Bacterial infection enhances the production of apoplastic vesicles carrying stress-response proteins in Arabidopsis.
Abstract
Exosomes are extracellular vesicles (EVs) that play a central role in intercellular signaling in mammals by transporting proteins and small RNAs. Plants are also known to produce EVs, particularly in response to pathogen infection. The contents of plant EVs have not been analyzed, however, and their function is unknown. Here, we describe a method for purifying EVs from the apoplastic fluids of Arabidopsis (Arabidopsis thaliana) leaves. Proteomic analyses of these EVs revealed that they are highly enriched in proteins involved in biotic and abiotic stress responses. Consistent with this finding, EV secretion was enhanced in plants infected with Pseudomonas syringae and in response to treatment with salicylic acid. These findings suggest that EVs may represent an important component of plant immune responses.
Eukaryotic cells secrete three main classes of extracellular vesicles (EVs), each with a distinct mechanism of biogenesis. Apoptotic bodies are the largest (1,000–5,000 nm in diameter) and most heterogenous of the three classes. They are products of membrane blebbing released from cells during the late stages of programmed cell death. The other two classes of EVs are microvesicles (100–1,000 nm in diameter) and exosomes (30–150 nm in diameter). Microvescicles are shed directly from the plasma membrane (PM), while exosomes are released from a cell after the fusion of a multivesicular body (MVB) with the PM (György et al., 2011; van der Pol et al., 2012; Akers et al., 2013)
Exosomes were originally thought to function as a waste disposal system, but more recent findings indicate that they mediate intercellular communication and are capable of modulating immune responses. Exosomes secreted by immune cells present antigens on their surface and contribute to adaptive immunity by activating T cells (Raposo et al., 1996; Théry et al., 2002; Giri and Schorey, 2008). In certain cases, antigen-bearing exosomes provide an effective means of vaccination (Wolfers et al., 2001; Aline et al., 2004; Altieri et al., 2004) and can serve as a source of diagnostic biomarkers for various diseases (Welton et al., 2010; Foulds et al., 2012; Saman et al., 2012). Exosomes also can down-regulate immune cells, facilitate normal growth and development (Hedlund et al., 2009), or promote the spread of tumors and viruses (Skog et al., 2008; Meckes et al., 2010; Hood and Wickline, 2012).
Exosomes also mediate intercellular communication by shuttling mRNAs and various species of small noncoding RNAs between cells. These molecules remain functional after delivery and can elicit effects in the recipient cell (Pegtel et al., 2010; Mittelbrunn et al., 2011; Ridder et al., 2014). The RNA content of exosomes varies widely depending on the cell type and state of pathology. For example, exosomes from malignant tumor cells have a microRNA content representative of metastatic tumors. Their RNA profiles can serve as an additional diagnostic biomarker and help distinguish between malignant and benign cells (Taylor and Gercel-Taylor, 2008; Pigati et al., 2010). Furthermore, the ability of exosomes to shuttle RNA from cell to cell suggests that they could be used therapeutically to administer nucleic acid drugs (Ohno et al., 2013).
Despite the many promising uses for exosomes, many basic biological questions regarding their biogenesis, loading, secretion, and uptake remain unanswered. To date, the majority of research on exosomes has been performed using mammalian cell cultures, with just a few studies describing exosome production in standard model organisms such as Caenorhabditis elegans (Liégeois et al., 2006) and Drosophila melanogaster (Beckett et al., 2013; Corrigan et al., 2014). Unfortunately, the plant kingdom has been largely absent from the field of exosome research, in spite of the fact that exosome release had been observed in plant cells 15 years before it was discovered in mammalian cells (Halperin and Jensen, 1967).
The small amount of data on plant exosomes comes mainly from studies using transmission electron microscopy (TEM). These studies show that MVBs proliferate in plant cells during pathogen attack and are frequently observed in various states of fusion with the PM. The majority of these fusion events occur at or near papillae, extracellular defense structures that block pathogen entry into the cell (Zeyen and Bushnell, 1979; An et al., 2006a, 2006b). GTPases that promote fusion between MVBs and the PM also accumulate around sites of infection and are required for the successful formation of papillae (Böhlenius et al., 2010; Ebine et al., 2011; Nielsen et al., 2012). Furthermore, TEM has shown that the papillary matrix contains small vesicles similar to those observed inside MVBs (Politis and Goodman, 1978). Combined, these data suggest that exosome secretion in plants contributes to the development of early defense structures in response to pathogens.
Plant exosomes appear to mediate the transport of various compounds and proteins into the extracellular space. Important defense compounds, such as hydrogen peroxide and callose (a β-1,3-Glc polymer), are trafficked to the PM inside MVBs and accumulate inside papillae (Xu and Mendgen, 1994; An et al., 2006a). The synthase responsible for producing papillary callose, POWDERY MILDEW RESISTANT4, also is embedded within papillae, together with the syntaxin AtSYP121/PENETRATION1 (PEN1) and the ATP-binding cassette transporter PEN3 (Meyer et al., 2009; Ellinger et al., 2013; Underwood and Somerville, 2013). Significantly, mutation of PEN1 delays the formation of papillae (Assaad et al., 2004). In addition, exosome-mediated transport may facilitate the secretion of many other proteins in plants. On average, 50% of extracellular proteins lack a signal peptide normally required for secretion through the standard secretory pathway (Agrawal et al., 2010). A number of these leaderless secretory proteins have antimicrobial activities and are released in response to pathogens.
Exosomes may also mediate the transport of small interfering RNAs from plant cells into fungal cells. During host-induced gene silencing, small RNAs expressed in a plant are able to reduce the expression of target transcripts in invading fungi, specifically in the haustoria (Nowara et al., 2010). Interestingly, TEM has revealed the presence of numerous vesicles in the extrahaustorial matrix (Micali et al., 2011). While it is unclear if these vesicles originated in the fungus or the plant, they may mediate the transfer of small RNAs between the two organisms. In support of this hypothesis, it was reported recently that fungal microRNAs target genes in host plant cells, indicating that there exists a mechanism for the secretion of fungal microRNAs that are then taken up by the host cell (Weiberg et al., 2013). Parasitic plants also form haustoria and can transport mRNAs into host tissues. Parasitic plant mRNA can travel large distances from the invading haustoria, suggesting that it may be packaged inside exosomes (Kim et al., 2014).
Mammalian exosomes are regularly isolated from cultured medium and biological fluids, including blood (Caby et al., 2005), saliva (Palanisamy et al., 2010), semen (Vojtech et al., 2014), urine (Pisitkun et al., 2004), milk (Admyre et al., 2007), cerebrospinal fluid (Street et al., 2012), synovial fluid (Skriner et al., 2006), bronchoalveolar fluid (Prado et al., 2008), amniotic fluid (Asea et al., 2008), and fecal matter (Koga et al., 2011). To our knowledge, however, only a single laboratory has attempted to isolate plant exosomes. Regente et al. (2009) observed vesicles 50 to 200 nm in diameter in the fluids collected from water-imbibed sunflower (Helianthus annuus) seeds. To expand the current knowledge of plant exosomes, we purified vesicles from the apoplastic fluid of Arabidopsis (Arabidopsis thaliana) rosettes. These EVs are enriched in proteins related to stress and defense, including PEN1. The secretion of these EVs is enhanced during infection with a virulent strain of the bacterial pathogen Pseudomonas syringae and in response to salicylic acid (SA). Our data suggest that exosomes contribute to innate immunity and may mediate intercellular communication in plants as well as in animals.
RESULTS AND DISCUSSION
Vesicle-Like Structures Are Present in Arabidopsis Apoplastic Fluid
EVs produced by mammalian cells are routinely isolated from cell culture media and numerous biological fluids. Isolation methods typically use a combination of filtration and differential centrifugation steps. We applied these same techniques to isolate EVs from the apoplastic fluid of Arabidopsis rosettes. Briefly, whole rosettes were harvested and vacuum infiltrated with a pH 6 MES buffer adapted from that described by Regente et al. (2009; see “Materials and Methods”). The infiltrated plants were carefully packaged inside syringes and centrifuged at a low speed. The resulting apoplastic wash was collected, filtered, and centrifuged successively at 10,000g, 40,000g, and 100,000g. The 40,000g and 100,000g pellets (P40 and P100 fractions, respectively) were retained and resuspended in buffer for further analysis.
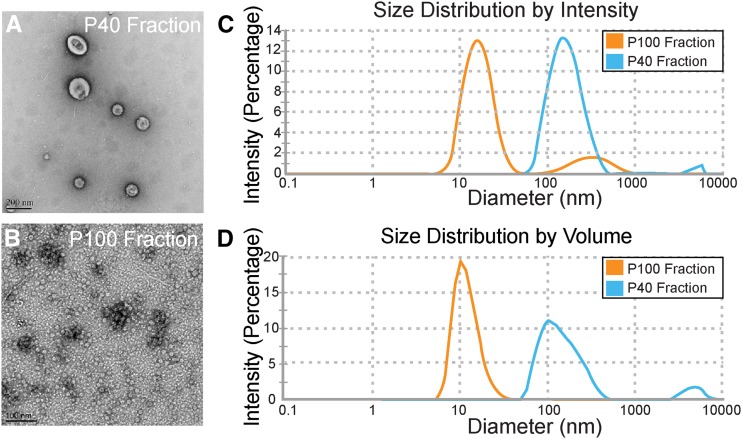
To determine if vesicles were present in the apoplastic fluid, we examined the P40 and P100 fractions using negative staining and TEM. The P40 fraction contained numerous cup-shaped structures (approximately 100 nm in diameter) reminiscent of vesicles (Fig. 1A). The cupped shape of these objects is an artifact of negative staining (Thery et al., 2006). The P100 fraction, however, was generally devoid of vesicle-like structures and instead was dominated by small (approximately 12 nm in diameter) circular objects (Fig. 1B).
Figure 1.
The apoplastic wash from Arabidopsis leaves contains vesicle-like structures. A and B, Negative staining and TEM of the P40 and P100 fractions derived from the apoplastic wash. C and D, Light-scattering charts showing peak intensities and percentages of differently sized particles in the P40 and P100 fractions. The experiments were repeated a minimum of three times with similar results.
We used light scattering to determine the size range of particles in each pellet prior to fixation. Particles in the P40 fraction ranged in size from approximately 50 to 300 nm in diameter (Fig. 1C), with the most abundant species around 150 nm in diameter (Fig. 1D). Particles in the P100 fraction were much smaller, ranging in size from approximately 10 to 17 nm in diameter (Fig. 1C), with the most abundant species around 12 nm in diameter (Fig. 1D). These results demonstrate that vesicle-like structures can be isolated from apoplastic fluid and that 40,000g is a sufficient speed for their isolation.
PEN1 Is Associated with Apoplastic Vesicles
Based on the mammalian literature, EV preparations contain a heterogenous mixture of vesicles originating from different sources and possessing diverse functions. In animal systems, protein markers are used to help distinguish among various classes of EVs and rule out contaminating cellular debris. For example, mammalian exosomes are enriched in a particular subset of proteins, including major histocompatibility complexes I and II, tetraspanins (CD9, CD63, CD81, and CD82), and heat shock proteins (HSC70 and HSP90; Wubbolts et al., 2003). In plants, the protein content of EVs has not been described; however, the syntaxin PEN1 has been shown to associate with extracellular membranous material, suggesting that it may be packaged into exosomes (Meyer et al., 2009; Nielsen et al., 2012).
