐PO770]. Journal of the American Society of Nephrology 2002;13:422A. [CENTRAL: CN‐00445075] [Google Scholar]
- Depner TA, Gotch F, Daugirdas JT, Greene T, Kaufman AM, Hemodialysis (HEMO) Study. Monitoring dialysis therapy using estimates of amount of urea removed and mean urea volume [abstract]. Journal of the American Society of Nephrology 1995;6(3):597. [CENTRAL: CN‐00483727] [Google Scholar]
- Depner TA, Gotch FA, Port FK, Wolfe RA, Lindsay RM, Blake PG, et al. How will the results of the HEMO study impact dialysis practice?. Seminars in Dialysis 2003;16(1):8‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Depner TA, Greene T, Gotch FA, Daugirdas JT, Keshaviah PR, Star RA, et al. Imprecision of the hemodialysis dose when measured directly from urea removal. Hemodialysis Study Group. Kidney International 1999;55(2):635‐47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Dwyer JT, Bergen C, Daugirdas J, Larive B, Leung J, Rocco M, et al. Baseline associations between estimated resting metabolic rate and energy intake in the hemodialysis (HEMO) study [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):355A. [CENTRAL: CN‐00445182] [Google Scholar]
- Dwyer JT, Chumlea WC, Frydrych A, Kusek J, Leung J, Paranandi L, et al. Better nutritional status and lower comorbidity associated with quality of life at baseline in the HEMO Study [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):206A. [CENTRAL: CN‐00445183] [Google Scholar]
- Dwyer JT, Cunniff PJ, Maroni BJ, Kopple JD, Burrowes JD, Powers SN, et al. The hemodialysis pilot study: nutrition program and participant characteristics at baseline. The HEMO Study Group.[Erratum appears in J Ren Nutr 1998 Oct;8(4):230]. Journal of Renal Nutrition 1998;8(1):11‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Dwyer JT, Kopple JD, Maroni BJ, Burrowes JD, Powers SN, Cockram DB, et al. Dietary intake and nutritional status in the HEMO pilot study population [abstract]. Journal of the American Society of Nephrology 1995;6(3):576. [CENTRAL: CN‐00483815] [Google Scholar]
- Dwyer JT, Larive B, Leung J, Rocco M, Burrowes JD, Chumlea WC, et al. Nutritional status affects quality of life in Hemodialysis (HEMO) Study patients at baseline. Journal of Renal Nutrition 2002;12(4):213‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Dwyer JT, Larive B, Leung J, Rocco MV, Greene T, Burrowes J, et al. Are nutritional status indicators associated with mortality in the Hemodialysis (HEMO) Study?. Kidney International 2005;68(4):1766‐76. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Dwyer JT, Leung J, Larive B, Rocco MV, Greene T, Burrowes J, et al. Are common nutritional risk factors among the strongest mortality predictors when also considering case‐mix? Results from the hemodialysis (HEMO) study [abstract no: SA‐PO368]. Journal of the American Society of Nephrology 2004;15(Oct):382A. [CENTRAL: CN‐00550516] [Google Scholar]
- Eknoyan G, Agodoa L, Beck G, Daugirdas J, Greene T, Kusek J, et al. Adequacy of delivered dialysis doses in the HEMO study: an interim report [abstract]. Journal of the American Society of Nephrology 1997;8(Program & Abstracts):282A. [CENTRAL: CN‐00445206] [Google Scholar]
- Eknoyan G, Beck GJ, Cheung AK, Daugirdas JT, Greene T, Kusek JW, et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. New England Journal of Medicine 2002;347(25):2010‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Eknoyan G, Beck GJ, Kusek JW, Levey AS, Levin NW, et al. Design, characterization of prevalent patients, and progress of the hemodialysis (HEMO) study [abstract]. Journal of the American Society of Nephrology 1995;6(3):598. [CENTRAL: CN‐00484324] [Google Scholar]
- Eknoyan G, Greene T, HEMO Study Group. Primary results from the HEMO study [abstract no: SA‐PO767]. Journal of the American Society of Nephrology 2002;13(Program & Abstracts):421A. [CENTRAL: CN‐00445207] [Google Scholar]
- Eknoyan G, HEMO Study Group. The US mortality and morbidity of haemodialysis (HEMO) study. Design and results of the pilot phase [abstract]. 6th Asian Pacific Congress of Nephrology; 1995 Dec 5‐9; Hong Kong. 1995:19. [CENTRAL: CN‐00460693]
- Flythe JE, Kimmel SE, Brunelli SM. Rapid fluid removal during dialysis is associated with cardiovascular morbidity and mortality. Kidney International 2011;79(2):250‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene T, Beck GJ, Gassman JJ, Gotch FA, Kusek JW, Levey AS, et al. Design and statistical issues of the hemodialysis (HEMO) study. Controlled Clinical Trials 2000;21(5):502‐25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Greene T, Daugirdas J, Depner T, Allon M, Beck G, Chumlea C, et al. Association of achieved dialysis dose with mortality in the hemodialysis study: an example of "dose‐targeting bias". Journal of the American Society of Nephrology 2005;16(11):3371‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Greene T, Kusek JK, Levin NW. Progress and adequacy of delivered dialysis doses in the hemodialysis (HEMO) study: interim results [abstract]. 35th Congress. European Renal Association. European Dialysis and Transplantation Association; 1998 Jun 6‐9; Rimini, Italy. 1998:253.