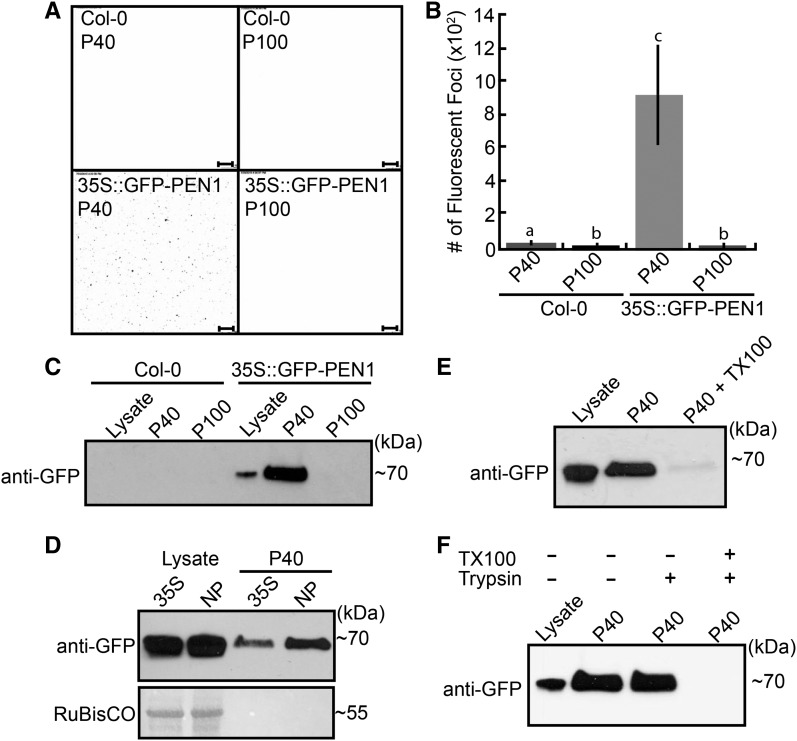
To determine if PEN1 is associated with the P40 fraction, we isolated vesicles from plants constitutively expressing GFP-PEN1. When we examined the P40 fraction using confocal fluorescence microscopy, we observed numerous fluorescent spots on the surface of the slide. This fluorescent signal was not present in the P100 fraction when using GFP-PEN1 plants or in either pellet when using nontransgenic plants (Fig. 2, A and B). In agreement with these findings, an immunoblot for GFP revealed the presence of GFP-PEN1 exclusively in the P40 fraction derived from GFP-PEN1 plants (Fig. 2C). Furthermore, GFP-PEN1 was present in the P40 fraction regardless of whether it was expressed under the control of a 35S or native promoter (Fig. 2D), indicating that its presence in the apoplast-derived pellet is not a result of overexpression.
Figure 2.
Arabidopsis EVs contain the syntaxin PEN1. A, Confocal microscopy images (inverted) of the P40 and P100 fractions derived from the apoplastic wash of nontransgenic (Columbia-0 [Col-0]) and GFP-PEN1 transgenic plants. Bars = 10 µm. B, Quantification of fluorescent foci from confocal microscopy images. Letters signify which values are significantly different from each other based on a two-tailed unpaired Student's t test (P < 0.05). C, Immunoblots of the P40 and P100 fractions confirm that GFP-PEN1 is present only in the P40 fraction of transgenic GFP-PEN1 plants. The lysate lane indicates whole-leaf protein extracts. D, GFP-PEN1 expressed under the control of a native promoter (NP) also accumulates in the P40 fraction. E, Detergent treatment removes GFP-PEN1 from the P40 fraction. P40 fractions derived from GFP-PEN1 plants were treated with buffer alone or buffer plus TX100 followed by recentrifugation. Treatment with TX100 removed GFP-PEN1 from the pellet. F, GFP-PEN1 in the P40 fraction is protected from trypsin degradation in the absence of detergent. All experiments were repeated a minimum of three times with similar results. Error bars in B indicate sd; n = 4; P < 0.05 using a two-tailed unpaired Student’s t test.
To test if the presence of GFP-PEN1 in the P40 fraction is dependent on membranous structures, we washed the pellet with either buffer alone or buffer containing Triton X-100 (TX100) and recentrifuged at the same speed. Treating the pellet with detergent before the second centrifugation greatly reduced the amount of detectable GFP-PEN1 (Fig. 2E). This suggests that GFP-PEN1 is pelleting in association with the observed vesicles.
Papillary GFP-PEN1 fluorescence lasts for days after its secretion, despite the unfavorably acidic conditions within the apoplast (Meyer et al., 2009). This observation suggests that PEN1 is protected inside the lumen of EVs. If GFP-PEN1 is associated with vesicles in the P40 fraction, then it should be largely shielded from protease digestion. To test this hypothesis, we performed a protease protection assay. When the P40 fraction was treated with trypsin alone, GFP-PEN1 was protected from digestion (Fig. 2F). Pretreating the pellet with TX100 followed by trypsin, however, completely eliminated the GFP-PEN1 signal, indicating that PEN1 is sheltered within the lumen of apoplastic vesicles.
Apoplastic Vesicles Are Enriched for PEN1
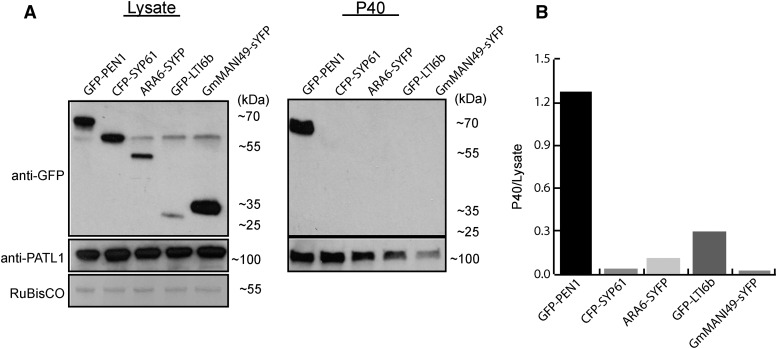
PEN1 was shown previously to mediate trafficking between the Golgi complex and the PM (Geelen et al., 2002). Therefore, GFP-PEN1-associated vesicles in the P40 fraction could be derived from intracellular vesicles released by cellular rupture that could potentially occur during vacuum infiltration or from reconstituted fragments of the PM. To test whether apoplastic vesicles might be artifacts produced by damaged cells, we isolated vesicles from plants expressing GFP-PEN1, the trans-Golgi network/early endosome (TGN/EE) marker SYNTAXIN PROTEIN61 (SYP61-CFP; Robert et al., 2008), the late endosome marker ARA6-sYFP (Ueda et al., 2004), a marker for Golgi bodies (GmManI49-sYFP; Gu and Innes, 2012), or the PM marker GFP-LOW TEMPERATURE INDUCED6b (LTI6b; Cutler et al., 2000). We used an immunoblot to probe each P40 fraction for its respective fusion protein and compared the intensity of the signals in the P40 fraction with those obtained using whole-cell protein extract. The results show that the P40 fraction is enriched for GFP-PEN1 and not the other markers tested (Fig. 3).
Figure 3.
Apoplastic EVs are enriched for PEN1. A, Transgenic lines constitutively expressing the indicated endomembrane and PM markers were subjected to EV isolation, and the P40 fractions were analyzed by immunoblot. The lysate blot indicates whole-leaf protein extracts. Only GFP-PEN1 was readily detectable in the P40 fraction. B, Quantification of marker proteins in the P40 fraction. Band intensities were quantified, and the ratio between the P40 and lysate bands was calculated for each protein. PATL1, an EV-localized protein that we identified by mass spectrometry (Supplemental Table S1), was used to normalize for EV concentrations in the P40 fractions. The specificity of the anti-PATL1 antibody is shown in Supplemental Figure S1. This experiment was repeated a minimum of three times with similar results.
While GFP-PEN1 localizes to the PM, the P40 fraction was not enriched for GFP-LTI6b, suggesting that the vesicles are not reconstituted fragments of PM, nor are they generated directly from the PM. Therefore, we surmise that their origins are endosomal in nature. The P40 fraction, however, was not enriched for the TGN/EE marker SYP61-CFP, the late endosome marker ARA6-sYFP, or the Golgi marker GmMANI49-sYFP, suggesting that the P40 vesicles are not liberated endosomes or Golgi (Fig. 3). The absence of SYP61 is particularly significant, because PEN1 and SYP61 colocalize and interact on early endosomes (Drakakaki et al., 2012; Hachez et al., 2014). Combined, our results suggest that the vesicles in the P40 fraction are enriched for the exosome marker PEN1 but not for markers that may indicate cellular damage. Although some cellular damage may have occurred during the isolation of apoplastic wash, it is most likely not significant enough to account for the presence of PEN1-rich vesicles.
Plant EVs Float in an Iodixanol Density Gradient
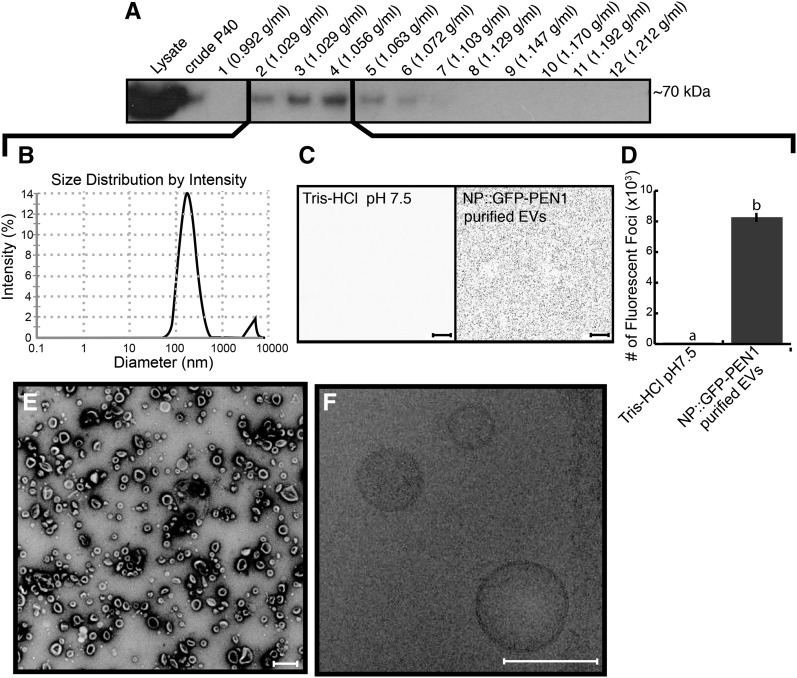
To further purify EVs and estimate their density, we used a discontinuous iodixanol (OptiPrep) density gradient to isolate apoplastic vesicles from GFP-PEN1 plants. After 17 h of centrifugation, we divided the gradient into 12 fractions. Each fraction was pelleted at 100,000g, washed, and pelleted again. We examined the pellets derived from each fraction using an immunoblot for GFP. The signal for GFP-PEN1 was spread out over several fractions (fractions 2–7) and was most concentrated at densities ranging from 1.029 to 1.056 g mL−1 (fractions 2–4; Fig. 4A). Light scattering detected particles within these fractions with an average diameter of 165 nm (Fig. 4B). We confirmed the presence of GFP-PEN1 fluorescence in fractions 2 to 4 using confocal microscopy (Fig. 4, C and D) and observed vesicles using both negative staining TEM and cryo-electron microscopy (Fig. 4, E and F). The latter confirmed the presence of a lipid bilayer (Fig. 4F).
Figure 4.
Density gradient purification of EVs. A, Immunoblot detection of GFP-PEN1 in fractions from the iodixanol density gradient. Fraction 1 consisted of 3.5 mL, fraction 2 was 1.7 mL, fractions 3 to 7 were 0.6 mL each, and fractions 8 to 12 were 0.7 mL each. B, Light-scattering charts showing peak intensities for pooled fractions 2 to 4. C, Confocal microscopy images (inverted) of Tris-HCl, pH 7.5, control (left) and pooled fractions 2 to 4 resuspended in Tris-HCl, pH 7.5 (right). Bars = 10 µm. D, Quantification of confocal images in C. Letters signify values that are significantly different from each other based on a two-tailed unpaired Student's t test (P < 0.0001). E, Negative staining and TEM of fractions 2 to 4. Bar = 10 µm. F, Cryo-electron microscopy of fractions 2 to 4. Note the lipid bilayer in the bottom right vesicle. Bar = 100 nm. All experiments were repeated at least two times with similar results. Error bars indicate sd; n = 3; P < << 0.001 using a two-tailed unpaired Student’s t test.
EV Recovery Is Not Affected by Treatment with Brefeldin A
The accumulation of GFP-PEN1 at powdery mildew infection sites can be inhibited by pretreating leaves with brefeldin A (BFA; Nielsen et al., 2012), a fungal toxin that inhibits endomembrane trafficking by targeting ADP ribosylation factor-GTP exchange factor proteins (Nebenführ et al., 2002). We thus investigated whether pretreatment of Arabidopsis rosette leaves with BFA would reduce the recovery of EVs in apoplastic washes. Infiltration of 300 µm BFA (the concentration used by Nielsen et al. [2012]) into leaves 20 h prior to EV isolation did not reduce EV recovery based on the detection of GFP-PEN1 (Supplemental Fig. S1). It is difficult to interpret this result, however, because we do not know the rate of EV turnover; thus, the EVs recovered after 20 h of BFA treatment could have been present prior to BFA treatment. In addition, PEN1 localization to fungal penetration sites appears to be mediated, at least in part, by the recycling of PEN1 from the PM (Nielsen and Thordal-Christensen, 2012). BFA is known to inhibit the endocytic pathway in plant cells (Emans et al., 2002) and, thus, might be expected to inhibit PEN1 relocalization by interfering with the recycling of PEN1 rather than by inhibiting the release of EVs.
Protein Content of Plant EVs
To better understand the protein content of plant EVs and gain insight into their potential roles, we analyzed density gradient-purified EVs from GFP-PEN1 plants using mass spectrometry. Our analysis identified 598 proteins from two replicates. One hundred seventy of these proteins were common to both replicates and had an average peptide count of at least two. Among the selected proteins, the chimeric GFP-PEN1 was one of the most prominent, confirming that GFP-PEN1-associated EVs are represented in the proteome (Supplemental Table S1).
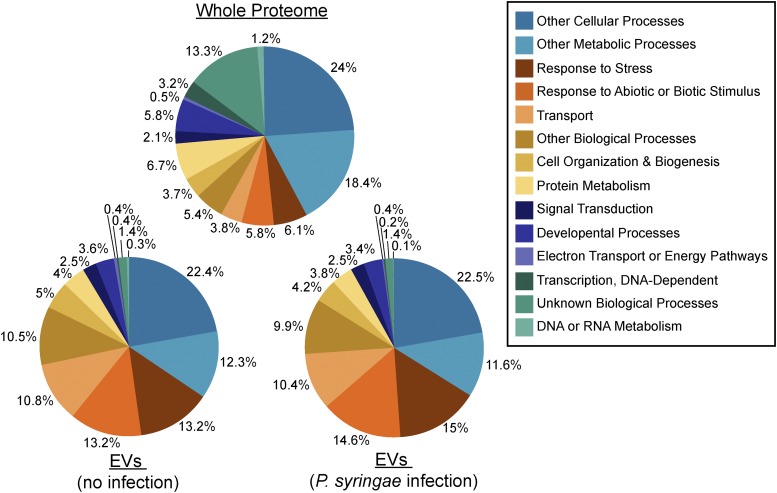
We next chose to analyze the EV proteome for Gene Ontology (GO) terms related to biological processes. Compared with the whole Arabidopsis proteome, the EV proteome was highly enriched for proteins involved in biotic and abiotic stress responses. Approximately 26% of the EV proteome was categorized under the GO terms responses to biotic or abiotic stimulus and response to stress. The genome-wide frequency for proteins with these GO terms is 11%, suggesting that EVs are specialized for roles in defense and stress adaptation (Fig. 5).
Figure 5.
Plant EVs are enriched for stress-response proteins. The entire Arabidopsis proteome (top), the EV proteome from uninfected plants (bottom left), and the EV proteome from P. syringae-infected plants (bottom right) were categorized based on GO terms through The Arabidopsis Information Resource Web site (www.arabidopsis.org). These analyses are based on the data given in Supplemental Table S1 and are derived from two biological replicates.
Among the defense-related proteins, we recognized several proteins involved in signal transmission, many of which are highly induced in response to stress and/or contribute to immunity. One of the most noteworthy of these proteins is RPM1-INTERACTING PROTEIN4 (RIN4). RIN4 interacts with the disease resistance proteins RESISTANCE TO PSEUDOMONAS SYRINGAE pv. MACULICOLA1 and RESISTANCE TO PSEUDOMONAS SYRINGAE2. Modification of RIN4 by bacterial effectors activates resistance protein signaling and initiates an immune response (Mackey et al., 2002, 2003). Interestingly, we also detected several proteins known to interact with RIN4, including ATPASE2 (AT4G30190), EARLY-RESPONSIVE TO DEHYDRATION4 (AT1G30360), REMORIN (AT3G61260), and DELTA(24)-STEROL REDUCTASE (AT3G19820; Mackey et al., 2002; Liu et al., 2009). The presence of RIN4 and other proteins involved in immune signaling suggests that EVs may play a role in microbe-associated molecular pattern- or effector-triggered immunity. It is tempting to speculate that, similar to mammalian exosomes, plant EVs modulate pathogen recognition by promoting the extracellular trafficking of key signaling proteins. Such trafficking may rapidly remove proteins from a cell to down-regulate signaling or spread signals to neighboring cells to amplify pathogen detection.
Other defense-related proteins included members of the myrosinase-glucosinolate system, such as the glucosinolate transporters PEN3 (AT1G78900; Stein et al., 2006; Meyer et al., 2009; Underwood and Somerville, 2013) and NRT1/PTR FAMILY2.10 (AT3G47960; Nour-Eldin et al., 2012) and the myrosinase EPITHIOSPECIFIC MODIFIER1 (AT3G14210; Zhang et al., 2006). Myrosinases and glucosinolates are localized to separate compartments in the cell and typically only interact after damage (Bones and Rossiter, 1996). Plant exosomes, however, may provide safe compartments for the storage and secretion of bioactive metabolites in intact cells (Underwood and Somerville, 2013). Once secreted, these EVs may function as concentrated packets of antimicrobial compounds. In this context, it is noteworthy that PEN3 is one of the most abundant EV proteins based on peptide counts (Supplemental Table S1) and has been shown to accumulate around powdery mildew haustoria (Underwood and Somerville, 2013).
The EV proteome also contained proteins involved in reactive oxygen species (ROS) signaling, such as PHOSPHOLIPASE Dα (PLDα; AT4G35790) and PLDδ (AT3G15730; Sang et al., 2001; Zhang et al., 2003), and oxidative stress responses, such as ANNEXIN1 (AT1G35720), ASCORBATE PEROXIDASE1 (AT1G07890), and GLUTATHIONE S-TRANSFERASE PHI2 (AT4G02520; Wagner et al., 2002; Davletova et al., 2005; Koussevitzky et al., 2008; Konopka-Postupolska et al., 2009). Combined, these proteins suggest that EVs may help modulate levels of ROS or contribute to ROS signaling. Glucosinolate metabolites induce extracellular ROS production (Hossain et al., 2013), so the pairing of proteins involved in the myrosinase-glucosinolate system and ROS signaling/protection in EVs may be functionally significant.
The EV proteome also was enriched for various membrane-trafficking proteins, including the syntaxins PEN1 (SYP121), SYP122 (AT3G52400), SYP132 (AT3G11820), and SYP71 (AT3G09740). PEN1, SYP122, and SYP132 belong to the same subfamily and facilitate transport to the PM. Significantly, all three have been shown to contribute to immunity (Nühse et al., 2003; Assaad et al., 2004; Kalde et al., 2007). Interestingly, SYP71 is localized mainly to the endoplasmic reticulum and is required for the infection of Arabidopsis by Turnip mosaic virus, where it plays a role in the fusion of virus-induced vesicles with chloroplasts (Wei et al., 2013). In Lotus japonicus, LjSYP71 contributes to symbiotic nitrogen fixation in nodules (Hakoyama et al., 2012).
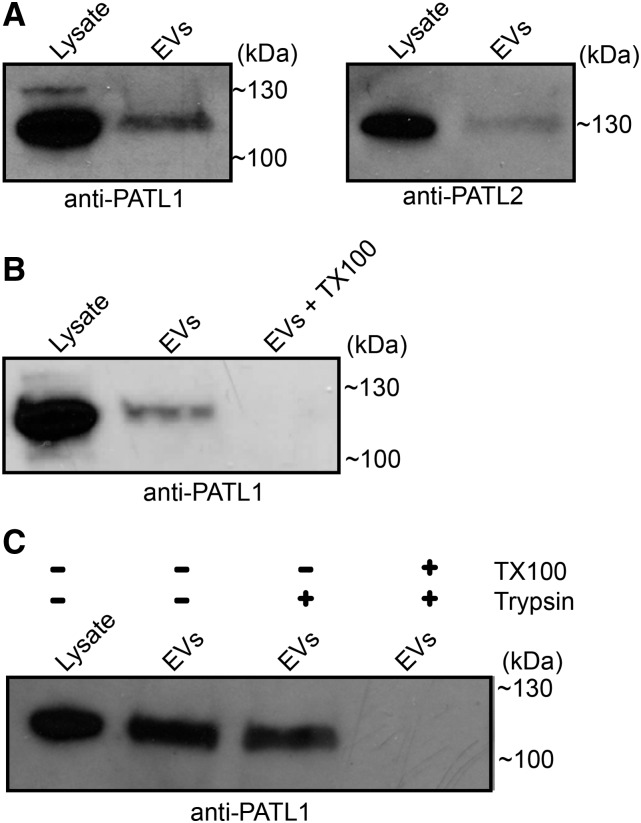
Other trafficking proteins included various RAB GTPases as well as PATELLIN1 (PATL1; AT1G72150) and PATL2 (AT1G22530). PATL1 and PATL2 both bind phosphoinositides and are thought to mediate vesicle transport and/or fusion (Petersen et al., 2014). PATL1 has been localized to the cell plate in dividing cells but also associates with PLASMODESMATA-LOCATED PROTEIN1 (PDLP1) during downy mildew infection and may colocalize with PDLP1 at the extrahaustorial membrane (Caillaud et al., 2014). We confirmed the presence of both PATL1 and PATL2 in the P40 fraction using anti-PATL antibodies (Peterman et al., 2004; Deng et al., 2007; Fig. 6A; Supplemental Fig. S1). We also showed that PATL1 is associated with membranous structures in the P40 fraction (Fig. 6B) and is protected from proteolytic digestion, which suggests that PATL1 is protected within the lumen of EVs (Fig. 6C). These findings not only validate our mass spectrometry data but also provide an additional marker for identifying EVs.