- HEMO Study Group. Comparison of methods to predict the equilibrated kt/v (eKTV) in the HEMO study [abstract]. Journal of the American Society of Nephrology 1995;6(3):596. [CENTRAL: CN‐00484323] [Google Scholar]
- Hemo Study Group, Eknoyan G, Beck GJ, Kusek JW, Levin NW. Progress of the hemodialysis (HEMO) study [abstract]. Nephrology 1997;3(Suppl 1):S419. [CENTRAL: CN‐00460926] [Google Scholar]
- Kang EW, Pike F, Ramer S, Abdel‐Kader K, Myaskovsky L, Dew MA, et al. The association of mental health over time with cardiac outcomes in HEMO study patients. Clinical Journal of the American Society of Nephrology: CJASN 2012;7(6):957‐64. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaysen GA, Greene T, Daugirdas JT, Kimmel PL, Schulman GW, Toto RD, et al. Longitudinal and cross‐sectional effects of C‐reactive protein, equilibrated normalized protein catabolic rate, and serum bicarbonate on creatinine and albumin levels in dialysis patients. American Journal of Kidney Diseases 2003;42(6):1200‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Leung J, Dwyer J, Cockram D, Rocco M, Larive B, Chumlea C, et al. Association of nutritional parameters with mortality depends on length of follow‐up [abstract no: SA‐PO928]. Journal of the American Society of Nephrology 2003;14(Nov):503A. [CENTRAL: CN‐00550731] [Google Scholar]
- Leung J, Larive B, Dwyer J, Hibberd P, Jacques P, Rand W, et al. Folic acid supplementation and cardiac and stroke mortality among hemodialysis patients. Journal of Renal Nutrition 2010;20(5):293‐302. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey A, Athienites NV, Gassman JJ, Martin AA, Ornt DB, Kusek JW, et al. Comorbidity assessment in the HEMO study: an interim report [abstract no: A0933]. Journal of the American Society of Nephrology 1997;8(Program & Abstracts):201A. [CENTRAL: CN‐00446339] [Google Scholar]
- Leypoldt JK, Cheung AK, Agodoa LY, Daugirdas JT, Greene T, Keshaviah PR, et al. Hemodialyzer mass transfer‐area coefficients for urea increase at high dialysate flow rates. The Hemodialysis (HEMO) Study. Kidney International 1997;51(6):2013‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Leypoldt JK, Cheung AK, Clark WR, Daugirdas JT, Gotch FA, Greene T, et al. Characterization of low and high flux dialyzers with reuse in the HEMO study: interim report [abstract no: A1345]. Journal of the American Society of Nephrology 1996;7(9):1518. [CENTRAL: CN‐00583522] [Google Scholar]
- Liang KV, Pike F, Argyropoulos C, Weissfeld L, Teuteberg J, Dew MA, et al. Heart failure severity scoring system and medical‐ and health‐related quality‐of‐life outcomes: The HEMO study. American Journal of Kidney Diseases 2011;58(1):84‐92. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Liang KV, Rosenbaum A, Stephenson R, Pike F, Weissfeld L, et al. Peripheral vascular disease severity impacts health outcomes and health‐related quality of life in maintenance hemodialysis patients in the HEMO Study. Nephrology Dialysis Transplantation 2012;27(7):2929‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Locatelli F. Effect of dialysis dose and membrane flux in maintenance hemodialysis. New England Journal of Medicine 2003;348(15):1491‐4. [MEDLINE: ] [PubMed] [Google Scholar]
- Lynch KE, Lynch R, Curhan GC, Brunelli SM. Altered taste perception and nutritional status among hemodialysis patients. Journal of Renal Nutrition 2013;23(4):288‐95. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch KE, Lynch R, Curhan GC, Brunelli SM. Prescribed dietary phosphate restriction and survival among hemodialysis patients. Clinical Journal of the American Society of Nephrology: CJASN 2011;6(3):620‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroni B, Burkart J, Burrowes J, Cockram D, Drabik M, Dwyer J, et al. Baseline nutritional characteristics of the HEMO study participants: interim report [abstract no: A1041]. Journal of the American Society of Nephrology 1996;7(9):1456. [CENTRAL: CN‐00583818] [Google Scholar]
- Maroni B, Burkhart J, Burrowes J, Dwyer J, Henry R, Kusek J, et al. Relationship between dialysis dose, membrane flux and indices of nutritional status at baseline in the HEMO study: an interm report [abstract no: A1322]. Journal of the American Society of Nephrology 1997;8(Program & Abstracts):288A. [CENTRAL: CN‐00446608] [Google Scholar]