Figure 6.
Plant EVs are associated with PATL1 and PATL2. A, Immunoblots of the P40 fraction and whole-leaf protein extracts using PATL1 and PATL2 antibodies. B, Detergent treatment removes PATL1 from the P40 fraction. P40 fractions derived from Col-0 plants were treated with buffer alone or buffer plus TX100. Following recentrifugation, the sample treated with TX100 contained much less PATL1. C, PATL1 in the P40 fraction is protected from trypsin degradation in the absence of detergent. All experiments were repeated a minimum of two times with similar results.
Finally, the EVs contained numerous proteins for the transport of ions, water, sugar, and other substrates. The abundance of proton pumps in the EV proteome suggests that they actively transport ions and may regulate their own membrane potentials. Alternatively, vacuolar ATPases could have a dual role in vesicle trafficking and EV secretion. In C. elegans, the V0-ATPase complex promotes exosome secretion by mediating the fusion of MVBs with the apical PM (Liégeois et al., 2006). Plant EVs contain nearly all of the proteins required to form a complete V1/V0-ATPase complex.
Comparisons with Other Proteomes
To better understand the potential origins and content of EVs, we compared the list of EV proteins with other published proteomes for various subcellular regions/compartments, including the PM, plasmodesmata, Golgi complex, TGN/EE, MVBs/late endosomes, tonoplast, and clathrin-coated vesicle pits (Alexandersson et al., 2004; Fernandez-Calvino et al., 2011; Floerl et al., 2012; Heard et al., 2015). Overall, the EV proteome was most similar to compartments associated with RABF2b/ARA7, a RAB GTPase distributed between the TGN/EE and MVBs. Approximately 59% of EV proteins were reported to be present in the TGN/EE/MVB proteome (Table I). This level of similarity is consistent with our hypothesis that EVs are derived from the intraluminal vesicles of MVBs and, thus, are equivalent to mammalian exosomes. The EV proteome had no more than approximately 54% overlap with the remaining proteomes (Table I). Notably, we identified 23 EV proteins that were absent from the other endomembrane proteomes (Supplemental Table S2). These may represent useful markers for future work on EVs.
Table I. Comparison of the EV proteome with other plant subcellular proteomes.
Shared protein values indicate the percentages of EV proteins that were found in the indicated published proteomes.
| Proteome Source | Shared Proteins | Publication Source |
|---|---|---|
| RABF2b/ARA7 (TGN/EE/late endosome) | 58.58% | Heard et al. (2015) |
| RABD2a/ARA5 (Golgi/TGN/EE/secretory vesicles) | 53.85% | Heard et al. (2015) |
| Plasmodesmata | 53.25% | Fernandez-Calvino et al. (2011) |
| RABG3f (LE/MVB/tonoplast) | 50.89% | Heard et al. (2015) |
| RABF1/ARA6 (LE/MVB) | 49.70% | Heard et al. (2015) |
| PM | 49.11% | Alexandersson et al. (2004) |
| CLC2 (clathrin-coated vesicle pits) | 47.93% | Heard et al. (2015) |
| GOT1 (Golgi) | 44.97% | Heard et al. (2015) |
| VAMP711 (tonoplast) | 44.38% | Heard et al. (2015) |
When we compared GO terms for the EV proteome with the other endomembrane proteomes, we found that the percentages for various biological processes remained similar across all proteomes. However, the EV proteome had a higher percentage of stress-response proteins (Supplemental Table S3), suggesting that EVs may play a role in adapting to biotic and abiotic stress.
We also used software to predict signal peptides in the EV proteome. We found that only 16% of the proteins in the EV proteome have a predicted signal peptide, which supports our hypothesis that EV proteins reach the apoplast via a nonconventional secretory pathway. Combined, the above analyses suggest that plant EVs constitute a distinct subcellular compartment.
The Plant EV Proteome Changes Little in Response to P. syringae Infection
Mammalian cells modify the composition and function of exosomes during stress (Clayton et al., 2005; Beninson et al., 2014). We questioned whether plant cells also might modify the protein content of EVs during bacterial infection. In parallel with our proteomic analysis of EVs from uninfected plants, we examined the protein content of EVs from plants infected with P. syringae pv tomato strain DC3000 (pVSP61:empty), which is virulent on Col-0 (Axtell et al., 2003). Three days after spray inoculating GFP-PEN1 plants with P. syringae, we isolated and purified EVs for mass spectrometry analysis. Our analysis identified 647 proteins from two replicates, 142 of which were common to both replicates and had an average peptide count of at least two. These were chosen for further analysis (Supplemental Table S1). We also analyzed our samples for proteins belonging to P. syringae to assess whether we had copurified bacterial outer membrane vesicles. However, we failed to reproducibly detect P. syringae proteins between the two replicates.
Similar to the EV proteome from uninfected plants, EVs from infected plants were enriched for proteins involved in stress and defense. Approximately 29% of the proteome had GO terms of responses to biotic or abiotic stimulus or response to stress, a slight increase from the first proteome (Fig. 5). However, when we compared the two proteomes for protein content, we found that they differed very little. When we took into consideration proteins that were identified in only one replicate for either treatment, the differences between the two proteomes essentially vanished. Only three proteins were unique to the EV proteome from infected plants, while eight proteins were unique to the EV proteome from uninfected plants. None of the proteins unique to either treatment have strong connections to immunity. While the overall identity of proteins associated with EVs did not vary significantly with treatment, some proteins did have a much higher average peptide count in samples from infected plants. This suggests that, while alterations to the EV protein content may occur under stress, these changes are slight and likely involve differences in quantity rather than identity.
EV Secretion Is Enhanced during Biotic Stress
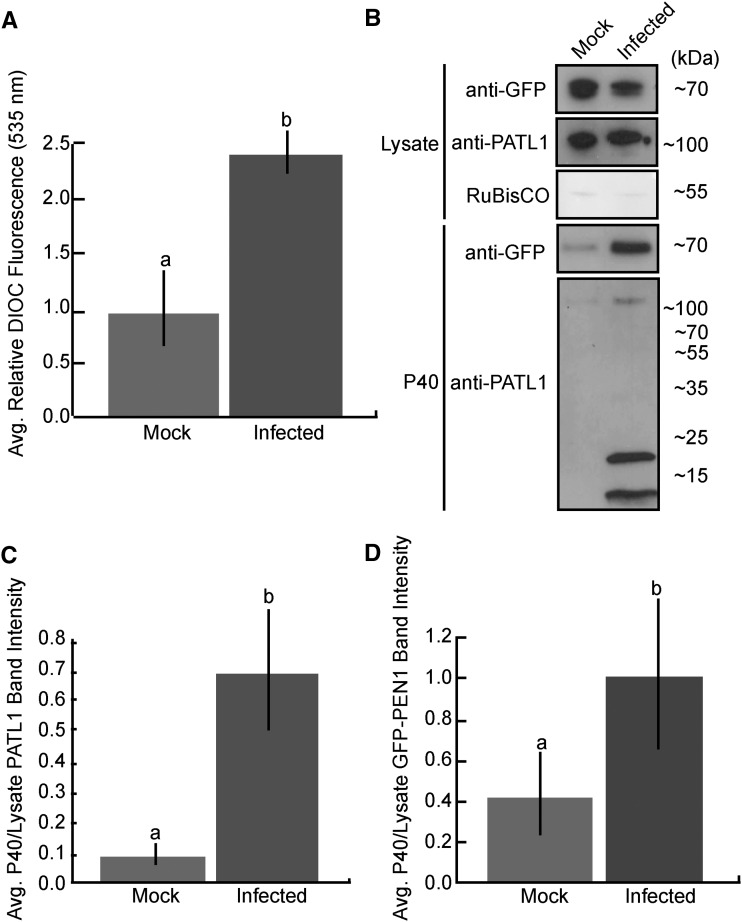
MVBs proliferate in plant cells challenged with pathogens, suggesting an increase in exosome secretion (An et al., 2006a, 2006b; Wang et al., 2014). Mammalian exosome secretion also is enhanced during stress (Clayton et al., 2005; Hedlund et al., 2011). To test whether plant EVs are secreted in greater abundance during infection, we infected GFP-PEN1 Arabidopsis with P. syringae. Three days after inoculation, we isolated EVs, quantified total membrane content in the P40 fraction, and probed it for GFP-PEN1 and PATL1 using an immunoblot. These analyses revealed a dynamic increase in EV secretion during P. syringae infection. Infected plants secreted over twice as many EVs as uninfected controls (Fig. 7A). This increase in vesicle content was corroborated by an increase in GFP-PEN1 and PATL1 signal in the P40 fraction of infected plants (Fig. 7, B–D).
Figure 7.
EV secretion is enhanced during P. syringae infection. A, Total membrane content in the P40 fraction increases over 2-fold following spray inoculation with a virulent P. syringae strain. Col-0 Arabidopsis plants were sprayed with either P. syringae DC3000 (pVPS61:empty) at an optical density at 600 nm of 0.2 or a control solution lacking bacteria. EVs were isolated 3 d after the initial infection from three sets of plants per treatment. Each sample of EVs was stained with 3,3′-dihexyloxacarbocyanine iodide (DiOC6), washed, and repelleted. Fluorescence intensity was quantified (error bars indicate sd; n = 3; P < < 0.001 using a two-tailed unpaired Student’s t test). B to D, PATL1 and GFP-PEN1 contents in the P40 fraction increase over 2-fold following P. syringae infection. B, Representative P40 fractions for both treatments, as well as samples of total cellular lysate, were immunoblotted for full-length PATL1. The levels of PATL1 are higher in the P40 fraction from infected plants compared with the P40 fraction from mock-infected plants, as indicated by a more intense band around 130 kD and the appearance of strong degradation products around 20 and 10 kD. C and D, Quantification of PATL1 and GFP-PEN1 band intensities from B. The experiment was repeated three times with similar results. The results of only one experiment are shown.