- Causland FR, Brunelli SM, Waikar SS. Dialysis dose and intradialytic hypotension: results from the HEMO study. American Journal of Nephrology 2013;38(5):388‐96. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Causland FR, Waikar SS, Brunelli SM. Increased dietary sodium is independently associated with greater mortality among prevalent hemodialysis patients. Kidney International 2012;82(2):204‐11. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K, Benz R, HEMO Study Group. Quality of life (QOL) outcomes in the HEMO study [abstract no: SA‐P0796]. Journal of the American Society of Nephrology 2002;13(Program & Abstracts):421A. [CENTRAL: CN‐00446759] [Google Scholar]
- Meyer KB, Paranandi L, Hays R, Benz R, Athienites N, Kusek J, et al. Clinical correlates of baseline quality of life in the HEMO study: an interim report [abstract]. Journal of the American Society of Nephrology 1997;8(Program & Abstracts):204A. [CENTRAL: CN‐00446757] [Google Scholar]
- Meyer KB, Paranandi L, Hays R, Benz R, Athienites N, Kusek J, et al. Quality of life in the HEMO study: an interim report [abstract]. Journal of the American Society of Nephrology 1997;8(Program & Abstracts):204A. [CENTRAL: CN‐00446758] [Google Scholar]
- Miskulin DC, Athienites NV, Yan G, Martin AA, Ornt DB, Kusek JW, et al. Comorbidity assessment using the Index of Coexistent Diseases in a multicenter clinical trial. Kidney International 2001;60(4):1498‐510. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ng Y, Unruh ML, Kusek JW, Meyer KB, Yan G, Rocco MV, et al. Hemodialysis timing, survival and cardiovascular outcomes in the HEMO study [abstract no: SU‐PO439]. Journal of the American Society of Nephrology 2004;15(Oct):630A. [CENTRAL: CN‐00550583] [Google Scholar]
- Ng YH, Meyer KB, Kusek JW, Yan G, Rocco MV, Kimmel PL, et al. Hemodialysis timing, survival, and cardiovascular outcomes in the Hemodialysis (HEMO) study. American Journal of Kidney Diseases 2006;47(4):614‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rao M, Guo D, Perianayagam MC, Jaber BL, Pereira BJ, Balakrishnan VS, et al. Comparative predictive power of CRP and IL‐6 for all‐cause and cardiovascular mortality in end‐stage renal disease [abstract no: F‐PO452]. Journal of the American Society of Nephrology 2004;15(Oct):166A. [CENTRAL: CN‐00583200] [Google Scholar]
- Rao M, Guo D, Perianayagam MC, Jaber BL, Pereira BJ, Balakrishnan VS, et al. Cytokine gene polymorphisms modulate acute phase response among hemodialysis patients [abstract no: F‐PO460]. Journal of the American Society of Nephrology 2004;15(Oct):167A. [CENTRAL: CN‐00583202] [Google Scholar]
- Rao M, Li L, Bertrand LJ, Pereira BJ, Balakrishnan VS, HEMO Study Group. Plasma adiponectin and clinical outcomes among hemodialysis patients [abstract no: 133]. American Journal of Kidney Diseases 2006;47(4):A52. [Google Scholar]
- Rao M, Li L, Menon V, Balakrishnan VS. Plasma adiponectin and clinical outcomes among hemodialysis patients [abstract no: F‐PO619]. Journal of the American Society of Nephrology 2006;17(Abstracts):468A. [CENTRAL: CN‐00583203] [Google Scholar]
- Rao M, Menon V, Balakrishnan VS. Determinants of adiponectin levels in hemodialysis patients [abstract no: F‐PO618]. Journal of the American Society of Nephrology 2006;17(Abstracts):468A. [CENTRAL: CN‐00602005] [Google Scholar]
- Rocco M, Benz R, Burkart J, Cheung A, Heyka R, Irving S, et al. Baseline blood pressure in HEMO study participants: an interim report [abstract no: A1153]. Journal of the American Society of Nephrology 1997;8(Program & Abstracts):250‐1A. [CENTRAL: CN‐00447425] [Google Scholar]
- Rocco M, Dwyer J, HEMO Study Group. Effect of dose and flux interventions on nutritional parameters: results from the hemodialysis study [abstract no: SA‐P0768]. Journal of the American Society of Nephrology 2002;13(Program & Abstracts):421A. [CENTRAL: CN‐00447426] [Google Scholar]
- Rocco M, Miskulin D, Unruh M, Yan G, Athienites N, Kusek J, et al. Modification of the ICED to improve its predictive ability for mortality in chronic hemodialysis patients: results from the hemodialysis (HEMO) study [abstract no: SA‐PO380]. Journal of the American Society of Nephrology 2004;15(Oct):385A. [CENTRAL: CN‐00550559] [Google Scholar]
- Rocco MV, Dwyer JT, Larive B, Greene T, Cockram DB, Chumlea WC, et al. The effect of dialysis dose and membrane flux on nutritional parameters in hemodialysis patients: results of the HEMO Study. Kidney International 2004;65(6):2321‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rocco MV, Paranandi L, Burrowes JD, Cockram DB, Dwyer JT, Kusek JW, et al. Nutritional status in the HEMO Study cohort at baseline. American Journal of Kidney Diseases 2002;39(2):245‐56. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rocco MV, Yan G, Gassman J, Lewis J, Ornt D, Weiss B, et al. A comparison of causes of death in the HEMO study with the HCFA death notification form [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):254A. [CENTRAL: CN‐00550561] [Google Scholar]