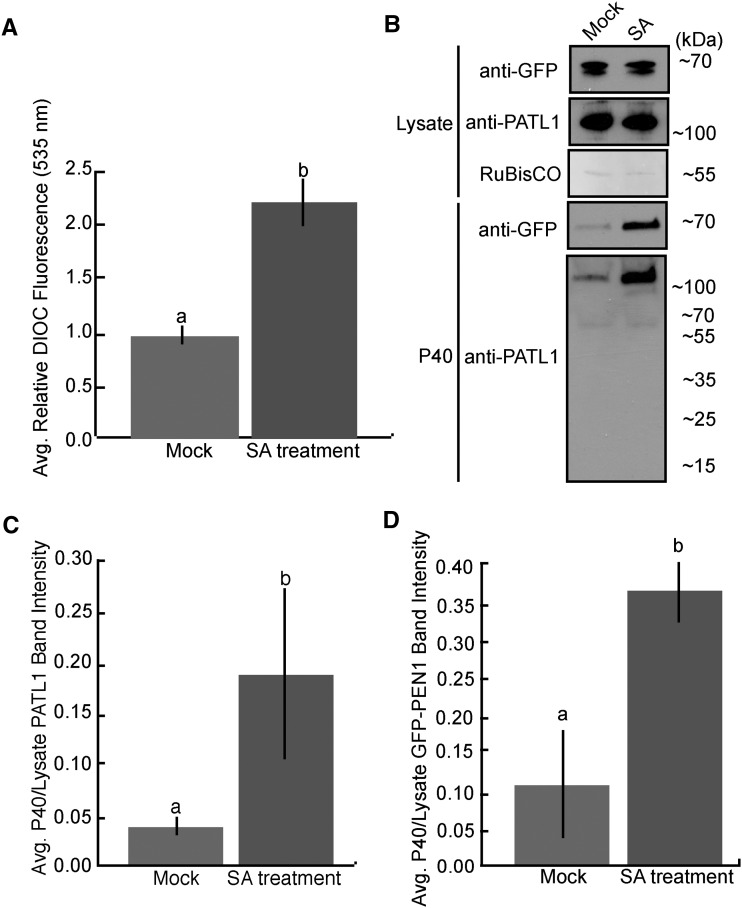
In order to determine if enhanced EV secretion is a general immune response or a specific reaction to P. syringae, we treated GFP-PEN1 plants with the defense hormone SA and isolated EVs 12 h later. Similar to P. syringae-infected plants, the P40 fraction derived from SA-treated plants contained twice as much membranous material as the P40 fraction from mock-treated plants (Fig. 8A). An immunoblot of the P40 fractions revealed a similar increase in GFP-PEN1 and PATL1 (Fig. 8, B–D). Interestingly, during an infection, the majority of the PATL1 signal in P40 fractions was present as degradation products of 10 and 20 kD. These bands were not present in the P40 fractions from SA-treated plants. Combined, the data suggest that enhanced EV secretion is a general immune response.
Figure 8.
EV secretion is enhanced after SA treatment. A, Total membrane content in the P40 fraction increases over 2-fold following treatment with SA. Col-0 Arabidopsis plants were sprayed with either 2 mm SA or a control solution lacking SA. EVs were isolated 12 h after spraying from three sets of plants per treatment. Each sample of EVs was stained with DiOC6, washed, and repelleted. Fluorescence intensity was quantified (error bars indicate sd; n = 3; P < < 0.001 using a two-tailed unpaired Student’s t test). B to D, PATL1 and GFP-PEN1 levels in the P40 fraction increase over 3-fold following SA treatment. B, Representative P40 fractions, as well as samples of total cellular lysate, were immunoblotted for full-length PATL1. The levels of PATL1 are higher in the P40 fraction from SA-treated plants, as indicated by a more intense band around 130 kD. C and D, Quantification of PATL1 and GFP-PEN1 band intensities from B. The experiment was repeated three times with similar results. The results of only one experiment are shown.
CONCLUSION
Reports of plant EVs date back to the late 1960s, but their function and composition remain poorly understood. To our knowledge, this is the first study to isolate and purify plant EVs from leaves. We also have established plant EV markers and methods for the quantification of secreted vesicles. Our analyses have revealed that plant EV production is enhanced in response to biotic stress and that they are enriched for defense/stress-related proteins. Based on these findings, we expect plant EVs to play a prominent role in immunity. Previous studies have shown that EVs contribute to the formation of defensive papillae. They also may function to deliver antimicrobial compounds into invading pathogens or possibly stimulate immune responses in neighboring cells. How exosomes pass through the plant cell wall is not clear, but the fact that we can isolate them from apoplastic wash fluids confirms that they do.
Past research has only been able to observe localized GFP-PEN1 secretion in the context of powdery mildew infections, but our findings suggest that GFP-PEN1 is constitutively secreted at some level inside EVs. The focal accumulation of GFP-PEN1 around fungal haustoria is easily observable using confocal microscopy. In contrast, EV secretion under normal circumstances is likely diffuse and, therefore, not as easy to detect. Constitutive EV secretion could provide some basal level of apoplastic defense. However, EV secretion may be a rapid process, and we cannot yet rule out the possibility that buffer infiltration somehow stimulates EV secretion.
The ability to isolate Arabidopsis exosomes represents an exciting opportunity to better understand plant immunity and intercellular signaling. In this study, we have laid the groundwork for asking bigger questions about plant EVs, such as how EVs contribute to plant immunity, which genetic pathways contribute to EV biogenesis, secretion, and uptake, and, most importantly, whether EVs play a role in intercellular RNA transport.
MATERIALS AND METHODS
Plant Lines and Growth Conditions
The following transgenic plant lines were used in this study (all Col-0 background): 35S::GFP-PEN1 (Meyer et al., 2009), native promoter::GFP-PEN1 (Yang et al., 2014), SYP61-CFP (Robert et al., 2008), ARA6-sYFP and GmMANI49-sYFP (Gu and Innes, 2012), and GFP-LTI6b (Cutler et al., 2000). Arabidopsis (Arabidopsis thaliana) seeds were surface sterilized with 50% bleach and plated on 0.5× Murashige and Skoog medium containing 0.8% agar. The seeds were vernalized for 2 d at 4°C before being moved to short-day conditions (9-h days, 22°C, and 150 µE m−2 s−1). After 1 week, the seedlings were transferred to Pro-Mix PGX. Plants were grown for 5 to 7 weeks before harvest.
Vesicle Isolation
Vesicles were isolated from the apoplastic wash of Arabidopsis rosettes. Whole rosettes were harvested at the root and vacuum infiltrated with vesicle isolation buffer (VIB; 20 mm MES, 2 mm CaCl2, and 0.1 m NaCl, pH 6). This buffer was based on that used by Regente et al. (2009), who isolated vesicles from imbibed sunflower (Helianthus annuus) seeds. We modified their buffer by switching from a Tris buffer at pH 7.5 to a MES buffer at pH 6, adding 2 mm CaCl2, and eliminating β-mercaptoethanol. These changes were made in order to more closely mimic native apoplastic fluids, with the expectation that we would do less damage to cell walls and cells. However, we observed no obvious difference in our ability to extract vesicles, and no further optimization was attempted.
Infiltrated plants were carefully blotted to remove excess fluid, placed inside 30-mL syringes, and centrifuged in 50-mL conical tubes at 700g for 20 min at 2°C (JA-14 rotor, Avanti J-20 XP centrifuge; Beckman Coulter). The resulting apoplastic wash was filtered through a 0.45-μm membrane and centrifuged successively at 10,000g for 30 min, 40,000g for 60 min, and 100,000g for 60 min at 2°C (TLA100.3, Optima TLX ultracentrifuge; Beckman Coulter). The 40,000g and 100,000g pellets (P40 and P100, respectively) were retained and resuspended in VIB for further analysis. For a majority of the experiments, only the 40,000g pellet was isolated. In these cases, the pellet was washed once with 3 mL of chilled VIB and recentrifuged at 40,000g for 60 min before final suspension in VIB.
Discontinuous OptiPrep Gradient Vesicle Purification
To prepare discontinuous iodixanol gradients (OptiPrep; Sigma-Aldrich), solutions of 40% (v/v), 20% (v/v), 10% (v/v), and 5% (v/v) iodixanol were created by diluting an aqueous 60% OptiPrep stock solution in VIB. The gradient was formed by carefully layering 3 mL of 40% solution, 3 mL of 20% solution, 3 mL of 10% solution, and 2 mL of 5% solution in a 14- × 89-mm ultra-clear centrifuge tube (Beckman Coulter). Approximately 0.5 mL of vesicles resuspended in VIB was layered on top of the gradient. Centrifugation was performed at 100,000g for 17 h at 2°C (SW41, Optima XPN-100 ultracentrifuge; Beckman Coulter). When the vesicles were initially purified to determine their density, 12 fractions were manually collected from the top of the gradient to the bottom. The first fraction consisted of 3.5 mL. The second fraction was 1.7 mL. Fractions 3 to 7 were 0.6 mL each, and fractions 8 to 12 were 0.7 mL each. The density of each fraction was determined using a mock density gradient as described by Schröder et al. (1997), except that the fractions were diluted 1:1,000 in deionized water and A244 was measured using a NanoDrop 2000 (Thermo Scientific). When vesicles were purified for mass spectrometry, the first 4.5 mL at the top of the gradient was discarded. After that, 3 volumes of 0.7 mL were collected. These fractions were each brought up to 3.5 mL with VIB and centrifuged at 100,000g for 60 min at 2°C (TLA100.3, Optima TLX ultracentrifuge; Beckman Coulter). The pellets were washed with 3.5 mL of VIB and repelleted using the same centrifugation conditions. The resulting pellets were combined by resuspending them in a total of 50 μL of VIB or 150 μL of 20 mm Tris-HCl, pH 7.8, for immunoblot and mass spectrometry analyses, respectively.
Pseudomonas syringae Infections and SA Treatment
P. syringae strain DC3000 (pVSP61:empty) was grown as a lawn on King’s Medium B agar overnight at 30°C. The bacterial lawn was scraped from the plate and resuspended to an optical density at 600 nm of 0.2 using 10 mm MgCl2 plus 0.01% Silwet L77. Col-0 Arabidopsis plants were sprayed with the bacterial solution or a control solution lacking bacteria. Plastic domes were placed over the plants overnight to maintain high humidity and removed the following morning. Three days after the initial infection, apoplastic wash was collected from three sets of plants of both infected and mock-infected plants. The samples from each set of plants were kept separate from each other during the course of the experiment to control for both biological and technical variations.
For SA treatment, plants were sprayed with either 20 mm MES, pH 6, + 0.01% Silwet (control solution) or 2 mm SA, 20 mm MES, pH 6, + 0.01% Silwet. Plastic domes were placed over the plants overnight to maintain high humidity. Twelve hours after SA treatment, apoplastic wash was collected from three sets of plants of both SA- and mock-treated plants. The samples were kept separate from each other to control for both biological and technical variations.