- Rocco MV, Yan G, Gassman J, Lewis JB, Ornt D, Weiss B, et al. Comparison of causes of death using HEMO Study and HCFA end‐stage renal disease death notification classification systems. The National Institutes of Health‐funded Hemodialysis. Health Care Financing Administration. American Journal of Kidney Diseases 2002;39(1):146‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rocco MV, Yan G, Heyka RJ, Benz R, Cheung AK, HEMO Study Group. Risk factors for hypertension in chronic hemodialysis patients: baseline data from the HEMO study. American Journal of Nephrology 2001;21(4):280‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sattar A, Argyropoulos C, Weissfeld L, Younas N, Fried L, Kellum JA, et al. All‐cause and cause‐specific mortality associated with diabetes in prevalent hemodialysis patients. BMC Nephrology 2012;13:130. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su CT, Yabes J, Pike F, Weiner DE, Beddhu S, Burrowes JD, et al. Changes in anthropometry and mortality in maintenance hemodialysis patients in the HEMO Study. American Journal of Kidney Diseases 2013;62(6):1141‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tangri N, Shastri S, Tighiouart H, Beck GJ, Cheung AK, Eknoyan G, et al. beta‐Blockers for prevention of sudden cardiac death in patients on hemodialysis: a propensity score analysis of the HEMO Study. American Journal of Kidney Diseases 2011;58(6):939‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Unruh M, Benz R, Greene T, Yan G, Beddhu S, DeVita M, et al. Effects of hemodialysis dose and membrane flux on health‐related quality of life in the HEMO Study. Kidney international 2004;66(1):355‐66. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Unruh M, Miskulin D, Yan G, Hays RD, Benz R, Kusek JW, et al. Racial differences in health‐related quality of life among hemodialysis patients. Kidney International 2004;65(4):1482‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Unruh M, Yan G, Radeva M, Hays RD, Benz R, Athienites NV, et al. Bias in assessment of health‐related quality of life in a hemodialysis population: a comparison of self‐administered and interviewer‐administered surveys in the HEMO study. Journal of the American Society of Nephrology 2003;14(8):2132‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Unruh ML, Newman AB, Larive B, Amanda DM, Miskulin DC, Greene T, et al. The influence of age on changes in health‐related quality of life over three years in a cohort undergoing hemodialysis. Journal of the American Geriatrics Society 2008;56(9):1608‐17. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uribarri J, Levin N, Delmez J, Depner T, Ornt D, Owen W, et al. Association of acidosis and nutritional parameters in hemodialysis (HD) patients [abstract]. Journal of the American Society of Nephrology 1997;8(Program & Abstracts):108A. [CENTRAL: CN‐00448101] [DOI] [PubMed] [Google Scholar]
- Uribarri J, Levin NW, Delmez J, Depner TA, Ornt D, Owen W, et al. Association of acidosis and nutritional parameters in hemodialysis patients. American Journal of Kidney Diseases 1999;34(3):493‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vlagopoulos P, Sarnak M, Yan G, Herzog C, Ornt D, Kusek J, et al. Risk factors for sudden death (SD) in chronic hemodialysis [abstract no: SU‐PO346]. Journal of the American Society of Nephrology 2004;15(Oct):609A. [CENTRAL: CN‐00550660] [Google Scholar]
- Waikar SS, Curhan GC, Brunelli SM. Mortality associated with low serum sodium concentration in maintenance hemodialysis. American Journal of Medicine 2011; Vol. 124, issue 1:77‐84. [MEDLINE: ] [DOI] [PMC free article] [PubMed]
- Wald R, Sarnak MJ, Tighiouart H, Cheung AK, Levey AS, Eknoyan G, et al. Disordered mineral metabolism in hemodialysis patients: an analysis of cumulative effects in the Hemodialysis (HEMO) Study. American Journal of Kidney Diseases 2008;52(3):531‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kennedy 1989 {published data only}
- Kennedy SH, Craven JL, Rodin GM, Roin GM. Major depression in renal dialysis patients: an open trial of antidepressant therapy.[Erratum appears in J Clin Psychiatry 1989 Apr;50(4):148 Note: Roin GM [corrected to Rodin GM]]. Journal of Clinical Psychiatry 1989;50(2):60‐3. [MEDLINE: ] [PubMed] [Google Scholar]
Koo 2005 {published data only}
- Koo JR, Yoon JY, Joo MH, Lee HS, Oh JE, Kim SG, et al. Treatment of depression and effect of antidepression treatment on nutritional status in chronic hemodialysis patients. American Journal of the Medical Sciences 2005;329(1):1‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kurella 2005 {published data only}
- Kurella M, Yaffe K, Shlipak MG, Wenger NK, Chertow GM. Chronic kidney disease and cognitive impairment in menopausal women. American Journal of Kidney Diseases 2005;45(1):66‐76. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Levy 1996 {published data only}
- Levy NB, Blumenfield M, Beasley CM Jr, Dubey AK, Solomon RJ, Todd R, et al. Fluoxetine in depressed patients with renal failure and in depressed patients with normal kidney function. General Hospital Psychiatry 1996;18(1):8‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lieh 2004 {published data only}
- Lieh Yeh T, Liang Huang C, Kuang Yang Y, Dar Lee Y, Cheng Chen C, See Chen P. The adjustment to illness in patients with generalized anxiety disorder is poorer than that in patients with end‐stage renal disease. Journal of Psychosomatic Research 2004;57(2):165‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Miller 2005 {published data only}
- Miller DK, Chibnall JT, Videen SD, Duckro PN. Supportive‐affective group experience for persons with life‐threatening illness: reducing spiritual, psychological, and death‐related distress in dying patients. Journal of Palliative Medicine 2005;8(2):333‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moug 2004 {published data only}
- Moug SJ, Grant S, Creed G, Boulton Jones M. Exercise during haemodialysis: West of Scotland pilot study. Scottish Medical Journal 2004;49(1):14‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pearlman 1988 {published data only}
- Pearlman C, Carson W, Metz A. Hemodialysis, chronic renal failure, and ECT. Convulsive Therapy 1988;4(4):332‐3. [PubMed] [Google Scholar]
Seabolt 2001 {published data only}
- Seabolt JL, Leon OA. Response to nefazodone in a depressed patient with end‐stage renal disease. General Hospital Psychiatry 2001;23(1):45‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sharp 2005 {published data only}
- Sharp J, Wild MR, Gumley AI, Deighan CJ. A cognitive behavioral group approach to enhance adherence to hemodialysis fluid restrictions: a randomized controlled trial. American Journal of Kidney Diseases 2005;45(6):1046–57. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
SMILE Study 2010 {published data only}
- Green JA, Mor MK, Shields AM, Sevick MA, Arnold RM, Palevsky PM, et al. Associations of health literacy with dialysis adherence and health resource utilization in patients receiving maintenance hemodialysis. American Journal of Kidney Diseases 2013;62(1):73‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Green JA, Mor MK, Shields AM, Sevick MA, Palevsky PM, Fine MJ, et al. Prevalence and demographic and clinical associations of health literacy in patients on maintenance hemodialysis. Clinical Journal of the American Society of Nephrology 2011;6(6):1354‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JA, Mor MK, Shields AM, Sevik MA, Palevsky PM, Fine MJ, et al. Renal provider perceptions and practice patterns regarding the management of pain, sexual dysfunction, and depression in hemodialysis patients. Journal of Palliative Medicine 2012;15(2):163‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mor MK, Sevick MA, Shields AM, Green JA, Palevsky PM, Arnold RM, et al. Sexual function, activity, and satisfaction among women receiving maintenance hemodialysis. Clinical Journal of the American Society of Nephrology 2014;9(1):128‐34. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisbord SD, Mor MK, Green JA, Sevick MA, Shields AM, Zhao X, et al. Comparison of symptom management strategies for pain, erectile dysfunction, and depression in patients receiving chronic hemodialysis: a cluster randomized effectiveness trial. Clinical Journal of the American Society of Nephrology: CJASN 2013;8(1):90‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisbord SD, Mor MK, Sevick MA, Shields AM, Rollman BL, Palevsky PM, et al. Associations of depressive symptoms and pain with dialysis adherence, health resource utilization, and mortality in patients receiving chronic hemodialysis. Clinical Journal of the American Society of Nephrology: CJASN 2014;9(9):1594‐602. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisbord SD, Shields AM, Mor MK, Sevick MA, Homer M, Peternel J, et al. Methodology of a randomized clinical trial of symptom management strategies in patients receiving chronic hemodialysis: the SMILE study. Contemporary Clinical Trials 2010;31(5):491‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Solak 2012 {published data only}