Immunoblots
For immunoblots, 40 µL of resuspended vesicles in VIB was combined with 10 µL of 5× SDS loading buffer (250 mm Tris-HCl, pH 6.8, 8% SDS, 0.1% Bromophenol Blue, 40% glycerol, and 400 mm dithiothreitol) and heated at 95°C for 5 min. Leaf lysate samples were used as positive controls. The lysate was prepared by freezing 250 mg of leaf tissue in liquid nitrogen and grinding with a mortar and pestle. Ground leaf tissue was extracted in 500 µL of protein extraction buffer (150 mm NaCl, 50 mm Tris HCl, pH 7.5, 0.1% Nonidet P-40, and 1% plant protease inhibitor cocktail [Sigma-Aldrich]) and centrifuged at 10,000 rpm for 5 min at 4°C to pellet debris. Forty microliters of leaf lysate was combined with 10 µL of 5× SDS loading buffer, and the mixture was heated at 95°C for 5 min. All samples were loaded on 4% to 20% Precise Protein Gels (Thermo Scientific) and separated at 100 V for 1 h in BupH Tris-HEPES-SDS running buffer (Thermo Scientific). The proteins were transferred to a nitrocellulose membrane (GE Water & Process Technologies). Ponceau staining was used to confirm the equal loading of samples and successful transfer. Membranes were washed with 1× Tris-buffered saline (50 mm Tris-Cl and 150 mm NaCl, pH 7.5) containing 0.1% Tween 20 (TBST) and blocked with 5% Difco Skim Milk (BD) overnight at 4°C. Membranes were incubated with monoclonal mouse anti-GFP or anti-mCherry antibody (Abcam) at a 1:3,000 dilution or polyclonal anti-PATL1 (Peterman et al., 2004; Deng et al., 2007) at a 1:5,000 dilution for 1 h, washed with TBST, and incubated with horseradish peroxidase-labeled goat anti-mouse or anti-rabbit antibody (Abcam) at a 1:5,000 dilution for 1 h. After a final wash in TBST, protein bands were imaged using Immuno-Star Reagents (Bio-Rad) or Supersignal West Femto Maximum Sensitivity Substrates (Thermo Scientific) and x-ray film. Band intensities were quantified using the FlourChem E system with AlphaView SA software (version 3.4.0; ProteinSimple).
Fluorometric Quantification of EVs
For fluorometric assays, P40 fractions were resuspended in 100 μL of 10 μm DiOC6 (ICN Biomedicals) diluted with MES buffer (20 mm MES, pH 6) plus 1% plant protease inhibitor cocktail (Sigma-Aldrich) and 2 mm 2,2′-dipyridyl disulfide. The resuspended P40 fractions were incubated at 37°C for 10 min, washed with 3 mL of MES buffer, repelleted (40,000g, 60 min, at 2°C; TLA100.3, Optima TLX Ultracentrifuge; Beckman Coulter), and resuspended in fresh MES buffer. DiOC6 fluorescence was recorded using an Appliskan plate reader (Thermo Electron) and 96-well, half-area plates (lot no. 16915037; Costar). Fluorescence intensity was measured at 485 nm excitation and 535 nm emission (using filters C00005X/E00035M) for 500 ms.
Dynamic Light Scattering
Light-scattering analyses were performed using a Zetasizer Nano-S (Malvern Instruments) and a 50-μL quartz cuvette. Samples were equilibrated at 20°C for 2 min followed by two readings consisting of 10 measurements each.
Confocal Microscopy and TEM
To detect vesicle-associated GFP fluorescence in ultracentrifuge fractions, resuspended pellets were examined using a Leica SP5 AOBS inverted confocal microscope (Leica Microsystems) equipped with 320 numerical aperture 0.7 and 363 numerical aperture 1.3 water objectives. GFP fluorescence was detected using an argon laser (488 nm excitation) and a 495- to 550-nm emission range.
For TEM, 6 μL of vesicles resuspended in VIB was applied to 3.05-mm copper Formvar-carbon-coated electron microscopy grids (Electron Microscopy Sciences). Prior to applying the samples, the grids were glow discharged for 45 s. Samples were wicked off using filter paper, and the grids were washed in 60 μL of 2% uranyl acetate. The grids were allowed to air dry and imaged at 80 kV using a JEM-1010 transmission electron microscope (JEOL USA).
For cryo-electron microscopy, 6 μL of vesicles was applied to copper Quantifoil grids (R1.2/1.3; Electron Microscopy Sciences). Prior to freezing, the grids were glow discharged for 45 s on each side. Grids were frozen using a Vitrobot (22°C, 100% humidity, blot time of 4 s, drain time of 2 s, and −2-mm offset to the grid’s position; FEI). The frozen samples were analyzed using a JEM 3200FS transmission electron microscope (JEOL USA).
Protease Protection Assay
To test if GFP-PEN1 is protected within the lumen of plant vesicles, P40 fractions derived from 35S::GFP-PEN1 plants were resuspended in 30 μL of Tris-HCl, pH 7.8. Resuspended vesicles received one of three different treatments: (1) no treatment; (2) 1% TX100; or (3) 1% TX100 followed by 1 μg mL−1 trypsin (Promega). For treatment with TX100, 6 μL of 6% TX100 was added. The samples were kept on ice for 30 min with occasional mixing. For trypsin digestion, 4 μL of 10 μg mL−1 trypsin in 1 mm HCl was added to TX100-treated samples. The samples were incubated at 37°C for 1 h before being processed for an immunoblot.
BFA Treatment
For BFA treatment, a 50 mm stock solution of BFA (ApexBio) was prepared using methanol. The stock solution was diluted in deionized water to create a working solution of 300 µm BFA. Arabidopsis plants were hand infiltrated with the working BFA solution or a mock solution containing an equivalent amount of methanol. The plants were covered with a dome after infiltration to maintain humidity. Twenty hours after infiltration, apoplastic wash was collected from three biological replicates for both treatments. EVs were isolated from equal amounts of apoplastic wash for each sample. The P40 fractions were probed for GFP-PEN1 using an immunoblot.
Mass Spectrometry
EVs were isolated from native promoter::GFP-PEN1 transgenic Arabidopsis that contained a T-DNA insertion in the native PEN1 gene (Collins et al., 2003). Samples were denatured in a solution of 8 m urea, then incubated for 45 min at 57°C with 2.1 mm dithiothreitol to reduce Cys residue side chains. These side chains were then alkylated with 4.2 mm iodoacetamide for 1 h in the dark at 21°C. The urea was diluted to 1 m, then 1 μg of trypsin was added and the samples were digested for 12 h at 37°C. The resulting peptides were desalted using a ZipTip (Millipore). The samples were dried down and injected into an Eksigent HPLC device coupled to an LTQ Velos mass spectrometer (Thermo Fisher Scientific) operating in top-eight data-dependent tandem mass spectrometry selection. The peptides were separated using a 75-μm, 15-cm column packed in house with C18 resin (Michrom Bioresources) at a flow rate of 300 nL min−1. A 2-h gradient was run from buffer A (2% acetonitrile and 0.1% formic acid) to 60% buffer B (100% acetonitrile and 0.1% formic acid). The resulting data were searched in Protein Prospector against the Arabidopsis proteome in UniProt (downloaded January 21, 2015). Carbamidomethylation of Cys residues was set as a fixed modification. Protein N-terminal acetylation, oxidation of Met, and pyro-Gln formation were set as variable modifications. A total of two variable modifications were allowed. The mass tolerance for parent and precursor ions was set to 0.6 D.
Proteins that were present in both replicates for a given treatment and had an average peptide count of no less than two were selected for further analysis. All proteomes were categorized based on GO annotation using The Arabidopsis Information Resource bulk data retrieval and analysis tools (https://www.arabidopsis.org/tools/bulk/go/index.jsp). Comparisons between the list of EV-associated proteins and other published proteomes were accomplished using the Bioinformatics & Evolutionary Genomics Venn Diagram software (bioinformatics.psb.ugent.be). Signal peptides were predicted using SignalP4.1 Server (http://www.cbs.dtu.dk/services/SignalP/; Petersen et al., 2011).
Accession Numbers
Arabidopsis Genome Initiative accession numbers for all proteins identified in our EVs are provided in Supplemental Table S1.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. The anti-PATL1 antibody is specific for PATL protein.
Supplemental Figure S2. Pretreatment with BFA does not affect EV recovery.
Supplemental Table S1. Plant EV proteome.
Supplemental Table S2. EV proteins not found in published endomembrane proteomes.
Supplemental Table S3. Comparison of GO terms among Arabidopsis subcellular proteomes.
Supplementary Material
Acknowledgments
We thank Dr. Claire Walczak for the use of equipment and Dr. Stephanie Ems-McClung for technical advice; Dr. Jonathan Trinidad and the Laboratory for Mass Spectrometry at Indiana University for the proteomic analysis and help describing the methods involved; Indiana University’s Electron Microscopy Center, especially Drs. Barry Stein and David Morgan, for help with TEM, as well as the Indiana University Light Microscopy Imaging Center for access to the Leica SP5 confocal microscope; Drs. William Underwood and Xangdou Wei for providing 35S::GFP-PEN1 and NP::GFP-PEN1 seeds, respectively; and Dr. Kaye Peterman for providing antisera to PATL1 and PATL2.
Glossary
- EV
extracellular vesicle
- MVB
multivesicular body
- PM
plasma membrane
- TEM
transmission electron microscopy
- TX100
Triton X-100
- TGN/EE
trans-Golgi network/early endosome
- BFA
brefeldin A
- GO
Gene Ontology
- ROS
reactive oxygen species
- Col-0
Columbia-0
- SA
salicylic acid
- VIB
vesicle isolation buffer
- TBST
Tris-buffered saline containing 0.1% Tween 20
- DiOC6
3,3′-dihexyloxacarbocyanine iodide
Footnotes
This work was supported by the National Institute of General Medical Sciences of the National Institutes of Heath (grant no. R01 GM063761 to R.W.I.), by the Bridge Funding program of the Indiana University Office of the Vice Provost for Research, and by a Miller Fellowship from the Indiana University Foundation (to B.D.R.).
Articles can be viewed without a subscription.