- Atalay H, Solak Y, Biyik Z, Gaipov A, Guney F, Turk S. Cross‐over, open‐label trial of the effects of gabapentin versus pregabalin on painful peripheral neuropathy and health‐related quality of life in haemodialysis patients. Clinical Drug Investigation 2013;33(6):401‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Biyik Z, Solak Y, Atalay H, Gaipov A, Guney F, Turk S. Gabapentin versus pregabalin in improving sleep quality and depression in hemodialysis patients with peripheral neuropathy: a randomized prospective crossover trial. International Urology & Nephrology 2013;45(3):831‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Solak Y, Biyik Z, Atalay H, Gaipov A, Guney F, Turk S, et al. Pregabalin versus gabapentin in the treatment of neuropathic pruritus in maintenance haemodialysis patients: a prospective, crossover study. Nephrology 2012;17(8):710‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tsay 2004a {published data only}
- Tsay SL. Acupressure and fatigue in patients with end‐stage renal disease‐a randomized controlled trial. International Journal of Nursing Studies 2004;41(1):99‐106. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tsay 2004b {published data only}
- Tsay SL, Cho YC, Chen ML. Acupressure and Transcutaneous Electrical Acupoint Stimulation in improving fatigue, sleep quality and depression in hemodialysis patients. American Journal of Chinese Medicine 2004;32(3):407‐16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tsay 2004c {published data only}
- Tsay SL, Hung LO. Empowerment of patients with end‐stage renal disease‐‐a randomized controlled trial. International Journal of Nursing Studies 2004;41(1):59‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tsay 2005 {published data only}
- Tsay SL, Lee YC, Lee YC. Effects of an adaptation training programme for patients with end‐stage renal disease. Journal of Advanced Nursing 2005;50(1):39‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Turk 2010 {published data only}
- Solak Y, Atalay H, Kan S, Kaynar M, Bodur S, Yeksan M, et al. Effects of sildenafil and vardenafil treatments on sleep quality and depression in hemodialysis patients with erectile dysfunction. International Journal of Impotence Research 2011;23(1):27‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Turk S, Solak Y, Kan S, Atalay H, Kilinc M, Agca E, et al. Effects of sildenafil and vardenafil on erectile dysfunction and health‐related quality of life in haemodialysis patients: a prospective randomized crossover study. Nephrology Dialysis Transplantation 2010;25(11):3729‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
van Vilsteren 2005 {published data only}
- Vilsteren MC, Greef MH, Huisman RM. The effects of a low‐to‐moderate intensity pre‐conditioning exercise programme linked with exercise counselling for sedentary haemodialysis patients in The Netherlands: results of a randomized clinical trial. Nephrology Dialysis Transplantation 2005;20(1):141‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wuerth 2001 {published data only}
- Wuerth D, Finkelstein SH, Ciarcia J, Peterson R, Kliger AS, Finkelstein FO. Identification and treatment of depression in a cohort of patients maintained on chronic peritoneal dialysis. American Journal of Kidney Diseases 2001;37(5):1011‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zetin 1980 {published data only}
- Zetin M, Stone RA. Effects of zinc in chronic hemodialysis. Clinical Nephrology 1980;13(1):20‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Zetin 1982 {published data only}
- Zetin M, Frost NR, Brumfield D, Stone RA. Amitriptyline stimulates weight gain in hemodialysis patients. Clinical Nephrology 1982;18(2):79‐82. [MEDLINE: ] [PubMed] [Google Scholar]
References to studies awaiting assessment
Gharekhani 2014 {published data only}
- Dashti‐Khavidaki S, Gharekhani A, Khatami MR, Miri ES, Khalili H, Razeghi E, et al. Effects of omega‐3 fatty acids on depression and quality of life in maintenance hemodialysis patients. American Journal of Therapeutics 2014;21(4):275‐87. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gharekhani A, Khatami MR, Dashti‐Khavidaki S, Razeghi E, Abdollahi A, Hashemi‐Nazari SS, et al. Effects of oral supplementation with omega‐3 fatty acids on nutritional state and inflammatory markers in maintenance hemodialysis patients. Journal of Renal Nutrition 2014;24(3):177‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gharekhani A, Khatami MR, Dashti‐Khavidaki S, Razeghi E, Noorbala AA, Hashemi‐Nazari SS, et al. The effect of omega‐3 fatty acids on depressive symptoms and inflammatory markers in maintenance hemodialysis patients: a randomized, placebo‐controlled clinical trial. European Journal of Clinical Pharmacology 2014;70(6):655‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Solak 2010 {published data only}
- Solak Y, Atalay H, Kan S, Kilinc M, Bodur S, Turk S. Effects of vardenafil and sildenafil on sleep quality and depression in impotent hemodialysis patients: a prospective cross‐over study [abstract no: SA595]. NDT Plus 2010;3(Suppl 3):iii242‐3. [EMBASE: 70484061] [Google Scholar]
References to ongoing studies
NCT02358343 {published data only}
- Mehrotra R. ASCEND: A trial of sertraline vs. CBT for end‐stage renal disease patients with depression. www.clinicaltrials.gov/ct2/show/NCT02358343 (accessed 20 January 2016).
NCT02407821 {published data only}
- Jassal V. Pilot study to evaluate the feasibility and safety of performing a double blind, placebo‐controlled, randomized controlled trial of the routine use of SSRI's at the initiation of end‐stage renal disease treatment Escitalopram. www.clinicaltrials.gov/ct2/show/NCT02407821 (accessed 20 January 2016).
Additional references
Abbas Tavallaii 2009
- Abbas Tavallaii S, Ebrahimnia M, Shamspour N, Assari S. Effect of depression on health care utilization in patients with end‐stage renal disease treated with hemodialysis. European Journal of Internal Medicine 2009;20(4):411‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Abram 1971
- Abram HS, Moore GL, Westervelt FB Jr. Suicidal behavior in chronic dialysis patients. American Journal of Psychiatry 1971;127(9):1199‐204. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Anderson 2001
- Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta‐analysis. Diabetes Care 2001;24(6):1069‐78. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
DiMatteo 2000
- DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta‐analysis of the effects of anxiety and depression on patient adherence. Archives of Internal Medicine 2000;160(14):2101‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Emerging Risk Factors Collaboration 2010
- Emerging Risk Factors Collaboration, Kaptoge S, Angelantonio E, Lowe G, Pepys MB, Thompson SG, et al. C‐reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta‐analysis. Lancet 2010;375(9709):132‐40. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
ERBP 2012
- Nagler EV, Webster AC, Vanholder R, Zoccali C. Antidepressants for depression in stage 3‐5 chronic kidney disease: a systematic review of pharmacokinetics, efficacy and safety with recommendations by European Renal Best Practice (ERBP). Nephrology Dialysis Transplantation 2012;27(10):3736‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Friend 1997
- Friend R, Hatchett L, Wadhwa NK, Suh H. Serum albumin and depression in end‐stage renal disease. Advances in Peritoneal Dialysis 1997;13:155‐7. [MEDLINE: ] [PubMed] [Google Scholar]
GRADE 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. Journal of Clinical Epidemiology 2011;64(4):380‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Haenel 1980
- Haenel T, Brunner F, Battegay R. Renal dialysis and suicide: occurrence in Switzerland and in Europe. Comprehensive Psychiatry 1980;21(2):140‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hedayati 2005
- Hedayati SS, Grambow SC, Szczech LA, Stechuchak KM, Allen AS, Bosworth HB. Physician‐diagnosed depression as a correlate of hospitalizations in patients receiving long‐term hemodialysis. American Journal of Kidney Diseases 2005;46(4):642‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hedayati 2012
- Hedayati SS, Yalamanchili V, Finkelstein FO. A practical approach to the treatment of depression in patients with chronic kidney disease and end‐stage renal disease. Kidney International 2012;81(3):247‐55. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Juergenson 1996
- Juergensen PH, Juergensen DM, Wuerth DB, Finkelstein SH, Steele TE, Kliger AS, et al. Psychosocial factors and incidence of peritonitis. Advances in Peritoneal Dialysis 1996;12:196‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Kimmel 1998
- Kimmel PL, Thamer M, Richard CM, Ray NF. Psychiatric illness in patients with end‐stage renal disease. American Journal of Medicine 1998;105(3):214‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Koo 2003
- Koo JR, Yoon JW, Kim SG, Lee YK, Oh KH, Kim GH, et al. Association of depression with malnutrition in chronic hemodialysis patients. American Journal of Kidney Diseases 2003;41(5):1037‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lacson 2012