References
- Admyre C, Johansson SM, Qazi KR, Filén JJ, Lahesmaa R, Norman M, Neve EP, Scheynius A, Gabrielsson S (2007) Exosomes with immune modulatory features are present in human breast milk. J Immunol 179: 1969–1978 [DOI] [PubMed] [Google Scholar]
- Agrawal GK, Jwa NS, Lebrun MH, Job D, Rakwal R (2010) Plant secretome: unlocking secrets of the secreted proteins. Proteomics 10: 799–827 [DOI] [PubMed] [Google Scholar]
- Akers JC, Gonda D, Kim R, Carter BS, Chen CC (2013) Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol 113: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandersson E, Saalbach G, Larsson C, Kjellbom P (2004) Arabidopsis plasma membrane proteomics identifies components of transport, signal transduction and membrane trafficking. Plant Cell Physiol 45: 1543–1556 [DOI] [PubMed] [Google Scholar]
- Aline F, Bout D, Amigorena S, Roingeard P, Dimier-Poisson I (2004) Toxoplasma gondii antigen-pulsed-dendritic cell-derived exosomes induce a protective immune response against T. gondii infection. Infect Immun 72: 4127–4137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altieri SL, Khan AN, Tomasi TB (2004) Exosomes from plasmacytoma cells as a tumor vaccine. J Immunother 27: 282–288 [DOI] [PubMed] [Google Scholar]
- An Q, Ehlers K, Kogel KH, van Bel AJ, Hückelhoven R (2006a) Multivesicular compartments proliferate in susceptible and resistant MLA12-barley leaves in response to infection by the biotrophic powdery mildew fungus. New Phytol 172: 563–576 [DOI] [PubMed] [Google Scholar]
- An Q, Hückelhoven R, Kogel KH, van Bel AJ (2006b) Multivesicular bodies participate in a cell wall-associated defence response in barley leaves attacked by the pathogenic powdery mildew fungus. Cell Microbiol 8: 1009–1019 [DOI] [PubMed] [Google Scholar]
- Asea A, Jean-Pierre C, Kaur P, Rao P, Linhares IM, Skupski D, Witkin SS (2008) Heat shock protein-containing exosomes in mid-trimester amniotic fluids. J Reprod Immunol 79: 12–17 [DOI] [PubMed] [Google Scholar]
- Assaad FF, Qiu JL, Youngs H, Ehrhardt D, Zimmerli L, Kalde M, Wanner G, Peck SC, Edwards H, Ramonell K, et al. (2004) The PEN1 syntaxin defines a novel cellular compartment upon fungal attack and is required for the timely assembly of papillae. Mol Biol Cell 15: 5118–5129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axtell MJ, Chisholm ST, Dahlbeck D, Staskawicz BJ (2003) Genetic and molecular evidence that the Pseudomonas syringae type III effector protein AvrRpt2 is a cysteine protease. Mol Microbiol 49: 1537–1546 [DOI] [PubMed] [Google Scholar]
- Beckett K, Monier S, Palmer L, Alexandre C, Green H, Bonneil E, Raposo G, Thibault P, Le Borgne R, Vincent JP (2013) Drosophila S2 cells secrete wingless on exosome-like vesicles but the wingless gradient forms independently of exosomes. Traffic 14: 82–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beninson LA, Brown PN, Loughridge AB, Saludes JP, Maslanik T, Hills AK, Woodworth T, Craig W, Yin H, Fleshner M (2014) Acute stressor exposure modifies plasma exosome-associated heat shock protein 72 (Hsp72) and microRNA (miR-142-5p and miR-203). PLoS ONE 9: e108748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhlenius H, Mørch SM, Godfrey D, Nielsen ME, Thordal-Christensen H (2010) The multivesicular body-localized GTPase ARFA1b/1c is important for callose deposition and ROR2 syntaxin-dependent preinvasive basal defense in barley. Plant Cell 22: 3831–3844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bones AM, Rossiter JT (1996) The myrosinase-glucosinolate system, its organisation and biochemistry. Physiol Plant 97: 194–208 [Google Scholar]
- Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C (2005) Exosomal-like vesicles are present in human blood plasma. Int Immunol 17: 879–887 [DOI] [PubMed] [Google Scholar]
- Caillaud MC, Wirthmueller L, Sklenar J, Findlay K, Piquerez SJ, Jones AM, Robatzek S, Jones JD, Faulkner C (2014) The plasmodesmal protein PDLP1 localises to haustoria-associated membranes during downy mildew infection and regulates callose deposition. PLoS Pathog 10: e1004496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton A, Turkes A, Navabi H, Mason MD, Tabi Z (2005) Induction of heat shock proteins in B-cell exosomes. J Cell Sci 118: 3631–3638 [DOI] [PubMed] [Google Scholar]
- Collins NC, Thordal-Christensen H, Lipka V, Bau S, Kombrink E, Qiu JL, Hückelhoven R, Stein M, Freialdenhoven A, Somerville SC, et al. (2003) SNARE-protein-mediated disease resistance at the plant cell wall. Nature 425: 973–977 [DOI] [PubMed] [Google Scholar]
- Corrigan L, Redhai S, Leiblich A, Fan SJ, Perera SM, Patel R, Gandy C, Wainwright SM, Morris JF, Hamdy F, et al. (2014) BMP-regulated exosomes from Drosophila male reproductive glands reprogram female behavior. J Cell Biol 206: 671–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler SR, Ehrhardt DW, Griffitts JS, Somerville CR (2000) Random GFP:cDNA fusions enable visualization of subcellular structures in cells of Arabidopsis at a high frequency. Proc Natl Acad Sci USA 97: 3718–3723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davletova S, Rizhsky L, Liang H, Shengqiang Z, Oliver DJ, Coutu J, Shulaev V, Schlauch K, Mittler R (2005) Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell 17: 268–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Z, Zhang X, Tang W, Oses-Prieto JA, Suzuki N, Gendron JM, Chen H, Guan S, Chalkley RJ, Peterman TK, et al. (2007) A proteomics study of brassinosteroid response in Arabidopsis. Mol Cell Proteomics 6: 2058–2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakakaki G, van de Ven W, Pan S, Miao Y, Wang J, Keinath NF, Weatherly B, Jiang L, Schumacher K, Hicks G, et al. (2012) Isolation and proteomic analysis of the SYP61 compartment reveal its role in exocytic trafficking in Arabidopsis. Cell Res 22: 413–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebine K, Fujimoto M, Okatani Y, Nishiyama T, Goh T, Ito E, Dainobu T, Nishitani A, Uemura T, Sato MH, et al. (2011) A membrane trafficking pathway regulated by the plant-specific RAB GTPase ARA6. Nat Cell Biol 13: 853–859 [DOI] [PubMed] [Google Scholar]
- Ellinger D, Naumann M, Falter C, Zwikowics C, Jamrow T, Manisseri C, Somerville SC, Voigt CA (2013) Elevated early callose deposition results in complete penetration resistance to powdery mildew in Arabidopsis. Plant Physiol 161: 1433–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emans N, Zimmermann S, Fischer R (2002) Uptake of a fluorescent marker in plant cells is sensitive to brefeldin A and wortmannin. Plant Cell 14: 71–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Calvino L, Faulkner C, Walshaw J, Saalbach G, Bayer E, Benitez-Alfonso Y, Maule A (2011) Arabidopsis plasmodesmal proteome. PLoS ONE 6: e18880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floerl S, Majcherczyk A, Possienke M, Feussner K, Tappe H, Gatz C, Feussner I, Kües U, Polle A (2012) Verticillium longisporum infection affects the leaf apoplastic proteome, metabolome, and cell wall properties in Arabidopsis thaliana. PLoS ONE 7: e31435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds P, Mann DM, Allsop D (2012) Phosphorylated α-synuclein as a potential biomarker for Parkinson’s disease and related disorders. Expert Rev Mol Diagn 12: 115–117 [DOI] [PubMed] [Google Scholar]
- Geelen D, Leyman B, Batoko H, Di Sansebastiano GP, Moore I, Blatt MR (2002) The abscisic acid-related SNARE homolog NtSyr1 contributes to secretion and growth: evidence from competition with its cytosolic domain. Plant Cell 14: 387–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri PK, Schorey JS (2008) Exosomes derived from M. bovis BCG infected macrophages activate antigen-specific CD4+ and CD8+ T cells in vitro and in vivo. PLoS ONE 3: e2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Innes RW (2012) The KEEP ON GOING protein of Arabidopsis regulates intracellular protein trafficking and is degraded during fungal infection. Plant Cell 24: 4717–4730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- György B, Szabó TG, Pásztói M, Pál Z, Misják P, Aradi B, László V, Pállinger E, Pap E, Kittel A, et al. (2011) Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci 68: 2667–2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachez C, Laloux T, Reinhardt H, Cavez D, Degand H, Grefen C, De Rycke R, Inzé D, Blatt MR, Russinova E, et al. (2014) Arabidopsis SNAREs SYP61 and SYP121 coordinate the trafficking of plasma membrane aquaporin PIP2;7 to modulate the cell membrane water permeability. Plant Cell 26: 3132–3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakoyama T, Oi R, Hazuma K, Suga E, Adachi Y, Kobayashi M, Akai R, Sato S, Fukai E, Tabata S, et al. (2012) The SNARE protein SYP71 expressed in vascular tissues is involved in symbiotic nitrogen fixation in Lotus japonicus nodules. Plant Physiol 160: 897–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin W, Jensen WA (1967) Ultrastructural changes during growth and embryogenesis in carrot cell cultures. J Ultrastruct Res 18: 428–443 [DOI] [PubMed] [Google Scholar]
- Heard W, Sklenář J, Tomé DF, Robatzek S, Jones AM (2015) Identification of regulatory and cargo proteins of endosomal and secretory pathways in Arabidopsis thaliana by proteomic dissection. Mol Cell Proteomics 14: 1796–1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund M, Nagaeva O, Kargl D, Baranov V, Mincheva-Nilsson L (2011) Thermal- and oxidative stress causes enhanced release of NKG2D ligand-bearing immunosuppressive exosomes in leukemia/lymphoma T and B cells. PLoS ONE 6: e16899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund M, Stenqvist AC, Nagaeva O, Kjellberg L, Wulff M, Baranov V, Mincheva-Nilsson L (2009) Human placenta expresses and secretes NKG2D ligands via exosomes that down-modulate the cognate receptor expression: evidence for immunosuppressive function. J Immunol 183: 340–351 [DOI] [PubMed] [Google Scholar]
- Hood JL, Wickline SA (2012) A systematic approach to exosome-based translational nanomedicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol 4: 458–467 [DOI] [PubMed] [Google Scholar]
- Hossain MS, Ye W, Hossain MA, Okuma E, Uraji M, Nakamura Y, Mori IC, Murata Y (2013) Glucosinolate degradation products, isothiocyanates, nitriles, and thiocyanates, induce stomatal closure accompanied by peroxidase-mediated reactive oxygen species production in Arabidopsis thaliana. Biosci Biotechnol Biochem 77: 977–983 [DOI] [PubMed] [Google Scholar]
- Kalde M, Nühse TS, Findlay K, Peck SC (2007) The syntaxin SYP132 contributes to plant resistance against bacteria and secretion of pathogenesis-related protein 1. Proc Natl Acad Sci USA 104: 11850–11855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G, LeBlanc ML, Wafula EK, dePamphilis CW, Westwood JH (2014) Genomic-scale exchange of mRNA between a parasitic plant and its hosts. Science 345: 808–811 [DOI] [PubMed] [Google Scholar]
- Koga Y, Yasunaga M, Moriya Y, Akasu T, Fujita S, Yamamoto S, Matsumura Y (2011) Exosome can prevent RNase from degrading microRNA in feces. J Gastrointest Oncol 2: 215–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka-Postupolska D, Clark G, Goch G, Debski J, Floras K, Cantero A, Fijolek B, Roux S, Hennig J (2009) The role of annexin 1 in drought stress in Arabidopsis. Plant Physiol 150: 1394–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koussevitzky S, Suzuki N, Huntington S, Armijo L, Sha W, Cortes D, Shulaev V, Mittler R (2008) Ascorbate peroxidase 1 plays a key role in the response of Arabidopsis thaliana to stress combination. J Biol Chem 283: 34197–34203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liégeois S, Benedetto A, Garnier JM, Schwab Y, Labouesse M (2006) The V0-ATPase mediates apical secretion of exosomes containing Hedgehog-related proteins in Caenorhabditis elegans. J Cell Biol 173: 949–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Elmore JM, Coaker G (2009) Investigating the functions of the RIN4 protein complex during plant innate immune responses. Plant Signal Behav 4: 1107–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey D, Belkhadir Y, Alonso JM, Ecker JR, Dangl JL (2003) Arabidopsis RIN4 is a target of the type III virulence effector AvrRpt2 and modulates RPS2-mediated resistance. Cell 112: 379–389 [DOI] [PubMed] [Google Scholar]
- Mackey D, Holt BF III, Wiig A, Dangl JL (2002) RIN4 interacts with Pseudomonas syringae type III effector molecules and is required for RPM1-mediated resistance in Arabidopsis. Cell 108: 743–754 [DOI] [PubMed] [Google Scholar]
- Meckes DG Jr, Shair KH, Marquitz AR, Kung CP, Edwards RH, Raab-Traub N (2010) Human tumor virus utilizes exosomes for intercellular communication. Proc Natl Acad Sci USA 107: 20370–20375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D, Pajonk S, Micali C, O’Connell R, Schulze-Lefert P (2009) Extracellular transport and integration of plant secretory proteins into pathogen-induced cell wall compartments. Plant J 57: 986–999 [DOI] [PubMed] [Google Scholar]
- Micali CO, Neumann U, Grunewald D, Panstruga R, O’Connell R (2011) Biogenesis of a specialized plant-fungal interface during host cell internalization of Golovinomyces orontii haustoria. Cell Microbiol 13: 210–226 [DOI] [PubMed] [Google Scholar]
- Mittelbrunn M, Gutiérrez-Vázquez C, Villarroya-Beltri C, González S, Sánchez-Cabo F, González MA, Bernad A, Sánchez-Madrid F (2011) Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun 2: 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebenführ A, Ritzenthaler C, Robinson DG (2002) Brefeldin A: deciphering an enigmatic inhibitor of secretion. Plant Physiol 130: 1102–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen ME, Feechan A, Böhlenius H, Ueda T, Thordal-Christensen H (2012) Arabidopsis ARF-GTP exchange factor, GNOM, mediates transport required for innate immunity and focal accumulation of syntaxin PEN1. Proc Natl Acad Sci USA 109: 11443–11448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen ME, Thordal-Christensen H (2012) Recycling of Arabidopsis plasma membrane PEN1 syntaxin. Plant Signal Behav 7: 1541–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nour-Eldin HH, Andersen TG, Burow M, Madsen SR, Jørgensen ME, Olsen CE, Dreyer I, Hedrich R, Geiger D, Halkier BA (2012) NRT/PTR transporters are essential for translocation of glucosinolate defence compounds to seeds. Nature 488: 531–534 [DOI] [PubMed] [Google Scholar]
- Nowara D, Gay A, Lacomme C, Shaw J, Ridout C, Douchkov D, Hensel G, Kumlehn J, Schweizer P (2010) HIGS: host-induced gene silencing in the obligate biotrophic fungal pathogen Blumeria graminis. Plant Cell 22: 3130–3141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nühse TS, Boller T, Peck SC (2003) A plasma membrane syntaxin is phosphorylated in response to the bacterial elicitor flagellin. J Biol Chem 278: 45248–45254 [DOI] [PubMed] [Google Scholar]
- Ohno S, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, Fujita K, Mizutani T, Ohgi T, Ochiya T, et al. (2013) Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther 21: 185–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanisamy V, Sharma S, Deshpande A, Zhou H, Gimzewski J, Wong DT (2010) Nanostructural and transcriptomic analyses of human saliva derived exosomes. PLoS ONE 5: e8577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MA, Hopmans ES, Lindenberg JL, de Gruijl TD, Würdinger T, Middeldorp JM (2010) Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci USA 107: 6328–6333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterman TK, Ohol YM, McReynolds LJ, Luna EJ (2004) Patellin1, a novel Sec14-like protein, localizes to the cell plate and binds phosphoinositides. Plant Physiol 136: 3080–3094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KE, Manangon E, Hood JL, Wickline SA, Fernandez DP, Johnson WP, Gale BK (2014) A review of exosome separation techniques and characterization of B16-F10 mouse melanoma exosomes with AF4-UV-MALS-DLS-TEM. Anal Bioanal Chem 406: 7855–7866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen TN, Brunak S, von Heijne G, Nielsen H (2011) SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods 8: 785–786 [DOI] [PubMed] [Google Scholar]
- Pigati L, Yaddanapudi SC, Iyengar R, Kim DJ, Hearn SA, Danforth D, Hastings ML, Duelli DM (2010) Selective release of microRNA species from normal and malignant mammary epithelial cells. PLoS ONE 5: e13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisitkun T, Shen RF, Knepper MA (2004) Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci USA 101: 13368–13373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politis DJ, Goodman RN (1978) Localized cell-wall appositions: incompatibility response of tobacco leaf-cells to Pseudomonas pisi. Phytopathology 68: 309–316 [Google Scholar]
- Prado N, Marazuela EG, Segura E, Fernández-García H, Villalba M, Théry C, Rodríguez R, Batanero E (2008) Exosomes from bronchoalveolar fluid of tolerized mice prevent allergic reaction. J Immunol 181: 1519–1525 [DOI] [PubMed] [Google Scholar]
- Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ (1996) B lymphocytes secrete antigen-presenting vesicles. J Exp Med 183: 1161–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regente M, Corti-Monzón G, Maldonado AM, Pinedo M, Jorrín J, de la Canal L (2009) Vesicular fractions of sunflower apoplastic fluids are associated with potential exosome marker proteins. FEBS Lett 583: 3363–3366 [DOI] [PubMed] [Google Scholar]
- Ridder K, Keller S, Dams M, Rupp AK, Schlaudraff J, Del Turco D, Starmann J, Macas J, Karpova D, Devraj K, et al. (2014) Extracellular vesicle-mediated transfer of genetic information between the hematopoietic system and the brain in response to inflammation. PLoS Biol 12: e1001874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert S, Chary SN, Drakakaki G, Li S, Yang Z, Raikhel NV, Hicks GR (2008) Endosidin1 defines a compartment involved in endocytosis of the brassinosteroid receptor BRI1 and the auxin transporters PIN2 and AUX1. Proc Natl Acad Sci USA 105: 8464–8469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saman S, Kim W, Raya M, Visnick Y, Miro S, Saman S, Jackson B, McKee AC, Alvarez VE, Lee NC, et al. (2012) Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J Biol Chem 287: 3842–3849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang Y, Cui D, Wang X (2001) Phospholipase D and phosphatidic acid-mediated generation of superoxide in Arabidopsis. Plant Physiol 126: 1449–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder M, Schäfer R, Friedl P (1997) Spectrophotometric determination of iodixanol in subcellular fractions of mammalian cells. Anal Biochem 244: 174–176 [DOI] [PubMed] [Google Scholar]
- Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT Jr, Carter BS, Krichevsky AM, Breakefield XO (2008) Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 10: 1470–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skriner K, Adolph K, Jungblut PR, Burmester GR (2006) Association of citrullinated proteins with synovial exosomes. Arthritis Rheum 54: 3809–3814 [DOI] [PubMed] [Google Scholar]
- Stein M, Dittgen J, Sánchez-Rodríguez C, Hou BH, Molina A, Schulze-Lefert P, Lipka V, Somerville S (2006) Arabidopsis PEN3/PDR8, an ATP binding cassette transporter, contributes to nonhost resistance to inappropriate pathogens that enter by direct penetration. Plant Cell 18: 731–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street JM, Barran PE, Mackay CL, Weidt S, Balmforth C, Walsh TS, Chalmers RT, Webb DJ, Dear JW (2012) Identification and proteomic profiling of exosomes in human cerebrospinal fluid. J Transl Med 10: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DD, Gercel-Taylor C (2008) MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol 110: 13–21 [DOI] [PubMed] [Google Scholar]
- Thery C, Amigorena S, Raposo G, Clayton A (2006) Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol Chapter 3: Unit 3.22 [DOI] [PubMed] [Google Scholar]
- Théry C, Duban L, Segura E, Véron P, Lantz O, Amigorena S (2002) Indirect activation of naïve CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol 3: 1156–1162 [DOI] [PubMed] [Google Scholar]
- Ueda T, Uemura T, Sato MH, Nakano A (2004) Functional differentiation of endosomes in Arabidopsis cells. Plant J 40: 783–789 [DOI] [PubMed] [Google Scholar]
- Underwood W, Somerville SC (2013) Perception of conserved pathogen elicitors at the plasma membrane leads to relocalization of the Arabidopsis PEN3 transporter. Proc Natl Acad Sci USA 110: 12492–12497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Pol E, Böing AN, Harrison P, Sturk A, Nieuwland R (2012) Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev 64: 676–705 [DOI] [PubMed] [Google Scholar]
- Vojtech L, Woo S, Hughes S, Levy C, Ballweber L, Sauteraud RP, Strobl J, Westerberg K, Gottardo R, Tewari M, et al. (2014) Exosomes in human semen carry a distinctive repertoire of small non-coding RNAs with potential regulatory functions. Nucleic Acids Res 42: 7290–7304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner U, Edwards R, Dixon DP, Mauch F (2002) Probing the diversity of the Arabidopsis glutathione S-transferase gene family. Plant Mol Biol 49: 515–532 [DOI] [PubMed] [Google Scholar]
- Wang F, Shang Y, Fan B, Yu JQ, Chen Z (2014) Arabidopsis LIP5, a positive regulator of multivesicular body biogenesis, is a critical target of pathogen-responsive MAPK cascade in plant basal defense. PLoS Pathog 10: e1004243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei T, Zhang C, Hou X, Sanfaçon H, Wang A (2013) The SNARE protein Syp71 is essential for turnip mosaic virus infection by mediating fusion of virus-induced vesicles with chloroplasts. PLoS Pathog 9: e1003378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiberg A, Wang M, Lin FM, Zhao H, Zhang Z, Kaloshian I, Huang HD, Jin H (2013) Fungal small RNAs suppress plant immunity by hijacking host RNA interference pathways. Science 342: 118–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welton JL, Khanna S, Giles PJ, Brennan P, Brewis IA, Staffurth J, Mason MD, Clayton A (2010) Proteomics analysis of bladder cancer exosomes. Mol Cell Proteomics 9: 1324–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfers J, Lozier A, Raposo G, Regnault A, Théry C, Masurier C, Flament C, Pouzieux S, Faure F, Tursz T, et al. (2001) Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med 7: 297–303 [DOI] [PubMed] [Google Scholar]
- Wubbolts R, Leckie RS, Veenhuizen PT, Schwarzmann G, Möbius W, Hoernschemeyer J, Slot JW, Geuze HJ, Stoorvogel W (2003) Proteomic and biochemical analyses of human B cell-derived exosomes: potential implications for their function and multivesicular body formation. J Biol Chem 278: 10963–10972 [DOI] [PubMed] [Google Scholar]
- Xu HX, Mendgen K (1994) Endocytosis of 1,3-beta-glucans by broad bean cells at the penetration site of the cowpea rust fungus (haploid stage). Planta 195: 282–290 [Google Scholar]
- Yang L, Qin L, Liu G, Peremyslov VV, Dolja VV, Wei Y (2014) Myosins XI modulate host cellular responses and penetration resistance to fungal pathogens. Proc Natl Acad Sci USA 111: 13996–14001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeyen RJ, Bushnell WR (1979) Papilla response of barley epidermal cells caused by Erysiphe graminis: rate and method of deposition determined by microcinematography and transmission electron microscopy. Can J Bot 57: 898–913 [Google Scholar]
- Zhang W, Wang C, Qin C, Wood T, Olafsdottir G, Welti R, Wang X (2003) The oleate-stimulated phospholipase D, PLDδ, and phosphatidic acid decrease H2O2-induced cell death in Arabidopsis. Plant Cell 15: 2285–2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Ober JA, Kliebenstein DJ (2006) The gene controlling the quantitative trait locus EPITHIOSPECIFIER MODIFIER1 alters glucosinolate hydrolysis and insect resistance in Arabidopsis. Plant Cell 18: 1524–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.