- Lacson E Jr, Li NC, Guerra‐Dean S, Lazarus M, Hakim R, Finkelstein FO. Depressive symptoms associate with high mortality risk and dialysis withdrawal in incident hemodialysis patients. Nephrology Dialysis Transplantation 2012;27(7):2921‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lopes 2002
- Lopes AA, Bragg J, Young E, Goodkin D, Mapes D, Combe C, et al. Depression as a predictor of mortality and hospitalization among hemodialysis patients in the United States and Europe. Kidney International 2002;62(1):199‐207. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Manns 2014
- Manns B, Hemmelgran B, Lillie E, Dip SC, Cyr A, Gladish M, et al. Setting research priorities for patients on or nearing dialysis. Clinical Journal of The American Society of Nephrology: CJASN 2014;9(10):1813‐21. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Martin 2000
- Martin CR, Thompson DR. Prediction of quality of life in patients with end‐stage renal disease. British Journal of Health Psychology 2000;5(1):41‐55. [EMBASE: 2000060174] [Google Scholar]
Mitchell 2011
- Mitchell AJ, Chan M, Bhatti H, Halton M, Grassi L, Johansen C, et al. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative‐care settings: a meta‐analysis of 94 interview‐based studies. Lancet Oncology 2001;12(2):160‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Murtagh 2007
- Murtagh FE, Addington‐Hall J, Higginson IJ. The prevalence of symptoms in end‐stage renal disease: a systematic review. Advances in Chronic Kidney Disease 2007;14(1):82‐99. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
O'Connor 2009
- O'Connor EA, Whitlock EP, Beil TL, Gaynes BN. Screening for depression in adult patients in primary care settings: a systematic evidence review. Annals of Internal Medicine 2009;151(11):793‐803. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Palmer 2013a
- Palmer S, Vecchio M, Craig JC, Tonelli M, Johnson DW, Nicolucci A, et al. Prevalence of depression in chronic kidney disease: systematic review and meta‐analysis of observational studies. Kidney International 2013;84(1):179‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Palmer 2013b
- Palmer SC, Vecchio M, Craig JC, Tonelli M, Johnson DW, Nicolucci A, et al. Association between depression and death in people with CKD: a meta‐analysis of cohort studies. American Journal of Kidney Diseases 2013;62(3):493‐505. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schunemann 2011a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schunemann 2011b
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Sensky 1996
- Sensky T, Leger C, Gilmour S. Psychosocial and cognitive factors associated with adherence to dietary and fluid restriction regimens by people on chronic haemodialysis. Psychotherapy & Psychosomatics 1996;65(1):36‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Steele 1996
- Steele TE, Baltimore D, Finkelstein SH, Jeurgenson P, Kliger AS, Finkelstein FO. Quality of life in peritoneal dialysis patients. Journal of Nervous & Mental Disease 1996;184(6):368‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tong 2008
- Tong A, Sainsbury P, Carter SM, Hall B, Harris DC, Walker RG, et al. Patients' priorities for health research: focus group study of patients with chronic kidney disease. Nephrology Dialysis Transplantation 2008;23(10):3206‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tsay 2002
- Tsay SL, Healstead M. Self‐care self‐efficacy, depression, and quality of life among patients receiving hemodialysis in Taiwan. International Journal of Nursing Studies 2002;39(3):245‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Turner 2008
- Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R. Selective publication of antidepressant trials and its influence on apparent efficacy. New England Journal of Medicine 2008;358(3):252‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vaccarino 2007
- Vaccarino V, Johnson BD, Sheps DS, Reis SE, Kelsey SF, Bittner V, et al. Depression, inflammation, and incident cardiovascular disease in women with suspected coronary ischemia: the National Heart, Lung, and Blood Institute‐sponsored WISE study. Journal of the American College of Cardiology 2007;50(21):2044‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Rabindranath 2003
- Rabindranath KS, Macleod AM, Daly C, Roderick P, Butler J, Wallace S. Physical measures for treating depression in dialysis patients. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD004541] [DOI] [PubMed] [Google Scholar]
Rabindranath 2005
- Rabindranath KS, Butler J, Roderick PJ, Wallace SA, Daly C, MacLeod AM. Physical measures for treating depression in dialysis patients. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD004541.pub2] [DOI] [PubMed] [Google Scholar]


